- 1Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of Management, Cancer Hospital of Huanxing, Beijing, China
- 3Department of Medical Oncology, Cancer Hospital of Huanxing, Beijing, China
- 4Department of Comprehensive Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 5Vascular Access Center, Hunan Cancer Hospital/The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China
- 6Department of Lymphoma and Hematology, Hunan Cancer Hospital/The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China
- 7Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Radiation Oncology, Peking University Cancer Hospital and Institute, Beijing, China
- 8Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
- 9Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 10State Key Laboratory of Oncology in South China, Sun Yat-sen University Cancer Center, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China
Background: Central venous catheters are convenient for drug delivery and improved comfort for cancer patients, but they also cause serious complications. The most common complication is catheter-related thrombosis (CRT).
Objectives: This study aimed to evaluate the incidence and risk factors for CRT in cancer patients and develop an effective prediction model for CRT in cancer patients.
Methods: The development of our prediction model was based on a retrospective cohort (n = 3,131) from the National Cancer Center. Our prediction model was confirmed in a prospective cohort from the National Cancer Center (n = 685) and a retrospective cohort from the Hunan Cancer Hospital (n = 61). The predictive accuracy and discriminative ability were determined by receiver operating characteristic (ROC) curves and calibration plots.
Results: Multivariate analysis demonstrated that sex, cancer type, catheter type, position of the catheter tip, chemotherapy status, and antiplatelet/anticoagulation status at baseline were independent risk factors for CRT. The area under the ROC curve of our prediction model was 0.741 (CI: 0.715–0.766) in the primary cohort and 0.754 (CI: 0.704–0.803) and 0.658 (CI: 0.470–0.845) in validation cohorts 1 and 2, respectively. The model also showed good calibration and clinical impact in the primary and validation cohorts.
Conclusions: Our model is a novel prediction tool for CRT risk that accurately assigns cancer patients into high- and low-risk groups. Our model will be valuable for clinicians when making decisions regarding thromboprophylaxis.
Introduction
With the rapid growth of medical research over the past decades, more advanced cancer treatments are available for cancer patients to improve their survival. With advances in cancer treatments, more complications, especially cardiovascular complications, have been widely seen in practice (1, 2). To provide better cardiovascular care for cancer patients, a new multidisciplinary field of cardio-oncology was established and has received appreciation and recognition worldwide (3).
Central venous catheters were considered a medical advance that brought convenience for drug delivery and improved comfort for patients, but it also introduced catheter-related complications (4, 5). Catheter-related thrombosis (CRT) is one of the major catheter-related complications affecting many cancer patients (5). The reported incidences of CRT range from 2.4 to 61.5% in cancer patients (4–10). Unlike cancer-associated venous thromboembolism, CRT is recognized as a unique entity because the incidence of CRT in cancer patients is correlated with cancer- and catheter-related risk factors for thrombus formation (11). Moreover, catheter-related risk factors play critical roles that cannot be neglected (6). The central venous catheter, one device, contributes to the three factors of venous thrombus formation described in Virchow's triad (12): the placement of a venous catheter can cause local vessel damage; the presence of a venous catheter changes the dynamics of blood flow (13); and protein and blood cell adhesion on the surface of the catheter increased hypercoagulability. Due to the high incidence of CRT in cancer patients, several clinical trials were initiated to test the efficacy of routine thromboprophylaxis for CRT prevention. However, the results were not conclusive or solid enough to support routine thromboprophylaxis for cancer patients with catheters (11). Therefore, the decision to apply preventive treatment with anticoagulants should be discussed case by case.
The previously published results of the AVERT trial and the CASSINI trial showed benefits of thromboprophylaxis in cancer patients with a high risk of venous thromboembolism (14, 15). Venous thromboembolism (VTE) risk stratification in these two trials was based on the well-validated risk prediction tool, the Khorana risk score (16). While routine thromboprophylaxis for VTE prevention in the general patient population is controversial (17), positive results from these trials have proven the applicability of risk stratification to identify patients who are most likely to benefit from thromboprophylaxis.
CRT is defined as VTE associated with the use of a central venous catheter (18). The same strategy of risk stratification could be applied to CRT prevention in cancer patients. However, first, a well-designed and validated risk prediction tool is needed. We conducted a large-scale observational cohort study of cancer patients with catheters to develop a CRT risk prediction model. In addition to the main cancer-related factors considered in the Khorana risk score, catheter-related factors were also largely investigated when developing our model. Both symptomatic and asymptomatic CRT were considered in our analysis because they have similar clinical impacts on the prognosis of cancer patients (19). Validation of this model was performed in two independent external cohorts.
Materials and Methods
Study Subjects and Study Design
The primary cohort consisted of cancer patients treated at the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College between January 1, 2015 and December 31, 2018. Patients in validation cohort 1 were recruited from the center mentioned above between January 1, 2019 and August 31, 2019, prospectively and independently. Patients in validation cohort 2 were recruited from Hunan Cancer Hospital, and their data were analyzed retrospectively.
The inclusion criteria of patients in our study were as follows: (1) adult and ambulatory patients who were pathologically diagnosed with malignant tumors and underwent successful catheterization, (2) patients who voluntarily participated and voluntarily reported their data in this study, and (3) at least one vascular ultrasound examination was performed during catheter placement. The exclusion criteria were as follows: (1) incomplete basic patient information, (2) the catheter had not been removed at the beginning of patient screening for the primary cohort and validation cohort 2 or at the end of follow-up for validation cohort 1, and (3) unknown location of the primary tumor.
According to the maximum duration of use of centrally inserted central catheters (CICCs) or peripherally inserted central catheters (PICCs), our primary endpoint was objectively confirmed CRT in patients with CICCs during the 3-months use period and in patients with PICCs during the 12-months use period. Patients in validation cohort 2 were under continuous follow-up, and all variables were recorded until extubation was performed upon the doctor's request or when thrombosis occurred (whichever occurred first). The diagnosis of CRT was made by vascular ultrasound.
