- Department of Radiology, Affiliated Hospital of North Sichuan Medical College, Nanchong, China
Purpose: This study aimed to develop a joint model combining T2-weighted imaging (T2WI) suppressed fat radiomics, and clinical parameters to predict the energy efficiency factor (EEF) required for high-intensity focused ultrasound (HIFU) ablation in patients with adenomyosis.
Materials and methods: This retrospective study included 169 adenomyosis patients who underwent HIFU ablation between September 2021 and May 2024. EEF values were calculated based on T2WI fat suppression (T2WI-FS) sequences, and radiomics features were extracted. Predictive features were selected using minimum redundancy maximum relevance (MRMR) and least absolute shrinkage and selection operator (LASSO) methods, and two joint—based on decision tree and random forest algorithms—models were developed for EEF prediction.
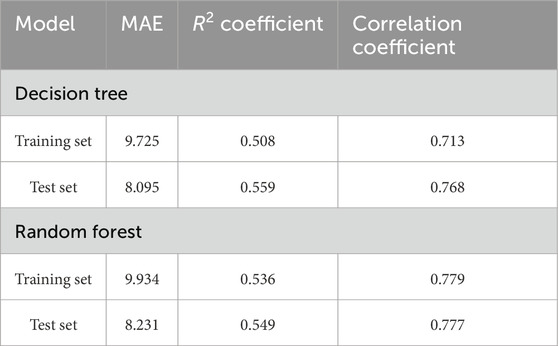
Results: The decision tree model achieved a mean absolute error (MAE) of 8.095 on the test set, while the random forest model exhibited an MAE of 8.231. The Wilcoxon rank-sum test for the test set revealed that the discrepancy in predictive performance between the two models was statistically significant (p < 0.05). The correlation coefficients were 0.768 and 0.777, and the R2 coefficients of the two models in the test set were 0.559 and 0.549, respectively.
Conclusion: The joint model integrating T2WI radiomics and clinical data effectively predicted EEF values for HIFU ablation in adenomyosis. This approach provides a foundation for optimizing HIFU dosing strategies and enhancing treatment safety and efficacy.
1 Introduction
Adenomyosis is a relatively common uterine disorder characterized by the abnormal invasion of endometrial epithelial cells and stromal fibroblasts into the myometrium (Zhai et al., 2020). Affected patients experience excessive uterine bleeding, pelvic pain, anemia, and infertility (Bulun et al., 2021), and the management of this condition often entails the use of surgical procedures, various drugs, uterine artery embolization, and high-intensity focused ultrasound (HIFU) ablation (Chinese experts' consensus on diagnosis and treatment of adenomyosis, 2020; Dason et al., 2023). However, pharmacological therapies are limited by incomplete efficacy and potential side effects (Shui et al., 2015). Surgical treatments may lead to endocrine disturbances and ovarian dysfunction, and hysterectomy is unsuitable for women desiring fertility (Li et al., 2021; Du et al., 2021). Furthermore, uterine artery embolization carries the risk of post-embolization syndrome (de Bruijn et al., 2016).
In patients, HIFU ablation offers particular utility as a fertility-preserving treatment option that is noninvasive and preserves fertility (Shui et al., 2015; Jeng et al., 2020; Siedek et al., 2019; Capezzuoli et al., 2024). HIFU treatment, however, is not an option for all patients, and differences in the amount of ultrasound required or the degree of therapeutic efficacy are evident among different adenomyosis pathological types (Gong et al., 2016; Yu et al., 2023). The energy necessary to ablate a unit volume (mm3) during HIFU procedures is referred to as the energy efficiency factor (EEF) (Gong et al., 2016; Li et al., 2006). Lower EEF values coincide with reduced energy requirements, more efficient ablation, and a shorter procedural duration, while higher EEF values necessitate more energy to achieve the same degree of ablation, resulting in longer procedures and increased risk of serious adverse events for patients (Wei, 2021). Precise control HIFU dosing strategies is thus vital for optimizing therapeutic efficacy and ensuring patient safety. When doses are not sufficient, a second treatment round may be necessary, whereas excessively high doses can cause nerve damage or cutaneous burns (Zhou, 2021).
