- 1Department of Natural Sciences, Faculty of Science and Engineering, Tokyo City University, Tokyo, Japan
- 2Graduate School of Biological Sciences, Tokyo Metropolitan University, Tokyo, Japan
- 3Graduate School of Integrative Science and Engineering, Tokyo City University, Tokyo, Japan
The invasion of plants into specialized environments requires acclimatory changes, which can simultaneously act as barriers to further invasion in these contexts. In coastal areas, vegetation is shaped by various stresses from the marine environment; therefore, these areas are predominantly inhabited by plant species that have evolved specific acclimations to these stresses, including many endemic species. In recent years, the invasive species Bidens pilosa L. (Asteraceae) has been reported in the coastal areas of Japan. We conducted comparative morphological analyses of coastal and inland populations of B. pilosa to elucidate the background of their acclimation to coastal areas. The large leaf area of B. pilosa is suggested to be involved in the capture of sand blown by the wind in the unstable soil composed of sea sand in its habitat. In addition, the acclimatory changes in B. pilosa were achieved without considerable alterations in the resource allocation ratio between stems and leaves. The coastal population of B. pilosa exhibited a significantly higher ratio of resources allocated to roots, indicating that this acclimationfacilitated stable establishment in coastal soils and enhanced moisture acquisition in coastal areas subjected to severe drought stress. In contrast to the increased ratio of resource allocation to roots, the coastal population of B. pilosa displayed a significantly lower investment in inflorescences. The findings suggest that once a population is established, it can sustain itself with minimal investment in inflorescences. This is likely attributable to the limited number of plants in coastal areas and reduced competitive pressures in these environments. These acclimatory changes in B. pilosa may lead to the expansion of its habitat in various coastal areas.
1 Introduction
The invasion of introduced vascular plants currently poses a major threat to natural ecosystems across nearly all continents, the global economy, and human health (Theoharides and Dukes, 2007). Natural ecosystems are often plagued by the invasion of alien species, which constantly threaten native plant populations (Morgan, 1998; Wilkerson, 2013). Recent studies indicate that roads and hiking trails in high mountain regions, which host numerous endemic species, serve as corridors facilitating the introduction of alien species from lowland species pools to those of the highlands (Koyama et al., 2024). Additionally, these pathways alter the abiotic environment and establish disturbance-resistant alien species (Fuentes-Lillo et al., 2021). Invasive species can cause numerous problems in diverse environments (Marshall et al., 2003; Ellstrand et al., 2010; Jauni and Hyvönen, 2010).
The existence of locally adapted and acclimated plants indicates that disruptive selection promotes alternative trait combinations across distinct environments. This implies that trade-offs arising from phenological, physiological, morphological, and genetic constraints are critical determinants of diversification. Variations in plant populations are frequently observed when water availability changes abruptly over short spatial scales (Jackson and Jobbgy, 2005). In coastal areas, the accumulation of salts in the soil causes a decrease in its osmotic potential and results in plant dehydration; therefore, salt stress is associated with water stress (Kaspari et al., 2009). Roots, stems, and leaves can store water, and numerous studies have reported environmentally induced morphological changes in response to drought conditions within coastal areas (Tunala et al., 2012; Ohga et al., 2013; Sunami et al., 2013; Shiba et al., 2021, 2022a, b; Takizawa et al., 2022, 2023). For example, Tunala et al. (2012) demonstrated that the epidermal cells of the coastal variety of Aster hispidus Thunb. var. insularis (Makino) Okuyama (Asteraceae), a variety known to occur in coastal regions, were larger in size but fewer in number than those of A. hispidus Thunb. var. hispidus, which typically grows in inland grasslands. They also showed that these cells were involved in the ability of succulent leaves to store water. Sunami et al. (2013) found a correlation between leaf hair on the abaxial side of leaves and stomatal density in this variety. They showed that fewer leaf hairs corresponded to lower stomatal density to mitigate transpirational water loss. Ohga et al. (2013) proposed that the coastal population of Adenophora triphylla var. japonica (Regel) H. Hara (Campanulaceae) evolved relatively thick leaves via a heterochronic process for water storage. Shiba et al. (2022c) reported that the coastal population of Eurya japonica Thunb. (Ternstroemiaceae) exhibited smaller stomata and larger epidermal cells on both the adaxial and abaxial sides to reduce transpiration during gas exchange and retain water in leaves, respectively. Similar results regarding the differentiation between inland and coastal populations were observed in Ligustrum japonicum Thunb. (Oleaceae) (Takizawa et al., 2022). In addition to drought conditions, wind-induced stress also plays an important role in the speciation of plants adapted to coastal areas. Shiba et al. (2023) reported that wind speed in coastal areas contributed to reducing the lamina area and petiole length per petiole cross-sectional area in Farfugium japonicum (L.) Kitamura (Asteraceae) based on wind speed data from the Automated Meteorological Data Acquisition System (AMeDAS) installed at approximately 1,300 locations across Japan. Moreover, Shiba et al. (2024a) revealed that F. japonicum exhibited dwarfed laminae, petioles, and scaves, indicating that constant strong winds in coastal areas restricted height growth. These observations indicate that coastal areas are environments where stress-adapted and acclimated plants can invade. Because of these stresses, although only 4% of all vascular plants in Japan inhabit coastal areas, approximately 30% of all endemic species are located in these areas (Sawada et al., 2007), emphasizing their significance in terms of conservation. Oka (2010) reported an increase in invasive plants in the coastal areas of Japan, while Mabuchi et al. (2020) indicated that invasive species occupied various parts of the coastal areas affected by the tsunami resulting from the Great East Japan Earthquake in 2011. Takizawa et al. (2023) reported that L. lucidum Aiton, introduced to Japan as a roadside tree in the mid-1800s, invaded dry coastal areas and displayed a decrease in stomatal size. These studies indicate that invasive plants are more prevalent in coastal areas than expected; therefore, their acclimations need to be clarified.
Bidens pilosa L. (Asteraceae) is an annual herbaceous plant species that can reach a height of approximately 2 m. It features pinnate leaves and flower heads consisting of approximately four to five broad white ray florets with numerous tubular yellow disc florets (Koyama, 1995). B. pilosa has been utilized in traditional medicine for the treatment of various ailments (Chih et al., 1995; Ubillas et al., 2000; Chang et al., 2001; Oliveira et al., 2004; Sundararajan et al., 2006; Yuan et al., 2008; Ashafa and Afolayan, 2009; Tobinaga et al., 2009; Dagawal and Ghorpade, 2011; Adia et al., 2014). B. pilosa is native to the Americas, but has been introduced to various regions globally, including Eurasia, Africa, Australia, and the Pacific Islands (Koyama, 1995). In Japan, B. pilosa is an invasive plant that proliferates in cultivated fields, roadsides, and disturbed lands in the urban areas of Honshu, Kyushu, and Ryukyu and often becomes weedy (Koyama, 1995; Asami et al., 1999). Recently, Abe (2021) reported the invasion of B. pilosa in the coastal areas of Japan. Our findings corroborated this, as we identified this species in several coastal areas in Japan (Figure 1A), where it coexisted with coastal endemic species, such as Setaria viridis (L.) P.Beauv. var. pachystachys (Franch. & Sav.) Makino & Nemoto (Poaceae), Ixeris repens (L.) A.Gray (Asteraceae), Canavalia lineata (Thunb.) DC. (Fabaceae) and Calystegia soldanella (L.) R.Br. (Convolvulaceae). Our preliminary survey also found a population of B. pilosa that grew sympatrically with Lysimachia mauritiana Lam. (Purimulaceae), an endemic species of coastal areas, along the coast (Figure 1). B. pilosa has been reported to produce allelochemicals that may adversely affect the growth of native plants (Arthur et al., 2012; Balah et al., 2024). This underscores the need to clarify the acclimation patterns of this species in coastal areas to conserve coastal vegetation. Previously, comparative cultivation analyses using open-top chambers (OTC) have revealed that B. pilosa has acclimated to the wind by altering its leaf area and stem length (Shiba et al., 2024b). However, strong stress in coastal areas is influenced by both wind and soil moisture (Nakajima and Yoshizaki, 2010; Ito and Yoshizaki, 2017, 2019). Consequently, this poses the following question: how can we analyze the acclimation patterns of B. pilosa to these multiple stresses in coastal areas?
