- Faculty of Medicine–Division of Biomedical Sciences–Memorial University of Newfoundland, St. John’s, NL, Canada
Ventricular tachycardias (VTs) and fibrillations (VFs) are frequent complications of ischemic myocardial infarction (MI). Because their initiation mechanism remains unknown, these arrhythmias are virtually unpredictable and often degenerate into cardiac arrest and syncope without immediate medical assistance. Electrical mapping and ablation techniques have located the origin of ischemic arrhythmias in the terminal arborizations of the cardiac conduction system, the Purkinje fibers. A classical model of MI in the dog has demonstrated that abnormal calcium (Ca2+) cycling in the Purkinje cells (Pcells) is the source of non-driven depolarizations (DADs) in the conduction tissue and is likely to create the pro-arrhythmic conditions of human ischemic heart. A better understanding of Ca2+ abnormalities in Pcells post infarction is an evident prerequisite for elucidating the mechanism of ischemic arrhythmias. Nevertheless, a unique Ca2+ handling system was discovered in Pcells, exhibiting fundamental differences compared with the well-known model of Excitation-Contraction coupling of ventricular cardiomyocytes. This cellular specificity of Purkinje fibers was observed in large mammalian species but not in murine hearts, where Purkinje cells are comparable to ventricular myocytes and designed to respond to 400–600 stimulations/min. The present report reviews the mechanism of Ca2+ arrhythmogenicity in Pcells of large mammalian hearts and documents the need for animal models that simulate the size and function of human hearts to study ischemic arrhythmias.
Introduction
Sudden cardiac death (SCD) accounts for 15%–20% of the mortality in adults worldwide. This dramatic issue is most frequently associated with ischemic heart disease (or coronary artery disease) and is caused by life-threatening ventricular tachycardia (VTs) and fibrillation (VFs). These tachyarrhythmias commonly arise in the setting of myocardial ischemia secondary to a coronary occlusion, particularly in the early stage of the myocardial infarction (MI) (Haissaguerre et al., 2016). The pathophysiology of those arrhythmias involves a complex interplay between ischemia-induced changes in ion channel function, cellular metabolism, and structural remodelling in the ventricular myocardium (Ter Keurs and Boyden, 2007). However, the Purkinje fibers have been increasingly recognized as key contributors to the specific arrhythmogenesis post-MI (Haissaguerre et al., 2016; Nogami, 2011; Benali et al., 2024). There is a consensual agreement that this arrhythmic risk originates from intracellular calcium dysregulation in Purkinje cells (Ter Keurs and Boyden, 2007). Intracellular Ca2+ concentration oscillations in these cells generate non-driven (non-sinusal) electric impulses in the fibers, which trigger focal ectopic activity and reentry mechanisms in the myocardium. Understanding these Ca2+ abnormalities in Purkinje cells is the key to identifying and treating a fundamental cause of SCD post myocardial infarction. As with most human diseases, animal models are indispensable for investigating the underlying mechanisms of arrhythmia. Nevertheless, many questions regarding the physiology of Purkinje fibers still make it difficult to choose a suitable model. Even though our understanding of Purkinje cells is incomplete, it already indicates that the classical model of cardiac “excitation-contraction” coupling (Bers, 2002) does not apply to those cells. In addition, numerous observations have demonstrated that Purkinje cells exhibit structural differences that result in their Ca2+ handling varying across species. Considering the consensual implication of Ca2+ in the triggered arrhythmias of ischemic heart (see (Ter Keurs and Boyden, 2007) for review), the choice of animal models is crucial for investigating the origins of ischemic arrhythmias post infarction in human patients. This brief review summarizes our current knowledge of Purkinje cells and highlights some key elements in selecting the most suitable animal model for the pathophysiology of human Purkinje fibers.
