- 1BASF Australia Ltd., Southbank, VIC, Australia
- 2BASF Belgium Coordination Center, Technologiepark-Zwijnaarde, Ghent, Belgium
In December 2022, the governments of 196 countries adopted the Kunming-Montreal Global Biodiversity Framework (KMGBF), a strategic plan to support and advance implementation of the objectives of the Convention on Biological Diversity (CBD) and its subsidiary agreements, including the Cartagena Protocol on Biosafety (Protocol). The KMGBF includes a “biosafety” target (Target 17), that reflects the CBD obligations for Parties to implement biosafety measures, and measures for handling biotechnology and distributing its benefits. The unprecedented inclusion of a biosafety target in the KMGBF, with explicit recognition of benefits and its placement amongst other targets for “tools and solutions for implementation and mainstreaming”, has ignited hope for renewed recognition of the potential for biotechnology to contribute to global environmental goals. This would mark a shift in this international forum that began with these intentions, but subsequently changed focus towards the potential adverse impacts of biotechnology and restrictive application of precaution. Simultaneously, a decade-long program of work on “synthetic biology” has been examining the implications of new developments in biotechnologies for the objectives of the CBD, with an emphasis on the scope and adequacy of existing biosafety measures, and more recently, “horizon scanning” for new technological developments. This review provides an overview of the status of biotechnology/synthetic biology policy developments under the CBD, focusing on the period from the drafting of the KMGBF in 2018 to current programs of work resulting from decisions made at the 2024 United Nations Biodiversity Conference. These are expected to have implications for biotechnology/synthetic biology capacity development and adoption, and implementation of the KMGBF. Relevant parallel policy developments under other international fora, including the International Union for the Conservation of Nature and Natural Resources (IUCN) and the Organisation for Economic Cooperation and Development (OECD), are also examined.
1 Introduction
The year 2025 marks the 50th anniversary of the 1975 Asilomar meeting on recombinant DNA (rDNA) technology, where the foundations for the safe use of biotechnology were established prior their practical application (Berg et al., 1975). Innovation in biotechnology expanded through the late 20th century, both driven by commercial interest and tempered by anti-biotechnology activism, and rDNA and other biotechnologies have since been widely adopted and applied in various sectors, including agriculture (e.g., ISAAA, 2020; Bennett et al., 2013; Mannion and Morse, 2012; also Roell and Zurbriggen, 2020), healthcare (e.g., Soozanipour et al., 2023; Assidi et al., 2022), and industry (e.g., Tang and Zhao, 2009; Soetaert and Vandamme, 2006), and in scientific fields including conservation (e.g., International Union for the Conservation of Nature and Natural Resources, 2019; Corlett, 2017; Cruz-Cruz et al., 2013). This growth was accompanied by the development of national regulatory policies, guidelines and frameworks aimed at addressing potential risks to human health and the environment associated with the technologies and/or the resulting organisms and products (Turnbull et al., 2021; also Herring and Paarlberg, 2016). These developments were compelled by recognition of the potential (and now demonstrated) benefits of biotechnological applications, including for national development strategies, and the need to safely facilitate innovation, but within regulatory frameworks that anticipate and manage potential adverse impacts (Ad Hoc Working Group of Experts on Biological Diversity, 1990).
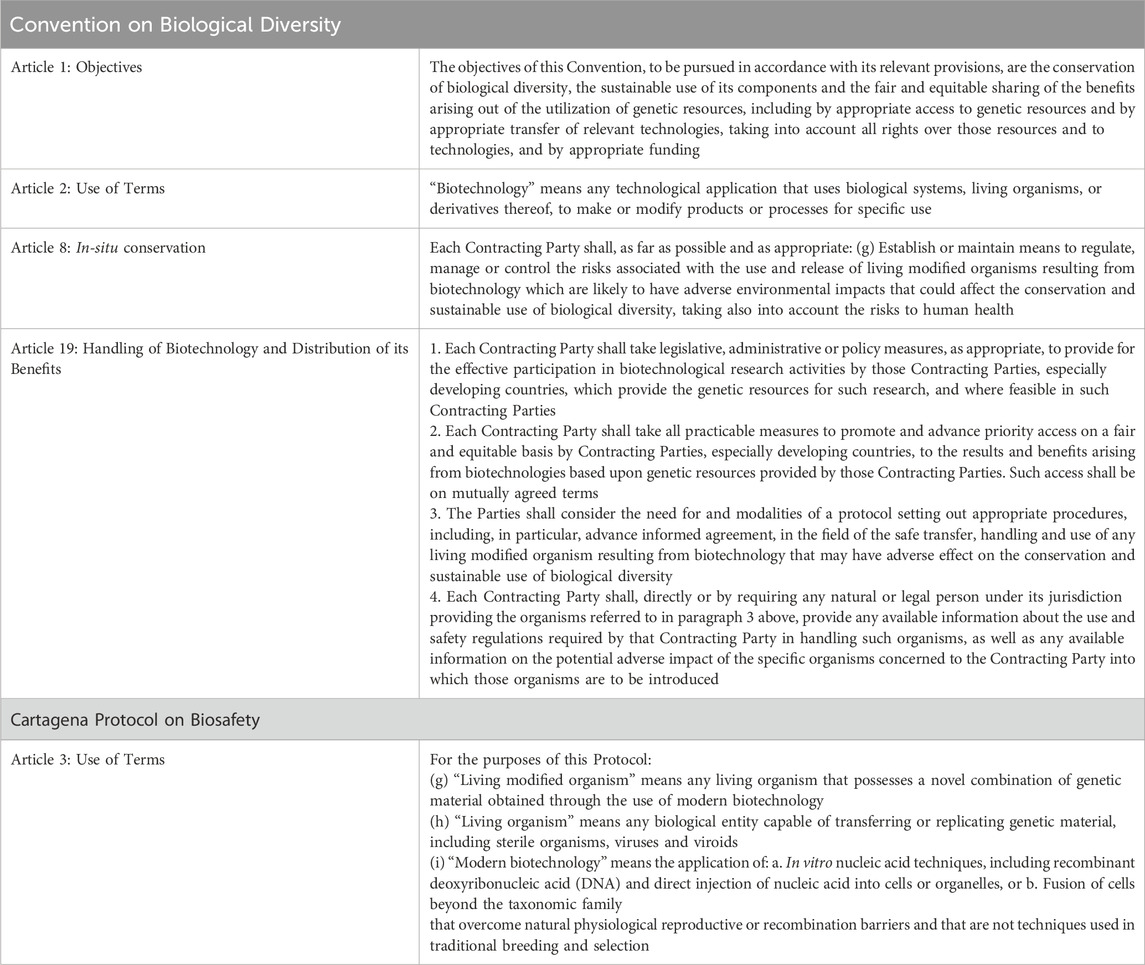
At the international level, “biosafety” regulation was provided for through provisions in the United Nations Convention on Biological Diversity1 (CBD), and more specifically where these involve transboundary movements, by its subsidiary agreement, the Cartagena Protocol on Biosafety (Protocol) (SCBD, 2000). This international regime takes a precautionary approach to “living modified organisms” (LMOs), setting out procedures for the assessment of potential adverse impacts (i.e., risk assessment) and their regulation, management or control (Glowka et al., 1994; CBD Article 8 (g); Protocol Article 1). In practice, for much of the past 3 decades regulatory procedures based on (or consistent with) this regime have predominantly applied to agricultural LMOs, e.g., the use and release into the environment (i.e., cultivation) of LM crops, and the handling and transport of agricultural commodities in international trade. More recently, other types of LMOs have received more attention in this forum, e.g., LM fish and LM insects for public health applications, particularly in the context of reviewing approaches to risk assessment.2
During the drafting of the CBD, the potential benefits of biotechnology were recognized (Ad Hoc Working Group of Experts on Biological Diversity, 1990), with its essential role in the attainment of the objectives of the CBD expressly recognized in its text (CBD Article 16), along with the need for “biosafety” regulation (CBD Article 8(g)). However, in subsequent work to support implementation, a near-exclusive focus on precaution has been maintained and continues to exert strong influence in this forum even today. In this paradigm of inherent risk associated with the use of biotechnology, anti-biotechnology activism became established and contributed to restricting efforts to facilitate biotechnology adoption and application (Herring and Paarlberg, 2016; Nature, 2017; Paarlberg, 2014; Strauss et al., 2009; De Greef, 2004; Arts and Mack, 2003). There is ample commentary on the agricultural focus of Protocol implementation and policy development, and the resulting detrimental impact on innovation, trade and food security (e.g., Ludlow et al., 2025), however this is not the scope of this review. Despite these policy challenges, the field of biotechnology has matured and various categories of biotechnological products have entered the market, supporting the view that any potential risks have been adequately addressed by the established regulatory approaches [e.g., environmental (agriculture) applications in Pellegrino et al. (2018), Klümper and Qaim (2014), also Voigt (2020)].
Since the turn of the century, with the emergence of “new” biotechnological tools and technologies and expansion of their potential applications, the international regulatory discourse has shown a gradual shift from the predominant focus on precautionary risk regulation to greater consideration of the potential benefits, including for the increasingly urgent global environmental concern of biodiversity decline (e.g., see Trump et al., 2023; OECD, 2014). This time however, while the debate remains contentious, a change in paradigm is supported by 4 decades of experience with environmental applications of biotechnology, an accumulated wealth of regulatory and scientific knowledge and understanding, as well as expertise in environmental risk assessment and risk management. Under the CBD, this shift is particularly evident with a new program of work (since 2024) aimed at developing and building capacity in “synthetic biology” in developing countries to support implementation of the CBD’s objectives and its current strategic plan, the Kunming-Montreal Global Biodiversity Framework (KMGBF).3 The evolving discourse under the CBD is examined in detail in this review, spanning the drafting of the KMGBF that started in 2018 through to recent decisions made on synthetic biology and related topics at the 2024 UN Biodiversity Conference. This review also considers relevant parallel policy developments in two other international fora, the International Union for the Conservation of Nature and Natural Resources (IUCN) and Organisation for Economic Co-operation and Development (OECD), that may influence the future evolution of the CBD discourse.
2 Biotechnology in the CBD
The Earth Summit (also known as the United Nations Conference on Environment and Development (UNCED)) held in Rio de Janeiro in 1992, was a landmark conference focused on sustainable development, and it resulted in several key outcomes including the adoption of Agenda 21 (United Nations Department of Public Information, 1993). This action plan outlined objectives for sustainable development and international cooperation, aimed at addressing the issues of the time and preparing for the challenges of the twenty-first century.4 Agenda 21 recognized the then-emerging field of biotechnology and its potential for enhancing environmental sustainability, with emphasis placed on improving food security and human health, and environmental protection, with the latter encompassing both beneficial applications and “environmentally sound” management.5 The regulatory aspects established in Agenda 21 are codified in the CBD, which was opened for signature at the Earth Summit then entered into force only 18 months later on 29 December 1993 (Ekardt et al., 2023; Glowka et al., 1994). With 196 member governments (“Parties”), the CBD is among the most ratified of the UN conventions.6
The text of the CBD reflects the growing global commitment to sustainable development, with express recognition of the need to balance conservation objectives with sustainable use (Glowka et al., 1994). It has three primary objectives: the conservation of biological diversity, the sustainable use of its components and the fair and equitable sharing of the benefits arising out of the utilization of genetic resources (Table 1; CBD Article 1). The third objective reflects a compromise struck between developing countries and developed countries intended to support the transfer of financial, scientific and technical (including biotechnology) resources from wealthy countries to developing countries to incentivize implementation of the first two objectives (Ekardt et al., 2023; Fredriksson, 2021). In support of these objectives, the CBD incorporated two new focus areas in biodiversity regulation: provisions for the safe use of “biotechnology” (CBD Article 8(g)), as mentioned above, and access and benefit sharing (CBD Article 15) provisions applicable to “genetic resources” (Glowka et al., 1994), the latter being the raw materials used in research and development including biotechnological applications. These provisions have produced two subsidiary agreements with more detailed regulatory procedures: the biosafety Protocol that entered into force 10 years after the CBD (11 September20037), and the Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization (SCBD, 2011) that entered into force 21 years later (12 October20148). The CBD remains the primary international forum for regulatory policy development in these two interconnected areas and the discussions and decisions made can influence technology adoption, research and development activities with biotechnology, and commercialization of its products.
In December 2022, the CBD’s 15th Conference of the Parties (COP-15; see Table 2) adopted the KMGBF.9 This occasion was widely reported as the “Paris moment” for nature (Slavin, 2022)10, with comparisons to the Paris Agreement of the CBD’s “sister” Earth Summit Convention, the United Nations Framework Convention on Climate Change.11 In essence, the KMGBF is a strategic plan for the implementation of the objectives of the CBD, and it builds on previous CBD strategic plans to present a revised and ambitious biodiversity agenda, with its mission to halt and reverse global biodiversity loss by 2030 and vision to be “Living in Harmony with Nature” by 2050 (KMGBF Section F) (Hughes and Grumbine, 2023). The KMGBF is the first CBD strategic plan to include a “biosafety target” (Target 17), with its text reflecting CBD provisions requiring Parties to implement biosafety measures (CBD Article 8(g)), and measures for handling biotechnology and distributing its benefits (CBD Article 19) (Table 1). The inclusion of a biosafety target in a CBD strategic plan signaled a possible shift in sentiment in this forum to supporting the implementation of biotechnologies towards advancing the CBD’s objectives. The development of this Target is examined in more detail in Section 3.2 below.
Table 2. Overview of CBD processes referred to in this review (SCBD, 2005).