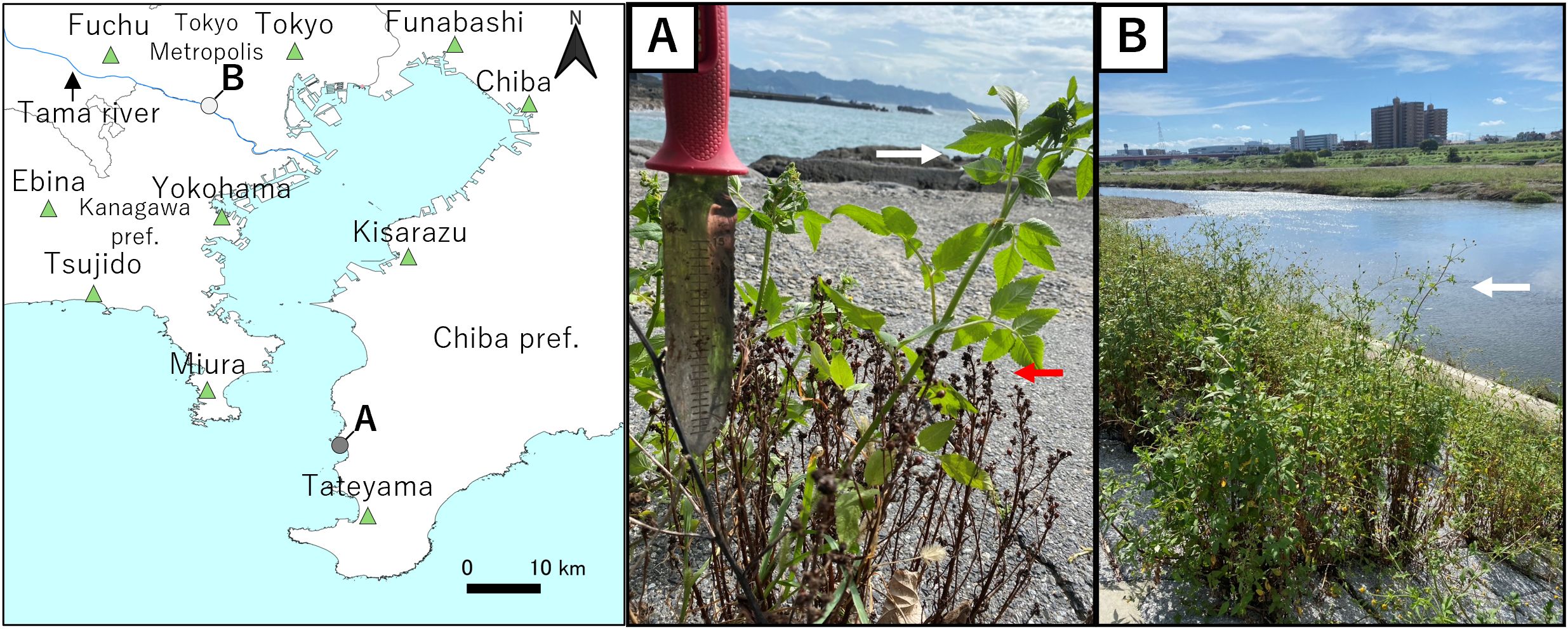
Figure 1. Sampling sites of Bidens pilosa and AMeDAS stations. AMeDAS locations are indicated by triangles. Coastal population of Bidens pilosa. (A) coastal, (B) inland. White arrow indicates B. pilosa, and red arrow indicates Lysimachia mauritiana.
Resource allocation is essential for plant development, yield formation, and tolerance to abiotic and biotic stresses. It also serves as a key indicator of plant growth and adaptation strategies and varies with environmental conditions (Kerkhoff et al., 2006; Reich et al., 2008; Sardans and Peñuelas, 2013; Shiba et al., 2024a, b; Shiba and Fukuda, 2024). Plant resource allocation reflects the trade-off in the distribution of aboveground and belowground biomass, which can be influenced by external environmental conditions (Fan et al., 2019; Poorter et al., 2012; Reich et al., 2014; Yang et al., 2010, 2018; Shiba et al., 2024b). Comparative analyses of resource allocation strategies among different environmental conditions are crucial in understanding plant adaptation or acclimation. These studies have been widely used to analyze biomass partitioning between aboveground and belowground organs (Enquist and Niklas, 2002; Cheng and Niklas, 2007; McCarthy and Enquist, 2007; Yang et al., 2010; Shiba et al., 2024b). Resource allocation patterns of five black spruce species planted along a latitudinal gradient in a boreal forest revealed that trees in the northern region budded earlier, grew less, and reached reproductive maturity earlier than those in the southern region. In addition, late frost damage affected growth capacity without seasonal adjustment in the subsequent year. This indicated that local adaptation of functional traits might lead to the inability of black spruce to adapt to future climate conditions associated with global warming or alternatively function as a powerful evolutionary force promoting rapid adaptation to changing environmental conditions (Silvestro et al., 2023). Therefore, analyzing resource allocation patterns is beneficial for research on the adaptation and acclimation of local populations to future climate changes. Consequently, plants serve as effective systems for examining environmental acclimation mechanisms. The acclimation pattern of B. pilosa to multiple stresses in coastal areas can clarify differences in resource allocation modes. Shiba et al. (2024b) indicated that B. pilosa reduced the allocation of resources to the ground under wind stress conditions. However, this study was solely conducted in a cultivated environment. Investigating how B. pilosa alters its resource allocation ratio in response to water stress and unstable soil due to sea sand and wind stress in outdoor coastal areas is pertinent. This study aims to clarify the changes in functional morphology and resource allocation during growth as a case study of the acclimation patterns of Bidens pilosa to coastal environments.
2 Materials and methods
On October 2, 2024, 58 individuals of the coastal B. pilosa population were collected from the coast of Kyonan Town, Awa District, Chiba Prefecture (35°06′04″ N, 139°49′31″ E). Subsequently, on October 19, 2024, 43 individuals of the inland B. pilosa population were collected from the Tamagawa Riverbed in Tamatsutsumi, Setagaya Ward, Tokyo (35°36′24″ N, 139°37′55″ E). During collection, slight damage was noted in individuals from the coastal population; however, both populations had developed inflorescences. The soil at the sampling sites consisted of marine sand and gravel for the coastal populations, while the inland populations were primarily found in areas with typical soil.
In addition, we presented monthly data for 2024 on temperature, precipitation, sunshine duration, wind speed, and humidity near the B. pilosa populations in this study using AMeDAS data from the Japan Meteorological Agency (Supplementary Materials 1; Figure 1). According to the AMeDAS Monthly Maximum Instantaneous Wind Speed data, the coastal populations (e.g., Tateyama, Miura, and Kisarazu) experienced wind speeds ranging from 19.6 to 22.7 m/s in August, while the inland populations (e.g., Fuchu, Tokyo, and Yokohama) experienced wind speeds ranging from 13.2 to 21.1 m/s, indicating that wind speeds tend to be higher in coastal areas(Supplementary Table 8).
2.1 Morphological measurements
Leaf area (cm²) was measured by selecting up to three fully expanded leaves per individual, photographing them, and analyzing the images with ImageJ software to calculate the average value. The number of nodes was counted for each individual as an indicator of the leaf number.
Stem length (mm) was measured using ImageJ software following the capture of an image of the entire individual. Stem diameter (mm) was measured at the base using a digital caliper (CD-15APX; Mitutoyo Corporation, Kawasaki, Japan) in triplicate, and the average value was calculated.
2.2 Biomass of roots, stems, leaves, and inflorescences
Following morphological measurements, the samples were divided into roots, stems, leaves, and inflorescences and subsequently dried in a drying oven (FS-405, Advantec Toyo Co., Ltd., Tokyo, Japan) at 75°C for at least 72 h. After drying, the samples were immediately removed from the oven, and the dry mass (g) of each organ was measured using an electronic balance (ATX224R, Shimadzu Corporation, Kyoto, Japan).
Additionally, the above/belowground ratio was calculated for each population by defining the dry mass of stems, leaves, and inflorescences as the aboveground biomass and the dry mass of roots as the belowground biomass.
2.3 Statistical analysis
Statistical analyses were conducted using R software (R Core Team, 2024). After confirming the normality of the data using the Shapiro–Wilk test, Levene’s test was used to assess the homogeneity of variances for the normally distributed data. A Student’s t-test was conducted under the assumption of equal variances. The Mann–Whitney U test was performed for data that did not conform to a normal distribution.
Scatter plots were generated for each environment to examine the relationship between the two variables. After performing a statistical test for correlation and the Shapiro–Wilk test, if normality was confirmed for one or both variables and a statistically significant correlation was found between the two environments, an analysis of covariance (ANCOVA) was performed. The relationship between the two variables was analyzed for stem basal diameter versus stem length and root versus inflorescence dry mass.
3 Results
In this study, the comparison data between the two groups included the node number, leaf area, and dry weight of various organs (roots, stems, leaves, and inflorescences), as well as the above/belowground ratio. Only the node number was compared using Student’s t-test. Other variables did not follow a normal distribution and were consequently compared using the Mann–Whitney U test. For stem basal diameter versus stem length, both environments showed a normal distribution and statistically significant correlation; therefore, an ANCOVA was performed. However, for root versus inflorescence dry mass, neither variable followed a normal distribution and no statistical correlation was found in the coastal population; therefore, an ANCOVA was not conducted.
3.1 Leaf and stem morphological measurements
A statistically significant difference was observed in the number of nodes between the coastal and inland populations (p < 0.001). The number of nodes in the coastal population was 17.34 ± 0.48 (mean ± SE), whereas those in the inland population were significantly higher at 20.05 ± 0.53 (Figure 2A). A statistically significant difference was also observed in the leaf area between the coastal and inland populations (p < 0.01). The leaf area in the coastal population was 2,141.12 ± 158.62 mm², while that of the inland population was significantly smaller at 1,500.00 ± 109.66 mm² (Figure 2B). These results suggest that the leaf morphology of the coastal population may represent an acclimation pattern characterized by larger but fewer leaves (Figure 2).
