- 1Graduate School of Biomedical Sciences, Health Sciences, Nagasaki University, Nagasaki, Japan
- 2Department of Occupational Therapy, School of Health Sciences, Sapporo Medical University, Sapporo, Japan
- 3Faculty of Medicine, Kagoshima University, Kagoshima, Japan
- 4Center for Environmental and Health Sciences, Hokkaido University, Sapporo, Japan
- 5Medical Center for Dementia, Ebetsu City Hospital, Ebetsu, Japan
- 6Department of Neuropsychiatry, School of Medicine, Sapporo Medical University, Sapporo, Japan
- 7Department of Physical Therapy, School of Health Sciences, Sapporo Medical University, Sapporo, Japan
Introduction: Early detection of objective cognitive impairment is essential to delay or prevent dementia; however, traditional in-person screening often faces practical barriers, including limited accessibility and substantial personnel demands. Web-based cognitive tools are promising for scalable screening. This study aimed to identify cognitive subgroups among community-dwelling older adults using latent class analysis (LCA) based on data collected through a freely accessible web-based cognitive screening platform that enables convenient participation anytime and anywhere using older adults' own mobile devices.
Methods: Between September and December 2024, adults aged ≥65 from Sapporo and Ebetsu, Japan, were recruited via newspaper insert flyers (92,290 households) and community posters. QR codes linked to the study website were optimized for various devices. After obtaining electronic consent, participants completed web-based demographic surveys and cognitive assessments of memory, attention, and processing speed. Subjective health and memory complaints were recorded. LCA identified cognitive subgroups based on performance, complaints, and sociodemographic factors.
Results: Among the 528 participants (mean age = 71.2; 57% female), most reported good health (86%) and daily conversations (92%). Cognitive function was generally preserved. LCA revealed four clusters: socially isolated females with high subjective memory complaints (SMCs); cohabiting males with high SMCs; cohabiting females with high health perception and preserved cognition; and older adults with cognitive decline.
Discussion: The combination of mass outreach and web-based screening is feasible and effective in identifying diverse cognitive profiles. These findings highlight the mismatch between subjective and objective cognition and the relationship between social context, supporting scalable, tailored approaches and cognitive health.
Introduction
The increasing prevalence of cognitive decline and dementia is a major public health challenge worldwide, particularly in rapidly aging societies. Given that early intervention strategies may delay or prevent progression to dementia, the timely identification of at-risk individuals has emerged as a paramount public health priority (1, 2). However, conventional cognitive screening approaches face substantial implementation barriers, which limit their population-level effectiveness. Traditional assessments typically require in-person attendance at clinical facilities or community centers, creating significant accessibility challenges for older adults with mobility limitations, transportation difficulties, or caregiving obligations (3, 4). Furthermore, venue-based screening may introduce a systematic selection bias, as participants tend to be more socially engaged, health-conscious, and physically capable compared to the broader older adult population (5, 6), potentially underestimating the true prevalence of cognitive impairment in the community.
Advances in digital and mobile health (mHealth) technologies have provided promising opportunities for overcoming these limitations. Although smartphone and Internet use among adults aged ≥65 years were limited a decade ago, recent surveys indicate that over 60% now own smartphones and over 70% regularly use the Internet (7–10). This digital transformation has enabled the development of web-based cognitive screening tools offering scalable, cost-effective, and geographically accessible alternatives to traditional assessment methods (11–19). These platforms can be deployed across diverse populations, including underserved communities with limited access to specialized geriatric services, while allowing participants to complete assessments in familiar environments at their convenience.
However, alternative recruitment approaches that can reach broader and more diverse older adult populations are urgently required, particularly due to population aging and the growing demand for inclusive public health interventions. Community-wide, large-scale recruitment strategies, such as newspaper insert flyers, local media campaigns, and widespread poster distributions, offer promising solutions by reaching individuals who may not actively seek health information or participate in organized activities (20). Furthermore, these methods are especially valuable for reaching socially isolated, technologically underserved, or otherwise marginalized older adults, who are often underrepresented in health research. By leveraging familiar and accessible communication channels, such as printed materials in community centers, supermarkets, and local newspapers, these strategies align with the public health principles of equity and population-based outreach. Their effectiveness may be further enhanced when combined with user-friendly digital platforms that minimize technological barriers and support broader participation across varying levels of digital literacy.
Despite these advancements, widespread adoption of web-based cognitive screening among older adults remains limited. Several factors may have contributed to this discrepancy. First, disparities in digital literacy and self-efficacy persist, with many older adults lacking confidence in navigating online platforms, even when device ownership is high (21, 22). Second, concerns about privacy, data security, and the potential misuse of cognitive health information can deter participation, particularly in cultures with strong preferences for in-person medical interactions (23). Third, limitations in physical, cognitive, or sensory abilities, such as visual impairment, reduced fine motor control, or mild cognitive impairment, may hinder the independent use of digital tools despite their user-centered design features, creating usability challenges (24, 25). Incorporating privacy-preserving online assessment environments and usability-focused design features may mitigate these barriers. Classifying older adults based on a combination of socio-demographic factors, cognitive performance measured via an online platform, and subjective health perceptions may provide valuable insights into designing tailored interventions and support strategies for an increasingly digital society.
The cognitive profiles of community-dwelling older adults may exhibit considerable heterogeneity when examined in relation to their subjective health perceptions and memory concerns, suggesting that objective cognitive performance alone may not fully capture the complexity of cognitive aging in real-world contexts. Accumulating evidence indicates that subjective health status is an important determinant of cognitive functioning, with individuals reporting better perceived health and demonstrating superior performance across multiple cognitive domains (26–28). Subjective memory complaints (SMCs) are another critical dimension that may modify the cognitive profiles of older adults, even in the absence of objectively detectable impairment. Studies have consistently demonstrated that individuals who report subjective memory difficulties exhibit distinct neuropsychological patterns (29–31). Moreover, SMCs are potential early indicators of future cognitive decline, with several prospective studies demonstrating that older adults with persistent memory complaints show accelerated rates of cognitive deterioration and an increased risk of progression to mild cognitive impairment and dementia (32–34). The multifaceted interplay between cognitive performance, subjective health, and memory concerns underscores the need for thorough profiling of cognitive aging trajectories in broadly sampled older populations.
