- 1 NASA Johnson Space Center, Human Health and Performance Directorate, Houston, TX, United States
- 2 Aegis Aerospace, Houston, TX, United States
- 3 Geocontrol Systems, Houston, TX, United States
- 4 JES Tech, Houston, TX, United States
- 5 KBR, Houston, TX, United States
- 6 Aerospace Medicine Division, School of Public and Population Health, University of Texas Medical Branch, Galveston, TX, United States
Introduction: Although the International Space Station provides a normoxic environment, deep space missions are expected to leverage a hypobaric, mildly hypoxic living environment to facilitate frequent extravehicular activities EVAs, aka spacewalks. Although hypoxia may be experienced terrestrially, it will be atypical for human physiology to live in hypobaric/hypoxic conditions yet frequently experience hyperoxic stress due to EVAs. It is well established that hypoxia induces dysregulation of the human immune system, in generally a sensitized/proinflammatory fashion. This is primarily evidenced from studies of individuals living at altitude.
Methods: To ascertain the effect of hypoxic/hyperoxic shifts on immunity, a series of 11 days hypobaric chamber studies were conducted at the Johnson Space Center. The living environment consisted of 8.2–9.6psi/28.5%–34% oxygen, and there were several simulated EVAs which were performed under hypobaric/hyperoxic conditions consisting of 4.3psi/85%–95% oxygen. For the current sub-study, biosamples were collected before and after simulated EVAs to ascertain the effects of hypoxia, decompression and hyperoxic stress on immunity. The sub-study consisted of 3 chamber tests, 23 total subjects.
Results: Shifts in leukocyte distribution, function, and plasma cytokine concentrations were associated with atmospheric shifts, primarily after the hypobaric/hyperoxic EVA activities. Astronauts already experience immune system dysregulation due to microgravity, stress, and other mission influences.
Discussion: These data indicate that, similar to living at high altitude, altered atmosphere exposure in a pressurized vehicle environment may dysregulate human immunity which may be exacerbated by EVAs. The additive effects of hypoxia, in concert with other spaceflight mission variables, on clinical risks for astronauts must be better characterized to enable future exploration class space missions.
Background
Orbital spaceflight onboard the Space Shuttle and the International Space Station (ISS) were operated in a normoxic, sea-level atmosphere, i.e., 14.7 psi and 21% oxygen. Spacewalks, or extravehicular activities (EVAs) are required to be performed in the lower pressure, mildly hyperoxic atmosphere, of a spacesuit. Typically, the EVA suit atmosphere was 4.3 psi and >95% oxygen. To avoid decompression sickness, the EVA required a denitrogenation (oxygen prebreathe) protocol that took several hours (Brady and Polk, 2011).
Future deep space exploration missions, particularly those on planetary surfaces, will entail a higher frequency of EVAs than on missions to low Earth orbit. Also, it is not desirous to require a prolonged oxygen prebreathe prior to the EVA. Instead, quick exit out of the habitat or pressurized rover would be beneficial, to enable frequent EVAs during exploration missions. The current concept of operations under consideration by the Artemis Program is an ambient vehicle/habitat ‘exploration atmosphere’ of 8.2 psi and 34% O2 (piO2 128 mmHg) (Norcross et al., 2013). This would allow for a shorter oxygen prebreathe (∼20 min), greatly shortening the logistics before exiting the vehicle for an EVA and greatly facilitating exploration operations. EVA suit atmosphere would still be hyperoxic (piO2 222 mmHg). Deep space explorers would therefore be frequently cycling between hypoxic and hyperoxic conditions. There is evidence that hyperoxia exposures influenced brain perfusion, cognition, and EEG activity (Damato et al., 2020; Damato et al., 2022b). There is very little existing research data regarding the effects of such atmospheric cycling on physiology.
Spaceflight mission stressors other than altered atmosphere, such as microgravity, circadian misalignment and physiologic stress, result in the persistent dysregulation of the human immune system (Krittanawong et al., 2022; Rooney et al., 2019). The phenomenon is characterized by reductions in T and NK cell function, altered cytokine profiles, and the reactivation of latent herpesviruses (Crucian et al., 2015; Bigley, 2019; Crucian et al., 2014; Mehta et al., 2017; Rooney et al., 2019). While these alterations have not caused widespread clinical issues, some crewmembers experience immune-related adverse events, including manifestations of symptomatic herpes viral reactivation, allergy, and respiratory distress (Crucian et al., 2016a IJGM). Three case reports have been published of astronauts regarding clinically relevant immune compromise, and dermatitis on orbit, two of which confirmed the involvement of latent herpesvirus reactivation (Crucian et al., 2016b; Mehta et al., 2022; Mehta et al., 2024). One ISS crewmember experienced zoster (laboratory confirmed by skin swab) on orbit, and the outbreak was correlated with peaks in both immune dysfunction and stress hormone concentrations (Mehta et al., 2024). Another case correlated dermatitis flares with operational stressors (Crucian et al., 2016a). In a third case, virus ‘evolution’ onboard ISS, defined as increasing micro-variants, was documented (Mehta et al., 2022). Such cases are important as they demonstrate that the immune dysregulation in astronauts can progress to operationally-impactful adverse clinical events during flight. It is reasonable to hypothesize that the immune dysregulation observed aboard ISS missions will intensify during longer missions in deep space, thereby placing crewmembers at elevated clinical risk (Crucian and Sams, 2009).
Other environments, such as saturation diving and Antarctic winterover, share many isolation and mission stressors with spaceflight, including station lifestyle, extreme environment, and 6–12 months deployments. Saturation diving can also include frequent changes to atmospheric pressure, extended duration living in altered atmospheric conditions, and challenges with humidity and sea life induced contamination (Brubakk et al., 2014). Findings from immune studies at Concordia Antarctic Station were surprisingly discordant from spaceflight findings. Whereas spaceflight generally results in a suppression of leukocyte function, at Concordia (innate) cellular function was generally sensitized, with functional responses elevated (Feuerecker et al., 2014; Feuerecker et al., 2019). The only confounding variable thought best to be responsible for this sensitization was the persistent hypobaric hypoxia as Antarctica is a mountain, and Concordia Station sits at 10,606 feet in elevation (piO2 ∼97 mmHg).
Hypoxia is known to regulate and/or influence immunity, inflammation, and physiology in a generally stimulatory fashion, as reviewed by Taylor et al. (Taylor and Colgan, 2017; Damato et al., 2022a). Hypoxia can influence immune cellular functional capacity (e.g., proliferation, development), or can function primarily via transcriptional changes mediated by hypoxia-inducible factor. While there is variability among altitude/hypoxia studies, generally, effects on immunity are stimulatory, and pro-inflammatory (Kubo et al., 1998; Hartmann et al., 2000; Coussons-Read et al., 2002; Facco et al., 2005; Ermolao et al., 2009), while other reports suggest lymphocytosis, or HPA axis mediated immune suppression (Siqués et al., 2007; Chen et al., 2012; Oliver et al., 2013; Kleessen et al., 2005) or innate cellular migration and inflammation (Pahl et al., 2006). Hypoxia also specifically influences anti-tumor immunity (Arnaiz and Harris, 2022). Physical activity or exercise at altitude in a hypoxic environment had differential influences (de Aquino Lemos et al., 2013; Mazzeo, 2005; Tiollier et al., 2005; Choukèr et al., 2005). A recent study suggests that normobaric hypoxia training and increased erythropoiesis impacts iron metabolism, which can challenge maintenance of immune function (Nolte et al., 2025). The effects of hypoxia on immunity appear highly biased, meaning some aspects of inflammation/immunity may be suppressed, whereas others may be heightened or sensitized. This effect may be exploited as a treatment for specific disease states related to an alteration in immune bias. For example, hypoxia can be beneficial for asthma allergy, or eczema, which are all considered to be ‘TH2’ diseases, or certain tumors resulting in intra-tumor localized hypoxia (Schultze-Werninghaus, 2008; Engst and Vocks, 2000; Hatfield et al., 2015).
Current denitrogenation prebreathe protocols performed prior to conducting a spacewalk on the ISS, from a normoxic vehicle to a hypobaric spacesuit, last about 4 h. EVAs will be much more frequent during upcoming planetary exploration. To optimize time spent exploring the Lunar surface and minimize prebreathe durations while maintaining a safe risk posture for decompression sickness risk prevention, an ‘exploration atmosphere’ is being planned for the Artemis program, where the vehicle will be at 8.2 psi and 34% oxygen (or similar), allowing for significantly shorter prebreathe durations prior to EVA decompression. There are varied literature reporting immune dysregulation associated with hypoxia, primarily derived from studies of people living at altitude, as well as decompression stress.
Immune dysregulation has also been well documented to be associated with other spaceflight stressors, such as microgravity, isolation, circadian misalignment, or altered diet. This has been observed in studies of astronauts during flight, as an amalgamation of all these mission stressors, and also in ground-space-analog studies, where such variables may be studied individually. Given that deep space missions will likely be conducted in a uniquely hypobaric/hypoxic environment with intermittent hypobaric/mildly hyperoxic EVAs, the current study was conducted in a hypobaric chamber housed at the Johnson Space Center. Subjects lived in the chamber for 11 days in a mildly hypoxic environment, and during several mission days participated in hyperoxic simulated ‘EVAs’. This operational scenario is a high-fidelity representation of the atmosphere planned for deep space missions. Biosampling was conducted on several days, both before and after EVA. The objective was to allow a determination of the effects of both hypoxia and hyperoxia (and cycling therein) on immunity, inflammation, and latent herpesvirus reactivation.
Methods
Subjects
Subjects were healthy volunteers, medically screened by the JSC Test Subject Facility, consented and enrolled to participate. Subject demographic information is presented in Table 1. For this study, there were three complete 11-day chamber missions, each with 8 participating subjects. Six were full subjects, participating in all simulated EVA activities, and the remaining two were ‘Doppler technicians’, involved in the collection of other study data during the EVAs. Of the 24 total mission participants, four individuals repeated in a second mission, therefore 20 individuals participated in one or more missions. Of the 20 participating individuals, 10 were male and 10 were female. The Doppler technicians lived in the chamber but did not participate in the physical activities associated with EVA. The Doppler Technicians did experience all altered atmosphere cycles, isolation, diet, circadian misalignment, and all other ‘chamber variables’, and performed Doppler imaging as part of their work requirement. One subject dropped during the third mission, resulting in a final study ‘n’ of 23 subjects.
