- 1Functional Genomics Unit, Dasman Diabetes Institute, Kuwait City, Kuwait
- 2Clinical Division, Dasman Diabetes Institute, Kuwait City, Kuwait
- 3Applied Oral Sciences, The Forsyth Institute, Cambridge, MA, United States
- 4Family Medicine and Pediatric Unit, Dasman Diabetes Institute, Kuwait City, Kuwait
- 5Faculty of Allied Health Sciences, Kuwait University, Kuwait City, Kuwait
- 6Biochemistry and Molecular Biology Unit, Dasman Diabetes Institute, Kuwait City, Kuwait
- 7Faculty of Medicine, Kuwait University, Kuwait City, Kuwait
- 8Immunology Unit, Dasman Diabetes Institute, Kuwait City, Kuwait
- 9National Dasman Diabetes Biobank, Dasman Diabetes Institute, Kuwait City, Kuwait
- 10Cell Therapy and Applied Genomics, King Hussein Cancer Center, Amman, Jordan
- 11Research Division, Dasman Diabetes Institute, Kuwait City, Kuwait
Caveolin-1 (CAV1) variants have been suggested to be associated with obesity and related metabolic disorders, but information based on human studies is limited. In the present study, we aimed to investigate the potential association between the CAV1 rs1997623 C/A variant and metabolic syndrome (MetS) in Kuwaiti children. DNA from saliva samples collected from 1313 Kuwaiti children (mean age: 12 years) were genotyped using the TaqMan SNP genotyping assay. The classification of MetS was based on the presence/absence of four indicators; (1) central obesity, (2) elevated systolic or diastolic blood pressure, (3) low salivary high-density lipoprotein cholesterol (HDLC), and (4) high salivary glucose. In this study, children with MetS scored ≥3, children in the intermediate metabolic group scored 1 or 2 and children without MetS scored 0. About one-third of the children were obese. A total of 246 children (18.7%) were classified as having MetS; 834 children (63.5%) were in the intermediate metabolic group, and 233 children (17.7%) had no indication of MetS. Obesity was highly prevalent in the MetS group (91.9%) while 26.8% of children were obese in the intermediate metabolic group. None of the children were obese in the group without MetS. Analysis of the CAV1 rs1997623 variant revealed a significant association of the A-allele (p = 0.01, Odds Ratio (OR) = 1.66) and the heterozygous CA-genotype (p = 0.005, OR = 1.88) with MetS. Consistently, the A-allele (p = 0.002, OR = 1.71) and CA-genotype (p = 0.005, OR = 1.70) also showed significant association with the intermediate metabolic group. Furthermore, the A-allele (p = 0.01, OR = 1.33) and the CA-genotype (p = 0.008, OR = 1.55) were associated with low levels of saliva HDLC. Individuals who were heterozygous or homozygous for the variant (CA/AA) showed significantly lower levels of high HDLC compared to those harboring the CC-genotype (p = 0.023). Our study revealed a novel association of the CAV1 rs1997623 variant with the MetS and with low saliva HDLC levels in young Kuwaiti children and indicated the need for further in-depth studies to unravel the role of CAV1 gene in the genetic etiology of MetS.
Introduction
The prevalence of obesity has dramatically increased over the past decades, affecting nearly half of the Kuwaiti population (ALNohair, 2014). Childhood obesity is considered one of the major public health concerns in many countries including Kuwait due to its long-term effects on health and wellbeing of the individuals in the population. Recent reports on Kuwait Nutrition Surveillance System indicates that 26.2% of school children (>5–19 years old) are obese according to the WHO Body Mass Index (BMI) for age z-scores, indicating a high prevalence of childhood obesity and its associated metabolic complications (MOH, 2015). Metabolic syndrome (MetS) refers to a cluster of interconnected factors that increase the risk of non-communicable diseases, typically defined based on clinical measurements such as blood pressure and abdominal obesity as well as fasting blood glucose, serum triglycerides, and high-density cholesterol concentrations. Very limited information is currently available on the metabolic health status of children in Kuwait (Elkum et al., 2016).
The etiology of obesity is multifactorial and includes both genetic and environmental factors. Global studies have reported 157 diverse genetic loci and other lifestyle-related susceptibility factors (Willer et al., 2013). In this post-genome-wide association study era, an enormous wealth of genomic data has become publicly available, enabling the fine-mapping and translation of genetic information into clinical practice. In this study, we focused on the caveolin-1 (CAV1) gene, located on chromosome 7q31.2, that acts as an integral component of the small plasma membrane invaginations known as caveolae (Nixon et al., 2007). Caveolae are implicated in endocytosis and in other signaling pathways involving eNOS, G Beta Gamma, G alpha, integrin, platelet-derived growth factor, and endocytic virus entry (Schwencke et al., 2006). Several animal cell model studies have elucidated the role of CAV1 in glucose and lipid metabolism. CAV1 tends to play an important role in blood glucose regulation either by directly interacting with the insulin receptor or by facilitating GLUT4-mediated glucose transport (Gonzalez-Munoz et al., 2009). Upregulation of CAV1 has been linked to a heightened state of oxidative stress and impaired diabetic wound healing (Bitar et al., 2013). CAV1-deficient mice have been reported to exhibit increased levels of triglycerides and free fatty acids, predisposing them to hyperlipidaemia and obesity (Park et al., 2002). Conflicting roles of CAV1 in promoting or negatively regulating HDLC uptake have also been reported in literature (Langmann et al., 1999; Matveev et al., 1999; Frank et al., 2002; Wang et al., 2003).
Caveolin-1 variants have been previously implicated in various cancers, such as breast, renal and nasopharyngeal carcinomas (Liu et al., 2011; Tsou et al., 2011; Chang et al., 2014). Nevertheless, very few studies have explored the significance of the association of CAV1 variants with metabolic traits (Pojoga et al., 2011; Baudrand et al., 2015; Chen et al., 2016). The CAV1 rs926198 SNP was reported to be associated with MetS in Caucasians and Hispanics (Baudrand et al., 2015). The rs926198 and rs3807989 SNPs were also shown to be associated with insulin resistance and hypertension in the same study population (Pojoga et al., 2011; Baudrand et al., 2015). Similarly, the rs3807989 SNP was reported to be associated with significant risk of coronary heart disease in the Chinese Han population (Chen et al., 2016). Nevertheless, no study has so far characterized the role of CAV1 rs1997623 variant with MetS.
Materials and Methods
Study Rationale
A review of the literature indicates several animal and cell model studies depicting the significance of CAV1 in the pathophysiology of diabetes and obesity. Our previous study has additionally shown the risk role of CAV1 in impaired diabetic wound healing (Bitar et al., 2013). In preparation for the present study, we shortlisted CAV1 rs1997623 single nucleotide polymorphism as a population-specific risk variant from our in-house exome database consisting of 156 adult Arabs. We were intrigued to note that the current genome wide association (GWA) studies did not highlight any role for CAV1 in MetS. A detailed search indicated that the most commonly used Illumina Human OmniExpress Bead Chip array does not include the tested rs1997623 variant. Therefore, we adopted a candidate gene approach to evaluate the risk association of the CAV1 rs1997623 SNP with indicators of metabolic complications associated with childhood obesity. Saliva samples were used as specimens to analyze the association of the candidate variant with metabolic complications related to obesity. Parallel validation studies have been reported in the literature indicating the possibility of saliva to be a non-invasive surrogate marker for blood glucose and blood lipid measures (Mascarenhas et al., 2014; Singh et al., 2014; Shi et al., 2015; Hartman et al., 2016). The study population consisted of a sub-sample of Kuwaiti children drawn from previous studies (Goodson et al., 2013, 2014; Shi et al., 2015; Hartman et al., 2016) and used information collected during the original study. The risk variant was investigated for its association with MetS based on indicators such as waist circumference, blood pressure (diastolic and systolic), saliva glucose concentration and saliva HDLC concentration.
Sample Collection
A cohort of Kuwaiti school children were recruited for the nationwide “Kuwait healthy lifestyle study” from all six Kuwaiti governorates covering 138 schools from 2011 to 2014 (Goodson et al., 2013, 2014, 2017; Shi et al., 2015; Hartman et al., 2016). A total of 8317 children were enrolled into the original study. The average number of schools visited was 23 schools per governorate (range 13–31, average of 46 children/school). For the present study, a subset of 1313 participant were chosen by random sampling. Written informed consent was obtained from the guardians/parents of all participants in the original study in accordance with the Declaration of Helsinki. Participant’s assent was obtained on the day of the visit. The original study as well as the present study were reviewed and approved by the institutional research ethics committees at Dasman Diabetes Institute.
