1
Swammerdam Institute for Life Sciences-Center for NeuroScience, University of Amsterdam, Amsterdam, The Netherlands
2
Department of Neuroscience, Erasmus Medical Center, Rotterdam, The Netherlands
Humans and rodents retain memories for stressful events very well. The facilitated retention of these memories is normally very useful. However, in susceptible individuals a variety of pathological conditions may develop in which memories related to stressful events remain inappropriately present, such as in post-traumatic stress disorder. The memory enhancing effects of stress are mediated by hormones, such as norepinephrine and glucocorticoids which are released during stressful experiences. Here we review recently identified molecular mechanisms that underlie the effects of stress hormones on synaptic efficacy and learning and memory. We discuss AMPA receptors as major target for stress hormones and describe a model in which norepinephrine and glucocorticoids are able to strengthen and prolong different phases of stressful memories.
Stress is a reaction to particular challenging or threatening events and is an essential part of our daily life. Stress increases our focus and alertness in order to cope with these situations and to prepare for similar future occasions. A little stress is therefore beneficial, but too much chronic, low-level stress can have rather deleterious consequences and is a major risk factor for mental diseases such as depression (e.g. Brown et al., 2004
).
Many studies have examined how stressful experiences modulate learning and memory (Joëls et al., 2006
). The literature yields a complex picture. There is ample evidence that prolonged stress hampers learning and memory processes in humans and rodents (Lupien et al., 2009
). Numerous studies show that (prolonged) exposure to mild stressors reduces hippocampal long-term potentiation (LTP) and facilitates long-term depression (LTD) (Artola et al., 2006
; Krugers et al., 2006
), two forms of synaptic plasticity believed to underlie learning and memory. On the other hand there is profound evidence that stress can facilitate, and might even be essential for, good learning and memory performance in rodents (Oitzl and de Kloet, 1992
; Sandi and Rose, 1994
; Roozendaal and McGaugh, 1996
; Sandi et al., 1997
; Oitzl et al., 2001
). Such, memory enhancing effects of stress have also been reported in humans (Lupien et al., 2002
; Smeets et al., 2009
). These apparently opposing effects of stress on memory may depend on time–stress and stress hormones mainly facilitate learning and memory processes when stress is experienced in the context and around the time of the event that needs to be remembered (Joëls et al., 2006
). Moreover, stress-hormones facilitate memory consolidation (Sandi and Rose, 1994
; Oitzl et al., 2001
), but suppress the retrieval of already stored information (Roozendaal et al., 2009
). Other factors that may play a role in the opposite effects of stress on memory are duration of stress (prolonged versus acute) as well as magnitude of stress (Alfarez et al., 2006
; Joëls et al., 2006
).
Identifying the molecular and cellular mechanisms how stress enhances learning and memory and synaptic function is a developing area of investigation. Recent studies have shown that hormones that are released during stress – like norepinephrine and corticosteroids – enhance hippocampal synaptic efficiency by differentially affecting AMPA receptor function. Norepinephrine and corticosterone rapidly but transiently affect AMPA receptor trafficking (Hu et al., 2007
; Groc et al., 2008
). In addition, glucocorticoids can also persistently modulate AMPA receptor dynamics (Groc et al., 2008
; Martin et al., 2009
). In this perspectives article we discuss these recently identified mechanisms and propose a new model in which these hormones via their effects on AMPA receptors promote synaptic efficiency and facilitate learning and memory processes. The influence of stress hormones on AMPA receptor trafficking might reshape our thinking about the basic machinery regulation plasticity at synapses.
In our daily lives we face many emotionally arousing experiences that can range from small displeasures to major life events such as accidents or loss of relatives. These potential threats of our bodily homeostasis are referred to as “stress”. One important function of stress is to induce long-term adaptive responses. Enhanced memory for stressful events is such a well-known highly adaptive phenomenon that helps to remember important information.
