- 1Biorobotics and Biomechanics Lab, Department of Mechanical Engineering, University of Maine, Orono, ME, United States
- 2School of Physical Therapy, Husson University, Bangor, ME, United States
Purpose: Age-related gait impairments are strongly associated with increased fall risk, disability, and mortality. While traditional rehabilitation focuses on the lower limbs, arm movements play a key role in stabilizing gait through interlimb neural coupling. This study investigates whether rhythmic haptic cueing of arm swing, which enhances gait, affects interlimb neuromuscular coordination in older adults.
Methods: Seventeen older adults (mean age = 73.2
Results: Rhythmic Cueing significantly increased arm ROM and gait speed compared to Baseline walking, with improvements comparable to Fast walking. Overall upper–lower limb coherence increased in the alpha and beta bands during Cueing compared to Baseline, with Cueing also exceeding Fast in the alpha band. In specific muscle pairings, significant alpha-band effects were observed in contralateral shoulder–leg pairs, specifically between the left anterior deltoid and right rectus femoris, and between the left posterior deltoid and right biceps femoris. Directionality analysis revealed dominant zero-lag coherence, reflecting shared subcortical and cortical drive in the alpha and beta/gamma bands, respectively, and greater forward-lag coherence during Cueing compared to Baseline, indicating enhanced cortical arm-to-leg influence.
Significance: These findings demonstrate that externally cued arm swing can modulate gait performance and potentially interlimb neural coupling, activating both subcortical and cortical pathways. Rhythmic haptic cueing shows promise as an intervention for older adults, supporting its potential integration into home-based gait rehabilitation programs.
1 Introduction
Gait impairments are a significant concern in the aging population, as slow or unstable walking is strongly associated with an increased risk of falls, disability, and even mortality (Nonnekes et al., 2025; Montero-Odasso et al., 2022; Verghese et al., 2006). Recent studies confirm that even small declines in gait speed or stability predict higher rates of hospitalization and loss of independence (Nonnekes et al., 2025; Montero-Odasso et al., 2022; Hardy et al., 2007). A combination of musculoskeletal, sensory, and neural changes causes these impairments. Age-related neurodegeneration, including early changes that precede Alzheimer’s disease, is now recognized as a key contributor to gait decline even in the absence of overt cognitive symptoms (Ali et al., 2025; Allali et al., 2016). Age-related reductions in interlimb coordination and balance are well documented, with older adults exhibiting greater instability and slower recovery after perturbations, reflecting diminished neural control and adaptability (Krasovsky et al., 2012; Krasovsky et al., 2014; Bruijn et al., 2010; Van Hoornweder et al., 2022; Rezaei et al., 2024; Noghani et al., 2025).
Traditional rehabilitation approaches primarily target the legs, but given the growing evidence that arm movements play a significant role in walking, integrating arm swing into gait rehabilitation warrants further investigation. Arm swing is not merely a passive result of trunk rotation; rather, it actively contributes to rhythm regulation, energy conservation, and body stabilization during gait (Meyns et al., 2013; Punt et al., 2015; Bruijn et al., 2010). Restricting arm swing has been shown to increase instability (Bloom and Hejrati, 2021), whereas enhancing it can improve gait stability and coordination, potentially reducing the risk of falls (Krasovsky et al., 2012; Lamontagne and Fung, 2004; Bruijn et al., 2010; Thompson et al., 2017).
Recent work shows that training interventions such as wearable devices can be used to drive arm swing with rhythmic haptic cues (Noghani et al., 2023). Our previous study using a wearable haptic cueing system to shorten arm swing cycle time (CT) by 20%, led to a 30.2% increase in arm range of motion (ROM) and an 18.2% increase in walking speed in older adults (Khiyara et al., 2025). These results suggest that cueing the arms can improve gait speed, symmetry, and perceived balance and coordination in older adults.
While these findings demonstrate the potential for external cueing to enhance arm swing and overall gait performance, the underlying neural mechanisms that mediate these improvements remain unclear. In particular, it is not yet known whether changes in arm movement directly influence leg motion through neural pathways or if both are modulated by a shared central drive. To investigate this, electromyography (EMG) can be used to explore the directionality and coordination of neural signals between the limbs during locomotion.
To better understand these potential neural pathways, directional coherence analysis of EMG signals offers a valuable tool. This technique not only assesses the frequency of muscle activation patterns but also identifies the directionality of neural influence between limbs. Specifically, coherence can be decomposed into three components: zero-lag coherence, indicating shared input; forward-lag coherence, suggesting arm-to-leg influence; and reverse-lag coherence, indicating leg-to-arm influence (Halliday, 2015; Weersink et al., 2021a). This partitioning allows researchers to determine whether limb coordination arises from a common neural source or reflects directional drive between arms and legs. For example, Weersink et al. (2021a) demonstrated that during gait, forward-lag coherence from arm to leg muscles reflects top-down cortical influence, suggesting a potential mechanism by which arm movement could modulate leg activity during walking.
While our previous study (Khiyara et al., 2025) demonstrated the benefits of arm swing training in older adults in terms of key spatiotemporal gait parameters, the current study aims to investigate the underlying neural mechanisms that may explain these improvements. Prior work has shown that arm swing is neurally coupled with leg movement during walking, involving shared cortical and subcortical control pathways, and that gait-related arm swing can drive lower limb muscles, as demonstrated by significant alpha and beta/gamma intermuscular coherence between upper and lower limbs (Weersink et al., 2021a). Alpha-band coherence, in particular, is often linked to subcortical rhythmic control and may be enhanced through synchronized arm swing driven by rhythmic cueing. Moreover, challenging walking tasks have been reported to increase intermuscular coherence (Da Silva Costa et al., 2024; Hüche Larsen et al., 2024). For instance, beta-band intermuscular coherence increased during more complex balance tasks such as beam walking (da Silva Costa et al., 2024; De Freitas et al., 2025). Similarly, proprioceptively challenging or proactive locomotor conditions are associated with increased EMG-EMG coherence, indicating augmented functional coupling under heightened task demands (De Freitas et al., 2025; Da Silva Costa et al., 2024). In addition, Kerkman et al. (2020) found that intermuscular coherence in the 4–22 Hz range increases during 1:1 arm–leg coordination (Kerkman et al., 2020), a pattern associated with faster walking speeds. While increased task difficulty has been associated with elevated intermuscular coherence, the specific effect of walking speed alone remains unclear. Faster walking in older adults may itself represent a more demanding task, potentially recruiting additional neural resources that support enhanced interlimb coupling.
We therefore propose that a wearable haptic cueing system which can increase arm swing rhythm and amplitude, as well as promote faster walking may influence interlimb neural coordination, potentially enhancing intermuscular coherence, particularly in the alpha (8–15 Hz) and beta (15–30 Hz) frequency bands. We hypothesize that both rhythmic cueing and fast walking can enhance intermuscular coherence and forward-lag coherence (arm
2 Methods
2.1 Participants
The data were collected during the human subject experiment previously reported in our study (Khiyara et al., 2025). Here, we analyzed the data of seventeen community-dwelling older adults (6 males/11 females; mean ± standard deviation, age: 73.2 ± 6.0 years; range: 65–92 years; height: 168.7 ± 8.9 cm; mass: 73.1 ± 18.5 kg) who self-reported being right-handed. Participants were required to be able to walk independently for at least 20 min continuously to meet the inclusion criteria. Exclusion criteria included self-reported conditions affecting gait, muscle function, such as peripheral neuropathy, Parkinson’s disease, cerebral palsy, multiple sclerosis, and stroke, reported via an online screening questionnaire. All procedures were approved by the University of Maine Institutional Review Board (IRB 2019-04-15). Written informed consent was obtained from all participants, and data were anonymized to ensure confidentiality.
2.2 Experimental protocol
Each participant completed four walking conditions on an indoor standard 200-m track: Baseline, Fast, and two trials with haptic cueing as previously described (Khiyara et al., 2025). The Baseline and Fast conditions were performed without any cueing. In the Baseline condition, participants walked at their self-selected comfortable pace. In the Fast condition, participants walked at their fastest comfortable pace without running. These two walking conditions were conducted on a straight 60-m segment of the track. The Cueing condition utilized a wearable vibrotactile system to deliver bilateral haptic cues, aimed at modulating the timing of arm swing. While there were two Cueing conditions, one to reduce and the other to increase arm swing cycle time (CT), here we focus on the condition to reduce CT by 20%. This condition aimed to increase the frequency of arm swing (i.e., equivalent to shortening the CT) and thereby increase walking speed in the older-adult participants. Participants were instructed to synchronize peak shoulder flexion with the onset of vibration on that arm. The Cueing condition was performed over a full 200-m lap around the indoor track, and the participants were familiarized with the rhythmic cueing on their arms before the experimental trial.
2.3 Data collection
As shown in Figure 1a, each participant’s arm was equipped with a haptic cueing unit secured with Velcro straps on the lateral side of each brachium, positioned midway between the shoulder and elbow. The location was chosen because it sits on soft tissue, avoids joints, and allows the device to remain comfortable and stable during walking, consistent with previous work (Noghani et al., 2023; Khiyara et al., 2025). Each haptic electronic unit consisted of an ESP8266 microcontroller, a battery, and a custom circuit board, which was connected to a haptic cell containing three vibrotactors. The haptic cell was oriented toward the front of the arm to deliver cues aligned with the forward motion of arm swing (Figure 1a). The vibrotactors vibrated at 240 Hz, a frequency that falls within the optimal response range of Pacinian mechanoreceptors, making the vibrations easily detectable and producing a clear tactile sensation (Noghani et al., 2021; Noghani et al., 2023; Khiyara et al., 2025; Sharafian et al., 2025). Each vibration cue lasted 100 ms, a duration previously shown to produce a distinct and easily perceived sensation during walking (Noghani et al., 2021; Noghani et al., 2023; Khiyara et al., 2025). An Inertial Measurement Unit (IMU) mounted on each arm recorded arm swing CT and arm range of motion (ROM). Additional IMUs, embedded in custom 3D-printed heel clips, were placed on the participants’ shoes to capture gait events such as heel strikes and toe-offs. Surface Electromyography (sEMG) sensors were placed on eight muscles (Figure 1b): the shoulder muscles [anterior (AD) and posterior deltoid (PD)] and the upper leg muscles [biceps femoris (BF), and rectus femoris (RF)] of both the left and right limbs, following SENIAM guidelines for electrode positioning (Hermens et al., 1999) and Delsys guidelines for sensor placement (Delsys Inc, 2025; D. Inc, 2023). These specific muscles were selected based on a prior work by Weersink et al. (2021a), who conducted intermuscular coherence analysis during gait between bilateral shoulder muscles (AD and PD) and both proximal and distal lower-limb muscles in healthy participants. They reported that the highest coherence values were observed between shoulder muscles and proximal leg muscles, specifically the BF and RF.
