- 1Faculty of Medicine, School of Health Sciences, University of Ioannina, Ioannina, Greece
- 2Department of Medical, Oncology, Greece Society for Study of Clonal Heterogeneity of Neoplasia (EMEKEN), University Hospital of Ioannina, Ioannina, Greece
- 3Associated Scientific Partner, Athens, Greece
As the incidence of malignancies in young adults is increasing, fertility preservation in cancer survivors arises as a major concern. Especially among female cancer patients, pregnancy rates are estimated to be 40% lower compared to women of the same age. Nowadays oncologists are to be preoccupied not only with their patients’ successful treatment, but also with the maintenance of the potential of the latter to conceive and obtain children. Chemotherapy associated ovarian failure (COF), refers to disruption of ovarian function both as an endocrine gland and as a reproductive organ, due to previous exposure to chemotherapy agents. Although the underlying mechanism is not fully understood, it is supposed that chemotherapy agents may induce either DNA damage of premature ovarian follicle or early activation and apoptosis of them, resulting into early exhaustion of available follicle deposit. Various chemotherapy agents have been associated with COF with the highest incidence being reported for patients undergoing combination regimens. Although a variety of alternatives in order to maintain ovarian function and fertility in female cancer survivors are available, adequately established practices to do so are lacking. Thus, it is of major importance to investigate further and collect sufficient evidence, aiming to guide patients and physicians in everyday clinical practice.
Introduction
Over 6.6 million women are estimated to be annually diagnosed with cancer, about 10% of them being younger than 40 years old (1). On the other hand, in modern Western societies, an increasing proportion of women delay their first pregnancy until the fourth decade of life (2). Notably, female cancer survivors are 40% less probable to become pregnant, compared to healthy women, with low pregnancy rates mainly reported among patients diagnosed with leukemia, cervical and breast cancer (3, 4). In this context, nowadays oncologists are not only to be preoccupied with their patients’ successful treatment, but also with major fertility preservation concerns. In the following paragraphs, we attempt to summarize the underlying mechanisms of COF, as well as the current therapeutic and preventive strategies addressing female fertility maintenance dilemmas.
COF—Definition and Associated Antineoplastic Agents
Chemotherapy associated ovarian failure (COF) refers to disruption of both endocrine and reproductive ovarian function, after exposure to chemotherapy. It is defined as either the absence of regular menses in premenopausal female patients or as increased FSH levels (>40 IU/L) (5).
In 2006, the American Association of clinical oncology attempted to sort antineoplastic regimens, according to the associated fertility compromise risk. Hematopoietic stem cell transplant (HSCT) initiation regimens steadily compromise patients’ fertility, while gonadotoxicity of adjuvant chemotherapy regimens against early breast cancer varies with duration of exposure and patient’s age. Characteristically, triple agent combinations, such as CMF (cyclophosphamide, methotrexate, fluorouracil), entail a high risk of infertility if administered for more than four cycles in women older than 40, whereas the risk is significantly reduced for younger patients. Notably, vincristine, methotrexate, and fluorouracil do not impose considerable fertility hazards, while there are no sufficient date regarding taxanes, oxaliplatin, and targeted treatments (6) (Table 1).
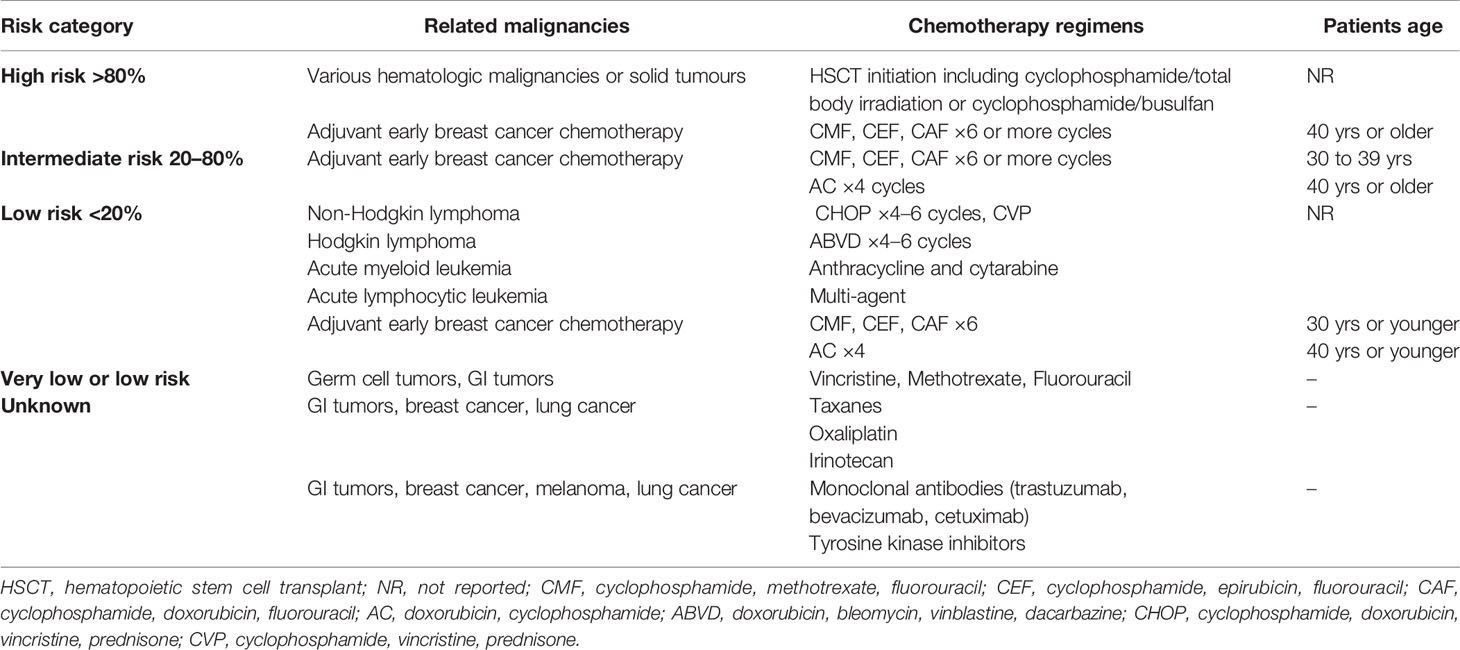
Table 1 Risk of infertility associated with antineoplastic systematic treatment [based on American Society of Clinical Oncology Recommendations on Fertility Preservation in Cancer Patients, (6).
