- 1Sensorimotor Neuroscience Laboratory, Cognitive and Information Sciences, University of California, Merced, Merced, CA, United States
- 2Ahmanson-Lovelace Brain Mapping Center, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, United States
- 3Semel Institute for Neuroscience and Human Behavior, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, United States
- 4Department of Psychiatry and Biobehavioral Sciences, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, United States
- 5Brain Research Institute, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, United States
Corticospinal excitability (CSE) in humans measured with Transcranial Magnetic Stimulation (TMS) is generally increased by the perception of other people’s actions. This perception can be unimodal (visual or auditory) or multimodal (visual and auditory). The increase in TMS-measured CSE is typically prominent for muscles involved in the perceived action (muscle specificity). There are two main classes of accounts for this phenomenon. One suggests that the motor system mirrors the actions that the observer perceives (the resonance account). The other suggests that the motor system predicts the actions that the observer perceives (the predictive account). To test these accounts (which need not be mutually exclusive), subjects were presented with four versions of three-note piano sequences: sound only, sight only, audiovisual, and audiovisual with sound lagging behind (the prediction violation condition). CSE was measured in two hand muscles used to play the notes. CSE increased reliably in one muscle only for the prediction violation condition, in line with the predictive account, while the other muscle demonstrated CSE increase for all conditions, in line with the resonance account. This finding supports both predictive coding accounts as well as resonance accounts of motor facilitation during action perception.
Introduction
Motor regions of the brain are traditionally defined by their primary role in motor control (i.e., coding goals, planning, coordinating, and executing actions) but motor areas additionally play a role in the perception of others’ actions (e.g., Hari et al., 1998; Buccino et al., 2001; Aziz-Zadeh et al., 2004; Fadiga et al., 2005). A common measurement used for detecting motor activation is transcranial magnetic stimulation (TMS) -induced motor-evoked potentials (MEPs), which reflects the level of corticospinal excitability (CSE) at the time of stimulation. MEPs have high temporal resolution, allowing for a precise measure of activity modulation. Increased CSE is found during visual perception of actions (Fadiga et al., 1995) as well as auditory perception of actions (Kohler et al., 2002; Aziz-Zadeh et al., 2004). This increase is thought to reflect the recruitment of the mirror neuron system (Gallese et al., 1996), which is active both during action observation and action execution for similar actions, suggesting its involvement in the understanding of others’ actions (e.g., Fogassi et al., 2005; Iacoboni et al., 2005; Kaplan and Iacoboni, 2007; Gallese, 2008; Kilner, 2011). Populations of mirror neurons have been uncovered in premotor cortex that discharge upon observation and execution of the same action (Rizzolatti et al., 2001; Ferrari et al., 2003). The increased excitability in action perception is additionally time-dependent and effector-specific relative to the action being observed. Gangitano et al. (2001) recorded MEPs from the first dorsal interosseous (FDI) muscle during observation of a cyclic hand movement, and found that at the time when the observed finger aperture was at its maximum, MEPs recorded from the observer’s FDI muscle were highest. When the observed finger aperture was at its minimum, MEPs were lowest. Thus, the cortical motor areas of an observer are recruited for motor simulation of others’ actions in synchrony with those actions, and this is specific to the same muscle involved in the action.
Motor activity during action observation is also referred to as motor resonance, due to its time-dependent and effector-specific nature. The motor system of the observer “resonates” with that of the actor, allowing the observer to use their own body to understand, from within, the action being performed. One unanswered question is whether action observation is an active and predictive top-down process or a more automatic, bottom-up process. Early accounts, such as the direct-matching hypothesis (Iacoboni et al., 1999), suggest that motor resonance arises by directly perceiving an action via automatic activation in the observer of the cortical areas that represent the execution of that action. More recent theories propose that the mirror neuron system may function as a prediction mechanism during observation of others’ actions (Kilner et al., 2007). This differs significantly from the traditional assumptions of simulation (Gallese and Goldman, 1998), where the body of the observer “resonates” with the observed action but does not actively predict the future states of the movements. Active prediction proposes that top-down mechanisms influence the increase in CSE during observation, as a way to follow along with and actively predict another’s movements. It may be that mirror system activity actually reflects this predictive process, as the brain uses what it knows about the motor system of the observed actor to project the future state of the actor’s body. Because this type of prediction is very similar to that in motor control, the same neural systems (i.e., the motor system, mirror system) will underlie this process. Essentially, an observer can predict the motor commands of an observed actor given the expectations about their goal, and the implemented kinematics of that movement can be predicted using the observer’s own motor system.
Increased activity in motor areas has been observed in pianists when listening to piano pieces and observing piano playing (e.g., Haueisen and Knösche, 2001; Bangert and Altenmüller, 2003; Meister et al., 2004; Haslinger et al., 2005; Bangert et al., 2006; D’Ausilio et al., 2006), suggesting involvement of the mirror neuron system during music perception. Researchers have thus proposed that music is not passively heard, but actively perceived as the expressive motor acts that caused the music and are instantiated in the mirror neuron system (Molnar-Szakacs and Overy, 2006; Wallmark et al., 2018). Music-making observation is also a good candidate domain for exploring prediction during action observation, due to its sequential, and thus predictive, nature. Candidi et al. (2012) found that when expert pianists observed a fingering error (a note played with the incorrect finger), CSE recorded from the muscle corresponding to the finger playing the note increased significantly compared to the correct fingering of the keys. Non-musicians who were visually trained to detect the errors did not show this muscle-specific increase in CSE during the fingering errors. The authors conclude that the experience of musically trained pianists provides their brains with simulative error monitoring systems. In other words, when participants were observing the fingering error, a prediction error occurred, leading to an increase in motor system activation. Furthermore, Stephan et al. (2018) showed in a recent study that auditory cues from a learned melody led to increased activation of the muscle that plays the following note of the melody, suggesting an anticipatory process occurring during melody processing in music perception.
