Corrigendum: Hypogonadism and Sexual Dysfunction in Testicular Tumor Survivors: A Systematic Review
- 1Section of Endocrinology, Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy
- 2Unit of Pediatric Hematology and Oncology, Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy
Testicular tumor is the most common malignancy in men of reproductive age. According to the tumor histology and staging, current treatment options include orchiectomy alone or associated with adjuvant chemo- and/or radiotherapy. Although these treatments have considerably raised the percentage of survivors compared to the past, they have been identified as risk factors for testosterone deficiency and sexual dysfunction in this subgroup of men. Male hypogonadism, in turn, predisposes to the development of metabolic and cardiovascular impairment that negatively affects general health. Accordingly, longitudinal studies report a long-term risk for cardiovascular diseases after radiotherapy and/or cisplatin-based chemotherapy in testicular tumor survivors. The aim of this review was to summarize the current evidence on hypogonadism and sexual dysfunction in long-term cancer survivors, including the epidemiology of cardiovascular and metabolic disorders, to increase the awareness that serum testosterone levels, sexual function, and general health should be evaluated during the endocrinological management of these patients.
Introduction
The testicular tumor is the most common solid malignancy in young adult men (aged 14–44 years) in Western countries and represents ~1.5% of all tumor diagnosis worldwide (1). Its incidence has risen over the last decades, especially in industrialized countries (2). Testicular tumor affects from <1 per 100,000 males in many African and Asian nations to >9 per 100,000 men in the highest-incidence areas of Northern and Western Europe. Despite the highest incidence in more developed countries and particularly in Europe, the incidence-to-mortality ratio is 26:1 in northern Europe compared with 2:1 in Southeast Asia, South–Central Asia, and Africa. This indicates the need to improve the treatment strategy in some non-European countries (3).
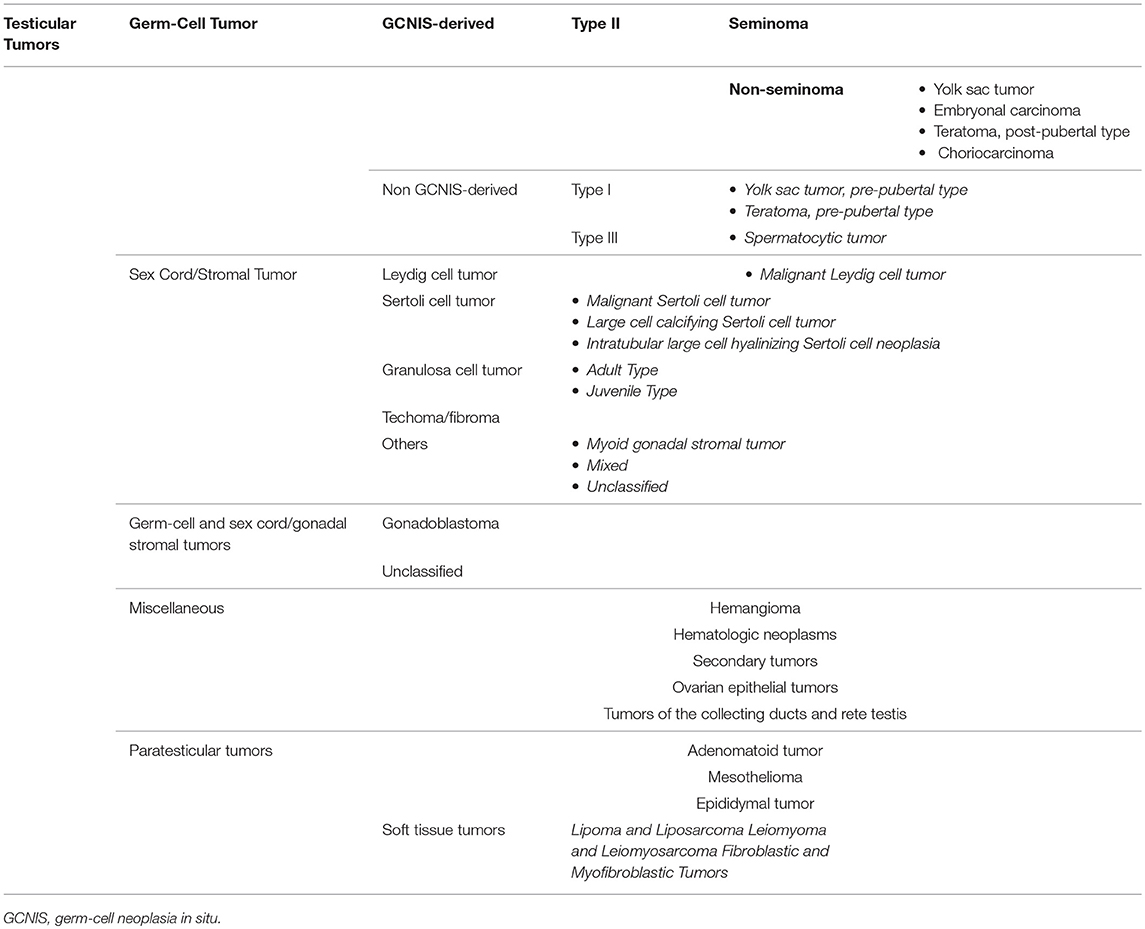
Over the years, a multitude of classifications have been proposed for testicular tumor, reflecting a progressive better understanding of its pathogenesis. Despite the testis being a relatively small organ, it consists of many different cell types; thus, it may give rise to a large variety of neoplasms (Table 1). Nonetheless, more than 95% of testicular tumors are testicular germ-cell tumors (TGCTs) derived from germ cells (4). Sex cord stromal tumors and other non-germ-cell tumors are exceedingly rare. The most recent WHO classification, which has been published in 2016, represents a transition from an exclusively morphological system into one that takes into account the histological composition, the age of onset, and the pathogenic mechanisms of testicular tumor development (5). This new classification recognizes two major types of TGCTs: those derived from germ-cell neoplasia in situ (GCNIS) and those unrelated to GCNIS (5).
Management of testicular tumor is controversial. After orchiectomy, subsequent management options include active surveillance, adjuvant chemotherapy or radiotherapy, and primary retroperitoneal lymphadenectomy (RPLND) (6). Treatment-related toxicity is crucial considering that the long-term survival rate of TGCTs is ~99%, regardless of treatment strategy (6). For this reason, the most recent guidelines focus on minimizing unnecessary treatments to avoid adverse effects that are associated with them and to customize treatment for each patient considering patient's individual risks and his individual wishes (7). Each patient should be informed about the potential advantages and disadvantages of surveillance and adjuvant therapy (7). While surveillance allows most patients to avoid additional treatment, adjuvant therapy significantly lowers the relapse rate (7). Over the years, enthusiasm for adjuvant radiotherapy has been markedly reduced by the risk of radiation-induced secondary cancers. An increasing evidence suggests that active surveillance post-orchiectomy is a suitable alternative to adjuvant regimens in both stage I seminomas and non-seminomas (6). In the treatment of advanced testicular tumor, the current standard of care includes the use of platinum-based chemotherapy [bleomycin, etoposide, and cisplatin (BEP)] (6). A clear dose relationship has been established for the following BEP sequelae: pulmonary toxicity, fertility (8), neurotoxicity, ototoxicity, nephrotoxicity, metabolic syndrome, and hypogonadism (9, 10).
Hypogonadism has been often reported in testicular tumor survivors. Indeed, testicular tumor may represent a feature of the so-called testicular dysgenesis syndrome (TDS) (11, 12). The possibility exists that TDS may somehow impair Leydig cell function. Accordingly, studies indicate that germ-cell malignancy itself may be associated with poorer gonadal function in the remaining testis prior to other treatments (13). Also, the occurrence of microlithiasis (a feature of TDS) in the remaining testis has been shown to predict the incoming of hypogonadism in testicular tumor survivors (14). In addition, because of the radio- and/or chemo-induced Leydig cell damage, adjuvant therapy rises the risk of hormonal deterioration that results in increasing serum luteinizing hormone (LH) levels and decreasing serum testosterone concentrations (15, 16).