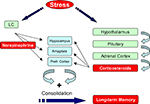
Exposure to stressful events activates multiple hormonal and neurotransmitter systems (Joëls and Baram, 2009
). Many of these systems have been reported to modulate memory processes, such as the Corticotropin-Releasing Factor (CRF) (Blank et al., 2003
). Being aware of this, we focus in this perspectives article on the roles of norepinephrine and corticosteroid hormones. During exposure to emotionally arousing events, norepinephrine (NE) is rapidly released in the brain by neurons that originate from the locus coeruleus (Figure 1
). The major targets of these projections include the hippocampus, prefrontal cortex, and amygdala which are critical for learning and memory (Joëls et al., 2006
). Exposure to arousing events also activates the Hypothalamo-Pituitary-Adrenal (HPA)-axis which results in a slower increased release of corticosterone (in most rodents) or cortisol (humans) from the adrenal glands (Figure 1
). These hormones enter the brain and bind to two subtypes of discretely localized receptors, the mineralocorticoid receptor (MR) and glucocorticoid receptor (GR). MRs are occupied when hormone levels are low and exert their effects classically via the genome (de Kloet et al., 2005
). Recent evidence shows that corticosteroid hormones can also exert rapid non-genomic effects via MRs (Karst et al., 2005
). In this way MRs could play a critical role during the initial phase of the stress response. GRs have a 10-fold lower affinity for corticosterone, become activated when hormone levels rise after stress and slowly exert genomic actions in the hippocampus (de Kloet et al., 2005
; Joëls et al., 2006
). Both MRs and GRs are localized in regions that are critical for memory formation such as hippocampus, amygdala, and prefrontal cortex.
Figure 1. Stress hormones enhance memory formation. Exposure to a fearful event rapidly activates the autonomic nervous system and enhances norepinephrine levels in the brain. At the same time the hypothalamo–pituitary-adrenal (HPA) axis is activated which causes a slow increase in plasma corticosterone levels. These hormones act via their receptors, which are present in regions that are critical for memory formation such as hippocampus, amygdala, and prefrontal cortex, to promote memory consolidation. For details see text. LC: Locus Coeruleus, Prefr. Cortex: Prefrontal Cortex.
Norepinephrine and corticosteroid hormones, via their receptors, play an important role in the memory enhancing effects of stress. NE enhances memory formation of emotional events via the brain β-adrenergic receptors (β-ARs) both in humans and rodents: post-training application of norepinephrine or β-ARs agonists promotes memory consolidation in different memory tasks such as inhibitory avoidance task, fear conditioning and in Morris water-maze learning (Hu et al., 2007
; Roozendaal et al., 2009
). Activation of α-adrenergic receptors also enhances memory, presumably by enhancing the actions of β-adrenergic actions (Ferry et al., 1999a
,b
). Corticosteroid hormones, via MRs have been implicated in the appraisal process and the onset of the stress response. Via GRs, these hormones promote long-term consolidation of information (de Kloet et al., 1999
; Roozendaal et al., 2009
). Accordingly, a point mutation in the mouse GR, which selectively prevents genomic actions, impairs spatial memory performance (Oitzl et al., 2001
). Importantly, recent literature suggests that both corticosteroids and norepinephrine work in concert for optimal memory performance both in humans and rodents (de Quervain et al., 2009
; Roozendaal et al., 2009
). Thus, noradrenergic activation is essential for the memory enhancing effects and glucocorticoids play a permissive role in noradrenergic actions thereby promoting memory formation (Roozendaal et al., 2009
).
The basolateral complex of the amygdala (BLA) is an important target for norepinephrine and corticosteroid hormones to facilitate memory for emotional events. Several studies suggest that hormonal changes in BLA-activity modulate memory consolidation by influencing plasticity in other brain regions that are critical for memory consolidation such as hippocampus and prefrontal cortex (Roozendaal et al., 2009
). This way, stress-hormones via the BLA can modulate different types (e.g. spatial) memory consolidation. However, norepinephrine and corticosteroid hormones can also influence hippocampal synaptic plasticity independent of BLA-input (Karst and Joëls, 2005
; Wiegert et al., 2006
; Hu et al., 2007
; Martin et al., 2009
). Hormonal regulation of hippocampal-dependent learning and memory processes therefore likely occurs via direct or indirect (via BLA) hormonal effects on hippocampal plasticity.
