- 1Sandwell and West Birmingham NHS Trust, West Bromwich, United Kingdom
- 2School of Health Sciences, College of Medicine and Health, University of Birmingham, Birmingham, United Kingdom
- 3Royal Wolverhampton NHS Trust, Wolverhampton, United Kingdom
- 4Institute of Clinical Sciences, University of Birmingham, Birmingham, United Kingdom
- 5Aston Medical School, Aston University, Birmingham, United Kingdom
Background: Osteoporosis, a condition marked by low bone mineral density (BMD) and structural deterioration, affects more women than men over 50 globally. In women, declining estrogen during the menopause accelerates bone resorption, heightening fracture risk. An association between osteoporosis and depression, frailty fractures and poor quality of life has been identified. Both menopause hormone therapy (MHT) and exercise are shown to improve BMD, with MHT reducing bone resorption and exercise promoting bone formation. This review examines the effectiveness of MHT, exercise, and their combination in managing menopausal osteoporosis.
Method: A multifactor scoping review was conducted to address osteoporosis and MHT, osteoporosis and exercise, and osteoporosis and MHT and exercise combined.
Results: Initial searches identified 15,158 studies, narrowed to 20 meeting the inclusion criteria. MHT and exercise are effective in preserving BMD in menopausal women. Combined estrogen and progesterone MHT is more effective than estrogen-only, with studies suggesting that MHT prescribed at low doses for longer durations more effectively preserves BMD. Resistance training (RT) completed 2–3 days per week at a moderate-to-high intensity combined with impact activity completed at a minimum of 3 days per week is optimal for improving BMD in menopausal women, while low-impact exercises provide supplemental benefits. Combining MHT with exercise enhances BMD more than either alone.
Conclusion: This review highlights that combining MHT and structured exercise is most effective for enhancing BMD in menopausal women. Given certain safety considerations surrounding MHT in some women, exercise remains a cornerstone for the prevention and management of osteoporosis as well as for promoting overall wellness.
Introduction
Osteoporosis is a disease characterized by bone structural deterioration and low bone mineral density (BMD), measured by dual-energy x-ray absorptiometry (DXA), to produce a T-score (1). BMD is categorized as normal when the T-score is greater than −1, osteopenia when it falls between −1 and −2.5, and osteoporosis when it is less than −2.5. Osteoporosis affects one in three women and one in five men over the age of 50 worldwide (2), with osteoporosis in women increasing from 6.8% at ages 50–59 to 25.7% at ages 70–79 and 34.9% in those over 80 (3). Bone remodeling occurs throughout life to maintain the strength and integrity of bone. This is achieved through osteoblasts, bone-forming cells that deposit new bone tissue, and osteoclasts, bone-resorbing cells that break down bone tissue. Estrogen plays an important role in the complex interplay of new bone formation and bone resorption. It enhances osteoblast activity, reducing osteocyte apoptosis (recruitment of osteoclasts to initiate bone resorption) and promoting osteoclast apoptosis (osteoclast cell death) to inhibit osteoclast function, suppressing bone resorption (4).
The menopause is a natural biological process marking the end of a woman’s reproductive years, in which the loss of ovarian follicular function results in decreased estrogen and progesterone. The perimenopause typically starts around the age of 45, whereby estrogen levels begin to fall, characterized by irregular periods and menopause symptoms. Menopause is then defined as 12 consecutive months without a menstrual period, with the following phase termed postmenopause. Therefore, as estrogen levels reduce during the menopausal transition, there is greater bone resorption than bone formation, resulting in decreased BMD and risk of osteoporotic fractures (5).
Menopause hormone therapy (MHT) can reduce excessive bone resorption by inhibiting osteoclast activity (6). The two main types of MHT are combined MHT and estrogen-only MHT (7). Combined MHT includes both estrogen and progestogen and is recommended for women with an intact uterus to protect the endometrium from the unopposed effects of estrogen (7). Estrogen-only MHT is suitable for women who have undergone hysterectomy, as there is no endometrium to protect.
Endogenous estrogens produced by the human body include estradiol, estrone, and estriol. In MHT, estrogen may be administered in the form of conjugated equine estrogens (CEOs) or synthetic bioidentical preparations that are structurally identical to these endogenous hormones. MHT may also include phytoestrogens—plant-derived compounds from sources such as soy or yams—which have a molecular structure different from human estrogens. While phytoestrogens can bind to estrogen receptors and exert mild estrogenic effects, they are not bioidentical and do not replicate the full biological activity of endogenous estrogens.
CEOs, derived from the urine of pregnant mares, contain a mixture of estrogen compounds such as estrone sulfate and equilin sulfate, which are metabolized into active estrogens in the body (8). CEOs have been widely used in MHT for the management of menopausal symptoms (9). However, their use has declined following the Women’s Health Initiative (WHI), which reported an increased risk of adverse outcomes, including breast cancer, thromboembolism, cardiovascular disease, and endometrial cancer (10).
Progestogens are an essential component of combined MHT to counteract the proliferative effects of estrogen on the endometrium. These are available in two main forms: synthetic and natural. Synthetic progestogens, also known as progestins, include medroxyprogesterone acetate (MPA), norethisterone, levonorgestrel, norgestrel, drospirenone, and dydrogesterone. MPA has been widely used in combined MHT but is associated with an increased risk of breast cancer (11), prompting a shift toward alternative progestogens with potentially better safety profiles.
Natural progestogens include micronized progesterone (e.g., Utrogestan), which is plant-derived and bioidentical to endogenous progesterone. This offers a more physiological and potentially more tolerable option for MHT.
Another form of MHT is Duavive®, which combines conjugated estrogens with bazedoxifene acetate, a selective estrogen receptor modulator (SERM) (12). Duavive is typically prescribed for postmenopausal women with a uterus when progestogen therapy is unsuitable or unnecessary. Bazedoxifene provides endometrial protection, eliminating the need for progestogen (13).
Mechanical loading applied during exercise leads to an osteogenic response, stimulating bone growth and increased BMD, through the mechanosensory role of osteocytes. When osteocytes receive signals of mechanical load, the subsequent mechanotransduction, biochemical and intracellular changes in response to mechanical stimuli, impact the function of osteoblasts and osteoclasts to modify homeostasis (14). Exercise prescriptions vary by type and intensity, including resistance training (RT), aerobic and impact training, and Tai Chi. RT intensity is measured as a percentage of one-repetition max (1RM), defined as the maximum amount of weight lifted for one repetition, while aerobic intensity uses a percentage of maximum heart rate (MHR), which is calculated as 220 minus age. Exercise intensity is often categorized based on percentages of MHR or perceived exertion. The American Heart Association (AHA) (15) defines moderate-intensity exercise as 50%–70% of MHR and vigorous-intensity exercise as 70%–85% of MHR.