Therefore, the aim of this review was to gather together the current evidence of hypogonadism and sexual dysfunction in long-term testicular tumor survivors, including the epidemiology of cardiovascular and metabolic disorders, to increase the awareness to evaluate serum testosterone, sexual function, and general health in testicular tumor survivors.
Methods
We performed a comprehensive review of the literature aimed at evaluating the occurrence of hypogonadism and its related complications, including cardiovascular, metabolic and bone mineralization impairment, and sexual dysfunction in testicular tumor survivors. A systematic search was made through PubMed, MEDLINE, Cochrane, Google Scholar, and Scopus databases. Data were independently extracted by RC and FB. The search strategy was based on the following keywords: “testicular cancer,” “testicular tumor,” “testosterone,” “hypogonadism,” “cardiovascular,” “diabetes,” “bone,” “osteoporosis,” “erectile dysfunction,” “premature ejaculation,” and “sexual dysfunction.” Additional manual searches were made using the reference lists of relevant studies.
No language restriction was used for any literature search. Information on the year of publication, country, continent, study design, and mean age of patients was collected. Studies that met the following inclusion criteria were included in the qualitative synthesis:
• Full-length articles (including longitudinal, retrospective, cross-sectional, case–control studies, review, and meta-analysis) published between 1990 and 2019;
• Studies carried out on patients with testicular tumor of any histological type and stage, whose treatment (surgery, radiotherapy, and/or chemotherapy) was clearly reported;
• Studies having at least one among gonadotropins, total testosterone, cardiovascular health, metabolic profile, or bone mineralization as main outcome, collected at baseline and or at the follow-up counseling.
Studies that did not met the above-mentioned inclusion criteria were excluded.
Hypogonadism
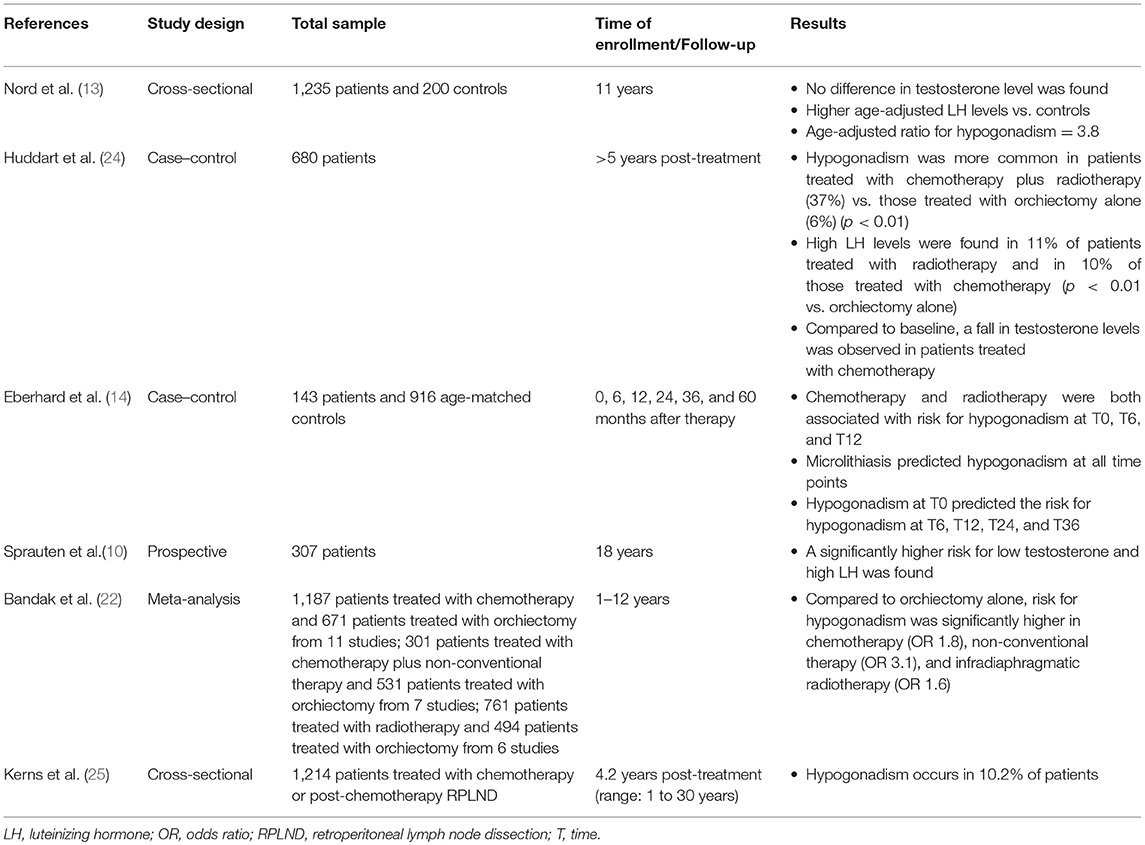
Several longitudinal studies have been carried out to assess the Leydig cell function in testicular tumor survivors. The evidence suggests the vulnerability of Leydig cells to platinum-based chemotherapy and radiotherapy. Animal studies have shown Leydig cell apoptosis (as well as in Sertoli and germ cells) induced by cisplatin both on single administration and on a cumulative manner (17–19). In addition, patients receiving more than 20-Gy dose of radiation at the testicular level need testosterone replacement therapy after 15 years of follow-up, as for a half of patients receiving 16-Gr dose of radiation (20). Interestingly, infra-diaphragmatic radiotherapy when administered at the dose of 30 Gy, corresponding to 0.09–0.32 Gy testicular irradiation (21), is associated also with a slightly greater risk for developing testosterone deficiency, according to a study of meta-analysis (22). These findings suggest that Leydig cells are susceptible to minimal irradiation doses (22, 23).
A number of studies compared chemotherapy-, radiotherapy-, and orchiectomy-alone-dependent toxicity. The results are influenced by the length of follow-up, since those having a longer time of surveillance allow drafting of conclusions on the Leydig cell functional reserve. A summary of the risk of developing hypogonadism in testicular tumor survivors is reported in Table 2.
A prospective multicenter study on 1,235 testicular tumor survivors (mean age 44 years) investigated the risk for hypogonadism after a 11-year-long follow-up. While no difference in serum testosterone levels was found among patients and controls (n = 200), age-adjusted LH levels were higher in the former. In greater detail, the age-adjusted OR of hypogonadism was 3.8 in testicular tumor survivors and showed to increase with treatment intensity being marginally high for surgery alone, 3.5 for radiotherapy, and 4.8 and 7.9 for low- and high-dose chemotherapy, respectively (13). These findings suggest the occurrence of an age-dependent deterioration in Leydig cell function of testicular tumor survivors, with a higher effect of chemotherapy compared to radiotherapy.
A longitudinal cohort study on 307 patients with testicular tumor reported lower testosterone levels at all surveillance time points, which were done after a mean of 9 years (range: 5–21 years; S1) and after a mean of 18 years (range: 13–28 years; S2) (10). At baseline, the risk of testosterone deficiency was higher in the orchiectomy-alone group (n = 69; OR = 4.7) than for radiotherapy (n = 130; OR = 2.6) and chemotherapy (n = 108; OR = 1.9), when compared to controls. At S2, the risk of low testosterone levels was significantly higher in patients receiving chemotherapy (OR = 5.2) than in those treated with radiotherapy (OR = 3.3) or surgery alone (OR = 2). Similar results were found for the risk of high LH serum levels. Therefore, in contrast to surgery alone, both groups receiving radio- and chemotherapy (with a higher effect in the latter) had a lower Leydig cell function with time. In addition, the cumulative platinum dose was significantly associated with the risk of increasing LH levels for each cycle. These results suggest a functional reserve decrement in testosterone production of the remaining testis, which makes testicular tumor survivors vulnerable to the aging-related decline of Leydig cell function (late-onset hypogonadism). Furthermore, residual long-term serum platinum levels and the consequent chronic exposure of the testicular tissue may contribute to hypogonadism as well and may explain the reason why the group treated with chemotherapy has worse Leydig cell function (10).
