- 1Department of Nuclear Medicine, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, China
- 2Department of Nuclear Medicine, The First Hospital of Jilin University, Changchun, China
- 3Department of Radiology, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China
Background: Repeated radioiodine (131I) treatment (RT) are commonly performed in patients with 131I-avid distant metastatic differentiated thyroid cancer (DM-DTC), but more precise indications remain indeterminate. This prospective study was conducted to explore predictors for biochemical response (BR) to next RT.
Methods: Totally thyroidectomized patients with 131I-avid DM-DTC demonstrated by initial post-therapeutic whole body scan (Rx-WBS) were consecutively recruited. Repeated RTs were performed at a fixed dose and a fixed interval, which was terminated once a decline in thyroid stimulating hormone-suppressed thyroglobulin (Tgon) could not be achieved or Rx-WBS was negative. BR was evaluated by change rate of Tgon level (ΔTgon%).
Results: After exclusion of 27 ineligible courses, a total of 166 neighboring course pairs from 77 patients were established and utilized. Univariate and multivariate analyses showed that the maximum target/background ratio (T/Bmax) on the whole body scan and ΔTgon% derived from the former RT were independently associated to the latter one. In predicting biochemical remission, the positive predictive value (PPV) and negative predictive value (NPV) of T/Bmax at the cut-off value of 8.1 were 79.1% and 84.0%, respectively; whereas the PPV and NPV of ΔTgon% at the cut-off value of 25.3% were 70.8% and 77.1%, respectively. Notably, the PPV of combined T/Bmax ≥ 8.1 and ΔTgon% ≥ 25.3% increased to 87.7%; while the NPV of T/Bmax ≥ 8.1 or ΔTgon% ≥ 25.3% reached as high as 97.7%.
Conclusions: This study revealed that combined use of the latest RT-derived T/Bmax and ΔTgon% may efficiently identify biochemical responders/non-responders to next RT, warranting management optimization of patients with 131I-avid DM-DTC.
Introduction
Papillary thyroid cancer and follicular thyroid cancer are collectively termed as differentiated thyroid cancer (DTC), which accounts for approximately 90% of thyroid cancer, and are generally indolent with relatively favorable prognosis (1, 2). Distant metastatic differentiated thyroid cancer (DM-DTC) occupying 4%–15% of all DTC cases at initial presentation or during subsequent follow-up, however, represents the main cause of disease-specific mortality (3). Traditionally targeted therapy using radioiodine (131I) with multiple courses has become a routine therapeutic strategy for 131I-avid DM-DTC for decades, with regard to 131I uptake demonstrated by post-therapeutic whole body scan (Rx-WBS) (4).
As is well known that the 2015 American Thyroid Association (ATA) management guidelines provide only general indication for repeated 131I treatment (RT) by suggesting that 131I-avid metastatic lesions may be treated with 131I and that RT may be repeated when an objective benefit is demonstrated (5). Due to difficulties in a detailed definition of so-called “objective benefit”, the guidelines failed to describe more precise indications for repeated RT of 131I-avid DM-DTC. Many centers continue to simply repeat RT as long as there is visible 131I-avid lesions on Rx-WBS and/or elevated thyroglobulin (Tg) (6), yielding a potential overuse of 131I in patients with radioiodine-refractory disease (7) and increased risk of side effects, such as salivary gland dysfunction (8), pulmonary fibrosis (9), bone marrow suppression (10), and secondary cancers (11). More importantly, an objective benefit from a latest course of RT does not necessarily mean that patient continues to benefit from next course, as varying responses to repeated RT have been noted in previous studies (12, 13).
Additionally, since patient-based retrospective qualitative studies merely demonstrate multiple factors possibly related to final outcome of accumulated RTs, they hardly provide more reliable evidences for or against next RT (14–16). We, therefore, conducted this prospective course-based study to identify predictors for biochemical response (BR) to next RT from clinicopathological features in patients with 131I-avid DM-DTC followed by efficacy assessment, aiming at optimizing indications for repeated RT and reducing potential abuse of 131I.
Materials and Methods
Study Conduct
All the patients provided written informed consent prior to the initiation of the study. The protocol was approved by the Ethics Committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital (Shanghai, P.R. China). The authors vouched for the completeness and accuracy of the data and analyses.
Study Populations
Totally thyroidectomized patients with 131I-avid DM-DTC demonstrated by initial Rx-WBS in a single institution were consecutively recruited from May 1st, 2014. Following low-iodine diet and levothyroxine withdrawal for 4 weeks as in preparation for initial RT, repeated RT was performed at a fixed 131I dose of 7.4 GBq with an interval of nearly 6 months between neighboring therapeutic 131I administrations (5, 17). RT was terminated once a decline in thyroid stimulating hormone (TSH)-suppressed Tg (Tgon) could not be achieved or Rx-WBS was negative. Nearly 1 month after RT, patients were visited and TSH, free triiodothyronine, and free thyroxine were examined to guide the adjustment of levothyroxine dosage.
Establishment of Eligible RT Course Pair
A course pair was constituted by neighboring two RTs (the former course and the latter course). For example, if a patient underwent three courses of RT, the second course acted as not only the latter course of the first course pair but also the former course of the second course pair, and so on. 131I-avid DM-DTC and baseline Tgon level just before former RT ≥ 10.0 ng/ml were the enrollment criteria of course pair. Course pairs possibly interfered by cancers beyond DTC, other therapeutics beyond RT, suppressed TSH level > 0.1 mIU/L (5), and anti-Tg antibody (TgAb) > 60.0 IU/ml were excluded. Eligible course pairs were then utilized in the univariate and multivariate analyses to identify factors associated with biochemical response (BR) to next RT.
Serologic Examinations
TSH, Tg, and TgAb levels were measured before, one and 4 months after RT by an electrochemiluminescent immunoassay on a Cobas analyzer (Roche Diagnostics Gmbh, Roche Ltd., Basel, Switzerland). The lower and upper detection limits of the TSH assay were 0.005 and 100 mIU/L, respectively, and TSH levels lower or higher than those were counted as 0.005 and 100 mIU/L, respectively. Similarly, Tg levels higher than 50,000 ng/ml were counted as 50,000 ng/ml, while TgAb levels lower than 10 IU/ml were counted as 10 IU/ml.
Post-Therapeutic Whole Body Scan
Rx-WBS was obtained 3 days after the oral administration of 7.4 GBq of 131I in liquid by a single photon emission computed tomography/computed tomography (SPECT/CT, GE Discovery NM/CT 670) equipped with detectors composed of NaI(Tl) crystal with 3/8 inch thickness and parallel-hole high-energy collimators. Anterior and posterior acquisitions were simultaneously performed at a speed of 10 cm/min. The body-contouring system was used to minimize the distance between the patient and the collimator. A low dose CT (voltage of 120keV) was added immediately to yield SPECT/CT images if Rx-WBS had shown inconclusive findings to achieve definitive diagnoses as previously described by our team (18). Semi-quantitative analysis of 131I uptake of metastatic foci in planar imaging was conducted using a region-of-interest (ROI) technique (19). Briefly, regions were drawn separately around each distant metastatic lesion and normal frontal cranial bone on anterior and posterior images, and the maximum count of each ROI was obtained and utilized to yield the maximum target/background ratio (T/Bmax) in each Rx-WBS, representing the only one most active metastatic lesion.
