- 1Department of Cellular and Integrative Physiology, University of Texas Health Science Center at San Antonio, San Antonio, TX, United States
- 2Glenn Biggs Institute for Alzheimer’s & Neurodegenerative Diseases, University of Texas Health Science Center at San Antonio, San Antonio, TX, United States
- 3Department of Pharmacology, University of Texas Health Science Center at San Antonio, San Antonio, TX, United States
Ferroptosis is an iron-dependent programmed cell death that plays an important role in neurodegenerative and neuropsychiatric diseases. In the present study, we have highlighted how different risk factors are involved in the induction of ferroptosis in brain cells. In addition, we also demonstrated how ferroptosis plays an important role in different brain diseases. In our study why we focused and elaborated on the mechanisms of ferroptosis only in brain cells (Neurons, oligodendrocytes, and microglia) because they are particularly vulnerable to such kind of cell death. Additionally, brain cells are more dependent on mitochondrial function, iron regulation, and high levels of polyunsaturated fatty acids (PUFAs) as compared to peripheral body cells. Highlighting ferroptosis is more important because it has demonstrated several important mechanisms of neuronal injury and dysfunction which provides a deep understanding of the etiology of various brain diseases that were not sufficiently described by other programmed cell death pathways. Therefore, it has led to the exploration of new therapeutic strategies against various brain diseases and thus targeting ferroptosis-related proteins opens a new therapeutic window for several incurable brain diseases, and various ferroptosis regulators are now under clinical trials. However, their validation as a preclinical therapeutic agent is needed. Interestingly, here in our study we also summarize the most recent potential therapeutic targets and promising interventions which will provide a beam of light for future therapies against major brain diseases.
Introduction
Ferroptosis is an iron-dependent programmed cell death that has bridging metabolic dysfunction with redox biology. Iron is pivotal in normal physiological conditions such as DNA synthesis, cell division, neurotransmission, cellular respiration, oxygen transport, and cellular metabolism. Ferroptosis is characterized by unique biochemical and morphological changes that distinguish it from other program cells death. The most important features of ferroptosis are alteration in redox balance, iron homeostasis and lipid metabolism. Transferrin, Metal Transporter, and Iron Response Element Binding Protein 2, are important regulators of ferroptosis that induces cell death and modulating intracellular and systemic iron homeostasis. Irons overload initiates oxidation of the acyl tail of unsaturated fatty acid via Fenton reactions which in turn leads to increase the formation of reactive oxygen species (ROS) and lipid peroxidation (Stockwell, 2022; Dix et al., 2024; Jiang et al., 2021; Li et al., 2020; Berndt et al., 2024). Therefore, higher the level of unsaturated fatty acid more will be the ROS production and vice versa. Primarily there are two antioxidant systems know as Glutathione (GSH)/Glutathione peroxidase 4 (GPX4) systems and the Coenzyme Q10 (CoQ10)/Ferroptosis Suppressor Protein 1 (FSP1) system which catalyzes the reduction of lipid peroxides. Change in the expression and activity of these molecules is very crucial to understand the fate of a cell. Ten years ago, ferroptosis was first documented as a program cell death of the body which is involved in variety of biological processes, including muscle atrophy, neuron loss, tumor growth, ischemia reperfusion and immune escape. It has been shown that ferroptosis play essential role in health maintaince and in the development of multiple human diseses (Supplementary Figure S1).
Unlike other forms of cell death, ferroptosis specifically affects neurons with high metabolic demands and polyunsaturated fatty acid-rich membranes, making them especially vulnerable under conditions of oxidative stress and inflammation. Recently, increase interest and development of ferroptosis related research have uncover numeriuos regulators to facilitate the clinical and precilical application of that agents and opend a new therapeutic window for feroptisis related neurodegenerative and psychiatric diseases (Zeng et al., 2023a; Pan et al., 2023; Liang et al., 2022; Tang et al., 2021; Li et al., 2023a; Dixon et al., 2012; Peng et al., 2022a; Liao et al., 2022; Qin et al., 2022). This review comprehensively elaborates the detail mechanisms, importance in brain diseases, regulation, therapeutic avenues and different risk factors of ferroptosis (Figure 1).
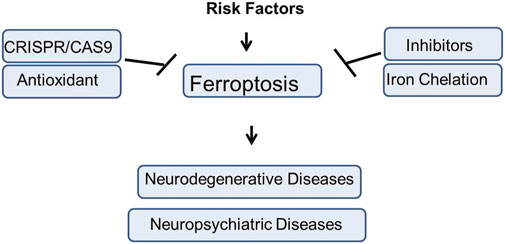
Figure 1. The graphical summery of the present study. The risk factors involved in the induction of Ferroptosis mediated neuropsychiatric and neurodegenerative disorders. Different therapeutic strategies against Ferroptosis mediated brain diseases such as use of inhibitors, antioxidant agents, genetic engineering approach, Iron Chelation, etc., has been elucidated in the present study.
We also highlight how to use CRISPR editing on brain disease risk genes in ferroptosis. Our finding could provide strategies for innovative treatments of ferroptosis-associated diseases, offering hope for addressing some of the most challenging biomedical conditions of our time.
Role and importance of ferroptosis in neurodegenerative, neuropsychatric diseases and its unifying model
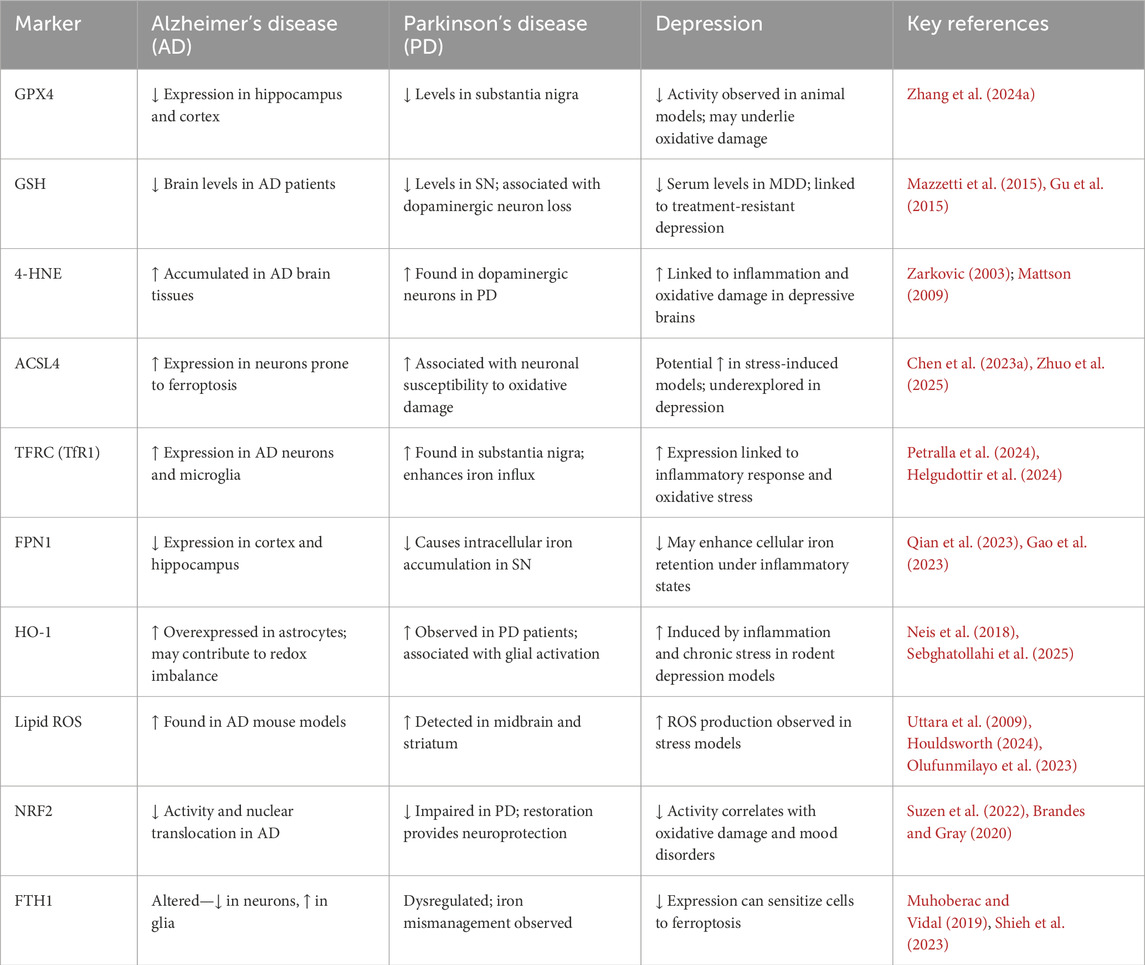
Iron absorption and transport can be disrupted by neurological disorders, which can result in excessive iron accumulation, elevated oxidative stress, and cellular ferroptosis. Ferroptosis is a major contribution to neurodegenerative disorders, according to growing study (Figure 2) (Wei et al., 2024; Long et al., 2023; Wang et al., 2024). In diseases such as alzheimer’s, parkinson’s, and multiple sclerosis, imbalances in iron metabolism and antioxidant protection result in oxidative stress, rendering neurons and glial cells particularly vulnerable to ferroptotic injury. Microglia and oligodendrocytes, responsible for regulating neuroinflammation and myelination, can experience ferroptosis due to chronic inflammation and oxidative stress, exacerbating neural injury (Li et al., 2024a). In diseases such as alzheimer’s, parkinson’s, and multiple sclerosis, imbalances in iron metabolism and antioxidant protection result in oxidative stress, rendering neurons and glial cells particularly vulnerable to ferroptotic injury. Microglia and oligodendrocytes, responsible for regulating neuroinflammation and myelination, can experience ferroptosis due to chronic inflammation and oxidative stress, exacerbating neural injury (Li et al., 2024a). We have described the ferroptosis drivers across diseases in the following table.
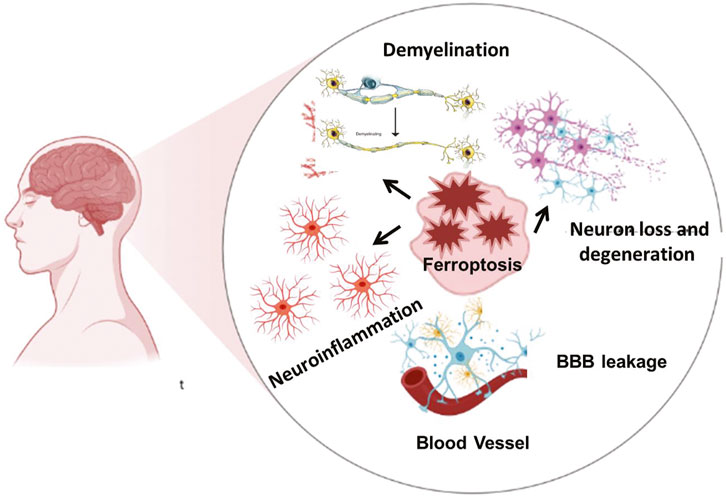
Figure 2. The Ferroptosis mediated brain diseases. Iron overload, ROS burden and oxidative stress are involved in demyelination, neuroinflammation, and neuronal loss which in turn lead to various neurodegenerative and neuropsychiatric diseases. Neuroinflammation is further actively participating in the blood brain barrier disruption.
Unifying model of ferroptosis drivers across diseases
Ferroptosis and alzheimer
Abnormal iron metabolism has been linked to the development of AD, according to a number of research. Ferroptosis-related characteristics, such as aberrant iron metabolism, glutamate excitotoxicity, and the buildup of lipid ROS, have been seen in the brain tissues of AD patients and AD model mice. Iron levels in the hippocampus, cortical lobe, and basal ganglia are also higher in AD patients than in control participants, according to research. The degree of amyloid deposition has also been linked to the levels of iron and ferritin in brain tissue. Furthermore, AD patients exhibit downregulated expression of GPX4 and increased levels of 4-HNE and Malondialdehyde in different parts of the brain. According to one study, patients with AD were able to live better lives after receiving an intramuscular injection of the iron-chelating drug deferoxami (Bulk et al., 2018; Lee and Lee, 2019; Yan and Zhang, 2019; Dare et al., 2020; Villalon-Garcia et al., 2023; Rogers and Lahiri, 2004; Lei et al., 2019; Zhang et al., 2022a). One known contributing element to the onset of AD is glutamate excitotoxicity. According to Zhang et al. (2020), AD may result from an increase in extracellular glutamate concentration brought on by system Xc–failure during ferroptosis. Additionally, Feng et al. (2023) found that the hippocampus of a mouse model of AD in which PSEN1 (Presenilin-1) had been knocked down had higher expression of ferroptosis-related proteins (GPX4, SLC7A11, ACSL4, Phosphatidylethanolamine Binding Protein 1) than did healthy mice (Feng et al., 2024a). Recent studies emphasize the importance of iron in controlling tau phosphorylation and aggregation, which may lead to the development of neurofibrillary tangles in neurodegenerative disorders like alzheimer’s disease. A notable interaction seems to exist between iron and tau, affecting disease progression and symptoms. Notably, tau binds to iron, resulting in its aggregation and potential accumulation as iron-rich tangles in the brains of individuals with AD. Additionally, increased iron concentrations may enhance tau phosphorylation in cultured neurons, indicating a possible connection between increased iron and abnormal tau in alzheimer’s disease (Mohammadi et al., 2024). Furthermore, Recent studies suggest that the Iron-driven production of reactive oxygen species (ROS) could result in protein misfolding and cell damage. The misfolding and aggregation of neuronal proteins like α-synuclein, Tau, amyloid beta (Aβ), TDP-43, or SOD1 is a prevalent characteristic of various neurodegenerative diseases, and iron has been demonstrated to promote protein aggregation (Joppe et al., 2019). This suggests that ferroptosis and neurodegenerative diseases like AD are closely related. Because of this, ferroptosis could be a key process in the development of AD. Ferroptosis, driven by iron accumulation, oxidative stress, and protein aggregation, plays a central role in the pathogenesis and progression of Alzheimer’s disease (Dare et al., 2020; Feng et al., 2024a; Zhang et al., 2020; Han et al., 2023).
Ferroptosis and parkinson
Ferroptosis and Parkinson’s disease are closely related, according to an increasing number of studies. Ferroptosis-like clinical features of parkinson’s disease (PD) include oxidative stress, LPO, GSH depletion, and abnormalities of iron metabolism (da Costa Caiado et al., 2025). Furthermore, the loss of dopaminergic neurones in the substantia nigra (SN) and striatum can be avoided by using the iron chelating drug DFP and the ferroptosis-specific inhibitor Ferrostatin-1 (Fer-1) Lipostatin-1. Ferroptosis was identified by Do Van et al. (2016) as a new type of cellular death in parkinson’s disease. In dopaminergic cells, Erastin causes cytotoxicity through activating Protein Kinase C (PKC), which sets off MEK signalling and promotes ferroptosis. Additionally, ferroptosis in PD can be reduced by PKC suppression. The 140 amino acid protein known as α-Syn is mostly expressed in the brain and is essential for several neuronal synaptic functions. One important pathological sign of parkinson’s disease (PD) is the aggregation of α-Syn, which is a prominent component of intracellular Lewy bodies. α-Syn produces LPO by producing ROS, which increases calcium influx and causes cell death (Dionisio et al., 2021; Thapa et al., 2022; Do Van et al., 2016; Chartier-Harlin et al., 2004; Angelova et al., 2020). Cumulative findings showed that lowering α-synuclein levels in dopaminergic neurons helps prevent ferroptosis, whereas enhanced α-synuclein levels in neuronal precursor cells from patients with SNCA triplication increases susceptibility to lipid peroxidation and ferroptosis (Mahoney-Sanchez et al., 2022). Ferroptosis has a role in the degenerative mechanism of parkinson’s disease (PD), as research has shown that elevated iron accumulation or decreased intracellular glutathione levels contribute to the abnormal aggregation of PD α-Syn (Angelova et al., 2020). Ferroptosis contributes significantly to Parkinson’s disease progression through iron accumulation, oxidative stress, and α-synuclein aggregation, making it a promising therapeutic target.
