- 1Department of Rehabilitation Medicine and Geriatrics, Chongqing Liangping District People’s Hospital, Chongqing, China
- 2Jiangsu Province Key Laboratory of Anesthesiology, Jiangsu Province Key Laboratory of Anesthesia and Analgesia Application Technology, NMPA Key Laboratory for Research and Evaluation of Narcotic and Psychotropic Drugs, School of Anesthesiology, Xuzhou Medical University, Xuzhou, Jiangsu, China
- 3Department of Anesthesiology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, China
Anesthetics have long been recognized as essential pharmacological agents for surgical procedures, primarily valued for their ability to induce unconsciousness and provide analgesia. However, emerging research over the past 3 decades has revealed an additional and potentially transformative property of certain anesthetics: their ability to protect the heart against ischemic injury. This comprehensive review examines the cardioprotective effects of both intravenous and volatile anesthetics, with particular focus on propofol, ketamine, isoflurane, and sevoflurane. We analyze the molecular mechanisms underlying their protective actions, including modulation of mitochondrial function, reduction of oxidative stress, and regulation of key survival pathways such as PI3K/Akt/GSK3βand p53 signaling. The review evaluates preclinical evidence from cellular and animal models, as well as clinical studies investigating anesthetic-mediated cardioprotection in cardiac surgery patients. Special attention is given to the phenomenon of anesthetic preconditioning and postconditioning, their comparative efficacy, and the challenges in translating these protective strategies into clinical practice. We also discuss emerging concepts such as the role of microRNAs in mediating anesthetic-induced protection and the potential cardioprotective benefits of anesthetic combinations. Finally, we identify critical gaps in current knowledge and propose future research directions that may enhance the clinical application of anesthetic-mediated cardioprotection.
1 Introduction
The recognition that anesthetic agents may confer cardioprotective benefits has fundamentally transformed our perspective on these pharmacological compounds (Lotz and Kehl, 2015). While anesthetics have been employed clinically since the mid-19th century, their capacity to actively safeguard the heart against ischemic injury has only emerged as a focus of rigorous scientific investigation in recent decades. This paradigm shift has created new opportunities to enhance outcomes in cardiac surgery and acute coronary syndrome management (Hendrix and Kramer, 2025).
The understanding of anesthetic-mediated cardioprotection stems from groundbreaking research on ischemic preconditioning, where scientists first demonstrated the heart’s remarkable ability to develop resistance to prolonged ischemia following brief ischemic episodes (Murry et al., 1986). This fundamental discovery unveiled the myocardium’s innate protective mechanisms and their potential for pharmacological stimulation. Further investigations revealed that specific anesthetic compounds could mimic these protective effects without requiring actual ischemic events, transforming our approach to both cardiovascular research and clinical anesthesia (Zhu et al., 2017; Shirakawa et al., 2014; Kobayashi et al., 2008; Ko et al., 1997a). Despite significant advances in medical technology, ischemia-reperfusion injury continues to pose major clinical challenges, particularly in cardiac surgery and acute coronary care settings (Xiang et al., 2024). The persistent occurrence of myocardial damage during procedures involving cardiopulmonary bypass remains a critical factor affecting postoperative recovery (Algoet et al., 2023). This ongoing clinical challenge highlights the importance of exploring anesthetic agents as potential therapeutic tools to reduce ischemia-reperfusion injury, offering a practical approach to improving patient outcomes using well-established medications (Kato and Foex, 2002).
This review offers a systematic examination of anesthetic-induced cardioprotection with three principal aims: first, to delineate the molecular mechanisms underlying the protective effects of various anesthetic agents; second, to assess the comparative efficacy of different anesthetics in both experimental and clinical settings; and third, to analyze the translational challenges and opportunities for optimizing patient outcomes. Our analysis focuses on propofol, ketamine, and volatile anesthetics (isoflurane, sevoflurane), which represent the most extensively studied agents in cardioprotection research. Building upon previous comprehensive evaluations (De Hert et al., 2005; Pagel and Crystal, 2018), this review provides an updated, comprehensive synthesis that significantly advances previous analyses. We integrate contemporary preclinical findings from cellular and animal models with robust clinical evidence, while addressing critical translational gaps identified in earlier reviews (Lin et al., 2021; Van Allen et al., 2012; Chiari and Fellahi, 2024; Tamura et al., 2025). Specifically, we examine patient-specific considerations (e.g., diabetes, aging) and propose practical strategies for clinical implementation. By emphasizing emerging biomarkers, combination therapies, and individualized application, this synthesis not only updates but significantly expands the current understanding of anesthetic-mediated cardioprotection.
The potential clinical impact of anesthetic cardioprotection is substantial. Successful translation of these effects could improve outcomes in cardiac surgery, enhance management of acute coronary syndromes, and potentially inform novel approaches to heart failure prevention. Furthermore, elucidation of these protective mechanisms may guide development of new pharmacological agents that provide cardioprotection independent of anesthetic effects, potentially benefiting non-surgical patients at risk of ischemic heart disease.
2 Mechanisms of anesthetic-induced cardioprotection
2.1 Propofol
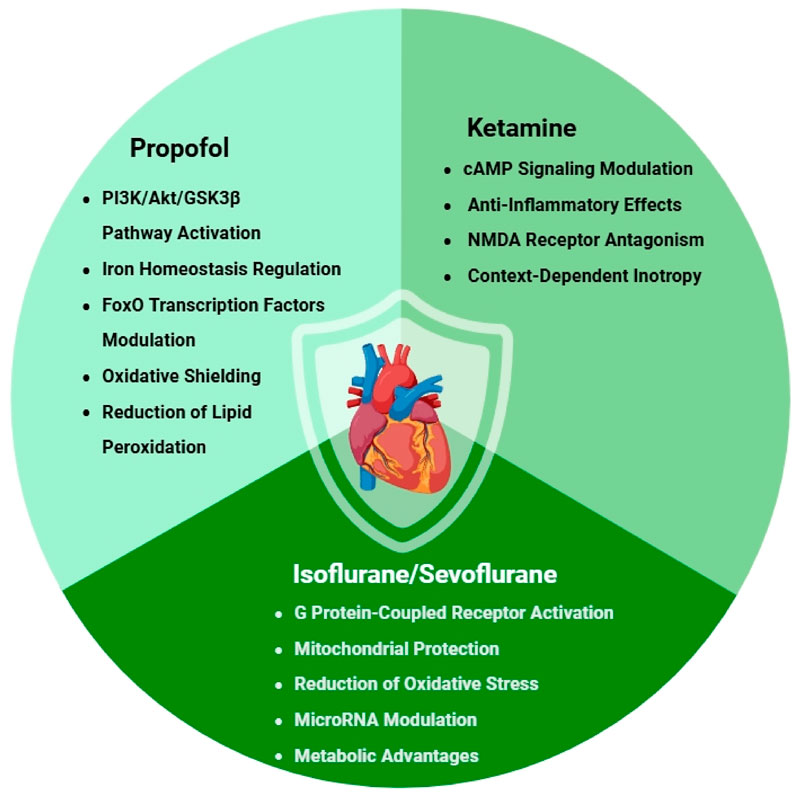
Propofol (2,6-diisopropylphenol) has emerged as one of the most clinically important intravenous anesthetics, with accumulating evidence demonstrating its remarkable cardioprotective properties (Figure 1) (H et al., 2021). Beyond its well-established anesthetic effects, extensive preclinical and clinical investigations have revealed that propofol exerts its protective influence through multiple, intricately interconnected molecular pathways, making it a particularly fascinating subject for ongoing cardiovascular research (Zhao et al., 2015).
The cardioprotective mechanisms of propofol are primarily mediated through activation of the PI3K/Akt/GSK3β signaling pathway, a crucial survival pathway in cardiomyocytes. Detailed mechanistic studies have shown that propofol significantly upregulates Caveolin-3 (Cav-3), an essential membrane scaffolding protein that plays pivotal roles in cardiomyocyte function, signal transduction, and survival. These investigations demonstrated that propofol exerts a protective effect by specifically inhibiting proteasomal degradation of Cav-3 during the critical phases of ischemia-reperfusion injury. This preservation of Cav-3 leads to markedly enhanced activation of the PI3K/Akt/GSK3β pathway (Zhu et al., 2017). The resulting signaling cascade initiates a powerful anti-apoptotic program that promotes cell survival under conditions of ischemic stress, while simultaneously inhibiting key mediators of cell death pathways.
Recent advances in our understanding of propofol’s cardioprotective effects have revealed another significant mechanism involving its regulation of iron homeostasis in cardiomyocytes (Zhang et al., 2019). Given that iron dysregulation and subsequent iron-catalyzed oxidative damage have been strongly implicated in the pathogenesis of ischemia-reperfusion injury (Sun et al., 2024; Pan et al., 2022), these findings take on particular clinical relevance. Cutting-edge research has demonstrated that propofol effectively inhibits pathological iron deposition in both H9c2 cardiomyoblast cells and in vivo mouse myocardium through sophisticated modulation of the AKT/p53 signaling pathway (Li et al., 2022). This iron-regulating effect appears to be especially important for reducing the burst of reactive oxygen species (ROS) generation that typically occurs during the reperfusion phase, thereby substantially limiting oxidative damage to critical cellular components including lipids, proteins, and DNA.
Further expanding our understanding of propofol’s multifaceted protective effects, studies have identified its significant influence on FoxO transcription factors, which serve as master regulators of cellular responses to oxidative stress. Comprehensive research has shown that propofol post-conditioning dramatically improves outcomes following hypoxia/reoxygenation-induced injury by reducing apoptosis and modulating autophagy in cardiac cells through upregulation of forkhead transcription factors (H et al., 2021; Zhang et al., 2022). This represents an additional, independent layer of cardioprotection that complements rather than merely duplicates the benefits mediated through the PI3K/Akt pathway.
The intrinsic antioxidant properties of propofol deserve particular emphasis in any discussion of its cardioprotective mechanisms. The drug’s unique chemical structure, featuring a phenolic hydroxyl group, confers exceptional free radical scavenging capabilities that make it particularly effective at mitigating oxidative stress during the critical reperfusion period (Marik, 2005). This direct antioxidant activity synergizes with its effects on cellular signaling pathways to create a comprehensive, multifaceted protective profile that addresses multiple aspects of ischemia-reperfusion injury (Hausburg et al., 2020). Rigorous experimental studies have consistently shown that propofol treatment reduces markers of lipid peroxidation, helps preserve mitochondrial membrane potential integrity, and maintains optimal cellular glutathione levels during ischemia-reperfusion scenarios (Ranjbar et al., 2014; Yoo et al., 1999; Xia et al., 2004; Shao et al., 2008).