MRI is often used for the assessment of adenomyosis as it provides high levels of soft-tissue resolution and is amenable to multiparametric multi-sequence imaging (O'Shea et al., 2020). Radiomics is a high-throughput, quantitative approach to processing large volumes of imaging data, enabling the detection of the heterogeneity of tissues of interest through feature extraction to support clinical decision-making (Lambin et al., 2017). So far, there have been relatively few studies exploring the use of T2-weighted imaging (T2WI) fat suppression (T2WI-FS) sequence-based radiomics features as a means of guiding HIFU dose delivery in adenomyosis patients. In this study, radiomics and clinical feature-based machine learning models were developed to predict EEF values in order to provide an imaging-based means of guiding dose delivery for the HIFU-based management of adenomyosis.
2 Materials and methods
2.1 Patient selection
The hospital Medical Ethics Committee provided retrospective approval for this study, with the requirement for informed consent having been waived. For this study, data from patients who underwent HIFU treatment for uterine adenomyosis between September 2021 and May 2024 in our hospital were collected retrospectively. Patient selection was based on predefined inclusion and exclusion criteria.
Inclusion criteria were as follows: (1) adenomyosis diagnosed based on clinical and imaging results; (2) the absence of any relevant history of surgery or other interventions; (3) >18 years of age; (4) MRI performed within 3 days of preoperative and postoperative examinations; (5) complete clinical data.
Exclusion criteria were as follows: (1) the presence of uterine fibroids; (2) any active pelvic inflammation, or suspected/confirmed pelvic malignancies; (3) missing or poor-quality images; (4) women who were breastfeeding or pregnant; (5) comorbid heart, liver, kidney, and other organ failure.
Based on these criteria, 169 patients were retained for further analysis.
2.2 MRI parameters
A 3.0T superconducting magnetic resonance imaging machine (UIH uMR790) with a 12-channel body-phased array coil was used for all MRI scanning. Each participant was positioned supine with the head facing forward, and a sandbag was placed on the abdomen to minimize respiratory artifacts. The T2-weighted fast spin-echo sequence with fat suppression (T2WI-FS) was used for adenomyosis lesion delineation and subsequent radiomics feature extraction. The key imaging parameters for the T2WI-FS sequence included repetition time (TR), echo time (TE), layer thickness, matrix, and field of view (FOV). Specifically, the T2WI-FS sequence used for this study had the following settings: TR = 2867 ms, TE = 93 ms, layer thickness = 4 mm, layer spacing = 2 mm, FOV = 240 mm × 240 mm, and matrix = 256 × 100. Contrast was injected at a rate of 2 mL/s, and arterial images corresponding to the early, intermediate, and late phases were collected at 15, 30, and 45 s, respectively, following contrast injection. The injection dose was 0.1 mmol/kg.
2.3 Preoperative data analysis
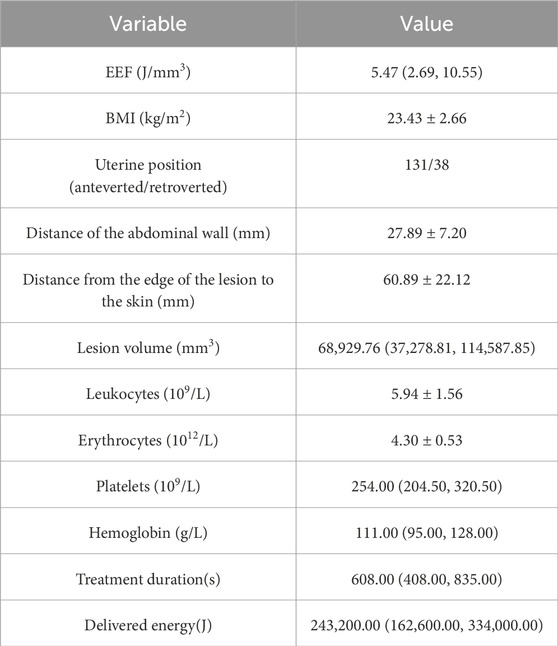
Preoperative MRI data for the enrolled patients were analyzed by two clinicians with over 5 years of imaging experience, with discussion and consensus in cases of disagreement. Analyzed clinical data included distance from the abdominal wall, distance from the leading edge of the lesion to the skin, uterus position (anterior/posterior), lesion volume, body mass index (BMI), age, leukocytes, erythrocytes, hemoglobin, platelets, treatment duration, and delivered energy. The EEF was defined as the amount of ultrasound energy required per unit volume of ablation and calculated as follows: EEF = treatment dose/volume of the non-perfused area (j/mm3) (Du et al., 2021).
