- National Clinical Research Center for Ocular Diseases, Eye Hospital, Wenzhou Medical University, Wenzhou, Zhejiang, China
Background: To develop a retinal age prediction model based on a foundation model using fundus images and to determine the association between gamma-glutamyl transferase (GGT) levels and the retinal age gap.
Methods: A total of 36,044 fundus images with reasonable quality from 9,752 participants in the Jidong Eye Cohort Study were included in this study. Of these images, 8,869 fundus images from 3,010 healthy individuals were used to train and validate the model based on the foundation model RETFound for age prediction using 10-fold cross-validation. A total of 4,081 fundus images from 4,081 participants who were enrolled from May to October 2023 had available GGT data, and these images were used to investigate the association between GGT levels and the retinal age gap.
Results: The trained model in this study achieved excellent performance, with a mean absolute error (MAE) of 2.42 ± 0.08 years. The mean age of the participants in the analysis dataset was 43.7 ± 10.4 years, and 1987 (48.7%) participants were women. The multivariable βs and 95% confidence intervals (CIs) of the retinal age gap in the second, third, and fourth GGT quartiles compared with the lowest GGT quartiles were 0.42 (0.08–0.77), 0.54 (0.15–0.92), and 0.72 (0.29–1.14) (P for trend = 0.001), respectively, in the fully adjusted model (adjusted for age, sex, current smoking status, current drinking status, body mass index, hypertension, diabetes, dyslipidemia, and serum uric acid).
Conclusion: Increased GGT levels were significantly associated with accelerated retinal aging as quantified by the retinal age gap. Our findings indicate that elevated GGT levels may have an adverse effect on the aging process.
Introduction
Gamma-glutamyl transferase (GGT), which is a ubiquitous enzyme that is critical for glutathione metabolism and oxidative stress regulation, has increasingly been identified as a biomarker in addition to its traditional role in hepatobiliary health (Emdin et al., 2005; Kunutsor, 2016; Brennan et al., 2022). Elevated GGT levels are closely correlated with systemic oxidative damage, chronic inflammation, and metabolic dysfunction, all of which play critical roles in the progression of aging (Ali et al., 2016; Corti et al., 2020; Chiyanika et al., 2025). Several studies have demonstrated that higher GGT levels are associated with various age-related chronic diseases, including cardiovascular disease, cognitive impairment, and all-cause/disease-specific mortality, suggesting its profound relevance for the aging process (Breitling et al., 2011; Jeon et al., 2020; Tang et al., 2021; Cho et al., 2023). Furthermore, emerging evidence suggests that GGT may mediate the effects of modifiable lifestyle factors, such as diet, sleep, and cardiovascular risk profiles, on aging (Liu et al., 2024, pp. 2005–2018; Wang X. et al., 2024; Xu et al., 2024). However, direct investigations into the association between GGT and biological aging remain scarce, highlighting a critical gap in understanding the use of GGT as a systemic aging marker.
The retina is an ideal window for assessing systemic aging (Zhu et al., 2023; Tan et al., 2024; Wang et al., 2025), given its shared embryological origins and microvascular features with vital organs (e.g., the brain, heart, and kidneys) (Patton et al., 2005; Flammer et al., 2013; Wong et al., 2014; Li et al., 2023); thus, retinal alterations reliably reflect systemic circulatory health and neurodegenerative processes. Importantly, retinal imaging allows the rapid, noninvasive, and cost-effective assessment of aging and presents a critical advantage in population-scale studies (Li et al., 2022). Recent advances in deep learning, particularly with convolutional neural networks, have revealed the potential to rapidly and accurately predict biological age based on retinal images (Grimbly et al., 2024). Additionally, advances in foundation model technology have further increased the accuracy of retinal age prediction, decreased training data volume, and reduced associated computational expenses (Zhou et al., 2023). The retinal age gap, that is, the difference between predicted retinal age and chronological age, has emerged as a reliable and promising indicator for quantifying aging acceleration (Grimbly et al., 2024). A positive retinal age gap indicates accelerated retinal aging (exceeding chronological age), whereas a negative retinal age gap signifies slower retinal aging, and the retinal age gap is correlated with mortality risk and diseases (e.g., Parkinson’s disease, cardiovascular disease, kidney failure, and diabetic retinopathy) (Zhu et al., 2023; Hu et al., 2022; Zhu et al., 2022; Zhang et al., 2023; Chen et al., 2023a). Thus, retinal age may provide new insight into GGT-related effects on aging. Although previous studies reported established roles of GGT in systemic aging and validated the retinal age gap as an available indicator of aging, the relationship between GGT levels and the retinal age gap remains unexplored. Therefore, this community-based study aimed to investigate the association between GGT levels and the retinal age gap using the foundation model of color fundus photography.
Materials and methods
Study design and population
This study was a part of the Jidong Eye Cohort Study (JECS). All the data that were analyzed in this study were collected from participants who were enrolled in the JECS. The detailed design and methodology of the JECS have been previously published (Yang et al., 2020). From August 2019 to October 2023, approximately 10,000 participants were recruited from the Jidong communities (Tangshan, Hebei, China). All the participants were subjected to comprehensive ophthalmic examinations, physical measurements, and biological sample collection, and all the participants completed detailed healthcare questionnaires. This study was approved by the Ethics Committee of the Staff Hospital of Jidong Oil-field of Chinese National Petroleum (approval number: 2018 YILUNZI 1) and the Ethics Committee of the Eye Hospital of Wenzhou Medical University (approval number: 2021-074-K-63-01). The study followed the guidelines of the Declaration of Helsinki, and all the participants provided written informed consent.
Assessment of color fundus photography
In this study, digital fundus images were captured using a 45° nonmydriatic fundus camera (CR2AF; Canon; Tokyo, Japan) without pupil dilation. A total of 43,558 images from 9,752 participants were collected from the JECS. After quality control, 36,044 images from 9,285 participants met the required standards. The quality control process, described in detail in previous publications (Wang J. et al., 2024; Zhou et al., 2022; 2023), utilized a collaboration between ophthalmologists and an automated retinal image analysis tool that included image quality grading. Color fundus photography with retinal disease (i.e., nerve fiber layer defects, abnormal cup-to-disco ratio, macular degeneration, retinal vein occlusion, diabetic retinopathy, and severe opacification of the refractive media) were ruled out, and only images that were classified as good or usable were considered acceptable for this study.