Given these methodological and public health challenges, this study aimed to address a new strategy for community-based cognitive screening by integrating large-scale recruitment strategies and web-based assessment tools. Specifically, we used community-wide newspaper flyers and poster displays to recruit a more representative sample of older adults, followed by a comprehensive digital cognitive evaluation accessible from the participants' homes. Our primary objective was to characterize the heterogeneity of cognitive aging within a broadly recruited community sample using latent class analysis (LCA).
Methods
Participants
Participants were recruited between September and December 2024 from Sapporo and Ebetsu in Hokkaido, Japan, using a two-pronged recruitment strategy designed to maximize population reach while ensuring methodological feasibility. First, recruitment flyers were distributed to 92,290 households through newspaper insert flyers in Hokkaido Shimbun, the most widely circulated daily newspaper in the region, providing broad geographic coverage in urban and suburban areas. Second, recruitment posters were strategically displayed in community centers located in six wards of Sapporo City (Kita, Higashi, Nishi, Shiroishi, Teine, and Atsubetsu) and three districts of Ebetsu City (Ebetsu, Oasa, and Nopporo). These locations were selected based on feasibility considerations in this study. This dual-modality approach was designed to reach individuals who primarily receive health information through traditional media channels and those who frequent community gathering spaces.
Procedures of digital-based assessment
Recruitment flyers and posters prominently featured a quick response code (QR code; a matrix barcode that encodes information in a two-dimensional pattern) that provided direct access to the study website, eliminating barriers associated with manual URL entry and facilitating a seamless transition from recruitment materials to study participation. The website was optimized for accessibility across multiple devices, including smartphones and tablet computers, with responsive design features to accommodate varying levels of technological proficiency among the participants. Upon accessing the site, potential participants were presented with a comprehensive informed consent page that detailed the study objectives, procedures, potential risks and benefits, data handling protocols, and participants' rights, including the ability to withdraw at any time without penalty.
Following electronic informed consent, the participants were automatically directed to complete a structured demographic questionnaire that captured key sociodemographic variables, followed immediately by a web-based cognitive assessments. The assessment platform incorporated user-friendly design elements, including large fonts, high-contrast displays, and intuitive navigation features. To ensure data integrity and participant privacy, all responses were encrypted during transmission and automatically stored on a secure server upon the completion of each assessment module.
Following the completion of all the cognitive assessments, the participants received immediate feedback displayed on the screen. Based on a large normative sample of 19,000 community-dwelling older adults maintained by the National Center for Geriatrics and Gerontology, each participant's performance was evaluated using age- and sex-specific means and standard deviations. This allowed the classification of cognitive function as either within the expected range or indicative of a potential decline. In cases in which a marked decline was detected, the system provided navigational guidance, recommending follow-up at specialized medical institutions that focused on dementia diagnosis and care. All contents of the study website intended for participants, including the electronic consent page, questionnaires, task instructions, and on-screen feedback, were presented in Japanese.
One advantage of this approach is that the entire cognitive assessment can be completed free of charge using a personal device, allowing participants to manage the process independently without necessarily disclosing the results to family members. When the cognitive function is preserved, feedback may enhance confidence in daily lifestyle habits. Conversely, when early signs of decline are detected, assessment can facilitate timely awareness and prevention, thereby supporting proactive engagement in cognitive health management.
Inclusion and exclusion criteria
Participants were considered eligible for inclusion based on a comprehensive set of criteria designed to capture a representative sample of community-dwelling older adults, while ensuring data quality and analytical validity. The inclusion criteria were as follows: (1) chronological age of ≥65 years at the time of assessment; (2) absence of self-reported history of major neurological or psychiatric conditions that could impact cognitive performance, such as stroke, Parkinson's disease, clinically diagnosed depression, dementia, or mild cognitive impairment (MCI); (3) functional independence in basic activities of daily living, including eating, grooming, ambulation, bathing, and stair climbing; (4) absence of formal certification for long-term care services under the Japan's Long-Term Care Insurance system; (5) cognitive test performance within acceptable statistical bounds, defined as scores within ±3.0 standard deviations (SD) from the sample mean to exclude extreme outliers; and (6) legitimate study participation, verified by excluding registrations with usernames containing the string “Test,” which likely represented practice entries or system testing. To maintain data integrity and ensure independent observations, duplicate entries from the same individual were identified and resolved by retaining only the earliest complete record for analysis. Figure 1 shows a flowchart of the participant selection process.
Ethical considerations
Prior to participation, all individuals were presented with an online informed consent form detailing the purpose, procedures, data handling, and voluntary nature of the study. Only those who provided electronic consent were permitted to participate in the demographic survey and cognitive assessment.
This study was approved by the Ethics Committee of Sapporo Medical University (Approval No. 6-1-20; August 20, 2024). All procedures were conducted in accordance with the ethical standards of the Institutional Research Committee and principles outlined in the Declaration of Helsinki.
Cognitive assessment
Cognitive function was assessed using a web-based version of the National Center for Geriatrics and Gerontology-Functional Assessment Tool (NCGG-FAT). The NCGG-FAT has been validated as a reliable screening tool for detecting cognitive decline in older adults, and its utility in large-scale community settings has been demonstrated by Makizako et al. (35). The web-based version offers several advantages, including compatibility with various platforms such as smartphones and tablets, regardless of the operating system. The new assessment tool evaluates three domains: memory, attention, and processing speed. Additionally, as long as Internet access is available, the assessment can be conducted anytime and anywhere, enhancing its accessibility and scalability for both clinical and community-based applications.
Memory
Memory was assessed using two components: immediate recognition and delayed recall. Participants were instructed to memorize a list of 10 target words, each displayed for 2 s on a tablet screen. Thereafter, they were presented with a set of 30 words, including 10 target words and 20 distractors, and asked to identify the original words. This task was repeated three times, and the total number of correct selections across the three trials (range: 0–30) was recorded as the immediate recognition score. Approximately 20 min later, the participants were asked to recall the 10 target words. Each word correctly recalled within 60 s was awarded one point, yielding a delayed recall score (range: 0–10). Immediate recognition and delayed recall scores were evaluated for potential decline based on a cutoff of 1.0 SD below the overall participants mean score. For memory classification, the participants were categorized into one of the following three groups: robust, impairment in either immediate recognition or delayed recall, or impairment in both domains.
