- 1Department of Statistics, North Carolina State University, Raleigh, NC, United States
- 2Department of Mathematics, North Carolina State University, Raleigh, NC, United States
- 3Department of Medical Research, Taichung Veterans General Hospital, Taichung, Taiwan
- 4Penn Neurodegeneration Genomics Center, Department of Pathology and Laboratory Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States
- 5Institute of Epidemiology and Preventive Medicine, National Taiwan University, Taipei, Taiwan
- 6Department of Statistics, National Cheng-Kung University, Tainan, Taiwan
The explosion of biobank data offers unprecedented opportunities for gene-environment interaction (GxE) studies of complex diseases because of the large sample sizes and the rich collection in genetic and non-genetic information. However, the extremely large sample size also introduces new computational challenges in G×E assessment, especially for set-based G×E variance component (VC) tests, which are a widely used strategy to boost overall G×E signals and to evaluate the joint G×E effect of multiple variants from a biologically meaningful unit (e.g., gene). In this work, we focus on continuous traits and present SEAGLE, a Scalable Exact AlGorithm for Large-scale set-based G×E tests, to permit G×E VC tests for biobank-scale data. SEAGLE employs modern matrix computations to calculate the test statistic and p-value of the GxE VC test in a computationally efficient fashion, without imposing additional assumptions or relying on approximations. SEAGLE can easily accommodate sample sizes in the order of 105, is implementable on standard laptops, and does not require specialized computing equipment. We demonstrate the performance of SEAGLE using extensive simulations. We illustrate its utility by conducting genome-wide gene-based G×E analysis on the Taiwan Biobank data to explore the interaction of gene and physical activity status on body mass index.
1 Introduction
Human complex diseases such as neurodegenerative diseases, psychiatric disorders, metabolic syndromes, and cancers are complex traits for which disease susceptibility, disease development, and treatment response are mediated by intricate genetic and environmental factors. Understanding the genetic etiology of these complex diseases requires collective consideration of potential genetic and environmental contributors. Studies of gene-environment interactions (G×E) enable understanding of the differences that environmental exposures may have on health outcomes in people with varying genotypes (Ottman, 1996; Hunter, 2005; McAllister et al., 2017). Examples include the impact of physical activity and alcohol consumption on the genetic risk for obesity-related traits (Sulc et al., 2020), the impact of air pollution on the genetic risk for cardio-metabolic and respiratory traits (Favé et al., 2018), and other examples reviewed in Ritz et al. (2017).
When assessing G×E effects, set-based tests are popular approaches to detecting interactions between an environmental factor and a set of single nucleotide polymorphism (SNPs) in a gene, sliding window, or functional region (Tzeng et al., 2011; Lin et al., 2013; Zhao et al., 2015; Lin et al., 2016; Su et al., 2017). Compared to single-SNP G×E tests, set-based G×E tests can enhance testing performance by reducing multiple-testing burden and by aggregating G×E signals over multiple SNPs that are of moderate effect sizes or of low frequencies.
Large-scale biobanks collect genetic and health information on hundreds of thousands of individuals. Their large sample sizes and rich data on non-genetic factors offer unprecedented opportunities for in-depth studies on G×E effects. While the explosion of biobank data collections provides great hopes for novel G×E discoveries, it also introduces computational challenges. In particular, many set-based G×E tests can be cast as variance component (VC) tests under a random effects modeling framework (Lin et al., 2013; Su et al., 2017), including kernel machine based tests (Wang et al., 2015a; Lin et al., 2016) and similarity regression based methods (Tzeng et al., 2011; Zhao et al., 2015). Hypothesis testing in this framework relies on computations with phenotypic variance matrices with dimension n × n (with n as the sample size) and may involve estimating nuisance variance components. When n is large, as in the case of biobank data, matrix computations whose operation counts scale with n3 are prohibitive in terms of computation time and storage.
A number of methods attempt to ease this computational burden by bypassing the estimation of nuisance variance components, either through approximation of the variance or kernel matrices (Marceau et al., 2015) or through approximation of the score-like test statistics (Wang et al., 2020). In the first case, approximating the kernel matrices still requires an expensive eigenvalue decomposition upfront, in addition to storage for the explicit formation of the n × n kernel matrices, thus lacking practical scalability. In the latter case, approximating the test statistics requires assumptions that may or may not be valid and are difficult to validate in practice. Our numerical studies in Section 3 show that the Type 1 error rates and power can be sub-optimal when data do not adhere to the required assumptions.
In this work, we focus on continuous traits and introduce a Scalable Exact AlGorithm for Large-scale set-based G×E tests (SEAGLE) for performing G×E VC tests on biobank data. Here “exact” refers to the fact that SEAGLE computes the original VC test statistic without any approximations, rather than the null distribution of the test statistic being asymptotic or exact. Exactness and scalability are achieved through the judicious use of modern matrix computations, allowing us to dispense with approximations and assumptions. Our numerical experiments illustrate that SEAGLE produces Type 1 error rates and power identical to those of the original GxE VC methods (Tzeng et al., 2011), but at a fraction of the speed. Additionally, SEAGLE can easily handle biobank-scale data with as many as n individuals in the order of 105, often at the same speed as state-of-the-art approximate methods (Wang et al., 2020). Compared with the state-of-the-art approximate method (Wang et al., 2020), SEAGLE can produce more accurate Type 1 error rates and power. Another advantage of SEAGLE is its user-friendliness; it can be run on ordinary laptops and does not require specialized or high performance computing equipment or parallelization. In fact, nearly all of our timing comparisons in Section 3 were performed on a 2013 Intel Core i5 laptop with a 2.70 GHz CPU and 16 GB RAM, specs that are standard for modern laptops. Therefore, SEAGLE makes it possible to run exact and scalable G×E VC tests on biobank-scale data with just a modicum of computational resources.
The rest of the paper proceeds as follows. Section 2 describes the standard mixed effects model for G×E effects, testing procedures, computational performance, and SEAGLE algorithm. Section 3 illustrates SEAGLE’s performance through numerical studies. Section 4 concludes with a brief summary of our contributions and avenues for future work.
2 Materials and Methods
We describe the standard mixed effects model for G×E effects and testing procedures (Section 2.1), the computational challenges for biobank-scale data (Section 2.2), the components of the SEAGLE algorithm (Section 2.3), and the SEAGLE algorithm as a whole (Section 2.3.4).
2.1 G×E Variance Component Tests for Continuous Traits
We present the standard mixed effects model for studying G×E effects, the score-like test statistic, and its p-value. Let
Consider the linear mixed effects model (Tzeng et al., 2011; Lin et al., 2013),
Here,
The SNP-set analysis models the G and G×E effects of the L SNPs as random effects rather than fixed effects. This choice avoids power loss for non-small L and numerical difficulties from correlated SNPs that can occur in a fixed effects model. To assess the presence of G×E effects with H0 : c = 0 in Model (1), one can apply a score-like test to the corresponding variance component with H0 : ν = 0.
To simplify the null model of (Eq. 1) in the score-like test, we consolidate and define
In (Eq. 2),
Given the λℓ’s, the p-value of T can be computed with the moment matching method in Liu et al. (2009) or the exact method in Davies (1980).
2.2 Computational Challenges in G×E Variance Component Tests for Biobank-Scale Data
We identify three computational bottlenecks.
