- 1Department of Internal Medicine 4, University of Erlangen-Nürnberg, Erlangen, Germany
- 2Department of Pathology (Section Nephropathology), Friedrich-Alexander University Erlangen, Erlangen, Germany
Cardiac vagal afferent neurons, located in the nodose ganglion, play a pivotal role in cardiopulmonary reflexes that link cardiac filling states to renal sympathetic outflow and the maintenance of circulatory homeostasis. Their excitability depends on a fine balance of depolarizing and repolarizing ion fluxes, yet the contribution of mechanosensitive (MS) ion channels to this regulation remains incompletely understood. While non-selective cation channels such as Piezo1/2 are established mediators of baroreceptor function, they are not directly responsible for repolarization. In contrast, mechanosensitive potassium channels are ideally suited to terminate action potentials and thereby shape afferent signaling from the heart. We, therefore, tested the hypothesis that MS potassium channels are functionally expressed in nodose ganglion neurons with cardiac projections. Using excised-patch recordings with stepwise suction, we identified two types of MS channels. One was inhibited by extracellular gadolinium (100 µM) and exhibited a higher unitary conductance, while the other was insensitive to gadolinium and showed a lower conductance. Both channel types were predominantly selective for K+ but also permeable to Na+, with a relative K+: Na+ permeability of ∼3.3–3.4. This mixed selectivity provides sufficient depolarization to activate voltage-gated Na+ channels and thereby initiate action potential firing. Our findings provide direct evidence for the presence of MS potassium channels in cardiac vagal afferent neurons and suggest that they may contribute critically to the mechanoelectric coupling and reflex control of cardiovascular function.
Introduction
The regulation of renal function is strongly coupled with cardiac sensory input (Feng et al., 2025; Johns, 2013). Stretch-sensitive receptors located in the atrial walls and great vessels detect changes in cardiac filling and wall tension and transmit this information via afferent neural pathways to central autonomic control centers (van Weperen and Vaseghi, 2023). Through this cardiopulmonary afferent signaling, the heart exerts a profound influence on renal sympathetic nerve activity and thereby modulates sodium and water excretion (Evans and Bie, 2016). In the setting of structural cardiac remodeling—such as in heart failure without overt systolic dysfunction (Hamo et al., 2024)—this reflex arc may become impaired, leading to dysregulated renal sympathetic outflow and inappropriate fluid retention (DiBona et al., 1996).
We recently demonstrated that in heart failure without overt systolic dysfunction, the regulation of sympathetic renal nerve activity via cardiac afferent pathways is impaired (Pickny et al., 2023). This disturbance appears to originate from dysfunctional sensory nerve endings embedded in the altered myocardial tissue (Chapleau et al., 2001; Moore, 2024; Pauziene et al., 2023). Primary afferent neurons located in the nodose ganglion, which relay signals from the heart, displayed increased excitability and elevated firing rates in vitro. However, this enhanced neuronal responsiveness did not restore afferent-mediated reflex function in vivo, suggesting that other mechanisms—beyond simple excitability—are involved in the breakdown of afferent control.
Cardiac afferents are predominantly unmyelinated C-fibers with chemo- and mechanosensitive (MS) properties (Veelken et al., 2003), and their excitability depends on the coordinated activity of ion channels mediating depolarization and repolarization. Although voltage-gated sodium channels such as Nav1.7 and Nav1.8 initiate action potentials (Catterall, 2023; Vasylyev et al., 2024; Ditting et al., 2016; Kwong et al., 2008), they are not the primary focus of the present study. Rather, we concentrated on the repolarization phase, which is shaped largely by potassium conductance and may be influenced by mechanical stimuli. MS potassium channels are strong candidates for dynamically adjusting afferent excitability in response to changes in cardiac wall tension.
Despite MS Piezo1 and Piezo2 channels having emerged as highly prominent targets of research (Lacroix and Wijerathne, 2025; Wang and Xiao, 2018) and having advanced our understanding of cardiovascular mechanotransduction and baroreceptor signaling (Zeng et al., 2018; McGrane et al., 2025), these non-selective cation channels primarily conducting calcium play only an indirect role in neuronal repolarization. In contrast, potassium channels activated by mechanical stress are directly suited to terminate action potentials and reset neuronal excitability.
However, while Piezo channels are an essential element for mechanoelectrical coupling, their role in neuronal repolarization is likely indirect. The role of Ca2+-sensitive potassium channels in action potential generation is considered modulatory in terms of neuronal excitability (Orfali and Albanyan, 2023). In contrast, potassium channels activated by mechanical stress are directly suited to terminate action potentials and reset neuronal excitability (Kanda et al., 2023). This distinction underlies the rationale of the present study, which focuses on identifying and characterizing MS potassium channels as potential key modulators of cardiac afferent function.
The present study was, therefore, designed to test whether MS potassium channels are functionally expressed in nodose ganglion neurons with cardiac projections. We used whole-cell patch-clamp recordings from primary cultures of nodose ganglion neurons and applied gadolinium—a known blocker of stretch-activated ion fluxes (Zhang et al., 2018)—to assess the presence and functional contribution of stretch-sensitive potassium conductance in these neurons. We focused specifically on cardiac afferent neurons because of their central role in modulating renal sympathetic nerve activity via cardiopulmonary reflex pathways (Feng et al., 2025; Johns, 2013; van Weperen and Vaseghi, 2023). Hence, a special method was used, as previously described, to identify neurons with cardiac axons in the nodose ganglion (Pickny et al., 2023). Understanding how cardiac sensory neurons process mechanical input is essential for elucidating the mechanisms that couple cardiac filling states to renal sodium and water excretion.
Methods
For the experiments, male Sprague–Dawley rats (Ivanovas, Kisslegg, Germany) weighting 250 g–300 g were maintained in cages at 24 ± 2 °C. They were fed a standard rat diet (no. C 1000, Altromin, Lage, FRG) containing 0.2% sodium by weight and were allowed free access to tap water. All procedures performed on the animals were carried out in accordance with the guidelines of the American Physiological Society and in compliance with the NIH Guide for Animal Care and Use in Laboratory Practice. They were approved by the local government agency (Regierung von Unterfranken). We retrogradely labeled cardiac neurons in twelve rats. The preparation failed in 5% of the animals. A total of 753 successful excised inside-out patch recordings out of a total of 820 patches were included in the data analysis.
Retrograde labeling of cardiac neurons
To identify cardiac baroreceptor neurons, these cells were labeled via intrapericardial application of the dicarbocyanine dye 1,1′ dioleyl-3,3,3′ tetramethylindocarbocyaline methansulfonate (DiI) (D9–DiI, Molecular Probes, Eugen, OR) at the junction of the great vessels with the heart in male Sprague–Dawley rats (4–8 weeks old, Ivanovas, Kisslegg, Germany), as previously described (Pickny et al., 2023; Linz and Veelken, 2002). Animals were anesthetized. Mechanical ventilation was established via a tracheal tube, and a high midline thoracotomy was performed. The lobes of the thymus were carefully separated from each other, exposing a small portion of the roof of the pericardial sac adherent to the thymus. The roof of the pericardial sac was slightly opened to insert a fine glass cannula filled with DiI for respective intrapericardial application (10 µL of DiI 50 mg/mL). Afterward, the roof of the pericardial sac was closed by apposing the two lobes of the thymus and sealing them together with polyacrylic glue. Finally, the thorax was closed in layers (Pickny et al., 2023; Veelken et al., 1990). We allowed 1 week for DiI to be transported back to the neuronal cells in the nodose ganglion.