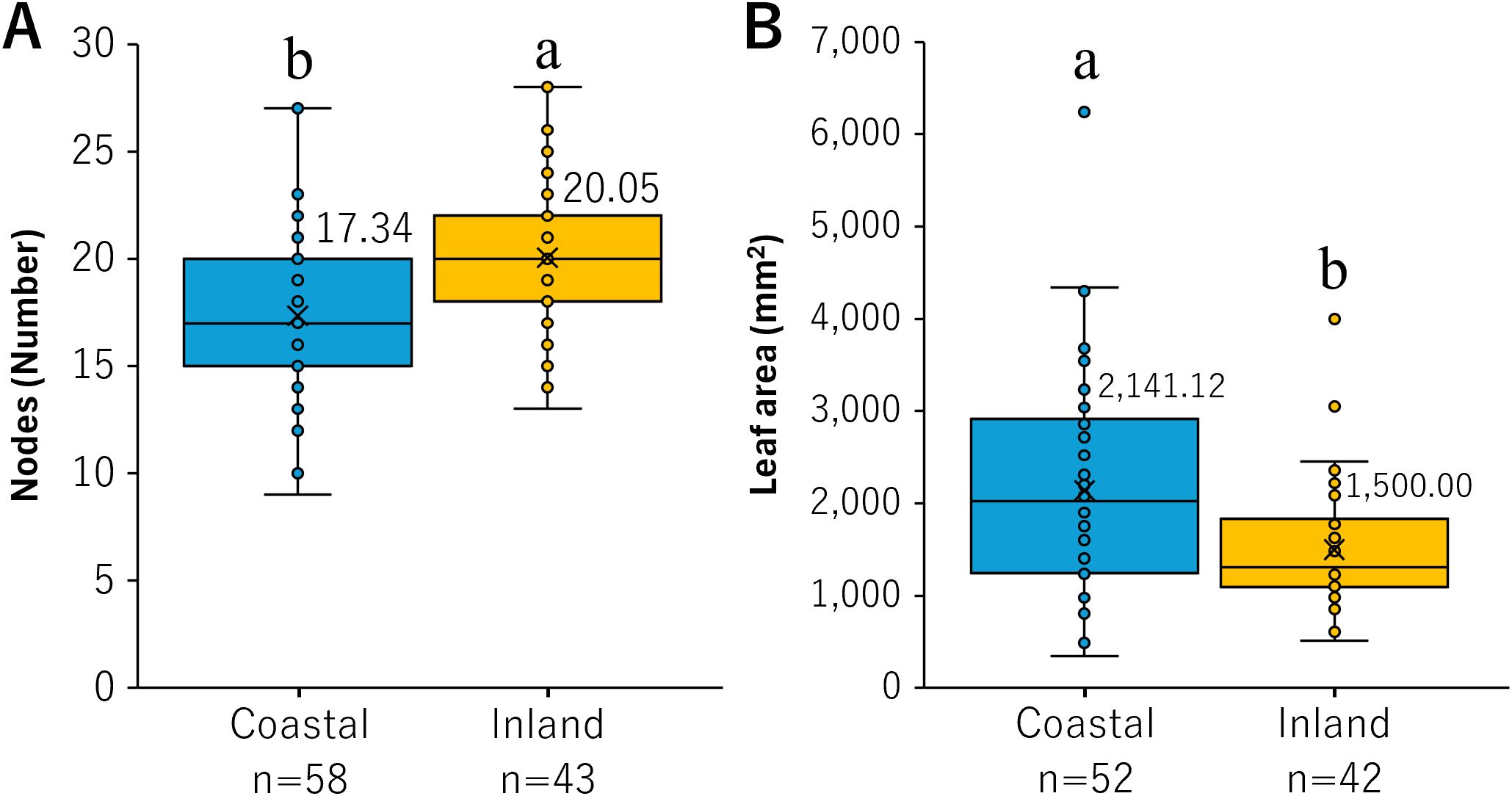
Figure 2. Comparison of (A) nodes and (B) leaf area. The values denoted by different letters in the box plot exhibit significant differences for nodes, as determined by the Student’s t-test, and for leaf area, as determined by the Mann–Whitney U test (p < 0.05). The means are plotted at cross marks, with the figures located in the top right corner of the diagram.
The relationship between basal stem diameter and stem length in B. pilosa demonstrated a statistically significant positive correlation in both populations (coastal: p < 0.001, inland: p < 0.001). In addition, the ANCOVA results revealed no interaction effect (p = 0.11). This suggests that the stem development of B. pilosa is not influenced by the environment, with a consistent addition of stem length corresponding to increases in stem diameter, irrespective of whether the plant is situated in a coastal or inland environment (Figure 3).
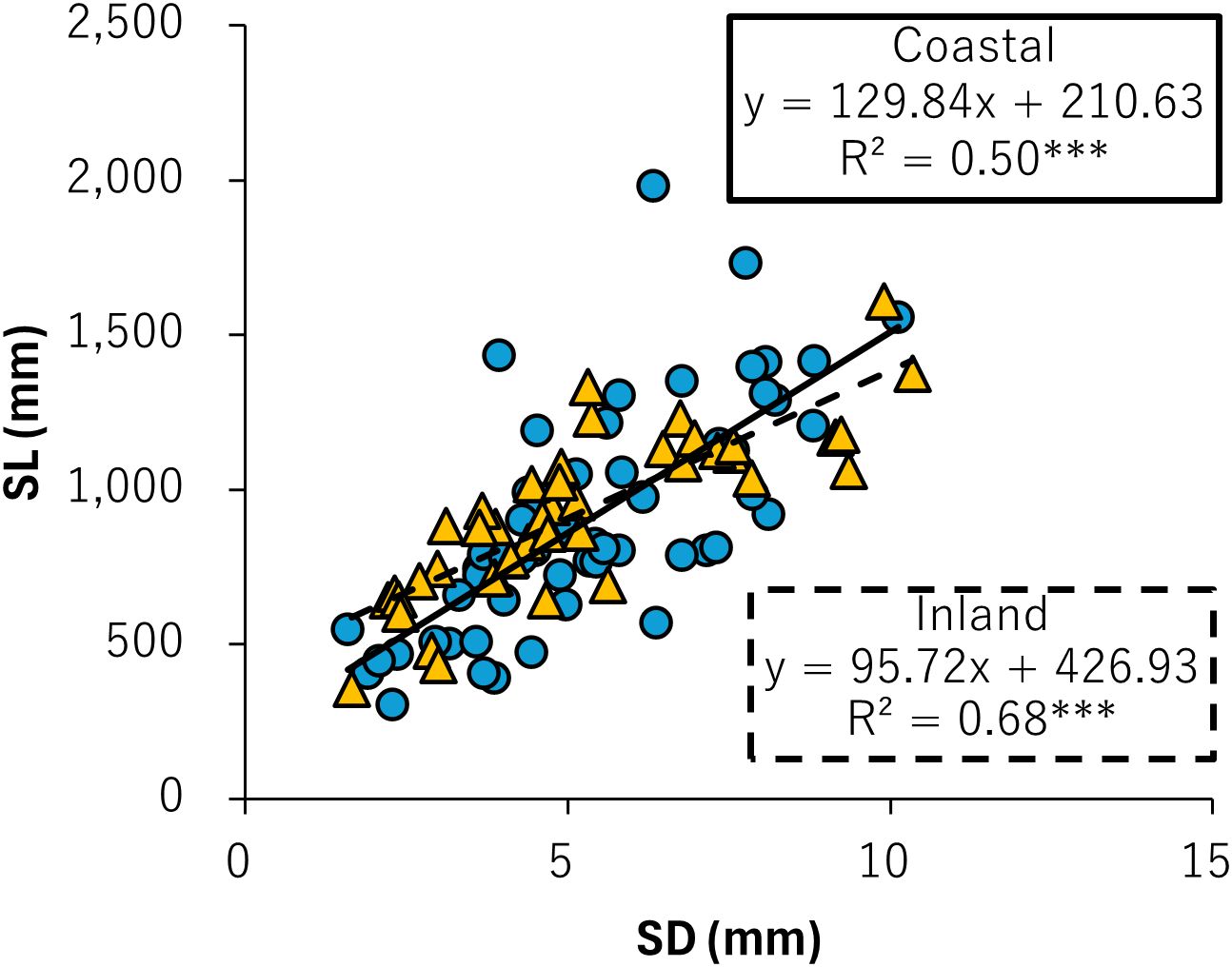
Figure 3. Stem diameter (SD) versus stem length (SL) for coastal (blue circle and solid line) and inland (orange triangle and dashed line) plant samples. The linear regression equation, coefficient of determination, and statistical evaluation of the correlation are presented. The significance of the correlation coefficient is indicated as follows: ***p < 0.001.
3.2 Resource allocation to each organ
The dry mass of each organ is shown in Figure 4. No significant difference was observed between the two groups regarding the dry mass of stems and leaves (stems: p = 0.44, leaves: p = 0.85; Figures 4B, C). However, significant differences were observed between roots and inflorescences (roots: p < 0.01, inflorescences: p < 0.001; Figures 4A, D). The dry mass of roots in the coastal population was 1.06 ± 0.15 g, whereas that in the inland population was significantly lower at 0.58 ± 0.01 g (Figure 4A). Additionally, the dry mass of inflorescences in the coastal population was 0.11 ± 0.01 g, whereas that in the inland population was significantly higher at 0.44 ± 0.08 g (Figure 4D). Furthermore, the above/belowground ratio was significantly different between the coastal and inland populations (p < 0.001). The ratio in the coastal population was 7.23 ± 0.41, whereas that in the inland population was significantly higher at 12.41 ± 0.65 (Figure 4E). These results suggest that B. pilosa in coastal environments allocates more resources to roots while maintaining similar allocations to stems and leaves, despite a decrease in resource allocation to inflorescences (Figure 4).