Article 8(g) of the CBD on biotechnology regulation is also the basis for a now decade-long program of work on “synthetic biology”. This work has involved consideration of the implications of “new” developments in biotechnologies and/or their application for the objectives of CBD (Keiper and Atanassova, 2020), and it has led to additional interrelated areas of work. One example is the development of additional voluntary guidance materials to support case-by-case risk assessments of LMOs containing engineered gene drives in accordance with the risk assessment provisions of the Protocol (Ad Hoc Technical Expert Group on Risk Assessment, 2024). It was also the origin of debate about broadening benefit sharing to cover not only the use of physical genetic resources, as stated in the third CBD objective, but also the information related to these resources (termed “digital sequence information” or DSI) (Ad Hoc Technical Expert Group on Synthetic Biology, 2015). The CBD synthetic biology program of work is examined in more detail in Section 4 below.
There is no consensus on what DSI constitutes (Ad Hoc Technical Expert Group on Digital Sequence Information on Genetic Resources, 2018; also Silvestri and Roig-Cerdeño, 2025), but it is generally recognised that it would capture genetic sequence information which is essential for biotechnological applications (Kreiken and Arts, 2024), and the link between DSI and synthetic biology is recognised in COP decisions.12 The considerations of the implications of synthetic biology for the objectives of the CBD have highlighted changing scientific practices enabled by developments in biotechnological tools and technologies, particularly the emerging trend of “dematerialisation”, which refers to the separation of physical matter (i.e., genetic resources) from associated information (Bond and Scott, 2020; Laird and Wynberg, 2018). Concerns about undermining the third objective of the CBD with increasing use and exchange of DSI–instead of the genetic resources that may have benefit sharing obligations attached to them–gathered momentum until DSI was decoupled from the work program on synthetic biology and a substantial program of work was established towards the development of a benefit sharing solution (see COP decisions13).
3 Development of the KMGBF and Target 17
3.1 A brief history
Action agendas supported by targets for global environmental governance have become increasingly common since the beginning of this century. Multilateral agreements, global goals (e.g., the Millennium Development Goals (United Nations, 2010), and the Sustainable Development Goals14), and previous CBD strategic plans have adopted targets to define a coherent action agenda, enable more visible measurement and reporting on progress, and support their credibility (Hagerman and Pelai, 2016). The KMGBF succeeds and replaces the CBD’s 2011–2020 Strategic Plan for Biodiversity (the “Aichi Targets”) and the preceding 2002–2010 strategic plan (the “2010 Biodiversity Target”).15 The implementation of earlier CBD strategic plans did not meet expectations, with the failure of Parties to deliver on most of the Aichi Targets reported widely (e.g., Maney et al., 2024; Ekardt et al., 2023; Dickie, 2022; Nature 2020). Learning from past experience, Parties developed the KMGBF with a greater focus on SMART (specific, measurable, ambitious, realistic, and time-bound) targets aligned with identified underlying drivers of biodiversity loss. It is also accompanied by enhanced monitoring mechanisms, with a “monitoring framework” consisting of indicators to measure progress and assist alignment with national strategies (Hughes, 2023; Hughes et al., 2022; Perino et al., 2021; Butchart et al., 2016).
Preparations for the current CBD strategic plan began back in 2016,16 with a process for its development adopted by COP-14 in 2018.17 A “zero-draft”18 was circulated in January 2020 (Tsioumani, 2020), followed by an “updated zero draft”19 in July 2020 and “first draft”20 in July 2021,21 with all versions developed though broad consultative input from Parties and other stakeholders. However, by the time of the COP-15 in 2022, much of the draft text remained unresolved with Parties reaching agreement on only about 20% of it. This was attributed in part to the loss of in-person negotiations at the height of the COVID-19 pandemic and a reduced willingness to compromise (Hughes, 2023). Ultimately, the KMGBF adopted at COP-15 consists of hard-fought compromises that give Parties flexibility in implementation, with trade-offs reflecting the tension between the SMART elements of “ambition” and “reality” (Streck, 2023, also Diaz, 2022).
The success of the KMGBF ultimately relies on CBD Parties mainstreaming and implementing it at national levels. Mechanisms to support this include the requirement for Parties to submit National Reports (their seventh National Reports are requested by February 202622) that include measures taken towards implementation of commitments they have made in National Biodiversity Strategies and Action Plans (NBSAPs). NBSAPs are a policy instrument capturing national strategies and specific actions that governments intend to take to achieve them (Maney et al., 2024; Carroll et al., 2022; Hagerman and Pelai, 2016). CBD Parties were expected to submit updated NBSAPs by COP-16 (October 2024) to reflect the new targets of the KMGBF (Carroll et al., 2022). At the time of writing - 2 years post KMGBF adoption23 - only 46 Parties have submitted NBSAPs aligned with the KMGBF, and 77 Parties have set national targets for every KMGBF target.24 For Target 17, 103 CBD Parties have set at least one national target, with these appearing to vary in their degree of alignment with the KMGBF target, with Parties self-reporting 57% of their targets to be highly aligned with Target 17, 23% with medium alignment, 10% with low alignment, and 13% not reported.25 This varying degree of alignment could complicate the effective implementation of national targets as tools for achieving the objectives of the KMGBF.
3.2 A new biosafety Target
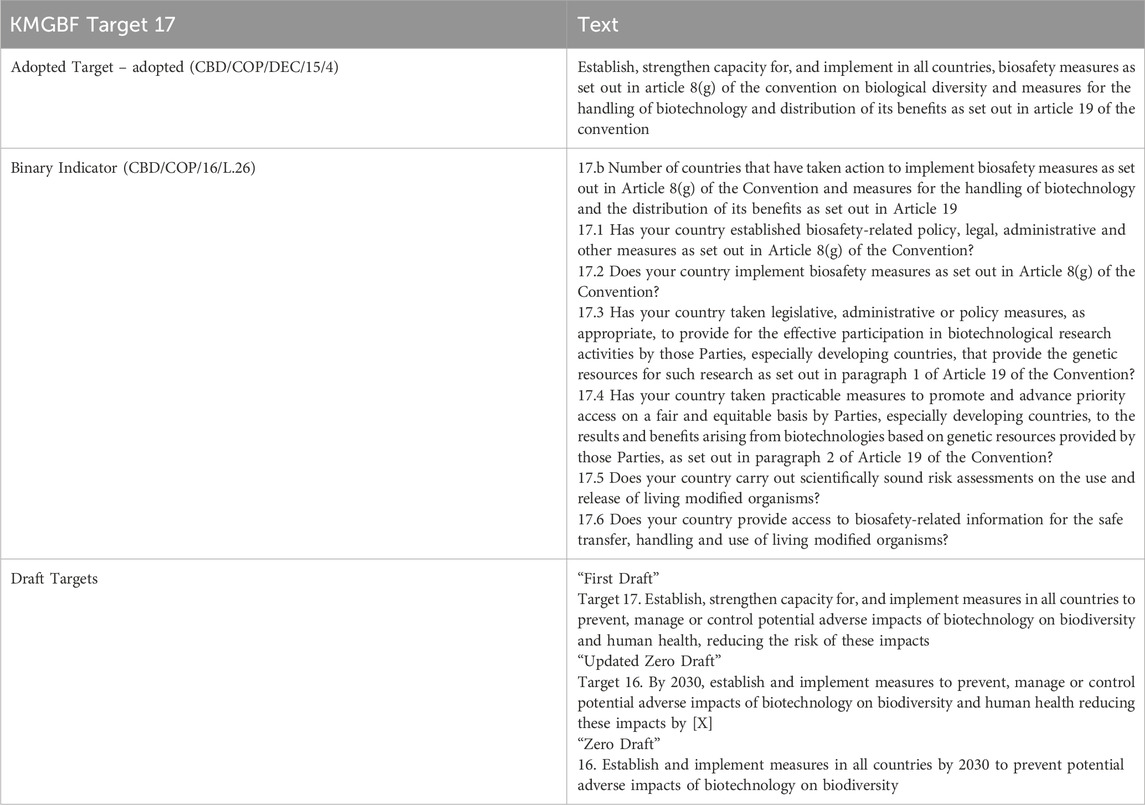
Target 17 reflects the CBD biotechnology provisions to establish and implement biosafety measures (CBD Article 8(g)) and measures for the handling of biotechnology and distribution of its benefits as set out in CBD Article 19 (see Table 1), with strengthening of capacity applicable to both areas also required. Historically, CBD capacity building resources have concentrated on the development of national biosafety frameworks, and early COP discussions on Article 8(g) were focused on one paragraph of Article 19 only–Article 19 (3) that provides the legal basis for the development of a protocol setting out procedures for the “safe transfer, handling and use” of LMOs, with this ultimately resulting in the Protocol (SCBD, 2005). Regulatory procedures are also supported by Article 19 (4) that requires information on use and safety regulations, and on potential adverse impacts where this information is available, to be provided to Parties where an LMO will be introduced.
Less attention has been paid to the first two paragraphs of CBD Article 19 about the participation in biotechnological research activities by Parties that provide the necessary genetic resources, especially developing countries [Article 19 (1)], and the priority fair and equitable access of such Parties to the results and benefits arising from biotechnology on mutually agreed terms [Article 19 (2)]. The inequitable participation of developing countries in the context of “synthetic biology” was highlighted in the outcomes of the most recent synthetic biology program of work, and the 2024 COP-16 decision established a new program of work aimed at addressing this issue (see Section 4.3 below). This decision is in line with the CBD provisions of Article 19 (1) and 19 (2) and is indicative of a shift towards policy action in support of biotechnology adoption. However, the development of Target 17 (see Table 3), and the parallel interconnected developments in the synthetic biology program of work (Section 4 below), exemplify the challenge to define meaningful and implementable targets in this area.
For most of the KMGBF targets, the monitoring framework includes either “headline” or “binary” indicators that Parties are requested to address in their National Reports, and optional “component” and “complimentary” indicators.26 Target 17 has a “binary indicator” that in essence enables a tally of countries that have taken actions relevant to its implementation (Table 3). The binary indicator is unpacked into a list of questions for Parties to respond to by selecting one of four options: “no,” “under development,” “partially,” and “fully.” These indicators faithfully adhere to the language of CBD Article 8(g), with the “establishment” (indicator 17.1) and “implementation” (indicator 17.2) elements of biosafety measures, and “distribution of its benefits” elements of Article 19, specifically the “effective participation in biotechnological research activities” [Article 19 (1); indicator 17.3], equitable access to the “results and benefits arising from biotechnologies” [Article 19 (2); indicator 17.4], and the sharing of biosafety-related information [Article 19 (4); indicator 17.6]. The requirement for Parties to address binary indicators in their National Reports may help address challenges with the collection of information that enables measurement of progress presented by the variable alignment of national commitments made in NBSAPs to the KMGBF Targets.
Indicator 17.5 is the only indicator that extends beyond the text of the CBD articles specified in Target 17. This addresses the conduct of scientifically sound risk assessments with the use and release of LMOs, and arguably this inclusion is warranted since risk assessment is fundamental to the “implementation” of biosafety measures (Protocol Article 15 and Annex III). Further, capacity building in this area has consistently been identified as a need by Parties, as reflected by the ongoing inquiry in the risk assessment program of work whereby Parties are invited to submit proposals on their “needs and priorities for further guidance materials on specific topics of risk assessment” by each COP-MOP.27 In recent years, submissions made via this process28 have generally included LMOs/applications that are under consideration in the CBD’s synthetic biology program of work (e.g., LMOs containing engineered gene drives and other genome-edited organisms), linking the two programs of work and raising concerns about the need for treaty programs be complementary and not duplicative (discussed in more detail in Section 4.4 below).
The “benefit” element of Target 17 is not elaborated further in the binary indicators, nor in any of the optional indicators. However, such benefits can be inferred, since effective participation in research and access to results and benefits should support participation in biotechnology in a broader sense, beyond the operational requirements of establishing and implementing biosafety measures. KMGBF guidance notes prepared by the CBD Secretariat See: https://www.cbd.int/gbf/targets/17 (accessed 17 June 2025) highlight that progress towards Target 17 could contribute to the achievement of all KMGBF goals (A, B, C, D), and several environmental targets: 4 (halt extinction), 6 (reduce invasive species), 7 (reduce pollution), 8 (mitigate climate change), and 10 (enhance sustainability of production systems). The contribution of Target 17 to the achievement of Target 13 is also noted: this target reflects the benefit sharing provisions of the CBD, namely, the fair and equitable sharing of benefits from genetic resources, and it expressly includes DSI. The potential contribution of biotechnology to achieving the KMGBF, including the specific targets listed above (as well as Target 17), is to be examined in the new program of work on synthetic biology established by the 2024 COP-16 decision (discussed in detail in Section 4.3 below).
The version of Target 17 in the early drafts (“Zero Draft,” “Updated Zero Draft,” “First Draft”) of the KMGBF did not refer to specific CBD provisions or “benefits”, rather the emphasis was on biosafety measures (per Article 8(g)) and reducing adverse impacts (Zero drafts) or risk (First Draft), consistent with the historical precautionary discourse on biotechnology in this forum. The “Updated Zero Draft” attempted to improve the SMART-ness of the target by adding a measure of reduced adverse impacts (“reducing these impacts by [X]”), however implementing such a measure would be challenging considering the lack of a baseline to improve upon regarding “adverse impacts” or “risk.” It is also unclear how this would be implemented by Parties that consider their established regulatory systems to already effectively manage risk and therefore would have difficulty measuring improvement. These considerations demonstrate challenges with crafting SMART targets where the specific area of regulation does not have a history of, and lacks agreed evidence of, “adverse impacts” to biodiversity. The eventual text in Target 17 of the adopted KMGBF did not retain these measures, and with the inclusion of additional relevant provisions from Article 19 it arguably has greater balance than earlier drafts, however it is unlikely to be regarded as a model “SMART” target in future analyses of the KMGBF.
