Abstract
Background: Dementia has a significant impact on quality of life of older individuals. Impaired proteostasis has been implicated as a potential cause of dementia, that can be therapeutically targeted to improve patient outcomes. This review aimed to collate all current evidence of the potential for targeting proteostasis with repurposed drugs as an intervention for age-related dementia and cognitive decline.
Methods: PubMed, Web of Science and Embase databases were searched from inception until 4th July 2017 for studies published in English. Interventional studies of repurposed proteostasis-modifying drugs in Alzheimer's disease (AD), Parkinson's disease (PD), Lewy Body disease, vascular dementia, and cognitive aging, in either animal models or humans with change in cognition as the outcome were included. The SYRCLE and Cochrane tools were used to assess risk of bias for included studies.
Results: Overall 47 trials, 38 animal and 9 human, were isolated for inclusion in this review. Drugs tested in animals and humans included lithium, rapamycin, rifampicin, and tyrosine kinase inhibitors. Drugs tested only in animals included Macrophage and Granulocyte-Macrophage Colony Stimulating Factors, methylene blue, dantrolene, geranylgeranylacetone, minocycline and phenylbutyric acid. Lithium (n = 10 animal, n = 6 human) and rapamycin (n = 12 animal, n = 1 human) were the most studied proteostasis modifying drugs influencing cognition. Nine of ten animal studies of lithium showed a statistically significant benefit in Alzheimer's models. Rapamycin demonstrated a significant benefit in models of vascular dementia, aging, and Alzheimer's, but may not be effective in treating established Alzheimer's pathology. Lithium and nilotinib had positive outcomes in human studies including Alzheimer's and Parkinson's patients respectively, while a human study of rifampicin in Alzheimer's failed to demonstrate benefit. Microdose lithium showed a strongly significant benefit in both animals and humans. While the risk of bias was relatively low in human studies, the risk of bias in animal studies was largely unclear.
Conclusion: Overall, the collective findings support the hypothesis that targeting proteostasis for treatment of dementia may be beneficial, and therefore future studies in humans with repurposed proteostasis modifying drugs are warranted. Larger human clinical trials focusing on safety, efficacy, tolerability, and reproducibility are required to translate these therapeutics into clinical practice.
Introduction
Dementias including Alzheimer's disease, vascular dementia, Parkinson's disease and Lewy body disease, have a significant impact on global health due to the increasing number of older individuals suffering from this disease (Prince et al., 2015). Developing effective methods for preventing, delaying or treating dementia are pressing priorities. The highest risk factor for dementia is chronological age, with an annual incidence of Alzheimer's disease doubling every 5 years past the age of 65 years (Bermejo-Pareja et al., 2008). Dementia subtypes share several pathological processes including abnormal accumulation of misfolded proteins such as; amyloid beta (Aβ) and tau in Alzheimer's disease, and alpha-synuclein in Parkinson's disease and Lewy Body disease (Ganguly et al., 2017). Loss of proteostasis is an important feature during the aging process (López-Otín et al., 2013), suggesting the age-related decline in the ability to refold or degrade damaged proteins may contribute to the exponential rise in dementia incidence observed with increasing age (Yerbury et al., 2016).
Several drugs already approved for their use in humans are known to enhance proteostasis including; lithium, mTOR inhibitors (sirolimus/rapamycin, everolimus), and tyrosine kinase inhibitors (nilotinib). The concept of modifying aging with a repurposed drug to prevent multiple diseases of aging will soon be tested in the Targeting Aging with Metformin (TAME) trial. TAME will examine Metformin's ability to prevent diseases of aging in non-diabetic elderly, including cognitive impairment (Barzilai et al., 2016), via targeting the deregulated nutrient sensing associated with aging. Applying a similar strategy to target the loss of proteostasis could be effective in preventing and/or treating age-related dementia.
This systematic review will examine the evidence for targeting proteostasis with repurposed drugs as an intervention for age-related dementia and cognitive decline.
Methods
Protocol registration and search strategy
The protocol of this systematic review was registered at PROSPERO International prospective register of systematic reviews (Reg #: CRD42018091645). PubMed, Web of Science and Embase databases were used for this search from inception until 4th July 2017. The complete search strategy is presented in Supplementary Data 1. Key search terms included; “vascular dementia,” “Alzheimer* disease” “Lewy Body Disease,” “Parkinson* disease,” “cognitive aging,” “autophag*,” “lysosom*,” “proteasome endopeptidase complex,” “molecular chaperone*,” “unfolded protein response,” “insulin*,” “mTOR,” “GSK-3,” “akt,” “PI3K,” “AMPK,” “sirtuin*,” “sirolimus,” “everolimus,” “temsirolimus,” “rapamycin,” “metformin,” “DPP-4,” “GLP-1,” “nicotinamide,” “NAD,” “spermidine,” “imatinib,” “nilotinib,” “dasatinib,” “bosutinib,” “ponatinib,” “bafetinib,” “lithium,” “heat-shock protein,” “calori* restriction,” “carbohydrate restricted diet,” “protein restricted diet”. In addition to the database search a “snowballing” method was used to identify relevant articles out of the reference section and PubMed citations of each included article. After duplicates were removed studies were then screened for inclusion using Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia).
Eligibility criteria
Type of studies
The search was designed to retrieve all published research studies that investigated the effect of modifying protein homeostasis or deregulated nutrient sensing (DNS) on cognitive function in age-related neurodegenerative disease and normal aging populations. To be included in this review the study had to report on one or more neuropsychological tests measuring change in cognitive function. To meet the criteria of modifying protein homeostasis or deregulated nutrient sensing, the intervention had to be previously demonstrated to modulate these pathways, or data had to be provided proving the intervention's effect on these pathways. Animal in vivo models and human trials were included in this review. The following Dementia populations/models were specifically targeted; Alzheimer's disease, Vascular Dementia, Parkinson's disease and Lewy Body Disease. In addition, normal aging populations, defined as a population not suffering from dementia and over the age of 18 years for human studies, as well as populations likely to have a higher pace of aging such as animal models with diabetes or obesity were included. Randomized controlled trials (RCTs) and non-randomized studies comparing outcomes to either retrospective or prospective controls met the inclusion criteria. Studies were excluded if they met the following criteria; observational studies, exercise as the sole intervention, in vitro data only, conference abstracts, reviews, editorials, letters to the editor, case reports with ≤5 population size, or published in a language other than English.
Outcome
In animals (using mice as an example), cognitive tests would include spatial memory tests (Morris water maze [MWM], radial arm water maze [RAWM], Barnes maze), associative learning tasks (passive avoidance, fear conditioning), alternation tasks (Y-Maze/T-Maze), recognition memory tasks (Novel Object Recognition), attentional tasks (3 and 5 choice serial reaction time), set-shifting tasks, and reversal learning tasks. In human studies examples of neuropsychological measures would be cognitive testing batteries commonly used in clinical or research settings to examine cognitive function, such as the Mini-Mental State Examination (MMSE), Rowland Universal Dementia Assessment Scale (RUDAS), Neuropsychiatry Unit Cognitive Assessment Tool (NUCOG), Montreal Cognitive Assessment (MOCA), Clinical Dementia Rating Scale Sum of Boxes (CDR-SoB), Addenbrooke's Cognitive Examination (ACE) or Alzheimer's Disease Assessment Scale-cognitive subscale (ADAS-Cog).
Study selection
Two review authors (DH and CT) independently screened the titles and abstracts and subsequently the full text articles of potentially relevant studies against the inclusion and exclusion criteria. A third reviewer (ABM) resolved any disagreements between the authors.
Included studies were separated into the following four groups for data extraction (1) proteostasis–repurposed drug, (2) proteostasis–novel intervention (defined as a novel molecule, botanical extract, or dietary manipulation), (3) DNS–repurposed drug and (4) DNS–novel intervention. Where an intervention is thought to modify both pathways (for example the mTOR inhibitor, rapamycin) it was included in the loss of proteostasis group. The current paper presents the results of the 1st group: proteostasis—repurposed drugs.
Data extraction and quality assessment
The following variables were extracted independently by two reviewers (DH and CT): author, year of publication, study design, species, animal model/population (dementia subtype or normal aging), sample size, age, sex, baseline cognition/stage of disease, duration of study, cognitive outcome, drug, comparator, setting, hallmark(s) of aging targeted by the intervention, and journal citation. For binary outcomes the number of events and total number in group, percentage of events or ratios with confidence intervals; for continuous outcomes, mean or median, standard deviation, standard error, confidence intervals or interquartile range, and number of participants; other reported results such as mean difference and p-values of measures of cognitive function.
Risk of bias was assessed by two reviewers (DH, CT) using the Cochrane Risk of Bias tool (Higgins et al., 2011) for human studies and SYRCLE's risk of bias tool for animal studies. The SYRCLE RoB tool is an adaptation of the Cochrane tool for use in systematic reviews of laboratory animal studies (Hooijmans et al., 2014).
Registered human trials
To establish the progress of repurposed drugs into human studies which have not yet been completed, clinicaltrials.gov was searched for registered studies of the drugs identified in our search in Alzheimer's disease, Parkinson's disease, Lewy Body Disease and Vascular Dementia.
Results
Study selection and characteristics
The literature search and selection process for this review is illustrated in Figure 1. After exclusion of duplicates the remaining 1,687 studies were screened for Title and Abstracts of which 413 underwent full text screening. An additional 22 studies were identified via snowballing. Overall, 47 articles specifically investigating a proteostasis intervention on Dementia and cognitive aging were included in this review. The repurposed drugs used in these intervention studies are outlined in Tables 1, 2 and Supplementary Table 1. The following drugs were found testing the modification of cognition in animal and human studies; lithium (n = 10 animal, n = 6 human), rapamycin (n = 12 animal, n = 1 human), rifampicin (n = 1 animal, n = 1 human), tyrosine kinase inhibitors (bosutinib n = 1 animal, nilotinib n = 1 human), Macrophage Colony Stimulating Factor (M-CSF; n = 1 animal), Granulocyte Macrophage Colony Stimulating Factor (GM-CSF; n = 1 animal), methylene blue (n = 4, animal), geranylgeranylacetone (GGA; n = 2 animal), dantrolene (n = 3 animal), minocycline (n = 2 animal) and phenylbutyric acid (n = 1 animal). Doxycycline was tested in a single human trial only. Of these drugs only lithium, rapamycin, rifampicin, and the tyrosine kinase inhibitors have been tested in both animal and human studies (Figure 2).
