- 1Department of Brain and Behavioral Sciences, University of Pavia, Pavia, Italy
- 2Department of Medicine and Health Sciences “V. Tiberio”, University of Molise, Campobasso, Italy
- 3Digital Neuroscience Center, IRCCS Mondino Foundation, Pavia, Italy
Cerebellar Purkinje cells are one of the most complex neurons in the central nervous system and are well known for their extensive dendritic tree dotted by dendritic spines. PC spines receive excitatory synapses from parallel and climbing fibers and, although their morphological properties are comparable to those of other neuronal types, they show distinct extracellular and intracellular regulatory properties. Purkinje cell spine protrusion and helical patterning do not require nearby axons, as e.g., in pyramidal cells. Instead, Purkinje cell spines require structural proteins located on parallel and climbing fibers for their stabilisation and maintenance. The total spine number is influenced by scaffold proteins and eventually reflects the total dendritic length and local spine density. Purkinje cell spines were supposed to range up to over 105 in rodents and 106 in humans, but recent experimental data show that spines are less numerous than initially thought. Instead, they are endowed with mechanisms designed to improve their efficiency and differentiation. Some spines are double-headed, thereby enhancing Purkinje cell responses when the companion parallel fiber is stimulated. Other spines are single-headed and presumably endowed with slow neurotransmission mechanisms. Latest experimental data showed that glial cells modulate spines activity after a task or learning. Eventually, these multiple mechanisms can make each spine crucial in its own way for synaptic pattern recognition. In this review, we present the most recent advancements on Purkinje cell spines spanning their biochemical, structural, and functional properties, both in mice and humans, and propose a recalculation of the effective complement of spines and their activation by parallel fibers.
Introduction
The first description of “protoplasmic processes,” currently called dendrites, was reported by Golgi in 1883, using his newly developed Golgi-method staining (Golgi, 1883; Bentivoglio et al., 2019). Five years later, the first image of cerebellar Purkinje cells (PC) spines was published by Santiago Ramón y Cajal but, until this seminal work, spines were discarded as staining artifacts (Ramón y Cajal, 1888; Yuste, 2015; Defelipe, 2025). Spines are small protrusions of a neuron cell membrane, canonically described with a mushroom-like shape, which can be observed on the dendrites and, sometimes, also on the soma (Lackey et al., 2024). These protrusions are usually elicited by a nearby axon and consolidated through synaptic activity, but there are exceptions to this rule. Spines are quite common in the central nervous system (CNS) and can be found on both excitatory neurons, such as pyramidal neurons (PN) (Davidson et al., 2020), and inhibitory neurons, such as the PC (Lee et al., 2004; Lee et al., 2005), striatal medium spiny neurons (Lanciego et al., 2012), and dopaminergic neurons (Jang et al., 2015). Their primary role is to create a confined space in which a tightly interconnected biochemical machinery can modulate the strength of the postsynaptic responses (Nimchinsky et al., 2002). Cerebellar PCs are inhibitory neurons most known for expressing a huge quantity of spines on their extensive dendritic tree. Their spines retain most of the properties common to other neuronal types, but they also exhibit critical differences. Spines in PN are primarily generated and consolidated through synaptic activity, while in PCs, internal and external control proteins are required. Moreover, the presence of 103–105 spines on PC dendrites brought about issues of how they are generated, maintained, consolidated, silenced, and raised hypotheses on mechanisms that might limit their extensive coding space (Marr, 1969; Albus, 1971). It was proposed that not all spines have a presynaptic partner, that some are silent or covered by glial processes, and that some others generate only slow responses mediated by metabotropic glutamate receptors 1 (mGluR1). Recent experiments showed that the number of spines is lower than previously thought, with 30–50 thousand synapses in mice and 300–500 thousand synapses in humans. However, several spines receive more than one parallel fiber (PF) and glial cells would not silence spines but make them more specific for certain activities. Therefore, PC spines are endowed with complex mechanisms that eventually fine-tune neurotransmission and synaptic plasticity. This review considers the most recent experimental advancements on PC spines and the plethora of proteins that are involved in balancing this delicate system.
Cerebellum: Purkinje and granule cells
The cerebellum is highly convoluted and, in humans, it can account for approximately 80% of the neocortex surface (Sereno et al., 2020). It is divided into 10 lobules, and each lobule is composed of 3 layers, termed granular (GL), molecular (ML) and Purkinje cell layer (PL). The GL contains the tightly packed granule cells (GrC) (Nguyen et al., 2023) and the sparser Golgi (GoC), Lugaro and Unipolar Brush cells, with the latter located primarily in the vestibular lobuli (lobuli IX and X). The PL contains the soma of PCs, the soma of Bergmann glia (BG) and the candelabrum cells (Osorno et al., 2022). The ML contains multiple types of inhibitory interneurons, the PFs, climbing fibers (CF) and the extensive dendritic arborisation of PCs (Figure 1). The ML is the location where the extensive territory occupied by PF synapses intermingles with the territory occupied by a few thousands of CF synapses.
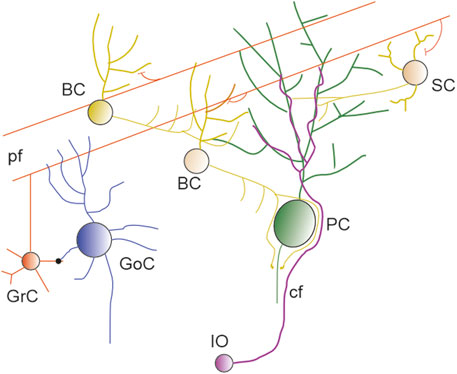
Figure 1. The cerebellar network. Schematics of the circuit showing GrC and PF (red), SC and BC (yellow and orange), PC (green), IO neuron and CF (violet), Golgi cells (blu).
Cerebellar GrCs, in conjunction with PCs, form the conserved primary input/output pathway of the cerebellar network (van der Heijden and Sillitoe, 2021). These neurons evolved millions of years before the first PN appeared in a nervous system and, during their long life, acquired characteristics that differentiate them from other neuronal cell types. One of these differences was recently highlighted by studying the amount of unmethylated DNA contained in their nuclei. This amount was unusually high for neurons, making them closer to glial cells (Tian et al., 2022; Tan et al., 2023).
The Purkinje cell
PCs were described in 1837 by Johannes Evangelista Purkinje (Purkinje, 1837; Zárský, 2012; VoŽeh, 2015), who used the most advanced optical microscope available in the nineteenth century. Unfortunately, the limitations in the staining process restricted his study to the somatic region. Only in 1888, Santiago Ramón y Cajal, using an improved Golgi staining, described PC dendritic tree and spines (Golgi, 1883; Ramón y Cajal, 1888; Yuste, 2015).
It is one of the oldest neurons of the CNS and appeared in cartilaginous fishes 400 million years ago (Hibi et al., 2017; Mokhtar, 2020). These neurons are mostly known for their extensive and elaborated dendritic trees dotted by tens of thousands of spines. Even though not all species exhibit highly intricate dendritic trees (O’Brien and Unwin, 2006), they all have in common the presence of dendritic spines. This means that, over time, certain morphological properties were adapted by the evolution, while others were improved to support complex behaviour. Recent technical advancement showed that PCs are not a single family limited to just Zebrin- and Zebrin+ but there are multiple variants in zebrafish (Magnus et al., 2023) and up to eleven PC subtypes in mice (Khouri-Farah et al., 2025). In humans, the morphological evolution led to up to three distinct trees attached to the same soma (Busch and Hansel, 2023).
The importance of PCs as a computational powerhouse, in connection with the excitatory input transmitted from GrCs and the remainder of the neurons forming the cerebellar network, can be summarised by the wide range of abilities showed by the cerebellum. It is a motor coordination system (movement, balance) (Morton and Bastian, 2004), it is involved in higher cognition (Schmahmann, 2019), fear responses (Liu et al., 2010), language (Desmond and Fiez, 1998), emotion and sociality (Van Overwalle, 2024). Spinocerebellar ataxias (SCAs), which are progressive, degenerative, genetic diseases, are linked to multiple DNA mutations leading to various degrees of PC malfunctioning. In some cases mutations are so extreme to cause PC death through suppression of their intrinsic firing by hyperexcited ML interneurons (SCA1), by a reduction in Cav2.1 calcium (Ca2+) channel activity (SCA3), or a reduction of potassium (K+) channels currents (SCA6) (Hills et al., 2013; Hoxha et al., 2018; Huang and Verbeek, 2019; Egorova et al., 2023; Zhu et al., 2024). The cerebellar involvement in neurodegeneration was reported in Alzheimer’s disease (Mavroudis et al., 2019) and in Parkinsonism, where PC axonal dysfunction contributes to essential tremor (Schmahmann, 2019). Numerous physiological, biochemical and morphological studies of single cerebellar cells have been performed extensively on rodents, both in healthy and diseased conditions. The same approach can be used in humans only in very specific cases for ethical and technical reasons. This limitation can be mitigated by using non-invasive techniques that, although not providing the same quality data as single-cell recordings, can provide information useful to compare mice and humans. Unfortunately, as recently reported, the cerebellum is not taken into consideration in neurodegenerative disorders and is often neglected in multiple imaging studies (Wang et al., 2025).
The granule cell, ascending axon and parallel fibers
Mice GrCs have a compact morphology with just three to six dendrites (Nguyen et al., 2023) and occasional branches (Houston et al., 2017). Some examples of the human GrC were reconstructed from ex vivo tissues, showing only three dendrites and a soma comparable in size with mice (Jacobs et al., 2014). The small dimension of the somato-dendritic sections makes them the most common neuron in the entire CNS (Tan et al., 2023). Their thin axon is split into an ascending axon (AA) followed by two PFs oriented in opposite directions (Palay and Chan-Palay, 1974b; Huang and Huang, 1998; Heck and Sultan, 2002). When an AA enters the ML, it can make synapses with multiple spines of the same PC, with AA/PF ratio in the order of 5%–10% (Lu et al., 2009). Instead, PFs can reach over 3 mm length in rats (Huang and Huang, 1998) and an average of 6.64 mm length in rhesus monkeys (Mugnaini, 1983). These fibers are canonically reported to make a single synaptic contact with a single spine of each PC they encounter. Recently it was shown that a single PF can make two or even more contacts with spines located in different locations of the same dendritic tree (Loschky et al., 2022). Even though PF intersect multiple PCs along their pathway, they only establish a stable connection with approximately half of them (Nguyen et al., 2023; Park et al., 2023).
There are few sources estimating the total number of PFs. An in vivo study using a sparse labelling method showed 540 PFs for a 200 × 200 μm2 section, accounting for just 0.38% of PFs in that section (Wilms and Häusser, 2015). The estimation for the total number of PF was about 142.000, which was in the same range as previously reported (Palay and Chan-Palay, 1974a). Another recent measurement performed on a 175 × 122 × 50 μm3 EM slab reported 33.900 (Park et al., 2023), which was suggested to account for 76.9% of the total number of PFs passing through the section. The total number of PF that can hypothetically pass through the section could reach 42.500 PFs. This variation in the number of PFs depends on the conformation of each lobulus and differences in the ML thickness between each sulcus, apex and the tissue in-between them. The changes in the ML thickness were studied in human sections showing that most lobuli have a thickness between 300 and 340 µm (Zheng et al., 2023). The smallest thickness was identified in lobule X (170 ± 80 µm), while lobules I and II showed the maximum thickness (360 ± 110 µm). In human sections of 200 μm × 160 μm, the estimated total number of PFs was 33.515 ± 36.261 (Kuo et al., 2011). Despite the large range of variation, the upper bound estimates are consistent with previous reports. More experiments will be required to elucidate the variability of PF number in each lobule, in both human and rodents as well as in health and disease.
Purkinje cell spine properties
PCs are endowed with tens of thousands of spines that follow a helical pattern along the dendrites (O’Brien and Unwin, 2006; Parajuli et al., 2020) (Figures 2A,B). During mice development and until P20, spines are also expressed on the soma. They direct the CFs in the translocation process from the soma to the dendrites and vice versa for BC collaterals (Ichikawa et al., 2011). The requirement of having an intrinsic system to manage the spine distribution is a reflection of a profound difference with other neuronal types. PC spines follow the “Sotelo model,” which states that spines are intrinsically generated during the first and second week of development and a nearby axon is not required for their protrusion (Sotelo and Dusart, 2009; Dusart and Flamant, 2012; Verslegers et al., 2014). In contrast, PNs and other neuronal types follow the “Millers–Peters model,” which states that a spine can be protruded only when an axon and a dendrite are within a certain distance (Yuste and Bonhoeffer, 2004). It is a critical difference since a PC generates tens of thousands of PF - spine pairs with the ability to elaborate a near-infinite number of synaptic patterns. This supposed limitless in input/output could interfere with the encoding/decoding process in deep cerebellar nuclei (DCN), vestibular nuclei, and their transmission to the red nucleus and thalamus (Pugh and Raman, 2005; Gilbert and Rasmussen, 2025).
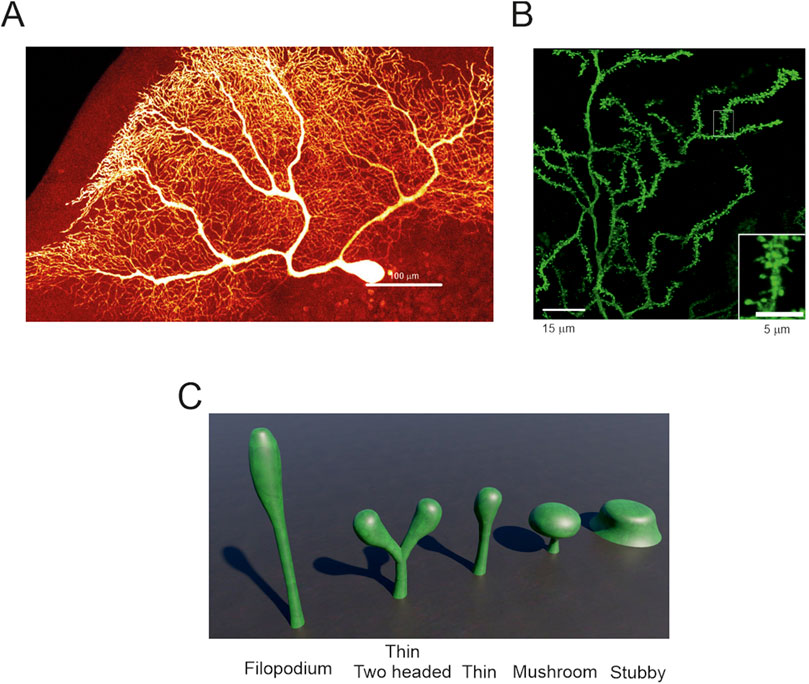
Figure 2. Purkinje cells spines – (A) Human PC reconstructed from post-mortem tissue (Masoli et al., 2024). At this resolution, spines are not evident. (B) Zoom-in of a portion the image in A showing spines decorating the dendrites. The inset further enlarges a dendrite crowded with many spines (modified from (Masoli et al., 2024)). (C) Typical spine shapes that can be found in PCs and in other neuronal types. The most common is the thin spine shape and, in PCs, 15% can have two heads (image built with Blender 3.6).
One neck, not always a single head
The most stereotyped spine shape is called “mushroom” since it looks like a mushroom. It is canonically used to illustrate the morphological, biochemical and biophysical properties of a spine because there is a clear distinction between two adjacent regions called “neck” and “head” (Risher et al., 2014). The neck is generated by the outward bending of the cellular membrane followed by the head, which forms its terminal part. The mushroom-like shape has a neck and a head of similar length, but the head is significantly wider than the neck. When the difference between neck and head diameters is small, and the neck is multiple times longer than the head, the spine is called “thin”. If the difference in diameter and length between neck and head are non-existent, the spine is called “stubby”. When a spine does not show a clear separation between neck and head, it is called a “filopodium” (Li et al., 2023) (Figure 2C). A recent clustering analysis showed that spines should not be classified in predefined categories because they are a “continuum of shapes” with multiple intermediate forms (Pchitskaya and Bezprozvanny, 2020).
In rats, 75% of PC spines were described “thin” and only 25% were stubby, mushroom-like or with more than one head (branched) (Lee et al., 2004). A similar proportion was identified in mouse and human morphologies, with a higher number of thin spines compared to stubby, mushroom, and branched spines (Busch and Hansel, 2025). A recent technical advancement allowed to discern that 15% of spines in awake mice, and 7% in sleeping mice, have two heads on a single neck and in rarer cases, even three heads for a single neck (Loschky et al., 2022). Branched spines with similar features were also described in mouse and human PCs (Busch and Hansel, 2025) and in mouse hippocampal neurons (Mohrmann et al., 2024) suggesting a possible conserved property. Moreover, a rare “spine cluster” was uncovered in human morphologies, in which a single giant head showed multiple swellings acting as single spine heads (Busch and Hansel, 2025). Currently, it is not known if each head of the “spine cluster” contains an active synapse.
The other important part of the spine, the neck, can be wrongly classified by the low resolution of two photon microscopy and optical microscopes. This issue can increase the total number of stubby spines compared to the other known types (Tønnesen et al., 2014). In the majority of neuronal types, spines, necks and heads lay on the same plane but, in PCs, some heads can reach a 60° angle compared to the neck (Parajuli et al., 2020). Post Synaptic Densities (PSDs) are usually placed at the top centre of the postsynaptic membrane, but PC spines can angle their heads so the PSD can switch position and be placed even on the side of the head. With this flexibility, they can generate more occasions to find a nearby PF to establish a contact (Parajuli et al., 2020).