Attention
To assess attentional function, digit-span tasks were administered in forward and backward formats. The test began with two-digit sequences. After the digits were presented sequentially on the screen, the participants were instructed to input the memorized sequence by tapping the digits on the screen. In the forward digit span task, participants tapped the digits in the same order as they were presented, whereas in the backward digit span task, they tapped the digits in a reverse order. When a participant correctly responded to two trials with the same digit length, the sequence length increased by one digit. The test was terminated if the participant failed two consecutive trials of the same length. The number of correctly completed trials was recorded separately for the forward and backward tasks and used as an index of attentional capacity. Forward and backward digit span scores were evaluated for potential attentional impairment using a cutoff of 1.0 SD below the overall participant mean for each task. For attentional classification, the participants were categorized into one of three groups: robust, impairment in either the forward or backward digit span, or impairment in both domains.
Processing speed
Processing speed was assessed using the Symbol Digit Substitution Task. Nine symbol–number pairs were displayed at the top of the screen. A target symbol appeared at the center, and the participants selected the corresponding number from the options shown below. The score was determined by the number of correct matches completed within 90 s, with one point awarded for each correct response. In the present study, processing speed impairment was defined as a score falling 1.0 SD below the overall participant mean.
Subjective health
Participants were asked to respond to a single item assessing subjective health status: “Do you consider yourself to be in good health?” Responses were recorded as either “Yes” or “No.” Those who responded “No” were classified as having subjective health complaints.
SMCs
SMCs were assessed using four self-report items commonly used in geriatric cognitive screening (36, 37). Participants were asked whether they felt that they had problems with their memory; whether they found themselves forgetting where they placed things more often than before; whether they sometimes forgot the names of close friends or acquaintances; and whether others had told them that they had become forgetful. Each item was answered with either “Yes” or “No”. The number of affirmative responses was summed to generate an SMC score of 0–4, with higher scores indicating greater perceived memory difficulty.
Sociodemographic Status
Participants provided information on their age, sex, living arrangements, and years of education. Age was categorized into six groups: 65–69, 70–74, 75–79, 80–84, 85–89, and ≥90 years. Sex was recorded as “female”, “male”, or “other/no response”. Living arrangements were assessed by asking the participants whether they lived alone or with others. As part of the sociodemographic assessment, participants were asked to respond to a single item: “Do you have conversations with someone every day?” Responses were recorded as either “Yes” or “No”. Regular social interactions, including daily conversations, are associated with better cognitive functioning in older adults (38). Besides, spontaneous everyday speech activates frontal brain regions and contributes to cognitive engagement (39). Given this evidence, daily conversation was included as a relevant variable in the cognitive health analysis. These variables were used to characterize the participants and included as covariates in the LCA to explore their associations with cognitive profiles and subjective perceptions of health and memory. Although educational level was recorded, it was not included as a classification variable in the LCA. This decision was based on the primary objective of the study, which was to identify subgroups derived from cognitive performance and key social and behavioral factors, rather than from background demographic characteristics such as education. Information on years of education was, however, used in subsequent post-hoc analyses to further characterize the identified clusters.
Statistical analysis
LCA was conducted to identify distinct subgroups of participants based on their cognitive performance, SMCs, and sociodemographic characteristics. LCA is a probabilistic technique that identifies unobserved subgroups (latent classes) based on shared response patterns. It is particularly well suited to datasets comprising discrete and binary variables, and it offers advantages over traditional clustering or factor analytic methods by accommodating non-continuous data structures (40). The following variables were included in the model: age, sex, living arrangement, subjective health complaints, daily conversations, number of SMCs, memory performance, working memory performance, and processing speed. Models with two to 10 latent classes were estimated. Model fit was evaluated using the Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), likelihood ratio chi-square statistic (G²), and clinical interpretability. The optimal number of classes was determined based on the lowest AIC and BIC values as well as the clinical relevance and distinctiveness of the identified subgroups. Participants were assigned to the cluster with the highest posterior probability of membership. Descriptive statistics were used to characterize each cluster, and class labels were determined from class-specific item-response profiles and descriptive statistics. Continuous variables are presented as medians with interquartile ranges (IQRs), whereas categorical variables are reported as counts and percentages. post-hoc analyses were also conducted to examine differences in years of education across the identified clusters. An overall group difference assessment was performed using a Kruskal–Wallis test, followed by a Dwass–Steel–Critchlow–Fligner test to identify differences between clusters.
All analyses were conducted using R ver. 4.4.0 (R Foundation for Statistical Computing, Vienna, Austria), with LCA models implemented via the “poLCA” package ver. 1.4.1 (41). The significance level was set at p < .05.
Results
Characteristics of the participants
In total, 528 older adults were included in this study. The mean age was 71.2 years (SD = 4.8), and 57% of the participants were female. Table 1 presents the participants' characteristics. Most participants lived with others (86%) and reported good health (86%). The majority engaged in daily conversations (92%), and SMCs were distributed across the sample, with 11% reporting no complaints and 11% reporting all four items.
Regarding cognitive performance, 72% of the participants showed no memory impairment, 21% had mild impairment in either immediate or delayed recall, and 6.3% had impairments in both. Working memory was intact in 79% of the participants, with 16% showing a mild decline and 5.5% showing impairment in both the forward and backward digit span tasks. Processing speed was preserved in 87% of the participants, whereas 13% demonstrated reduced performance.
Identification of clusters via LCA
LCA identified a four-cluster model as the optimal solution based on model fit indices and clinical interpretability (Table 2). The four-cluster model demonstrated the lowest AIC and favorable goodness-of-fit statistics compared with models with fewer or more classes. Furthermore, clinical interpretability was supported by distinct and coherent profiles observed across the four clusters, each reflecting meaningful combinations of cognitive performance, SMCs, and sociodemographic characteristics. Each participant was assigned to the cluster with the highest posterior probability of membership.
Table 3 summarizes the characteristics of each cluster after the participants were classified into the cluster with the highest posterior probability based on the LCA results. The labeling of the clusters was determined by examining the most salient sociodemographic, behavioral, and cognitive features of each cluster.
Cluster 1: socially isolated females with high SMCs
This small but distinct subgroup consisted predominantly of women (84%) who lived alone (100%). Although most participants reported good self-rated health (84%), this cluster exhibited the highest prevalence of daily social isolation, with 79% not engaging in daily conversations. Notably, participants reported elevated levels of SMCs, despite showing largely preserved objective cognitive performance. A mild decline in attention was observed (24%) but did not reach the levels seen in Cluster 4.