Unexpected fingering errors cause one kind of prediction error that reflects an error of intent: the observer assumes that the player will play with one finger, and this prediction is violated when the player uses another. There are also purely sensory errors that can give rise to prediction errors, such as a multimodal stimulus with a misalignment between the auditory and visual components. Sensory errors that are not tied to specific effector movement error may not use the motor prediction system, as the prediction might differ from motor control prediction. On the other hand, existing research (Schubotz, 2007) has found that prediction of non-human-created sensory states (i.e., pitch prediction, object prediction) also relies on the motor regions of the brain. This suggests that we may see increased predictive activity in motor areas during a sensory prediction error as well.
We aimed in our study to explore CSE modulation when there is no error in human movement production, but the sensory consequences of observed movements are temporally misaligned, resulting in a sensory prediction error. Specifically, the auditory correlate of a piano key press is delayed so that it begins 550 ms after the visual key press occurs. As the incoming visual signal of the motor act is perceived, a prediction of the corresponding auditory consequence is made. When the auditory signal is delayed so that onset is 200 ms into the video, the sensory prediction is violated. If the motor system is involved in sensory prediction, this stimulus should increase CSE due to error detection. Systems that use predictive coding mechanisms work optimally by using low resources when predictions are closer to the actual observed state, and increasing resource use during large discrepancies or errors (Mlynarski and Hermundstad, 2018). If effector-specific motor regions are recruited for generating sensory predictions, we can expect to observe an increase of CSE in the observed muscle during sensory discrepancies, but not an increase in other muscles (effector-specificity). For example, if a participant is observing a key played with the actor’s index finger, we should see increased excitability recorded from the participant’s index finger muscle, but not from their pinky muscle. Furthermore, while detection of fingering errors increases CSE in experienced musicians only, the prediction error in our study should give rise to facilitation in both musicians and non-musicians, as no training is needed to understand the relationship between the observed action and the timing of its sensory consequences (c.f. Candidi et al., 2012).
In addition, while it is known that both visual and auditory action observation leads to increased CSE, the differential influences of each of these modalities on their own remains unclear. It is also unclear whether multimodal action perception will lead to additive activity in motor cortex summing over both visual and auditory contributions to motor regions. If motor involvement is primarily a bottom-up, automatic phenomenon, we might expect additive effects of multimodal presentation on CSE. The active inference framework, however, predicts that multimodal presentation will not lead to increased CSE over single-modality, as the information is redundant for predictive purposes. Thus, if we see summative effects, we have evidence for the resonance account. On the other hand, if we do not see additive properties, we cannot rule out either theory as there may also be ceiling effects or over-dominance by one of the sensory modalities. Therefore, an additional objective of the present work was to explore the modulation of CSE during auditory, visual, and multimodal music perception to explore the potentially different effects of modalities on motor system activity.
Materials and Methods
Subjects
Forty subjects were recruited for this study (15 males, mean age = 22 ± 2.6, range = 18–26). Due to excessive noise in the EMG signal or a participant having difficulty maintaining wakefulness, we excluded two subjects. Twenty-three of the final participants were non-musicians and had never played the piano, five had played for less than 3 years and 10 for more than 3 years. All subjects were right-handed and had normal or corrected-to-normal vision and no hearing impairments. Subjects were screened for contraindications for TMS and previous medical problems that would be risk factors for TMS. The UC Merced Institutional Review Board approved the study, and written consent was obtained for all subjects. The experiment took about 1 h, and subjects received two research credits that can be used for credit in some undergraduate courses.
Stimuli
Stimuli consisted of video recordings of a three-note piano sequence played using the right hand. Half of the stimuli were recordings of the thumb finger playing one note, followed by the index finger playing the next note twice. The finger movement that produces this action involves the FDI muscle. The other half had the same pattern but played by the ring finger followed by the fifth digit (pinky). This second half was played one scale higher than the keys used in the first half, so that the auditory cue for each could be distinguished. The finger movement that produces this action involves the flexor digiti minimi (FDM) muscle. For each of these two patterns, we created auditory-only (video blacked out), visual-only (audio silenced), multimodal, and multimodal time-lag versions, using iMovie. The time-lag version was created by starting the audio for the video 550 ms after the visual stimulus began. Eight different stimuli videos were used, resulting in a 2 (finger movement: thumb-index-index, ring-pinky-pinky) by 4 [modality: Auditory (A), Visual (V), Audiovisual (AV), AV-lag] design. The videos were filmed using an iPhone 7 Plus camera (resolution 1080p; 60 frames per second) and were edited using iMovie. Each video was played 10 times in a randomized order, leading to a total 80 stimulations during the experiment. TMS stimulation was triggered by our presentation software, Paradigm, at the time in which the index or pinky finger began its second down press, 1400 ms into the video. In between trials, a crosshair appeared on the screen where participants were instructed to maintain focus. Figure 1 displays a visual depiction of the trial sequence.
