- 1IRCCS Ospedale Policlinico San Martino, Genoa, Italy
- 2IRCCS Istituto Giannina Gaslini, Genoa, Italy
- 3Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
- 4Pediatric Palliative Care Service, VIDAS ODV, Milan, Italy
- 5Ospedale dei Bambini Vittore Buzzi, Milan, Italy
- 6Italian Association for Congenital Central Hypoventilation Syndrome (A.I.S.I.C.C.), Firenze, Italy
Introduction: Heterozygous mutations in the paired-like homeobox 2b (PHOX2B) gene cause congenital central hypoventilation syndrome (CCHS). While polyalanine expansions are almost exclusively associated with isolated CCHS, missense, nonsense, and frameshift mutations are mainly identified in syndromic CCHS, presenting with Hirschsprung disease (CCHS + HSCR) alone and/or together with neuroblastoma (CCHS + HSCR + NB). CCHS-associated missense mutations occur in the PHOX2B homeodomain, where impaired transcriptional activity has been suggested as their functional effect. However, the molecular pathogenesis underlying their association with HSCR- and/or NB-associated CCHS has not been investigated to date.
Methods: we applied an in silico analysis and a minigene approach in vitro to test the effect of the PHOX2B c.428A>G missense variant on the splicing of intron 2.
Results: we demonstrate that the missense c.428A>G variant, reported by us and others in a set of CCHS + HSCR cases but never associated with NB, not only causes the amino acid change p.Q143R change but also disrupts the intron 2 splice donor site, producing an aberrant mRNA transcript and likely a hypomorphic, dysfunctional protein.
Discussion: We, therefore, propose that in the presence of splicing defects of PHOX2B, a loss-of-function mechanism may underlie CCHS + HSCR and potentially explain the absence of neural-crest-derived tumors.
1 Introduction
Heterozygous mutations in PHOX2B, a gene encoding a transcription factor expressed during the differentiation of neural crest cells (NCCs) into autonomic nervous system precursor cells (Pattyn et al., 1999), are associated with congenital central hypoventilation syndrome (CCHS). CCHS patients are characterized by an abnormal ventilatory response to hypoxia and hypercapnia, requiring treatment with ventilatory support, tracheostomy, or diaphragmatic pacers, especially during sleep.
The vast majority of mutations consist of expansions, ranging from +4 to +13 residues, of a 20-residue polyalanine stretch in exon 3, whose pathogenic effect is expressed through the partial or total retention of PHOX2B in the cytoplasmic compartment, in a soluble or aggregated form, thus compromising its role in the transcriptional regulation of target genes exerted in the nucleus (Bachetti et al., 2005a; Bachetti and Ceccherini, 2020). However, modulation of the cellular response using Hsp90 inhibitors and histone deacetylase (HDAC) inhibitors has shown beneficial effects in vitro (Di Zanni et al., 2012; Africano et al., 2024).
Life-threatening breathing deficiencies in CCHS can be accompanied by Hirschsprung disease (HSCR)—a condition marked by intestinal aganglionosis due to defective migration of NCC precursors of enteric ganglion cells—NCC-derived tumors such as neuroblastoma (NB) (Trochet et al., 2005), and several other autonomic nervous system dysfunctions (Trang et al., 2020). CCHS can, therefore, manifest as isolated or syndromic, with the latter most often characterized by the co-occurrence of HSCR with or without NB. Interestingly, PHOX2B mutations are uniquely responsible for syndromic forms of CCHS, in contrast to atypical/isolated presentations, which may be associated with different genetic alterations (Spielmann et al., 2017; Hernandez-Miranda et al., 2018).
While polyalanine repeat mutations (PARMs) are mainly associated with isolated CCHS, syndromic CCHS—particularly CCHS + HSCR or CCHS + HSCR + NB—is more frequently associated with non-PARMs (NPARMs), including missense (MS), nonsense (NS), and frameshift (FS) mutations (Bachetti and Ceccherini, 2020).
Frameshift mutations are differently distributed along the PHOX2B gene in patients with CCHS + HSCR, with or without NB, depending on the type of translational frame disruption. This disruption affects the severity of the resulting transcriptional dysfunction and the predisposition to isolated or syndromic CCHS (Di Lascio et al., 2018). Moreover, both loss-of-function and gain-of-function effects, with the latter characterized by aberrant target gene acquisition such as SOX10 and GFAP (Nagashimada et al., 2012; Di Lascio et al., 2018) and the failure of sympathoadrenal progenitor cells to differentiate into the neuronal lineage (Ponzoni et al., 2022), have been proposed for NB occurrence in the presence of FS mutations.
Conversely, most missense mutations affect the homeodomain region, leading to different syndromic CCHS associations, whose underlying molecular mechanisms are still undisclosed.
Starting from the molecular and clinical characterization of two patients carrying the PHOX2B c.428A>G (p.Q143R) variant, affected by CCHS and intestinal abnormal phenotypes, in this work, we have investigated how the nucleotide change and substitutions at neighbor positions, rather than the amino acid replacement itself, could influence the clinical expressivity of homeodomain-affecting PHOX2B variants.
2 Methods
2.1 CCHS molecular diagnosis
The molecular analysis of the PHOX2B gene was performed at IRCCS Giannina Gaslini.
For patient 1 (MN), the coding region of the PHOX2B gene was analyzed for mutations by direct DNA sequencing using the BigDye Terminator Cycle Sequencing Kit (Applied Biosystems) on an ABI 3100 DNA Automated Sequencer (Matera et al., 2004), according to a protocol we have already reported.
For patient 2 (SM), whole-exome sequencing was performed, as already reported (Di Rocco et al., 2018), using the Ion Torrent technology with coverage >100X (Ion-Proton, Thermo Fisher Scientific) on DNA extracted from peripheral blood. The PHOX2B variant thus detected was confirmed by direct DNA sequencing, as described above.
In both patients, the c.428A>G variant in exon 2 of the PHOX2B gene was detected in heterozygosity.
2.2 In silico splicing prediction software
In silico prediction of splicing at the PHOX2B gene was performed using both the Alternative Splice Site Predictor (ASSP) (http://wangcomputing.com/assp/) and the SD-score algorithm for mutation simulation (https://www.med.nagoya-u.ac.jp/neurogenetics/SD_Score/sd_score.htmli). The ASSP is a sequence analysis tool for the prediction and classification of splice sites. The ASSP identifies putative splice sites using pre-processing models and subsequently classifies them as either constitutive or alternative isoform/cryptic splice sites, combining several statistics, such as position-specific score matrices for the splice sites, GC content, and oligonucleotide frequency models (Wang and Marin, 2006). The classification performance of ASSP, i.e., the distinction between constitutive or alternative isoform/cryptic splice sites, is 67.45% for the acceptor sites and 71.23% for the donor sites. The SD-score algorithm is a practical tool to study the splicing consequences of mutations affecting the 5′splice site in silico, predicting aberrant splicing when the SD score for the mutant allele is lower than that for the wild-type (WT) allele (Sahashi et al., 2007). The SD score is accompanied by the information content (Ri), which improves predictive accuracy. Differences between two sequences are expressed as “delta,” with ΔSD < −0.34 and ΔRi < −1.45 indicating aberrant splicing (Sahashi et al., 2007).
