- Department of Cell Biology and Physiology and The Kidney Institute, University of Kansas Medical Center, Kansas City, KS, United States
In the late 1950’s, Na,K-ATPase (NKA) was discovered as the active transport system that establishes and maintains the transmembrane Na+ and K+ gradients necessary for cell survival and function. Almost 70 years later, a novel unexpected function for NKA was unveiled, when it was shown that NKA has the amazing versatility of playing a role beyond its classical “ion pumping” function to also serve as the receptor and signal transducer for the effects of cardiotonic steroids (CTS) in cells. Since then, the field of NKA research expanded into a new dimension. The additional unexpected finding that CTS are commonly present in the body fluids of mammals inspired investigators to further study the CTS-induced and NKA-mediated pathway, its mechanisms of action, effects in cells, and importance to tissue and body physiology. Therefore, a vast amount of information has accumulated in recent years. In this article, we attempt to review the most current information available, focusing on the effects of CTS and NKA signaling in physiological and pathological states. We also discuss controversies, unsolved issues, and future directions of this fascinating area of research.
Introduction
In addition to its classical role in ion transport, a novel function for NKA was unveiled when it was found that it also serves as a plasma membrane receptor and a signal transducer for the effect of cardiotonic steroids (CTS) in different cells and tissues. In this review, we discuss various aspects of this fascinating role of NKA and its importance in physiologic and pathologic states. This field of research has grown very rapidly in the last several years as investigators tried to understand the mechanisms of action and effects of CTS in different cells and tissues. The vast literature makes it challenging to cover all articles available. Therefore, we would like to suggest the reader to also visit a series of excellent reviews on this topic (Xie, 2001; Harwood and Yaqoob, 2005; Pierre and Xie, 2006; Aperia, 2007; Tian and Xie, 2008; Bagrov et al., 2009; Riganti et al., 2011; Silva and Soares-da-Silva, 2012; Aperia et al., 2016; Askari, 2019; Orlov et al., 2021; Blaustein and Hamlyn, 2024; Gao et al., 2025).
Na,K-ATPase as an ion transporter
The Na,K-ATPase (NKA) was discovered in 1957 as the cell plasma membrane ion transport system that maintains the low Na+ and high K+ concentrations inside the cells compared to the extracellular medium (Skou, 1957). These transmembrane gradients are essential for the secondary transport of other ions, nutrients, and water in and out of the cell, maintenance of cell volume, and generation of the cell resting membrane potential, which allows for the development of action potentials in excitable tissues (Féraille and Doucet, 2001; Contreras et al., 2024).
NKA is composed of at least two major subunits, α and β and, in some tissues, these proteins are joined by a third subunit, which belong to a family of polypeptides that have the common consensus sequence Phe-X-Tyr-Asp or FXYD (Sweadner, 1989; Lingrel and Kuntzweiler, 1994; Kaplan, 2002; Jorgensen et al., 2003; Garty and Karlish, 2005; Geering, 2005; Clausen et al., 2017). NKA subunits assemble in a 1:1:1 stoichiometry, but these complexes can also oligomerize forming dimers or higher order molecular structures at the cell plasma membrane (Lopina, 2000; Taniguchi et al., 2001; Laughery et al., 2004; Martin, 2005; Lindzen et al., 2006; Barwe et al., 2012; Yoneda et al., 2016). The NKA α subunit, also called the catalytic subunit, is a multi-spanning membrane protein directly involved in the translocation of the ions across the cell membrane. It contains the binding sites for Na+ and K+ and harbors the site for ATP hydrolysis, which fuels ion active transport. In addition, the extracellular face of the α subunit holds the molecular pocket where cardiotonic steroids (CTS) dock (Andersen and Vilsen, 1995; Lingrel et al., 1998; Jorgensen et al., 2003; Dyla et al., 2020). The NKA β subunit is a single membrane pass glycosylated membrane protein necessary for stabilizing the folding of the NKA α subunit during synthesis and for the intracellular trafficking and delivery of the whole enzyme to the cell plasma membrane (Ackermann and Geering, 1990; Geering, 1990; Geering, 1991; Chow and Forte, 1995; Ueno et al., 1997; Rajasekaran et al., 2004; Vagin et al., 2007). In epithelia and the nervous system, the NKA β subunit has been shown to also function as an adhesion molecule that participates in cell-cell interaction (Müller-Husmann et al., 1993; Tokhtaeva et al., 2011; Cereijido et al., 2012; Vagin et al., 2012). The FXYD subunit is a small type I membrane polypeptide that comprises several members, the first of which was discovered in the kidney and called the γ subunit (Therien and Blostein, 2000). While not essential for NKA enzymatic activity, the FXYD polypeptide is a regulator of NKA activity (Geering et al., 2003; Sweadner et al., 2003; Yap et al., 2021).
In mammals, NKA is expressed as a series of isozymes, which result from the association of different molecular variants or isoforms of each of its subunits (Blanco and Mercer, 1998; Mobasheri et al., 2000). The NKA α subunit exists as four isoforms (NKA α1-4) which have a tissue-specific distribution and different functional properties. NKA α1 is expressed in all tissues and is the isoform that maintains the basal Na+ and K+ transmembrane gradients in the cell (Lingrel, 1992; Blanco, 2005; Matchkov and Krivoi, 2016). NKA α2 is expressed primarily in cardiac, smooth, and skeletal muscles where it is essential for the contractility of the myofibers (He et al., 2001; Dostanic et al., 2003; Shelly et al., 2004; Swift et al., 2007; Aronsen et al., 2013; McKenna et al., 2024). NKA α2 is also expressed in other tissues; in the brain NKA α2 is present in glia and contributes to K+ homeostasis and neurotransmitter release in the synaptic cleft (Moseley et al., 2003; Kawakami and Ikeda, 2006; Larsen et al., 2016). In the lung, NKA α2 is important for alveolar fluid clearance (Ridge et al., 2003). In adipose tissue (Coppi and Guidotti, 1997), NKA α2 contributes to insulin regulated K+ uptake. The NKA α3 isoform is primarily expressed in neurons, where it re-establishes cell membrane potential after depolarization (Dobretsov and Stimers, 2005; Holm et al., 2016). NKA α3 was also identified in the adult heart, contributing there to the electrical activity of the heart (Sweadner, 1989; Kaplan, 2002; Lingrel, 2010). Finally, NKA α4 is the NKA isoform with the most restricted pattern of expression, being limited to the male germ cells of the testis and sperm, where it is essential for male fertility (Woo et al., 1999; Blanco et al., 2000; Wagoner et al., 2005).
Regarding the NKA β isoforms, three different polypeptides were found (NKA β1-3) which also have a tissue-specific distribution. NKA β1 is ubiquitously expressed, NKA β2 is present in muscle and glia, and NKA β3 is found in the retina, liver, lung, and testes (Blanco and Mercer, 1998). All three β isoforms associate with the various α polypeptides in different combinations when expressed in vitro, though these interactions are more limited in vivo since they depend on the particular subset of isoforms expressed in each tissue (Eakle et al., 1994; Blanco et al., 1995a; Blanco et al., 1995b).
With respect to the FXYD polypeptides, seven different variants have been described in humans, which like the NKA α and β subunits are distributed in a tissue-specific manner. While FXYD polypeptides have been shown to modulate the ion affinity and maximal activity of NKA, their functions in vivo are not yet well characterized (Yap et al., 2021).
As an ion transporter, NKA continuously cycles between two different main conformations: the E1 state (where in the presence of Na+ and ATP the NKA α subunit becomes phosphorylated) and the E2 state (where in the presence of K+ and ATP the NKA α subunit dephosphorylates) (Glynn, 1956; Apell et al., 1998; Kaplan, 2002; Martin, 2005). In each pumping cycle, NKA moves 3 Na+ out and 2 K+ into the cell, while one molecule of ATP is hydrolyzed (Glynn, 1956; Post and Jolly, 1957). Under the physiological environment of the cell, the Na+ and ATP levels are critical for the regulation of NKA activity (Bertorello and Katz, 1993; Yu, 2003). Several hormones also regulate NKA activity by mechanisms that include: 1) changes in the activity of preexisting NKA molecules at the cell membrane; 2) modification of the expression of NKA molecules through changes in gene transcription and translation (Horowitz et al., 1990; Orlowski and Lingrel, 1990; Féraille and Doucet, 2001); or 3) post-translational phosphorylation of the α subunit, which regulates NKA insertion and retrieval from the cell plasma membrane (McDonough and Farley, 1993; Ewart and Klip, 1995; Gao et al., 1999; Therien and Blostein, 2000; Figtree et al., 2009; Poulsen et al., 2010; Cordeiro et al., 2024).
Na,K-ATPase as a receptor and signal transducer
The initial observation that NKA had effects that go beyond its function of maintaining the transmembrane gradient for Na+ and K+ in the cell was suggested by experiments performed in the early 1970s, which showed that chronic treatment of cultured cells with ouabain upregulates the expression of the genes that encode for the NKA α and β subunits (Pressley, 1988). This intriguing finding suggested that NKA could somehow communicate with the cell nucleus to regulate gene transcription and protein synthesis through induction of early response proto-oncogenes and activation of transcription factors. Further work expanded the list of genes that were regulated by ouabain and showed that this compound also enhanced cell growth. It was also realized that those effects were separate from changes in intracellular ions due to NKA inhibition (Xie and Askari, 2002).
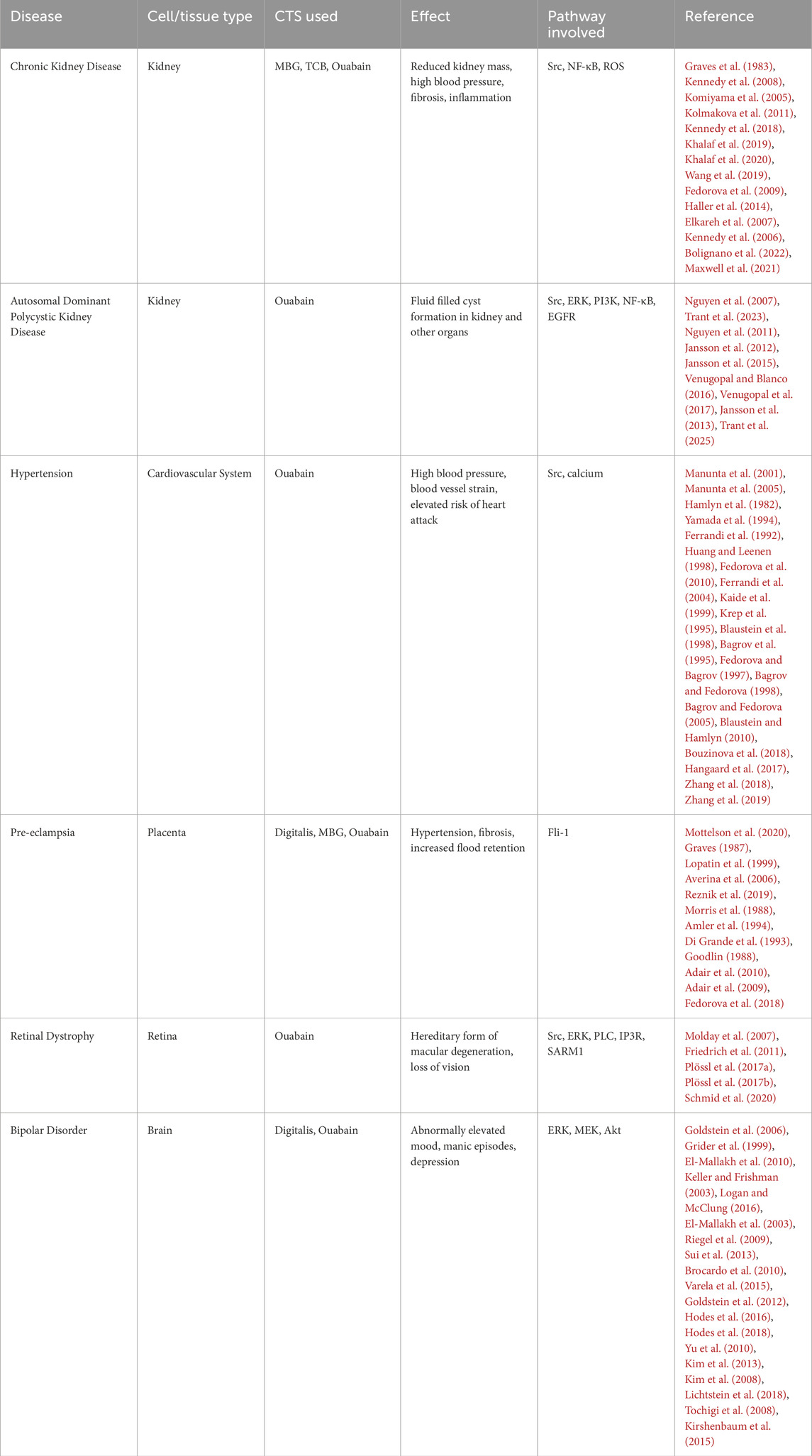
The modulatory effects of ouabain on gene expression went dormant for some time until the early 1990s, when studies spearheaded by Askari and Xie showed that ouabain treatment of neonatal myocardial cells in culture activated downstream pathways in the cells and activated gene transcription in a fashion that resembled the effect of growth factors and hormones (Peng et al., 1996; Huang et al., 1997; Xie et al., 1999; Haas et al., 2000; Aizman et al., 2001; Xie and Askari, 2002; Miyakawa-Naito et al., 2003; Khundmiri et al., 2006). CTS modulate gene expression in tissues different than the heart; for example, using differential display analysis to compare mRNA levels, it was shown that ouabain, bufalin and norbufalin downregulate expression of the signalin protein 14-3-3 in rat lens (McGowan et al., 1999). These results represented the starting point of what we know today as the “non-pumping” or “signaling” function of NKA. In this way, the concept evolved that ouabain is on the one hand an inhibitor of NKA transport activity, which modifies ion concentrations inside the cell; and on the other, an activator of NKA mediated signal transduction, which modulates gene and protein expression. Both events, shown in Figure 1, play a critical role in overall cell function. The fascinating discovery of a downstream signaling pathway activated by CTS gave a new dimension to the role of NKA in cells and opened new unexpected avenues of research for this ion transporter.
Figure 1. Scheme showing the control of cell function by CTS via the NKA ion transport and signaling functions. The right side of the diagram shows the Inhibition of NKA pumping mode by CTS, which directly raises intracellular Na+ levels. This in turn secondarily augments cytosolic Ca2+ via the Na/Ca exchanger (NCX) to elicit the “cardiotonic effect”. The left side of the figure shows the activation of NKA signaling by CTS, which stimulates a series of downstream intracellular intermediates that will ultimately modulate gene expression. (CTS) cardiotonic steroid, (α and β) subunits of the Na-K-ATPase. See the text for additional definitions.
Cardiotonic steroids; the ligands that trigger NKA signaling
CTS include two main types of structures: cardenolides, of which ouabain, digoxin, and digitalis are the most widely studied members (Schoner and Scheiner-Bobis, 2007b; Botelho et al., 2019; Patocka et al., 2020), and bufadienolides, which have bufalin and marinobufagenin as the main representative compounds (Bagrov et al., 2009; Agrawal et al., 2012; Asrorov et al., 2023). Structurally, these chemical scaffolds consist of 1) a steroidal backbone, with A/B and C/D rings in a cis conformation; 2) a lactone ring attached to C17, which in cardenolides is a five-member unsaturated butyrolactone and in bufadienolides a six-member pyrone ring; and 3) a sugar moiety at C3, which is highly variable depending on the CTS considered (Botelho et al., 2019). A characteristic of CTS is their vast natural chemical diversity, with important differences in their steroid skeleton, lactone moiety and sugar group. The best-known naturally occurring CTS include the cardenolides ouabain, digoxin, digitoxin, and oleandrin; and the bufadienolides bufalin, hellebrin, and marinobufagenin.
Ouabain was one of the first CTS found to have a practical application. Initially, it was used in low amounts as herbal remedies and in high concentrations as a poison (Goldman, 2001; Wachtel-Galor and Benzie, 2011). In the laboratory setting due to its hydrophilic property and high specificity, ouabain has been classically employed to distinguish NKA ion transport and activity from the function of other ATPases. Ouabain-NKA interaction requires the presence of Na+ and ATP and is antagonized by K+ (Schultheis et al., 1993). Once bound, ouabain interferes with NKA reaction cycle, locking the enzyme in the E2 conformation (Hansen, 1982).
When used at relatively high concentrations, ouabain and other CTS target and inhibit NKA activity. Digitalis and digoxin have found important application in modern medicine, inducing a beneficial increase in cardiac inotropy, chronotropy, and hypertrophy, which have made them useful agents for the treatment of congestive heart failure and atrial fibrillation (Ehle et al., 2011; Sethi et al., 2018; Whayne, 2018; Meijler, 1985; Bessen, 1986). These CTS, however, have a different therapeutic profile than ouabain (Fuerstenwerth, 2014). Despite their widespread clinical usage, the mechanisms of action of CTS remained elusive for decades. It is now known that the inhibition of NKA activity by CTS causes a primary increase in intracellular Na+ ([Na+]i) which, through the Na/Ca exchanger, secondarily raises Ca2+ concentrations in the cardiac cells (Blaustein et al., 2016). This increase in cytosolic Ca2+ favors heart contractility, the main effect for which CTS have received their distinct name. In addition to increasing heart contractility through partial inhibition of NKA ion transport, CTS can also exert this effect via NKA signaling (Buzaglo et al., 2016; Buzaglo et al., 2019). Both mechanisms of action of CTS effects mediated by NKA ion transport and signaling are shown in Figure 1. The cardiotonic effect is thought to be mediated by the NKA α2 isoform, highly expressed in the cardiac T-tubules, whereby it functionally interacts with the Na/Ca exchanger. While excellent as positive inotropic agents, the clinical use of CTS declined over the years due to their narrow therapeutic window and adverse effects (Patocka et al., 2020).
Originally, it was thought that CTS were only the products of plants and amphibians and that they only appeared in mammals from exogenous sources in the diet. In the early 1980s, some data was obtained that suggested that an agent which inhibited NKA was present in the serum of experimental rat models (de Wardener and Clarkson, 1985). It took almost another decade to identify the circulating compound as ouabain or an isomer of it Hamlyn et al. (1991). However, several other endogenous steroids, collectively termed digitalis-like compounds (DLC), were also identified in human tissues. There is ample evidence now that CTS circulate in the bloodstream of mammals including ouabain, which appears to be endogenously produced in tissues, such as the adrenal glands and hypothalamus and that its secretion is regulated by different nervous system and humoral factors (Blaustein, 1996; Ferrandi et al., 1997; Manunta et al., 2001; Schoner, 2002; Manunta et al., 2005; Schoner and Scheiner-Bobis, 2007a; Hamlyn and Manunta, 2011). The discovery that ouabain itself or ouabain-like substances are present in plasma of humans and could function as hormones, along with the results indicating the signaling role of NKA, sparked curiosity for the physiologic implications of this new pathway in cell regulation and its potential pharmacologic relevance. A general discussion of the actions of CTS in different tissues follows.
NKA signaling in normal physiology
Currently, a breath of information has been gathered showing that CTS have pleiotropic effects, triggering a variety of responses in different cells and tissues. Earlier studies showed that low doses of ouabain led to the growth of myocardial cells and that this could contribute to the beneficial effect of cell hypertrophy in cardiac insufficiency (Kometiani et al., 1998; Liu et al., 2000). More recently, it was shown that ouabain also delayed and even prevented the cardiac remodeling that follows pressure overload in mouse hearts. These effects are particularly important to understand the consequences of cardiac injury and the mechanisms involved in the development of heart failure (Belliard et al., 2016; Lopatina et al., 2016; Buzaglo et al., 2019). Moreover, ouabain administered prior to sustained ischemia confers protection against cardiac ischemia-reperfusion injury (Belliard et al., 2016).
Ouabain also has effects beyond the heart, causing cell proliferation of vascular smooth muscle cells (Aydemir-Koksoy et al., 2001; Abramowitz et al., 2003; Allen et al., 2003; Kotova et al., 2006b), human endothelial cells (Tverskoi et al., 2016), rat and opossum kidney tubular epithelial cells (Dmitrieva and Doris, 2003; Khundmiri et al., 2006), and fibroblasts (Winnicka et al., 2010). Endogenous ouabain also regulates viability of a series of cell lines in culture (Dvela et al., 2012). In addition, ouabain protects renal cells from undergoing apoptosis (Dmitrieva and Doris, 2003; Golden and Martin, 2006; Khundmiri et al., 2006; Li et al., 2006; Aperia, 2012), and promotes epithelial cell migration and ciliogenesis (Larre et al., 2011; Verdejo-Torres et al., 2019). Importantly, ouabain-induced NKA signaling regulates cell-cell adhesion and influences the polarized phenotype of renal epithelial cells in culture. In MDCK cells, nanomolar concentrations of ouabain enhance tight-, adherens-, and gap-junctional proteins, promoting the estable structure of the epithelium. In contrast, in the same cells, micromolar concentrations of ouabain induces cell detachment (Larre et al., 2010; Castillo et al., 2019; Contreras et al., 2024). In LLC-PK1 renal cells, NKA signaling regulates the metabolic capacity of the cells (Kutz et al., 2021). In the whole kidney, NKA signaling activated by ouabain controls vascular tone and sodium homeostasis (Cai H. et al., 2008; Nesher et al., 2009; Blaustein and Hamlyn, 2010). Ouabain also has a protective role against the harmful effects of serum deprivation and Shiga toxin infection in rat kidney tubular cells (Li et al., 2006; Li et al., 2010).
In smooth muscle cells of vessels, ouabain induces overall contraction and potentiates vascular tone and the contractile response to agonists (Zhang et al., 2018). In those cells, ouabain also regulates intercellular communication by modulating gap junctions and connexin levels (Hangaard et al., 2017). In vascular endothelial cells, ouabain promotes endothelin-1 and nitric oxide production, which further supports the notion that ouabain is a regulator of vascular tone (Scheiner-Bobis et al., 2006).
In the gastrointestinal tract, NKA plays a primary role in nutrient and water absorption by creating the Na+ gradient that is used by secondary transport systems (Field, 2003; Nepal et al., 2021). Also, by secondarily maintaining intracellular Ca2+ concentrations, NKA affects intestinal contractility (Daniel et al., 2009). Circulating ouabain regulates intestinal tight junctions by modulating claudin expression, likely via Rho-associated coiled-coil forming kinase (ROCK) signaling (Markov et al., 2020; Cho et al., 2025). Ouabain also reduces blood flow and oxygen consumption in the intestine by acting on blood vessels (Pawlik et al., 1974). Moreover, chronic exposure to ouabain appears to have a protective effect on the epithelial gut barrier (Markov et al., 2023).
Within the nervous system, ouabain increases the growth and survivability of retinal cells, neurons, and astrocytes and stimulates neuronal branching in hippocampal cells (Murata et al., 1996; Dvela et al., 2012; Li and Zhou, 2015; Lopachev et al., 2016; Salles von-Held-Ventura et al., 2016; Orellana et al., 2018).
In the lung, ouabain is involved in airway remodeling and facilitates injury by inducing proinflammatory cytokine production (Silva et al., 2023).
NKA signaling also seems to play a role in the regulation of the immune system, as CTS can affect the function and proliferation of lymphocytes and monocytes (Hammarstrom and Smith, 1979; Brodie et al., 1995; Olej et al., 1998; Esteves et al., 2005; Rodrigues-Mascarenhas et al., 2009; Kinoshita et al., 2014; Nascimento et al., 2014; Teixeira and Rumjanek, 2014). Ouabain also stimulates the secretion of interleukin in the brain (Pirkmajer et al., 2020).
Within skeletal muscle, ouabain works synergistically with insulin to enhance glycogen synthesis (Kotova et al., 2006a) and prevents injury-associated cell depolarization (Kravtsova et al., 2020; Kravtsova et al., 2021).