Each participant provided a sample of saliva (3 mL) after an overnight fast and biochemical analyses were performed as described previously (Goodson et al., 2013, 2014, 2017; Shi et al., 2015; Hartman et al., 2016). DNA was extracted using the QIAamp® DNA extraction kit from Qiagen (Hilden, Germany) according to the manufacturer’s instructions. Previously recorded anthropometric, clinical and biochemical parameters included body height (m) and weight (kg), BMI (kg/m2), heart rate (HR, beats/min), self-reported hours of sleep, saliva flow rate (SFR, mL/h), waist circumference (WC, cm), systolic blood pressure (SBP, mmHg), diastolic blood pressure (DBP, mmHg), saliva glucose concentration (mg/dL), and saliva HDLC concentration (mg/dL) are presented in (Table 1). Study participants fitness was assessed based on the degree of HR elevation following 3 min of standardized exercise. All methods and cut-offs are presented by Shi et al. (2015). Obesity was defined as BMI for age z-scores ≥95th percentile according to the WHO. Central obesity was based on WC ≥90th percentile. Elevated blood pressure was based on either SBP (>130 mmHg) or DBP (>85 mmHg), high salivary glucose was defined as ≥1.13 mg/dL; equivalent to an extrapolated blood concentration of 100 mg/dL (Mascarenhas et al., 2014) and low salivary HDLC was defined as ≤0.6 mg/dL; equivalent to an extrapolated blood concentration of 40 mg/dL (Singh et al., 2014).
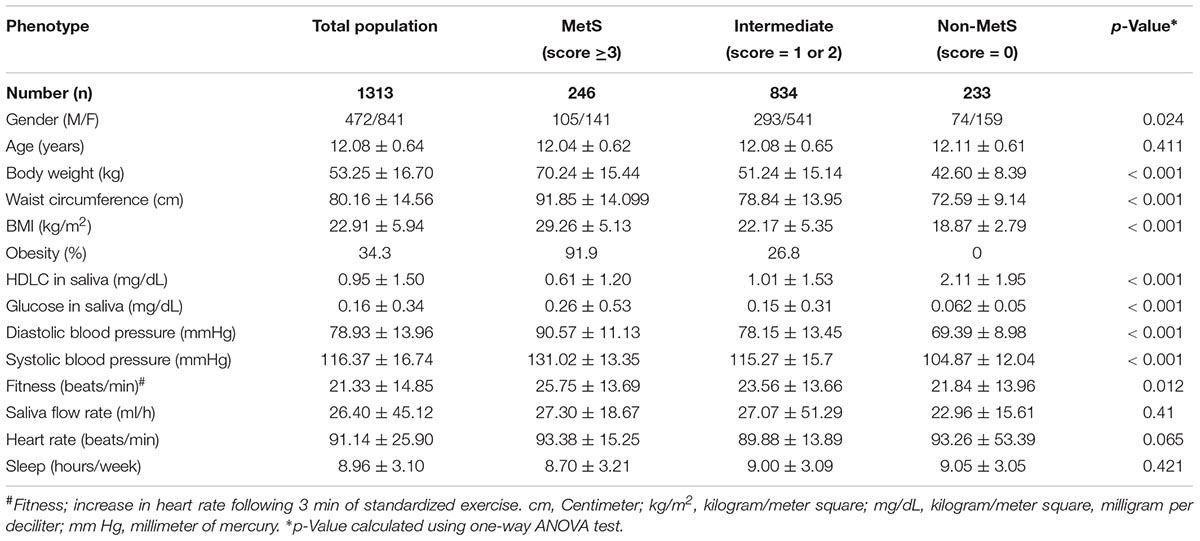
Table 1. Characteristics of study subjects based on the scoring system for metabolic syndrome (MetS).
Metabolic Syndrome Score
Metabolic syndrome was first recognized as a syndrome delineating the co-occurrence of common metabolic traits such as obesity, insulin resistance, hypertension, impaired glucose tolerance or diabetes, hyperinsulinemia, and dyslipidemia (Reaven, 1988). Although definitions of MetS remain controversial, a method adopted in National Health and Nutrition Examination Survey (NHANES) III, defines MetS as the presence of any 3 of these 5 clinical diagnostic traits (Ford et al., 2002). These characteristics were adapted in 2007 to 10 to 16-year-old adolescents by a consensus group of the International Diabetes Foundation as (1) abdominal girth (≥90th percentile in waist circumference), (2) high blood pressure (SBP ≥ 130 mmHg or DBP ≥ 85 mmHg), (3) reduction in HDLC (<40 mg/dL), (4) high fasting glucose (≥100 mg/dL), and (5) high fasting triglyceride (≥150 mg/dL) (Zimmet et al., 2007). In our study cohorts, MetS was defined based on the presence of at least three of the following four risk factors, (1) increased WC (≥90th percentile); (2) raised blood pressure (SBP ≥130 or DBP ≥85; (3) reduced salivary HDLC level (<0.6 mg/dL); and (4) elevated salivary glucose (≥1.13 mg/dL). We have previously shown that the salivary measures of HDLC (Shi et al., 2015) and glucose (Hartman et al., 2016) correlates with the plasma measurements. Since salivary triglycerides were not observed to correlate with plasma triglycerides, this was not measured. Following the criterion (Reaven, 1997; Ford et al., 2002; Zimmet et al., 2007; Hartman et al., 2015; Shi et al., 2015), a total of 246 children were classified as MetS (a score ≥3). About 99.1% of children in this group are obese with two or more additional metabolic conditions. A subset of 233 children who had no indications of MetS were considered as non-MetS (score = 0).
A subgroup of 834 children who failed to fulfill the comprehensive definition of MetS were classified as intermediate metabolic/ unclassified group. The children in this group majorly differs from the MetS by the presence of one or two metabolic risk factors (score = 1 or 2) and comprises of 26.8% of obese, 35.5% of SBP/DBP and 61.5% of low HDLC children. Hence, the intermediate risk group was considered as a separate subgroup for subsequent analysis. It is not justified to label this group as MetS (given the strict criteria), nor as non-MetS, as they are characterized by the presence of at least one or two metabolic risk factors and may fulfill the criteria for MetS later in life as they grow older. Given the fact that MetS is age-dependent, we opted for a thorough and more scientifically transparent approach. Both MetS and intermediate MetS groups were separately compared to the group children with no metabolic complications (Non-MetS).
SNP Genotyping Using Real-Time PCR
Caveolin-1 rs1997623 SNP genotyping was conducted using the TaqMan SNP genotyping assay (Applied Biosystems, Foster City, CA, United States) and the ABI 7500 real-time PCR system (Applied Biosystems). Each PCR reaction contained 10 ng of genomic DNA, 5× FIREPol Master Mix (Solis BioDyne, Tartu, Estonia, Europe) and 1 μl of 20× TaqMan SNP Genotyping Assay. Thermal cycling conditions were as follows: 60°C for 1 min, 95°C for 15 min, 40 cycles of 95°C for 15 s and 60°C for 1 min. The genotypes ascribed by real-time PCR were confirmed by direct sequencing of the PCR products for selected cases of homozygotes and heterozygotes. Sequencing reactions were performed using the BigDye terminator cycle sequencing FS ready reaction kit (Applied Biosystems) according to manufacturer’s instructions on an ABI PRISM 3730 Xl genetic analyzer (Applied Biosystems).
Statistical Analysis
Genetic association analysis was conducted using the Statistical Package for the Social Sciences, SPSS version 25.0’ (IBM Corp., Armonk, NY, United States). Deviation from the Hardy–Weinberg equilibrium (HWE) was tested using the GenePop software1. Differences in genotype and allele frequencies between the study groups were assessed using the chi-square (χ2) test p ≤ 0.05 was considered statistically significant. Genotype-based ORs and 95% confidence interval (CI) were calculated to measure potential risk factors using individuals homozygous for the non-susceptible allele as a reference. To further analyze the overall effect of rs1997623 variant on studied phenotype individuals carrying the mutant allele (CA and AA) were combined. For continuous variables, comparisons of the means between study subjects were assessed using the Student’s t-test or ANOVA for normally distributed data and the non-parametric Mann–Whitney U-test for non-normally distributed data. To assess the effect of rs1997623 genotype on continuous variable linear regression analysis was adopted. Binary logistic regression analysis was used to identify the risk of different genotypes of rs1997623 variant for MetS and low measures of HDLC after adjustment for the confounding effect of age and gender. The rs1997623 variant was considered as a predictor variable and binary outcome measures of metabolic status (presence/absence) as a predicted variable. The wildtype CC genotype was used as the reference category to calculate the adjusted odds ratio and 95% CI. Two-way ANOVA was carried out using GraphPad prism software2. Differences in the allelic frequency distribution of rs1997623 variant between Kuwaiti and other ethnic population from GnomAD exome population database3 were carried out using chi-square test (2 × 2, 1df).
