- 1Department of Biochemistry and Biotechnology, Vasyl Stefanyk Carpathian National University, Ivano-Frankivsk, Ukraine
- 2Research and Development University, Ivano-Frankivsk, Ukraine
Post-traumatic stress disorder (PTSD) is a complex psychiatric condition characterized by behavioral, cognitive, immunological, and neurochemical disturbances following traumatic experiences. Despite various therapeutic approaches, effective long-term treatments remain limited, highlighting the need for preventive strategies that enhance stress resilience. In this study, we evaluated the impact of long-term kefir consumption on behavioral, hematological, and biochemical parameters in a mouse model displaying some PTSD-like features, particularly fear- and anxiety-related behaviors induced by acute inescapable stress. Male C57BL/6J mice received kefir daily for 2 months before stress induction via electric foot shocks and continued supplementation for five additional months during recovery. Behavioral testing demonstrated that kefir-fed mice exhibited reduced anxiety-like behaviors, including increased exploration in the open field, elevated plus maze, and light/dark box tests. These mice also showed fewer freezing episodes in the aversive context test, indicating attenuated fear memory. Hematological analysis revealed a modest reduction in erythrocyte count and monocytes, alongside elevated paraoxonase (PON) activity, suggesting enhanced antioxidant defense and a shift toward anti-inflammatory immune responses. RT-qPCR analysis of the cerebral cortex showed increased steady-state transcript levels of genes involved in oxidative stress response and neuroprotection (TXNRD1, UGDH, HSPB8, GADD45B, PPARGC1A) and decreased levels of the pro-inflammatory cytokine gene IL6 transcript. These results indicate that long-term kefir intake mitigates stress-induced behavioral and physiological alterations, likely through modulation of immune and oxidative stress pathways. Taken together, our findings support the potential of kefir as a functional dietary intervention for promoting stress resilience and alleviating PTSD-like symptoms, possibly via mechanisms involving the gut-brain axis.
1 Introduction
Post-traumatic stress disorder (PTSD) is a multifaceted psychiatric condition that arises following exposure to traumatic events. It is characterized by intrusive memories, mood disturbances, cognitive impairments, hyperarousal, avoidance behavior, and a persistent sense of threat (Miao et al., 2018; Dmytriv et al., 2023; Pinna et al., 2023; Balatskyi et al., 2025). Individuals with PTSD are at increased risk of developing depression neurodegenerative diseases, substance use disorders, and various comorbidities. A similar pattern has recently been observed in the Ukrainian population affected by the ongoing Russian-Ukrainian war (Lushchak et al., 2023). Although preventive strategies are regarded as the most effective means of addressing PTSD, existing approaches lack sufficient evidence to support widespread clinical implementation (Bisson et al., 2021). Consequently, the development of novel, accessible interventions to promote stress resilience remains a research priority.
Dietary interventions targeting mental health, particularly PTSD, are still underexplored, despite growing recognition of the gut-brain axis as a central regulator of stress responses and emotional wellbeing (Yin et al., 2014; Kearney et al., 2022; Rook, 2024). Functional foods, particularly those rich in probiotics and bioactive compounds, have demonstrated the potential to modulate brain function through microbial metabolites and neuroactive compounds (Gomez-Pinilla and Gomez, 2011; Gradus et al., 2017). Fermented dairy products (FDPs) are of particular interest due to their ability to influence the composition and function of the gut microbiota. It is also known that kefir consumption by mice had a positive effect on inflammation modulation by reducing the level of pro-inflammatory cytokines and increasing the level of anti-inflammatory cytokines, as well as modulating oxidative stress (Gogineni, 2013; Albuquerque Pereira et al., 2024; Mariana et al., 2024). Experimental studies have shown that peptides derived from fermented milk can reduce anxiety-like behavior and mitigate brain damage in stress-exposed mice (Joung et al., 2021; 2023). Moreover, human studies report associations between FDP consumption and lower anxiety levels (Sousa et al., 2022). These benefits are largely attributed to modulation of the gut-brain axis–a – bidirectional communication system linking the gastrointestinal tract and central nervous system via immune, neural, and endocrine pathways (Dmytriv et al., 2024a; Dmytriv et al., 2024b; Loh et al., 2024). However, the long-term effects of FDPs on stress-related behavior and their underlying molecular mechanisms remain poorly characterized.
While many studies have focused on the microbial or metabolic mechanisms of fermented products, the initial step in our research was to determine whether specific kefir as a whole could induce measurable behavioral and physiological benefits in a murine PTSD-like model. This approach allowed us to assess the integrated biological impact of kefir before dissecting the contributions of its individual microbial or biochemical components. Subsequent studies will focus on identifying the specific active factors and pathways underlying these effects.
In this study, we investigated whether prolonged kefir consumption could attenuate stress-induced behavioral, hematological, and molecular alterations in a mouse model of PTSD. Our findings provide compelling evidence that kefir may serve as a functional dietary intervention to enhance stress resilience and mitigate the symptoms of PTSD.
2 Materials and methods
2.1 Experimental design
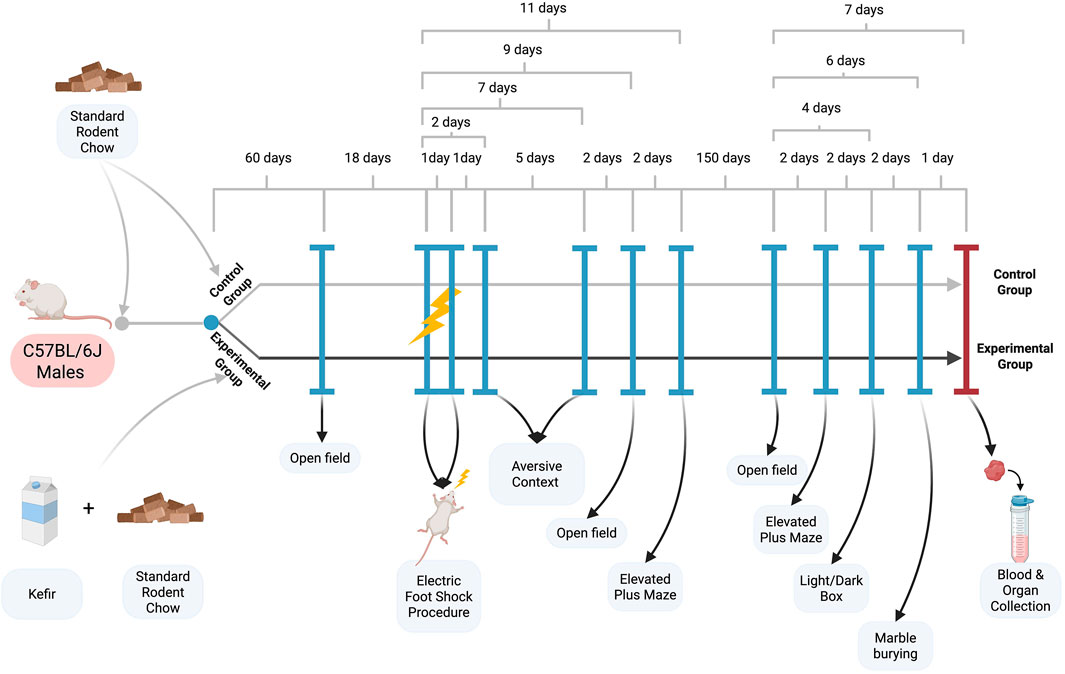
Male C57BL/6J mice aged 8–11 months were housed under controlled laboratory conditions with a 12-h light/dark cycle (6 a.m.–6 p.m.), ambient temperature of 22 °C ± 2 °C, and relative humidity of 50%–60%. Mice were randomly assigned to control and experimental groups (five to seven animals per group). Control animals received standard rodent chow (21.8% protein, 4.8% fat, 69.1% carbohydrates, 3.9% fiber), while the experimental group received unlimited access to chow and kefir in separate dishes. Food, water, and kefir were provided ad libitum.
Kefir (Molokiya, Ukraine) was prepared from normalized cow’s milk with kefir starter culture. Nutritional values per 100 g: carbohydrates – 3.9 g (including 3.9 g sugars), proteins – 2.9 g, fats – 2.5 g (1.58 g saturated), salt – 0.05 g; energy value – 209 kJ (50 kcal).