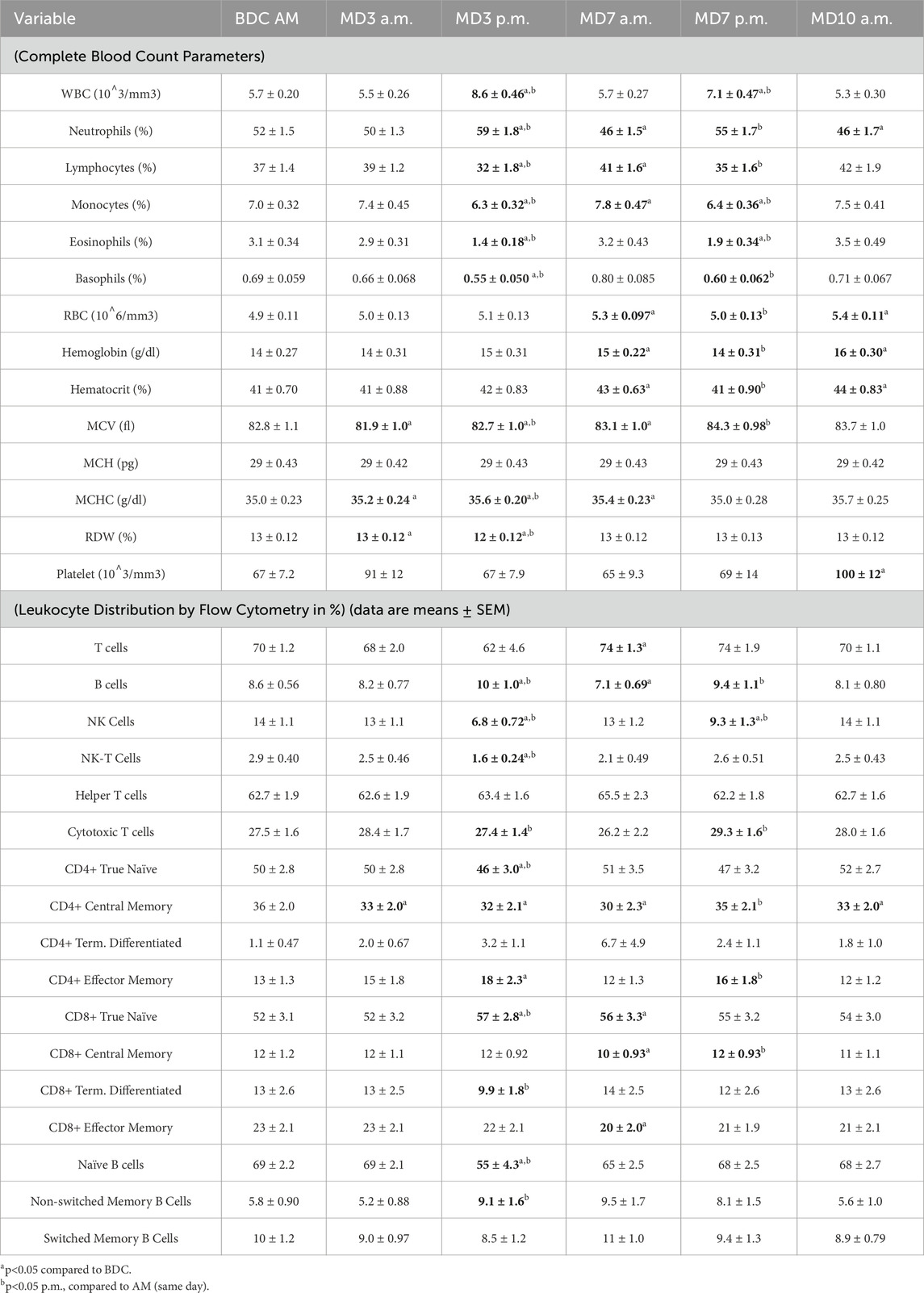
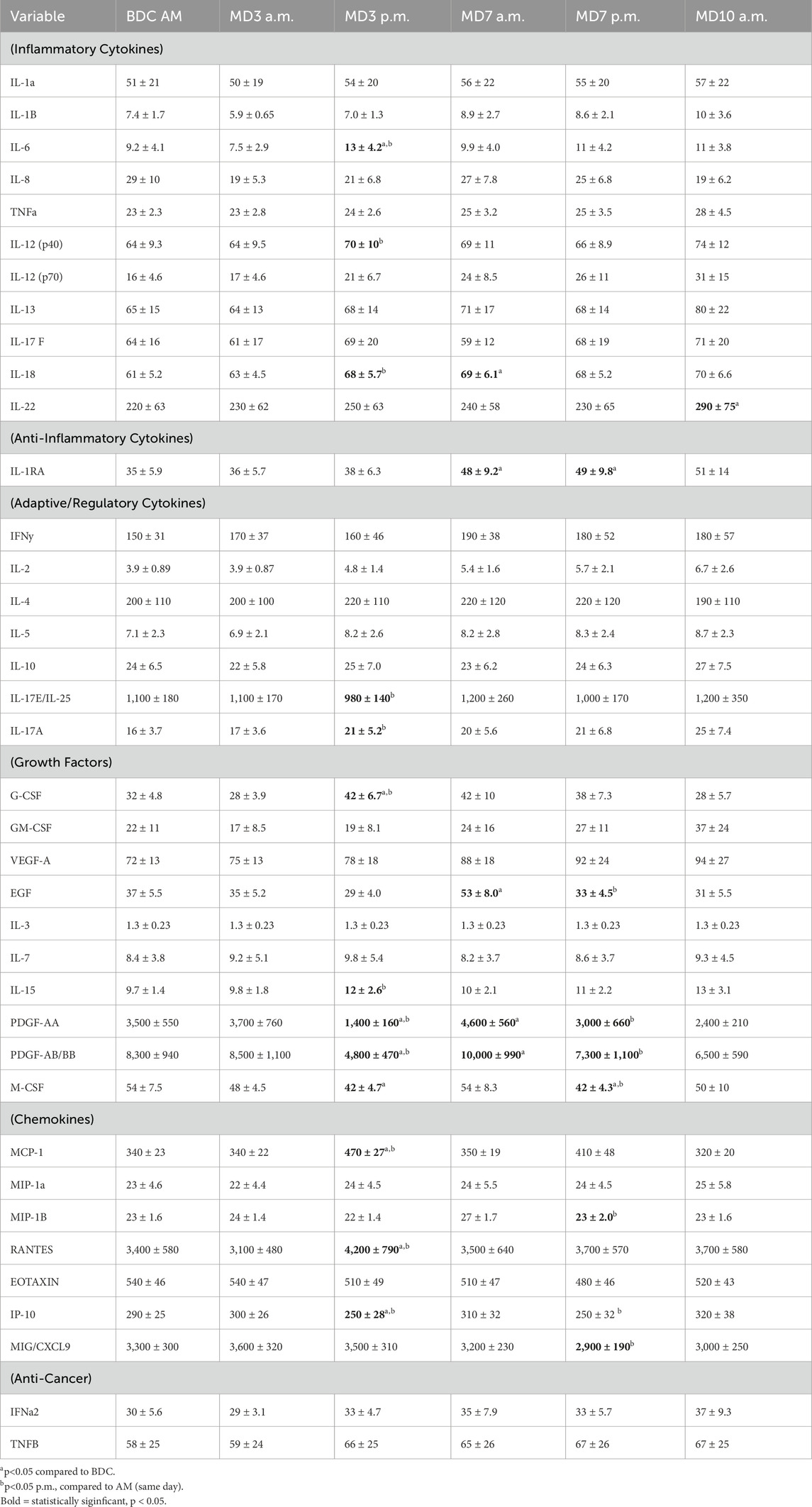
Table 1. Complete blood count parameters and peripheral leukocyte distribution by flow cytometry (data are means ± SEM).
Samples
Sampling schedule is described in Figure 1. In brief, samples consisted of 1.0 mL saliva, sampled early morning on all collection days, which occurred at baseline (5–7 days prior to entering the chamber) and each mission day, for stress hormone and latent virus DNA measurements. Saliva was collected via passive drool, and frozen immediately until batch analysis. Ambient blood was collected in a 6.0 mL heparin anticoagulated monovette at baseline and 5 times inside the chamber during the mission. The mission blood collections were scheduled to allow some interpretation of the effects of hypoxia, hyperoxic EVA and decompression stress, and cycling between the two as indicated (Figure 1). Urine samples were collected over a 24 h period during baseline data collection (BDC), and on mission days (MD) 3, 7, and 10. A 5 mL aliquot was removed from the 24 h pool and frozen at −80°C until batch processing.
