- 1Recovery Ecology, Conservation Science and Wildlife Health, San Diego Zoo Wildlife Alliance, Escondido, CA, United States
- 2Texas Parks and Wildlife Department, Austin, TX, United States
- 3Conservation Genetics, Conservation Science and Wildlife Health, San Diego Zoo Wildlife Alliance, Escondido, CA, United States
- 4Department of Ecology and Evolutionary Biology, University of California, Los Angeles, Los Angeles, CA, United States
Introduction: Effective conservation breeding requires having husbandry and breeding protocols which maximize reproductive outputs. However, often times critical information is missing about endangered species’ breeding habitats, which hampers the formation of best practice for breeding management. Females of many mammal species are suspected to mate with multiple males within a given estrous cycle, thus, we hypothesized that structuring mating opportunities for multimale mating would improve reproductive outcomes in a conservation breeding population of the critically endangered Pacific pocket mouse (Perognathus longimembris pacificus). Pocket mice possess a number of traits that suggest sperm competition is likely, and that there may be benefits to facilitating multimale mating.
Methods: We experimentally manipulated mating opportunities to determine if there where reproductive benefits to females with multimale vs single male mating.
Results: Although we did not find a greater likelihood of pregnancy, litter size or pup weight for multi mated females, we documented multiple paternity and a first mating advantage which suggest multimale mating may have benefits in specific cases.
Discussion: In determining that only a narrow application of multimale mating would benefit Pacific pocket mice, we explore how using an experimental framework to test different mating strategies can prevent wasting resources and increase the efficiency of breeding programs.
1 Introduction
Many conservation breeding programs face difficulties in producing sufficient animals for sustainability and recovery (Bowkett, 2009; Conde et al., 2013; Collar and Butchart, 2014; Powell et al., 2019). Even small inefficiencies in breeding protocols can have serious consequences for the long-term viability of species. Basic management decisions in these programs include determining when and how to offer mating opportunities, balancing best practices against available resources. Strategies to maximize reproductive outputs and genetic diversity vary widely by species, depending on their mating system. However, the mating systems and breeding behavior of many threatened and endangered animals remain poorly understood, limiting the effectiveness of these programs. Efforts to uncover and test species-relevant breeding strategies can be crucial for filling these information gaps and improving breeding protocols.
In many mammal mating systems, females mate with multiple males within an estrous cycle (Firman and Simmons, 2008; Lane et al., 2008; Hoogland, 2013; Steinwald et al., 2013; Gromov, 2024). Sperm competition in these species is expected, and may improve breeding outcomes. Sperm competition has been particularly well studied over the past few decades, with several traits, such as relative testes size, sperm morphology, copulatory plugs, and genital morphology, identified as indicators of sperm competition in a species (Mangels et al., 2016; Comizzoli and Holt, 2022). For instance, copulatory plugs are formed by seminal fluid proteins in the male ejaculate, and have primarily evolved as an adaptation to prevent or delay subsequent inseminations of the female by additional males (Parker, 1970; Schneider et al., 2016). As a result, copulatory plugs function as a type of passive mate guarding and are prominent in species with higher levels of sperm competition (Dean, 2013; Schneider et al., 2016). Sperm competition and cryptic female choice allow a male or female to influence paternity on a post-copulatory level with evidence for these mechanisms found across genera including insects, mammals, fish and birds (Parker, 1970; McCreight et al., 2011; Comizzoli and Holt, 2022). It was previously thought that multimale mating and the resulting sperm competition was only beneficial for subsequent males, but research over the past few decades has provided evidence that females can gain both direct and indirect benefits from mating with multiple males which positively influences female reproductive fitness (Firman and Simmons, 2008; Hoogland, 2013). Given these potential fitness benefits, incorporating multimale mating strategies in ex-situ conservation breeding programs could improve outcomes for species where sperm competition is thought to occur in the wild.
We tested whether offering females the opportunity to mate with multiple males improved breeding outcomes in a conservation breeding population of Pacific pocket mice (Perognathus longimembris pacificus). P.l. pacificus are a critically endangered small mammal in the heteromyid family, native to the coastal region of southern California, USA. With only three small extant populations remaining, they have been the focus of an ex-situ conservation breeding and reintroduction program since 2012. P.l. pacificus are aggressive towards conspecifics outside of breeding contexts (Eisenberg and Isaac, 1963), and therefore, current breeding protocols at the conservation breeding facility stage single-male mating through closely managed interactions. However, several characteristics of P.l. pacificus’s ecology and morphology suggest that promiscuous or polyandrous mating may occur. A lack of traditional male secondary sexual traits, such as ornaments or armaments, lack of sexual dimorphism, and their solitary nature (Hayden et al., 1966) suggest minimal pre-copulatory competition between males for access to females (García-Navas, 2017). However, the presence of copulatory plugs after mating (Shier et al., 2025) and sperm morphology characteristics, such as sperm head hookedness (SDZWA, unpublished data; Shier et al., 2016) suggests that post-copulatory competition could occur and that females may mate with multiple males (Parker, 1970; Šandera et al., 2013; Sutter et al., 2016). Related species, such as the banner-tailed kangaroo rat (Dipodomys spectabilis) and giant kangaroo rat (Dipodymys ingens) have also been shown to engage in promiscuous mating in the wild, which can result in litters exhibiting mixed paternity (Randall et al., 2002; Waser et al., 2006; Steinwald et al., 2013).
By providing the opportunity for female P.l. pacificus to mate with more than one male as part of an experimental framework, we aimed to determine if there were differences in reproductive fitness between females that mated multiply versus singly. Based on the species’ suspected tendency to mate multiply in the wild, we expected to find benefits of multiple mating across several potential reproductive outcomes, including: greater rates of pregnancy, larger litters, or pups with larger birth weights. We also investigated whether litters showed evidence of multiple paternity and explored how this could influence management of the species.
2 Methods
2.1 Subjects
Animals were housed at the San Diego Zoo Wildlife Alliances’ Pacific pocket mice conservation breeding facility located in Escondido, California. As a species that shows intraspecific aggression, P.l. pacificus were housed singly, in semi-social enclosures (30 x 12 x 30 cm). Enclosures were made of Plexiglass with removable slotted inserts between enclosures, allowing some olfactory, visual and tactile contact between neighbors. This type of social contact facilitates estrous cycling while limiting physical interaction, as demonstrated in other heteromyids (Yoerg, 1999). Animals were housed next to neighbors of the opposite sex during breeding season (February 1-September 30) where possible. We provided animals with ½ tsp of finch seed daily, which was supplemented by greens (for water), mealworms, and native seed enrichments. All animals are individually marked at the base of the tail with a light-activated microtransponder (500 x 500 x 100 micron P-chip; PharmaSeq, LLC, Monmouth Junction, NJ, USA).
Between 2017 and 2022, we gave female P.l. pacificus who came into estrus and displayed vaginal perforation opportunities to mate (reproductive cycle tracking described Shier et al., 2025). If females remained perforate after a copulation and if timing allowed with the remaining nocturnal hours, they were included in this study (N = 59 females, 81 breedings; details below). Due to the limited number of experimental animals, the females included were a mix of virgin females, those who had mated previously and those who had given birth previously. Previous analyses of the breeding program suggested that females who had given birth in prior seasons were not at a reproductive advantage to those who had not (Shier et al., 2025). Females in the study had a mean age of 1.83 years and the oldest participating female was five years old. Although five is beyond the typical lifespan of the species in the wild, they can live to nearly 10 years in human care, and historical analyses on age found no difference in reproductive outcomes between adult P.l. pacificus and mice >4 years old (Shier et al., 2025). As the breeding and reintroduction program is first and foremost designed to produce and introduce genetically healthy animals back into the wild, males used in pairings were chosen based on genetic compatibility and genetic representation in the captive population using the program PMx (Ballou et al., 2023), as well as reproductive condition at the time of the breeding attempt (scrotal or partially scrotal vs non-scrotal).