Biochemical Response Assessment
BR was evaluated by the comparison between post-therapeutic Tgon approximately 4 months post RT and pre-therapeutic Tgon just before levothyroxine withdrawal. ΔTgon%, change rate of Tgon level, was defined as follows: [(pre-therapeutic Tgon level–post-therapeutic Tgon level)/pre-therapeutic Tgon level] × 100%.
In determining the categorization of BR to the latter course of RT, the following standards were used:
ΔTgon% ≥ 25.0% indicated effective RT (biochemical remission), while ΔTgon% < 25.0% meant non-effective RT, including biochemical stabilization (-25.0% ≤ ΔTgon% < 25.0%) and biochemical progression (ΔTgon% < -25.0%) (4, 20).
Statistical Analysis
Continuous variables with normal distributions are presented as means ± standard deviations, whereas continuous variables with non-normal distribution are presented as medians with ranges. Categorical variables are reported as numbers with percentages. Unpaired Student’s t-tests, Mann-Whitney U-tests, Fisher’s tests, or chi-square tests were used for univariate analyses as needed, and significant variables were included in the subsequent multivariate logistic regression analysis. The optimum cut-off values for independent predictors were calculated using receiver operating characteristic (ROC) curves. Statistical analyses were performed using SPSS software (v. 24.0). All p values were two-sided; p values < 0.05 were considered statistically significant.
Results
Clinical Characteristics
From May 1st, 2014 through November 31st, 2018, a total of 193 course pairs were consecutively enrolled. After exclusion of 8 course pairs interfered by cancers beyond DTC, 6 course pairs interfered by other therapeutics including operation, radiotherapy, targeted therapy, and 13 course pair with inappropriate suppressed TSH level or elevated TgAb, a total of 166 eligible course pairs from 77 patients (age, 42.4 ± 11.5 years) were finally established and utilized.
Regarding the continuous variables of the 166 former courses of interest, the median baseline Tgon and TSH-stimulated Tg (Tgoff) levels just before RT were 199.0 ng/ml (range, 11.2–29,980.0 ng/ml) with a median TSH of 0.04 mIU/L (range, 0.005–0.07 mIU/L) and 1116.0 ng/ml (range, 35.4–50,000.0 ng/ml) with a median TSH of 100.0 mIU/L (range, 33.8–100.0 mIU/L), respectively. At 4 months post RT, the median Tgon declined to 134.0 ng/ml (range, 10.3–22,498.0 ng/ml) with a median TSH of 0.06 mIU/L (range, 0.005–0.09 mIU/L), yielding a median ΔTgon% of 31.7% (range, -367.3%–96.8%). The median T/Bmax was 11.0 (range, 1.0–317.5). Besides, the categorical clinicopathological characteristics of eligible former courses of RT from 77 patients are shown in Table 1.
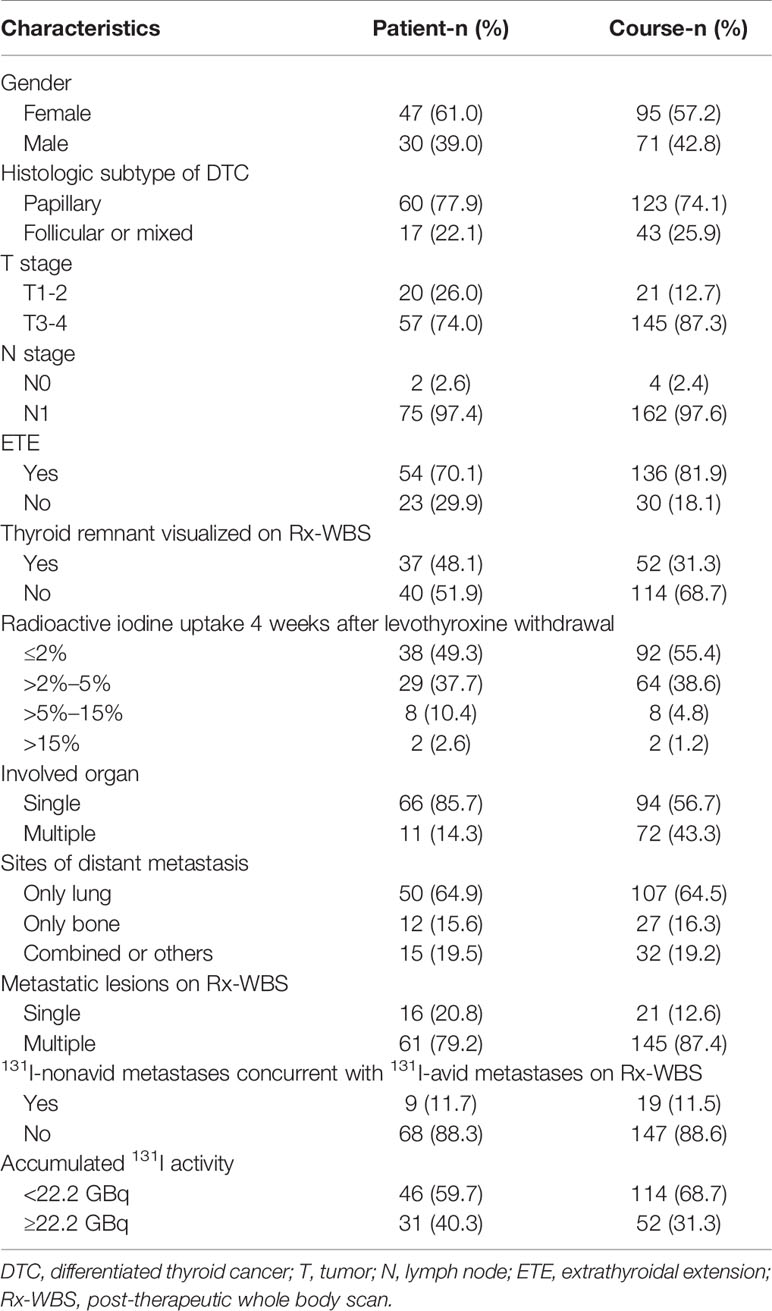
Table 1 Categorical clinicopathological characteristics of eligible former 131I therapeutic courses (N = 166) from 77 patients.
Out of 166 paired latter courses, effective RT (biochemical remission) was obtained in 84 (50.6%) courses; while 49.4% courses yielded non-effective RT, including biochemical stabilization and biochemical progression in 30.2% and 19.2% of all courses, respectively.
