- Department of Burns, First People’s Hospital of Shangqiu City, Shangqiu, Henan, China
Chronic scars and pain following burns not only impair patients’ quality of life but also resist current empirical treatments, highlighting an urgent need for mechanism-based therapies. Early studies have characterized key mediators of scar fibrosis and nociception, yet integration of molecular and neural pathways remains limited. Here, we comprehensively review 1 molecular and cellular drivers of burn scar formation—particularly transforming growth factor-β (TGF-β)–induced fibroblast activation and extracellular matrix remodeling; 2 bidirectional interactions between scar tissue and nerve regeneration via neuropeptides (Nerve growth factor, Substance P, calcitonin gene-related peptide); 3 mechanisms underpinning long-term scar pain, including peripheral/central sensitization through TRPV1/Nav channels and neuroinflammation; and 4 emerging treatments—such as laser, extracorporeal shock wave therapy (ESWT), regenerative injections, and transient receptor potential (TRP) antagonists—that target these pathways. We conclude that a detailed understanding of scar–nerve crosstalk at the molecular level is pivotal for developing targeted interventions and improving long-term outcomes.
1 Introduction
Burn scars are the fibrotic tissue that forms during the healing of skin and subcutaneous injuries caused by thermal, chemical, electrical, or radiation exposure (Hettiaratchy and Dziewulski, 2004a). Depending on the healing trajectory, wounds may be classified as acute—characterized by timely epithelialization within a few weeks—or chronic, in which the repair process is prolonged (>12 weeks) and often results in excessive collagen deposition and structural remodeling (Peña and Martin, 2024). Such scars not only alter skin architecture but can also lead to functional impairment, cosmetic concerns, and persistent pain (Moi et al., 2016). The initial severity and depth of the burn injury critically influence both the likelihood and extent of scar formation and the persistence of pain symptoms. Clinically, burns are classified based on depth into superficial (involving only the epidermis), partial-thickness (superficial and deep dermal layers), full-thickness (extending through the entire dermis), and fourth-degree burns (involving underlying tissues such as muscle and bone) (Hettiaratchy and Dziewulski, 2004b; Jeschke et al., 2020). Deeper burns, especially full-thickness and fourth-degree injuries, are more prone to delayed healing, nerve damage, and consequent neuropathic pain, contractures, and long-term functional or cosmetic deficits (Jeschke et al., 2020).
Studies have estimated that 32%–72% of burn patients develop hypertrophic scars (Tyack et al., 2015), while the incidence of scar contractures at the time of discharge ranges from 38% to 54% (Téot et al., 2020). A substantial body of evidence indicates that survivors of extensive or deep burns often experience functional impairments due to scar contractures, reduced skin elasticity, and sensory abnormalities, which negatively impact daily activities and social participation (Téot et al., 2020). In addition to their disfiguring appearance, burn scars can cause symptoms such as pain, pruritus, and sleep disturbances, further diminishing quality of life. Chronic pain associated with burn scars persists in 25%–68% of patients and is frequently accompanied by neuropathic features such as tingling, burning sensations, and allodynia, posing significant challenges for clinical management (Bijlard et al., 2017). Neuropathological mechanisms including peripheral sensitization (Roy et al., 2023), neurogenic inflammation (Shahabi et al., 2009), and central sensitization (Stanton et al., 2024) are believed to underlie burn scar-related pain, with transient receptor potential vanilloid 1 (TRPV1), Nav channels, and neuropeptides playing pivotal roles in the transmission of pain signals.
Despite the availability of various analgesic and anti-scar interventions—such as pressure therapy, silicone dressings, laser treatments, and pharmacological approaches—the efficacy of these methods remains limited due to significant inter-individual variability and the lack of precise therapeutic targets. As a result, current strategies often fail to meet the diverse needs of burn patients. Therefore, elucidating the mechanisms underlying the interaction between scar formation and pain perception, identifying novel therapeutic targets, and developing multidimensional objective assessment tools are of critical importance for optimizing personalized treatment strategies (Faour et al., 2023). This review will explore the molecular and cellular mechanisms, neuropathological pathways, and the interplay between scar tissue and nerve regeneration. By integrating the latest research advances, we aim to identify potential translational points between mechanistic understanding and clinical application, thereby providing a theoretical foundation and research framework for future precision therapies. See Table 1 for a full list of abbreviations used.
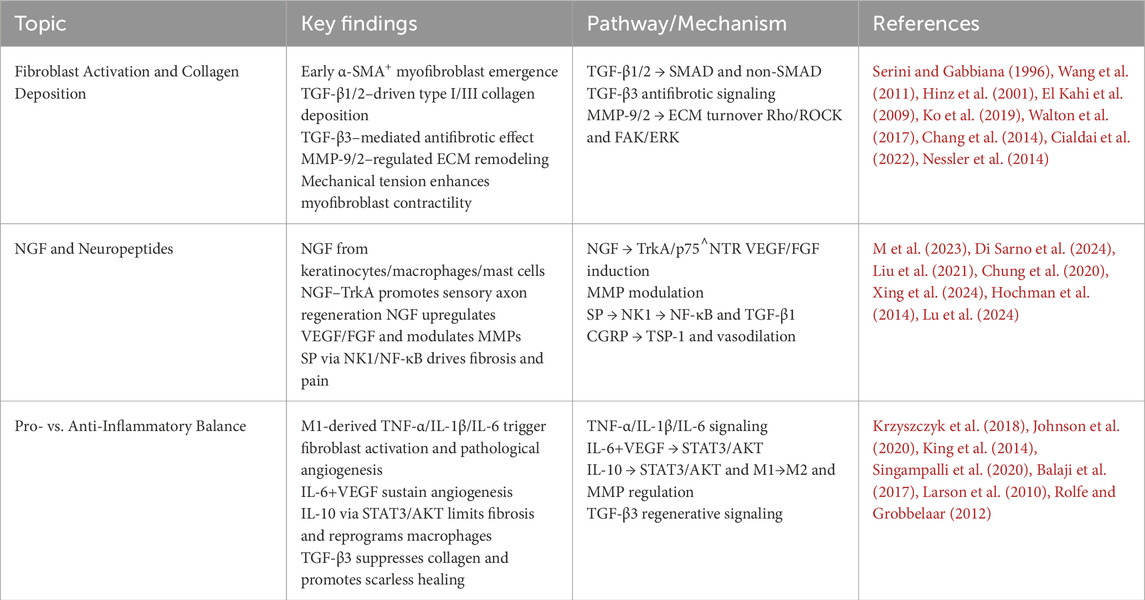
To guide readers through the organization of this review, we have provided a roadmap in Figure 1, which outlines the four main themes: Mechanisms of burn scar formation; Crosstalk between scar tissue and nerve regeneration; Pathophysiology of chronic burn scar pain; and Advances in therapeutic strategies for burn scar pain management. The following sections will address each of these topics in turn.
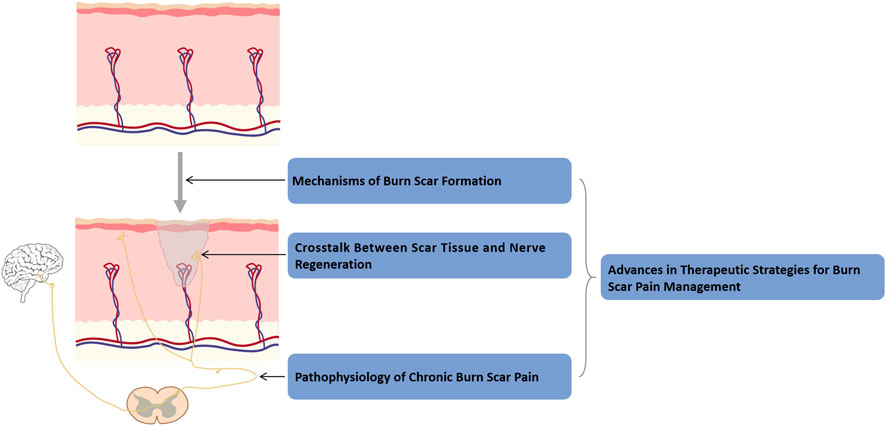
Figure 1. Roadmap of this review. This roadmap illustrates the organization of the review into four interconnected sections: mechanisms of burn scar formation, scar–nerve interactions, chronic scar pain pathophysiology, and advances in pain management strategies.
2 Molecular and cellular mechanisms of burn scar formation
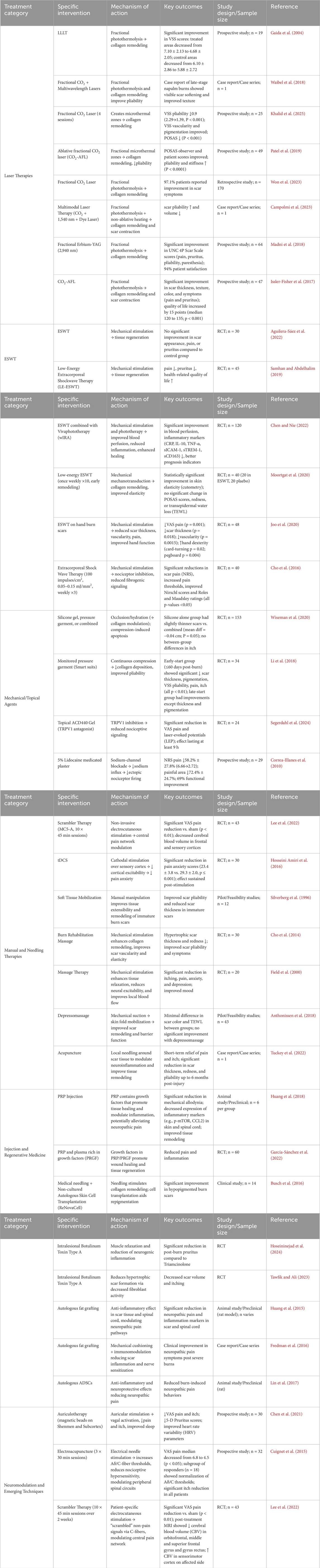
Burn scar formation is a highly dynamic and multi-layered process that begins with an acute inflammatory response at the site of injury, followed by a proliferative phase, and culminates in a remodeling phase where relatively stable scar tissue is formed (Werner and Grose, 2003). During this progression, fibroblasts transdifferentiate into myofibroblasts under the combined influence of transforming growth factor-β (TGF-β) signaling and mechanical tension (Tomasek et al., 2002), producing excessive type I and III collagen that contributes to the development of a fibrotic extracellular matrix (Wynn, 2008). Nerve growth factor (NGF) and neuropeptides such as Substance P (SP) and calcitonin gene-related peptide (CGRP) not only facilitate nerve and vascular remodeling but also modulate pain sensitivity within scar regions (Ji et al., 2014). The balance between early pro-inflammatory cytokines (e.g., TNF-α, IL-1β, IL-6) and later anti-inflammatory mediators (e.g., IL-10, TGF-β3) plays a critical role in regulating the activity of matrix metalloproteinases (MMPs), which in turn governs collagen degradation and remodeling efficiency—ultimately influencing the texture and functional quality of the resulting scar tissue (Page-McCaw et al., 2007). Figure 2 illustrates the main cellular and molecular mechanisms of the burn scar formation process. Table 2 summarizes the major cellular and molecular pathways involved in burn scar formation and pain modulation.