The cellular mechanisms via which NE and corticosterone facilitate learning and memory processes remain largely unknown. Via activation of β-ARs, NE can activate PKA and CaMKII (Wang et al., 2004
; Hu et al., 2007
). In agreement with their memory enhancing effects, NE, via activation of β-ARs promotes the induction of hippocampal long-term potentiation (LTP) (Winder et al., 1999
; Hu et al., 2007
). Corticosterone has been reported to rapidly increase hippocampal glutamate release probability (Karst et al., 2005
) and at the same time rapidly promotes the induction of hippocampal LTP (Wiegert et al., 2006
). However, corticosterone is best known for it’s slowly and prolonged effects via the genomic pathway. Upon activation, GR translocate to the nucleus and modify transcription of responsive genes, either through DNA binding of homodimers or through protein–protein interactions with other transcription factors (de Kloet et al., 2005
; Joëls et al., 2006
). In this way, neurons induce slow but persistent changes in protein expression and facilitate cell physiology changes over the time course of hours (Morsink et al., 2006
). Consistently, GR activation, via transcriptional regulation and the mitogen-activated protein kinase (MAPK) signaling pathway appears to be critically involved in the memory enhancement of contextual information by corticosteroid hormones (Revest et al., 2005
).
Despite these studies on second messenger systems that are modulated by stress hormones in parallel to their memory enhancing effects, our understanding of a molecular mechanism that can explain the memory enhancing effects of stress hormones is very limited. While glucocorticoids enhance the excitability of principal BLA cells by increasing their intrinsic excitability and decreasing the impact of GABA(A) Inhibitory Postsynaptic Potentials (IPSPs) (Duvarci and Paré, 2007
), recent findings indicate that hippocampal excitatory synapses are directly targeted by stress hormones (Karst and Joëls, 2005
; Hu et al., 2007
; Groc et al., 2008
; Martin et al., 2009
). We hypothesize that these stress-induced alterations in glutamatergic hippocampal synapses may explain – at least in part – how stress facilitates hippocampus-dependent learning and memory processes.
Synaptic plasticity at excitatory synapses is thought to be critical for information processing in the brain and to underlie many complex behaviors such as learning and memory. The best-studied forms of synaptic plasticity are long-term potentiation (LTP) and long-term depression (LTD) of excitatory synaptic transmission (Malinow and Malenka, 2002
; Bredt and Nicoll, 2003
). LTP involves the activity-dependent recruitment of AMPA receptors to the postsynaptic membrane and a concurrent increase in AMPA-mediated transmission whereas LTD reflects a decrease in synaptic AMPA receptor function.
The number and subunit compositions of synaptic AMPA receptors are carefully regulated in order to modify synaptic strength during plasticity (Shepherd and Huganir, 2007
). Although concentrated at the postsynaptic membrane, AMPA receptors are not static components of excitatory synapses. Instead, surface AMPA receptors are highly dynamic – they are continuously delivered and removed in and out of synapses in response to neuronal activity. Some synaptic membrane receptors can exchange with extrasynaptic receptors even in tens of milliseconds thereby underpinning the potential role of these receptors in tuning synaptic transmission (Heine et al., 2009
).
AMPA receptors are composed of four types of subunits, designated as GluR1 (GluA1), GluR2 (GluA2), GluR3 (GluA3), and GluR4 (GluA4), which combine to form tetramers. The subunit composition of AMPA receptors has been proposed to dictate the mode of AMPA receptor trafficking and their biophysical properties (Cull-Candy et al., 2006
). In the mature hippocampus, most AMPA receptors are heterotetrameric, consisting of symmetric ‘dimer of dimers’ of GluR2 and either GluR1 or GluR3 (Song and Huganir, 2002
; Mayer, 2005
). The leading model for constitutive and activity-dependent AMPA receptor trafficking is that basal synaptic transmission is primarily mediated by GluR2/3 heteromers, which undergo constitutive cycling into and out of synapses (Malinow and Malenka, 2002
). However, a recent study suggests that ∼80% of synaptic AMPA receptors in the hippocampus are GluR1/2 heteromers, and most of the remaining receptors are GluR2/3 heteromers (Lu et al., 2009
). Studies using overexpression of GluR1 in CA1 pyramidal cells reveal that synaptic insertion of GluR1 homomeric receptors is activity-dependent and can be elicited by LTP-inducing stimuli (Hayashi et al., 2000
). Likewise, heteromeric GluR1/2 receptors are also synaptically inserted during plasticity (Shi et al., 2001
; Kessels and Malinow, 2009
). Thus, activity dependent (and LTP-induced) delivery of AMPA receptors is believed to depend on the GluR1 subunit (Kessels and Malinow, 2009
). Whether LTP increases synaptic incorporation of endogenous AMPA receptors that consist of homomeric GluR1/1 receptors (Plant et al., 2006
) remains to be verified (Adesnik and Nicoll, 2007
). Once arrived at synapses, GluR1/2 heteromers are believed to be gradually replaced by the cycling GluR2/3 heteromers after LTP induction (Shi et al., 2001
; Plant et al., 2006
).
