- 1Department of Environmental Engineering and Water Technology, UNESCO-IHE Institute for Water Education, Delft, Netherlands
- 2Department of Biotechnology, Delft University of Technology, Delft, Netherlands
- 3Winogradsky Institute of Microbiology, Research Center of Biotechnology, Russian Academy of Sciences (RAS), Moscow, Russia
The objective of this study was to investigate the ability of a culture highly enriched with the polyphosphate-accumulating organism, “Candidatus Accumulibacter phosphatis” clade IIC, to adjust their metabolism to different phosphate availabilities. For this purpose the biomass was cultivated in a sequencing batch reactor with acetate and exposed to different phosphate/carbon influent ratios during six experimental phases. Activity tests were conducted to determine the anaerobic kinetic and stoichiometric parameters as well as the composition of the microbial community. Increasing influent phosphate concentrations led to increased poly-phosphate content and decreased glycogen content of the biomass. In response to higher biomass poly-phosphate content, the biomass showed higher specific phosphate release rates. Together with the phosphate release rates, acetate uptake rates also increased up to an optimal poly-phosphate/glycogen ratio of 0.3 P-mol/C-mol. At higher poly-phosphate/glycogen ratios (obtained at influent P/C ratios above 0.051 P-mol/C-mol), the acetate uptake rates started to decrease. The stoichiometry of the anaerobic conversions clearly demonstrated a metabolic shift from a glycogen dominated to a poly-phosphate dominated metabolism as the biomass poly-phosphate content increased. FISH and DGGE analyses confirmed that no significant changes occurred in the microbial community, suggesting that the changes in the biomass activity were due to different metabolic behavior, allowing the organisms to proliferate under conditions with fluctuating phosphate levels.
Introduction
To prevent the receiving waters from eutrophication, the Enhanced Biological Phosphorus Removal (EBPR) process is a cost-effective and environmentally-friendly process for phosphorus removal in wastewater treatment activated sludge systems. The organisms responsible for EBPR are called phosphate-accumulating organisms (PAO). Under anaerobic conditions, PAO are able to take up volatile fatty acids (VFA), such as acetate (HAc) and propionate (HPr), and store them intracellularly as poly-β-hydroxyalkanoates (PHA) (Wentzel et al., 1985; Comeau et al., 1986; Mino et al., 1987). The VFA uptake and storage processes require energy and reducing power. For instance, acetate is taken up by active transport, activated to acetyl-CoA, followed by condensation of two acetyl-CoA molecules into acetoacetyl-CoA and subsequently reduced to poly-β-hydroxybutyrate. The required reducing equivalents (NADH) are obtained from glycolysis (Smolders et al., 1994), while required ATP is obtained partly from the glycolysis supplemented by polyphosphate conversion. Cleavage of intracellular polyphosphate (poly-P), and subsequent release of ortho-phosphate into the liquid phase, is assumed the main pathway for all energy generation. Poly-P is converted by either the combined action of polyphosphate: AMP phosphotransferase [(poly-P)n +AMP ➔ (poly-P)n-1 + ADP] and adenylate kinase [2 ADP ➔ ATP + AMP)] or the action of polyphosphate kinase [(poly-P)n + ADP ➔ (poly-P)n-1 + ATP] (Van Groenestijn et al., 1987; Van Niel et al., 1998; Martín et al., 2006; Wilmes et al., 2008). Release of orthophosphate is considered to potentially generate a proton motive force through a PIT system (Van Veen et al., 1994; Saunders et al., 2007; Burow et al., 2008). Glycolysis of intracellular glycogen is considered the main reducing power source (Mino et al., 1987; Smolders et al., 1994), besides this process generates additional energy. However, several studies have shown that glycolysis in PAO can also function as the main energy generating pathway when the energy production pathway from poly-P is limiting (Brdjanovic et al., 1998; Hesselman et al., 2000; Erdal et al., 2008; Zhou et al., 2008; Acevedo et al., 2012; Welles et al., 2014). Under aerobic conditions, PAO are able to grow and to take up and store ortho-phosphate in excess as intracellular polyphosphate, leading to P-removal from the bulk liquid by wastage of activated sludge (Mino et al., 1998).
The stoichiometry and kinetic rates of EBPR anaerobic conversions (HAc + Poly-P + Gly ➔ PHB/PHV + CO2 + ortho-P) are still controversial. Reported anaerobic P-release/HAc-uptake ratios range from 0.15 up to 0.93 P-mol/C-mol (Wentzel et al., 1987; Smolders et al., 1994; Pereira et al., 1996; Hesselman et al., 2000; Kisoglu et al., 2000) and kinetic rates range from 1 up to 7 [C-mmol HAc/(gVSS.h)] (Smolders et al., 1994; Liu et al., 1997; Sudiana et al., 1999; Filipe et al., 2001; Schuler and Jenkins, 2003b). Such differences have often been explained based on differences on microbial composition and operational conditions. Presence of glycogen-accumulating organisms (GAO) has been suggested as a microbial factor affecting the anaerobic stoichiometry (Mino et al., 1987). GAO compete with PAO for substrate but neither release phosphate anaerobically nor store it under aerobic conditions. Besides GAO, the enrichment of different PAO clades is another factor since PAO I mainly relies on poly-P as energy source for VFA uptake, while PAO II utilizes a mixed PAO-GAO metabolism where glycogen generates a significant part of the energy required for VFA uptake (Welles et al., 2015b). Regarding the operational conditions, pH, nature of the carbon source, sodium and calcium concentration affect the anaerobic stoichiometry (Satoh et al., 1992; Smolders et al., 1994; Oehmen et al., 2005; Barat and van Loosdrecht, 2006; Barat et al., 2008; Welles et al., 2014).
In addition, it has been suggested that the PAO biomass P-content affects the anaerobic stoichiometry. In this regard, at short-term, enriched PAO cultures can shift from a poly-P dependent toward a glycogen dependent metabolism when the biomass P-content decreases (Brdjanovic et al., 1998; Hesselman et al., 2000; Erdal et al., 2008; Zhou et al., 2008; Acevedo et al., 2012). Using highly enriched PAO I and II cultures, Welles et al. (2015b) demonstrated that the poly-P depletion led to a seven and two-fold decrease in the anaerobic kinetic rates of PAO I and II, respectively. Although these studies clearly indicated that the PAO possess metabolic flexibility, the results only represent the short-term response to changes in the biomass P-content. Furthermore, in most of the studies the specific PAO clades were either not reported or the biomass consisted of a mixture of different PAO clades (Brdjanovic et al., 1998; Hesselman et al., 2000; Zhou et al., 2008; Erdal et al., 2008; Acevedo et al., 2012). Finally, not all studies covered a broad range of different biomass P-contents (Welles et al., 2015b). Therefore, it remains unclear how the kinetic rates and stoichiometry of specific PAO clades I and II are affected by a wide range of different biomass P-contents during long-term operation.
In two long-term studies, Liu et al. (1997) and Schuler and Jenkins (2003a) observed a gradual shift from a PAO metabolism to a GAO metabolism when the P-influent concentrations and intracellular poly-P content decreased. In particular, Schuler and Jenkins (2003b) observed higher HAc-uptake rates when the metabolism shifted from a GAO- to a PAO-metabolism. To explain their observations, Liu et al. (1997) suggested that a PAO-GAO competition might have taken place; whereas, Schuler and Jenkins (2003a,b) suggested that the PAO and GAO metabolisms could be either two unique metabolisms in separate groups of organisms or two components of one metabolism in one single group of organisms. In those studies; however, no microbial identification analyses were performed. Kong et al. (2002), in a similar study, observed a shift from Betaproteobacteria (the subdivision to which most “Candidatus Accumulibacter phosphatis” belong) at a high P/C influent ratio to Alphaproteobacteria (the subdivision to which “Defluviicoccus” belong) and Gammaproteobacteria (the subdivisions to which most “Candidatus Competibacter phosphatis” belong) at a low P/C influent ratio. Recent studies have demonstrated that PAO I and II have very different characteristics in terms of morphology, stoichiometry and kinetic rates, the ability to denitrify and possibly tolerance to stress conditions as well (Carvalho et al., 2007; Flowers et al., 2009; Slater et al., 2010; Welles et al., 2015b). These differences may lead to the prevalence of specific clades under certain conditions and consequently differences in the metabolic conversions. Considering the metabolic differences, prevalence of specific clades may significantly affect EBPR processes. For instance, differences in the anaerobic stoichiometry may affect the P-release efficiency in combined chemical and biological P-removal and P-recovery processes. Therefore it becomes important to study the metabolism of the specific clades separately to get a better understanding about the conditions favoring the specific clades. Furthermore, considering the large differences in kinetic rates (four times higher for PAO II at poly-P depleted conditions), the different PAO clades can no longer be considered as one organism in modeling approaches (Welles et al., 2015b).
Therefore, the objective of this study was to assess at long-term how the anaerobic kinetics and stoichiometry of a highly enriched PAO II culture are affected by a wide range of different biomass P-contents. This will provide a more quantitative insight into the relationship between storage polymers and anaerobic metabolic pathways, contributing to explain the wide range of different P/HAc ratios and kinetic rates observed in previous studies. From a practical perspective, this important understanding will ultimately help to improve the existing metabolic models, leading to better design and operation of the EBPR processes and, in particular, of combined chemical and biological phosphorus removal and recovery systems where the P-contents in general are lower.
Materials and Methods
PAO Enrichment and SBR Operation
The PAO culture was enriched as described in previous studies (Welles et al., 2014, 2015a,b) in a 2.5 L double-jacketed laboratory sequencing batch reactor (SBR). The SBR was operated and controlled automatically in a sequential mode by an Applikon ADI controller also used for data acquisition and storage (e.g., pH and O2) using BioXpert software (Applikon, The Netherlands, Schiedam). The reactor was inoculated with activated sludge from a municipal wastewater treatment plant with a 5-stage Bardenpho configuration (Hoek van Holland, The Netherlands).
