- School of Mechanical and Power Engineering, Harbin University of Science and Technology, Harbin, China
The clinical reliability of implantable cardiovascular assist devices (CADs) necessitates rigorous verification by the Mock Circulatory Loop (MCL) to assess their hemodynamic performance, encompassing key parameters such as head, flow, and hemolytic properties. In this paper, we undertake a systematic review of the evolution of this technology system and propose a three-level classification model based on bibliometric analysis (n = 130), in which the dual-circulatory system accounts for 47.27% of the total, to reveal its physiological synergistic mechanism and the innovative application of multi-circulatory configurations in complex clinical scenarios. The study indicates that the prevailing technological impediments pertain to: (i) deviation of 3D-printed vascular mechanical properties (anatomical fidelity loss), (ii) decline in long-term shear force simulation accuracy, and (iii) paucity of module interface compatibility. From an interdisciplinary integration perspective, the present study indicates that adaptive closed-loop hybrid-MCL systems represent a key direction for technological evolution: their architecture, which couples real-time digital twins with physical loops, can dynamically adjust blood flow parameters. When combined with multi-scale simulation optimization, this approach significantly enhances the reliability of long-term shear stress predictions. Furthermore, the integration of personalized digital twins establishes a high-fidelity patient-specific validation platform, thereby providing a theoretical framework for precise evaluation of cardiovascular devices.
Introduction
The MCL serves as a foundational experimental platform for cardiovascular research, capable of precise reproduction of physiological parameters such as blood pressure (BP), heart rate (HR), vascular compliance (VC), and resistance (PR), among others. This capability stems from the construction of sub-systems dedicated to body circulation, pulmonary circulation, and coronary circulation (Yang and Wang, 2023; Masoud et al., 2023). An analysis of the system architecture reveals that the MCL consists of two modules: the driver unit and the fluidic circuit. The primary functions of the MCL include performance validation of CADs, dynamic simulation of physiological and pathological states, and in vitro pre-evaluation of therapeutic regimens. Although heart transplantation remains the gold standard therapy for end-stage heart failure, CAD implantation has become an important alternative due to donor shortages and surgical risks (Huang, 2013; Liang, 2016; Libera et al., 2022; Yang and Wang, 2023). In this context, the MCL provides a controlled and reproducible in vitro validation environment for CADs, and is particularly irreplaceable in hemodynamic optimization and device stability testing.
The conceptual prototype of MCL originated with the development of the first artificial heart-lung device by Gibbon in 1935 (Silvay and Castillo, 2013; Flick, 1957; Böttcher and Alexi-Meskishvili, 2006; Pastuszko and EdieGibbon, 2004), and the early systems were based on nonpulsatile flow simulation, focusing on the mechanical reproduction of vascular resistance characteristics (Makinouchi et al., 1994; Yoshino et al., 2001). Typical representatives, such as the primary model constructed by Yoshizawa et al. (2002) used a reservoir vessel to simulate venous return and regulated peripheral resistance through a pinch stop valve. Such systems are only capable of evaluating the basic performance of blood pumps due to the lack of a cardiac pacing module and the limitation of anatomical accuracy, and have been gradually iterated as research needs have increased.
A categorization of MCL-related studies, as depicted in Figure 1, is proposed based on the history of MCL development and the literature cited in the article. The categorization system includes four types: simple MCLs, MCLs with body circulation only, MCLs with body circulation and pulmonary circulation, and specially designed MCLs. The latter category has significantly expanded the boundaries of clinical applications of MCLs through the introduction of technological innovations such as 3D printing and smart materials. The developmental timeline of MCLs is illustrated in Figure 1.
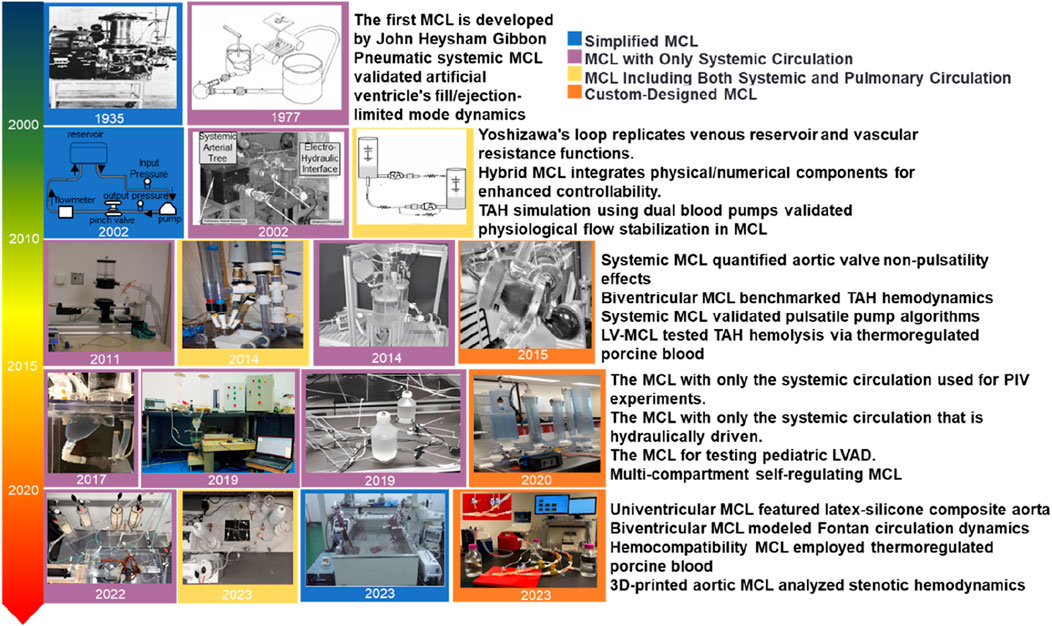
Figure 1. Overview of the different stages of MCL development (Ferrari et al., 2002; Geselowitz et al., 1977; Pastuszko and EdieGibbon, 2004; Yoshizawa et al., 2002; Khalil et al., 2008; Tuzun et al., 2011; Jahren et al., 2014; Love et al., 2014; Gräf et al., 2015; Reider et al., 2017; Pugovkin et al., 2019; Gregory et al., 2020; Alavi et al., 2022; Hong et al., 2023; Li et al., 2023; Rasooli et al., 2023; Khienwad et al., 2019).
The MCL structure is composed primarily of a driver and a circulatory loop. The driver serves as the power source for the heart’s pumping of blood and is frequently employed to simulate the atria and ventricles when integrated with the chambers. The circulatory loop is utilized to simulate an array of components external to the heart, such as blood vessels and valves, within the context of blood circulation. The amalgamation of the drive system and the circulatory loop facilitates a comprehensive simulation of the human body. The integration of these systems enables the full simulation of blood circulation within the human body. Through the modification of the parameters that define the components of the MCL, the simulation of diverse physiological states, including sedentary, exercise, and hemodynamic states associated with cardiovascular diseases such as heart failure and single ventricle, can be achieved. The evolution of the MCL, influenced by the advancement of related fields such as materials science, has led to modifications in its hardware and structural materials. The integration of these components often facilitates the simulation of atrial ventricles. The classification of components has undergone several modifications, with the components now being categorized into three distinct types: traditional, innovative, and other. The development of the MCL’s structure is outlined in Table 1.
The prevailing focus of MCL research has undergone a shift towards the latter three types of systems. Early models, which were simple in nature and based on venous fluid storage and simple resistance regulation, have limited research and clinical value. This is due to their inability to effectively simulate complex cardiovascular disease states and comprehensively test modern CADs. The extant review literature has clear limitations. For instance, Agrafiotis et al. (2024). concentrated exclusively on total artificial hearts (TAH) and left ventricular assist devices (LVAD). Moreover, the mechanical/numerical/hybrid three-part rule proposed by Cappon et al. (2021) lacks systematic adaptation analysis for different types of CADs.
In order to address this gap, this study proposes and adopts a “CAD type-oriented” review framework as a novel approach. The objective of this framework is to methodically trace the developmental trajectory of MCL technology and elucidate the fundamental compatibility relationships between diverse MCL systems (e.g., systemic circulation only, systemic-pulmonary circulation, special designs) and mainstream CADs (including ventricular assist devices (VADs), intra-aortic balloon pumps (IABP), TAH, etc.). This compatibility analysis framework has been used to identify three cutting-edge directions that are required to overcome the current technical limitations. Firstly, there is a necessity for adaptive closed-loop hybrid-MCL systems that solve dynamic response bottlenecks through digital-physical real-time coupling. Secondly, multi-scale simulation accuracy optimization is required, which combines computational fluid dynamics and organ-on-a-chip technology to improve the reliability of long-term shear force prediction. Thirdly, there is a requirement for personalized digital twin integration that relies on patient imaging data to build a high-fidelity verification platform. This framework provides a systematic solution for the precise evaluation and clinical translation of cardiovascular devices.
MCLs for CADs testing
The validation of the performance and stability of VADs—a critical medical device for enhancing cardiac output in patients with heart failure—is contingent on the MCL (Li S. et al., 2020; Rosalia et al., 2021; Xu, 2017; Ochsner et al., 2013; A et al., 2023). A standard MCL comprises left ventricular chamber, aortic valve, mitral valve, and arterial compliance modules, which can be utilized to emulate the physiological and pathological states of the cardiovascular system (CVS). This capability facilitates the assessment of VAD functionality and the optimization of control strategies. In vitro MCL testing offers a reproducible validation platform for device performance while ensuring patient safety, in comparison to in vivo experimentation, which carries ethical risks and financial costs. A review of the literature indicates the effectiveness of existing MCL studies in evaluating the hydrodynamic properties of CADs under multiple cyclic loads. Despite the discrepancy between MCL-generated pressure waveforms and human data, its in vitro test results are valuable in guiding the preclinical validation of CADs. It is important to note that the integration of a hemodynamic monitoring module, additive manufacturing technology, and a multi-sensor fusion system enables the MCL to enhance the ability to dynamically resolve the working state of CADs and optimize the device control algorithm. This chapter will systematically explore the application characteristics of different MCL systems in device performance testing and control strategy development, based on the CADs classification framework.