2.2 Breeding protocols
We developed detailed protocols for breeding since the inception of the program in 2012 (detailed in Shier et al., 2025). In summary, we staged breeding opportunities in a dedicated breeding arena (30x50x100cm) with females that visually presented as perforate. We observed pairings for aggression and courtship behavior, truncating the session if undue stress occurred. We gave pairs a maximum of 90 minutes to copulate. If females failed to copulate, and remained perforate, they were given additional breeding opportunities with other males. If copulation occurred, we noted whether a copulatory plug was present, but it is possible that females may have groomed out a plug before a visual assessment could be made.
2.3 Multimale mating procedure
We originally aimed to determine whether differences in reproductive success could be due to the act of mating with different males or due to increased sperm numbers in the vaginal tract from mating more than once. Therefore, after an initial copulation females were assigned to one of two mating conditions: same male mating (SMM, N = 19), or multiple male mating (MMM, N = 62). Before being offered another set of mating opportunities, we transferred the female to a temporary enclosure (23 x 15 x 15 cm) including sand, seed and her nest jar from her home enclosure. We kept the female in this temporary enclosure for 30-60 minutes to allow her to recuperate and groom out the copulatory plug (if present). At the end of the rest phase, we noted whether a copulatory plug was still present.
After their rest period, females in the SMM condition were re-paired with the same male for a single session, regardless of whether a copulatory plug was present. After the breeding trials concluded, we returned the male to their home enclosure and moved the female into an enclosure in the pregnancy room at the conclusion of the trial. In the MMM condition, we paired the female with a new male that she did not previously encounter or copulate with during that estrous cycle. If the female did not copulate with this new male, we removed him from the arena and added another male. This procedure continued until the female was no longer perforate, mated, or until three males had been paired with the female. If the female failed to mate despite being given the opportunity to with multiple males, she was considered a failed multiple mating.
2.4 Post-copulation
Regardless of experimental group, after the breeding trials were finished, we moved all females into a separate room without adult males, housing other potentially pregnant females and nursing dams. Their housing setup, lactation diet and frequency of reproductive checks were the same for all experimental pocket mice as all other potentially pregnant pocket mice at the facility (see Shier et al., 2025). Females that started cycling or did not give birth after the normal gestation period had passed were returned to their original home enclosures.
We tracked litter size and pup growth via a 21 day weight check of all pups in the litter. Earlier weight checks were not possible since the species is sensitive to disturbance in the first weeks following parturition. We also used a weight check taken at 30 days post-weaning (roughly 60 days of age) to determine whether any potential weight differences remained once pups were independent.
2.5 Parentage identification
To perform genetic analyses, we took a tissue sample from all P.l. pacificus in our care either upon collection, after weaning, or at death if the individual died before a sample was taken. We used a standard ear snip protocol (Metcalf et al., 2001; Alexander and Riddle, 2005; Loew et al., 2006; Waser et al., 2006; Hendricks et al., 2020; Shier et al., 2021). Ear snips can be as small as a pencil point and still provide ample genetic data for analysis of parentage and genetic relationships (Waser et al., 2006). Ear snips were obtained by sterilizing scissors with 200 proof ethanol, holding the scissors on a tangent from the edge of the pinna, and snipping a sliver (~0.5mm) off the edge of the pinna. Tissue samples were then transferred to a vial with 200 proof ethanol and stored in -20°C freezer until processed. We sterilized scissors between animals. DNA from tissue samples were extracted using the QIAamp DNA Mini Kit (Qiagen Inc.) following the manufacturer’s protocol.
Seventeen microsatellite markers specifically designed for P.l. pacificus were used for genetic testing. PCRs were performed using the Qiagen Multiplex PCR Kit (Qiagen Inc.) followed by verification of products on a 1.5% TBE agarose gel. Fragment analysis was performed using capillary electrophoresis on an ABI 3130 genetic analyzer (Applied Biosystems) and alleles were scored relative to an internal size standard (500 ROX) using GeneMapper 3.0 (Applied Biosystems).
To determine the true sire of multiple paternity litters, we used the program Cervus (Kalinowski, et al., 2007) to estimate the allele frequency for all captive-bred individuals. The program runs a simulation of parentage analysis with known sexes for 10,000 dams and 10,000 sires to provide statistical power throughout the process of parentage assignments. Finally, we conducted a parent pair analysis with known sexes using LOD (Logarithm of the odds) scores to determine the most likely candidate parents based on the delta of likelihood values.
2.6 Analysis
We investigated: 1) whether female P.l. pacificus will mate multiply, 2) whether there are any fitness benefits to females that mate multiply, and 3) whether multimale mating results in multiple paternity, including any biases towards siring based on copulation order. We addressed these topics with a series of questions, listed below.
2.6.1 Will female P.l. pacificus mate multiply and does this differ based on whether she is offered the same or a different male?
We examined the number of successes and failures of 2nd copulations for females offered different males or the same male, using a Fisher’s exact test. We also investigated whether the presence of a copulatory plug after the first copulation reduced the likelihood of a second copulation, with a Fisher’s exact test.
2.6.2 Does mating multiply increase female reproductive fitness?
We had too few females that mated more than once with the same male (N = 2) for statistical analysis. Therefore, we investigated the influence of multimale mating between the three remaining treatment groups, which varied in the type of breeding attempts offered and their outcomes. These groups were: females that mated multiply with a different male than their first copulation, females that did not mate multiply with a different male despite having the opportunity (i.e. failed multimale mating), and females that did not mate multiply with the same male despite having the opportunity. We determined the effect of these three treatments on several metrics of fitness: likelihood of pregnancy from pairing (Y/N), likelihood of giving birth to a litter from pairing (Y/N), litter size for known pregnancies, female weight gain during pregnancy, the weight of pups produced at their youngest standard check (21 days) and the weight of pups 30 days post-weaning.
We used generalized linear mixed models (GLMMs) with the lme4 package in R (Bates et al., 2015; R Core Team, 2021) to examine the effect of treatment on each metric of fitness, allowing us to account for potential non-random variation in the data between breeding years and between different dams. We included study year and Dam ID as random effects initially in all models, dropping either or both if they failed to contribute to variation within the model. If neither random effect accounted for variance in the model, we instead used a Generalized Linear Model (GLM). For each model where year was included, we examined whether 2019 was an outlier year, since it has previously been excluded from other reproductive analyses (Shier et al., 2025), due to a diet change that radically impacted weight, pregnancy, and young of year survival.
We analyzed pregnancy (Y/N) and the production of a litter (Y/N) with a binomial error distribution and a logit-link function, litter size with a Poisson error distribution and the three models on body weight with a Gaussian error distribution (female weight gain during pregnancy, pup weight at 21 days since birth and 30 days since weaning). For each binomial model we constructed binned plots and for all other models we examined the appropriateness of the data distribution and model fit using the DHARMa package (Hartig, 2021), to make quantile-quantile plots and test for issues of over dispersion, outliers and zero inflation.
