- 1Department of Respiratory and Critical Care Medicine, Affiliated Cixi Hospital, Wenzhou Medical University, Ningbo, Zhejiang, China
- 2Department of Respiratory and Critical Care Medicine, Affiliated People’s Hospital, Ningbo University, Ningbo, Zhejiang, China
The TCA cycle is a central pathway for oxidative phosphorylation in eukaryotic mitochondria, plays a role in energy production and biosynthesis, and can alter cellular function in a variety of ways. As our understanding of the TCA cycle has improved, there is increasing evidence that it is inextricably linked to lung disease. This article summarizes the relationship between the TCA cycle and different types of lung disease and focuses on the specific mechanisms by which the TCA cycle and its intermediate metabolites influence lung disease progression.
1 Introduction
Mitochondria play a pivotal role in eukaryotes, facilitating cellular energy production and biosynthesis; they regulate the energy metabolism essential for cell growth, primarily through the tricarboxylic acid cycle (TCA cycle) and oxidative phosphorylation (Koranteng et al., 2024). In the cytoplasm, glucose is converted to pyruvate through glycolysis. In the presence of sufficient oxygen, pyruvate enters the mitochondria and is completely oxidized to carbon dioxide and water through the TCA cycle, generating high-energy electrons and intermediary metabolites of the TCA cycle (Wu et al., 2024).
The TCA cycle and its metabolic intermediates are involved in a variety of key biological processes and can influence biological pathophysiology by regulating NADH/NADPH homeostasis, scavenging reactive oxygen species (ROS), and generating ATP through phosphorylation at the substrate level (MacLean et al., 2023). Further studies have indicated that the TCA cycle may play a role in the progression of lung diseases. This article provides a review of the role of the TCA cycle in the development of lung diseases and the related mechanisms, with the goal of providing new insights into the diagnosis and treatment of lung diseases.
1.1 The TCA cycle and its intermediate metabolites
The TCA cycle, also referred to as the citric acid cycle, commences with the incorporation of acetyl coenzyme A (acetyl-CoA) into the cycle via glycolysis, fatty acid beta-oxidation, or amino acid metabolism; this represents the principal mechanism through which mitochondrial energy is produced (Wu et al., 2024). Acetyl-CoA enters the circulation and combines with oxaloacetate to form citric acid in the presence of citrate synthase. The conversion of citric acid to its isomer isocitrate is subsequently catalyzed by cis-aconitase. The cycle continues with two oxidative decarboxylation reactions in which isocitrate is converted into five-carbon α-ketoglutarate (α-KG) and subsequently into four-carbon succinyl coenzyme A with the release of two molecules of CO2 and the generation of two NADH molecules. Subsequently, succinyl-CoA hydrolyses the thioester bond to produce succinate by thiokinase, releasing GTP to produce ATP. In the subsequent phase, succinate is oxidized to fumarate by succinate dehydrogenase (SDH). Succinate oxidation leads to FADH2 reduction in SDHA, which transfers electrons to iron sulfur clusters in SDHB, this electrons will then travel across electron transport chain (ETC.). Following the conversion of fumarate to malic acid, the latter subsequently undergoes further dehydrogenation, resulting in the production of NADPH and oxaloacetic acid, which then enter the tricarboxylic acid cycle once more. The TCA cycle is a metabolic pathway that not only generates energy and electron carriers (such as NADH2 AND FADH2) but also produces a variety of intermediate metabolites. These metabolites play a role in controlling the chromatin modification of proteins, DNA methylation, and posttranslational modification, which can alter cellular function (Martínez-Reyes and Chandel, 2020). The TCA cycle metabolites that influence cellular function include citrate, α-KG, succinate, fumarate, and itaconate.
Citrate plays a role in protein acetylation and fatty acid synthesis on the one hand and in regulating energy metabolism on the other hand (Icard et al., 2023). Mutations in SDH result in the accumulation of succinate, a compound commonly found in a variety of cancers. Consequently, succinate is regarded as a cancer metabolite (Dalla Pozza et al., 2020). Furthermore, succinate plays a pivotal role in the regulation of innate immunity. Metabolic analyses of LPS-treated macrophages have revealed a considerable abundance of succinate within these cells (Mills et al., 2016). α-KG is generated from isocitrate via the action of isocitrate dehydrogenase and can serve as substrate for glutamine synthesis, forming a crucial link between the TCA cycle and glutamine metabolism (Park et al., 2023). Furthermore, α-KG serves as a pivotal regulator of the hypoxic response (Tennant et al., 2009) and plays a significant role in the inflammatory response (Liu et al., 2017). Fumarates promote tumor growth through multiple signal transduction pathways and act as immunomodulators, exerting their effects by controlling chromatin modifications (Martínez-Reyes and Chandel, 2020). Itaconate is derived from the decarboxylation of cis-aconitic acid and serves as an important immunomodulator and antimicrobial agent (Bambouskova et al., 2018). Itaconate activates the downstream pathway of the antioxidant transcription factor NRF2, thereby contributing to its anti-inflammatory properties in activated macrophages (Mills et al., 2018).
In lung disease, The TCA cycle metabolites that influence lung disease include citrate, α-KG, succinate, fumarate, and itaconate. The present paper sets out the findings that elevated levels of succinate, fumarate and malate have been detected in patients suffering from chronic obstructive pulmonary disease (COPD), tuberculosis (TB) and lung cancer (LC) (Pinto-Plata et al., 2019; Collins et al., 2021; Alosaimi et al., 2024) It has been observed that levels of fumarate and malate decrease in cases of pulmonary hypertension, while citrate and succinate levels increase (Sakao et al., 2021; Zhao et al., 2014). The detailed exposition of the specific impact mechanisms under consideration will be provided in the following section.
1.2 The TCA cycle and chronic inflammatory airway disease
Chronic inflammatory airway disease is defined as a chronic condition in which there is inflammation of the upper and/or lower airways. The hallmark symptoms of this disease are airway inflammation, obstruction and remodeling. The most common forms of chronic inflammatory airway disease are asthma and COPD (Mannion et al., 2023).
1.2.1 The influence between the TCA cycle and bronchial asthma
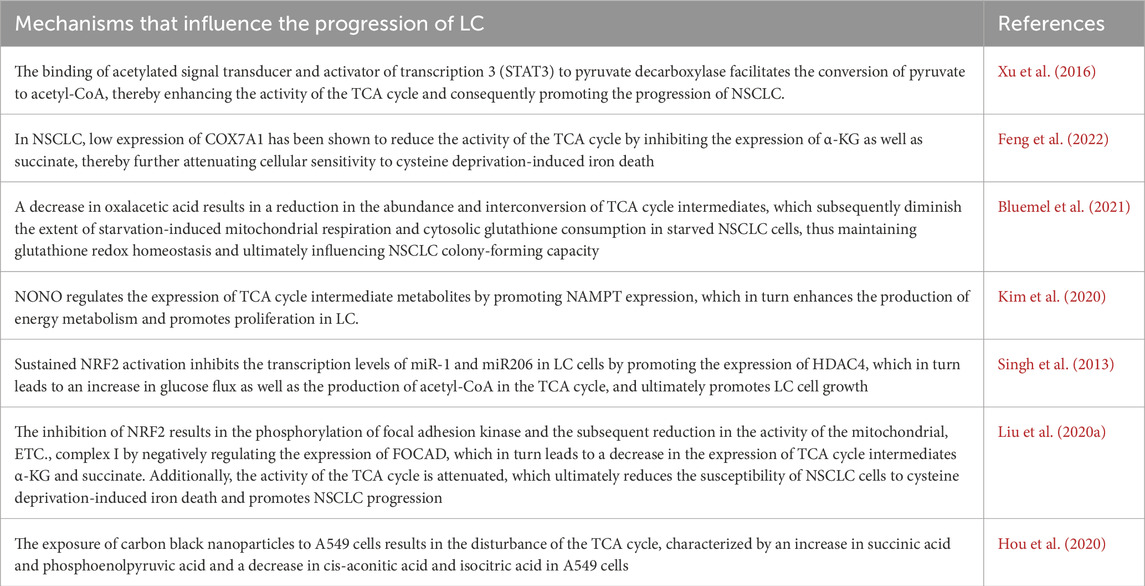
Bronchial asthma is a chronic respiratory disease that is characterized by four main features: airway inflammation, bronchial hyperresponsiveness, increased mucus production and varying degrees of airflow limitation (Barosova et al., 2023). On the basis of the presence or absence of eosinophil recruitment, bronchial asthma can be classified into two main categories: a type-2 (T2 or Th2)-high endotype with a type 2 immune response and eosinophil recruitment and a type-2 (T2 or Th2) endotype with activation-mediated activation of Th1 and/or Th17 cells, which is considered a low or nontype-2 endotype (Kuruvilla et al., 2019). Research has indicated a potential correlation between bronchial asthma and the TCA cycle. Metabolomic analyses of ovalbumin (OVA)-induced asthmatic mice have demonstrated that organic acid metabolism is altered in these mice compared with that observed in control mice (Seo et al., 2017). Furthermore, metabolomic analysis of urine from children with asthma revealed a potential correlation between the TCA cycle and the pathogenesis of asthma (Li S. et al., 2020). Further mechanistic studies have demonstrated that elevated expression of isocitrate dehydrogenase (IDH) results in the increased expression of α-KG within the TCA cycle, which in turn facilitates the activation of ten-eleven translocation (TET) enzymes. The activation of TET enzymes has been shown to induce the hypomethylation of specific genes, including transforming growth factor-β2 (TGF-β2) and type III collagen (COL3A), which are characteristic of smooth muscle cells in the airways of individuals with asthma (Huang, 2020). Furthermore, it has been demonstrated that house dust mites (HDMs) alone or STING pathway stimulation can progressively activate the expression of immune responsive gene 1 (IRG1) in dendritic cells via the mitochondrial superoxide-dependent pathway. Additionally, IRG1 has been shown to catalyze the production of the TCA cycle intermediate itaconate. The IRG1/itaconate pathway, on the one hand, attenuates the allergen uptake and antigen presentation capacity of DCs to CD4+ T cells; on the other hand, it can promote the expression of superoxide dismutase 1 (superoxide radical scavenger) and inhibit the expression of NADPH oxidase 1, a superoxide-generating oxidase, through redox metabolism, thus reducing the production of mitochondrial superoxide. In a previous study, exogenous itaconate (4-octyl itaconate) was administered via the intrapulmonary route, and 4-OI attenuated the features of allergic asthma manifestations, including HDM sensitization, Th2-mediated eosinophilic airway inflammation, and mucosal cellular hyperplasia (Jaiswal et al., 2022) (Figure 1).