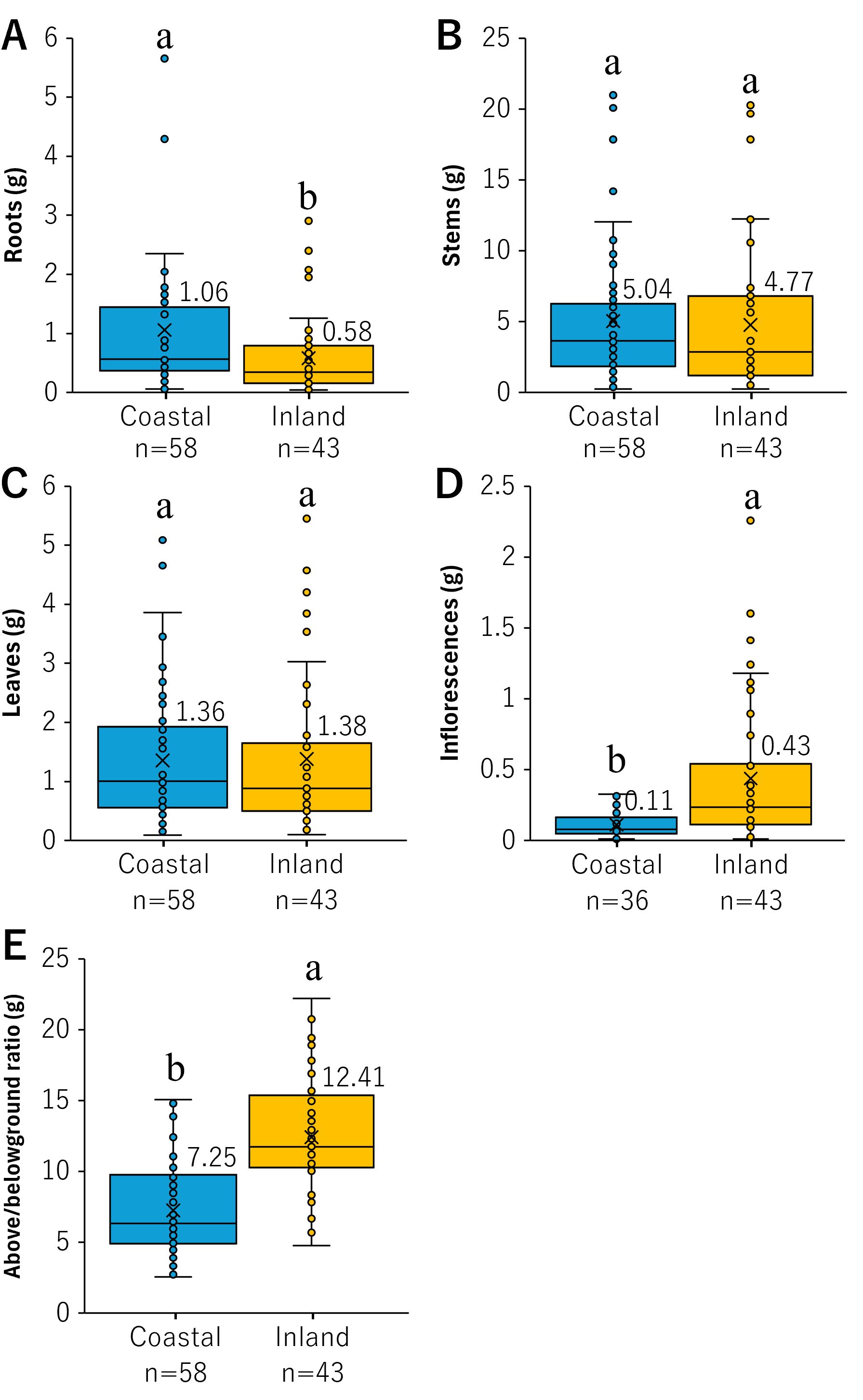
Figure 4. Comparison of (A) roots, (B) stems, (C) leaves, (D) inflorescences, (E) above/belowground biomass ratio. The values denoted by different letters in the box plot exhibit significant differences, as determined by the Mann–Whitney U test (p < 0.05). The means are plotted at cross marks, with the figures located in the top right corner of the diagram.
Because the dry mass of roots and inflorescences varied between environments, an analysis of their relationship revealed a statistically significant positive correlation in B. pilosa from the inland population (coastal: p = 0.27, inland: p < 0.001; Figure 5). This suggests that B. pilosa from inland environments increases resource allocation to roots and inflorescences, whereas B. pilosa from coastal environments continues to invest resources in roots, with minimal investment in inflorescences (Figure 5).
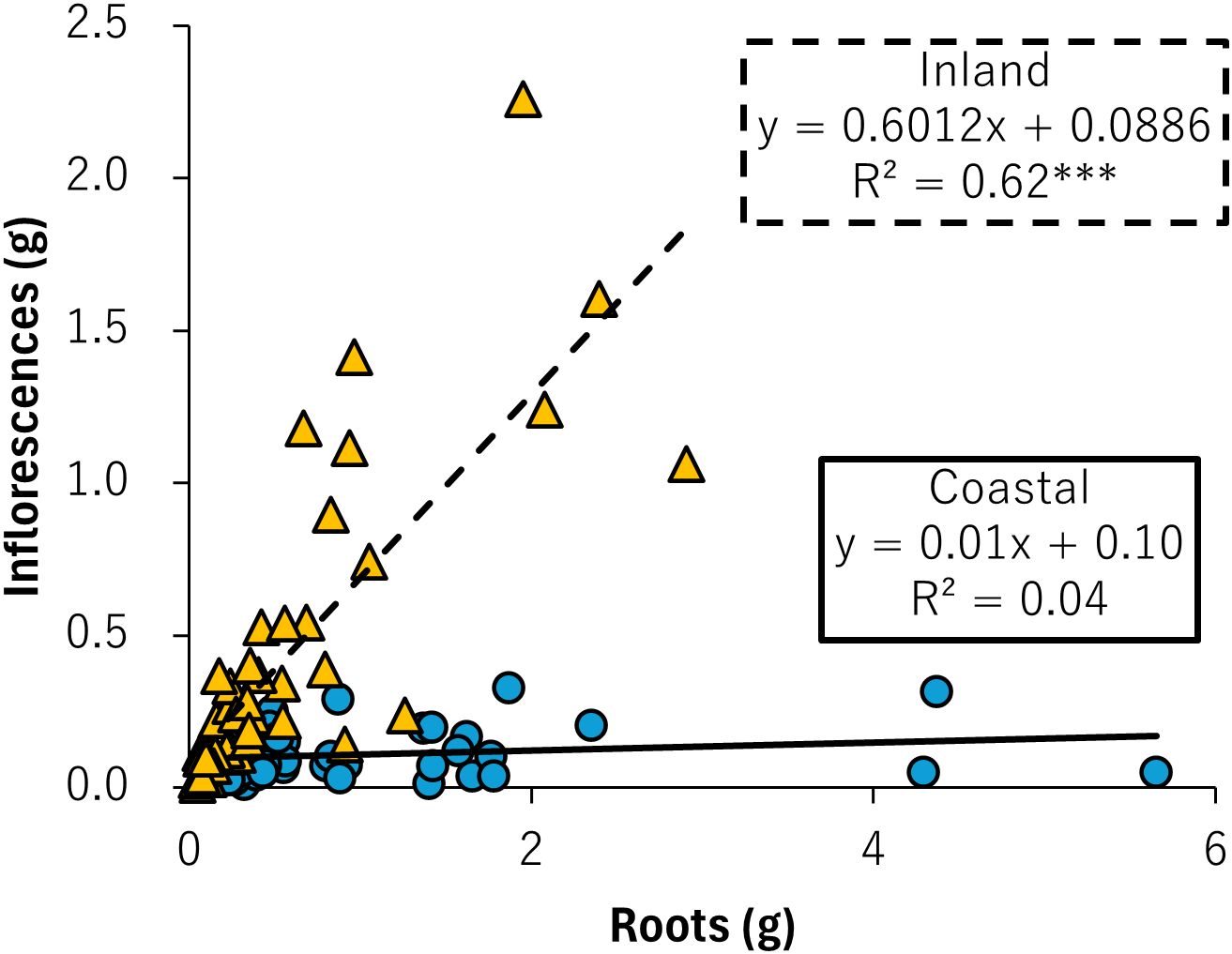
Figure 5. Root biomass versus inflorescence biomass for coastal (blue circle and solid line) and inland (orange triangle and dashed line) plant samples. The linear regression equation, coefficient of determination, and statistical evaluation of the correlation are presented. The significance of the correlation coefficient is indicated as follows: ***p < 0.001.