MCLs for ventricular assist device testing
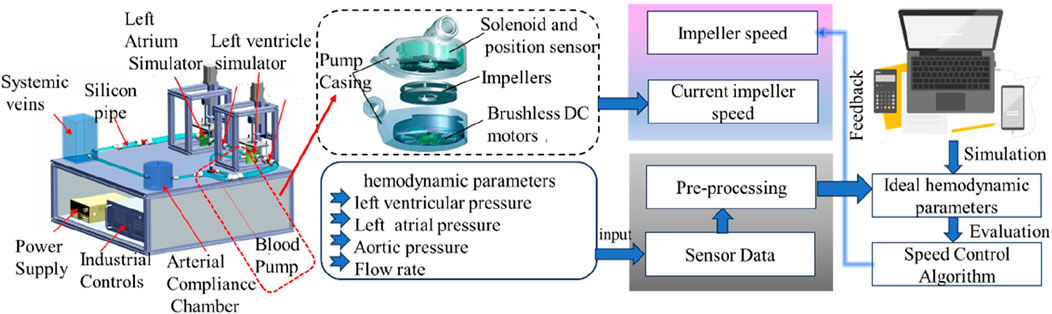
LVAD is a core intervention for the treatment of left heart failure by driving the cyclic flow of blood from the left ventricle to the aorta via an impeller (Leopaldi et al., 2015), and the internal state of the heart in heart failure with the cross-section of the heart after LVAD implantation is shown in Figure 3a. MCL-based in vitro testing of LVADs includes three main directions: hydrodynamic performance evaluation, control algorithm optimization and complication simulation studies. Notably, clinical complications such as thrombosis, ventricular pumping and valvular regurgitation may be induced after LVAD implantation due to the altered hemodynamic environment, which makes the value of MCL in the study of pathomechanisms particularly prominent (Rogers et al., 2017). The content and flow of the MCL workflow for testing LVAD is shown in Figure 2.
In the domain of LVAD performance assessment, Xu (2017) successfully simulated the hemodynamic characteristics of LVAD in dilated heart failure by modifying the Windkessel four-component MCL model while preserving the physiological function of the right heart-pulmonary circulation and optimized the axial-flow pump characteristic curves accordingly. Shu Li’s team (Li S. et al., 2020) proposed the concept of systematic validation, constructed the test matrix that included 12 physiological-pathological conditions, and systematically analyzed the interaction mechanism between LVAD and the circulatory system through the integration of the MCL platform of the body/pulmonary dual circulation. In a related study, Wilson et al. (2023) and Tozzi et al. (2017) employed a glass-blown, transparent left ventricle model in conjunction with particle image velocimetry (PIV) technology to visualize and monitor the end-flow field (Reider et al., 2017; Roldán-Alzate et al., 2015; Goto et al., 2021; Tozzi et al., 2017). Furthermore, certain studies have utilized isolated porcine hearts as an alternative to conventional hydraulic actuators, a method that can more accurately replicate the mechanical characteristics of the heart. However, its implementation and advancement remain constrained due to the intricacy involved in acquiring and maintaining biological specimens (van Dort et al., 2020; van Dort et al., 2022; Granegger et al., 2013; Meskin et al., 2024). Figure 3b presents a comparison between an isolated porcine heart and a 3D-printed heart.
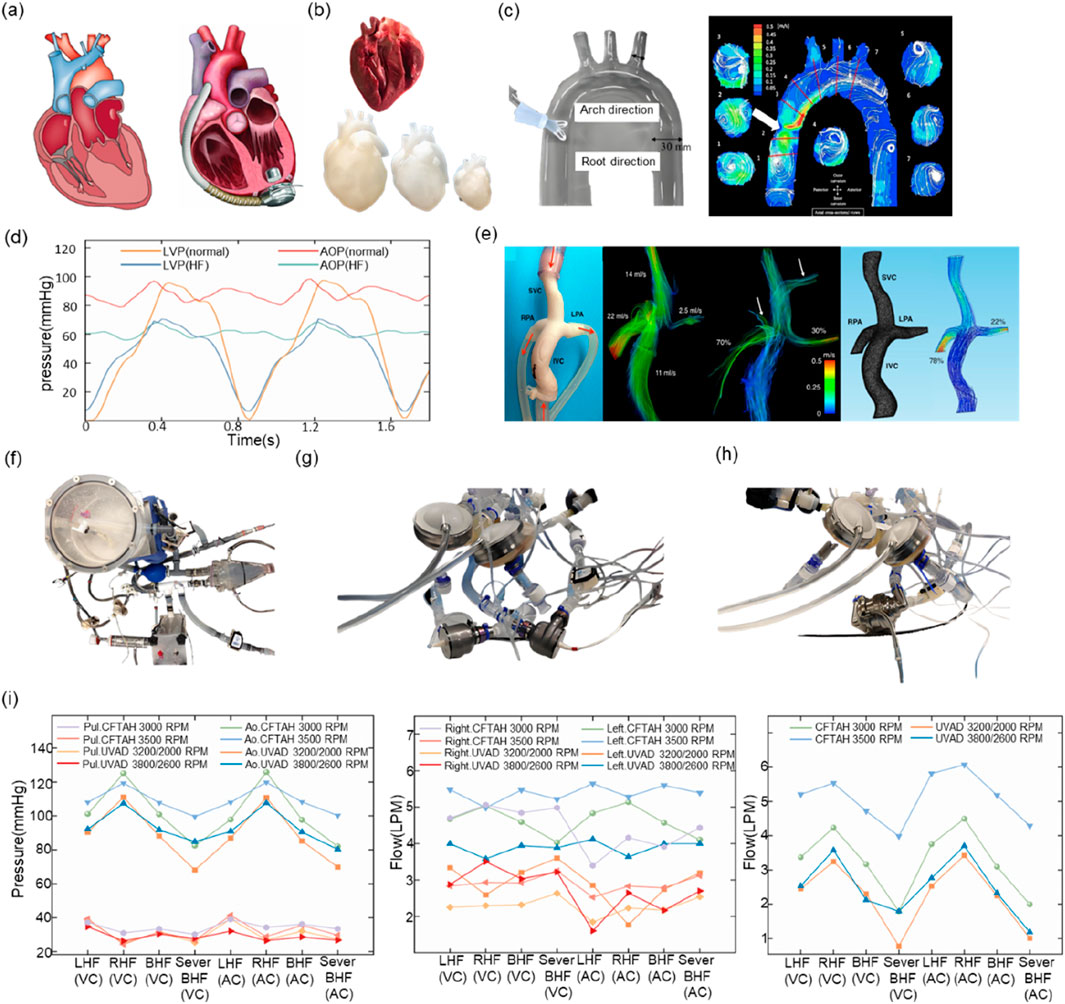
Figure 3. (a) Heart failure heart vs. heart implanted with LVAD (b) Isolated porcine heart vs. 3D-printed heart (c) PIV experiment (d) Left ventricular pressure vs. aortic pressure in normal vs. heart failure state (e) Fontan circulatory model vs. 4D Flow MRI effects (f) MCL used to test IABP (g) MCL used to test BiVAD (h) MCL used to MCL for testing TAH (i) BiVAD vs. TAH at the same MCL.
Existing studies have primarily centered on the utilization of the MCL to emulate particular hemodynamic conditions or to examine the mechanisms through which the LVAD interacts with the circulatory system. However, exhaustive investigations of complications subsequent to LVAD implantation frequently necessitate reconfiguration of the MCL hardware or adaptation of control strategies. Thrombosis is closely related to the flow field perturbation inside the LVAD, so most studies have used transparent flow channel design combined with PIV technology for flow field visualization (Goto et al., 2021; Reider et al., 2017; Roldán-Alzate et al., 2015), as shown in Figure 3c for the experimental image of PIV (Roldán-Alzate et al., 2015). In terms of hemolytic effect assessment, researchers have predominantly employed quantitative analysis of blood damage by integrating microscopic imaging and spectrophotometric detection modules (Giridharan et al., 2011; Leme et al., 2011; Woelke et al., 2020). Furthermore, LVAD is susceptible to inducing ventricular pumping or pulmonary circulation stasis due to its inherent characteristics of sluggish preload response and enhanced afterload sensitivity. To mitigate these risks, closed-loop control algorithms are necessary (Ochsner et al., 2013; Gregory et al., 2020; Rocchi et al., 2023a). Tuzun et al. (2011) revealed the dynamic effects of LVAD operating parameters on aortic valve function based on the MCL platform, and the results of Y. Tanaka’s team (Tanaka et al., 2020) further used a silicone LVAD model to validate the effectiveness of the pump speed-blood pressure synergistic optimization strategy for the intervention of severe aortic regurgitation.
It is noteworthy that epidemiological data on heart failure in children indicates that approximately 12,000–35,000 children require cardiac support each year in the United States. Their high readmission rates and treatment costs underscore the urgency of pediatric LVAD development. Given the anatomical structure and hemodynamic characteristics of the cardiovascular system in children, which are significantly different from those of adults, the development of a dedicated pediatric MCL system is essential to meet the needs of device testing. Gregory et al. (2020), Vandenberghe et al. (2011), and Huang et al. (2013) the authors successfully achieved the in vitro functional validation of pediatric ventricular assist devices by constructing scaled-down MCLs. These MCLs have the advantages of channel sizing and low-flow simulation accuracy, and have the potential to provide a more accurate and efficient device. Tuning and low-flow simulation accuracy with unique technical advantages.