Fine-tuning the foundation model for age prediction
Following previous studies (Zhu et al., 2023; Grimbly et al., 2024; Wang J. et al., 2024), chronological age was assumed to match biological age in normally aging individuals. To establish a reliable reference for biological age prediction, the healthy dataset for model training and validation included JECS participants without clinical diagnoses of hypertension, diabetes, chronic kidney disease, cardiovascular disease, or stroke. Subclinical cases were excluded via imaging examinations, physical examinations, and laboratory tests (e.g., fasting blood glucose), ensuring only individuals with normal results were included.
In this study, all the data were divided into two parts: the data from JECS 2023 were utilized as the analysis dataset only, and the data from JECS 2019 to JECS 2021 were used as the training and validation dataset. To prevent leakage in the analysis set, all the participants who had follow-up data in 2023 were removed from the training and validation datasets. In detail, among the 9,285 participants with fundus images of acceptable quality, we first identified 4,377 individuals who had follow-up data from 2023 (May to October 2023) in the JECS, and these data were included in the analysis dataset. Fundus images from right eyes were primarily used for retinal age calculation, and left-eye images were substituted when right-eye images were unavailable. A total of 4,081 fundus images of the 4,081 participants had available GGT data, and these images were used to investigate the association between GGT levels and the retinal age gap (images and other data were all acquired from May to October 2023). For the remaining 4,908 participants, a total of 8,869 fundus images from 3,010 healthy individuals were selected to form the healthy dataset for model training and validation. This dataset was further divided using 10-fold cross-validation to ensure robust model development (Figure 1).
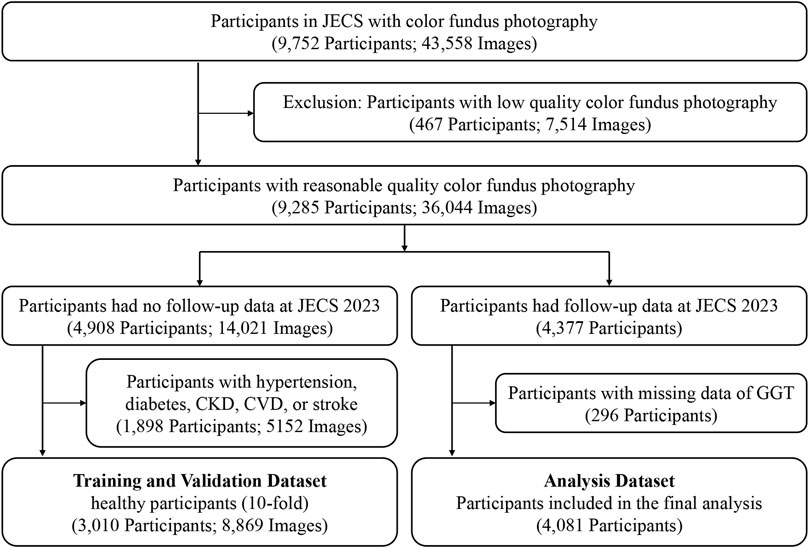
Figure 1. Flow Chart of the Study Participants and Images. JECS, Jidong Eye Cohort Study; CKD, chronic kidney disease; CVD, cardiovascular disease; GGT, gamma-glutamyl transferase.
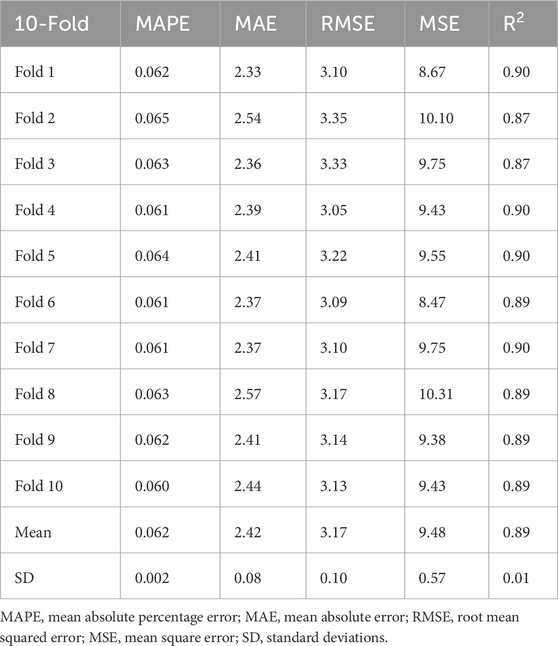
We fine-tuned the foundation model of color fundus photography RETFound (Zhou et al., 2023), which is a state-of-the-art architecture that was pretrained on large-scale retinal image datasets (904,170 unlabeled retinal fundus images), to develop and validate the model for age prediction (Figure 2). Briefly, all the fundus images were preprocessed by resizing to a resolution of 224 × 224 pixels and normalized using the mean and standard deviation of the retinal image dataset. Data augmentation, including random erasing and DeiT-style random augmentation, was applied during training (Zhou et al., 2023). The model was optimized using the L2 loss between the predicted and chronological ages. Training utilized the AdamW optimizer with a 10-epoch warm-up and a cosine learning rate decay policy with an initial learning rate of 0.001. When implemented on an NVIDIA 4090 GPU with a batch size of 64, the model was trained for 100 epochs using PyTorch. Performance was evaluated using the mean absolute error (MAE), mean absolute percentage error (MAPE), root mean squared error (RMSE), and R2 between the predicted retinal age and chronological age.