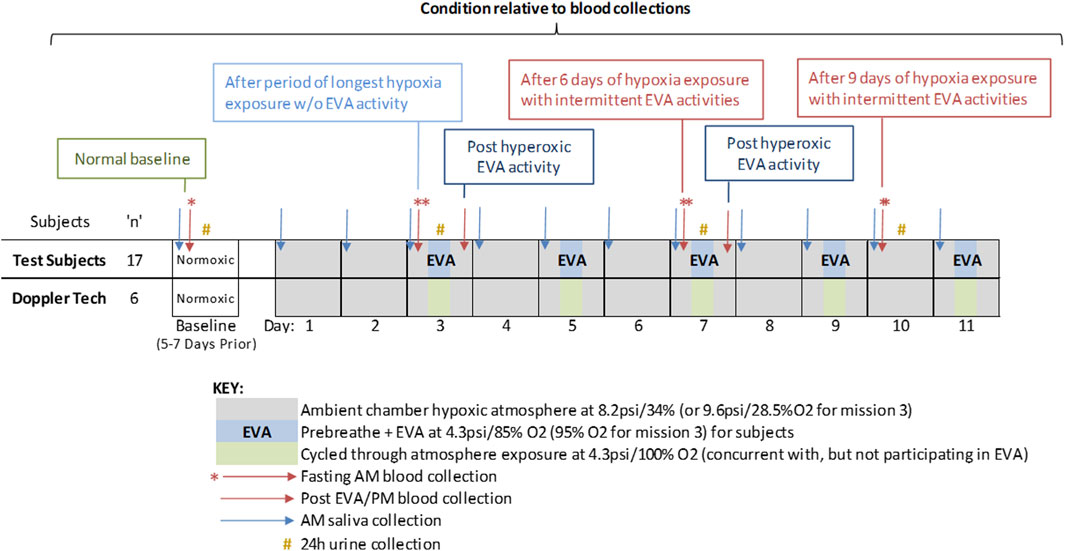
Figure 1. Study design including duration, atmosphere composition, EVA days, and biosample collections. The study was designed to assess the effects of mild hypoxia (baseline and Day 3 – AM); hyperoxic EVA activity (Day 3 and 7, post EVA), and living in mild hypoxia interspersed with hyperoxic challenging EVAs (Day 7 and 10, a.m.). Doppler technicians live inside the chamber and also experienced the hypoxia conditions during EVAs, but did not participate in the physical activities.
Chamber and atmosphere
A livable 20-foot diameter hypobaric chamber was outfitted with furniture and equipment to support multiple subjects living in the chamber for 11 days (Figure 2). This was a program level evaluation for NASA with multiple objectives. Prior to depressing the chamber to the exploration atmosphere, subjects underwent a 180 min 100% Oxygen prebreathe. Thereafter, they lived in the chamber for 11 days at the exploration atmosphere: 8.2psi/34%O2 for missions 1 and 2 and 9.6psi/28.5%O2 for mission 3. The atmosphere was changed for mission 3 to address a NASA desire to explore options for programs and vehicles that could not readily achieve >30%O2 due to flammability limitations. Simulated EVAs were conducted on MD 3, 5, 7, 9, and 11. Prior to simulated EVAs, subjects underwent a 85% O2 - 15%N2 (95%O2 - 5% N2 when coming from the 9.6psi atmosphere) prebreathe and the chamber pressure was then reduced to 4.3psi to simulate spacesuit pressure for the simulated EVA. The simulated EVAs were conducted shirt sleeve (not in a spacesuit) and subjects conducted various activities representative of the metabolic workloads expected during Lunar surface EVAs for 6 h with a targeted average metabolic workload of ∼40% of VO2max. Simulated EVAs and associated physical activities were identical across the simulated EVAs on MD3, 5, 7, 9, and 11.
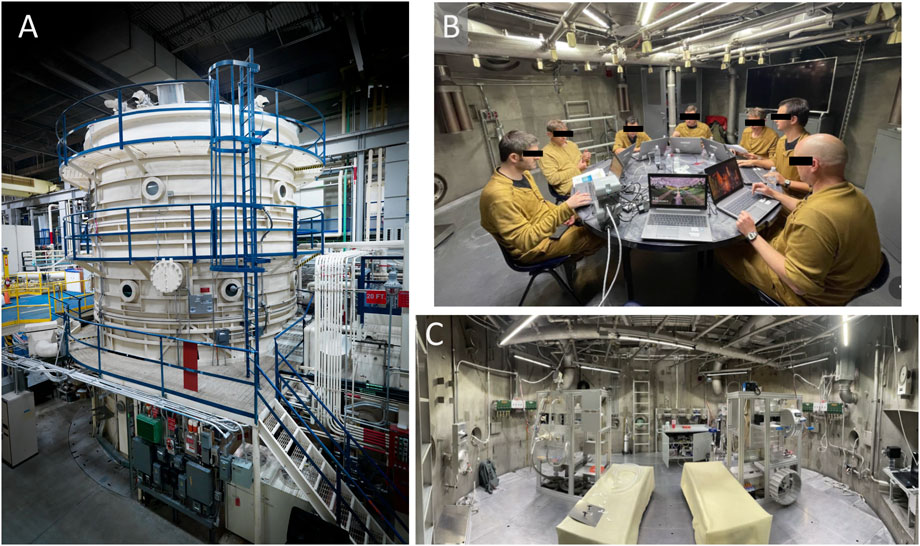
Figure 2. (A) Image of the hypobaric chamber at the NASA Johnson Space Center; (B) crewmembers inside the chamber during the study; (C) chamber EVA simulation stations. Photo credits to NASA.
The Doppler technicians lived at the same ambient chamber atmosphere, but during the EVA activities were masked to 100% oxygen as a protective measure and were tasked with monitoring venous gas emboli in the active subjects; thus did not partake in the metabolic load of the simulated EVA activities (remained at a resting metabolic rate) but underwent the same decompression profile. After each EVA, the hypobaric chamber was re-established to the exploration atmosphere (8.2 psi/34% O2 or 9.6psi/28.5%O2) prior to removing their breathing masks.
As no differences were observed between mission 3 and missions 1,2 (data not shown), the hypoxia exposure is similar (mission 3: pp O2 141 mmHg; missions 1, 2: pp O2 144 mmHg) and the hypobaric/hyperoxic EVA exposures were similar (mission 3: 4.3psi, 95% O2, missions 1, 2: 4.3psi, 85% O2) (Figure 3), for purposes of this product and to increase statistical power, the three chamber missions were grouped as a single cohort. Similarly, no differences were observed between the prime EVA subjects and the Doppler technicians, who during the EVA were on a slightly different hyperoxic atmosphere (Figure 3). Therefore, for purposes of these analyses all 23 subject across 3 missions were analyzed as a single cohort.
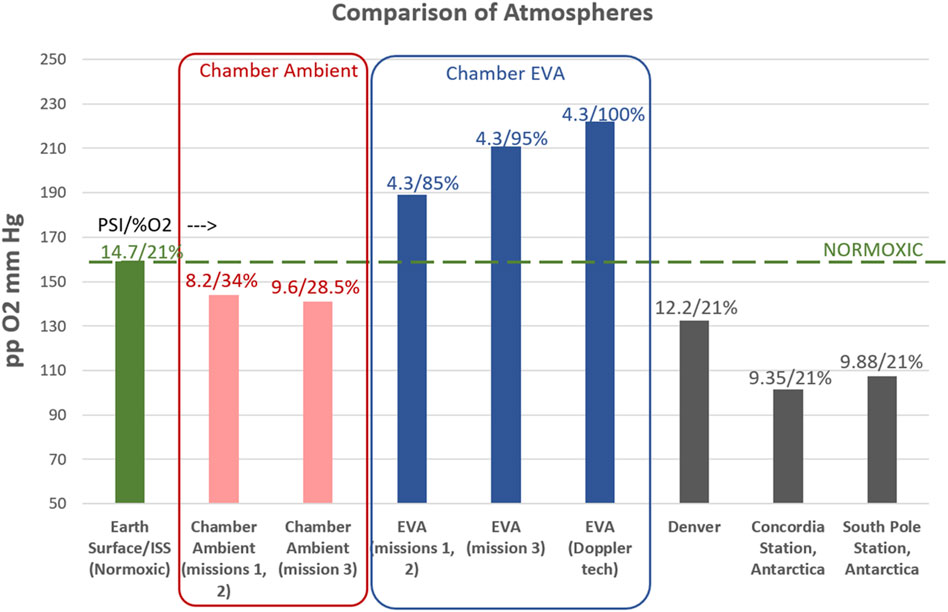
Figure 3. Comparison of Atmospheres between Earth (sea level), International Space Station (ISS), chamber conditions for this study, and relevant terrestrial locations/altitudes. A comparison of the partial pressure of O2, in mmHg, is represented (Y-axis), while the atmospheric pressure (psi) and percent oxygen are indicated above each data point. Note that for the chamber conditions, the ambient living atmosphere is mildly hypoxic for all three missions. Mission 3 had a slightly different atmosphere, but a similar level of mild hypoxia. The EVA conditions were hyperoxic as indicated for all missions. During EVA, regular subjects experienced the 4.3psi/85%–95% O2 atmosphere and participated in physical activities associated with spacewalks, where the Doppler technicians were masked to 100% O2 and did not participate in the physical activities. As no differences were seen between the missions, or the EVA/Doppler Technician groups, data remain grouped as a single (8 subjects enrolled per mission, 23 completed subjects total) cohort.
Complete blood count
A complete blood count, WBC, bulk leukocyte subsets via potentiometric measurement (no positive identification via surface marker expression), and RBC parameters was performed using standard techniques using a Sysmex XM-10 (Kobe, Hyogo, Japan) hematology analyzer according to the manufacturer’s instructions.
Flow cytometry/leukocyte subsets
A comprehensive 5-color flow cytometry antibody matrix, up to 10 colors per analysis tube, was formulated for peripheral blood immunophenotype analysis. Fluorescent labeled antibodies were obtained from Cytex (Amsterdam The Netherlands). The specific fluorescent antibody matrix setup, relevant leukocyte subsets identified, as well as blood sample staining and lysis protocols, and flow cytometry analysis were set up and performed on a Beckman Coulter ‘Gallios’ flow cytometer as previously described (Crucian et al., 2013). Specific antibodies utilized were as follows:
Tube A: IgD (FITC), CD56 (PE), CD19 (PC5), CD14 (PC7), CD45 (APC), CD27 (APC-Cy7), CD3 (V500).
Tube B: CD57 (FITC), CD62L (PE), CD28 (PC5), CCR7 (PC7), CD45RA (APC), CD4 (APC-Cy7), CD8 (Pac Blue), CD3 (V500).
This panel assessed the major leukocyte and lymphocyte subsets, T-cell subsets, memory/naïve and central memory T-cell subsets, and constitutively activated T-cell percentages. Whole blood, without any artificial cellular enhancement or purification, was stained for analysis.