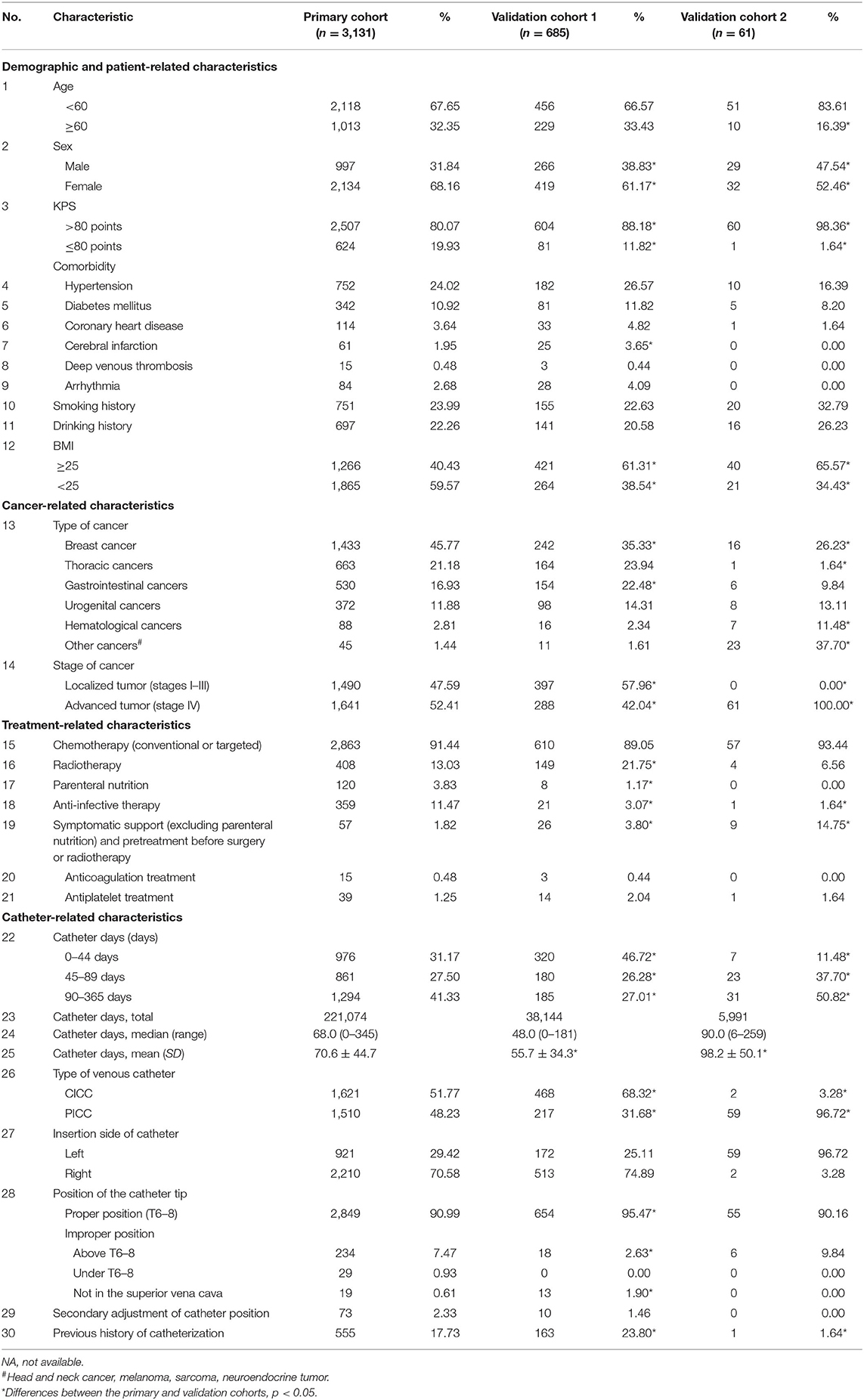
Thirty-six variables were recorded, and these variables included (but were not limited to) the following: general information (age, sex, body mass index, and smoking and drinking habits); past or concomitant diseases [hypertension, diabetes mellitus, coronary heart disease, cerebral infarction, deep venous thrombosis (DVT), and arrhythmia]; cancer status (tumor type, stage, Karnofsky performance score); baseline treatment information; catheter-related information (purpose of catheterization, catheterization history, catheter type, insertion side, position of the catheter tip, and secondary adjustment of the catheter); and baseline examination (routine blood test, D-dimer test). All 36 variables are listed in Table 1 and Supplementary Table 1 (baseline examination).
All patients received routine catheter care provided by a professional team once or twice per week. During follow-up visits, patients in validation cohort 2 were asked about their general situation and whether they had experienced any adverse events or complications since the last visit. Information about complications was continuously recorded.
This study did not interfere with any doctor's decision-making and did not change or delay any treatments.
Catheterization Method
Modified Seldinger technique with ultrasound guidance was used for CICC and PICC placement. Detailed catheterization methods are shown in the Supplementary Textual Material. All CICCs were non-tunneled subclavian catheters.
After catheterization, a chest X-ray examination (including the upper limb and neck on the ipsilateral side of the catheter) was performed to confirm the catheter's direction and the position of the catheter tip. All X-ray films were evaluated by a physician specializing in venous catheterization and at least one radiologist responsible for chest X-ray reports. The tip of the catheter was typically located at the lower third of the superior vena cava, the cavoatrial junction, or the upper third of the right atrium. If the vertebrae were used as a reference, being at thoracic vertebra segment 6–8 (T6–T8) was considered the proper position; otherwise, it was deemed improper.
Doppler Ultrasound
Ultrasound with Doppler and color imaging (GE LOGIQTM E9) was performed at extubation and if any clinical symptoms suggesting CRT were noted.
Every ultrasound report was evaluated by the same radiologist team at the National Cancer Center. CRT was diagnosed after finding a thrombus with partial or total occlusion of the vessel.
Statistical Analysis
General Characteristics and Incidence Reports
The incidence of CRT was calculated as the total number of catheter-related thromboses divided by the total number of catheters placed (%) or divided by 1,000 catheter days (/1,000 days of use).
Pearson's x2 test was used to compare categorical variables, and an independent samples t-test was used to compare continuous variables. All statistical tests were two-sided, and p < 0.05 were considered statistically significant. Pearson's x2 test and the independent samples t-test were performed with SPSS software (Version 23, SPSS Inc., IBM, NY, USA).
Development of the Prediction Model
Univariable and multivariable analyses were performed to identify the significant independent risk factors for CRT. Variables with p < 0.25 in the univariate analysis were included in the multivariate analysis (20). The results are presented as the adjusted odds ratios (ORs) with 95% confidence intervals (CIs). The prediction model was developed based on the results of the multivariate analysis by binary logistic regression and further optimized by stepwise forward and backward selection. A relatively optimal model was ultimately achieved. A nomogram was formulated to illustrate our prediction model by using the rms package (version 5.1-4) in R software (http://www.r-project.org/, The R Foundation for Statistical Computing v3.6.0). The univariable, multivariable, and binary logistic regression analyses were performed with SPSS software (Version 23, SPSS Inc., IBM, NY, USA).
Assessment of the Prediction Model
The performance of the model was assessed in terms of discrimination, calibration, and clinical impact by using the packages rms (version 5.1-4), pROC (version 1.15.3), and rmda (version 1.6) in R software (http://www.r-project.org/, The R Foundation for Statistical Computing v3.6.0).
Discrimination was measured by the area under the receiver operating characteristic (ROC) curve. Calibration was measured by calibration plots, the coefficient of determination (R2), and the Hosmer–Lemeshow (H-L) test (21). The cutoff value of our model between the high- and low-risk groups was derived from the maximum Youden index. Furthermore, clinical impact was assessed by decision curve analysis (22).
Results
Patient Characteristics
From January 1, 2015 to December 31, 2018, we enrolled 3,860 cancer patients who received a catheter and underwent a vascular ultrasound examination. After screening, 3,131 patients were enrolled in the primary cohort. The total number of catheter days was 221,074, and the median number of catheter days per patient was 68.0 (range, 0–345). For validation cohort 1, 2,909 patients were recorded, and 685 patients were ultimately enrolled. Validation cohort 2 consisted of 61 patients. The flow chart of patient enrollment is shown in Supplementary Figure 1.