The classification of VADs is determined by the therapeutic objective, with LVADs, right ventricular assist devices (RVADs), biventricular assist devices (BiVADs), and interventional ventricular assist devices (PVADs) being the primary categories. The evaluation of RVADs and BiVADs necessitates the simulation of both the physical and pulmonary circulations, due to the heterogeneity of the disease conditions targeted. Right heart failure is frequently a secondary condition resulting from LV dysfunction, and its pathogenesis involves multiple factors, including altered venous return, septal dyskinesia, and elevated pulmonary artery pressures due to LVAD. As an intervention for right heart failure, the Fontan procedure can achieve direct vena cava-pulmonary artery anastomosis through the creation of a Total Cavopulmonary Connection (TCPC). While this approach enables the direct vena cava-pulmonary artery anastomosis, it also introduces the vulnerability of Fontan circulation failure due to the absence of right heart pump function. To address this limitation, the clinical implementation of an RVAD has emerged as a therapeutic strategy to manage or alleviate Fontan circulatory failure. Roldán-Alzate et al. (2015) employed 4D flow magnetic resonance to compare the flow field characteristics of real blood vessels with those of a 3D-printed model, thereby providing a validation basis for the numerical simulation of TCPC. A representative 4D Flow MRI image of the TCPC configuration is shown in Figure 3e. Gregory et al. (2020) demonstrated by the RVAD-MCL system that the effectiveness of the device to reduce inferior vena cava pressure, but the study failed to fully evaluate the effect of blood composition on device performance due to the rheological differences between experimental fluids and real blood.
In the case of biventricular failure, the available clinical options for mechanical support include the BiVAD and the TAH. In order to enhance the fidelity of blood utilized in testing (Kado et al., 2020), the ANASTASIOS PETROU team (Petrou et al., 2019) employed a bovine blood-human blood mixture as the circulating medium, in conjunction with a hydraulic/pneumatic composite drive system, to effectively simulate the effects of biventricular assist in complex pathologies such as aortic regurgitation. Notably, Nguyen And et al. (2004) have pioneered the integration of the SynCardia TAH single-pump chamber into the Fontan circulating MCL, thereby demonstrating the feasibility of the device to substitute for right heart function and offering novel insights into single ventricle assist strategies.
PVADs have been shown to offer distinct advantages in the context of short-term circulatory support, typically ranging from 0 to 15 days, due to their unique characteristics related to percutaneous implantation, which is typically accomplished via the femoral or brachial artery route. To address the challenges posed by the complex flow field characteristics within the PVAD, Gupta et al. (2020) have developed a body-circulating MCL with an integrated PIV module, in conjunction with a 3D-printed pump casing, for the purpose of visual monitoring. This module enables the visualization of the flow field. Monreal’s team established a PVAD testing platform that is clinically verifiable by adjusting the system resistance through Hoffman clips in conjunction with a constant-temperature blood-simulating fluid (Monreal et al., 2023). In vivo experimental data were in good agreement with the MCL test results. These studies provide an important in vitro validation tool for optimizing the performance of VADs with different interventional pathways.
The application of MCLs in different types of VADs demonstrates that traditional MCLs have been more developed, and today’s MCLs can be flexibly assembled or replaced with powerful functional modules, such as PIV, artificial heart, and VADs modules, according to different needs. However, there is a paucity of studies related to MCLs developed for children. Nevertheless, it is hypothesized that this phenomenon will promote the development of related MCLs in the future, as the number of children with cardiovascular diseases is increasing year by year. A review of the literature reveals that specially designed MCLs will be more emphasized in future clinical applications to accomplish more complex requirements.
MCL for intra-aortic balloon counterpulsation testing
IABP is a CAD that is commonly used to assist cardiac recovery, improve coronary circulation, and reestablish the balance of oxygen supply and demand (Ferrari et al., 2001; Ferrari et al., 2011; Ferrari et al., 2005). It does so by improving coronary perfusion and regulating the balance of myocardial oxygen supply and demand through time-phased balloon contraction and relaxation. The intervention is typically performed by positioning a balloon catheter via the femoral or external iliac artery in the descending aortic segment between the distal 1–2 cm of the left subclavian artery and the proximal renal artery. Hemodynamic regulation of diastolic pressurization and systolic decompression is achieved by an extracorporeal counterpulsation controller (Patterson et al., 2014; Khan and Siddiqui, 2022; Xu et al., 2023). Wave intensity analysis, a pivotal parameter for assessing the efficacy of IABP, can differentiate between forward propagating waves and reverse reflected waves by employing time-domain features, thereby providing a quantitative basis for device optimization (De Lazzari et al., 2023; Capoccia et al., 2015; Kolyva et al., 2009; Lu et al., 2011; Parker and Jones, 1990; Bleasdale et al., 2003).
Since the introduction of IABP into the clinic by Capoccia et al. (2015) and Kantrowitz et al. (1993) in the 1960s, it is in vitro testing technology has continued to evolve. The first MCL, established in 1971, had basic the evolution of IABP-MCL testing capabilities has been driven by the demand for developing devices such as LVADs. This demand has led to the development of modern IABP-specific MCLs, which have gradually incorporated coronary artery circulation simulation, silicone 3D-printed aortic models, and other bionic components. In contrast to other test platforms for ventricular assist devices, IABP-MCL requires the integration of an artificial aortic module and a synchronized drive (Xu et al., 2023). While earlier systems only simulated vascular compliance and resistance parameters through elastic lumens and pipelines, current state-of-the-art models have enabled multiscale simulation from the atrial ventricle to the systemic-coronary circulation, which significantly improves the biofidelity of hemodynamic simulation. Similar to other VADs, IABP-MCL requires a closed-loop control system to precisely regulate the timing of balloon inflation and deflation to ensure optimal mechanical coupling with the cardiac cycle.
Mechanical implantable devices, including LVAD, IABP, and IARBP, have been utilized as adjunctive therapy for patients with heart failure. Beyond the fundamental components of the MCL, including the drive system, the atria, and the valves, Wang et al. (2017) and Cappon et al. (2021) have explored the potential of concurrently testing multiple CAD models. This approach was undertaken to enhance the comparability of the data by developing an MCL that incorporated the brachiocephalic trunk, the subclavian artery, the common carotid artery, and the coronary arteries. Building upon this approach, Ferrari et al. (2001) refined the MCL, incorporating it into LVAD and BiVAD testing to enhance its compatibility with IABP evaluation. In their model, the aorta of the MCL was replicated by a conical rubber tube. Due to the complexity of the human body’s positioning, it is often necessary to evaluate the performance of the IABP in various states after implantation procedures. To address this issue, Swalen et al. (2005) studied the performance of the IABP in the tilted state, the IABP was placed in a real-sized latex aorta in an MCL operated horizontally at an angle of 10°, by tilting the artificial aorta to achieve this. More recently, Kolyva et al. (2016) and Kolyva et al. (2012) designed an MCL that simulated and tested IABP performance in multiple patient positions by modeling the aorta with a 12-branch polyurethane composite aorta and with all branches linked at the capillary and other vascular structures, The end resistance and end compliance of the model were provided by the aforementioned branches, respectively. In contrast, Corazza et al. (2014) and Corazza et al. (2013) departed from the traditional idea of the MCL for testing IABP by innovatively incorporating an external reservoir to amplify balloon pulsations induced by physiologic arterial pressure pulses.
In the context of evaluating mechanical implantable assist devices, it is imperative to acknowledge the inherent differences in hemodynamic parameters, such as aortic pressure and flow, between children and adults. Consequently, MCLs must be deliberated upon in consideration of diverse application populations. Pantalos et al. (2010) introduced a 5 cc pediatric IABP catheter through an O-ring sealed connector in their designed MCL and into the thoracic descending aorta through an iliac bifurcation Y connector to simulate the introduction and location of IABP in the aorta of pediatric patients (Wang et al., 2017; Cappon et al., 2021; Pantalos et al., 2010).
By examining the evolution of the MCL utilized for IABP testing from its early iterations to the contemporary MCL, it becomes evident that the MCL employed for IABP testing has evolved in a manner analogous to the broader development of the MCL with respect to its structural composition, and the hardware can be upgraded according to the updating of the materials of the aorta and other components of the MCL. At the practical application level, the performance of the IABP is limited in two aspects due to its structural characteristics. Firstly, the diameter of the balloon catheter must be sufficiently small due to surgical constraints. Secondly, the compressibility of the gas, from which the IABP is less efficient, is another factor (De Lazzari et al., 2023; Capoccia et al., 2015). Consequently, in the actual treatment process The IABP is commonly used as a short-term means of support to create the conditions for the ventricle to recover benign circulation, thereby eliminating the need for long-term support (Xu et al., 2023).
MCL for testing total artificial hearts
TAH is distinguished from VADs in that it comprises four components: the VADs, driver, monitoring system, and energy source. The TAH has the capacity to substitute for the right and left ventricles following implantation in the human body, thereby artificially facilitating both body and pulmonary circulation. Consequently, the MCL must encompass both body circulation and pulmonary circulation (Kuroda et al., 2023). In terms of application targets, the total artificial heart and BiVAD have similarities and are more suitable for application in patients with right ventricular failure caused by LVAD implantation. In order to understand the facilitating effect of the total artificial heart and BiVAD on patients with heart failure, respectively, Karimov et al. (2023) compared the hemodynamic effects of the total artificial heart and the BiVAD by establishing two different types of MCLs. The hemodynamic performance of the BiVAD is illustrated in Figures 3g,h.