2.3 Generation of WT (c.428A) and mutant (c.428G) pSPL3 constructs
After evaluating the in silico splicing prediction for the c.428A/G alleles, a 261 bp region in the PHOX2B gene—encompassing exon 2 and 45 bp upstream and 28 bp downstream of flanking intronic sequences—was amplified from the gDNA of a patient heterozygous for the c.428A>G variant using the AccuPrime GC-Rich Kit (Life Technologies) and primers Ph2F 5′-TGCCGGCTGATTTGCTCAC-3′ and Ph2R 5′-AGCGGGGTCGGTTTCCAGG-3’. PCR products were then cloned into the pCR2.1 vector (TOPO TA cloning, Life Technologies) to separate the A and G alleles. The A and G fragments were EcoRI-digested from TOPO TA plasmids to be inserted into the EcoRI site of the pSPL3 plasmid to generate the two pSPL3-A and pSPL3-G minigenes.
2.4 Cell cultures and transfection
Although not of neural crest origin, HEK293T cells were selected to test the effect of the “G” allele on PHOX2B gene splicing because i. they are characterized by high transfection efficiency, which is necessary for performing functional studies using transient transfections, and ii. splicing is a ubiquitous mechanism, which does not require a specific cellular background. This cell line was grown in DMEM supplemented with 10% FBS, 1% glutamine, and 1% penicillin/streptomycin. Cells were plated at 70% confluency the day before transfection, which was performed by mixing 5 μg of DNA from pSPL3-A and pSPL3-G constructs with FuGENE HD (Promega) at a 3:1 ratio.
2.5 Splicing minigene assay
Five micrograms of pSPL3-A and pSPL3-G plasmids were transiently transfected into HEK293T cells, and total RNA was extracted 24 h later (RNeasy Plus mini Kit, QIAGEN). cDNA was obtained starting from 1 μg RNA (iScript cDNA Synthesis Kit, Bio-Rad). Polymerase chain reaction (PCR) on cDNA was performed using primers SD6 (5′-TCT GAG TCA CCT GGA CAA CC-3′) and SA2 5’-(ATC TCA GTG GTA TTT GTG AGC-3′) and AccuPrime GC-Rich DNA Polymerase Kit (Thermo Fisher Scientific) in 25 μL reaction mixture through 40 cycles at 95 °C for 10′, 95 °C for 30″, 55 °C for 45″, and 72 °C for 3′, followed by one cycle at 72 °C for 7’. The PCR products were then analyzed via electrophoresis on a 1.5% agarose gel and characterized by Sanger sequencing using the SD and SA2 primers.
2.6 Quantification and statistical analysis
The percentage of correctly or aberrantly spliced transcripts was estimated by quantifying the bands from the electrophoresis analysis of PCR products using ImageJ, particularly using the ratio between the intensity of the target band and the combined intensity of the upper and lower bands of the gel. The results were analyzed using GraphPad Prism. Comparisons of the means between the two groups were performed using two-way ANOVA, followed by Sidak’s multiple testing correction.
3 Results
3.1 Clinical evaluation of CCHS patients carrying the PHOX2B c.428A>G variant
Patient 1. MN, a female child born prematurely at 34+1 weeks via cesarean section, presented with central hypoventilation and hypotonia at birth associated with pulmonary hypertension. She was immediately transferred to the Neonatal Intensive Care Unit at Buzzi Hospital due to severe respiratory acidosis and was treated with intubation, mechanical ventilation, and, after a month of hospitalization, tracheostomy. At the age of 1 month, the identification of the c.428A>G variant in the PHOX2B gene, leading to the amino acid change p.Q143R, confirmed the molecular diagnosis of CCHS. The mutation occurred de novo, being absent in both parents. Moreover, preliminary observations of severe constipation and abdominal distension suggested rectal biopsies that confirmed the lack of ganglionic structures in the submucosa, thus leading to the diagnosis of co-occurring HSCR. Currently, after several hospitalizations for the removal of the intestinal aganglionic tract, hypoglycemia, and intestinal perforation due to necrotizing enterocolitis (NEC), she is under continuous respiratory, nutritional, and neurological monitoring. Finally, she suffered from essential arterial hypertension, which was treated with captopril, and severe hypermetropia.
Patient 2. SM, a baby girl born at 36 weeks by cesarean section for alterations in cardiotocographic tracing, was immediately transferred to the Neonatal Intensive Care Unit of the Rho Hospital (Milan) for the absence of spontaneous respiratory activity and hypotonia and subsequently to the Neonatal Intensive Care Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico (Milan). She presented with early feeding difficulties and delayed neurological development, with episodes of withdrawal of contact, sialorrhea, generalized hypertonicity, and limb clonus. The EEG during sleep showed a “slow trend compatible with epileptiform episodes,” which subsequently normalized without requiring continuous anti-epileptic therapy. The child was then taken to the pediatrics department of the De Marchi Clinic in Milan, and at 4 months, she was treated with a tracheostomy to allow mechanical ventilation during sleep. During the first year of life, the child also experienced recurrent hyperinsulinemic hypoglycemic episodes, as confirmed by a pathological glucagon test. In the subsequent years, hyperglycemia occurred during intercurrent infections. Concomitant to the metabolic imbalance, the child presented signs of intestinal dysmotility with alternate alvo. Detection of the c.428A>G variant in the PHOX2B gene confirmed that the child had CCHS. Again, the mutation had occurred de novo, being absent in both parents. After a few years, she was admitted to the Buzzi Hospital in Milan for signs of intestinal dysmotility, such as marked constipation with some sub-occlusive episodes. She presented with abdominal pain and a distended abdomen in the upper quadrants. Furthermore, her body growth was delayed. Two years later, she was evaluated for gastrointestinal disorders due to severe episodes of diarrhea; following two aspiration biopsies, the quality of which did not allow excluding or confirming the presence of ganglia, focal hypertrophy of cholinergic fibers in the muscularis mucosae and lamina propria was observed, ultimately suggesting ultra-short HSCR disease. The metabolic changes gradually resolved as intestinal motility and growth improved.