4 Discussion
Coastal ecosystems play many important roles in coastal defense, such as attenuating high wind stress (Hesp, 1989, 1991; Carter, 1991; Morris et al., 2018; Hanley et al., 2020). However, introduciung alien plant species severely threatens local biodiversity, ecosystem services, and environmental quality (Pejchar and Mooney, 2009; Pyšek and Richardson, 2010; Jones and McDermott, 2018). Therefore, it is imperative to elucidate the mechanisms by which invasive plants acclimate to coastal areas. Our analysis revealed that the number of leaves of B. pilosa in the coastal area was lower than that in the inland areas (Figure 2A). Shiba et al. (2024b) demonstrated that wind stress reduced the number of leaves in B. pilosa. This suggests that high wind speeds in coastal areas reduce the number of leaves of this species to avoid breakage by alleviating the load on the leaves and supporting stems. Interestingly, the leaf area of B. pilosa was significantly greater in coastal areas (Figure 2B), while no significant differences were observed in the relationship between stem basal diameter and height across environments (Figure 3). Therefore, allocation of resources to stems and leaves in the coastal population of B. pilosa did not significantly differ from that in the inland population (Figures 4B, C). Shiba et al. (2024b) showed that B. pilosa exhibited a reduced number of leaves, leaf area, and stem height in windy areas. In contrast, our results contradict the findings of Shiba et al. (2024b), suggesting that stress other than wind may contribute to the observed increase in leaf area in coastal areas. Why is there a contradiction, and what stresses other than wind affect coastal populations? For example, Ohga et al. (2013) showed that coastal of Adenophora triphylla var. japonica had increased leaf area by enlarging leaf cells, allowing them to retain water, and similar results have been shown for Aster hispidus var. insularis (Tunala et al., 2012). Nonetheless, since our study did not directly measure environmental factors such as drought or salinity, causal relationships between these factors and leaf morphology could not be determined. Meanwhile, it is known that sand carried by the wind accumulates around plants in coastal regions (Hesp, 2002; Zarnetske et al., 2015; Hesp et al., 2019), and plant height and leaf size are thought to influence their sand-trapping capacity (Hesp, 2002; Feagin et al., 2015; Zarnetske et al., 2015). As the frequency and intensity of strong winds increase due to climate change, the enlargement of leaf area in B. pilosa in such environments may play a role in trapping sand for moisture retention and plant anchorage. These findings suggest that coastal populations of B. pilosa reduce the number of leaves in response to strong winds, while elongating stems to maximize leaf production and stabilize growth by increasing leaf area. Such acclimation likely reflects a structural strategy that differs significantly from that of inland populations in terms of resource allocation to stems and leaves. Yoshizaki et al. (2023) showed that the leaves of coastal plants became increasingly susceptible to desiccation and salt intrusion after the leaf surface was damaged by blown sand. This implies that the coastal population of B. pilosa faces challenges related to establishment and damage caused by blowing sand. Mechanical stress on plants in coastal areas is considerably influenced by wind pressure, which is the primary environmental factor responsible for stem lodging. This phenomenon occurs when the forces exerted on plants exceed the maximum force their stems can withstand before breaking. Cooper and Mendiola (2004) noted that dwarfing could enhance plant species resistance to strong winds and lodging resistance. This suggests that this response can be adaptive because these plants are less susceptible to wind damage. Shiba et al. (2024b) demonstrated that an increase in wind speed correlated with a decrease in the rate of resource investment in stems and leaves. Consequently, based on the acclimation patterns revealed in our study, B. pilosa is unlikely to invade coastal areas characterized by elevated wind speeds, as wind pressure can likely cause lodging, given the resource investment rate in stems and leaves revealed in our study. In addition to wind stress, salt stress is also a major abiotic factor in coastal environments. Salt spray can damage leaf surfaces, reduce leaf area, increase water stress, and impair photosynthetic performance, thereby compounding the challenges faced by plants in these environments (Du and Hesp, 2020). Although coastal habitats may share general features such as soil type, local variations in wind conditions and salinity levels could lead to distinct acclimation patterns in other coastal populations of B. pilosa. Therefore, future studies should compare B. pilosa populations across coastal sites with differing wind regimes and salinity gradients to better understand the diversity of acclimation patterns.
The importance of the aboveground structures of plants in coastal areas is well established; however, considerable uncertainty remains regarding their belowground structures. Research has examined the differences in above- and belowground resource allocation patterns in response to nutrients and sand burial in coastal areas (Day, 1996; Brown and Zinnert, 2018; Dech and Maun, 2021). Belowground resource in coastal areas are critical for sediment stabilization and erosion mitigation (Charbonneau et al., 2017; Bryant et al., 2019; Feagin et al., 2019; De Battisti and Griffin, 2020). Our study revealed that the coastal population of B. pilosa exhibited significantly higher root weights and resource investment rates than those of the inland population (Figures 4A, E). Strong winds enhance plant anchorage by promoting root development (Danjon et al., 2005; Tamasi et al., 2005; Štofko and Kodrık, 2008). Several studies have shown that wind affects root growth and biomass allocation to roots (Cleugh et al., 1998; Poorter et al., 2012; Gardiner et al., 2016; Feng et al., 2019). Shiba et al. (2024b) reported that there was no significant difference in root biomass between wind-exposed and control populations of B. pilosa. This result suggests that the significant increase in resource investment in roots observed in the coastal population (Figure 4A) cannot be explained by strong winds and that other environmental factors in coastal areas may be involved. Other environmental factors in coastal soils—such as burial, nutrient limitation, and salt stress—should also be considered. Roots play an important role in sediment dynamics (Gyssels et al., 2005; Reubens et al., 2007; Comino et al., 2010). Several studies have reported that roots provide physical reinforcement through sediment entanglement, sediment particle incorporation into their tissue, and sediment transport resistance (Gregory, 2006; Reubens et al., 2007; Feagin et al., 2015). Additionally, root structures can provide anchoring forces for plants and biomechanical reinforcement in coastal areas (Gregory, 2006; Reubens et al., 2007; Klimešová et al., 2018). Coastal soil erosion, driven by strong winds, consistently undermines plant growth and establishment. However, Davidson et al. (2020) found that increased investment in deepening root systems enhanced plant stability under unstable conditions. The increased investment in roots observed in this study may also be attributed to such functional roles. However, these factors were not directly examined in this study, and further experimental investigation is required to identify the causes of the increased root biomass.
Plants can adjust their resource allocation to prioritize growth or reproduction in different environments, thereby optimizing their fitness (Mironchenko and Kozlowski, 2014). Moreover, reproductive allocation varies considerably among populations of a species growing under different environmental conditions. Semelparous annual species allocate a greater proportion of their resources to reproduction than that of iteroparous perennials (Weiner, 2004). It is questionable whether coastal populations of B. pilosa can invest resources in their reproductive organs due to ongoing investment in their roots. A previous study showed that B. pilosa maintained consistent resource allocation to inflorescences despite exposure to strong wind conditions (Shiba et al., 2024b). Our results suggest that the coastal population of B. pilosa invests significantly fewer resources in inflorescences (Figure 4D). The inland population of B. pilosa exhibited a larger increase in inflorescences corresponding to an increase in root mass, whereas the coastal population allocated more resources to root development at the expense of inflorescences (Figure 5). Reduced height growth is the most common response to wind stress because diminished stature enhances the ability of plants to resist forces and experience reduced drag (Jaffe et al., 1984; Liu et al., 2007). Studies have indicated that plants exposed to wind are shorter than those grown under wind-still conditions (Russell and Grace, 1978; Telewski and Jaffe, 1986). This suggests that this response may be adaptive, as shorter plants are less susceptible to wind damage. However, as mentioned above, coastal populations of B. pilosa maintain a stem height comparable to inland populations but reduce leaf number while increasing leaf area, which may enhance sand capture, stabilize the plant base, and slightly reduce wind impact. Nevertheless, it remains unclear whether sand capture is effective in mitigating broader environmental stresses, and further investigation is needed. This shift in resource allocation is expected to reduce seed production in the coastal population and may substantially affect their fitness. Coastal vegetation is a unique ecosystem, characterized by high morphological, ecological, and dynamic diversity. In such environments, plants may acclimate more readily due to reduced competition. Consequently, B. pilosa is one of the plant species that has acclimated to coastal areas and may thrive in this environment despite a limited number of seeds. In addition to these ecological traits, the thorny awns of the fruit of this species get caught on people’s clothing and are artificially dispersed (anthropochory), expanding the distribution range of this plant throughout urban areas in Japan (Shiba et al., 2024b). The coastal areas we surveyed is shallow and safe for small children to play in, so it is a popular spot with many people coming and going, attracting many swimmers and anglers from nearby prefectures and areas (Chiba Prefectural Government, 2025; Chiba Prefecture Tourism Information, n.d). Therefore, the high level of human traffic in this coastal area may have contributed to the invasion of this species. This study provides the first report demonstrating that, in addition to its known remarkable reproductive capacity and epizoochorous dispersal strategy, B. pilosa has also invaded coastal environments—typically resistant to inland species—by altering its resource allocation and functional morphology through phenotypic plasticity.