The initial MCL utilized for the study of TAH was developed by Kolff et al. (1959) this initial model lacked the capacity to regulate resistance, thereby constraining the scope of TAH testing (Kolff et al., 1959). Subsequently, Laumen et al. (2013) employed electrically adjustable components in the MCL. This MCL was designed to assess the hemodynamic performance of TAH to simulate physiological, pathological, and particularly changing circulatory conditions. A bronchial shunt was incorporated into the MCL bypass, and the overall performance of the TAH was evaluated in vitro with this configuration. Notably, no thrombus was generated during the prolonged testing period. To achieve an optimal left-right blood flow balance, Ng et al. (2017) developed an adaptive starlink-like controller based on a four-element MCL. This controller incorporated an adaptive mechanism to minimize the risk of pulmonary congestion and atrial pumping, while meeting the cardiac pumping demands. In contrast to the MCL design by Vignali et al. (2022) the proposed controller utilizes a technical atrial shunt (TIAS) to enable independent testing of the left and right outputs of the TAH. This TIAS compensates for volume shifts between the pulmonary and somatic circulations due to potential blood flow imbalances.
As with MCLs for testing VADs, MCLs for testing TAHs can similarly be assembled and replaced with different modules to simulate more human states. For instance, as reported by Love et al. (2014) the circulatory loop was designed to include a number of novel features, including pressure-regulated slots for These include the incorporation of pressure-regulated slots to simulate exercise conditions, the inclusion of adjustable hardware parameters, and the incorporation of pulmonary vascular resistance to simulate changes in human exercise status and left-right flow imbalance during respiration. Additionally, a left atrial suction valve has been incorporated. Garrison et al. (1994b) reported that left ventricular blood from TAH has a higher hemolysis rate than ventricular blood from the pulmonary circulation. Consequently, Gräf et al. (2015) investigated hemolysis in the left ventricle of TAH by designing an MCL for the observation of blood compatibility. In this model, porcine blood was used as the fluid, and the atria were connected to the ventricles to This approach was undertaken to minimize the effects of fluid inertia. In contrast to the majority of MCLs, which do not incorporate a non-invasive pressure chamber into their structural design, this study’s MCL was equipped with such a chamber, ensuring the measurement of flow or pressure without direct blood contact. The interior of the MCL was constructed with blood-compatible materials, thereby preventing direct contact between the blood and the air. The study culminated in a final test of blood compatibility with TAH support, which was performed to assess the effectiveness of the designed MCL.
According to the literature review, the key aspects of testing TAH include adequate cardiac output, balanced left and right flows, performance testing, and blood compatibility. However, there are currently fewer studies related to blood compatibility for TAH. TAH for the other testing aspects. It is hypothesized that in the future, there will be more MCL experiments on blood compatibility for TAH due to the increasing emphasis on the blood-destructive nature of the mechanical implants. Blood compatibility will become a major concern.
Hybrid-MCL for personalized CAD assessment
In addition to the aforementioned fully physical MCLs, Hybrid-MCLs have emerged in recent years, revolutionizing testing of cardiovascular devices by combining real-time computational models with physical fluid circuits (Bardi et al., 2022). This architecture dynamically couples lumped-parameter digital twins to hydraulic interfaces via high-bandwidth sensors, extending beyond personalized hemodynamic tuning to encompass dynamic pathological event simulation—particularly critical arrhythmias affecting over 50% of VAD patients (Hong et al., 2025; Cappon et al., 2021; Korn et al., 2018).
Fresiello’s foundational work demonstrated patient-specific tuning across clinical phenotypes with <10% error (Fresiello et al., 2015), while recent advances by Rapp et al. (2022) have addressed the critical gap in transient pathology modeling through ECG-driven approaches. By processing fiducial points from clinical ECGs (PhysioNet MGH/MF database), their system modulates ventricular elastance functions to replicate atrial/ventricular fibrillation, achieving <4% pressure tracking error during VAD-supported arrhythmias. This multi-method framework combines: real-time CVS parameter adjustment for acute stenosis induction; clinical PLV data tracing for atrial fibrillation; and F-wave injection (8 Hz mean frequency) for electrophysiological fidelity.
Building on these personalized and pathological simulation capabilities, Ochsner et al. (2012) engineered a standardized numerical-hydraulic interface operating at 5 kHz. This enables real-time assessment of mixed-flow VADs under pulmonary hypertension (PH) and exercise conditions without physical reconfiguration. Concurrently, Bardi et al. (2024) expanded this paradigm to anatomical fidelity: their PID-controlled Windkessel outlet generates patient-specific waveforms, while LED-PIV technology captures 3D velocity fields in a compliant aneurysm model, thereby demonstrating that hybrid-MCL can simultaneously resolve global hemodynamics and local fluid dynamics. In the context of complex biventricular applications, Perra et al. (2025) implemented a preload-responsive controller in a TAH-guided hybrid-MCL. This controller autonomously adjusts impulse parameters during sleep-rest-exercise transitions while maintaining flow balance during pulmonary artery pressure fluctuations. In a recent study, Nair et al. (2025) validated the applicability to congenital diseases. The pressure drops in a compliant aortic stenosis model matched 3D fluid-structure simulation results with an average error of only 1.6 mmHg, demonstrating robustness in pediatric motion states.
It is evident that these advancements establish hybrid-MCL as a unified platform that integrates computational adaptability and physical realism. This ultimately accelerates the optimization of patient-specific devices through repeatable in vitro tuning and clinical validation.
Discussion
The MCL has become the primary human hemodynamic simulation system for evaluating cardiovascular implantable devices, including ventricular assist devices and total artificial hearts. The simulation of hemodynamic changes following device implantation, as conducted by MCL, is a critical step in validating device performance prior to surgical intervention. It is evident that the implementation of continuous improvements to the MCL’s structural framework has resulted in a notable expansion of its application scope, thereby encompassing complex clinical scenarios with increasing complexity. The integration of modular designs and Hybrid-MCLs has emerged as a pivotal technological catalyst for this progress. For instance, by replacing or expanding modules (e.g., the aorta, ventricular assist device, or total artificial heart), MCL can adapt to the testing requirements of different devices. Furthermore, hybrid systems facilitate dynamic simulation of pathological states by real-time coupling of digital cardiovascular models with physical circuits. It is important to note that total artificial hearts require replacement of both left and right ventricular structures, while ventricular assist devices retain partial cardiac function. This discrepancy leads to substantial variations in the extraction of circulatory parameters across different devices. The digital twin architecture of hybrid-MCLs provides a unified solution by adjusting digital model parameters, thereby enabling the rapid switching of device testing scenarios and circumventing the hardware reconfiguration bottleneck inherent in traditional MCLs (Malone et al., 2022).
Since the advent of MCL, an analysis of prevailing trends has revealed that the complexity of MCL manifests a distinct hierarchical structure. In the analysis of the 130 studies included, it was found that approximately 16.36% of MCL models incorporated only the systemic circulation, rendering them suitable for basic parameter testing of left ventricular assist devices. In addition, 47.27% of MCL models integrated both the systemic and pulmonary circulations, thereby enabling simulation of the coupled effects of ventricular assist devices on the cardiopulmonary system. The remaining 36.36% of MCL models further incorporated subsystems such as the coronary circulation, cerebral blood supply, and renal circulation. It is noteworthy that recently emerging hybrid-MCLs integrate multi-circulatory physiological interactions (e.g., cerebral blood flow autoregulation coupled with systemic circulation) through digital modelling, thereby significantly reducing hardware complexity. Despite the prevailing focus on dual-circulatory MCLs in current research, there has been an annual increase in the proportion of multi-circulatory MCLs and hybrid-MCLs. This trend may be closely related to clinical concerns about the long-term safety of devices, such as the potential for blood cell damage caused by impeller rotation in ventricular assist devices, as well as complex cases, such as the customized needs of amputees for extracorporeal simulation. The primary benefit of hybrid systems is the ability to achieve “virtual multi-circuit coupling” through the utilization of digital twin technology. This approach circumvents the occurrence of physical interface compatibility issues while concurrently supporting personalized hemodynamic validation for complex cases, such as those involving patients with arrhythmia. Furthermore, although the number of publications in 2025 has slightly decreased, the overall annual publication trend continues to show a linear increase, indicating sustained growth in research activity in this field.
However, current MCL research still faces numerous technical challenges. The first is the compatibility issue of modular design. The fluid dynamics parameters of different circulatory loops must be dynamically matched. For example, when replacing circulatory modules for organs such as the brain or kidneys, pressure adjustments are necessary to prevent system imbalance. The brain circulatory system is just one typical scenario; similar challenges exist in subsystems such as the liver and kidneys. Additionally, the interface sealing and anticoagulant properties of 3D-printed organ models and MCL hardware require further optimization (Xu et al., 2023). Notably, the FDA-led Round Robin study (aimed at harmonizing testing protocols for mechanical circulatory support devices) will provide a critical framework for addressing multi-center data comparability, long-term performance evaluation, and module interface standardization, and is expected to guide revisions to the next-generation of the ISO 14708-5 standard (Fresiello et al., 2015; Fresiello et al., 2024; Rocchi et al., 2023b; Fresiello et al., 2014). Hybrid-MCL offers an innovative approach—digital models assume parameter coordination functions (e.g., Fresiello’s closed-loop regulation), while physical circuits only need to maintain basic interfaces, significantly reducing hardware adaptation complexity. Second is the limitation of long-term performance evaluation. Existing MCLs are primarily used for short-term testing, but ventricular assist devices must operate continuously for years after implantation. How to simulate long-term wear through accelerated aging experiments remains a challenge. Hybrid systems can simulate pathological evolution processes spanning months or even years by integrating digital degradation models (e.g., Perra’s physiological controller). Finally, there is the complexity of multi-loop coupling. For example, the introduction of brain blood flow autoregulation mechanisms may disrupt system stability. Next-generation hybrid-MCLs use intelligent algorithms (such as Rapp’s ECG-driven elastic function (Rapp et al., 2022)) to dynamically adjust digital-physical interactions in real time, significantly enhancing system robustness.