Among 3,131 patients in the primary cohort, 2,134 (68.16%) were women, and the mean patient age (standard deviation, SD) was 53.7 (±11.1) years. Among 685 patients in validation cohort 1, 419 (61.17%) were women, and the mean patient age (SD) was 54.2 (±11.7) years. Among 61 patients in validation cohort 2, 32 were women, and the mean patient age (SD) was 59.2 (±11.0) years. The characteristics of patients, such as chemotherapy and antiplatelet or anticoagulation status at baseline, were balanced across the primary and validation cohorts. However, as shown in Table 1, the percentage of female patients was significantly higher in the primary cohort than in the validation cohorts (68.16 vs. 61.17% in validation cohort 1 and 52.46% in validation cohort 2). The proportion of breast cancers was also higher in the primary cohort (45.77%) than in the validation cohorts (35.33% in validation cohort 1 and 26.23% in validation cohort 2). The three populations differed notably concerning the type of venous catheter used and the position of the catheter tip. The D-dimer concentration was not requested when collecting data on the patients in the validation cohorts because it was not a significant risk factor according to our primary analysis. For the same reason, results of routine blood examinations were not requested for individuals in validation cohort 2. The detailed baseline characteristics of patients in the primary and validation cohorts are listed in Table 1 and Supplementary Table 1.
CRT in the Primary Cohort
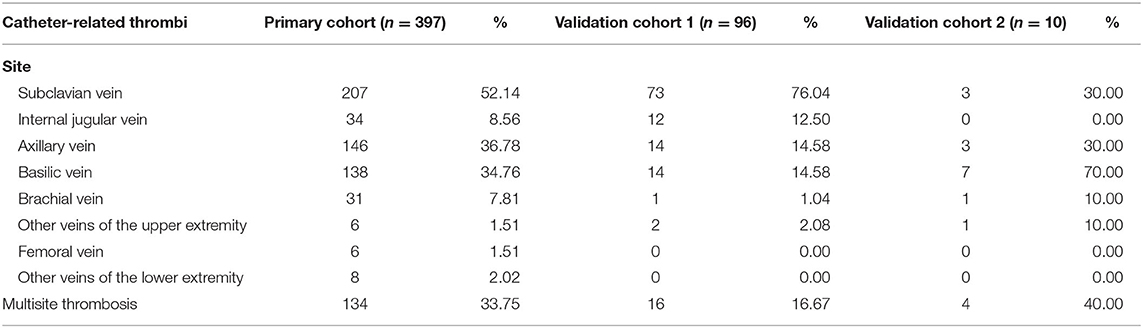
In total, 397 cases (12.7%) of CRT were recorded in our study, with an incidence of 1.80 per 1,000 catheter days. The most common CRT site was the subclavian vein, which accounted for 52.14% (207/397) of the CRT cases. Moreover, 33.75% (134/397) of the CRT cases involved multisite thrombosis. Detailed information on the distribution of thrombosis is shown in Table 2.
Development of the CRT Prediction Model
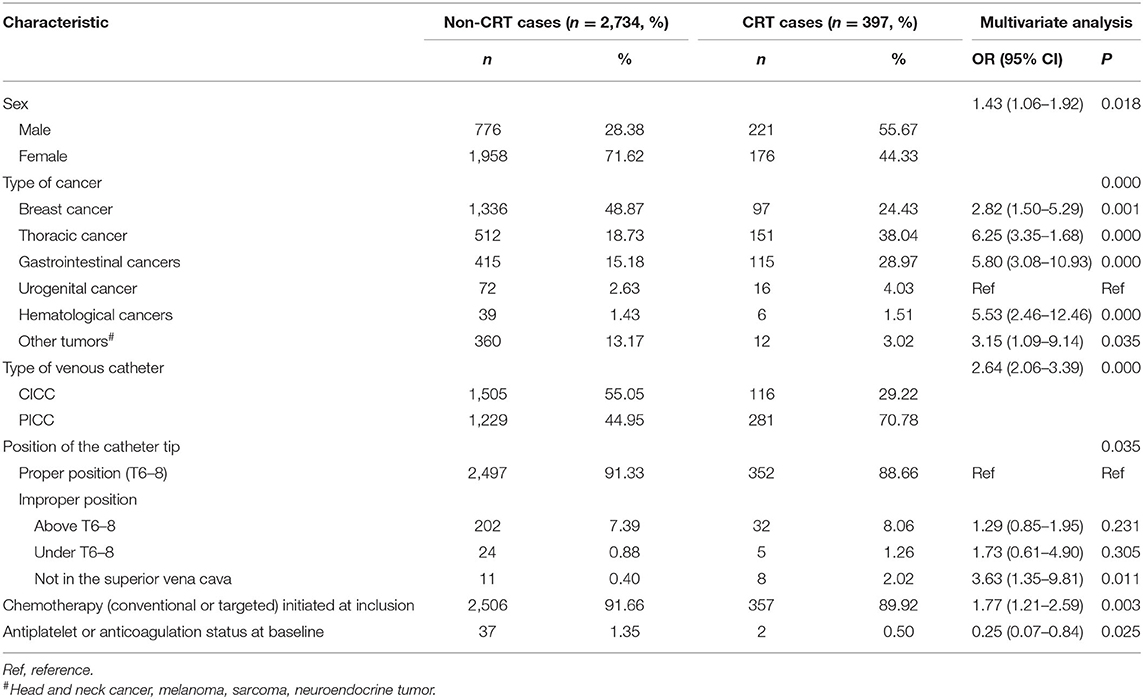
The results of the univariate analysis are listed in Supplementary Table 2. The multivariate analysis combined the stepwise forward and backward selection techniques and demonstrated that sex (male vs. female), type of cancer, type of venous catheter used (CICC vs. PICC), position of the catheter tip, chemotherapy (conventional or targeted) initiated at inclusion, and antiplatelet or anticoagulation status at baseline were independent risk factors for CRT (Table 3). These six factors that were verified by the multivariate analysis were included in our new prediction model. The prognostic nomogram that integrated the six independent factors is shown in Figure 1, and the score of the nomogram is shown in Supplementary Table 3.
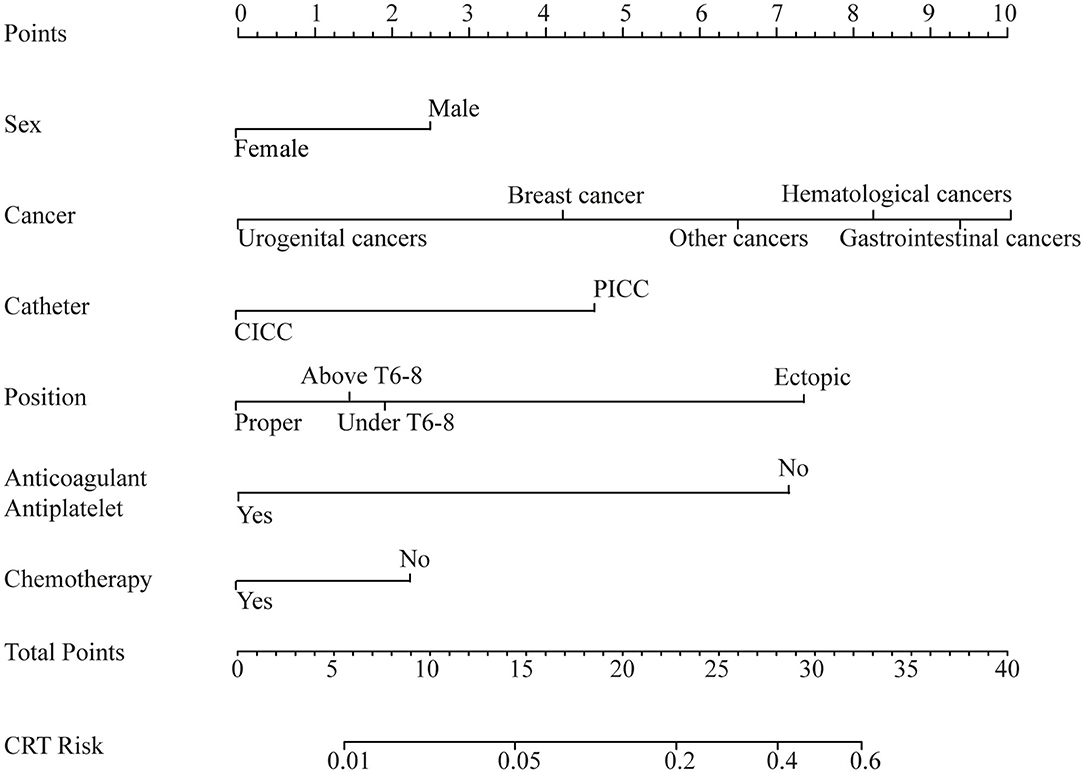
Figure 1. Nomogram. Nomogram of the predicted catheter-related thrombosis (CRT) risk in cancer patients.