The SBR was operated in cycles of 6 h (2.25 h anaerobic, 2.25 aerobic and 1.5 settling phase) following similar operating conditions used in previous studies (Smolders et al., 1994; Brdjanovic et al., 1997; Welles et al., 2014). The pH was maintained at 7.0 by dosing 0.4 M HCl and 0.4 M NaOH and temperature was controlled at 20 ± 1°C. Each cycle started with a 5 min sparging phase with nitrogen gas at a flow rate of 30 L/h to create anaerobic conditions. After the first 5 min, 1.25 L of synthetic substrate was fed to the SBR over a period of 5 min and nitrogen gas sparging continued throughout the anaerobic phase. In the aerobic phase, compressed air was sparged to the SBR at a flow rate of 60 L/h. Mixing was provided at 500 rpm, except during settling and decant phases when mixing was switched off.
The SBR was controlled at a biomass retention time (SRT) of 8 days, not taking into account the potential loss of solids in the effluent during removal and biofilm removal during regular cleaning. At the end of the settling period, the supernatant was pumped out from the reactor, leaving 1.25 L of mixed liquor in the reactor. This resulted in a total hydraulic retention time (HRT) of 12 h.
Experimental Phases and Medium
To investigate the effect of the storage polymers on the on the kinetic rates and stoichiometry of the anaerobic conversions, six long-term experimental phases were designed with the aim to obtain an EBPR biomass with different P-contents. The SBR was fed with the same medium in all the experimental phases. The only difference was the orthophosphate concentration. The respective orthophosphate concentrations (provided with the addition of NaH2PO4.H2O) are shown in Table 1.
The concentrated medium was prepared with demineralised water. In the beginning of every cycle, 250 mL of concentrated substrate together with 1000 mL demi water were fed to the reactor. After dilution, the influent contained per liter: 860 mg CH3COONa·3H2O (12.6 C-mmol/L, 405 mg COD/L), 107 mg NH4Cl (2 N-mmol/L), 120 mg MgSO4.7H2O, 14 mg CaCl2.2H2O, 48 mg KCl, 2 mg of allyl-N-thiourea (ATU) to inhibit nitrification, 0.3 mL/L trace element solution, and a defined concentration of NaH2PO4.H2O different in each experimental phase as previously described. The trace element solution was prepared as described by Smolders et al. (1994). Prior to use, both concentrated solutions were autoclaved at 110°C for 1 h. In phase 0, the biomass culture was enriched, which required a long operation time to obtain a high grade enrichment culture dominated by a specific PAO clade. This phase was not continuously monitored. In phase 1, multiple experiments were done to characterize the biomass performance and microbial community in short-term experiments (Welles et al., 2015b). The remaining phases were about 2-3 SRT each with the aim to be long enough for obtaining stable performance and short enough to avoid gradual changes in the microbial community structure.
SBR Monitoring
The performance of the SBR was regularly monitored by measuring ortho-phosphate (-P), acetate (HAc), total suspended solids (TSS) and volatile suspended solids (VSS). Stable performance in the reactor was confirmed by daily observation of the aforementioned parameters as well as by the pH and DO online data. At the end of each experimental phase, (except for phase 0), a cycle test was conducted to determine the anaerobic stoichiometric and kinetic parameters. In the cycle tests, polyhydroxyalkanoate (PHA) and glycogen concentrations were determined in addition to the above described parameters. Furthermore, the composition of the microbial community was characterized by fluorescence in situ hybridization (FISH) analysis and denaturing gradient gel electrophoresis (DGGE) analysis.
Kinetic Rates and Stoichiometric Values
The PO4-release rates and HAc-uptake rates were determined using the PO4 and HAc profiles observed in the cycle tests and expressed as maximum active biomass specific rates as described by Smolders et al. (1994) and Brdjanovic et al. (1997). For the maintenance activity (in the absence of HAc), only phosphate release was determined and no glycogen consumption. In order to see potential changes in the glycogen content due to maintenance activity, that are significant enough to be measured, different type of tests would need to be conducted (Zeng et al., 2003). As the maintenance activity was not the main focus of this study, no additional tests were conducted. The stoichiometric parameters of interest were: P/HAc, PHV/HAc, PHB/HAc, PHV/PHB, gly/HAc and gly/PHB.
Analyses
Determination of TSS, VSS and -P concentrations were performed in accordance with Standard Methods (A.P.H.A., 1995). HAc was determined using a Varian 430-GC Gas Chromatograph (GC) equipped with a split injector (split ratio 1:10), a WCOT Fused Silica column with a FFAP-CB coating (25 m × 0.53 mm × 1 μm), and coupled to a FID detector. Helium gas was used as carrier gas. Temperature of the injector, column and detector were 200°, 105°, and 300°C, respectively. PHB and PHV contents of freeze dried biomass were determined by gas chromatography after a digestion, esterification and extraction step following the method described by Smolders et al. (1994). Glycogen content of freeze dried biomass was determined by HPLC after digestion according to the method described by Smolders et al. (1994) and Dircks et al. (2001) but with an extended digestion of 5 h in 5 mL 0.9 M HCl, using 5 mg of freeze-dried biomass as described by Lanham et al. (2012).
Characterization of Microbial Populations
An estimation of the biomass fractions of the populations of interest (PAO Type I, PAO type II and GAO) was based on FISH analyses, following the procedure described by Winkler et al. (2011). All bacteria were targeted by the EUB338 mix (general bacteria probe) (Amann et al., 1990; Amann, 1995; Daims et al., 1999). “Candidatus Accumulibacter phosphatis” and “Candidatus Competibacter phosphatis” were targeted by PAOMIX probe (mixture of probes PAO462, PAO651, and PAO846) (Crocetti et al., 2000) and GAOMIX probe (mixture of probes GAOQ431 and GAOQ989) (Crocetti et al., 2002), respectively. PAO I (clade IA and other type I clades) and PAO II (clade IIA, IIC, and IID) were targeted by the probes Acc-1-444 and Acc-2-444 (Flowers et al., 2009), respectively. Hybridized samples were examined with Zeiss Axioplan-2 epifluorescence microscope. The quantification of the PAO and GAO biomass fractions (of the entire bacterial community) and the PAO I and PAO II fractions (of the PAO community) in the biomass was carried out via FISH image analysis in a previous study (Welles et al., 2015b).
To confirm the FISH observations and to identify potential changes in the microbial populations at the sub-clade level, 16S-rDNA-PCR DGGE was applied. Samples were collected at the end of each experimental phase. DNA extraction, PCR amplification, DGGE, band isolation, sequencing and identification of microorganisms were carried out according to the procedures described by Bassin et al. (2011). To double confirm the specific PAO clade, ppk1 gene fragments were recovered and analyzed. A direct PCR was done on the gDNA of the biomass sample from experimental stage 1 using the primers for Accumulibacter sp. like bacteria and amplifying the near full length polyphosphate kinase I gene, ACCppk1-254F and ACCppk1-1376R (McMahon et al., 2007). The product from PCR was sequenced using both primers. Both reads were assembled using Codoncode aligner software v4.2.7 (Codoncode corp. USA) and submitted for BLASTn (NCBI) analysis.
Microscopy
For thin sectioning electron microscopy, the cells were first fixed in 3% (v/v) glutaraldehyde for 1 h on ice, then post-fixed in 1% (w/v) OsO4 + 0.5 M NaCl for 3 h at room temperature, washed and stained overnight with 1% (w/v) uranyl acetate, dehydrated in ethanol series and embedded in Epoxy resin. The thin sections were finally stained with 1% lead acetate.
Active Biomass
The active biomass concentration was determined as MLVSS excluding PHB, PHV and glycogen (active biomass = MLVSS – PHB – PHV – glycogen). Unbiodegradable particulate endogenous residue, shown to be another non-active biomass component of the MLVSS (Wentzel et al., 1988, 1989a,b) was neglected for the sake of simplicity, and marginal contribution to the MLVSS in these enrichment cultures. The active biomass concentration was expressed in C-mol units by taking into account the experimentally determined composition of PAO (CH2.09O0.54N0.20P0.015) (Smolders et al., 1994).
Estimation of Poly-P
The concentration of PAO Poly-P was estimated on the basis of the inorganic suspended solids to total suspended solids (ISS/TSS) ratio and confirmed using steady-state mass balances as described in Welles et al. (2015b). Equation 1 was developed using the ISS/TSS ratio of the biomass, assuming that (i) the ISS/TSS ratio associated with active biomass in non-EBPR biomass (ISSb) was 0.025 mg ISS/mg TSS (as observed in this study after poly-P depletion), (ii) a poly-P composition of (PO3)3MgK with a P-content (fP, ppASH) of 0.31 mg P/mg ISS and, (iii) negligible chemical precipitation. Equation 2, derived from the steady-state mass balance of phosphorus, assuming that (i) the solids in the effluent were negligible, (ii) a ratio of non poly-P phosphorus per VSS (fP, bVSS) equal to the P-content of non-EBPR biomass at around 0.023 mg P/mg VSS (Metcalf and Eddy, 2003) and, (iii) absence of chemical precipitation. A detailed description of the development of the equations can be found in Supplementary file.
where;
VSS: Concentration of volatile suspended solids
ISS: Concentration of inorganic suspended solids
Poly-P: Concentration of poly-phosphate
TP, i: concentration of total phosphorus in the influent
TP, e: Concentration of total phosphorus in the effluent
fP, bVSS: Ratio of non poly-P phosphorus per VSS
fISSb, TSS: ISS/TSS ratio associated with active biomass
fP, ppISS: P-content of poly-P
HRT: Hydraulic retention time
SRT: Solids retention time
Results
EBPR Performance at Different P/C Influent Ratios
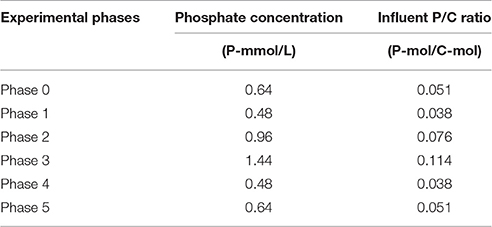
An enriched EBPR culture was cultivated with different influent ortho-phosphate concentrations during six experimental phases (phases 0 to 5). The increase in influent phosphate concentration resulted in higher P-release and consequently higher ortho-phosphate concentrations at the end of the anaerobic phase (Figure 1A) and higher biomass ISS/TSS ratio at the end of the aerobic phase (Figure 1B). A decrease in the influent phosphate concentrations decreased both the biomass ISS/TSS ratio content and ortho-phosphate concentrations at the end of the anaerobic phase, showing that the observed patterns were reversible. The P-release/HAc-uptake ratio fluctuated between 0.15 and 0.6 P-mol/C-mol, depending on the influent ortho-phosphate concentration, showing the highest P-release in phase 3 at the highest influent ortho-phosphate concentration. Full P-removal was observed in all phases except in phase 3, leaving an average ortho-phosphate concentration of 0.20 P-mmol/L in the effluent.