After 2 months of kefir consumption, mice underwent baseline behavioral testing (open field test) before stress induction. Mice then received two sessions of electric foot shocks over 2 days to induce PTSD-like symptoms and continued kefir intake for five additional months. Behavioral tests were conducted at several time points (see Figure 1): the aversive context test (days 2 and 7), open field test (day 9), and elevated plus maze (day 11). Long-term behavioral effects were assessed 5 months later using the open field, elevated plus maze, light/dark box, and marble burying tests.
All procedures were approved by the Animal Experiments Committee of Vasyl Stefanyk Precarpathian National University and conducted under Directive 2010/63/EU on the protection of animals used for scientific purposes.
2.2 Stress induction procedure and aversive context test
Stress was induced using a metal-grid floor shock chamber connected to a stimulus generator. This acute footshock paradigm is widely used to model trauma-induced fear and anxiety in rodents and is often referred to as a PTSD-like or contextual fear model (Martinho et al., 2021). It does not reproduce all parameters of human PTSD, but allows the assessment of persistent fear memory and stress reactivity. Each mouse was placed in the chamber for 7 min: 2 min of acclimatization followed by 15 electric shocks (0.8 mA, 10 s duration, 10 s intervals) over 5 min, repeated on two consecutive days (Martinho et al., 2022). On days 2 and 7, mice were re-exposed to the same chamber without shocks for the aversive context test. Freezing behavior, defined as immobility except for breathing for ≥3 s, was scored from video recordings (Martinho et al., 2021).
2.3 Open field test
The open field test is commonly used to measure locomotor and anxiety-like behavior in mice (Seibenhener and Wooten, 2015). In this work behavioral activity was assessed in a 40 × 40 cm polyvinyl chloride chamber divided into 16 squares (10 × 10 cm). Locomotion and anxiety-related behaviors were recorded and analyzed using ToxTrac software (v2.98) from 10-min video recordings (Rodriguez et al., 2018; https://sourceforge.net/projects/toxtrac/). Outcomes included average movement speed, time spent in the central squares (inner zone), and number of fecal boli.
2.4 Elevated plus maze test
Anxiety-related behavior was evaluated using a standard elevated plus maze (EPM) with two open and two closed arms intersecting at a central platform. Mice were allowed to explore the maze for 10 min. The time spent in open and closed arms was recorded (Walf and Frye, 2007).
2.5 Light/dark box test
Mice were placed in a divided glass box (30 × 30 × 40 cm per compartment) with one dark and one illuminated zone. After being placed in the dark zone and closing the lid, mice were observed for 10 min. The number of entries into the light zone, time spent there, and latency to first entry were recorded (Bourin and Hascoët, 2003; Crawley and Goodwin, 1980).
2.6 Marble burying test
Each cage was filled with 5 cm of wood shavings, and 20 marbles were arranged in four rows. Mice were placed in the cage for 30 min, and the number of marbles buried ≥75% was counted (Angoa-Pérez et al., 2013; Sampson, 2024).
2.7 Hematological parameters
Blood was collected after a 12-h fast via retro-orbital puncture under CO2 anesthesia. Half the sample was centrifuged (1500 g, 15 min, 4 °C) for plasma; the remainder was used for hematological analyses.
Hemoglobin was measured using Drabkin’s reagent (Genesis LLC, Ukraine) at 540 nm. Hematocrit was assessed using microcapillary tubes centrifuged at 2000 g for 20 min. Erythrocyte and leukocyte counts were performed using Goryaev’s chamber after dilution with 3% NaCl or 5% acetic acid + methylene blue, respectively. Leukocyte differentials were determined from blood smears stained by Romanowski or May-Grunewald-Giemsa methods, counting 200 cells per animal at 1000× magnification. The cells were classified according to standard protocols (O’Connell et al., 2015), and the percentages of various leukocyte types were determined.
2.8 Assays of activities of paraoxonase, myeloperoxidase, and levels of total protein and glucose in blood plasma
Plasma paraoxonase (PON) activity was measured spectrophotometrically at 405 nm using 4-nitrophenyl acetate as a substrate. The reaction mixture consisted of 50 mM potassium phosphate buffer (pH 7.0), 1 mM CaCl2, and 3.2 mM 4-nitrophenylacetate. The extinction coefficient of p-nitrophenol 14,000 M-1 cm-1, was used to calculate the PON activity (Vatashchuk et al., 2023). Myeloperoxidase (MPO) activity was measured as H2O2-dependent oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB), and the absorbance was measured at 450 nm using a Multiskan MCC/340 microplate reader (Yadav et al., 2014). Plasma glucose and total protein levels were assessed using standard kits and the Bradford assay (Bradford, 1976).
2.9 Polymerase chain reaction (RT-qPCR)
Total RNA isolation, RNA quantification, and real-time quantitative polymerase chain reaction (RT-qPCR) were performed as previously described (Demianchuk et al., 2024). The AriaMx system (Agilent Technologies, Inc.) was used for RT-qPCR. Relative fold change in messenger RNA (mRNA) levels was calculated using the 2–ΔΔCq method (Livak and Schmittgen, 2001), using Cq values for the expression of the RPL27 gene (encoding ribosomal protein L27) as a reference.
Total RNA was extracted from cerebral cortex samples as previously described (Demianchuk et al., 2024). RT-qPCR was performed using the AriaMx system (Agilent Technologies). Relative transcript levels of genes of interest gene expression were calculated using the 2–ΔΔCq method (Livak and Schmittgen, 2001), normalized to RPL27 as a reference gene. Oligonucleotide sequences (see Table 1) were received from Metabion International AG (Steinkirchen, Germany).
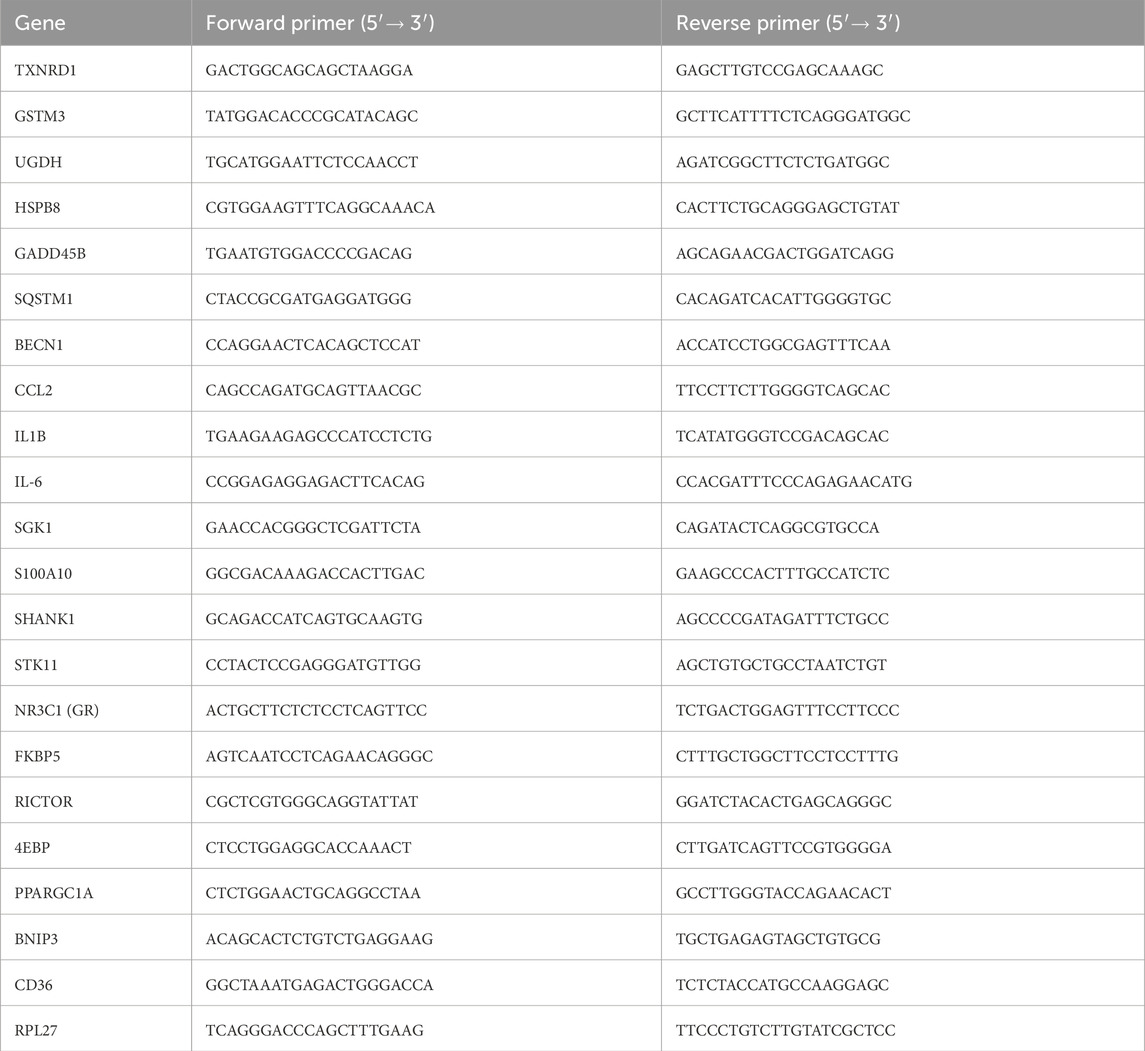
Table 1. Oligonucleotide sequences used in the study to analyze the levels of mRNA by quantitative real-time polymerase chain reaction.