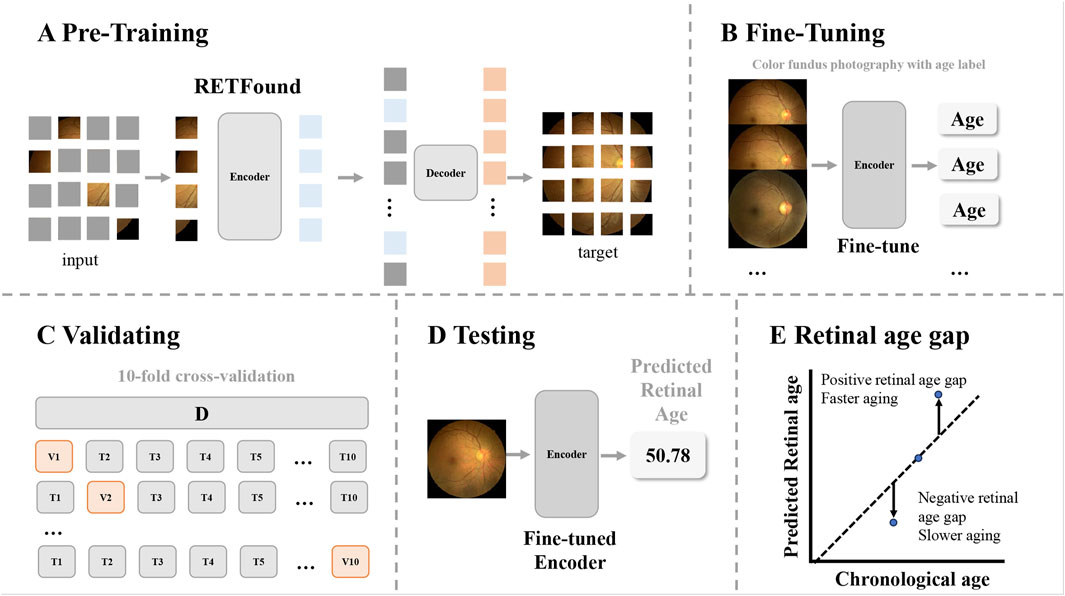
Figure 2. Overview of the study workflow. The workflow illustrates how retinal age gaps were calculated from the color fundus images. (A) The pretrained foundation model of color fundus photography used in this study was a Masked Autoencoders architecture. (B) The encoder from RETFound was fine-tuned using fundus images of healthy participants, with chronological age as the target (using a linear head). (C) Model validation was performed using 10-fold cross-validation. (D) The fine-tuned model predicted retinal ages for participants in the analysis dataset. (E) The retinal age gap was defined as the difference between predicted retinal age and chronological age, with positive values indicating faster aging and negative values indicating slower aging.
Definition of the retinal age gap
The retinal age gap was calculated as the difference between the predicted retinal age according to the fundus images and the chronological age. A positive retinal age gap indicated faster retinal aging than chronological age, while a negative retinal age gap suggested slower retinal aging.
Assessment of general variables
In this study, we collected the participants’ basic information through clinical examinations, laboratory tests, and standardized questionnaires about their demographic features, current smoking and alcohol consumption statuses, and medical history (Su et al., 2022). The average monthly income was classified into two levels: “< ¥3000” and “≥ ¥3000”. Education level was defined as “illiterate, primary or middle school” and “college graduate and above”. In this study, diabetes was defined as a fasting blood glucose (FBG) level ≥7.0 mmol/L, self-reported history of diabetes, or current use of antidiabetic drugs. Hypertension was defined as a systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, self-reported history of hypertension, or current use of antihypertensive medications. Dyslipidemia was defined as low-density lipoprotein (LDL-C) ≥ 3.3 mmol/L, high-density lipoprotein (HDL-C) < 1.04 mmol/L, total cholesterol (TC) ≥ 5.18 mmol/L, triglyceride (TG) ≥ 1.7 mmol/L, the use of lipid-lowering medications, or a self-reported history of dyslipidemia.
Assessment of gamma-glutamyl transferase
Fasting venous blood samples were obtained from the elbow in the morning after the participants had fasted from food and drink for at least 8 h, and the samples were stored in vacuum tubes containing ethylenediaminetetraacetic acid. The levels of GGT and serum uric acid (SUA) were measured by an autoanalyzer (Hitachi, Tokyo, Japan) via the uricase‒peroxidase method at the Central Laboratory of Jidong Oil-Field Hospital (Tang et al., 2021). The participants in this study were stratified into quartiles based on the GGT levels (Q1: ≤16 U/L; Q2: 17–23 U/L; Q3: 24–37 U/L; and Q4: ≥38 U/L).
Statistical analysis
Continuous variables are presented as means and standard deviations (SD), whereas categorical variables are presented as frequencies and percentages. To analyze the differences among different GGT quartile groups, we applied a one-way ANCOVA test for normally distributed continuous variables. For categorical variables, we used chi-square tests or Fisher’s exact tests.
Multivariable generalized linear models were used to assess the relationship between GGT quartiles and the retinal age gap. We treated the GGT quartiles as a continuous ordinal variable to test for trends. The multivariable generalized linear models were adjusted for different sets of covariates: age and sex (Model 1); age, sex, current smoking status, current drinking status, body mass index (BMI), hypertension, diabetes, dyslipidemia, and SUA (Model 2). Furthermore, we examined the associations of a 1 standard deviation change in GGT level with the retinal age gap. Additionally, by adding interaction terms in the adjusted models, we investigated whether the associations between GGT and the retinal age gap varied according to sex, hypertension, diabetes, dyslipidemia, and current drinking status.
The associations are expressed as βs and 95% confidence intervals (CIs). In generalized linear models, βs estimate the absolute change in retinal age gap: for GGT quartiles, they represent the difference relative to the lowest quartile, while for continuous GGT, they reflect the change per 1 SD increase. A positive β indicates an increase in the retinal age gap, and a negative β indicates a decrease. In all the analyses, statistical significance was set to a 2-tailed P value <0.05. All the statistical analyses were carried out using SAS software (version 9.4; SAS Institute Inc., Cary, NC, United States).
Results
Performance of the trained model for predicting age
The model was trained and validated on data from 3,010 healthy participants; these participants had a mean age of 40.6 ± 10.9 years at baseline, and 1773 (58.9%) were female. As shown in Table 1, the trained model in this study achieved strong performance on the healthy dataset, with an MAE of 2.42 ± 0.08 years, an RMSE of 3.17 ± 0.10, and an R2 of 0.89 ± 0.01; thus, this model is better than those described in previous studies (Ahadi et al., 2023; Zhu et al., 2023; Grimbly et al., 2024; Wang J. et al., 2024).
Baseline characteristics of the participants included in the analysis dataset
A total of 4,081 participants from the Jidong communities, who were recruited between May and October 2023, were ultimately included in the analysis. The mean age of the included participants was 43.7 ± 10.4 years, 1987 (48.7%) were female, and the mean retinal age gap was −0.5 ± 4.18 years. Table 2 summarizes the baseline characteristics of the participants in different quartiles of GGT levels. Participants with higher GGT levels were more likely to be male (P < 0.001), current smokers (P < 0.001), and current drinkers (P < 0.001). They were also more likely to have a higher BMI (P < 0.001), higher SUA (P < 0.001), and a higher prevalence of hypertension (P < 0.001), diabetes (P < 0.001), and dyslipidemia (P < 0.001).
