- 1Department of Anesthesiology, University of Nebraska Medical Center, Omaha, NE, United States
- 2Department of Surgery, University of Nebraska Medical Center, Omaha, NE, United States
- 3Department of Cellular and Integrative Physiology, University of Nebraska Medical Center, Omaha, NE, United States
Introduction: Each year, over 1.8 million people die from hemorrhagic shock, and, since the median time from onset to death is only two hours, early recognition is the cornerstone of management. The sympathetic nervous system is the fastest physiological hemodynamic compensatory mechanism, and we have developed a novel measure of sympathetic vascular control called sympathetic vasomotion which could serve as an early marker of hemorrhage.
Methods: We performed unilateral renal denervation on six rabbits and instrumented these rabbits with bilateral renal flow probes and arterial pressure telemeters to allow for measurement of sympathetic vasomotion in paired vascular beds that differed only by sympathetic innervation. After a two-week recovery period, conscious rabbits then underwent controlled blood withdrawal via an auricular arterial catheter to simulate hemorrhage.
Results: Vasomotion differences between innervated and denervated kidneys in admittance gain, phase shift, and coherence increased significantly prior to increases in heart rate or decreases in blood pressure.
Discussion: These data suggest that sympathetic vasomotion could be a useful physiologically based biomarker for the early detection of hemorrhage. Further studies are needed to evaluate the utility of monitoring the sympathetic nervous system in clinical settings.
Introduction
Hemorrhagic shock is a significant source of morbidity and mortality. Over 1.8 million people die annually from hemorrhagic shock, accounting for over 86 million years of life lost (Cannon, 2018). The median time from bleeding onset to death is only 2 h. Therefore, early recognition of blood loss is the cornerstone of management (Fox et al., 2017).
Because of the clinical importance of the early detection of hemorrhage, several measures of hemodynamic instability have been developed (Hinojosa-Laborde et al., 2016; Yadav et al., 2017; Maheshwari et al., 2020; Massalha et al., 2022; Benson et al., 2023; Latimer et al., 2023). Some of the leading measures are widely available clinically but are based on opaque machine learning algorithms, providing clinicians with values of unclear significance and provenance, and, despite significant industry investment, the added value of these measures in pragmatic clinical trials remains unclear (Maheshwari et al., 2020; Massalha et al., 2022; Mulder et al., 2024; Rellum et al., 2025). A physiologically based measure to detect hemodynamic instability and facilitate anticipatory management of hemorrhage could address the shortcomings of current measures, decrease clinical uncertainty, and improve patient outcomes.
The sympathetic nervous system is the master regulator of short-term circulatory homeostasis, integrating multimodal sensory input and coordinating changes in venous capacitance, arteriolar tone, cardiac contractility, heart rate, and humoral activation to buffer a wide range of hemodynamic stressors (Zucker and Gilmore, 1991). In the case of hemorrhage, low-pressure baroreceptors sense decreases in effective circulating volume, resulting in increases in sympathetic outflow that work to maintain organ perfusion (Chien, 1967). The role of the sympathetic nervous system as the first responder to circulatory challenges makes it an ideal physiological marker for the early detection of hemorrhage. Unfortunately, from a practical standpoint, its utility is limited by the fact that clinicians do not currently have a reliable way to measure sympathetic nerve activity.
Our recent work has identified a novel method that leverages simultaneous recordings of blood pressure and blood flow to quantify sympathetic outflow, termed sympathetic vasomotion (Pellegrino et al., 2020; 2025). By enabling clinicians to measure sympathetic vasomotion non-invasively, this technology could allow for earlier treatment and better management of hemorrhage. Our previous work with sympathetic vasomotion has shown that sympathetic vasomotion is decreased by a broad array of sympatholytic stimuli, including surgical denervation, catheter-based radiofrequency denervation, intrathecal blockade, and ganglionic blockade, but we have never tested the effect of a sympathoexcitatory stimulus, like hemorrhage, on sympathetic vasomotion (Sundlöf and Wallin, 1978).
We hypothesized that hemorrhage would increase sympathetic vasomotion. We tested this hypothesis in a graded model of hemorrhage using a conscious, chronically instrumented rabbit model to determine the sensitivity of sympathetic vasomotion to blood loss and to eliminate the potentially confounding effects of general anesthesia. These chronically instrumented rabbits underwent bilateral renal blood flow probe implantation and unilateral surgical denervation. The bilateral nature of the kidneys and the isolated autonomic innervation of this organ allows for an elegant model in which two analogous vascular beds can differ only by their innervation status without complicating animal factors like sensorimotor loss and debility.
Methods
All methods are further detailed in the Online Supplement.
Data and materials availability
Data and source code used for this study are available on figshare (https://doi.org/10.6084/m9.figshare.29737739). The corresponding author is responsible for the maintenance of this repository.
Rabbit instrumentation
Experiments were carried out on adult male New Zealand White rabbits. All experiments were reviewed and approved by our Institutional Animal Care and Use Committee and carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Six rabbits were instrumented with arterial pressure (AP) telemeters and bilateral renal blood flow (RBF) probes and underwent unilateral surgical renal denervation under general anesthesia. This left one kidney fully innervated (INV) with the contralateral denervated (DNx) kidney exposed to the same hemodynamic input, perfusion pressure, and circulating neurohumoral environment (Figure 1). Functional renal denervation was validated by demonstrating an abrogation of the renovascular response to the nasopharyngeal reflex to cigarette smoke (Mousa et al., 2005; Burke et al., 2016).