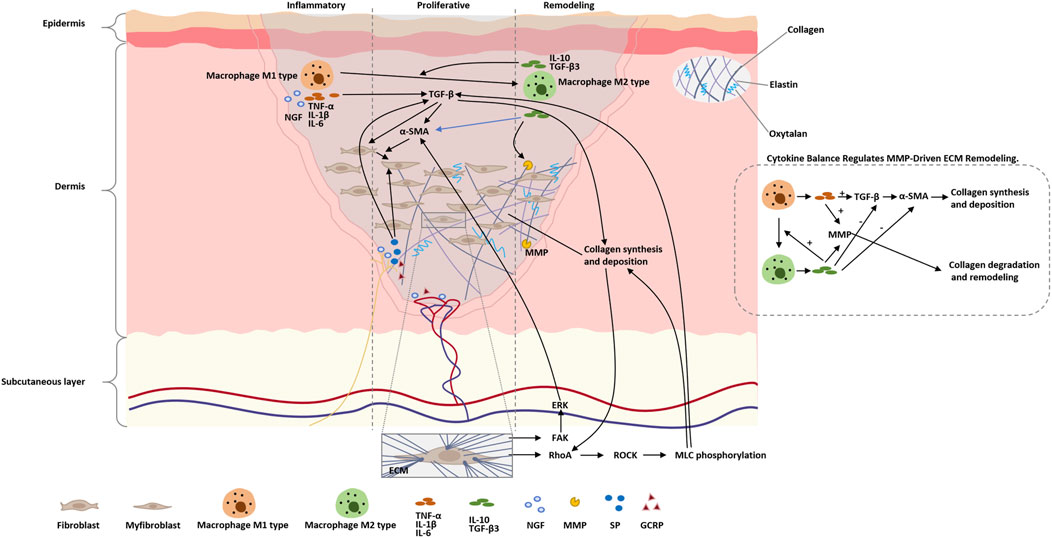
Figure 2. Schematic of molecular and cellular events in burn scar formation. The figure highlights the sequential phases (inflammation → proliferative → remodeling), key mediators (cytokines, growth factors, MMPs, neuropeptides), mechanotransduction pathways (Rho/ROCK, FAK/ERK) that drive myofibroblast activation, and the balance between pro- and anti-inflammatory signals which regulates MMP activity and final scar quality.
2.1 Fibroblast activation and collagen deposition
In burn scar formation, the transdifferentiation of fibroblasts into myofibroblasts—characterized by α-smooth muscle actin (α-SMA) expression—is a key initiating event for scar contraction and excessive tissue repair. Studies have shown that myofibroblasts emerge early during wound healing, with α-SMA-positive cells detectable as early as days 4–6 post-injury (Serini and Gabbiana, 1996). Their numbers increase significantly within 1–3 weeks, peaking around the second week, and while they gradually decline thereafter, they can persist in pathological scars (Wang et al., 2011). During this transdifferentiation, growth factors such as TGF-β1 and TGF-β2 activate both SMAD-dependent and non-SMAD signaling pathways to induce α-SMA expression in fibroblasts, endowing them with strong contractile capabilities and making them central players in scar contraction (Hinz et al., 2001). TGF-β1 is markedly upregulated in wound sites, promoting fibroblast proliferation, α-SMA expression, and the upregulation of pro-collagen and fibronectin genes via SMAD-dependent and independent mechanisms, thereby driving excessive type I and III collagen deposition (El Kahi et al., 2009). TGF-β2 is also elevated in the early repair phase, recruiting and activating fibroblasts, and enhancing the early secretion and crosslinking of type I/III collagen, which contributes to increased scar volume and stiffness (Ko et al., 2019). In contrast, TGF-β3 is highly expressed during early wound healing and has demonstrated antifibrotic properties across various tissues. It can inhibit collagen synthesis and promote scarless healing. For instance, Occleston et al. found that local delivery of TGF-β3 significantly reduced scar volume and suppressed type I/III collagen deposition, resulting in nearly scar-free tissue repair (Walton et al., 2017). Both animal models and clinical trials have shown that exogenous TGF-β3 can modulate the early inflammatory microenvironment and suppress fibroblast activation, offering new therapeutic insights into scar regression (Chang et al., 2014).
The remodeling of fibrotic matrix begins approximately 5–6 weeks post-injury and plays a pivotal role in determining the final texture and function of the scar (Cialdai et al., 2022). During this phase, MMPs, particularly MMP-2 and MMP-9, undergo activity changes that mediate partial degradation and reorganization of the extracellular matrix. MMP-9 levels increase rapidly within hours of injury, peaking on day 1 and gradually declining thereafter. MMP-2 levels rise between days 3–7, peaking around day 7 and remaining relatively stable to support sustained collagen degradation and reorganization (Nessler et al., 2014). In addition to biochemical signaling, mechanical tension serves as a critical trigger for fibroblast activation. In vitro and animal studies have demonstrated that external mechanical stretching can significantly enhance α-SMA expression and contractile force in myofibroblasts through Rho/ROCK and FAK/ERK pathways, exacerbating scar contraction and fibrotic matrix remodeling. Mechanical tension also promotes the proliferation of nerve fibers and the directional alignment of collagen in scar tissue, sensitizing nociceptive neurons and further amplifying scar-associated pain (Gallucci et al., 2006).
2.2 Nerve growth factor (NGF) and neuropeptides
During the early phase following burn injury, large amounts of NGF are secreted by various cell types, including keratinocytes, macrophages, and mast cells (M et al., 2023; Di Sarno et al., 2024). NGF signals through both the high-affinity receptor tropomyosin receptor kinase A (TrkA) and the low-affinity receptor p75 neurotrophin receptor (p75NTR), which together coordinate target cell proliferation, survival, and apoptosis (M et al., 2023). NGF-TrkA signaling stimulates the regeneration and collateral sprouting of sensory nerve axons post-injury, thereby accelerating the re-establishment of cutaneous neural networks and contributing to the restoration of sensory function (Liu et al., 2021). During the proliferative phase, NGF promotes angiogenesis by upregulating vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) expression in endothelial cells and enhances re-epithelialization through increased proliferation and migration of epidermal stem cells (M et al., 2023).
Beyond its reparative functions, NGF in scar tissue has been shown to induce myofibroblast differentiation and modulate MMP activity, thereby attenuating excessive collagen deposition and scar contracture, leading to improved scar quality (Liu et al., 2021). Concurrently, neuropeptides released from C-fiber terminals—such as SP and CGRP—play dual roles in scar tissue by modulating nociception and pruritus, as well as altering the microenvironment through effects on vascular permeability and fibroblast proliferation (Chung et al., 2020). SP, a key mediator of neuropathic pain and post-injury itch, increases sensory nerve terminal sensitivity within scars, contributing to symptoms like burning, stinging, and hyperesthesia (Téot et al., 2020). It activates downstream inflammatory pathways (e.g., NF-κB) via the neurokinin 1 (NK1) receptor, upregulates profibrotic cytokines such as TGF-β1, and directly stimulates fibroblast proliferation and collagen synthesis, thereby promoting excessive scar tissue formation (Xing et al., 2024). CGRP, while also involved in pain transmission, predominantly modulates vasodilation and immune cell function (Téot et al., 2020). During the proliferative phase, CGRP enhances fibroblast proliferation and migration, and upregulates type I collagen synthesis, supporting granulation tissue maturation and wound closure (Xing et al., 2024; Hochman et al., 2014). Under certain conditions, CGRP exerts anti-inflammatory effects by inducing thrombospondin-1 release, suppressing excessive immune cell recruitment, and promoting immune cell apoptosis. Its potent vasodilatory capacity further improves local microcirculation and preserves capillary architecture (Xing et al., 2024; Lu et al., 2024).
2.3 Balance between pro- and anti-inflammatory mediators
In the early stages of wound healing, pro-inflammatory M1 macrophages release high levels of cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6), which promote fibroblast proliferation, angiogenesis, and keratinocyte migration, yet also lay the groundwork for excessive fibrosis (Krzyszczyk et al., 2018). These cytokines stimulate fibroblast proliferation and collagen secretion and act synergistically with angiogenic factors like VEGF to facilitate neovascularization. Moreover, they modulate basement membrane components and chemotactic signals, enhancing keratinocyte migration and accelerating re-epithelialization. IL-6, in cooperation with VEGF, sustains endothelial cell proliferation and migration, thereby maintaining pathological angiogenesis that supports prolonged fibroblast activity and continuous collagen deposition (Johnson et al., 2020).
As the wound enters the resolution phase, anti-inflammatory mediators such as IL-10 and TGF-β3 are upregulated, helping to suppress excessive inflammatory responses and facilitate extracellular matrix (ECM) remodeling (King et al., 2014). IL-10 activates downstream signal transducer and activator of transcription 3 (STAT3) and Protein Kinase B (AKT) pathways in fibroblasts, downregulating the expression of type I/III collagen and α-SMA, thus inhibiting the contractile phenotype of myofibroblasts and slowing the progression of fibrosis (Singampalli et al., 2020). IL-10 also reprograms macrophages from the M1 to the M2 phenotype, promoting immature vessel formation and regulating MMP activity to support organized ECM deposition and tissue repair (King et al., 2014; Balaji et al., 2017). In fetal wounds, TGF-β3 is more abundantly expressed compared to TGF-β1/β2. TGF-β3 suppresses collagen synthesis and modulates the inflammatory milieu, enabling regenerative, nearly scarless healing. As such, it is considered a key factor in scar reversal (Larson et al., 2010). Multiple animal studies have demonstrated that exogenous delivery of TGF-β3 significantly reduces scar formation and facilitates smooth, functionally superior tissue regeneration (Rolfe and Grobbelaar, 2012).
3 Interaction between scar tissue and nerve regeneration
During the later stages of burn wound healing, the mechanical rigidity of scar tissue and the biochemical remodeling of the ECM form a dual barrier to the regeneration of new nerve fibers. At the same time, neurotrophic factors and neuropeptides secreted by nerve axons activate fibroblasts in return, establishing a vicious feedback loop that collectively drives and maintains chronic neuropathic pain. Several studies have highlighted that mechanotransduction pathways, particularly Yes-associated protein and transcriptional coactivator with PDZ-binding motif (YAP/TAZ), act as key molecular integrators between ECM stiffness and fibroblast or neuronal responses (Dupont et al., 2011; Meng et al., 2018). The crosslinking of collagen fibers by lysyl oxidase (LOX), activation of integrins, and Hippo pathway inactivation lead to nuclear translocation of YAP/TAZ, which cooperatively regulate genes related to fibrosis and pain sensitization (Qin et al., 2018). Additionally, aligned ECM components like collagen I and laminin guide axons, whereas aberrant proteins such as Tenascin-C (Jiang et al., 2020; Guimarães et al., 2023), along with miRNA dysregulation (e.g., miR-29, miR-124), further disrupt regeneration and promote pain (van Rooij et al., 2008; Hassan et al., 2024; Shao et al., 2016).
3.1 Mechanical obstruction of scar matrix
LOX-mediated collagen crosslinking critically increases extracellular matrix stiffness and constitutes the principal mechanical barrier to nerve regeneration in burn scars (Tomasek et al., 2002). In burn scars, the activity of the LOX family of enzymes is significantly elevated, which promotes excessive crosslinking between type I/III collagen molecules. This results in an increase in the Young’s modulus of the scar tissue by 20%–50% compared to normal dermis, physically creating a “rigid cage” around the nerve growth cones. This increased rigidity significantly impedes the lateral extension of axons and induces persistent mechanical sensitization of local nerve endings (Cai et al., 2017; Chaudhari et al., 2023). The Hippo–YAP/TAZ pathway plays a central role in sensing this increased ECM stiffness. Under high mechanical stress, YAP and TAZ are dephosphorylated, translocate to the nucleus, and interact with TEA domain family member (TEAD) transcription factors to drive myofibroblast activation, α-SMA expression, and pro-fibrotic gene transcription (Dupont et al., 2011; Meng et al., 2018). YAP/TAZ also cooperate with TGF-β/SMAD signaling—through induction of SMAD7 and modulation of SMAD3 activity—to amplify extracellular matrix gene expression and reinforce tissue rigidity and reinforcing the mechanical barrier to nerve regeneration (Qin et al., 2018; Kuehlmann et al., 2020; Yan and Cui, 2023; Taskinen et al., 1995). Additionally, the mechanosensitive channel piezo-type mechanosensitive ion channel component 1 (Piezo1) is upregulated in both scar fibroblasts and sensory nerve terminals. Calcium influx mediated by Piezo1 activates the Rho/ROCK pathway, which not only promotes myofibroblast contraction and matrix remodeling but also induces repetitive firing in adjacent neurons, maintaining a state of mechanical hypersensitivity (He et al., 2024; Xu et al., 2023).