Figure 1
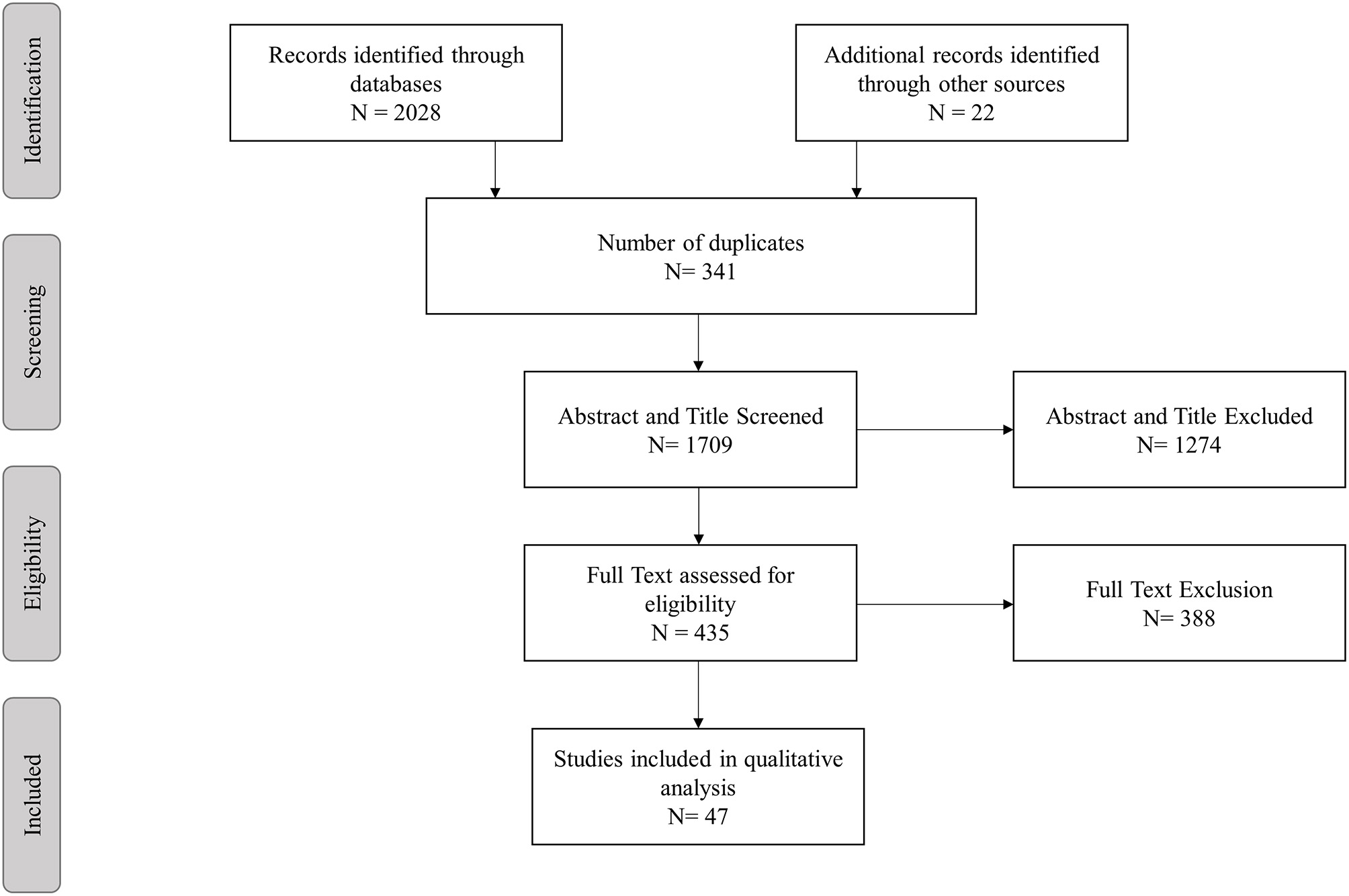
Study selection process.
Table 1
| Author, Year | Species | Model | Sample size (n) | Age | Sex (%F) | Baseline cognition | Duration | Dose | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Rx | Ctrl | |||||||||
| a | Caccamo et al., 2007 | Mouse | AD (3xTg) | Wt: 10Tg: 10 | Wt: 10Tg: 10 | 15m | NR | Est | 4w | 300μl of 0.6mol/L/d IP |
| a | Rockenstein et al., 2007 | Mouse | AD (Tg hAPP) | WT: 6Tg: 6 | WT: 6Tg: 6 | 3m | NR | Est | 3m | 20mg/kg/d IP |
| a | Fiorentini et al., 2010 | Mouse | AD (TgCRND8) | Ear: 8Est: 8 | Ear: 8Est: 8 | 2m6m | Mix | EarEst | 5w | 0.223mEq/L IP |
| a | Toledo and Inestrosa, 2010 | Mouse | AD (Tg APP- PS1) | 3-≥6 | 3-≥6 | 9m | NR | Est | 12w | 0.2–1.5 meq/L |
| a | Sy et al., 2011 | Mouse | AD (3xTg) | Na = 6LPS = 6 | Na = 6LPS = 6 | 11–13m | 67 | Est | 6w | 6–10mg/d food |
| a | Nunes et al., 2015 | Mouse | AD (Cg- Tg(PDGFB- APPSwInd) 20Lms/2J) | Pre: 8Est: 7WT: NR. | WT: 12TG: 7 | 2m10m | 0 | PreEst | 16m8m | 0.25mg/kg/d (H2O) |
| a | Nocjar et al., 2007 | Rat | Aging (Sprague-Dawley) | 16 | 14 | 2m | 0 | Pre | 80d | 0.72mEq/l food |
| a | Wilson et al., 2017 | Rat | AD (Tg McGill- R-Thy1-APP) | WT: ≥5Tg:≥5 | WT: ≥5Tg:≥5 | 3m | Mix | Ear | 2m | Li 40μg/kg/d PR |
| a | Nery et al., 2014 | Zebrafish | AD (ICV Aβ) | No inj: 10Veh: 10 AB: 10 | No inj: 10Veh: 10 AB: 10 | 5d | Mix | Pre | 5d | 100μm (H2O) |
| a | McBride et al., 2010 | Drosophila | AD (Tg psn[B3]/+, psn[I2]/+) PD (Tg 30Y-GAL4:UAS-Syn) | Pre: 72 | Pre: 70 | 30d | 0 | Pre | 25d | 5mM Li food |
| Est: 74 | Est: 75 | 45d | Est | 15d | ||||||
| PD: 39 | PD: NR | |||||||||
| b | Spilman et al., 2010 | Mouse | AD (Tg hAPP) Aging (C57BL/6J) | 12 10 | 12 10 | 7m | 0 | Ear YA | 3m | 14mg/kg food |
| b | Majumder et al., 2011 | Mouse | AD (3xTg) Aging (C57BL6/ 129svj) | 4040 | 20 20 | 18m | NR | PreEst | 16m3m | 14mg/kg food |
| b | Halloran et al., 2012 | Mouse | Aging (C57BL/6J) | 9–14 | 9–14 | 12m25m | Mix | MAOA | 40w | 14mg/kg food |
| b | Majumder et al., 2012 | Mouse | Aging (C57BL/6/ 129svj) | 2020 | 20 | 18m | NR | YAMA | 16m3m | 14mg/kg food |
| b | Lin et al., 2013 | Mouse | AD (Tg hAPP) | Tg: 10 | Tg: 10 | 7m | 0 | Est | 16w | 14mg/kg food |
| WT: 18 | WT: 17 | YA | ||||||||
| b | Neff et al., 2013 | Mouse | Aging (C57BL/6Jrj) | YA: 20 | YA: 20 | 4m | 0 | YA | 12m | 14mg/kg food |
| MA: 21 | MA: 21 | 13m | MA | |||||||
| OA: 27 | OA: 27 | 20–22m | OA | |||||||
| b | Wang et al., 2014 | Mouse | Aging (C57BL/6J, stz diabetic) | 9 | 9 | 3m | 0 | Est | 45d | 2.24mg/kg/d PO |
| b | Lin et al., 2015 | Mouse | AD (APOE4 Tg) | 15 | 15 | 7m | 100 | Pre | 6m | 14mg/kg food |
| b | Jahrling et al., 2017 | Mouse | VD (LDL-R–/– HFD) | 10 | 10 | 12m | 0 | Est | 16w | 14mg/kg food |
| b | Zhang et al., 2017 | Mouse | AD (3xTg) | 10 | 10 | 7m | 50 | Ear | 2m | 1mg/kg/d PO |
| b | Wang et al., 2016 | Rat - Sprague Dawley | AD (ICV Aβ) | 18 | 20 | 6m | 0 | Pre | 2w | 500 microg ICV/2w |
| b | Zhu et al., 2014 | Rat | AD (scop Wistar) | 10 | 10 | NR | 0 | Pre | 14d | 3.5mg/kg/d IP |
| c | Umeda et al., 2016 | Mouse | AD (Tg APPOSK), (tau609) Aging (WT) | APPOSK 12m 0.5mg: 9 18m 0.5mg: 10 1mg: 10 Tau609 8m 0.5mg: 8 15m 1mg: 7 | APPOSK 12m: 9 18m: 10 WT 8m: 10 12m: 10 15m: 11 18m: 16 Tau609 8m: 9 15m: 7 | APP 11m 17m Tau 7m 14m | 0 | Est | 1m | [5pt]0.5mg/d (APP12m, APP18m, tau8m) 1mg/d PO (APP18m, tau15m) |
| d | Lonskaya et al., 2013 | Mouse | AD (ICV lentiviral Aβ42, C57BL6) AD (Tg APP model) | Aβ42: 12 Tg: 12 | Aβ42: 12 Tg: 12 | 11m | NR | Est | 3w | 5mg/kg/d IP |
Characteristics of animal studies testing the effect of lithium (a), rapamycin (b), rifampicin (c), bosutinib (d) on cognition.
3xTg, triple transgenic; Aβ, amyloid beta; AD, Alzheimer's dementia; Ctrl, control; d, days; Ear, early disease; Est, established; F, female; hAPP, human amyloid precursor J20; HFD, High fat diet; ICV, intracerebroventricular; Inj, injection; IP, intra-peritoneal; LDL-R–/–, low density lipoprotein receptor knockout; Li, lithium; LPS, lipopolysaccharide; m, months; MA, middle age; NR, not reported; OA, Old Age; PD, Parkinson's disease dementia; PO, per oral; PR, per rectum; Pre, presymptomatic; Rapa, rapamycin; Scop, scopolamine; stz, streptozocin induced; Tg, transgenic; VD, vascular dementia; w, weeks; WT, wild type; YA, young adult.