In agreement with these findings, in a more than 5-year-long follow-up prospective study on 680 patients, low testosterone levels were found in 11% of the group of patients undergoing orchiectomy (n = 169), while a significantly higher portion of patients with low testosterone levels was found in patients receiving both radiotherapy and chemotherapy (37%, n = 81). Irradiated patients (n = 158) and those who received chemotherapy (n = 272) showed abnormally high LH levels in the 11% and in the 10% of cases, respectively. The results of this study confirmed that gonadal dysfunction is common in testicular tumor survivors even when managed with orchiectomy alone. Chemotherapy seems to result in an additional risk of testicular failure (24).
A meta-analysis of cohort studies definitively confirmed the occurrence of a higher risk for testosterone deficiency in TGCT patients treated with standard chemotherapy (≤4 platinum-based for chemotherapy cycle; OR 1.8), non-conventional chemotherapy (platinum-based combination chemotherapy with double dose of cisplatin, >4 cycles of platinum-based combination chemotherapy, or both chemotherapy and radiotherapy; OR 3.1), and radiotherapy (OR 1.6) when compared to patients with orchiectomy alone (22). The follow-up time of the studies included in this meta-analysis (22) ranged from only 2 months to 12 years, and some of them reported Leydig cell recovery in the years following the treatment. Accordingly, when patients are monitored for <5 years, the occurrence of hypogonadism is less frequently reported. In fact, a study carried out in 143 TGCT patients found a higher risk for hypogonadism in patients treated with radiotherapy or with three to four chemotherapy cycles when compared to adjuvant chemotherapy (≤2 cycles) at the 6 and 12th post-therapy month. Adjuvant chemotherapy consisted of no more than two cycles of combined therapy with bleomycin plus cisplatin plus etoposide or vinblastin, or carboplatin single administration, and it was offered to patients with a clinical stage I testicular tumor. High-dose chemotherapy consisted of three to four cycles, and it was administered to patients with more advanced disease. By contrast, no difference was found in further surveillance time points (24, 36, and 60 months). High doses of chemotherapy or radiotherapy seem to be, therefore, more harmful than the adjuvant chemotherapy on Leydig cell function, at least during the first-year post-treatment (14). This study also investigated whether any predictor of testosterone deficiency development in testicular tumor survivors does exist. Interestingly, while testicular volume, consistency, age, androgen receptor polymorphisms, and tumor stage have not been found to correlate with risk of hypogonadism, both mycrolithiasis in the remaining testis and the presence of low testosterone levels after orchiectomy but prior to any other treatment predicted the risk of developing hypogonadism (14). The reason why mycrolithiasis may somehow be associated with a higher risk of Leydig cell failure might be inherent to the possible existence of a tumor-dependent mechanism of Leydig cell damage. Testicular mycrolithiasis belongs to the TDS spectrum, the latter syndrome being considered to be involved in testicular tumor pathogenesis (26, 27).
Framing together these results, Leydig cell vulnerability to chemotherapy and radiotherapy results in an impaired function in the first post-treatment year (14), with an apparent restoration of the Leydig cell function after at least 5 years from the treatment (14). The subsequent later decline of the function, due to subtler damage of Leydig cell function, seems to initially arise with a first phase of subclinical hypogonadism, consisting of increased LH and normal testosterone levels (13, 24), until the full onset of testosterone deficiency (10). This more likely happens in older patients, due to greater susceptibility of Leydig cells to the aging-induced damage in testicular tumor survivors, as previously suggested (10, 13) (Figure 1).
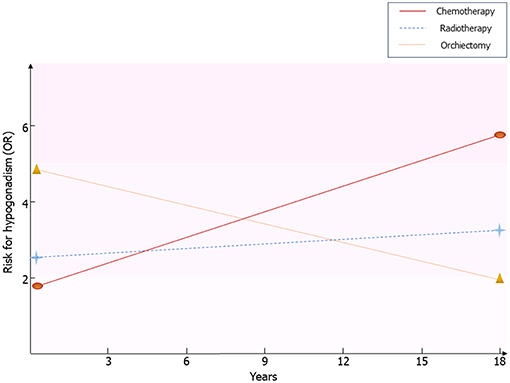
Figure 1. Risk for hypogonadism in testicular tumor survivors. Results coming from the available follow-up studies are resumed. Risk of hypogonadism has been calculated in comparison with healthy men. OR, odds ratio.
Testicular Tumor Survivors: Long-Term Complications
Cardiovascular Diseases
The number of testicular tumor survivors has markedly increased through the decades. A multicenter study carried out on 1,214 testicular tumor patients treated with platinum-based chemotherapy has recently investigated the prevalence of adverse health outcomes (the so-called “Platinum Study”), in an attempt to assess long-term platinum-dependent toxicities. Mean age of patients was 37 years (range: 18–74 years), and the mean time from chemotherapy completion was 4.2 years (range: 1–30 years). Hypertension, peripheral artery disease, and a thromboembolic event were reported in the 9.4, 4.6, and 7.2% of cases, respectively. Coronary artery disease and cardiovascular events such as transient ischemic attack and stroke were negligible (1.6, 0.7, and 0.5%, respectively). Interestingly, the Reynaud phenomenon occurred in 33.4% of patients (25). In support of these findings, ongoing endothelial cell and vascular damage and hypertension could be related to the long-term serum platinum levels (28, 29).
The 10-year cardiovascular risk assessed by the Framingham Risk Score (3%) and the Systemic Coronary Risk Evaluation (1.7%) algorithms was comparable to controls and was independent of the treatment (30). By contrast, a greater risk of developing cardiovascular disease was found after 10.2 years of observation in 992 testicular tumor survivors (31). Other studies confirmed these findings (32). Similarly, a 20-year follow-up study carried out in 990 testicular tumor survivors and 990 age-matched controls more recently found a 5.7-fold higher risk for coronary artery disease in patients treated with chemotherapy (BEP) alone (n = 364) compared with surgery alone (n = 206) and a 3.1-fold higher risk for myocardial infarction in survivors treated with chemotherapy alone compared with controls. Both groups of patients receiving chemotherapy and radiotherapy (n = 386) showed an increased prevalence of administration with antihypertensive and antidiabetic drugs compared with controls. Atherosclerosis was observed only in 8% of patients, despite an increased risk for atherosclerotic disease observed in the chemotherapy and radiotherapy groups (both single and combined administration) compared with surgery alone. The risk was greater in the case of combined chemotherapy and radiotherapy (33). Summary of available data from studies on cardiovascular risk factors and diseases in testicular cancer survivors is described in Table 3.