Results
Study Characteristics
Genetic profiling of ancestry was not carried out to due to limitations with the salivary DNA samples. Ethnic bias within the population studied was minimized by excluding non-native children from Asia and adjacent Arab countries. This was in turn facilitated by conducting a detailed interview of the study participants to understand their ethnic background and ancestry. DNA samples of native Kuwaiti children who attended public schools where included in this study. A total of 1,313 children were genotyped to study the potential association of the CAV1 rs1997623 C/A SNP with specific metabolic traits. The number of live births in Kuwait in 2002 was 43,490 (United Nations Demographic Yearbooks). Therefore, the 1,313 children in this study represent approximately 3% of the target population of native Kuwaiti children, who were 10-years old in 2012.
The characteristics of study participants based on their metabolic status are detailed in Table 1. About a third of the children (34.3%), were obese. Two hundred and forty-six children (18.7%) were classified as having MetS; 834 children (63.5%) were in the intermediate metabolic risk group and 233 children (17.7%) had no indication of MetS. Obesity was highly prevalent in the MetS group (91.9%), while 26.8% of children were obese in the intermediate metabolic group. None of the children were obese in the group without MetS. As expected, significantly increased measures of body weight, WC, BMI, HDLC, glucose, DBP, SBP, and fitness were observed in the MetS group followed by intermediate MetS in comparison to non-MetS group, by one-way ANOVA analysis (Table 1). Other tested parameters such as age, saliva flow rate, heart rate, and weekly sleeping hours failed to show any significant differences across the three study groups.
We further observed a significant correlation between various tested phenotypes such as weight, BMI, waist circumference, SBP, DBP and fitness. Both glucose and HDLC failed to show any significant correlation with the aforementioned phenotypes, though a weak positive correlation was observed to exist between glucose and HDLC (p < 0.001, r = 19.4). The analysis stratified by sex failed to show any significant differences in the distribution of obesity, hypertension, HDLC and glucose between boys and girls. No significant deviation from HWE was found for the tested SNP (p > 0.05); allele-A represents the minor allele with a frequency of 0.15 in the tested population. Studying the genotype–phenotype relationships revealed significant differences in the distribution of WC, weight, BMI, DBP, SBP, fitness, glucose levels, and HDLC levels between MetS, intermediate MetS and the group without MetS, as determined using two-way ANOVA (p < 0.0003, Supplementary Table 1).
Association of CAV1 rs1997623 SNP With MetS
We further investigated the risk role of rs1997623 variant by linear regression analysis with the MetS score as a continuous dependent variable and genotype as the independent variable (CC, CA/AA) controlling for other covariates such as age and gender. Analysis revealed significant association of rs1997623 variant with MetS (F = 4.43, p = 0.004). A subgroup of children with and without MetS, excluding the intermediate group, also revealed significantly increased measures of body weight (p = 0.002), BMI (p = 0.001), WC (p = 0.004), DBP (p = 0.001), and SBP (p = 0.02) in the mutant CA/AA genotypes compared with those carrying the wild-type (CC) genotype (Figure 1).
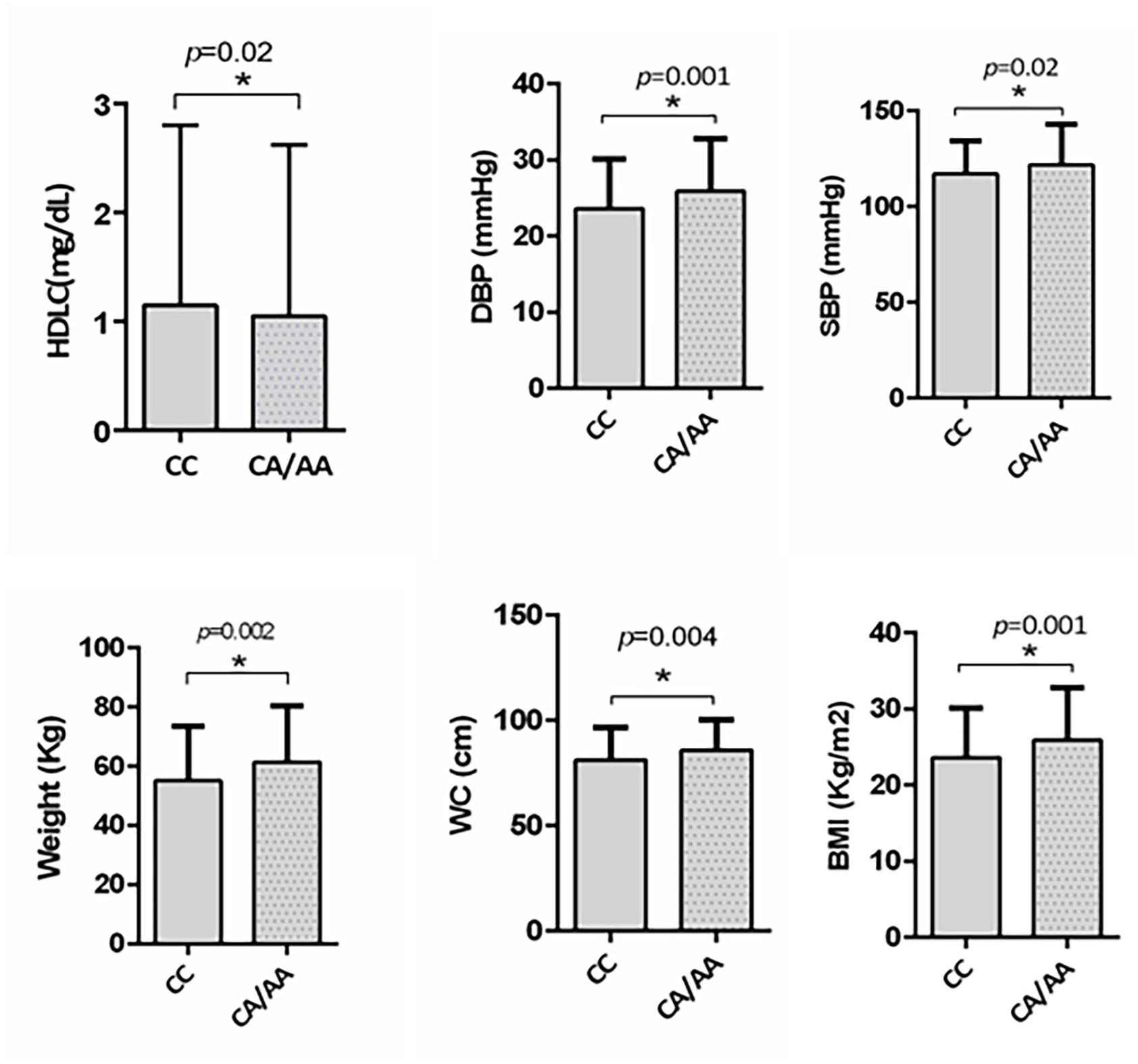
Figure 1. Depicts significantly increased measures of weight (p = 0.002), BMI (p = 0.001), waist circumference (p = 0.004), DBP (p = 0.007), and SBP (p = 0.02) in subjects carrying mutant CA/AA genotype (n = 115) compared to those with wildtype CC genotype (n = 358) in a subgroup of subjects with and without MetS by independent t-test. A significant reduction in HDLC (p = 0.02) was also observed in subjects carrying mutant CA/AA genotype compared to the wildtype CC genotype by Mann–Whitney test.
Categorical analysis revealed a significant difference in the distribution of the rs1997623 variant across the three study groups (χ2 = 11.64, p = 0.02; df = 4). The rs1997623 variant was observed to be significantly associated with susceptibility to MetS; the frequency of the A-allele was higher in the MetS group (15%) than that in the non-MetS group (10%), with a significant χ2 value of 6.24 (p = 0.01) and an OR of 1.66 at 95% CI 1.11–2.46 (Table 2). The heterozygous rs1997623 CA genotype showed significant association with MetS compared with the non-MetS group (p = 0.005, OR = 1.88 at 95% CI 1.21–2.93). Lack of association between MetS and mutant AA genotype could be due to limited frequency of AA genotype in the study population. We further observed significant associations of the rs1997623 A-allele (p = 0.002, OR = 1.71 at 95% CI 1.22–2.40) and CA genotype (p = 0.005, OR = 1.70 at 95% CI 1.17–2.47) with the intermediate metabolic group compared with the non-MetS group (Table 2). The overall distribution of genotype frequencies (CA + AA) versus CC) also revealed significant association of the rs1997623 SNP with MetS (p = 0.01, OR = 1.75 at 95% CI 1.13–2.68) and with the intermediate metabolic group (p = 0.004, OR = 1.7 at 95% CI 1.18–2.44).