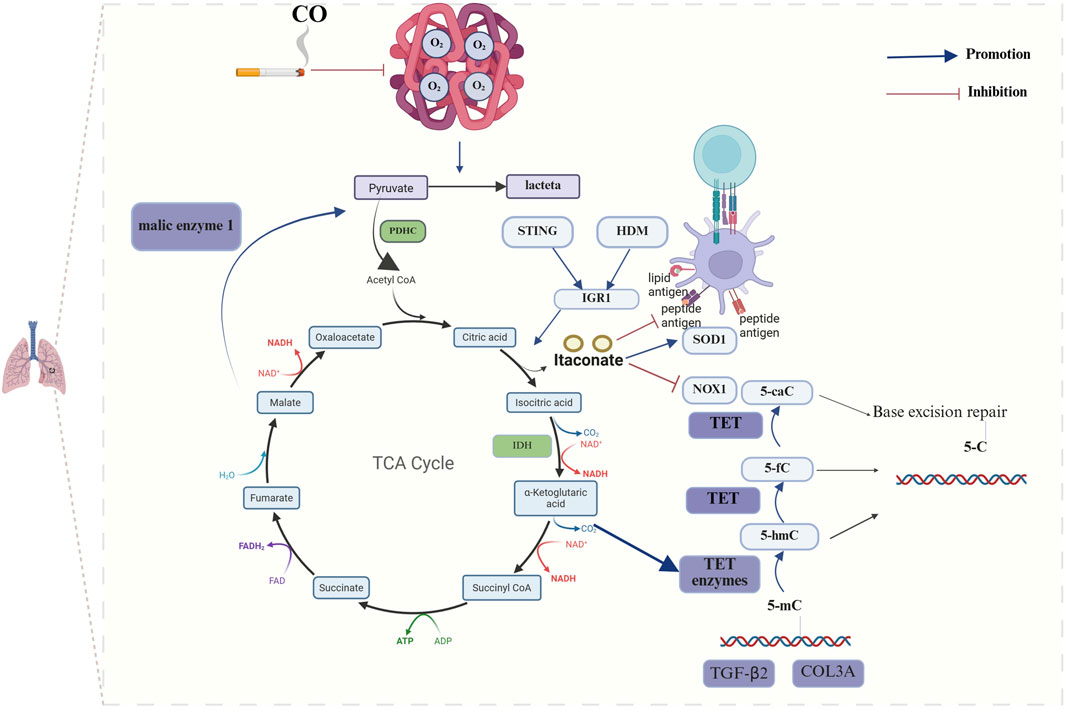
Figure 1. Mechanisms of TCA cycle metabolites in chronic airway inflammatory diseases. The development of bronchial asthma significantly influences disease progression, primarily through its impact on the abundance of itaconic acid and α-KG within the TCA cycle. Elevated expression of IDH results in the increased expression of α-KG, which in turn facilitates the activation of TET enzymes and ultimately induces the hypomethylation of specific genes, including TGF-β2 and COL3A. HDMs or STING pathway stimulation catalyzes the production of the itaconate by inducing the expression of IRG1. The IRG1/itaconate pathway decreases the allergen uptake and antigen presentation capacity of DCs to CD4+ T cells and influences the expression of SOD1 and NOX1. The progression of COPD is significantly influenced by the interplay between pyruvate and oxidative stress. CO reduces the oxygen-binding capacity of hemoglobin, which leads to a reduction in the overall activity of aerobic pathways and an increase in anaerobic glycolysis activity. (The blue arrows represent promotion, and the red arrows represent inhibition; created using BioRender.com).
1.2.2 The influence between the TCA cycle and COPD
COPD is characterized by chronic bronchitis, mucosal ciliary dysfunction, emphysema and a progressive decline in lung function. Tobacco smoke exposure represents the primary environmental risk factor for the development of COPD. However, bacterial and viral infections frequently lead to acute exacerbations (Mammen and Sethi, 2016; Schleich et al., 2023). Research has demonstrated that the activation of the TCA cycle results in increased ATP production and that elevated extracellular ATP levels within the airway lumen are linked to the pathogenesis of COPD (Naz et al., 2017). Furthermore, the TCA cycle intermediates have been shown to influence the survival of COPD patients. Plasma metabolomics sequencing data from COPD patients suggests that α-KG, succinate, fumarate, and malate are significantly higher in patients who succumb to COPD than in those who survive the disease (Pinto-Plata et al., 2019). To gain deeper insight into the interrelationship between the TCA cycle and COPD, researchers have conducted further mechanistic studies and discovered, through RNA sequencing, that the expression of malic enzyme 1 (ME1) transcripts is downregulated in alveolar macrophages (AMs) of COPD patients compared with healthy donors. ME1 catalyzes the reversible oxidative decarboxylation of malate to pyruvate, thereby replenishing TCA cycle intermediates and converting NADP + to NADPH. Conversely, ME1 deletion results in a lower oxygen consumption rate (OCR)/extracellular acidification rate (ECAR) ratio and a reduction in TCA cycle intermediates (Ryan et al., 2023). Smoking represents a significant environmental factor in the development of COPD. Carbon monoxide in smoke significantly reduces the oxygen-binding capacity of hemoglobin. Additionally, lung parenchymal injury due to oxidative stress and a chronic inflammatory response cause respiratory and locomotor muscles to atrophy to varying degrees, which reduces the capacity for oxygen uptake. Compared with that in healthy controls, the combination of these two factors results in a hypoxic state in individuals with COPD who smoke; this leads to a reduction in the overall level of aerobic pathways and an increase in anaerobic glycolysis, which in turn results in dysregulation of the TCA cycle. The degree of this dysregulation is correlated with the severity of the disease (Xue et al., 2021) (Figure 1).
The literature indicates that the TCA cycle plays a significant role in the progression and treatment of chronic airway inflammatory diseases, including bronchial asthma and COPD. However, few studies have explored the mechanisms through which the TCA cycle affects chronic airway inflammatory diseases. Therefore, further studies focusing on the mechanism of the TCA cycle in chronic airway inflammatory diseases are needed to provide new insights and ideas for the treatment of such diseases.
1.3 The TCA cycle and infectious diseases of the lungs
Lung infections represent a significant global health burden, with considerable impacts on both lung health and the exacerbation of other chronic inflammatory airway diseases, including asthma and COPD. Additionally, they can lead to the development of other systemic diseases, such as cardiovascular diseases. Both bacterial and viral result in a wide range of lung infections, varying in severity (Hambo and Harb, 2023; Fitzpatrick and Busse, 2022). Lung infections progress through distinct stages. Upon the establishment of an infectious focus, bacteria undergo metabolic alterations and virulence regulation to increase their ability to survive in the nutrient-limited and oxidative environment of the airways (Tomlinson et al., 2021).
1.3.1 The influence between the TCA cycle and bacterial infection
The TCA cycle is a crucial metabolic pathway in mitochondria. Previous studies have investigated the metabolomic profile of urine from mice infected with Streptococcus pneumoniae, a bacterium that commonly causes lung infections. These studies revealed a notable reduction in TCA cycle intermediates in the urine of mice treated with S. pneumoniae (Slupsky et al., 2009). Additionally, TCA cycle remodeling was identified in patients with both active and untreated TB; this was characterized by elevated plasma concentrations of the TCA cycle intermediates succinate, fumarate and malate and reduced concentrations of itaconate. Furthermore, this effect was more pronounced in patients with multidrug-resistant TB. However, the administration of appropriate antituberculosis treatment was observed to reverse this proinflammatory response to a certain extent. Further mechanistic studies have demonstrated that TCA cycle remodeling is a key driver of inflammation, whereby the upregulation of IL-1β expression and downregulation of granulocyte–macrophage colony-stimulating factor (GM-CSF) expression facilitate the production of proinflammatory arachidonic acid (Collins et al., 2021). Furthermore, in a mouse model of sepsis, the polarization of pulmonary M1-type macrophages is accompanied by a reduction in PRDX3 expression (Huang et al., 2024). The present study investigates the function of PRDX3 in relation to mitochondrial hydrogen peroxide metabolism, with the aim of elucidating its role in oxidative stress and cellular damage (Cox et al., 2009; Cadenas, 2004). Further investigation into the effects of PRDX3 revealed an increase in circulating metabolites in TCA and a shift in M1-type macrophages towards a M2-type macrophages. (Huang et al., 2024). In addition to its impact on the progression of lung infections, the TCA cycle influences the efficacy of subsequent antibiotic therapy in the context of bacterial infections. ROS produced by macrophages can inactivate the TCA cycle by inactivating essential TCA cycle enzymes, such as aconitase and succinate dehydrogenase. This leads to a decrease in respiration and ATP production, which ultimately increases the antibiotic resistance of Staphylococcus aureus (Rowe et al., 2020) (Figure 2).