Neuronal cell culture
Primary neuronal cultures of the nodose ganglia was established following previously described protocols (Pickny et al., 2023; Linz and Veelken, 2002; Ditting et al., 2003). Rats were anesthetized with hexobarbital. Both nodose ganglia were dissected and treated with a solution of collagenase (type 1A, 2 mg/mL, Sigma) in high-glucose Dulbecco’s modified Eagle’s medium (DMEM, Biochrom) on a stirring platform in a 5% CO2 incubator at 37 °C. After 1 h, ganglia were transferred into PBS solution containing trypsin (type III, 2 mg/mL, Sigma) and incubated for 10 min. Enzymatic activity was terminated using DMEM containing 10% FCS (Gibco, BRL). The softened tissue was triturated using siliconized pipettes. After centrifugation for 5 min, the supernatant was drawn off, and the cells were resuspended in a medium containing high-glucose DMEM, 10% FCS, and penicillin/streptomycin. The cells were cultured on poly-L-lysine-coated coverslips for 6–7 days until they were used for the studies.
We used only DiI-labeled neurons of the nodose ganglion that were unequivocally of cardiac origin. For this purpose, a small laser beam (a wavelength of 532 nm) powered by a storage battery was mounted to the patch-clamp recording setup. This equipment allowed for the detection of DiI-stained nodose ganglion cells, facilitating the identification of proper neurons for single-channel recordings.
Single-channel recordings
Cells were transferred into a 1-mL recording chamber. The bath solution contained (in mM) 140 KCl, 1 MgCl2, and 10 HEPES. The pH was adjusted to 7.35 with KOH. In some experiments, the concentration of chloride was reduced by the use of potassium sulfate instead of potassium chloride to identify chloride conductances. Sodium conductances were identified by substituting KCl with NaCl in both the extracellular and intracellular solutions. Patch pipettes had resistances of 4 MΩ–5 MΩ and were filled with a solution containing (in mM) 140 KCl, 2 CaCl2, 1 MgCl2, and 10 HEPES. The pH was adjusted to 7.35 with KOH. Tetraethylammonium chloride (TEA, 40 mM) was used to block voltage-activated currents, especially potassium-activated currents. The trivalent lanthanide salt gadolinium chloride (100 µM) was used to inhibit MS-channels (see Zhang et al., 2018; Hamill and McBride, 1996).
The activity of MS channels was recorded from excised inside-out patches using an Axopatch 200B Patch-Clamp Amplifier (Axon Instruments, California) and low-pass filtered at 2 kHz. Seal resistances ranged from 10 GΩ to 20 GΩ. The membrane was voltage clamped at −60 mV, which is the typical resting membrane potential for the types of neurons investigated (Bobryshev et al., 2012; Ikeda et al., 1986). To obtain the current–voltage (I–V) relationship from each channel, the voltage was stepwise increased from −100 to +40 mV, and channel currents were measured.
In eukaryotic systems, Piezo and TRP channels exhibit distinct biophysical properties that allow for clear differentiation—such as differences in unitary conductance and ion selectivity (Coste et al., 2012; Nilius and Honoré, 2012). Hence, the classification of channels found in our experiments was based on those well-established, previously described single-channel parameters.
Application of suction
To activate MS channels, suction was applied to the pipette holder. A mercury-calibrated manometer was used to monitor pressure steps, which were achieved using a syringe. The steps reached their plateau and remained stable within 0.2 ms. They ranged from 0 to 60 mmHg negative pressure. The change per step was 20 mmHg, and each step was held for 10 s. After activation, the channels were recorded once more at 0 mmHg to demonstrate the release properties of the openings.
Change of solutions
In some experiments, the pipette solution was exchanged using the pipette-perfusion technique (Tang et al., 1990). For this purpose, a thin glass perfusion pipette (diameter 100 µm) with a tip opening of approximately 10 µm was placed within the patch-pipette at a distance of 50 µm–100 µm away from the patch. The distance was necessary to prevent disruption caused by the perfusion stream. At a perfusion pressure of 100 mmHg, the new solution reached the patch within several hundred microseconds, as demonstrated by the blockade of TEA-sensitive potassium channels perfused with 40 mM TEA. Pharmacological agents can be washed out with this method within several seconds. To prevent an out-streaming of the solution during the suction protocol, the perfusion pipette was only placed within the patch pipette when it was used immediately before and after the inside-out measurements. When MS- and voltage-gated potassium-channels were found simultaneously in a patch, TEA-block was used to study MS-conductances.
Data analysis
Recordings were digitized at 5 kHz and analyzed off-line using pCLAMP software (version 8.0.1., Axon Instruments).
In all cases within a single patch, we reported the product NPo, where N is the number of channels and Po is the open probability of a single channel. This approach is the standard in single-channel analysis when the exact number of channels in a patch cannot be determined with certainty (England et al., 1993). Across a large number of studies (Kosakai et al., 2015; Dove et al., 1998; Shoemaker and Worrell, 1991; Gu et al., 2014; Kolesnikov et al., 2021; Meng et al., 2022), NPo provided a consistent, quantitative index of how experimental manipulations (alternative nucleotides, kinases, second messengers, mechanical forces, pharmacological agents, disease states, or genetic variants) alter the activity of single ion channels. Because NPo inherently incorporates both the number of channels in a patch and their individual gating behavior, it is especially well-suited to reveal changes in open probability, frequency of openings, lifetime of open or closed states, and the recruitment of previously silent channels.
Since N was not directly accessible in excised-patch recordings without further manipulations (e.g., non-stationary noise analysis or full activation protocols) in our experiments, and such procedures were not feasible in our experimental paradigm, we chose the NPo metric as described above as the most reliable and interpretable parameter (for more details, see below). This allows for meaningful comparisons across conditions without requiring assumptions regarding the absolute number of channels.
To express the activity of MS channels, the product NPo was calculated, where N represents the number of single MS ion channels in the patch and Po represents the open probability of each channel. Po cannot be expressed directly, as outlined above, because the number of active channels in patch-clamp-experiments is not known. NPo was determined by the events list method. Each opening and closing event was identified, and the sum of the open intervals was divided by the total duration of the recording period.
Negative currents represented a flow of positive charges from the inside to the outside of the membrane according to the general convention. All data are expressed as the means ± SEM, unless otherwise stated. Data were compared using ANOVA and respective post hoc tests. A value of p < 0.05 was considered statistically significant.
Results
MS channel activity in cardiac nodose neurons
To determine whether cardiac-projecting vagal sensory neurons express MS ion channels, we performed a total of 753 excised inside-out patch recordings: 332 under control conditions with 2 mM Ca2+ in the pipette and 421 under Ca2+-free conditions (10 mM EGTA). The application of negative pipette pressure elicited distinct single-channel openings that disappeared immediately after the release of suction (Figure 1A). Channel activity increased stepwise with rising suction and, on the expanded timescale, displayed the rapid “flickering” openings that are characteristic of MS channels (Figure 1B). In some patches, channels of smaller amplitude but higher overall activity (NPo) were detected, often with multiple distinct conductance levels resolved (Figure 1B).