In the future, hybrid-MCL will become the key paradigm for overcoming technical limitations. The digital architecture of the system is of a modular nature, thus supporting the concept of “virtual plug-and-play.” To provide an example, in the case of amputees, it is only necessary to adjust the digital circulation parameters, whilst the simulation of intracranial vascular lesions can be achieved through the hybrid integration of 3D-printed models and PID controllers. Of greater significance is the enhancement of MCL, which transitions from a hardware testing instrument to a clinical decision-making platform. This transformation is exemplified by the integration of patient imaging data to generate digital twins and the simulation of the hemodynamic response subsequent to device implantation (for instance, the efficacy of aortic valve stenosis intervention). Furthermore, cross-scale simulation (for example, computational fluid dynamics integrated with hybrid-MCL) and machine learning optimization will propel personalized validation into a new phase. Despite the fact that research on these fusion technologies is still in its infancy, their value in reducing clinical risks and accelerating device translation is becoming increasingly apparent.
Conclusion
The evolution of MCL has been predominantly driven by the demands of CADs, with a shift from single-function validation to multi-organ physiological simulation. A review of the literature from the past decade reveals that MCLs encompassing body circulation and pulmonary circulation remain the prevailing standard. However, there has been an observed annual increase in the prevalence of multi-circulation MCLs. This trend mirrors the clinical concern regarding the long-term safety of CADs and the impact of multi-organ interactions. The integration of 3D-printed organ models with multimodal sensors, such as PIV and 4D Flow MRI, is facilitated by modular design in MCLs. However, significant technical challenges persist. Insufficient fluid compatibility of multi-loop circuits (i.e., dynamic matching capability of pressure-flow parameters between subsystems), such as the conflict between cerebral circulation autoregulation and other circulatory systems, remains a substantial hurdle. Simulation and mechanical pumping control; second, the existing MCLs are difficult to simulate the biocompatibility problems caused by long-term blood flow shear, such as the VAD impeller’s blood cell damage; and third, low matching of individualized physiological characteristics, which restricts the efficiency of clinical translation. In response to these challenges, research indicates that adaptive closed-loop hybrid-MCL systems represent a critical breakthrough pathway. These systems dynamically coordinate multi-loop conflicts through real-time digital twin coupling with physical loops. Simultaneously, multi-scale simulation accuracy optimization significantly enhances the reliability of long-term shear force predictions. This is achieved through a computational fluid dynamics and organ-on-a-chip integrated platform. The integration of personalized digital twins establishes a high-fidelity validation environment by integrating patient imaging and postoperative data, thereby systematically addressing issues pertaining to physiological feature matching. The overarching objective of MCL has exceeded the confines of conventional in vitro validation frameworks, metamorphosing into a physiological digital twin that serves as a nexus between engineering design and clinical decision-making. This transformation necessitates interdisciplinary collaboration to drive technological innovation and standardized integration.
Author contributions
HL: Writing – original draft. YW: Writing – review and editing, Funding acquisition, Data curation. XW: Writing – review and editing, Supervision, Methodology, Formal Analysis. LZ: Software, Validation, Investigation, Writing – review and editing, Project administration. LL: Conceptualization, Visualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by National Natural Science Foundation of China (51875143) and Natural Science Foundation of Heilongjiang Province of China (LH2024E085).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
A G. D., Jean R., Moustafa M., Crusan A., Richardson E., Shinnar M., et al. (2023). Using a mock circulatory loop as a regulatory science tool to simulate different heart failure conditions. J. Biomechanical Eng. 146 (1), 011004. doi:10.1115/1.4063746
Agrafiotis E., Geith M. A., Golkani M. A., Hergesell V., Sommer G., Spiliopoulos S., et al. (2021). An active approach of pressure waveform matching for stress-based testing of arteries. Artif. Organs 45 (12), 1562–1575. doi:10.1111/aor.14064
Agrafiotis E., Zimpfer D., Mächler H., Holzapfel G. A. (2024). Review of systemic mock circulation loops for evaluation of implantable cardiovascular devices and biological tissues. J. Endovascular Ther. 2024, 15266028241235876. doi:10.1177/15266028241235876
Alavi R., Aghilinejad A., Wei H., Niroumandi S., Wieman S., Pahlevan N. M. (2022). A coupled atrioventricular-aortic setup for in-vitro hemodynamic study of the systemic circulation: design, fabrication, and physiological relevancy. PLoS ONE 17 (11), e0267765. doi:10.1371/journal.pone.0267765
Amabili M., Balasubramanian P., Ferrari G., Franchini G., Giovanniello F., Tubaldi E. (2020). Identification of viscoelastic properties of Dacron aortic grafts subjected to physiological pulsatile flow. J. Mech. Behav. Biomed. Mater. 110, 103804. doi:10.1016/j.jmbbm.2020.103804
Bardi F., Gasparotti E., Vignali E., Avril S., Celi S. (2022). A hybrid mock circulatory loop for fluid dynamic characterization of 3D anatomical phantoms. IEEE Trans. Biomed. Eng. 70 (5), 1651–1661. doi:10.1109/TBME.2022.3224581
Bardi F., Gasparotti E., Vignali E., Antonuccio M. N., Storto E., Avril S., et al. (2024). A hybrid mock circulatory loop integrated with a LED-PIV system for the investigation of AAA compliant phantoms. Front. Bioeng. Biotechnol. 12, 1452278. doi:10.3389/fbioe.2024.1452278
Baturalp T. B. (2016). Design and development of a systemic mock circulation loop with a novel beating left ventricular simulator.
Bazan O., Ortiz J. P. (2016). Experimental validation of a cardiac simulator for in vitro evaluation of prosthetic heart valves. Braz. J. Cardiovasc. Surg. 31, 151–157. doi:10.5935/1678-9741.20160041
Bender M., Escher A., Röhrich M. (2023). An atraumatic mock loop for realistic hemocompatibility assessment of blood pumps.
Bleasdale R. A., Parker K. H., Jones C. J. H. (2003). Chasing the wave. Unfashionable but important new concepts in arterial wave travel. Am. J. Physiology-Heart Circulatory Physiology 284 (6), H1879–H1885. doi:10.1152/ajpheart.00070.2003
Böttcher W., Alexi-Meskishvili V. V. (2006). The first operations on the valves of the heart with the aid of an artificial heart pump. Z. für Herz- Thorax-und Gefäßchirurgie 20, 75–81. doi:10.1007/s00398-006-0532-6
Brunner B. S., Thierij A., Jakob A., Tengler A., Grab M., Thierfelder N., et al. (2022). 3D-printed heart models for hands-on training in pediatric cardiology–the future of modern learning and teaching? GMS J. Med. Educ. 39 (2), Doc23. doi:10.3205/zma001544
Capoccia M., Bowles C. T., Pepper J. R., Banner N. R., Simon A. R. (2015). Evidence of clinical efficacy of counterpulsation therapy methods. Heart Fail. Rev. 20, 323–335. doi:10.1007/s10741-014-9468-1
Cappon F., Wu T., Papaioannou T., Du X., Hsu P. L., Khir A. W. (2021). Mock circulatory loops used for testing cardiac assist devices: a review of computational and experimental models. Int. J. Artif. Organs 44 (11), 793–806. doi:10.1177/03913988211045405
Cardiovascular Mathematics (2010). Modeling and simulation of the circulatory system. Springer Science & Business Media.
Chen H., Wang W., Liu D., Cao Z., Yang Y., He Y., et al. (2023). The effect of terminal impedance on aortic morphology and hemodynamics: an in vitro phantom study using flow field visualization. Front. Bioeng. Biotechnol. 11, 1175916. doi:10.3389/fbioe.2023.1175916
Choi S. W., Nam K. W., Lim K. M., Shim E. B., Won Y. S., Woo H. M., et al. (2014). Effect of counter-pulsation control of a pulsatile left ventricular assist device on working load variations of the native heart. Biomed. Eng. online 13, 35–11. doi:10.1186/1475-925X-13-35
Corazza I., Melandri G., Nanni S., Marcelli E., Cercenelli L., Bianchini D., et al. (2013). Passive counterpulsation: biomechanical rationale and clinical validation. J. Mech. Med. Biol. 13 (05), 1340004. doi:10.1142/s0219519413400046
Corazza I., Bianchini D., Marcelli E., Cercenelli L., Zannoli R. (2014). Passive aortic counterpulsation: biomechanical rationale and bench validation. J. Biomechanics 47 (7), 1618–1625. doi:10.1016/j.jbiomech.2014.03.001
De Lazzari B., Capoccia M., Badagliacca R., Bozkurt S., De Lazzari C. (2023). IABP versus Impella support in cardiogenic shock: in silico study. J. Cardiovasc. Dev. Dis. 10 (4), 140. doi:10.3390/jcdd10040140
Fajdek B., Golnik A. (2010). Modelling and simulation of human circulatory system[C]//2010 15th International Conference on methods and models in automation and robotics. IEEE, 399–404. doi:10.1109/MMAR.2010.5587199
Ferrari G., Górczyńska K., Mimmo R., De Lazzari C., Clemente F., Tosti G., et al. (2001). IABP assistance: a test bench for the analysis of its effects on ventricular energetics and hemodynamics. Int. J. Artif. Organs 24 (5), 274–280. doi:10.1177/039139880102400505
Ferrari G., De Lazzari C., Kozarski M., Clemente F., Górczyńska K., Mimmo R., et al. (2002). A hybrid mock circulatory system: testing a prototype under physiologic and pathological conditions. ASAIO J. 48 (5), 487–494. doi:10.1097/00002480-200209000-00009
Ferrari G., Kozarski M., De Lazzari C., Górczyńska K., Tosti G., Darowski M. (2005). Development of a hybrid (numerical-hydraulic) circulatory model: prototype testing and its response to IABP assistance. Int. J. Artif. Organs 28 (7), 750–759. doi:10.1177/039139880502800714
Ferrari G., Khir A. W., Fresiello L., Di Molfetta A., Kozarski M. (2011). Hybrid model analysis of intra-aortic balloon pump performance as a function of ventricular and circulatory parameters. Artif. organs 35 (9), 902–911. doi:10.1111/j.1525-1594.2011.01244.x
Ferrari G., Balasubramanian P., Tubaldi E., Giovanniello F., Amabili M. (2019). Experiments on dynamic behaviour of a Dacron aortic graft in a mock circulatory loop. J. Biomechanics 86, 132–140. doi:10.1016/j.jbiomech.2019.01.053
Flick J. B. (1957). Memoir of John Heysham Gibbon (1871-1956). Trans. Stud. Coll. Physicians Phila. 25 (2), 116–118.