Identification of Predictors for BR to Next RT
Upon analyzing the association of potential clinicopathologic features with BR to next RT, a total of 16 former course-derived factors were involved in the univariate analysis. We found that thyroid remnant visualized on Rx-WBS, ΔTgon%, T/Bmax, and T stage were associated with BR (p < 0.05). In the multivariable logistic regression analysis, however, only T/Bmax and ΔTgon% were remained as independent predictors for biochemical remission with Odds ratios of 3.809 and 1.044, respectively (Table 2).
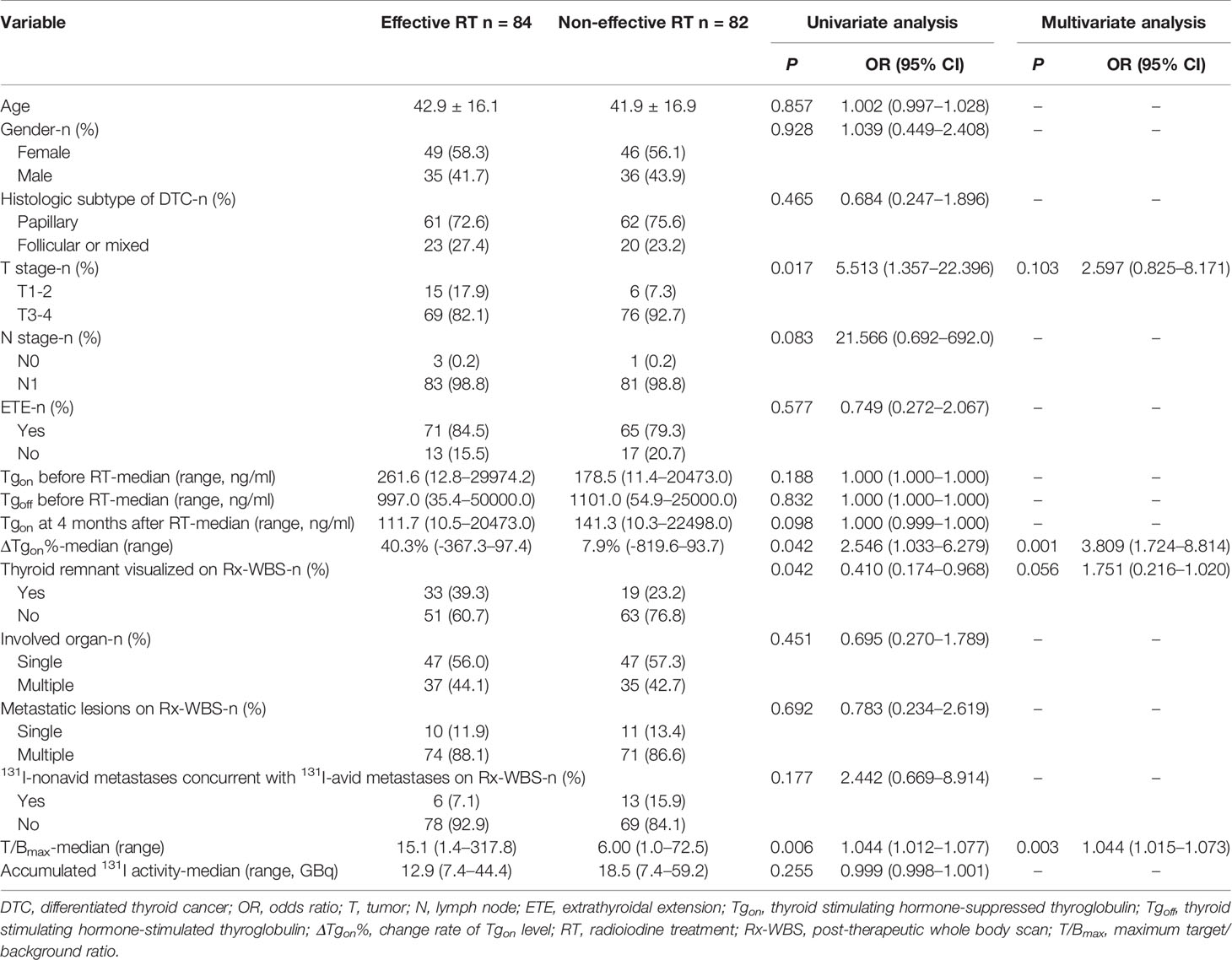
Table 2 Univariate analysis of factors potentially associated with biochemical response to next radioiodine treatment in 166 former courses.
Efficacy of T/Bmax and/or ΔTgon%
Cut-off values of T/Bmax at 8.1 and ΔTgon% at 25.3% were obtained by ROC analyses to optimally differentiate effective RT from non-effective RT. The predictive efficacy of a T/Bmax of 8.1 and/or ΔTgon% of 25.3% were further calculated and are listed in Table 3. The specificity of T/Bmax ≥ 8.1 and ΔTgon% ≥ 25.3% was 90.2%, and the sensitivity of T/Bmax ≥ 8.1 or ΔTgon% ≥ 25.3% was 98.8%. Moreover, the positive predictive value (PPV) and negative predictive value (NPV) of T/Bmax ≥ 8.1 were higher than those of ΔTgon% ≥ 25.3% (79.1% vs. 70.8%; 84.0% vs. 77.1%). Notably, the highest PPV of 87.7% was achieved by a criterion of combined T/Bmax ≥ 8.1 and ΔTgon% ≥ 25.3%, whereas a criterion of T/Bmax ≥ 8.1 or ΔTgon% ≥ 25.3% yielded the highest NPV of 97.7%.
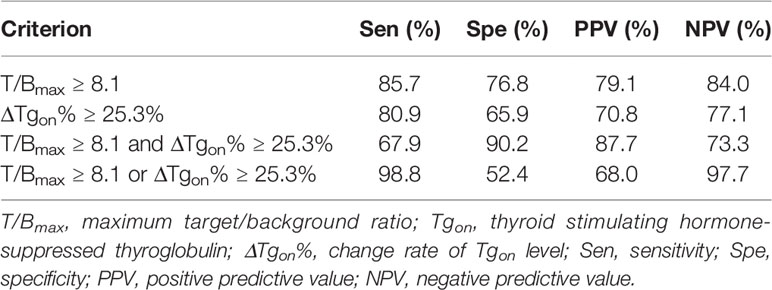
Table 3 Efficacy of T/Bmax of 8.1 and/or ΔTgon% of 25.3% in predicting biochemical response to next radioiodine treatment.