Prior to the “draft” documents of the KMGBF, the early text compilations in the development of Target 17 included a wide range of elements collected through consultation from Parties and stakeholders that were ultimately not retained. These included a broader notion of recognizing and encouraging potential benefits towards achieving the objectives of the CBD and relevant sustainable development goals, references to synthetic biology and new genetic technologies, as well as horizon scanning, monitoring and assessment as a preventative measure, in connection with implementation of Article 8(g) (Open-Ended Working Group on the Post-2020 Global Biodiversity Framework, 2022). This text was ultimately lost through streamlining efforts, despite the need for “horizon scanning, monitoring and assessment” agreed by CBD Parties at COP-1429 and apparently paving the way for establishing such a process. This was instead allocated to the synthetic biology program of work by the Parties at COP-15,30 with one cycle of the process undertaken in the COP-15/16 intersessional period (discussed in Section 4.2 below). This has been highlighted as an example of the weakening of the KMGBF text as it was negotiated, with the assertion that such frameworks should have the ambition of preventing issues before they occur rather than only solving them (Hughes, 2023). However, such a process was unproven at the time, and the utility of such a process was put to the test in the context of synthetic biology and at COP-16 the CBD Parties did not decide to continue it for further iterations. Arguably, the implementation of effective biosafety frameworks is already preventative as opposed to rectifying.
4 Synthetic biology
4.1 A brief history
Synthetic biology has been on the CBD COP agenda since 2014 when COP-12 decided to establish a dedicated program of work for the topic.31 This decision, and subsequent decisions to extend work by COP-13,32 COP-14,33 COP-15,34 and COP-16,35 have been contentious for several reasons. The Parties exhibit broadly diverging views on what “synthetic biology” is and the need for a stand-alone program of work on it (i.e., whether or not it is a “new and emerging issue” (NEI) identified according an established COP process–see below), concerns regarding the process followed (or not followed) to justify the ongoing work (i.e., the absence of a complete NEI analysis), and concerns regarding the lack of a clear scope, direction and deliverables for the work, with repetition in the mandates of the Ad Hoc Technical Expert Groups (AHTEG) in each intersessional period. The lack of financial, human and technical capacity to enable effective participation in the programs of work is also a constant theme. In Keiper and Atanassova (2020), we reviewed these developments from the early CBD discussions on synthetic biology in the context of its consideration as a “new and emerging issue relating to the conservation and sustainable use of biological diversity”36 by COP-10,37 COP-11,38 and COP-12, and the subsequent establishment and continuation of dedicated programs of work, to the outcomes of this work and expectations for COP-15, which was then expected to occur in 2020.
In the eventual COP-15 that occurred in 2022, delayed due to the COVID-19 pandemic, the synthetic biology decision redirected and restructured the program of work to support a new process: “broad and regular horizon scanning, monitoring and assessing of the most recent technological developments” in synthetic biology. As noted above, the need for such a process was agreed by COP-14 in 2018, and this was included (but not retained) in the early text compiled in the drafting of KMGBF Target 17. The COP-15 decision established a new type of AHTEG, with expansion intended to include broader “multidisciplinary” expertise (termed “m-AHTEG”), and a mandate that marked a substantial change from previous work. Up to that point, the synthetic biology programs of work had consisted of, inter alia, developing an “operational definition”, considering the potential beneficial and adverse implications vis-a-vis the objectives of the CBD, considering the scope and ongoing applicability of existing regulatory frameworks, regulatory definitions (e.g., LMO per Protocol Article 3) and approaches to detection and identification, and application of the COP-defined criteria for identifying a NEI.39
The issues of the operational definition and NEI analysis have not been resolved and remain outstanding after more than a decade of work on synthetic biology, and they were raised again by Parties for consideration by COP-16 in 2024. Prior to COP-16, the twenty-sixth meeting of the CBD’s Subsidiary Body on Scientific Technical and Technological Advice (SBSTTA-26) considered the outcomes of the program of work mandated by the COP-15 decision and produced a recommendation for the COP-16 decision on future work.40 That recommendation reflects the contentious nature of the discussions, with much of the document containing bracketed text that could not be resolved in the SBSTTA’s negotiations. This includes an extensive list of potential tasks for an AHTEG/m-AHTEG, including revisiting the operational definition and a “final assessment” of synthetic biology against the NEI criteria. Ultimately, the COP-16 decision did not take forward these elements of the SBSTTA-26 recommendations.
4.2 Intractable issues–definition, NEI analysis and horizon scanning
While there are many challenging issues that have not been resolved in the CBD synthetic biology program of work, three remain particularly difficult to conclude due to lack of consensus among Parties. Since the early work on this topic more than 10 years ago, Parties have not been able to reach agreement on an operational definition of synthetic biology, and an acceptable outcome is unlikely to be found in this forum. The first AHTEG established by the COP-12 decision was mandated to “work towards an operational definition of synthetic biology”, and their 2015 report proposed one (Ad Hoc Technical Expert Group on Synthetic Biology, 2015). However, this definition was not supported by Parties at a subsequent meeting of SBSTTA-20, with calls for reopening and negotiation of the AHTEG’s text (e.g., to add “inclusion and exclusion criteria”), and it was bracketed in the resulting recommendation for resolution at COP-13.41 Ultimately, the AHTEG’s operational definition was not endorsed at COP-13, with the decision instead acknowledging it as an outcome of the AHTEG’s work and describing it as “useful as a starting point” in the context of deliberations on the topic under the CBD.42 Despite this delicately drafted compromise text to reflect its unresolved status, the operational definition has been cited in subsequent work in other fora as though it was endorsed (e.g., International Union for the Conservation of Nature and Natural Resources, 2024b; International Union for the Conservation of Nature and Natural Resources, 2019).
A second long-standing issue is the lack of a complete and conclusive NEI analysis. The continued emphasis on the need for this analysis reflects the fundamental and persistent differences between Parties on the topic of synthetic biology. This is evident in the 2019 AHTEG report, where the criteria for identifying an NEI were applied but agreement was reached for one of the seven criteria only: that the topic was relevant to the implementation of the CBD (Ad Hoc Technical Expert Group on Synthetic Biology, 2019). Synthetic biology was introduced to the CBD agenda via a collection of submissions from civil society organizations (i.e., at its onset this issue was not Party-driven) to the NEI process in 2011.43 This CBD-defined process involves invitation for proposals to be considered by the SBSTTA, which then makes recommendations to COP regarding decisions on any further work. The first SBSTTA consideration of the initial synthetic biology proposals was inconclusive, and subsequently COP-11 invited further submissions of information addressing the criteria for identifying NEIs. The following SBSTTA reached similar inconclusive outcomes and COP-12 decided to establish a dedicated program of work on synthetic biology that has continued since.44
Some Parties have questioned the establishment and repeated continuation of programs of work on synthetic biology in the absence of a conclusion from a completed NEI process that clearly establishes the need for it. These Parties share the general view that the technologies and applications referred to as “synthetic biology” remain within existing relevant regulatory mechanisms applicable to biotechnology applications (e.g., submissions made by Australia,45 and the United Kingdom46). There has also been examination of the NEI process (specifically the criteria), triggered by questions about application initiated by members of AHTEGs (Ad Hoc Technical Expert Group on Synthetic Biology, 2019; also Ad Hoc Technical Expert Group on Synthetic Biology, 2017). In a review,47 Parties submitted that the criteria should be considered as a whole to identify issues significant enough to warrant a place on the CBD agenda (e.g., submissions by Australia,48 and New Zealand49), and conversely, that they provide guidance only and it is not necessary for all criteria to be met to qualify as an NEI (e.g., submission by the European Union50). There is also the view that irrespective of the criteria and/or outcomes of NEI analyses, there is a need for the CBD agenda to include new biotechnological developments because these are perceived to be occurring at a rapid pace with potential implications for the CBD’s objectives (e.g., submission by Norway51). The criterion that synthetic biology is relevant to the CBD may be a rare point on which broad consensus is possible. Some participants have also pointed to the need for a more pragmatic approach than that attempted thus far, with application of the NEI criteria to specific foreseeable applications on a case-by-case basis, rather attempting to address an entire undefined field of vast potential application (e.g., submissions by the Global Industry Coalition,52 and Outreach Network for Gene Drive Research53). It is argued that such a targeted approach would enable identification of specific potential concerns relevant to the CBD and focus any further work on the topic on the needs of Parties.
The contention regarding the outstanding NEI analysis appeared to be resolved by the 2022 COP-15 decision where Parties crafted compromise text recognizing that the analysis had proved challenging and inconclusive, and that no further analysis would be required–meaning that there would be no further attempts to complete it. The COP-15 decision then continues with the establishment of a new process for “broad and regular horizon scanning monitoring and assessment” to be supported by a new m-AHTEG, with the outcomes of one cycle of this process subject to review by the SBSTTA and requiring a new COP decision to extend it for additional iterations. Notably, this new “horizon scanning” process did not involve criteria like the COP-defined criteria for the identification of NEIs. Instead, a synthetic biology “development” (or “trend” or “issue”) could be submitted in the initial information gathering step and where supported by an m-AHTEG member(s) it could progress further in the process (see the process set out in Multidisciplinary Ad Hoc Technical Expert Group, 2023). This relative lack of stringency proved concerning for some Parties who emphasized the need for CBD processes to be “Party-driven” and renewed calls for completion of a NEI analysis (IISD, 2024a).
The outcomes of “horizon scanning” undertaken in the COP-15/16 intersessional period were first considered by CBD Parties at SBSTTA-26 in 2024, where a divergence of views was evident regarding the merits of the process and its outcomes (IISD, 2024a; IISD, 2024d). The debate ranged from the novelty of the “trends and issues” identified, the need to revise the process, whether to extend the mandate of the m-AHTEG for another iteration of the process, or to revise its mandate and/or reestablish it (IISD, 2024b; IISD, 2024c). Some Parties also raised concerns regarding duplication with the Protocol, since topics identified and “assessed” in the process are within the scope of its regulatory mechanisms (IISD, 2024c), recalling the need for a coordinated, complementary and non-duplicative approach on issues related to synthetic biology.54 The final document (“conference room paper”) adopted by the SBSTTA-26 meeting contained 65 sets of brackets (IISD, 2024b), reflecting the polarized nature of the negotiations, and the substantial task of finding an acceptable way forward on this agenda item that was deferred to COP-16.
4.3 A new direction–capacity building and KMGBF implementation
The COP-16 synthetic biology decision does not include returning to the outstanding issues of the operational definition or NEI analysis, nor does it continue the horizon scanning process in the extensive manner undertaken by the m-AHTEG in the COP-15/16 intersessional period (Multidisciplinary Ad Hoc Technical Expert Group, 2023; Multidisciplinary Ad Hoc Technical Expert Group, 2024). Notably, it includes consideration of the “most recent technological developments” in a broad sense, similar to previous synthetic biology decisions, but no ongoing work for any of the specific “trends and issues” identified and prioritized by the m-AHTEG. Instead, a primary focus of the COP-16 decision is the development of a “thematic action plan to support capacity-building and development, access to and transfer of technology and knowledge-sharing in the context of synthetic biology … ”55 with this aimed at supporting implementation of CBD objectives and the KMGBF in developing countries.56 This is consistent with one of the “trends and issues” identified and prioritized in the horizon scanning process by the m-AHTEG, namely, the “inequity in the participation of developing countries in the context of synthetic biology” (Multidisciplinary Ad Hoc Technical Expert Group, 2024). It also addresses the repeatedly conveyed challenges with implementing biodiversity targets that arise by virtue of their technical nature. Analyses of previous strategic plans have reported that lacking scientific knowledge, capability and capacity impact both the implementation of technical indicators to measure progress towards targets, and the use of tools and technologies that promote their implementation (Hagerman and Pelai, 2016).
The development of a thematic action plan originates from proposals made and/or supported by developing countries at SBSTTA-26 (e.g., statements by Argentina,57 and Brazil58), and this need has been consistently raised throughout the synthetic biology programs of work (e.g., Multidisciplinary Ad Hoc Technical Expert Group, 2024; Multidisciplinary Ad Hoc Technical Expert Group, 2023; Ad Hoc Technical Expert Group on Synthetic Biology, 2019; Ad Hoc Technical Expert Group on Synthetic Biology, 2017). These requests are broader than the development and implementation of national biosafety frameworks, which has been the focus of capacity building efforts under the CBD and Protocol. Rather, Parties wish to develop domestic capacity for adoption and application of biotechnology towards realization of the potential benefits, consistent with the original spirit of the CBD. Accordingly, this new work has been publicized as helping Parties to assess and apply synthetic biology technologies, and foster innovation while also safeguarding biodiversity (emphasis added).59 This new direction constitutes an expansion of the relatively narrow approach to capacity building and development historically supported in the CBD forum, with developing country parties that have established national biosafety frameworks seeking to be empowered with the tools and capabilities to address domestic priorities.
The COP-16 synthetic biology decision also includes a mandate for a new AHTEG, which no longer includes the additional “multidisciplinary” element in its composition.60 While the mandate is a simplified version of the extensive (but unresolved) list of tasks resulting from SBSTTA-26, it remains substantial. Its tasks include supporting the preparation of the thematic action plan61 and providing advice in relation to implementation of the CBD’s objectives and the KMGBF.62 Towards this, the AHTEG will consider a scientific study (to be commissioned by the SCBD) on research that is relevant to achieving the KMGBF, in particular its Targets 4, 6, 7, 8, 10, 13 and 17.63 The AHTEG will also identify potential benefits and potential impacts (positive and negative) related to the CBD objectives and implementation of the KMGBF64 which repeats elements of past AHTEG mandates. Collectively the new mandate has been publicized as a “unique opportunity to explore synthetic biology in relation to the CBD’s three fundamental objectives and in implementing the KMGBF” (emphasis added).65 In comparison to previous CBD programs of work on synthetic biology this marks a shift towards potentially delivering informative and actionable outcomes for Parties. The outcomes of this work will be reviewed by SBSTTA-28 (expected in mid-2026) prior to the next decision on this topic at COP-17.66
While not specifically mentioned in the synthetic biology program of work, there are other targets that are relevant in the context of supporting participation in synthetic biology towards implementation of the KMGBF. These include: Target 20 for the strengthening of capacity-building and development, access to and transfer of technology, and promoting development of and access to innovation and technical and scientific cooperation; and Target 21 regarding ensuring the best available data, information and knowledge, are accessible to decision makers, practitioners and the public to guide biodiversity action. These have been described as social targets that are values-based rather than data-driven, meaning that they will be challenging to measure and monitor (Hughes and Grumbine, 2023). Target 20 is however linked to the CBD’s long-term strategic framework for capacity-building and development67 that is referred to in the COP-16 decision on synthetic biology in regard to ensuring alignment and non-duplication. Our reading of Target 21 is that it aligns with calls to address science-policy knowledge deficiencies that hamper progress in achieving targets (e.g., Xu et al., 2021).