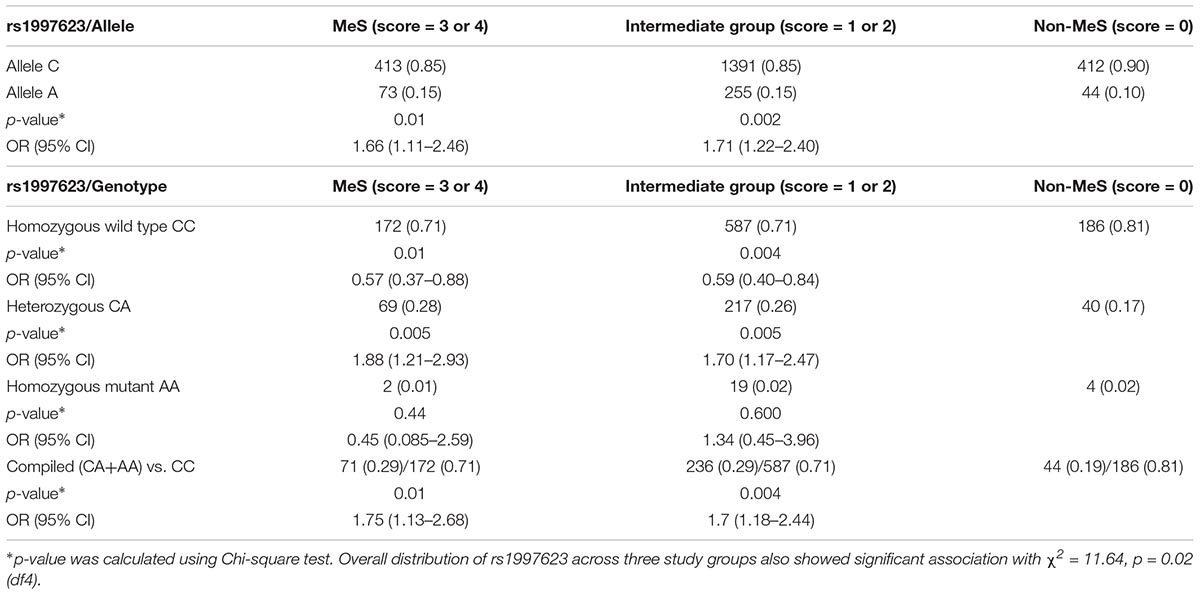
Table 2. Allele and genotypic distribution of the CAV1 rs1997623 SNP based on metabolic syndrome risk score.
We additionally carried out logistic regression analysis to ascertain the effects of rs1997623, age and gender on the like-hood of children having MetS. Our analysis indicates that only gender contributed significantly to model with a p = 0.006. Compared to wildtype CC genotype, children with CA genotype showed increased susceptibility to MetS with a p = 0.004 and adjusted odds ratio (AOR) of 1.938 at 95% CI 1.24–3.029 (Table 3). To further analyze the overall effect of rs1997623 variant on the tested phenotype, we combined children carrying the mutant allele (CA and AA). Compared to wildtype CC, children carrying the mutant genotype showed increased susceptibility to MetS with a p = 0.008 and adjusted odds ratio (AOR) of 1.806 at 95% CI 1.170–2.789.
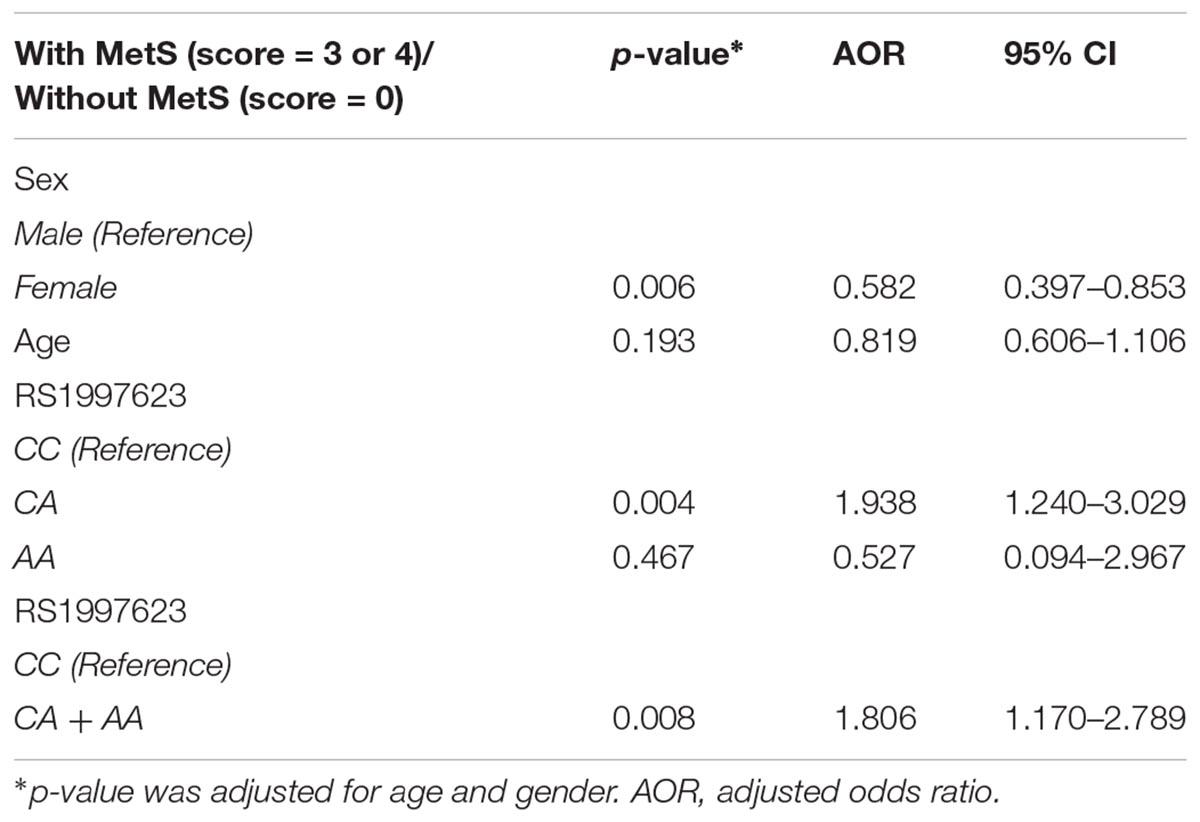
Table 3. Logistic regression analysis of the rs1997623 variant in a group of subjects with and without metabolic syndrome.
Association of CAV1 rs1997623 SNP With Low Salivary High-Density Lipoprotein Cholesterol (HDLC)
We further investigated the association of the rs1997623 SNP with metabolic traits such as saliva HDLC of the study participants. Our analysis revealed a significant association of rs1997623 with low saliva HDLC in Kuwaiti children. Subjects carrying the heterozygous or mutant genotype CA/AA (mean rank 597.05, n = 342) had a significantly lower HDLC level compared with those carrying the CC wildtype (mean rank 649.67, n = 928), (p = 0.023; Figure 2). The linear regression analysis failed to show a statistically significant association between HDLC and rs1997623 SNP.