The prefrontal cortex was selected for molecular analysis due to its key involvement in emotional regulation, cognitive control, and extinction of fear responses, which are central to PTSD pathology. This region also provides sufficient tissue quantity for reproducible RNA isolation and reliable RT-qPCR measurements.
2.10 Statistical analysis
Data are presented as mean ± SEM. Statistical analyses were conducted using GraphPad Prism v10.0.0 (GraphPad Software, Boston, MA, USA). Differences between groups were assessed using unpaired Student’s t-test, Holm-Sidak test. Linear mixed effects model approach implemented in GraphPad Prism was applied to evaluate time-dependent influence of the treatment. Multiple comparisons were conducted using t-test followed by p-value adjustment by Benjamini-Krieger-Yekutieli procedure.
Sample size was evaluated using Robin Ristl’s sample size calculator (Medical University of Vienna, https://homepage.univie.ac.at/robin.ristl/samplesize.php?test=anova), for two groups with unequal sample sizes, with mean difference 1.5, standard deviation of 0.6, significance level 0.05, and power 0.8. This calculation gave us a sample size of four and six individuals.
3 Results
3.1 Body mass and Fur condition of mice
Before the first open field test induction (conducted prior to the foot shock procedure), kefir-fed mice had significantly higher body mass–approximately 8% (p = 0.01) greater–compared to control animals (Figure 2A). However, by the end of the experiment, body mass differences were no longer statistically significant (Figure 2B). Kefir-treated mice also exhibited a noticeably improved fur conditions, including increased physical activity, livelier behavior, and shinier fur compared to controls (Figure 2C).
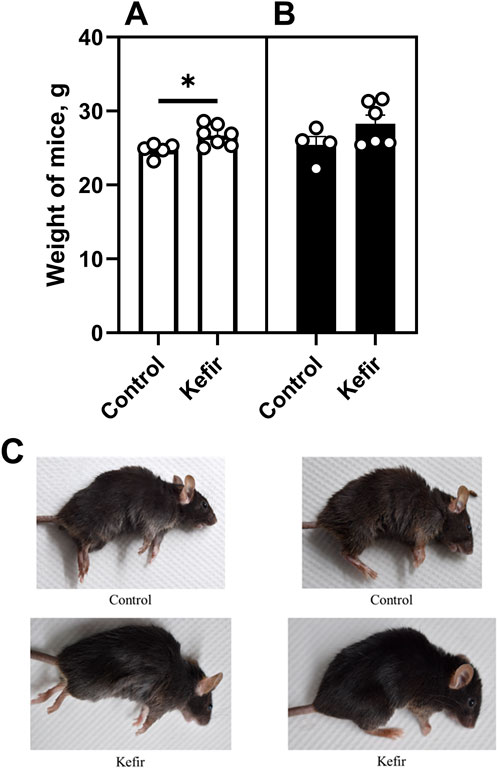
Figure 2. Body mass of mice (A) before the foot shock procedure, (B), at the end of the experiment, and (C) fur conditions of animals from the control and kefir-consuming group under anesthesia. Data are presented as mean ± SEM, n = 4-6. *Significantly different from the control group (P < 0.05) according to unpaired Student’s t-test.
3.2 Behavioral tests
The open field test was conducted three times: before stress exposure, 1 week post-stress, and 5 months after stress induction (Figure 1). Control mice displayed lower locomotor activity after stress, with average speed decreased by 56% (p < 0.001) in the second trial and 44% (p < 0.001) in the third compared to the first. Kefir-treated mice also exhibited a 33% (p = 0.005) lower speed in the second test compared to pre-stress levels, but their behavior remained more consistent over time (Figure 3A).
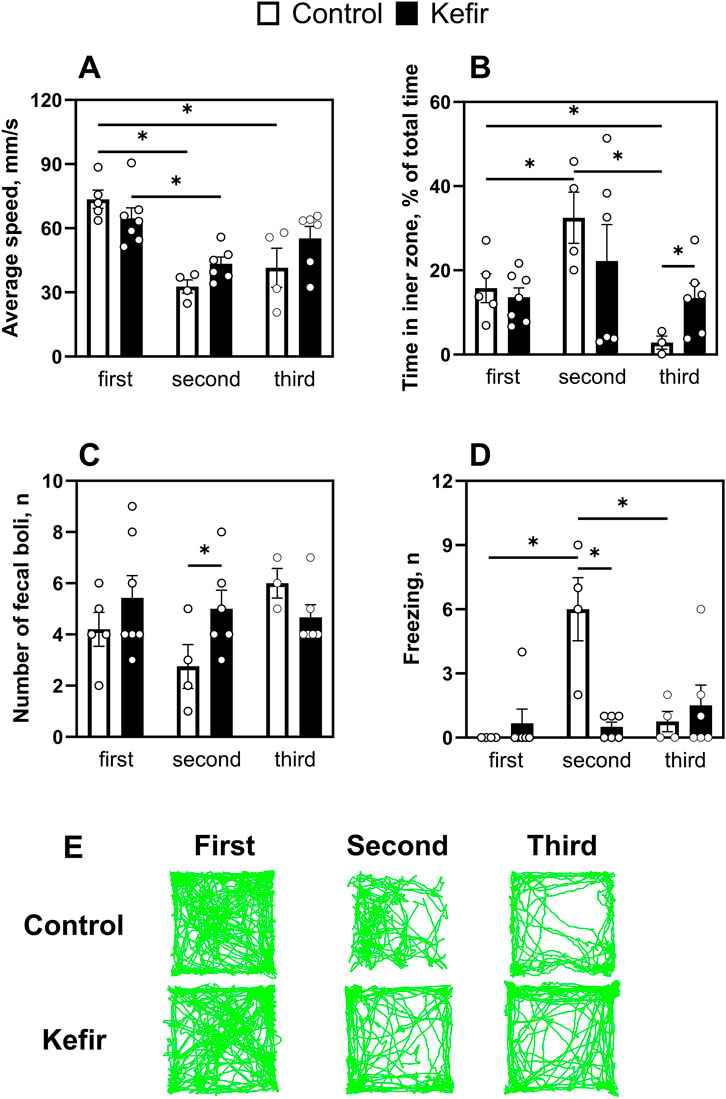
Figure 3. Comparison of results of the first (before foot shock), second (1 week after foot shock), and third (5 months after foot shock) open field test trails. (A) An average speed of mice, (B) time spent in the central squares of the open field, (C) number of fecal boli, (D) number of freezings of mice, (E) representative trails of mouse movement. Data are presented as mean ± SEM, n = 3-7. *Significant difference (P < 0.05) between groups according to the mixed effect model approach, followed by pairwise comparisons with Benjamini-Krieger-Yekutieli adjustment of p-values for multiple testing.