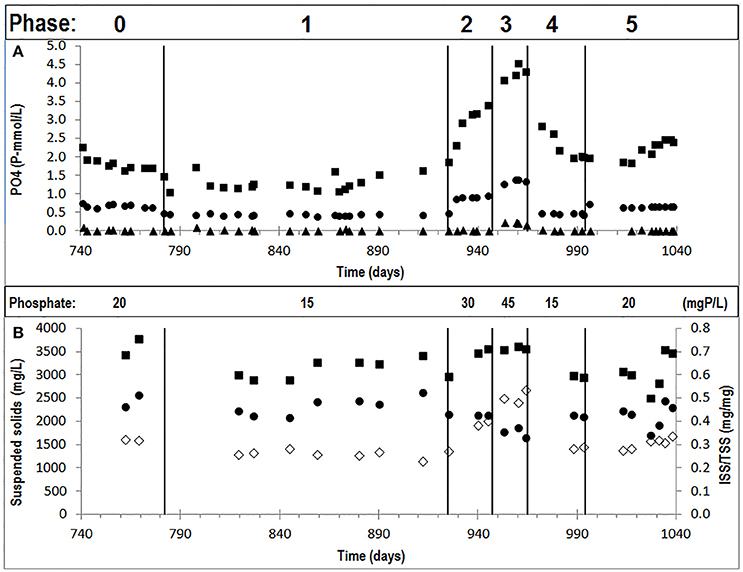
Figure 1. Concentrations observed in the different cycles of the experimental phases studied: (A) orthophosphate concentrations in the influent (•), end of the anaerobic phase (■), and end of the aerobic phase (▲); and, (B) TSS concentrations in the end of the aerobic phase (■), VSS concentrations in the end of the aerobic phase (•) and ISS/TSS ratios (♢).
Microbial Community at Different Influent P/C Ratios
FISH analysis and quantification showed that PAO were highly dominant in experimental phase 1, 3, and 5 (PAO biomass fraction of 99 ± 3% in phase 1, (Welles et al., 2015b) and more specifically that the PAO population consisted of PAO clade II with minor traces of PAO clade I (biomass fractions of 99 ± 6% PAO II and 1 ± 2% PAO I in phase 1, Welles et al., 2015b) (Figures 2A,D) (raw FISH images are shown in Supplementary Figures 1–24, Supplementary File). GAO were not detected and only minor traces of PAO clade I were observed. In the execution of the FISH microscopic analysis, GAO biomass samples were used as a positive control for the Competibacter probes and as negative control for the PAO probes, which confirmed that there were no problems with the probes or analytical procedures to detect GAO presence or PAO absence in the samples. Light microscopy confirmed the dominance of bacteria with the PAO morphology, but also showed that bacteria with smaller dimensions and different morphology were present as well (Figures 2G,H).
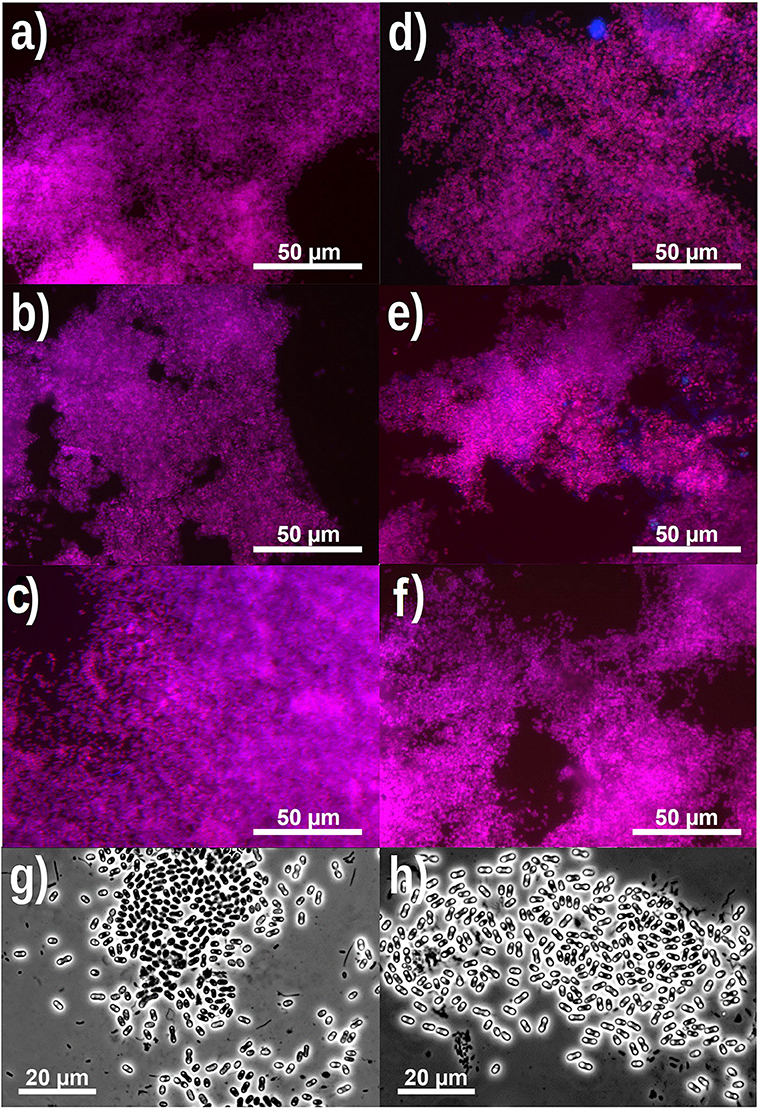
Figure 2. Representative FISH microscopic images (A–F) showing the distribution of bacterial populations in biomass samples collected at the end of phase 1 (A,D), phase 3 (B,E) and phase 5 (C,F) on day 890, 964 and 1034, respectively. In (A–C); blue: EUB mix (Cy5); purple (superposition of blue and red): PAO mix (Cy3); and cyan green (superposition of blue and green): GAO mix (Fluos). In (D–F); blue: PAO mix (Cy5), purple (superposition of blue and red): PAO clade II (Cy3), and cyan green (superposition of blue and green): PAO clade I (Fluos). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.) Phase contrast images (G,H) showing the microbial distribution at the end of phase 5.
Based on FISH analyses, no significant changes occurred in the relative quantities of PAO I, PAO II, and GAO throughout the execution of the experimental phases (Figures 2B,C,E,F) and therefore quantification was not considered useful in the other experimental phases. 16S-rRNA gene based DGGE profiles were obtained from samples collected at the end of each experimental phase (Figure 3). From the DNA derived DGGE patterns, 24 bands were selected, covering all the unique bands observed in the different phases. The phylogenetic analysis of selected band sequences from all experimental phases was conducted in this study (Table 2). The bacterial groups detected were: (i) Betaproteobacteria closely related to “Candidatus Accumulibacter phosphatis” (bands 7, 21, and 22); (ii) Betaproteobacteria not closely related to “Candidatus Accumulibacter phosphatis” (band 9); (iii) Deltaproteobacteria (band 13); (iv) Alphaproteobacteria (bands 06 and 04); (v) Armatimonadetes were also detected (band 20), as well as (vi) bacteria belonging to Bacteroidetes (bands 14, 17, 2, 1, 15, 18, 16, 12, 3, and 11). Bands 05, 08, 10, 19, 23, and 24 could not provide sufficient DNA of the required quality for sequencing and the quality of the sequence obtained from band 9 was insufficient for submission to the GenBank A phylogenetic tree analysis of the 16S rRNA-gene sequences obtained from the biomass sample collected at the end of experimental phase 1 was presented in a previous study (Welles et al., 2015b) and revealed that the PAO belonged to “Candidatus Accumulibacter phosphatis” clade IIC/D. To confirm the specific PAO clade, the polyphosphate kinase I gene was sequenced and a phylogenetic analysis was performed (Figure 4). This analysis confirmed that the PAO belonged to “Candidatus Accumulibacter phosphatis” clade IIC.
Figure 3. Microbial identification analysis: 16S rRNA bands obtained by DGGE. M = marker and Number 1 to 5 correspond to biomass samples collected at the end of the experimental phases on day 890 (phase 1), day 945 (phase 2), day 964 (phase 3), day 993 (phase 4), and day 1034 (phase 5).
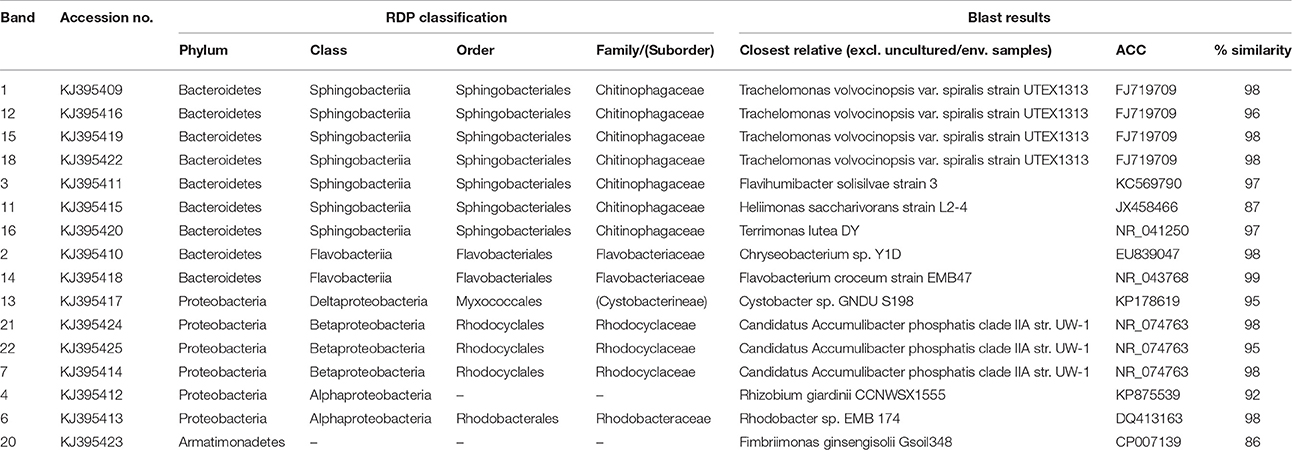
Table 2. Microbial identification analysis: phylogenetic analysis of 16S rRNA bands obtained by DGGE.