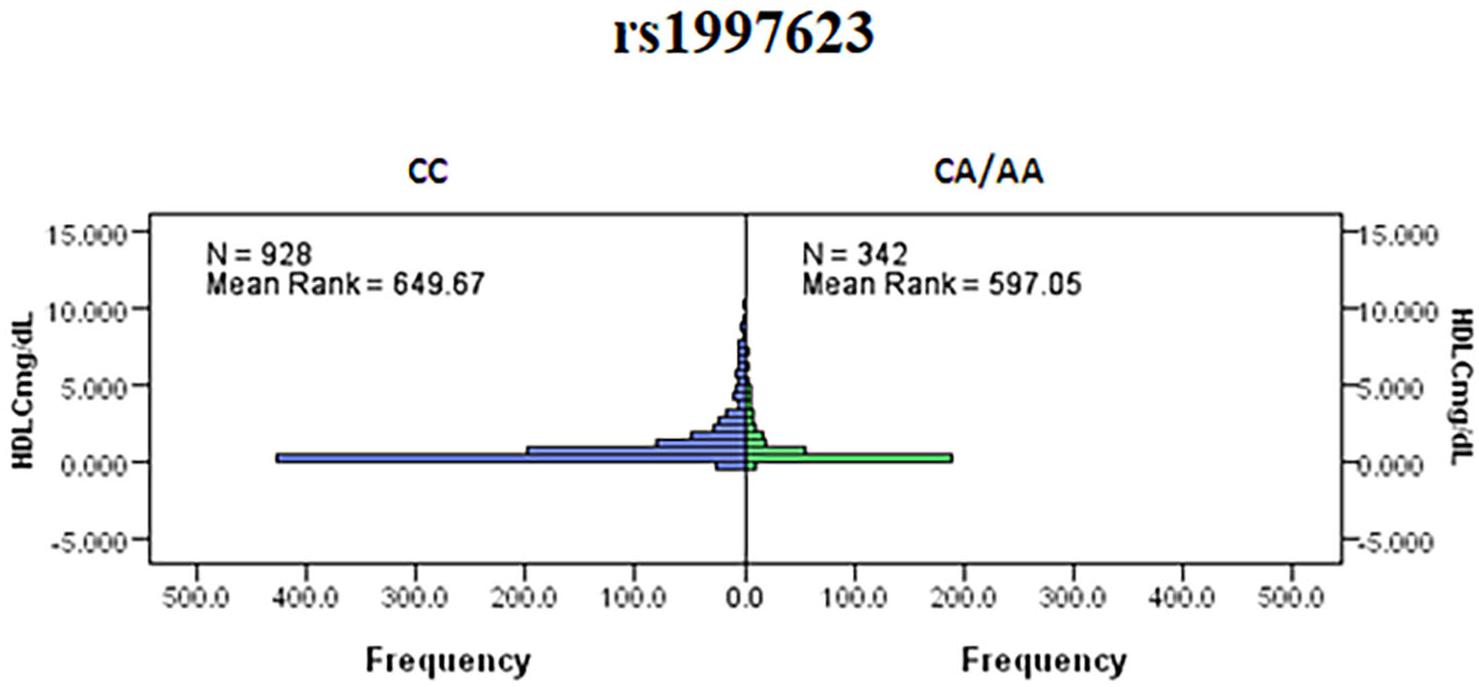
Figure 2. Indicates a significant association of rs1997623 with low HDLC in Kuwaiti children. Subjects carrying the heterozygous or mutant genotype showed significantly lower level of HDLC when compared to those with the wildtype genotype by Mann–Whitney U-test (p = 0.023).
We further verified the association of the rs1997623 SNP with low HDLC (Table 4) by stratifying the participants into clinical subtypes as follows: those with a low HDLC level (n = 724) and those with a normal HDLC level (n = 572). The frequency of the A-allele was higher in subjects with low HDLC (16%) than those in the control group (13%), χ2 = 6.08; (p = 0.01) and an OR = 1.33 (95% CI 1.05–1.66). Similarly, the rs1997623 heterozygous CA genotype showed significant association with predisposition toward low HDLC (p = 0.0008; OR = 1.55; 95% CI 1.20–2.01). The overall distribution of genotype frequencies (CA + AA)/CC, also showed significant association (p = 0.003, OR = 1.47, 95% CI 1.14–1.89) with low HDLC. The strength of this association significantly improved after adjustment for age and sex using logistic regression analysis (Table 5). The children carrying CA genotype showed increased odds of low HDLC level compared to those carrying CC genotype (p = 0.001, AOR = 1.569, 95% CI 1.209–2.1037). Combined analysis also indicated association of mutant genotype with reduced measured of HDLC (p = 0.002, AOR = 1.489, 95% CI 1.157–1.916).
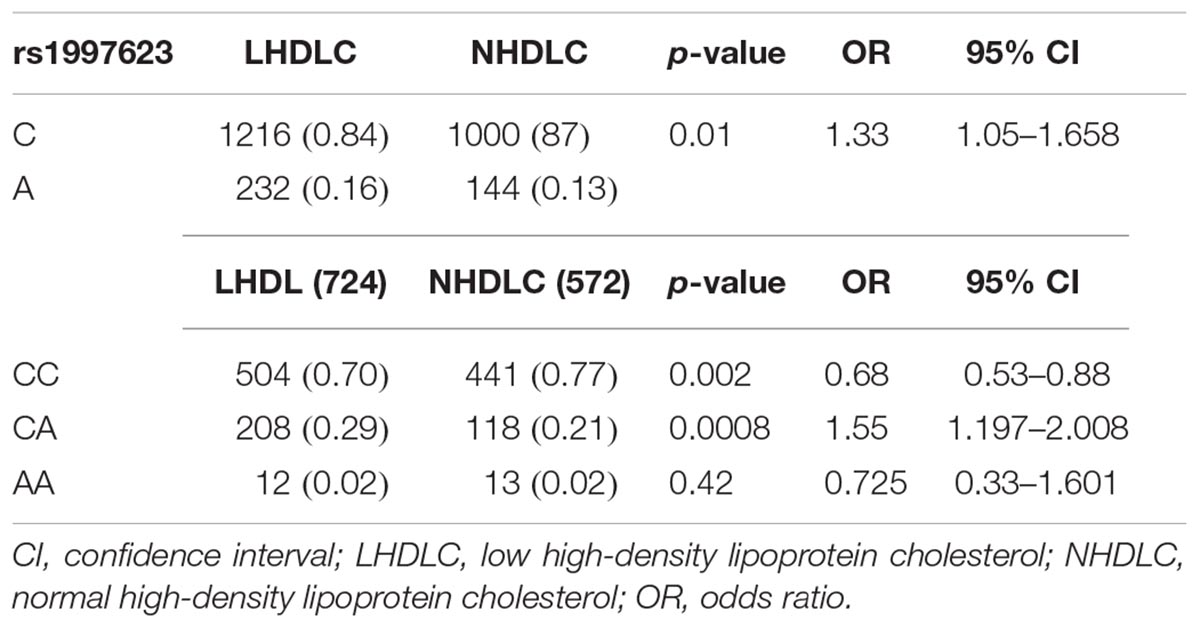
Table 4. Allele and genotypic distribution of the CAV1 rs1997623 SNP in low HDLC subjects compared to the control group.
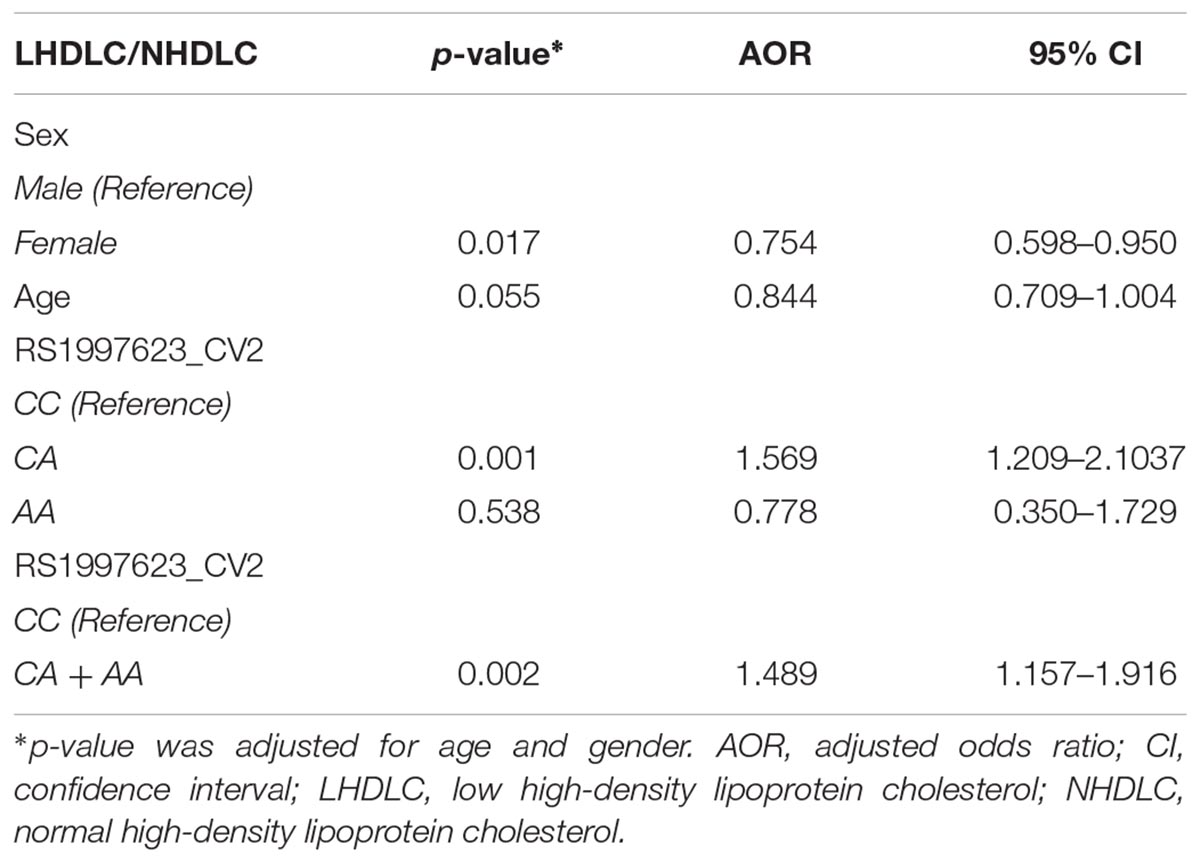
Table 5. Logistic regression analysis of the rs1997623 variant in a group of subjects with low HDLC and normal HDLC.