Multiple biochemical pathways can regulate the trafficking of synaptic receptors (Sheng and Kim, 2002
; Kennedy et al., 2005
). Recent studies demonstrate that AMPA receptor trafficking is regulated by both exocytotic and endocytotic processes and by their surface lateral diffusion in the plasma membrane (Choquet and Triller, 2003
; Kennedy and Ehlers, 2006
; Shepherd and Huganir, 2007
; Newpher and Ehlers, 2008
). Both GluR1/2 and GluR2/3 containing AMPA receptors are formed in the endoplasmic reticulum, move to the Golgi compartment where they become glycosylated and traffic to the postsynaptic membrane were they are anchored by PDZ-domain-containing scaffolding proteins (Kennedy and Ehlers, 2006
; Sheng and Hoogenraad, 2007
). Although, the precise molecular mechanisms that govern synaptic AMPA receptor incorporation remains unknown, recent findings shed new light on the underlying cellular processes. First, it was shown that endocytosis is important for the number of AMPA receptors at the membrane surface and recycling endosomes supply AMPA receptors for LTP (Park et al., 2004
). Recent evidence suggests that receptor recycling from postsynaptic endocytic zones – which are adjacent to the postsynaptic density – is crucial for maintaining a mobile population of surface AMPA receptors that can be synaptically inserted to increase synaptic strength (Blanpied et al., 2003
; Lu et al., 2007
; Petrini et al., 2009
). Second, although all AMPA receptor subunits contain PDZ-binding motifs, they interact with specific synaptic scaffold proteins (Bredt and Nicoll, 2003
; Kim and Sheng, 2004
). These synaptic scaffold proteins are thought to specifically regulate the synaptic clustering of different AMPA-type receptor and participate in their specific dynamic exocytosis, endocytosis and surface trafficking routes (Kennedy et al., 2005
). Other synaptic proteins required for the trafficking of AMPA receptors are stargazin and its related family of transmembrane AMPA receptor regulatory proteins (TARPs) (Nicoll et al., 2006
) and the recently identified mammalian cornichon homolog (CNIH) family of small transmembrane proteins (Schwenk et al., 2009
). Third, several studies show that LTP-dependent trafficking of GluR1 containing AMPA receptors is strictly controlled by postsynaptic signaling pathways. Phosphorylation of GluR1 at serine 818 (S818) by PKC is a critical event in the plasticity-driven synaptic incorporation of AMPA receptors (Boehm et al., 2006
), while phosphorylation of S831 by CaMKII and protein kinase C (PKC) and phosphorylation of S845 by cAMP-dependent protein kinase (PKA) affects the open-channel probability of the receptor but may also regulate the synaptic incorporation of AMPA receptor on the surface (Esteban et al., 2003
). Recently, it was found that PKC phosphorylation of the serine 816 (S816) and S818 residues of GluR1 enhanced binding of an actin-binding protein 4.1N to GluR1, facilitates extrasynaptic GluR1 insertion and is critical for expression of LTP (Lin et al., 2009
). Importantly, genetically modified mice with knock-in mutations that block GluR1 phosphorylation, show that S831 and S845 are critical for synaptic plasticity and memory (Lee et al., 2003
). In agreement, mice lacking the GluR1 subunit express deficits in short-term working memory (Reisel et al., 2002
).