Considering the finite number of follicles available in the ovaries and their co-existence in different stages of development, variable pathophysiologic mechanisms have been proposed to underlie chemotherapy induced ovarian failure (see Table 2). These include:
a. “Accelerated” ovarian follicle maturation: Chemotherapy agents induce apoptosis of mature, functioning ovarian follicles, resulting in depression of estrogen and anti-müllerian hormone negative feedback on the gonadotropic cells of the anterior pituitary. Constantly elevated gonadotropins may accelerate maturation of premature ovarian follicles, which, in their turn, enter apoptosis under systematic chemotherapy, thus the gradual exhaustion of ovarian follicles deposit (5, 7, 8). Supporting evidence comes from histology studies of murine ovarian tissue, in cyclophosphamide treated mice, showing increased population of early growing follicles, in parallel with elimination of the quiescent ones (8). The enhanced phosphorylation of proteins involved in the maturation of primordial follicles seems to be mediated via the PI3K/PTEN/Akt signaling pathway, which may also be activated due to a direct effect of chemotherapy on oocytes and on pregranulosa cells supporting them (7–9).
b. Direct quiescent follicle DNA damage: Non-cell cycle specific chemotherapeutics, such as alkylating agents and doxorubicin, can induce formation of cross-links in the DNA of non-dividing, dormant oocytes. The subsequent accumulation of DNA strand breaks activates the pro-apoptotic intracellular pathways, leading to apoptosis of the affected ovarian follicles (10). Relevant supporting evidence derives from studies of human oocyte in vitro cultures and human ovarian xenograft murine models, exposed to doxorubicin (11) and cyclophosphamide (12), revealing double strand breaks and features of apoptotic death in premature oocytes.
c. Disrupted ovarian vascularization: Chemotherapy may compromise the functionality of ovarian vasculature and stroma supporting the gonadal cells. Local vascular spasm reducing ovarian blood flow, fibrosis of the ovarian cortex affecting blood vessel formation, inhibition of angiogenesis, are some of the described associated mechanisms. Relative evidence has been found in in vitro and murine xenograft studies of human ovarian tissue, as well as mouse ovaries, exposed to doxorubicin (10, 13).
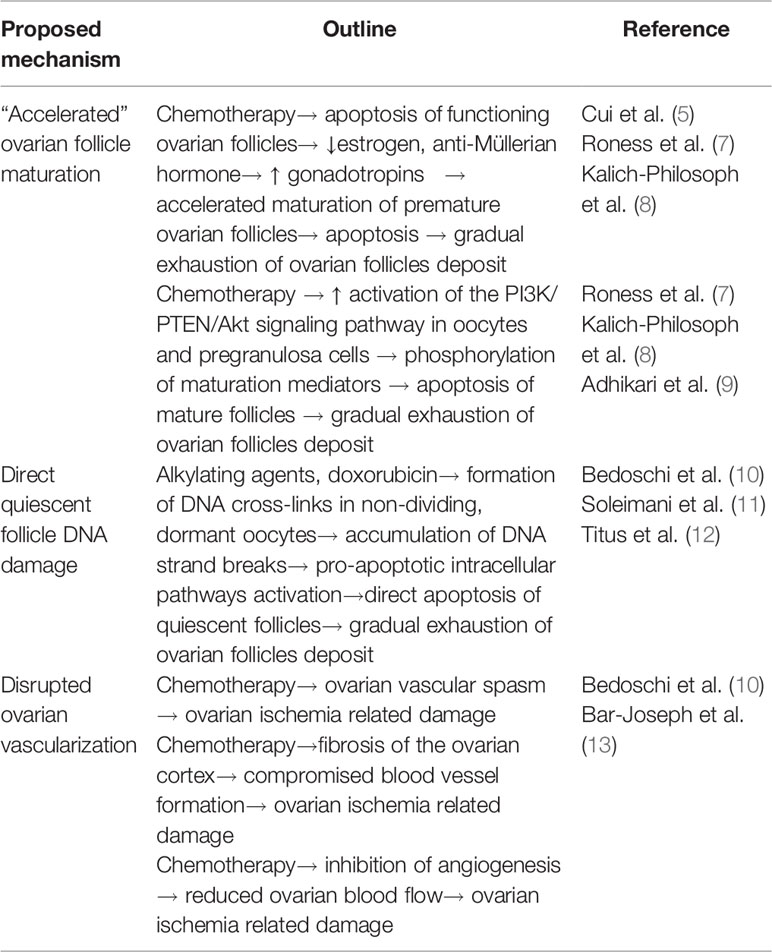
Table 2 Summary of the suggested underlying mechanisms by which chemotherapy compromises follicular ovarian reserve (5, 7–13).