3.2 In silico prediction of PHOX2B splicing defects due to c.428A>G
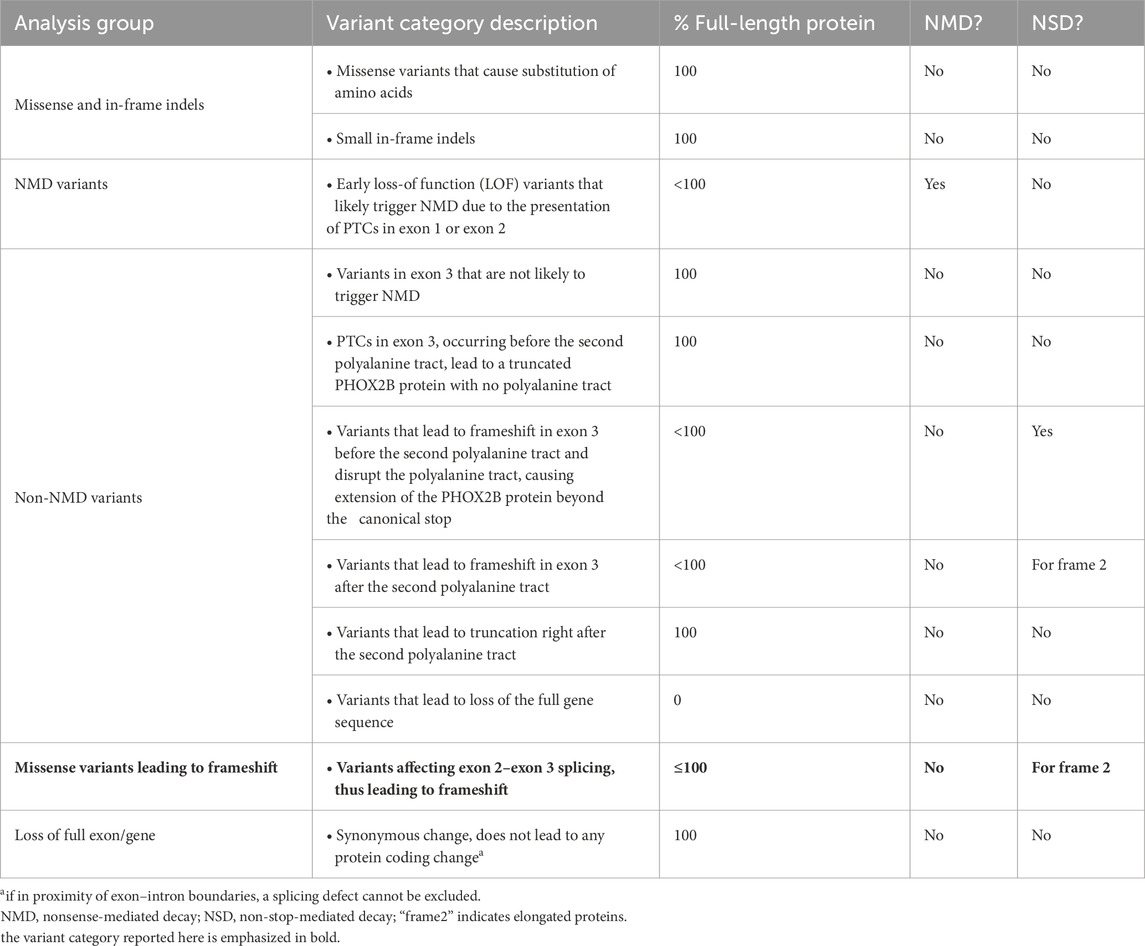
After observing that the c.428 nucleotide represents the second-last position of exon 2, namely, -2 nt with respect to the GT donor site of intron 2 (Figure 1), we hypothesized a role for the c.428A>G variant in the regulation of the splicing process. At the -2 nt position, A is present in 63.3% of cases, while G has been found only in 11.8% of cases (Sahashi et al., 2007; Sibley et al., 2016) (Figure 1).
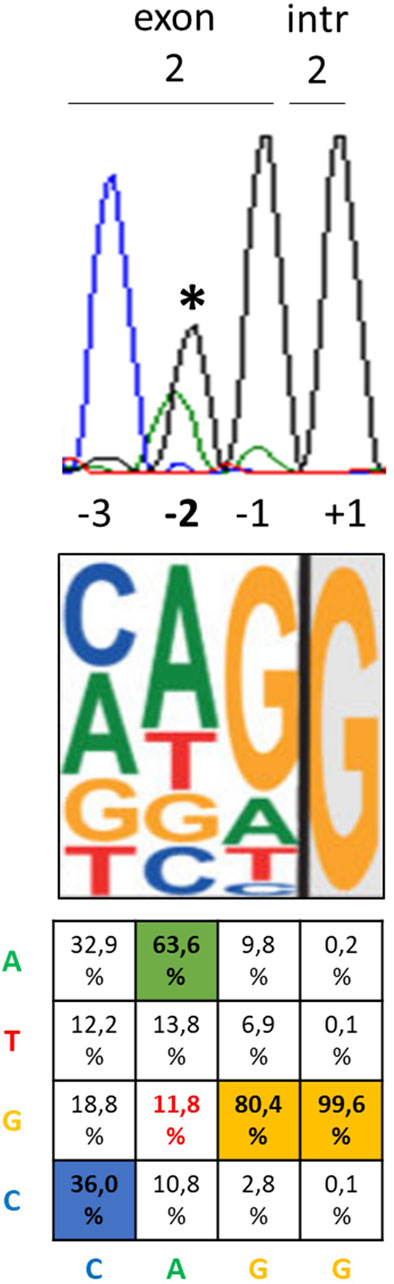
Figure 1. Position of the c.428A>G PHOX2B variant relative to the exon–intron junction and most likely nucleotides. From top to bottom, the three pictures represent the electropherogram showing the heterozygous c.428A>G variant in the DNA sequence of the CCHS + HSCR patient (asterisk) with the position of each nucleotide with respect to the exon 2–intron 2 boundary, the most probable consensus matrix of the 5′ splicing site (modified by Sibley et al., 2016), and the probability (%) to find A, T, G, and C nucleotides in each position from −3 to +1 (Sahashi et al., 2007).
To investigate whether the c.428G allele could interfere with the splicing process, the entire PHOX2B exon 2, flanked by portions of introns 1 and 2, underwent in silico analyses using the ASSP and SD-score algorithms. Both programs suggested aberrant splicing in the presence of the c.428G allele compared to the A allele. In particular, the ASSP detected a lower splicing score for the G allele than that for the A allele, in addition to an increased value of activation of a cryptic splicing site (Table 1). The SD score showed ΔSD = −1,009 and ΔRi = −2,456, which are largely below the threshold of the values predicting aberrant splicing, namely, ΔSD < −0.34 and ΔRi < −1.45 (Table 1). In addition, ESEfinder 2.0 further confirmed the results obtained using the two other software applications (not shown).
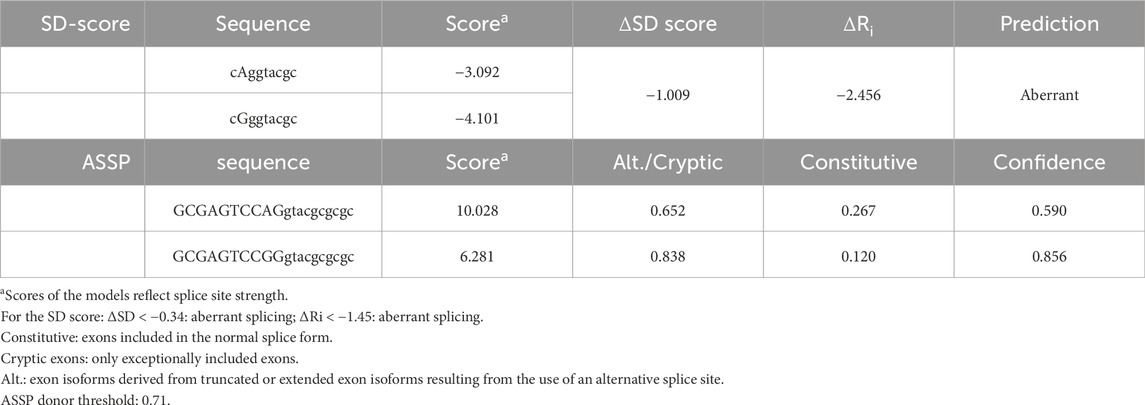
Table 1. In silico analysis, through the ASSP and SD-score algorithms, of the possible effect on splicing of the c.428A>G PHOX2B variant.