In terms of anxiety-like behavior, control mice spent twice as much time in the inner zone during the second test compared to the first (p = 0.03). However, their time in the center markedly decreased in the third trial - to 82% (p = 0.044) and 91.3% (p = 0.04) of the values observed in the first and second tests, respectively. In contrast, kefir-fed mice maintained stable exploration of the central area across all trials and spent nearly five times more time (p = 0.03) in the inner zone than controls during the third test (Figure 3B), suggesting reduced anxiety. The number of fecal boli did not differ significantly, except during the second trial, when the kefir group produced nearly twice as many (p = 0.04) (Figure 3C). Freezing behavior peaked in control animals during the second trial, while kefir-fed mice exhibited consistently lower freezing, including significantly fewer episodes than controls during the second test (p < 0.001) (Figure 3D).
In the aversive context test, kefir-fed mice showed markedly lower fear responses, with 76% fewer freezing episodes (p = 0.03) on day 2% and 91% fewer on day 7 (p = 0.02) compared to control mice (Figures 4A,B), indicating sustained attenuation of fear-related responses.
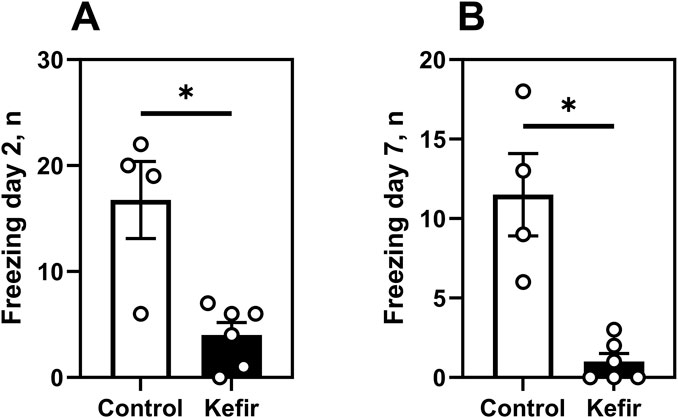
Figure 4. Aversive context test. (A) number of freezing events of mice in the aversive context on day 2, (B) number of freezing events of mice in the aversive context on day 7. Data are presented as mean ± SEM, n = 4-6. *Significantly different from the control group (P < 0.05) according to unpaired Student’s t-test.
In the first round of elevated plus maze (EPM) and marble burying tests (1 week after foot shock), no significant differences were observed between groups (Figures 5A–C). However, in the second round (5 months post-stress), kefir-fed mice spent 12 times more time (p = 0.004) in the open arms of the EPM compared to controls (Figure 5D), indicating reduced anxiety. The number of entries into closed arms did not differ (Figure 5E), nor did marble burying behavior (Figure 5F).
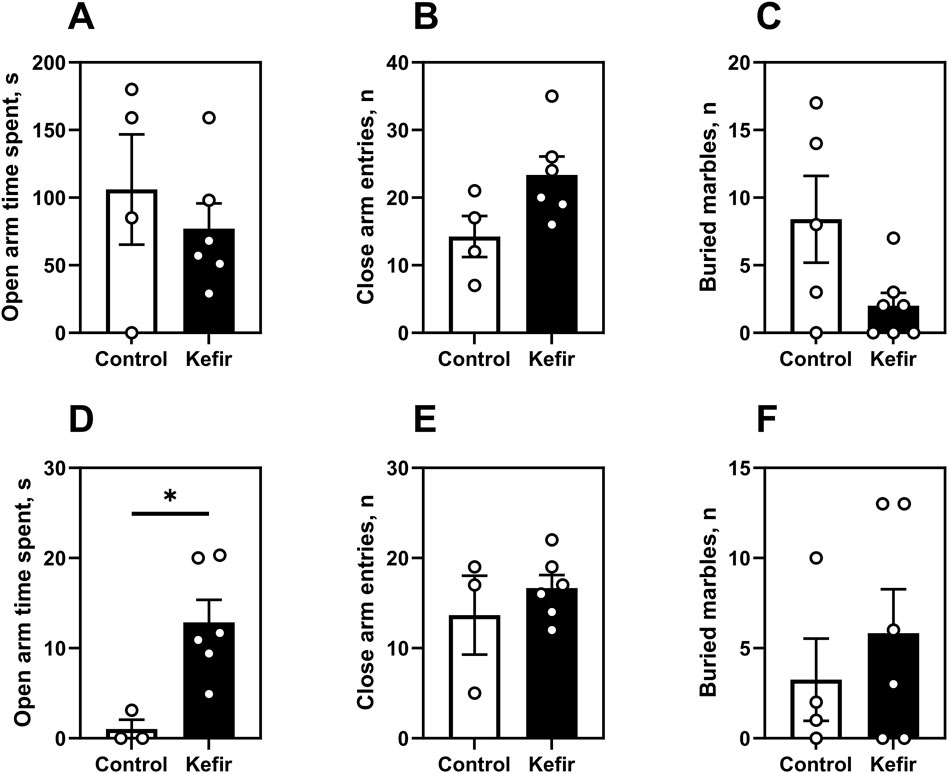
Figure 5. Elevated plus maze and marble burying tests. This figure shows data from two trials of the elevated plus maze test. The first trial was conducted 1 month after the foot shock (A–C), and the second 5 months after the foot shock (D,E and F). First trial. (A) Time spent in the open arms of the elevated plus maze, (B) number of closed arm entries of the elevated plus maze, (C) number of buried marbles in the marbles burying test. Second trial. (D) time spent in the open arms of the elevated plus maze, (E) number of entries into the closed arms of the elevated plus maze, (F) number of balls buried in the ball burial test. Data are presented as mean ± SEM, n = 3-7. *Significantly different from the control group (P < 0.05) according to unpaired Student’s t-test.
In the light/dark box test (5 months post-stress), kefir-fed mice made significantly more entries (p = 0.003) into the light zone and exhibited shorter latency to enter it (p = 0.002), compared to controls (Figures 6A,B). These mice also defecated 43% less often during the test (p = 0.04) (Figure 6C), further supporting an anxiolytic effect of kefir.
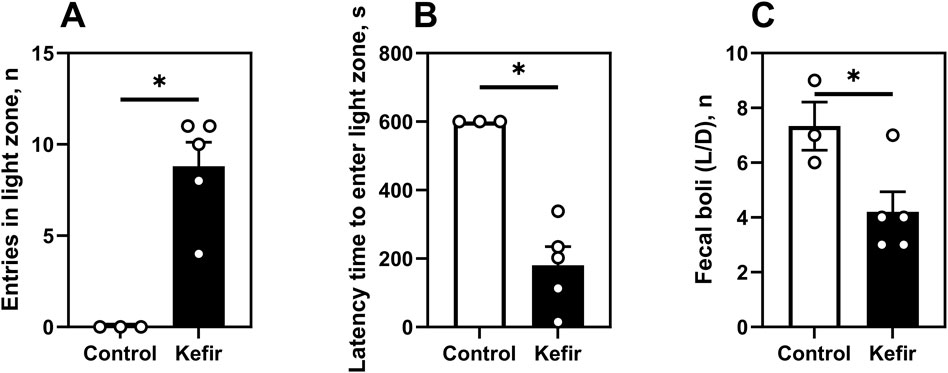
Figure 6. Light/dark box test (5 months after foot shock). (A) number of entries in the light zone of the light/dark box, (B) latency time (time spent by mice to enter the light zone for the first time), (C) number of mice defecating in the light/dark box. Data are presented as mean ± SEM, n = 4-6. *Significantly different from the control group (P < 0.05) according to unpaired Student’s t-test.
3.3 Hematological parameters
Among hematological parameters, only red blood cell count was significantly lower (by 17%) in kefir-consuming mice compared to controls (p = 0.001) (Table 2). No significant differences were found in hemoglobin concentration, hematocrit, or total leukocyte count between the groups.