In addition to treatment, we included testes position and longest mount as covariates in the pregnancy model. These covariates were selected because testes position and longest mount have been shown to influence the likelihood of pregnancy in a wider analyses on P.l. pacificus reproductive success (Shier et al., 2025). With females who mated multiply, we used the best testes position (i.e., fully scrotal or partially scrotal) and the longest mount between the two matings. To investigate the likelihood of producing a litter, we used only the subset of data where females had been confirmed pregnant. In the models investigating pup weight, we included litter size as a covariate alongside treatment.
Since covariates were chosen in a hypothesis-based way for all models, we did not pursue model selection and instead interpreted the full models.
2.6.3 Does multimale mating result in multiple paternity?
To better craft management advice, we aimed to determine several outcomes that may influence the circumstances under which multimale mating may be an appropriate recommendation. First, we investigated if litters produced after multimale mating showed multiple paternity, and if so, whether there was an effect of mating order. We used an exact test of goodness of fit, assuming a 50:50 distribution of paternity between both males. We also investigated whether multimale mating was less likely to result in multiple paternity if a copulatory plug was present after the first mating and after the second mating, using Fisher’s exact tests.
3 Results
3.1 Will female P.l. pacificus mate multiply and does this differ based on whether she is offered the same or a different male?
Females mated more than once in ~40% of the trials (32 out of 81), with either the same or a different male, confirming that pocket mice will mate multiply. Females were more likely to mate with a different male than the same one a second time (Fisher’s exact test; p = 0.003), with only two of 19 same male pairing attempts resulting in a second copulation. The pocket mice were not less likely to mate a second time if they were observed with a copulatory plug (Fisher’s exact test; p = 1.00). As multiply mating with the same male only occurred twice, it was dropped from further analysis.
3.2 Does mating multiply increase fitness?
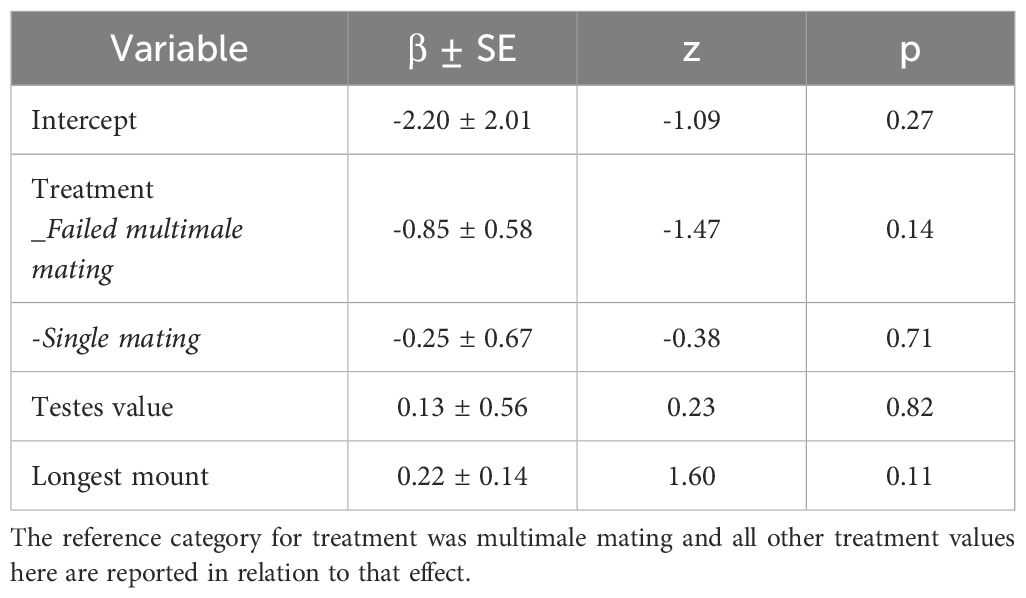
Fifty-six percent of the multimale and single mating breedings resulted in pregnancy (N = 44 of 79). We did not detect an effect of mating multiply on the likelihood of becoming pregnant (Binomial GLMM; N = 79 pairings; Single mating vs multimale mating, β ± SE = -0.25 ± 0.67, z = -0.38, p = 0.71; Figure 1). No other covariates predicted pregnancy either (Table 1). Year was also excluded as a random effect because it had 0 variance in the model.
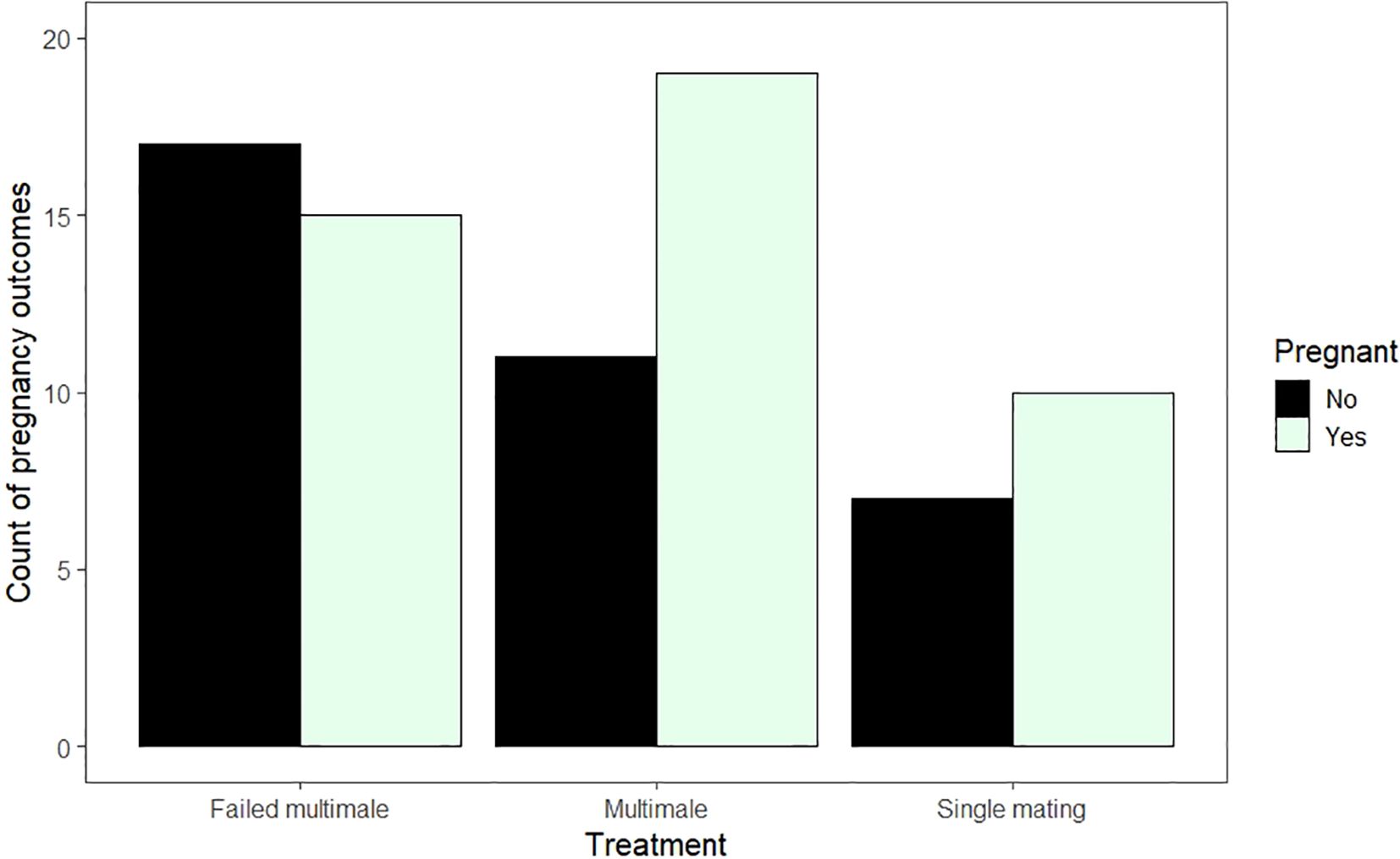
Figure 1. Raw counts of copulation outcomes (the occurrence of pregnancy) by different mating treatment groups. In the failed multimale group the females were offered the opportunity to mate with either the same or a different male after a copulation but did not mate again. Multimale mating includes only females who mated with a different male during the 2nd mating opportunity and single mating included only females who were not given an additional opportunity to mate with a different male after a copulation.