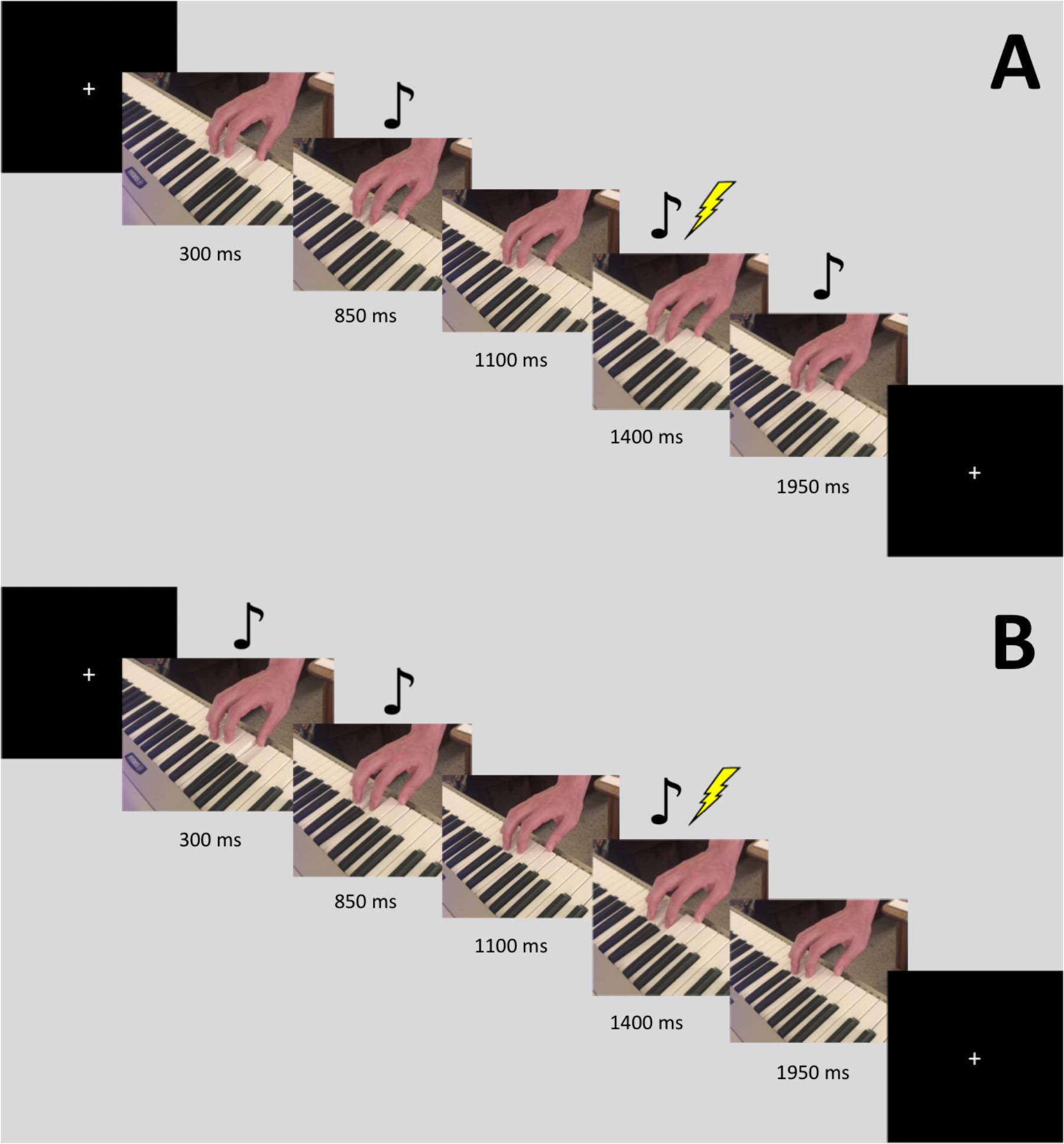
FIGURE 1. Sequence for a single trial with the FDI stimulus. The images are slices taken from the video. Stimulation occurred 1400 ms into the video. The music notes denote when the auditory tone was heard. Panel (A) displays the sequence for the AV-lag condition, while panel (B) displays the sequence for the regular AV condition.
TMS and EMG Recording
Corticospinal excitability was measured by the peak-to-peak amplitude of motor evoked potentials (MEPs) recorded using electromyography (EMG) on two muscles of the right hand. Two bipolar surface electrodes were placed on the belly of the participants’ right FDI muscle. Two additional electrodes were placed over the FDM muscle. A ground electrode was placed on a bone near the elbow of the subject. In order to obtain optimal EMG signal, we abraded and cleaned the skin under the electrodes, and secured the electrodes with medical tape. A bandpass filter (50–1000 Hz) was applied to the EMG signal, which was digitized at 1024 Hz for offline analysis. MEPs were elicited by applying single-pulse TMS to the region of the left motor cortex that induced MEPs in both FDI and FDM. If a location that induced MEPs in both muscles could not be determined, we used the FDI hotspot and thus did not record MEPs from the FDM muscle. Pulses were delivered using a Magstim Rapid2 TM with an attached 70 mm figure-of-eight coil positioned over the optimal scalp location with the handle pointing backward at 45° from the midline. The motor hotspot localization procedure was as follows. Subjects were fitted with a swim cap that was covered by a grid of dots 1 cm apart. Optimal scalp position was determined by moving the coil in 1 cm intervals until the location eliciting the best MEPs in both muscles was identified. We were unable to find the shared hotspot position for six subjects, and thus only have data from FDI for these subjects. The optimal location was marked on the swim cap worn by the participant. Resting motor threshold was determined as the percent of machine output that produced 3 out of 6 MEPs of at least 50 mV peak-to-peak amplitude. The stimulation intensity during the experiment was set to 120% of a participant’s resting motor threshold. The coil was held steady at the optimal position throughout the experiment. The inter-pulse interval between each stimulation was between 9 and 10 s. Subjects were instructed to keep their head still and remain relaxed with their right hand on their lap for the duration of the experiment, while attending to the videos as they appeared.