Distribution of the rs1997623 Variant in the Kuwaiti Population Compared With Other Ethnicities
We further compared the distribution of the rs1997623 variant in Kuwaitis with its distribution in the GnomAD exome population database (see text footnote 3). The comparison of allele frequency (Figure 3) was carried out using a χ2 (2 × 2, 1df). The minor allele frequency of rs1997623 was observed to be 0.15 in the Kuwaiti population. The rs1997623 A-allele was found to be the minor allele in all the tested populations. African population showed the highest frequency of the A-allele (0.29), while the East Asians were reported to have the lowest frequency (0.05). A comparative analysis indicated a highly significant difference in allele frequency distribution between Kuwaitis and Africans (p < 0.0001), Americans (p < 0.0001), Ashkenazi Jews (p = 0.001), East Asians (p < 0.0001), and Finns (p < 0.0001). However, non-Finn Europeans (p = 0.123) and South Asians (p = 0.225) failed to show any significant differences when compared with Kuwaitis. Similarly, the Avon Longitudinal Study of Parents and Children (ALSPAC) (p = 0.862) and TwinsUK cohorts (p = 0.152) revealed no significant differences when compared with the Kuwaiti population.
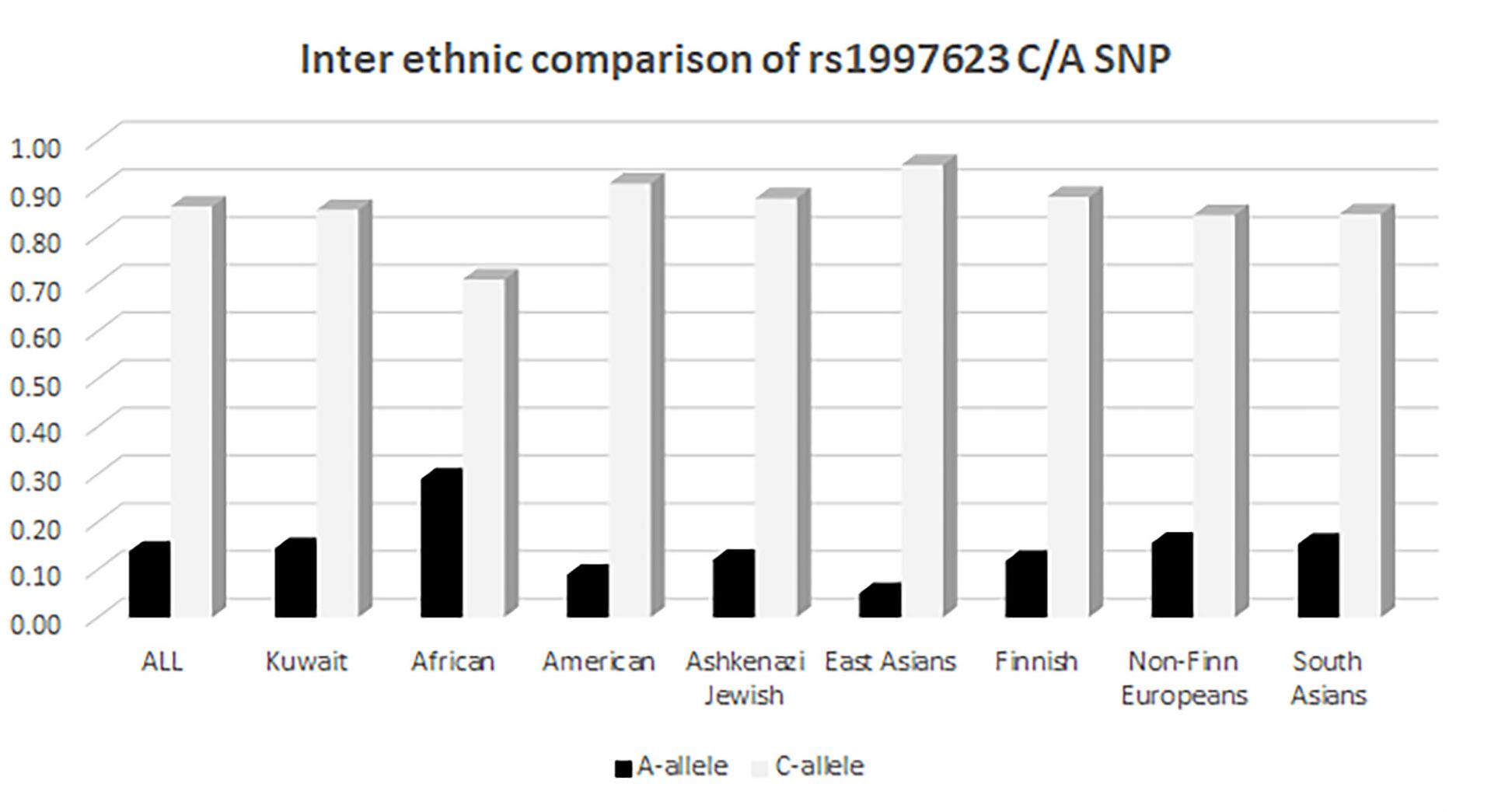
Figure 3. Inter ethnic comparison of rs1997623 C/A SNP in Kuwaitis with its distribution in GnomAD exome population database (http://gnomad.broadinstitute.org).
Discussion
Our exome variant database hinted toward a possible association between the rs1997623 and obesity. However, the data was too limited to reach any meaningful conclusion, which prompted us to undertake a more extensive study based on 1313 children from all Governorates of Kuwait. In this study, saliva samples were used as specimens to analyze the association of the CAV1 rs1997623 SNP with metabolic complications related to obesity.
Variations in the CAV1 gene have been recently associated with metabolic complications including cardiovascular outcomes (Pojoga et al., 2011; Baudrand et al., 2015; Chen et al., 2016). To our knowledge no study has so far characterized the role of CAV1 rs1997623 variant with MetS. In the presented study, we report a novel association of CAV1 rs1997623 variant with the MetS and with low saliva HDLC levels in Kuwaiti children. The heterozygous rs1997623 variant leads to significantly lower levels of HDLC in the study population. HDLC is considered as the “good cholesterol,” that act as a scavenger mediating the transport of low density lipoprotein from the arteries to the liver. Apart from its anti-oxidant, anti-thrombotic and anti-inflammatory effects, HDLC tends to promote pancreatic beta cell function and glucose metabolism (Drew et al., 2009; Fryirs et al., 2010). The lack of association of homozygous mutant AA genotype with decreased odds of MetS could be due to limited frequency of AA genotype in the study population. Further analysis need to be carried out in a relatively large cohort to estimate the effect of mutant genotype. Mitigating our selection, we additionally detected a significant association of CAV1 rs1997623 variant with intermediate metabolic group. This possibly reflects the association of rs1997623 variant with individual predictive markers of MetS, further signifying the need to address the children with fewer metabolic complications.