Ovarian Function Preservation Approaches
GnRH Analogs: Attempting to Block Premature Follicle Activation
Constant GnRH analogs administration during chemotherapy has been thought to inhibit early ovarian follicle recruitment, by desensitizing hypophysis to the innate GnRH effect (7). GnRH analogs have been mostly employed for fertility preservation in early breast cancer and lymphoma female patients, with ambiguous results.
About 20.038 women aged between 15 and 44 years are yearly diagnosed with early breast cancer in the US, and 97% of them face a risk of infertility due to adjuvant chemotherapy, while half of them wish to have children (14). The potential protective effect of GnRHa administration concurrently with adjuvant chemotherapy, in order to protect ovarian function, has been addressed in several clinical trials (15–17).
A metanalysis of seven placebo-controlled, randomized clinical trials, recruiting 1,047 patients, conducted between 1975 and 2015 seems to favor GnRH administration (15). GnRH analogs employed included goserelin (three trials), triptorelin (three trials), and leuprolide (one trial), while chemotherapy consisted mainly of anthracyclines, cyclophosphamide, and taxanes. Use of tamoxifen was reported in six trials (0% in three trials and about 70% in another three trials). GnRHa administration seemed to double rates of regular menstruation, compared to placebo, at 6 (OR = 2.41, 95% CI 1.40–4.15, p = 0.002) and 12 months (OR = 1.85, 95% CI 1.33–2.59, p = 0.0003) after chemotherapy withdrawal. Patients on GnRHa during adjuvant chemotherapy also seemed to have almost twice the chance of pregnancy, compared to the untreated women (OR 1.85, 95% IC 1.02–3.36, p = 0.04) (15, 17).
In a more recent report of the PROMISE-GIM6 trial, included in the above metanalysis, neither recovery of menstruation (HR of 1.28, 95% CI 0.98–1.68, p = 0.071) nor pregnancy rates (2.56, 95% CI 0.68–9.6, p = 0.142) were found significantly higher in triptorelin treated patients, at 7 years of follow-up (18). Nonetheless, patients’ age may act as a confounding factor; in the OPTION trial (19), goserelin administration conferred an advantage in patients younger than 40 years old, as COF was observed at 2.6% of goserelin treated patients and at 20% (p = 0.038) of placebo treated patients, while no benefit was established in the total trial population (COF incidence in 18.5 vs. 34.8% in the goserelin and control arm, respectively, p = 0.048).
Hodgkin and non-Hodgkin lymphomas are estimated to affect up to 18 women aged between 15 and 39 years old, per 100.000 of population (20, 21). A metanalysis of three randomized clinical trials and four case-control series, including a total of 434 lymphoma patients under systematic chemotherapy, deduced that GnRHa treatment seemed to decrease the incidence of COF, defined as increased FSH level, by 68% (OR 0.32 95% CI, 0.13–0.77, p = 0.01) (22). In contrast, spontaneous pregnancy rates were not significantly affected with 13.5 and 11% of survivors getting pregnant in GnRHa and placebo treated groups, respectively (OR = 1.11, 95% CI 0.55–2.26, p = 0.75). Nevertheless, the most recent, randomized, multicenter clinical trial addressing COF in female patients having undergone chemotherapy for Hodgkin or non-Hodgkin lymphomas suggests otherwise (23). Among 67 evaluable lymphoma female patients treated with alkylating agents between 2002 and 2008, COF (defined as at least one measurement of FSH level >40 IU/L) occurred in 19.5 and 25% of patients in the triptorelin and control arm respectively. Triptorelin administration was not an independent prognostic factor for patient protection from COF, in the multivariate analysis (OR = 0.7, 95% CI 0.15–3.24, p = 0.651); in this trial, occurrence of COF after chemotherapy was found to be increased 70-fold after an initiation regimen for hematopoietic stem cell transplant, and 10-fold by administration of a cumulative dose of cyclophosphamide greater than 5 g/m2. In addition, both groups achieved similar pregnancy rates (53% in the triptorelin treated patients, and 43% in the placebo, p = 0.467), three pregnancies occurring among placebo treated women diagnosed with protocol defined COF.