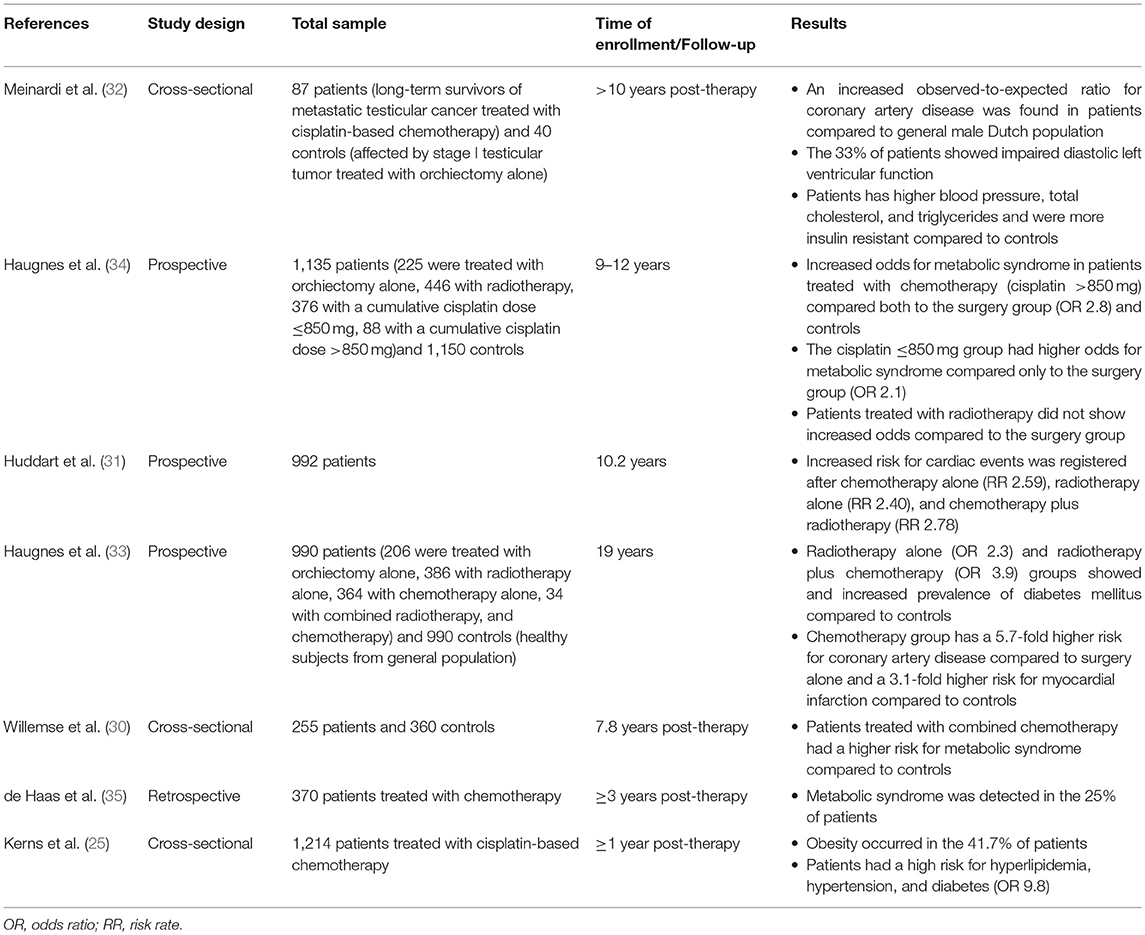
Table 3. Summary of available data from studies on cardiovascular risk factors and cardiovascular diseases in testicular cancer survivors.
In summary, these findings suggest the presence of a greater risk of developing cardiovascular diseases in testicular tumor survivors, especially after chemotherapy. Two hypotheses have been proposed to explain this association. The direct one suggests a chemotherapy-induced damage at the endothelial level. The indirect hypothesis ascribes the risk to the increased incidence of cardiovascular risk factors, such as hypertension, dyslipidemia, metabolic syndrome, and diabetes, which, in turn, raise the susceptibility to cardiovascular diseases (28).
Metabolic Diseases
According to the findings of the “Platinum Study,” which investigated 1,214 testicular cancer survivors, the most frequent adverse outcome observed 4.2 years after chemotherapy completion was obesity, with a prevalence of 71.5%. Diabetes and hypertriglyceridemia rarely occurred (3 and 0.5%, respectively), and hypercholesterolemia was reported in 8% of cases (25).
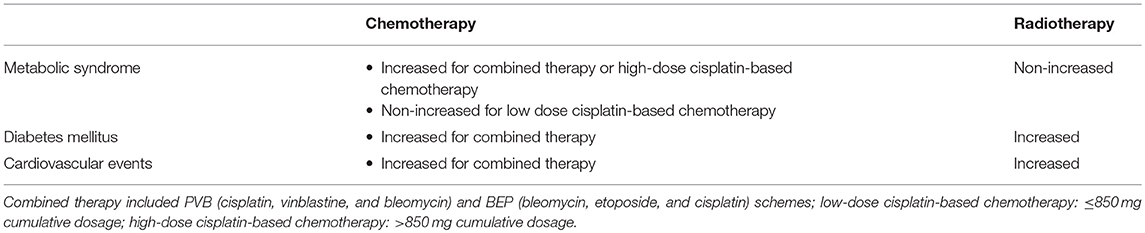
A follow-up study (1998–2002) on 1,135 testicular tumor survivors younger than 60 years assessed the association between metabolic syndrome (the modified National Cholesterol Education Program definition was used) and type of testicular tumor treatment. The sample studied included patients treated with surgery alone (n = 225), radiotherapy (n = 446), and cumulative cisplatinum dose ≤850 mg (n = 376) and >850 mg (n = 88). A greater risk for metabolic syndrome was found in both groups of patients receiving chemotherapy compared with those who underwent to surgery alone. The group treated with the higher cisplatinum cumulative dose showed a greater risk compared to controls (n = 1150), even after adjusting for testosterone levels, thus suggesting that this risk is not dependent on hypogonadism but is due to cisplatinum-induced damage (34). However, other studies have shown that serum testosterone levels <15 nmol/L are associated with a greater risk for developing metabolic syndrome in testicular tumor survivors (35). Indeed, after a median follow-up of 5 years, testicular tumor survivors treated with chemotherapy showed a 2.2-fold higher risk of developing metabolic syndrome compared with controls, whereas the risk increased up to 4.1-fold in survivors whose testosterone levels were <15 nmol/L. Furthermore, among the entire cohort of patients, overweight, and hypercholesterolemia were both found in 24% of cases (35). Similar findings were also reported in a study showing a higher risk of metabolic syndrome in a cohort of 255 testicular tumor survivors 7.8 years after chemotherapy (36). The risk was 2.5-fold higher in survivors with hypogonadism (30).
In conclusion, several reports have found the presence of different dysmetabolic diseases [obesity, metabolic syndrome, and diabetes mellitus (DM)], hypogonadism, and other cardiovascular risk factors. Their early diagnosis and proper treatment are of paramount relevance to lower the long-term cardiovascular risk in testicular tumor survivors.
Bone Density
The occurrence of a decreased bone mineral density (BMD) has been suggested in testicular tumor survivors. A prospective study on 63 germ-cell testicular tumor patients (mean age: 33 years; range: 16–70 years) showed a significant bone loss (lumbar spine BMD: −1.52%; total hip BMD: −2.05%) after 1 year from combination chemotherapy in patients with metastatic testicular tumor (n = 36), with no sign of recovery up to 5 years of follow-up. The decrease in BMD was not related with gonadal function, vitamin D levels, cisplatin cumulative dose, or corticosteroid administration. In contrast, stage I patients with no evidence of metastasis, treated with surgery alone or combined with a single dose of adjuvant chemotherapy, did not show any significant difference in BMD (37). In addition, lower BMD was observed in testicular germ-cell tumor patients treated with unilateral orchiectomy (n = 125) compared to age-matched controls (n = 41), despite the absence of hypogonadism (38). A cross-sectional study in 199 long-term testicular tumor survivors evaluated after a mean of 7.4 years from unilateral orchiectomy and in 45 newly diagnosed testicular tumor patients 3 months after orchiectomy showed an increased prevalence of mild and moderate vertebral fractures (40.2 and 31.1%, respectively) by the Genant's semi-quantitative method, independently of BMD, type of treatment, and gonadal function (39). Furthermore, osteopenia or osteoporosis was found in 43–51% of cases among a cohort of 1,249 long-term testicular tumor survivors. Hypogonadism more frequently occurred in patients with reduced BMD, but all survivors with osteopenia or osteoporosis showed lower testosterone levels. The patients treated with radiotherapy did not show a significantly worse BMD compared with those who received chemotherapy or surgery alone (23). Accordingly, the 9-year-long follow-up in 91 testicular tumor survivors (mean age: 31 years) revealed a significantly 6–8% lower hip BMD in both untreated and treated hypogonadal survivors compared to eugonodal ones and a significant 8% lower spinal BMD in untreated hypogonadal compared to eugonodal survivors (40), thus suggesting the increased risk of impaired bone health in hypogonadal testicular tumor survivors. By contrast, a single study on only 39 testicular tumor (TT) patients after a follow-up time ranging from 5 to 28 years did not find abnormal BMD in patients treated with surgery alone or with chemotherapy (41). Summary of available data from studies on bone mineralization in testicular cancer survivors is described in Table 4.
