- 1MOE, Shanghai Key Laboratory of Children's Environmental Health, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Department of Nuclear Medicine, Shanghai Tenth People's Hospital, Tongji University, Shanghai, China
- 3School of Public Health, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 4The Women and Children's Health Care Department, Shanghai First Maternity and Infant Hospital, Tongji University School of Medicine, Shanghai, China
Subclinical hypothyroidism (SCH) is a mild form of hypothyroidism that is common among women of childbearing age. The impact of SCH on adverse perinatal outcomes is unclear and universal screening for thyroid function before or during pregnancy is also much debated. In the present retrospective cohort study on 7,587 women from Shanghai, we assessed whether SCH was associated with adverse perinatal outcomes. The relationship between the risks of adverse outcomes and the time of screening and LT4 treatment status for SCH were also evaluated. SCH was associated with hypertensive disorders of pregnancy (HDP) [odds ratio (OR): 4.04; 95% confidence interval (CI): 1.85–8.84; P = 0.000]. After classification into four different groups based on the time of screening for thyroid function, the increased likelihood of HDP persisted in those diagnosed with SCH in the first and second trimesters (OR: 9.69; 95% CI: 1.73–54.48; P = 0.01 and OR: 3.66; 95% CI: 1.07–12.57, P = 0.03, respectively). The diagnosis of SCH in the preconception period and the third trimester was not significantly associated with HDP and other adverse perinatal outcomes. Five out of 120 (5/120) treated women (4.17%) vs. 4/45 untreated women (8.89%) developed HDP, 4/5 were treated after conception. The results indicate that during pregnancy, SCH conferred an increased risk of HDP, particularly in women diagnosed with the disorder in the first and second trimesters.
Introduction
Despite the well-known deleterious effects of overt thyroid dysfunction in women of reproductive age, the impact of subclinical hypothyroidism (SCH), a mild form of hypothyroidism defined as elevated thyroid stimulating hormone (TSH) greater than the upper limit of the reference range with normal free thyroxine (FT4) levels, on perinatal outcomes remains unclear (1, 2). On a worldwide scale, numerous studies with variable methodological quality have found inconsistent associations between SCH in pregnancy and adverse obstetrical outcomes including miscarriage, fetal death, preterm delivery, gestational diabetes, hypertensive disorders of pregnancy (HDP), placental abruption, low birth weight, 5 min Apgar scores <7 and lower IQ in childhood (3–6). The diagnostic criteria for SCH in pregnancy have changed over the years and vary between countries. These inconsistencies may be attributed to the differences in the definition of SCH (different TSH cut-offs), timing of TSH evaluation, and bias during enrolment of subjects and selection of end-point events (5, 7). Therefore, more studies including large samples and diverse populations are required to further evaluate the impact of SCH on prenatal outcomes.
To date, universal screening for thyroid dysfunction, both before and during pregnancy, is debated. The updated American Thyroid Association (ATA) guidelines published in 2017 concluded that there was insufficient evidence to make recommendations on the universal screening of thyroid dysfunction in the preconception phase or during early pregnancy (8). This may partly be due to the uncertainty regarding the impact of thyroid hormone replacement on maternal and neonatal outcomes. Two well-designed randomized controlled trials reported that maternal treatment for SCH did not result in improved cognitive function in their children (9, 10). However, since fetal thyroid hormones originate almost exclusively from the maternal system before 12–14 weeks of gestation, these results may have been influenced by delays in antenatal screening and initiation of treatment for hypothyroidism (in the second trimester) (11). Few studies have considered the impact of the timing of screening for SCH on perinatal outcomes. However, this is crucial for deciding the need and timing of routine screening of thyroid function in women who are trying to conceive.
In the present retrospective cohort study, we included 7,587 pregnant women to determine whether SCH is related to adverse perinatal outcomes. We also investigated the relationship between adverse perinatal outcomes and the timing of the first thyroid function test.