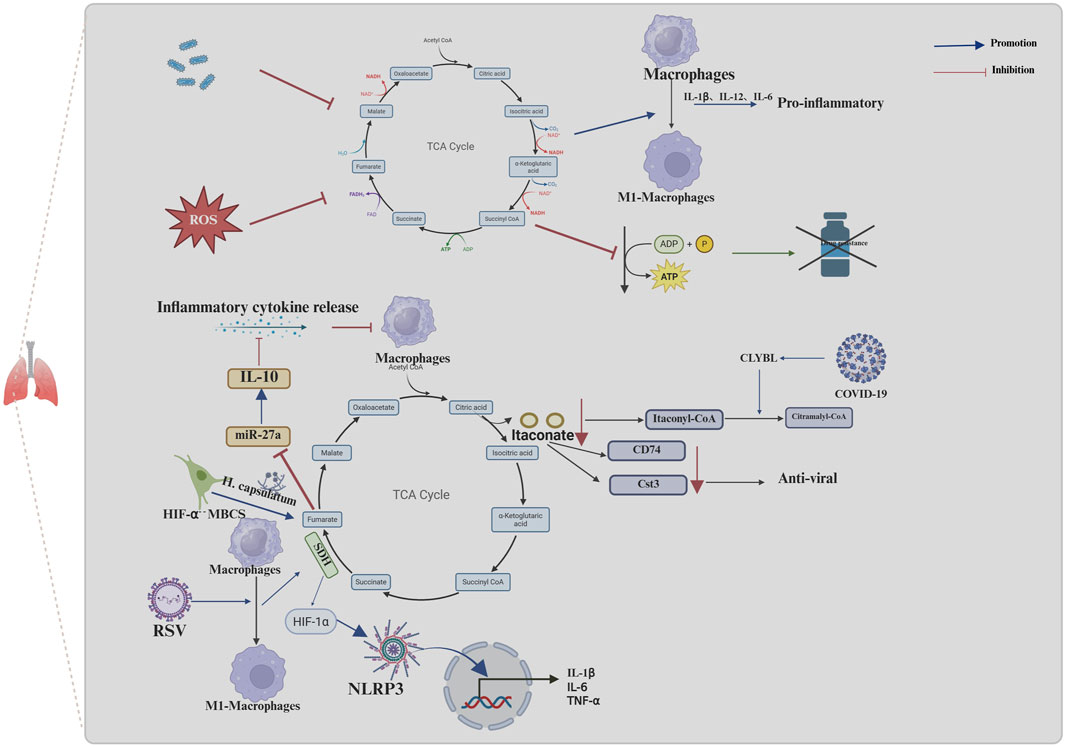
Figure 2. Mechanisms of the TCA cycle and its metabolites in infectious diseases of the lungs. Macrophage effects on the TCA cycle in infectious lung disease promote infection progression and drug resistance. The inhibition of the TCA cycle by TB and ROS, promotes the release of inflammatory factors through promoting the conversion of macrophages to pro-inflammatory macrophages (M1-macrophages) and inhibits the production of ATP, which can cause resistance to drugs. COVID-19 leads to greater conversion of itaconyl-CoA to citramalyl-CoA mediated by upregulation of CLYBL, which in turn, promotes the conversion of itaconate to itaconyl-CoA and results in a decrease in itaconate. The decrease of itaconate decreases the expression of CD74 and Cst3, in macrophages; this, in turn, suppresses the antiviral effect. RSV promotes the conversion of macrophages to M1-macrophages and then stimulates the activity of SDH in the TCA cycle, which in turn facilitates the release of cytokines, such as IL-1β, through HIF-1α/NLRP3 pathway and contributes to the host defense response, stimulating lung tissue inflammation or injury. In the absence of HIF-1α, lung infection by Histoplasma capsulatum in MBCS resulted in the accumulation of fumarate, which further promoted IL-10 protein production by downregulating miR-27a expression IL-10 accumulation inhibits the ability of macrophages to respond to proinflammatory cytokines. (The blue arrows represent promotion, and the red arrows represent inhibition; created using BioRender.com).
1.3.2 The influence between the TCA cycle and viral infection
Viruses represent a significant additional cause of lung infections. Severe viral infections can result in excessive inflammation, lung damage, acute respiratory distress syndrome and, in the most severe cases, death (Gautam et al., 2024). Coronavirus disease 2019 (COVID-19) has become one of the most prevalent viruses in recent years. Metabolomic analyses of plasma samples from patients with prolonged symptoms following COVID-19 infection revealed alterations primarily associated with increased TCA cycle activity (Martínez et al.). Further mechanistic studies revealed that in patients with severe manifestations of COVID-19 infection, the specific upregulation of Citramalyl-CoA lyase (CLYBL) leads to greater conversion of itaconyl-CoA to citramalyl-CoA by promoting the cleavage of citramalyl-CoA. This, in turn, promotes the conversion of itaconate to itaconyl-CoA, which results in a decrease in itaconate (Liu et al., 2022). In contrast, itaconate has been shown to upregulate the expression of proteins with antiviral and immune functions, including CD74 and cystatin 3 (Cst3), in macrophages; this, in turn, has been shown to exert an inhibitory effect on viral entry into cells, regulate immunity and provide cytoprotection (Bruchez et al., 2020; Jakoš et al., 2019; Li R. et al., 2020). In addition to the effects of the COVID-19 virus, respiratory syncytial virus (RSV) has been shown to promote the M1 phenotype in AMs during the acute phase of infection; it then stimulates the activation of SDH in the TCA cycle. This, in turn, facilitates the release of cytokines, such as IL-1β, through the HIF-1α/NLRP3 pathway and contributes to the host defense response, stimulating lung tissue inflammation or injury. Conversely, quercetin inhibits SDH activity by promoting itaconic acid anabolism, thus acting to ameliorate lung inflammation (An et al., 2024) (Figure 2).
1.3.3 The influence between the TCA cycle and fungal infection
Fungal infections are relatively uncommon in individuals with intact immune systems. However, Cryptococcus gattii has been reported to cause pneumonia in such individuals. Additionally, the decreased expression of proteins associated with the TCA cycle in the lungs of rats infected with C. gattii indicates that infections with this fungus may result in the dysregulation of the TCA cycle (Rosa et al., 2019). It has been demonstrated that conditions such as immunodeficiency or the frequent use of antibiotics can promote fungal infections. A study involving mice (Lyz2cre HIF1αFL/FL) revealed that following Histoplasma capsulatum infection, those lacking hypoxia-inducible factor 1α (HIF-1α) expression in bone marrow cells began to exhibit increased fungal loads on the third day of infection and ultimately died from the sublethal inoculum of H. capsulatum (Fecher et al., 2016). Further mechanistic studies revealed that in the absence of HIF-1α, lung infection by H. capsulatum resulted in the accumulation of the TCA-circulating intermediate fumarate, which further promoted IL-10 protein production by inhibiting JmjC domain-containing histone demethylase 5 (KDM5)-mediated histone demethylation. This process downregulates miR-27a expression and further promotes IL-10 protein production, leading to the alleviation of IL-10 transcript degradation. As an anti-inflammatory cytokine, IL-10 accumulation inhibits the ability of macrophages to respond to proinflammatory cytokines which in turn facilitates uninhibited fungal growth and is a primary contributor to elevated host mortality rates (Evans et al., 2021).
In conclusion, the TCA cycle plays a significant role in the progression of infectious lung diseases, their treatment, and posttreatment resistance. However, the mechanism of action of the TCA cycle varies for different pathogens, necessitating further in-depth studies on the basis of the main prevalent pathogens according to local epidemiological statistics.