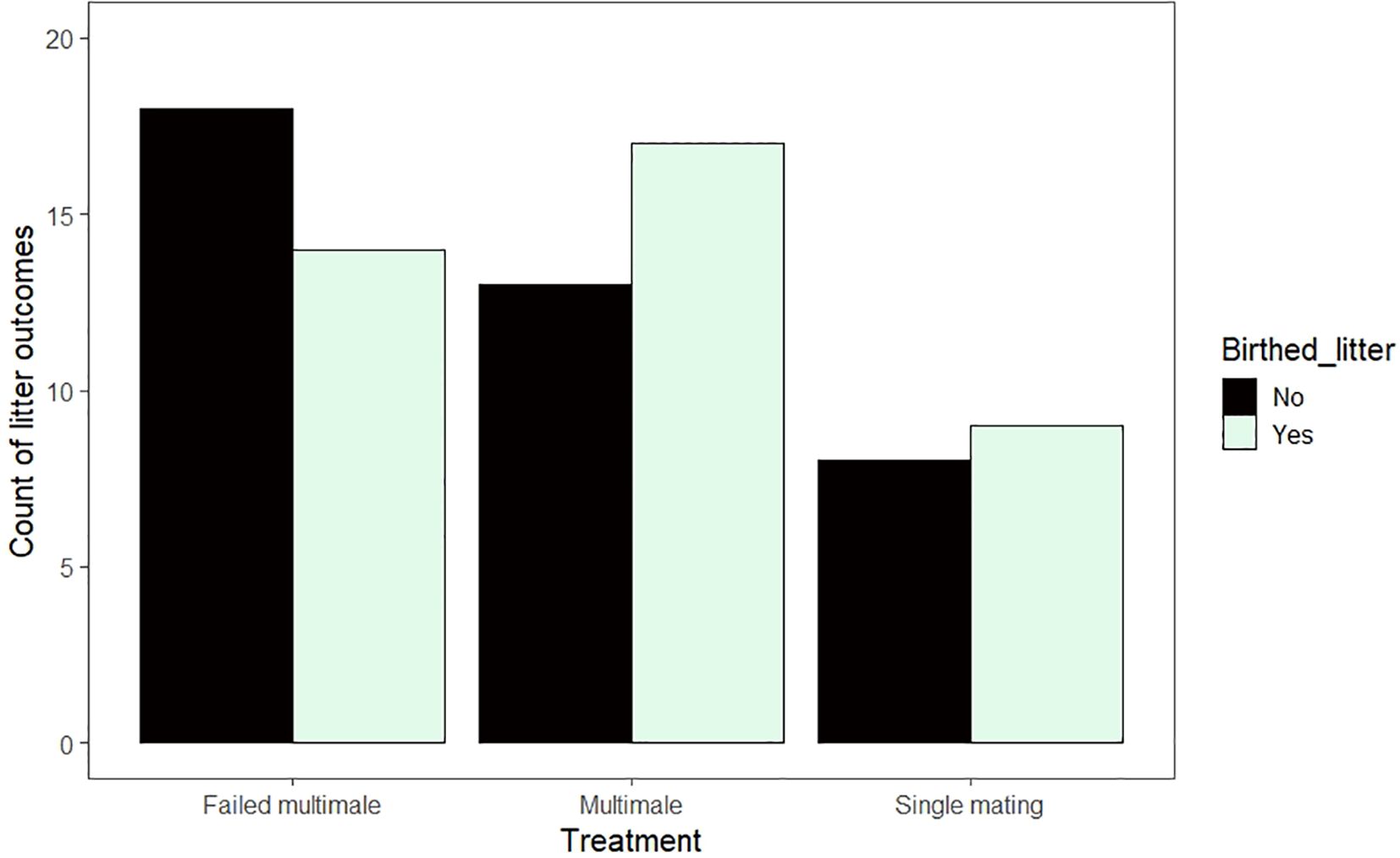
Of the 79 pairings, 48% resulted in a litter of at least one pup. We did not detect an effect of mating multiply on the likelihood of birthing a litter (Binomial GLMM; N = 79 pairings; Single mating vs multimale mating, β ± SE = -0.15 ± 0.64, z = -0.23, p = 0.82; Figure 2). Year was also excluded as a random effect because it had 0 variance in the model.
Forty-two pregnancies had litters where the number of pups produced could be verified, including four where no pups were born. We observed a mean litter size of 3.10 pups across the 42 pregnancies. There was no difference in litter size between treatment groups (Poisson GLM; N = 42, Single mating vs multimale mating, β ± SE = 0.06 ± 0.22, z = 0.29, p = 0.77, Failed multimale mating vs multimale mating, β ± SE = 0.05 ± 0.20, z = 0.23, p = 0.82; Figure 3).
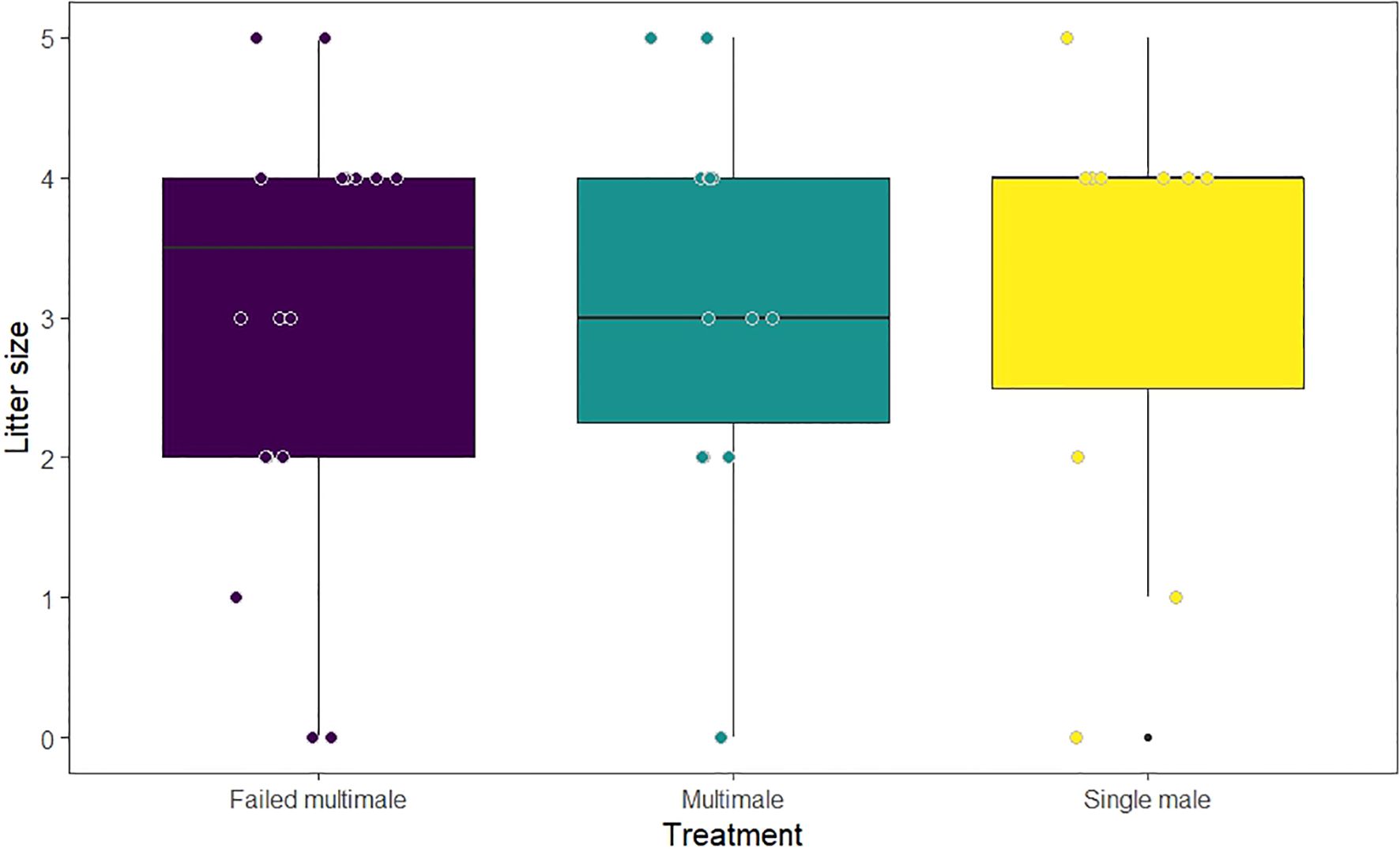
Figure 3. Litter size by treatment. Colored dots depict raw litter size and have been jittered to vary slightly to avoid visual overlap. Boxplots show the median value (horizontal line) with the upper and lower quartiles of the data contained within the box and outliers (black dots).