Materials and Methods
Study Population
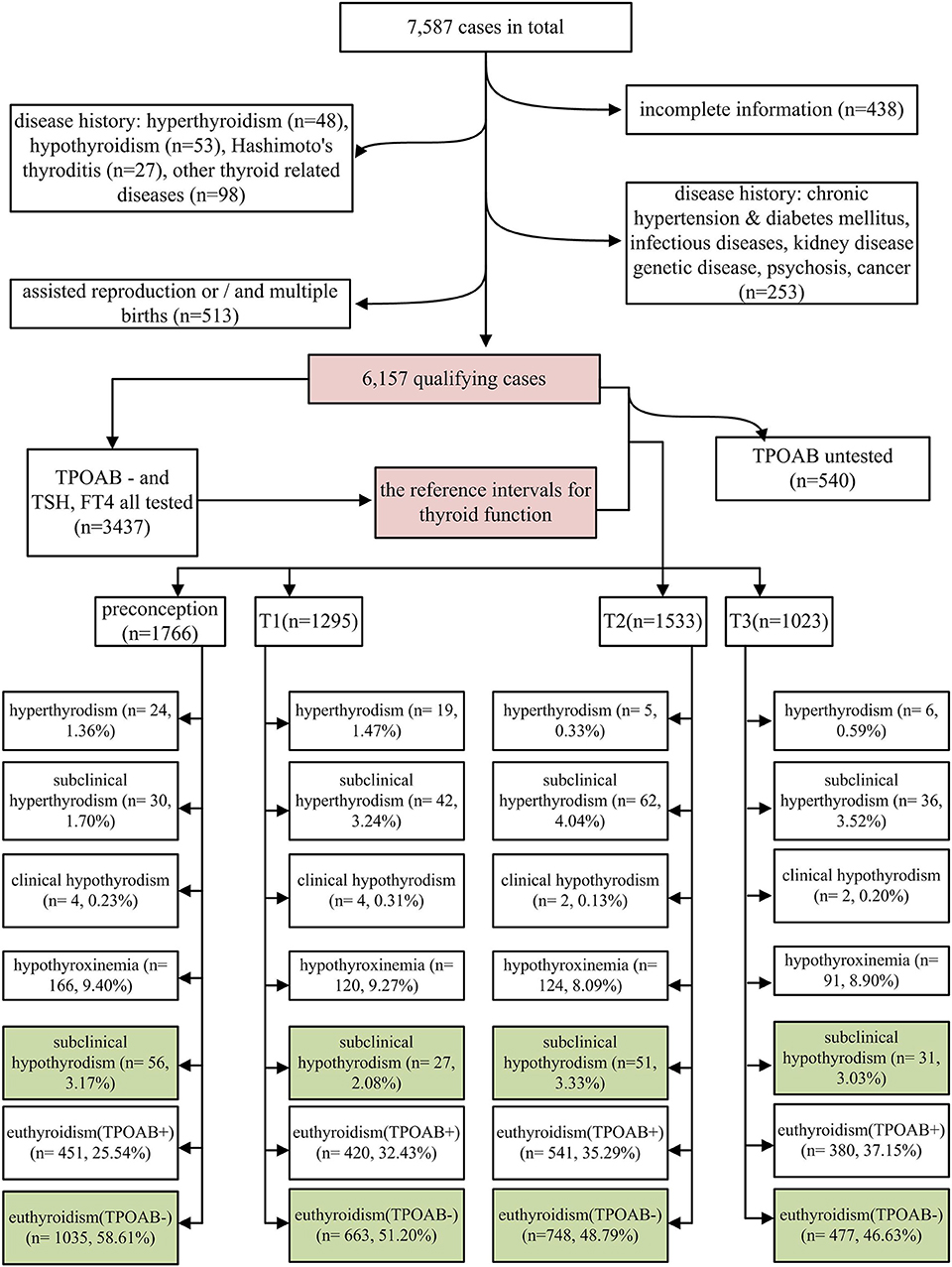
The study subjects were selected from a retrospective cohort study, which was conducted at the Shanghai First Maternity and Infant Hospital. The subjects were women who delivered at this hospital between January 1, 2015 and December 31, 2015. Each woman underwent thyroid function tests at least once during the 2 years prior to childbirth. A total of 7,587 women were identified as potential participants. A total of 438, 48, 53, 27, and 98 women with an incomplete history, hyperthyroidism, hypothyroidism, Hashimoto thyroiditis, and other thyroid diseases, respectively, were excluded in addition to 253 cases with a history of disease with possible impact on perinatal outcomes (including 10, 6, 3, 5, 12, 10, 201, and 6 cases with immune disorders, nephropathy, diabetes, hypertension, hereditary disease, cancer, infectious diseases, and other mental illness, respectively). Among the remaining 6,670 cases, 348, 290, and 125 cases of in-vitro fertilization (IVF), multiple births, and both IVF and multiple births, were excluded. Finally, 6,157 cases were eligible for analysis (Figure 1).
Group Classification
According to the earliest time of thyroid function testing, the subjects were categorized into 4 different groups, namely preconception (within 2 years before pregnancy), first trimester (<13 weeks), second trimester ≥13 and <28 weeks), and third trimester (≥28 weeks).
Trimester-Specific Reference Intervals of Thyroid Function
The present study established trimester-specific reference intervals of thyroid function according to the recommendations of the National Academy of Clinical Biochemistry (12) and the American Thyroid Association guidelines (8). The reference intervals of trimester-specific thyroid function are detailed in Tables S1, S2.
