- Cape Peninsula University of Technology, Cape Town, South Africa
Background: Individuals with Down syndrome (DS) are born with or develop many physical limitations which affect their quality of life. Although studies involving cerebral oxygenation in the general population have been performed, such a study is yet to be conducted on individuals with DS.
Method: Fifty-four participants (DS:27; non-DS:27) were tested for cerebral oxygenation during an incremental exercise test. Participants (38.9 ± 5.9 years) were tested for ΔHbO2 and ΔHHb using near-infrared spectroscopy. V̇O2 peak determination was performed with a standardised incremental treadmill protocol and the ventilatory threshold (VT) and respiratory compensation point (RCP) were determined.
Results: There were no significant differences for
Conclusion: A significant different evolution for ΔHbO2 over exercise intensity (80% VT, VT, RCP, VO2 peak) between sedentary adults with and without DS was found. This ΔHbO2 may possibly reflect previously reported executive function limitations and/or the many physiological limitations that individuals with DS are born with or develop over time. Such speculations would need to be tested with future cause-effect studies.
1 Introduction
Down syndrome (DS) is a genetic condition affecting chromosomes, with a prevalence of 1 in 650 live births in developing countries (DSSA, 2023). Individuals with DS have a unique set of physical characteristics such as short stature, small low-set ears, flat nasal bridge, small mouth, and many other unique characteristics (Antonarakis et al., 2020). They have physical conditions that hamper their ability to perform submaximal and maximal exercise such as muscle hypotonicity, ligamentous laxity, congenital heart defects and chronotropic incompetence (Foley and Killeen, 2019; Zahari et al., 2019; Fernhall et al., 2013; Guerra et al., 2003). Chronotropic incompetence may be caused by an inadequate catecholamines response to maximal exercise in individuals with DS (Fernhall et al., 2009).
A variety of parameters associated with maximal exercise can be improved with structured aerobic or anaerobic, or combined training interventions consisting of walking, running, interval training, cycling, swimming, resistance training and many other modalities (Boer, 2020; Hardee and Fetters, 2017; Boer and Moss, 2016; Kerstiens and Green, 2015; Li et al., 2013; Mendonca et al., 2013; Shields et al., 2013; Mendonca et al., 2011; Mendonca and Pereira, 2009; Carmeli et al., 2002). However, some aerobic exercise intervention studies show limited improvement in peak oxygen consumption (Varela et al., 2001; Millar et al., 1993).
During exercise, the demand for oxygen by the body increases to meet the metabolic needs of the working muscles. The brain, like other organs, also requires oxygen to function properly (Ainslie et al., 2016; Hall et al., 2012). Maintaining adequate cerebral oxygenation during exercise is crucial for maintaining cognitive function and preventing neurological injury (Mekari et al., 2015; Raichle and Gusnard, 2002).
Several factors affect cerebral oxygenation during exercise, including the intensity, duration, and type of exercise, as well as the individual’s fitness level and health status (Oussaidene et al., 2015). During high-intensity exercise, there is a reduction in cerebral oxygenation due to increased demand for oxygen by the working muscles and decreased blood flow to the brain (Mekari et al., 2015; Drigny et al., 2014). This can result in symptoms such as dizziness, light-headedness, and in extreme cases loss of consciousness. However, regular exercise training can improve cerebral oxygenation by increasing blood flow to the brain and promoting the growth of new blood vessels (Coetsee and Terblanche, 2017). Additionally, certain types of exercise, such as aerobic exercise, have been shown to improve cognitive function and reduce the risk of neurological disorders such as dementia and Alzheimer’s disease which are conditions commonly affecting individuals with DS (Fortea et al., 2021). It is also known that individuals with DS have pronounced limitations in executive function (EF) as reported in a meta-analytic study (Tungate and Conners, 2021). However, EF and cognition may be improved with exercise as EF is associated with cerebral oxygenation (Goenarjo et al., 2020; Mekari et al., 2019; Ogoh and Tarumi, 2019; Ogoh, 2017; Dupuy et al., 2015). Although these studies were conducted in the general population, it has been demonstrated that cognition (Merzbach et al., 2023; Ptomey et al., 2018) and EF (Ringenbach et al., 2021) can be significantly improved for individuals with DS, which is important since most individuals with DS exhibit cognitive impairment (Moncaster et al., 2010).
Monitoring cerebral oxygenation during exercise can be done through non-invasive techniques such as near-infrared spectroscopy (NIRS), which measures changes in blood oxygenation levels in the brain. Typically, cerebral oxygenation is monitored during rest and a maximal aerobic incremental test and assessed at specific breakpoint intensities (such as the ventilatory threshold and the respiratory compensation point (RCP)) and at peak or maximum intensity (Kojima et al., 2021; Stevens et al., 2018; Tempest et al., 2017; Oussaidene et al., 2015; Tempest and Parfitt, 2016; Jung et al., 2015). In most cases the change in cerebral oxygenation increases from rest to the ventilatory threshold and RCP and either plateaus until peak exercise (Tempest et al., 2017; Tempest and Parfitt, 2016; Jung et al., 2015) or increases (Stevens et al., 2018; Tempest and Parfitt, 2016) or decreases (Kojima et al., 2021; Oussaidene et al., 2015). The discrepancy between RCP and peak exercise could be related to fitness (De Wachter et al., 2021).
Such a study remains to be conducted in individuals with DS and could provide further insight into the physical limitations experienced by these individuals during submaximal and peak exercise. Consequently, the aim with such an exploratory study was to assess the cerebral oxygenation and deoxygenation in sedentary adults with DS compared to an age-matched non-DS sedentary control group.
2 Methods
2.1 Participants
A total of 54 sedentary adults (DS: 27; 27 non-DS) with a mean age of 38.9 years (±5.9) were tested for body mass, stature, and cerebral oxygenation during a standardised incremental VO2 peak test. Testing was conducted within a 4-week period. Information about the study and the consent form were distributed to the Executive Managers of three Intellectually Disabled Care Centres (IDCC) who redistributed the information and consent forms to the parents/guardians of adults with DS. Information about the study was also distributed to adults in the general population within the same area(s) of the three IDCC via social media platforms and the local newspapers.
Before participating in the study, participants or the legal guardians (for adults with DS) completed a consent form, an adapted consent form with plain language (for adults with DS) and an adapted physical activity readiness questionnaire (aPARQ).
Individuals of 25–50 years of age were eligible to participate in the study. Furthermore, adults diagnosed with DS (for the DS group) who had the cognitive ability to perform and understand all testing instructions were eligible for the study. Individuals who had not taken part in any structural physical activity (structured aerobic [including walking], anaerobic or resistance training activity) during the 6 months prior to the study were included. Any individuals suffering from congenital heart disease or any other chronic physical or mental conditions contraindicative to the demand of physical activity, or those who did not answer ‘yes’ to all the questions in the aPARQ were excluded. The DS and non-DS groups were not purposely matched for exact physical activity profile or body mass index. This information was collected by the primary guardian of the participant (for the DS group). Adults who could not walk on the treadmill without holding onto the handrails were also excluded.
2.2 Procedures
Ethical approval for this study was granted by the Faculty of Health Sciences Research Ethical Committee from the University of Pretoria (269/2023).
