- School of Physical Education, Hubei University of Technology, Wuhan, Hubei, China
Background: Various exercise interventions have been widely applied to enhance site-specific bone mineral density in menopausal females. This network meta-analysis aims to assess and compare the impact of these interventions on improving BMD in this demographic.
Methods: A systematic search of PubMed, EMBASE, Cochrane Library, and Web of Science was made up to 4 December 2024 to detect randomized controlled trials (RCTs) comparing continuous endurance training whole-body vibration resistance training multicomponent training mind-body training intermittent training and combined training against control interventions. Primary outcomes included lumbar spine bone mineral density and femoral neck bone mineral density while secondary outcomes covered whole body bone mineral density and total hip bone mineral density A Bayesian random-effects NMA was performed.
Results: Fifty-five RCTs involving 3,453 participants were included. Compared with the control group, MCT demonstrated greater efficacy in improving FNBMD (mean difference [MD] 0.02; 95% credible interval [CrI] [0.01, 0.04]). Based on the surface under the cumulative ranking (SUCRA), MBT ranked highest for LSBMD (75.9%), CT for WBBMD (77.6%), and MBT for THBMD (60.7%), suggesting potential benefits of these interventions.
Conclusion: This study suggests that MBT, MCT, and CT may contribute to improving BMD in elderly postmenopausal women. However, further rigorously designed RCTs are warranted to validate these findings.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42025636067.
1 Background
With the growing aging population, the bone health of postmenopausal women has become a critical public health concern, particularly among the old population (Tartibian et al., 2011). The drop in estrogen levels during menopause catalyzes an accelerated process of bone resorption. This process ultimately leads to a marked depletion of bone mineral density (BMD) (Guadalupe-Grau et al., 2009). The resultant decline in BMD amplifies the susceptibility of osteoporotic fractures and associated complications, predisposing postmenopausal women to osteoporosis (OP) (Fu et al., 2011; De Aguiar et al., 2023). Notably, OP has been reported to impair functional ability and quality of life in this demographic (Ma et al., 2016; Benzinger et al., 2019; Autier et al., 2000), which is also strongly associated with elevated mortality rates, healthcare expenditures, and socioeconomic burdens on individuals, families, and society.
Currently, antiresorptive agents, especially bisphosphonates (BPPs), remain the mainstay pharmacological treatment for OP. However, due to their mechanism of binding to bone matrix and inhibiting bone resorption, they may also interfere with normal bone remodeling and reduce bone flexibility (Li et al., 2021; Russell et al., 2007). In contrast, exercise interventions have demonstrated promising benefits in improving BMD, preventing fractures, and mitigating OP progression in postmenopausal women, with minimal adverse effects (Tarantino et al., 2017; Kemmler et al., 2020). Accumulating evidence supports that exercise interventions, such as continuous endurance training (CET), resistance training (RT), mind-body training (MBT), whole-body vibration (WBV), combined training (CT), and multicomponent training (MCT), may effectively improve BMD in elderly postmenopausal women and further enhance their quality of life (de Oliveira et al., 2019; Aboarrage Junior et al., 2018; Marques et al., 2011a; Wen et al., 2017; Ammann and Rizzoli, 2003; Zou et al., 2017; Hejazi et al., 2022; Mohammad et al., 2020). Nevertheless, sustaining sufficient intensity and duration of exercise interventions may pose challenges for this demographic, making the identification of optimal exercise intervention essential (Burton et al., 2017; Sherrington et al., 2020).
Existing research on exercise interventions for BMD improvement in elderly postmenopausal females has produced a mosaic of inconsistent findings. For example, a meta-analysis by Hejazi et al. after incorporating 53 randomized controlled trials (RCTs) reported that RT, CT, and MBT significantly improved femoral neck bone mineral density (FNBMD), while CET and CT were effective in enhancing lumbar spine bone mineral density (LSBMD). Conversely, Mohammad Rahimi et al. (Mohammad et al., 2020) observed no significant effects of CET on LSBMD or FNBMD. Notably, WBV appeared to outperform CET, RT, and CT in improving LSBMD (Mohammad et al., 2020). Additionally, the analysis of Zehnacker et al. on 20 RCTs (Zehnacker and Bemis-Dougherty, 2007) further highlighted that site-specific high-intensity weight-bearing training improved BMD in the spine and hip among menopausal females (Zehnacker and Bemis-Dougherty, 2007). These discrepancies may be ascribed to variations in exercise interventions, intervention durations, intensities, demographic characteristics, and study designs. Furthermore, the scarcity of high-quality trials directly comparing different exercise interventions limits definitive conclusions regarding the most efficacious intervention (Hejazi et al., 2022). To date, systematic reviews and meta-analyses consistently report that exercise interventions help mitigate the loss of BMD in elderly postmenopausal women. However, the relative efficacy of various exercise interventions across demographics with osteoporosis, osteopenia, or normal bone mass remains unclear.
Network meta-analysis (NMA) facilitates the concurrent comparison of multiple exercise interventions, even when direct head-to-head trials are unavailable. Moreover, NMA allows for the ranking of interventions by efficacy outcome, thereby offering comprehensive evidence to inform clinical decision-making. Therefore, the objective of systematic review and NMA in this study is to elucidate the impacts of diverse exercise interventions on BMD among elderly postmenopausal women by synthesizing comparative evidence either directly or indirectly. The findings will provide scientifically grounded recommendations for designing exercise strategies specifically tailored to improve BMD in this demographic.
2 Methods
2.1 Design and registration
The NMA adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Guidelines. The study protocol was prospectively registered in the International Prospective Register of Systematic Reviews (PROSPERO; Registration No. CRD42025636067).
2.2 Inclusion and exclusion criteria
The inclusion criteria were defined using the PICOS framework (Participants, Interventions, Comparators, Outcomes, Study Design) as follows: (1) Participants: Postmenopausal women aged ≥60 years with non-adherence to exercise guidelines (defined as engaging in less than 120 min of weekly physical activity). (2) Interventions: At least one form of exercise intervention (regardless of form or duration), including CET, RT, CT, WBV, INT, MBT, or MCT. These exercise interventions are among the most extensively investigated modalities for improving BMD in older postmenopausal women (de Oliveira et al., 2019; Aboarrage Junior et al., 2018; Marques et al., 2011a; Wang et al., 2015; Li et al., 2023; Riaz et al., 2024). (3) Comparator: Non-exercise control groups. (4) Outcomes: At least one of the following outcomes: Primary outcomes included LSBMD and FNBMD, while secondary outcomes covered whole body bone mineral density (WBBMD) and total hip bone mineral density (THBMD). (5) Study Design: Randomized controlled trials (RCTs) only. (6) Language: Studies published solely in English.
The exclusion criteria comprised: (1) Non-postmenopausal cohorts; (2) Studies with undefined intervention protocols; (3) Non-RCT designs, such as cohort studies, review articles, case reports, descriptive studies, opinion pieces, or conference abstracts; (4) Studies with incomplete, inaccurate, or irretrievable data.
2.3 Search strategy
Two investigators (M.M.Y. and L.D.S.) independently performed a comprehensive literature search in PubMed, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), and Web of Science up to 4 December 2024. No restrictions were imposed regarding publication type, date, or status. The search combined Medical Subject Headings (MeSH) and free-text keywords, covering all known variants (postmenopausal) AND (exercise OR physical activity OR continuous endurance training OR mind–body training OR intermittent training OR resistance training OR multicomponent training OR combined training) AND (RCT). To minimize the risk of missing eligible studies, reference lists of relevant articles were also manually screened by both investigators. (The full search strategy is detailed in Supplementary Appendix A).
2.4 Study selection
Using the predefined inclusion and exclusion criteria, two investigators (M.M.Y and L.D.S) independently screened studies. All potentially relevant records were input into EndNote X9, with duplicates removed. Title/abstract screening was made to exclude irrelevant studies, followed by full-text evaluations. Discrepancies were settled via discussion or consultation with a third investigator (S.W.T).
2.5 Data extraction and quality assessment
The information was gathered via a standardized data extraction form as follows: (1) Study characteristics, including the first author’s name, publication year, and country; (2) Participant characteristics, including age, sample size of intervention and control groups, and demographic type (healthy vs osteoporotic); (3) Intervention details, including exercise intervention measures and duration; (4) Reported outcomes. Data were extracted by one investigator (M.M.Y) and cross-validated by another investigator (L.D.S).