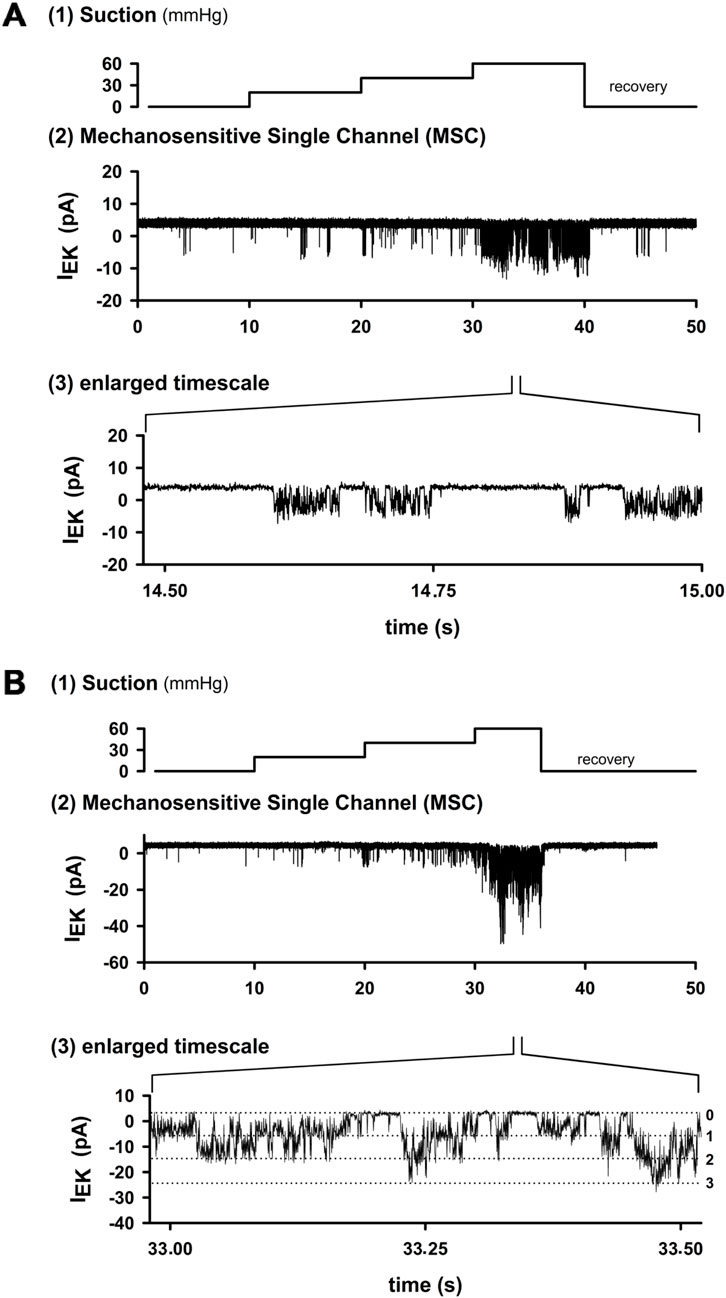
Figure 1. Activation of MS channels in cardiac nodose neurons. (A) Example trace from an excised inside-out patch. Stepwise increases in negative pipette pressure (10 s per step) evoked channel openings, which returned to baseline after suction release. At higher resolution, channel activity showed rapid “flickering” openings typical of MS channels. (B) Another patch with channels of smaller amplitude but higher overall activity (higher NPo). Three distinct conductance levels can be resolved on an expanded timescale. Hence, mechanosensitive channels were observed in 28.8% of patches under 2 mM Ca2+ in the pipette (134/332) and only 3.7% of patches under EGTA (16/421). These data demonstrate that cardiac nodose neurons express stretch-activated ion channels whose activity increases with mechanical stimulation.
MS channel activity was detected in 134/332 patches (28.8%) under control conditions, while the remaining 198 patches (71.2%) showed no mechanosensitivity. In contrast, under Ca2+-free conditions (10 mM EGTA), activity was only detected in 16/421 patches (3.7%). Thus, the presence of MS channels in cardiac projecting nodose neurons depends strongly on intracellular Ca2+ buffering, and not all retrogradely labeled neurons contain these channels. These results provide the first direct evidence that this subset of vagal afferents possesses membrane channels that can be directly activated by mechanical stretch.
In patch-clamp experiments, inward cation currents are defined as negative currents. Opening properties of the MS channels—including voltage dependence and conductance—are comprehensively analyzed and presented in Figures 2–4, where representative single-channel traces and quantitative summaries are displayed.
Two MS channel populations defined by gadolinium sensitivity
We next tested whether MS channels in cardiac nodose neurons could be distinguished pharmacologically by the sensitivity to gadolinium (Gd3+), a broad inhibitor of MS currents. Of the 30 patches tested with 100 μM Gd3+, 16 were strongly inhibited, while 14 were unaffected (Figures 2A, B). Group data confirmed a significant reduction in open probability (NPo) in the sensitive group (p < 0.05), whereas no effect was observed in the insensitive group (Figure 2C).
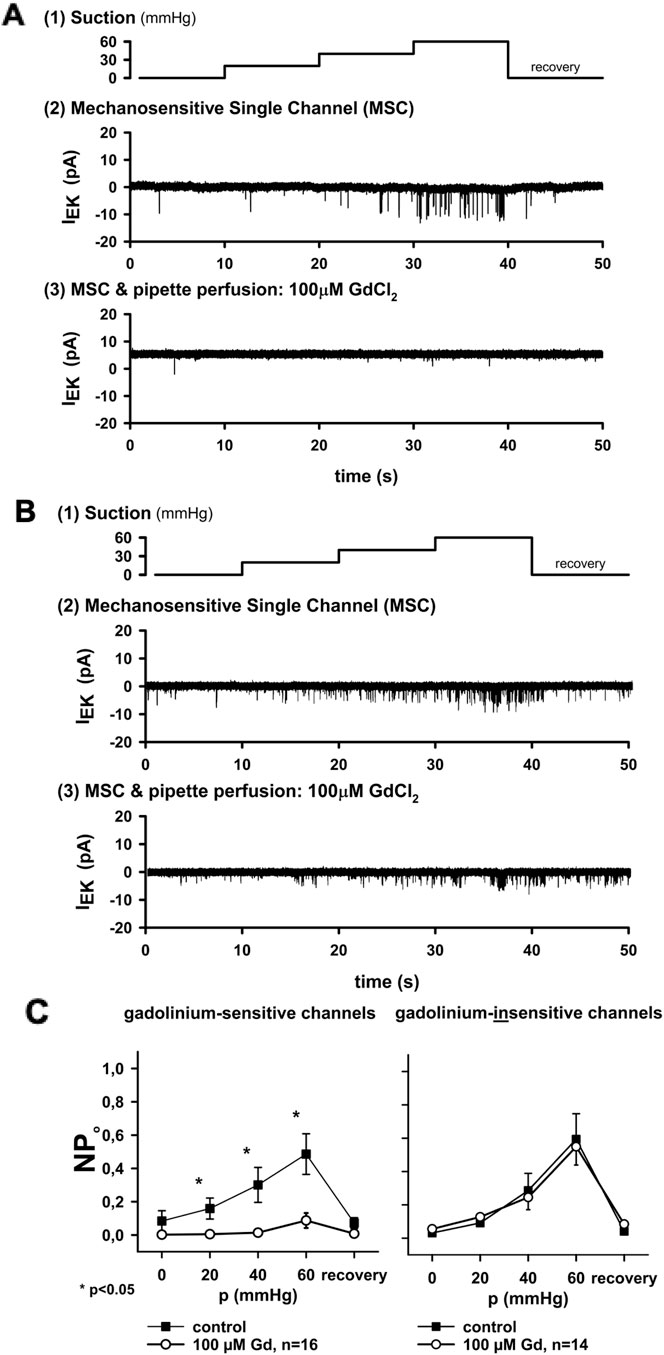
Figure 2. Gadolinium distinguishes two types of MS channels. (A) In one group of channels, activity was strongly inhibited by extracellular 100 μM Gd3+, a known blocker of MS currents. (B) In another group, channel activity was unaffected by Gd3+. (C) Summary plots of open probability (NPo) versus applied pressure. Inhibition by Gd3+ was significant in sensitive channels (n = 16, *p < 0.05) but not in insensitive channels (n = 14). Thus, two distinct channel populations could be differentiated in cardiac nodose neurons: Gd3+-sensitive and Gd3+-insensitive. Conductance analysis was only performed in the open (i.e., unblocked) state of the channel.