Evaluation of Maternal Thyroid Function and LT4 Treatment Status
Maternal thyroid function was defined as any of the following: (1) overt hyperthyroidism: serum TSH <0.1 mIU/L and FT4 above the trimester-specific reference interval (97.5th percentile), excluding gestational hyperthyroidism; (2) subclinical hyperthyroidism: serum TSH concentration below the statistically defined lower limit of the trimester-specific reference range with serum FT4 concentrations within trimester-specific reference ranges; (3) overt hypothyroidism: TSH concentration above the trimester-specific reference interval (97.5th percentile), with a decreased FT4 (<2.5th percentile of the trimester-specific reference interval) or TSH concentration above 10.0 mIU/L irrespective of the level of FT4; (4) hypothyroxinemia: normal TSH concentration (between the 2.5th and 97.5th percentiles) in conjunction with FT4 concentrations in the lower 10th percentile of the reference range; (5) SCH: TSH higher than the 97.5th percentile and FT4 between the 2.5th and 97.5th percentiles; (6) euthyroidism with thyroid peroxidase antibody positivity (TPOAb+): both serum TSH and FT4 within the reference range (2.5th−97.5th) and anti-TPOAb levels higher than the upper limit of the reference value provided by the test kit (60 IU/mL in this study); and (7) euthyroidism with thyroid peroxidase antibody negativity (TPOAb–): both serum TSH and FT4 within the reference range (2.5th−97.5th) and anti-TPOAb levels lower than the upper limit of the reference value provided by the test kit. In current study, we focused on SCH; euthyroid and TPOAb– women were considered as the reference group (CON: labeled green in Figure 1). LT4 treatment status was identified from the medical history of the 165 SCH women.
Evaluation of Adverse Perinatal Outcomes
The adverse perinatal outcomes were based on the diagnoses at discharge as per the medical records. We acquired data with a focus on the following adverse maternal and neonatal outcomes: gestational diabetes, hypertensive disorders of pregnancy (HDP), intrahepatic cholestasis of pregnancy (ICP), preterm labor, placenta previa, preterm premature rupture of membranes (PROM), fetal growth restriction (FGR), fetal distress, fetal death, low Apgar score (<7) at 5 min, low birth weight (birth weight <2,500 g), and macrosomia (birth weight >4,000 g). Factors that potentially impact the relationship between SCH and perinatal outcomes were chosen as potential confounders. We defined maternal age, gestational age, race, gravidity, parity, and delivery modality as possible confounding factors based on data from both, previous studies and our cohort.
Statistical Analysis
All data were expressed as means ± standard deviations or numbers and percentages. The Student's t- and chi-squared tests were used to compare continuous and categorical variables, respectively. The risks of adverse outcomes in patients with SCH were determined by the chi-squared test and were presented as odds ratios (ORs) with 95% confidence intervals (CIs). After adjusting for confounders, multivariable logistic regression analysis was used to assess the associations between maternal SCH and obstetric outcomes. Statistical analysis was performed using the SPSS 19.0 software (SPSS Inc., Chicago, IL, USA) package. P < 0.05 was considered to be statistically significant.
Results
Characteristics of Participants
Among 6,157 eligible cases, the overall incidence of SCH was 2.68%; the highest and lowest incidences of SCH were observed in the second (3.33%; 51 of 1,533 cases) and first (2.08%; 27 of 1,295 cases) trimesters, respectively (Figure 1). Notably, abnormal thyroid function was detected in about 50% of the cases (Figure 1). In all stages of the perinatal period, euthyroidism with TPOAb positivity was found to be the most common type of thyroid dysfunction (25.54–37.15%), followed by hypothyroxinemia (8.09–9.40%) (Figure 1). Out of the 165 women with SCH, 120 received LT4 treatment and 45 did not. Therefore, 5/120 treated women (4.17%) vs. 4/45 untreated women (8.89%) developed HDP, and even from the treated women, 4/5 were actually started on treatment late in pregnancy, while only 1/5 was started before pregnancy.
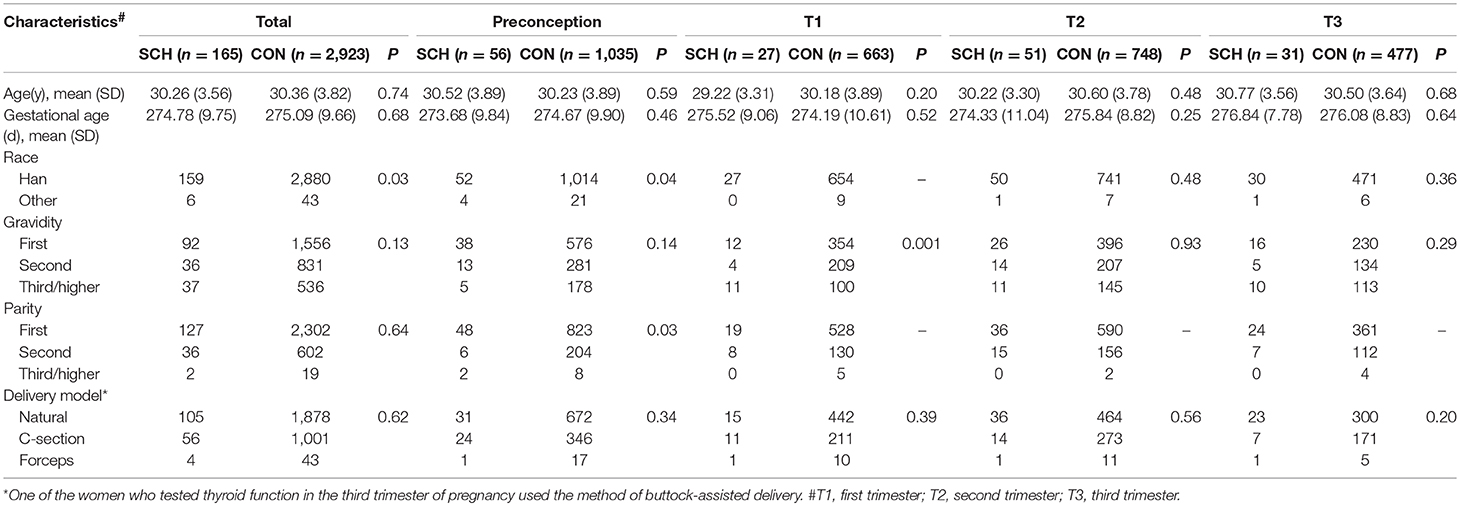
The general demographic characteristics of the participants are shown in Table 1, categorized as the SCH (n = 165) and CON (n = 2,923) groups. Prior to grouping by different prenatal stages, race (P = 0.03) was found to be statistically different between the two groups (Table 1). After grouping according to the phases of the perinatal period, there were statistically significant differences between the groups with regard to race (P = 0.04) and parity (P = 0.03) in the preconception period. In the first trimester, gravidity (P = 0.001) was significantly different between the two groups. No differences were found in the general characteristics between the SCH and CON groups in both, the preconception and third trimester periods.