Plasma cytokine concentrations (30 plex)
Plasma was aliquoted from 7.5 mL Lithium Heparin Sarstedt blood tube after centrifugation at 974 RCF for 15 min. Plasma samples were vortexed and centrifuged at 10,621 X RCF for 10 min. Samples were ran neat. Cytokine concentration was measured using a Milliplex Human Cytokine/Chemokine MAGNETIC BEAD Premixed 30 Plex Kit (Millipore Sigma, Chicago, IL, United States) according to manufacturer’s instructions. Samples were analyzed on a Luminex Magpix instrument with xMAP INTELLIFLEX software (Luminex, Austin, TX, United States). The 30 cytokines analyzed were IL-1a, IL-1b, TNFa, IL-6, IL1ra, IL-8, IL-2, IFNg, IL-4, IL-5, IL-10, IL-17a, CCL2/MCP-1, CCL3/MIP-1 alpha, CCL4/MIP-1 beta, CCL5/RANTES, GCSF, GM-CSF, VEGF, IL-3, IL-7, EGF, Eotaxin, IL-12 (p40), IL-12 (p70), IL-15, IP-10, IL-13, IFN-a2 and TNFb.
Leukocyte function (mitogen stimulated cytokine profiles)
Whole blood culture setup, mitogen stimulation, and maintenance was performed as previously described (Feuerecker et al., 2019). Supernatant from cell cultures were collected after 48 h of culture time. Samples were vortexed and centrifuged at 10,621 X RCF for 10 min. Samples were diluted 3:1 with provided Assay Buffer. Cytokine concentration was measured using a MILLIPLEX MAP Human High Sensitivity T Cell Magnetic Bead 13 Plex Panel assay (Millipore Sigma, Chicago, IL, United States) according to manufacturer’s instructions. Samples were analyzed on a Luminex Magpix instrument with xMAP INTELLIFLEX software (Luminex, Austin, TX, United States). The 13 cytokines analyzed were GMCSF, IFNy, IL-10, IL-12 (p70), IL-13, IL-1B, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8 and TNFa.
Saliva cortisol
Frozen saliva samples were thawed at room temperature, vortexed, and up to 1 mL was aliquoted for analysis. All samples were centrifuged at 18,000 x g for 20 min. Up to 800 uL of supernatant was removed and saved for salivary cortisol analysis. Analysis of salivary cortisol from the saliva supernatant was performed using Salimetrics Expanded Range High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit (State College, PA; Cat. No. 1–3,002) per manufacturer’s instructions.
Saliva latent herpesvirus DNA
Following saliva separation for cortisol analysis, the remaining 200ul (cell pellet) was DNA extracted using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany; Cat. No. 51106), per manufacturer’s instructions. Viral loads (copies/mL saliva) for EBV, HSV-1, and VZV were determined for each sample by standard curve analysis using the QuantStudio 3 Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA). Primers and probes for each virus have been previously published (Mehta et al., 2014; Mehta et al., 2022). DNA concentration was determined by Qubit 4 Fluorometer (Invitrogen, Thermo Fisher Scientific, Waltham, MA) using Invitrogen™ QuantiT™ Qubit™ dsDNA HS Assay Kit (Invitrogen, Thermo Fisher Scientific, Waltham, MA, Cat. No. Q32854) per the manufacturer’s instructions. PCR on GAPDH was used as a quality control. GAPDH primers and probe have been published previously (Cohrs et al., 2008).
Urine latent herpesvirus DNA
Urine samples were then thawed, homogenized, and a 2 mL aliquot was removed for DNA extraction using Qiagen Viral RNA Mini Kit (Qiagen, Hilden, Germany, Cat. No. 52906). Briefly, 2 mL of urine was centrifuge at 14,000 rpm for 10 min and then 1860 µL of supernatant was removed leaving 140 µL urine and the cell pellet. DNA extraction on the cell pellet was performed according to the manufacturer’s instructions. DNA concentration was determined by Qubit 4 Fluorometer (Thermo Fisher Scientific, Waltham, MA) and CMV qPCR was conducted using the QuantStudio 7 Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA). CMV primers and probes have been published previously (Mehta et al., 2024). PCR on GAPDH was used as a quality control. GAPDH primers and probe have been published previously.
Statistical analysis
Mixed models were chosen for testing for flexibility in accounting for the repeated measures design and inherent imbalances across factors, like PM only being measured on MD3 and MD7. Each immune measure was analyzed using linear mixed-effects models defined in terms of the interaction of Mission Day (BDC, MD3, MD7, or MD10) and time (AM or PM). Random effects included the within-subject repeated measures (subject-specific intercepts), and the nesting of subjects within missions to fully address the data dependences. Additionally, models included robust standard errors to allow for non-homogenous variance across conditions. Marginal means were used for estimation and pairwise comparisons with simulation-method p-value adjustments. When the overall F-test was significant, pairwise comparisons were conducted to assess difference from baseline (BDC) for all other timepoints, and between AM and PM on MD3 and MD7. Analyses were conducted in SAS v9.4 using the GLIMMIX procedure and LSMEANS/LSMESTIMATE statements.
Results
CBC/peripheral leukocyte distribution
In mission, whole blood cells (WBCs) were significantly elevated after the EVA activities on MD3 and MD7, both in comparison to the BDC as well as to the AM timepoint of each day (Table 2). The post-EVA elevation in WBC was driven by an increase in the relative percentage of neutrophils which became significantly elevated after each EVA activity (Table 2). In what may be a rebound effect, the neutrophil percentage was significantly reduced on the AM samplings of MD7 and MD10 when compared to BDC. There was a concurrent opposite response in the percentage of lymphocytes, monocytes, eosinophils and basophils that tracked with the neutrophil changes (Table 2).
Red blood cell parameters were also responsive to the altered atmosphere exposure. At MD3 a.m., the mild hypoxia induced an increase in mean corpuscular hemoglobin concentration (MCHC) and red cell distribution width (RDW) but a decrease in mean corpuscular volume (MCV) when compared to BDC (Table 2). The hyperoxic EVA recovered the MCV but an increase continued through to MD10. By MD7, increases in the RBC, hemoglobin and hematocrit were evident, and these alterations persisted through MD10 (Table 2). Platelet counts were generally stable throughout the mission, but achieved a statistically significant increase on MD10.
‘Fine’ leukocyte subsets were assessed, positively identified by surface marker expression, by flow cytometry analysis. Although mostly unaltered through the MD3 a.m. sampling, broad alterations were evident following the hyperoxic exposures. These included increases in total B cells and decreases in NK and NKT percentages (Table 2). A significant decrease in the cytotoxic T cell subset was evident following MD3 hyperoxic EVA, but this reversed and increased following MD7 hyperoxic EVA. No parallel changes in the helper T percentage was detected (Table 2). An increase in the maturation state of the helper-T population was evident with shifts towards CD4+ Effector Memory cells. However, this was not detected in the cytotoxic subset, where a significant increase in the ‘naïve’ subset was observed (Table 2).
Plasma cytokine concentrations
No significant alterations were observed following the initial period of hypoxia exposure (MD3 a.m. vs BDC). Following the MD3 hyperoxic EVA, significant alterations were observed in the plasma concentration of several cytokines: IL-6 (↑), IL-12p40 (↑), IL-18 (↑), IL17E (↓), IL-17A (↑), G-CSF (↑), IL-15 (↑), M-CSF (↓), MCP-1 (↑), RANTES (↑) and IP-10 (↓) (Table 3). By the AM sample of MD7, IL-18, IL-1RA, EGF, PDGF-AA and PDGF-AB/BB were significantly elevated compared to BDC (Table 3). Following the MD7 hyperoxic EVA, the plasma concentration of several cytokines decreased compared to MD7 a.m. timepoint: EGF, PDGF-AA, PDGF-AB/BB, M-CSF, MIP-1B, IP-10, and MIG/CXCL9 (Table 3). Only two cytokines were consistent between the two EVA mission days with significant decreases seen in M-CSF and IP-10. At the AM sampling on MD10, 9 of the 10 categorized inflammatory cytokines were elevated, however only IL-22 was significantly elevated (Table 3). IL-1ra was also elevated at MD10a.m., however, this was not statistically significant.
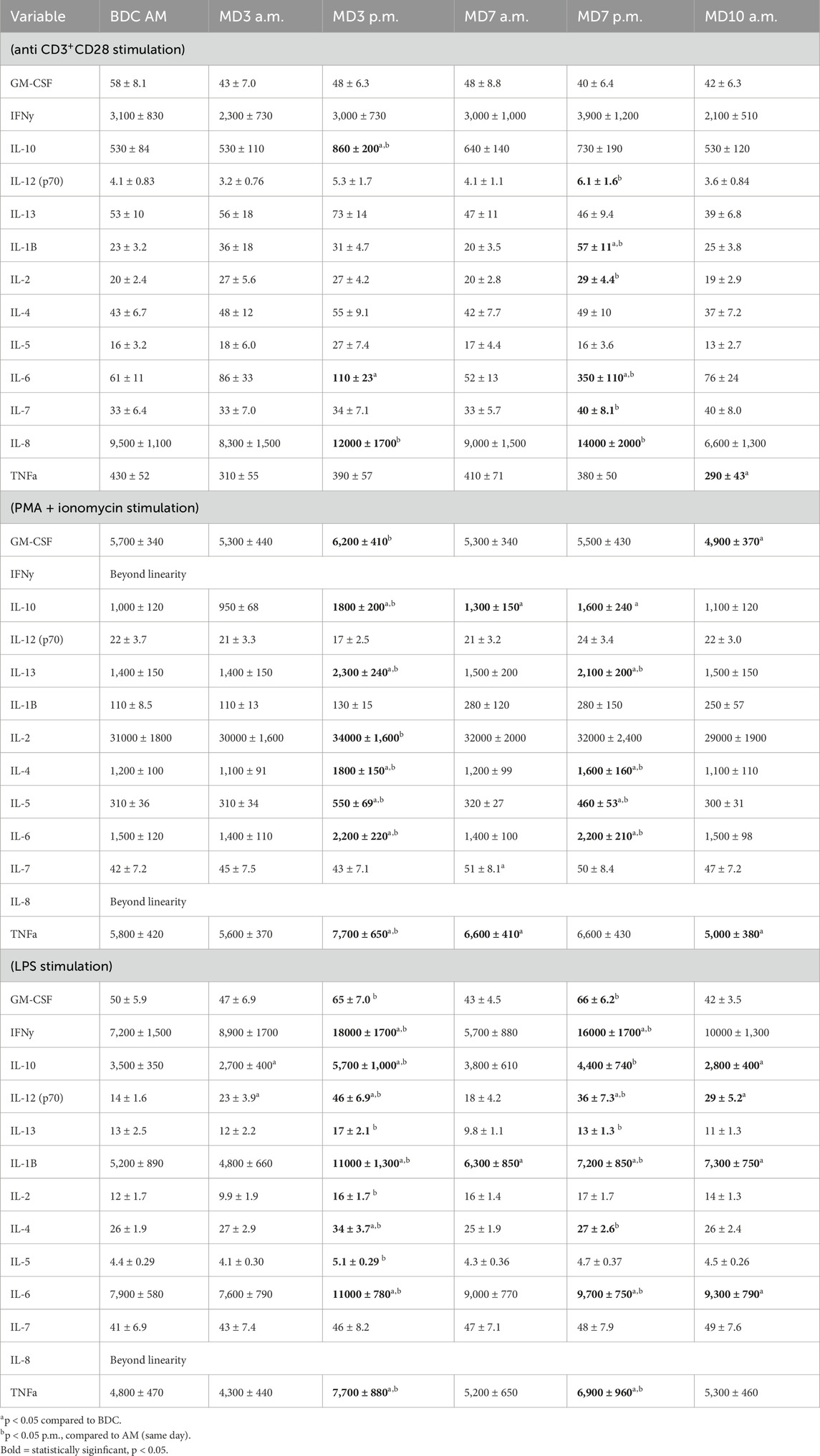
Table 3. Leukocyte function assessed via mitogen stimulated (48h culture) cytokine profiles (pg/ml supernatant) (data are means ± SEM).
