- 1Department of Aquaculture, Faculty of Agriculture, Universiti Putra Malaysia, Serdang, Selangor Darul Ehsan, Malaysia
- 2International Institute of Aquaculture and Aquatic Sciences (I-AQUAS), Universiti Putra Malaysia, Port Dickson, Negeri Sembilan, Malaysia
- 3Department of Crop Science, Faculty of Agricultural and Forestry Sciences, Universiti Putra Malaysia Sarawak, Bintulu, Sarawak, Malaysia
- 4Retired, Kuala Lumpur, Malaysia
Seagrass ecosystems in Malaysia are increasingly threatened by coastal development, resulting in widespread habitat degradation. A decade-long monitoring program in the Sungai Pulai estuary (2015–2025) documented trends in species composition, water quality, and habitat recovery following reclamation activities. Thirteen seagrass species were recorded, indicating high diversity compared to the seventeen known in Malaysian waters. Four species, i.e., Halophila decipiens, H. major, H. beccarii, and H. nipponica, were newly documented, with H. nipponica as a new national record for Malaysia. Seagrass percentage cover showed a moderate negative correlation with conductivity (r = –0.622, p < 0.05) in Merambong A (MA), as well as conductivity (r = –0.594), total dissolved solids (r = –0.500), and salinity (r = –0.519) in Merambong C (MC). It also showed a moderate negative correlation with DO (r = –0.545) and salinity (r = –0.502) in Tanjung Adang Laut (TAL). In response to habitat degradation, a rehabilitation program was carried out at the Merambong shoal using Enhalus acoroides seedlings as stabilizer species, along with cover species such as H. ovalis, H. major, and H. spinulosa. A total of 8,591 seedlings were transplanted across 324-square-meter plots, achieving survival rates of 63.39% at MA and 66.07% at Merambong B (MB), surpassing the success of direct seeding and vegetative transplant methods. Cover studies showed that MB consistently had more E. acoroides coverage during the early (10–30 months) and late (30–60 months) stages, peaking at 86.08% in certain plots. The inclusion of mixed species improved sediment stabilization and facilitated rapid vegetative recovery. These findings demonstrate the effectiveness of seedling-based rehabilitation strategies, emphasize the importance of species complementarity, and highlight the need for ongoing monitoring to protect tropical seagrass habitats from human pressures.
1 Introduction
Seagrasses play an essential ecological role in coastal marine environments by stabilizing sediments, cycling nutrients, improving water quality, supporting high biodiversity, and providing vital nursery habitats for marine species. However, these productive ecosystems face increasing threats from human activities, especially in rapidly urbanizing coastal zones (Nordlund et al., 2017; Waycott et al., 2009; Rozaimi et al., 2017; Ambo-Rappe, 2020; Unsworth et al., 2021; Lima et al., 2022, 2023; Stankovic et al., 2023). Coastal development, including land reclamation, dredging, port construction, and sediment discharge, has caused widespread degradation and fragmentation of seagrass habitats, especially in Southeast Asia (Waycott et al., 2009; Short et al., 2011). A global review of 94 studies also showed that seagrass habitats reliably improve recruitment and abundance of commercially important species (Herrera et al., 2022). Supporting this, Lima et al. (2023) highlighted that nursery provisioning remains one of the most consistently reported ecosystem services of seagrass meadows, especially in tropical regions. These findings highlight the cascading effects of seagrass loss, including decreased fishery productivity and compromised food security, emphasizing the need for conservation and restoration in tropical coastal areas. Restoration and rehabilitation of seagrass meadows have gained momentum as global awareness increases regarding their ecological and economic significance.
Restoration refers to returning seagrass ecosystems to near-original conditions, while rehabilitation focuses on improving degraded meadows without necessarily replicating the original species composition or functions (Gordon, 1996; Seddon, 2004; Ganassin and Gibs, 2008). Since the 1960s, various transplanting methods, both vegetative (e.g., plugs, rhizomes, sods) and generative (e.g., seeds, seedlings), have been tested with varying levels of success (Fonseca et al., 1998; Calumpong and Fonseca, 2001). While vegetative methods provide immediate cover, they can be labor-intensive and cause damage to donor beds. Conversely, seedling-based approaches are increasingly favored because of their lower cost, scalability, and minimal impact on source populations (Christensen et al., 2004).
Despite the global rise in restoration studies, tropical seagrass rehabilitation remains underrepresented compared to temperate systems. However, species like Enhalus acoroides, which produce large and abundant seeds, are suitable for seed-based restoration. Studies have demonstrated promising outcomes using seedling tanks, hessian bags, and even seeding machines (Orth et al., 2006; Harwell and Orth, 1999; Nixon et al., 2002). According to Ambo-Rappe and Moore (2019), seed-based transplanting may provide a viable long-term solution for tropical systems, particularly when donor meadows are limited.
In Malaysia, seagrasses such as E. acoroides and Halophila ovalis were once abundant along shallow coasts (Ridley, 1924; Henderson, 1954). Over time, however, degradation due to natural and human disturbances such as dredging, boat anchoring, and coastal development has resulted in habitat fragmentation and loss of vegetated areas (Japar Sidik et al., 2007, 2018; Abu et al., 2022; Emmclan et al., 2022). Fragmentation decreases ecological resilience, as patchy beds with bare gaps become more vulnerable to macroalgae overgrowth, sediment instability, and further decline (Serra et al., 2020; Hemminga and Duarte, 2000). The Sungai Pulai estuary in southern Johor, Malaysia, is known for its rich biological diversity and ecological value, especially because of its extensive seagrass and macroalgal communities (Japar Sidik et al., 2006, 2014, 2020; Japar Sidik and Muta Harah, 2011a). Despite its ecological significance, the Sungai Pulai estuary has experienced considerable degradation over the past twenty years due to intense human activities (Japar Sidik and Muta Harah, 2011b; Japar Sidik et al., 2018).
Despite its ecological importance, the Sungai Pulai estuary has faced significant degradation over the past two decades due to intensive human activities (Muta Harah and Japar Sidik, 2011; Japar Sidik et al., 2018). A major development activity in the region started in 2014 with the launch of the Forest City reclamation project in Mukim Tanjung Kupang, Johor Bahru district. The original plan, covering about 5,000 acres, included construction near the Merambong shoal, a subtidal seagrass meadow valued for its ecological importance. During Phase 1, activities such as sand mining, landfilling, and infrastructure construction result in habitat loss, reduced seagrass cover, and declining water quality due to increased sedimentation. Approximately 9.95 hectares of seagrass habitat were directly lost (Figure 1). These impacts not only reduce the size of seagrass beds but also disrupt their natural structure, causing shifts in species composition and negatively affecting ecosystem health. This indicates a fundamental shift in the dominance of primary producers and ecosystem function (Muta Harah et al., 2016). Observations in the Merambong shoal have revealed a transition from seagrass dominance to an increase in opportunistic macroalgae, such as Ulva reticulata and Amphiroa fragilissima (Abu et al., 2022; Emmclan et al., 2022), indicating early signs of ecological imbalance and the potential for prolonged damage. Following environmental assessments and consultations, the Department of Environment (DOE) revised the development layout to minimize further ecological impact. Since then, collaborative efforts have been initiated with project developers to support long-term monitoring and restoration of seagrass habitats in the area. In response to this loss, Universiti Putra Malaysia (UPM), in collaboration with Country Garden Pacificview Sdn. Bhd., launched a long-term (2015–2025) monitoring and assisted recovery program focusing on the most affected zones, especially Merambong A and B. The program involved systematic monitoring of species composition, seagrass coverage, macroalgae growth, and environmental factors, along with active transplanting efforts using both seedlings of E. acoroides and ramets of Halophila species.
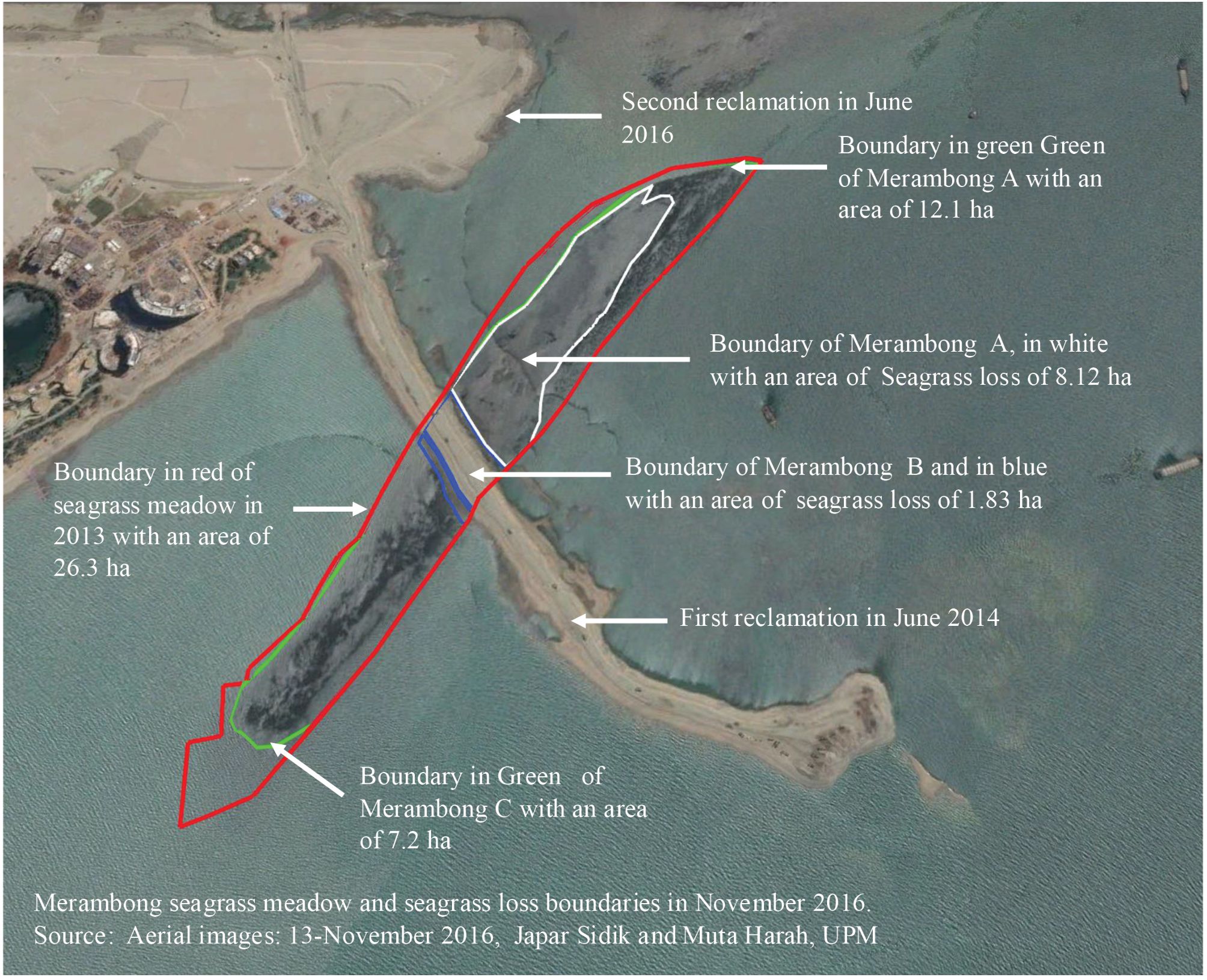
Figure 1. Merambong seagrass meadow. Note that Merambong A exhibits bare areas and low seagrass cover due to the second reclamation. In contrast, Merambong C exhibited healthy seagrass cover, experiencing minimal impact from the second reclamation. Source: Aerial images: 13-November 2016, Japar Sidik and Muta Harah, UPM.
Global concern over seagrass degradation has led to the formation of initiatives such as the Global Conservation of Vulnerable, Threatened and Endangered Seagrass, aimed at fostering coordinated restoration programs (UNEP-WCMC, 2021; IOC-UNESCO, 2020). The Sungai Pulai estuary exemplifies this crisis, where habitat modification, sedimentation, and eutrophication have caused substantial losses.
This program tracks temporal trends in seagrass growth, community composition, and overall ecosystem health. An assisted recovery initiative was also conducted, involving the transplanting of stabilizer species, Enhalus acoroides, and cover species (Halophila ovalis, H. major, H. spinulosa) into the barren areas of Merambong A and the reclaimed area of Merambong B to promote sediment stabilization and succession. The objectives were to evaluate planting materials and techniques, monitor survival and growth across transplant plots, and assess rehabilitation outcomes in relation to site characteristics and disturbance history. This research provides valuable empirical data to advance tropical seagrass restoration science and guide best practices across Malaysia and the broader Indo-Pacific region. The objectives were to evaluate planting materials and techniques, monitor survival and growth across transplant plots, and assess rehabilitation outcomes in relation to site characteristics and disturbance history. Hence, this study presents the first successful long-term documentation (2015–2025) of tropical seagrass rehabilitation in Malaysia amid active coastal development. It offers empirical evidence supporting seedling-based restoration as an affordable and ecologically sustainable strategy in donor-limited systems. The integration of ecological monitoring with practical rehabilitation provides a replicable framework for managing and restoring seagrass meadows across tropical Indo-Pacific regions.
2 Materials and methods
2.1 Ecological setting of the Merambong shoal
Located at the mouth of the Sungai Pulai estuary on the southeast coast of Peninsular Malaysia, the Merambong shoal (1°19’45.6” N to 1°20’21.49” N and 103°35’51.9” E to 103°36’13.91” E) is recognized as one of the region’s most extensive and species-rich seagrass beds. This subtidal shoal extends from 150 m to 1.2 km in length and 100 m to 150 m in width, resting on calcareous sandy mud. It is submerged throughout most tidal phases, with seagrasses exposed only during the lowest spring tides. The area is subject to dual monsoonal influence, with seasonal variability driven by the Northeast and Southeast Monsoons (Akhir and Chuen, 2011) and is shaped by hydrodynamic exchanges between the Straits of Malacca and the Straits of Johor. During the reclamation activity in February 2014, Merambong seagrass shoal was partitioned into three segments: Merambong A (MA, facing the Second Link), Merambong B (MB, the shoal’s reclaimed area), Merambong C (MC, facing the Tanjung Adang Laut seagrass shoal), and Tanjung Adang Laut seagrass shoal as shown in Figure 2.