In contrast to candidate gene studies, none of the GWA studies implicated in the literature have detected the significance of CAV1 variants with respect to metabolic traits. To the best of our knowledge, the most commonly used Illumina Human OmniExpress Bead Chip genome wide array does not include the tested rs1997623 variant. Apparently, a vast majority of GWA studies were also performed in European, African-American and Asian population and relatively little information is available from the State of Kuwait (Hebbar et al., 2017, 2018). The genetic divergence of Kuwaiti population has largely been evidenced by the differences in disease prevalence and risk allele frequencies. The regions socio economic status post-hydrocarbon boom and sedentary lifestyle practices have attributed to the increase in prevalence of metabolic disorders including dyslipidemia (70.3%), obesity (48.2%), hypertension (25.3%), and diabetes (17.9%) (Alarouj et al., 2013). Although differences are small, a comparative analysis of allelic frequency distribution of rs1997623 variant in Kuwaiti population, at this locus, is significantly different from African, American, Finnish, Jewish, and East Asian populations, while being closer to South Asian and non-Finnish European populations.
Not much is known regarding the functional consequences of the rs1997623 SNP. The rs1997623 varint is part of the exon 1 transcript (NM_001172895.1) that undergoes nonsense-mediated decay. We are currently performing functional studies on this variant in our laboratory, specifically addressing whether the variant alters the expression of caveolin in vitro. Several animal and cell model studies have elucidated the role of CAV1 in lipid metabolism. CAV1 knockout mice were reported to have hyper-triglyceridaemia with adipocyte abnormalities (Razani et al., 2002). Parallel to this, significantly decreased expression of the CAV1 gene positively correlates with reduced lipogenic gene expression in the visceral adipose tissue of obese subjects (Fernandez-Real et al., 2010). The role of CAV1 in HDLC metabolism was further evidenced by increased levels of plasma HDLC in mouse liver overexpressing CAV1 (Frank et al., 2001). Given the fact that the rs1997623 variant is predicted to be associated with transcript-dependent promoter loss, we hypothesize that the magnitude of effect caused by the rs1997623 SNP would largely rely on transcript-dependent expression of CAV1. In addition to the allelic variations of the rs1997623 SNP, the variable ratio of the various CAV1 isoforms may also be important in understanding its regulatory role in lipid metabolism.
Conclusion
We investigated the genetic associations of the rs1997623 variant for its potential association with metabolic traits in a large cohort of Kuwaiti children. Our study revealed, for the first time, an association of the rs1997623 variant with MetS in Kuwaiti children with high prevalence of obesity. Heterozygosity of the rs1997623 variant was associated with lower levels of HDLC in this study group. This study calls for further in-depth population-based studies to unravel the genetics of obesity and related metabolic complications in the Kuwaiti/Arab populations.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
RN and FA-M designed the study. FA-M, RN, and JG directed the work. OA, JG, SD, and M-LH directed study participant recruitment, sample processing, and data collection. FA-M, RN, HaA, and MB shortlisted the gene/variant. RN, MM, GA, and NA performed the experiment. FA-M, RN, and PS carried out statistical analysis and interpretation. EA-O, LD, HeA, M-LH, MT, HoA, and FA-R carried out clinical data analysis and interpretation. RN and FA-M wrote the manuscript. EA-O, JT, LD, JG, AA, FA-R, SS, MQ, MA-F, JA, M-LH, PS, MB, HaA, and OA reviewed and edited the manuscript. FA-M, EA-O, and JT critically revised and approved the manuscript.
Funding
This study was supported by the Dasman Diabetes Institute Research Administration grant RA-2011-005A. FA-M was supported by Kuwait Foundation for the Advancement of Sciences (KFAS) grant number P116-13MG-02.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We greatly appreciate the efforts of all the members of the National Dasman Diabetes Biobank for processing the samples and Dr. Diana Marouco for language editing and proofreading.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2018.00689/full#supplementary-material
Footnotes
- ^ http://genepop.curtin.edu.au
- ^ https://www.graphpad.com/scientific-software/prism
- ^ http://gnomad.broadinstitute.org
References
Alarouj, M., Bennakhi, A., Alnesef, Y., Sharifi, M., and Elkum, N. (2013). Diabetes and associated cardiovascular risk factors in the State of Kuwait: the first national survey. Int. J. Clin. Pract. 67, 89–96. doi: 10.1111/ijcp.12064
Baudrand, R., Goodarzi, M. O., Vaidya, A., Underwood, P. C., Williams, J. S., Jeunemaitre, X., et al. (2015). A prevalent caveolin-1 gene variant is associated with the metabolic syndrome in Caucasians and Hispanics. Metabolism 64, 1674–1681. doi: 10.1016/j.metabol.2015.09.005
Bitar, M. S., Abdel-Halim, S. M., and Al-Mulla, F. (2013). Caveolin-1/PTRF upregulation constitutes a mechanism for mediating p53-induced cellular senescence: implications for evidence-based therapy of delayed wound healing in diabetes. Am. J. Physiol. Endocrinol. Metab. 305, E951–E963. doi: 10.1152/ajpendo.00189.2013
Chang, W. S., Tsai, C. W., Wang, S. M., Wang, S. W., Wu, H. C., Ji, H. X., et al. (2014). Association of caveolin-1 genotypes with renal cell carcinoma risk in Taiwan. Chin. J. Physiol. 57, 220–226. doi: 10.4077/cjp.2014.bac213
Chen, S., Wang, X., Wang, J., Zhao, Y., Wang, D., Tan, C., et al. (2016). Genomic variant in CAV1 increases susceptibility to coronary artery disease and myocardial infarction. Atherosclerosis 246, 148–156. doi: 10.1016/j.atherosclerosis.2016.01.008
Drew, B. G., Duffy, S. J., Formosa, M. F., Natoli, A. K., Henstridge, D. C., Penfold, S. A., et al. (2009). High-density lipoprotein modulates glucose metabolism in patients with type 2 diabetes mellitus. Circulation 119, 2103–2111. doi: 10.1161/circulationaha.108.843219
Elkum, N., Al-Arouj, M., Sharifi, M., Shaltout, A., and Bennakhi, A. (2016). Prevalence of childhood obesity in the state of Kuwait. Pediatr. Obes. 11, e30–e34. doi: 10.1111/ijpo.12090
Fernandez-Real, J. M., Catalan, V., Moreno-Navarrete, J. M., Gomez-Ambrosi, J., Ortega, F. J., Rodriguez-Hermosa, J. I., et al. (2010). Study of caveolin-1 gene expression in whole adipose tissue and its subfractions and during differentiation of human adipocytes. Nutr. Metab. 7:20. doi: 10.1186/1743-7075-7-20
Ford, E. S., Giles, W. H., and Dietz, W. H. (2002). Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 287, 356–359.
Frank, P. G., Marcel, Y. L., Connelly, M. A., Lublin, D. M., Franklin, V., Williams, D. L., et al. (2002). Stabilization of caveolin-1 by cellular cholesterol and scavenger receptor class B type I. Biochemistry 41, 11931–11940.
Frank, P. G., Pedraza, A., Cohen, D. E., and Lisanti, M. P. (2001). Adenovirus-mediated expression of caveolin-1 in mouse liver increases plasma high-density lipoprotein levels. Biochemistry 40, 10892–10900.