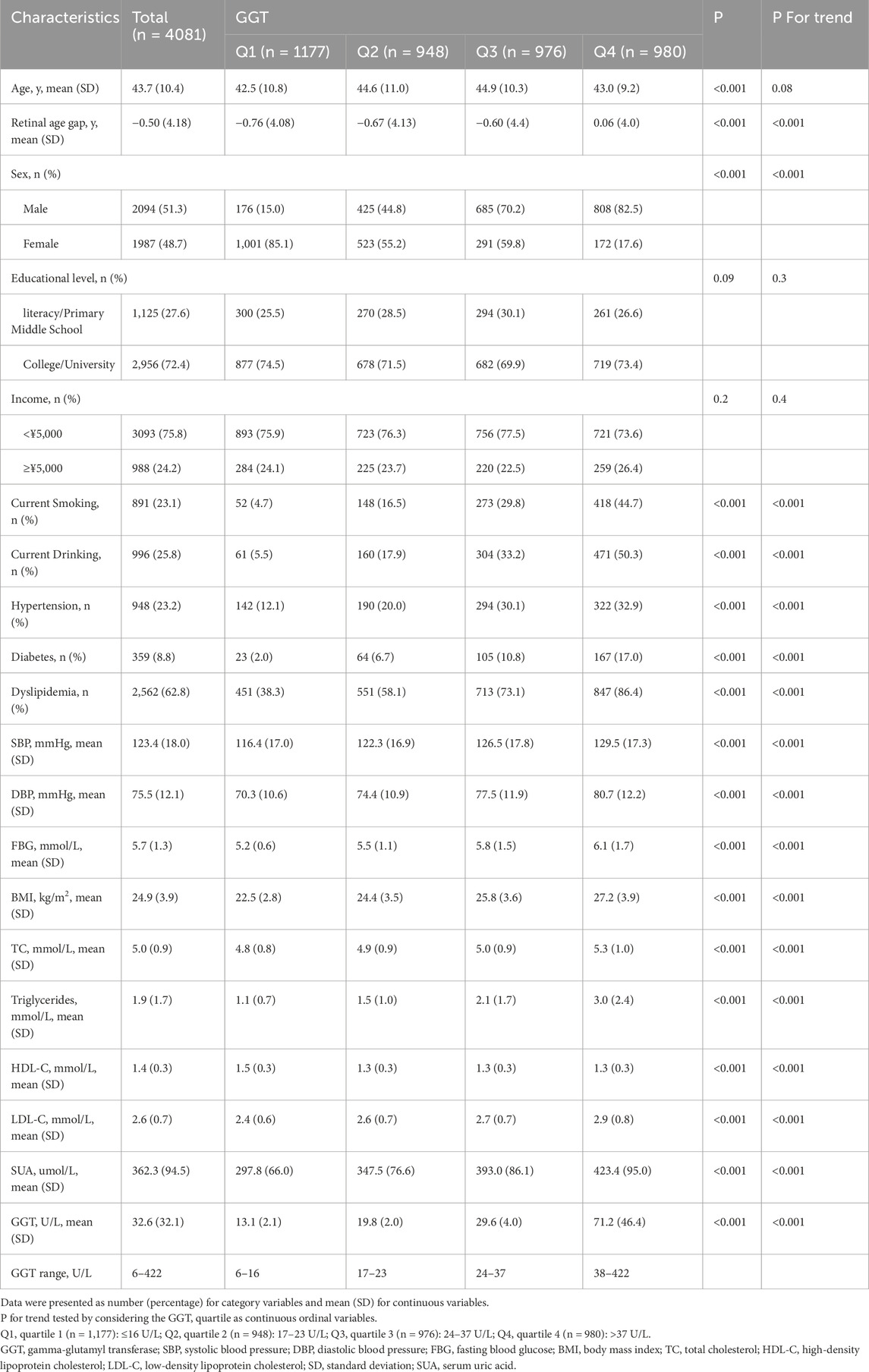
Table 2. Baseline characteristics of participants grouped according to gamma-glutamyl transferase quartiles.
Table 2 also presents the retinal age gap in different GGT quartile groups for the baseline characteristics. The retinal age gap increased from −0.76 years in Q1 to 0.06 years in Q4 (P for trend <0.001).
Associations between GGT levels and the retinal age gap
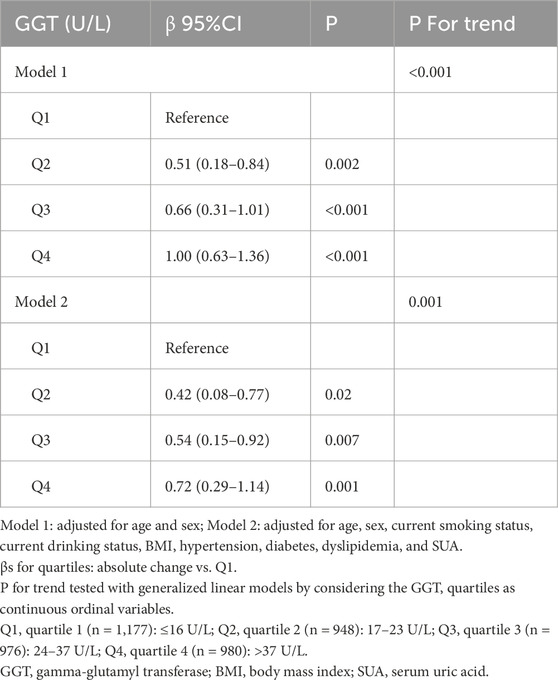
The associations between GGT quartiles and the retinal age gap are shown in Table 3. The participants in the highest GGT quartile showed a significant increase in the retinal age gap (β = 1.00, 95% CI = 0.63–1.36, P < 0.001), even after fully adjusting for potential confounding factors. Moreover, compared with those for the participants in the lowest quartile, the fully adjusted βs and 95% CIs for the participants in the second, third, and fourth GGT quartiles were 0.42 (0.08–0.77), 0.54 (0.15–0.92), and 0.72 (0.29–1.14), respectively. Additionally, a significant trend (P for trend ≤0.001) was observed across all quartiles in both models.