The participants visited the laboratory on three occasions. Upon the first visit, the participants’ body mass and stature were assessed. Participants wore a shirt and light weight trunks only. The body mass and stature measurements were used to calculate body mass index (BMI). The participants were familiarised with the cerebral oxygenation and maximal oxygen uptake equipment (first and second visit) as stipulated by Fernhall et al. (1990). They practiced walking on the treadmill using the standardised protocol adapted for adults with and without DS using gas exchange equipment (Boer, 2023; Fernhall et al., 1990). Upon the third visit, all participants performed the peak oxygen uptake test in association with cerebral oxygenation. More familiarisation sessions were conducted if deemed necessary.
All participants were tested using the Cosmed K5 CPET testing system (Cosmed, Rome, Italy) using a standardised treadmill protocol. The treadmill protocol started with an initial velocity of 4 km/h at an incline of 0%. After two-minute intervals, the treadmill incline increased by 2.5% until it reached an incline of 15%, whereafter the speed increased by 1.6 km/h after every minute until exhaustion was reached (Boer, 2023; Fernhall et al., 1990). The treadmill was calibrated, and the running belt moved freely with no obstructions. The
Changes in oxyhaemoglobin (HbO2) and deoxyhaemoglobin (HHb) was measured by a two-channel near-infrared spectrometer (NIRO 200NX, Hamamatsu, Japan) at rest and during completion of the peak oxygen uptake test. Prior to any measurements being taken, the participant’s forehead was cleaned with alcohol swabs to avoid false measurements caused by make-up, or other potential substances on the surface of the skin. Measurements were made with the sampling time set at 5 Hz (Hz) and at wavelengths of 735, 810, and 850 nm as determined by the manufacturer. The position of the measurement probes was calculated using the international 10–20 system for EEG electrode placement (Jurcak et al., 2007). The light emitter sensor was placed on the medial side of the forehead for the prefrontal cortex measurements, at Fp1 and Fp2 for the left- and right-hand side, respectively, with the detectors being placed between positions F3 and F7 on the left-hand side, and F4 and F8 on the right-hand side. The distance between each emitter and detector was 4 cm.
Before the exercise test was performed, participants were instructed to sit quietly for 5 min with their eyes closed while resting values were obtained. Seeing that it is not possible to assess optical path lengths with NIRS, relative changes in HbO2 and HHb with respect to baseline were analysed. The concentration of HbO2 represents the balance between oxygen distribution and oxygen use; HHb reflects oxygen extraction by the tissue (Coetsee and Terblanche, 2017). Furthermore, a white Velcro headband was placed around the head to ensure that the wires of the two measurement channels did not impede the participant in any way. Markers were placed and the time at the beginning and end of each event (end of rest or increase in treadmill incline or speed in maximal incremental test) in the testing procedure was recorded to ensure accuracy of start and end times. All cerebral hemodynamic recordings were averaged during the last 30 s of a 5-minute seated resting period and during the final 30 s of each incremental exercise stage including up to the point of peak exercise.
2.3 Statistical analysis
Data were analysed with the Statistical Package for the Social Sciences (SPSS 30.0, Chicago, IL, United States). A significance level of p < 0.05 was used. A sample size of 10 or more afforded adequate statistical power for between-group differences, using an alpha of 0.05 and a power of 0.80 from standard deviations in other studies where untrained individuals were tested (Stevens et al., 2018; Tempest and Parfitt, 2016). All data were tested for normality and homogeneity of variance with the Shapiro-Wilk statistic and the Levene’s test respectively. Data are presented as mean and standard deviation. Differences between groups at baseline, were evaluated using an independent t-Test for demographic, anthropometrical, submaximal and peak oxygen uptake.
A two-way ANOVA with repeated measures (80% VT, VT, RCP and End) and between groups (DS vs. non-DS) was used to identify the interaction effect of exercise intensity and group on ΔHbO2 and ΔHHb separately as conducted by Stevens et al. (2018) and Brugniaux et al. (2014). Consequently, the analysis involved eight separate ANOVA models, stratified by sex (male and female), haemoglobin species (HbO2 and HHb), and NIRS probe location (left and right prefrontal cortex). Greenhouse-Geisser corrections were applied if the assumption of sphericity was not met. Furthermore, a two-way ANOVA with repeated measures (80% VT, VT, RCP and End) and between groups (DS vs. non-DS) was used to identify the interaction effect of PETCO2 for males and females separately. When statistical significance was detected through the two-way ANOVA, all pairwise differences were identified using Bonferroni adjusted pairwise comparisons. Effect sizes associated with the F statistics were expressed as partial eta-squared (np2) defined as small (0.01), medium (0.06) and large (0.14) (Cohen et al., 1990).
3 Results
All 54 participants completed the
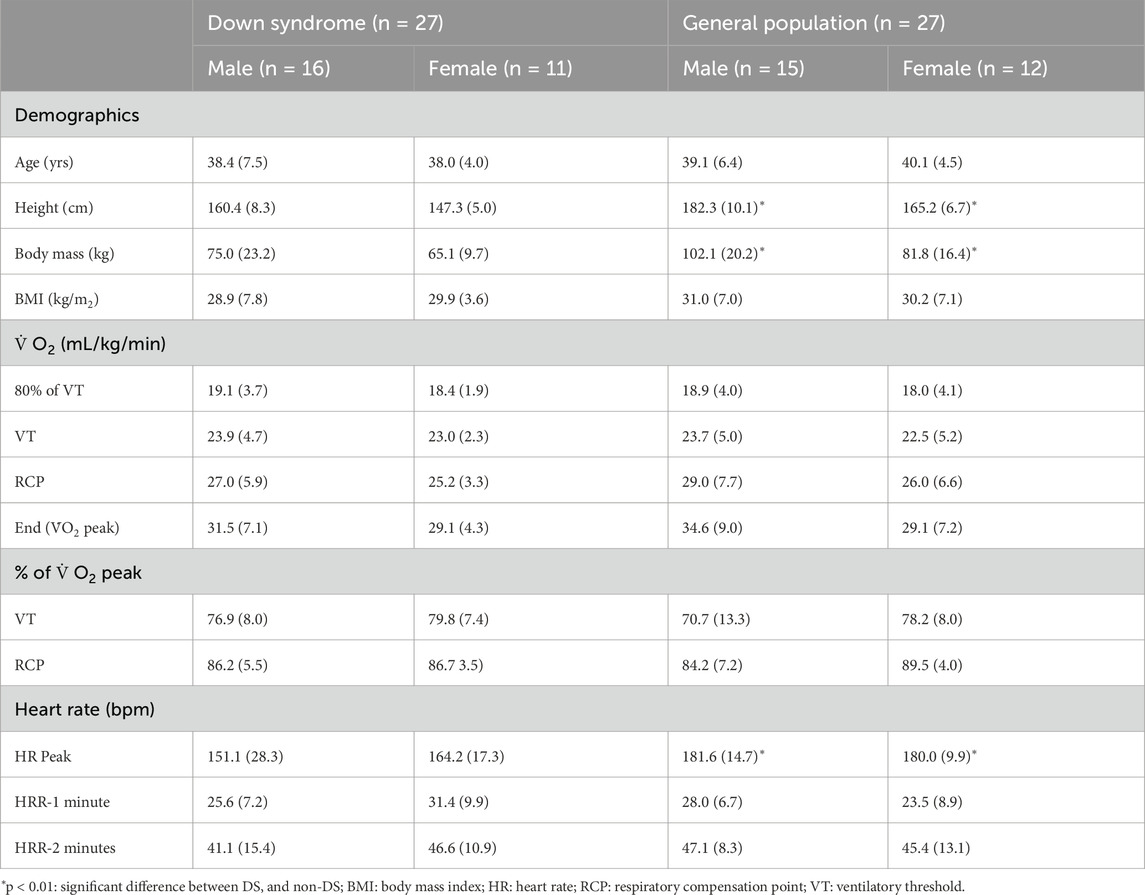
Table 1. Participant demographics and average 30-s oxygen uptake (
To determine whether any significant difference in submaximal and peak
3.1 Change in HbO2
Regarding the males, there was a significant interaction effect for group (DS vs. non-DS) and time (over exercise intensity) (F (2.558; 69.079) = 4.528; p = 0.009; np2 = 0.144) for the left prefrontal cortex (LPC) and the right prefrontal cortex (RPC) (F (2.123; 61.563) = 3.238; p = 0.026; np2 = 0.104). Similarly, there was a significant interaction effect for group and time (F (1.6; 33.604) = 5.636; p = 0.012; np2 = 0.212) for the LPC and the RPC (F (1.558; 29.593) = 6.305; p = 0.009; np2 = 0.249) for the females. All effect sizes were large (medium for males for RPC) for the interaction model.