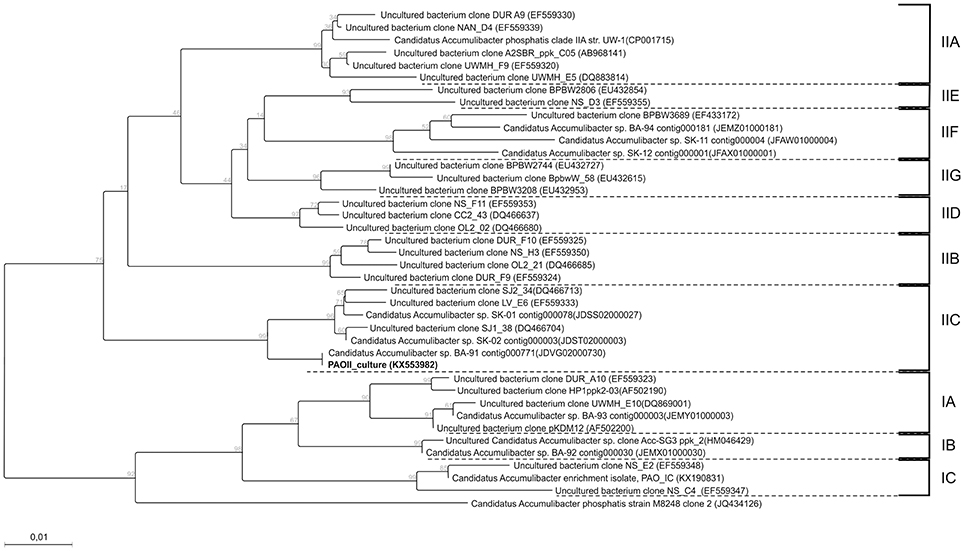
Figure 4. Phylogenetic tree constructed using the neighbor joining method implemented in the CLC genomic workbench v7.5.1 package. The distance between sequences were measured using the kimura model. 332 amino-acid positions were used for calculation which correspondsg to a near complete ppk1 gene. The sequence of the ppk1 gene from Rhodocyclus tenuis (AF502199) was used as an outgroup and afterwards pruned from the tree. Also bootstrap (1000 rounds) was performed and the resulting values were displayed on the branching points. All clades are indicated by the text IA till IC and IIA till IIG. The scale bar represent one percent difference in amino-acid composition. The sequence obtained in this study is indicated in bold.
Among the sequenced bands, no “Candidatus Competibacter phosphatis” nor Defluviicoccus (another known GAO) sequences were detected. There were also no sequences detected that were related to Gammaproteobacteria, the subdivision to which “Candidatus Competibacter phosphatis” belong. In the different experimental phases, no differences were observed in the dominant bands (band 1, 4, and 7), representing bacteria closely related to Bacteroidetes, “Candidatus Accumulibacter phosphatis” and Alphaproteobacteria, respectively. From the minor bands, band 3, representing Bacteroidetes (closely related to Flavihumibacter petaseus) and band 6, representing an Alphaproteobacteria (closely related to Rhodobacter capsulatus), showed slightly higher intensity during phases 2 and 3 which were the phases with higher influent ortho-phosphate concentrations (0.97 and 1.45 P-mmol/L, respectively), but these bands were not detected when the influent ortho-phosphate concentration decreased in phase 4 and 5 to 0.48 and 0.65 P-mmol/L, respectively.
Effect of Influent P/C Ratio on Intracellular Storage Polymers
The effect of the influent P/C ratio on the ISS/TSS ratio of the biomass and storage polymers is shown in Figures 5A,B. As the influent P/C ratio increased, the ISS/TSS ratio increased linear proportionally. The influent P/C ratio data point that corresponds to zero was obtained in a batch experiment where the poly-P was depleted from the enriched biomass (Welles et al., 2015b). At higher biomass ISS/TSS ratios, the poly-P/active biomass ratio and poly-P/gly ratio increased while the glycogen/active biomass ratio decreased. To get a better understanding of the intracellular organization of storage polymers, thin sections were prepared from the PAO cells taken at the end of the anaerobic phase in experimental phase 2 and analyzed by electron microscopy (Figure 5C). In this phase, with an influent phosphorus concentration of 0.97 P-mmol/L the biomass ISS/TSS ratio reached about 0.4 at the end of the aerobic phase. The microscopy showed that poly-P appeared as 1 or 2 large dense inclusions. Smaller electron transparent inclusions surrounded by a membrane were observed and assumed to correspond to PHA, as it is known to be surrounded by a membrane (Liebergesell et al., 1994; Pieper-Fürst et al., 1994; Steinbüchel et al., 1995; Mayer et al., 1996) while the white dispersed spots were considered to be glycogen, which is known to be freely dispersed in the cytosol (Braña et al., 1980; Kamio et al., 1981).
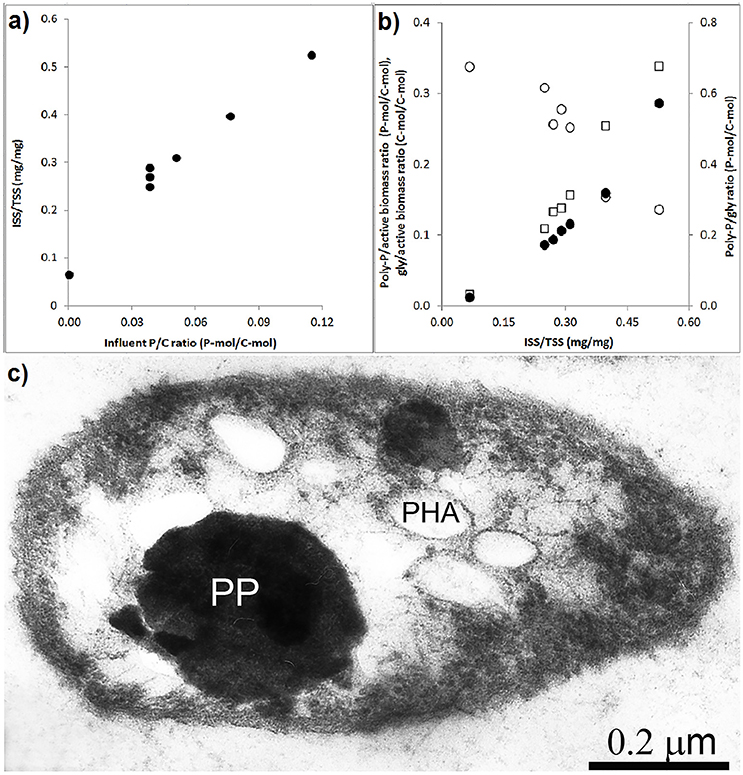
Figure 5. Storage polymers in enriched PAO biomass samples collected at the end of the experimental phases on day 881 and 890 (phase 1), day 945 (phase 2), day 964 (phase 3), day 993 (phase 4), and day 1034 (phase 5): (A) ash/TSS ratio as a functions of the influent P/C ratio at the end of the aerobic phase; (B) the relationship between the ash/TSS ratio at the end of the aerobic phase and (•) estimated poly-P/active biomass ratio, (○) glycogen/active biomass ratio and (□) poly-P/gly ratio; and, (C) electron microscope image of a thin section showing the poly-P, PHA and glycogen organization in the enriched PAO II cell at the end of the anaerobic phase.
Effect of P-Content on PAO Kinetics
The specific P-release rates for acetate uptake increased strongly when the poly-P/active biomass ratio increased from 0 to 0.2 P-mol/C-mol, above which the rates seemed to level off (Figure 6A). Also, the endogenous P-release rate increased with the increasing poly-P/active biomass ratio. When the P-release rate increased, the HAc-uptake rate also increased. A maximum HAc-uptake rate of 0.20 C-mol/C-mol.h was observed when the P-release rate reached 0.07 P-mol/C-mol.h at a poly-P/gly ratio of around 0.3 P-mol/C-mol (Figure 6B). Above this poly-P/gly ratio, the HAc-uptake rate decreased.
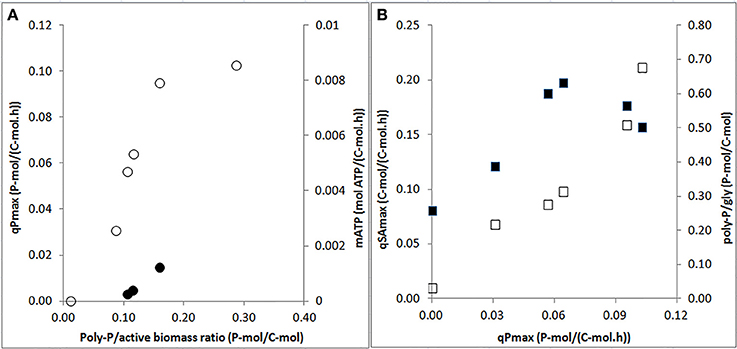
Figure 6. Effects of poly-P contents and P-release rates on specific biomass kinetic rates at the end of the experimental phases on day 881 and 890 (phase 1), day 945 (phase 2), day 964 (phase 3), day 993 (phase 4), and day 1034 (phase 5): (A) specific P-release rates during HAc uptake (○: qPmax) and endogenous maintenance activity (•: mATP) as a function of the poly-P/active biomass ratio; and, (B) HAc-uptake rates (■: qSAmax) and poly-P/gly ratio (□: poly-P/gly) vs. P-release rates.