Leukocyte function/mitogen stimulated cytokine profiles
Supernatant cytokine concentrations following mitogenic stimulation of whole blood cells was used as a functional assessment of immunocytes. Different mitogens allowed a snapshot of the functional capacity of different leukocyte subsets. Following stimulation with all mitogens, no functional alterations were observed following the initial exposure to mild hypoxia (MD3 a.m.). Following the MD3 hyperoxic EVA, stimulation with antibodies to CD3 and CD28, triggered a significant increase in IL-10, IL-6 and IL-8 (Table 4). No alterations were evident in the MD7 a.m. sample, but after the MD7 EVA, increases were detected for six cytokines, with IL-8 consistently elevated across both EVAs (Table 4). On the morning of MD10, no alterations were observed compared to baseline sans a decrease in TNFa.
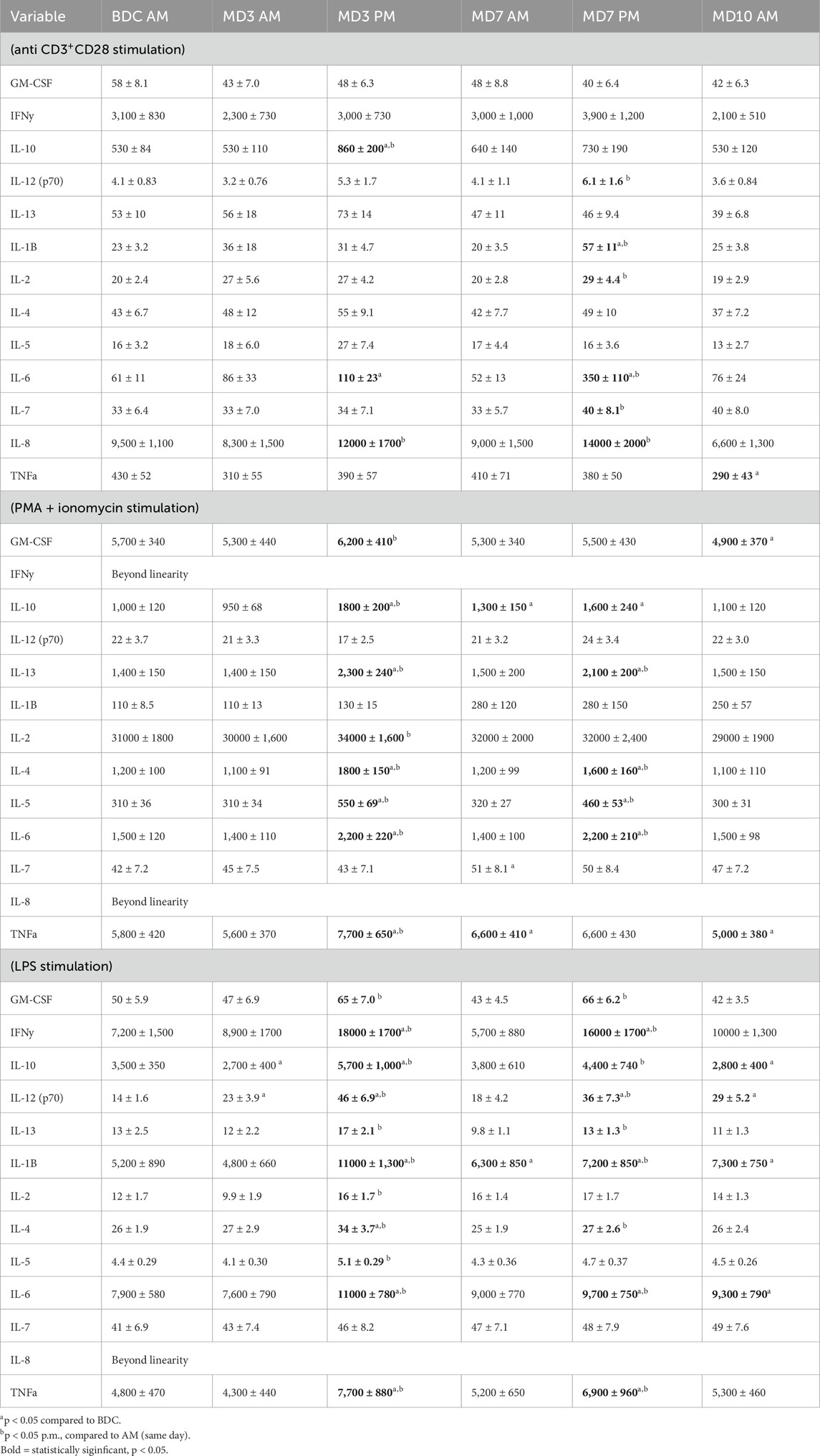
Table 4. Leukocyte function assessed via mitogen stimulated (48 h culture) cytokine profiles (pg/ml supernatant).
Stimulation with PMA and ionomycin is a broader, more powerful pharmacological stimulus that bypasses intracellular signaling pathways. It acts on all the leukocyte subsets. Following PMA/I stimulation, no alterations were observed in the MD3 a.m. sampling, but following the MD3 EVA increases were observed in 8 different cytokines (Table 4). Similarly, after the MD7 a.m. sampling there was only a lingering increase in IL-10, IL-7, and TNFa compared to BDC, but following the MD7 EVA sampling there was a significant increase in four different cytokines (IL-13, IL-4, IL-5, and IL-6) compared to MD7 a.m. (Table 4). These four cytokines were significantly increased after both hyperoxic EVA missions days. On the morning of MD10 both GM-CSF and TNFa were reduced compared to the BDC timepoint.
Stimulation with LPS is a targeted stimulus that primarily activates monocytes via their CD14 cell surface receptor. Following LPS stimulation, no alterations were observed in the MD3 a.m. sampling, but following the MD3 EVA (timepoint MD3 p.m.) significant increases were observed in 11 of the 12 measurable cytokines when compared to MD3 a.m. (Table 4). Similarly, after the MD7 a.m. sampling only IL-1b was increased when compared to baseline, but following the MD7 EVA sampling there was a significant increase in 9 of the 12 measurable cytokines when compared to MD7 a.m. (Table 4). Only IL-2 and IL-5 did not respond in the same manner to both EVA mission days. On the morning of MD10, IL-10 was significantly decreased while IL-12 (p70), IL-1B, and IL-6 were significantly increased compared to BDC.
Saliva cortisol
Mean saliva cortisol was not elevated across any of the 11 study days (Table 5). Values ranged from 0.39 to 0.52 µg/dL.
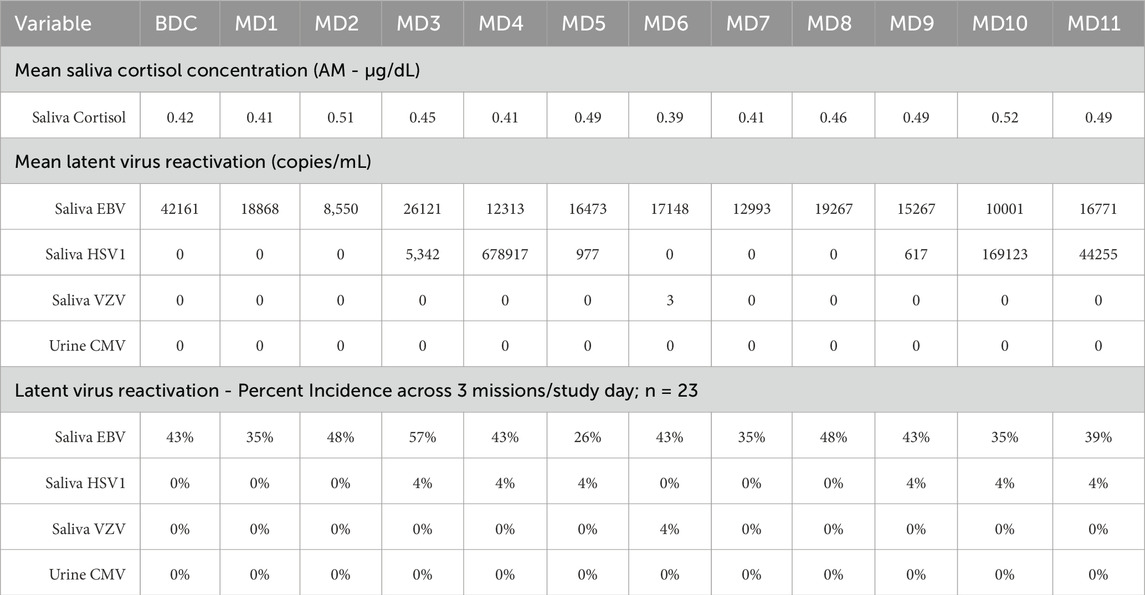
Table 5. Saliva Cortisol (µg/dL), Latent Herpesvirus DNA (copies/mL saliva), incidence per 3 missions; (n = 23).