3.2 ECM’s role in nerve fiber guidance
In an ideal repair microenvironment, type I collagen and laminin guide axonal growth through integrin-ECM interactions, providing adhesion sites and directional guidance for the axons. Clinical studies have demonstrated that implantation of type I collagen conduits at facial nerve defects significantly enhances functional recovery, confirming the scaffold role of organized ECM in nerve regeneration (Yao et al., 2022). Decellularized fibroblast-derived matrices retain natural adhesion ligands and neurotrophic factor binding sites, significantly increasing the number and speed of axons crossing the injury gap in peripheral nerve injury animal models, further emphasizing the importance of biochemical scaffolds in promoting regeneration (Xu et al., 2023; Kim and Granstein, 2021). However, in pathological scars, the high expression of Tenascin-C and its multi-domain crosslinking networks at high concentrations misdirect nerve fibers, causing ectopic branching and disrupting nerve pathways within the fibrotic region. This misrouting leads to ectopic discharges, which become one of the sources of chronic pain (Jiang et al., 2020; Guimarães et al., 2023). The imbalance between mechanical and biochemical signaling is also amplified by the Piezo1 channel, whose calcium influx in response to mechanical stress in high-stiffness ECM disrupts biochemical signaling in nerve growth cones, triggering pathological pain responses (He et al., 2024; Xu et al., 2023). Furthermore, the imbalance in the expression of miR-29 family and miR-124 in fibrotic tissue and neurons not only regulates collagen synthesis genes but also influences the transcription of TRPV1 and Nav channels, molecularly cooperating to maintain scar-associated neuropathic pain (van Rooij et al., 2008; Hassan et al., 2024; Shao et al., 2016).
3.3 Nerve-mediated scar remodeling feedback
The abundant secretion of NGF from regenerating nerve terminals activates SMAD and mitogen-activated protein kinase (MAPK) signaling pathways in fibroblasts through the TrkA and p75NTR receptors. This significantly upregulates the expression of collagen type I alpha 1 (COL1A1), collagen type III alpha 1 (COL3A1), and α-SMA, promoting myofibroblast transdifferentiation and collagen deposition, which in turn increases scar stiffness and sustains a positive feedback loop of nerve sensitization (M et al., 2023; Micera et al., 2001). SP, released by C-fiber terminals and binding to the NK1 receptor, activates pro-inflammatory pathways such as nuclear factor-kappa B (NF-κB), enhancing neuronal excitability. It also directly promotes fibroblast proliferation and collagen synthesis, thereby maintaining a high activity pro-fibrotic and pro-nociceptive microenvironment at the scar edge (Zaarour et al., 2022). The continuous release of CGRP facilitates myofibroblast differentiation and matrix remodeling through both SMAD-dependent and -independent mechanisms. Its vasodilation and increased permeability effects further promote the infiltration of pro-inflammatory cells and nerve sensitization, strengthening the persistence of chronic pain under high-concentration conditions (Kim and Granstein, 2021). Additionally, pro-inflammatory macrophages in the ganglia and scar tissue interact through IL-1β/brain-derived neurotrophic factor (BDNF), activating microglial cells and sustaining p38 MAPK and epigenetic modifications in the central nervous system, thus constructing pain “memory” and consolidating long-term scar-related pain states (Guimarães et al., 2023; Zhang et al., 2023).
4 Pathophysiology of chronic burn scar pain
Long-term pain arising from burn scars is multifactorial, encompassing peripheral nerve remodeling, ion channel sensitization, central neuroplastic changes, neuro-immune interactions, and the unique biomechanical properties of scar tissue. As summarized in Figure 3, burn scar pain arises from four converging mechanisms—aberrant nerve fiber regrowth and tethering within a rigid collagen matrix, neurogenic inflammation via SP/CGRP-driven mast cell activation, central sensitization through astrocyte/microglial release of IL-1β/COX-2/iNOS, and persistent biomechanical stress from stiff, inelastic scar tissue. The heterogeneity of pain phenotypes and enduring neural adaptations should be recognized to fully understand and manage chronic burn scar pain.
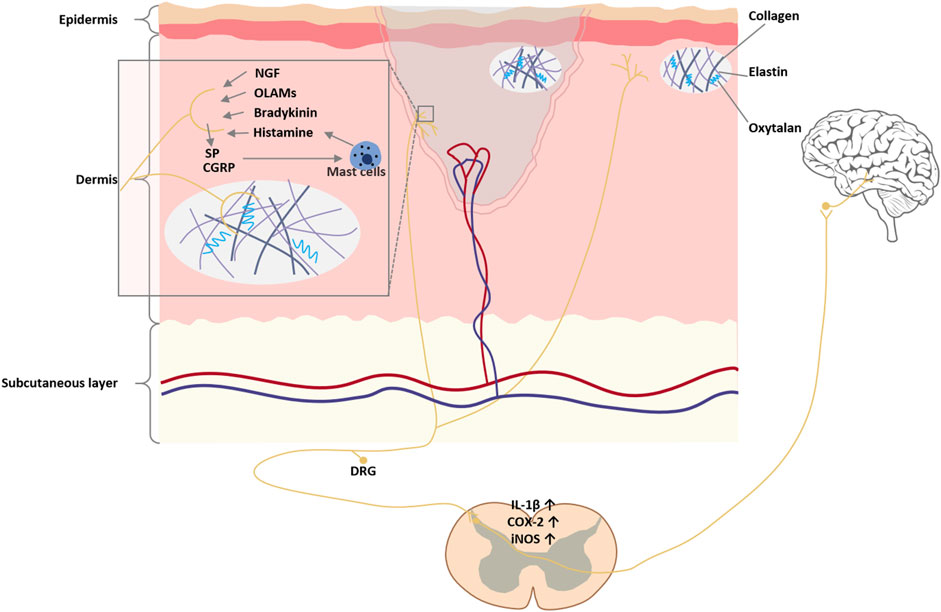
Figure 3. Pathways of burn scar pain. Aberrant nerve fiber regeneration within the scar, driven by elevated NGF, produces neuroma-like clusters and C-fiber hyperinnervation that lower pain thresholds and elicit spontaneous firing, while regenerated fibers tethered in rigid scar tissue generate traction neuropathy. Persistent peripheral nociceptor activation releases SP and CGRP, triggering mast cell degranulation and pro-inflammatory cytokine release, which in turn recruit immune cells and sensitize nociceptors, and sustained input activates spinal astrocytes and microglia to elevate IL-1β, cycloxygenase-2 (COX-2), and iNOS via NF-κB and c-Jun N-terminal kinase (JNK) pathways, producing central “wind-up”. Finally, excessive collagen deposition and cross-linking stiffen the scar—leading to mechanical stress, local ischemia, and microtrauma–inflammation feedback loops around joints or nerve pathways that perpetuate chronic pain.
4.1 Peripheral nerve remodeling and pain phenotype heterogeneity
Burn scars often heal with dysregulated reinnervation, leading to neuroma-like clusters and hyperinnervation that lower mechanical and thermal pain thresholds (Palanivelu et al., 2018). Elevated levels of NGF within the scar promote aberrant sprouting of C-fibers, exacerbating characteristic neuropathic sensations—burning, shooting, or electric shock–like pain—long after wound closure (Bijlard et al., 2017; Cuignet et al., 2015; Adenzato et al., 2018; Barrett et al., 2019). Moreover, regenerating axons may become entrapped within rigid scar matrices, creating traction neuropathy: scar-nerve adhesions restrict normal nerve gliding, so movement of surrounding tissues triggers ectopic discharges perceived as spontaneous or movement-evoked pain (Adenzato et al., 2018; Mewa Kinoo and Singh, 2017). Clinical observations reveal that burn scar pain is not monolithic. Patients exhibit diverse pain phenotypes—mechanical allodynia, spontaneous burning pain, and hyperalgesia—reflecting differential involvement of Aβ, Aδ, and C-fiber subsets and their specific receptor expression profiles (TRPV1, TRPA1, Nav1.7) within the scar (Green et al., 2013; Gouin et al., 2017; Bagood and Isseroff, 2021). Psychological factors and individual coping strategies further shape these phenotypes, as pediatric survivors who rely on internalizing coping exhibit higher long-term anxiety and pain levels (Griffin et al., 2015).
4.2 Ion channel sensitization and peripheral-central neuroplasticity
At the molecular level, burn-induced pain involves upregulation and sensitization of key ion channels on primary afferents. TRPV1 channels are overexpressed and activated by lipid mediators in injured skin, and transient receptor potential ankyrin 1 (TRPA1) contributes to mechanical hypersensitivity; voltage-gated sodium channels such as Nav1.7 are also overproduced, lowering activation thresholds and enabling spontaneous firing. Inflammatory mediators (e.g., bradykinin, TNF-α, IL-1β) amplify these effects via protein kinase C (PKC), NF-κB, and JNK pathways, perpetuating heightened nociceptor excitability (Green et al., 2013; Gouin et al., 2017; Bagood and Isseroff, 2021).
Continuous peripheral nociceptive input drives central sensitization. In rodent models, non-severe burns cause selective loss of large (Type A) dorsal root ganglia (DRG) neurons—with a relative increase in Type B (pain/itch) neurons—indicating lasting DRG remodeling that favors nociceptive signaling (Palanivelu et al., 2018). In the dorsal horn, sustained input activates microglia and astrocytes, elevating COX-2, iNOS, and pro-inflammatory cytokines, which further lower central pain thresholds and produce “wind-up” phenomena characteristic of chronic neuropathic pain (Lee et al., 2022; You et al., 2025).
4.3 Neuro-immune interactions and neurogenic inflammation
Sensory neurons in the scar release SP and CGRP, triggering mast cell degranulation and release of histamine, proteases, and cytokines. This neurogenic inflammation sensitizes nociceptors and recruits additional immune cells, creating a self-sustaining inflammatory loop at the scar site (Abd-Elsayed et al., 2022; Lee et al., 2022).
4.4 Scar biomechanics and sustained mechanical stress
Biomechanical alterations of burn scars drive and sustain chronic pain through multilevel interactions (Morgan et al., 2018; Figure 4). In the course of burn wound remodeling, fibroblasts undergo phenotypic transition into myofibroblasts, leading to overproduction of densely cross-linked collagen. This excessive matrix cross-linking substantially augments scar stiffness and tissue adhesiveness, imposing continuous localized mechanical loads during skin traction or bodily movement, thereby activating peripheral nociceptors (Yin et al., 2022). The resultant mechanical milieu further drives fibrosis through canonical mechanotransduction cascades—integrin/focal adhesion-FAK, Rho/ROCK, and FAK/ERK—promoting sustained myofibroblast contractility and augmented matrix deposition. These processes reinforce each other to form a self-sustaining tension-fibrosis positive feedback loop (Yin et al., 2022). Furthermore, mechanical stimuli activate mechanosensitive ion channels—including the Piezo family and selected TRP channels—on peripheral sensory neurons and other cells, inducing neuronal depolarization and mechanical sensitization, which in turn amplify mechanical pain perception (He et al., 2021). Abnormal patterns of nerve regeneration are frequently observed within scar tissue, characterized by excessive proliferation and misdirected growth of nerve fibers, or entrapment within the fibrotic extracellular matrix, resulting in neuroma formation or small-fiber dysfunction. These alterations are accompanied by upregulation of neurotrophic factors such as NGF, SP, and CGRP, as well as neuroinflammatory mediators, which collectively exacerbate aberrant peripheral discharges and sustain neuropathic pain (Yang et al., 2025). In parallel, impaired microcirculation and focal hypoxia within the scar maintain a state of low-grade chronic inflammation. Repetitive and intense peripheral inputs may, via spinal and supraspinal pathways, induce or aggravate central sensitization, thereby establishing a chronic pain state driven by peripheral–central interplay (Yang et al., 2025).