Structural and electrophysiological characteristics of Purkinje cells
The Purkinje fibers
As initially described by Sunao Tawara (Akiyama, 2010), the Purkinje fibers (Figure 1A) form the terminal arborizations of the conduction system, branching in every region of the ventricles as prolongations of the His-Purkinje bundles. They are responsible for transmitting the nodal impulses to the working cardiomyocytes and coordinating the contraction of the ventricular chambers during the heartbeat. The Purkinje tissue is present in the ventricles as free-running strands in the subendocardial region and transmural fibers in the endocardium (Pauziene et al., 2017; Boyden et al., 1989). To date, no evidence has been found to support fundamental differences between the two types of fibers. However, the ratio of free strands to transmural varies among species. This ratio determines the accessibility of these fibers in animal models, such as the rat, which has a high density of intramural fibers, or the dog, which has a high density of free strands in the subendocardial region. The fibers are connected to the myocardium by transitional cells (Martinez-Palomo et al., 1970). Although very little is known about transitional cells, a possible role in the regulation of the conduction at the Purkinje-myocardium interface has been proposed, and some studies have implicated these cells as potential sources of arrhythmogenicity (Blackwell et al., 2022; Behradfar et al., 2014).
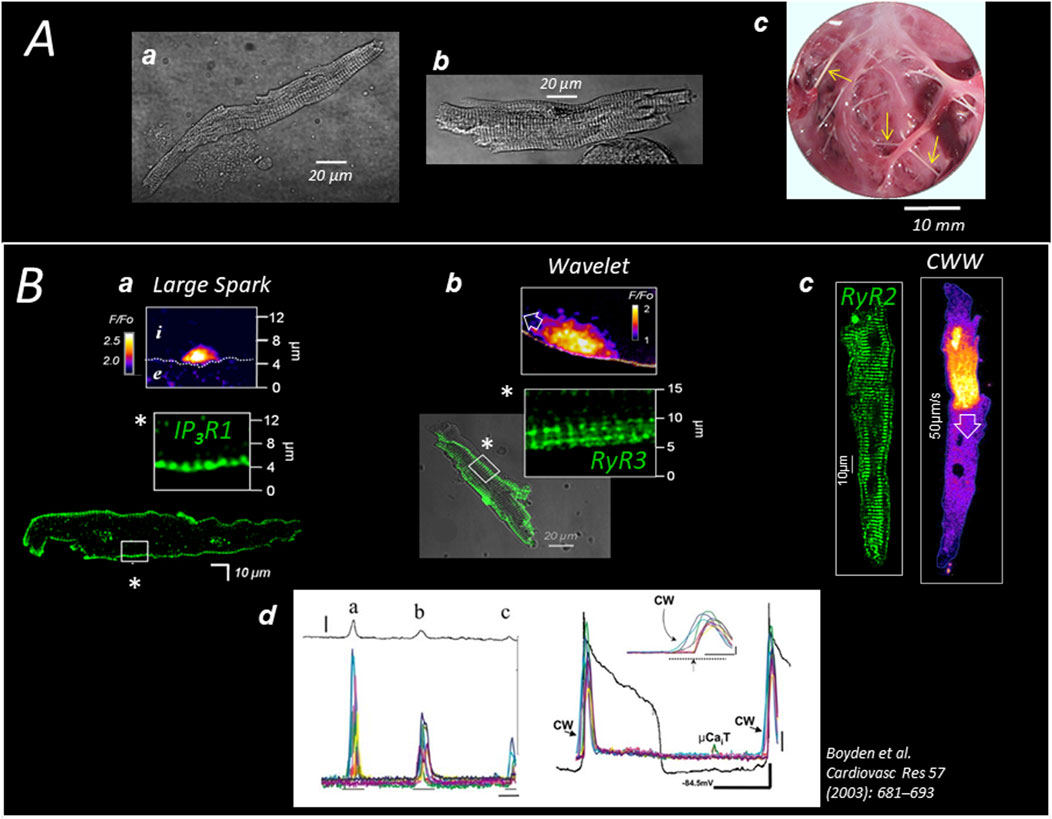
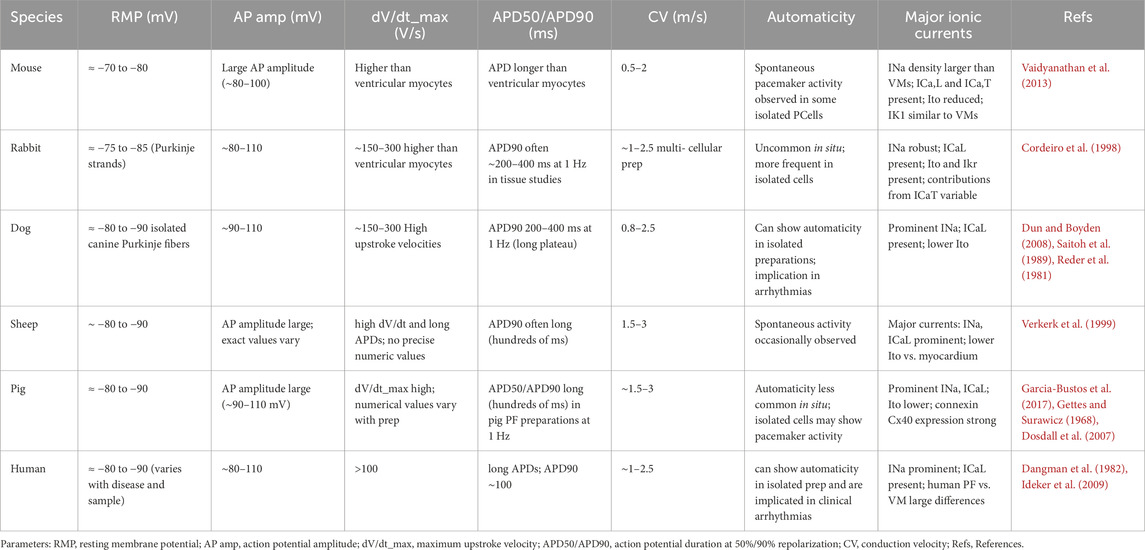
Figure 1. Specific Ca2+ handling of cardiac Purkinje cells in large mammalian species (A) Pig (a) and human (b) Purkinje cells are enzymatically dispersed from sub-endocardial (free-running) Purkinje strands (c; see arrows in pig heart). (B) The expression of 3 different channels (here in dog heart) delimits three concentric regions of SR-Ca2+ release; the first region (a) extends 2–3 µm under the sarcolemma (SL) and expresses the Inositol Phosphate Receptor IP3R1 which generates large Ca2+ sparks; the second layer (b) extends 5–10 µm under the SL and expresses the (non-cardiac) ryanodine receptor RyR3; the RyR3 region produces small waves (Wavelets) which propagate on short distances exclusively at the cell periphery and are of the same amplitude than sparks; the third layer (c) fills most of the cell core, expressing the typical cardiac ryanodine receptor RyR2 which produces large cell-wide waves (CWWs); CWWs (d) induce membrane depolarizations (DADs) proportional to the density and amplitude of the waves present in the cell or in the cell aggregate (left panel) and, occasionally, can trigger a full action potential (right panel).
The cardiac Purkinje cells: structure and morphology
Purkinje cells and ventricular cardiomyocytes share the same rod shape and sarcomeric striation due to an organized myofibrillar system (Figure 1A). Although their density and arrangement in Purkinje cells vary among species (see Table 1), the myofibrils give both cell types a similar macroscopic appearance. This explains why the Purkinje cells are still referred to as “Purkinje myocytes” and have been considered a model for cardiac cell physiology in many past studies. In both cell types, the sarcomeres shorten when the surrounding Ca2+ concentration rises, but with apparently slower kinetics in Purkinje cells, suggesting differences in the protein composition of the sarcomeres. The reasons Purkinje cells encompass a functional contractile machinery remains unclear. It could be to coordinate the position of the Purkinje and myocardial fibers during the contraction.
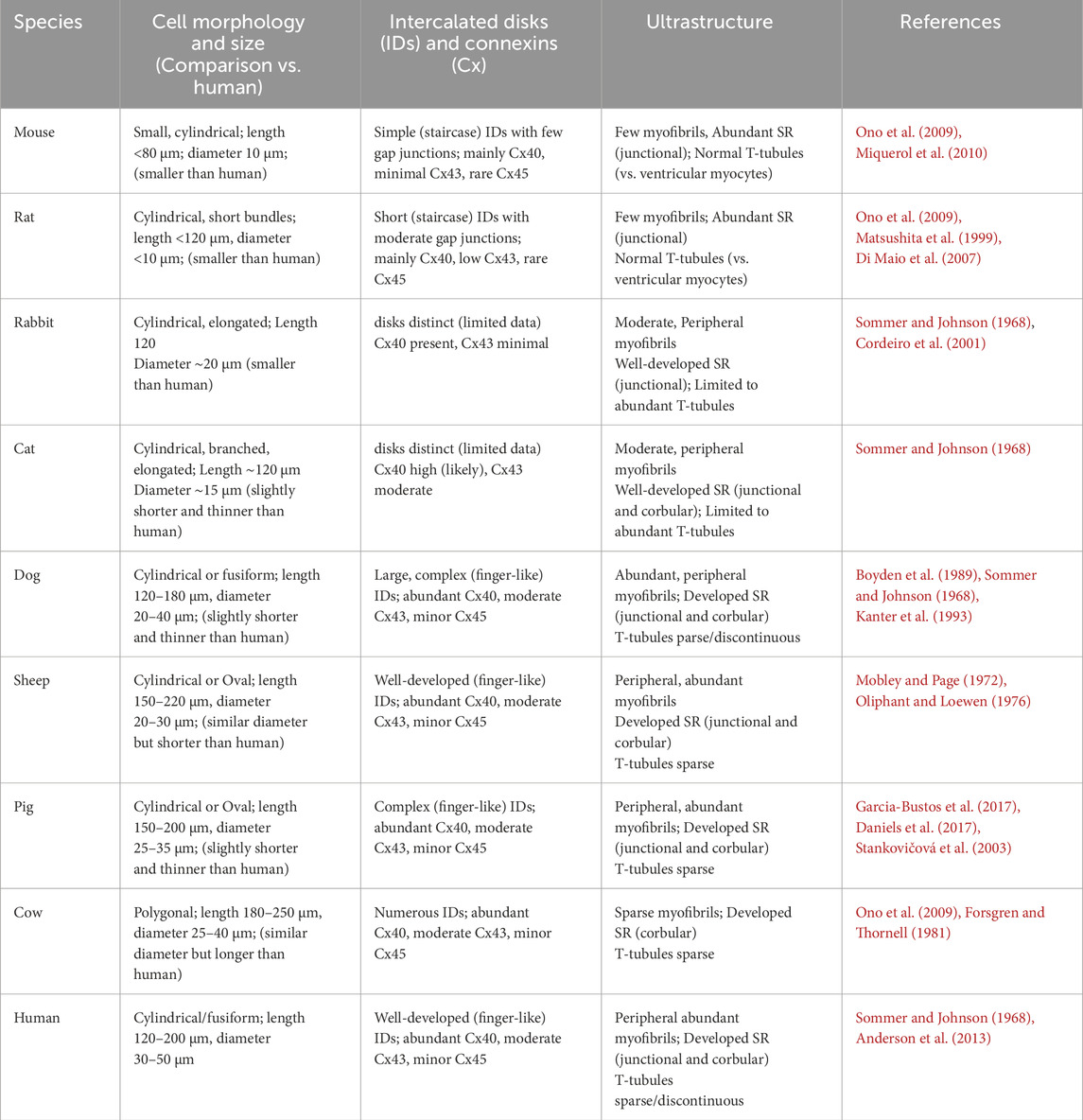
Table 1. This table summarizes the comparative morphology and ultrastructure of cardiac Purkinje cells across several mammalian species.