Saliva/urine herpesvirus DNA
Saliva was collected first thing in the morning at BDC, and each of the 11 mission days. No shedding of CMV, in urine, was detected across any of the study days. Only a single positive sample was observed for saliva VZV, and only 3 copies/mL (Table 5). HSV DNA in saliva was observed repeatedly in a single subject, on MD3-5 and MD9-11 (Table 5). EBV reactivation in saliva was observed in more subjects, ranging from 25% to 54% positive samples from the total 23 subject count, however no statistically significant increase was observed in any of the 11 mission days, compared to baseline (Table 5).
Discussion
There are reports that hypoxia may have benefit as a prophylactic, or therapeutic, for some disease states, and that the addition of intermittent hyperoxia during the exposures may heighten the magnitude of benefit (Uzun et al., 2023). A mechanism of action may be the upregulation of reactive oxygen species and hypoxia-inducible genes. Other reports suggest that hyperoxia exposures are well tolerated (Netzer et al., 2024; Walker et al., 2018). There is, however, no broad evidence base informative of the effect of routine cycling between hypoxia and hyperoxia on aspects physiology. The current study was conducted at the Johnson Space Center, to assess the condition of living in a habitat at mild hypoxia, with periodic decompression with hyperoxic EVA activity simulation (atmospheres being defined by vehicle and mission constraints), on multiple aspects of physiology. The goal was to ascertain if the atmospheric cycling increased any specific clinical risks. The immune assessment was part of that parent study, and utilized a battery of assays previously validated to monitor multiple aspects of immune dysregulation observed in astronauts during spaceflight. Blood samples were collected as described in the schedule above, timed to specifically assess the effect of mild hypoxia, hyperoxic EVA activity, decompression stress, and alternating between the two environments. These assays include peripheral leukocyte distribution as a measure of in-vivo mobilization of immune subsets generally related to immune responses, plasma cytokine concentrations as an indicator of the in-vivo status of the hormonal regulation of immunity between particular immune ‘biases’, and immune cell functional capacity assessed following mitogenic stimulation. Additionally, saliva stress hormone concentrations and the subclinical reactivation of latent herpesviruses were monitored daily.
The data revealed minimal alterations, immune, stress, etc., resulting from the mildly hypoxic cabin environment, primarily associated with the MD3 a.m. sampling (Table 1). At this point, the subjects had experienced almost 3 continuous days in the exploration atmosphere environment. On the surface, this would seem somewhat discordant with our previous finding of profound immune sensitization and latent virus reactivation while living at Concordia Station, Antarctica. These results, as they themselves were somewhat polar opposite from the spaceflight findings, were generally attributed to the confounding variable of persistent hypobaric hypoxia. However, it is relevant that the hypoxia experienced at Concordia Station is profound, with an atmosphere pp O2 of 101.6 mmHg (with an ambient pressure of 522 mmHg), as compared to 159 mmHg at sea level (760 mmHg ambient). This chamber study was NOT designed to assess impactful hypoxia, and was only adjusted to 141–144 mmHg pp O2 but in a significantly lower pressure than Concordia (422/493 mmHg ambient), to coincide with the planned deep space atmosphere. The authors believe this is why noteworthy effects of hypoxia, despite a more pronounced hypobaric stress, were not observed in this chamber study.
The post-hyperoxic EVA findings however, primarily MD3 and MD7 p.m. samplings, were consistent. A redistribution of leukocytes was evident, primarily an increase in neutrophils, with a relative decrease in lymphocytes, monocytes, basophils and eosinophils (Table 2). The RBC, hematocrit and hemoglobin were all increased, and RBC morphology was altered, with an increase in corpuscular volume and hemoglobin (Table 2). Lymphocyte subsets were also altered post EVA, with an increase in the T cell percentage, a reduction in cytotoxic T cells (without a corresponding increase in helper T cells), and an increase in the maturation state of the helper T cell subsets, but a converse reduction in the maturation state of the cytotoxic compartment (Table 2). B cell subsets also moved towards a increased maturation state. Plasma cytokine dysregulation was also evident post- EVA, with an increase in the concentration of several cytokines associated with inflammation (IL-6, IL-12, IL-18) and chemokines also associated with inflammation (MCP-1, IP-10) (Table 3). By MD7, IL-1ra was also increased, confirming a pro-inflammatory state in the subjects. Alterations were also observed for several other cytokines, including RANTES, PDGF, and others, that likely point to disruption of other immunoregulatory processes (Table 3). Minimal alterations in plasma cytokines were observed by MD10, indicating that the previous alterations were indeed associated only with the hyperoxic EVA exposures and not the cabin hypoxia. Leukocyte function, as determined by supernatant cytokine production following blood leukocyte mitogenic stimulation in culture (48 h) was dramatically altered post EVA exposure (Table 4). Increases in multiple cytokines were observed after the EVA activities on MD3 and MD7, with far less alterations at the ‘cycling’ timepoints on the mornings of MD7 and MD10. These results confirm a ‘sensitization’ or increase in immune function was evident, moreso seemingly in the innate populations (LPS stimulation) and broadly (PMA-I stimulation), than was observed in following the T cell specific stimulation (anti-3/28) (Table 4).
The primary finding from this study is that, while living in a mildly hypoxic exploration atmosphere did not have a profound impact on immunity, the participation in hypobaric/hyperoxic EVA activities impacted numerous and varied immune parameters. A summation of the primary significant impacts of hyperoxic EVA are represented in Figure 4. This study was designed to be an applied, clinical research assessment, that monitored four basic categories of outputs: (A) leukocyte distribution, (B) plasma cytokine concentrations, (C) leukocyte function, and (D) latent virus reactivation. We have previously discussed how these parameters generally provide a good snapshot of immune mobilization (A), immunological response biases (B), environmental impacts on function (C), and an adverse clinical outcome that may be quantitated (D). This is insufficient for a detailed biological pathway analysis, however is sufficient for a broad assessment of subject immune function, determining the nature of any dysregulation, and projections of clinical risk. The significant findings confirm immune mobilization of primarily innate cells, a generally pro-inflammatory sensitization, and profound environmental impacts in reducing leukocyte function, without a concurrent rise in latent virus reactivation as is commonly observed in astronauts (Figure 4). Most of the cytokines that were increased in the subject’s plasma, although not to a profound level, are indeed associated with inflammation. The reductions in cell function were most remarkable in both the consistency across the 13 cytokines measures, and across the 3 distinct stimuli. PMA and ionomycin stimulates the nucleus directly and bypass the intracellular signaling pathways required for activation in-vivo, yet it yielded the most consistent reduction in function (11/12 cytokines). In previous stress-analog studies a reduction in cellular function is almost always associated with both stress and the reactivation of latent herpesviruses. The most likely reason for the absence of latent virus reactivation in this study, and the unchanging cortisol levels, seems to be the relatively short duration of the chamber habitation, and the intermittent ‘rest’ days allowing a degree of recovery, something that does not happen during spaceflight of any duration.
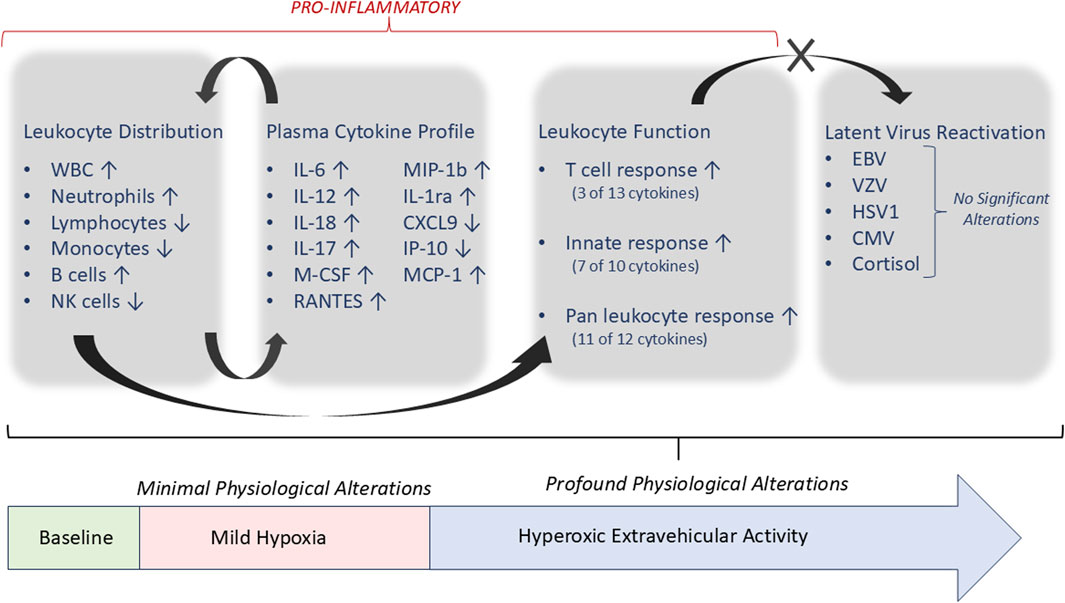
Figure 4. Summary of significant alterations associated with hyperoxic EVA activity, and immunological associations. All parameters listed with ‘↑/↓’ were significantly altered as described, compared to baseline, except where noted.