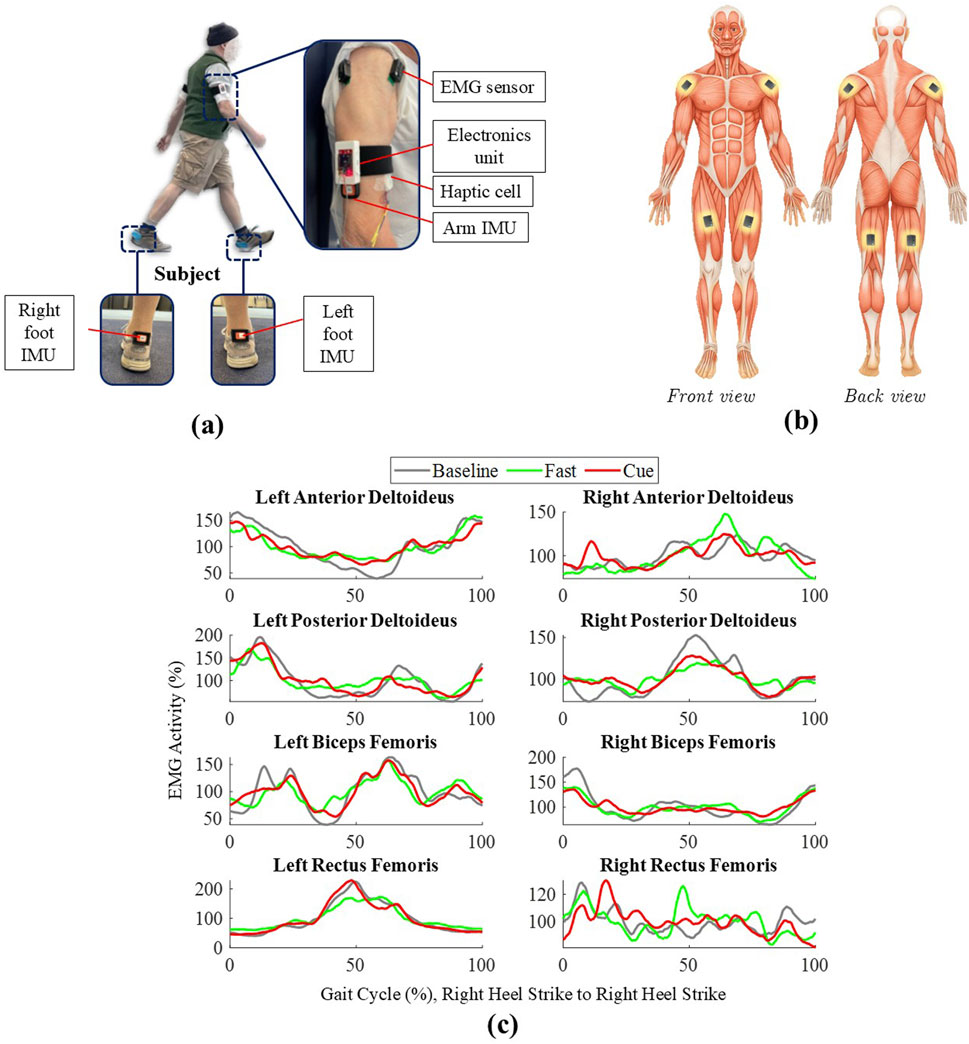
Figure 1. (a) Participant with the haptic cueing system, including arm sEMG/IMU sensors, electronics unit, haptic cell, and foot IMUs. (b) Schematic of EMG sensor placement on AD, PD, RF, and BF (bilaterally). (c) Grand-averaged EMG envelopes over one normalized gait cycle, all segmented by the right heel strike (RHS, 0%–100%) for Baseline, Fast, and Cueing conditions.
The rhythmic haptic cues were controlled by a custom Android application (Noghani et al., 2021; Noghani et al., 2023) that received real-time foot IMU data at 60 Hz and detected heel strikes based on sagittal foot angle trajectories. The application managed vibrotactor activation by calculating a delay
where
Coefficients
sEMG signals were recorded at a sampling rate of 1926 Hz using the Trigno Wireless Biofeedback System by D. Inc. (2023) with the manufacturer’s built-in 20–450 Hz band-pass filter. Although this setting attenuates sub-20 Hz power, coherence remains mathematically preserved when the same linear time-invariant filter is applied to both channels used in analysis; therefore,
2.4 Spatiotemporal and kinematic data processing
Kinematic parameters, including walking speed, arm ROM, and arm ROM symmetry ratio, were calculated from the IMU data recorded on the arms and feet. Sagittal plane angles of the upper limbs were derived from the arms’ IMUs. Arm ROM was computed as the angular difference between maximum shoulder flexion and extension within a gait cycle. Arm ROM symmetry was calculated as the ratio of right arm ROM divided by left arm ROM, where values greater than 1 indicate a larger right arm ROM, values less than 1 indicate a larger left arm ROM, and values closer to 1 indicate greater symmetry (Khiyara et al., 2025). Walking speed was calculated by integrating linear acceleration data from foot-mounted IMUs using a zero-velocity update algorithm (Dadashi et al., 2013; Hossain et al., 2023). To ensure analysis of steady-state walking, the first 10 gait cycles of each walking condition were discarded, and the subsequent 30 consecutive gait cycles were analyzed (Khiyara et al., 2025; Noghani et al., 2023).
2.5 Preprocessing EMG data
The preprocessing for intermuscular coherence and directionality analysis closely followed the process described by Weersink et al. (2021a), with adjustments to account for different experimental conditions and the implementation of algorithms using MATLAB 2024b (MathWorks Inc. 2024). The raw sEMG signals were first high-pass filtered at 5 Hz using a finite impulse response (FIR) filter. The FIR filters were selected for their stability and linear phase response, which preserves the temporal structure of the signal (Litwin, 2000). MATLAB’s designfilt and filtfilt functions were used to implement the filter and ensure zero-phase distortion. This process effectively removed the low-frequency noise, including baseline drift and movement artifacts commonly introduced during gait. Following filtering, the sEMG signals were rectified using full-wave rectification, which converted all negative values to positive (Konrad, 2005; Weersink et al., 2021a). The filtered and rectified signals were segmented into individual gait cycles based on right heel strikes identified from the Xsens IMU data, consistent with prior EMG studies that used right-heel-strike segmentation (Kim et al., 2016; Kwon et al., 2023; Weersink et al., 2021a). The first 10 steps of each condition were excluded to ensure the analysis of steady-state walking, and 30 gait cycles were analyzed for each participant (Sharafian et al., 2025; Khiyara et al., 2025). For consistency, the 30 analyzed gait cycles during the cueing condition were extracted from the same straight 60-m portion of the 200-m track as used in the Baseline and Fast conditions.
The duration of each gait cycle was calculated, and the sEMG data were time-warped using linear interpolation to align each cycle to the individual’s average gait cycle duration. The time-warped sEMG envelopes were normalized by expressing them as a percentage of the mean activity of the individual within each condition (Weersink et al., 2021a). The normalized sEMG envelopes were smoothed using a moving average filter with a 10-ms window to further refine the signal. This preprocessing pipeline, which included time-warping, normalization, and smoothing, was applied separately for each condition, exclusively for visualization purposes, to generate the grand-averaged EMG envelopes shown in Figure 1c. For intermuscular coherence analysis, only the sEMG signals high-pass filtered at 5 Hz and rectified were used (Weersink et al., 2021a; 2022a). The signals were segmented into individual gait cycles based on right heel strikes (Weersink et al., 2021a); no time-warping was applied, and all original gait cycle durations were retained to avoid frequency distortion. Time-warping to 0%–100% of the gait cycle was applied only after the spectral analysis, strictly for coherence heatmap visualization.
2.6 Coherence and directionality analysis
Time-dependent intermuscular coherence was computed using a sliding-window Fourier analysis, following the framework of Halliday et al. (1995) as also implemented in more recent gait studies (Weersink et al., 2021a; Weersink et al., 2022a). The sEMG signals from the upper and lower limbs were segmented with a 200 ms window sliding in 50 ms steps across each gait cycle. This produced a series of time offsets (i.e., the window positions) relative to the heel-strike event. For each offset, auto-spectral densities
This procedure yields a time-dependent coherence spectrum at each offset,
For each participant, coherence was computed individually and later pooled across participants to derive group-level coherence estimates. Coherence estimates were averaged over gait cycles and time offsets within each condition. Following the approach by Halliday et al. (Halliday et al., 1995) and Weersink et al. (Weersink et al., 2021a), significant coherence values
To assess the directionality of coupling, we applied the non-parametric decomposition method (Halliday, 2015), which separates coherence into forward, reverse, and zero-lag components. First, the sEMG signals were pre-whitened to remove autocorrelation structure while preserving their coherence. Each signal’s Fourier transform was divided by its amplitude spectrum (i.e., the square root of its autospectrum), yielding whitened processes with flat unit spectra
The resulting time-domain function,
These components reflect direction-specific coherence: forward-lag indicates upper-limb influence on lower limb, reverse-lag indicates lower-limb influence on upper limb, and zero-lag reflects common input. Directional coherence was computed for each participant and condition, and the results were used to quantify task-specific modulation of interlimb neural coupling. All computations were implemented in MATLAB 2024a.
