- 1Institute of Applied Biology, Faculty of Biotechnology and Food Sciences, Slovak University of Agriculture in Nitra, Nitra, Slovakia
- 2Institute of Biotechnology, Faculty of Biotechnology and Food Sciences, Slovak University of Agriculture in Nitra, Nitra, Slovakia
- 3Institute of Nutrition and Genomics, Faculty of Agrobiology and Food Resources, Slovak University of Agriculture in Nitra, Nitra, Slovakia
- 4Institute of Food Sciences, Faculty of Biotechnology and Food Sciences, Slovak University of Agriculture in Nitra, Nitra, Slovakia
Trace elements are essential for a number of physiological functions including oxygen transfer, enzymatic reactions and antioxidant protection of the animal organism. Elevated concentrations outside the physiological optimum, on the other hand, can cause undesirable health complications, disrupt metabolic pathways, reproductive capacity, or oxidative balance. The negative anthropogenic impacts on the environment are alarming and the impacts on the aquatic environment have been increasing disproportionately in recent years. Against this background, all potential threats to biota need to be explained and better understood, the possible risks need to be better informed and understood, and a balance needs to be struck between the fundamental nature and the harmful effects of these metals. This mini-review examines the roles of potentially toxic metals including cobalt (Co), copper (Cu), iron (Fe), manganese (Mn), molybdenum (Mo) and zinc (Zn) in fish physiology. This document also elucidates the mechanisms underlying the assessment of regulatory processes, the potential negative consequences of overexposure, the interactions of these metals on fish health, and in the environmental context.
1 Introduction
Aquatic environment is complex system, however, human activities, such as industrial discharge, agricultural runoff, and urban wastewater, are increasingly disrupting this delicate balance. This leads to an accumulation of many types of environmental pollutants in the water and sediment (Edo et al., 2024; Khallaf et al., 2018; Kolarova and Napiórkowski, 2021; Kumar et al., 2010). One group of these pollutants are metals, or trace elements. Heavy metals, or potentially toxic elements, are fairly well studied (Dash and Kalamdhad, 2021; Deb and Fukushima, 1999; Kumar et al., 2010). On the other hand, we have the group of potentially toxic metals. These elements (e.g., cobalt, Co, copper, Cu, iron, Fe, manganese, Mn, molybdenum, Mo, or zinc, Zn) are essential for many basic physiological functions of organisms, such as oxygen transport, enzymatic reactions, and the maintenance of antioxidant defence mechanisms (Aliko et al., 2018; Inomata, 2024; Pouil et al., 2016; Wang et al., 2024). They also play a critical role in maintaining the physiological health of fish and are involved in cellular processes and metabolic pathways (Bagheri et al., 2024; Lall, 2003; Zhao et al., 2014). The presence of potentially toxic metals alone does not determine their effects on aquatic animals; the concentration levels and types of interactions they experience in an ecological context also play a role. While some metals may be necessary in small amounts, excessive accumulation above a certain threshold can have adverse toxicological effects (Bury et al., 2003; Chen et al., 2024; Clearwater et al., 2002; Naz et al., 2023). The situation is further complicated by the many interactions between different metals, which may be antagonistic or synergistic in their activities. Elevated metal concentrations can have cascading consequences, including bioaccumulation, oxidative stress, reproductive dysfunction, and impaired biochemical and/or immune systems in fish (de Oliveira et al., 2018; Helczman et al., 2024; Kovacik et al., 2023; Passos et al., 2022; Kovacik et al., 2019). One metal may affect the toxicity or uptake of another metal, creating a dynamic system whose effects on fish populations are unpredictable.
This mini-review aims to summarize the current knowledge about the effects of potentially toxic metals on fish health, focusing on the following points.
• Physiological roles of potentially toxic metals in fish
•Mechanisms of absorption and regulation
•Possible adverse effects of overexposure
•Interactions of these metals the aquatic environment
•Implications for ecological risk assessment and management of aquatic ecosystems
By examining these variables, we can provide a comprehensive and up-to-date summary of recent research on the function of potentially toxic metals in fish health-a broader and more integrative approach than many previous studies, which have focused primarily on contamination levels or toxicological effects. We also aim to identify areas of current ignorance and suggest possible directions for future research based on data from multiple studies. Previous reviews have largely focused on heavy metals as toxic pollutants affecting fish physiology and on the human health risks associated with metal contamination in fish tissues. The emphasis of this review on the physiological functions and regulatory mechanisms of potentially toxic metals, together with an assessment of ecological risks, represents a significant and novel contribution to the field.
2 Sources of potentially toxic metals in fish
The accumulation of potentially toxic metals such in the aquatic environment and in fish can be attributed to both natural and anthropogenic sources. Understanding these sources is essential to mitigate the risks associated with metal bioaccumulation in fish and to ensure the sustainability of aquatic ecosystems. The reported concentrations of selected metals in freshwater ecosystem and fish are presented in Supplementary Material (Supplementary Table S1).
2.1 Natural sources
The main natural sources of metals in the aquatic environment include geological weathering of rocks, erosion and biological processes in ecosystems. These metals subsequently reach the water and sediments, from where they can naturally, through various forms of transport, enter the body of fish and accumulate there. Fe and Mn are widely distributed elements in the earth’s crust (Jia et al., 2018; Kumar et al., 2010). They are naturally and readily released into aquatic ecosystems by weathering of rocks and soil erosion. They are found mainly in sediments, along with Zn and Cu. Fish may accumulate metals through their diet and interactions with the environment. Some fish species feed on sediments and the organisms residing in them, which facilitates the transfer of metals such as Fe and Mn from sediments into the fish body (Campos et al., 2018; Rajkowska and Protasowicki, 2013). These metals also accumulate in phytoplankton and zooplankton from where they are further transferred to higher trophic levels, including fish (Dash and Kalamdhad, 2021; Kumar et al., 2010). Oxygen levels in water due to seasonal changes can affect the availability of metals in the aquatic environment. Under low O2 conditions, there is increased mobility of Fe and Mn in sediments, leading to higher bioaccumulation in fish (Boota et al., 2024).
2.2 Anthropogenic sources
The main anthropogenic sources of metals in the aquatic ecosystem include industrial discharges, agricultural runoff, mining activities and wastewater. High levels of metals such as Cu, Zn, and Mn are often found in wastewater from industry. Numerous studies from the Indus River and Nile River channels have confirmed elevated concentrations of these metals in fish due to nearby industrial activities in the area (Boota et al., 2024; Khallaf et al., 2018). The widespread use of fertilizers and pesticides contributes significantly to environmental pollution and the release of metals such as Zn, Cu and Mn into water. Fe, Zn and Cu are often found in high levels in wastewater whether as a result of corrosion of pipelines or processing of various materials in the metallurgical or electro technical industries. Waste and leachate from mining activities is one of the most significant contributors to pollution of watercourses. Metals such as Fe and Mn enter the aquatic ecosystem through mine water discharges. In the Xiang River in China, mining activities have been identified as the primary source of metal contamination, with fish species showing elevated levels of Zn, Fe and Cu (Jia et al., 2018).