3.3 Functional effect of c.428A>G on the splicing process
The PHOX2B exon 2, flanked by its intronic regions, was cloned into the pSPL3 expression vector between the two artificial splice donor (SD) and splice acceptor (SA) sites, thus making the minigenes pSPL3-A and pSPL3-G suitable for testing the splicing efficiency of the subcloned region. In particular, we transfected the human HEK293T cell line, characterized by high transfection efficiency, with the constructs bearing the wild-type PHOX2B allele c.428A or the variant c.428G, in addition to the empty pSPL3 construct, containing only the SD- and SA-flanked endogenous exon and used as the positive control to assess the efficiency of the in vitro splicing process. Following RNA extraction and cDNA synthesis, PCR amplification of the transcripts was performed using primers that map to the vector (Figure 2A).
Figure 2. Evaluation of the splicing effect of the c.428A>G variant by gel electrophoresis following the pSPL3 minigene assay. (A) Cloning strategy of the genomic region in pSPL3 (the position of the c.428A>G variant is indicated by a red star). (B) Agarose gel shows the SD6-SA2 PCR products derived from HEK293T cells transfected with the empty pSPL3 vector (lane 1), the pSPL3-A (c.428A allele, lane 2) and pSPL3-G (c.428G allele, lane 3) constructs, in addition to untransfected cells (lane 4). The size of the PCR products is indicated on the right. Correct splicing of exon 2 generates a 445 bp fragment. (C) Quantification of the bands corresponding to the aberrant and correct splice products for each of the two alleles (A and G) is shown below.
Gel electrophoresis analysis of PCR products showed that, while no transcript could be detected by the cDNA analysis of the HEK293T not-transfected cells, the empty pSPL3 was able to correctly perform the splicing of the sequence lying between SD and SA, as demonstrated by the 257 bp product derived from the SD–SA joining fragment (Figure 2B). Moreover, while the presence of the wild-type c.428A allele allowed mostly correct splicing, generating the expected 445 bp transcript, the c.428G allele produced a larger transcript fragment in the absence of the wild-type fragment, thus suggesting the occurrence of an aberrant splicing. Quantification of gel electrophoresis bands of three different biological replicates confirmed that almost 80% of the pSPL3-A vector underwent correct splicing, with only 20% of the transcripts using a cryptic donor site; in contrast, in the presence of the pSPL3-G plasmid, more than 90% of the transcripts were generated using the cryptic donor site, and in less than 10% of the cases, the splicing process occurred correctly (Figure 2C). The sequencing analysis eventually confirmed that the presence of the c.428G allele caused the inactivation of the functional intron 2 splice donor site and the aberrant inclusion of part of the PHOX2B intron 2 into the final transcript (Figure 3).
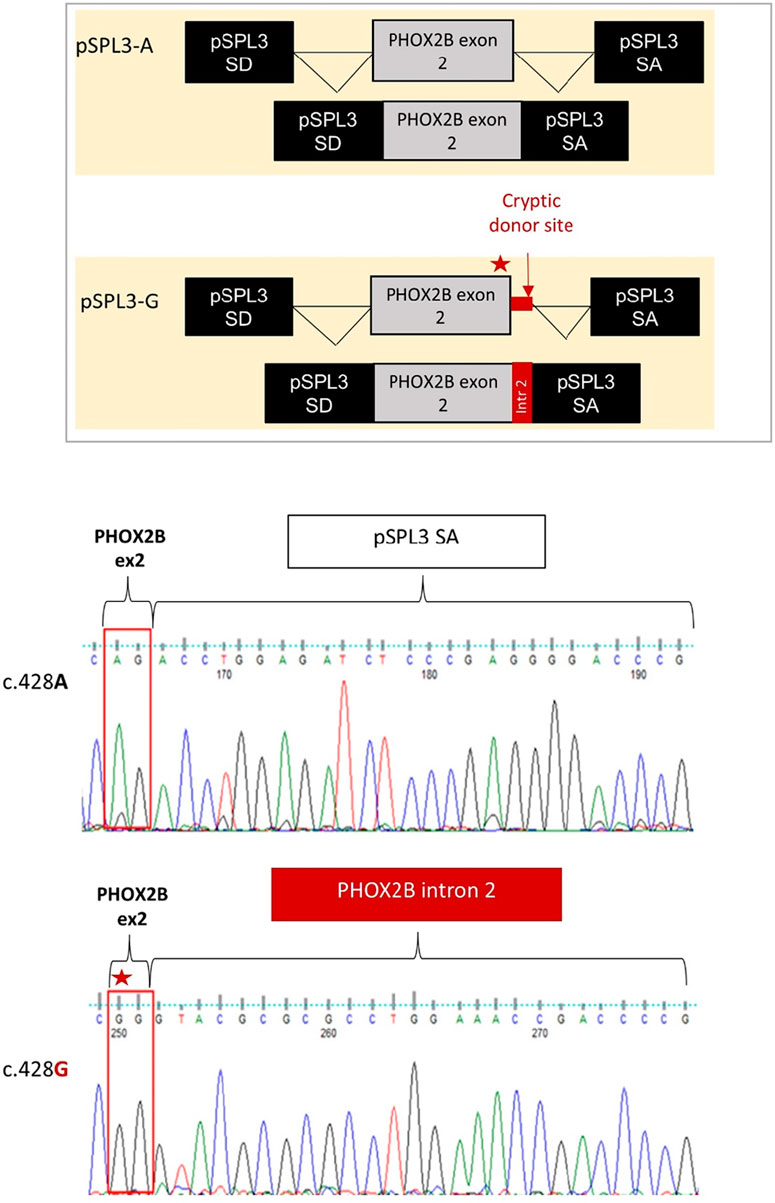
Figure 3. Evaluation of the splicing effect of the c.428A>G variant by Sanger sequencing following the pSPL3 minigene assay. From top to bottom it is schematized i) the correct joining between PHOX2B exon 2 and the downstream pSPL3 exon SA for the c.428A allele (pSPL3-A); ii) the aberrant splicing between PHOX2B exon 2 and the downstream pSPL3 intron, generated by the abolition of the canonical donor site with the subsequent activation of a criptic GT donor site within the intron; iii) the electropherogram of the splice fragment sequences showing the splicing products induced by both the c.428A and c.428G alleles in the pSPL3 minigene assay.
4 Discussion
In this work, we report for the first time the functional consequence of a PHOX2B c.428A>G (p.Q143R) variant, an NPARM found in two patients from our cohort, associated with syndromic CCHS co-occurring with intestinal dysfunctions. In particular, we show that the missense variant masked a splicing defect and its consequences.
In contrast to the vast majority of missense mutations affecting exon 2, which are associated with neural crest cell tumors (NCTs), such as neuroblastoma, either isolated or occurring with CCHS, the CCHS patients in this study showed no evidence of tumors. Furthermore, several CCHS patients carrying the same missense substitution c.428A>G with co-occurrence of only HSCR have already been reported (Table 2) (Trochet et al., 2005; Berry-Kravis et al., 2006; Nabila et al., 2016; Kasi et al., 2021; Balakrishnan et al., 2021), thus confirming that no NCT associated with this PHOX2B mutation has ever been found, with the exception of one benign ganglioneuroma that had to be resected (Balakrishnan et al., 2021).