2.7 Statistical analysis
Walking speed, arm ROM, and arm ROM ratio were analyzed using linear mixed-effects models in SPSS v29 (IBM Corp., Armonk, NY, USA), similar to the statistical approach used in our previous work (Khiyara et al., 2025). Walking condition (Baseline, Fast, Cueing) was included as a fixed main effect, with participant ID as a random effect to account for repeated measurements within participants. Gender was modeled as a fixed factor, and age and BMI were included as covariates. BMI was calculated from measured height and body mass using the standard formula: body mass (kg) divided by height squared (m2). Two-way interaction terms between gender, age, and BMI were included in the models to assess potential moderating effects on gait outcomes. Post hoc pairwise comparisons between walking conditions were performed with Bonferroni-adjusted confidence intervals (CI) to control for multiple comparisons. All statistical analyses were conducted at a significance level of
3 Results
3.1 Gait speed and arm swing metrics
Figure 2 shows group means and standard deviations for walking speed, arm ROM, and arm ROM ratio across the Baseline, Fast, and Cueing conditions. The pairwise comparison results are displayed in Figure 2, in the form of asterisks for the
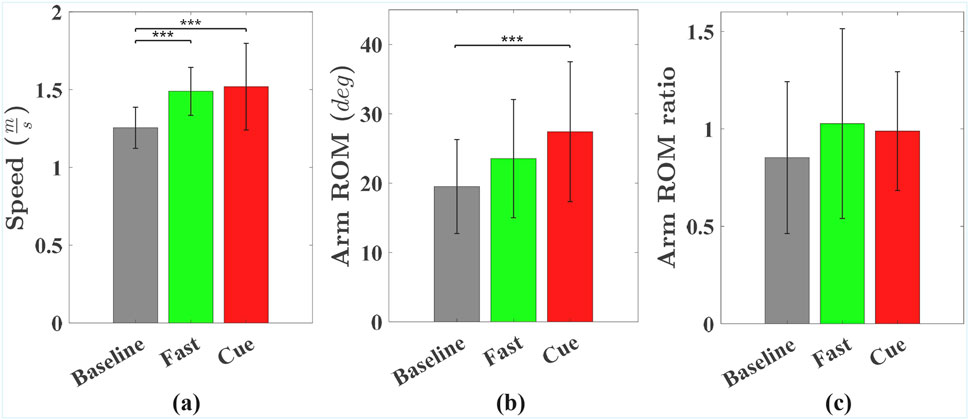
Figure 2. Group means and standard deviations for (a) gait speed, (b) arm ROM, and (c) arm ROM ratio across Baseline, Fast, and Cueing conditions. Significant pairwise differences are indicated by asterisks: *
As shown in Figure 2b, arm ROM was significantly affected by walking condition (
Finally, as shown in Figure 2c, there was a trend indicating that walking condition may have affected the arm ROM ratio (
3.2 Intermuscular coherence
Time-dependent intermuscular coherence was computed between the anterior deltoid (AD) and posterior deltoid (PD) muscles (left and right), as well as the bilateral biceps femoris (BF) and rectus femoris (RF) muscles. Figures 3, 4 show time–frequency coherence heatmaps for the Baseline, Fast, and Cueing walking conditions. Coherence is plotted across the normalized gait cycle percentage.
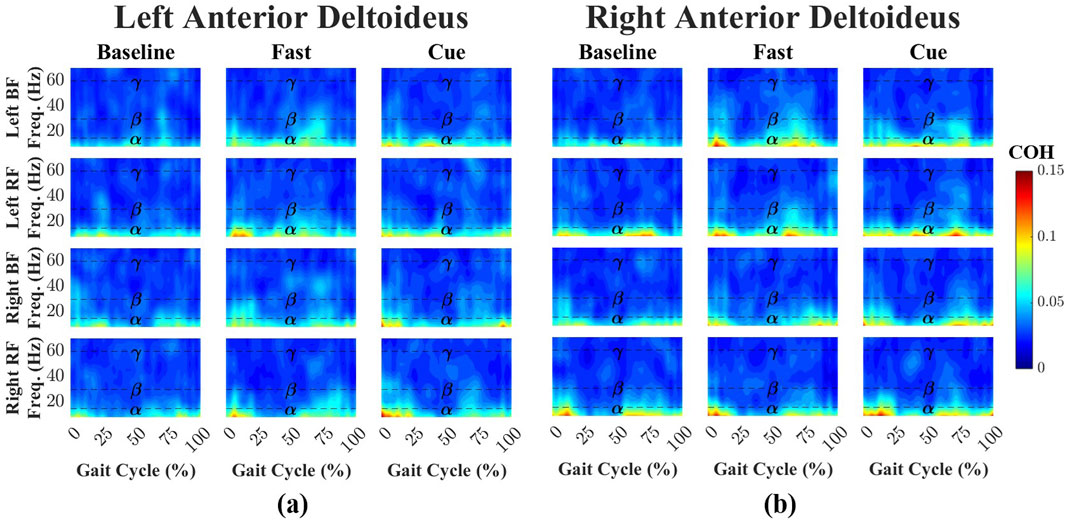
Figure 3. Time–frequency coherence heatmaps between the left (a) and right (b) AD and bilateral lower-limb muscles (BF and RF) across three walking conditions: Baseline, Fast, and Cueing. Coherence is plotted over the normalized gait cycle based on the right heel strike (x-axis) and frequency range 0–70 Hz (y-axis). Frequency bands are denoted by dashed lines:
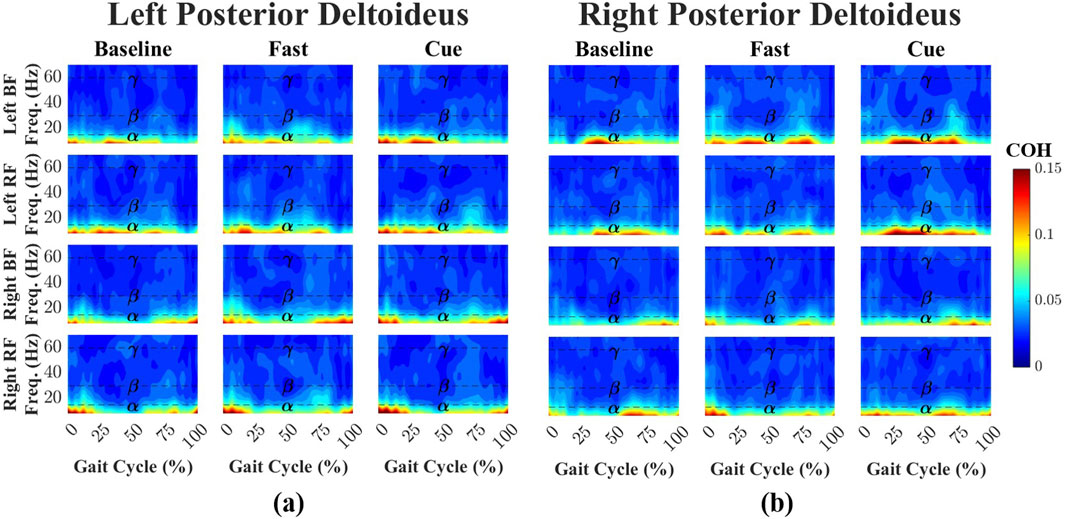
Figure 4. Time–frequency coherence heatmaps between the left (a) and right (b) PD and bilateral lower-limb muscles across Baseline, Fast, and Cueing conditions. Coherence is plotted over the normalized gait cycle (x-axis) and frequency range 0–70 Hz (y-axis). Frequency bands are denoted by dashed lines:
For the left AD-leg muscle pairs (Figure 3a), coherence during Baseline walking was primarily confined to the
For the left PD-leg muscle pairs (Figure 4a), coherence during Baseline walking was dominated by the
Across all conditions and frequency bands, PD–leg coherence values were consistently higher than AD–leg coherence, although none of these differences reached statistical significance. This trend was most apparent in the
Finally, as shown in Figure 5, when coherence values across all upper–lower limb muscle pairs were analyzed using Friedman’s ANOVA, significant effects of walking condition were found in all three frequency bands. In the
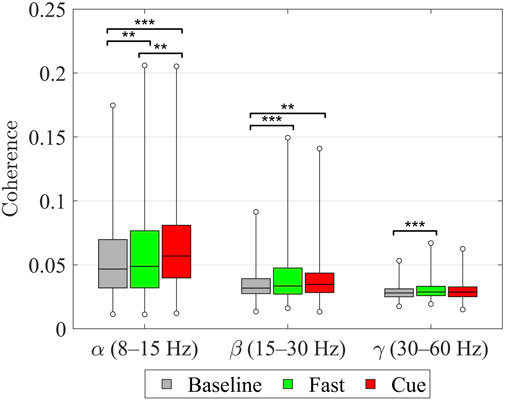
Figure 5. Intermuscular coherence between upper and lower limb muscle pairs within the
3.3 Directionality coherence
To determine the direction of neural influence, coherence was decomposed into forward-lag (
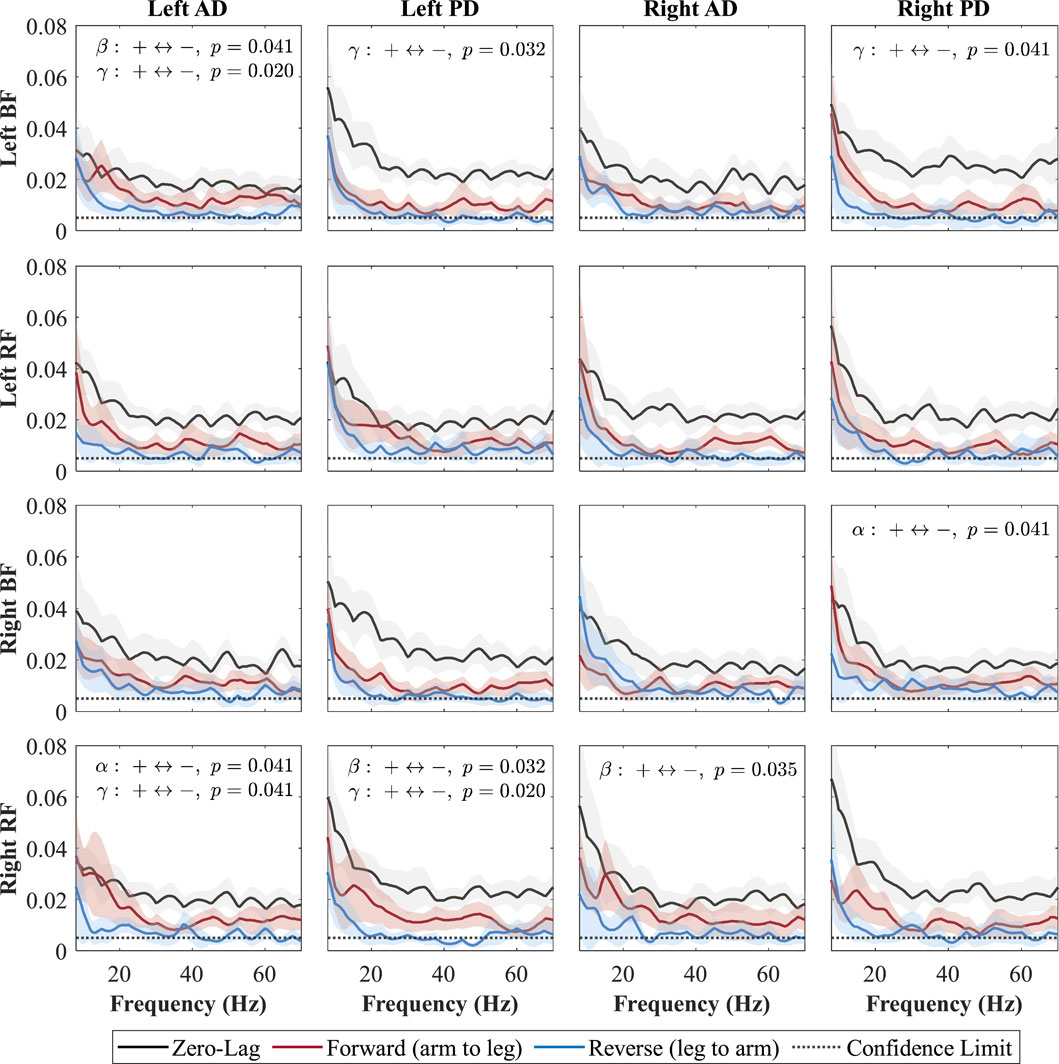
Figure 6. Directional coherence spectra for Baseline between upper- and lower-limb muscles. Subplots (4
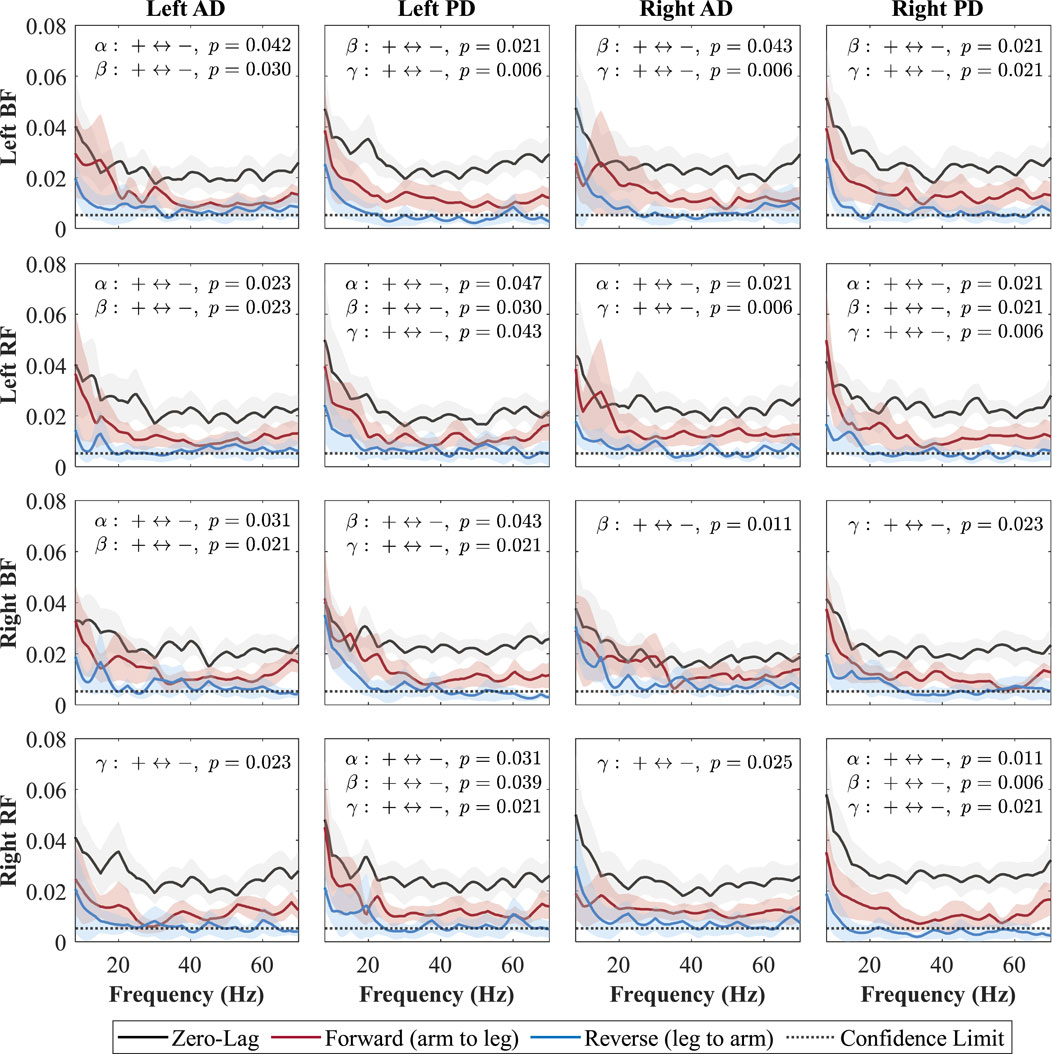