Table 2
| Author, year | Design | Condition | Sample size (n) | Age (yrs) | Female (%) | Baseline cognition | Duration | Dose | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rx | Ctrl | Rx | Ctrl | ||||||||
| a | Pomara et al., 1983 | OL(pre-post) | AD | 7 | NA | “Geriatric” | NR | NR | 6w | 0.53 mmol/L (mean at 6w) | |
| a | Macdonald et al., 2008 | OL (match ctrl) | AD | 22 | 44 | 80.9 ± 7.9 | 81.2 | 59 | MMSE 12–24 | 12m | 0.3–0.8 mmol/L |
| a | Hampel et al., 2009 | RCT | AD | 33 | 38 | 68.2 ± 7.2 | 68.9 ± 8.3 | 52 | MMSE 21–26 | 10w | 0.5–0.8 mmol/l |
| a | Leyhe et al., 2009 | RCT | AD | 13 | 14 | 71.0 ± 9.0 | 69.4 ± 8.5 | 59 | MMSE 21–26 | 10w | 0.5–0.8 mmol/L |
| a | Forlenza et al., 2011 | RCT | AD-MCI | 23 | 22 | 70.9 ± 5.3 | 74.2 ± 6.5 | NR | MCI | 12m | 0.25–0.5 mmol/L |
| a | Nunes et al., 2013 | RCT | AD | 58 | 55 | 77.0 ± 0.1 | 78.0 ± 0.76 | 66 | MMSE 12–24 | 15m | 300 μg/d |
| b | Kraig et al., 2018 | RCT | Aging | 11 | 14 | 80.4 ± 8.6 | 80.6 ± 7.9 | 28 | OA | 8w | 1mg/d PO |
| c | Molloy et al., 2013 | RCT | AD | Rif: 101 Dox: 102 Rif + dox: 101 | 102 | Rif:78.6 (73.5–82.3) Dox: 78.7 (74.1–83.6) Rif+dox:79.2 (74.4–83.5) | 78.6 (72.4–83) | 50 | MMSE 20–25 | 12m | Rif: 300mg/d Dox: 100mg BD Rif + dox: 300mg/d + 100mg BD |
| d | Pagan et al., 2016 | OL (pre-post) | PD | 12 | NA | 71.8 (49–89) | NA | 25 | MoCA 9–28 | 6m | Nilo 150mg or 300mg/d |
Characteristics of human studies testing the effect of lithium (a), rapamycin (b), rifampicin & doxycycline (c) and nilotinib (d) on cognition.
OL, open label; RCT, randomized controlled trial; AD, Alzheimer's Disease, PD, Parkinson's disease; LBD, Lewy Body Disease; MMSE, Mini mental state exam; MoCA, Montreal Cognitive Assessment; NR, not reported; SD, standard deviation; MCI, Mild Cognitive Impairment; Li, lithium; Ctrl, control; NA, Not applicable; D, days; W, weeks; M, months; Rx, treatment; Rif, rifampicin; Dox, doxycycline; PL, placebo; Nilo, nilotinib; Age, refers to age at baseline of study.
Figure 2
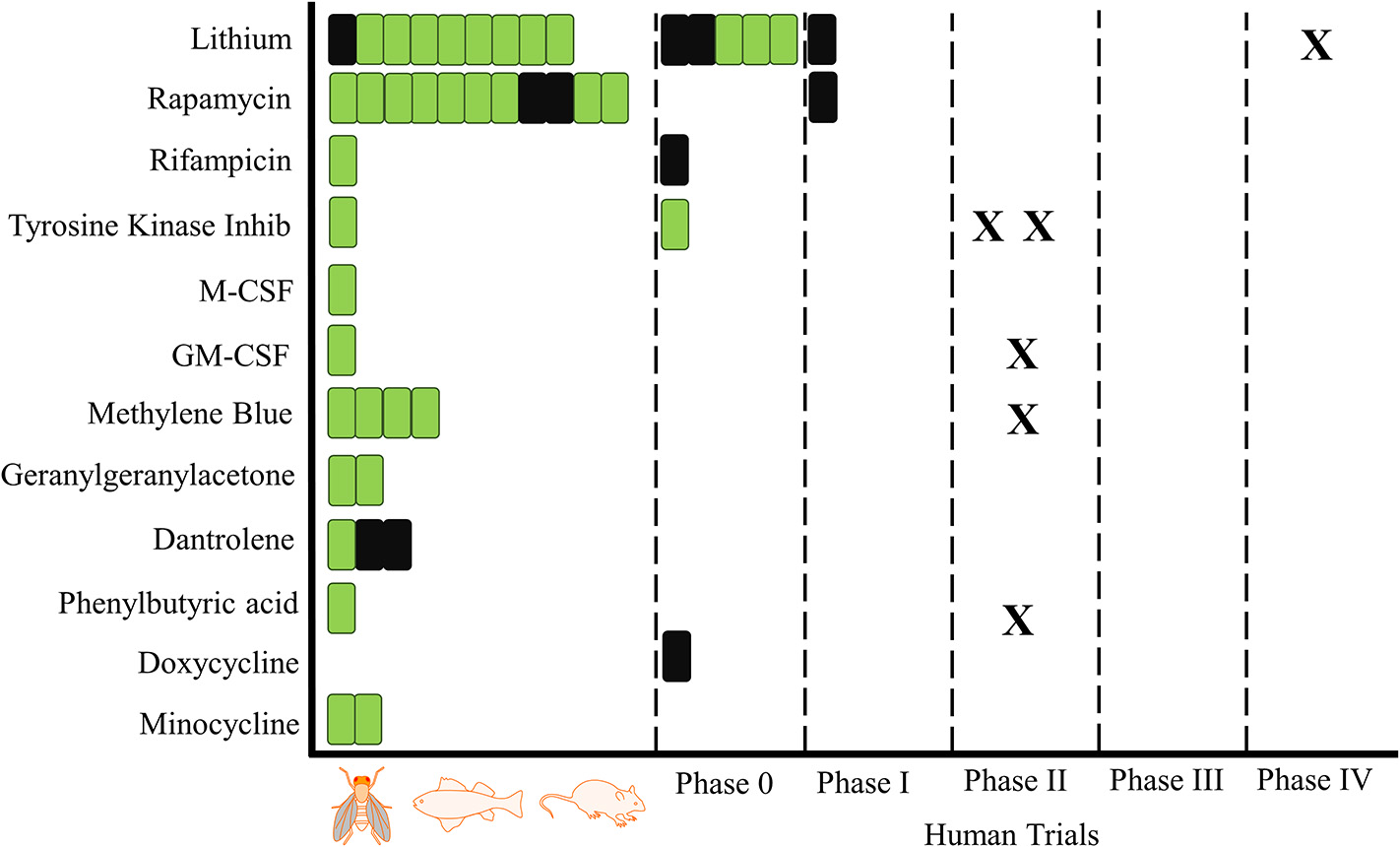
Proteostasis Drugs and Cognitive Outcomes: From Animals to Humans. A schematic overview of trials investigating the influence of proteostasis drugs on cognitive outcomes. One bar is equal to one trial, green indicates a positive result for at least one cognitive outcome and black is indicative of no positive outcomes. X represents a registered trial. Trials are arranged in chronological order.
Lithium and cognitive aging
Lithium was the most investigated proteostasis modulator for cognitive aging. Overall 16 studies (6 humans, 6 mice, 2 rats, 1 drosophila, and 1 zebrafish) investigating the influence of lithium on cognitive aging were found. The majority of these studies utilized an Alzheimer's animal model or were conducted in an Alzheimer's population (Tables 1, 2). The findings of these studies were largely positive with all animal studies, bar one (Caccamo et al., 2007), reporting the use of lithium as having a statistically significant beneficial impact on at least one cognitive outcome irrespective of treatment duration (Table 3). The findings of the animal studies are consistent with the lithium human studies (Table 4), with three randomized controlled studies showing a statistically significant benefit of the use on lithium on either the ADAS-Cog or MMSE (Leyhe et al., 2009; Forlenza et al., 2011; Nunes et al., 2013). The other three studies, two of which were open-label (Pomara et al., 1983; Macdonald et al., 2008) and one RCT (Hampel et al., 2009), did not show cognitive benefits after treatment with lithium.
Table 3
| Author, year | Cognitive tests | Outcomes | Significance | |
|---|---|---|---|---|
| a | Caccamo et al., 2007 | T-maze (Alternation %) | Wt Li−66.67 (8.7), ctrl−72.15 (2.4) | ± |
| Tg Li−55.71 (5.6), ctrl−55.21 (5.8) | ± | |||
| a | Nocjar et al., 2007 | Hole-board spatial discrimination task: | ||
| Search time (s) session 6 | Li−6 (1), Ctrl−20 (3) | + | ||
| Repeat visits (# lower = better) | Li−0.6 (0.1), Ctrl−1.5 (0.4) | + | ||
| Number of errors (# lower = better) | Li−1.6 (0.2), Ctrl−1.9 (0.2) | ± | ||
| T-maze delayed alternation task: | ||||
| Sessions to reach criterion (#) | Li−13.5 (1), Ctrl−20 (1.5) | + | ||
| Social conditioned place preference: | ||||
| Percent correct (%) | 1min Li−75 (2.5), Ctrl−72.5 (5) | ± | ||
| 3min Li−70 (3), Ctrl−58 (4) | + | |||
| 5min Li−65 (2.5), Ctrl−60 (2.5) | ± | |||
| Run time (min) | 1min Li−3.7 (1), Ctrl−1.9 (0.2) | NR | ||
| 3min Li−2.7 (0.5), Ctrl−2.8 (0.5) | ± | |||
| 5min Li−2.9 (0.5), Ctrl−3.2 (0.5) | ± | |||
| Preference for social chamber (s): | Li−280 (100), Ctrl−175 (100) | + | ||
| a | Rockenstein et al., 2007 | Morris water maze: | ||
| Meters to reach platform day 7 | Tg Li−3 (0.5), ctrl−11.5 (3) | + | ||
| Wt Li−3.25 (0.5), ctrl−3 (0.5) | ||||
| Platform crosses (#) | Tg Li−7 (1.5), ctrl−6 (1) | ± | ||
| Wt Li−6 (2), ctrl−7 (1) | ||||
| Time in target quadrant (s) | Tg Li−16 (3), ctrl−18 (2) | ± | ||
| Wt Li−15 (3), ctrl−16 (3) | ||||
| a | Fiorentini et al., 2010 | Morris water maze: | Early stage disease (3 months) | |
| Escape latency day 4 (s) | Li 35s (5s), ctrl 55s (2s) | +++ | ||
| Time in target section (%) | Li 7.4% (2.25%), ctrl 1.25% (1.25%) | ++ | ||
| Inhibitory avoidance test (s) | Li 27 (3), ctrl 9.5 (2) | +++ | ||
| Late stage disease (7 months) | ||||
| Escape latency day 4 (s) | Li 50 (2), ctrl 59 (1) | NR | ||
| Time in target section (%) | Li 1.5 (0.5), ctrl 1.5 (0.5) | ± | ||
| Inhibitory avoidance test | Li 14 (3.5), ctrl 8 (4) | ± | ||
| a | Toledo and Inestrosa, 2010 | Morris water maze: | ||
| Escape latency day 5 (s) | Tg Li−45 (9), ctrl−35 (7.5) | ± | ||
| WT ctrl−27 (10) | ||||
| Memory flexibility test: | ||||
| No. of trials to criterion (#) | Tg Li−7 (0.5), ctrl 12 (0.25) | + | ||
| WT ctrl−5 (0.25) | ||||
| a | Sy et al., 2011 | Morris water maze: | ||
| Escape latency during day 7 (s) | Li + Na−25 (2), ctrl + Na−17 (5) | NR | ||
| Li + LPS−26 (3), ctrl + LPS−20 (3) | NR | |||
| Probe trial (24 h) | ||||
| Time spent in target quadrant (s) | Li + Na−17.5 (4.5), ctrl + Na−22 (4) | ± | ||
| Li + LPS−19 (2), ctrl + LPS−13 (4) | ± | |||
| Latency to platform (s) | Li + Na−24 (6), ctrl + Na−17.5 (5) | ± | ||
| Li + LPS−26 (5), ctrl + LPS−47.5 (7.5) | + | |||
| Number of platform location crosses (#) | Li + Na−5 (1.3), ctrl + Na−5.4 (1) | ± | ||
| Li + LPS−3 (0.25), ctrl + LPS−1 (0.5) | + | |||
| a | Nunes et al., 2015 | Barnes maze: | Treated before deficits | |
| Escape latency (s; mean) | Li−40 (3), ctrl−75 (7) | + | ||
| Time in target quadrant (%) | Li−52.3 (6.8) Ctrl−22.8 (4.9) | +++ | ||
| Aversive memory test session (s) | Li−299 (298/300), Ctrl−216 (137/298) | ++ | ||
| Barnes maze: | Treated after deficits | |||
| Escape latency (s; mean) | Li – 25 (2), Ctrl – 75 (7) | + | ||
| Time in target quadrant (%) | Li−32 (4) Ctrl−22.8 (4.9) | ++ | ||
| Aversive memory test session (s): | Li−298 (139/298), Ctrl−216 (137/298) | + | ||
| a | Wilson et al., 2017 | Novel object recognition (preference ratio) | WT Li−0.39 (0.04), veh−0.43 (0.04) Tg AD Li−0.39 (0.02) veh−0.28 (0.02) | NR + |