Results
The EMG data was exported from Visor2 (ANT Neuro), and we ran a custom Python script to extract MEPs (peak-to-peak amplitudes). We also calculated area under the curve, but as these values correlated over 98% with the peak-to-peak amplitudes, we did not use both measures. In order to use inter-individual comparisons, z-scores were calculated separately for each muscle from each participant. Trials in which MEP amplitudes were larger than 2.5 standard deviations from the mean and those less than 50 μV were excluded as outliers. Less than 5% of all data were excluded. Statistical analyses were carried out in R.
A repeated-measures analysis of variance (ANOVA) was conducted on MEPs from each muscle to assess the significance of the effect of our experimental conditions on the MEP amplitudes. We had a repeated-measures 2 × 4 design with two finger movements (FDI, FDM) and four modality conditions (A, V, AV, AV-lag).
The amplitude of MEPs recorded from FDI was significantly modulated by the modality of the observed action [main effect: F(3,111) = 3.52; p = 0.01] (Figure 2A). This effect is due to the AV-lag condition (the prediction violation condition) inducing significantly larger MEPs (z-score = 0.103 ± standard deviation: 0.055) than the other modalities (z-scores; A: -0.067 ± 0.052, V: -0.032 ± 0.05, AV: 0.016 ± 0.05). We also observed an interaction between finger movement and modality [F(3,111) ± 4.93, p < 0.01], meaning that we did see muscle-specificity in MEP modulation in some conditions but none or less in others. We observed no additional main effect of the finger movement condition. Post hoc multiple comparisons using Tukey’s honest significant differences revealed that when observing the index finger pressing the key, the AV-lag condition (the prediction violation condition) produced larger MEPs in the FDI (z-score = 0.223 ± 0.055) than while observing the pinky finger pressing the key (-0.017), [t(68) = 0.391, p < 0.05], while there were no significant differences in the other conditions. The post hoc multiple comparisons for all of the analyses can be found in Supplementary Tables S1–S4.
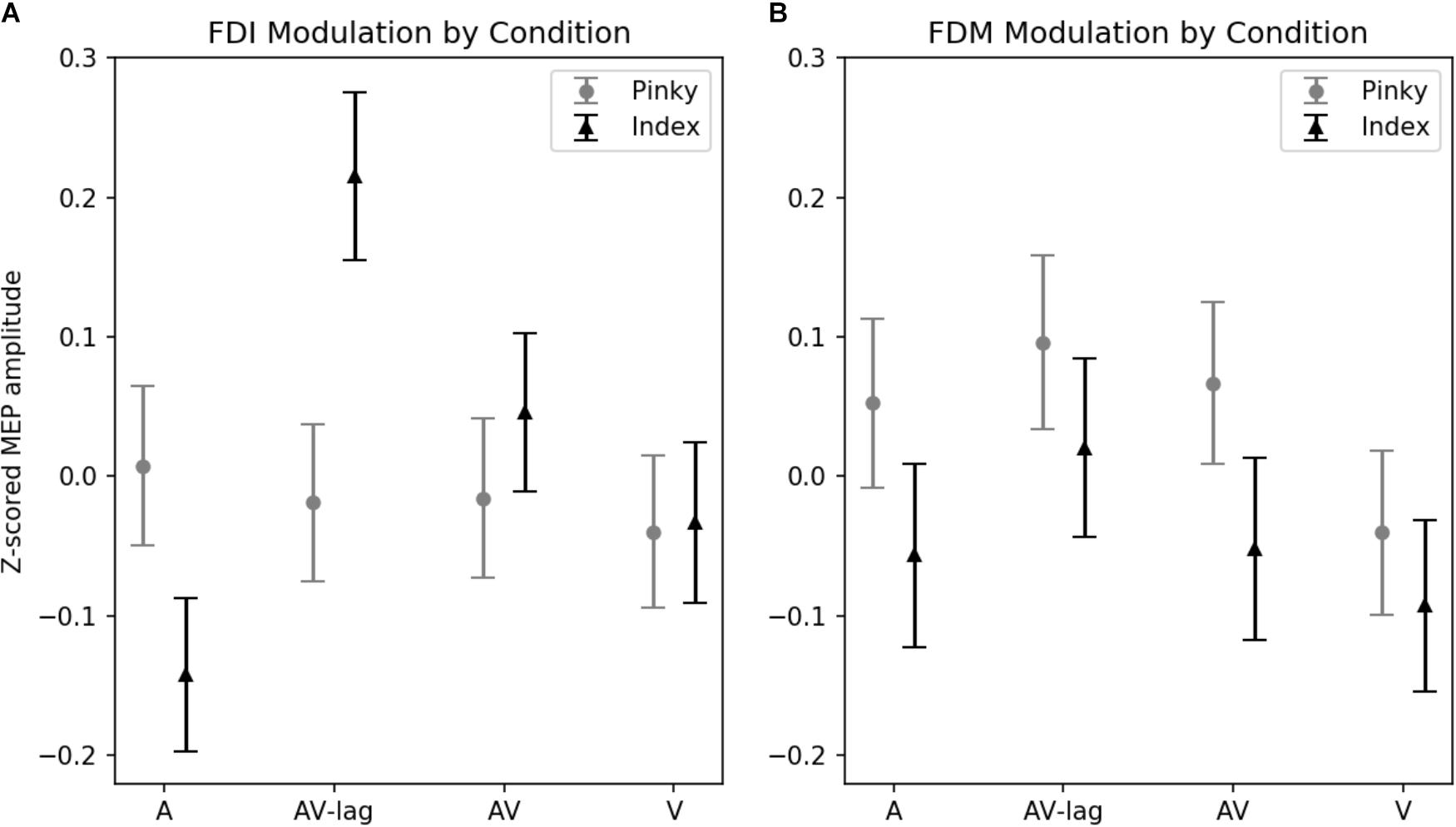
FIGURE 2. Z-scored MEP amplitudes for each modality and finger movement. Data from all subjects. Vertical bars denote standard error of means. (A) MEPs recorded from FDI. Motor evoked potentials in the index-press AV-lag condition show the largest facilitation. (B) MEPs recorded from FDM.