Figure 7. Directional coherence spectra for Fast between upper- and lower-limb muscles. Subplots (4
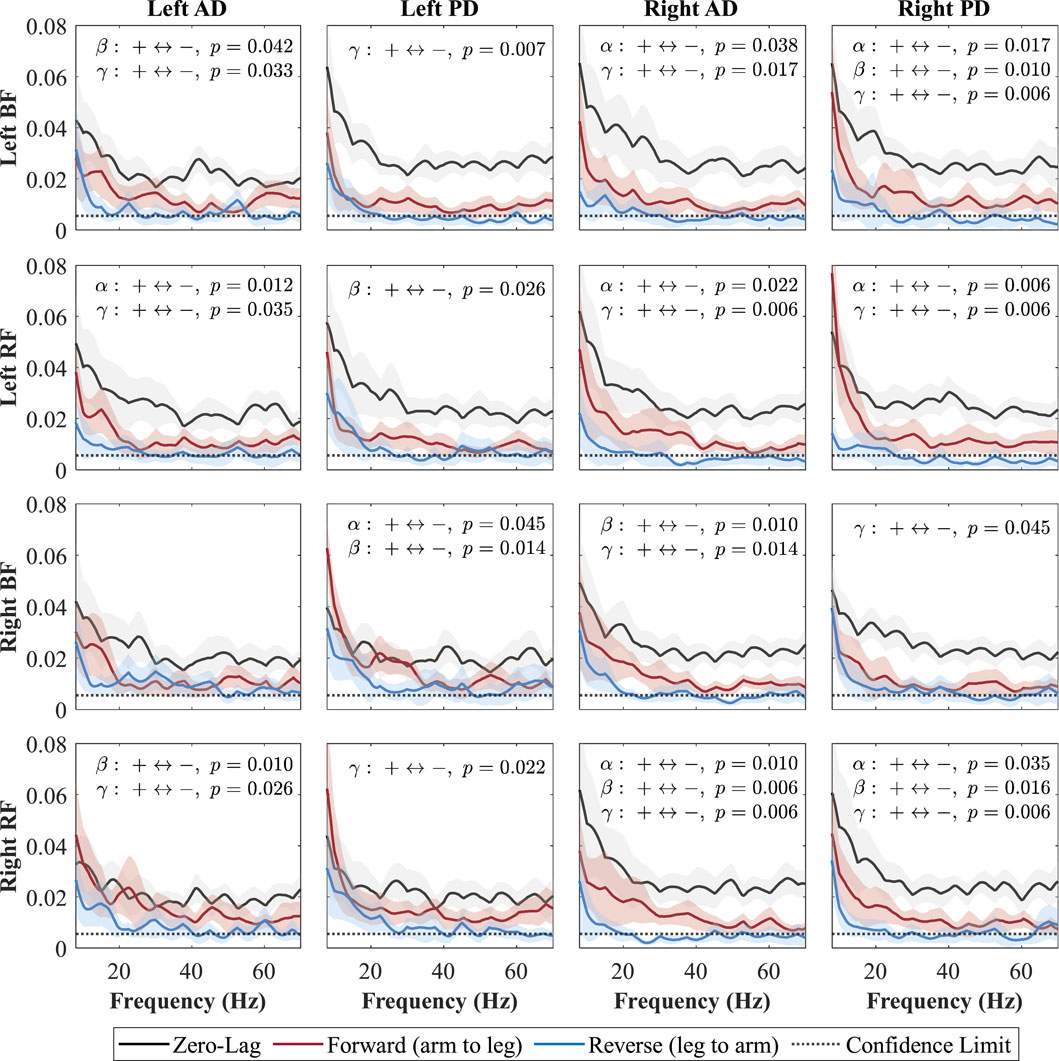
Figure 8. Directional coherence spectra for Cueing between upper- and lower-limb muscles. Subplots (4
Each subplot shows a muscle pair with zero-lag (0, black line; shared neural drive), forward-lag (+, red line; arm-to-leg influence), and reverse-lag (–, blue line; leg-to-arm influence) coherence components. Shaded areas denote 95% CIs and the dotted horizontal line marks significance/confidence limit. Consistent with Weersink et al. (Weersink et al., 2021a), only the FDR-corrected Wilcoxon
During the Baseline condition (Figure 6), zero-lag coherence was present in every shoulder–leg combination, confirming a strong shared drive. After FDR correction, the forward-lag component exceeded the reverse-lag component in several specific pairs, indicating a top-down arm
In the Fast condition (Figure 7), forward-lag coherence consistently exceeded reverse-lag coherence for all shoulder–leg combinations, with significant effects occurring in at least one of the frequency bands (alpha, beta, or gamma) for each pair. In the Cueing condition (Figure 8), a larger number of shoulder–leg pairs reached significance in the forward-versus reverse-lag comparison compared to Baseline, further reflecting a strengthened top-down influence during rhythmic haptic cueing, which was used to synchronize arm swing to a target rhythm designed to increase walking speed and arm ROM. Significant forward-lag coherence was especially prevalent in the gamma band, with multiple ipsilateral and contralateral muscle pairings surpassing the confidence limit and showing statistical significance after correction. As shown in Figure 8, the detailed
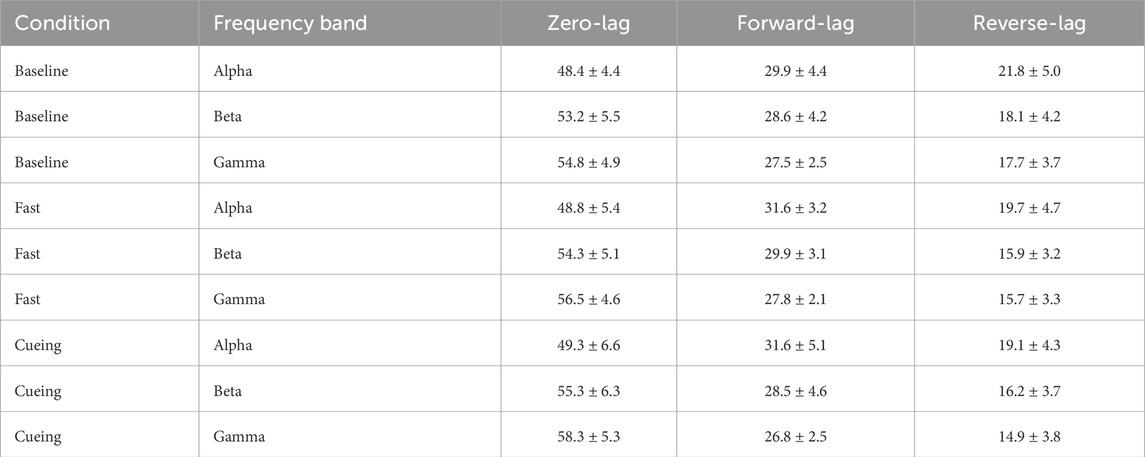
For every subject–pair combination, the AUC of the zero-, forward-, and reverse-lag spectra was integrated within each frequency band. Each AUC was then expressed as a percentage of the sum of the three components. These percentages were averaged across participants within a muscle pair and subsequently across all the pairs; the resulting grand means and standard deviations are reported in Table 1.
Across all conditions, zero-lag coherence accounted for approximately half of the total coherence, indicating that interlimb coupling was primarily driven by a common (zero-lag) input. Forward-lag coherence was consistently greater than reverse-lag coherence, reflecting a predominant arm
Building on the pair-level averages in Table 1; Figure 9 presents box plots including all participants’ data in each frequency across the conditions. For each participant, coherence values were averaged across all shoulder–leg pairs to provide an overall upper-versus lower-limb value in each frequency band and condition. Then, the data of all participants were used to calculate the box plots for each frequency band for each condition. Across all conditions, the separation between forward- and reverse-lag coherence was evident across all frequency bands. In Baseline, forward- and reverse-lag coherence were the closest, differing by less than 10% across all frequency bands. In contrast, both Fast and Cueing showed a larger separation between forward and reverse components in every band, indicating a clearer predominance of arm
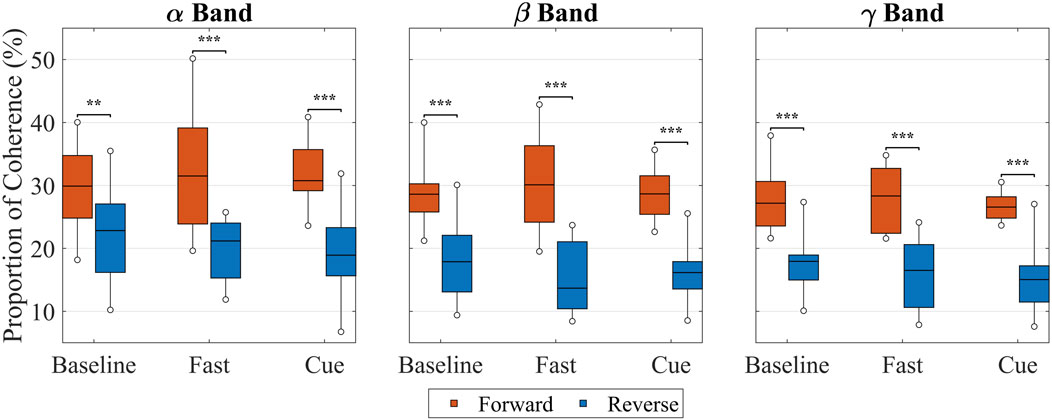
Figure 9. Proportion of forward (red) and reverse (blue) intermuscular coherence in the
4 Discussion
4.1 Overview of findings
This study demonstrates that rhythmic haptic cueing of arm swing significantly influences gait performance and enhances neuromuscular coordination in older adults. Specifically, the Cueing condition, in which arm swing cycle time was reduced, resulted in a significant increase in arm range of motion (ROM) and a corresponding significant improvement in gait speed compared to Baseline. A similar trend was observed in the Fast condition, where gait speed increased significantly, though increases in arm ROM did not reach significance. Although improvements in arm ROM ratio were not statistically significant at the group level, individual-level enhancements were observed. These behavioral improvements coincided with increased intermuscular coherence in the alpha and beta bands, and a notable presence of gamma-band coherence in directionality analysis in the forward-lag (arm to leg) component. Together, these results suggest that the cueing intervention activated both subcortical and cortical pathways to enhance interlimb coordination.