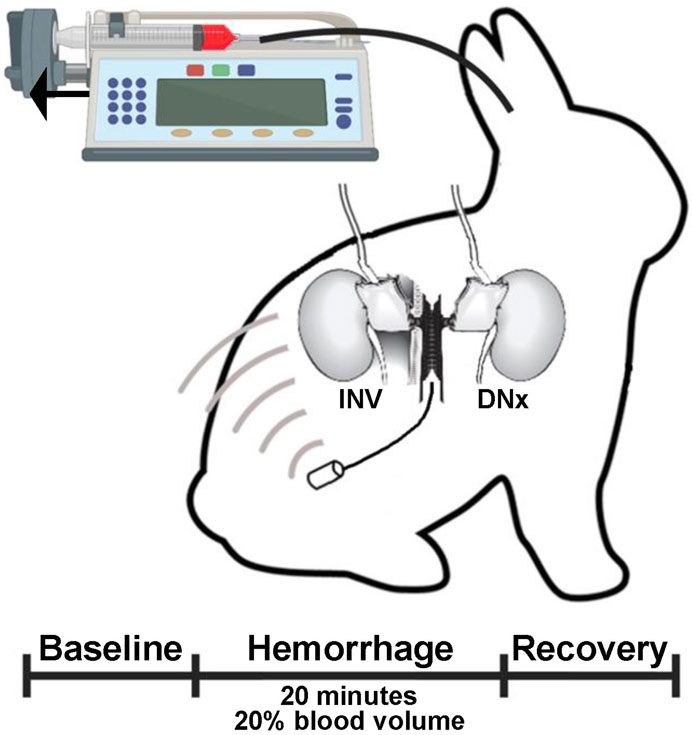
Figure 1. Experimental paradigm. Rabbits underwent unilateral surgical denervation and instrumentation with an arterial pressure telemeter and bilateral volumetric renal blood flow probes. At least 2 weeks later, rabbits underwent controlled hemorrhage via an auricular artery catheter that was connected to a syringe pump run in reverse to remove 1% estimated blood volume per minute for 20 min.
Hemorrhage experiments
After a 2-week recovery period during which the rabbits were acclimated to a quiet, dimly lit procedure room, catheters were placed in the central auricular artery and the marginal ear vein under local anesthesia with 1% lidocaine. As a control, an automated syringe pump was run at a constant rate disconnected from both the intravenous and intraarterial catheters for at least 15 min; the last 10 min of data from this were used as baseline data. The auricular arterial catheter was then connected to a syringe containing heparin that was loaded on the automated pump in reverse, resulting in a constant removal of arterial blood. This setup was used to remove 1% of the estimated blood volume (60 mL/kg) per minute for 20 min, resulting in removal of 20% of the estimated blood volume to mimic Class II normotensive shock. The syringe pump was then stopped and another 10 min of data were acquired and are reported as the recovery period. At the end of the recovery period, data acquisition was terminated, and the withdrawn blood was administered via intermittent intravenous boluses.
Data analysis
Analysis of hemodynamic data was performed in MATLAB (MathWorks, Inc., Natick, Massachusetts, United States). Heart rate variability analysis was performed using the intervals between the maximum of the derivative of arterial pressure with respect to time as described previously (Pellegrino et al., 2014). Sympathetic vasomotion analysis was performed as described previously (Pellegrino et al., 2020; 2025). In brief, a time-varying two-element Windkessel model of the renal circulation was used to extract the beat-to-beat resistive RBF time series, which was in turn used to calculate the AP-resistive RBF time-varying transfer function through bispectral wavelet analysis. Based on the physical relationship between pressure and flow, the three components of the AP-resistive RBF time-varying transfer function each have a unique physiological interpretation. Specifically, admittance gain reflects the magnitude of transduction of pressure into flow with lower admittance gain reflecting active vasoconstrictive behavior. Phase shift indicates timing differences between oscillations in pressure and flow, with autoregulatory mechanisms creating a positive phase shift, baroreflex-mediated vascular control resulting in negative phase shift, and passive Poiseuille flow resulting in zero phase shift. Coherence reflects the consistency of the pressure-flow relationship over time and is thus inversely related to the amount of active vascular control. Occurrence histograms of admittance gain, phase shift, and coherence were calculated for each kidney in the baseline state as well as over four different time periods during hemorrhage (0–5 min, 5–10 min, 10–15 min, and 15–20 min) and in the recovery state. These individual occurrence histograms were used for subsequent group analysis, and the mean group data are displayed as contour plots. Vasomotion difference maps between the INV and DNx kidneys were computed in two ways. The first set calculated t-statistics for each independent variable pair (e.g., frequency-admittance gain bin, frequency-phase shift bin) using two-tailed, paired t-tests intended to convey directionality, magnitude, and consistency of differences but not statistical significance per se. The second set of difference maps were calculated as the occurrence difference between INV and DNx kidneys. Both were used to calculate the sympathetic vasomotion magnitude for admittance gain, phase shift, and coherence. This was performed by summing the total occurrence difference between INV and DNx kidneys at each bin where |t| > 2.571 (P < 0.05 for n = 6) in the t-statistic difference map. Total renal sympathetic vasomotion magnitude was calculated as the square root of the sum of squares for the admittance gain, phase shift, and coherence.
Statistical testing
All data are displayed as mean ± the standard error of the mean. Statistical testing of simple, normally distributed measures (e.g., arterial pressure, heart rate, renal blood flow, and sympathetic vasomotion magnitude) was conducted as a repeated measures ANOVA with appropriate within-subjects factors (e.g., state, innervation status) with Greenhouse-Geisser correction for sphericity and α = 0.05. Post-hoc statistical testing versus the baseline state was performed, and the Holm-Bonferroni method for multiple comparisons was used. Consistent with our prior work, for cumulative sympathetic vasomotion, we also performed non-parametric cumulative difference mass testing using t-statistic maps with a threshold of 2.571, which allows for a priori significance testing of non-independent multidimensional data while addressing the multiple comparisons issues with such data (Maris and Oostenveld, 2007). The shuffled null data are displayed as violin plots alongside the actual cumulative t-statistics along with the non-parametric P values.