4.4 Duplication with the protocol
The COP-14 decision on synthetic biology emphasizes the need for “a coordinated, complementary and non-duplicative approach on issues related to synthetic biology” under the CBD and its Protocols, as well as among other treaties and relevant organizations and initiatives.68 This is emphasized again in the subsequent deliberations of the AHTEG (Ad Hoc Technical Expert Group on Synthetic Biology, 2019) and m-AHTEG (Multidisciplinary Ad Hoc Technical Expert Group, 2023). This reflected growing concerns about the expanding mandates of the synthetic biology programs of work over time, with these including cross-cutting regulatory and technical topics with existing programs of work under the Cartagena Protocol, and the need for efficient use of Party resources, particularly developing country Parties with resource constraints.
The COP-16 decision on synthetic biology also refers to alignment and non-duplication, but in the context of existing plans and strategies for capacity building and development under the CBD and its Protocols.69 The Cartagena Protocol includes provisions on capacity building (Article 22), and specifically the “strengthening of human resources and institutional capacities in biosafety” for the purpose of its effective implementation (Article 22 (1)).70 This has been supported by the CBD, for example, by grants from the Global Environment Facility for the development and implementation of National Biosafety Frameworks since the late 1990s, prior to the entry into force of the Protocol.71 The emphasis on capacity building for the development of national biosafety frameworks is evident from the first Protocol COP-MOP where the first “Action Plan” was adopted,72 with consecutive versions adopted through to 2020.73 More recently, in parallel with the development of the KMGBF, a new “Capacity-Building Action Plan for the Cartagena Protocol on Biosafety”74 was drafted to span the period up to 2030, and this was adopted at COP-MOP1075 that occurred concurrently with COP-15 and the adoption of the KMGBF. This latest action plan is aligned with an “Implementation Plan for the Cartagena Protocol” which is “anchored in and complementary to” the KMGFB.76
Consistent with previous plans, the current plans for the Protocol concentrate on implementation of its provisions and the establishment of functional national biosafety frameworks. Capacity building towards supporting participation in biotechnology, rather than focusing solely on biosafety assessment and regulation, is more contentious in this forum, despite developing countries consistently calling for this. This broader notion of capacity building is captured by the prioritization of “inequity in the participation of developing countries in the context of synthetic biology” via the horizon scanning process by the m-AHTEG (emphasis added). Submissions made by representatives of developing countries in online discussions held on this theme in the COP-15/16 intersessional period included capacity building in biosafety, as well as in creating enabling environments that foster research and development, and developing innovation capacity to address local biodiversity issues and support social and economic development.77 The m-AHTEG considered these submissions and produced a table of options in their report, and consistent with historical trends, this emphasizes biosafety assessment and regulation. However, the report also acknowledges broader elements of the full “technology cycle”, including technical needs for research and development, and supporting activities such as facilitating access to scientific information, increasing scientific cooperation, and providing education and training opportunities, and technical support (Multidisciplinary Ad Hoc Technical Expert Group, 2024). The new direction of the synthetic biology program of work appears to be aimed at a more expansive consideration of capacity-building issues, and to avoid duplication and ensure complementarity to the Protocol action plans, the focus should extend beyond biosafety.
Assertions of duplication with the Protocol have also arisen in the synthetic biology programs of work because the subject of regulation, namely, LMOs, are within its scope. The first AHTEG on synthetic biology concluded that LMOs “developed through current and near future applications of synthetic biology are similar to LMOs as defined in the Cartagena Protocol” (Ad Hoc Technical Expert Group on Synthetic Biology, 2015). Parties have made submissions throughout the programs of work on the topic supporting this conclusion and confirming that their national regulatory frameworks (consisting of Protocol-aligned provisions but also other relevant regulations) remain applicable (e.g., submissions by Australia,78 Brazil,79 and Canada,80 peer reviewing the Ad Hoc Technical Expert Group on Synthetic Biology (2017) report). More recently, the m-AHTEG generated an extensive list of “trends and issues” (see Multidisciplinary Ad Hoc Technical Expert Group, 2024) through the horizon scanning process comprising many types of LMOs/applications that would be within the scope of existing regulatory mechanisms (e.g., LMOs containing engineered gene drives), as exemplified by cases that have already been assessed and authorized for use in one or more Protocol Parties (e.g., self-limiting LM insects, metabolically engineered crops, certain genome edited plants), with these and many others under discussion in the past programs of work now spanning more than a decade (e.g., various engineered organisms for bioproduction). This overlap with the Protocol was a major reason for questioning the utility of the process as a mechanism for scanning the “horizon” for “new” developments and trends; other reasons included, for example, the conflation of issues (e.g., integration of artificial intelligence in synthetic biology processes) and issues that strayed beyond scope (e.g., inclusion of “contained uses”) (e.g., submission by United Kingdom81 peer reviewing the Multidisciplinary Ad Hoc Technical Expert Group (2024) report).
A specific example of duplication is the m-AHTEG’s identification and prioritization of LMOs containing “engineered gene drives to control vector-borne diseases and invasive species”. This has arguably been the predominant LMO/application debated throughout the synthetic biology programs of work, as reflected in COP decisions82 and civil society campaigns for moratoria on releases into the environment (e.g., Civil Society Working Group on Gene Drives, 2016;83 see also Callaway, 2018; Callaway, 2016). However, these are LMOs within the scope of the Protocol, where they were assessed via a COP-MOP-defined process for the “identification and prioritization of specific issues regarding risk assessment” that may warrant consideration by the COP-MOP.84 This led to the COP-MOP10 decision to develop relevant supporting materials to support the conduct of case-by-case risk assessment in accordance with the Cartagena Protocol framework,85 and these were drafted by an AHTEG on Risk Assessment in the COP-MOP10/11 intersessional period. The finished product - additional voluntary guidance materials (Ad Hoc Technical Expert Group on Risk Assessment, 2024) - was “welcomed” by COP-MOP11 in 2024.86 This provides an example of how established treaty processes may be used to identify and address specific topics and enable appropriate action. If the broader category “synthetic biology” was assessed according to the same criteria, it would likely be inconclusive like the NEI analysis.
5 Other international processes
While this review highlights the policy developments under the CBD, it is also important to acknowledge relevant parallel, and sometimes interdependent work in other international fora on biotechnology/synthetic biology. This is in line with the role of the SCBD to coordinate with other relevant bodies [CBD Article 24(d)]. One such body is the International Union for the Conservation of Nature and Natural Resources (IUCN) which has had a program of work on synthetic biology since 2016 (see Keiper and Atanassova, 2020), and which is currently developing a policy on the implications of synthetic biology in nature conservation.87 Given their complementary conservation objectives, the IUCN has had long-term engagement in CBD processes through the provision of conservation-relevant technical and policy advice.88 Another body is the Organisation for Economic Co-operation and Development (OECD), which has been supporting the governance of biotechnology in initiatives that predate developments under the CBD. Collaboration amongst these fora and the CBD has the potential to facilitate the sharing of best practices and enhance capacity building, and provide insights that could strengthen CBD processes and contribute to the implementation of KMGBF Target 17. The ongoing synthetic biology work in both of these fora is discussed in more detail in the following sections.
5.1 International Union for the Conservation of Nature and Natural Resources (IUCN)
The IUCN was created in 1948 and today has a broad membership that includes national and subnational governments, non-governmental organizations, indigenous peoples’ organisations, scientific and academic institutions, and business associations.89 IUCN members meet every 4 years at the IUCN World Congress to debate policy issues and produce resolutions and recommendations,90 with these outcomes potentially influencing subsequent CBD COP discussions. The first such outcome for synthetic biology was a resolution at the 2016 IUCN World Congress for the development of an IUCN policy on biodiversity conservation and synthetic biology.91 Controversially, the resolution was publicized as imposing a de facto moratorium92 on research with LMOs containing engineered gene drives until an urgent assessment was undertaken on the potential impacts on the conservation and sustainable use of biological diversity as well as equitable sharing of benefits arising from genetic resources–reflecting all three CBD objectives. Moratoria campaigns then followed at COP-13 (2016) and COP-14 (2018) but these were rejected by CBD Parties (Callaway, 2018; Callaway, 2016).
To inform the work towards development of the IUCN policy, an assessment was commissioned on the state of science and policy around synthetic biology techniques as they relate to biodiversity. This assessment aimed to identify, based on the best available evidence, synthetic biology applications with the potential to impact, both positively and negatively, the conservation and sustainable use of biodiversity. The resulting report published in 2019 links developments under the CBD, and indicates that similar tensions are present in both fora, with polarization of views regarding the safety and potential impacts of LMOs (International Union for the Conservation of Nature and Natural Resources, 2019). However, the report attempts to take a nuanced approach by considering applications on a case-by-case basis, rather than conflating them for summary judgement–an approach that we have argued above as necessary for meaningful application of the CBD NEI process. It also diverges from CBD processes by qualifying the level of confidence in elements of the assessment by indicating the level of agreement against the quantity and quality of evidence (“established but incomplete,” “speculative,” “well established,” “competing explanations”). This approach was adapted from systems used in other international fora: the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services, 2016) and the Intergovernmental Panel on Climate Change (Moss and Schneider, 2000; International Union for the Conservation of Nature and Natural Resources, 2019). Such a system may have been beneficial to incorporate into the methodology of the horizon scanning process developed (Multidisciplinary Ad Hoc Technical Expert Group, 2023) and undertaken (Multidisciplinary Ad Hoc Technical Expert Group, 2024) in the COP-15/16 CBD synthetic biology program of work.
The next IUCN World Congress was held in 2021, delayed 1 year due to the COVID-19 pandemic, and in another synthetic biology Resolution a process was established for the development of the policy.93 This Resolution requests “an inclusive and participatory process to develop an IUCN policy on the implications of the use of synthetic biology in nature conservation”, with the outcomes to be deliberated at the next IUCN World Congress (to be held in October 202594). The Resolution sets out a process that was subsequently deliberated at two IUCN Council meetings: in November 2022, a detailed process for implementation of the Resolution was decided (International Union for the Conservation of Nature and Natural Resources Council Programme and Policy Committee Res 123 Working Group, 2023), and in another decision in May 2023 this process was revised.95 Key elements of the process include a “Citizens’ Assembly” and the appointment of a Policy Development Working Group, with training to be provided to support participants in the process (International Union for the Conservation of Nature and Natural Resources Council Programme and Policy Committee Res 123 Working Group, 2023). Publicly available outcomes of the policy development process thus far include “Recommendations of the IUCN Citizens’ Assembly on Synthetic Biology in Relation to Nature Conservation”, in January 2024 (International Union for the Conservation of Nature and Natural Resources, 2024a), and a briefing document developed as part of the training for participants (International Union for the Conservation of Nature and Natural Resources, 2024b).
The Recommendations of the IUCN Citizens’ Assembly explain that this forum is a type of participatory and anticipatory assessment method typically used to address polarized societal questions, and it alludes to the polarization of views on synthetic biology in the conservation community. Its sixteen members were selected at random from the IUCN membership evenly representing governments and non-governmental organizations (civil society), with balanced regional and gender representation (International Union for the Conservation of Nature and Natural Resources, 2024a). Their Recommendations list principles and associated recommendations spanning six topics: stockpiling resources and knowledge gaps, synthetic biology definition and policy scope, assessing risks and benefits, indigenous peoples and local communities (IPLCs) involvement and rights, awareness-raising and trust, and access and benefit-sharing. Contentious areas are evident, for example, regarding the extent to which precautionary principle should be recognized in developing risk assessment policy. There is also disagreement as to whether the development of a definition of synthetic biology is an appropriate task for the Policy Development working group (International Union for the Conservation of Nature and Natural Resources, 2024a).
The issues of divergence apparent from review of the Recommendations, and the content of the topics more generally, show consistency with the discussions and information contributed to the CBD synthetic biology programs of work, and in particular with the contributions to that work from civil society organizations. A case in point is the greater emphasis on the precautionary principle in the IUCN materials, which has been a contentious issue since the drafting of the Protocol and throughout its subsequent implementation (Marchant, 2001; also Keiper and Atanassova, 2020). Another example in the Recommendations is the proposed scope of the policy, with this expanded to non-conservation applications, with agriculture specifically mentioned. The recommendations that are ultimately addressed and incorporated by the Policy Development Working Group remain to be seen; the developing policy is reported as having undergone two rounds of IUCN-wide peer review, with a revised third draft to be submitted to the IUCN Council, who will then transmit it to the 2025 IUCN World Conservation Congress for debate and potential adoption by IUCN Members (International Union for the Conservation of Nature and Natural Resources Council Programme and Policy Committee Res 123 Working Group, 2023).