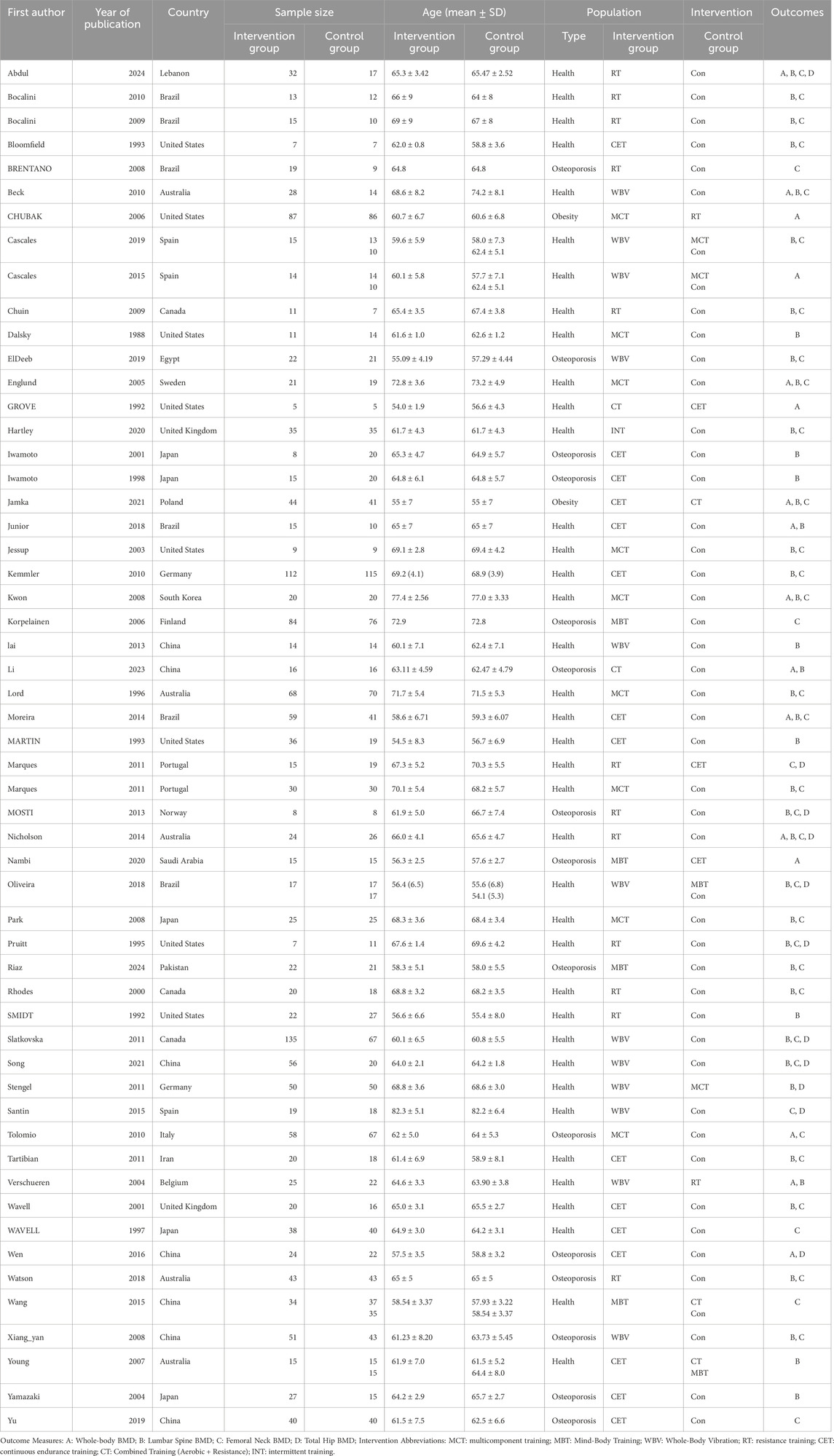
Two investigators (M.M.Y. and L.D.S.) independently examined the potential risk of bias for every included study via the Cochrane Risk of Bias 2.0 (ROB 2.0) tool (Sterne et al., 2019; McGuinness and Higgins, 2021). Any discrepancies were settled via discussion. The tool evaluates six domains: (1) Randomization process; (2) Deviations from intended interventions; (3) Missing outcome data; (4) Measurement of the outcome; (5) Selection of the reported result; (6) Overall bias. Each domain was assigned one of three ratings: “low risk,” “some concerns,” or “high risk.” The overall bias classification followed a hierarchical structure: “low risk” (all domains rated as low), “some concerns” (at least one domain with some concerns but none with high risk), or “high risk” (at least one domain rated as high risk). Discrepancies were resolved via discussion or, if necessary, consultation with a third investigator.
2.6 Data synthesis and statistical analysis
Statistical models employing Bayesian frameworks were built via JAGS software (gemtc 0.8-2 and rjags 4–10 packages) in R (version 4.4.3). Continuous outcomes were analyzed using mean differences (MDs) with 95% credible interval (CrI) to quantify effect sizes. Random-effects models addressed clinical heterogeneity across studies (e.g., country, exercise interventions, participant health status, intervention duration, calcium supplementation) throughout NMA. Four Markov Chain Monte Carlo (MCMC) chains were run for each outcome, with 50,000 iterations per chain, discarding the first 20,000 iterations as burn-in. Convergence was evaluated via trace plots and the Gelman–Rubin–Brooks diagnostic statistic (Brooks and Gelman, 1998). Relative intervention rankings by outcome were estimated through surface under the cumulative ranking (SUCRA) (Veron et al., 2016). Higher SUCRA values mean better intervention rankings. Model fit and consistency were assessed by comparing the Deviance Information Criterion (DIC). A DIC difference of less than 5 was considered indicative of good consistency, in which case the consistency model was retained (Dempster, 1997). Local inconsistencies in closed loops were analyzed via node-splitting. The presence of publication bias was systematically explored using adjusted funnel plots. Network plots and adjusted funnel plots were generated using Stata version 15.0.
3 Results
3.1 Search results
The PRISMA flow diagram (Figure 1) details the study selection process. The initial search identified 13,300 records from the four databases. Following the removal of 4,869 duplicates, 8,192 studies were excluded via title/abstract screening. Full-text reviews excluded 193 studies (non-RCT designs, irrelevant populations, or incomplete data) (details provided in Figure 1). An additional 9 records were identified through reference screening, resulting in 55 eligible studies eventually incorporated into the NMA.
3.2 Characteristics of included studies
Table 1 sets out the characteristics and detailed information of the studies incorporated into the NMA. Between 1998 and 2024, fifty-five RCTs fulfilled the inclusion criteria. Geographically, seventeen studies were conducted in the Americas (de Oliveira et al., 2019; Aboarrage Junior et al., 2018; Chubak et al., 2006; Moreira et al., 2014; Bocalini et al., 2010; Bocalini et al., 2009; Martin and Notelovitz, 1993; Bloomfield et al., 1993; Smidt et al., 1992; Grove and Londeree, 1992; Slatkovska et al., 2011; Jessup et al., 2003; Brentano et al., 2008; Dalsky et al., 1988; Chuin et al., 2009; Pruitt et al., 1995; Rhodes et al., 2000), seventeen in Asia (Tartibian et al., 2011; Wen et al., 2017; Wang et al., 2015; Li et al., 2023; Riaz et al., 2024; Abdul-Al et al., 2024; Brooke-Wavell et al., 1997; Lai et al., 2013; Song and Yang, 2021; Kwon et al., 2008; Iwamoto et al., 2001; Iwamoto et al., 1998; Ruan et al., 2008; Yamazaki et al., 2004; Yu et al., 2019; Nambi et al., 2020; Park et al., 2008), fifteen in Europe (Marques et al., 2011a; Jamka et al., 2021; Hartley et al., 2020; Marín-Cascales et al., 2019; Marin-Cascales et al., 2015; Brooke-Wavell et al., 2001; von Stengel et al., 2011; Verschueren et al., 2004; Englund et al., 2005; Marques et al., 2011b; Kemmler et al., 2010; Korpelainen et al., 2006; Mosti et al., 2013; Tolomio et al., 2010; Santin-Medeiros et al., 2015), five in Oceania (Nicholson et al., 2015; Young et al., 2007; Watson et al., 2018; Beck and Norling, 2010; Lord et al., 1996), and one in Africa (ElDeeb and Abdel-Aziem, 2020). Collectively, these RCTs encompassed 3,453 menopause women aged between 54.1 and 82.3 years, with sample sizes between 14 and 227 participants. Among the participants, 2,292 were healthy, 258 were classified as overweight or obese, and 903 were diagnosed with osteoporosis or osteopenia. The interventions varied across studies: seventeen studies involved CET (Aboarrage Junior et al., 2018; Wen et al., 2017; Moreira et al., 2014; Martin and Notelovitz, 1993; Bloomfield et al., 1993; Grove and Londeree, 1992; Brooke-Wavell et al., 1997; Iwamoto et al., 2001; Iwamoto et al., 1998; Yamazaki et al., 2004; Yu et al., 2019; Nambi et al., 2020; Jamka et al., 2021; Brooke-Wavell et al., 2001; Marques et al., 2011b; Kemmler et al., 2010; Tartibian et al., 2011), fourteen studies focused on RT (Chubak et al., 2006; Bocalini et al., 2010; Bocalini et al., 2009; Smidt et al., 1992; Brentano et al., 2008; Chuin et al., 2009; Pruitt et al., 1995; Rhodes et al., 2000; Abdul-Al et al., 2024; Verschueren et al., 2004; Marques et al., 2011b; Mosti et al., 2013; Nicholson et al., 2015; Watson et al., 2018), sixteen studies implemented MCT (Marques et al., 2011a; Chubak et al., 2006; Jessup et al., 2003; Dalsky et al., 1988; Lai et al., 2013; Kwon et al., 2008; Park et al., 2008; Marín-Cascales et al., 2019; Marin-Cascales et al., 2015; von Stengel et al., 2011; Englund et al., 2005; Kemmler et al., 2010; Korpelainen et al., 2006; Tolomio et al., 2010; Nicholson et al., 2015; Lord et al., 1996), twelve studies utilized WBV (de Oliveira et al., 2019; Slatkovska et al., 2011; Lai et al., 2013; Song and Yang, 2021; Ruan et al., 2008; Marín-Cascales et al., 2019; Marin-Cascales et al., 2015; von Stengel et al., 2011; Verschueren et al., 2004; Santin-Medeiros et al., 2015; Beck and Norling, 2010; ElDeeb and Abdel-Aziem, 2020), five studies employed CT (Wang et al., 2015; Li et al., 2023; Grove and Londeree, 1992; Jamka et al., 2021; Young et al., 2007), six studies incorporated MBT (de Oliveira et al., 2019; Wang et al., 2015; Riaz et al., 2024; Nambi et al., 2020; Korpelainen et al., 2006; Young et al., 2007), two studies utilized high-intensity INT (Smidt et al., 1992; Hartley et al., 2020). Intervention protocols are detailed in Supplementary Table S1.
