- 1Department of Otolaryngology-Head and Neck Surgery, Johns Hopkins University, Baltimore, MD, United States
- 2Laboratory of Behavioral Neuroscience, National Institute on Aging, Baltimore, MD, United States
- 3Department of Radiology and Radiological Sciences, Johns Hopkins University, Baltimore, MD, United States
- 4Department of Otolaryngology-Head and Neck Surgery, Division of Otology, Neurotology, and Skull Base Surgery, Johns Hopkins University, Baltimore, MD, United States
Beta-amyloid (Aβ) plaque deposition is a key feature of Alzheimer’s disease (AD), and occurs years before the onset of symptoms. Aβ plaque deposition has been shown to be present in ~30% of cognitively normal older adults using amyloid C-11 labeled Pittsburgh Compound B (11C-PiB) Positron Emission Tomography (PET) imaging. Prior studies have reported a link between reduced vestibular function and poorer cognition in healthy older adults. It is unknown whether vestibular impairment occurs in association with AD pathology among individuals in the preclinical phase of AD, which could contribute to the observed association between vestibular and cognitive function in healthy older adults. Using the Baltimore Longitudinal Study of Aging (BLSA), we analyzed the association between a comprehensive set of vestibular function measures and PiB status in 98 healthy participants with a mean age of 77.3 (±8.26). We did not observe a significant relationship between any vestibular function measure and PiB status in cognitively-intact older adults in the BLSA. This finding suggests that Aβ deposition does not explain the observed association between reduced vestibular function and poorer cognition in healthy older adults.
Introduction
Alzheimer’s disease (AD) is a neurodegenerative pathology that leads to memory loss and behavioral changes, and is ultimately fatal (Alzheimer’s Association, 2016). Beta-amyloid (Aβ) plaque deposition is a key feature of AD that occurs years before the onset of symptoms and is associated with brain cell death (Jack et al., 2018). One hypothesis for the pathogenesis of AD is that excessive extracellular Aβ accumulation causes synaptic dysfunction due to either a breakdown of Aβ clearance or Aβ overproduction (Hardy and Selkoe, 2002; Barage and Sonawane, 2015). However, this amyloid cascade hypothesis is controversial (Hardy and Selkoe, 2002; van Dyck, 2018).
Postmortem studies and cerebral spinal fluid (CSF) analysis were the initial methods of investigating Aβ deposition, but the detection of Aβ in living patients with radiotracer ligands have increased in popularity over the last decade (Thal et al., 2006; Grimmer et al., 2009). C-11 labeled Pittsburgh Compound B (11C-PiB) was the first successful Positron Emission Tomography (PET) radiotracer allowing imaging of brain Aβ (Klunk et al., 2004; Rowe et al., 2007; Rabinovici and Jagust, 2009; Quigley et al., 2011; Clark et al., 2012; Driscoll et al., 2012). The increased use of PiB imaging has led to the observation that Aβ is also present in approximately one-third of cognitively-normal older adults (Mintun et al., 2006; Resnick et al., 2010; Aizenstein et al., 2008). Increased Aβ deposition in cognitively normal adults has been associated with increased risk of decline on cognitive measures of visuospatial function, episodic and semantic memory, and mental status and increased risk of progression to AD (Mintun et al., 2006; Pike et al., 2007; Resnick et al., 2010; Vlassenko et al., 2011; Rowe et al., 2013; Baker et al., 2016).
Numerous studies suggest that impairment of the vestibular (inner ear balance) system is associated with poorer cognitive function in healthy older adults (Bigelow and Agrawal, 2015; Semenov et al., 2016). The vestibular system plays a critical role in balance, gait, and spatial orientation, and reduced vestibular function in healthy older adults has been linked to poorer spatial cognitive abilities (Schautzer et al., 2003; Agrawal et al., 2009, 2013; Bigelow and Agrawal, 2015; Bigelow et al., 2015). Moreover, recent evidence has shown that patients with AD are significantly more likely to have vestibular impairment relative to age-matched controls (Previc, 2013; Nakamagoe et al., 2015; Harun et al., 2016; Cronin et al., 2017). Similarly, AD patients with vestibular impairment are more likely to have impaired spatial cognition, as measured by neurocognitive tests as well as behaviors suggestive of impaired spatial cognition, such as difficulty driving (Liu et al., 1991; Binetti et al., 1998; Rainville et al., 2002; Leandri et al., 2009; Birdane et al., 2012; Versijpt et al., 2017). Vestibular loss may be specifically related to a “spatial” subtype of AD (Henderson et al., 1989).
