- 1Department of Sports Medicine, Wuhan Sports University, Wuhan, China
- 2Institute of Intelligent Sport and Proactive Health, Department of Health and Physical Education, Jianghan University, Wuhan, China
With the intensification of population aging, sarcopenia in older adults has become a significant public health issue affecting quality of life. Sarcopenia is a progressive and systemic skeletal muscle disorder characterized by reduced muscle mass, decreased muscle strength, and diminished physical function. Although conventional exercise interventions have shown some efficacy in managing sarcopenia, their effects are limited and often insufficient to effectively halt disease progression. Therefore, exploring more efficient exercise interventions is of great importance. Blood flow restriction training (BFRT), as an emerging exercise intervention, has garnered increasing attention in recent years for its application in sarcopenia among older adults. Studies suggest that, compared to traditional resistance exercise, BFRT demonstrates superior effectiveness in improving muscle strength and mass in older adults, potentially serving as a viable alternative to conventional training methods. However, BFRT also presents certain limitations, including potential risks such as cardiovascular responses and muscle injury. Therefore, careful consideration of appropriate application scenarios and exercise loads is crucial during its implementation. This study reviews the biological mechanisms of BFRT in the intervention of sarcopenia and proposes tailored training protocols and application models for older adults. Furthermore, it thoroughly examines the potential risks and applicability of BFRT, aiming to provide theoretical foundations and practical guidance for clinical application. Additionally, the limitations of current research are analyzed, offering recommendations for future research directions.
1 Introduction
Skeletal muscle, one of the largest organs in the human body, accounts for approximately 40% of total body weight and plays a crucial role in controlling daily movement, thermoregulation, physical strength, and metabolic homeostasis (Yadav et al., 2022; Jing et al., 2022). Maintaining skeletal muscle health—including muscle strength, muscle mass, and muscle function (Cawthon et al., 2022)—is a critical prerequisite for extending healthy lifespan (McLeod et al., 2016; Tieland et al., 2018).
However, with advancing age, skeletal muscle strength and mass progressively decline at annual rates of 2%–3% and 0.5%–1% respectively (Goodpaster et al., 2006). This pathological condition is formally defined as sarcopenia—a geriatric syndrome characterized by progressive, generalized loss of skeletal muscle mass, strength, and physical function (Cruz-Jentoft and Sayer, 2019). The European Working Group on Sarcopenia in Older People (EWGSOP2) specifically emphasized in 2018 that reduced muscle strength should serve as the primary diagnostic criterion (Cruz-Jentoft et al., 2019). Etiologically, sarcopenia can be classified into two categories: primary (age-related) and secondary (associated with comorbidities such as rheumatoid arthritis, systemic sclerosis, and osteoarthritis) (Cruz-Jentoft et al., 2019; Bauer et al., 2019). Sarcopenia impairs physical function and increases risks of falls, disability, and mortality, severely impacting elderly health and quality of life (Evans et al., 2024). It also raises healthcare costs, creating substantial individual and societal burdens (Tournadre et al., 2019). Effective interventions are therefore essential to manage this age-related condition and improve health outcomes (Scott et al., 2021).
Currently, resistance training is widely recognized as the most effective intervention for sarcopenia (Dent et al., 2018; Fragala et al., 2019). However, its application may be limited due to its intensity characteristics. High-load resistance training (>70% 1RM) may increase the risk of exercise-related injuries due to its high mechanical loading properties (Schutzer, 2004). Furthermore, this training modality often results in reduced compliance and adherence among older adults with comorbidities (e.g., frailty, sarcopenia), rehabilitation patients, and individuals experiencing acute pain episodes (Fragala et al., 2019; Lavallee and Balam, 2010; Lees et al., 2005). Therefore, exploring safe, efficient, and low-load alternative interventions is urgently needed.
In recent years, blood flow restriction training (BFRT) has garnered significant attention as an emerging therapeutic intervention (Slysz et al., 2016). BFRT involves applying external pressure to the proximal limb using compression devices (e.g., tourniquets or inflatable cuffs) during exercise, partially restricting arterial inflow and fully occluding venous outflow. This creates an ischemic and hypoxic environment within the muscle tissue (Beckwée et al., 2019), triggering a cascade of physiological processes related to tissue adaptation. BFRT affects skeletal muscle primarily by promoting the secretion of anabolic hormones, protein synthesis, recruitment of type II muscle fibers, cellular swelling, and the generation of reactive oxygen species and their derivatives [e.g., nitric oxide (NO) and heat shock proteins (HSPs)] (Yuan et al., 2023).
BFRT significantly enhances muscle strength and physical function in elderly populations by inducing muscle hypertrophy (Baker et al., 2020). Multiple studies confirm that BFRT achieves comparable outcomes to conventional resistance training for sarcopenia management in older adults (Fabero-Garrido et al., 2022; Kong et al., 2023; Mallmann et al., 2024), with superior efficacy particularly observed in strength improvement (Kong et al., 2023). Consequently, for elderly patients with comorbidities such as degenerative joint disorders or cardiovascular diseases, BFRT emerges as a viable alternative intervention that simultaneously mitigates injury risks associated with high-load training while achieving equivalent strength gains (Patterson et al., 2019; Hughes et al., 2017; Ladlow et al., 2018; Zhang T. et al., 2022; Mallmann et al., 2024).
However, existing research still shows inconsistencies or requires further exploration in several key areas: the biological mechanisms, optimal training protocols (including resistance, cuff width, load, repetitions, sets, frequency, etc.), application effects (such as BFR applied alone or combined with aerobic exercise or resistance training), and safety concerns. Therefore, the aim of this review is to systematically integrate existing evidence, with a focus on elucidating the biological mechanisms of BFRT for sarcopenia intervention in older adults. Based on these mechanisms, we will propose tailored training protocols and application models suitable for elderly sarcopenia patients. Furthermore, we will comprehensively evaluate the safety profiles and potential risks of BFRT, thereby providing an evidence-based foundation for its long-term clinical application and broader implementation in geriatric sarcopenia management.
2 Biological mechanisms of BFRT intervention in sarcopenia
The pathogenesis of sarcopenia involves multiple factors, including nutritional deficiencies, chronic inflammation, hypogonadism, abnormal myokine secretion, hormonal alterations, insulin resistance, motor neuron loss, impaired neuromuscular junction function, inadequate muscle blood flow, mitochondrial dysfunction, and satellite cell senescence (Zhang X. et al., 2022). Numerous studies have demonstrated that BFRT can induce muscle hypertrophy, thereby enhancing muscle strength and improving physical function in older adults (Baker et al., 2020; Fabero-Garrido et al., 2022; Kong et al., 2023; Mallmann et al., 2024). However, the specific mechanisms underlying these effects remain incompletely understood. In general, the mechanisms by which BFRT addresses sarcopenia in the elderly primarily include promoting the secretion of anabolic hormones, regulating protein synthesis, accelerating the recruitment of type II muscle fibers, inducing cellular swelling, and stimulating the production of reactive oxygen species and their derivatives (e.g., NO and HSPs) (Yuan et al., 2023). A deeper understanding of the biological mechanisms of BFRT in treating sarcopenia is essential for developing physiologically informed, personalized training protocols for elderly patients. The following sections will elaborate on these mechanisms, as illustrated in Figure 1.
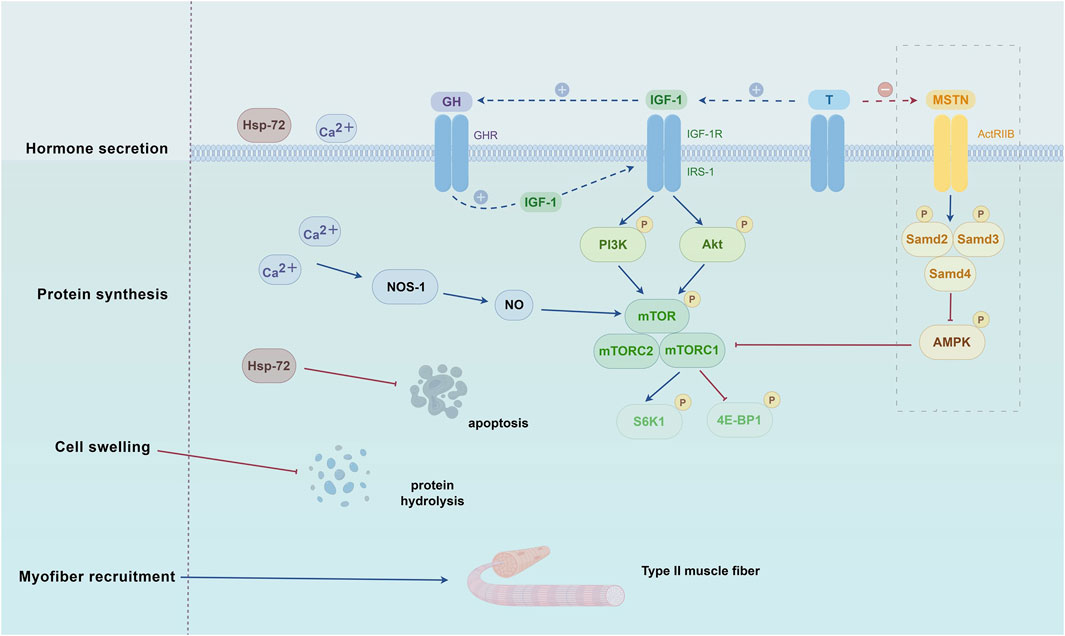
Figure 1. Biological Mechanisms of BFRT Intervention in Sarcopenia. The mechanisms by which BFRT addresses sarcopenia in the elderly primarily include promoting the secretion of anabolic hormones, regulating protein synthesis, accelerating the recruitment of type II muscle fibers, inducing cellular swelling, and stimulating the production of reactive oxygen species and their derivatives (e.g., NO and HSPs). The illustration was created with figdraw (figdraw.com). Abbreviations: 4E-BP1, eukaryotic initiation factor 4E-binding protein 1; ActRIIB, activin receptor type-2B; Akt, protein kinase B; HSP-72, heat shock protein-72; IGF-1, insulin-like growth factor-1; IGF-1R, insulin-like growth factor 1 receptor; IRS-1, insulin receptor substrate-1; MSTN, myostatin; mTOR, mammalian target of rapamycin; NOS-1, nitric oxide synthase-1; PI3K, phosphatidylinositol 3-kinase; S6K1, ribosomal protein S6 kinase 1; T, testosterone.
2.1 BFRT promotes the secretion of anabolic hormones
2.1.1 Growth hormone
Growth hormone (GH) and insulin-like growth factor-1 (IGF-1) play critical roles in maintaining skeletal muscle strength and mass. In the circulatory system, the secretion of GH stimulates the release of IGF-1, which in turn promotes GH secretion (Le Roith et al., 2001). Both hormones activate protein synthesis and inhibit protein degradation through the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling pathway, thereby influencing muscle mass (Bian et al., 2020).
GH is a peptide hormone with multiple functions, including maintaining cardiac function, glucose homeostasis, bone mineralization, the balance of lipogenesis and lipolysis, and skeletal muscle anabolism. With aging, GH secretion gradually declines (Hage and Salvatori, 2023), and its deficiency leads to reduced muscle strength and mass. Research suggests that BFRT can increase GH secretion and potentially improve body composition and muscle performance. For instance, the study by Shimizu et al. (2016) demonstrated that in 040 older adults who underwent 4 weeks of BFRT (20% 1RM), GH levels significantly increased (from 0.9 ± 0.7 ng/mL to 3.1 ± 1.3 ng/mL, p < 0.05). However, since the participants were healthy older adults, the findings have limited applicability to elderly patients with sarcopenia. Additionally, compared to younger males, older males exhibit a blunted GH response following BFRT (p = 0.02) (Manini et al., 2012), suggesting that age may be a critical factor influencing the incidence and extent of muscle hypertrophy induced by BFRT.
2.1.2 Insulin-like growth factor-1
In males aged 60 and above, the secretion of IGF-1 decreases with age, which is closely associated with the development of sarcopenia and muscle weakness (Chen et al., 2017). Research indicates that during aging (Musarò et al., 2001) and in animal models of neuromuscular diseases (Bosch-Marcé et al., 2011), the overexpression of IGF-1 in skeletal muscle can regulate protein synthesis and promote body growth. When IGF-1 binds to its receptor (insulin-like growth factor 1 receptor, IGF-1R), IGF-1R phosphorylates the intracellular adaptor protein insulin receptor substrate-1 (IRS-1), which then recruits and phosphorylates PI3K, subsequently activating Akt (Yoshida and Delafontaine, 2020). IGF-1 induces protein synthesis and muscle hypertrophy through the PI3K/Akt pathway, and the activation of Akt increases fiber size in the muscles of regenerating and adult rats while preventing denervation atrophy (Pallafacchina et al., 2002). In a case report, a 91-year-old male patient with sarcopenia showed a significant increase in plasma IGF-1 concentration after 3 months of low-intensity BFRT (30% 1RM) (Grutter Lopes et al., 2019). Additionally, Barjaste et al. (2021) found that a single session of BFR walking training led to an increase in IGF-1 levels. These findings suggest that BFRT enhances IGF-1 secretion, which may promote muscle hypertrophy and offer potential therapeutic benefits for sarcopenia.
However, a self-controlled trial by Ozaki et al. (2014) found that a single session of BFR walking exercise with a pressure of 240 mmHg and an intensity of 50% maximal oxygen uptake (VO2max) did not induce changes in IGF-1 levels. This contrasts with findings from (Barjaste et al., 2021) in a pre-post controlled study, which reported significant IGF-1 elevation after bilateral BFR training. The discrepancy may be attributed to differences in experimental design, Barjaste et al. applied bilateral BFR (i.e., restricting blood flow to both legs simultaneously), whereas Ozaki et al. used unilateral BFR (i.e., restricting blood flow to only one leg, with the other serving as a control). Unilateral BFR may not have generated sufficient metabolic stress or localized hypoxia to significantly stimulate IGF-1 secretion. Furthermore, a subsequent randomized controlled trial by Ozaki et al. (2017) demonstrated that changes in anabolic hormones (such as GH, insulin, and norepinephrine) were not significantly correlated with muscle hypertrophy induced by BFR walking. These preliminary results suggest that the elevation of anabolic hormones induced by BFR walking may have limited impact on muscle growth. In this regard, animal studies have indicated that when other factors, such as myostatin, heat shock protein-72 (HSP-72), and nitric oxide synthase-1 (NOS-1), undergo changes favorable to muscle growth, IGF-1 may not be essential for muscle hypertrophy (Kawada and Ishii, 2005).
2.1.3 Testosterone
Testosterone is one of the most important sex hormones in plasma, playing a critical role in protein, carbohydrate, and fat metabolism. It is essential for maintaining muscle mass and function, bone mass, and body composition (MacLean et al., 2008). After the age of 30, blood levels of testosterone decline by 1%–2% annually (Tajar et al., 2010), accompanied by a reduction in muscle mass, strength, and physical function (Perry et al., 2000). Therefore, testosterone is considered a key factor in preserving muscle mass and function during aging (Galvão et al., 2008).
The positive effects of testosterone on skeletal muscle involve several mechanisms. First, testosterone-induced increases in muscle volume are associated with concentration-dependent increases cross-sectional areas (CSA) of both type I and type II muscle fibers and myonuclear number (Sinha-Hikim et al., 2002). Second, testosterone promotes the activation and proliferation of satellite cells, enhancing muscle regeneration in both young and aged mice (Serra et al., 2013). Additionally, testosterone upregulates IGF-1 expression (Ferrando et al., 2002) and downregulates the gene expression of myostatin (Dubois et al., 2014). Studies have shown that low-intensity BFRT effectively increases levels of GH, IGF-1, and testosterone in young men, thereby enhancing muscle anabolic potential and promoting hormone secretion (Yinghao et al., 2021). However, since these studies focused solely on young individuals, their findings have limited applicability to older adults. In conclusion, while testosterone secretion can effectively improve muscle mass and strength in elderly patients, its specific effects on muscle function remain unclear. Therefore, testosterone cannot be considered a unique mechanism through which BFRT mitigates sarcopenia.
In summary, BFRT may improve sarcopenia by modulating hormone levels during exercise. However, it should be noted that current evidence for BFRT-induced hormonal responses (e.g., GH, IGF-1, testosterone) in elderly populations with sarcopenia remains limited, with most studies conducted in healthy older adults or younger individuals. Furthermore, Laurentino et al. (2022) found that although BFRT significantly increased muscle mass and strength, along with elevated levels of GH, IGF-1, and testosterone, statistical analysis revealed no significant correlation between these improvements and hormonal changes (p > 0.05). This implies that the primary mechanisms of BFRT may operate independently of systemic hormonal regulation, particularly in the understudied sarcopenic population. Further studies targeting hormonal pathways in sarcopenia cohorts are needed to clarify their precise role.