1. The test statistic T and the p-value computation depend on
2. The REML EM algorithm (Supplementary Appendix S1) estimates the nuisance variance components τ and σ in V under the null hypothesis. Each iteration requires products with the orthogonal projector
3. Computing the p-values requires two eigenvalue decompositions: 1) an eigenvalue decomposition of V to compute
2.3 Components of the SEAGLE Algorithm for Biobank-Scale GxE Variance Component Test
We present our approach for overcoming the three computational challenges in the previous section: Multiplication with V−1 without explicit formation of the inverse (Section 2.3.1), a scalable REML EM algorithm (Section 2.3.2), and a scalable algorithm for computing the eigenvalues of C (Section 2.3.3). The idea is to replace explicit formation of inverses by low-rank updates and linear system solutions; and to replace n × n eigenvalue decompositions with L × L ones.
2.3.1 Multiplication by V−1 Without Explicit Formation of V−1
The test statistic T and its p-value calculation depend on V−1. We avoid the explicit formation of the inverse by viewing V = τGGT +σIn as the low-rank update of a diagonal matrix, and then applying the Sherman-Morrison-Woodbury formula below to reduce the dimension of the computed inverse from n to L where L ≪ n.
LEMMA 1 [Section 2.1.4 in Golub and Van Loan (2013)]. Let
Applying Lemma 1 to the product of the inverse of
which reduces the dimension of the inverse from n to L. The explicit computation of the inverse of
2.3.2 Scalable Restricted Maximum Likelihood Expectation-Maximization Algorithm
We present the scalable version of the REML EM algorithm in Supplementary Appendix S1 that avoids explicit formation of the orthogonal projector and the inverses.
We assume throughout that
where
In iteration t + 1 of the algorithm, define
Then the updates in Supplementary Eq. S5 and Supplementary Eq. S6 from Supplementary Appendix S1 can be expressed as
The two bottlenecks in the REML EM algorithm are the computation of the non-symmetric “square-root” A in (Eq. 5), and products with
To avoid explicit formation of the full matrix in (Eq. 5), we compute instead the QR decomposition
where
Thus, A = Q2 represents the trailing n − P columns of the orthogonal matrix Q in the QR factorization of
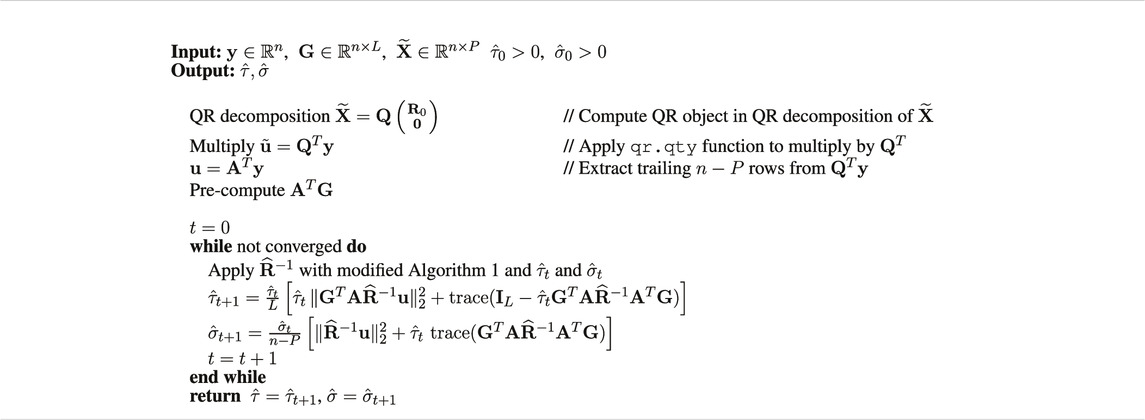
Algorithm 2 shows pseudocode for the REML EM algorithm. Since A = Q2 occurs only as AT in matrix-vector or matrix-matrix multiplications, we do not compute A explicitly. Instead, we compute the full QR decomposition in (Eq. 7) where the “QR object”
Furthermore, we apply
2.3.3 Scalable Algorithm for Computing the Eigenvalues of C
Computation of the p-values requires the eigenvalues of
The explicit formation of P is avoided by computing instead products
2.3.4 The SEAGLE Algorithm
Combining the algorithms from Section 2.3.1, Section 2.3.2, and Section 2.3.3 gives the SEAGLE Algorithm 3 for computing the score-like test statistic T and its p-value. SEAGLE is implemented in the publicly available R package SEAGLE.
Algorithm 3 consists of three parts. Part I computes
3 Results
3.1 Simulation Study
We evaluate the performance of our proposed method SEAGLE using simulation studies from two settings. In the first, we simulate data from a random effects genetic model according to Model (1). This enables us to evaluate SEAGLE’s estimation and testing performance. We include experiments with a smaller n = 5,000 to enable comparisons with existing G×E VC tests as well as larger n values (i.e., 20,000 and 100,000) to demonstrate SEAGLE’s effectiveness on biobank-scale data. In the second, we simulate data from a fixed effects genetic model with larger n = 20,000 and n = 100,000 observations. This enables us to evaluate the testing performance when the data do not follow our modeling assumptions.
In each setting, we study the Type 1 error rate and power. We consider three baseline approaches: i) the original G×E VC test (referred to as OVC) (Tzeng et al., 2011; Wang et al., 2015b), as implemented in the SIMreg R package (https://www4.stat.ncsu.edu/˜jytzeng/software_simreg.php); ii) FastKM (Marceau et al., 2015), as implemented in the FastKM R package; and iii) MAGEE (Wang et al., 2020), as implemented in the MAGEE R package. MAGEE is the state-of-the-art scalable G×E VC test with demonstrated superior performance compared to several set-based GxE methods.
In all simulations, we obtain the genotype design matrix
3.1.1 Random Effects Simulation Study
Given the n × L genotype design matrix G (where L = 100 for n = 5, 20, and 100 k, and L = 400 for n = 20 and 100 k) and the n × P covariate design matrix
We begin by examining the Type 1 error rate for SEAGLE with N = 20,000,000 replicates. Table 1 depicts the SEAGLE Type 1 error rates and shows that SEAGLE provides reasonable control over the Type 1 error rate at varying α-levels with p-values from Davies (1980). Next, under H0: ν = 0 and τ = σ = 1 with N = 1,000 replicates, we compare the testing results of SEAGLE with OVC. Table 2 shows the bias and the mean square error (MSE) of the estimated values for τ and σ obtained from the SEAGLE and OVC REML EM algorithms. Both algorithms produce very small bias and MSE for τ and σ. The left and right panels in Figure 1 depicts scatter plots of the score-like test statistics and p-values, respectively, produced by SEAGLE and OVC. The panels show that SEAGLE and OVC produce identical test statistics and p-values, hence the “exactness” of the SEAGLE algorithm.
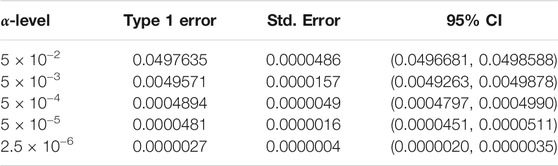
TABLE 1. Type 1 error rate with 95% confidence intervals (CIs) for SEAGLE with Davies p-values over N = 20,000,000 replicates for n = 5,000 observations, L = 100 loci, and variance components τ = σ = 1 and ν = 0.