3.3 Quality assessment
The risk of bias across the included RCTs was examined via standardized criteria. Out of the fifty-five studies, twenty-five (45.5%) were assessed as having some concerns regarding bias, twenty-three (41.8%) were deemed low risk, and seven (12.7%) were considered high risk. Most RCTs adequately described their randomization procedures. However, the primary sources of bias stemmed from insufficient reporting on allocation concealment and blinding of outcome assessors. A comprehensive risk of bias assessment for every study is available in Supplementary Figure S1. Figure 2 sums up the risk of bias assessments.
3.4 NMA
3.4.1 Primary outcomes
3.4.1.1 LSBMD
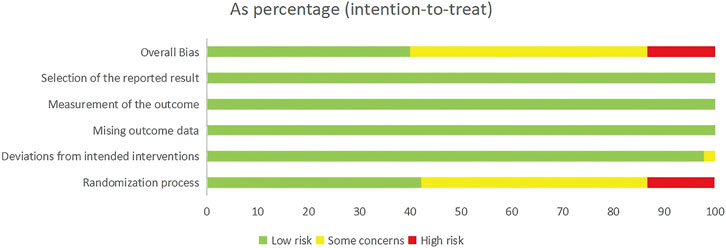
A total of forty-one RCTs evaluated the effects of seven exercise interventions on LSBMD in postmenopausal women. NMA results (Figure 3A) revealed that, compared to the control group, WBV (MD: 0.03; 95% CrI [0.01, 0.04]), MCT (MD: 0.02; 95% CrI [0.00, 0.05]), and RT (MD: 0.01; 95% CrI [0.00, 0.02]) significantly increased LSBMD. Conversely, CET (MD: 0.03; 95% CrI [0.01, 0.05]) was associated with a significant increase in LSBMD (Figure 3B). SUCRA analysis indicated that MBT ranked highest for enhancing LSBMD (75.9%), suggesting it may be a particularly promising intervention (Figure 3C).
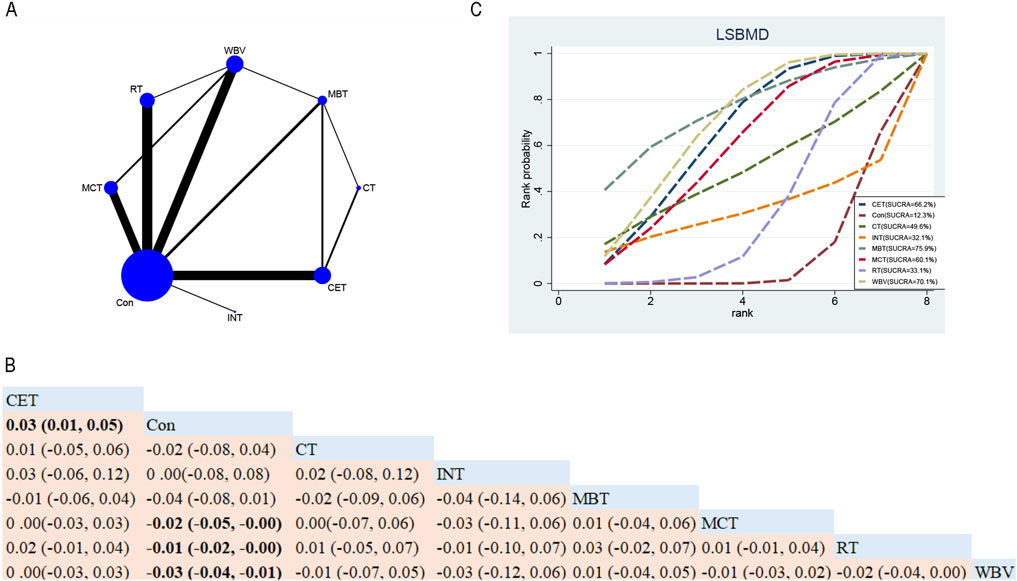
Figure 3. Network Diagram and NMA Results. (A) Network diagram for LSBMD (g/cm2). (B) Relative efficacy of diverse exercise interventions on LSBMD. Note: Estimates are expressed as MD with 95% CrI (in parentheses). Read comparisons between interventions from left to right. Effectiveness estimates are at the intersection of exercise intervention columns and rows. Significant results are shown in bold. (C) SUCRA for different exercise interventions on LSBMD. The exercise interventions in the plot are: MCT–Multicomponent training, MBT–Mind-body training, WBV–Whole-body vibration, RT–Resistance training, CET–Continuous endurance training, CT–Combined training, INT–Intermittent training, Con–Control group.
3.4.1.2 FNBMD
Overall, thirty-eight RCTs assessed the impacts of seven exercise interventions on FNBMD in postmenopausal women (Figure 4A). NMA results (Figure 4B) showed that compared with the control group, MCT (MD 0.02; 95% CrI [0.00, 0.04]) and RT (MD 0.01; 95% CrI [0.01, 0.02]) significantly increased FNBMD (Figure 4B). In contrast, compared to CET, the control group (MD 0.02; 95% CrI [0.00, 0.04]) indicated that CET significantly reduced FNBMD in postmenopausal women (Figure 4B). Based on SUCRA, MCT achieved the highest ranking for improving FNBMD (77.3%), supporting its potential as the most effective intervention (Figure 4C).
Figure 4. Network Diagram and NMA Results. (A) Network diagram for FNBMD (g/cm2). (B) Relative effects of exercise interventions on FNBMD. Note: Estimates are presented as MD with 95% CrI (in parentheses). Read comparisons between interventions from left to right. Effectiveness estimates are at the intersection of exercise intervention columns and rows. Significant results are shown in bold. Continuous Endurance Training; Multicomponent Training; Resistance Training. (C) SUCRA for different exercise interventions on FNBMD. The exercise interventions in the plot are: MCT–Multicomponent training, MBT–Mind-body training, WBV–Whole-body vibration, RT–Resistance training, CET–Continuous endurance training, CT–Combined training, INT–Intermittent training, Con–Control group.
3.4.2 Secondary outcomes
3.4.2.1 WBBMD
In total, fifteen RCTs explored the efficacy of five exercise interventions on WBBMD in postmenopausal women (Figure 5A). NMA results (Figure 5B) revealed no statistically significant disparities among the different exercise interventions (Figure 5B). SUCRA identified CT as the top-ranked intervention for WBBMD (77.6%), indicating its strong potential efficacy (see Figure 5C).