Cluster 2: cohabiting males with high SMCs
The largest cluster was composed mainly of cohabiting men (71%) with high rates of daily conversation (99%) and self-reported good health (86%). Objective cognitive performance, including processing speed and memory, was relatively preserved. However, this group reported frequent SMCs (95% of participants reported one or more SMCs), suggesting a potential dissociation between perceived and actual cognitive functioning.
Cluster 3: cohabiting females with high subjective health and SMCs
This subgroup included only women (100%), the majority of whom lived with others (89%). Participants in this cluster demonstrated the most favorable objective cognitive scores across all domains. They also had the highest levels of self-rated health (92%) and the lowest levels of SMCs compared to the other clusters (21% of individuals in this cluster did not report any SMCs).
Cluster 4: older adults with cognitive decline
This cluster was characterized by the oldest age distribution, with the majority of participants aged 75 years and older (83.1%). Although most lived with others (88%), they had the lowest levels of self-rated health (69%) and the highest prevalence of processing speed impairment (75%). Memory and attention impairments were also more common in this group (60%).
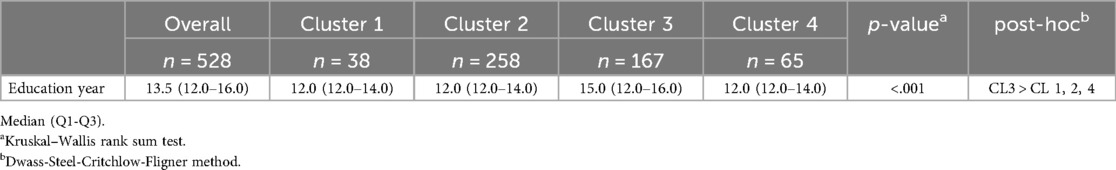
A post-hoc analysis was conducted to examine differences in years of education across the four identified clusters. The results indicated that Cluster 3 had significantly more years of education compared to Clusters 1, 2, and 4 (p < .01), whereas no significant differences were observed among Clusters 1, 3, and 4. Detailed results are presented in Table 4.
Discussion
Our findings demonstrated that web-based cognitive assessment tools can be successfully completed by a wide range of community-dwelling older adults and can hold considerable potential as an accessible and scalable approach for screening cognitive decline. Using LCA, we classified participants based on their objective cognitive performance, subjective health and memory complaints, and sociodemographic factors. The largest subgroup consisted of cohabiting males with frequent SMCs but preserved objective cognition. Another group comprised socially isolated females with high subjective complaints despite relatively intact cognitive scores. The third cluster included cohabiting females with high perceived health and good cognitive performance. The fourth group represented the oldest participants, showing marked impairments in processing speed and memory function. This study highlights diverse cognitive aging patterns and a mismatch between perceived and actual memory. Social contexts are associated with cognition and self-awareness. This recruitment method may have provided access to a representative older population.
Characteristics of study participants in context
The participants in this study were community-dwelling older adults who voluntarily responded to large-scale recruitment via newspaper insert flyers and posters. Passive recruitment through widely distributed newspaper insert flyers and publicly posted flyers may have facilitated outreach to diverse segments of community-dwelling older adults, including those not actively engaged in formal care or research networks. The mean age was 71.2 years, with a predominance of females (57%) and a high proportion of women reporting good subjective health (86%). Most of the participants lived with others (86%) and engaged in daily conversations (92%), suggesting a relatively high level of social integration. Cognitive performance was generally preserved, with 72% of participants showing no memory impairment and 79% demonstrating intact working memory. These figures suggest that the study population included diverse older adults, including those with subtle cognitive concerns but without overt impairment. Compared to nationally representative cohorts in Japan (42–44), our sample appeared slightly younger, with fewer individuals reporting functional limitations or poor health status. Importantly, the inclusion criteria excluded individuals diagnosed with neuropsychiatric disorders or long-term care certification, which may have further shaped the sample toward relatively independent and cognitively intact individuals. Nonetheless, the diversity in SMCs and sociodemographic factors observed across the latent classes suggests meaningful heterogeneity within this seemingly healthy population.
Interpretation of clusters
Cluster 1: socially isolated females with high SMCs
This small but distinct group consisted entirely of women living alone, with limited daily conversations and elevated SMCs. The overrepresentation of women aligns with prior findings that older women report more memory complaints than men even when cognitive scores are comparable (45–47). Social isolation may play a significant role in this cluster, with evidence linking limited engagement with cognitive decline and increased subjective complaints (48–52). The bi-directional relationship between isolation and depression may have exacerbated concerns (53–55). In this predominantly female cluster, heightened health consciousness may be associated with an increased frequency of SMCs. Previous research has indicated that women tend to be more attuned to their health status than men (56, 57), which may lead to a greater awareness of subtle cognitive changes and, consequently, heightened concern or anxiety regarding memory function. Clinically, this group may be overlooked by screenings that focus solely on objective performance. Therefore, healthcare professionals should consider integrating subjective assessments and sociodemographic contexts into routine evaluations to better identify at-risk individuals. Persistent subjective complaints are associated with an increased risk of future decline (32, 33), underscoring the need for multidimensional assessment and proactive support.
Cluster 2: cohabiting males with high SMCs
The predominance of this cluster may reflect the relatively younger age and common living arrangements of older adults, making cohabiting environments more typical in this demographic. Additionally, younger individuals may have been more likely to access web-based assessment platforms via mobile devices, contributing to their overrepresentation in this group.
This cluster consisted mainly of cohabiting older men with frequent SMCs despite intact objective cognition. This challenges prior findings that women report more memory concerns (45), suggesting that broader community samples may reveal underrecognized patterns among men. Cohabiting older men may engage in heightened self-monitoring and feel internal pressure to uphold their cognitive competence, especially after retirement (58–60). These dynamics could amplify SMCs despite intact cognition, thus underscoring gendered patterns in cognitive self-perception.
Interventions should target psychological mechanisms underlying these concerns. Psychoeducation, which normalizes age-related changes and distinguishes them from pathological changes, may reduce anxiety (61, 62). Given their intact objective cognition and relatively younger age, individuals in this cluster may benefit from health promotion strategies aimed at maintaining their current cognitive and physical functioning. Preventive interventions that reinforce self-efficacy and address subjective concerns may be particularly effective in supporting long-term well-being.