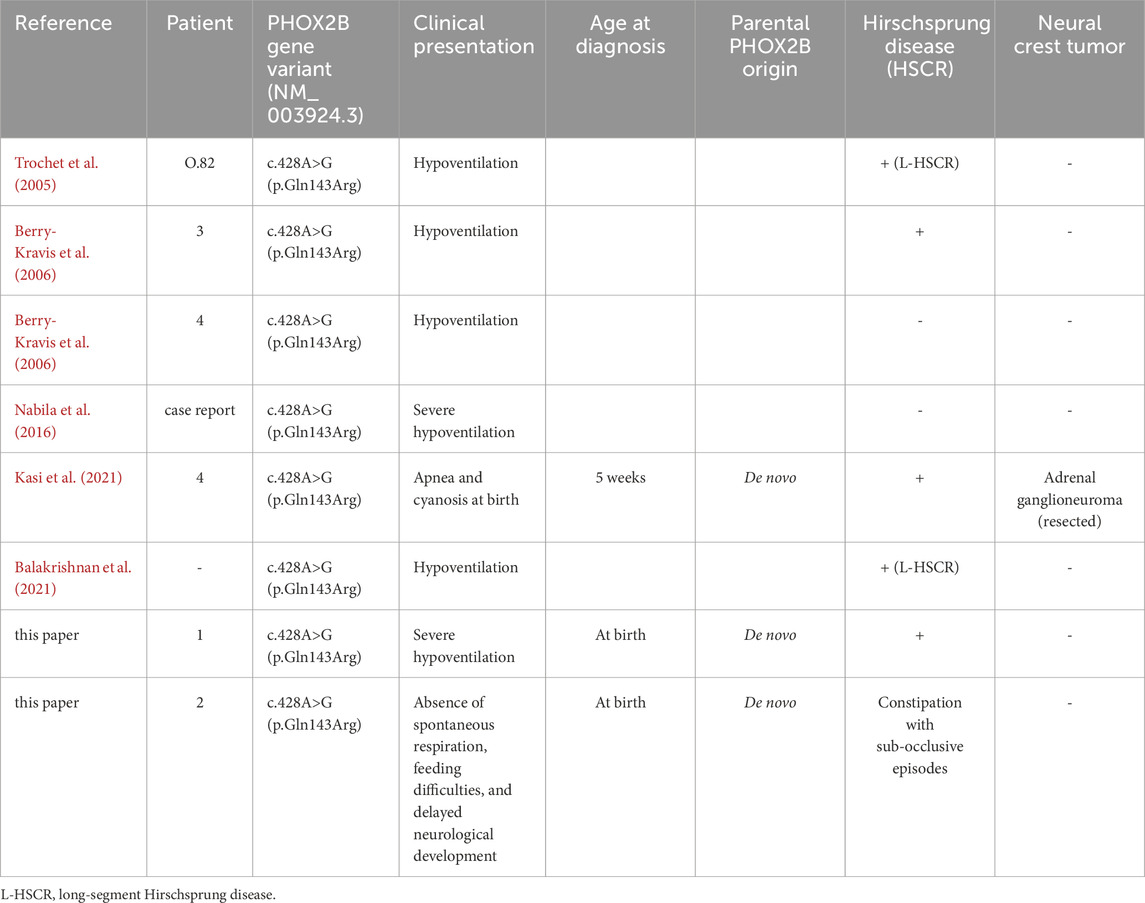
Table 2. Patients already reported to carry the c.428A>G PHOX2B variant with associated symptoms and references.
The hypothesis of an involvement of the c.428A>G variant in the splicing process was postulated when NPARMs were first associated with syndromic CCHS (Berry-Kravis et al., 2006), but it has never been demonstrated to date. Our results show that the c.428G allele, due to its proximity (−2) to the end of exon 2, can alter the splicing process, thus impairing the production of correct mutant mRNA. However, in contrast to what was postulated by Berry-Kravis et al. (2006), we suggest that the aberrant mRNA product may produce a hypomorphic protein, likely leading to a loss-of-function rather than a toxic gain-of-function effect. Unfortunately, given the unavailability of CCHS target tissues, we were unable to verify whether the same aberrant splicing also occurs in patient cells. Furthermore, we were unable to characterize the resulting abnormal protein in vitro or assess whether mRNA surveillance mechanisms could downregulate or suppress mutant mRNA levels.
Given the well-known role of RET haploinsufficiency in HSCR pathogenesis, caused by RET mutations with loss-of-function or downregulation of RET gene expression (Edery et al., 1994), and the role of PHOX2B in promoting and regulating RET expression, the latter can probably be compromised in the presence of PHOX2B mutations that are no longer able to transactivate the RET promoter (Pattyn et al., 1999; Bachetti et al., 2005b; Di Zanni et al., 2017), namely, in the presence of a hypomorphic PHOX2B protein.
Therefore, one could hypothesize that a loss-of-function mechanism due to reduced function/expression, rather than a gain-of-function mechanism, underlies the PHOX2B NPARMs effects in the absence of NCC-derived tumors. This is in agreement with a recent report of a three-generational familial case in which a mutation in intron 1, identified in three patients with CCHS + HSCR, was predicted to alter the splicing process (Pace et al., 2020). Although authors claim the possibility that, similarly to nonsense mutations in exon 1, a cryptic translational start codon is used (Cain et al., 2017), they suggest that production of a hypomorphic protein, rather than haploinsufficiency due to nonsense-mediated decay, may occur. However, since they do not analyze the consequence of such a splicing variant in intron 1 with a minigene, they cannot exclude that nonsense-mediated decay may partially eliminate the mutant gene product.
It is interesting to note that the variant c.422G>A (p.R141Q) is located within the sequence coding for the homeodomain at position (−8) with respect to the last nucleotide of exon 2; therefore, it is not close enough to be predicted by the software analysis to interfere with splicing, and it has been found in several patients suffering from syndromic CCHS, including neuroblastoma (Trochet et al., 2005; Lombardo et al., 2018). This observation seems to support the hypothesis of different pathogenetic mechanisms underlying NB occurrence in syndromic CCHS.
The position of the c.428A>G variant and the results presented in this study, suggesting an FS mutation masked by MS, are in accordance with the reported “regional” distribution of FS mutations underlying CCHS + HSCR or CCHS + HSCR + NCC tumors. We have already observed that CCHS + HSCR alone mostly associates with changes in the reading frame arising upstream of the polyAla region, while the triple phenotype is predominantly associated with variants affecting the polyAla region (CCHS + HSCR ± NB) and is almost exclusively associated with variants located downstream of the polyAla tract (CCHS + HSCR + NB) (Di Lascio et al., 2018), with an NB-promoting gradient in this distal region (Figure 4A).