2.4 Image segmentation and radiomics feature extraction
Two radiologists with extensive diagnostic experience used 3D Slicer software to outline lesions in a layer-by-layer manner in the SPAIR sequence using preoperative T2WI-FS MRI results. In cases of adenomyosis involving the entire uterine wall, the edge of the region of interest (ROI) was maintained at an appropriate distance from the endometrium and perimetrium layer. “PyRadiomics” software, integrated into the 3D Slicer platform, was used to extract 1,223 features from T2WI-FS sequence ROIs, including various texture, shape, and first-order features. One-third of all objects were randomly extracted from T2WI-FS sequences, and the corresponding ROIs were re-contoured, with the interclass correlation coefficient (ICC) parameter used to examine consistency among investigators. These features with an ICC ≥0.75 were retained.
2.5 Feature selection and model development
The uAI Research Portal (v. 730) was used to analyze radiomics and clinical features separately and develop a predictive model. Initially, patients in the study population were randomized at a 7:3 ratio into the training (n = 118) and testing (n = 51) cohorts. Radiomics feature data analyzed via ICC were Z-score-normalized, and those features that were highly correlated with the EEF but not redundant were retained via minimum redundancy maximum relevance (MRMR), while the least absolute shrinkage and selection operator (LASSO) method was used to screen the retained features, thereby improving the model’s generalizability. Subsequently, L2 norm regularization was applied to the screened features during processing, and the final radiomics and combined regression models were developed using the decision tree and random forest methods. Model performance was assessed based on the R2 coefficient, correlation coefficient, and mean absolute error (MAE) values.
2.6 Statistical analyses
Data were analyzed using R (version 4.3.2) and SPSS 27.0. Normally distributed and skewed continuous data were reported as x ± s and M (Q25, Q75), respectively, with count data instead being reported as the number of cases or the corresponding ratio. MAE values were compared among methods using the Wilcoxon rank-sum test.
3 Results
3.1 Patient data
In total, this study included 169 patients with a median age of 43 years (range: 21–56 years). Other baseline clinical data for these patients are presented in Table 1.