The GluR2 subunit determines many of the major biophysical properties of the receptor. GluR2-containing AMPAR are almost impermeable for Ca2+, caused by their editing (Sommer et al., 1991
; Cull-Candy et al., 2006
; Isaac et al., 2007
). A number of studies suggest that this subunit dictates the removal of AMPA receptors from the synapse during LTD (Bredt and Nicoll, 2003
) and controls recycling and degradation of AMPA receptors after internalization (Lee et al., 2004
). The clathrin adaptor AP2 which is critical for endocytosis may be relevant in this respect since it specifically binds to GluR2. Recent work demonstrates a role for the extracellular domain of GluR2 in promoting spine and synapse formation (Passafaro et al., 2002
; Saglietti et al., 2007
). Moreover, the GluR2 subunit also has behaviorally relevance since spatial memory is hampered in mice with a forebrain specific loss of GluR2 (Shimshek et al., 2006
).
Evidence is accumulating that stress hormones like norepinephrine and glucocorticoids affect various aspects of AMPA receptor function. The nature of these effects indicates that they include both rapid non-genomic effects and slow genomic and protein synthesis dependent actions.
Rapid Effects
First, norepinephrine (NE) – which is released during the initial phase of the stress–response – induces phosphorylation of GluR1 at S845/S831 (Hu et al., 2007
). These non-genomic effects are rapidly induced, reversible and occur via activation of β-ARs and involve a PKA and CaMKII-dependent mechanism (Hu et al., 2007
). Moreover, phosphorylation of GluR1 at the S845/S831 site lowers the threshold for LTP, and facilitates emotional memory formation (Hu et al., 2007
). During this initial phase corticosteroid hormone levels start to rise. Via MR activation, these hormones rapidly and reversibly (via non-genomic actions) increase miniature excitatory postsynaptic currents (mEPSCs) frequency presumably reflecting increased presynaptic release of glutamate (Karst et al., 2005
) and increase lateral diffusion of predominantly GluR2 containing AMPA receptors (Groc et al., 2008
). At the same time MR activation promotes activity dependent synaptic increase in GluR2 containing AMPA receptors (Groc et al., 2008
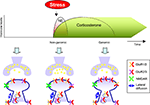
). Thus, a concept emerges showing that norepinephrine and MR activation rapidly but transiently via non-genomic actions promote synaptic GluR1/2-containing AMPA receptors (Figure 2
). This might contribute to a rapid non-genomic enhanced induction of LTP by both NE and corticosterone as reported before (Wiegert et al., 2006
; Hu et al., 2007
). Moreover, the increase in AMPA receptor mediated synaptic transmission might be relevant for the memory enhancing effects of these stress hormones. Associative fearful learning has been reported to drive GluR1-containing AMPA receptors to the membrane, which also underlies this form of learning (Rumpel et al., 2005
) and NE induced phosphorylation of GluR1 promotes contextual fear conditioning (Hu et al., 2007
). These studies are in agreement with behavioral studies in mutant mice showing that GluR1-containing AMPA receptors are relevant for short-term, but not necessarily long-term memory processes (Zamanillo et al., 1999
; Reisel et al., 2002
).
Figure 2. Differential effects of stress hormones on AMPA receptor trafficking. During the initial phase after stress exposure when norepinephrine (NE) are rapidly and reversible released activation of β-ARs and corticosteroid hormones via MRs modulate synaptic function; release probability, lateral diffusion, synaptic incorporation of GluR1/2 containing AMPA receptors, and (threshold for) LTP is enhanced. These effects require non-genomic actions. Second, corticosteroid levels slowly rise, return to baseline, but these hormones can have persistent genomic actions via GR activation. Via GR activation these hormones increase synaptic incorporation of GluR2/3 containing AMPA receptors, increase lateral diffusion, and suppress LTP.