Results
Nasopharyngeal reflex
Functional renal denervation was verified by the nasopharyngeal reflex (Schiller et al., 2016). Perinasal administration of a noxious stimulus in rabbits results in increases in both parasympathetic and sympathetic outflow, manifesting as bradycardia and a sympathetically mediated decrease in renal blood flow in INV kidneys (Supplementary Figure S1). This renal vasoconstrictive response is absent in DNx kidneys.
Hemodynamics
Hemodynamics for the hemorrhage experiment are shown in Figure 2. Hemorrhage resulted in a statistically significant decrease in renal blood flow (P < 0.001), and there was a statistically significant interaction between hemorrhage and innervation status (P < 0.001) with blood flow decreasing more in DNx kidneys than their INV counterparts. While this reached statistical significance, it should be noted that the magnitude of this difference (7 mL/min decrease for INV vs. 8 mL/min for DNx) is physiologically insignificant. Heart rate increased significantly (P < 0.001), although not until 10–15 min of hemorrhage. Mean arterial pressure did not significantly change over the course of the experiment, consistent with the targeted normotensive (Class II) hemorrhage.

Figure 2. Hemodynamics. (A) Hemodynamic measures, including renal blood flow of the innervated (INV) and denervated (DNx) kidneys, heart rate, and arterial pressure for one representative rabbit. (B) Mean renal blood flow decreased over the course of the experiment for both INV and DNx kidneys with a statistically greater change in DNx kidneys. (C) Heart rate increased significantly with hemorrhage, but not until 10–15 min of blood withdrawal. (D) Mean arterial pressure did not change significantly over the course of the experiment. Statistical testing was performed via repeated measures ANOVA with one (C,D) or two (B) factors, and post hoc testing versus baseline was performed with Holm-Bonferroni-corrected paired t-tests, n = 6. *P < 0.05, **P < 0.01, *** P < 0.005 vs. baseline, †† P < 0.01 vs. baseline for DNx. RBF, renal blood flow.
Heart rate variability
Heart rate variability has been studied in more severe models of hemorrhage (Kawase et al., 2000; Batchinsky et al., 2007b; 2007a). We performed linear, non-linear, and spectral analysis over the course of hemorrhage (Supplementary Figure S5). Overall linear time-domain heart rate variability, quantified as the standard-deviation of the normal-to-normal intervals, trended down with hemorrhage, but this did not reach statistical significance (Supplementary Figure S5A, Pstate = 0.310). Approximate entropy, a non-linear measure of heart rate complexity, changed significantly over the experiment (P < 0.05), increasing at 0–5 min of hemorrhage and decreasing thereafter (Supplementary Figure S5B). Post-hoc testing did not show statistical differences for any of the states from baseline. Spectral analysis did not show any statistically significant effects on raw power (Supplementary Figure S5C–S5E) although high-frequency power trended down with hemorrhage. The low-frequency to high-frequency power ratio, a normalized spectral measure, increased with hemorrhage (Supplementary Figure S5F, P < 0.05). This increase did not reach statistical significance until 15–20 min of hemorrhage.
Admittance gain
Admittance gain quantifies the magnitude of the transduction of arterial pressure oscillations into renal blood flow with respect to passive Poiseuille flow. Lower admittance gain indicates active buffering of arterial pressure oscillations by vasoconstrictive or autoregulatory mechanisms. At baseline, there was more low admittance gain behavior in INV kidneys and more high admittance gain behavior in DNx kidneys as would be expected due to sympathetic vasoconstriction (Figure 3A; Supplementary Figure S2). These differences increased with hemorrhage, reaching statistical significance at 0–5 min of hemorrhage and peaking at 5–10 min of hemorrhage (Figures 3B–D).

Figure 3. Admittance Gain. (A) Representative time-frequency plots showing admittance gain of the innervated (INV) and denervated (DNx) kidneys for one representative rabbit. (B) Admittance gain sympathetic vasomotion magnitude increased with hemorrhage. (C) Vasomotion difference maps of t-statistics between INV and DNx kidneys and (D) vasomotion difference maps of mean occurrence between INV and DNx kidneys show that hemorrhage increases low admittance gain behavior. Statistical testing was performed on the data in B using a repeated measures ANOVA with post hoc testing versus baseline using Holm-Bonferroni-corrected paired t-tests, n = 6. *P < 0.05, **P < 0.01, ***P < 0.005 vs. baseline.
Phase shift
Phase shift quantifies the temporal relationship between oscillations in blood pressure and blood flow and provides insight into the directionality of vascular control mechanisms. Passive Poiseuille flow is characterized by synchrony between blood pressure and flow and thus a phase shift of zero. Negative phase shift behavior indicates that arterial pressure oscillations precede renovascular modulation, consistent with baroreflex-mediated vascular control, while positive phase shift behavior indicates that blood flow precedes renovascular modulation, consistent with autoregulatory vascular control. At baseline, there was more negative phase shift behavior in INV kidneys and more zero and positive phase shift behavior in DNx kidneys (Figure 4A; Supplementary Figure S3). Hemorrhage significantly increased these differences, reaching statistical significance at 5–10 min of hemorrhage and remaining statistically significant for the remainder of the experiment (Figures 4B–D).

Figure 4. Phase Shift. (A) Representative time-frequency plots showing phase shift of the innervated (INV) and denervated (DNx) kidneys for one representative rabbit. (B) Phase shift sympathetic vasomotion magnitude increased with hemorrhage. (C) Vasomotion difference maps of t-statistics between INV and DNx kidneys and (D) vasomotion difference maps of mean occurrence between INV and DNx kidneys show that hemorrhage increases negative phase shift behavior in INV kidneys. Statistical testing was performed on the data in B using a repeated measures ANOVA with post hoc testing versus baseline using Holm-Bonferroni-corrected paired t-tests, n = 6. *P < 0.05, **P < 0.01, ***P < 0.005 vs. baseline. P = 0.91.