In summary, bone remodeling is maintained by a balance between osteoclastic resorption and osteoblastic formation. In menopause, reduced estrogen levels disrupt this equilibrium—enhancing resorption through increased receptor activator of nuclear factor kappa-β ligand (RANKL) and decreased osteoprotegerin—contributing to osteoporosis. Regular exercise promotes osteoblastic activity and bone strength, while hormonal therapy can mitigate the effects of estrogen deficiency, highlighting the critical interplay between mechanical loading and hormonal regulation in maintaining bone health.
Research demonstrates the impact of the menopausal transition on women’s mental health and quality of life (16). Bromberger et al. (17) identified that peri-menopausal women and early postmenopausal women (PMW) are two to four times more likely to experience a significant depressive episode. Due to physiological changes such as weight gain and muscle loss during the menopause transition, women often experience low self-efficacy and body dissatisfaction (18). Further to this, growing evidence highlights the bidirectional relationship between postmenopausal osteoporosis and mental health disorders (19). A systematic review and meta-analysis by Wang et al. (20) found that PMW with osteoporosis are significantly more likely to experience depressive symptoms compared to those without osteoporosis. In addition, research by Smith et al. (21) demonstrated that chronic arthralgia, common among osteoporotic 121 patients, is associated with poorer mood, lower quality of life, and heightened depression scores.
Osteoporosis-related frailty fractures have a profound impact on health-related quality of life (HRQoL), leading to chronic pain, reduced mobility, loss of independence, and an increased healthcare burden (22). These factors contribute to social isolation and emotional distress, further exacerbating psychological symptoms in PMW. Research has consistently demonstrated a strong association between low BMD, osteoporotic fractures, and a decline in self-reported mental health (23, 24). Alarmingly, osteoporosis-related physical limitations have been linked to higher rates of suicidal ideation in PMW, highlighting the urgent need for effective management strategies (25). The psychological impact of osteoporosis is likely driven by a combination of biological, psychological, and social factors, including chronic pain, reduced mobility, and diminished quality of life.
MHT has been widely recognized as an effective intervention for mitigating BMD loss. Beyond its skeletal benefits, MHT may also contribute to improved mental wellbeing, as stabilizing estrogen levels has been shown to slow bone resorption while positively influencing mood regulation and cognitive function (26). Similarly, exercise plays a critical role in osteoporosis management, not only by enhancing BMD but also by modulating the hypothalamic–pituitary–adrenal (HPA) axis, leading to reduced cortisol secretion (27). Since elevated cortisol levels are associated with sleep disturbances, anxiety, and mood fluctuations (62), exercise may offer both physical and psychological benefits for osteoporotic PMW. Moreover, a previous scoping review has highlighted the significant impact of regular exercise on the overall quality of life in this population (28).
These findings emphasize the need for a holistic management approach in postmenopausal osteoporosis, addressing both physical symptoms and psychological wellbeing. Given the consequences of osteoporosis on physical and mental health, it is essential to establish the most effective management strategies to preserve BMD and prevent osteoporosis in menopausal women. While both MHT and exercise independently show promise in maintaining bone density, their combined effects remain less explored. This review focuses on evaluating the direct impact of these interventions on BMD, as identifying the most effective strategy for bone health is a crucial first step before considering secondary outcomes such as psychological wellbeing. Although mental health is undeniably linked to osteoporosis, it was not included in this review, as the primary aim was to assess the physiological effects of MHT and exercise on BMD. Therefore, this scoping review aims to evaluate the effectiveness of MHT alone, exercise alone, and their combination in improving BMD in menopausal women, providing valuable insights into optimizing treatment approaches for this population.
2 Methods
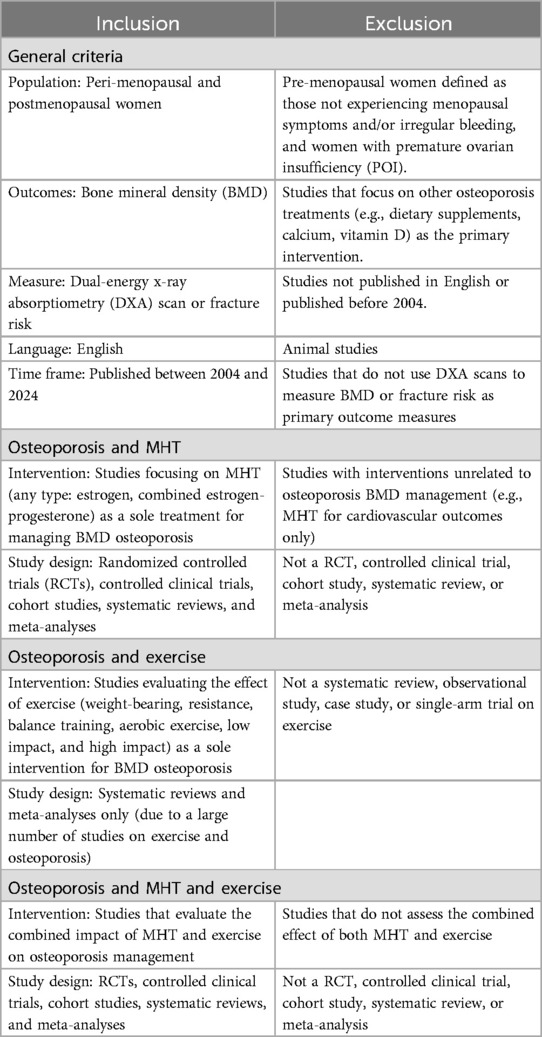
The Arksey and O’Malley (29) framework was used to conduct this scoping review, with the PRISMA-ScR checklist to report the results. The research question guiding this systematic review is: What is the impact of MHT alone, exercise alone, and their combination compared to no intervention or a placebo on BMD in menopausal women? Addressing multiple interventions posed challenges in evaluating outcomes beyond BMD. While our initial intent was to assess secondary outcomes, such as the impact on mental health, we focused on BMD to maintain methodological rigor and raise awareness of the significant impact on mental health. This decision highlights the need for further research into the under-investigated area of these interventions’ effects on mental health, suggesting a dedicated systematic review on this topic. To define the research question and guide the study selection process, we used the PICO framework. The population was menopausal women, including both perimenopausal and postmenopausal women; the intervention involved MHT alone, exercise alone, and MHT and exercise combined; the comparison included no intervention or placebo; and the outcome assessed improvements in BMD measured by DXA scans, shown as a T-score. Given the research question, a multifactor search was required to address osteoporosis and MHT, osteoporosis and exercise, and osteoporosis and MHT and exercise combined.