Cluster 3: cohabiting females with high health perception and preserved cognition
This cluster, composed of older cohabiting women with high subjective health, minimal SMCs, and good cognitive performance, may represent a resilient aging phenotype and a model for protective factors in cognitive health. Women tend to exhibit heightened awareness and engagement in health management and lifestyle practices (63), which may contribute to the preservation of cognitive function.
Beyond cognitive health, future interventions should address preventive measures against functional decline, sociodemographic adaptation to life events such as bereavement or children's departure from the household, and the management of sex-specific health concerns such as osteoporosis, which are particularly prevalent among older women.
Cluster 4: older adults with cognitive decline and processing speed impairment
This cluster included the oldest participants and showed the most pronounced cognitive impairment, particularly in terms of processing speed. Age-related declines in processing speed are well documented and often precede other cognitive impairments (64–66). Despite living with others and engaging in conversations, these individuals may require more intensive cognitive support and caregiver involvement. Their profile underscores the need for age-sensitive interventions and monitoring. Importantly, cohabitants may not recognize these subtle cognitive changes, especially when the decline is gradual and masked by routine interactions. This highlights the need for broader cognitive screening efforts that extend beyond clinical settings and incorporate inputs from formal and informal caregivers. A previous study showed that even among individuals who do not live alone, the rate of cognitive decline tends to be faster when cohabiting with someone other than a spouse (67). Therefore, cognitive screening from a more objective perspective, particularly by individuals outside of the family, is becoming increasingly important.
Limitations and strengths
This study has some limitations that warrant consideration. First, its cross-sectional design precludes causal inferences regarding the relationships between cognitive performance, subjective health, and SMCs. Longitudinal follow-up is necessary to examine cognitive trajectories and transitions between clusters over time. Second, although mobile device ownership has been rapidly increasing among older adults in Japan, participants may still be biased toward relatively healthy and technologically literate individuals because participation requires access to digital devices and the ability to complete web-based assessments. As a result, individuals with lower digital literacy or more pronounced cognitive or functional decline may have been underrepresented. However, this also highlights a unique strength of the study. Because the participants predominantly consisted of digitally active older adults, the findings may reflect subtle cognitive differences that emerge before overt cognitive decline becomes evident. This interpretation is supported by recent meta-analysis showing that older adults who regularly engage with digital technologies tend to exhibit better cognitive functioning than those who do not (68). Identifying these early cognitive differences in a high-functioning, digitally connected population may offer valuable opportunities for early detection and preventive interventions. Third, although recruitment via newspaper insert flyers and posters broadened the outreach, the actual number of participants was relatively modest. One likely factor was the need to scan a QR code to access the study website, which may have posed a barrier for some older adults. This highlights a key challenge for future recruitment strategies, and future efforts should include multiple access options to promote inclusivity. Lastly, all data were self-reported or digitally assessed without clinical verification, which may have limited diagnostic precision.
Despite these limitations, this study had several strengths. This study demonstrates the feasibility of web-based cognitive screening in a community setting and introduces a novel, low-cost recruitment strategy that enhances ecological validity. The use of LCA allowed for the nuanced identification of cognitive and sociodemographic subgroups, offering insights into the diversity of ageing experiences. These findings can inform targeted interventions and public health strategies aimed at promoting cognitive wellbeing among older adults in this digitized society. Collaboration with local governments is essential for turning these findings into practical dementia prevention tools.
Conclusions
This study identified four distinct cognitive and sociodemographic subgroups of community-dwelling older adults using LCA. By employing a web-based cognitive assessment tool, we sought to identify participants who may be inaccessible through traditional venue-based screening approaches, thereby enhancing the diversity and ecological validity of the study sample. The findings revealed that SMCs do not always align with objective cognitive performance, highlighting the need to promote accessible tools and supportive environments that enable individuals to undergo objective cognitive screening with ease. These insights underscore the importance of personalized approaches to cognitive health, particularly in community settings. Web-based screening combined with inclusive outreach strategies may serve as a scalable method for early detection and tailored support. Future research should explore the longitudinal changes within these subgroups and evaluate interventions that address both cognitive function and well-being.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Sapporo Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Instead of providing written informed consent, participants indicated their agreement to take part in the study by tapping the consent button on the web-based consent screen, which was displayed prior to accessing the demographic questionnaire and cognitive assessments.
Author contributions
SS: Writing – review & editing, Formal analysis, Writing – original draft, Methodology, Conceptualization, Investigation, Data curation. KY: Data curation, Investigation, Resources, Formal analysis, Writing – review & editing. KM: Formal analysis, Data curation, Writing – review & editing, Investigation, Resources. NI: Writing – review & editing. KM: Investigation, Writing – review & editing, Resources. TK: Writing – review & editing, Investigation, Resources. CK: Writing – review & editing, Resources, Investigation. HI: Project administration, Formal analysis, Methodology, Data curation, Resources, Supervision, Investigation, Writing – review & editing, Funding acquisition, Conceptualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by research grants from the Ministry of Health, Labour and Welfare of Japan (Grant No. 24GB2001) and the Grants-in-Aid for Scientific Research in Japan (Grant Nos. KAKENHI 24KJ1846 and 25K21134).
Acknowledgments
We would like to thank all participants in the study and the research staff for their contributions to the study. We would like to thank Editage (https://www.editage.jp) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
LCA, latent class analysis; SMC, subjective memory complaints; MCI, mild cognitive impairment; AIC, Akaike Information Criterion; BIC, Bayesian Information Criterion.