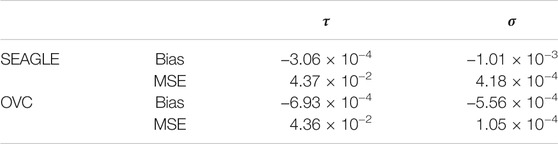
TABLE 2. SEAGLE vs. original G×E VC (OVC) results based on N = 1,000 replicates for n = 5,000 observations and L = 100 loci under H0: no G×E effect (ν = 0). Table shows the bias and mean square error (MSE) of the estimated τ and σ values, compared to the true values τ = σ = 1.
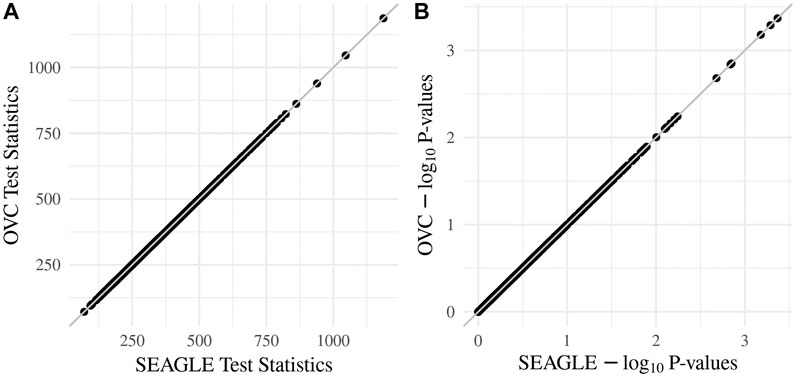
FIGURE 1. SEAGLE vs. original G×E VC (OVC) results based on N = 1, 000 replicates for n = 5,000 observations and L = 100 loci under H0: no G×E effect (ν = 0). The (A,B) show the scatter plots of the testing results computed from SEAGLE vs. those from OVC, depicting the “exact” relationship between SEAGLE and OVC; (A) depicts test statistics T and (B) depicts the p-values.
Since the data are generated from a random effects model under H0: ν = 0, we can compute the “true” score-like test statistic T by evaluating (Eq. 2) at the true τ and σ values, and obtaining the corresponding p-value. We refer to this as the “Truth” and include it as a baseline approach. Supplementary Figure S1 depicts quantile-quantile plots (QQ plots) of the p-values from different methods, each over N = 1,000 replicates. We use the Kolmogorov-Smirnov (KS) test to examine whether or not these observed p-values follow the expected null distribution, i.e., to test for H0: the observed p-values follows Uniform (0,1). With τ = σ = 1, all methods exhibit similar p-value behavior and follow Uniform (0,1) except MAGEE (Supplementary Figure S1A). In fact, the points for SEAGLE (red) and OVC (light blue) overlap. The corresponding p-values of the KS test are 0.3599, 0.4303, 0.4303, 0.3529, and 3.33e-15 for Truth, SEAGLE, OVC, FastKM, and MAGEE, respectively. The deviation of MAGEE’s p-value distribution is likely due to the non-negligible genetic main effects as reported in Wang et al. (2020). To confirm, we repeat the same simulation with τ = 0.01 and σ = 1 and examine the p-values of MAGEE. The results based on N = 1,000 replicates show that the MAGEE p-values behavior similarly to Uniform (0,1) (Supplementary Figure S1B). The p-values of the KS test are 0.7244, 0.4945, and 0.1426 for Truth, SEAGLE and MAGEE, respectively.
In Table 3, we compute the MSE of the p-values obtained from SEAGLE, OVC, FastKM, and MAGEE, compared to the Truth p-values at τ = σ = 1. We observe that MAGEE produces p-values with larger MSE than the other methods. Supplementary Figure S2 shows the corresponding absolute relative error of the p-values for each method, computed by first taking the absolute difference between a method’s p-value and the Truth p-value, then dividing it by the Truth p-value. The boxplots suggest that MAGEE exhibits higher bias and greater variance than SEAGLE, OVC and FastKM at τ = σ = 1.

TABLE 3. Mean squared error (MSE) of the p-values obtained from different G×E VC tests, compared to the “Truth” p-values. Results are obtained with τ = σ = 1 under H0: ν = 0 over N = 1,000 replicates with n = 5,000 observations and L = 100 loci.
Regarding the computational cost, the left panel in Figure 2 shows boxplots of the computation time in seconds required to obtain a single p-value for each of the methods over N = 1,000 replicates with τ = σ = 1 and ν = 0. The right panel shows the same boxplots for just SEAGLE and MAGEE. Results show that at n = 5,000 observations and L = 100 loci, SEAGLE is faster than MAGEE on average. All replicates were computed on a 2013 Intel Core i5 laptop with a 2.70 GHz CPU and 16 GB RAM.
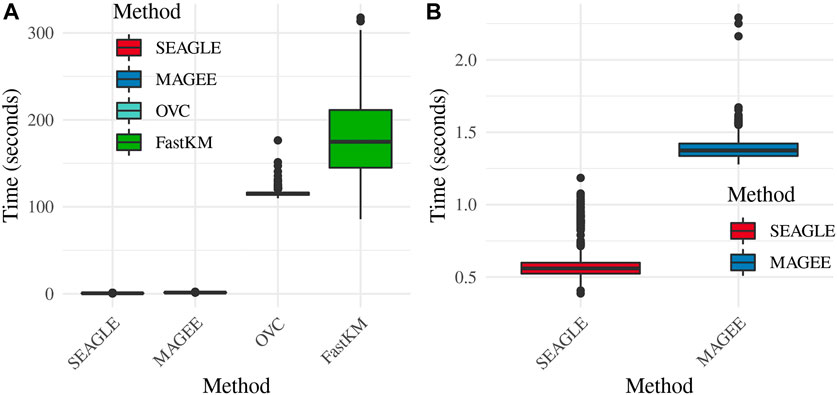
FIGURE 2. (A) Box plots of computation time in seconds to obtain a single p-value over N = 1,000 replicates for n = 5,000 observations and L = 100 loci. (B) Computation time in seconds to obtain a single p-value for SEAGLE and MAGEE.
Figure 3 shows the Type 1 error rate for each method under H0: ν = 0. SEAGLE performs identically to OVC with respect to Type 1 error rate at α = 0.05 while requiring only a fraction of the computation time, as demonstrated in Figure 2. By contrast, MAGEE is nearly as fast as SEAGLE for n = 5,000 and L = 100 but produces much more conservative p-values at τ = σ = 1.
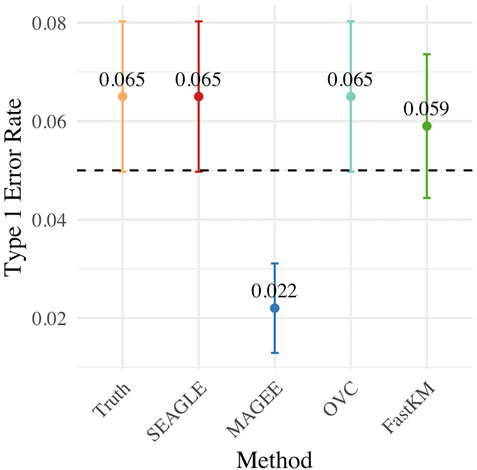
FIGURE 3. Type 1 error at α = 0.05 level for N = 1,000 replicates with n = 5,000 observations and L = 100 loci with τ = σ = 1 and ν = 0.