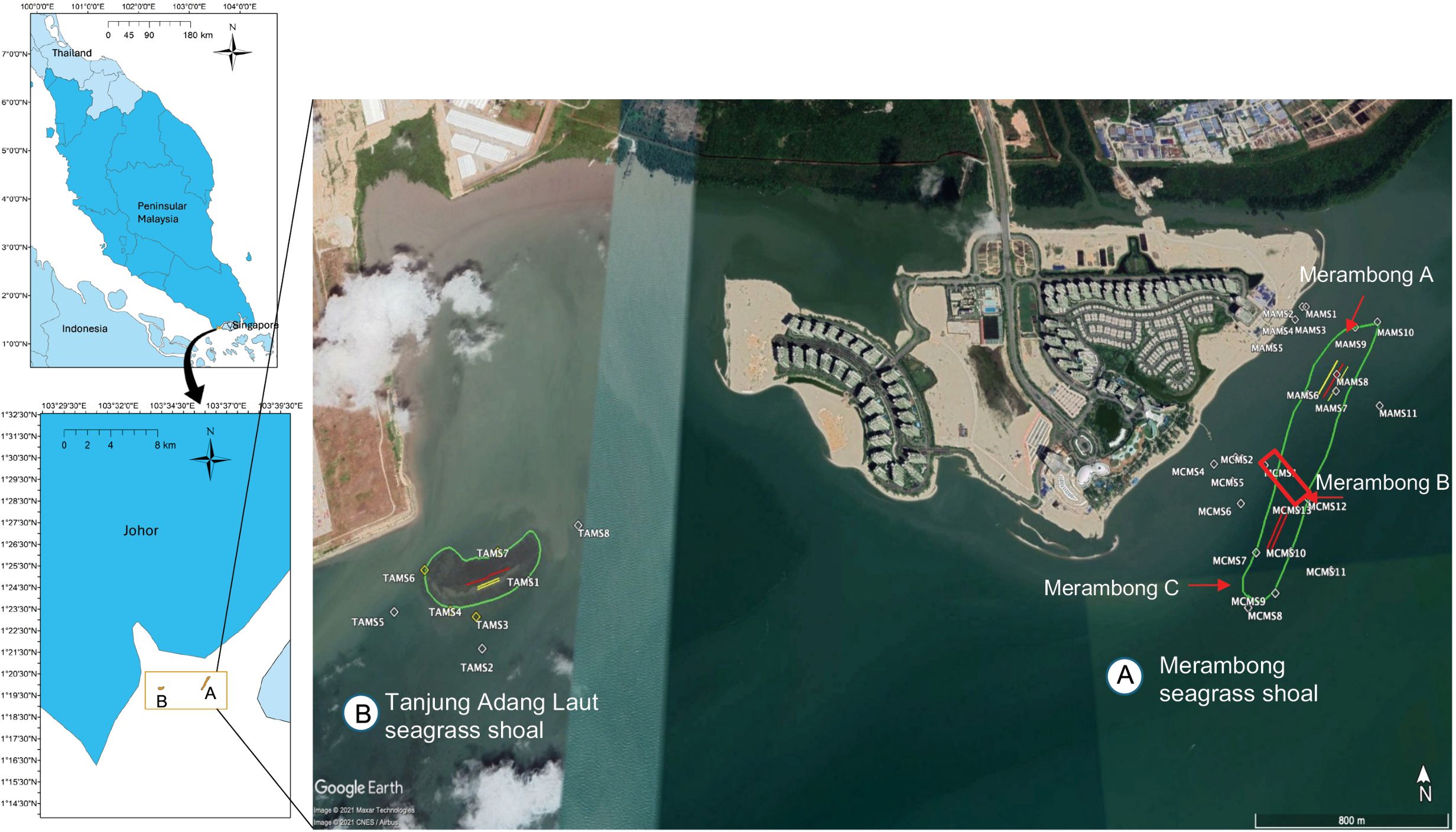
Figure 2. Seagrass location at Merambong and Tanjung Adang Laut shoals, Sungai Pulai Estuary, Johor, Malaysia. (A) Merambong seagrass shoal showing divisions into three sections: Merambong A (facing the Second Link, including Transects A, B, and C; monitoring stations MAMS 1-11), Merambong B (the shoal’s reclaimed area, excavated in February 2018, indicated by a red rectangle), and Merambong C (including Transects A, B, facing the Tanjung Adang Laut seagrass shoal, with monitoring stations MCMS 1-13). (B) The Tanjung Adang Laut seagrass shoal features permanent transects (A, B) and water quality monitoring stations, TAMS 1-8. Map Source: Google Earth-Image @ 2021 Maxar Technologies/CNES/Airbus.
2.2 Seagrass monitoring
Monitoring of the seagrass bed was conducted monthly from February 2015 to March 2024, with some months skipped (February, June, and October) due to weather-related and logistical constraints, as well as COVID-19 restrictions in 2020. As site conditions gradually stabilized, the monitoring frequency was adjusted to quarterly between 2021 and 2022. From 2023 onward, assessments were conducted biannually to continue tracking long-term recovery trends while accommodating site stability. Due to national COVID-19 restrictions and safety limitations between 2020 and 2021, scheduled seagrass monitoring was disrupted, leading to a decrease in sampling frequency. No interpolation or imputation methods were used to estimate missing data. To ensure consistency and reduce bias, only data from the corresponding sampling months, February, June, and October, were used for temporal comparisons across years. This approach ensured that observed patterns were based on actual field data rather than predictions from models. Site selection remained consistent throughout the study, and before the pandemic, these locations showed relatively stable seagrass presence and habitat conditions, supporting the validity of long-term trend analysis.
At each zone of the shoal, a fixed 200-meter transect line was established, with 50 cm × 50 cm quadrats placed at 10-meter intervals along the transect. Quadrat images were captured using a camera equipped with a Global Positioning System (GPS). Data on seagrass species, the presence of macroalgae, and the percentage of area coverage were recorded during each survey. Coverage of seagrass, macroalgae, and substrate was estimated using Photoquad v1.4 software. Seagrass, macroalgae, and substrate coverages were assigned Braun-Blanquet (B-B) scale values (Table 1, Jupp et al., 1996, as cited in Japar Sidik et al., 2001).
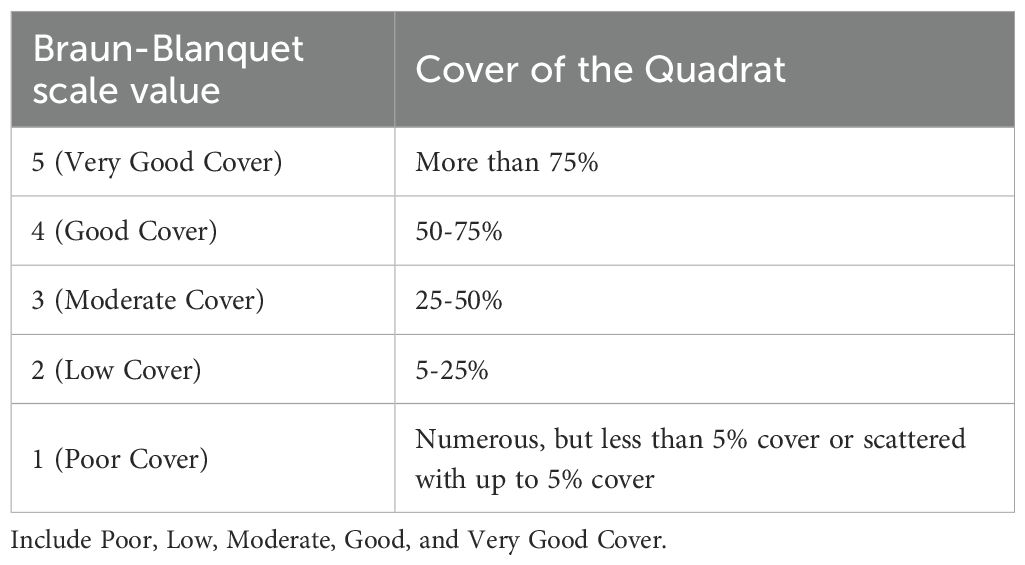
Table 1. A cover-abundance according to modified Braun-Blanquet (B-B) scale values presented below, as mentioned in Jupp et al. (1996, in Japar Sidik et al., 2001).
2.3 Donor sites and transplant materials
Planting materials for rehabilitation were collected from the ecologically stable Tanjung Adang Laut (TAL) and MC shoals. The dominance of E. acoroides in both beds facilitated the targeted collection of fruits and shoots without significantly compromising donor populations. Fully ripened fruits of E. acoroides exceeding 3.6 cm in diameter were harvested and transported to a semi-outdoor cultivation facility at the Institute of Aquaculture and Aquatic Sciences (I-AQUAS), Universiti Putra Malaysia. Seeds extracted from these fruits germinated in a 2.4 m × 1.2 m × 1 m seawater tank under natural light and monitored water parameters (temperature: 29–30°C; salinity: 30–37 ppt; DO: 4.6–5.7 mg/L; pH: 7.3–7.9). Germination progressed through defined phases, from cotyledon emergence to juvenile seedling development. Halophila ovalis, H. major, and H. spinulosa were sourced from Merambong A for transplanting in the bare area of Merambong A and from Merambong A and C for transplanting in the reclaimed area of Merambong B.
2.4 Assisted seagrass recovery program: transplanting of seagrasses at Merambong A and B
Initial transplant trials were conducted at MA between December 2016 and March 2017. A total of 35 plots were established using 100 cm × 100 cm quadrats, excluding additional transplants performed directly on bare substrate without quadrat framing, which were implemented opportunistically based on site conditions. Each quadrat contained nine planting units, arranged based on the type of Material: individual shoots (trimmed to 2.5 cm), clusters of three seeds, or groups of three seedlings aged 2 to 3 months. Shoots and seedlings were manually inserted into the substrate at a depth of approximately 5 cm.
Following the pilot trials, seedlings exhibited the highest establishment success and were selected for large-scale transplantation from April 2017 to September 2023. A 50 m to 100 m transect line and quadrat (50 m x 50 m) were used as the markers for transplanting seagrass in bare areas or within H. ovalis of H. major of the shoal at Merambong A and B. Two- to three-month-old laboratory-germinated Enhalus acoroides (as Stabilizer Species) seedlings were planted at each assigned point in the 100 cm x 100 cm plot in bare areas or among H. ovalis, H. major. A 10 cm x 10 cm patch of Halophila ovalis, H. major, or H. spinulosa (as Cover Species) was also collected from adjacent areas. These were transplanted at each point in the 100 cm x 100 cm plot, specifically in bare spots (Figure 3A). A total of 28 transplant batches (approximately 275 quadrats) were carried out at MA and MB. The transplants were monitored monthly for survival, growth, and expansion. In areas where the transplants did not survive, they were replaced (Figure 3B).
Figure 3. (A) Planting plot measuring 100 cm x 100 cm, with each point consisting of three- to two-month-old Enhalus acoroides laboratory-germinated seedlings as stabilizer species, along with Halophila ovalis, H. major, or H. spinulosa as cover species, collected from the adjacent area, were planted in 10 cm x 10 cm plots at each designated point. (B) A total of 28 transplant batches (approximately 275 quadrats) were conducted at MA and MB.
2.5 Water quality and nutrient conditions
Monthly water quality monitoring was conducted across 31 stations, comprising 11 at Merambong A (MA), 12 at Merambong C (MC), and 8 at Tanjung Adang Laut (TAL). Parameters measured in situ included temperature, dissolved oxygen, conductivity, total dissolved solids (TDS), salinity, and pH using a YSI Professional Plus handheld multi-parameter instrument (YSI Inc., USA). Water visibility was assessed using a 30 cm diameter Secchi disc, following the method described by Abal and Dennison (1996). Water visibility was measured using a 30 cm diameter Secchi disc (Abal and Dennison, 1996). Total suspended solids (TSS) were determined in the laboratory according to EPA Method 160.2 (United States Environmental Protection Agency, 1999). Nutrients contents were determined using HI97105 Marine Master Multiparameter Photometer (HANNA Instruments, Woonsocket, USA), including nitrate (NO3−) (Zinc Reduction Method), nitrite (NO2−) (EPA Diazotization method 354.1), orthophosphate (PO4−) (Standard Methods for the Examination of Water and Wastewater, 20th Edition, Ascorbic Method) and ammonia (NH3−) (Salicylate method) (Hach Company, 2004).
2.6 Statistical analysis
Statistical analysis was performed to assess temporal and spatial variations in water quality and nutrient dynamics across the three shoals. A two-way repeated measures ANOVA was conducted to examine the effects of location (MA, MC, TAL) and time/period (Phase 1: February 2015–February 2018; Phase 2: June 2018–February 2024) on the measured parameters. Tests of sphericity and Wilks’ Lambda were utilized to evaluate assumptions and multivariate significance. Post hoc comparisons using Tukey’s HSD test provided additional insights into pairwise differences across locations and time frames. Pearson’s correlation analyses were employed to examine the relationships between environmental variables and seagrass percentage cover during the monitoring period. Pearson’s correlation analyses (p<0.05) were performed between seagrass percentage cover and water quality variables, with missing values estimated. Significant Pearson correlation coefficients are shown in tabular form and interpreted as very high (>0.90), high (0.70 to 0.89), moderate (0.50 to 0.69), low (0.30 to 0.49), and negligible (<0.30) for positive correlations, and vice versa (Hinkle et al., 2003).
3 Results
3.1 Seagrass status in Sungai Pulai estuary from 1996 to 2025
Historically, ten seagrass species were recorded at Merambong shoal in 1996, including Cymodocea rotundata. However, since systematic monitoring began in 2013, C. rotundata has not been observed at the site to date (Table 2). Between 2015 and 2025, three new seagrass species were documented for the Sungai Pulai estuary, along with one new national distribution record, increasing the total number of species recorded in the estuary to 13 (Table 2, Figure 4). Halophila decipiens was first discovered in August 2015 at Merambong A, while H. major was identified in June 2015 at Merambong C and Tanjung Adang Laut. Halophila beccarii was also confirmed before April 2015 at Sungai Pendas.
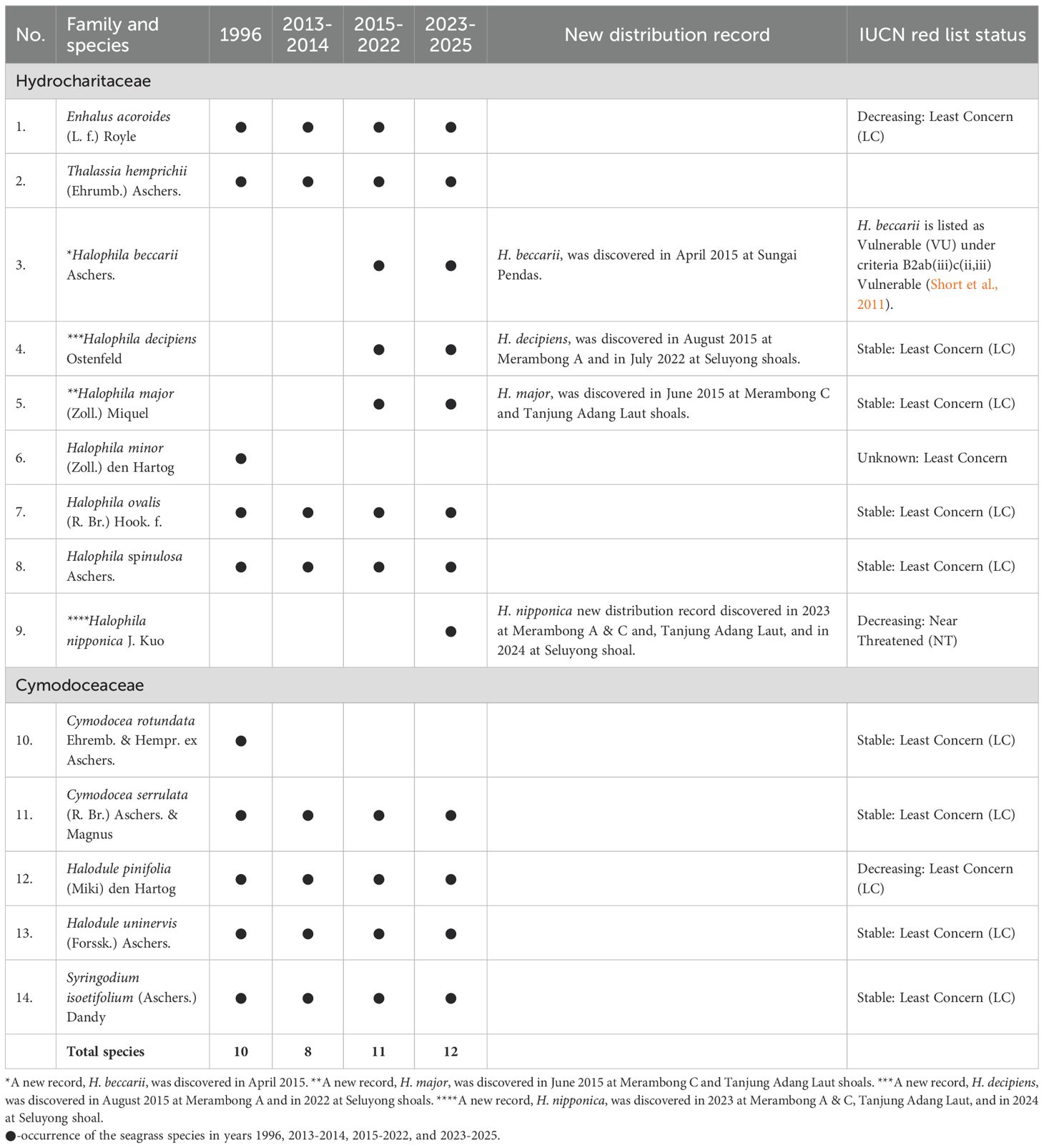
Table 2. Seagrass species occurrence at the Sungai Pulai estuary seagrass shoals, Johor, from 1996 to 2025.