As shown in Table 1, the morphology of Purkinje cells varies among mammalian species, but the major differences are observed between small and large hearts, likely due to distinct electrophysiological constraints (see Table 2).
The cardiac Purkinje cells: electrophysiology
The impulse velocity in the fibers varies with the heart size, likely related to the propagation distance, ranging from 1–2 m/s in small rodents to more than 4 m/s in larger animals (see Table 2), i. e., approximately tenfold larger compared to myocardium (0.3–0.4 m/s) (Durrer et al., 1970). The low resistance of Purkinje fibers (compared to myocardium) facilitates the rapid conduction of nodal impulses across the ventricle. Gap junctions in the intercalated disks participate in this low resistance. Electric and Ca2+ signals propagate cell-to-cell in the Purkinje fiber through the gap junctions, which, like those in ventricular myocytes, contain channels composed of connexins 40 (Cx40) and 43 (Cx43) (Table 1). The conductance of the Cx40 channel is twice as high as that of the Cx43 channel, and Cx40 is three times more concentrated in Purkinje cells compared to ventricular cells (Sivagangabalan et al., 2014; Kanter et al., 1993). This predominance of C x 40 is likely to facilitate the rapid intercellular current flow and contribute to the high conduction velocity in the Purkinje fibers. Connexin 45 forms low-conductance gap junction channels and is also expressed in the Purkinje cells, possibly modulating the conduction in the fibers (Dun and Boyden, 2008).
At the cellular level, presumably still related to the conduction function of Purkinje tissue, there are significant differences in the electrophysiology of Purkinje cells compared to ventricular myocytes, as thoroughly reviewed in (Dun and Boyden, 2008; Boyden et al., 2010). In brief, the Purkinje cells have a longer action potential with a prominent phase 1 of repolarization, longer APD50 and APD90, a more negative plateau, and larger AP amplitude than ventricular cells (Dun and Boyden, 2008). Although two levels of resting membrane potential (RMP) have been reported in Purkinje fibers (Gadsby and Cranefield, 1977), it is widely recognized that, under normal physiological conditions, the RP (∼-80 mV) is comparable to that of ventricular myocytes (Boyden et al., 2010). However, a slow diastolic depolarization due to the presence of IF current can arise in Purkinje fibers in the absence of overdrive suppression by the sinus rhythm (Dun and Boyden, 2008). Although the regular activity of Purkinje fibers in the heart is triggered, the presence of IF current attributes the Purkinje tissue with a natural tendency towards automaticity, ranging from 20 to 40 beats per minute in large mammalian species, including humans (Vassalle, 1977).
Ca2+ handling and Ca2+ mobilization of Purkinje cells
The primary function of Purkinje cells is to generate an AP in response to evoked depolarization from adjacent cells in the fiber. The membrane depolarization and formation of action potential in Purkinje cells involve voltage-gated channels, with specific isoform profiles explicitly expressed in those cells as listed in (Dun and Boyden, 2008). Nearly as a side effect of the electrical transmission function of Purkinje cells, the depolarization is accompanied by an influx of Ca2+ in the cytosol due to the activation of two voltage gated Ca2+ channels: the L-type Ca2+ channel (LTCC) with the two isoforms Cav1.2 and Cav1.3, and the T-type Ca2+ channel (TTCC) with the three isoforms Cav3.1, Cav3.2, and Cav3.3 (Dun and Boyden, 2008; Rosati et al., 2007). Unlike ventricular myocytes, a large representation of T-type Ca2+ current (ICaT) compared to L-type Ca2+ current (ICaL) has been reported in Purkinje fibers (Tseng and Boyden, 1989). ICa,T activates at more hyperpolarizing potentials than the high-voltage ICa,L, which is predominant in cardiomyocytes. Strongly expressed in nodal cells and Purkinje cells, a role in pacemaker activity has been attributed to ICa,T (Mangoni and Nargeot, 2008). These Ca2+ currents trigger further Ca2+ release from the intracellular Ca2+ compartment, the sarcoplasmic reticulum (SR), in a process called “Ca2+ induced Ca2+ Release” (CICR) (Fabiato, 1983). In ventricular cardiomyocytes, the SR is arranged around the myofibrils. Tubular invaginations of the sarcolemma, called transverse tubules (T tubules), extend deep in the cell at the level of every Z-disc in the myofibrils. In this region, referred to as the “dyadic cleft”, the T-tubules are close to the terminal cisternae of the SR (junctional SR) so that L-type Ca2+ channels in the membrane of the tubules face clusters of Ca2+ channels, RyR2, in the SR membrane. This arrangement is ubiquitously distributed in cardiomyocytes and is crucial for the uniform CICR and synchronous contraction of those cells. Ca2+ is released from the junctional SR in the dyadic cleft, but it can also occur outside, from isolated extremities of the SR called corbular SR (Franzini-Armstrong et al., 1999).
In large mammalian species, such as dogs, sheep, pigs, and humans (see Table 1), Purkinje cells are devoid of organized transverse tubular system (Sommer and Johnson, 1968; Cordeiro et al., 2001; Daniels et al., 2017; Sommer and Johnson, 1970) and exhibit an internal structure comparable to that of atrial myocytes (Bootman et al., 2011; Mackenzie et al., 2004). In this condition, the intracellular Ca2+ mobilization in Purkinje cells primarily relies on the release of Ca2+ by the corbular SR in the core and, to a lesser extent, by the junctional SR under the membrane (Ter Keurs and Boyden, 2007). Upon electric stimulation, Ca2+ enters the cell mostly across the peripheral membrane through the voltage-gated inward Ca2+ currents. The absence of T tubules in the core predicts that, in Purkinje cells, the influence of Na-Ca exchange (NCX) is limited to a restricted space under the sarcolemma, known as the “SubSL” compartment (Stuyvers et al., 2005), and Ca2+ in the center is modulated by diffusion and SR Ca2+ transport systems.
In large mammalian species, the absence of T tubules reduces the total membrane surface area of the cell, which likely also contributes to the low total membrane resistance and rapid conduction of Purkinje fibers. The functional consequence of this structural particularity in Purkinje cells is a non-uniform and slower intracellular Ca2+ mobilization compared to that of ventricular cardiomyocytes (Boyden et al., 2003). A biphasic Ca2+ response to electrical stimulation was reported in Purkinje cells, based on the aequorin signal, by Wier 45 years ago (Gil and Hess, 1984). Consistently, using more recent Ca2+ probes and advanced Ca2+ imaging techniques, we found that stimulation induces a first release of Ca2+ under the sarcolemma, probably from junctional SR in the SubSL, followed by the progression of a front of elevated Ca2+ toward the cell center (Haq et al., 2013); see Figure 2. Sarcomere shortening is observed at the end of the progression, when the cytosol is fully loaded with Ca2+. A specific model of Ca2+ mobilization demonstrated that this “centripetal” propagation results from a combination of Ca2+ diffusion and consecutive Ca2+ release (by CICR) from concentric layers of the SR (Dun and Boyden, 2008; Boyden et al., 2010); Figure 2B. Presumably supporting this centripetal Ca2+ mobilization and, more generally, possibly compensating for the absence of T tubules, Purkinje cells were shown to express in canine heart three types of SR-Ca2+ channels (Daniels et al., 2017; Stuyvers et al., 2005) (Figures 1B, 2A): IP3R1 under the sarcolemma, RyR3 deeper but still in the peripheral region of the SR, and the “cardiac” RyR2 in the central SR. The distinct localization of these channels defines three specific regions of SR-Ca2+ release (Stuyvers et al., 2005). So far, we have observed the same arrangement of channels and the same centripetal Ca2+ mobilization in dog, sheep, pig, and human Purkinje cells.