The Mediterranean region, as a single region, is the world’s largest tourist destination, attracting about one-third of the world’s international tourists and generating more than one-quarter of international tourism revenues, and international tourist arrivals in the Mediterranean region are expected to reach 500 million by 2030 (UNWTO, 2012). Several reports have stated that Bidens pilosa species complex has already invaded countries surrounding the Mediterranean (Rojas-Sandoval, 2018; El Mokni et al., 2022; Plants of the World Online, 2025; Portal to the flora of Italy, 2025). In particular, the characteristic of B. pilosa fruits is that they can spread by adhering to people’s clothes, etc (Shiba et al., 2024b), so it is expected that the distribution of B. pilosa will continue to expand in the Mediterranean region in the future. Furthermore, our study showed that B. pilosa have the characteristics to acclimate to special environments such as coastal areas, so there is a concern that B. pilosa will spread regardless of the environment in the Mediterranean region. B. pilosa also have the troublesome property of increasing the number of seeds in places where wind speed is reduced due to urban buildings, structures, and buildings (Shiba et al., 2024b). In addition, Fan et al. (2025) showed based on future climate scenarios that although populations of B. pilosa will decrease in tropical regions, the higher the latitude, the more adaptive the environment for this species will be, suggesting that the Mediterranean region is changing into an environment suitable for B. pilosa in the future. Unless proactive measures are taken to cull B. pilosa, which grows by acclimating its morphology to a variety of locations, from mountains to the sea, and from cities to the countryside, it is feared that an irreparable tragedy may befall the Mediterranean region, the world’s largest tourist destination.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
UE: Investigation, Methodology, Project administration, Software, Validation, Writing – original draft. MS: Conceptualization, Funding acquisition, Methodology, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. TF: Funding acquisition, Investigation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by JSPS KAKENHI Grant Numbers JP24KJ2045, JP25K09763.
Acknowledgments
We thank Drs. Hara S, Ishihara Y, Kameda H, Tokuyama K, Yajima I, Izawa H, Kurosu S, and Kurotaki K for advising of our study.
Conflict of interest
The authors declare that this study was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2025.1604666/full#supplementary-material
References
Abe S. (2021). Comparison of traditional and automated approaches in classification of Japanese coastal beech and dune vegetation. Veg. Sci. 38, 67–80. doi: 10.15031/vegsci.38.67
Adia M. M., Anywar G., Byamukama R., Kamatenesi-Mugisha M., Sekagya Y., Kakudidi E. K., et al. (2014). Medicinal plants used in malaria treatment by Prometra herbalists in Uganda. J. Ethnopharmacol. 155, 580–588. doi: 10.1016/j.jep.2014.05.060
Arthur G. D., Naidoo K. K., and Coopoosamy R. M. (2012). Bidens pilosa L: Agricultural and pharmaceutical importance. J. Med. Plants Res. 6, 3282–3287. doi: 10.5897/JMPR12.195
Asami K., Yamato M., Hattori T., Akamatsu H., and Takeda Y. (1999). Floristic composition and process of establishment of the Bldens pilosa var. minor-Imperata cyilndrica community maintained by cutting on nonarable land in Okinawa Prefecture. J. Veg. Sci. 16, 1–11. doi: 10.15031/vegsci.16.1
Ashafa A. O. T. and Afolayan A. J. (2009). Screening the root extracts from Bidens pilosa L. var. radiata (Asteraceae) for antimicrobial potentials. J. Med. Plants Res. 3, 568–572.
Balah M. A., Al-Andal A., Radwan A. M., and Donia A. E. M. (2024). Unveiling allelopathic dynamics and impacts of invasive Erigeron bonariensis and Bidens pilosa on plant communities and soil parameters. Sci. Rep. 14, 10159. doi: 10.1038/s41598-024-57552-7
Brown J. K. and Zinnert J. C. (2018). Mechanisms of surviving burial: dune grass interspecific differences drive resource allocation after sand deposition. Ecosphere 9, e02162. doi: 10.1002/ecs2.2162
Bryant D. B., Bryant M. A., Sharp J. A., Bell G. L., and Moore C. (2019). The response of vegetated dunes to wave attack. Coast. Eng. 152, 1–8. doi: 10.1016/j.coastaleng.2019.103506
Carter R. W. G. (1991). Near-future seal level impacts on coastal dune landscapes. Landscape Ecol. 6, 29–39. doi: 10.1007/BF00157742
Chang J. S., Chiang L. C., Chen C. C., Liu L. T., Wang K. C., and Lin C. C. (2001). Antileukemic activity of Bidens pilosa L. var. minor (Blume) sherff and Houttuynia cordata Thunb. Am. J. Chin. Med. 29, 303–312. doi: 10.1142/S0192415X01000320
Charbonneau B. R., Wootton L. S., Wnek J. P., Langley J. A., and Posner M. A. (2017). A species effect on storm erosion: invasive sedge stabilized dunes more than native grass during hurricane sandy. J. Appl. Ecol. 54, 1385–1394. doi: 10.1111/1365-2664.12846
Cheng D. L. and Niklas K. J. (2007). Above- and below-ground biomass relationships across 1534 forested communities. Ann. Bot. 99, 95–102. doi: 10.1093/aob/mcl206
Chiba Prefectural Government (2025).Survey results on tourism in Chiba Prefecture in FY2023. Available online at: https://www.pref.chiba.lg.jp/kankou/press/2024/r5-2.html (Accessed June 7, 2025).
Chiba Prefecture Tourism Information Iwai coast (Tateyama City). Available online at: https://maruchiba.jp/spot/detail_11019.html (Accessed June 7, 2025).
Chih H. W., Lin C. C., and Tang K. S. (1995). Anti-inflammatory activity of Taiwan folk medicine “ham-hong-chho” in rats. Am. J. Chin. Med. 23, 273–278. doi: 10.1142/S0192415X95000328
Cleugh H. A., Miller J. M., and Böhm M. (1998). Direct mechanical effects of wind on crops. Agrofor. Syst. 41, 85–112. doi: 10.1023/A:1006067721039
Comino E., Marengo P., and Rolli V. (2010). Root reinforcement effect of different grass species: a comparison between experimental and models results. Soil Tillage Res. 110, 60–118. doi: 10.1016/j.still.2010.06.006
Cooper R. L. and Mendiola T. (2004). Registration of 10 determinate semidwarf soybean germplasm lines. Crop Sci. 44, 699–700. doi: 10.2135/cropsci2004.6990
Dagawal M. J. and Ghorpade D. S. (2011). Antimicrobial activity of an ethnomedicinal plant Bidens pilosa L. Int. J. Pharm. Sci. Res. 2, 2237–2238. doi: 10.13040/IJPSR.0975-8232.2(8).2237-38
Danjon F., Fourcaud T., and Bert D. (2005). Root architecture and wind-firmness of mature Pinus pinaster. New Phytol. 168, 387–400. doi: 10.1111/j.1469-8137.2005.01497.x
Davidson S. G., Hesp P. A., and da Silva G. M. (2020). Controls on dune scarping. Prog. Phys. Geogr. 44, 923–947. doi: 10.1177/0309133320932880
Day F. P. (1996). Effects of nitrogen availability on plant biomass along a barrier island dune chronosequence. Castanea. 61, 369–381.