Importantly, the maximum NPo values differed between the two groups: 0.26 ± 0.05 in Gd3+-sensitive channels versus 0.48 ± 0.07 in Gd3+-insensitive channels (p < 0.05). This indicates that gadolinium not only blocks one population but also distinguishes channels with different activity profiles.
Biophysical properties distinguish Gd3+-sensitive and -insensitive MS channels
To further characterize the two channel populations, we examined single-channel currents during voltage steps from −100 to +40 mV under constant suction (Figure 3A). At negative potentials, channel openings carried inward currents, while at depolarized potentials, outward currents were observed.
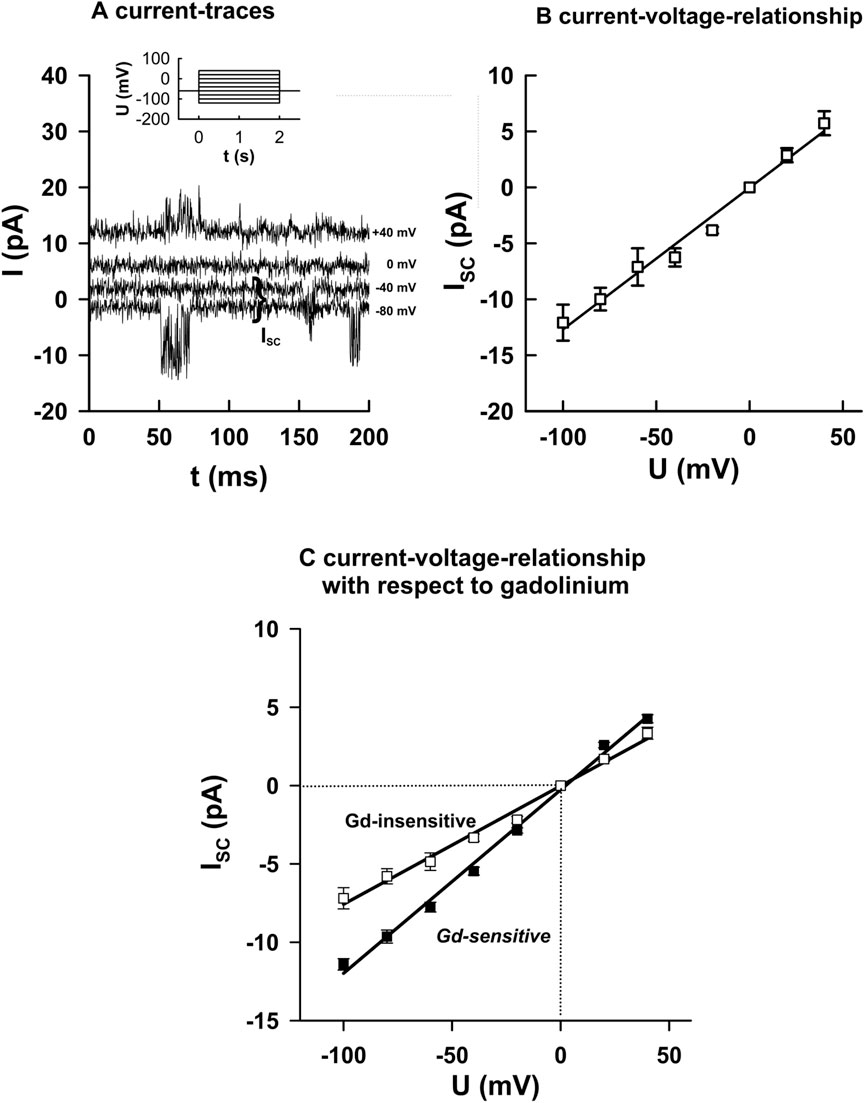
Figure 3. Biophysical properties of Gd3+-sensitive and -insensitive MS channels. (A) Example of currents recorded during voltage steps (−100 to +40 mV, 2 s duration) under constant suction (20 mmHg–40 mmHg). (B) Corresponding current–voltage relationships from individual openings (mean ± SEM, 10 events each). (C) Summary data: Gd3+-sensitive channels exhibited higher slope conductance (116.4 ± 5.3 pS, n = 16) than Gd3+-insensitive channels (79.1 ± 6.5 pS, n = 14; p < 0.05). Both groups reversed near 0 mV under symmetrical K+ conditions, indicating predominant K+ selectivity. These results identified two functional classes of MS channels differing in single-channel conductance. The slopes of the regression lines were significantly different from each other (p < 0.05).
I–V relationships were constructed from individual openings (Figure 3B). Gd3+-sensitive channels exhibited a significantly higher slope conductance (116.4 ± 5.3 pS, n = 16) than Gd3+-insensitive channels (76.1 ± 6.5 pS, n = 14; p < 0.05; Figure 3C). Both groups reversed close to 0 mV under symmetrical K+ conditions, confirming predominant K+ selectivity. These data show that the two MS channel populations differ not only in pharmacology and open probability but also in their single-channel conductance.
MS channels do not conduct chloride ions
To test for Cl− permeability, extracellular chloride was replaced with impermeant sulfate. The I–V relationship and reversal potential of MS channels were unchanged in both Gd3+-sensitive (n = 3) and Gd3+-insensitive channels (n = 3; Figure 4). This excludes chloride as a relevant permeant ion and confirms that both channel populations predominantly conduct cations.
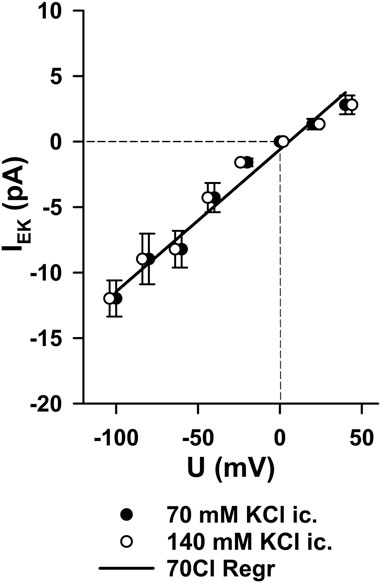
Figure 4. Lack of chloride permeability in MS channels. When extracellular chloride was replaced by sulfate, the current–voltage relationship of MS channels was unchanged. Reversal potentials remained close to 0 mV in both Gd3+-sensitive channels (n = 3) and Gd3+-insensitive channels (n = 3). This confirms that the identified MS channels conduct mainly cations and exclude Cl− as a significant permeant ion.
According to standard electrophysiological convention, inward cation currents (flow of positive charge into the cell) are plotted as negative currents. In our recordings, this refers to single-channel openings at negative membrane potentials. The linear I–V relationship refers to the voltage dependence of the current across a broader range, confirming the ohmic behavior of the channels without voltage gating. These observations are not contradictory but reflect the compatibility of ohmic linearity with inward currents at negative potentials, depending on the ionic driving forces.
Sodium permeability of MS channels
Finally, we examined whether the MS channels conduct sodium ions. When K+ was replaced by Na+ on both sides of the membrane, slope conductance was reduced in both channel types (Figures 5A, B). The calculated permeability ratios of K+: Na+ were 3.3–3.4 in both groups (n = 4 each, p < 0.05).