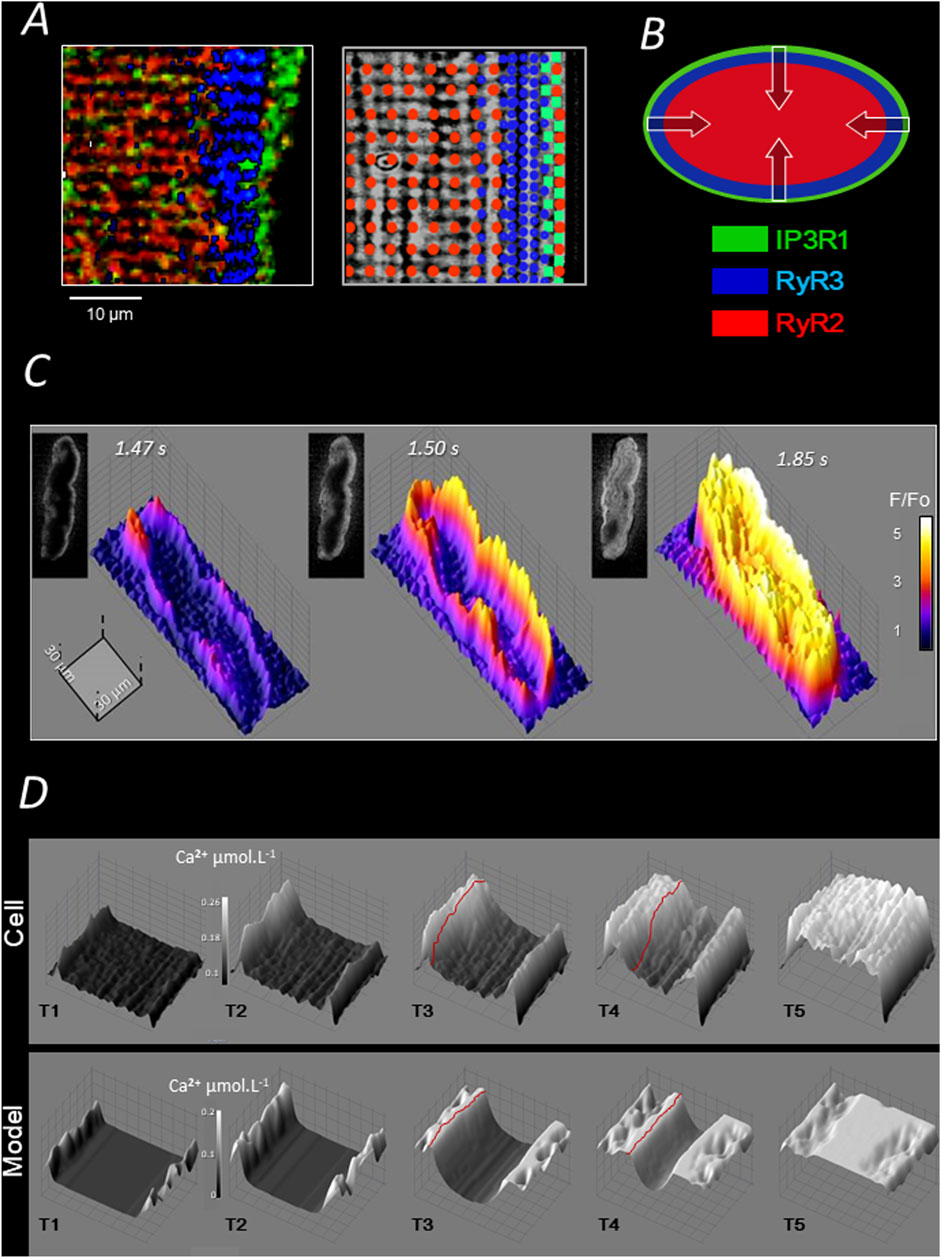
Figure 2. Ca2+ handling and electrically evoked Ca2+ mobilization of pig Purkinje cells. (A) Each of the three channel regions described in Figure 1B is stained with a specific antibody in the left panel (color legend is represented in B); the three regions partially overlap over a few micrometers; as shown in the right panel, the intermediate expression of RyR3 overlaps with both RyR2 and IP3R regions and some RyR2 are expressed in the IP3R region (Stuyvers et al., 2005); (B) the layered arrangement of SR Ca2+ channels shown in A allows for centripetal “layer-to-layer” activation by CICR and Ca2+ diffusion (Daniels et al., 2017). (C) 2D and 3D illustrations of three representative sequences of the (AP-mediated) centripetal Ca2+ propagation in pig Purkinje cells (see text). (D) Computational modelling of the centripetal propagation of Ca2+ in pig Purkinje cells; red lines underline the Ca2+ front moving toward the cell centre by CICR and diffusion (Haq et al., 2013).
In summary, as shown in Figure 2D, the electric stimulation of Purkinje cells mediates a typical centripetal Ca2+ mobilization, which is produced by the consecutive activation of IP3R1-, RyR3-, and RyR2-Ca2+ release regions. A computational model of this mechanism has been proposed in (Haq et al., 2013).
Like RyR2 in the ventricular myocytes, the three SR-Ca2+ release channels of the Purkinje cell spontaneously generate stochastic spark- and wave-like events (Stuyvers et al., 2005), as illustrated in Figure 1B. Because of different biophysical properties, the three channels open at distinct Ca2+ concentrations and with different kinetics, creating three regions of specific spontaneous Ca2+ events (Figure 1B); (Stuyvers et al., 2005; Daniels et al., 2017): large asymmetrical Ca2+ sparks under the sarcolemma, small Ca2+ waves (“wavelets”) that propagate over short distances in the peripheral RyR3-region, and large Ca2+ waves (“Cell-Wide-Waves” or “CWWs”) spanning the entire width of the cell and travelling in the cell and cell-to-cell in the longitudinal direction of the fibers. The CWWs are of sufficient magnitude to depolarize the membrane through NCX activation and are the Ca2+ events underlying the DADs in Purkinje cells (Boyden et al., 2000); see Panel B in Figure 1. Wavelets are thought to be the triggering events (by CICR) of the CWWs in the cell periphery (Stuyvers et al., 2005). Regular Ca2+ sparks were observed in the three regions of Ca2+ release (Stuyvers et al., 2005; Hirose et al., 2006).
Ca2+ arrhythmogenicity of Purkinje fibers post infarction
Delayed afterdepolarization (DAD) is the electrical event that initiates triggered arrhythmias in the Purkinje fibers. DADs are observed in all cardiac cells and are caused by spontaneous rises in cytoplasmic Ca2+ concentration (Figure 1B), commonly resulting from SR Ca2+ release (Hirose et al., 2006), and, occasionally in cardiomyocytes, by sudden Ca2+ demobilization from the myofibrillar troponin C (Ter Keurs et al., 2006). The increase of cytosolic Ca2+ activates NCX, generating a forward mode INCX current, as well as other Ca2+−sensitive currents, such as chloride currents and non-selective cationic currents (Ter Keurs and Boyden, 2007). The resulting ionic imbalance depolarizes the membrane, generating a DAD. Technically, the amplitude of the DAD depends on the size of the resting K+ conductance, mainly determined by the inward-rectifier K+-current IK1, relative to INCX amplitude (Landstrom et al., 2017). A spontaneous AP can arise when the DAD amplitude reaches the threshold of the inward INa current (Figure 1B). The spontaneous APs in Purkinje fibers activate the surrounding myocardium, producing a premature ventricular beat/contraction (PVB/PVC), the first marker of a more severe tachycardic occurrence (Reder et al., 1981; Dosdall et al., 2007). The frequency and amplitude of DADs and PVBs determine the onset of the tachyarrhythmia in the ventricle.