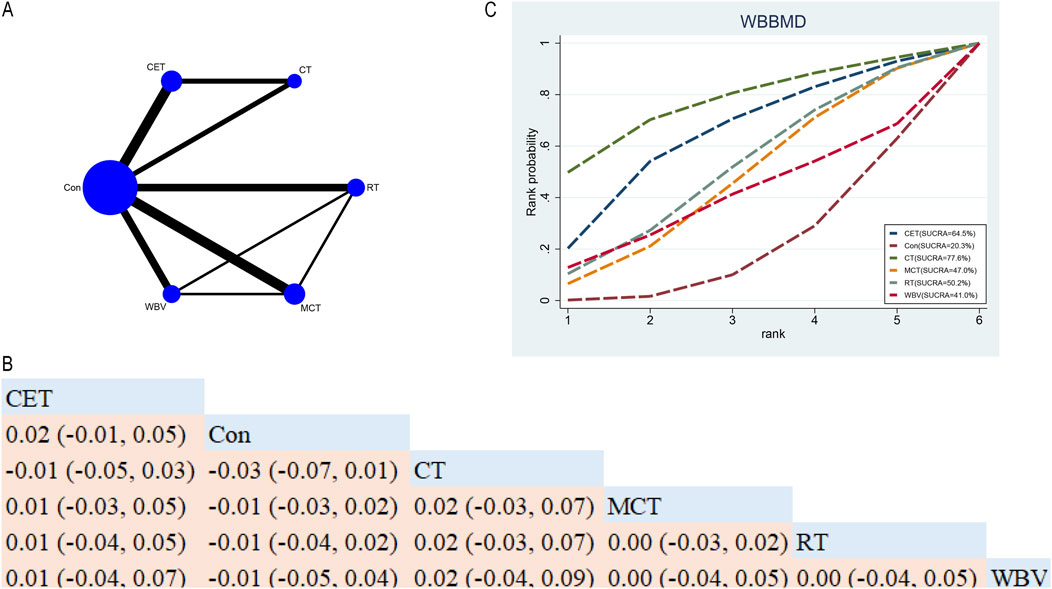
Figure 5. Network Diagram and NMA Results (A) Network diagram for WBBMD (g/cm2). (B) Relative effects of different exercise interventions on WBBMD. Note: Estimates are presented as MD with 95% CrI (in parentheses). Read comparisons between interventions from left to right. Effectiveness estimates are at the intersection of exercise intervention columns and rows. Significant results are shown in bold. (C) SUCRA for different exercise interventions on WBBMD. The exercise interventions in the plot are: MCT–Multicomponent training, WBV–Whole-body vibration, RT–Resistance training, CET–Continuous endurance training, CT–Combined training, Con–Control group.
3.4.2.2 THBMD
In total, ten RCTs probed into the roles of five exercise interventions on THBMD in postmenopausal older women (Figure 6A). NMA results (Figure 6B) demonstrated that no statistically significant differences were found among the various exercise interventions (Figure 6B). SUCRA indicated that MBT ranked highest for increasing THBMD (60.7%), suggesting it may be a promising intervention (see Figure 6C).
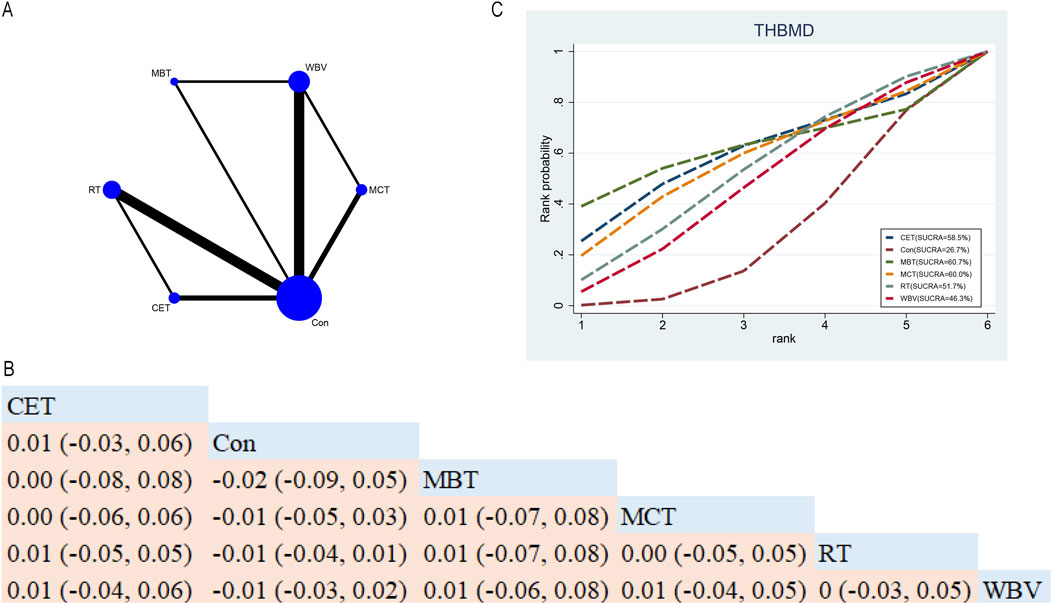
Figure 6. Network Diagram and NMA Results. (A) Network diagram for THBMD (g/cm2). (B) Relative effects of different exercise interventions on total hip bone mineral density (THBMD). Note: Estimates are presented as MD with 95% CrI (in parentheses). Read comparisons between interventions from left to right. Effectiveness estimates are at the intersection of exercise intervention columns and rows. Significant results are shown in bold. (C) SUCRA for different exercise interventions on THBMD. The exercise interventions in the plot are: MCT–Multicomponent training, MBT–Mind-body training, WBV–Whole-body vibration, RT–Resistance training, CET–Continuous endurance training, Con–Control group.
3.5 Subgroup and sensitivity analyses
Only LSBMD and FNBMD met the criteria for subgroup analyses. For LSBMD, stratification by health status revealed that CET was significantly effective in healthy participants (MD 0.03; 95% CrI [0, 0.07]), whereas WBV was significantly effective in those with osteoporosis (MD 0.05; 95% CrI [0.02, 0.09]). SUCRA rankings also identified CET (78.9%) and WBV (81.0%) as top interventions. This suggests that exercise interventions exert beneficial effects across different health statuses, although the optimal modality may vary. For FNBMD, CET (MD 0.02; 95% CrI [0, 0.04]) and MCT (MD 0.03; 95% CrI [0.01, 0.05]) were both significantly effective in healthy participants. No statistically significant differences were observed in osteoporotic participants, but SUCRA rankings placed MCT (79.2%) and WBV (76.4%) at the top. Notably, the ranking of WBV differed substantially from the overall results, highlighting the need for further validation with high-quality RCTs (see Supplementary Figure S2A–C, S3A–C, S4A–C,S5A–C).
Due to the limited number of eligible studies, subgroup analyses were not performed for WBBMD and THBMD; instead, sensitivity analyses were conducted. For WBBMD, exclusion of osteoporotic participants revealed no great differences among interventions, although SUCRA ranking placed CT (67.6%) as the leading intervention, consistent with the overall findings. This implies that health status has only a modest influence on whole-body bone mass. For THBMD, exclusion of osteoporotic participants likewise revealed no significant differences, but SUCRA rankings positioned MBT (61.8%) at the top, again aligning with the overall results. These findings imply that health status may have little impact on hip bone density, though the conclusions remain constrained by the limited evidence base. In summary, the results for WBBMD and THBMD should be interpreted cautiously, and additional high-quality RCTs are needed to substantiate these observations. (See Supplementary Figure S6A–C, S7A–C).
3.6 Consistency and publication bias assessment
DIC served as a tool to compare consistency and inconsistency models. The variation in DIC values across all closed-loop models was lower than 5, indicating good model consistency with DIC. Node-splitting analysis was performed to assess and explore local inconsistencies in the closed loops, and the results showed no evidence of local inconsistency, as listed in Table 2. Regarding publication bias, adjusted funnel plots (Supplementary Figures S8-S17) showed no traces of publication bias.
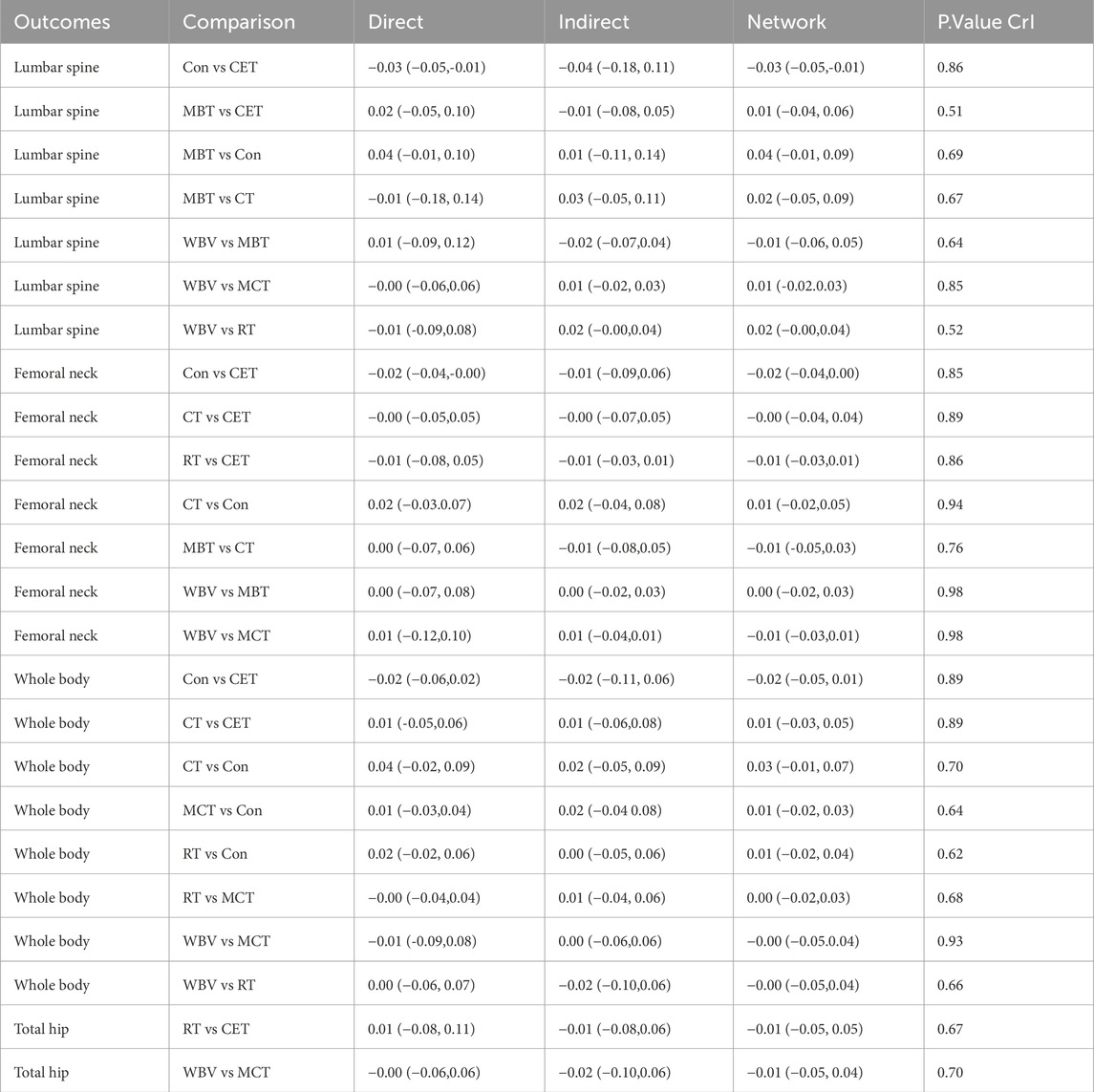
Table 2. Local inconsistency evaluation and exploration for closed-loop results using node splitting method.