The area under the ROC curve of our new prediction model was 0.741 (CI: 0.715–0.766) in the primary cohort (Figure 2A). The coefficient of determination (R2) of 0.138 and the results of the Pearson goodness-of-fit test and Hosmer–Lemeshow goodness of fit test were not significant (P = 0.138, df = 6). The calibration plot for CRT risk showed optimal agreement between our model's prediction and the actual observation (Figure 2B).
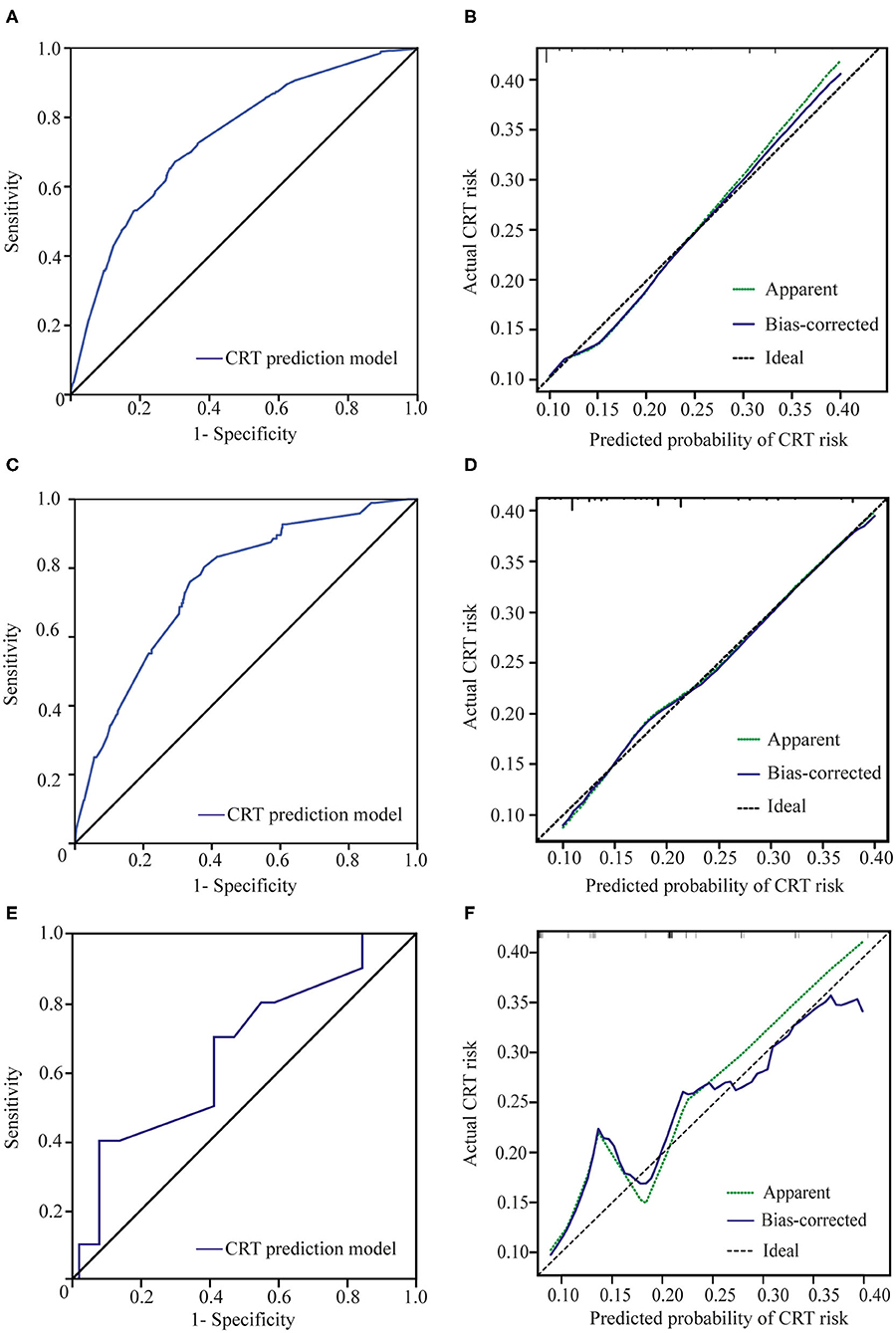
Figure 2. Receiver operating characteristic (ROC) curves and calibration plots. (A) ROC curve of the CRT prediction model in the primary cohort. (B) The calibration plot for the risk of CRT in the primary cohort showed optimal agreement between the prediction and actual observation. The apparent line is the in-sample calibration. The bias-corrected line is derived via 1,000 repetitions of bootstrapping. The ideal line represents a perfect prediction (the predicted probability equals the observed probability). (C) ROC curve of our prediction model in validation cohort 1. (D) The calibration plot in validation cohort 1 also showed optimal agreement between the prediction and actual observation. The apparent line is the in-sample calibration. The bias-corrected line is derived via 100 repetitions of bootstrapping. The ideal line represents a perfect prediction (the predicted probability equals the observed probability). (E) ROC curve of the CRT prediction model in validation cohort 2. (F) The calibration plot for the risk of CRT in validation cohort 2 showed good agreement between the prediction and actual observation. The apparent line is the in-sample calibration. The bias-corrected line is derived via 40 repetitions of bootstrapping. The ideal line represents a perfect prediction (the predicted probability equals the observed probability).
The maximum Youden index was 0.371 (sensitivity, 67.3%; specificity, 69.8%) at a score of 19.6. Patients with a score higher than 19.6 were considered at high risk of CRT. The incidence of CRT in the high-risk group was 24.5% (267/1,092), which was significantly higher than that in the low-risk group (6.4%, 130/2,039) (p < 0.001) (Figure 3).
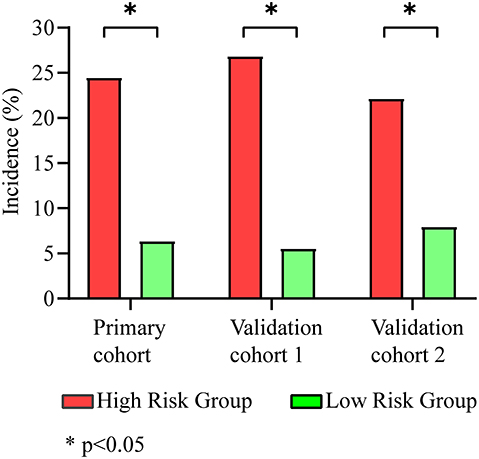
Figure 3. Incidence of CRT in different groups. The high-risk group had a higher incidence of CRTs than the low-risk group in the primary cohort (24.5 vs. 6.4%), validation cohort 1 (26.8 vs. 5.6%), and validation cohort 2 (22.2 vs. 8.0%).