Total surface area and dendritic length
In mammals, the width covered by PC dendritic trees passes from an average of 180 µm in P27 mice (Wilms and Häusser, 2015), to 300 µm in P90 Guinea pigs (Rapp et al., 1994) and to an average of 700 µm in 50–90 years old humans (Jacobs et al., 2014; Masoli et al., 2024; Busch and Hansel, 2025). The extensive dendritic tree in conjunction with rather large soma, averaging 20 µm in P27 mice and 35 µm in adult humans (Masoli et al., 2024), restricts the number of PCs to about 0.5% of all the neurons in the cerebellum (Tan et al., 2023). The total dendritic length, along with the linear spine density, is widely used to calculate the total spine number, which can vary among the cells. Since PCs are embedded in a 3D space, they do not occupy it entirely but are constrained by multiple parameters. The location in a lobule (apex or sulcus) (Nedelescu and Abdelhack, 2013) and the thickness of the ML (Liu et al., 2021; Zheng et al., 2023) dictate the overall shape of the dendrites and their extension. The same space contains other neurons (Stellate cells, Basket cells, candelabrum cells), fibers (PFs and CFs) and glial cells (Bergmann glia). Moreover, the cerebellar microvasculature contributes to the overall reduction of the space available for dendritic expansion. These factors can all limit the extension of their dendritic tree and the total number of spines. As summarized in Table 1, the total dendritic length can range from an average of 2,782.59 ± 671.12 µm in P27 mice (Masoli et al., 2024) to 7,900 µm in P63 mice (Gao et al., 2011; Takeo et al., 2021). In humans, it can range from 9,507 ± 1,053.13 µm (Mavroudis et al., 2021) to 63,645 ± 4,572 µm (Busch and Hansel, 2025). In all cases, the reported dendritic length may be underestimated due to the incomplete reconstruction of some thin dendrites. According to the data in Table 1, the variability reported above, and the type of technique used to reconstruct dendrites and spines (Li et al., 2023), the most common dendritic length ranges between 4,000 and 7,000 µm in mice and between 30,000 and 70,000 µm in humans. The human datasets are still very limited, and there are cases in which the total length amounts to only 10,000 μm, despite the use of good quality tissue sources and technique (Masoli et al., 2024).
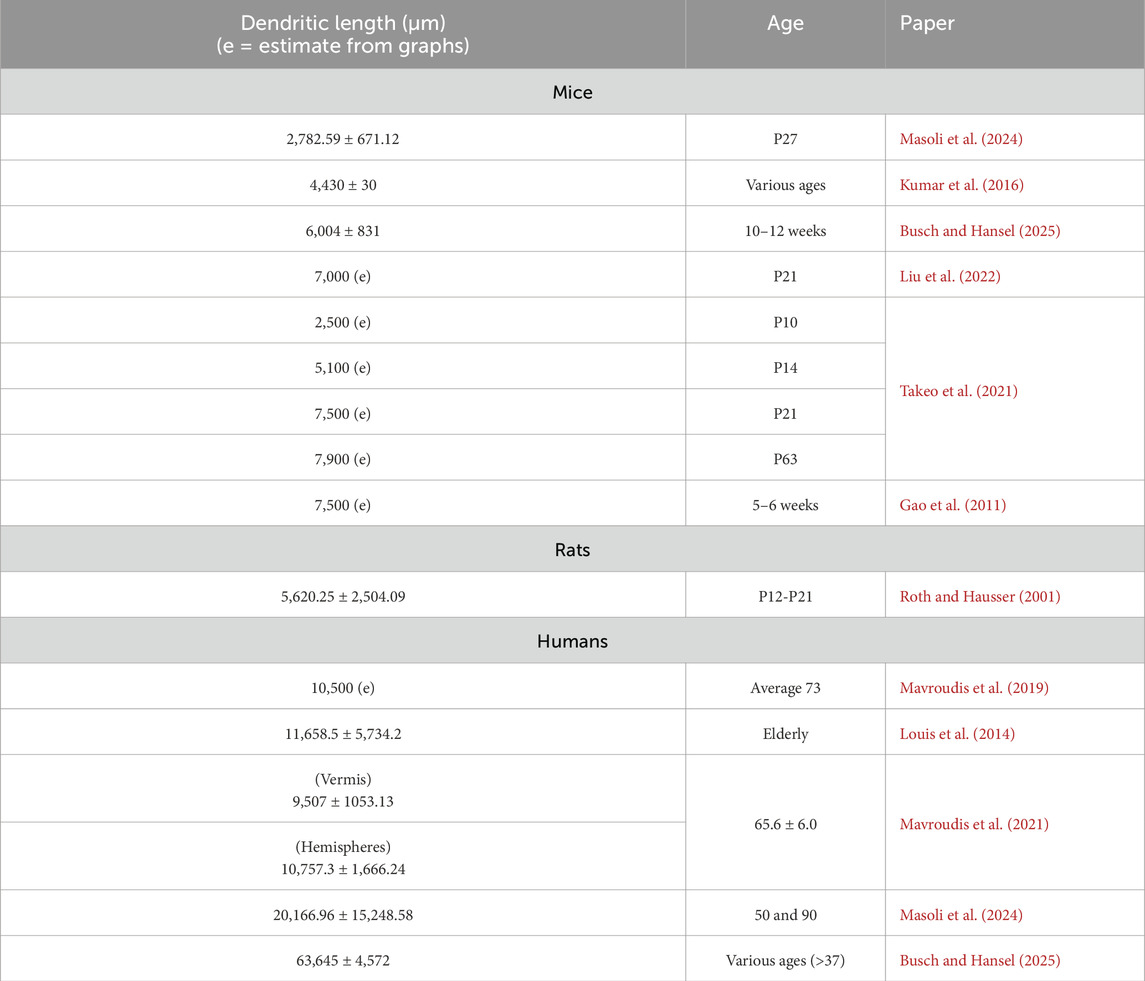
Table 1. Total dendritic length and age. The table shows the age and the total dendritic length, which in some cases were estimated from graphs. The range is rather variable from 2 mm to 8 mm in mice. The variability is similar in human but on an order of magnitude more ranging from 9 mm to 67 mm.
Spine number per unit length
The number of spines for linear micron can vary between different studies, depending on the overall quality of the tissue and/or the techniques used. Usually, the number of spines is calculated from single dendritic branches using an optical microscope or from digitised images using confocal microscopes (Li et al., 2023). The stacked images, with the aid of specific software, can be reconstructed into a file and visualised with a computer to better study the distribution of spines in a 3D space (Gao et al., 2011; Busch and Hansel, 2025). The most cited estimates reported 4.5 spines/µm in feline PCs (Palkovits et al., 1971) and 17.2 spines/µm in rats (Napper and Harvey, 1988). While most reconstructions reported an average of 2 spines/µm, other studies reported 1.4 spines/µm (Gao et al., 2011) or up to 5.1 ± 0.61 (Busch and Hansel, 2025) or 7.1 ± 1.693 spines/µm (Parajuli et al., 2020). The spine number per unit length did not show associations with mice treatment, learning tasks or enriched environment, which are factors known to stimulate spinogenesis (Gelfo et al., 2016; Stevenson et al., 2021). Neither age nor animal strain appeared to exert an effect. Similar values were reported in human PCs, although with a limited number of investigations showing an average of 2 spines/µm. Recent human reconstructions showed an average of 6.9 ± 0.77 spines/μm (Busch and Hansel, 2025). The average distribution of spines, taken from various publications, is summarised in Table 2. The spine number per unit length obtained with most recent techniques and high-quality tissue can range between 4 and 8 spines/µm. In human, as previously discussed, the range was shown to range between 6 and 7 spines/µm (Busch and Hansel, 2025). It is not yet possible to define if 6 spines/μm is the lowest value since similar analyses were performed on high-quality tissue and yielded 2 spines/µm (Masoli et al., 2024). The human datasets do not yet cover the entire cerebellum, and regional differences can be a lot more critical compared to mice.
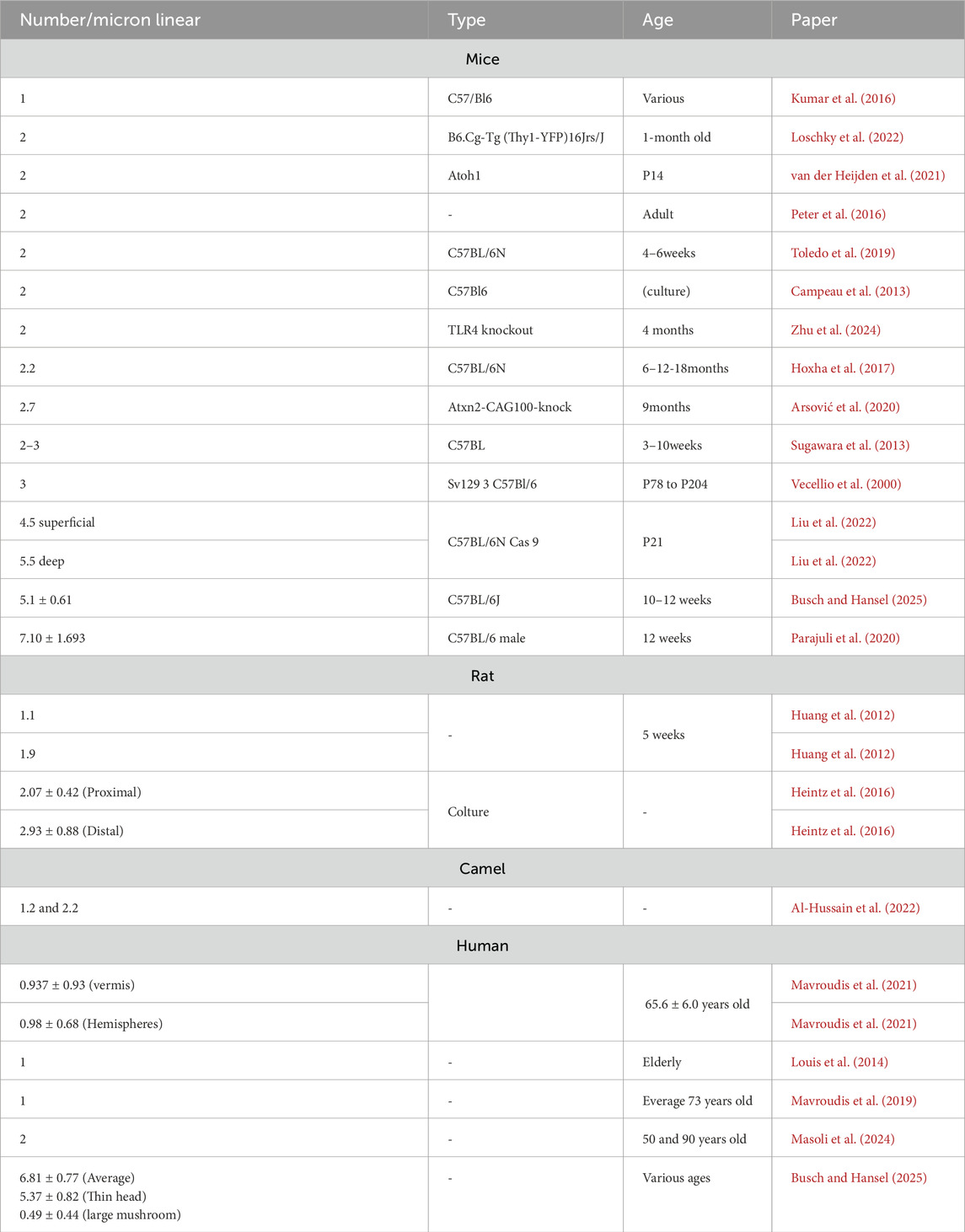
Table 2. Spines distribution in literature. The table contains experimental values collected in the literature about the average number of spines, the animal type and the age. Not all these data was available in the mentioned papers.
Spine number estimates
Early estimates of PC spines
One of the first estimates of the number of PC spines ranged between 80,000 and 100,000 spines in the feline cerebellum (Palkovits et al., 1971). This value was estimated considering 4.5 spines/µm, which matches current studies on rodents. However, the total dendritic tree length was not provided, making it difficult to assess the number of spines. Another estimate was performed on rat PCs and proposed 17.2 spines/µm. This value was multiplied by a total dendritic length of 9,941.5 µm, which was an average value obtained from a previous study (Palay and Chan-Palay, 1974a) yielding 175,000 spines per PC (Napper and Harvey, 1988). Compared to common spine estimation techniques, this approach was not based on the number of spines/µm but on an equation using spine volume densities as the main parameter (Napper and Harvey, 1988). This estimation, which is often used for reference, does not match the majority of experimental recordings and, even in the best cases, it is six-seven times larger than reality. Based on these numbers, many authors estimated that human PCs could reach up to one million spines or even more (Huang et al., 2014), but this value has recently been disproven (Busch and Hansel, 2025).
Estimates based on the latest experimental data
As detailed in Table 1, the most common quantification of the spine density in mice and humans is 2 spines/µm. The average mouse dendritic length is approximately 5,000 μm, which, with 2 spines/µm, gives space to a maximum of 10,000 spines. Based on recent detailed morphological reconstructions, the spine number per unit length in mice can range up to 5.1 ± 0.61 (Busch and Hansel, 2025) or even 7.1 ± 1.693 (Parajuli et al., 2020) spines/µm. Even in these cases, with the same 5,000 µm dendritic length, the maximum number of spines increases up to a value ranging between 25,000 and 35,000 spines. Taking into consideration the longest recorded mouse dendritic tree (7,900 µm) (Takeo et al., 2021), and the highest number of spines/µm (7 ± 1.693) (Parajuli et al., 2020), the total number would reach 55,300 spines. Using the maximum spine density (6.81 ± 0.77 spines/µm) (Busch and Hansel, 2025) and the maximum dendritic length (63,645 ± 4,572 µm) (Busch and Hansel, 2025) reported in the human tissue, a similar calculation yields ∼470,000 spines, which is still only 47% of the proposed estimate of about one million. The estimates provided in Table 1 are primarily obtained from pieces of spiny dendrites and overlook differences in spine distribution due to regional variability or the absence of spines on main dendritic trunks. The most recent experimental data (Busch and Hansel, 2025) showed that human spines cover more dendritic length (95%) compared to mice (87%). This could mean that human PC have more spiny dendrites even in presence of multiple main trunks stemming from the soma.
Fewer spines than expected but more critical than hypothesised
Based on the information given above, the total spine number is lower than the most cited estimations, but this reduction may not be a negative factor after all. The question turns into how synaptic integration over fewer spines can generate an effective response able to modulate DCN and the vestibular nucleus (Gilbert and Rasmussen, 2025). Due to the initial estimate of a very high number of spines, one argument used to reduce their total number was that ∼90% of them had no presynaptic partner, i.e., they were silent (Isope and Barbour, 2002). This hypothesis has recently been challenged by the discovery that 92.7% of spines do present a synapse (Loschky et al., 2022). Thus, the number of spines is lower than initially thought, but the number of spines featuring a synaptic connection is significantly higher. This evidence is in accordance with recent estimates leveraging advanced recording techniques to show that, in mice, there are up to 42,000 PFs (Park et al., 2023), which would yield about 40,000 synaptic pairs (PF–spine) if 93% of them synapsed with a PC. In close agreement, a recent estimate of spine density and total dendritic length (Liu et al., 2022) allowed to calculate the number of ∼35,000 spines, which we will use for all subsequent calculations (Figure 3).
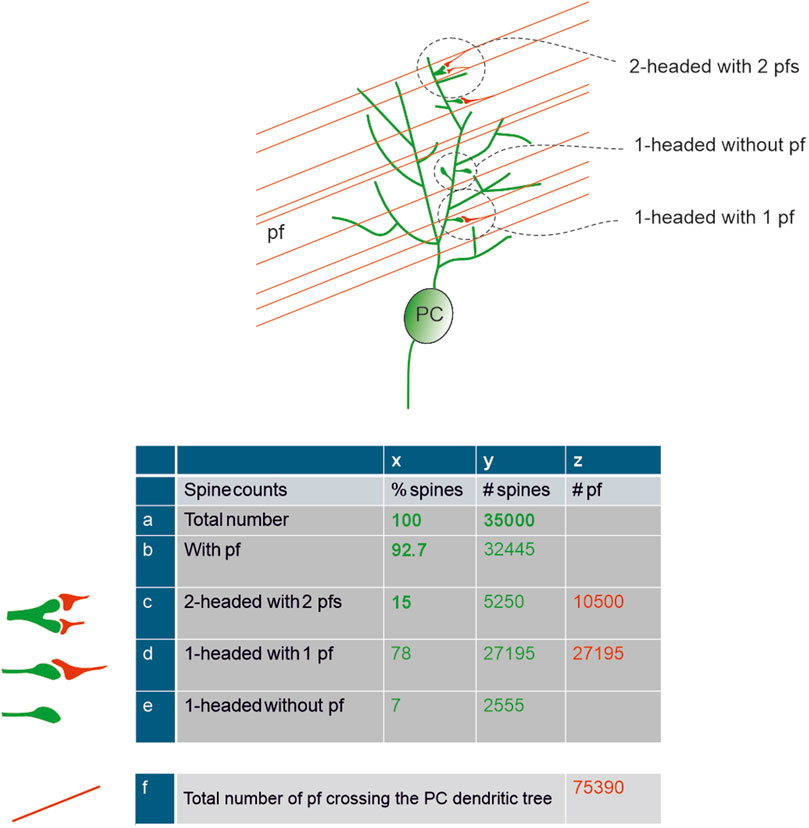
Figure 3. Total spine number and parallel fibers interaction – Schematics of PFs passing through the PC dendritic tree and of their connectivity with the PC spines. The insets illustrate the three main types of spine contacts. The table shows calculations of the number of spines (green) and PFs (red). Numbers in bold are derived from literature. The total number of spines, 35000, was taken from (Liu et al., 2022), the percentage of spines contacted by PFs and of double-headed spines was taken from (Loschky et al., 2022). The number of PFs crossing the PC dendritic tree is calculated assuming a contact rate of 50% (Park et al., 2023).
A critical point is that not all the connected spines appear to have the same functional properties. Approximately 15.1% ± 3.6% of all spines are double-headed, receive one PF per head, and have been suggested to be more “eloquent” compared to the typical single headed spine (Loschky et al., 2022). The response of two or more PFs on the same spine can be elicited by the synchronous activation of GrCs, increasing their overall postsynaptic current and potentially resulting in a somatic response. Moreover, 16% of single-headed spines, which express mGluR1 mediated slow responses, may be critical in the overall synaptic pattern recognition. By combining this observation, 7% of synapses would remain orphan and therefore fully silent, 15% double-headed and fast-responding, and 78% single-headed, either slow-responding (16%) or partially silenced by glial cells (see below). This picture yields an estimate of up to a maximum of 85% putative silent synapses, approaching earlier estimates of 90% (Isope and Barbour, 2002; Brunel et al., 2004), but redefining their nature to include, in addition to null responses, also slow and partial responses.
As a special morphological feature of PCs, each spine is surrounded by BG, which forms multiple types of peri-synaptic astrocytic processes (PAPs) (Tao-Cheng, 2025). This affects the computation of active spines since glial cells can remodel spine structure by nibbling pieces of the spine membrane, a process that was reported to modulate their activity after learning (Morizawa et al., 2022). This is in contrast to another hypothesis, claiming that glia covered spines to make them unresponsive (Lippman Bell et al., 2010).