Adverse Perinatal Outcomes in Women With SCH
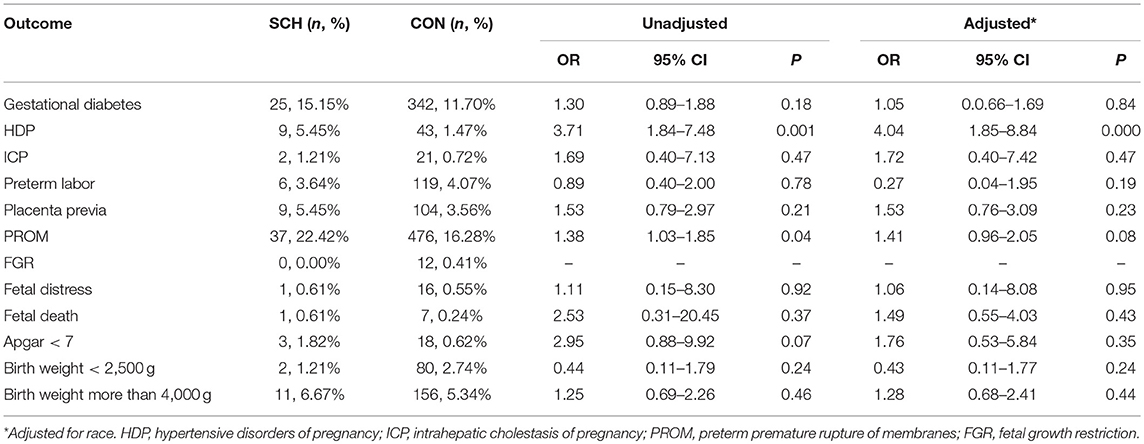
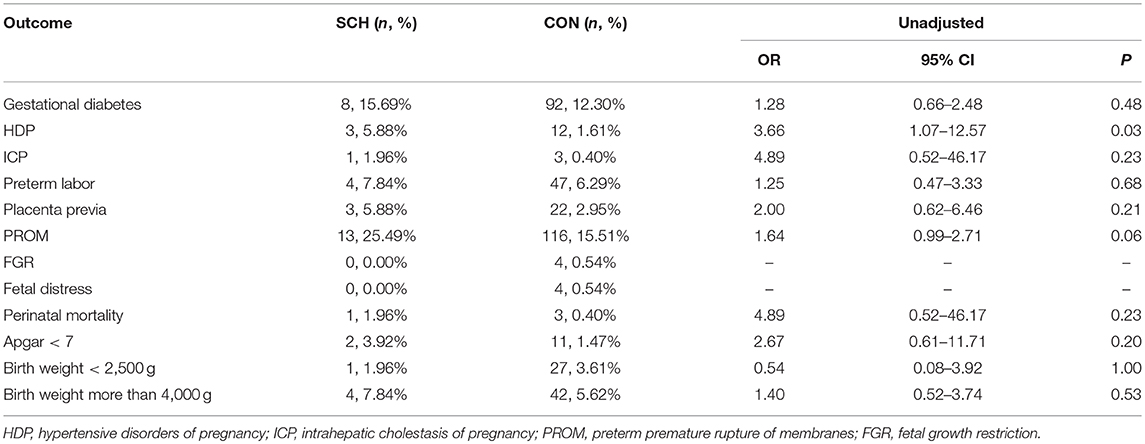
The adverse perinatal outcomes in the SCH and CON groups are demonstrated in Table 2. No significant differences were found in the incidence of gestational diabetes, ICP, preterm labor, placenta previa, FGR, fetal distress, fetal death, Apgar score <7, birth weight <2,500 g, and birth weight more than 4,000 g. In contrast, HDP (5.45% vs. 1.47%; OR: 3.71; 95% CI: 1.84–7.48; P = 0.001) and PROM (22.42%% vs. 16.28%; OR: 1.38; 95% CI: 1.03–1.85; P = 0.04) were significantly higher in the SCH group. However, after controlling for confounding factors, statistical difference between the two groups was noted only for HDP (OR: 4.04; 95% CI: 1.85–8.84; P = 0.000).