Additionally, H. nipponica, a new distribution record for Malaysia, was observed in 2023 at Tanjung Adang Laut and in 2024 at Merambong and Seluyong shoals (Figure 5). These recent discoveries, made during the project’s active monitoring period, highlight the ecological significance of the Sungai Pulai estuarine complex and contribute to an updated Malaysian seagrass inventory. The findings underscore the importance of continuous monitoring to detect previously undetected or emerging species and better understand seagrass distribution in response to environmental changes.

Figure 5. New record of seagrasses during 2015-2024. (A) H. beccarii, (B) H. decipiens, (C) H. major and (D) H. nipponica.
The conservation status of each species was assessed using the IUCN Red List (2023). Halophila beccarii is classified as Vulnerable B2ab(iii)c(ii,iii) under the IUCN Red List Categories and Criteria Version 3.1 (Short et al., 2011; IUCN, 2023) due to its restricted and fragmented distribution, small population size, and ongoing decline. Halophila nipponica, newly recorded in Malaysia, is listed as a decreasing Near Threatened (NT), reflecting increasing regional pressures. Most other species, including Enhalus acoroides, Cymodocea rotundata, Halophila decipiens, Halodule uninervis, Halophila ovalis, and Syringodium isoetifolium, are listed as Least Concern (LC). However, some show signs of local instability, underscoring the need for site-specific conservation and long-term monitoring strategies.
3.2 Trends in seagrass diversity from 2015 to 2024
Seagrass and seaweed abundance were assessed as percentage cover using fixed transects and classified using the Braun-Blanquet (B-B) scale. The spatial and temporal trends in cover at Merambong A (MA) are illustrated in Figures 6A–C. Seagrass coverage was documented near the previously reclaimed area along Transect Lines B and C, as well as farther away at Transect Line A. Across these transects, coverage ranged from Good Cover (B-B Scale 4, 50–75%) to Very Good Cover (B-B Scale 5, >75%). Merambong A exhibited generally Good Cover, dominated by Halophila major, H. ovalis, and H. spinulosa from both transplant efforts and natural regeneration. Additional naturally occurring species included Halodule pinifolia, H. uninervis, Thalassia hemprichii, and Cymodocea serrulata. Initial monitoring in 2015–2016 revealed localized seagrass losses, with coverage declining by 0.75% to 18.83%, primarily attributed to extensive reclamation activities. Seaweed percentage cover was high during 2015-2016, with values ranging from 7.75% to 74.96%. Recovery signs emerged between August 2015 and January 2016, with regrowth observed along all transects. During the mid-phase of monitoring (2017–2019), seagrass cover increased, ranging from Moderate Cover (B-B Scale 3, 25–50%) to Very Good Cover (>75%), with a peak recorded in June 2018 at 79.25% in Merambong A. Seaweed percentage cover showed high variability, with significant peaks in June 2017 and June 2019. Concurrently, substrate cover declined as seagrasses became more established.
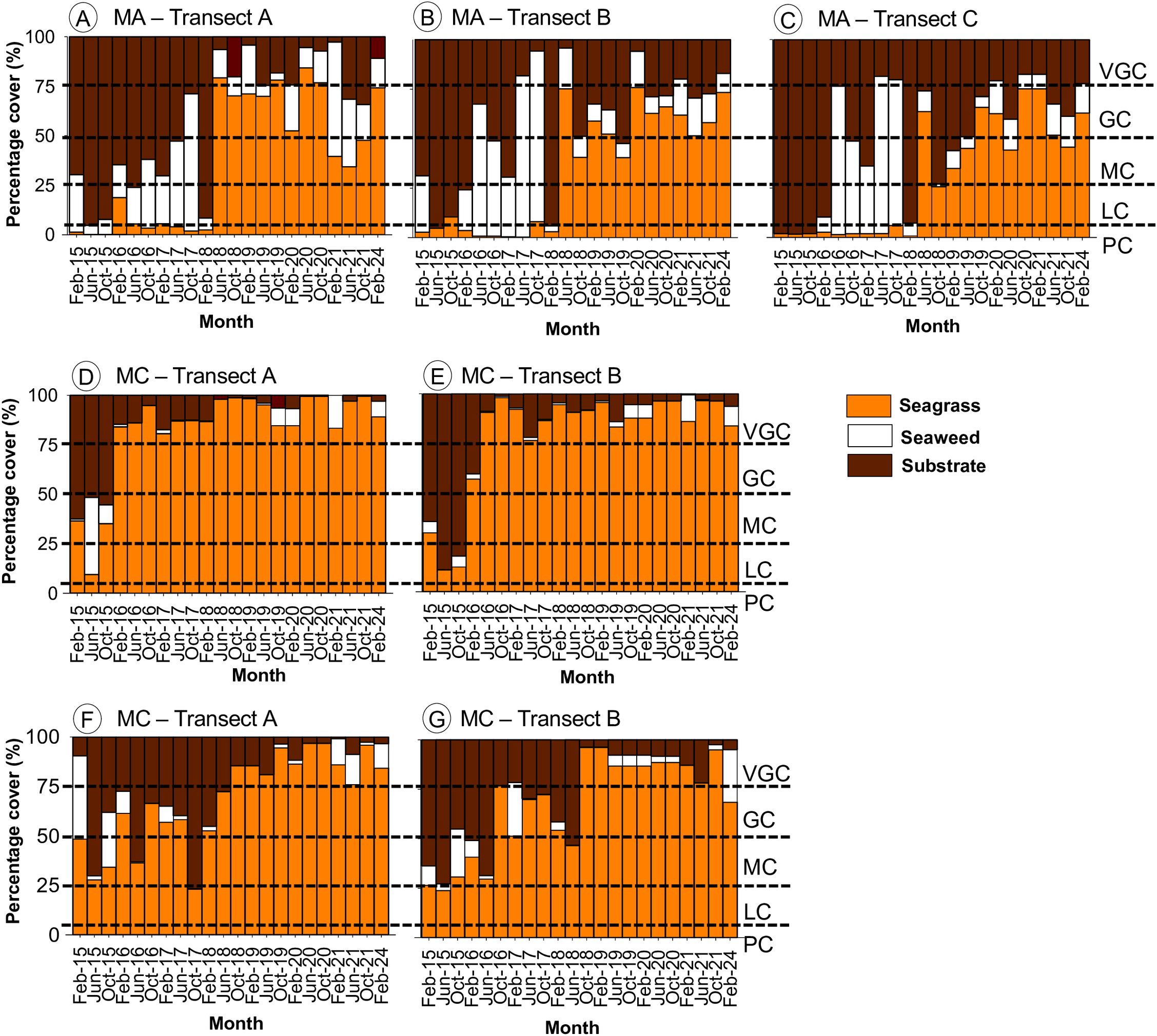
Figure 6. Cover percentage of seagrass, seaweed, and substrate over nine years (2015-2024) for three locations: (A–C) Merambong A (MA), (D, E) Merambong C (MC), and (F, G) Tanjung Adang Laut (TAL). The gray dotted line represents the Braun-Blanquet scale values: 5 - Very Good Cover (VGC) with more than 75% cover, 4 - Good Cover (GC) with 50-75% cover, 3 - Moderate Cover (MC) with 25-50% cover, 2 - Low Cover (LC) with 5-25%, and 1 - Poor Cover (PC) with numerous, but less than 5% cover or scattered with up to 5% cover.
At Merambong C (MC), a relatively less disturbed reference site, seagrass communities rebounded more rapidly and consistently (Figures 6D, E). However, a marked decline in cover was recorded between March and August 2015. In June 2015, macroalgal cover reached 39.08%, dominated by Amphiroa fragilissima along Transect Line A. Recovery began in November 2015, with seagrass expanding from the shoal margins into deeper areas. Key species included H. ovalis, H. major, H. spinulosa, and C. serrulata. Macroalgal interference remained limited, with only sporadic patches of Caulerpa manorensis reaching high density.
At the TAL shoal, seaweed coverage along Transects A and B was relatively low, classified as B-B Scale 2 (5–25%). A noticeable decline in seagrass cover was observed beginning in October 2015, with coverage dropping to Moderate Cover (B-B Scale 3, 25–50%) (Figures 6F, G), accompanied by increased substrate exposure and a moderate presence of seaweed, particularly A. fragilissima. Seagrass cover initially remained low but began to recover by October 2017. From 2017 to 2020, a significant recovery phase occurred, during which seagrass coverage increased and stabilized within the Moderate to Very Good Cover range (B-B Scale 3–5, 25–100%), suggesting successful regrowth. Throughout this period, seaweed cover remained consistently low, showing minimal variation until June 2018. Concurrently, substrate cover declined as seagrass cover improved, indicating a positive inverse relationship. In subsequent years, seagrass coverage showed intermittent fluctuations, likely reflecting seasonal changes or ongoing disturbances, with an overall positive trajectory by the end of the monitoring period (B-B Scale 4–5; 50–>75%).
In Merambong A, however, seagrass recovery was inconsistent and often suppressed, remaining within Poor (B-B Scale 1; <5%) to Low Cover (Scale 2; 5–25%) due to the proliferation of the green drift macroalga Ulva reticulata (Figure 7A). In early 2017, U. reticulata formed dense, free-floating mats, significantly reducing light penetration to underlying seagrass canopies. A notable mass accumulation of U. reticulata was recorded in early 2017, forming dense, free-floating mats that restricted light penetration to the seagrass below. Macroalgae such as Caulerpa manorensis, C. sertularioides, Hydropuntia edulis, Ulva reticulata, and Amphiroa fragilissima were observed co-occurring with H. major and H. ovalis in Merambong A (Figures 7B–F). Certain patches exhibited coverage of C. manorensis exceeding 60%. In the later monitoring phase (2020–2024), seagrass cover stabilized, reaching a peak of 84.33% in June 2020, while seaweed presence continued to fluctuate, remaining prominent during specific periods. However, at Tanjung Adang Laut shoal, the edge of the shoal was densely colonized by free-floating macroalgae, including U. intestinalis, U. reticulata, A. fragilissima (Figures 7G–I), and H. edulis, coexisting with seagrasses.

Figure 7. Seaweed colonization and substrate exposure in Merambong A (A–F) and Tanjung Adang Laut (G–I) show ecological responses likely influenced by sedimentation and land reclamation activities. (A) dense bloom of U. reticulata covering seagrass bed, (B) scattered patches of Hydropuntia edulis (arrows) on exposed sediment, (C) co-occurrence of H. edulis (arrows) and U. reticulata forming clumps over seagrass, (D) drift patch of Caulerpa manorensis along transect line, (E) mixed growth of C. manorensis (arrow) and U. reticulata within seagrass, (F) presence of C. sertularoides, (G) early-year monitoring shows visible substrate and red mat of A. fragilissima and patches of C. manorensis (arrow) (H) extensive spread of A. fragilissima across shoal and (I) thick mat of A. fragilissima covering H ovalis (arrow), indicating light competition.
The transient growth of macroalgae A. fragilissima and H. edulis at TAL did not result in significant habitat degradation. This contrast highlights the significance of local hydrodynamics and sediment quality in mitigating algal overgrowth and facilitating natural recovery. A total of 41 seaweed species were recorded at Merambong and 38 at Tanjung Adang shoal. The overall composition was largely consistent across sites, contributing to a more comprehensive species inventory for the Sungai Pulai estuary. This richness, together with the decline in macroalgal blooms and the increase in seagrass coverage, indicates that successful restoration depends not only on the planting strategy but also on long-term environmental management. Sustained efforts to control sediment inflow and nutrient loading from surrounding developments will be essential to ensuring the sustainability of restored habitats.
3.3 Seasonal reproductive patterns and propagule viability
Field observations revealed that the flowering and fruiting phases of Enhalus acoroides were consistently present at both shoals throughout the year (Figure 8). Elevated reproductive activity was recorded during two main periods: January to March and October to December. The emergence of female flower buds was first observed in November, December, and January, coinciding with the appearance of fully mature female flowers, which were last detected in January. Anthesis is inferred to have occurred between November and January, as well as from July to November. During these periods, a higher abundance of both female and male flowers, along with a substantial release of pollen, was documented, particularly between February and March and December and January. The production of female and male flowers was notably higher at Tanjung Adang Laut (TAL) (Figures 8A–D), with mean densities of 5.87 ± 1.83/m² and 4.70 ± 3.34/m², respectively, compared to Merambong shoal (Figures 8E–H), which recorded 3.87 ± 2.33/m² for female flowers and 4.00 ± 4.10/m² for male flowers.