Significant increases over exercise intensity (see # in Figure 1) with large effects sizes were reported for the males (LPC: F (2.558; 69.079) = 39.525, p < 0.001; np2 = 0.594; RPC: F (2.123; 61.563) = 26.762; p < 0.001; np2 = 0.489) and for the females (LPC: F (1.6; 33.604) = 6.917; p = 0.005; np2 = 0.248; RPC: F (1.558; 29.593) = 5.253; p = 0.044; np2 = 0.146). The post hoc Bonferroni analysis reported no significant differences (p > 0.05) from RCP to peak exercise for both hemispheres and both genders for DS and non-DS group (ΔHbO2 continued to increase for all males and for females with DS; but not for females in the non-DS group). Significant post hoc differences between DS and non-DS for RCP and peak exercise are reported for the males only (LPC and RPC) (See * in Figure 1). Significant within group post hoc increases are reported for exercise intensity versus VT80% (See $ in Figure 1).
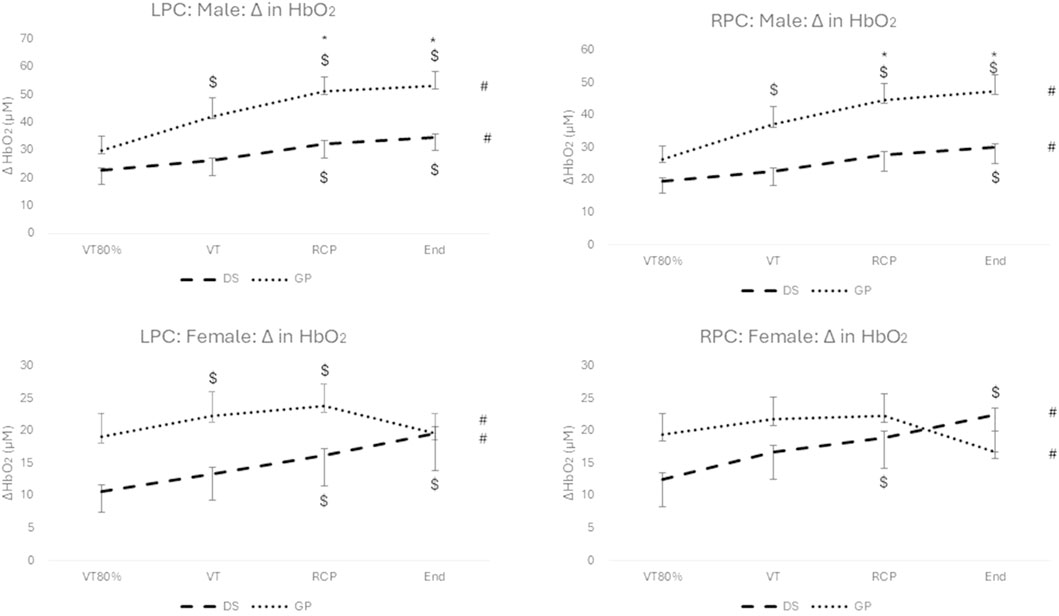
Figure 1. Change in HbO2 for males (top) and females (bottom), and for the LPC (left) and RPC (right) for the DS group (dashed line) and the non-DS group (dotted line). #: p < 0.05 for within group main effect of exercise intensity; *: p < 0.05 between DS and non-DS; $: p < 0.05 for exercise intensity versus VT80%.
3.2 Change in HHb
No significant interaction effect for group (DS vs. non-DS) and time (over exercise intensity) (F (1.886; 50.929) = 2.897; p = 0.067; np2 = 0.097) for the LPC was found for the males. Likewise, there was no significant interaction effect for group and time (F (1.465; 30.763) = 1.482; p = 0.241; np2 = 0.066) for the LPC and the RPC (F (1.434; 27.242) = 0.700; p = 0.460; np2 = 0.036) for the females. However, for the males a marginal significant interaction effect with a medium effect size (F (1.559; 45.208) = 3.659; p = 0.044; np2 = 0.112) for the RPC was found.
Significant increases over exercise intensity (see # in Figure 2) with large effects sizes were reported for the males (LPC: F (1.886; 50.929) = 31.966; p < 0.001; np2 = 0.542; RPC: F (1.559; 45.208) = 33.949; p < 0.001; np2 = 0.538) and for the females (LPC: F (1.465; 30.763) = 33.399; p < 0.001; np2 = 0.614; RPC: F (1.434; 27.242) = 26.090; p < 0.001; np2 = 0.579). Although, ΔHHb continued to increase from RCP to peak exercise for both groups, both genders and both hemispheres, only the change for the non-DS group was significant (p < 0.05) (See and in Figure 2). Significant post hoc differences between DS and non-DS for RCP and peak exercise are reported for the males only (LPC and RPC) (See * in Figure 2). Significant within group post hoc increases are reported for exercise intensity versus VT80% (See $ in Figure 2).