Ferroptosis and depression
According to research, neuroinflammation is the immune response of the central nervous system (CNS) that is mostly the result of astrocytes and microglia in the hippocampus. A possible link between iron and neuroinflammation has been suggested by the association of microglia, which are recognised for having a high iron content, with depression linked to aberrant glial activation and iron overload. It is unknown, therefore, exactly how iron overload upsets neurotransmitter balance and causes anxiety and depressed symptoms (Li et al., 2024a; Lee and Hyun, 2023; Uzungil et al., 2022; Zeng et al., 2023b). Recent studies shown that In depression and associated neuropsychiatric conditions, inflammatory mechanisms are crucial in promoting ferroptosis. Increased concentrations of pro-inflammatory cytokines—like TNF-α, IL-6, and IL-1β—can interfere with glutathione metabolism and inhibit antioxidant systems such as GPX4, making neurons more susceptible to oxidative damage. At the same time, inflammation causes iron imbalances, raising intracellular free iron by enhancing DMT1 expression and degrading Ferritin, which further promotes lipid peroxidation. These mechanisms establish a feedback loop in which persistent neuroinflammation drives ferroptosis, leading to neuronal degeneration and the underlying causes of depression (Liu et al., 2025; Feng et al., 2025; Dang et al., 2022).
Studies have demonstrated that Brain-derived Neurotrophic Factor (BDNF) signal transduction is essential for synaptic plasticity in depression, and that BDNF downregulation may have neurotoxic consequences. According to Li et al., iron overload may cause BDNF to be downregulated through the iron urin BDNF pathway, which could result in injury to the hippocampus. Additionally, Gao et al. demonstrated that iron deposition in hippocampal microglia is directly linked to neuronal death and degeneration in a Chronic Unexpected Mild Stress (CUMS) animal model. Furthermore, Zeng et al. emphasised how important Nrf2 is as an anti-inflammatory mediator in controlling iron deposition and neuroinflammatory reactions in depression. Cao et al. found clear changes in protein expression between normal mice and CUMS model animals in a comparative research employing hippocampus proteomics, showing significant iron deposition and neuronal necrosis activation in the hippocampus, which encourages the development of depression. Zhang et al. recently found that CUMS model mice had considerably higher expression levels of different inflammatory markers, but that this neuropathological alteration was successfully reversed by treatment with the iron chelating drug deferoxamine (DFO). All of these results point to a possible connection between the onset of depression and the neurotoxicity brought on by iron overload (Zeng et al., 2023b; Shkundin and Halaris, 2023; Li et al., 2023b; Gao et al., 2019; Cao et al., 2021; Zhang et al., 2022b; Lima Giacobbo et al., 2019; Williams et al., 2022). Iron overload–induced neuroinflammation and ferroptosis play a central role in the pathogenesis of depression by disrupting antioxidant defenses, impairing BDNF signaling, and triggering neuronal degeneration. In order to elaborate the ferroptosis related marker and their correlation with neurodegenerative and neuropschychtric diseases, we have provided a summarized table.
Mechanisms of ferroptosis
Aging and metabolic impairment could affect iron’s normal physiological function in the body, increasing the risks of iron-linked neurodegenerative diseases. Specifically, the free intracellular divalent iron (Fe2+) is highly reactive, as it promotes the generation of Reactive Oxygen Species (ROS) via Fenton reactions. These ROS catalyze the peroxidation of polyunsaturated fatty acids, resulting in cellular membrane damage. Aging-dependent iron accumulation in the brain promotes direct ferroptosis and enzyme-mediated redox reactions such as lipid peroxidation (Dixon et al., 2012; Li et al., 2021; Benarroch, 2023; Zhou et al., 2020a; Rochette et al., 2022; Sato et al., 2022; Zhou et al., 2023). Ferroptosis-linked Neurodegenerative Diseases (NDDs) encompass a complex group of conditions associated with neuronal cell death and functional decline (Sun et al., 2022). Advances in proteomic, genomic, animal, and cellular approaches have identified several novel targets recently approved for treating alzheimer’s Disease (AD), Amyotrophic Lateral Sclerosis (ALS), and other NDDs (Emerson and Swarup, 2023; Amartumur et al., 2024). Several convincing studies have revealed that iron accumulation in affected brain regions and multiple neurodegenerative diseases share pathological links. The concept that iron overload significantly accelerates neurodegeneration and acts as a driver of disease progression is increasingly supported by research. (Stockwell, 2022; Dixon et al., 2012; Sato et al., 2022; Ndayisaba et al., 2019; D’Mello and Kindy, 2020; Mitchell et al., 1973; Wiernicki et al., 2020; Kurian and Hayflick, 2013).
In alzheimer’s disease, iron dysregulation contributes to amyloid-beta plaque aggregation and tau hyperphosphorylation. Both processes exacerbate oxidative stress and neuronal damage, ultimately resulting in progressive cognitive decline (Tamagno et al., 2021). Iron deposition in the hippocampus and basal ganglia of AD patients correlates with disease severity and is linked to impaired memory and executive function (Wang et al., 2023a; Liu et al., 2018). Similarly, ALS is characterized by motor neuron degeneration, muscle atrophy, and eventual paralysis (Wijesekera and Leigh, 2009). Iron accumulation in motor neurons has been shown to contribute to mitochondrial dysfunction and oxidative stress, key drivers of disease progression (Cheng et al., 2022). Elevated ferritin levels in the cerebrospinal fluid of ALS patients suggest disrupted iron metabolism as a significant pathological feature (Muyderman and Chen, 2014; Hemerkova and Valis, 2021; Paydarnia et al., 2021; Zheng et al., 2017).
In parkinson’s disease (PD), excess iron in the substantia nigra is associated with dopaminergic neuronal loss and the aggregation of alpha-synuclein, a hallmark of PD pathology. Iron-induced oxidative stress exacerbates neuroinflammation, further promoting neuronal death and motor dysfunction. Therapeutic strategies targeting iron accumulation, such as chelation therapy, show promise in reducing oxidative stress and improving motor symptoms in PD (Yi et al., 2022; Srinivasan et al., 2021).
Neurodegeneration with Brain Iron Accumulation (NBIA) disorders, such as Pantothenate Kinase-Associated Neurodegeneration PKAN, are rare conditions involving abnormal iron deposition in the basal ganglia. Clinically, these disorders present with progressive movement abnormalities, dystonia, parkinsonism, and cognitive decline. The excessive iron in these regions triggers ferroptosis, leading to neuronal degeneration and the observed neurological deficits (Tonekaboni and Mollamohammadi, 2014; Dusek et al., 2022).
Understanding the interplay between ferroptosis and these neurodegenerative diseases is crucial for developing therapeutic strategies aimed at preventing ferroptosis-mediated neuronal damage. By targeting iron dysregulation, oxidative stress, and lipid peroxidation, researchers hope to mitigate the progression of these debilitating conditions.
Recent research indicates lipid and amino acid metabolism provides a foundation for ferroptosis. For example, in 1973, Jerry Mitchell revealed that acetaminophen induces hepatic necrosis in rats dependent on Cysteine and glutathione (GSH). Similarly, the polyunsaturated fatty acids in the membrane lipids were identified as an essential peroxidation substrate for ferroptosis (Stockwell, 2022; Mitchell et al., 1973). However, ferroptosis has multiple molecular regulators, and it is still unclear whether it is programmed cell death because the molecular pathway in normal physiology has not yet been well explored. Multiple pathways need to be explored. However, the most recent and studied mechanisms involved in regulating ferroptosis are loss of the antioxidant system of the cell, iron dyshomeostasis, and lipid peroxidation (Stockwell, 2022; Dixon et al., 2012; Wiernicki et al., 2020).
Redox reaction imbalance and ferroptosis
Active factors disrupting the antioxidant system-mediated ferroptosis are aging, Type 2 Diabetes (T2D), chronic obesity, etc. (Deng et al., 2023; Li et al., 2023c). Aging could negatively affect the transfer of RNA between cells (Dluzen et al., 2017; Hamdan et al., 2021), factors involved in protein syntheses such as Translation Elongation Factor 2 (TEF2), and the level of C-Glycosyl Tryptophan. Increased glycosylation of tryptophan and increased (C-gly Trp) strongly correlate with aging (Li et al., 2023c; Anisimova et al., 2018; Parrado et al., 1999; Menni et al., 2013; Schmidt-Sommerfeld et al., 1992; Il’yasova et al., 2012; Cindric et al., 2021). Translation Elongation Factor 2 (TEF2) is relatively less active and more fragmented with age, which results in the decline of protein synthesis. This phenomenon induces ROS, and, in turn, this reactive oxygen species inhibits the activation of TEF-2 (Parrado et al., 1999; Li et al., 2024b; Ball et al., 2021). Dysregulated RNA transfer is related to the poor quantity and quality of MicroRNAs (miRNAs). All these aging-related impairments ultimately affect cell growth, cell survival, protein synthesis, and antioxidant defense mechanisms of cells. Recent research revealed that metabolic syndrome and metabolic panel dysregulation are strongly linked with diabetes and obesity (Ball et al., 2021; Iorio and Croce, 2012; Bonomini et al., 2015; Zhang et al., 2023a). T2D and obesity could trigger multiple pathways, such as the inflammatory pathways that include activation and translocation of Necrotic Factor Kappa B (NF-kB), TNFα, INOS, IL-1β, INF-γ, leukocyte infiltration, MCP-1, etc (Mahmoud and Abdel-Rasheed, 2023; Marunaka, 2023; Khan et al., 2021). Increased free fatty acid and hyperglycemia are the two most common worst conditions in obese diabetic patients. This could activate both insulin-resistant and oxidative stress pathways (Khan et al., 2021; Tangvarasittichai, 2015; Fryk et al., 2021; Chandrasekaran and Weiskirchen, 2024). Aberrant activation of the JAK/STAT pathway is linked with the induction of inflammatory cytokines, producing Superoxide Anions (SA) and Advanced Glycation End Products (AGEs) (Simon et al., 1998). Stress Kinase (JNK) and transcription factor Nuclear Factor kappa B (NF-κB) activation and phosphorylation actively induce insulin resistance via Insulin Receptor Substrate 1 (IRS1) disruption (Solinas and Becattini, 2017; Yung and Giacca, 2020; Baker et al., 2011). Collectively, Age, T2D, and obesity-related signaling pathways end at induction of oxidative stress and disruption of first-line defense Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT), and Glutathione Peroxidase (GPX) (Novak et al., 1996; Wang and Zhang, 2024; Zgutka et al., 2023; Promyos et al., 2023; Gusti et al., 2021). On the other hand, it can also regulate BTB and CNC Homology 1 (BACH1) and Heme oxygenase-1 (HO-1) (Kondo et al., 2013; Jin et al., 2023; Ryter, 2022). BACH1 promotes ferroptosis by repressing gene transcription that regulates Glutathione (GSH) synthesis and intracellular labile iron metabolism (Irikura et al., 2023; Soni et al., 2024). At the same time, HO-1 can act as a mediator of ferroptosis. HO-1 can increase the labile iron pool and promote lipid peroxidation, leading to ferroptosis (Han et al., 2022; Chen et al., 2023b). Ferroptosis in the brain works very closely with the demyelination of the neuronal cells, which is dependent on T Cell Receptor (TCR) signaling (Luoqian et al., 2022; Qin et al., 2023). Aging, Type 2 Diabetes, and obesity converge on oxidative stress and inflammatory pathways that disrupt antioxidant defenses and promote ferroptosis, contributing to neurodegeneration and demyelination (Figure 3).
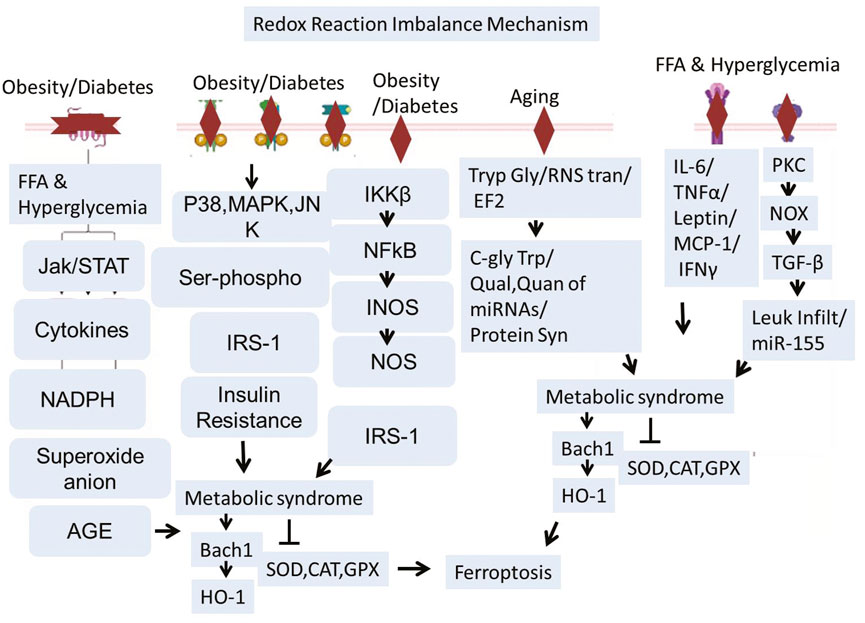
Figure 3. Schematic representation illustrating how aging, Type 2 Diabetes (T2D), and obesity lead to ferroptosis and neurodegeneration. Aging, T2D, and obesity hinder antioxidant defenses by affecting RNA transfer, lowering TEF2 activity, and triggering chronic inflammation. These conditions trigger oxidative stress pathways such as JAK/STAT, NF-κB, and JNK, resulting in decreased activity of essential antioxidant enzymes (SOD, CAT, GPX). Increased free fatty acids, AGEs, and cytokines intensify ROS generation and insulin resistance. Ferroptosis is facilitated by BACH1-driven inhibition of GSH production alongside HO-1–triggered iron accumulation and lipid peroxidation. The consequent ferroptotic cell death aids in neurodegeneration and demyelination via TCR signaling.
Abnormal iron metabolism and ferroptosis
There are several well known health condition which aguments iron dyshomeostasis and activates different pathways that leads to iron load and lipids peroxidation in the central nervous system. Among them, Cardiovascular diseases, high cholesterol, smoking, diabetes, and high blood pressure are the common abnormilities that could induces cerebral ischemia and stroke. These factors lead to the building of plaques and clots in the arteries, which lead to a lack of blood flow to the brain (Ekker et al., 2023). The other causes like many babies develop hypoxic conditions in their brains if the oxygen is not distributed correctly to the brain immediately after birth. This sometimes creates a group of conditions collectively known as cerebral palsy (CP) (Paul et al., 2022). Therefore, the proper distribution of oxygen in the body is necessary. Poor, insufficient, or lack of oxygen supply to the organ results in hypoxia, infarction, or ischemia, which aggravates several cell death pathways mediated through lipid peroxidation and iron overload. Cerebral infarction and brain ischemia mediate Ubiquitin-Specific Protease 14 (USP14), Cyclic Guanosine Monophosphate–Adenosine Monophosphate Synthase cGAS-STING, JAK-STAT3, HIF-1α, and Nrf-2 signaling (Zhang et al., 2023b; Ma et al., 2023; Huang et al., 2022; Li et al., 2023d; Liu et al., 2024a; Hu et al., 2022). Recent studies showed that USP14 activation is involved in iron overload, while inhibition enhances mitophagy and normalizes the mitochondrial defects of Parkin KO human neurons (Bernardo et al., 2024). Hypoxic damage in the brain could also activate HIF-1α, which is a transcription complex. HIF-1α can increase iron levels in the brain via upregulating Transferrin Receptor 1 (TfR1) (Baranova et al., 2007; Vela, 2018). TfR1 is involved in transporting iron in the cell (Vela, 2018; Ding et al., 2011; Wang et al., 2020a; Fillebeen et al., 2019). Besides cerebral ischemia, Traumatic Brain Injury (TBI) is a leading cause of brain damage and paralysis. Kids and Athletic people are more vulnerable to such kinds of injuries. TBI not only causes acute brain damage but also sometimes leads to chronic and long-term disabilities. Recently, several studies have explored the mechanisms involved in TBI-related brain pathologies. Besides other pathologies, TBI is also actively involved in brain iron dyshomeostasis and overload via inhibition of the TrkB/PI3K/Akt/Nrf2 signaling pathway. Other conditions like metabolic disorders could induce iron overload via endoplasmic reticulum stress, ROS, and suppression of cytokine signaling three expressions. A recent study highlighted the critical role of hypothalamic iron in obesity development. This study revealed that reducing iron overload in AgRP neurons inhibits AgRP neuron activity, endoplasmic reticulum stress, Suppressor of Cytokine Signaling 3 (SOCS3), oxidative stress, and NF-κB signaling. This mechanism works like a feedback loop where iron overload induces obesity, and on the other hand, obesity and metabolic disorders will accelerate iron overload (Zhang et al., 2024b; Li et al., 2023e). The aging process could connected with increased hepcidin, while increased hepcidin is associated with increased ubiquitination. This could significantly reduce the iron exporters known as Ferroportin-1 (FPN1) (Sato et al., 2022). Various health conditions—such as cardiovascular disease, cerebral ischemia, hypoxia, traumatic brain injury, metabolic disorders, and aging—converge on iron dyshomeostasis and oxidative stress pathways, promoting ferroptosis and contributing to neurodegeneration.