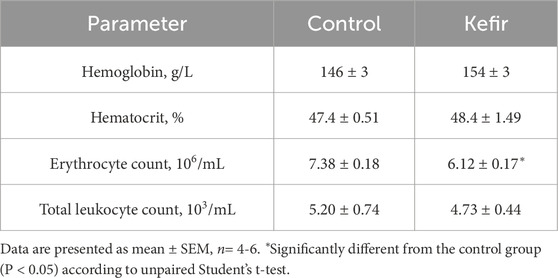
Table 2. Hematological parameters in peripheral blood from mice of the control and kefir-fed groups.
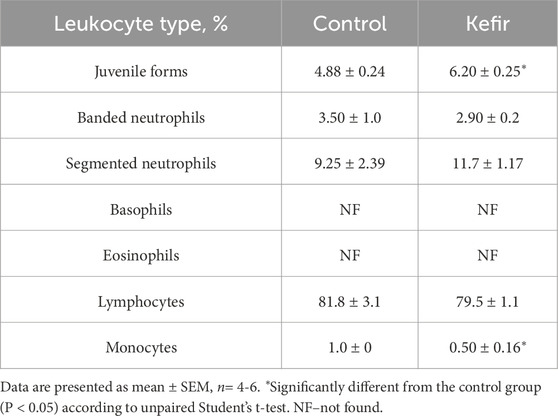
Differential leukocyte analysis showed a 50% lower monocyte percentage (p = 0.03) and a 27% higher juvenile leukocyte form (p = 0.01) in the kefir-fed group (Table 3). Percentages of other leukocyte subtypes (lymphocytes, segmented and banded neutrophils) did not differ between groups.
3.4 Biochemical and metabolic parameters of blood plasma
No significant intergroup differences were detected in blood glucose, total plasma protein, or myeloperoxidase (MPO) activity (Figures 7A–C). However, paraoxonase (PON) activity was 22% higher in the kefir group (p = 0.03), indicating enhanced antioxidant capacity (Figure 7D).
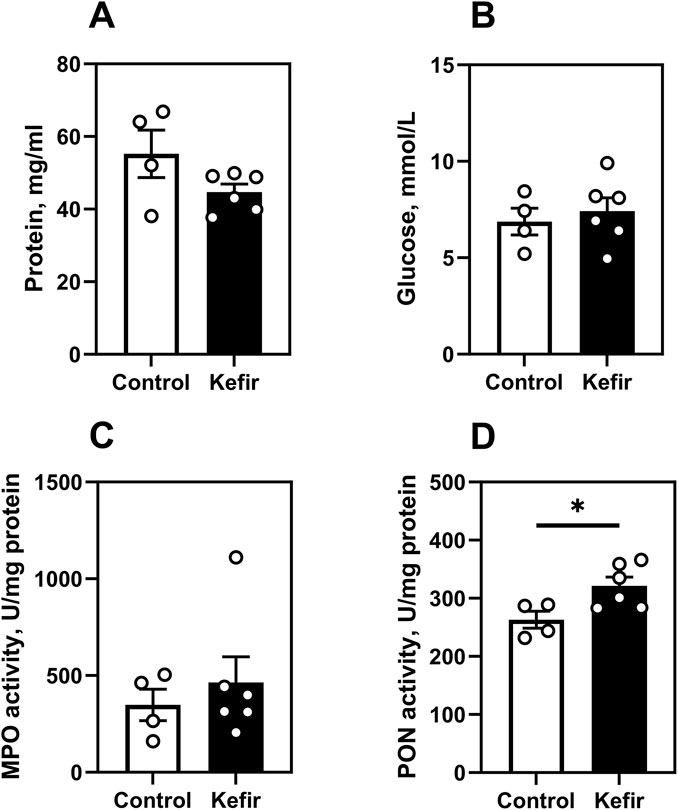
Figure 7. Metabolic and biochemical blood parameters. (A) total protein, (B) glucose level, (C) myeloperoxidase activity, (D) paraoxonase activity. Data are presented as mean ± SEM, n = 4-6. *Significantly different from the control group (P < 0.05) according to unpaired Student’s t-test.
3.5 mRNA levels in the mouse cerebral cortex
RT-qPCR analysis of cerebral cortex tissue revealed significant transcriptional differences between control and kefir-fed groups in transcript levels of genes involved in oxidative stress response, inflammation, neuroplasticity, autophagy, and energy metabolism (Table 4). Among antioxidant defense genes, TXNRD1 and UGDH were upregulated by 34% (p = 0.049) and 40% (p < 0.001), respectively. Expression of GSTM3 remained unchanged.
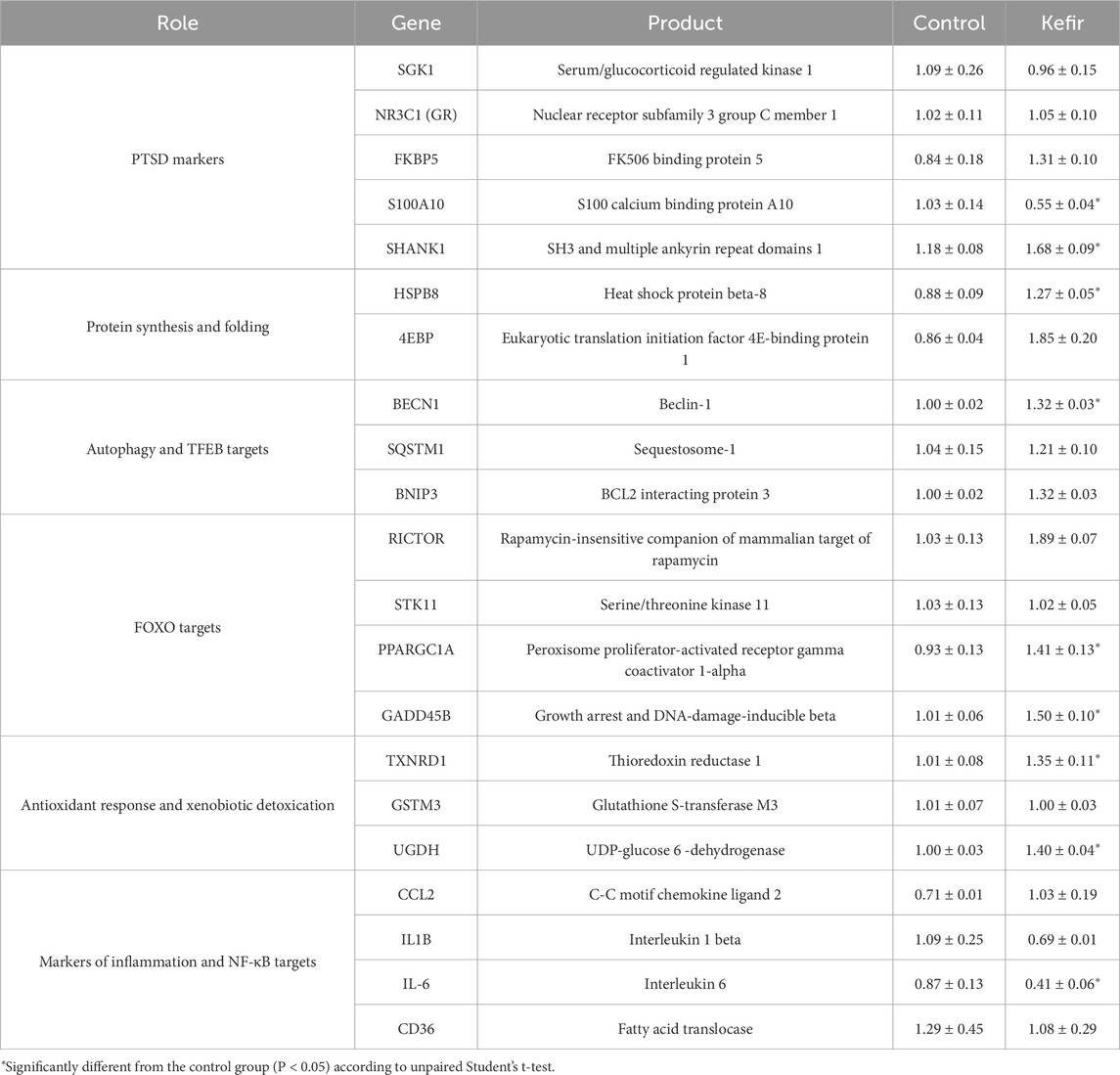
Table 4. mRNA transcripts in the cerebral cortex of mice. Data are presented as mean ± SEM, n = 3-5.