Relationship Between Group-Specific SCH and Adverse Perinatal Outcomes
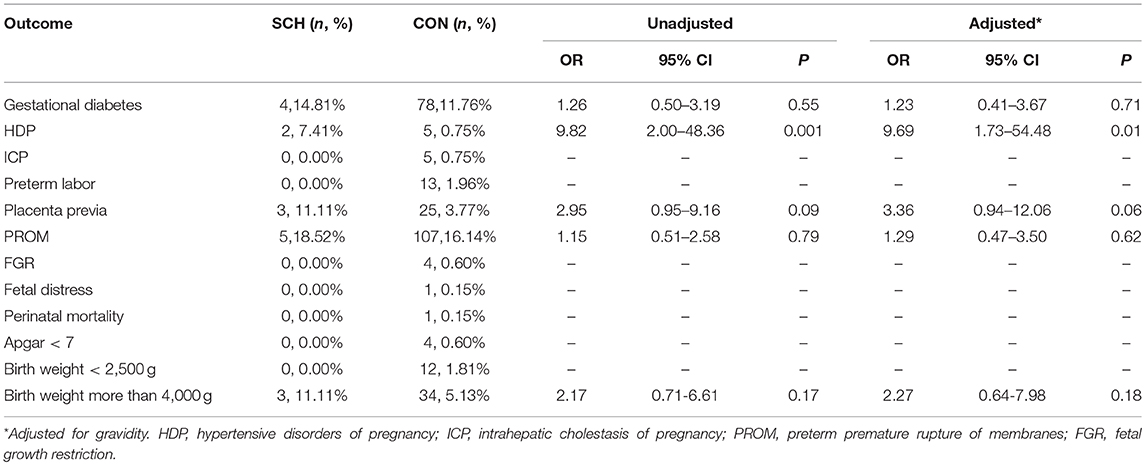
Based on the different stages of the perinatal period, SCH was not associated with an increased risk of pregnancy-related complications or adverse fetal growth outcomes in either the preconception or third trimester periods. However, in the first and second trimester, SCH was associated with HDP (OR: 9.69; 95% CI: 1.73–54.48; P = 0.01, and OR: 3.66; 95% CI: 1.07–12.57; P = 0.03, respectively) (Tables 3, 4). This indicates that pregnant women diagnosed with SCH in these stages were more likely to develop hypertension during the remainder of their pregnancy.
Discussion
Main Findings
In the present study, the overall incidence of SCH was 2.68%. In total, 5/120 treated women (4.17%) vs. 4/45 untreated women (8.89%) developed HDP, and from the treated women 4/5 were actually started on treatment late in pregnancy. Except for race, the demographic indices were similar between the SCH and CON groups. SCH in pregnancy was associated with a significantly increased risk of HDP, particularly in those who were diagnosed with SCH in the first and second trimester. Conversely, in women who were tested for thyroid function before conception and the third trimester, a detrimental effect of SCH on the risk of maternal and neonatal outcomes was not observed.
Data Interpretation and Comparisons to Findings in Previous Studies
The diagnostic criteria for SCH in pregnancy have changed over the years and vary between countries. Since the daily intakes of iodine, prevalence of thyroid autoimmunity, genetic backgrounds, and environmental factors vary between different populations, and the gestational age affects TSH levels, it is crucial to use a laboratory- or population-based trimester-specific TSH reference range for the diagnosis of SCH in pregnancy (8). Advances in the assessment of thyroid function have indicated that the interpretation of thyroid function tests depends on the stage of pregnancy (13). To facilitate precise evaluation of thyroid function during pregnancy, we used 4 different reference ranges for TSH and FT4 according to the 4 different perinatal stages. We also used population-derived 2.5th and 97.5th percentiles as reference intervals for the diagnosis of thyroid disorders, based on the overall analysis in this cohort. On the basis of these reference ranges, the incidence of SCH ranged from 2.08 to 3.33%. Studies using the same 97.5th percentile for the cut-off value for TSH as ours, have reported a similar prevalence of SCH (with an overall pooled-prevalence estimate of 3.47%) (1).
Reports suggest that SCH during pregnancy is associated with adverse perinatal outcomes, including miscarriage, preterm delivery, gestational diabetes, eclampsia, PROM, intrauterine growth restriction, and low birth weight (5, 14). Data from a few studies have also demonstrated that preconception SCH may be associated with a risk of infertility (15, 16). In the present study, we found a significant correlation between SCH and HDP; this finding concurs with that of many previous studies (11, 17, 18). However, published data have also suggested that SCH has no adverse impact on the outcomes of pregnancy (19, 20). The inconsistent results on the association between SCH and adverse pregnancy outcomes may be attributable to multiple factors, including the year the study was performed, the variable criteria used to identify the disease, and the gestational age at thyroid function screening.