While we found some support to suggest that females had different patterns of weight gain depending on whether they had been singly or multiply mated (Figure 4), litter size had a large impact and their interaction was not significant (Gaussian GLMM, N = 42, Treatment multimale vs single: Litter size interaction, β ± SE = 0.30 ± 0.17, t = -1.75, p = 0.09).
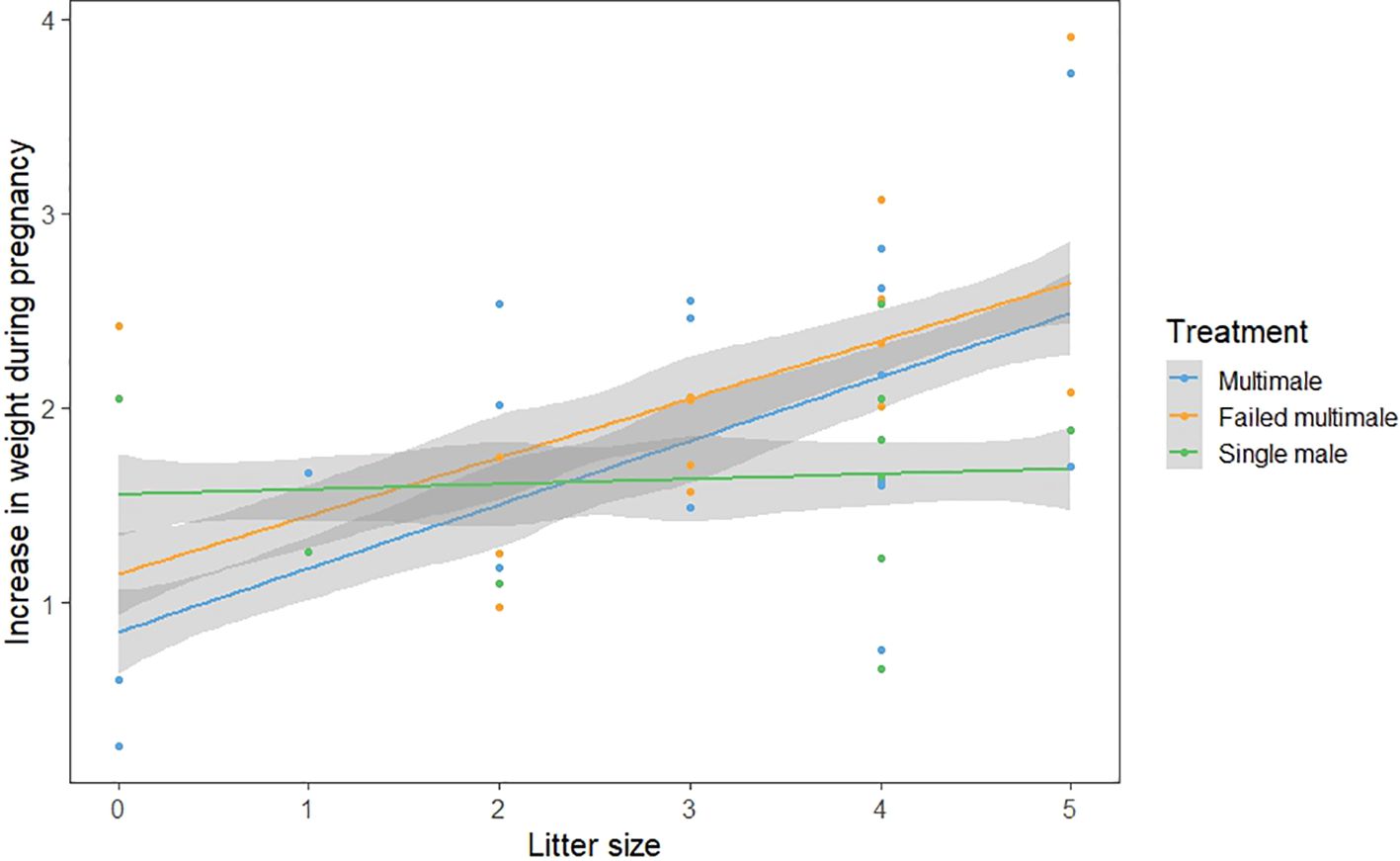
Figure 4. Changes in weight over pregnancy based on litter size and treatment. Lines represented predicted values from the GLMM model and points show raw data. Shaded regions are 95% confidence intervals calculated from the predicted values.
We recorded a 21-day weight for 117 pups produced during the experiment. Examining the random effects showed that 2019 was an outlier year and was therefore excluded from the weight data (N = 9 pups). Pups in larger litters were more likely to weigh less (Gaussian GLMM; N = 108, β ± SE = -0.32 ± 0.10, t = -3.22, p < 0.01). Pocket mice in the singly mated group had higher weights than those in the multiply mated group (Single mating vs multimale mating, β ± SE = 0.49 ± 0.16, t = 3.06, p <0.01; Figure 5). However, there was no difference in weight between pups whose mother had been multiply mated and those where multimale mating was attempted but failed (β ± SE = -0.08 ± 0.24, t = -0.35, p = 0.73), suggesting the lower weights may have been an effect of the experience of interacting with other males post-mating, not one of mating itself.