Furthermore, to verify the reliability of our results, we also examined the relationships between a 1 SD change in GGT and the retinal age gap. We found similar relationships. In two models (Model 1: adjusted for age and sex; Model 2: adjusted for age, sex, current smoking status, current drinking status, BMI, hypertension, diabetes, dyslipidemia, and SUA.), the βs values and 95% CIs were 0.31 (0.19–0.43) and 0.19 (0.07–0.33), respectively.
Subgroup analysis
Subgroup analyses by sex, hypertension, diabetes, dyslipidemia, and current drinking status are presented in Table 4. A significant interaction was found between non-hypertension and continuous GGT quartiles for the retinal age gap. The βs of the continuous GGT quartile items in participants with or without hypertension were 0.18 and 0.22, respectively (P for interaction = 0.04). However, no significant interactions were observed for sex, diabetes, dyslipidemia, or current drinking status with the continuous GGT quartiles for the retinal age gap.
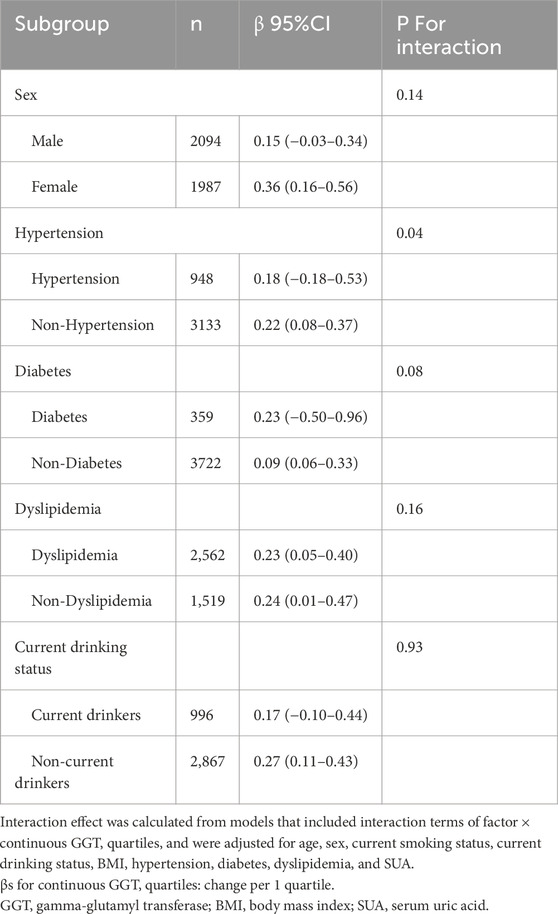
Table 4. Subgroup analyses of the associations between gamma-glutamyl transferase levels and the retinal age gap were performed on the basis of sex, hypertension, diabetes, dyslipidemia, and current drinking status.
Discussion
This study provides compelling evidence linking elevated GGT levels with accelerated retinal aging, as quantified by the retinal age gap, in a relatively large community-based population. The multivariable generalized linear model analysis revealed that the participants with GGT levels in the highest quartile had a significantly greater retinal age gap independent of potential confounders, and a significant trend was observed across all quartiles.
In this study, we developed and validated a deep learning model for predicting retinal age using fundus images, and the model achieved strong performance with an MAE of 2.42 years, outperforming most of the previous models in predicting age using fewer training samples. Previous studies have shown MAEs of 2.8–3.5 years for retinal age prediction (Zhu et al., 2023; Ahadi et al., 2023; Chen et al., 2023b; Abreu-Gonzalez et al., 2023), and MAEs of 3.26–3.65 years for multimodal biological age estimation including fundus images (Wang J. et al., 2024). The improvement in the performance and training efficiency of the retinal age prediction model occurred due to the use of the foundation model that was pretrained on 904,170 unlabeled retinal fundus images (Zhou et al., 2023). After being pretrained on a large-scale retinal image dataset, the foundation model learned how to detect and analyze the characteristics of retinal images. Thus, we only needed to fine-tune the model on a relatively small dataset to achieve even better performance (Sevgi et al., 2025).
To the best of our knowledge, this study represents one of the first attempts to demonstrate the concept of an association between GGT levels and the retinal age gap. In our study, we found that higher GGT levels are associated with accelerated retinal aging, as quantified by the retinal age gap. Several previous studies have demonstrated that elevated GGT levels are associated with several age-related diseases, which support our findings. For example, Cho et al. (Cho et al., 2014, pp. 2010–2011; Lei et al., 2020; Kim et al., 2022) previously reported an association between elevated GGT levels and the risk of age-related ocular diseases, including age-related macular degeneration (AMD), primary glaucoma, and ocular motor cranial nerve palsy. Moreover, GGT was proven to be associated with the risk of cardiovascular disease (Kunutsor et al., 2015), Alzheimer’s disease (Kunutsor and Laukkanen, 2016; Kunutsor et al., 2018), Parkinson’s disease (Yoo et al., 2020), and metabolic abnormalities (Ya et al., 2017). Our study results may help explain why people with higher GGT levels are more likely to have age-related diseases. The accelerated aging of participants with higher GGT levels may be explained by the potential proinflammatory and pro-oxidative effects of GGT (Lee and Jacobs, 2005; Turgut et al., 2006; Turgut and Tandogan, 2011; Sarli et al., 2013). In addition, GGT levels are directly involved in the formation of atheromatous plaques, which have been implicated in the mechanism underlying the pathogenesis of vascular aging (Franzini et al., 2009).
In addition, some prior studies have explored the mediating effect of GGT in the aging process, which is supportive of our findings. For example, Liu et al. (Liu et al., 2024, pp. 2005–2018) showed that GGT, along with bilirubin and uric acid, collectively mediated the relationship between Life’s Essential 8 scores and PhenoAgeAccel, which was measured using clinical laboratory blood chemistries. Moreover, two studies reported that GGT plays an important role in the association of sleep and blood benzene with accelerated aging (Wang X. et al., 2024; Yang et al., 2024). Another study using data from the United Kingdom Biobank revealed that the relationships between plant protein and four biological aging measures were mediated by GGT (Xu et al., 2024). The results of these previous studies suggest that GGT has a crucial effect on the aging process, and our findings directly implicate GGT as an independent driver of biological aging.