Figure 4 shows the power for each method under the alternative hypothesis that ν > 0. SEAGLE again performs identically to OVC while requiring a fraction of the computation time. By contrast, MAGEE is nearly as fast as SEAGLE for n = 5,000 and L = 100 but has lower power when τ = σ = 1 and ν = 0.04.
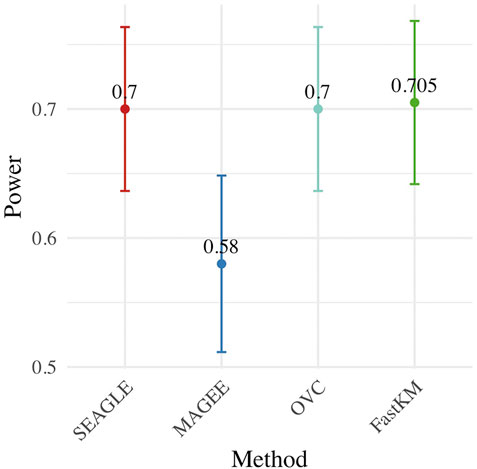
FIGURE 4. Power at α = 0.05 level over N = 200 replicates with n = 5,000 observations, L = 100 loci, and ν = 0.04.
Figures 5–7 show the comparisons of SEAGLE and MAGEE under the large-n simulations (i.e., n = 20,000 and n = 100,000 individuals) with L = 100 and L = 400 loci and by setting τ = σ = 1. Figure 5 shows the Type 1 error rate for Truth, SEAGLE, and MAGEE under H0 : ν = 0. SEAGLE performs almost identically to Truth and OVC, and provides adequate coverage at the α = 0.05 level. Meanwhile, MAGEE produces conservative p-values. Figure 6 depicts boxplots of the computation time in seconds required to obtain a single p-value for SEAGLE and MAGEE under H0 : ν = 0. SEAGLE is faster than MAGEE at both n = 20,000 and n = 100,000 when L = 100 and requires comparable computation time to MAGEE when L = 400. These timing results were obtained on a single core of an Intel Xeon Gold 6226 R (2.90 GHz) machine with 8 GB of RAM. Figure 7 shows the power for SEAGLE and MAGEE under HA: ν > 0 (i.e., ν = 0.007 for n = 20,000 and ν = 0.002 for n = 100,000). SEAGLE exhibits greater power than MAGEE in all four scenarios considered.
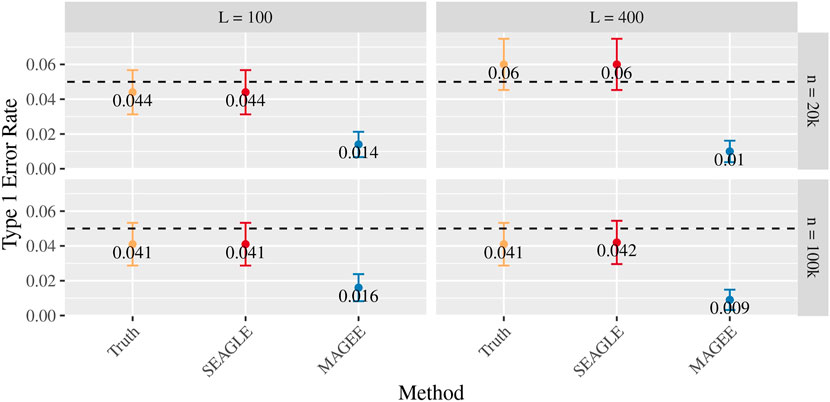
FIGURE 5. Type 1 error at α = 0.05 for random effects simulations with N = 1,000 replicates for n = 20,000 and n = 100,000 observations, L = 100 and L = 400 loci, and τ = σ = 1 and ν = 0.
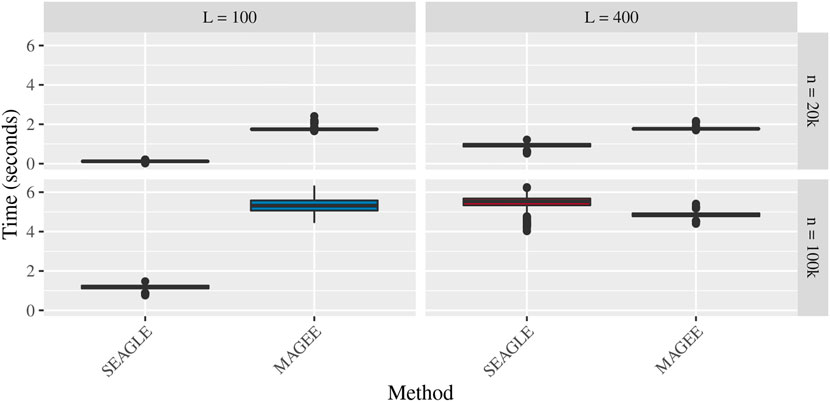
FIGURE 6. Time in seconds for random effects simulations with N = 1,000 replicates for n = 20,000 and n = 100,000 observations, L = 100 and L = 400 loci, and τ = σ = 1 and ν = 0. Replicates computed on a single core of an Intel Xeon Gold 6226R (2.90 GHz) machine with 8 GB of RAM.
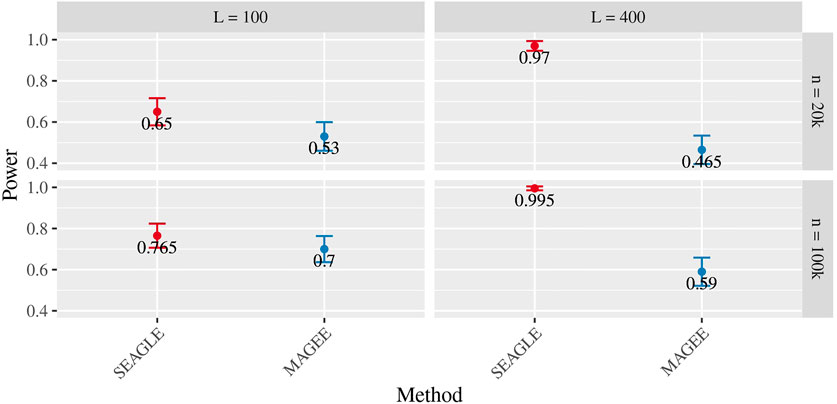
FIGURE 7. Power at α = 0.05 level over N = 200 replicates with n = 20,000 (ν = 0.007) and n = 100,000 (ν = 0.002) observations, L = 100 and L = 400 loci, and τ = σ = 1.
3.1.2 Fixed Effects Simulation Study
To study the performance of our proposed method when the data may not adhere to our model assumptions, we follow previous work (Marceau et al., 2015; Wang et al., 2020) and simulate data according to the fixed effects model with a given
where
We first evaluate the Type 1 error of SEAGLE by simulating N = 1,000 replicates with L = 100 for both n = 20,000 and 100,000, and setting γGE = 0 for all loci while letting the first ℓ = 40 loci to have non-zero γG. For both sample sizes, the SNR based on Var(GγG)/Var(e) of Eq. 8 is 0.015, 0.061 and 0.138 for γG = 0.5, γG = 1 and γG = 1.5, respectively. Figure 8 depicts the Type 1 error rate at α = 0.05 over varying values for γG. While the Type 1 error rate for SEAGLE remains relatively unaffected by different γG values, MAGEE produces more conservative p-values as γG increases. This is consistent with the MAGEE assumption requiring a small G main effect (Wang et al., 2020). Supplementary Figure S3 shows the corresponding quantile-quantile plots for the p-values obtained from SEAGLE and MAGEE.