The amplitude of MEPs recorded from FDM is significantly modulated by the observed finger playing the note, [F(1,31) = 4.32, p < 0.05] (Figure 2B). This muscle-specificity in MEP modulation is driven by larger MEPs when observing the pinky finger pressing the key (z-score = 0.40 ± 0.064) compared to while observing the index finger pressing the key (z-score = 0.33 ± 0.053). Modality was marginally significant [F(3,93) = 2.22, p = 0.08], with the AV-lag condition resulting in larger MEPs (z-score = 0.076 ± 0.07) than the other conditions (z-scores; A: 0.008 ± 0.065, V: -0.083 ± 0.06, AV: -0.01 ± 0.06). We did not obtain an interaction between modality and finger movement.
Average normalized MEP amplitudes for each modality condition and finger movement can be seen in Figure 2. Overlaid example FDI MEPs from the two finger movement conditions (index and pinky) in the AV-lag modality are given in Figure 3.
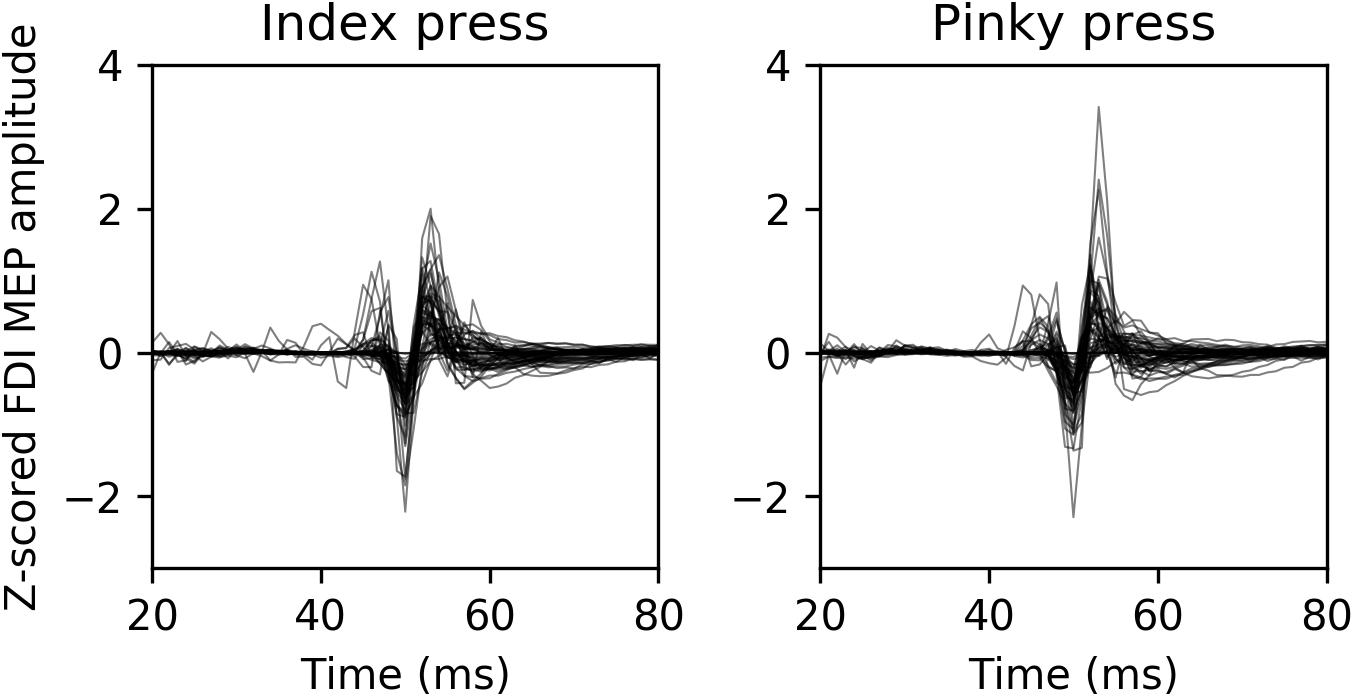
FIGURE 3. Representative example MEPs recorded from FDI from 13 randomly selected participants during the AV-lag trials. The left image contains MEPs from index press observation condition, and the right image contains MEPs from pinky press observation condition.
Discussion
In this study, we tested whether the increase in CSE during action perception is driven by bottom up resonance or by top down predictive coding. A selective increase in muscle specific CSE for the AV-lag (the prediction violation) condition that violate sensory expectations, would support the top down, predictive coding hypothesis. The FDI data support the predictive account, by showing an interaction between finger movement and modality, with larger MEPs for the AV-lag index movement stimuli compared to the AV-lag pinky movement stimuli. The FDM results, on the other hand, are consistent with the motor resonance account, as we observed muscle-specific modulation in all conditions, and no interaction between observed finger movement and condition, with and without sensory expectations violation.