HDP, including preeclampsia, gestational hypertension, chronic hypertension, and superimposed preeclampsia, has been shown to be a leading cause of maternal and perinatal morbidity and mortality in a multitude of large epidemiological studies worldwide (21–23). In our study, we observed that SCH was associated with HDP, particularly among women diagnosed with SCH in the first and second trimesters. However, the exact cause of HDP remains unknown. In addition to SCH, multiple factors including hormonal disorders, imbalances of angiogenic factors, and placental hypoxia also contribute to high blood pressure (24). In women with SCH, the mechanism for HDP may be based on decreased nitric oxide secretion and impairment of vasodilation in endothelial tissues (25). Hypercoagulability, increments in blood viscosity, and lipid abnormalities in patients with SCH potentially increase the risk for atherosclerosis; the increase of blood pressure in SCH may be related to these factors (26). However, the exact mechanism remains unclear.
In addition to the short-term impact on the mother and fetus, HDP also leads to an increase in the risk of other diseases later in life, including anxiety disorders in adolescent offspring and maternal cardiovascular diseases after pregnancy (27, 28). So early screening and treatment of SCH are both important. In our study, 5/9 women who developed HDP were intervened by LT4, but only one of them started before pregnancy. So it suggested that post-pregnancy treatment may reduce the chance for LT4 to have a beneficial effect. And the latest meta-analysis shows that LT4 replacement therapy can reduce blood pressure in SCH patients (29). But to date, the universal screening for thyroid function in childbearing women has been much debated in the scientific literature (6). Data on the role of thyroid screening on perinatal outcomes is limited, particularly for SCH. As the key parameter for SCH, the ATA guidelines of 2017 recommended screening for abnormal TSH concentrations in women planning assisted reproduction or known to have TPOAb positivity (8). Data on the role of thyroid screening in perinatal outcomes are limited, particularly with respect to SCH. Spencer et al. (30) suggested that compared with case finding or no screening, the universal screening of women before and during pregnancy was likely to be more effective in identifying women with thyroid dysfunction. In a large population-based cohort study in China, TSH elevation prior to conception was reportedly associated with an increased risk of adverse pregnancy outcomes, even in mild cases (7). Although the cases of SCH were not categorized in their study, the evidence suggests the need for perinatal screening of thyroid function; it also suggests that screening for TSH levels is particularly meaningful.
Compared to the adverse consequences in later life, screening for SCH in pregnancy is expected to be a cost-effective strategy. In the USA, analysis for cost-effectiveness has demonstrated that for every 100,000 pregnant women screened for SCH, $8,356,383 is saved, and 589.3 QALYs (marginal cost per quality-adjusted life year) are gained (31). Another study also reported that universal screening instead of high risk screening would result in an annual saving of €2,653,854 for the Spanish National Health System (32). The findings of this study suggest that if pregnancy is planned, universal screening should ideally be performed in the preconception period. However, in developing countries, the implementation of universal preconception thyroid screening may be hindered by economic constraints. The national preconception health examination project in China, which offers access to free preconception medical examinations, including thyroid hormone assays for rural couples planning a pregnancy, may be a good exemplary solution (33).
Strengths and Limitations
The large sample size and the inclusion of women in the preconception period are some of the strengths of the present study. However, it also has some limitations. First, it was a retrospective study; therefore, the data analyzed were from women who had already delivered. Consequently, pregnancy loss caused by various factors (including SCH) before delivery was not considered during analysis. Second, the study focused on two time points, namely, that of the earliest thyroid function test and the time of delivery. Although we were able to identify the women who received LT4 treatment, detailed information (drug dosage and effect) on the specific interventions for SCH were not available, since at our center, most women with abnormal thyroid function are referred to endocrinology clinics for optimal treatment. Third, after grouping, the number of HDP cases in each SCH sub-group during pregnancy was very small; this may have reduced the power of the test. Further prospective trials with larger samples are needed to elucidate the relationship between SCH in the preconception period and perinatal outcomes in the Chinese population. The role of LT4 supplements also needs further evaluation.
Conclusion
SCH during pregnancy, particularly when detected in the first and the second trimester, is associated with an increased risk of HDP, compared to euthyroid TPOAb- controls. Our findings suggest that routine thyroid function screening is necessary during pregnancy, particularly during the first and second trimesters.
Data Availability
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
This study complies with the guidelines of the Declaration of Helsinki. All data were processed anonymously.