Perhaps most intriguingly, clinical and experimental observations have revealed that propofol’s cardioprotective effects follow a clear dose-response relationship (Shao et al., 2008; Vanlersber et al., 2008). While moderate, clinically relevant doses provide significant protection against ischemia-reperfusion injury, very high concentrations may paradoxically produce negative inotropic effects and impair cardiac function, particularly in immature or developing hearts. This biphasic dose-response curve, first systematically characterized in preclinical models, carries important implications for optimizing dosing strategies across different patient populations and clinical scenarios (Shirakawa et al., 2014). The recognition of this dose-dependent effect has prompted more nuanced approaches to propofol administration in cardiac patients and those at risk of perioperative ischemia.
2.2 Ketamine
Ketamine has emerged as a uniquely versatile intravenous anesthetic with multifaceted cardioprotective properties that distinguish it from other agents in its class (Figure 1) (Trimmel et al., 2018; Molojavyi et al., 2001; Hirota and Lambert, 2011; Zanos et al., 2018; Hudetz and Pagel, 2010). While it shares certain protective pathways with propofol, particularly in modulating cellular survival signals (Cope et al., 1997), ketamine’s distinct pharmacological profile - combining NMDA receptor antagonism with sympathomimetic and anti-inflammatory effects - confers specific clinical advantages that may be particularly valuable in high-risk cardiac scenarios (Ko et al., 1997b; Han et al., 2002). Beyond its direct cardioprotective roles, these properties also align with evolving perioperative strategies aimed at modulating surgical stress responses and reducing systemic inflammation through multimodal analgesic approaches (Zanos et al., 2018; Zhang et al., 2024; Jian et al., 2018).
The drug’s cardioprotective mechanisms operate through an integrated network of pathways that address multiple aspects of ischemic and inflammatory myocardial injury. A particularly noteworthy mechanism involves ketamine’s modulation of cAMP signaling, which serves as a crucial second messenger system in cardiomyocytes (Peña and Wolska, 2005). Detailed cellular studies have demonstrated that ketamine not only enhances basal cAMP levels in cardiac cells but also effectively counteracts the pathological suppression of cAMP induced by pro-inflammatory cytokines like TNF-α and IL-1β (Hill et al., 1998). This dual action helps maintain critical intracellular signaling during the inflammatory storms that frequently accompany cardiac surgery and ischemia-reperfusion injury, potentially preserving myocardial contractility and metabolic function when they are most compromised.
Ketamine’s robust anti-inflammatory properties constitute another major component of its cardioprotective arsenal (Natoli, 2021; Ibrahim et al., 2017). The drug exerts a multimodal immunomodulatory effect, simultaneously suppressing the production of damaging pro-inflammatory cytokines (including TNF-α, IL-6, and IL-1β) while enhancing the release of protective anti-inflammatory mediators like IL-10. These effects are achieved through several complementary mechanisms: direct inhibition of the NF-κB signaling pathway (a master regulator of inflammatory gene expression), modulation of the NLRP3 inflammasome complex, and potential effects on toll-like receptor signaling (Bi et al., 2024; Santos et al., 2025). Such comprehensive anti-inflammatory activity may be especially beneficial in clinical conditions where systemic inflammation directly contributes to myocardial dysfunction, such as in sepsis-induced cardiomyopathy, post-cardiac arrest syndrome, or the systemic inflammatory response following cardiopulmonary bypass (Zhang et al., 2021).
The NMDA receptor antagonism that forms the basis of ketamine’s anesthetic and analgesic properties also contributes meaningfully to its cardioprotective profile (Tyagi et al., 2009). During ischemic episodes, excessive glutamate release leads to sustained activation of myocardial NMDA receptors (particularly those containing GluN2B subunits), resulting in pathological calcium influx and subsequent activation of cell death pathways (Abbaszadeh et al., 2018). Ketamine’s potent blockade of these receptors helps break this vicious cycle, reducing calcium-mediated injury during both the ischemic and reperfusion phases (Lisek et al., 2020; Iacobucci and Popescu, 2024). Interestingly, this mechanism may complement the drug’s other protective effects, as NMDA receptor overactivation has been linked to both inflammatory signaling and oxidative stress in cardiomyocytes.
Ketamine’s effects on myocardial contractility present a fascinating paradox that underscores the context-dependent nature of its actions (Kunst et al., 1999). While the drug can produce mild negative inotropic effects in healthy myocardium (likely through L-type calcium channel modulation), numerous clinical observations suggest it may actually better preserve ventricular function in failing or stressed hearts compared to alternative anesthetics (Hanouz et al., 2004; Sprung et al., 1998). This apparent paradox may reflect ketamine’s unique ability to maintain sympathetic tone while simultaneously providing cellular protection against ischemia and inflammation - a combination particularly suited to compromised myocardium.
The convergence of these diverse mechanisms - spanning metabolic regulation, inflammatory control, receptor modulation, and functional preservation - establishes ketamine as an exceptionally versatile cardioprotective agent. Its multifaceted action profile makes it particularly valuable in complex clinical scenarios where myocardial ischemia coexists with systemic inflammation, autonomic instability, or pre-existing cardiac dysfunction. Looking forward, important research priorities include the development of optimized dosing protocols for specific high-risk populations (such as patients with septic cardiomyopathy or advanced heart failure), investigation of potential synergistic effects when combined with other cardioprotective strategies (including remote ischemic preconditioning or targeted temperature management), and exploration of its role in emerging applications like donor heart preservation for transplantation.
2.3 Volatile anesthetics
Volatile anesthetics, including isoflurane, sevoflurane, and desflurane, have been extensively documented to exert significant cardioprotective effects across both preclinical and clinical investigations (Van Allen et al., 2012; Tanaka et al., 2004; Pratt et al., 2006; Suda and Uka, 2022). These pharmacological agents mimic the protective mechanisms of ischemic preconditioning while offering the distinct advantage of being pharmacologically inducible without necessitating actual ischemic events (Hara, 2006; Riess et al., 2004). The cardioprotective properties of volatile anesthetics are mediated through multifaceted interactions involving diverse cellular targets (Figure 1) (Agarwal et al., 2014a; Kikuchi et al., 2015; Zhang T. et al., 2025), with their effects being particularly pronounced when administered during the preconditioning phase.
Isoflurane demonstrates comprehensive cardioprotection through several synergistic mechanisms: substantial reduction of oxidative stress, preservation of mitochondrial structural and functional integrity, and optimization of intracellular calcium homeostasis (An et al., 2007; Agarwal et al., 2014b; Liu and Liu, 2018; Lang et al., 2013). Experimental evidence has demonstrated that isoflurane pretreatment significantly attenuates cardiac oxidative damage following ischemia-reperfusion injury, as quantified by reductions in established biomarkers of lipid peroxidation and protein oxidation (Li et al., 2012). This antioxidant effect is mediated through both direct free radical scavenging and upregulation of endogenous antioxidant defense systems. The understanding of isoflurane’s cardioprotective mechanisms has been substantially advanced by recent discoveries in molecular cardiology. Research has revealed that isoflurane exerts its protective effects not only through immediate pharmacological actions but also by inducing lasting epigenetic modifications. Studies examining cardiac cells exposed to hypoxia/reoxygenation injury demonstrate that isoflurane pretreatment significantly alters microRNA-363-3p expression patterns (Ge et al., 2022). This microRNA modulation initiates a comprehensive cellular defense program that simultaneously regulates apoptotic pathways through Bcl-2 family proteins, enhances antioxidant defenses via Nrf2 pathway activation, and strengthens pro-survival signaling through Akt and ERK pathway modulation. These findings fundamentally expand our comprehension of volatile anesthetic-mediated protection by demonstrating its capacity to establish persistent epigenetic changes that confer myocardial resilience. The sustained alteration of microRNA expression profiles provides a molecular basis for the long-lasting cardioprotective effects observed following isoflurane exposure, extending well beyond the acute perioperative period.
Sevoflurane has emerged as a clinically promising cardioprotective agent, exerting its effects through multiple synergistic mechanisms. The anesthetic enhances myocardial ischemic tolerance primarily by activating protein kinase C (PKC) and mitochondrial ATP-sensitive potassium (KATP) channels, thereby preserving mitochondrial function during ischemia-reperfusion injury (Hara et al., 2001; Jiang et al., 2016; Bouwman et al., 2007; de Ruijter et al., 2003; Bouwman et al., 2006). During ischemic conditioning procedures, sevoflurane exposure induces significant molecular changes in cardiomyocytes, including upregulation of cardioprotective microRNAs and cytokines, while simultaneously suppressing mediators of cellular damage (Guerrero-Orriach et al., 2024). Additionally, sevoflurane exerts cardioprotective effects against hypoxia/reoxygenation-induced myocardial injury by downregulating lncRNA LINC00265, which functions as a molecular sponge to inhibit miR-370-3p, thereby reducing apoptosis, and inflammatory cytokine release (IL-6, TNF-α) (Shao et al., 2025). Notably, sevoflurane preconditioning activates the PI3K/AKT/GSK3β pathway to upregulate Syntaxin1a, significantly reducing myocardial apoptosis in murine ischemia-reperfusion models (Liu et al., 2025). These multi-targeted actions - encompassing ion channel modulation, kinase signaling activation, and epigenetic regulation - collectively contribute to sevoflurane’s superior clinical performance in cardiac protection, particularly in surgical preconditioning protocols and potential applications for acute coronary syndromes.
These molecular effects translate to measurable clinical benefits, as demonstrated in randomized trials showing sevoflurane’s superiority over propofol in patients undergoing cardiopulmonary bypass. Specifically, sevoflurane-treated patients exhibit reduced postoperative troponin release and improved ventricular functional recovery (Marcos-Vidal et al., 2014; Likhvantsev et al., 2016). Its rapid onset/offset pharmacokinetics make it especially suitable for preconditioning protocols (Beukers et al., 2025). Desflurane, while less studied, shows comparable cardioprotective potential (Qin and Zhou, 2023; Ozarslan et al., 2012). Its low blood solubility allows precise titration, and evidence suggests it may be particularly effective when administered during early reperfusion. However, its strong sympathetic activation effects require careful hemodynamic management (Landoni et al., 2007).