Validation of the Clinical Prediction Model
Using our new prediction model, we first tested the performance of the model in a prospective validation cohort (validation cohort 1). The ROC curve and calibration plot of validation cohort 1 are shown in Figures 2C,D. The area under the ROC curve was 0.754 (CI: 0.704–0.803), the coefficient of determination (R2) was 0.214, and the results of the Pearson and Hosmer–Lemeshow goodness-of-fit tests were not significant (p = 0.875, df = 7). These results indicate that our model shows good discrimination and calibration. The incidence of thrombosis in the low-risk group was 5.6% (23/413), which was significantly lower than that in the high-risk group (26.8%, 73/272) (p < 0.001) (Figure 3).
Validation cohort 2 was a smaller cohort, and the area under the ROC curve also reached 0.658 (CI: 0.470–0.845). The calibration curve showed good agreement with the actual CRT risk (Figures 2E,F). The incidences of thrombosis in the low- and high-risk groups were 8.0% (2/25) and 22.2% (8/36), respectively (p < 0.001) (Figure 3).
Comparison of the CRT Prediction Model and the Khorana Risk Score Model
The Khorana risk score model is commonly used as a prediction tool for VTE in cancer patients. It is composed of five risk factors: the site of cancer, prechemotherapy platelet count, hemoglobin level (and/or use of erythropoiesis-stimulating agents), prechemotherapy leukocyte count, and body mass index (BMI) (16). The site of cancer (the type of cancer) was the only risk factor included in our analysis and incorporated into our new model for predicting CRT risk. However, in terms of predicting CRT risk in cancer patients, the Khorana risk score model did not perform very well. In our primary cohort, the area under the ROC curve of the Khorana risk score model was 0.539 (CI: 0.509–0.569), and the area under the ROC curve of our CRT prediction model was 0.741 (CI: 0.715–0.766), which was significantly higher than that of the Khorana risk score model (p < 0.001) (Figure 4A). The same result was obtained in validation cohort 1; the area under the ROC curve of the Khorana risk score model was 0.551 (CI: 0.490–0.611), and the area under the ROC curve of our CRT prediction model was 0.755 (CI: 0.705–0.804) (p < 0.001) (Figure 4B). However, the advantages of our new model over the Khorana risk score model were not statistically significant in validation cohort 2 (Figure 4C).
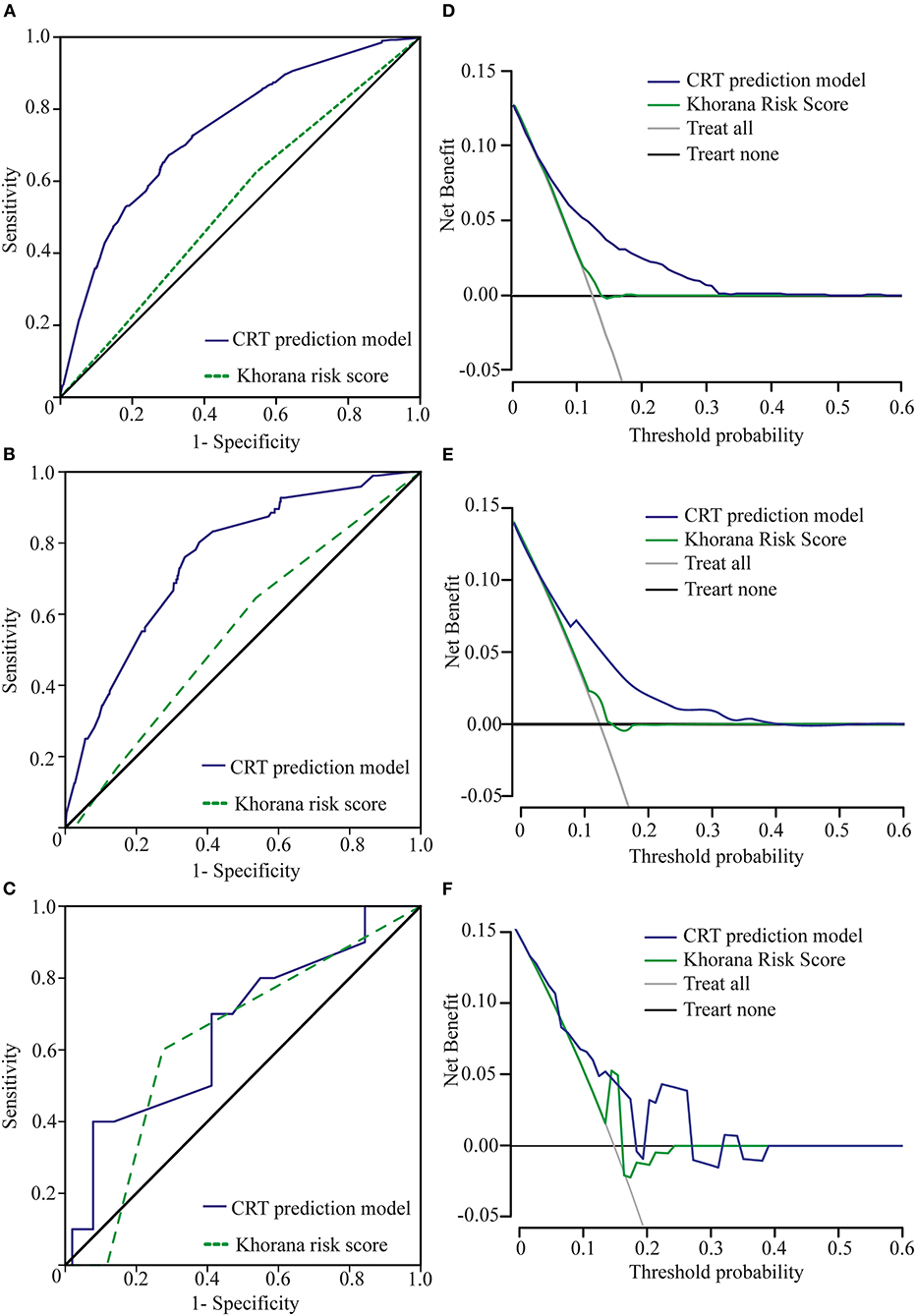
Figure 4. Comparison of ROC curves and net benefits between the Khorana risk score model and the new CRT prediction model. (A) ROC curve of the CRT prediction model and the Khorana risk score model in the primary cohort. (B) Our new model is the higher line on the decision curve, which indicates that the CRT prediction model leads to a higher net benefit than the Khorana risk score model in the primary cohort. (C) ROC curve of the CRT prediction model and the Khorana risk score model in validation cohort 1. (D) Our new model is the higher line on the decision curve, which indicates that the CRT prediction model leads to a higher net benefit than the Khorana risk score model in validation cohort 1. (E) ROC curve of the CRT prediction model and the Khorana risk score model in validation cohort 2. Due to the small sample size of validation cohort 2, there was no significant difference in the area under the ROC between the two groups. (F) Due to the small sample size of cohort 2, our new model is basically the higher line on the decision curve.