Coherence
Coherence quantifies the consistency in the pressure-flow relationship over time and correlates inversely with active vascular modulation. Surgical denervation removes an active vascular control mechanism, and, accordingly, DNx kidneys displayed more high coherence behavior than INV kidneys (Figure 5A; Supplementary Figure S4). These differences increased with hemorrhage, reaching statistical significance in the first 5 min of hemorrhage and remaining elevated for the remainder of the experiment (Figures 5B–D).

Figure 5. Coherence. (A) Representative time-frequency plots showing coherence of the innervated (INV) and denervated (DNx) kidneys for one representative rabbit. (B) Coherence sympathetic vasomotion magnitude increased with hemorrhage. (C) Vasomotion difference maps of t-statistics between INV and DNx kidneys and (D) vasomotion difference maps of mean occurrence between INV and DNx kidneys show that hemorrhage increases low coherence behavior in INV kidneys. Statistical testing was performed on the data in B using a repeated measures ANOVA with post hoc testing versus baseline using Holm-Bonferroni-corrected paired t-tests, n = 6. *P < 0.05, **P < 0.01, ***P < 0.005 vs. baseline.
Total sympathetic vasomotion
The data from admittance gain, phase shift, and coherence of the arterial pressure-resistive blood flow time-varying transfer function were aggregated in two measures: total renal sympathetic vasomotion magnitude and the total vasomotion profile difference. Total renal sympathetic vasomotion, the square root of the sum of squares for the transfer function components, is displayed in Figure 6A. Total renal sympathetic vasomotion difference increases in the first 5 minutes of hemorrhage and remains statistically higher throughout the remainder of the experiment. The total vasomotion profile difference is a statistical group measure that reflects the statistical differences across all components of the time-varying pressure-resistive flow transfer function (Figure 6B). The total vasomotion profile difference was statistically significant at baseline, and the magnitude of this difference increased with hemorrhage, peaking between 10 and 15 min of hemorrhage. The difference between INV and DNx kidneys was statistically significant for all states (from baseline to recovery).

Figure 6. Total Vasomotion. Hemorrhage increased (A) total renal sympathetic vasomotion magnitude and (B) the total vasomotion profile difference. Statistical testing was performed on the data in A using a repeated measures ANOVA with post hoc testing versus baseline using Holm-Bonferroni-corrected paired t-tests. Statistical testing was performed on the data in B using non-parametric cumulative difference mass testing using t-statistic maps with a threshold of 2.571, n = 6. *P < 0.05, **P < 0.01, ***P < 0.005 vs. baseline. n = 6.
Discussion
These data show that renal sympathetic vasomotion increases in response to hemorrhage. Moreover, increases in sympathetic vasomotion occur prior to increases in heart rate or decreases in blood pressure. Sympathetic vasomotion remains elevated over the course of hemorrhage. These data are consistent with the physiological role of the sympathetic nervous system as the first responder to hemodynamic stress and indicate that sympathetic vasomotion may be a useful early marker of hemorrhage.
Some of the nuances in the presented data are best interpreted after a discussion of the neural mechanisms underlying the sympathetic response to hemorrhage. Hemorrhage initially decreases effective circulating volume, which is sensed by the low-pressure baroreceptors in the atria, ventricles, and pulmonary circulation, and eventually decreases cardiac output, which is sensed by arterial baroreceptors in the aorta and carotid sinus. These baroreceptors transmit this information to key autonomic nuclei like the nucleus tractus solitarius, rostral ventrolateral medulla, and the paraventricular nucleus of the hypothalamus (Carlson et al., 1986; Badoer et al., 1993) (18, 19). Integration occurs at these key autonomic nuclei with distant modulation from higher-order structures, including the thalamus, the insular cortices, the anterior cingulate gyrus, and the orbitofrontal cortex of the cortical autonomic network (Kimmerly et al., 2005). This results in increased sympathetic outflow, mobilizing blood volume via venoconstriction, constricting arterioles to maintain perfusion pressure, and increasing heart rate and cardiac contractility.
The time delay between the activation of low-pressure and high-pressure baroreceptors may explain why admittance gain and coherence differences increase within the first 5 min of hemorrhage while phase shift differences did not increase until 5–10 min of hemorrhage. Admittance gain and coherence measure active vasoconstriction and vascular control, which would be increased by unloading of the low-pressure baroreceptors. For phase shift, the observed increases are instead indicative of increased baroreflex-mediated renovascular control, which would not occur until the arterial baroreceptors are unloaded later in hemorrhage. Additionally, admittance gain peaked at 5–10 min of hemorrhage and was no longer statistically different from baseline during the recovery phase (P = 0.07). Because of how admittance gain is normalized to mean renovascular conductance, this measure likely reflects the rate of change in sympathetically mediated vascular tone rather than the sympathetic vascular tone per se. Similarly, conflicting neural reflexes may underpin the delayed tachycardia despite clear increases in renal sympathetic tone. Decreases in venous return due to hemorrhage may unload the atrial receptors and inhibit the Bainbridge reflex, which would oppose the expected tachycardia from increases in sympathetic outflow. Subsequent experiments involving measurement of cardiac filling pressures and cardiac output and manipulation of the neural circuitry involved in the response to hemorrhage, including sinoaortic denervation, could better characterize the mechanism of these differences. One unexpected finding was that hemorrhage resulted in statistically greater decreases in RBF in DNx kidneys compared to INV kidneys. This effect, while statistically significant, was physiologically insignificant, but one might have reasonably expected the increased renal sympathetic tone to result in greater renal vasoconstriction of the INV kidney. Renal denervation-induced increases in sensitivity to circulating catecholamines, which notably are not released rhythmically in the renal vascular bed and thus affect renovascular resistance without affecting renal sympathetic vasomotion, may have mediated this counterintuitive effect (Hansen et al., 1994).