Volatile anesthetics also appear to influence myocardial metabolism in ways that may enhance ischemic tolerance (van den Brom et al., 2013; Stowe and Kevin, 2004). Several studies have reported that these agents promote a shift toward more efficient energy utilization during ischemia, potentially by modulating substrate selection and improving mitochondrial coupling. These metabolic effects may complement the direct protective actions on signaling pathways and ion channels (Stowe and Kevin, 2004; Yamanaka and Hayashi, 2009; Zhang et al., 2023). Beyond their metabolic effects, volatile anesthetics modulate ion transporter activity, notably that of the K+-Cl- cotransporter 2 (KCC2). While KCC2 has traditionally been studied for its role in maintaining neuronal chloride homeostasis and facilitating emergence from anesthesia (Hu et al., 2023; Song and Hu, 2024), emerging evidence also supports its functional significance in the heart (Modi et al., 2023). It is thus hypothesized that during ischemia-reperfusion injury, volatile anesthetics may influence cardiac KCC2 activity, thereby potentially contributing to the regulation of cell volume and ionic balance. Importantly, the cardioprotective benefits of volatile anesthetics may be significantly enhanced through synergistic combination with lung-protective ventilation strategies. By employing lower tidal volumes, optimal PEEP, and careful avoidance of hyperoxia, anesthesiologists can reduce ventilator-induced lung injury and the subsequent inflammatory cross-talk between the lung and heart, thereby creating a more favorable environment for the myocardial protective effects of volatile agents to manifest (Zou et al., 2024; Ferrando et al., 2015). Clinical implementation continues to evolve, with current evidence supporting volatile use throughout cardiac procedures rather than limited to preconditioning phases (Bonanni et al., 2020). Ongoing research explores optimal combinations with other protective strategies and applications in non-cardiac surgeries for high-risk patients (Zhang et al., 2023; Park et al., 2020).
3 Comparative cardioprotective efficacy of anesthetic agents
3.1 Intravenous vs. volatile anesthetics
The comparative cardioprotective efficacy between intravenous and volatile anesthetic agents has been extensively studied in both laboratory and clinical settings, with a primary focus on patients undergoing cardiac surgery (Bonanni et al., 2020; Bein et al., 2005). A key clinical trial evaluating propofol-based total intravenous anesthesia versus sevoflurane anesthesia in coronary artery bypass graft (CABG) procedures demonstrated better cardioprotection with the volatile anesthetic, as indicated by improved cardiac functional parameters and lower troponin release (De Hert et al., 2002). Subsequent comprehensive reviews and pooled analyses have largely supported these initial findings, showing a consistent, albeit modest, cardioprotective benefit favoring volatile agents in cardiac surgical contexts. However, a more detailed analysis reveals several important considerations. Propofol appears to be particularly effective when used as a postconditioning intervention rather than as the primary maintenance anesthetic (He et al., 2008; Liu et al., 2021). Growing evidence indicates that combining propofol with volatile anesthetics may produce enhanced protective effects, although the ideal dosing protocols for such combined approaches need further clarification (Schumacher et al., 2009; Diz et al., 2010; Wolf et al., 2021). These findings highlight the critical need for selecting anesthetic techniques based on specific clinical circumstances to optimize myocardial protection during cardiac procedures.
The differential effects likely stem from distinct molecular mechanisms: volatile anesthetics primarily act through preconditioning pathways involving mitochondrial KATP channels and protein kinase C activation, while propofol’s benefits are more related to its antioxidant properties and direct effects on cellular survival pathways. This mechanistic diversity suggests potential advantages for either approach depending on the specific clinical scenario and patient characteristics.
3.2 Age and comorbidity considerations
The effectiveness of anesthetic-mediated cardioprotection demonstrates significant variability across different patient populations, with age and comorbid conditions emerging as key determinants of therapeutic response (van den Brom et al., 2013; Ruiz-Meana et al., 2020; Domene et al., 2025). Developmental stage represents a particularly important consideration, as evidenced by research demonstrating heightened vulnerability of immature myocardium to potential adverse effects at elevated propofol concentrations (Shirakawa et al., 2014). This age-dependent sensitivity may reflect differences in drug metabolism, receptor expression patterns, or cellular stress responses during cardiac development.
Advanced age similarly influences anesthetic cardioprotection, with experimental studies showing attenuated protective effects in senescent animal models for both volatile anesthetics and propofol (Ruiz-Meana et al., 2020; Suleiman et al., 2015; Sniecinski and Liu, 2004). The underlying mechanisms likely involve age-associated alterations in cellular signaling cascades, mitochondrial bioenergetics, and redox homeostasis that collectively impair preconditioning responses. These findings have important implications for geriatric patients undergoing cardiac procedures. Diabetes mellitus constitutes another critical modifier of anesthetic cardioprotective efficacy (Liu and Xia, 2012; Drenger et al., 2011; Canfield et al., 2016). The pathological hyperglycemic milieu appears to disrupt multiple protective signaling pathways, including those mediated by adenosine receptors and protein kinase C isoforms. Clinical observations parallel preclinical findings, demonstrating reduced cardioprotection in diabetic patients that may necessitate modified anesthetic strategies (Amour et al., 2010; Canfield et al., 2012). Potential approaches include tighter perioperative glycemic control or the use of adjunctive agents to restore protective signaling.
These collective findings emphasize the necessity of developing personalized anesthetic regimens that carefully consider multiple patient-specific factors including developmental stage and chronological age, the presence and severity of metabolic comorbidities (particularly renal and hepatic function), baseline myocardial function, and the anticipated surgical stress and ischemic burden (Zhu et al., 2024; Abraham et al., 2023; Zhu et al., 2023). Current research in this area would benefit from incorporating large-scale human genetic and epidemiological insights to better understand patient heterogeneity and refine personalized approaches (Chen et al., 2025; Zhu SQ. et al., 2025; Yurkovich et al., 2024). Such comprehensive patient profiling enables clinicians to optimize cardioprotection while minimizing potential adverse effects, particularly in vulnerable populations where standard anesthetic approaches may prove less effective. The integration of these considerations into clinical decision-making represents an important step toward precision medicine in perioperative cardioprotection, requiring careful evaluation of how age-related physiological changes, comorbid conditions, and procedural factors interact to influence anesthetic efficacy. Future research should focus on developing validated clinical algorithms that systematically incorporate these multidimensional patient characteristics to guide anesthetic selection and dosing in cardiac surgery populations.
4 Clinical applications and challenges
4.1 Cardiac surgery applications
The most compelling clinical application of anesthetic cardioprotection lies in cardiac surgery, where ischemia-reperfusion injury remains an unavoidable consequence of cardiopulmonary bypass and aortic cross-clamping (Torregroza et al., 2020; Landoni et al., 2019). A substantial body of evidence now demonstrates that anesthetic selection can significantly impact key postoperative outcomes, including ventricular function recovery, incidence of arrhythmias, and magnitude of cardiac enzyme release (Uhlig et al., 2016). Volatile anesthetics, particularly sevoflurane and desflurane, have emerged as preferred agents in many cardiac centers, with numerous studies demonstrating their superiority over propofol-based total intravenous anesthesia in preserving myocardial function following coronary artery bypass grafting (CABG) (De Hert et al., 2002; Zangrillo et al., 2015; Zhang Y. et al., 2025). Meta-analyses of randomized controlled trials consistently show approximately 20%–30% reductions in troponin release with volatile-based regimens, along with improved early postoperative ejection fraction and reduced inotropic requirements (Yu and Beattie, 2006; Li and Yuan, 2015). The protective effects appear most pronounced in isolated CABG procedures, where ischemic times are typically shorter, though benefits have also been documented in more complex valve surgeries requiring longer cardioplegic arrest (Zangrillo et al., 2022; Piriou et al., 2000). Mechanistically, these clinical observations align with laboratory findings demonstrating volatile anesthetics’ ability to preserve mitochondrial function, reduce oxidative stress, and attenuate calcium overload during reperfusion (De Hert et al., 2008). However, the implementation of volatile-based cardiac anesthesia requires careful consideration of several practical factors, including the need for specialized vaporizers in the bypass circuit and potential interactions with cardioplegia solutions (Landoni et al., 2013). Furthermore, the optimal dosing strategy - whether to administer volatiles throughout surgery or concentrate exposure during specific preconditioning or postconditioning phases - remains an active area of investigation (Meybohm et al., 2015). Interestingly, beyond direct pharmacological conditioning, emerging evidence suggests that regional analgesic techniques such as novel fascial plane blocks (e.g., erector spinae plane or parasternal blocks) may also contribute to systemic anti-inflammatory and cardioprotective effects by modulating neuroimmune pathways and reducing surgical stress responses (Bagnol et al., 2024; Sandeep et al., 2022). This multimodal approach—combining volatile anesthetics with regional techniques—may offer complementary benefits for cardioprotection. Recent studies suggest that combining volatile anesthetics with remote ischemic preconditioning may provide additive protective benefits (Chen et al., 2022), while others have explored the potential of pharmacologic postconditioning with volatile agents during the critical reperfusion period (Lurati Buse et al., 2012). The ongoing debate about the clinical significance of these protective effects continues to drive research into optimal anesthetic protocols for various cardiac surgical populations (Yu, 2011).
4.2 Expanding applications in non-cardiac surgery
While the cardioprotective effects of anesthetics are most extensively studied in cardiac surgery, emerging evidence suggests potential applications in high-risk non-cardiac procedures (Van Allen et al., 2012; Landoni et al., 2009a). Vascular surgery patients, who frequently have significant underlying coronary disease, may represent a particularly promising population for anesthetic-mediated protection (Bas et al., 2012). Preliminary studies indicate that volatile anesthetic use during major vascular procedures may reduce postoperative cardiac complications by 15%–25%, though these findings require confirmation in larger, multicenter trials (Landoni et al., 2009a). The physiological rationale for this protection stems from the frequent hemodynamic fluctuations and potential ischemic episodes during vascular surgery, creating conditions where anesthetic preconditioning could theoretically attenuate myocardial stunning (Harris, 2022; Chen et al., 2018). Other surgical populations that might benefit include patients undergoing major orthopedic procedures (who often have cardiovascular comorbidities) (Kvarda et al., 2022) and those receiving solid organ transplants (where ischemia-reperfusion injury affects both the graft and potentially the heart) (Eltzschig and Eckle, 2011; Jahn et al., 2022). However, several unique challenges arise when considering anesthetic cardioprotection outside cardiac surgery (Landoni et al., 2009b; Hovaguimian et al., 2014). First, the duration and magnitude of ischemic insults are typically less predictable than in controlled cardiac procedures, making optimal timing of protective strategies more difficult (Kalogeris et al., 2012; Zaugg et al., 2014). Second, the balance between potential cardiac benefits and other considerations (such as effects on cerebral or renal perfusion) becomes more complex in heterogeneous non-cardiac surgeries (Stoppe et al., 2017; Petersen et al., 2018). Third, practical constraints like operating room workflow and equipment availability may limit volatile anesthetic use in some non-cardiac settings (Habte et al., 2024). Despite these challenges, the high incidence of perioperative cardiac events in vulnerable populations continues to drive interest in expanding anesthetic cardioprotection strategies beyond traditional cardiac surgery applications (Landoni et al., 2009b; Wong and Irwin, 2016). Future research should focus on identifying which non-cardiac surgical patients stand to benefit most (Hong et al., 2019), developing protocols that integrate seamlessly with diverse surgical workflows (Schild et al., 2019), and determining whether brief exposure to protective anesthetics (rather than maintenance throughout surgery) might suffice for risk reduction (Yoo et al., 2024; Orriach et al., 2020).