4 Discussion
4.1 Key findings
A comprehensive literature search identified 55 RCTs involving 3,454 participants, and a Bayesian NMA was conducted to evaluate the comparative effects of seven exercise interventions—namely CET, RT, MCT, MBT, WBV, INT, and CT—on BMD in postmenopausal women. Outcomes were analyzed across multiple skeletal sites, including the lumbar spine, femoral neck, total hip, and whole body. The results suggested MBT as a promising intervention for attenuating the loss of LSBMD, while MCT appeared potentially beneficial for improving FNBMD. In contrast, CET, CT, and INT showed no significant effects on the primary outcomes of BMD. For the secondary outcomes, SUCRA analysis ranked CT highest for WBBMD and MBT for THBMD, implying relative advantages of these modalities, although no definitive statistical superiority was established.
4.1.1 Effects of different exercise interventions on primary BMD outcomes
4.1.1.1 LSBMD
NMA revealed that MBT, primarily consisting of practices such as Baduanjin, Tai Chi, Yijinjing, and yoga, had the highest probability of improving LSBMD in postmenopausal women. These findings align with previous research by Li et al. (2023). Prior research reported that MBTs like Baduanjin may enhance musculoskeletal strength and flexibility, systematically engage joints and muscles, and improve balance, cardiopulmonary function, and mental wellbeing, thereby achieving holistic mind–body benefits (Liu et al., 2015). Some researchers hypothesize that these exercises could modulate endocrine function—for example, by elevating serum vitamin D levels, reducing parathyroid hormone, and increasing intestinal calcium absorption—mechanisms that could facilitate bone formation (Zhao et al., 2017). However, these mechanisms were not directly assessed in the present NMA and should therefore be interpreted as potential explanations rather than empirical evidence. Tai Chi, characterized by slow and fluid movements, has been reported to help prevent bone loss at the lumbar spine and proximal femoral neck in menopausal women (Zou et al., 2017). This benefit may be related to two mechanisms: first, engagement of the lumbar spine creates shear stress; second, repeated weight transfer between legs produces ground reaction forces (Wu and Hitt, 2005; Chang et al., 2014). Both mechanisms may stimulate osteogenesis in weight-bearing bones. Similarly, Yi Jin Jing, through stretching muscles and tendons, may enhance flexibility and joint mobility, potentially offering therapeutic benefits for bone health and overall wellbeing. Zou et al. (2017) noted that the unique low-impact nature of MBTs, such as Tai Chi and Baduanjin, not only supports bone metabolism and increases BMD but also minimizes injury risk, making them particularly suitable for elderly postmenopausal women (Shen et al., 2010). Considering physical, psychological, and social factors, MBTs such as Baduanjin and Tai Chi appear to be potentially valuable strategies for improving LSBMD in this demographic.
4.1.1.2 FNBMD
This study indicated that MCT appears to be a potentially effective option for enhancing FNBMD in elderly postmenopausal women. MCT typically incorporates a combination of RT, CET, balance training, and flexibility exercises. Each component contributes independently, yet they act synergistically to improve both BMD and overall health. RT delivers mechanical loading that stimulates bone formation. CET enhances cardiovascular function and metabolic health. Balance training reduces the risk of falls, and flexibility exercises improve joint mobility and muscle coordination. Together, these components interact to facilitate gains in bone mineral density and overall physical wellbeing. Beyond these direct effects, potential biological mechanisms underlying the benefits of MCT warrant further attention. Previous studies suggest that comprehensive exercise programs can enhance blood circulation to bone tissue, thereby improving nutrient and oxygen delivery and supporting osteoblast activity (Renno et al., 2007). In addition, vascular endothelial cells can release local regulators such as nitric oxide, interleukin-6, and endothelin, which stimulate osteoblast differentiation and inhibit osteoclast activity. Importantly, recent experimental evidence highlights that mixed aerobic and anaerobic training protocols may also elicit endocrine responses that favor bone remodeling. For instance, Vasto et al. reported that high-intensity trampoline-based training increased circulating GLP-1 and GIP levels in adult women. These peptides are known to influence glucose metabolism and bone turnover (Vasto et al., 2022). Although such mechanisms were not directly assessed in our NMA, they provide valuable insights suggesting that the effects of MCT on bone health may extend beyond mechanical loading to include hormonal and metabolic pathways.
Nevertheless, these mechanisms remain speculative in the context of this analysis. Future RCTs incorporating both BMD outcomes and biomarker assessments are essential to elucidate whether these endocrine pathways mediate the observed benefits of MCT on skeletal health in postmenopausal women.
4.1.2 Effects of different exercise interventions on secondary outcomes
SUCRA ranking identified CT and MBT as the most promising interventions for slowing the decline in WBBMD and THBMD, or the reduction of bone mass in postmenopausal women. A combination of CET and RT stimulates osteoblast activity through mechanical loading, promoting bone formation. Additionally, CET improves blood circulation and metabolism, and provides enhanced nourishment to the bones, collectively slowing the loss of WBBMD in postmenopausal women. MBTs, such as Baduanjin, Yijinjing, yoga, and Tai Chi, offer gentle mechanical loading to the bones, activating osteoblast activity and promoting bone formation. Furthermore, these exercises focus on balance, coordination, and muscle control, so that they are conducive to enhancing body stability and muscle strength, alleviating the risk of falls, and indirectly protecting bone health. Moreover, MBTs help alleviate stress, improve endocrine balance, and further attenuate the loss of THBMD in elderly postmenopausal women.
4.2 Differences in interventions and participants and their impact
Although exercise modalities were categorized as CET, RT, MCT, MBT, WBV, INT, and CT, the specific intervention protocols across studies varied in intensity, frequency, duration, loading pattern, and supervision (Supplementary Table S1). This heterogeneity may, in part, explain the differences in effect sizes. For example, WBV varied significantly in frequency and acceleration, potentially leading to inconsistent skeletal responses. Mind-body training, while generally producing lower impact loads, may improve lumbar spine stability and reduce fall risk, explaining its relative advantage in improving LSBMD rather than the hip. Intervention duration and adherence also influenced the results. Hence, these differences should be considered when interpreting the pooled results. More standardized reporting and protocol design is needed in future trials.
Collectively, the subgroup and sensitivity analyses indicate that participant characteristics have limited influence on the overall conclusions. Specifically, CET and WBV appear particularly beneficial in improving LSBMD among osteoporotic women, while MCT demonstrates relative advantages in enhancing FNBMD, especially in healthy participants. These findings are consistent with the exercise characteristics of osteoporotic populations: given their reduced bone mass and fragile skeletal architecture, osteoporotic women are typically unsuitable for high-impact or high-load exercise. Instead, moderate-intensity, continuous aerobic exercise (e.g., CET) offers a safe option that stimulates bone metabolism and slows bone loss (Papaioannou et al., 2010; Yagami et al., 2014). Moreover, this population often experiences decreased muscle strength and balance, leading to elevated fall risk. WBV provides skeletal loading under low mechanical stress and also improves lower-limb strength and balance, thereby enhancing bone density and reducing fall risk simultaneously (Rayman et al., 2011; Triunfo et al., 2013; Author anonymous, 2023). In summary, these results underscore the differential effects of exercise interventions across populations and highlight the need for future studies to design exercise prescriptions for osteoporotic women that are both safer and more effective.