The renal circulation was used for these experiments for two reasons. First, as stated previously, as a bilateral visceral organ, the kidneys offer an elegant model where two circulatory beds can differ strictly by innervation status while being exposed to the same systemic stimulus, perfusion pressure, and circulating humoral milieu. Second, the renal circulation and its innervation are of significant clinical interest. In the inpatient setting, renal circulatory changes are a driving factor in the most common causes of acute kidney injury, including sepsis and hemorrhage (Prowle et al., 2012; Wang et al., 2017). On a chronic basis, hypertension and diabetes profoundly impact the renal circulation on a structural and functional level, and, in doing so, lead to renal dysfunction (Carlström et al., 2015). Moreover, elevated renal sympathetic outflow plays a causal role in human hypertension, which underpins the minimally invasive renal denervation technologies employed clinically, and in the renal dysfunction seen in chronic heart failure (Esler et al., 1989; Böhm et al., 2020; Azizi et al., 2022; 2023; Xia et al., 2022).
Our finding that renal sympathetic vasomotion is an early marker of hemorrhage highlights the broader principle that sympathetic control of other vascular beds could also provide distinct, valuable sensors for hemodynamic stress. This first responder role of the sympathetic nervous system has been shown to be easier to detect in peripheral tissues (Hirsch et al., 1989). For example, cutaneous blood flow is under tonic sympathetic inhibition and would be expected to exhibit intense vasomotion at the earliest onset of hemorrhage as the body shunts blood centrally (Bovaira et al., 2023). A similar early response may be measured in the limb vasculature, where sympathetic vasomotion could be measured in the femoral, brachial, or radial arteries. In contrast, the cerebral circulation, at the level of the carotid or middle cerebral artery, may display even stronger autoregulatory mechanisms than those observed in the kidney (Medow et al., 2014). Observing diverging patterns of vasomotion across different beds could provide individualized signatures for different shock states and hemodynamic stressors, providing clinicians with more useful information.
The ability to predict impending hemodynamic collapse is critical in the perioperative, critical care, and emergency settings, and other measures have been developed for this purpose. The most widely available measure in the clinical environment is the hypotension prediction index, which is based on proprietary algorithms derived from machine learning and is integrated into Acumen patient monitoring equipment (Becton, Dickinson and Company, Franklin Lakes, NJ, United States). This index has shown mixed results in clinical practice and has recently been criticized as a surrogate for mean arterial pressure (Maheshwari et al., 2020; Li et al., 2022; Mohammadi et al., 2024; Mulder et al., 2024; Rellum et al., 2025). Another measure in development is the compensatory reserve index, which is also a measure that was derived from machine learning analysis of plethysmography data and has shown promise in experimental and early clinical work (Hinojosa-Laborde et al., 2016; Latimer et al., 2023). Both measures are based on opaque artificial neural networks trained on rudimentary features from subject waveforms and are not informed by autonomic neural networks or vascular physiology. While many other such measures have been proposed, sympathetic vasomotion is unique in that it is the only measure that is derived from the known physiological role of the sympathetic nervous system as the body’s first responder to hemodynamic stress.
While this study highlights the potential for sympathetic vasomotion for the early detection of hemorrhage, it does not directly demonstrate the utility of sympathetic vasomotion as an endpoint to guide treatment for bleeding patients as we did not monitor the rabbits while we retransfused the withdrawn blood. Hemorrhagic shock is primarily managed with a combination of bleeding control and volume resuscitation, ideally with whole blood (Brill et al., 2022). Overly aggressive resuscitation may lead to multiple secondary injuries, including pulmonary edema, cardiogenic shock, electrolyte disturbances, and coagulopathy while inadequate resuscitation will lead to hypoperfusion and potentially multiorgan failure (Malbrain et al., 2020). The potential for sympathetic vasomotion to serve as a marker of return to circulatory homeostasis after hemorrhage may be nearly as important as its role in the early detection of hemorrhage and warrants further study.
Other clinical studies have studied the effects of sympathoexcitatory stimuli on the renal vasculature. One impressive set of experiments exposed subjects to escalating levels of lower body negative pressure while measuring hemodynamics and neurotransmitter dynamics in the renal circulation (Tidgren et al., 1990). This showed that renal norepinephrine overflow increased at low levels of low body negative pressure, prior to the development of tachycardia and hypotension, mirroring our findings in rabbits. Furthermore, they saw that renal norepinephrine overflow peaked at magnitudes of lower body negative pressure that produced tachycardia and began to fall at magnitudes of lower body negative pressure that produced hypotension, which again matches our rabbit data. Other clinical studies have shown sympathetically mediated renovascular responses to handgrip exercise and mental stress (Tidgren and Hjemdahl, 1989; Momen et al., 2005; Teixeira et al., 2025) although mental stress in particular exhibits significant interindividual variability.
On a more fundamental level, this study highlights the ability of sympathetic vasomotion to reflect increases, not just decreases, in sympathetic outflow. This is crucial not only for hemorrhage and other states of hemodynamic instability, but also for prevalent conditions like cardiovascular disease, chronic kidney disease, and diabetes where elevated sympathetic tone is a powerful pathophysiological driver (Malpas, 2010; Scott-Solomon et al., 2021). The ability to measure and thoughtfully intervene on maladaptive sympathoexcitation could improve patient outcomes for these chronic conditions. For example, the ability to identify patients with elevated sympathetic tone could improve patient selection for autonomic therapies like renal denervation for hypertension or cardiac sympathetic denervation for ventricular arrhythmias (Vaseghi et al., 2014; Saxena et al., 2022).