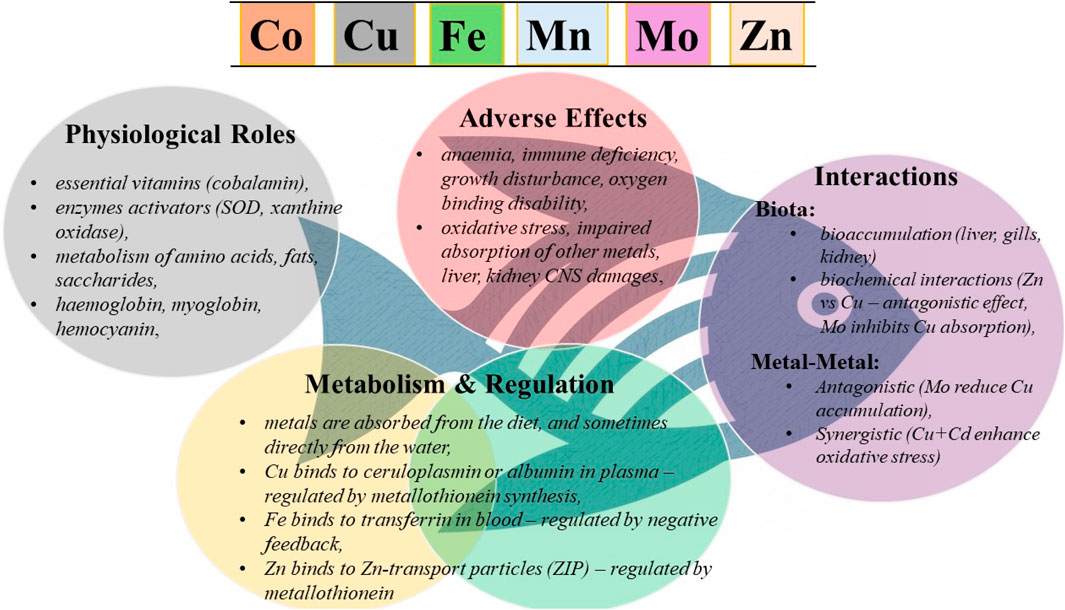
3 Biological role of potentially toxic metals in freshwater fish
Essential metals play specific and crucial roles in the fish organism. They are involved in the immune response, physiological processes, antioxidant protection, enzymatic activity as well as overall growth and development (Table 1).
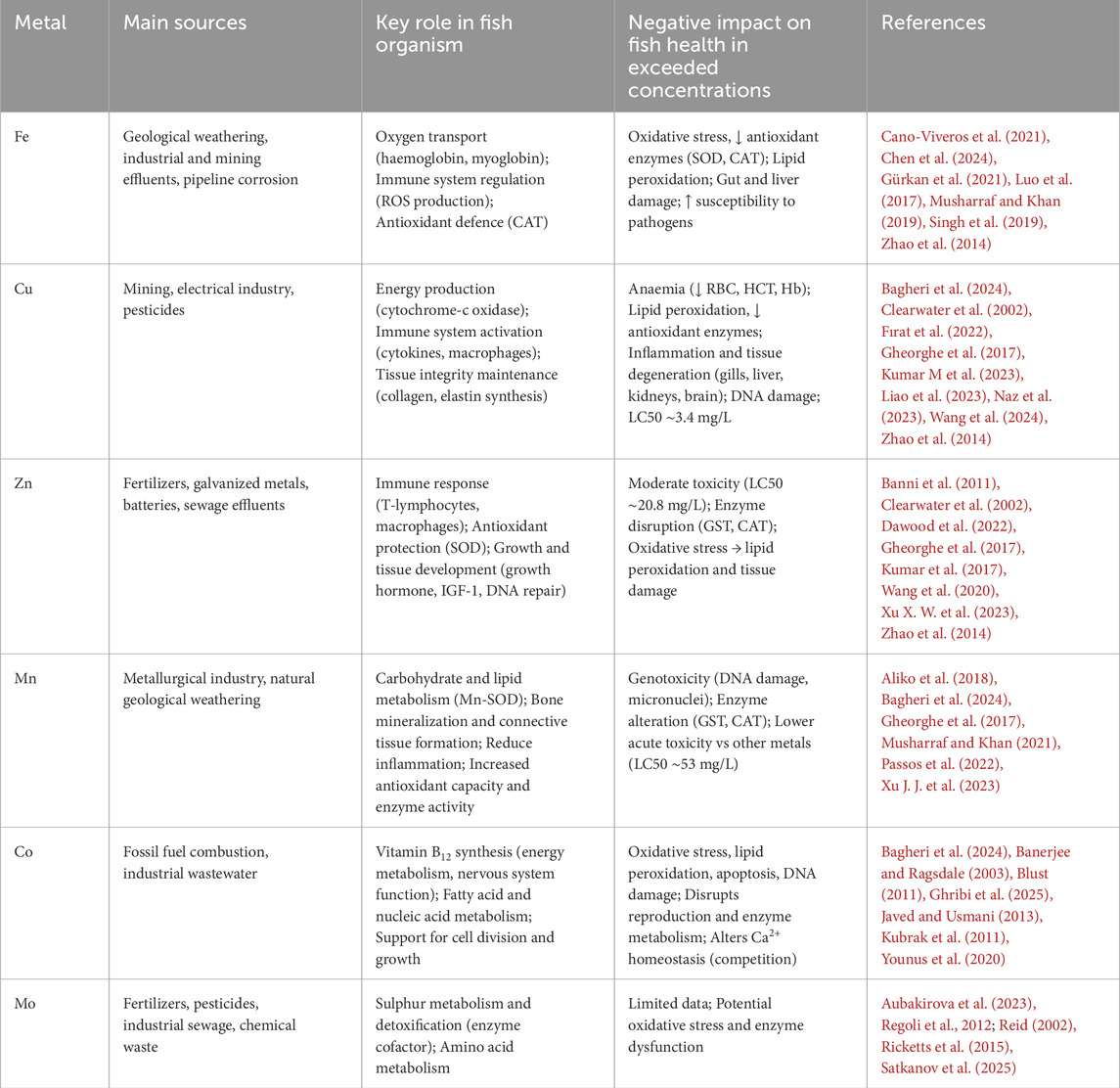
Table 1. Summary of sources, key roles in the organism and negative effects of selected metals on fish health.