4.2 Interpretation of gait metrics
The significant increase in gait speed observed in the Cueing condition reinforces the hypothesis that modulating arm swing can influence lower-limb output through interlimb coupling, as reported previously (Khiyara et al., 2025). While Fast walking also increased gait speed, the Cueing condition achieved comparable improvements without consciously trying to walk faster, highlighting the potential of externally driven arm swing training for older adults with reduced mobility. The significant 40.6% increase in arm ROM during Cueing compared to Baseline, along with a 16.5% increase relative to Fast (not significant), suggests that rhythmic cueing can elicit greater upper-limb engagement than both normal and voluntary fast walking. Previous studies have highlighted the importance of arm swing amplitude in enhancing trunk stability and reducing energy expenditure during gait (Ortega et al., 2008; Meyns et al., 2013). Our findings are consistent with this body of research, supporting the view that increased arm swing amplitude contributes to both biomechanical and neuromuscular efficiency (Huang and Ferris, 2004; Umberger, 2008). Although changes in arm ROM ratio did not reach statistical significance at the group level
4.3 Intermuscular coherence
Intermuscular coherence is a neurophysiological measure that quantifies the correlation in the frequency domain between EMG signals recorded from two distinct muscles, reflecting the common neural input shared between their motor unit pools (Dos Santos et al., 2020; De Freitas et al., 2025; Weersink, 2021). This shared neural input may originate from cortical, subcortical, or spinal pathways that collectively coordinate muscle activation during rhythmic tasks such as walking (Farmer et al., 1993; Grosse et al., 2004; Weersink et al., 2021a; De Freitas et al., 2025). Intermuscular coherence is typically stronger between muscle pairs with close anatomical and functional relationships, making it a valuable tool for exploring neural circuitry involved in motor control and detecting impairments in these pathways (Farina et al., 2004).
The present coherence results indicate that both normal walking (Baseline and Fast) and rhythmic haptic cueing engage multiple levels of the central nervous system. At the group level (Figure 5), walking condition significantly modulated intermuscular coherence between upper and lower limbs in all bands: in the alpha-band, coherence increased stepwise (Fast
The beta-band increases observed in both Fast and Cue compared to Baseline are consistent with previous findings that more challenging or coordinated walking tasks increase EMG–EMG coherence. These beta-band effects likely reflect added corticospinal contributions for sensorimotor integration and adaptive control under higher demand and tighter timing constraints (De Freitas et al., 2025; Da Silva Costa et al., 2024; Kerkman et al., 2020). Similarly, Kerkman et al. (2020) reported increased intermuscular coherence in the alpha and lower beta bands during 1:1 arm–leg coordination. In our study, Fast walking likely promoted this natural 1:1 coupling, as typically seen at higher speeds (Tester et al., 2012), whereas the Cueing system enforced it by delivering rhythmic arm vibrations in synchrony with the contralateral heel strike (Noghani et al., 2021; Noghani et al., 2023; Khiyara et al., 2025). This precise synchronization likely strengthened the interlimb coupling that contributed to the observed increases in alpha- and low beta-band coherence.