5 Strengths and limitations
To the best of our knowledge, this NMA marks a pioneering endeavor to comprehensively evaluate and compare the diverse impacts of exercise interventions on BMD in elderly postmenopausal women, while simultaneously establishing a comprehensive ranking of these interventions based on their efficacy. This study unveils profound insights into the optimal interventions for fortifying BMD in this demographic. However, several limitations must be acknowledged. First, the relatively constrained sample sizes and the paucity of included studies could potentially compromise the precision and generalizability of the findings. Second, the considerable variability in exercise protocols, participant profiles, as well as the intensity, frequency, and duration of physical activity across studies introduces an element of heterogeneity. Further stratification by baseline BMD status or detailed age categories was not feasible due to insufficient data. Nevertheless, differences in baseline BMD levels and age distributions across studies may have influenced the observed outcomes. However, the limited reporting prevented more comprehensive exploration. In addition, the exclusive reliance on English-language literature may introduce potential selection bias. Therefore, more high-quality RCTs are necessary to validate the results of this study.
6 Conclusion
Currently, no single exercise intervention has been shown to unequivocally optimize BMD across all skeletal sites in elderly postmenopausal women. MBT emerged as a potentially effective intervention for slowing BMD decline and preserving bone mass in the lumbar spine and total hip joint. MCT appeared most promising for enhancing FNBMD, while CT showed potential benefits in sustaining WBBMD in postmenopausal women. Given the inherent constraints of current clinical research, future research should focus on larger cohorts, extended follow-up durations, and more stringent research frameworks to substantiate these promising yet preliminary findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
MM: Writing – original draft, Software, Data curation, Conceptualization, Methodology, Validation. WS: Visualization, Investigation, Writing – review and editing, Supervision. DL: Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2025.1633913/full#supplementary-material
References
Abdul-Al O., Zunquin G., El Hage R. (2024). Effects of two types of resistance training modalities (Hypertrophy vs. contrast training) on bone parameters in a group of healthy elderly women. J. Clin. Densitom. 27 (4), 101521. doi:10.1016/j.jocd.2024.101521
Aboarrage Junior A. M., Teixeira CVLS, Dos Santos R. N., Machado A. F., Evangelista A. L., Rica R. L., et al. (2018). A high-intensity Jump-based aquatic exercise program improves bone mineral density and functional fitness in postmenopausal women. Rejuvenation Res. 21 (6), 535–540. doi:10.1089/rej.2018.2069
Ammann P., Rizzoli R. (2003). Bone strength and its determinants. Osteoporos. Int. 14, 13–18. doi:10.1007/s00198-002-1345-4
Author anonymous (2023). Correction to: (082) more than 75% of female sexual dysfunctions can be prevented if the partner is treated, but that's the main problem. J. Sex. Med. 20 (8), 1139. doi:10.1093/jsxmed/qdad084
Autier P., Haentjens P., Bentin J., Baillon J. M., Grivegnee A.-R., Closon M. C., et al. (2000). Costs induced by hip fractures: a prospective controlled study in Belgium. Belgian hip fracture study group. Osteoporos. Int. 11, 373–380. doi:10.1007/s001980070102
Beck B. R., Norling T. L. (2010). The effect of 8 mos of twice-weekly low- or higher intensity whole body vibration on risk factors for postmenopausal hip fracture. Am. J. Phys. Med. Rehabil. 89 (12), 997–1009. doi:10.1097/PHM.0b013e3181f71063
Benzinger P., Riem S., Bauer J., Jaensch A., Becker C., Buechele G., et al. (2019). Risk of institutionalization following fragility fractures in older people. Osteoporos. Int. 30, 1363–1370. doi:10.1007/s00198-019-04922-x
Bloomfield S. A., Williams N. I., Lamb D. R., Jackson R. D. (1993). Non-weightbearing exercise May increase lumbar spine bone mineral density in healthy postmenopausal women. Am. J. Phys. Med. Rehabil. 72 (4), 204–209. doi:10.1097/00002060-199308000-00006
Bocalini D. S., Serra A. J., dos Santos L., Murad N., Levy R. F. (2009). Strength training preserves the bone mineral density of postmenopausal women without hormone replacement therapy. J. Aging Health 21 (3), 519–527. doi:10.1177/0898264309332839
Bocalini D. S., Serra A. J., Dos Santos L. (2010). Moderate resistive training maintains bone mineral density and improves functional fitness in postmenopausal women. J. Aging Res. 2010, 760818. doi:10.4061/2010/760818
Brentano M. A., Cadore E. L., Da Silva E. M., Ambrosini A. B., Coertjens M., Petkowicz R., et al. (2008). Physiological adaptations to strength and circuit training in postmenopausal women with bone loss. J. Strength Cond. Res. 22 (6), 1816–1825. doi:10.1519/JSC.0b013e31817ae3f1
Brooke-Wavell K., Jones P. R., Hardman A. E. (1997). Brisk walking reduces calcaneal bone loss in post-menopausal women. Clin. Sci. (Lond). 92 (1), 75–80. doi:10.1042/cs0920075
Brooke-Wavell K., Jones P. R., Hardman A. E., Tsuritan Y. Y. (2001). Commencing, continuing and stopping brisk walking: effects on bone mineral density, quantitative ultrasound of bone and markers of bone metabolism in postmenopausal women. Osteoporos. Int. 12 (7), 581–587. doi:10.1007/s001980170081
Brooks S. P., Gelman A. (1998). General methods for monitoring convergence of iterative simulations. J. Comput. Graph. statistics 7 (4), 434–455. doi:10.1080/10618600.1998.10474787
Burton E., Hill A.-M., Pettigrew S., Lewin G., Bainbridge L., Farrier K., et al. (2017). Why do seniors leave resistance training programs? Clin. interventions aging 12, 585–592. doi:10.2147/CIA.S128324
Chang Y.-T., Chang J.-H., Huang C.-F. (2014). Ground reaction force characteristics of Tai chi push hand. J. Sports Sci. 32 (18), 1698–1703. doi:10.1080/02640414.2014.915419
Chubak J., Ulrich C. M., Tworoger S. S., Sorensen B., Yasui Y., Irwin M. L., et al. (2006). Effect of exercise on bone mineral density and lean mass in postmenopausal women. Med. Sci. Sports Exerc 38 (7), 1236–1244. doi:10.1249/01.mss.0000227308.11278.d7
Chuin A., Labonté M., Tessier D., Khalil A., Bobeuf F., Doyon C. Y., et al. (2009). Effect of antioxidants combined to resistance training on BMD in elderly women: a pilot study. Osteoporos. Int. 20 (7), 1253–1258. doi:10.1007/s00198-008-0798-5
Dalsky G. P., Stocke K. S., Ehsani A. A., Slatopolsky E., Lee W. C., Birge S. J. (1988). Weight-bearing exercise training and lumbar bone mineral content in postmenopausal women. Ann. Intern. Med. 108 (6), 824–828. doi:10.7326/0003-4819-108-6-824
De Aguiar E.D.O.G, Marconi E. M., Monteiro-Oliveira B. B., Gomes-Santos A. C., Oliveira A. C. C., Paineiras-Domingos L. L., et al. (2023). Whole-body vibration exercise improves the functionality in postmenopausal women: a systematic review. Iran. J. Public Health 52 (3), 476. doi:10.18502/ijph.v52i3.12131
de Oliveira L. C., de Oliveira R. G., de Almeida Pires-Oliveira D. A. (2019). Effects of whole-body vibration versus pilates exercise on bone mineral density in postmenopausal women: a randomized and controlled clinical trial. J. Geriatr. Phys. Ther. 42 (2), E23–e31. doi:10.1519/jpt.0000000000000184
Dempster A. P. (1997). The direct use of likelihood for significance testing. Statistics Comput. 7, 247–252. doi:10.1023/a:1018598421607
ElDeeb A. M., Abdel-Aziem A. A. (2020). Effect of whole-body vibration exercise on power profile and bone mineral density in postmenopausal women with osteoporosis: a randomized controlled trial. J. Manip. Physiol. Ther. 43 (4), 384–393. doi:10.1016/j.jmpt.2019.12.003
Englund U., Littbrand H., Sondell A., Pettersson U., Bucht G. (2005). A 1-year combined weight-bearing training program is beneficial for bone mineral density and neuromuscular function in older women. Osteoporos. Int. 16 (9), 1117–1123. doi:10.1007/s00198-004-1821-0
Fu X., Ma X., Lu H., He W., Wang Z., Zhu S. (2011). Associations of fat mass and fat distribution with bone mineral density in pre-and postmenopausal Chinese women. Osteoporos. Int. 22, 113–119. doi:10.1007/s00198-010-1210-9