Iron overload has been implicated in the development of neurodegenerative diseases such as alzheimer’s via increased tau phosphorylation and abnormal cleavage of amyloid precursor proteins, as shown in Wang et al. (2023a), Wang et al. (2022), Wang et al. (2020b) (Figure 4).
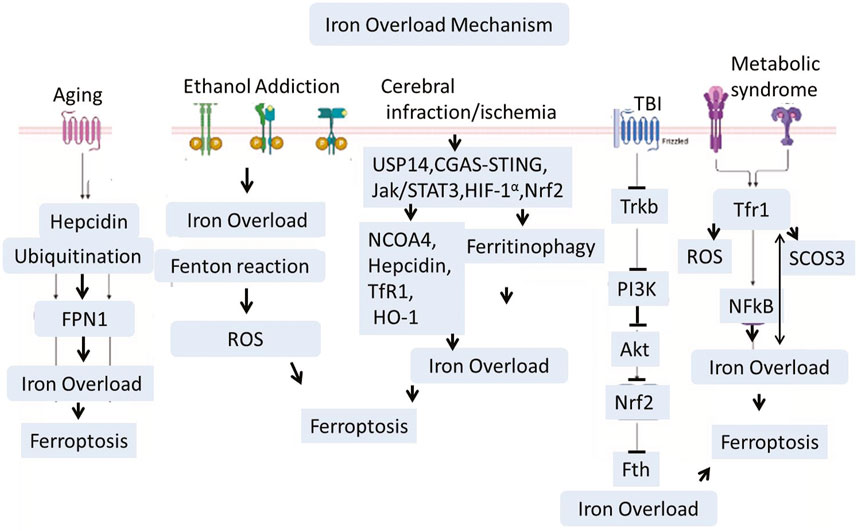
Figure 4. Traumatic brain injury (TBI), Ischemia, Aging, and ethanol addiction interfere with iron metabolism via signaling pathways including USP14, HIF-1α, and JAK/STAT3. Iron accumulation is worsened by heightened expression of Transferrin Receptor 1 (TfR1) and decreased iron export through Ferroportin-1 (FPN1), especially in older individuals. Furthermore, the buildup of iron in the hypothalamus linked to obesity encourages oxidative stress and inflammation via SOCS3 and NF-κB. Together, these mechanisms promote lipid peroxidation and ferroptosis, connecting systemic issues to neurodegeneration in conditions like Alzheimer’s through tau hyperphosphorylation and amyloid dysregulation.
Lipid metabolism and ferroptosis
Many types of brain injury, insult, or stress could induce the production of Reactive Oxygen Species (ROS). During the oxidative phosphorylation process within the mitochondria, many electrons leak from the electron transport chain, interacting with the oxygen molecule and producing superoxide radicals (O2-). They are byproducts of normal metabolism in the body. These superoxide radicals are converted into other ROS like hydrogen peroxide (H2O2) and Hydroxyl Radicals (HO-). When the cellular antioxidant system cannot control and balance this reactive species, it will generate oxidative stress (Pizzino et al., 2017; Brieg et al., 2012). These free radicals are very reactive and attack unsaturated fatty acids in a cell membrane, known as lipid peroxidation. Lipid peroxidation is a chain reaction that damages the cell membranes (Pre, 1991). Lipid peroxidation causes ferroptosis by activating or inhibiting several signaling pathways. Recent studies have shown that LPO inhibits the PI3K/AKT/mTOR signaling pathway. Inhibiting the PI3K/AKT/mTOR pathway can increase autophagy. Excessive autophagy can lead to iron accumulation and higher oxidative stress levels, amplifying ferroptosis (Butler et al., 2017; Yang et al., 2023; Zhang et al., 2023c). It is essential to know that LPO-mediated oxidative stress burden can trigger the expression of Regulator of Calcineurin 1 (RCAN1) (originally called Adapt78) and Cyclin-Dependent Kinase 5 (CDK5). In old animals, it has been observed that CDK5 over-activation significantly triggers the GSK3 beta activities, which in turn leads to Tau hyperphosphorylation (Ermak et al., 2011; Guo et al., 2018; Engmann and Giese, 2009; Plattner et al., 2006; Lloret et al., 2011). It is well-recognized that lipid peroxidation also activates other stress-related pathways that come with ferroptosis. The most relevant is the phosphorylated JNK pathway. JNK activates the transcription factors such as NF-κB and initiates the release of cytokines and chemokines. These cytokines work in both ways, i.e., on one side, they disrupt the antioxidant system, while on the other, they induce microgliosis, astrocytosis, and neuroinflammation. LPO induces ferroptosis, which is involved in amyloid beta aggregation and neurodegeneration (Figure 5) (Banji et al., 2022; Khan et al., 2016).
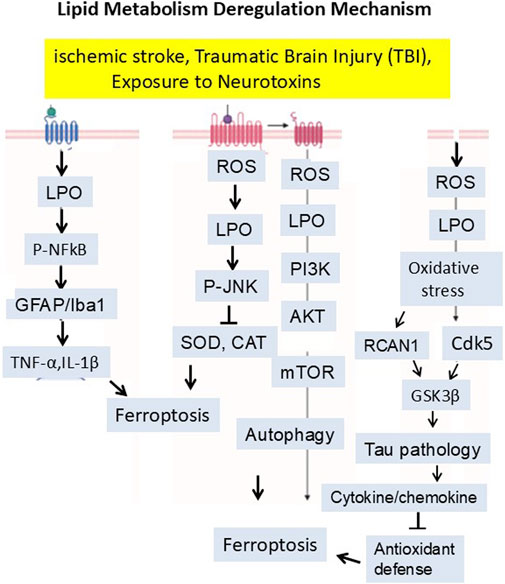
Figure 5. Mitochondria-derived reactive oxygen species (ROS) initiate lipid peroxidation, which disrupts membrane integrity and activates signaling pathways—such as PI3K/AKT/mTOR inhibition, CDK5–GSK3β–Tau hyperphosphorylation, and JNK–NF-κB-mediated neuroinflammation—ultimately promoting ferroptosis and neurodegeneration. The signaling mechanism has been summarized in the manuscript.
Besides these commen ferrosptosis mechanisms, there are several well documented studies which indicated that the cellular iron is mainly processed in the cytoplasm, mitochondria, and endosomes, where it experiences uptake, utilization, storage, and regulation. Iron is taken up by the cell through transferrin receptors, released in endosomes where it is reduced from Fe3+ to Fe2+, and then moved into the cytoplasm by divalent metal transporter 1 (DMT1). In the cytoplasm, surplus iron is securely stored in ferritin, while mitochondria use iron for producing heme and iron-sulfur (Fe-S) clusters, crucial for cellular respiration and enzyme functions. Iron dysregulation and build-up significantly affect mitochondrial performance, as mitochondria are key consumers and controllers of cellular iron. An overload of iron in mitochondria stimulates the production of reactive oxygen species (ROS) via Fenton reactions, resulting in oxidative damage to mitochondrial DNA, proteins, and lipids. This hinders electron transport chain function, lowers ATP synthesis, and interferes with mitochondrial membrane potential. With time, these alterations can induce mitochondrial dysfunction, facilitating cell death mechanisms like ferroptosis. Additionaly, Iron dysregulation and excess significantly affect mitochondrial dynamics, resulting in disrupted movement, fusion/fission balance, and mitophagy. This disturbs the mitochondrial membrane potential and impacts motor proteins crucial for correct mitochondrial transport along axons and dendrites. Moreover, impaired mitochondria do not efficiently undergo mitophagy as a result of oxidative changes to mitophagy receptors and hindered autophagosome development. The buildup of impaired mitochondria leads to energy shortages, neuroinflammation, and the advancement of neurodegeneration, as observed in conditions like parkinson’s and alzheimer’s diseases (Tang et al., 2021; Cheng et al., 2022; Bharat et al., 2023; Ru et al., 2024; Onukwufor et al., 2022; Zhao et al., 2024a; Duan et al., 2022a; Chen and Chan, 2009; Chen et al., 2023c; Zong et al., 2024; Beal, 1995; Wang et al., 2023b; Mishra et al., 2022; Wen et al., 2025; Feng et al., 2023; Fang et al., 2023).
Methodological approaches
This review article aims to summarize the findings of studies on ferroptosis’s mechanism and therapeutic approach. The motivation for preparing this review was based on our previous studies on ferroptosis. Here, we searched for potential research articles on ferroptosis and its mechanisms. In addition, to identify studies on the mechanism and therapeutic strategies of ferroptosis, we conducted searches using the keywords “ferroptosis,” ferroptosis mechanisms,” and “therapeutic strategies” in all available and independent databases. The abstracts were thoroughly studied, and the main findings were recorded to understand these studies clearly. All studies covering animal and cellular models were included.
Potential therapeutic strategies
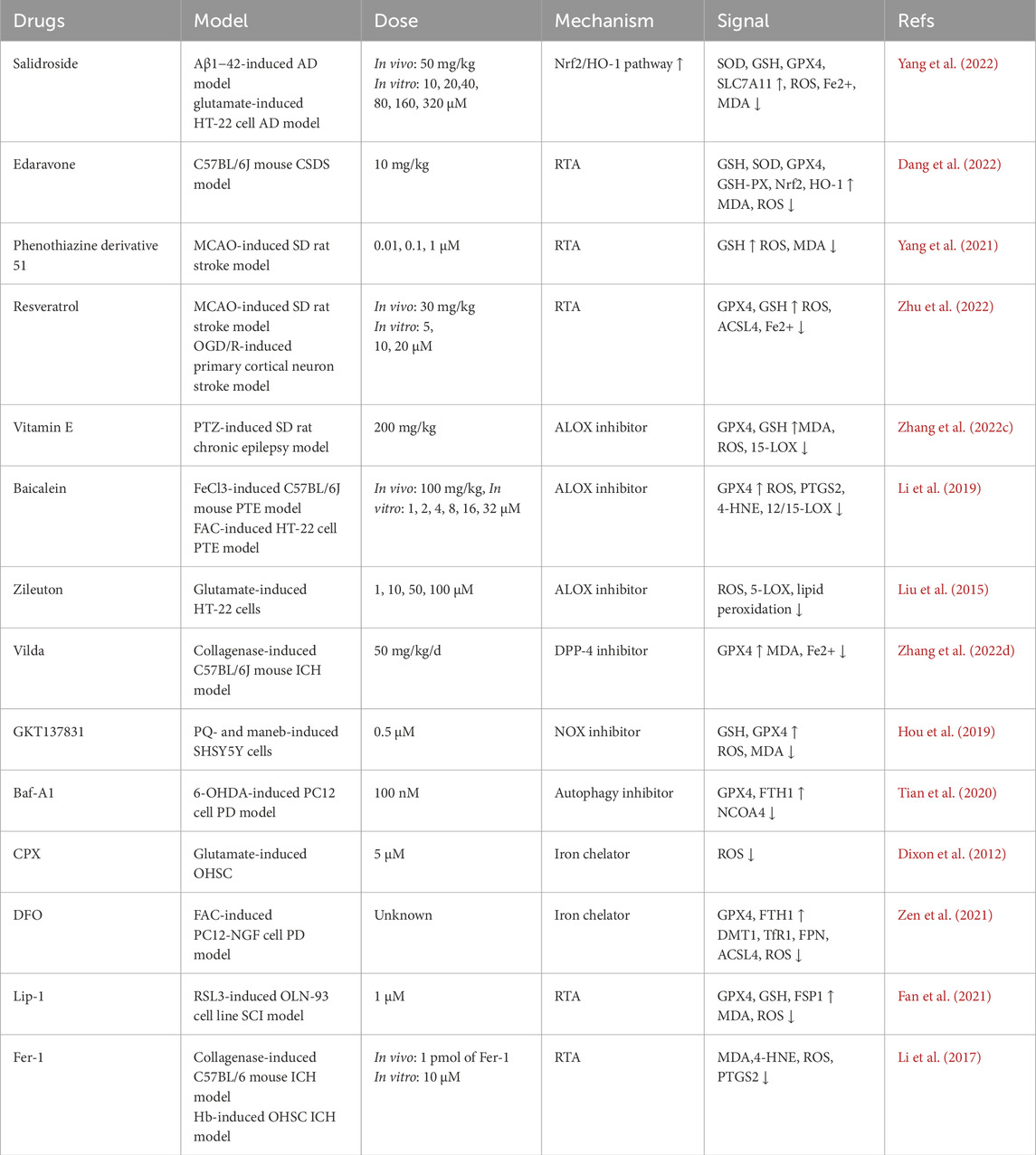
It has been reported that artesunate prevents brain damage at low doses by blocking ferroptosis, and iron chelators such as DFO and DFP have demonstrated positive effects against iron-related neurodegenerative disorders. In tauopathy, Dihydroartemisinin (DHA) may have a neuroprotective effect via interacting with O-GlcNAcylation and phosphorylation, pointing to a possible treatment for tau pathology-related learning and memory impairments (Xia et al., 2021). In mice fed a high-fat diet, grape seed extract lowers calcium and iron levels and acts as an antioxidant to prevent ferroptosis. Iron regulatory proteins are crucial for preserving mitochondrial and cellular iron homeostasis. By blocking the iron regulating protein in dopaminergic neurones, BJP-IVb lowers iron content to stop parkinson’s disease. Additionally, rapamycin lessens the substantia nigra’s dopamine neurone loss via controlling ferroptosis and ferritinophagy. By inhibiting ferroptosis, the iron absorption inhibitor ferristatin II offers neuroprotection, while HBED therapy reduces secondary damage following TBI by attaching to Fe2+ and changing it into Fe3+. Only DFO, DPF, and DFX are presently authorised for clinical usage. (Xia et al., 2021; Kong et al., 2019; Tsurusaki et al., 2019; Guan et al., 2021; Du et al., 2019; Du et al., 2021; El Ayed et al., 2017; Manolova et al., 2019; Li et al., 2024c; Liu et al., 2023a; Khalaf et al., 2018; Yan et al., 2021; Ge et al., 2021).
Sulfasalazine’s neuroprotective properties can be used therapeutically to prevent catastrophic neuronal death (Ryu et al., 2003). By regulating neuroinflammation and ferroptosis through the Nrf2/HO-1 signalling pathway, astragaloside IV reduces stroke-induced early brain damage (Zhang et al., 2023c). By preventing ferroptosis, curcumin protects against disease by upregulating Nrf2 expression and its downstream targets, HO-1 and GPX4, in hepatocytes, cardiomyocytes, neurones, renal tubule cells, and chondrocytes. Eriodictyol inhibits ferroptosis by stimulating the Nrf2/HO-1 pathway, which greatly improves cognitive impairments. Similar processes are used by forsythiin A, salidroside, tetrahydroxy stilbene glycoside, and spermidine to prevent ferroptosis in AD, PD, and myocardial I/R injury. By triggering the Nrf2 pathway and upregulating the expression of GPX4 and SLC7A11. morroniside prevents ferroptosis in dopaminergic neurones in parkinson’s disease. Through the SIRT1/Nrf2 signalling pathway, edaravone prevents ferroptosis and may be used as a treatment for depression, traumatic brain injury, and stroke. By activating Nrf2, Tertiary butylhydroquinone (TBHQ) and hinokitiol have also been demonstrated to have neuroprotective effects (Zhang et al., 2023c; Pardieu et al., 2022; Luo and Zhang, 2021; Peng et al., 2022b; Wang et al., 2023c; Du et al., 2023; Wang et al., 2021).
By downregulating ACSL4 expression independently of PPAR-γ, rosiglitazone prevents ferroptosis and lessens MASH brought on by arsenic. Nicorandil may prevent ferroptosis and the translocation of ACSL4 into the mitochondria. By preventing ACSL4 activity, triacsin C can alleviate parkinson’s disease (PD); and clausenamide can also alleviate behavioural impairments in PD animal models by preventing the nuclear translocation of ALOX5. Methyl ferulic acid controls the expression of ACSL4 to reduce neuropathic pain in mice. By reducing the expression of ACSL4 and ALOX15 in spinal cord tissue, proanthocyanidin therapy dramatically improves spinal cord damage (Wei et al., 2020; Guo et al., 2023; Li et al., 2023f; Chen et al., 2024; Huang et al., 2024; Duan et al., 2022b; Iqbal et al., 2023; Tang et al., 2023; Li et al., 2023g; Liu et al., 2023b; Zhou et al., 2020b; Cheng et al., 2020). To summarize the potentiali therapeutic agents against ferroptosis in various neurodegenerativ and neuropsychatric diseases, we provided a representative table below.