At present, the nature of the association between vestibular loss, cognitive impairment and AD is unknown. Peripheral vestibular loss has been hypothesized to cause cognitive decline and AD, given the dense cholinergic inputs from the peripheral vestibular system to the medial temporal region and hippocampus, which are among the first to be degraded in AD (Previc, 2013; Besnard et al., 2015; Semenov et al., 2016). Alternatively, AD neuropathology (e.g., Aβ deposition), which is present in AD patients as well as in a subset of cognitively-intact adults, could be a common factor that explains both cognitive decline and vestibular physiologic abnormalities (e.g., by disruption of central vestibular pathways; Rodrigue et al., 2009; Braskie et al., 2010). However, it is unclear if there are any molecular or cellular links of vestibular function and Aβ deposition. In this study, we sought to answer this question by evaluating the cross-sectional association between vestibular function and Aβ deposition in a cohort of healthy older adults in the Baltimore Longitudinal Study of Aging (BLSA).
Materials and Methods
Participants
Ninety-eight participants were selected from the BLSA, a long-running study of aging supported by the National Institute on Aging. There were 51 female and 47 male participants with a mean age of 77.3 (±8.26). PiB-PET scans were initiated in 2005 as part of a neuroimaging sub-study within the BLSA (Resnick et al., 2000). This sub-study enrolled BLSA participants with no known brain disease (dementia, mental illness, stroke, seizures), severe cardiac or pulmonary disease, or metastatic cancer. Vestibular Function Testing was initiated in 2013. Eligible participants were age ≥55 years and had PiB-PET scan and Vestibular Physiologic Testing performed at the same study visit. All participants provided written informed consent, and the BLSA study protocol was approved by the National Institute of Environmental Health Sciences Institutional Review Board and the PET studies were approved by the Johns Hopkins Medicine Institutional Review Board.
Image Acquisition and Processing
Dynamic PiB-PET scans were obtained using a GE Advance scanner in 3D mode directly after 15 mCi of [11C]-PiB was injected intravenously (Bilgel et al., 2016). Participants wore a thermoplastic head mask to decrease motion during the scan. PET scans were acquired according to the following protocol for frame duration: 4 × 0.25, 8 × 0.5, 9 × 1, 2 × 3, 10 × 5 min (70 min total, 33 frames). Filtered back-projection with a ramp filter, which yielded a spatial resolution of approximately 4.5 mm full width at half max at the center of the field of view (image matrix = 128 × 128, 35 slices, pixel size = 2 × 2 mm, slice thickness = 4.25 mm), was used to reconstruct images. Magnetization prepared rapid gradient echo (MPRAGE) images were obtained using a 3T scanner [Philips Achieva, repetition time (TR) = 6.8 ms, echo time (TE) = 3.2 ms, flip angle 8°, image matrix = 256 × 256, 170 slices, pixel size = 1 × 1 mm, slice thickness = 1.2 mm]. For each participant, a concurrent or closest-in-time MRI scan was matched with each PiB-PET image. Anatomical labels were obtained for each MRI scan using Multi-atlas region Segmentation using Ensembles of registration algorithms and parameters (MUSE; Doshi et al., 2016).
Each dynamic PET scan was aligned to the mean of the first 2 min of the scan to adjust for movement (Jenkinson et al., 2002). The average of the first 20 min of PET scans was rigidly registered onto the corresponding MRI, and the MUSE label image was transformed from MRI to PET space. Distribution volume ratio (DVR) images were computed in PET native space using a simplified reference tissue model (Zhou et al., 2007) with cerebellar gray matter as the reference region. Mean cortical β-amyloid burden was calculated as the average of the DVR values in cingulate, frontal, parietal (including precuneus), lateral temporal, and lateral occipital cortical regions, excluding the sensorimotor strip.