Our study revealed a stronger association between GGT levels and the retinal age gap in non-hypertensive participants. The associations of GGT levels with the retinal age gap remained consistent across sex, diabetes, dyslipidemia, and current drinking status subgroups. These findings suggest that the absence of hypertension may enhance the association of GGT levels with the retinal age gap. However, further studies are needed to confirm this interplay.
Our findings have several significant implications for public health and clinical practice. First, we investigated the relationship between high GGT levels and accelerated retinal aging, as qualified by the retinal age gap. Our findings extend the utility of GGT beyond its traditional hepatobiliary applications. Second, the retinal age gap, as quantified through advanced imaging analytics, provides a novel framework for assessing aging processes that is low-cost, widely available, noninvasive, and time efficient. Third, our study further shows the advantages of fine-tuning based on the foundation model. This approach not only improves performance but also further reduces training costs.
The main strengths of this study were the process of capturing high-quality fundus images and performing detailed blood biochemical examinations in a relatively large community-based study, adjustments that were made for several potential confounding factors, including lifestyle and metabolic confounders, and the use of sensitivity analyses and subgroup analyses to ensure the robustness of the results. However, several limitations in our study should also be acknowledged. First, the cross-sectional design precludes causal inference. Although we propose that elevated GGT levels drive retinal aging through oxidative pathways, reverse causation remains theoretically possible. Longitudinal studies tracking GGT trajectories and retinal aging progression are needed to establish temporal relationships, and a future study objective may include further follow-up to the JECS. Second, the study participants were all from the Jidong community, and validation in diverse populations is needed to confirm the universal applicability of these findings. Finally, some potential confounders, such as hormone levels and cell factors that may affect GGT levels, the retinal age gap, and residual confounders, were not included in the analysis.
Conclusion
In conclusion, this study revealed that higher GGT levels were associated with accelerated retinal aging, as quantified by the retinal age gap. Given the accelerating aging of the global population, our findings emphasize that GGT, which serves as a potential systemic biomarker for oxidative stress, may exert a detrimental effect on the aging process, thereby highlighting the importance of integrating GGT assessment into evaluations related to aging.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Staff Hospital of Jidong Oil-field of Chinese National Petroleum and the Ethics Committee of the Eye Hospital of Wenzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
KY: Conceptualization, Investigation, Methodology, Formal Analysis, Writing – original draft, Data curation. XZ: Writing – original draft, Methodology, Investigation, Data curation, Validation. ZL: Data curation, Writing – review and editing. WL: Data curation, Writing – review and editing. JY: Writing – review and editing, Data curation. SD: Writing – review and editing, Data curation. ZW: Data curation, Writing – review and editing. YW: Data curation, Writing – review and editing. JA: Data curation, Writing – review and editing. ZG: Writing – review and editing, Data curation. BS: Data curation, Writing – review and editing. JQ: Resources, Writing – review and editing. FL: Resources, Project administration, Methodology, Conceptualization, Writing – review and editing. LC: Resources, Conceptualization, Project administration, Methodology, Funding acquisition, Writing – review and editing, Supervision, Data curation, Formal Analysis. ML: Project administration, Methodology, Conceptualization, Formal Analysis, Data curation, Supervision, Funding acquisition, Writing – review and editing, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Zhejiang Provincial Natural Science Foundation of China, No. LTGY23H120002 (to ML) and LY22H120007 (to LC); the National Natural Science Foundation of China, No. 82271047 (to LC). The funding bodies played no role in the study design, collection, analysis, and interpretation of data.
Acknowledgments
We thank Kang Zhang for critical comments and wisdom throughout the study. The authors are grateful to the Jidong community for its collaboration, especially the dedicated participants and all research staff involved in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
GGT, gamma-glutamyl transferase; JECS, Jidong Eye Cohort Study; MAE, mean absolute error; MAPE, mean absolute percentage error; RMSE, root mean squared error; FBG, fasting blood glucose; LDL-C, low-density lipoprotein; HDL-C, high-density lipoprotein; TC, total cholesterol; TG, triglyceride; SUA, serum uric acid; SD, standard deviation; BMI, body mass index; CIs, confidence intervals; AMD, age-related macular degeneration.
References
Abreu-Gonzalez R., Rodríguez-Martín J. N., Quezada-Peralta G., Rodrigo-Bello J. J., Gil-Hernández M. A., Bermúdez-Pérez C., et al. (2023). Retinal age as a predictive biomarker of the diabetic retinopathy grade. Arch. la Soc. Española Oftalmol. (English Ed.) 98, 265–269. doi:10.1016/j.oftale.2023.04.008
Ahadi S., Wilson K. A., Babenko B., McLean C. Y., Bryant D., Pritchard O., et al. (2023). Longitudinal fundus imaging and its genome-wide association analysis provide evidence for a human retinal aging clock. eLife 12, e82364. doi:10.7554/eLife.82364