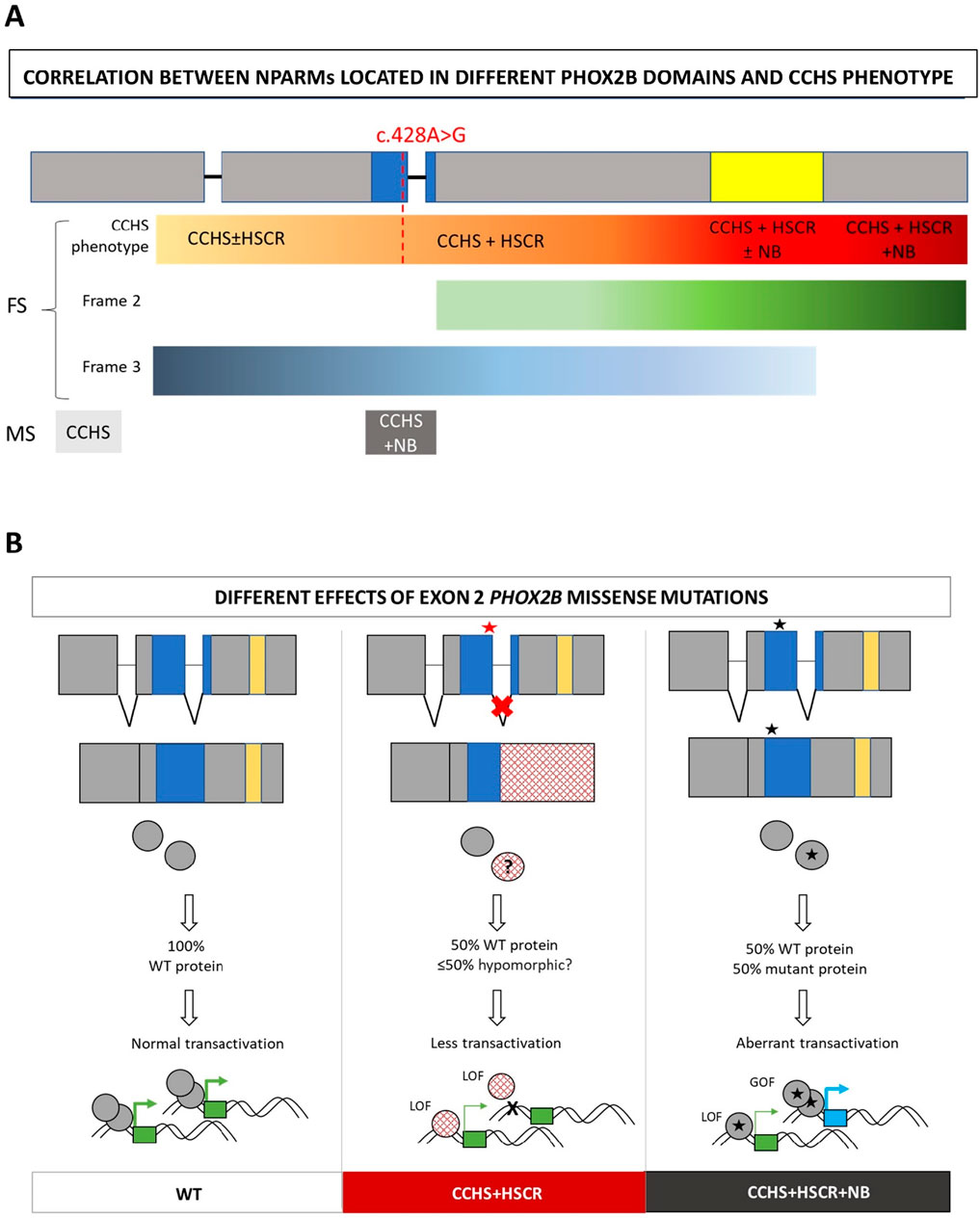
Figure 4. Hypothesis on the mechanism of action of splicing mutations in syndromic CCHS. (A) Distribution along the gene domains of frameshift (FS) and missense (MS) PHOX2B variants in association with the different phenotypes. The symbol ± indicates whether or not you are likely to have the phenotype. The intensity of the color from yellow to dark red indicate the increasing severity of CCHS phenotypes, starting from isolated CCHS to syndromic with different degrees of severity. The red line correspond to the location of the c.428A>G mutation and its corresponding phenotype in the hypothesis of a frameshift effect of the splicing defect. Moreover, the intensity of the green (frame 2) and blue (frame 3) bars correlates with the distribution of FS with frame 2 and frame 3, respectively. For missense (MS), isolated CCHS and CCHS + NB are indicated in light and dark grey, respectively. (B) From left to right: correct splicing of exon 2, aberrant splicing of exon 2 mediated by MS variants in CCHS+HSCR, correct splicing of exon 2 in the presence of MS variants inducing CCHS + HSCR + NB. The molecular consequences of the three cases are shown. Red star: splicing defect induced by MS mutation; Black star: no splicing defect induced by MS mutation. Blue box: homeodomain; yellow box: polyalanine region. LOF: loss-of-function; GOF: gain-of-function. Squared circles (mid panel): disrupted mutant protein due to aberrant splicing, likely reduced with respect to the WT due to quality control systems (nonsense mediated decay for mRNA or ribosome quality control for elongated mutant peptides); star circles (right panel): full length mutant protein carrying the MS mutation. The possible effects of the WT and mutant products on target gene transactivation are also shown. Green boxes: target genes regularly transactivated by the WT; blue box: aberrantly acquired target gene; thick arrows: active promoter transactivation; thin arrows: lower promoter transactivation.
However, because we were unable to characterize the fragments resulting from aberrant splicing of the PHOX2B c.428G allele in terms of protein production, elongation (frame 2), or truncation (frame 3), we cannot provide a definitive conclusion regarding the consequences of this PHOX2B variant. Due to its location in the penultimate exon, we were unable to include exon 3 in the cassette cloned in the pSPL3 minigene, as it is not flanked at the 3′ end by an intronic sequence that would have allowed the evaluation of splicing between exon 2 and the pSPL3 acceptor site. Nonetheless, we cannot rule out partial no-stop-mediated mRNA decay in the case of “frame 2,” resulting in protein elongation and the subsequent degradation of the mutant mRNA allele.
This would be consistent with no known associations with NCC-derived tumors in patients carrying PHOX2B variants associated with the activation of mRNA surveillance (Zhou et al., 2021).
However, we cannot know a priori whether the mRNA of the mutant allele will be partially or completely degraded or, alternatively, fully produced in case the quality control is completely bypassed, nor in what type of translational context this might occur. We have summarized these hypotheses in a table showing the possible consequences of different classes of mutations of the PHOX2B protein (Table 3).
In any case, regardless of the modality and extent of this quality control, the defective splicing observed in association with the present PHOX2B mutation partially affects the homeodomain encoded between exons 2 and 3. This could have consequences in terms of the transactivation efficiency of target genes and, ultimately, could strengthen the hypothesis of a loss-of-function (LOF) mechanism attributed to this mutation (Figure 4B).
Furthermore, different mechanisms underlying the co-occurrence or non-occurrence of neural crest tumors with CCHS and HSCR still require additional experimental confirmation. In contrast to the missense mutation in exon 2 not close to exon–intron boundaries, which are mostly associated with neural crest tumors, many FS mutations in the last exon 3 are associated with the CCHS + HSCR phenotype, probably because the aberrant hypomorphic protein can be produced when the degradation mediated by mRNA surveillance is partially or completely escaped (Figure 4B). Conversely, the incidence of neural crest-derived tumors increases with the presence of FS mutations toward the end of the gene (Figure 5) (Di Lascio et al., 2018), although the underlying mechanism remains unclear.