Pittsburgh Compound B (PiB) Status
PiB imaging has been commonly dichotomized into positive and negative status using a mean cortical distribution volume (cDVR; Klunk et al., 2004; Lopresti et al., 2005; Villeneuve et al., 2015; Bilgel et al., 2016). Mean cDVR was calculated as the average of the DVR values in cingulate, frontal, parietal (including precuneus), lateral temporal, and lateral occipital cortical regions, excluding the sensorimotor strip. PiB positive vs. negative status was determined using a two-class Gaussian mixture model fitted to the baseline mean cDVR data. The DVR corresponding to the intersection of the probability density functions of the two classes was used to categorize subjects into PiB+ and PiB−. The cutoff for separating PiB+ and PiB− was 1.064 in this study.
Vestibular Function Testing
Vestibular Physiologic Testing included assessment of saccular function using the cervical vestibular-evoked myogenic potential (cVEMP) test and utricular function using the ocular vestibular-EMP (oVEMP) test. Video head-impulse testing (VHIT) was used to assess semicircular canal function to determine a vestibular-ocular reflex (VOR) gain. Test procedures are discussed briefly in Supplementary Material S1.
Statistical Analysis
Baseline demographics and Vestibular Testing were compared between PiB+ and PiB− groups using t-tests for continuous variables and Fisher’s exact tests for binary variables. Multiple linear and logistic regression models adjusted for age and sex were used. Linear regression models were used for continuous outcomes (e.g., VEMP amplitudes, VOR gain) while logistic regression models were used for binary outcomes (e.g., present vs. absent VEMP responses). Statistical analyses were conducted using R version 3.4.11. We used an alpha-level of 0.05 to determine statistical significance.
Results
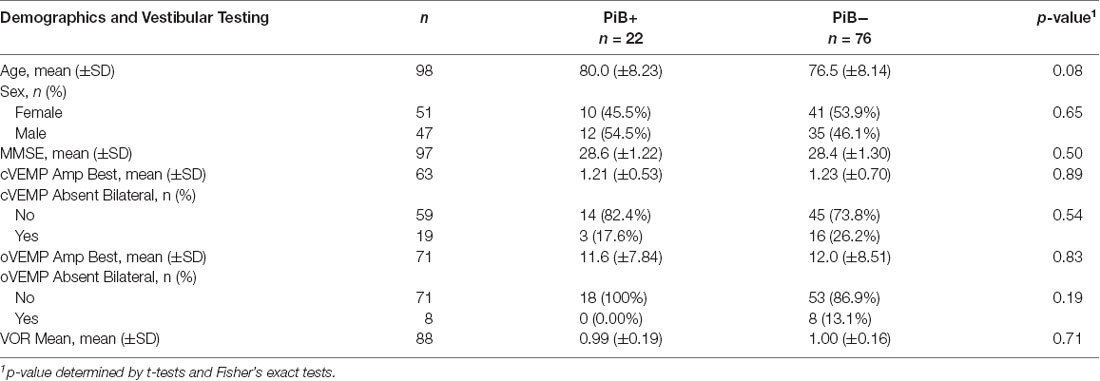
The cohort included 98 participants with PiB-PET scans and Vestibular Testing (Table 1). There were 22 PiB+ (22.4%) and 76 PiB− (77.6%) participants. There were no significant differences in demographic characteristics between PiB+ and PiB− participants. Mental status (measured by the MMSE score) also did not differ significantly between PiB+ and PiB− participants (28.6 ±1.22 in PiB+ and 28.4 ±1.30 in PiB−). Additionally, there were no significant differences in vestibular test results in univariate comparisons between the PiB+ and PiB− groups (Table 1).