Effect of P-Content on PAO Stoichiometry
A clear relationship was observed between the P-content and stoichiometry of the anaerobic conversions. The PO4-release/HAc-uptake ratio increased drastically when the poly-P/active biomass ratio increased (Figure 7A). Consequently, the gly/HAc ratio, PHV/gly ratio, PHV/HAc ratio, PHB/HAc ratio and PHV/PHB ratio decreased when the PO4/HAc ratio increased (Figures 7B–F).
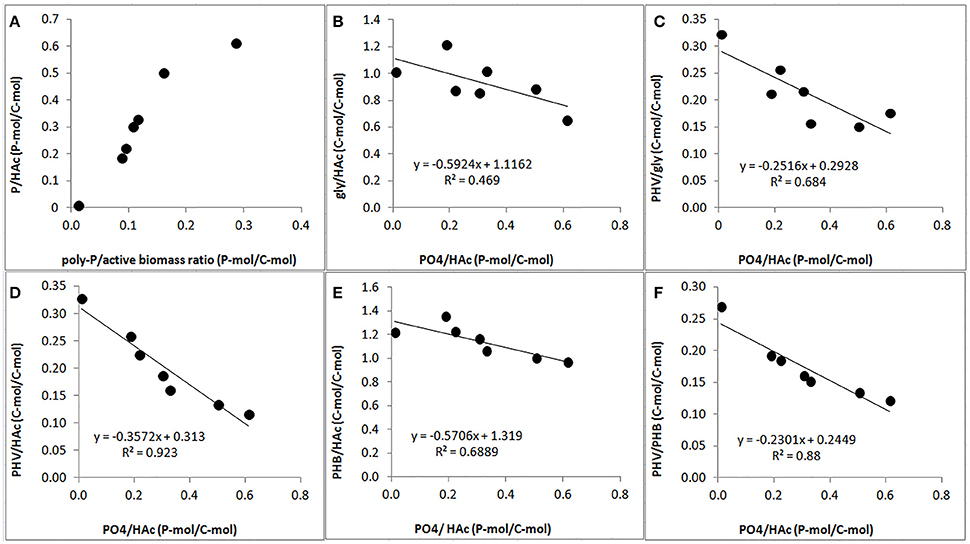
Figure 7. Effect of poly-P content on anaerobic PAO stoichiometric parameters at the end of the experimental phases on day 881 and 890 (phase 1), day 945 (phase 2), day 964 (phase 3), day 993 (phase 4), and day 1034 (phase 5): (A) correlation of PO4/HAc ratio (filled circle) and estimated poly-P/active biomass ratio; (B) correlation of gly/HAc ratio (filled circle) and PO4/HAc ratio; (C) correlation of PHV/gly (filled circle) and PO4 /HAc ratio; (D) correlation of PHV/HAc (filled circle) and PO4/HAc ratio; (E) correlation of PHB/HAc ratio (filled circle) and PO4/HAc ratio; and, (F) correlation of PHV/PHB ratio (filled circle) and PO4/HAc ratio.
Discussion
Effect of Influent P/C Ratio on the Microbial Population, Storage Polymers and EBPR Performance
FISH analysis showed in experimental phase 1, that the population was highly dominated by “Candidatus Accumulibacter phosphatis” clade II. In a previous study (Welles et al., 2015b), with the same biomass culture (SBR-L) in the same experimental phase, the dominance of Betaproteobacteria (subdivision to which most “Candidatus Accumulibacter phosphatis” belong) was double confirmed by an additional FISH analysis, confirming that the well-known GAO, “Candidatus Competibacter phosphatis” and Defluviicoccus (belonging to Gammaproteobacteria and Alphaproteobacteria, respectively) were not present in detectable quantities. In addition, a poly-P staining confirmed that practically all bacteria contained poly-P and thus had the PAO morphology (Welles et al., 2015b). Phylogenetic analysis of the 16S rRNA gene in a previous study (Welles et al., 2015b) and the ppk1 gene analysis in this study, confirmed that the PAO enriched was clade IIC. As FISH analysis demonstrated that the biomass was highly dominated by PAO II and both FISH and DGGE analysis demonstrated that in the different experimental phases, the composition of the microbial community did not change over time, further quantification by FISH microscopy in each experimental phase was not considered useful.
As the poly-P content of the biomass increased, the glycogen content decreased, indicating that poly-P is the preferred storage polymer by PAO IIC which results in higher poly-P/glycogen ratios when the influent phosphate concentrations becomes less limiting at higher influent P/HAc ratios. Still glycogen formation was never completely eliminated. When the poly-P content increased, the metabolism shifted from a mixed PAO-GAO metabolism to a typical PAO metabolism, i.e., P-release/HAc-uptake ratio higher than 0.5 P-mol/C-mol in experimental phase 3. In this experimental phase, the biomass culture was able to remove about 1.45 P-mmol/L, resulting in high poly-P contents (0.54 mg ISS/mg TSS, 0.32 mg P/mg VSS). This removal capacity and poly-P contents are in the range of the highest contents reported in literature (Wentzel et al., 1988; Schuler and Jenkins, 2003a), confirming that the culture was highly enriched with PAO.
While FISH analysis demonstrated that a major fraction of the biomass comprised of PAO (99%), The DGGE profiles showed in addition to the “Candidatus Accumulibacter phosphatis” clade IIC/D band two other intense bands belonging to Bacteroidetes and Alphaproteobacteria. This discrepancy may be explained by the differences in the analytical methods. While the intensity of the DGGE bands represents the quantity of PCR amplified 16s rRNA gene products from specific micro-organisms, the bacterial fractions determined by FISH quantification represent the relative surface areas of bacterial flocs in which the target 16S rRNA is present. A more detailed microscopic analysis of the PAO II dominated flocs (Figures 2G,H) showed that other bacteria of a smaller size were homogeneously enmeshed in each PAO cluster. The smaller bacteria were on a volume or surface area basis present as a minor population (hardly observed by FISH), but their presence was more abundant on a cell-counting basis. In the DGGE analysis the DNA is extracted from the biomass. Considering the large difference in size of the bacteria (about one order of magnitude) and the fact that each bacteria has a genome, the fraction of extracted template DNA belonging to Bacteroidetes and Alphaproteobacteria in comparison to the fraction of extracted template DNA belonging to PAO may have been much larger than the dry weight biomass fractions. Other factors that may have contributed to the differences in the bacterial quantities obtained from FISH and DGGE data between these organisms may have been: (i) differences in DNA extraction efficiency, (ii) different copy numbers of the 16S rRNA gene in the genomes (only 2 for “Candidatus Accumulibacter phosphatis”) and, (iii) differences in PCR amplification efficiency of the 16S rRNA genes. In the perception of the authors, FISH microscopy is more reliable to quantify the bacterial fractions in the microbial community, while DGGE is more reliable for identification of the specific microorganisms. Therefore, the microbial populations other than “Candidatus Accumulibacter phosphatis” clade IIC, observed by DGGE, were considered as minor populations, which were not responsible for the major function of the biomass.
Effect of Storage Polymers on Kinetic Rates and Stoichiometry
The shift in the stoichiometry of the anaerobic conversions for HAc-uptake indicated that the changes in kinetic P-release and HAc-uptake rates were associated with a shift in the relative ratio of the metabolic fluxes from the different energy generating pathways (glycogen conversion and poly-P degradation), which may have been triggered by a change in the level of the different intracellular storage polymers. A schematic overview of the proposed mechanism that regulates the metabolic shift is shown in Figure 8. When HAc uptake took place to a major extent through a glycogen dependent metabolism in experimental phase 1, the estimated available intracellularly stored poly-P (5.1 P-mmol/L) was about four times higher than the poly-P actually used for HAc uptake (1.3 P-mmol/L). This indicates that the gradual shift in the metabolism of PAO is not driven by a stoichiometric limitation of the available poly-P. Considering that poly-P was stored as large inclusions (Figure 5C), the rate of poly-P consumption may have been poly-P surface area related. This has also been seen in past studies for PHA (Smolders et al., 1995; Murnleitner et al., 1996). Thus, when the poly-P content of the biomass is low, possibly the rate of energy production from poly-P consumption is limited, which then needs to be topped up by energy production from glycogen conversion. The consumption of glycogen allows the cell to harvest 0.5 mol ATP/C-mol glycogen (Zeng et al., 2003), while poly-P conversion generates 1 mol ATP/P-mol poly-P (Van Groenestijn et al., 1987; Smolders et al., 1994).
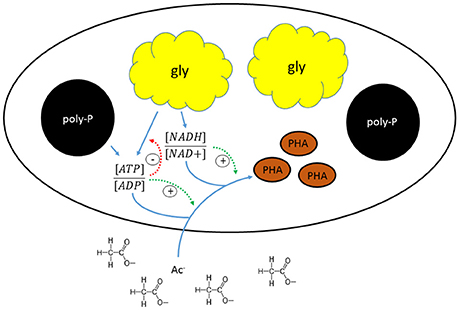
Figure 8. Schematic representation of the mechanism regulating the metabolic shift in “Candidatus Accumulibacter phosphatis” clade IIC.