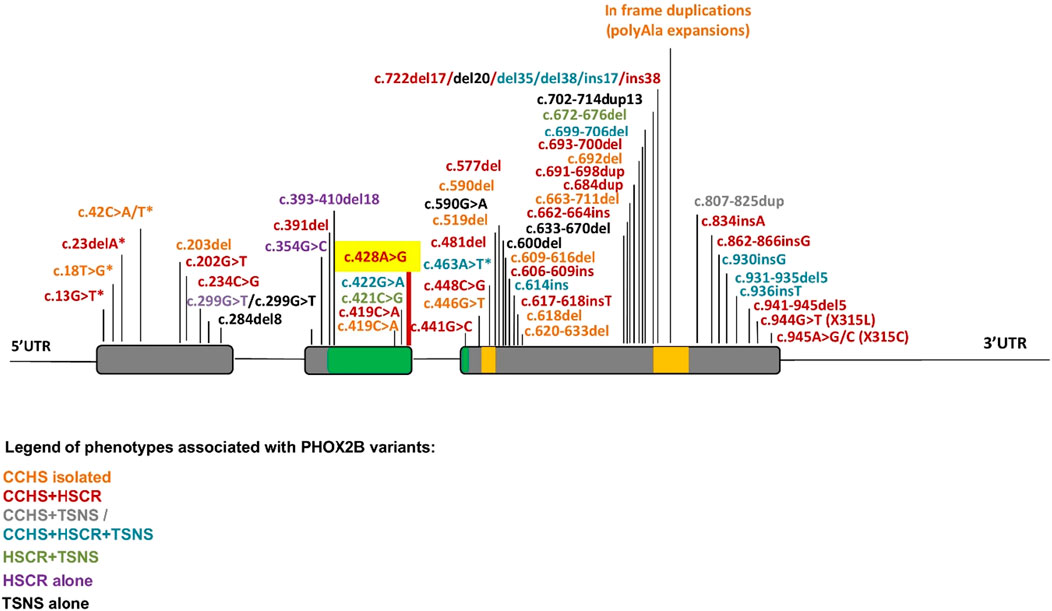
Figure 5. Summary of PHOX2B variants and associated phenotypes. The location of mutations along the PHOX2B gene identified in CCHS, HSCR, and neural crest-derived tumors (NCTs), and their possible combinations, are indicated by a color scale. Asterisks indicate a MS mutation leading to a premature stop codon. Gray: exons; green: homeodomain; yellow: 9 and 20 polyalanine domains.
5 Conclusion
In conclusion, we propose that when a missense mutation in the homeodomain does not affect splicing, the PHOX2B mutant protein can be fully produced, thus being competent to exert its pathogenic, gain-of-function effects, which are probably ascribable to downstream aberrant transcriptional regulation and/or modified interactomes. On the other hand, when the splicing mechanism is compromised, another pathogenic mechanism, likely producing an aberrant hypomorphic protein, could account for the sole HSCR co-occurrence. Further studies are required to address these unresolved questions.
Data availability statement
The full data set presented in this article is not immediately available because much of the data does not address the purpose for which it was generated, namely, obtaining a molecular diagnosis at the PHOX2B locus. Requests for access to the data set should be directed to the corresponding author, Isabella Ceccherini.
Ethics statement
These studies involving humans were approved by Comitato di Etica per la Ricerca Scientifica e Bioetica e per la Sperimentazione Clinica of the Istituto Giannina Gaslini, Genova, Italy. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for molecular PHOX2B testing was provided by the participants’ legal guardians/next of kin.
Author contributions
TB: formal analysis, writing – original draft, data curation, writing – review and editing, conceptualization, methodology, and investigation. SB: writing – review and editing, investigation, validation, and formal analysis. GS: resources, validation, and writing – review and editing. MB: writing – review and editing and data curation. FM: writing – review and editing and data curation. ICe: data curation and writing – review and editing. LS: data curation and writing – review and editing. FC: data curation and writing – review and editing. FM: writing – review and editing and data curation. ICa: project administration, writing – review and editing, conceptualization, and supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Italian Ministry of Health through both “Ricerca Corrente” n. 2776400 to I.C. and “5x mille” (5M-2018-23680426 TO ICe) and “Ricerca Corrente” FA706 to TB.
Acknowledgments
The authors are grateful to the family association “Associazione Italiana per la Syndrome da Ipoventilazione Centrale Congenita” (AISICC) for their support and encouragement. Dr. Francesca Menni is a member of the European Reference Network for Hereditary Metabolic Disorders (MetabERN).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Africano C., Bachetti T., Uva P., Pitollat G., Del Zotto G., Giacopelli F., et al. (2024). Identification of a histone deacetylase inhibitor as a therapeutic candidate for congenital central hypoventilation syndrome. Mol. Ther. Nucleic Acids 35 (4), 102319. doi:10.1016/j.omtn.2024.102319
Bachetti T., Ceccherini I. (2020). Causative and common PHOX2B variants define a broad phenotypic spectrum. Clin. Genet. 97, 103–113. doi:10.1111/cge.13633
Bachetti T., Matera I., Borghini S., Di Duca M., Ravazzolo R., Ceccherini I. (2005a). Distinct pathogenetic mechanisms for PHOX2B associated polyalanine expansions and frameshift mutations in congenital central hypoventilation syndrome. Hum. Mol. Genet. 14 (13), 1815–1824. doi:10.1093/hmg/ddi188
Bachetti T., Borghini S., Ravazzolo R., Ceccherini I. (2005b). An in vitro approach to test the possible role of candidate factors in the transcriptional regulation of the RET proto-oncogene. Gene Expr. 12 (3), 137–149. doi:10.3727/000000005783992106
Balakrishnan K., Perez I. A., Keens T. G., Sicolo A., Punati J., Danialifar T. (2021). Hirschsprung disease and other gastrointestinal motility disorders in patients with CCHS. Eur. J. Pediatr. 180 (2), 469–473. doi:10.1007/s00431-020-03848-5
Berry-Kravis E. M., Zhou L., Rand C. M., Weese-Mayer D. E. (2006). Congenital central hypoventilation syndrome: PHOX2B mutations and phenotype. Am. J. Respir. Crit. Care Med. 174, 1139–1144. doi:10.1164/rccm.200602-305OC
Cain J. T., Kim D. I., Quast M., Shivega W. G., Patrick R. J., Moser C., et al. (2017). Nonsense pathogenic variants in exon1 of PHOX2B lead to translational reinitiation in congenital central hypoventilation syndrome. Am. J. Med. Genet. A 173, 1200–1207. doi:10.1002/ajmg.a.38162
Di Lascio S., Benfante R., Di Zanni E., Cardani S., Adamo A., Fornasari D., et al. (2018). Structural and functional differences in PHOX2B frameshift mutations underlie isolated or syndromic congenital central hypoventilation syndrome. Hum. Mutat. 39, 219–236. doi:10.1002/humu.23365
Di Rocco M., Rusmini M., Caroli F., Madeo A., Bertamino M., Marre-Brunenghi G., et al. (2018). Novel spondyloepimetaphyseal dysplasia due to UFSP2 gene mutation. Clin. Genet. 93, 671–674. doi:10.1111/cge.13134
Di Zanni E., Bachetti T., Parodi S., Bocca P., Prigione I., Di Lascio S., et al. (2012). In vitro drug treatments reduce the deleterious effects of aggregates containing polyAla expanded PHOX2B proteins. Neurobiol. Dis. 45 (1), 508–518. doi:10.1016/j.nbd.2011.09.007