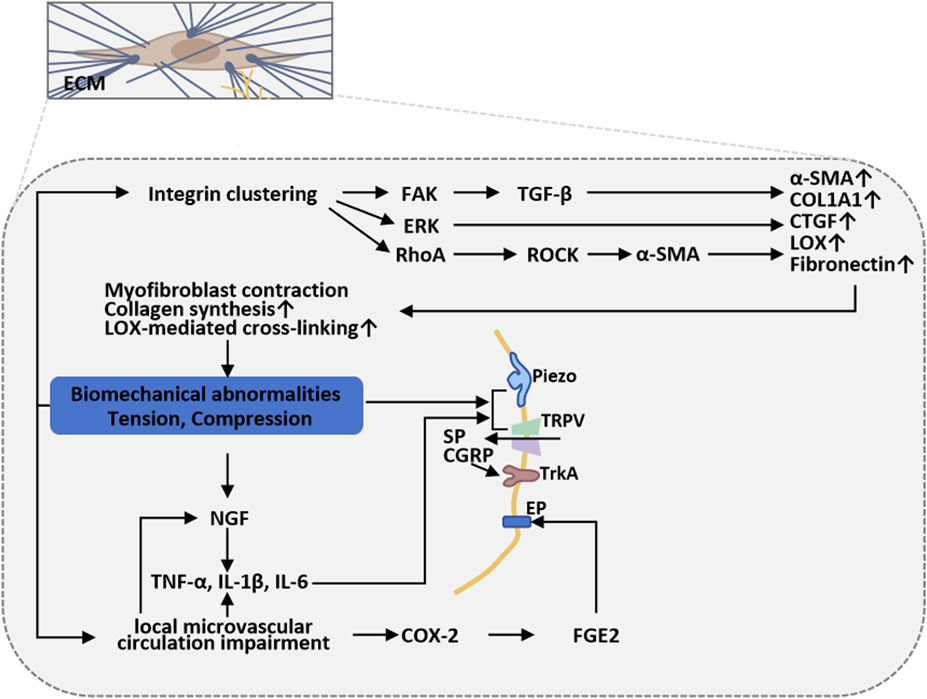
Figure 4. Scar biomechanics and sustained mechanical stress in burn-scar-related chronic pain. Myofibroblast-driven collagen cross-linking increases scar stiffness and adhesiveness, imposing sustained mechanical loads that activate nociceptors and mechanotransduction cascades (integrin/FAK, Rho/ROCK, FAK/ERK), reinforcing fibrosis. Concurrent activation of Piezo/TRP channels, abnormal nerve regeneration, neuroinflammation, and microvascular hypoxia sustains peripheral sensitization and drives central sensitization, maintaining chronic pain.
Based on these mechanisms, clinical interventions that modify the mechanical environment (e.g., tension-reducing sutures, pressure therapy, silicone sheeting) or attenuate traction and neuronal excitability (e.g., local botulinum toxin injection, targeted inhibition of neurotrophic factors, or blockade of mechanosensitive channels) can partially alleviate scar-associated pain. These observations also highlight Piezo channels, the integrin–FAK/Rho signaling axis, and the NGF–neuroinflammation pathway as promising therapeutic targets for future intervention (Tiskratok et al., 2025; Kuehlmann et al., 2020).
5 Research progress of burn scar pain treatment
Burn-scar pain therapies have evolved from single-modality palliation to multimodal, mechanism-based regimens addressing fibrosis, aberrant innervation, and neuroinflammation. These approaches fall into six interrelated categories—laser therapies, ESWT, mechanical supports/topicals, manual and needling therapies, injection/regenerative medicine, and neuromodulation—converge on collagen remodeling, inflammation modulation, and nociceptor desensitization. To provide a clearer overview of current interventions targeting burn scar pain, Table 3 summarizes key treatment categories, specific interventions, mechanisms of action, clinical outcomes, and supporting evidence.
5.1 Laser therapies
Non-pharmacologic modalities leveraging photobiomodulation and mechanotransduction have shown notable efficacy in controlled studies. Low-level laser therapy (LLLT) at 400 mW, 670 nm decreased visual analog scale (VAS) pain scores and improved scar pliability for up to 3 months post-treatment, mechanistically linked to mitochondrial chromophore absorption that enhances adenosine triphosphate (ATP) synthesis, modulates reactive oxygen species, and downregulates pro-inflammatory cytokines via NF-κB inhibition (Gaida et al., 2004; Mansouri et al., 2020). Waibel et al. reported a case of multi-wavelength fractional CO2 laser treatment performed over four decades after napalm burns, resulting in noticeable scar softening, improved texture, and better pliability (Waibel et al., 2018). While the report highlights the long-term potential of laser interventions, it remains a single-patient case study, underscoring the need for larger trials to confirm reproducibility. In a prospective cohort study involving 49 children undergoing 180 laser sessions, significant improvements were observed in both observer- and patient-rated patient and observer scar assessment Scale (POSAS) scores, with marked reductions in stiffness and improved pliability (Patel et al., 2019). Fractional CO2 laser produces microthermal treatment zones within hypertrophic burn scars, promoting controlled collagen remodeling and downregulating TGF-β1 and IL-6, which translates into significant reductions in scar thickness and a 97.1% patient-reported improvement rate in appearance, pliability, and pain (Won et al., 2023). A recent prospective study involving 25 patients with skin of color reported significant reductions in vancouver scar scale (VSS) pliability scores after four treatment sessions, along with improvements in pigmentation and patient-reported outcomes (Khalid et al., 2025). Its high reported patient satisfaction underscores its clinical promise, yet objective measures of pain and randomized control data are lacking. Larger-scale, blinded trials incorporating sensory profiling and neurophysiological correlates are needed to validate and refine these findings.
Multimodal regimens combining fractional CO2, non-ablative 1,540 nm, and 595 nm dye lasers synergistically soften scar bulk, target neovasculature, and modulate pro-inflammatory cytokines, sustaining comfort and reducing scar thickness for at least 3 months (Campolmi et al., 2023). The combination approach targets multiple scar features simultaneously, enhancing efficacy. Regimen complexity and cost may hinder widespread clinical adoption. Comparative studies evaluating cost-effectiveness, treatment sequencing, and long-term analgesic durability across different scar pain phenotypes are warranted. Adjunctive Erbium-YAG (2940 nm) and low-level soft-laser (670 nm) photobiomodulation further enhance collagen reorganization and mitochondrial function to alleviate neuropathic pain (Gaida et al., 2004; Madni et al., 2018; Issler-Fisher et al., 2017). These adjunctive modalities may optimize outcomes through synergistic mitochondrial and extracellular matrix effects. Nonetheless, their additive benefit over monotherapy remains insufficiently quantified.
5.2 Extracorporeal shock wave therapy (ESWT)
ESWT, reduced scar pain by overstimulating and “defunctionalizing” peripheral nociceptors, promoting fibroblast mechanotransduction through AKT signaling, and inducing hyaluronan-rich vesicle release that remodels extracellular matrix and decreases tissue stiffness (Cao et al., 2025). This mechanism-based approach directly targets mechanical contributors to neuropathic pain, offering an advantage in scars with high rigidity. But the underlying nociceptor subtypes affected remain poorly characterized. A single randomized trial suggests ESWT can improve scar appearance, pain, and pruritus over 5 months when added to standard care, but direct comparisons to standard care alone were not statistically significant (Aguilera-Sáez et al., 2022). Larger, longer-term randomized controlled trials (RCTs) with standardized dosing and patient stratification are needed to confirm and optimize ESWT’s clinical benefits in burn scar management. Repetitive micro-mechanical stresses activate fibroblast and endothelial mechanotransduction, upregulating endothelial nitric oxide synthase (eNOS)-mediated angiogenesis and downregulating SP, CGRP, and IL-6, which transiently desensitizes hyperexcitable nociceptors and diminishes neurogenic inflammation (Aguilera-Sáez et al., 2022; Samhan and Abdelhalim, 2019; Chen and Nie, 2022; Moortgat et al., 2020; Joo et al., 2020; Cho et al., 2016). The mechanotransduction effects provide a dual benefit for both scar remodeling and pain relief, supported by animal and human data. However, the analgesic duration is often transient, typically lasting several weeks. Longitudinal trials with extended follow-up are needed to assess sustained desensitization and recurrence risk.
Meta-analyses confirm significant VAS pain reductions (SMD = −0.59; p < 0.0001) and itch relief (SMD = −0.94; p = 0.004) versus standard care (Samhan and Abdelhalim, 2019). This quantitative synthesis strengthens the clinical relevance of ESWT. Heterogeneity across protocols (e.g., intensity, frequency, anatomical site) limits interpretability. Standardized treatment regimens and responder subgroup analyses should be integrated into future RCTs. Combining ESWT with water-filtered infrared-A (wIRA) photobiomodulation further augments IL-10 and suppresses TNF-α and prostaglandin E2 (PGE2) to optimize the healing milieu (Chen and Nie, 2022). This synergistic protocol expands therapeutic impact to both inflammatory and regenerative pathways. Yet, controlled head-to-head trials comparing ESWT alone vs. ESWT+wIRA are lacking. Site-specific protocols for hand scars report superior functional outcomes and scar pliability over sham (Joo et al., 2020), though longer follow-up RCTs highlight the need to refine dosing parameters (Aguilera-Sáez et al., 2022). Targeted anatomical protocols allow for precision medicine applications and better functional recovery. However, reproducibility across different patient populations remains uncertain. More robust multicenter trials are needed to optimize dose, site, and patient-specific customization.
5.3 Mechanical support and topical agents
Silicone gel forms an occlusive, hydrating film that normalizes transepidermal water loss, preventing keratinocyte-driven overproduction of collagen via cytokine-mediated keratinocyte–fibroblast signaling. Pressure garments (15–25 mmHg) apply uniform compression, inducing localized hypoxia that promotes fibroblast apoptosis and realigns collagen fibers along normal skin tension lines, yielding modest reductions in scar height (−0.04 cm; P = 0.05) but inconsistent analgesic benefit (Wiseman et al., 2020; Harris et al., 2024; Li et al., 2018). These results highlight the limited and inconsistent analgesic and anti-hypertrophic effects of current mechanical approaches. While widely prescribed and integrated into burn rehabilitation protocols, clinical studies have reported variable adherence and limited sustained pain relief. Pressure therapy’s analgesic mechanisms remain poorly understood, necessitating mechanistic trials exploring its interaction with local nociceptor function and inflammatory modulation.
Recent efforts to modulate TRP channels have produced compelling early-phase data supporting their role in scar-associated nociception. Topical application of the selective TRPV1 antagonist ACD-440 gel resulted in significant reductions in visual analogue scale (VAS) scores and pinprick pain responses over 4 weeks in a Phase 2a trial, with minimal systemic exposure, likely by inhibiting TRPV1-mediated cation influx and subsequent neurogenic inflammation (Segerdahl et al., 2024). This agent represents a novel, targeted approach to peripheral sensitization with a favorable safety profile. However, the small sample size and short trial duration limit extrapolation. Preclinical work on TRPA1 antagonists such as LY3526318 has further confirmed the potential of targeting multiple TRP family members to attenuate chronic pain, underscoring the value of ion-channel–targeted approaches for neuropathic components of burn pain (Green et al., 2016; Salas et al., 2017; Mellado Lagarde et al., 2024). These mechanistic insights open avenues for multi-target topical therapies. Translational gaps persist as human trials of TRPA1 antagonists remain scarce. Topical 5% lidocaine plasters inhibit voltage-gated sodium channels in peripheral nerve terminals, reducing numerical rating scale (NRS) pain scores by 58.2% ± 27.8% and decreasing painful surface area by 72.4% ± 24.7%, with 69% of patients reporting functional gains (Correa-Illanes et al., 2010). This modality offers rapid, localized analgesia with a favorable tolerability profile and minimal systemic side effects. However, long-term use may be limited by cost, adherence, and tolerance development. Comparative studies assessing lidocaine plasters versus ion-channel antagonists would help determine optimal first-line topical therapy.
5.4 Manual and needling therapies
Neuromodulation approaches targeting central pain networks have produced encouraging preliminary results. Scrambler therapy—using algorithmically varied electrocutaneous stimuli to “scramble” pain signals transmitted via C-fibers—yielded significant pain reductions in chronic burn patients over 2 weeks. Concurrent functional magnetic resonance imaging (MRI) revealed normalization of activity within the pain matrix, supporting its central neuromodulatory mechanism (Lee et al., 2022). This non-pharmacologic technique shows promise for central desensitization, but current evidence stems from small, uncontrolled cohorts. Transcranial direct current stimulation (tDCS) over the primary motor cortex decreased pain anxiety and improved pain thresholds in burn patients, likely through enhancement of descending inhibitory pathways and modulation of cortical excitability, although effects on itch and pain intensity warrant further study (Hosseini Amiri et al., 2016; Lefaucheur et al., 2017). The method is non-invasive and well-tolerated, but optimal stimulation parameters, duration of effect, and responder profiles remain to be defined. Mechanical therapies also play a role.