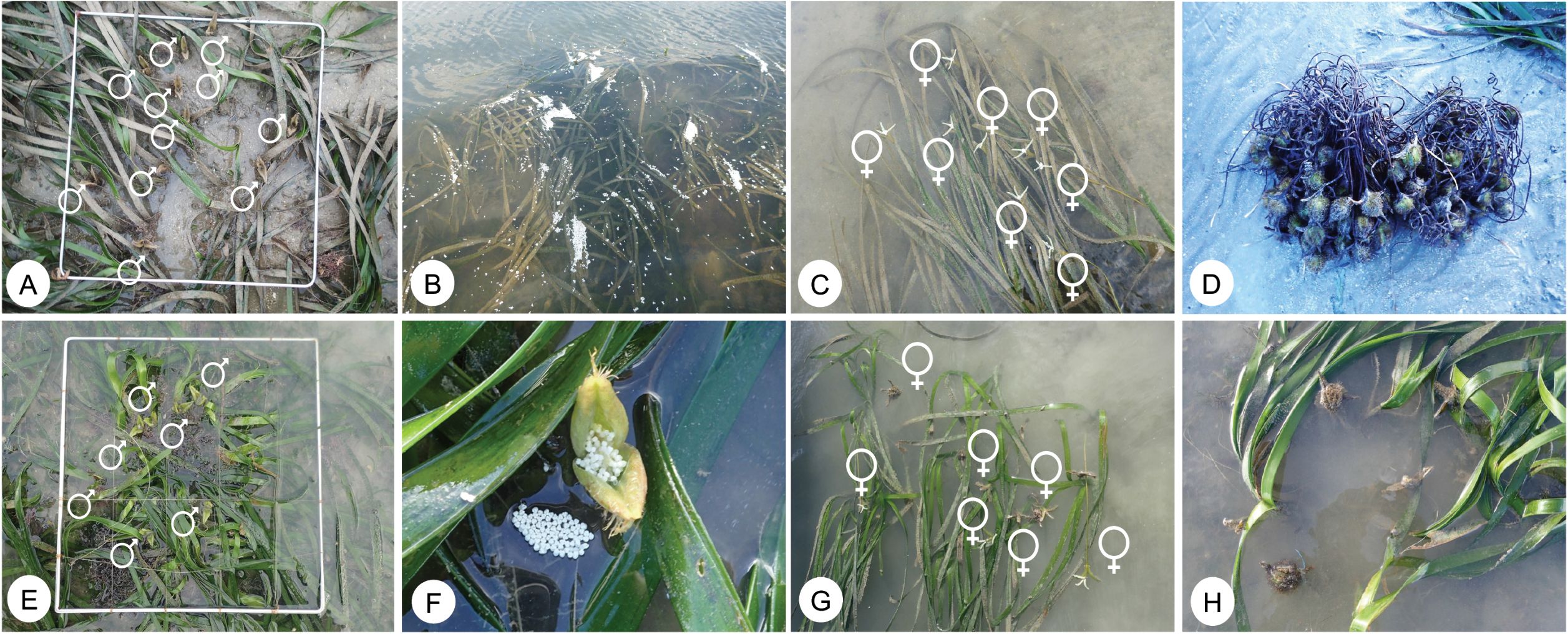
Figure 8. Phenology of E. acoroides in TAL (A–D) in February to March and (E–H) in MC from August to September. (A, E)-Male flower exposed during low tide, (B)-Pollen of male flowers on the water surface, (C, G)-Female flower, (D)-fruits, (F)-Male inflorescence with many flowers, and (H)-Matured and developing fruits.
Tanjung Adang Laut yielded a greater number of mature fruits (propagules), with a peak of 153 propagules harvested in November 2016 (Figure 9). Across both shoals, over 2,900 viable propagules were collected and subsequently cultured for seedling production. Morphologically, propagules collected from TAL exhibited rounder forms with an average seed count of 11.1 (range: 7–18) per fruit, while those from Merambong were more elongated and carried slightly more seeds on average (12.5 seeds, range: 8–19). Seed size metrics also varied, with TAL propagules being significantly larger in both length (13.57 ± 1.05 mm) and diameter (14.52 ± 1.08 mm) compared to their Merambong counterparts (12.39 ± 1.0 mm in length; 12.86 ± 1.41 mm in diameter).
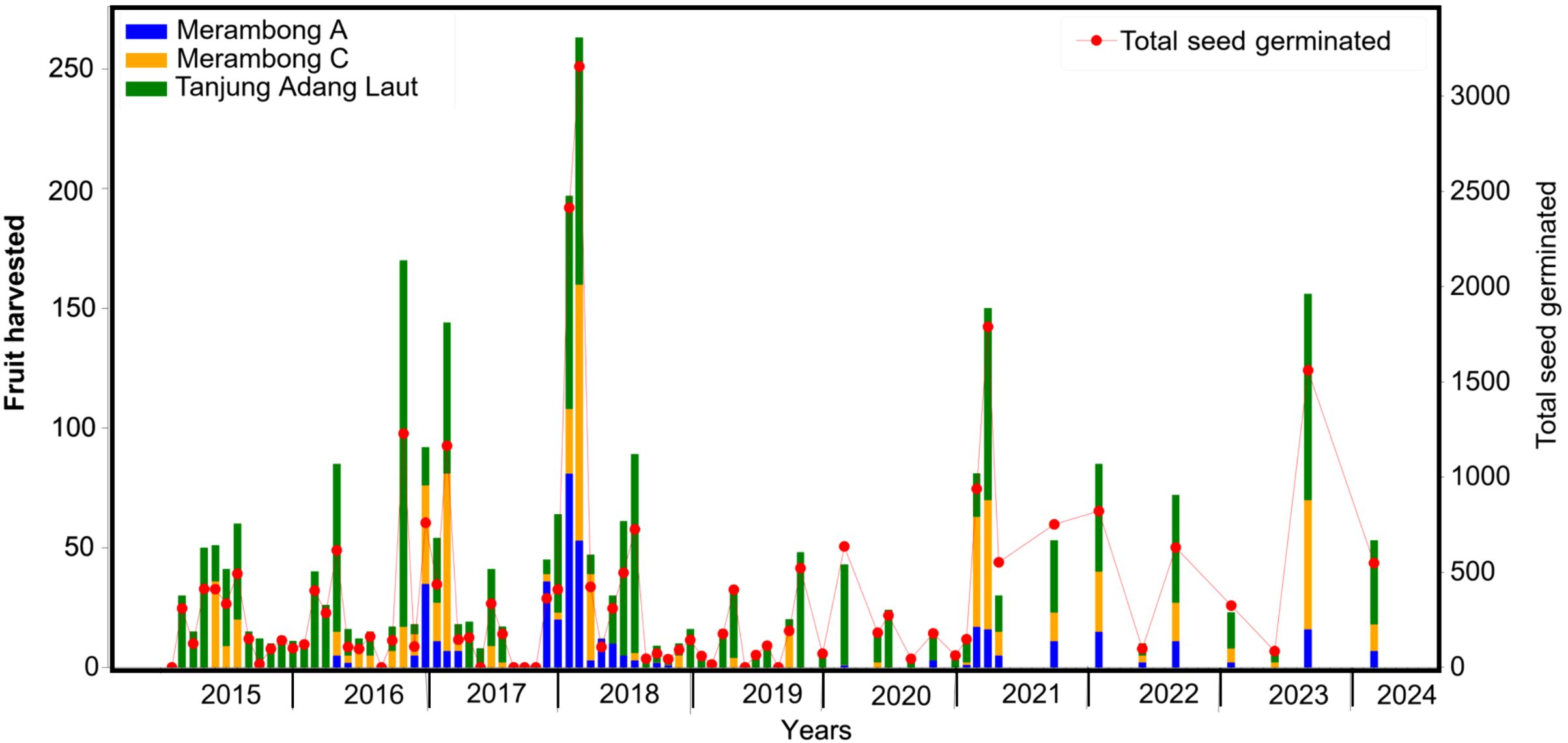
Figure 9. The bar graph depicts the number of Enhalus acoroides propagules collected from the Merambong A and C, and Tanjung Adang Laut shoals. The line plot represents the number of seedlings that germinated in the tank culture.
3.4 Germination, seedling establishment, and growth performance of seagrass transplanting
Germination trials conducted under controlled tank culture conditions revealed that nearly half of the Enhalus acoroides propagules had already ruptured upon arrival, mainly due to the time-sensitive nature of transport (3–5 days). Despite this, the seedlings progressed through three distinct developmental phases over 12 weeks, including germination, seedling establishment, and juvenile growth, characterized by the formation of adventitious roots, multiple shoots, and elongated leaves. Following the propagation of viable E. acoroides seedlings, experimental planting trials revealed distinct patterns of establishment and survival. Initial pilot efforts at MA tested three planting materials: vegetative shoots, direct seeds, and seedlings aged two to three months.
The results indicated stark differences in viability. While seedlings recorded survival rates ranging from 44.4% to 63% over four months, the survival rate of transplanted shoots remained below 22.2%, and none of the directly sown seeds lasted beyond the first month. These findings prompted a strategic shift to prioritize seedlings as the core planting material. This approach not only enhanced success rates but also minimized pressure on donor beds by utilizing the natural fecundity of E. acoroides fruits, which contain 8–19 seeds each. Seedlings also demonstrated better resilience during transplanting and produced stable rooting structures, reducing the risk of dislodgement. Between 2017 and 2023, a total of 8,591 seedlings were transplanted into 324 one-square-meter plots across MA and MB. The majority (6,934) were established in MA, with MB receiving 1,657. Transplants were supported by selected cover species (H. ovalis, H. major, and H. spinulosa) to create a composite habitat matrix. Throughout the monitoring period, seedling survival remained consistent after 8 to 9 months, with MB showing slightly higher rates (66.07%) than MA (63.39%) (Figure 10A). Percentage cover analyses reinforced this trend: MB consistently maintained higher E. acoroides cover across both early (10–30 months) and late (30–60 months) phases, reaching 86.08% in some plots (Figure 10B). MA showed more variable coverage, attributed mainly to early-stage substrate instability caused by prior reclamation activity. Nonetheless, a gradual improvement in shoot density and lateral expansion was observed. Notably, signs of megafaunal interaction, specifically dugong feeding trails, have been observed within transplanted plots, suggesting a positive ecological response and re-engagement of higher trophic levels with the recovering habitat (Figure 11).
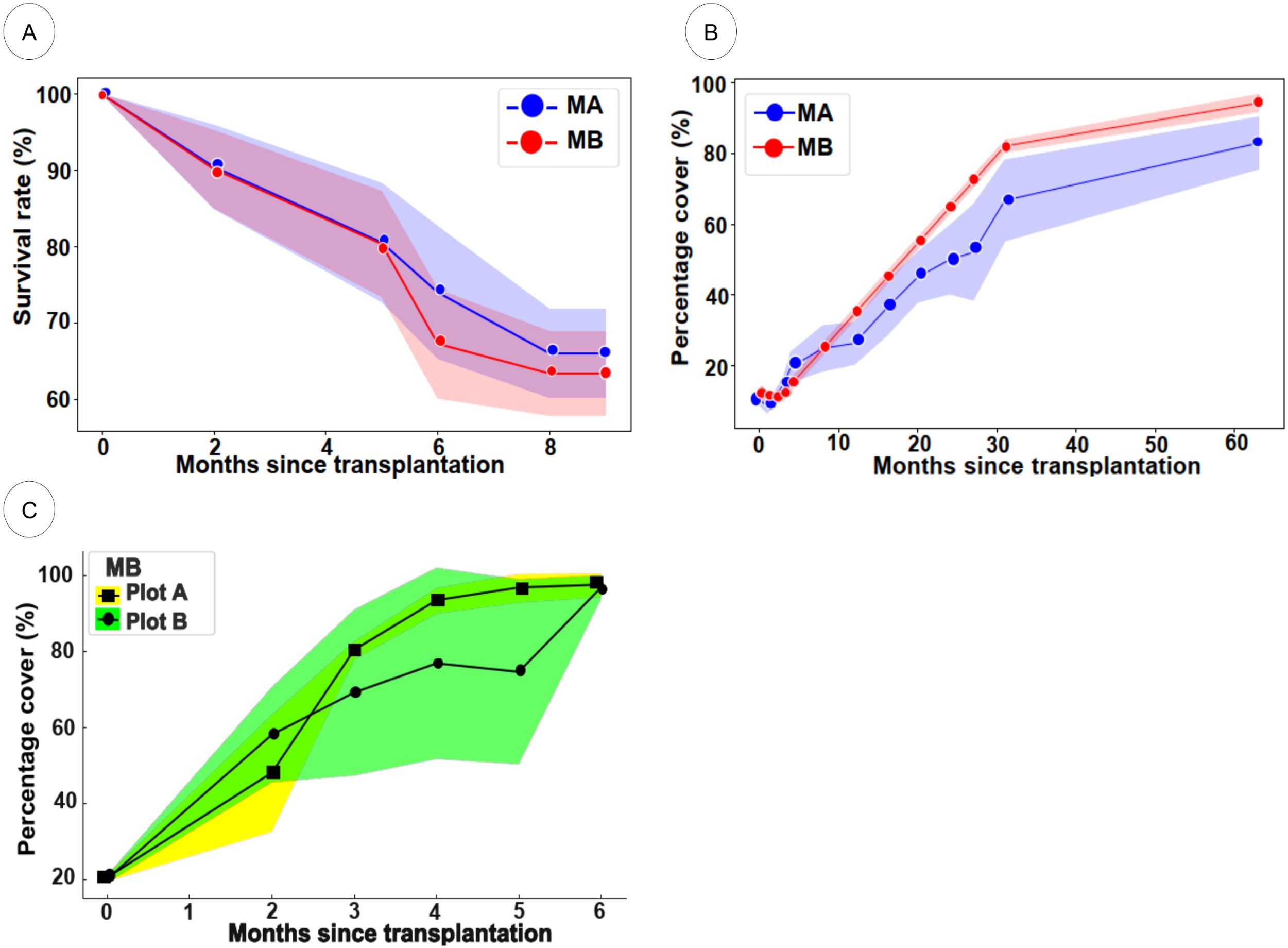
Figure 10. Survival rate and growth of transplanted seagrasses. (A) The survival rate of E. acoroides seedlings at MA and MB after 8 months, (B) the percentage cover of E. acoroides at MA and MB after 60 months, and (C) the percentage cover of mixed species, including E. acoroides, H. ovalis, H. major, and H. spinulosa at MB after 6 months.