Grove K. A., Londeree B. R. (1992). Bone density in postmenopausal women: high impact vs low impact exercise. Med. Sci. Sports Exerc 24 (11), 1190–1194. doi:10.1249/00005768-199211000-00002
Guadalupe-Grau A., Fuentes T., Guerra B., Calbet J. A. (2009). Exercise and bone mass in adults. Sports Med. 39, 439–468. doi:10.2165/00007256-200939060-00002
Hartley C., Folland J. P., Kerslake R., Brooke-Wavell K. (2020). High-Impact exercise increased femoral neck bone density with no adverse effects on imaging markers of knee Osteoarthritis in postmenopausal women. J. Bone Min. Res. 35 (1), 53–63. doi:10.1002/jbmr.3867
Hejazi K., Askari R., Hofmeister M. (2022). Effects of physical exercise on bone mineral density in older postmenopausal women: a systematic review and meta-analysis of randomized controlled trials. Archives Osteoporos. 17 (1), 102. doi:10.1007/s11657-022-01140-7
Iwamoto J., Takeda T., Otani T., Yabe Y. (1998). Effect of increased physical activity on bone mineral density in postmenopausal osteoporotic women. Keio J. Med. 47 (3), 157–161. doi:10.2302/kjm.47.157
Iwamoto J., Takeda T., Ichimura S. (2001). Effect of exercise training and detraining on bone mineral density in postmenopausal women with osteoporosis. J. Orthop. Sci. 6 (2), 128–132. doi:10.1007/s007760100059
Jamka M., Mądry E., Bogdański P., Kryściak J., Mądry R., Lisowska A., et al. (2021). The effect of endurance and endurance-strength training on bone mineral density and content in abdominally Obese postmenopausal women: a randomized trial. Healthc. (Basel) 9 (8), 1074. doi:10.3390/healthcare9081074
Jessup J. V., Horne C., Vishen R., Wheeler D. (2003). Effects of exercise on bone density, balance, and self-efficacy in older women. Biol. Res. Nurs. 4 (3), 171–180. doi:10.1177/1099800402239628
Kemmler W., von Stengel S., Engelke K., Haeberle L., Kalender W. A. (2010). Exercise effects on bone mineral density, Falls, coronary risk factors, and health care costs in older women the Randomized controlled senior fitness and prevention (SEFIP) Study. Archives Intern. Med. 170 (2), 179–185. doi:10.1001/archinternmed.2009.499
Kemmler W., Shojaa M., Kohl M., von Stengel S. (2020). Effects of different types of exercise on bone mineral density in postmenopausal women: a systematic review and meta-analysis. Calcif. tissue Int. 107, 409–439. doi:10.1007/s00223-020-00744-w
Korpelainen R., Keinänen-Kiukaanniemi S., Heikkinen J., Väänänen K., Korpelainen J. (2006). 35.Effect of impact exercise on bone mineral density in elderly women with low BMD: a population-based randomized controlled 30-month intervention. Osteoporos. Int. 17 (1), 109–118. doi:10.1007/s00198-005-1924-2
Kwon Y., Park S., Kim E., Park J. (2008). The effects of multi-component exercise training on VO2max, muscle mass, whole bone mineral density and fall risk in community-dwelling elderly women. Jpn. J. Phys. Fit. Sports Med. 57 (3), 339–348. doi:10.7600/jspfsm.57.339
Lai C. L., Tseng S. Y., Chen C. N., Liao W. C., Wang C. H., Lee M. C., et al. (2013). Effect of 6 months of whole body vibration on lumbar spine bone density in postmenopausal women: a randomized controlled trial. Clin. Interv. Aging 8, 1603–1609. doi:10.2147/cia.S53591
Li S.-S., He S.-H., Xie P.-Y., Li W., Zhang X.-X., Li T.-F., et al. (2021). Recent progresses in the treatment of osteoporosis. Front. Pharmacol. 12, 717065. doi:10.3389/fphar.2021.717065
Li J., Gu Q., Li R., Wang R., Cai Y., Huang Y., et al. (2023). Effect of Yi Jin Jing exercise plus Elastic Band Resistance exercise on overall bone mineral density in postmenopausal women. J. Sci. Med. Sport 26 (2), 87–92. doi:10.1016/j.jsams.2023.01.006
Liu B.-X., Chen S.-P., Li Y.-D., Wang J., Zhang B., Lin Y., et al. (2015). The effect of the modified eighth section of eight-section brocade on osteoporosis in postmenopausal women: a prospective randomized trial. Medicine 94 (25), e991. doi:10.1097/MD.0000000000000991
Lord S. R., Ward J. A., Williams P., Zivanovic E. (1996). The effects of a community exercise program on fracture risk factors in older women. Osteoporos. Int. 6 (5), 361–367. doi:10.1007/BF01623009
Ma C., Liu A., Sun M., Zhu H., Wu H. (2016). Effect of whole-body vibration on reduction of bone loss and fall prevention in postmenopausal women: a meta-analysis and systematic review. J. Orthop. Surg. Res. 11, 24–10. doi:10.1186/s13018-016-0357-2
Marin-Cascales E., Rubio-Arias J. A., Romero-Arenas S., Alcaraz P. E. (2015). Effect of 12 weeks of whole-body vibration versus multi-component training in post-menopausal women. Rejuvenation Res. 18 (6), 508–516. doi:10.1089/rej.2015.1681
Marín-Cascales E., Rubio-Arias J. Á., Alcaraz P. E. (2019). Effects of two different neuromuscular training protocols on regional bone mass in postmenopausal women: a randomized controlled trial. Front. Physiology 10, 846. doi:10.3389/fphys.2019.00846
Marques E. A., Mota J., Machado L., Sousa F., Coelho M., Moreira P., et al. (2011a). Multicomponent training program with weight-bearing exercises elicits favorable bone density, muscle strength, and balance adaptations in older women. Calcif. tissue Int. 88, 117–129. doi:10.1007/s00223-010-9437-1
Marques E. A., Wanderley F., Machado L., Sousa F., Viana J. L., Moreira-Goncalves D., et al. (2011b). Effects of resistance and aerobic exercise on physical function, bone mineral density, OPG and RANKL in older women. Exp. Gerontol. 46 (7), 524–532. doi:10.1016/j.exger.2011.02.005
Martin D., Notelovitz M. (1993). Effects of aerobic training on bone mineral density of postmenopausal women. J. Bone Min. Res. 8 (8), 931–936. doi:10.1002/jbmr.5650080805
McGuinness L. A., Higgins J. P. (2021). Risk-of-bias VISualization (robvis): an R package and shiny web app for visualizing risk-of-bias assessments. Res. synthesis methods 12 (1), 55–61. doi:10.1002/jrsm.1411
Mohammad R. G. R., Smart N. A., Liang M. T., Bijeh N., Albanaqi A. L., Fathi M., et al. (2020). The impact of different modes of exercise training on bone mineral density in older postmenopausal women: a systematic review and meta-analysis research. Calcif. tissue Int. 106, 577–590. doi:10.1007/s00223-020-00671-w
Moreira L. D. F., Fronza F.C.A.O, Dos Santos R. N., Zach P. L., Kunii I. S., Hayashi L. F., et al. (2014). The benefits of a high-intensity aquatic exercise program (HydrOS) for bone metabolism and bone mass of postmenopausal women. J. Bone Mineral Metabolism 32 (4), 411–419. doi:10.1007/s00774-013-0509-y
Mosti M. P., Kaehler N., Stunes A. K., Hoff J., Syversen U. (2013). Maximal strength training in postmenopausal women with osteoporosis or osteopenia. J. Strength Cond. Res. 27 (10), 2879–2886. doi:10.1519/JSC.0b013e318280d4e2
Nambi G., Abdelbasset W. K., Moawd S. A., Sakr H. R., Elnegamy T. E., George J. S. (2020). Effect of virtual reality training on post-menopausal osteoporotic women. Archives Pharm. Pract. 11 (2), 6–12. doi:10.51847/npC61ufZin
Nicholson V. P., McKean M. R., Slater G. J., Kerr A., Burkett B. J. (2015). Low-Load very high-repetition resistance training attenuates bone loss at the lumbar spine in active post-menopausal women. Calcif. Tissue Int. 96 (6), 490–499. doi:10.1007/s00223-015-9976-6
Papaioannou A., Morin S., Cheung A. M., Atkinson S., Brown J. P., Feldman S., et al. (2010). 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. Cmaj 182 (17), 1864–1873. doi:10.1503/cmaj.100771
Park H., Kim K. J., Komatsu T., Park S. K., Mutoh Y. (2008). Effect of combined exercise training on bone, body balance, and gait ability: a randomized controlled study in community-dwelling elderly women. J. bone mineral metabolism 26, 254–259. doi:10.1007/s00774-007-0819-z
Pruitt L. A., Taaffe D. R., Marcus R. (1995). Effects of a one-year high-intensity versus low-intensity resistance training program on bone mineral density in older women. J. bone mineral Res. 10 (11), 1788–1795. doi:10.1002/jbmr.5650101123
Rayman M. P., Stranges S., Griffin B. A., Pastor-Barriuso R., Guallar E. (2011). Effect of supplementation with high-selenium yeast on plasma lipids: a randomized trial. Ann. Intern Med. 154 (10), 656–665. doi:10.7326/0003-4819-154-10-201105170-00005