References
1. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. (2020) 396(10248):413–46. doi: 10.1016/S0140-6736(20)30367-6
2. Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. (2015) 385(9984):2255–63. doi: 10.1016/S0140-6736(15)60461-5
3. Saha T, Lee K, Hyun KK, Cassidy J, Jang S. Understanding travel behaviors and mobility challenges faced by older adults during the COVID-19 pandemic. J Ageing Longev. (2024) 4(3):177–87. doi: 10.3390/jal4030012
4. Yin L, Caceres M, Skowronski J, Muramatsu N. Assessing motor function in frail older adults in their home settings: challenges, strategies and recommendations. Int J Environ Res Public Health. (2023) 20(15):6515. doi: 10.3390/ijerph20156515
5. Banack HR, Kaufman JS, Wactawski-Wende J, Troen BR, Stovitz SD. Investigating and remediating selection bias in geriatrics research: the selection bias toolkit. J Am Geriatr Soc. (2019) 67(9):1970–6. doi: 10.1111/jgs.16022
6. Gaertner B, Seitz I, Fuchs J, Busch MA, Holzhausen M, Martus P, et al. Baseline participation in a health examination survey of the population 65 years and older: who is missed and why? BMC Geriatr. (2016) 16:21. doi: 10.1186/s12877-016-0185-6
7. Faverio M. Share of those 65 and older who are tech users has grown in the past decade. Pew Res Center. (2022). Available online at: https://www.pewresearch.org/fact-tank/2022/01/13/share-of-those-65-and-older-who-are-tech-users-has-grown-in-the-past-decade/ (Accessed August 17, 2022).
8. Hunsaker A, Hargittai E. A review of internet use among older adults. New Media Soc. (2018) 20(10):3937–54. doi: 10.1177/1461444818787348
9. Marler W, Hargittai E. Division of digital labor: partner support for technology use among older adults. New Media Soc. (2024) 26(2):978–94. doi: 10.1177/14614448211068437
10. Minisry of Internal Affairs and Communications. MIC ICT Policy (2023). White Paper. Available online at: https://www.soumu.go.jp/main_sosiki/joho_tsusin/eng/whitepaper/index.html (Accessed October 6, 2023).
11. Morrissey S, Gillings R, Hornberger M. Feasibility and reliability of online vs in-person cognitive testing in healthy older people. PLoS One. (2024) 19(8):e0309006. doi: 10.1371/journal.pone.0309006
12. Belleville S, LaPlume AA, Purkart R. Web-based cognitive assessment in older adults: where do we stand? Curr Opin Neurol. (2023) 36(5):491. doi: 10.1097/WCO.0000000000001192
13. Van Mierlo LD, Wouters H, Sikkes SAM, Van der Flier WM, Prins ND, Bremer JAE, et al. Screening for mild cognitive impairment and dementia with automated, anonymous online and telephone cognitive self-tests. J Alzheimers Dis. (2017) 56(1):249–59. doi: 10.3233/JAD-160566
14. Huynh D, Patterson M, Huang B. Clinical efficacy of a digital cognitive assessments in detecting cognitive impairments: a pooled analysis. Innov Aging. (2024) 8(Suppl 1):1230. doi: 10.1093/geroni/igae098.3936
15. Haas M, Zuber S, Framorando D, Khawli EE, Scheibe S, Kliegel M. Online assessment of cognitive functioning: a reliable alternative to laboratory testing? Innov Aging. (2020) 4(Suppl 1):576–7. doi: 10.1093/geroni/igaa057.1917
16. Thompson LI. Digital assessment approaches to overcome barriers to cognitive screening and monitoring for older adults in primary care settings. Alzheimers Dement. (2025) 20(Suppl 8):e095393. doi: 10.1002/alz.095393
17. Samonte MJC, Donato LEF, Fallarme KAL, Ocampo NRP. Memorecall: a senior friendly web application for holistic intervention of early memory lapses in geriatric individuals. Proceeding of the 2024 6th International Conference on Information Technology and Computer Communications; New York, NY, USA: Association for Computing Machinery (2025). p. 50–6. (ITCC ‘24). doi: 10.1145/3704391.3704399 (Accessed July 27, 2025)
18. Sokołowski DR, Pani J, Hansen TI, Håberg AK. Participation and engagement in online cognitive testing. Sci Rep. (2024) 14(1):14800. doi: 10.1038/s41598-024-65617-w
19. Dorociak KE, Mattek N, Lee J, Leese MI, Bouranis N, Imtiaz D, et al. The survey for memory, attention, and reaction time (SMART): development and validation of a brief web-based measure of cognition for older adults. Gerontology. (2021) 67(6):740–52. doi: 10.1159/000514871
20. Mackenzie-Stewart R, de Lacy-Vawdon C, Murphy N, Smith BJ. Engaging adults in organized physical activity: a scoping review of recruitment strategies. Health Promot Int. (2023) 38(3):daad050. doi: 10.1093/heapro/daad050
21. An J, Zhu X, Wan K, Xiang Z, Shi Z, An J, et al. Older adults’ self-perception, technology anxiety, and intention to use digital public services. BMC Public Health. (2024) 24:3533. doi: 10.1186/s12889-024-21088-2
22. Aslan A, Mold F, van Marwijk H, Armes J. What are the determinants of older people adopting communicative e-health services: a meta-ethnography. BMC Health Serv Res. (2024) 24:60. doi: 10.1186/s12913-023-10372-3
23. Sugawara Y, Hirakawa Y, Iwagami M, Kuroki H, Mitani S, Inagaki A, et al. Issues in the adoption of online medical care: cross-sectional questionnaire survey. J Med Internet Res. (2024) 26(1):e64159. doi: 10.2196/64159
24. Cornejo R, Tentori M, Favela J. Enriching in-person encounters through social media: a study on family connectedness for the elderly. Int J Hum Comput Stud. (2013) 71(9):889–99. doi: 10.1016/j.ijhcs.2013.04.001
25. Schroeder T, Dodds L, Georgiou A, Gewald H, Siette J. Older adults and new technology: mapping review of the factors associated with older adults’ intention to adopt digital technologies. JMIR Aging. (2023) 6(1):e44564. doi: 10.2196/44564
26. Chamberlain JD, Sprague BN, Ross LA. Age- and time-varying associations between subjective health and episodic memory in older adults. J Gerontol B Psychol Sci Soc Sci. (2022) 77(4):673–82. doi: 10.1093/geronb/gbab142