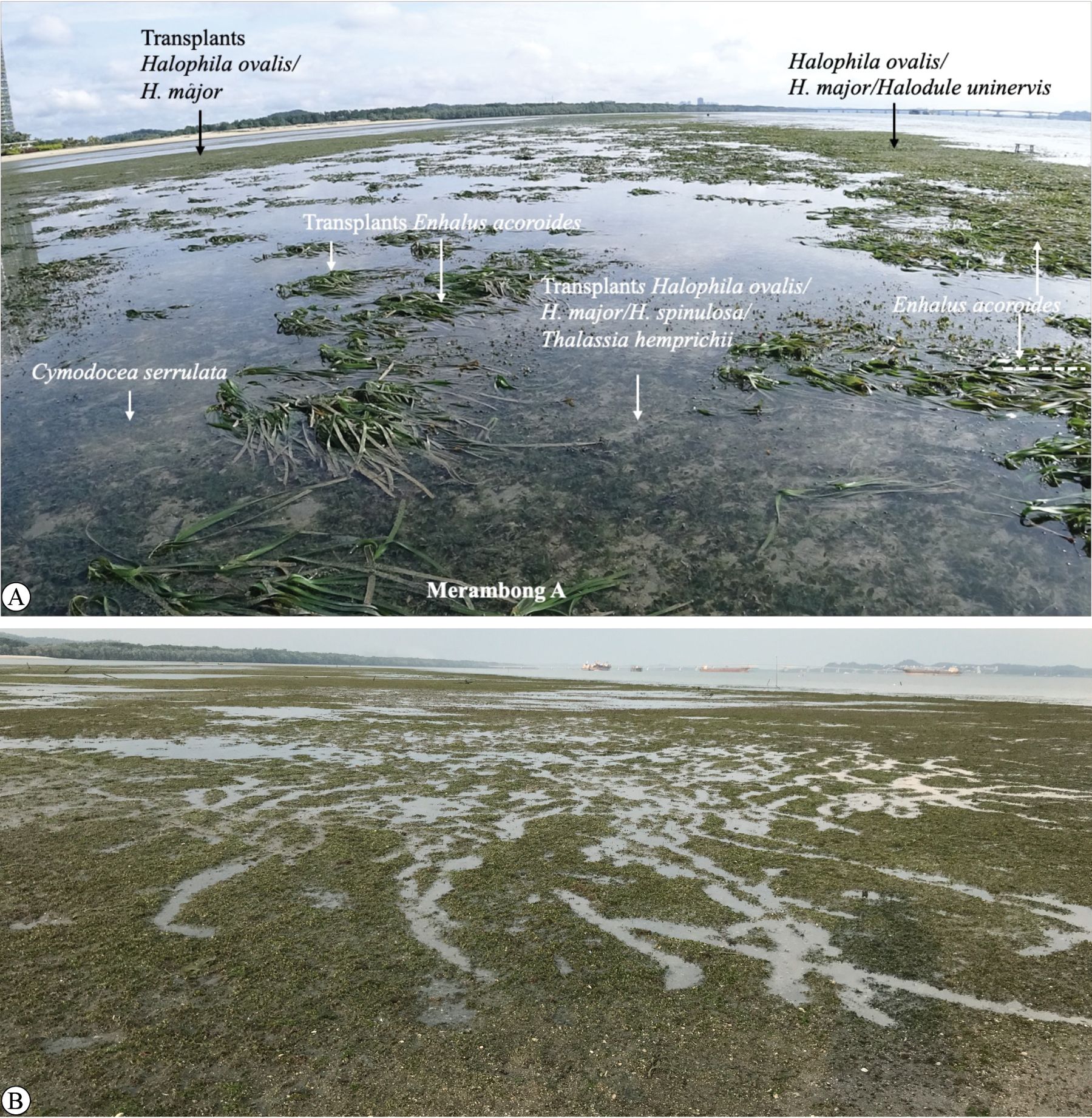
Figure 11. The recovery program in Merambong shoal shows positive results with healthy seagrass growth. (A) The coverage includes a mix of H. major, H. ovalis, H. spinulosa, T. hemprichii, and H. uninervis, along with E. acoroides. (B) Dugong trails were observed at Merambong A and B, where the transplanting activity took place.
Among the supporting cover species, H. ovalis and H. major demonstrated rapid expansion, significantly contributing to overall vegetative cover. While E. acoroides exhibited slower horizontal spread, it maintained a consistent and persistent presence. Two representative plots at MB illustrated contrasting development patterns (Figure 10C). Plot A exhibited a steady increase in cover from ~20% to full coverage by month six, while Plot B showed initial lag followed by rapid expansion. In both plots, H. ovalis and H. major were dominant contributors, with E. acoroides providing structural stability. In contrast, H. spinulosa performed poorly at MA, with coverage declining from 6.80% to 3.28% over a two-month period, likely due to substrate instability and high exposure. These results highlight that mixed-species planting schemes can enhance plot stability, suppress macroalgal overgrowth, and promote sediment retention, particularly in dynamic shoal environments. Ultimately, the use of community-based, multispecies restoration strategies appears promising for enhancing transplant success and supporting long-term habitat recovery.
As transplanting efforts expanded, observations from mixed-species plots offered valuable insights into species dynamics and functional complementarity. Beginning in 2018, selected plots at MA and MB shoals incorporated patches of fast-growing cover species, H. ovalis, H. major, and H. spinulosa, alongside E. acoroides seedlings. This approach aimed to replicate natural community compositions and promote early-stage sediment stabilization. Results from MB demonstrated a particularly successful establishment of mixed assemblages. By the sixth month post-transplantation, plots containing both stabilizer (E. acoroides) and cover species reached percentage cover values approaching full saturation, with values exceeding 90% in some cases (Figure 12).

Figure 12. Percentage cover of transplanting plot using PhotoQuad analysis: green line represents E. acoroides, blue is Halophila sp. and yellow is seaweed Ulva reticulata. (A–C) Percentage cover of E. acoroides seedlings in MA shoal, (D) matured fruits in transplanted area, (E–G) percentage cover of E. acoroides, H. ovalis, H. major, and H. spinulosa at MB shoal, and (H) male flower (in circle) observed at transplanting plot.
3.5 Environmental parameters and their relationship to seagrass recovery
Table 3 compares water quality parameters and nutrient concentrations across three sites during two monitoring phases. Phase 1 (February 2015 to February 2018) coincided with major land reclamation activities, while Phase 2 (June 2018 to February 2024) followed the removal of the MB causeway. The assessment refers to the ASEAN Marine Water Quality Criteria (AMWQC) (Secretariat ASEAN, 2008) and the Malaysian Marine Water Quality Standard (MMWQS) (Department of Environment, 2021). Class 1 represents high-quality waters in marine parks, coral reefs, and seagrass beds, whereas Class 3 encompasses areas exposed to pollution from human activities, such as coastal development and land reclamation. Overall, apparent differences were found between sites and phases. Parameters such as temperature, salinity, total suspended solids (TSS), and ammonia showed significant variation, especially in Phase 1.
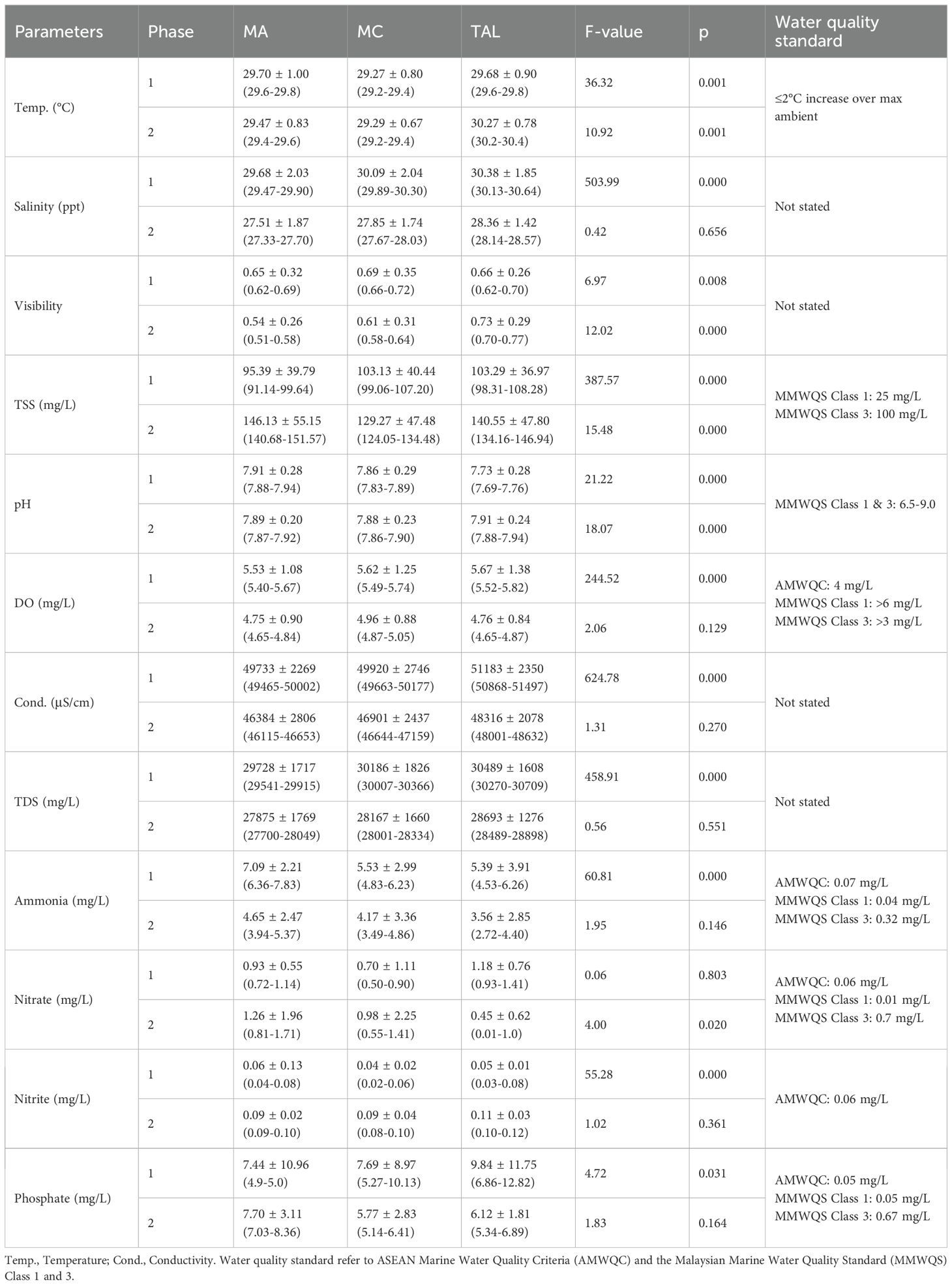
Table 3. Water quality parameters and nutrient contents are presented as means ± standard deviations and ranges across locations (MA, MC, and TAL) and phases (Phase 1: February 2015 to February 2018; Phase 2: June 2018 to February 2024).
The temperature was relatively stable across locations but showed slight differences. In Phase 1, temperatures ranged from 29.27°C at MC to 29.70°C at MA (F = 36.32, p = 0.001). In Phase 2, TAL recorded the highest temperature (30.27°C), followed by MA (29.47°C) and MC (29.29°C). Salinity also showed notable differences in Phase 1, ranging from 29.68 ppt at MA to 30.38 ppt at TAL. In Phase 2, salinity dropped slightly at all sites, but TAL remained the highest at 28.36 ppt, with no significant differences between sites.
TSS exhibited significant differences between both sites and phases. In Phase 1, TAL recorded the highest TSS at 103.29 mg/L, followed closely by MC at 103.13 mg/L and MA at 95.39 mg/L, with a highly significant F-value of 387.57. During Phase 2, TSS increased at all sites, reaching 146.13 mg/L at MA, 140.55 mg/L at TAL, and 129.27 mg/L at MC. The minimum and maximum values exceeded 25 mg/L for Class 1, Sensitive Marine Habitats and all values surpassed 100 mg/L under Class 3, which includes areas susceptible to pollution from human activities such as coastal development and land reclamation.
Ammonia levels were significantly higher in Phase 1, particularly at MA (7.09 mg/L), compared to MC (5.53 mg/L) and TAL (5.39 mg/L) (F = 60.81, p = 0.000). In Phase 2, levels decreased but remained highest at MA (4.65 mg/L); however, the differences were not statistically significant (F = 1.95, p = 0.146). Other parameters, such as pH, nitrate, and phosphate, showed differences only in certain phases or locations. Some values exceeded the MMWQS Class 3 (ammonia: 0.32 mg/L) and AMWQC (ammonia: 0.07 mg/L; nitrite: 0.05 mg/L) standards, particularly for ammonia (4.83-7.83 mg/L) and nitrite (0.02-0.08 mg/L) during Phase 1. TSS levels often surpassed Class 1 and 3 limits during Phase 2, ranging from 124.05 to 151.57 mg/L. Higher TSS in TAL and MA may indicate increased sedimentation from reclamation activities or runoff during the monsoon season.
Pearson’s correlation analysis, visualized in the combined heatmap (Figure 13), revealed clear spatial variability in the relationships between seagrass percentage cover and water quality parameters across the three study sites. At MA, seagrass cover showed moderate negative correlations with conductivity (r = –0.622, p < 0.05), salinity (r = –0.451, p < 0.05), and TDS (r = –0.430, p < 0.05). At MC, similar patterns were observed, with significant negative correlations for conductivity (r = –0.594, p < 0.05), salinity (r = –0.519, p < 0.05), and TDS (r = –0.500, p < 0.05). At TAL, moderate negative correlations were found with DO (r = –0.545, p < 0.05), salinity (r = –0.502, p < 0.05), and TDS (r = –0.453, p < 0.05). Other parameters, such as visibility, temperature, and TSS, showed weaker and more variable relationships across sites. Overall, the heatmap highlights conductivity, salinity, and TDS as consistently important factors negatively associated with seagrass cover, with DO emerging as an additional key driver at TAL.
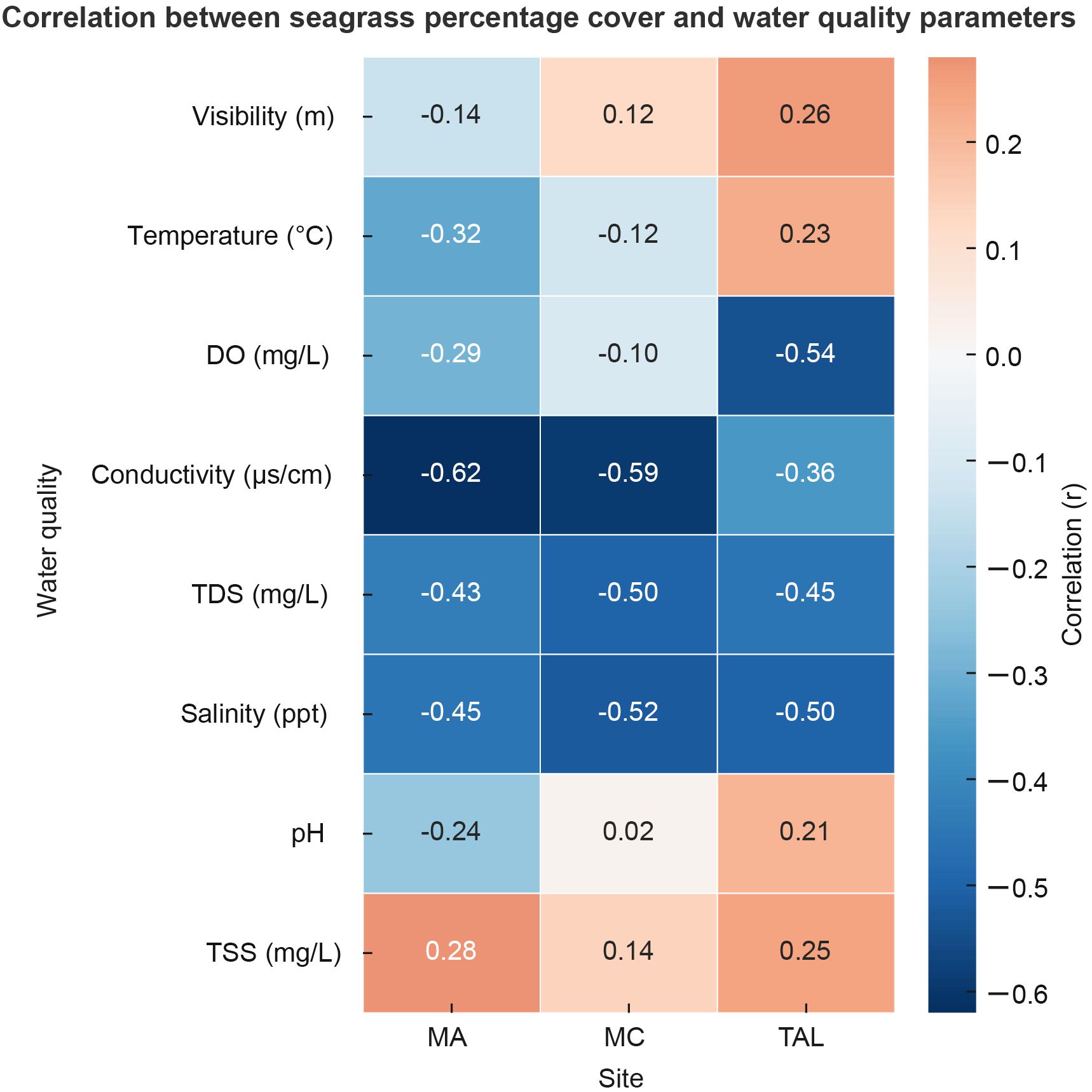
Figure 13. Pearson’s correlation relationships between seagrass percentage cover and water quality parameters across the MA, MC, and TAL.