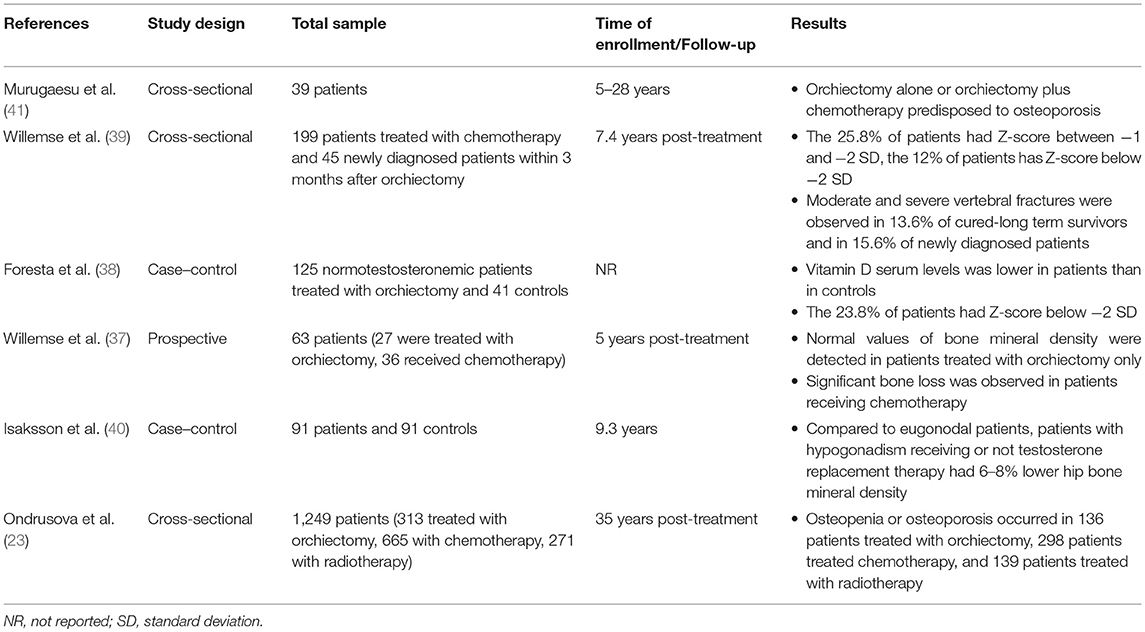
Table 4. Summary of available data from studies on bone mineralization in testicular cancer survivors.
In conclusion, vertebral fractures and impaired BMD occur in testicular tumor survivors, but it is still unclear whether it is related to hypogonadism or to cancer therapy-induced bone damage. Osteological examination should be considered in the follow-up of these patients.
Sexual Function
Sexual dysfunction is often experienced by testicular tumor survivors. The available evidence on this topic is summarized in Table 5.
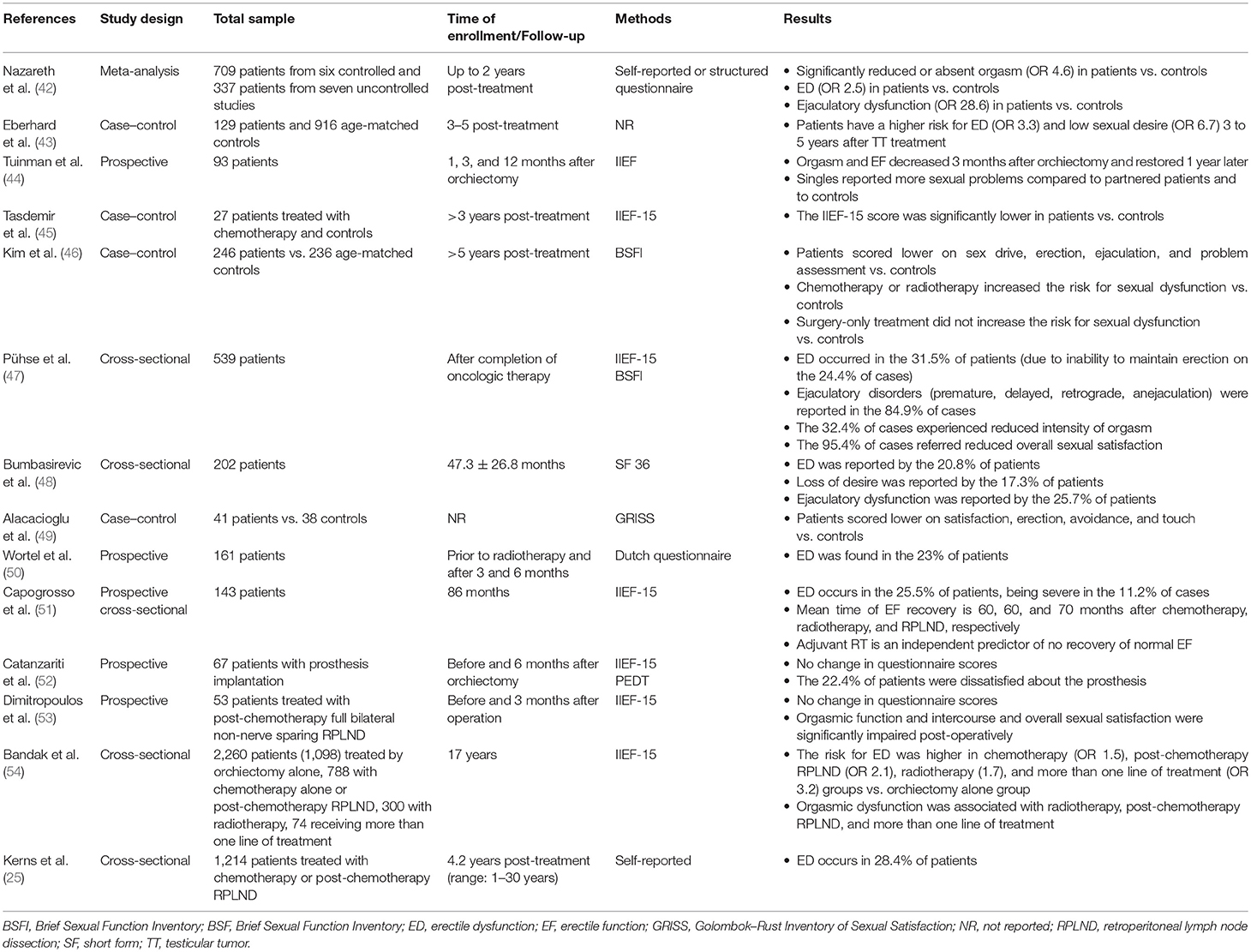
Table 5. Summary of available data from studies on sexual dysfunction in testicular tumor survivors.
Erection
A number of reports have evaluated the erectile function among testicular tumor survivors (36, 46). A multicenter study encompassing more than 1,200 survivors reported a 4.2-fold higher risk of erectile dysfunction (ED) in testicular tumor survivors compared with controls (25). The prevalence of ED has been esteemed to range from 30 to 40% (45–47, 55) in testicular tumor survivors, mainly assessed by the International Index of Erectile Function (IIEF) questionnaire and largely due to the incapacity to maintain the erection (47).
A meta-analysis of controlled studies found a ~2.5-fold greater risk of ED up to 2 years after treatment (42). Data from a longitudinal study showed a median time of erectile function recovery of 60, 60, and 70 months in patients receiving radiotherapy, chemotherapy, and RPLND, respectively, after a ~7.5-year-long follow-up in 143 Caucasian-European testicular tumor survivors. Only adjuvant radiotherapy emerged as an independent predictor of non-recovery (51). Accordingly, the Childhood Cancer Survivor Study indicated a negative impact of radiotherapy on erectile function, since a ≥10-Gr testicular irradiation dose was associated with a greater risk of ED (RR 3.55) among a cohort of 1,622 male cancer survivors (mean age: 37.2 years) (56). However, chemotherapy is also capable of negatively influencing sexual function. Data from a controlled study reported worse scores at the IIEF-15 and the Beck Anxiety questionnaire in patients receiving chemotherapy more than 3 years before evaluation compared to the age-matched controls who did not undergo to chemotherapy. The absence of any significant difference in serum gonadotropin and testosterone levels between the two groups suggests that the greater risk of ED is independent from hypogonadism. However, the small sample size (n = 27) limits the reliability of the study results (45). In addition, a longitudinal, cross-sectional study from 202 Serbian testicular tumor survivors followed-up for at least 1 year after platinum-based chemotherapy reported ED in 20.8% of cases (using the SF questionnaire). No patient of this cohort underwent testicular prosthesis implantation due to their socioeconomic background (48). Testicular prosthesis does not seem to affect sexual function per se; a part patients complain about is its consistence (52).