One potential explanation for this difference is that motor cortex representations of muscles that are used more often and for actions that require more skillful movements may have a higher predictive role during action observation. The FDI muscle is a heavily used muscle for object-oriented, manipulative, skilled actions. On the other hand, the FDM is used in a significantly less specific way. Indeed most humans are unable to move their pinky finger without coactivating at least the ring finger. Further studies may test this hypothesis.
Corticospinal excitability increased the most in FDI for the AV-lag FDI stimulus, suggesting that the sensory error was detected and processed in motor regions. Prior work (Candidi et al., 2012) has shown that the motor system in musicians enables simulation of observed piano playing, and that activation increases when a fingering error by the pianist is observed. Here we provide evidence that motor activity also increases during the observation of a non-movement-related sensory error during action observation, which is present even in our participant pool, comprised primarily of non-musician subjects. The delayed onset of the auditory component resulted in a sensory prediction error, and a corresponding increase in CSE. This provides evidence for a general predictive process taking place in motor areas of the brain at multiple levels, from intention prediction to sensory consequence prediction.
Active inference or “action-oriented predictive processing” has gained much interest over the last few years as a potential framework of how the brain instantiates perception, action, and cognition (Miall, 2003; Friston et al., 2011; Clark, 2015). Instead of considering the brain as a passive processor of bottom-up sensory information, these theories suggest that the brain is undergoing top-down active inference in order to predict incoming sensory information. Sensory information that is received provides feedback for top-down predictions to adjust predictive models in order to decrease prediction error in the future. Under this framework, prediction happens at multiple levels. At each level, generative models are created to predict information about the upcoming state of a lower level. Generative models calculate a prediction error based on a comparison of expected to actual sensory state. The prediction error is sent up the hierarchy, so that top-down mechanisms can adjust future predictions. This recurs until prediction error of the system is minimized. An important conceptual distinction in active inference theories is that motor processing is no different from sensory processing, as both are involved in top-down processing/prediction. A resulting idea from this is the existence of a single action-perception process that attempts to predict sensory input from all modalities. For action, the modality being predicted is proprioceptive input. The primary goal is to minimize surprise and thus minimize prediction errors.
In active prediction, neurons that are typically known to represent particular actions also represent the causes of sensory input (the same idea underlies ideomotor theory; Prinz, 2005; Hommel, 2013). In other words, perception and action share a common neural code. As such, Friston et al. (2011) suggest that the mirror neuron system can also be explained with active inference and predictive coding. Active inference implies a circular causality, whereby actions are deployed in order to fulfill predictions prescribed by perception, which updates these predictions using information obtained via actions. During action observation, the same process is instantiated, but without the corresponding proprioceptive feedback that occurs during action. This means that the same neuronal ensembles that encode an action during movement will encode that same action during observation. This naturally allows for the formation of mirror neurons, which will underlie this predictive process (Kilner et al., 2007) for both action observation and action execution. Neurons with mirroring properties have now been described in multiple systems of the primate brain. Beyond the original findings in fronto-parietal circuits for grasping (Rizzolatti and Craighero, 2004), mirroring response have been recorded in dorsal premotor and primary motor cortex for reaching movements (Dushanova and Donoghue, 2010), in the lateral intraparietal area LIP for eye gaze (Shepherd et al., 2009), in the ventral intraparietal area VIP for touch (Ishida et al., 2010), and in human SMA and medial temporal cortex for grasping actions and facial expressions (Mukamel et al., 2010). This pervasive mirroring machinery seems ideal for generating predictive models during action observation. Higher-level generative models will make predictions about intentions and goals, while levels lower in the hierarchy will be involved in prediction of observed low level muscle movements.
This kind of predictive mirror neuron system can explain our results as well as the increased motor activation during fingering errors reported in Candidi et al. (2012), where error detection caused increased activation in predictive models to account for perceived error. When the visual component of the AV-lag stimulus begins with no auditory counterpart, the prediction is that the given trial is a visual stimulus only trial. At the auditory component onset, this prediction is violated and there are misaligned sensory representations of an ongoing observed action. Future studies may manipulate experimentally the number of trials that violate expectations within experimental blocks to further test the predictive coding hypothesis, as previously done for action preparation (Bestmann et al., 2008).
In summary, sensory error detection during action observation leads to increased activity in FDI. This facilitation likely results from prediction error caused by a mismatch between expected sensory consequence and actual sensory input. Sensory prediction errors may be generated in motor regions, and potentially rely on mirror neurons for this predictive process. This suggests that reconsidering the mirror system not only as a passive simulation mechanism, but also as supporting predictive mechanisms, may help improve our understanding of the functions of this system. On the other hand, FDM modulation seemed to reflect motor resonance, as muscle-specificity was the only significant predicting variable. This is not surprising, since there is no reason to think that motor resonance and predictive coding cannot co-exist. Indeed, a previous study by Gangitano et al. (2004) reported results more in support of a pure motor resonance function of the mirror system, in which the “surprising” conditions violating expectations did not induce any modulation of MEPs.
Future studies may want to determine under which conditions the mirror system and/or motor areas employ active prediction or motor resonance. Our experiment used music as a tool to explore this question. It is possible that music is particularly special in its multimodal, sequential nature. It remains to be tested whether different kinds of sensory prediction also involve active prediction in the motor system, or if other contexts (i.e., novel actions) may invoke a more passive, resonant role. Future work is warranted to investigate other kinds of sensory prediction and involvement of the motor system in these domains.
Ethics Statement
This study was carried out in accordance with the recommendations of the Ethical Guidelines for Biomedical Research Involving Human Subjects, with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the University of California, Merced Institutional Review Board.
Author Contributions
CG, MI, and RB initiated this work following workshops for the UC MERCI (Music experience research community initiative) group. All of these authors contributed substantially to this work, and approved it for publication. CG performed the experiments, completed the analyses, and made the graphs. RB and MI contributed both to the experiment design and to the intellectual components of the work.