Mechanical manipulation disrupts fibrotic architecture and gates nociceptive signaling. Soft-tissue mobilization (STM) applies sustained shear and compression to break aberrant collagen cross-links, realign fibers, and enhance microvascular perfusion. Pilot RCTs note within-group ROM gains and subjective pain relief, albeit without significant between-group differences. While STM is safe and low-cost, its additive benefit in multimodal protocols is unclear and requires larger trials with objective outcome metrics (Silverberg et al., 1996). Scar massage (30 min twice weekly) employs effleurage and Petrissage to stimulate Aβ fibers, invoking spinal gate-control analgesia and achieving an additional 1.2-point VAS reduction versus controls (Cho et al., 2014; Field et al., 2000). Randomized trial (Anthonissen et al.) has rigorously evaluated a manual/needling modality—depressomassage—in addition to standard physiotherapy. Over 6 months, adding depressomassage did not improve scar colour, transepidermal water loss, or pain (VAS) compared with physiotherapy alone (Anthonissen et al., 2018). No controlled trials of soft-tissue mobilization, scar massage, acupuncture, or neuromodulatory techniques (e.g., scrambler therapy, tDCS) were identified, highlighting a critical need for well-designed RCTs to establish their efficacy in burn-scar pain management. Perilesional acupuncture combined with scar massage activates deqi-related central analgesic pathways, producing sustained NRS pain decreases for up to 6 months post-treatment (Tuckey et al., 2022). This integrative regimen demonstrates durability, but sample sizes were small and control groups varied. Overall, mechanical manipulation can plausibly disrupt fibrotic architecture and gate nociceptive signaling. However, high-quality, adequately powered RCTs with objective outcome measures (for example, blinded ROM assessment, validated pain scales, perfusion or ECM imaging, and MMP/TIMP biomarkers) are needed to define the magnitude, durability, and optimal role of these interventions in burn-scar pain management.
5.5 Injection and regenerative medicine
Bioactive injections target both scar tissue and nociceptive pathways. Bioactive injections target both scar tissue and nociceptive pathways. Rodent models show a 45% reduction in collagen deposition and increased mechanical withdrawal thresholds at weeks 7–8 (Huang et al., 2018), suggesting concurrent anti-fibrotic and analgesic effects. In a randomized intra-patient trial, platelet-rich plasma (PRP) accelerated donor-site healing (55% vs. 20% epithelialization by day 8, p = 0.036) and improved pain/scar outcomes versus hydrocolloid dressings (García-Sánchez et al., 2022). These findings underscore the dual benefits of injectable strategies in modulating both mechanical and sensory properties of scar tissue. The translation of these results to humans remains preliminary, highlighting the need for dose-escalation studies and long-term safety data. Separately, combining medical needling with non-cultured autologous skin cell suspension achieved significant repigmentation in 85% of participants at 12 months (Busch et al., 2016). Botulinum toxin A (BTX-A) cleaves synaptosomal-associated protein, 25 kDa (SNAP-25) to block acetylcholine and neuropeptide release, and inhibits fibroblast proliferation, producing greater scar-thickness and pruritus reductions than triamcinolone acetonide (P = 0.0287; P = 0.0482) (Hoseininejad et al., 2024; Tawfik and Ali, 2023). While these results mirror preclinical dual anti-fibrotic and analgesic effects, the field lacks randomized, placebo-controlled, long-term trials for agents like botulinum toxin A, adipose-derived stem cells, and cytokine modulators.
Anti-inflammatory modulation within the scar microenvironment has emerged as another promising avenue. Autologous fat grafting into burn scars, rich in adipose-derived stem cells, significantly downregulated pro-inflammatory cytokines (IL-1β, TNF-α) and enzymes (COX-2, iNOS, nNOS) within both scar tissue and spinal dorsal horns, correlating with reduced mechanical allodynia and thermal hyperalgesia in animal models (Huang et al., 2015; Fredman et al., 2016; Lin et al., 2017). The mechanism involves suppression of NF-κB and JNK signaling pathways, leading to decreased neuroinflammation and spinal neuronal apoptosis. This dual peripheral-central mechanism suggests that ADSC-based therapy may interrupt chronic pain circuits beyond the scar. There is a need for larger-scale, placebo-controlled human trials to evaluate durability and consistency of the effect. Building on these preclinical data, small open-label trials of the IL-1 receptor antagonist anakinra in patients with chronic hypertrophic scars are underway, aiming to attenuate peripheral nociceptor sensitization and central neuroinflammatory cascades (Huang et al., 2018; Lin et al., 2017). The rationale is strong, given the role of IL-1β in both pain initiation and maintenance. However, human data are currently limited to early-phase exploratory studies.
Regenerative cell therapies seek to restore normal dermal architecture and nerve patterning while mitigating fibrosis. Mesenchymal stem cell (MSC)-based therapies aim to restore dermal structure, promote ordered nerve regeneration, and mitigate fibrosis. A Phase 1 dose-escalation study of intravenous mesenchymal stem cells (MSCs) in acute burn patients reported accelerated wound closure and reduced scar thickness. Secondary analyses noted lower patient-reported pain scores during rehabilitation, likely reflecting MSC-mediated paracrine release of anti-fibrotic (HGF, IL-10) and neurotrophic factors that promote orderly reinnervation (Lin et al., 2017). While this represents a promising avenue for mechanism-based repair, regulatory inconsistencies and cost barriers currently limit widespread implementation. Autologous fat grafting, through the adipose-derived stem cells (ADSCs), promotes angiogenesis, reduces inflammation, and regulates immune responses by releasing growth factors and cytokines, thereby alleviating scar-related pain. By reducing extracellular matrix stiffness and improving tissue compliance, it may reduce mechanical strain on regenerating nerve fibers—thereby attenuating pain (Ahmad et al., 2024). This mechanical-biological synergy is appealing in post-surgical scar management. However, variability in graft take and resorption rates poses a challenge to reproducibility. Optimization of delivery techniques and integration with other regenerative methods may enhance outcomes.
5.6 Neuromodulation and emerging techniques
Targeted electrical or magnetic stimuli directly modulate pain circuits. Auricular magnetic-bead stimulation of Shenmen and Subcortex enhances parasympathetic tone, reducing VAS pain from 4.8 ± 1.2 to 2.6 ± 1.0, though effects partially revert by 1 month (Chen et al., 2021). This technique is low-cost, non-invasive, and easily repeatable, offering a valuable option for patients with contraindications to systemic therapy. However, its effects appear transient, with partial symptom rebound within 1 month. Electroacupuncture elevates Aδ and C-fiber thresholds and yields significant pain-score reductions in responders (Cuignet et al., 2015). Its analgesic effects likely involve spinal segmental modulation and endorphin release, offering a dual peripheral–central mechanism.
Spinal cord stimulation offers opioid-sparing analgesia in refractory burn pain and permits permanent implantation with cessation of opioid use. Its long-term implantation capability and central targeting offer advantages in persistent neuropathic pain states. Its invasive nature, high cost, and risk of complications (e.g., infection, lead migration) limit its use to select, treatment-resistant patients. Scrambler therapy algorithmically “scrambles” C-fiber input to normalize aberrant pain signaling, demonstrating significant, durable VAS reductions and central network modulation on MRI (Lee et al., 2022). Its central desensitization mechanism is particularly relevant to chronic burn pain, which often involves spinal sensitization. Though promising, scrambler therapy currently lacks large-scale validation, and its optimal treatment schedule and durability beyond several months remain unclear.
5.7 Biomarker-driven stratification and personalized approaches
Burn scar pain represents a multifaceted clinical challenge, with patients exhibiting predominantly inflammatory-driven pain, peripheral neuropAthic pain, or centrally sensitized pain. Biomarker-driven stratification offers the potential to categorize patients based on dominant pain mechanisms, thereby guiding individualized, mechanism-specific interventions. For instance, patients exhibiting elevated levels of pro-inflammatory mediators such as IL-1β and TNF-α in scar tissue and plasma may represent an inflammatory-dominant phenotype. These patients could benefit preferentially from anti-inflammatory and immunomodulatory therapies, such as IL-1 receptor antagonists or adipose-derived stem cell (ADSC) grafting, which have been shown to downregulate inflammatory cytokines and alleviate neuroimmune sensitization both peripherally and within the spinal cord (Huang et al., 2015). This subset may also respond favorably to regenerative biologics with anti-fibrotic properties targeting NF-κB and JNK pathways (Fredman et al., 2016; Lin et al., 2017).
Upregulation of ion channels—particularly TRPV1 and TRPA1—has been observed in patients with peripheral sensitization phenotypes, marked by thermal hyperalgesia and mechanical allodynia. In such cases, TRPV1 antagonists like ACD-440 have demonstrated significant reductions in VAS pain scores in early-phase clinical trials, with favorable tolerability and minimal systemic exposure (Segerdahl et al., 2024). Targeting these ion channels with topical or injectable formulations could yield personalized relief in patients identified through molecular or sensory profiling (Segerdahl et al., 2024; Green et al., 2016; Salas et al., 2017).
For patients whose pain persists despite peripheral intervention—and in whom neuroimaging reveals sustained cortical hyperactivity or altered pain network connectivity—a centrally sensitized phenotype may be inferred. These individuals may benefit more from neuromodulatory interventions such as tDCS, Scrambler therapy, or spinal cord stimulation (Cuignet et al., 2015; Lee et al., 2022; Hosseini Amiri et al., 2016; Lefaucheur et al., 2017). Functional MRI and quantitative sensory testing (QST) can assist in identifying central amplification patterns, enabling early selection of central nervous system (CNS)-directed therapies.
Despite these advances, implementation remains limited by several barriers. Most biomarker studies to date are exploratory, underpowered, and lack standardization. Multicenter prospective trials with defined mechanistic endpoints are needed to validate stratification frameworks and assess predictive utility. Moreover, integration of these biomarkers into real-time clinical workflows will require user-friendly platforms, possibly augmented by artificial intelligence and digital health technologies.
Several critical gaps persist in burn-scar pain research despite recent advances. Most clinical trials remain small and use heterogeneous outcome measures, limiting meta-analytic synthesis and generalizability (Deflorin et al., 2020; Nguyen et al., 2025). There is no consensus on validated, burn-specific pain assessment scales—particularly for pediatric and darker-skinned populations—leading to underrepresentation and measurement bias (Nguyen et al., 2025; Jeschke et al., 2020). Long-term follow-up beyond 1 year is uncommon, hindering evaluation of durability and potential late-emerging adverse effects of emerging therapies (Jeschke et al., 2020; Dobson et al., 2024). Correlative studies linking molecular or imaging biomarkers to clinical pain outcomes are scarce, obstructing biomarker-driven patient stratification and mechanism-based treatment optimization (Siu et al., 2025; Atiyeh et al., 2025). Head-to-head comparisons of TRP-channel antagonists, cell-based regeneration, photobiomodulation, and neuromodulation are lacking, precluding evidence-based selection among modalities (Siu et al., 2025; Amini-Nik et al., 2018). Regulatory and manufacturing inconsistencies in cell therapies impede reproducibility across centers and raise cost-effectiveness concerns that remain largely unaddressed (Siu et al., 2025), ENREF77 (Atiyeh et al., 2025). Moreover, the potential of digital health platforms and artificial intelligence for personalized pain management in burn scars remains unexplored, representing a promising yet untapped frontier.
6 Conclusion
Scar formation after burn injury is a multi-phase, multifactorial process regulated by complex interactions. The TGF-β signaling pathway plays a central role by promoting the transdifferentiation of fibroblasts into myofibroblasts and enhancing collagen I/III deposition, thereby contributing to the excessive fibrotic matrix. NGF and neuropeptides such as SP and CGRP not only facilitate peripheral nerve regeneration but also exert bidirectional regulatory effects within the scar microenvironment by modulating angiogenesis and fibroblast proliferation.