It should be noted that although stimulations during cell culture were utilized to assess immunocyte functional capacity, these cultures occurred during normoxic and normobaric laboratory conditions. An excellent indicator of direct hypoxia/hyperoxia effects on cell function would be inclusion of altered-atmosphere cell culture. In such a study, alterations may be ‘induced’ by the altered atmosphere culture conditions, even in healthy individuals, essentially creating an artifactual dysregulation that is not representative of the in-vivo condition. That was not the goal of this study. Instead, the goal was to assess dysregulation induced in-vivo, by performing ‘normal’ stimulations ex-vivo and observing functional decrements. This is analogous to spaceflight investigations of astronauts with cellular function altered by microgravity. Dysregulation may be induced in healthy subjects’ cells by culturing in modeled-microgravity conditions, in a bioreactor or clinostat device. For functional assessment of astronauts during spaceflight however, blood samples are collected onboard ISS, and returned for terrestrial ‘static’ (normal, 1xG) cell culture to monitor function. While altered culture conditions are informative for basic cell biology studies, to assess an individual’s immune function in a clinical fashion is not desirous to induce artifactual decrements by culturing in unusual conditions.
Although the changes described above associated with hyperoxic simulated EVAs are noteworthy, they are generally associated with mobilization, inflammation, and cell sensitization. We are therefore unsurprised that in only an 11 days study, there was no increase in stress hormones or any clinically relevant reactivation of latent herpesviruses. Such reactivation, as is commonly observed during spaceflight, is generally associated with immune suppression, and is commonly observed in astronauts during spaceflight (Rooney et al., 2019). Immune suppression was not observed during this study. Nor does this study represent the creation of a true ground-based Space Flight analog in terms of immune response. The goal was not to recreate mission stressors but to create a high-fidelity situation to assess the effect of altered atmospheres in parallel with the physical activity of EVA.
The results from this study confirm previous observations that altered oxygen partial pressures in the atmosphere can profoundly influence immunity. Limitations may include an imperfect analog relevance to spaceflight, as other mission associated stressor (microgravity, radiation, etc.) are absent. Generally, the literature regarding hypoxia exposure is derived from assessments of individuals living at altitude, and the findings generally indicate that such hypoxia exposure is immune sensitizing (Kubo et al., 1998; Hartmann et al., 2000; Coussons-Read et al., 2002; Facco et al., 2005; Ermolao et al., 2009). The previous study at Concordia station confirmed this scenario but using a more sophisticated panel of assays as defined by immune alterations in astronauts (Feuerecker et al., 2014; Feuerecker et al., 2019). The effect of very mild hypoxia in conjunction with intermittent hyperoxic EVA on immunity was unknown. Decompression stress is also known to trigger an inflammatory response. Particularly following hyperbaric (vs hypobaric) exposures, decompression also causes elevation of inflammatory markers even without clinical signs or symptoms of decompression illness (Mitchell, 2024; Mrakic-Sposta et al., 2024). Although similar studies in hypobaric exposures are sparse, blood markers for hypobaric exposures show elevation of inflammatory markers (Connolly et al., 2023). The data seem to indicate that the mild hypoxia condition, even less than hypoxia experienced in an equivalent altitude of Denver, is not disruptive. The hyperoxia and decompression stress of EVAs result in a profound mobilization inflammation and sensitization of immunity as clearly evident in the samples taken after the simulated EVAs (PM). Humans during deep space missions will be a complicated applied experiment consisting of multiple mission stressors and influencing variables, including microgravity stress circadian misalignment, altered diet, and all this in a much more limited habitable volume with communication delays. Altered atmosphere simply adds yet another variable that can influence human physiology. We suggest further characterization is warranted as scenarios with different atmospheres are tested with more EVAs, as well as an ability to monitor immunity and crew members during deep space missions. Having a better understanding of immune changes during atmospheric cycling will allow for development of broad biomedical countermeasures capable of restoring immune function.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: Data available at the NASA Life Sciences Data Archive (LSDA) upon request.
Ethics statement
The studies involving humans were approved by National Aeronautics and Space Administration Johnson Space Center IRB. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
BC: Formal Analysis, Funding acquisition, Writing – original draft, Project administration, Supervision, Validation, Investigation, Methodology, Data curation, Conceptualization, Writing – review and editing, Resources. DD: Writing – original draft, Formal Analysis, Methodology, Data curation. AG: Formal Analysis, Writing – review and editing, Funding acquisition, Resources, Writing – original draft, Project administration, Conceptualization, Data curation, Visualization, Validation, Investigation. CG: Formal Analysis, Writing – original draft, Methodology, Data curation, Investigation, Writing – review and editing. SB-L: Writing – original draft, Formal Analysis, Data curation, Investigation, Methodology. AC: Methodology, Writing – original draft, Investigation, Data curation, Formal Analysis, Writing – review and editing. MY: Writing – original draft, Writing – review and editing, Formal Analysis. SS: Methodology, Investigation, Conceptualization, Data curation, Formal Analysis, Writing – review and editing. SZ: Methodology, Data curation, Investigation, Validation, Writing – review and editing, Writing – original draft, Formal Analysis. TO: Writing – review and editing, Writing – original draft, Formal Analysis. MYH-Y: Investigation, Conceptualization, Writing – review and editing, Project administration, Writing – original draft. PE: Conceptualization, Project administration, Writing – review and editing, Formal Analysis, Data curation, Writing – original draft, Investigation. KM-G: Writing – original draft, Project administration. SM: Writing – review and editing, Methodology, Supervision, Writing – original draft, Conceptualization, Project administration, Formal Analysis, Investigation, Data curation, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The overall chamber study as a Program level evaluation was funded by the NASA JSC Extravehicular Activity and Human Surface Mobility Program. This included the immunological sub-study, for all immediate analyses. Additional funding for analysis of all frozen biosamples was provided by the NASA Human Research Program, Human Health and Countermeasures Element.
Acknowledgments
The authors express their gratitude to all the subjects, across all three missions, who participated in this study, as well as the mission organizers from the NASA JSC EVA and Environmental Physiology Laboratory and NASA Crew and Thermal Systems division. The authors are also grateful for study support from the NASA JSC Clinical Laboratory.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Arnaiz E., Harris A. L. (2022). Role of hypoxia in the interferon response. Front. Immunol. 13. doi:10.3389/fimmu.2022.821816
Bigley A. B., Agha N. H., Baker F. L., Spielmann G., Kunz H. E., Mylabathula P. L., et al. (2019). NK cell function is impaired during long-duration spaceflight. J. Appl. Physiol. (1985) 126 (4), 842–853. doi:10.1152/japplphysiol.00761.2018
Brady T. K., Polk J. D. (2011). Polk. In-Suit light exercise (ISLE) prebreathe protocol peer review assessment. Washinton, DC: NASA. NASA/TM-2011-217062/Volume I NESC-RP-10-00659. Available online at: https://ntrs.nasa.gov/api/citations/20110007150/downloads/20110007150.pdf.
Brubakk A. O., Ross J. A., Thom S. R. (2014). Saturation diving; physiology and pathophysiology. Compr. Physiol. 4 (3), 1229–1272. doi:10.1002/cphy.c130048
Chen X. Q., Kong F. P., Zhao Y., Du J. Z. (2012). High-altitude hypoxia induces disorders of the brain-endocrine-immune network through activation of corticotropin-releasing factor and its type-1 receptors. Zhongguo Ying Yong Sheng Li Xue Za Zhi 28 (6), 481–487.