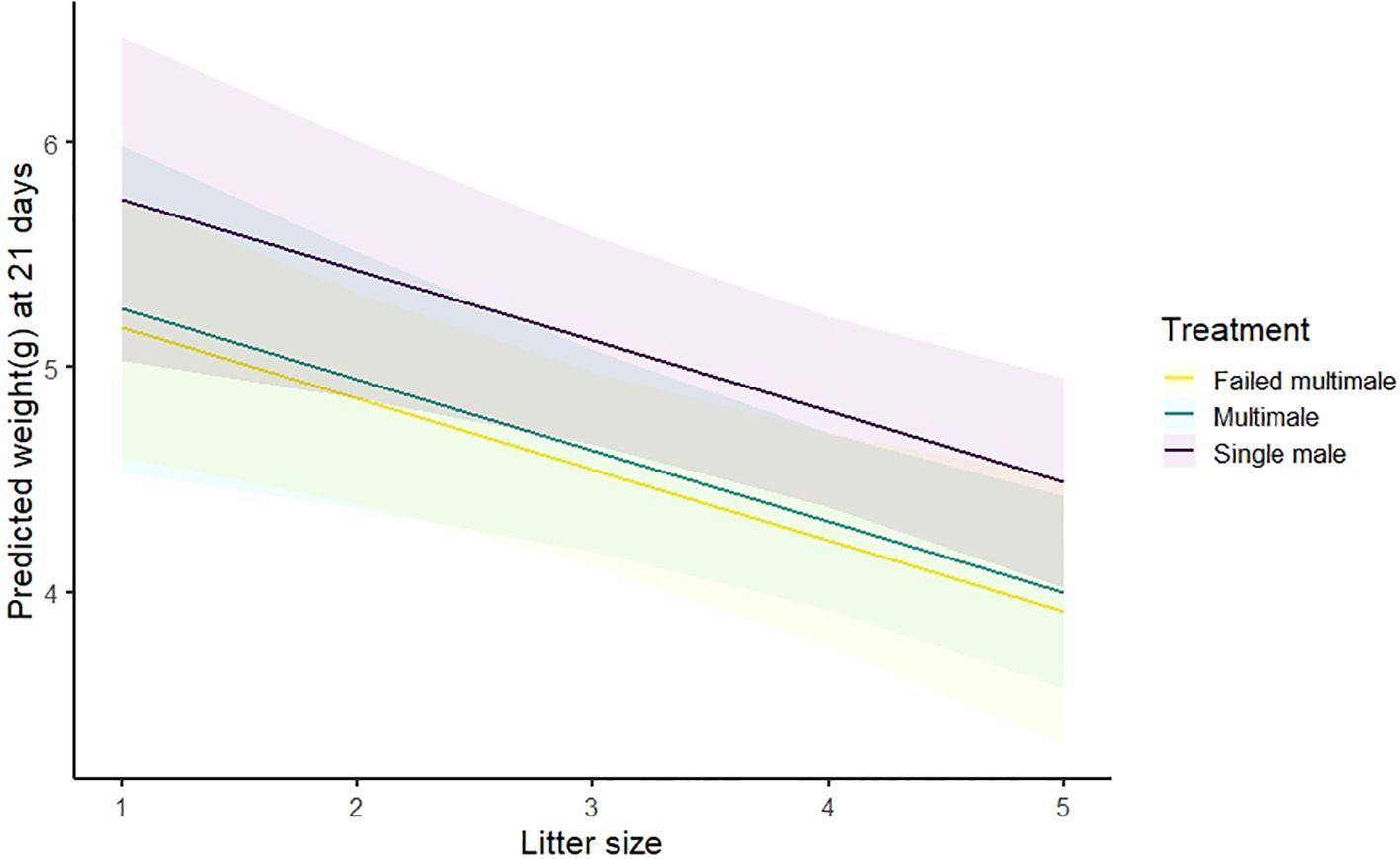
Figure 5. Weight of pups at 21 days old, predicted by treatment and litter size. We obtained the predicted values from a GLMM including treatment and litter size as covariates, with year and dam ID as random effects. Shaded regions show the upper and lower confidence intervals along the predicted values.
In examining weight 30 days post-weaning, we did not need to exclude 2019 since it was not an outlier year for pups that survived to that age class. By roughly 60 days of age, when pups hit 30 days post-wean, there was no longer an effect of treatment on weight. Specifically, there was no difference in weight between pups born to multiply mated and single mated litters (Gaussian GLMM, N = 117 pups, β ± SE = 0.05 ± 0.20, t = 0.26, p = 0.80), nor any difference between multiply mated and failed multiply mated litters (β ± SE = -0.04 ± 0.19, t = -0.19, p = 0.85). Additionally, there was no difference in weight between pups based on litter size (β ± SE = -0.10 ± 0.45, t = -1.05, p = 0.30).
We found a trend suggesting a negative impact of offering more matings. The more opportunities we offered females to mate after an initial copulation, the lower her resulting pups’ weights were at 21 days (β ± SE = -0.32 ± 0.16, t = -2.03, p = 0.054).
3.3 Does multimale mating result in multiple paternity?
We found evidence for multiple paternity in litters where there were copulations with multiple males (44% had multiple paternity, N = 7 of 16 litters). Overall, there was a bias towards the first copulated male in siring pups (75%, N = 33 of 44 pups sired by first male; Exact goodness of fit test, 0.75 (CI = 0.60 - 0.87), p=0.001). A copulatory plug after the first (Fisher’s exact test, p = 1.00) or after the second copulation (p = 0.24) did not influence the likelihood of multiple paternity.
4 Discussion
Conservation breeding programs can benefit from understanding the mating system of the target, imperiled species. Based on what we knew of the morphology and natural history of P.l. pacificus and closely related species (Randall, 1991; Randall et al., 2002), we aimed to determine whether offering multiple breeding opportunities to females would improve reproductive fitness and, thus, breeding outcomes. We did not find significant improvements in the likelihood of pregnancy, litter size or pup condition that would warrant the adoption of this approach across the breeding program. The only difference we saw between experimental groups was in the weight of pups at 21 days, which suggested a potential drawback, not benefit, of offering females post-copulatory mating opportunities. Despite the lack of positive impact on fitness, there may be circumstances in which using multimale mating would be advantageous in this system since we found that multiply mated litters had multiple paternity.
Although we had predicted to find a positive impact of conducting multimale mating, the null results are equally as important in developing a robust conservation breeding program. Conservation breeding is a challenge, and many programs struggle to maintain sustainable populations or meet recovery goals (Collar and Butchart, 2014; Powell et al., 2019). With time and resource restrictions, often programs are run based on best guesses, or trial and error. Based on the available evidence, it would be plausible to consider conducting multimale matings for pocket mice, but it was not guaranteed to help, since multimale mating is not always beneficial (e.g., no impact on pregnancy or litter size in North American red squirrels, Tamiasciurus hudsonicus, Lane et al., 2008). Had we done so without the strict experimentation this study afforded, it would have been easy to adopt a time-intensive and costly intervention unnecessarily. Reporting null results is important to prevent others from repeating efforts not likely to improve outcomes. In this case, caution should be taken before incorporating assumed wild-type mating patterns without further testing.
Although we found no evidence of increased female reproductive fitness via multimale mating, nearly 40% of females given the opportunity would mate with an additional male, whilst less than 11% of females offered the opportunity to remate with their same male chose to do so. This result does suggest some possible benefit to females mating with multiple males that was not captured in our study. For example, research with house mice suggest that females benefit from polyandry by producing sons that achieve high reproductive success (Firman, 2011). Alternatively, females that mate with multiple males may receive indirect benefits that are not able to be captured by our study due to the artificial housing conditions. For instance, in banner-tailed kangaroo rats, females were significantly more likely to be captured on mounds belonging to neighboring males in the breeding season compared to the nonbreeding season (Steinwald et al., 2013). Although the authors posit that this change in mobility may serve as a means of seeking competing mates, multimale mating could indirectly benefit the female’s fitness by an opportunity to widen her foraging grounds through allowing matings with neighboring males.