| Morris water maze: | ||||
| Escape latency training day 5 (s) | WT Li−31 (7), veh−15 (5) | NR | ||
| Tg Li−40 (10) AD veh−33 (8) | ± | |||
| Time in target quadrant (%) | WT Li−44 (5), veh−48 (5) | ± | ||
| Tg AD Li−45 (3), veh−50 (8) | ± | |||
| Auditory fear conditioning task: | ||||
| Contextual (% freezing) | WT Li−60 (15), veh−79 (11) | ± | ||
| Tg AD Li−60 (10) veh−55 (10) | ± | |||
| Cued recall (% freezing) | WT Li−55 (15), veh−85 (10) | NR | ||
| Tg AD Li−63 (7), veh−30 (5) | + | |||
| a | Nery et al., 2014 | Avoidance behavior | ||
| % animals in non-stimulus area | Aβ inj Li 65 (2), ctrl: 55 (1) | +++ | ||
| a | McBride et al., 2010 | Treated before deficits | Alzheimer's Tg: | |
| Learning during training (%) | ||||
| psn[B3]/+ flies | Li 75(5) -> 45(10), ctrl 62.5(7.5) -> 51 (9) | +++ | ||
| psn[I2]/+ flies | Li 65(7.5) -> 30(10) ctrl 63(8) -> 52(8) | +++ | ||
| Short term memory (%) | ||||
| psn[B3]/+ flies | Li–Naive 90(2), trained 70(5) | ++ | ||
| Ctrl–Naive 76(6), trained 75(6) | ± | |||
| psn[I2]/+ flies | Li–Naive 88(2), trained 72(5) | + | ||
| Ctrl–Naive 83(5), trained 85 (3) | ± | |||
| Treated after deficits | ||||
| Learning during training (%) | ||||
| psn[B3]/+ flies | Li 65(7.5) -> 18(7), ctrl 70(5) -> 62.5(7.5) | +++ | ||
| psn[I2]/+ flies | Li 76(5) -> 18(7), ctrl 47.5(7.5) -> 35(7.5) | +++ | ||
| Short term memory (%) | ||||
| psn[B3]/+ flies | Li–Naive 90(4), trained 62.5(7.5) | ++ | ||
| Ctrl–Naive 70(8), trained 75(7) | ± | |||
| psn[I2]/+ flies | Li–Naive 84(6), trained 62.5(7.5) | ++ | ||
| Ctrl–Naive 57.5(7.5), trained 63(8) | ± | |||
| Treated before deficits | ||||
| Short term memory | Parkinson's Tg: | |||
| Li 80(4) -> 75(5), ctrl 82.5(5) -> 78(4) | ± | |||
| b | Spilman et al., 2010 | Morris water maze: | ||
| Escape latency day 4 (s) | Tg Rapa 32(3), ctrl 42(5) | + | ||
| WT Rapa 15(2.5), ctrl 32.5(3) | NR | |||
| Platform crosses (#) | Tg Rapa 2.5(0.5), ctrl 0.9(0.1) | +/± | ||
| WT Rapa 5(1), ctrl 3.1(0.4) | NR | |||
| b | Majumder et al., 2011 | Morris water maze: | ||
| Escape latency day 5 (s) | Pre-AD–Rapa 26.9(2.1), ctrl 37.96(2.9) | + | ||
| Est AD – Rapa 36(2), ctrl 37.96(2.9) | ± | |||
| YA–Rapa 20.7(1.05), ctrl 29.1(2.7) | + | |||
| MA–Rapa 32.5(1.5), ctrl 29.1(2.7) | ± | |||
| Trial time in target quadrant (s) | Pre-AD–Rapa 22.5(2.5), ctrl 15(2.5) | + | ||
| Est AD–Rapa 17.5(1.5), ctrl 15(2.5) | ± | |||
| YA–Rapa 29(2), ctrl 21.5(1.5) | + | |||
| MA–Rapa 21(1.5), ctrl 21.5(1.5) | ± | |||
| MWM platform crosses (#) | Pre-AD–Rapa 3.5(0.5), ctrl 1.95(0.25) | + | ||
| Est AD–Rapa 1.75(0.2), ctrl 1.95(0.25) | ± | |||
| YA–Rapa 5.25(0.3), ctrl 3.8 (0.25) | + | |||
| MA–Rapa 3.5 (0.2), ctrl 3.8 (0.25) | ± | |||
| Novel object recognition | Pre-AD–Rapa 65 (7), ctrl 50 (5) | + | ||
| Est AD–Rapa 55 (2.5), ctrl 50 (5) | ± | |||
| YA–Rapa 67.5(2.5), ctrl 70 (4) | ± | |||
| MA–Rapa 60 (5), ctrl 70 (4) | ± | |||
| b | Halloran et al., 2012 | Passive avoidance test (s) | MA–Rapa 200(40), ctrl 160(40) | ± |
| OA–Rapa 200(30), ctrl 100(20) | + | |||
| b | Majumder et al., 2012 | Morris water maze: | ||
| Escape latency day 5 (s) | YA–Rapa 21(1), ctrl 30(2.5) | + | ||
| MA–Rapa 31(2), ctrl 30(2.5) | ± | |||
| Time in target quadrant (s) | YA–Rapa 28.73(1.65), ctrl 21.3(1.24) | ++ | ||
| MA–Rapa 20.97(1.18), ctrl 21.3(1.24) | ± | |||
| Latency to platform (s) | YA–Rapa 20(2), ctrl 27(3) | + | ||
| MA–Rapa 31(3), ctrl 27(3) | ± | |||
| Platform crosses | YA–Rapa 5.3(0.2), ctrl 3.9(0.15) | +++ | ||
| MA–Rapa 3.5(0.25), ctrl 3.9 (0.15) | ± | |||
| b | Lin et al., 2013 | Morris water maze: | ||
| Escape latency day 5 training (s) | WT–rapa 28(4), ctrl 25(3) | |||
| AD–rapa 35(9), ctrl 40(4) | ± | |||
| Platform crosses (#) | WT rapa−3.1(0.5), ctrl 3.9(0.6) | |||
| AD rapa−2.1 (0.5), ctrl−0.75 (0.25) | + | |||
| b | Neff et al., 2013 | Object place recognition (s) | YA rapa–novel 22(3), known 12(2) | ± |
| YA veh–novel 24(4), known 14(2) | ||||
| MA rapa–novel 15(3), known 10(2) | ± | |||
| MA veh–novel 17(2), known 15(4) | ||||
| Morris water maze: | ||||
| Escape latency day 5 (s) | YA rapa 30(1), veh 41 (2) | +/± | ||
| MA rapa 37 (3), veh 39 (1) | +/± | |||
| Time in target quadrant (s) | YA rapa 25(2), veh 21 (2) | ++ | ||
| MA rapa 25 (2), veh 20 (3) | ++ | |||
| Target crossings (#) | YA rapa 2.3(0.2), veh 1.3(0.2) | ++ | ||
| MA rapa 1.5 (0.3), veh 1.5 (0.2) | ± | |||
| Context fear conditioning: | ||||
| Activity suppression (ratio) | YA rapa 0.2 (0.02), veh 0.21 (0.02) | ++ | ||
| MA rapa 0.195 (0.01), veh 0.28 (0.02) | ++ | |||
| OA rapa 0.195 (0.01), veh 0.25 (0.04) | ++ | |||
| b | Wang et al., 2014 | Morris water maze: | ||
| Escape latency day 4 (s) | Rapa 25(3), ctrl 35(4) | + | ||
| Escape latency trial (s) | Rapa 16(5), ctrl 32(2.5) | ++ | ||
| Time in target quadrant (s) | Rapa 26(3.5), ctrl 12.5(1) | ++ | ||
| b | Zhu et al., 2014 | Morris water maze: | ||
| Escape latency (s) | Scop + rapa−50 (7.5) | − | ||
| Scop + saline−38 (5) | ++ | |||
| Saline only−55 (7) | ||||
| Scop + rapa + MAD−39 (4) | + | |||
| Time in target quadrant (%) | Scop + rapa−65 (7) | − | ||
| Scop + saline−80 (8) | + | |||
| Saline only−62 (6) | ||||
| Scop + rapa + MAD−75 (7.5) | + | |||
| b | Lin et al., 2015 | Morris water maze: | ||
| Escape latency (s) | Rapa 25(1), ctrl 19(2) | ± | ||
| Platform crosses (#) | Rapa 1.6(0.25), ctrl 1.75(0.25) | ± | ||
| b | Wang et al., 2016 | Y-maze (alternation %): | ||
| 4wks post infusion | Rapa 39(6), ctrl 62(10) | − | ||
| 8 wks post infusion | Rapa 48(7), ctrl 53(8) | ± | ||
| b | Jahrling et al., 2017 | Morris water maze: | ||
| Escape latency day 4 (s) | Rapa 30(4), ctrl 40(5) | +++ | ||
| Trial time in target quadrant (%) | Rapa 32(6), ctrl 13(2) | + | ||
| Spatial Novelty (>0.33 = intact) | Rapa 0.44 (0.02), ctrl 0.34 (0.02) | +++ | ||
| b | Zhang et al., 2017 | Morris water maze: | ||
| Escape latency day 5 (s) | Rapa−32.5(5), veh 67(13) | +++ | ||
| Time in target quadrant (%) | Rapa−42.5(7.5), veh 22.5(7.5) | + | ||
| Number of platform crossings (#) | Rapa−3.75 (5.5), veh−1.5 (0.5) | + | ||
| c | Umeda et al., 2016 | Morris water maze: | ||
| Escape latency day 5 (s) | 12m APP rif−19(5), veh−35(6) | ++ | ||
| 18m APP veh−36(5) | ||||
| 18m APP rif0.5mg−29(5) | ± | |||
| 18m APP rif1mg−17.5(5) | ++ | |||
| 8m Tau609 rif0.5mg 14(2.3), veh−41(8) | + | |||
| 15m Tau609 rif1mg 29(7), veh 43(7) | ± | |||
| Time in target quadrant (%) | 12m APP rif−45(5), veh−29(3) | ± | ||
| 18m APP veh−29(6) | ||||
| 18m APP rif0.5mg−37(6) | ± | |||
| 18m APP rif1mg−49(4) | + | |||
| 8m Tau609 rif0.5mg 30(4), veh−16(7) | + | |||
| 15m Tau609 rif0.5mg 42(10), veh−21(9) | ± | |||
| d | Lonskaya et al., 2013 | Morris water maze: | ||
| Time in target quadrant (%) | Aβ icv bosu 29(1), ctrl 19 (1) | + | ||
| Time in target quadrant (% of WT) | Tg bosu 87.5(15) ctrl 75(10) | + | ||
| Platform crosses (#) | Aβ icv bosu 5.5 (0.5), ctrl 4 (0.25) | + | ||
| Platform crosses (% WT) | Tg bosu 147.5(7.5), ctrl 80(5) | + |
Results of animal studies testing the effect of lithium (a), rapamycin (b), rifampicin (c) and bosutinib (d) on cognition.