1.4 The TCA cycle and pulmonary vascular disease
Pulmonary vascular diseases encompass a range of conditions, including PH, pulmonary embolism (PE) and pulmonary infarction. PH, in particular, is a pulmonary vascular disease typified by intricate all-vessel pathology, primarily manifested as extensive remodeling and obstruction of the lumen of small arteries (Maron et al., 2021). PH progression involves the aberrant proliferation and dysregulation of multiple cell types, coupled with the development of inflammatory and fibrotic processes throughout the vascular system (Hu et al., 2020). An elevation in pulmonary artery pressure can impose a significant hemodynamic burden on the right ventricle, potentially leading to right ventricular hypertrophy and, in some cases, progression to right heart failure (Provencher et al., 2024). Nevertheless, the etiology and specific pathogenesis of PH remain incompletely understood in the current literature. A metabolomic analysis of hypertrophied right ventricles in male rats from previous studies revealed a trend toward decreased levels of alanine, argininosuccinic acid, and downstream TCA cycle intermediates (including fumaric acid and malic acid), as well as reduced activation of the TCA cycle in rats with PH compared with control rats (Sakao et al., 2021). In contrast, in humans, metabolite analyses of lung samples obtained from patients with PH at the time of lung transplantation versus normal lung tissue obtained from cancer patients undergoing surgical lobectomy revealed higher levels of the TCA cycle intermediates citrate and succinate in PH lung tissue than in normal lung tissue (Zhao et al., 2014). In the initial stages of PH, the overexpression of Mitofusin 1 (Mfn1) in pulmonary endothelial cells results in an excessive degree of mitochondrial fusion, which in turn leads to a reduction in the accumulation of TCA cycle intermediates, including succinate, α-KG, and others, leading to a decrease in basal ATP and an increase in glycolytic ATP, resulting in a Warburg effect, which can lead to the activation of mt-ROS-mediated HIF-1α proteins, ultimately influencing the progression of PH (Yegambaram et al., 2024). After progression to severe PH, the activity of aconitase (the enzyme that catalyzes the formation of cis-aconitate from citrate) in the TCA cycle increases, thereby promoting the conversion of citrate to isocitrate and consequently increasing the concentration of metabolic intermediates downstream of citrate. Concurrently, other TCA metabolites, including succinate and succinylcarnitine, are also elevated in PH, thereby promoting the TCA cycle. This results in the continuous translocation of metabolic intermediates of the TCA cycle from the mitochondria to the cytoplasm, which in turn increases fatty acid synthesis and thereby promotes vascular remodeling (Zhao et al., 2014). Nevertheless, in the context of prolonged hypoxia, Sirtuin 4 (SIRT4) is activated in pulmonary artery smooth muscle cells (PSMCs), leading to the inhibition of its downstream regulators, pyruvate dehydrogenase (PDH) and glutamate dehydrogenase 1 (GLUD1). This, in turn, results in a reduction in amino acids, as well as acetyl-CoA and α-KG. Succinyl-CoA, fumarate, and oxaloacetate, which enter the TCA cycle, result in decreased TCA cycle activity and mitochondrial dysfunction, which ultimately leads to the hyperproliferation of PASMCs and the formation and progression of PH. Conversely, exosomes secreted by human-derived mesenchymal stem cells (MSCs) are rich in metabolic proteins and genes and can alleviate PH through the inhibition of SIRT4 and the reestablishment of the TCA cycle and mitochondrial metabolism (Hogan et al., 2019). PE represents the third most prevalent form of cardiovascular disease, following coronary artery disease and stroke (Huang et al., 2025). There are notable disparities in short-term mortality rates across different risk categories for PE (Chowdhury et al., 2023). Metabolomic analysis of serum from patients with different levels of risk for PE revealed significant differences in the levels of the circulating TCA intermediates α-KG, malate, isocitrate, fumarate and cis-aconitate between low-risk and intermediate-high-risk PE patients, with α-KG showing the most significant changes (Zeleznik et al., 2018) (Figure 3).
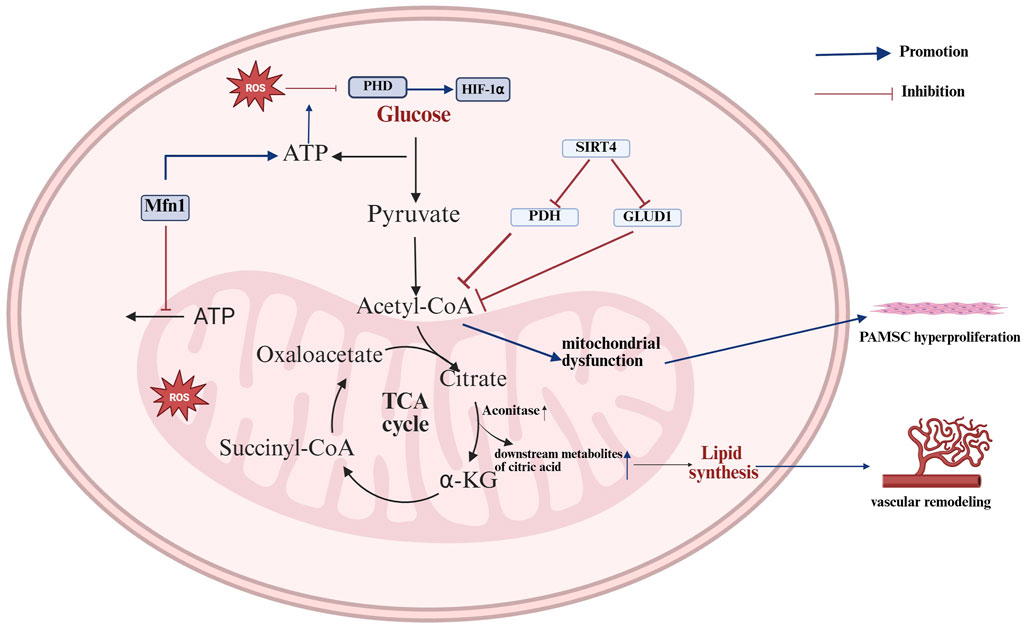
Figure 3. Mechanisms of the TCA cycle in pulmonary vascular disease. The TCA cycle promotes vascular remodeling and smooth muscle hyperproliferation in pulmonary arterial hypertension, primarily by affecting energy production and ROS accumulation, thereby influencing disease progression. Mfn1 results in an excessive degree of mitochondrial fusion, which in turn leads to a decrease in basal ATP and an increase in glycolytic ATP; increased glycolytic ATP production promotes the inhibitory effect of ROS on PHD expression, which further promotes HIF-1α expression and affects PH progression. The increase in aconitase activity results in increased levels of downstream metabolites of citric acid, including isocitrate, succinate, and succinylcarnitine. These metabolites are continuously transported from the mitochondria to the cytoplasm, which in turn increases fatty acid synthesis and thereby promotes vascular remodeling. In chronic hypoxia, the activation of SIRT4 results in the inhibition of the expression of its downstream regulators, PDH and GLUD1. This, in turn, reduces the amount of amino acids entering the TCA cycle, leading to a reduction in TCA cycling activity and mitochondrial dysfunction. Ultimately, this process promotes the over-proliferation of PMSCs (The blue arrows represent promotion, and the red arrows represent inhibition; created using BioRender.com).
In addition to the aforementioned studies, recent research on the impact of the TCA cycle on pulmonary vascular diseases has focused primarily on PH. The findings indicate that the TCA cycle has a considerable influence on the progression of PH, suggesting novel insights into the mitigation of PH progression and associated therapeutic approaches. Nevertheless, the number of studies conducted on PE and pulmonary infarction is relatively limited, and further investigations into the progression of these two diseases are warranted.
1.5 The TCA cycle and lung cancer
Lung cancer (LC) is the leading cause of cancer-related mortality on a global scale (Siegel et al., 2024). Despite significant advancements in therapeutic interventions, including surgery, chemotherapy, targeted therapies, immunotherapy and radiotherapy, the overall prognosis of patients with LC remains poor. Imbalances in redox homeostasis and disruptions in redox signaling are common features of tumors and play crucial roles in malignant progression and treatment resistance (Mendes et al., 2024). By analyzing microarray datasets and performing metabolomic analyses of plasma from non-small cell lung cancer (NSCLC) patients and healthy individuals, researchers were able to ascertain that NSCLC is primarily associated with the TCA cycle, RNA degradation, and pyrimidine metabolism (Zhao et al., 2020). Patients with NSCLC exhibit elevated plasma levels of oxaloacetate and malate (Cang et al., 2022). Additionally, research has revealed a correlation between mutations in succinate dehydrogenase, fumarate hydratase, and isocitrate and the progression and prognosis of NSCLC (Guo et al., 2015).