2.2 BFRT regulates protein synthesis
Muscle growth primarily occurs through enhancing anabolic signals or inhibiting catabolic pathways to promote protein synthesis. Among the key mechanisms regulating muscle mass and protein synthesis, the mammalian target of rapamycin (mTOR) signaling pathway plays a central role (Mukund and Subramaniam, 2020). As a highly conserved serine/threonine protein kinase, mTOR is crucial for skeletal muscle hypertrophy. mTOR exists in two distinct functional complexes: mTORC1 and mTORC2 (Marcotte et al., 2015).
Specifically, mTORC1 is pivotal in regulating protein synthesis and muscle mass. Research indicates that mTORC1 promotes protein synthesis and skeletal muscle hypertrophy by activating its downstream target ribosomal protein S6 kinase 1 (S6K1) and inhibiting eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) (Goodman, 2013). Furthermore, mTOR serves as a critical node in the protein synthesis signaling pathway, with its activity regulated by various upstream signaling molecules. For example, IGF-1 activates mTOR through the PI3K/Akt pathway, thereby inducing skeletal muscle hypertrophy (Barbé et al., 2015). Consequently, mTOR acts as a bridge in the regulation of skeletal muscle hypertrophy, capable of both receiving upstream signals and influencing downstream pathways to comprehensively participate in the regulation of muscle hypertrophy. Additionally, the upregulation of reactive oxygen species and their derivatives (e.g., NO and HSPs) and the downregulation of myostatin may also positively influence protein synthesis.
2.2.1 BFRT promotes protein synthesis
BFRT enhances mTOR signaling through activation of the PI3K/Akt pathway (Mukund and Subramaniam, 2020), thereby stimulating muscle protein synthesis and inducing skeletal muscle hypertrophy. In elderly populations prone to sarcopenia, low-intensity BFRT has been shown to effectively activate the mTORC1 signaling pathway and promote muscle protein synthesis. A study by Fry et al. (2010) demonstrated that in older adults, muscle protein synthesis increased significantly by 56% at 3 h post-BFRT exercise, accompanied by a notable rise in the phosphorylation levels of S6K1 and Akt. This indicates enhanced mTORC1 signaling following BFRT. This indicates enhanced mTORC1 signaling following BFRT. The strengthening of mTORC1 signaling suggests an enhancement in translation initiation, which may explain how BFRT promotes muscle protein synthesis. Moreover, S6K1 phosphorylation, a key regulator of exercise-induced muscle protein synthesis, has been shown to increase with BFRT (Loenneke et al., 2010). Fujita’s research revealed that in young men, S6K1 phosphorylation levels were significantly higher in the BFRT group (20% 1RM, 200 mmHg) compared to the non-BFR control group at 3 h post-exercise, with a 46% increase in muscle protein synthesis (Fujita et al., 2007). However, these findings are limited to young males, necessitating further research to elucidate the specific biological mechanisms by which BFRT regulates protein synthesis. The phosphorylation of the 4E-BP1 protein is also closely associated with the mTORC1 signaling pathway (Böhm et al., 2021). Research by Le Bacquer et al. (2019) demonstrated that knockout of the 4E-BP1 gene enhanced muscle protein synthesis in mice, accompanied by increased muscle strength (assessed via standardized grip test) and muscle mass (P < 0.05). These findings suggest that 4E-BP1 deficiency may improve muscle mass and function in aging mice, potentially by mitigating energy metabolism disorders. This implies that 4E-BP1 phosphorylation could be a therapeutic target for sarcopenia. BFRT, by inhibiting 4E-BP1 phosphorylation, may play a positive role in mitigating sarcopenia.
Research has also shown that during BFRT, elevated intracellular Ca2+ concentrations or blood flow reperfusion can activate neuronal nitric oxide synthase (NOS) and produce NO (Howard et al., 1995). NO production is associated with mTOR activation and linked to protein synthesis (Ito et al., 2013). Furthermore, NO mediates the activation of satellite cells (Anderson, 2000). Previous research has observed a significant increase in NOS expression following BFRT (Larkin et al., 2012). Therefore, NO may play a crucial role in the muscle adaptations induced by BFRT.
HSPs are induced under hypoxic, ischemic reperfusion, and acidotic conditions and function as chaperones to prevent misfolding or aggregation of proteins under metabolic stress caused by compression training (Loenneke et al., 2010). Research has shown that HSP-72 not only protects against muscle protein loss but also mitigates the decline in protein synthesis caused by disuse muscle atrophy and inhibits apoptosis, thereby reducing muscle mass loss (Naito et al., 2000). Kawada and Ishii were the first to report a significant increase in HSP-72 in the plantar muscles of rats after 2 weeks of BFRT (Kawada and Ishii, 2005). These findings are associated with significant muscle hypertrophy, suggesting that the upregulation of HSP-72 may be a potential mechanism by which BFRT promotes skeletal muscle hypertrophy and attenuates atrophy. However, Fry et al. found no significant increase in Heat shock protein-70 (HSP-70) levels after BFRT (20% 1RM, 200 mmHg) (Fry et al., 2010). These conflicting data may indicate that only specific HSPs, such as HSP-72, play a role in muscle hypertrophy, while others, like HSP-70, do not exhibit similar effects. To better understand the mechanisms of BFRT-induced muscle hypertrophy, further research is needed to investigate different heat shock protein subtypes and identify those with significant post-exercise anabolic roles.
In summary, the activation of the mTOR signaling pathway, increased levels of NOS, and elevated expression of HSP-72 following BFRT may serve as key mechanisms that contribute to the enhanced muscle protein synthesis and subsequent skeletal muscle hypertrophy induced by BFRT.
2.2.2 BFRT inhibits protein breakdown
In addition to promoting protein synthesis, BFRT also prevents skeletal muscle atrophy by inhibiting the expression of proteins associated with protein degradation. Myostatin, also known as growth differentiation factor 8, is primarily expressed in skeletal muscle (Baczek et al., 2020) and acts as a negative regulator of muscle mass and development (Guo et al., 2020). Myostatin binds to the activin receptor type-2B (ActRIIB), leading to the phosphorylation of Smad2 and Smad3, which then form a heterodimer with Smad4 and translocate to the nucleus to regulate gene transcription, thereby negatively regulating muscle growth. This process not only activates genes involved in muscle protein degradation but also suppresses protein synthesis by inhibiting the IGF-1/Akt/mTOR pathway (Bilski et al., 2022). Research has shown that myostatin levels increase with age and muscle mass loss (Yarasheski et al., 2002). For instance, patients with sarcopenia exhibit significantly elevated myostatin levels alongside reduced Akt phosphorylation efficiency. Specifically, myostatin mRNA and protein levels in sarcopenia patients increased by 2-fold and 1.4-fold, respectively, while Akt phosphorylation efficiency decreased by 30% (Léger et al., 2008). This suggests that elevated myostatin levels may be a key factor in the development of age-related sarcopenia. Therefore, inhibiting the expression of myostatin -related proteins is of significant importance for treating sarcopenia in the elderly.
Studies have also demonstrated that BFRT can reduce myostatin expression, thereby positively impacting the improvement of sarcopenia. For example, Laurentino et al. (2012) found that after 8 weeks of BFR knee extension training (20% 1RM), myostatin mRNA expression decreased by 45%. This indicates that low myostatin expression may play a role in the muscle hypertrophy induced by BFRT.
2.3 BFRT accelerates type II muscle fiber recruitment
Multiple studies have demonstrated significant type II muscle fiber atrophy in the skeletal muscles of older adults (Larsson, 1978; Verdijk et al., 2007; Martel et al., 2006; Snijders et al., 2009). Given that type II fast-twitch fibers predominantly contribute to high-intensity contractions, their atrophy may lead to decreased muscle strength in the elderly, ultimately promoting the development and progression of sarcopenia (Cruz-Jentoft et al., 2019). BFRT enhances type II fiber recruitment, representing a key mechanism for its muscle growth promotion. Research indicate that muscle hypertrophy induced by BFRT involves significant increases in CSA of both type I and II muscle fibers (Libardi et al., 2024; Wang et al., 2023). Loenneke et al. (2011a) confirmed that compared to conventional training, low-intensity BFRT significantly improves motor unit recruitment efficiency, particularly in activating type II fibers. In elderly populations, a 6-week BFRT intervention (30% 1RM) increased type I/II fiber CSA by approximately 20% (P < 0.05), while significantly enhancing maximal strength and endurance (Wang et al., 2023).
At the molecular level, BFRT-induced metabolic stress modulates calcium ion dynamics (release/reuptake), thereby strengthening actin-myosin interactions (Berchtold et al., 2000) and facilitating type II fiber activation. Given type II fibers’ fast-twitch properties and high-force output characteristics, their preferential recruitment enables greater force production under lower loads, which critically contributes to strength development and hypertrophy. These findings establish BFRT as an effective intervention for age-related declines in skeletal muscle mass and function (e.g., sarcopenia) (Conceição and Ugrinowitsch, 2019; Hughes et al., 2017). However, the cellular adaptation mechanisms to BFRT in aging individuals remain incompletely elucidated.
2.4 BFRT induces cell swelling
Current evidence suggests that BFRT-induced increases in muscle mass and strength may be associated with acute cell swelling mechanisms. Loenneke et al. proposed that BFR triggers cell swelling by increasing intracellular osmotic pressure, thereby activating the mTOR pathway to promote protein synthesis and muscle hypertrophy (Loenneke et al., 2012c). This hypothesis is supported by counterevidence: studies show that cellular dehydration downregulates mTOR signaling and impairs protein metabolism regulation (Schliess et al., 2006). However, it should be noted that this mechanism was initially based on hepatocyte research, and its translatability to human skeletal muscle remains unverified.
Recent experiments have challenged the swelling hypothesis: Nyakayiru et al. (2019) found that BFR alone (without muscle contraction) did not significantly enhance myofibrillar protein synthesis. This suggests that if cell swelling does exert anabolic effects, its mechanism may involve inhibiting protein breakdown (e.g., by downregulating MURF1 expression) (Kakehi et al., 2020). Alternatively, the study’s experimental conditions (resting state in healthy young males) may have limited observable effects, with BFR benefits potentially more pronounced under immobilization or other physiological stressors.
Furthermore, the relationship between BFR pressure gradients and the degree of cell swelling warrants investigation. Theoretically, moderate external pressure may induce cell swelling, activating mTOR and other anabolic pathways to promote protein accretion, whereas insufficient pressure may fail to provide adequate stimulus, and excessive pressure could cause ischemic injury or disrupt cell volume regulation, thereby diminishing efficacy (Loenneke et al., 2011b). However, direct evidence establishing a dose-response relationship between BFR pressure levels and cell swelling remains lacking, necessitating further mechanistic studies.
Despite extensive evidence of post-BFRT cell swelling, research on this mechanism remains limited, and no studies have confirmed whether swelling-induced suppression of catabolism contributes to muscle growth. Future studies should clarify the relationship between BFR intensity and cell swelling, and determine whether cellular edema plays a pivotal role in human muscle hypertrophy signaling. These findings would hold significant clinical implications for populations with exercise contraindications.
In summary, although multiple potential mechanisms of BFRT for intervening in age-related sarcopenia have been proposed, the mechanisms underlying BFRT-induced muscle adaptation remain incompletely understood. The primary mechanisms by which BFRT stimulates muscle growth include: 1) increasing the secretion of anabolic hormones (e.g., GH, IGF-1, and testosterone); 2) enhancing mTOR signaling through the PI3K/Akt pathway and suppressing the expression of proteins associated with protein degradation (e.g., myostatin), thereby promoting protein synthesis; 3) altering motor unit recruitment patterns to accelerate the recruitment of type II muscle fibers; 4) activating the mTOR pathway through induced cell swelling, promoting protein synthesis and muscle hypertrophy. Additionally, studies have shown that BFRT can influence the expression of HSP-72 and NOS-1. In conclusion, low-intensity BFRT appears to exert its effects through multiple mechanisms. However, research on these mechanisms remains incomplete, and further studies are needed to clarify the specific mechanisms by which BFRT intervenes in sarcopenia and to determine the impact of related factors on muscle activation.
3 Training protocols for BFRT intervention in sarcopenia
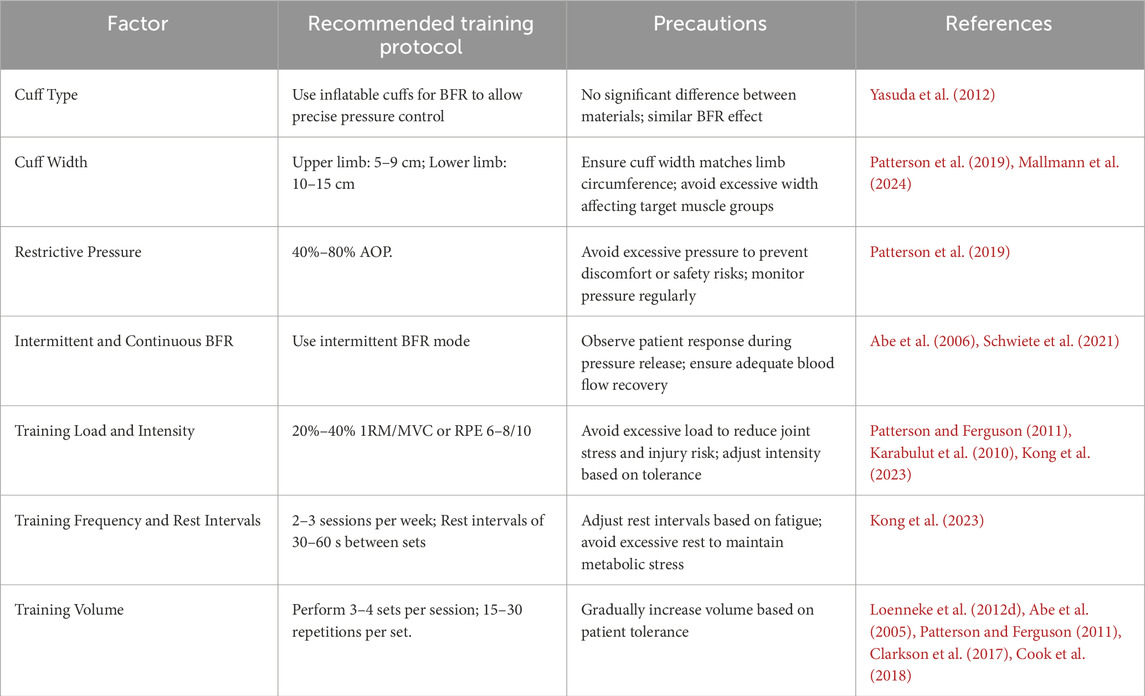
The efficacy of BFRT is influenced by various training parameters, making the development of scientifically sound training protocols crucial for the prevention and treatment of sarcopenia in older adults. However, several challenges and research limitations persist in practical applications. For instance, there is a lack of consistency in the applied restrictive pressure across studies, and research on BFRT in older adults with sarcopenia remains limited. Therefore, based on existing literature, this section discusses the impact of six key factors—cuff type and width, restrictive pressure, intermittent versus continuous application, training load and intensity, training frequency, and inter-set rest intervals and training volume—on the effectiveness of BFRT in sarcopenia intervention. Additionally, recommendations for BFRT training protocols are proposed (see Table 1).
3.1 Cuff type and width
The selection of cuff type is important for BFRT. The cuffs used in BFRT can be categorized into inflatable and non-inflatable types. Inflatable cuffs include specialized elastic inflatable cuffs (Yasuda et al., 2012) and nylon inflatable cuffs (non-elastic) (Freitas et al., 2017), while non-inflatable cuffs consist of elastic types [e.g., elastic straps with buckles (Yamanaka et al., 2012) and elastic knee braces (Luebbers et al., 2014)] and non-elastic types [e.g., tourniquets (Shinohara et al., 1997)]. Different cuff types vary in material, width, and pressure quantifiability. Studies indicate comparable efficacy between elastic and nylon cuffs under matched width and pressure conditions (Loenneke et al., 2014), offering flexibility in material selection. However, pressure must be individualized to avoid excessive occlusion risks (Dankel et al., 2017).
Current guidelines recommend that pressure protocols should be individualized based on both cuff characteristics and anthropometric measurements (Patterson et al., 2019). Cuff width significantly influences the required occlusion pressure, with wider cuffs (e.g., 12 cm) necessitating lower pressures compared to narrower counterparts (e.g., 5 cm) (Jessee et al., 2016; Loenneke et al., 2012c). Additionally, larger limb circumscriptions require proportionally higher occlusion pressures (Loenneke et al., 2015a; Loenneke et al., 2012 J.).
To optimize safety and efficacy, cuff selection should be tailored to individual characteristics and training objectives. For athletes, narrower cuffs are recommended as they can reduce muscle growth inhibition beneath the cuff (Ellefsen et al., 2015; Jakobsgaard et al., 2018; Kacin and Strazar, 2011), thereby enhancing training outcomes and athletic performance (Wortman et al., 2021). Additionally, narrower cuffs help minimize discomfort during upper-limb training (Spitz et al., 2019; Fitzgibbons et al., 2012).