+++ favoring intervention, highly significant p < 0.001. ++ favoring intervention, significant p < 0.01. + favoring intervention, significant p < 0.05. +/± trend favoring intervention, p < 0.1. ± not significant. +/± trend favoring control, p < 0.1. –favoring control, significant p < 0.05. – favoring control, significant p < 0.01. — favoring control, highly significant p < 0.001. LPS, lipopolysaccharide; MA, treated from middle age; MAD, 3-methyladenine; NR, p-value not reported; OA, treated from old age; Scop, scopolamine; YA, treated from young adulthood.
Table 4
| Author, year | Cognitive tests | Outcome | Significance | |
|---|---|---|---|---|
| a | Pomara et al., 1983 | Buschke selective reminding test | [2pt]No quantitative data reported—“None of the psychometric measures showed either consistent, significant increases or decreases” | ± |
| Digit span/supraspan test | ||||
| Sperling test of iconic memory | ||||
| Word fluency tasks | ||||
| Wechsler Memory Scale | ||||
| a | Macdonald et al., 2008 | Change in MMSE | Li−4.8 (5.5), ctrl−4.0 (5.0) | ± |
| a | Hampel et al., 2009 | MMSE | Li−23.6 (1.6) -> 22.6 (3.5) | ± |
| PBO−23.6 (1.7) -> 23.2 (2.7) | ||||
| ADAS-Cog | Li−15.8 (4.2) -> 15.6 (4.4) | ± | ||
| PBO−5.4 (5)-> 16.6 (5.1) | ||||
| ADAS-Cog % with improvement >4 points | Li−28.6%, PBO−14.3% | NR | ||
| a | Leyhe et al., 2009 | ADAS-Cog | Li 19.2 (5.7) -> 17.7 (5.8) | + |
| PBO 16.5 (5.1) -> 18.0 (5.1) | ||||
| a | Forlenza et al., 2011 | ADAS-Cog | Li 11.0(6.7)->12.6(6.6), PBO 10.7(5.1)-> 13.9(8.5) | + |
| CDR–SoB | Li 1.4(1.3) -> 2.2(1.8), PBO 1.9(1.4) -> 2.8(2.3) | ± | ||
| Delayed recall | Li 4.8(2.1) -> 4.8(2.2), PBO 4.2(2.3) -> 4.5(2.3) | ± | ||
| Figure recall | Li 2.3(1.2) -> 2.0(1.3), PBO 1.9(1.1) -> 1.6(1.2) | ± | ||
| Sequence letters & numbers | Li 6.4(2.1) -> 6.0(2.9), PBO 6.3(2.6) -> 5.1(2.6) | + | ||
| Trail making test A (s) | Li 69.1(44.2) -> 62.8(31.5), PBO 89.9(67.4) -> 63.6(41.9) | ± | ||
| Trail making test B | Li 171.8(83.9) -> 184.9(78.1), PBO 207.1 (79.6) -> 190.7 (92.8) | ± | ||
| Conversion MCI->AD | Li (n = 20) Stable = 16, Progress = 4 | ± | ||
| PBO (n = 20) Stable = 13, Progress = 7 | ||||
| MCI->AD converters CDR-SoB | Li 3.3(1.3) -> 4.4(1.5), PBO 3.4(1.4) -> 5.6(1.5) | + | ||
| a | Nunes et al., 2013 | MMSE | Li 19.48 (0.67) -> 19.82 (0.9) | +++ |
| PBO 17.95 (0.73) -> 14 (1.326) | ||||
| b | Kraig et al., 2018 | Pre-post test change | ||
| EXIT25 | PBO 0.38 (-1.84, 2.61), rapa−0.1 (-3.31, 3.11) | ± | ||
| SLUMS | PBO 0.38 (-2.03, 1.26), rapa−0.8 (-3.92, 2.32) | ± | ||
| TAPS | PBO−1 (-3.18, 1.18), rapa 1.44 (-1.68, 4.57) | ± | ||
| c | Molloy et al., 2013 | SADAS-Cog | Rif−0m = 22, 12m = 27.5 | — |
| Doxy – 0m = 21, 12m = 25.5 | — | |||
| Rif + Doxy−0m = 22, 12m = 28 | — | |||
| PBO−0m = 21, 12m = 25 | ||||
| CDR-SoB mean | Rif−0m = 6, 12m = 8.5 | ± | ||
| Non-Rif−0m = 5.75, 12m = 7.75 | ||||
| Doxy – 0m = 6, 12m = 8.5 | ± | |||
| Non-Doxy – 0m = 5.75, 12m = 7.8 | ||||
| SMMSE | ns vs. placebo, data NR | ± | ||
| Qmci | Rif worse than PBO, data NR | — | ||
| d | Pagan et al., 2016 | MMSE (change 0w->24w) | 150mg–+3.85, 300mg–+3.5 | NR |
| SCOPA-Cog (change 0w->24w) | 150mg–+1.85, 300mg–+2.00 |
Results of human studies testing the effect of lithium (a), rapamycin (b), rifampicin & doxycycline (c) and nilotinib (d) on cognition.
+++ favoring intervention, highly significant p < 0.001. ++ favoring intervention, significant p < 0.01. + favoring intervention, significant p < 0.05. +/± trend favoring intervention, p < 0.1. ± not significant. +/± trend favoring control, p < 0.1. - favoring control, significant p < 0.05. – favoring control, significant p < 0.01. — favoring control, highly significant p < 0.001. EXIT25, Executive interview; NR, p-value not reported; PBO, placebo; RoB, Cochrane risk of bias; SLUMS, St Louis University Memory Status; TAPS, Texas Assessment of Processing Speed.
Rapamycin and cognitive aging
Rapamycin has been identified as the second most frequently investigated (n = 13 studies) proteostasis modulator. Twelve animal studies (10 mice, 2 rats), predominately using an Alzheimer's disease model, investigated the influence of rapamycin on cognitive aging (Table 1). Overall, nine out of twelve animal studies reported a statistically significant benefit on at least one cognitive outcome, irrespective of treatment duration (Table 3). One human study investigating safety, efficacy and tolerability of rapamycin in humans (Kraig et al., 2018), did not report any significant benefit to overall cognition in an older population (Table 4).
Rifampicin, tetracycline antibiotics, tyrosine kinase inhibitors and cognitive aging
Rifampicin, tetracycline antibiotics and tyrosine kinase inhibitors have been tested in both animal and human models (Tables 1, 2). The two rifampicin studies (1 mouse, 1 human; Tables 3, 4) investigating its therapeutic effects for Alzheimer disease had opposite findings, with positive cognitive outcomes in mice (Umeda et al., 2016), but no benefit found in the human study (Molloy et al., 2013).
Studies of tetracycline antibiotics showed similar results to rifampicin, with minocycline showing cognitive benefits in two studies using rat and chicken models of Alzheimer disease (Supplementary Table 1, Supplementary Table 2), while another tetracycline, doxycycline, showed no benefit in human AD patients, either alone or in combination with rifampicin (Molloy et al., 2013).
The tyrosine kinase inhibitor, bosutinib, was reported in mouse models of Alzheimer's disease as statistically beneficial to cognitive function, and another tyrosine kinase inhibitor, nilotinib, was found to improve scores on the MMSE and SCOPA-Cog in an open-label study in patients with Parkinson's disease, however the statistical significance was not reported (Tables 3, 4).
Other proteostasis-modifying drugs
There were six proteostasis modulators that have been tested to improve cognitive outcomes in animal models but are yet to be studied in human populations—M-CSF, GM-CSF, methylene blue, GGA, dantrolene, and phenylbutyric acid (Supplementary Table 1). Both of the M/GM-CSF studies indicated beneficial outcomes with the use of these therapeutics in mouse models of Alzheimer's disease, as was the case with methylene blue, GGA and phenylbutyric acid (Supplementary Table 2). Studies of dantrolene to improve cognitive outcomes showed a statistically significant improvement in one mouse model of Alzheimer's (Peng et al., 2012) but no benefit to cognition in another (Wu et al., 2015), with one study in aged rats indicating a trend toward benefit on Morris water maze performance.
Risk of bias
Table 5 shows the SYRCLE risk of bias ratings for animal studies. The majority of animal studies had an unclear risk of bias, as specific details of randomization and blinding were often not provided. Most studies provided information on the baseline characteristics of animals, and some studies did specify that the investigator performing behavioral assessments of the animals was blinded to the treatment status of the animal, indicating low risk of bias where this was the case. Overall the risk of bias was similar across studies regardless of the drug being tested.