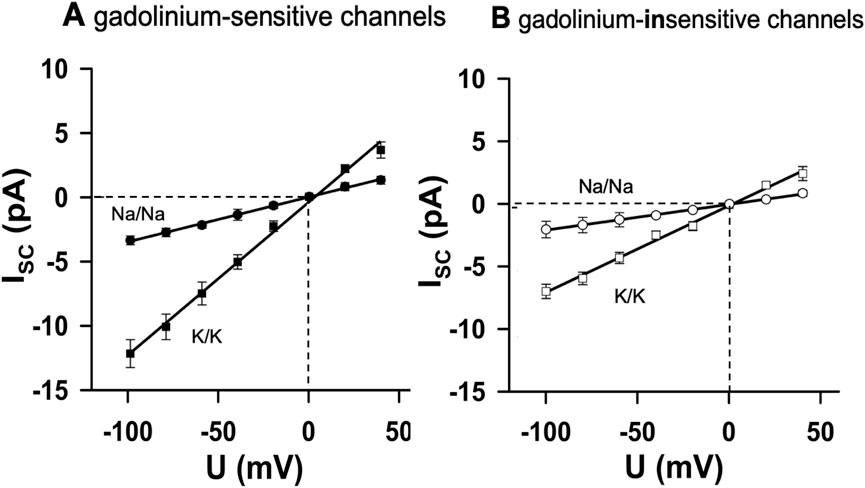
Figure 5. Sodium permeability of MS channels. Replacement of K+ with Na+ on both sides of the membrane reduced slope conductance in both channel types. (A) Gd3+-sensitive channels (n = 4). (B) Gd3+-insensitive channels (n = 4). The calculated permeability ratios of K+: Na+ were ∼3.3–3.4 in both groups (p < 0.05 between the regression slopes). Thus, cardiac nodose MS channels were predominantly K+ -selective but also allowed Na+ permeation, sufficient to depolarize neurons toward the threshold for action potential generation.
Thus, although both channel populations are predominantly K+-selective, they also permit Na+ entry. This mixed selectivity implies that the activation of MS channels by mechanical stimuli can depolarize cardiac-projecting nodose neurons toward the threshold for action potential initiation, thereby providing a plausible mechanism for mechanoelectric coupling in vagal afferents.
Discussion
We hypothesized that neurons from the nodose ganglion with cardiac afferents should contain MS potassium channels that, among other effects, will support proper repolarization and post-hyperpolarization phases during action potential generation.
This paper could eventually demonstrate that in nodose ganglion neurons with cardiac afferents, there must exist at least two types of stretch-activated MS ion channels with predominant potassium conductance besides other proposed putative mechanotransducing mechanisms (Zeng et al., 2018). One exhibited a slope conductance of 116 pS and was blocked by 100 μM Gd3+, a known inhibitor of MS currents (Zhang et al., 2018). It has likely been described using cell-attached measurements previously (Hamill and Martinac, 2001). The author showed that in a co-culture with aortic endothelial cells, the number of patches on neurons containing these MS channels increased This points to a diffusible factor from endothelial cells, which might regulate the expression of these channels. The author was able to block MS activity when 20 µM of gadolinium was applied.
The other channel we could characterize was insensitive to extracellular application of 100 μM Gd3+ and had a smaller conductance of 76 pS. Interestingly, the channel with lower conductivity and, therefore, smaller channel width was insensitive to Gd3+, for the action of this substance is sometimes observed as mainly obstructing the pore. In a simple model, it would be easier to obstruct a small pore than a wide pore. Hence, not only the diameter but also the geometry or electrostatic properties of the pore may have an influence on the attachment and blocking effect of Gd3+. The insensitive channel shared properties of a 54 pS stretch-activated channel in colon sensory neurons (Su et al., 2000), which was also unaffected by 100 μM Gd3+, but in contrast to this channel, the MS channels of nodose neurons were not influenced by 40 mM TEA either on the extracellular side of the membrane.
Although gadolinium is considered a general inhibitor, several studies have shown that not all cellular mechano-transduction depended on Gd3-sensitive channels, so a classical Gd3 blockade of stretch-activated or MS cation channels failed to inhibit a mechanically evoked response (Sakamoto et al., 2010; Malek and Izumo, 1996; Steffensen et al., 1991).
Hence, gadolinium can help—as in our study—to distinguish between MS cation channels. The exact meaning of this finding is not yet understood.
Stretch-inactivated channels that were described in other preparations have not been observed at any patch on nodose neurons with cardiac afferents (Matsumoto et al., 2011). The existence of a mechanical notch filter that limits the range of the cell potential by two antagonizing channel types can, therefore, be assumed to be rather unlikely (Sackin, 1995a; Murthy, 2023).
The channels were predominantly selective for potassium and sodium and can, therefore, be classified as cation channels. In our measurements, the relative permeability of potassium versus sodium was 3.4 in Gd3+-sensitive channels and 3.3 in Gd3+-insensitive channels. Taking the Goldman equation into account with typical ion concentrations ([Na]ec/[Na]ic = [K]ic/[K]ec = 140 mM/5 mM), the reversal potential of the channels could depolarize the neurons to −33 mV, where voltage-sensitive sodium conductances are already activated.
Chloride or calcium conductances could not be detected in the two types of channels in our experiments. The calcium gradient used did not shift the reversal potential significantly from 0 mV. Therefore, the eventual permeability for this ion must be very low. Sharma et al. (1995) detected Gd3+-sensitive increases in intracellular Ca2+ of approximately 500 nM using the fura-2 technique when nodose neurons were mechanically stimulated. However, until now, it has not been possible to determine special membrane channels responsible for this increase.
When symmetrical potassium solutions were used, the two channel types showed the same reversal potential (0 mV) and a linear I–V relationship. The open probabilities (Bennett et al., 2000) were independent of voltage, suggesting that no voltage sensor is present in the channel proteins. So, they are unlikely to be influenced during the generation of action potentials in the neurons. Voltage insensitivity is a common feature of many MS ion channels in different preparations (Su et al., 2000; Sackin, 1995a).
The number of active MS channels in a patch decreased when extracellular calcium was buffered with EGTA. This suggests a new gating mechanism for neuronal MS channels as calcium normally acts from the intracellular side of the membrane on channel proteins, as observed in calcium-activated potassium channels. Extracellular Ca2+-dependent inhibition is known from the hair bundles in vertebrate hair cells, which are involved in the process of hearing. Here, mechanotransduction is abolished when reduced Ca2+ disrupts the tip links (Hamill and Martinac, 2001; Bennett et al., 2000). Calcium may modulate the structure of the channel protein, rendering it stretch-sensitive or inducing an active conformational state. On the other hand, EGTA (Kra et al., 1998) might be involved in the interaction itself.
The activity of MS ion channels in cardiac nodose neurons increased when the patch-clamp mode was changed from cell-attached to inside-out. Activation by the force of pipette retraction is not likely because microscopy analysis of patches showed purely fixed membranes (Hamill and Martinac, 2001; Gil et al., 1999). There is more evidence that membrane–cytoskeleton interactions are involved in the activation, as other authors have reported (Kra et al., 1998; Cunningham et al., 1997). Here, cytochalasin D and colchicine, which disrupted filaments of the cytoskeleton, are potent enough to increase channel activity in cell-attached patches (Lesage et al., 2000; Maingret et al., 1999). So, these channels may be inactivated by intracellular components in the parent cell.