Application of T/Bmax of 8.1 Combined With ΔTgon% of 25.3%
According to the results of T/Bmax and ΔTgon% obtained from the 166 former courses and the categorical criteria (T/Bmax ≥ 8.1 and ΔTgon% ≥ 25.3%, T/Bmax ≥ 8.1 and ΔTgon% < 25.3%, T/Bmax < 8.1 and ΔTgon% ≥ 25.3%, and T/Bmax < 8.1 and ΔTgon% < 25.3%), the BR distribution of the 166 paired latter courses are shown in Figure 1. Upon the 166 latter courses, consistent (T/Bmax ≥ 8.1 and ΔTgon% ≥ 25.3%; T/Bmax < 8.1 and ΔTgon% < 25.3%) and inconsistent (T/Bmax ≥ 8.1 and ΔTgon% < 25.3%; T/Bmax < 8.1 and ΔTgon% ≥ 25.3%) conditions were found in 109 (65.7%) and 57 (35.3%) courses, respectively, with biochemical remission rates of 53.2% (58/109) and 45.6% (28/57), respectively. Representative cases with effective RT or non-effective RT are illustrated in Figures 2 and 3, respectively. Additionally, four patients with T/Bmax ≥ 8.1 and ΔTgon% ≥ 25.3% who obtained excellent benefits from the former RT exhibited subtle Tgon levels before and negative Rx-WBS after the latter course of RT (Figure 4).
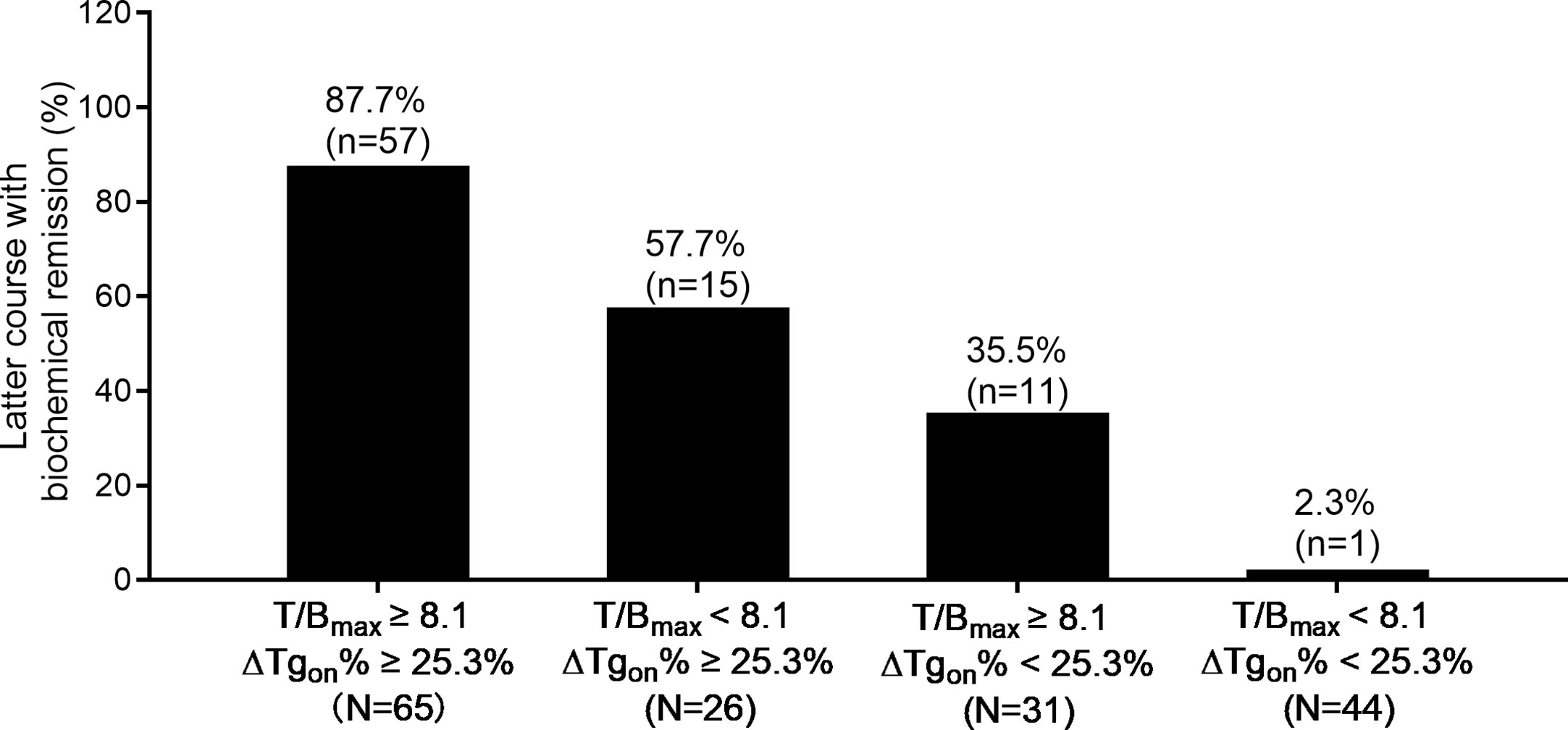
Figure 1 Distribution of biochemical remission in 166 paired latter courses of RT with regard to the results of T/Bmax and ΔTgon% obtained from the former courses. RT, radioiodine treatment; T/Bmax, maximum target-to-background ratio; Tgon, thyroid stimulating hormone-suppressed thyroglobulin; ΔTgon%, change rate of Tgon level.