4.3 Translational challenges
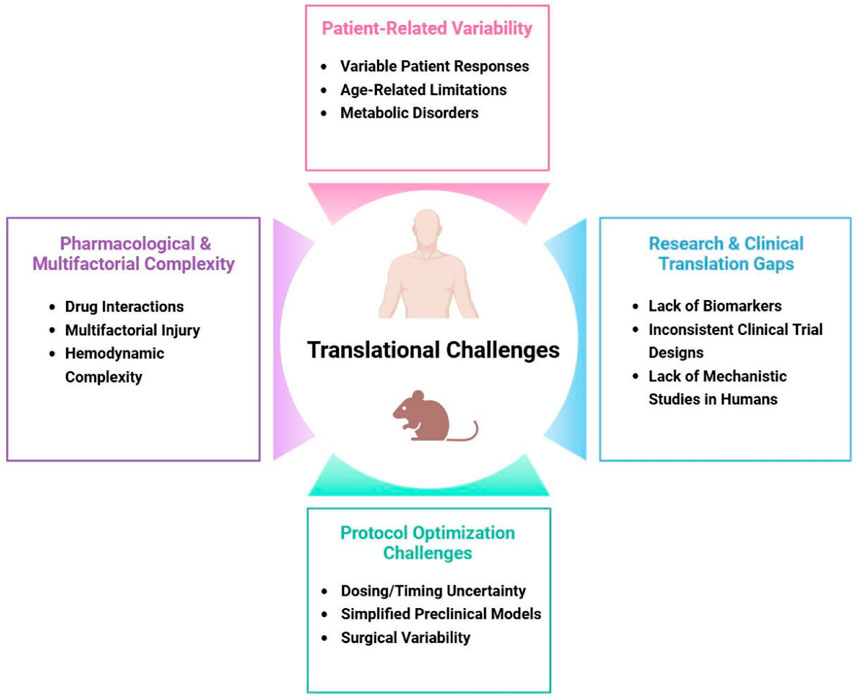
Despite compelling preclinical evidence demonstrating the cardioprotective potential of various anesthetics (Li et al., 2024), translating these findings into consistent clinical benefits has proven challenging (Figure 2) (Lin et al., 2021; Pagel, 2009). One major obstacle is the significant variability in patient responses to protective strategies, influenced by factors such as age, genetic background, and comorbidities like diabetes or heart failure (Kikuchi et al., 2015). For instance, studies suggest that the efficacy of anesthetic preconditioning may be attenuated in elderly or diabetic patients due to age-related mitochondrial dysfunction or metabolic disturbances that impair protective signaling pathways (Mio et al., 2008). Another critical challenge lies in determining the optimal dosing and timing protocols for anesthetic-induced cardioprotection (Frässdorf et al., 2009). While animal studies often use standardized ischemia-reperfusion models (Alemany et al., 2023), clinical scenarios present complex variables including differing surgical durations (Glance et al., 2018), varying ischemic insults (Sandroni et al., 2021), and heterogeneous patient physiologies (See, 2023) that complicate protocol standardization (Kahol et al., 2011; Zho et al., 2016). Additionally, the interactions between anesthetic agents and other perioperative medications—such as beta-blockers, statins, or vasopressors—may either potentiate or interfere with cardioprotective mechanisms (Riess, 2009), adding another layer of complexity to clinical application. Perhaps most fundamentally, the multifactorial nature of perioperative myocardial injury means that anesthetic strategies alone cannot address all potential contributors to cardiac damage (Priebe, 2005), including surgical trauma, systemic inflammation, and hemodynamic instability (Chiari and Fellahi, 2024). These translational gaps highlight the need for more sophisticated clinical research approaches that account for real-world variability while maintaining scientific rigor.
5 Future directions
To overcome current limitations and fully realize the clinical potential of anesthetic-mediated cardioprotection, several key research directions should be prioritized. First, there is an urgent need to develop validated biomarkers—using genomic, proteomic, or metabolomic profiling—that can identify patients most likely to benefit from specific anesthetic strategies. Integrating these biomarkers with emerging perioperative precision monitoring platforms, such as continuous hemodynamic and metabolic tracking, would enable dynamic, physiology-guided personalization of anesthetic regimens tailored to individual patient characteristics and real-time surgical demands. Second, investigating rational combinations of anesthetics—such as pairing volatile agents with propofol or adjuncts like dexmedetomidine—may yield synergistic effects that maximize protection while minimizing adverse outcomes; this approach should be systematically evaluated in both preclinical models and clinical trials. Third, exploring non-anesthetic drugs that target the same protective pathways (e.g., PI3K/Akt activators or mPTP inhibitors) could lead to novel cardioprotective therapies applicable to non-surgical settings like acute coronary syndromes. Finally, large-scale, multicenter clinical trials employing standardized outcome measures are essential to establish evidence-based protocols for different patient populations and surgical contexts. These trials should incorporate advanced monitoring techniques to assess cardioprotection in real-time and employ long-term follow-up to evaluate lasting clinical benefits. Importantly, as recent advances highlight the need to integrate organ protection with patient-centered recovery outcomes, future studies should simultaneously evaluate both cardioprotective efficacy and functional recovery metrics—as demonstrated in non-cardiac settings where anesthetic selection directly influences quality of recovery—to comprehensively optimize perioperative care (Zhu S. et al., 2025). By addressing these priorities, future research can bridge the gap between promising laboratory findings and meaningful improvements in patient care.
6 Conclusion
The cardioprotective effects of anesthetics represent an exciting convergence of anesthesiology and cardiovascular science. While challenges remain in translating these effects into consistent clinical benefits, the accumulated evidence strongly supports the concept that anesthetic choice can meaningfully influence cardiac outcomes. As our understanding of the underlying mechanisms continues to grow, so too will our ability to harness these effects for patient benefit.
Author contributions
TF: Conceptualization, Writing – review and editing, Writing – original draft. XJ: Conceptualization, Writing – review and editing, Writing – original draft, Funding acquisition. CT: Writing – original draft, Writing – review and editing, Visualization. DY: Writing – review and editing, Funding acquisition. HZ: Writing – original draft, Funding acquisition. XW: Writing – review and editing, Resources. SL: Writing – review and editing, Supervision. KW: Writing – review and editing, Writing – original draft, Supervision, Funding acquisition, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 82401511); the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (No. 24KJA320007); Jiangsu Province Innovative and Entrepreneurial Team Program (No. JSSCTD202451); College Student Innovation and Entrepreneurship Training Program (No. 202410313002Z); and Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. KYCX24_3115).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbaszadeh S., Javidmehr A., Askari B., Janssen P. M. L., Soraya H. (2018). Memantine, an NMDA receptor antagonist, attenuates cardiac remodeling, lipid peroxidation and neutrophil recruitment in heart failure: a cardioprotective agent? Biomed. Pharmacother. 108, 1237–1243. doi:10.1016/j.biopha.2018.09.153
Abraham A. S., Elliott C. W., Abraham M. S., Ahuja S. (2023). Intra-operative anesthetic induced myocardial protection during cardiothoracic surgery: a literature review. J. Thorac. Dis. 15 (12), 7042–7049. doi:10.21037/jtd-23-1101
Agarwal B., Stowe D. F., Dash R. K., Bosnjak Z. J., Camara A. K. (2014a). Mitochondrial targets for volatile anesthetics against cardiac ischemia-reperfusion injury. Front. Physiol. 5, 341. doi:10.3389/fphys.2014.00341
Agarwal B., Dash R. K., Stowe D. F., Bosnjak Z. J., Camara A. K. (2014b). Isoflurane modulates cardiac mitochondrial bioenergetics by selectively attenuating respiratory complexes. Biochim. Biophys. Acta 1837 (3), 354–365. doi:10.1016/j.bbabio.2013.11.006
Alemany V. S., Recco D. P., Emani S. M., del Nido P. J., McCully J. D. (2023). Model of ischemia and reperfusion injury in rabbits. Jove-Journal Vis. Exp. 201. doi:10.3791/64752
Algoet M., Janssens S., Himmelreich U., Gsell W., Pusovnik M., Van den Eynde J., et al. (2023). Myocardial ischemia-reperfusion injury and the influence of inflammation. Trends Cardiovasc Med. 33 (6), 357–366. doi:10.1016/j.tcm.2022.02.005
Amour J., Brzezinska A. K., Jager Z., Sullivan C., Weihrauch D., Du J. H., et al. (2010). Hyperglycemia adversely modulates endothelial nitric oxide synthase during anesthetic preconditioning through Tetrahydrobiopterin- and heat shock protein 90-mediated mechanisms. Anesthesiology 112 (3), 576–585. doi:10.1097/ALN.0b013e3181cded1f
An J., Bosnjak Z. J., Jiang M. T. (2007). Myocardial protection by isoflurane preconditioning preserves Ca2+ cycling proteins independent of sarcolemmal and mitochondrial KATP channels. Anesth. Analg. 105 (5), 1207–1213. doi:10.1213/01.ane.0000281053.13929.d0
Bagnoli L., Fabbri N., Ventura M., De Nardus A., Greco S., Righini E. (2024). Miracle twins: erector spinae plane block and quadratus lumborum block, what can we learn from their comparison. Anesth. Periop Sci. 2 (1), 1. doi:10.1007/s44254-023-00044-0
Bassuoni A. S., Amr Y. M. (2012). Cardioprotective effect of sevoflurane in patients with coronary artery disease undergoing vascular surgery. Saudi J. Anaesth. 6 (2), 125–130. doi:10.4103/1658-354X.97024
Bein B., Renner J., Caliebe D., Scholz J., Paris A., Fraund S., et al. (2005). Sevoflurane but not propofol preserves myocardial function during minimally invasive direct coronary artery bypass surgery. Anesth. Analg. 100 (3), 610–616. doi:10.1213/01.ANE.0000145012.27484.A7