5.2 Organisation for Economic Co-operation and Development (OECD)
The OECD is an intergovernmental organization founded in 1961 and today has 39 member countries. It serves as a forum for the development of international standards, and a knowledge hub for the exchange of experiences and best-practices in public policy in the context of sustainable economic development.96 In recognition of biotechnology as a driver of economic growth and development, the OECD has been at the forefront of monitoring technological advancements and in supporting biosafety policy initiatives since the early 1980s (OECD, 2016; Kearns et al., 2021). Early seminal outcomes include the 1986 OECD Council Recommendation concerning Safety Considerations for Applications of Recombinant DNA Organisms in Industry, Agriculture and the Environment (OECD, 1986a), which was recently updated to reflect the now almost 4 decades of experience with such applications (OECD, 2025a). When developed, it was intended that this Recommendation, and the accompanying “Blue Book” (OECD, 1986b), would promote international understanding and consensus on biosafety issues (Kearns et al., 2021). These resources were followed by Safety Considerations for Biotechnology (OECD, 1992) and Safety Considerations for Biotechnology: Scale-up of Crop Plants (OECD, 1993), and collectively they established fundamental principles of risk assessment that were later codified in the Protocol. These include the case-by-case and comparative nature of the assessment, the concept of familiarity, and consideration of the characteristics of the organism, the introduced trait, the receiving environment, and the interactions among them (OECD, 2023a).
The recognition by OECD members of the need for and value of international co-operation and harmonization in safety assessment led to the establishment of two closely related programs: the Working Party on Harmonisation of Regulatory Oversight in Biotechnology (WP-HROB) (established in 1995) to address environmental risk/safety assessment and regulation of organisms produced though modern biotechnology, and the Working Party on the Safety of Novel Foods and Feeds (established in 1999) to address the safety assessment of novel foods and feeds, especially products of modern biotechnology. These groups have provided a forum for exchanging approaches and experiences to support high standards of safety assessment, mutual understanding of regulatory processes across different countries, and reduce the potential for non-tariff trade barriers (Kearns et al., 2011). Their key outputs include a series of “consensus documents” providing biological information for host organisms (e.g., plants, micro-organisms) that can be used directly to address certain environmental assessment requirements,97 and technical compositional information to support food/feed safety assessment.98 The WP-HROB also developed the universally recognized Unique Identifier (UI) system to facilitate access to information about the associated transgenic plant (OECD, 2006). This is utilized by the OECD’s BioTrack Product Database99 which has interoperability with the information exchange platform established under the Protocol, the Biosafety Clearing House (SCBD, 2004)100.
A notable recent outcome of a long-running project of the WP-HROB was the publication of a Consensus Document on Environmental Considerations for the Release of Transgenic Plants (OECD, 2023a). This builds upon the foundational principles established in the early OECD biosafety work to describe an approach to planning and structuring an environmental risk/safety assessment termed “problem formulation” (OECD, 2023a). Notably, this work strongly influenced the subsequent recent development of additional voluntary guidance materials under the Protocol to support the case-by-case risk assessment of LMOs containing engineered gene drives. This was the first additional voluntary guidance materials developed under the Protocol to exemplify a problem formulation approach in case-by-case implementation of the Protocol’s risk assessment provisions, and the first to be “welcomed” by the COP-MOP.101 In contrast, previous programs of work on risk assessment for the development of guidance materials failed to produce outcomes that were acceptable for Protocol Parties (Hokanson, 2019).
The long-running work in support of biosafety at the OECD is part of a broader, multifaceted biotechnology policy agenda in the context of science, technology and innovation (STI) and governance (OECD, 2018) supported since 2015 by the Working Party on Biotechnology, Nanotechnology and Converging Technologies (WP-BNCT). The WP-BNCT helps to address policy issues related to biotechnology, nanotechnology and their emergence and convergence with other technologies, and advances ethical and responsible innovation for more resilient economies. A focus of their work is to ensure that policies impacting technologies and innovations - from support for research and development, to their adoption and use - remain “fit for purpose” over time (OECD, 2023b). The WP-BNCT also collaborates with the Global Forum on Technology (GFTech) (see below) launched in 2023 as a platform for dialogue to promote collaboration on the design, development, and deployment of emerging technologies in an ethical, sustainable, and inclusive way.102
OECD policy work specifically addressing synthetic biology predates the CBD and IUCN (e.g., see discussions with scientific bodies in 2008; Organisation for Economic Co-operation and Development Working Party on Biotechnology, 2009), and it is of broader scope, however there is a partial overlap in the areas of governance and regulation and also the benefits considered. Their publication, Emerging Policy Issues in Synthetic Biology (OECD, 2014), recognises its potential in several important economic sectors, that it has a basis in several decades of biotechnology research and development, and that it benefits from established biosafety regulation and governance developed for transgenic (LMO) organisms. This contrasts with the fragmented/absence of comprehensive regulation rhetoric evident in the CBD discourse at that time (e.g., Ad Hoc Technical Expert Group on Synthetic Biology, 2015), with the existing foundations generally overlooked in favour of new and more precautionary regulation. Notably, the OECD publication points out that like biotechnology, the adoption of synthetic biology may be hindered in some parts of the world due to over-regulation, and that policy flexibility is necessary that includes recognition of the potential societal benefits (OECD, 2014). These observations underscore the broader consideration of OECD initiatives, which extend beyond the precautionary biosafety emphasis of the CBD.
The GFTech was launched with the aim of informing policymakers of key areas where action is needed to foster a strong bioeconomy whilst mitigating potential risks (Robinson and Nadal, 2025). It began its work by forming an expert focus group on the strategic area of synthetic biology, which aimed to take a forward-look at emerging technology trends and where they will impact, to articulate the main policy and governance challenges, and identify key policy issues of concern that could be further explored in future work (Robinson and Nadal, 2025). The expert focus group recently published a working paper providing a synthesis of their activities in 2023–2024. These included a “horizon scan” of technology advancements categorised according to their expected timeframe: short (1–5 years), medium (5–10 years), and long (10+ years). Five areas described as having the potential for transformative change in the economy and in society were identified: human health and life sciences, food security and soil regeneration, circularity and emissions reduction, synthetic biology convergence with artificial intelligence and automation, and decentralised and distributed manufacturing. While the scope of this horizon scan was broader than that undertaken under the CBD–which was framed by its three objectives–the approaches taken differ markedly in the first step of collecting information. The GFTech process involved surveying its 66 members, with the group predominantly consisting of representatives of the scientific community and industry (Robinson and Nadal, 2025); by contrast, the synthetic biology AHTEGs and m-AHTEG have had limited participation by its practitioners. Further, the basis of the “horizon scan” undertaken by the m-AHTEG was information previously submitted in the CBD programs of work (Multidisciplinary Ad Hoc Technical Expert Group, 2023; Multidisciplinary Ad Hoc Technical Expert Group, 2024). We (Keiper and Atanassova, 2020) and others (e.g., Lai et al., 2019) have emphasised the need for stronger engagement by the scientific community in work to promote better outcomes for the Parties.
The report of the GFTech synthetic biology focus group identifies five policy and governance themes across the five application areas, including: strong and resilient innovation ecosystems, skills and workforce development, equity and access, biosecurity and biosafety, and anticipatory governance and responsible innovation. It also identifies a role for the OECD in leveraging its expertise to develop a “Recommendation on the responsible development of synthetic biology”, supported by the sharing of best practices in technology appraisal for governance, the development of advanced metrics and indicators, and anticipating the convergence with artificial intelligence, automation and robotics (Robinson and Nadal, 2025). Such an initiative would be in line with the OECD’s work in the field of responsible innovation103 and technology governance (OECD, 2024a), e.g., Recommendation on Artificial Intelligence (OECD, 2019), Recommendation on Responsible Innovation in Neurotechnology (OECD, 2025b), and Recommendation of the Council for Agile Regulatory Governance to Harness Innovation (OECD, 2025c) which provides guidance for policymakers to design agile regulations that can address the regulatory challenges and opportunities arising from emerging technologies.
As technological advancements and the convergence of different fields may present new governance challenges, the OECD has highlighted the need for adaptive regulatory strategies, with opportunities particularly in the areas of building trust in biotechnology/synthetic biology, and building cultures of responsibility. These themes have also emerged in the early CBD synthetic biology discourse but have not been explored further, with an emphasis instead on precaution as the primary solution. As noted in the 2014 policy publication (OECD, 2014), and also the 2023 GFTech brief (Organisation for Economic Co-operation and Development–Global Forum on Technology, 2023), concerns have been expressed regarding over-regulation and its impact of stifling innovation. In the CBD context, this translates to limiting the use of new tools and technologies that could advance the implementation of CBD objectives and the KMGBF. The OECD points to elements of adaptivity to new technologies including, e.g., the development of soft law (non-binding principles, standards, guidelines, and codes of practice) that complement the existing formal regulatory approaches (OECD, 2024a; OECD, 2024b). Towards this the OECD emphasises the need for broad input from the research community, governments, academia, industry and non-governmental organisations.104 This approach to technology governance aligns with the proposal made by practitioners in the field for a “Global forum on synthetic biology” to connect policymakers with practitioners for building confidence and for discussion of the future of engineering biology (Dixon et al., 2022).
6 Conclusion
In the development of the KMGBF, it was evident that CBD Parties paid attention to the failures of previous strategic plans, and many elements of the KMGBF were intentionally developed to support improved implementation at national levels. However, unlike most of the KMGBF’s targets, Target 17 did not have a predecessor to improve upon. The historical focus in this forum on the implementation of biosafety frameworks evidently influenced its drafting, with this ultimately balanced by the incorporation of a “benefits” component late in the KMGBF drafting process. While this outcome is consistent with the foundational CBD provisions on biotechnology, we theorize that more recent developments under the CBD had a strong impact on the adopted “benefits” text. Specifically, increased pressure from Parties for the long-running work on synthetic biology to produce more tangible and actionable outcomes, and strong political will for the KMGBF to succeed translating to support for enabling tools (including biotechnology) for implementation, and the interlinked issue of DSI and aspirations for the benefit sharing mechanism to generate resources for implementation.
In future analyses of the progress and success of the KMGBF, Target 17 may be critiqued for its lack of SMART-ness, however, this could not be attributed to want of effort. Attempts to incorporate measurability proved challenging due to the preventative nature of the regulatory obligation, i.e., it is difficult to quantify improvement to biodiversity resulting from managing or reducing impacts of biotechnology. We contend that the foundational but unverified assumption of risk associated with biotechnology should be called into question when next considering a biosafety target (and indicators) for a CBD strategic plan, and that proposed initiatives based on such a paradigm, e.g., “horizon scanning”, merit the scrutiny evident at SBSTTA-26 and COP-16. We further contend that measures to promote biotechnology adoption and implementation warrant deeper consideration beyond merely reciting elements of the CBD treaty text, with proactive initiatives that will deliver measurable outcomes. The thematic action plan of the current synthetic biology program of work may provide one basis for such initiatives.
The parallel programs of work on synthetic biology in other international fora may also provide valuable insights for the implementation of Target 17, as well as other KMGBF targets, and the thematic action plan. While the work of the IUCN and OECD is framed by different objectives, there is some overlap with the CBD in scope, including their considerations of potential impacts and governance. However, these organizations have adopted different approaches to how these considerations are informed, with the varying perspectives and interests of the stakeholders participating in each influencing the breadth, quality and the overall impact of the resulting work. CBD Parties should identify and leverage best practices in these (and other) fora. For example, the IUCN looked to the work of other international environmental organizations that deal with contentious technical issues and adopted qualifiers to indicate confidence in their key messages–such tools are also crucial for the polarized CBD synthetic biology work and would have benefited the horizon scanning process. We also consider that the broader OECD policy scope can complement the more precautionary approach adopted by the CBD, particularly in regard to more balanced consideration of benefits, and initiatives for agile and adaptive governance of new technologies that support their realization.
Author contributions
FK: Conceptualization, Writing – original draft, Writing – review and editing. AA: Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
Authors FK and AA were employed by BASF, a global research and development company with a diverse range of chemistry-based business segments, and an agricultural business that includes biotech seed products. They participate in the CBD programs of work mentioned in this review via the Global Industry Coalition and CropLife International. FK has participated in the AHTEGs/m-AHTEG on Synthetic Biology, and the 2023–2024 AHTEG on Risk Assessment.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1Convention on Biological Diversity, Adopted 5 June 1992, 1760 UNTS 69 (entered into force 29 December 1993).
2See “Risk assessment studies as per Decision CP-9/13” at: https://bch.cbd.int/protocol/studies.shtml.
3Decision of the 16th Conference of the Parties (COP-16) to the CBD: CBD/COP/DEC/16/21, available at: https://www.cbd.int/doc/decisions/cop-16/cop-16-dec-21-en.pdf.
4Agenda 21, Chapter 1 (preambular text).
5Agenda 21, Chapter 16.
6See: List of Parties at https://www.cbd.int/information/parties.shtml (accessed 6 January 2025); also see https://treaties.un.org/Pages/ViewDetails.aspx?src=TREATY&mtdsg_no=XXVII-8&chapter=27&clang=_en.
7See: https://bch.cbd.int/protocol/parties (accessed 7 January 2025).
8See: https://www.cbd.int/abs/nagoya-protocol/signatories (accessed 7 January 2025).
9The KMGBF is contained within COP-15 decision CBD/COP/DEC/15/4 Annex I, available at: https://www.cbd.int/doc/decisions/cop-15/cop-15-dec-04-en.pdf.
10See also, e.g.,.: “New international biodiversity agreement strengthens climate action” available at: https://unfccc.int/news/new-international-biodiversity-agreement-strengthens-climate-action; “Can businesses and governments turn the tide on nature loss at COP16?” available at: https://www.weforum.org/stories/2024/10/cop16-nature-government-business/.
11United Nations Framework Convention on Climate Change, Adopted 9 May 1992, 1771 UNTS 107 (entered into force 21 March 1994).
12See, e.g., document CBD/COP/DEC/15/31; available at: https://www.cbd.int/doc/decisions/cop-15/cop-15-dec-31-en.pdf.