Co is one of the less studied trace elements for fish health but plays several known functions. It is a key component of vitamin B12, which is essential for energy metabolism and proper nervous system function. Acts as a cofactor for enzymes involved in fatty acid synthesis and nucleic acid metabolism (Banerjee and Ragsdale, 2003; Blust, 2011). Supports cell division and differentiation and is essential for normal growth and development of cyprinid fish Tor putitora, especially in the early stages of life (Younus et al., 2020).
Cu is a cofactor of several enzymes such as cytochrome-c oxidase and superoxide dismutase (Cu/Zn-SOD - in the cytosol), which play a role in energy production and antioxidant defence (Clearwater et al., 2002; Wang et al., 2024). It plays a role in cytokine production, and is involved in the activation of immune cells such as macrophages and lymphocytes (Bagheri et al., 2024). It is an essential component for the synthesis of elastin and collagen, critical for tissue integrity and wound healing (Wang et al., 2024). Participates in the nervous system health, neurotransmitter synthesis and synaptic function in stressed zebrafish (Green et al., 2024).
Fe is a vital element for various biological processes in fish, including oxygen transport, enzyme function, and immune response. As a key component of haemoglobin and myoglobin, Fe is essential for oxygen transport and energy metabolism in fish (de Oliveira et al., 2018). It is involved in the regulation of immune cells and the production of reactive oxygen species (ROS) required for defence against pathogens (de Oliveira et al., 2018; Musharraf and Khan, 2019). It is part of the antioxidant capacity, as a component of catalase (CAT), an iron-containing haemoprotein that breaks down hydrogen peroxide (H2O2) into water and oxygen, thereby protecting cells from oxidative damage (de Oliveira et al., 2018; Luo et al., 2017). Dietary Fe improves growth performance, haematology and gut health of fish Labeo rohita (Musharraf and Khan, 2019), especially when provided in a nanoform that increases bioavailability.
Mn is a cofactor of enzymes involved in carbohydrate metabolism, lipid metabolism. Plays role in antioxidant protection as part of the superoxide dismutase (Mn-SOD) (de Oliveira et al., 2018; Musharraf and Khan, 2021). Mn could impact bone mineralization and connective tissue protein synthesis in salmonids (Baeverfjord et al., 2019). It is important for the growth performance, fatty acid uptake or triglycerides deposition. Mn also could reduce inflammation, increased antioxidant capacity and enzyme activity in Carassiud auratus or Pelteobagrus fulvidraco (Aliko et al., 2018; Xu J. J. et al., 2023).
Mo is a cofactor of enzymes involved in sulphur metabolism and detoxification processes (Inomata, 2024; Lall, 2003). It is essential for the metabolism of amino acids and other biomolecules (Lall, 2003). Recent studies suggest a significant biological relevance, namely in the context of nitric oxide production from nitrate and nitrite by increasing the activity of the molybdoenzymes xanthine oxidase (XO) and aldehyde oxidase (AO) in the liver in Silurus glanis (Aubakirova et al., 2023). Another study provides important insights into the ability of a Mo cofactor (Mo-co) isolated from the protein fraction of fish liver extract to restore NADPH-nitrate reductase (NADPH-NR) activity (Satkanov et al., 2025).
Zn is one of the best-studied elements in fish nutrition and physiology. It is critical for the activation and function of T-lymphocytes and macrophages and is involved in the production of antibodies. Participates in the regulation of immune response-related gene expression in Oreochromis niloticus (El-Sayed et al., 2023). Acts as a cofactor for antioxidant enzymes such as SOD, and helps protect cells from oxidative damage in zebrafish or Pangasius hypophthalmus (Banni et al., 2011; Kumar et al., 2017). Regulates endocrine signalling pathways (growth hormone and IGF-1) that influence body growth, tissue differentiation and bone ossification. It is part of enzymes that regulate important biological functions including DNA repair, RNA formation and amino acid metabolism (Dawood et al., 2022; Wang et al., 2020). It is required for the activity of enzymes involved in DNA replication, and its absence causes growth inhibition and abnormalities (Clearwater et al., 2002), Specifically, increased growth performance, improved antioxidant capacity and inflammatory responses were confirmed in P. fulvidraco (Xu X. W. et al., 2023).
4 Key pathways and uptake mechanisms in the accumulation of metals in fish
The accumulation of potentially toxic metals in freshwater fish involves a complex interplay of uptake pathways and biological mechanisms (Figure 1). The main uptake pathways include uptake through the food and water, and indirect uptake through the skin and mucous membranes.
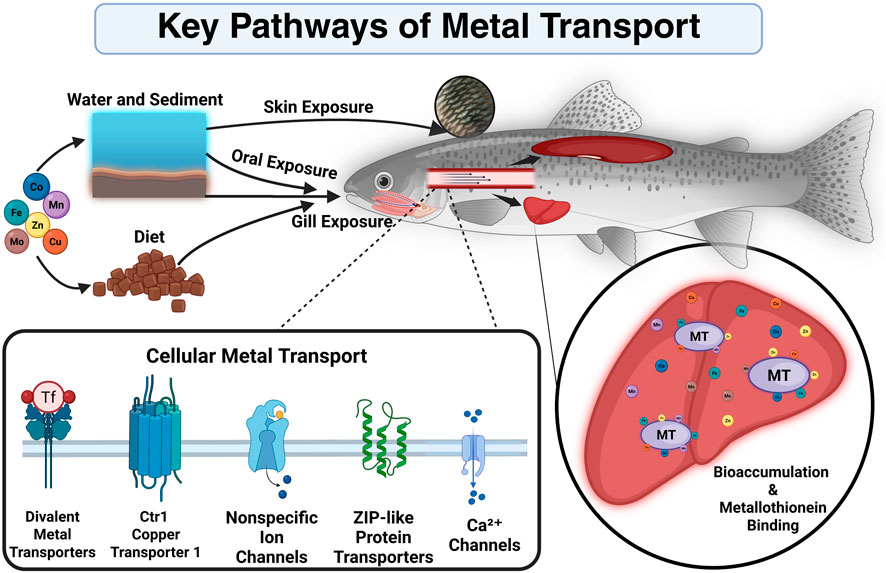
Figure 1. Key exposure routes, cellular uptake mechanisms, and intracellular interactions of metals in fish (Created in BioRender. Helczman, 2025).