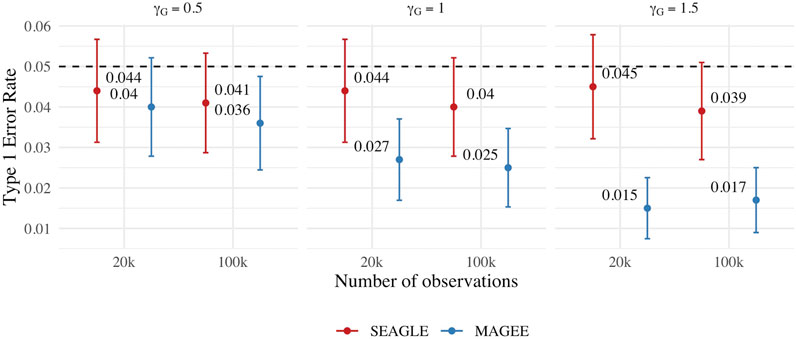
FIGURE 8. Type 1 error at α = 0.05 for fixed effects simulations with N = 1,000 replicates with n = 20,000 and n = 100,000 observations, and L = 100 loci with γGE = 0 and varying values of γG.
Figure 9 depicts boxplots of the computation time in seconds required to obtain a single p-value over the N = 1,000 replicates for n = 20,000 and n = 100,000, and L = 100, over varying values for γG. All replicates were computed on a 2013 Intel Core i5 laptop with a 2.70 GHz CPU and 16 GB RAM. For n = 20,000, SEAGLE is faster than MAGEE at larger values of γG even though MAGEE computes an approximation to the test statistic T and bypasses the traditional REML EM algorithm. At smaller values of γG, however, SEAGLE requires a few seconds more than MAGEE. This is because smaller γG values result in smaller τ, and the REML EM algorithm converges slowly for τ values close to 0. Supplementary Figure S4 illustrates this empirically for n = 20,000 with the estimated values of τ produced by the REML EM algorithm at different γG values. These trends persist for n = 100,000 observations.
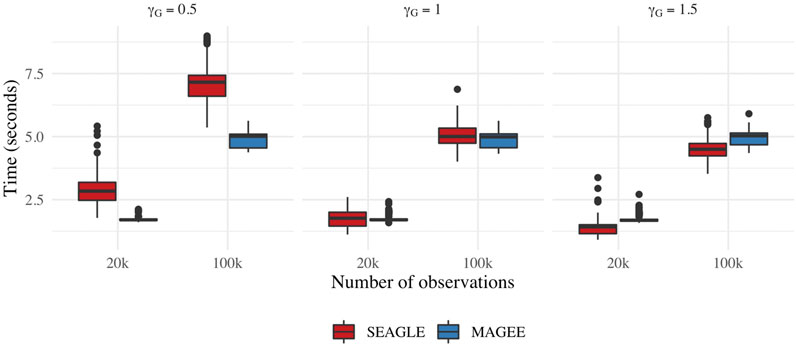
FIGURE 9. Computation time in seconds for fixed effects simulations with N = 1,000 replicates with n = 20,000 observations and L = 100 loci.
For power evaluation, we simulate N = 200 replicates with L = 100 and 400, and let the first ℓ causal loci to have non-zero γG and γGE. For L = 100 loci, we set γGE for the first ℓ = 40 causal loci to be 0.1 or 0.15. For L = 400 loci, we set γGE for the first ℓ = 120 causal loci to be 0.075 or 0.1. These γGE values are determined so that the power for n = 20,000 at α = 0.05 is not close to 1. When L = 100, the SNR for the G×E effect based on Var(diag(E)GγGE)/Var(e) of (Eq. 8) is 0.0006 and 0.0015 for γGE = 0.1 and 0.15, respectively. When L = 400, the SNR for the GxE effect is 0.0014 and 0.0024 for γGE = 0.075 and 0.1, respectively. The values of γG for the ℓ causal loci are set to be 0.5, 1.0, and 1.5 as before. For L = 100, the SNR for the G main effect is 0.015, 0.061 and 0.138 for γG = 0.5, γG = 1 and γG = 1.5, respectively. For L = 400, the SNR for the G main effect is 0.050, 0.201 and 0.453 for γG = 0.5, γG = 1 and γG = 1.5, respectively.
Figure 10 shows the power for L = 100 loci. At n = 20,000, SEAGLE exhibits better power than MAGEE at all combinations of γG and γGE. Moreover, the difference in power increases for larger values of γG since MAGEE relies on the assumption that the G main effect size is small. At n = 100,000 and the same values of γGE, we report the power at α = 0.001 instead of 0.05 because the power at α = 0.05 is near 1 for both methods. We see that both methods produce similar results although SEAGLE still outperforms MAGEE at slightly smaller values of γGE. Figure 11 shows the power for L = 400 loci. Similar patterns of relative power performance are observed as in the case of L = 100, except that the power difference between SEAGLE and MAGEE is more pronounced in L = 400.
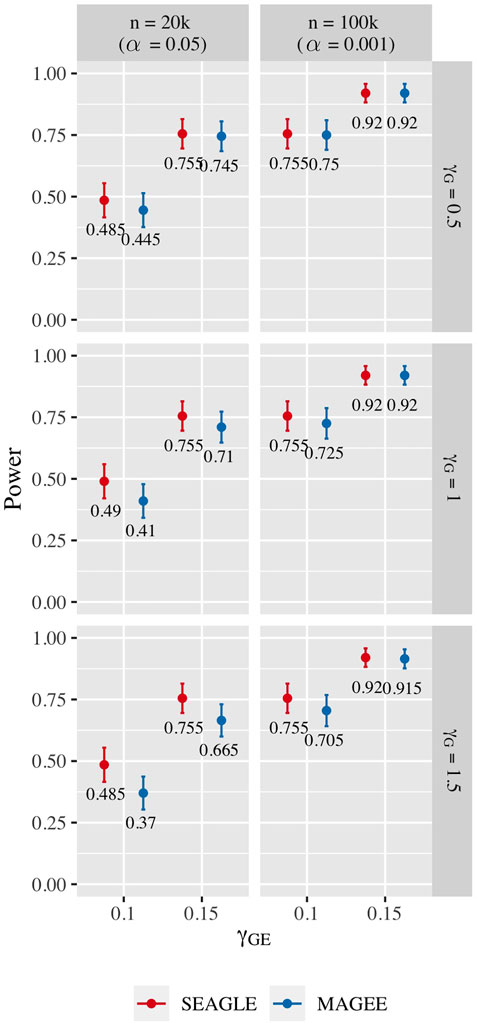
FIGURE 10. Power at α = 0.05 and α = 0.001 for p-values from fixed genetic effects model over N = 200 replicates with n = 20,000 and n = 100,000 observations, respectively, and L = 100 loci.