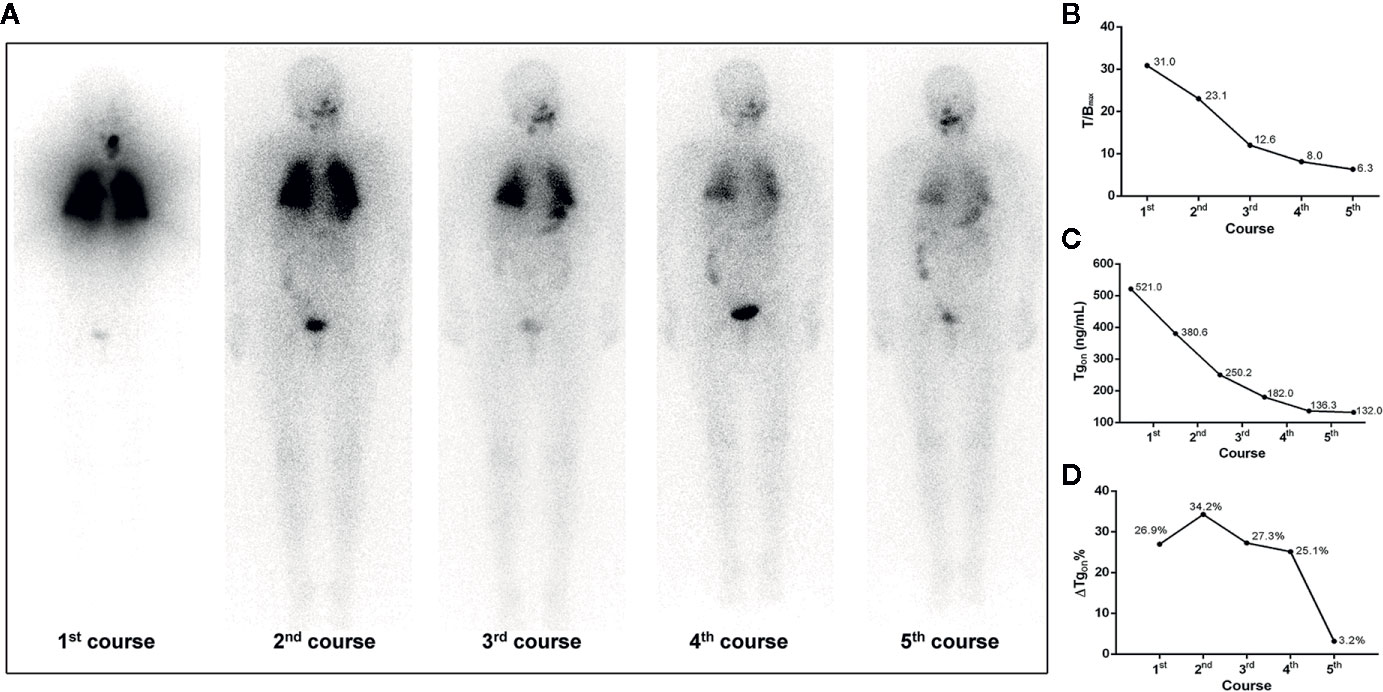
Figure 2 A 68-year-old female differentiated thyroid cancer patient with 131I-avid pulmonary metastases responding to RT. The T/Bmax and ΔTgon% resulting from the first, second, and third course of RT were more than 8.1 (31.0, 23.1, and 12.6) and 25.3% (26.9%, 34.2%, and 27.3%), respectively, indicating biochemical remission was achievable in the paired latter courses, which were confirmed by the significant decrease in Tgon resulting from the second, third, and fourth course of RT, respectively. After the fourth administration of 131I, nevertheless, T/Bmax decreased to 8.0 and ΔTgon% declined to 25.1%, indicating a non-effective RT was achievable in the paired latter courses, which was confirmed by the subtle decrease in Tgon (3.2%) resulting from the fifth course of RT. (A) Whole body scans (anterior) at 3 days after the oral administration of 7.4 GBq of 131I; (B) T/Bmax; (C) Tgon; (D) ΔTgon%. RT, radioiodine treatment; T/Bmax, maximum target-to-background ratio; Tgon, thyroid stimulating hormone-suppressed thyroglobulin; ΔTgon%, change rate of Tgon level.
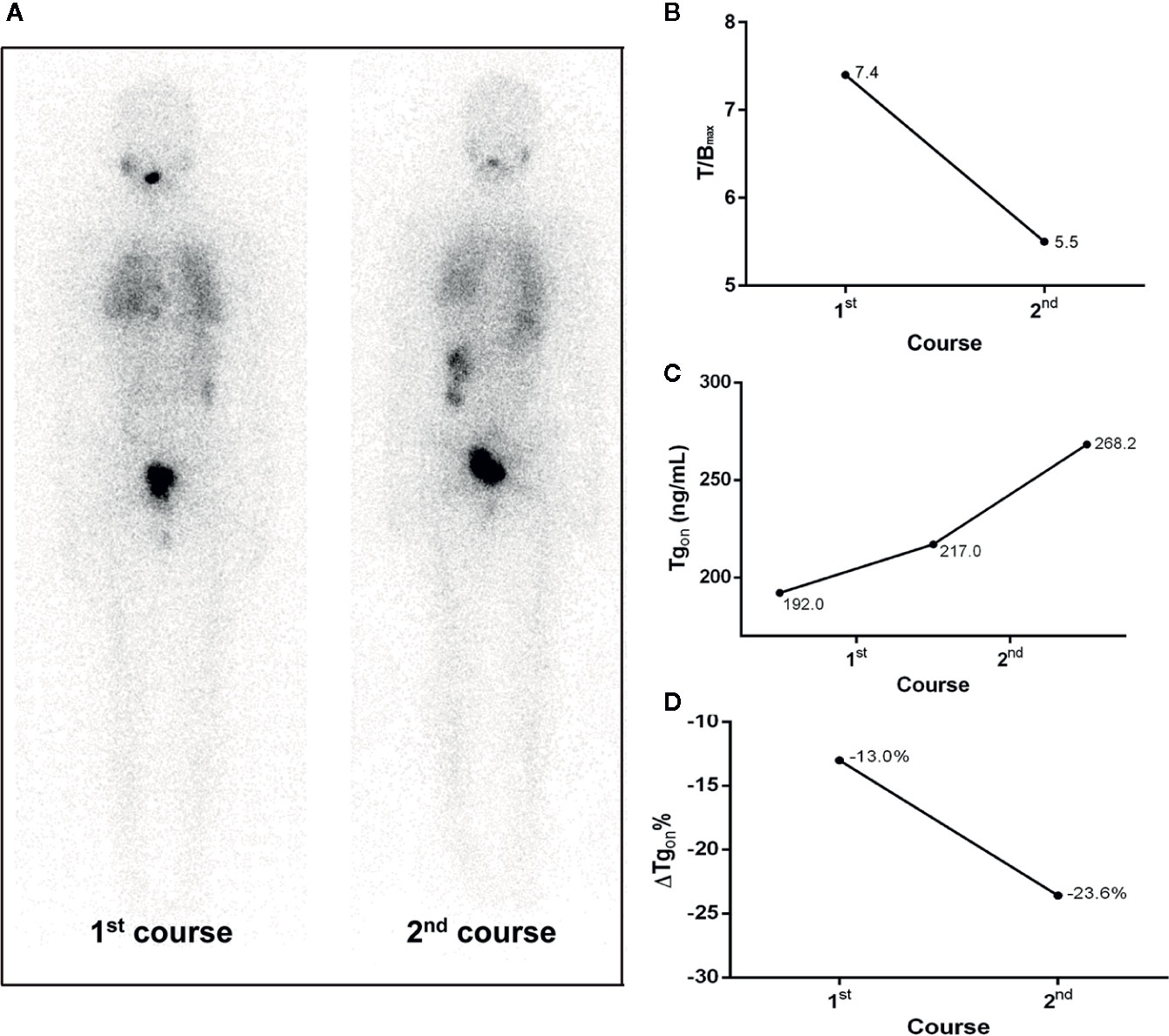
Figure 3 A 20-year-old female differentiated thyroid cancer patient with 131I-avid pulmonary metastases not responding to RT. The T/Bmax and ΔTgon% resulting from the initial course of RT were less than 8.1 and 25.3%, respectively, indicating a non-effective RT was achievable in the paired latter courses, which was confirmed by the increase in Tgon (23.6%) at 4 months after the second course of RT. (A) Whole body scans (anterior) at 3 days after the oral administration of 7.4 GBq of 131I; (B) T/Bmax; (C) Tgon; (D) ΔTgon%. RT, radioiodine treatment; T/Bmax, maximum target-to-background ratio; Tgon, thyroid stimulating hormone-suppressed thyroglobulin; ΔTgon%, change rate of Tgon level.