Gills are considered to be the primary source of uptake of metals from the aquatic environment into the fish. Gills play a critical role in ion regulation and gas exchange, and are directly and continuously exposed (Deb and Fukushima, 1999; Zia and McDonald, 1994). Chloride cells found in the gills of fish are specialized cells that are involved in ion transport, especially Ca2+. They may also play a role in the uptake of some metals, especially when present as divalent cations (Zia and McDonald, 1994).
Through ionic bonds or covalent bonds with cytosolic compounds, these metals can bind to the gill surface. The affinity is different in this process, with Cu showing a higher affinity compared to Fe or Zn.
Fish accumulate high levels of metals through ingestion of food or sediment (Bury et al., 2003; Pouil et al., 2016). Assimilatory efficiency plays an important role in the transport of metals across the intestinal wall, which varies greatly depending on the specific metal. For example, Zn and Cu have a higher assimilative efficiency than Co or Mo, which may lead to their more efficient absorption in the gut and consequently higher accumulation in the body of Oncorhynchus mykiss (Ojo and Wood, 2007).
Particularly, in the presence of high concentrations of metals in the aquatic environment, absorption of metals also occurs through the skin and mucous membranes of fish (Deb and Fukushima, 1999). This process can be influenced by factors such as the chemical form of the metal, water temperature, pH and the physiological state of the fish.
Metals enter fish cells through ion channels and transporters. While Cu and Zn can enter cells through Ca2+ channels, whereas Fe uptake is mediated by specific iron transporters such as DMT1 (divalent metal transporter 1) or transferrin receptors (Bury et al., 2003; Deb and Fukushima, 1999). Metals can interact with each other in their absorption and biological effects. For instance, excess zinc (Zn) may reduce copper (Cu) absorption by stimulating the production of metallothionein’s, which bind copper and prevent its absorption. Similarly, iron (Fe) can increase the toxicity of manganese (Mn) by promoting its accumulation in the body, especially in the brain, which can lead to neurotoxic effects (Clearwater et al., 2002; de Oliveira et al., 2018).
Specific proteins and metallothionein (MT) play the most important role in maintaining metal homeostasis and detoxification of the body (Deb and Fukushima, 1999). MT´s are cysteine-rich proteins that bind metals via thiol groups. They are involved in the detoxification of metals such as Cu and Zn. Metals are bound by these mechanisms after entering the fish body and subsequently either stored or excreted for proper regulation.
The bioavailability of metals is influenced by the chemical parameters of the water (pH, hardness and the presence of ligands). The toxicity of some metals such as Cu is influenced by the formation of hydroxide complexes in circumneutral waters (Liao et al., 2023).
The gills, as an organ constantly exposed to direct contact with water and food, and the liver, as a major detoxification organ, are among the primary tissues of metal accumulation. Numerous studies have demonstrated high accumulation of metals such as Fe, Cu and Zn in the liver (Javed and Usmani, 2013; Jia et al., 2017; Spanopoulos-Zarco et al., 2017). Kidney is also one of the organs with high accumulation, as it plays an important role in excretion of excess metals. However, the efficiency of excretion varies depending on both the metal and the degree of accumulation (Deb and Fukushima, 1999; Javed and Usmani, 2013).
5 Potential toxicity of metals in fish
Although the monitored elements are biogenic and play various roles in the fish organism, maintaining their optimal levels is crucial for the health of the organism. Their deficiency or excessive accumulation can lead to physiological and biochemical disturbances, that may have toxic effects on cellular and systemic processes. In general, excessive levels of metals can cause oxidative stress, DNA damage and disruption of enzyme activity, leading to impaired growth, reproduction and survival (de Oliveira et al., 2018; Naz et al., 2023). Also, metal speciation in water, i.e. the different chemical forms in which these metals occur in the environment, directly affects their effectiveness and uptake/absorption into the organism. Free metal ions are more bioavailable, and fish tissues/mucous membranes absorb them much more easily. On the other hand, metals in organic or inorganic compounds are less bioavailable (De Paiva Magalhães et al., 2015; Millero, 2001). Metal speciation, and thus of course bioavailability, is also affected by environmental changes (e.g. pH, salinity, hardness, and oxygen or carbon content) (Dahlberg et al., 2025; Pierrot and Millero, 2017; Rouleau et al., 1996); for example, significant changes in pH (either decrease or increase) combined with factors such as water hardness and the presence of different metals, influence metal speciation by maintaining metals in their free ionic forms, which can increase absorption and associated toxicity in fish (De Paiva Magalhães et al., 2015; Namieśnik and Rabajczyk, 2010).
Excessive dietary Fe exposure can lead to oxidative stress, decreased activity of antioxidant enzymes (e.g., superoxide dismutase, catalase), and increased lipid peroxidation (Cano-Viveros et al., 2021; Gürkan et al., 2021), specifically in the liver of Prochilodus lineatus (de Oliveira et al., 2018). This suppression of immunity makes fish more susceptible to pathogens such as Aeromonas hydrophila in Micropterus salmoides (Chen et al., 2024). High Fe levels can also damage intestinal, gills and liver tissues in Labeo rohita and alter the composition and function of the gut microbiota (Chen et al., 2024; Singh et al., 2019), specifically in M. salmoides.
Exposure to copper sulphate or copper oxide nanoparticles (CuO-NPs) decreases erythrocyte count, haematocrit, and haemoglobin levels, indicating anaemia. Cu exposure increases lipid peroxidation and decreases the activity of antioxidant enzymes (e.g., SOD, CAT). It also causes inflammation and degenerative changes in gills, liver, kidney and brain tissues (Fırat et al., 2022; Naz et al., 2023), and DNA damage (Fırat et al., 2022; Kumar M et al., 2023; Passos et al., 2022) in L. rohita, Oreochromis niloticus, and/or Channa punctata.
The 96-h LC50 for Zn in freshwater fish (e.g., Cyprinus carpio) is approximately 20.8 mg/L, indicating moderate toxicity (Gheorghe et al., 2017). Zn toxicity affects the activity of enzymes involved in detoxification, including increased expression of glutathione-S-transferase (GST) and catalase (CAT) (Passos et al., 2022). This response can lead to oxidative stress, which causes lipid peroxidation and subsequent tissue damage.
Mn in combination with Fe induces genotoxic effects including DNA damage and micronucleus formation in O. niloticus (Passos et al., 2022). It also alters the activity of thyroid function, hepatic, GST and CAT enzymes (Hoseini et al., 2014; Kumar N et al., 2023). The LC50 for Mn in freshwater fish C. carpio is relatively high (>53 mg/L), indicating lower acute toxicity compared to other metals (Gheorghe et al., 2017).