Beukers A., Breel J., van den Brom C., Saatpoor A., Kluin J., Eleveld D., et al. (2025). Pharmacokinetics and pharmacodynamics of analgesic and anesthetic drugs in patients during cardiac surgery with cardiopulmonary bypass: a narrative review. Anesth. Analg. doi:10.1213/ANE.0000000000007564
Bi Z., Kong L., Zhao J., Song D., Duan F. (2024). Positive effects of low-dose S-ketamine on preventing myocardial injury after thoracoscopic lobectomy in patients aged 70 to 85. BMC Anesthesiol. 24 (1), 103. doi:10.1186/s12871-024-02491-z
Bonanni A., Signori A., Alicino C., Mannucci I., Grasso M. A., Martinelli L., et al. (2020). Volatile anesthetics versus propofol for cardiac surgery with cardiopulmonary bypass: meta-Analysis of randomized trials. Anesthesiology 132 (6), 1429–1446. doi:10.1097/ALN.0000000000003236
Bouwman R. A., Salic K., Padding F. G., Eringa E. C., van Beek-Harmsen B. J., Matsuda T., et al. (2006). Cardioprotection via activation of protein kinase C-delta depends on modulation of the reverse mode of the Na+/Ca2+ exchanger. Circulation 114, I226–I232. doi:10.1161/CIRCULATIONAHA.105.000570
Bouwman R. A., Musters R. J. P., van Beek-Harmsen B. J., de Lange J. J., Lamberts R. R., Loer S. A., et al. (2007). Sevoflurane-induced cardioprotection depends on PKC-α activation via production of reactive oxygen species. Br. J. Anaesth. 99 (5), 639–645. doi:10.1093/bja/aem202
Canfield S. G., Sepac A., Sedlic F., Muravyeva M. Y., Bai X. W., Bosnjak Z. J. (2012). Marked hyperglycemia attenuates anesthetic preconditioning in human-induced pluripotent stem cell-derived cardiomyocytes. Anesthesiology 117 (4), 735–744. doi:10.1097/ALN.0b013e3182655e96
Canfield S. G., Zaja I., Godshaw B., Twaroski D., Bai X., Bosnjak Z. J. (2016). High glucose attenuates anesthetic cardioprotection in stem-cell-derived cardiomyocytes: the role of reactive oxygen species and mitochondrial fission. Anesth. Analg. 122 (5), 1269–1279. doi:10.1213/ANE.0000000000001254
Chen S., Lotz C., Roewer N., Broscheit J. A. (2018). Comparison of volatile anesthetic-induced preconditioning in cardiac and cerebral system: molecular mechanisms and clinical aspects. Eur. J. Med. Res. 23 (1), 10. doi:10.1186/s40001-018-0308-y
Chen H. S., Cui Y., Li X. Q., Wang X. H., Ma Y. T., Zhao Y., et al. (2022). Effect of remote ischemic conditioning vs usual care on neurologic function in patients with acute moderate ischemic stroke: the RICAMIS randomized clinical trial. JAMA 328 (7), 627–636. doi:10.1001/jama.2022.13123
Chen C., Zhu S., Zheng Z., Ding X., Shi W., Xia T., et al. (2025). A genome-wide study on the genetic and causal effects of smoking in neurodegeneration. J. Transl. Med. 23 (1), 743. doi:10.1186/s12967-025-06688-9
Chiari P., Fellahi J. L. (2024). Myocardial protection in cardiac surgery: a comprehensive review of current therapies and future cardioprotective strategies. Front. Med. (Lausanne) 11, 1424188. doi:10.3389/fmed.2024.1424188
Cope D. K., Impastato W. K., Cohen M. V., Downey J. M. (1997). Volatile anesthetics protect the ischemic rabbit myocardium from infarction. Anesthesiology 86 (3), 699–709. doi:10.1097/00000542-199703000-00023
De Hert S. G., ten Broecke P. W., Mertens E., Van Sommeren E. W., De Blier I. G., Stockman B. A., et al. (2002). Sevoflurane but not propofol preserves myocardial function in coronary surgery patients. Anesthesiology 97 (1), 42–49. doi:10.1097/00000542-200207000-00007
De Hert S. G., Turani F., Mathur S., Stowe D. F. (2005). Cardioprotection with volatile anesthetics: mechanisms and clinical implications. Anesth. Analg. 100 (6), 1584–1593. doi:10.1213/01.ANE.0000153483.61170.0C
De Hert S. G., Longrois D., Yang H., Fleisher L. A. (2008). Does the use of a volatile anesthetic regimen attenuate the incidence of cardiac events after vascular surgery ? Acta Anaesthesiol. Belg. 59 (1), 19–25.
de Ruijter W., Musters R. J. P., Boer C., Stienen G. J. M., Simonides W. S., de Lange J. J. (2003). The cardioprotective effect of sevoflurane depends on protein kinase C activation, opening of mitochondrial K(+)(ATP) channels, and the production of reactive oxygen species. Anesth. Analg. 97 (5), 1370–1376. doi:10.1213/01.ane.0000081786.74722.da
Diz J. C., Del Río R., Lamas A., Mendoza M., Durán M., Ferreira L. M. (2010). Analysis of pharmacodynamic interaction of sevoflurane and propofol on bispectral index during general anaesthesia using a response surface model. Br. J. Anaesth. 104 (6), 733–739. doi:10.1093/bja/aeq081
Domene S. S., Fulginiti D., Thompson A., Vargas V. P. S., Rodriguez L. C., Colon M. D. T., et al. (2025). Inhalation anesthesia and total intravenous anesthesia (TIVA) regimens in patients with obesity: an updated systematic review and meta-analysis of randomized controlled trials. J. Anesth. Analg. Crit. Care 5 (1), 15. doi:10.1186/s44158-025-00234-1
Drenger B., Ostrovsky I. A., Barak M., Nechemia-Arbely Y., Ziv E., Axelrod J. H. (2011). Diabetes blockade of sevoflurane postconditioning is not restored by insulin in the rat heart: phosphorylated signal transducer and activator of transcription 3- and phosphatidylinositol 3-kinase-mediated inhibition. Anesthesiology 114 (6), 1364–1372. doi:10.1097/ALN.0b013e31820efafd
Eltzschig H. K., Eckle T. (2011). Ischemia and reperfusion--from mechanism to translation. Nat. Med. 17 (11), 1391–1401. doi:10.1038/nm.2507
Ferrando C., Soro M., Belda F. J. (2015). Protection strategies during cardiopulmonary bypass: ventilation, anesthetics and oxygen. Curr. Opin. Anaesthesiol. 28 (1), 73–80. doi:10.1097/ACO.0000000000000143
Frässdorf J., Borowski A., Ebel D., Feindt P., Hermes M., Meemann T., et al. (2009). Impact of preconditioning protocol on anesthetic-induced cardioprotection in patients having coronary artery bypass surgery. J. Thorac. Cardiovasc. Surg. 137 (6), 1436–1442.e14422. doi:10.1016/j.jtcvs.2008.04.034
Ge Y. H., Wang C. B., Cui B. Q., Liu Y. G., Lin D. M., Zhang L., et al. (2022). Isoflurane preconditioning may attenuate cardiomyocyte injury induced by hypoxia/reoxygenation possibly by regulating miR-363-3p. Neurotox. Res. 40 (6), 1895–1901. doi:10.1007/s12640-022-00584-6
Glance L. G., Dutton R. P., Feng C. Y., Li Y., Lustik S. J., Dick A. W. (2018). Variability in case durations for common surgical procedures. Anesth. Analgesia 126 (6), 2017–2024. doi:10.1213/ANE.0000000000002882
Guerrero-Orriach J. L., Carmona-Luque M. D., Quesada Munoz G., Rodriguez Capitan M. J. (2024). miRNA expression: I/R cardiomyocyte and sevoflurane. Biomolecules 14 (12), 1554. doi:10.3390/biom14121554
Han R. H., Huang H. M., Han H., Chen H., Zeng F., Xie X., et al. (2021). Propofol postconditioning ameliorates hypoxia/reoxygenation induced H9c2 cell apoptosis and autophagy via upregulating forkhead transcription factors under hyperglycemia. Mil. Med. Res. 8 (1), 58. doi:10.1186/s40779-021-00353-0
Habte M. F., Tegegne B. A., Alemayehu T. Y. (2024). Anesthetics drug wastage and preventive strategies: systematic review. PLoS One 19 (7), e0306933. doi:10.1371/journal.pone.0306933
Han J., Kim N., Joo H., Kim E. (2002). Ketamine abolishes ischemic preconditioning through inhibition of K(ATP) channels in rabbit hearts. Am. J. Physiol. Heart Circ. Physiol. 283 (1), H13–H21. doi:10.1152/ajpheart.01064.2001
Hanouz J. L., Persehaye E., Zhu L., Lammens S., Lepage O., Massetti M., et al. (2004). The inotropic and lusitropic effects of ketamine in isolated human atrial myocardium: the effect of adrenoceptor blockade. Anesth. Analg. 99 (6), 1689–1695. doi:10.1213/01.ANE.0000136466.85913.3C
Hara T., Tomiyasu S., Sungsam C., Fukusaki M., Sumikawa K. (2001). Sevoflurane protects stunned myocardium through activation of mitochondrial ATP-Sensitive potassium channels. Anesth. Analgesia 92 (5), 1139–1145. doi:10.1097/00000539-200105000-00012
Harris D. E. (2022). Perioperative acute myocardial infarction and ischemia after noncardiac surgery: pathophysiology, prevention, and nursing implications. AORN J. 116 (6), 517–531. doi:10.1002/aorn.13826
Hausburg M. A., Banton K. L., Roman P. E., Salgado F., Baek P., Waxman M. J., et al. (2020). Effects of propofol on ischemia-reperfusion and traumatic brain injury. J. Crit. Care 56, 281–287. doi:10.1016/j.jcrc.2019.12.021
He W., Zhang F. J., Wang S. P., Chen G., Chen C. C., Yan M. (2008). Postconditioning of sevoflurane and propofol is associated with mitochondrial permeability transition pore. J. Zhejiang University-Science B 9 (2), 100–108. doi:10.1631/jzus.B0710586
Hendrix J. M., Kramer J. (2025). “Anesthesia inhalation agents and their cardiovascular effects,” in StatPearls. Treasure island (FL) with ineligible companies. Disclosure: Jeremy kramer declares no relevant financial relationships with ineligible companies.