Franzetti G., Díaz-Zuccarini V., Balabani S. (2019). Design of an in vitro mock circulatory loop to reproduce patient-specific vascular conditions: toward precision medicine. J. Eng. Sci. Med. Diagnostics Ther. 2 (4), 041004. doi:10.1115/1.4044488
Fresiello L., Zieliński K., Jacobs S., Di Molfetta A., Pałko K. J., Bernini F., et al. (2014). Reproduction of continuous flow left ventricular assist device experimental data by means of a hybrid cardiovascular model with baroreflex control. Artif. organs 38 (6), 456–468. doi:10.1111/aor.12178
Fresiello L., Ferrari G., Di Molfetta A., Zieliński K., Tzallas A., Jacobs S., et al. (2015). A cardiovascular simulator tailored for training and clinical uses. J. Biomed. Inf. 57, 100–112. doi:10.1016/j.jbi.2015.07.004
Fresiello L., Hermens J. A. J., Pladet L., Meuwese C. L., Donker D. W. (2024). The physiology of venoarterial extracorporeal membrane oxygenation-A comprehensive clinical perspective. Perfusion 39 (1_Suppl), 5S–12S. doi:10.1177/02676591241237639
Garrison L. A., Lamson T. C., Deutsch S., Geselowitz D. B., Gaumond R. P., Tarbell J. M. (1994a). An in-vitro investigation of prosthetic heart valve cavitation in blood. J. heart valve Dis. 3, S8–S24.
Garrison L. A., Frangos J. A., Geselowitz D. B., Lamson T. C., Tarbell J. M. (1994b). A new mock circulatory loop and its application to the study of chemical additive and aortic pressure effects on hemolysis in the Penn State electric ventricular assist device. Artif. Organs 18 (5), 397–407. doi:10.1111/j.1525-1594.1994.tb02222.x
Gehron J., Zirbes J., Bongert M., Schäfer S., Fiebich M., Krombach G., et al. (2019). Development and validation of a life-sized mock circulatory loop of the human circulation for fluid-mechanical studies. Asaio J. 65 (8), 788–797. doi:10.1097/MAT.0000000000000880
Geselowitz D. B., Miller G. E., Phillips W. M. (1977). Dynamic model of a sac-type pneumatically driven artificial ventricle. J. Biomech. Eng. 99, 14–19. doi:10.1115/1.3426261
Giridharan G. A., Sobieski M. A., Ising M., Slaughter M. S., Koenig S. C. (2011). Blood trauma testing for mechanical circulatory support devices. Biomed. Instrum. Technol. 45 (4), 334–339. doi:10.2345/0899-8205-45.4.334
Goto T., Fukuda I., Inamura T., Shirota M., Minakawa M. (2021). Flow analysis during mock circulation in normal and aortic arch aneurysm models through an aortic cannula toward the aortic arch and root. J. Artif. Organs 24 (4), 442–449. doi:10.1007/s10047-021-01267-3
Gräf F., Finocchiaro T., Laumen M., Mager I., Steinseifer U. (2015). Mock circulation loop to investigate hemolysis in a pulsatile total artificial heart. Artif. Organs 39 (5), 416–422. doi:10.1111/aor.12399
Granegger M., Mahr S., Horvat J., Aigner P., Roehrich M., Stoiber M., et al. (2013). Investigation of hemodynamics in the assisted isolated porcine heart. Int. J. Artif. Organs 36 (12), 878–886. doi:10.5301/ijao.5000257
Gregory S. D., Pauls J. P., Wu E. L., Stephens A., Steinseifer U., Tansley G., et al. (2020). An advanced mock circulation loop for in vitro cardiovascular device evaluation. Artif. Organs 44 (6), E238–E250. doi:10.1111/aor.13636
Guo Z. (2022). Research on the design and development of in vitro simulation cycle testing system based on user study. Shandong Province, China: Shandong University.
Gupta S., Balakrishnan K. R., Kumar R. K. (2020). Design of a percutaneous left ventricular assist device, 298–305.
Hildebrand S., Groß-Hardt S., Schmitz-Rode T., Steinseifer U., Jansen S. V. (2022). In-vitro performance of a single-chambered total artificial heart in a fontan circulation. J. Artif. Organs 25 (1), 1–8. doi:10.1007/s10047-021-01273-5
Hong W., Yu H., Chen J., Talamantes J., Rollins D. M., Fang X., et al. (2023). A mock circulation loop to characterize in vitro hemodynamics in human systemic arteries with stenosis. Fluids 8 (7), 198. doi:10.3390/fluids8070198
Hong W., Tewari V., Chen J., Sawchuk A. P., Yu H. (2025). A comprehensive review of mock circulation loop systems for experimental hemodynamics of cardiovascular diseases. Fluids 10 (7), 166. doi:10.3390/fluids10070166
Huang H. (2013). The state key laboratory of fluid power transmission and control. Hangzhou, China: Zhejiang University and Surgery Department, Zhejiang Provincial People’s Hospital.
Huang F., Ruan X., Zou J., Qian W., Fu X. (2013). A fast building and effective hydraulic pediatric mock circulatory system for the evaluation of a left ventricular assist device. Asaio J. 59 (6), 575–585. doi:10.1097/MAT.0b013e3182a78e08
Jahren S. E., Ochsner G., Shu F., Amacher R., Antaki J. F., Vandenberghe S. (2014). Analysis of pressure head-flow loops of pulsatile rotodynamic blood pumps. Artif. organs 38 (4), 316–326. doi:10.1111/aor.12139
Jeong J. H., Kim Y. M., Lee B., Hong J., Woo S. Y., et al. (2020). Design and evaluation of enhanced mock circulatory platform simulating cardiovascular physiology for medical palpation training. Appl. Sci. 10 (16), 5433. doi:10.3390/app10165433
Jeong J. H., Lee B., Hong J., Yang T. H., Park Y. H. (2023). Reproduction of human blood pressure waveform using physiology-based cardiovascular simulator. Sci. Rep. 13 (1), 7856. doi:10.1038/s41598-023-35055-1
Kadem L., Knapp Y., Pibarot P., Bertrand E., Garcia D., Durand L. G., et al. (2005). A new experimental method for the determination of the effective orifice area based on the acoustical source term. Exp. Fluids 39 (6), 1051–1060. doi:10.1007/s00348-005-0038-0
Kado Y., Miyamoto T., Horvath D. J., Gao S., Fukamachi K., Karimov J. H. (2020). Development of a circulatory mock loop for biventricular device testing with various heart conditions. Int. J. Artif. Organs 43 (9), 600–605. doi:10.1177/0391398820903316
Kado Y., Polakowski A. R., Kuban B. D., Horvath D. J., Miyamoto T., Karimov J. H., et al. (2021). Left atrial assist device function at various heart rates using a mock circulation loop. Int. J. Artif. Organs 44 (7), 465–470. doi:10.1177/0391398820977508
Kantrowitz A., Cardona R. R., Freed P. S. (1993). Weaning from the intraaortic balloon pump. Comprehensive intraaortic ballon counterpulsation. Mosby Year Book, 398–410.
Karimov J. H., Miyagi C., Flick C. R., Polakowski A. R., Kuban B. D., Kuroda T., et al. (2023). Biventricular circulatory support using single-device and dual-device configurations: initial pump characterization in simulated heart failure model. Front. Cardiovasc. Med. 10, 1045656. doi:10.3389/fcvm.2023.1045656
Khalil H. A., Kerr D. T., Franchek M. A., Metcalfe R. W., Benkowski R. J., Cohn W. E., et al. (2008). Continuous flow total artificial heart: modeling and feedback control in a mock circulatory system. Asaio J. 54 (3), 249–255. doi:10.1097/MAT.0b013e3181739b70
Khienwad T., Wannawat P., Naiyanetr P. (2016). Assessment of artificial heart valve using dynamic mock circulatory system. Int. J. Appl. Biomed. Eng. 9 (1), 21–26.
Khienwad T., Wannawat P., Naiyanetr P. (2019). Novel design of a Frank-Starling physiological mock circulatory loop for in-vitro testing of rotary blood pump. Int. J. Appl. 12 (1).