Co toxicity can induce oxidative stress leading to lipid peroxidation, cell apoptosis and DNA damage in fish tissues (e.g. in Carassius auratus) (Blust, 2011; Kubrak et al., 2011). High levels interfere with fish reproductive health, enzyme metabolism and calcium balance due to competition (Bagheri et al., 2024; Ghribi et al., 2025).
In the case of Mo, there is insufficient information on its effects on fish health in excessive accumulation, but it is suggested that oxidative stress and dysfunction of some enzymes may occur. The 96-h LC50 values range from >50 to >10,000 mg L-1, with these values varying widely depending on the species (Reid, 2002; Ricketts et al., 2015). Exceeding toxic levels can lead to increased Mo accumulation in tissues and potential toxicity (Regoli et al., 2012). Despite its low toxicity, physiological changes have been observed even at sublethal concentrations, including accelerated breathing, loss of balance after physical exertion, and tissue damage such as fused gill lamellae and haemorrhages in the digestive tract (Reid, 2002; Ricketts et al., 2015). Interesting are also the interactions of Mo in nanoforms, e.g. MoS2, which can increase the toxicity of other metals, such as antimony, through oxidative stress and disruption of fatty acid metabolism in algae (Zou et al., 2024).
6 Future research and recommendations
Research on trace metals in fish is a key area for understanding their dual impact as essential elements and potential toxicants. The first priority should be to develop advanced, more sensitive and cost-effective methods for realistic monitoring of metal concentrations in the aquatic ecosystem. The use of modern techniques and artificial intelligence appears to be a promising step. Advanced AI algorithms can process large monitoring datasets, identify patterns of biomarker changes based on metal concentrations and environmental conditions, and generate predictive models for the early detection of risk and real-time prognosis of toxic effects. It is also important to identify new biomarkers and establish early warning systems to detect exposure and toxicity. Promising new biomarkers may include microRNAs that regulate stress pathways, as well as transcriptomic and metabolomic profiles that are sensitive to microelements. Proteomic analyses can identify proteins that enable the detection of sublethal toxicity before traditional oxidative stress symptoms appear. Furthermore, it is important to investigate the interactive effects of metals, which may have synergistic but also antagonistic effects on fish physiology. Developing models to predict these combined effects under different conditions would provide a deeper understanding of the complex interactions. Given changes in climatic conditions (temperature change, ocean acidification, shifts in hydrological cycles), it is essential to investigate the influence of these factors on the dynamics of metal toxicity.
7 Conclusion
This review discusses the intricate relations between potentially toxic metals in freshwater fish and their significance to fish physiology along with the possible detrimental effects of their accumulation. The interaction of their beneficial and adverse effects emphasizes the necessity of integrated management strategies in aquatic environments.
The role of cobalt, copper, iron, zinc, molybdenum and manganese in fish physiological processes and the precarious balance between natural and man-made sources of metals in aquatic systems are considerations of fundamental importance. The potential toxicity of even the essential elements, the determinants of metal toxicity and bioavailability, and the long-term ecological implications of metal accumulation in fish are also among the major conclusions of this study.
Future research should be directed predominantly at enhancing monitoring and early warning systems, examining the impacts of metal mixtures as well as their interactions with environmental stressors, developing novel solutions to pollution remediation, exploring the genetic and epigenetic impacts of chronic metal exposure, and refining risk model prediction associated with human health.
In conclusion, the regulation of potentially toxic metals in freshwater systems requires an integrative interdisciplinary strategy. Enabling a clearer understanding of such complex interactions will allow us to more effectively develop methodologies aimed at protecting the aquatic ecosystem, healthy fish populations, and protecting human health in an environment of increasing environmental pressures. The findings of this review can be translated into environmental monitoring and regulatory frameworks by identifying the most sensitive biomarkers of metal exposure, integrating AI-driven predictive models to detect early signs of toxicity, and informing evidence-based thresholds for water quality standards that account for sublethal and synergistic effects of metals.
Author contributions
AK: Conceptualization, Funding acquisition, Investigation, Writing – original draft, Writing – review and editing. MH: Conceptualization, Investigation, Writing – original draft. MT: Conceptualization, Writing – original draft. TJ: Writing – review and editing, Funding acquisition, Visualization. EK: Writing – review and editing. JA: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by The Ministry of Education, Research, Development and Youth of the Slovak Republic under the project VEGA 1/0571/23 and by the Slovak Research and Development Agency under the contract No. APVV-21-0168.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fphys.2025.1717005.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. During the preparation of this work, the authors used Perplexity Pro (© 2025 Perplexity AI) and DeepL translator (DeepL SE) to translate or to check the grammar of the text prepared by the authors to improve the readability and the academic quality of the processed text. After using this tool, the authors checked and edited the content and take full responsibility for the content of the publication.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2025.1609555/full#supplementary-material
References
Aliko V., Qirjo M., Sula E., Morina V., Faggio C. (2018). Antioxidant defense system, immune response and erythron profile modulation in gold fish, Carassius auratus, after acute manganese treatment. Fish. Shellfish Immunol. 76, 101–109. doi:10.1016/j.fsi.2018.02.042