If anything, we found that there could be a minor fitness cost to multimale mating if it were adopted across the board in our program. We found a decrease in pup weight at 21 days based on the mating setup. However, the effect was present both in pups whose mothers had mated multiply, and those whose mothers had been given merely the opportunity to mate multiply. This suggests that the process of being socialized with another male, post-copulation, influenced something about pup development which reduced the mean pup size, even when litter size was accounted for. While stress could feasibly be a mechanism, there is not good evidence from the literature that a single stressful event at the time of copulation would influence downstream reproductive outcomes since most research into the effects of stress on pregnancy in rodents examines reoccurring or chronic stressors (e.g., Meek et al., 2001). Regardless of the cause, the effect disappeared by the day 30 post-wean weight checks, suggesting that the pups and dams are able to compensate for whatever impact multimale mating caused.
Although it appears inadvisable to mate P.l. pacificus multiply for most breedings, there are some instances where it could be beneficial. The species is genetically imperiled, with the remaining wild diversity coming from three small extant populations (Wilder et al., 2020, 2022). Effective genetic management of the species requires special consideration of population origin because admixed individuals fare better and one population (Dana Point) appears to carry deleterious alleles (Wilder et al., 2020). To enhance genetic diversity within the time frame of single litters, multiple mating could be a useful strategy. As estrous cycling is rarer in founding females from Dana Point (Shier, unpublished data), it may be advantageous to capitalize on their mating opportunities when they arise. In these cases since the first mated male seems to gain the fitness advantage, the order of matings should prioritize the most valuable males first. This finding is particularly notable since, unlike P.l. pacificus, other species of small mammals, such as deer mice (Peromyscus maniculatus, Dewsbury, 1985) do not show the same fitness differential between first and subsequent mates. Therefore, for unrepresented founders whose genetic material is crucial to the breeding program, multimale mating should be considered as a strategy to maximize genetic diversity more rapidly.
We also gained valuable insights into pocket mice natural history. The presence of a copulatory plug between matings did not influence the likelihood of a second mating. This is consistent with findings from diverse other species where remating is unaffected by the placement of a copulatory plug (e.g. nematodes, Timmermeyer et al., 2010; lizards, Moreira and Birkhead, 2003). Also the presence of a copulatory plug did not influence the likelihood of multiple paternity, which aligns with research from other species suggesting that copulatory plugs also do not prevent the presence of multiple males’ DNA in females’ reproductive tracts (e.g. banner-tailed kangaroo rats (McCreight et al., 2011). As prior research suggested no impact of the presence of a copulatory plug in P.l. pacificus on downstream pregnancy outcomes (Shier et al., 2025), their evolutionary function in the species is still unknown.
As we continue to refine protocols and improve conservation breeding outputs for the Pacific Pocket Mouse Conservation Breeding and Reintroduction Program, we are better able to produce the numbers and genetic diversity needed for species recovery. This process often requires strict experimentation, which can be challenging given small sample sizes and the competing priorities of management and research. By developing evidence-based breeding recommendations, we hope to provide lessons that can be adopted by other small mammal breeding programs and contribute to an adaptive management framework.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The animal study was approved by SDZWA’s IACUC (15-005, 18-015, and 21-010). Permits for the holding and breeding of P.l. pacificus were authorized by the United States Fish and Wildlife Service (10A1A 142435-6 and ESPER 0002526), and California Department of Fish and Wildlife (SC-002508). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
AG: Formal Analysis, Project administration, Writing – original draft, Supervision. SL: Data curation, Writing – review & editing, Methodology, Conceptualization, Investigation. SK: Project administration, Data curation, Supervision, Investigation, Writing – review & editing. SH: Investigation, Writing – review & editing, Data curation. DS: Funding acquisition, Resources, Conceptualization, Writing – review & editing, Project administration, Methodology, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was funded by a California Traditional Section 6 grant to DM Shier and RR Swaisgood, and a cooperative agreement N62473-20-2-0016 from the U.S Navy. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of our funders.
Acknowledgments
We want to thank Erin Drum, Rachel Gosselin, Ashley Flanders, Amy Harris, Cora Dysin and Jamie Chang for help in conducting breedings and P.l. pacificus husbandry over the course of this experiment, and to the many volunteers who helped in the breeding facility.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2025.1667557/full#supplementary-material
References
Alexander L. F. and Riddle B. R. (2005). Phylogenetics of the new world rodent family heteromyidae. J. Mammalogy 86, 366–379. doi: 10.1644/BER-120.1
Ballou J. D., Lacy R. C., Pollak J. P., Callicrate T., and Ivy J. (2023). Software for demographic and genetic analysis and management of pedigreed populations. Available online at: http://www.scti.tools.
Bates D., Maechler M., Bolker B., and Walker S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Software 67, 1–48. doi: 10.18637/jss.v067.i01
Bowkett A. E. (2009). Recent captive-breeding proposals and the return of the ark concept to global species conservation. Conserv. Biol. 23, 773–776. doi: 10.1111/j.1523-1739.2008.01157.x
Collar N. J. and Butchart S. H. M. (2014). Conservation breeding and avian diversity: chances and challenges. Int. Zoo Yearbook 48, 7–28. doi: 10.1111/izy.12039
Comizzoli P. and Holt W. V. (2022). Recent progress in spermatology contributing to the knowledge and conservation of rare and endangered species. Annu. Rev. Anim. Biosci. 10, 469–490. doi: 10.1146/annurev-animal-020420-040600
Conde D. A., Colchero F., Gusset M., Pearce-Kelly P., Byers O., Flesness N., et al. (2013). Zoos through the lens of the IUCN red list: A global metapopulation approach to support conservation breeding programs. PloS One 8, e80311. doi: 10.1371/journal.pone.0080311
Dean M. D. (2013). Genetic disruption of the copulatory plug in mice leads to severely reduced fertility. PloS Genet. 9, e1003185. doi: 10.1371/journal.pgen.1003185
Dewsbury D. A. (1985). Interactions between males and their sperm during multi-male copulatory episodes of deer mice (Peromyscus maniculatus). Anim. Behav. 33, 1266–1274. doi: 10.1016/S0003-3472(85)80186-X
Eisenberg J. F. and Isaac D. E. (1963). The reproduction of heteromyid rodents in captivity. J. Mammalogy 44, 61. doi: 10.2307/1377168
Firman R. C. (2011). Polyandrous females benefit by producing sons that achieve high reproductive success in a competitive environment. Proc. R. Soc B. 278, 2823–2831. doi: 10.1098/rspb.2010.2791
Firman R. C. and Simmons L. W. (2008). Polyandry, sperm competition, and reproductive success in mice. Behav. Ecol. 19, 695–702. doi: 10.1093/beheco/arm158
García-Navas V. (2017). Lack of evolution of sexual size dimorphism in heteromyidae (Rodentia): the influence of resource defense and the trade-off between pre- and post-copulatory trait investment. Evol. Biol. 44, 56–68. doi: 10.1007/s11692-016-9390-7
Gromov V. S. (2024). Multiple mating by females and multiple paternity in rodents: cross-species comparative analysis. Rus.J.Theriol. 23, 57–72. doi: 10.15298/rusjtheriol.23.1.07
Hartig F. (2021). DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R package version 0.4.1. Available online at: https://CRAN.R-project.org/package=DHARMa (Accessed Feburary 1, 2023).