4 Discussions
4.1 New records and biogeographic implications
The diversity and evolving composition of seagrass species in the Sungai Pulai estuary reflects broader global patterns of species turnover, range shifts, and local extirpations associated with coastal development, sedimentation, and environmental change (Waycott et al., 2009; Duarte et al., 2022). The apparent disappearance of Cymodocea rotundata, a species once common in 1996 but absent in recent surveys, reflects trends observed in other tropical and subtropical regions, where sediment stress and habitat fragmentation have led to local declines of vulnerable taxa. Similarly, the emergence of new species such as Halophila nipponica in Malaysian waters highlights the importance of sustained monitoring to detect biogeographic expansions, possibly linked to climate change, regional dispersal via currents, or anthropogenic alterations that modify habitat suitability (Short et al., 2011; Che Alias et al., 2024).
Globally, H. nipponica has been reported in temperate to subtropical latitudes, and its successful establishment in sheltered, muddy substrates at Merambong, Tanjung Adang Laut, and Seluyong shoals suggests niche overlap with resident Halophila species, such as H. ovalis and H. major. This aligns with global findings that Halophila species often dominate early successional or disturbed habitats due to their clonal growth and high reproductive output (Rasheed et al., 2014). At the same time, their sensitivity to substrate instability and water quality positions them as valuable ecological indicators of early recovery stages or transitional meadow dynamics.
Additionally, H. nipponica, a new distribution record for Malaysia, was observed in 2023 at Tanjung Adang Laut and in 2024 at Merambong and Seluyong shoals (Figure 5). These recent discoveries, made during the project’s active monitoring period, highlight the ecological importance of the Sungai Pulai estuarine complex and contribute to an updated Malaysian seagrass inventory. The findings emphasize the need for continuous monitoring to detect previously overlooked or emerging species and improve understanding of seagrass distribution in response to environmental changes. This may also be due to advancements in species identification methods rather than recent colonization alone. Earlier assessments often grouped several morphologically similar species under the Halophila ovalis complex because of overlapping traits, as noted in past morphological studies (Annaletchumy et al., 2005). However, with the help of molecular techniques such as DNA barcoding (ITS region), recent work by Che Alias et al. (2024) successfully confirmed the presence of H. major, H. decipiens, and H. nipponica, which were previously misclassified or unnoticed. This shows that the perceived increase in species richness is partly due to better taxonomic resolution. Incorporating molecular tools into long-term monitoring improves our ability to accurately document biodiversity and detect indirect ecological changes in seagrass ecosystems.
The co-occurrence of multiple Halophila species in mixed beds observed in this study echoes restoration and biodiversity patterns seen in Southeast Asia, northern Australia, and East Asia, regions characterized by high seagrass species richness but also growing coastal pressures (Fortes et al., 2018; Ambo-Rappe, 2022). These parallels highlight the importance of local restoration data in supporting broader conservation efforts within frameworks such as the UN Decade on Ecosystem Restoration (2021–2030). Ensuring the persistence of less resilient species (e.g., H. beccarii) within such mixed assemblages will require careful consideration of sediment quality, hydrodynamics, and long-term nutrient regulation.
4.2 Potential recovery in disturbed tropical shoals
Findings from this multi-year restoration effort highlight the divergent recovery trajectories of tropical seagrass meadows subjected to varying degrees of disturbance. Of the three study sites, MA was initially the most degraded, primarily due to direct impacts from coastal reclamation activities. Fluctuations in seagrass cover at the study sites mainly resulted from a combination of human disturbances and macroalgal overgrowth, with environmental variability further impacting recovery. Human stressors such as sedimentation, increased turbidity from land reclamation, and changes in hydrology worsened declines in seagrass cover by blocking sunlight and physically disturbing the seabed. These pressures hindered seedling growth and reduced meadow resilience, often creating a feedback loop where poor conditions encouraged macroalgal dominance, which then suppressed seagrass regrowth. Colonization by macroalgae, especially Ulva reticulata and Amphiroa fragilissima, formed thick, persistent mats that further blocked sunlight from reaching the seagrass canopy. This aligns with findings in other coastal areas where macroalgae outcompete seagrasses for light and space, leading to large-scale die-offs (Terrados et al., 1999; Garcias-Bonet et al., 2008).
Light availability is a key limiting factor for seagrass survival, with most species needing at least 6–10 mol photon m-2 d-1 for sustained growth (Collier et al., 2016). Extended light deprivation is known to reduce shoot density, biomass, and survival (Longstaff and Dennison, 1999; Collier et al., 2007; Suykerbuyk et al., 2018). In Merambong A, for example, extensive macroalgal coverage, especially U. reticulata, was directly linked to significant seagrass die-off. This observation aligns with experimental evidence from Zuo et al. (2025), who found that macroalgal blooms alter sediment oxygen levels and increase hypoxic stress in seagrass beds. A prior study by Emmclan et al. (2022) on seagrasses in the Merambong Shoal during times of intense reclamation supports this, showing that seagrass decline was worst in disturbed areas with high sedimentation and low water flow. In these zones, light-sensitive species such as Halophila ovalis, H. major, and H. spinulosa were more heavily affected. However, their short life spans and quick colonization abilities (Cabaço et al., 2008) suggest these species could recover if macroalgal pressure lessens and environmental conditions become stable.
Furthermore, fluctuations in seagrass cover across the Merambong Shoal are closely linked to spatial and temporal variations in substrate composition, especially near the river mouth and reclamation zones. Waheeda et al. (2023) found that while most sites had sandy sediment, areas closer to the mainland and affected by reclamation (MA) showed significant silt and clay accumulation. This shift toward finer sediments coincided with reduced seagrass cover, indicating a substrate-driven suppression of seagrass establishment and growth. This pattern aligns with broader research showing that higher sediment mud content (< 63 µm) decreases underwater light penetration, lowers sediment porosity, and creates adverse rhizosphere conditions such as anoxia, which results in reduced net primary production and lower shoot and biomass density (Zabarte-Maeztu et al., 2020). Globally, fine sediments have been shown to hinder seagrass seedling anchorage and restrict rhizome expansion, thus reducing meadow resilience and recovery potential (Potouroglou et al., 2017). Similarly, high mud content decreases photosynthetic efficiency in unvegetated sediments and is associated with lower productivity even when light levels seem sufficient (Flowers et al., 2024). Species-specific responses are also important: tropical species such as E. acoroides and H. ovalis favor sandy or mixed sand-mud substrates for optimal rhizome anchorage and growth (Jiang et al., 2022; Størdal et al., 2023). Additionally, sediments impacted by reclamation often change organic carbon storage and redox profiles, further degrading seagrass habitat quality (Rahayu et al., 2025).
Along with the observed botanical changes, related research conducted under the same rehabilitation project documented promising signs of faunal recovery. A study by Waheeda et al. (2023) at Merambong Shoal reported increased abundance and diversity of macrobenthic organisms after seagrass re-establishment and sand bund removal. Dominant taxa included amphipods, isopods, hermit crabs, nereidid polychaetes, and burrowing bivalves, indicating a recovering benthic ecosystem. These results support the idea that seagrass restoration positively affects not only the plant structure but also the associated animal communities, boosting the ecological value of restored habitats. However, the site showed significant recovery over time, especially in areas where active transplantation coincided with stabilized substrates and less macroalgal overgrowth. This recovery highlights the natural resilience of tropical seagrass ecosystems and their ability to bounce back when favorable environmental conditions support restoration efforts.
In contrast, MC and TAL, both less directly impacted by development, exhibited more consistent recovery and natural regeneration throughout the monitoring period. These locations maintained higher levels of seagrass cover and were less vulnerable to algal overgrowth, offering strong evidence of ecological resilience where baseline conditions remain intact. This comparative spatial analysis reinforces a critical insight into tropical seagrass rehabilitation: the level of initial disturbance and the rate of post-disturbance stabilization play key roles in shaping long-term outcomes. Rehabilitation strategies that align with the environmental capacity of each site, such as initiating interventions following sediment resettlement, are more likely to yield sustainable outcomes. The positive results observed at MA and MB further demonstrate that active interventions, particularly the use of pre-cultivated seedlings and mixed-species assemblages, can effectively bridge recovery gaps even under challenging environmental conditions (van Katwijk et al., 2016; Ambo-Rappe, 2022).
4.3 Effectiveness of seedling-based rehabilitation strategies
Among the transplanting techniques evaluated, the use of seedlings proved to be the most effective method in this study. Compared to vegetative shoots or direct seeding, E. acoroides seedlings demonstrated higher survival rates and stronger post-transplant establishment. These results align with previous research highlighting the advantages of seed-derived planting materials, especially in donor-limited environments, due to their superior rooting stability and enhanced genetic diversity (Reynolds et al., 2012; Shen et al., 2023). Seedlings propagated under controlled tank conditions not only provided a reliable source of planting material but also allowed for early-stage monitoring of viability and growth. Their compact form, established roots, and capacity for lateral expansion enabled them to anchor even in dynamic subtidal substrates successfully. By contrast, bare-rooted shoots had low retention rates, and direct seeds failed to survive beyond the first-month post-transplantation. Furthermore, the reproductive biology of E. acoroides, which yields up to 19 seeds per fruit, makes seedling-based strategies particularly advantageous for rehabilitation programs requiring scalability. This study demonstrates that with proper preparation and post-planting care, the use of tank-cultured seedlings offers a practical, ecologically sound, and ethically preferable strategy for rehabilitating degraded tropical seagrass meadows. These findings contribute to a growing consensus in tropical restoration science that seedling-based methodologies outperform traditional adult plant transplants in terms of sustainability, resilience, and scalability (Short et al., 2002; Creencia et al., 2023).
In particular, E. acoroides acts as a key stabilizer species in dynamic subtidal environments because of its strong rhizome system and large canopy. While it does not form dense root mats, its thick rhizomes extend deeply into soft substrates, anchoring the plant and helping to prevent lateral sediment displacement (Twomey et al., 2021). The wide leaf blades also slow water flow and promote sediment buildup, improving microtopographic stability at the transplant site (Potouroglou et al., 2017). These biophysical traits enable E. acoroides seedlings to stabilize the substrate, support community succession, and reduce the impact of hydrodynamic disturbance, making them especially suited for high-energy shoals like those at Merambong Shoal.
Although seedling-based transplanting proved highly effective ecologically in this study, deploying it on a larger scale will require careful assessment of economic viability. Factors such as labor demands, resource availability, and long-term maintenance costs need to be balanced against traditional methods like direct seeding or vegetative transplants. While this study did not include a cost analysis, future research should include detailed economic evaluations to enable scalable and cost-effective restoration planning.
4.4 Functional roles of mixed-species assemblages
The introduction of fast-growing cover species such as H. ovalis, H. major, and H. spinulosa into transplant plots alongside E. acoroides seedlings significantly enhanced plot stabilization and overall rehabilitation outcomes. These rapidly colonizing species, known for their clonal expansion and horizontal spread, provided adequate ground cover during the critical early stages of transplant establishment. Their presence facilitated root anchoring, sediment retention, and the creation of favorable microhabitat conditions. At MB shoal, this mixed-species strategy proved especially successful, with cover species quickly occupying bare substrates and supporting the establishment of slower-growing E. acoroides. The structural and functional diversity within these assemblages probably reduced drift macroalgal overgrowth and helped buffer against environmental fluctuations, aligning with findings that emphasize the functional benefits of biodiversity in coastal ecosystems (Nordlund et al., 2017; Ambo-Rappe, 2020). In contrast, plots with only H. spinulosa showed lower survival rates and patchy recovery, especially in areas with unstable substrates, highlighting the importance of combining species interactions. These results stress the importance of strategic species selection and assemblage design to improve transplant success, particularly during high-disturbance or high-sedimentation conditions. Using multispecies assemblages not only speeds up early cover and sediment stabilization but also enhances ecological resilience, reflecting natural successional dynamics observed in healthy tropical seagrass ecosystems.
4.5 Water quality influence on seagrass rehabilitation
Water quality monitoring across the three shoals revealed spatial and temporal differences likely influencing seagrass rehabilitation outcomes. Merambong A, located nearest to active reclamation zones, exhibited increased turbidity, higher total suspended solids, and fluctuating nutrient concentrations during the study’s early phase. In contrast, Tanjung Adang Laut consistently recorded clearer water, more stable salinity, and higher dissolved oxygen levels, providing relatively favorable conditions for both natural and assisted recovery.
These findings suggest that while transplant materials and techniques are essential, the quality of the surrounding water, particularly in terms of nutrient concentrations, also significantly contributes to the success of seagrass rehabilitation. Improvements in water conditions at Merambong A after the cessation of reclamation activities in 2018 were accompanied by increases in seagrass cover and transplant survival, reinforcing the importance of environmental stabilization in supporting the long-term recovery of tropical seagrass ecosystems (McMahon et al., 2013).
The success of seagrass restoration relies on local water quality, which directly affects plant growth, survival, and resilience. In this study, Pearson’s correlation analysis consistently showed negative relationships between seagrass cover and conductivity, salinity, and total dissolved solids (TDS) across all sites. Some site-specific effects included a moderate negative correlation between cover and dissolved oxygen (DO) at TAL. Higher salinity, ionic concentration, and TDS can cause osmotic stress, reduce photosynthetic efficiency, and alter sediment nutrient dynamics, all of which can hinder seagrass establishment (Orth et al., 2020; Huang et al., 2012; York et al., 2022). High variability in DO, often linked to eutrophication and algal blooms, may further decrease seagrass productivity by limiting light availability and promoting epiphyte overgrowth (Burkholder et al., 2007).
Hydrodynamic conditions also played a critical role at the study sites. The construction of the sand-filled embankment (MB) at MA and MC changed tidal flow and wave exposure (Emmclan et al., 2022). MA, being sheltered, experienced less wind-driven wave stress, increased sediment deposition, and conditions favorable for the growth of Ulva reticulata, which can further reduce wave energy. In contrast, MC remained exposed to strong tidal currents, affecting nutrient exchange and sediment stability (Lanuru et al., 2018). While moderate hydrodynamic energy can help prevent sediment anoxia and promote nutrient delivery, too much exposure can decrease nutrient retention and uproot newly established shoots (El-Hacen et al., 2019; Schanz and Asmus, 2003).