For elderly patients with sarcopenia, inflatable elastic cuffs are preferred due to their precise pressure control (Yasuda et al., 2012), reducing cardiovascular risks. The recommended cuff width is 5–9 cm for the upper limbs and 10–15 cm for the lower limbs to ensure even pressure distribution and effective blood flow restriction (Patterson et al., 2019; Mallmann et al., 2024). Close monitoring of adverse reactions such as pain and fatigue during compression is also advised (Wernbom et al., 2006; Rossow et al., 2012).
3.2 Restrictive pressure
In the early stages of BFRT research, studies commonly applied uniform cuff pressures without accounting for individual variations (Loenneke et al., 2013). These absolute pressures ranged from as low as 50 mmHg (Kubota et al., 2011) to as high as 300 mmHg (Cook et al., 2007). Although most studies demonstrated that using uniform absolute pressures could still produce beneficial muscular adaptations, higher BFR pressures may potentiate cardiovascular responses and are frequently accompanied by significant discomfort (Jessee et al., 2017; Mattocks et al., 2017).
Current guidelines emphasize that pressure settings should be based on both cuff specifications and individual participant characteristics (Patterson et al., 2019). As mentioned earlier, cuff width (Jessee et al., 2016; Loenneke et al., 2012c) and limb dimensions (Loenneke et al., 2015a; Loenneke et al., 2012 J.) significantly influence pressure setting. Additionally, gender (Jessee et al., 2016), limb dominance (Tafuna’i et al., 2021), and body position (Karanasios et al., 2022) represent important influencing factors. However, these effects are generally minimal in most individuals and often indistinguishable from measurement error. Theoretically, these variables can be accounted for through individualized arterial occlusion pressure (AOP) measurement, which is considered the gold standard for pressure prescription. AOP refers to the minimum pressure threshold required to completely restrict blood flow in a limb and can be determined directly using methods such as Doppler ultrasound or palpation of distal pulses (Loenneke et al., 2012b). This is done by applying a pressure cuff at the proximal limb (e.g., upper arm or thigh root), monitoring pulse signals at the distal artery (e.g., radial artery or dorsalis pedis artery) using a Doppler flowmeter, gradually increasing cuff pressure until complete disappearance of pulse signals, at which point the recorded pressure reading represents the individualized AOP value (Loenneke et al., 2025).
In addition to direct AOP measurement, alternative methods exist to quantify the degree of blood flow restriction or estimate occlusion pressure. The placement of devices such as pulse oximeters distal to the cuff can objectively (though indirectly) assess vascular occlusion by quantifying reductions in peripheral oxygen saturation (SpO2) levels, reflecting impaired arterial inflow and/or venous outflow (Beaume et al., 2025). Furthermore, near-infrared spectroscopy (NIRS) can be employed to measure oxygenation status in muscle tissue of the occluded limb, providing an indirect evaluation of both the degree and effectiveness of blood flow restriction (Simpson et al., 2025). When occlusion is effectively achieved, characteristic patterns typically include decreased tissue oxygenation index and elevated deoxygenated hemoglobin levels (Simpson et al., 2025).
Several studies have proposed pressure prescription methods based on a percentage of systolic blood pressure (SBP) (Brandner et al., 2015; Kim et al., 2017; Cook et al., 2007). For instance, in the study by Kim et al. (Kim et al., 2017), BFR pressure was set at 130% of individual SBP. Previous research supports this approach, demonstrating that 130% SBP effectively induces muscular adaptations and fatigue while maintaining safety (Cook et al., 2007). However, although this SBP-based method accounts for individual blood pressure variations, it still results in significant inter-individual differences in the degree of vascular occlusion. This approach is notably less precise than individualized AOP measurement (Younger et al., 2004).
For elastic bands where objective pressure quantification is unattainable, a subjective pressure rating of 7 out of 10 (with 10 indicating maximal tolerable pressure without discomfort or pain) may serve as the BFRT pressure standard (Wilson et al., 2013). Doppler ultrasound validation has confirmed that this 7/10 standard achieves venous occlusion without complete arterial flow blockade, thereby ensuring safety (Wilson et al., 2013). However, this method presents practical limitations due to inter-individual variability in pressure perception and its dependence on operator expertise.
Therefore, it is currently recommended to set BFR training pressure based on measured AOP, with the range of 40%–80% AOP being empirically supported (Patterson et al., 2019).
3.3 Intermittent and continuous BFR
In BFRT-related studies, the use of cuffs during exercise can be categorized into intermittent application (releasing restrictive pressure during rest intervals) and continuous application (maintaining restrictive pressure during rest intervals). However, whether restrictive pressure should be released during rest intervals remains controversial. Some studies support maintaining pressure during rest intervals to sustain BFR effects, while others suggest that releasing pressure can reduce discomfort and potential risks. Currently, most studies recommend maintaining pressure during rest intervals in BFRT (Hackney et al., 2012). Continuous application of restrictive pressure helps sustain BFR, increase cellular swelling in muscle cells, and positively impact interventions for sarcopenia patients (Naylor et al., 2005). Research indicates that continuous-pressure BFRT significantly enhances muscle protein synthesis rates and GH levels (Fujita et al., 2007). Yasuda et al. (2015) found that continuous-pressure BFRT during low-load training significantly increased muscle CSA and strength. However, compared to continuous pressure, intermittent BFR application reduces discomfort and improves safety. A randomized controlled trial by Schwiete et al. (2021) revealed significant differences in subjective experience and compliance between intermittent and continuous BFR. Intermittent BFR reported lower discomfort (VAS score 3.2/10) compared to continuous BFR (5.8/10, p < 0.05) and higher training compliance (92% vs. 85%). This difference may stem from the reduction in nerve compression and metabolite accumulation due to intermittent pressure release. Moreover, releasing pressure during rest intervals facilitates local blood flow recovery, potentially enhancing muscle recovery and adaptive responses (Abe et al., 2006).
Older adults with sarcopenia often suffer from multiple chronic conditions and functional decline, resulting in lower tolerance to BFRT. Based on these findings, intermittent BFR offers significant advantages in safety, tolerance, and muscle recovery, making it more suitable for older adults with sarcopenia.
3.4 Training load and intensity
Training load and intensity are critical considerations in BFRT interventions for sarcopenia. Commonly used intensity indicators in BFRT-related studies include percentage of 1RM, maximal voluntary contraction (MVC), and rating of perceived exertion (RPE). Among these, 1RM serves as the most frequently utilized intensity indicator.
A recent meta-analysis showed that BFRT had the greatest effect on muscle hypertrophy and strength when the intensity was 15%–30% 1RM/MVC (Loenneke et al., 2012d). For instance, Laurentino et al. (2012) demonstrated that 8 weeks of BFR combined with low-load (20% 1RM) knee extension training resulted in a 40.1% increase in maximal knee extension strength and a 6.3% increase in quadriceps CSA. Compared to traditional training at the same intensity, BFRT achieves significant improvements in muscle mass and strength at lower intensities, with an optimal range of 20%–40% 1RM (Lixandrão et al., 2015). However, these studies primarily focused on younger populations, limiting their applicability to older adults.
For older adults, Patterson and Ferguson, (2011) used a 25% 1RM load for BFRT and observed significant strength improvements and increased post-occlusive calf blood flow. Karabulut et al. (2010) found that low-intensity BFRT (20% 1RM) enhanced leg muscle strength to a level comparable to high-intensity resistance exercise (80% 1RM). Additionally, Vechin et al. (2015) confirmed that low-intensity BFRT (20%–30% 1RM) significantly promotes muscle strength and mass in older adults. Recently, Kong et al. (2023) conducted a systematic review and meta-analysis on the effects of BFRT on muscle strength and mass in older adults, with included studies utilizing training loads ranging from 20% to 50% 1RM and reporting no adverse events. However, higher training intensities may not always be beneficial. Studies suggest that higher intensities (e.g., 50% 1RM vs. 30% 1RM) may not result in significant differences in acute responses (Jessee et al., 2018) or muscle hypertrophy (Madarame et al., 2008). Furthermore, training intensities exceeding 40%–50% 1RM/MVC may cause cuff pressure to surpass SBP, leading to arterial occlusion (Yamada et al., 2004). Training under such conditions not only increases pain but may also pose safety risks (Loenneke et al., 2011b).
In summary, based on current evidence, a training load of 20%–40% 1RM or an RPE of 6–8 (on a 10-point scale) is recommended for older adults with sarcopenia (Yasuda et al., 2015). Low-load BFRT significantly enhances muscle protein synthesis, increases muscle mass and strength, and reduces joint load and injury risk.
3.5 Training frequency and inter-set rest intervals
For older adults with sarcopenia, training frequency and inter-set rest intervals are critical factors influencing training efficacy and recovery. However, there is no consensus on the optimal BFRT training frequency and rest intervals for this population. Generally, a training frequency of 2-3 sessions per week is sufficient to achieve significant improvements in muscle strength and mass (Kong et al., 2023). For example, Loenneke et al. (2012d) found that older women performing BFRT twice weekly experienced significant increases in knee extension strength and muscle thickness. However, some studies suggest that higher training frequencies (e.g., 4 sessions per week) may yield greater benefits. Clarkson et al. (2017) demonstrated that older men performing BFRT four times weekly showed superior improvements in muscle strength and function compared to a control group training twice weekly.
Since metabolic byproducts accumulated during training are partially cleared during rest intervals, the duration of inter-set rest plays a crucial role in modulating metabolic stress (de Freitas et al., 2017). Loenneke et al. (2015b) suggested that shorter rest intervals during BFRT increase metabolic stress, promoting muscle growth. However, for older adults with sarcopenia, excessively short rest intervals may lead to fatigue accumulation and increased injury risk. Studies indicate that rest intervals of 30–60 s are safe and effective for this population. For instance, Cook et al. (2018) found that a BFRT protocol with 30-s rest intervals significantly improved muscle strength and function in older women.
In summary, for older adults with sarcopenia, a BFRT frequency of 2-3 sessions per week with 30–60 s of inter-set rest is recommended. Excessive training frequency may hinder recovery, while prolonged rest intervals may reduce training efficiency.
3.6 Training volume
Training volume, comprising the number of sets and repetitions, is primarily influenced by training load, intensity, restriction pressure, and inter-set rest periods. For elderly patients with sarcopenia, determining the optimal number of sets and repetitions is vital to ensure training efficacy while avoiding the risks of overtraining.
Research indicates that 3-4 sets are safe and effective for elderly sarcopenia patients (Patterson and Ferguson, 2011). For instance, Abe et al. (2005) found that elderly men following a 3-set BFRT regimen showed significant improvements in muscle strength and thickness. However, some studies suggest that a higher number of sets may yield greater benefits. Cook et al. (2018) observed that elderly women on a 5-set BFRT regimen experienced superior improvements in muscle strength and functionality compared to those on a 3-set regimen. Regarding repetitions, a study demonstrated that a 15-repetition BFRT regimen significantly enhanced knee extension strength and muscle thickness in elderly women (Loenneke et al., 2012d). Conversely, Clarkson et al. (2017) found that elderly men on a 30-repetition BFRT regimen showed better improvements in muscle strength and functionality than those on a 15-repetition regimen. However, it is important to note that more repetitions are not always better, as excessively prolonging training duration or increasing repetitions can lead to overtraining. Additionally, beginners may struggle to meet the required repetition counts initially (Wernbom et al., 2012). Therefore, for elderly sarcopenia patients with decreased muscle mass, strength, and functionality, adjustments such as reducing exercise load, decreasing restriction pressure, and extending inter-set rest periods can help achieve the desired repetition counts. In conclusion, individuals new to BFRT should gradually adapt to the training stimulus before incrementally increasing training volume to ensure feasibility and safety.
In summary, significant progress has been made in the development of BFRT protocols. BFRT offers the unique advantage of achieving greater muscular adaptive responses at lower load intensities, making it particularly suitable for the rehabilitation of elderly patients with sarcopenia. When implementing BFRT, it is recommended to use inflatable cuffs with quantifiable pressure, with cuff widths of 5–9 cm for the upper limbs and 10–15 cm for the lower limbs. The restriction pressure should be individualized, typically set at 40%–80% AOP. An intermittent pressure mode should be adopted to avoid continuous compression, with inter-set rest periods of 30–60 s. The training load intensity should be 20%–40% of 1RM or controlled at 6-8 on the RPE scale (out of 10). The recommended training frequency is 2-3 sessions per week, with 3-4 sets per session and 15–30 repetitions per set. Additionally, BFR can be used alone or in combination with aerobic or resistance exercise. However, it is important to note that combining BFR with resistance exercise (Vechin et al., 2015) or aerobic exercise (Sugimoto et al., 2021) at the same intensity may lead to a more pronounced increase in heart rate and blood pressure. The following sections will provide a detailed discussion on the application modes of BFRT in the intervention of sarcopenia.
4 Application models of BFR in sarcopenia intervention
With the deepening understanding of the biological mechanisms and effects of BFRT, its application has gradually expanded from the field of sports and fitness to clinical rehabilitation, and its target population has shifted from healthy individuals and athletes to patients with clinical conditions. The primary characteristics of sarcopenia in the elderly include a significant decline in skeletal muscle strength (Cruz-Jentoft et al., 2019), as well as reduced stability and coordination of the musculoskeletal system (Lo et al., 2020). Studies have shown that BFR is applied during both voluntary resistance exercise (RE-BFR) (Chen et al., 2021; Parkington et al., 2022; Zhang et al., 2024) and aerobic exercise (AE-BFR) (Ozaki et al., 2011a; 2011b; Clarkson et al., 2017; Ferreira et al., 2017), and also passively without exercise (P-BFR) (Slysz et al., 2021; Fuchs et al., 2024). The multimodal application characteristics of BFRT provide clinicians with flexible protocol options. Rehabilitation professionals can precisely select appropriate intervention modalities based on patients’ functional status, rehabilitation goals, and clinical scenarios (Hackney et al., 2018; Valenzuela et al., 2018). The following section will systematically summarize the effects, relevant therapeutic parameters, and safety of BFR combined with aerobic exercise, resistance exercise, and standalone application, aiming to provide guidance for clinical practice.
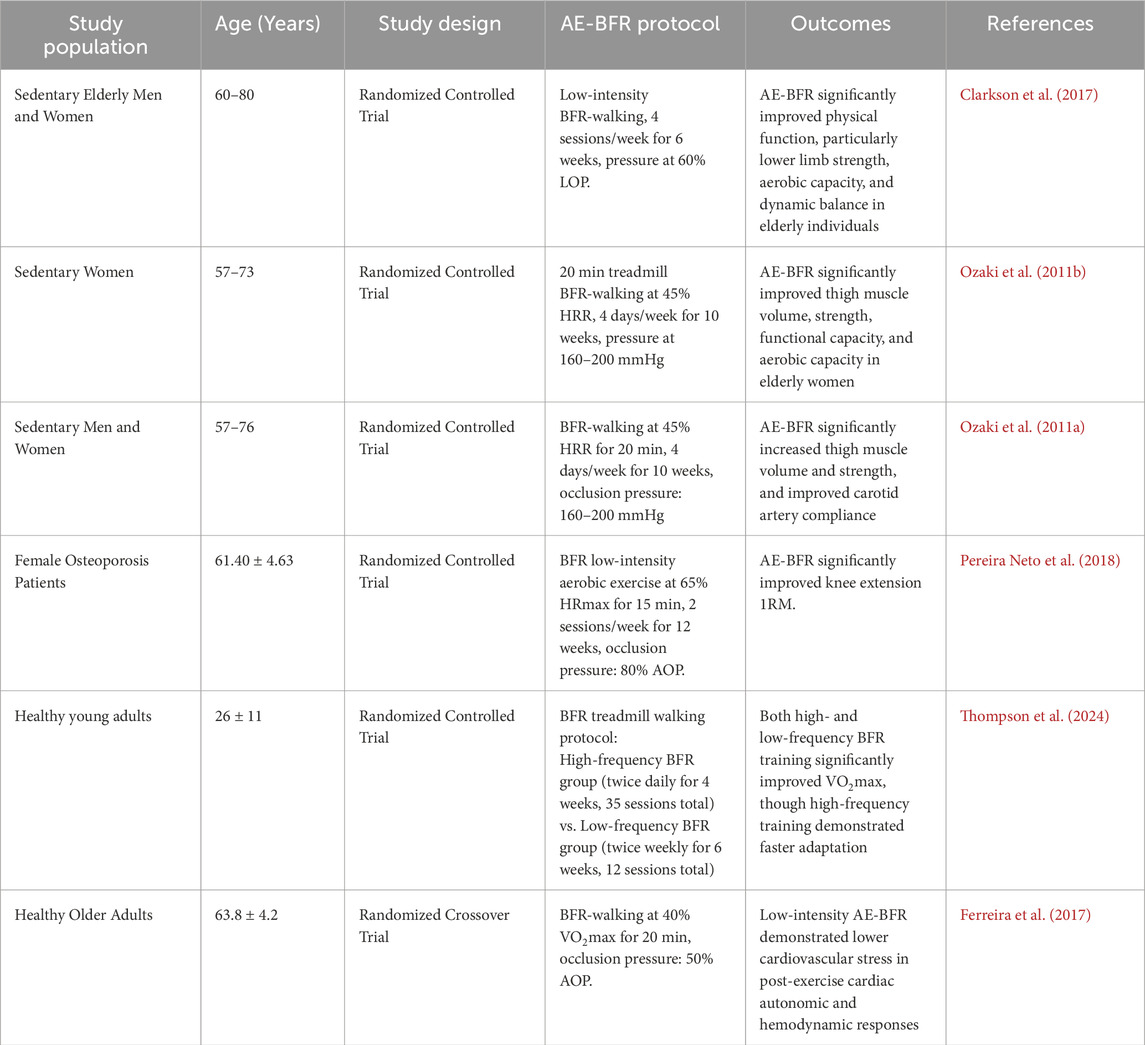
4.1 AE-BFR
AE-BFR refers to a training method that involves the use of external pressure devices (such as tourniquets, elastic wraps, or inflatable cuffs) applied to the proximal limb during aerobic activities (e.g., walking, running, or cycling). This technique partially restricts venous return and reduces arterial blood flow, thereby promoting muscle hypertrophy, enhancing muscle strength, and improving cardiopulmonary function (Silva et al., 2019). This training method is highly adaptable and requires minimal equipment, making it suitable for most elderly patients with sarcopenia.