De Battisti D. and Griffin J. N. (2020). Below-ground biomass of plants, with a key contribution of buried shoots, increases foredune resistance to wave swash. Ann. Bot. 125, 325–333. doi: 10.1093/aob/mcz125
Dech J. P. and Maun M. A. (2021). Adventitious root production and plastic resource allocation to biomass determine burial tolerance in woody plants from central Canadian coastal dunes. Ann. Bot. 98, 1095–1105. doi: 10.1093/aob/mcl196
Du J. and Hesp P. A. (2020). Salt spray distribution and its impact on vegetation zonation on coastal dunes: a review. Estuaries Coast. 43, 1–14. doi: 10.1007/s12237-020-00820-2
Ellstrand N. C., Heredia S. M., Leak-Garcia J. A., Heraty J. M., Burger J. C., Yao L., et al. (2010). Crops gone wild: evolution of weeds and invasives from domesticated ancestors. Evol. Appl. 3, 494–504. doi: 10.1111/j.1752-4571.2010.00140.x
El Mokni R., Iamonico D., Véla E., Verloove F., and Domina G. (2022). New records of Asteraceae for the non-native flora of Tunisia and north Africa with some nomenclatural remarks. Mediterr. Bot. 43, 72688. doi: 10.5209/mbot.73688
Enquist B. J. and Niklas K. J. (2002). Global allocation rules for patterns of biomass partitioning in seed plants. Science 295, 1517–1520. doi: 10.1126/science.1066360
Fan L., Ding J., Ma X., and Li Y. (2019). Ecological biomass allocation strategies in plant species with different life forms in a cold desert, China. J. Arid Land. 11, 729–739. doi: 10.1007/s40333-019-0062-1
Fan L., Mi C., Li J., Zhang Y., Zhang H., Zhang G., et al. (2025). Projecting global shifts in the invasive potential of Bidens pilosa L. under climate change using species distribution models. Front. Plant Sci. 16. doi: 10.3389/fpls.2025.1580278
Feagin R. A., Figlus J., Zinnert J. C., Sigren J., Martínez M. L., Silva R., et al. (2015). Going with the flow or against the grain? The promise of vegetation for protecting beaches, dunes, and barrier islands from erosion. Front. Ecol. Environ. 13, 203–210. doi: 10.1890/140218
Feagin R. A., Furman M., Salgado K., Martinez M. L., Innocenti R. A., Eubanks K., et al. (2019). The role of beach and sand dune vegetation in mediating wave run up erosion. Estuar. Coast. Shelf Sci. 219, 97–106. doi: 10.1016/j.ecss.2019.01.018
Feng J., Huang P., and Wan X. (2019). Interactive effects of wind and light on growth and architecture of poplar saplings. Ecol. Res. 34, 94–105. doi: 10.1111/1440-1703.1013
Fuentes-Lillo E., Lembrechts J. J., Cavieres L. A., Jiménez A., Haider S., Barros A., et al. (2021). Anthropogenic factors overrule local abiotic variables in determining non-native plant invasions in mountains. Biol. Invasions. 23, 3671–3686. doi: 10.1007/s10530-021-02602-8
Gardiner B., Berry P., and Moulia B. (2016). Review: wind impacts on plant growth, mechanics and damage. Plant Sci. 245, 94–118. doi: 10.1016/j.plantsci.2016.01.006
Gregory P. J. (2006). Roots, rhizosphere and soil: the route to a better understanding of soil science? Eur. J. Soil Sci. 57, 2–12. doi: 10.1111/j.1365-2389.2005.00778.x
Gyssels G., Poensen J., Bochet E., and Li Y. (2005). Impact of plant roots on the resistance of soils to erosion by water: a review. Prog. Phys. Geogr. 29, 189–217. doi: 10.1191/0309133305pp443ra
Hanley M. E., Bouma T. J., and Mossman H. L. (2020). The gathering storm: optimizing management of coastal ecosystems in the face of a climate-driven threat. Ann. Bot. 125, 197–212. doi: 10.1093/aob/mcz204
Hesp P. (1989). A review of biological and geomorphological processes involved in the initiation and development of incipient foredunes. Proc. R. Soc Edinburgh Section B Biol. Sci. 96, 181–201. doi: 10.1017/S0269727000010927
Hesp P. A. (1991). Ecological processes and plant adaptations on coastal dunes. J. Arid Environ. 21, 165–191. doi: 10.1016/S0140-1963(18)30681-5
Hesp P. (2002). Foredunes and blowouts: initiation, geomorphology and dynamics. Geomorphology 48, 245–268. doi: 10.1016/S0169-555X(02)00184-8
Hesp P. A., Dong Y., Cheng H., and Booth J. L. (2019). Wind flow and sedimentation in artificial vegetation: field and wind tunnel experiments. Geomorphology 337, 165–182. doi: 10.1016/j.geomorph.2019.03.020
Ito H. and Yoshizaki S. (2017). Effects of salt water immersion on seed germination of Pinus thunbergii Parl. and Pinus densiflora Sieb. et Zucc. J. Jpn. Soc Coast. Forest. 16, 15–19. doi: 10.60398/kaiganrin.16.2_15
Ito H. and Yoshizaki S. (2019). Effect of short-term salt water immersion on water absorption and germination of seeds in Japanese black pine (Pinus thunbergii Parl.). J. Jpn. Soc Revegetat. Technol. 45, 260–263. doi: 10.7211/jjsrt.45.260
Jackson R. B. and Jobbgy E. G. (2005). From icy roads to salty streams. Proc. Natl. Acad. Sci. U. S. A. 102, 14487–14488. doi: 10.1073/pnas.0507389102
Jaffe M. J., Telewski F. W., and Cooke P. W. (1984). Thigmomorphogenesis: on the mechanical properties of mechanically perturbed bean plants. Physiol. Plant 62, 73–78. doi: 10.1111/j.1399-3054.1984.tb05925.x
Jauni M. and Hyvönen T. (2010). Invasion level of alien plants in semi-natural agricultural habitats in boreal region. Agric. Ecosyst. Environ. 138, 109–115. doi: 10.1016/j.agee.2010.04.007
Jones B. A. and McDermott S. M. (2018). Health impacts of invasive species through an altered natural environment: assessing air pollution sinks as a causal pathway. Environ. Resour. Econ (Dordr). 71, 23–43. doi: 10.1007/s10640-017-0135-6
Kaspari M., Yanoviak S. P., Dudley R., Yuan M., and Clay N. A. (2009). Sodium shortage as a constraint on the carbon cycle in an inland tropical rainforest. Proc. Natl. Acad. Sci. U.S.A. 106, 19405–19409. doi: 10.1073/pnas.0906448106
Kerkhoff A. J., Fagan W. F., Elser J. J., and Enquist B. J. (2006). Phylogenetic and growth form variation in the scaling of nitrogen and phosphorus in the seed plants. Am. Nat. 168, 103–122. doi: 10.1086/507879
Klimešová J., Martínková J., and Ottaviani G. (2018). Belowground plant functional ecology: towards an integrated perspective. Funct. Ecol. 32, 2115–2126. doi: 10.1111/1365-2435.13145
Koyama H. (1995). Flora of Japan IIIb. in B. L. Eds. Iwatsuki K., Yamazaki T., Boufford D. E., and Ohba H. (Tokyo: Kodansha), 29–31.
Koyama A., Egawa C., and Akasaka M. (2024). Decline in alien plant species turnover among geographically isolated mountains with ropeway corridors. Glob. Ecol. Conserv. 56, e03282. doi: 10.1016/j.gecco.2024.e03282
Liu Y., Schieving F., Stuefer J. F., and Anten N. P. R. (2007). The effects of mechanical stress and spectral shading on the growth and allocation of ten genotypes of a stoloniferous plant. Ann. Bot. 99, 121–130. doi: 10.1093/aob/mcl230
Mabuchi S., Yamanouchi T., and Kurosawa T. (2020). Investigating the flora and vegetation of a coastal forest soon after mounding and afforestation following the Great East Japan Earthquake. Jpn. J. Conservat. Ecol. 25, 249–263. doi: 10.18960/hozen.2009
Marshall E. J. P., Brown V. K., Boatman N. D., Lutman P. J. W., Squire G. R., and Ward L. K. (2003). The role of weeds in supporting biological diversity within crop fields. Weed Res. 43, 77–89. doi: 10.1046/j.1365-3180.2003.00326.x
McCarthy M. C. and Enquist B. J. (2007). Consistency between an allometric approach and optimal partitioning theory in global patterns of plant biomass allocation. Funct. Ecol. 21, 713–720. doi: 10.1111/j.1365-2435.2007.01276.x
Mironchenko A. and Kozlowski J. (2014). Optimal allocation patterns and optimal seed mass of a perennial plant. J. Theor. Biol. 354, 12–24. doi: 10.1016/j.jtbi.2014.03.023
Morgan J. W. (1998). Patterns of invasion of an urban remnant of a species-rich grassland in southeastern Australia by non-native plant species. J. Veg. Sci. 9, 181–190. doi: 10.2307/3237117
Morris R. L., Konlechner T. M., Ghisalberti M., and Swearer S. E. (2018). From grey to green: efficacy of eco-engineering solutions for nature-based coastal defense. Glob. Change Biol. 24, 1827–1842. doi: 10.1111/gcb.14063
Nakajima Y. and Yoshizaki S. (2010). The comparison of tolerance to salt adhesion among 5 broad leaf trees species which grow near the coast. J. Jpn. Soc Revegetat. Technol. 36, 219–222. doi: 10.7211/jjsrt.36.219
Ohga K., Muroi M., Hayakawa H., Yokoyama J., Ito K., Tebayashi S., et al. (2013). Coastal adaptation of Adenophora triphylla var. japonica (Campanulaceae). Am. J. Plant Sci. 4, 596–601. doi: 10.4236/ajps.2013.43078
Oka K. (2010). Coastal environment and biodiversity: case study of conservation and restoration of coastal vegetation. J. Jpn. Soc Revegetat. Technol. 35, 503–507.
Oliveira F. Q., Andrade-Neto V., Krettli A. U., and Brandão M. G. (2004). New evidences of antimalarial activity of Bidens pilosa roots extract correlated with polyacetylene and flavonoids. J. Ethnopharmacol. 93, 39–42. doi: 10.1016/j.jep.2004.03.026
Pejchar L. and Mooney H. A. (2009). Invasive species, ecosystem services and human well-being. Trends Ecol. Evol. 24, 497–504. doi: 10.1016/j.tree.2009.03.016
Plants of the World Online (2025).Bidens pilosa L. Available online at: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:32564-2 (Accessed June 18, 2025).
Poorter H., Niklas K. J., Reich P. B., Oleksyn J., Poot P., and Mommer L. (2012). Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 193, 30–50. doi: 10.1111/j.1469-8137.2011.03952.x
Portal to the flora of Italy (2025).Bidens pilosa L. Available online at: https://dryades.units.it/florItaly/index.php?procedure=taxon_page&tipo=all&id=5549 (Accessed June 18, 2025).
Pyšek P. and Richardson D. M. (2010). Invasive species, environmental change and management, and health. Annu. Rev. Environ. Resour. 35, 25–55. doi: 10.1146/annurev-environ-033009-095548
R Core Team (2024). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available online at: https://www.R-project.org/ (Accessed March 21, 2025).