Renno A. C. M., Gomes A. R. S., Nascimento R. B., Salvini T., Parizoto N. (2007). Effects of a progressive loading exercise program on the bone and skeletal muscle properties of female osteopenic rats. Exp. Gerontol. 42 (6), 517–522. doi:10.1016/j.exger.2006.11.014
Rhodes E., Martin A., Taunton J., Donnelly M., Warren J., Elliot J. (2000). Effects of one year of resistance training on the relation between muscular strength and bone density in elderly women. Br. J. sports Med. 34 (1), 18–22. doi:10.1136/bjsm.34.1.18
Riaz S., Shakil Ur Rehman S., Hafeez S., Hassan D. (2024). Effects of kinect-based virtual reality training on bone mineral density and fracture risk in postmenopausal women with osteopenia: a randomized controlled trial. Sci. Rep. 14 (1), 6650. doi:10.1038/s41598-024-57358-7
Ruan X.-Y., Jin F.-Y., Liu Y.-L., Peng Z.-L., Sun Y.-G. (2008). Effects of vibration therapy on bone mineral density in postmenopausal women with osteoporosis. Chin. Med. J. 121 (13), 1155–1158. doi:10.1097/00029330-200807010-00001
Russell R. G. G., Xia Z., Dunford J. E., Oppermann U., Kwaasi A., Hulley P. A., et al. (2007). Bisphosphonates: an update on mechanisms of action and how these relate to clinical efficacy. Ann. N. Y. Acad. Sci. 1117 (1), 209–257. doi:10.1196/annals.1402.089
Santin-Medeiros F., Santos-Lozano A., Pablo Rey-Lopez J., Garatachea N. (2015). Effects of eight months of whole body vibration training on hip bone mass in older women. Nutr. Hosp. 31 (4), 1654–1659. doi:10.3305/nh.2015.31.4.8441
Shen C.-L., Chyu M.-C., Pence B. C., Yeh J. K., Zhang Y., Felton C. K., et al. (2010). Green tea polyphenols supplementation and Tai Chi exercise for postmenopausal osteopenic women: safety and quality of life report. BMC Complementary Altern. Med. 10, 76–10. doi:10.1186/1472-6882-10-76
Sherrington C., Fairhall N., Wallbank G., Tiedemann A., Michaleff Z. A., Howard K., et al. (2020). Exercise for preventing falls in older people living in the community: an abridged Cochrane systematic review. Br. J. sports Med. 54 (15), 885–891. doi:10.1136/bjsports-2019-101512
Slatkovska L., Alibhai S. M. H., Beyene J., Hu H., Demaras A., Cheung A. M. (2011). Effect of 12 months of whole-body vibration therapy on bone density and structure in postmenopausal women: a randomized trial. Ann. Intern. Med. 155 (10), 668–679. doi:10.7326/0003-4819-155-10-201111150-00005
Smidt G. L., Lin S. Y., O'Dwyer K. D., Blanpied P. R. (1992). The effect of high-intensity trunk exercise on bone mineral density of postmenopausal women. Spine (Phila Pa 1976) 17 (3), 280–285. doi:10.1097/00007632-199203000-00007
Song W., Yang Y. (2021). The effect of whole-body vibration training with different amplitudes on bone mineral density in elderly women. Isokinet. Exerc. Sci. 29 (4), 413–418. doi:10.3233/IES-200271
Sterne J. A., Savović J., Page M. J., Elbers R. G., Blencowe N. S., Boutron I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. bmj 366, l4898. doi:10.1136/bmj.l4898
Tarantino U., Iolascon G., Cianferotti L., Masi L., Marcucci G., Giusti F., et al. (2017). Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J. Orthop. Traumatology 18, 3–36. doi:10.1007/s10195-017-0474-7
Tartibian B., Hajizadeh Maleki B., Kanaley J., Sadeghi K. (2011). Long-term aerobic exercise and omega-3 supplementation modulate osteoporosis through inflammatory mechanisms in post-menopausal women: a randomized, repeated measures study. Nutr. Metabolism 8, 71. doi:10.1186/1743-7075-8-71
Tolomio S., Ermolao A., Lalli A., Zaccaria M. (2010). The effect of a multicomponent dual-modality exercise program targeting osteoporosis on bone health status and physical function capacity of postmenopausal women. J. Women Aging. 22 (4), 241–254. doi:10.1080/08952841.2010.518866
Triunfo S., Rosati P., Ferrara P., Gatto A., Scambia G. (2013). Fetal cholelithiasis: a diagnostic update and a literature review. Clin. Med. Insights Case Rep. 6, 153–158. doi:10.4137/CCRep.S12273
Vasto S., Amato A., Proia P., Baldassano S. (2022). Is the secret in the gut? SuperJump activity improves bone remodeling and glucose homeostasis by GLP-1 and GIP peptides in eumenorrheic women. Biol. (Basel). 11 (2), 296. doi:10.3390/biology11020296
Veroniki A. A., Straus S. E., Fyraridis A., Tricco A. C. (2016). The rank-heat plot is a novel way to present the results from a network meta-analysis including multiple outcomes. J. Clin. Epidemiol. 76, 193–199. doi:10.1016/j.jclinepi.2016.02.016
Verschueren S. M. P., Roelants M., Delecluse C., Swinnen S., Vanderschueren D., Boonen S. (2004). Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J. Bone Mineral Res. 19 (3), 352–359. doi:10.1359/JBMR.0301245
von Stengel S., Kemmler W., Engelke K., Kalender W. A. (2011). Effects of whole body vibration on bone mineral density and falls: results of the randomized controlled ELVIS study with postmenopausal women. Osteoporos. Int. 22 (1), 317–325. doi:10.1007/s00198-010-1215-4
Wang H., Yu B., Chen W., Lu Y., Yu D. (2015). Simplified Tai Chi resistance training versus traditional Tai Chi in slowing bone loss in postmenopausal women. Evid. Based Complement. Altern. Med. 2015, 379451. doi:10.1155/2015/379451
Watson S. L., Weeks B. K., Weis L. J., Harding A. T., Horan S. A., Beck B. R. (2018). High-Intensity resistance and impact training improves bone mineral density and physical function in postmenopausal women with osteopenia and osteoporosis: the LIFTMOR randomized controlled trial. J. Bone Min. Res. 33 (2), 211–220. doi:10.1002/jbmr.3284
Wen H. J., Huang T. H., Li T. L., Chong P. N., Ang B. S. (2017). Effects of short-term step aerobics exercise on bone metabolism and functional fitness in postmenopausal women with low bone mass. Osteoporos. Int. 28 (2), 539–547. doi:10.1007/s00198-016-3759-4
Wu G., Hitt J. (2005). Ground contact characteristics of Tai Chi gait. Gait and posture 22 (1), 32–39. doi:10.1016/j.gaitpost.2004.06.005
Yagami T., Yamamoto Y., Koma H. (2014). The role of secretory phospholipase A2 in the central nervous system and neurological diseases. Mol. Neurobiol. 49 (2), 863–876. doi:10.1007/s12035-013-8565-9
Yamazaki S., Ichimura S., Iwamoto J., Takeda T., Toyama Y. (2004). Effect of walking exercise on bone metabolism in postmenopausal women with osteopenia/osteoporosis. J. bone mineral metabolism 22, 500–508. doi:10.1007/s00774-004-0514-2
Young C. M., Weeks B. K., Beck B. R. (2007). Simple, novel physical activity maintains proximal femur bone mineral density, and improves muscle strength and balance in sedentary, postmenopausal Caucasian women. Osteoporos. Int. 18 (10), 1379–1387. doi:10.1007/s00198-007-0400-6
Yu P. A., Hsu W. H., Hsu W. B., Kuo L. T., Lin Z. R., Shen W. J., et al. (2019). The effects of high impact exercise intervention on bone mineral density, physical fitness, and quality of life in postmenopausal women with osteopenia: a retrospective cohort study. Med. (United States) 98 (11), e14898. doi:10.1097/MD.0000000000014898
Zehnacker C. H., Bemis-Dougherty A. (2007). Effect of weighted exercises on bone mineral density in post menopausal women a systematic review. J. geriatric Phys. Ther. 30 (2), 79–88. doi:10.1519/00139143-200708000-00007
Zhao R., Zhang M., Zhang Q. (2017). The effectiveness of combined exercise interventions for preventing postmenopausal bone loss: a systematic review and meta-analysis. J. Orthop. and sports Phys. Ther. 47 (4), 241–251. doi:10.2519/jospt.2017.6969
Keywords: mind-body training, multicomponent training, combined training, postmenopausal, bone mineral density, network meta-analysis
Citation: Ma M, Su W and Liu D (2025) Effects of different exercise interventions on bone mineral density in elderly postmenopausal women: a network meta-analysis. Front. Physiol. 16:1633913. doi: 10.3389/fphys.2025.1633913
Received: 23 May 2025; Accepted: 05 September 2025;
Published: 25 September 2025.
Edited by:
Jose A. Parraca, Universidade de Évora, PortugalReviewed by:
Alessandra Amato, University of Catania, ItalyTomohiro Mitoma, Okayama University, Japan
Copyright © 2025 Ma, Su and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wentao Su, MzE0MDkwODBAcXEuY29t
 Mingyu Ma
Mingyu Ma Wentao Su
Wentao Su Dansong Liu
Dansong Liu