AE-BFR has been demonstrated to significantly enhance muscle strength and mass in older adults (De Lemos Muller et al., 2024). Clarkson et al. (Clarkson et al., 2017) found that BFR combined with walking training significantly improved knee extension strength and muscle thickness in elderly women. Ozaki et al. (2011b) further confirmed that after 10 weeks of BFR walking training, older participants experienced a 3.1% increase in thigh muscle CSA, a 3.7% increase in muscle volume, a 5.9% improvement in maximal isometric strength, and up to a 22% increase in isokinetic strength. Additionally, this training improved peak oxygen uptake (VO2peak) and carotid artery compliance, potentially reducing the risk of cerebrovascular events. Similar findings were reported in another study by Ozaki et al. (2011a). For elderly patients with osteoporosis, AE-BFR also demonstrated significant benefits, increasing the 1RM of knee extension (Pereira Neto et al., 2018). Moreover, AE-BFR positively impacted the cardiovascular system, enhancing cardiorespiratory endurance (Bennett and Slattery, 2019). Research indicates that BFR walking training not only significantly enhances VO2peak (Ozaki et al., 2011b) and VO2max (Thompson et al., 2024) but also improves carotid artery compliance in older adults (Ozaki et al., 2011a). Compared to high-intensity aerobic exercise (70% VO2max), low-intensity BFR walking training (40% VO2max) exerted less cardiovascular stress on older adults (Ferreira et al., 2017).
Regarding training parameters, AE-BFR typically involves exercise intensities of 40%–80% VO2max, 50%–70% maximum heart rate (HRmax), or 30%–45% heart rate reserve (HRR), with durations of 5–20 min, performed 2–3 times per week for 3–12 weeks. The occlusion pressure is usually set at 40%–80% AOP. This parameter configuration ensures training efficacy while minimizing cardiovascular risks. For patients with sarcopenia, it is recommended to start with lower pressures (40%–50% AOP) and shorter durations (5–10 min), gradually increasing intensity. Although AE-BFR has been demonstrated to be safe and effective in improving cardiovascular function while mitigating age-related physiological decline (Yasuda et al., 2015), special precautions must be taken when applying this intervention to elderly populations with cardiovascular diseases. Strict contraindication screening (including, but not limited to, abnormal D-dimer levels and history of active thrombosis) must be conducted prior to implementation. Continuous blood pressure and heart rate monitoring should also be maintained throughout the training session to ensure safety (Yasuda et al., 2017).
In conclusion, existing research indicates that AE-BFR promotes muscle hypertrophy, increases muscle strength, prevents muscle atrophy, enhances walking ability, and improves arterial compliance in older adults, while reducing post-exercise cardiovascular stress. However, further studies are needed to optimize training parameters and evaluate long-term efficacy and safety. Table 2 summarizes the effects of AE-BFR based on relevant studies.
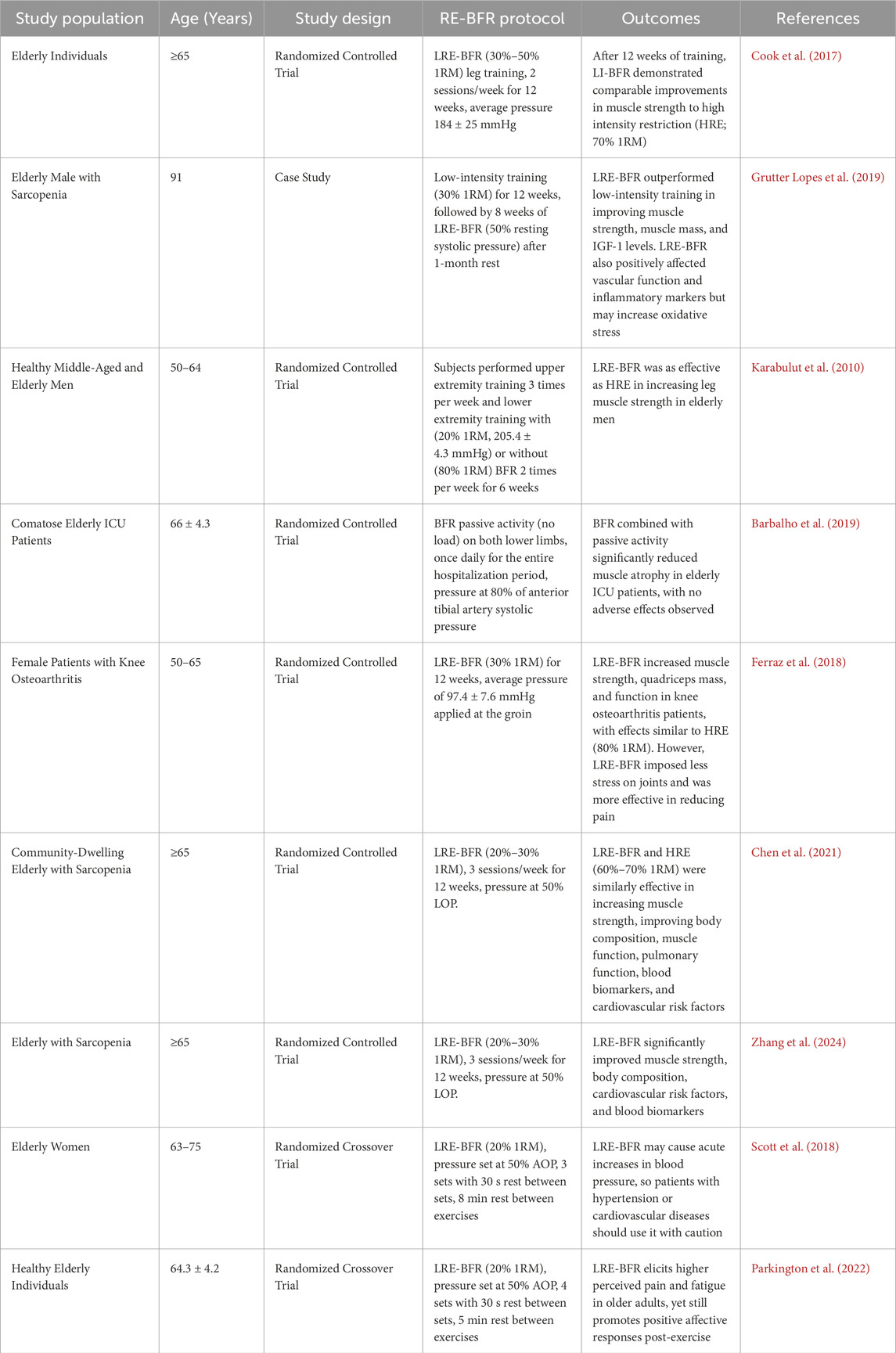
4.2 RE-BFR
While aerobic exercise can enhance muscle strength, it requires long-term adherence to achieve significant results. In contrast, RE-BFR can significantly increase muscle strength and volume in the trained limbs within a shorter period (Lo et al., 2020). Among these, BFR combined with low-intensity resistance exercise (LRE-BFR) is particularly suitable for elderly patients with sarcopenia and those with limited mobility due to underlying conditions, as it involves lower loads and does not result in adverse effects such as prolonged muscle function decline, muscle soreness, or muscle damage (Hughes et al., 2017).
Research has shown that LRE-BFR can significantly increase muscle CSA and strength in elderly patients with sarcopenia. For example, a case report by Grutter Lopes et al. (2019) demonstrated that after 3 months of LRE-BFR (30% 1RM), an elderly patient with sarcopenia experienced a 17.9% increase in grip strength, a 4.6% increase in maximal isokinetic knee extension strength, and a 2.1% increase in limb skeletal muscle mass. Additionally, LRE-BFR yields comparable or even superior results to traditional high-intensity resistance exercise. Cook et al. (2017) found that both LRE-BFR (30%–50% 1RM) and high-intensity resistance exercise (70% 1RM) improved muscle CSA in elderly individuals, although the effects of high-intensity resistance exercise were more pronounced initially. However, after 12 weeks of training, the differences between the two approaches diminished. Karabulut et al. (2010) also confirmed this, showing that LRE-BFR (20% 1RM) was nearly as effective as high-intensity resistance exercise (80% 1RM) in increasing muscle strength in elderly men. Overall, LRE-BFR (20%–30% 1RM) can produce similar muscular adaptations as high-intensity resistance exercise (70%–80% 1RM) while reducing joint stress (Fabero-Garrido et al., 2022). Furthermore, studies have found no adverse events such as muscle function decline, severe soreness, or increased muscle damage in sarcopenia patients following LRE-BFR (Barbalho et al., 2019). These characteristics make LRE-BFR particularly suitable for elderly individuals and patients with sarcopenia, as it maximizes training benefits while minimizing injury risks. RE-BFR not only prevents sarcopenia but also treats muscle loss caused by other conditions, such as knee osteoarthritis. Ferraz et al. (2018) demonstrated that LRE-BFR (30% 1RM) increased muscle CSA and improved physical function (e.g., timed up-and-go test and sit-to-stand test) in elderly patients with knee osteoarthritis, with effects similar to those of high-intensity resistance exercise (80% 1RM).
Regarding training parameters, it is recommended to use 40%–80% LOP combined with low-load resistance exercise (20%–30% 1RM), performing 3-4 sets of 15–20 repetitions per session, with 30–60 s of rest between sets, 2–3 times per week (Chen et al., 2021). For patients with sarcopenia, it is advisable to start with lower pressure (40%–50% LOP) and fewer sets (2-3 sets), gradually increasing intensity (Zhang et al., 2024). Safety assessments indicate that BFRT may be an alternative to reduce muscle wasting in the elderly population, but it should be used with caution in patients with hypertension or impaired cardiovascular function (Scott et al., 2018). In addition, the possibility of pain and discomfort should still be noted (Parkington et al., 2022). Therefore, it is recommended to conduct a comprehensive musculoskeletal assessment before training and adhere to the principle of progressive overload (e.g., gradually increasing training intensity, repetitions, or occlusion pressure) (Lixandrão et al., 2018) during the training process, while closely monitoring subjective discomfort to ensure both safety and effectiveness of the training.
In summary, RE-BFR provides a safe and effective training modality for elderly individuals and patients with sarcopenia, enhancing muscle strength and improving physical function while reducing joint stress, with effects comparable to high-intensity resistance exercise. However, further research is needed to optimize training parameters and evaluate long-term efficacy and safety. Table 3 summarizes the findings from relevant studies on RE-BFR.
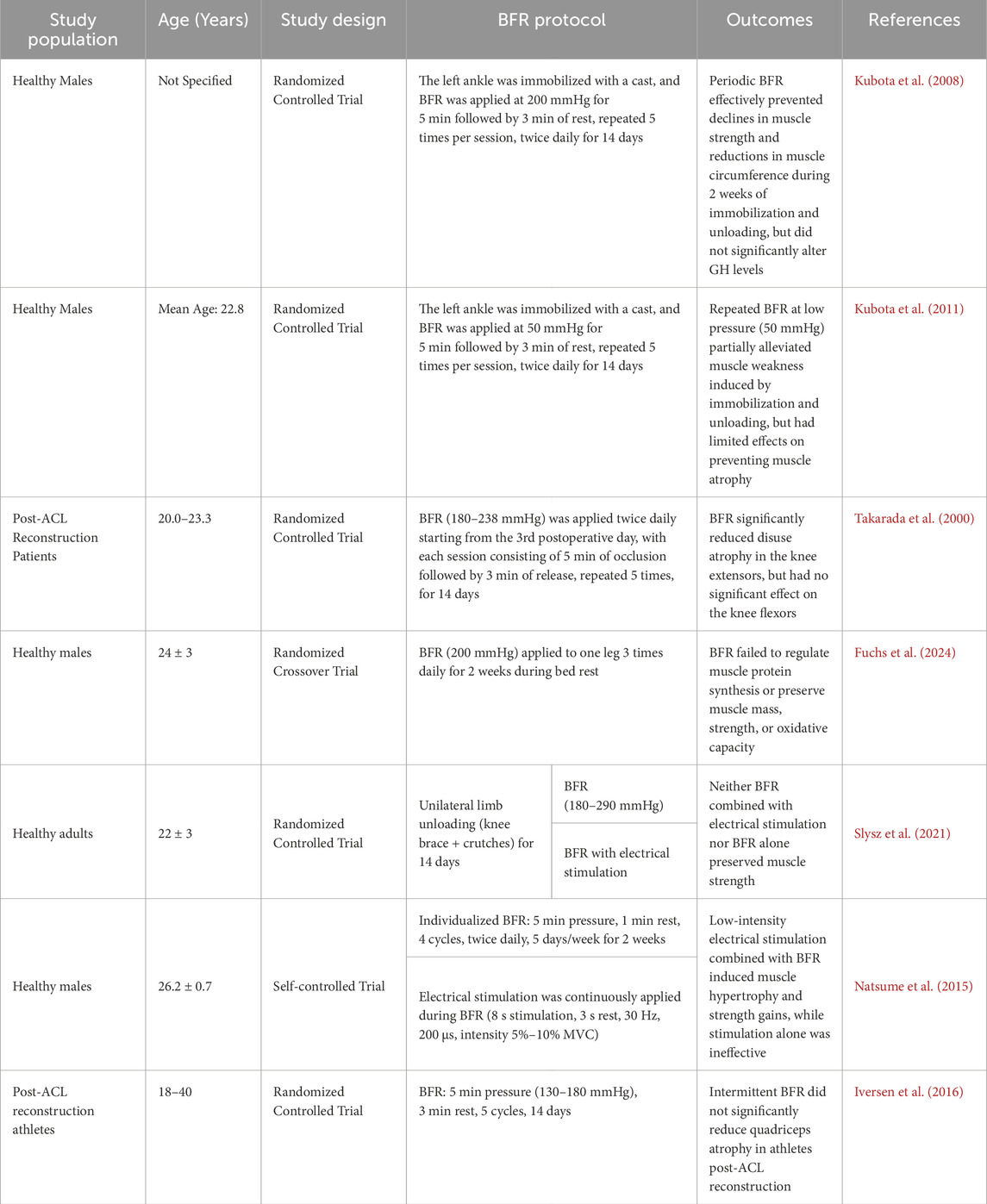
4.3 P-BFR
During periods of illness, injury, surgery, joint immobilization, or prolonged bed rest, reduced weight-bearing activities lead to significant declines in muscle strength and mass, resulting in functional impairments (Parry and Puthucheary, 2015). Studies have shown that after 30 days of bed rest, quadriceps strength decreases by 20%–60% (Stevens et al., 2003), and the muscle mass of the knee extensors decreases by an average of 11%–16% (Cook et al., 2010). Therefore, for elderly patients with sarcopenia or those unable to perform early load-bearing exercises post-surgery, early rehabilitation interventions using P-BFR can be used to mitigate the loss of strength and muscle size due to discontinuation (Hughes et al., 2017).
The mechanism of BFR without muscle contraction resembles ischemic preconditioning, involving cycles of ischemia-reperfusion, with some researchers considering them functionally equivalent (Patterson et al., 2019). In a follow-up study involving healthy subjects with cast immobilization, Kubota et al. (2008) found that applying BFR for 14 days effectively prevented disuse-induced weakness in knee extensors and flexors and reduced declines in thigh and calf circumference. Another study on cast immobilization demonstrated that repeated BFR at 50 mmHg cuff pressure reduced chronic unloading-induced muscle weakness (Kubota et al., 2011). Additionally, Takarada et al. (2000) found that applying blood flow restriction (BFR) (200–260 mmHg, twice daily, with 5 min of inflation followed by 3 min of rest, repeated 5 times) significantly decreased quadriceps atrophy after anterior cruciate ligament (ACL) surgery (9.4% vs. 20.7%). These findings suggest BFR may suppress muscle atrophy by downregulating MuRF1 expression (Kakehi et al., 2020). However, two recent studies failed to observe BFR’s benefits against bed rest (Fuchs et al., 2024) or knee brace immobilization (Slysz et al., 2021).