Table 5
| Author, year | Sequence generation | Baseline characteristics | Allocation concealment | Random Housing | Blinding of personnel & participants | Random outcome assessment | Incomplete outcome data | Selective outcome reporting |
|---|---|---|---|---|---|---|---|---|
| LITHIUM | ||||||||
| Caccamo et al., 2007 | Unclear | Low | Unclear | Unclear | Unclear | Unclear | Low | Low |
| Nocjar et al., 2007 | Unclear | Low | Unclear | Unclear | Low | Unclear | Low | Low |
| Rockenstein et al., 2007 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low |
| Fiorentini et al., 2010 | Unclear | Low | Unclear | Unclear | Unclear | Unclear | Low | Low |
| Toledo and Inestrosa, 2010 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low |
| Sy et al., 2011 | Unclear | Low | Unclear | Unclear | Unclear | Unclear | Low | High |
| Nunes et al., 2015 | Unclear | Low | Unclear | Unclear | Unclear | Unclear | Low | Low |
| Wilson et al., 2017 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low |
| Nery et al., 2014 | Unclear | Low | Unclear | Unclear | Unclear | Low | Low | Low |
| McBride et al., 2010 | Unclear | Low | Unclear | Unclear | Low | Unclear | Low | Low |
| RAPAMYCIN | ||||||||
| Spilman et al., 2010 | Unclear | Low | Unclear | Unclear | Unclear | Unclear | Low | Low |
| Majumder et al., 2011 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low |
| Halloran et al., 2012 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low |
| Majumder et al., 2012 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low |
| Lin et al., 2013 | Unclear | Low | Unclear | Unclear | Low | Unclear | Low | Low |
| Neff et al., 2013 | Unclear | Low | Unclear | Unclear | Low | Unclear | Low | Low |
| Wang et al., 2014 | Unclear | Low | Unclear | Unclear | Unclear | Unclear | Low | Low |
| Zhu et al., 2014 | Low | Low | Unclear | Unclear | Unclear | Unclear | Low | Low |
| Lin et al., 2015 | Unclear | Low | Unclear | Unclear | Low | Unclear | Low | Low |
| Wang et al., 2016 | Unclear | Low | Unclear | Unclear | Unclear | Unclear | Low | Low |
| Jahrling et al., 2017 | Unclear | Low | Unclear | Unclear | Low | Unclear | Low | Low |
| Zhang et al., 2017 | Unclear | Low | Unclear | Unclear | Unclear | Unclear | Low | Low |
| RIFAMPICIN | ||||||||
| Umeda et al., 2016 | Unclear | Low | High | Unclear | High | Unclear | Low | Low |
| BOSUTINIB | ||||||||
| Lonskaya et al., 2013 | Unclear | Low | Unclear | Unclear | Low | Unclear | Low | High |
| M-CSF | ||||||||
| Boissonneault et al., 2009 | Unclear | Low | Unclear | Unclear | Low | Unclear | High | High |
| GM-CSF | ||||||||
| Boyd et al., 2010 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | High |
| METHYLENE bLUE | ||||||||
| Deiana et al., 2009 | Low | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low |
| Hochgräfe et al., 2015 | Unclear | Low | Unclear | Unclear | Unclear | Unclear | Low | Low |
| Medina et al., 2011 | Unclear | Low | Unclear | Unclear | Unclear | Unclear | Low | High |
| Stack et al., 2014 | Low | Low | Unclear | Unclear | Unclear | Unclear | Low | Low |
| GGA | ||||||||
| Hoshino et al., 2013 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low |
| Sun et al., 2017 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low |
| DANTROLENE | ||||||||
| Hopp et al., 2014 | Unclear | Low | Unclear | Unclear | Unclear | Unclear | Low | Low |
| Peng et al., 2012 | Unclear | Low | Unclear | Unclear | Unclear | Unclear | Low | Low |
| Wu et al., 2015 | Unclear | Low | Unclear | Unclear | Unclear | Unclear | Low | Low |
| PHENYLBUTYRIC ACID | ||||||||
| Wiley et al., 2011 | Unclear | Low | Unclear | Unclear | Low | Unclear | Low | Low |
| MINOCYCLINE | ||||||||
| Choi et al., 2007 | Unclear | Low | Unclear | Unclear | Unclear | Unclear | Low | High |
| Gibbs and Gibbs, 2013 | Unclear | Low | Unclear | Unclear | Unclear | Unclear | Low | Low |
SYRCLE Risk of Bias for animal studies.
Table 6 shows the Cochrane risk of bias rating for human studies. There was significant heterogeneity among lithium studies, with (Pomara et al., 1983; Macdonald et al., 2008) scoring high risk of bias across most or all domains due to an open label design and not reporting all quantitative outcome data. (Hampel et al., 2009) had an intermediate risk of bias as investigators were aware of patient treatment status. Studies by Leyhe et al. (2009), Forlenza et al. (2011) and Nunes et al. (2013) scored a lower risk of bias due to randomized, double-blind designs, though details of sequence generation and allocation concealment were not reported. Studies of rapamycin (Kraig et al., 2018) and rifampicin (Molloy et al., 2013) scored a low risk of bias across most domains. The study using nilotinib (Pagan et al., 2016) was rated as having a high risk of bias across most domains, due to an open label design.
Table 6
| Author, year | Sequence generation | Allocation concealment | Blinding of personnel & participants | Blinding of outcome assessment | Incomplete outcome data | Selective outcome reporting |
|---|---|---|---|---|---|---|
| LITHIUM | ||||||
| Pomara et al., 1983 | High | High | High | High | High | Unclear |
| Macdonald et al., 2008 | High | High | High | High | High | High |
| Hampel et al., 2009 | Low | Unclear | Low | High | Low | Low |
| Leyhe et al., 2009 | Unclear | Unclear | Low | Unclear | Low | Low |
| Forlenza et al., 2011 | Unclear | Unclear | Low | Low | Low | Low |
| Nunes et al., 2013 | Unclear | Unclear | Low | Low | Low | Low |
| RAPAMYCIN | ||||||
| Kraig et al., 2018 | Low | Unclear | Low | Low | Low | Low |
| RIFAMPICIN | ||||||
| Molloy et al., 2013 | Low | Low | Low | Low | Low | Low |
| NILOTINIB | ||||||
| Pagan et al., 2016 | High | High | High | High | Low | Low |
Cochrane Risk of Bias for human studies.
Registered human trials
Figure 2 shows the progress of the drugs identified in our search from animal into human studies, including planned or ongoing studies registered on clinicaltrials.gov. Nilotinib has two phase 2 studies registered, one in Alzheimer's disease and one in Parkinson's disease. Lithium has one phase 4 study registered, while GM-CSF and phenylbutyric acid each have one phase 2 study registered in Alzheimer's disease.
Discussion
In this review we have summarized all current animal and human research studies that have investigated the effect of proteostasis modulators on cognitive function. Overall, the therapeutic alteration of proteostasis pathways using repurposed drugs is a promising approach to the treatment of dementia and age-related cognitive decline with a reasonable research translation between animal and human studies showing similar conclusions observed across the studies.
Lithium
Lithium was the most studied proteostasis modifying drug identified in this review, and the furthest progressed in translation to treatment of age-related dementia. All except one of the animal studies included in this review showed a benefit to at least one cognitive outcome despite heterogeneity in species, model, dose, stage of disease at intervention and duration of treatment. Three of six human studies also found benefit, and consistent with the results in mice the largest effect was observed with microdoses and long duration of treatment (Nunes et al., 2013). The only negative RCT used a high dose for a short duration (Hampel et al., 2009). Taken together, these studies show lithium has a consistent benefit in Alzheimer's disease in animal models and humans, which appears more pronounced with lower doses, longer durations of treatment, and when commenced at an earlier stage of disease.
The apparent superiority of lower doses of lithium is encouraging, as at standard psychiatric dose it can have renal and thyroid-related side effects that limit tolerability and is toxic at levels only slightly above the therapeutic range (Timmer and Sands, 1999). Higher lifetime exposure to natural microlevels of lithium in drinking water is associated with a reduced incidence of dementia (Kessing et al., 2017), a finding which adds plausibility to the idea that microdoses of lithium rather than the current therapeutic doses might be beneficial for the treatment of dementia.
A possible explanation for lithium's non-linear dose response involves the interaction between lithium's autophagy-enhancing inhibition of inositol triphosphate receptor (IP3R) signaling, and its autophagy-reducing inhibition of GSK-3B, a tau-phosphorylating kinase (Sarkar et al., 2008). Genetic reduction of IP3R signaling in drosophila rescues Alzheimer's disease phenotypes in the same way lithium does (McBride et al., 2010), suggesting lithium's inhibition of IP3R signaling via reducing the formation of inositol triphosphate (IP3) may be sufficient to cause its benefits. This is mechanistically plausible, as inhibition of IP3R signaling enhances autophagy through decreasing calcium release from the endoplasmic reticulum, enhancing proteostasis (Sarkar et al., 2005). However; lithium's inhibition of GSK3B has also been postulated to have beneficial effects in Alzheimer's disease via reducing tau phosphorylation and neurofibrillary tangle formation as well as inhibiting autophagy via increasing mTOR signaling (Sarkar et al., 2008). Although, this latter theory is less likely to explain the positive influence of microdoses of lithium as lower levels of lithium do not inhibit GSK-3B in mice (Nunes et al., 2015). However, this GSK-3B mechanism may explain the reduced efficacy of lithium observed with increasing doses. Increased mTOR signaling due to GSK3B inhibition counteracts the autophagy-enhancing effects of reduced IP3R activation beyond a certain dose (Sarkar et al., 2009). If increased mTOR activity limits the effective dose of lithium, this raises the question of whether an mTOR inhibitor such as rapamycin combined with lithium would have beneficial synergistic effects in dementia or cognitive aging. A synergistic benefit of lithium and rapamycin has been demonstrated in a drosophila Huntington's disease model (Sarkar et al., 2008), but to our knowledge this has not been explored in models of Alzheimer's disease.
Rapamycin
Predictably, rapamycin the known inhibitor of mTOR was also one of the most studied proteostasis modulators investigated, more so in animal models then in humans. Overall, the trials indicated a positive therapeutic effect of rapamycin on cognitive outcomes. Cognitive benefits via rapamycin was demonstrated in models of normal aging (Majumder et al., 2011, 2012; Neff et al., 2013; Wang et al., 2014), vascular dementia (Jahrling et al., 2017), and transgenic Alzheimer's disease models (Spilman et al., 2010; Majumder et al., 2011; Lin et al., 2013; Zhang et al., 2017), which suggests rapamycin has potential to rescue cognitive decline caused by a range of pathologies. This breadth of efficacy is a promising characteristic, as autopsy studies have demonstrated that mixed pathology is common in dementia sufferers (Nelson et al., 2016).
Majumder et al. (2011) reported a possible mechanism explaining their findings that rapamycin while effective in preventing cognitive decline before pathology develops, fails as a treatment once Alzheimer's pathology is established. They found rapamycin induces autophagy strongly both before and after Alzheimer's pathology is present. However, increased autophagy induction fails to reduce levels of amyloidβ in mice with established disease and leads to accumulation of enlarged autophagosomes containing undigested material. This finding suggests deficient substrate clearance and is consistent with previous findings that autophagy in Alzheimer's disease is principally defective at the stage of autolysosomal proteolysis (Nixon and Yang, 2011; Bordi et al., 2016). Therefore, to be effective in established Alzheimer's dementia rapamycin may need to be combined with a drug that can enhance autophagy at the stage of autolysosomal digestion.
Rapamycin's demonstrated ability to improve phenotypes of aging in animals has recently led to human trials assessing safety and efficacy in older adults (Kraig et al., 2018). Despite demonstrating low-dose rapamycin can be used safely in older adults, they were unable to show significant enhancement of cognition. However, larger trials are required to determine the potential benefit of rapamycin's for cognitive aging in humans.
Rifampicin, tetracycline antibiotics and tyrosine kinase inhibitors
Rifampicin was more effective when started earlier in the disease process, and when used at a higher dose (Umeda et al., 2016). Cohorts treated with the lower dose at a later stage of disease did not show an improved cognitive benefit, a finding which is relevant to interpreting the DARAD study of rifampicin in Alzheimer's disease by Molloy et al. (2013).
In the DARAD study, rifampicin was tested in Alzheimer's patients at a low dose and showed either no benefit or on some measures a significant worsening of cognition compared to placebo. A possible explanation for this failure is an insufficient dose and treatment duration. Data supporting this view is provided by Iizuka et al. (2017), who in an observational study determined that a minimum dose rifampicin of 450 mg/day for at least 12 months was required before any cognitive improvement was observed (Iizuka et al., 2017).