On the decision curve, our new model was the higher line, indicating that our new prediction model leads to a higher net benefit than the Khorana risk score model for cancer patients with catheters (22). The net benefit represented the balance between CRT risk and the potential cost of unnecessary thromboprophylaxis (Figures 4D–F).
Discussion
According to the Global Cancer Statistics, 18.1 million new cancer cases and 9.6 million cancer-related deaths occurred globally in 2018, and Asians accounted for nearly 50% of new cancer cases and nearly 70% of cancer-related deaths (23). In line with this global report, 4.3 million new cancer cases were reported in China in 2018 (24). Concerning the treatment of tumors, most patients inevitably need to use a venous catheter, resulting in a very large number of cancer patients at risk of CRT.
Several CRT prediction models have been reported but not externally validated (25, 26), and they did not give special attention to the cancer status/type. A number of prediction models for cancer-associated venous thromboembolism have been proposed in recent years (16, 27, 28). However, the catheter-related risk factors were not taken into consideration when developing these models. A CRT risk analysis in breast cancer patients and a CRT predictor analysis in cancer patients with central venous ports have also been reported in the literature (29, 30). However, without a scoring system to stratify individuals into different risk groups, knowing only the risk factors will make it difficult to put the knowledge into practice. Moreover, limited to a specific type of cancer or a certain type of catheter may increase the accuracy of a prediction model for a certain group of patients, but at the same time, it could be a significant trade-off of the scope of applications.
The duration of the present study was 4.5 years. In total, 3,877 patients were enrolled, and 36 variables of cancer patients were analyzed. All patients underwent vascular ultrasound to avoid false negatives, and all data were carefully documented. We developed a new clinical prediction model for CRT in cancer patients. Our novel prediction model was externally validated in two independent cohorts. Above all, we consider our risk prediction model to be reliable, and this approach is worth popularizing in clinical practice.
Potentially serious and life-threatening complications of CRT could lead to an inability to obtain blood samples, delays in therapy, prolonged hospitalization, frequent catheter replacement, and even death (31). Catheter-associated thrombosis is one of the most important complications. The incidence of CRT in cancer patients from different series varies from 2.4 to 61.5%, and the incidence of symptomatic thrombi also varies widely, from 0.3 to 28% (4, 5). Our study revealed that the total incidence of CRT was 12.7% (1.80/1,000 catheter days), consistent with previous research.
The risk factors for CRT reported in previous studies were numerous and controversial. A meta-analysis noted that none of the 25 studies included the same high-risk factors for CRT (32), showing how difficult it is to establish a CRT prediction model. Some scholars classified CRT risk factors as follows: patient, biomarker, treatment, catheter, technical, and vessel related (33). We found in our research that sex, the type of cancer, the type of venous catheter used, the CRT position of the catheter tip, chemotherapy initiated at inclusion, and the antiplatelet or anticoagulation status at baseline are closely related to CRT.
Regarding the incidence of CRT in patients with different types of cancer, we found that patients with thoracic cancer, gastrointestinal cancers, and hematological cancers were at a relatively high risk of thrombosis, while the lowest incidence was observed in patients with urogenital cancer. Lung cancer, gastrointestinal cancer, and lymphoma were associated with a high risk of CRT, which was in agreement with previous findings. However, the risk of CRT in patients with urogenital cancer was not consistent with that reported in the literature (16), which may be related to the inconsistency in the enrollment criteria and the methods of thrombus detection used.
The advantages and disadvantages of different catheters have been discussed for a long time. At present, the merits and demerits of PICCs and CICCs are still controversial. Owing to the limited evidence available, there is no clear preference between PICCs and CICCs for treatment in clinical guidelines (4, 5). In most studies, the use of PICCs was associated with higher rates of thrombosis (34–36). However, Cai et al. reported the opposite conclusion (37). Consistent with most previous studies, our multivariate analysis showed that patients with PICCs are more than twice as likely to develop thrombosis than patients with CICCs. Implanted access ports (PORTs) have been another commonly used device in recent years. The safety of the infusion port has been widely recognized. Compared with PORTs, PICCs are associated with a higher risk for catheter-related DVT and other adverse events (38, 39).
The relationship between the exact tip position of the catheter and thrombosis has rarely been reported. The tip of the catheter should be located at the lower third of the superior vena cava, at the cavoatrial junction, or the upper third of the right atrium. Improper positioning of the catheter tip is associated with a high risk of malfunction, venous thrombosis, vessel erosion, visceral complications, and other complications (34, 40, 41). Our study found that abnormal catheter positioning was closely related to thrombosis. Not locating the catheter tip in the superior vena cava posed three times greater CRT risk than locating the tip in a proper location. Currently, postoperative standard chest X-ray is an economical and reliable way to determine the location of the catheter (4, 5). However, even if the initial position is correct, mispositioning may occur later for many reasons, such as obesity, body movements, breathing movements, or variations in the venous anatomy (congenital or acquired) (42).
We found that chemotherapy was a risk factor for CRT, which is consistent with a few previous studies (43, 44). Some articles have shown that the CRT incidence in patients with parenteral nutrition is significantly high (45), but a similar conclusion was not reached in our study. No associations between radiotherapy, anti-infective therapy, and CRT were observed.
Our study also found that short catheter days may be protective for CRT. However, this finding could be biased. Because the catheter was removed ahead of time if a thrombus was detected, this reduced catheter days. Thus, we did not include catheter days in the multivariate analyses.
Some high-risk factors that were reported in previous studies were not identified in this study. For example, BMI, platelet count, and hemoglobin, which were included in the Khorana risk score as risk factors for VTE (16), were not associated with CRT risk in our model. A few recognized high-risk factors for chronic cardiovascular diseases, such as smoking, hypertension, and diabetes, have not been identified as high-risk factors for CRT (46). One possible explanation is that, unlike cardiovascular diseases, which are caused mainly by long-term chronic conditions, for patients who have a relatively short catheterization duration, their risk is closely related to the primary tumor and characteristics of the catheter itself. Surprisingly, the D-dimer concentration had no effect on predicting CRT. Although many studies have agreed that the D-dimer concentration is positively correlated with symptomatic DVT, the performance of the CRT prediction model based on the D-dimer concentration was poor in cancer patients (47). The main reason for this finding was that the D-dimer concentration is generally high in cancer patients, especially in patients with terminal-stage cancer.
As mentioned above, the Khorana risk score is widely used to predict the risk of VTE in cancer patients. As shown in our study, the use of the Khorana risk score in assessing CRT risk is extremely limited. Our risk prediction model showed good discrimination and calibration. Cancer patients were accurately divided into a high- or low-risk group. External validation also confirmed the reliability of our model.
Current guidelines do not recommend routine prophylaxis with anticoagulants to prevent CRT. The approach of systemic anticoagulation has not shown any solid evidence of decreasing CRT incidence (4, 5). Our next research goal may be to evaluate the benefits of routine prophylaxis in high-risk patients selected by our model.
This study has several limitations undermining its generalizability. First, the primary cohort in this open-label study was from a single center. It is possible that our study does not reflect the full spectrum of cancer patients. Second, this study was observational, and whether early interventions, such as antiplatelet therapy, would have changed the clinical outcomes is still unknown. Further investigations should be carried out.