Choukèr A., Demetz F., Martignoni A., Smith L., Setzer F., Bauer A., et al. (2005). Strenuous physical exercise inhibits granulocyte activation induced by high altitude. J. Appl. Physiol. (1985) 98 (2), 640–647. doi:10.1152/japplphysiol.00036.2004
Cohrs R. J., Mehta S. K., Schmid D. S., Gilden D. H., Pierson D. L., et al. (2008). Asymptomatic reactivation and shed of infectious varicella zoster virus in astronauts. J. Med. Virol. 80 (6), 1116–1122. doi:10.1002/jmv.21173
Connolly D. M., Madden L. A., Edwards V. C., D'Oyly T. J., Harridge S. D. R., Smith T. G., et al. (2023). Early human pathophysiological responses to exertional hypobaric decompression stress. Aerosp. Med. Hum. Perform. 94 (10), 738–749. doi:10.3357/AMHP.6247.2023
Coussons-Read M. E., Mazzeo R. S., Whitford M. H., Schmitt M., Moore L. G., Zamudio S. (2002). High altitude residence during pregnancy alters cytokine and catecholamine levels. Am. J. Reprod. Immunol. 48 (5), 344–354. doi:10.1034/j.1600-0897.2002.01078.x
Crucian B., Sams C. (2009). Immune system dysregulation during spaceflight: clinical risk for exploration-class missions. J. Leukoc. Biol. 86 (5), 1017–1018. doi:10.1189/jlb.0709500
Crucian B., Stowe R., Mehta S., Uchakin P., Quiriarte H., Pierson D., et al. (2013). Immune system dysregulation occurs during short duration spaceflight on board the space shuttle. J. Clin. Immunol. 33 (2), 456–465. doi:10.1007/s10875-012-9824-7
Crucian B. E., Zwart S. R., Mehta S., Uchakin P., Quiriarte H. D., Pierson D., et al. (2014). Plasma cytokine concentrations indicate that in vivo hormonal regulation of immunity is altered during long-duration spaceflight. J. Interferon Cytokine Res. 34 (10), 778–786. doi:10.1089/jir.2013.0129
Crucian B., Stowe R. P., Mehta S., Quiriarte H., Pierson D., Sams C. (2015). Alterations in adaptive immunity persist during long-duration spaceflight. NPJ Microgravity 1, 15013. doi:10.1038/npjmgrav.2015.13
Crucian B., Babiak-Vazquez A., Johnston S., Pierson D. L., Ott C. M., Sams C. (2016a). Incidence of clinical symptoms during long-duration orbital spaceflight. Int. J. Gen. Med. 9, 383–391. doi:10.2147/IJGM.S114188
Crucian B., Johnston S., Mehta S., Stowe R., Uchakin P., Quiriarte H., et al. (2016b). A case of persistent skin rash and rhinitis with immune system dysregulation onboard the International space Station. J. Allergy Clin. Immunol. Pract. 4 (4), 759–762. doi:10.1016/j.jaip.2015.12.021
Damato E. G., Flak T. A., Mayes R. S., Strohl K. P., Ziganti A. M., Abdollahifar A., et al. (2020). Neurovascular and cortical responses to hyperoxia: enhanced cognition and electroencephalographic activity despite reduced perfusion. J. Physiol. 598, 3941–3956. doi:10.1113/JP279453
Damato E. G., Fillioe S. J., Margevicius S. P., Mayes R. S., Somogyi J. E., Vannix I. S., et al. (2022a). Increased serum levels of proinflammatory cytokines are accompanied by fatigue in military T-6A Texan II instructor pilots. Front. Physiol. 13, 876750. doi:10.3389/fphys.2022.876750
Damato E. G., Fillioe S. J., Vannix I. S., Norton L. K., Margevicius S. P., Beebe J. L., et al. (2022b). Characterizing the dose response of hyperoxia with brain perfusion. Aerosp. Med. Hum. Perform. 93, 493–498. doi:10.3357/AMHP.6056.2022
de Aquino Lemos V., dos Santos R. V. T., Lira F. S., Rodrigues B., Tufik S., de Mello M. T. (2013). Can high altitude influence cytokines and sleep? Mediat. Inflamm. 2013 (1), 279365. doi:10.1155/2013/279365
Engst R., Vocks E. (2000). High-mountain climate therapy for skin diseases and allergies-- mode of action, therapeutic results, and immunologic effects. Rehabil. (Stuttg) 39 (4), 215–222. doi:10.1055/s-2000-5897
Ermolao A., Travain G., Facco M., Zilli C., Agostini C., Zaccaria M. (2009). Relationship between stress hormones and immune response during highaltitude exposure in women. J. Endocrinol. Invest. 32 (11), 889–894. doi:10.1007/BF03345767
Facco M., Zilli C., Siviero M., Ermolao A., Travain G., Baesso I., et al. (2005). Modulation of immune response by the acute and chronic exposure to high altitude. Med. Sci. Sports Exerc 37 (5), 768–774. doi:10.1249/01.mss.0000162688.54089.ce
Feuerecker M., Crucian B., Salam A. P., Rybka A., Kaufmann I., Moreels M., et al. (2014). Early adaption to the antarctic environment at dome C: consequences on stress-sensitive innate immune functions. High. Alt. Med. Biol. 15 (3), 341–348. doi:10.1089/ham.2013.1128
Feuerecker M., Crucian B. E., Quintens R., Buchheim J. I., Salam A. P., Rybka A., et al. (2019). Immune sensitization during 1 year in the Antarctic high-altitude concordia Environment. Allergy 74 (1), 64–77. doi:10.1111/all.13545
Hartmann G., Tschöp M., Fischer R., Bidlingmaier C., Riepl R., Tschöp K., et al. (2000). High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine 12 (3), 246–252. doi:10.1006/cyto.1999.0533
Hatfield S. M., Kjaergaard J., Lukashev D., Schreiber T. H., Belikoff B., Abbott R., et al. (2015). Immunological mechanisms of the antitumor effects of supplemental oxygenation. Sci. Transl. Med. 7 (277), 277ra30. doi:10.1126/scitranslmed.aaa1260
Norcross J., Norsk P., Law J., Arias D., Conkin J., Perchonok M., et al. (2013). Effects of the 8 psia/32% O2 atmosphere on the human in the spaceflight environment. Washington, DC: NASA Johnson Space Center: NASA Technical Reports Server.
Kleessen B., Schroedl W., Stueck M., Richter A., Rieck O., Krueger M. (2005). Microbial and immunological responses relative to high-altitude exposure in mountaineers. Med. Sci. Sports Exerc 37 (8), 1313–1318. doi:10.1249/01.mss.0000174888.22930.e0
Krittanawong C., Singh N. K., Scheuring R. A., Urquieta E., Bershad E. M., Macaulay T. R., et al. (2022). Human health during space travel: State-Of-the-art review. Cells 12 (1), 40. doi:10.3390/cells12010040
Kubo K., Hanaoka M., Hayano T., Miyahara T., Hachiya T., Hayasaka M., et al. (1998). Inflammatory cytokines in BAL fluid and pulmonary hemodynamics in highaltitude pulmonary edema. Respir. Physiol. 111 (3), 301–310. doi:10.1016/s0034-5687(98)00006-1
Mehta S. K., Laudenslager M. L., Stowe R. P., Crucian B. E., Sams C. F., Pierson D. L., et al. (2014). Multiple latent viruses reactivate in astronauts during Space Shuttle missions. Brain Behav. Immun. 41 (2), 210–217.
Mehta S. K., Laudenslager M. L., Stowe R. P., Crucian B. E., Feiveson A. H., Sams C. F., et al. (2017). Latent virus reactivation in astronauts on the international space station. NPJ Microgravity 3, 11. doi:10.1038/s41526-017-0015-y
Mehta S. K., Szpara M. L., Rooney B. V., Diak D. M., Shipley M. M., Renner D. W., et al. (2022). Dermatitis during spaceflight associated with HSV-1 reactivation. Viruses 14 (4), 789. doi:10.3390/v14040789
Mehta S. K., Suresh R., Brandt K., Diak D. M., Smith S. M., Zwart S. R., et al. (2024). Immune system dysregulation preceding a case of laboratory-confirmed zoster/dermatitis on board the International space station. J. Allergy Clin. Immunol. Glob. 3 (2), 100244. doi:10.1016/j.jacig.2024.100244
Mitchell S. J. (2024). Decompression illness: a comprehensive overview. Diving hyperbaric Med. 54 (1Suppl. l), 1–53. doi:10.28920/dhm54.1.suppl.1-53
Mrakic-Sposta S., Brizzolari A., Vezzoli A., Graci C., Cimmino A., Giacon T. A., et al. (2024). Decompression illness after technical diving session in Mediterranean Sea: oxidative stress, inflammation, and HBO therapy. Int. J. Mol. Sci. 25 (21), 11367. doi:10.3390/ijms252111367
Netzer N. C., Jaekel H., Popp R., Gostner J. M., Decker M., Eisendle F., et al. (2024). Oxidative stress reaction to hypobaric-hyperoxic civilian flight conditions. Biomolecules 14 (4), 481. doi:10.3390/biom14040481
Nolte S., Malhan D., Klemmer A., Kastner T., Walter N., Fleckenstein D., et al. (2025). Training in normobaric hypoxia induces hematological changes that affect iron metabolism and immunity. Sci. Rep. 15 (1), 17757. doi:10.1038/s41598-025-01542-w
Oliver S. J., Macdonald J. H., Harper Smith A. D., Lawley J. S., Gallagher C. A., Di Felice U., et al. (2013). High altitude impairs in vivo immunity in humans. High. Alt. Med. Biol. 14 (2), 144–149. doi:10.1089/ham.2012.1070
Pahl A., Szelenyi S., Brune K. (2006). Hypoxia induced chemokine expression in nasal epithelial cells: development of an in vitro model for chronic rhinosinusitis. Altex 23 (2), 59–63.
Rooney B. V., Crucian B. E., Pierson D. L., Laudenslager M. L., Mehta S. K., et al. (2019). Herpes virus reactivation in astronauts during spaceflight and its application on Earth. Front. Microbiol. 10. doi:10.3389/fmicb.2019.00016
Schultze-Werninghaus G. (2008). Effects of high altitude on bronchial asthma. Pneumologie 62 (3), 170–176. doi:10.1055/s-2007-1016442
Siqués P., Brito J., León-Velarde F., Barrios L., De La Cruz J. J., López V., et al. (2007). Hematological and lipid profile changes in sea-level natives after exposure to 3550-m altitude for 8 months. High. Alt. Med. Biol. 8 (4), 286–295. doi:10.1089/ham.2007.8405
Taylor C. T., Colgan S. P. (2017). Regulation of immunity and inflammation by hypoxia in immunological niches. Nat. Rev. Immunol. 17 (12), 774–785. doi:10.1038/nri.2017.103
Tiollier E., Schmitt L., Burnat P., Fouillot J. P., Robach P., Filaire E., et al. (2005). Living high-training low altitude training: effects on mucosal immunity. Eur. J. Appl. Physiol. 94 (3), 298–304. doi:10.1007/s00421-005-1317-4
Uzun A. B., Iliescu M. G., Stanciu L. E., Ionescu E. V., Ungur R. A., Ciortea V. M., et al. (2023). Effectiveness of intermittent hypoxia-hyperoxia therapy in different pathologies with possible metabolic implications. Metabolites 13 (2), 181. doi:10.3390/metabo13020181
Keywords: immunity, spaceflight, hypoxia, hyperoxia, extravehicular activity
Citation: Crucian B, Diak DM, Garbino A, Gutierrez C, Bustos-Lopez S, Colorado A, Young M, Smith SM, Zwart SR, Oswald TM, Hew-Yang MY, Estep P, Marshall-Goebel K and Mehta S (2025) Effects of hypoxia/hyperoxia exposure on immune function – results from a spacecraft-relevant hypobaric chamber study. Front. Physiol. 16:1637834. doi: 10.3389/fphys.2025.1637834
Received: 29 May 2025; Accepted: 01 August 2025;
Published: 09 September 2025.
Edited by:
Bridgette Rooney, Eagle Integrated Services, United StatesReviewed by:
Laura Veronica Gonzalez Bosc, University of New Mexico, United StatesSalih Murat Egi, Galatasaray University, Türkiye
Nikolaus Christoph Netzer, University of Innsbruck, Austria
Copyright © 2025 Crucian, Diak, Garbino, Gutierrez, Bustos-Lopez, Colorado, Young, Smith, Zwart, Oswald, Hew-Yang, Estep, Marshall-Goebel and Mehta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brian Crucian, YnJpYW4uY3J1Y2lhbi0xQG5hc2EuZ292
 Brian Crucian
Brian Crucian Douglass M. Diak
Douglass M. Diak Alejandro Garbino3
Alejandro Garbino3 Cody Gutierrez
Cody Gutierrez Scott M. Smith
Scott M. Smith Sara R. Zwart
Sara R. Zwart Karina Marshall-Goebel
Karina Marshall-Goebel Satish Mehta
Satish Mehta