The significant, though visually small, gamma increase with Fast (but not Cue) suggests that high-frequency neural coupling is more influenced by the effort and focus needed for faster walking than by the timing effects of external cueing. Gamma-band coherence is typically observed during rapid or changing movements and is thought to reflect brief, task-specific bursts of corticospinal drive (Mima et al., 2000; Brown et al., 1998; Borhanazad et al., 2024). The greater gamma coherence in the Fast condition likely reflects the additional corticospinal engagement required for rapid, voluntary gait adjustments. For older adults, this heightened neural demand may have contributed to the greater physical and cognitive effort of walking faster without cueing, whereas rhythmic cueing may have reduced reliance on such high-frequency drive by shifting control toward alpha-band mechanisms, thereby lowering the perceived difficulty. This interpretation aligns with prior work showing that greater task difficulty is associated with increased higher-band coherence (Da Silva Costa et al., 2024; De Freitas et al., 2025).
Overall, these band-specific changes suggest a distributed control framework in which spinal and brainstem circuits (including CPGs and reticulospinal pathways) provide rhythmic patterning and interlimb coupling, while corticospinal contributions scale with the demands of speed and external timing, consistent with established evidence of arm–leg coupling during gait (Weersink et al., 2021a; Kerkman et al., 2020; De Freitas et al., 2025).
When looking at specific muscle pairs (Supplementary Figures S1–S3), significant changes clustered in contralateral (diagonal) shoulder–leg combinations: Left AD–Right RF (
Across all conditions, the posterior deltoid (PD) muscle showed a consistent (but non-significant) trend toward higher alpha-band coherence with proximal leg muscles compared to the anterior deltoid (AD) muscle. This contrasts with findings from Weersink et al. (2021a); Weersink (2021), who showed comparable coherence values between AD and PD with proximal leg muscles in older adults, suggesting a similar level of neural coupling. Our results instead suggest a preferential coupling between the PD and leg muscles, particularly in the alpha-band range, which may reflect greater reliance on subcortical pathways. Given the biomechanical role in shoulder extension during the backward swing phase of gait and its function as a postural stabilizer (Pontzer et al., 2009; Barthelemy and Nielsen, 2010; La Scaleia et al., 2014), the stronger coherence with leg muscles may reflect enhanced interlimb coordination via reticulospinal pathways. This supports the interpretation of our results that the PD contributes more substantially than the AD to rhythmic interlimb coordination during steady-state walking. Given that the present AD–PD contrasts did not reach significance (
4.4 Directional coherence
The decomposition of coherence into directional components revealed distinct differences in neural influence between the Baseline, Fast, and Cueing conditions. A prominent zero-lag component was consistently observed across all shoulder–leg muscle pairs, suggesting a shared presynaptic drive likely originating from both subcortical sources (e.g., reticulospinal pathways) and cortical contributions (Weersink et al., 2021a). This pattern supports the role of common neural input, possibly stemming from the CPGs, reticulospinal pathways, and cortical (corticospinal) contributions, in coordinating rhythmic upper and lower-limb activity during gait. Because this zero-lag component reflects shared rather than directional influence, no statistical comparisons were performed on it, and the analysis instead focused on the directional forward- and reverse-lag components, following the approach of Weersink et al. (Weersink et al., 2021a).
During the Baseline condition (Figure 6), forward-lag coherence (arm
Both increasing walking speed and applying rhythmic vibration cueing enhanced forward-lag coherence (arm
In the Cueing condition, forward-lag coherence was also greater than reverse-lag coherence for nearly all muscle pairs, with only one pair (left AD–right BF) not reaching significance. Compared to Baseline, Cueing increased the number of significant pairs and produced effects in the alpha, beta, and gamma band. This pattern likely reflects the combined contributions of bilateral subcortical drive, providing a shared rhythmic signal between arms and legs, and phase-specific cortical adjustments that fine-tune muscle activation during the gait cycle (Charalambous and Hadjipapas, 2022; Weersink et al., 2021a; Grosse and Brown, 2003; Conway et al., 1995; Salenius et al., 1997; Fisher et al., 2012). The vibration-based rhythm may help synchronize arm swing more precisely, reinforcing the shared rhythmic drive while enhancing the accuracy of swing-phase.
Across both Fast and Cueing conditions, forward-lag coherence increased in the alpha, beta, and gamma bands, indicating the parallel involvement of complementary descending pathways. This pattern suggests that increasing walking speed or applying rhythmic haptic cueing strengthens top-down arm-to-leg drive by simultaneously enhancing subcortical pathways (alpha-band coherence) and cortical pathways (beta-/gamma-band coherence). The alpha-band coherence is associated with bilateral, automatic drive from corticoreticulospinal and reticulospinal projections (Charalambous and Hadjipapas, 2022; Weersink et al., 2021a) and may also reflect coordinated output from spinal CPGs that link cervical and lumbar locomotor networks via propriospinal connections (Zehr and Duysens, 2004; Dietz, 2003; Klarner and Zehr, 2018). Beta-/gamma-band coherence reflects corticospinal contributions for phase-specific, goal-directed control (Petersen et al., 2012; Fisher et al., 2012; Lemon, 2008). These pathways work together during walking, with alpha-band and CPG-related drives providing a bilateral framework for rhythmic coordination, and beta/gamma-band activity refining movement precision. Weersink et al. (2021a) reported this coexistence of alpha and beta coherence during normal walking. In our study, this pattern was present at Baseline but was amplified in the Fast and Cueing conditions, with greater magnitude and broader spatial distribution, suggesting that increasing walking speed or providing rhythmic arm swing stimulation can enhance both the shared rhythmic drive and the precise cortical modulation that together coordinate upper–lower limb interactions.
Reverse-lag coherence did not statistically exceed forward-lag coherence in any pair or frequency band in either condition. This asymmetry supports the interpretation of a predominantly descending neural influence from the arm to leg muscles. Weersink et al. (2021a) similarly found no cases where reverse-lag coherence significantly exceeded forward-lag coherence and reported comparable forward and reverse coherence in some muscle combinations during normal walking—consistent with our Baseline results. Our results build on these findings by showing that speed and externally modulated arm swing increase forward-directed coherence. These results reinforce the notion that upper-limb motion is not merely a passive component of gait but can actively drive lower-limb activity through distributed neural circuits involving both the brainstem and motor cortex (Weersink et al., 2021a).
4.5 Relationship between gait metrics and intermuscular coherence
To place these neural effects in behavioral context, cueing elicited the largest increase in arm swing, 40.6% relative to Baseline and an additional 16.5% relative to Fast, and the largest increase in alpha-band intermuscular coherence, consistent with stronger sensory inputs that modulates spinal locomotor circuits (Dietz, 2003; Zehr and Duysens, 2004; Weersink et al., 2021a). Both Fast and Cueing conditions increased arm range of motion, which paralleled increases in forward-lag coherence, indicating a stronger top-down influence from arms to legs. Both conditions also increased gait speed and beta-band coherence, whereas only the Fast condition increased gamma-band coherence, suggesting that high-frequency coupling relates more to the effort of voluntary speeding than to speed alone (De Freitas et al., 2025; Da Silva Costa et al., 2024; Kerkman et al., 2020). Overall, cueing may alter the mechanism by which speed is achieved, favoring subcortical/spinal common drive (higher
4.6 Clinical and rehabilitation implications
The directional coherence findings carry important implications for gait rehabilitation in older adults and individuals with neurological disorders such as Parkinson’s disease. Cueing that reduces arm swing CT can enhance interlimb neural coupling and increase gait speed without requiring conscious control of walking speed, making it a promising intervention for individuals with fatigue or impaired motor control. Previous studies have shown that passive or externally guided arm swing can improve gait initiation and coordination in individuals with Parkinsonian gait (Weersink et al., 2020; Weersink et al., 2021b; Weersink et al., 2022a; Weersink et al., 2022b; Kawashima et al., 2008). Moreover, the observed increases in beta- and gamma-band coherence suggest that rhythmic cueing may engage cortical pathways and promote neuroplasticity. These findings raise the possibility that long-term cueing interventions could yield lasting improvements in motor control, an area that warrants further investigation in future research.
This dual engagement of subcortical and cortical pathways has important implications for rehabilitation strategies. Rhythmic cueing interventions may be effective precisely because they engage multiple levels of the motor hierarchy simultaneously, potentially facilitating both immediate motor adjustments and longer-term motor learning. In clinical populations with impaired cortical function, the preservation of subcortical responses to rhythmic cues may provide an alternative pathway for motor rehabilitation.
4.7 Limitations and future directions
Despite the promising findings, several limitations should be acknowledged. The sample size (N = 17) could limit statistical power, especially for detecting subtle effects in arm swing symmetry. The participants only underwent a short period of walking, completing one lap around the track (200 m) while receiving haptic stimuli during the Cueing condition. In the present study, we focused on upper and proximal lower limb muscles (AD, PD, BF, RF), consistent with prior work by Weersink et al. (2021a), Weersink et al. (2022b), which reported the strongest coherence in these upper–lower limb muscle pairs. This selection of proximal lower-limb muscles was further based on studies employing arm–thigh kinematic coupling to investigate interlimb coordination during gait (Hejrati et al., 2017; Carpinella et al., 2010). However, future work should also examine distal lower-limb muscles, such as the medial gastrocnemius (MG) and tibialis anterior (TA), to provide a more comprehensive view of gait control. Moreover, while EMG analysis offers indirect evidence of neural coupling, it cannot localize specific neural generators. Integrating EEG and EMG could provide greater specificity in identifying cortical contributions. Additionally, this study focused on the acute effects of rhythmic cueing; future work should evaluate the long-term effects of such cueing on gait parameters, neuromuscular coordination, and fall risk. Exploring individual differences in responsiveness to cueing may also support the development of adaptive, personalized gait training systems.
It is worth noting that the distinction between subcortical and cortical contributions based solely on frequency bands should be interpreted with caution. While alpha-band activity is predominantly subcortical, and beta/gamma activity is predominantly cortical, there is likely considerable overlap and interaction between these systems that may not be fully captured by frequency-domain analysis alone.