A dynamic balance between pro-inflammatory cytokines (e.g., IL-6, TNF-α, IL-1β) and anti-inflammatory cytokines (e.g., IL-10, TGF-β3) is maintained throughout the early, middle, and late stages of scar development, jointly influencing matrix remodeling and scar maturation. In peripheral sensitization, upregulation of TRPV1 and Nav channels leads to reduced pain thresholds, while neurogenic inflammation and central sensitization—mediated through neuropeptide release, microglial activation, and epigenetic modifications—sustain and amplify chronic pain signaling. The high stiffness and organized collagen structure of scar ECM act not only as mechanical barriers but also, under specific conditions, provide biochemical cues for nerve fiber guidance. The balance between these opposing properties is crucial for nerve regeneration and pain perception.
In parallel with these mechanistic insights, burn scar pain management has evolved toward multimodal, mechanism-based strategies. Contemporary interventions include laser therapy and ESWT to promote collagen remodeling and reduce scar stiffness, as well as adjunctive topical agents and pressure garments to modulate inflammation and attenuate hyperalgesia. Regenerative injection therapies (such as stem cell or platelet-rich plasma injections) aim to reverse fibrosis and foster tissue regeneration. Neuromodulation techniques and TRP-channel antagonists (for example, TRPV1 inhibitors) are being applied to desensitize nociceptors and modulate central pain networks. In addition, biomarker-driven stratification also offers new opportunities to tailor burn scar pain treatments based on individual pain mechanisms. These modalities collectively address the peripheral and central contributors to chronic scar pain. However, robust outcome measures and long-term efficacy for these treatments require further validation.
Author contributions
MZ: Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Shangqiu Science and Technology Project (2024116).
Acknowledgments
We would like to thank Scientific Research Program of Shangqiu City for their assistance with this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abd-Elsayed A., Pope J., Mundey D. A., Slavin K. V., Falowski S., Chitneni A., et al. (2022). Diagnosis, treatment, and management of painful scar: a narrative review. J. pain Res. 15, 925–937. doi:10.2147/JPR.S355096
Adenzato M., Mauck M. C., Shupp J. W., Williams F., Villard M. A., Jones S. W., et al. (2018). Hypertrophic scar severity at autograft sites is associated with increased pain and itch after major thermal burn injury. J. neural Transm. 39 (4), 536–544. doi:10.1093/jbcr/irx012
Aguilera-Sáez J., Dos Santos B. P., Serracanta J., Monte-Soldado A., Bosacoma P., Rivas-Nicolls D., et al. (2022). The effect of Extracorporeal Shock Wave Therapy in the treatment of burn scars: a prospective, randomized, controlled trial. Burns J. Int. Soc. Burn Inj. 48 (3), 577–584. doi:10.1016/j.burns.2021.06.006
Ahmad N., Anker A., Klein S., Dean J., Knoedler L., Remy K., et al. (2024). Autologous fat grafting-A panacea for scar tissue therapy? Cells 13 (16), 1384. doi:10.3390/cells13161384
Amini-Nik S., Yousuf Y., Jeschke M. G. (2018). Scar management in burn injuries using drug delivery and molecular signaling: current treatments and future directions. Adv. drug Deliv. Rev. 123, 135–154. doi:10.1016/j.addr.2017.07.017
Anthonissen M., Meirte J., Moortgat P., Maertens K., Daly D., Fieuws S., et al. (2018). Influence on clinical parameters of depressomassage (part I): the effects of depressomassage on color and transepidermal water loss rate in burn scars: a pilot comparative controlled study. Burns J. Int. Soc. Burn Inj. 44 (4), 877–885. doi:10.1016/j.burns.2017.11.004
Atiyeh B., El Hachem T. F., Chalhoub R., Emsieh S. E. (2025). Have the recent advancements in wound repair and scar management technology improved the quality of life in burn patients? Burns J. Int. Soc. Burn Inj. 51 (4), 107443. doi:10.1016/j.burns.2025.107443
Bagood M. D., Isseroff R. R. (2021). TRPV1: role in skin and skin diseases and potential target for improving wound healing. Int. J. Mol. Sci. 22 (11), 6135. doi:10.3390/ijms22116135
Balaji S., Wang X., King A., Le L. D., Bhattacharya S. S., Moles C. M., et al. (2017). Interleukin-10-mediated regenerative postnatal tissue repair is dependent on regulation of hyaluronan metabolism via fibroblast-specific STAT3 signaling. FASEB J. 31 (3), 868–881. doi:10.1096/fj.201600856R
Barrett L. W., Fear V. S., Waithman J. C., Wood F. M., Fear M. W. (2019). Understanding acute burn injury as a chronic disease. Burns and trauma 7, 23. doi:10.1186/s41038-019-0163-2
Bijlard E., Uiterwaal L., Kouwenberg C. A., Mureau M. A., Hovius S. E., Huygen F. J. (2017). A systematic review on the prevalence, etiology, and pathophysiology of intrinsic pain in dermal scar tissue. Pain physician 20, 1–13.
Busch K. H., Bender R., Walezko N., Aziz H., Altintas M. A., Aust M. C. (2016). Combination of medical needling and non-cultured autologous skin cell transplantation (ReNovaCell) for repigmentation of hypopigmented burn scars. J. Cosmet. dermatology 42 (7), 1556–1566. doi:10.1016/j.burns.2016.04.009
Cai L., Xiong X., Kong X., Xie J. (2017). The role of the lysyl oxidases in tissue repair and remodeling: a concise review. Tissue Eng. Regen. Med. 14 (1), 15–30. doi:10.1007/s13770-016-0007-0
Campolmi P., Quintarelli L., Fusco I. (2023). A multimodal approach to laser treatment of extensive hypertrophic burn scar: a case report. Am. J. case Rep. 24, e939022. doi:10.12659/AJCR.939022
Cao B., Tang X., Liu C., Xu G., Lei M., Wu F., et al. (2025). Unlocking new Frontiers: the cellular and molecular impact of extracorporeal shock wave therapy (ESWT) on central nervous system (CNS) disorders and peripheral nerve injuries (PNI). Exp. Neurol. 384, 115052. doi:10.1016/j.expneurol.2024.115052
Chang Z., Kishimoto Y., Hasan A., Welham N. V. (2014). TGF-β3 modulates the inflammatory environment and reduces scar formation following vocal fold mucosal injury in rats. Dis. models and Mech. 7 (1), 83–91. doi:10.1242/dmm.013326
Chaudhari N., Findlay A. D., Stevenson A. W., Clemons T. D., Yao Y., Joshi A., et al. (2023). Author Correction: topical application of an irreversible small molecule inhibitor of lysyl oxidases ameliorates skin scarring and fibrosis. Nat. Commun. 14 (1), 135. doi:10.1038/s41467-023-35849-x
Chen J., Nie J. (2022). Effects of extracorporeal shock wave combined with vivaphototherapy on Blood perfusion, inflammatory response, and prognosis in burn patients. Comput. Math. methods Med. 2022, 1386875. doi:10.1155/2022/1386875
Chen C. C., Chen S. P., Lyu S. Y., Hsu C. H. (2021). Application of auriculotherapy for post-burn scar syndrome in Young adults with major burns. J. Acupunct. meridian Stud. 14 (4), 127–136. doi:10.51507/j.jams.2021.14.4.127
Cho Y. S., Jeon J. H., Hong A., Yang H. T., Yim H., Cho Y. S., et al. (2014). The effect of burn rehabilitation massage therapy on hypertrophic scar after burn: a randomized controlled trial. Burns J. Int. Soc. Burn Inj. 40 (8), 1513–1520. doi:10.1016/j.burns.2014.02.005
Cho Y. S., Joo S. Y., Cui H., Cho S. R., Yim H., Seo C. H. (2016). Effect of extracorporeal shock wave therapy on scar pain in burn patients: a prospective, randomized, single-blind, placebo-controlled study. Medicine 95 (32), e4575. doi:10.1097/MD.0000000000004575
Chung B. Y., Kim H. B., Jung M. J., Kang S. Y., Kwak I. S., Park C. W., et al. (2020). Post-burn pruritus. Int. J. Mol. Sci. 21 (11), 3880. doi:10.3390/ijms21113880
Cialdai F., Risaliti C., Monici M. (2022). Role of fibroblasts in wound healing and tissue remodeling on Earth and in space. Front. Bioeng. Biotechnol. 10, 958381. doi:10.3389/fbioe.2022.958381
Correa-Illanes G., Calderón W., Roa R., Piñeros J. L., Dote J., Medina D. (2010). Treatment of localized post-traumatic neuropathic pain in scars with 5% lidocaine medicated plaster. Local regional Anesth. 3, 77–83. doi:10.2147/LRA.S13082
Cuignet O., Pirlot A., Ortiz S., Rose T. (2015). The effects of electroacupuncture on analgesia and peripheral sensory thresholds in patients with burn scar pain. J. burn care and Res. 41 (6), 1298–1305. doi:10.1016/j.burns.2015.03.002
Deflorin C., Hohenauer E., Stoop R., van Daele U., Clijsen R., Taeymans J. (2020). Physical management of scar tissue: a systematic review and meta-analysis. J. Altern. complementary Med. (New York, N.Y.) 26 (10), 854–865. doi:10.1089/acm.2020.0109
Dobson G. P., Morris J. L., Letson H. L. (2024). Pathophysiology of severe burn injuries: new therapeutic opportunities from a systems perspective. J. burn care and Res. 45 (4), 1041–1050. doi:10.1093/jbcr/irae049
Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., et al. (2011). Role of YAP/TAZ in mechanotransduction. Nature 474 (7350), 179–183. doi:10.1038/nature10137
Faour S., Farahat M., Aijaz A., Jeschke M. G. (2023). Fibrosis in burns: an overview of mechanisms and therapies. Am. J. physiology. Cell physiology 325 (6), C1545–C1557. doi:10.1152/ajpcell.00254.2023
Field T., Peck M., Hernandez-Reif M., Krugman S., Burman I., Ozment-Schenck L., et al. (2000). Postburn itching, pain, and psychological symptoms are reduced with massage therapy. J. burn care and rehabilitation 21 (3), 189–193. doi:10.1067/mbc.2000.105087
Fredman R., Edkins R. E., Hultman C. S. (2016). Fat grafting for neuropathic pain after severe burns. Ann. plastic Surg. 76 (Suppl. 4), S298–S303. doi:10.1097/SAP.0000000000000674
Gaida K., Koller R., Isler C., Aytekin O., Al-Awami M., Meissl G., et al. (2004). Low Level Laser Therapy--a conservative approach to the burn scar? Burns J. Int. Soc. Burn Inj. 30 (4), 362–367. doi:10.1016/j.burns.2003.12.012
Gallucci R. M., Lee E. G., Tomasek J. J. (2006). IL-6 modulates alpha-smooth muscle actin expression in dermal fibroblasts from IL-6-deficient mice. J. investigative dermatology 126 (3), 561–568. doi:10.1038/sj.jid.5700109
García-Sánchez J. M., Mirabet Lis V., Ruiz-Valls A., Pérez-Plaza A., Sepúlveda Sanchis P., Pérez-Del-Caz M. D. (2022). Platelet rich plasma and plasma rich in growth factors for split-thickness skin graft donor site treatment in the burn patient setting: a randomized clinical trial. Burns J. Int. Soc. Burn Inj. 48 (7), 1662–1670. doi:10.1016/j.burns.2021.10.001