Ali S. S., Oni E. T., Blaha M. J., Veledar E., Feiz H. R., Feldman T., et al. (2016). Elevated gamma-glutamyl transferase is associated with subclinical inflammation independent of cardiometabolic risk factors in an asymptomatic population: a cross-sectional study. Nutr. Metab. (Lond) 13, 37. doi:10.1186/s12986-016-0097-7
Breitling L. P., Claessen H., Drath C., Arndt V., Brenner H. (2011). Gamma-glutamyltransferase, general and cause-specific mortality in 19,000 construction workers followed over 20 years. J. Hepatology 55, 594–601. doi:10.1016/j.jhep.2010.12.029
Brennan P. N., Dillon J. F., Tapper E. B. (2022). Gamma-glutamyl transferase (γ-GT) – an old dog with new tricks? Liver Int. 42, 9–15. doi:10.1111/liv.15099
Chen R., Chen Y., Zhang J., Wang W., Hu W., He M., et al. (2023a). Retinal age gap as a predictive biomarker for future risk of clinically significant diabetic retinopathy. Acta Diabetol. 61, 373–380. doi:10.1007/s00592-023-02199-5
Chen R., Wang Y., Zhang S., Bulloch G., Zhang J., Liao H., et al. (2023b). Biomarkers of ageing: current state-of-art, challenges, and opportunities. MedComm – Future Med. 2, e50. doi:10.1002/mef2.50
Chiyanika C., Shumbayawonda E., Pansini M., Liu K. H., Yip T. C., Wong V. W., et al. (2025). Gamma-glutamyl transferase: a potential biomarker for pancreas steatosis in patients with concurrent obesity, insulin resistance and metabolic dysfunction-associated steatotic liver disease. Clin. Obes. 15, e12712. doi:10.1111/cob.12712
Cho B.-J., Heo J. W., Kim T. W., Ahn J., Chung H. (2014). Prevalence and risk factors of age-related macular degeneration in Korea: the Korea national health and nutrition examination survey 2010–2011. Invest. Ophthalmol. Vis. Sci. 55, 1101–1108. doi:10.1167/iovs.13-13096
Cho E. J., Jeong S.-M., Chung G. E., Yoo J.-J., Cho Y., Lee K., et al. (2023). Gamma-glutamyl transferase and risk of all-cause and disease-specific mortality: a nationwide cohort study. Sci. Rep. 13, 1751. doi:10.1038/s41598-022-25970-0
Corti A., Belcastro E., Dominici S., Maellaro E., Pompella A. (2020). The dark side of gamma-glutamyltransferase (GGT): pathogenic effects of an ‘antioxidant’ enzyme. Free Radic. Biol. Med. 160, 807–819. doi:10.1016/j.freeradbiomed.2020.09.005
Emdin M., Pompella A., Paolicchi A. (2005). Gamma-glutamyltransferase, atherosclerosis, and cardiovascular disease: triggering oxidative stress within the plaque. Circulation 112, 2078–2080. doi:10.1161/CIRCULATIONAHA.105.571919
Flammer J., Konieczka K., Bruno R. M., Virdis A., Flammer A. J., Taddei S. (2013). The eye and the heart. Eur. Heart J. 34, 1270–1278. doi:10.1093/eurheartj/eht023
Franzini M., Corti A., Martinelli B., Del Corso A., Emdin M., Parenti G. F., et al. (2009). Gamma-glutamyltransferase activity in human atherosclerotic plaques-biochemical similarities with the circulating enzyme. Atherosclerosis 202, 119–127. doi:10.1016/j.atherosclerosis.2008.03.023
Grimbly M. J., Koopowitz S.-M., Chen R., Sun Z., Foster P. J., He M., et al. (2024). Estimating biological age from retinal imaging: a scoping review. BMJ Open Ophth 9, e001794. doi:10.1136/bmjophth-2024-001794
Hu W., Wang W., Wang Y., Chen Y., Shang X., Liao H., et al. (2022). Retinal age gap as a predictive biomarker of future risk of Parkinson’s disease. Age Ageing 51, afac062. doi:10.1093/ageing/afac062
Jeon J., Kim D. H., Kim W., Choi D.-W., Jung K. J., Jang S.-I. (2020). Dose-response relationship between gamma-glutamyltransferase and the risk of atherosclerotic cardiovascular diseases in Korean adults. Atherosclerosis 292, 152–159. doi:10.1016/j.atherosclerosis.2019.11.004
Kim J., Han K., Yoo J., Park K.-A., Oh S. Y. (2022). Liver enzymes and risk of ocular motor cranial nerve palsy: a nationwide population-based study. Neurol. Sci. 43, 3395–3405. doi:10.1007/s10072-021-05735-9
Kunutsor S. K. (2016). Gamma-glutamyltransferase—Friend or foe within? Liver Int. 36, 1723–1734. doi:10.1111/liv.13221
Kunutsor S. K., Bakker S. J. L., Kootstra-Ros J. E., Gansevoort R. T., Dullaart R. P. F. (2015). Circulating gamma glutamyltransferase and prediction of cardiovascular disease. Atherosclerosis 238, 356–364. doi:10.1016/j.atherosclerosis.2014.12.045
Kunutsor S. K., Laukkanen J. A. (2016). Gamma glutamyltransferase and risk of future dementia in middle-aged to older Finnish men: a new prospective cohort study. Alzheimer’s and Dementia 12, 931–941. doi:10.1016/j.jalz.2016.03.003
Kunutsor S. K., Laukkanen J. A., Burgess S. (2018). Genetically elevated gamma-glutamyltransferase and Alzheimer’s disease. Exp. Gerontol. 106, 61–66. doi:10.1016/j.exger.2018.03.001
Lee D.-H., Jacobs D. R. (2005). Association between serum gamma-glutamyltransferase and C-reactive protein. Atherosclerosis 178, 327–330. doi:10.1016/j.atherosclerosis.2004.08.027
Lei Y., Gao Y., Song M., Cao W., Sun X. (2020). Retrospective case-control data of serum nitrotyrosine level and clinical biomedical indices in primary glaucoma patients. Data Brief 31, 105706. doi:10.1016/j.dib.2020.105706
Li H., Gao M., Song H., Wu X., Li G., Cui Y., et al. (2023). Predicting ischemic stroke risk from atrial fibrillation based on multi-spectral fundus images using deep learning. Front. Cardiovasc. Med. 10, 1185890. doi:10.3389/fcvm.2023.1185890
Li J., Wang L., Gao Y., Liang Q., Chen L., Sun X., et al. (2022). Automated detection of myopic maculopathy from color fundus photographs using deep convolutional neural networks. Eye Vis 9, 13. doi:10.1186/s40662-022-00285-3
Liu W., Wang J., Wang M., Hou H., Ding X., Ma L., et al. (2024). Oxidative stress factors mediate the association between life’s essential 8 and accelerated phenotypic aging: NHANES 2005–2018. Journals Gerontology Ser. A 79, glad240. doi:10.1093/gerona/glad240