Ouabain participates in promoting the differentiation of different cell types, including bone marrow cells, murine stem cells (Lee et al., 2011; Sayed et al., 2014). In addition, ouabain has different effects in male and female reproductive tissues, placenta and the embryo (Thundathil et al., 2006; Uddin et al., 2008; Giannatselis et al., 2011; Lucas et al., 2012; Dvela-Levitt et al., 2015; Upmanyu et al., 2016; Upmanyu et al., 2019).
Besides ouabain, the CTS marinobufagenin has been shown to activate collagen-1 production in heart fibroblasts, stimulate epithelial to mesenchymal transition of renal epithelial cells, and promote the growth of liver tissue explants (Fedorova et al., 2009; Penniyaynen et al., 2015).
These examples show the wide variety of actions that ouabain and other CTS exert. Since most of these effects are related to stimulation of cell growth, one could consider endogenous CTS as growth hormones. However, CTS effects go beyond its cell hyperplastic and hypertrophic effects and influence other important aspects of cell physiology, including cell metabolism, migration, differentiation, apoptosis, and tissue fibrosis. A brief summary of some of the CTS actions is presented in Table 1.
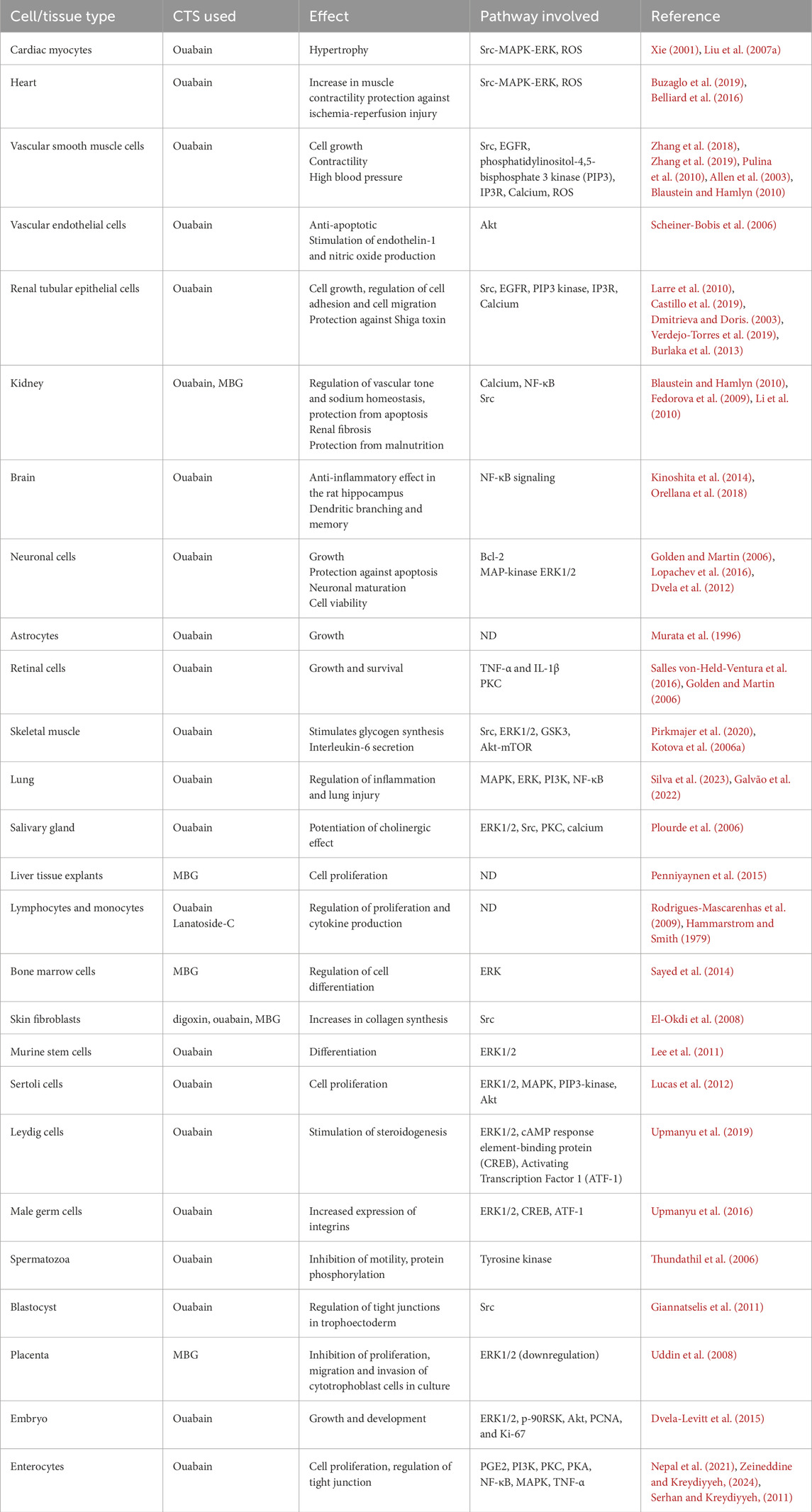
Table 1. NKA signaling effects and intracellular pathways activated by CTS in different normal cells and organs.
It is important to note that the effects of CTS are seen at concentrations that are within those at which these compounds circulate in the bloodstream of mammals (Hamlyn, 2014; Blaustein and Hamlyn, 2024). This highlights the physiologic relevance of NKA signaling for cells and tissues. The dose-dependent effects of CTS, with high relatively concentrations being deleterious and relatively low amounts inducing signaling show the functional duality of NKA, as an ion transporter essential for maintaining life and as a signaling molecule that regulates cell physiology (Blaustein, 2018). This versatility of NKA function, also shows the complex mechanisms by which NKA controls cell physiology. Below, we discuss some of the cellular pathways which have been identified as downstream effectors of CTS binding to NKA.
Downstream effectors of the NKA signaling pathway
Src/EGFR/Ras/Raf/MEK/ERK pathway
The first intracellular messengers noted to be involved in the hypertrophic effects elicited by ouabain in myocardiocytes corresponded to a common signal transduction cascade that included Ras and the mitogen-activated kinase/extracellularly regulated kinase 1/2 (MAPK/ERK1/2) (Kometiani et al., 1998). Further work expanded this pathway to include the upstream activation of the protooncogene Src kinase and the phosphorylation of the epidermal growth factor receptor (EGFR) (Haas et al., 2000; Pratt et al., 2018). Of particular interest was the finding that ouabain stimulated these pathways not just in rat cardiomyocytes but also in A7r5, L929, and importantly, HeLa cell lines at doses consistent with the relatively higher affinity for ouabain exhibited by human NKA (Askari, 2019). This suggested that NKA signaling was not only a phenomenon of rodent cells but was shared by human cell models. Xie and Askari additionally showed that the ouabain-induced effects occurred independently of changes in Na+ and Ca2+ concentrations and were mimicked when cells were incubated in medium without K+, which highly reduces the transport function of NKA (Liu et al., 2000), suggesting that these changes were due to pathways outside of ion transport inhibition. At the time, this newly identified intracellular signaling pathway, named the NKA signalosome, represented a breakthrough for our understanding for the mechanisms of action of CTS.
In studying the NKA/Src signaling cascade, important focus was placed on the mechanisms by which NKA activates Src kinase. It was reported that NKA and Src establish a direct interaction through two molecular domains. These include the Src SH2 domain, which binds to the CD2 region of NKA α1 and the Src kinase domain, which binds to the NKA α1 CD3 motif (Tian et al., 2006; Li et al., 2009; Ye et al., 2011; Yu et al., 2018). In this model, while NKA is in the E1 conformational state, Src remains bound to NKA through both of its interacting sites, which keeps it in an inactive status. Upon CTS binding, the stabilization of NKA in the E2 conformation frees the kinase domain of Src, which allows Src autophosphorylation and activation (Banerjee et al., 2015; Yu et al., 2018). The activated Src then phosphorylates EGFR and stimulates downstream effectors that lead to regulation of gene transcription. While there is evidence that NKA and Src colocalize and interact with one another (Liu et al., 2003; Wang et al., 2004; Kotova et al., 2006b; Liang et al., 2006; Tian et al., 2006; Nie et al., 2020), this interaction has not been found in all experimental models (Weigand et al., 2012; Clifford and Kaplan, 2013; Yosef et al., 2016). In this latter context, NKA and Src may be indirectly linked and it has been proposed that this could depend on other intermediary proteins or though changes in the ATP/ADP ratio putatively connected to NKA ion pumping in the cell (Weigand et al., 2012; Gable et al., 2014). Based on the hypothesis of a direct association between NKA and Src, a small peptide, pNaKtide, was developed (Riegel et al., 2009). This peptide contains the 20 amino acid sequence of the CD3 N-terminal domain of NKA α1 that was described to bind to Src and has been shown to block the ouabain-induced activation of Src, ERK, and hypertrophy in cardiac myocytes, but not the activation of the kinase by other ligands, such as insulin-like growth factor 1 (IGF-1). Since its initial synthesis, pNaKtide has been used in a number of studies where it has been reported to dampen the activation of the NKA/Src signaling complex in different cell and animal models (Liu et al., 2016; Hangaard et al., 2017; Drummond et al., 2018; Li et al., 2018; Cheng et al., 2019; Khalaf et al., 2019; Sodhi et al., 2023; Zong et al., 2023). Further investigation is needed to ascertain whether NKA-Src interaction can be explained by a direct or indirect model.
Caveolae in NKA signaling
Following the discovery of the involvement of Src, EGFR, and ERK in ouabain-induced and NKA-mediated signaling, a specific caveolin-binding motif in the sequence of NKA α1 was identified. This suggested that NKA could be interacting with its signaling partners within the caveolar microdomains of the plasma membrane (Xie and Askari, 2002). Caveolae, flask-shaped invaginations of the cell membrane rich in cholesterol, are centers that harbor a series of ligand receptors and related signaling molecules (Cohen et al., 2004; Patel et al., 2008; Lamaze et al., 2017; Parton, 2018). Therefore, it was theorized that NKA may be sharing the microenvironment of caveolae with other receptors as clusters for cell signaling (Fielding, 2001; Parton et al., 2018). This was supported by the use of cholesterol-depleting agents, which showed that caveolar integrity was necessary for the downstream actions of ouabain in cardiac myocytes, including NKA/Src colocalization and activation of ERK (Liu et al., 2003; Wang et al., 2004). Additionally, it was shown that ouabain-induced endocytosis of NKA alongside Src and EGFR occurred via clathrin-coated vesicles in a manner requiring caveolae. This ouabain-dependent NKA endocytosis agrees with the common mechanism of receptor signaling inactivation (Liu, 2005; Liu et al., 2005). Moreover, knock out of caveolin-1, an essential structural component of caveolae, interfered with the ouabain-induced activation of Src, ERK, collagen synthesis, and proliferation, but did not affect Src and ERK basal activation (Quintas et al., 2010). Further, cardiac cells obtained from caveolin-1 knockout mice exhibited reduced ouabain-induced activation of PI3K/Akt, ERK, and cardiac hypertrophy, validating the idea of the need of caveolae for NKA signaling (Bai et al., 2016).
Based on the importance of caveolae in NKA signaling, it was proposed that two separate pools of NKA existed at the cell plasma membrane: one corresponding to the signaling NKA localized in caveolae and the other representing the “pumping” NKA, which performed the classical Na+/K+ exchange at the general plasma membrane (Liang et al., 2007). The relationship between NKA and caveolae was further supported by the reduction of caveolin-1 expression at the cell plasma membrane and its shift to intracellular stores when NKA expression was reduced (Cai T. et al., 2008). Additional evidence for a NKA-caveolae connection was suggested by the increase in NKA activity observed in models where cell caveolae were disrupted, which presumably indicated that the NKA free from caveolae is then dedicated to ion transport (Quintas et al., 2010). Conversely, graded reduction of NKA expression in pig kidney LLC-PK1 cells resulted in the loss of the capacity of the cells to respond to ouabain-induced signaling in favor of the more vital ion transport function of NKA (Liang et al., 2007). However, a clear difference between the signaling and pumping actions of NKA has not been supported by other reports, which have shown that NKA from both caveolar and non-caveolar preparations retain ion transport activity and reside with signaling partners (Liu et al., 2011); therefore, it remains unclear whether caveolae are strictly the place from which the NKA signaling apparatus operates or if the caveolar NKA is also involved in pumping ions.
Interestingly, isolated caveolar preparations showed that NKA α1 has a higher affinity for ouabain than preparations of general cell plasma membrane, where NKA α1 typically shows a lower capacity for ouabain binding. These findings led to the provoking idea that increased ouabain affinity of the caveolar NKA would facilitate the binding and downstream effects of ouabain (Dmitrieva and Doris, 2003; Ferrandi et al., 2004). However, another report failed to show caveolar-specific changes in the kinetics of NKA toward ouabain (Liu et al., 2011). Notably, the change in ouabain affinity was seen in caveolar fractions obtained from detergent-free methods, while the contrasting study used sodium-dodecyl-sulfate (SDS) to enrich for NKA following the classical protocol developed by Jørgensen (Jørgensen, 1986). It is possible that within the environment of cell caveolae, NKA has an increased capacity to bind CTS, but this may be disrupted by harsh methods of purification with detergents. In summary, the NKA signaling apparatus requires the association of several proteins including caveolin and the caveolae microdomains of the plasma membrane where other receptor kinases are also found (Lamaze et al., 2017).
Reactive oxygen species (ROS)
Reactive oxygen species (ROS) were identified as other intermediates of the CTS/NKA signaling pathway of cardiac cells. It was shown that the antioxidant n-acetylcysteine (NAC) inhibited the transcriptional and translational changes of growth related genes normally increased by ouabain in cardiac myocytes, including skeletal muscle actin (skACT) and atrial natriuretic factor (ANF) (Chen et al., 2017). Notably, in these cells NAC also partially ablated the increase in ERK phosphorylation induced by ouabain, suggesting some overlap for the signaling pathways downstream of CTS binding to NKA (Aizman et al., 2001). The link between NKA signaling and ROS production was also established in human pluripotent stem cells induced to develop as myocardiocytes and expressing a mutant form of the NKA α1 that cannot signal through Src. Those cells showed significant reduction in ROS but also had reduced basal and maximal rates of mitochondrial respiration, spare respiratory capacity, and ATP production, indicating the importance of ROS as part of NKA signaling and the role of this pathway in cell metabolism (Cai et al., 2023). In the whole heart, low doses of ouabain exert cardioprotective effects and ischemia pre- and post-conditioning (Fuerstenwerth, 2014; Duan et al., 2018; Marck and Pierre, 2018). It has recently been proposed that this is mediated by NKA signaling and opening of the mitochondrial ATP sensitive channel (mitoKATP), which causes the increase in ROS and secondary stimulation of the mitochondrial protein kinase C epsilon. This in turn inhibits the opening of the mitochondrial permeability transition pore (mPTP), protecting the tissue from cell death. Interestingly, it has been proposed that the connection between NKA signaling and mitochondria occurs via vesicular signaling complexes; however, this is currently a hypothesis that remains to be confirmed (Garlid et al., 2009).
ROS generation via NKA signaling activated by ouabain was found to occur in various other cell types (rat cardiomyocytes, A7r5, and HeLa cells) and to be independent from changes in intracellular Na+ and Ca2+ concentrations, further supporting the notion that NKA signaling is independent from the ion transport function of NKA (Liu et al., 2000). ROS appears to function upstream of MEK and ERK as NAC partially ablated the increases in ERK phosphorylation (Aizman et al., 2001; Yan et al., 2013).
Additionally, it was shown that NAC pretreatment of LLC-PK1 cells ablated the normal ouabain-induced increase of phosphorylation of Src, suggesting that ROS may play an essential role in NKA signaling in other tissues, such as the kidney (Yan et al., 2013). Interestingly, ROS seem to activate NKA signaling in the absence of CTS or at least act on similar pathways (Liu et al., 2006; Liu et al., 2012; Wang et al., 2014). This activation of NKA signaling by ROS results in a positive feedback mechanism, known as the ROS amplification loop, which leads to oxidative stress. This interplay between NKA signaling and ROS has been linked to worsening of chronic conditions, such as obesity, diabetes, dyslipidemia, and atherosclerosis (Srikanthan et al., 2016). This effect of ROS may be associated with redox modifications of NKA and Src. It has been shown that Src kinase can be oxidized and also glutathionylated depending on redox state and this affects Src activity (Yang et al., 2020). NKA also undergoes redox-dependent modifications through glutathionylation of its α and β subunits (Figtree et al., 2009; Petrushanko et al., 2012). Interestingly, according to modeling data, the glutathionylation of the NKA α subunit can affect the interaction of Src kinase and Na,K-ATPase (Petrushanko et al., 2017).
Calcium oscillations and protein kinase C (PKC)
An important additional intermediate of NKA signaling was identified by the group of Aperia in kidney epithelial cells. These researchers found that partially inhibitory concentrations of ouabain in rat kidney tubular cells induced regular, low-frequency calcium oscillations that caused the activation and nuclear localization of the transcription factor NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) (Aizman et al., 2001). Their study also supported the involvement of phospholipase C as part of the (PLC)/inositol triphosphate receptor (IP3R) pathway. PLC had also been implicated in the effects of ouabain in rat cardiac myocytes (Kometiani et al., 1998). Studies using preparations from rat kidney confirmed that PLC-1 and the IP3R isoforms 2 and 3 were coenriched with NKA, caveolin-1 and Src in light density fractions of the cell membranes. It was also found that the major intracellular domain of NKA interacts with PLC-γ1, while the N-terminus binds to IP3R, which suggested that NKA may tether PLC-γ1 and IP3 receptors to form a calcium regulatory complex (Zhang et al., 2006). NKA and IP3R interaction is stabilized by ankyrin (Liu et al., 2008; Aperia et al., 2020) and this is enhanced by the presence of ouabain (Miyakawa-Naito et al., 2003). Importantly, ouabain stimulated the phosphorylation of PLC-γ1 and IP3 in a Src dependent manner and the release of Ca2+ from intracellular stores in LLC-PK1 cells (Yuan et al., 2005). The direct association between NKA and IP3R also suggested that NKA served to bring together the ER and cell plasma membrane to facilitate calcium signaling to discrete regions of the cell (Chen et al., 2008).
The involvement of calcium as a messenger in the NKA signaling pathway was also found in neurons. Thus, ouabain enhances dendritic growth of rat cortical brain cells via calcium oscillations, MAP kinase, Ca-calmodulin dependent protein kinase, and activation of gene transcription regulated by cAMP response element binding protein (CREB) (Desfrere et al., 2009). Ouabain also generates calcium oscillations in glial cells, activating the IP3R and NF-κB in hippocampal astrocytes in culture (Liu X. L. et al., 2007). In conclusion, the experimental evidence available indicates that ouabain-induced and NKA-mediated signaling activates IP3R in two ways: one through stimulation of PLC, and the other by a direct interaction of NKA with IP3R. It is important to note that that the effects of CTS involve NKA control of intracellular calcium levels through both inhibition of NKA ion transport activity and signaling. These mechanisms cooperate in fine tuning intracellular calcium by decreasing its export out of the cell and favoring its movement from intracellular stores (Tian and Xie, 2008). Another point to consider is that calcium signaling results from a complex interplay between activation and inactivation of intracellular and cell surface calcium permeable channels, each subjected to specific regulation (Dupont et al., 2011; Parkash and Asotra, 2012; Smedler and Uhlen, 2014). Therefore, CTS represent additional regulators, which along with others control the development and amplitude of calcium spikes, as well as the frequency of calcium oscillations. It is interesting to propose that the low frequency calcium changes induced by ouabain represent a signature of the action of CTS that leads to cell effects that are different from those caused by other stimuli.
PI3K/Akt/mTOR pathway
The effects of ouabain on cell hypertrophy and proliferation suggested that protein kinase B (Akt), a mediator known to influence cell growth, could be involved in NKA signal transduction. Working with opossum kidney proximal tubular cells, it was found that nanomolar concentrations of ouabain stimulated Akt in a manner that required phosphatidylinositol 3 kinase (PI3K), PLC, and Ca2+ (Khundmiri et al., 2006). These authors also showed that Akt inhibition suppressed the ouabain-induced increase in cell proliferation. Shortly after, experiments performed in neonatal and adult rat cardiac myocytes, as well as in adult rat Langendorff-perfused hearts, showed that ouabain stimulated the phosphorylation of Akt and its substrates, mammalian target of rapamycin (mTOR) and glycogen synthase kinase (GSK) in a manner that required PI3K 1A (Liu L. et al., 2007). It was also found that this pathway required Src but not EGFR or MEK, suggesting that the activation of Src by ouabain stimulates PI3K and Akt signaling independently from EGFR and ERK. However, a later study using SYF cells, a line deficient for the kinases Src, Yes, and Fyn, and the same cells where Src has been reintroduced, showed that ouabain-induced activation of Akt was independent from the presence of Src (Wu et al., 2013). This study also provided evidence for a direct interaction between NKA and PI3K 1A. This indicated that NKA signaling uses downstream pathways that are separate from the activation of Src.
Regulation of NKA signaling
In the short term, ouabain and other CTS at circulating concentrations do not cause a measurable change in NKA pumping activity; however, it has been noticed that chronic treatment with CTS decrease NKA pumping activity and, in the kidney, it reduces Na+ absorption; interestingly, this occurs without significant changes in intracellular Na+ (Liu et al., 2002; Liu, 2005). The ouabain-induced decrease in NKA activity was shown to be due to endocytosis of NKA via a clathrin-coated pit and endosome-dependent process, which requires Src, PI3K, and caveolin-1 (Liu et al., 2004; Liu et al., 2005). Interestingly, not only NKA but also other components of the NKA signaling complex are internalized, as suggested by the finding of EGFR and Src, along with NKA, in endosomes (Liu, 2005). Once brought into the cell, the fate of the NKA signaling complex is its degradation in lysosomes. This process is of physiological relevance, since it serves to regulate NKA signaling to avoid overstimulation of the NKA signaling pathway (Cherniavsky-Lev et al., 2014). At present, the mechanisms that lead to NKA internalization are unclear. It has been suggested that this process may be due to a conformational change in NKA itself, caused by the binding of CTS, rather than activation of additional intracellular signaling cascades. This is supported by evidence showing that several stimuli other that CTS, such as hypoxia and hypercapnia, also cause NKA internalization (Chibalin et al., 1997; Zhang et al., 1999; Dada et al., 2003; Lecuona et al., 2009; Welch et al., 2010). It is possible that the conformational state of NKA depends on the type of CTS bound. In particular, ouabain and marinobufagenin binding drives NKA into different conformations, which could help explain the diversity of cellular effects that different CTS have (Klimanova et al., 2015). Thus, ouabain and marinobufagenin, at similar concentration, equally inhibit NKA ion transport in renal epithelial cells; however, they induce cell death at different IC50 values (Akimova et al., 2005). While further investigation is needed, it is clear that NKA internalization and degradation represents a mechanism important to not only control NKA signaling but also influence overall NKA expression levels and activity at the cell plasma membrane. This is another indication of the complex crosstalk that exists between CTS-induced NKA signaling events and NKA pumping activity.
A factor that modulates NKA signaling is hypoxia. It has been shown that under low oxygen conditions, the activation of mediators of NKA signaling, such as Src and ERK is abrogated in human embryonic kidney cells (HEK293 cells) and mouse fibroblast cells (SC1 cells) (Lakunina et al., 2017; Petrushanko et al., 2017). Therefore, hypoxia makes the cells less sensitive to CTS, reducing the effects of these substances. Conversely, ouabain protects SC1 cells from hypoxia (Lakunina et al., 2017) and this effect has also been observed in neuronal slice cultures (Oselkin et al., 2010). Endogenous CTS play an important role in adaptation to hypoxia and their level increases in human divers and diving animals (Manfrini et al., 2022). Under conditions of chronic hypoxia, such as in patients with idiopathic pulmonary arterial hypertension, endogenous CTS are increased (Manfrini et al., 2023). Moreover, endogenous CTS are also elevated in myocardial and renal ischemia (Strauss et al., 2019). Altogether, this shows that ouabain prevents the damage caused by low oxygen and ischemia and therefore, CTS may provide a beneficial effect in the adaptation of cells to hypoxia.