3.2 Feature selection
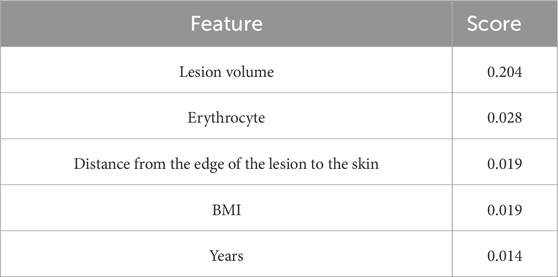
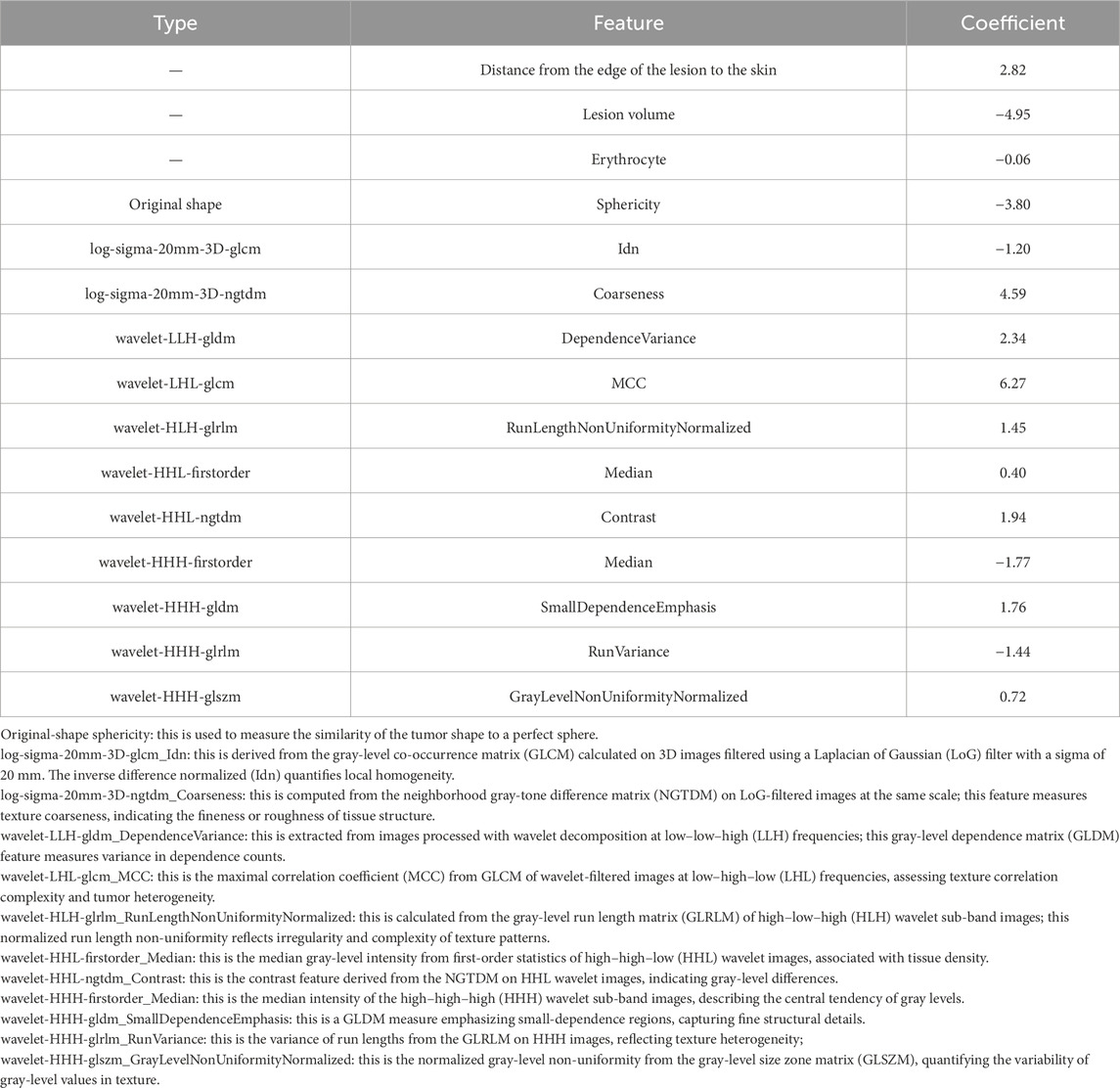
Based on ICC analyses, 1,040 T2WI-FS sequence features were retained for analysis (ICC > 0.75). Using the MRMR method for preliminary feature selection (Table 2), followed by the LASSO method for further refinement, the most important radiomics and clinical features for predicting HIFU ablation doses were selected, resulting in the retention of 12 radiomics features and 3 clinical features, namely, lesion edge-to-skin distance, lesion volume, and red blood cell count (Table 3).