A comprehensive search was performed on Embase, EMCARE, MEDLINE, CINHAL, and the Cochrane Library Database of Systematic Reviews. Due to the large volume of studies generated from the initial search, a second, more refined search was carried out. Only records that met the following criteria were included: English language, published between 2004 and 2024; and study types including randomized control trials (RCTs), cohort studies, case-control studies, systematic reviews, meta-analyses, cross-sectional studies, or qualitative studies.
The initial search on osteoporosis and exercise yielded a substantial number of studies. As a result, the search was further narrowed to include only systematic reviews and meta-analyses, or systematic reviews that included meta-analyses. Studies were then exported into RefWorks®, where titles and abstracts were screened. The inclusion and exclusion criteria were applied to identify the most relevant studies (Table 1).
The search terms were adjusted to align with the indexing systems of each database. In EMCARE and MEDLINE, Medical Subject Headings (MeSH) and keyword variations were used, including: [“Osteoporosis” OR “Bone density” (MeSH)] AND [“Menopause” OR “Postmenopause” OR “Perimenopause” (MeSH) OR “Post-menopause” OR “Peri-menopause” OR “Menopause*” (Keywords)] AND [“Hormone replacement therapy” OR “Estrogen Replacement Therapy” (MeSH) OR “HRT” (Keyword)] OR [“Exercise” (MeSH) OR “Physical activity” (Keyword)].
In CINAHL, the equivalent CINAHL Subject Headings were applied along with keywords to capture relevant studies, such as: [“Osteoporosis” OR “Bone Density” (CINAHL heading)] AND [“Menopause” OR “Postmenopause” OR “Perimenopause” (CINAHL headings) OR “Post-menopause” OR “Peri-menopause” OR “Menopaus*” (Keywords)] AND [“Hormone replacement therapy” (CINAHL headings) OR “HRT” OR “oestrogen replacement therapy” (Keywords)] OR [“Exercise” OR “Physical Activity” (CINAHL headings)].
The Cochrane Library Database of Systematic Reviews was searched using broad keyword variations without MeSH terms to maximize retrieval, ensuring coverage of systematic reviews related to osteoporosis management in menopausal women. Boolean operators and truncation were consistently used across all databases to refine the search and capture relevant literature. Key terms used across the databases included: (“Osteoporosis” OR “Bone density”) AND (“Postmenopause” OR “Post-menopause” OR “Perimenopause” OR “Menopause*”) AND (“Hormone replacement therapy” OR “HRT” OR “oestrogen replacement therapy”) AND (“Exercise” OR “Physical activity”).
Relevant information from the selected studies was documented in a Microsoft Word document under the following headings: author(s), year of publication, study design, study aim, intervention, and key findings. The data were then analyzed and synthesized to align with the research findings.
3 Results
3.1 Study selection
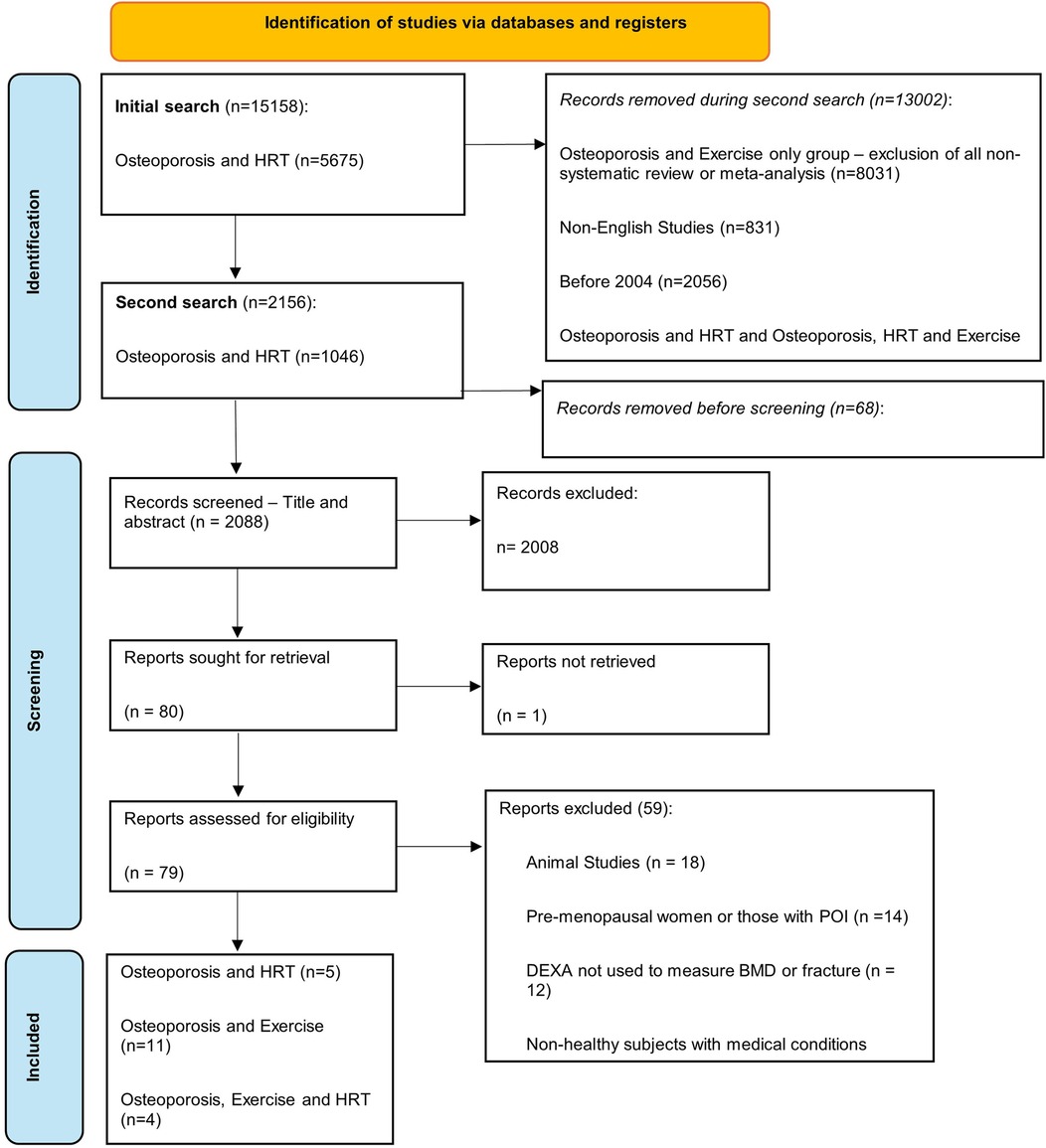
A total of 15,158 studies were identified for osteoporosis and MHT (n = 5,675), osteoporosis and exercise (n = 8,551), and osteoporosis and MHT and exercise combined (n = 932). The second circumscribed search identified 2,156 studies, of which 68 were removed due to being duplicates. Moreover, 2,008 studies were excluded after title and abstract screening, with 80 sought for retrieval. Furthermore, 79 studies were assessed for eligibility, with 59 excluded according to our eligibility criteria. Thus, 20 studies met the inclusion criteria and were included in this review. There were five studies for osteoporosis and MHT, 11 studies for osteoporosis and exercise, and four studies for osteoporosis and MHT and exercise combined (Figure 1).