13COP-13 decision: CBD/COP/XIII/16 (available at: https://www.cbd.int/doc/decisions/cop-13/cop-13-dec-16-en.pdf); COP-14 decision: CBD/COP/14/20 (available at: https://www.cbd.int/doc/decisions/cop-14/cop-14-dec-20-en.pdf); COP-15 decision: CBD/COP/15/9 (available at: https://www.cbd.int/doc/decisions/cop-15/cop-15-dec-09-en.pdf); COP-16 decision: CBD/COP/16/2 (available at: https://www.cbd.int/doc/decisions/cop-16/cop-16-dec-02-en.pdf).
14See “The 17 Goals” at: https://sdgs.un.org/goals.
15Document UNEP/CBD/COP/6/20 decision VI/26; available at https://www.cbd.int/doc/decisions/cop-06/full/cop-06-dec-en.pdf.
16COP-13 decision CBD/COP/DEC/XIII/1; available at: https://www.cbd.int/doc/decisions/cop-13/cop-13-dec-01-en.pdf.
17COP-14 decision CBD/COP/DEC/14/34; available at: https://www.cbd.int/doc/decisions/cop-14/cop-14-dec-34-en.pdf.
18Document CBD/WG2020/2/3; available at: https://www.cbd.int/doc/c/da8c/9e95/9e9db02aaf68c018c758ff14/wg2020-02-03-en.pdf.
19Document CBD/POST2020/PREP/2/1; available at https://www.cbd.int/doc/c/3064/749a/0f65ac7f9def86707f4eaefa/post2020-prep-02-01-en.pdf.
20Document CBD/WG2020/3/3; available at: https://www.cbd.int/doc/c/abb5/591f/2e46096d3f0330b08ce87a45/wg2020-03-03-en.pdf.
21See: https://www.cbd.int/conferences/post2020 (accessed 19 February 2025).
22COP-15 decision CBD/COP/DEC/15/6; available at: https://www.cbd.int/doc/decisions/cop-15/cop-15-dec-06-en.pdf; see also https://www.cbd.int/nbsap/post-cop15.shtml.
23See: “Remarks by Astrid Schomaker, Executive Secretary of the Convention on Biological Diversity on the occasion of the second anniversary of the adoption of the Kunming-Montreal Global Biodiversity Framework”, available at: https://www.cbd.int/article/kmgbf-two-years-2024.
24See the “Online Reporting Tool for NBSAPs and National Reports”, available at: https://ort.cbd.int/?_gl=1*1kv5u3r*_ga*MTUyMTA1ODk1Ny4xNzEzMjIyNjIy*_ga_7S1TPRE7F5*MTczNDU2OTUyNi40MzAuMS4xNzM0NTcyMDg0LjYwLjAuMA.#0.4/0/0 (accessed 3 February 2025).
25Available at: https://ort.cbd.int/national-targets/analyzer?globalTargets=GBF-TARGET-17#0.5/2/-32.9 (accessed 3 February 2025).
26See: https://gbf-indicators.org/ (accessed 19 February 2025).
27For example, COP-MOP11 decision CBD/CP/MOP/DEC/11/7 paragraph 8; available at: https://www.cbd.int/doc/decisions/cp-mop-11/cp-mop-11-dec-07-en.pdf.
28For example, past submissions can be accessed via the Biosafety Clearing House at: https://bch.cbd.int/en/portals/risk-assessment/submission/previous (accessed 17 February 2025).
29COP-14 decision CBD/COP/DEC/14/19 paragraph 3.
30COP-15 decision CBD/COP/DEC/15/9.
31COP-12 decision CBD/COP/DEC/XII/24, available at: https://www.cbd.int/doc/decisions/cop-12/cop-12-dec-24-en.pdf.
32COP-13 decision CBD/COP/DEC/XIII/1, available at: https://www.cbd.int/doc/decisions/cop-13/cop-13-dec-17-en.pdf.
33COP-14 decision CBD/COP/DEC/14/19, available at: https://www.cbd.int/doc/decisions/cop-14/cop-14-dec-19-en.pdf.
34COP-15 decision CBD/COP/DEC/15/31, available at: https://www.cbd.int/doc/decisions/cop-15/cop-15-dec-31-en.pdf.
35COP-16 decision CBD/COP/DEC/16/21, available at: https://www.cbd.int/doc/decisions/cop-16/cop-16-dec-21-en.pdf.
36See: https://www.cbd.int/emerging (accessed 14 February 2025).
37COP-10 decision CBD/COP/DEC/X/13, available at: https://www.cbd.int/doc/decisions/cop-10/cop-10-dec-13-en.pdf.
38COP-11 decision CBD/COP/DEC/XI/11, available at: https://www.cbd.int/doc/decisions/cop-11/cop-11-dec-11-en.pdf.
39The NEI criteria are contained in COP-9 decision CBD/COP/DEC/IX/29, paragraphs 11 and 12; available at: https://www.cbd.int/decision/cop/default.shtml?id=11672.
40SBSTTA-26 recommendation CBD/SBSTTA/REC/26/4, available at: https://www.cbd.int/doc/recommendations/sbstta-26/sbstta-26-rec-04-en.pdf.
41SBSTTA-20 recommendation CBD/SBSTTA/REC/XX/8, available at: https://www.cbd.int/doc/recommendations/sbstta-20/sbstta-20-rec-08-en.pdf.
42COP-13 decision CBD/COP/DEC/XIII/17.
43See “Proposals for new and emerging issues for SBSTTA-16 and COP-11” at https://www.cbd.int/emerging.
44See “Proposals for new and emerging issues for SBSTTA-17 and COP-12” at https://www.cbd.int/emerging.
45Available at: https://bch.cbd.int/en/database/BCH-SUB-SCBD-107999-1 (accessed 18 February 2025).
46Available at: https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwww.cbd.int%2Fdoc%2Femerging-issues%2FUK-overviewofcomments-SB-2013-10-en.docx&wdOrigin=BROWSELINK (accessed 24 February 2025).
47See: CBD Notification 2017–054 requesting submissions of views, available at: https://www.cbd.int/notifications/2017-054; and the resulting submissions under “Review of proposals for new and emerging issues received after COP-13” available at: https://www.cbd.int/emerging (accessed 26 February 2025).
48Available at: https://www.cbd.int/doc/emerging-issues/Australia-submission-2017-054-en.pdf (accessed 24 February 2025).
49Available at: https://www.cbd.int/doc/emerging-issues/NewZealand-submission-2017-054-en.pdf (accessed 24 February 2025).
50Available at: https://www.cbd.int/doc/emerging-issues/European-Union-submission-2017-054-en.pdf (accessed 24 February 2025).
51Available at: https://www.cbd.int/doc/emerging-issues/ntf-2019-041-submission-norway-en.pdf (accessed 24 February 2025).
52Available at: https://bch.cbd.int/en/database/SUB/BCH-SUB-SCBD-114285 (accessed 26 February 2025).
53Available at: https://bch.cbd.int/en/database/BCH-SUB-SCBD-114306-1 (accessed 26 February 2025).
54See, e.g., COP-14 decision CBD/COP/DEC/14/19 paragraph 7.
55COP-16 decision CBD/COP/DEC/16/21, paragraph 9(a).
56COP-16 decision CBD/COP/DEC/16/21, paragraph 1.
57Available at: https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwww.cbd.int%2Fdoc%2Finterventions%2F66420d84206cd9c8e4be9452%2FARGENTINA_AgendItem5_SynBio.docx&wdOrigin=BROWSELINK (accessed 24 February 2025).
58Available at: https://www.cbd.int/doc/interventions/66431a584e6b257221223acf/statement%20brazil%20synthetic%20biology.pdf (accessed 24 February 2025).
59See: https://www.un.org/sustainabledevelopment/blog/2024/11/biodiversity-cop-16-important-agreement-reached-towards-goal-of-making-peace-with-nature-2/#:∼:text=After%20roughly%2012%20hours%20of,venue%20to%20complete%20the%20agenda (accessed December 2024).
60COP-16 decision CBD/COP/DEC/16/21, paragraph 1, Annex paragraph 3(a).
61COP-16 decision CBD/COP/DEC/16/21, paragraph 7.
62COP-16 decision CBD/COP/DEC/16/21, Annex paragraph 9(f); see also https://www.un.org/sustainabledevelopment/blog/2024/11/biodiversity-cop-16-important-agreement-reached-towards-goal-of-making-peace-with-nature-2/#:∼:text=After%20roughly%2012%20hours%20of,venue%20to%20complete%20the%20agenda (accessed 22 December 2024).
63COP-16 decision CBD/COP/DEC/16/21, paragraph 9(b), Annex paragraph 3(a).
64COP-16 decision CBD/COP/DEC/16/21, Annex paragraph 3(c) (d) (e).
65See: https://www.un.org/sustainabledevelopment/blog/2024/11/biodiversity-cop-16-important-agreement-reached-towards-goal-of-making-peace-with-nature-2/#:∼:text=After%20roughly%2012%20hours%20of,venue%20to%20complete%20the%20agenda (accessed 22 December 2024).
66COP-16 decision CBD/COP/DEC/16/21, paragraph 10.
67COP-15 decision CBD/COP/DEC/15/8, available at: https://www.cbd.int/doc/decisions/cop-15/cop-15-dec-08-en.pdf.
68See, e.g., CBD/COP/DEC/14/19, paragraph 7, available at: https://www.cbd.int/doc/decisions/cop-14/cop-14-dec-19-en.pdf.
69COP-16 decision CBD/COP/DEC/16/21, paragraph 5.
70See: https://bch.cbd.int/protocol/cpb_art22.shtml (accessed 24 February 2025).
71See https://www.cbd.int/financial/biosafety.shtml#:∼:text=The%20GEF%20approved%2037%20projects%20for%20the%20Implementation,%2443.0%20million%20for%20a%20total%20of%20%2478.2%20million; see also https://www.thegef.org/what-we-do/topics/biodiversity/biosafety (accessed 6 January 2025).
72“Action Plan for Building Capacities for the Effective Implementation of the Protocol” adopted by COP-MOP 1 in decision BS-I/15, and contained in Annex I.
73Updated version adopted by COP-MOP3 in decision BS-III/3; “Capacity-Building for the Effective Implementation of the Cartagena Protocol on Biosafety” adopted by COP-MOP6 in decision BS-VI/3 and retained by COP-MOP8 to 2020 in decision CBD/CP/MOP/DEC/9/3.
74Available at: https://bch.cbd.int/protocol/post2020/capacity-building/text.shtml (accessed 6 January 2025).
75COP-MOP10 decision CBD/CP/MOP/DEC/10/4; available at: https://www.cbd.int/doc/decisions/cp-mop-10/cp-mop-10-dec-04-en.pdf.
76COP-MOP10 decision CBD/CP/MOP/DEC/10/3; available at: https://www.cbd.int/doc/decisions/cp-mop-10/cp-mop-10-dec-03-en.pdf.
77See the online discussions held 6–22 November 2023: “Topic 1: Trends and issues in synthetic biology identified for more detailed assessment” thread “5: Inequity in the participation of developing countries in the context of synthetic biology”, available at: https://www.cbd.int/synbio/current_activities/open-ended_online_forum/november_2023?threadid=3037; and “Topic 2: Capacity-building, technology-transfer and knowledge-sharing needs”, available at: https://www.cbd.int/synbio/current_activities/open-ended_online_forum/november_2023?threadid=3042.
78Available at: https://bch.cbd.int/en/database/113246 (accessed 28 February 2025).
79Available at: https://bch.cbd.int/en/database/SUB/BCH-SUB-BR-263815 (accessed 28 February 2025).
80Available at: https://bch.cbd.int/en/database/113245 (accessed 28 February 2025).
81Available at: https://bch.cbd.int/en/database/SUB/BCH-SUB-GB-267067 (accessed 28 February 2025).
82See: COP-15 decision CBD/COP/DEC/15/31; COP-14 decision CBD/COP/DEC/14/19; COP-13 decision CBD/COP/DEC/XIII/17
83See also, e.g.,: https://www.etcgroup.org/content/160-global-groups-call-moratorium-new-genetic-extinction-technology-un-convention (accessed 28 February 2025).
84COP-MOP9 decision CBD/CP/MOP/DEC/9/13 Annex I; available at: https://www.cbd.int/doc/decisions/cp-mop-09/cp-mop-09-dec-13-en.pdf.
85COP-MOP10 decision CBD/CP/MOP/DEC/10/10; available at: https://www.cbd.int/doc/decisions/cp-mop-10/cp-mop-10-dec-10-en.pdf.
86COP-MOP11 decision CBD/CP/MOP/DEC/11/7; available at: https://www.cbd.int/doc/decisions/cp-mop-11/cp-mop-11-dec-07-en.pdf.
87See: https://iucn.org/our-work/informing-policy/setting-conservation-priorities/synthetic-biology-and-nature-conservation#:∼:text=Recent%20technological%20advancements%20in%20synthetic%20biology%20create%20both,and%20coherent%20policy%20to%20guide%20its%20potential%20applications (accessed 14 February 2025).
88See: https://iucn.org/our-work/informing-policy/international-policy/un-convention-biological-diversity-cbd (accessed 14 February 2025).
89See “Our Union” at: https://iucn.org/our-union (accessed 14 January 2025).
90See “Our work” at: https://iucn.org/our-work (accessed 14 January 2025).
91Resolution WCC-2016-Res-086: Development of IUCN Policy on Biodiversity Conservation and Synthetic Biology. Available at: https://portals.iucn.org/library/node/46503.
92E.g., see: https://foe.org/news/2016-08-genetic-extinction-technology-rejected-by-international-group-of-scientists/.
93Resolution WCC-2020-Res-123-EN: Towards development of an IUCN policy on synthetic biology in relation to nature conservation. Available at: https://portals.iucn.org/library/sites/library/files/resrecfiles/WCC_2020_RES_123_EN.pdf.
94See: https://iucncongress2025.org/.
95See: Decisions of the “109th Meeting of the IUCN Council” available at: https://iucn.org/sites/default/files/2023-06/decisions-of-the-109th-meeting-of-the-iucn-council-draft-26.05_en-final_0.pdf (accessed 6 January 2025).