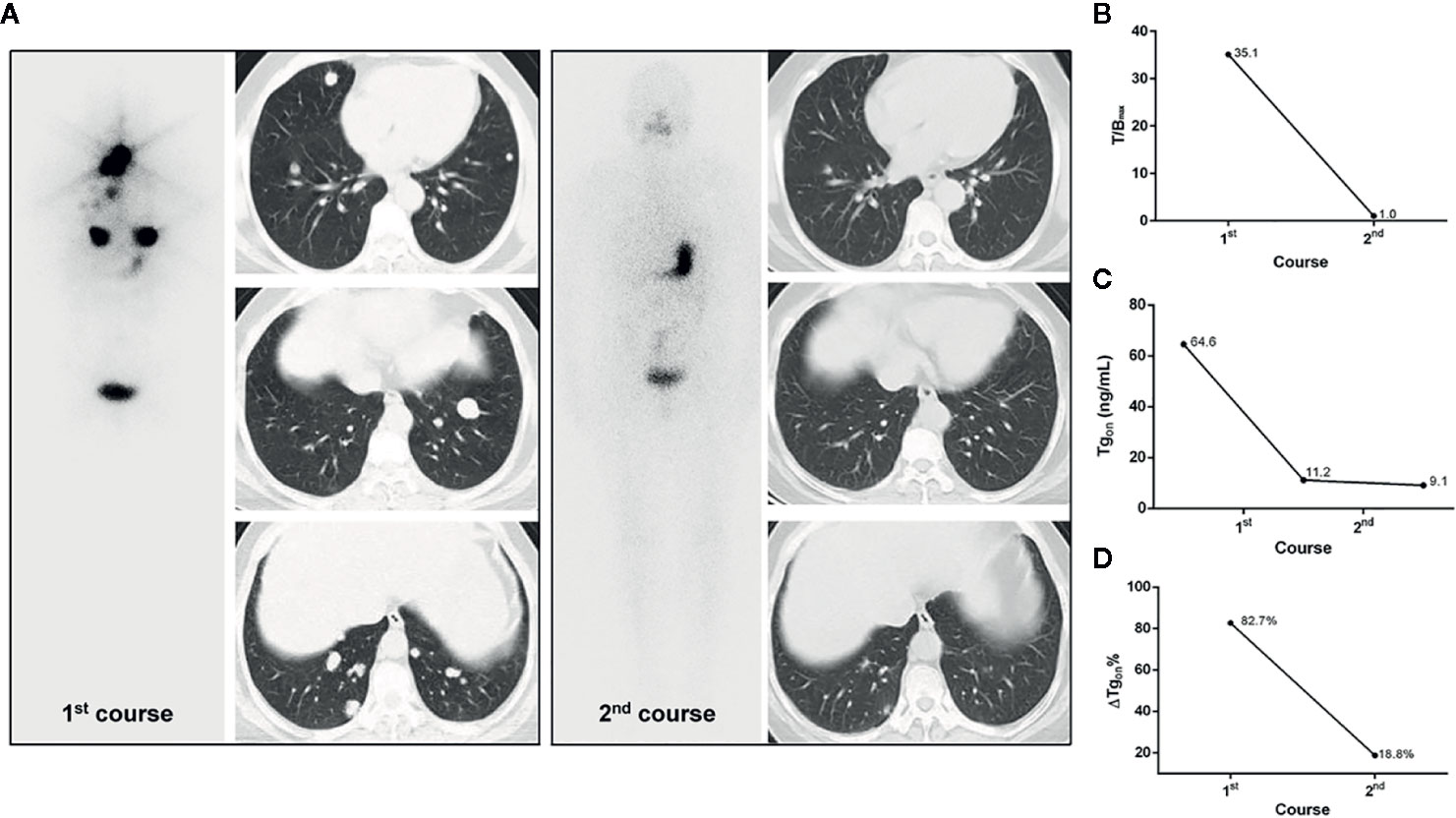
Figure 4 A 53-year-old female differentiated thyroid cancer patient with robust 131I-avid pulmonary metastases achieving excellent benefit from the initial RT. The T/Bmax and ΔTgon% resulting from the initial course of RT were 35.1 and 82.7%, respectively. In the second course of RT, unexpectedly, post therapeutic whole body scans were negative and the size of lesions were dramatically decreased on chest computed tomography, along with a Tgon of 9.1 ng/ml, indicating an excellent benefit from the initial course of RT, which was demonstrated by a continuous decrease in Tgon to 0.9 ng/ml during the 3-year follow-up. (A) Whole body scans (anterior) at 3 days after the oral administration of 7.4 GBq of 131I; (B) T/Bmax; (C) Tgon; (D) ΔTgon%. RT, radioiodine treatment; T/Bmax, maximum target-to-background ratio; Tgon, thyroid stimulating hormone-suppressed thyroglobulin; ΔTgon%, change rate of Tgon level.
Additionally, 131I-nonavid metastasis concurrent with 131I-avid metastasis was found in 11 patients with a total of 19 former courses. In this entity, 83.3% (5/6) of the latter courses of RT yielded biochemical remission if T/Bmax ≥ 8.1 and ΔTgon% ≥ 25.3% were met in the former course, while no biochemical remission (0/9) was obtained from the latter RT course if the former course of RT created T/Bmax < 8.1 and ΔTgon% < 25.3%. The distribution of biochemical remission in all the paired latter courses is shown in Figure 5.