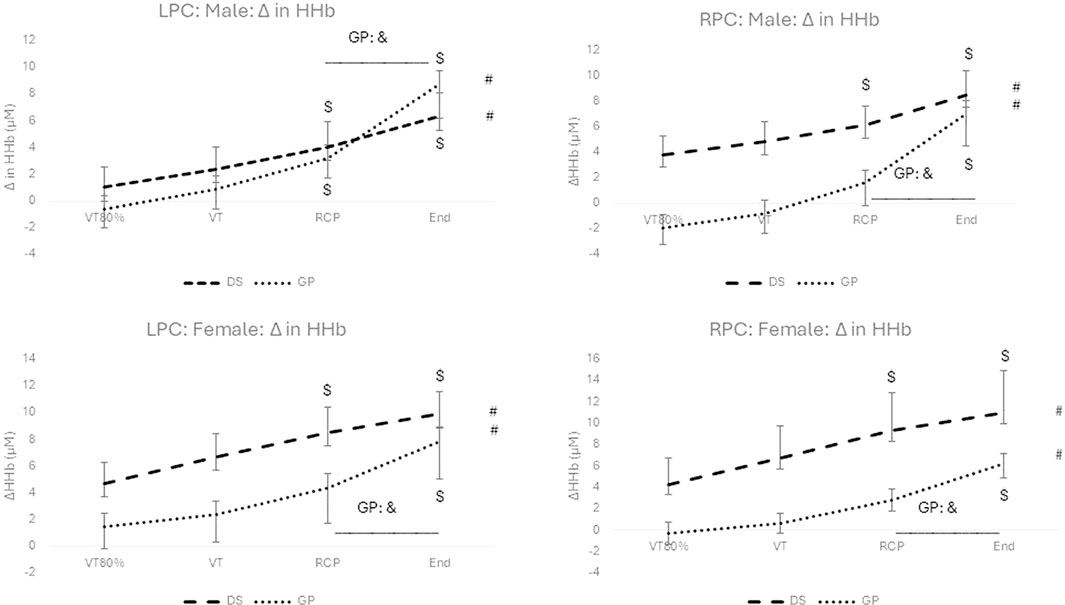
Figure 2. Change in HHb for males (top) and females (bottom), and for the LPC (left) and RPC (right) for the DS group (dashed line) and the non-DS group (dotted line). #: p < 0.05 for within group main effect of exercise intensity; *: p < 0.05 between DS and non-DS; $: p < 0.05 for exercise intensity versus VT80%; &:p < 0.05 significant increase from RCP to peak exercise.
3.3 Change in PETCO2
No significant interaction effect (Figure 3) for group (DS vs. non-DS) and time (over exercise intensity) was reported for the males (F (1.779; 52.181) = 0.356; p = 0.680) and females (F (1.513; 31.775) = 1.389; p = 0.260). The only significant post hoc differences (p < 0.05) between DS and non-DS are reported for the females at the exercise intensity of the RCP and peak exercise (See * in Figure 3).
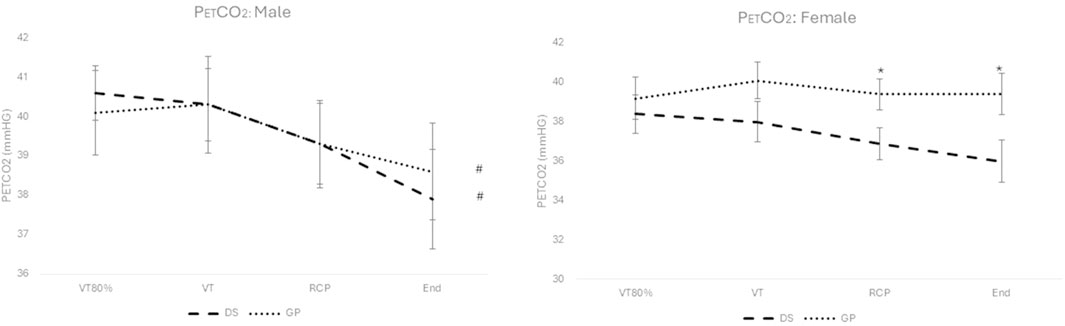
Figure 3. PETCO2 for males (left) and females (right), for the DS group (dashed line) and the non-DS group (dotted line). #: p < 0.05 for within group main effect of exercise intensity; *: p < 0.05 between DS and non-DS.
A significant decrease for the main effect over exercise intensity (see # in Figure 3) is reported for the males (F (1.799; 52.181) = 7.186; p = 0.002), but not for the females (F (1.513; 31.775) = 1.989; p = 0.162).
4 Discussion
The primary purpose of this exploratory study was to assess the change in HbO2 and HHb between sedentary adults with and without DS during an incremental VO2 peak test. A shared cerebral haemodynamic response between both groups of participants in the current study was that an increased change in the main effect of exercise intensity for the change in HbO2 and HHb for both hemispheres were reported as other studies conducted in the general population for sedentary and active individuals (Kojima et al., 2021; Stevens et al., 2018; Tempest et al., 2017; Oussaidene et al., 2015; Tempest and Parfitt, 2016; Jung et al., 2015) and confirmed by a systematic review (De Wachter et al., 2021). Similarly both male groups demonstrated a significant decrease over exercise intensity for PETCO2 (main effect over time) but not for either group of females.
4.1 Profile of the change in HbO2 and HHb between individuals with DS and non-DS
Although there were no significant differences between DS and non-DS in submaximal (80% VT, VT and RCP) and
4.1.1 Possible speculative mechanisms associated with the altered profiles for ΔHbO2 and ΔHHb
The mechanisms for these findings remain unexplained as functional magnetic resonance imaging (fMRI), positron emission topography and the assessment of other contributing molecular and physiological mechanisms such as insulin-like growth factor, vascular endothelial growth factor, vascular availability of nitric oxide, brain derived neurotrophic factor, neural connectivity and mitochondrial density were not studied (Brugniaux et al., 2014; Hayes et al., 2013). A large percentage of adults with DS develop Alzheimer’s disease (Zammit et al., 2024; Rubenstein et al., 2024; Fortea et al., 2021) and although none of the participants in the current study were diagnosed as having Alzheimer’s, it could be speculated that some of these sedentary and mostly overweight participants with a mean age of 38.2 years (56% older than 40 years) could have potentially developed some form of neurological degeneration, cerebral amyloid angiopathy, haemorrhagic lesions, white matter microstructural abnormalities or enlarged perivascular spaces (Rizvi et al., 2022; Fortea et al., 2021; Hasina et al., 2022). Amyloid plaques and tau neurofibrillary tangles are present in almost all individuals with DS by age 40 years (Zammit et al., 2024; Helman et al., 2019; Mann, 1988). There is evidence that the accumulation of amyloid plaque may cause damage to cerebral vasculature and brain cells in persons with Alzheimer’s disease (Lim et al., 2014; Jack et al., 2009; Buckner et al., 2005). However, these cause-effect speculations would need to be confirmed in an exclusive DS population.
A multitude of other factors could also be hypothesized to be related to this ΔHbO2 group difference seeing that most adults with DS suffer from chronotropic incompetence (Leti et al., 2015), muscle hypotonicity and ligamentous laxity (Mendonca et al., 2010), obesity and related conditions (Bertapelli et al., 2016), mitochondrial dysfunction (Tan et al., 2023), congenital heart disease (Santoro and Steffensen, 2021), thyroid disease (Capone et al., 2018), and blunted catecholamine response during submaximal and maximal exercise (Fernhall et al., 2009). The significantly ΔHbO2 profile could possibly be associated with the pronounced EF limitations for adults with DS as reported by a meta-analysis of 57 studies comparing DS to a typically developed mental age-matched group (Tungate and Conners, 2021). The prefrontal cortex is involved where most of the EFs occur (Bishop et al., 2010). Furthermore, cerebral oxygenation is important in influencing cognitive function (Ogoh and Tarumi, 2019; Ogoh, 2017) and several studies report the impact of cerebral oxygenation on EF during exercise and resting conditions (Goenarjo et al., 2020; Mekari et al., 2019; Dupuy et al., 2015).