Signaling of NKA α isoforms
Most studies of the signaling properties of NKA have focused on the NKA α1, which is the NKA α isoform expressed in most cell types (Blanco and Mercer, 1998). Initial studies focused on the use of relatively high concentrations of ouabain; however, as studies on NKA signaling developed, lower doses of ouabain were used, which better reflected the CTS levels normally found in the bloodstream of mammals (in the picomolar to nanomolar range). The relatively low affinity of NKA α1 for ouabain in rodent models posed the controversy as to how this NKA isoform could respond to the low amounts of ligand used in some experiments. As will be discussed below, this was explained by the requirement of only a few molecules of ouabain to bind to NKA and that this will result in an amplification through the activation of the downstream mediators. However, this hypothesis has not yet been unequivocally demonstrated. Treatment of adult or neonatal cardiac myocytes with ouabain, in doses lower that those used in initial experiments, recapitulated the observations originally obtained. Since in addition to NKA α1, myocytes also express NKA α2 and NKA α3 isoforms (Blanco and Mercer, 1998), focus was directed to the role of NKA α isoforms different from NKA α1. This was logical, considering that the higher affinity of NKA α2 and NKA α3 would allow these isoforms to more readily respond to lower concentrations of ouabain. However, it was shown that NKA signaling in cardiac cells was primarily mediated via NKA α1, while the changes in intracellular Ca2+ due to ouabain ion transport inhibition of NKA α2 was responsible for the inotropic effects of CTS (Blaustein and Hamlyn, 2020). Additional studies performed in Sf9 insect cells expressing NKA α2 suggested that this NKA isoform was not involved in signaling. In addition, in this cell model, NKA α1, NKA α3, and NKA α4 responded to ouabain by increasing the phosphorylation of ERK in a dose-dependent manner and in line with the respective affinity that each NKA isoform has for ouabain (Pierre et al., 2008). In agreement with the findings in Sf9 cells, LLC-PK1 cells with severe knock down of NKA α1, and rescued with rat NKA α2, were able to recover ion transport function but failed to reestablish ouabain-stimulated signaling (Xie et al., 2015). This suggested that the inability of NKA α2 to signal was not an artifact produced in the insect cells but also occurs in mammalian cells. Different from these results, in smooth muscle cells of the vasculature, NKA α2 responds to ouabain not only reducing ion transport, but also by interacting with Src and activating downstream signal transduction pathways (Kotova et al., 2006b; Bouzinova et al., 2018). Therefore, it appears that the ability of NKA α2 to signal is tissue and cell type specific.
An interesting consideration when analyzing the involvement of NKA α2 in signaling CTS effects is that the putative Src interaction domain present in NKA α1 is absent in NKA α2 (Xie et al., 2015). Interestingly, when NKA α2 was mutated to express the Src interacting domain of NKA α1 in kidney epithelial cells, ouabain was then able to activate ERK in the cells (Yu et al., 2018). It is therefore possible that NKA α2 does have signaling capacity, but it uses downstream effectors that are different from those activated by the other NKA isoforms.
Unlike NKA α2, NKA α3 has been found to increase ERK phosphorylation in response to sub-inhibitory concentrations of ouabain in systems where NKA α3 is either the only or the predominant α isoform expressed (Pierre et al., 2008; Madan et al., 2017). In the LLC-PK1 model, in which pig NKA α1 is greatly knocked down, expression of rat NKA α3, reestablishes ouabain induced phosphorylation of ERK and Akt. Interestingly, this response did not involve Src and instead relied on the activation of PKC and PI3K. In this manner, unlike NKA α1, NKA α3 signaling is not Src-based (Madan et al., 2017). Studies in neuroblastoma cells, which contain both NKA α1 and NKA α3, have demonstrated an increase of ERK activation with ouabain administration. When these two isoforms were individually silenced with small interfering RNA (siRNA), ERK activation was abolished only when NKA α3 but not NKA α1 was silenced. (Karpova L. et al., 2010). In another study, treatment of rat cerebellar cells with relatively low ouabain concentrations led to activation of the MAPK, PKC and PIP3 kinases, causing cell apoptosis. Instead, higher concentrations of ouabain stimulated Src and MAPK and reduced apoptosis. Based on the differences in ouabain affinity for NKA isoforms, it was interpreted that that NKA α1 and NKA α3 mediate ouabain effects using different pathways (Karpova L. V. et al., 2010). Differences in NKA α3 and NKA α1 signaling were also suggested by the transcriptomic changes triggered by ouabain in rat cerebellar granule cells (Smolyaninova et al., 2019). Altogether, these results show the selective role that different NKA isoforms exert in mediating ouabain effects. The fact that NKA α3, similar to NKA α2, lacks the putative Src-binding domain present in NKA α1 supports the idea that NKA α3 uses other downstream effector and that this may be occurring in a cell type specific manner (Madan et al., 2017).
Finally, the signaling capacity of NKA α4, an isoform which is only expressed in male germ cells of the testis and primarily in differentiated sperm, has also been explored. Exposure of murine spermatogenic cells (GC-2) to ouabain increased ERK phosphorylation, the transcription factors CREB, ATF-1, and the expression of integrins in the cells. This response was abolished when NKA α4 expression in those cells was reduced via siRNA (Upmanyu et al., 2016). Studies performed in differentiated spermatozoa from bull showed that ouabain stimulated the phosphorylation of a series of proteins at tyrosine residues and aided in the process of sperm capacitation, an event that is important for sperm fertilizing capacity (Thundathil et al., 2006; Rajamanickam et al., 2017). However, these authors used concentrations of ouabain which are significantly higher than the ouabain concentrations detected in body fluids, which makes uncertain whether this is a physiological regulatory mechanism for sperm. Because bull spermatozoa contain the NKA α1, NKA α2, and NKA α3 isoforms in addition to NKA α4, dissecting the contribution of each NKA isoform to ouabain’s effects is difficult. However, NKA α4 signaling capacity is supported by its interaction with caveolin-1 and EGFR in the lipid raft fractions, and with SRC, EGFR, and ERK in the non-raft fractions isolated from the membranes of those cells (Rajamanickam et al., 2017).
Altogether these results suggests that each NKA α isoform uses different downstream effectors to influence cell function in a specific manner. The specific signaling pathways known to date for each NKA α isoform are illustrated in Figure 2.
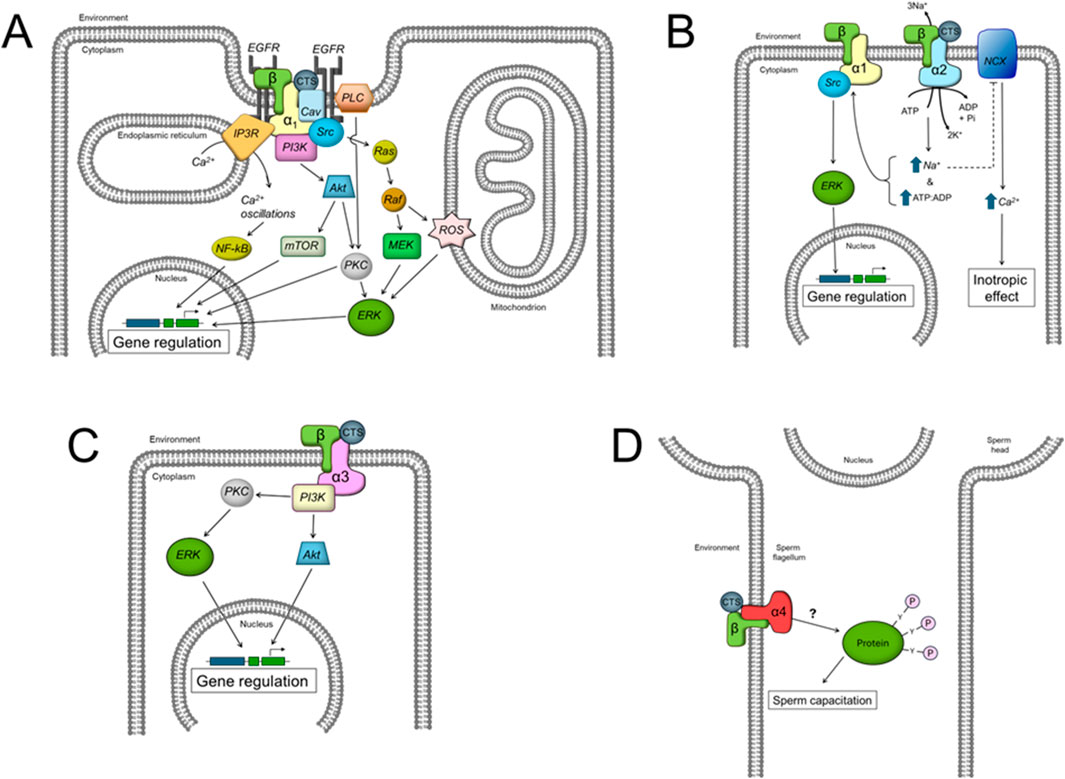
Figure 2. Scheme showing the different intracellular signaling pathways described to date for the effects of CTS by the different NKA α isoforms. (A) α1 isoform, (B) α2 isoform, (C) α3 isoform, and (D) α4 isoform. See the text for additional definitions.
NKA signaling in disease
As NKA signaling was further explored, focus was placed not only on the effects of CTS in normal states, but also under different pathological situations. Below, we summarize conditions in which CTS induced and NKA mediated signaling is altered, either due to altered levels of circulating CTS or to abnormal response of the diseased cells (see Table 2).
Chronic kidney disease
In the 1980s, it was noted that patients with renal failure or chronic kidney disease (CKD) had elevated circulating levels of a digoxin-like substances (Graves et al., 1983). This finding remained unexplained for a number of years, until the discovery of the function of NKA as a signaling system. By performing partial nephrectomy in mice, as a model for uremic cardiomyopathy, it was found that the reduction in kidney function was accompanied by an increase in blood pressure (Kennedy et al., 2008). Later, it was reported that patients with CKD, as well as animal models of renal failure, had elevated levels of MBG and another CTS, telocinobufagenin (TCB) (Komiyama et al., 2005; Kolmakova et al., 2011). Further investigation indicated that these substances contributed to the kidney damage that accompanies renal failure in CKD, with an increase in fibrosis as the central cause. This was demonstrated in epithelial renal cell lines and in rodent models of CKD, where fibrosis required Src and an intact NKA signaling complex (Kennedy et al., 2018). TCB also augmented inflammatory cytokines, macrophage oxidative burst, and activated NF-κB (Khalaf et al., 2019). TCB effects were prevented if mice were manipulated to reduce expression of NKA α1 or when cell lines were pretreated with the NKA/Src complex inhibitor pNaKtide or the Src inhibitor PP2. TCB also increased macrophage adhesion to cultured renal cells and upregulated inflammatory adhesion molecules in immune and endothelial cells, effects which were blocked by pNaKtide and NKA α1 knockdown (Khalaf et al., 2020). Ouabain, similar to TCB, also worsened renal damage in nephrectomized rats through activation of the Src signaling pathway, increase in oxidative stress, and stimulation of the inflammasome (Wang et al., 2019).
The role of MBG was also studied in the development of the cardiovascular damage that follows renal failure (Kolmakova et al., 2011). MBG increases the nuclear translocation of the EMT transcription factor Snail in rat hearts. This also occurred in LLC-PK1 cells, where MBG increased the expression of other EMT proteins, including collagen I, fibronectin, and vimentin (Fedorova et al., 2009). Studies in rats have shown that in renal insufficiency secondary to partial nephrectomy, treatment with a monoclonal antibody against MBG or with Digifab, a digoxin antibody and nonspecific CTS binder, reduced kidney damage (Haller et al., 2014). These results suggest that elevated levels of CTS in the setting of renal failure exacerbate renal damage, especially through inflammation and fibrosis.
MBG is also implicated in the development of uremic cardiomyopathy due to renal failure in rats. Thus, administration of low doses of MBG caused the same extent of uremic cardiomyopathy as partial nephrectomy (Elkareh et al., 2007). MBG increased blood pressure and heart size, impaired diastolic relaxation, and enhanced cardiac fibrosis. In this model, pre-immunization against MBG attenuated the cardiac hypertrophy, impairment of diastolic function, cardiac fibrosis, and systemic oxidant stress seen with partial nephrectomy without a significant effect on blood pressure (Kennedy et al., 2006). MBG also directly affected isolated cardiac fibroblasts, increasing collagen expression and procollagen stability, which was ablated by the addition of inhibitors of Src, tyrosine phosphorylation, EGFR, and ROS formation (Elkareh et al., 2007). In agreement with the data described above, a correlation between MBG and cardiomyopathy was observed in patients with end-stage kidney disease (Bolignano et al., 2022). It has been further shown that erythrocytes from CKD patients had lower NKA activity, potentially due to the reduction in NKA at the cell surface do to internalization caused by the chronically increased circulating levels of CTS (Kolmakova et al., 2011). This NKA retrieval from the cell plasma membrane is dependent on the increased production of ROS, through NKA signaling (Maxwell et al., 2021). It is possible that this phenomenon contributes to the anemic phenotype that accompanies CKD.
In conclusion, the elevation of CTS in CKD is detrimental to patients with this condition, exacerbating renal insufficiency and the associated cardiomyopathy. Progression of these conditions appears to depend on an aberrantly activated NKA signaling apparatus in the setting of CKD. Therefore, it is conceivable that treatments directed toward sequestering the excess circulating CTS or that can dampen NKA-based signaling could alleviate CKD symptoms.
Autosomal dominant polycystic kidney disease
In the process of exploring the role of NKA in autosomal dominant polycystic kidney disease (ADPKD), our laboratory discovered that ouabain induced NKA signaling significantly contributes to the progression of this disease. ADPKD is the most common hereditary disease of the kidney, characterized by the formation and growth of fluid-filled cysts in the kidney and other organs. In human primary ADPKD cells isolated from renal cysts of patients with the disease and in renal tissue from mouse models of ADPKD, we discovered that a subpopulation of NKA has a significantly higher affinity for ouabain (Nguyen et al., 2007; Trant et al., 2023). This subset of NKA, which is constituted by the NKA α1 isoform, is characterized by an IC50 for ouabain that is 2-3 orders of magnitude lower than the NKA α1 from the normal kidney. Based on its response to ouabain, we estimated that this NKA population comprises between 15%–20% of the total NKA present in the sample (Nguyen et al., 2007). This higher-than-normal affinity for ouabain allows for relatively low doses of ouabain (in the nM range) to stimulate cell proliferation of human ADPKD cells in culture, but not in normal human kidney (NHK) cells (Nguyen et al., 2011). Besides increasing ADPKD cell growth, ouabain also stimulated the cyclic AMP (cAMP)-dependent increase in fluid secretion in ADPKD monolayers, another key event in the formation of ADPKD cysts (Jansson et al., 2012; Jansson et al., 2015). Interestingly, other cell parameters known to be accelerated in ADPKD, such as cell apoptosis (though to a lesser degree than proliferation) and epithelial to mesenchymal transition (EMT), are also promoted by ouabain (Venugopal and Blanco, 2016; Venugopal et al., 2017). These effects are mediated by activation of the classical NKA signaling pathway, involving Src, EGFR, MEK, and downregulation of cell cycle dependent kinases, which are already abnormally active in ADPKD (Jansson et al., 2013). In this manner, ouabain is a factor that further impacts the intracellular growth pathway that promote ADPKD cystogenesis. These data represented one of the first pieces of evidence for NKA signaling as a mechanism that, when altered, can exacerbate the progression of disease.
We have also investigated the effects of ouabain-induced NKA signaling in slowly progressive mouse models of ADPKD. We have shown that low doses of ouabain significantly increase cyst progression in vivo and that ouabain-induced cyst progression depends on the ouabain affinity of NKA, as cyst progression drastically increases in a mouse model where all NKA α1 has been genetically engineered to have a high affinity for ouabain (Trant et al., 2023). Recently, we have shown that these ouabain effects also require cell caveolae, and that NKA signaling in ADPKD is abrogated when caveolae are not present (Trant et al., 2025). Therefore, ouabain at levels such as those circulating in blood, and through the higher-than-normal affinity of NKA of ADPKD cells, promotes renal cyst formation and development.
Hypertension
Increased circulating concentration of CTS have been reported both in rodent models with volume expansion and high blood pressure and in patients with essential hypertension (Hamlyn et al., 1982; Ferrandi et al., 1992; Yamada et al., 1994; Huang and Leenen, 1998; Manunta et al., 2001; Manunta et al., 2005; Fedorova et al., 2010). Moreover, intravenous infusion of ouabain into rats caused a significant increase in blood pressure (Ferrandi et al., 2004). This hypertensive state could be prevented if the animals were pre-treated with Digifab (Krep et al., 1995; Kaide et al., 1999). These observations prompted investigations to study the mechanisms by which CTS and NKA signaling affect the vasculature. The role of ion changes and the typical increase in calcium via the NKA and Na/Ca exchanger link was shown to occur in vascular smooth muscle following a mechanism similar to that of cardiac cells. Blaustein showed that NKA α2 is the main NKA α isoform involved in controlling Na+ and Ca2+ concentrations in the restricted space between the cell plasma membrane and the sarcoplasmic reticulum, which he called the plasmerosome (Blaustein et al., 1998). Bagrov found that not only ouabain but also marinobufagenin exerts a similar effect in rat aorta and that this depends on the action on NAK α1 from the smooth muscle and NAK α3 isoform from nerve terminals arriving to the vessel (Bagrov et al., 1995; Fedorova and Bagrov, 1997). Moreover, ouabain and marinobufagenin can also regulate the tone of mesenteric arteries (Bagrov and Fedorova, 1998), showing that the coordinated action of both NKA ligands controls blood pressure (Bagrov and Fedorova, 2005). This explained how circulating ouabain, by preferentially inhibiting the pumping action of the ouabain-sensitive NKA α2 causes hypertension (Blaustein and Hamlyn, 2010). However, mechanisms besides NKA pumping action are also involved. Of interest was the observation that ouabain potentiates vascular tone and the contraction of vessels induced by vasoconstrictor agonists (Bouzinova et al., 2018). This effect is mediated through NKA signaling within vascular smooth muscle cells. Ouabain increases input resistance in the arterial wall of vascular smooth muscle cells which is antagonized by inhibitors of tyrosine phosphorylation, Src, and the NKA/Src complex. It also increases Src autophosphorylation, which is antagonized by pNaKtide (Hangaard et al., 2017; Zhang et al., 2018), and sensitizes the contractile apparatus of the cells to calcium through phosphorylation of myosin phosphatase targeting protein 1 (MYPT1) (Bouzinova et al., 2018). The effect of ouabain on the potentiation to noradrenaline sensitivity correlated with arterial size and ouabain-induced Src activation (but not total Src levels). The vasoconstriction appears to be due to sensitization of the muscle contractile machinery rather than changes in intracellular calcium. In addition, arterial size also correlated with a higher number of high affinity ouabain binding sites as measured with the fluorescent marker BODIPY FL ouabain, which implicates NKA α2 over NKA α1 as the main isoform involved in this process. Considering that relatively low doses of ouabain were used in the study, which would inhibit the ion transport of NKA α2 more than NKA α1, the authors concluded that local but not general changes in Na+ concentration, due to NKA α2 inhibition, may be mediating the effect (Bouzinova et al., 2018; Zhang et al., 2019). Without a doubt, NKA and the regulation of both its pumping and signaling functions by endogenous as well as exogenous CTS play a major role in the control of blood pressure.
Pre-eclampsia
There are many physiological changes that occur during pregnancy, including fluid and Na+ retention, which increases overall plasma volume by 50% by the end of gestation (Mottelson et al., 2020). This change prompted several lines of research to explore the role of CTS in pregnancy as regulators of vascular resistance and renal salt and fluid balance. It was shown that a moderate increase in digitalis-like compounds occurs in a healthy pregnancy and that this increase was more drastic in pregnancies that developed with hypertension (Graves, 1987). It was later shown that the levels of marinobufagenin (MBG) in plasma correlated to severity of hypertension in pre-eclamptic patients (Lopatin et al., 1999; Averina et al., 2006; Reznik et al., 2019). The involvement of CTS in pre-eclampsia was strengthened by the finding that the placenta itself produces CTS (Morris et al., 1988). In addition, NKA expression in the placenta of pre-eclamptic/hypertensive patients was found to be increased and its affinity to ouabain slightly augmented (Amler et al., 1994). Further studies showed that Digibind decreased pre-eclamptic placental arterial tone (Di Grande et al., 1993), decreased the blood pressure of hypertensive patients during pregnancy (Goodlin, 1988; Adair et al., 2010), and ameliorated postpartum pre-eclampsia (Adair et al., 2009). It is currently thought that the increase NKA signaling worsens or even causes pre-eclampsia itself due to an increase in fibrosis, as happens in cardiac, renal, and vascular tissues. Indeed, it has been shown that MBG at elevated levels in the placenta can increase placental and umbilical collagen-1 protein as a result of dampened expression of the transcription factor Fli-1, a mechanism shared with other tissues (Fedorova et al., 2018). Despite promising results in clinical trials with CTS immunoneutralization as a treatment for pre-eclampsia, little research has been done on the downstream pathway mechanisms for NKA signaling in the placenta.
Retinal dystrophy
Interest in the involvement of NKA in a hereditary form of retinal dystrophy, X-linked juvenile retinoschisis (XLRS), was fostered after discovering that the deficient protein in the disease, retinoschisin, directly interacts with the retinal NKA α3β2 isoenzyme (Molday et al., 2007). It was later found that the interaction requires the NKA β2 subunit and that this phenomenon is important for the anchoring of retinoschisin to retinal cell membranes (Friedrich et al., 2011; Plössl et al., 2017a). When it was discovered that retinoschisin itself regulated ERK activation and its downstream target genes, interest was directed into the involvement of NKA signaling specifically in this disease (Plössl et al., 2017b). When retinoschisin was re-introduced into a model deficient in this protein, the colocalization between NKA and its signaling partners, including Src, caveolin-1, PLC, and IP3R, was increased, and NKA localization, which is altered in retinoschisin knockout mice (Friedrich et al., 2011) was restored in the photoreceptor inner segments (Plössl et al., 2017a). It has been further shown that ouabain can displace retinoschisin from NKA, though only at high µM to mM concentrations (Schmid et al., 2020). The authors of these studies proposed that retinoschisin normally binds to and suppresses NKA signaling in the retina, and that when retinoschisin is mutated or knocked out, NKA signaling is increased; this is demonstrated by decreased activations of Src and ERK in retinoschisin knockout cells in which the protein has been reintroduced (Plössl et al., 2017a). These results suggest a regulatory effect of retinoschisin on NKA signaling and reduction in retinoschisin expression could represent an initial step in XLRS pathogenesis.
Bipolar disorder
Bipolar disorder (BD) is a genetic and highly debilitating mental health disorder characterized by extreme mood swings, including manic episodes of abnormally elevated mood alternating with depressive episodes. As in other conditions mentioned above, BD appears to be accompanied by abnormally high circulating levels of CTS. However, patients with BD have a specific increase of CTS within the parietal cortex (Goldstein et al., 2006), while the overall level of CTS in plasma is low (Grider et al., 1999; El-Mallakh et al., 2010). Interestingly, digitalis toxicity can be accompanied by manic-depressive symptoms in humans (Keller and Frishman, 2003). It has been shown that intracerebroventricular (ICV) injections of ouabain in rats induced mania and mimicked the manic phenotypes of bipolar disorder (El-Mallakh et al., 2003; Riegel et al., 2009; Sui et al., 2013; Logan and McClung, 2016). These effects were reduced using the common BD mood stabilizers, lithium and valproic acid (Brocardo et al., 2010; Varela et al., 2015). In addition, ICV injections of anti-ouabain antibodies lowered CTS concentrations in the brain, caused anti-depressive effects (Goldstein et al., 2006; Goldstein et al., 2012), and blocked the hyperactivation produced by amphetamine (Hodes et al., 2016; Hodes et al., 2018). ICV administration of ouabain in the frontal cortex, striatum, and hippocampus mediate these effects through classic NKA signaling pathways, including Akt and its substrates (Yu et al., 2010; Kim et al., 2013), as well as MEK and ERK (Kim et al., 2008). The increase of these second messengers was also seen after administration of the stimulant amphetamine and interestingly, anti-ouabain antibodies block the effects of amphetamine (Lichtstein et al., 2018). It is clear that the activation of downstream effectors of CTS-NKA interaction modifies neuronal activity and neurotransmission, altering behavior and inducing BD (El-Mallakh et al., 2022). Interestingly, injection of digoxin specific Fab fragments (Digibind), which antagonizes CTS effects, resulted in a transient significant beneficial effect on depression symptoms in BD patients (Zilberstein et al., 2023).