3.2 Study characteristics
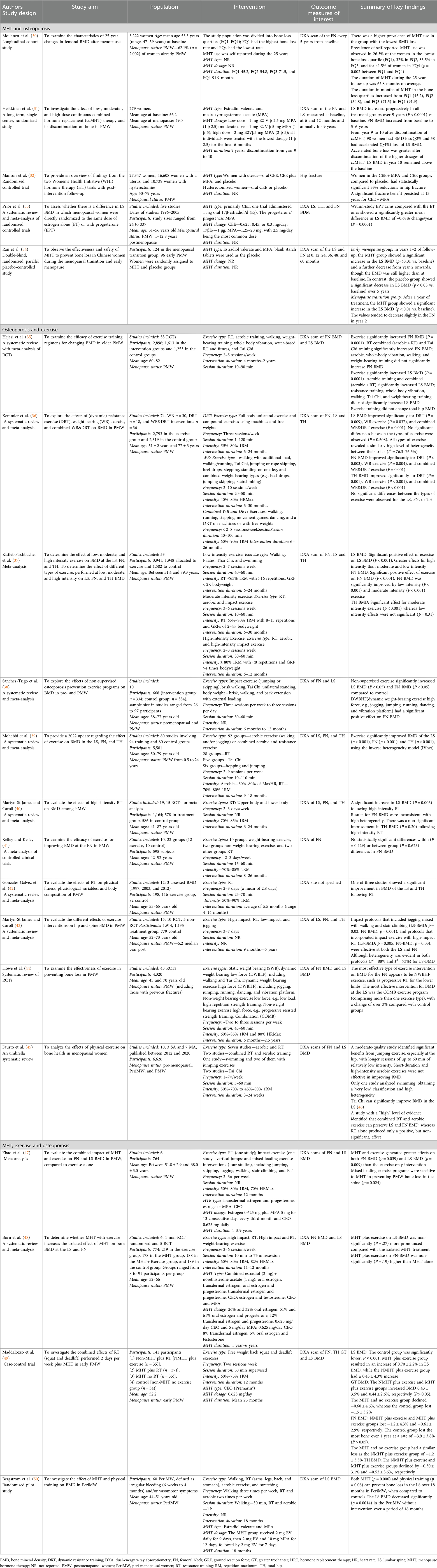
Of the 20 studies, the study designs included cohort studies (n = 1), case-control studies (n = 1), RCTs (n = 4), systematic reviews (n = 2), meta-analyses (n = 3), and systematic reviews with meta-analyses (n = 9). DXA was used in all studies, with one study also measuring the incidence of hip fractures. Among sites measured, 17 assessed lumbar spine (LS) BMD, 17 measured femoral neck (FN) BMD, one study measured the greater trochanter (GT), and one study did not specify the site of measurement. The study characteristics and results are described in detail in Table 2 and summarized in the following sections.
3.3 Osteoporosis and MHT
Evidence supports the use of MHT in the prevention of osteoporosis. A 25-year cohort study, involving 3,222 women, identified a negative correlation between MHT duration and BMD loss, with greater bone loss in PMW on MHT for 3.75 years compared to 7.66 years (30). Similarly, a 10-year study of 279 PMW examined the effect of continuous combined MHT [Estradiol valerate (E2 V) and MPA] at a low (1 mg E2 V þ 2.5 mg MPA (1 þ 2.5)], medium [1 mg E2 V þ 5 mg MPA (1 þ 5)], and high dose [2 mg E2Vþ5 mg MPA (2 þ 5)] on BMD, and the effect 1-year post-discontinuation after 9 years (31). Long-term low-dose MHT maintained FN BMD for 5–6 years and LS BMD for at least 9 years (31), with high dosages accelerating bone loss after discontinuation.
Combined MHT has been widely studied for its impact on BMD (32–34). The Women’s Health Initiative (WHI) RCT (32) involving 16,608 women with a uterus found a 33% reduction in hip fractures for those on CEO + MPA or CEO compared to placebo, with fracture benefits persisting at 13 years. A meta-analysis (33) involving studies with the number of participants ranging from 24 to 337 showed that combined MHT (predominantly CEO and MPA) had a greater effect on LS BMD than estrogen alone in PMW. Similarly, Ran et al. (34) confirmed that estradiol valerate and MPA increased or maintained BMD in 96 early PMW (aged 40–55 years old).
3.4 Osteoporosis and exercise
The impact of exercise on BMD has been widely investigated, with evidence supporting the use of exercise in managing BMD in menopausal women. DXA scans have confirmed the positive effect of exercise on the LS, FN, and TH T-scores (35–40). In contrast, studies have identified that prescribed exercise does not result in changes to TH BMD (35, 40) and the FN (41), though the latter was of lower quality, lacking quality appraisal and sufficient sample sizes.
3.4.1 Exercise types
RT has been extensively investigated, though results have varied. Hejazi et al. (35) identified that RT alone did not significantly increase the BMD of the LS and FN in 2,896 PMW. Gonzales-Galvez et al. (42) support this as two of three studies assessing RT alone had no significant improvement in BMD in 198 PMW, though most studies lasted only 6 months. Whereas RT alone, completed over 6 months, significantly preserved BMD in 1,164 PWM (40), suggesting that the duration of RT is an important factor.
Martyn-St James and Carroli (43) found that combining impact loading (running or jumping) and high-intensity RT with low-impact exercises (stairs and walking) can maintain LS and FN BMD in 1,914 PMW, though study quality was low as blinding of participants is difficult. Further studies (35, 44) have also shown that combined exercise programs significantly improved BMD in the LS, FN, and TH. Kemmler et al. (36) found no significant differences between RT and combined RT on the LS, FN and TH in 2,793 exercising participants; possibly due to participants being 8 years postmenopause, with already low BMD, making it more challenging for interventions to show significant improvements.