Funding
University of California Music Experience Research Community Initiative (UC MERCI) supported this work by the grant number CA-15-328736.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2018.00736/full#supplementary-material.
References
Aziz-Zadeh, L., Iacoboni, M., Zaidel, E., Wilson, S., and Mazziotta, J. (2004). Left hemisphere motor facilitation in response to manual action sounds. Eur. J. Neurosci. 19, 2609–2612. doi: 10.1111/j.0953-816X.2004.03348.x
Bangert, M., and Altenmüller, E. O. (2003). Mapping perception to action in piano practice: a longitudinal DC-EEG study. BMC Neurosci. 4:26. doi: 10.1186/1471-2202-4-26
Bangert, M., Peschel, T., Schlaug, G., Rotte, M., Drescher, D., Hinrichs, H., et al. (2006). Shared networks for auditory and motor processing in professional pianists: evidence from fMRI conjunction. Neuroimage 30, 917–926. doi: 10.1016/j.neuroimage.2005.10.044
Bestmann, S., Harrison, L. M., Blankenburg, F., Mars, R. B., Haggard, P., Friston, K. J., et al. (2008). Influence of uncertainty and surprise on human corticospinal excitability during preparation for action. Curr. Biol. 18, 775–780. doi: 10.1016/j.cub.2008.04.051
Buccino, G., Binkofski, F., Fink, G. R., Fadiga, L., Fogassi, L., Gallese, V., et al. (2001). Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur. J. Neurosci. 13, 400–404.
Candidi, M., Maria Sacheli, L., Mega, I., and Aglioti, S. M. (2012). Somatotopic mapping of piano fingering errors in sensorimotor experts: TMS studies in pianists and visually trained musically naives. Cereb. Cortex 24, 435–443. doi: 10.1093/cercor/bhs325
Clark, A. (2015). “Embodied prediction,” in Open MIND, eds T. Metzinger and J. Windt (Frankfurt: MIND Group).
D’Ausilio, A., Altenmüller, E., Olivetti Belardinelli, M., and Lotze, M. (2006). Cross-modal plasticity of the motor cortex while listening to a rehearsed musical piece. Eur. J. Neurosci. 24, 955–958. doi: 10.1111/j.1460-9568.2006.04960.x
Dushanova, J., and Donoghue, J. (2010). Neurons in primary motor cortex engaged during action observation. Eur. J. Neurosci. 31, 386–398. doi: 10.1111/j.1460-9568.2009.07067.x
Fadiga, L., Craighero, L., and Olivier, E. (2005). Human motor cortex excitability during the perception of others’ action. Curr. Opin. Neurobiol. 15, 213–218. doi: 10.1016/j.conb.2005.03.013
Fadiga, L., Fogassi, L., Pavesi, G., and Rizzolatti, G. (1995). Motor facilitation during action observation: a magnetic stimulation study. J. Neurophysiol. 73, 2608–2611. doi: 10.1152/jn.1995.73.6.2608
Ferrari, P. F., Gallese, V., Rizzolatti, G., and Fogassi, L. (2003). Mirror neurons responding to the observation of ingestive and communicative mouth actions in the monkey ventral premotor cortex. Eur. J. Neurosci. 17, 1703–1714. doi: 10.1046/j.1460-9568.2003.02601.x
Fogassi, L., Ferrari, P. F., Gesierich, B., Rozzi, S., Chersi, F., and Rizzolatti, G. (2005). Parietal lobe: from action organization to intention understanding. Science 308, 662–667. doi: 10.1126/science.1106138
Friston, K., Mattout, J., and Kilner, J. (2011). Action understanding and active inference. Biol. Cybern. 104, 137–160. doi: 10.1007/s00422-011-0424-z
Gallese, V. (2008). Mirror neurons and the social nature of language: the neural exploitation hypothesis. Soc. Neurosci. 3, 317–333. doi: 10.1080/17470910701563608
Gallese, V., Fadiga, L., Fogassi, L., and Rizzolatti, G. (1996). Action recognition in the premotor cortex. Brain 119, 593–609. doi: 10.1093/brain/119.2.593
Gallese, V., and Goldman, A. (1998). Mirror neurons and the simulation theory of mind-reading. Trends Cogn. Sci. 2, 493–501. doi: 10.1016/S1364-6613(98)01262-5
Gangitano, M., Mottaghy, F. M., and Pascual-Leone, A. (2001). Phase-specific modulation of cortical motor output during movement observation. Neuroreport 12, 1489–1492. doi: 10.1097/00001756-200105250-00038
Gangitano, M., Mottaghy, F. M., and Pascual-Leone, A. (2004). Modulation of premotor mirror neuron activity during observation of unpredictable grasping movements. Eur. J. Neurosci. 20, 2193–2202. doi: 10.1111/j.1460-9568.2004.03655.x
Hari, R., Forss, N., Avikainen, S., Kirveskari, E., Salenius, S., and Rizzolatti, G. (1998). Activation of human primary motor cortex during action observation: a neuromagnetic study. Proc. Natl. Acad. Sci. U.S.A. 95, 15061–15065. doi: 10.1073/pnas.95.25.15061
Haslinger, B., Erhard, P., Altenmüller, E., Schroeder, U., Boecker, H., and Ceballos-Baumann, A. O. (2005). Transmodal sensorimotor networks during action observation in professional pianists. J. Cogn. Neurosci. 17, 282–293. doi: 10.1162/0898929053124893
Haueisen, J., and Knösche, T. R. (2001). Involuntary motor activity in pianists evoked by music perception. J. Cogn. Neurosci. 13, 786–792. doi: 10.1162/08989290152541449
Hommel, B. (2013). “Ideomotor action control: on the perceptual grounding of voluntary actions and agents,” in Action Science: FOUNDATIONS of an Emerging Discipline, eds W. Prinz, M. Beisert, and A. Herwig (Cambridge, MA: MIT Press), 113–136.