Hayden P., Gambino J. J., and Lindberg R. G. (1966). Laboratory breeding of the little pocket mouse, perognathus longimembris. J. Mammalogy 47, 412–423. doi: 10.2307/1377682
Hendricks S., Navarro A. Y., Wang T., Wilder A., Ryder O. A., and Shier D. M. (2020). Patterns of genetic partitioning and gene flow in the endangered San Bernardino kangaroo rat (Dipodomys merriami parvus) and implications for conservation management. Conserv. Genet. 21, 819–833. doi: 10.1007/s10592-020-01289-z
Hoogland J. L. (2013). Why do female prairie dogs copulate with more than one male?—Insights from long-term research. J. Mammalogy 94, 731–744. doi: 10.1644/12-MAMM-A-291.1
Kalinowski S. T., Taper M. L., and Marshall T. C. (2007). Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol. 16 (5), 1099–1106. doi: 10.1111/j.1365-294X.2007.03089.x
Lane J. E., Boutin S., Gunn M. R., Slate J., and Coltman D. W. (2008). Female multiple mating and paternity in free-ranging North American red squirrels. Anim. Behav. 75, 1927–1937. doi: 10.1016/j.anbehav.2007.10.038
Loew S. S., Williams D. F., Ralls K., Pilgrim K., and Fleischer R. C. (2006). Population structure and genetic variation in the endangered Giant Kangaroo Rat (Dipodomys ingens). Conserv. Genet. 6, 495–510. doi: 10.1007/s10592-005-9005-9
Mangels R., Tsung K., Kwan K., and Dean M. D. (2016). Copulatory plugs inhibit the reproductive success of rival males. J. Evolutionary Biol. 29, 2289–2296. doi: 10.1111/jeb.12956
McCreight J. C., DeWoody J. A., and Waser P. M. (2011). DNA from copulatory plugs can give insights into sexual selection. J. Zoology 284, 300–304. doi: 10.1111/j.1469-7998.2011.00806.x
Meek L. R., Dittel P. L., Sheehan M. C., Chan J. Y., and Kjolhaug S. R. (2001). Effects of stress during pregnancy on maternal behavior in mice. Physiol. Behav. 72, 473–479. doi: 10.1016/S0031-9384(00)00431-5
Metcalf A. E., Nunney L., and Hyman B. C. (2001). Geographic patterns of genetic differentiation within the restricted range of the endangered Stephen’s kangaroo rat Dipodomys stephensi. Evolution 55, 1233–1244. doi: 10.1111/j.0014-3820.2001.tb00643.x
Moreira P. L. and Birkhead T. R. (2003). Copulatory plugs in the Iberian Rock Lizard do not prevent insemination by rival males. Funct. Ecol. 17, 796–802. doi: 10.1111/j.1365-2435.2003.00789.x
Parker G. A. (1970). Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 45, 525–567. doi: 10.1111/j.1469-185X.1970.tb01176.x
Powell D. M., Dorsey C. L., and Faust L. J. (2019). Advancing the science behind animal program sustainability: An overview of the special issue. Zoo Biol. 38, 5–11. doi: 10.1002/zoo.21474
Randall J. (1991). Mating strategies of a nocturnal, desert rodent (Dipodomys spectabilis). Behav. Ecol. Sociobiol 28, 215–220. doi: 10.1007/BF00172173
Randall J. A., Hekkala E. R., Cooper L. D., and Barfield J. (2002). Familiarity and flexible mating strategies of a solitary rodent, Dipodomys ingens. Anim. Behav. 64, 11–21. doi: 10.1006/anbe.2002.3029
R Core Team (2021). R: A language and environment for statistical computing. Available online at: https://www.R-project.org/ (Accessed March 31, 2022).
Šandera M., Albrecht T., and Stopka P. (2013). Variation in apical hook length reflects the intensity of sperm competition in murine rodents. PloS One 8, e68427. doi: 10.1371/journal.pone.0068427
Schneider M. R., Mangels R., and Dean M. D. (2016). The molecular basis and reproductive function(s) of copulatory plugs. Mol. Reprod. Dev. 83, 755–767. doi: 10.1002/mrd.22689
Shier D. M., King S. N. D., Leivers S. J., and Greggor A. L. (2025). Using retrospective analyses to adaptively manage conservation breeding of an endangered rodent. Conserv. Sci. Pract. 7, e13307. doi: 10.1111/csp2.13307
Shier D., Leivers S., King S., Chock R., Navarro A., and Montagne J. (2016). Captive breeding, anti-predator behavior and reintroduction of the Pacific pocket mouse (Perognathus longimembris pacificus). (Escondido, CA: San Diego Zoo Wildlife Alliance). 10a1A annual report to CDFW.
Shier D. M., Navarro A. Y., Tobler M., Thomas S. M., King S. N. D., Mullaney C. B., et al. (2021). Genetic and ecological evidence of long-term translocation success of the federally endangered Stephens’ kangaroo rat. Conservat Sci. Prac 3, e478. doi: 10.1111/csp2.478
Steinwald M. C., Swanson B. J., Doyle J. M., and Waser P. M. (2013). Female mobility and the mating system of the banner-tailed kangaroo rat (Dipodomys spectabilis). J. Mammal 94, 1258–1265. doi: 10.1644/13-MAMM-A-124
Sutter A., Simmons L. W., Lindholm A. K., and Firman R. C. (2016). Function of copulatory plugs in house mice: mating behavior and paternity outcomes of rival males. BEHECO 27, 185–195. doi: 10.1093/beheco/arv138
Timmermeyer N., Gerlach T., Guempel C., Knoche J., Pfann J. F., Schliessmann D., et al. (2010). The function of copulatory plugs in Caenorhabditis remanei: hints for female benefits. Front. Zool 7, 28. doi: 10.1186/1742-9994-7-28
Waser P. M., Busch J. D., McCORMICK C. R., and Dewoody J. A. (2006). Parentage analysis detects cryptic precapture dispersal in a philopatric rodent. Mol. Ecol. 15, 1929–1937. doi: 10.1111/j.1365-294X.2006.02893.x
Wilder A. P., Dudchenko O., Curry C., Korody M., Turbek S. P., Daly M., et al. (2022). A chromosome-length reference genome for the endangered pacific pocket mouse reveals recent inbreeding in a historically large population. Genome Biol. Evol. 14, evac122. doi: 10.1093/gbe/evac122
Wilder A. P., Navarro A. Y., King S. N. D., Miller W. B., Thomas S. M., Steiner C. C., et al. (2020). Fitness costs associated with ancestry to isolated populations of an endangered species. Conserv. Genet. 21, 589–601. doi: 10.1007/s10592-020-01272-8
Keywords: breeding protocols, captive breeding, conservation breeding program, conservation physiology, polygynandry, small mammal
Citation: Greggor AL, Leivers SJ, King SND, Hunjan S and Shier D (2025) Testing multimale mating as a strategy for improving the reproductive output of an endangered small mammal. Front. Conserv. Sci. 6:1667557. doi: 10.3389/fcosc.2025.1667557
Received: 16 July 2025; Accepted: 28 August 2025;
Published: 22 September 2025.
Edited by:
Nucharin Songsasen, Smithsonian Conservation Biology Institute (SI), United StatesReviewed by:
Chase A. LaDue, Oklahoma City Zoo and Botanical Garden, United StatesMicaela Gunther, Cal Poly Humboldt, United States
Copyright © 2025 Greggor, Leivers, King, Hunjan and Shier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alison L. Greggor, YWdyZWdnb3JAc2R6d2Eub3Jn
 Alison L. Greggor
Alison L. Greggor Samantha J. Leivers1,2
Samantha J. Leivers1,2 Sumitha Hunjan
Sumitha Hunjan