Kim M. S., Lee W., Kim K. B., Lim H. G., Kim Y. J. (2021). A preclinical trial of perventricular pulmonary valve implantation: pericardial versus aortic porcine valves mounted on self-expandable stent. Artif. Organs 45 (5), E89–E100. doi:10.1111/aor.13845
Kolff W. J., Akutsu T., Dreyer B. (1959). Artificial heart in the chest and use of polyurethane for making hearts, valves and aortas. ASAIO J. 5 (1), 298–303. doi:10.1088/0031-9155/3/4/108
Kolyva C., Pantalos G. M., Giridharan G. A., Pepper J. R., Khir A. W. (2009). Discerning aortic waves during intra-aortic balloon pumping and their relation to benefits of counterpulsation in humans. J. Appl. Physiology 107 (5), 1497–1503. doi:10.1152/japplphysiol.00413.2009
Kolyva C., Biglino G., Pepper J. R., Khir A. W. (2012). A mock circulatory system with physiological distribution of terminal resistance and compliance: application for testing the intra-aortic balloon pump. Artif. Organs 36 (3), E62–E70. doi:10.1111/j.1525-1594.2010.01071.x
Kolyva C., Pepper J. R., Khir A. W. (2016). Newly shaped intra-aortic balloons improve the performance of counterpulsation at the semirecumbent position: an in vitro study. Artif. organs 40 (8), E146–E157. doi:10.1111/aor.12791
Korn L., Rüschen D., Zander N., Leonhardt S., Walter M. (2018). Real-time ecg simulation for hybrid mock circulatory loops. Artif. Organs 42 (2), 131–140. doi:10.1111/aor.13000
Kung E. O., Taylor C. A. (2011). Development of a physical Windkessel module to re-create in vivo vascular flow impedance for in vitro experiments. Cardiovasc. Eng. Technol. 2, 2–14. doi:10.1007/s13239-010-0030-6
Kuroda T., Miyagi C., Fukamachi K., Karimov J. H. (2023). Biventricular assist devices and total artificial heart: strategies and outcomes. Front. Cardiovasc. Med. 9, 972132. doi:10.3389/fcvm.2022.972132
Laumen M., Finocchiaro T., Cuenca E., Guersoy D., Spiliopoulos S., Tenderich G., et al. (2013). A novel total artificial heart for destination therapy: in-vitro and in-vivo study. Biomed. Engineering/Biomedizinische Tech. 58. doi:10.1515/bmt-2013-4373
Leme J., Fonseca J., Bock E., da Silva C., da Silva B. U., Dos Santos A. E., et al. (2011). A new model of centrifugal blood pump for cardiopulmonary bypass: design improvement, performance, and hemolysis tests. Artif. Organs 35 (5), 443–447. doi:10.1111/j.1525-1594.2011.01254.x
Leopaldi A. M., Vismara R., Lemma M., Valerio L., Cervo M., Mangini A., et al. (2012). In vitro hemodynamics and valve imaging in passive beating hearts. J. biomechanics 45 (7), 1133–1139. doi:10.1016/j.jbiomech.2012.02.007
Leopaldi A. M., Vismara R., Van Tuijl S., Redaelli A., van de Vosse F. N., Fiore G. B., et al. (2015). A novel passive left heart platform for device testing and research. Med. Eng. Phys. 37 (4), 361–366. doi:10.1016/j.medengphy.2015.01.013
Li B., Wang H., Li G., Liu J., Zhang Z., Gu K., et al. (2020a). A patient-specific modelling method of blood circulatory system for the numerical simulation of enhanced external counterpulsation. J. Biomechanics 111, 110002. doi:10.1016/j.jbiomech.2020.110002
Li S., Hsu P. L., Hao Y., Ren H. (2020b). Study on in vitro performance verification protocol for left ventricular assist device. Int. J. Artif. Organs 43 (4), 242–251. doi:10.1177/0391398819882701
Li P., Mei X., Ge W., Wu T., Zhong M., Huan N., et al. (2023). A comprehensive comparison of the in vitro hemocompatibility of extracorporeal centrifugal blood pumps. Front. Physiology 14, 1136545. doi:10.3389/fphys.2023.1136545
Liang X. (2016). Design optimization and control of left ventricular assist device. Shanghai, China: Shanghai Jiao Tong University.
Libera F., Kavitha M., Kaatje G., Hayward C., Rocchi M., Bezy M., et al. (2022). Initial clinical validation of a hybrid in silico—in vitro cardiorespiratory simulator for comprehensive testing of mechanical circulatory support systems. Front. Physiology 13, 967449. doi:10.3389/fphys.2022.967449
Liu G. M., Hou J. F., Wei R. J., Hu S. S. (2021). A 3-dimensional printed left ventricle model incorporated into a mock circulatory loop to investigate hemodynamics inside a severely failing ventricle supported by a blood pump. Artif. Organs 45 (2), 143–150. doi:10.1111/aor.13802
Love H. C., Timms D. L., Nestler F., Frazier O. H., Cohn W. E. (2014). A mock circulatory loop for designing and evaluating total artificial hearts. IEEE, 2014, 5667–5670. doi:10.1109/EMBC.2014.6944913
Lu P. J., Yang C. F. J., Wu M. Y., Hung C. H., Chan M. Y., Hsu T. C. (2011). Wave energy patterns of counterpulsation: a novel approach with wave intensity analysis. J. Thorac. Cardiovasc. Surg. 142 (5), 1205–1213. doi:10.1016/j.jtcvs.2011.02.018
Luo M. (2016). Contract for an in vitro hemodynamic characterization device. Nanchang, China: Nanchang University.
Makinouchi K., Ohara Y., Sakuma I., Damm G., Mizuguchi K., Jikuya T., et al. (1994). Internal hydraulic loss in a seal-less centrifugal gyro pump. Artif. Organs 18 (1), 25–31. doi:10.1111/j.1525-1594.1994.tb03295.x
Malone A., Gallagher S., Saidi J., Rizq G., O'Dowd E., Vallence D., et al. (2022). In vitro benchtop mock circulatory loop for heart failure with preserved ejection fraction emulation. Front. Cardiovasc. Med. 9, 910120. doi:10.3389/fcvm.2022.910120
Marassi M., Castellini P., Pinotti M., Scalise L. (2004). Cardiac valve prosthesis flow performances measured by 2D and 3D-stereo particle image velocimetry. Exp. Fluids 36, 176–186. doi:10.1007/s00348-003-0693-y
Masoud F., Erika B., D'Souza G. A., Herbertson L. H., Scully C. G. (2023). Mock circulatory loop generated database for dynamic characterization of pressure-based cardiac output monitoring systems. Comput. Biol. Med. 160, 106979. doi:10.1016/j.compbiomed.2023.106979
Meskin M., Starkey P. A., Kaspersen A. E., Ringgaard S., Sand S. G., Nygaard J. V., et al. (2024). Investigating the importance of left atrial compliance on fluid dynamics in a novel mock circulatory loop. Sci. Rep. 14 (1), 1864. doi:10.1038/s41598-024-52327-6
Mi J., Zhao Z., Wang H., Tang H. (2023). Study of the relationship between pulmonary artery pressure and heart valve vibration sound based on mock loop. Bioengineering 10 (8), 985. doi:10.3390/bioengineering10080985
Monreal G., Koenig S. C., Taskin M. E., Shambaugh C., LaRose J. A., Slaughter M. S. (2023). Feasibility testing of the RT cardiac systems percutaneous mechanical circulatory support device. ASAIO J. 69 (6), 519–526. doi:10.1097/MAT.0000000000001887
Nair P. J., Perra E., McElhinney D. B., Marsden A. L., Ennis D. B., Dual S. A. (2025). Experiments and simulations to assess exercise-induced pressure drop across aortic coarctations. J. Biomechanical Eng. 147 (7), 071007. doi:10.1115/1.4068716
Nestler F., Bradley A. P., Wilson S. J., Timms D. L., Frazier O. H., Cohn W. E. (2014). A hybrid mock circulation loop for a total artificial heart. Artif. organs 38 (9), 775–782. doi:10.1111/aor.12380
Ng B. C., Smith P. A., Nestler F., Timms D., Cohn W. E., Lim E. (2017). Application of adaptive starling-like controller to total artificial heart using dual rotary blood pumps. Ann. Biomed. Eng. 45, 567–579. doi:10.1007/s10439-016-1706-3
Nguyen And T. T., Biadillah Y., Mongrain R., Brunette J., Tardif J. C., Bertrand O. F. (2004). A method for matching the refractive index and kinematic viscosity of a blood analog for flow visualization in hydraulic cardiovascular models. J. Biomech. Eng. 126 (4), 529–535. doi:10.1115/1.1785812
Ochsner G., Amacher R., Amstutz A., Plass A., Schmid Daners M., Tevaearai H., et al. (2012). A novel interface for hybrid mock circulations to evaluate ventricular assist devices. IEEE Trans. Biomed. Eng. 60 (2), 507–516. doi:10.1109/TBME.2012.2230000
Ochsner G., Amacher R., Daners M. S. (2013). Emulation of ventricular suction in a hybrid mock circulation[C]//2013 european control conference (ECC). IEEE, 3108–3112. doi:10.23919/ECC.2013.6669530
Pantalos G. M., Ionan C., Koenig S. C., Gillars K. J., Horrell T., Sahetya S., et al. (2010). Expanded pediatric cardiovascular simulator for research and training. ASAIO J. 56 (1), 67–72. doi:10.1097/MAT.0b013e3181c838ae
Parker K. H., Jones C. J. H. (1990). Forward and backward running waves in the arteries: analysis using the method of characteristics. J. Biomech. Eng. 112, 322–326. doi:10.1115/1.2891191
Pastuszko P., EdieGibbon R. N. J. H. (2004). John H. Gibbon, Jr., the inventor of the first successful heart-lung machine. J. cardiac Surg. 19 (1), 65–73. doi:10.1111/j.0886-0440.2004.04018.x
Patterson T., Perera D., Redwood S. R. (2014). Intra-aortic balloon pump for high-risk percutaneous coronary intervention. Circ. Cardiovasc. Interv. 7 (5), 712–720. doi:10.1161/CIRCINTERVENTIONS.114.001258
Perra E., Jonasson D., Zaman S. F., Brynedal Ignell N., Broomé M., Finocchiaro T., et al. (2025). Physiological control of realheart total artificial heart. Artif. Organs, aor.15036. doi:10.1111/aor.15036
Petrou A., Granegger M., Meboldt M., Schmid Daners M. (2019). A versatile hybrid mock circulation for hydraulic investigations of active and passive cardiovascular implants. Asaio J. 65 (5), 495–502. doi:10.1097/MAT.0000000000000851
Pugovkin A. A., Markov A. G., Selishchev S. V., Korn L., Walter M., Leonhardt S., et al. (2019). Advances in hemodynamic analysis in cardiovascular diseases investigation of energetic characteristics of adult and pediatric sputnik left ventricular assist devices during mock circulation support. Cardiol. Res. Pract. 2019, 1–15. doi:10.1155/2019/4593174
Rapp E. S., Pawar S. R., Longoria R. G. (2022). Hybrid mock circulatory loop simulation of extreme cardiac events. IEEE Trans. Biomed. Eng. 69 (9), 2883–2892. doi:10.1109/TBME.2022.3156963
Rasooli R., Holmstrom H., Giljarhus K. E. T. (2013). In vitro hemodynamic performance of a blood pump for self-powered venous assist in univentricular hearts.