Collectively, BFR alone may be insufficient to fully prevent muscle loss. Notably, methodological heterogeneity (e.g., immobilization protocols, pressure parameters) complicates comparisons. Although there may be differences in the progression of atrophy due to different braking modalities, some form of muscle contraction appears to be essential for maintaining skeletal muscle tissue (Cook et al., 2010). This contraction may not be active, as studies have found that electrical stimulation combined with BFR has equally protective effects (Slysz et al., 2021; Natsume et al., 2015). However Iversen et al. (2016) found no atrophy mitigation even with combined BFR and contraction. Moreover, special attention must be paid to population-specific protocols for P-BFR therapy in clinical practice. For instance, in orthopedic postoperative patients, the compression area should strictly avoid cast or external fixator sites, with implementation delayed for at least 72 h post-surgery to prevent active bleeding (Takarada et al., 2000). Table 4 summarizes BFR effects in the absence of exercise.
In summary, comparisons between different exercise modalities indicate that RE-BFR is superior to AE-BFR in promoting muscle hypertrophy, while AE-BFR offers greater advantages in improving cardiorespiratory function (Centner et al., 2019). Additionally, P-BFR is more suitable for individuals who are bedridden or unable to perform load-bearing exercises. For elderly patients with sarcopenia, it is recommended to select the appropriate exercise modality based on individual conditions to achieve comprehensive improvements in muscle strength and cardiorespiratory fitness.
5 Safety and risks of BFRT intervention in sarcopenia
5.1 Safety
In the elderly population, BFRT has emerged as an effective intervention for treating age-related sarcopenia. This method not only promotes muscle strength but also reduces the risks associated with high-intensity training. Additionally, BFRT is cost-effective and easy to implement (Bahamondes-Ávila et al., 2020). However, in clinical applications, besides considering the benefits of BFRT, it is essential to prioritize its safety and potential adverse effects in elderly individuals with sarcopenia.
Clinical safety studies indicate that the incidence of adverse reactions to BFRT is low. A meta-analysis by Patterson et al. (2019) involving 47 studies reported that the incidence of BFRT-related adverse events was only 0.05%, primarily manifesting as transient limb numbness and other mild symptoms. A nationwide survey by Yasuda et al. (2017) further confirmed the safety of BFRT, revealing no serious risks such as paralysis due to nerve compression, pulmonary embolism, venous thrombosis, rhabdomyolysis, cerebral infarction, cerebral hemorrhage, or pulmonary infarction. Based on these findings, BFRT is considered a relatively safe intervention, although it should be conducted under professional supervision to minimize potential adverse effects (Bilski et al., 2022).
Moreover, BFRT does not negatively affect arterial stiffness. Yasuda et al. (2015) found that in healthy elderly individuals, hemodynamic parameters (e.g., heart rate and blood pressure), arterial stiffness index, vascular endothelial function, coagulation factors (fibrin degradation products [FDP] and D-dimer), and muscle damage markers (creatine kinase levels) showed no significant changes before and after BFRT, and no cardiovascular adverse events or muscle damage were observed. Ozaki et al. (2013) also reported that BFRT had no significant impact on arterial stiffness.
BFRT can also improve vascular endothelial function and peripheral circulation in healthy elderly individuals. Shimizu et al. (2016) demonstrated that after 4 weeks of LRE-BFR (20% 1RM), vascular endothelial growth factor and reactive hyperemia index significantly increased (both P < 0.01), while von Willebrand factor significantly decreased (P < 0.05), indicating improved vascular endothelial function.
Specialized studies on the elderly population further validate the safety of BFRT. Karabulut et al. (2013) found that 6 weeks of LRE-BFR (20% 1RM) did not increase resting inflammatory markers (IL-6) or muscle damage markers (CK). Additionally, BFRT has demonstrated dual benefits in chronic pain and rehabilitation populations, effectively alleviating pain while maintaining high safety (Song et al., 2021). For example, BFRT significantly improved pain, function, and joint mobility in patients with knee osteoarthritis, outperforming traditional resistance exercise with good safety (Bryk et al., 2016).
In summary, BFRT exhibits high safety and minimal adverse effects in cardiovascular, musculoskeletal, and special populations, making it a safe intervention for the elderly. However, individualized assessment and monitoring remain crucial, particularly for patients with severe cardiovascular diseases or bleeding tendencies. Table 5 summarizes relevant studies on the safety of BFRT.
5.2 Risks
BFRT offers certain advantages in the intervention of sarcopenia in the elderly, but its potential risks cannot be overlooked, and safety concerns require careful attention (Manini and Clark, 2009). The primary effects of BFRT on limbs are the reduction of arterial blood flow and the accumulation of venous blood, leading to a state of relative ischemia and hypoxia (Yasuda et al., 2010). During this process, metabolic byproducts such as lactic acid cannot be effectively cleared (Yasuda et al., 2015), resulting in a significant increase in metabolic stress. Current research on the safety of BFRT primarily focuses on thrombosis, blood pressure elevation, microvascular dysfunction, endothelial cell apoptosis and damage, and neuromuscular dysfunction.
Thrombosis is a key area of safety research in BFRT. Nakajima et al. (Nakajima et al., 2007) were the first to systematically evaluate the effects of BFRT on hemostatic function, finding no significant changes in thrombosis markers (e.g., D-dimer and FDP) after training, while tissue plasminogen activator (tPA) significantly increased (a decrease in tPA may indicate a risk of thrombosis). Although oxygen saturation decreased in a low-pressure chamber, the study found no serious adverse effects related to hypoxia, providing preliminary evidence for the safety of BFRT. Subsequently, Zaar et al. (2009) further elucidated the mechanism of external pressure on the coagulation system through simulated negative pressure environments. Although an increase in thrombin generation markers such as thrombin-antithrombin III complex (TAT) was observed, the levels were far below pathological thresholds, and D-dimer levels remained unchanged, indicating no stable thrombus formation. This early activation of the coagulation system may be related to physiological responses in mild to moderate blood loss states. Building on this, Madarame et al. (2010) specifically studied LRE-BFR and found that it did not significantly increase thrombin generation markers (e.g., prothrombin fragment 1 + 2 [PTF] and TAT)) or intravascular clot formation markers (e.g., D-dimer and FDP), indicating no significant thrombosis risk under these training conditions. Although BFRT led to a significant decrease in plasma volume, no activation of the coagulation system was observed, further supporting the safety of BFRT. These studies suggest that scientifically applied BFRT does not induce thrombosis, but further research is needed to assess thrombosis risks in postoperative patients undergoing BFRT rehabilitation. Studies by Bond et al. (2019) and Hughes et al. (2019) showed that even in orthopedic postoperative patients, BFRT did not increase the risk of venous thromboembolism, with safety profiles comparable to those of healthy populations. Additionally, long-term safety studies (Cook et al., 2017; Neto et al., 2017) further support this conclusion, indicating that BFRT interventions lasting more than 12 weeks do not cumulatively increase thrombosis risk. Although these studies differ in methods and populations, they consistently conclude that BFRT does not significantly increase thrombosis risk. However, caution is still advised when using BFRT in elderly individuals with cardiovascular diseases or other health issues (Centner et al., 2019).
Recent studies have demonstrated that BFRT induces acute blood pressure elevation, primarily attributed to the accumulation of metabolic byproducts (Yamada et al., 2025). Compared to equivalent training without BFR, BFRT elicits more pronounced blood pressure fluctuations (Brandner et al., 2015; Downs et al., 2014). However, these hemodynamic changes remain within normal exercise-induced physiological ranges, with blood pressure returning to baseline levels within 5–10 min post-exercise (Rossow et al., 2012). The current evidence regarding the effects of repetitive BFRT on resting blood pressure remains insufficient (Wong et al., 2022). Notably, a recent large-scale study found no observable changes in resting blood pressure following repeated BFRT interventions (Spitz et al., 2024).
After BFRT, the reperfusion process may lead to cellular damage, manifesting as microvascular dysfunction, endothelial cell apoptosis and damage, and neuromuscular dysfunction. During reperfusion following BFRT, skeletal muscles acutely release inflammatory molecules and reactive oxygen species, impairing microvascular function (Hughes et al., 2017). Additionally, repeated reperfusion injury can affect endothelial function, increasing membrane permeability and generating reactive oxygen species, leading to endothelial cell damage (Madarame et al., 2013). In a study by Jenkins et al. (2013), BFRT (220 mmHg) was applied to 10 young males, revealing a significant increase in CD62E+ endothelial microparticles at 10 and 20 min (P < 0.05), indicating vascular endothelial activation. Simultaneously, CD31+/CD42b− endothelial microparticles significantly increased (P < 0.05), suggesting endothelial cell apoptosis. This study demonstrates that excessive BFRT pressure can cause vascular endothelial activation, apoptosis, and damage in local tissues. Therefore, Da Cunha Nascimento et al. (2020) recommend setting the vascular occlusion pressure to 40%–60% AOP in practical use, achieving transient ischemia and hypoxia in local muscle groups while avoiding endothelial damage due to excessive pressure. Furthermore, ischemia-reperfusion may cause neuromuscular dysfunction, but related studies have only been conducted in mouse models, requiring further validation for human applicability (Wilson et al., 2019).
In conclusion, BFRT may pose potential risks to the human body, necessitating greater attention to safety during its application. However, given the limited number of high-quality clinical studies, small sample sizes, and lack of homogeneity in study populations—particularly the scarcity of reports on safety and adverse effects in elderly sarcopenia patients—further validation of these findings is required. Table 6 summarizes relevant studies on the potential risks of BFRT.
6 Conclusions and future perspectives
BFRT presents a promising and innovative approach for counteracting sarcopenia in older adults. Current evidence supports its efficacy in enhancing muscle strength and function, underpinned by multifaceted mechanisms including anabolic hormone release, mTOR pathway activation, cellular swelling, and preferential type II fiber recruitment. For clinical implementation, individualized pressure prescription using appropriate cuff widths and low-load exercise intensities is paramount. BFR demonstrates versatility, being effective when combined with resistance or aerobic exercise, and shows potential benefit even in passive applications for immobilized individuals. While generally safe under proper guidance, vigilance for potential vascular, neurological, or muscular adverse effects is warranted, especially given the paucity of safety data specific to sarcopenic populations.
Despite providing practical recommendations based on current literature, key knowledge gaps persist. Future research must prioritize elucidating the precise biological mechanisms in the aging muscle, establishing optimal, individualized protocols, and expanding evidence within diverse sarcopenic cohorts, including those with varied etiologies beyond primary age-related loss. Crucially, the long-term functional outcomes and potential physiological trade-offs associated with BFRT in this vulnerable population require rigorous investigation to fully realize its therapeutic potential for improving mobility and quality of life (Loenneke et al., 2025).
Author contributions
WL: Writing – original draft, Writing – review and editing. MH: Writing – review and editing, Data curation. QY: Conceptualization, Writing – review and editing. YL: Investigation, Writing – review and editing. LC: Supervision, Writing – review and editing. QR: Conceptualization, Writing – review and editing, Validation. GX: Conceptualization, Writing – review and editing, Methodology, Funding acquisition. YW: Writing – review and editing, Investigation, Supervision, Funding acquisition, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by National Natural Science Foundation of China (82071970, 82272123), Science Fund for Distinguished Young Scholars of Hubei Province (2023AFA109) and Science and Technology Project of Jianghan University (2022XKZX008).
Acknowledgments
The illustration was created with figdraw (figdraw.com).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
4E-BP1, eukaryotic initiation factor 4E-binding protein 1; ACL, Anterior Cruciate Ligament; ActRIIB, activin receptor type-2B; AE-BFR, blood flow restriction combined with aerobic exercise; Akt, protein kinase B; AOP, arterial occlusion pressure; BFR, blood flow restriction; BFRT, blood flow restriction training; CSA, cross-sectional areas; FDP, fibrin degradation products; HRE, high-intensity restriction; HSP-70, heat shock protein-70; HSP-72, heat shock protein-72; HSPs, heat shock proteins; HRmax, maximum heart rate; HRR, heart rate reserve; IGF-1, insulin-like growth factor-1; IGF-1R, insulin-like growth factor 1 receptor; IRS-1, insulin receptor substrate-1; LOP, limb occlusion pressure; LRE-BFR, blood flow restriction combined with low-intensity resistance exercise; mTOR, mammalian target of rapamycin; MVC, maximal voluntary contraction; NO, nitric oxide; NOS, nitric oxide synthase; NOS-1, nitric oxide synthase-1; P-BFR, blood flow restriction (without exercise); PI3K, phosphatidylinositol 3-kinase; RE-BFR, blood flow restriction combined with resistance exercise; RPE, rating of perceived exertion; S6K1, ribosomal protein S6 kinase 1; SBP, resting systolic blood pressure; TAT, thrombin-antithrombin III complex; VO2max, maximal oxygen uptake; VO2peak, peak oxygen uptake.
References
Abe T., Yasuda T., Midorikawa T., Sato Y., Kearns C. F., Inoue K., et al. (2005). Skeletal muscle size and circulating IGF-1 are increased after two weeks of twice daily “KAATSU” resistance training. Int. J. KAATSU Train. Res. 1 (1), 6–12. doi:10.3806/ijktr.1.6
Abe T., Kearns C. F., Sato Y. (2006). Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, Kaatsu-walk training. J. Appl. Physiology 100 (5), 1460–1466. doi:10.1152/japplphysiol.01267.2005
Anderson J. E. (2000). A role for nitric oxide in muscle repair: nitric oxide-mediated activation of muscle satellite cells. Mol. Biol. Cell 11 (5), 1859–1874. doi:10.1091/mbc.11.5.1859
Baczek J., Silkiewicz M., Wojszel Z. B. (2020). Myostatin as a biomarker of muscle wasting and other pathologies-state of the art and knowledge gaps. Nutrients 12 (8), 2401. doi:10.3390/nu12082401
Bahamondes-Ávila C., Ponce-Fuentes F., Chahin-Inostroza N., Bracho-Milic F., Navarrete-Hidalgo C. (2020). Strenght training with partial restriction of blood flow in older adults with sarcopenia. Rev. Cubana Salud Pública 46 (3), e1105. Available online at: https://www.scielosp.org/article/rcsp/2020.v46n3/e1105/es/.