Slowly Developing Effects
After the initial phase of a stressor, NE levels return to baseline and they no longer affect phosphorylation of AMPA receptors and induction of LTP (Hu et al., 2007
). However, plasma corticosterone levels still rise, and via slow genomic action can persistently affect neuronal function. Via GRs these hormones slowly increase synaptic incorporation, lateral diffusion and mobility of GluR2 containing AMPA receptors (Groc et al., 2008
; Martin et al., 2009
) along with slowly increasing AMPA receptor synaptic transmission (Karst and Joëls, 2005
; Martin et al., 2009
). Immunofluorescent studies demonstrate that corticosterone, via GR activation – and in a protein synthesis dependent manner – increases surface labeling of GluR2 containing AMPA receptors (rather than changing GluR1 levels). This suggests that glucocorticoids slowly – via genomic effects enhances synaptic levels of GluR2/3-containing receptors (Figure 2
). We hypothesize that this may be relevant for conserving synaptic strength. First, the exchange of GluR1/2 AMPA receptors with GluR2/3 containing AMPA receptors has been implicated in maintaining synaptic strength and thereby provides a possible mechanism how these hormones persistently increase synaptic strength (Shi et al., 2001
). Second, GluR2 containing AMPA receptors have been reported to promote spine formation which might provide a long-term structural adaptation to increase the capacity to store information (Passafaro et al., 2002
; Saglietti et al., 2007
). Third, after exposure to emotionally arousing events, via a mechanism that requires GR activation, the ability to induce LTP is reduced (Kim and Diamond, 2002
; Wiegert et al., 2005
). The precise mechanism(s) underlying this suppressive effect may involve occlusion of synaptic plasticity (Alfarez et al., 2006
; Groc et al., 2008
). An important functional implication could be that impairing LTP induction prevents the ability to overwrite network information. At the longer term the slow increase in synaptic GluR2/3 levels induced by stress hormones might therefore promote memory consolidation (Shimshek et al., 2006
), e.g. by increasing the number of synapses or altering synaptic properties (Passafaro et al., 2002
; Isaac et al., 2007
; Saglietti et al., 2007
).
Recent findings indicate that stress hormones like norepinephrine and corticosterone both rapidly and slowly increase AMPA receptors mediated synaptic transmission. Here, we hypothesize that differential effects on AMPA receptor trafficking may provide a cellular mechanism that underlies the memory enhancing effects of these hormones.
The fast effects of the norepinephrine and corticosterone mediated increase of surface AMPA receptor levels are most likely due to increased AMPA receptor trafficking (Hu et al., 2007
; Groc et al., 2008
). An important question that remains to be addressed is – what are the underlying molecular mechanisms? PKA appears to be involved in norepinephrine-induced trafficking of AMPA receptors (Hu et al., 2007
) and MR activation rapidly (via a non-genomic pathway) alters mEPSC frequency via the ERK pathway (Olijslagers et al., 2008
). However it remains to be investigated how MR activation alters lateral diffusion of AMPA receptors as well as the activity-dependent increase in synaptic AMPA receptors. Important candidates that mediate these rapid effects are the second messengers involved in rapid phosphorylation of AMPA receptors like PKA, CaMKII or PKC. In addition, it remains to be addressed how corticosterone slowly increases synaptic AMPA receptor levels. The 3-h delay in onset of GR-induced GluR2 surface expression and the dependence on protein synthesis suggests a mechanism involving transcriptional regulation of GR-affected genes. It is tempting to speculate that GR activation alters genes encoding proteins involved in regulating GluR2/3 delivery and/or membrane anchoring. On one hand, there are multiple potential candidates that could directly influence AMPA receptor trafficking, including auxiliary subunits of AMPA receptors, such as CNIH and TARPs (Nicoll et al., 2006
; Schwenk et al., 2009
), scaffolding proteins, such as PICK1 and GRIP (Terashima et al., 2004
; Lu and Ziff, 2005
) and trafficking regulators NSF and AP2 (Lee et al., 2002
; Kastning et al., 2007
). However, no effect was seen in total AP2 levels in corticosterone treated hippocampal neurons (Martin et al., 2009
).
On the other hand, there are probably even more candidate proteins and regulatory mechanism that could more indirectly effect AMPA trafficking. For example, an increase in surface GluR2/3 receptors could be achieved by slowing down endocytosis or increasing the recycling of internalized receptors by affecting the general trafficking machinery or one of the multiple synaptic signaling pathways. Moreover, AMPA receptor surface mobility is known to be regulated by several Ca2+-dependent processes such as depolarization, glutamate treatment, and tetanic stimulation (Tardin et al., 2003
; Groc et al., 2004
; Newpher and Ehlers, 2008
; Heine et al., 2009
). It is very well possible that changes in Ca2+ signals contributes to the described increase in GluR2 surface mobilization. Norepinephrine and corticosterone could regulate Ca2+ influx by modulating glutamate receptors or voltage-sensitive Ca2+ channel activity or affect downstream kinases and phosphates signalling (Bloodgood and Sabatini, 2007
). Importantly, corticosteroid hormones have been shown to be potent regulators of calcium channels and currents (Van Gemert et al., 2009
). It is also possible that corticosterone affects the diffusion barrier that normally limits AMPA receptor diffusion in the membrane (Martin et al., 2009
). Some answers about the molecular mechanism behind the slow GR-induced GluR2 surface expression may come from the several hundred recently identified GR-affected target genes in a micro array survey using corticosterone-treated hippocampal slices of rats (Morsink et al., 2006
).