4.1.2 Fitness, physical activity, exercise and cerebral oxygenation
Although the mechanisms for these group differences are not known, exercise or being physically active would be important for both sedentary groups of the current investigation. It has been reported that exercise improves the density and integrity of grey and white matter (d’Arbeloff, 2020; Kundu et al., 2021) and other known cerebrovascular benefits such as an improvement in angiogenesis, neurovascular plasticity and oxygen saturation in brain regions related to cognitive performance such as the prefrontal cortices and hippocampus (Song et al., 2024; Stimpson et al., 2018). As reported by numerous other studies on the general population, the cerebral oxygenation profile improved after an exercise intervention (Marillier et al., 2022; Caen et al., 2019; Oussaidene et al., 2015) and this was confirmed by a recent systematic review (Salzman et al., 2022). This finding has also been shown in a cross-sectional study where a physical active group was compared to a sedentary group (Brugniaux et al., 2014). Future studies need to be conducted to confirm whether the cerebral oxygenation status could improve prefrontal cortex oxygenation for adults with DS with exercise or for those who are more physically active. If so, such interventions should be conducted as most adults with DS live a sedentary lifestyle as reported by a systematic review of 17 studies (Agiovlasitis et al., 2020) and age prematurely (Boer, 2024). Although this population ages prematurely, individuals with DS older than 40 years are a rapidly growing population due to improvements in medical care and the management of co-occurring illnesses, although these medical interventions do not help to improve the quality of life in older age (de Graaf et al., 2017). Fortunately, the positive effect of exercise for this population has been shown for health (Kerstiens and Green, 2015), fitness (Montalva-Valenzuela et al., 2024), functional ability and quality of life (Hardee and Fetters, 2017), cognition (Merzbach et al., 2023; Ptomey et al., 2018) and executive function (Ringenbach et al., 2021).
4.2 Change in HbO2 and HHb from low intensity exercise to peak exercise intensity
From low to submaximal exercise intensities, the change in cerebral oxygenation and deoxygenation is expected to increase (Jung et al., 2015). A significant main effect increase for ΔHbO2 and ΔHHb over exercise intensity was shown for both genders and groups with large effect sizes for both the LPC and RPC (p < 0.05). Most studies with trained and untrained participants have reported increased cerebral oxygenation from rest to VT and VT to RCP, where some showed a continued improvement to VO2 peak (Stevens et al., 2018; Tempest and Parfitt, 2016), or a plateau (Tempest et al., 2017; Tempest and Parfitt, 2016; Jung et al., 2015) or a decrease (Kojima et al., 2021; Oussaidene et al., 2015). Our study showed increases in the ΔHbO2 from 80% VT to VT and VT to RCP, but differences with respect to gender and group from RCP to peak VO2. However, ΔHbO2 ameliorations from RCP to peak exercise were not significant for both genders and for both the LPC and RPC (p > 0.05). In studies comparing trained versus untrained individuals a decreased cerebral oxygenation profile from RCP to peak exercise is reported for trained individuals (Oussaidene et al., 2015; Brugniaux et al., 2014) but only Oussaidene et al. (2015) reported significance. It is possible that for untrained individuals, this effect may not be reached due to a sedentary lifestyle (Stevens et al., 2018; Jung et al., 2015; Tempest and Parfitt, 2016). To study the discrepancies between studies, the direct analysis of cerebral blood flow and cerebral blood volume and other molecular mechanisms would need to be analysed at exercise intensities ranging from RCP to peak exercise as cerebral blood flow would decrease if metabolic acidosis was reached due to the increased ventilatory response resulting in a response to reduced PaCO2, known as hypocapnia (Caen et al., 2019). The current study did report a main effect of significant decrease over PETCO2 for both groups of male participants over exercise intensity (p < 0.01) but not between RCP and peak exercise. There was no significant decrease over exercise intensity nor specifically between RCP and peak exercise for both groups of female participants for PETCO2 although post hoc analyses reported significant differences between DS and non-DS females at RCP and peak exercise (Figure 3). What is apparent from these complex findings is that the observed differences in the ΔHbO2 cannot simply be ascribed to differences in the PETCO2 kinetic given that both the hyper and hypocapneic profile responses to exercise were comparable across groups (especially for the males).
Regarding the ΔHHb, other studies conducted on trained and untrained individuals in the general population have also reported increases in the ΔHHb from rest to VT, VT to RCP and RCP to peak exercise (Kojima et al., 2021; Stevens et al., 2018; Tempest and Parfitt, 2016; Oussaidene et al., 2015). Although these studies demonstrated increases, they did not always report on statistical significance. The current study also reported increases in the change of HHb from 80%VT to VT, VT to RCP and from RCP to peak exercise for both genders and for the LPC and the RPC. Considering increases from RCP to peak exercise in both the LPC and the RPC, significance was reported for both genders for the non-DS group (p < 0.05) but not for the DS group. Mixed results are reported regarding this finding for other studies conducted on untrained individuals in the general population. One study reported significant increases (Tempest et al., 2017), some reported no significant increases (Caen et al., 2019; Oussaidene et al., 2015) and some did not report on significance (Stevens et al., 2018). We cannot attribute physiological and molecular mechanisms for these findings, and this is made more difficult due to discrepancies in other untrained general population studies, and the possible influencing factors regarding the many physiological and developed limitations faced by the DS group and their pronounced limitations in EF and cognition. Future studies need to be conducted to further analyse these findings, and the use of fMRI and positron emission topography could provide evidence of changes in the direct measurement of cerebral blood volume and cerebral blood flow.
4.3 Conclusion, limitations and future studies
The conclusion of the current study is that a significant different evolution of the ΔHbO2 existed for this group of sedentary adults with DS when conducting an incremental exercise test, although no significant between group differences with respect to VT, RCP, peak exercise and VT/
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethical approval for this study was granted by the Faculty of Health Sciences Research Ethical Committee from the University of Pretoria (269/2023). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’; legal guardians/next of kin.
Author contributions
P-HB: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Research was supported by the NRF (CSRP23041392731).