Globally, successful seagrass restoration depends on reducing environmental stressors before planting. Poor water quality, especially from nutrient enrichment and sediment loading, is among the main causes of restoration failure (van Katwijk et al., 2016). High nutrient levels can lead to phytoplankton blooms and epiphyte growth, which compete with seagrasses for light and space (Burkholder et al., 2007). Increased turbidity and suspended sediments also limit light penetration, reducing photosynthesis and growth (Cabaço et al., 2008). In Merambong Shoal, it is vital to prevent nutrient runoff from agriculture, aquaculture effluent, and untreated wastewater, as well as to avoid physical disturbances like dredging, propeller scarring, and sediment burial, all of which can significantly decrease shoot density and carbohydrate reserves (Cabaço and Santos, 2007, 2012). Extreme changes in coastal hydrodynamics caused by structures should also be minimized to prevent alterations in current patterns and sediment movement that could destabilize newly planted beds. Additionally, sediment tends to accumulate on seagrass beds because they slow current flow and reduce wave energy (Gambi et al., 1990). The presence of seagrasses can also encourage macroalgae growth, especially U. reticulata, on soft sediments (Buapet et al., 2008).
4.6 Implications for coastal rehabilitation under global change
The outcomes of this rehabilitation effort contribute to broader discussions on coastal habitat resilience in the context of global environmental change. Tropical seagrass ecosystems are increasingly threatened by compounded anthropogenic pressures, including urban expansion, land reclamation, eutrophication, and climate-related stressors such as sea-level rise and intensified storm activity (Waycott et al., 2009; IPCC, 2022). As key habitats for carbon sequestration, shoreline protection, and biodiversity support, seagrass meadows must be prioritized in both regional and international conservation frameworks. The success of this transplanting initiative in a heavily impacted and rapidly developing coastal area highlights the potential for recovery when rehabilitation efforts are tailored to local conditions and supported by continuous monitoring. Using various planting techniques, including seedling propagation and mixed-species designs, can enhance ecological performance and strengthen adaptive capacity in uncertain future conditions. This research also emphasizes the importance of long-term datasets in restoration science. Monitoring activities that extend over years or decades provide essential insights into system dynamics, recovery thresholds, and feedback mechanisms. Such information is vital for refining management strategies and improving predictive ecological models, especially as habitat restoration becomes a central part of the UN Decade on Ecosystem Restoration and broader climate adaptation efforts.
However, restoring seagrass beds to restore full ecological function and provide ongoing benefits such as nursery habitat for marine life and contributions to local fisheries requires long-term conservation strategies. Active protection against human pressures like sedimentation, eutrophication, and physical disturbances (e.g., anchoring and dredging) is necessary to support habitat stabilization and ecosystem development (Ralph et al., 2006; Unsworth et al., 2015; Potouroglou et al., 2017). Strategically incorporating restored beds into marine spatial planning and stakeholder engagement frameworks can further enhance recovery, ensuring that rehabilitation efforts lead to conservation-scale gains (Lester et al., 2020; Lima et al., 2023). Aligning local restoration with broader governance frameworks will help ensure seagrass meadows not only recover but also provide lasting benefits for biodiversity, carbon storage, and coastal community resilience (Rifai et al., 2022, 2023; Boström-Einarsson et al., 2020). Ultimately, ecosystem-based restoration and rehabilitation of tropical seagrass meadows, grounded in ecological insights, adaptive management, and localized strategies, offer scalable and resilient solutions for conserving marine biodiversity and sustaining coastal communities in an era of rapid environmental change.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
MZ: Data curation, Resources, Visualization, Conceptualization, Formal Analysis, Project administration, Validation, Software, Writing – review & editing, Methodology, Supervision, Investigation, Writing – original draft, Funding acquisition. NS: Data curation, Methodology, Writing – original draft, Investigation, Software, Formal Analysis. SR: Writing – review & editing, Data curation. JSB: Writing – review & editing, Writing – original draft, Methodology, Data curation, Conceptualization, Validation, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by an Industry Grant, Country Garden Pacificview Sdn. Bhd.
Acknowledgments
We would like to thank the Vice-Chancellor of Universiti Putra Malaysia for his encouragement and the facilities provided. The authors also thank the academic editor and three anonymous reviewers, whose constructive comments and input significantly improved the article.
Conflict of interest
The authors declare that this study received funding from Country Garden Pacificview Sdn. Bhd. The funder had the following involvement in the study: technical support and assistance. the funder had no other role in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2025.1646399/full#supplementary-material
Supplementary Table 1 | Percentage cover of seagrasses, seaweeds and substrate in MA, MC, and TAL.
Supplementary Table 2 | Propagules of E. acoroides collected from MA, MC, and TAL shoals and seed germination.
Supplementary Table 3 | (a) Survival rate of E. acoroides seedlings, (b) Percentage cover of transplanted E. acoroides and (c) Percentage cover transplanting of mixed species.
Supplementary Table 4 | (a) Water quality and nutrients in MA, MC and TAL, (b) Pearson Correlation of percentage cover and water quality in MA, MC and TAL.
References
Abal E. G. and Dennison W. C. (1996). Seagrass depth range and water quality in southern Moreton Bay, Queensland, Australia. Mar. Freshw. Res. 47, 763–771. doi: 10.1071/MF9960763
Abu N. J., Bujang J. S., Zakaria M. H., and Zulkifly S. (2022). Use of Ulva reticulata as a growth supplement for tomato (Solanum lycopersicum). PLoS One 17, e0270604. doi: 10.1371/journal.pone.0270604
Akhir M. F. M. and Chuen Y. J. (2011). Seasonal variation of water characteristics during inter-monsoon along the east coast of Johor. J. Sustainability Sci. Manage. 6, 206–214. Available online at: http://dac.umt.edu.my:8080/xmlui/handle/123456789/6904. (Accessed May 26, 2025).
Ambo-Rappe R. (2020). “Seagrass meadows for fisheries in Indonesia: A preliminary study,” in Proceedings of the 3rd International Symposium on Marine and Fisheries (ISMF 2020), South Sulawesi, Indonesia, 5–6 June 2020. IOP Conference Series: Earth and Environmental Science, vol. 564,. (Bristol, United Kingdom: IOP Publishing Ltd.), 012017. doi: 10.1088/1755-1315/564/1/012017
Ambo-Rappe R. (2022). The success of seagrass restoration using Enhalus acoroides. Seeds is correlated with substrate and hydrodynamic conditions. J. Environ. Manage. 310, 114692. (Cambridge, MA: Academic Press) doi: 10.1016/j.jenvman.2022.114692
Ambo-Rappe R. and Moore A. M. (2019). “Seagrass Restoration in Indonesia: Lessons Learned from Past Failures and Future Prospects” in World Seas: An Environmental Evaluation, Volume II: The Indian Ocean to the Pacific. Ed C. Sheppard. (Cambridge, MA: Academic Press). doi: 10.1016/B978-0-08-100853-9.00032-4
Annaletchumy L., Japar Sidik B., Muta Harah Z., and Arshad A. (2005). Morphology of Halophila ovalis (R. Br.) Hook. f. from peninsular and east Malaysia. Pertanika J. Trop. Agric. Sci. (Malaysia) 28, 1–11. Available online at: https://www.researchgate.net/publication/279684914_Morphology_of_Halophila_ovalis_RBr_Hook_f_from_Peninsular_and_East_Malaysia (Accessed August 1, 2025).
Boström-Einarsson L., Babcock R. C., Bayraktarov E., Ceccarelli D., Cook N., Ferse S. C., et al. (2020). Coral restoration–A systematic review of current methods, successes, failures and future directions. PloS One 15, e0226631. doi: 10.1371/journal.pone.0226631
Buapet P., Hiranpan R., Ritchie R. J., and Prathep A. (2008). Effect of nutrient inputs on growth, chlorophyll, and tissue nutrient concentration of Ulva reticulata from a tropical habitat. Sci. Asia 34, 245–252. doi: 10.2306/scienceasia1513-1874.2008.34.245
Burkholder J. M., Tomasko D. A., and Touchette B. W. (2007). Seagrasses and eutrophication. J. Exp. Mar. Biol. Ecol. 350, 46–72. doi: 10.1016/j.jembe.2007.06.024
Cabaço S. and Santos R. (2007). Effects of burial and erosion on the seagrass Zostera noltii. J. Exp. Mar. Bio. Ecol. 340, 204–212. doi: 10.1016/j.jembe.2006.09.003
Cabaco S. and Santos R. (2012). Seagrass reproductive effort as an ecological indicator of disturbance. Ecol. Indic. 23, 116–122. doi: 10.1016/j.ecolind.2012.03.022
Cabaço S., Santos R., and Duarte C. M. (2008). The impact of sediment burial and erosion on seagrasses: a review. Estuarine Coast. Shelf Sci. 79, 354–366. doi: 10.1016/j.ecss.2008.04.021
Calumpong H. P. and Fonseca M. S. (2001). “Seagrass transplantation,” in Global Seagrass Research Methods. Eds. Short F. T., Coles R. G., and Short C. A. (Elsevier, Amsterdam).
Che Alias M. A., Zakaria M. H., Ramaiya S. D., Esa Y., Ab. Ghani N. I., and Bujang J. S. (2024). Morphological and genetic identification of Halophila species and a new distribution record of Halophila nipponica at the Tanjung Adang Laut shoal, Johor, Malaysia. PloS One 19, e0309143. doi: 10.1371/journal.pone.0309143
Christensen P. B., Almela E. D., and Diekmann O. (2004). “Can transplanting accelerate the recovery of seagrasses,” in European Seagrasses: An Introduction to Monitoring and Management. Eds. Borum J., Duarte C. M., Krause-Jensen D., and Greve T. M. (EU project Monitoring and Managing of European Seagrasses (M&MS, Denmark).
Collier C. J., Adams M. P., Langlois L., Waycott M., O’Brien K. R., Maxwell P. S., et al. (2016). Thresholds for morphological response to light reduction for four tropical seagrass species. Ecol. Indic. 67, 358–366. doi: 10.1016/j.ecolind.2016.02.050
Collier C. J., Lavery P. S., Masini R. J., and Ralph P. J. (2007). Morphological, growth and meadow characteristics of the seagrass Posidonia sinuosa along a depth-related gradient of light availability. Mar. Ecol. Prog. Ser. 337, 103–115. doi: 10.3354/meps337103
Creencia G. B. A., Manolis P. L. D., and Sedigo J. T. (2023). Transplantation and survival rate of seagrass (Enhalus acoroides) in the coastal areas of Ternate Cavite. SDSSU Multidiscip. Res. J. 11, 11–16. Available online at: https://smrj.nemsu.edu.ph/index.php/SMRJ/article/view/225/153 (Accessed August 1, 2025).
Duarte C. M., Bruhn A., and Krause-Jensen D. (2022). A seaweed aquaculture imperative to meet global sustainability targets. Nat. Sustainability 5, 185–193. doi: 10.1038/s41893-021-00773-9
El-Hacen E. H., Bouma T. J., Govers L. L., Piersma T., and Olff H. (2019). Seagrass sensitivity to collapse along a hydrodynamic gradient: evidence from a pristine subtropical intertidal ecosystem. Ecosystems 22, 1–17. doi: 10.1007/s10021-018-0319-0
Emmclan L. S. H., Zakaria M. H., Ramaiya S. D., Natrah I., and Bujang J. S. (2022). Morphological and biochemical responses of tropical seagrasses (Family: Hydrocharitaceae) under colonization of the macroalgae Ulva reticulata Forsskal. PeerJ 10, e12821. doi: 10.7717/peerj.12821
Flowers G. J., Needham H. R., Bulmer R. H., Lohrer A. M., and Pilditch C. A. (2024). The effect of sediment mud content on primary production in seagrass and unvegetated intertidal flats. Estuaries Coasts 47, 1544–1560. doi: 10.1007/s12237-024-01403-1
Fonseca M. S., Kenworthy W. J., and Thayer G. W. (1998). Guidelines for the Conservation and Restoration of Seagrasses in the United States and Adjacent Waters (Silver Spring, Maryland: U.S. Department of Commerce, National Oceanic and Atmospheric Administration).
Fortes M. D., Ooi J. L. S., Tan Y. M., Prathep A., Bujang J. S., and Yaakub S. M. (2018). Seagrass in Southeast Asia: A Review of status and knowledge gaps, and a road map for conservation. Botanica Marina 61, 269–288. doi: 10.1515/bot-2018-0008
Gambi M. C., Nowell A. R. M., and Jumars P. A. (1990). Flume observations on flow dynamics in Zostera marina (eelgrass) beds. Mar. Biol. Prog. Ser. 61, 159–169. Available online at: https://www.jstor.org/stable/24842256 (Accessed May 26, 2025).
Ganassin C. and Gibbs P. J. (2008). A Review of Seagrass Planting as a Means of Habitat Compensation Following Loss of Seagrass Meadow. Cronulla, NSW: NSW Department of Primary Industries, Fisheries Final Report Series No. 96, January 2008. ISBN 1449-9967. https://handsoffpointperon.com/wp-content/uploads/2016/06/NSW_seagrass_replanting_failure_in_2008.pdf (Accessed August 4, 2025).
Garcias-Bonet N., Marbà N., Holmer M., and Duarte C. M. (2008). Effects of sediment sulfides on seagrass Posidonia oceanica meristematic activity. Mar. Ecol. Prog. Ser. 372, 1–6. doi: 10.3354/meps07714
Gordon D. M. (1996). Status of Seagrass Restoration: Review of international Literature (Perth, Western Australia: LeProvost, Dames and Moore).
Harwell M. C. and Orth R. J. (1999). Eelgrass (Zostera marina L.) seed protection for field experiments and implications for large-scale restoration. Aquatic Botany 64, 51–61. doi: 10.1016/S0304-3770(99)00008-X
Henderson M. R. (1954). Malayan Wild Flowers: Monocotyledon (Kuala Lumpur: The Malayan Nature Society).
Herrera M., Tubío A., Pita P., Vázquez E., Olabarria C., Duarte C. M., et al. (2022). Trade-offs and synergies between seagrass ecosystems and fishing activities: A global literature review. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.781713
Hinkle D. E., Wiersma W., and Jurs S. G. (2003). Multiple-comparison procedures. Appl. Statistic Behav. Sci. 2003, 370–399.
Huang X., Borthakur D., and Ni H. (2012). Photosynthetic activity and antioxidative response of seagrass Thalassia hemprichii to trace metal stress. Acta Oceanologica Sin. 31, 98–108. doi: 10.1007/s13131-012-0210-3
Intergovernmental Panel on Climate Change (IPCC) (2022). “Climate change 2022: impacts, adaptation and vulnerability,” in Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Eds. Pörtner H. O., Roberts D. C., and Tignor M. (Cambridge University Press, Cambridge, UK). doi: 10.1017/9781009325844
IOCUNESCO (2020). Global Ocean Science Report 2020 – Charting Capacity for Ocean Sustainability. Ed. Isensee K. (Paris: UNESCO Publishing). IOC Policy Series No. 2020.
IUCN (2023). IUCN Red List Categories and Criteria: Version 3.1. 2nd ed. (Gland, Switzerland and Cambridge, UK: IUCN).
Japar Sidik B. and Muta Harah Z. (2011a). “Seagrasses- diversity, values and why they are declining,” in Malaysia’s Marine Biodiversity: Inventory and Current Status. Eds. Kamaruddin I., Mohamed C. A. R., Rozaimi M. J., Kee Alfian B. A. A., Fitra A. Z., and Lee J. N. (Department of Marine Park Malaysia, Putrajaya).