In aggregate, the count of active spines on the PC dendritic tree is complicated not just by the anatomical connectivity but also by specific processes that can regulate their effectiveness. To summarise, even though the average number of spines is probably ∼35,000 in mice and ∼360,000 in humans, 93% are connected to a presynaptic partner, 15% (double-headed) have high efficiency, while 78% (single-headed) have low efficiency (either modulated by glial PAPs or generating slow metabotropic responses). Similarly, the number of PFs effectively conveying information to a PC is also difficult to establish. It has been reported that PCs make synapses only with about half of the PFs traversing their dendrites (Nguyen et al., 2023; Park et al., 2023), amounting to ∼76,000 PFs crossing the PC dendritic tree, with ∼27,000 synapsing on double-headed spines and ∼10,000 synapsing on single-headed synapses (Figure 3).
Molecular properties of Purkinje cell spines
Even thou the total spine number is lower than the original estimates, it does not detract from the fact that each spine is covered and contains multiple protein types and enzymes. These can be broadly subdivided into: a) structural proteins, which are fundamental to preserve the connection with the presynaptic side (i.e., PF and CF) through a series of transmembrane and secreted proteins; b) ionic channels and synaptic receptors, which allow the generation of action potentials and local increases in the intracellular Ca2+ concentration; and c) structural proteins and enzymes involved in the control of synaptic plasticity, Ca2+ buffering and receptors turnover.
Structural proteins between spines and parallel fibers
The tripartite complex that stabilises PF-PC spines require: a) the GluD2 receptor on the spine surface, b) the neuropeptide cerebellin (Cbln1), and c) the presynaptic cell adhesion protein neurexin (Nrxn) on the PF membrane (Paul et al., 2024).
The GluD2 receptor
A critical structural protein is the GluD2 receptor (Kakegawa et al., 2009; Burada et al., 2022), whose structure was recently reconstructed with cryo-EM microscopy (Burada et al., 2020). GluD2 is a member of the glutamate receptor (iGluR) family encoded by the GRID2 gene and has long been regarded as an “orphan” receptor, as it is not gated by glutamate (Naur et al., 2007; Yuzaki and Aricescu, 2017; Brunetti et al., 2024). It can act as an ionotropic receptor only in the Lurcher mutation (p.Ala654Thr), in which the protein quaternary structure is twisted in a constitutively open state (Selimi et al., 2003). This abnormal open state can be closed by D-serine or Glycine (Itoh and Yuzaki, 2024) and enhanced by extracellular Ca2+ (Hansen et al., 2009). In both cases, the alteration in GluD2 activity can push PCs into a hyper-excited state that ultimately leads to cell death in about two post-natal weeks. When the cerebellum is still immature, D-serine released by BG is critical to generate long-term depression (LTD) because GluD2 regulates the trafficking of AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionate) receptors (Kakegawa et al., 2011). The receptor is primarily expressed in cerebellar PCs (Itoh and Yuzaki, 2024), while it is less abundant in cerebral and hippocampal neurons (Konno et al., 2014). In all cases, GluD2 promotes synaptogenesis (Khan, 2017), thereby increasing the number of spines (Spanaki et al., 2024). GluD2 stabilises spines and promotes postsynaptic LTD both in the immature and mature cerebellum (Kakegawa et al., 2011). Deletion of GRID2 causes ataxia in humans (Hills et al., 2013) and can be rescued in mouse cultures by injections of GluD1 in the PC soma (Ryu et al., 2012). Another critical activity performed by GluD2 is the separation of territories occupied by PF synapses and CF synapses. Its absence causes the aberrant development of CF collaterals and synapses in the PF territory (Ichikawa et al., 2016).
The adaptor protein complex 2 (AP-2) and Glutamate Receptor Delta 2 Interacting Protein 1 (GRID2IP) were recently found to be critical for the balance of PF-CF territories. Loss of the two AP-2 isogenes, i.e., Ap2a1 and Ap2a2, in PCs causes the degradation of GRID2IP and an increased expression of GluD2. This reduces the CF-PC territory and increases the PF-PC territory, making PCs more excitable. This leads to morphological degeneration, early PC death and Spino Cerebellar Ataxia type 1 (SCA1) (Tolve et al., 2025).
Cerebellin, an adaptor protein
There are four variants of the secreted protein Cbln, which are encoded by four genes (Cbln1-4) (Südhof, 2023). They can interact with both GluD1 and GluD2 and with the various Nrxn isoforms only if these contain an insert in the alternatively spliced sequence 4 (SS4) (Uemura et al., 2010). The first Cbln, as suggested by the name, was discovered in the cerebellum: it is secreted by cerebellar GrCs and interacts with Nrxn to form the tripartite complex that stabilizes PF-PC synapses. Cbln1 expression is quite low at birth, but it undergoes a 20-fold increase and thereby becomes the most expressed cerebellar isoform during the postnatal development. Conversely, Cbln2 is highly expressed before birth but is strongly downregulated during the postnatal phase and reaches a very low expression level in adulthood (Seigneur and Südhof, 2017). Cbln4 has a slim expression in the cerebellum, but it was recently shown that it is the first Cbln isoform downregulated in SCA2 (Arsović et al., 2020) followed by Cbln3, which is highly critical for the maintenance of PF-PC synapses. Cbln3 cannot be secreted from PFs unless it associates with Cbln1 and, when it reaches the synaptic cleft, accumulates and modulates Cbln1 activity. This activity was explored in mice with a KO for Cbln3, which increased sevenfold the expression of Cbln1. Instead, the KO of Cbln1 completely eliminated Cbln3 from the synaptic cleft (Bao et al., 2006; Iijima et al., 2007; Larsen, 2021). Cbln1 is also critical for downregulating the formation of inhibitory synapses, mainly from SCs, on the PC dendritic tree (Ito-Ishida et al., 2014b) (see the inhibitory section). It has recently been discovered that Cbln1 and GluD2 are not only critical for maintaining spines and their presynaptic partners, but even for the dendritic tree development. A total KO of GluD2 does not influence the shape of PC trees, but a sparse KO or an increase in Clbn1 and GluD2 proteins disrupts the morphological properties of PCs (Takeo et al., 2021).
Neurexin, type I cell adhesion protein
Three isoforms of Nrxn are encoded by three genes (Nrxn1-3) but they are highly rearranged through alternative splicing (Fuccillo et al., 2015; Südhof, 2017). Nrxns are so important for the survival of GrCs that a KO of these proteins is fatal even in cell cultures. This condition was reversed by the application of brain derived nerve factor (BDNF) and partially rescued by insulin-like growth factor-1 (IGF-1) (Uemura et al., 2022). BDNF is a neurotrophic factor that is postulated to have an autocrine or paracrine activity on GrCs axons. This substance is released by GrCs axon under control of Nrxns. They are critical for the creation of the presynaptic machinery, which is activity-induced through action potential-dependent Ca2+ entry. Multiple combinations of Nrxn KO showed that the different isoforms are interchangeable since the cerebellum shows no structural defects when only two out of three Nrxn isoforms (Nrxn1/2, Nrxn2/3 and Nrxn1/3) are genetically deleted (Uemura et al., 2022). Nrxn2 was found to be critically involved with Cbln1 in the regulation of GrCs axonal guidance and growth. These proteins act as cues during development and elongation of the axon in an autocrine manner (Han et al., 2022) Figure 4A.
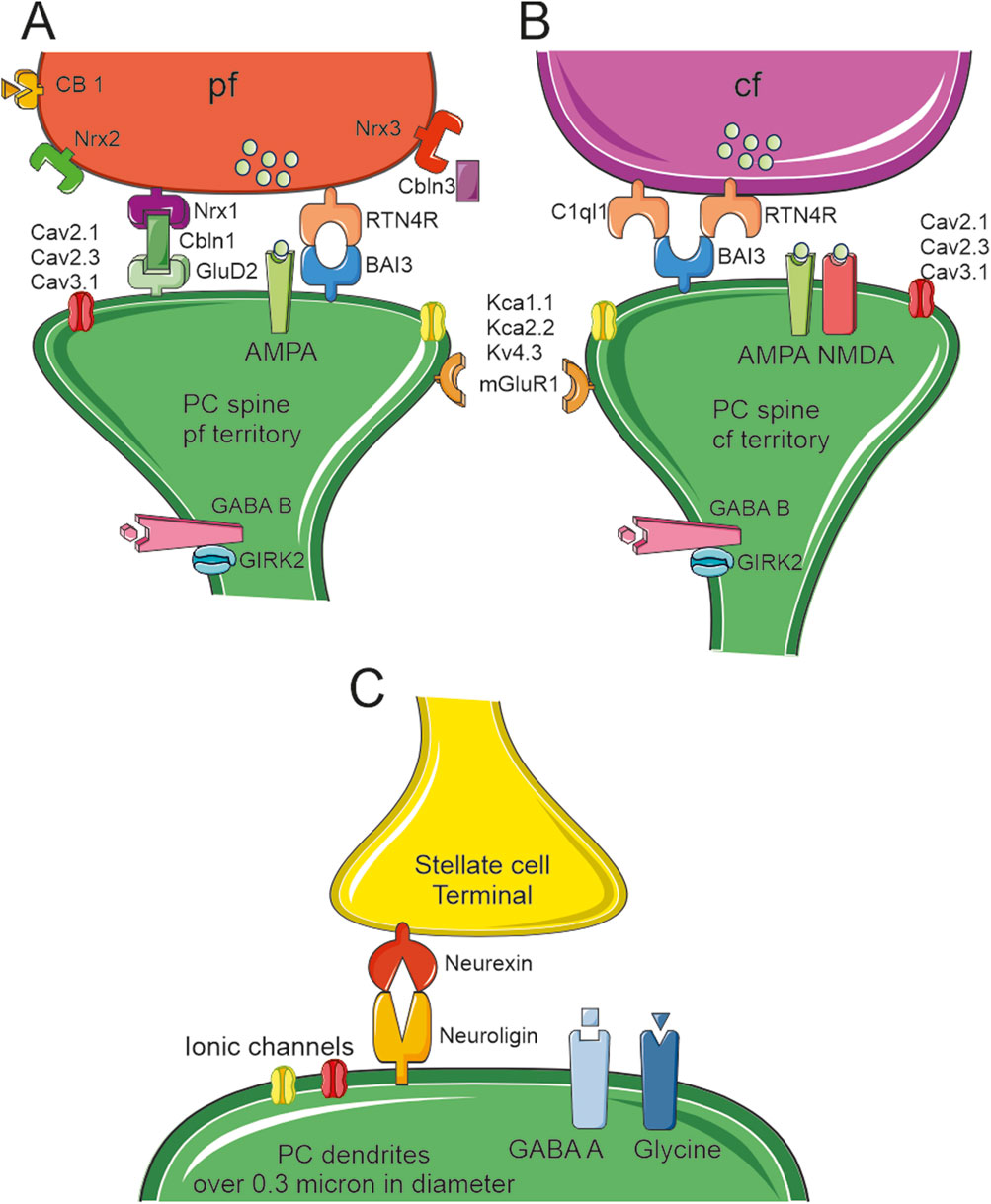
Figure 4. Proteins linking the pre and post synaptic sides. Schematic drawing of the main molecular components of PC spine synapses. (A)The PF terminal expresses three types of neurexin (nrx1-2–3) with nrx1 linked to the postsynaptic GluD2 through cerebellin (Cbln3). This is the main system that keeps PF and PC spines connected together. A second system, RTN4R and BAI3, can be found in some PF–PC synapses (although this complex is more typically expressed in CF–PC synapses). These spines expressed AMPA receptors, three types of Ca2+channels (Cav2.1, Cav2.3, Cav3.1), and three types of K+channels (KCa1.1, KCa2.2 and Kv4.3). The GABAB receptor is expressed on the spine neck along with GIRK2. (B)The CF terminal expresses RTN4R and C1ql1 linked to the postsynaptic BAI3. This is the main system that keeps CF and PC spines connected together. The CF spines express both AMPA and NMDA receptors, and (possibly) the same three types of Ca2+and K+channels as the PF spines. As in PF spines, CF spines express the GABA B receptor and GIRK2 on the spine neck. (C)The stellate cell synaptic terminals end on PCs dendrites and they are kept in place by presynaptic Neurexin and postsynaptic Neuroligin. The postsynaptic side hosts GABAA and Glycine receptors. PCs dendrites have multiple types of ionic channels that can change depending on their diameters [for reference supplemental materials (Masoli et al., 2024)].
Scaffold proteins between spines and climbing fibers
CFs are the terminal part of axons belonging to neurons located in the inferior olive nucleus. These fibers are critical to deliver the graded control correction signals capable of changing PC activity through the activation of PF-PC synaptic plasticity (Hansel et al., 2001; Coesmans et al., 2004; Jörntell and Hansel, 2006; Hoxha et al., 2016; Boele et al., 2018). The majority of PCs in mice shows a single trunk stemming from the soma, but PCs with two trunks stemming from the soma can be found in lobuli IX and X (Nedelescu et al., 2018). This is even more evident in human reconstructions, where three distinct branches were frequently observed (Busch and Hansel, 2023; 2025; Masoli et al., 2024). Similar to the previously defined tripartite complex, a complex of two proteins is required to stabilise a CF on PC spines: a) the secreted C1ql1 complement family protein and b) the brain angiogenesis inhibitor 3 (BAI3/ADGRB3) protein, which is an orphan receptor of the adhesion G-protein-coupled receptors (GPCR) (Sigoillot et al., 2015). Both GluD2 and BAI3 can be found in the immature PCs when the PF and CF synaptic territories are not yet defined. After the stabilisation of the synaptic territories, some spines belonging to the PF territory keep BAI3 on their membrane surface and connect RTN4R located on the PF presynaptic side (Paul et al., 2024). The presence of a single winner CF has been proved wrong for humans (Busch and Hansel, 2023) since there can be more than one depending on the number of main trunks. In some cases, more than one CF has been observed in rodents too (Nishiyama and Linden, 2004; Piochon et al., 2014). To guide the rodent CFs shift, transient somatic spines are generated lasting only until P20 and used by both CF and BC axon collaterals (Ichikawa et al., 2011). After P21, only the winner CF can be found on dendritic spines, showing a larger volume, extensive PSD and more AMPA receptors compared to somatic spines and dendritic spines of the looser CF. This process is coordinated by the Rab3-interacting molecule RIM, which can also be found in PFs (Nitta et al., 2025). Progranulin release by PCs acts as a retrograde signal activating sort1, which increases the release probability of the presynaptic terminals of the CF that have translocated from the soma to the dendrites (Uesaka et al., 2018). Dysregulation of either C1ql1 or BAI3 in the adult allows the formation of new synaptic contacts between nearby CF branches and the upper part of PC dendritic tree (Aimi et al., 2023). The KO of either Cbln1 or C1ql1 causes the disruption of at least 50% of PF and CF synapses (Paul et al., 2024). As observed upon the KO of GluD2, the absence of Cbln1 disrupts the CF territory, increasing its presence in territories normally occupied by PF synapses Figure 4B.
How structural proteins control inhibitory synapses
Early work described that BC collaterals interact with PC somatic spines for 2 weeks until P20 (Ichikawa et al., 2011). After this point in the development, no other evidence of inhibitory interneurons synapsing with PC spines was provided, not even SCs, which make synapses directly with dendrites. This is different compared with the morphological reconstructions and electrophysiological recordings performed in PNs, in which some spines are dedicated to receiving inhibitory synapses (Boivin and Nedivi, 2018). The absence of SC synapses on PC spines is due to the same Cbln1 that controls the PF territory. The number of SC and CF synapses was significantly increased in cerebellar slices from Clbn1/DluD2-deficient mice (Ito-Ishida et al., 2014a). This investigation further showed that the lack of Cbln1 also increased the density of the Vesicular Gaba Transporter (VGAT)-positive puncta, which are a marker of GABA- and glycine-containing inhibitory terminals (although with some regional differences in P11 mice). Since Cbln1-GluD2 signalling can control the territory occupied by VGAT, this finding confirms that the PF-PC synapses can hetero-synaptically control the generation and stabilisation of molecular layer interneurons (MLI)-PC synapses (Ito-Ishida et al., 2014a). Cbln1 finely regulates synaptogenesis through the Src-family protein tyrosine kinase (SFK) pathway (Ito-Ishida et al., 2014b). PC-expressed Neuroligin can interact with Nrxn expressed by MLI to generate the complex that stabilises these synapses (Südhof, 2008; Zhang et al., 2015; Park et al., 2018). The absence of Neuroligin or Nrxn can impair mature synapses but is not involved in synaptogenesis, which has been recently attributed to Dystroglycan. SC synapses cannot be found on spines since they do not co-localise with markers of excitatory synapses (Jahncke et al., 2025). Global KO of Neuroligin 2 reduced the inhibitory input from MLIs to PCs and suppressed pruning of CF synapses (Suk et al., 2025) (Figure 4C).
Ionic channels and receptors in PC spines
The majority of synaptic receptors, ionic channels and internal biochemical pathways which control the postsynaptic plasticity are within the conglomerate of scaffolding proteins forming the PSD (Harris and Stevens, 1988; Cramer and Gao, 2013; Chen et al., 2022).
Ionic channels
PCs are endowed with multiple voltage-dependent ionic channels distributed over the different cell compartments (Masoli et al., 2015). Some channels have an axosomatic expression, but the majority are somato-dendritic. Some have a higher expression on the proximal part of dendritic trees, whereas others cover the entire tree, including dendritic spines. The most known ionic channel, the P-type high voltage-activated (HVA) Ca2+ channel (Cav2.1) can be found everywhere and can act alone or cluster with big (BK, KCa1.1) and small conductance Ca2+-dependent K+ channels (SK2, KCa2.2) (Indriati et al., 2013; Luján et al., 2018a). The modulation of the spike amplitude is under control of A-type K+ channel (Kv4.3) and the rebound excitation from negative potential is modulated by a low voltage-activated (LVA) Ca2+ channel (Cav3.1) (Otsu et al., 2014; Alfaro-Ruíz et al., 2020). The R-type HVA Ca2+ channel (Cav2.3) is found in spines, but is not critical in controlling the overall PC electrical responses (Otsu et al., 2014).
The physical length and the absence of voltage-dependent sodium or Ca2+ channels from the necks (Araya et al., 2006) can generate a local filtering system. The G-protein inward-rectifier K+ channels 2 (GIRK2), expressed on both necks and heads (Luján et al., 2018b), could also act as a modulatory system. It can shunt the forward propagation of weak signals from the spine to the rest of the dendrite and, at the same, filter the back propagating spikes from the axosomatic compartments by promoting membrane hyperpolarization.