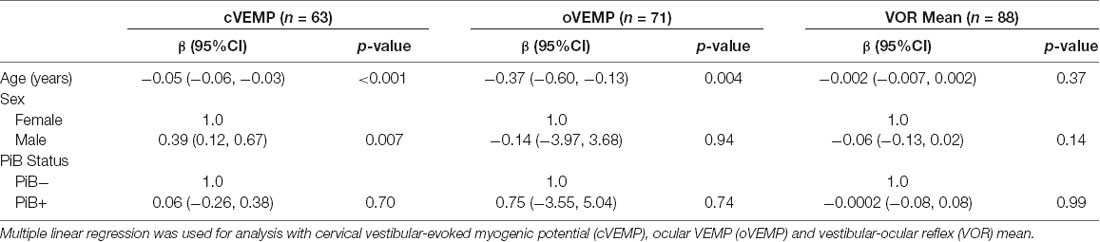
Next, we used multiple linear regression models adjusting for age and sex to evaluate the association between PiB status as the independent variable of interest and vestibular physiologic function as the outcome of interest (Table 2). Neither cVEMP amplitude, oVEMP amplitude, nor VOR gain was significantly related to PiB status. In logistic regression analyses, the odds of having bilaterally absent cVEMPs was not significantly different between PiB+ and PiB− participants (OR 0.83, p = 0.28; data not shown). We could not complete an analysis of bilaterally absent oVEMPs because there were no PiB+ participants with bilaterally absent oVEMPs.
Discussion
We did not observe a significant relationship between PiB status and vestibular function in 98 cognitively intact older adults in the BLSA. This finding suggests that CNS pathology due to Aβ deposition may not explain the observed association between reduced vestibular function and poorer cognition in healthy older adults. In prior work, we found that patients with AD were significantly more likely to have vestibular loss compared to healthy older adults (Harun et al., 2016). Although we did not evaluate AD patients in this study, it is possible that vestibular loss may be related to the broader category of AD and related dementias (ADRD) via mechanisms independent of AD pathology (specifically Aβ deposition).
Limitations of this study include the small number of PiB+ participants, although the prevalence is in line with prior studies of healthy older adults (Aizenstein et al., 2008; Resnick et al., 2010; Johnson et al., 2013). Although the vestibular function testing we used has been used in numerous studies, it is possible that the Vestibular Testing used in this study may not be sensitive enough to detect vestibular dysfunction in this patient population. Although to our knowledge this cohort of 98 participants with both PiB imaging and Vestibular Physiologic Testing is the largest to date, future studies in larger numbers of participants will provide more definitive evidence. This study suggests that there is insufficient evidence linking vestibular loss and AD pathology (specifically Aβ deposition) in healthy older adults. Other possible mediators of the observed association between vestibular function and AD could include Tau or other age-related co-morbidities.
Author Contributions
RK and YA provided substantial contributions to the conception or design of the work and interpretation of data for the work, drafting it, and revising it for intellectual content. MB and SR provided substantial contributions to the acquisition, analysis or interpretation of data for the work, and revising it for intellectual content. DW provided substantial contributions to the acquisition of data for the work.
Funding
YA is supported by NIH NIDCD K23-DC013056. The Baltimore Longitudinal Study of Aging (BLSA) is supported by NIA award (1ZIAAG000015-57). This work was supported in part by the Intramural Research Program of the National Institute on Aging, NIH.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2018.00408/full#supplementary-material
References
Agrawal, Y., Ward, B. K., and Minor, L. B. (2013). Vestibular dysfunction: prevalence, impact and need for targeted treatment. J. Vestib. Res. 23, 113–117. doi: 10.3233/VES-130498
Agrawal, Y., Carey, J. P., Della Santina, C. C., Schubert, M. C., and Minor, L. B. (2009). Disorders of balance and vestibular function in US adults: data from the national health and nutrition examination survey, 2001–2004. Arch. Intern. Med. 169, 938–944. doi: 10.1001/archinternmed.2009.66