1.5.1 The influence of the TCA cycle on the progression of lung cancer
The rapid proliferation of tumor cells necessitates the production of large quantities of ATP, nucleotides, lipids and proteins, which in turn results in metabolic reprogramming (Paes de Araújo et al., 2019). The rapid uptake of glucose for energy and macromolecules is a crucial aspect of NSCLC progression. The TCA cycle plays a pivotal role in cellular oxidative phosphorylation (Zhang et al., 2024a). Additionally, glycolysis and glucose oxidation via the PDH and TCA cycles are increased in NSCLC relative to neighboring benign lungs (Hensley et al., 2016). Nevertheless, in the absence of glucose, glucose-independent NSCLC cells can activate Rac-Pak signaling via the PI3K signaling pathway and further internalize extracellular proteins through megacellular effluent. Additionally, alanine generated from the degradation of internalized proteins can be converted to pyruvate via alanine transaminase 2 (ALT2), promoting the TCA cycle and gluconeogenesis, which in turn supports NSCLC cell growth and progression (Hodakoski et al., 2019). Furthermore, the downregulation of Postsynaptic density-Discs large-Zonula occludens-1 (PDZ) and Lin11, Isl-1 and Mec-3 (LIM) domain 2 (PDMIL2) expression can result in the impairment of SDH expression and mitochondrial dysfunction within the TCA cycle through the activation of the NF-κB signaling pathway, which in turn leads to the accumulation of succinate and mt-ROS (Yang et al., 2024). The accumulation of mt-ROS and cancer metabolites can additionally facilitate the progression of NSCLC through the inactivation of prolyl hydroxylase (PHD) and the activation of HIF-1α (Dalla Pozza et al., 2020; Yang et al., 2024). Additionally, DEAD-box helicase 5 (DDX5) has been shown to increase mitochondrial respiration by facilitating the accumulation of succinate, an intermediate in the TCA cycle. This, in turn, has been shown to increase the energy requirements of small cell lung cancer, thereby promoting its growth and metastasis (Xing et al., 2020) (Figure 4) The TCA cycle plays a dual role in LC progression, exerting a promoting effect when its activity is increased and a restraining influence when its activity is reduced. Bone morphogenetic protein 2 (BMP2) is markedly expressed in 98% of NSCLC cases. BMP signaling has been shown to increase cell survival, migration, cancer stem cell self-renewal and metastasis, whereas increased BMP expression is associated with poorer survival outcomes (Langenfeld et al., 2003; Huang et al., 2021). BMP signaling can inhibit AMPK signaling by suppressing liver kinase B1 (LKB1); this results in a reduction in TCA cycle intermediates and amino acid expression in the lung adenocarcinoma (LUAD) cell line H1299, which in turn decreases the activity of the TCA cycle and ultimately promotes the progression of NSCLC (Vora et al., 2022). There are numerous mechanisms by which the TCA cycle influences the progression of LC, including those described above; the other potential mechanisms are outlined in Table 1.
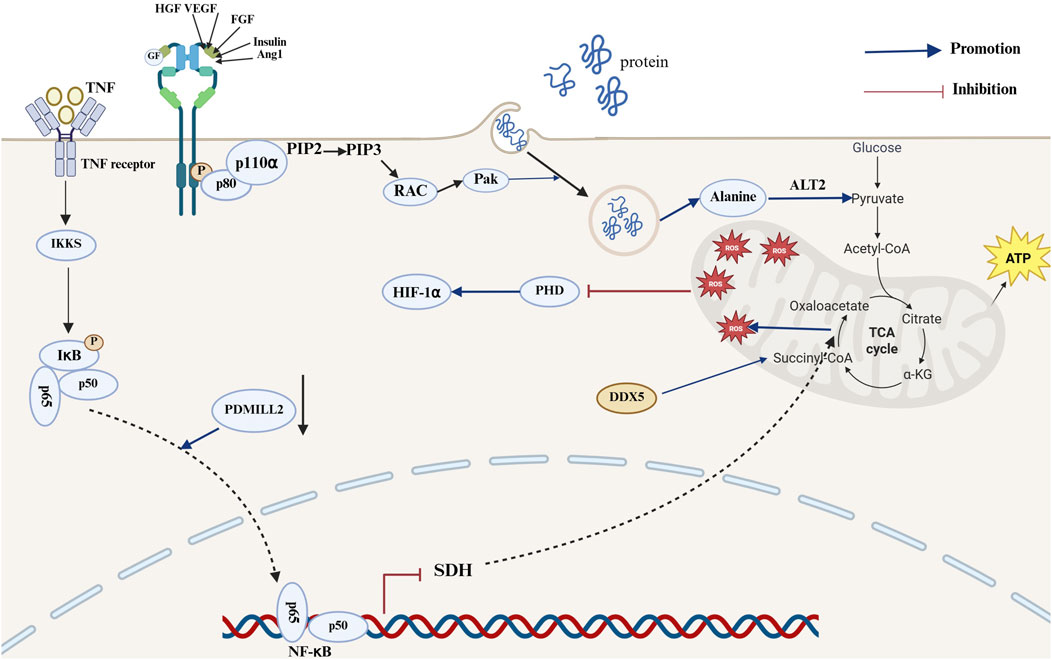
Figure 4. Mechanisms of the TCA cycle in the development of lung cancer. In the mechanism of lung cancer progression, the NF-κB and PI3K signaling pathways can promote lung cancer development by affecting succinate and ROS accumulation and pyruvate production. Activation of the PI3K signaling pathway promotes activation of the Rac-Pak pathway, thereby promoting the megacytosis of extracellular proteins. Proteins transported into the cell are degraded by internalization, resulting in the production of alanine, which can be converted to pyruvate by ALT2 and promote the TCA cycle and gluconeogenesis for energy production. PDMIL2 downregulation promotes impaired SDH expression and increased mt-ROS production following NF-κB pathway activation. The accumulation of mt-ROS results in the inactivation of PHD, which then leads to the activation of HIF-1α. This ultimately promotes the progression of NSCLC. DDX5 promotes succinyl accumulation to promote mitochondrial respiration (The blue arrows represent promotion, and the red arrows represent inhibition; created using BioRender.com).
1.5.2 The influence of the TCA cycle on the treatments of lung cancer
Despite the growing number of early diagnostic and therapeutic options for LC, LC remains the leading cause of cancer-related deaths worldwide. Consequently, further research into therapeutic modalities is essential. Immunotherapy represents a significant advancement in the treatment of LC. In patients treated with immunotherapy as a first-line approach, the difference in TCA cycle activity was most pronounced between the long-term and short-term survival groups, with the greatest disparity observed between citrate and cis-aconitate (Xu Y. et al., 2024). Studies have demonstrated that the levels of the TCA cycle intermediates succinate, fumarate, and malate are elevated in LC cells and that their abundance is reduced by the immunotherapeutic agent nivolumab (Alosaimi et al., 2024). Chemotherapy represents a key instrument in the treatment of LC. A metabolomic analysis of the relationship between pemetrexed and LC revealed substantial metabolomic alterations in the TCA cycle pathway in newly diagnosed NSCLC patients compared with healthy controls. Furthermore, significant changes in the pyruvate metabolic pathway have been observed in pemetrexed-treated patients who respond effectively to pemetrexed therapy compared with newly diagnosed NSCLC patients (Sun et al., 2023). Further mechanistic studies revealed that malic acid, a cyclic intermediate of the TCA cycle, can inhibit glucose-6-phosphate (G6PD) activity by binding to malate dehydrogenase 2 (MDH2) and further increase intracellular ROS levels and inhibit DNA synthesis via the pentose phosphate pathway (PPP), which in turn promotes the growth of LC. While dimethyl malate (DMM) can increase the sensitivity of LC cells to chemotherapy, the simultaneous treatment of LC cells with DMM and cisplatin (CDDP) can more significantly inhibit the growth and colony formation of tumor cells, reduce the concentration of chemotherapeutic drugs and decrease the side effects of drugs (Sun et al., 2024). Other mechanisms of the TCA cycle for lung cancer treatment are shown in Table 2.
1.5.3 The influence of TCA cycle on the resistance of lung cancer treatments
The high mortality rate of LC is significantly associated with drug resistance. Consequently, delaying drug resistance can markedly improve the prognosis of LC patients. Erlotinib is a targeted drug for epidermal growth factor receptor (EGFR) mutations. However, in comparison with H292 cells, erlotinib-resistant H292 cells demonstrated a marked dependence on glutamine in the production of TCA-related intermediates, particularly α-KG and citrate (Kim et al., 2022). Mechanistic studies have demonstrated that the expression of miR-147b in LUAD cells results in the disruption of the TCA cycle and activation of the pseudohypoxic response; this subsequently activates a tolerance strategy to defend against EGFR inhibition, ultimately leading to an EGFR-tyrosine kinase inhibitor (EGFR-TKI)-tolerant state (Zhang et al., 2019). The TCA cycle has been shown to promote LUAD homologous recombination (HR) activity by mediating SHFM1, which has been shown to result in a poor prognosis in patients with lung adenocarcinoma and a reduction in immunotherapy efficacy (Xu Z. et al., 2024). Furthermore, exogenous glutamine has been shown to generate lipid-based ROS through the TCA cycle, thereby sensitizing cells to erastin-induced iron death (Gao et al., 2019). While arginine succinate synthetase (ASS1) overexpression inhibits the oxidative TCA cycle and disrupts oxidative pathways in NSCLC cells, it also promotes reduced glutamine carboxylation with transamination, which in turn leads to a reduction in mitochondrial lipid ROS production. This ultimately results in resistance to erastin-induced iron death (Hu et al., 2023).
LC has attracted global attention because of its high incidence and mortality rates. The above studies have demonstrated that there has been a notable increase in research activity focused on the role of the TCA cycle in the development and treatment of lung cancer. Intermediate metabolites of the TCA cycle can be metabolized by the human body, having fewer side effects than other chemotherapy and immunotherapy treatments do. Consequently, there is a clear need for further research to explore the role of TCA cycle metabolites in the treatment of LC. One of the reasons for the high incidence and mortality of LC is the difficulty in early diagnosis. There are relatively few studies on the role of the TCA cycle in the early diagnosis of LC; thus, further research in this area is warranted.