Baker B. S., Stannard M. S., Duren D. L., Cook J. L., Stannard J. P. (2020). Does blood flow restriction therapy in patients older than age 50 result in muscle hypertrophy, increased strength, or greater physical function? A systematic review. Clin. Orthop. and Relat. Res. 478 (3), 593–606. doi:10.1097/CORR.0000000000001090
Barbalho M., Rocha A. C., Seus T. L., Raiol R., Del Vecchio F. B., Coswig V. S. (2019). Addition of blood flow restriction to passive mobilization reduces the rate of muscle wasting in elderly patients in the intensive care unit: a within-patient randomized trial. Clin. Rehabil. 33 (2), 233–240. doi:10.1177/0269215518801440
Barbé C., Kalista S., Loumaye A., Ritvos O., Lause P., Ferracin B., et al. (2015). Role of IGF-I in follistatin-induced skeletal muscle hypertrophy. Am. J. Physiology-Endocrinology Metabolism 309 (6), E557–E567. doi:10.1152/ajpendo.00098.2015
Barjaste A., Mirzaei B., Rahmani-nia F., Haghniyaz R., Brocherie F. (2021). Concomitant aerobic- and hypertrophy-related skeletal muscle cell signaling following blood flow-restricted walking. Sci. and Sports 36 (2), e51–e58. doi:10.1016/j.scispo.2020.03.006
Bauer J., Morley J. E., Schols A. M. W. J., Ferrucci L., Cruz-Jentoft A. J., Dent E., et al. (2019). Sarcopenia: a time for action. An SCWD position paper. J. Cachexia, Sarcopenia Muscle 10 (5), 956–961. doi:10.1002/jcsm.12483
Beaume J., Di Domenico H., Bowen M., Hintzy F., Millet G. Y., Pageaux B., et al. (2025). Neuromuscular fatigue induced by cycling at a fixed level of perceived effort: effects of different purported hypoxic methods. Scand. J. Med. and Sci. Sports 35 (2), e70021. doi:10.1111/sms.70021
Beckwée D., Delaere A., Aelbrecht S., Baert V., Beaudart C., Bruyere O., et al. (2019). Exercise interventions for the prevention and treatment of sarcopenia. A systematic umbrella review. J. Nutr. health aging 23 (6), 494–502. doi:10.1007/s12603-019-1196-8
Bennett H., Slattery F. (2019). Effects of blood flow restriction training on aerobic capacity and performance: a systematic review. J. Strength Cond. Res. 33 (2), 572–583. doi:10.1519/JSC.0000000000002963
Berchtold M. W., Brinkmeier H., Müntener M. (2000). Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol. Rev. 80 (3), 1215–1265. doi:10.1152/physrev.2000.80.3.1215
Bian A., Ma Y., Zhou X., Guo Y., Wang W., Zhang Y., et al. (2020). Association between sarcopenia and levels of growth hormone and insulin-like growth factor-1 in the elderly. BMC Musculoskelet. Disord. 21 (1), 214. doi:10.1186/s12891-020-03236-y
Bilski J., Pierzchalski P., Szczepanik M., Bonior J., Zoladz J. (2022). Multifactorial mechanism of sarcopenia and sarcopenic obesity. Role of physical exercise, microbiota and myokines. Cells 11 (1), 160. doi:10.3390/cells11010160
Böhm R., Imseng S., Jakob R. P., Hall M. N., Maier T., Hiller S. (2021). The dynamic mechanism of 4E-BP1 recognition and phosphorylation by mTORC1. Mol. Cell 81 (11), 2403–2416.e5. doi:10.1016/j.molcel.2021.03.031
Bond C. W., Hackney K. J., Brown S. L., Noonan B. C. (2019). Blood flow restriction resistance exercise as a rehabilitation modality following orthopaedic surgery: a review of venous thromboembolism risk. J. Orthop. and Sports Phys. Ther. 49 (1), 17–27. doi:10.2519/jospt.2019.8375
Bosch-Marcé M., Wee C. D., Martinez T. L., Lipkes C. E., Choe D. W., Kong L., et al. (2011). Increased IGF-1 in muscle modulates the phenotype of severe SMA mice. Hum. Mol. Genet. 20 (9), 1844–1853. doi:10.1093/hmg/ddr067
Brandner C. R., Kidgell D. J., Warmington S. A. (2015). Unilateral bicep curl hemodynamics: Low-pressure continuous vs high-pressure intermittent blood flow restriction. Scand. J. Med. and Sci. Sports 25 (6), 770–777. doi:10.1111/sms.12297
Bryk F. F., Dos Reis A. C., Fingerhut D., Araujo T., Schutzer M., Cury R. d. P. L., et al. (2016). Exercises with partial vascular occlusion in patients with knee osteoarthritis: a randomized clinical trial. Knee Surg. Sports Traumatol. Arthrosc. 24 (5), 1580–1586. doi:10.1007/s00167-016-4064-7
Cawthon P. M., Visser M., Arai H., Ávila-Funes J. A., Barazzoni R., Bhasin S., et al. (2022). Defining terms commonly used in sarcopenia research: a glossary proposed by the Global Leadership in Sarcopenia (GLIS) Steering Committee. Eur. Geriatr. Med. 13 (6), 1239–1244. doi:10.1007/s41999-022-00706-5
Centner C., Wiegel P., Gollhofer A., König D. (2019). Effects of blood flow restriction training on muscular strength and hypertrophy in older individuals: a systematic review and meta-analysis. Sports Med. 49 (1), 95–108. doi:10.1007/s40279-018-0994-1
Chen H., Chung Y., Chen Y., Ho S. Y., Wu H. J. (2017). Effects of different types of exercise on body composition, muscle strength, and IGF-1 in the elderly with sarcopenic obesity. J. Am. Geriatrics Soc. 65 (4), 827–832. doi:10.1111/jgs.14722
Chen N., He X., Zhao G., Lu L., Ainsworth B. E., Liu Y., et al. (2021). Efficacy of low-load resistance training combined with blood flow restriction vs. high-load resistance training on sarcopenia among community-dwelling older Chinese people: study protocol for a 3-arm randomized controlled trial. Trials 22 (1), 518. doi:10.1186/s13063-021-05495-z
Clarkson M. J., Conway L., Warmington S. A. (2017). Blood flow restriction walking and physical function in older adults: a randomized control trial. J. Sci. Med. Sport 20 (12), 1041–1046. doi:10.1016/j.jsams.2017.04.012
Conceição M. S., Ugrinowitsch C. (2019). Exercise with blood flow restriction: an effective alternative for the non-pharmaceutical treatment for muscle wasting. J. Cachexia, Sarcopenia Muscle 10 (2), 257–262. doi:10.1002/jcsm.12397
Cook S. B., Clark B. C., Ploutz-Snyder L. L. (2007). Effects of exercise load and blood-flow restriction on skeletal muscle function. Med. and Sci. Sports and Exerc. 39 (10), 1708–1713. doi:10.1249/mss.0b013e31812383d6
Cook S. B., Brown K. A., DeRuisseau K., Kanaley J. A., Ploutz-Snyder L. L. (2010). Skeletal muscle adaptations following blood flow-restricted training during 30 days of muscular unloading. J. Appl. Physiology 109 (2), 341–349. doi:10.1152/japplphysiol.01288.2009
Cook S. B., LaRoche D. P., Villa M. R., Barile H., Manini T. M. (2017). Blood flow restricted resistance training in older adults at risk of mobility limitations. Exp. Gerontol. 99, 138–145. doi:10.1016/j.exger.2017.10.004
Cook S. B., Scott B. R., Hayes K. L., Murphy B. G. (2018). Neuromuscular adaptations to low-load blood flow restricted resistance training. J. Sports Sci. and Med. 17 (1), 66–73. Available online at: https://pmc.ncbi.nlm.nih.gov/articles/PMC5844210/.
Cruz-Jentoft A. J., Sayer A. A. (2019). Sarcopenia. Lancet 393 (10191), 2636–2646. doi:10.1016/S0140-6736(19)31138-9
Cruz-Jentoft A. J., Bahat G., Bauer J., Boirie Y., Bruyére O., Cederholm T., et al. (2019). Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48 (1), 16–31. doi:10.1093/ageing/afy169
Da Cunha Nascimento D., Schoenfeld B. J., Prestes J. (2020). Potential implications of blood flow restriction exercise on vascular health: a brief review. Sports Med. 50 (1), 73–81. doi:10.1007/s40279-019-01196-5
Dankel S., Buckner S., Counts B., Jessee M. B., Mouser J. G., Mattocks K. T., et al. (2017). The acute muscular response to two distinct blood flow restriction protocols. Physiol. Int. 104 (1), 64–76. doi:10.1556/2060.104.2017.1.1
de Freitas M. C., Gerosa-Neto J., Zanchi N. E., Lira F. S., Rossi F. E. (2017). Role of metabolic stress for enhancing muscle adaptations: practical applications. World J. Methodol. 7 (2), 46–54. doi:10.5662/wjm.v7.i2.46
De Lemos Muller C. H., Farinha J. B., Leal-Menezes R., Ramis T. R. (2024). Aerobic training with blood flow restriction on muscle hypertrophy and strength: systematic review and meta-analysis. J. Strength and Cond. Res. 38 (7), 1341–1349. doi:10.1519/JSC.0000000000004800
Dent E., Morley J. E., Cruz-Jentoft A. J., Arai H., Kritchevsky S. B., Guralnik J., et al. (2018). International Clinical Practice Guidelines for Sarcopenia (ICFSR): screening, diagnosis and management. J. Nutr. Health Aging 22 (10), 1148–1161. doi:10.1007/s12603-018-1139-9
Downs M. E., Hackney K. J., Martin D., Caine T. L., Cunningham D., O'Connor D. P., et al. (2014). Acute vascular and cardiovascular responses to blood flow-restricted exercise. Med. and Sci. Sports and Exerc. 46 (8), 1489–1497. doi:10.1249/MSS.0000000000000253
Dubois V., Laurent M. R., Sinnesael M., Cielen N., Helsen C., Clinckemalie L., et al. (2014). A satellite cell-specific knockout of the androgen receptor reveals myostatin as a direct androgen target in skeletal muscle. FASEB J. 28 (7), 2979–2994. doi:10.1096/fj.14-249748
Ellefsen S., Hammarström D., Strand T. A., Zacharoff E., Whist J. E., Rauk I., et al. (2015). Blood flow-restricted strength training displays high functional and biological efficacy in women: a within-subject comparison with high-load strength training. Am. J. Physiology-Regulatory, Integr. Comp. Physiology 309 (7), R767–R779. doi:10.1152/ajpregu.00497.2014
Evans W. J., Guralnik J., Cawthon P., Appleby J., Landi F., Clarke L., et al. (2024). Sarcopenia: no consensus, no diagnostic criteria, and no approved indication-how did we get here? GeroScience 46 (1), 183–190. doi:10.1007/s11357-023-01016-9
Fabero-Garrido R., Gragera-Vela M., Del Corral T., Izquierdo-García J., Plaza-Manzano G., López-de-Uralde-Villanueva I. (2022). Effects of low-load blood flow restriction resistance training on muscle strength and hypertrophy compared with traditional resistance training in healthy adults older than 60 years: systematic review and meta-analysis. J. Clin. Med. 11 (24), 7389. doi:10.3390/jcm11247389
Ferrando A. A., Sheffield-Moore M., Yeckel C. W., Gilkison C., Jiang J., Achacosa A., et al. (2002). Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am. J. Physiology-Endocrinology Metabolism 282 (3), E601–E607. doi:10.1152/ajpendo.00362.2001
Ferraz R. B., Gualano B., Rodrigues R., Kurimori C. O., Fuller R., Lima F. R., et al. (2018). Benefits of resistance training with blood flow restriction in knee osteoarthritis. Med. and Sci. Sports and Exerc. 50 (5), 897–905. doi:10.1249/MSS.0000000000001530
Ferreira M. L. V., Sardeli A. V., Souza G. V. D., Bonganha V., Santos L. D. C., Castro A., et al. (2017). Cardiac autonomic and haemodynamic recovery after a single session of aerobic exercise with and without blood flow restriction in older adults. J. Sports Sci. 35 (24), 2412–2420. doi:10.1080/02640414.2016.1271139
Fitzgibbons P. G., DiGiovanni C., Hares S., Akelman E. (2012). Safe tourniquet use: a review of the evidence. J. Am. Acad. Orthop. Surg. 20 (5), 310–319. doi:10.5435/JAAOS-20-05-310
Fragala M. S., Cadore E. L., Dorgo S., Izquierdo M., Kraemer W. J., Peterson M. D., et al. (2019). Resistance training for older adults: position statement from the national strength and conditioning Association. J. Strength Cond. Res. 33 (8), 2019–2052. doi:10.1519/JSC.0000000000003230
Freitas E., Poole C., Miller R., Heishman A. D., Kaur J., Bemben D. A., et al. (2017). Time course change in muscle swelling: High-Intensity vs. blood flow restriction exercise. Int. J. Sports Med. 38 (13), 1009–1016. doi:10.1055/s-0043-118342
Fry C. S., Glynn E. L., Drummond M. J., Timmerman K. L., Fujita S., Abe T., et al. (2010). Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J. Appl. Physiology 108 (5), 1199–1209. doi:10.1152/japplphysiol.01266.2009
Fuchs C. J., Hermans W. J. H., Nyakayiru J., Weijzen M. E. G., Smeets J. S. J., Aussieker T., et al. (2024). Daily blood flow restriction does not preserve muscle mass and strength during 2 weeks of bed rest. J. Physiology, JP286065. doi:10.1113/JP286065
Fujita S., Abe T., Drummond M. J., Cadenas J. G., Dreyer H. C., Sato Y., et al. (2007). Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J. Appl. Physiology 103 (3), 903–910. doi:10.1152/japplphysiol.00195.2007
Galvão D. A., Spry N. A., Taaffe D. R., Newton R. U., Stanley J., Shannon T., et al. (2008). Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int. 102 (1), 44–47. doi:10.1111/j.1464-410X.2008.07539.x
Goodman C. A. (2013). “The role of mTORC1 in regulating protein synthesis and skeletal muscle mass in response to various mechanical stimuli,” B. Nilius, T. Gudermann, R. Jahn, R. Lill, S. Offermanns, and O. H. Petersen 166, 43–95. doi:10.1007/112_2013_17
Goodpaster B. H., Park S. W., Harris T. B., Kritchevsky S. B., Nevitt M., Schwartz A. V., et al. (2006). The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. Journals Gerontology Ser. A Biol. Sci. Med. Sci. 61 (10), 1059–1064. doi:10.1093/gerona/61.10.1059
Grutter Lopes K., Bottino D. A., Farinatti P., de Souza M. d. G. C., Maranhão P. A., de Araujo C. M. S., et al. (2019). Strength training with blood flow restriction – a novel therapeutic approach for older adults with sarcopenia? A case report. Clin. Interventions Aging 14, 1461–1469. doi:10.2147/CIA.S206522
Guo A., Li K., Xiao Q. (2020). Sarcopenic obesity: myokines as potential diagnostic biomarkers and therapeutic targets? Exp. Gerontol. 139, 111022. doi:10.1016/j.exger.2020.111022
Hackney K. J., Everett M., Scott J. M., Ploutz-Snyder L. (2012). Blood flow-restricted exercise in space. Extreme Physiology and Med. 1 (1), 12. doi:10.1186/2046-7648-1-12
Hackney K. J., Brown L. W. J., Stone K. A., Tennent D. J. (2018). The role of blood flow restriction training to mitigate Sarcopenia, Dynapenia, and enhance clinical recovery. Tech. Orthop. 33 (2), 98–105. doi:10.1097/BTO.0000000000000271
Hage C., Salvatori R. (2023). Growth hormone and aging. Endocrinol. Metabolism Clin. N. Am. 52 (2), 245–257. doi:10.1016/j.ecl.2022.10.003
Howard A. B., Alexander R. W., Taylor W. R. (1995). Effects of magnesium on nitric oxide synthase activity in endothelial cells. Am. J. Physiology-Cell Physiology 269 (6), C612–C618. doi:10.1152/ajpcell.1995.269.3.C612
Hughes L., Paton B., Rosenblatt B., Gissane C., Patterson S. D. (2017). Blood flow restriction training in clinical musculoskeletal rehabilitation: a systematic review and meta-analysis. Br. J. Sports Med. 51 (13), 1003–1011. doi:10.1136/bjsports-2016-097071
Hughes L., Patterson S. D., Haddad F., Rosenblatt B., Gissane C., McCarthy D., et al. (2019). Examination of the comfort and pain experienced with blood flow restriction training during post-surgery rehabilitation of anterior cruciate ligament reconstruction patients: a UK National health Service trial. Phys. Ther. Sport 39, 90–98. doi:10.1016/j.ptsp.2019.06.014
Ito N., Ruegg U. T., Kudo A., Miyagoe-Suzuki Y., Takeda S. (2013). Activation of calcium signaling through Trpv1 by nNOS and peroxynitrite as a key trigger of skeletal muscle hypertrophy. Nat. Med. 19 (1), 101–106. doi:10.1038/nm.3019
Iversen E., Røstad V., Larmo A. (2016). Intermittent blood flow restriction does not reduce atrophy following anterior cruciate ligament reconstruction. J. Sport Health Sci. 5 (1), 115–118. doi:10.1016/j.jshs.2014.12.005
Jakobsgaard J. E., Christiansen M., Sieljacks P., Wang J., Groennebaek T., de Paoli F., et al. (2018). Impact of blood flow-restricted bodyweight exercise on skeletal muscle adaptations. Clin. Physiology Funct. Imaging 38 (6), 965–975. doi:10.1111/cpf.12509
Jenkins N. T., Padilla J., Boyle L. J., Credeur D. P., Laughlin M. H., Fadel P. J. (2013). Disturbed blood flow acutely induces activation and apoptosis of the human vascular endothelium. Hypertension 61 (3), 615–621. doi:10.1161/HYPERTENSIONAHA.111.00561
Jessee M. B., Buckner S. L., Dankel S. J., Counts B. R., Abe T., Loenneke J. P. (2016). The influence of Cuff Width, sex, and race on arterial occlusion: implications for blood flow restriction research. Sports Med. 46 (6), 913–921. doi:10.1007/s40279-016-0473-5
Jessee M., Dankel S., Buckner S., Mouser J. G., Mattocks K. T., Loenneke J. P. (2017). The cardiovascular and perceptual response to very low load blood flow restricted exercise. Int. J. Sports Med. 38 (08), 597–603. doi:10.1055/s-0043-109555
Jessee M. B., Mouser J. G., Buckner S. L., Dankel S. J., Mattocks K. T., Abe T., et al. (2018). Effects of load on the acute response of muscles proximal and distal to blood flow restriction. J. Physiological Sci. 68 (6), 769–779. doi:10.1007/s12576-018-0593-9
Jing Y., Zuo Y., Yu Y., Sun L., Yu Z., Ma S., et al. (2022). Single-nucleus profiling unveils a geroprotective role of the FOXO3 in primate skeletal muscle aging. Protein and Cell 14 (7), pwac061. doi:10.1093/procel/pwac061
Kacin A., Strazar K. (2011). Frequent low-load ischemic resistance exercise to failure enhances muscle oxygen delivery and endurance capacity. Scand. J. Med. and Sci. Sports 21 (6), e231–e241. doi:10.1111/j.1600-0838.2010.01260.x
Kakehi S., Tamura Y., Kubota A., Takeno K., Kawaguchi M., Sakuraba K., et al. (2020). Effects of blood flow restriction on muscle size and gene expression in muscle during immobilization: a pilot study. Physiol. Rep. 8 (14), e14516. doi:10.14814/phy2.14516
Karabulut M., Abe T., Sato Y., Bemben M. G. (2010). The effects of low-intensity resistance training with vascular restriction on leg muscle strength in older men. Eur. J. Appl. Physiology 108 (1), 147–155. doi:10.1007/s00421-009-1204-5
Karabulut M., Sherk V. D., Bemben D. A., Bemben M. G. (2013). Inflammation marker, damage marker and anabolic hormone responses to resistance training with vascular restriction in older males. Clin. Physiology Funct. Imaging 33 (5), 393–399. doi:10.1111/cpf.12044
Karanasios S., Koutri C., Moutzouri M., Xergia S. A., Sakellari V., Gioftsos G. (2022). The effect of body position and the reliability of upper limb arterial occlusion pressure using a handheld doppler ultrasound for blood flow restriction training. Sports Health A Multidiscip. Approach 14 (5), 717–724. doi:10.1177/19417381211043877
Kawada S., Ishii N. (2005). Skeletal muscle hypertrophy after chronic restriction of venous blood flow in rats. Med. and Sci. Sports and Exerc. 37 (7), 1144–1150. doi:10.1249/01.mss.0000170097.59514.bb
Kim J., Lang J. A., Pilania N., Franke W. D. (2017). Effects of blood flow restricted exercise training on muscular strength and blood flow in older adults. Exp. Gerontol. 99, 127–132. doi:10.1016/j.exger.2017.09.016
Kong J., Li Z., Zhu L., Li L., Chen S. (2023). Comparison of blood flow restriction training and conventional resistance training for the improvement of sarcopenia in the older adults: a systematic review and meta-analysis. Sports Med. Health Sci. 5 (4), 269–276. doi:10.1016/j.smhs.2022.12.002
Kubota A., Sakuraba K., Sawaki K., Sumide T., Tamura Y. (2008). Prevention of disuse muscular weakness by restriction of blood flow. Med. and Sci. Sports and Exerc. 40 (3), 529–534. doi:10.1249/MSS.0b013e31815ddac6
Kubota A., Sakuraba K., Koh S., Ogura Y., Tamura Y. (2011). Blood flow restriction by low compressive force prevents disuse muscular weakness. J. Sci. Med. Sport 14 (2), 95–99. doi:10.1016/j.jsams.2010.08.007
Ladlow P., Coppack R. J., Dharm-Datta S., Conway D., Sellon E., Patterson S. D., et al. (2018). Low-Load resistance training with blood flow restriction improves clinical outcomes in musculoskeletal rehabilitation: a single-blind randomized controlled trial. Front. Physiology 9, 1269. doi:10.3389/fphys.2018.01269
Larkin K. A., Macneil R. G., Dirain M., Sandesara B., Manini T. M., Buford T. W. (2012). Blood flow restriction enhances post–resistance exercise angiogenic gene expression. Med. and Sci. Sports and Exerc. 44 (11), 2077–2083. doi:10.1249/MSS.0b013e3182625928
Larsson L. (1978). Morphological and functional characteristics of the ageing skeletal muscle in man. A cross-sectional study. Acta Physiol. Scand. Suppl. 457, 1–36. Available online at: https://pubmed.ncbi.nlm.nih.gov/281113/.