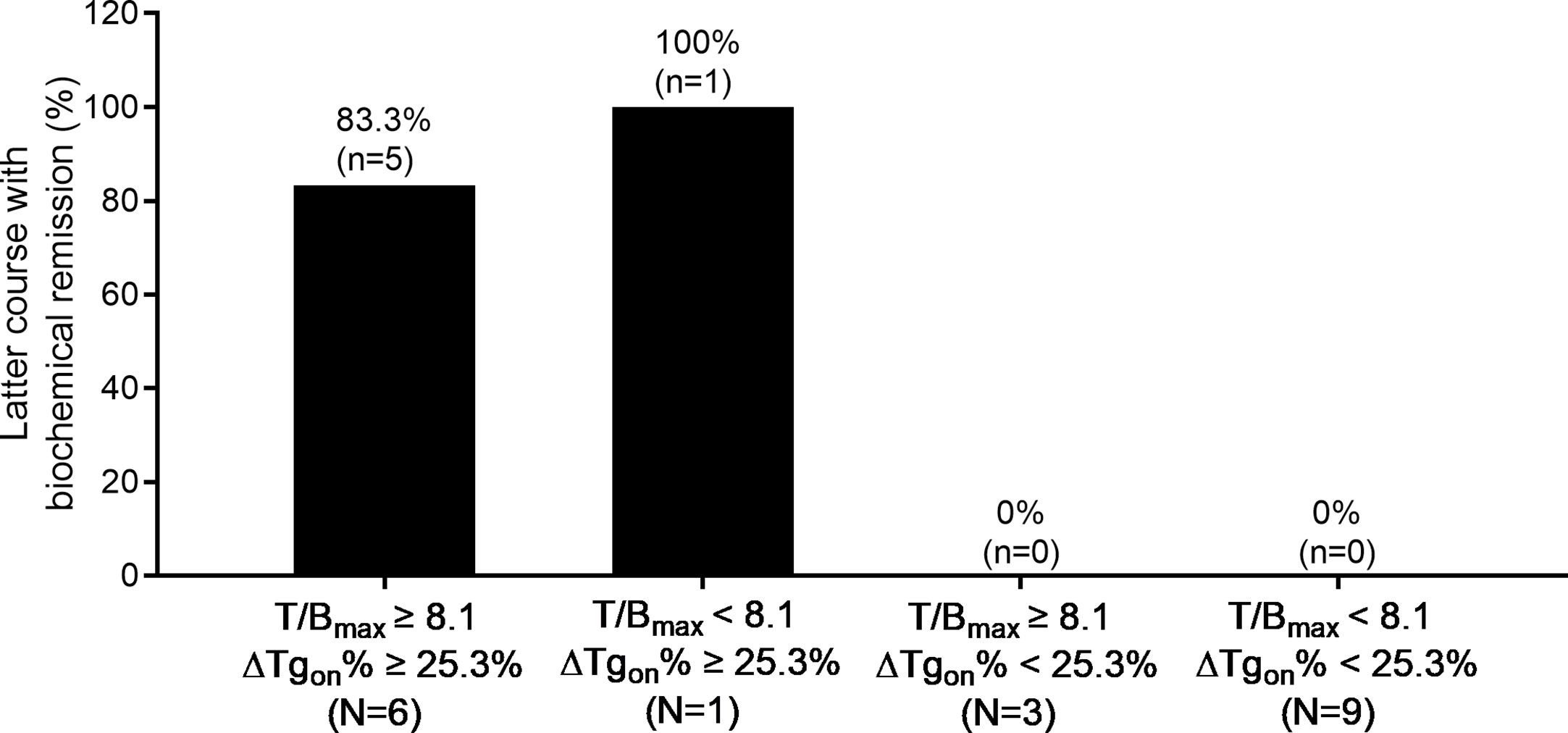
Figure 5 Distribution of biochemical remission in 19 paired latter courses of RT in patients with 131I-nonavid metastases concomitant with 131I-avid metastases with regard to the results of T/Bmax and ΔTgon% obtained from the former courses. RT, radioiodine treatment; T/Bmax, maximum target-to-background ratio; Tgon, thyroid stimulating hormone-suppressed thyroglobulin; ΔTgon%, change rate of Tgon level.
Moreover, accumulated 131I activity ≥ 22.2 GBq was administered in 20 patients with a total of 52 former courses. In this entity, 87.5% (14/16) of the latter courses of therapy brought biochemical remission if T/Bmax ≥ 8.1 and ΔTgon% ≥ 25.3% were met in the former course, while only 5.6% (1/18) of the latter courses of RT resulted in biochemical remission if the former course of RT had yielded in T/Bmax < 8.1 and ΔTgon% < 25.3%. The distribution of biochemical remission in the paired latter courses is shown in Figure 6.
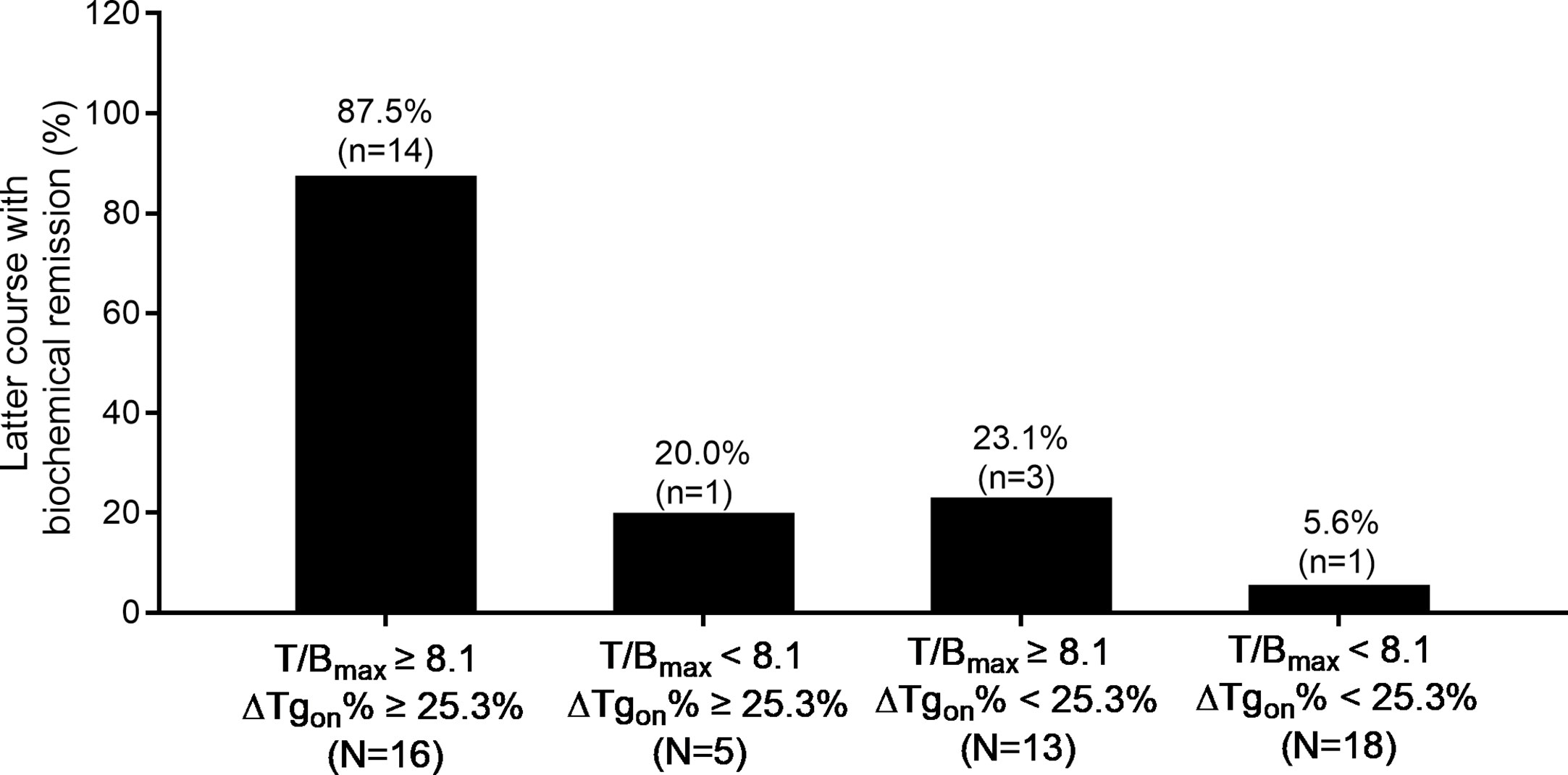
Figure 6 Distribution of biochemical remission in 52 paired latter courses of RT in patients with accumulated 131I activity ≥ 22.2 GBq with regard to the results of T/Bmax and ΔTgon% obtained from the former courses. RT, radioiodine treatment; T/Bmax, maximum target-to-background ratio; Tgon, thyroid stimulating hormone-suppressed thyroglobulin; ΔTgon%, change rate of Tgon level.