Patton N., Aslam T., MacGillivray T., Pattie A., Deary I. J., Dhillon B. (2005). Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J. Anat. 206, 319–348. doi:10.1111/j.1469-7580.2005.00395.x
Sarli B., Baktir A. O., Saglam H., Arinc H., Kurtul S., Akpek M., et al. (2013). The relation of serum γ-glutamyl transferase levels and coronary collateral circulation in patients with chronic coronary total occlusion. Coron. Artery Dis. 24, 298–302. doi:10.1097/MCA.0b013e32835f301d
Sevgi M., Ruffell E., Antaki F., Chia M. A., Keane P. A. (2025). Foundation models in ophthalmology: opportunities and challenges. Curr. Opin. Ophthalmol. 36, 90–98. doi:10.1097/ICU.0000000000001091
Su B., Zhu X., Yang K., Xiao Y., Li C., Shi K., et al. (2022). Age- and sex-related differences in the retinal capillary plexus in healthy Chinese adults. Eye Vis 9, 38. doi:10.1186/s40662-022-00307-0
Tan Y. Y., Kang H. G., Lee C. J., Kim S. S., Park S., Thakur S., et al. (2024). Prognostic potentials of AI in ophthalmology: systemic disease forecasting via retinal imaging. Eye Vis 11, 17. doi:10.1186/s40662-024-00384-3
Tang Z., Chen X., Zhang W., Sun X., Hou Q., Li Y., et al. (2021). Association between gamma-glutamyl transferase and mild cognitive impairment in Chinese women. Front. Aging Neurosci. 13, 630409. doi:10.3389/fnagi.2021.630409
Turgut O., Tandogan I. (2011). Gamma-glutamyltransferase to determine cardiovascular risk: shifting the paradigm forward. JAT 18, 177–181. doi:10.5551/jat.6189
Turgut O., Yilmaz A., Yalta K., Karadas F., Birhan Yilmaz M. (2006). gamma-Glutamyltransferase is a promising biomarker for cardiovascular risk. Med. Hypotheses 67, 1060–1064. doi:10.1016/j.mehy.2006.04.010
Wang J., Gao Y., Wang F., Zeng S., Li J., Miao H., et al. (2024). Accurate estimation of biological age and its application in disease prediction using a multimodal image transformer system. Proc. Natl. Acad. Sci. U.S.A. 121, e2308812120. doi:10.1073/pnas.2308812120
Wang J., Wang Y. X., Zeng D., Zhu Z., Li D., Liu Y., et al. (2025). Artificial intelligence-enhanced retinal imaging as a biomarker for systemic diseases. Theranostics 15, 3223–3233. doi:10.7150/thno.100786
Wang X., Yan X., Li M., Cheng L., Qi X., Zhang J., et al. (2024). U-shaped association between sleep duration and biological aging: evidence from the UK biobank study. Aging Cell 23, e14159. doi:10.1111/acel.14159
Wong C. W., Wong T. Y., Cheng C.-Y., Sabanayagam C. (2014). Kidney and eye diseases: common risk factors, etiological mechanisms, and pathways. Kidney Int. 85, 1290–1302. doi:10.1038/ki.2013.491
Xu X., Hu J., Pang X., Wang X., Xu H., Yan X., et al. (2024). Association between plant and animal protein and biological aging: findings from the UK biobank. Eur. J. Nutr. 63, 3119–3132. doi:10.1007/s00394-024-03494-9
Ya Z., Fei L., Yue Z., Dan L., Neng-bo L., Yi L., et al. (2017). Association between serum gamma-glutamyl transferase and serum uric acid levels in Chinese females: a cross-sectional study. Endocr. Res. 42, 296–301. doi:10.1080/07435800.2017.1300809
Yang B., Jia Y., Yan M., Zhao X., Gu Z., Qin Y., et al. (2024). Moderate BMI accumulation modified associations between blood benzene, toluene, ethylbenzene and xylene (BTEX) and phenotypic aging: mediating roles of inflammation and oxidative stress. Environ. Pollut. 360, 124669. doi:10.1016/j.envpol.2024.124669
Yang K., Cui L., Zhang G., Wang X., Zhu X., Xiao Y., et al. (2020). The jidong eye cohort study: objectives, design, and baseline characteristics. Eye Vis 7, 58. doi:10.1186/s40662-020-00223-1
Yoo D., Kim R., Jung Y. J., Han K., Shin C. M., Lee J.-Y. (2020). Serum gamma-glutamyltransferase activity and Parkinson’s disease risk in men and women. Sci. Rep. 10, 1258. doi:10.1038/s41598-020-58306-x
Zhang S., Chen R., Wang Y., Hu W., Kiburg K. V., Zhang J., et al. (2023). Association of retinal age gap and risk of kidney failure: a UK biobank study. Am. J. Kidney Dis. 81, 537–544.e1. doi:10.1053/j.ajkd.2022.09.018
Zhou Y., Chia M. A., Wagner S. K., Ayhan M. S., Williamson D. J., Struyven R. R., et al. (2023). A foundation model for generalizable disease detection from retinal images. Nature 622, 156–163. doi:10.1038/s41586-023-06555-x
Zhou Y., Wagner S. K., Chia M. A., Zhao A., Woodward-Court P., Xu M., et al. (2022). AutoMorph: automated retinal vascular morphology quantification via a deep learning pipeline. Trans. Vis. Sci. Tech. 11, 12. doi:10.1167/tvst.11.7.12
Zhu Z., Chen Y., Wang W., Wang Y., Hu W., Shang X., et al. (2022). Association of retinal age gap with arterial stiffness and incident cardiovascular disease. Stroke 53, 3320–3328. doi:10.1161/STROKEAHA.122.038809
Keywords: retinal age gap, gamma-glutamyl transferase, foundation model, aging acceleration, non-invasive screening
Citation: Yang K, Zhu X, Li Z, Lian W, Yan J, Ding S, Wang Z, Wang Y, Ai J, Guo Z, Su B, Qu J, Lu F, Cui L and Li M (2025) Association between gamma-glutamyl transferase levels and the retinal age gap. Front. Physiol. 16:1601093. doi: 10.3389/fphys.2025.1601093
Received: 27 March 2025; Accepted: 15 July 2025;
Published: 26 August 2025.
Edited by:
Christina Maria Pabelick, Mayo Clinic, United StatesReviewed by:
Xiangyu Fu, West China Hospital, Sichuan University, ChinaSerkan Sugeçti, Bülent Ecevit University, Türkiye
Copyright © 2025 Yang, Zhu, Li, Lian, Yan, Ding, Wang, Wang, Ai, Guo, Su, Qu, Lu, Cui and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Li, bG1AZXllLmFjLmNu; Lele Cui, Y2xsQGV5ZS5hYy5jbg==; Fan Lu, bHVmYW5AZXllLmFjLmNu
†These authors have contributed equally to this work
 Kai Yang†
Kai Yang† Jia Qu
Jia Qu Lele Cui
Lele Cui Ming Li
Ming Li