2 Conclusion
As the TCA cycle plays an important role in energy production and biosynthesis, increasing attention is being given to the TCA cycle and its metabolic intermediates, with a focus on defining their relationship with disease progression, exploring potential mechanisms of action and providing insight into therapeutic options. Lung disease has attracted worldwide attention as a disease of high morbidity, and a thorough understanding of its pathogenesis is beneficial for subsequent treatment and early prevention. Therefore, an increasing number of researchers are focusing on the relationship between the TCA cycle and lung diseases, with LC as the main research category. Studies have shown that the TCA cycle has an important relationship with LC progression, treatment and drug resistance, providing an important basis for improving the prognosis of LC patients. We hope that the mechanism of action and therapeutic aspects identified in this study will be translated into clinical practice in the future. Moreover, relatively few studies have investigated the relationships between the TCA cycle and other lung diseases, and treatment guidelines are relatively limited. Therefore, we hope that in the future, while we continue to research the relationship between the TCA cycle and LC, we will also make breakthroughs in other lung diseases, such as lung infections and chronic airway inflammation, to improve the prognosis and quality of life of patients.
Author contributions
XL: Investigation, Writing – original draft. AZ: Writing – review and editing. HW: Funding acquisition, Writing – review and editing. XX: Visualization, Writing – review and editing. LC: Funding acquisition, Visualization, Writing – review and editing. LL: Conceptualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by “Ningbo Clinical Research Center for Respiratory Diseases, grant number 2022L004” and “Ningbo Natural Science Foundation, Grant number 2022J031” and “Medical Science and Technology Project of Zhejiang Province, Grant number 2024KY370”.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alosaimi M. E., Alotaibi B. S., Abduljabbar M. H., Alnemari R. M., Almalki A. H., Serag A. (2024). Unveiling the altered metabolic pathways induced by nivolumab in non-small cell lung cancer via GC-MS metabolomics approach coupled with multivariate analysis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1240, 124144. doi:10.1016/j.jchromb.2024.124144
An L., Zhai Q., Tao K., Xiong Y., Ou W., Yu Z., et al. (2024). Quercetin induces itaconic acid-mediated M1/M2 alveolar macrophages polarization in respiratory syncytial virus infection. Phytomedicine 130, 155761. doi:10.1016/j.phymed.2024.155761
Bambouskova M., Gorvel L., Lampropoulou V., Sergushichev A., Loginicheva E., Johnson K., et al. (2018). Electrophilic properties of itaconate and derivatives regulate the IκBζ-ATF3 inflammatory axis. Nature 556, 501–504. doi:10.1038/s41586-018-0052-z
Barosova R., Baranovicova E., Hanusrichterova J., Mokra D. (2023). Metabolomics in animal models of bronchial asthma and its translational importance for clinics. Int. J. Mol. Sci. 25, 459. doi:10.3390/ijms25010459
Bluemel G., Planque M., Madreiter-Sokolowski C. T., Haitzmann T., Hrzenjak A., Graier W. F., et al. (2021). PCK2 opposes mitochondrial respiration and maintains the redox balance in starved lung cancer cells. Free Radic. Biol. Med. 176, 34–45. doi:10.1016/j.freeradbiomed.2021.09.007
Bruchez A., Sha K., Johnson J., Chen L., Stefani C., McConnell H., et al. (2020). MHC class II transactivator CIITA induces cell resistance to ebola virus and SARS-like coronaviruses. Science 370, 241–247. doi:10.1126/science.abb3753
Cadenas E. (2004). Mitochondrial free radical production and cell signaling. Mol. Asp. Med. 25, 17–26. doi:10.1016/j.mam.2004.02.005
Cang S., Liu R., Mu K., Tang Q., Cui H., Bi K., et al. (2022). Assessment of plasma amino acids, purines, tricarboxylic acid cycle metabolites, and lipids levels in NSCLC patients based on LC-MS/MS quantification. J. Pharm. Biomed. Anal. 221, 114990. doi:10.1016/j.jpba.2022.114990
Chowdhury J. M., Brown P., Kasarabada A. (2023). Risk stratification of pulmonary embolism. Curr. Opin. Pulm. Med. 29, 363–369. doi:10.1097/MCP.0000000000000998
Collins J. M., Jones D. P., Sharma A., Khadka M., Liu K. H., Kempker R. R., et al. (2021). TCA cycle remodeling drives proinflammatory signaling in humans with pulmonary tuberculosis. PLoS Pathog. 17, e1009941. doi:10.1371/journal.ppat.1009941
Cox A. G., Winterbourn C. C., Hampton M. B. (2009). Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochem. J. 425, 313–325. doi:10.1042/BJ20091541
Dai W., Wang G., Chwa J., Oh M. E., Abeywardana T., Yang Y., et al. (2020). Mitochondrial division inhibitor (mdivi-1) decreases oxidative metabolism in cancer. Br. J. Cancer 122, 1288–1297. doi:10.1038/s41416-020-0778-x
Dalla Pozza E., Dando I., Pacchiana R., Liboi E., Scupoli M. T., Donadelli M., et al. (2020). Regulation of succinate dehydrogenase and role of succinate in cancer. Semin. Cell Dev. Biol. 98, 4–14. doi:10.1016/j.semcdb.2019.04.013
Evans H. M., Schultz D. F., Boiman A. J., McKell M. C., Qualls J. E., Deepe G. S. (2021). Restraint of fumarate accrual by HIF-1α preserves miR-27a-Mediated limitation of interleukin 10 during infection of macrophages by Histoplasma capsulatum. mBio 12, e0271021. doi:10.1128/mBio.02710-21
Fecher R. A., Horwath M. C., Friedrich D., Rupp J., Deepe G. S. (2016). Inverse correlation between IL-10 and HIF-1α in macrophages infected with Histoplasma capsulatum. J. Immunol. 197, 565–579. doi:10.4049/jimmunol.1600342
Feng Y., Xu J., Shi M., Liu R., Zhao L., Chen X., et al. (2022). COX7A1 enhances the sensitivity of human NSCLC cells to cystine deprivation-induced ferroptosis via regulating mitochondrial metabolism. Cell Death Dis. 13, 988. doi:10.1038/s41419-022-05430-3
Fitzpatrick A. M., Busse W. W. (2022). Perspectives in respiratory infections and the lung. J. Allergy Clin. Immunol. Pract. 10, 694–696. doi:10.1016/j.jaip.2022.01.026
Gao M., Yi J., Zhu J., Minikes A. M., Monian P., Thompson C. B., et al. (2019). Role of Mitochondria in ferroptosis. Mol. Cell 73, 354–363. doi:10.1016/j.molcel.2018.10.042
Gautam A., Boyd D. F., Nikhar S., Zhang T., Siokas I., Van de Velde L. A., et al. (2024). Necroptosis blockade prevents lung injury in severe influenza. Nature 628, 835–843. doi:10.1038/s41586-024-07265-8
Guo X., Li D., Wu Y., Chen Y., Zhou X., Wang X., et al. (2015). Genetic variants in genes of tricarboxylic acid cycle key enzymes are associated with prognosis of patients with non-small cell lung cancer. Lung Cancer 87, 162–168. doi:10.1016/j.lungcan.2014.12.005
Hambo S., Harb H. (2023). Extracellular vesicles and their role in lung infections. Int. J. Mol. Sci. 24, 16139. doi:10.3390/ijms242216139
Hensley C. T., Faubert B., Yuan Q., Lev-Cohain N., Jin E., Kim J., et al. (2016). Metabolic heterogeneity in human lung tumors. Cell 164, 681–694. doi:10.1016/j.cell.2015.12.034
Hodakoski C., Hopkins B. D., Zhang G., Su T., Cheng Z., Morris R., et al. (2019). Rac-Mediated macropinocytosis of extracellular protein promotes glucose Independence in non-small cell lung cancer. Cancers (Basel) 11, 37. doi:10.3390/cancers11010037
Hogan S. E., Rodriguez Salazar M. P., Cheadle J., Glenn R., Medrano C., Petersen T. H., et al. (2019). Mesenchymal stromal cell-derived exosomes improve mitochondrial health in pulmonary arterial hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 316, L723-L737–l737. doi:10.1152/ajplung.00058.2018
Hou L., Guan S., Jin Y., Sun W., Wang Q., Du Y., et al. (2020). Cell metabolomics to study the cytotoxicity of carbon black nanoparticles on A549 cells using UHPLC-Q/TOF-MS and multivariate data analysis. Sci. Total Environ. 698, 134122. doi:10.1016/j.scitotenv.2019.134122