5 Conclusion
Rhythmic haptic cueing that targets arm swing cycle time enhances arm range of motion, gait speed, and interlimb neural coupling in older adults. These behavioral and neural improvements support the incorporation of upper limb-focused cueing systems into gait rehabilitation protocols. While our previous study (Khiyara et al., 2025) demonstrated the benefit of arm swing training in older adults in terms of key spatiotemporal gait parameters, the current study investigated the underlying neural mechanism to explain those improvements. The observed increase in alpha- and beta-band coherence and forward-lag directional influence highlights the active role of the arms in driving coordinated lower-limb activity, advancing our understanding of interlimb neural dynamics during walking. Collectively, these results support the hypothesis that rhythmic arm movement actively contributes to gait coordination through coupled subcortical and corticospinal mechanisms. Targeting this interlimb coupling through rhythmic arm cueing offers a promising rehabilitation strategy for improving gait performance and stability, particularly in older adults and individuals with gait impairments.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The University of Maine Institutional Review Board (IRB 2019-04-15). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
IK: Formal analysis, Investigation, Data curation, Writing – review and editing, Writing – original draft, Conceptualization, Visualization, Methodology, Software. BS: Writing – review and editing. BH: Writing – review and editing, Conceptualization, Funding acquisition, Supervision, Project administration, Resources, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Science Foundation under Grant 2145177.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2025.1657092/full#supplementary-material
References
Ali F., Syrjanen J. A., Figdore D. J., Kremers W. K., Mielke M. M., Jack C. R., et al. (2025). Association of plasma biomarkers of alzheimer’s pathology and neurodegeneration with gait performance in older adults. Commun. Med. 5, 19. doi:10.1038/s43856-024-00713-6
Allali G., Ayers E. I., Verghese J. (2016). Motoric cognitive risk syndrome subtypes and cognitive profiles. Journals Gerontology Ser. A Biomed. Sci. Med. Sci. 71, 378–384. doi:10.1093/gerona/glv092
Baker M. R., Baker S. N. (2003). The effect of diazepam on motor cortical oscillations and corticomuscular coherence studied in man. J. physiology 546, 931–942. doi:10.1113/jphysiol.2002.029553
Barthelemy D., Nielsen J. B. (2010). Corticospinal contribution to arm muscle activity during human walking. J. physiology 588, 967–979. doi:10.1113/jphysiol.2009.185520
Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 57, 289–300. doi:10.1111/j.2517-6161.1995.tb02031.x
Benjamini Y., Yekutieli D. (2001). The control of the false discovery rate in multiple testing under dependency. Ann. statistics 29, 1165–1188. doi:10.1214/aos/1013699998
Bloom J., Hejrati B. (2021). The effects of forearm movements on human gait during walking with various self-selected speeds. Hum. Mov. Sci. 79, 102835. doi:10.1016/j.humov.2021.102835
Borhanazad M., van Wijk B. C., Buizer A. I., Kerkman J. N., Bekius A., Dominici N., et al. (2024). Lateralized modulation of cortical beta power during human gait is related to arm swing. Iscience 27, 110301. doi:10.1016/j.isci.2024.110301
Brown P., Salenius S., Rothwell J. C., Hari R. (1998). Cortical correlate of the piper rhythm in humans. J. neurophysiology 80, 2911–2917. doi:10.1152/jn.1998.80.6.2911
Bruijn S. M., Meijer O. G., Beek P. J., Van Dieen J. H. (2010). The effects of arm swing on human gait stability. J. Exp. Biol. 213, 3945–3952. doi:10.1242/jeb.045112
Carpinella I., Crenna P., Rabuffetti M., Ferrarin M. (2010). Coordination between upper-and lower-limb movements is different during overground and treadmill walking. Eur. J. Appl. physiology 108, 71–82. doi:10.1007/s00421-009-1168-5
Charalambous C. C., Hadjipapas A. (2022). Is there frequency-specificity in the motor control of walking? the putative differential role of alpha and beta oscillations. Front. Syst. Neurosci. 16, 922841. doi:10.3389/fnsys.2022.922841
Chen Y.-T., Li S., Magat E., Zhou P., Li S. (2018). Motor overflow and spasticity in chronic stroke share a common pathophysiological process: analysis of within-limb and between-limb emg-emg coherence. Front. neurology 9, 795. doi:10.3389/fneur.2018.00795
Chen M., Lu Z., Zhou P. (2022). A dilemma for coherence calculation: should preprocessing filters be applied? Front. Neurosci. 16, 838627. doi:10.3389/fnins.2022.838627
Conway B., Halliday D., Farmer S., Shahani U., Maas P., Weir A., et al. (1995). Synchronization between motor cortex and spinal motoneuronal pool during the performance of a maintained motor task in man. J. physiology 489, 917–924. doi:10.1113/jphysiol.1995.sp021104
D. Inc (2023). Trigno wireless Biofeedback system user’s guide. Natick, MA, USA.Version MAN-031-1-7, MP1135H
Da Silva Costa A. A., Moraes R., den Otter R., Gennaro F., Bakker L., Dos Santos P. C. R., et al. (2024). Corticomuscular and intermuscular coherence as a function of age and walking balance difficulty. Neurobiol. Aging 141, 85–101. doi:10.1016/j.neurobiolaging.2024.05.004
Dadashi F., Mariani B., Rochat S., Büla C. J., Santos-Eggimann B., Aminian K. (2013). Gait and foot clearance parameters obtained using shoe-worn inertial sensors in a large-population sample of older adults. Sensors 14, 443–457. doi:10.3390/s140100443
De Freitas S., Dal Maso F., Fradet L., Arvisais D., Cherni Y. (2025). Age and task-dependent modulations in emg-emg coherence during gait: a scoping review. Preprint Repository Name [Preprint]. Available online at: https://persistent-url (Accessed March 15, 2025).
Dietz V. (2003). Spinal cord pattern generators for locomotion. Clin. Neurophysiol. 114, 1379–1389. doi:10.1016/s1388-2457(03)00120-2
Dos Santos P. C. R., Lamoth C. J., Barbieri F. A., Zijdewind I., Gobbi L. T. B., Hortobágyi T. (2020). Age-specific modulation of intermuscular beta coherence during gait before and after experimentally induced fatigue. Sci. Rep. 10, 15854. doi:10.1038/s41598-020-72839-1
Farina D., Merletti R., Enoka R. M. (2004). The extraction of neural strategies from the surface emg. J. Appl. physiology 96, 1486–1495. doi:10.1152/japplphysiol.01070.2003
Farmer S., Bremner F., Halliday D., Rosenberg J., Stephens J. (1993). The frequency content of common synaptic inputs to motoneurones studied during voluntary isometric contraction in man. J. physiology 470, 127–155. doi:10.1113/jphysiol.1993.sp019851
Fisher K. M., Zaaimi B., Williams T. L., Baker S. N., Baker M. R. (2012). Beta-band intermuscular coherence: a novel biomarker of upper motor neuron dysfunction in motor neuron disease. Brain 135, 2849–2864. doi:10.1093/brain/aws150
Grosse P., Brown P. (2003). Acoustic startle evokes bilaterally synchronous oscillatory emg activity in the healthy human. J. neurophysiology 90, 1654–1661. doi:10.1152/jn.00125.2003
Grosse P., Edwards M., Tijssen M., Schrag A., Lees A. J., Bhatia K., et al. (2004). Patterns of emg–emg coherence in limb dystonia. Mov. Disord. 19, 758–769. doi:10.1002/mds.20075
Halliday D. M. (2015). Nonparametric directionality measures for time series and point process data. J. Integr. Neurosci. 14, 253–277. doi:10.1142/S0219635215300127
Halliday D., Rosenberg J., Amjad A., Breeze P., Conway B., Farmer S. (1995). A framework for the analysis of mixed time series/point process data—theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog. biophysics Mol. Biol. 64, 237–278. doi:10.1016/s0079-6107(96)00009-0
Halliday D., Conway B., Christensen L., Hansen N., Petersen N., Nielsen J. (2003). Functional coupling of motor units is modulated during walking in human subjects. J. neurophysiology 89, 960–968. doi:10.1152/jn.00844.2002
Hardy S. E., Perera S., Roumani Y. F., Chandler J. M., Studenski S. A. (2007). Improvement in usual gait speed predicts better survival in older adults. J. Am. Geriatrics Soc. 55, 1727–1734. doi:10.1111/j.1532-5415.2007.01413.x
Hejrati B., Chesebrough S., Foreman K. B., Abbott J. J., Merryweather A. S. (2016). Comprehensive quantitative investigation of arm swing during walking at various speed and surface slope conditions. Hum. Mov. Sci. 49, 104–115. doi:10.1016/j.humov.2016.06.001
Hejrati B., Merryweather A. S., Abbott J. J. (2017). Generating arm-swing trajectories in real-time using a data-driven model for gait rehabilitation with self-selected speed. IEEE Trans. Neural Syst. Rehabilitation Eng. 26, 115–124. doi:10.1109/TNSRE.2017.2740060
Hermens H. J., Freriks B., Disselhorst-Klug C., Rau G. (1999). SENIAM: European recommendations for surface electromyography. Tech. Rep.