Ionotropic receptors (AMPA, NMDA)
The majority of neurons express both AMPA and NMDA receptors on their spines, whereas PCs express a majority of spines with only AMPA receptors. The absence of postsynaptic NMDA receptors can be due their presynaptic expression on PF (Schonewille et al., 2021). Postsynaptic NMDA receptors are instead expressed by spines belonging to the CF territory (Piochon et al., 2007; 2010). The AMPA receptor subtype expressed by human PC spines comprises all the known subunits (GluR1 – GluR4) in their flip and flop splice variants (Tomiyama et al., 1999). The highest expression was reported for GluR1 (Castejón and Dailey, 2009), GluR2 (Liu et al., 2010) and GluR3 (Loschky et al., 2022). AMPA receptors in PCs are almost impermeable to Ca2+ ions, since they contain the GluR2 subunit. This subunit is critical for the AMPA assembly since it controls receptor kinetics, conductance of single-channel, and Ca2+ permeability. The passage of Ca2+ is limited by the presence of an arginine residue at position 607 (R607) that introduces an additional positive charge in the pore (Isaac et al., 2007). According to recent experiments, Ca2+ permeability can be modulated by the transmembrane AMPAR regulatory protein (TARP) and cornichon auxiliary subunits, modifying the known properties of GluR2 subunits (Miguez-Cabello et al., 2025). The paired pulse facilitation of AMPA receptors differ depending on the source of the presynaptic innervation: AMPA receptors expressed by spines belonging to the PF territory present a strong facilitation (Schmidt, 2019), whereas those expressed in the CF territory showed a strong depression after a single pulse (Zhang et al., 2020). This difference has been proven, not only with electrophysiological approaches but also by assessing the expression of the glutamate transporters Vglut1 and Vglut2. The former is associated with the majority of PFs and the latter only with CFs (Mao et al., 2022). These AMPA receptors are assisted primarily by NMDA GluN2A subunits and, to a lesser extent, by GluN2B subunits (Renzi and Cull-Candy, 2007). GluN2A has a high opening probability that facilitates Ca2+ entry, whereas GluN2B has half the value of GluN2A opening probability but shows longer openings (Santucci and Raghavachari, 2008). These receptors, along with Cav2.1 Ca2+ channels, play a critical role in Ca2+-dependent facilitation and depression (Kim et al., 2008; Benton and Raman, 2009; Adams et al., 2010).
PCs synthesise and release glutamate from their dendrites until the fourth postnatal week (Crépe et al., 2011). This autocrine activity on spine receptors is useful for depolarisation-induced suppression of excitation (DSE), and for depolarisation-induced potentiation of inhibition (DPI) (Crépe et al., 2011). Glial cells are usually in charge of clearing the excessive glutamate from the cleft, and this is one of the multiple activities performed by BG, which expresses the glutamate transporter EAAT2. Contrary to other neuronal types, PCs express the EAAT4 transporter in the spine perisynaptic region (Dehnes et al., 1998; Tao-Cheng, 2025). In postischemic mice, the low expression of this transporter causes excitotoxicity and cell death (Yamashita et al., 2006).
Three members of the ionotropic P2X receptors (P2X2, P2X4, and P2X6), which are non-selective cation channels gated by ATP, have been detected on spines belonging only to PF territory (Rubio and Soto, 2001). These ionotropic receptors mediate both membrane depolarization and Ca2+ influx in some regions of the CNS (Mut-Arbona and Sperlágh, 2023), but their physiological role in cerebellar PCs is yet to be determined.
Metabotropic receptors (GABAB, mGluR1)
Compared to PNs, PCs do not have spines capable of receiving inhibitory inputs from GABAergic interneurons. However, spines belonging to both PF and CF territories express extra synaptic GABAB receptors, which can cluster with GIRK2 channels on spine necks and with Cav2.1. The activation of GABAB receptors enhances the depression of the synaptic currents (AMPA-mediated fast synaptic currents and mGluR-mediated slow synaptic currents) induced by glutamate in spine heads (Tabata and Kano, 2006) and is responsible for PC hyperpolarization (Luján et al., 2018b). The presence of GABAB receptors, GIRK2 ionic channels, and the correlation between neck lengths and electrical activity (Araya et al., 2006) could generate a filtering property that could reduce the noise to signal ratio of each spine.
Another major player in generating slow responses is mGluR1. The CF that wins the competition and becomes stabilised on PC specific main trunk is under control of mGluR1 located on spines, AMPA receptors located on PFs and NMDA receptors located on MLI (Nakayama et al., 2024). The cannabinoid receptor CB1 is located on the presynaptic PF (Buceta et al., 2020) and is stimulated by mGluR1 through a signalling cascade that generates 2-arachidonoylglycerol (2-AG) and anandamide as retrograde messengers (Marcaggi, 2015; Hoxha et al., 2016). This pathway is critical because it reduces the release probability of PF in a Ca2+- and glutamate-dependent manner (Safo et al., 2006). Accordingly, mGluR1 stimulates phospholipase Cβ (PLCβ) to cleave phosphatidylinositol-4,5-bisphosphate (PIP2) into inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) (Negri et al., 2020). DAG is hydrolyzed into 2-AG by DAG lipase and can thus serve as a retrograde messenger to reduce glutamate release from PFs (Safo et al., 2006). While DAG promotes the inhibitory inputs at the PF-PC synapse, the other branch of the signalling cascade, i.e., IP3, maintains the presynaptic function by inducing the secretion of BDNF, which acts as a retrograde messenger to increase the glutamate release probability (Furutani et al., 2006). The chronic suppression of mGluR1 and IP3 profoundly reduce the release probability. A similar activity can be induced by applications of BDNF (Furutani et al., 2006). The weight of DAG vs. IP3 signalling at the PF-PC synapse could depend on their different rates of degradation upon PLCβ activation (Raghu et al., 2019; Joensuu et al., 2020).
Inside a PC spine
The intracellular molecular mechanisms of PC spines are highly specialized and include several enzymatic cascades, molecular motors, and a specialization of the endoplasmic reticulum (ER) called spine apparatus. These are instrumental in ensuring spine neurotransmission, plasticity, and motility.
Cytoplasmic molecules and the spine apparatus
The ER in PC dendritic spines is central to Ca2+ dynamics and synaptic plasticity. It regulates intracellular Ca2+ homeostasis, which is essential for synaptic function and plasticity. The ER network extends into the dendrites and spines with specialised sub domains, such as spine-associated ER and smooth ER tubules, contribute to localised Ca2+ dynamics. The ER serves as a major Ca2+ store, modulating LTD and other forms of synaptic plasticity in PCs. Ryanodine receptors (RyRs) and IP3 receptors (IP3Rs) play key roles in Ca2+ release from the ER. This Ca2+ regulation is crucial for the function of cerebellar circuits, impacting motor learning, since the ER interacts with synaptic receptors, including AMPA and mGluRs (Konietzny et al., 2023). Synaptopodin is an actin-associated protein highly expressed in neuronal dendritic spines. It is known to organise the spine apparatus, a special form of the ER inside dendritic spines. It plays an important role in Ca2+ signalling and synaptic plasticity. Synaptopodin is mainly found in cortical PNs (especially in the hippocampus and neocortex), but it is not expressed in PCs. PCs have other types of ER structures in their spines (such as spine smooth ER) (Mundel et al., 1997; Deller et al., 2000; Vlachos et al., 2009; Wagner et al., 2011).
A recent investigation revealed that PIP2 can be primarily located in PC spines and GrC presynaptic active zones (Eguchi et al., 2023). As explained above, during glutamatergic stimulation, mGluR1 stimulates PLCβ to cleave PIP2 into DAG and IP3, which releases ER Ca2+ by activating IP3Rs. IP3-induced ER Ca2+ release can then be amplified by Ca2+-induced Ca2+ release (CICR) through RyRs and lead to a dramatic reduction in the ER Ca2+ concentration ([Ca2+]ER). Stromal interaction molecule (STIM) proteins, namely, STIM1 and STIM2, can, respectively, detect large and small decreases in the [Ca2+]ER; once activated, STIM proteins oligomerize and translocate to ER-plasma membrane junctions, known as puncta, where they bind to and gate the Ca2+-permeable channel, Orai1. This mechanism is known as store-operated Ca2+ entry (SOCE) and is primarily responsible for refilling ER Ca2+ in neurons (Moccia et al., 2015). STIM1 is abundantly expressed in cerebellar PCs (Klejman et al., 2009) and a recent investigation reported that it is preferentially localized in the dendritic subsurface cisterns of the ER in mouse PCs (Nomura et al., 2025). Orai1 is also highly expressed in cerebellar PCs from several species, including human, rat and Cynomolgus monkey (Guzman et al., 2014), but it is still unclear whether it contributes to SOCE. In this view, PCs are also enriched with Orai2 (Skibinska-Kijek et al., 2009), which may serve as a dominant negative regulator of Orai1 (Kito et al., 2015; Yoast et al., 2020), thereby strongly limiting Orai1-mediated Ca2+ entry in PCs. However, STIM1 can interact with many other components of the Ca2+ handling machinery (Moccia et al., 2015), including Cav1.2 channels (Wang et al., 2010), NMDA receptors (Gruszczynska-Biegala et al., 2020), AMPA receptors (Gruszczynska-Biegala et al., 2016), and members of Transient Receptor Potential (TRP) superfamily of non-selective cation channels, such as TRP Canonical 1 (TRPC1) and TRPC3 (Zeng et al., 2008; Lee et al., 2014). TRPC1 and TRPC3 are both expressed in PCs, but only TRPC3 can be gated by STIM1 in response to IP3-dependent ER Ca2+ release (Hartmann et al., 2008). A series of investigations has unambiguously demonstrated that STIM1-gated TRPC3-containing channels mediated mGluR1-dependent slow synaptic excitatory postsynaptic currents (EPSPslow) in cerebellar PCs (Hartmann et al., 2014; Gui et al., 2024). STIM1 maintains the ER Ca2+ pool that it mobilized during dendritic mGluR1 signalling to ensure motor coordination (Hartmann et al., 2014), regulates PC intrinsic excitability by interacting with Sarco-Endoplasmic Reticulum Ca2+-ATPase (SERCA) to clear intracellular Ca2+ and fine-tune the recruitment of Ca2+-dependent conductances (Ryu et al., 2017), and is crucial for the memory consolidation of the vestibulo-ocular reflex (Jang et al., 2020). The major expression of STIM1 and SERCA2 was detected at the dendritic level with little to no presence on spines. Conversely, two other critical receptors for the release of Ca2+ from the smooth ER (SER), RyR1 and IP3R1, were expressed on spines. The former had a lower spine expression compared to the somato-dendrites compartments, while the latter was highly expressed in spines (Nomura et al., 2025). An early study showed that the rapid replenishment of the ER Ca2+ store within the spine is driven by the intraluminal redistribution of dendritic Ca2+ (Okubo et al., 2015). This observation suggests that the ER within the spine neck does not represent a significant barrier to Ca2+ diffusion and that the absence of STIM1 impairs the overall ER Ca2+ dynamics in PCs. The neuronal ER functions act as an intracellular tunnel to redistribute stored Ca2+ within the neurons and as a leaky integrator of Ca2+ spike-inducing synaptic inputs (Okubo et al., 2015). This separation can lead to two distinct levels of synaptic plasticity; one strictly located on the dendritic level and one confined in each spine. This compartmentalized Ca2+ regulation is critical for cerebellar function and motor coordination.
Cytoskeleton
The cytoskeleton within the dendritic spines of PCs is primarily composed of filamentous actin (F-actin), which provides structural support and facilitates synaptic plasticity. Several key proteins regulate the organization and dynamics of this actin cytoskeleton: 1) Myosin XVI is a motor protein that interacts with the WAVE Regulatory Complex (WRC) to modulate actin dynamics in PC spines. Inhibition of the WRC accelerates F-actin turnover, resulting in altered spine morphology and reduced structural plasticity (Roesler et al., 2019). 2) Cortactin is predominantly localized near the postsynaptic density and sub-membrane regions of PC spines, and plays a role in actin filament branching and stabilization. Its distribution in these spines differs from that in forebrain neurons, suggesting region-specific functions in synaptic architecture (Szabó et al., 2021). 3) CaMKIIβ (Ca2+/Calmodulin-Dependent Protein Kinase II Beta) is the most abundant protein in the PSD and it is involved in synaptic plasticity through the phosphorylation of multiple NMDA subunits (Kennedy, 2000). This kinase also promotes spine formation and elongation through its F-actin binding activity (Okamoto et al., 2007). Activation of group I mGluRs, i.e., mGluR1 and mGluR5, triggers protein kinase C (PKC)-mediated phosphorylation of CaMKIIβ, leading to its dissociation from F-actin. This mechanism prevents excessive spine development and maintains proper spine morphology in mature PCs (Sugawara et al., 2017). 4) Myosin-Va is a motor protein responsible for transporting the ER into dendritic spines of PCs. The presence of ER in spines is essential for synaptic plasticity, and myosin-Va facilitates this process by pulling the ER into spines along actin filaments (Wagner et al., 2011).
These proteins collectively contribute to the dynamic regulation of the actin cytoskeleton in PC spines, influencing their structure and function in cerebellar synaptic plasticity (for a comparison, see Table 3).
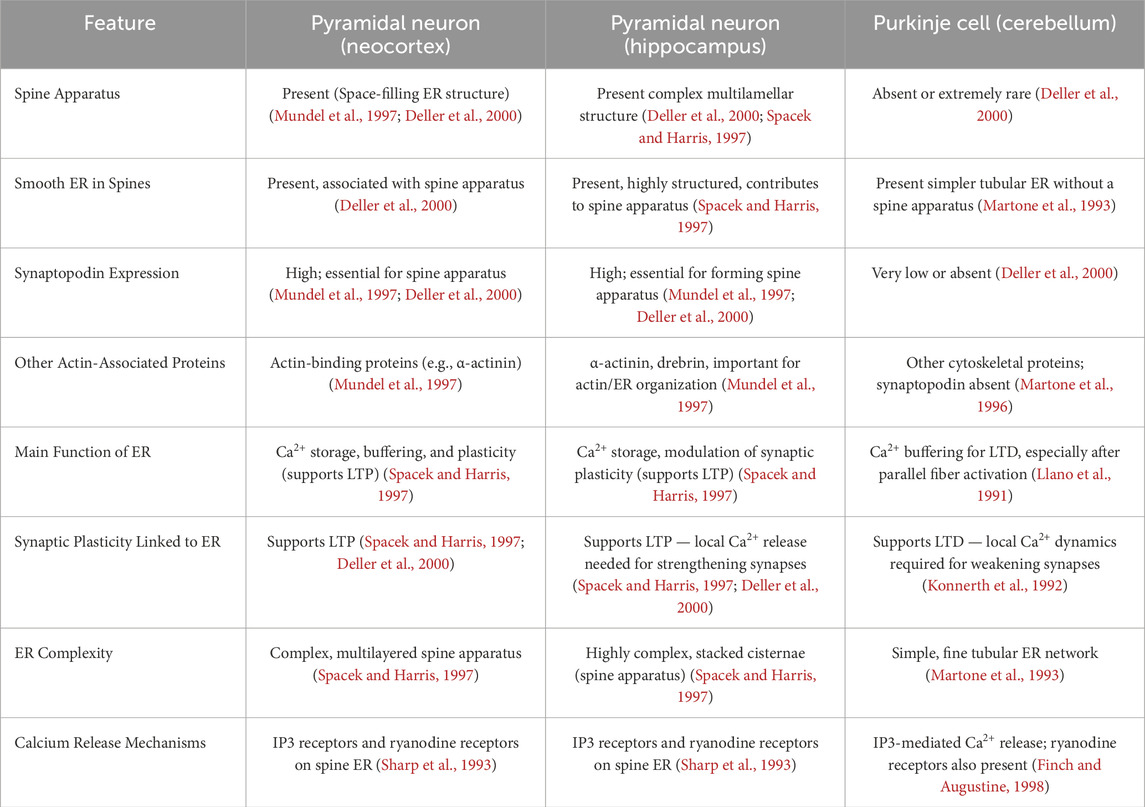
Table 3. Comparison of ER related properties. Differences between cortex, hippocampus and Purkinje cells.
Purkinje cell spine regulation
Parallel fibers (anti-Hebbian) and climbing fibers (Hebbian) long-term potentiation and depression
PC dendrites receive excitatory inputs from PFs and CFs and the spines are instrumental in generating specific forms of long-term synaptic plasticity, including long-term potentiation (LTP) and LTD. While synaptic plasticity at CF – spine synapses follows the Hebbian rules, the PF – spine synapse present both LTD and LTP based on a non-Hebbian plasticity rule (Roberts and Leen, 2010; Piochon et al., 2012; Runge et al., 2020). The general synaptic plasticity rule (Lisman, 1989; Shouval et al., 2002; Pali et al., 2025) dictates that LTP is generated by low Ca2+ concentrations and LTD by high concentrations. LTD at PF - PC synapses consists of an activity-dependent long-lasting reduction in synaptic strength (Roberts and Leen, 2010; Nishiyama and Yasuda, 2015). Coincidence of PF stimulation (glutamate release) and CF activation (membrane depolarisation and Ca2+ influx) triggers LTD (Piochon et al., 2012; Daida et al., 2024). This leads to an influx of Ca2+ via voltage-gated Ca2+ channels and to Ca2+ release from the endogenous ER stores through the CICR process (Harvey-Girard et al., 2010). Glutamate released from PFs activates mGluR1 to produce IP3 production, thereby promoting IP3-induced Ca2+ release from the spine apparatus (Hartmann et al., 2011). A high localised Ca2+ concentration, together with PKC activation, induces AMPA receptor (GluA2 subunit) internalisation from the postsynaptic membrane, weakening synaptic transmission (Lisman, 1989). LTD is essential for motor learning, such as eye-blink conditioning and adaptation of the vestibulo-ocular reflex (Sharp et al., 1993; Finch and Augustine, 1998; Hansel et al., 2001; Ito, 2001). Moderate PF activation without strong CF co-activation leads to protein kinase A (PKA) stimulation and enhances AMPA receptor phosphorylation, promoting their insertion or stabilisation at the postsynaptic membrane, involving the activation of phosphatases, such as protein phosphatase 1 (PP1) and PP2B (calcineurin) (Lewis and Maler, 2002). The nitric oxide (NO)/soluble guanylyl cyclase/cyclinc guanosine monophosphate (cGMP) signalling pathway has also been implicated (Lev-Ram et al., 2002). Unlike LTD, where a large, spatially localised Ca2+ rise triggers depression, LTP requires smaller, slower Ca2+ elevations that fails to engage the higher threshold LTD pathway. LTP may help counterbalance LTD, maintaining synaptic homeostasis and contributing to fine-tuning of motor commands (Martone et al., 1993; Spacek and Harris, 1997; Lev-Ram et al., 2002; Coesmans et al., 2004). The presynaptic protein RIM1, in connection with Rab3-interacting molecule, is necessary for LTP to occur between PFs and PC (Uriu et al., 2010). It should also be noted that also presynaptic forms of synaptic plasticity also exist at the PF-PC synapse but are not considered here (Hansel et al., 2001).