Hu Y., Chi L., Kuebler W. M., Goldenberg N. M. (2020). Perivascular inflammation in pulmonary arterial hypertension. Cells 9, 2338. doi:10.3390/cells9112338
Hu Q., Dai J., Zhang Z., Yu H., Zhang J., Zhu X., et al. (2023). ASS1-Mediated reductive carboxylation of Cytosolic Glutamine confers ferroptosis resistance in cancer cells. Cancer Res. 83, 1646–1665. doi:10.1158/0008-5472.CAN-22-1999
Huang S. K. (2020). A fresh take on the “TCA” cycle: tets, citrate, and asthma. Am. J. Respir. Cell Mol. Biol. 63, 1–3. doi:10.1165/rcmb.2020-0101ED
Huang F., Cao Y., Wang C., Lan R., Wu B., Xie X., et al. (2021). PNMA5 promotes bone metastasis of non-small-cell lung cancer as a target of BMP2 signaling. Front. Cell Dev. Biol. 9, 678931. doi:10.3389/fcell.2021.678931
Huang W., Wang L., Huang Z., Sun Z., Zheng B. (2024). Peroxiredoxin 3 has a crucial role in the macrophage polarization by regulating mitochondrial homeostasis. Respir. Res. 25, 110. doi:10.1186/s12931-024-02739-9
Huang K., Zhu J., Sun K., Wang H., Peng Y., Peng B. (2025). Frontier and hotspot evolution in pulmonary embolism: a bibliometric analysis from 2004 to 2023. Med. Baltim. 104, e42747. doi:10.1097/MD.0000000000042747
Icard P., Simula L., Zahn G., Alifano M., Mycielska M. E. (2023). The dual role of citrate in cancer. Biochim. Biophys. Acta Rev. Cancer 1878, 188987. doi:10.1016/j.bbcan.2023.188987
Jaiswal A. K., Yadav J., Makhija S., Mazumder S., Mitra A. K., Suryawanshi A., et al. (2022). Irg1/itaconate metabolic pathway is a crucial determinant of dendritic cells immune-priming function and contributes to resolute allergen-induced airway inflammation. Mucosal Immunol. 15, 301–313. doi:10.1038/s41385-021-00462-y
Jakoš T., Pišlar A., Jewett A., Kos J. (2019). Cysteine cathepsins in tumor-associated immune cells. Front. Immunol. 10, 2037. doi:10.3389/fimmu.2019.02037
Kim S. J., Ju J. S., Park S. S., Suh Y. A., Yoo H. J., Choi E. K., et al. (2020). An RNA-binding-protein, NONO governs energy metabolism by regulating NAMPT in lung cancer. Biochem. Biophys. Res. Commun. 528, 376–382. doi:10.1016/j.bbrc.2020.01.011
Kim J. H., Kim J., Im S. S., Lee J. H., Hwang S., Chang E. J., et al. (2021). BIX01294 inhibits EGFR signaling in EGFR-mutant lung adenocarcinoma cells through a BCKDHA-mediated reduction in the EGFR level. Exp. Mol. Med. 53, 1877–1887. doi:10.1038/s12276-021-00715-7
Kim S., Jeon J. S., Choi Y. J., Baek G. H., Kim S. K., Kang K. W. (2022). Heterogeneity of glutamine metabolism in acquired-EGFR-TKI-resistant lung cancer. Life Sci. 291, 120274. doi:10.1016/j.lfs.2021.120274
Koranteng J., Chung K. F., Michaeloudes C., Bhavsar P. (2024). The role of mitochondria in eosinophil function: implications for severe asthma pathogenesis. Front. Cell Dev. Biol. 12, 1360079. doi:10.3389/fcell.2024.1360079
Kuruvilla M. E., Lee F. E., Lee G. B. (2019). Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin. Rev. Allergy Immunol. 56, 219–233. doi:10.1007/s12016-018-8712-1
Langenfeld E. M., Calvano S. E., Abou-Nukta F., Lowry S. F., Amenta P., Langenfeld J. (2003). The mature bone morphogenetic protein-2 is aberrantly expressed in non-small cell lung carcinomas and stimulates tumor growth of A549 cells. Carcinogenesis 24, 1445–1454. doi:10.1093/carcin/bgg100
Li S., Liu J., Zhou J., Wang Y., Jin F., Chen X., et al. (2020a). Urinary metabolomic profiling reveals biological pathways and predictive signatures associated with childhood asthma. J. Asthma Allergy 13, 713–724. doi:10.2147/JAA.S281198
Li R., Zhang P., Wang Y., Tao K. (2020b). Itaconate: a metabolite regulates inflammation response and oxidative stress. Oxid. Med. Cell Longev. 2020, 5404780. doi:10.1155/2020/5404780
Liu P. S., Wang H., Li X., Chao T., Teav T., Christen S., et al. (2017). α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 18, 985–994. doi:10.1038/ni.3796
Liu P., Wu D., Duan J., Xiao H., Zhou Y., Zhao L., et al. (2020a). NRF2 regulates the sensitivity of human NSCLC cells to cystine deprivation-induced ferroptosis via FOCAD-FAK signaling pathway. Redox Biol. 37, 101702. doi:10.1016/j.redox.2020.101702
Liu M., Tong Z., Ding C., Luo F., Wu S., Wu C., et al. (2020b). Transcription factor c-Maf is a checkpoint that programs macrophages in lung cancer. J. Clin. Invest 130, 2081–2096. doi:10.1172/JCI131335
Liu Y., Song L., Zheng N., Shi J., Wu H., Yang X., et al. (2022). A urinary proteomic landscape of COVID-19 progression identifies signaling pathways and therapeutic options. Sci. China Life Sci. 65, 1866–1880. doi:10.1007/s11427-021-2070-y
MacLean A., Legendre F., Appanna V. D. (2023). The tricarboxylic acid (TCA) cycle: a malleable metabolic network to counter cellular stress. Crit. Rev. Biochem. Mol. Biol. 58, 81–97. doi:10.1080/10409238.2023.2201945
Mammen M. J., Sethi S. (2016). COPD and the microbiome. Respirology 21, 590–599. doi:10.1111/resp.12732
Mannion J. M., McLoughlin R. M., Lalor S. J. (2023). The airway Microbiome-IL-17 axis: a critical regulator of chronic inflammatory disease. Clin. Rev. Allergy Immunol. 64, 161–178. doi:10.1007/s12016-022-08928-y
Maron B. A., Abman S. H., Elliott C. G., Frantz R. P., Hopper R. K., Horn E. M., et al. (2021). Pulmonary arterial hypertension: diagnosis, treatment, and novel advances. Am. J. Respir. Crit. Care Med. 203, 1472–1487. doi:10.1164/rccm.202012-4317SO
Martínez S., Albóniga O. E., López-Huertas M. R., Gradillas A., Barbas C. (2024). Reinforcing the evidence of mitochondrial dysfunction in long COVID patients using a multiplatform mass spectrometry-based metabolomics approach. J. Proteome Res. 23, 3025–3040. doi:10.1021/acs.jproteome.3c00706
Martínez-Reyes I., Chandel N. S. (2020). Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 11, 102. doi:10.1038/s41467-019-13668-3
Mendes C., Lemos I., Hipólito A., Abreu B., Freitas-Dias C., Martins F., et al. (2024). Metabolic profiling and combined therapeutic strategies unveil the cytotoxic potential of selenium-chrysin (SeChry) in NSCLC cells. Biosci. Rep. 44. doi:10.1042/BSR20240752
Mills E. L., Kelly B., Logan A., Costa A. S. H., Varma M., Bryant C. E., et al. (2016). Succinate dehydrogenase supports metabolic repurposing of Mitochondria to drive inflammatory macrophages. Cell 167, 457–470. doi:10.1016/j.cell.2016.08.064
Mills E. L., Ryan D. G., Prag H. A., Dikovskaya D., Menon D., Zaslona Z., et al. (2018). Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 556, 113–117. doi:10.1038/nature25986
Mondal A., Jia D., Bhatt V., Akel M., Roberge J., Guo J. Y., et al. (2022). Ym155 localizes to the mitochondria leading to mitochondria dysfunction and activation of AMPK that inhibits BMP signaling in lung cancer cells. Sci. Rep. 12, 13135. doi:10.1038/s41598-022-17446-y
Naz S., Kolmert J., Yang M., Reinke S. N., Kamleh M. A., Snowden S., et al. (2017). Metabolomics analysis identifies sex-associated metabotypes of oxidative stress and the autotaxin-lysoPA axis in COPD. Eur. Respir. J. 49, 1602322. doi:10.1183/13993003.02322-2016
Nunn K., Yanxiang Guo J. (2023). Transient systemic autophagy ablation irreversibly inhibits lung tumor cell metabolism and promotes T-cell mediated tumor killing. Autophagy 19, 1879–1881. doi:10.1080/15548627.2022.2141534
Paes de Araújo R., Bertoni N., Seneda A. L., Felix T. F., Carvalho M., Lewis K. E., et al. (2019). Defining metabolic rewiring in lung squamous cell carcinoma. Metabolites 9, 47. doi:10.3390/metabo9030047
Park S. J., Yoo H. C., Ahn E., Luo E., Kim Y., Sung Y., et al. (2023). Enhanced glutaminolysis drives hypoxia-induced chemoresistance in pancreatic cancer. Cancer Res. 83, 735–752. doi:10.1158/0008-5472.CAN-22-2045
Pinto-Plata V., Casanova C., Divo M., Tesfaigzi Y., Calhoun V., Sui J., et al. (2019). Plasma metabolomics and clinical predictors of survival differences in COPD patients. Respir. Res. 20, 219. doi:10.1186/s12931-019-1167-y
Provencher S., Mai V., Bonnet S. (2024). Managing pulmonary arterial hypertension with cardiopulmonary comorbidities. Chest 165, 682–691. doi:10.1016/j.chest.2023.08.023