While the etiology of BD is unknown, numerous genes have been associated to this condition; among them is the NKA α3 isoform, in which expression is reduced in the prefrontal cortex of post-mortem patient brain samples (Tochigi et al., 2008). In agreement with NKA α3 playing a role in BD is the finding that the Myshkin mice, a genetic model carrying a missense inactivating mutation of NKA α3, shows a mood-related behavioral profile that is similar to that of bipolar patients in the manic state (Kirshenbaum et al., 2015). Moreover, like in the manic BD induced by ouabain, Myshkin mice show activation of Akt and ERK in the hippocampus, supporting the notion that the mechanism underlying this condition depends on either higher level of endogenous ouabain, or abnormally increased NKA α3 signaling. Interestingly, this could represent a case in which NKA has deficient pumping activity but hyperactivated signaling function.
CTS and cancer
The role of NKA in cancer is a broad topic, which is outside the scope of this review; however, we would like to include a general view here. For a more detailed discussion of research in this area, we direct interested readers to several excellent reviews on the topic (Al-Ghoul and Valdes, 2008; Mijatovic et al., 2012; Slingerland et al., 2013; Alevizopoulos et al., 2014; Silva et al., 2021).
The idea of using CTS as anti-cancer agents developed from two main observations: one was the increase in NKA ion transport and activity that accompanies some type of cancer cells, and the other is the property of CTS to stimulate cell apoptosis. In addition, cancer cells show changes in expression of the NKA α and β subunits, alteration in the composition of its isoforms, and an increase in NKA sensitivity to CTS (Weidemann, 2005). Altogether, these data led to the idea of using NKA as a potential target for cancer therapy and a biomarker for cancer detection. However, studies correlating CTS use in cancer in patients gave conflicting results. While some studies observed that patients treated with digitalis have reduced cancer incidence and less recurrences of pre-existing cancers compared with patients not taking digitalis, others have shown no protective effects or a lack of improvement with respect to mortality (Al-Ghoul and Valdes, 2008; Osman et al., 2017; Zhang et al., 2017; Geng et al., 2020).
Early work showed that the CTS oleandrin decreased tumor development in skin cancer (Kanwal et al., 2020; Francischini et al., 2022). A variety of other cardenolides, including digitalis, digitoxin, hellebrin and ouabain have been shown to have similar effects in other cancer cell lines (Ayogu and Odoh, 2020). Moreover, besides natural compounds, some synthetic CTS have been tested for their use as anti-cancer agents (de Oliveira et al., 2021). While CTS present anti-proliferative effects on cancer cells, it is important to note that this is different from their growth effects in noncancerous cells. While not clear yet, it has been hypothesized that the difference in response depends on the pattern of NKA isoform expression or the dissimilar signaling pathways activated by CTS in normal versus cancer cells. It is important to note that most of the studies have tested the effect of different CTS on cells in culture, including breast, pancreatic, lung, prostate, melanoma, leukemia, neuroblastoma, and renal adenocarcinoma. In general, CTS mainly function on tumor cells as sensitizers of apoptosis and anoikis, or as inducers of autophagy-like death. For example, digoxin stimulated the activity of caspases 3/7 in gastric cancer cells, increasing anoikis and reducing the number of metastatic cells. This effect is mediated by the NKAα3 isoform (Numata et al., 2025). Digoxin also reduced circulating tumor cell clusters in patients with metastatic breast cancer (Kurzeder et al., 2025).
While the signaling function of NKA plays a role in cancer and exert some anti-cancer effect, the mechanisms of CTS action have not been completely elucidated. Among the various intracellular pathways that CTS activate, the most relevant to tumorigenesis is the downregulation of several transcription factors, such as c-Myc, NF-κB, and the hypoxia-inducible factor (HIF-1α), which are directly involved with cell growth, proliferation, and apoptosis (Mijatovic and Kiss, 2013). CTS downregulation of c-Myc also activates the expression of the multiple drug-resistant gene, which appears to be the mechanism by which CTS helps maintain the sensitivity of cancer cells to different drugs (Mijatovic and Kiss, 2013). The inhibition of HIF-1α production reduces the expression of proangiogenic genes, which will diminish blood flow to the tumor and consequently its development. CTS also upregulate the expression of the cell cycle inhibitor p21, inducing cell cycle arrest. Moreover, CTS have a radio sensitizing effect on malignant cancer cell lines, an event that depends on the downregulation of topoisomerases (Winnicka et al., 2006). Another pathway regulated by CTS is the PI3K-Akt pathway, which is involved in the activation of caspases and apoptosis of cancer cells (Yin et al., 2012). CTS have also been shown to solidify tight junctions in cancer cell, a mechanism that is important to reduce the metastatic nature of tumors (de Souza et al., 2014; Rocha et al., 2014). In addition, a loss of Na/K-ATPase-mediated Src regulation leads to stimulation of the Warburg phenotype in cancer, implying that that NKA signaling works as a suppressor of the abnormal metabolic changes in cancer cells (Banerjee et al., 2018). Recently, artificial intelligence, molecular docking and molecular dynamic simulations have been used to identify the mechanisms of action of bufalin. This approach predicted that the estrogen receptor (ESR1) is a potential target of bufalin. Further experimental analysis confirmed that bufalin interacts and stimulates degradation of ESR1. These data suggested that the anti-cancer effects of bufalin are related to its ability to overcome tumor endocrine resistance (Jiang et al., 2025).
It is important to point out that we are here providing a broad view of the actions of CTS. However, there are marked differences in the effect, potencies, and mechanism of actions of CTS and that this occurs in a cancer-type dependent manner. In addition, while the signaling role of NKA is important for the anti-cancer effect of CTS, it appears that the increase in intracellular calcium that results from the inhibition of NKA pumping also plays a role in inducing cancer cell apoptosis (Winnicka et al., 2006). In any case, targeting NKA with CTS has emerged as an attractive approach for the treatment of cancer. One issue with the use of CTS is the toxicity that these compounds present, which limits their use in the relatively high doses required to stop cell growth of cancer cells. Improving the therapeutic index, as well as enhancing the selectivity of effect of CTS, represent unmet goals which need to be achieved before they can be applied in cancer treatment.
Conclusions and prospects
Following the captivating discovery of the signaling role of NKA as a receptor and a signal transduction molecule for CTS effects nearly 30 years ago, a significant amount of data and important progress has been made. However, as has happened with other dazing findings, the road has been challenging. The novel non-canonical role of NKA posed a number of questions and led to heated, controversial debates. While some points have been clarified, many others still remain to be elucidated. At earlier stages in the search for endogenous CTS, a point of disagreement revolved around the nature and origin of the circulating compounds. While mass spectrometry showed that ouabain itself or a ouabain isomer was the CTS agent present in blood, its endogenous nature was challenged. This was based on the fact that in mammals, steroids display the typical trans and not cis conformation, and that mammals do not have the capability to synthesize the rhamnose sugar present in ouabain. These criticisms have been justified, arguing that different components of the CTS molecule may come premade from exogenous sources. Unfortunately, our understanding of the biosynthetic pathways involved in the synthesis of ouabain are at present incomplete. While it is known that cholesterol is the main substrate for the synthesis of ouabain and that the first reaction steps are the cleavage of the side chain and 3β hydroxylation of cholesterol, the more distal reactions in the biosynthetic pathway are unknown (Lichtstein et al., 2012). Despite this, it seems clear that CTS, CTS-like substances and ouabain are present in body fluids; however, distinguishing their identity has been difficult. While the use of immunoassay techniques have shown the presence of CTS in plasma, the assumption that they correspond to ouabain has not always been confirmed by mass spectroscopy (Baecher et al., 2014). This shows that the various immunoassays developed to detect CTS may not yet have the selectivity needed to clearly distinguish between different cardenolides. Moreover, the difficulties in measuring the low levels of CTS in extracellular fluids, which showed high variability depending on the sample type and method used, has complicated their study. Despite these caveats, it is now well accepted that different CTS, including ouabain, are circulating in body fluids (Blaustein and Hamlyn, 2024), but more accurate and straightforward methods for CTS determination need to be developed.
Another point relevant to the effects of CTS has been the possibility that these compounds could be acting on a receptor different from the NKA. While several other targets of CTS have been suggested, evidence for a direct interaction of CTS with those targets have not been fully proven. Experiments in mice in which the ouabain affinity of NKA has been modified to alter their binding to ouabain show a good correlation between the binding capacity of NKA and CTS cell effects, suggesting that if not the only receptor, NKA is the main receptor for CTS (Lingrel, 2010). The affinity of the NKA for CTS varies depending on the species and NKA isoform considered. This raises new questions as to the role of different NKA isoforms in the response. In rodents, the differences in ouabain affinities of NKA isoforms (Blanco and Mercer, 1998), led to the assumption that in cells where various isoforms are expressed, the effect may be mediated by the isoform with the highest affinity for ouabain. However, in species different from rodents, the smaller differences in ouabain binding between isoforms makes it difficult to ascertain which NKA is mediating the effects. As mentioned above, the low ouabain affinity in rodents implies that NKA α1 would not be able to respond to the relatively low levels of CTS found in circulation. A mechanism that has been proposed to explain this inconsistency is the interaction among NKA αβ protomers that may allow occupation of a small fraction of NKA by CTS which may be sufficient to cause a large amplification of the downstream signaling effects (Liu and Askari, 2006; Orlov et al., 2021). While this topic merits further study, the current experimental evidence shows that, at physiologic levels, CTS can activate NKA signaling and it is possible that the differences in NKA ouabain affinity may have developed to differentially respond to these compounds in a cell specific manner (Takeyasu et al., 2001; Herbertz et al., 2024).
Another point regards the differences in effect of CTS, which vary depending on the amount of CTS used. While it is clear that relatively high doses of CTS inhibit NKA activity and low ones activate signaling, it has been reported that nanomolar or subnanomolar concentrations of ouabain can also stimulate NKA activity. This stimulation has been observed in whole homogenates prepared from rat brain, where the effect was ascribed to a displacement of an endogenous ouabain-like compound from NKA or changes induced by ouabain on the environment surrounding NKA (Lichtstein et al., 1985). An activation of NKA activity by ouabain was also reported in cardiac preparations, in which the effect appears to be mainly involve the NKA α2 isoform (Gao et al., 1999). However, these results remain unclear and further investigations are needed to find an explanation for this puzzling response.
Additional uncertainties over NKA signaling revolved around the underlying mechanism of NKA signaling, which to date is not completely clear. This particularly refers to the localization of the NKA signaling complex, which is unclear if it resides in only caveolae, the general cell plasma membrane, or in both. In addition, while there is consensus that Src is a key mediator of the downstream effects of ouabain, the way in which NKA cross talks with Src is still the matter of debate. Thus, there is evidence indicating a direct association between NKA and Src (Li and Xie, 2009) and using microscale thermophoresis, a dissociation constant (Kd) for the NKA-Src complex was calculated to be about 0.2 µM (Petrushanko et al., 2022). On the other hand, other studies, using immunoprecipitation and blue native gels were unable to detect a stable Src-NKA complex (Li and Xie, 2009; Yosef et al., 2016). These differences may reflect the different type of preparations used for each study. In addition, it is unknown whether Src and NKA form a functional complex, or if their interaction is transient. Besides the upstream interactions of the NKA receptor, experimental evidence shows that downstream mechanisms of NKA signaling are dependent on the NKA isoform targeted by CTS and this may explain, at least in part, the cell specific actions of CTS (Madan et al., 2017; Yu et al., 2018). Overall, this highlights the complexity of mechanisms of action for NKA signaling and emphasizes the biological relevance of NKA isoforms.
Another interesting point is the difficulty in dissecting the effects of CTS that depend on the action on NKA ion transport from those resulting from the activation of NKA signaling function. The simultaneous action between those two roles of NKA and their crosstalk show the complex mechanisms that cells have developed to maintain their homeostasis and regulate their function. The multifaceted function of NKA, as well as the intriguing effects of CTS are strong drivers to continue investigating in this field of research. This will help us to develop pharmacologic approaches that, by interfering with CTS-induced and NKA-mediated signaling, or enhancing it, can be used to correct conditions in which this pathway is dysregulated.
Author contributions
JT: Writing – original draft, Writing – review and editing. JL: Writing – review and editing. PM-S: Writing – review and editing. GB: Conceptualization, Project administration, Resources, Supervision, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abramowitz J., Dai C., Hirschi K. K., Dmitrieva R. I., Doris P. A., Liu L., et al. (2003). Ouabain- and marinobufagenin-induced proliferation of human umbilical vein smooth muscle cells and a rat vascular smooth muscle cell line, A7r5. Circulation 108 (24), 3048–3053. doi:10.1161/01.Cir.0000101919.00548.86
Ackermann U., Geering K. (1990). Mutual dependence of Na,K-ATPase alpha- and beta-subunits for correct posttranslational processing and intracellular transport. FEBS Lett. 269 (1), 105–108. doi:10.1016/0014-5793(90)81130-g
Adair C. D., Luper A., Rose J. C., Russell G., Veille J. C., Buckalew V. M. (2009). The hemodynamic effects of intravenous digoxin-binding fab immunoglobulin in severe preeclampsia: a double-blind, randomized, clinical trial. J. Perinatol. 29 (4), 284–289. doi:10.1038/jp.2008.224
Adair C. D., Buckalew V. M., Graves S. W., Lam G. K., Johnson D. D., Saade G., et al. (2010). Digoxin immune fab treatment for severe preeclampsia. Am. J. Perinatol. 27 (8), 655–662. doi:10.1055/s-0030-1249762
Agrawal A. A., Petschenka G., Bingham R. A., Weber M. G., Rasmann S. (2012). Toxic cardenolides: chemical ecology and coevolution of specialized plant-herbivore interactions. New Phytol. 194 (1), 28–45. doi:10.1111/j.1469-8137.2011.04049.x
Aizman O., Uhlén P., Lal M., Brismar H., Aperia A. (2001). Ouabain, a steroid hormone that signals with slow calcium oscillations. Proc. Natl. Acad. Sci. 98 (23), 13420–13424. doi:10.1073/pnas.221315298
Akimova O. A., Bagrov A. Y., Lopina O. D., Kamernitsky A. V., Tremblay J., Hamet P., et al. (2005). Cardiotonic steroids differentially affect intracellular Na+ and [Na+]i/[K+]i-independent signaling in C7-MDCK cells. J. Biol. Chem. 280 (1), 832–839. doi:10.1074/jbc.M411011200
Al-Ghoul M., Valdes R. (2008). Mammalian cardenolides in cancer prevention and therapeutics. Ther. Drug Monit. 30 (2), 234–238. doi:10.1097/FTD.0b013e31816b90ff
Alevizopoulos K., Calogeropoulou T., Lang F., Stournaras C. (2014). Na+/K+ ATPase inhibitors in cancer. Curr. Drug Targets 15 (10), 988–1000. doi:10.2174/1389450115666140908125025
Allen J. C., Abramowitz J., Koksoy A. (2003). Low concentrations of ouabain activate vascular smooth muscle cell proliferation. Ann. N. Y. Acad. Sci. 986, 504–508. doi:10.1111/j.1749-6632.2003.tb07235.x
Amler E., Cester N., Salvolini E., Staffolani R., Burkhard M., Mazzanti L., et al. (1994). Human hypertensive placenta contains an increased amount of Na, K-ATPase with higher affinity for cardiac glycosides. Cell Biol. Int. 18 (7), 723–727. doi:10.1006/cbir.1994.1101
Andersen J. P., Vilsen B. (1995). Structure-function relationships of cation translocation by Ca(2+)- and Na+, K(+)-ATPases studied by site-directed mutagenesis. FEBS Lett. 359 (2-3), 101–106. doi:10.1016/0014-5793(95)00019-6
Apell H. J., Schneeberger A., Sokolov V. S. (1998). Partial reactions of the Na, K-ATPase: kinetic analysis and transport properties. Acta Physiol. Scand. Suppl. 643, 235–245.
Aperia A. (2007). New roles for an old enzyme: na,k-atpase emerges as an interesting drug target. J. Intern Med. 261 (1), 44–52. doi:10.1111/j.1365-2796.2006.01745.x
Aperia A. (2012). 2011 Homer Smith award: to serve and protect: classic and novel roles for Na+, K+ -adenosine triphosphatase. J. Am. Soc. Nephrol. 23 (8), 1283–1290. doi:10.1681/asn.2012010102
Aperia A., Akkuratov E. E., Fontana J. M., Brismar H. (2016). Na+-K+-ATPase, a new class of plasma membrane receptors. Am. J. Physiol. Cell Physiol. 310 (7), C491–C495. doi:10.1152/ajpcell.00359.2015
Aperia A., Brismar H., Uhlén P. (2020). Mending fences: na, K-ATPase signaling via Ca(2+) in the maintenance of epithelium integrity. Cell Calcium 88, 102210. doi:10.1016/j.ceca.2020.102210
Aronsen J. M., Swift F., Sejersted O. M. (2013). Cardiac sodium transport and excitation-contraction coupling. J. Mol. Cell Cardiol. 61, 11–19. doi:10.1016/j.yjmcc.2013.06.003
Askari A. (2019). The other functions of the sodium pump. Cell Calcium 84, 102105. doi:10.1016/j.ceca.2019.102105
Asrorov A. M., Kayumov M., Mukhamedov N., Yashinov A., Mirakhmetova Z., Huang Y., et al. (2023). Toad venom bufadienolides and bufotoxins: an updated review. Drug Dev. Res. 84 (5), 815–838. doi:10.1002/ddr.22072
Averina I. V., Tapilskaya N. I., Reznik V. A., Frolova E. V., Fedorova O. V., Lakatta E. G., et al. (2006). Endogenous Na/K-ATPase inhibitors in patients with preeclampsia. Cell Mol. Biol. (Noisy-le-grand) 52 (8), 19–23.