How to modulate a spine: presynaptic release probability
There are various substances that can module the overall synaptic strength without the need to physically eliminate the PF-spine synapse. The endocannabinoids, which are produced by PCs and act as a retrograde signal, interact with the presynaptic side, reducing the release probability through CB1 receptors (Safo et al., 2006). A critical presynaptic protein termed RIM1 is important in the control and recruitment of presynaptic Ca2+ channels (Kaeser and Regehr, 2014). This protein is activated by progranulin generated by PCs and, acting as a diffusible signal, leads instead to an increase in release probability. This strengthening of the synaptic activity was recorded during the stage in which a CF becomes the winner with its translocation from somatic to dendritic spines (Uriu et al., 2010; Nitta et al., 2025). The presynaptic NMDA receptors are involved in the production of NO, which, compared to many other neuronal types, is not produced by the postsynaptic side (D’Angelo, 2014; Mapelli et al., 2017). NO may influence the postsynaptic Ca2+ dynamics and thereby change the overall strength of the presynaptic side, pushing the synapse into LTP (Schonewille et al., 2021). The structural proteins between PF and spines are critical, but the axon itself can define if a presynaptic active site needs to be stabilized or abolished (Aiken and Holzbaur, 2024). Based on the type of signals that need to be elaborated by a specific PCs, it is possible that the synapse is initially established between PF and PC and a certain point in the development, the presynaptic side itself is pruned.
How to modulate a spine: postsynaptic properties
As previously defined (see chapter “Spine number estimates”), to reduce the impact of the large number of spines and their low response it was assumed the absence of fast AMPA receptor-mediated responses, and the presence of a majority of mGluR1/TRPC3-mediated slow synaptic currents (Jin et al., 2007). These slow EPSC, lasting up to hundreds of milliseconds, were recently shown to change greatly depending on the lobuli (Thomas et al., 2024). Slow responses could be elicited in spines with only one head to preserve the PF-spine synapse, to convey support information, or to maintain the synapse active when no relevant information is transmitted. This model is supported by the recent discovery that branched spines are more “eloquent” compared to single-headed spines (Loschky et al., 2022). Another way to reduce the number spines, without physical deletion, is through GABAB receptors located peri synaptically and on spine necks. These receptors are connected with GIRK2 channels that can act as a filter for small intensity presynaptic activation or by slow responses elicited by mGluR1. The PF – spine synapse follows an anti-Hebbian rule to generate short and long-term potentiation (Lev-Ram et al., 2002; Piochon et al., 2012). This is in agreement with the evidence that postsynaptic Ca2+ needs to remain low to generate LTP, while it must increase by coincident activation of CFs to generate LTD.
Dynamic changes in morphological conformation
A property that was studied in layer 5 PN showed that the length of spine necks electrically isolates the heads from the dendrites. This activity was recorded using Spine Uncaging Potentials and showed a correlation between the neck lengths and electrical activity recorded at the somatic level. Longer necks had more impact on the activity, even reaching a complete silencing of the post-synaptic potentials, where shorted neck allowed post-synaptic potential transmission (Araya et al., 2006). When a synaptic contact is established, its shape does not change even during LTD activity (Sdrulla and Linden, 2007). This view was recently challenged with a new experimental procedure showing that, besides changing their shape, the entire dendritic spine can be retracted and regenerated along the day/night cycle (Loschky et al., 2022). An in vivo experimental procedure further showed that PC spine size can be changed by a process that required the endocytosis of some spine membrane by BG (Morizawa et al., 2022). This process was marked by increased activity of BGs after training and learning.
Spine modification in diseases
Spine properties can be greatly modified in the presence of mutations that dysregulate various signalling pathways, thereby resulting in severe neurological diseases. In many cases, the PC dendritic tree can change its shape, branching points and overall arborisation. The dendritic trees are subject to marked modifications as exemplified by the atrophy observed in PCs of Weaver mice and in ectopic PCs of Reeler mice. In both mouse models, the presence of spines is unaffected by the mutations, but their linear count is lower compared to controls (Yuste and Bonhoeffer, 2004). In essential tremor, a human parkinsonism characterized by localised axon swelling, there is a reduction in the complexity of PC dendritic tree with just a small reduction in spine number per unit length (Louis et al., 2014). In Staggerer mice, which are missing the retinoid-related orphan receptor α (RORα), the animal is ataxic (SCA1) and PCs show stunted trees with parts of them completely devoid of spines (Mitsumura et al., 2011). Some remnants of the PF-PC connectivity can be observed with excitatory synapses made directly on the dendritic surface. Another ataxia (SCA2) is caused by polyglutamine expansion in Ataxin-2 (ATXN2) and its activity on CaMKIIα and CaMKIV signalling with a reduction in spine length and spine density (Arsović et al., 2020). A point mutation in the protein kinase C gamma (PKCγ), involved in SCA14, showed that it has a limited impact on spinogenesis except if it is upregulated. In the latter case, it causes the reduction of the number of spines, their length and overall maturity (Sziber et al., 2025). In human schizophrenia, a decrease in spine density was observed, but with no information about changes to the spine shape (Mavroudis et al., 2017). This specific mutation is yet to be replicated in animal models.
Conclusions and computational implications
Although the number of spines appears to be lower than initially thought, this is not expected to hamper the encoding capabilities of PCs. Indeed, instead of having ∼90% silent synapses out of 100000, there would be a maximum of 85% inactive or poorly active synapse out of 35,000. The more “eloquent” double-headed spines would be 15% of the total, i.e., ∼5,000 in mouse and ∼40,000 in human PCs contribute more to the modularity of cerebellar organisation (Streng et al., 2025) compared to the single-headed spines. We recently compared PCs in mice and humans (Masoli et al., 2024), showing that the human/mouse spine head ratio (7.5) could determine the computing capability of the neurons. This number compared well with other metrics like the dendritic surface ratio (5.5) and dendritic complexity index (6.5), as well as with dendritic transfer impedance computed for clusters of spines that can effectively impact spike generation in the PC axonal initial segment (6.5) with 1-ms time resolution. This suggested that the increased number of contacts was almost entirely transformed into effective combinations of input patterns that can regulate spike generation in the soma, akin to the linear encoding in a perceptron (Brunel et al., 2004; Walter et al., 2009). The maximum computational capacity, which depends on the number of alternative states established by the dendrites, turned out to be 28 for mice and 251 for human (Masoli et al., 2024). It remains to be determined whether these figures would change by making assumptions about spine efficiency, e.g., following the arguments reported here. The electrical isolation generated by the spine neck, the large number of thin spines, and the hyperpolarizing activity of GABAB/GIRK2 channels suggest that each spine may individually influence the overall neuronal encoding activity. This is because of the reduction in the noise/signal ratio, allowing the transmission of strong excitatory activity concentrated on few spines. It should be noted that, owing to the redundancy of dendritic combinations, some output spike patterns may be mutually indistinguishable on the temporal resolution scale of the neuron. Ad hoc simulations using PC computational models with spines may allow the calculation of the combinatorial capacity in human and mouse PCs under more realistic assumptions, for example, that segments are not fully active or inactive or that spine independence is incomplete, or that individual spines have specific and differentiated neurotransmission properties reflecting modulatory, plastic, or pathological states (Rieke, 1999; London et al., 2002; Arleo et al., 2010).
Author contributions
SM: Conceptualization, Writing – original draft, Writing – review and editing. MR: Writing – original draft, Writing – review and editing. FM: Writing – original draft, Writing – review and editing. ED’A: Funding acquisition, Writing – original draft, Writing – review and editing, Validation, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. SM - National Recovery and Resilience Plan (NRRP), project IR00011-EBRAINS-Italy funded by the European Union–NextGenerationEU (Project IR0000011, CUP B51E22000150006, “EBRAINS-Italy”).
Acknowledgments
MFR - The Virtual Brain Twin Project has received funding from the European Union’s Research and Innovation Program Horizon Europe under grant agreement No. 101137289. ED’A - Work supported by #NEXTGENERATIONEU (NGEU) and funded by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), project MNESYS (PE0000006) – A Multiscale integrated approach to the study of the nervous system in health and disease (DN. 1553 11.10.2022). The European Union’s Horizon Europe Programme under the Specific Grant Agreement No. 101147319 (EBRAINS 2.0 Project). Figure 4 - Image provided by Servier Medical Art (https://smart.servier.com/), licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adams P., Rungta R., Garcia E., van den Maagdenberg A. M. J. M., MacVicar B. A., Snutch T. P. (2010). Contribution of calcium-dependent facilitation to synaptic plasticity revealed by migraine mutations in the P/Q-type calcium channel. Proc. 107, 18694–18699. doi:10.1073/pnas.1009500107
Aiken J., Holzbaur E. L. F. (2024). Spastin locally amplifies microtubule dynamics to pattern the axon for presynaptic cargo delivery. Curr. Biol. 34, 1687–1704.e8. doi:10.1016/j.cub.2024.03.010
Aimi T., Matsuda K., Yuzaki M. (2023). C1ql1-Bai3 signaling is necessary for climbing fiber synapse formation in mature Purkinje cells in coordination with neuronal activity. Mol. Brain 16, 61–17. doi:10.1186/s13041-023-01048-4
Al-Hussain S. M., Yousuf M. S., Hani A. B., Zaqout S., Djouhri L., Mustafa A. G. (2022). A Golgi study of neurons in the camel cerebellum (Camelus dromedarius). Anat. Rec. 305, 1264–1276. doi:10.1002/ar.24742
Albus J. S. (1971). A theory of cerebellar function. Math. Biosci. 10, 25–61. doi:10.1016/0025-5564(71)90051-4
Alfaro-Ruíz R., Aguado C., Martín-Belmonte A., Moreno-Martínez A. E., Luján R. (2020). Cellular and subcellular localisation of kv4-associated kchip proteins in the rat cerebellum. Int. J. Mol. Sci. 21, 6403–6419. doi:10.3390/ijms21176403
Araya R., Jiang J., Eisenthal K. B., Yuste R. (2006). The spine neck filters membrane potentials. Proc. Natl. Acad. Sci. U. S. A. 103, 17961–17966. doi:10.1073/pnas.0608755103
Arleo A., Nieus T., Bezzi M., D’Errico A., D’Angelo E., Coenen O. (2010). How synaptic release probability shapes neuronal transmission: information-theoretic analysis in a cerebellar granule cell. Neural comput. 22, 2031–2058. doi:10.1162/NECO_a_00006-Arleo
Arsović A., Halbach M. V., Canet-Pons J., Esen-Sehir D., Döring C., Freudenberg F., et al. (2020). Mouse ataxin-2 expansion downregulates camkii and other calcium signaling factors, impairing granule—purkinje neuron synaptic strength. Int. J. Mol. Sci. 21, 6673–36. doi:10.3390/ijms21186673
Bao D., Pang Z., Morgan M. A., Parris J., Rong Y., Li L., et al. (2006). Cbln1 is essential for interaction-dependent secretion of Cbln3. Mol. Cell. Biol. 26, 9327–9337. doi:10.1128/mcb.01161-06
Bentivoglio M., Cotrufo T., Ferrari S., Tesoriero C., Mariotto S., Bertini G., et al. (2019). The original histological slides of camillo golgi and his discoveries on neuronal structure. Front. Neuroanat. 13, 3–13. doi:10.3389/fnana.2019.00003
Benton M. D., Raman I. M. (2009). Stabilization of Ca current in Purkinje neurons during high-frequency firing by a balance of Ca-dependent facilitation and inactivation. Channels (Austin). 3, 393–401. doi:10.4161/chan.3.6.9838
Boele H. J., Peter S., Ten Brinke M. M., Verdonschot L., Ijpelaar A. C. H., Rizopoulos D., et al. (2018). Impact of parallel fiber to Purkinje cell long-term depression is unmasked in absence of inhibitory input. Sci. Adv. 4, eaas9426–eaas9429. doi:10.1126/sciadv.aas9426
Boivin J. R., Nedivi E. (2018). Functional implications of inhibitory synapse placement on signal processing in pyramidal neuron dendrites. Curr. Opin. Neurobiol. 51, 16–22. doi:10.1016/j.conb.2018.01.013
Brunel N., Hakim V., Isope P., Nadal J. P., Barbour B. (2004). Optimal information storage and the distribution of synaptic weights: perceptron versus Purkinje cell. Neuron 43, 745–757. doi:10.1016/j.neuron.2004.08.023
Brunetti V., Soda T., Berra-Romani R., De Sarro G., Guerra G., Scarpellino G., et al. (2024). Two signaling modes are better than one: flux-independent signaling by ionotropic glutamate receptors is coming of age. Biomedicines 12, 880. doi:10.3390/biomedicines12040880
Buceta I., Elezgarai I., Rico-Barrio I., Gerrikagoitia I., Puente N., Grandes P. (2020). Deletion of the cannabinoid CB1 receptor impacts on the ultrastructure of the cerebellar parallel fiber-Purkinje cell synapses. J. Comp. Neurol. 528, 1041–1052. doi:10.1002/cne.24808
Burada A. P., Vinnakota R., Kumar J. (2020). The architecture of GluD2 ionotropic delta glutamate receptor elucidated by cryo-EM. J. Struct. Biol. 211, 107546. doi:10.1016/j.jsb.2020.107546
Burada A. P., Vinnakota R., Bharti P., Dutta P., Dubey N., Kumar J. (2022). Emerging insights into the structure and function of ionotropic glutamate delta receptors. Br. J. Pharmacol. 179, 3612–3627. doi:10.1111/bph.15313
Busch S. E., Hansel C. (2023). Climbing fiber multi-innervation of mouse Purkinje dendrites with arborization common to human. Science 381, 420–427. doi:10.1126/science.adi1024
Busch S. E., Hansel C. (2025). eLife Assessment: non-allometric expansion and enhanced compartmentalization of Purkinje cell dendrites in the human cerebellum. doi:10.7554/eLife.105013.2.sa3
Campeau J. L., Wu G., Bell J. R., Rasmussen J., Sim V. L. (2013). Early increase and late decrease of Purkinje cell dendritic spine density in prion-infected organotypic mouse cerebellar cultures. PLoS One 8, e81776–e81777. doi:10.1371/journal.pone.0081776
Castejón O. J., Dailey M. E. (2009). Immunohistochemistry of GluR1 subunits of AMPA receptors of rat cerebellar nerve cells. Biocell 33, 71–80. doi:10.32604/biocell.2009.33.071
Chen X., Du Y., Broussard G. J., Kislin M., Yuede C. M., Zhang S., et al. (2022). Transcriptomic mapping uncovers Purkinje neuron plasticity driving learning. Nature 605, 722–727. doi:10.1038/s41586-022-04711-3
Coesmans M., Weber J. T., De Zeeuw C. I., Hansel C. (2004). Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron 44, 691–700. doi:10.1016/j.neuron.2004.10.031
Cramer S., Gao W., Chen G., Ebner T. J. (2013). Reevaluation of the beam and radial hypotheses of parallel fiber action in the cerebellar cortex. J. Neurosci. 33, 11412–11424. doi:10.1523/JNEUROSCI.0711-13.2013
Crépe F., Galante M., Habbas S., McLean H., Danie H. (2011). Role of the vesicular transporter VGLUT3 in retrograde release of glutamate by cerebellar Purkinje cells. J. Neurophysiol. 105, 1023–1032. doi:10.1152/jn.00736.2010
Daida A., Kurotani T., Yamaguchi K., Takahashi Y., Ichinohe N. (2024). Different numbers of conjunctive stimuli induce LTP or LTD in mouse cerebellar purkinje cell. Cerebellum 23, 2297–2307. doi:10.1007/s12311-024-01726-6
Davidson A. M., Mejiá-Gómez H., Jacobowitz M., Mostany R. (2020). Dendritic spine density and dynamics of layer 5 pyramidal neurons of the primary motor cortex are elevated with aging. Cereb. Cortex 30, 767–777. doi:10.1093/cercor/bhz124
Defelipe J. (2025). Cajal and the discovery of the Golgi method: a neuroanatomist ’ s dream. Anat. Sci. Int. doi:10.1007/s12565-025-00840-7
Dehnes Y., Chaudhry F. A., Ullensvang K., Lehre K. P., Storm-Mathisen J., Danbolt N. C. (1998). The glutamate transporter EAAT4 in rat cerebellar Purkinje cells: a glutamate-gated chloride channel concentrated near the synapse in parts of the dendritic membrane facing astroglia. J. Neurosci. 18, 3606–3619. doi:10.1523/jneurosci.18-10-03606.1998
Deller T., Merten T., Roth S. U., Mundel P., Frotscher M. (2000). Actin-associated protein synaptopodin in the rat hippocampal formation: localization in the spine neck and close association with the spine apparatus of principal neurons. J. Comp. Neurol. 418, 164–181. doi:10.1002/(SICI)1096-9861(20000306)418:2<164::AID-CNE4>3.0.CO;2-0
Desmond J. E., Fiez J. A. (1998). Neuroimaging studies of the cerebellum: language, learning and memory. Trends Cogn. Sci. 2, 355–362. doi:10.1016/S1364-6613(98)01211-X
Dusart I., Flamant F. (2012). Profound morphological and functional changes of rodent Purkinje cells between the first and the second postnatal weeks: a metamorphosis? Front. Neuroanat. 6, 11. doi:10.3389/fnana.2012.00011
D’Angelo E. (2014). The organization of plasticity in the cerebellar cortex: from synapses to control. 1st ed. Elsevier B.V. doi:10.1016/B978-0-444-63356-9.00002-9
Egorova P. A., Marinina K. S., Bezprozvanny I. B. (2023). Chronic suppression of STIM1-mediated calcium signaling in Purkinje cells rescues the cerebellar pathology in spinocerebellar ataxia type 2. Biochim. Biophys. Acta - Mol. Cell Res. 1870, 119466. doi:10.1016/j.bbamcr.2023.119466
Eguchi K., Le Monnier E., Shigemoto R. (2023). Nanoscale phosphoinositide distribution on cell membranes of mouse cerebellar neurons. J. Neurosci. 43, 4197–4216. doi:10.1523/JNEUROSCI.1514-22.2023
Finch E. A., Augustine G. J. (1998). Local calcium signalling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature 396, 753–756. doi:10.1038/25541
Fuccillo M. V., Földy C., Gökce Ö., Rothwell P. E., Sun G. L., Malenka R. C., et al. (2015). Single-cell mRNA profiling reveals cell-type-specific expression of neurexin isoforms. Neuron 87, 326–340. doi:10.1016/j.neuron.2015.06.028
Furutani K., Okubo Y., Kakizawa S., Iino M. (2006). Postsynaptic inositol 1,4,5-trisphosphate signaling maintains presynaptic function of parallel fiber-Purkinje cell synapses via BDNF. Proc. Natl. Acad. Sci. U. S. A. 103, 8528–8533. doi:10.1073/pnas.0600497103
Gao Y., Perkins E. M., Clarkson Y. L., Tobia S., Lyndon A. R., Jackson M., et al. (2011). β-III spectrin is critical for development of purkinje cell dendritic tree and spine morphogenesis. J. Neurosci. 31, 16581–16590. doi:10.1523/JNEUROSCI.3332-11.2011
Gelfo F., Florenzano F., Foti F., Burello L., Petrosini L., De Bartolo P. (2016). Lesion-induced and activity-dependent structural plasticity of Purkinje cell dendritic spines in cerebellar vermis and hemisphere. Brain Struct. Funct. 221, 3405–3426. doi:10.1007/s00429-015-1109-5
Gilbert M., Rasmussen A. (2025). The cerebellar deep nuclei: a patch for rate codes? Front. Neural Circuits 19, 1548123–14. doi:10.3389/fncir.2025.1548123
Golgi C. (1883). Sulla fina anatomia degli organi centrali del sistema nervoso. Riv. Sper. Freniatr.