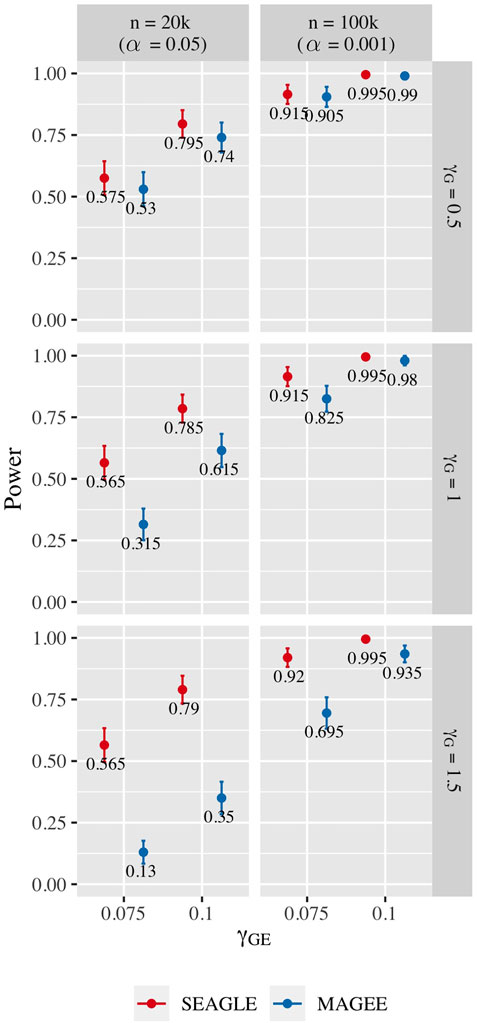
FIGURE 11. Power at α = 0.05 and α = 0.001 for p-values from fixed genetic effects model over N = 200 replicates with n = 20,000 and n = 100,000 observations, respectively, and L = 400 loci.
3.2 Application to the Taiwan Biobank Data
To illustrate the scalability of the G×E VC test using SEAGLE, we apply SEAGLE and MAGEE to the Taiwan Biobank (TWB) data. TWB is a nationwide biobank project initiated in 2012 and has recruited more than 15,995 individuals. Peripheral blood specimens were extracted and genotyped using the Affymetrix Genomewide Axiom TWB array, which was designed specifically for a Taiwanese population. We conduct the gene-based G×E analysis and evaluate the interaction between gene and physical activity (PA) status on body mass index (BMI), adjusting for age, sex and the top 10 principal components for population substructure. The PA status is a binary indicator for with/without regular physical activity. Our G×E analyses focuses on a subset of 11,664 unrelated individuals who have non-missing phenotype and covariate information. After PLINK quality control (i.e., removing SNPs with call rates < 0.95 or Hardy-Weinberg Equilibrium p-value < 10−6), there are 589,867 SNPs remaining, which are mapped to genes according to the gene range list “glist-hg19” from the PLINK Resources page at https://www.cog-genomics.org/plink/1.9/resources. There are a total of 13,260 genes containing > 1 SNPs for G×PA interaction analysis.
The median run time of SEAGLE and MAGEE is 2.4 and 1.3 s, respectively, Both SEAGLE and MAGEE do not find any significant G×PA interactions at the genome-wide Bonferroni threshold 0.05/13,260 = 3.77 × 10−6. We hence discuss the results using a less stringent threshold, i.e., 5 × 10−4 and summarize the results in Figure 12 and Supplementary Table S4. SEAGLE and MAGEE identify 8 and 6 G×PA interactions, respectively, among which 5 G×PA results are identified by both methods (Figure 12). The observation that SEAGLE identifies slightly more G×PA effects than MAGEE generally agrees with the simulation findings. We use the GeneCards Human Gene Database (www.genecards.org) (Stelzer et al., 2016) to explore the relevance of the identified genes with BMI or PA (see Supplementary Table S4). Two of the 5 commonly identified genes, i.e., FCN2 and OCM, have non-zero relevance scores (i.e., 0.56 and 0.91, respectively). For the 3 genes identified by SEAGLE only, i.e., ALOX5AP, BCLAF1 and PCDH17, their relevance scores are 6.16, 0.26 and 1.54, respectively. The expression of ALOX5AP has also been found associated with obesity and insulin resistance (Kaaman et al., 2006) as well as exercise-induced stress (Hilberg et al., 2005). On the other hand, TBPL1 (identified by MAGEE only) is not in the GeneCards relevance list with BMI or PA.
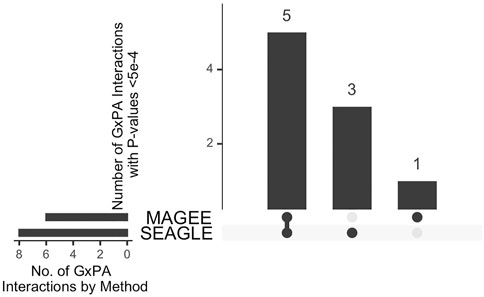
FIGURE 12. Upset plot of significant G×PA interactions identified by SEAGLE and MAGEE in the Taiwan Biobank at the 5 × 10−4 nominal level.
4 Discussion
We introduced SEAGLE, a scalable exact algorithm for performing set-based G×E VC tests on large-scale biobank data. We achieve scalability and accuracy by applying modern numerical analysis techniques, which include avoiding the explicit formation of products and inverses of large matrices. Our numerical experiments illustrate that SEAGLE produces Type 1 error rates and power that are identical to those of the original VC test (Tzeng et al., 2011), while requiring a fraction of the computational cost. Moreover, SEAGLE is well-equipped to handle the very large dimensions required for analysis of large-scale biobank data.
State-of-the-art computational approaches such as MAGEE bypass the traditional time-consuming REML EM algorithm, and instead compute an approximation to the score-like test statistic by assuming that the G main effect size is small. In practice, however, the G main effect size is often unknown. Our numerical experiments illustrate that SEAGLE generally achieves better Type 1 error and power with comparable computation time.
In general, computational time differences between SEAGLE and MAGEE depend primarily on the effect size of the genetic main effect (G effect) and the size of the data, particularly the number of loci L. This is due to the fact that SEAGLE computes the score-like test statistic exactly and therefore requires an EM algorithm to estimate the main effect parameter τ and the noise effect parameter σ. The EM algorithm requires more time to converge when τ is small, particularly when τ is very close to zero. Furthermore, the EM algorithm also requires more computational time for data with larger dimensions, particularly as L grows larger. Despite these differences, the computational time for SEAGLE is at least comparable to that of MAGEE in all the many scenarios considered in this manuscript.
We highlight the fact that most of our timing experiments were performed on a 2013 Intel Core i5 laptop with a 2.70 GHz CPU and 16 GB RAM. Therefore, SEAGLE performs efficient and exact set-based G×E tests on biobank-scale data with n = 20,000 and n = 100,000 observations on ordinary laptops, without any need for high performance computational platforms. This makes SEAGLE broadly accessible to all researchers. Software for SEAGLE is publicly available as the SEAGLE package on GitHub (https://github.com/jocelynchi/SEAGLE), and is also available on the Comprehensive R Archive Network.