Acknowledgments
We would like to thank God and all participants who were part of this research.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agiovlasitis S., Choi P., Allred A. T., Xu J., Motl R. W. (2020). Systematic review of sedentary behaviour in people with Down syndrome across the lifespan: a clarion call. J. Appl. Res. Intellect. Disabil. 33 (2), 146–159. doi:10.1111/jar.12659
Ainslie P. N., Hoiland R. L., Bailey D. M. (2016). Lessons from the laboratory; integrated regulation of cerebral blood flow during hypoxia. Exp. Physiol. 101 (9), 1160–1166. doi:10.1113/EP085671
Antonarakis S. E., Skotko B. G., Rafii M. S., Strydom A., Pape S. E., Bianchi D. W., et al. (2020). Down syndrome. Nat. Rev. Dis. Prim. 6 (1), 9–20. doi:10.1038/s41572-019-0143-7
Bertapelli F., Pitetti K., Agiovlasitis S., Guerra-Junior G. (2016). Overweight and obesity in children and adolescents with Down syndrome—prevalence, determinants, consequences, and interventions: a literature review. Res. Dev. Disabil. 57, 181–192. doi:10.1016/j.ridd.2016.06.018
Bishop N. A., Lu T., Yankner B. A. (2010). Neural mechanisms of ageing and cognitive decline. Nature 464 (7288), 529–535. doi:10.1038/nature08983
Boer P. H. (2020). The effect of 8 weeks of freestyle swim training on the functional fitness of adults with Down syndrome. J. Intellect. Disabil. Res. 64 (10), 770–781. doi:10.1111/jir.12768
Boer P. H. (2023). A slightly adapted treadmill protocol for the determination of maximal oxygen uptake in adults with Down syndrome. J. Appl. Res. Intellect. Disabil. 36 (5), 1162–1168. doi:10.1111/jar.13138
Boer P. H. (2024). Functional fitness of adults with Down syndrome: a longitudinal study. J. Intellect. Disabil. Res. 68 (3), 237–247. doi:10.1111/jir.13107
Boer P. H., Moss S. J. (2016). Effect of continuous aerobic vs. interval training on selected anthropometrical, physiological and functional parameters of adults with Down syndrome. J. Intellect. Disabil. Res. 60 (4), 322–334. doi:10.1111/jir.12251
Brugniaux J. V., Marley C. J., Hodson D. A., New K. J., Bailey D. M. (2014). Acute exercise stress reveals cerebrovascular benefits associated with moderate gains in cardiorespiratory fitness. J. Cereb. Blood Flow and Metabolism 34 (12), 1873–1876. doi:10.1038/jcbfm.2014.142
Buckner R. L., Snyder A. Z., Shannon B. J., LaRossa G., Sachs R., Fotenos A. F., et al. (2005). Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J. Neurosci. 25 (34), 7709–7717. doi:10.1523/JNEUROSCI.2177-05.2005
Caen K., Vermeire K., Pogliaghi S., Moerman A., Niemeijer V., Bourgois J. G., et al. (2019). Aerobic interval training impacts muscle and brain oxygenation responses to incremental exercise. Front. Physiology 10, 1195–1205. doi:10.3389/fphys.2019.01195
Capone G. T., Chicoine B., Bulova P., Stephens M., Hart S., Crissman B., et al. (2018). Co-occurring medical conditions in adults with Down syndrome: a systematic review toward the development of health care guidelines. Am. J. Med. Genet. Part A 176 (1), 116–133. doi:10.1002/ajmg.a.38512
Carmeli E., Kessel S., Coleman R., Ayalon M. (2002). Effects of a treadmill walking program on muscle strength and balance in elderly people with Down syndrome. J. Gerontol. A Biol. Sci. Med. Sci. 57 (2), M106–M110. doi:10.1093/gerona/57.2.M106
Coetsee C., Terblanche E. (2017). Cerebral oxygenation during cortical activation: the differential influence of three exercise training modalities. A randomized controlled trial. Eur. J. Appl. Physiology 117, 1617–1627. doi:10.1007/s00421-017-3651-8
Cohen J. D., Dunbar K., McClelland J. L. (1990). On the control of automatic processes: a parallel distributed processing account of the stroop effect. Psychol. Rev. 97 (3), 332–361. doi:10.1037/0033-295x.97.3.332
d’Arbeloff T. (2020). Cardiovascular fitness and structural brain integrity: an update on current evidence. GeroScience 42 (5), 1285–1306. doi:10.1007/s11357-020-00244-7
de Graaf G., Buckley F., Dever J., Skotko B. G. (2017). Estimation of live birth and population prevalence of Down syndrome in nine U.S. states. J. Med. Genet A 173, 2710–2719. doi:10.1002/ajmg.a.38402
De Wachter J., Proost M., Habay J., Verstraelen M., Díaz-García J., Hurst P., et al. (2021). Prefrontal cortex oxygenation during endurance performance: a systematic review of functional near-infrared spectroscopy studies. Front. Physiology 12, 761232–22. doi:10.3389/fphys.2021.761232
Drigny J., Gremeaux V., Dupuy O., Gayda M., Bherer L., Juneau M., et al. (2014). Effect of interval training on cognitive functioning and cerebral oxygenation in obese patients: a pilot study. J. Rehabilitation Med. 46 (10), 1050–1054. doi:10.2340/16501977-1905
DSSA (2023). Down syndrome South Africa. Available online at: http://www.downsyndrome.org.za (Accessed March 30, 2023).
Dupuy O., Gauthier C. J., Fraser S. A., Desjardins-Crèpeau L., Desjardins M., Mekary S., et al. (2015). Higher levels of cardiovascular fitness are associated with better executive function and prefrontal oxygenation in younger and older women. Front. Hum. Neurosci. 9 (66), 66–12. doi:10.3389/fnhum.2015.00066
Fernhall B., Baynard T., Collier S. R., Figueroa A., Goulopoulou S., Kamimori G. H., et al. (2009). Catecholamine response to maximal exercise in persons with Down syndrome. Am. J. Cardiol. 103 (5), 724–726. doi:10.1016/j.amjcard.2008.10.036
Fernhall B., Millar A. L., Tymeson G. T., Burkett L. N. (1990). Maximal exercise testing of mentally retarded adolescents and adults: reliability study. Archives Phys. Med. Rehabilitation 71, 1065–1068.