96See: “The OECD: Better policies for better lives” available at: https://www.oecd.org/en/about.html (accessed 6 January 2025).
97See: “Consensus documents: harmonisation of regulatory oversight in biotechnology” available at: https://www.oecd.org/en/topics/sub-issues/biosafety-novel-food-and-feed-safety/consensus-documents-work-on-harmonisation-of-regulatory-oversight-in-biotechnology.html#:∼:text=These%20consensus%20documents%20comprise%20technical%20information%20for%20use,to%20be%20mutually%20recognised%20among%20OECD%20Member%20countries (accessed 4 February 2025).
98See: “Consensus documents: safety of novel foods and feeds” available at: https://www.oecd.org/en/topics/sub-issues/biosafety-novel-food-and-feed-safety/consensus-documents-on-the-safety-of-novel-foods-and-feeds.html (accessed 4 February 2025).
99Available at: https://biotrackproductdatabase.oecd.org/byOrganism.aspx (accessed 27 February 2025).
100Available at: https://bch.cbd.int/en/ (accessed 24 February 2025).
101COP-MOP11 decision CBD/CP/MOP/DEC/11/7; available at: https://www.cbd.int/doc/decisions/cp-mop-11/cp-mop-11-dec-07-en.pdf.
102See: https://www.oecd.org/en/networks/global-forum-on-technology.html (accessed 18 February 2025).
103See: https://www.oecd.org/en/topics/responsible-innovation.html (accessed 18 February 2025).
104See: https://www.oecd.org/en/networks/global-forum-on-technology.html (accessed 24 January 2025).
105See: 2024 Biodiversity Conference website: https://www.cbd.int/conferences/2024 (accessed 10 February 2025).
106Described, for example, in the report of the m-AHTEG (Multidisciplinary Ad Hoc Technical Expert Group, 2023).
References
Ad Hoc Technical Expert Group on Digital Sequence Information on Genetic Resources [AHTEG] (2018). Report of the ad hoc technical expert group on digital sequence information on genetic resources. Document CBD/SBSTTA/22/INF/4 CBD/DSI/AHTEG/2018/1/4. Available online at: https://www.cbd.int/doc/c/f99e/e90a/71f19b77945c76423f1da805/dsi-ahteg-2018-01-04-en.pdf (accessed December, 2024).
Ad Hoc Technical Expert Group on Risk Assessment [AHTEG] (2024). Additional voluntary guidance materials to support case-by-case risk assessments of living modified organisms containing engineered gene drives. Doc. CBD/CP/MOP/11/9. Available online at: https://www.cbd.int/doc/c/175e/90b0/89c0c71660cccc1539adf34f/cp-mop-11-09-en.pdf.
Ad Hoc Technical Expert Group on Synthetic Biology [AHTEG] (2015). Report of the ad hoc technical expert group on synthetic biology. Doc. UNEP/CBD/SYNBIO/AHTEG/2015/1/3. Available online at: https://www.cbd.int/kb/record/meetingDocument/106031 (accessed December, 2024).
Ad Hoc Technical Expert Group on Synthetic Biology [AHTEG] (2017). Report of the ad hoc technical expert group on synthetic biology. Doc. CBD/SYNBIO/AHTEG/2017/1/3. Available online at: https://www.cbd.int/doc/c/aa10/9160/6c3fcedf265dbee686715016/synbio-ahteg-2017-01-03-en.pdf (accessed February, 2025).
Ad Hoc Technical Expert Group on Synthetic Biology [AHTEG] (2019). Report of the ad hoc technical expert group on synthetic biology. Doc. CBD/SYNBIO/AHTEG/2019/1/3. Available online at: https://www.cbd.int/doc/c/2074/26e7/a135b1b57dabe8e8ed669324/synbio-ahteg-2019-01-03-en.pdf (accessed December, 2024).
Ad Hoc Working Group of Experts on Biological Diversity (1990). Final report of the sub-working group on biotechnology. Document UNEP/Bio.Div/SWGB.1/S/Rev.1. Available online at: https://www.cbd.int/doc/meetings/iccbd/swgb-01/official/swgb-01-05-rev1-en.pdf (accessed December, 2024).
Arts, B., and Mack, S. (2003). Environmental NGOs and the biosafety protocol: a case study on political influence. Environ. Policy Gov. 13, 19–33. doi:10.1002/eet.309
Assidi, M., Buhmeida, A., and Budowle, B. (2022). Medicine and health of 21st Century: not just a high biotech-driven solution. Med 7, 67. doi:10.1038/s41525-022-00336-7
Bennett, A. B., Chi-Ham, C., Barrows, G., Sexton, S., and Zilberman, D. (2013). Agricultural biotechnology: economics, environment, ethics, and the future. Ann. Rev. Environ. Resour. 16, 249–279. doi:10.1146/annurev-environ-050912-124612
Berg, P., Baltimore, D., Brenner, S., Roblin, R. O., and Singer, M. F. (1975). Summary statement of the Asilomar conference on recombinant DNA molecules, Proc. Natl. Acad. Sci. U. S. A., 72, 1981–1984. doi:10.1073/pnas.72.6.1981
Bond, M. R., and Scott, D. (2020). Digital biopiracy and the (dis)assembling of the Nagoya Protocol. Geoforum 117, 24–32. doi:10.1016/j.geoforum.2020.09.001
Butchart, S. H. M., Di Marco, M., and Watson, J. E. M. (2016). Formulating smart commitments on biodiversity: lessons from the Aichi targets. Conserv. Lett. 9, 457–468. doi:10.1111/conl.12278
Callaway, E. (2016). Open peer review finds more takers. Nature 539, 343. doi:10.1038/nature.2016.20969
Callaway, E. (2018). Ban on ‘gene drives’ is back on the UN’s agenda — worrying scientists. Nature 563, 454–455. doi:10.1038/d41586-018-07436-4
Carroll, C., Rohlf, D. J., and Epstein, Y. (2022). Mainstreaming the ambition, coherence, and comprehensiveness of the Post-2020 Global Biodiversity Framework into conservation policy. Front. Conserv. Sci. 3, 906699. doi:10.3389/fcosc.2022.906699
Civil Society Working Group on Gene Drives (2016). The case for a global moratorium on genetically-engineered gene drives. Available online at: https://www.etcgroup.org/sites/www.etcgroup.org/files/files/cbd_cop_13_gene_drive_moratorium_briefing.pdf.
Corlett, R. T. (2017). A bigger toolbox: biotechnology in biodiversity conservation. Trends Biotechnol. 35, 55–65. doi:10.1016/j.tibtech.2016.06.009
Cruz-Cruz, C. A., González-Arnao, M. T., and Engelmann, F. (2013). Biotechnology and conservation of plant biodiversity. Resources 2, 73–95. doi:10.3390/resources2020073
De Greef, W. (2004). The Cartagena Protocol and the future of agbiotech. Nature 22, 811–812. doi:10.1038/nbt0704-811
Diaz, S. (2022). COP15 biodiversity plan risks being alarmingly diluted. Nature 612, 9. doi:10.1038/d41586-022-04154-w
Dickie, G. (2022). ‘Explainer: why did past targets to protect nature fail over the last decade?’ Reuters, online. Available online at: https://www.reuters.com/business/environment/why-did-past-targets-protect-nature-fail-over-last-decade-2022-12-09/.
Dixon, T. A., Freemont, P. S., Johnson, R. A., and Pretorius, I. S. (2022). A global forum on synthetic biology: the need for international engagement. Nat. Commun. 13, 3516. doi:10.1038/s41467-022-31265-9
Ekardt, F., Gunther, P., Hagemann, K., Garske, B., Heyl, K., and Weyland, R. (2023). Legally binding and ambitious biodiversity protection under the CBD, the global biodiversity framework, and human rights law. Environ. Sci. Eur. 35, 80. doi:10.1186/s12302-023-00786-5
Fredriksson, M. (2021). Dilemmas of protection: decolonising the regulation of genetic resources as cultural heritage. Int. J. Herit. Stud. 27, 720–733. doi:10.1080/13527258.2020.1852295
Glowka, L., Burhenne-Guilmin, F., Synge, H., McNeely, J. A., and Gündling, L. (1994). A Guide to the Convention on biological diversity. Gland: IUCN.
Hagerman, S. M., and Pelai, R. (2016). “As far as possible and as appropriate”: implementing the Aichi biodiversity targets. Conserv. Lett. 9, 469–478. doi:10.1111/conl.12290
Herring, R., and Paarlberg, R. (2016). The political economy of biotechnology. Ann. Rev. Resour. Econ. 8, 397–416. doi:10.1146/annurev-resource-100815-095506
Hokanson, K. (2019). When policy meets practice: the dilemma for guidance on risk assessment under the Cartagena protocol on biosafety. Front. Bioeng. Biotechnol. 7, 82. doi:10.3389/fbioe.2019.00082
Hughes, A., Shen, X., Corlett, R., Li, L., Luo, M., Woodley, S., et al. (2022). Challenges and possible solutions to creating an achievable and effective Post-2020 Global Biodiversity Framework. Ecosyst. Health Sust. 8, 2124196. doi:10.1080/20964129.2022.2124196
Hughes, A. C. (2023). The Post-2020 Global Biodiversity Framework: how did we get here, and where do we go next? Integr. Conserv. 2, 1–9. doi:10.1002/inc3.16
Hughes, A. C., and Grumbine, R. E. (2023). The Kunming-Montreal Global Biodiversity Framework: what it does and does not do, and how to improve it. Front. Environ. Sci. 11, 1281536. doi:10.3389/fenvs.2023.1281536
Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services [IPBES] (2016). Summary for policymakers of the assessment report on the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on pollinators, pollination and food production. Bonn: IPBES.
International Institute for Sustainable Development [IISD] (2024a). Earth negotiations bulletin, summary report for the 26th meeting of the CBD subsidiary body on scientific, technical and technological advice (SBSTTA-26) and 4th meeting of the subsidiary body on implementation (SBI-4): 13-29 may 2024, 9. Winnipeg: IISD, 836. Available online at: https://enb.iisd.org/sites/default/files/2024-12/enb09836e.pdf (accessed December, 2024).
International Institute for Sustainable Development [IISD] (2024b). Earth negotiations bulletin, daily report for the 26th meeting of the CBD subsidiary body on scientific, technical and technological advice (SBSTTA-26) and 4th meeting of the subsidiary body on implementation (SBI-4): 18 may 2024, 9. Winnipeg: IISD, 828. Available online at: https://enb.iisd.org/sites/default/files/2024-12/enb09828e.pdf (accessed December, 2024).
International Institute for Sustainable Development [IISD] (2024d). “Earth negotiations bulletin, daily report for the 26th meeting of the CBD subsidiary body on scientific, technical and technological advice (SBSTTA-26) and 4th meeting of the subsidiary body on implementation (SBI-4): 13 may 2024,”, 9. Winnipeg: IISD, 823. Available online at: https://enb.iisd.org/sites/default/files/2024-12/enb09823e.pdf (accessed December, 2024).
International Institute for Sustainable Development [IISD] (2024c). “Earth negotiations bulletin, daily report for the 26th meeting of the CBD subsidiary body on scientific, technical and technological advice (SBSTTA-26) and 4th meeting of the subsidiary body on implementation (SBI-4): 14 may 2024,”, 9. Winnipeg: IISD, 824. Available online at: https://enb.iisd.org/sites/default/files/2024-12/enb09824e.pdf (accessed December, 2024).
International Service for the Acquisition of Agri-biotech Applications [ISAAA] (2020). Brief 55: global status of commercialised biotech/GM crops: 2019. Available online at: https://www.isaaa.org/resources/publications/briefs/55/default.asp.
International Union for the Conservation of Nature and Natural Resources Council Programme and Policy Committee Res 123 Working Group [IUCN Working Group] (2023). Process for the implementation of WCC 2020 Res 123 towards development of an IUCN policy on synthetic biology in relation to nature conservation. Available online at: https://iucn.org/sites/default/files/2023-03/2023_synbio_policy_dev_process_en.pdf.
International Union for the Conservation of Nature and Natural Resources [IUCN] (2019). Genetic frontiers for conservation: an assessment of synthetic biology and biodiversity conservation, synthesis and key messages. Gland: IUCN. doi:10.2305/IUCN.CH.2019.04.en
International Union for the Conservation of Nature and Natural Resources [IUCN] (2024a). Recommendations of the IUCN Citizens’ assembly on synthetic biology in relation to nature conservation. Gland: IUCN.
International Union for the Conservation of Nature and Natural Resources [IUCN] (2024b). Synthetic biology in relation to nature conservation: briefing document. Gland: IUCN.
Kearns, P., Fukase, Y., and Dagallier, B. (2011). “The biotechnology and biosafety activities at the OECD,” in Challenges for agricultural research (Paris: OECD). doi:10.1787/9789264090101-23-en
Kearns, P. W. E., Kleter, G. A., Bergmans, H. E. N., and Kuiper, H. A. (2021). Biotechnology and biosafety policy at OECD: future trends. Trends Biotechnol. 39, 965–969. doi:10.1016/j.tibtech.2021.03.001
Keiper, F. J., and Atanassova, A. (2020). Regulation of synthetic biology: developments under the convention on biological diversity and its protocols. Front. Bioeng. Biotechnol. 8, 310. doi:10.3389/fbioe.2020.00310
Klümper, W., and Qaim, M. (2014). A meta-analysis of the impacts of genetically modified crops. PLOS One 9, e111629. doi:10.1371/journal.pone.0111629
Kreiken, B. E., and Arts, B. J. M. (2024). Disruptive data: how access and benefit-sharing discourses structured ideas and decisions during the Convention on Biological Diversity negotiations over digital sequence information from 2016 to 2022. Glob. Environ. 87, 102892. doi:10.1016/j.gloenvcha.2024.102892
Lai, H.-E., Canavan, C., Cameron, L., Moore, S., Danchenko, M., Kuiken, T., et al. (2019). Synthetic biology and the united Nations. Trends Biotechnol. 37, 1146–1151. doi:10.1016/j.tibtech.2019.05.011
Laird, S. A., and Wynberg, R. P. (2018). Fact-finding and scoping study on digital sequence information on genetic resources in the context of the Convention on Biological Diversity and the Nagoya Protocol. Document CBD/SBSTTA/22/INF/3 CBD/DSI/AHTEG/2018/1/3.