Ren J. G., Seth P., Ye H., Guo K., Hanai J. I., Husain Z., et al. (2017). Citrate suppresses tumor growth in multiple models through inhibition of glycolysis, the tricarboxylic acid cycle and the IGF-1R pathway. Sci. Rep. 7, 4537. doi:10.1038/s41598-017-04626-4
Rosa R. L., Berger M., Santi L., Driemeier D., Barros Terraciano P., Campos A. R., et al. (2019). Proteomics of rat lungs infected by Cryptococcus gattii reveals a potential warburg-like effect. J. Proteome Res. 18, 3885–3895. doi:10.1021/acs.jproteome.9b00326
Rowe S. E., Wagner N. J., Li L., Beam J. E., Wilkinson A. D., Radlinski L. C., et al. (2020). Reactive oxygen species induce antibiotic tolerance during systemic Staphylococcus aureus infection. Nat. Microbiol. 5, 282–290. doi:10.1038/s41564-019-0627-y
Ryan E. M., Sadiku P., Coelho P., Watts E. R., Zhang A., Howden A. J. M., et al. (2023). NRF2 activation reprograms defects in oxidative metabolism to restore macrophage function in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 207, 998–1011. doi:10.1164/rccm.202203-0482OC
Sakao S., Kawakami E., Shoji H., Naito A., Miwa H., Suda R., et al. (2021). Metabolic remodeling in the right ventricle of rats with severe pulmonary arterial hypertension. Mol. Med. Rep. 23, 227. doi:10.3892/mmr.2021.11866
Schleich F., Bougard N., Moermans C., Sabbe M., Louis R. (2023). Cytokine-targeted therapies for asthma and COPD. Eur. Respir. Rev. 32, 220193. doi:10.1183/16000617.0193-2022
Seo C., Hwang Y. H., Lee H. S., Kim Y., Shin T. H., Lee G., et al. (2017). Metabolomic study for monitoring of biomarkers in mouse plasma with asthma by gas chromatography-mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1063, 156–162. doi:10.1016/j.jchromb.2017.08.039
Siegel R. L., Giaquinto A. N., Jemal A. (2024). Cancer statistics. CA Cancer J. Clin. 74, 12–49. doi:10.3322/caac.21820
Singh A., Happel C., Manna S. K., Acquaah-Mensah G., Carrerero J., Kumar S., et al. (2013). Transcription factor NRF2 regulates miR-1 and miR-206 to drive tumorigenesis. J. Clin. Invest 123, 2921–2934. doi:10.1172/JCI66353
Slupsky C. M., Cheypesh A., Chao D. V., Fu H., Rankin K. N., Marrie T. J., et al. (2009). Streptococcus pneumoniae and Staphylococcus aureus pneumonia induce distinct metabolic responses. J. Proteome Res. 8, 3029–3036. doi:10.1021/pr900103y
Sun H., Piao H., Qi H., Yan M., Liu H. (2019). Study on the metabolic reprogramming of lung cancer cells regulated by docetaxel based on metabolomics. Zhongguo Fei Ai Za Zhi 22, 208–215. doi:10.3779/j.issn.1009-3419.2019.04.02
Sun R., Fei F., Wang M., Jiang J., Yang G., Yang N., et al. (2023). Integration of metabolomics and machine learning revealed tryptophan metabolites are sensitive biomarkers of pemetrexed efficacy in non-small cell lung cancer. Cancer Med. 12, 19245–19259. doi:10.1002/cam4.6446
Sun M., Feng Q., Yan Q., Zhao H., Wang H., Zhang S., et al. (2024). Malate, a natural inhibitor of 6PGD, improves the efficacy of chemotherapy in lung cancer. Lung Cancer 190, 107541. doi:10.1016/j.lungcan.2024.107541
Tennant D. A., Frezza C., MacKenzie E. D., Nguyen Q. D., Zheng L., Selak M. A., et al. (2009). Reactivating HIF prolyl hydroxylases under hypoxia results in metabolic catastrophe and cell death. Oncogene 28, 4009–4021. doi:10.1038/onc.2009.250
Tomlinson K. L., Prince A. S., Wong Fok Lung T. (2021). Immunometabolites drive bacterial adaptation to the airway. Front. Immunol. 12, 790574. doi:10.3389/fimmu.2021.790574
Vora M., Mondal A., Jia D., Gaddipati P., Akel M., Gilleran J., et al. (2022). Bone morphogenetic protein signaling regulation of AMPK and PI3K in lung cancer cells and C. elegans. Cell Biosci. 12, 76. doi:10.1186/s13578-022-00817-3
Wu J., Liu N., Chen J., Tao Q., Li Q., Li J., et al. (2024) The tricarboxylic acid cycle metabolites for cancer: friend or enemy. Research (Wash D C), 12 (7), 0351. doi:10.34133/research.0351
Xing Z., Russon M. P., Utturkar S. M., Tran E. J. (2020). The RNA helicase DDX5 supports mitochondrial function in small cell lung cancer. J. Biol. Chem. 295, 8988–8998. doi:10.1074/jbc.RA120.012600
Xu Y. S., Liang J. J., Wang Y., Zhao X. J., Xu L., Xu Y. Y., et al. (2016). STAT3 undergoes acetylation-dependent mitochondrial translocation to regulate pyruvate metabolism. Sci. Rep. 6, 39517. doi:10.1038/srep39517
Xu Y., Ding K., Peng Z., Ding L., Li H., Fan Y. (2024a). Evaluating for correlations between specific metabolites in patients receiving first-line or second-line immunotherapy for metastatic or recurrent NSCLC: an exploratory Study based on two cohorts. Mol. Cancer Ther. 23, 733–742. doi:10.1158/1535-7163.mct-23-0459
Xu Z., He D., Huang L., Deng K., Jiang W., Qin J., et al. (2024b). Metabolic reprogramming-driven homologous recombination and TCA cycle dysregulation contribute to poor prognoses in lung adenocarcinoma. J. Cell Mol. Med. 28, e18406. doi:10.1111/jcmm.18406
Xue M., Zeng Y., Lin R., Qu H. Q., Zhang T., Zhang X. D., et al. (2021). Metabolomic profiling of anaerobic and aerobic energy metabolic pathways in chronic obstructive pulmonary disease. Exp. Biol. Med. (Maywood) 246, 1586–1596. doi:10.1177/15353702211008808
Yang J. X., Chuang Y. C., Tseng J. C., Liu Y. L., Lai C. Y., Lee A. Y., et al. (2024). Tumor promoting effect of PDLIM2 downregulation involves mitochondrial ROS, oncometabolite accumulations and HIF-1α activation. J. Exp. Clin. Cancer Res. 43, 169. doi:10.1186/s13046-024-03094-9
Yegambaram M., Sun X., Lu Q., Jin Y., Ornatowski W., Soto J., et al. (2024). Mitochondrial hyperfusion induces metabolic remodeling in lung endothelial cells by modifying the activities of electron transport chain complexes I and III. Free Radic. Biol. Med. 210, 183–194. doi:10.1016/j.freeradbiomed.2023.11.008
Zeleznik O. A., Poole E. M., Lindstrom S., Kraft P., Van Hylckama Vlieg A., Lasky-Su J. A., et al. (2018). Metabolomic analysis of 92 pulmonary embolism patients from a nested case-control study identifies metabolites associated with adverse clinical outcomes. J. Thromb. Haemost. 16, 500–507. doi:10.1111/jth.13937
Zhang W. C., Wells J. M., Chow K. H., Huang H., Yuan M., Saxena T., et al. (2019). miR-147b-mediated TCA cycle dysfunction and pseudohypoxia initiate drug tolerance to EGFR inhibitors in lung adenocarcinoma. Nat. Metab. 1, 460–474. doi:10.1038/s42255-019-0052-9
Zhang W., Ding M., Feng Y., Cai S., Luo Z., Shan J., et al. (2024a). Modulation of cellular metabolism and alleviation of bacterial dysbiosis by Aconiti Lateralis Radix Praeparata in non-small cell lung cancer treatment. Phytomedicine 126, 155099. doi:10.1016/j.phymed.2023.155099
Zhang W., Cai S., Qin L., Feng Y., Ding M., Luo Z., et al. (2024b). Alkaloids of Aconiti Lateralis Radix Praeparata inhibit growth of non-small cell lung cancer by regulating PI3K/Akt-mTOR signaling and glycolysis. Commun. Biol. 7, 1118. doi:10.1038/s42003-024-06801-6
Zhao Y., Peng J., Lu C., Hsin M., Mura M., Wu L., et al. (2014). Metabolomic heterogeneity of pulmonary arterial hypertension. PLoS One 9, e88727. doi:10.1371/journal.pone.0088727
Keywords: TCA cycle, asthma, chronic obstructive pulmonary disease (COPD), lung infection, pulmonary hypertension (PH), lung cancer
Citation: Lu X, Zhang A, Wang H, Xu X, Chen L and Luo L (2025) Emerging role of the TCA cycle and its metabolites in lung disease. Front. Physiol. 16:1621013. doi: 10.3389/fphys.2025.1621013
Received: 30 April 2025; Accepted: 04 August 2025;
Published: 15 August 2025.
Edited by:
Martina Di Rienzo, National Institute for Infectious Diseases Lazzaro Spallanzani (IRCCS), ItalyReviewed by:
Antoni Olona Ferrer, Duke-NUS Medical School, SingaporeChidozie Okoye, University of Nigeria, Nigeria
Copyright © 2025 Lu, Zhang, Wang, Xu, Chen and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingfang Luo, OTk3ODExMTM5QHFxLmNvbQ==
 Xinan Lu
Xinan Lu Aijun Zhang1
Aijun Zhang1 Xinjia Xu
Xinjia Xu Liping Chen
Liping Chen