27. Fernández-Ballbé Ó, Martin-Moratinos M, Saiz J, Gallardo-Peralta L, Barrón López de Roda A. The relationship between subjective aging and cognition in elderly people: a systematic review. Healthcare (Basel). (2023) 11(24):3115. doi: 10.3390/healthcare11243115
28. Li G, Xie X. Longitudinal relationship between cognitive decline and subjective well-being in older adults based on the random-intercept cross-lagged panel model. Health Soc Care Community. (2024) 2024(1):7312477. doi: 10.1155/2024/7312477
29. Hayes JM, Tang L, Viviano RP, van Rooden S, Ofen N, Damoiseaux JS. Subjective memory complaints are associated with brain activation supporting successful memory encoding. Neurobiol Aging. (2017) 60:71–80. doi: 10.1016/j.neurobiolaging.2017.08.015
30. Webster-Cordero F, Giménez-Llort L. A systematic review on subjective cognitive complaints: main neurocognitive domains, myriad assessment tools, and new approaches for early detection. Geriatrics (Basel). (2025) 10(3):65. doi: 10.3390/geriatrics10030065
31. Rodini M, Bonarota S, Serra L, Caltagirone C, Carlesimo GA. Could accelerated long-term forgetting be a feature of the higher rate of memory complaints associated with subjective cognitive decline? An exploratory study. J Alzheimer’s Dis. (2024) 100(4):1165–82. doi: 10.3233/JAD-240218
32. Mendonça MD, Alves L, Bugalho P. From subjective cognitive complaints to dementia: who is at risk?: a systematic review. Am J Alzheimers Dis Other Demen. (2016) 31(2):105–14. doi: 10.1177/1533317515592331
33. Abner EL, Kryscio RJ, Caban-Holt AM, Schmitt FA. Baseline subjective memory complaints associate with increased risk of incident dementia: the PREADVISE trial. J Prev Alzheimers Dis. (2015) 2(1):11–6. doi: 10.14283/jpad.2015.37
34. Melikyan ZA, Kawas CH, Paganini-Hill A, Al-Darsani Z, Jiang L, Bukhari SA, et al. Subjective memory complaints and neuropathologic changes at age 90 and older: the 90+ study. Alzheimer’s Demen. (2024) 20(S1):e089868. doi: 10.1002/alz.089868
35. Makizako H, Shimada H, Park H, Doi T, Yoshida D, Uemura K, et al. Evaluation of multidimensional neurocognitive function using a tablet personal computer: test-retest reliability and validity in community-dwelling older adults. Geriatr Gerontol Int. (2013) 13(4):860–6. doi: 10.1111/ggi.12014
36. Smart CM, Karr JE, Areshenkoff CN, Rabin LA, Hudon C, Gates N, et al. Non-pharmacologic interventions for older adults with subjective cognitive decline: systematic review, meta-analysis, and preliminary recommendations. Neuropsychol Rev. (2017) 27(3):245–57. doi: 10.1007/s11065-017-9342-8
37. Ikeda Y, Ogawa N, Yoshiura K, Han G, Maruta M, Hotta M, et al. Instrumental activities of daily living: the processes involved in and performance of these activities by Japanese community-dwelling older adults with subjective memory complaints. Int J Environ Res Public Health. (2019) 16(14):2617. doi: 10.3390/ijerph16142617
38. Neff P, Demiray B, Martin M, Röcke C. Cognitive abilities predict naturalistic speech length in older adults. Sci Rep. (2024) 14(1):31031. doi: 10.1038/s41598-024-82144-w
39. Fukaya Y, Kawaguchi M, Kitamura T. Does everyday conversation contribute to cognitive functioning? A comparison of brain activity during task-oriented and life-worldly communication using near-infrared spectroscopy. Gerontol Geriatr Med. (2020) 6:2333721420980309. doi: 10.1177/2333721420980309
40. Weller BE, Bowen NK, Faubert SJ. Latent class analysis: a guide to best practice. J Black Psychol. (2020) 46(4):287–311. doi: 10.1177/0095798420930932
41. Linzer DA, Lewis JB. poLCA: an R package for polytomous variable latent class analysis. J Stat Softw. (2011) 42:1–29. doi: 10.18637/jss.v042.i10
42. Sugita A, Ling L, Tsuji T, Kondo K, Kawachi I. Cultural engagement and incidence of cognitive impairment: a 6-year longitudinal follow-up of the Japan gerontological evaluation study (JAGES). J Epidemiol. (2021) 31(10):545–53. doi: 10.2188/jea.JE20190337
43. Minohara T, Ohara T, Nakazawa T, Hirabayashi N, Furuta Y, Shibata M, et al. Association of impaired olfactory identification with prevalent mild cognitive impairment and regional brain atrophy: the hisayama study. Psychiatry Clin Neurosci. (2025) 79(7):370–7. doi: 10.1111/pcn.13813
44. Nishita Y, Tange C, Tomida M, Otsuka R, Ando F, Shimokata H. Personality and global cognitive decline in Japanese community-dwelling elderly people: a 10-year longitudinal study. J Psychosom Res. (2016) 91:20–5. doi: 10.1016/j.jpsychores.2016.10.004
45. Li J, Hao W, Fu C, Zhou C, Zhu D. Sex differences in memory: do female reproductive factors explain the differences? Front Endocrinol (Lausanne). (2022) 13:837852. doi: 10.3389/fendo.2022.837852
46. Sharma M, Goswami I. Multivariate decomposition of gender differentials in cognitive impairment among older adults in India based on longitudinal ageing study in India, 2017–2018. BMC Psychiatry. (2025) 25(1):385. doi: 10.1186/s12888-025-06811-6
47. Chen NX, Corrada MMM, Gilsanz P, Farias ST, George KM, Posis AIB, et al. Gender differences in the association between subjective and objective cognitive decline in a diverse cohort: life after 90 study. Alzheimer’s Dement. (2024) 20(S3):e091848. doi: 10.1002/alz.091848
48. Jang H, Hill NL, Turner JR, Bratlee-Whitaker E, Jeong M, Mogle J. Poor-quality daily social encounters, daily stress, and subjective cognitive decline among older adults. Innov Aging. (2024) 8(6):igae038. doi: 10.1093/geroni/igae038
49. Evans IEM, Llewellyn DJ, Matthews FE, Woods RT, Brayne C, Clare L, et al. Social isolation, cognitive reserve, and cognition in healthy older people. PLoS One. (2018) 13(8):e0201008. doi: 10.1371/journal.pone.0201008