Gruszczynska-Biegala J., Sladowska M., Kuznicki J. (2016). AMPA receptors are involved in store-operated calcium entry and interact with STIM proteins in rat primary cortical neurons. Front. Cell. Neurosci. 10, 251. doi:10.3389/fncel.2016.00251
Gruszczynska-Biegala J., Strucinska K., Maciag F., Majewski L., Sladowska M., Kuznicki J. (2020). STIM protein-NMDA2 receptor interaction decreases NMDA-dependent calcium levels in cortical neurons. Cells 9, 160. doi:10.3390/cells9010160
Gui L., Tellios V., Xiang Y.-Y., Feng Q., Inoue W., Lu W.-Y. (2024). Neuronal nitric oxide synthase regulates cerebellar parallel fiber slow EPSC in purkinje neurons by modulating STIM1-gated TRPC3-containing channels. Cerebellum 23, 1867–1881. doi:10.1007/s12311-024-01683-0
Guzman R., Valente E. G., Pretorius J., Pacheco E., Qi M., Bennett B. D., et al. (2014). Expression of ORAII, a plasma membrane resident subunit of the CRAC channel, in rodent and non-rodent species. J. Histochem. Cytochem. 62, 864–878. doi:10.1369/0022155414554926
Han P., She Y., Yang Z., Zhuang M., Wang Q., Luo X., et al. (2022). Cbln1 regulates axon growth and guidance in multiple neural regions. PLOS Biol. 20, e3001853. doi:10.1371/journal.pbio.3001853
Hansel C., Linden D. J., D’Angelo E. (2001). Beyond parallel fiber LTD: the diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat. Neurosci. 4, 467–475. doi:10.1038/87419
Hansen K. B., Naur P., Kurtkaya N. L., Kristensen A. S., Gajhede M., Kastrup J. S., et al. (2009). Modulation of the dimer interface at ionotropic glutamate-like receptor delta2 by D-serine and extracellular calcium. J. Neurosci. 29, 907–917. doi:10.1523/JNEUROSCI.4081-08.2009
Harris K. M., Stevens J. K. (1988). Dendritic spines of rat cerebellar Purkinje cells: serial electron microscopy with reference to their biophysical characteristics. J. Neurosci. 8, 4455–4469. doi:10.1523/JNEUROSCI.08-12-04455.1988
Hartmann J., Dragicevic E., Adelsberger H., Henning H. A., Sumser M., Abramowitz J., et al. (2008). TRPC3 channels are required for synaptic transmission and motor coordination. Neuron 59, 392–398. doi:10.1016/j.neuron.2008.06.009
Hartmann J., Henning H. A., Konnerth A. (2011). mGluR1/TRPC3-mediated synaptic transmission and calcium signaling in mammalian central neurons. Cold Spring Harb. Perspect. Biol. 3, a006726–16. doi:10.1101/cshperspect.a006726
Hartmann J., Karl R. M., Alexander R. P. D., Adelsberger H., Brill M. S., Rühlmann C., et al. (2014). STIM1 controls neuronal Ca2+ signaling, mGluR1-dependent synaptic transmission, and cerebellar motor behavior. Neuron 82, 635–644. doi:10.1016/j.neuron.2014.03.027
Harvey-Girard E., Lewis J., Maler L. (2010). Burst-induced anti-hebbian depression acts through short-term synaptic dynamics to cancel redundant sensory signals. J. Neurosci. 30, 6152–6169. doi:10.1523/JNEUROSCI.0303-10.2010
Heck D., Sultan F. (2002). Cerebellar structure and function: making sense of parallel fibers. Hum. Mov. Sci. 21, 411–421. doi:10.1016/s0167-9457(02)00123-9
Heintz T. G., Eva R., Fawcett J. W. (2016). Regional regulation of purkinje cell dendritic spines by integrins and Eph/ephrins. PLoS One 11, e0158558–15. doi:10.1371/journal.pone.0158558
Hibi M., Matsuda K., Takeuchi M., Shimizu T., Murakami Y. (2017). Evolutionary mechanisms that generate morphology and neural-circuit diversity of the cerebellum. Dev. Growth Differ. 59, 228–243. doi:10.1111/dgd.12349
Hills L. B., Masri A., Konno K., Kakegawa W., Lam A. T. N., Lim-Melia E., et al. (2013). Deletions in GRID2 lead to a recessive syndrome of cerebellar ataxia and tonic upgaze in humans. Neurology 81, 1378–1386. doi:10.1212/WNL.0b013e3182a841a3
Houston C. M., Diamanti E., Diamantaki M., Kutsarova E., Cook A., Sultan F., et al. (2017). Exploring the significance of morphological diversity for cerebellar granule cell excitability. Sci. Rep. 7, 46147. doi:10.1038/srep46147
Hoxha E., Tempia F., Lippiello P., Miniaci M. C. (2016). Modulation, plasticity and pathophysiology of the parallel fiber-purkinje cell synapse. Front. Synaptic Neurosci. 8, 35–16. doi:10.3389/fnsyn.2016.00035
Hoxha E., Gabriele R. M. C., Balbo I., Ravera F., Masante L., Zambelli V., et al. (2017). Motor deficits and cerebellar atrophy in Elovl5 knock out mice. Front. Cell. Neurosci. 11, 343–11. doi:10.3389/fncel.2017.00343
Hoxha E., Balbo I., Miniaci M. C., Tempia F. (2018). Purkinje cell signaling deficits in animal models of ataxia. Front. Synaptic Neurosci. 10, 6–17. doi:10.3389/fnsyn.2018.00006
Huang C. ming, Huang R. H. (1998). Measuring parallel fiber length in the rat cerebellum. Brain Res. 801, 211–215. doi:10.1016/S0006-8993(98)00444-2
Huang C., Gammon S. J., Dieterle M., Huang R. H., Likins L., Ricklefs R. E., et al. (2014). Dramatic increases in number of cerebellar granule-cell-Purkinje-cell synapses across several mammals. Mamm. Biol.79, 163–169. doi:10.1016/j.mambio.2013.12.003
Huang M., Verbeek D. S. (2019). Why do so many genetic insults lead to Purkinje Cell degeneration and spinocerebellar ataxia? Neurosci. Lett. 688, 49–57. doi:10.1016/j.neulet.2018.02.004
Huang T.-Y., Lin L.-S., Cho K.-C., Chen S.-J., Kuo Y., Yu L., et al. (2012). Chronic treadmill exercise in rats delicately alters the Purkinje cell structure to improve motor performance and toxin resistance in the cerebellum. J. Appl. Physiol. 113, 889–895. doi:10.1152/japplphysiol.01363.2011
Ichikawa R., Yamasaki M., Miyazaki T., Konno K., Hashimoto K., Tatsumi H., et al. (2011). Developmental switching of perisomatic innervation from climbing fibers to basket cell fibers in cerebellar Purkinje cells. J. Neurosci. 31, 16916–16927. doi:10.1523/JNEUROSCI.2396-11.2011
Ichikawa R., Sakimura K., Watanabe M. (2016). GluD2 endows parallel fiber-purkinje cell synapses with a high regenerative capacity. J. Neurosci. 36, 4846–4858. doi:10.1523/JNEUROSCI.0161-16.2016
Iijima T., Miura E., Matsuda K., Kamekawa Y., Watanabe M., Yuzaki M. (2007). Characterization of a transneuronal cytokine family Cbln - regulation of secretion by heteromeric assembly. Eur. J. Neurosci. 25, 1049–1057. doi:10.1111/j.1460-9568.2007.05361.x
Indriati D. W., Kamasawa N., Matsui K., Meredith A. L., Watanabe M., Shigemoto R. (2013). Quantitative localization of Cav2.1 (P/Q-type) voltage-dependent calcium channels in Purkinje cells: somatodendritic gradient and distinct somatic coclustering with calcium-activated potassium channels. J. Neurosci. 33, 3668–3678. doi:10.1523/JNEUROSCI.2921-12.2013
Isaac J. T. R. R., Ashby M., McBain C. J. (2007). The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 54, 859–871. doi:10.1016/j.neuron.2007.06.001
Isope P., Barbour B. (2002). Properties of unitary granule cell Purkinje cell synapses in adult rat cerebellar slices. J. Neurosci. 22, 9668–9678. doi:10.1523/JNEUROSCI.22-22-09668.2002
Ito M. (2001). Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol. Rev. 81, 1143–1195. doi:10.1152/physrev.2001.81.3.1143
Ito-Ishida A., Kakegawa W., Kohda K., Miura E., Okabe S., Yuzaki M. (2014a). Cbln1 downregulates the formation and function of inhibitory synapses in mouse cerebellar Purkinje cells. Eur. J. Neurosci. 39, 1268–1280. doi:10.1111/ejn.12487
Ito-Ishida A., Okabe S., Yuzaki M. (2014b). The role of Cbln1 on Purkinje cell synapse formation. Neurosci. Res. 83, 64–68. doi:10.1016/j.neures.2014.01.009
Itoh M., Yuzaki M. (2024). The hidden face of GluD1 at inhibitory synapses. Cell Res. 34, 405–406. doi:10.1038/s41422-024-00931-6
Jacobs B., Johnson N. L., Wahl D., Schall M., Maseko B. C., Lewandowski A., et al. (2014). Comparative neuronal morphology of the cerebellar cortex in afrotherians, carnivores, cetartiodactyls, and primates. Front. Neuroanat. 8, 24. doi:10.3389/fnana.2014.00024
Jahncke J. N., Schnell E., Wright K. M. (2025). Distinct functional domains of Dystroglycan regulate inhibitory synapse formation and maintenance in cerebellar Purkinje cells. Commun. Biol. 8, 878. doi:10.1038/s42003-025-08323-1
Jang M., Bum Um K., Jang J., Jin Kim H., Cho H., Chung S., et al. (2015). Coexistence of glutamatergic spine synapses and shaft synapses in substantia nigra dopamine neurons. Sci. Rep. 5, 14773–16. doi:10.1038/srep14773
Jang D. C., Shim H. G., Kim S. J. (2020). Intrinsic plasticity of cerebellar purkinje cells contributes to motor memory consolidation. J. Neurosci. 40, 4145–4157. doi:10.1523/JNEUROSCI.1651-19.2020
Jin Y., Kim S. J., Kim J., Worley P. F., Linden D. J. (2007). Long-term depression of mGluR1 signaling. Neuron 55, 277–287. doi:10.1016/j.neuron.2007.06.035
Joensuu M., Wallis T. P., Saber S. H., Meunier F. A. (2020). Phospholipases in neuronal function: a role in learning and memory? J. Neurochem. 153, 300–333. doi:10.1111/jnc.14918
Jörntell H., Hansel C. (2006). Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-purkinje cell synapses. Neuron 52, 227–238. doi:10.1016/j.neuron.2006.09.032
Kaeser P. S., Regehr W. G. (2014). Molecular mechanisms for synchronous, asynchronous, and spontaneous neurotransmitter release. Annu. Rev. Physiol. 76, 333–363. doi:10.1146/annurev-physiol-021113-170338
Kakegawa W., Miyazaki T., Kohda K., Matsuda K., Emi K., Motohashi J., et al. (2009). The N-terminal domain of GluD2 (GluRdelta2) recruits presynaptic terminals and regulates synaptogenesis in the cerebellum in vivo. J. Neurosci. 29, 5738–5748. doi:10.1523/JNEUROSCI.6013-08.2009
Kakegawa W., Miyoshi Y., Hamase K., Matsuda S., Matsuda K., Kohda K., et al. (2011). D-Serine regulates cerebellar LTD and motor coordination through the δ glutamate receptor. Nat. Neurosci. 14, 603–613. doi:10.1038/nn.2791
Kennedy M. B. (2000). Signal-processing machines at the postsynaptic density. Sci. 80 290, 750–754. doi:10.1126/science.290.5492.750
Khan M. Z. (2017). Ionotropic glutamate receptors (iGluRs) of the delta family (GluD1 and GluD2) and synaptogenesis. Alex. J. Med. 53, 201–206. doi:10.1016/j.ajme.2016.09.003
Khouri-Farah N., Guo Q., Perry T. A., Dussault R., Li J. Y. H. (2025). FOXP genes regulate Purkinje cell diversity and cerebellar morphogenesis. Nat. Neurosci. 4, 1–21. doi:10.1038/s41593-025-02042-w
Kim E. Y., Rumpf C. H., Fujiwara Y., Cooley E. S., Van Petegem F., Minor D. L. (2008). Structures of CaV2 Ca2+/CaM-IQ domain complexes reveal binding modes that underlie calcium-dependent inactivation and facilitation. Structure 16, 1455–1467. doi:10.1016/j.str.2008.07.010
Kito H., Yamamura H., Suzuki Y., Yamamura H., Ohya S., Asai K., et al. (2015). Regulation of store-operated Ca2+ entry activity by cell cycle dependent up-regulation of Orai2 in brain capillary endothelial cells. Biochem. Biophys. Res. Commun. 459, 457–462. doi:10.1016/j.bbrc.2015.02.127
Klejman M. E., Gruszczynska-Biegala J., Skibinska-Kijek A., Wisniewska M. B., Misztal K., Blazejczyk M., et al. (2009). Expression of STIM1 in brain and puncta-like co-localization of STIM1 and ORAI1 upon depletion of Ca(2+) store in neurons. Neurochem. Int. 54, 49–55. doi:10.1016/j.neuint.2008.10.005
Konietzny A., Wegmann S., Mikhaylova M. (2023). The endoplasmic reticulum puts a new spin on synaptic tagging. Trends Neurosci. 46, 32–44. doi:10.1016/j.tins.2022.10.012
Konnerth A., Dreessen J., Augustine G. J. (1992). Brief dendritic calcium signals initiate long-lasting synaptic depression in cerebellar Purkinje cells. Proc. Natl. Acad. Sci. U. S. A. 89, 7051–7055. doi:10.1073/pnas.89.15.7051
Konno K., Matsuda K., Nakamoto C., Uchigashima M., Miyazaki T., Yamasaki M., et al. (2014). Enriched expression of GluD1 in higher brain regions and its involvement in parallel fiber-interneuron synapse formation in the cerebellum. J. Neurosci. 34, 7412–7424. doi:10.1523/JNEUROSCI.0628-14.2014
Kumar A., Paeger L., Kosmas K., Kloppenburg P., Noegel A. A., Peche V. S. (2016). Neuronal actin dynamics, spine density and neuronal dendritic complexity are regulated by CAP2. Front. Cell. Neurosci. 10, 180–17. doi:10.3389/fncel.2016.00180
Kuo S.-H., Faust P. L., Vonsattel J.-P. G., Ma K., Louis E. D. (2011). Parallel fiber counts and parallel fiber integrated density are similar in essential tremor cases and controls. Acta Neuropathol. 121, 287–289. doi:10.1007/s00401-010-0776-9
Lackey E. P., Moreira L., Norton A., Hemelt M. E., Osorno T., Nguyen T. M., et al. (2024). Specialized connectivity of molecular layer interneuron subtypes leads to disinhibition and synchronous inhibition of cerebellar Purkinje cells. Neuron 112, 2333–2348.e6. doi:10.1016/j.neuron.2024.04.010
Lanciego J. L., Luquin N., Obeso J. A. (2012). Functional neuroanatomy of the basal ganglia. Cold Spring Harb. Perspect. Med. 2, a009621–20. doi:10.1101/cshperspect.a009621
Larsen K. (2021). The porcine cerebellin gene family. Gene 799, 145852. doi:10.1016/j.gene.2021.145852
Lee K. J., Kim H., Kim T. S., Park S. H., Rhyu I. J. (2004). Morphological analysis of spine shapes of Purkinje cell dendrites in the rat cerebellum using high-voltage electron microscopy. Neurosci. Lett. 359, 21–24. doi:10.1016/j.neulet.2004.01.071
Lee K. J., Kim H., Rhyu I. J. (2005). The roles of dendritic spine shapes in Purkinje cells. Cerebellum 4, 97–104. doi:10.1080/14734220510007842
Lee K. P., Choi S., Hong J. H., Ahuja M., Graham S., Ma R., et al. (2014). Molecular determinants mediating gating of Transient Receptor Potential Canonical (TRPC) channels by stromal interaction molecule 1 (STIM1). J. Biol. Chem. 289, 6372–6382. doi:10.1074/jbc.M113.546556
Lev-Ram V., Wong S. T., Storm D. R., Tsien R. Y. (2002). A new form of cerebellar long-term potentiation is postsynaptic and depends on nitric oxide but not cAMP. Proc. Natl. Acad. Sci. U. S. A. 99, 8389–8393. doi:10.1073/pnas.122206399
Lewis J. E., Maler L. (2002). Dynamics of electrosensory feedback: short-term plasticity and inhibition in a parallel fiber pathway. J. Neurophysiol. 88, 1695–1706. doi:10.1152/jn.2002.88.4.1695
Li B. Z., Sumera A., Booker S. A., McCullagh E. A. (2023). Current best practices for analysis of dendritic spine morphology and number in neurodevelopmental disorder research. ACS Chem. Neurosci. 14, 1561–1572. doi:10.1021/acschemneuro.3c00062
Lippman Bell J. J., Lordkipanidze T., Cobb N., Dunaevsky A. (2010). Bergmann glial ensheathment of dendritic spines regulates synapse number without affecting spine motility. Neuron Glia Biol. 6, 193–200. doi:10.1017/S1740925X10000165
Lisman J. (1989). A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc. Natl. Acad. Sci. U. S. A. 86, 9574–9578. doi:10.1073/pnas.86.23.9574