The openings of nodose MS channels exhibited a flickering behavior, indicating an extremely low open-state time constant that could not be resolved by standard patch-clamp amplifiers. Based on amplifier specifications, the time constant must be faster than 0.1 ms. This limitation impacted both the accuracy of current measurements and the precise calculation of channel conductance. The flickering states were integrated into longer-lasting bursts on the millisecond timescale. In MS channels of chicken muscle cells, Guharay and Sachs (1984) showed that only this state was influenced by the cytoskeleton. Using cytochalasin, all other states remained unchanged (Sackin, 1995a; Sackin, 1995b).
Recent high-resolution electrophysiological studies have confirmed the presence of such rapid gating events in MS ion channels, emphasizing the need for advanced methods such as high-speed patch-clamp and fluorescence-based imaging to resolve these fast transitions (Cox et al., 2018; Wu et al., 2017; Jiang et al., 2021). The flickering states are typically integrated into longer bursts lasting several milliseconds.
In line with earlier findings, recent research confirms that cytoskeletal elements selectively influence particular gating states of MS channels. In particular, actin filament disruption using agents such as cytochalasin D alters prolonged open states, while rapid flicker transitions remain largely unaffected. These observations support a model in which mechanical force is transduced not only via membrane tension but also through tethering to the cytoskeletal network, particularly actin filaments (Jiang et al., 2021; Ingber, 2006).
In summary, the Gd-sensitive and -insensitive potassium channel types of cardiac nodose ganglion neurons shared many equivalent properties, with the exception of the conductivity. Further work is needed to elucidate the extent to which crosstalk between these channels and voltage-gated sodium channels influences the sensitivity and activity of cardiac afferent pathways in both healthy and diseased conditions.
Beyond the focus of this investigation, a large variety of stretch-activated channels (Webster et al., 2025) can be found in the nodose ganglion in general: Piezo1/2 were identified as directly stretch-activated cation channels essential for baroreceptor function, and their deletion abolished reflex-mediated activity (Zeng et al., 2018). In hypertensive rats, activation of Piezo1 by Yoda1 lowered the blood pressure through increased afferent firing, an effect prevented by GsMTx4, while SERCA2 inhibition attenuated these currents, indicating Ca2+-dependent gating (Cui et al., 2024; Zhao et al., 2025). TTN3 contributed to dynamic pressure sensing and baroreflex maintenance (Lu et al., 2020). Among TRP channels, TRPV4/TRPA1 were diet-sensitive, TRPC1/3 localized to low-threshold complexes, and TRPA1 and TRPV1 mediated visceral and gastro-esophageal mechanosensitivity, respectively. ENaC/ASIC2 deficits impaired baroreflex integration, while HCN channels, nitric oxide, and cytoskeleton-dependent stretch conductances further shaped mechanosensory excitability (Sharma et al., 1995; Kra et al., 1998; Li et al., 2016; Lu et al., 2009; Doan et al., 2004; Li et al., 1998). In addition to Piezo and TRP channels, members of the K2P family, such as TREK-1, have also been described in nodose ganglion neurons as MS potassium channels (Park et al., 2023). Although our data cannot exclude their contribution, the biophysical properties we observed (unitary conductance, Na+ permeability, and Gd3+ sensitivity) suggest that at least two distinct populations of MS K+ channels coexist. Future studies will be required to determine whether TREK1 or related K2P channels account for one of the populations described here.
A more definitive classification of the channels described in this publication would ideally involve genetic or structural analyses. Such experiments, however, were beyond the scope and feasibility of the present study. Instead, we adopted a functional classification approach based on comparisons with established mechanotransducers. Although this provides valuable insights into their properties, future studies employing genetic or structural methods will be required to confirm their molecular identity and further refine their mechanistic understanding.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because neurons were harvested upon euthanasia of the animals.
Author contributions
PL: Writing – original draft, Writing – review and editing. EH: Writing – review and editing, Conceptualization. TD: Formal analysis, Writing – original draft. MS: Writing – review and editing. KA: Writing – review and editing. KH: Writing – review and editing. RV: Writing – review and editing, Writing – original draft. KR: Writing – review and editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study received the grant-in-aid of the Deutsche Forschungsgemeinschaft.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bennett M. R., Farnell L., Gibson W. G. (2000). The probability of quantal secretion near a single calcium channel of an active zone. BiophysJ 78 (5), 2201–2221. doi:10.1016/S0006-3495(00)76769-5
Bobryshev A. Y., Petrushenko E. A., Krishtal O. A. (2012). Effect of of ATP on neurons of the rat intact nodose ganglion. Neurophysiology. 43 (6), 432–436. doi:10.1007/s11062-012-9247-3
Catterall W. A. (2023). Voltage gated sodium and calcium channels: discovery, structure, function, and pharmacology. Pharmacol. Channels (Austin). 17 (1), 2281714. doi:10.1080/19336950.2023.2281714
Chapleau M. W., Li Z., Meyrelles S. S., Ma X., Abboud F. M. (2001). Mechanisms determining sensitivity of baroreceptor afferents in health and disease. AnnNYAcadSci 940, 1–19. doi:10.1111/j.1749-6632.2001.tb03662.x
Coste B., Xiao B., Santos J. S., Syeda R., Grandl J., Spencer K. S., et al. (2012). Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483 (7388), 176–181. doi:10.1038/nature10812
Cox C. D., Bavi N., Martinac B. (2018). Bacterial mechanosensors. Annu. Rev. Physiol. 80, 71–93. doi:10.1146/annurev-physiol-021317-121351
Cui C. P., Xiong X., Zhao J. X., Fu D. H., Zhang Y., Ma P. B., et al. (2024). Piezo1 channel activation facilitates baroreflex afferent neurotransmission with subsequent blood pressure reduction in control and hypertension rats. Acta Pharmacol. Sin. 45 (1), 76–86. doi:10.1038/s41401-023-01154-y
Cunningham J. T., Wachtel R. E., Abboud F. M. (1997). Mechanical stimulation of neurites generates an inward current in putative aortic baroreceptor neurons in vitro. Brain Res. 757 (1), 149–154. doi:10.1016/s0006-8993(97)00153-4