Table of potential therapeutic candidtes
One popular technique for enhancing the bioavailability and retention duration of bioactive substances is the use of nanoparticles. Quercetin’s bioavailability is a significant concern and a major barrier to its application in AD treatment. Liu et al. created a smart nanoparticle (TQCN) to treat AD by addressing ferroptosis. It was made from quercetin and modified with triphenylphosphine. TQCN, a specific type of nanomedicine efficiently chelates iron by spontaneous coordination mediated by plant polyphenols and self-assemble metal-phenol nanocomplexes in situ, reducing iron overload and related free radical outburst by utilizing advantageous brain targeting and mitochondrial localization features. TQCN also lowers cellular lipid peroxidation, restores iron metabolism balance, and activates the Nrf2 endogenous defence system. Due to its multimodal modulation of the pathogenic process that causes ferroptosis, TQCN therapy may alleviate severe cognitive impairment in AD mice and relieve a variety of neurodegenerative illnesses associated with brain iron buildup (Liu et al., 2024b; Herpich and Rincon, 2020). Neurotrophin, nerve growth factor, and edaravone are examples of neuroprotective medications that protect the brain from ferroptosis and oxidative stress. However, because of their short circulation half-life and limited BBB permeability, these neuroprotective medications frequently fall short of the anticipated therapeutic effect. Zhang et al. used the acidic pathological features of ischaemic tissue to build a pH/GSH-supported polyamino acid nanogel (NG/EDA). To increase the neuroprotective effects of edaravone, NG/EDA is triggered by the acidic and edaravone-induced high levels of GSH microenvironment. This allows for the selective and prolonged release of edaravone at the site of ischaemic injury. The findings demonstrated that in rats with pMCAO, NG/EDA could effectively accumulate at the site of cerebral ischaemia damage and cross the blood-brain barrier. By preventing ferroptosis, NG/EDA dramatically increases the survival rate of OGD neurones while also considerably lowering the infarct volume and neurobehavioral score of pMCAO mice. A novel and promising model for neuroprotection in cerebral I/R injury and other illnesses of the central nervous system may be offered by this pH/GSH dual-responsive nanoplatform. Inflammatory cytokine production has a key role in the pathophysiology of disorders involving I/R damage. A class of copper-based, neutrophil membrane-coated nanoparticles (N-Cu5.4O@DFO NPs) with excellent stability and biocompatibility was described by Ding et al. By efficiently scavenging iron and exhibiting strong antioxidant qualities, these nanoparticles reduce oxidative damage and inflammatory reactions, thereby enhancing I/R damage (Herpich and Rincon, 2020; Jin et al., 2017; Zhang et al., 2024c; Zhuge et al., 2024; Ding et al., 2023). A flavonoid glycoside obtained from locust plants, rutin has strong antioxidant properties and has been widely used to treat neurological and cardiovascular conditions. To get rid of ROS and stop ferroptosis, Feng et al., created rutin-loaded polydopamine nanoparticles (PEG-PDA@rutin NPs). PEG-PDA@rutin NPs have a diameter of roughly 100 nm and demonstrate both ROS-triggered drug release and superior ROS clearance capabilities. PEG-PDA@rutin NPs have the ability to efficiently enter cells, stop ferroptosis, remove ROS, and heal mitochondrial damage. Ren et al. developed a ROS-responsive drug nanocore, mPEG-b-Lys-BECI-TCO, for SCI repair, and combined MSCs with Fer-1 to create a synergistic drug release nanoparticle system (Niu et al., 2021; Muvhulawa et al., 2022; Negahdari et al., 2021; Feng et al., 2024b; Zhang et al., 2021). Following SCI, this multimodal therapy approach may prevent inflammation and ferroptosis and provide a fresh approach to building drug-synergistic cell treatment systems that target ferroptosis. An innovative flavonoid glycoside with potent antioxidant properties is apigenin-7-O-glucoside (AGL). By selectively binding to HO-1 and monoamine oxidase b, AGL helps to avoid ferroptosis and preserve mitochondrial function by preventing the buildup of Fe2+ and the generation of ROS. However, AGL’s limited practical use is due to its weak water solubility. Zhao et al. created two amphiphilic compounds, mPEG-TK-DA and DTPA-N10-10, with ROS-scavenging properties in order to get around this restriction. They also self-assembled AGL through hydrophobic and hydrophilic contacts, creating multi-site ROS-scavenging nanoparticles known as PDN@AGL. By lowering ROS levels and lipid peroxidation, PDN@AGL prevents ferroptosis, and it is thought that the ATF3/SLC7A11 pathway is a key player in this process. The possible use of PDN@AGL to treat human disorders is supported by the control of ATF3/SLC7A11-mediated ferroptosis. PDN@AGL offers a promising treatment approach for conditions marked by ferroptosis and oxidative stress by resolving the solubility problem and boosting AGL’s antioxidant capability (Yao et al., 2023; Feng et al., 2022; Zhao et al., 2024b; Katz et al., 2021).
CRISPR/CAS9 based therapeutic strategies
A novel therapeutic approach for both central and peripheral disorders is gene level editing. Gene editing techniques like CRISPR/CAS9 can be used to treat diseases caused by genetic mutations, according to a number of well-known studies. For example, Transferrin Receptor Protein 1 gene, after downloading the FASTA sequence (mRNA) from NCBI and copying the exon (the coding sequence), sgRNA was created to the crosponding exon. In the present review, we provide a research direction that by deleting a tiny amount of DNA from the relevant exon, the gene of interest will be knocked down, thereby preventing ferrosptsis-mediated degeneration (Konstantinidis et al., 2022; Wadhwani et al., 2019; Kolanu, 2024; Abdelnour et al., 2021; Deneault, 2024) (Figure 6).
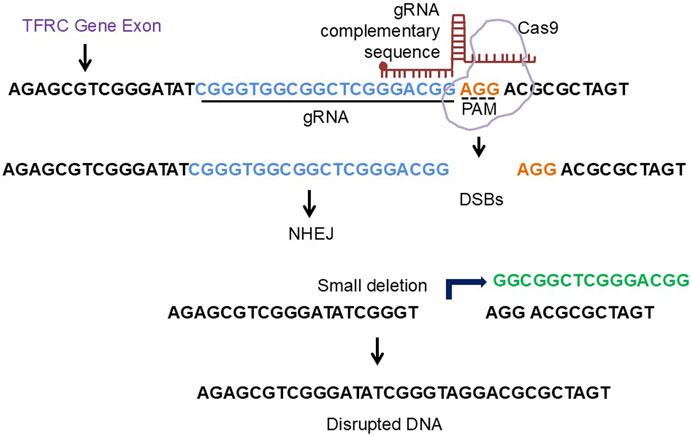
Figure 6. The diagrammatic representation of crispr/cas9 approach to engineer ferroptosis related gene. The TFRC gene exon FASTA sequence was copied from NCBI and the respective gRNA were designed using CHOP CHOP software. The deletion of the nucleotide sequence from the corresponding exon will lead to knockdown of the TFRC gene.
Challenges and future directions
Despite the promise of ferroptosis inhibition in neuroprotection, several challenges remain. One major obstacle is the complexity of ferroptosis regulation in the brain and its intricate interplay with other cell death pathways, including apoptosis, autophagy, and necroptosis. Dissecting the precise molecular mechanisms underlying ferroptosis and its pathological role in neurodegeneration remains a significant research priority.
Iron dysregulation is central to ferroptosis, and therapies targeting iron homeostasis, such as chelation, have shown mixed clinical success. Iron chelators like deferoxamine and deferiprone reduce labile iron and mitigate reactive oxygen species (ROS), yet they face limitations in crossing the BBB effectively. Emerging strategies, such as nanoparticle-based delivery systems, could improve BBB penetration, allowing targeted chelation therapies to reach affected brain regions (Nunez and Chana-Cuevas, 2018; Popescu and Nichol, 2011). Additionally, mapping brain metals using advanced imaging techniques, such as quantitative susceptibility mapping (QSM), offers a promising non-invasive approach to identify iron deposition and monitor therapeutic responses in diseases like AD (Uchida et al., 2022). However, challenges remain in standardizing QSM and interpreting regional brain iron concentrations across diverse neurodegenerative disorders (Ward et al., 2014). Genetic factors, such as PRMT1 expression, also complicate ferroptosis regulation. PRMT1 promotes ferroptosis by suppressing key antioxidant systems, including solute carrier family 7-member 11 (SLC7A11), and its inhibition could provide dual therapeutic benefits—enhancing neuroprotection while improving treatment responses in conditions like gliomas (Li et al., 2024d). Advanced genome-editing approaches, particularly CRISPR/Cas9-based techniques, hold significant promise for precisely targeting ferroptosis-related genes to attenuate neurodegeneration (Nouri Nojadeh et al., 2023). However, ensuring the safety and specificity of CRISPR-based interventions in the central nervous system (CNS) remains a challenge. Delivery systems such as adeno-associated viruses (AAVs) offer a potential solution for CNS-specific targeting of ferroptosis regulators but require further optimization and validation in preclinical models. Another challenge is the intersection of ferroptosis with neuroinflammatory pathways. Excess iron accumulation in the substantia nigra, as seen in PD, exacerbates oxidative stress and neuroinflammation, further driving dopaminergic neuronal loss. Neuroinflammatory cytokines and immune activation pathways, such as NF-κB signaling, may synergize with ferroptosis to amplify neurodegeneration. Addressing both iron dysregulation and inflammatory processes will require combination therapies that target multiple pathological pathways simultaneously.
In amyotrophic lateral sclerosis (ALS), elevated ferritin and transferrin receptor levels in cerebrospinal fluid have been associated with reduced survival, suggesting that disrupted iron metabolism may serve as both a biomarker and a therapeutic target. However, identifying patient-specific factors, such as genetic predispositions or iron regulatory gene polymorphisms, will be crucial for tailoring ferroptosis inhibitors to individual patients. Biomarkers like serum ferritin, oxidative stress markers, and QSM-based iron mapping may aid in predicting therapeutic responses and monitoring disease progression. Lastly, syndromes of neurodegeneration with brain iron accumulation (NBIA), such as pantothenate kinase-associated neurodegeneration (PKAN), exemplify the devastating clinical effects of ferroptosis driven by excessive iron deposition. NBIA disorders often present with progressive dystonia, parkinsonism, and cognitive decline, highlighting the urgent need for therapies that prevent ferroptosis-mediated neuronal loss (Ward et al., 2014; Ayton et al., 2017; Nadjar et al., 2012; Belaidi and Bush, 2016; Schneider and Bhatia, 2012). Overcoming these challenges requires a multifaceted approach involving novel drug delivery systems, advanced imaging modalities, and genetic targeting technologies to optimize therapeutic efficacy and safety.
Conclusion
Ferroptosis represents a novel and distinct form of regulated cell death, characterized by iron dysregulation, lipid peroxidation, and oxidative stress. Its involvement in neurodegeneration with brain iron accumulation highlights its pathological significance. The evidence linking ferroptosis to neuronal loss underscores its potential as a critical driver of neurodegeneration in regions of the brain where iron accumulation and oxidative damage are pronounced (Ayton et al., 2017; Belaidi and Bush, 2016; Schneider and Bhatia, 2012). A sophisticated understanding of the molecular mechanisms regulating ferroptosis—including disruptions in the antioxidant defense system, iron homeostasis, and lipid metabolism has paved the way for identifying therapeutic targets to mitigate neurodegeneration and preserve neuronal function.
Current therapeutic approaches targeting ferroptosis hold promise for neuroprotection. Strategies such as iron chelation therapy, antioxidant supplementation, and small-molecule ferroptosis inhibitors have shown efficacy in preclinical models by reducing oxidative stress, limiting lipid peroxidation, and restoring iron homeostasis. Advanced therapies, such as CRISPR/Cas9-based gene editing, offer a precise means to target ferroptosis-related genes (Li et al., 2024d; Zhang et al., 2024d). Furthermore, innovations in nanoparticle-based drug delivery systems and brain-penetrant chelators address longstanding challenges related to therapeutic access across the blood-brain barrier. These advancements suggest that a combination of pharmacological, genetic, and nanotechnological approaches may offer synergistic benefits for slowing or halting disease progression (Ashok et al., 2022; Zhou et al., 2024).
Despite these promising developments, significant challenges remain in translating ferroptosis-targeting therapies to clinical practice. The complex interplay between ferroptosis and other cell death pathways, such as apoptosis, autophagy, and neuroinflammation, necessitates further investigation. Identifying robust biomarkers, such as serum ferritin levels, oxidative stress markers, and advanced neuroimaging techniques like quantitative susceptibility mapping (QSM), will be necessary for patient stratification and therapeutic monitoring (Ward et al., 2014; Ayton et al., 2017; Nadjar et al., 2012). Additionally, understanding the heterogeneity of ferroptosis mechanisms across different neurodegenerative diseases and patient populations will enable the development of tailored therapies.
In conclusion, targeting ferroptosis provides a promising therapeutic avenue for combating neurodegenerative diseases marked by iron dysregulation and oxidative damage. Continued research into the molecular mechanisms of ferroptosis, coupled with advancements in therapeutic delivery and biomarker development, is essential for realizing its clinical potential. By integrating multidisciplinary approaches and addressing current challenges, ferroptosis-targeted strategies hold the potential to transform the treatment landscape for neurodegenerative disorders, ultimately improving outcomes and quality of life for affected individuals.
Author contributions
MK: Writing – original draft. QH: Writing – review and editing. KO: Writing – review and editing. LM: Writing – review and editing. JB: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors would like to acknowledge HL138093 (JCB), T32 HL007446 (JCB), AARG-NTF-22-971669, NIAP24_1273876, NIA K01AG084813, R01 AG059421, P30 AG066546.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2025.1641323/full#supplementary-material
References
Abdelnour S. A., Xie L., Hassanin A. A., Zuo E., Lu Y. (2021). The potential of CRISPR/Cas9 gene editing as a treatment strategy for inherited diseases. Front. Cell Dev. Biol. 9, 699597. doi:10.3389/fcell.2021.699597
Amartumur S., Nguyen H., Huynh T., Kim T. S., Woo R. S., Oh E., et al. (2024). Neuropathogenesis-on-chips for neurodegenerative diseases. Nat. Commun. 15 (1), 2219. doi:10.1038/s41467-024-46554-8
Angelova P. R., Choi M. L., Berezhnov A. V., Horrocks M. H., Hughes C. D., De S., et al. (2020). Alpha synuclein aggregation drives ferroptosis: an interplay of iron, calcium and lipid peroxidation. Cell Death Differ. 27 (10), 2781–2796. doi:10.1038/s41418-020-0542-z
Anisimova A. S., Alexandrov A. I., Makarova N. E., Gladyshev V. N., Dmitriev S. E. (2018). Protein synthesis and quality control in aging. Aging (Albany NY) 10 (12), 4269–4288. doi:10.18632/aging.101721
Ashok A., Andrabi S. S., Mansoor S., Kuang Y., Kwon B. K., Labhasetwar V. (2022). Antioxidant therapy in oxidative stress-induced neurodegenerative diseases: role of nanoparticle-based drug delivery systems in clinical translation. Antioxidants (Basel) 11 (2), 408. doi:10.3390/antiox11020408
Ayton S., Fazlollahi A., Bourgeat P., Raniga P., Ng A., Lim Y. Y., et al. (2017). Cerebral quantitative susceptibility mapping predicts amyloid-beta-related cognitive decline. Brain 140 (8), 2112–2119. doi:10.1093/brain/awx137
Baker R. G., Hayden M. S., Ghosh S. (2011). NF-κB, inflammation, and metabolic disease. Cell Metab. 13 (1), 11–22. doi:10.1016/j.cmet.2010.12.008
Ballard D. J., Peng H. Y., Das J. K., Kumar A., Wang L., Ren Y., et al. (2021). Insights into the pathologic roles and regulation of eukaryotic elongation Factor-2 kinase. Front. Mol. Biosci. 8, 727863. doi:10.3389/fmolb.2021.727863
Banji O. J. F., Banji D., Makeen H. A., Alqahtani S. S., Alshahrani S. (2022). Neuroinflammation: the role of anthocyanins as neuroprotectants. Curr. Neuropharmacol. 20 (11), 2156–2174. doi:10.2174/1570159X20666220119140835
Baranova O., Miranda L. F., Pichiule P., Dragatsis I., Johnson R. S., Chavez J. C. (2007). Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J. Neurosci. 27 (23), 6320–6332. doi:10.1523/JNEUROSCI.0449-07.2007
Beal M. F. (1995). Mitochondrial dysfunction and oxidative damage in neurodegenerative diseases. Neuroscience intelligence unit. Austin: Landes Co, 128.