3.3 Model results
The combined models based on 12 imaging features and 3 clinical features were developed using decision tree and random forest methods. These two models demonstrated MAE values of 8.095 and 8.231, respectively, in the testing cohort; the Wilcoxon rank-sum test revealed a statistically significant difference in predictive efficacy between the models in both the training and testing cohorts (p < 0.05). The Pearson correlation coefficients for the two models in the testing cohort were 0.768 and 0.777, respectively. Both models showed a strong correlation between the predicted and actual EEF values, indicating that the developed EEF dose prediction models have practical value (Table 4).
4 Discussion
As a noninvasive treatment modality, HIFU has emerged as an important strategy for adenomyosis treatment as it can help preserve patient fertility (Jeng et al., 2020; Lee, 2021; Wei et al., 2023). The energy dose used for HIFU ablation is closely correlated with procedural efficacy, and the EEF, which measures the energy necessary to ablate a unit volume of the target lesion, is a key index associated with HIFU therapeutic dosing (Yu et al., 2023; Xu et al., 2023). Precise control of patient doses is essential to ensure that treatments are safe and efficacious. If the dose is insufficient, the ablation effect may be inadequate, potentially requiring an additional round of treatment, whereas excessive dosing may result in adverse outcomes such as cutaneous burns or nerve damage (Wei, 2021). To ensure that the appropriate amount of energy is deposited in the target area, EEF thus needs to be precisely predicted and controlled, allowing for the optimization of therapeutic efficacy while preventing complications. In this study, a combined model based on 12 radiomics features derived from T2WI-FS images and 3 clinical features was developed using the decision tree and random forest methods to facilitate EEF prediction in the context of the HIFU ablation-based treatment of adenomyosis. Both of these models performed well in the training and testing cohorts, with the combined model exhibiting preferable predictive ability such that it may aid clinicians in their efforts to develop a reasonable approach to treating adenomyosis patients.
In some reports, researchers have demonstrated the utility of clinical and imaging features as predictors of HIFU ablation dose in patients with uterine diseases. Gong et al. (2016) determined that the ventral distance from the skin of uterine adenomyosis foci, T1WI enhancement type, and foci volume were linearly associated with EEF such that they may be leveraged for a predictive index for the HIFU-based treatment of uterine adenomyosis. Peng et al. (2015) explored the factors that impact ultrasound dose and developed a HIFU dosing model for uterine fibroid treatment, ultimately determining that the ventral distance from the skin to the fibroids and T2WI signal intensity could serve as effective dosimetric predictors in this context. Conventional MRI features derived from visual image inspection, however, are susceptible to inter-observer variability due to differences in experience, compromising the accuracy and stability of these results. Radiomics strategies, in contrast, can enable the high-throughput extraction of quantitative imaging data and the detection of minute differences among images not visible to the naked eye that can be used for further analyses (Lambin et al., 2017; Gillies et al., 2016). Accordingly, a combined model was developed in this study based on MR radiomics features and clinical features to guide the prediction of EEF values for the HIFU-based treatment of adenomyosis. The results are expected to offer guidance for therapeutic dosing efforts. Relative to the T1WI sequences, T2WI-FS sequences do not necessitate the use of contrast, which can damage the kidneys and liver, and signal changes for this sequence are related to the pathological features of uterine adenomyosis (Du et al., 2021; Yu et al., 2023). The T2WI-FS sequence is also closely associated with the pathophysiological changes observed in adenomyotic foci, prompting the selection of this sequence for radiomics feature extraction in the present study.