There is only one small, randomized clinical trial addressing the effectiveness of GnRHa in patients treated for ovarian cancer, with conservative surgery and adjuvant chemotherapy. Thirty patients aged between 12 and 45 years, among whom 20 were diagnosed with germ cell tumors, were 1:1 randomized to receive the GnRHa diphereline or nothing, during chemotherapy. Employed regimens included BEP (bleomycin, etoposide, cisplatin) (13 in GnRHa arm/9 in control arm), Carboplatin plus paclitaxel (2 in GnRHa arm/4 in control arm), cisplatin plus paclitaxel (0 in GnRHa arm/1 in control arm), VAC (0 in GnRHa arm/1 in control arm). COF was defined as permanent absence of menses and FSH higher than 20 mIU/ml at 6 months after chemotherapy completion. All patients receiving diphereline experienced recovery of menses, and had premenopausal FSH and estradiol values, whereas one third of patients in the control group had permanent cessation of menses, high FSH, and low estradiol levels. Remarkably, cyclophosphamide and cisplatin, the two most gonadotoxic agents, were administered only in two patients, both of them in the control group, while it is not reported if these two patients were among the five ones experiencing permanent COF (24).
Moreover, in a small study pre- and post-menarchal patients treated for Hodgkin and non-Hodgkin lymphoma, thymoma, acute myeloid, and lymphoid leukemia, GnRH treatment confined a more notable benefit in preserving menstruation and fertility in postpubertal patients, whereas prepubertal girls seemed to be at less risk of COF, even in the absence of GnRH treatment (25).
In conclusion, GnRH analog treatment is not adequately established and it is not currently suggested as a reliable measure of fertility preservation by international guidelines, although it appears to have some protective effect, especially in younger patients. More studies and more long-term results of the already conducted trials are needed to further investigate this question.
Oocyte/Embryo Cryopreservation
Oocyte or embryo cryopreservation may be recommended to premenopausal women affected by any type of malignancy (4). Oocyte cryopreservation is performed by ovarian hyperstimulation by gonadotropins and freezing of the transvaginally retrieved mature oocytes. The embryo cryopreservation protocols include in vitro insemination of the collected oocytes before storage. When conception is desired, either defrosted in vitro fertilized oocytes or defrosted embryos are introduced in the patient (26). Little is known about the potential of the ovarian stimulation to promote growth of hormone-driven neoplasms, suggesting that this strategy should probably be withheld for aggressive and hormone sensitive disease (4, 27).
The oocyte cryopreservation protocol (26) begins with controlled ovarian stimulation of the patient, by administration of FSH, follitropin alpha, lutropin alfa, and urofolitropin, starting at 2–3 days after the onset of menstruation. Mature oocytes then transvaginally collected under ultrasound guidance, after hCG administration. Oocyte insemination for embryo preservation is achieved via in vitro intracytoplasmic sperm injection (ICSI). Oocytes or embryos are then exposed to an ethyl glycol and dimethylsulphoxide solution and inserted in storage straws, within which they are frozen by immersion in liquid nitrogen.
Frozen eggs and embryos can be rewarmed by insertion in culture dishes, within sucrose-based culture media. Next, in vitro fertilized oocytes or embryos are re-introduced in the patient, after sufficient preparation with systematic and transvaginal estradiol administration.
Encouragingly, frozen oocytes are equally prone to in vitro fertilization compared to fresh ones (70 vs 72%), and even more fruitful considering embryo implantation rates (43 vs 35%) as well as clinical pregnancies achieved per transfer (57 vs 44%) (28). Besides, among 900 children born by 2009, employing cryopreservation methods, congenital anomalies rate did not differ significantly from the general population (29). However, the effectiveness of cryopreservation among female cancer survivors has not been systematically recorded (30). In a retrospective trial, performed in a tertiary care referral center, only 11 of 252 premenopausal female cancer patients attempted fertilization after cancer remission, four of them achieving pregnancies, and two ending up with a healthy delivery (31). Accordingly, oncologic female patients tend to accomplish lower implantation rates (32.5 vs 42.6%) as well as fewer pregnancies (35.7 vs 57.7%) and live deliveries (41.1 vs 68.8%) compared to age matched controls. In spite of these limitations, oocyte/embryo cryopreservation in cancer patients should be encouraged, as they may offer the patient a fair chance of preserving their fertility (32).
Cryopreservation of Ovarian Tissue
Cryopreservation of ovarian tissue, aspires to fully recover the ovarian endocrine and reproductive function, after being re-transplanted to the patient. Markedly, it is applicable to prepubescent girls, while not requiring potentially harmful hormonal pretreatment (33).
Indeed, 130 live births have been described worldwide, resulting from transplantation of cryopreserved ovarian tissue (33). Normal ovarian function is restored in 64% of patients undergoing autotransplantation, 58% of them achieving uncomplicated childbearing and delivery (34).
The procedure consists of laparoscopic ovariectomy, followed by dissection and vitrification of the obtained ovarian tissue (35). When restoration of the ovarian reproductive function is desired, vitrificated ovarian tissue is warmed, inoculated in vitro with Akt stimulators, and laparoscopically inserted in the subserosa of the fallopian tubes. After ultrasonographic confirmation of follicle maturation, the latter are transvaginally collected, in vitro fertilized, and re-introduced to the patient (35). Unfortunately, there are no valid biomarkers to assess the residual follicles deposit in the preserved tissue, in order to predict the expected patient’s potential to produce mature follicles (33, 35).