3.4.2 Exercise intensity
Studies have investigated the impact of different exercise intensities on BMD. Kistler-Fischbacher et al. (37) found high-intensity exercise more impactful on LS BMD than FN BMD in 3,941 participants, whereas Sanchez-Trigo et al. (38) showed that dynamic high-force weight-bearing exercises (e.g., jogging, jumping, running, and dancing) significantly improved FN BMD, but not LS BMD in 668 participants. Despite these results, both studies only included a small number of high-intensity studies, possibly due to the perceived risk to health; therefore, the between-group analysis may be underpowered (37, 38).
Kistler-Fischbacher et al. (37) identified that FN BMD significantly improved with low-intensity (P < 0.001) and moderate-intensity (P < 0.001) exercise. Moderate-intensity exercise was most effective for improving TH BMD, though again, there was insufficient data to meta-analyze the impact of high-intensity exercise, reducing the reliability of this conclusion. Evidence on low-intensity Tai Chi is conflicting. Yeh et al. (46) found that Tai Chi significantly improves LS BMD, while Hejazi et al. (35) reported significant increases in FN and TH BMD but not LS. In contrast, Sanchez-Trigo et al. (38) reported no significant effect and Polidoulis et al. (51) a low effect on BMD.
3.5 Osteoporosis and MHT and exercise combined
A meta-analysis by Zhao et al. (47) identified that MHT significantly increased the effects of exercise on LS (p = 0.009) and FN (p = 0.039) BMD compared with exercise alone in 764 PMW. Further analysis supported that combined high-impact activities (jumping, skipping, dancing, and hopping) with high-intensity RT are more responsive to both estrogen-only and combined MHT (47). In contrast, another meta-analysis by Born et al. (48) identified no significant difference between the effect of MHT and exercise vs. MHT alone on the FN and LS in 774 PMW.
Zhao et al. (47) included participants of varying ages, while Born et al. (48) focused on early PMW (within 10 years of menopause). Maddalozzo et al. (49) found that in 141 early PMW (within 3 years), completing only squats and deadlifts was more effective than MHT alone for preserving LS BMD. Bergström et al. (50) found that MHT and exercise, including walking and RT, prevented LS BMD loss in 60 perimenopausal women over 18 months, though a high dropout rate limited long-term conclusions. Thus, these studies suggest that exercise is most effective during the perimenopause and early postmenopause.
4 Discussion
Research has highlighted the benefit of using MHT in preventing osteoporosis in menopausal women. MHT prescribed for longer durations resulted in less bone loss (30), with low-dose MHT preferred, as higher doses accelerate bone loss after discontinuation (31). Furthermore, previous research supports the use of combined MHT rather than estrogen alone in preserving BMD (32–34), though this is only applicable in women with a uterus and when progesterone is tolerated. Exercise and MHT combined, compared to exercise alone and MHT alone, significantly improved BMD in menopausal women (47), demonstrating a positive estrogenic response to mechanical loading during exercise on BMD (52). This thus suggests that MHT should be considered as an adjunct to exercise.
None of the included studies explicitly examined the effect of MHT initiation at different time points after menopause, highlighting a gap in the current literature. The timing of MHT initiation post-menopause is a crucial factor in determining its effectiveness in preserving BMD. Research suggests that earlier initiation, typically within the first 10 years of menopause, may provide the greatest skeletal benefits (53). However, our review did not identify consistent data on the impact of delayed MHT initiation. This highlights the need for further research to clarify whether later initiation still offers protective effects or if there is a critical window beyond which benefits diminish.
Despite the effectiveness of MHT in preserving BMD, there are risks associated with its use. The most common estrogen used in these interventions was CEO, administered orally (32, 33, 47, 48), though given the risks reported by the WHI, there has been a significant shift toward transdermal estrogen. Transdermal estrogen, absorbed through the skin via patches, gels, or sprays, has been associated with a lower risk of venous thromboembolism and stroke (54, 55), as it can be prescribed in lower doses as it bypasses the enterohepatic circulation. Furthermore, the most common progesterone used in these studies was MPA (31–34, 47, 48), which, again, has been associated with adverse outcomes. Furthermore, a large meta-analysis by Garthlehner et al. (63), using CEO and MPA, presented similar risks to the WHI, including an increased risk of breast cancer, venous thromboembolism, and stroke when using combined MHT. Consequently, studies that primarily used CEO and MPA may not be fully generalizable to the current population, as prescribing patterns and clinical guidelines have evolved to favor safer delivery methods and formulations.
The International Menopause Society (56) and the British Menopause Society [BMS (57)] advocate the use of MHT as the treatment of choice for osteoporosis prevention; however, this is not supported by further national or international societies (64). The American Association of Clinical Endocrinologists [AACE; (58)] states that in line with FDA licensing guidelines, estrogen should be used for the prevention of postmenopausal osteoporosis in women at significant risk of osteoporosis and when non-estrogen options are unsuitable. The AACE also emphasizes that estrogen should only be prescribed for menopausal symptoms at the lowest dose for the shortest duration (58). Most guidelines prioritize bisphosphonates or denosumab as first-line treatments, recommending MHT only if these are unsuitable, in patients who are under 60 years old, or in those <10 years postmenopause and without previous myocardial infarction, stroke, or breast cancer (2, 59). These guidelines typically evaluate a wide range of evidence, providing strong evidence-based recommendations. Therefore, although MHT can successfully preserve BMD and reduce the risk of osteoporotic fractures, given the risks associated, the initiation of MHT in the prevention of osteoporosis is inconclusive. Further research is required to assess the impact of combined MHT using transdermal estrogen and micronized progesterone on BMD, as these display less harmful characteristics.
This review highlights the importance of exercise in maintaining BMD and managing postmenopausal osteoporosis without hormone supplementation (35–40). Despite these studies displaying heterogeneity between exercise intensity, type, duration, and frequency, the consensus is that PMW should complete combined regimes, including RT and impact activity (also referred to as weight-bearing exercise). To preserve BMD, RT needs to be at an intensity of 70%–85% 1RM completed at least twice per week for over 6 months, with longer interventions likely to produce better results. Impact activity, including jogging and jumping, should be completed at least 3 times per week. Low-impact activity, including Tai Chi, walking, and Pilates, can also be useful in addition to combined training, but should not be used as the sole intervention. Existing guidance from the Bone Health and Osteoporosis Foundation (60) and the National Osteoporosis Guideline Group (61) strongly recommends combined exercise in those at risk of osteoporosis.
Given that the optimal management for preserving BMD in menopausal women involves a combination of MHT and exercise, future research should build on this foundation by examining its broader implications—particularly its impact on mental health. Strong evidence links osteoporosis-related bone loss to psychological distress, including depression, anxiety, and suicidal ideation. A systematic review by Manning et al. (25), published in the British Journal of General Practice, found that osteoporosis and fractures, particularly vertebral fractures, are significantly associated with an increased risk of self-harm and suicide. Raising awareness of this dual burden is essential for optimizing patient care. Despite these findings, research has predominantly focused on skeletal outcomes, often relegating mental health to a secondary concern.