Recent studies suggested incorporating functional annotations can further enhance power and accuracy in set-based association analysis [e.g., STAAR (Li et al., 2020) and GAMBIT (Quick et al., 2020)], as a variant’s functional importance could better reflect the causal/null status of the variants than its MAF. The variant-specific annotation weights derived in these methods can also be incorporated into set-based GxE test (and hence SEAGLE). To do so, consider an L × 1 annotation weight vector wj based on annotation class j, j = 1, …, J with J the total number of annotation classes, such as the scores derived based on tissue-specific regulatory annotations in Quick et al. (2020) or the estimated probability of being causal derived from the epigenetic annotation PCs in Li et al. (2020). We can first replace G by diag(wj)G in SEAGLE and obtain the association p-value of the target variant set for annotation class j (denoted by pj), j = 1, …, J. Then we combine these pj’s across annotation classes using the Cauchy Combination Test (Liu and Xie, 2020) or the Harmonic Mean p-value (Wilson, 2019), and obtain the final p-value of the variant set.
We conclude with a discussion of possible avenues for future extensions. First, the current SEAGLE framework only allows for quantitative traits. Extension to binary traits can be based on developing a scalable algorithm for the G×E VC test described in Zhao et al. (2015) using similar numerical analysis techniques, although the extension can be intricate due to the complexity of the EM algorithm for estimating the nuisance VCs involved with binary traits.
Second is the extension from a single environmental factor to a set of factors represented by
Here
Third is the extension from unrelated samples to family samples. With family samples, Model (1) will include an additional nuisance VC associated with kinship matrix to account for the within-family correlation. Similar scalable algorithms as for the multi-E analysis would also be useful for analyzing family samples.
The last is the extension to other types of kernels (Broadaway et al., 2015; Wang et al., 2015b) of the current random effects framework, which can be viewed as a special case of kernel machine regression with linear kernels. As in Lumley et al. (2018) and Wu and Sankararaman (2018), we will explore the potential of randomized numerical linear algebra, by drawing on the authors’ long standing expertise in the development of numerically stable, accurate and efficient randomized matrix algorithms (Eriksson-Bique et al., 2011; Ipsen and Wentworth, 2014; Wentworth and Ipsen, 2014; Holodnak and Ipsen, 2015; Saibaba et al., 2017; Holodnak et al., 2018; Drineas and Ipsen, 2019; Chi and Ipsen, 2021).
Data Availability Statement
The datasets for this article are not publicly available but requests to access the data from the Taiwan Biobank can be emailed to YmlvYmFua0BnYXRlLnNpbmljYS5lZHUudHc=.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethical committee at Taichung Veterans General Hospital (IRB TCVGH No. CE16270B-2). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
JC, II and J-YT conceived the presented ideas and study design. JC implemented the methods and performed the numerical studies under the supervision of II and J-YT. JC, T-HH, C-HL, L-SW, W-PL, T-PL, and J-YT contributed to data processing, analysis and result interpretation of the real data analysis. JC, II and J-YT drafted the manuscript with input from T-HH, C-HL, L-SW, W-PL, and T-PL. All authors helped shape the research, discussed the results and contributed to the final manuscript.
Funding
This work has been partially supported by National Science Foundation Grant DMS-1760374 (to JC and II), National Science Foundation Grant DMS-2103093 (to JC), National Institutes of Health Grants U54 AG052427 (to L-SW and W-PL), U24 AG041689 (L-SW, W-PL and J-YT), and P01 CA142538 (to J-YT), and Taiwan Ministry of Science and Technology Grants MOST 106-2314-B-002-097-MY3 (to T-PL) and MOST 109-2314-B-002-152 (to T-PL).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank the reviewers for their helpful feedback, thank Yueyang Huang for his help with the example data and PLINK1.9 code for the SEAGLE R package tutorials, and thank Caizhi Huang for his help in the simulations for the revision.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2021.710055/full#supplementary-material
References
Broadaway, K. A., Duncan, R., Conneely, K. N., Almli, L. M., Bradley, B., Ressler, K. J., et al. (2015). Kernel Approach for Modeling Interaction Effects in Genetic Association Studies of Complex Quantitative Traits. Genet. Epidemiol. 39, 366–375. doi:10.1002/gepi.21901
Chi, J. T., and Ipsen, I. C. F. (2021). A Projector-Based Approach to Quantifying Total and Excess Uncertainties for Sketched Linear Regression. Inf. Inference, 1–23. doi:10.1093/imaiai/iaab016
Davies, R. B. (1980). Algorithm AS 155: The Distribution of a Linear Combination of χ 2 Random Variables. Appl. Stat. 29, 323–333. doi:10.2307/2346911
Drineas, P., and Ipsen, I. C. F. (2019). Low-Rank Matrix Approximations Do Not Need a Singular Value Gap. SIAM J. Matrix Anal. Appl. 40, 299–319. doi:10.1137/18m1163658
Eriksson-Bique, S., Solbrig, M., Stefanelli, M., Warkentin, S., Abbey, R., and Ipsen, I. C. F. (2011). Importance Sampling for a Monte Carlo Matrix Multiplication Algorithm, with Application to Information Retrieval. SIAM J. Sci. Comput. 33, 1689–1706. doi:10.1137/10080659x
Favé, M. J., Lamaze, F. C., Soave, D., Hodgkinson, A., Gauvin, H., Bruat, V., et al. (2018). Gene-by-environment Interactions in Urban Populations Modulate Risk Phenotypes. Nat. Commun. 9, 1–12. doi:10.1038/s41467-018-03202-2
Golub, G. H., and Van Loan, C. F. (2013). Matrix Computations 4th Edition, Vol. 4. Baltimore, Maryland, United States: The Johns Hopkins University Press.
Higham, N. J. (2002). Accuracy and Stability of Numerical Algorithms. second edn. Philadelphia, PA: Society for Industrial and Applied Mathematics (SIAM).