When the P-release rates increased, the HAc-uptake rates increased up to a maximum value above which the HAc-uptake rate started to decrease again. The increase in the HAc-uptake rate coupled to an increase in the P-release rate suggests that the energy production could have been the rate limiting step for HAc-uptake and that at high poly-P content the energy production from poly-P was faster than the energy production from glycogen. Differences in the energy production rates from poly-P and glycogen may be explained by differences in the number of metabolic conversions of each energy generating pathway. The metabolic pathway for ATP production using poly-P requires only two biochemical conversions and subsequent release of ortho-phosphate over the membrane accompanied by counterions (Van Groenestijn et al., 1987; Saunders et al., 2007). Although the exact pathway for energy production from glycogen remains unclear, such as the type of glycolytic pathways, it probably requires at least 10 biochemical conversions (Satoh et al., 1994) to convert glycogen into PHA and avoid the net-production of reduction equivalents. When the biomass P-content becomes high, the P-release and its associated energy production rate from poly-P conversion become high as well. However, at high biomass P-content, the glycogen content appears to be low, although glycogen consumption for anaerobic substrate uptake was never eliminated in the anaerobic conversions and therefore seems to be essential for the production of reduction equivalents, which was also observed in the study of Schuler and Jenkins (2003a). Possibly the production rate of reduction equivalents is also surface area related. Therefore, a decrease in the glycogen content may trigger a transition where the rate limiting step of the HAc-uptake process changes from the energy production rate to the production rate of reduction equivalents. Thus, at a high biomass P-content, the HAc uptake rate starts to decrease possibly due to limited supply rate of reduction equivalents by glycogen. An optimal HAc uptake rate seems to occur at a poly-P/gly ratio of 0.3 P-mol/C-mol, which corresponds with an ISS/TSS ratio of 0.3 mg ash/mg TSS obtained at an influent P/C ratio of 0.05 P-mol/C-mol. This is roughly the influent P/C ratio at which the PAO culture was originally enriched in this study.
The anaerobic P-release rate for maintenance energy production (determined in the absence of acetate) seemed to also increase when the biomass P-content increased. Assuming that the maintenance coefficient (biomass specific energy requirements per unit of time) of a microorganism is more or less constant under defined environmental conditions (Herbert, 1958; Pirt, 1965), the drastic change in the P-release rate for maintenance energy production suggests that for the production of maintenance energy also a change in metabolism occurred. Possibly, the changes in the P-release rates for anaerobic maintenance energy production may have been compensated by changes in glycogen consumption rates. This hypothesis could have only been verified by additional tests with a longer duration as the potential glycogen consumption for maintenance energy production is very little and the glycogen measurements are not very accurate (Zeng et al., 2003). Due to potential changes in the long term experiments, the prolonged maintenance tests were not performed.
Assessment of the Anaerobic Stoichiometric Parameters against Values Reported in Literature
Table 3 shows a comparison between the anaerobic kinetic rates and stoichiometric parameters obtained in this study and previously reported values (Liu et al., 1997; Schuler and Jenkins, 2003a,b; among others with highly enriched PAO I, PAO II, and GAO cultures). Similar to the findings of Liu et al. (1997) and Schuler and Jenkins (2003b), the P/HAc stoichiometry increased when the biomass P-content increased but the dependency of the stoichiometry was different. In this study, the P/HAc stoichiometry at a medium initial estimated P/TSS ratio of 0.07 mg/mg was 0.17 P-mol/C-mol against 0.32 P-mol/C-mol reported by Schuler and Jenkins (2003a). This discrepancy can be explained by the findings of a recent study that showed at a de fined poly-P/VSS ratio (0.125 mg/mg), that the anaerobic P/HAc stoichiometry of PAO I was reported to be 2.4 times higher than the stoichiometry of PAO II (Welles et al., 2015b). Possibly the biomass enriched by Schuler and Jenkins (2003a,b,c) may have been dominated by PAO I.
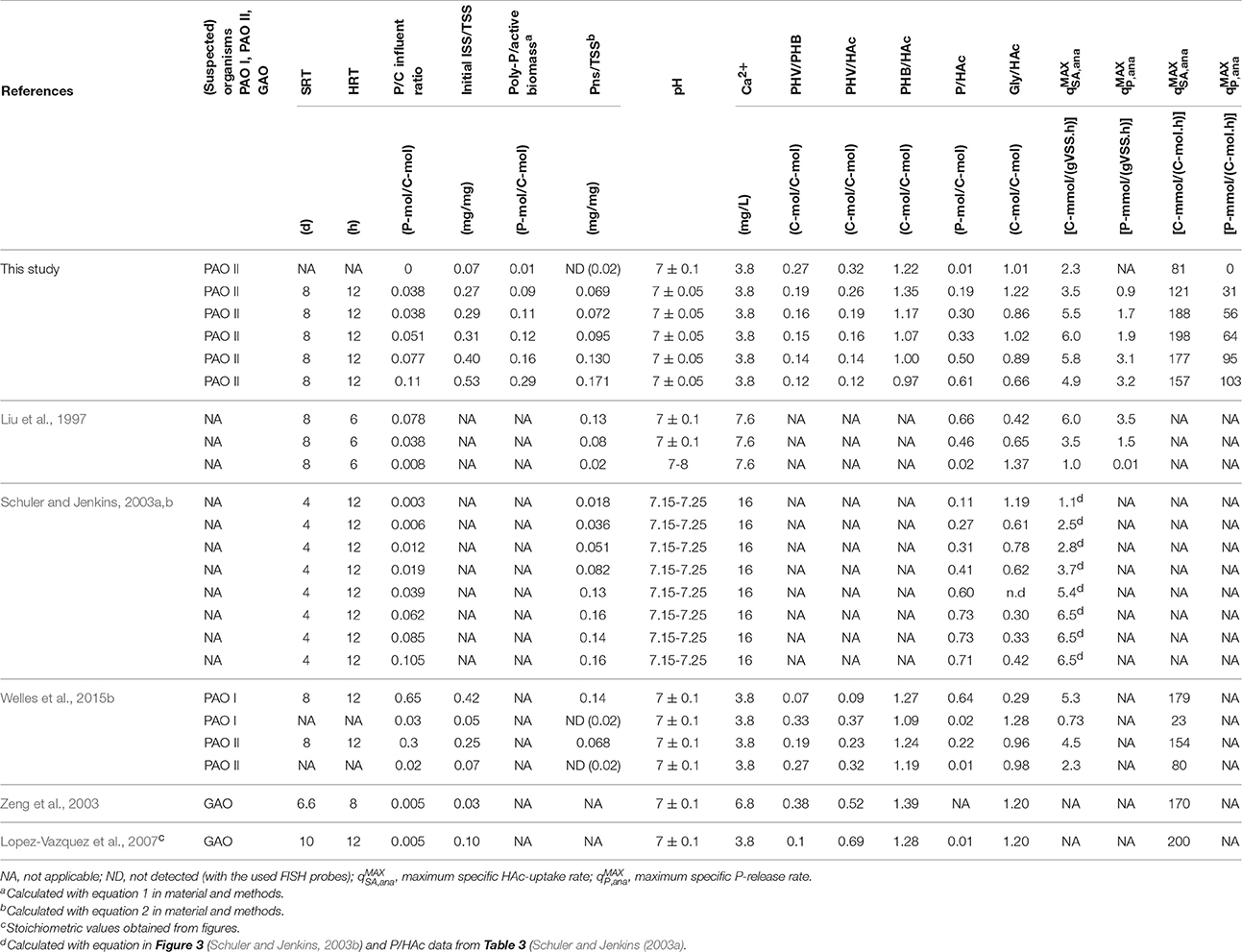
Table 3. Comparison of kinetic and stoichiometric values obtained in this study and previous studies with enriched EBPR cultures.
Implications on EBPR Performance and Modeling
The findings drawn in this study suggest that in wastewaters containing high VFA concentrations from hydrolysis and fermentation processes in the sewerage, the anaerobic kinetic rates of PAO are highly dependent on the poly-P content of PAO. The kinetic rates and associated competitiveness of certain PAO clades, increase as the P-content of the biomass increases. In the anaerobic phase of WWTP's treating such wastewaters, PAO perform their best at a medium range P-content (like observed in this study on PAO IIC) or at a high P-content (Schuler and Jenkins, 2003a,b,c). If, in a certain system, GAO become dominant, the availability of P per PAO biomass, and there with the PAO specific P-content, increases. Consequently, the HAc uptake rate may increase. However, as the P-content increases due to high influent P/C ratios, the return of phosphorus from the sludge line to the water line or if GAO prevail, the aerobic P-uptake ability of PAO is reduced because PAO become saturated with poly-P. In addition, the endogenous P-release activity of PAO increases, which may lead to higher secondary P-release processes in an anaerobic zone that follows the aerated stage. Dependent on different factors such as the climate, the type of sewerage system and the dynamics of wastewater in the sewerage, the hydrolysis and fermentation processes in the sewerage and the associated VFA production, may be limited. In such cases, the PAO in the activated sludge systems may rely for a major extend on the VFA production from fermentation processes in the anaerobic stage of the activated sludge system, which are in general much slower than the VFA consumption processes by PAO I, II and GAO when fed with high VFA concentrations. In such cases, the competition between the different microbial communities will not be determined by the maximum rates of PAO I, II and GAO, but instead the competition between the organisms will be majorly be determined by the Ks values of the VFA uptake processes of the respective microbial communities.
The observed relationship between the anaerobic stoichiometry and the sludge P-content implies that a determination of the anaerobic HAc-uptake stoichiometry at pH 7.0 under strict anaerobic conditions at a temperature of 20°C may help to estimate the poly-P content of the PAO present in the activated sludge. Without any microbial characterization, a stoichiometric P/HAc value in the range of 0.65-0.72 P-mol/C-mol in activated sludge at the end of the anaerobic phase, would indicate that the PAO present in the sludge are saturated with poly-P and that GAO are not present. In such cases, the net ortho-phosphate uptake capacity of the activated sludge may be limited due to incapability of PAO to take up additional phosphate during the anoxic or aerobic phase.
Author Contributions
All authors contributed to the design of the experiments. LW performed the experiments and drafted the manuscript. BA conducted the microbial analysis. All authors critically read and contributed to the final version of the manuscript. All authors read and approved the final manuscript.
Funding
This research project was financed by UNESCO-IHE internal research fund.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was carried out as part of the SALINE project led by UNESCO-IHE Institute for Water Education and consortium partners KWR Watercycle Research Institute, Delft University of Technology, University of Cape Town, The Hong Kong University of Science and Technology, Polytechnic University José Antonio Echeverría and Birzeit University. Thanks to Mitchell Geleijnse who supported this study by conducting the phylogenetic analysis using the ppk1 gene.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.02121/full#supplementary-material
References
Acevedo, B., Oehmen, A., Carvalho, G., Seco, A., Borras, L., and Barat, R. (2012). Metabolic shift of polyphosphate-accumulating organisms with different levels of poly-phosphate storage. Water Res. 46, 1889–1900. doi: 10.1016/j.watres.2012.01.003
Amann, R. I. (1995). “In situ identification of microorganisms by whole cell hybridization with rRNA-targeted nucleic acid probes,” in Molecular Microbial Ecology Manual, eds A. D. L. Akkermans, J. D. van Elsas, and F. J. de Bruijn (London: Kluwer Academic Publisher), 1–15.