Therefore, Ca2+ is the principal player in the generation of DADs, and the abnormal spontaneous Ca2+ activity in the Purkinje cells is widely recognized as a cause of tachyarrhythmias in the ischemic heart (Haissaguerre et al., 2016).
Although the fundamental alteration that leads to the “electrogenic” Ca2+ release in Purkinje cells remains unknown, several hypotheses can be discussed from our current understanding of Purkinje cells. First, inspired by the alteration of the RyR2 reported in inherited tachycardic diseases, such as CPVT (Priori and Chen, 2011; Herron et al., 2010; Kang et al., 2010), aberrant SR-Ca2+ release has been proposed to explain the arrhythmogenicity of Purkinje cells post-infarction (Hirose et al., 2006; Hirose et al., 2008). Similarly, an upregulation of SR-Ca2+ release by the reticular protein CASQ2 (Chen et al., 2013) or a reduction of its Ca2+ buffering capacity due, e.g., to an alteration similar to the mutation discussed in (Faggioni and Knollmann, 2012; Galimberti and Knollmann, 2011), could increase the Ca2+ liberation by the SR. Alternatively, a potentiation of SR-Ca2+ uptake would be expected to accelerate the transfer of Ca2+ from Ca2+ pumps to the Ca2+ release channels, possibly causing the abnormal increase in SR-Ca2+ release in Purkinje cells.
Beyond the alteration underlying the pro-arrhythmic augmentation of spontaneous Ca2+ release, another striking question is how ischemia in the ventricular myocardium induces the Ca2+ arrhythmogenicity in the Purkinje fibers.
It is well known that Ca2+ overload arises in cells directly exposed to ischemia, mainly due to the depletion of cellular energy and depression of ATP-dependent Ca2+ extrusion from the cytosol. In addition to loading the SR with Ca2+ and increasing the spontaneous SR-Ca2+ release (“SR Ca2+ leak”), the excess of Ca2+ in the cell triggers multiple reactions involved in apoptosis, hibernation, and cell death (Webster, 2012; Lüss et al., 1998). This is the case in ventricular myocytes of the infarction area. However, the early evidence of increased spontaneous Ca2+ activity in Purkinje cells after MI has been found in free-running Purkinje fibers spanning the subendocardial region. Except at the Purkinje-myocardium interface, these fibers are anatomically independent of the ventricular myocardium and are frequently connected to the endocardium outside the ischemic area. In this situation, it is logical to anticipate that these free-running strands primarily rely on O2 and nutrients from the surrounding blood flow in the chamber (Janse and Wit, 1989) and are not directly exposed to the ischemic conditions affecting the myocardium. Interestingly, this may suggest a potential release of bioactive (paracrine) agents by the ischemic myocardial cells and “remote” impact on the intracellular Ca2+ handling of subendocardial Purkinje strands.
Purkinje fibers are involved in many different types of cardiac arrhythmias, with a majority related to myocardial ischemia and infarction, as reviewed in (Nogami et al., 2023). The mechanisms of these arrhythmias evolve with the different stages of the MI, but the exact time course of Purkinje-mediated arrhythmicity from the onset of an acute ischemic attack to the healed MI and scar formation is not clearly established in human patients. In the dog model of coronary ligation, the Purkinje arrhythmogenicity has been reported under the form of Purkinje-mediated triggers of VTs and VFs after reperfusion during the acute phase 1b (∼30 min) (Xing and Martins, 2001; Arnar and Martins, 2002). After the scar formation, in the long-term chronic phase 3 (weeks, months) post MI, Purkinje fibers surviving in the healed MI area, remain excitable but more prone to Ca2+-mediated DADs. The Purkinje focal activity could occasionally trigger monomorphic VTs or participate in reentrant circuits in a minority of patients (less than 5%) (Bogun et al., 2006). Ablation of these foci usually abolishes those late and often recurrent arrhythmias (Charton et al., 2023). Nevertheless, animal models have shown that Ca2+-mediated arrhythmogenicity is a specific feature of Purkinje fibers located in the border zone of the infarct and arises during the subacute phase 2 (within 48–72 h) while the infarct is still evolving (Haissaguerre et al., 2016). Despite the known large prevalence of VTs and VFs, likely due to abnormal Ca2+ handling-induced DADs in Purkinje cells, and the high risk of sudden cardiac death during this phase (Frampton et al., 2023), no precise quantification of phase 2 arrhythmic incidence has been reported in humans (Sattler et al., 2019).
Importance of animal models for the study of Ca2+ arrhythmogenicity
Animal models are indispensable for providing not only mechanistic insight into the Ca2+ arrhythmogenicity of Purkinje fibers in humans but also assisting the development of targeted antiarrhythmic therapies. Pursuing these goals requires high-resolution tools and invasive techniques that further justify the use of animal models of the human heart.
Nevertheless, the differences identified so far between Purkinje cells and ventricular myocytes have been observed in the hearts of large mammalian species. In small rodents, Purkinje cells are comparable to ventricular myocytes as both cell types contain the same arrangement of myofibrils, abundant transverse tubules, and well-developed sarcoplasmic reticulum (Di Maio et al., 2007). Interestingly, the presence of T tubules in mouse and rat Purkinje cells suggests that Ca2+ handling and Ca2+ mobilization in small rodents do not show the specific characteristics found in cells of large animals, potentially including humans. Supposing that these differences of Purkinje fibers compared to myocardium found in large-sized hearts are to facilitate the conduction, their absence in mice and rats is not surprising, since the impulse propagation distance is shorter and, therefore, the need for low-resistance fibers is less than in larger hearts.
To date, experimental and clinical data support the conclusion that the post-MI risk of ventricular fibrillation and cardiac arrest in humans is linked to a deficient component of Ca2+ handling in Purkinje cells (Haissaguerre et al., 2016). Models addressing this deficiency must consider the interspecies differences, specifically the discrepancy in Ca2+ handling systems and Ca2+ mobilization between small and large animals. For instance, the hypothesis of aberrant SR Ca2+ release as the source of arrhythmogenicity may ultimately apply to mouse or rat models in which Purkinje cells are likely to express only one SR Ca2+ channel (RyR2). The same hypothesis is less likely in large animal species. Increased SR-Ca2+ release has been evidenced 48 h after coronary ligation in canine Purkinje cells, in the three regions expressing distinct channels (Hirose et al., 2006). The simultaneous alteration of the three channels in distinct subcellular regions within 2 days is improbable. As another example, the IP3R1 channel has been recently implicated in the arrhythmogenesis of the human heart (Sun et al., 2025). However, confirmation of this implication has been achieved by inducing IP3R1 expression in a mouse model of Purkinje cells, which are expected to express a radically different Ca2+ handling system compared to that predicted in human Purkinje cells, where IP3R1 is likely to play a role in the centripetal Ca2+ mobilization.
Our current knowledge of Purkinje cells in large animal species strongly suggests that the subcellular foundations of Ca2+ arrhythmogenicity differ between mouse and human Purkinje cells. Considering the arguments supporting abnormal Ca2+ handling as a probable source of Purkinje pro-arrhythmicity in ischemic human hearts, only cell models with a Ca2+ management system consistent with that of common large mammalian species will be suitable for identifying the molecular origins of triggered arrhythmias in humans.