In this study, the distance from the leading lesion edge to the skin and the lesion volume were identified as predictors of the EEF for HIFU ablation, in line with prior studies (Gong et al., 2016). The greater the distance between the lesion’s leading edge and the skin, the greater the degree to which ultrasound waves will be subjected to reflection, absorption, and scattering during propagation, resulting in attenuation that can adversely affect ablation therapy (Gong et al., 2016; Baker et al., 2001). Deeper focal points have a greater effect on ultrasound during propagation, particularly affecting the tissues in front of the focal point, resulting in the attenuation of the ultrasound energy. Deeper foci require a larger amount of ultrasound energy to achieve the same volume of necrosis relative to superficial lesions with the same ablation rate. This is because tissue interfaces in the acoustic channel may result in greater energy attenuation owing to the absorption, reflection, and scattering of ultrasound waves with increasing volume in front of the lesion (Peng et al., 2015; Liu et al., 2018). As adenomyotic lesions become larger, a lower dose is necessary to ablate a given target tissue volume. This is attributable to the larger lesions occupying a larger amount of the acoustic pathway, minimizing sciatic nerve pain, and decreasing the interval between acoustic treatments such that greater amounts of acoustic energy can be delivered in a given amount of time, thereby preventing the treatment area from cooling. As necrotic tissues have a dynamic impact on the acoustic conditions within the target lesion, this may also play a role in ultrasound energy deposition (Liu et al., 2018; Chen et al., 1997). Additionally, this study found a significant negative correlation between erythrocyte count and the EEF required for HIFU treatment of adenomyosis. Specifically, higher erythrocyte counts were associated with a lower required EEF. We hypothesize a possible mechanism for this phenomenon: an increase in the erythrocyte content alters the acoustic properties of tissue, particularly the acoustic impedance and the propagation path of ultrasound waves in blood-rich tissues. As erythrocyte density increases, the absorption and focusing efficiency of ultrasound energy within the tissue are enhanced, thereby improving energy utilization and reducing the total energy required to achieve therapeutic effects. Furthermore, a high erythrocyte concentration may alter the way heat is deposited in the local microenvironment, making it easier for the treatment area to reach the desired temperature threshold, thus improving the efficiency of the treatment outcome.
The advantage of this study is the development of a clinically applicable predictive model that may facilitate the optimization of ultrasound energy dosing by enabling preoperative adjustment of treatment parameters, thereby improving procedural efficiency and reducing the likelihood of prolonged treatment durations or the need for repeat ablations due to under-dosing. By providing a more precise estimate of the energy required to ablate a specific lesion volume, the model also helps prevent excessive energy delivery, which, in turn, reduces the risk of complications and enhances overall treatment safety. Importantly, by striking an appropriate balance between sufficient and excessive dosing, this approach not only safeguards patient wellbeing but also promotes greater treatment compliance, satisfaction, and long-term clinical benefit.
This study has several limitations. The retrospective design may introduce selection bias. Furthermore, the lack of external validation and the relatively small sample size constrain the generalizability of the findings. Although the model achieved moderate predictive performance (R2 = 0.50–0.55), providing a clinically meaningful benchmark, significant room for improvement remains in HIFU’s EEF modeling. Additionally, variations in MRI equipment and acquisition protocols across institutions pose a major challenge to the reproducibility of radiomics features, potentially compromising the model’s clinical applicability. To address these limitations, future research will focus on expanding the sample size and conducting multicenter studies to evaluate the model’s generalizability and robustness across diverse populations and clinical settings. We will also explore advanced ensemble learning methods, such as XGBoost and LightGBM, to further enhance predictive accuracy. Moreover, efforts will be undertaken to standardize MRI protocols and calibrate equipment consistently across all participating centers. These initiatives aim to enhance the model’s reliability and clinical utility, enabling its use as a tool to guide clinical decision-making in HIFU-based treatments for adenomyosis.