Aizenstein, H. J., Nebes, R. D., Saxton, J. A., Price, J. C., Mathis, C. A., Tsopelas, N. D., et al. (2008). Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch. Neurol. 65, 1509–1517. doi: 10.1001/archneur.65.11.1509
Alzheimer’s Association. (2016). 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 12, 459–509. doi: 10.1016/j.jalz.2016.03.001
Baker, J. E., Lim, Y. Y., Pietrzak, R. H., Hassenstab, J., Snyder, P. J., Masters, C. L., et al. (2016). Cognitive impairment and decline in cognitively normal older adults with high amyloid-β: a meta-analysis. Alzheimers Dement. 6, 108–121. doi: 10.1016/j.dadm.2016.09.002
Barage, S. H., and Sonawane, K. D. (2015). Amyloid cascade hypothesis: pathogenesis and therapeutic strategies in Alzheimer’s disease. Neuropeptides 52, 1–18. doi: 10.1016/j.npep.2015.06.008
Besnard, S., Lopez, C., Brandt, T., Denise, P., and Smith, P. F. (2015). Editorial: the vestibular system in cognitive and memory processes in mammalians. Front. Integr. Neurosci. 9:55. doi: 10.3389/fnint.2015.00055
Bigelow, R. T., and Agrawal, Y. (2015). Vestibular involvement in cognition: visuospatial ability, attention, executive function, and memory. J. Vestib. Res. 25, 73–89. doi: 10.3233/VES-150544
Bigelow, R. T., Semenov, Y. R., Trevino, C., Ferrucci, L., Resnick, S. M., Simonsick, E. M., et al. (2015). Association between visuospatial ability and vestibular function in the baltimore longitudinal study of aging. J. Am. Geriatr. Soc. 63, 1837–1844. doi: 10.1111/jgs.13609
Bilgel, M., An, Y., Zhou, Y., Wong, D. F., Prince, J. L., Ferrucci, L., et al. (2016). Individual estimates of age at detectable amyloid onset for risk factor assessment. Alzheimers Dement. 12, 373–379. doi: 10.1016/j.jalz.2015.08.166
Binetti, G., Cappa, S. F., Magni, E., Padovani, A., Bianchetti, A., and Trabucchi, M. (1998). Visual and spatial perception in the early phase of Alzheimer’s disease. Neuropsychology 12, 29–33. doi: 10.1037/0894-4105.12.1.29
Birdane, L., Incesulu, A., Gurbuz, M. K., and Ozbabalik, D. (2012). Sacculocolic reflex in patients with dementia: is it possible to use it for early diagnosis? Neurol. Sci. 33, 17–21. doi: 10.1007/s10072-011-0595-3
Braskie, M. N., Klunder, A. D., Hayashi, K. M., Protas, H., Kepe, V., Miller, K. J., et al. (2010). Plaque and tangle imaging and cognition in normal aging and Alzheimer’s disease. Neurobiol. Aging 31, 1669–1678. doi: 10.1016/j.neurobiolaging.2008.09.012
Clark, V. H., Resnick, S. M., Doshi, J., Beason-Held, L. L., Zhou, Y., Ferrucci, L., et al. (2012). Longitudinal imaging pattern analysis (SPARE-CD Index) detects early structural and functional changes before cognitive decline in healthy older adults. Neurobiol. Aging 33, 2733–2745. doi: 10.1016/j.neurobiolaging.2012.01.010
Cronin, T., Arshad, Q., and Seemungal, B. M. (2017). Vestibular deficits in neurodegenerative disorders: balance, dizziness, and spatial disorientation. Front. Neurol. 8:538. doi: 10.3389/fneur.2017.00538
Doshi, J., Erus, G., Ou, Y., Resnick, S. M., Gur, R. C., Gur, R. E., et al. (2016). MUSE: MUlti-atlas region segmentation utilizing ensembles of registration algorithms and parameters, and locally optimal atlas selection. Neuroimage 127, 186–195. doi: 10.1016/j.neuroimage.2015.11.073
Driscoll, I., Troncoso, J. C., Rudow, G., Sojkova, J., Pletnikova, O., Zhou, Y., et al. (2012). Correspondence between in vivo 11C-PiB-PET amyloid imaging and postmortem, region-matched assessment of plaques. Acta Neuropathol. 124, 823–831. doi: 10.1007/s00401-012-1025-1