In addition, the anatomy of the Purkinje tissue influences the cardiac conduction (Vigmond and Stuyvers, 2016) and also displays notable differences among animals. For example, horses have more abundant and thicker Purkinje fibers compared to dogs. Like human and rabbit Purkinje fibers, most dog Purkinje fibers extend in the subendocardial region as free strands with many subendocardial connections with the myocardium. On the contrary, most pig Purkinje fibers are transmural and connect with the myocardium throughout the ventricular wall (Gómez-Torres et al., 2021; Šolc, 2007; Lelovas et al., 2014). Overall, the pig heart is currently recognized as one of the most effectiv translational models for cardiovascular diseases (Mackenzie et al., 2004; Stuyvers et al., 2005), while the dog heart is widely used in cardiac electrophysiology (Willis and Oliveira, 2018). Nevertheless, the Purkinje cells of both species appear to exhibit the same features currently predicted in human cells. This aspect may also be considered when selecting translational models for ischemic arrhythmias.
Finally, while murine models are well adapted to study a specific protein expression the distinctive features of the heart, including the Purkinje system, in the small animals should be considered in the mechanistic studies of Purkinje-induced Ca2+ arrhythmia in humans.
Conclusion
Purkinje fibers, once considered passive conductors, are now clinically recognized as active arrhythmogenic agents in the ischemic and infarcted heart. Animal models strongly suggest that the unique Ca2+ handling of Purkinje cells is involved in the spontaneous depolarizations that initiate lethal ventricular tachyarrhythmias upon ischemic myocardial infarction. The discovery of specific features in the Ca2+ handling of Purkinje cells is relatively recent. Numerous questions remain concerning the evoked and spontaneous activation of these cells in the current translational animal models, and the applicability of many findings to humans, although highly probable, has not yet been formally established. However, the level of our knowledge is sufficient to indicate that the choice of animal models for human ischemic arrhythmias must integrate the unique features recently discovered in the Purkinje cells of large mammalian species.
Intracellular Ca2+ manipulations have already been considered for treating the ischemic arrhythmic risk (Ter Keurs and Boyden, 2007; Boyden and Ter Keurs, 2005). Still, the lethal arrhythmias will remain unpredictable as long as the exact origin of Ca2+ dysfunctions in Purkinje fibers of ischemic heart is not clarified.
Author contributions
BS: Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akiyama T. (2010). Sunao tawara: discoverer of the atrioventricular conduction system of the heart. Cardiol. J. 17, 428–434.
Anderson R. H., Boyett M. R., Dobrzynski H., Moorman A. F. M. (2013). The anatomy of the conduction system: implications for the clinical cardiologist. J. Cardiovasc Transl. Res. 6, 187–196. doi:10.1007/s12265-012-9433-0
Arnar D. O., Martins J. B. (2002). Purkinje involvement in arrhythmias after coronary artery reperfusion. Am. J. Physiol. Hear Circ. Physiol. 282, 1189–1196. doi:10.1152/ajpheart.00227.2001
Behradfar E., Nygren A., Vigmond E. J. (2014). The role of purkinje-myocardial coupling during ventricular arrhythmia: a modeling study. PLoS One 9, e88000. doi:10.1371/journal.pone.0088000
Benali K., Vigmond E. J., Haissaguerre M. (2024). Identifying Purkinje involvement in ventricular fibrillation substrate. JACC Clin. Electrophysiol. 10, 1791–1793. doi:10.1016/j.jacep.2024.05.036
Bers D. M. (2002). Cardiac excitation-contraction coupling. Nature 415, 198–205. doi:10.1038/415198a
Blackwell D. J., Faggioni M., Wleklinski M. J., Gomez-Hurtado N., Venkataraman R., Gibbs C. E., et al. (2022). The Purkinje-myocardial junction is the anatomic origin of ventricular arrhythmia in CPVT. JCI Insight 7, e151893. doi:10.1172/jci.insight.151893
Bogun F., Good E., Reich S., Elmouchi D., Igic P., Tschopp D., et al. (2006). Role of Purkinje fibers in post-infarction ventricular tachycardia. J. Am. Coll. Cardiol. 48, 2500–2507. doi:10.1016/j.jacc.2006.07.062
Bootman M. D., Smyrnias I., Thul R., Coombes S., Roderick H. L. (2011). Atrial cardiomyocyte calcium signalling. Biochim. Biophys. Acta. Mol. Cell Res. 1813, 922–934. doi:10.1016/j.bbamcr.2011.01.030
Boyden P. A., Ter Keurs H. (2005). Would modulation of intracellular Ca2+ be antiarrhythmic? Pharmacol. Ther. 108, 149–179. doi:10.1016/j.pharmthera.2005.03.011
Boyden P. A., Albala A., Dresdner K. P. (1989). Electrophysiology and ultrastructure of canine subendocardial Purkinje cells isolated from control and 24-hour infarcted hearts. Circ. Res. 65, 955–970. doi:10.1161/01.RES.65.4.955
Boyden P. A., Pu J., Pinto J., Ter Keurs HEDJ (2000). Ca2+ transients and Ca2+ waves in purkinje cells: role in action potential initiation. Circ. Res. 86, 448–455. doi:10.1161/01.RES.86.4.448
Boyden P. A., Barbhaiya C., Lee T., Ter Keurs HEDJ (2003). Nonuniform Ca 2+ transients in arrhythmogenic Purkinje cells that survive in the infarcted canine heart. Cardiovasc Res. 57, 681–693. doi:10.1016/s0008-6363(02)00725-3
Boyden P. A., Hirose M., Dun W. (2010). Cardiac Purkinje cells. Hear Rhythm 7, 127–135. doi:10.1016/j.hrthm.2009.09.017
Charton J., Tixier R., Sacher F., Hocini M., Haissaguerre M., Duchateau J. (2023). Stepwise ablation strategy for post–myocardial infarction ventricular fibrillation: from arrhythmia suppression to ablation. Hear Case Rep. 9, 133–137. doi:10.1016/j.hrcr.2022.10.021
Chen H., Valle G., Furlan S., Nani A., Gyorke S., Fill M., et al. (2013). Mechanism of calsequestrin regulation of single cardiac ryanodine receptor in normal and pathological conditions. J. Gen. Physiol. 142, 127–136. doi:10.1085/jgp.201311022
Cordeiro J. M., Spitzer K. W., Giles W. R. (1998). Repolarizing K+ currents in rabbit heart Purkinje cells. J. Physiol. 508, 811–823. doi:10.1111/j.1469-7793.1998.811bp.x
Cordeiro J. M., Spitzer K. W., Giles W. R., Ershler P. E., Cannell M. B., Bridge J. H. B. (2001). Location of the initiation site of calcium transients and sparks in rabbit heart Purkinje cells. J. Physiol. 531, 301–314. doi:10.1111/j.1469-7793.2001.0301i.x
Dangman K. H., Danilo P., Hordof A. J., Mary-Rabine L., Reder R. F., Rosen M. R. (1982). Electrophysiologic characteristics of human ventricular and Purkinje fibers. Circulation 65, 362–368. doi:10.1161/01.cir.65.2.362
Daniels R. E., Haq K. T., Miller L. S., Chia E. W., Miura M., Sorrentino V., et al. (2017). Cardiac expression of ryanodine receptor subtype 3; a strategic component in the intracellular Ca2 + release system of Purkinje fibers in large mammalian heart. J. Mol. Cell Cardiol. 104, 31–42. doi:10.1016/j.yjmcc.2017.01.011
Di Maio A., Ter K. H. E., Franzini-Armstrong C. (2007). T-tubule profiles in Purkinje fibres of mammalian myocardium. J. Muscle Res. Cell Motil. 28, 115–121. doi:10.1007/s10974-007-9109-6