Pro-inflammatory IL6 expression was lower by 53% (p = 0.046), while IL1B showed a non-significant 37% decrease (p = 0.2) (Table 4). CCL2 levels were 45% higher (p = 0.18), possibly reflecting a compensatory immune mechanism. No significant differences were observed in SGK1, NR3C1, or FKBP5 (glucocorticoid signaling pathway).
Neuroplasticity-related genes showed notable differences: SHANK1 and GADD45B were upregulated by 43% (p = 0.006) and 50% (p = 0.004), respectively, while S100A10 was downregulated (p = 0.04) by 47%. Among autophagy-related genes, BECN1 was higher by 32% (p < 0.001), while SQSTM1 and BNIP3 levels remained unchanged (Table 4).
For protein homeostasis, HSPB8 was 44% higher (p = 0.03). Genes related to mTOR signaling and metabolic regulation also responded to kefir intake: RICTOR was 84% higher (p = 0.002), EIF4EBP1 nearly doubled, and PPARGC1A was elevated by 52% (p = 0.04). No significant differences were observed in STK11 or CD36 (Table 4).
4 Discussion
Fermented dairy products, particularly kefir, are increasingly recognized for their multifunctional health benefits, including immune modulation, antioxidant enhancement, and potential neuroprotective effects (Moineau and Goulet, 1991; Mitsuoka, 2014; Silva et al., 2023). These effects are largely attributed to kefir’s ability to modulate the gut microbiota and influence gut-brain axis communication (Perdigón et al., 2002; Kim et al., 2016; Kumar et al., 2021; Chakrabarti et al., 2022). The present study demonstrates that long-term kefir consumption exerts beneficial effects on behavior, physiology, and brain gene expression in a mouse model of post-traumatic stress disorder (PTSD).
In our work, kefir-fed mice exhibited improved fur conditions, and their body weight was also 8% higher compared to the control group. Therefore, we believe that this may be due to both the addition of kefir to their diet and the fact that the experimental group received more calories, which could have led to an improvement in their appearance. The mice also showed enhanced stress resilience, as indicated by stable exploratory activity and attenuated anxiety-like behavior across multiple behavioral tests, including the open field, elevated plus maze, light/dark box, and aversive context tests. In contrast, control mice displayed significantly lower locomotion and higher signs of anxiety, such as reduced central exploration and elevated freezing responses. These behavioral improvements align with previous reports showing that kefir modulates central neurotransmission through gut microbiota-derived metabolites, particularly those affecting GABAergic and serotonergic systems (Van De Wouw et al., 2020; Icer et al., 2024). Kefir peptides have also been shown to activate BDNF/TrkB signaling and reduce stress-induced hyperthermia, further supporting its role in stress resilience (Chen et al., 2021; Balasubramanian et al., 2024). An interesting effect of kefir on the number of defecations by mice in an open field test was also observed, since only in the second test (after stress) did the number of defecations increase in mice that consumed kefir. Although it was previously noted that kefir did not affect intestinal motility (Van De Wouw et al., 2020). This can be explained by the combination of kefir consumption and stress. For example, kefir lowers the pH in the large intestine due to the presence of short-chain fatty acids (butyric, propionic, and acetic acids), which, in turn, increases the secretion of corticosteroids in mice due to stress, and which can affect intestinal motility (Unsal and Balkay, 2012). Together, this can increase peristalsis, which is why mice had a higher level of defecation compared to the control group. However, after a certain period of time, the number of defecations normalized due to adaptation.
However, we did not observe any differences in the marble ball digging test, which is used to determine the presence of compulsive behavior. These results may be related to the involvement of different neural circuits (Shin and Liberzon, 2010): anxiety and fear are mainly associated with hyperactivation of the amygdala, while obsessive-compulsive disorder has a different localization in brain circuits, particularly the cortico-striato-thalamo-cortical loops (Li and Mody, 2016), and kefir may not have an effect on these regulatory areas of the brain. It may also be due to insufficient sensitivity of the test or an insufficient sample size for this test.
At the systemic level, kefir supplementation resulted in a modest reduction in erythrocyte counts without affecting hemoglobin or hematocrit, indicating preserved oxygen transport. Lower levels in monocytes and higher in juvenile leukocytes suggest a shift toward a less inflammatory immune profile, consistent with kefir’s known immunomodulatory effects (Karaffová et al., 2021; Ben Taheur et al., 2022).
Biochemical analyses showed that kefir did not affect either plasma glucose and protein levels, nor MPO activity, suggesting no acute systemic inflammatory responses. However, higher paraoxonase (PON1) activity in kefir-treated mice points to enhanced antioxidant defense and lipid metabolism, which may contribute to both cardiovascular and neuroprotective effects (Jakubowski, 2024).
In the mouse cerebral cortex, kefir consumption affected the expression of several genes related to oxidative stress response, inflammation, synaptic plasticity, and cellular metabolism. We observed an increase in mRNA levels of TXNRD1 and UGDH, both associated with the Nrf2 pathway, which regulates cellular antioxidant defense and metabolic adaptation (Tonelli et al., 2018; Wu et al., 2012). These transcriptional changes may indicate activation of protective molecular responses rather than direct antioxidant effects.
A moderate reduction in IL6 expression could reflect attenuation of neuroinflammatory signaling, possibly mediated by Nrf2–NF-κB crosstalk (Saha et al., 2020). In parallel, changes in BECN1, HSPB8, and GADD45B transcripts suggest a potential modulation of autophagy and stress adaptation pathways, although the functional implications remain to be clarified.
Genes involved in neuronal plasticity and energy metabolism, such as SHANK1, RICTOR, EIF4EBP1, and PPARGC1A, also showed altered transcripts. Since these genes participate in mTORC2 and mitochondrial biogenesis pathways, modulation of their expression might reflect compensatory cellular responses to the stress (Costa-Mattioli and Monteggia, 2013; Huang and Fingar, 2014).
Overall, the observed transcriptional changes suggest that kefir may influence molecular networks linked to antioxidant defense, inflammation, and synaptic regulation. However, as mRNA levels do not necessarily reflect protein abundance or enzymatic activity, these findings should be interpreted cautiously and verified in future studies at the protein or functional level.
The molecular analyses were limited to the prefrontal cortex because of its central role in cognitive control and emotional regulation in PTSD. Other regions, such as the hippocampus and amygdala, were not examined in the present series due to limited tissue availability. Nonetheless, future studies will include these regions to provide a more comprehensive neuroanatomical understanding of kefir’s effects.
Taken together, our findings indicate that long-term kefir consumption exerts broad protective effects in a PTSD-like mouse model by improving behavioral responses, modulating immune and oxidative stress markers, and enhancing the expression of genes involved in neuroplasticity and metabolic regulation. These results support the potential of kefir consumption as a functional dietary strategy for enhancing stress resilience and mitigating trauma-related disorders, likely through mechanisms involving the gut-brain axis. However, these findings also demonstrate the need for further research into optimized dosing, strain selection, and combinatorial therapies.
Further research on this topic should determine whether there is a direct correlation between kefir modulation of the microbiome and the results we obtained. 16S rRNA sequencing should be performed to identify the diversity of the microbiota, verify the expression of tight junction proteins occludin and ZO-1 in colon tissue, and study intestinal metabolites such as short-chain fatty acids and the tryptophan metabolite 5-hydroxytryptamine. In addition, our study determined the overall effect of kefir as a dietary supplement. Therefore, future research will aim to identify specific strains and metabolites responsible for the observed effects, which is critical for translational applications.
5 Conclusion and perspectives
This study demonstrates that long-term kefir consumption significantly attenuates stress-induced behavioral alterations and favorably modulates physiological and molecular parameters in mice exposed to traumatic stress. Kefir-treated mice exhibited reduced anxiety-like behavior across multiple validated tests, including the open field, elevated plus maze, light/dark box, and aversive context paradigms. In addition to behavioral improvements, kefir intake led to beneficial changes in hematological and biochemical markers, including increased paraoxonase activity and reduced monocyte levels, indicating enhanced antioxidant defense and lower systemic inflammation. At the molecular level, in the cerebral cortex, kefir modulated the expression of key genes associated with oxidative stress resistance, neuroprotection, synaptic plasticity, autophagy, and metabolic regulation. These findings suggest that kefir promotes stress resilience through the activation of adaptive pathways in both the immune and nervous systems, potentially mediated by the gut-brain axis. Collectively, our data support the potential of kefir as a functional dietary intervention to prevent or mitigate PTSD-related symptoms. While the present results are preliminary and limited to an animal model, they raise the possibility that kefir generally, and particular kefir Molokia, could contribute to nutrition-based strategies aimed at supporting human mental health and resilience.