Hill G. E., Anderson J. L., Lyden E. R. (1998). Ketamine inhibits the proinflammatory cytokine-induced reduction of cardiac intracellular cAMP accumulation. Anesth. Analgesia 87 (5), 1015–1019. doi:10.1097/00000539-199811000-00006
Hirota K., Lambert D. G. (2011). Ketamine: new uses for an old drug? Br. J. Anaesth. 107 (2), 123–126. doi:10.1093/bja/aer221
Hong B., Lee S., Kim Y., Lee M., Youn A. M., Rhim H., et al. (2019). Anesthetics and long-term survival after cancer surgery-total intravenous versus volatile anesthesia: a retrospective study. BMC Anesthesiol. 19 (1), 233. doi:10.1186/s12871-019-0914-4
Hovaguimian F., Schlapfer M., Beck-Schimmer B. (2014). Organ protection in allograft recipients: anesthetic strategies to reduce postoperative morbidity and mortality. Curr. Opin. Organ Transpl. 19 (2), 121–130. doi:10.1097/MOT.0000000000000062
Hu J. J., Liu Y., Yao H., Cao B., Liao H., Yang R., et al. (2023). Emergence of consciousness from anesthesia through ubiquitin degradation of KCC2 in the ventral posteromedial nucleus of the thalamus. Nat. Neurosci. 26 (5), 751–764. doi:10.1038/s41593-023-01290-y
Hudetz J. A., Pagel P. S. (2010). Neuroprotection by ketamine: a review of the experimental and clinical evidence. J. Cardiothorac. Vasc. Anesth. 24 (1), 131–142. doi:10.1053/j.jvca.2009.05.008
Iacobucci G. J., Popescu G. K. (2024). Calcium- and calmodulin-dependent inhibition of NMDA receptor currents. Biophys. J. 123 (3), 277–293. doi:10.1016/j.bpj.2023.12.018
Ibrahim T. H., Abdelrahman H. S., Alharbi M. A., Zabani I. A., Ismail M. F., Kary H. (2017). Effect of ketamine on pro- and anti-inflammatory cytokine response in paediatric cardiac surgery: a prospective randomised controlled study. Indian J. Anaesth. 61 (7), 549–555. doi:10.4103/ija.IJA_607_16
Jahn N., Volker M. T., Laudi S., Stehr S., Schneeberger S., Brandacher G., et al. (2022). Analysis of volatile anesthetic-induced organ protection in simultaneous pancreas-kidney transplantation. J. Clin. Med. 11 (12), 3385. doi:10.3390/jcm11123385
Jian W., Rejaei D., Shihab A., Alston T. A., Wang J. (2018). The role of multimodal analgesia in preventing the development of chronic postsurgical pain and reducing postoperative opioid use. J. Opioid Manag. 14 (6), 453–461. doi:10.5055/jom.2018.0478
Jiang J. J., Li C., Li H., Zhang L., Lin Z. H., Fu B. J., et al. (2016). Sevoflurane postconditioning affects post-ischaemic myocardial mitochondrial ATP-sensitive potassium channel function and apoptosis in ageing rats. Clin. Exp. Pharmacol. Physiology 43 (5), 552–561. doi:10.1111/1440-1681.12565
Kahol K., Vankipuram M., Patel V. L., Smith M. L. (2011). Deviations from protocol in a complex trauma environment: errors or innovations? J. Biomed. Inf. 44 (3), 425–431. doi:10.1016/j.jbi.2011.04.003
Kalogeris T., Baines C. P., Krenz M., Korthuis R. J. (2012). Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 298, 229–317. doi:10.1016/B978-0-12-394309-5.00006-7
Kato R., Foex P. (2002). Myocardial protection by anesthetic agents against ischemia-reperfusion injury: an update for anesthesiologists. Can. J. Anaesth. 49 (8), 777–791. doi:10.1007/BF03017409
Kikuchi C., Dosenovic S., Bienengraeber M. (2015). Anaesthetics as cardioprotectants: translatability and mechanism. Br. J. Pharmacol. 172 (8), 2051–2061. doi:10.1111/bph.12981
Ko S. H., Yu C. W., Lee S. K., Choe H., Chung M. J., Kwak Y. G., et al. (1997a). Propofol attenuates ischemia-reperfusion injury in the isolated rat heart. Anesth. Analg. 85 (4), 719–724. doi:10.1097/00000539-199710000-00002
Ko S. H., Lee S. K., Han Y. J., Choe H., Kwak Y. G., Chae S. W., et al. (1997b). Blockade of myocardial ATP-sensitive potassium channels by ketamine. Anesthesiology 87 (1), 68–74. doi:10.1097/00000542-199707000-00010
Kobayashi I., Kokita N., Namiki A. (2008). Propofol attenuates ischaemia-reperfusion injury in the rat heart in vivo. Eur. J. Anaesthesiol. 25 (2), 144–151. doi:10.1017/S0265021507001342
Kunst G., Martin E., Graf B. M., Hagl S., Vahl C. F. (1999). Actions of ketamine and its isomers on contractility and calcium transients in human myocardium. Anesthesiology 90 (5), 1363–1371. doi:10.1097/00000542-199905000-00021
Kvarda P., Puelacher C., Clauss M., Kuehl R., Gerhard H., Mueller C., et al. (2022). Perioperative myocardial injury and mortality after revision surgery for orthopaedic device-related infection. Bone Jt. J. 104-B (6), 696–702. doi:10.1302/0301-620X.104B6.BJJ-2021-1486.R1
Landoni G., Biondi-Zoccai G. G. L., Zangrillo A., Bignami E., D'Avolio S., Marchetti C., et al. (2007). Desflurane and sevoflurane in cardiac surgery:: a meta-analysis of randomized clinical trials. J. Cardiothorac. Vasc. Anesth. 21 (4), 502–511. doi:10.1053/j.jvca.2007.02.013
Landoni G., Turi S., Bignami E., Zangrillo A. (2009a). Organ protection by volatile anesthetics in non-coronary artery bypass grafting surgery. Future Cardiol. 5 (6), 589–603. doi:10.2217/fca.09.52
Landoni G., Fochi O., Bignami E., Calabro M. G., D'Arpa M. C., Moizo E., et al. (2009b). Cardiac protection by volatile anesthetics in non-cardiac surgery? A meta-analysis of randomized controlled studies on clinically relevant endpoints. HSR Proc. Intensive Care Cardiovasc Anesth. 1 (4), 34–43.
Landoni G., Greco T., Biondi-Zoccai G., Nigro Neto C., Febres D., Pintaudi M., et al. (2013). Anaesthetic drugs and survival: a Bayesian network meta-analysis of randomized trials in cardiac surgery. Br. J. Anaesth. 111 (6), 886–896. doi:10.1093/bja/aet231
Landoni G., Lomivorotov V. V., Neto C. N., Monaco F., Pasyuga V. V., Bradic N., et al. (2019). Volatile anesthetics versus total intravenous anesthesia for cardiac surgery. N. Engl. J. Med. 380 (13), 1214–1225. doi:10.1056/nejmoa1816476
Lang X. E., Wang X., Jin J. H. (2013). Mechanisms of cardioprotection by isoflurane against I/R injury. Front. Biosci. Landmark Ed. 18 (1), 387–393. doi:10.2741/4109
Li F., Yuan Y. (2015). Meta-analysis of the cardioprotective effect of sevoflurane versus propofol during cardiac surgery. Bmc Anesthesiol. 15, 128. doi:10.1186/s12871-015-0107-8
Li T., Wu W., You Z., Zhou R. H., Li Q., Zhu D., et al. (2012). Alternative use of isoflurane and propofol confers superior cardioprotection than using one of them alone in a dog model of cardiopulmonary bypass. Eur. J. Pharmacol. 677 (1-3), 138–146. doi:10.1016/j.ejphar.2011.12.030
Li T., Tan Y., Ouyang S., He J., Liu L. L. (2022). Resveratrol protects against myocardial ischemia-reperfusion injury via attenuating ferroptosis. Gene 808, 145968. doi:10.1016/j.gene.2021.145968
Li T., Li Y., Zeng Y., Zhou X., Zhang S., Ren Y. (2024). Construction of preclinical evidence for propofol in the treatment of reperfusion injury after acute myocardial infarction: a systematic review and meta-analysis. Biomed. Pharmacother. 174, 116629. doi:10.1016/j.biopha.2024.116629
Likhvantsev V. V., Landoni G., Levikov D. I., Grebenchikov O. A., Skripkin Y. V., Cherpakov R. A. (2016). Sevoflurane versus total intravenous anesthesia for isolated coronary artery bypass surgery with cardiopulmonary bypass: a randomized trial. J. Cardiothorac. Vasc. Anesth. 30 (5), 1221–1227. doi:10.1053/j.jvca.2016.02.030
Lin S., Neelankavil J., Wang Y. (2021). Cardioprotective effect of anesthetics: translating science to practice. J. Cardiothorac. Vasc. Anesth. 35 (3), 730–740. doi:10.1053/j.jvca.2020.09.113
Lisek M., Zylinska L., Boczek T. (2020). Ketamine and calcium Signaling-A crosstalk for neuronal physiology and pathology. Int. J. Mol. Sci. 21 (21), 8410. doi:10.3390/ijms21218410
Liu H. J., Liu B. (2018). Inhibition of MicroRNA-23 contributes to the isoflurane-mediated cardioprotection against oxidative stress. Cardiovasc Toxicol. 18 (5), 450–458. doi:10.1007/s12012-018-9455-1
Liu K. X., Xia Z. (2012). Potential synergy of antioxidant N-acetylcysteine and insulin in restoring sevoflurane postconditioning cardioprotection in diabetes. Anesthesiology 116 (2), 488–489. doi:10.1097/ALN.0b013e31823fd063
Liu H., Chen B., Guo B., Deng X., Wang B., Dou X. (2021). Postconditioning with sevoflurane or propofol alleviates lipopolysaccharide-induced neuroinflammation but exerts dissimilar effects on the NR2B subunit and cognition. Mol. Neurobiol. 58 (9), 4251–4267. doi:10.1007/s12035-021-02402-0
Liu M., Xu F., Lv J., Liu X., Wang E. (2025). Sevoflurane preconditioning protects against myocardial ischemia reperfusion injury in mice via PI3K/AKT/GSK3β-mediated upregulation of Syntaxin1a. J. Biochem. Mol. Toxicol. 39 (5), e70260. doi:10.1002/jbt.70260
Lotz C., Kehl F. (2015). Volatile anesthetic-induced cardiac protection: molecular mechanisms, clinical aspects, and interactions with nonvolatile agents. J. Cardiothorac. Vasc. Anesth. 29 (3), 749–760. doi:10.1053/j.jvca.2014.11.012
Lurati Buse G. A., Schumacher P., Seeberger E., Studer W., Schuman R. M., Fassl J., et al. (2012). Randomized comparison of sevoflurane versus propofol to reduce perioperative myocardial ischemia in patients undergoing noncardiac surgery. Circulation 126 (23), 2696–2704. doi:10.1161/CIRCULATIONAHA.112.126144
Marcos-Vidal J. M., González R., Garcia C., Soria C., Galiana M., De Prada B. (2014). Sedation with sevoflurane in postoperative cardiac surgery: influence on troponin T and creatinine values. Heart Lung Vessels 6 (1), 33–42.