Dosdall D. J., Cheng K.-A., Huang J., Allison J. S., Allred J. D., Smith W. M., et al. (2007). Transmural and endocardial Purkinje activation in pigs before local myocardial activation after defibrillation shocks. Hear Rhythm 4, 758–765. doi:10.1016/j.hrthm.2007.02.017
Dun W., Boyden P. A. (2008). The Purkinje cell; 2008 style. J. Mol. Cell Cardiol. 45, 617–624. doi:10.1016/j.yjmcc.2008.08.001
Durrer D., van Dam R. T., Freud G. E., Janse M. J., Meijler F. L., Arzbaecher R. C. (1970). Total excitation of the isolated human heart. Circulation 41, 899–912. doi:10.1161/01.CIR.41.6.899
Fabiato A. (1983). Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am. J. Physiol. Cell Physiol. 245, C1–C14. doi:10.1152/ajpcell.1983.245.1.C1
Faggioni M., Knollmann B. C. (2012). Calsequestrin 2 and arrhythmias. Am. J. Physiol. Hear Circ. Physiol. 302, 1250–1260. doi:10.1152/ajpheart.00779.2011
Forsgren S., Thornell L.-E. (1981). The development of Purkinje fibres and ordinary myocytes in the bovine fetal heart. An ultrastructural study. Anat. Embryol. 162, 127–136. doi:10.1007/BF00306485
Frampton J., Ortengren A. R., Zeitler E. P. (2023). Arrhythmias after acute myocardial infarction. Yale J. Biol. Med. 96, 83–94. doi:10.59249/LSWK8578
Franzini-Armstrong C., Protasi F., Ramesh V. (1999). Shape, size, and distribution of Ca2+ release units and couplons in skeletal and cardiac muscles. Biophys. J. 77, 1528–1539. doi:10.1016/S0006-3495(99)77000-1
Gadsby D. C., Cranefield P. F. (1977). Two levels of resting potential in cardiac purkinje fibers. J. Gen. Physiol. 70, 725–746. doi:10.1085/jgp.70.6.725
Galimberti E. S., Knollmann B. C. (2011). Efficacy and potency of class I antiarrhythmic drugs for suppression of Ca 2+ waves in permeabilized myocytes lacking calsequestrin. J. Mol. Cell Cardiol. 51, 760–768. doi:10.1016/j.yjmcc.2011.07.002
Garcia-Bustos V., Sebastian R., Izquierdo M., Molina P., Chorro F. J., Ruiz-Sauri A. (2017). A quantitative structural and morphometric analysis of the Purkinje network and the Purkinje–myocardial junctions in pig hearts. J. Anat. 230, 664–678. doi:10.1111/joa.12594
Gettes L., Surawicz B. (1968). Effects of low and high concentrations of potassium on the simultaneously recorded Purkinje and ventricular action potentials of the perfused pig moderator band. Circ. Res. 23, 717–729. doi:10.1161/01.res.23.6.717
Gil W. W., Hess P. (1984). Excitation-contraction coupling in cardiac purkinje fibers: effects of cardiotonic steroids on the intracellular [Ca2+] transient, membrane potential, and contraction. J. Gen. Physiol. 83, 395–415. doi:10.1085/jgp.83.3.395
Gómez-Torres F. A., Estupiñán H. Y., Ruíz-Saurí A. (2021). Morphometric analysis of cardiac conduction fibers in horses and dogs, a comparative histological and immunohistochemical study with findings in human hearts. Res. Vet. Sci. 135, 200–216. doi:10.1016/j.rvsc.2021.02.013
Haissaguerre M., Vigmond E., Stuyvers B., Hocini M., Bernus O. (2016). Ventricular arrhythmias and the his-Purkinje system. Nat. Rev. Cardiol. 13, 155–166. doi:10.1038/nrcardio.2015.193
Haq K. T., Daniels R. E., Miller L. S., Miura M., ter Keurs HEDJ, Bungay S. D., et al. (2013). Evoked centripetal Ca2+ mobilization in cardiac Purkinje cells: insight from a model of three Ca2+ release regions. J. Physiol. 591, 4301–4319. doi:10.1113/jphysiol.2013.253583
Herron T. J., Milstein M. L., Anumonwo J., Priori S. G., Jalife J. (2010). Purkinje cell calcium dysregulation is the cellular mechanism that underlies catecholaminergic polymorphic ventricular tachycardia. Hear Rhythm 7, 1122–1128. doi:10.1016/j.hrthm.2010.06.010
Hirose M., Stuyvers B. D., Dun W., Ter Keurs H. E., Boyden P. A. (2006). Increased spontaneous Ca2+ release in arrhythmogenic Purkinje cells from infarcted canine heart is due to a lowered threshold of Ca2+ release channels. Circulation 114, 9–10.
Hirose M., Stuyvers B., Dun W., ter Keurs HEDJ, Boyden P. A. P. (2008). Function of Ca2+ release channels in Purkinje cells that survive in the infarcted canine heart: a mechanism for triggered Purkinje ectopy. Circ. Arrhythm. Electrophysiol. 1, 387–395. doi:10.1161/CIRCEP.107.758110
Ideker R. E., Kong W., Pogwizd S. (2009). Purkinje fibers and arrhythmias. Pacing Clin. Electrophysiol. 32, 283–285. doi:10.1111/j.1540-8159.2008.02232.x
Janse M. J., Wit A. L. (1989). Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol. Rev. 69, 1049–1169. doi:10.1152/physrev.1989.69.4.1049
Kang G., Giovannone S. F., Liu N., Liu F. Y., Zhang J., Priori S. G., et al. (2010). Purkinje cells from RyR2 mutant mice are highly arrhythmogenic but responsive to targeted therapy. Circ. Res. 107, 512–519. doi:10.1161/CIRCRESAHA.110.221481
Kanter H. L., Laing J. G., Beau S. L., Beyer E. C., Saffitz J. E. (1993). Distinct patterns of connexin expression in canine Purkinje fibers and ventricular muscle. Circ. Res. 72, 1124–1131. doi:10.1161/01.res.72.5.1124
Landstrom A. P., Dobrev D., Wehrens X. H. T. (2017). Calcium signaling and cardiac arrhythmias. Circ. Res. 120, 1969–1993. doi:10.1161/CIRCRESAHA.117.310083
Lelovas P. P., Kostomitsopoulos N. G., Xanthos T. T. (2014). A comparative anatomic and physiologic overview of the porcine heart. J. Am. Assoc. Lab. Anim. Sci. 53, 432–438.
Lüss H., Boknıék P., Heusch G., Müller F. U., Neumann J., Schmitz W., et al. (1998). Expression of calcium regulatory proteins in short-term hibernation and stunning in the in situ porcine heart1. Cardiovasc Res. 37, 606–617. doi:10.1016/S0008-6363(97)00238-1
Mackenzie L., Roderick H. L., Berridge M. J., Conway S. J., Bootman M. D. (2004). The spatial pattern of atrial cardiomyocyte calcium signalling modulates contraction. J. Cell Sci. 117, 6327–6337. doi:10.1242/jcs.01559
Mangoni M. E., Nargeot J. (2008). Genesis and regulation of the heart automaticity. Physiol. Rev. 88, 919–982. doi:10.1152/physrev.00018.2007
Martinez-Palomo A., Alanis J., Benitez D. (1970). Transitional cardiac cells of the conductive system of the dog heart: distinguishing morphological and electrophysiological features. J. Cell Biol. 47, 1–17. doi:10.1083/jcb.47.1.1
Matsushita T., Oyamada M., Fujimoto K., Yasuda Y., Masuda S., Wada Y., et al. (1999). Remodeling of cell-cell and cell-extracellular matrix interactions at the border zone of rat myocardial infarcts. Circ. Res. 85, 1046–1055. doi:10.1161/01.RES.85.11.1046
Miquerol L., Moreno-Rascon N., Beyer S., Dupays L., Meilhac S. M., Buckingham M. E., et al. (2010). Biphasic development of the mammalian ventricular conduction system. Circ. Res. 107, 153–161. doi:10.1161/CIRCRESAHA.110.218156
Mobley B. A., Page E. (1972). The surface area of sheep cardiac Purkinje fibres. J. Physiol. 220, 547–563. doi:10.1113/jphysiol.1972.sp009722
Nogami A. (2011). Purkinje-related arrhythmias. J. Arrhythmia 27, 6–27. doi:10.1016/s1880-4276(11)80004-9
Nogami A., Komatsu Y., Talib A. K., Phanthawimol W., Naeemah Q. J., Haruna T., et al. (2023). Purkinje-related ventricular tachycardia and ventricular fibrillation: solved and unsolved questions. JACC Clin. Electrophysiol. 9, 2172–2196. doi:10.1016/j.jacep.2023.05.040
Oliphant L., Loewen R. (1976). Filament systems in Purkinje cells of the sheep heart: possible alterations of myofibrillogenesis. J. Mol. Cell Cardiol. 8, 76AD.