Our study has several limitations. Additionally, while our work established the sensitivity of renal sympathetic vasomotion in the detection of hemorrhage, we did not test the specificity of sympathetic vasomotion for hemorrhage compared to other sympathoexcitatory stimuli. This is important in clinical contexts where pain and other modes of shock (e.g., cardiogenic, obstructive) may also increase sympathetic tone. Furthermore, as detailed previously, other circulations beyond the renal circulation may be important and more accessible sources for clinical hemodynamic monitoring. Finally, frequency-based measures from a small cohort of rabbits, which have physiological (e.g., cardiac, respiratory, and sympathetic) frequencies that are quite different from humans, must be reproduced in large animals prior to translating them to the clinic.
In rabbits, hemorrhage increases renal sympathetic vasomotion prior to changes in heart rate or blood pressure, which is consistent with the physiological role of the sympathetic nervous system as the first responder to hemodynamic stress. Sympathetic vasomotion could form the basis for the novel physiologically based marker of hemodynamic instability, helping clinicians recognize hemorrhage earlier and optimize resuscitation. Subsequent work will focus on translation of this work to large animals and humans.
Perspectives: Sympathetic vasomotion, a novel marker of sympathetic outflow, increases prior to other hemodynamic changes in hemorrhage. Sympathetic vasomotion could serve as an early detection tool for hemorrhage that facilitates prompt and precise resuscitation.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://doi.org/10.6084/m9.figshare.29737739.
Ethics statement
The animal study was approved by University of Nebraska Medical Center Institutional Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
PRP: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing. AMS: Conceptualization, Investigation, Methodology, Writing – review and editing. IIP: Conceptualization, Writing – review and editing. IHZ: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Writing – review and editing. H-JW: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. National Institutes of Health, National Institute on Aging, AG077803, and Department of Veterans Affairs, RX004632 to IIP. National Institutes of Health, National Heart Lung and Blood Institute, HL153176 and HL172029 and Theodore Hubbard Foundation to IHZ. National Institutes of Health, National Heart Lung and Blood Institute, HL171602, HL169205, HL152160, HL172029, and HL170127 to HJW.
Acknowledgements
Preprint is available on bioRxiv at https://doi.org/10.1101/2025.08.23.671352.
Conflict of interest
PRP, IHZ, HJW, and AMS have patents related to this work (U.S. Patents #10,881,303 and #11,317,889).
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2025.1712566/full#supplementary-material
References
Azizi M., Mahfoud F., Weber M. A., Sharp A. S. P., Schmieder R. E., Lurz P., et al. (2022). Effects of renal denervation vs sham in resistant hypertension after medication escalation: prespecified analysis at 6 months of the RADIANCE-HTN TRIO randomized clinical trial. JAMA Cardiol. 7, 1244–1252. doi:10.1001/JAMACARDIO.2022.3904
Azizi M., Saxena M., Wang Y., Jenkins J. S., Devireddy C., Rader F., et al. (2023). Endovascular ultrasound renal denervation to treat hypertension: the RADIANCE II randomized clinical trial. JAMA 329, 651–661. doi:10.1001/JAMA.2023.0713
Badoer E., McKinley M. J., Oldfield B. J., McAllen R. M. (1993). A comparison of hypotensive and non-hypotensive hemorrhage on Fos expression in spinally projecting neurons of the paraventricular nucleus and rostral ventrolateral medulla. Brain Res. 610, 216–223. doi:10.1016/0006-8993(93)91403-F
Batchinsky A. I., Cooke W. H., Kuusela T. A., Jordan B. S., Wang J. J., Cancio L. C. (2007a). Sympathetic nerve activity and heart rate variability during severe hemorrhagic shock in sheep. Auton. Neurosci. 136, 43–51. doi:10.1016/j.autneu.2007.03.004
Batchinsky A. I., Cooke W. H., Kuusela T., Cancio L. C. (2007b). Loss of complexity characterizes the heart rate response to experimental hemorrhagic shock in swine. Crit. Care Med. 35, 519–525. doi:10.1097/01.CCM.0000254065.44990.77
Benson B., Belle A., Lee S., Bassin B. S., Medlin R. P., Sjoding M. W., et al. (2023). Prediction of episode of hemodynamic instability using an electrocardiogram based analytic: a retrospective cohort study. BMC Anesthesiol. 23, 324–10. doi:10.1186/s12871-023-02283-x
Böhm M., Kario K., Kandzari D. E., Mahfoud F., Weber M. A., Schmieder R. E., et al. (2020). Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED pivotal): a multicentre, randomised, sham-controlled trial. Lancet 395, 1444–1451. doi:10.1016/S0140-6736(20)30554-7
Bovaira M., Cañada-Soriano M., García-Vitoria C., Calvo A., De Andrés J. A., Moratal D., et al. (2023). Clinical results of lumbar sympathetic blocks in lower limb complex regional pain syndrome using infrared thermography as a support tool. Pain Pract. 23, 713–723. doi:10.1111/PAPR.13236