A key question about cryopreservation is the establishment of an optimal freezing protocol, as too slow and too rapid freezing procedures may cause osmotic cell dehydration and intracellular water crystal formation, respectively, both being detrimental to the ovarian tissue. Thus, most protocols include the use of cryoprotectants, such as glycerol, DMSO, and ethylene glycol, although at high concentrations such substances also exert a toxic effect on the ovarian tissue, creating another concern for clinical practice (33).
Vitrification is an alternative cryopreservation method consisting in the conversion of the resected ovarian tissue to a preservable glass-like solid, by ultrafast cooling in the presence of high levels of cryoprotectants (33). Despite appearing as a promising choice it has not been adequately evaluated in clinical practice. In a series of 37 patients undergoing vitrification for primary ovarian insufficiency (POI), published in 2015, IVF and embryo transfer were finally performed in four of them, resulting in three pregnancies, two of which leading to live births and one ending up with a miscarriage (35). In an earlier series of 27 POI patients, one live delivery was noted, among three patients undergoing IVF and embryo transfer (36).
In conclusion, cryopreservation of ovarian tissue is an alternative solution for fertility preservation, applicable to prepubertal patients, which should be further investigated, in order to overcome technical obstacles and obtain relevant clinical experience (33, 35).
Alternative Therapeutic Approaches—Preclinical Data
Except from the GnRH analogs, other pharmaceutical agents have been explored in the preclinical setting within the last 20 years, in the context of fertility preservation (see Table 3). These include:
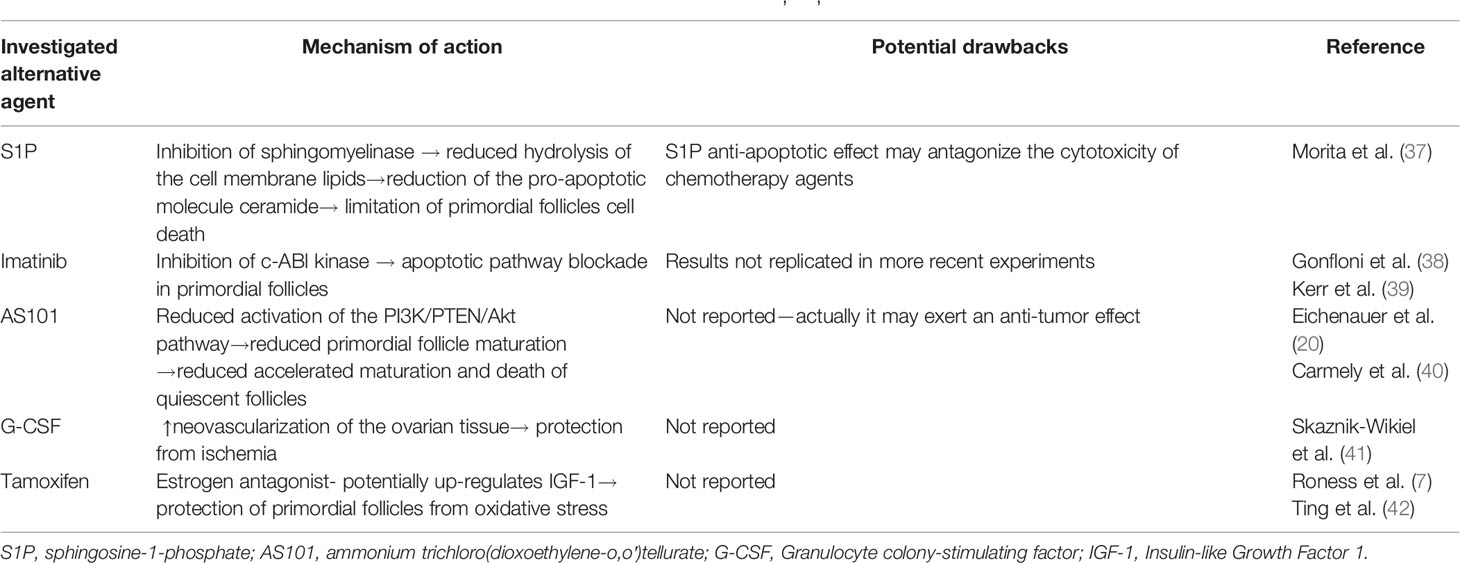
Table 3 Summary of the alternative therapeutic agents under preclinical investigation (7, 20, 37–42).
Sphingosine-1-phosphate (S1P): The sphingomyelinase pathway may mediate the activation of cell death in primordial follicles, via accumulation of ceramide, an apoptotic molecular messenger, produced by sphingomyelinase catalyzed hydrolysis of the cellular membrane. Indeed, murine oocytes in which the sphingomyelinase gene has been either knocked down or inhibited by the molecule S1P resisted normal developmental apoptosis during gametogenesis. Similarly, in murine models treated with S1P, primordial ovarian follicles also resisted radiation induced apoptosis. Consequently, S1P may be a promising agent to be further investigated in future studies, although its anti-apoptotic effect may potentially compromise the cytotoxicity of chemotherapy agents (37).