Fernhall B. O., Mendonca G. V., Baynard T. (2013). Reduced work capacity in individuals with Down syndrome: a consequence of autonomic dysfunction? Exerc. Sport Sci. Rev. 41 (3), 138–147. doi:10.1097/JES.0b013e318292f408
Foley C., Killeen O. G. (2019). Musculoskeletal anomalies in children with Down syndrome: an observational study. Archives Dis. Child. 104 (5), 482–487. doi:10.1136/archdischild-2018-315751
Fortea J., Zaman S. H., Hartley S., Rafii M. S., Head E., Carmona-Iragui M. (2021). Alzheimer's disease associated with Down syndrome: a genetic form of dementia. Lancet Neurology 20 (11), 930–942. doi:10.1016/S1474-4422(21)00245-3
Goenarjo R., Bosquet L., Berryman N., Metier V., Perrochon A., Fraser S. A., et al. (2020). Cerebral oxygenation reserve: the relationship between physical activity level and the cognitive load during a stroop task in healthy young males. Int. J. Environ. Res. Public Health 17 (4), 1406–1420. doi:10.3390/ijerph17041406
Guerra M., Llorens N., Fernhall B. (2003). Chronotropic incompetence in persons with Down syndrome. Archives Phys. Med. Rehabilitation 84 (11), 1604–1608. doi:10.1053/S0003-9993(03)00342-3
Hall C. N., Klein-Flugge M. C., Howarth C., Attwell D. (2012). Oxidative phosphorylation, not glycolysis, powers presynaptic and postsynaptic mechanisms underlying brain information processing. J. Neuro Sci. 32, 8940–8951. doi:10.1523/JNEUROSCI.0026-12.2012
Hardee J. P., Fetters L. (2017). The effect of exercise intervention on daily life activities and social participation in individuals with Down syndrome: a systematic review. Res. Dev. Disabil. 62, 81–103. doi:10.1016/j.ridd.2017.01.011
Hasina Z., Wang N., Wang C. C. (2022). Developmental neuropathology and neurodegeneration of Down syndrome: current knowledge in humans. Front. Cell Dev. Biol. 10, 877711. doi:10.3389/fcell.2022.877711
Hayes S. M., Hayes J. P., Cadden M., Verfaellie M. (2013). A review of cardiorespiratory fitness-related neuroplasticity in the aging brain. Front. Aging Neurosci. 5, 31–47. doi:10.3389/fnagi.2013.00031
Helman A. M., Siever M., McCarty K. L., Lott I. T., Doran E., Abner E. L., et al. (2019). Microbleeds and cerebral amyloid angiopathy in the brains of people with Down syndrome with Alzheimer’s disease. J. Alzheimer’s Dis. 67 (1), 103–112. doi:10.3233/JAD-180589
Jack C. R., Lowe V. J., Weigand S. D., Wiste H. J., Senjem M. L., Knopman D. S., et al. (2009). Serial PIB and MRI in normal, mild cognitive impairment and alzheimer's disease: implications for sequence of pathological events in alzheimer's disease. Brain 132 (5), 1355–1365. doi:10.1093/brain/awp062
Jung R., Moser M., Baucsek S., Dern S., Schneider S. (2015). Activation patterns of different brain areas during incremental exercise measured by near-infrared spectroscopy. Exp. Brain Res. 233, 1175–1180. doi:10.1007/s00221-015-4201-4
Jurcak V. D., Tsuzuki D., Dan I. (1953). 10/20, 10/10, and 10/5 systems revisited: their validity as relative head-surface-based positioning systems. Neuroimage 34 (4), 1600–1611.
Kerstiens R. L., Green M. (2015). Exercise in individuals with Down syndrome: a brief review. Int. J. Exerc. Sci. 8 (2), 192–201. doi:10.70252/DCTG6034
Kojima S., Morishita S., Hotta K., Qin W., Kato T., Oyama K., et al. (2021). “Relationship between decrease of oxygenation during incremental exercise and partial pressure end-tidal carbon dioxide: near-infrared spectroscopy vector analysis,” in Oxygen transport to tissue XLII (Cham: Springer), 119–124.
Kundu S., Huang H., Erickson K. I., McAuley E., Kramer A. F., Rohde G. K. (2021). Investigating impact of cardiorespiratory fitness in reducing brain tissue loss caused by ageing. Brain Commun. 3 (4), fcab228–16. doi:10.1093/braincomms/fcab228
Leti T., Guinot M., Favre-Juvin A., Bricout V. A. (2015). Difference of catecholamine responses to exercise in men with trisomy 21, with or without chronotropic incompetence. Physiology and Behav. 142, 97–103. doi:10.1016/j.physbeh.2015.02.007
Li C., Chen S., How Y. M., Zhang A. L. (2013). Benefits of physical exercise intervention on fitness of individuals with Down syndrome: a systematic review of randomized-controlled trials. Int. J. Rehabilitation Res. 36 (3), 187–195. doi:10.1097/MRR.0b013e3283634e9c
Lim Y. Y., Maruff P., Pietrzak R. H., Ellis K. A., Darby D., Ames D., et al. (2014). Aβ and cognitive change: examining the preclinical and prodromal stages of alzheimer's disease. Alzheimer's and Dementia 10 (6), 743–751.e1. doi:10.1016/j.jalz.2013.11.005
Mann D. M. (1988). The pathological association between Down syndrome and alzheimer disease. Mech. Ageing Dev. 43 (2), 99–136. doi:10.1016/0047-6374(88)90041-3
Marillier M., Borowik A., Chacaroun S., Baillieul S., Doutreleau S., Guinot M., et al. (2022). High-intensity interval training to promote cerebral oxygenation and affective valence during exercise in individuals with obesity. J. Sports Sci. 40 (13), 1500–1511. doi:10.1080/02640414.2022.2086658
Mekari S., Dupuy O., Martins R., Evans K., Kimmerly D. S., Fraser S., et al. (2019). The effects of cardiorespiratory fitness on executive function and prefrontal oxygenation in older adults. Geroscience 41 (5), 681–690. doi:10.1007/s11357-019-00128-5
Mekari S., Fraser S., Bosquet L., Bonnéry C., Labelle V., Pouliot P., et al. (2015). The relationship between exercise intensity, cerebral oxygenation and cognitive performance in young adults. Eur. J. Appl. Physiology 115, 2189–2197. doi:10.1007/s00421-015-3199-4
Mendonca G. V., Pereira F. D. (2009). Influence of long-term exercise training on submaximal and peak aerobic capacity and locomotor economy in adult males with Down's syndrome. Med. and Sci. Monit. 15, 33–39. doi:10.12659/MSM.947100
Mendonca G. V., Pereira F. D., Fernhall B. (2011). Effects of combined aerobic and resistance exercise training in adults with and without Down syndrome. Archives Phys. Med. and Rehabilitation 92, 37–45. doi:10.1016/j.apmr.2010.09.015
Mendonca G. V., Pereira F. D., Fernhall B. (2013). Heart rate recovery and variability following combined aerobic and resistance exercise training in adults with and without Down syndrome. Res. Dev. Disabil. 34 (1), 353–361. doi:10.1016/j.ridd.2012.08.023
Mendonca G. V., Pereira F. D., Fernhall B. O. (2010). Reduced exercise capacity in persons with Down syndrome: cause, effect, and management. Ther. Clin. Risk Manag. 6, 601–610. doi:10.2147/TCRM.S10235
Merzbach V., Ferrandino M., Gernigon M., Marques Pinto J., Scruton A., Gordon D. (2023). Impact of prescribed exercise on the physical and cognitive health of adults with Down syndrome: the MinDSets study. Int. J. Environ. Res. Public Health 20 (23), 7121. doi:10.3390/ijerph20237121
Millar A. L., Fernhall B. O., Burkett L. N. (1993). Effects of aerobic training in adolescents with Down syndrome. Med. Sci. Sports Exerc. 25 (2), 270–274. doi:10.1249/00005768-199302000-00018
Miyazawa T., Horiuchi M., Komine H., Sugawara J., Fadel P. J., Ogoh S. (2013). Skin blood flow influences cerebral oxygenation measured by near-infrared spectroscopy during dynamic exercise. Eur. J. Appl. Physiology 113, 2841–2848. doi:10.1007/s00421-013-2723-7
Moncaster J. A., Pineda R., Moir R. D., Lu S., Burton M. A., Ghosh J. G., et al. (2010). Alzheimer's disease amyloid-beta links lens and brain pathology in Down syndrome. PloS one 5 (5), e10659. doi:10.1371/journal.pone.0010659
Montalva-Valenzuela F., Castillo-Paredes A., Farias-Valenzuela C., Andrades-Ramirez O., Concha-Cisternas Y., Guzmán-Muñoz E. (2024). Effects of exercise, physical activity, and sports on physical fitness in adults with Down syndrome: a systematic review. AIMS Public Health 11 (2), 577–600. doi:10.3934/publichealth.2024029
Ogoh S. (2017). Relationship between cognitive function and regulation of cerebral blood flow. J. Physiol Sci. 67 (3), 345–351. doi:10.1007/s12576-017-0525-0
Ogoh S., Tarumi T. (2019). Cerebral blood flow regulation and cognitive function: a role of arterial baroreflex function. J. Physiol Sci. 69 (6), 813–823. doi:10.1007/s12576-019-00704-6
Oussaidene K., Prieur F., Tagougui S., Abaidia A., Matran R., Mucci P. (2015). Aerobic fitness influences cerebral oxygenation response to maximal exercise in healthy subjects. Respir. Physiology and Neurobiol. 205, 53–60. doi:10.1016/j.resp.2014.10.009
Ozemek C., Bonikowske A., Christle J., Gallo P. (2025). ACSM's guidelines for exercise testing and prescription. 12th Edition. Lippincott Williams and Wilkins.