DiBona G. F., Sawin L. L., Jones S. Y. (1996). Characteristics of renal sympathetic nerve activity in sodium-retaining disorders. Am. J. Physiology 271 (1 Pt 2), R295–R302. doi:10.1152/ajpregu.1996.271.1.R295
Ditting T., Linz P., Hilgers K. F., Jung O., Geiger H., Veelken R. (2003). Putative role of epithelial sodium channels (ENaC) in the afferent limb of cardiorenal reflexes in rats. BRC 98, 388–400. doi:10.1007/s00395-003-0426-7
Ditting T., Freisinger W., Rodionova K., Schatz J., Lale N., Heinlein S., et al. (2016). Impaired excitability of renal afferent innervation after exposure to the inflammatory chemokine CXCL1. Am. J. Physiol. Ren. Physiol. 310 (5), F364–F371. doi:10.1152/ajprenal.00189.2015
Doan T. N., Stephans K., Ramirez A. N., Glazebrook P. A., Andresen M. C., Kunze D. L. (2004). Differential distribution and function of hyperpolarization-activated channels in sensory neurons and mechanosensitive fibers. J. Neurosci. 24 (13), 3335–3343. doi:10.1523/JNEUROSCI.5156-03.2004
Dove L. S., Abbott L. C., Griffith W. H. (1998). Whole-cell and single-channel analysis of P-type calcium currents in cerebellar Purkinje cells of leaner mutant mice. J. Neurosci. 18 (19), 7687–7699. doi:10.1523/JNEUROSCI.18-19-07687.1998
England S. K., Wooldridge T. A., Stekiel W. J., Rusch N. J. (1993). Enhanced single-channel K+ current in arterial membranes from genetically hypertensive rats. Am. J. Physiol. 264 (5 Pt 2), H1337–H1345. doi:10.1152/ajpheart.1993.264.5.H1337
Evans R. G., Bie P. (2016). Role of the kidney in the pathogenesis of hypertension: time for a neo-guytonian paradigm or a paradigm shift? Am. J. physiology Regul. Integr. Comp. physiology 310 (3), R217–R229. doi:10.1152/ajpregu.00254.2015
Feng E. Y., Pan S., Verma H., Zheng H., Plata A. A., Zubcevic J., et al. (2025). Central nervous system mechanisms of salt-sensitive hypertension. Physiol. Rev. 105 (4), 1989–2032. doi:10.1152/physrev.00035.2024
Gil Z., Silberberg S. D., Magleby K. L. (1999). Voltage-induced membrane displacement in patch pipettes activates mechanosensitive channels. ProcNatlAcadSciUSA 96 (25), 14594–14599. doi:10.1073/pnas.96.25.14594
Gu X. Q., Pamenter M. E., Siemen D., Sun X., Haddad G. G. (2014). Mitochondrial but not plasmalemmal BK channels are hypoxia-sensitive in human glioma. Glia 62 (4), 504–513. doi:10.1002/glia.22620
Guharay F., Sachs F. (1984). Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. JPhysiol(Lond) 352, 685–701. doi:10.1113/jphysiol.1984.sp015317
Hamill O. P., Martinac B. (2001). Molecular basis of mechanotransduction in living cells. Physiol. Rev. 81 (2), 685–740. doi:10.1152/physrev.2001.81.2.685
Hamill O. P., McBride D. W. (1996). The pharmacology of mechanogated membrane ion channels. PharmacolRev 48 (2), 231–252. doi:10.1016/s0031-6997(25)06942-x
Hamo C. E., DeJong C., Hartshorne-Evans N., Lund L. H., Shah S. J., Solomon S., et al. (2024). Heart failure with preserved ejection fraction. Nat. Rev. Dis. Prim. 10 (1), 55. doi:10.1038/s41572-024-00540-y
Ikeda S. R., Schofield G. G., Weight F. F. (1986). Na+ and Ca2+ currents of acutely isolated adult rat nodose ganglion cells. J. Neurophysiol. 55 (3), 527–539. doi:10.1152/jn.1986.55.3.527
Ingber D. E. (2006). Cellular mechanotransduction: putting all the pieces together again. FASEB J. 20 (7), 811–827. doi:10.1096/fj.05-5424rev
Jiang Y., Yang X., Jiang J., Xiao B. (2021). Structural designs and mechanogating mechanisms of the mechanosensitive Piezo channels. Trends Biochem. Sci. 46 (6), 472–488. doi:10.1016/j.tibs.2021.01.008
Johns E. J. (2013). Autonomic regulation of kidney function. Handb. Clin. Neurol. 117, 203–214. doi:10.1016/B978-0-444-53491-0.00017-1
Kanda H., Noguchi K., Dai Y. (2023). Axonal membrane stretch suppresses neuronal excitability by activating mechanosensitive K2P channels at the node of ranvier. Mol. Brain 16 (1), 8. doi:10.1186/s13041-023-01000-6
Kolesnikov D., Perevoznikova A., Gusev K., Glushankova L., Kaznacheyeva E., Shalygin A. (2021). Electrophysiological properties of endogenous single Ca(2+) activated Cl(-) channels induced by local Ca(2+) entry in HEK293. Int. J. Mol. Sci. 22 (9), 4767. doi:10.3390/ijms22094767
Kosakai K., Tsujiuchi Y., Yoshino M. (2015). Nitric oxide augments single Ca(2+) channel currents via cGMP-dependent protein kinase in kenyon cells isolated from the mushroom body of the cricket brain. J. Insect Physiol. 78, 26–32. doi:10.1016/j.jinsphys.2015.04.009
Kraske S., Cunningham J. T., Hajduczok G., Chapleau M. W., Abboud F. M., Wachtel R. E. (1998). Mechanosensitive ion channels in putative aortic baroreceptor neurons. Am. J. Physiology 275 (4 Pt 2), H1497–H1501. doi:10.1152/ajpheart.1998.275.4.H1497
Kwong K., Carr M. J., Gibbard A., Savage T. J., Singh K., Jing J., et al. (2008). Voltage-gated sodium channels in nociceptive versus non-nociceptive nodose vagal sensory neurons innervating guinea pig lungs. J. Physiol. 586 (5), 1321–1336. doi:10.1113/jphysiol.2007.146365
Lacroix J. J., Wijerathne T. D. (2025). PIEZO channels as multimodal mechanotransducers. Biochem. Soc. Trans. 53 (1), 293–302. doi:10.1042/BST20240419
Lesage F., Maingret F., Lazdunski M. (2000). Cloning and expression of human TRAAK, a polyunsaturated fatty acids-activated and mechano-sensitive K(+) channel. FEBS Lett. 471 (2-3), 137–140. doi:10.1016/s0014-5793(00)01388-0
Li Z., Chapleau M. W., Bates J. N., Bielefeldt K., Lee H. C., Abboud F. M. (1998). Nitric oxide as an autocrine regulator of sodium currents in baroreceptor neurons. Neuron 20 (5), 1039–1049. doi:10.1016/s0896-6273(00)80484-5
Li Y. L., Zhang D., Tu H., Muelleman R. L. (2016). Altered ENaC is associated with aortic baroreceptor dysfunction in chronic heart failure. Am. J. Hypertens. 29 (5), 582–589. doi:10.1093/ajh/hpv141
Linz P., Veelken R. (2002). Serotonin 5-HT(3) receptors on mechanosensitive neurons with cardiac afferents. AmJPhysiol Heart CircPhysiol 282 (5), H1828–H1835. doi:10.1152/ajpheart.00708.2000
Lu Y., Ma X., Sabharwal R., Snitsarev V., Morgan D., Rahmouni K., et al. (2009). The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation. Neuron 64 (6), 885–897. doi:10.1016/j.neuron.2009.11.007
Lu H. J., Nguyen T. L., Hong G. S., Pak S., Kim H., Kim H., et al. (2020). Tentonin 3/TMEM150C senses blood pressure changes in the aortic arch. J. Clin. investigation 130 (7), 3671–3683. doi:10.1172/JCI133798
Maingret F., Patel A. J., Lesage F., Lazdunski M., Honore E. (1999). Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J. Biol. Chem. 274 (38), 26691–26696. doi:10.1074/jbc.274.38.26691
Malek A. M., Izumo S. (1996). Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. J. Cell Sci. 109 (Pt 4), 713–726. doi:10.1242/jcs.109.4.713