Author Contributions
M-QW, JL, Y-QW, YY, C-HY, and JH contributed to study design and acquisition of research data. M-QW and JL conducted the data analysis. M-QW drafted the manuscript. All authors contributed to improvements of the manuscript for important intellectual content and approved the final version for publication.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 81703248], National Key R&D Program of China [grant number 2017YFC1600500] and Shanghai Municipal Health Bureau [grant number 20164Y0002]. The funders had no role in the conduct of the study, the analysis or interpretation of data, and the preparation, review, or approval of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are indebted to the excellent professional work of the research staff at Shanghai First Maternity and Infant Hospital.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2019.00522/full#supplementary-material
Abbreviations
CI, confidence interval; FGR, fetal growth restriction; FT4, free thyroxine; TPOAb+, thyroid peroxidase antibody positive; HDP, hypertensive disorders of pregnancy; ICP, intrahepatic cholestasis of pregnancy; IVF, in-vitro fertilization; LT4, levothyroxine; OR, odds ratio; PROM, preterm premature rupture of membranes; SCH, subclinical hypothyroidism; T1, first trimester; T2, second trimester; T3, third trimester; TPOAb–, thyroid peroxidase antibody negative; TSH, thyroid stimulating hormone.
References
1. Dong AC, Stagnaro-Green A. Differences in diagnostic criteria mask the true prevalence of thyroid disease in pregnancy: a systematic review and meta-analysis. Thyroid. (2019) 29:278–89. doi: 10.1089/thy.2018.0475
2. Korevaar TIM. The upper limit for tsh during pregnancy: why we should stop using fixed limits of 2.5 or 3.0 mu/l. Thyroid Res. (2018) 11:5. doi: 10.1186/s13044-018-0048-7
3. Yamamoto JM, Benham JL, Nerenberg KA, Donovan LE. Impact of levothyroxine therapy on obstetric, neonatal and childhood outcomes in women with subclinical hypothyroidism diagnosed in pregnancy: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. (2018) 8:e022837. doi: 10.1136/bmjopen-2018-022837
4. Maraka S, Ospina NM, O'Keeffe DT, Espinosa De Ycaza AE, Gionfriddo MR, Erwin PJ, et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid. (2016) 26:580–90. doi: 10.1089/thy.2015.0418
5. Maraka S, Singh Ospina NM, Mastorakos G, O'Keeffe DT. Subclinical hypothyroidism in women planning conception and during pregnancy: who should be treated and how? J Endocr Soc. (2018) 2:533–46. doi: 10.1210/js.2018-00090
6. Velasco I, Taylor P. Identifying and treating subclinical thyroid dysfunction in pregnancy: emerging controversies. Eur J Endocrinol. (2018) 178:D1–12. doi: 10.1530/eje-17-0598
7. Chen S, Zhou X, Zhu H, Yang H, Gong F, Wang L, et al. Preconception tsh and pregnancy outcomes: a population-based cohort study in 184 611 women. Clin Endocrinol. (2017) 86:816–24. doi: 10.1111/cen.13329
8. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. (2017) 27:315–89. doi: 10.1089/thy.2016.0457
9. Lazarus JH, Bestwick JP, Channon S, Paradice R, Maina A, Rees R, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. (2012) 366:493–501. doi: 10.1056/NEJMoa1106104
10. Casey BM, Thom EA, Peaceman AM, Varner MW, Sorokin Y, Hirtz DG, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. (2017) 376:815–25. doi: 10.1056/NEJMoa1606205
11. Chen LM, Du WJ, Dai J, Zhang Q, Si GX, Yang H, et al. Effects of subclinical hypothyroidism on maternal and perinatal outcomes during pregnancy: a single-center cohort study of a chinese population. PLoS ONE. (2014) 9:e109364. doi: 10.1371/journal.pone.0109364
12. Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. (2003) 13:3–126. doi: 10.1089/105072503321086962
13. Lazarus JH. Thyroid function in pregnancy. Br Med Bull. (2011) 97:137–48. doi: 10.1093/bmb/ldq039
14. Nelson DB, Casey BM, McIntire DD, Cunningham FG. Subsequent pregnancy outcomes in women previously diagnosed with subclinical hypothyroidism. Am J Perinatol. (2014) 31:77–84. doi: 10.1055/s-0033-1334457
15. Abalovich M, Mitelberg L, Allami C, Gutierrez S, Alcaraz G, Otero P, et al. Subclinical hypothyroidism and thyroid autoimmunity in women with infertility. Gynecol Endocrinol. (2007) 23:279–83. doi: 10.1080/09513590701259542
16. Feldthusen AD, Pedersen PL, Larsen J, Toft Kristensen T, Ellervik C, Kvetny J. Impaired fertility associated with subclinical hypothyroidism and thyroid autoimmunity: the Danish general suburban population study. J Pregnancy. (2015) 2015:132718. doi: 10.1155/2015/132718