Aubakirova K., Satkanov M., Kulataeva M., Assylbekova G., Kambarbekova A., Alikulov Z. (2023). Molybdoenzymes isolated from S. glanis liver can produce nitric oxide from nitrates and nitrites. Czech J. Anim. Sci. 68, 222–230. doi:10.17221/206/2022-CJAS
Baeverfjord G., Antony Jesu Prabhu P., Fjelldal P. G., Albrektsen S., Hatlen B., Denstadli V., et al. (2019). Mineral nutrition and bone health in salmonids. Rev. Aquac. 11, 740–765. doi:10.1111/raq.12255
Bagheri S., Gholamhosseini A., Hoseinifar S. H., Banaee M. (2024). Investigation of the effects of heavy metals (copper, cobalt, manganese, selenium, and zinc) on fish immune systems – an overview. Ann. Anim. Sci. 24, 1025–1035. doi:10.2478/aoas-2024-0017
Banerjee R., Ragsdale S. W. (2003). The many faces of vitamin B12: catalysis by cobalamin-dependent enzymes. Annu. Rev. Biochem. 72, 209–247. doi:10.1146/annurev.biochem.72.121801.161828
Banni M., Chouchene L., Said K., Kerkeni A., Messaoudi I. (2011). Mechanisms underlying the protective effect of zinc and selenium against cadmium-induced oxidative stress in zebrafish Danio rerio. BioMetals 24, 981–992. doi:10.1007/s10534-011-9456-z
Blust R. (2011). Cobalt, In: Fish physiology. Amsterdam, Netherlands: Elsevier. p. 291–326. doi:10.1016/S1546-5098(11)31006-0
Boota M. W., Soomro S., Xia H., Qin Y., Kakakhel M. A., Yan C., et al. (2024). Distribution and bioaccumulation of trace elements in two Cyprinidae fish species in the Indus river, Pakistan, including the impact of hydraulic structure on macroinvertebrates’ biodiversity. Environ. Res. 252, 118882. doi:10.1016/j.envres.2024.118882
Bury N. R., Walker P. A., Glover C. N. (2003). Nutritive metal uptake in teleost fish. J. Exp. Biol. 206, 11–23. doi:10.1242/jeb.00068
Campos S. A. B., Dal-Magro J., De Souza-Franco G. M. (2018). Metals in fish of different trophic levels in the area of influence of the AHE Foz do Chapecó reservoir, Brazil. Environ. Sci. Pollut. Res. 25, 26330–26340. doi:10.1007/s11356-018-2522-0
Cano-Viveros S., Galar-Martínez M., Gasca-Pérez E., García-Medina S., Ruiz-Lara K., Gómez-Oliván L. M., et al. (2021). The relationship between embryotoxicity and oxidative stress produced by aluminum, iron, mercury, and their mixture on Cyprinus carpio. Water. Air. Soil Pollut. 232, 376. doi:10.1007/s11270-021-05312-y
Chen X., Liu H., Liu S., Zhang Z., Li X., Mao J. (2024). Excessive dietary iron exposure increases the susceptibility of largemouth bass (Micropterus salmoides) to Aeromonas hydrophila by interfering with immune response, oxidative stress, and intestinal homeostasis. Fish. Shellfish Immunol. 147, 109430. doi:10.1016/j.fsi.2024.109430
Clearwater S. J., Farag A. M., Meyer J. S. (2002). Bioavailability and toxicity of dietborne copper and zinc to fish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 132, 269–313. doi:10.1016/S1532-0456(02)00078-9
Dahlberg A. D., Waller D. L., Severson T. J., Barbour M. T., Meulemans M., Wise J. K., et al. (2025). Using bioavailability modeling to refine copper treatments for zebra mussel control and better understanding risks to non-target species. Sci. Rep. 15, 29333. doi:10.1038/s41598-025-09231-4
Dash S., Kalamdhad A. S. (2021). Understanding the dynamics of heavy metals in a freshwater ecosystem through their toxicity and bioavailability assay. Environ. Dev. Sustain. 23, 16381–16409. doi:10.1007/s10668-021-01349-5
Dawood M. A. O., Alagawany M., Sewilam H. (2022). The role of zinc microelement in aquaculture: a review. Biol. Trace Elem. Res. 200, 3841–3853. doi:10.1007/s12011-021-02958-x
de Oliveira L. F., Santos C., Risso W. E., dos Reis Martinez C. B. (2018). Triple-mixture of Zn, Mn, and Fe increases bioaccumulation and causes oxidative stress in freshwater neotropical fish. Environ. Toxicol. Chem. 37, 1749–1756. doi:10.1002/etc.4133
De Paiva Magalhães D., Da Costa Marques M. R., Baptista D. F., Buss D. F. (2015). Metal bioavailability and toxicity in freshwaters. Environ. Chem. Lett. 13, 69–87. doi:10.1007/s10311-015-0491-9
Deb S. C., Fukushima T. (1999). Metals in aquatic ecosystems: mechanisms of uptake, accumulation and release-Ecotoxicological perspectives. Int. J. Environ. Stud. 56, 385–417. doi:10.1080/00207239908711212
Edo G. I., Itoje-akpokiniovo L. O., Obasohan P., Ikpekoro V. O., Samuel P. O., Jikah A. N., et al. (2024). Impact of environmental pollution from human activities on water, air quality and climate change. Ecol. Front. 44, 874–889. doi:10.1016/j.ecofro.2024.02.014
El-Sayed A.-F. M., Figueiredo-Silva C., Zeid S. M. S., Makled S. O. (2023). Metal–amino acid complexes (Zn, Se, Cu, Fe, and Mn) enhance immune response, antioxidant capacity, liver function enzymes, and expression of cytokine genes in Nile Tilapia reared under field conditions. J. Aquat. Anim. Health 35, 248–262. doi:10.1002/aah.10194
Fırat Ö., Erol R., Fırat Ö. (2022). An investigation on freshwater fish Oreochromis niloticus (linnaeus, 1758): assessing hemotoxic effects of different copper compounds used as nanomaterial or pesticide. Bull. Environ. Contam. Toxicol. 108, 549–554. doi:10.1007/s00128-021-03320-6
Gheorghe S., Vasile G. G., Gligor C., Lucaciu I. E., Lazar M. N. (2017). Metallic elements (Cu, Zn, Ni and Mn) toxicity effects determination on a fresh water fish Cyprinus carpio (common carp) laboratory acclimatized. Rev. Chim. 68, 1711–1715. doi:10.37358/RC.17.8.5750
Ghribi F., Bejaoui S., Chetoui I., Trabelsi W., Belhassen D., Ben Fayala C., et al. (2025). Toxicological effects of cobalt on common carp: oxidative stress, ionic imbalance, fatty acid disruption, and gill histopathology. Histopathol. Environ. Geochem. Health 47, 98. doi:10.1007/s10653-025-02407-x
Green S. L., Silvester E., Dworkin S., Shakya M., Klein A., Lowe R., et al. (2024). Molecular variations to the proteome of zebrafish larvae induced by environmentally relevant copper concentrations. Aquat. Toxicol. 272, 106963. doi:10.1016/j.aquatox.2024.106963
Gürkan M., Gürkan S. E., Yılmaz S., Ateş M. (2021). Comparative toxicity of alpha and gamma iron oxide nanoparticles in rainbow trout: histopathology, hematology, accumulation, and oxidative stress. Water. Air. Soil Pollut. 232, 37. doi:10.1007/s11270-021-04988-6
Helczman M., Tomka M., Arvay J., Tvrda E., Andreji J., Fik M., et al. (2024). Selected micro- and macro-element associations with oxidative status markers in common carp (Cyprinus carpio) blood serum and ejaculate: a correlation study. J. Toxicol. Environ. Health A 87, 999–1014. doi:10.1080/15287394.2024.2406429
Helczman M. (2025). Key exposure routes, cellular uptake mechanisms, and intracellular interactions of metals in fish. Available online at: https://BioRender.com/5f377r8.