Imatinib: a widely used tyrosine kinase inhibitor, has been thought to exert an anti-apoptotic effect in primordial ovarian follicles, through inhibition of c-ABl kinase mediated apoptotic pathway. Imatinib co-administration with cisplatin to rodent models can limit death of primordial follicles, preserving reproductive ovarian function (38), although these results were not replicated (39).
AS101: AS101 acts as a modulator of the PI3K/PTEN/Akt pathway, mediating primordial follicle activation under chemotherapy. Supportively, when administered to female rodents under cyclophosphamide treatment, AS101 was found to reduce activation and subsequent exhaustion of ovarian quiescent follicles, thus preserving fertility (20) without compromising the effectiveness of antineoplastic treatment (40).
G-CSF: Interestingly, Granulocyte colony stimulating factors, frequently used against chemotherapy induced myelotoxicity, can maintain ovarian function in mice models under treatment alkylating factors, by promotion of neovascularization of the ovarian tissue (41), what may protect the ovaries from chemotherapy related ischemia.
Tamoxifen: Tamoxifen, an estrogen antagonist used in hormone-dependent breast cancer, has been also explored as a potential fertility preservation agent. As it has been shown in rodent studies, co-administration of tamoxifen with cyclophosphamide and doxorubicin seems to preserve ovarian follicle deposit (42). Although the underlying mechanism has not been clarified, it has been suggested that tamoxifen upregulates IGF-1 (Insulin-like Growth Factor 1), which protects primordial follicles from oxidative stress (7).
Conclusion—Further Questions
Although a variety of alternatives in order to maintain ovarian function and fertility in female cancer survivors, diagnosed and undergoing chemotherapy at a young age, adequately established practices to do so are lacking. Notably, study of the applicable literature reveals a relative lack of clinical evidence regarding preservation of patient fertility among a variety of malignancies mostly affecting children, adolescents, and young adults of both genders, such as CNS tumors, germ cell neoplasms, osseous and soft tissue sarcomas. Similarly, fertility preservation in young patients affected by cancer types more frequent in older ages, such as early stage colon cancer, has not been investigated sufficiently either.
Although current oncofertility guidelines are universal among different tumor types and patient profiles (43), potential disparities between patients due to age, chemotherapy agents employed, and the malignancy itself may also interfere with fertility preservation practices. Consequently, a more methodical investigation of fertility preservation strategies, considering the above parameters, is required, in order to adequately establish the most efficient practices for each patient group.
Especially regarding young female cancer survivors, in an era that age of pregnancy is pushed even after the age of 40, it is of major importance to further investigate and collect sufficient evidence, aiming to safely guide patients and physicians in everyday clinical practice. Until then, oncologists should not neglect this domain of life of their female, younger patients; female cancer patients have to be encouraged to express their concerns and wishes, regarding fertility and pregnancy after antineoplastic treatment completion, in order to organize a plan of action that will allow them to maintain a normal endocrine function as well as the possibility to create a family.
Author Contributions
All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Salama M, Woodruff T. Anticancer treatments and female fertility: clinical concerns and role of oncologists in oncofertility practice. Expert Rev Anticancer Ther (2017) 17(8):687–92. doi: 10.1080/14737140.2017.1335199
2. Bellieni C. The Best Age for Pregnancy and Undue Pressures. J Family Reprod Health (2016) 10(3):104–7.
3. Stensheim H, Cvancarova M, Møller B, Fosså SD. Pregnancy after adolescent and adult cancer: a population-based matched cohort study. Int J Cancer (2011) 129:1225–36. doi: 10.1002/ijc.26045
4. Peccatori F, Azim H, Orecchia R, Hoekstra H, Pavlidis N, Kesic V, et al. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2013) 24:vi160–70. doi: 10.1093/annonc/mdt199
5. Cui W, Stern C, Hickey M, Goldblatt F, Anazodo A, Stevenson WS, et al. Preventing ovarian failure associated with chemotherapy. Med J Aust (2018) 209(9):412–6. doi: 10.5694/mja18.00190
6. Lee S, Schover L, Partridge A, Patrizio P, Wallace W, Hagerty K, et al. American Society of Clinical Oncology Recommendations on Fertility Preservation in Cancer Patients. J Clin Oncol (2006) 24(18):2917–31. doi: 10.1200/JCO.2006.06.5888
7. Roness H, Kashi O, Meirow D. Prevention of chemotherapy-induced ovarian damage. Fertil Steril (2016) 105(1):20–9. doi: 10.1016/j.fertnstert.2015.11.043
8. Kalich-Philosoph L, Roness H, Carmely A, Fishel-Bartal M, Ligumsky H, Paglin S, et al. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci Transl Med (2013) 5(185):185ra62. doi: 10.1126/scitranslmed.3005402
9. Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev (2009) 30(5):438–64. doi: 10.1210/er.2008-0048
10. Bedoschi G, Navarro PA, Oktay K. Chemotherapy-induced damage to ovary: mechanisms and clinical impact. Future Oncol (2016) 12(20):2333–44. doi: 10.2217/fon-2016-0176
11. Soleimani R, Heytens E, Darzynkiewicz Z, Oktay K. Mechanisms of chemotherapy-induced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY) (2011) 3(8):782–93. doi: 10.18632/aging.100363
12. Titus S, Oktay K. Mechanisms of chemotherapy-induced primordial follicle death in human. Presented at: Society for the Study of Reproduction. Grand Rapids, MI, USA (2014). 19–23 July 2014 (Abstract 325796).