Liu Y., Formisano L., Savtchouk I., Takayasu Y., Szabo G., Zukin R. S., et al. (2010). A single fear-inducing stimulus induces a transcription-dependent switch in synaptic AMPAR phenotype. Nat. Neurosci. 13, 223–231. doi:10.1038/nn.2474
Liu C. J., Ammon W., Siless V., Fogarty M., Wang R., Atzeni A., et al. (2021). Quantification of volumetric morphometry and optical property in the cortex of human cerebellum at micrometer resolution. Neuroimage 244, 118627. doi:10.1016/j.neuroimage.2021.118627
Liu Z., Jiang M., Liakath-Ali K., Sclip A., Ko J., Zhang R. S., et al. (2022). Deletion of Calsyntenin-3, an atypical cadherin, suppresses inhibitory synapses but increases excitatory parallel-fiber synapses in cerebellum. Elife 11, e70664–35. doi:10.7554/eLife.70664
Llano I., Dreessen J., Kano M., Konnerth A. (1991). Intradendritic release of calcium induced by glutamate in cerebellar Purkinje cells. Neuron 7, 577–583. doi:10.1016/0896-6273(91)90370-f
London M., Schreibman A., Haäusser M., Larkum M. E., Segev I. (2002). The information efficacy of a synapse. Nat. Neurosci. 5, 332–340. doi:10.1038/nn826
Loschky S. S., Spano G. M., Marshall W., Schroeder A., Nemec K. M., Schiereck S. S., et al. (2022). Ultrastructural effects of sleep and wake on the parallel fiber synapses of the cerebellum. Elife 11, e84199–26. doi:10.7554/ELIFE.84199
Louis E. D., Lee M., Babij R., Ma K., Cortés E., Vonsattel J. P. G., et al. (2014). Reduced Purkinje cell dendritic arborization and loss of dendritic spines in essential tremor. Brain 137, 3142–3148. doi:10.1093/brain/awu314
Lu H., Esquivel A. V., Bower J. M. (2009). 3D electron microscopic reconstruction of segments of rat cerebellar Purkinje cell dendrites receiving ascending and parallel fiber granule cell synaptic inputs. J. Comp. Neurol. 514, 583–594. doi:10.1002/cne.22041
Luján R., Aguado C., Ciruela F., Arus X. M., Martín-Belmonte A., Alfaro-Ruiz R., et al. (2018a). SK2 channels associate with mGlu1α receptors and CaV2.1 channels in purkinje cells. Front. Cell. Neurosci. 12, 311–316. doi:10.3389/fncel.2018.00311
Luján R., Aguado C., Ciruela F., Cózar J., Kleindienst D., de la Ossa L., et al. (2018b). Differential association of GABAB receptors with their effector ion channels in Purkinje cells. Brain Struct. Funct. 223, 1565–1587. doi:10.1007/s00429-017-1568-y
Magnus G., Xing J., Zhang Y., Han V. Z. (2023). Diversity of cellular physiology and morphology of Purkinje cells in the adult zebrafish cerebellum. J. Comp. Neurol. 531, 461–485. doi:10.1002/cne.25435
Mao H., Mediavilla T., Estévez-Silva H., Marcellino D., Sultan F. (2022). Increase of vesicular glutamate transporter 2 co-expression in the deep cerebellar nuclei related to skilled reach learning. Brain Res. 1782, 1–8. doi:10.1016/j.brainres.2022.147842
Mapelli L., Gagliano G., Soda T., Laforenza U., Moccia F., D’Angelo E. U. (2017). Granular layer neurons control cerebellar neurovascular coupling through an NMDA receptor/NO-dependent system. J. Neurosci. 37, 1340–1351. doi:10.1523/JNEUROSCI.2025-16.2016
Marcaggi P. (2015). Cerebellar endocannabinoids: retrograde signaling from purkinje cells. Cerebellum 14, 341–353. doi:10.1007/s12311-014-0629-5
Marr D. (1969). A theory of cerebellar cortex. J. Physiol. 202, 437–470. doi:10.1113/jphysiol.1969.sp008820
Martone M. E., Zhang Y., Simpliciano V. M., Carragher B. O., Ellisman M. H. (1993). Three-dimensional visualization of the smooth endoplasmic reticulum in purkinje cell dendrites. J. Neurosci. 13, 4636–4646. doi:10.1523/jneurosci.13-11-04636.1993
Martone M. E., Pollock J. A., Jones Y. Z., Ellisman M. H. (1996). Ultrastructural localization of dendritic messenger RNA in adult rat hippocampus. J. Neurosci. 16, 7437–7446. doi:10.1523/jneurosci.16-23-07437.1996
Masoli S., Solinas S., D’Angelo E. (2015). Action potential processing in a detailed Purkinje cell model reveals a critical role for axonal compartmentalization. Front. Cell. Neurosci. 9, 47–22. doi:10.3389/fncel.2015.00047
Masoli S., Sanchez-Ponce D., Vrieler N., Abu-Haya K., Lerner V., Shahar T., et al. (2024). Human Purkinje cells outperform mouse Purkinje cells in dendritic complexity and computational capacity. Commun. Biol. 7, 5. doi:10.1038/s42003-023-05689-y
Mavroudis I. A., Petrides F., Manani M., Chatzinikolaou F., Ciobică A. S., Pădurariu M., et al. (2017). Purkinje cells pathology in schizophrenia. A morphometric approach. Rom. J. Morphol. Embryol. 58, 419–424.
Mavroudis I., Petridis F., Kazis D., Njau S. N., Costa V., Baloyannis S. J. (2019). Purkinje cells pathology in Alzheimer’s disease. Am. J. Alzheimers. Dis. Other Demen. 34, 439–449. doi:10.1177/1533317519859200
Mavroudis I., Kazis D., Petridis F., Chatzikonstantinou S., Karantali E., Njau S., et al. (2021). Morphological and morphometric changes in the Purkinje cells of patients with essential tremor. Exp. Ther. Med. 23, 167–168. doi:10.3892/etm.2021.11090
Miguez-Cabello F., Wang X.-T., Yan Y., Brake N., Alexander R. P. D., Perozzo A. M., et al. (2025). GluA2-containing AMPA receptors form a continuum of Ca2+-permeable channels. Nature 641, 537–544. doi:10.1038/s41586-025-08736-2
Mitsumura K., Hosoi N., Furuya N., Hirai H. (2011). Disruption of metabotropic glutamate receptor signalling is a major defect at cerebellar parallel fibre-Purkinje cell synapses in staggerer mutant mice. J. Physiol. 589, 3191–3209. doi:10.1113/jphysiol.2011.207563
Moccia F., Zuccolo E., Soda T., Tanzi F., Guerra G., Mapelli L., et al. (2015). Stim and Orai proteins in neuronal Ca(2+) signaling and excitability. Front. Cell. Neurosci. 9, 153. doi:10.3389/fncel.2015.00153
Mohrmann L., Seebach J., Missler M., Rohlmann A. (2024). Distinct alterations in dendritic spine morphology in the absence of β-neurexins. Int. J. Mol. Sci. 25, 1285. doi:10.3390/ijms25021285
Mokhtar D. M. (2020). Patterns of organization of cerebellum and spinal cord of the red-tail shark (Epalzeorhynchos bicolor): histological, morphometrical, and immunohistochemical studies. Microsc. Microanal. 26, 1255–1263. doi:10.1017/S1431927620024563
Morizawa Y. M., Matsumoto M., Nakashima Y., Endo N., Aida T., Ishikane H., et al. (2022). Synaptic pruning through glial synapse engulfment upon motor learning. Nat. Neurosci. 25, 1458–1469. doi:10.1038/s41593-022-01184-5
Morton S. M., Bastian A. J. (2004). Cerebellar control of balance and locomotion. Neuroscientist 10, 247–259. doi:10.1177/1073858404263517
Mugnaini E. (1983). The length of cerebellar parallel fibers in chicken and rhesus monkey. J. Comp. Neurol. 220, 7–15. doi:10.1002/cne.902200103
Mundel P., Reiser J., Borja A. Z. M., Pavenstädt H., Davidson G. R., Kriz W., et al. (1997). Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp. Cell Res. 236, 248–258. doi:10.1006/excr.1997.3739
Mut-Arbona P., Sperlágh B. (2023). P2 receptor-mediated signaling in the physiological and pathological brain: from development to aging and disease. Neuropharmacology 233, 109541. doi:10.1016/j.neuropharm.2023.109541
Nakayama H., Miyazaki T., Abe M., Yamazaki M., Kawamura Y., Choo M., et al. (2024). Direct and indirect pathways for heterosynaptic interaction underlying developmental synapse elimination in the mouse cerebellum. Commun. Biol. 7, 806–813. doi:10.1038/s42003-024-06447-4
Napper R. M. A., Harvey R. J. (1988). Quantitative study of the Purkinje cell dendritic spines in the rat cerebellum. J. Comp. Neurol. 274, 158–167. doi:10.1002/cne.902740203
Naur P., Hansen K. B., Kristensen A. S., Dravid S. M., Pickering D. S., Olsen L., et al. (2007). Ionotropic glutamate-like receptor delta2 binds D-serine and glycine. Proc. Natl. Acad. Sci. U. S. A. 104, 14116–14121. doi:10.1073/pnas.0703718104
Nedelescu H., Abdelhack M. (2013). Comparative morphology of dendritic arbors in populations of purkinje cells in mouse sulcus and apex. Neural Plast. 2013, 948587. doi:10.1155/2013/948587
Nedelescu H., Abdelhack M., Pritchard A. T. (2018). Regional differences in Purkinje cell morphology in the cerebellar vermis of male mice. J. Neurosci. Res. 96, 1476–1489. doi:10.1002/jnr.24206
Negri S., Faris P., Pellavio G., Botta L., Orgiu M., Forcaia G., et al. (2020). Group 1 metabotropic glutamate receptors trigger glutamate-induced intracellular Ca2+ signals and nitric oxide release in human brain microvascular endothelial cells. Cell. Mol. Life Sci. 77, 2235–2253. doi:10.1007/s00018-019-03284-1
Nguyen T. M., Thomas L. A., Rhoades J. L., Ricchi I., Yuan X. C., Sheridan A., et al. (2023). Structured cerebellar connectivity supports resilient pattern separation. Nature 613, 543–549. doi:10.1038/s41586-022-05471-w
Nimchinsky E. A., Sabatini B. L., Svoboda K. (2002). Structure and function of dendritic spines. Annu. Rev. Physiol. 64, 313–353. doi:10.1146/annurev.physiol.64.081501.160008
Nishiyama H., Linden D. J. (2004). Differential maturation of climbing fiber innervation in cerebellar vermis. J. Neurosci. 24, 3926–3932. doi:10.1523/JNEUROSCI.5610-03.2004
Nishiyama J., Yasuda R. (2015). Biochemical computation for spine structural plasticity. Neuron 87, 63–75. doi:10.1016/j.neuron.2015.05.043
Nitta A., Yamasaki M., Miyazaki T., Konno K., Yoshimura H., Watanabe M. (2025). Molecular and anatomical strengthening of “winner” climbing fiber synapses in developing mouse purkinje cells. J. Neurosci. 45, e2156242025. doi:10.1523/JNEUROSCI.2156-24.2025
Nomura S., Yamasaki M., Miyazaki T., Konno K., Watanabe M. (2025). Preferential localization of STIM1 to dendritic subsurface ER structures in mouse purkinje cells. J. Neurosci. 45, e1829242025. doi:10.1523/JNEUROSCI.1829-24.2025
Okamoto K. I., Narayanan R., Lee S. H., Murata K., Hayashi Y. (2007). The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc. Natl. Acad. Sci. U. S. A. 104, 6418–6423. doi:10.1073/pnas.0701656104
Okubo Y., Suzuki J., Kanemaru K., Nakamura N., Shibata T., Iino M. (2015). Visualization of Ca2+ filling mechanisms upon synaptic inputs in the endoplasmic reticulum of cerebellar purkinje cells. J. Neurosci. 35, 15837–15846. doi:10.1523/JNEUROSCI.3487-15.2015
Osorno T., Rudolph S., Nguyen T., Kozareva V., Nadaf N. M., Norton A., et al. (2022). Candelabrum cells are ubiquitous cerebellar cortex interneurons with specialized circuit properties. Nat. Neurosci. 25, 702–713. doi:10.1038/s41593-022-01057-x
Otsu Y., Marcaggi P., Feltz A., Isope P., Kollo M., Nusser Z., et al. (2014). Activity-dependent gating of calcium spikes by A-type K+ channels controls climbing fiber signaling in purkinje cell dendrites. Neuron 84, 137–151. doi:10.1016/j.neuron.2014.08.035
O’Brien J., Unwin N. (2006). Organization of spines on the dendrites of Purkinje cells. Proc. Natl. Acad. Sci. U. S. A. 103, 1575–1580. doi:10.1073/pnas.0507884103
Palay S. L., Chan-Palay V. (1974a). Cerebellar cortex. Berlin, Heidelberg: Springer Berlin Heidelberg. doi:10.1007/978-3-642-65581-4
Palay S. L., Chan-Palay V. (1974b). “Granule cells,” in Cerebellar cortex: cytology and organization (Berlin, Heidelberg: Springer Berlin Heidelberg), 63–99. doi:10.1007/978-3-642-65581-4_3
Pali E., Masoli S., Di Domenico D., Sorbo T., Prestori F., D’Angelo E. (2025). Coincidence detection between apical and basal dendrites drives STDP in cerebellar Golgi cells. Commun. Biol. 8, 731–16. doi:10.1038/s42003-025-08153-1
Palkovits M., Magyar P., Szentágothai J. (1971). Quantitative histological analysis of the cerebellar cortex in the cat. 3. Structural organization of the molecular layer. Brain Res. 34, 1–18. doi:10.1016/0006-8993(71)90347-7
Parajuli L. K., Urakubo H., Takahashi-Nakazato A., Ogelman R., Iwasaki H., Koike M., et al. (2020). Geometry and the organizational principle of spine synapses along a dendrite. eNeuro 7, 0248–20.2020. doi:10.1523/ENEURO.0248-20.2020
Park D., Bae S., Yoon T. H., Ko J. (2018). Molecular mechanisms of synaptic specificity: spotlight on hippocampal and cerebellar synapse organizers. Mol. Cells 41, 373–380. doi:10.14348/molcells.2018.0081
Park C., Gim J., Bahn S., Kim G. H., Im Y., Lee S.-H., et al. (2023). A cerebellar disinhibitory circuit supports synaptic plasticity. Bioarxiv. doi:10.1101/2023.09.15.557147
Paul M. A., Sigoillot S. M., Marti L., Urra Quiroz F. J., Delagrange M., Cheung H. W., et al. (2024). Stepwise molecular specification of excitatory synapse diversity onto cerebellar Purkinje cells. Nat. Neurosci. 28, 308–319. doi:10.1038/s41593-024-01826-w
Pchitskaya E., Bezprozvanny I. (2020). Dendritic spines shape analysis—classification or clusterization? Perspective. Front. Synaptic Neurosci. 12, 31. doi:10.3389/fnsyn.2020.00031
Peter S., Ten Brinke M. M., Stedehouder J., Reinelt C. M., Wu B., Zhou H., et al. (2016). Dysfunctional cerebellar Purkinje cells contribute to autism-like behaviour in Shank2-deficient mice. Nat. Commun. 7, 12627. doi:10.1038/ncomms12627
Piochon C., Irinopoulou T., Brusciano D., Bailly Y., Mariani J., Levenes C. (2007). NMDA receptor contribution to the climbing fiber response in the adult mouse Purkinje cell. J. Neurosci. 27, 10797–10809. doi:10.1523/JNEUROSCI.2422-07.2007
Piochon C., Levenes C., Ohtsuki G., Hansel C. (2010). Purkinje cell NMDA receptors assume a key role in synaptic gain control in the mature cerebellum. J. Neurosci. 30, 15330–15335. doi:10.1523/JNEUROSCI.4344-10.2010
Piochon C., Kruskal P., Maclean J., Hansel C. (2012). Non-Hebbian spike-timing-dependent plasticity in cerebellar circuits. Front. Neural Circuits 6, 124. doi:10.3389/fncir.2012.00124
Piochon C., Kloth A. D., Grasselli G., Titley H. K., Nakayama H., Hashimoto K., et al. (2014). Cerebellar plasticity and motor learning deficits in a copy-number variation mouse model of autism. Nat. Commun. 5, 5586. doi:10.1038/ncomms6586
Pugh J. R., Raman I. M. (2005). GABAA receptor kinetics in the cerebellar nuclei: evidence for detection of transmitter from distant release sites. Biophys. J. 88, 1740–1754. doi:10.1529/biophysj.104.055814
Purkinje jan E. (1837). Neueste Untersuchungen aus der Nerven-und Hirnanatomie. Amtlicher Ber. über Versamml. Ges. Dtsch. Naturforscher Aerzte, 177–180.
Raghu P., Joseph A., Krishnan H., Singh P., Saha S. (2019). Phosphoinositides: regulators of nervous system function in health and disease. Front. Mol. Neurosci. 12, 208. doi:10.3389/fnmol.2019.00208
Ramón y Cajal S. (1888). Estructura de los centros nerviosos de las aves. Rev. Trimest. Histol. Norm. Patològica 1, 1–10.