Belaidi A. A., Bush A. I. (2016). Iron neurochemistry in Alzheimer’s disease and Parkinson’s disease: targets for therapeutics. J. Neurochem. 139 (Suppl. 1), 179–197. doi:10.1111/jnc.13425
Benarroch E. (2023). What is the role of ferroptosis in neurodegeneration? Neurology 101 (7), 312–319. doi:10.1212/WNL.0000000000207730
Bernardo G., Prado M. A., Dashtmian A. R., Favaro M., Mauri S., Borsetto A., et al. (2024). USP14 inhibition enhances Parkin-independent mitophagy in iNeurons. Pharmacol. Res. 210, 107484. doi:10.1016/j.phrs.2024.107484
Berndt C., Alborzinia H., Amen V. S., Ayton S., Barayeu U., Bartelt A., et al. (2024). Ferroptosis in health and disease. Redox Biol. 75, 103211. doi:10.1016/j.redox.2024.103211
Bharat V., Durairaj A. S., Vanhauwaert R., Li L., Muir C. M., Chandra S., et al. (2023). A mitochondrial inside-out iron-calcium signal reveals drug targets for Parkinson’s disease. Cell Rep. 42 (12), 113544. doi:10.1016/j.celrep.2023.113544
Bonomini F., Rodella L. F., Rezzani R. (2015). Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 6 (2), 109–120. doi:10.14336/AD.2014.0305
Brandes M. S., Gray N. E. (2020). NRF2 as a therapeutic target in neurodegenerative diseases. ASN Neuro 12, 1759091419899782. doi:10.1177/1759091419899782
Brieger K., Schiavone S., Miller F. J., Krause K. H. (2012). Reactive oxygen species: from health to disease. Swiss Med. Wkly. 142, w13659. doi:10.4414/smw.2012.13659
Bulk M., Kenkhuis B., van der Graaf L. M., Goeman J. J., Natté R., van der Weerd L. (2018). Postmortem T2*- weighted MRI imaging of cortical iron reflects severity of Alzheimer’s disease. J. Alzheimers Dis. 65 (4), 1125–1137. doi:10.3233/JAD-180317
Butler D. E., Marlein C., Walker H. F., Frame F. M., Mann V. M., Simms M. S., et al. (2017). Inhibition of the PI3K/AKT/mTOR pathway activates autophagy and compensatory Ras/Raf/MEK/ERK signalling in prostate cancer. Oncotarget 8 (34), 56698–56713. doi:10.18632/oncotarget.18082
Cao H., Zuo C., Huang Y., Zhu L., Zhao J., Yang Y., et al. (2021). Hippocampal proteomic analysis reveals activation of necroptosis and ferroptosis in a mouse model of chronic unpredictable mild stress-induced depression. Behav. Brain Res. 407, 113261. doi:10.1016/j.bbr.2021.113261
Chandrasekaran P., Weiskirchen R. (2024). Effects of probiotics on gut microbiota: an overview. Int. J. Mol. Sci. 25 (3), 6022. doi:10.3390/ijms25116022
Chartier-Harlin M. C., Kachergus J., Roumier C., Mouroux V., Douay X., Lincoln S., et al. (2004). Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 364 (9440), 1167–1169. doi:10.1016/S0140-6736(04)17103-1
Chen H., Chan D. C. (2009). Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum. Mol. Genet. 18 (R2), R169–R176. doi:10.1093/hmg/ddp326
Chen F., Kang R., Liu J., Tang D. (2023a). The ACSL4 network regulates cell death and autophagy in diseases. Biology (Basel) 12 (6), 864. doi:10.3390/biology12060864
Chen Y., Guo X., Zeng Y., Mo X., Hong S., He H., et al. (2023b). Oxidative stress induces mitochondrial iron overload and ferroptotic cell death. Sci. Rep. 13 (1), 15515. doi:10.1038/s41598-023-42760-4
Chen W., Zhao H., Li Y. (2023c). Mitochondrial dynamics in health and disease: mechanisms and potential targets. Signal Transduct. Target Ther. 8 (1), 333. doi:10.1038/s41392-023-01547-9
Chen Z., Li S., Liu M., Yin M., Chen J., Li Y., et al. (2024). Nicorandil alleviates cardiac microvascular ferroptosis in diabetic cardiomyopathy: role of the mitochondria-localized AMPK-Parkin-ACSL4 signaling pathway. Pharmacol. Res. 200, 107057. doi:10.1016/j.phrs.2024.107057
Cheng J., Fan Y. Q., Liu B. H., Zhou H., Wang J. M., Chen Q. X. (2020). ACSL4 suppresses glioma cells proliferation via activating ferroptosis. Oncol. Rep. 43 (1), 147–158. doi:10.3892/or.2019.7419
Cheng R., Dhorajia V. V., Kim J., Kim Y. (2022). Mitochondrial iron metabolism and neurodegenerative diseases. Neurotoxicology 88, 88–101. doi:10.1016/j.neuro.2021.11.003
Cindric A., Krištić J., Martinić Kavur M., Pezer M. (2021). Glycosylation and aging. Adv. Exp. Med. Biol. 1325, 341–373. doi:10.1007/978-3-030-70115-4_17
D’Mello S. R., Kindy M. C. (2020). Overdosing on iron: elevated iron and degenerative brain disorders. Exp. Biol. Med. (Maywood) 245 (16), 1444–1473. doi:10.1177/1535370220953065
da Costa Caiado M. J., Dolga A. M., den Dunnen W. F. A. (2025). Iron(ing) out parkinsonisms: the interplay of proteinopathy and ferroptosis in Parkinson’s disease and tau-related parkinsonisms. Redox Biol. 79, 103478. doi:10.1016/j.redox.2024.103478
Dang R., Wang M., Li X., Wang H., Liu L., Wu Q., et al. (2022). Edaravone ameliorates depressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4 pathway. J. Neuroinflammation 19 (1), 41. doi:10.1186/s12974-022-02400-6
Dare L. R., Garcia A., Soares C. B., Lopes L., Neves B. H. S., Dias D. V., et al. (2020). The reversal of memory deficits in an Alzheimer’s disease model using physical and cognitive exercise. Front. Behav. Neurosci. 14, 152. doi:10.3389/fnbeh.2020.00152
Deneault E. (2024). Recent therapeutic gene editing applications to genetic disorders. Curr. Issues Mol. Biol. 46 (5), 4147–4185. doi:10.3390/cimb46050255
Deng Q., Zhu Y., Zhang M., Fei A., Liang J., Zheng J., et al. (2023). Ferroptosis as a potential new therapeutic target for diabetes and its complications. Endocr. Connect. 12 (3), e220419. doi:10.1530/EC-22-0419
Ding H., Yan C. Z., Shi H., Zhao Y. S., Chang S. Y., Yu P., et al. (2011). Hepcidin is involved in iron regulation in the ischemic brain. PLoS One 6 (9), e25324. doi:10.1371/journal.pone.0025324
Ding C., Wang B., Zheng J., Zhang M., Li Y., Shen H. H., et al. (2023). Neutrophil membrane-inspired nanorobots act as antioxidants ameliorate ischemia reperfusion-induced acute kidney injury. ACS Appl. Mater Interfaces 15 (34), 40292–40303. doi:10.1021/acsami.3c08573
Dionisio P. A., Amaral J. D., Rodrigues C. M. P. (2021). Oxidative stress and regulated cell death in Parkinson's disease. Ageing Res. Rev. 67, 101263. doi:10.1016/j.arr.2021.101263
Dixon S. J., Olzmann J. A. (2024). The cell biology of ferroptosis. Nat. Rev. Mol. Cell Biol. 25 (6), 424–442. doi:10.1038/s41580-024-00703-5
Dixon S. J., Lemberg K. M., Lamprecht M. R., Skouta R., Zaitsev E. M., Gleason C. E., et al. (2012). Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149 (5), 1060–1072. doi:10.1016/j.cell.2012.03.042
Dluzen D. F., Noren Hooten N., Evans M. K. (2017). Extracellular RNA in aging. Wiley Interdiscip. Rev. RNA 8 (2), e1385. doi:10.1002/wrna.1385
Do Van B., Gouel F., Jonneaux A., Timmerman K., Gelé P., Pétrault M., et al. (2016). Ferroptosis, a newly characterized form of cell death in Parkinson’s disease that is regulated by PKC. Neurobiol. Dis. 94, 169–178. doi:10.1016/j.nbd.2016.05.011
Du J., Wang T., Li Y., Zhou Y., Wang X., Yu X., et al. (2019). DHA inhibits proliferation and induces ferroptosis of leukemia cells through autophagy dependent degradation of ferritin. Free Radic. Biol. Med. 131, 356–369. doi:10.1016/j.freeradbiomed.2018.12.011
Du J., Wang X., Li Y., Ren X., Zhou Y., Hu W., et al. (2021). DHA exhibits synergistic therapeutic efficacy with cisplatin to induce ferroptosis in pancreatic ductal adenocarcinoma via modulation of iron metabolism. Cell Death Dis. 12 (7), 705. doi:10.1038/s41419-021-03996-y
Du Y. W., Li X. K., Wang T. T., Zhou L., Li H. R., Feng L., et al. (2023). Cyanidin-3-glucoside inhibits ferroptosis in renal tubular cells after ischemia/reperfusion injury via the AMPK pathway. Mol. Med. 29 (1), 42. doi:10.1186/s10020-023-00642-5
Duan G., Li J., Duan Y., Zheng C., Guo Q., Li F., et al. (2022a). Mitochondrial iron metabolism: the crucial actors in diseases. Molecules 28 (1), 29. doi:10.3390/molecules28010029
Duan J., Wang Z., Duan R., Yang C., Zhao R., Feng Q., et al. (2022b). Therapeutic targeting of hepatic ACSL4 ameliorates NASH in mice. Hepatology 75 (1), 140–153. doi:10.1002/hep.32148
Dusek P., Hofer T., Alexander J., Roos P. M., Aaseth J. O. (2022). Cerebral iron deposition in neurodegeneration. Biomolecules 12 (5), 714. doi:10.3390/biom12050714
Ekker M. S., Verhoeven J. I., Schellekens M. M. I., Boot E. M., van Alebeek M. E., Brouwers P. J. A. M., et al. (2023). Risk factors and causes of ischemic stroke in 1322 young adults. Stroke 54 (2), 439–447. doi:10.1161/STROKEAHA.122.040524
El Ayed M., Kadri S., Smine S., Elkahoui S., Limam F., Aouani E. (2017). Protective effects of grape seed and skin extract against high-fat-diet-induced lipotoxicity in rat lung. Lipids Health Dis. 16 (1), 174. doi:10.1186/s12944-017-0561-z
Emerson N. E., Swarup V. (2023). Proteomic data advance targeted drug development for neurogenerative diseases. Biol. Psychiatry 93 (9), 754–755. doi:10.1016/j.biopsych.2023.02.003
Engmann O., Giese K. P. (2009). Crosstalk between Cdk5 and GSK3beta: implications for Alzheimer’s disease. Front. Mol. Neurosci. 2, 2. doi:10.3389/neuro.02.002.2009
Ermak G., Pritchard M. A., Dronjak S., Niu B., Davies K. J. A. (2011). Do RCAN1 proteins link chronic stress with neurodegeneration? FASEB J. 25 (10), 3306–3311. doi:10.1096/fj.11-185728
Fan B. Y., Pang Y. L., Li W. X., Zhao C. X., Zhang Y., Wang X., et al. (2021). Liproxstatin-1 is an effective inhibitor of oligodendrocyte ferroptosis induced by inhibition of glutathione peroxidase 4. Neural Regen. Res. 16 (3), 561–566. doi:10.4103/1673-5374.293157
Fang X., Ardehali H., Min J., Wang F. (2023). The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat. Rev. Cardiol. 20 (1), 7–23. doi:10.1038/s41569-022-00735-4
Feng Y. D., Ye W., Tian W., Meng J. R., Zhang M., Sun Y., et al. (2022). Old targets, new strategy: apigenin-7-O-beta-d-(-6''-p-coumaroyl)-glucopyranoside prevents endothelial ferroptosis and alleviates intestinal ischemia-reperfusion injury through HO-1 and MAO-B inhibition. Free Radic. Biol. Med. 184, 74–88. doi:10.1016/j.freeradbiomed.2022.03.033
Feng S., Tang D., Wang Y., Li X., Bao H., Tang C., et al. (2023). The mechanism of ferroptosis and its related diseases. Mol. Biomed. 4 (1), 33. doi:10.1186/s43556-023-00142-2
Feng L., Sun J., Xia L., Shi Q., Hou Y., Zhang L., et al. (2024a). Ferroptosis mechanism and Alzheimer's disease. Neural Regen. Res. 19 (8), 1741–1750. doi:10.4103/1673-5374.389362
Feng W., Zhu N., Xia Y., Huang Z., Hu J., Guo Z., et al. (2024b). Melanin-like nanoparticles alleviate ischemia-reperfusion injury in the kidney by scavenging reactive oxygen species and inhibiting ferroptosis. iScience 27 (4), 109504. doi:10.1016/j.isci.2024.109504
Feng X., Zhang W., Liu X., Wang Q., Dang X., Han J., et al. (2025). Ferroptosis-associated signaling pathways and therapeutic approaches in depression. Front. Neurosci. 19, 1559597. doi:10.3389/fnins.2025.1559597
Fillebeen C., Charlebois E., Wagner J., Katsarou A., Mui J., Vali H., et al. (2019). Transferrin receptor 1 controls systemic iron homeostasis by fine-tuning hepcidin expression to hepatocellular iron load. Blood 133 (4), 344–355. doi:10.1182/blood-2018-05-850404
Fryk E., Olausson J., Mossberg K., Strindberg L., Schmelz M., Brogren H., et al. (2021). Hyperinsulinemia and insulin resistance in the obese may develop as part of a homeostatic response to elevated free fatty acids: a mechanistic case-control and a population-based cohort study. EBioMedicine 65, 103264. doi:10.1016/j.ebiom.2021.103264
Gao W., Wang W., Liu G., Zhang J., Yang J., Deng Z. (2019). Allicin attenuated chronic social defeat stress induced depressive-like behaviors through suppression of NLRP3 inflammasome. Metab. Brain Dis. 34 (1), 319–329. doi:10.1007/s11011-018-0342-z
Gao G., You L., Zhang J., Chang Y. Z., Yu P. (2023). Brain iron metabolism, Redox balance and neurological diseases. Antioxidants (Basel) 12 (6), 1289. doi:10.3390/antiox12061289
Ge C., Zhang S., Mu H., Zheng S., Tan Z., Huang X., et al. (2021). Emerging mechanisms and disease implications of ferroptosis: potential applications of natural products. Front. Cell Dev. Biol. 9, 774957. doi:10.3389/fcell.2021.774957
Gu F., Chauhan V., Chauhan A. (2015). Glutathione redox imbalance in brain disorders. Curr. Opin. Clin. Nutr. Metab. Care 18 (1), 89–95. doi:10.1097/MCO.0000000000000134
Guan D., Li C., Li Y., Li Y., Wang G., Gao F., et al. (2021). The DpdtbA induced EMT inhibition in gastric cancer cell lines was through ferritinophagy-mediated activation of p53 and PHD2/hif-1α pathway. J. Inorg. Biochem. 218, 111413. doi:10.1016/j.jinorgbio.2021.111413
Guo D., Xie W., Xiong P., Li H., Wang S., Chen G., et al. (2018). Cyclin-dependent kinase 5-mediated phosphorylation of chloride intracellular channel 4 promotes oxidative stress-induced neuronal death. Cell Death Dis. 9 (10), 951. doi:10.1038/s41419-018-0983-1
Guo T., Yan W., Cui X., Liu N., Wei X., Sun Y., et al. (2023). Liraglutide attenuates type 2 diabetes mellitus-associated non-alcoholic fatty liver disease by activating AMPK/ACC signaling and inhibiting ferroptosis. Mol. Med. 29 (1), 132. doi:10.1186/s10020-023-00721-7
Gusti A. M. T., Qusti S. Y., Alshammari E. M., Toraih E. A., Fawzy M. S. (2021). Antioxidants-Related superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), Glutathione-S-Transferase (GST), and nitric oxide synthase (NOS) gene variants analysis in an Obese population: a preliminary case-control study. Antioxidants (Basel) 10 (4), 595. doi:10.3390/antiox10040595
Hamdan Y., Mazini L., Malka G. (2021). Exosomes and micro-RNAs in aging process. Biomedicines 9 (8), 968. doi:10.3390/biomedicines9080968