Marik P. E. (2005). Propofol: an immunomodulating agent. Pharmacotherapy 25 (5 Pt 2), 28S-33S–33S. doi:10.1592/phco.2005.25.5_part_2.28s
Meybohm P., Bein B., Brosteanu O., Cremer J., Gruenewald M., Stoppe C., et al. (2015). A multicenter trial of remote ischemic preconditioning for heart surgery. N. Engl. J. Med. 373 (15), 1397–1407. doi:10.1056/NEJMoa1413579
Mio Y., Bienengraeber M. W., Marinovic J., Gutterman D. D., Rakic M., Bosniak Z. J., et al. (2008). Age-related attenuation of isoflurane preconditioning in human atrial cardiomyocytes - roles for mitochondrial respiration and sarcolemmal adenosine triphosphate-sensitive potassium channel activity. Anesthesiology 108 (4), 612–620. doi:10.1097/ALN.0b013e318167af2d
Modi A. D., Khan A. N., Cheng W. Y. E., Modi D. M. (2023). KCCs, NKCCs, and NCC: potential targets for cardiovascular therapeutics? A comprehensive review of cell and region specific expression and function. Acta histochem. 125 (4), 152045. doi:10.1016/j.acthis.2023.152045
Molojavyi A., Preckel B., Comfère T., Müllenheim J., Thämer V., Schlack W. (2001). Effects of ketamine and its isomers on ischemic preconditioning in the isolated rat heart. Anesthesiology 94 (4), 623–629. doi:10.1097/00000542-200104000-00016
Murry C. E., Jennings R. B., Reimer K. A. (1986). Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74 (5), 1124–1136. doi:10.1161/01.cir.74.5.1124
Natoli S. (2021). The multiple faces of ketamine in anaesthesia and analgesia. Drugs Context 10, 1–14. doi:10.7573/dic.2020-12-8
Orriach J. L. G., Belmonte J. J. E., Aliaga M. R., Fernandez A. R., Ponferrada A. R., Navarro M. R., et al. (2020). Anesthetic-induced myocardial conditioning: molecular fundamentals and scope. Curr. Med. Chem. 27 (13), 2147–2160. doi:10.2174/0929867325666180926161427
Ozarslan N. G., Ayhan B., Kanbak M., Celebioglu B., Demircin M., Ince C., et al. (2012). Comparison of the effects of sevoflurane, isoflurane, and desflurane on microcirculation in coronary artery bypass graft surgery. J. Cardiothorac. Vasc. Anesth. 26 (5), 791–798. doi:10.1053/j.jvca.2012.03.019
Pagel P. S. (2009). Cardioprotection by volatile anesthetics: established scientific principle or lingering clinical uncertainty? J. Cardiothorac. Vasc. Anesth. 23 (5), 589–593. doi:10.1053/j.jvca.2009.07.001
Pagel P. S., Crystal G. J. (2018). The discovery of myocardial preconditioning using volatile anesthetics: a history and contemporary clinical perspective. J. Cardiothorac. Vasc. Anesth. 32 (3), 1112–1134. doi:10.1053/j.jvca.2017.12.029
Pan Y. H., Wang X. K., Liu X. W., Shen L. H., Chen Q. X., Shu Q. (2022). Targeting ferroptosis as a promising therapeutic strategy for ischemia-reperfusion injury. Antioxidants 11 (11), 2196. doi:10.3390/antiox11112196
Park J., Lee S. H., Lee J. H., Min J. J., Kwon J. H., Oh A. R., et al. (2020). Volatile versus total intravenous anesthesia for 30-day mortality following non-cardiac surgery in patients with preoperative myocardial injury. PLoS One 15 (9), e0238661. doi:10.1371/journal.pone.0238661
Peña J. R., Wolska B. M. (2005). Differential effects of isoflurane and ketamine/inactin anesthesia on cAMP and cardiac function in FVB/N mice during basal state and β-adrenergic stimulation. Basic Res. Cardiol. 100 (2), 147–153. doi:10.1007/s00395-004-0503-6
Petersen C., Wetterslev J., Meyhoff C. S. (2018). Perioperative hyperoxia and post-operative cardiac complications in adults undergoing non-cardiac surgery: systematic review protocol. Acta Anaesthesiol. Scand. 62 (7), 1014–1019. doi:10.1111/aas.13123
Piriou V., Chiari P., Knezynski S., Bastien O., Loufoua J., Lehot J. J., et al. (2000). Prevention of isoflurane-induced preconditioning by 5-hydroxydecanoate and gadolinium -: possible involvement of mitochondrial adenosine triphosphate-sensitive potassium and stretch-activated channels. Anesthesiology 93 (3), 756–764. doi:10.1097/00000542-200009000-00025
Pratt P. F., Wang C., Weihrauch D., Bienengraeber M. W., Kersten J. R., Pagel P. S., et al. (2006). Cardioprotection by volatile anesthetics: new applications for old drugs? Curr. Opin. Anaesthesiol. 19 (4), 397–403. doi:10.1097/01.aco.0000236139.31099.b5
Priebe H. J. (2005). Perioperative myocardial infarction--aetiology and prevention. Br. J. Anaesth. 95 (1), 3–19. doi:10.1093/bja/aei063
Qin H., Zhou J. (2023). Myocardial protection by desflurane: from basic mechanisms to clinical applications. J. Cardiovasc. Pharmacol. 82 (3), 169–179. doi:10.1097/FJC.0000000000001448
Ranjbar A., Sharifzadeh M., Karimi J., Tavilani H., Baeeri M., Shayesteh T. H., et al. (2014). Propofol attenuates toxic oxidative stress by CCl4 in liver mitochondria and blood in rat. Iran. J. Pharm. Res. 13 (1), 253–262.
Riess M. L. (2009). The rocky road from bench to bedside: beta-blockers and anesthetic postconditioning. Anesthesiology 110 (3), 451–452. doi:10.1097/ALN.0b013e318198160b
Riess M. L., Stowe D. F., Warltier D. C. (2004). Cardiac pharmacological preconditioning with volatile anesthetics: from bench to bedside? Am. J. Physiol. Heart Circ. Physiol. 286 (5), H1603–H1607. doi:10.1152/ajpheart.00963.2003
Ruiz-Meana M., Boengler K., Garcia-Dorado D., Hausenloy D. J., Kaambre T., Kararigas G., et al. (2020). Ageing, sex, and cardioprotection. Br. J. Pharmacol. 177 (23), 5270–5286. doi:10.1111/bph.14951
Sandeep B., Huang X., Li Y., Xiong D., Zhu B., Xiao Z. (2022). A comparison of regional anesthesia techniques in patients undergoing video-assisted thoracic surgery: a network meta-analysis. Int. J. Surg. 105, 106840. doi:10.1016/j.ijsu.2022.106840
Sandroni C., Cronberg T., Sekhon M. (2021). Brain injury after cardiac arrest: pathophysiology, treatment, and prognosis. Intensive Care Med. 47 (12), 1393–1414. doi:10.1007/s00134-021-06548-2
Santos L., Dos Santos Petry F., Saibro-Girardi C., Hansen J., Martins D., Frohlich N., et al. (2025). Neuroprotective role of ketamine in reducing neuroinflammation and enhancing neuroplasticity against a cortisol-induced in vitro stress model. Mol. Neurobiol. doi:10.1007/s12035-025-05114-x
Schild S., Sedlmayr B., Schumacher A. K., Sedlmayr M., Prokosch H. U., St Pierre M., et al. (2019). German cognitive aid working G: A dagital cognitive aid for anesthesia to support intraoperative crisis management: results of the user-centered design Process. JMIR Mhealth Uhealth 7 (4), e13226. doi:10.2196/13226
Schumacher P. M., Dossche J., Mortier E. P., Luginbuehl M., Bouillon T. W., Struys MMRF (2009). Response surface modeling of the interaction between propofol and sevoflurane. Anesthesiology 111 (4), 790–804. doi:10.1097/ALN.0b013e3181b799ef
See K. C. (2023). Personalizing care for critically ill adults using omics: a concise review of potential clinical applications. Cells 12 (4), 541. doi:10.3390/cells12040541
Shao H., Li J., Zhou Y., Ge Z., Fan J., Shao Z., et al. (2008). Dose-dependent protective effect of propofol against mitochondrial dysfunction in ischaemic/reperfused rat heart: role of cardiolipin. Br. J. Pharmacol. 153 (8), 1641–1649. doi:10.1038/bjp.2008.45
Shao Y., Gu Q., Yuan Y., Wang L., Yu T. (2025). The preconditioning with sevoflurane alleviates hypoxia-reoxygenation-induced myocardial cell injury by regulating the lncRNA LINC00265/miR-370-3p axis. Cardiovasc Toxicol. 25 (5), 778–789. doi:10.1007/s12012-025-09984-4
Shirakawa M., Imura H., Nitta T. (2014). Propofol protects the immature rabbit heart against ischemia and reperfusion injury: impact on functional recovery and histopathological changes. Biomed. Res. Int. 2014, 601250. doi:10.1155/2014/601250
Sniecinski R., Liu H. (2004). Reduced efficacy of volatile anesthetic preconditioning with advanced age in isolated rat myocardium. Anesthesiology 100 (3), 589–597. doi:10.1097/00000542-200403000-00019
Song X. J., Hu J. J. (2024). Neurobiological basis of emergence from anesthesia. Trends Neurosci. 47 (5), 355–366. doi:10.1016/j.tins.2024.02.006
Sprung J., Schuetz S. M., Stewart R. W., Moravec C. S. (1998). Effects of ketamine on the contractility of failing and nonfailing human heart muscles in vitro. Anesthesiology 88 (5), 1202–1210. doi:10.1097/00000542-199805000-00010
Stoppe C., Meybohm P., Benstoem C., Goetzenich A. (2017). Remote ischemic preconditioning in cardiac anesthesia: a review focusing on translation. Minerva Anestesiol. 83 (6), 610–623. doi:10.23736/S0375-9393.17.11756-6
Stowe D. F., Kevin L. G. (2004). Cardiac preconditioning by volatile anesthetic agents: a defining role for altered mitochondrial bioenergetics. Antioxidants and Redox Signal. 6 (2), 439–448. doi:10.1089/152308604322899512
Suda Y., Uka T. (2022). The NMDA receptor antagonist ketamine impairs and delays context-dependent decision making in the parietal cortex. Commun. Biol. 5 (1), 690. doi:10.1038/s42003-022-03626-z
Suleiman M. S., Underwood M., Imura H., Caputo M. (2015). Cardioprotection during adult and pediatric open heart surgery. Biomed. Res. Int. 2015, 712721. doi:10.1155/2015/712721
Sun F., He Y. L., Yang Z. Q., Xu G. H., Wang R. G., Juan Z. D., et al. (2024). Propofol pretreatment inhibits ferroptosis and alleviates myocardial ischemia-reperfusion injury through the SLC16A13-AMPK-GPX4 pathway. Biomed. and Pharmacother. 179, 117345. doi:10.1016/j.biopha.2024.117345
Tamura T., Yoshikawa Y., Ogawa S., Ida M., Hirata N. (2025). New insights in cardiovascular anesthesia: a dual focus on clinical practice and research. J. Anesth. 39 (1), 117–122. doi:10.1007/s00540-024-03421-6
Tanaka K., Ludwig L. M., Kersten J. R., Pagel P. S., Warltier D. C. (2004). Mechanisms of cardioprotection by volatile anesthetics. Anesthesiology 100 (3), 707–721. doi:10.1097/00000542-200403000-00035
Torregroza C., Raupach A., Feige K., Weber N. C., Hollmann M. W., Huhn R. (2020). Perioperative cardioprotection: general mechanisms and pharmacological approaches. Anesth. Analgesia 131 (6), 1765–1780. doi:10.1213/ANE.0000000000005243
Trimmel H., Helbok R., Staudinger T., Jaksch W., Messerer B., Schochl H., et al. (2018). S(+)-ketamine: current trends in emergency and intensive care medicine. Wien Klin. Wochenschr 130 (9-10), 356–366. doi:10.1007/s00508-017-1299-3
Tyagi N., Mishra P. K., Tyagi S. C. (2009). Homocysteine, hydrogen sulfide (H2S) and NMDA-Receptor in heart failure. Indian J. Biochem. Biophys. 46 (6), 441–446.