Aydemir-Koksoy A., Abramowitz J., Allen J. C. (2001). Ouabain-induced signaling and vascular smooth muscle cell proliferation. J. Biol. Chem. 276 (49), 46605–46611. doi:10.1074/jbc.M106178200
Ayogu J. I., Odoh A. S. (2020). Prospects and therapeutic applications of cardiac glycosides in cancer remediation. ACS Comb. Sci. 22 (11), 543–553. doi:10.1021/acscombsci.0c00082
Baecher S., Kroiss M., Fassnacht M., Vogeser M. (2014). No endogenous ouabain is detectable in human plasma by ultra-sensitive UPLC-MS/MS. Clin. Chim. Acta 431, 87–92. doi:10.1016/j.cca.2014.01.038
Bagrov A. Y., Fedorova O. V. (1998). Effects of two putative endogenous digitalis-like factors, marinobufagenin and ouabain, on the Na+, K+-pump in human mesenteric arteries. J. Hypertens. 16 (12 Pt 2), 1953–1958. doi:10.1097/00004872-199816121-00015
Bagrov A. Y., Fedorova O. V. (2005). Cardenolide and bufadienolide ligands of the sodium pump. How they work together in NaCl sensitive hypertension. Front. Biosci. 10, 2250–2256. doi:10.2741/1694
Bagrov A. Y., Roukoyatkina N. I., Pinaev A. G., Dmitrieva R. I., Fedorova O. V. (1995). Effects of two endogenous Na+, K(+)-ATPase inhibitors, marinobufagenin and ouabain, on isolated rat aorta. Eur. J. Pharmacol. 274 (1-3), 151–158. doi:10.1016/0014-2999(94)00735-p
Bagrov A. Y., Shapiro J. I., Fedorova O. V. (2009). Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol. Rev. 61 (1), 9–38. doi:10.1124/pr.108.000711
Bai Y., Wu J., Li D., Morgan E. E., Liu J., Zhao X., et al. (2016). Differential roles of caveolin-1 in ouabain-induced Na+/K+-ATPase cardiac signaling and contractility. Physiol. Genomics 48 (10), 739–748. doi:10.1152/physiolgenomics.00042.2016
Banerjee M., Duan Q., Xie Z. (2015). SH2 ligand-like effects of second cytosolic domain of Na/K-ATPase α1 subunit on src kinase. PLoS One 10 (11), e0142119. doi:10.1371/journal.pone.0142119
Banerjee M., Cui X., Li Z., Yu H., Cai L., Jia X., et al. (2018). Na/K-ATPase Y260 phosphorylation-mediated src regulation in control of aerobic glycolysis and tumor growth. Sci. Rep. 8 (1), 12322. doi:10.1038/s41598-018-29995-2
Barwe S. P., Skay A., McSpadden R., Huynh T. P., Langhans S. A., Inge L. J., et al. (2012). Na,K-ATPase beta-subunit cis homo-oligomerization is necessary for epithelial lumen formation in mammalian cells. J. Cell Sci. 125 (Pt 23), 5711–5720. doi:10.1242/jcs.108795
Belliard A., Gulati G. K., Duan Q., Alves R., Brewer S., Madan N., et al. (2016). Ischemia/reperfusion-induced alterations of enzymatic and signaling functions of the rat cardiac Na+/K+-ATPase: protection by ouabain preconditioning. Physiol. Rep. 4 (19), e12991. doi:10.14814/phy2.12991
Bertorello A. M., Katz A. I. (1993). Short-term regulation of renal Na-K-ATPase activity: physiological relevance and cellular mechanisms. Am. J. Physiol. 265 (6 Pt 2), F743–F755. doi:10.1152/ajprenal.1993.265.6.F743
Bessen H. A. (1986). Therapeutic and toxic effects of digitalis: William Withering, 1785. J. Emerg. Med. 4 (3), 243–248. doi:10.1016/0736-4679(86)90048-x
Blanco G. (2005). Na,K-ATPase subunit heterogeneity as a mechanism for tissue-specific ion regulation. Semin. Nephrol. 25 (5), 292–303. doi:10.1016/j.semnephrol.2005.03.004
Blanco G., Mercer R. W. (1998). Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am. J. Physiol. 275 (5), F633–F650. doi:10.1152/ajprenal.1998.275.5.F633
Blanco G., Koster J. C., Sánchez G., Mercer R. W. (1995a). Kinetic properties of the alpha 2 beta 1 and alpha 2 beta 2 isozymes of the Na,K-ATPase. Biochemistry 34 (1), 319–325. doi:10.1021/bi00001a039
Blanco G., Sánchez G., Mercer R. W. (1995b). Comparison of the enzymatic properties of the Na,K-ATPase alpha 3 beta 1 and alpha 3 beta 2 isozymes. Biochemistry 34 (31), 9897–9903. doi:10.1021/bi00031a011
Blanco G., Sanchez G., Melton R. J., Tourtellotte W. G., Mercer R. W. (2000). The alpha4 isoform of the Na,K-ATPase is expressed in the germ cells of the testes. J. Histochem Cytochem 48 (8), 1023–1032. doi:10.1177/002215540004800801
Blaustein M. P. (1996). Endogenous ouabain: role in the pathogenesis of hypertension. Kidney Int. 49 (6), 1748–1753. doi:10.1038/ki.1996.260
Blaustein M. P. (2018). The pump, the exchanger, and the holy spirit: origins and 40-year evolution of ideas about the ouabain-Na(+) pump endocrine system. Am. J. Physiol. Cell Physiol. 314 (1), C3–C26. doi:10.1152/ajpcell.00196.2017
Blaustein M. P., Hamlyn J. M. (2010). Signaling mechanisms that link salt retention to hypertension: endogenous ouabain, the Na(+) pump, the Na(+)/Ca(2+) exchanger and TRPC proteins. Biochim. Biophys. Acta 1802 (12), 1219–1229. doi:10.1016/j.bbadis.2010.02.011
Blaustein M. P., Hamlyn J. M. (2020). Ouabain, endogenous ouabain and ouabain-like factors: the Na(+) pump/ouabain receptor, its linkage to NCX, and its myriad functions. Cell Calcium 86, 102159. doi:10.1016/j.ceca.2020.102159
Blaustein M. P., Hamlyn J. M. (2024). Sensational site: the sodium pump ouabain-binding site and its ligands. Am. J. Physiol. Cell Physiol. 326 (4), C1120–c1177. doi:10.1152/ajpcell.00273.2023
Blaustein M. P., Juhaszova M., Golovina V. A. (1998). The cellular mechanism of action of cardiotonic steroids: a new hypothesis. Clin. Exp. Hypertens. 20 (5-6), 691–703. doi:10.3109/10641969809053247
Blaustein M. P., Chen L., Hamlyn J. M., Leenen F. H., Lingrel J. B., Wier W. G., et al. (2016). Pivotal role of α2 Na+ pumps and their high affinity ouabain binding site in cardiovascular health and disease. J. Physiol. 594 (21), 6079–6103. doi:10.1113/JP272419
Bolignano D., De Rosa S., Greco M., Presta P., Patella G., Crugliano G., et al. (2022). Marinobufagenin, left ventricular geometry and cardiac dysfunction in end-stage kidney disease patients. Int. Urol. Nephrol. 54 (10), 2581–2589. doi:10.1007/s11255-022-03161-0
Botelho A. F. M., Pierezan F., Soto-Blanco B., Melo M. M. (2019). A review of cardiac glycosides: structure, toxicokinetics, clinical signs, diagnosis and antineoplastic potential. Toxicon 158, 63–68. doi:10.1016/j.toxicon.2018.11.429
Bouzinova E. V., Hangaard L., Staehr C., Mazur A., Ferreira A., Chibalin A. V., et al. (2018). The α2 isoform Na,K-ATPase modulates contraction of rat mesenteric small artery via cSrc-dependent Ca2+ sensitization. Acta Physiol. 224 (1), e13059. doi:10.1111/apha.13059
Brocardo P. S., Budni J., Pavesi E., Franco J. L., Uliano-Silva M., Trevisan R., et al. (2010). Folic acid administration prevents ouabain-induced hyperlocomotion and alterations in oxidative stress markers in the rat brain. Bipolar Disord. 12 (4), 414–424. doi:10.1111/j.1399-5618.2010.00827.x
Brodie C., Tordai A., Saloga J., Domenico J., Gelfand E. W. (1995). Ouabain induces inhibition of the progression phase in human T-cell proliferation. J. Cell Physiol. 165 (2), 246–253. doi:10.1002/jcp.1041650205
Burlaka I., Liu X. L., Rebetz J., Arvidsson I., Yang L., Brismar H., et al. (2013). Ouabain protects against Shiga toxin-triggered apoptosis by reversing the imbalance between Bax and Bcl-xL. J. Am. Soc. Nephrol. 24 (9), 1413–1423. doi:10.1681/ASN.2012101044
Buzaglo N., Rosen H., Ben Ami H. C., Inbal A., Lichtstein D. (2016). Essential opposite roles of ERK and Akt signaling in cardiac steroid-induced increase in heart contractility. J. Pharmacol. Exp. Ther. 357 (2), 345–356. doi:10.1124/jpet.115.230763
Buzaglo N., Golomb M., Rosen H., Beeri R., Ami H. C., Langane F., et al. (2019). Augmentation of ouabain-induced increase in heart muscle contractility by Akt inhibitor MK-2206. J. Cardiovasc Pharmacol. Ther. 24 (1), 78–89. doi:10.1177/1074248418788301
Cai H., Wu L., Qu W., Malhotra D., Xie Z., Shapiro J. I., et al. (2008a). Regulation of apical NHE3 trafficking by ouabain-induced activation of the basolateral Na+-K+-ATPase receptor complex. Am. J. Physiol. Cell Physiol. 294 (2), C555–C563. doi:10.1152/ajpcell.00475.2007
Cai T., Wang H., Chen Y., Liu L., Gunning W. T., Quintas L. E. M., et al. (2008b). Regulation of caveolin-1 membrane trafficking by the Na/K-ATPase. J. Cell Biol. 182 (6), 1153–1169. doi:10.1083/jcb.200712022
Cai L., Pessoa M. T., Gao Y., Strause S., Banerjee M., Tian J., et al. (2023). The Na/K-ATPase α1/Src signaling axis regulates mitochondrial metabolic function and redox signaling in human iPSC-Derived cardiomyocytes. Biomedicines 11 (12), 3207. doi:10.3390/biomedicines11123207
Castillo A., Ortuno-Pineda C., Flores-Maldonado C., Larre I., Martinez Rendon J., Hinojosa L., et al. (2019). Ouabain modulates the Adherens junction in renal epithelial cells. Cell Physiol. Biochem. 52 (6), 1381–1397. doi:10.33594/000000097
Cereijido M., Contreras R. G., Shoshani L., Larre I. (2012). The Na+-K+-ATPase as self-adhesion molecule and hormone receptor. Am. J. Physiol. Cell Physiol. 302 (3), C473–C481. doi:10.1152/ajpcell.00083.2011
Chen Y., Cai T., Yang C., Turner D. A., Giovannucci D. R., Xie Z. (2008). Regulation of inositol 1,4,5-trisphosphate receptor-mediated calcium release by the Na/K-ATPase in cultured renal epithelial cells. J. Biol. Chem. 283 (2), 1128–1136. doi:10.1074/jbc.M708025200
Chen Y., Huang W., Yang M., Xin G., Cui W., Xie Z., et al. (2017). Cardiotonic steroids stimulate macrophage inflammatory responses through a pathway involving CD36, TLR4, and Na/K-ATPase. Arterioscler. Thromb. Vasc. Biol. 37 (8), 1462–1469. doi:10.1161/atvbaha.117.309444
Cheng X., Song Y., Wang Y. (2019). pNaKtide ameliorates renal interstitial fibrosis through inhibition of sodium-potassium adenosine triphosphatase-mediated signaling pathways in unilateral ureteral obstruction mice. Nephrol. Dial. Transpl. 34 (2), 242–252. doi:10.1093/ndt/gfy107
Cherniavsky-Lev M., Golani O., Karlish S. J., Garty H. (2014). Ouabain-induced internalization and lysosomal degradation of the Na+/K+-ATPase. J. Biol. Chem. 289 (2), 1049–1059. doi:10.1074/jbc.M113.517003
Chibalin A. V., Katz A. I., Berggren P. O., Bertorello A. M. (1997). Receptor-mediated inhibition of renal Na(+)-K(+)-ATPase is associated with endocytosis of its alpha- and beta-subunits. Am. J. Physiol. 273 (5), C1458–C1465. doi:10.1152/ajpcell.1997.273.5.C1458
Cho Y., Taniguchi A., Kubo A., Ikenouchi J. (2025). Rho-ROCK liberates sequestered claudin for rapid de novo tight junction formation. Elife 13. doi:10.7554/eLife.102794
Chow D. C., Forte J. G. (1995). Functional significance of the beta-subunit for heterodimeric P-type ATPases. J. Exp. Biol. 198 (Pt 1), 1–17. doi:10.1242/jeb.198.1.1
Clausen M. V., Hilbers F., Poulsen H. (2017). The structure and function of the Na,K-ATPase isoforms in health and disease. Front. Physiol. 8, 371. doi:10.3389/fphys.2017.00371
Clifford R. J., Kaplan J. H. (2013). Human breast tumor cells are more resistant to cardiac glycoside toxicity than non-tumorigenic breast cells. PLoS One 8 (12), e84306. doi:10.1371/journal.pone.0084306
Cohen A. W., Hnasko R., Schubert W., Lisanti M. P. (2004). Role of caveolae and caveolins in health and disease. Physiol. Rev. 84 (4), 1341–1379. doi:10.1152/physrev.00046.2003
Contreras R. G., Torres-Carrillo A., Flores-Maldonado C., Shoshani L., Ponce A. (2024). Na+/K+-ATPase: more than an electrogenic pump. Int. J. Mol. Sci. 25 (11), 6122. doi:10.3390/ijms25116122
Coppi M. V., Guidotti G. (1997). The alpha2L111R,N122D isoform of the Na,K-ATPase expressed in HeLa cells does not undergo an adipocyte-like increase in activity in response to insulin. Biochem. Biophys. Res. Commun. 236 (2), 444–448. doi:10.1006/bbrc.1997.6981
Cordeiro B. M., Leite Fontes C. F., Meyer-Fernandes J. R. (2024). Molecular basis of Na, K-ATPase regulation of diseases: Hormone and FXYD2 interactions. Int. J. Mol. Sci. 25 (24), 13398. doi:10.3390/ijms252413398
Dada L. A., Chandel N. S., Ridge K. M., Pedemonte C., Bertorello A. M., Sznajder J. I. (2003). Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-zeta. J. Clin. Invest. 111 (7), 1057–1064. doi:10.1172/jci16826
Daniel E. E., Eteraf T., Sommer B., Cho W. J., Elyazbi A. (2009). The role of caveolae and caveolin 1 in calcium handling in pacing and contraction of mouse intestine. J. Cell Mol. Med. 13 (2), 352–364. doi:10.1111/j.1582-4934.2008.00667.x
de Oliveira G. C., Rocha S. C., da Silva Lopes M. A., Paixao N., Alves S. L. G., Pessoa M. T. C., et al. (2021). Implications of synthetic modifications of the cardiotonic steroid lactone ring on cytotoxicity. J. Membr. Biol. 254 (5-6), 487–497. doi:10.1007/s00232-021-00186-x
de Souza W. F., Barbosa L. A., Liu L., de Araujo W. M., de-Freitas-Junior J. C., Fortunato-Miranda N., et al. (2014). Ouabain-induced alterations of the apical junctional complex involve α1 and β1 Na,K-ATPase downregulation and ERK1/2 activation independent of caveolae in colorectal cancer cells. J. Membr. Biol. 247 (1), 23–33. doi:10.1007/s00232-013-9607-y
de Wardener H. E., Clarkson E. M. (1985). Concept of natriuretic hormone. Physiol. Rev. 65 (3), 658–759. doi:10.1152/physrev.1985.65.3.658
Desfrere L., Karlsson M., Hiyoshi H., Malmersjo S., Nanou E., Estrada M., et al. (2009). Na,K-ATPase signal transduction triggers CREB activation and dendritic growth. Proc. Natl. Acad. Sci. U. S. A. 106 (7), 2212–2217. doi:10.1073/pnas.0809253106
Di Grande A., Boura A. L., Read M. A., Malatino L. S., Walters W. A. (1993). Release of a substance from the human placenta having digoxin-like immunoreactivity. Clin. Exp. Pharmacol. Physiol. 20 (9), 603–607. doi:10.1111/j.1440-1681.1993.tb01747.x
Dmitrieva R. I., Doris P. A. (2003). Ouabain is a potent promoter of growth and activator of ERK1/2 in ouabain-resistant rat renal epithelial cells. J. Biol. Chem. 278 (30), 28160–28166. doi:10.1074/jbc.M303768200
Dobretsov M., Stimers J. R. (2005). Neuronal function and alpha3 isoform of the Na/K-ATPase. Front. Biosci. 10, 2373–2396. doi:10.2741/1704
Dostanic I., Lorenz J. N., Schultz Jel J., Grupp I. L., Neumann J. C., Wani M. A., et al. (2003). The alpha2 isoform of Na,K-ATPase mediates ouabain-induced cardiac inotropy in mice. J. Biol. Chem. 278 (52), 53026–53034. doi:10.1074/jbc.M308547200
Drummond C. A., Fan X., Haller S. T., Kennedy D. J., Liu J., Tian J. (2018). Na/K-ATPase signaling mediates miR-29b-3p regulation and cardiac fibrosis formation in mice with chronic kidney disease. PLoS One 13 (5), e0197688. doi:10.1371/journal.pone.0197688
Duan Q., Xu Y., Marck P. V., Kalisz J., Morgan E. E., Pierre S. V. (2018). Preconditioning and postconditioning by cardiac glycosides in the mouse heart. J. Cardiovasc Pharmacol. 71 (2), 95–103. doi:10.1097/FJC.0000000000000549
Dupont G., Combettes L., Bird G. S., Putney J. W. (2011). Calcium oscillations. Cold Spring Harb. Perspect. Biol. 3 (3), a004226. doi:10.1101/cshperspect.a004226
Dvela M., Rosen H., Ben-Ami H. C., Lichtstein D. (2012). Endogenous ouabain regulates cell viability. Am. J. Physiol. Cell Physiol. 302 (2), C442–C452. doi:10.1152/ajpcell.00336.2011
Dvela-Levitt M., Cohen-Ben Ami H., Rosen H., Ornoy A., Hochner-Celnikier D., Granat M., et al. (2015). Reduction in maternal circulating ouabain impairs offspring growth and kidney development. J. Am. Soc. Nephrol. 26 (5), 1103–1114. doi:10.1681/ASN.2014020130
Dyla M., Kjaergaard M., Poulsen H., Nissen P. (2020). Structure and mechanism of P-Type ATPase ion pumps. Annu. Rev. Biochem. 89, 583–603. doi:10.1146/annurev-biochem-010611-112801
Eakle K. A., Kabalin M. A., Wang S. G., Farley R. A. (1994). The influence of beta subunit structure on the stability of Na+/K(+)-ATPase complexes and interaction with K+. J. Biol. Chem. 269 (9), 6550–6557. doi:10.1016/s0021-9258(17)37407-0
Ehle M., Patel C., Giugliano R. P. (2011). Digoxin: clinical highlights: a review of digoxin and its use in contemporary medicine. Crit. Pathw. Cardiol. 10 (2), 93–98. doi:10.1097/HPC.0b013e318221e7dd
El-Mallakh R. S., El-Masri M. A., Huff M. O., Li X. P., Decker S., Levy R. S. (2003). Intracerebroventricular administration of ouabain as a model of mania in rats. Bipolar Disord. 5 (5), 362–365. doi:10.1034/j.1399-5618.2003.00053.x
El-Mallakh R. S., Stoddard M., Jortani S. A., El-Masri M. A., Sephton S., Valdes R. (2010). Aberrant regulation of endogenous ouabain-like factor in bipolar subjects. Psychiatry Res. 178 (1), 116–120. doi:10.1016/j.psychres.2009.03.032
El-Mallakh R. S., Sampath V. P., Horesh N., Lichtstein D. (2022). Endogenous cardiac steroids in bipolar disorder: state of the art. Int. J. Mol. Sci. 23 (3), 1846. doi:10.3390/ijms23031846
Elkareh J., Kennedy D. J., Yashaswi B., Vetteth S., Shidyak A., Kim E. G. R., et al. (2007). Marinobufagenin stimulates fibroblast collagen production and causes fibrosis in experimental uremic cardiomyopathy. Hypertension 49 (1), 215–224. doi:10.1161/01.hyp.0000252409.36927.05
El-Okdi N., Smaili S., Raju V., Shidyak A., Gupta S., Fedorova L., et al. (2008). Effects of cardiotonic steroids on dermal collagen synthesis and wound healing. J Appl. Physiol. 105 (1), 30–36. doi:10.1152/japplphysiol.00119.2008
Esteves M. B., Marques-Santos L. F., Affonso-Mitidieri O. R., Rumjanek V. M. (2005). Ouabain exacerbates activation-induced cell death in human peripheral blood lymphocytes. An Acad Bras Cienc 77 (2), 281–292. doi:10.1590/s0001-37652005000200008
Ewart H. S., Klip A. (1995). Hormonal regulation of the Na(+)-K(+)-ATPase: mechanisms underlying rapid and sustained changes in pump activity. Am. J. Physiol. 269 (2 Pt 1), C295–C311. doi:10.1152/ajpcell.1995.269.2.C295
Fedorova O. V., Bagrov A. Y. (1997). Inhibition of Na/K ATPase from rat aorta by two Na/K pump inhibitors, ouabain and marinobufagenin: evidence of interaction with different alpha-subunit isoforms. Am. J. Hypertens. 10 (8), 929–935. doi:10.1016/s0895-7061(97)00096-4
Fedorova L. V., Raju V., El-Okdi N., Shidyak A., Kennedy D. J., Vetteth S., et al. (2009). The cardiotonic steroid hormone marinobufagenin induces renal fibrosis: implication of epithelial-to-mesenchymal transition. Am. J. Physiol. Ren. Physiol. 296 (4), F922–F934. doi:10.1152/ajprenal.90605.2008
Fedorova O. V., Shapiro J. I., Bagrov A. Y. (2010). Endogenous cardiotonic steroids and salt-sensitive hypertension. Biochim. Biophys. Acta 1802 (12), 1230–1236. doi:10.1016/j.bbadis.2010.03.011
Fedorova O. V., Ishkaraeva V. V., Grigorova Y. N., Reznik V. A., Kolodkin N. I., Zazerskaya I. E., et al. (2018). Antibody to Marinobufagenin reverses placenta-induced fibrosis of umbilical arteries in preeclampsia. Int. J. Mol. Sci. 19 (8), 2377. doi:10.3390/ijms19082377
Féraille E., Doucet A. (2001). Sodium-potassium-adenosinetriphosphatase-dependent sodium transport in the kidney: hormonal control. Physiol. Rev. 81 (1), 345–418. doi:10.1152/physrev.2001.81.1.345
Ferrandi M., Minotti E., Salardi S., Florio M., Bianchi G., Ferrari P. (1992). Ouabainlike factor in Milan hypertensive rats. Am. J. Physiol. 263 (4 Pt 2), F739–F748. doi:10.1152/ajprenal.1992.263.4.F739
Ferrandi M., Manunta P., Balzan S., Hamlyn J. M., Bianchi G., Ferrari P. (1997). Ouabain-like factor quantification in mammalian tissues and plasma: comparison of two independent assays. Hypertension 30 (4), 886–896. doi:10.1161/01.hyp.30.4.886
Ferrandi M., Molinari I., Barassi P., Minotti E., Bianchi G., Ferrari P. (2004). Organ hypertrophic signaling within caveolae membrane subdomains triggered by ouabain and antagonized by PST 2238. J. Biol. Chem. 279 (32), 33306–33314. doi:10.1074/jbc.M402187200
Field M. (2003). Intestinal ion transport and the pathophysiology of diarrhea. J. Clin. Invest. 111 (7), 931–943. doi:10.1172/JCI18326
Fielding C. J. (2001). Caveolae and signaling. Curr. Opin. Lipidol. 12 (3), 281–287. doi:10.1097/00041433-200106000-00007
Figtree G. A., Liu C. C., Bibert S., Hamilton E. J., Garcia A., White C. N., et al. (2009). Reversible oxidative modification: a key mechanism of Na+-K+ pump regulation. Circ. Res. 105 (2), 185–193. doi:10.1161/circresaha.109.199547
Francischini C. R. D., Mendonca C. R., Barcelos K. A., Silva M. A. M., Botelho A. F. M. (2022). Antitumor effects of oleandrin in different types of cancers: systematic review. Toxicon 216, 15–27. doi:10.1016/j.toxicon.2022.06.010
Friedrich U., Stöhr H., Hilfinger D., Loenhardt T., Schachner M., Langmann T., et al. (2011). The Na/K-ATPase is obligatory for membrane anchorage of retinoschisin, the protein involved in the pathogenesis of X-linked juvenile retinoschisis. Hum. Mol. Genet. 20 (6), 1132–1142. doi:10.1093/hmg/ddq557
Fuerstenwerth H. (2014). On the differences between ouabain and digitalis glycosides. Am. J. Ther. 21 (1), 35–42. doi:10.1097/MJT.0b013e318217a609
Gable M. E., Abdallah S. L., Najjar S. M., Liu L., Askari A. (2014). Digitalis-induced cell signaling by the sodium pump: on the relation of Src to Na(+)/K(+)-ATPase. Biochem. Biophys. Res. Commun. 446 (4), 1151–1154. doi:10.1016/j.bbrc.2014.03.071
Galvão J. G. F. M., Cavalcante-Silva L. H. A., de Almeida Lima É., Carvalho D. C. M., Alves A. F., Mascarenhas S. R. (2022). Ouabain modulates airway remodeling caused by Th2-high asthma in mice. Int. Immunopharmacol. 109, 108808. doi:10.1016/j.intimp.2022.108808
Gao J., Wymore R., Wymore R. T., Wang Y., McKinnon D., Dixon J. E., et al. (1999). Isoform-specific regulation of the sodium pump by alpha- and beta-adrenergic agonists in the guinea-pig ventricle. J. Physiol. 516 (Pt 2), 377–383. doi:10.1111/j.1469-7793.1999.0377v.x
Gao Y., Xu Y., Bai F., Puri R., Tian J., Liu J. (2025). Factors that influence the Na/K-ATPase signaling and function. Front. Pharmacol. 16, 1639859. doi:10.3389/fphar.2025.1639859
Garlid K. D., Costa A. D., Quinlan C. L., Pierre S. V., Dos Santos P. (2009). Cardioprotective signaling to mitochondria. J. Mol. Cell Cardiol. 46 (6), 858–866. doi:10.1016/j.yjmcc.2008.11.019
Garty H., Karlish S. J. (2005). FXYD proteins: tissue-specific regulators of the Na,K-ATPase. Semin. Nephrol. 25 (5), 304–311. doi:10.1016/j.semnephrol.2005.03.005
Geering K. (1990). Subunit assembly and functional maturation of Na,K-ATPase. J. Membr. Biol. 115 (2), 109–121. doi:10.1007/bf01869450
Geering K. (1991). The functional role of the beta-subunit in the maturation and intracellular transport of Na,K-ATPase. FEBS Lett. 285 (2), 189–193. doi:10.1016/0014-5793(91)80801-9
Geering K. (2005). Function of FXYD proteins, regulators of Na, K-ATPase. J. Bioenerg. Biomembr. 37 (6), 387–392. doi:10.1007/s10863-005-9476-x
Geering K., Beguin P., Garty H., Karlish S., Fuzesi M., Horisberger J. D., et al. (2003). FXYD proteins: new tissue- and isoform-specific regulators of Na,K-ATPase. Ann. N. Y. Acad. Sci. 986, 388–394. doi:10.1111/j.1749-6632.2003.tb07219.x
Geng M., Lin A., Nguyen T. P. (2020). Revisiting antiarrhythmic drug therapy for atrial fibrillation: reviewing lessons learned and redefining therapeutic paradigms. Front. Pharmacol. 11, 581837. doi:10.3389/fphar.2020.581837
Giannatselis H., Calder M., Watson A. J. (2011). Ouabain stimulates a Na+/K+-ATPase-mediated SFK-activated signalling pathway that regulates tight junction function in the mouse blastocyst. PLoS One 6 (8), e23704. doi:10.1371/journal.pone.0023704
Glynn I. M. (1956). Sodium and potassium movements in human red cells. J. Physiol. 134 (2), 278–310. doi:10.1113/jphysiol.1956.sp005643
Golden W. C., Martin L. J. (2006). Low-dose ouabain protects against excitotoxic apoptosis and up-regulates nuclear Bcl-2 in vivo. Neuroscience 137 (1), 133–144. doi:10.1016/j.neuroscience.2005.10.004
Goldman P. (2001). Herbal medicines today and the roots of modern pharmacology. Ann. Intern Med. 135 (8 Pt 1), 594–600. doi:10.7326/0003-4819-135-8_part_1-200110160-00010
Goldstein I., Levy T., Galili D., Ovadia H., Yirmiya R., Rosen H., et al. (2006). Involvement of Na(+), K(+)-ATPase and endogenous digitalis-like compounds in depressive disorders. Biol. Psychiatry 60 (5), 491–499. doi:10.1016/j.biopsych.2005.12.021
Goldstein I., Lax E., Gispan-Herman I., Ovadia H., Rosen H., Yadid G., et al. (2012). Neutralization of endogenous digitalis-like compounds alters catecholamines metabolism in the brain and elicits anti-depressive behavior. Eur. Neuropsychopharmacol. 22 (1), 72–79. doi:10.1016/j.euroneuro.2011.05.007
Goodlin R. C. (1988). Antidigoxin antibodies in eclampsia. N. Engl. J. Med. 318 (8), 518–519. doi:10.1056/nejm198802253180815
Graves S. W. (1987). The possible role of digitalislike factors in pregnancy-induced hypertension. Hypertension 10 (5 Pt 2), I84–I86. doi:10.1161/01.hyp.10.5_pt_2.i84
Graves S. W., Brown B., Valdes R. (1983). An endogenous digoxin-like substance in patients with renal impairment. Ann. Intern Med. 99 (5), 604–608. doi:10.7326/0003-4819-99-5-604
Grider G., El-Mallakh R. S., Huff M. O., Buss T. J., Miller J., Valdes R. (1999). Endogenous digoxin-like immunoreactive factor (DLIF) serum concentrations are decreased in manic bipolar patients compared to normal controls. J. Affect Disord. 54 (3), 261–267. doi:10.1016/s0165-0327(98)00208-0
Haas M., Askari A., Xie Z. (2000). Involvement of Src and epidermal growth factor receptor in the signal-transducing function of Na+/K+-ATPase. J. Biol. Chem. 275 (36), 27832–27837. doi:10.1074/jbc.M002951200
Haller S. T., Drummond C. A., Yan Y., Liu J., Tian J., Malhotra D., et al. (2014). Passive immunization against Marinobufagenin attenuates renal fibrosis and improves renal function in experimental renal disease. Am. J. Hypertens. 27 (4), 603–609. doi:10.1093/ajh/hpt169
Hamlyn J. M. (2014). Natriuretic hormones, endogenous ouabain, and related sodium transport inhibitors. Front. Endocrinol. (Lausanne) 5, 199. doi:10.3389/fendo.2014.00199
Hamlyn J. M., Manunta P. (2011). Endogenous ouabain: a link between sodium intake and hypertension. Curr. Hypertens. Rep. 13 (1), 14–20. doi:10.1007/s11906-010-0161-z
Hamlyn J. M., Ringel R., Schaeffer J., Levinson P. D., Hamilton B. P., Kowarski A. A., et al. (1982). A circulating inhibitor of (Na++ K+) ATPase associated with essential hypertension. Nature 300 (5893), 650–652. doi:10.1038/300650a0
Hamlyn J. M., Blaustein M. P., Bova S., DuCharme D. W., Harris D. W., Mandel F., et al. (1991). Identification and characterization of a ouabain-like compound from human plasma. Proc. Natl. Acad. Sci. U. S. A. 88 (14), 6259–6263. doi:10.1073/pnas.88.14.6259
Hammarstrom L., Smith C. I. (1979). Species restriction of the mitogenicity induced by lanatoside C. Lymphocyte activation by digitalis glycosides is confined to cells from digitalis resistant species. Immunology 37 (2), 389–396.