Iacoboni, M., Molnar-Szakacs, I., Gallese, V., Buccino, G., Mazziotta, J. C., and Rizzolatti, G. (2005). Grasping the intentions of others with one’s own mirror neuron system. PLoS Biol. 3:e79. doi: 10.1371/journal.pbio.0030079
Iacoboni, M., Woods, R. P., Brass, M., Bekkering, H., Mazziotta, J. C., and Rizzolatti, G. (1999). Cortical mechanisms of human imitation. Science 286, 2526–2528. doi: 10.1126/science.286.5449.2526
Ishida, H., Nakajima, K., Inase, M., and Murata, A. (2010). Shared mapping of own and others’ bodies in visuotactile bimodal area of monkey parietal cortex. J. Cogn. Neurosci. 22, 83–96. doi: 10.1162/jocn.2009.21185
Kaplan, J. T., and Iacoboni, M. (2007). Multimodal action representation in human left ventral premotor cortex. Cogn. Proc. 8, 103–113. doi: 10.1007/s10339-007-0165-z
Kilner, J. M. (2011). More than one pathway to action understanding. Trends Cogn. Sci. 15, 352–357. doi: 10.1016/j.tics.2011.06.005
Kilner, J. M., Friston, K. J., and Frith, C. D. (2007). Predictive coding: an account of the mirror neuron system. Cogn. Proc. 8, 159–166. doi: 10.1007/s10339-007-0170-2
Kohler, E., Keysers, C., Umilta, M. A., Fogassi, L., Gallese, V., and Rizzolatti, G. (2002). Hearing sounds, understanding actions: action representation in mirror neurons. Science 297, 846–848. doi: 10.1126/science.1070311
Meister, I. G., Krings, T., Foltys, H., Boroojerdi, B., Müller, M., Töpper, R., et al. (2004). Playing piano in the mind—an fMRI study on music imagery and performance in pianists. Cogn. Brain Res. 19, 219–228. doi: 10.1016/j.cogbrainres.2003.12.005
Miall, R. C. (2003). Connecting mirror neurons and forward models. Neuroreport 14, 2135–2137. doi: 10.1097/00001756-200312020-00001
Mlynarski, W., and Hermundstad, A. M. (2018). Adaptive coding for dynamic sensory inference. bioRxiv [Preprint].
Molnar-Szakacs, I., and Overy, K. (2006). Music and mirror neurons: from motion to‘e’motion. Soc. Cogn. Affect. Neurosci. 1, 235–241. doi: 10.1093/scan/nsl029
Mukamel, R., Ekstrom, A. D., Kaplan, J., Iacoboni, M., and Fried, I. (2010). Single-neuron responses in humans during execution and observation of actions. Curr. Biol. 20, 750–756. doi: 10.1016/j.cub.2010.02.045
Prinz, W. (2005). “An ideomotor approach to imitation,” in Perspectives on Imitation: From neuroscience to social science, Vol. 1, eds S. Hurley and N. Chater (Cambridge, MA: MIT Press), 141–156.
Rizzolatti, G., and Craighero, L. (2004). The mirror-neuron system. Annu. Rev. Neurosci. 27, 169–192. doi: 10.1146/annurev.neuro.27.070203.144230
Rizzolatti, G., Fogassi, L., and Gallese, V. (2001). Neurophysiological mechanisms underlying the understanding and imitation of action. Nat. Rev. Neurosci. 2:661. doi: 10.1038/35090060
Schubotz, R. I. (2007). Prediction of external events with our motor system: towards a new framework. Trends Cogn. Sci. 11, 211–218. doi: 10.1016/j.tics.2007.02.006
Shepherd, S. V., Klein, J. T., Deaner, R. O., and Platt, M. L. (2009). Mirroring of attention by neurons in macaque parietal cortex. Proc. Natl. Acad. Sci. U.S.A. 106, 9489–9494. doi: 10.1073/pnas.0900419106
Stephan, M. A., Lega, C., and Penhune, V. B. (2018). Auditory prediction cues motor preparation in the absence of movements. Neuroimage 174, 288–296. doi: 10.1016/j.neuroimage.2018.03.044
Keywords: corticospinal excitability, action observation, music perception, motor prediction, motor resonance, transcranial magnetic stimulation, mirror neuron system
Citation: Gordon CL, Iacoboni M and Balasubramaniam R (2018) Multimodal Music Perception Engages Motor Prediction: A TMS Study. Front. Neurosci. 12:736. doi: 10.3389/fnins.2018.00736
Received: 18 July 2018; Accepted: 25 September 2018;
Published: 18 October 2018.
Edited by:
Daniela Sammler, Max-Planck-Institut für Kognitions- und Neurowissenschaften, GermanyReviewed by:
Eleonora Bartoli, Baylor College of Medicine, United StatesOlivia Morgan Lapenta, Western Sydney University, Australia
Copyright © 2018 Gordon, Iacoboni and Balasubramaniam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ramesh Balasubramaniam, cmFtZXNoQHVjbWVyY2VkLmVkdQ==
 Chelsea L. Gordon
Chelsea L. Gordon Marco Iacoboni
Marco Iacoboni Ramesh Balasubramaniam
Ramesh Balasubramaniam