Gouin O., L'Herondelle K., Lebonvallet N., Le Gall-Ianotto C., Sakka M., Buhé V., et al. (2017). TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: pro-inflammatory response induced by their activation and their sensitization. Protein and cell 8 (9), 644–661. doi:10.1007/s13238-017-0395-5
Green D. P., Ruparel S., Roman L., Henry M. A., Hargreaves K. M. (2013). Role of endogenous TRPV1 agonists in a postburn pain model of partial-thickness injury. Pain 154 (11), 2512–2520. doi:10.1016/j.pain.2013.07.040
Green D., Ruparel S., Gao X., Ruparel N., Patil M., Akopian A., et al. (2016). Central activation of TRPV1 and TRPA1 by novel endogenous agonists contributes to mechanical allodynia and thermal hyperalgesia after burn injury. Mol. pain 12, 1744806916661725. doi:10.1177/1744806916661725
Griffin B., Rimmer R. B., Alam N. B., Bay R. C., Sadler I. J., Foster K. N., et al. (2015). The reported pain coping strategies of pediatric burn survivors-does a correlation exist between coping style and development of anxiety disorder? BMJ open 36 (2), 336–343. doi:10.1097/BCR.0000000000000109
Guimarães R. M., Aníbal-Silva C. E., Davoli-Ferreira M., Gomes F. I. F., Mendes A., Cavallini M. C. M., et al. (2023). Neuron-associated macrophage proliferation in the sensory ganglia is associated with peripheral nerve injury-induced neuropathic pain involving CX3CR1 signaling. eLife 12, e78515. doi:10.7554/eLife.78515
Harris I. M., Lee K. C., Deeks J. J., Moore D. J., Moiemen N. S., Dretzke J. (2024). Pressure-garment therapy for preventing hypertrophic scarring after burn injury. Cochrane database Syst. Rev. 1 (1), Cd013530. doi:10.1002/14651858.CD013530.pub2
Hassan M., Shahzadi S., Yasir M., Chun W., Kloczkowski A. (2024). Therapeutic implication of miRNAs as an active regulatory player in the management of pain: a review. A Rev. 15 (8), 1003. doi:10.3390/genes15081003
He J., Fang B., Shan S., Xie Y., Wang C., Zhang Y., et al. (2021). Mechanical stretch promotes hypertrophic scar formation through mechanically activated cation channel Piezo1. Cell Death Dis. 12 (3), 226. doi:10.1038/s41419-021-03481-6
He J., Cheng X., Fang B., Shan S., Li Q. (2024). Mechanical stiffness promotes skin fibrosis via Piezo1-Wnt2/Wnt11-CCL24 positive feedback loop. Cell death and Dis. 15 (1), 84. doi:10.1038/s41419-024-06466-3
Hettiaratchy S., Dziewulski P. (2004a). ABC of burns. Introduction. BMJ Clin. Res. ed. 328 (7452), 1366–1368. doi:10.1136/bmj.328.7452.1366
Hettiaratchy S., Dziewulski P. (2004b). ABC of burns: pathophysiology and types of burns. BMJ Clin. Res. ed. 328 (7453), 1427–1429. doi:10.1136/bmj.328.7453.1427
Hinz B., Celetta G., Tomasek J. J., Gabbiani G., Chaponnier C. (2001). Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol. Biol. cell 12 (9), 2730–2741. doi:10.1091/mbc.12.9.2730
Hochman B., Tucci-Viegas V. M., Monteiro P. K. P., França J. P., Gaiba S., Ferreira L. M. (2014). The action of CGRP and SP on cultured skin fibroblasts. Central Eur. J. Biol. 9, 717–726. doi:10.2478/s11535-014-0301-6
Hoseininejad S. S., Rahbar R., Farhadi M., Godarzi S. (2024). Comparative evaluation of intralesional injection of botulinum toxin type A versus triamcinolone acetonide in treating post-burn pruritus. Maedica 19 (4), 763–768. doi:10.26574/maedica.2024.19.4.763
Hosseini Amiri M., Tavousi S. H., Mazlom S. R., Manzari Z. S. (2016). Effect of transcranial direct current stimulation on pain anxiety during burn wound care. Burns J. Int. Soc. Burn Inj. 42 (4), 872–876. doi:10.1016/j.burns.2016.01.006
Huang S. H., Wu S. H., Lee S. S., Chang K. P., Chai C. Y., Yeh J. L., et al. (2015). Fat grafting in burn scar alleviates neuropathic pain via anti-inflammation effect in scar and spinal cord. PloS one 10 (9), e0137563. doi:10.1371/journal.pone.0137563
Huang S. H., Wu S. H., Lee S. S., Lin Y. N., Chai C. Y., Lai C. S., et al. (2018). Platelet-rich plasma injection in burn scar areas alleviates neuropathic scar pain. Int. J. Med. Sci. 15 (3), 238–247. doi:10.7150/ijms.22563
Issler-Fisher A. C., Fisher O. M., Smialkowski A. O., Li F., van Schalkwyk C. P., Haertsch P., et al. (2017). Ablative fractional CO(2) laser for burn scar reconstruction: an extensive subjective and objective short-term outcome analysis of a prospective treatment cohort. Burns J. Int. Soc. Burn Inj. 43 (3), 573–582. doi:10.1016/j.burns.2016.09.014
Jeschke M. G., van Baar M. E., Choudhry M. A., Chung K. K., Gibran N. S., Logsetty S. (2020). Burn injury. Nat. Rev. Dis. Prim. 6 (1), 11. doi:10.1038/s41572-020-0145-5
Ji R. R., Xu Z. Z., Gao Y. J. (2014). Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 13 (7), 533–548. doi:10.1038/nrd4334
Jiang D., Christ S., Correa-Gallegos D., Ramesh P., Kalgudde Gopal S., Wannemacher J., et al. (2020). Injury triggers fascia fibroblast collective cell migration to drive scar formation through N-cadherin. Nat. Commun. 11 (1), 5653. doi:10.1038/s41467-020-19425-1
Johnson B. Z., Stevenson A. W., Prêle C. M., Fear M. W., Wood F. M. (2020). The role of IL-6 in skin fibrosis and cutaneous wound healing. Biomedicines 8 (5), 101. doi:10.3390/biomedicines8050101
Joo S. Y., Lee S. Y., Cho Y. S., Seo C. H. (2020). Clinical utility of extracorporeal shock wave therapy on hypertrophic scars of the hand caused by burn injury: a prospective, randomized, double-blinded study. J. Clin. Med. 9 (5), 1376. doi:10.3390/jcm9051376
El Kahi C. G., Atiyeh B. S., Abdallah Hajj Hussein I., Jurjus R., Dibo S. A., Jurjus A., et al. (2009). Modulation of wound contracture alpha-smooth muscle actin and multispecific vitronectin receptor integrin alphavbeta3 in the rabbit's experimental model. Int. wound J. 6 (3), 214–224. doi:10.1111/j.1742-481X.2009.00597.x
Khalid S., Sanabria B., Tan I., Khan S., Khan H., Rao B. (2025). Treatment of postburn hypertrophic scaring in skin of color with fractional CO2 laser - a prospective cohort study. JAAD Int. 19, 35–42. doi:10.1016/j.jdin.2025.01.001
Kim Y. J., Granstein R. D. (2021). Roles of calcitonin gene-related peptide in the skin, and other physiological and pathophysiological functions. Brain, Behav. and Immun. - health 18, 100361. doi:10.1016/j.bbih.2021.100361
King A., Balaji S., Le L. D., Crombleholme T. M., Keswani S. G. (2014). Regenerative wound healing: the role of interleukin-10. Adv. wound care 3 (4), 315–323. doi:10.1089/wound.2013.0461
Ko U. H., Choi J., Choung J., Moon S., Shin J. H. (2019). Physicochemically tuned myofibroblasts for wound healing strategy. Sci. Rep. 9 (1), 16070. doi:10.1038/s41598-019-52523-9
Krzyszczyk P., Schloss R., Palmer A., Berthiaume F. (2018). The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front. physiology 9, 419. doi:10.3389/fphys.2018.00419
Kuehlmann B., Bonham C. A., Zucal I., Prantl L., Gurtner G. C. (2020). Mechanotransduction in wound healing and fibrosis. J. Clin. Med. 9 (5), 1423. doi:10.3390/jcm9051423
Larson B. J., Longaker M. T., Lorenz H. P. (2010). Scarless fetal wound healing: a basic science review. Plastic Reconstr. Surg. 126 (4), 1172–1180. doi:10.1097/PRS.0b013e3181eae781
Lee S. Y., Park C. H., Cho Y. S., Kim L., Yoo J. W., Joo S. Y., et al. (2022). Scrambler therapy for chronic pain after burns and its effect on the cerebral pain network: a prospective, double-blinded, randomized controlled trial. J. Clin. Med. 11 (15), 4255. doi:10.3390/jcm11154255
Lefaucheur J. P., Antal A., Ayache S. S., Benninger D. H., Brunelin J., Cogiamanian F., et al. (2017). Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. neurophysiology 128 (1), 56–92. doi:10.1016/j.clinph.2016.10.087
Li P., Li-Tsang C. W. P., Deng X., Wang X., Wang H., Zhang Y., et al. (2018). The recovery of post-burn hypertrophic scar in a monitored pressure therapy intervention programme and the timing of intervention. Burns J. Int. Soc. Burn Inj. 44 (6), 1451–1467. doi:10.1016/j.burns.2018.01.008
Lin C. H., Wu S. H., Lee S. S., Lin Y. N., Kuo Y. R., Chai C. Y., et al. (2017). Autologous adipose-derived stem cells reduce burn-induced neuropathic pain in a rat model. Int. J. Mol. Sci. 19 (1), 34. doi:10.3390/ijms19010034
Liu Z., Wu H., Huang S. (2021). Role of NGF and its receptors in wound healing (Review). Exp. Ther. Med. 21 (6), 599. doi:10.3892/etm.2021.10031
Lu Y. Z., Nayer B., Singh S. K., Alshoubaki Y. K., Yuan E., Park A. J., et al. (2024). CGRP sensory neurons promote tissue healing via neutrophils and macrophages. Nature 628 (8008), 604–611. doi:10.1038/s41586-024-07237-y
M G. E. B., Dosh L., Haidar H., Gerges A., Baassiri S., Leone A., et al. (2023). Nerve growth factor and burn wound healing: update of molecular interactions with skin cells. Burns J. Int. Soc. Burn Inj. 49 (5), 989–1002. doi:10.1016/j.burns.2022.11.001
Madni T. D., Nakonezny P. A., Imran J. B., Clark A. T., Cunningham H. B., Hoopman J. E., et al. (2018). Patient satisfaction after fractional ablation of burn scar with 2940nm wavelength Erbium-Yag laser. Burns J. Int. Soc. Burn Inj. 44 (5), 1100–1105. doi:10.1016/j.burns.2018.02.004
Mansouri V., Arjmand B., Rezaei Tavirani M., Razzaghi M., Rostami-Nejad M., Hamdieh M. (2020). Evaluation of efficacy of low-level laser therapy. J. lasers Med. Sci. 11 (4), 369–380. doi:10.34172/jlms.2020.60
Mellado Lagarde M. M., Wilbraham D., Martins R. F., Zhao H. S., Jackson K., Johnson K. W., et al. (2024). Clinical proof-of-concept results with a novel TRPA1 antagonist (LY3526318) in 3 chronic pain states. Pain 166, 1497–1518. doi:10.1097/j.pain.0000000000003487
Meng Z., Qiu Y., Lin K. C., Kumar A., Placone J. K., Fang C., et al. (2018). RAP2 mediates mechanoresponses of the Hippo pathway. Nature 560 (7720), 655–660. doi:10.1038/s41586-018-0444-0
Mewa Kinoo S., Singh B. (2017). Complex regional pain syndrome in burn pathological scarring: a case report and review of the literature. Burns J. Int. Soc. Burn Inj. 43 (3), e47–e52. doi:10.1016/j.burns.2017.02.007
Micera A., Vigneti E., Pickholtz D., Reich R., Pappo O., Bonini S., et al. (2001). Nerve growth factor displays stimulatory effects on human skin and lung fibroblasts, demonstrating a direct role for this factor in tissue repair. Proc. Natl. Acad. Sci. U. S. A. 98 (11), 6162–6167. doi:10.1073/pnas.101130898
Moi A. L., Haugsmyr E., Heisterkamp H. (2016). Long-term study of health and quality of life after burn injury. Ann. burns fire disasters 29 (4), 295–299.