Reich P. B., Luo Y., Bradford J. B., Poorter H., Perry C. H., and Oleksyn J. (2014). Temperature drives global patterns in forest biomass distribution in leaves, stems, and roots. Proc. Natl. Acad. Sci. U. S. A. 111, 13721–13726. doi: 10.1073/pnas.1216053111
Reich P. B., Tjoelker M. G., Pregitzer K. S., Wright I. J., Oleksyn J., and MaChado J. L. (2008). Scaling of respiration to nitrogen in leaves, stems and roots of higher land plants. Ecol. Lett. 11, 793–801. doi: 10.1111/j.1461-0248.2008.01185.x
Reubens B., Poesen J., Danjon F., Geudens G., and Muys B. (2007). The role of fine and coarse roots in shallow slope stability and soil erosion control with a focus on root system architecture: a review. Trees (Berl. West). 21, 385–402. doi: 10.1007/s00468-007-0132-4
Rojas-Sandoval J. (2018). Bidens pilosa (blackjack) (Wallingford, UK: CABI Compendium), 9148. doi: 10.1079/cabicompendium.9148
Russell G. and Grace J. (1978). The effect of wind-speed on the growth of grasses. J. Appl. Ecol. 16, 507–514. doi: 10.2307/2402525
Štofko P. and Kodrık M. (2008). Comparison of the root system architecture between windthrown and undamaged spruces growing in poorly drained sites. J. For. Sci. 54, 150–160. doi: 10.17221/3101-JFS
Sardans J. and Peñuelas J. (2013). Tree growth changes with climate and forest type are associated with relative allocation of nutrients, especially phosphorus, to leaves and wood. Glob. Ecol. Biogeogr. 22, 494–507. doi: 10.1111/geb.12015
Sawada Y., Nakanishi H., Oshida K., and Hattori T. (2007). A check list of coastal plants in Japan. Hum. Nat. 17, 85–101.
Shiba M., Arihara S., Harada S., and Fukuda T. (2024a). Impact on the scape of Farfugium japonicum var. japonicum (Asteraceae) under strong wind conditions based on morphological and mechanical analyses. Front. Plant Sci. 15, 1407127. doi: 10.3389/fpls.2024.1407127
Shiba M. and Fukuda T. (2024). Rheophytic Osmunda lancea (Osmundaceae) exhibits large flexibility in the petiole. Sci. Rep. 14, 2866. doi: 10.1038/s41598-024-53406-4
Shiba M., Kobayashi N., Harada S., and Fukuda T. (2024b). Decrease in wind stress leads to an increase in the above ground morphology and number of seeds of an invasive alien species, Bidens pilosa (Asteraceae). Front. Plant Sci. 15, 1445437. doi: 10.3389/fpls.2024.1445437
Shiba M., Mizuno T., and Fukuda T. (2023). Effect of strong wind on laminas and petioles of Farfugium japonicum (L.) Kitam. var. japonicum (Asteraceae). Front. Plant Sci. 14, 1182266. doi: 10.3389/fpls.2023.1182266
Shiba M., Tate T., and Fukuda T. (2021). Rheophytic adaptation of Eurya japonica Thunb. (Ternstroemiaceae). Int. J. Biol. 13, 65–73. doi: 10.5539/ijb.v13n2p65
Shiba M., Tate T., and Fukuda T. (2022a). Adaptative leaf morphology of Eurya japonica Thunb. (Ternstroemiaceae) in serpentine areas. J. Plant Stud. 11, 10–18. doi: 10.5539/jps.v11n1p10
Shiba M., Tate T., and Fukuda T. (2022b). Serpentine adaptation of Ligustrum japonicum Thunb. (Oleaceae) based on morphological and anatomical approaches. Int. J. Biol. 14, 10–18. doi: 10.5539/ijb.v14n2p10
Shiba M., Tate T., and Fukuda T. (2022c). Leaf anatomical adaptations of Eurya japonica Thunb. (Pentaphylacaceae) in coastal habitats. J. Plant Stud. 11, 31–41. doi: 10.5539/jps.v11n1p31
Silvestro R., Mura C., Alano Bonacini D., de Lafontaine G., Faubert P., Mencuccini M., et al. (2023). Local adaptation shapes functional traits and resource allocation in black spruce. Sci. Rep. 13, 21257. doi: 10.1038/s41598-023-48530-6
Sunami T., Ohga K., Muroi M., Hayakawa H., Yokoyama J., Ito K., et al. (2013). Comparative analyses of hairless-leaf and hairy-leaf type individuals in Aster hispidus var. insularis (Asteraceae). J. Plant Stud. 2, 1–6. doi: 10.5539/jps.v2n1p1
Sundararajan P., Dey A., Smith A., Doss A. G., Rajappan M., and Natarajan S. (2006). Studies of anticancer and antipyretic activity of Bidens pilosa whole plant. Afr. Health Sci. 6, 27–30. doi: 10.5555/afhs.2006.6.1.27
Takizawa E., Shiba M., Yoshizaki S., and Fukuda T. (2023). Stomatal study of introduced species, Ligustrum lucidum Aiton (Oleaceae), in coastal areas of Japan. J. Plant Stud. 12(1), 24–36. doi: 10.5539/jps.v12n1p24
Takizawa E., Tate T., Shiba M., Ishii C., Yoshizaki S., and Fukuda T. (2022). Coastal adaptation of Ligustrum japonicum Thunb. (Oleaceae). J. Jpn. Soc Coast. Forest. 21, 1–8. doi: 10.60398/kaiganrin.21.1_1
Tamasi E., Stokes A., Lasserre B., Danjon F., Berthier S., Fourcaud T., et al. (2005). Influence of wind loading on root system development and architecture in oak (Quercus robur L.) seedlings. Trees 19, 374–384. doi: 10.1007/s00468-004-0396-x
Telewski F. W. and Jaffe M. J. (1986). Thigmomorphogenesis: field and laboratory studies of Abies fraseri in response to wind or mechanical perturbation. Physiol. Plant 66, 211–218. doi: 10.1111/j.1399-3054.1986.tb02411.x
Theoharides K. A. and Dukes J. S. (2007). Plant invasion across space and time: factors affecting nonindigenous species success during four stages of invasion. New Phytol. 176, 256–273. doi: 10.1111/j.1469-8137.2007.02207.x
Tobinaga S., Sharma M. K., Aalbersberg W. G., Watanabe K., Iguchi K., Narui K., et al. (2009). Isolation and identification of a potent antimalarial and antibacterial polyacetylene from Bidens pilosa. Planta Med. 75, 624–628. doi: 10.1055/s-0029-1185377
Tunala, Hayakawa H., Minamiya Y., Gale S. W., Yokoyama J., Arakawa R., et al. (2012). Foliar adaptations in Aster hispidus var. insularis (Asteraceae). J. Plant Stud. 1, 19–25. doi: 10.5539/jps.v1n2p19
Ubillas R. P., Mendez C. D., Jolad S. D., Luo J., King S. R., Carlson T. J., et al. (2000). Antihyperglycemic acetylenic glucosides from Bidens pilosa. Planta Med. 66, 82–83. doi: 10.1055/s-0029-1243117
Weiner J. (2004). Allocation, plasticity and allometry in plants. Perspect. Plant Ecol. Evol. Syst. 6, 207–215. doi: 10.1078/1433-8319-00083
Wilkerson M. L. (2013). Invasive plants in conservation linkages: a conceptual model that addresses an underappreciated conservation issue. Ecography 36, 1319–1330. doi: 10.1111/j.1600-0587.2013.00182.x
Yang Y., Dou Y., An S., and Zhu Z. (2018). Abiotic and biotic factors modulate plant biomass and root/shoot (R/S) ratios in grassland on the Loess Plateau, China. Sci. Total Environ. 636, 621–631. doi: 10.1016/j.scitotenv.2018.04.260
Yang Y., Fang J., Ma W., Guo D., and Mohammat A. (2010). Large-scale pattern of biomass partitioning across China’s grasslands. Glob. Ecol. Biogeogr. 19, 268–277. doi: 10.1111/j.1466-8238.2009.00502.x
Yoshizaki S., Kawabata A., Tsuchiya T., and Seki H. (2023). Damage to tree leaves caused by wind-blown sand impact: trial of sandblasting experiment using a self-made simple apparatus. J. Jpn. Soc Coast. Forest. 22, 1–7. doi: 10.60398/kaiganrin.22.1_1
Yuan L. P., Chen F. H., Ling L., Dou P. F., Bo H., Zhong M. M., et al. (2008). Protective effects of total flavonoids of Bidens pilosa L. (TFB) on animal liver injury and liver fibrosis. J. Ethnopharmacol. 116, 539–546. doi: 10.1016/j.jep.2008.01.010
Keywords: resource allocation, leaf size, root, blown sand, coastal ecotype, aboveground morphology
Citation: Endo U, Shiba M and Fukuda T (2025) The invasive alien species Bidens pilosa (Asteraceae) has successfully invaded and acclimated to coastal areas. Front. Conserv. Sci. 6:1604666. doi: 10.3389/fcosc.2025.1604666
Received: 02 April 2025; Accepted: 23 June 2025;
Published: 10 July 2025.
Edited by:
Lina Podda, University of Cagliari, ItalyReviewed by:
Gianniantonio Domina, University of Palermo, ItalyEmanuele Del Guacchio, University of Naples Federico II, Italy
Pere Fraga Arguimbau, Fundació Privada Carl Faust, Spain
Copyright © 2025 Endo, Shiba and Fukuda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masayuki Shiba, bXN5a3NoaWJhNDhAZ21haWwuY29t
 Uta Endo
Uta Endo Masayuki Shiba
Masayuki Shiba Tatsuya Fukuda3
Tatsuya Fukuda3