Laurentino G. C., Ugrinowitsch C., Roschel H., Aoki M. S., Soares A. G., Neves M., et al. (2012). Strength training with blood flow restriction diminishes Myostatin gene expression. Med. and Sci. Sports and Exerc. 44 (3), 406–412. doi:10.1249/MSS.0b013e318233b4bc
Laurentino G., Loenneke J., Ugrinowitsch C., Aoki M., Soares A., Roschel H., et al. (2022). Blood-Flow-Restriction-Training-Induced hormonal response is not associated with gains in muscle size and strength. J. Hum. Kinet. 83, 235–243. doi:10.2478/hukin-2022-0095
Lavallee M. E., Balam T. (2010). An overview of strength training injuries: acute and chronic. Curr. Sports Med. Rep. 9 (5), 307–313. doi:10.1249/JSR.0b013e3181f3ed6d
Le Bacquer O., Combe K., Patrac V., Ingram B., Combaret L., Dardevet D., et al. (2019). 4E-BP1 and 4E-BP2 double knockout mice are protected from aging-associated sarcopenia. J. Cachexia, Sarcopenia Muscle 10 (3), 696–709. doi:10.1002/jcsm.12412
Le Roith D., Bondy C., Yakar S., Liu J. L., Butler A. (2001). The Somatomedin Hypothesis: 2001. Endocr. Rev. 22 (1), 53–74. doi:10.1210/edrv.22.1.0419
Lees F. D., Clark P. G., Nigg C. R., Newman P. (2005). Barriers to exercise behavior among older adults: a focus-group study. J. Aging Phys. Activity 13 (1), 23–33. doi:10.1123/japa.13.1.23
Léger B., Derave W., De Bock K., Hespel P., Russell A. P. (2008). Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res. 11 (1), 163–175B. doi:10.1089/rej.2007.0588
Libardi C. A., Godwin J. S., Reece T. M., Ugrinowitsch C., Herda T. J., Roberts M. D. (2024). Effects of low-load resistance training with blood flow restriction on muscle fiber myofibrillar and extracellular area. Front. Physiology 15, 1368646. doi:10.3389/fphys.2024.1368646
Lixandrão M. E., Ugrinowitsch C., Laurentino G., Libardi C. A., Aihara A. Y., Cardoso F. N., et al. (2015). Effects of exercise intensity and occlusion pressure after 12 weeks of resistance training with blood-flow restriction. Eur. J. Appl. Physiology 115 (12), 2471–2480. doi:10.1007/s00421-015-3253-2
Lixandrão M. E., Ugrinowitsch C., Berton R., Vechin F. C., Conceição M. S., Damas F., et al. (2018). Magnitude of muscle strength and mass adaptations between high-load resistance training versus low-load resistance training associated with blood-flow restriction: a systematic review and meta-analysis. Sports Med. 48 (2), 361–378. doi:10.1007/s40279-017-0795-y
Lo J. H., U K. P., Yiu T., Ong M. T. Y., Lee W. Y. W. (2020). Sarcopenia: current treatments and new regenerative therapeutic approaches. J. Orthop. Transl. 23, 38–52. doi:10.1016/j.jot.2020.04.002
Loenneke J. P., Wilson G. J., Wilson J. M. (2010). A mechanistic approach to blood flow occlusion. Int. J. Sports Med. 31 (01), 1–4. doi:10.1055/s-0029-1239499
Loenneke J. P., Fahs C. A., Wilson J. M., Bemben M. G. (2011a). Blood flow restriction: the metabolite/volume threshold theory. Med. Hypotheses 77 (5), 748–752. doi:10.1016/j.mehy.2011.07.029
Loenneke J. P., Wilson J. M., Wilson G. J., Pujol T. J., Bemben M. G. (2011b). Potential safety issues with blood flow restriction training. Scand. J. Med. and Sci. Sports 21 (4), 510–518. doi:10.1111/j.1600-0838.2010.01290.x
Loenneke J., Fahs C., Thiebaud R., Rossow L. M., Abe T., Ye X., et al. (2012a). The acute muscle swelling effects of blood flow restriction. Acta Physiol. Hung. 99 (4), 400–410. doi:10.1556/APhysiol.99.2012.4.4
Loenneke J. P., Fahs C. A., Rossow L. M., Sherk V. D., Thiebaud R. S., Abe T., et al. (2012b). Effects of cuff width on arterial occlusion: implications for blood flow restricted exercise. Eur. J. Appl. Physiology 112 (8), 2903–2912. doi:10.1007/s00421-011-2266-8
Loenneke J. P., Fahs C. A., Rossow L. M., Abe T., Bemben M. G. (2012c). The anabolic benefits of venous blood flow restriction training may be induced by muscle cell swelling. Med. Hypotheses 78 (1), 151–154. doi:10.1016/j.mehy.2011.10.014
Loenneke J. P., Wilson J. M., Marín P. J., Zourdos M. C., Bemben M. G. (2012d). Low intensity blood flow restriction training: a meta-analysis. Eur. J. Appl. Physiology 112 (5), 1849–1859. doi:10.1007/s00421-011-2167-x
Loenneke J. P., Fahs C. A., Rossow L. M., Thiebaud R. S., Mattocks K. T., Abe T., et al. (2013). Blood flow restriction pressure recommendations: a tale of two cuffs. Front. Physiology 4, 249. doi:10.3389/fphys.2013.00249
Loenneke J., Thiebaud R. S., Fahs C. A., Rossow L. M., Abe T., Bemben M. G. (2014). Blood flow restriction: effects of cuff type on fatigue and perceptual responses to resistance exercise. Acta Physiol. Hung. 101 (2), 158–166. doi:10.1556/APhysiol.101.2014.2.4
Loenneke J. P., Allen K. M., Mouser J. G., Thiebaud R. S., Kim D., Abe T., et al. (2015a). Blood flow restriction in the upper and lower limbs is predicted by limb circumference and systolic blood pressure. Eur. J. Appl. Physiology 115 (2), 397–405. doi:10.1007/s00421-014-3030-7
Loenneke J. P., Kim D., Fahs C. A., Thiebaud R. S., Abe T., Larson R. D., et al. (2015b). Effects of exercise with and without different degrees of blood flow restriction on torque and muscle activation. Muscle and Nerve 51 (5), 713–721. doi:10.1002/mus.24448
Loenneke J. P., Hammert W. B., Kataoka R., Yamada Y., Abe T. (2025). Twenty-five years of blood flow restriction training: what we know, what we don’t, and where to next? J. Sports Sci., 1–18. doi:10.1080/02640414.2025.2474329
Luebbers P. E., Fry A. C., Kriley L. M., Butler M. S. (2014). The effects of a 7-week practical blood flow restriction program on well-trained collegiate athletes. J. Strength Cond. Res. 28 (8), 2270–2280. doi:10.1519/JSC.0000000000000385
MacLean H. E., Chiu W. S. M., Notini A. J., Axell A. M., Davey R. A., McManus J. F., et al. (2008). Impaired skeletal muscle development and function in male, but not female, genomic androgen receptor knockout mice. FASEB J. 22 (8), 2676–2689. doi:10.1096/fj.08-105726
Madarame H., Neya M., Ochi E., Nakazato K., Sato Y., Ishii N. (2008). Cross-transfer effects of resistance training with blood flow restriction. Med. and Sci. Sports and Exerc. 40 (2), 258–263. doi:10.1249/mss.0b013e31815c6d7e
Madarame H., Kurano M., Takano H., Iida H., Sato Y., Ohshima H., et al. (2010). Effects of low-intensity resistance exercise with blood flow restriction on coagulation system in healthy subjects. Clin. Physiology Funct. Imaging 30 (3), 210–213. doi:10.1111/j.1475-097X.2010.00927.x
Madarame H., Kurano M., Fukumura K., Fukuda T., Nakajima T. (2013). Haemostatic and inflammatory responses to blood flow-restricted exercise in patients with ischaemic heart disease: a pilot study. Clin. Physiology Funct. Imaging 33 (1), 11–17. doi:10.1111/j.1475-097X.2012.01158.x
Mallmann A. L. S., Santos L. P. D., Doria L. D., Ferreira L. F., Ramis T. R., Da Rosa L. H. T. (2024). Blood flow restriction resistance training as an alternative to resistance training alone to improve strength in elderly: a systematic review with meta-analysis. Saúde Desenvolv. Hum. 12 (1). doi:10.18316/sdh.v12i1.10821
Manini T. M., Clark B. C. (2009). Blood flow restricted exercise and skeletal muscle health. Exerc. Sport Sci. Rev. 37 (2), 78–85. doi:10.1097/JES.0b013e31819c2e5c
Manini T. M., Yarrow J. F., Buford T. W., Clark B. C., Conover C. F., Borst S. E. (2012). Growth hormone responses to acute resistance exercise with vascular restriction in young and old men. Growth Hormone and IGF Res. 22 (5), 167–172. doi:10.1016/j.ghir.2012.05.002
Marcotte G. R., West D. W. D., Baar K. (2015). The molecular basis for load-induced skeletal muscle hypertrophy. Calcif. Tissue Int. 96 (3), 196–210. doi:10.1007/s00223-014-9925-9
Martel G. F., Roth S. M., Ivey F. M., Lemmer J. T., Tracy B. L., Hurlbut D. E., et al. (2006). Age and sex affect human muscle fibre adaptations to heavy-resistance strength training. Exp. Physiol. 91 (2), 457–464. doi:10.1113/expphysiol.2005.032771
Mattocks K. T., Jessee M. B., Counts B. R., Buckner S. L., Grant Mouser J., Dankel S. J., et al. (2017). The effects of upper body exercise across different levels of blood flow restriction on arterial occlusion pressure and perceptual responses. Physiology and Behav. 171, 181–186. doi:10.1016/j.physbeh.2017.01.015
McLeod M., Breen L., Hamilton D. L., Philp A. (2016). Live strong and prosper: the importance of skeletal muscle strength for healthy ageing. Biogerontology 17 (3), 497–510. doi:10.1007/s10522-015-9631-7
Mukund K., Subramaniam S. (2020). Skeletal muscle: a review of molecular structure and function, in health and disease. WIREs Syst. Biol. Med. 12 (1), e1462. doi:10.1002/wsbm.1462
Musarò A., McCullagh K., Paul A., Houghton L., Dobrowolny G., Molinaro M., et al. (2001). Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat. Genet. 27 (2), 195–200. doi:10.1038/84839
Naito H., Powers S. K., Demirel H. A., Sugiura T., Dodd S. L., Aoki J. (2000). Heat stress attenuates skeletal muscle atrophy in hindlimb-unweighted rats. J. Appl. Physiology 88 (1), 359–363. doi:10.1152/jappl.2000.88.1.359
Nakajima T., Takano H., Kurano M., Iida H., Kubota N., Yasuda T., et al. (2007). Effects of KAATSU training on haemostasis in healthy subjects. Int. J. KAATSU Train. Res. 3 (1), 11–20. doi:10.3806/ijktr.3.11
Natsume T., Ozaki H., Saito A. I., Abe T., Naito H. (2015). Effects of electrostimulation with blood flow restriction on muscle size and strength. Med. and Sci. Sports and Exerc. 47 (12), 2621–2627. doi:10.1249/MSS.0000000000000722
Naylor L. H., Weisbrod C. J., O’Driscoll G., Green D. J. (2005). Measuring peripheral resistance and conduit arterial structure in humans using Doppler ultrasound. J. Appl. Physiology 98 (6), 2311–2315. doi:10.1152/japplphysiol.01047.2004
Neto G. R., Novaes J. S., Dias I., Brown A., Vianna J., Cirilo-Sousa M. S. (2017). Effects of resistance training with blood flow restriction on haemodynamics: a systematic review. Clin. Physiology Funct. Imaging 37 (6), 567–574. doi:10.1111/cpf.12368
Nyakayiru J., Fuchs C. J., Trommelen J., Smeets J. S. J., Senden J. M., Gijsen A. P., et al. (2019). Blood flow restriction only increases myofibrillar protein synthesis with exercise. Med. and Sci. Sports and Exerc. 51 (6), 1137–1145. doi:10.1249/MSS.0000000000001899
Ozaki H., Miyachi M., Nakajima T., Abe T. (2011a). Effects of 10 weeks walk training with leg blood flow reduction on carotid arterial compliance and muscle size in the elderly adults. Angiology 62 (1), 81–86. doi:10.1177/0003319710375942
Ozaki H., Sakamaki M., Yasuda T., Fujita S., Ogasawara R., Sugaya M., et al. (2011b). Increases in thigh muscle volume and strength by walk training with leg blood flow reduction in older participants. Journals Gerontology Ser. A Biol. Sci. Med. Sci. 66A (3), 257–263. doi:10.1093/gerona/glq182
Ozaki H., Yasuda T., Ogasawara R., Sakamaki-Sunaga M., Naito H., Abe T. (2013). Effects of high-intensity and blood flow-restricted low-intensity resistance training on carotid arterial compliance: role of blood pressure during training sessions. Eur. J. Appl. Physiology 113 (1), 167–174. doi:10.1007/s00421-012-2422-9
Ozaki H., Kakigi R., Kobayashi H., Loenneke J. P., Abe T., Naito H. (2014). Effects of walking combined with restricted leg blood flow on mTOR and MAPK signalling in young men. Acta Physiol. 211 (1), 97–106. doi:10.1111/apha.12243
Ozaki H., Loenneke J. P., Abe T. (2017). Blood flow-restricted walking in older women: does the acute hormonal response associate with muscle hypertrophy? Clin. Physiology Funct. Imaging 37 (4), 379–383. doi:10.1111/cpf.12312
Pallafacchina G., Calabria E., Serrano A. L., Kalhovde J. M., Schiaffino S. (2002). A protein kinase B-dependent and rapamycin-sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc. Natl. Acad. Sci. 99 (14), 9213–9218. doi:10.1073/pnas.142166599
Parkington T., Maden-Wilkinson T., Klonizakis M., Broom D. (2022). Comparative perceptual, affective, and cardiovascular responses between resistance exercise with and without blood flow restriction in older adults. Int. J. Environ. Res. Public Health 19 (23), 16000. doi:10.3390/ijerph192316000
Parry S. M., Puthucheary Z. A. (2015). The impact of extended bed rest on the musculoskeletal system in the critical care environment. Extreme Physiology and Med. 4 (1), 16. doi:10.1186/s13728-015-0036-7
Patterson S. D., Ferguson R. A. (2011). Enhancing strength and postocclusive calf blood flow in older people with training with blood-flow restriction. J. Aging Phys. Activity 19 (3), 201–213. doi:10.1123/japa.19.3.201
Patterson S. D., Hughes L., Warmington S., Burr J., Scott B. R., Owens J., et al. (2019). Blood flow restriction exercise: considerations of methodology, application, and safety. Front. Physiology 10, 533. doi:10.3389/fphys.2019.00533
Pereira Neto E. A., Bittar S. T., Silva J. C. G. D., Pfeiffer P. A. S., Santos H. H. D., Sousa M. D. S. C. D. (2018). Walking with blood flow restriction improves the dynamic strength of women with osteoporosis. Rev. Bras. Med. do Esporte 24 (2), 135–139. doi:10.1590/1517-869220182402175290
Perry H. M., Miller D. K., Patrick P., Morley J. E. (2000). Testosterone and leptin in older African-American men: relationship to age, strength, function, and season. Metabolism 49 (8), 1085–1091. doi:10.1053/meta.2000.7710
Rossow L. M., Fahs C. A., Loenneke J. P., Thiebaud R. S., Sherk V. D., Abe T., et al. (2012). Cardiovascular and perceptual responses to blood-flow-restricted resistance exercise with differing restrictive cuffs. Clin. Physiology Funct. Imaging 32 (5), 331–337. doi:10.1111/j.1475-097X.2012.01131.x