5 Conclusion
In summary, the T2WI-FS radiomics and clinical feature-based model developed in this study was able to reliably predict the EEF value necessary for the HIFU ablation of uterine adenomyosis. This tool can thus effectively guide the clinical assessment of the difficulty of HIFU-based uterine adenomyosis treatment, providing a reference for HIFU dose selection while improving the safety and efficacy of this interventional approach.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Ethics statement
The Affiliated Hospital of North Sichuan Medical College’s Medical Ethics Committee eliminated the need for informed consent after approving the research plan (IRB no. 2024ER282-1). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZaL: Data curation, Writing – original draft, Investigation, Methodology. ZiL: Methodology, Writing – original draft. YW: Conceptualization, Writing – original draft. XW: Software, Writing – original draft. XH: Conceptualization, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2025.1602866/full#supplementary-material
References
Baker K. G., Robertson V. J., Duck F. A. (2001). A review of therapeutic ultrasound: biophysical effects. Phys. Ther. 81, 1351–1358. doi:10.1093/ptj/81.7.1351
Bulun S. E., Yildiz S., Adli M., Wei J. J. (2021). Adenomyosis pathogenesis: insights from next-generation sequencing. Hum. Reprod. Update 27, 1086–1097. doi:10.1093/humupd/dmab017
Capezzuoli T., Toscano F., Ceccaroni M., Roviglione G., Stepniewska A., Fambrini M., et al. (2024). Conservative surgical treatment for adenomyosis: new options for looking beyond uterus removal. Best. Pract. Res. Clin. Obstet. Gynaecol. 95, 102507. doi:10.1016/j.bpobgyn.2024.102507
Chen L., ter Haar G., Hill C. R. (1997). Influence of ablated tissue on the formation of high-intensity focused ultrasound lesions. Ultrasound Med. Biol. 23, 921–931. doi:10.1016/s0301-5629(97)00016-1
Chinese experts' consensus on diagnosis and treatment of adenomyosis (2020). Zhonghua Fu Chan Ke Za Zhi 55, 376–383. doi:10.3760/cma.j.cn112141-20200228-00150
Dason E. S., Maxim M., Sanders A., Papillon-Smith J., Ng D., Chan C., et al. (2023). Guideline no. 437: diagnosis and management of adenomyosis. J. Obstet. Gynaecol. Can. 45, 417–429.e1. doi:10.1016/j.jogc.2023.04.008
de Bruijn A. M., Ankum W. M., Reekers J. A., Birnie E., van der Kooij S. M., Volkers N. A., et al. (2016). Uterine artery embolization vs hysterectomy in the treatment of symptomatic uterine fibroids: 10-Year outcomes from the randomized EMMY trial. Am. J. Obstet. Gynecol. 215, 745.e1–745.e12. doi:10.1016/j.ajog.2016.06.051
Du C. C., Wang Y. Q., Qu D. C., Jiang L. P., Yu X., Zhou H. G. (2021). Magnetic resonance imaging T2WI hyperintense foci number and the prognosis of adenomyosis after high-intensity focused ultrasound treatment. Int. J. Gynaecol. Obstet. 154, 241–247. doi:10.1002/ijgo.13587
Gillies R. J., Kinahan P. E., Hricak H. (2016). Radiomics: images are more than pictures, they are data. Radiology 278, 563–577. doi:10.1148/radiol.2015151169
Gong C., Yang B., Shi Y., Liu Z., Wan L., Zhang H., et al. (2016). Factors influencing the ablative efficiency of high intensity focused ultrasound (HIFU) treatment for adenomyosis: a retrospective study. Int. J. Hyperth. 32, 496–503. doi:10.3109/02656736.2016.1149232
Jeng C. J., Ou K. Y., Long C. Y., Chuang L., Ker C. R. (2020). 500 cases of high-intensity focused ultrasound (HIFU) ablated uterine fibroids and adenomyosis. Taiwan J. Obstet. Gynecol. 59, 865–871. doi:10.1016/j.tjog.2020.09.013
Lambin P., Leijenaar R. T. H., Deist T. M., Peerlings J., de Jong E. E. C., van Timmeren J., et al. (2017). Radiomics: the bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 14, 749–762. doi:10.1038/nrclinonc.2017.141