Hangaard L., Bouzinova E. V., Staehr C., Dam V. S., Kim S., Xie Z., et al. (2017). Na-K-ATPase regulates intercellular communication in the vascular wall via cSrc kinase-dependent connexin43 phosphorylation. Am. J. Physiol. Cell Physiol. 312 (4), C385–c397. doi:10.1152/ajpcell.00347.2016
Hansen O. (1982). Studies on ouabain-complexed (Na++ K+)-ATPase carried out with vanadate. Biochim. Biophys. Acta 692 (2), 187–195. doi:10.1016/0005-2736(82)90520-x
Harwood S., Yaqoob M. M. (2005). Ouabain-induced cell signaling. Front. Biosci. 10, 2011–2017. doi:10.2741/1676
He S., Shelly D. A., Moseley A. E., James P. F., James J. H., Paul R. J., et al. (2001). The alpha(1)- and alpha(2)-isoforms of Na-K-ATPase play different roles in skeletal muscle contractility. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281 (3), R917–R925. doi:10.1152/ajpregu.2001.281.3.R917
Herbertz M., Dalla S., Wagschal V., Turjalei R., Heiser M., Dobler S. (2024). Coevolutionary escalation led to differentially adapted paralogs of an insect’s Na,K-ATPase optimizing resistance to host plant toxins. Mol. Ecol. 33 (14), e17041. doi:10.1111/mec.17041
Hodes A., Rosen H., Deutsch J., Lifschytz T., Einat H., Lichtstein D. (2016). Endogenous cardiac steroids in animal models of mania. Bipolar Disord. 18 (5), 451–459. doi:10.1111/bdi.12413
Hodes A., Lifschytz T., Rosen H., Cohen Ben-Ami H., Lichtstein D. (2018). Reduction in endogenous cardiac steroids protects the brain from oxidative stress in a mouse model of mania induced by amphetamine. Brain Res. Bull. 137, 356–362. doi:10.1016/j.brainresbull.2018.01.016
Holm R., Toustrup-Jensen M. S., Einholm A. P., Schack V. R., Andersen J. P., Vilsen B. (2016). Neurological disease mutations of α3 Na+,K+-ATPase: structural and functional perspectives and rescue of compromised function. Biochim. Biophys. Acta 1857 (11), 1807–1828. doi:10.1016/j.bbabio.2016.08.009
Horowitz B., Hensley C. B., Quintero M., Azuma K. K., Putnam D., McDonough A. A. (1990). Differential regulation of Na,K-ATPase alpha 1, alpha 2, and beta subunit mRNA and protein levels by thyroid hormone. J. Biol. Chem. 265 (24), 14308–14314. doi:10.1016/s0021-9258(18)77301-8
Huang B. S., Leenen F. H. (1998). Both brain angiotensin II and “ouabain” contribute to sympathoexcitation and hypertension in Dahl S rats on high salt intake. Hypertension 32 (6), 1028–1033. doi:10.1161/01.hyp.32.6.1028
Huang L., Li H., Xie Z. (1997). Ouabain-induced hypertrophy in cultured cardiac myocytes is accompanied by changes in expression of several late response genes. J. Mol. Cell. Cardiol. 29 (2), 429–437. doi:10.1006/jmcc.1996.0320
Jansson K., Nguyen A. N., Magenheimer B. S., Reif G. A., Aramadhaka L. R., Bello-Reuss E., et al. (2012). Endogenous concentrations of ouabain act as a cofactor to stimulate fluid secretion and cyst growth of in vitro ADPKD models via cAMP and EGFR-Src-MEK pathways. Am. J. Physiol. Ren. Physiol. 303 (7), F982–F990. doi:10.1152/ajprenal.00677.2011
Jansson K., Magenheimer B. S., Maser R. L., Calvet J. P., Blanco G. (2013). Overexpression of the polycystin-1 C-tail enhances sensitivity of M-1 cells to ouabain. J. Membr. Biol. 246 (7), 581–590. doi:10.1007/s00232-013-9573-4
Jansson K., Venugopal J., Sanchez G., Magenheimer B. S., Reif G. A., Wallace D. P., et al. (2015). Ouabain regulates CFTR-mediated anion secretion and Na,K-ATPase transport in ADPKD cells. J. Membr. Biol. 248 (6), 1145–1157. doi:10.1007/s00232-015-9832-7
Jiang S., Liu K., Jiang T., Li H., Wei X., Wan X., et al. (2025). Harnessing artificial intelligence to identify Bufalin as a molecular glue degrader of estrogen receptor alpha. Nat. Commun. 16 (1), 7854. doi:10.1038/s41467-025-62288-7
Jørgensen P. L. (1986). Structure, function and regulation of Na,K-ATPase in the kidney. Kidney Int. 29 (1), 10–20. doi:10.1038/ki.1986.3
Jorgensen P. L., Hakansson K. O., Karlish S. J. (2003). Structure and mechanism of Na,K-ATPase: functional sites and their interactions. Annu. Rev. Physiol. 65, 817–849. doi:10.1146/annurev.physiol.65.092101.142558
Kaide J., Ura N., Torii T., Nakagawa M., Takada T., Shimamoto K. (1999). Effects of digoxin-specific antibody fab fragment (Digibind) on blood pressure and renal water-sodium metabolism in 5/6 reduced renal mass hypertensive rats. Am. J. Hypertens. 12 (6), 611–619. doi:10.1016/s0895-7061(99)00029-1
Kanwal N., Rasul A., Hussain G., Anwar H., Shah M. A., Sarfraz I., et al. (2020). Oleandrin: a bioactive phytochemical and potential cancer killer via multiple cellular signaling pathways. Food Chem. Toxicol. 143, 111570. doi:10.1016/j.fct.2020.111570
Kaplan J. H. (2002). Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 71, 511–535. doi:10.1146/annurev.biochem.71.102201.141218
Karpova L., Eva A., Kirch U., Boldyrev A., Scheiner-Bobis G. (2010a). Sodium pump alpha1 and alpha3 subunit isoforms mediate distinct responses to ouabain and are both essential for survival of human neuroblastoma. FEBS J. 277 (8), 1853–1860. doi:10.1111/j.1742-4658.2010.07602.x
Karpova L. V., Bulygina E. R., Boldyrev A. A. (2010b). Different neuronal Na(+)/K(+)-ATPase isoforms are involved in diverse signaling pathways. Cell Biochem. Funct. 28 (2), 135–141. doi:10.1002/cbf.1632
Kawakami K., Ikeda K. (2006). Modulation of neural activities by Na, K-ATPase alpha 2 subunit through functional coupling with transporters. Cell Mol. Biol. (Noisy-le-grand) 52 (8), 92–96.
Keller S., Frishman W. H. (2003). Neuropsychiatric effects of cardiovascular drug therapy. Cardiol. Rev. 11 (2), 73–93. doi:10.1097/01.CRD.0000053453.89776.2D
Kennedy D. J., Vetteth S., Periyasamy S. M., Kanj M., Fedorova L., Khouri S., et al. (2006). Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension 47 (3), 488–495. doi:10.1161/01.hyp.0000202594.82271.92
Kennedy D. J., Elkareh J., Shidyak A., Shapiro A. P., Smaili S., Mutgi K., et al. (2008). Partial nephrectomy as a model for uremic cardiomyopathy in the mouse. Am. J. Physiol. Ren. Physiol. 294 (2), F450–F454. doi:10.1152/ajprenal.00472.2007
Kennedy D. J., Khalaf F. K., Sheehy B., Weber M. E., Agatisa-Boyle B., Conic J., et al. (2018). Telocinobufagin, a novel cardiotonic steroid, promotes renal fibrosis via Na⁺/K⁺-ATPase profibrotic signaling pathways. Int. J. Mol. Sci. 19 (9), 2566. doi:10.3390/ijms19092566
Khalaf F. K., Dube P., Kleinhenz A. L., Malhotra D., Gohara A., Drummond C. A., et al. (2019). Proinflammatory effects of cardiotonic steroids mediated by NKA α-1 (Na+/K+-ATPase α-1)/Src complex in renal epithelial cells and immune cells. Hypertension 74 (1), 73–82. doi:10.1161/hypertensionaha.118.12605
Khalaf F. K., Tassavvor I., Mohamed A., Chen Y., Malhotra D., Xie Z., et al. (2020). Epithelial and endothelial adhesion of immune cells is enhanced by cardiotonic steroid signaling through Na(+)/K(+)-ATPase-α-1. J. Am. Heart Assoc. 9 (3), e013933. doi:10.1161/jaha.119.013933
Khundmiri S. J., Metzler M. A., Ameen M., Amin V., Rane M. J., Delamere N. A. (2006). Ouabain induces cell proliferation through calcium-dependent phosphorylation of Akt (protein kinase B) in opossum kidney proximal tubule cells. Am. J. Physiol. Cell Physiol. 291 (6), C1247–C1257. doi:10.1152/ajpcell.00593.2005
Kim S. H., Yu H. S., Park H. G., Jeon W. J., Song J. Y., Kang U. G., et al. (2008). Dose-dependent effect of intracerebroventricular injection of ouabain on the phosphorylation of the MEK1/2-ERK1/2-p90RSK pathway in the rat brain related to locomotor activity. Prog. Neuropsychopharmacol. Biol. Psychiatry 32 (7), 1637–1642. doi:10.1016/j.pnpbp.2008.05.027
Kim S. H., Yu H. S., Park H. G., Ha K., Kim Y. S., Shin S. Y., et al. (2013). Intracerebroventricular administration of ouabain, a Na/K-ATPase inhibitor, activates mTOR signal pathways and protein translation in the rat frontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry 45, 73–82. doi:10.1016/j.pnpbp.2013.04.018
Kinoshita P. F., Yshii L. M., Vasconcelos A. R., Orellana A. M., Lima Lde S., Davel A. P., et al. (2014). Signaling function of Na,K-ATPase induced by ouabain against LPS as an inflammation model in hippocampus. J. Neuroinflammation 11, 218. doi:10.1186/s12974-014-0218-z
Kirshenbaum G. S., Dachtler J., Roder J. C., Clapcote S. J. (2015). Characterization of cognitive deficits in mice with an alternating hemiplegia-linked mutation. Behav. Neurosci. 129 (6), 822–831. doi:10.1037/bne0000097
Klimanova E. A., Petrushanko I. Y., Mitkevich V. A., Anashkina A. A., Orlov S. N., Makarov A. A., et al. (2015). Binding of ouabain and marinobufagenin leads to different structural changes in Na,K-ATPase and depends on the enzyme conformation. FEBS Lett. 589 (19 Pt B), 2668–2674. doi:10.1016/j.febslet.2015.08.011
Kolmakova E. V., Haller S. T., Kennedy D. J., Isachkina A. N., Budny G. V., Frolova E. V., et al. (2011). Endogenous cardiotonic steroids in chronic renal failure. Nephrol. Dial. Transpl. 26 (9), 2912–2919. doi:10.1093/ndt/gfq772
Kometiani P., Li J., Gnudi L., Kahn B. B., Askari A., Xie Z. (1998). Multiple signal transduction pathways link Na+/K+-ATPase to growth-related genes in cardiac myocytes. The roles of Ras and mitogen-activated protein kinases. J. Biol. Chem. 273 (24), 15249–15256. doi:10.1074/jbc.273.24.15249
Komiyama Y., Dong X. H., Nishimura N., Masaki H., Yoshika M., Masuda M., et al. (2005). A novel endogenous digitalis, telocinobufagin, exhibits elevated plasma levels in patients with terminal renal failure. Clin. Biochem. 38 (1), 36–45. doi:10.1016/j.clinbiochem.2004.08.005
Kotova O., Al-Khalili L., Talia S., Hooke C., Fedorova O. V., Bagrov A. Y., et al. (2006a). Cardiotonic steroids stimulate glycogen synthesis in human skeletal muscle cells via a Src- and ERK1/2-dependent mechanism. J. Biol. Chem. 281 (29), 20085–20094. doi:10.1074/jbc.M601577200
Kotova O., Galuska D., Essén-Gustavsson B., Chibalin A. V. (2006b). Metabolic and signaling events mediated by cardiotonic steroid ouabain in rat skeletal muscle. Cell Mol. Biol. (Noisy-le-grand) 52 (8), 48–57.
Kravtsova V. V., Bouzinova E. V., Matchkov V. V., Krivoi I. I. (2020). Skeletal muscle Na,K-ATPase as a target for circulating ouabain. Int. J. Mol. Sci. 21 (8), 2875. doi:10.3390/ijms21082875
Kravtsova V. V., Paramonova I. I., Vilchinskaya N. A., Tishkova M. V., Matchkov V. V., Shenkman B. S., et al. (2021). Chronic ouabain prevents Na,K-ATPase dysfunction and targets AMPK and IL-6 in disused Rat soleus muscle. Int. J. Mol. Sci. 22 (8), 3920. doi:10.3390/ijms22083920
Krep H., Price D. A., Soszynski P., Tao Q. F., Graves S. W., Hollenberg N. K. (1995). Volume sensitive hypertension and the digoxin-like factor. Reversal by a Fab directed against digoxin in DOCA-salt hypertensive rats. Am. J. Hypertens. 8 (9), 921–927. doi:10.1016/0895-7061(95)00181-n
Kurzeder C., Nguyen-Strauli B. D., Krol I., Ring A., Castro-Giner F., Nuesch M., et al. (2025). Digoxin for reduction of circulating tumor cell cluster size in metastatic breast cancer: a proof-of-concept trial. Nat. Med. 31 (4), 1120–1124. doi:10.1038/s41591-024-03486-6
Kutz L. C., Cui X., Xie J. X., Mukherji S. T., Terrell K. C., Huang M., et al. (2021). The Na/K-ATPase α1/Src interaction regulates metabolic reserve and Western diet intolerance. Acta Physiol. (Oxf) 232 (3), e13652. doi:10.1111/apha.13652
Lakunina V. A., Burnysheva K. M., Mitkevich V. A., Makarov A. A., Petrushanko I. Y. (2017). Changes in the receptor function of Na,K-ATPase during hypoxia and ischemia. Mol. Biol. Mosk. 51 (1), 148–154. doi:10.1134/s0026893317010101
Lamaze C., Tardif N., Dewulf M., Vassilopoulos S., Blouin C. M. (2017). The caveolae dress code: structure and signaling. Curr. Opin. Cell Biol. 47, 117–125. doi:10.1016/j.ceb.2017.02.014
Larre I., Lazaro A., Contreras R. G., Balda M. S., Matter K., Flores-Maldonado C., et al. (2010). Ouabain modulates epithelial cell tight junction. Proc. Natl. Acad. Sci. U. S. A. 107 (25), 11387–11392. doi:10.1073/pnas.1000500107
Larre I., Castillo A., Flores-Maldonado C., Contreras R. G., Galvan I., Muñoz-Estrada J., et al. (2011). Ouabain modulates ciliogenesis in epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 108 (51), 20591–20596. doi:10.1073/pnas.1102617108
Larsen B. R., Stoica A., MacAulay N. (2016). Managing brain extracellular K(+) during neuronal activity: the physiological role of the Na(+)/K(+)-ATPase subunit isoforms. Front. Physiol. 7, 141. doi:10.3389/fphys.2016.00141
Laughery M., Todd M., Kaplan J. H. (2004). Oligomerization of the Na,K-ATPase in cell membranes. J. Biol. Chem. 279 (35), 36339–36348. doi:10.1074/jbc.M402778200
Lecuona E., Sun H., Vohwinkel C., Ciechanover A., Sznajder J. I. (2009). Ubiquitination participates in the lysosomal degradation of Na,K-ATPase in steady-state conditions. Am. J. Respir. Cell Mol. Biol. 41 (6), 671–679. doi:10.1165/rcmb.2008-0365OC
Lee Y. K., Ng K. M., Lai W. H., Man C., Lieu D. K., Lau C. P., et al. (2011). Ouabain facilitates cardiac differentiation of mouse embryonic stem cells through ERK1/2 pathway. Acta Pharmacol. Sin. 32 (1), 52–61. doi:10.1038/aps.2010.188
Li Z., Xie Z. (2009). The Na/K-ATPase/Src complex and cardiotonic steroid-activated protein kinase cascades. Pflugers Arch. 457 (3), 635–644. doi:10.1007/s00424-008-0470-0
Li Y., Zhou G. M. (2015). Interneuron regeneration after ouabain treatment in the adult mammalian retina. Neuroreport 26 (12), 712–717. doi:10.1097/wnr.0000000000000414
Li J., Zelenin S., Aperia A., Aizman O. (2006). Low doses of ouabain protect from serum deprivation-triggered apoptosis and stimulate kidney cell proliferation via activation of NF-kappaB. J. Am. Soc. Nephrol. 17 (7), 1848–1857. doi:10.1681/asn.2005080894
Li Z., Cai T., Tian J., Xie J. X., Zhao X., Liu L., et al. (2009). NaKtide, a Na/K-ATPase-derived peptide Src inhibitor, antagonizes ouabain-activated signal transduction in cultured cells. J. Biol. Chem. 284 (31), 21066–21076. doi:10.1074/jbc.M109.013821
Li J., Khodus G. R., Kruusmägi M., Kamali-Zare P., Liu X. L., Eklöf A. C., et al. (2010). Ouabain protects against adverse developmental programming of the kidney. Nat. Commun. 1 (4), 42. doi:10.1038/ncomms1043
Li H., Yin A., Cheng Z., Feng M., Zhang H., Xu J., et al. (2018). Attenuation of Na/K-ATPase/Src/ROS amplification signal pathway with pNaktide ameliorates myocardial ischemia-reperfusion injury. Int. J. Biol. Macromol. 118 (Pt A), 1142–1148. doi:10.1016/j.ijbiomac.2018.07.001
Liang M., Cai T., Tian J., Qu W., Xie Z. J. (2006). Functional characterization of Src-interacting Na/K-ATPase using RNA interference assay. J. Biol. Chem. 281 (28), 19709–19719. doi:10.1074/jbc.M512240200
Liang M., Tian J., Liu L., Pierre S., Liu J., Shapiro J., et al. (2007). Identification of a pool of non-pumping Na/K-ATPase. J. Biol. Chem. 282 (14), 10585–10593. doi:10.1074/jbc.M609181200
Lichtstein D., Samuelov S., Bourrit A. (1985). Characterization of the stimulation of neuronal Na(+), K(+)-ATPase activity by low concentrations of ouabain. Neurochem. Int. 7 (4), 709–715. doi:10.1016/0197-0186(85)90069-5
Lichtstein D., Rosen H., Dvela M. (2012). Cardenolides and bufadienolides as hormones: what is missing? Am. J. Physiol. Ren. Physiol. 302 (8), F957–F958. doi:10.1152/ajprenal.00042.2012
Lichtstein D., Ilani A., Rosen H., Horesh N., Singh S. V., Buzaglo N., et al. (2018). Na+, K+-ATPase signaling and bipolar disorder. Int. J. Mol. Sci. 19 (8), 2314. doi:10.3390/ijms19082314
Lindzen M., Gottschalk K. E., Füzesi M., Garty H., Karlish S. J. (2006). Structural interactions between FXYD proteins and Na+,K+-ATPase: alpha/beta/FXYD subunit stoichiometry and cross-linking. J. Biol. Chem. 281 (9), 5947–5955. doi:10.1074/jbc.M512063200
Lingrel J. B. (1992). Na,K-ATPase: isoform structure, function, and expression. J. Bioenerg. Biomembr. 24 (3), 263–270. doi:10.1007/BF00768847
Lingrel J. B. (2010). The physiological significance of the cardiotonic steroid/ouabain-binding site of the Na,K-ATPase. Annu. Rev. Physiol. 72, 395–412. doi:10.1146/annurev-physiol-021909-135725
Lingrel J. B., Kuntzweiler T. (1994). Na+,K(+)-ATPase. J. Biol. Chem. 269 (31), 19659–19662. doi:10.1016/s0021-9258(17)32067-7
Lingrel J. B., Croyle M. L., Woo A. L., Arguello J. M. (1998). Ligand binding sites of Na,K-ATPase. Acta Physiol. Scand. Suppl. 643, 69–77.
Liu J. (2005). Ouabain-induced endocytosis and signal transduction of the Na/K-ATPase. Front. Biosci. 10, 2056–2063. doi:10.2741/1681
Liu L., Askari A. (2006). On the importance and mechanism of amplification of digitalis signal through Na+/K+-ATPase. Cell Mol. Biol. (Noisy-le-grand) 52 (8), 28–30.