Reider C., Moon J., Ramesh V., Montes R., Campos J., Herold B., et al. (2017). Intraventricular thrombus formation in the LVAD-assisted heart studied in a mock circulatory loop. Meccanica 52, 515–528. doi:10.1007/s11012-016-0433-z
Reil J. C., Saisho H., Jockwer A., Fujita B., Paluszkiewicz L., Reil G. H., et al. (2023). Impact of heart rate, aortic compliance and stroke volume on the aortic regurgitation fraction studied in an ex vivo pig model. Open Heart 10 (2), e002319. doi:10.1136/openhrt-2023-002319
Rocchi M., Gross C., Moscato F., Schlöglhofer T., Meyns B., Fresiello L. (2023a). An in vitro model to study suction events by a ventricular assist device: validation with clinical data. Front. Physiology 14, 1155032. doi:10.3389/fphys.2023.1155032
Rocchi M., Ingram M., Claus P., D'hooge J., Meyns B., Fresiello L. (2023b). Use of 3D anatomical models in mock circulatory loops for cardiac medical device testing. Artif. organs 47 (2), 260–272. doi:10.1111/aor.14433
Rogers J. G., Pagani F. D., Tatooles A. J., Bhat G., Slaughter M. S., Birks E. J., et al. (2017). Intrapericardial left ventricular assist device for advanced heart failure. N. Engl. J. Med. 376 (5), 451–460. doi:10.1056/NEJMoa1602954
Roka-Moiia Y., Li M., Ivich A., Muslmani S., Kern K. B., Slepian M. J. (2020). Impella 5.5 versus centrimag: a head-to-head comparison of device hemocompatibility. Asaio J. 66 (10), 1142–1151. doi:10.1097/MAT.0000000000001283
Roldán-Alzate A., García-Rodríguez S., Anagnostopoulos P. V., Srinivasan S., Wieben O., François C. J. (2015). Hemodynamic study of TCPC using in vivo and in vitro 4D flow MRI and numerical simulation. J. Biomechanics 48 (7), 1325–1330. doi:10.1016/j.jbiomech.2015.03.009
Rosalia L., Ozturk C., Shoar S., Fan Y., Malone G., Cheema F. H., et al. (2021). Device-based solutions to improve cardiac physiology and hemodynamics in heart failure with preserved ejection fraction. Basic Transl. Sci. 6 (9-10), 772–795. doi:10.1016/j.jacbts.2021.06.002
Schampaert S., van't Veer M., Rutten M. C. M., van Tuijl S., de Hart J., van de Vosse F. N., et al. (2013). Autoregulation of coronary blood flow in the isolated beating pig heart. Artif. Organs 37 (8), 724–730. doi:10.1111/aor.12065
Shehab S., Allida S. M., Newton P. J., Robson D., Macdonald P. S., Davidson P. M., et al. (2019). Valvular regurgitation in a biventricular mock circulatory loop. Asaio J. 65 (6), 551–557. doi:10.1097/MAT.0000000000000852
Silvay G., Castillo J. G. (2013). John Heysham Gibbon and the 60th anniversary of the first successful heart-lung machine: brief notes about the development of cardiac surgery in Europe and Slovakia. Bratisl. Lek. Listy 114 (5), 247–250. doi:10.1053/j.jvca.2012.11.004
Swalen M. J., Segers P., Verdonck P., Khir A. W. (2005). Hemodynamic parameters of the IABP in the ascending aorta of a tilted mock circulation. ASAIO J. 51 (2), 30A. doi:10.1097/00002480-200503000-00119
Tanaka Y., Vu V., Fischer I., Nakajima T., Jocker B., May-Newman K., et al. (2020). The impact of pump speed and blood pressure optimization on severe aortic valve insufficiency with left ventricular assist device: a mock loop study. J. Heart Lung Transplant. 39 (4), S404–S405. doi:10.1016/j.healun.2020.01.153
Tanné D., Bertrand E., Kadem L., Pibarot P., Rieu R. (2010). Assessment of left heart and pulmonary circulation flow dynamics by a new pulsed mock circulatory system. Exp. fluids 48, 837–850. doi:10.1007/s00348-009-0771-x
Tozzi P., Maertens A., Emery J., Joseph S., Kirsch M., Avellan F. (2017). An original valveless artificial heart providing pulsatile flow tested in mock circulatory loops. Int. J. Artif. Organs 40 (12), 683–689. doi:10.5301/ijao.5000634
Tuzun E., Rutten M., Dat M., van de Vosse F., Kadipasaoglu C., de Mol B. (2011). Continuous-flow cardiac assistance: effects on aortic valve function in a mock loop. J. Surg. Res. 171 (2), 443–447. doi:10.1016/j.jss.2010.05.040
van Dort D. I. M., Thannhauser J., Gommans F. D. H., Ten Cate T. J., Duncker D. J., Suryapranata H., et al. (2020). Proof of principle of a novel co-pulsating intra-ventricular membrane pump. Artif. organs 44 (12), 1267–1275. doi:10.1111/aor.13757
van Dort D. I. M., Thannhauser J., Morshuis W. J., Geuzebroek G. S., Duncker D. J. (2022). A novel intra-ventricular assist device enhances cardiac performance in normal and acutely failing isolated porcine hearts. Int. J. Artif. Organs 45 (4), 388–396. doi:10.1177/03913988211003912
Vandenberghe S., Shu F., Arnold D. K., Antaki J. F. (2011). A simple, economical, and effective portable paediatric mock circulatory system. Proc. Institution Mech. Eng. Part H J. Eng. Med. 225 (7), 648–656. doi:10.1177/0954411911402287
Vignali E., Gasparotti E., Mariotti A., Haxhiademi D., Ait-Ali L., Celi S. (2022). High-versatility left ventricle pump and aortic mock circulatory loop development for patient-specific hemodynamic in vitro analysis. ASAIO J. 68 (10), 1272–1281. doi:10.1097/MAT.0000000000001651
Wang Y., Koenig S. C., Sobieski M. A., Slaughter M. S., Giridharan G. A. (2017). Hemodynamic benefits of counterpulsation, implantable, percutaneous, and intraaortic rotary blood pumps: an in-silico and in vitro study. Cardiovasc. Eng. Technol. 8, 439–452. doi:10.1007/s13239-017-0314-1
Wilson S. I., Ingram K. E., Oh A., Moreno M. R., Kassi M. (2023). The role of innovative modeling and imaging techniques in improving outcomes in patients with LVAD. Front. Cardiovasc. Med. 10, 1248300. doi:10.3389/fcvm.2023.1248300
Woelke E., Klein M., Mager I., Schmitz-Rode T., Steinseifer U., Arens J., et al. (2020). Miniaturized test loop for the assessment of blood damage by continuous-flow left-ventricular assist devices. Ann. Biomed. Eng. 48, 768–779. doi:10.1007/s10439-019-02404-z
Xu Q. (2017). Research on blood circulation system based on left heart assistance. Jiangsu Province, China: Jiangsu University.
Xu K. W., Gao Q., Wan M., Zhang K. (2023). Mock circulatory loop applications for testing cardiovascular assist devices and in vitro studies. Front. Physiology 14, 1175919. doi:10.3389/fphys.2023.1175919
Yang Z., Wang C. (2023). Can we expect a transgenic pig heart? Progress of xenograft research. J. Fudan Univ. (Medical Edition) 50 (04). doi:10.3969/j.issn.1672-8467.2023.04.018
Yilmaz S., Sedef H. (2023). Enhancing control of dynamic flow systems through innovator controller design and parametric polynomial modeling.
Yoshino M., Uemura M., Takahashi K., Watanabe N., Hoshi H., Ohuchi K., et al. (2001). Design and evaluation of a single-pivot supported centrifugal blood pump. Artif. organs 25 (9), 683–687. doi:10.1046/j.1525-1594.2001.06856.x
Keywords: mock circulatory loop, cardiovascular assist devices, hemodynamics, systemic circulation, pulmonary circulation, hybrid-MCL
Citation: Li H, Wang Y, Wu X, Zhou L and Liu L (2025) Technological evolution of in vitro mock circulatory loops for advanced cardiovascular assist device evaluation. Front. Physiol. 16:1610040. doi: 10.3389/fphys.2025.1610040
Received: 11 April 2025; Accepted: 20 August 2025;
Published: 02 September 2025.
Edited by:
Geoffrey A. Head, Baker Heart and Diabetes Institute, AustraliaReviewed by:
Ian Carr, United States Food and Drug Administration, United StatesCaio Francisco Ternus De Abreu, Universidade do Vale do Rio dos Sinos - UNISINOS, Brazil
Copyright © 2025 Li, Wang, Wu, Zhou and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yiwen Wang, d3l3QGhyYnVzdC5lZHUuY24=
 Hongyu Li
Hongyu Li Yiwen Wang
Yiwen Wang Xuefeng Wu
Xuefeng Wu Lijie Zhou
Lijie Zhou