Future systematic reviews should therefore prioritize mental health as a primary outcome, investigating the combined effects of MHT and exercise on both skeletal and psychological wellbeing. By adopting this integrated approach, research can provide a more comprehensive understanding of how these interventions contribute to overall wellbeing in menopausal women, ultimately reinforcing the urgency of a comprehensive, multidisciplinary management approach and informing more holistic and effective management strategies.
4.1 Strengths and limitations
Although not a systematic review, a comprehensive search strategy was implemented, using the PRISMA-ScR framework. To our knowledge, this is the first review to scope literature from the last 20 years addressing the impact on osteoporosis of MHT, exercise, and MHT and exercise combined. However, we acknowledge that numerous guidelines from international societies and national bodies all consider the information contained in this review as part of their guidance. There is emerging evidence to which this review has added to existing knowledge.
This scoping review has several limitations. First, the inclusion of only systematic reviews and meta-analyses in the exercise and osteoporosis group may introduce bias from lower-quality studies and risk studies being missed. Additionally, the heterogeneity of exercise interventions and MHT preparations across studies, such as varying types, intensities, and durations, makes it difficult to compare outcomes and draw definitive conclusions, reducing the overall reliability and generalizability of the findings. Pharmacological treatments such as bisphosphonates and denosumab were not included, as the aim was to explore non-bisphosphonate strategies. However, we acknowledge this as a limitation, as pharmacological treatments remain essential in osteoporosis management. Future research comparing multiple treatment modalities, including pharmacological approaches, would provide a more comprehensive understanding of osteoporosis management in menopausal women. Finally, the small number of studies included that examined the combined effects on osteoporosis of MHT and exercise (n = 4) may impact the generalizability and validity of the results, as a broader body of evidence is needed to draw more robust conclusions about the combined interventions. Furthermore, the variability in study designs, methodologies, and outcome measures may contribute to uncertainty in the overall findings.
5 Conclusion
This review highlights that a combination of MHT and structured exercise offers the most effective approach for increasing BMD in menopausal women. For those with a uterus, combined estrogen and progestogen MHT has shown the greatest benefit in preserving bone health. However, due to ongoing debate surrounding the long-term safety of MHT for BMD preservation, exercise remains a critical and universally applicable strategy in the prevention and management of postmenopausal osteoporosis. Specifically, combined RT performed two to three times per week at an intensity of 70%–85% of 1RM, along with impact-loading activities such as jogging, jumping, or hopping at least three times per week, has been shown to be optimal for improving BMD in postmenopausal women. These interventions should be maintained for a minimum of 6 months and progress gradually in intensity and complexity to sustain their effectiveness.
In conclusion, the management of osteoporosis during menopause requires a personalized and multi-faceted approach. While MHT and exercise independently support bone health, their combined use may offer synergistic benefits. Clinical decision-making should weigh individual risk profiles and current evidence to guide effective and safe interventions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
OP: Writing – original draft, Writing – review & editing. JB: Writing – review & editing. SB: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to express our deepest gratitude to Professor Rachel Shaw, whose expertise in psychology has been invaluable to this manuscript. Their insightful feedback and guidance have enhanced the quality of our work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. NICE. Osteoporosis—prevention of fragility fractures: What are osteoporosis and osteoporotic fractures? (2023). Available at: https://cks.nice.org.uk/topics/osteoporosis-prevention-of-fragility-fractures/background-information/definition/ (Accessed August 2, 2024).
2. International Osteoporosis Foundation. Epidemiology of osteoporosis and fragility fractures (2024). Available at: https://www.osteoporosis.foundation/facts-statistics/epidemiology-of-osteoporosis-and-fragility-fractures (Accessed August 2, 2024).
3. Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. (2014) 29(11):2520–6. doi: 10.1002/jbmr.2269
4. Almeida M, Laurent MR, Dubois V, Claessens F, O'brien CA, Bouillon R, et al. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol Rev. (2017) 97(1):135–87. doi: 10.1152/physrev.00033.2015
5. Silva BC, Hipólito Rodrigues MA. Estrogen hormone therapy and postmenopausal osteoporosis: does it really take two to tango? Women Health. (2023) 63(10):770–3. doi: 10.1080/03630242.2023.2278211
6. Shah N, Ariel D. The role of menopausal hormone therapy in the prevention and treatment of low bone density in perimenopausal and postmenopausal women. Curr Opin Obstet Gynecol. (2023) 35(2):141–9. doi: 10.1097/GCO.0000000000000858
7. NICE. Menopause: Hormone replacement therapy (MHT) (2024). Available at: https://cks.nice.org.uk/topics/menopause/prescribing-information/hormone-replacement-therapy-MHT/ (Accessed August 5, 2024).
8. Chang WC, Wang JH, Ding DC. Conjugated equine estrogen used in postmenopausal women associated with a higher risk of stroke than estradiol. Sci Rep. (2021) 11(1):10801. doi: 10.1038/s41598-021-90357-6
9. Bhavnani BR, Stanczyk FZ. Pharmacology of conjugated equine estrogens: efficacy, safety and mechanism of action. J Steroid Biochem Mol Biol. (2014) 142:16–29. doi: 10.1016/j.jsbmb.2013.10.011
10. Rossouw JE, Anderson GL, Prentice RL, Lacroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. (2002) 288(3):321–33. doi: 10.1001/jama.288.3.321
11. Asi N, Mohammed K, Haydour Q, Gionfriddo MR, Vargas OLM, Prokop LJ, et al. Progesterone vs. synthetic progestins and the risk of breast cancer: a systematic review and meta-analysis. Syst Rev. (2016) 5(1):121. doi: 10.1186/s13643-016-0294-5
12. Goldberg T, Fidler B. Conjugated estrogens/bazedoxifene (Duavee): a novel agent for the treatment of moderate-to-severe vasomotor symptoms associated with menopause and the prevention of postmenopausal osteoporosis. Pharm Ther. (2015) 40(3):178–82.
13. Pinkerton JV, Harvey JA, Lindsay R, Pan K, Chines AA, Mirkin S, et al. Effects of bazedoxifene/conjugated estrogens on the endometrium and bone: a randomized trial. J Clin Endocrinol Metab. (2014) 99(2):E189–98. doi: 10.1210/jc.2013-1707
14. Chang X, Xu S, Zhang H. Regulation of bone health through physical exercise: mechanisms and types. Front Endocrinol (Lausanne). (2022) 13:1029475. doi: 10.3389/fendo.2022.1029475
15. American Heart Association. Target Heart Rates Chart. Dallas, TX: American Heart Association (n.d.). Available at: https://www.heart.org/en/healthy-living/fitness/fitness-basics/target-heart-rates (Accessed February 26, 2025).