Liu J., Tian J., Haas M., Shapiro J. I., Askari A., Xie Z. (2000). Ouabain interaction with cardiac Na+/K+-ATPase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. J. Biol. Chem. 275 (36), 27838–27844. doi:10.1074/jbc.M002950200
Liu J., Periyasamy S. M., Gunning W., Fedorova O. V., Bagrov A. Y., Malhotra D., et al. (2002). Effects of cardiac glycosides on sodium pump expression and function in LLC-PK1 and MDCK cells. Kidney Int. 62 (6), 2118–2125. doi:10.1046/j.1523-1755.2002.00672.x
Liu L., Mohammadi K., Aynafshar B., Wang H., Li D., Liu J., et al. (2003). Role of caveolae in signal-transducing function of cardiac Na+/K+-ATPase. Am. J. Physiol. Cell Physiol. 284 (6), C1550–C1560. doi:10.1152/ajpcell.00555.2002
Liu J., Kesiry R., Periyasamy S. M., Malhotra D., Xie Z., Shapiro J. I. (2004). Ouabain induces endocytosis of plasmalemmal Na/K-ATPase in LLC-PK1 cells by a clathrin-dependent mechanism. Kidney Int. 66 (1), 227–241. doi:10.1111/j.1523-1755.2004.00723.x
Liu J., Liang M., Liu L., Malhotra D., Xie Z., Shapiro J. I. (2005). Ouabain-induced endocytosis of the plasmalemmal Na/K-ATPase in LLC-PK1 cells requires caveolin-1. Kidney Int. 67 (5), 1844–1854. doi:10.1111/j.1523-1755.2005.00283.x
Liu L., Li J., Liu J., Yuan Z., Pierre S. V., Qu W., et al. (2006). Involvement of Na+/K+-ATPase in hydrogen peroxide-induced hypertrophy in cardiac myocytes. Free Radic. Biol. Med. 41 (10), 1548–1556. doi:10.1016/j.freeradbiomed.2006.08.018
Liu L., Zhao X., Pierre S. V., Askari A. (2007a). Association of PI3K-Akt signaling pathway with digitalis-induced hypertrophy of cardiac myocytes. Am. J. Physiology-Cell Physiology 293 (5), C1489–C1497. doi:10.1152/ajpcell.00158.2007
Liu X. L., Miyakawa A., Aperia A., Krieger P. (2007b). Na,K-ATPase generates calcium oscillations in hippocampal astrocytes. Neuroreport 18 (6), 597–600. doi:10.1097/WNR.0b013e3280b07bc9
Liu X., Špicarová Z., Rydholm S., Li J., Brismar H., Aperia A. (2008). Ankyrin B modulates the function of Na,K-ATPase/Inositol 1,4,5-Trisphosphate receptor signaling microdomain. J. Biol. Chem. 283 (17), 11461–11468. doi:10.1074/jbc.M706942200
Liu L., Ivanov A. V., Gable M. E., Jolivel F., Morrill G. A., Askari A. (2011). Comparative properties of caveolar and noncaveolar preparations of kidney Na+/K+-ATPase. Biochemistry 50 (40), 8664–8673. doi:10.1021/bi2009008
Liu J., Kennedy D. J., Yan Y., Shapiro J. I. (2012). Reactive oxygen species modulation of Na/K-ATPase regulates fibrosis and renal proximal tubular sodium handling. Int. J. Nephrol. 2012, 381320. doi:10.1155/2012/381320
Liu J., Tian J., Chaudhry M., Maxwell K., Yan Y., Wang X., et al. (2016). Attenuation of Na/K-ATPase mediated oxidant amplification with pNaKtide ameliorates experimental uremic cardiomyopathy. Sci. Rep. 6, 34592. doi:10.1038/srep34592
Logan R. W., McClung C. A. (2016). Animal models of bipolar mania: the past, present and future. Neuroscience 321, 163–188. doi:10.1016/j.neuroscience.2015.08.041
Lopachev A. V., Lopacheva O. M., Osipova E. A., Vladychenskaya E. A., Smolyaninova L. V., Fedorova T. N., et al. (2016). Ouabain-induced changes in MAP kinase phosphorylation in primary culture of rat cerebellar cells. Cell Biochem. Funct. 34 (5), 367–377. doi:10.1002/cbf.3199
Lopatin D. A., Ailamazian E.K., Dmitrieva R. I., Shpen V. M., Fedorova O. V., Doris P. A., et al. (1999). Circulating bufodienolide and cardenolide sodium pump inhibitors in preeclampsia. J. Hypertens. 17 (8), 1179–1187. doi:10.1097/00004872-199917080-00018
Lopatina E. V., Kipenko A. V., Pasatetskaya N. A., Penniyaynen V. A., Krylov B. V. (2016). Modulation of the transducer function of Na(+),K(+)-ATPase: new mechanism of heart remodeling. Can. J. Physiol. Pharmacol. 94 (10), 1110–1116. doi:10.1139/cjpp-2015-0577
Lopina O. D. (2000). Na+,K+-ATPase: structure, mechanism, and regulation. Membr. Cell Biol. 13 (6), 721–744.
Lucas T. F., Amaral L. S., Porto C. S., Quintas L. E. (2012). Na+/K+-ATPase α1 isoform mediates ouabain-induced expression of cyclin D1 and proliferation of rat sertoli cells. Reproduction 144 (6), 737–745. doi:10.1530/REP-12-0232
Madan N., Xu Y., Duan Q., Banerjee M., Larre I., Pierre S. V., et al. (2017). Src-independent ERK signaling through the rat α3 isoform of Na/K-ATPase. Am. J. Physiol. Cell Physiol. 312 (3), C222–c232. doi:10.1152/ajpcell.00199.2016
Manfrini V., Poscia R., Messaggio E., Proietti S., Palumbo S., Fiorucci L., et al. (2022). Endogenous ouabain in human and animal models of hypoxia. Aquat. Mamm. 48, 182–194. doi:10.1578/AM.48.2.2022.182
Manfrini V., Badagliacca R., Messaggio E., Poscia R., Torre R., Manunta P., et al. (2023). Exploratory study on the endogenous ouabain in idiopathic pulmonary arterial hypertension patients. Ann. Ist. Super. Sanita 59 (1), 76–79. doi:10.4415/ANN_23_01_11
Manunta P., Messaggio E., Ballabeni C., Sciarrone M. T., Lanzani C., Ferrandi M., et al. (2001). Plasma ouabain-like factor during acute and chronic changes in sodium balance in essential hypertension. Hypertension 38 (2), 198–203. doi:10.1161/01.HYP.38.2.198
Manunta P., Iacoviello M., Forleo C., Messaggio E., Hamlyn J. M., Lucarelli K., et al. (2005). High circulating levels of endogenous ouabain in the offspring of hypertensive and normotensive individuals. J. Hypertens. 23 (9), 1677–1681. doi:10.1097/01.hjh.0000177049.38417.67
Marck P. V., Pierre S. V. (2018). Na/K-ATPase signaling and cardiac pre/postconditioning with cardiotonic steroids. Int. J. Mol. Sci. 19 (8), 2336. doi:10.3390/ijms19082336
Markov A. G., Fedorova A. A., Kravtsova V. V., Bikmurzina A. E., Okorokova L. S., Matchkov V. V., et al. (2020). Circulating ouabain modulates expression of claudins in Rat intestine and cerebral blood vessels. Int. J. Mol. Sci. 21 (14), 5067. doi:10.3390/ijms21145067
Markov A. G., Livanova A. A., Fedorova A. A., Kravtsova V. V., Krivoi I. I. (2023). Chronic ouabain targets pore-forming Claudin-2 and ameliorates radiation-induced damage to the rat intestinal tissue barrier. Int. J. Mol. Sci. 25 (1), 278. doi:10.3390/ijms25010278
Martin D. W. (2005). Structure-function relationships in the NA+,K+-pump. Semin. Nephrol. 25 (5), 282–291. doi:10.1016/j.semnephrol.2005.03.003
Matchkov V. V., Krivoi I. I. (2016). Specialized functional diversity and interactions of the Na,K-ATPase. Front. Physiol. 7, 179. doi:10.3389/fphys.2016.00179
Maxwell K. D., Chuang J., Chaudhry M., Nie Y., Bai F., Sodhi K., et al. (2021). The potential role of Na-K-ATPase and its signaling in the development of anemia in chronic kidney disease. Am. J. Physiol. Ren. Physiol. 320 (2), F234–f242. doi:10.1152/ajprenal.00244.2020
McDonough A. A., Farley R. A. (1993). Regulation of Na,K-ATPase activity. Curr. Opin. Nephrol. Hypertens. 2 (5), 725–734. doi:10.1097/00041552-199309000-00006
McGowan M. H., Russell P., Carper D. A., Lichtstein D. (1999). Na+, K+-ATPase inhibitors down-regulate gene expression of the intracellular signaling protein 14-3-3 in rat lens. J. Pharmacol. Exp. Ther. 289 (3), 1559–1563. doi:10.1016/s0022-3565(24)38306-5
McKenna M. J., Renaud J. M., Ortenblad N., Overgaard K. (2024). A century of exercise physiology: effects of muscle contraction and exercise on skeletal muscle Na(+),K(+)-ATPase, Na(+) and K(+) ions, and on plasma K(+) concentration-historical developments. Eur. J. Appl. Physiol. 124 (3), 681–751. doi:10.1007/s00421-023-05335-9
Meijler F. L. (1985). An “account” of digitalis and atrial fibrillation. J. Am. Coll. Cardiol. 5 (5 Suppl. A), 60a–68a. doi:10.1016/s0735-1097(85)80464-2
Mijatovic T., Kiss R. (2013). Cardiotonic steroids-mediated Na+/K+-ATPase targeting could circumvent various chemoresistance pathways. Planta Med. 79 (3-4), 189–198. doi:10.1055/s-0032-1328243
Mijatovic T., Dufrasne F., Kiss R. (2012). Na+/K+-ATPase and cancer. Pharm. Pat. Anal. 1 (1), 91–106. doi:10.4155/ppa.12.3
Miyakawa-Naito A., Uhlén P., Lal M., Aizman O., Mikoshiba K., Brismar H., et al. (2003). Cell signaling microdomain with Na,K-ATPase and inositol 1,4,5-trisphosphate receptor generates calcium oscillations. J. Biol. Chem. 278 (50), 50355–50361. doi:10.1074/jbc.M305378200
Mobasheri A., Avila J., Cózar-Castellano I., Brownleader M. D., Trevan M., Francis M. J., et al. (2000). Na+, K+-ATPase isozyme diversity; comparative biochemistry and physiological implications of novel functional interactions. Biosci. Rep. 20 (2), 51–91. doi:10.1023/a:1005580332144
Molday L. L., Wu W. W., Molday R. S. (2007). Retinoschisin (RS1), the protein encoded by the X-linked retinoschisis gene, is anchored to the surface of retinal photoreceptor and bipolar cells through its interactions with a Na/K ATPase-SARM1 complex. J. Biol. Chem. 282 (45), 32792–32801. doi:10.1074/jbc.M706321200
Morris J. F., Poston L., Wolfe C. D., Hilton P. J. (1988). A comparison of endogenous digoxin-like immunoreactivity and sodium transport inhibitory activity in umbilical arterial and venous serum. Clin. Sci. (Lond) 75 (6), 577–579. doi:10.1042/cs0750577
Moseley A. E., Lieske S. P., Wetzel R. K., James P. F., He S., Shelly D. A., et al. (2003). The Na,K-ATPase alpha 2 isoform is expressed in neurons, and its absence disrupts neuronal activity in newborn mice. J. Biol. Chem. 278 (7), 5317–5324. doi:10.1074/jbc.M211315200
Mottelson M. N., Lundsgaard C. C., Møller S. (2020). Mechanisms in fluid retention - towards a mutual concept. Clin. Physiol. Funct. Imaging 40 (2), 67–75. doi:10.1111/cpf.12615
Müller-Husmann G., Gloor S., Schachner M. (1993). Functional characterization of beta isoforms of murine Na,K-ATPase. The adhesion molecule on glia (AMOG/beta 2), but not beta 1, promotes neurite outgrowth. J. Biol. Chem. 268 (35), 26260–26267. doi:10.1016/s0021-9258(19)74309-9
Murata Y., Matsuda T., Tamada K., Hosoi R., Asano S., Takuma K., et al. (1996). Ouabain-induced cell proliferation in cultured rat astrocytes. Jpn. J. Pharmacol. 72 (4), 347–353. doi:10.1254/jjp.72.347
Nascimento C. R., Valente R. C., Echevarria-Lima J., Fontes C. F., de Araujo-Martins L., Araujo E. G., et al. (2014). The influence of Ouabain on human dendritic cells maturation. Mediat. Inflamm. 2014, 494956. doi:10.1155/2014/494956
Nepal N., Arthur S., Haynes J., Palaniappan B., Sundaram U. (2021). Mechanism of Na-K-ATPase inhibition by PGE2 in intestinal epithelial cells. Cells 10 (4), 752. doi:10.3390/cells10040752
Nesher M., Dvela M., Igbokwe V. U., Rosen H., Lichtstein D. (2009). Physiological roles of endogenous ouabain in normal rats. Am. J. Physiology-Heart Circulatory Physiology 297 (6), H2026–H2034. doi:10.1152/ajpheart.00734.2009
Nguyen A. N., Wallace D. P., Blanco G. (2007). Ouabain binds with high affinity to the Na,K-ATPase in human polycystic kidney cells and induces extracellular signal-regulated kinase activation and cell proliferation. J. Am. Soc. Nephrol. 18 (1), 46–57. doi:10.1681/ASN.2006010086
Nguyen A. N., Jansson K., Sanchez G., Sharma M., Reif G. A., Wallace D. P., et al. (2011). Ouabain activates the Na-K-ATPase signalosome to induce autosomal dominant polycystic kidney disease cell proliferation. Am. J. Physiol. Ren. Physiol. 301 (4), F897–F906. doi:10.1152/ajprenal.00095.2011
Nie Y., Bai F., Chaudhry M. A., Pratt R., Shapiro J. I., Liu J. (2020). The Na/K-ATPase α1 and c-Src form signaling complex under native condition: a crosslinking approach. Sci. Rep. 10 (1), 6006. doi:10.1038/s41598-020-61920-4
Numata Y., Fujii T., Toda C., Okumura T., Manabe T., Takeda N., et al. (2025). Digoxin promotes anoikis of circulating cancer cells by targeting Na+/K+-ATPase α3-isoform. Cell Death Dis. 16 (1), 373. doi:10.1038/s41419-025-07703-z
Olej B., dos Santos N. F., Leal L., Rumjanek V. M. (1998). Ouabain induces apoptosis on PHA-activated lymphocytes. Biosci. Rep. 18 (1), 1–7. doi:10.1023/a:1022259832207
Orellana A. M., Leite J. A., Kinoshita P. F., Vasconcelos A. R., Andreotti D. Z., de Sa Lima L., et al. (2018). Ouabain increases neuronal branching in hippocampus and improves spatial memory. Neuropharmacology 140, 260–274. doi:10.1016/j.neuropharm.2018.08.008
Orlov S. N., Tverskoi A. M., Sidorenko S. V., Smolyaninova L. V., Lopina O. D., Dulin N. O., et al. (2021). Na,K-ATPase as a target for endogenous cardiotonic steroids: what's the evidence? Genes Dis. 8 (3), 259–271. doi:10.1016/j.gendis.2020.01.008
Orlowski J., Lingrel J. B. (1990). Thyroid and glucocorticoid hormones regulate the expression of multiple Na,K-ATPase genes in cultured neonatal rat cardiac myocytes. J. Biol. Chem. 265 (6), 3462–3470. doi:10.1016/s0021-9258(19)39790-x
Oselkin M., Tian D., Bergold P. J. (2010). Low-dose cardiotonic steroids increase sodium-potassium ATPase activity that protects hippocampal slice cultures from experimental ischemia. Neurosci. Lett. 473 (2), 67–71. doi:10.1016/j.neulet.2009.10.021
Osman M. H., Farrag E., Selim M., Osman M. S., Hasanine A., Selim A. (2017). Cardiac glycosides use and the risk and mortality of cancer; systematic review and meta-analysis of observational studies. PLoS One 12 (6), e0178611. doi:10.1371/journal.pone.0178611
Parkash J., Asotra K. (2012). Calcium oscillations and waves in cells. Adv. Exp. Med. Biol. 740, 521–529. doi:10.1007/978-94-007-2888-2_23
Parton R. G. (2018). Caveolae: structure, function, and relationship to disease. Annu. Rev. Cell Dev. Biol. 34, 111–136. doi:10.1146/annurev-cellbio-100617-062737
Parton R. G., Tillu V. A., Collins B. M. (2018). Caveolae. Curr. Biol. 28 (8), R402–R405. doi:10.1016/j.cub.2017.11.075
Patel H. H., Murray F., Insel P. A. (2008). Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu. Rev. Pharmacol. Toxicol. 48, 359–391. doi:10.1146/annurev.pharmtox.48.121506.124841
Patocka J., Nepovimova E., Wu W., Kuca K. (2020). Digoxin: pharmacology and toxicology-A review. Environ. Toxicol. Pharmacol. 79, 103400. doi:10.1016/j.etap.2020.103400
Pawlik W., Shepherd A. P., Mailman D., Jacobson E. D. (1974). Effects of ouabain on intestinal oxygen consumption. Gastroenterology 67 (1), 100–106. doi:10.1016/s0016-5085(19)32930-0
Peng M., Huang L., Xie Z., Huang W.-H., Askari A. (1996). Partial inhibition of Na+/K+-ATPase by ouabain induces the Ca2+-dependent expressions of early-response genes in cardiac myocytes. J. Biol. Chem. 271 (17), 10372–10378. doi:10.1074/jbc.271.17.10372
Penniyaynen V. A., Kipenko A. V., Lopatina E. V., Bagrov A. Y., Krylov B. V. (2015). The effect of marinobufagenin on the growth and proliferation of cells in the organotypic culture. Dokl. Biol. Sci. 462, 164–166. doi:10.1134/S0012496615030096
Petrushanko I. Y., Yakushev S., Mitkevich V. A., Kamanina Y. V., Ziganshin R. H., Meng X., et al. (2012). S-glutathionylation of the Na,K-ATPase catalytic alpha subunit is a determinant of the enzyme redox sensitivity. J. Biol. Chem. 287 (38), 32195–32205. doi:10.1074/jbc.M112.391094
Petrushanko I. Y., Mitkevich V. A., Lakunina V. A., Anashkina A. A., Spirin P. V., Rubtsov P. M., et al. (2017). Cysteine residues 244 and 458-459 within the catalytic subunit of Na,K-ATPase control the enzyme’s hydrolytic and signaling function under hypoxic conditions. Redox Biol. 13, 310–319. doi:10.1016/j.redox.2017.05.021
Petrushanko I. Y., Tverskoi A. M., Barykin E. P., Petrovskaya A. V., Strelkova M. A., Leonova O. G., et al. (2022). Na,K-ATPase acts as a beta-amyloid receptor triggering src kinase activation. Cells 11 (17), 2753. doi:10.3390/cells11172753
Pierre S. V., Xie Z. (2006). The Na,K-ATPase receptor complex: its organization and membership. Cell Biochem. Biophys. 46 (3), 303–316. doi:10.1385/cbb:46:3:303
Pierre S. V., Sottejeau Y., Gourbeau J. M., Sanchez G., Shidyak A., Blanco G. (2008). Isoform specificity of Na-K-ATPase-mediated ouabain signaling. Am. J. Physiol. Ren. Physiol. 294 (4), F859–F866. doi:10.1152/ajprenal.00089.2007
Pirkmajer S., Bezjak K., Matkovic U., Dolinar K., Jiang L. Q., Mis K., et al. (2020). Ouabain suppresses IL-6/STAT3 signaling and promotes cytokine secretion in cultured skeletal muscle cells. Front. Physiol. 11, 566584. doi:10.3389/fphys.2020.566584
Plössl K., Royer M., Bernklau S., Tavraz N. N., Friedrich T., Wild J., et al. (2017a). Retinoschisin is linked to retinal Na/K-ATPase signaling and localization. Mol. Biol. Cell 28 (16), 2178–2189. doi:10.1091/mbc.E17-01-0064
Plössl K., Weber B. H., Friedrich U. (2017b). The X-linked juvenile retinoschisis protein retinoschisin is a novel regulator of mitogen-activated protein kinase signalling and apoptosis in the retina. J. Cell Mol. Med. 21 (4), 768–780. doi:10.1111/jcmm.13019
Plourde D., Soltoff S. P. (2006). Ouabain potentiates the activation of ERK1/2 by carbachol inparotid gland epithelial cells; inhibition of ERK1/2 reduces Na(+)-K(+)-ATPase activity. Am. J. Physiol. Cell Physiol. 290 (3), C702–C710. doi:10.1152/ajpcell.00213.2005
Post R. L., Jolly P. C. (1957). The linkage of sodium, potassium, and ammonium active transport across the human erythrocyte membrane. Biochim. Biophys. Acta 25 (1), 118–128. doi:10.1016/0006-3002(57)90426-2
Poulsen H., Morth P., Egebjerg J., Nissen P. (2010). Phosphorylation of the Na+,K+-ATPase and the H+,K+-ATPase. FEBS Lett. 584 (12), 2589–2595. doi:10.1016/j.febslet.2010.04.035
Pratt R. D., Brickman C. R., Cottrill C. L., Shapiro J. I., Liu J. (2018). The Na/K-ATPase signaling: from specific ligands to general reactive oxygen species. Int. J. Mol. Sci. 19 (9), 2600. doi:10.3390/ijms19092600
Pressley T. A. (1988). Ion concentration-dependent regulation of Na,K-pump abundance. J. Membr. Biol. 105 (3), 187–195. doi:10.1007/BF01870996
Pulina M. V., Zulian A., Berra-Romani R., Beskina O., Mazzocco-Spezzia A., Baryshnikov S. G., et al. (2010). Upregulation of Na+ and Ca2+ transporters in arterial smooth muscle from ouabain-induced hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 298 (1), H263–H274. doi:10.1152/ajpheart.00784.2009
Quintas L. E., Pierre S. V., Liu L., Bai Y., Liu X., Xie Z. J. (2010). Alterations of Na+/K+-ATPase function in caveolin-1 knockout cardiac fibroblasts. J. Mol. Cell Cardiol. 49 (3), 525–531. doi:10.1016/j.yjmcc.2010.04.015
Rajamanickam G. D., Kastelic J. P., Thundathil J. C. (2017). Na/K-ATPase regulates bovine sperm capacitation through raft- and non-raft-mediated signaling mechanisms. Mol. Reprod. Dev. 84 (11), 1168–1182. doi:10.1002/mrd.22879
Rajasekaran S. A., Gopal J., Willis D., Espineda C., Twiss J. L., Rajasekaran A. K. (2004). Na,K-ATPase beta1-subunit increases the translation efficiency of the alpha1-subunit in MSV-MDCK cells. Mol. Biol. Cell 15 (7), 3224–3232. doi:10.1091/mbc.e04-03-0222
Reznik V. A., Kashkin V. A., Agalakova N. I., Adair C. D., Bagrov A. Y. (2019). Endogenous bufadienolides, fibrosis and preeclampsia. Cardiol. Res. Pract. 2019, 5019287. doi:10.1155/2019/5019287
Ridge K. M., Olivera W. G., Saldias F., Azzam Z., Horowitz S., Rutschman D. H., et al. (2003). Alveolar type 1 cells express the alpha2 Na,K-ATPase, which contributes to lung liquid clearance. Circ. Res. 92 (4), 453–460. doi:10.1161/01.RES.0000059414.10360.F2
Riegel R. E., Valvassori S. S., Elias G., Réus G. Z., Steckert A. V., de Souza B., et al. (2009). Animal model of mania induced by ouabain: evidence of oxidative stress in submitochondrial particles of the rat brain. Neurochem. Int. 55 (7), 491–495. doi:10.1016/j.neuint.2009.05.003
Riganti C., Campia I., Kopecka J., Gazzano E., Doublier S., Aldieri E., et al. (2011). Pleiotropic effects of cardioactive glycosides. Curr. Med. Chem. 18 (6), 872–885. doi:10.2174/092986711794927685
Rocha S. C., Pessoa M. T., Neves L. D., Alves S. L., Silva L. M., Santos H. L., et al. (2014). 21-Benzylidene digoxin: a proapoptotic cardenolide of cancer cells that up-regulates Na,K-ATPase and epithelial tight junctions. PLoS One 9 (10), e108776. doi:10.1371/journal.pone.0108776
Rodrigues-Mascarenhas S., Da Silva de Oliveira A., Amoedo N. D., Affonso-Mitidieri O. R., Rumjanek F. D., Rumjanek V. M. (2009). Modulation of the immune system by ouabain. Ann. N. Y. Acad. Sci. 1153, 153–163. doi:10.1111/j.1749-6632.2008.03969.x
Salles von-Held-Ventura J., Mázala-de-Oliveira T., Cândida da Rocha Oliveira A., Granja M. G., Gonçalves-de-Albuquerque C. F., Castro-Faria-Neto H. C., et al. (2016). The trophic effect of ouabain on retinal ganglion cells is mediated by IL-1β and TNF-α. Biochem. Biophys. Res. Commun. 478 (1), 378–384. doi:10.1016/j.bbrc.2016.07.043
Sayed M., Drummond C. A., Evans K. L., Haller S. T., Liu J., Xie Z., et al. (2014). Effects of Na/K-ATPase and its ligands on bone marrow stromal cell differentiation. Stem Cell Res. 13 (1), 12–23. doi:10.1016/j.scr.2014.04.002
Scheiner-Bobis G., Eva A., Kirch U. (2006). Signalling pathways involving sodium pump stimulate endothelin-1 secretion and nitric oxide production in endothelial cells. Cell Mol. Biol. (Noisy-le-grand) 52 (8), 58–63.