Hoseini S. M., Hedayati A., Ghelichpour M. (2014). Plasma metabolites, ions and thyroid hormones levels, and hepatic enzymes׳ activity in Caspian roach (Rutilus rutilus caspicus) exposed to waterborne manganese. Ecotoxicol. Environ. Saf. 107, 84–89. doi:10.1016/j.ecoenv.2014.05.002
Inomata T. (2024). Metal complexes relating to biological functions. In: Tanase, T., and Ishii, Y., editors. Coordination Chemistry: molecular science of organic–inorganic complexes. Burlington House, London: Royal Society of Chemistry. p. 367–393. doi:10.1039/9781837673254-00367
Javed M., Usmani N. (2013). Assessment of heavy metal (Cu, Ni, Fe, Co, Mn, Cr, Zn) pollution in effluent dominated rivulet water and their effect on glycogen metabolism and histology of Mastacembelus armatus. SpringerPlus 2, 390. doi:10.1186/2193-1801-2-390
Jia Y., Wang L., Qu Z., Wang C., Yang Z. (2017). Effects on heavy metal accumulation in freshwater fishes: species, tissues, and sizes. Environ. Sci. Pollut. Res. 24, 9379–9386. doi:10.1007/s11356-017-8606-4
Jia Y., Wang L., Cao J., Li S., Yang Z. (2018). Trace elements in four freshwater fish from a mine-impacted river: spatial distribution, species-specific accumulation, and risk assessment. Environ. Sci. Pollut. Res. 25, 8861–8870. doi:10.1007/s11356-018-1207-z
Khallaf E. A., Authman M. M. N., Alne-na-ei A. A. (2018). Contamination and ecological hazard assessment of heavy metals in freshwater sediments and Oreochromis niloticus (linnaeus, 1758) fish muscles in a Nile River canal in Egypt. Environ. Sci. Pollut. Res. 25, 13796–13812. doi:10.1007/s11356-018-1521-5
Kolarova N., Napiórkowski P. (2021). Trace elements in aquatic environment. Origin, distribution, assessment and toxicity effect for the aquatic biota. Ecohydrol. Hydrobiol. 21, 655–668. doi:10.1016/j.ecohyd.2021.02.002
Kovacik A., Tvrda E., Miskeje M., Arvay J., Tomka M., Zbynovska K., et al. (2019). Trace metals in the freshwater fish Cyprinus carpio: effect to serum biochemistry and oxidative status markers. Biol. Trace Elem. Res. 188, 494–507. doi:10.1007/s12011-018-1415-x
Kovacik A., Tvrda E., Tomka M., Revesz N., Arvay J., Fik M., et al. (2023). Seasonal assessment of selected trace elements in grass carp (Ctenopharyngodon idella) blood and their effects on the biochemistry and oxidative stress markers. Environ. Monit. Assess. 195, 1522. doi:10.1007/s10661-023-12152-2
Kubrak O. I., Husak V. V., Rovenko B. M., Storey J. M., Storey K. B., Lushchak V. I. (2011). Cobalt-induced oxidative stress in brain, liver and kidney of goldfish Carassius auratus. Chemosphere 85, 983–989. doi:10.1016/j.chemosphere.2011.06.078
Kumar B., Senthil Kumar K., Priya M., Mukhopadhyay D., Shah R. (2010). Distribution, partitioning, bioaccumulation of trace elements in water, sediment and fish from sewage fed fish ponds in eastern Kolkata, India. Toxicol. Environ. Chem. 92, 243–260. doi:10.1080/02772240902942394
Kumar N., Krishnani K. K., Kumar P., Jha A. K., Gupta S. K., Singh N. P. (2017). Dietary zinc promotes immuno-biochemical plasticity and protects fish against multiple stresses. Fish. Shellfish Immunol. 62, 184–194. doi:10.1016/j.fsi.2017.01.017
Kumar M., Singh S., Dwivedi S., Trivedi A., Dubey I., Trivedi S. P. (2023). Copper-induced genotoxicity, oxidative stress, and alteration in transcriptional level of autophagy-associated genes in snakehead fish Channa punctatus. Biol. Trace Elem. Res. 201, 2022–2035. doi:10.1007/s12011-022-03301-8
Kumar N., Thorat S. T., Reddy K. S. (2023). Multi biomarker approach to assess manganese and manganese nanoparticles toxicity in Pangasianodon hypophthalmus. Sci. Rep. 13, 8505. doi:10.1038/s41598-023-35787-0
Lall S. P. (2003). 5 - the minerals. In: Halver, J. E., and Hardy, R. W., editors. Fish nutrition. San Diego: Academic Press. Third Edition. p. 259–308. doi:10.1016/B978-012319652-1/50006-9
Liao W., Zhu Z., Feng C., Yan Z., Hong Y., Liu D., et al. (2023). Toxicity mechanisms and bioavailability of copper to fish based on an adverse outcome pathway analysis. J. Environ. Sci. 127, 495–507. doi:10.1016/j.jes.2022.06.002
Luo Z., Zou G.-Y., Gao Y., Ye H.-M., Xi W.-Q., Liu X. (2017). Effect of dietary iron (Fe) levels on growth performance, hepatic lipid metabolism and antioxidant responses in juvenile yellow catfish Pelteobagrus fulvidraco. Aquac. Nutr. 23, 1475–1482. doi:10.1111/anu.12523
Millero F. (2001). Speciation of metals in natural waters. Geochem. Trans. 2, 57. doi:10.1186/1467-4866-2-57
Musharraf M., Khan M. A. (2019). Requirement of fingerling Indian major carp, Labeo rohita (Hamilton) for dietary iron based on growth, whole body composition, haematological parameters, tissue iron concentration and serum antioxidant status. Aquaculture 504, 148–157. doi:10.1016/j.aquaculture.2019.02.002
Musharraf M., Khan M. A. (2021). Dietary manganese requirement of fingerling Indian major carp, Labeo rohita (Hamilton) estimated by growth, tissue manganese concentration and hepatic manganese-superoxide dismutase activity. Aquaculture 540, 736734. doi:10.1016/j.aquaculture.2021.736734
Namieśnik J., Rabajczyk A. (2010). The speciation and physico-chemical forms of metals in surface waters and sediments. Chem. Speciat. Bioavailab. 22, 1–24. doi:10.3184/095422910X12632119406391
Naz S., Hussain R., Guangbin Z., Chatha A. M. M., Rehman Z. U., Jahan S., et al. (2023). Copper sulfate induces clinico-hematological, oxidative stress, serum biochemical and histopathological changes in freshwater fish rohu (Labeo rohita). Front. Vet. Sci. 10, 1142042. doi:10.3389/fvets.2023.1142042