Fryirs, M. A., Barter, P. J., Appavoo, M., Tuch, B. E., Tabet, F., Heather, A. K., et al. (2010). Effects of high-density lipoproteins on pancreatic beta-cell insulin secretion. Arterioscler. Thromb. Vasc. Biol. 30, 1642–1648. doi: 10.1161/atvbaha.110.207373
Gonzalez-Munoz, E., Lopez-Iglesias, C., Calvo, M., Palacin, M., Zorzano, A., and Camps, M. (2009). Caveolin-1 loss of function accelerates glucose transporter 4 and insulin receptor degradation in 3T3-L1 adipocytes. Endocrinology 150, 3493–3502. doi: 10.1210/en.2008-1520
Goodson, J. M., Hartman, M. L., Shi, P., Hasturk, H., Yaskell, T., Vargas, J., et al. (2017). The salivary microbiome is altered in the presence of a high salivary glucose concentration. PLoS One 12:e0170437. doi: 10.1371/journal.pone.0170437
Goodson, J. M., Kantarci, A., Hartman, M. L., Denis, G. V., Stephens, D., Hasturk, H., et al. (2014). Metabolic disease risk in children by salivary biomarker analysis. PLoS One 9:e98799. doi: 10.1371/journal.pone.0098799
Goodson, J. M., Tavares, M., Wang, X., Niederman, R., Cugini, M., Hasturk, H., et al. (2013). Obesity and dental decay: inference on the role of dietary sugar. PLoS One 8:e74461. doi: 10.1371/journal.pone.0074461
Hartman, M. L., Goodson, J. M., Barake, R., Alsmadi, O., Al-Mutawa, S., Ariga, J., et al. (2015). Salivary glucose concentration exhibits threshold kinetics in normal-weight, overweight, and obese children. Diabetes Metab. Syndr. Obes. 8, 9–15. doi: 10.2147/dmso.s72744
Hartman, M. L., Goodson, J. M., Shi, P., Vargas, J., Yaskell, T., Stephens, D., et al. (2016). Unhealthy phenotype as indicated by salivary biomarkers: glucose, insulin, VEGF-A, and IL-12p70 in obese kuwaiti adolescents. J. Obes. 2016:6860240. doi: 10.1155/2016/6860240
Hebbar, P., Alkayal, F., Nizam, R., Melhem, M., Elkum, N., John, S. E., et al. (2017). The TCN2 variant of rs9606756 [Ile23Val] acts as risk loci for obesity-related traits and mediates by interacting with Apo-A1. Obesity 25, 1098–1108. doi: 10.1002/oby.21826
Hebbar, P., Nizam, R., Melhem, M., Alkayal, F., Elkum, N., John, S. E., et al. (2018). Genome-wide association study identifies novel recessive genetic variants for high TGs in an Arab population. J. Lipid Res. 59, 1951–1966. doi: 10.1194/jlr.P080218
Langmann, T., Klucken, J., Reil, M., Liebisch, G., Luciani, M. F., Chimini, G., et al. (1999). Molecular cloning of the human ATP-binding cassette transporter 1 (hABC1): evidence for sterol-dependent regulation in macrophages. Biochem. Biophys. Res. Commun. 257, 29–33. doi: 10.1006/bbrc.1999.0406
Liu, L. C., Su, C. H., Wang, H. C., Tsai, C. W., Chang, W. S., Ho, C. Y., et al. (2011). Significant association of caveolin-1 (CAV1) genotypes with breast cancer in Taiwan. Anticancer Res. 31, 3511–3515.
Mascarenhas, P., Fatela, B., and Barahona, I. (2014). Effect of diabetes mellitus type 2 on salivary glucose–a systematic review and meta-analysis of observational studies. PLoS One 9:e101706. doi: 10.1371/journal.pone.0101706
Matveev, S., van der Westhuyzen, D. R., and Smart, E. J. (1999). Co-expression of scavenger receptor-BI and caveolin-1 is associated with enhanced selective cholesteryl ester uptake in THP-1 macrophages. J. Lipid Res. 40, 1647–1654.
MOH (2015). Ministry of Health. https://www.moh.gov.kw/en/Ministry-Statistics
Nixon, S. J., Carter, A., Wegner, J., Ferguson, C., Floetenmeyer, M., Riches, J., et al. (2007). Caveolin-1 is required for lateral line neuromast and notochord development. J. Cell Sci. 120(Pt 13), 2151–2161. doi: 10.1242/jcs.003830
Park, D. S., Woodman, S. E., Schubert, W., Cohen, A. W., Frank, P. G., Chandra, M., et al. (2002). Caveolin-1/3 double-knockout mice are viable, but lack both muscle and non-muscle caveolae, and develop a severe cardiomyopathic phenotype. Am. J. Pathol. 160, 2207–2217. doi: 10.1016/s0002-9440(10)61168-6
Pojoga, L. H., Underwood, P. C., Goodarzi, M. O., Williams, J. S., Adler, G. K., Jeunemaitre, X., et al. (2011). Variants of the caveolin-1 gene: a translational investigation linking insulin resistance and hypertension. J. Clin. Endocrinol. Metab. 96, E1288–E1292. doi: 10.1210/jc.2010-2738
Razani, B., Combs, T. P., Wang, X. B., Frank, P. G., Park, D. S., Russell, R. G., et al. (2002). Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J. Biol. Chem. 277, 8635–8647. doi: 10.1074/jbc.M110970200
Reaven, G. M. (1988). Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 37, 1595–1607.
Reaven, G. M. (1997). Banting lecture 1988. Role of insulin resistance in human disease. 1988. Nutrition 13, 65; discussion 64–66.
Schwencke, C., Braun-Dullaeus, R. C., Wunderlich, C., and Strasser, R. H. (2006). Caveolae and caveolin in transmembrane signaling: implications for human disease. Cardiovasc. Res. 70, 42–49. doi: 10.1016/j.cardiores.2005.11.029
Shi, P., Goodson, J. M., Hartman, M. L., Hasturk, H., Yaskell, T., Vargas, J., et al. (2015). Continuous metabolic syndrome scores for children using salivary biomarkers. PLoS One 10:e0138979. doi: 10.1371/journal.pone.0138979
Singh, S., Ramesh, V., Oza, N., Balamurali, P. D., Prashad, K. V., and Balakrishnan, P. (2014). Evaluation of serum and salivary lipid profile: a correlative study. J. Oral Maxillofac. Pathol. 18, 4–8. doi: 10.4103/0973-029x.131881
Tsou, Y. A., Tsai, C. W., Tsai, M. H., Chang, W. S., Li, F. J., Liu, Y. F., et al. (2011). Association of caveolin-1 genotypes with nasopharyngeal carcinoma susceptibility in Taiwan. Anticancer Res. 31, 3629–3632.
Wang, L., Connelly, M. A., Ostermeyer, A. G., Chen, H. H., Williams, D. L., and Brown, D. A. (2003). Caveolin-1 does not affect SR-BI-mediated cholesterol efflux or selective uptake of cholesteryl ester in two cell lines. J. Lipid Res. 44, 807–815. doi: 10.1194/jlr.M200449-JLR200
Willer, C. J., Schmidt, E. M., Sengupta, S., Peloso, G. M., Gustafsson, S., Kanoni, S., et al. (2013). Discovery and refinement of loci associated with lipid levels. Nat. Genet. 45, 1274–1283. doi: 10.1038/ng.2797
Keywords: CAV1, HDLC, metabolic syndrome, Kuwaiti children, obesity
Citation: Nizam R, Al-Ozairi E, Goodson JM, Melhem M, Davidsson L, Alkhandari H, Al Madhoun A, Shamsah S, Qaddoumi M, Alghanim G, Alhasawi N, Abu-Farha M, Abubaker J, Shi P, Hartman M-L, Tavares M, Bitar M, Ali H, Arefanian H, Devarajan S, Al-Refaei F, Alsmadi O, Tuomilehto J and Al-Mulla F (2018) Caveolin-1 Variant Is Associated With the Metabolic Syndrome in Kuwaiti Children. Front. Genet. 9:689. doi: 10.3389/fgene.2018.00689
Received: 29 May 2018; Accepted: 11 December 2018;
Published: 21 December 2018.
Edited by:
Daniel Shriner, National Human Genome Research Institute (NHGRI), United StatesReviewed by:
Fasil Tekola Ayele, National Institutes of Health (NIH), United StatesCheryl L. Thompson, Case Western Reserve University, United States
Copyright © 2018 Nizam, Al-Ozairi, Goodson, Melhem, Davidsson, Alkhandari, Al Madhoun, Shamsah, Qaddoumi, Alghanim, Alhasawi, Abu-Farha, Abubaker, Shi, Hartman, Tavares, Bitar, Ali, Arefanian, Devarajan, Al-Refaei, Alsmadi, Tuomilehto and Al-Mulla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rasheeba Nizam, cmFzaGVlYmEuaXFiYWxAZGFzbWFuaW5zdGl0dXRlLm9yZw== Fahd Al-Mulla, ZmFoZC5hbG11bGxhQGRhc21hbmluc3RpdHV0ZS5vcmc=; ZmFoZEBhbC1tdWxsYS5vcmc=
 Rasheeba Nizam
Rasheeba Nizam Ebaa Al-Ozairi2
Ebaa Al-Ozairi2 Jo Max Goodson
Jo Max Goodson Motesam Melhem
Motesam Melhem Lena Davidsson
Lena Davidsson Ashraf Al Madhoun
Ashraf Al Madhoun Sara Shamsah
Sara Shamsah Ghazi Alghanim
Ghazi Alghanim Mohamed Abu-Farha
Mohamed Abu-Farha Jehad Abubaker
Jehad Abubaker Hamad Ali
Hamad Ali Hossein Arefanian
Hossein Arefanian Sriraman Devarajan
Sriraman Devarajan Jaakko Tuomilehto11
Jaakko Tuomilehto11 Fahd Al-Mulla
Fahd Al-Mulla