Amann, R. I., Binder, B. J., Olson, R. J., Chisholm, S. W., Devereux, R., and Stahl, D. A. (1990). Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56, 1919–1925.
A.P.H.A. (1995). Standard Methods for the Examination of Water and Waste Water, 17th Edn. Washington, DC: American Public Health Association.
Barat, R., Montoya, T., Borrás, L., Ferrer, J., and Seco, A. (2008). Interactions between calcium precipitation and the polyphosphate-accumulating bacteria metabolism. Water Res. 42, 3415–3424. doi: 10.1016/j.watres.2008.05.003
Barat, R., and van Loosdrecht, M. C. M. (2006). Potential phosphorus recovery in a WWTP with the BCFS process: interactions with the biological process. Water Res. 40, 3507–3516. doi: 10.1016/j.watres.2006.08.006
Bassin, J. P., Pronk, M., Muyzer, G., Kleerebezem, R., Dezotti, M., and van Loosdrecht, M. C. M. (2011). Effect of elevated salt concentrations on the aerobic granular sludge process: linking microbial activity with microbial community structure. Appl. Environ. Microbiol. 77, 7942–7953. doi: 10.1128/AEM.05016-11
Braña, A. F., Manzanal, M. B., and Hardisson, C. (1980). Occurrence of polysaccharide granules in sporulating hyphae of Streptomyces viridochromogenes. J. Bacteriol. 144, 1139–1142.
Brdjanovic, D., van Loosdrecht, M. C. M., Hooijmans, C. M., Alaerts, G. J., and Heijnen, J. J. (1997). Temperature effects on physiology of biological phosphorus removal. J. Environ. Eng-ASCE 123, 144–154. doi: 10.1061/(ASCE)0733-9372(1997)123:2(144)
Brdjanovic, D., van Loosdrecht, M. C. M., Hooijmans, C. M., Mino, T., Alaerts, G. J., and Heijnen, J. J. (1998). Effect of polyphosphate limitation on the anaerobic metabolism of phosphorus-accumulating microorganisms. Appl. Microbiol. Biotechnol. 50, 273–276. doi: 10.1007/s002530051289
Burow, L. C., Mabbett, A. N., and Blackall, L. L. (2008). Anaerobic glyoxylate cycle activity during simultaneous utilization of glycogen and acetate in uncultured Accumulibacter enriched in enhanced biological phosphorus removal communities. ISME J. 2, 1040–1051. doi: 10.1038/ismej.2008.45
Carvalho, G., Lemos, P. C., Oehmen, A., and Reis, M. A. (2007). Denitrifying phosphorus removal: linking the process performance with the microbial community structure. Water Res. 41, 4383–4396. doi: 10.1016/j.watres.2007.06.065
Comeau, Y., Hall, K. J., Hancock, R. E. W., and Oldham, W. K. (1986). Biochemical-model for enhanced biological phosphorus removal. Water Res. 20, 1511–1521. doi: 10.1016/0043-1354(86)90115-6
Crocetti, G. R., Banfield, J. F., Keller, J., Bond, P. L., and Blackall, L. L. (2002). Glycogen accumulating organisms in laboratory-scale and full-scale wastewater treatment processes. Microbiology 148, 3353–3364. doi: 10.1099/00221287-148-11-3353
Crocetti, G. R., Hugenholtz, P., Bond, P. L., Schuler, A., Keller, J., Jenkins, D., et al. (2000). Identification of polyphosphate-accumulating organisms and design of 16S rRNA-directed probes for their detection and quantitation. Appl. Environ. Microbiol. 66, 1175–1182. doi: 10.1128/AEM.66.3.1175-1182.2000
Daims, H., Brühl, A., Amann, R., Amann, R., Schleifer, K. H., and Wagner, M. (1999). The domain-specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Syst. Appl. Microbiol. 22, 345–352. doi: 10.1016/S0723-2020(99)80053-8
Dircks, K., Henze, M., van Loosdrecht, M. C., Mosbæk, H., and Aspegren, H. (2001). Storage and degradation of poly-β-hydroxybutyrate in activated sludge under aerobic conditions. Water Res. 35, 2277–2285. doi: 10.1016/S0043-1354(00)00511-X
Ekama, G. A., and Wentzel, M. C. (2004). A predictive model for the reactor inorganic suspended solids concentration in activated sludge systems. Water Res. 38, 4093–4106. doi: 10.1016/j.watres.2004.08.005
Erdal, U. G., Erdal, Z. K., Daigger, G. T., and Randall, C. W. (2008). Is it PAO-GAO competition or metabolic shift in EBPR system? Evidence form an experimental study. Water Sci. Technol. 58, 1329–1334. doi: 10.2166/wst.2008.734
Filipe, C. D., Daigger, G. T., and Grady, C. P. Jr. (2001). A metabolic model for acetate uptake under anaerobic conditions by glycogen accumulating organisms: Stoichiometry, Kinetics and the effect of pH. Biotechnol. Bioeng. 76, 17–31. doi: 10.1002/bit.1022
Flowers, J. J., He, S., Yilmaz, S., Noguera, D. R., and McMahon, K. D. (2009). Denitrification capabilities of two biological phosphorus removal sludges dominated by different ‘Candidatus Accumulibacter’ clades. Environ. Microbiol. Rep. 1, 583–588. doi: 10.1111/j.1758-2229.2009.00090.x
Herbert, D. E. N. I. S. (1958). Some principles of continuous culture. Recent Prog. Microbiol. 3, 381–396.
Hesselman, R. P. X., von Rummell, R., Resnick, S. M., Hany, R., and Zehnder, A. J. B. (2000). Anaerobic metabolism of bacteria performing enhanced biological phosphate removal. Water Res. 34, 3487–3494. doi: 10.1016/S0043-1354(00)00092-0
Kamio, Y., Terawaki, Y., Nakajima, T., and Matsuda, K. (1981). Structure of glycogen produced by Selenomonas ruminantium. Agric. Biol. Chem. 45, 209–216. doi: 10.1271/bbb1961.45.209
Kisoglu, Z., Erdal, U., and Randall, C. W. (2000). “The effect of COD/TP ratio on intracellular storage materials, system performance and kinetic parameters in a BNR system,” in Proceedings of the 73rd Annual Water Environment Federation Technical Exposition and Conference (California, CA).
Kong, Y. H., Beer, M., Rees, G. N., and Seviour, R. J. (2002). Functional analysis of microbial communities in aerobic-anaerobic sequencing batch reactors fed with different phosphorus/carbon)P/C) ratios. Microbiology 148, 2299–2307. doi: 10.1099/00221287-148-8-2299
Lanham, A. B., Ricardo, A. R., Coma, M., Fradinho, J., Carvalheira, M., Oehmen, A., et al. (2012). Optiomisation of glycogen quantification in mixed microbial cultures. Biores. Technol. 118, 518–525. doi: 10.1016/j.biortech.2012.05.087
Liebergesell, M., Sonomoto, K., Madkour, M., Mayer, F., and Steinbüchel, A. (1994). Purification and characterization of the poly (hydroxyalkanoic acid) synthase from Chromatium vinosum and localization of the enzyme at the surface of poly (hydroxyalkanoic acid) granules. Eur. J. Biochem. 226, 71–80. doi: 10.1111/j.1432-1033.1994.tb20027.x
Liu, W. T., Nakamura, K., Matsuo, T., and Mino, T. (1997). Internal energy-based competition between poly-phosphate- and glycogen-accumulating bacteria in biological phosphorus removal reactors-effect of P/C feeding ratio. Water Res. 31, 1430–1438. doi: 10.1016/S0043-1354(96)00352-1
Lopez-Vazquez, C. M., Song, Y. I., Hooijmans, C. M., Brdjanovic, D., Moussa, M. S., Gijzen, H. J., et al. (2007). Short-term temperature effect on the anaerobic metabolism of glycogen accumulating organisms. Biotechnol. Bioeng. 97, 483–495. doi: 10.1002/bit.21302
Martín, H. G., Ivanova, N., Kunin, V., Warnecke, F., Barry, K. W., McHardy, A. C., et al. (2006). Metagenomic analysis of two enhanced biological phosphorus removal (EBPR) sludge communities. Nat. Biotechnol. 24, 1263–1269. doi: 10.1038/nbt1247
Mayer, F., Madkour, M. H., Pieper-Furst, U., Wieczorek, R., Liebergesell, M., and Steinbuchel, A. (1996). Electron microscopic observations on the macromolecular organization of the boundary layer of bacterial PHA inclusion bodies. J. Gen. Appl. Microbiol. 42, 445–455. doi: 10.2323/jgam.42.445
McMahon, K. D., Yilmaz, S., He, S., Gall, D. L., Jenkins, D., and Keasling, J. D. (2007). Polyphosphate kinase genes from full-scale activated sludge plants. Appl. Microbiol. Biotechnol. 77, 167–173. doi: 10.1007/s00253-007-1122-6
Metcalf Eddy, Inc. (2003). Wastewater Engineering - Treatment and Reuse, 4th Edn. New York, NY: Mc Graw Hill.
Mino, T., Arun, V., Tsuzuki, Y., and Matsuo, T. (1987). “Effect of phosphorus accumulation on acetate metabolism in the biological phosphorus removal process,” in Biological Phosphate Removal from Wastewaters, Advances in Water Pollution Control, R. Ramadori (Oxford: Pergamon Press), 27–38.