Ptomey L. T., Szabo A. N., Willis E. A., Gorczyca A. M., Greene J. L. ., Danon J. C., et al. (2018). Changes in cognitive function after a 12-week exercise intervention in adults with Down syndrome. Disabil. Health J. 11 (3), 486–490. doi:10.1016/j.dhjo.2018.02.003
Raichle M. E., Gusnard D. A. (2002). Appraising the brain's energy budget. Proc. Natl. Acad. Sci. U. S. A. 99 (16), 10237–10239. doi:10.1073/pnas.172399499
Ringenbach S., Arnold N., Myer B., Hayes C., Nam K., Chen C. C. (2021). Executive function improves following acute exercise in adults with Down syndrome. Brain Sci. 11 (5), 620–632. doi:10.3390/brainsci11050620
Rizvi B., Head E., Brickman A. M. (2022). “The role of cerebrovascular disease in aging and alzheimer's disease among people with Down syndrome,” in The neurobiology of aging and alzheimer disease in Down syndrome. Editors E. Head, and I. Lott (Academic Press), 63–73. doi:10.1016/B978-0-12-818845-3.00012-8
Rubenstein E., Tewolde S., Michals A., Weuve J., Fortea J., Fox M. P., et al. (2024). Alzheimer dementia among individuals with Down syndrome. JAMA Netw. Open 7 (9), e2435018. doi:10.1001/jamanetworkopen.2024.35018
Salzman T., Dupuy O., Fraser S. A. (2022). Effects of cardiorespiratory fitness on cerebral oxygenation in healthy adults: a systematic review. Front. Physiology 13, 838450–15. doi:10.3389/fphys.2022.838450
Santoro S. L., Steffensen E. H. (2021). Congenital heart disease in Down syndrome–A review of temporal changes. J. Congenit. Cardiol. 5, 1–14. doi:10.1186/s40949-020-00055-7
Shields N., Taylor N. F., Wee E., Wollersheim D., O'Shea S. D., Fernhall B. (2013). A community-based strength training programme increases muscle strength and physical activity in young people with Down syndrome: a randomised controlled trial. Res. Dev. Disabil. 34 (12), 4385–4394. doi:10.1016/j.ridd.2013.09.022
Song B. X., Azhar L., Koo G. K. Y., Marzolini S., Gallagher D., Swardfager W., et al. (2024). The effect of exercise on blood concentrations of angiogenesis markers in older adults: a systematic review and meta-analysis. Neurobiol. Aging 135, 15–25. doi:10.1016/j.neurobiolaging.2023.12.004
Stevens D., Halaki M., Chow C. M., O’Dwyer N. (2018). The effects of multi-stage exercise with and without concurrent cognitive performance on cardiorespiratory and cerebral haemodynamic responses. Eur. J. Appl. Physiology 118, 2121–2132. doi:10.1007/s00421-018-3942-8
Stimpson N. J., Davison G., Javadi A. H. (2018). Joggin’ the noggin: towards a physiological understanding of exercise-induced cognitive benefits. Neurosci. and Biobehav. Rev. 88, 177–186. doi:10.1016/j.neubiorev.2018.03.018
Stone M. R., Gibson A. S. C., Thompson K. G. (2016). Asymmetry of cerebral hemodynamic response to incremental cycling exercise. Int. J. Sports Physiology Perform. 11 (2), 273–275. doi:10.1123/ijspp.2015-0168
Tan K. L., Lee H. C., Cheah P. S., Ling K. H. (2023). Mitochondrial dysfunction in Down syndrome: from pathology to therapy. Neuroscience 511, 1–12. doi:10.1016/j.neuroscience.2022.12.003
Tempest G., Parfitt G. (2016). Self-reported tolerance influences prefrontal cortex hemodynamics and affective responses. Cogn. Affect Behav. Neurosci. 16, 63–71. doi:10.3758/s13415-015-0374-3
Tempest G. D., Davranche K., Brisswalter J., Perrey S., Radel R. (2017). The differential effects of prolonged exercise upon executive function and cerebral oxygenation. Brain Cognition 113, 133–141. doi:10.1016/j.bandc.2017.02.001
Tungate A. S., Conners F. A. (2021). Executive function in Down syndrome: a meta-analysis. Res. Dev. Disabil. 108, 103802–103818. doi:10.1016/j.ridd.2020.103802
Varela A. M., Bettencount Sardinha L., Pitetti K. H. (2001). Effects of an aerobic rowing training regimen in young adults with Down syndrome. Am. J. Ment. Retard. 106 (2), 135–144. doi:10.1352/0895-8017(2001)106<0135:EOAART>2.0.CO;2
Wasserman K. (1984). The anaerobic threshold measurement to evaluate exercise performance. Am. Rev. Respir. Dis. 129 (2P2), S35–S40. doi:10.1164/arrd.1984.129.2P2.S35
Zahari N., Mat Bah M. N., A. Razak H., Thong M. K. (2019). Ten-year trend in prevalence and outcome of Down syndrome with congenital heart disease in a middle-income country. Eur. J. Pediatr. 178, 1267–1274. doi:10.1007/s00431-019-03403-x
Keywords: Prefrontal Cortex, brain oxygenation, cognition, NIRS, HHB, HbO2, VO2 peak
Citation: Boer P-H (2025) Cerebral oxygenation during submaximal and peak exercise for sedentary adults with and without down syndrome. Front. Physiol. 16:1595710. doi: 10.3389/fphys.2025.1595710
Received: 18 March 2025; Accepted: 27 June 2025;
Published: 09 July 2025.
Edited by:
Susanna Rampichini, University of Milan, ItalyReviewed by:
Paulo A. S. Armada-da-Silva, University of Lisbon, PortugalMarcin Sikora, Akademii Wychowania Fizycznego im. Jerzego Kukuczki w Katowicach, Poland
Copyright © 2025 Boer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pieter-Henk Boer, Ym9lcnAwMTFAZ21haWwuY29t
 Pieter-Henk Boer
Pieter-Henk Boer