13. Bar-Joseph H, Ben-Aharon I, Tzabari M, Tsarfaty G, Stemmer SM, Shalgi R. In vivo bioimaging as a novel strategy to detect doxorubicin-induced damage to gonadal blood vessels. PloS One (2011) 6(9):e23492. doi: 10.1371/journal.pone.0023492
14. Trivers KF, Fink AK, Partridge AH, Oktay K, Ginsburg ES, Li C, et al. Estimates of young breast cancer survivors at risk for infertility in the U.S. Oncologist (2014) 19(8):814–22. doi: 10.1634/theoncologist.2014-0016
15. Munhoz RR, Pereira AA, Sasse AD, Hoff PM, Traina TA, Hudis CA, et al. Gonadotropin-Releasing Hormone Agonists for Ovarian Function Preservation in Premenopausal Women Undergoing Chemotherapy for Early-Stage Breast Cancer: A Systematic Review and Meta-analysis. JAMA Oncol (2016) 2(1):65–73. doi: 10.1001/jamaoncol.2015.3251
16. Del Mastro L, Boni L, Michelotti A, Gamucci T, Olmeo N, Gori S, et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. JAMA (2011) 306:269–76. doi: 10.1001/jama.2011.991
17. Moore HC, Unger JM, Phillips K, Boyle F, Hitre E, Porter D, et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med (2015) 372:923–32. doi: 10.1056/NEJMoa1413204
18. Lambertini M, Boni L, Michelotti A, Gamucci T, Scotto T, Gori S, et al. Long-term outcome results of the phase III PROMISE-GIM6 study evaluating the role of LHRH analog (LHRHa) during chemotherapy as a strategy to reduce ovarian failure in early breast cancer patients. Ann Oncol (2015) 26:p.vi1. doi: 10.1093/annonc/mdv335.01
19. Leonard RC, Adamson DJ, Bertelli G, Mansi J, Yellowlees A, Dunlop G, et al. GnRHa agonist for protection against ovarian toxicity during chemotherapy for early breast cancer: the Anglo Celtic Group OPTION trial. Ann Oncol (2017) 28:1811–6. doi: 10.1093/annonc/mdx184
20. Eichenauer D, Aleman B, André M, Federico M, Hutchings M, Illidge T, et al. Hodgkin lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2018) 29:iv19–29. doi: 10.1093/annonc/mdy080
21. Surveillance, Epidemiology and End Results programme (SEER), supported by the National Cancer Institute (NCI) of the USA. Available at: seer.cancer.gov.
22. Zhang Y, Xiao Z, Wang Y, Luo S, Li X, Li S, et al. Gonadotropin-releasing hormone for preservation of ovarian function during chemotherapy in lymphoma patients of reproductive age: a summary based on 434 patients. PloS One (2013) 8:e80444. doi: 10.1371/journal.pone.0080444
23. Demeestere I, Brice P, Peccatori FA, Kentos A, Dupuis J, Zachee P, et al. No evidence for the benefit of gonadotropin-releasing hormone agonist in preserving ovarian function and fertility in lymphoma survivors treated with chemotherapy: Final long-term report of a prospective randomized trial. J Clin Oncol (2016) 34:2568–74. doi: 10.1200/JCO.2015.65.8864
24. Gilani M, Hasanzadeh M, Ghaemmaghami F, Ramazanzadeh F. Ovarian preservation with gonadotropin-releasing hormone analog during chemotherapy. Asia Pacif J Clin Oncol (2007) 3(2):79–83. doi: 10.1111/j.1743-7563.2007.00089.x
25. Pacheco B, Méndez Ribas J, Milone G, Fernández I, Kvicala R, Mila T, et al. Use of GnRH Analogs for Functional Protection of the Ovary and Preservation of Fertility during Cancer Treatment in Adolescents: A Preliminary Report. Gynecol Oncol (2001) 81(3):391–7. doi: 10.1006/gyno.2001.6181
26. Wang CT, Liang L, Witz C, Williams D, Griffith J, Skorupski J, et al. Optimized protocol for cryopreservation of human eggs improves developmental competence and implantation of resulting embryos. J Ovarian Res (2013) 6(1):15. doi: 10.1186/1757-2215-6-15
27. Oktay K. Further evidence on the safety and success of ovarian stimulation with letrozole and tamoxifen in breast cancer patients undergoing in vitro fertilization to cryopreserve their embryos for fertility preservation. J Clin Oncol (2005) 23:3858–9. doi: 10.1200/JCO.2005.04.011
28. Doyle J, Richter K, Lim J, Stillman R, Graham J, Tucker MJ, et al. Successful elective and medically indicated oocyte vitrification and warming for autologous in vitro fertilization, with predicted birth probabilities for fertility preservation according to number of cryopreserved oocytes and age at retrieval. Fertil Steril (2016) 105(2):459–66.e2. doi: 10.1016/j.fertnstert.2015.10.026