Uhlig C., Bluth T., Schwarz K., Deckert S., Heinrich L., De Hert S., et al. (2016). Effects of volatile anesthetics on mortality and postoperative pulmonary and other complications in patients undergoing surgery: a systematic review and Meta-analysis (vol 124, pg 1230, 2016). Anesthesiology 125 (5), 1078. doi:10.1097/ALN.0000000000001120
Van Allen N. R., Krafft P. R., Leitzke A. S., Applegate R. L., Tang J., Zhang J. H. (2012). The role of volatile anesthetics in cardioprotection: a systematic review. Med. Gas. Res. 2 (1), 22. doi:10.1186/2045-9912-2-22
van den Brom C. E., Bulte C. S. E., Loer S. A., Bouwman R. A., Boer C. (2013). Diabetes, perioperative ischaemia and volatile anaesthetics: consequences of derangements in myocardial substrate metabolism. Cardiovasc. Diabetol. 12, 42. doi:10.1186/1475-2840-12-42
Vanlersberghe C., Camu F. (2008). Propofol. Handb. Exp. Pharmacol. 182, 227–252. doi:10.1007/978-3-540-74806-9_11
Wolf A., Selpien H., Haberl H., Unterberg M. (2021). Does a combined intravenous-volatile anesthesia offer advantages compared to an intravenous or volatile anesthesia alone: a systematic review and meta-analysis. Bmc Anesthesiol. 21 (1), 52. doi:10.1186/s12871-021-01273-1
Wong S. S., Irwin M. G. (2016). Peri-operative cardiac protection for non-cardiac surgery. Anaesthesia 71 (Suppl. 1), 29–39. doi:10.1111/anae.13305
Xia Z., Godin D. V., Ansley D. M. (2004). Application of high-dose propofol during ischemia improves postischemic function of rat hearts: effects on tissue antioxidant capacity. Can. J. Physiol. Pharmacol. 82 (10), 919–926. doi:10.1139/y04-097
Xiang Q., Yi X., Zhu X. H., Wei X., Jiang D. S. (2024). Regulated cell death in myocardial ischemia-reperfusion injury. Trends Endocrinol. Metab. 35 (3), 219–234. doi:10.1016/j.tem.2023.10.010
Yamanaka H., Hayashi Y. (2009). Myocardial preconditioning in anesthesia: from bench to bedside. Masui 58 (3), 279–287.
Yoo K. Y., Yang S. Y., Lee J., Im W. M., Jeong C. Y., Chung S. S., et al. (1999). Intracoronary propofol attenuates myocardial but not coronary endothelial dysfunction after brief ischaemia and reperfusion in dogs. Br. J. Anaesth. 82 (1), 90–96. doi:10.1093/bja/82.1.90
Yoo S. H., Cho S., Won Y., Lee J. W. (2024). Exploration of the interaction between remote ischemic preconditioning and anesthetic-induced preconditioning using sevoflurane in isolated perfused rabbit heart. Ewha Med. J. 47 (4), e68. doi:10.12771/emj.2024.e68
Yu H. P. (2011). Role of anesthetic agents on cardiac and immune systems. Shock 36 (6), 532–541. doi:10.1097/SHK.0b013e3182357054
Yu C. H., Beattie W. S. (2006). The effects of volatile anesthetics on cardiac ischemic complications and mortality in CABG: a meta-analysis. Can. J. Anaesthesia-Journal Can. D Anesth. 53 (9), 906–918. doi:10.1007/BF03022834
Yurkovich J. T., Evans S. J., Rappaport N., Boore J. L., Lovejoy J. C., Price N. D., et al. (2024). The transition from genomics to phenomics in personalized population health. Nat. Rev. Genet. 25 (4), 286–302. doi:10.1038/s41576-023-00674-x
Zangrillo A., Musu M., Greco T., Di Prima A. L., Matteazzi A., Testa V., et al. (2015). Additive effect on survival of anaesthetic cardiac protection and remote ischemic preconditioning in cardiac surgery: a bayesian network meta-analysis of randomized trials. Plos One 10 (7), e0134264. doi:10.1371/journal.pone.0134264
Zangrillo A., Lomivorotov V. V., Pasyuga V. V., Belletti A., Gazivoda G., Monaco F., et al. (2022). Effect of volatile anesthetics on myocardial infarction after coronary artery surgery: a post hoc analysis of a randomized trial. J. Cardiothorac. Vasc. Anesth. 36 (8), 2454–2462. doi:10.1053/j.jvca.2022.01.001
Zanos P., Moaddel R., Morris P. J., Riggs L. M., Highland J. N., Georgiou P., et al. (2018). Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol. Rev. 70 (3), 621–660. doi:10.1124/pr.117.015198
Zaugg M., Lucchinetti E., Behmanesh S., Clanachan A. S. (2014). Anesthetic cardioprotection in clinical practice from proof-of-concept to clinical applications. Curr. Pharm. Des. 20 (36), 5706–5726. doi:10.2174/1381612820666140204120829
Zhang Y., Zuo Y., Li B., Xie J., Ma Z., Thirupathi A., et al. (2019). Propofol prevents oxidative stress and apoptosis by regulating iron homeostasis and targeting JAK/STAT3 signaling in SH-SY5Y cells. Brain Res. Bull. 153, 191–201. doi:10.1016/j.brainresbull.2019.08.018
Zhang J., Ma L., Hashimoto Y., Wan X., Shan J., Qu Y., et al. (2021). (R)-Ketamine ameliorates lethal inflammatory responses and multi-organ injury in mice induced by cecum ligation and puncture. Life Sci. 284, 119882. doi:10.1016/j.lfs.2021.119882
Zhang X. B., Wang X. K., Liu X. F., Luo W. H., Zhao H. W., Yin Y. Q., et al. (2022). Myocardial protection of propofol on apoptosis induced by anthracycline by PI3K/AKT/Bcl-2 pathway in rats. Ann. Transl. Med. 10 (10), 555. doi:10.21037/atm-22-1549
Zhang C., He C., Chen Z., Chen X., Qin J., Xu Y., et al. (2023). The effects of volatile anesthetics and propofol in patients undergoing off-pump coronary artery bypass grafting: a systematic review and meta-analysis. Front. Cardiovasc Med. 10, 1271557. doi:10.3389/fcvm.2023.1271557
Zhang Q. W., Wang X., Wang Z. Y., Sun H. L. (2024). Low-dose esketamine improves acute postoperative pain in patients undergoing thoracoscopic surgery. Anesth. Periop Sci. 2 (1), 5. doi:10.1007/s44254-023-00039-x
Zhang T., Chung W., Orser B. A. (2025a). Revisiting anesthesia-induced preconditioning for neuroprotection in the aging brain: a narrative review. Korean J. Anesthesiol. 78 (3), 187–198. doi:10.4097/kja.25073
Zhang Y., Li X., Sun T. (2025b). Identification of mitochondria-related genes associated with anesthetics in patients undergoing off-pump coronary artery bypass grafting surgery. Front. Surg. 12, 1515732. doi:10.3389/fsurg.2025.1515732
Zhao D., Li Q., Huang Q., Li X., Yin M., Wang Z., et al. (2015). Cardioprotective effect of propofol against oxygen glucose deprivation and reperfusion injury in H9c2 cells. Oxid. Med. Cell Longev. 2015, 184938. doi:10.1155/2015/184938
Zhou Z. B., Meng L., Gelb A. W., Lee R., Huang W. Q. (2016). Cerebral ischemia during surgery: an overview. J. Biomed. Res. 30 (2), 83–87. doi:10.7555/JBR.30.20150126
Zhu A., Wei X., Zhang Y., You T., Yao S., Yuan S., et al. (2017). Propofol provides cardiac protection by suppressing the proteasome degradation of Caveolin-3 in ischemic/reperfused rat hearts. J. Cardiovasc Pharmacol. 69 (3), 170–177. doi:10.1097/FJC.0000000000000454
Zhu S., Lu P., Liu Z., Li S., Li P., Wei B., et al. (2023). Longitudinal hemoglobin trajectories and acute kidney injury in patients undergoing cardiac surgery: a retrospective cohort study. Front. Cardiovasc Med. 10, 1181617. doi:10.3389/fcvm.2023.1181617
Zhu S. Q., Zheng Z. Y., Wang L. N., Luo G., Zhang Y., Jia T., et al. (2024). Association between loop diuretics and mortality in patients with cardiac surgery-associated acute kidney injury: a retrospective propensity score-weighted analysis. Anesth. Analgesia 139 (1), 124–134. doi:10.1213/ANE.0000000000006748
Zhu S. Q., Bo J. H., Xia T. J., Gu X. P. (2025a). Temporal patterns of cognitive decline after hypertension onset among middle-aged and older adults in China. Sci. Rep-Uk 15 (1), 16300. doi:10.1038/s41598-025-98267-7
Zhu S., Wu M., Ding X., Zhang W., Xia T., Bo J., et al. (2025b). Effects of etomidate versus propofol for total intravenous anaesthesia on postoperative quality of recovery in patients undergoing day-case laparoscopic cholecystectomy: protocol for a multicentre, randomised controlled non-inferiority trial. BMJ Open 15 (9), e098584. doi:10.1136/bmjopen-2024-098584
Keywords: general anesthesia, propofol, ketamine, isoflurane, cardiology
Citation: Fu T, Jia X, Tang C, Yu D, Zhou H, Wang X, Liu S and Wu K (2025) Anesthetic-mediated cardioprotection: from molecular mechanisms to clinical translation challenges. Front. Physiol. 16:1688142. doi: 10.3389/fphys.2025.1688142
Received: 18 August 2025; Accepted: 08 September 2025;
Published: 17 September 2025.
Edited by:
Angela J. Grippo, Northern Illinois University, United StatesReviewed by:
Shouqiang Zhu, Nanjing University, ChinaCopyright © 2025 Fu, Jia, Tang, Yu, Zhou, Wang, Liu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Su Liu, bGl1c3UteHptdUAxNjMuY29t; Kunwei Wu, a3Vud2VpLnd1QHh6aG11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Tingting Fu1†
Tingting Fu1† Kunwei Wu
Kunwei Wu