Di Zanni E., Adamo A., Belligni E., Lerone M., Martucciello G., Mattioli G., et al. (2017). Common PHOX2B poly-alanine contractions impair RET gene transcription, predisposing to hirschsprung disease. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 1770–1777. doi:10.1016/j.bbadis.2017.04.017
Edery P., Lyonnet S., Mulligan L. M., Pelet A., Dow E., Abel L., et al. (1994). Mutations of the RET proto-oncogene in hirschsprung's disease. Nature 367, 378–380. doi:10.1038/367378a0
Hernandez-Miranda L. R., Ibrahim D. M., Ruffault P. L., Larrosa M., Balueva K., Müller T., et al. (2018). Mutation in LBX1/Lbx1 precludes transcription factor cooperativity and causes congenital hypoventilation in humans and mice. Proc. Natl. Acad. Sci. U. S. A. 115 (51), 13021–13026. doi:10.1073/pnas.1813520115
Kasi A. S., Li H., Jurgensen T. J., Guglani L., Keens T. G., Perez I. A. (2021). Variable phenotypes in congenital central hypoventilation syndrome with PHOX2B nonpolyalanine repeat mutations. J. Clin. Sleep. Med. 17 (10), 2049–2055. doi:10.5664/jcsm.9370
Lombardo R. C., Porollo A., Cnota J. F., Hopkin R. J. (2018). Congenital heart disease and aortic arch variants associated with mutation in PHOX2B. Genet. Med. 20, 1538–1543. doi:10.1038/gim.2018.34
Matera I., Bachetti T., Puppo F., Di Duca M., Morandi F., Casiraghi G. M., et al. (2004). PHOX2B mutations and polyalanine expansions correlate with the severity of the respiratory phenotype and associated symptoms in both congenital and late onset central hypoventilation syndrome. J. Med. Genet. 41, 373–380. doi:10.1136/jmg.2003.015412
Nabila M., Farah J., Jamal F., Khalil M., Khalid A., Wafaa B., et al. (2016). First application of the exome sequencing in Morocco in a case of congenital central hypoventilation syndrome with a mutation of the PHOX2B gene. Int J Eng and Sci. Res 4 (4), 108–114.
Nagashimada M., Ohta H., Li C., Nakao K., Uesaka T., Brunet J.-F., et al. (2012). Autonomic neurocristopathy-associated mutations in PHOX2B dysregulate Sox10 expression. J. Clin. Invest. 122, 3145–3158. doi:10.1172/JCI63401
Pace N. P., Pace Bardon M., Borg I. (2020). A Respiratory/hirschsprung phenotype in a three-generation family associated with a novel pathogenic PHOX2B splice donor mutation. Mol. Genet. Genomic Med. 8 (12), e1528. doi:10.1002/mgg3.1528
Pattyn A., Morin X., Cremer H., Goridis C., Brunet J.-F. (1999). The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature 399, 366–370. doi:10.1038/20700
Ponzoni M., Bachetti T., Corrias M. V., Brignole C., Pastorino F., Calarco E., et al. (2022). Recent advances in the developmental origin of neuroblastoma: an overview. J. Exp. Clin. Cancer Res. 41, 92. doi:10.1186/s13046-022-02281-w
Sahashi K., Masuda A., Matsuura T., Shinmi J., Zhang Z., Takeshima Y., et al. (2007). In vitro and in silico analysis reveals an efficient algorithm to predict the splicing consequences of mutations at the 5' splice sites. Nucleic Acids Res. 35, 5995–6003. doi:10.1093/nar/gkm647
Sibley C. R., Blazquez L., Ule J. (2016). Lessons from non-canonical splicing. Nat. Rev. Genet. 17, 407–421. doi:10.1038/nrg.2016.46
Spielmann M., Hernandez-Miranda L. R., Ceccherini I., Weese-Mayer D. E., Kragesteen B. K., Harabula I., et al. (2017). Mutations in MYO1H cause a recessive form of central hypoventilation with autonomic dysfunction. J. Med. Genet. 54 (11), 754–761. doi:10.1136/jmedgenet-2017-104765
Trang H., Samuels M., Ceccherini I., Frerick M., Garcia-Teresa M. A., Peters J., et al. (2020). Guidelines for diagnosis and management of congenital central hypoventilation syndrome. Orphanet J. Rare Dis. 15 (1), 252. doi:10.1186/s13023-020-01460-2
Trochet D., O'Brien L. M., Gozal D., Trang H., Nordenskjöld A., Laudier B., et al. (2005). PHOX2B genotype allows for prediction of tumor risk in congenital central hypoventilation syndrome. Am. J. Hum. Genet. 76, 421–426. doi:10.1086/428366
Wang M., Marin A. (2006). Characterization and prediction of alternative splice sites. Gene 366, 219–227. doi:10.1016/j.gene.2005.07.015
Zhou A., Rand C. M., Hockney S. M., Niewijk G., Reineke P., Speare V., et al. (2021). Paired-like homeobox gene (PHOX2B) nonpolyalanine repeat expansion mutations (NPARMs): genotype-phenotype correlation in congenital central hypoventilation syndrome (CCHS). Genet. Med. 23 (9), 1656–1663. doi:10.1038/s41436-021-01178-x
Keywords: paired-like homeobox 2b, transcript splicing, Hirschsprung disease, neural crest-derived tumors, loss-of-function
Citation: Bachetti T, Bagnasco S, Santamaria G, Bedeschi MF, Menni F, Catalano I, Spaccini L, Cavigioli F, Morandi F and Ceccherini I (2025) The PHOX2B c.428A>G missense variant affects post-transcriptional regulation and may explain the absence of neural crest-derived tumors in congenital central hypoventilation syndrome. Front. Physiol. 16:1616994. doi: 10.3389/fphys.2025.1616994
Received: 23 April 2025; Accepted: 22 July 2025;
Published: 22 September 2025.
Edited by:
Luis R. Hernandez-Miranda, Charité University Medicine Berlin, GermanyReviewed by:
Xiongwei Wu, The Affiliated Hospital of Qingdao University, ChinaYiling Xia, Charité University Medicine Berlin, Germany
Copyright © 2025 Bachetti, Bagnasco, Santamaria, Bedeschi, Menni, Catalano, Spaccini, Cavigioli, Morandi and Ceccherini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Isabella Ceccherini, aXNhYmVsbGFjZWNjaGVyaW5pQGdhc2xpbmkub3Jn
 Tiziana Bachetti
Tiziana Bachetti Simona Bagnasco
Simona Bagnasco Giuseppe Santamaria2
Giuseppe Santamaria2 Francesca Menni
Francesca Menni Isabella Ceccherini
Isabella Ceccherini