Japar Sidik B. and Muta Harah Z. (2011b). “Seagrasses in Malaysia,” in Seagrasses: Resource Status and Trends in Indonesia, Japan, Malaysia, Thailand and Vietnam. Eds. Ogawa H., Japar Sidik B., and Muta Harah Z. (Department of Marine Park Malaysia, Putrajaya).
Japar Sidik B., Muta Harah Z., and Arshad A. (2006). Distribution and significance of seagrass ecosystems in Malaysia. Aquat. Ecosystem Health Manage. 9, 203–214. doi: 10.1080/14634980600705576
Japar Sidik B., Muta Harah Z., and Arshad A. (2007). Seagrass resources and their declining in Malaysia. MIMA Bull. 14, 36–42. doi: 10.1007/978-94-007-4001-3_268
Japar Sidik B., Muta Harah Z., and Arshad A. (2014). Seagrass shoals of Sungai Pulai estuary, Johor. Malayan Nat. J. 66, 1–14. Available online at: https://myjurnal.mohe.gov.my/public/article-view.php?id=94192 (Accessed May 26, 2025).
Japar Sidik B., Muta Harah Z., Nur Leena W. W. S., and Arshad A. (2020). Ecology report on the seagrass meadows and reef ecosystems at Sungai Pulai estuary for forest city development: report-20 November 2019–January 2020. Unpublished report, 101 pp.
Japar Sidik B., Muta Harah Z., and Short F. T. (2018). “Seagrass in Malaysia: issues and challenges ahead,” in The Wetland Book II. Eds. Finlayson C., Milton G., Prentice R., and Davidson N. (Springer, Dordrecht), 975–1883. doi: 10.1007/978-94-007-4001-3_268
Japar Sidik B., Salomao O., and Milchakova N. A. (2001). “Methods to measure macroalgal biomass and abundance in seagrass meadows,” in Global Seagrass Research Methods. Eds. Short F. T. and Coles R. G. (Elsevier Science B.V., Amsterdam), 223–235.
Jiang Z., Liu S., Cui L., He J., Fang Y., Premarathne C., et al. (2022). Sand supplementation favors tropical seagrass Thalassia hemprichii in eutrophic bay: implications for seagrass restoration and management. BMC Plant Biol. 22, 296. doi: 10.1186/s12870-022-03647-0
Jupp B. P., Durako M. J., Kenworthy W. J., Thayer G. W., and Schilla L. (1996). Distribution, abundance and species composition of seagrasses at several sites in Oman. Aquat. Bot. 53, 199–213. doi: 10.1016/0304-3770(96)01023-6
Lanuru M., Ambo-Rappe R., Amri K., and Williams S. L. (2018). Hydrodynamics in Indo-Pacific seagrasses with a focus on short canopies. Botanica Marina 61, 1–8. doi: 10.1515/bot-2017-0037
Lester S. E., Dubel A. K., Hernán G., McHenry J., and Rassweiler A. (2020). Spatial planning Principles for marine ecosystem restoration. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00328
Lima M. D. A. C., Bergamo T. F., Ward R. D., and Joyce C. B. (2023). A review of seagrass ecosystem services: providing nature-based solutions for a changing world. Hydrobiologia 850, 2655–2670. doi: 10.1007/s10750-023-05244-0
Lima M. D. A. C., Ward R. D., Joyce C. B., Kauer K., and Sepp K. (2022). Carbon stocks in Southern England’s intertidal seagrass meadows. Estuarine Coast. Shelf Sci. 275, 107947. doi: 10.1016/j.ecss.2022.107947
Longstaff B. J. and Dennison W. C. (1999). Seagrass survival during pulsed turbidity events: the effects of light deprivation on the seagrasses Halodule pinifolia and Halophila ovalis. Aquat. Bot. 65, 105–121. doi: 10.1016/S0304-3770(99)00035-2
McMahon K., Collier C. J., Lavery P. S., and Rasheed M. A. (2013). Seagrass resilience to disturbance: A conceptual framework. Mar. pollut. Bull. 83, 605–614. doi: 10.1016/j.marpolbul.2013.06.016
Muta Harah Z. and Japar Sidik B. (2011). “Disturbances in seagrass ecosystem in Malaysia,” in Seagrasses: Resource Status and Trends in Indonesia, Japan, Malaysia, Thailand and Vietnam. Eds. Ogawa H., Japar Sidik B., and Muta Harah Z. (Department of Marine Park Malaysia, Putrajaya).
Muta Harah Z., Japar Sidik B., Ramaiya S. D., Farahin N. N. S., and Emmclan L. S. H. (2016). “Seascape alterations in Malaysian seagrass ecosystems,” in Proceeding 7th International Agriculture Congress 2016: Enhancing Green Agriculture. Ed. Yusop M. R. (Universiti Putra Malaysia Press, Bangi, Putrajaya), 321–324.
Nixon S. W., Maynard B., Granger S., and Schwartz M. (2002). Density-Dependent Effects on GrazingaAnd Success of Seed Generated Seagrass (Zostera marina L.) Plants (Narragansett, Rhode Island: NOAA/UNH Cooperative Institute for Coastal and Estuarine Environmental Technology (CICEET).
Nordlund L. M., Koch E. W., Barbier E. B., and Creed J. C. (2017). Seagrass ecosystem services and their variability across genera and geographical regions. PloS One 12, e0169942. doi: 10.1371/journal.pone.0163091
Orth R. J., Carruthers T. J., Dennison W. C., Duarte C. M., Fourqurean J. W., Heck K. L., et al. (2006). A global crisis for seagrass ecosystems. Bioscience 56, 987–996. doi: 10.1641/0006-3568(2006)56[987:AGCFSE]2.0
Orth R. J., Lefcheck J. S., McGlathery K. S., Aoki L., Luckenbach M. W., Moore K. A., et al. (2020). Restoration of seagrass habitat leads to rapid recovery of coastal ecosystem services. Sci. Adv., 6, eabc6434. doi: 10.1126/sciadv.abc6434
Potouroglou M., Bull J. C., Krauss K. W., Kennedy H. A., Fusi M., Daffonchio D., et al. (2017). Measuring the role of seagrasses in regulating sediment surface elevation. Sci. Rep. 7, 11917. doi: 10.1038/s41598-017-12354-y
Rahayu Y. P., Kendrick G. A., Masqué P., Kiswara W., Salim H. L., Lubis A. A., et al. (2025). Impacts of dredging and restoration on sedimentary carbon stocks in seagrass meadows of Pari Island, Indonesia. Sci. Rep. 15, 25551. doi: 10.1038/s41598-025-03870-3
Ralph P. J., Tomasko D. A., Moore K. A., Seddon S., and Macinnis-Ng C. M. (2006). “Human impacts on seagrasses: eutrophication, sedimentation, and contamination,” in Seagrasses: Biology, Ecology and Conservation. Eds. Larkum A. W. D., Orth R. J., and Duarte C. M. (Springer, Tokyo), 567–593. doi: 10.1007/978-1-4020-2983-7_24
Rasheed M. A., McKenna S., Carter A., and Coles R. G. (2014). Contrasting recovery of shallow and deep water seagrass communities following climate associated losses in tropical north Queensland, Australia. Mar. pollut. Bull. 83, 491–499. doi: 10.1016/j.marpolbul.2014.02.013
Reynolds L. K., McGlathery K. J., and Waycott M. (2012). Genetic diversity enhances restoration success by augmenting ecosystem services. PloS One 7, e38397. doi: 10.1371/journal.pone.0038397
Ridley H. N. (1924). The Flora of the Malay Peninsula: Monocotyledones (UK: L. Reeve & Company, Limited).
Rifai H., Hernawan U. E., Zulpikar F., Sondakh C. F., Ambo-Rappe R., Sjafrie N. D., et al. (2022). Strategies to improve management of Indonesia’s blue carbon seagrass habitats in marine protected areas. Coast. Manag., 50, 93–105. doi: 10.1080/08920753.2022.2022948
Rifai H., Quevedo J. M. D., Lukman K. M., Sondak C. F., Risandi J., Hernawan U. E., et al. (2023). Potential of seagrass habitat restorations as nature-based solutions: Practical and scientific implications in Indonesia. Ambio 52, 546–555. doi: 10.1007/s13280-022-01811-2
Rozaimi M., Fairoz M., Hakimi T. M., Hamdan N. H., Omar R., Ali M. M., et al. (2017). Carbon stores from a tropical seagrass meadow in the midst of anthropogenic disturbance. Mar. pollut. Bull. 119, 253–260. doi: 10.1016/j.marpolbul.2017.03.073
Schanz A. and Asmus H. (2003). Impact of hydrodynamics on development and morphology of intertidal seagrasses in the Wadden Sea. Mar. Ecol. Prog. Ser. 261, 123–134. doi: 10.3354/meps261123
Seddon S. (2004). Going with the flow: Facilitating seagrass rehabilitation. Ecol. Manage. Restor. 5, 167–176. doi: 10.1111/j.1442-8903.2004.00205.x
Serra T., Gracias N., and Hendriks I. E. (2020). Fragmentation in seagrass canopies can alter hydrodynamics and sediment deposition rates. Water 12, 3473. doi: 10.3390/w12123473
Shen J., Yin L., Zhang J., Jia S., Wang Y., Wang D., et al. (2023). Restoration performance of Thalassia hemprichii and Enhalus acoroides at Gaolong Bay and Xincun Lagoon, Hainan Island, China. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1294779
Short F. T., Kopp B. S., Gaeckle J., and Tamaki H. (2002). Seagrass ecology and estuarine mitigation: a low-cost method for eelgrass restoration. Fisheries Sci. 68, 1759–1762. doi: 10.2331/fishsci.68.sup2_1759
Short F. T., Polidoro B., Livingstone S. R., Carpenter K. E., Bandeira S., Bujang J. S., et al. (2011). Extinction risk assessment of the world’s seagrass species. Biol. Conserv. 144, 1961–1971. doi: 10.1016/j.biocon.2011.04.010
Stankovic M., Mishra A. K., Rahayu Y. P., Lefcheck J., Murdiyarso D., Friess D., et al. (2023). Blue carbon assessments of seagrass and mangrove ecosystems in South and Southeast Asia: Current progress and knowledge gaps. Sci. Total Environ. 904, 166618. doi: 10.1016/j.scitotenv.2023.166618
Størdal I. F., Uleberg E. V., Tsegaye D., and Colman J. E. (2023). Restoration of seagrass habitats: Effects of artificial and natural sediments on the development of transplanted eelgrass (Zostera marina). Aquat. Bot. 188, 103677. doi: 10.1016/j.aquabot.2023.103677
Suykerbuyk W., Govers L. L., van Oven W. G., Giesen K., Giesen W. B., de Jong D. J., et al. (2018). Living in the intertidal: desiccation and shading reduce seagrass growth, but high salinity or population of origin have no additional effect. PeerJ 6, e5234. doi: 10.7287/peerj.preprints.26509v1
Terrados J., Duarte C. M., Kamp-Nielsen L., Agawin N. S. R., Gacia E., Lacap D., et al. (1999). Are seagrass growth and survival constrained by the reducing conditions of the sediment? Aquat. Bot. 65, 175–197. doi: 10.1016/S0304-3770(99)00039-X
Twomey A. J., Saunders M. I., Callaghan D. P., Bouma T. J., Han Q., and O’Brien K. R. (2021). Lateral sediment erosion with and without the non-dense root-mat forming seagrass Enhalus acoroides. Estuarine Coast. Shelf Sci. 253, 107316. doi: 10.1016/j.ecss.2021.107316
UNEP−WCMC (2021). The Area of Influence of Site−Based Operations – Direct Impacts (Cambridge, UK: UNEP−WCMC).
United States Environmental Protection Agency (1999).Standard operating procedure for the analysis of residue, nonfilterable (suspended solids), water, method 160.2 NS (Gravimetric, 103-105°C). Available online at: http://www.epa.gov/rmdcrl/sop/sopdoc/AIG018.pdf (Accessed May 12 2014).
Unsworth R. K., Collier C. J., Waycott M., Mckenzie L. J., and Cullen-Unsworth L. C. (2015). A framework for the resilience of seagrass ecosystems. Mar. pollut. Bull. 100, 34–46. doi: 10.1016/j.marpolbul.2015.08.016
Unsworth R. K., Higgs A., Walter B., Cullen-Unsworth L. C., Inman I., and Jones B. L. (2021). Canopy accumulation: Are seagrass meadows a sink of microplastics? Oceans 2, 162–178. doi: 10.3390/oceans2010010
van Katwijk M. M., Thorhaug A., Marbà N., Orth R. J., Duarte C. M., Kendrick G. A., et al. (2016). Global analysis of seagrass restoration: the importance of large-scale planting. J. Appl. Ecol. 53, 567–578. doi: 10.1111/1365-2664.12562
Waheeda A. I. K., Teh J. C., Arshad A., and Wong N. L. W. (2023). Impact of sand bund removal on seagrass ecosystems: A study of macrobenthic community structure and correlation with macrophytes cover and sediments. Mar. pollut. Bull. 192, 115111. doi: 10.1016/j.marpolbul.2023.115111
Waycott M., Duarte C. M., Carruthers T. J., Orth R. J., Dennison W. C., Olyarnik S., et al. (2009). Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. 106, 12377–12381. doi: 10.1073/pnas.0905620106
York P. H., Bryant C. V., and Rasheed M. A. (2022). Post-Flood Seagrass Monitoring in Hervey Bay - May 2022 (Cairns: JCU Centre for Tropical Water & Aquatic Ecosystem Research Publication).
Zabarte-Maeztu I., Matheson F. E., Manley-Harris M., Davies-Colley R. J., Oliver M., and Hawes I. (2020). Effects of fine sediment on seagrass meadows: a case study of Zostera muelleri in Pāuatahanui Inlet, New Zealand. J. Mar. Sci. Eng. 8, 645. doi: 10.3390/jmse8090645
Keywords: seagrass rehabilitation, transplanting, coastal development, long-term monitoring, seagrass recovery, Sungai Pulai estuary
Citation: Zakaria MH, Syed NNF, Ramaiya SD and Bujang JS (2025) Seagrass assisted recovery and long-term monitoring in the Sungai Pulai estuary, Johor, Malaysia. Front. Conserv. Sci. 6:1646399. doi: 10.3389/fcosc.2025.1646399
Received: 13 June 2025; Accepted: 08 October 2025;
Published: 29 October 2025.
Edited by:
David W. Inouye, University of Maryland, College Park, United StatesReviewed by:
Guanglong Qiu, Guangxi Academy of Marine Sciences (Guangxi Mangrove Research Center), ChinaHusain Latuconsina, Universitas Islam Malang, Indonesia
Copyright © 2025 Zakaria, Syed, Ramaiya and Bujang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muta Harah Zakaria, bXV0YUB1cG0uZWR1Lm15
 Muta Harah Zakaria
Muta Harah Zakaria Nurul Nur Farahin Syed
Nurul Nur Farahin Syed Shiamala Devi Ramaiya
Shiamala Devi Ramaiya Japar Sidik Bujang
Japar Sidik Bujang