Grimmer, T., Riemenschneider, M., Förstl, H., Henriksen, G., Klunk, W. E., Mathis, C. A., et al. (2009). β amyloid in Alzheimer’s disease: increased deposition in brain is reflected in reduced concentration in cerebrospinal fluid. Biol. Psychiatry 65, 927–934. doi: 10.1016/j.biopsych.2009.01.027
Hardy, J., and Selkoe, D. J. (2002). The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356. doi: 10.1126/science.1072994
Harun, A., Oh, E. S., Bigelow, R. T., Studenski, S., and Agrawal, Y. (2016). Vestibular impairment in dementia. Otol. Neurotol. 37, 1137–1142. doi: 10.1097/MAO.0000000000001157
Henderson, V. W., Mack, W., and Williams, B. W. (1989). Spatial disorientation in Alzheimer’s disease. Arch. Neurol. 46, 391–394. doi: 10.1001/archneur.1989.00520400045018
Jack, C. R. Jr., Bennett, D. A., Blennow, K., Carrillo, M. C., Dunn, B., Haeberlein, S. B., et al. (2018). NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14, 535–562. doi: 10.1016/j.jalz.2018.02.018
Jenkinson, M., Bannister, P., Brady, M., and Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841. doi: 10.1016/s1053-8119(02)91132-8
Johnson, K. A., Sperling, R. A., Gidicsin, C. M., Carmasin, J. S., Maye, J. E., Coleman, R. E., et al. (2013). Florbetapir (F18-AV-45) PET to assess amyloid burden in Alzheimer’s disease dementia, mild cognitive impairment, and normal aging. Alzheimers Dement. 9, S72–S83. doi: 10.1016/j.jalz.2012.10.007
Klunk, W. E., Engler, H., Nordberg, A., Wang, Y., Blomqvist, G., Holt, D. P., et al. (2004). Imaging brain amyloid in Alzheimer’s disease with pittsburgh compound-B. Ann. Neurol. 55, 306–319. doi: 10.1002/ana.20009
Leandri, M., Cammisuli, S., Cammarata, S., Baratto, L., Campbell, J., Simonini, M., et al. (2009). Balance features in Alzheimer’s disease and amnestic mild cognitive impairment. J. Alzheimers Dis. 16, 113–120. doi: 10.3233/jad-2009-0928
Liu, L., Gauthier, L., and Gauthier, S. (1991). Spatial disorientation in persons with early senile dementia of the Alzheimer type. Am. J. Occup. Ther. 45, 67–74. doi: 10.5014/ajot.45.1.67
Lopresti, B. J., Klunk, W. E., Mathis, C. A., Hoge, J. A., Ziolko, S. K., Lu, X., et al. (2005). Simplified quantification of pittsburgh compound B amyloid imaging PET studies: a comparative analysis. J. Nucl. Med. 46, 1959–1972.
Mintun, M. A., Larossa, G. N., Sheline, Y. I., Dence, C. S., Lee, S. Y., Mach, R. H., et al. (2006). [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 67, 446–452. doi: 10.1212/01.wnl.0000228230.26044.a4
Nakamagoe, K., Fujimiya, S., Koganezawa, T., Kadono, K., Shimizu, K., Fujizuka, N., et al. (2015). Vestibular function impairment in Alzheimer’s disease. J. Alzheimers Dis. 47, 185–196. doi: 10.3233/JAD-142646
Pike, K. E., Savage, G., Villemagne, V. L., Ng, S., Moss, S. A., Maruff, P., et al. (2007). β-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain 130, 2837–2844. doi: 10.1093/brain/awm238
Previc, F. H. (2013). Vestibular loss as a contributor to Alzheimer’s disease. Med. Hypotheses 80, 360–367. doi: 10.1016/j.mehy.2012.12.023
Quigley, H., Colloby, S. J., and O’Brien, J. T. (2011). PET imaging of brain amyloid in dementia: a review. Int. J. Geriatr. Psychiatry 26, 991–999. doi: 10.1002/gps.2640
Rabinovici, G. D., and Jagust, W. J. (2009). Amyloid imaging in aging and dementia: testing the amyloid hypothesis in vivo. Behav. Neurol. 21, 117–128. doi: 10.3233/BEN-2009-0232
Rainville, C., Marchand, N., and Passini, R. (2002). Performances of patients with a dementia of the Alzheimer type in the standardized road-map test of direction sense. Neuropsychologia 40, 567–573. doi: 10.1016/s0028-3932(01)00133-6
Resnick, S. M., Goldszal, A. F., Davatzikos, C., Golski, S., Kraut, M. A., Metter, E. J., et al. (2000). One-year age changes in MRI brain volumes in older adults. Cereb. Cortex 10, 464–472. doi: 10.1093/cercor/10.5.464