Schliess F., Richter L., Vom Dahl S., Häussinger D. (2006). Cell hydration and mTOR-dependent signalling. Acta Physiol. 187 (1–2), 223–229. doi:10.1111/j.1748-1716.2006.01547.x
Schutzer K., Graves B. S. (2004). Barriers and motivations to exercise in older adults. Prev. Med. 39 (5), 1056–1061. doi:10.1016/j.ypmed.2004.04.003
Schwiete C., Franz A., Roth C., Behringer M. (2021). Effects of resting vs. continuous blood-flow restriction-training on strength, fatigue resistance, muscle thickness, and perceived discomfort. Front. Physiology 12, 663665. doi:10.3389/fphys.2021.663665
Scott B. R., Peiffer J. J., Thomas H. J., Marston K. J., Hill K. D. (2018). Hemodynamic responses to low-load blood flow restriction and unrestricted high-load resistance exercise in older women. Front. Physiology 9, 1324. doi:10.3389/fphys.2018.01324
Scott A. J., Ellison M., Sinclair D. A. (2021). The economic value of targeting aging. Nat. Aging 1 (7), 616–623. doi:10.1038/s43587-021-00080-0
Serra C., Tangherlini F., Rudy S., Lee D., Toraldo G., Sandor N. L., et al. (2013). Testosterone improves the regeneration of old and young mouse skeletal muscle. Journals Gerontology Ser. A Biol. Sci. Med. Sci. 68 (1), 17–26. doi:10.1093/gerona/gls083
Shimizu R., Hotta K., Yamamoto S., Matsumoto T., Kamiya K., Kato M., et al. (2016). Low-intensity resistance training with blood flow restriction improves vascular endothelial function and peripheral blood circulation in healthy elderly people. Eur. J. Appl. Physiology 116 (4), 749–757. doi:10.1007/s00421-016-3328-8
Shinohara M., Kouzaki M., Yoshihisa T., Fukunaga T. (1997). Efficacy of tourniquet ischemia for strength training with low resistance. Eur. J. Appl. Physiology Occup. Physiology 77 (1–2), 189–191. doi:10.1007/s004210050319
Silva J. C. G., Pereira Neto E. A., Pfeiffer P. A. S., Neto G. R., Rodrigues A. S., Bemben M. G., et al. (2019). Acute and chronic responses of aerobic exercise with blood flow restriction: a systematic review. Front. Physiology 10, 1239. doi:10.3389/fphys.2019.01239
Simpson C. W. C., Moore K. S., Smith H. K., Coskun B., Hamlin M. J. (2025). Tissue oxygenation in response to low-load and high-load back squats with continuous blood flow restriction in athletes. J. Sports Sci., 1–10. doi:10.1080/02640414.2025.2457859
Sinha-Hikim I., Artaza J., Woodhouse L., Gonzalez-Cadavid N., Singh A. B., Lee M. I., et al. (2002). Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am. J. Physiology-Endocrinology Metabolism 283 (1), E154–E164. doi:10.1152/ajpendo.00502.2001
Slysz J., Stultz J., Burr J. F. (2016). The efficacy of blood flow restricted exercise: a systematic review and meta-analysis. J. Sci. Med. Sport 19 (8), 669–675. doi:10.1016/j.jsams.2015.09.005
Slysz J. T., Boston M., King R., Pignanelli C., Power G. A., Burr J. F. (2021). Blood flow restriction combined with electrical stimulation attenuates thigh muscle disuse atrophy. Med. and Sci. Sports and Exerc. 53 (5), 1033–1040. doi:10.1249/MSS.0000000000002544
Snijders T., Verdijk L. B., Van Loon L. J. C. (2009). The impact of sarcopenia and exercise training on skeletal muscle satellite cells. Ageing Res. Rev. 8 (4), 328–338. doi:10.1016/j.arr.2009.05.003
Song J. S., Spitz R. W., Yamada Y., Bell Z. W., Wong V., Abe T., et al. (2021). Exercise-induced hypoalgesia and pain reduction following blood flow restriction: a brief review. Phys. Ther. Sport 50, 89–96. doi:10.1016/j.ptsp.2021.04.005
Spitz R. W., Chatakondi R. N., Bell Z. W., Wong V., Dankel S. J., Abe T., et al. (2019). The impact of cuff width and biological sex on cuff preference and the perceived discomfort to blood-flow-restricted arm exercise. Physiol. Meas. 40 (5), 055001. doi:10.1088/1361-6579/ab1787
Spitz R. W., Wong V., Yamada Y., Kataoka R., Song J. S., Hammert W. B., et al. (2024). The effect of isometric handgrip training with and without blood flow restriction on changes in resting blood pressure. Res. Q. Exerc. Sport, 1–8. doi:10.1080/02701367.2024.2418567
Stevens J. E., Mizner R. L., Snyder-Mackler L. (2003). Quadriceps strength and volitional activation before and after total knee arthroplasty for osteoarthritis. J. Orthop. Res. 21 (5), 775–779. doi:10.1016/S0736-0266(03)00052-4
Sugimoto T., Suga T., Tomoo K., Dora K., Mok E., Tsukamoto H., et al. (2021). Blood flow restriction improves executive function after walking. Med. and Sci. Sports and Exerc. 53 (1), 131–138. doi:10.1249/MSS.0000000000002446
Tafuna’i N. D., Hunter I., Johnson A. W., Fellingham G. W., Vehrs P. R. (2021). Differences in femoral artery occlusion pressure between sexes and dominant and non-dominant legs. Medicina 57 (9), 863. doi:10.3390/medicina57090863
Tajar A., Forti G., O’Neill T. W., et al. (2010). Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J. Clin. Endocrinol. and Metabolism 95 (4), 1810–1818. doi:10.1210/jc.2009-1796
Takarada Y., Takazawa H., Ishii N. (2000). Applications of vascular occlusion diminish disuse atrophy of knee extensor muscles. Med. and Sci. Sports and Exerc. 32 (12), 2035–2039. doi:10.1097/00005768-200012000-00011
Thompson K. M. A., Gamble A. S. D., Kontro H., Lee J. B., Burr J. F. (2024). Low- and high-volume blood-flow restriction treadmill walking both improve maximal aerobic capacity independently of blood volume. Scand. J. Med. and Sci. Sports 34 (1), e14534. doi:10.1111/sms.14534
Tieland M., Trouwborst I., Clark B. C. (2018). Skeletal muscle performance and ageing. J. Cachexia, Sarcopenia Muscle 9 (1), 3–19. doi:10.1002/jcsm.12238
Tournadre A., Vial G., Capel F., Soubrier M., Boirie Y. (2019). Sarcopenia. Jt. Bone Spine 86 (3), 309–314. doi:10.1016/j.jbspin.2018.08.001
Valenzuela P. L., Morales J. S., Pareja-Galeano H., Izquierdo M., Emanuele E., de la Villa P., et al. (2018). Physical strategies to prevent disuse-induced functional decline in the elderly. Ageing Res. Rev. 47, 80–88. doi:10.1016/j.arr.2018.07.003
Vechin F. C., Libardi C. A., Conceição M. S., Damas F. R., Lixandrão M. E., Berton R. P. B., et al. (2015). Comparisons between low-intensity resistance training with blood flow restriction and high-intensity resistance training on quadriceps muscle mass and strength in elderly. J. Strength Cond. Res. 29 (4), 1071–1076. doi:10.1519/JSC.0000000000000703
Verdijk L. B., Koopman R., Schaart G., Meijer K., Savelberg H. H. C. M., van Loon L. J. C. (2007). Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am. J. Physiology-Endocrinology Metabolism 292 (1), E151–E157. doi:10.1152/ajpendo.00278.2006
Wang J., Mogensen A.-M. G., Thybo F., Brandbyge M., Brorson J., Van Hall G., et al. (2023). Low-load blood flow-restricted resistance exercise produces fiber type-independent hypertrophy and improves muscle functional capacity in older individuals. J. Appl. Physiology 134 (4), 1047–1062. doi:10.1152/japplphysiol.00789.2022
Wernbom M., Augustsson J., Thomeé R. (2006). Effects of vascular occlusion on muscular endurance in dynamic knee extension exercise at different submaximal loads. J. Strength Cond. Res. 20 (2), 372. doi:10.1519/R-16884.1
Wernbom M., Paulsen G., Nilsen T. S., Hisdal J., Raastad T. (2012). Contractile function and sarcolemmal permeability after acute low-load resistance exercise with blood flow restriction. Eur. J. Appl. Physiology 112 (6), 2051–2063. doi:10.1007/s00421-011-2172-0
Wilson J. M., Lowery R. P., Joy J. M., Loenneke J. P., Naimo M. A. (2013). Practical blood flow restriction training increases acute determinants of hypertrophy without increasing indices of muscle damage. J. Strength Cond. Res. 27 (11), 3068–3075. doi:10.1519/JSC.0b013e31828a1ffa
Wilson R. J., Drake J. C., Cui D., Ritger M. L., Guan Y., Call J. A., et al. (2019). Voluntary running protects against neuromuscular dysfunction following hindlimb ischemia-reperfusion in mice. J. Appl. Physiology 126 (1), 193–201. doi:10.1152/japplphysiol.00358.2018
Wong V., Song J. S., Bell Z. W., Yamada Y., Spitz R. W., Abe T., et al. (2022). Blood flow restriction training on resting blood pressure and heart rate: a meta-analysis of the available literature. J. Hum. Hypertens. 36 (8), 738–743. doi:10.1038/s41371-021-00561-0
Wortman R. J., Brown S. M., Savage-Elliott I., Finley Z. J., Mulcahey M. K. (2021). Blood flow restriction training for athletes: a systematic review. Am. J. Sports Med. 49 (7), 363546520964454–1944. doi:10.1177/0363546520964454
Yadav A., Yadav S. S., Singh S., Dabur R. (2022). Natural products: potential therapeutic agents to prevent skeletal muscle atrophy. Eur. J. Pharmacol. 925, 174995. doi:10.1016/j.ejphar.2022.174995
Yamada E., Kusaka T., Tanaka S., Mori S., Norimatsu H., Itoh S. (2004). Effects of vascular occlusion on surface electromyography and muscle oxygenation during isometric contraction. J. Sport Rehabilitation 13 (4), 287–299. doi:10.1123/jsr.13.4.287
Yamada Y., Hammert W. B., Kataoka R., Song J. S., Kang A., Loenneke J. P. (2025). Limb dominance does not have a meaningful impact on arterial occlusion pressure. Clin. Physiology Funct. Imaging 45 (1), e12906. doi:10.1111/cpf.12906
Yamanaka T., Farley R. S., Caputo J. L. (2012). Occlusion training increases muscular strength in division IA football players. J. Strength Cond. Res. 26 (9), 2523–2529. doi:10.1519/JSC.0b013e31823f2b0e
Yarasheski K. E., Bhasin S., Sinha-Hikim I., Pak-Loduca J., Gonzalez-Cadavid N. F. (2002). Serum myostatin-immunoreactive protein is increased in 60-92 year old women and men with muscle wasting. J. Nutr. Health and Aging 6 (5), 343–348. Available online at: https://pubmed.ncbi.nlm.nih.gov/12474026/.
Yasuda T., Abe T., Brechue W. F., Iida H., Takano H., Meguro K., et al. (2010). Venous blood gas and metabolite response to low-intensity muscle contractions with external limb compression. Metabolism 59 (10), 1510–1519. doi:10.1016/j.metabol.2010.01.016
Yasuda T., Loenneke J. P., Thiebaud R. S., Abe T. (2012). Effects of blood flow restricted low-intensity concentric or eccentric training on muscle size and strength. PLoS ONE 7 (12), e52843. doi:10.1371/journal.pone.0052843
Yasuda T., Fukumura K., Uchida Y., Koshi H., Iida H., Masamune K., et al. (2015). Effects of Low-Load, elastic band resistance training combined with blood flow restriction on muscle size and arterial stiffness in older adults. Journals Gerontology Ser. A Biol. Sci. Med. Sci. 70 (8), 950–958. doi:10.1093/gerona/glu084
Yasuda T., Meguro M., Sato Y., Nakajima T. (2017). Use and safety of KAATSU training: results of a national survey in 2016. Int. J. KAATSU Train. Res. 13 (1), 1–9. doi:10.3806/ijktr.13.1
Yinghao L., Jing Y., Yongqi W., Jianming Z., Zeng G., Yiting T., et al. (2021). Effects of a blood flow restriction exercise under different pressures on testosterone, growth hormone, and insulin-like growth factor levels. J. Int. Med. Res. 49 (9), 030006052110395. doi:10.1177/03000605211039564
Yoshida T., Delafontaine P. (2020). Mechanisms of IGF-1-Mediated regulation of skeletal muscle hypertrophy and atrophy. Cells 9 (9), 1970. doi:10.3390/cells9091970
Younger A. S. E., McEwen J. A., Inkpen K. (2004). Wide contoured thigh cuffs and automated limb occlusion measurement allow lower tourniquet pressures. Clin. Orthop. and Relat. Res. 428 (428), 286–293. doi:10.1097/01.blo.0000142625.82654.b3
Yuan J., Wu L., Xue Z., Xu G., Wu Y. (2023). Application and progress of blood flow restriction training in improving muscle mass and strength in the elderly. Front. Physiology 14, 1155314. doi:10.3389/fphys.2023.1155314
Zaar M., Johansson P. I., Nielsen L. B., Crandall C. G., Shibasaki M., Hilsted L., et al. (2009). Early activation of the coagulation system during lower body negative pressure. Clin. Physiology Funct. Imaging 29 (6), 427–430. doi:10.1111/j.1475-097X.2009.00890.x
Zhang T., Wang X., Wang J., Xu G., Wu L., Li Y., et al. (2022a). Effect of blood flow restriction combined with low-intensity training on the lower limbs muscle strength and function in older adults: a meta-analysis. Exp. Gerontol. 164, 111827. doi:10.1016/j.exger.2022.111827
Zhang X., Xie W., Chen L., Xu G., Wu L., Li Y., et al. (2022b). Blood flow restriction training for the intervention of Sarcopenia: current stage and future perspective. Front. Med. 9, 894996. doi:10.3389/fmed.2022.894996
Zhang M., Song Y., Zhu J., Ding P., Chen N. (2024). Effectiveness of low-load resistance training with blood flow restriction vs. conventional high-intensity resistance training in older people diagnosed with sarcopenia: a randomized controlled trial. Sci. Rep. 14 (1), 28427. doi:10.1038/s41598-024-79506-9
Keywords: sarcopenia, aging, blood flow restriction training, muscle mass, muscle strength
Citation: Li W, Hu M, Yin Q, Liu Y, Chen L, Ru Q, Xu G and Wu Y (2025) Blood flow restriction training: a new approach for preventing and treating sarcopenia in older adults. Front. Physiol. 16:1616874. doi: 10.3389/fphys.2025.1616874
Received: 23 April 2025; Accepted: 01 August 2025;
Published: 25 August 2025.
Edited by:
Giuseppe D’Antona, University of Pavia, ItalyReviewed by:
Junlin Yuan, Chinese Academy of Sciences (CAS), ChinaAndré Luiz Mallmann, Hospital de Clínicas de Porto Alegre, Brazil
Copyright © 2025 Li, Hu, Yin, Liu, Chen, Ru, Xu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuxiang Wu, eXh3dUBqaHVuLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Wei Li
Wei Li Mingzhen Hu1,2†
Mingzhen Hu1,2† Qin Ru
Qin Ru Yuxiang Wu
Yuxiang Wu