Lee K. W. (2021). The Asian perspective on HIFU. Int. J. Hyperth. 38, 5–8. doi:10.1080/02656736.2021.1889697
Li F., Wang Z., Du Y., Ma P., Bai J., Wu F., et al. (2006). Study on therapeutic dosimetry of HIFU ablation tissue. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 23, 839–843. doi:10.3321/j.issn:1001-5515.2006.04.035
Li Z., Zhang J., Song Y., Yin X., Chen A., Tang N., et al. (2021). Utilization of radiomics to predict long-term outcome of magnetic resonance-guided focused ultrasound ablation therapy in adenomyosis. Eur. Radiol. 31, 392–402. doi:10.1007/s00330-020-07076-1
Liu Z., Gong C., Liu Y., Zhang L. (2018). Establishment of a scoring system for predicting the difficulty level of high-intensity focussed ultrasound ablation of uterine fibroids. Int. J. Hyperth. 34, 77–86. doi:10.1080/02656736.2017.1325015
O'Shea A., Figueiredo G., Lee S. I. (2020). Imaging diagnosis of adenomyosis. Semin. Reprod. Med. 38, 119–128. doi:10.1055/s-0040-1719017
Peng S., Zhang L., Hu L., Chen J., Ju J., Wang X., et al. (2015). Factors influencing the dosimetry for high-intensity focused ultrasound ablation of uterine fibroids: a retrospective study. Medicine 94, e650. doi:10.1097/MD.0000000000000650
Shui L., Mao S., Wu Q., Huang G., Wang J., Zhang R., et al. (2015). High-intensity focused ultrasound (HIFU) for adenomyosis: two-year follow-up results. Ultrason. Sonochem 27, 677–681. doi:10.1016/j.ultsonch.2015.05.024
Siedek F., Yeo S. Y., Heijman E., Grinstein O., Bratke G., Heneweer C., et al. (2019). Magnetic resonance-guided high-intensity focused ultrasound (MR-HIFU): technical background and overview of current clinical applications (part 1). Rofo 191, 522–530. doi:10.1055/a-0817-5645
Wei C. (2021). “Prediction of pathological classification, ablation difficulty and immediate ablation rate of uterine fibroids treated by HIFU based on conventional MRI and T2WI-radiomics features,” doi:10.26921/d.cnki.ganyu.2021.000015
Wei J., Wang L., Tao H., Wang X., Zheng F., He P., et al. (2023). Comparison of pregnancy outcomes in infertile patients with different types of adenomyosis treated with high-intensity focused ultrasound. Int. J. Hyperth. 40, 2238140. doi:10.1080/02656736.2023.2238140
Xu F., Lin Z., Wang Y., Gong C., He M., Guo Q., et al. (2023). Comparison of high-intensity focused ultrasound for the treatment of internal and external adenomyosis based on magnetic resonance imaging classification. Int. J. Hyperth. 40, 2211268. doi:10.1080/02656736.2023.2211268
Yu J. W., Yang M. J., Jiang L., Su X. Y., Chen J. Y. (2023). Factors influencing USgHIFU ablation for adenomyosis with NPVR ≥ 50. Int. J. Hyperth. 40, 2211753. doi:10.1080/02656736.2023.2211753
Zhai J., Vannuccini S., Petraglia F., Giudice L. C. (2020). Adenomyosis: mechanisms and pathogenesis. Semin. Reprod. Med. 38, 129–143. doi:10.1055/s-0040-1716687
Keywords: adenomyosis, magnetic resonance imaging, high-intensity focused ultrasound, energy efficiency factor, prediction
Citation: Liu Z, Liu Z, Wang Y, Wan X and Huang X (2025) Machine learning-based predictive analysis of energy efficiency factors necessary for the HIFU treatment of adenomyosis. Front. Physiol. 16:1602866. doi: 10.3389/fphys.2025.1602866
Received: 09 April 2025; Accepted: 14 July 2025;
Published: 15 August 2025.
Edited by:
Jiu Chen, Nanjing University, ChinaReviewed by:
Onur Can Bayrak, Yildiz Technical University, TürkiyeNa Tang, Shanghai First People’s Hospital, China
Copyright © 2025 Liu, Liu, Wang, Wan and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohua Huang, MTUwODI3OTc1NTNAMTYzLmNvbQ==
 Ziyan Liu
Ziyan Liu Ziyi Liu
Ziyi Liu Yuan Wang
Yuan Wang Xiyao Wan
Xiyao Wan Xiaohua Huang
Xiaohua Huang