These results suggest that the type of testicular tumor has clear implications in the erectile function. Orchiectomy alone may be preferred to other treatment strategies, when possible. Moreover, following an initial post-therapy damage, the erectile function seems to re-establish itself 6 years after the treatment (51). However, a longer time of observation suggests different conclusions. Very recently, a comprehensive prospective study carried out in a cohort of 2,260 testicular tumor survivors reported an increased risk of ED after a 17-year-long follow-up. In greater detail, the study population included 1,098 patients who underwent orchiectomy alone, 788 treated with chemotherapy (BEP) alone or post-retroperitoneal surgery, 300 patients treated with radiotherapy, and 74 receiving more than one treatment. ED was assessed by the IIEF-15 questionnaire. Compared to orchiectomy alone, the survey showed an increased risk of ED in patients who received chemotherapy (OR 1.5), chemotherapy plus post chemotherapy testicular surgery (OR 2.1), RT (OR 1.7), or more than one type of treatment (OR 3.2) (54), thus showing that additional treatments negatively impact the erectile function. Accordingly, data from other reports agree with the worse impact of RPLND following chemotherapy on erectile function (57, 58).
Orgasm and Ejaculation
About one third of testicular tumor survivors experience ejaculation dysfunction (45). In addition, a ~2.3 higher risk of impaired ejaculation has been reported in these patients compared with controls, being even higher (OR 3.06) in non-seminoma patients (47). A meta-analysis of controlled studies found a decreased or absent orgasmic sensation associated with ejaculatory dysfunction in testicular tumor survivors up to 2 years after the treatment (59). After 17 years of follow-up, orgasmic dysfunction seems to persist and to associate with radiotherapy, chemotherapy plus post-chemotherapy RLND, and more than one line of treatment in 2,260 testicular tumor survivors (52).
Treatment options may also influence the ejaculatory function in testicular tumor survivors. Chemotherapy showed a greater risk of delayed ejaculation compared to radiotherapy and surgery (46). Full bilateral, non-nerve-sparing RLND may associate with ejaculatory disorders compared to other treatments, probably due to a damage on the sympathetic nerve fibers that control ante-grade ejaculation (53). Accordingly, despite no difference in erectile function following post-chemotherapy RLND observed, orgasmic function and satisfaction were significantly impaired post-operatively, compared to pre-operative function in a cohort of 53 patients (53).
Conclusion
Since the introduction of platinum-based chemotherapy and radiotherapy, the 10-year survival rate of patients with testicular tumor has exceeded 97%. The choice of treatment, especially in stage I, where treatment options include surveillance, adjuvant chemotherapy, or adjuvant radiotherapy (6), should take into consideration the risk for long-term complications. Longitudinal studies have revealed a higher negative impact of chemotherapy on Leydig cell function than radiotherapy or orchiectomy alone, leading to a higher risk for hypogonadism. Compared to orchiectomy alone, combined or high-dose chemotherapy and radiotherapy increase the risk for metabolic syndrome, DM, and cardiovascular events (Table 6). Furthermore, the long-term risk for ED is higher in patients treated with combined treatments, chemotherapy plus RPLND, radiotherapy, and chemotherapy compared to orchiectomy alone (Figure 2). On this account, orchiectomy and clinical surveillance should be preferred. Finally, management of testicular tumor survivors should include the evaluation of gonadal function, cardiovascular and metabolic profiles, BMD, and sexual function to timely detect any possible impairment.
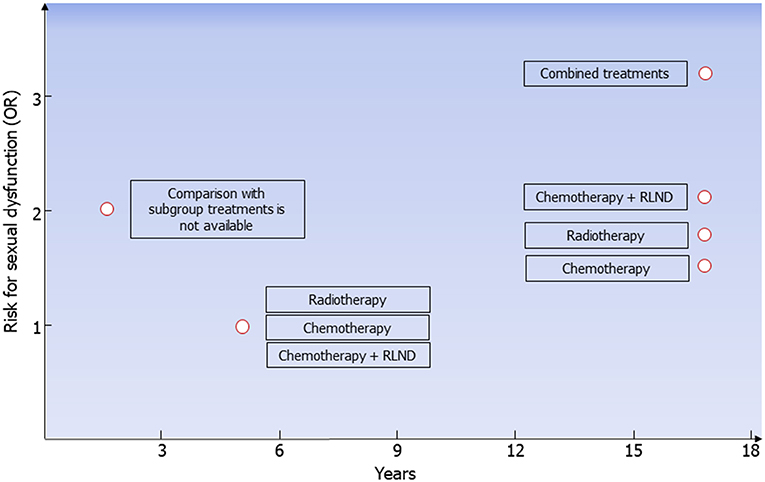
Figure 2. Risk for erectile dysfunction in testicular tumor survivors. According to data coming from all the available follow-up studies, risk for erectile dysfunction (ED) is higher 2 years after treatment in testicular tumor survivors. At the fifth year following radiotherapy, chemotherapy, or chemotherapy plus retroperitoneal lymph node dissection (RLND), the erectile function is apparently restored. The risk for ED is higher in patients treated with chemotherapy (OR 1.5), radiotherapy (OR 1.7), chemotherapy plus RLND (OR 2.1), and combined treatments (OR 3.2) compared to those treated with surgery only.
Author Contributions
RCa and RCo conceived the work and wrote the paper. RCa, FB, YD, GB, and MC identified the articles. AD and SL revised the paper critically and gave final approval. All authors read and approved the final manuscript.
Funding
This study has been partly funded by the University of Catania (Contribution for University Research–Research Plan 2016/2018). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN. Int J Cancer. (2015) 136:E359–86. doi: 10.1002/ijc.29210
2. Ghazarian AA, Trabert B, Devesa SS, McGlynn KA. Recent trends in the incidence of testicular germ cell tumors in the United States. Andrology. (2015) 3:13–8. doi: 10.1111/andr.288
3. Znaor A, Lortet-Tieulent J, Jemal A, Bray F. International variations and trends in testicular cancer incidence and mortality. Eur Urol. (2014) 65:1095–106. doi: 10.1016/j.eururo.2013.11.004
4. Moch H, Humphrey P, Ulbright T, Reuter V. WHO Classification of Tumors of the Urinary System and Male Genital Organs. 4th ed. Lyon: IARC (2016).