Schmid V., Plössl K., Schmid C., Bernklau S., Weber B. H. F., Friedrich U. (2020). Retinoschisin and cardiac glycoside crosstalk at the retinal Na/K-ATPase. Invest. Ophthalmol. Vis. Sci. 61 (5), 1. doi:10.1167/iovs.61.5.1
Schoner W. (2002). Endogenous cardiac glycosides, a new class of steroid hormones. Eur. J. Biochem. 269 (10), 2440–2448. doi:10.1046/j.1432-1033.2002.02911.x
Schoner W., Scheiner-Bobis G. (2007a). Endogenous and exogenous cardiac glycosides and their mechanisms of action. Am. J. Cardiovasc Drugs 7 (3), 173–189. doi:10.2165/00129784-200707030-00004
Schoner W., Scheiner-Bobis G. (2007b). Endogenous and exogenous cardiac glycosides: their roles in hypertension, salt metabolism, and cell growth. Am. J. Physiol. Cell Physiol. 293 (2), C509–C536. doi:10.1152/ajpcell.00098.2007
Schultheis P. J., Wallick E. T., Lingrel J. B. (1993). Kinetic analysis of ouabain binding to native and mutated forms of Na,K-ATPase and identification of a new region involved in cardiac glycoside interactions. J. Biol. Chem. 268 (30), 22686–22694. doi:10.1016/s0021-9258(18)41582-7
Serhan M. F., Kreydiyyeh S. I. (2011). Insulin targets the Na(+)/K(+) ATPase in enterocytes via PI3K, PKC, and MAPKS. J. Recept. Signal. Transduct. Res. 31 (4), 299–306. doi:10.3109/10799893.2011.587821
Sethi N. J., Nielsen E. E., Safi S., Feinberg J., Gluud C., Jakobsen J. C. (2018). Digoxin for atrial fibrillation and atrial flutter: a systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. PLoS One 13 (3), e0193924. doi:10.1371/journal.pone.0193924
Shelly D. A., He S., Moseley A., Weber C., Stegemeyer M., Lynch R. M., et al. (2004). Na(+) pump alpha 2-isoform specifically couples to contractility in vascular smooth muscle: evidence from gene-targeted neonatal mice. Am. J. Physiol. Cell Physiol. 286 (4), C813–C820. doi:10.1152/ajpcell.00389.2003
Silva E., Soares-da-Silva P. (2012). New insights into the regulation of Na+,K+-ATPase by ouabain. Int. Rev. Cell Mol. Biol. 294, 99–132. doi:10.1016/B978-0-12-394305-7.00002-1
Silva C. I. D., Gonçalves-de-Albuquerque C. F., Moraes B. P. T., Garcia D. G., Burth P. (2021). Na/K-ATPase: their role in cell adhesion and migration in cancer. Biochimie 185, 1–8. doi:10.1016/j.biochi.2021.03.002
Silva A. R., de Souza E. S. K. F. C., Souza T. B., Younes-Ibrahim M., Burth P., de Castro Faria Neto H. C., et al. (2023). The Na/K-ATPase role as a signal transducer in lung inflammation. Front. Immunol. 14, 1287512. doi:10.3389/fimmu.2023.1287512
Skou J. C. (1957). The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochimica Biophysica Acta 23, 394–401. doi:10.1016/0006-3002(57)90343-8
Slingerland M., Cerella C., Guchelaar H. J., Diederich M., Gelderblom H. (2013). Cardiac glycosides in cancer therapy: from preclinical investigations towards clinical trials. Invest. New Drugs 31 (4), 1087–1094. doi:10.1007/s10637-013-9984-1
Smedler E., Uhlen P. (2014). Frequency decoding of calcium oscillations. Biochim. Biophys. Acta 1840 (3), 964–969. doi:10.1016/j.bbagen.2013.11.015
Smolyaninova L. V., Shiyan A. A., Kapilevich L. V., Lopachev A. V., Fedorova T. N., Klementieva T. S., et al. (2019). Transcriptomic changes triggered by ouabain in rat cerebellum granule cells: role of α3-and α1-Na+,K+-ATPase-mediated signaling. PLoS One 14 (9), e0222767. doi:10.1371/journal.pone.0222767
Sodhi K., Maxwell K., Yan Y., Liu J., Chaudhry M. A., Xie Z., et al. (2023). pNaKtide inhibits Na/K-ATPase signaling and attenuates obesity. J. Clin. Med. Sci. 7 (4), 1000238.
Srikanthan K., Shapiro J. I., Sodhi K. (2016). The role of Na/K-ATPase signaling in oxidative stress related to obesity and cardiovascular disease. Molecules 21 (9), 1172. doi:10.3390/molecules21091172
Strauss M., Smith W., Fedorova O. V., Schutte A. E. (2019). The Na(+)K(+)-ATPase inhibitor Marinobufagenin and early cardiovascular risk in humans: a review of recent evidence. Curr. Hypertens. Rep. 21 (5), 38. doi:10.1007/s11906-019-0942-y
Sui L., Song X. J., Ren J., Ju L. H., Wang Y. (2013). Intracerebroventricular administration of ouabain alters synaptic plasticity and dopamine release in rat medial prefrontal cortex. J. Neural Transm. (Vienna) 120 (8), 1191–1199. doi:10.1007/s00702-013-0973-5
Sweadner K. J. (1989). Isozymes of the Na+/K+-ATPase. Biochim. Biophys. Acta 988 (2), 185–220. doi:10.1016/0304-4157(89)90019-1
Sweadner K. J., Arystarkhova E., Donnet C., Wetzel R. K. (2003). FXYD proteins as regulators of the Na,K-ATPase in the kidney. Ann. N. Y. Acad. Sci. 986, 382–387. doi:10.1111/j.1749-6632.2003.tb07218.x
Swift F., Tovsrud N., Enger U. H., Sjaastad I., Sejersted O. M. (2007). The Na+/K+-ATPase alpha2-isoform regulates cardiac contractility in rat cardiomyocytes. Cardiovasc Res. 75 (1), 109–117. doi:10.1016/j.cardiores.2007.03.017
Takeyasu K., Okamura H., Yasuhara J. C., Ogita Y., Yoshimura S. H. (2001). P-type ATPase diversity and evolution: the origins of ouabain sensitivity and subunit assembly. Cell Mol. Biol. (Noisy-le-grand) 47 (2), 325–333.
Taniguchi K., Kaya S., Abe K., Mardh S. (2001). The oligomeric nature of Na/K-transport ATPase. J. Biochem. 129 (3), 335–342. doi:10.1093/oxfordjournals.jbchem.a002862
Teixeira M. P., Rumjanek V. M. (2014). Ouabain affects the expression of activation markers, cytokine production, and endocytosis of human monocytes. Mediat. Inflamm. 2014, 760368. doi:10.1155/2014/760368
Therien A. G., Blostein R. (2000). Mechanisms of sodium pump regulation. Am. J. Physiol. Cell Physiol. 279 (3), C541–C566. doi:10.1152/ajpcell.2000.279.3.C541
Thundathil J. C., Anzar M., Buhr M. M. (2006). Na+/K+ATPase as a signaling molecule during bovine sperm capacitation. Biol. Reproduction 75 (3), 308–317. doi:10.1095/biolreprod.105.047852
Tian J., Xie Z. J. (2008). The Na-K-ATPase and calcium-signaling microdomains. Physiol. (Bethesda) 23, 205–211. doi:10.1152/physiol.00008.2008
Tian J., Cai T., Yuan Z., Wang H., Liu L., Haas M., et al. (2006). Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol. Biol. Cell 17 (1), 317–326. doi:10.1091/mbc.e05-08-0735
Tochigi M., Iwamoto K., Bundo M., Sasaki T., Kato N., Kato T. (2008). Gene expression profiling of major depression and suicide in the prefrontal cortex of postmortem brains. Neurosci. Res. 60 (2), 184–191. doi:10.1016/j.neures.2007.10.010
Tokhtaeva E., Sachs G., Souda P., Bassilian S., Whitelegge J. P., Shoshani L., et al. (2011). Epithelial junctions depend on intercellular trans-interactions between the Na,K-ATPase β1 subunits. J. Biol. Chem. 286 (29), 25801–25812. doi:10.1074/jbc.M111.252247
Trant J., Sanchez G., McDermott J. P., Blanco G. (2023). Ouabain enhances renal cyst growth in a slowly progressive mouse model of autosomal dominant polycystic kidney disease. Am. J. Physiology-Renal Physiology 325 (6), F857–F869. doi:10.1152/ajprenal.00056.2023
Trant J., Sanchez G., McDermott J. P., Blanco G. (2025). The cystogenic effects of ouabain in autosomal dominant polycystic kidney disease require cell caveolae. Exp. Cell Res. 444 (1), 114356. doi:10.1016/j.yexcr.2024.114356
Tverskoi A. M., Sidorenko S. V., Klimanova E. A., Akimova O. A., Smolyaninova L. V., Lopina O. D., et al. (2016). Effects of ouabain on proliferation of human endothelial cells correlate with Na+,K+-ATPase activity and intracellular ratio of Na+ and K. Biochem. (Mosc) 81 (8), 876–883. doi:10.1134/S0006297916080083
Uddin M. N., Horvat D., Glaser S. S., Danchuk S., Mitchell B. M., Sullivan D. E., et al. (2008). Marinobufagenin inhibits proliferation and migration of cytotrophoblast and CHO cells. Placenta 29 (3), 266–273. doi:10.1016/j.placenta.2007.12.009
Ueno S., Takeda K., Noguchi S., Kawamura M. (1997). Significance of the beta-subunit in the biogenesis of Na+/K(+)-ATPase. Biosci. Rep. 17 (2), 173–188. doi:10.1023/a:1027333529412
Upmanyu N., Dietze R., Kirch U., Scheiner-Bobis G. (2016). Ouabain interactions with the α4 isoform of the sodium pump trigger non-classical steroid hormone signaling and integrin expression in spermatogenic cells. Biochim. Biophys. Acta 1863 (11), 2809–2819. doi:10.1016/j.bbamcr.2016.09.001
Upmanyu N., Dietze R., Bulldan A., Scheiner-Bobis G. (2019). Cardiotonic steroid ouabain stimulates steroidogenesis in Leydig cells via the α3 isoform of the sodium pump. J. Steroid Biochem. Mol. Biol. 191, 105372. doi:10.1016/j.jsbmb.2019.04.021
Vagin O., Sachs G., Tokhtaeva E. (2007). The roles of the Na,K-ATPase beta 1 subunit in pump sorting and epithelial integrity. J. Bioenerg. Biomembr. 39 (5-6), 367–372. doi:10.1007/s10863-007-9103-0
Vagin O., Dada L. A., Tokhtaeva E., Sachs G. (2012). The Na-K-ATPase α1β1 heterodimer as a cell adhesion molecule in epithelia. Am. J. Physiol. Cell Physiol. 302 (9), C1271–C1281. doi:10.1152/ajpcell.00456.2011
Varela R. B., Valvassori S. S., Lopes-Borges J., Mariot E., Dal-Pont G. C., Amboni R. T., et al. (2015). Sodium butyrate and mood stabilizers block ouabain-induced hyperlocomotion and increase BDNF, NGF and GDNF levels in brain of Wistar rats. J. Psychiatr. Res. 61, 114–121. doi:10.1016/j.jpsychires.2014.11.003
Venugopal J., Blanco G. (2016). Ouabain enhances ADPKD cell apoptosis via the intrinsic pathway. Front. Physiol. 7, 107. doi:10.3389/fphys.2016.00107
Venugopal J., McDermott J., Sanchez G., Sharma M., Barbosa L., Reif G. A., et al. (2017). Ouabain promotes partial epithelial to mesenchymal transition (EMT) changes in human autosomal dominant polycystic kidney disease (ADPKD) cells. Exp. Cell Res. 355 (2), 142–152. doi:10.1016/j.yexcr.2017.04.001
Verdejo-Torres O., Flores-Maldonado C., Padilla-Benavides T., Campos-Blazquez J. P., Larre I., Lara-Lemus R., et al. (2019). Ouabain accelerates collective cell migration through a cSrc and ERK1/2 sensitive metalloproteinase activity. J. Membr. Biol. 252 (6), 549–559. doi:10.1007/s00232-019-00066-5
Wachtel-Galor S., Benzie I. F. F. (2011). “Herbal medicine: an introduction to its history, usage, regulation, current trends, and research needs,” in Herbal medicine: biomolecular and clinical aspects (Boca Raton, FL: CRC Press/Taylor & Francis).
Wagoner K., Sanchez G., Nguyen A. N., Enders G. C., Blanco G. (2005). Different expression and activity of the alpha1 and alpha4 isoforms of the Na,K-ATPase during rat male germ cell ontogeny. Reproduction 130 (5), 627–641. doi:10.1530/rep.1.00806
Wang H., Haas M., Liang M., Cai T., Tian J., Li S., et al. (2004). Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J. Biol. Chem. 279 (17), 17250–17259. doi:10.1074/jbc.M313239200
Wang Y., Ye Q., Liu C., Xie J. X., Yan Y., Lai F., et al. (2014). Involvement of Na/K-ATPase in hydrogen peroxide-induced activation of the Src/ERK pathway in LLC-PK1 cells. Free Radic. Biol. Med. 71, 415–426. doi:10.1016/j.freeradbiomed.2014.03.036
Wang J., Ullah S. H., Li M., Zhang M., Zhang F., Zheng J., et al. (2019). DR region specific antibody ameliorated but ouabain worsened renal injury in nephrectomized rats through regulating Na,K-ATPase mediated signaling pathways. Aging (Albany NY) 11 (4), 1151–1162. doi:10.18632/aging.101815
Weidemann H. (2005). Na/K-ATPase, endogenous digitalis like compounds and cancer development -- a hypothesis. Front. Biosci. 10, 2165–2176. doi:10.2741/1688
Weigand K. M., Swarts H. G., Fedosova N. U., Russel F. G., Koenderink J. B. (2012). Na,K-ATPase activity modulates Src activation: a role for ATP/ADP ratio. Biochim. Biophys. Acta 1818 (5), 1269–1273. doi:10.1016/j.bbamem.2012.01.015
Welch L. C., Lecuona E., Briva A., Trejo H. E., Dada L. A., Sznajder J. I. (2010). Extracellular signal-regulated kinase (ERK) participates in the hypercapnia-induced Na,K-ATPase downregulation. FEBS Lett. 584 (18), 3985–3989. doi:10.1016/j.febslet.2010.08.002
Whayne T. F. (2018). Clinical use of digitalis: a state of the art review. Am. J. Cardiovasc Drugs 18 (6), 427–440. doi:10.1007/s40256-018-0292-1
Winnicka K., Bielawski K., Bielawska A. (2006). Cardiac glycosides in cancer research and cancer therapy. Acta Pol. Pharm. 63 (2), 109–115.
Winnicka K., Bielawski K., Bielawska A., Miltyk W. (2010). Dual effects of ouabain, digoxin and proscillaridin A on the regulation of apoptosis in human fibroblasts. Nat. Prod. Res. 24 (3), 274–285. doi:10.1080/14786410902991878
Woo A. L., James P. F., Lingrel J. B. (1999). Characterization of the fourth alpha isoform of the Na,K-ATPase. J. Membr. Biol. 169 (1), 39–44. doi:10.1007/pl00005899
Wu J., Akkuratov E. E., Bai Y., Gaskill C. M., Askari A., Liu L. (2013). Cell signaling associated with Na(+)/K(+)-ATPase: activation of phosphatidylinositide 3-kinase IA/Akt by ouabain is independent of Src. Biochemistry 52 (50), 9059–9067. doi:10.1021/bi4011804
Xie Z. (2001). Ouabain interaction with cardiac Na/K-ATPase reveals that the enzyme can act as a pump and as a signal transducer. Cell Mol. Biol. (Noisy-le-grand) 47 (2), 383–390.
Xie Z., Askari A. (2002). Na(+)/K(+)-ATPase as a signal transducer. Eur. J. Biochem. 269 (10), 2434–2439. doi:10.1046/j.1432-1033.2002.02910.x
Xie Z., Kometiani P., Liu J., Li J., Shapiro J. I., Askari A. (1999). Intracellular reactive oxygen species mediate the linkage of Na+/K+-ATPase to hypertrophy and its marker genes in cardiac myocytes. J. Biol. Chem. 274 (27), 19323–19328. doi:10.1074/jbc.274.27.19323
Xie J., Ye Q., Cui X., Madan N., Yi Q., Pierre S. V., et al. (2015). Expression of rat Na-K-ATPase α2 enables ion pumping but not ouabain-induced signaling in α1-deficient porcine renal epithelial cells. Am. J. Physiol. Cell Physiol. 309 (6), C373–C382. doi:10.1152/ajpcell.00103.2015
Yamada K., Goto A., Nagoshi H., Hui C., Yagi N., Sasabe M., et al. (1994). Participation of ouabainlike compound in reduced renal mass-saline hypertension. Hypertension 23 (1 Suppl. l), I110–I113. doi:10.1161/01.hyp.23.1_suppl.i110
Yan Y., Shapiro A. P., Haller S., Katragadda V., Liu L., Tian J., et al. (2013). Involvement of reactive oxygen species in a feed-forward mechanism of Na/K-ATPase-mediated signaling transduction. J. Biol. Chem. 288 (47), 34249–34258. doi:10.1074/jbc.M113.461020
Yang Y., Dong X., Zheng S., Sun J., Ye J., Chen J., et al. (2020). GSTpi regulates VE-cadherin stabilization through promoting S-glutathionylation of Src. Redox Biol. 30, 101416. doi:10.1016/j.redox.2019.101416
Yap J. Q., Seflova J., Sweazey R., Artigas P., Robia S. L. (2021). FXYD proteins and sodium pump regulatory mechanisms. J. Gen. Physiol. 153 (4), e202012633. doi:10.1085/jgp.202012633
Ye Q., Li Z., Tian J., Xie J. X., Liu L., Xie Z. (2011). Identification of a potential receptor that couples ion transport to protein kinase activity. J. Biol. Chem. 286 (8), 6225–6232. doi:10.1074/jbc.M110.202051
Yin P. H., Liu X., Qiu Y. Y., Cai J. F., Qin J. M., Zhu H. R., et al. (2012). Anti-tumor activity and apoptosis-regulation mechanisms of bufalin in various cancers: new hope for cancer patients. Asian Pac J. Cancer Prev. 13 (11), 5339–5343. doi:10.7314/apjcp.2012.13.11.5339
Yoneda J. S., Scanavachi G., Sebinelli H. G., Borges J. C., Barbosa L. R., Ciancaglini P., et al. (2016). Multimeric species in equilibrium in detergent-solubilized Na,K-ATPase. Int. J. Biol. Macromol. 89, 238–245. doi:10.1016/j.ijbiomac.2016.04.058
Yosef E., Katz A., Peleg Y., Mehlman T., Karlish S. J. (2016). Do src kinase and Caveolin interact directly with Na,K-ATPase? J. Biol. Chem. 291 (22), 11736–11750. doi:10.1074/jbc.M116.721084
Yu S. P. (2003). Na(+), K(+)-ATPase: the new face of an old player in pathogenesis and apoptotic/hybrid cell death. Biochem. Pharmacol. 66 (8), 1601–1609. doi:10.1016/s0006-2952(03)00531-8
Yu H. S., Kim S. H., Park H. G., Kim Y. S., Ahn Y. M. (2010). Activation of Akt signaling in rat brain by intracerebroventricular injection of ouabain: a rat model for mania. Prog. Neuropsychopharmacol. Biol. Psychiatry 34 (6), 888–894. doi:10.1016/j.pnpbp.2010.04.010
Yu H., Cui X., Zhang J., Xie J. X., Banerjee M., Pierre S. V., et al. (2018). Heterogeneity of signal transduction by Na-K-ATPase alpha-isoforms: role of Src interaction. Am. J. Physiol. Cell Physiol. 314 (2), C202–C210. doi:10.1152/ajpcell.00124.2017
Yuan Z., Cai T., Tian J., Ivanov A. V., Giovannucci D. R., Xie Z. (2005). Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex. Mol. Biol. Cell 16 (9), 4034–4045. doi:10.1091/mbc.e05-04-0295
Zeineddine S., Kreydiyyeh S. (2024). The signaling pathway through which Procyanidin B2 increases the activity of the Na+/K+ ATPase in Caco-2 cells. J. Funct. Foods. 119, 106301. doi:10.1016/j.jff.2024.106301
Zhang Y., Norian J. M., Magyar C. E., Holstein-Rathlou N. H., Mircheff A. K., McDonough A. A. (1999). In vivo PTH provokes apical NHE3 and NaPi2 redistribution and Na-K-ATPase inhibition. Am. J. Physiol. 276 (5), F711–F719. doi:10.1152/ajprenal.1999.276.5.F711
Zhang S., Malmersjo S., Li J., Ando H., Aizman O., Uhlen P., et al. (2006). Distinct role of the N-terminal tail of the Na,K-ATPase catalytic subunit as a signal transducer. J. Biol. Chem. 281 (31), 21954–21962. doi:10.1074/jbc.M601578200
Zhang C., Xie S. H., Xu B., Lu S., Liu P. (2017). Digitalis use and the risk of breast cancer: a systematic review and meta-analysis. Drug Saf. 40 (4), 285–292. doi:10.1007/s40264-016-0484-z
Zhang L., Aalkjaer C., Matchkov V. V. (2018). The Na,K-ATPase-Dependent src kinase signaling changes with mesenteric artery diameter. Int. J. Mol. Sci. 19 (9), 2489. doi:10.3390/ijms19092489
Zhang L., Staehr C., Zeng F., Bouzinova E. V., Matchkov V. V. (2019). The Na,K-ATPase in vascular smooth muscle cells. Curr. Top. Membr. 83, 151–175. doi:10.1016/bs.ctm.2019.01.007
Zilberstein A., Krivoy N., Horesh N., Klein E., Lichtstein D. (2023). Beneficial effect of digoxin-specific Fab fragments in bipolar disorder-a preliminary study. J. Affect. Disord. Rep. 13, 100600. doi:10.1016/j.jadr.2023.100600
Keywords: Na,K-ATPase isoforms, cardiotonic steroid, cardenolide, bufadienolide, ouabain, intracellular signaling
Citation: Trant J, Lowery JB, Morales-Sosa P and Blanco G (2025) Na,K-ATPase mediated and cardiotonic induced signaling in health and disease. Front. Physiol. 16:1694027. doi: 10.3389/fphys.2025.1694027
Received: 27 August 2025; Accepted: 07 October 2025;
Published: 14 November 2025.
Edited by:
Alexei Bagrov, Padakonn Pharma, EstoniaReviewed by:
David Lichtstein, Hebrew University of Jerusalem, IsraelIrina Yurievna Petrushanko, Engelhardt Institute of Molecular Biology (RAS), Russia
Copyright © 2025 Trant, Lowery, Morales-Sosa and Blanco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gustavo Blanco, Z2JsYW5jb0BrdW1jLmVkdQ==
 Jordan Trant
Jordan Trant Jenna Beth Lowery
Jenna Beth Lowery Pedro Morales-Sosa
Pedro Morales-Sosa Gustavo Blanco
Gustavo Blanco