Mino, T., van Loosdrecht, M. C. M., and Heijnen, J. J. (1998). Microbiology and biochemistry of the enhanced biological phosphate removal process. Water Res. 32, 3193–3207. doi: 10.1016/S0043-1354(98)00129-8
Murnleitner, E., Kuba, T., van Loosdrecht, M. C. M., and Heijnen, J. J. (1996). An integrated metabolic model for aerobic and denitrifying biological phosphorus removal. Biotechnol. Bioeng. 54, 434–450. doi: 10.1002/(SICI)1097-0290(19970605)54:5<434::AID-BIT4>3.0.CO;2-F
Oehmen, A., Zeng, R. J., Yuan, Z., and Keller, J. (2005). Anaerobic metabolism of propionate by polyphosphate-accumulating organisms in enhanced biological phosphorus removal systems. Biotechnol. Bioeng. 91, 43–53. doi: 10.1002/bit.20480
Pereira, H., Lemos, P. C., Reis, M. A. M., Crespo, J. P. S. G., Carrondo, M. J. T., and Santos, H. (1996). Model for carbon metabolism in biological phosphorus removal processes based on in vivo C13-NMR labelling experiments. Water Res. 30:2128. doi: 10.1016/0043-1354(96)00035-8
Pieper-Fürst, U., Madkour, M. H., Mayer, F., and Steinbüchel, A. (1994). Purification and characterization of a 14-kilodalton protein that is bound to the surface of polyhydroxyalkanoic acid granules in Rhodococcus ruber. J. Bacteriol. 176, 4328–4337. doi: 10.1128/jb.176.14.4328-4337.1994
Pirt, S. J. (1965). The maintenance energy of bacteria in growing cultures. Proc. R. Soc. Lond. B. Biol. Sci. 163, 224–231. doi: 10.1098/rspb.1965.0069
Satoh, H., Mino, T., and Matsuo, T. (1992). Uptake of organic substrates and accumulation of polyhydroxyalkanoates linked with glycolysis of intracellular carbohydrates under anaerobic conditions in the biological excess phosphate removal processes. Water Sci. Technol. 26, 933–942.
Satoh, H., Mino, T., and Matsuo, T. (1994). Deterioration of Enhanced Biological Phosphorus removal by the domination of microorganisms without polyphosphate accumulation. Wat. Sci. Technol. 30, 203–211.
Saunders, A. M., Mabbett, A. N., McEwan, A. G., and Blackall, L. L. (2007). Proton motive force generation from stored polymers for the uptake of acetate under anaerobic conditions. FEMS Microbiol. Lett. 274, 245–251. doi: 10.1111/j.1574-6968.2007.00839.x
Schuler, A. J., and Jenkins, D. (2003a). Enhanced biological phosphorus removal from wastewater by biomass with different phosphorus contents, part 1: experimental results and comparison with metabolic models. Water Environ. Res. 75, 485–498. doi: 10.2175/106143003X141286
Schuler, A. J., and Jenkins, D. (2003b). Enhanced biological phosphorus removal from wastewater by biomass with different phosphorus contents, part 2: anaerobic adenosine triphosphate utilization and acetate uptake rates. Water Environ. Res. 75, 499–511. doi: 10.2175/106143003X141295
Schuler, A. J., and Jenkins, D. (2003c). Enhanced biological phosphorus removal from wastewater by biomass with different phosphorus contents, part III: anaerobic sources of reducing equivalents. Water Environ. Res. 75, 512–522.
Slater, F. R., Johnson, C. R., Blackall, L. L., Beiko, R. G., and Bond, P. L. (2010). Monitoring associations between clade-level variation, overall community structure and ecosystem function in enhanced biological phosphorus removal (EBPR) systems using terminal-restriction fragment length polymorphism (T-RFLP). Water Res. 44, 4908–4923. doi: 10.1016/j.watres.2010.07.028
Smolders, G. J. F., Van der Meij, J., Van Loosdrecht, M. C. M., and Heijnen, J. J. (1994). Model of the anaerobic metabolism of the biological phosphorus removal process: stoichiometry and pH influence. Biotechnol. Bioeng. 43, 461–470. doi: 10.1002/bit.260430605
Smolders, G. J. F., van Loosdrecht, M. C. M., and Heijnen, J. J. (1995). A metabolic model for the biological phosphorus removal process. Water Sci. Technol. 31, 79–97. doi: 10.1016/0273-1223(95)00182-M
Steinbüchel, A., Aerts, K., Babel, W., Föllner, C., Liebergesell, M., Madkour, M. H., et al. (1995). Considerations on the structure and biochemistry of bacterial polyhydroxyalkanoic acid inclusions. Can. J. Microbiol. 41, 94–105. doi: 10.1139/m95-175
Sudiana, I., Mino, T., Satoh, H., Nakamura, K., and Matsuo, T. (1999). Metabolism of enhanced biological phosphorus removal and non-enhanced biological phosphorus removal sludge with acetate and glyucose as carbon source. Water Sci. Technol. 39:29. doi: 10.1016/S0273-1223(99)00141-9
Van Groenestijn, J. W., Deinema, M. H., and Zehnder, A. J. B. (1987). ATP production from polyphosphate in Acinetobacter strain 210A. Arch. Microbiol. 148, 14–19. doi: 10.1007/BF00429640
Van Niel, E. W. J., Appeldoorn, K. J., Zehnder, A. J. B., and Kortstee, G. J. J. (1998). Inhibition of anaerobic phosphate release by nitric oxide in activated sludge. Appl. Environ. Microbiol. 64, 2925–2930.
Van Veen, H. W., Abee, T., Kortstee, G. J., Pereira, H., Konings, W. N., and Zehnder, A. J. (1994). Generation of a proton motive force by the excretion of metal-phosphate in the polyphosphate-accumulating Acinetobacter johnsonii strain 210A. J. Biol. Chem. 269, 29509–29514.
Welles, L., Lopez-Vazquez, C. M., Hooijmans, C. M., Van Loosdrecht, M. C. M., and Brdjanovic, D. (2014). Impact of salinity on the anaerobic metabolism of phosphate-accumulating organisms (PAO) and glycogen-accumulating organisms (GAO). Appl. Microbiol. Biotechnol. 98, 7609–7622. doi: 10.1007/s00253-014-5778-4
Welles, L., Lopez-Vazquez, C. M., Hooijmans, C. M., van Loosdrecht, M. C. M., and Brdjanovic, D. (2015a). Impact of salinity on the aerobic metabolism of phosphate-accumulating organisms. Appl. Microbiol. Biotechnol. 99, 3659–3672. doi: 10.1007/s00253-014-6287-1
Welles, L., Tian, W. D., Saad, S., Abbas, B., Lopez-Vazquez, C. M., Hooijmans, C. M., et al. (2015b). Accumulibacter clades Type I. and II performing kinetically different glycogen-accumulating organisms metabolisms for anaerobic substrate uptake. Water Res. 83, 354–366. doi: 10.1016/j.watres.2015.06.045
Wentzel, M. C., Dold, P. L., Ekama, G. A., and Marais, G. (1989b). Enhanced polyphosphate organism cultures in activated sludge systems. Part III: kinetic model. Water, S. A. 15, 89–102.
Wentzel, M. C., Dold, P. L., Ekama, G. A., and Marais, G. V. R. (1985). Kinetics of biological phosphorus release. Water Sci. Technol. 17, 57–71.
Wentzel, M. C., Dold, P. L., Loewenthal, R. E., Ekama, G. A., and Marais, G. V. R. (1987). “Experiments towards establishing the kinetics of biological excess phosphorus removal,” in Advances in Water Pollution Control: Biological Phosphate Removal from Wastewaters, ed R. Ramadori (Oxford: Pergamon Press), 79–91.
Wentzel, M. C., Ekama, G. A., Loewenthal, R. E., Dold, P. L., and Marais, G. (1989a). Enhanced polyphosphate organism cultures in activated sludge systems. Part II: experimental behaviour. Water S. A. 15, 71–88.
Wentzel, M. C., Loewenthal, R. E., Ekama, G. A., and Marais, G. V. R. (1988). Enhanced polyphosphate organism cultures in activated sludge systems- Part 1: enhanced culture development. Water S.A. 14, 81–92.
Wilmes, P., Andersson, A. F., Lefsrud, M. G., Wexler, M., Shah, M., Zhang, B., et al. (2008). Community proteogenomics highlights microbial strain-variant protein expression within activated sludge performing enhanced biological phosphorus removal. ISME J. 2, 853–864. doi: 10.1038/ismej.2008.38
Winkler, M. K., Bassin, J. P., Kleerebezem, R., De Bruin, L. M., Van den Brand, T. P. H., and Van Loosdrecht, M. C. M. (2011). Selective sludge removal in a segregated aerobic granular biomass system as a strategy to control PAO–GAO competition at high temperatures. Water Res. 45, 3291–3299. doi: 10.1016/j.watres.2011.03.024
Zeng, R. J., van Loosdrecht, M. C. M., Yuan, Z., and Keller, J. (2003). Metabolic model for glycogen-accumulating organisms in anaerobic/aerobic activated sludge systems. Biotechnol. Bioeng. 81, 92–105. doi: 10.1002/bit.10455
Keywords: polyphosphate-accumulating organisms (PAO), glycogen-accumulating organisms (GAO), phosphate limitation, metabolic response, microbial population dynamics
Citation: Welles L, Abbas B, Sorokin DY, Lopez-Vazquez CM, Hooijmans CM, van Loosdrecht MCM and Brdjanovic D (2017) Metabolic Response of “Candidatus Accumulibacter Phosphatis” Clade II C to Changes in Influent P/C Ratio. Front. Microbiol. 7:2121. doi: 10.3389/fmicb.2016.02121
Received: 19 August 2016; Accepted: 15 December 2016;
Published: 05 January 2017.
Edited by:
William James Hickey, University of Wisconsin-Madison, USAReviewed by:
Naresh Singhal, University of Auckland, New ZealandDaniel Puyol, King Juan Carlos University, Spain
Copyright © 2017 Welles, Abbas, Sorokin, Lopez-Vazquez, Hooijmans, van Loosdrecht and Brdjanovic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laurens Welles, laurenswelles@gmail.com
 Laurens Welles
Laurens Welles Ben Abbas2
Ben Abbas2 Dimitry Y. Sorokin
Dimitry Y. Sorokin Mark C. M. van Loosdrecht
Mark C. M. van Loosdrecht