Resnick, S. M., Sojkova, J., Zhou, Y., An, Y., Ye, W., Holt, D. P., et al. (2010). Longitudinal cognitive decline is associated with fibrillar amyloid-β measured by [11C]PiB. Neurology 74, 807–815. doi: 10.1212/WNL.0b013e3181d3e3e9
Rodrigue, K. M., Kennedy, K. M., and Park, D. C. (2009). β-amyloid deposition and the aging brain. Neuropsychol. Rev. 19, 436–450. doi: 10.1007/s11065-009-9118-x
Rowe, C. C., Bourgeat, P., Ellis, K. A., Brown, B., Lim, Y. Y., Mulligan, R., et al. (2013). Predicting Alzheimer disease with β-amyloid imaging: results from the australian imaging, biomarkers, and lifestyle study of ageing. Ann. Neurol. 74, 905–913. doi: 10.1002/ana.24040
Rowe, C. C., Ng, S., Ackermann, U., Gong, S. J., Pike, K., Savage, G., et al. (2007). Imaging β-amyloid burden in aging and dementia. Neurology 68, 1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea
Schautzer, F., Hamilton, D., Kalla, R., Strupp, M., and Brandt, T. (2003). Spatial memory deficits in patients with chronic bilateral vestibular failure. Ann. N Y Acad. Sci. 1004, 316–324. doi: 10.1196/annals.1303.029
Semenov, Y. R., Bigelow, R. T., Xue, Q. L., du Lac, S., and Agrawal, Y. (2016). Association between vestibular and cognitive function in U.S. adults: data from the national health and nutrition examination survey. J. Gerontol. A Biol. Sci. Med. Sci. 71, 243–250. doi: 10.1093/gerona/glv069
Thal, D. R., Capetillo-Zarate, E., Del Tredici, K., and Braak, H. (2006). The development of amyloid β protein deposits in the aged brain. Sci. Aging Knowledge Environ. 2006:re1. doi: 10.1126/sageke.2006.6.re1
van Dyck, C. H. (2018). Anti-amyloid-β monoclonal antibodies for Alzheimer’s disease: pitfalls and promise. Biol. Psychiatry 83, 311–319. doi: 10.1016/j.biopsych.2017.08.010
Versijpt, J., Tant, M., Beyer, I., Bier, J. C., Cras, P., De Deyn, P. P., et al. (2017). Alzheimer’s disease and driving: review of the literature and consensus guideline from belgian dementia experts and the belgian road safety institute endorsed by the belgian medical association. Acta Neurol. Belg. 117, 811–819. doi: 10.1007/s13760-017-0840-5
Villeneuve, S., Rabinovici, G. D., Cohn-Sheehy, B. I., Madison, C., Ayakta, N., Ghosh, P. M., et al. (2015). Existing pittsburgh compound-b positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain 138, 2020–2033. doi: 10.1093/brain/awv112
Vlassenko, A. G., Mintun, M. A., Xiong, C., Sheline, Y. I., Goate, A. M., Benzinger, L. T. S., et al. (2011). Amyloid-β plaque growth in cognitively normal adults: longitudinal PIB data. Ann. Neurol. 70, 857–861. doi: 10.1002/ana.22608
Keywords: vestibular function, Pittsburgh compound B, PiB, PET, Alzheimer’s disease, BLSA, older adults, beta-amyloid
Citation: Kamil RJ, Bilgel M, Wong DF, Resnick SM and Agrawal Y (2018) Vestibular Function and Beta-Amyloid Deposition in the Baltimore Longitudinal Study of Aging. Front. Aging Neurosci. 10:408. doi: 10.3389/fnagi.2018.00408
Received: 23 July 2018; Accepted: 28 November 2018;
Published: 11 December 2018.
Edited by:
Daniel Ortuño-Sahagún, Universidad de Guadalajara, MexicoReviewed by:
Ramesh Kandimalla, Texas Tech University Health Sciences Center, United StatesBrian J. Lopresti, University of Pittsburgh, United States
Copyright © 2018 Kamil, Bilgel, Wong, Resnick and Agrawal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rebecca J. Kamil, cmthbWlsMUBqaG1pLmVkdQ==
 Rebecca J. Kamil
Rebecca J. Kamil Murat Bilgel
Murat Bilgel Dean F. Wong
Dean F. Wong Susan M. Resnick
Susan M. Resnick Yuri Agrawal
Yuri Agrawal