Conclusions
In conclusion, we developed and validated a new risk prediction model for CRT in cancer patients. It is easy to use and available as a paper-based nomogram. This simple model was able to accurately discriminate patients at high and low risks of CRT. The use of this model could help clinicians make decisions regarding prophylaxis in cancer patients and provide clues for the early monitoring and detection of thrombotic events.
“A stitch in time may save nine.”—Thomas Fuller's Gnomologia, Adagies and Proverbs, Wise Sentences and Witty Sayings, Ancient and Modern, Foreign and British, 1732.
Data Availability Statement
All datasets presented in this study are included in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Boards of Cancer Hospital, Chinese Academy of Medical Sciences. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
BL, FM, and YZ conceptualized and designed the study. JX and ZYu were responsible for the catheterization. JX, YZ, BL, XS, XL, and YW acquired the data. BL, ZH, and ZYi analyzed the data. JX and ZYi gave administrative, technical, or material support. BL drafted the manuscript. All authors were responsible for the critical revision of the manuscript and reviewing.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2020.571227/full#supplementary-material
Supplementary Figure 1. Flow chart. Flow chart of patient enrollment.
Supplemental Table 1. Baseline examination of cases.
References
1. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JWW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. (2013) 49:1374–403. doi: 10.1016/j.ejca.2012.12.027
2. Sturgeon KM, Lei D, Bluethman SM, Shouhao Z, Trifiletti DM, Changchuan J, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. (2019) 40:3889–97. doi: 10.1093/eurheartj/ehz766
3. Herrmann J. From trends to transformation: where cardio-oncology is to make a difference. Eur Heart J. (2019) 40:3898–900. doi: 10.1093/eurheartj/ehz781
4. Schiffer CA, Mangu PB, Wade JC, Camp-Sorrell D, Cope DG, El-Rayes BF, et al. Central venous catheter care for the patient with cancer: American society of clinical oncology clinical practice guideline. J Clin Oncol. (2013) 31:1357–70. doi: 10.1200/JCO.2012.45.5733
5. Sousa B, Furlanetto J, Hutka M, Gouveia P, Wuerstlein R, Mariz JM, et al. Central venous access in oncology: ESMO clinical practice guidelines. Ann Oncol. (2015) 26:v152–68. doi: 10.1093/annonc/mdv296
6. Khorana AA, Mones J, Soff GA. Preventing venous thromboembolism in patients with cancer. Reply. N Engl J Med. (2019) 380:2181. doi: 10.1056/NEJMc1904003
7. Karthaus M, Kretzschmar A, Kröning H, Biakhov M, Irwin D, Marschner N, et al. Dalteparin for prevention of catheter-related complications in cancer patients with central venous catheters: final results of a double-blind, placebo-controlled phase III trial. Ann Oncol. (2006) 17:289–96. doi: 10.1093/annonc/mdj059
8. Lavau-Denes S, Lacroix P, Maubon A, Preux PM, Genet D, Vénat-Bouvet L, et al. Prophylaxis of catheter-related deep vein thrombosis in cancer patients with low-dose warfarin, low molecular weight heparin, or control: a randomized, controlled, phase III study. Cancer Chemother Pharmacol. (2013) 72:65–73. doi: 10.1007/s00280-013-2169-y
9. Niers TM, Di Nisio M, Klerk CP, Baarslag HJ, Büller HR, Biemond BJ. Prevention of catheter-related venous thrombosis with nadroparin in patients receiving chemotherapy for hematologic malignancies: a randomized, placebo-controlled study. J Thromb Haemost. (2007) 5:1878–82. doi: 10.1111/j.1538-7836.2007.02660.x
10. Verso M, Agnelli G, Kamphuisen PW, Ageno W, Bazzan M, Lazzaro A, et al. Risk factors for upper limb deep vein thrombosis associated with the use of central vein catheter in cancer patients. Intern Emerg Med. (2008) 3:117–22. doi: 10.1007/s11739-008-0125-3
11. Kahale LA, Tsolakian IG, Hakoum MB, Matar CF, Barba M, Yosuico VE, et al. Anticoagulation for people with cancer and central venous catheters. Cochrane Database Syst Rev. (2018) 6:CD006468. doi: 10.1002/14651858.CD006468.pub6
12. Kumar DR, Hanlin E, Glurich I, Mazza JJ, Yale SH. Virchow's contribution to the understanding of thrombosis and cellular biology. Clin Med Res. (2010) 8:168–72. doi: 10.3121/cmr.2009.866
13. Piper R, Carr PJ, Kelsey LJ, Bulmer AC, Keogh S, Doyle BJ. The mechanistic causes of peripheral intravenous catheter failure based on a parametric computational study. Sci Rep. (2018) 8:3441. doi: 10.1038/s41598-018-21617-1
14. Carrier M, Abou-Nassar K, Mallick R, Tagalakis V, Shivakumar S, Schattner A, et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. (2019) 380:711–9. doi: 10.1056/NEJMoa1814468
15. Khorana AA, Soff GA, Kakkar AK, Vadhan-Raj S, Riess H, Wun T, et al. Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med. (2019) 380:720–8. doi: 10.1056/NEJMoa1814630
16. Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. (2008) 111:4902–7. doi: 10.1182/blood-2007-10-116327
17. Carrier M, Khorana AA, Moretto P, Le Gal G, Karp R, Zwicker JI. Lack of evidence to support thromboprophylaxis in hospitalized medical patients with cancer. Am J Med. (2014) 127:82–6. doi: 10.1016/j.amjmed.2013.09.015
18. Citla SD, Abou-Ismail MY, Ahuja SP. Central venous catheter-related thrombosis in children and adults. Thromb Res. (2020) 187:103–12. doi: 10.1016/j.thromres.2020.01.017
19. Dentali F, Ageno W, Giorgi PM, Imberti D, Malato A, Nitti C, et al. Prognostic relevance of an asymptomatic venous thromboembolism in patients with cancer. J Thromb Haemost. (2011) 9:1081–3. doi: 10.1111/j.1538-7836.2011.04259.x
20. Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. (2008) 3:17. doi: 10.1186/1751-0473-3-17
21. Heus P, Reitsma JB, Collins GS, Damen J, Scholten R, Altman DG, et al. Transparent reporting of multivariable prediction models in journal and conference abstracts: TRIPOD for Abstracts. Ann Intern Med. (2020). doi: 10.7326/M20-0193. [Epub ahead of print].