17. Ramtahal R, Dhanoo A. Subclinical hypothyroidism causing hypertension in pregnancy. J Am Soc Hypertens. (2016) 10:691–3. doi: 10.1016/j.jash.2016.06.039
18. Wilson KL, Casey BM, McIntire DD, Halvorson LM, Cunningham FG. Subclinical thyroid disease and the incidence of hypertension in pregnancy. Obstet Gynecol. (2012) 119:315–20. doi: 10.1097/AOG.0b013e318240de6a
19. Cleary-Goldman J, Malone FD, Lambert-Messerlian G, Sullivan L, Canick J, Porter TF, et al. Maternal thyroid hypofunction and pregnancy outcome. Obstet Gynecol. (2008) 112:85–92. doi: 10.1097/AOG.0b013e3181788dd7
20. Mannisto T, Vaarasmaki M, Pouta A, Hartikainen AL, Ruokonen A, Surcel HM, et al. Thyroid dysfunction and autoantibodies during pregnancy as predictive factors of pregnancy complications and maternal morbidity in later life. J Clin Endocrinol Metab. (2010) 95:1084–94. doi: 10.1210/jc.2009-1904
21. Heath LJ, Hyde H, Miller C, Norris JM. Investigation of elevation as a risk factor for hypertensive disorders of pregnancy among colorado women between 2007 and 2015. Hypertens Pregnancy. (2019) 38:1–12. doi: 10.1080/10641955.2018.1538378
22. Mustafa R, Ahmed S, Gupta A, Venuto RC. A comprehensive review of hypertension in pregnancy. J Pregnancy. (2012) 2012:105918. doi: 10.1155/2012/105918
23. Ying W, Catov JM, Ouyang P. Hypertensive disorders of pregnancy and future maternal cardiovascular risk. J Am Heart Assoc. (2018) 7:e009382. doi: 10.1161/jaha.118.009382
24. Braunthal S, Brateanu A. Hypertension in pregnancy: pathophysiology and treatment. SAGE Open Med. (2019) 7:2050312119843700. doi: 10.1177/2050312119843700
25. Cakmak BD, Turker UA, Temur M, Ustunyurt E. Pregnancy outcomes of antibody negative and untreated subclinical hypothyroidism. J Obstet Gynaecol Res. (2019) 45:810–16. doi: 10.1111/jog.13925
26. Liu D, Jiang F, Shan Z, Wang B, Wang J, Lai Y, et al. A cross-sectional survey of relationship between serum tsh level and blood pressure. J Hum Hypertens. (2010) 24:134–8. doi: 10.1038/jhh.2009.44
27. Riise HKR, Sulo G, Tell GS, Igland J, Egeland G, Nygard O, et al. Hypertensive pregnancy disorders increase the risk of maternal cardiovascular disease after adjustment for cardiovascular risk factors. Int J Cardiol. (2019) 282:81–7. doi: 10.1016/j.ijcard.2019.01.097
28. Dachew BA, Scott JG, Mamun A, Alati R. Hypertensive disorders of pregnancy and the risk of anxiety disorders in adolescence: findings from the avon longitudinal study of parents and children. J Psychiatr Res. (2019) 110:159–65. doi: 10.1016/j.jpsychires.2019.01.001
29. He W, Li S, Zhang JA, Zhang J, Mu K, Li XM. Effect of levothyroxine on blood pressure in patients with subclinical hypothyroidism: a systematic review and meta-analysis. Front Endocrinol. (2018) 9:454. doi: 10.3389/fendo.2018.00454
30. Spencer L, Bubner T, Bain E, Middleton P. Screening and subsequent management for thyroid dysfunction pre-pregnancy and during pregnancy for improving maternal and infant health. Cochrane Database Syst Rev. (2015) Cd011263. doi: 10.1002/14651858.CD011263.pub2
31. Thung SF, Funai EF, Grobman WA. The cost-effectiveness of universal screening in pregnancy for subclinical hypothyroidism. Am J Obstet Gynecol. (2009) 200:267.e261–7. doi: 10.1016/j.ajog.2008.10.035
32. Donnay Candil S, Balsa Barro JA, Alvarez Hernandez J, Crespo Palomo C, Perez-Alcantara F, Polanco Sanchez C. [cost-effectiveness analysis of universal screening for thyroid disease in pregnant women in spain]. Endocrinol Nutr. (2015) 62:322–30. doi: 10.1016/j.endonu.2015.03.007
Keywords: subclinical hypothyroidism, pregnancy, hypertensive disorders of pregnancy, thyroid function, thyroid function screening
Citation: Wu M-Q, Liu J, Wang Y-Q, Yang Y, Yan C-H and Hua J (2019) The Impact of Subclinical Hypothyroidism on Adverse Perinatal Outcomes and the Role of Thyroid Screening in Pregnancy. Front. Endocrinol. 10:522. doi: 10.3389/fendo.2019.00522
Received: 06 May 2019; Accepted: 16 July 2019;
Published: 06 August 2019.
Edited by:
Alex Stewart Stagnaro-Green, University of Illinois at Chicago, United StatesReviewed by:
Spyridoula Maraka, University of Arkansas for Medical Sciences, United StatesMohd Shazli Shazli Draman, Cardiff University, United Kingdom
Copyright © 2019 Wu, Liu, Wang, Yang, Yan and Hua. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chong-Huai Yan, yanchonghuai@xinhuamed.com.cn; Jing Hua, szhuaj@hotmail.com
†These authors have contributed equally to this work
 Mei-Qin Wu
Mei-Qin Wu Jin Liu
Jin Liu Ya-Qian Wang
Ya-Qian Wang Ying Yang
Ying Yang Chong-Huai Yan
Chong-Huai Yan Jing Hua
Jing Hua