This study highlights kefir’s potential as a functional dietary intervention for enhancing stress resilience. Future research should focus on identifying the specific microbial strains and metabolites responsible for its effects, as well as determining optimal dosage and duration. Kefir may contribute to improved stress resilience in mice, which warrants further exploration in the context of mental health. Additionally, combining kefir with other nutritional or therapeutic strategies may further enhance its benefits. Overall, kefir represents a promising, accessible tool for supporting mental health through gut-brain axis modulation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Vasyl Stefanyk Carpathian National University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
VB: Methodology, Investigation, Writing – original draft, Visualization, Data curation, Formal Analysis, Validation. TD: Investigation, Writing – original draft. AD: Writing – original draft, Visualization. VL: Conceptualization, Resources, Writing – review and editing, Funding acquisition, Project administration, Validation, Data curation, Methodology, Supervision, Formal Analysis.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgements
The authors thank H. Cherevata for animal care, and students I. Labych, V. Derkachov, A. Tkachyk, N. Stefanyshyn, I. Granas, and O. Vasylyshyn for their valuable assistance with tissue collection and blood parameter measurements. We are also grateful to R. Shuliar, M. Mishchur, and T. Prysiazhniuk for constructing the chamber used for stress induction in mice. The authors thank two reviewers whose highly professional and constructive comments helped us substantially improve the quality of the paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Generative AI was used for polishing of the text.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Albuquerque Pereira M. D. F., Matias Albuini F., Gouveia Peluzio M. D. C. (2024). Anti-inflammatory pathways of kefir in murine model: a systematic review. Nutr. Rev. 82, 210–227. doi:10.1093/nutrit/nuad052
Angoa-Pérez M., Kane M. J., Briggs D. I., Francescutti D. M., Kuhn D. M. (2013). Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. JoVE 82, 50978. doi:10.3791/50978
Balasubramanian R., Schneider E., Gunnigle E., Cotter P. D., Cryan J. F. (2024). Fermented foods: harnessing their potential to modulate the microbiota-gut-brain axis for mental health. Neurosci. Biobehav. Rev. 158, 105562. doi:10.1016/j.neubiorev.2024.105562
Balatskyi V., Strilbytska O., Abrat O., Tkachyk A., Lylyk M., Lushchak V., et al. (2025). Behavioral and metabolic effects of escapable electric foot shock stress in Male mice. BMC Res. Notes. 18, 127. doi:10.1186/s13104-025-07189-0
Ben Taheur F., Mansour C., Mechri S., Skhiri S. S., Jaouadi B., Mzoughi R., et al. (2022). Does probiotic kefir reduce dyslipidemia, hematological disorders and oxidative stress induced by zearalenone toxicity in wistar rats? Toxicon 14, 100121. doi:10.1016/j.toxcx.2022.100121
Bisson J. I., Wright L. A., Jones K. A., Lewis C., Phelps A. J., Sijbrandij M., et al. (2021). Preventing the onset of post traumatic stress disorder. Clin. Psychol. Rev. 86, 102004. doi:10.1016/j.cpr.2021.102004
Bourin M., Hascoët M. (2003). The mouse light/dark box test. Eur. J. Pharmacol. 463, 55–65. doi:10.1016/s0014-2999(03)01274-3
Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. doi:10.1006/abio.1976.9999
Chakrabarti A., Geurts L., Hoyles L., Iozzo P., Kraneveld A. D., La Fata G., et al. (2022). The microbiota–gut–brain axis: pathways to better brain health. Perspectives on what we know, what we need to investigate and how to put knowledge into practice. Cell. Mol. Life Sci. 79, 80. doi:10.1007/s00018-021-04060-w
Chen H.-L., Lan Y.-W., Tu M.-Y., Tung Y.-T., Chan M. N.-Y., Wu H.-S., et al. (2021). Kefir peptides exhibit antidepressant-like activity in mice through the BDNF/TrkB pathway. J. Dairy Sci. 104, 6415–6430. doi:10.3168/jds.2020-19222
Costa-Mattioli M., Monteggia L. M. (2013). mTOR complexes in neurodevelopmental and neuropsychiatric disorders. Nat. Neurosci. 16, 1537–1543. doi:10.1038/nn.3546
Crawley J., Goodwin F. K. (1980). Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol. Biochem. Beh. 13, 167–170. doi:10.1016/0091-3057(80)90067-2
Demianchuk O., Vatashchuk M., Gospodaryov D., Hurza V., Ivanochko M., Derkachov V., et al. (2024). High-fat high-fructose diet and alpha-ketoglutarate affect mouse behavior that is accompanied by changes in oxidative stress response and energy metabolism in the cerebral cortex. BBA - General Subj. 1868, 130521. doi:10.1016/j.bbagen.2023.130521
Dmytriv T. R., Tsiumpala S. A., Semchyshyn H. M., Storey K. B., Lushchak V. I. (2023). Mitochondrial dysfunction as a possible trigger of neuroinflammation at post-traumatic stress disorder (PTSD). Front. Physiol. 14, 1222826. doi:10.3389/fphys.2023.1222826
Dmytriv T. R., Duve K. V., Storey K. B., Lushchak V. I. (2024a). Vicious cycle of oxidative stress and neuroinflammation in pathophysiology of chronic vascular encephalopathy. Front. Physiol. 15, 1443604. doi:10.3389/fphys.2024.1443604
Dmytriv T. R., Storey K. B., Lushchak V. I. (2024b). Intestinal barrier permeability: the influence of gut microbiota, nutrition, and exercise. Front. Physiol. 15, 1380713. doi:10.3389/fphys.2024.1380713
Gogineni V. K. (2013). Probiotics: history and evolution. J. Anc. Dis. Prev. Rem. 1, 2. doi:10.4172/2329-8731.1000107
Gomez-Pinilla F., Gomez A. G. (2011). The influence of dietary factors in central nervous system plasticity and injury recovery. PM&R 6, 11–16. doi:10.1016/j.pmrj.2011.03.001
Gradus J. L., Farkas D. K., Svensson E., Ehrenstein V., Lash T. L., Toft Sørensen H. (2017). Posttraumatic stress disorder and gastrointestinal disorders in the Danish population. Epidemiology 28, 354–360. doi:10.1097/ede.0000000000000622
Huang K., Fingar D. C. (2014). Growing knowledge of the mTOR signaling network. Seminars Cell. Dev. Biol. 36, 79–90. doi:10.1016/j.semcdb.2014.09.011
Icer M. A., Sarikaya B., Kocyigit E., Atabilen B., Çelik M. N., Capasso R., et al. (2024). Contributions of gamma-aminobutyric acid (GABA) produced by lactic acid bacteria on food quality and human health: current applications and future prospects. Foods 13, 2437. doi:10.3390/foods13152437
Jakubowski H. (2024). The molecular bases of anti-oxidative and anti-inflammatory properties of paraoxonase 1. Antioxidants 13, 1292. doi:10.3390/antiox13111292
Joung J. Y., Song J. G., Kim H. W., Oh N. S. (2021). Protective effects of milk casein on the brain function and behavior in a mouse model of chronic stress. J. Agric. Food Chem. 69, 1936–1941. doi:10.1021/acs.jafc.0c07292
Joung J. Y., Song J. G., Lee B., Kim H. W., Oh N. S. (2023). Preventive effect of peptides derived from fermented milk on chronic stress-induced brain damage and intestinal dysfunction in mice. J. Dairy Sci. 106, 8287–8298. doi:10.3168/jds.2023-23320