Han S., Lin F., Qi Y., Liu C., Zhou L., Xia Y., et al. (2022). HO-1 contributes to luteolin-triggered ferroptosis in clear cell renal cell carcinoma via increasing the labile iron pool and promoting lipid peroxidation. Oxid. Med. Cell Longev. 2022, 3846217. doi:10.1155/2022/3846217
Han Q., Sun L., Xiang K. (2023). Research progress of ferroptosis in Alzheimer disease: a review. Medicine (Baltimore) 102 (36), e35142. doi:10.1097/MD.0000000000035142
Helgudottir S. S., Johnsen K. B., Routhe L. G., Rasmussen C. L. M., Thomsen M. S., Moos T. (2024). Upregulation of transferrin receptor 1 (TfR1) but not glucose transporter 1 (GLUT1) or CD98hc at the blood-brain barrier in response to valproic acid. Cells 13 (14), 1181. doi:10.3390/cells13141181
Hemerkova P., Valis M. (2021). Role of oxidative stress in the pathogenesis of amyotrophic lateral sclerosis: antioxidant metalloenzymes and therapeutic strategies. Biomolecules 11 (3), 437. doi:10.3390/biom11030437
Herpich F., Rincon F. (2020). Management of acute ischemic stroke. Crit. Care Med. 48 (11), 1654–1663. doi:10.1097/CCM.0000000000004597
Hou L., Huang R., Sun F., Zhang L., Wang Q. (2019). NADPH oxidase regulates paraquat and maneb-induced dopaminergic neurodegeneration through ferroptosis. Toxicology 417, 64–73. doi:10.1016/j.tox.2019.02.011
Houldsworth A. (2024). Role of oxidative stress in neurodegenerative disorders: a review of reactive oxygen species and prevention by antioxidants. Brain Commun. 6 (1), fcad356. doi:10.1093/braincomms/fcad356
Hu X., Zhang H., Zhang Q., Yao X., Ni W., Zhou K. (2022). Emerging role of STING signalling in CNS injury: inflammation, autophagy, necroptosis, ferroptosis and pyroptosis. J. Neuroinflammation 19 (1), 242. doi:10.1186/s12974-022-02602-y
Huang R., Shi Q., Zhang S., Lin H., Han C., Qian X., et al. (2022). Inhibition of the cGAS-STING pathway attenuates lung Ischemia/Reperfusion injury via regulating endoplasmic reticulum stress in alveolar epithelial type II cells of rats. J. Inflamm. Res. 15, 5103–5119. doi:10.2147/JIR.S365970
Huang Q., Ru Y., Luo Y., Luo X., Liu D., Ma Y., et al. (2024). Identification of a targeted ACSL4 inhibitor to treat ferroptosis-related diseases. Sci. Adv. 10 (13), eadk1200. doi:10.1126/sciadv.adk1200
Il’yasova D., Scarbrough P., Spasojevic I. (2012). Urinary biomarkers of oxidative status. Clin. Chim. Acta 413 (19-20), 1446–1453. doi:10.1016/j.cca.2012.06.012
Iorio M. V., Croce C. M. (2012). Causes and consequences of microRNA dysregulation. Cancer J. 18 (3), 215–222. doi:10.1097/PPO.0b013e318250c001
Iqbal S., Jabeen F., Kahwa I., Omara T. (2023). Suberosin alleviates thiazolidinedione-induced cardiomyopathy in diabetic rats by inhibiting ferroptosis via modulation of ACSL4-LPCAT3 and PI3K-AKT signaling pathways. Cardiovasc Toxicol. 23 (9-10), 295–304. doi:10.1007/s12012-023-09804-7
Irikura R., Nishizawa H., Nakajima K., Yamanaka M., Chen G., Tanaka K., et al. (2023). Ferroptosis model system by the re-expression of BACH1. J. Biochem. 174 (3), 239–252. doi:10.1093/jb/mvad036
Jiang X., Stockwell B. R., Conrad M. (2021). Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 22 (4), 266–282. doi:10.1038/s41580-020-00324-8
Jin Q., Cai Y., Li S., Liu H., Zhou X., Lu C., et al. (2017). Edaravone-encapsulated agonistic micelles rescue ischemic brain tissue by tuning blood-brain barrier permeability. Theranostics 7 (4), 884–898. doi:10.7150/thno.18219
Jin J., He Y., Guo J., Pan Q., Wei X., Xu C., et al. (2023). BACH1 controls hepatic insulin signaling and glucose homeostasis in mice. Nat. Commun. 14 (1), 8428. doi:10.1038/s41467-023-44088-z
Joppe K., Roser A. E., Maass F., Lingor P. (2019). The contribution of iron to protein aggregation disorders in the central nervous system. Front. Neurosci. 13, 15. doi:10.3389/fnins.2019.00015
Katz J. N., Arant K. R., Loeser R. F. (2021). Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA 325 (6), 568–578. doi:10.1001/jama.2020.22171
Khalaf S., Ahmad A. S., Chamara K. V. D. R., Doré S. (2018). Unique properties associated with the brain penetrant iron chelator HBED reveal remarkable beneficial effects after brain trauma. J. Neurotrauma 36 (1), 43–53. doi:10.1089/neu.2017.5617
Khan M. S., Ali T., Kim M. W., Jo M. H., Jo M. G., Badshah H., et al. (2016). Anthocyanins protect against LPS-Induced oxidative stress-mediated neuroinflammation and neurodegeneration in the adult mouse cortex. Neurochem. Int. 100, 1–10. doi:10.1016/j.neuint.2016.08.005
Khan M. S., Ikram M., Park T. J., Kim M. O. (2021). Pathology, risk factors, and oxidative damage related to type 2 diabetes-mediated Alzheimer’s disease and the rescuing effects of the potent antioxidant anthocyanin. Oxid. Med. Cell Longev. 2021, 4051207. doi:10.1155/2021/4051207
Kolanu N. D. (2024). CRISPR-Cas9 gene editing: curing genetic diseases by inherited epigenetic modifications. Glob. Med. Genet. 11 (1), 113–122. doi:10.1055/s-0044-1785234
Kondo K., Ishigaki Y., Gao J., Yamada T., Imai J., Sawada S., et al. (2013). Bach1 deficiency protects pancreatic beta-cells from oxidative stress injury. Am. J. Physiol. Endocrinol. Metab. 305 (5), E641–E648. doi:10.1152/ajpendo.00120.2013
Kong Z., Liu R., Cheng Y. (2019). Artesunate alleviates liver fibrosis by regulating ferroptosis signaling pathway. Biomed. Pharmacother. 109, 2043–2053. doi:10.1016/j.biopha.2018.11.030
Konstantinidis E., Molisak A., Perrin F., Streubel-Gallasch L., Fayad S., Kim D. Y., et al. (2022). CRISPR-Cas9 treatment partially restores amyloid-beta 42/40 in human fibroblasts with the Alzheimer’s disease PSEN 1 M146L mutation. Mol. Ther. Nucleic Acids 28, 450–461. doi:10.1016/j.omtn.2022.03.022
Kurian M. A., Hayflick S. J. (2013). Pantothenate kinase-associated neurodegeneration (PKAN) and PLA2G6-associated neurodegeneration (PLAN): review of two major neurodegeneration with brain iron accumulation (NBIA) phenotypes. Int. Rev. Neurobiol. 110, 49–71. doi:10.1016/B978-0-12-410502-7.00003-X
Lee J., Hyun D. H. (2023). The interplay between intracellular iron homeostasis and neuroinflammation in neurodegenerative diseases. Antioxidants (Basel) 12 (4), 918. doi:10.3390/antiox12040918
Lee J. H., Lee M. S. (2019). Brain iron accumulation in atypical parkinsonian syndromes: in vivo MRI evidences for distinctive patterns. Front. Neurol. 10, 74. doi:10.3389/fneur.2019.00074
Lei P., Bai T., Sun Y. (2019). Mechanisms of ferroptosis and relations with regulated cell death: a review. Front. Physiol. 10, 139. doi:10.3389/fphys.2019.00139
Li Q., Han X., Lan X., Gao Y., Wan J., Durham F., et al. (2017). Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight 2 (7), e90777. doi:10.1172/jci.insight.90777
Li Q., Li Q. Q., Jia J. N., Sun Q. Y., Zhou H. H., Jin W. L., et al. (2019). Baicalein exerts neuroprotective effects in FeCl(3)-Induced posttraumatic epileptic seizures via suppressing ferroptosis. Front. Pharmacol. 10, 638. doi:10.3389/fphar.2019.00638
Li J., Cao F., Yin H. L., Huang Z. J., Lin Z. T., Mao N., et al. (2020). Ferroptosis: past, present and future. Cell Death Dis. 11 (2), 88. doi:10.1038/s41419-020-2298-2
Li B., Xia M., Zorec R., Parpura V., Verkhratsky A. (2021). Astrocytes in heavy metal neurotoxicity and neurodegeneration. Brain Res. 1752, 147234. doi:10.1016/j.brainres.2020.147234
Li W., Liang L., Liu S., Yi H., Zhou Y. (2023a). FSP1: a key regulator of ferroptosis. Trends Mol. Med. 29 (9), 753–764. doi:10.1016/j.molmed.2023.05.013
Li E., Yin H., Su M., Li Q., Zhao Y., Zhang L., et al. (2023b). Inhibition of ferroptosis alleviates chronic unpredictable mild stress-induced depression in mice via tsRNA-3029b. Brain Res. Bull. 204, 110773. doi:10.1016/j.brainresbull.2023.110773
Li L., Dai Y., Ke D., Liu J., Chen P., Wei D., et al. (2023c). Ferroptosis: new insight into the mechanisms of diabetic nephropathy and retinopathy. Front. Endocrinol. (Lausanne) 14, 1215292. doi:10.3389/fendo.2023.1215292
Li B., Wang W., Li Y., Wang S., Liu H., Xia Z., et al. (2023d). cGAS-STING pathway aggravates early cerebral ischemia-reperfusion injury in mice by activating NCOA4-mediated ferritinophagy. Exp. Neurol. 359, 114269. doi:10.1016/j.expneurol.2022.114269
Li Y., Qin M., Zhong W., Liu C., Deng G., Yang M., et al. (2023e). RAGE promotes dysregulation of iron and lipid metabolism in alcoholic liver disease. Redox Biol. 59, 102559. doi:10.1016/j.redox.2022.102559
Li X., Li Z., Dong X., Wu Y., Li B., Kuang B., et al. (2023f). Astragaloside IV attenuates myocardial dysfunction in diabetic cardiomyopathy rats through downregulation of CD36-mediated ferroptosis. Phytother. Res. 37 (7), 3042–3056. doi:10.1002/ptr.7798
Li K., Wang M., Huang Z. H., Wang M., Sun W. Y., Kurihara H., et al. (2023g). ALOX5 inhibition protects against dopaminergic neurons undergoing ferroptosis. Pharmacol. Res. 193, 106779. doi:10.1016/j.phrs.2023.106779
Li Z., Zhang Y., Ji M., Wu C., Zhang Y., Ji S. (2024a). Targeting ferroptosis in neuroimmune and neurodegenerative disorders for the development of novel therapeutics. Biomed. Pharmacother. 176, 116777. doi:10.1016/j.biopha.2024.116777
Li S., Zhang G., Hu J., Tian Y., Fu X. (2024b). Ferroptosis at the nexus of metabolism and metabolic diseases. Theranostics 14 (15), 5826–5852. doi:10.7150/thno.100080
Li Q. M., Xu T., Zha X. Q., Feng X. W., Zhang F. Y., Luo J. P. (2024c). Buddlejasaponin IVb ameliorates ferroptosis of dopaminergic neuron by suppressing IRP2-mediated iron overload in Parkinson’s disease. J. Ethnopharmacol. 319 (Pt 1), 117196. doi:10.1016/j.jep.2023.117196
Li H., Qi X., Yang H., Ju H. (2024d). PRMT1 promotes radiotherapy resistance in glioma stem cells by inhibiting ferroptosis. Jpn. J. Radiol. 43, 129–137. doi:10.1007/s11604-024-01651-y
Liang D., Minikes A. M., Jiang X. (2022). Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol. Cell 82 (12), 2215–2227. doi:10.1016/j.molcel.2022.03.022
Liao M., Qin R., Huang W., Zhu H. P., Peng F., Han B., et al. (2022). Targeting regulated cell death (RCD) with small-molecule compounds in triple-negative breast cancer: a revisited perspective from molecular mechanisms to targeted therapies. J. Hematol. Oncol. 15 (1), 44. doi:10.1186/s13045-022-01260-0
Lima Giacobbo B., Doorduin J., Klein H. C., Dierckx R. A. J. O., Bromberg E., de Vries E. F. J. (2019). Brain-derived neurotrophic factor in brain disorders: focus on neuroinflammation. Mol. Neurobiol. 56 (5), 3295–3312. doi:10.1007/s12035-018-1283-6
Liu Y., Wang W., Li Y., Xiao Y., Cheng J., Jia J. (2015). The 5-lipoxygenase inhibitor zileuton confers neuroprotection against glutamate oxidative damage by inhibiting ferroptosis. Biol. Pharm. Bull. 38 (8), 1234–1239. doi:10.1248/bpb.b15-00048
Liu J. L., Fan Y. G., Yang Z. S., Wang Z. Y., Guo C. (2018). Iron and Alzheimer’s disease: from pathogenesis to therapeutic implications. Front. Neurosci. 12, 632. doi:10.3389/fnins.2018.00632
Liu T., Wang P., Yin H., Wang X., Lv J., Yuan J., et al. (2023a). Rapamycin reverses ferroptosis by increasing autophagy in MPTP/MPP(+)-induced models of Parkinson’s disease. Neural Regen. Res. 18 (11), 2514–2519. doi:10.4103/1673-5374.371381
Liu T., Wang R., Qi W., Jia L., Ma K., Si J., et al. (2023b). Methyl ferulic acid alleviates neuropathic pain by inhibiting Nox4-induced ferroptosis in dorsal root ganglia neurons in rats. Mol. Neurobiol. 60 (6), 3175–3189. doi:10.1007/s12035-023-03270-6
Liu X., Xie C., Wang Y., Xiang J., Chen L., Yuan J., et al. (2024a). Ferritinophagy and ferroptosis in cerebral ischemia reperfusion injury. Neurochem. Res. 49 (8), 1965–1979. doi:10.1007/s11064-024-04161-5
Liu Y., Zhao D., Yang F., Ye C., Chen Z., Chen Y., et al. (2024b). In situ self-assembled phytopolyphenol-coordinated intelligent nanotherapeutics for multipronged management of ferroptosis-driven Alzheimer’s disease. ACS Nano 18 (11), 7890–7906. doi:10.1021/acsnano.3c09286
Liu X., Luo Q., Zhao Y., Ren P., Jin Y., Zhou J. (2025). The ferroptosis-mitochondrial axis in depression: unraveling the feedforward loop of oxidative stress, metabolic homeostasis dysregulation, and neuroinflammation. Antioxidants (Basel) 14 (5), 613. doi:10.3390/antiox14050613
Lloret A., Badia M. C., Giraldo E., Ermak G., Alonso M. D., Pallardó F. V., et al. (2011). Amyloid-beta toxicity and tau hyperphosphorylation are linked via RCAN1 in Alzheimer's disease. J. Alzheimers Dis. 27 (4), 701–709. doi:10.3233/JAD-2011-110890
Long H., Zhu W., Wei L., Zhao J. (2023). Iron homeostasis imbalance and ferroptosis in brain diseases. MedComm (2020) 4 (4), e298. doi:10.1002/mco2.298
Luo H., Zhang R. (2021). Icariin enhances cell survival in lipopolysaccharide-induced synoviocytes by suppressing ferroptosis via the Xc-/GPX4 axis. Exp. Ther. Med. 21 (1), 72. doi:10.3892/etm.2020.9504
Luoqian J., Yang W., Ding X., Tuo Q. Z., Xiang Z., Zheng Z., et al. (2022). Ferroptosis promotes T-cell activation-induced neurodegeneration in multiple sclerosis. Cell Mol. Immunol. 19 (8), 913–924. doi:10.1038/s41423-022-00883-0
Ma X., Xin D., She R., Liu D., Ge J., Mei Z. (2023). Novel insight into cGAS-STING pathway in ischemic stroke: from pre-to post-disease. Front. Immunol. 14, 1275408. doi:10.3389/fimmu.2023.1275408
Mahmoud M., Abdel-Rasheed M. (2023). Influence of type 2 diabetes and obesity on adipose mesenchymal stem/stromal cell immunoregulation. Cell Tissue Res. 394 (1), 33–53. doi:10.1007/s00441-023-03801-6