Moortgat P., Anthonissen M., Van Daele U., Vanhullebusch T., Maertens K., De Cuyper L., et al. (2020). The effects of shock wave therapy applied on hypertrophic burn scars: a randomised controlled trial. Scars, burns and Heal. 6, 2059513120975624. doi:10.1177/2059513120975624
Morgan M., Deuis J. R., Frøsig-Jørgensen M., Lewis R. J., Cabot P. J., Gray P. D., et al. (2018). Burn pain: a systematic and critical review of epidemiology, pathophysiology, and treatment. Pain Med. (Malden, Mass.) 19 (4), 708–734. doi:10.1093/pm/pnx228
Nessler M. B., Puchała J., Chrapusta A., Nessler K., Drukała J. (2014). Levels of plasma matrix metalloproteinases (MMP-2 and MMP-9) in response to INTEGRA® dermal regeneration template implantation. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 20, 91–96. doi:10.12659/MSM.889135
Nguyen A., Duckworth E., Abu-Romman A., Melnick B., Coles B., Galiano R. D. (2025). Exploring the gaps: a scoping review of burn injury research in skin of colour. Wound repair Regen. 33 (1), e13252. doi:10.1111/wrr.13252
Page-McCaw A., Ewald A. J., Werb Z. (2007). Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. cell Biol. 8 (3), 221–233. doi:10.1038/nrm2125
Palanivelu V., Maghami S., Wallace H. J., Wijeratne D., Wood F. M., Fear M. W. (2018). Loss of Type A neuronal cells in the dorsal root ganglion after a non-severe full-thickness burn injury in a rodent model. Burns J. Int. Soc. Burn Inj. 44 (7), 1792–1800. doi:10.1016/j.burns.2018.04.008
Patel S. P., Nguyen H. V., Mannschreck D., Redett R. J., Puttgen K. B., Stewart F. D. (2019). Fractional CO2 laser treatment outcomes for pediatric hypertrophic burn scars. J. burn care and Res. 40 (4), 386–391. doi:10.1093/jbcr/irz046
Peña O. A., Martin P. (2024). Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 25 (8), 599–616. doi:10.1038/s41580-024-00715-1
Qin Z., Xia W., Fisher G. J., Voorhees J. J., Quan T. (2018). YAP/TAZ regulates TGF-β/Smad3 signaling by induction of Smad7 via AP-1 in human skin dermal fibroblasts. Cell Commun. Signal. CCS 16 (1), 18. doi:10.1186/s12964-018-0232-3
Rolfe K. J., Grobbelaar A. O. (2012). A review of fetal scarless healing. ISRN Dermatol. 2012, 698034. doi:10.5402/2012/698034
van Rooij E., Sutherland L. B., Thatcher J. E., DiMaio J. M., Naseem R. H., Marshall W. S., et al. (2008). Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. U. S. A. 105 (35), 13027–13032. doi:10.1073/pnas.0805038105
Roy T. K., Uniyal A., Tiwari V. (2023). Correction to: multifactorial pathways in burn injury-induced chronic pain: novel targets and their pharmacological modulation. Mol. Biol. Rep. 50 (6), 5525. doi:10.1007/s11033-023-08369-6
Salas M. M., Clifford J. L., Hayden J. R., Iadarola M. J., Averitt D. L. (2017). Local resiniferatoxin induces long-lasting analgesia in a rat model of full thickness thermal injury. Pain Med. (Malden, Mass.) 18 (12), 2453–2465. doi:10.1093/pm/pnw260
Samhan A. F., Abdelhalim N. M. (2019). Impacts of low-energy extracorporeal shockwave therapy on pain, pruritus, and health-related quality of life in patients with burn: a randomized placebo-controlled study. Burns J. Int. Soc. Burn Inj. 45 (5), 1094–1101. doi:10.1016/j.burns.2019.02.007
Di Sarno L., Eftimiadi G., Chiaretti A., Manni L., Genovese O., Soligo M., et al. (2024). Increased serum levels of proNGF, mature NGF and interleukins in burn-injured children. Eur. Rev. Med. Pharmacol. Sci. 28 (11), 3787–3795. doi:10.26355/eurrev_202406_36385
Segerdahl M., Rother M., Halldin M. M., Popescu T., Schaffler K. (2024). Topically applied novel TRPV1 receptor antagonist, ACD440 Gel, reduces evoked pain in healthy volunteers, a randomized, double-blind, placebo-controlled, crossover study. Eur. J. pain London, Engl. 28 (10), 1656–1673. doi:10.1002/ejp.2299
Serini G., Gabbiana G. (1996). Modulation of alpha-smooth muscle actin expression in fibroblasts by transforming growth factor-beta isoforms: an in vivo and in vitro study. Wound repair Regen. 4 (2), 278–287. doi:10.1046/j.1524-475X.1996.40217.x
Shahabi S., Hassan Z. M., Jazani N. H. (2009). Post heat shock tolerance: a neuroimmunological anti-inflammatory phenomenon. J. Inflamm. Lond. Engl. 6, 7. doi:10.1186/1476-9255-6-7
Shao J., Cao J., Wang J., Ren X., Su S., Li M., et al. (2016). MicroRNA-30b regulates expression of the sodium channel Nav1.7 in nerve injury-induced neuropathic pain in the rat. Mol. pain 12, 1744806916671523. doi:10.1177/1744806916671523
Silverberg R., Johnson J., Moffat M. (1996). The effects of soft tissue mobilization on the immature burn scar: results of a pilot study. J. burn care and rehabilitation 17 (3), 252–259. doi:10.1097/00004630-199605000-00013
Singampalli K. L., Balaji S., Wang X., Parikh U. M., Kaul A., Gilley J., et al. (2020). The role of an IL-10/hyaluronan Axis in dermal wound healing. Front. cell Dev. Biol. 8, 636. doi:10.3389/fcell.2020.00636
Siu W. S., Ma H., Leung P. C. (2025). Review on current advancements in facilitation of burn wound healing. Bioeng. Basel, Switz. 12 (4), 428. doi:10.3390/bioengineering12040428
Stanton E., Won P., Manasyan A., Gurram S., Gilllenwater T. J., Yenikomshian H. A. (2024). Neuropathic pain in burn patients - a common problem with little literature: a systematic review. Burns J. Int. Soc. Burn Inj. 50 (5), 1053–1061. doi:10.1016/j.burns.2024.02.013
Taskinen H. S., Heino J., Röyttä M. (1995). The dynamics of beta 1 integrin expression during peripheral nerve regeneration. Acta neuropathol. 89 (2), 144–151. doi:10.1007/BF00296358
Tawfik A. A., Ali R. A. (2023). Evaluation of botulinum toxin type A for treating post burn hypertrophic scars and keloid in children: an intra-patient randomized controlled study. J. Cosmet. dermatology 22 (4), 1256–1260. doi:10.1111/jocd.15634
L. Téot, T. A. Mustoe, E. Middelkoop, and G. G. Gauglitz (2020). “Textbook on scar management: state of the art management and emerging technologies,” This book is an open access publication.
Tiskratok W., Chuinsiri N., Limraksasin P., Kyawsoewin M., Jitprasertwong P. (2025). Extracellular matrix stiffness: mechanotransduction and mechanobiological response-driven strategies for biomedical applications targeting fibroblast inflammation. Polym. (Basel). 17 (6), 822. doi:10.3390/polym17060822
Tomasek J. J., Gabbiani G., Hinz B., Chaponnier C., Brown R. A. (2002). Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. cell Biol. 3 (5), 349–363. doi:10.1038/nrm809
Tuckey C. R., Kohut S. H., Edgar D. W. (2022). Case study: pilot testing of a local acupuncture intervention protocol for burn scars. Scars, burns and Heal. 8, 20595131211058430. doi:10.1177/20595131211058430
Tyack Z., Ziviani J., Kimble R., Plaza A., Jones A., Cuttle L., et al. (2015). Measuring the impact of burn scarring on health-related quality of life: development and preliminary content validation of the Brisbane Burn Scar Impact Profile (BBSIP) for children and adults. Burns J. Int. Soc. Burn Inj. 41 (7), 1405–1419. doi:10.1016/j.burns.2015.05.021
Waibel J. S., Phuc Phan Thi K., Hoenig L. J. (2018). Laser treatment performed more than 4 decades after napalm burns: healing the scars of the napalm girl. JAMA dermatol. 154 (10), 1228–1229. doi:10.1001/jamadermatol.2018.2774
Walton K. L., Johnson K. E., Harrison C. A. (2017). Targeting TGF-β mediated SMAD signaling for the prevention of fibrosis. Front. Pharmacol. 8, 461. doi:10.3389/fphar.2017.00461
Wang X. Q., Kravchuk O., Winterford C., Kimble R. M. (2011). The correlation of in vivo burn scar contraction with the level of α-smooth muscle actin expression. Burns J. Int. Soc. Burn Inj. 37 (8), 1367–1377. doi:10.1016/j.burns.2011.07.018
Werner S., Grose R. (2003). Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 83 (3), 835–870. doi:10.1152/physrev.2003.83.3.835
Wiseman J., Ware R. S., Simons M., McPhail S., Kimble R., Dotta A., et al. (2020). Effectiveness of topical silicone gel and pressure garment therapy for burn scar prevention and management in children: a randomized controlled trial. Clin. Rehabil. 34 (1), 120–131. doi:10.1177/0269215519877516
Won P., Cooper M., Gillenwater T. J., Yenikomshian H. A. (2023). Treatment of hypertrophic burn scars with laser therapy: a review of adverse events. Ann. plastic Surg. 91 (6), 715–719. doi:10.1097/SAP.0000000000003712
Wynn T. A. (2008). Cellular and molecular mechanisms of fibrosis. J. pathology 214 (2), 199–210. doi:10.1002/path.2277
Xing L., Chen B., Qin Y., Li X., Zhou S., Yuan K., et al. (2024). The role of neuropeptides in cutaneous wound healing: a focus on mechanisms and neuropeptide-derived treatments. Front. Bioeng. Biotechnol. 12, 1494865. doi:10.3389/fbioe.2024.1494865
Xu Y., Huang Y., Cheng X., Hu B., Jiang D., Wu L., et al. (2023). Mechanotransductive receptor Piezo1 as a promising target in the treatment of fibrosis diseases. Front. Mol. Biosci. 10, 1270979. doi:10.3389/fmolb.2023.1270979
Yan L., Cui Z. (2023). Integrin β1 and the repair after nervous system injury. Eur. Neurol. 86 (1), 2–12. doi:10.1159/000526690
Yang E., Xu R., Zhang H., Xia W., Huang X., Zan T. (2025). Deciphering pain and pruritus in keloids from the perspective of neurological dysfunction: where are we now? Biomedicines 13 (3), 663. doi:10.3390/biomedicines13030663
Yao Y., Findlay A., Stolp J., Rayner B., Ask K., Jarolimek W. (2022). Pan-lysyl oxidase inhibitor PXS-5505 ameliorates multiple-organ fibrosis by inhibiting collagen crosslinks in rodent models of systemic sclerosis. Int. J. Mol. Sci. 23 (10), 5533. doi:10.3390/ijms23105533
Yin J., Zhang S., Yang C., Wang Y., Shi B., Zheng Q., et al. (2022). Mechanotransduction in skin wound healing and scar formation: potential therapeutic targets for controlling hypertrophic scarring. Front. Immunol. 13, 1028410. doi:10.3389/fimmu.2022.1028410
You Z., Jain S., Shen S., Mao J., Martyn J. A. J. (2025). Pathophysiology and management of burn injury-induced pain. Burns open Int. open access J. burn Inj. 10, 100396. doi:10.1016/j.burnso.2025.100396
Zaarour R. F., Saha D., Dey R., Dutta A., Kumar P., Rana I., et al. (2022). The neuropeptide Substance P facilitates the transition from an inflammatory to proliferation phase-associated responses in dermal fibroblasts. Exp. Dermatol. 31 (8), 1188–1201. doi:10.1111/exd.14573
Keywords: burns, management, pain, scars (cicatrix), treatment
Citation: Zhao M (2025) Burn scar pain: from mechanisms to treatments. Front. Physiol. 16:1627798. doi: 10.3389/fphys.2025.1627798
Received: 13 May 2025; Accepted: 08 September 2025;
Published: 24 September 2025.
Edited by:
Jacobo Elies, University of Bradford, United KingdomReviewed by:
Zoltan Meszar, University of Debrecen, HungaryArchita Sharma, Texas A and M University, United States
Debajyoti Pal, College of Veterinary Sciences and Animal Husbandry, India
Copyright © 2025 Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minjuan Zhao, MTY2NTA2MzA5MzNAMTYzLmNvbQ==
 Minjuan Zhao
Minjuan Zhao