Ludlow, K., Falck-Zepeda, J., and Smyth, S. J. (2025). Risk-appropriate, science-based innovation regulations are important. Trends Biotechnol. 43, 502–510. doi:10.1016/j.tibtech.2024.11.004
Maney, C., Guaras, D., Harrison, J., Guizar-Coutiño, A., Harfoot, M. B. J., Hill, S. L. L., et al. (2024). National commitments to Aichi targets and their implications for monitoring the kunming-montreal global biodiversity framework. NPJ Biodivers. 3, 6. doi:10.1038/s44185-024-00039-5
Mannion, A. M., and Morse, S. (2012). Biotechnology in agriculture: agronomic and environmental considerations and reflections based on 15 years of GM crops. Prog. Phys. Geogr. 36, 747–763. doi:10.1177/0309133312457109
Marchant, G. (2001). The precautionary principle: an 'unprincipled' approach to biotechnology regulation. J. Risk Res. 4, 143–157. doi:10.1080/136698701750128088
Moss, R., and Schneider, S. (2000). “Uncertainties in the IPCC TAR: recommendations to lead authors for more consistent assessment and reporting,” in Guidance papers on the cross cutting issues of the third assessment report of the IPCC. Editors R. Pachauri, T. Taniguchi, and K. Tanaka (Geneva: World Meteorological Organization), 33–51.
Multidisciplinary Ad Hoc Technical Expert Group [m-AHTEG] (2023). Report of the multidisciplinary ad hoc technical expert group on synthetic biology to support the process for broad and regular horizon scanning, monitoring and assessment. Doc. CBD/SYNBIO/AHTEG/2023/1/3. Available online at: https://www.cbd.int/doc/c/7327/0b5e/32328cd7a318c34e7849321e/synbio-ahteg-2023-01-03-en.pdf.
Multidisciplinary Ad Hoc Technical Expert Group [m-AHTEG] (2024). Report of the multidisciplinary ad hoc technical expert group on synthetic biology to support the process for broad and regular horizon scanning, monitoring and assessment on its second meeting. Doc. CBD/SYNBIO/AHTEG/2024/1/3. Available online at: https://www.cbd.int/doc/c/a26e/a6dc/571dd825eb08eef3865f85de/synbio-ahteg-2024-01-03-en.pdf.
Nature (2017). Gene-drive technology needs thorough scrutiny. Nature 552, 6. doi:10.1038/d41586-017-08214-4
Nature (2020). The United Nations must get its new biodiversity targets right, 578, 337–338. doi:10.1038/d41586-020-00450-5
Open-Ended Working Group on the Post-2020 Global Biodiversity Framework (2022). Outcomes of the work of the informal groups on the post-2020 global biodiversity framework. Doc. CBD/WG2020/5/2. Available online at: https://www.cbd.int/doc/c/dfeb/e742/b936c09eae9dd558c1310b5b/wg2020-05-02-en.pdf.
Organisation for Economic Co-operation and Development – Global Forum on Technology [OECD-GFTech] (2023). Inaugural event brief Technology deep drives – synthetic biology. Available online at: https://www.oecd.org/content/dam/oecd/en/events/2023/6/gftech-inaugural-event-brief-synthetic-biology-june-2023.pdf.
Organisation for Economic Co-operation and Development [OECD] (1986b). Recombinant DNA safety considerations (“Blue Book”). Paris: OECD Publishing.
Organisation for Economic Co-operation and Development [OECD] (1986a). Recommendation of the Council concerning safety considerations for applications of recombinant DNA organisms in industry, agriculture and the environment 1986 version. Paris: OECD Publishing.
Organisation for Economic Cooperation and Development [OECD] (1992). Safety considerations for biotechnology. Paris: OECD Publishing.
Organisation for Economic Cooperation and Development [OECD] (1993). Safety considerations for biotechnology: Scale up of crop plants. Paris: OECD Publishing.
Organisation for Economic Co-operation and Development [OECD] (2006). Revised 2006: OECD Guidance for the Designation of a unique Identifier for transgenic plants. Document ENV/JM/MONO(2002)7/REV1. Available online at: https://www.fao.org/fileadmin/user_upload/gmfp/resources/1_OECDGuidance_UI.pdf#:∼:text=This%20guidance%20for%20a%20unique%20identifier%20for%20transgenic,Group%20on%20Harmonisation%20of%20Regulatory%20Oversight%20in%20Biotechnology.
Organisation for Economic Co-operation and Development [OECD] (2014). “Emerging policy issues in synthetic biology,”. Paris: OECD Publishing. doi:10.1787/9789264208421-en
Organisation for Economic Co-operation and Development [OECD] (2016). OECD science, technology and innovation outlook 2016. Paris: OECD Publishing. doi:10.1787/sti_in_outlook-2016-en
Organisation for Economic Co-operation and Development [OECD] (2018). OECD science, technology and innovation outlook 2018: adapting to technological and societal disruption. Paris: OECD Publishing. doi:10.1787/sti_in_outlook-2018-en
Organisation for Economic Co-operation and Development [OECD] (2019). “Recommendation of the Council on artificial intelligence,” in Document OECD/LEGAL/0449.
Organisation for Economic Co-operation and Development [OECD] (2023a). “OECD consensus document on environmental considerations for the release of transgenic plants,”. Paris: OECD Publishing. doi:10.1787/62ed0e04-en
Organisation for Economic Co-operation and Development [OECD] (2023b). “OECD science, technology and innovation outlook 2023: enabling transitions in times of disruption,”. Paris: OECD Publishing. doi:10.1787/0b55736e-en
Organisation for Economic Co-operation and Development [OECD] (2024a). “Framework for anticipatory governance of emerging technologies, OECD science, technology and industry policy papers No. 165,”Paris: OECD Publishing. doi:10.1787/0248ead5-en
Organisation for Economic Co-operation and Development [OECD] (2024b). “OECD agenda for transformative science, technology and innovation policies, OECD science, technology and industry policy papers No. 164,”Paris: OECD Publishing. doi:10.1787/ba2aaf7b-en
Organisation for Economic Co-operation and Development [OECD] (2025a). “Recommendation of the Council concerning safety considerations for applications of recombinant DNA organisms in industry, agriculture and the environment,” in Document OECD/LEGAL/0225.
Organisation for Economic Co-operation and Development [OECD] (2025b). Recommendation of the Council for agile regulatory governance to harness innovation. Doc. OECD/LEGAL/0464.
Organisation for Economic Co-operation and Development [OECD] (2025c). Recommendation of the Council on responsible innovation in Neurotechnology. Doc. OECD/LEGAL/0457.
Organisation for Economic Co-operation and Development Working Party on Biotechnology [OECD-WPB] (2009). Summary record of the 24thsession of the working Party on biotechnology (WPB). Doc. DSTI/STP/BIO/M(2008)7, Item 16 “Synthetic Biol. Available online at: https://one.oecd.org/document/DSTI/STP/BIO/M(2008)7/en/pdf.
Paarlberg, R. (2014). A dubious success: the NGO campaign against GMOs. Gm. Crops Food 5, 223–228. doi:10.4161/21645698.2014.952204
Pellegrino, E., Bedini, S., Nuti, M., and Ercoli, L. (2018). Impact of genetically engineered maize on agronomic, environmental and toxicological traits: a meta-analysis of 21 years of field data. Sci. Rep. 8, 3113. doi:10.1038/s41598-018-21284-2
Perino, A., Pereira, H. M., Felipe-Lucia, M., Kim, H. J., Kühl, H. S., Marselle, M. R., et al. (2021). Biodiversity post-2020: closing the gap between global targets and national-level implementation. Conserv. Lett. 15, e12848. doi:10.1111/conl.12848
Robinson, D., and Nadal, D. (2025). “Synthetic biology in focus: policy issues and opportunities in engineering life,” in OECD science, technology and industry working papers 2025/03. Paris: OECD Publishing. doi:10.1787/3e6510cf-en
Roell, M.-S., and Zurbriggen, M. D. (2020). The impact of synthetic biology for future agriculture and nutrition. Curr. Opin. Biotechnol. 61, 102–109. doi:10.1016/j.copbio.2019.10.004
Secretariat of the Convention on Biological Diversity [SCBD] (2000). Cartagena protocol on biosafety to the convention on biological diversity: text and annexes. Montreal: Secretariat of the Convention on Biological Diversity.
Secretariat of the Convention on Biological Diversity [SCBD] (2004). The biosafety clearing House of the Cartagena protocol on biosafety: a guide to the BCH. Montreal: Secretariat of the Convention on Biological Diversity.
Secretariat of the Convention on Biological Diversity [SCBD] (2005). Handbook of the convention on biological diversity including its Cartagena protocol on biosafety. 3rd edition. Montreal: Secretariat of the Convention on Biological Diversity. Available online at: https://www.cbd.int/doc/handbook/cbd-hb-all-en.pdf (accessed December, 2024).
Secretariat of the Convention on Biological Diversity [SCBD] (2011). Nagoya protocol on access to genetic resources and the fair and equitable sharing of benefits arising from their utilisation to the convention on biological diversity. Montreal: Secretariat of the Convention on Biological Diversity.
Silvestri, L. C., and Roig-Cerdeño, M. (2025). Genetic resources are, above all, information: perspectives from law, biology and economics. Int. Environ. Agreem. Polit. Law Econ. 25, 127–143. doi:10.1007/s10784-025-09665-1
Slavin, T. (2022). ‘How Elizabeth Mrema is striving to affect a ‘Paris moment’ for nature’ Reuters. Available online at: https://www.reuters.com/business/sustainable-business/how-elizabeth-mrema-is-striving-affect-paris-moment-nature-2022-10-25/(accessed December, 2024).
Soetaert, W., and Vandamme, E. (2006). The impact of industrial biotechnology. Biotechnol. J. 1, 756–769. doi:10.1002/biot.200600066
Soozanipour, A., Ejeian, F., Boroumand, Y., Rezayat, A., and Moradi, S. (2023). Biotechnological advancements towards water, food and medical healthcare: a review. Chemosphere 312, 137185. doi:10.1016/j.chemosphere.2022.137185
Strauss, S. H., Tan, H., Boerjan, W., and Sedjo, R. (2009). Strangled at birth? Forest biotech and the convention on biological diversity. Nat. Biotechnol. 27, 519–527. doi:10.1038/nbt0609-519
Streck, C. (2023). Synergies between the Kunming-Montreal Global Biodiversity Framework and the Paris Agreement: the role of policy milestones, monitoring frameworks and safeguards. Clim. Policy. 23, 800–811. doi:10.1080/14693062.2023.2230940
Tang, W. L., and Zhao, H. (2009). Industrial biotechnology: tools and applications. Biotechnol. J. 4, 1725–1739. doi:10.1002/biot.200900127
Trump, B., Cummings, C., Klasa, K., Galaitsi, S., and Linkov, I. (2023). Governing biotechnology to provide safety and security and address ethical, legal, and social implications. Front. Genet. 13, 1052371. doi:10.3389/fgene.2022.1052371
Tsioumani, E. (2020). Convention on biological diversity: a review of the post-2020 global biodiversity framework working group negotiations. Environ. Policy Law. 50, 55–59. doi:10.3233/EPL-200207
Turnbull, C., Lillemo, M., and Hvoslef-Eide, T. A. K. (2021). Global regulation of genetically modified crops amid the gene edited crop boom – a review. Front. Plant Sci. 12, 630396. doi:10.3389/fpls.2021.630396
United Nations (2010). The Millennium development goals report. New York: United Nations. Available online at: https://www.undp.org/sites/g/files/zskgke326/files/migration/rw/Millenium-Development-Goals_report_2010.pdf (accessed February, 2025).
United Nations Department of Public Information (1993). Agenda 21: programme of action for sustainable development, Rio Declaration on Environment and Development, statement of forest principles: the final text of agreements negotiated by Governments at the United Nations Conference on Environment and Development (UNCED). Available online at: https://digitallibrary.un.org/record/170126#:∼:text=Title%20Agenda%2021%20%3A%20programme%20of%20action%20for,and%20Development%20%28UNCED%29%2C%203-14%20June%201992%2C%20Rio%20d.
Voigt, C. A. (2020). Synthetic biology 2020–2030: six commercially-available products that are changing our world. Nat. Commun. 11, 6379. doi:10.1038/s41467-020-20122-2
Keywords: Convention on biological diversity, Cartagena Protocol on Biosafety, Kunming-Montreal Global Biodiversity Framework, KMGBF Target 17, biotechnology regulation, synthetic biology policy, OECD biotechnology and biosafety policy
Citation: Keiper F and Atanassova A (2025) International synthetic biology policy developments and implications for global biodiversity goals. Front. Synth. Biol. 3:1585337. doi: 10.3389/fsybi.2025.1585337
Received: 28 February 2025; Accepted: 26 May 2025;
Published: 30 June 2025.
Edited by:
Alicia Sanchez-Gorostiaga, Instituto Madrileño de Investigación y Desarrollo Rural, Agrario y Alimentario, SpainReviewed by:
Ali Samy Abdelaal, Damietta University, EgyptNeil E. Hoffman, United States Department of Agriculture (USDA), United States
Copyright © 2025 Keiper and Atanassova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Felicity Keiper, ZmVsaWNpdHkua2VpcGVyQGJhc2YuY29t
 Felicity Keiper
Felicity Keiper Ana Atanassova
Ana Atanassova