Mahoney-Sanchez L., Bouchaoui H., Boussaad I., Jonneaux A., Timmerman K., Berdeaux O., et al. (2022). Alpha synuclein determines ferroptosis sensitivity in dopaminergic neurons via modulation of ether-phospholipid membrane composition. Cell Rep. 40 (8), 111231. doi:10.1016/j.celrep.2022.111231
Manolova V., Nyffenegger N., Flace A., Altermatt P., Varol A., Doucerain C., et al. (2019). Oral ferroportin inhibitor ameliorates ineffective erythropoiesis in a model of beta-thalassemia. J. Clin. Invest 130 (1), 491–506. doi:10.1172/JCI129382
Marunaka Y. (2023). Molecular mechanisms of obesity-induced development of insulin resistance and promotion of amyloid-beta accumulation: dietary therapy using weak organic acids via improvement of lowered interstitial fluid pH. Biomolecules 13 (5), 779. doi:10.3390/biom13050779
Mattson M. P. (2009). Roles of the lipid peroxidation product 4-hydroxynonenal in obesity, the metabolic syndrome, and associated vascular and neurodegenerative disorders. Exp. Gerontol. 44 (10), 625–633. doi:10.1016/j.exger.2009.07.003
Mazzetti A. P., Fiorile M. C., Primavera A., Lo Bello M. (2015). Glutathione transferases and neurodegenerative diseases. Neurochem. Int. 82, 10–18. doi:10.1016/j.neuint.2015.01.008
Menni C., Kastenmüller G., Petersen A. K., Bell J. T., Psatha M., Tsai P. C., et al. (2013). Metabolomic markers reveal novel pathways of ageing and early development in human populations. Int. J. Epidemiol. 42 (4), 1111–1119. doi:10.1093/ije/dyt094
Mishra J., Bhatti G. K., Sehrawat A., Singh C., Singh A., Reddy A. P., et al. (2022). Modulating autophagy and mitophagy as a promising therapeutic approach in neurodegenerative disorders. Life Sci. 311 (Pt A), 121153. doi:10.1016/j.lfs.2022.121153
Mitchell J. R., Jollow D. J., Potter W. Z., Gillette J. R., Brodie B. B. (1973). Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J. Pharmacol. Exp. Ther. 187 (1), 211–217. doi:10.1016/s0022-3565(25)29666-5
Mohammadi S., Ghaderi S., Fatehi F. (2024). Iron accumulation/overload and Alzheimer's disease risk factors in the precuneus region: a comprehensive narrative review. Aging Med. (Milton) 7 (5), 649–667. doi:10.1002/agm2.12363
Muhoberac B. B., Vidal R. (2019). Iron, ferritin, hereditary ferritinopathy, and neurodegeneration. Front. Neurosci. 13, 1195. doi:10.3389/fnins.2019.01195
Muvhulawa N., Dludla P. V., Ziqubu K., Mthembu S. X. H., Mthiyane F., Nkambule B. B., et al. (2022). Rutin ameliorates inflammation and improves metabolic function: a comprehensive analysis of scientific literature. Pharmacol. Res. 178, 106163. doi:10.1016/j.phrs.2022.106163
Muyderman H., Chen T. (2014). Mitochondrial dysfunction in amyotrophic lateral sclerosis - a valid pharmacological target? Br. J. Pharmacol. 171 (8), 2191–2205. doi:10.1111/bph.12476
Nadjar Y., Gordon P., Corcia P., Bensimon G., Pieroni L., Meininger V., et al. (2012). Elevated serum ferritin is associated with reduced survival in amyotrophic lateral sclerosis. PLoS One 7 (9), e45034. doi:10.1371/journal.pone.0045034
Ndayisaba A., Kaindlstorfer C., Wenning G. K. (2019). Iron in neurodegeneration - cause or consequence? Front. Neurosci. 13, 180. doi:10.3389/fnins.2019.00180
Negahdari R., Bohlouli S., Sharifi S., Maleki Dizaj S., Rahbar Saadat Y., Khezri K., et al. (2021). Therapeutic benefits of rutin and its nanoformulations. Phytother. Res. 35 (4), 1719–1738. doi:10.1002/ptr.6904
Neis V. B., Rosa P. B., Moretti M., Rodrigues A. L. S. (2018). Involvement of heme oxygenase-1 in neuropsychiatric and neurodegenerative diseases. Curr. Pharm. Des. 24 (20), 2283–2302. doi:10.2174/1381612824666180717160623
Niu B., Liao K., Zhou Y., Wen T., Quan G., Pan X., et al. (2021). Application of glutathione depletion in cancer therapy: enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials 277, 121110. doi:10.1016/j.biomaterials.2021.121110
Nouri Nojadeh J., Bildiren Eryilmaz N. S., Erguder B. I. (2023). CRISPR/Cas9 genome editing for neurodegenerative diseases. EXCLI J. 22, 567–582. doi:10.17179/excli2023-6155
Novak U., Ward A. C., Hertzog P. J., Hamilton J. A., Paradiso L. (1996). Aberrant activation of JAK/STAT pathway components in response to G-CSF, interferon-alpha/beta and interferon-gamma in NFS-60 cells. Growth Factors 13 (3-4), 251–260. doi:10.3109/08977199609003226
Nunez M. T., Chana-Cuevas P. (2018). New perspectives in iron chelation therapy for the treatment of neurodegenerative diseases. Pharmaceuticals (Basel) 11 (4), 109. doi:10.3390/ph11040109
Olufunmilayo E. O., Gerke-Duncan M. B., Holsinger R. M. D. (2023). Oxidative stress and antioxidants in neurodegenerative disorders. Antioxidants (Basel) 12 (2), 517. doi:10.3390/antiox12020517
Onukwufor J. O., Dirksen R. T., Wojtovich A. P. (2022). Iron dysregulation in mitochondrial dysfunction and Alzheimer's disease. Antioxidants (Basel) 11 (4), 692. doi:10.3390/antiox11040692
Pan J., Xiong W., Zhang A., Zhang H., Lin H., Gao L., et al. (2023). The imbalance of p53-Park7 signaling axis induces iron homeostasis dysfunction in doxorubicin-challenged cardiomyocytes. Adv. Sci. (Weinh) 10 (15), e2206007. doi:10.1002/advs.202206007
Pardieu B., Pasanisi J., Ling F., Dal Bello R., Penneroux J., Su A., et al. (2022). Cystine uptake inhibition potentiates front-line therapies in acute myeloid leukemia. Leukemia 36 (6), 1585–1595. doi:10.1038/s41375-022-01573-6
Parrado J., Bougria M., Ayala A., Castaño A., Machado A. (1999). Effects of aging on the various steps of protein synthesis: fragmentation of elongation factor 2. Free Radic. Biol. Med. 26 (3-4), 362–370. doi:10.1016/s0891-5849(98)00202-0
Paul S., Nahar A., Bhagawati M., Kunwar A. J. (2022). A review on recent advances of cerebral palsy. Oxid. Med. Cell Longev. 2022, 2622310. doi:10.1155/2022/2622310
Paydarnia P., Mayeli M., Shafie M., Agah E., Hasani S. A., Jazani M. R., et al. (2021). Alterations of the serum and CSF ferritin levels and the diagnosis and prognosis of amyotrophic lateral sclerosis. eNeurologicalSci 25, 100379. doi:10.1016/j.ensci.2021.100379
Peng F., Liao M., Qin R., Zhu S., Peng C., Fu L., et al. (2022a). Regulated cell death (RCD) in cancer: key pathways and targeted therapies. Signal Transduct. Target Ther. 7 (1), 286. doi:10.1038/s41392-022-01110-y
Peng C. Y., Hu L., Wu Z. J., Wang J., Cai R. L. (2022b). Effects of moxibustion on p53, SLC7A11, and GPX4 expression in synovial tissues of rats with adjuvant arthritis. Zhen Ci Yan Jiu 47 (1), 21–26. doi:10.13702/j.1000-0607.20210837
Petralla S., Saveleva L., Kanninen K. M., Oster J. S., Panayotova M., Fricker G., et al. (2024). Increased expression of transferrin receptor 1 in the brain cortex of 5xFAD mouse model of alzheimer’s disease is associated with activation of HIF-1 signaling pathway. Mol. Neurobiol. 61 (9), 6383–6394. doi:10.1007/s12035-024-03990-3
Pizzino G., Irrera N., Cucinotta M., Pallio G., Mannino F., Arcoraci V., et al. (2017). Oxidative stress: harms and benefits for human health. Oxid. Med. Cell Longev. 2017, 8416763. doi:10.1155/2017/8416763
Plattner F., Angelo M., Giese K. P. (2006). The roles of cyclin-dependent kinase 5 and glycogen synthase kinase 3 in tau hyperphosphorylation. J. Biol. Chem. 281 (35), 25457–25465. doi:10.1074/jbc.M603469200
Popescu B. F., Nichol H. (2011). Mapping brain metals to evaluate therapies for neurodegenerative disease. CNS Neurosci. Ther. 17 (4), 256–268. doi:10.1111/j.1755-5949.2010.00149.x
Promyos N., Phienluphon P. P., Wechjakwen N., Lainampetch J., Prangthip P., Kwanbunjan K. (2023). Inverse correlation of superoxide dismutase and catalase with type 2 diabetes among rural thais. Nutrients 15 (9), 2071. doi:10.3390/nu15092071
Qian Z. M., Li W., Guo Q. (2023). Ferroportin1 in the brain. Ageing Res. Rev. 88, 101961. doi:10.1016/j.arr.2023.101961
Qin R., You F. M., Zhao Q., Xie X., Peng C., Zhan G., et al. (2022). Naturally derived indole alkaloids targeting regulated cell death (RCD) for cancer therapy: from molecular mechanisms to potential therapeutic targets. J. Hematol. Oncol. 15 (1), 133. doi:10.1186/s13045-022-01350-z
Qin D., Li D., Wang C., Guo S. (2023). Ferroptosis and central nervous system demyelinating diseases. J. Neurochem. 165 (6), 759–771. doi:10.1111/jnc.15831
Rochette L., Dogon G., Rigal E., Zeller M., Cottin Y., Vergely C. (2022). Lipid peroxidation and iron metabolism: two corner stones in the homeostasis control of ferroptosis. Int. J. Mol. Sci. 24 (1), 449. doi:10.3390/ijms24010449
Rogers J. T., Lahiri D. K. (2004). Metal and inflammatory targets for Alzheimer’s disease. Curr. Drug Targets 5 (6), 535–551. doi:10.2174/1389450043345272
Ru Q., Li Y., Chen L., Wu Y., Min J., Wang F. (2024). Iron homeostasis and ferroptosis in human diseases: mechanisms and therapeutic prospects. Signal Transduct. Target Ther. 9 (1), 271. doi:10.1038/s41392-024-01969-z
Ryter S. W. (2022). Heme Oxygenase-1: an anti-inflammatory effector in cardiovascular, lung, and related metabolic disorders. Antioxidants (Basel) 11 (3), 555. doi:10.3390/antiox11030555
Ryu B. R., Lee Y. A., Won S. J., Noh J. H., Chang S. Y., Chung J. M., et al. (2003). The novel neuroprotective action of sulfasalazine through blockade of NMDA receptors. J. Pharmacol. Exp. Ther. 305 (1), 48–56. doi:10.1124/jpet.102.042606
Sato T., Shapiro J. S., Chang H. C., Miller R. A., Ardehali H. (2022). Aging is associated with increased brain iron through cortex-derived hepcidin expression. Elife 11, e73456. doi:10.7554/eLife.73456
Schmidt-Sommerfeld E., Penn D., Rinaldo P., Kossak D., Li B. U., Huang Z. H., et al. (1992). Urinary medium-chain acylcarnitines in medium-chain acyl-CoA dehydrogenase deficiency, medium-chain triglyceride feeding and valproic acid therapy: sensitivity and specificity of the radioisotopic exchange/high performance liquid chromatography method. Pediatr. Res. 31 (6), 545–551. doi:10.1203/00006450-199206000-00002
Schneider S. A., Bhatia K. P. (2012). Syndromes of neurodegeneration with brain iron accumulation. Semin. Pediatr. Neurol. 19 (2), 57–66. doi:10.1016/j.spen.2012.03.005
Sebghatollahi Z., Yogesh R., Mahato N., Kumar V., Mohanta Y. K., Baek K. H., et al. (2025). Signaling pathways in oxidative stress-induced neurodegenerative diseases: a review of phytochemical therapeutic interventions. Antioxidants (Basel) 14 (4), 457. doi:10.3390/antiox14040457
Shieh J. T., Tintos-Hernandez J. A., Murali C. N., Penon-Portmann M., Flores-Mendez M., Santana A., et al. (2023). Heterozygous nonsense variants in the ferritin heavy-chain gene FTH1 cause a neuroferritinopathy. HGG Adv. 4 (4), 100236. doi:10.1016/j.xhgg.2023.100236
Shkundin A., Halaris A. (2023). Associations of BDNF/BDNF-AS SNPs with depression, schizophrenia, and bipolar disorder. J. Pers. Med. 13 (9), 1395. doi:10.3390/jpm13091395
Simon A. R., Rai U., Fanburg B. L., Cochran B. H. (1998). Activation of the JAK-STAT pathway by reactive oxygen species. Am. J. Physiol. 275 (6), C1640–C1652. doi:10.1152/ajpcell.1998.275.6.C1640
Solinas G., Becattini B. (2017). JNK at the crossroad of obesity, insulin resistance, and cell stress response. Mol. Metab. 6 (2), 174–184. doi:10.1016/j.molmet.2016.12.001
Soni P., Ammal Kaidery N., Sharma S. M., Gazaryan I., Nikulin S. V., Hushpulian D. M., et al. (2024). A critical appraisal of ferroptosis in Alzheimer’s and Parkinson’s disease: new insights into emerging mechanisms and therapeutic targets. Front. Pharmacol. 15, 1390798. doi:10.3389/fphar.2024.1390798
Srinivasan E., Chandrasekhar G., Chandrasekar P., Anbarasu K., Vickram A. S., Karunakaran R., et al. (2021). Alpha-synuclein aggregation in Parkinson's disease. Front. Med. (Lausanne) 8, 736978. doi:10.3389/fmed.2021.736978
Stockwell B. R. (2022). Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell 185 (14), 2401–2421. doi:10.1016/j.cell.2022.06.003
Sun Y., Xia X., Basnet D., Zheng J. C., Huang J., Liu J. (2022). Mechanisms of ferroptosis and emerging links to the pathology of neurodegenerative diseases. Front. Aging Neurosci. 14, 904152. doi:10.3389/fnagi.2022.904152
Suzen S., Tucci P., Profumo E., Buttari B., Saso L. (2022). A pivotal role of Nrf2 in neurodegenerative disorders: a new way for therapeutic strategies. Pharmaceuticals (Basel) 15 (6), 692. doi:10.3390/ph15060692
Tamagno E., Guglielmotto M., Vasciaveo V., Tabaton M. (2021). Oxidative stress and beta amyloid in Alzheimer’s disease. Which comes first: the chicken or the egg? Antioxidants (Basel) 10 (9), 1479. doi:10.3390/antiox10091479
Tang D., Chen X., Kang R., Kroemer G. (2021). Ferroptosis: molecular mechanisms and health implications. Cell Res. 31 (2), 107–125. doi:10.1038/s41422-020-00441-1
Tang F., Zhou L. Y., Li P., Jiao L.-L., Chen K., Guo Y. J., et al. (2023). Inhibition of ACSL4 alleviates parkinsonism phenotypes by reduction of lipid reactive oxygen species. Neurotherapeutics 20 (4), 1154–1166. doi:10.1007/s13311-023-01382-4
Tangvarasittichai S. (2015). Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 6 (3), 456–480. doi:10.4239/wjd.v6.i3.456
Thapa K., Khan H., Kanojia N., Singh T. G., Kaur A., Kaur G. (2022). Therapeutic insights on ferroptosis in Parkinson’s disease. Eur. J. Pharmacol. 930, 175133. doi:10.1016/j.ejphar.2022.175133
Tian Y., Lu J., Hao X., Li H., Zhang G., Liu X., et al. (2020). FTH1 inhibits ferroptosis through ferritinophagy in the 6-OHDA model of Parkinson’s disease. Neurotherapeutics 17 (4), 1796–1812. doi:10.1007/s13311-020-00929-z
Tonekaboni S. H., Mollamohammadi M. (2014). Neurodegeneration with brain iron accumulation: an overview. Iran. J. Child. Neurol. 8 (4), 1–8.