5. Williamson SR, Delahunt B, Magi-Galluzzi C, Algaba F, Egevad L, Ulbright TM, et al. The World Health Organization 2016. Classification of testicular germ cell tumours: a review and update from the International Society of Urological Pathology Testis Consultation Panel. Hystopathology. (2017) 70:335–46. doi: 10.1111/his.13102
6. Aparicio J, Terrasa J, Durán I, Germà-Lluch JR, Gironés R, González-Billalabeitia E, et al. SEOM clinical guidelines for the management of germ cell testicular cancer (2016). Clin Transl Oncol. (2016) 18:1187–6. doi: 10.1007/s12094-016-1566-1
7. Oldenburg J, Aparicio J, Beyer J, Cohn-Cedermark G, Cullen M, Gilligan T, et al. On behalf of: SWENOTECA (Swedish Norwegian Testicular Cancer group), the Italian Germ Cell Cancer Group (IGG), Spanish Germ Cell Cancer Group (SGCCG). Personalizing, not patronizing: the case for patient autonomy by unbiased presentation of management options in stage I testicular cancer. Ann Oncol. (2015) 26:833–8. doi: 10.1093/annonc/mdu514
8. De Palma A, Vicari E, Palermo I, D'Agata R, Calogero AE. Effects of cancer and anti-neoplastic treatment on the human testicular function. J Endocrinol Invest. (2000) 23:690–6. doi: 10.1007/BF03343795
9. Brydoy M, Fossa SD, Dahl O, Bjøro T. Gonadal dysfunction and fertility problems in cancer survivors. Acta Oncol. (2007) 46:480–9. doi: 10.1080/02841860601166958
10. Sprauten M, Brydøy M, Haugnes HS, Cvancarova M, Bjøro T, Bjerner J, et al. Longitudinal serum testosterone, luteinizing hormone, and follicle-stimulating hormone levels in a population-based sample of long-term testicular cancer survivors. J Clin Oncol. (2014) 32:571–8. doi: 10.1200/JCO.2013.51.2715
11. Joensen UN, Jørgensen N, Rajpert-De Meyts E, Skakkebaek NE. Testicular dysgenesis syndrome and Leydig cell function. Basic Clin Pharmacol Toxicol. (2008) 102:155–61. doi: 10.1111/j.1742-7843.2007.00197
12. La Vignera S, Calogero AE, Condorelli R, Marziani A, Cannizzaro MA, Lanzafame F, et al. Cryptorchidism and its long-term complications. Eur Rev Med Pharmacol Sci. (2009) 13:351–6.
13. Nord C, Bjøro T, Ellingsen D, Mykletun A, Dahl O, Klepp O, et al. FossåSD. Gonadal hormones in long-term survivors 10 years after treatment for unilateral testicular cancer. Eur Urol. (2003) 44:322–8. doi: 10.1016/S0302-2838(03)00263-X
14. Eberhard J, Ståhl O, Cwikiel M, Cavallin-Ståhl E, Giwercman Y, Salmonson EC. GiwercmanA. Risk factors for post-treatment hypogonadism in testicular cancer patients. Eur J Endocrinol. (2008) 158:561–70. doi: 10.1530/EJE-07-0684
15. Bandak M, Jorgensen N, Juul A, Lauritsen J, Kier MGG, Mortensen MS, et al. Longitudinal 282 changes in serum levels of testosterone and luteinizing hormone in testicular cancer patients after 283 orchiectomy alone or bleomycin, etoposide, and cisplatin. EurUrol Focus. (2018) 4:591–8. doi: 10.1016/j.euf.2016.11.018
16. Bandak M, Aksglaede L, Juul A, Rorth M, Daugaard G. The pituitary–Leydig cell axis before and 290 after orchiectomy in patients with stage I testicular cancer. Eur J Cancer. (2011) 47:2585–91. doi: 10.1016/j.ejca.2011.05.026
17. Huang HF, Pogach LM, Nathan E, Giglio W. Acute and chronic effects of cisplatinum upon testicular function in the rat. J Androl. (1990) 11:436–45.
18. Maines MD, Sluss PM, Iscan M. Cis-platinum-mediated decrease in serum testosterone is associated with depression of luteinizing hormone receptors and cytochrome P-450scc in rat testis. Endocrinology. (1990) 126:2398–406. doi: 10.1210/endo-126-5-2398
19. Huddart RA, Titley J, Robertson D, Williams GT, Horwich A, Cooper CS. Programmed cell death in response to chemotherapeutic agents in human germ cell tumour lines. Eur J Cancer. (1995) 31A:739–46.
20. Bang AK, Petersen JH, Petersen PM, Andersson AM, Daugaard G, Jørgensen N. Testosterone production is better preserved after 16 than 20 Gray irradiation treatment against testicular carcinoma in situ cells. Int J Radiat Oncol Biol Phys. (2009) 75:672–6. doi: 10.1016/j.ijrobp.2008.11.057
21. Jacobsen KD, Olsen DR, Fosså K, Fosså SD. External beam abdominal radiotherapy in patients with seminoma stage I: field type, testicular dose, and spermatogenesis. Int J Radiat Oncol Biol Phys. (1997) 38:95–102. doi: 10.1016/S0360-3016(96)00597-4
22. Bandak M, Jørgensen N, Juul A, Vogelius IR, Lauritsen J, Kier MG, et al. Testosterone deficiency in testicular cancer survivors—a systematic review and meta-analysis. Andrology. (2016) 4:382–8. doi: 10.1111/andr.12177
23. Ondrusova M, Spanikova B, Sevcikova K, Ondrus D. Testosterone deficiency and bone metabolism damage in testicular cancer survivors. Am J Mens Health. (2018) 12:628–3. doi: 10.1177/1557988316661986
24. Huddart RA, Norman A, Moynihan C, Horwich A, Parker C, Nicholls E, et al. Fertility, gonadal and sexual function in survivors of testicular cancer. Br J Cancer. (2005) 93:200–7. doi: 10.1038/sj.bjc.6602677
25. Kerns SL, Fung C, Monahan PO, Ardeshir-Rouhani-Fard S, Abu Zaid MI, Williams AM, et al. Platinum Study Group. Cumulative burden of morbidity among testicular cancer survivors after standard cisplatin-based chemotherapy: a multi-institutional study. J Clin Oncol. (2018) 36:1505–12. doi: 10.1200/JCO.2017.77.0735
26. La Vignera S, Condorelli R, Vicari E, D'Agata R, Calogero AE. Testicular microlithiasis: analysis of prevalence and associated testicular cancer in central–eastern Sicilian andrological patients. Andrologia. (2012) 44:295–9. doi: 10.1111/j.1439-0272.2011.01180.x
27. Dantsev IS, Ivkin EV, Tryakin AA, Godlevski DN, Latyshev OY, Rudenko VV, et al. Genes associated with testicular germ cell tumors and testicular dysgenesis in patients with testicular microlithiasis. Asian J Androl. (2018) 20:593–9. doi: 10.4103/aja.aja_54_18
28. Feldman DR, Schaffer WL, Steingart RM. Late cardiovascular toxicity following chemotherapy for germ cell tumors. J Natl Compr Canc Netw. (2012) 10:537–44. doi: 10.6004/jnccn.2012.0051
29. Boer H, Proost JH, Nuver J, Bunskoek S, Gietema JQ, Geubels BM, et al. Long-term exposure to circulating platinum is associated with late effects of treatment in testicular cancer survivors. Ann Oncol. (2015) 26:2305–10. doi: 10.1093/annonc/mdv369
30. Willemse PM, Burggraaf J, Hamdy NA, Weijl NI, Vossen CY, van Wulften L, et al. Prevalence of the metabolic syndrome and cardiovascular disease risk in chemotherapy-treated testicular germ cell tumour survivors. Br J Cancer. (2013) 109:60–7. doi: 10.1038/bjc.2013.226
31. Huddart RA, Norman A, Shahidi M, Horwich A, Coward D, Nicholls J, et al. Cardiovascular disease as a long-term complication of treatment for testicular cancer. J Clin Oncol. (2003) 21:1513–23. doi: 10.1200/JCO.2003.04.173
32. Meinardi MT, Gietema JA, van der Graaf WT, van Veldhuisen DJ, Runne MA, Sluiter WJ, et al. Cardiovascular morbidity in long-term survivors of metastatic testicular cancer. J Clin Oncol. (2000) 18:1725–32. doi: 10.1200/JCO.2000.18.8.1725
33. Haugnes HS, Wethal T, Aass N, Dahl O, Klepp O, Langberg CW, et al. Cardiovascular risk factors and morbidity in long-term survivors of testicular cancer: a 20-year follow-up study. J Clin Oncol. (2010) 28:4649–57. doi: 10.1200/JCO.2010.29.9362