Ono N., Yamaguchi T., Ishikawa H., Arakawa M., Saikawa T., Shimada T., et al. (2009). Morphological varieties of the Purkinje fiber network in mammalian hearts, as revealed by light and electron microscopy. Arch. Histol. Cytol. 72, 139–149. doi:10.1679/aohc.72.139
Pauziene N., Rysevaite-Kyguoliene K., Alaburda P., Pauza A. G., Skukauskaite M., Masaityte A., et al. (2017). Neuroanatomy of the pig Cardiac ventricles. A stereomicroscopic, confocal and electron microscope study. Anat. Rec. 300, 1756–1780. doi:10.1002/ar.23619
Priori S. G., Chen S. R. W. (2011). Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ. Res. 108, 871–883. doi:10.1161/CIRCRESAHA.110.226845
Reder R. F., Miura D. S., Danilo P., Rosen M. R. (1981). The electrophysiological properties of normal neonatal and adult canine cardiac Purkinje fibers. Circ. Res. 48, 658–668. doi:10.1161/01.res.48.5.658
Rosati B., Dun W., Hirose M., Boyden P. A., McKinnon D. (2007). Molecular basis of the T- and L-type Ca2+ currents in canine Purkinje fibres. J. Physiol. 579, 465–471. doi:10.1113/jphysiol.2006.127480
Saitoh H., Bailey J. C., Surawicz B. (1989). Action potential duration alternans in dog Purkinje and ventricular muscle fibers. Further evidence in support of two different mechanisms. Circulation 80, 1421–1431. doi:10.1161/01.cir.80.5.1421
Sattler S. M., Skibsbye L., Linz D., Lubberding A. F., Tfelt-Hansen J., Jespersen T. (2019). Ventricular arrhythmias in first acute myocardial infarction: epidemiology, mechanisms, and interventions in large animal models. Front. Cardiovasc Med. 6, 158. doi:10.3389/fcvm.2019.00158
Sivagangabalan G., Nazzari H., Bignolais O., Maguy A., Naud P., Farid T., et al. (2014). Regional ion channel gene expression heterogeneity and ventricular fibrillation dynamics in human hearts. PLoS One 9, e82179. doi:10.1371/journal.pone.0082179
Šolc D. (2007). The heart and heart conducting system in the kingdom of animals: a comparative approach to its evolution. Exp. Clin. Cardiol. 12, 113–118.
Sommer J. R., Johnson E. A. (1968). Cardiac muscle. A comparative study of Purkinje fibers and ventricular fibers. J. Cell Biol. 36, 497–526. doi:10.1083/jcb.36.3.497
Sommer J. R., Johnson E. A. (1970). Comparative ultrastructure of cardiac cell membrane specializations. A review. Am. J. Cardiol. 25, 184–194. doi:10.1016/0002-9149(70)90578-3
Stankovičová T., Bito V., Heinzel F., Mubagwa K., Sipido K. R. (2003). Isolation and morphology of single Purkinje cells from the porcine heart. Gen. Physiol. Biophys. 22, 329–340.
Stuyvers B. D., Dun W., Matkovich S., Sorrentino V., Boyden P. A., Ter Keurs HEDJ (2005). Ca2+ sparks and waves in canine Purkinje cells: a triple layered system of Ca2+ activation. Circ. Res. 97, 35–43. doi:10.1161/01.res.0000173375.26489.fe
Sun B., Ni M., Li Y., Song Z., Wang H., Zhu H. L., et al. (2025). Inositol 1,4,5-Trisphosphate receptor 1 gain-of-function increases the risk for cardiac arrhythmias in mice and humans. Circulation 151, 847–862. doi:10.1161/CIRCULATIONAHA.124.070563
Ter Keurs HEDJ, Boyden P. A. (2007). Calcium and arrhythmogenesis. Physiol. Rev. 87, 457–506. doi:10.1152/physrev.00011.2006
Ter Keurs HEDJ, Wakayama Y., Miura M., Shinozaki T., Stuyvers B. D., Boyden P. A., et al. (2006). Arrhythmogenic Ca2+ release from cardiac myofilaments. Prog. Biophys. Mol. Biol. 90, 151–171. doi:10.1016/j.pbiomolbio.2005.07.002
Tseng G. N., Boyden P. A. (1989). Multiple types of Ca2+ currents in single canine Purkinje cells. Circ. Res. 65, 1735–1750. doi:10.1161/01.RES.65.6.1735
Vaidyanathan R., O’Connell R. P., Deo M., Milstein M. L., Furspan P., Herron T. J., et al. (2013). The ionic bases of the action potential in isolated mouse cardiac Purkinje cell. Hear Rhythm 10, 80–87. doi:10.1016/j.hrthm.2012.10.002
Vassalle M. (1977). The relationship among cardiac pacemakers. Overdrive suppression. Circ. Res. 41, 269–277. doi:10.1161/01.RES.41.3.269
Verkerk A. O., Veldkamp M. W., Abbate F., Antoons G., Bouman L. N., Ravesloot J. H., et al. (1999). Two types of action potential configuration in single cardiac Purkinje cells of sheep. Am. J. Physiol. Hear Circ. Physiol. 277, H1299–H1310. doi:10.1152/ajpheart.1999.277.4.h1299
Vigmond E. J., Stuyvers B. D. (2016). Modeling our understanding of the his-Purkinje system. Prog. Biophys. Mol. Biol. 120, 179–188. doi:10.1016/j.pbiomolbio.2015.12.013
Webster K. A. (2012). Mitochondrial membrane permeabilization and cell death during myocardial infarction: roles of calcium and reactive oxygen species. Future Cardiol. 8, 863–884. doi:10.2217/fca.12.58
Willis R., Oliveira P. (2018). Guide to canine and feline electrocardiography. Hoboken, New Jersey: Wiley.
Keywords: calcium, arrhythmia, vt, VF, Purkinje fiber, Purkinje cell, myocardial ischemia, myocardial infarction
Citation: Stuyvers BD (2025) Calcium arrhythmogenicity of Purkinje fibers: importance of the animal model. Front. Physiol. 16:1676701. doi: 10.3389/fphys.2025.1676701
Received: 30 July 2025; Accepted: 15 October 2025;
Published: 30 October 2025.
Edited by:
Federico Landra, University of Siena, ItalyReviewed by:
Hussam Ali, MultiMedica Holding SpA (IRCCS), ItalyCopyright © 2025 Stuyvers. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bruno D. Stuyvers, c3R1eXZlcnNAbXVuLmNh
 Bruno D. Stuyvers
Bruno D. Stuyvers