Karaffová V., Mudroňová D., Mad’ar M. ,, Hrčková G., Faixová D., Gancarčíková S., et al. (2021). Differences in immune response and biochemical parameters of mice fed by kefir milk and Lacticaseibacillus paracasei isolated from the kefir grains. Microorganisms 9, 831. doi:10.3390/microorganisms9040831
Kearney D. J., Kamp K. J., Storms M., Simpson T. L. (2022). Prevalence of gastrointestinal symptoms and irritable bowel syndrome among individuals with symptomatic posttraumatic stress disorder. J. Clin. Gastroenterol. 56, 592–596. doi:10.1097/mcg.0000000000001670
Kim B., Hong V. M., Yang J., Hyun H., Im J. J., Hwang J., et al. (2016). A review of fermented foods with beneficial effects on brain and cognitive function. JFN 21, 297–309. doi:10.3746/pnf.2016.21.4.297
Kumar M. R., Yeap S. K., Mohamad N. E., Abdullah J. O., Masarudin M. J., Khalid M., et al. (2021). Metagenomic and phytochemical analyses of kefir water and its subchronic toxicity study in BALB/c mice. BMC Complement. Med. Ther. 21, 183. doi:10.1186/s12906-021-03358-3
Li B., Mody M. (2016). Cortico-Striato-Thalamo-Cortical circuitry, working memory, and obsessive-compulsive disorder. Front. Psychiatry 7, 78. doi:10.3389/fpsyt.2016.00078
Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. doi:10.1006/meth.2001.1262
Loh J. S., Mak W. Q., Tan L. K. S., Ng C. X., Chan H. H., Yeow S. H., et al. (2024). Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases. Sig. Transduct. Target Ther. 9, 37. doi:10.1038/s41392-024-01743-1
Lushchak O., Orru M., Strilbytska O., Berezovskyi V., Cherkas A., Storey K. B., et al. (2023). Metabolic and immune dysfunctions in post-traumatic stress disorder: what can we learn from animal models? EXCLI J. 22, 928–945. doi:10.17179/excli2023-6391
Mariana de F., Albuquerque P., Matias Albuini F. (2024). Anti-inflammatory pathways of kefir in murine model: a systematic review. Nutr. Rev. 82 (2), 210–227. doi:10.1093/nutrit/nud052
Martinho R., Correia G., Seixas R., Oliveira A., Silva S., Serrão P., et al. (2021). Treatment with nepicastat decreases contextual traumatic memories persistence in post-traumatic stress disorder. Front. Mol. Neurosci. 14, 745219. doi:10.3389/fnmol.2021.745219
Martinho R., Seixas R., Azevedo M., Oliveira A., Serrão P., Moreira-Rodrigues M. (2022). Sotalol treatment may interfere with retrieval, expression, and/or reconsolidation processes thus disrupting traumatic memories in a post-traumatic stress disorder mice model. Front. Pharmacol. 12, 809271. doi:10.3389/fphar.2021.809271
Miao X.-R., Chen Q.-B., Wei K., Tao K.-M., Lu Z.-J. (2018). Posttraumatic stress disorder: from diagnosis to prevention. Mil. Med. Res. 5, 32. doi:10.1186/s40779-018-0179-0
Moineau S., Goulet J. (1991). Effect of fermented milks on humoral immune response in mice. Inter. Dairy J. 1, 231–239. doi:10.1016/0958-6946(91)90016-2
O’Connell K. E., Mikkola A. M., Stepanek A. M., Vernet A., Hall C. D., Sun C. C., et al. (2015). Practical murine hematopathology: a comparative review and implications for research. Comp. Med. 65, 96–113. doi:10.3121/cmr.2014.1284
Perdigón G., Maldonado Galdeano C., Valdez J., Medici M. (2002). Interaction of lactic acid bacteria with the gut immune system. Eur. J. Clin. Nutr. 56, 21–26. doi:10.1038/sj.ejcn.1601658
Pinna G., Kmita H., Lushchak V. I. (2023). Editorial: role of mitochondria in post-traumatic stress disorder (PTSD). Front. Physiol. 14, 1341204. doi:10.3389/fphys.2023.1341204
Rodriguez A., Zhang H., Klaminder J., Brodin T., Andersson P. L., Andersson M. (2018). ToxTrac: a fast and robust software for tracking organisms. Methods Ecol. Evol. 9 (3), 460–464. doi:10.1111/2041-210x.12874
Rook G. A. W. (2024). Evolution and the critical role of the microbiota in the reduced mental and physical health associated with low socioeconomic status (SES). Neurosci. Biobeh. Rev. 161, 105653. doi:10.1016/j.neubiorev.2024.105653
Saha S., Buttari B., Panieri E., Profumo E., Saso L. (2020). An overview of Nrf2 signaling pathway and its role in inflammation. Molecules 25 (22), 5474. doi:10.3390/molecules25225474
Seibenhener M. L., Wooten M. C. (2015). Use of the open field maze to measure locomotor and anxiety-like behavior in mice. JoVE 96, 52434. doi:10.3791/52434
Shin L. M., Liberzon I. (2010). The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35, 169–191. doi:10.1038/npp.2009.83
Silva A. O., Ribeiro J. M., Patrocínio T. B., Amorim G. E., Pereira-Júnior A. A., Ângelo M. L., et al. (2023). Protective effects of kefir against unpredictable chronic stress alterations in mice central nervous system, heart, and kidney. Probiotics and Antimicro Prot 15, 411–423. doi:10.1007/s12602-022-10031-9
Sousa R. J. M., Baptista J. A. B., Silva C. C. G. (2022). Consumption of fermented dairy products is associated with lower anxiety levels in Azorean university students. Front. Nutr. 9, 930949. doi:10.3389/fnut.2022.930949
Tonelli C., Chio I. I. C., Tuveson D. A. (2018). Transcriptional regulation by Nrf2. Antioxidants and Redox Signal. 29, 1727–1745. doi:10.1089/ars.2017.7342
Unsal H., Balkay M. (2012). “Glucocorticoids and the intestinal environment,” in Glucocorticoids - new recognition of our familiar friend. Editor X. Qian (London, United Kingdom: InTech). doi:10.5772/51977
Van De Wouw M., Walsh A. M., Crispie F., Van Leuven L., Lyte J. M., Boehme M., et al. (2020). Distinct actions of the fermented beverage kefir on host behaviour, immunity and microbiome gut-brain modules in the mouse. Microbiome 8, 67. doi:10.1186/s40168-020-00846-5
Vatashchuk M., Hurza V., Bayliak M. (2023). Підбір умов для спектрофотометричного визначення активності параоксонази з 4-нітрофенілацетатом у плазмі та печінці мишей. JPNU 9, 6–14. doi:10.15330/jpnu.9.4.6-14
Walf A. A., Frye C. A. (2007). The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2, 322–328. doi:10.1038/nprot.2007.44
Wu K. C., Cui J. Y., Klaassen C. D. (2012). Effect of graded Nrf2 activation on phase I and II drug-metabolizing enzymes and transporters in mouse liver. PLoS ONE 7 (8), e39006. doi:10.1371/journal.pone.0039006
Yadav M. K., Pradhan P. K., Sood N., Chaudhary D. K., Verma D. K., Debnath C., et al. (2014). Innate immune response of Indian major carp, Labeo rohita infected with oomycete pathogen Aphanomyces invadans. Fish and Shellfish Immunol 39, 524–531. doi:10.1016/j.fsi.2014.06.005
Keywords: stress, anxiety, behavior, PTSD, open field test, foot shock, inflammation
Citation: Balatskyi VA, Dmytriv TR, Divnych A and Lushchak VI (2025) Kefir enhances stress resilience and mitigates PTSD-related behavioral and hematological changes in mice. Front. Physiol. 16:1682807. doi: 10.3389/fphys.2025.1682807
Received: 09 August 2025; Accepted: 27 October 2025;
Published: 14 November 2025.
Edited by:
Xiangrong Cheng, Jiangnan University, ChinaCopyright © 2025 Balatskyi, Dmytriv, Divnych and Lushchak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Volodymyr I. Lushchak, dm9sb2R5bXlyLmx1c2hjaGFrQGNudS5lZHUudWE=
 Vitalii A. Balatskyi1
Vitalii A. Balatskyi1 Volodymyr I. Lushchak
Volodymyr I. Lushchak