Hossain M. T., Noghani M. A., Sidaway B., Hejrati B. (2023). Investigating the efficacy of a tactile feedback system to increase the gait speed of older adults. Hum. Mov. Sci. 90, 103103. doi:10.1016/j.humov.2023.103103
Huang H. J., Ferris D. P. (2004). Neural coupling between upper and lower limbs during recumbent stepping. J. Appl. Physiology 97, 1299–1308. doi:10.1152/japplphysiol.01350.2003
Hüche Larsen H., Justiniano M. D., Frisk R. F., Lundbye-Jensen J., Farmer S. F., Nielsen J. B. (2024). Task difficulty of visually guided gait modifications involves differences in central drive to spinal motor neurons. J. Neurophysiology 132, 1126–1141. doi:10.1152/jn.00466.2023
Kawashima N., Nozaki D., Abe M. O., Nakazawa K. (2008). Shaping appropriate locomotive motor output through interlimb neural pathway within spinal cord in humans. J. neurophysiology 99, 2946–2955. doi:10.1152/jn.00020.2008
Kerkman J. N., Bekius A., Boonstra T. W., Daffertshofer A., Dominici N. (2020). Muscle synergies and coherence networks reflect different modes of coordination during walking. Front. physiology 11, 751. doi:10.3389/fphys.2020.00751
Khiyara I., Sidaway B., Hejrati B. (2025). Utilizing rhythmic haptic cueing in arm swing training to improve gait speed among older adults. Ann. Biomed. Eng. 53, 855–866. doi:10.1007/s10439-024-03669-9
Kim Y., Bulea T. C., Damiano D. L. (2016). Novel methods to enhance precision and reliability in muscle synergy identification during walking. Front. Hum. Neurosci. 10, 455. doi:10.3389/fnhum.2016.00455
Klarner T., Zehr E. P. (2018). Sherlock holmes and the curious case of the human locomotor central pattern generator. J. neurophysiology 120, 53–77. doi:10.1152/jn.00554.2017
Krasovsky T., Baniña M. C., Hacmon R., Feldman A. G., Lamontagne A., Levin M. F. (2012). Stability of gait and interlimb coordination in older adults. J. Neurophysiology 107, 2560–2569. doi:10.1152/jn.00950.2011
Krasovsky T., Lamontagne A., Feldman A. G., Levin M. F. (2014). Effects of walking speed on gait stability and interlimb coordination in younger and older adults. Gait and posture 39, 378–385. doi:10.1016/j.gaitpost.2013.08.011
Kwon Y., Chilton L. K., Kim H., Franz J. R. (2023). The effect of prolonged walking on leg muscle activity patterns and vulnerability to perturbations. J. Electromyogr. Kinesiol. 73, 102836. doi:10.1016/j.jelekin.2023.102836
La Scaleia V., Sylos-Labini F., Hoellinger T., Wang L., Cheron G., Lacquaniti F., et al. (2014). Control of leg movements driven by emg activity of shoulder muscles. Front. Hum. Neurosci. 8, 838. doi:10.3389/fnhum.2014.00838
Lamontagne A., Fung J. (2004). Faster is better: implications for speed-intensive gait training after stroke. Stroke 35, 2543–2548. doi:10.1161/01.STR.0000144685.88760.d7
Lemon R. N. (2008). Descending pathways in motor control. Annu. Rev. Neurosci. 31, 195–218. doi:10.1146/annurev.neuro.31.060407.125547
Meyns P., Bruijn S. M., Duysens J. (2013). The how and why of arm swing during human walking. Gait and posture 38, 555–562. doi:10.1016/j.gaitpost.2013.02.006
Mima T., Steger J., Schulman A. E., Gerloff C., Hallett M. (2000). Electroencephalographic measurement of motor cortex control of muscle activity in humans. Clin. Neurophysiol. 111, 326–337. doi:10.1016/s1388-2457(99)00229-1
Montero-Odasso M., van der Velde N., Martin F. C., Petrovic M., Tan M. P., Ryg J., et al. (2022). World guidelines for falls prevention and management for older adults: a global initiative. Age ageing 51, afac205. doi:10.1093/ageing/afac205
Noghani M. A., Shahinpoor M., Hejrati B. (2021). Design and validation of a smartphone-based haptic feedback system for gait training. IEEE Robotics Automation Lett. 6, 6593–6600. doi:10.1109/lra.2021.3094502
Noghani M. A., Hossain M. T., Hejrati B. (2023). Modulation of arm swing frequency and gait using rhythmic tactile feedback. IEEE Trans. Neural Syst. Rehabilitation Eng. 31, 1542–1553. doi:10.1109/TNSRE.2023.3249628
Noghani M. A., Sharafian E., Sidaway B., Hejrati B. (2025). Increasing thigh extension with haptic feedback affects leg coordination in young and older adult walkers. J. Biomechanics 181, 112525. doi:10.1016/j.jbiomech.2025.112525
Nonnekes J., Post E., Imbalzano G., Bloem B. R. (2025). Gait changes with aging: an early warning sign for underlying pathology. J. Neurology 272, 257. doi:10.1007/s00415-025-12995-4
Ortega J. D., Fehlman L. A., Farley C. T. (2008). Effects of aging and arm swing on the metabolic cost of stability in human walking. J. biomechanics 41, 3303–3308. doi:10.1016/j.jbiomech.2008.06.039
Petersen T. H., Willerslev-Olsen M., Conway B. A., Nielsen J. B. (2012). The motor cortex drives the muscles during walking in human subjects. J. physiology 590, 2443–2452. doi:10.1113/jphysiol.2012.227397
Pontzer H., Holloway 4th J. H., Raichlen D. A., Lieberman D. E. (2009). Control and function of arm swing in human walking and running. J. Exp. Biol. 212, 523–534. doi:10.1242/jeb.024927
Punt M., Bruijn S. M., Wittink H., van Dieën J. H. (2015). Effect of arm swing strategy on local dynamic stability of human gait. Gait and posture 41, 504–509. doi:10.1016/j.gaitpost.2014.12.002
Rezaei A., Bhat S. G., Cheng C.-H., Pignolo R. J., Lu L., Kaufman K. R. (2024). Age-related changes in gait, balance, and strength parameters: a cross-sectional study. Plos one 19, e0310764. doi:10.1371/journal.pone.0310764
Salenius S., Portin K., Kajola M., Salmelin R., Hari R. (1997). Cortical control of human motoneuron firing during isometric contraction. J. neurophysiology 77, 3401–3405. doi:10.1152/jn.1997.77.6.3401
Sharafian M. E., Ellis C., Sidaway B., Hayes M., Hejrati B. (2025). The effects of real-time haptic feedback on gait and cognitive load in older adults. IEEE Trans. Neural Syst. Rehabilitation Eng. 33, 2335–2344. doi:10.1109/TNSRE.2025.3578865
Tester N. J., Barbeau H., Howland D. R., Cantrell A., Behrman A. L. (2012). Arm and leg coordination during treadmill walking in individuals with motor incomplete spinal cord injury: a preliminary study. Gait and posture 36, 49–55. doi:10.1016/j.gaitpost.2012.01.004
Thompson E., Agada P., Wright W. G., Reimann H., Jeka J. (2017). Spatiotemporal gait changes with use of an arm swing cueing device in people with Parkinson’s disease. Gait and posture 58, 46–51. doi:10.1016/j.gaitpost.2017.07.001
Umberger B. R. (2008). Effects of suppressing arm swing on kinematics, kinetics, and energetics of human walking. J. biomechanics 41, 2575–2580. doi:10.1016/j.jbiomech.2008.05.024
van Asseldonk E. H., Campfens S. F., Verwer S. J., van Putten M. J., Stegeman D. F. (2014). Reliability and agreement of intramuscular coherence in tibialis anterior muscle. PLoS One 9, e88428. doi:10.1371/journal.pone.0088428
Van Hoornweder S., Mora D. A. B., Depestele S., Frieske J., van Dun K., Cuypers K., et al. (2022). Age and interlimb coordination complexity modulate oscillatory spectral dynamics and large-scale functional connectivity. Neuroscience 496, 1–15. doi:10.1016/j.neuroscience.2022.06.008
Verghese J., LeValley A., Hall C. B., Katz M. J., Ambrose A. F., Lipton R. B. (2006). Epidemiology of gait disorders in community-residing older adults. J. Am. Geriatrics Soc. 54, 255–261. doi:10.1111/j.1532-5415.2005.00580.x
Weersink J. (2021). Arm swing in healthy and Parkinsonian gait: explorations on brain, muscle and movement level. Groningen, Netherlands: University of Groningen. doi:10.33612/diss.190721932
Weersink J. B., Gefferie S. R., van Laar T., Maurits N. M., de Jong B. M. (2020). Pre-movement cortico-muscular dynamics underlying improved Parkinson gait initiation after instructed arm swing. J. Parkinson’s Dis. 10, 1675–1693. doi:10.3233/JPD-202112
Weersink J. B., de Jong B. M., Halliday D. M., Maurits N. M. (2021a). Intermuscular coherence analysis in older adults reveals that gait-related arm swing drives lower limb muscles via subcortical and cortical pathways. J. physiology 599, 2283–2298. doi:10.1113/JP281094
Weersink J. B., Maurits N. M., van Laar T., de Jong B. M. (2021b). Enhanced arm swing improves parkinsonian gait with eeg power modulations resembling healthy gait. Park. and Relat. Disord. 91, 96–101. doi:10.1016/j.parkreldis.2021.09.011
Weersink J. B., de Jong B. M., Maurits N. M. (2022a). Neural coupling between upper and lower limb muscles in parkinsonian gait. Clin. Neurophysiol. 134, 65–72. doi:10.1016/j.clinph.2021.11.072
Weersink J. B., Maurits N. M., Halliday D. M., de Jong B. M. (2022b). Time-dependent directional intermuscular coherence analysis reveals that forward and backward arm swing equally drive the upper leg muscles during gait initiation. Gait and posture 92, 290–293. doi:10.1016/j.gaitpost.2021.11.036
Keywords: coordination, interlimb, neural coupling, intermuscular coherence, aging, haptic cueing, arm swing, wearable system
Citation: Khiyara I, Sidaway B and Hejrati B (2025) Modulating arm swing via haptic cueing alters interlimb neural coupling in older adults. Front. Physiol. 16:1657092. doi: 10.3389/fphys.2025.1657092
Received: 30 June 2025; Accepted: 04 September 2025;
Published: 22 September 2025.
Edited by:
Yury Ivanenko, Santa Lucia Foundation (IRCCS), ItalyReviewed by:
Michael James MacLellan, University of Prince Edward Island, CanadaAtsushi Oshima, The University of Tokyo, Japan
Copyright © 2025 Khiyara, Sidaway and Hejrati. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ines Khiyara, aW5lcy5raGl5YXJhQG1haW5lLmVkdQ==
 Ines Khiyara
Ines Khiyara Ben Sidaway
Ben Sidaway Babak Hejrati
Babak Hejrati