16. Hooper SC, Marshall VB, Becker CB, Lacroix AZ, Keel PK, Kilpela LS. Mental health and quality of life in postmenopausal women as a function of retrospective menopause symptom severity. Menopause. (2022) 29(6):707–13. doi: 10.1097/GME.0000000000001961
17. Bromberger JT, Kravitz HM, Chang YF, Cyranowski JM, Brown C, Matthews KA. Major depression during and after the menopausal transition: Study of Women's Health Across the Nation (SWAN). Psychol Med. (2011) 41(9):1879–88. doi: 10.1017/S003329171100016X
18. Rubinstein HR, Foster JL. I don't know whether it is to do with age or to do with hormones and whether it is do with a stage in your life': making sense of menopause and the body. J Health Psychol. (2013) 18(2):292–307. doi: 10.1177/1359105312454040
19. Mollard E, Bilek L, Waltman N. Emerging evidence on the link between depressive symptoms and bone loss in postmenopausal women. Int J Women’s Health. (2017) 10:1–9. doi: 10.2147/IJWH.S147006
20. Wang Y, Li J, Zhang J. Association between osteoporosis and depression in postmenopausal women: a systematic review and meta-analysis. J Affect Disord. (2021) 282:159–67.
21. Smith A, Johnson P, Davis R. The impact of chronic arthralgia on mood and quality of life in osteoporotic patients. Rheumatol Int. (2020) 40(3):405–12.31606775
22. Gao S, Zhao Y. Quality of life in postmenopausal women with osteoporosis: a systematic review and meta-analysis. Qual Life Res. (2023) 32(6):1551–65. doi: 10.1007/s11136-022-03281-1
23. Aktaş Ö, Kaplan S, Sezer N. An assessment of the relation between bone mineral density and clinic-demographic properties and life quality during postmenopausal period. J Back Musculoskeletal Rehabil. (2018) 31(5):803–10. doi: 10.3233/BMR-170933
24. Chen F, Fu T, Lin Y, Fan C. Correlation of quality of life with risk factors for first-incident hip fracture in postmenopausal women. J Obstet Gynaecol Res. (2018) 44(6):1126–33. doi: 10.1111/jog.13637
25. Manning FM, Mughal F, Ismail HA, Baines LM, Chew-Graham CA, Paskins Z, et al. Osteoporosis and fracture as risk factors for self-harm and suicide: a systematic review and meta-analysis. Br J Gen Pract. (2023) 73(735):e735–43. doi: 10.3399/BJGP.2023.0035
26. Duralde ER, Sobel TH, Manson JE. Management of perimenopausal and menopausal symptoms. Br Med J. (2023) 382:e072612. doi: 10.1136/bmj-2022-072612
27. Mahindru A, Patil P, Agrawal V. Role of physical activity on mental health and well-being: a review. Cureus. (2023) 15(1):e33475. doi: 10.7759/cureus.33475
28. Anupama DS, Norohna JA, Acharya KK, Ravishankar George A. Effect of exercise on bone mineral density and quality of life among postmenopausal women with osteoporosis without fracture: a systematic review. Int J Orthop Trauma Nurs. (2020) 39:100796. doi: 10.1016/j.ijotn.2020.100796
29. Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. (2005) 8(1):19–32. doi: 10.1080/1364557032000119616
30. Moilanen A, Kopra J, Kröger H, Sund R, Rikkonen T, Sirola J. Characteristics of long-term femoral neck bone loss in postmenopausal women: a 25-year follow-up. J Bone Miner Res. (2020) 37(2):173–8. doi: 10.1002/jbmr.4444
31. Heikkinen J, Vaheri R, Haapalahti J, Timonen U. A 10-year follow-up of the effect of continuous-combined hormone replacement therapy and its discontinuation on bone in postmenopausal women. Br Menopause Soc J. (2008) 12(2):70–7. doi: 10.1258/MI.2008.008008
32. Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the women’s health initiative randomized trials. JAMA. (2013) 310(13):1353–68. doi: 10.1001/jama.2013.278040
33. Prior JC, Seifertklauss VR, Giustini D, Adachi JD, Kalyan S, Goshtasebi A. Estrogen-progestin therapy causes a greater increase in spinal bone mineral density than estrogen therapy—a systematic review and meta-analysis of controlled trials with direct randomization. J Musculoskeletal Neuronal Interact. (2017) 17(3):146–54.
34. Ran SY, Yu Q, Chen Y, Lin SQ. Prevention of postmenopausal osteoporosis in Chinese women: a 5-year, double-blind, randomized, parallel placebo-controlled study. Climacteric. (2017) 20(4):391–6. doi: 10.1080/13697137.2017.1325459
35. Hejazi K, Askari R, Hofmeister M. Effects of physical exercise on bone mineral density in older postmenopausal women: a systematic review and meta-analysis of randomized controlled trials. Arch Osteoporos. (2022) 17(1):102. doi: 10.1007/s11657-022-01140-7
36. Kemmler W, Shojaa M, Kohl M, Von Stengel S. Effects of different types of exercise on bone mineral density in postmenopausal women: a systematic review and meta-analysis. Calcif Tissue Int. (2020) 107(5):409–39. doi: 10.1007/s00223-020-00744-w
37. Kistler-Fischbacher M, Weeks BK, Beck BR. The effect of exercise intensity on bone in postmenopausal women (part 2): a meta-analysis. Bone. (2021) 143:115697. doi: 10.1016/j.bone.2020.115697
38. Sanchez-Trigo H, Rittweger J, Sañudo B. Effects of non-supervised exercise interventions on bone mineral density in adult women: a systematic review and meta-analysis. Osteoporos Int. (2022) 33(7):1415–27. doi: 10.1007/s00198-022-06357-3
39. Mohebbi R, Shojaa M, Kohl M, von Stengel S, Jakob F, Kerschan-Schindl K, et al. Exercise training and bone mineral density in postmenopausal women: an updated systematic review and meta-analysis of intervention studies with emphasis on potential moderators. Osteoporos Int. (2023) 34(7):1145–78. doi: 10.1007/s00198-023-06682-1
40. Martyn-St James M, Carroll S. High-intensity resistance training and postmenopausal bone loss: a meta-analysis. Osteoporos Int. (2006) 17(8):1225–40. doi: 10.1007/s00198-006-0083-4
41. Kelley GA, Kelley KS. Exercise and bone mineral density at the femoral neck in postmenopausal women: a meta-analysis of controlled clinical trials with individual patient data. Am J Obstet Gynecol. (2006) 194(3):760–7. doi: 10.1016/j.ajog.2005.09.006.