Under basal conditions the corticosterone-invoked increase in freely mobile GluR2 facilitates recruitment and increases synaptic efficacy (Martin et al., 2009
). In this way, corticosterone could potentially promote consolidation of information. Furthermore, it has been described that enhanced AMPA receptor levels a few hours after corticosterone application occlude LTP (Groc et al., 2008
). Interestingly, corticosterone also facilitates NMDA receptor-invoked endocytosis of both synaptic and extra-synaptic GluR2 under conditions that weaken synaptic transmission (Martin et al., 2009
). An important functional implication of these effects could be that corticosterone on the longer term accentuates synaptic efficacy (Martin et al., 2009
). A behaviorally very relevant question is therefore how and whether AMPA receptors mediate the memory enhancing effects of stress hormones like corticosterone and norepinephrine. Studies using mice carrying mutations in the GluR1 phosphorylation sites indicate that norepinephrine-regulated phosphorylation of GluR1 facilitates emotional memory (Hu et al., 2007
). Additional studies using AMPA receptors with deletion of functional GluR2 and/or GluR3 subunits will be required to establish whether and which AMPA receptors are essential for stress (hormone) induced facilitation of learning and memory. Temporal erasure of functional AMPA receptors may reveal whether regulation of AMPA receptor function is critical for stress-induced facilitation of acquisition of information, consolidation of information or both.
Finally, there are several different compounds that are released upon exposure to stress such as neurotransmitters (e.g. norepinephrine), neuropeptides and steroid hormones (e.g. corticosteroid hormones) (Joëls and Baram, 2009
). Functionally, these individual mediators may act together for optimal memory performance (de Quervain et al., 2009
; Roozendaal et al., 2009
) and cellular plasticity (Pu et al., 2007
). Therefore it will be extremely relevant to precisely examine whether and how different stress-mediators together affect AMPA receptor function and modulate learning and memory processes.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Harmen J. Krugers is supported by the Royal Dutch Academy of Arts and Sciences (05CDP013 and 08CDP004), Dutch Brain Foundation (13F05.02 and 2008 (1).07). Casper C. Hoogenraad is supported by Netherlands Organization for Scientific Research (NWO-ALW and NWO-ECHO), Netherlands Organization for Health Research and Development (ZonMw-VIDI, ZonMw-TOP), Human Frontier Science Program Career Development Award (HFSP-CDA) and European Science Foundation (European Young Investigators (EURYI) Award).
Blank, T., Nijholt, I., Grammatopoulos, D. K., Randeva, H. S., Hillhouse, E. W., and Spiess, J. (2003). Corticotropin-releasing factor receptors couple to multiple G-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: role in neuronal excitability and associative learning. J. Neurosci. 23, 700–707.
Lee, H. K., Takamiya, K., Han, J. S., Man, H., Kim, C. H., Rumbaugh, G., Yu, S., Ding, L., He, C., Petralia, R. S., Wenthold, R. J., Gallagher, M., and Huganir, R. L. (2003). Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 112, 631–643.
Van Gemert, N. G., Carvalho, D. M., Karst, H., van der Laan, S., Zhang, M., Meijer, O. C., Hell, J. W., and Joëls, M. (2009). Dissociation between rat hippocampal CA1 and dentate gyrus cells in their response to corticosterone: effects on calcium channel protein and current. Endocrinology [Epub ahead of print].
Zamanillo, D., Sprengel, R., Hvalby, O., Jensen, V., Burnashev, N., Rozov, A., Kaiser, K. M., Köster, H. J., Borchardt, T., Worley, P., Lübke, J., Frotscher, M., Kelly, P. H., Sommer, B., Andersen, P., Seeburg, P. H., and Sakmann, B. (1999). Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science 284, 1805–1811.