Rapp M., Segev I., Yarom Y. (1994). Physiology, morphology and detailed passive models of Guinea-pig cerebellar Purkinje cells. J. Physiol. 474, 101–118. doi:10.1113/jphysiol.1994.sp020006
Renzi F., Cull-Candy S. G. (2007). Climbing-fibre activation of NMDA receptors in Purkinje cells of adult mice. J. Physiol. 585, 91–101. doi:10.1113/jphysiol.2007.141531
Risher W. C., Ustunkaya T., Alvarado J. S., Eroglu C. (2014). Rapid golgi analysis method for efficient and unbiased classification of dendritic spines. PLoS One 9, e107591. doi:10.1371/journal.pone.0107591
Roberts P. D., Leen T. K. (2010). Anti-Hebbian spike-timing-dependent plasticity and adaptive sensory processing. Front. Comput. Neurosci. 4, 156–11. doi:10.3389/fncom.2010.00156
Roesler M. K., Lombino F. L., Freitag S., Schweizer M., Hermans-Borgmeyer I., Schwarz J. R., et al. (2019). Myosin XVI regulates actin cytoskeleton dynamics in dendritic spines of purkinje cells and affects presynaptic organization. Front. Cell. Neurosci. 13, 330. doi:10.3389/fncel.2019.00330
Roth A., Hausser M. (2001). Compartmental models of rat cerebellar Purkinje cells based on simultaneous somatic and dendritic patch-clamp recordings. J. Physiol. 535, 445–472. doi:10.1111/j.1469-7793.2001.00445.x
Rubio M. E., Soto F. (2001). Distinct localization of P2X receptors at excitatory postsynaptic specializations. J. Neurosci. 21, 641–653. doi:10.1523/jneurosci.21-02-00641.2001
Runge K., Cardoso C., de Chevigny A. (2020). Dendritic spine plasticity: function and mechanisms. Front. Synaptic Neurosci. 12, 36. doi:10.3389/fnsyn.2020.00036
Ryu K., Yokoyama M., Yamashita M., Hirano T. (2012). Induction of excitatory and inhibitory presynaptic differentiation by GluD1. Biochem. Biophys. Res. Commun. 417, 157–161. doi:10.1016/j.bbrc.2011.11.075
Ryu C., Jang D. C., Jung D., Kim Y. G., Shim H. G., Ryu H.-H., et al. (2017). STIM1 regulates somatic Ca2+ signals and intrinsic firing properties of cerebellar purkinje neurons. J. Neurosci. 37, 8876–8894. doi:10.1523/JNEUROSCI.3973-16.2017
Safo P., Cravatt B., Regehr W. (2006). Retrograde endocannabinoid signaling in the cerebellar cortex. Cerebellum 5, 134–145. doi:10.1080/14734220600791477
Santucci D. M., Raghavachari S. (2008). The effects of NR2 subunit-dependent NMDA receptor kinetics on synaptic transmission and CaMKII activation. PLoS Comput. Biol. 4, e1000208. doi:10.1371/journal.pcbi.1000208
Schmahmann J. D. (2019). The cerebellum and cognition. Neurosci. Lett. 688, 62–75. doi:10.1016/j.neulet.2018.07.005
Schmidt H. (2019). Control of presynaptic parallel fiber efficacy by activity-dependent regulation of the number of occupied release sites. Front. Syst. Neurosci. 13, 30–36. doi:10.3389/fnsys.2019.00030
Schonewille M., Girasole A. E., Rostaing P., Mailhes-Hamon C., Ayon A., Nelson A. B., et al. (2021). NMDARs in granule cells contribute to parallel fiber-Purkinje cell synaptic plasticity and motor learning. Proc. Natl. Acad. Sci. U. S. A. 118, e2102635118–e2102635119. doi:10.1073/pnas.2102635118
Sdrulla A. D., Linden D. J. (2007). Double dissociation between long-term depression and dendritic spine morphology in cerebellar Purkinje cells. Nat. Neurosci. 10, 546–548. doi:10.1038/nn1889
Seigneur E., Südhof T. C. (2017). Cerebellins are differentially expressed in selective subsets of neurons throughout the brain. J. Comp. Neurol. 525, 3286–3311. doi:10.1002/cne.24278
Selimi F., Lohof A. M., Heitz S., Lalouette A., Jarvis C. I., Bailly Y., et al. (2003). Lurcher GRID2-induced death and depolarization can Be dissociated in cerebellar Purkinje cells. Neuron 37, 813–819. doi:10.1016/S0896-6273(03)00093-X
Sereno M. I., Diedrichsen J., Tachrount M., Testa-Silva G., D Arceuil H., De Zeeuw C. (2020). The human cerebellum has almost 80% of the surface area of the neocortex. Proc. Natl. Acad. Sci. U. S. A. 117, 19538–19543. doi:10.1073/pnas.2002896117
Sharp A. H., McPherson P. S., Dawson T. M., Aoki C., Campbell K. P., Snyder S. H. (1993). Differential immunohistochemical localization of inositol 1,4,5-trisphosphate- and ryanodine-sensitive Ca2+ release channels in rat brain. J. Neurosci. 13, 3051–3063. doi:10.1523/jneurosci.13-07-03051.1993
Shouval H. Z., Bear M. F., Cooper L. N. (2002). A unified model of NMDA receptor-dependent bidirectional synaptic plasticity. Proc. Natl. Acad. Sci. U. S. A. 99, 10831–10836. doi:10.1073/pnas.152343099
Sigoillot S. M., Iyer K., Binda F., González-Calvo I., Talleur M., Vodjdani G., et al. (2015). The secreted protein C1QL1 and its receptor Bai3 control the synaptic connectivity of excitatory inputs converging on cerebellar purkinje cells. Cell Rep. 10, 820–832. doi:10.1016/j.celrep.2015.01.034
Skibinska-Kijek A., Wisniewska M. B., Gruszczynska-Biegala J., Methner A., Kuznicki J. (2009). Immunolocalization of STIM1 in the mouse brain. Acta Neurobiol. Exp. (Wars). 69, 413–428. doi:10.55782/ane-2009-1753
Sotelo C., Dusart I. (2009). Intrinsic versus extrinsic determinants during the development of Purkinje cell dendrites. Neuroscience 162, 589–600. doi:10.1016/j.neuroscience.2008.12.035
Spacek J., Harris K. M. (1997). Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J. Neurosci. 17, 190–203. doi:10.1523/jneurosci.17-01-00190.1997
Spanaki C., Sidiropoulou K., Petraki Z., Diskos K., Konstantoudaki X., Volitaki E., et al. (2024). Glutamate-specific gene linked to human brain evolution enhances synaptic plasticity and cognitive processes. iScience 27, 108821. doi:10.1016/j.isci.2024.108821
Stevenson M. E., Nazario A. S., Czyz A. M., Owen H. A., Swain R. A. (2021). Motor learning rapidly increases synaptogenesis and astrocytic structural plasticity in the rat cerebellum. Neurobiol. Learn. Mem. 177, 107339. doi:10.1016/j.nlm.2020.107339
Streng M. L., Carter R. E., Kottke B. W., Togneri K., Wasserman E., Rajendran V., et al. (2025). Purkinje cell spatial correlation dynamics are key to cerebellar cortical contributions to behavior. J. Neurosci. 45, e1915242025. doi:10.1523/JNEUROSCI.1915-24.2025
Südhof T. C. (2008). Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455, 903–911. doi:10.1038/nature07456
Südhof T. C. (2017). Synaptic neurexin complexes: a molecular code for the logic of neural circuits. Cell 171, 745–769. doi:10.1016/j.cell.2017.10.024
Südhof T. C. (2023). Cerebellin–neurexin complexes instructing synapse properties. Curr. Opin. Neurobiol. 81, 102727. doi:10.1016/j.conb.2023.102727
Sugawara T., Hisatsune C., Le T. D., Hashikawa T., Hirono M., Hattori M., et al. (2013). Type 1 inositol trisphosphate receptor regulates cerebellar circuits by maintaining the spine morphology of purkinje cells in adult mice. J. Neurosci. 33, 12186–12196. doi:10.1523/JNEUROSCI.0545-13.2013
Sugawara T., Hisatsune C., Miyamoto H., Ogawa N., Mikoshiba K. (2017). Regulation of spinogenesis in mature Purkinje cells via mGluR/PKC-mediated phosphorylation of CaMKII. Proc. Natl. Acad. Sci. U. S. A. 114, E5256-E5265–E5265. doi:10.1073/pnas.1617270114
Suk E., Lai K., Uesaka N. (2025). Reduced GABAergic inhibition and impaired synapse elimination by neuroligin-2 deletion from Purkinje cells of the developing cerebellum, 1–18. doi:10.3389/fncir.2025.1530141
Szabó L. E., Marcello G. M., Süth M., Sótonyi P., Rácz B. (2021). Distribution of cortactin in cerebellar Purkinje cell spines. Sci. Rep. 11, 1375–11. doi:10.1038/s41598-020-80469-w
Sziber Z., Torrents-Solé P., Kovacevic A., Kapfhammer J. P. (2025). Protein kinase C gamma regulates Purkinje cell dendritic spine development in a mouse model of spinocerebellar ataxia. Exp. Neurol. 393, 115377. doi:10.1016/j.expneurol.2025.115377
Tabata T., Kano M. (2006). GABAB receptor-mediated modulation of glutamate signaling in cerebellar Purkinje cells. Cerebellum 5, 127–133. doi:10.1080/14734220600788911
Takeo Y. H., Shuster S. A., Jiang L., Hu M. C., Luginbuhl D. J., Rülicke T., et al. (2021). GluD2-and Cbln1-mediated competitive interactions shape the dendritic arbors of cerebellar Purkinje cells. Neuron 109, 629–644.e8. doi:10.1016/j.neuron.2020.11.028
Tan L., Shi J., Moghadami S., Parasar B., Wright C. P., Seo Y., et al. (2023). Lifelong restructuring of 3D genome architecture in cerebellar granule cells. Science 381, 1112–1119. doi:10.1126/science.adh3253
Tao-Cheng J.-H. (2025). Ultrastructural characterization of peri-synaptic astrocytic processes around cerebellar Purkinje spines under resting and stimulated conditions. Mol. Brain 18, 28. doi:10.1186/s13041-025-01198-7
Thomas R. E., Mudlaff F., Schweers K., Farmer W. T., Suvrathan A. (2024). Heterogeneity in slow synaptic transmission diversifies purkinje cell timing. J. Neurosci. 44, e0455242024. doi:10.1523/JNEUROSCI.0455-24.2024
Tian W., Zhou J., Bartlett A., Zeng Q., Liu H., Castanon R. G., et al. (2022). Epigenomic complexity of the human brain revealed by single-cell DNA methylomes and 3D genome structures. bioRxiv 11.30, 518285. doi:10.1101/2022.11.30.518285
Toledo A., Lang F., Doengi M., Morrison H., Stein V., Baader S. L. (2019). Merlin modulates process outgrowth and synaptogenesis in the cerebellum. Brain Struct. Funct. 224, 2121–2142. doi:10.1007/s00429-019-01897-7
Tolve M., Tutas J., Özer-Yildiz E., Klein I., Petzold A., Fritz V. J., et al. (2025). The endocytic adaptor AP-2 maintains Purkinje cell function by balancing cerebellar parallel and climbing fiber synapses. Cell Rep. 44, 115256. doi:10.1016/j.celrep.2025.115256
Tomiyama M., Palacios J. M., Cortés R., Mengod G. (1999). Flip and flop variants of AMPA receptor subunits in the human cerebellum: implication for the selective vulnerability of Purkinje cells. Synapse 31, 163–167. doi:10.1002/(SICI)1098-2396(199902)31:2<163::AID-SYN10>3.0.CO;2-H
Tønnesen J., Katona G., Rózsa B., Nägerl U. V. (2014). Spine neck plasticity regulates compartmentalization of synapses. Nat. Neurosci. 17, 678–685. doi:10.1038/nn.3682
Uemura T., Lee S. J., Yasumura M., Takeuchi T., Yoshida T., Ra M., et al. (2010). Trans-synaptic interaction of GluRdelta2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell 141, 1068–1079. doi:10.1016/j.cell.2010.04.035
Uemura T., Suzuki-Kouyama E., Kawase S., Kurihara T., Yasumura M., Yoshida T., et al. (2022). Neurexins play a crucial role in cerebellar granule cell survival by organizing autocrine machinery for neurotrophins. Cell Rep. 39, 110624. doi:10.1016/j.celrep.2022.110624
Uesaka N., Abe M., Konno K., Yamazaki M., Sakoori K., Watanabe T., et al. (2018). Retrograde signaling from progranulin to Sort1 counteracts synapse elimination in the developing cerebellum. Neuron 97, 796–805. doi:10.1016/j.neuron.2018.01.018
Uriu Y., Kiyonaka S., Miki T., Yagi M., Akiyama S., Mori E., et al. (2010). Rab3-interacting molecule γ isoforms lacking the rab3-binding domain induce long lasting currents but block neurotransmitter vesicle anchoring in voltage-dependent P/Q-type Ca2+ channels. J. Biol. Chem. 285, 21750–21767. doi:10.1074/jbc.M110.101311
van der Heijden M. E., Sillitoe R. V. (2021). Interactions between purkinje cells and granule cells coordinate the development of functional cerebellar circuits. Neuroscience 462, 4–21. doi:10.1016/j.neuroscience.2020.06.010
van der Heijden M. E., Lackey E. P., Perez R., Işleyen F. S., Brown A. M., Donofrio S. G., et al. (2021). Maturation of Purkinje cell firing properties relies on neurogenesis of excitatory neurons. Elife 10, e68045–37. doi:10.7554/eLife.68045
Van Overwalle F. (2024). Social and emotional learning in the cerebellum. Nat. Rev. Neurosci. 25, 776–791. doi:10.1038/s41583-024-00871-5
Vecellio M., Schwaller B., Meyer M., Hunziker W., Celio M. R. (2000). Alterations in Purkinje cell spines of calbindin D-28 k and parvalbumin knock-out mice. Eur. J. Neurosci. 12, 945–954. doi:10.1046/j.1460-9568.2000.00986.x
Verslegers M., Van Hove I., Dekeyster E., Gantois I., Hu T. T., D’Hooge R., et al. (2014). MMP-2 mediates Purkinje cell morphogenesis and spine development in the mouse cerebellum. Brain Struct. Funct. 220, 1601–1617. doi:10.1007/s00429-014-0747-3
Vlachos A., Korkotian E., Schonfeld E., Copanaki E., Deller T., Segal M. (2009). Synaptopodin regulates plasticity of dendritic spines in hippocampal neurons. J. Neurosci. 29, 1017–1033. doi:10.1523/JNEUROSCI.5528-08.2009
VoŽeh F. (2015). Jan Evangelista Purkyně and the cerebellum then and now. Physiol. Res. 64, S567–S584. doi:10.33549/physiolres.933231
Wagner W., Brenowitz S. D., Hammer J. A. (2011). Myosin-Va transports the endoplasmic reticulum into the dendritic spines of Purkinje neurons. Nat. Cell Biol. 13, 40–48. doi:10.1038/ncb2132
Walter J. T., Dizon M.-J., Khodakhah K. (2009). The functional equivalence of ascending and parallel fiber inputs in cerebellar computation. J. Neurosci. 29, 8462–8473. doi:10.1523/JNEUROSCI.5718-08.2009
Wang Y., Deng X., Mancarella S., Hendron E., Eguchi S., Soboloff J., et al. (2010). The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science 330, 105–109. doi:10.1126/science.1191086
Wang B., Lebel A., Mello A. M. D. (2025). Trends in Cognitive Sciences Ignoring the cerebellum is hindering progress in neuroscience. Trends Cogn. Sci. xx, 1–13. doi:10.1016/j.tics.2025.01.004
Wilms C. D., Häusser M. (2015). Reading out a spatiotemporal population code by imaging neighbouring parallel fibre axons in vivo. Nat. Commun. 6, 6464. doi:10.1038/ncomms7464
Yamashita A., Makita K., Kuroiwa T., Tanaka K. (2006). Glutamate transporters GLAST and EAAT4 regulate postischemic Purkinje cell death: an in vivo study using a cardiac arrest model in mice lacking GLAST or EAAT4. Neurosci. Res. 55, 264–270. doi:10.1016/j.neures.2006.03.007
Yoast R. E., Emrich S. M., Zhang X., Xin P., Johnson M. T., Fike A. J., et al. (2020). The native ORAI channel trio underlies the diversity of Ca2+ signaling events. Nat. Commun. 11, 2444. doi:10.1038/s41467-020-16232-6
Yuste R. (2015). The discovery of dendritic spines by Cajal. Front. Neuroanat. 9, 18–6. doi:10.3389/fnana.2015.00018
Yuste R., Bonhoeffer T. (2004). Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat. Rev. Neurosci. 5, 24–34. doi:10.1038/nrn1300
Yuzaki M., Aricescu A. R. (2017). A GluD coming-of-age story. Trends Neurosci. 40, 138–150. doi:10.1016/j.tins.2016.12.004
Zárský V. (2012). Jan Evangelista Purkyně/Purkinje (1787-1869) and the establishment of cellular physiology--Wrocław/Breslau as a central European cradle for a new science. Protoplasma 249, 1173–1179. doi:10.1007/s00709-012-0407-5
Zeng W., Yuan J. P., Kim M. S., Choi Y. J., Huang G. N., Worley P. F., et al. (2008). STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol. Cell 32, 439–448. doi:10.1016/j.molcel.2008.09.020
Zhang B., Chen L. Y., Liu X., Maxeiner S., Lee S.-J., Gokce O., et al. (2015). Neuroligins sculpt cerebellar purkinje-cell circuits by differential control of distinct classes of synapses. Neuron 87, 781–796. doi:10.1016/j.neuron.2015.07.020
Zhang R. S., Liakath-Ali K., Südhof T. C. (2020). Latrophilin-2 and latrophilin-3 are redundantly essential for parallel-fiber synapse function in cerebellum. Elife 9, e54443–21. doi:10.7554/eLife.54443
Zheng J., Yang Q., Makris N., Huang K., Liang J., Ye C., et al. (2023). Three-Dimensional digital reconstruction of the cerebellar cortex: lobule thickness, surface area measurements, and layer architecture. Cerebellum 22, 249–260. doi:10.1007/s12311-022-01390-8
Keywords: Purkinje cell, cerebellum, spines, synapses, history, parallel fibers
Citation: Masoli S, Rizza MF, Moccia F and D’Angelo E (2025) The spiny relationship between parallel fibers, climbing fibers, and Purkinje cells. Front. Physiol. 16:1671271. doi: 10.3389/fphys.2025.1671271
Received: 22 July 2025; Accepted: 19 September 2025;
Published: 09 October 2025.
Edited by:
Roberto Piacentini, Catholic University of the Sacred Heart, ItalyReviewed by:
Christian Hansel, The University of Chicago, United StatesTaegon Kim, Korea Institute of Science and Technology, Republic of Korea
Copyright © 2025 Masoli, Rizza, Moccia and D’Angelo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefano Masoli, c3RlZmFuby5tYXNvbGlAdW5pcHYuaXQ=; Egidio D’Angelo, ZGFuZ2Vsb0B1bmlwdi5pdA==
 Stefano Masoli
Stefano Masoli Martina Francesca Rizza
Martina Francesca Rizza Francesco Moccia
Francesco Moccia Egidio D’Angelo
Egidio D’Angelo