34. Haugnes HS, Aass N, Fosså SD, Dahl O, Klepp O, Wist EA, et al. Components of the metabolic syndrome in long-term survivors of testicular cancer. Ann Oncol. (2007) 18:241–8. doi: 10.1093/annonc/mdl372
35. de Haas EC, Altena R, Boezen HM, Zwart N, Smit AJ, Bakker SJ, et al. Early development of the metabolic syndrome after chemotherapy for testicular cancer. Ann Oncol. (2013) 24:749–55. doi: 10.1093/annonc/mds527
36. Carpentier MY, Fortenberry JD. Romantic and sexual relationships, body image, and fertility in adolescent and young adult testicular cancer survivors: a review of the literature. J Adolesc Health. (2010) 47:115–25. doi: 10.1016/j.jadohealth.2010.04.005
37. Willemse PM, Hamdy NA, de Kam ML, Burggraaf J. Osanto S. Changes in bone mineral density in newly diagnosed testicular cancer patients after anticancer treatment. J Clin Endocrinol Metab. (2014) 99:4101–8. doi: 10.1210/jc.2014-1722
38. Foresta C, Selice R, De Toni L, Di Mambro A, Carraro U, Plebani M, et al. Altered bone status in unilateral testicular cancer survivors: role of CYP2R1 and its luteinizing hormone-dependency. J Endocrinol Invest. (2013) 36:379–84. doi: 10.3275/8650
39. Willemse PM, Hamdy NA, van Wulften L, van Steijn-van Tol AQ, Putter H, Osanto S. Prevalence of vertebral fractures independent of BMD and anticancer treatment in patients with testicular germ cell tumors. J Clin Endocrinol Metab. (2010) 95:4933–42. doi: 10.1210/jc.2010-0093
40. Isaksson S, Bogefors K, Åkesson K, Egund L, Bobjer J, Leijonhufvud I, et al. Risk of low bone mineral density in testicular germ cell cancer survivors: association with hypogonadism and treatment modality. Andrology. (2017) 5:898–904. doi: 10.1111/andr.12383
41. Murugaesu N, Powles T, Bestwick J, Oliver RT, Shamash J. Long-term follow-up of testicular cancer patients shows no predisposition to osteoporosis. Osteoporos Int. (2009) 20:1627–30. doi: 10.1007/s00198-008-0793-x
42. Nazareth I, Lewin J, King M. Sexual dysfunction after treatment for testicular cancer: a systematic review. J Psychosom Res. (2001) 51:735–43. doi: 10.1016/S0022-3999(01)00282-3
43. Eberhard J, Ståhl O, Cohn-Cedermark G, Cavallin-Ståhl E, Giwercman Y, Rylander L, et al. Sexual function in men treated for testicular cancer. J Sex Med. (2009) 6:1979–89. doi: 10.1111/j.1743-6109.2009.01298.x
44. Tuinman MA, Hoekstra HJ, Vidrine DJ, Gritz ER, Sleijfer DT, Fleer J, et al. Sexual function, depressive symptoms and marital status in nonseminoma testicular cancer patients: a longitudinal study. Psychooncology. (2010) 19:238–47. doi: 10.1002/pon.1560
45. Tasdemir C, Firdolas F, Harputluoglu H, Altintas R, Gunes A. Erectile dysfunction in testicular cancer patients treated with chemotherapy. Andrologia. (2012) 44:226–9. doi: 10.1111/j.1439-0272.2011.01271.x
46. Kim C, McGlynn KA, McCorkle R, Li Y, Erickson RL, Ma S, et al. Sexual functioning among testicular cancer survivors: a case–control study in the U.S. J Psychosom Res. (2012) 73:68–73. doi: 10.1016/j.jpsychores.2012.02.011
47. Pühse G, Wachsmuth JU, Kemper S, Husstedt IW, Evers S, Kliesch S. Chronic pain has a negative impact on sexuality in testis cancer survivors. J Androl. (2012) 33:886–93. doi: 10.2164/jandrol.110.012500
48. Bumbasirevic U, Bojanic N, Pekmezovic T, Janjic A, Janicic A, Milojevic B, et al. Health-related quality of life, depression, and sexual function in testicular cancer survivors in a developing country: a Serbian experience. Support Care Cancer. (2013) 21:757–63. doi: 10.1007/s00520-012-1577-6
49. Alacacioglu A, Ulger E, Varol U, Yavuzsen T, Akyol M, Yildiz Y, et al. Sexual satisfaction, anxiety, depression and quality of life in testicular cancer survivors. Med Oncol. (2014) 31:43. doi: 10.1007/s12032-014-0043-3
50. Wortel RC, Ghidey Alemayehu W, Incrocci L. Orchiectomy and radiotherapy for stage I–II testicular seminoma: a prospective evaluation of short-term effects on body image and sexual function. J Sex Med. (2015) 12:210–8. doi: 10.1111/jsm.12739
51. Capogrosso P, Boeri L, Ferrari M, Ventimiglia E, La Croce G, Capitanio U, et al. Long-term recovery of normal sexual function in testicular cancer survivors. Asian J Androl. (2016) 18:85–9. doi: 10.4103/1008-682X.149180
52. Catanzariti F, Polito B, Polito M. Testicular prosthesis: patient satisfaction and sexual dysfunctions in testis cancer survivors. Arch Ital Urol Androl. (2016) 88:186–8. doi: 10.4081/aiua.2016.3.186
53. Dimitropoulos K, Karatzas A, Papandreou C, Daliani D, Zachos I, Pisters LL, et al. Sexual dysfunction in testicular cancer patients subjected to post-chemotherapy retroperitoneal lymph node dissection: a focus beyond ejaculation disorders. Andrologia. (2016) 48:425–30. doi: 10.1111/and.12462
54. Bandak M, Lauritsen J, Johansen C, Kreiberg M, Skøtt JW, Agerbaek M, et al. Sexual function in a nationwide cohort of 2,260 survivors of testicular cancer after 17 years of followup. J Urol. (2018) 200:794–800. doi: 10.1016/j.juro.2018.04.077
55. Lackner JE, Koller A, Schatzl G, Marberger M. KratzikC. Androgen deficiency symptoms in testicular cancer survivors are associated with sexual problems but not with serum testosterone or therapy. Urology. (2009) 74:825–9. doi: 10.1016/j.urology.2009.03.051
56. Ritenour CW, Seidel KD, Leisenring W, Mertens AC, Wasilewski-Masker K, Shnorhavorian M, et al. Erectile dysfunction in male survivors of childhood cancer—a report from the childhood cancer survivor study. J Sex Med. (2016) 13:945–54. doi: 10.1016/j.jsxm.2016.03.367
57. Rudberg L, Nilsson S, Wikblad K. Health-related quality of life in survivors of testicular cancer 3 to 13 years after treatment. J Psychosoc Oncol. (2000) 18:19–31. doi: 10.1300/J077v18n03_02
58. Ozen H, Sahin A, Toklu C, Rastadoskouee M, Kilic C, Gogus A, et al. Psychosocial adjustment after testicular cancer treatment. J Urol. (1998) 159:1947–50.
Keywords: hypogonadism, testicular tumor, testosterone, sexual dysfunction, cardiovascular risk
Citation: La Vignera S, Cannarella R, Duca Y, Barbagallo F, Burgio G, Compagnone M, Di Cataldo A, Calogero AE and Condorelli RA (2019) Hypogonadism and Sexual Dysfunction in Testicular Tumor Survivors: A Systematic Review. Front. Endocrinol. 10:264. doi: 10.3389/fendo.2019.00264
Received: 29 January 2019; Accepted: 09 April 2019;
Published: 07 May 2019.
Edited by:
Andrea Garolla, University of Padova, ItalyReviewed by:
Ranjith Ramasamy, University of Miami, United StatesGiancarlo Balercia, Polytechnical University of Marche, Italy
Copyright © 2019 La Vignera, Cannarella, Duca, Barbagallo, Burgio, Compagnone, Di Cataldo, Calogero and Condorelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sandro La Vignera, sandrolavignera@unict.it.
†These authors have contributed equally to this work
 Sandro La Vignera
Sandro La Vignera Rossella Cannarella
Rossella Cannarella Ylenia Duca
Ylenia Duca Federica Barbagallo1
Federica Barbagallo1 Andrea Di Cataldo
Andrea Di Cataldo Aldo E. Calogero
Aldo E. Calogero Rosita A. Condorelli
Rosita A. Condorelli