42. González-Gálvez N, Moreno-Torres JM, Vaquero-Cristóbal R. Resistance training effects on healthy postmenopausal women: a systematic review with meta-analysis. Climacteric. (2024) 27(3):296–304. doi: 10.1080/13697137.2024.2310521
43. Martyn-St James M, Carroll S. A meta-analysis of impact exercise on postmenopausal bone loss: the case for mixed loading exercise programmes. Br J Sports Med. (2009) 43(12):898–908. doi: 10.1136/bjsm.2008.052704
44. Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. (2011) 2011(7):CD000333. doi: 10.1002/14651858.CD000333.pub2
45. Fausto DY, Martins JBB, Machado AC, Saraiva PS, Pelegrini A, Guimarães ACA. What is the evidence for the effect of physical exercise on bone health in menopausal women? An umbrella systematic review. Climacteric. (2023) 26(6):550–9. doi: 10.1080/13697137.2023.2249819
46. Yeh M, Liao R, Hsu C, Chung Y, Lin J. Exercises improve body composition, cardiovascular risk factors and bone mineral density for menopausal women: a systematic review and meta-analysis of randomized controlled trials. Appl Nurs Res. (2018) 40:90–8. doi: 10.1016/j.apnr.2017.12.011
47. Zhao R, Xu Z, Zhao M. Effects of oestrogen treatment on skeletal response to exercise in the hips and spine in postmenopausal women: a meta-analysis. Sports Med. (2015) 45(8):1163–73. doi: 10.1007/s40279-015-0338-3
48. Born C, Jakob F, Shojaa M, Kohl M, von Stengel S, Kerschan-Schindl K, et al. Effects of hormone therapy and exercise on bone mineral density in healthy women-a systematic review and meta-analysis. J Clin Endocrinol Metab. (2022) 107(8):2389–401. doi: 10.1210/clinem/dgac180
49. Maddalozzo GF, Widrick JJ, Cardinal BJ, Winters-Stone KM, Hoffman MA, Snow CM. The effects of hormone replacement therapy and resistance training on spine bone mineral density in early postmenopausal women. Bone. (2007) 40(5):1244–51. doi: 10.1016/j.bone.2006.12.059
50. Bergström I, Freyschuss B, Landgren B. Physical training and hormone replacement therapy reduce the decrease in bone mineral density in perimenopausal women: a pilot study. Osteoporos Int. (2005) 16(7):823–8. doi: 10.1007/s00198-004-1758-3
51. Polidoulis I, Beyene J, Cheung AM. The effect of exercise on pQCT parameters of bone structure and strength in postmenopausal women—a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int. (2012) 23(1):39–51. doi: 10.1007/s00198-011-1734-7
52. Komht WM. Aging and the osteogenic response to mechanical loading. Int J Sport Nutr Exerc Metab. (2001) 11(s1):S137–42. doi: 10.1123/ijsnem.11.s1.s137
53. Hodis HN, Mack WJ. Menopausal hormone replacement therapy and reduction of all-cause mortality and cardiovascular disease. Cancer J. (2022) 28(3):208–23. doi: 10.1097/PPO.0000000000000591
54. Renoux C, Dell'Aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. Br Med J. (2010) 340:c2519. doi: 10.1136/bmj.c2519
55. Scarabin PY, Oger E, Plu-Bureau G. Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk. Lancet. (2003) 362(9382):428–32. doi: 10.1016/S0140-6736(03)14066-4
56. Baber RJ, Panay N, Fenton A. 2016 IMS recommendations on women’s midlife health and menopause hormone therapy. Climacteric. (2016) 19(2):109–50. doi: 10.3109/13697137.2015.1129166
57. Stevenson J, Collaboration with the Medical Advisory Council of the British and Menopause Society. Prevention and treatment of osteoporosis in post menopausal women (2022). Available at: https://thebms.org.uk/wp-content/uploads/2023/10/06-BMS-ConsensusStatement-Prevention-and-treatment-of-osteoporosis-in-women-SEPT2023-A.pdf (Accessed October 11, 2024).
58. Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, et al. American Association of clinical endocrinologists/American college of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract. (2020) 26(Suppl 1):1–46. doi: 10.4158/GL-2020-0524SUPPL
59. Shoback D, Rosen CJ, Black DM, Cheung AM, Murad MH, Eastell R. Pharmacological management of osteoporosis in postmenopausal women: an endocrine society guideline update. J Clin Endocrinol Metab. (2020) 105(3):587–94. doi: 10.1210/clinem/dgaa048
60. BHOF. Be Bone Strong™—Exercise/Safe Movement. Arlington, VA: Bone Health and Osteoporosis Foundation (2023). Available at: https://www.bonehealthandosteoporosis.org/patients/treatment/exercisesafe-movement/ (Accessed November 28, 2024).
61. NOGG. Section 5: Non-Pharmacological Management of Osteoporosis. United Kingdom: National Osteoporosis Guideline Group (2021). Available at: https://www.nogg.org.uk/full-guideline/section-5-non-pharmacological-management-osteoporosis (Accessed November 28, 2024).
62. Jones C, Gwenin C. Cortisol level dysregulation and its prevalence-is it nature's alarm clock?. Physiol Rep.. (2021) 8(24):e14644. doi: 10.14814/phy2.14644
63. Gartlehner G, Patel SV, Feltner C, Weber RP, Long R, Mullican K, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: evidence report and systematic review for the US Preventive Services Task Force. JAMA. (2017) 318(22):2234–49. doi: 10.1001/jama.2017.16952
Keywords: menopause, menopause hormone therapy, exercise, bone mineral density, osteoporosis
Citation: Platt O, Bateman J and Bakour S (2025) Impact of menopause hormone therapy, exercise, and their combination on bone mineral density and mental wellbeing in menopausal women: a scoping review. Front. Reprod. Health 7:1542746. doi: 10.3389/frph.2025.1542746
Received: 10 January 2025; Accepted: 21 April 2025;
Published: 12 May 2025.
Edited by:
Mihnea-Alexandru Găman, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Mohammad Tohidul Amin, Noakhali Science and Technology University, BangladeshSofia Lider, C.I. Parhon National Institute of Endocrinology, Romania
Kousalya Prabahar, University of Tabuk, Saudi Arabia
Copyright: © 2025 Platt, Bateman and Bakour. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olivia Platt, T2xpdmlhLnBsYXR0M0BuaHMubmV0
†ORCID:
James Bateman
orcid.org/0000-0003-1952-2821
Shagaf Bakour
orcid.org/0000-0002-9205-1555
 Olivia Platt
Olivia Platt James Bateman
James Bateman Shagaf Bakour
Shagaf Bakour