Hilberg, T., Deigner, H. P., Möller, E., Claus, R. A., Ruryk, A., Gläser, D., et al. (2005). Transcription in Response to Physical Stress-Clues to the Molecular Mechanisms of Exercise‐induced Asthma. FASEB j. 19, 1492–1494. doi:10.1096/fj.04-3063fje
Holodnak, J. T., and Ipsen, I. C. F. (2015). Randomized Approximation of the Gram Matrix: Exact Computation and Probabilistic Bounds. SIAM J. Matrix Anal. Appl. 36, 110–137. doi:10.1137/130940116
Holodnak, J. T., Ipsen, I. C. F., and Smith, R. C. (2018). A Probabilistic Subspace Bound with Application to Active Subspaces. SIAM J. Matrix Anal. Appl. 39, 1208–1220. doi:10.1137/17m1141503
Hunter, D. J. (2005). Gene-environment Interactions in Human Diseases. Nat. Rev. Genet. 6, 287–298. doi:10.1038/nrg1578
Ipsen, I. C. F., and Wentworth, T. (2014). The Effect of Coherence on Sampling from Matrices with Orthonormal Columns, and Preconditioned Least Squares Problems. SIAM J. Matrix Anal. Appl. 35, 1490–1520. doi:10.1137/120870748
Kaaman, M., Rydén, M., Axelsson, T., Nordström, E., Sicard, A., Bouloumié, A., et al. (2006). Alox5ap Expression, but Not Gene Haplotypes, Is Associated with Obesity and Insulin Resistance. Int. J. Obes. 30, 447–452. doi:10.1038/sj.ijo.0803147
Li, X., Li, Z., Zhou, H., Gaynor, S. M., Liu, Y., Chen, H., et al. (2020). Dynamic Incorporation of Multiple In Silico Functional Annotations Empowers Rare Variant Association Analysis of Large Whole-Genome Sequencing Studies at Scale. Nat. Genet. 52, 969–983. doi:10.1038/s41588-020-0676-4
Lin, X., Lee, S., Christiani, D. C., and Lin, X. (2013). Test for Interactions between a Genetic Marker Set and Environment in Generalized Linear Models. Biostatistics 14, 667–681. doi:10.1093/biostatistics/kxt006
Lin, X., Lee, S., Wu, M. C., Wang, C., Chen, H., Li, Z., et al. (2016). Test for Rare Variants by Environment Interactions in Sequencing Association Studies. Biometrics 72, 156–164. doi:10.1111/biom.12368
Liu, H., Tang, Y., and Zhang, H. H. (2009). A New Chi-Square Approximation to the Distribution of Non-negative Definite Quadratic Forms in Non-central normal Variables. Comput. Stat. Data Anal. 53, 853–856. doi:10.1016/j.csda.2008.11.025
Liu, Y., and Xie, J. (2020). Cauchy Combination Test: a Powerful Test with Analytic P-Value Calculation under Arbitrary Dependency Structures. J. Am. Stat. Assoc. 115, 393–402. doi:10.1080/01621459.2018.1554485
Lumley, T., Brody, J., Peloso, G., Morrison, A., and Rice, K. (2018). Fastskat: Sequence Kernel Association Tests for Very Large Sets of Markers. Genet. Epidemiol. 42, 516–527. doi:10.1002/gepi.22136
Marceau, R., Lu, W., Holloway, S., Sale, M. M., Worrall, B. B., Williams, S. R., et al. (2015). A Fast Multiple-Kernel Method with Applications to Detect Gene-Environment Interaction. Genet. Epidemiol. 39, 456–468. doi:10.1002/gepi.21909
McAllister, K., Mechanic, L. E., Amos, C., Aschard, H., Blair, I. A., Chatterjee, N., et al. (2017). Current Challenges and New Opportunities for Gene-Environment Interaction Studies of Complex Diseases. Am. J. Epidemiol. 186, 753–761. doi:10.1093/aje/kwx227
Ottman, R. (1996). Gene-Environment Interaction: Definitions and Study Design. Prev. Med. 25, 764–770. doi:10.1006/pmed.1996.0117
Quick, C., Wen, X., Abecasis, G., Boehnke, M., and Kang, H. M. (2020). Integrating Comprehensive Functional Annotations to Boost Power and Accuracy in Gene-Based Association Analysis. Plos Genet. 16, e1009060. doi:10.1371/journal.pgen.1009060
Ritz, B. R., Chatterjee, N., Garcia-Closas, M., Gauderman, W. J., Pierce, B. L., Kraft, P., et al. (2017). Lessons Learned from Past Gene-Environment Interaction Successes. Am. J. Epidemiol. 186, 778–786. doi:10.1093/aje/kwx230
Saibaba, A. K., Alexanderian, A., and Ipsen, I. C. F. (2017). Randomized Matrix-free Trace and Log-Determinant Estimators. Numer. Math. 137, 353–395. doi:10.1007/s00211-017-0880-z
Schaffner, S. F., Foo, C., Gabriel, S., Reich, D., Daly, M. J., and Altshuler, D. (2005). Calibrating a Coalescent Simulation of Human Genome Sequence Variation. Genome Res. 15, 1576–1583. doi:10.1101/gr.3709305
Stelzer, G., Rosen, N., Plaschkes, I., Zimmerman, S., Twik, M., Fishilevich, S., et al. (2016). The Genecards Suite: from Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinformatics 54, 1–33. doi:10.1002/cpbi.5
Su, Y.-R., Di, C.-Z., and Hsu, L. (2017). A Unified Powerful Set-Based Test for Sequencing Data Analysis of Gxe Interactions. Biostat 18, 119–131. doi:10.1093/biostatistics/kxw034
Sulc, J., Winkler, T. W., Heid, I. M., Kutalik, Z., Wood, A. R., Frayling, T. M., et al. (2020). Heterogeneity in Obesity: Genetic Basis and Metabolic Consequences. Curr. Diab Rep. 20, 1–13. doi:10.1007/s11892-020-1285-4
Tzeng, J.-Y., Zhang, D., Pongpanich, M., Smith, C., McCarthy, M. I., Sale, M. M., et al. (2011). Studying Gene and Gene-Environment Effects of Uncommon and Common Variants on Continuous Traits: a Marker-Set Approach Using Gene-Trait Similarity Regression. Am. J. Hum. Genet. 89, 277–288. doi:10.1016/j.ajhg.2011.07.007
Wang, X., Lim, E., Liu, C. T., Sung, Y. J., Rao, D. C., Morrison, A. C., et al. (2020). Efficient Gene-Environment Interaction Tests for Large Biobank‐scale Sequencing Studies. Genet. Epidemiol. 44, 908–923. doi:10.1002/gepi.22351
Wang, Z., Maity, A., Luo, Y., Neely, M. L., and Tzeng, J.-Y. (2015a). Complete Effect-Profile Assessment in Association Studies with Multiple Genetic and Multiple Environmental Factors. Genet. Epidemiol. 39, 122–133. doi:10.1002/gepi.21877
Wang, Z., Maity, A., Luo, Y., Neely, M. L., and Tzeng, J.-Y. (2015b). Complete Effect-Profile Assessment in Association Studies with Multiple Genetic and Multiple Environmental Factors. Genet. Epidemiol. 39, 122–133. doi:10.1002/gepi.21877
Wentworth, T., and Ipsen, I. C. F. (2014). Kappa_SQ: A Matlab Package for Randomized Sampling of Matrices with Orthonormal Columns. arXiv.
Wilson, D. J. (2019). The Harmonic Mean P-Value for Combining Dependent Tests. Proc. Natl. Acad. Sci. USA 116, 1195–1200. doi:10.1073/pnas.1814092116
Wu, Y., and Sankararaman, S. (2018). A Scalable Estimator of Snp Heritability for Biobank-Scale Data. Bioinformatics 34, i187–i194. doi:10.1093/bioinformatics/bty253
Keywords: gene-based GxE test for biobank data, GxE collapsing test for biobank data, GxE test for large-scale sequencing data, scalable GEI test, gene-environment variance component test, gene-environment kernel test, regional-based gene-environment test
Citation: Chi JT, Ipsen ICF, Hsiao T-H, Lin C-H, Wang L-S, Lee W-P, Lu T-P and Tzeng J-Y (2021) SEAGLE: A Scalable Exact Algorithm for Large-Scale Set-Based Gene-Environment Interaction Tests in Biobank Data. Front. Genet. 12:710055. doi: 10.3389/fgene.2021.710055
Received: 15 May 2021; Accepted: 13 September 2021;
Published: 02 November 2021.
Edited by:
Ching-Ti Liu, Boston University, United StatesCopyright © 2021 Chi, Ipsen, Hsiao, Lin, Wang, Lee, Lu and Tzeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jung-Ying Tzeng, anl0emVuZ0BuY3N1LmVkdQ==
 Jocelyn T. Chi
Jocelyn T. Chi Ilse C. F. Ipsen
Ilse C. F. Ipsen Tzu-Hung Hsiao
Tzu-Hung Hsiao Ching-Heng Lin
Ching-Heng Lin Li-San Wang4
Li-San Wang4 Wan-Ping Lee
Wan-Ping Lee Tzu-Pin Lu
Tzu-Pin Lu Jung-Ying Tzeng
Jung-Ying Tzeng