Ojo A. A., Wood C. M. (2007). In vitro analysis of the bioavailability of six metals via the gastro-intestinal tract of the rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 83, 10–23. doi:10.1016/j.aquatox.2007.03.006
Passos L. S., Coppo G. C., Pereira T. M., Teixeira B. C., Bona A. M., Merçon J., et al. (2022). Do manganese and iron in association cause biochemical and genotoxic changes in Oreochromis niloticus (teleostei: cichlidae)? Bull. Environ. Contam. Toxicol. 108, 708–715. doi:10.1007/s00128-021-03382-6
Pierrot D., Millero F. J. (2017). The speciation of metals in natural waters. Aquat. Geochem. 23, 1–20. doi:10.1007/s10498-016-9292-4
Pouil S., Warnau M., Oberhänsli F., Teyssié J., Bustamante P., Metian M. (2016). Influence of food on the assimilation of essential elements (Co, Mn, and Zn) by turbot Scophthalmus maximus. Mar. Ecol. Prog. Ser. 550, 207–218. doi:10.3354/meps11716
Rajkowska M., Protasowicki M. (2013). Distribution of metals (Fe, Mn, Zn, Cu) in fish tissues in two lakes of different trophy in Northwestern Poland. Environ. Monit. Assess. 185, 3493–3502. doi:10.1007/s10661-012-2805-8
Regoli L., Van Tilborg W., Heijerick D., Stubblefield W., Carey S. (2012). The bioconcentration and bioaccumulation factors for molybdenum in the aquatic environment from natural environmental concentrations up to the toxicity boundary. Sci. Total Environ. 435–436, 96–106. doi:10.1016/j.scitotenv.2012.06.020
Reid S. D. (2002). Physiological impact of acute molybdenum exposure in juvenile kokanee salmon (Oncorhynchus nerka). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 133, 355–367. doi:10.1016/S1532-0456(02)00121-7
Ricketts C. D., Bates W. R., Reid S. D. (2015). The effects of acute waterborne exposure to sublethal concentrations of molybdenum on the stress response in rainbow trout, Oncorhynchus mykiss. PLOS ONE 10, e0115334. doi:10.1371/journal.pone.0115334
Rouleau C., Tjälve H., Gottofrey J. (1996). Effects of low ph on the uptake and distribution of 54Mn(II) in brown trout (Salmo trutta). Environ. Toxicol. Chem. 15, 708–710. doi:10.1002/etc.5620150514
Satkanov M., Nurbekova Z., Bilyalov A., Tazhibay D., Zhaksylyk M., Kulatayeva M., et al. (2025). Biochemical properties of molybdenum cofactor isolated from fish liver. Fish. Physiol. Biochem. 51, 62. doi:10.1007/s10695-025-01473-3
Singh M., Barman A. S., Devi A. L., Devi A. G., Pandey P. K. (2019). Iron mediated hematological, oxidative and histological alterations in freshwater fish Labeo rohita. Ecotoxicol. Environ. Saf. 170, 87–97. doi:10.1016/j.ecoenv.2018.11.129
Spanopoulos-Zarco P., Ruelas-Inzunza J., Aramburo-Moran I. S., Bojórquez-Leyva H., Páez-Osuna F. (2017). Differential tissue accumulation of copper, iron, and zinc in bycatch fish from the Mexican pacific. Biol. Trace Elem. Res. 176, 201–206. doi:10.1007/s12011-016-0800-6
Wang Y., Zhao H., Liu Y., Nie X., Xing M. (2020). Zinc exerts its renal protection effect on arsenic-exposed common carp: a signaling network comprising Nrf2, NF-κB and MAPK pathways. Fish. Shellfish Immunol. 104, 383–390. doi:10.1016/j.fsi.2020.06.031
Wang L., Wang H., Gao C., Wang C., Yan Y., Zhou F. (2024). Dietary copper for fish: homeostasis, nutritional functions, toxicity, and affecting factors. Aquaculture 587, 740875. doi:10.1016/j.aquaculture.2024.740875
Xu J. J. J.-J., Jia B.-Y., Zhao T., Tan X.-Y., Zhang D.-G., Song C.-C., et al. (2023). Influences of five dietary manganese sources on growth, feed utilization, lipid metabolism, antioxidant capacity, inflammatory response and endoplasmic reticulum stress in yellow catfish intestine. Aquaculture 566, 739190. doi:10.1016/j.aquaculture.2022.739190
Xu X. W. X.-W., Song C.-C., Tan X.-Y., Zhong C.-C., Luo Z. (2023). Effects of dietary zinc (Zn) levels on growth performance, nutrient composition, muscle development, antioxidant and inflammatory responses in yellow catfish muscle. Aquac. Rep. 31, 101640. doi:10.1016/j.aqrep.2023.101640
Younus N., Zuberi A., Rashidpour A., Metón I. (2020). Dietary cobalt supplementation improves growth and body composition and induces the expression of growth and stress response genes in Tor putitora. Fish. Physiol. Biochem. 46, 371–381. doi:10.1007/s10695-019-00723-5
Zhao L., Xia Z., Wang F. (2014). Zebrafish in the sea of mineral (iron, zinc, and copper) metabolism. Front. Pharmacol. 5, 33. doi:10.3389/fphar.2014.00033
Zia S., McDonald D. G. (1994). Role of the gills and gill chloride cells in metal uptake in the freshwater-adapted rainbow trout, Oncorhynchus mykiss. Can. J. Fish. Aquat. Sci. 51, 2482–2492. doi:10.1139/f94-247
Keywords: trace elements, potential toxicity, health, ecotoxicology, fish, biomarker
Citation: Kovacik A, Helczman M, Tomka M, Jambor T, Kovacikova E and Arvay J (2025) The biological relevance of potentially toxic metals in freshwater fish. Front. Physiol. 16:1609555. doi: 10.3389/fphys.2025.1609555
Received: 14 April 2025; Accepted: 02 September 2025;
Published: 18 September 2025; Corrected: 28 October 2025.
Edited by:
Samad Rahimnejad, University of Murcia, SpainReviewed by:
Palash Kumar Pal, University of Calcutta, IndiaCopyright © 2025 Kovacik, Helczman, Tomka, Jambor, Kovacikova and Arvay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anton Kovacik, YW50b24ua292YWNpa0B1bmlhZy5zaw==
†These authors have contributed equally to this work
 Anton Kovacik
Anton Kovacik Marek Helczman1†
Marek Helczman1† Eva Kovacikova
Eva Kovacikova Julius Arvay
Julius Arvay