22. Kerr KF, Brown MD, Zhu K, Janes H. Assessing the clinical impact of risk prediction models with decision curves: guidance for correct interpretation and appropriate use. J Clin Oncol. (2016) 34:2534–40. doi: 10.1200/JCO.2015.65.5654
23. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
24. Feng R, Zong Y, Cao S, Xu R. Current cancer situation in China: good or bad news from the 2018 global cancer statistics? Cancer Commun. (2019) 39:22. doi: 10.1186/s40880-019-0368-6
25. Hao N, Xie X, Zhou Z, Li J, Kang L, Wu H, et al. Nomogram predicted risk of peripherally inserted central catheter related thrombosis. Sci Rep. (2017) 7:6344. doi: 10.1038/s41598-017-06609-x
26. Chopra V, Kaatz S, Conlon A, Paje D, Grant PJ, Rogers MAM, et al. The michigan risk score to predict peripherally inserted central catheter-associated thrombosis. J Thromb Haemost. (2017) 15:1951–62. doi: 10.1111/jth.13794
27. Pabinger I, van Es N, Heinze G, Posch F, Riedl J, Reitter EM, et al. A clinical prediction model for cancer-associated venous thromboembolism: a development and validation study in two independent prospective cohorts. Lancet Haematol. (2018) 5:e289–98. doi: 10.1016/S2352-3026(18)30063-2
28. Ferroni P, Zanzotto FM, Scarpato N, Riondino S, Guadagni F, Roselli M. Validation of a machine learning approach for venous thromboembolism risk prediction in oncology. Dis Markers. (2017) 2017:8781379. doi: 10.1155/2017/8781379
29. Tan L, Sun Y, Zhu L, Lei X, Liang D, Rao N, et al. Risk factors of catheter-related thrombosis in early-stage breast cancer patients: a single-center retrospective study. Cancer Manag Res. (2019) 11:8379–89. doi: 10.2147/CMAR.S212375
30. Hohl MC, Périard D, Grueber A, Hayoz D, Magnin JL, André P, et al. Predictors of venous thromboembolic events associated with central venous port insertion in cancer patients. J Oncol. (2014) 2014:743181. doi: 10.1155/2014/743181
31. Journeycake JM, Buchanan GR. Catheter-related deep venous thrombosis and other catheter complications in children with cancer. J Clin Oncol. (2006) 24:4575–80. doi: 10.1200/JCO.2005.05.5343
32. Leung A, Heal C, Perera M, Pretorius C. A systematic review of patient-related risk factors for catheter-related thrombosis. J Thromb Thrombolys. (2015) 40:363–73. doi: 10.1007/s11239-015-1175-9
33. Boersma RS, Jie KSG, Verbon A, van Pampus ECM, Schouten HC. Thrombotic and infectious complications of central venous catheters in patients with hematological malignancies. Ann Oncol. (2008) 19:433–42. doi: 10.1093/annonc/mdm350
34. Murray J, Precious E, Alikhan R. Catheter-related thrombosis in cancer patients. Brit J Haematol. (2013) 162:748–57. doi: 10.1111/bjh.12474
35. Günther SC, Schwebel C, Hamidfar-Roy R, Bonadona A, Lugosi M, Ara-Somohano C, et al. Complications of intravascular catheters in ICU: definitions, incidence and severity. A randomized controlled trial comparing usual transparent dressings versus new-generation dressings (the ADVANCED study). Intensive Care Med. (2016) 42:1753–65. doi: 10.1007/s00134-016-4582-2
36. Refaei M, Fernandes B, Brandwein J, Goodyear MD, Pokhrel A, Wu C. Incidence of catheter-related thrombosis in acute leukemia patients: a comparative, retrospective study of the safety of peripherally inserted vs. centrally inserted central venous catheters. Ann Hematol. (2016) 95:2057–64. doi: 10.1007/s00277-016-2798-4
37. Fracchiolla NS, Todisco E, Bilancia A, Gandolfi S, Orofino N, Guidotti F, et al. Clinical management of peripherally inserted central catheters compared to conventional central venous catheters in patients with hematological malignancies: a large multicenter study of the REL GROUP (rete ematologica lombarda - lombardy hematologic network, Italy). Am J Hematol. (2017) 92:E656–9. doi: 10.1002/ajh.24903
38. Taxbro K, Hammarskjöld F, Thelin B, Lewin F, Hagman H, Hanberger H, et al. Clinical impact of peripherally inserted central catheters vs implanted port catheters in patients with cancer: an open-label, randomised, two-centre trial. Br J Anaesth. (2019) 122:734–41. doi: 10.1016/j.bja.2019.01.038
39. Decousus H, Bourmaud A, Fournel P, Bertoletti L, Labruyère C, Presles E, et al. Cancer-associated thrombosis in patients with implanted ports: a prospective multicenter French cohort study (ONCOCIP). Blood. (2018) 132:707–16. doi: 10.1182/blood-2018-03-837153
40. Rosovsky RP, Kuter DJ. Catheter-related thrombosis in cancer patients: pathophysiology, diagnosis, and management. Hematol Oncol Clin North Am. (2005) 19:183–202. doi: 10.1016/j.hoc.2004.09.007
41. Pittiruti M, Bertollo D, Briglia E, Buononato M, Capozzoli G, De Simone L, et al. The intracavitary ECG method for positioning the tip of central venous catheters: results of an Italian multicenter study. J Vasc Access. (2012) 13:357–65. doi: 10.5301/JVA.2012.9020
42. Roldan CJ, Paniagua L. Central venous catheter intravascular malpositioning: causes, prevention, diagnosis, and correction. West J Emerg Med. (2015) 16:658–64. doi: 10.5811/westjem.2015.7.26248
43. Bertoglio S, Faccini B, Lalli L, Cafiero F, Bruzzi P. Peripherally inserted central catheters (PICCs) in cancer patients under chemotherapy: a prospective study on the incidence of complications and overall failures. J Surg Oncol. (2016) 113:708–14. doi: 10.1002/jso.24220
44. Chopra V, Anand S, Hickner A, Buist M, Rogers MA, Saint S, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. (2013) 382:311–25. doi: 10.1016/S0140-6736(13)60592-9
45. Reitzel RA, Rosenblatt J, Chaftari AM, Raad II. Epidemiology of infectious and noninfectious catheter complications in patients receiving home parenteral nutrition: a systematic review and meta-analysis. JPEN J Parenter Enteral Nutr. (2019) 43:832–51. doi: 10.1002/jpen.1609
46. Wang Y, Liu J, Wang W, Wang M, Qi Y, Xie W, et al. Lifetime risk for cardiovascular disease in a Chinese population: the Chinese multi-provincial cohort study. Eur J Prev Cardiol. (2015) 22:380–8. doi: 10.1177/2047487313516563
47. Nañez-Terreros H, Jaime-Perez JC, Muñoz-Espinoza LE, Camara-Lemaroy CR, Ornelas-Cortinas GE, Ramos-Dena RD, et al. D-dimer from central and peripheral blood samples in asymptomatic central venous catheter-related thrombosis in patients with cancer. Phlebology. (2019) 34:52–7. doi: 10.1177/0268355518772171
Keywords: catheters, thrombosis, nomogram, risk factor, cancer
Citation: Liu B, Xie J, Sun X, Wang Y, Yuan Z, Liu X, Huang Z, Wang J, Mo H, Yi Z, Guan X, Li L, Wang W, Li H, Ma F and Zeng Y (2020) Development and Validation of a New Clinical Prediction Model of Catheter-Related Thrombosis Based on Vascular Ultrasound Diagnosis in Cancer Patients. Front. Cardiovasc. Med. 7:571227. doi: 10.3389/fcvm.2020.571227
Received: 02 July 2020; Accepted: 18 September 2020;
Published: 26 October 2020.
Edited by:
Carlo Gabriele Tocchetti, University of Naples Federico II, ItalyReviewed by:
Giuseppina Novo, University of Palermo, ItalyChristian Cadeddu Dessalvi, University of Cagliari, Italy
Copyright © 2020 Liu, Xie, Sun, Wang, Yuan, Liu, Huang, Wang, Mo, Yi, Guan, Li, Wang, Li, Ma and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Ma, ZHJtYWZlaUAxMjYuY29t; Yixin Zeng, emVuZ3l4QHN5c3VjYy5vcmcuY24=
 Binliang Liu1
Binliang Liu1 Hongnan Mo
Hongnan Mo Xiuwen Guan
Xiuwen Guan Lixi Li
Lixi Li Fei Ma
Fei Ma