Brill J. B., Tang B., Hatton G., Mueck K. M., McCoy C. C., Kao L. S., et al. (2022). Impact of incorporating whole blood into hemorrhagic shock resuscitation: analysis of 1,377 consecutive trauma patients receiving emergency-release uncrossmatched blood products. J. Am. Coll. Surg. 234, 408–418. doi:10.1097/XCS.0000000000000086
Burke S. L., Lim K., Moretti J. L., Head G. A. (2016). Comparison of sympathetic nerve activity normalization procedures in conscious rabbits. Am. J. Physiol. Heart Circ. Physiol. 310, H1222–H1232. doi:10.1152/AJPHEART.00866.2015
Carlson D. E., Burchard K. W., Gann D. S. (1986). Right atrial volume during hemorrhage in the dog. Am. J. Physiol. 250, H1136–H1144. doi:10.1152/ajpheart.1986.250.6.H1136
Carlström M., Wilcox C. S., Arendshorst W. J. (2015). Renal autoregulation in health and disease. Physiol. Rev. 95, 405–511. doi:10.1152/physrev.00042.2012
Chien S. (1967). Role of the sympathetic nervous system in hemorrhage. Physiol. Rev. 47, 214–288. doi:10.1152/PHYSREV.1967.47.2.214
Esler M., Jennings G., Lambert G. (1989). Noradrenaline release and the pathophysiology of primary human hypertension. Am. J. Hypertens. 2, 140S-146S–146S. doi:10.1093/ajh/2.3.140S
Fox E. E., Holcomb J. B., Wade C. E., Bulger E. M., Tilley B. C.PROPPR Study Group (2017). Earlier endpoints are required for hemorrhagic shock trials among severely injured patients. Shock 47, 567–573. doi:10.1097/SHK.0000000000000788
Hansen J. M., Abildgaard U., Fogh-Andersen N., Kanstrup I. L., Bratholm P., Plum I., et al. (1994). The transplanted human kidney does not achieve functional reinnervation. Clin. Sci. (Lond) 87, 13–20. doi:10.1042/CS0870013
Hinojosa-Laborde C., Howard J. T., Mulligan J., Grudic G. Z., Convertino V. A. (2016). Comparison of compensatory reserve during lower-body negative pressure and hemorrhage in nonhuman primates. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310, R1154–R1159. doi:10.1152/ajpregu.00304.2015
Hirsch A. T., Levenson D. J., Cutler S. S., Dzau V. J., Creager M. A. (1989). Regional vascular responses to prolonged lower body negative pressure in normal subjects. Am. J. Physiol. 257, H219–H225. doi:10.1152/AJPHEART.1989.257.1.H219
Kawase M., Komatsu T., Nishiwaki K., Kimura T., Fujiwara Y., Takahashi T., et al. (2000). Heart rate variability during massive hemorrhage and progressive hemorrhagic shock in dogs. Can. J. Anaesth. 47, 807–814. doi:10.1007/BF03019486
Kimmerly D. S., O’Leary D. D., Menon R. S., Gati J. S., Shoemaker J. K. (2005). Cortical regions associated with autonomic cardiovascular regulation during lower body negative pressure in humans. J. Physiology 569, 331–345. doi:10.1113/JPHYSIOL.2005.091637
Latimer A. J., Counts C. R., Van Dyke M., Bulger N., Maynard C., Rea T. D., et al. (2023). The compensatory reserve index for predicting hemorrhagic shock in prehospital trauma. Shock 60, 496–502. doi:10.1097/SHK.0000000000002188
Li W., Hu Z., Yuan Y., Liu J., Li K. (2022). Effect of hypotension prediction index in the prevention of intraoperative hypotension during noncardiac surgery: a systematic review. J. Clin. Anesth. 83. doi:10.1016/j.jclinane.2022.110981
Maheshwari K., Shimada T., Yang D., Khanna S., Cywinski J. B., Irefin S. A., et al. (2020). Hypotension prediction index for prevention of hypotension during Moderate-to high-risk noncardiac surgery: a pilot randomized trial. Anesthesiology 133, 1214–1222. doi:10.1097/ALN.0000000000003557
Malbrain M. L. N. G., Langer T., Annane D., Gattinoni L., Elbers P., Hahn R. G., et al. (2020). Intravenous fluid therapy in the perioperative and critical care setting: executive summary of the international fluid academy (IFA). Ann. Intensive Care 10, 64. doi:10.1186/S13613-020-00679-3
Malpas S. C. (2010). Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol. Rev. 90, 513–557. doi:10.1152/physrev.00007.2009
Maris E., Oostenveld R. (2007). Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 164, 177–190. doi:10.1016/j.jneumeth.2007.03.024
Massalha M., Faranish R., Romano S., Salim R. (2022). Decreased inferior vena cava diameter as an early marker in postpartum hemorrhage. Ultrasound Obstetrics Gynecol. 59, 234–240. doi:10.1002/UOG.23695
Medow M. S., Del Pozzi A. T., Messer Z. R., Terilli C., Stewart J. M. (2014). Altered oscillatory cerebral blood flow velocity and autoregulation in postural tachycardia syndrome. Front. Physiol. 5 JUN 5, 234. doi:10.3389/FPHYS.2014.00234
Mohammadi I., Firouzabadi S. R., Hosseinpour M., Akhlaghpasand M., Hajikarimloo B., Tavanaei R., et al. (2024). Predictive ability of hypotension prediction index and machine learning methods in intraoperative hypotension: a systematic review and meta-analysis. J. Transl. Med. 22, 725. doi:10.1186/S12967-024-05481-4
Momen A., Bower D., Leuenberger U. A., Boehmer J., Lerner S., Alfrey E. J., et al. (2005). Renal vascular response to static handgrip exercise: sympathetic vs. autoregulatory control. Am. J. Physiol. Heart Circ. Physiol. 289, H1770–H1776. doi:10.1152/AJPHEART.01213.2004
Mousa T. M., Gao L., Cornish K. G., Zucker I. H. (2005). Effects of angiotensin II on autonomic components of nasopharyngeal stimulation in male conscious rabbits. J. Appl. Physiol. 98, 1607–1611. doi:10.1152/JAPPLPHYSIOL.01322.2004