Doxycycline also failed to produce benefit for human Alzheimer's disease patients in the DARAD study (Molloy et al., 2013), despite positive animal studies with closely related tetracycline antibiotic minocycline (Choi et al., 2007; Gibbs and Gibbs, 2013). It is difficult to know what implications this has for minocycline's repurposing potential, however currently there are no upcoming human studies registered on clinicaltrials.gov for either doxycycline or minocycline in dementia.
Lonskaya et al examined bosutinib in two mouse models of established Alzheimer's disease, and demonstrated statistically significant benefits after 3 weeks of treatment. Beneficial effects on cognition were also observed in human Parkinson's disease patients by Pagan et al. although the small size and open label design of the study mean the results require confirmation in larger randomized trials. These promising findings have led to significant interest in repurposing these drugs, to the extent phase 2 clinical trials are currently assessing the effect of these drugs in Alzheimer's and Parkinson's disease cohorts.
Other proteostasis-modifying drugs
Three other proteostasis-modifying drugs have excited interest in translating promising animal study findings into humans—GM-CSF, methylene blue and phenylbutyric acid have registered phase 2 studies on clinicaltrials.gov to test their use in Alzheimer's disease.
Dantrolene is a drug of interest due to similarities it shares with lithium, in that it enhances autophagy by reducing calcium efflux from the endoplasmic reticulum (Wang et al., 2017). Unlike lithium however it acts by inhibition of the ryanodine receptor rather than IP3R signaling, raising the possibility of a complementary mechanism of action (Vervliet et al., 2017). This suggests dantrolene is worth testing in human trials to determine whether it can provide similar benefits to lithium, and in combination with lithium in animal models for potential synergistic effects. Currently however, no human trials are registered on clinicaltrials.gov for dantrolene in dementia.
Limitations
Our study has several limitations. Our search strategy was based primarily on key terms related to the mechanisms of proteostasis, with the addition of a selection of drugs well known to modulate these processes. Therefore, our search may have missed studies that examine proteostasis-modifying drugs not named in our search and not mentioning proteostasis related key terms. However, we addressed this by adding relevant articles by snowballing.
Secondly, because the present study's focus is restricted to approved drugs, it does not provide an adequate overview of the translational pipeline where a repurposed drug is used as the basis for novel molecules that proceed into later stage studies.
Third, we cannot exclude publication bias, particularly in animal studies which are unlikely to be registered beforehand and may be less likely to be published if results are negative.
Conclusions
The results of this review support the concept of a translational approach to repurposing proteostasis modifying drugs for the treatment of age-related dementia and cognitive decline. However, larger clinical trials assessing the influence of these drugs particularly, lithium and rapamycin are required before they are ready for the clinic. In addition, animal models assessing whether the combination of proteostasis modulators can act in synergy to improve cognitive outcomes are required. A translational strategy based on systematic screening of rational drug combinations starting in simple model organisms such as C. elegans may provide a pipeline of novel candidate therapies to advance into human studies.
Statements
Author contributions
DH: Search strategy, screening, data extraction, drafting manuscript; CT: Search strategy screening, data extraction, drafting manuscript; NL: Search strategy, drafting manuscript; AM: Search strategy, conflict resolution, drafting manuscript.
Funding
An unrestricted grant by the University of Melbourne supported the work.
Acknowledgments
Thanks to Patrick Condron from the Brownless Biomedical Library, University of Melbourne, for his assistance with our search strategy.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2018.01520/full#supplementary-material
References
1
BarzilaiN.CrandallJ. P.KritchevskyS. B.EspelandM. A. (2016). Metformin as a tool to target aging. Cell Metab.23, 1060–1065. 10.1016/j.cmet.2016.05.011
2
Bermejo-ParejaF.Benito-LeónJ.VegaS.MedranoM. J.RománG. C. (2008). Incidence and subtypes of dementia in three elderly populations of central Spain. J. Neurol. Sci.264, 63–72. 10.1016/j.jns.2007.07.021
3
BoissonneaultV.FilaliM.LessardM.ReltonJ.WongG.RivestS. (2009). Powerful beneficial effects of macrophage colony-stimulating factor on beta-amyloid deposition and cognitive impairment in Alzheimer's disease. Brain132, 1078–1092. 10.1093/brain/awn331
4
BordiM.BergM. J.MohanP. S.PeterhoffC. M.AlldredM. J.CheS.et al. (2016). Autophagy flux in CA1 neurons of Alzheimer hippocampus: Increased induction overburdens failing lysosomes to propel neuritic dystrophy. Autophagy12, 2467–2483. 10.1080/15548627.2016.1239003
5
BoydT. D.BennettS. P.MoriT.GovernatoriN.RunfeldtM.NordenM.et al. (2010). GM-CSF upregulated in rheumatoid arthritis reverses cognitive impairment and amyloidosis in Alzheimer mice. J. Alzheimers. Dis.21, 507–518. 10.3233/JAD-2010-091471
6
CaccamoA.OddoS.TranL. X.LaFerlaF. M. (2007). Lithium reduces Tau phosphorylation but not Aβ or working memory deficits in a transgenic model with both plaques and tangles. Am. J. Pathol.170, 1669–1678. 10.2353/ajpath.2007.061178
7
ChoiY.KimH. S.ShinK. Y.KimE. M.KimM.KimH. S.et al. (2007). Minocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer's Disease models. Neuropsychopharmacology32, 2393–2404. 10.1038/sj.npp.1301377
8
DeianaS.HarringtonC. R.WischikC. M.RiedelG. (2009). Methylthioninium chloride reverses cognitive deficits induced by scopolamine: comparison with rivastigmine. Psychopharmacology202, 53–65. 10.1007/s00213-008-1394-2
9
FiorentiniA.RosiM. C.GrossiC.LuccariniI.CasamentiF. (2010). Lithium improves hippocampal neurogenesis, neuropathology and cognitive functions in APP mutant mice. Zars, T., ed. PLoS ONE5:e14382. 10.1371/journal.pone.0014382
10
ForlenzaO. V.DinizB. S.RadanovicM.SantosF. S. (2011). Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br. J. Psychiatry198, 351–356. 10.1192/bjp.110.080044
11
GangulyG.ChakrabartiS.ChatterjeeU.SasoL. (2017). Proteinopathy, oxidative stress and mitochondrial dysfunction: cross talk in Alzheimer's disease and Parkinson's disease. Drug Des. Devel. Ther.11, 797–810. 10.2147/DDDT.S130514
12
GibbsM. E.GibbsC. L. (2013). Deleterious effects of soluble beta amyloid on cognition, antagonism by saline and noradrenaline, a role for microglia. Neuroscience230, 62–71. 10.1016/j.neuroscience.2012.10.070
13
HalloranJ.HussongS.BurbankR.PodlutskayaN.FischerK.SloaneL.et al. (2012). Chronic inhibition of mTOR by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience223, 102–113. 10.1016/j.neuroscience.2012.06.054
14
HampelH.EwersM.BürgerK.AnnasP.MörtbergA.BogstedtA.et al. (2009). Lithium trial in Alzheimer's Disease: a randomized, single-blind, placebo-controlled, multicenter 10-week study. J. Clin. Psychiatry70, 922–931.
15
HigginsJ. P. T.AltmanD. G.GøtzscheP. C.JüniP.MoherD.OxmanA. D.et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trialsBMJ343:d5928. 10.1136/bmj.d5928
16
HochgräfeK.SydowA.MateniaD.CadinuD.KönenS.PetrovaO.et al. (2015). Preventive methylene blue treatment preserves cognition in mice expressing full-length pro-aggregant human Tau. Acta Neuropathol. Commun.3:25. 10.1186/s40478-015-0204-4
17
HooijmansC. R.RoversM. M.de VriesR. B.LeenaarsM.Ritskes-HoitingaM.LangendamM. W. (2014). SYRCLE's risk of bias tool for animal studies. BMC Med. Res. Methodol.14:43. 10.1186/1471-2288-14-43
18
HoppS. C.D'AngeloH. M.RoyerS. E.KaercherR. M.AdzovicL.WenkG. L. (2014). Differential rescue of spatial memory deficits in aged rats by L-type voltage-dependent calcium channel and ryanodine receptor antagonism. Neuroscience280, 10–18. 10.1016/j.neuroscience.2014.09.007
19
HoshinoT.SuzukiK.MatsushimaT.YamakawaN.SuzukiT.MizushimaT. (2013). Suppression of Alzheimer's disease-related phenotypes by geranylgeranylacetone in mice. PLoS ONE8:e76306. 10.1371/journal.pone.0076306
20
IizukaT.MorimotoK.SasakiY.KameyamaM.KurashimaA.HayasakaK.et al. (2017). preventive effect of rifampicin on alzheimer disease needs at least 450 mg daily for 1 year: An FDG-PET follow-up study. Dement. Geriatr. Cogn. Dis. Extra. 7, 204–214. 10.1159/000477343
21
JahrlingJ. B.LinA. L.DeRosaN.HussongS. A.Van SkikeC. E.GirottiM.et al. (2017). mTOR drives cerebral blood flow and memory deficits in LDLR-/- mice modeling atherosclerosis and vascular cognitive impairment. J. Cereb. Blood Flow Metab.38, 58–74. 10.1177/0271678X17705973
22
KessingL. V.GerdsT. A.KnudsenN. N.JørgensenL. F.KristiansenS. M.VoutchkovaD.et al. (2017). Association of lithium in drinking water with the incidence of dementia. JAMA Psychiatry74, 1005–1010. 10.1001/jamapsychiatry.2017.2362
23
KraigE.LinehanL. A.LiangH.RomoT. Q.LiuQ.WuY.et al. (2018). A randomized control trial to establish the feasibility and safety of rapamycin treatment in an older human cohort: Immunological, physical performance, and cognitive effects. Exp. Gerontol.105, 53–69. 10.1016/j.exger.2017.12.026
24
LeyheT.EschweilerG. W.StranskyE.GasserT.AnnasP.BasunH.et al. (2009). Increase of BDNF Serum Concentration in Lithium Treated Patients with Early Alzheimer's Disease. Journal of Alzheimer's Disease16, 649–656. 10.3233/JAD-2009-1004
25