Tsurusaki S., Tsuchiya Y., Koumura T., Nakasone M., Sakamoto T., Matsuoka M., et al. (2019). Hepatic ferroptosis plays an important role as the trigger for initiating inflammation in nonalcoholic steatohepatitis. Cell Death Dis. 10 (6), 449. doi:10.1038/s41419-019-1678-y
Uchida Y., Kan H., Sakurai K., Oishi K., Matsukawa N. (2022). Quantitative susceptibility mapping as an imaging biomarker for Alzheimer’s disease: the expectations and limitations. Front. Neurosci. 16, 938092. doi:10.3389/fnins.2022.938092
Uttara B., Singh A. V., Zamboni P., Mahajan R. T. (2009). Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 7 (1), 65–74. doi:10.2174/157015909787602823
Uzungil V., Tran H., Aitken C., Wilson C., Opazo C. M., Li S., et al. (2022). Novel antidepressant-like properties of the iron chelator deferiprone in a mouse model of depression. Neurotherapeutics 19 (5), 1662–1685. doi:10.1007/s13311-022-01257-0
Vela D. (2018). Hepcidin, an emerging and important player in brain iron homeostasis. J. Transl. Med. 16 (1), 25. doi:10.1186/s12967-018-1399-5
Villalon-Garcia I., Povea-Cabello S., Álvarez-Córdoba M., Talaverón-Rey M., Suárez-Rivero J. M., Suárez-Carrillo A., et al. (2023). Vicious cycle of lipid peroxidation and iron accumulation in neurodegeneration. Neural Regen. Res. 18 (6), 1196–1202. doi:10.4103/1673-5374.358614
Wadhwani A. R., Affaneh A., Van Gulden S., Kessler J. A. (2019). Neuronal apolipoprotein E4 increases cell death and phosphorylated tau release in alzheimer disease. Ann. Neurol. 85 (5), 726–739. doi:10.1002/ana.25455
Wang N., Zhang C. (2024). Oxidative stress: a culprit in the progression of diabetic kidney disease. Antioxidants (Basel) 13 (4), 455. doi:10.3390/antiox13040455
Wang S., He X., Wu Q., Jiang L., Chen L., Yu Y., et al. (2020a). Transferrin receptor 1-mediated iron uptake plays an essential role in hematopoiesis. Haematologica 105 (8), 2071–2082. doi:10.3324/haematol.2019.224899
Wang L., Yin Y. L., Liu X. Z., Shen P., Zheng Y. G., Lan X. R., et al. (2020b). Current understanding of metal ions in the pathogenesis of Alzheimer’s disease. Transl. Neurodegener. 9, 10. doi:10.1186/s40035-020-00189-z
Wang Y., Quan F., Cao Q., Lin Y., Yue C., Bi R., et al. (2021). Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J. Adv. Res. 28, 231–243. doi:10.1016/j.jare.2020.07.007
Wang F., Wang J., Shen Y., Li H., Rausch W. D., Huang X. (2022). Iron dyshomeostasis and ferroptosis: a new Alzheimer’s disease hypothesis? Front. Aging Neurosci. 14, 830569. doi:10.3389/fnagi.2022.830569
Wang J., Ma Y., Li J., Yang X., Hua G., Cai G., et al. (2023a). MiR-199a-3p regulates the PTPRF/β-Catenin axis in hair follicle development: insights into the pathogenic mechanism of alopecia areata. Int. J. Mol. Sci. 24 (22), 17632. doi:10.3390/ijms242417632
Wang S., Long H., Hou L., Feng B., Ma Z., Wu Y., et al. (2023b). The mitophagy pathway and its implications in human diseases. Signal Transduct. Target Ther. 8 (1), 304. doi:10.1038/s41392-023-01503-7
Wang X., Li S., Yu J., Wang W., Du Z., Gao S., et al. (2023c). Saikosaponin B2 ameliorates depression-induced microglia activation by inhibiting ferroptosis-mediated neuroinflammation and ER stress. J. Ethnopharmacol. 316, 116729. doi:10.1016/j.jep.2023.116729
Wang Y., Li H., He Q., Zou R., Cai J., Zhang L. (2024). Ferroptosis: underlying mechanisms and involvement in neurodegenerative diseases. Apoptosis 29 (1-2), 3–21. doi:10.1007/s10495-023-01902-9
Ward R. J., Zucca F. A., Duyn J. H., Crichton R. R., Zecca L. (2014). The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 13 (10), 1045–1060. doi:10.1016/S1474-4422(14)70117-6
Wei S., Qiu T., Wang N., Yao X., Jiang L., Jia X., et al. (2020). Ferroptosis mediated by the interaction between Mfn2 and IREα promotes arsenic-induced nonalcoholic steatohepatitis. Environ. Res. 188, 109824. doi:10.1016/j.envres.2020.109824
Wei Z., Yu H., Zhao H., Wei M., Xing H., Pei J., et al. (2024). Broadening horizons: ferroptosis as a new target for traumatic brain injury. Burns Trauma 12, tkad051. doi:10.1093/burnst/tkad051
Wen H., Deng H., Li B., Chen J., Zhu J., Zhang X., et al. (2025). Mitochondrial diseases: from molecular mechanisms to therapeutic advances. Signal Transduct. Target Ther. 10 (1), 9. doi:10.1038/s41392-024-02044-3
Wiernicki B., Dubois H., Tyurina Y. Y., Hassannia B., Bayir H., Kagan V. E., et al. (2020). Excessive phospholipid peroxidation distinguishes ferroptosis from other cell death modes including pyroptosis. Cell Death Dis. 11 (10), 922. doi:10.1038/s41419-020-03118-0
Wijesekera L. C., Leigh P. N. (2009). Amyotrophic lateral sclerosis. Orphanet J. Rare Dis. 4, 3. doi:10.1186/1750-1172-4-3
Williams R. A., Johnson K. W., Lee F. S., Hemmings H. C., Platholi J. (2022). A common human brain-derived neurotrophic factor polymorphism leads to prolonged depression of excitatory synaptic transmission by isoflurane in hippocampal cultures. Front. Mol. Neurosci. 15, 927149. doi:10.3389/fnmol.2022.927149
Xia L., Pang Y., Li J., Wu B., Du Y., Chen Y., et al. (2021). Dihydroartemisinin induces O-GlcNAcylation and improves cognitive function in a mouse model of tauopathy. J. Alzheimers Dis. 84 (1), 239–248. doi:10.3233/JAD-210643
Yan N., Zhang J. (2019). Iron metabolism, ferroptosis, and the links with Alzheimer’s disease. Front. Neurosci. 13, 1443. doi:10.3389/fnins.2019.01443
Yan H. F., Zou T., Tuo Q. Z., Xu S., Li H., Belaidi A. A., et al. (2021). Ferroptosis: mechanisms and links with diseases. Signal Transduct. Target Ther. 6 (1), 49. doi:10.1038/s41392-020-00428-9
Yang W., Liu X., Song C., Ji S., Yang J., Liu Y., et al. (2021). Structure-activity relationship studies of phenothiazine derivatives as a new class of ferroptosis inhibitors together with the therapeutic effect in an ischemic stroke model. Eur. J. Med. Chem. 209, 112842. doi:10.1016/j.ejmech.2020.112842
Yang S., Xie Z., Pei T., Xiong Q., Wei H., Wang Y., et al. (2022). Salidroside attenuates neuronal ferroptosis by activating the Nrf2/HO1 signaling pathway in Abeta(1-42)-induced Alzheimer’s disease mice and glutamate-injured HT22 cells. Chin. Med. 17 (1), 82.
Yang C. Z., Wang S. H., Zhang R. H., Lin J. H., Tian Y. H., Yang Y. Q., et al. (2023). Neuroprotective effect of astragalin via activating PI3K/Akt-mTOR-mediated autophagy on APP/PS1 mice. Cell Death Discov. 9 (1), 15. doi:10.1038/s41420-023-01324-1
Yao S., Pang M., Wang Y., Wang X., Lin Y., Lv Y., et al. (2023). Mesenchymal stem cell attenuates spinal cord injury by inhibiting mitochondrial quality control-associated neuronal ferroptosis. Redox Biol. 67, 102871. doi:10.1016/j.redox.2023.102871
Yi S., Wang L., Wang H., Ho M. S., Zhang S. (2022). Pathogenesis of alpha-synuclein in Parkinson’s disease: from a neuron-glia crosstalk perspective. Int. J. Mol. Sci. 23 (23), 14753. doi:10.3390/ijms232314753
Yung J. H. M., Giacca A. (2020). Role of c-Jun N-terminal kinase (JNK) in obesity and type 2 diabetes. Cells 9 (3), 706. doi:10.3390/cells9030706
Zarkovic K. (2003). 4-hydroxynonenal and neurodegenerative diseases. Mol. Asp. Med. 24 (4-5), 293–303. doi:10.1016/s0098-2997(03)00024-4
Zeng X., An H., Yu F., Wang K., Zheng L., Zhou W., et al. (2021). Benefits of iron chelators in the treatment of Parkinson's disease. Neurochem. Res. 46 (5), 1239–1251. doi:10.1007/s11064-021-03262-9
Zeng F., Nijiati S., Tang L., Ye J., Zhou Z., Chen X. (2023a). Ferroptosis detection: from approaches to applications. Angew. Chem. Int. Ed. Engl. 62 (35), e202300379. doi:10.1002/anie.202300379
Zeng T., Li J., Xie L., Dong Z., Chen Q., Huang S., et al. (2023b). Nrf2 regulates iron-dependent hippocampal synapses and functional connectivity damage in depression. J. Neuroinflammation 20 (1), 212. doi:10.1186/s12974-023-02875-x
Zgutka K., Tkacz M., Tomasiak P., Tarnowski M. (2023). A role for advanced glycation end products in molecular ageing. Int. J. Mol. Sci. 24 (12), 9881. doi:10.3390/ijms24129881
Zhang L., Liu W., Liu F., Wang Q., Song M., Yu Q., et al. (2020). IMCA induces ferroptosis mediated by SLC7A11 through the AMPK/mTOR pathway in colorectal cancer. Oxid. Med. Cell Longev. 2020, 1675613. doi:10.1155/2020/1675613
Zhang Y., Ren X., Wang Y., Chen D., Jiang L., Li X., et al. (2021). Targeting ferroptosis by polydopamine nanoparticles protects heart against ischemia/reperfusion injury. ACS Appl. Mater Interfaces 13 (45), 53671–53682. doi:10.1021/acsami.1c18061
Zhang Y., Wang M., Chang W. (2022a). Iron dyshomeostasis and ferroptosis in Alzheimer’s disease: molecular mechanisms of cell death and novel therapeutic drugs and targets for AD. Front. Pharmacol. 13, 983623. doi:10.3389/fphar.2022.983623
Zhang W., Yu M., Zhang Q., Yang Z., Zhang T. (2022b). DFO treatment protects against depression-like behaviors and cognitive impairment in CUMS mice. Brain Res. Bull. 187, 75–84. doi:10.1016/j.brainresbull.2022.06.016
Zhang X., Wu S., Guo C., Guo K., Hu Z., Peng J., et al. (2022c). Vitamin E exerts neuroprotective effects in pentylenetetrazole kindling epilepsy via suppression of ferroptosis. Neurochem. Res. 47 (3), 739–747. doi:10.1007/s11064-021-03483-y
Zhang Y., Zhang X., Wee Yong V., Xue M. (2022d). Vildagliptin improves neurological function by inhibiting apoptosis and ferroptosis following intracerebral hemorrhage in mice. Neurosci. Lett. 776, 136579. doi:10.1016/j.neulet.2022.136579
Zhang K., Ma Y., Luo Y., Song Y., Xiong G., Ma Y., et al. (2023a). Metabolic diseases and healthy aging: identifying environmental and behavioral risk factors and promoting public health. Front. Public Health 11, 1253506. doi:10.3389/fpubh.2023.1253506
Zhang W., Xu M., Chen F., Su Y., Yu M., Xing L., et al. (2023b). Targeting the JAK2-STAT3 pathway to inhibit cGAS-STING activation improves neuronal senescence after ischemic stroke. Exp. Neurol. 368, 114474. doi:10.1016/j.expneurol.2023.114474
Zhang C., Shi Z., Xu Q., He J., Chen L., Lu Z., et al. (2023c). Astragaloside IV alleviates stroke-triggered early brain injury by modulating neuroinflammation and ferroptosis via the Nrf2/HO-1 signaling pathway. Acta Cir. Bras. 38, e380723. doi:10.1590/acb380723
Zhang W., Liu Y., Liao Y., Zhu C., Zou Z. (2024a). GPX4, ferroptosis, and diseases. Biomed. Pharmacother. 174, 116512. doi:10.1016/j.biopha.2024.116512
Zhang Y., Chen L., Xuan Y., Zhang L., Tian W., Zhu Y., et al. (2024b). Iron overload in hypothalamic AgRP neurons contributes to obesity and related metabolic disorders. Cell Rep. 43 (3), 113900. doi:10.1016/j.celrep.2024.113900
Zhang Y., Zou Z., Liu S., Chen F., Li M., Zou H., et al. (2024c). Edaravone-loaded poly(amino acid) nanogel inhibits ferroptosis for neuroprotection in cerebral ischemia injury. Asian J. Pharm. Sci. 19 (2), 100886. doi:10.1016/j.ajps.2024.100886
Zhang X., Duan Y., Li S., Zhang Z., Peng L., Ma X., et al. (2024d). CRISPR screening identifies PRMT1 as a key pro-ferroptotic gene via a two-layer regulatory mechanism. Cell Rep. 43 (9), 114662. doi:10.1016/j.celrep.2024.114662
Zhao Y., Yang M., Liang X. (2024a). The role of mitochondria in iron overload-induced damage. J. Transl. Med. 22 (1), 1057. doi:10.1186/s12967-024-05740-4
Zhao X., Wang Z., Wu G., Yin L., Xu L., Wang N., et al. (2024b). Apigenin-7-glucoside-loaded nanoparticle alleviates intestinal ischemia-reperfusion by ATF3/SLC7A11-mediated ferroptosis. J. Control Release 366, 182–193. doi:10.1016/j.jconrel.2023.12.038
Zheng Y., Gao L., Wang D., Zang D. (2017). Elevated levels of ferritin in the cerebrospinal fluid of amyotrophic lateral sclerosis patients. Acta Neurol. Scand. 136 (2), 145–150. doi:10.1111/ane.12708
Zhou B., Liu J., Kang R., Klionsky D. J., Kroemer G., Tang D. (2020a). Ferroptosis is a type of autophagy-dependent cell death. Semin. Cancer Biol. 66, 89–100. doi:10.1016/j.semcancer.2019.03.002
Zhou H., Yin C., Zhang Z., Tang H., Shen W., Zha X., et al. (2020b). Proanthocyanidin promotes functional recovery of spinal cord injury via inhibiting ferroptosis. J. Chem. Neuroanat. 107, 101807. doi:10.1016/j.jchemneu.2020.101807
Zhou D., Lu P., Mo X., Yang B., Chen T., Yao Y., et al. (2023). Ferroptosis and metabolic syndrome and complications: association, mechanism, and translational applications. Front. Endocrinol. (Lausanne) 14, 1248934. doi:10.3389/fendo.2023.1248934
Zhou Q., Meng Y., Le J., Sun Y., Dian Y., Yao L., et al. (2024). Ferroptosis: mechanisms and therapeutic targets. MedComm (2020) 5 (12), e70010. doi:10.1002/mco2.70010
Zhu H., Huang J., Chen Y., Li X., Wen J., Tian M., et al. (2022). Resveratrol pretreatment protects neurons from oxygen-glucose deprivation/reoxygenation and ischemic injury through inhibiting ferroptosis. Biosci. Biotechnol. Biochem. 86 (6), 704–716. doi:10.1093/bbb/zbac048
Zhuge X., Tang R., Jiang Y., Lin L., Yang H. (2024). A multifunctional nanoplatform for chemotherapy and nanocatalytic synergistic cancer therapy achieved by amplified lipid peroxidation. Acta Biomater. 184, 419–430. doi:10.1016/j.actbio.2024.06.029
Zhuo B., Qin C., Deng S., Jiang H., Si S., Tao F., et al. (2025). The role of ACSL4 in stroke: mechanisms and potential therapeutic target. Mol. Cell Biochem. 480 (4), 2223–2246. doi:10.1007/s11010-024-05150-6
Keywords: ferroptosis, lipid peroxidation, oxidative stress, neurodegeneration with brain iron accumulation (NBIA), aceruloplasminemia, alzheimer’s disease, parkinson’s disease
Citation: Khan MS, Hu Q, Okeibunor K, Ma L and Bopassa JC (2025) Targeting ferroptosis for neuroprotection: potential therapeutic avenues in neurodegenerative and neuropsychiatric diseases. Front. Physiol. 16:1641323. doi: 10.3389/fphys.2025.1641323
Received: 04 June 2025; Accepted: 04 August 2025;
Published: 28 August 2025.
Edited by:
Yin Hua Zhang, Seoul National University, Republic of KoreaReviewed by:
Katarzyna Dzik, University of Gdansk, PolandSenlin Ji, Nanjing Drum Tower Hospital, China
Copyright © 2025 Khan, Hu, Okeibunor, Ma and Bopassa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jean C. Bopassa, Ym9wYXNzYUB1dGhzY3NhLmVkdQ==; Liang Ma, bWFsMUB1dGhzY3NhLmVkdQ==
 Muhammad S. Khan
Muhammad S. Khan Qichan Hu2
Qichan Hu2 Liang Ma
Liang Ma Jean C. Bopassa
Jean C. Bopassa