Matsumoto S., Yoshida S., Ikeda M., Kadoi J., Takahashi M., Tanimoto T., et al. (2011). Effects of acetazolamide on transient K+ currents and action potentials in nodose ganglion neurons of adult rats. CNS Neurosci. Ther. 17 (1), 66–79. doi:10.1111/j.1755-5949.2010.00133.x
McGrane A., Murray M., Bartoli F., Giannoudi M., Conning-Rowland M., Stewart L., et al. (2025). PIEZO force sensors and the heart. Cold Spring Harb. Perspect. Biol., a041806. doi:10.1101/cshperspect.a041806
Meng X. X., Zhang H., Meng G. L., Jiang S. P., Duan X. P., Wang W. H., et al. (2022). The effect of high-dietary K(+) (HK) on Kir4.1/Kir5.1 and ROMK in the distal convoluted tubule (DCT) is not affected by gender and Cl(-) content of the diet. Front. Physiol. 13, 1039029. doi:10.3389/fphys.2022.1039029
Moore J. P. (2024). Interoceptive signals from the heart and coronary circulation in health and disease. Auton. Neurosci. 253, 103180. doi:10.1016/j.autneu.2024.103180
Murthy S. E. (2023). Deciphering mechanically activated ion channels at the single-channel level in dorsal root ganglion neurons. J. Gen. Physiol. 155 (6), e202213099. doi:10.1085/jgp.202213099
Nilius B., Honoré E. (2012). Sensing pressure with ion channels. Trends Neurosci. 35 (8), 477–486. doi:10.1016/j.tins.2012.04.002
Orfali R., Albanyan N. (2023). Ca(2+)-Sensitive potassium channels. Molecules 28 (2), 885. doi:10.3390/molecules28020885
Park S. J., Zides C. G., Beyak M. J. (2023). Mechanical activation of vagal afferents involves opposing cation and TREK1 currents and NO regulation. Can. J. Physiol. Pharmacol. 101 (10), 521–528. doi:10.1139/cjpp-2022-0345
Pauziene N., Ranceviene D., Rysevaite-Kyguoliene K., Inokaitis H., Saburkina I., Plekhanova K., et al. (2023). Comparative analysis of intracardiac neural structures in the aged rats with essential hypertension. Anat. Rec. Hob. 306 (9), 2313–2332. doi:10.1002/ar.25109
Pickny L., Hindermann M., Ditting T., Hilgers K. F., Linz P., Ott C., et al. (2023). Myocardial infarction with a preserved ejection fraction-the impaired function of the cardio-renal baroreflex. Front. physiology 14, 1144620. doi:10.3389/fphys.2023.1144620
Sackin H. (1995a). Stretch-activated ion channels. Kidney Int. 48 (4), 1134–1147. doi:10.1038/ki.1995.397
Sackin H. (1995b). Mechanosensitive channels. AnnuRevPhysiol 57, 333–353. doi:10.1146/annurev.ph.57.030195.002001
Sakamoto Y., Ishijima M., Kaneko H., Kurebayashi N., Ichikawa N., Futami I., et al. (2010). Distinct mechanosensitive Ca2+ influx mechanisms in human primary synovial fibroblasts. J. Orthop. Res. 28 (7), 859–864. doi:10.1002/jor.21080
Sharma R. V., Chapleau M. W., Hajduczok G., Wachtel R. E., Waite L. J., Bhalla R. C., et al. (1995). Mechanical stimulation increases intracellular calcium concentration in nodose sensory neurons. Neuroscience 66 (2), 433–441. doi:10.1016/0306-4522(94)00560-r
Shoemaker R. L., Worrell R. T. (1991). Ca2(+)-sensitive K+ channel in aortic smooth muscle of rats. Proc. Soc. Exp. Biol. Med. 196 (3), 325–332. doi:10.3181/00379727-196-43196
Steffensen I., Bates W. R., Morris C. E. (1991). Embryogenesis in the presence of Blockers of Mechanosensitive ion channels: (embryogenesis/mechanosensitive ion channels/channel blockers/Xenopus/ascidians). Dev. Growth and Differ. 33 (5), 437–442. doi:10.1111/j.1440-169X.1991.00437.x
Su X., Wachtel R. E., Gebhart G. F. (2000). Mechanosensitive potassium channels in rat colon sensory neurons. JNeurophysiol 84 (2), 836–843. doi:10.1152/jn.2000.84.2.836
Tang J. M., Wang J., Quandt F. N., Eisenberg R. S. (1990). Perfusing pipettes. Pflugers Arch. 416 (3), 347–350. doi:10.1007/BF00392072
van Weperen V. Y. H., Vaseghi M. (2023). Cardiac vagal afferent neurotransmission in health and disease: review and knowledge gaps. Front. Neurosci. 17, 1192188. doi:10.3389/fnins.2023.1192188
Vasylyev D. V., Zhao P., Schulman B. R., Waxman S. G. (2024). Interplay of Nav1.8 and Nav1.7 channels drives neuronal hyperexcitability in neuropathic pain. J. Gen. Physiol. 156 (11), e202413596. doi:10.1085/jgp.202413596
Veelken R., Sawin L. L., DiBona G. F. (1990). Epicardial serotonin receptors in circulatory control in conscious Sprague-Dawley rats. Am. J. Physiology 258, H466–H472. doi:10.1152/ajpheart.1990.258.2.H466
Veelken R., Stetter A., Dickel T., Hilgers K. F. (2003). Bimodality of cardiac vagal afferent C-fibres in the rat. Pflugers Arch. 446 (5), 516–522. doi:10.1007/s00424-003-1078-z
Wang Y., Xiao B. (2018). The mechanosensitive Piezo1 channel: structural features and molecular bases underlying its ion permeation and mechanotransduction. J. Physiol. 596 (6), 969–978. doi:10.1113/JP274404
Webster S., Brynn R., Poole K. (2025). Evaluating the roles of ion channels in cellular force sensing. J. Cell Sci. 138 (15), jcs264038. doi:10.1242/jcs.264038
Wu J., Lewis A. H., Grandl J. (2017). Touch, tension, and transduction - the function and regulation of piezo ion channels. Trends Biochem. Sci. 42 (1), 57–71. doi:10.1016/j.tibs.2016.09.004
Zeng W. Z., Marshall K. L., Min S., Daou I., Chapleau M. W., Abboud F. M., et al. (2018). PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science 362 (6413), 464–467. doi:10.1126/science.aau6324
Zhang H., Walcott G. P., Rogers J. M. (2018). Effects of gadolinium on cardiac mechanosensitivity in whole isolated swine hearts. Sci. Rep. 8 (1), 10506. doi:10.1038/s41598-018-28743-w
Keywords: mechanosensitive potassium channels, cardiac baroreflex, vagal afferent, nodose ganglion, neuron
Citation: Linz P, Hutter E, Ditting T, Schiffer M, Amann K, Hilgers KF, Veelken R and Rodionova K (2025) Mechanosensitive potassium channels in neurons projecting cardiac axons of the nodose ganglion in rats. Front. Physiol. 16:1644488. doi: 10.3389/fphys.2025.1644488
Received: 10 June 2025; Accepted: 12 September 2025;
Published: 08 October 2025.
Edited by:
Eduardo Colombari, Departamento de Fisiologia e Patologia da Faculdade de Odontologia da Universidade Estadual Paulista, BrazilReviewed by:
Ana Campos-Ríos, University of Vigo, SpainKerly Shamyra Da Silva-Alves, State University of Ceará, Brazil
Copyright © 2025 Linz, Hutter, Ditting, Schiffer, Amann, Hilgers, Veelken and Rodionova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roland Veelken, cm9sYW5kLnZlZWxrZW5AdWstZXJsYW5nZW4uZGU=
†Grant-in-aid from the Deutsche Forschungsgemeinschaft (248553778)
‡Interdisciplinary Center for Clinical Research (IZKF) at the University Hospital of the University of Erlangen-Nuremberg (Project F6)
 Peter Linz
Peter Linz Eva Hutter1
Eva Hutter1 Mario Schiffer
Mario Schiffer Kerstin Amann
Kerstin Amann Karl F. Hilgers
Karl F. Hilgers Roland Veelken
Roland Veelken