29. Noyes N, Porcu E, Borini A. Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reprod BioMed Online (2009) 18(6):769–76. doi: 10.1016/S1472-6483(10)60025-9
30. The Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril (2013) 99:37–43. doi: 10.1016/j.fertnstert.2012.09.028
31. Specchia C, Baggiani A, Immediata V, Ronchetti C, Cesana A, Smeraldi A, et al. Oocyte Cryopreservation in Oncological Patients: Eighteen Years Experience of a Tertiary Care Referral Center. Front Endocrinol (2019) 10:600. doi: 10.3389/fendo.2019.00600
32. Cobo A, García-Velasco J, Domingo J, Pellicer A, Remohí J. Elective and Onco-fertility preservation: factors related to IVF outcomes. Hum Reprod (2018) 33(12):2222–31. doi: 10.1093/humrep/dey321
33. Rivas Leonel EC, Lucci CM, Amorim CA. Cryopreservation of Human Ovarian Tissue: A Review. Transfus Med Hemother (2019) 46(3):173–81. doi: 10.1159/000499054
34. Pacheco F, Oktay K. Current Success and Efficiency of Autologous Ovarian Transplantation: A Meta-Analysis. Reprod Sci (2017) 24(8):1111–20. doi: 10.1177/1933719117702251
35. Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, et al. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod (2015) 30(3):608–15. doi: 10.1093/humrep/deu353
36. Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci USA (2013) 110(43):17474–9. doi: 10.1073/pnas.1312830110
37. Morita Y, Perez GI, Paris F, et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med (2000) 6(10):1109–14. doi: 10.1038/80442
38. Gonfloni S, DiTella L, Caldarola S, Cannata SM, Klinger FG, Di Bartolomeo C, et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med (2009) 15:1179–85. doi: 10.1038/nm.2033
39. Kerr JB, Hutt KJ, Michalak EM, Cook M, Vandenberg CJ, Liew SH, et al. DNA Damage-Induced Primordial Follicle Oocyte Apoptosis and Loss of Fertility Require TAp63-Mediated Induction of Puma and Noxa. Mol Cell (2012) 48:343–52. doi: 10.1016/j.molcel.2012.08.017
40. Carmely A, Meirow D, Peretz A, Albeck M, Bartoov B, Sredni B. Protective effect of theimmunomodulator AS101 against cyclophosphamide-induced testicular damage inmice. Hum Reprod (2009) 24:1322–9. doi: 10.1093/humrep/den481
41. Skaznik-Wikiel ME, McGuire MM, Sukhwani M, Donohue J, Chu T, Krivak TC, et al. Granulocyte colony-stimulating factor with or without stemcell factor extends time to premature ovarian insufficiency in female mice treatedwith alkylating chemotherapy. Fertil Steril (2013) 99:2045–2054 e2043. doi: 10.1016/j.fertnstert.2013.01.135
42. Ting AY, Petroff BK. Tamoxifen decreases ovarian follicular loss from experimentaltoxicant DMBA and chemotherapy agents cyclophosphamide and doxorubicin inthe rat. J Assist Reprod Genet (2010) 27:591–7. doi: 10.1007/s10815-010-9463-y
43. Dolmans M, Lambertini M, Macklon K, Almeida Santos T, Ruiz-Casado A, Borini A, et al. EUropeanREcommendations for female FERtility preservation (EU-REFER): A joint collaboration between oncologists and fertility specialists. Crit Rev Oncol/Hematol (2019) 138:233–40. doi: 10.1016/j.critrevonc.2019.03.010
Keywords: ovarian reserve, ovarian failure, sterility, cancer, chemotherapy
Citation: Mauri D, Gazouli I, Zarkavelis G, Papadaki A, Mavroeidis L, Gkoura S, Ntellas P, Amylidi A-L, Tsali L and Kampletsas E (2020) Chemotherapy Associated Ovarian Failure. Front. Endocrinol. 11:572388. doi: 10.3389/fendo.2020.572388
Received: 14 June 2020; Accepted: 02 November 2020;
Published: 08 December 2020.
Edited by:
Nikolaos P. Polyzos, Dexeus University Hospital, SpainReviewed by:
Giuliano Marchetti Bedoschi, University of São Paulo, BrazilGeorgios Tsironis, Nicosia General Hospital, Cyprus
Copyright © 2020 Mauri, Gazouli, Zarkavelis, Papadaki, Mavroeidis, Gkoura, Ntellas, Amylidi, Tsali and Kampletsas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Davide Mauri, dvd.mauri@gmail.com
 Davide Mauri
Davide Mauri Ioanna Gazouli
Ioanna Gazouli Georgios Zarkavelis
Georgios Zarkavelis Alexandra Papadaki2
Alexandra Papadaki2 Stefania Gkoura
Stefania Gkoura Panagiotis Ntellas
Panagiotis Ntellas