50. Bland AR, Roiser JP, Mehta MA, Sahakian BJ, Robbins TW, Elliott R. The impact of COVID-19 social isolation on aspects of emotional and social cognition. Cogn Emot. (2022) 36(1):49–58. doi: 10.1080/02699931.2021.1892593
51. Hajek A, Riedel-Heller SG, König HH. Perceived social isolation and cognitive functioning. Longitudinal findings based on the German ageing survey. Int J Geriatr Psychiatry. (2020) 35(3):276–81. doi: 10.1002/gps.5243
52. Steijvers LC, Brinkhues S, Tilburg T, Hoebe CJ, Stijnen MM, Vries N, et al. Changes in structure and function of social networks of independently living middle-aged and older adults in diverse sociodemographic subgroups during the COVID-19 pandemic: a longitudinal study. BMC Public Health. (2022) 22(1):2253. doi: 10.1186/s12889-022-14500-2
53. Groarke JM, McGlinchey E, McKenna-Plumley PE, Berry E, Graham-Wisener L, Armour C. Examining temporal interactions between loneliness and depressive symptoms and the mediating role of emotion regulation difficulties among UK residents during the COVID-19 lockdown: longitudinal results from the COVID-19 psychological wellbeing study. J Affect Disord. (2021) 285:1–9. doi: 10.1016/j.jad.2021.02.033
54. Cooper HTJ, De Souza S. The effects of loneliness and isolation on depressive disorder: a narrative review. Eur Psychiatry. (2023) 66(Suppl 1):S832–3. doi: 10.1192/j.eurpsy.2023.1763
55. Sbarra DA, Ramadan FA, Choi KW, Treur JL, Levey DF, Wootton RE, et al. Loneliness and depression: bidirectional Mendelian randomization analyses using data from three large genome-wide association studies. Mol Psychiatry. (2023) 28(11):4594–601. doi: 10.1038/s41380-023-02259-w
56. Deeks A, Lombard C, Michelmore J, Teede H. The effects of gender and age on health related behaviors. BMC Public Health. (2009) 9:213. doi: 10.1186/1471-2458-9-213
57. Phillips SP, O’Connor M, Vafaei A. Women suffer but men die: survey data exploring whether this self-reported health paradox is real or an artefact of gender stereotypes. BMC Public Health. (2023) 23(1):94. doi: 10.1186/s12889-023-15011-4
58. Meng A, Nexø MA, Borg V. The impact of retirement on age related cognitive decline—a systematic review. BMC Geriatr. (2017) 17(1):160. doi: 10.1186/s12877-017-0556-7
59. Rohwedder S, Willis RJ. Mental retirement. J Econ Perspect. (2010) 24(1):119–38. doi: 10.1257/jep.24.1.119
60. Lahdenperä M, Virtanen M, Myllyntausta S, Pentti J, Vahtera J, Stenholm S. Psychological distress during the retirement transition and the role of psychosocial working conditions and social living environment. J Gerontol B Psychol Sci Soc Sci. (2022) 77(1):135–48. doi: 10.1093/geronb/gbab054
61. da Silva TBL, Dos Santos G, Moreira APB, Ishibashi GA, Verga CER, de Moraes LC, et al. Cognitive interventions in mature and older adults, benefits for psychological well-being and quality of life: a systematic review study. Dement Neuropsychol. (2021) 15(4):428–39. doi: 10.1590/1980-57642021dn15-040002
62. Shrestha S, Robertson S, Stanley MA. Innovations in research for treatment of late-life anxiety. Aging Ment Health. (2011) 15(7):811–21. doi: 10.1080/13607863.2011.569487
63. Nikbakht Nasrabadi A, Sabzevari S, Negahban Bonabi T. Women empowerment through health information seeking: a qualitative study. Int J Community Based Nurs Midwifery. (2015) 3(2):105–15.26005690
64. Ebaid D, Crewther SG, MacCalman K, Brown A, Crewther DP. Cognitive processing speed across the lifespan: beyond the influence of motor speed. Front Aging Neurosci. (2017) 9:62. doi: 10.3389/fnagi.2017.00062
65. Deary IJ, Ritchie SJ. Processing speed differences between 70- and 83-year-olds matched on childhood IQ. Intelligence. (2016) 55:28–33. doi: 10.1016/j.intell.2016.01.002
66. Kerchner GA, Racine CA, Hale S, Wilheim R, Laluz V, Miller BL, et al. Cognitive processing speed in older adults: relationship with white matter integrity. PLoS One. (2012) 7(11):e50425. doi: 10.1371/journal.pone.0050425
67. Yu Y, Lv J, Liu J, Chen Y, Chen K, Yang Y. Association between living arrangements and cognitive decline in older adults: a nationally representative longitudinal study in China. BMC Geriatr. (2022) 22(1):843. doi: 10.1186/s12877-022-03473-x
Keywords: cognitive impairment, web-based screening, latent class analysis, dementia, subjective memory complaints
Citation: Shimokihara S, Yokoyama K, Makino K, Ikeda N, Matsuyama K, Kashiwagi T, Kawanishi C and Ihira H (2025) Mapping cognitive diversity in older adults through community-based digital screening via mobile devices: a cross-sectional latent class analysis. Front. Digit. Health 7:1719318. doi: 10.3389/fdgth.2025.1719318
Received: 6 October 2025; Accepted: 10 November 2025;
Published: 25 November 2025.
Edited by:
Janel Hanmer, University of Pittsburgh, United StatesReviewed by:
Tatiana Karpouzian-Rogers, Northwestern University, United StatesSiti Anom Ahmad, Putra Malaysia University, Malaysia
Copyright: © 2025 Shimokihara, Yokoyama, Makino, Ikeda, Matsuyama, Kashiwagi, Kawanishi and Ihira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suguru Shimokihara, c2hpbW9raWhhcmFAbmFnYXNha2ktdS5hYy5qcA==
†ORCID:
Suguru Shimokihara
orcid.org/0000-0002-7235-4479
Kazuki Yokoyama
orcid.org/0000-0001-9865-7200
Keitaro Makino
orcid.org/0000-0001-9252-8256
Nozomu Ikeda
orcid.org/0009-0005-7888-6010
Tomonori Kashiwagi
orcid.org/0009-0004-2371-6586
Chiaki Kawanishi
orcid.org/0000-0003-3464-3787
Hikaru Ihira
orcid.org/0009-0008-1973-5644
 Suguru Shimokihara
Suguru Shimokihara Kazuki Yokoyama2,†
Kazuki Yokoyama2,† Keitaro Makino
Keitaro Makino Nozomu Ikeda
Nozomu Ikeda Kiyoji Matsuyama
Kiyoji Matsuyama Tomonori Kashiwagi
Tomonori Kashiwagi