LinA. L.JahrlingJ. B.ZhangW.DeRosaN.BakshiV.RomeroP.et al. (2015). Rapamycin rescues vascular, metabolic and learning deficits in apolipoprotein E4 transgenic mice with pre-symptomatic Alzheimer's disease. J. Cereb. Blood Flow Metab.37, 217–226. 10.1177/0271678X15621575
26
LinA. L.ZhengW.HalloranJ. J.BurbankR. R.HussongS. A.HartM. J.et al. (2013). Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer's disease. J. Cereb. Blood Flow Metab. 33, 1412–1421. 10.1038/jcbfm.2013.82
27
LonskayaI.HebronM. L.DesforgesN. M.FranjieA.MoussaC. E. (2013). Tyrosine kinase inhibition increases functional parkin-Beclin-1 interaction and enhances amyloid clearance and cognitive performance. EMBO Mol. Med.5, 1247–1262. 10.1002/emmm.201302771
28
López-OtínC.BlascoM. A.PartridgeL.SerranoM.KroemerG. (2013). The hallmarks of aging. Cell53, 1194–1217. 10.1016/j.cell.2013.05.039
29
MacdonaldA.BriggsK.PoppeM.HigginsA.VelayudhanL.LovestoneS. (2008). A feasibility and tolerability study of lithium in Alzheimer's disease. Int. J. Geriatr. Psychiatry23, 704–711. 10.1002/gps.1964
30
MajumderS.CaccamoA.MedinaD. X.BenavidesA. D.JavorsM. A.KraigE.et al. (2012). Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1beta and enhancing NMDA signaling. Aging Cell11, 326–335. 10.1111/j.1474-9726.2011.00791.x
31
MajumderS.RichardsonA.StrongR.OddoS. (2011). Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLoS ONE6:e25416. 10.1371/journal.pone.0025416
32
McBrideS. M.ChoiC. H.SchoenfeldB. P.BellA. J.LiebeltD. A.FerreiroD.et al. (2010). Pharmacological and genetic reversal of age dependent cognitive deficits due to decreased presenilin function. J. Neurosci. 30, 9510–9522. 10.1523/JNEUROSCI.1017-10.2010
33
MedinaD. X.CaccamoA.OddoS. (2011). Methylene blue reduces aβ levels and rescues early cognitive deficit by increasing proteasome activity. Brain Pathol.21, 140–149. 10.1111/j.1750-3639.2010.00430.x
34
MolloyD. W.StandishT. I.ZhouQ.GuyattG.DARAD Study Group. (2013). A multicenter, blinded, randomized, factorial controlled trial of doxycycline and rifampin for treatment of Alzheimer's disease: the DARAD trial. Int. J. Geriatr. Psychiatry28, 463–470. 10.1002/gps.3846
35
NeffF.Flores-DominguezD.RyanD. P.HorschM.SchröderS.AdlerT.et al. (2013). Rapamycin extends murine lifespan but has limited effects on aging. J. Clin. Invest.123, 3272–3291. 10.1172/JCI67674
36
NelsonP. T.TrojanowskiJ. Q.AbnerE. L.Al-JanabiO. M.JichaG. A.SchmittF. A.et al. (2016). “New old pathologies”: AD, PART, and cerebral age-related TDP-43 with sclerosis (CARTS). J. Neuropathol. Exp. Neurol.75, 482–498. 10.1093/jnen/nlw033
37
NeryL. R.EltzN. S.HackmanC.FonsecaR.AltenhofenS.GuerraH. N.et al. (2014). Brain intraventricular injection of amyloid-β in zebrafish embryo impairs cognition and increases tau phosphorylation, effects reversed by lithium. LaksJ. ed. PLoS ONE9:e105862. 10.1371/journal.pone.0105862
38
NixonR. A.YangD.-S. (2011). Autophagy failure in Alzheimer's disease – locating the primary defect. Neurobiol. Dis.43, 38–45. 10.1016/j.nbd.2011.01.021
39
NocjarC.HammondsM. D.ShimS. S. (2007). chronic lithium treatment magnifies learning in rats. Neuroscience150, 774–788. 10.1016/j.neuroscience.2007.09.063
40
NunesM. A.SchöweN. M.Monteiro-SilvaK. C.Baraldi-TornisieloT.SouzaS. I.BalthazarJ.et al. (2015). Chronic Microdose Lithium Treatment Prevented Memory Loss and Neurohistopathological Changes in a Transgenic Mouse Model of Alzheimer's Disease. HolscherC. ed. PLoS ONE10:e0142267. 10.1371/journal.pone.0142267
41
NunesM. A.VielT. A.BuckH. S. (2013). Microdose lithium treatment stabilized cognitive impairment in patients with Alzheimer's Disease. Curr. Alzheimer Res.10, 104–107.
42
PaganF.HebronM.ValadezE. H.Torres-YaghiY.HuangX.MillsR. R.et al. (2016). Nilotinib effects in Parkinson's disease and dementia with lewy bodies. J. Parkinsons. Dis.6, 503–517. 10.3233/JPD-160867
43
PengJ.LiangG.InanS.WuZ.JosephD. J.MengQ.et al. (2012). Dantrolene ameliorates cognitive decline and neuropathology in Alzheimer triple transgenic mice. Neurosci. Lett.516, 274–279. 10.1016/j.neulet.2012.04.008
44
PomaraN.Banay-SchwartzM.BlockR.StanleyM.GershonS. (1983). Elevation of RBC glycine and choline levels in geriatric patients treated with lithium. Am. J. Psychiatry140, 911–913. 10.1176/ajp.140.7.911
45
PrinceM.WimoA.GuerchetM.AliG.WuY.PrinaM.et al. (2015). The World Alzheimer Report 2015, The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends. Alzheimer's Disease International. Available online at: https://www.alz.co.uk/research/world-report-2015
46
RockensteinE.TorranceM.AdameA.ManteM.Bar-onP.RoseJ. B.et al. (2007). Neuroprotective effects of regulators of the glycogen synthase kinase-3β signaling pathway in a transgenic model of alzheimer's disease are associated with reduced amyloid precursor protein phosphorylation. J. Neurosci.27, 1981–1991. 10.1523/JNEUROSCI.4321-06.2007
47
SarkarS.FlotoR. A.BergerZ.ImarisioS.CordenierA.PascoM.et al. (2005). Lithium induces autophagy by inhibiting inositol monophosphatase. J. Cell Biol. 170, 1101–1111. 10.1083/jcb.200504035
48
SarkarS.KrishnaG.ImarisioS.SaikiS.O'KaneC. J.RubinszteinD. C. (2008). A rational mechanism for combination treatment of Huntington's disease using lithium and rapamycin. Hum. Mol. Genet.17, 170–178. 10.1093/hmg/ddm294
49
SarkarS.RavikumarB.FlotoR. A.RubinszteinD. C. (2009). Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ.16, 46–56. 10.1038/cdd.2008.110
50
SpilmanP.PodlutskayaN.HartM. J.DebnathJ.GorostizaO.BredesenD.et al. (2010). Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PLoS ONE5:e9979. 10.1371/journal.pone.0009979
51
StackC.JainuddinS.ElipenahliC.GergesM.StarkovaN.StarkovA.et al. (2014). Methylene blue upregulates Nrf2/ARE genes and prevents tau-related neurotoxicity. Hum. Mol. Genet. 23, 3716–3732. 10.1093/hmg/ddu080
52
SunY.ZhangJ. R.ChenS. (2017). Suppression of Alzheimer's disease-related phenotypes by the heat shock protein 70 inducer, geranylgeranylacetone, in APP/PS1 transgenic mice via the ERK/p38 MAPK signaling pathway. Exp. Ther. Med.14, 5267–5274. 10.3892/etm.2017.5253
53
SyM.KitazawaM.MedeirosR.WhitmanL.ChengD.LaneT. E.et al. (2011). Inflammation induced by infection potentiates tau pathological features in transgenic mice. Am. J. Pathol.178, 2811–2822. 10.1016/j.ajpath.2011.02.012
54
TimmerR. T.SandsJ. M. (1999). Lithium Intoxication. J. Am. Soc. Nephrol.10, 666–674.
55
ToledoE. M.InestrosaN. C. (2010). Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1DE9 mouse model of Alzheimer's disease. Mol. Psychiatry15, 272–285. 10.1038/mp.2009.72
56
UmedaT.OnoK.SakaiA.YamashitaM.MizuguchiM.KleinW. L.et al. (2016). Rifampicin is a candidate preventive medicine against amyloid-beta and tau oligomers. Brain139(Pt 5): 1568–1586. 10.1093/brain/aww042
57
VervlietT.PintelonI.WelkenhuyzenK.BootmanM. D.BannaiH.MikoshibaK.et al. (2017). Basal ryanodine receptor activity suppresses autophagic flux. Biochem. Pharmacol.132, 133–142. 10.1016/j.bcp.2017.03.011
58
WangS.ZhouS. L.MinF. Y.MaJ. J.ShiX. J.BereczkiE.et al. (2014). mTOR-mediated hyperphosphorylation of tau in the hippocampus is involved in cognitive deficits in streptozotocin-induced diabetic mice. Metab. Brain Dis.29, 729–736. 10.1007/s11011-014-9528-1
59
WangX.LiG. J.HuH. X.MaC.MaD. H.LiuX. L.et al. (2016). Cerebral mTOR signal and pro-inflammatory cytokines in Alzheimer's disease rats. Transl. Neurosci.7, 151–157. 10.1515/tnsci-2016-0022
60
WangY.ShiY.WeiH. (2017). Calcium dysregulation in Alzheimer's Disease: a target for new drug development. J. Alzheimers. Dis. Parkinsonism7:510.4172/2161-0460.1000374
61
WileyJ. C.Pettan-BrewerC.LadigesW. C. (2011). Phenylbutyric acid reduces amyloid plaques and rescues cognitive behavior in AD transgenic mice. Aging Cell10, 418–428. 10.1111/j.1474-9726.2011.00680.x
62
WilsonE. N.Do CarmoS.IulitaM. F.HallH.DucatenzeilerA.MarksA. R.et al. (2017). BACE1 inhibition by microdose lithium formulation NP03 rescues memory loss and early stage amyloid neuropathology. Transl. Psychiatry7:e1190. 10.1038/tp.2017.169
63
WuZ.YangB.LiuC.LiangG.EckenhoffM. F.LiuW.et al. (2015). Long-term dantrolene treatment reduced intraneuronal amyloid in aged Alzheimer triple transgenic mice. Alzheimer Dis. Assoc. Disord.29, 184–191. 10.1097/WAD.0000000000000075
64
YerburyJ. J.OoiL.DillinA.SaundersD. N.HattersD. M.BeartP. M.et al. (2016). Walking the tightrope: proteostasis and neurodegenerative disease. J. Neurochem.137, 489–505. 10.1111/jnc.13575
65
ZhangL.WangL.WangR.GaoY.CheH.PanY.et al. (2017). Evaluating the Effectiveness of GTM-1, Rapamycin, and Carbamazepine on Autophagy and Alzheimer Disease. Med. Sci. Monit.23, 801–808. 10.12659/MSM.898679
66
ZhuB.YangC.DingL. C.LiuN. (2014). 3-methyladenine, an autophagic inhibitor, attenuates therapeutic effects of sirolimus on scopolamine-induced cognitive dysfunction in a rat model. Int. J. Clin. Exp. Med.7, 3327–3332.
Summary
Keywords
aging, alzheimer's disease, dementia, lithium, proteostasis, rapamycin
Citation
Heard DS, Tuttle CSL, Lautenschlager NT and Maier AB (2018) Repurposing Proteostasis-Modifying Drugs to Prevent or Treat Age-Related Dementia: A Systematic Review. Front. Physiol. 9:1520. doi: 10.3389/fphys.2018.01520
Received
30 July 2018
Accepted
09 October 2018
Published
30 October 2018
Volume
9 - 2018
Edited by
Anis Larbi, Singapore Immunology Network (A*STAR), Singapore
Reviewed by
Carsten Merkwirth, Ferring Research Institute, Inc., United States; Michael Petrascheck, The Scripps Research Institute, United States
Updates
Copyright
© 2018 Heard, Tuttle, Lautenschlager and Maier.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea B. Maier andrea.maier@unimelb.edu.au
This article was submitted to Integrative Physiology, a section of the journal Frontiers in Physiology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.