Mulder M. P., Harmannij-Markusse M., Fresiello L., Donker D. W., Potters J. W. (2024). Hypotension prediction index is equally effective in predicting intraoperative hypotension during noncardiac surgery compared to a mean arterial pressure threshold: a prospective observational study. Anesthesiology 141, 453–462. doi:10.1097/ALN.0000000000004990
Pellegrino P. R., Schiller A. M., Zucker I. H. (2014). Validation of pulse rate variability as a surrogate for heart rate variability in chronically instrumented rabbits. Am. J. Physiol. Heart Circ. Physiol. 307, H97–H109. doi:10.1152/ajpheart.00898.2013
Pellegrino P. R., Zucker I. H., Chatzizisis Y. S., Wang H. J., Schiller A. M. (2020). Quantification of renal sympathetic vasomotion as a novel end point for renal denervation. Hypertension 76, 1247–1255. doi:10.1161/HYPERTENSIONAHA.120.15325
Pellegrino P. R., Zucker I. H., Chatzizisis Y. S., Wang H. J., Schiller A. M. (2025). Sympathetic vasomotion reflects catheter-based radiofrequency renal denervation. Hypertension 82, 1261–1270. doi:10.1161/HYPERTENSIONAHA.125.24980
Prowle J. R., Molan M. P., Hornsey E., Bellomo R. (2012). Measurement of renal blood flow by phase-contrast magnetic resonance imaging during septic acute kidney injury: a pilot investigation. Crit. Care Med. 40, 1768–1776. doi:10.1097/CCM.0B013E318246BD85
Rellum S. R., Noteboom S. H., van der Ster B. J. P., Schuurmans J., Kho E., Vlaar A. P. J., et al. (2025). The hypotension prediction index versus mean arterial pressure in predicting intraoperative hypotension: a clinical perspective. Eur. J. Anaesthesiol. 42, 527–535. doi:10.1097/EJA.0000000000002150
Saxena M., Schmieder R. E., Kirtane A. J., Mahfoud F., Daemen J., Basile J., et al. (2022). Predictors of blood pressure response to ultrasound renal denervation in the RADIANCE-HTN SOLO study. J. Hum. Hypertens. 36, 629–639. doi:10.1038/S41371-021-00547-Y
Schiller A. M., Pellegrino P. R., Zucker I. H. (2016). Renal nerves dynamically regulate renal blood flow in conscious, healthy rabbits. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310, R156–R166. doi:10.1152/ajpregu.00147.2015
Scott-Solomon E., Boehm E., Kuruvilla R. (2021). The sympathetic nervous system in development and disease. Nat. Rev. Neurosci. 22, 685–702. doi:10.1038/S41583-021-00523-Y
Sundlöf G., Wallin B. G. (1978). Effect of lower body negative pressure on human muscle nerve sympathetic activity. J. Physiol. 278, 525–532. doi:10.1113/JPHYSIOL.1978.SP012322
Teixeira A. L., Lee J. B., Nardone M., Burr J. F., Millar P. J. (2025). Interindividual variability in renal and muscle sympathetic responses to mental stress: contributions to blood pressure regulation. J. Appl. Physiol. 139, 27–36. doi:10.1152/JAPPLPHYSIOL.00575.2024
Tidgren B., Hjemdahl P. (1989). Renal responses to mental stress and epinephrine in humans. Am. J. Physiol. 257, F682–F689. doi:10.1152/AJPRENAL.1989.257.4.F682
Tidgren B. O., Hjemdahl P., Theodorsson E., Nussberger J. (1990). Renal responses to lower body negative pressure in humans. Am. J. Physiol. 259, F573–F579. doi:10.1152/AJPRENAL.1990.259.4.F573
Vaseghi M., Gima J., Kanaan C., Ajijola O. A., Marmureanu A., Mahajan A., et al. (2014). Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and long-term follow-up. Heart rhythm. 11, 360–366. doi:10.1016/j.hrthm.2013.11.028
Wang L., Song J., Buggs J., Wei J., Wang S., Zhang J., et al. (2017). A new mouse model of hemorrhagic shock-induced acute kidney injury. Am. J. Physiol. Ren. Physiol. 312, F134-F142–F142. doi:10.1152/ajprenal.00347.2016
Xia Z., Vellichirammal N. N., Han L., Gao L., Boesen E. I., Schiller A. M., et al. (2022). Cardiac spinal afferent denervation attenuates renal dysfunction in rats with cardiorenal syndrome type 2. JACC Basic Transl. Sci. 7, 582–596. doi:10.1016/J.JACBTS.2022.02.008
Yadav K., Singh A., Badhwar S., Jaryal A. K., Coshic P., Chatterjee K., et al. (2017). Decreased spontaneous baroreflex sensitivity as an early marker for progression of haemorrhage. High Blood Press. Cardiovasc. Prev. 24, 275–281. doi:10.1007/S40292-017-0205-4
Keywords: blood pressure, hemorrhage, renal circulation, signal processing, digital, sympathetic nervous system, homeostasis, biomarker, autonomic denervation
Citation: Pellegrino PR, Schiller AM, Pipinos II, Zucker IH and Wang H-J (2025) Sympathetic vasomotion as an early marker of hemorrhage. Front. Physiol. 16:1712566. doi: 10.3389/fphys.2025.1712566
Received: 24 September 2025; Accepted: 28 October 2025;
Published: 27 November 2025.
Edited by:
Gaetano Santulli, Albert Einstein College of Medicine, United StatesReviewed by:
Alexandre Kanashiro, University of Wisconsin, United StatesMarcos Paulo Rocha Alves, University of Copenhagen, Denmark
Copyright © 2025 Pellegrino, Schiller, Pipinos, Zucker and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter Ricci Pellegrino, cGV0ZXIucGVsbGVncmlub0B1bm1jLmVkdQ==
 Peter Ricci Pellegrino
Peter Ricci Pellegrino Alicia M. Schiller
Alicia M. Schiller Iraklis I. Pipinos2
Iraklis I. Pipinos2 Irving H. Zucker
Irving H. Zucker Han-Jun Wang
Han-Jun Wang