Discussion
In the era of precision medicine, a justification of RT is critical for the management of DTC, especially in patients with 131I-avid DM-DTC who possibly need multiple RT courses. Optimization of indications is a key step to enhance therapeutic efficacy and avoid inadequate or invalid 131I administrations, and appropriate predictors for responders/non-responders are anticipated to distinguish patients who would receive next RT from those who should turn to other management timely. To our knowledge, this is the first prospective quantitative study to predict BR to next RT by combined use of T/Bmax and ΔTgon% derived from the latest RT, providing a practical evidence for or against next RT in a course-based manner, warranting the justification of repeating RT or not.
Since that Rx-WBS reveals functioning metastases secondary to DTC, positive findings have been used to guide subsequent RT (21, 22). However, 131I uptake reflected by the latest Rx-WBS does not necessarily mean that the patient will respond to next RT due to a neglect of absorbed radiation dose to metastatic lesions, and the PPV of Rx-WBS in predicting final beneficial BR has been reported to be only 60.0% in patients with DM-DTC (23), which is in line with our present study demonstrating that a high rate as 49.4% (82/166) of latter RTs failed to achieve biochemical remission (Figure 1). Of note, T/Bmax derived from the Rx-WBS of the former RT was found to be an independent predictor with PPV and NPV of 79.1% and 84.0% at the cut-off value 8.1, respectively. It means that a higher T/Bmax obtained from the former Rx-WBS is associated with biochemical remission.
Tg, a reliable DTC-specific marker, positively correlates with tumor burden in totally thyroidectomized patients. Tgon > 10.0 ng/ml may be sufficient to ensure the existence of DTC in patients who have undergone total thyroidectomy (24, 25), which became one of the two enrollment criteria in our analysis. Unexpectedly, neither baseline Tgoff nor baseline Tgon of the former RT were associated with BR to the latter RT, whereas ΔTgon% turned out to be an independent predictor. It seems that a higher ΔTgon% derived from the former course correlates with biochemical remission. Unfortunately, predictive value of the ΔTgon% was relatively limited, as the PPV and NPV of ΔTgon% at 25.3% in our study were merely 70.8% and 77.1%, respectively (Table 3). This may be due to that synthesis of Tg does not accurately reflect the iodine consumption in DM-DTC lesions, which lays critical foundation for biochemical remission (26).
As mentioned above, the value of T/Bmax or ΔTgon% alone in predicting BR to the next course of RT was not sufficiently high. Inspiringly, the combined use of semi-quantitative T/Bmax and quantitative ΔTgon% displayed a robustly enhanced PPV and NPV compared with that of either T/Bmax or ΔTgon% alone. In the present study, T/Bmax ≥ 8.1 and ΔTgon% ≥ 25.3% possessed the best PPV, while T/Bmax ≥ 8.1 or ΔTgon% ≥ 25.3% yielded the optimal NPV (Table 3). These findings suggest that biochemical response to next RT can be confidently expected in patients whose latest RT has yielded a T/Bmax ≥ 8.1 and a ΔTgon% ≥ 25.3%, whereas patients whose latest RT has yielded a T/Bmax < 8.1 and a ΔTgon% < 25.3% would hardly benefit from next RT. Such stratification based on T/Bmax combined with ΔTgon% derived from the latest RT may be useful in justifying the acceptance or refusal of the next course. By the way, difficulties in predicting BR were encountered in approximately one third of former courses, implying that other predictors are still needed in decision-making of such inconsistent situations (T/Bmax ≥ 8.1 and ΔTgon% < 25.3%, or T/Bmax < 8.1 and ΔTgon% ≥ 25.3%).
Notably, excellent benefits were achieved in four former RT courses in the group of T/Bmax ≥ 8.1 and ΔTgon% ≥ 25.3%. Tgon levels subtly higher than 10.0 ng/ml and negative Rx-WBS findings were found in these four patients before and after RT, respectively. Such excellent benefits from former courses may compromise the PPV of T/Bmax ≥ 8.1 and ΔTgon% ≥ 25.3%, to a certain degree. This might be due to that a greater than 25% decrease in Tgon was hardly achievable by the latter RT, since the tumor burden after the initial RT was relieved substantially or even disappeared (Figure 4).
Moreover, in patients with 131I concentrations in some foci but not in others, 83.33% (5/6) of the courses of RT brought biochemical remission if the criterion of T/Bmax ≥ 8.1 and ΔTgon% ≥ 25.3% was met (Figure 5), indicating that such patients should not be hastily labelled as radioiodine refractoriness and repeated RT should not be arbitrarily terminated (7). Conversely, a T/Bmax < 8.1 and ΔTgon% < 25.3% in this setting implies that further RT should be avoided or a 131I dose of 7.4 GBq may be insufficient. Similar findings were obtained in patients with accumulated 131I activity over 22.2 GBq (Figure 6). Therefore, the combined use of both predictors with optimal cut-off values establishes an indispensable tool for decision-making in the above both entities.
It is well known that the role of RT may be influenced by adopted efficacy assessment criteria. For instance, the routinely used Response Evaluation Criteria in Solid Tumors are usually impractical in the assessment of responses to RT in DM-DTC patients, as non-measurable lesions are commonly found (27–29). Moreover, morphological changes of lesions are not easily perceived by anatomical imaging during a relatively short interval, since 131I-avid DM-DTC is well differentiated and indolent in nature, and repeated RT is recommended to be administered every 6–12 months. Fortunately, Tg level is closely associated with prognosis of patients with DM-DTC irrespective of age, initial staging and risk-stratification (30) and the ΔTgon% has been confirmed to reflect the response to RT and the degree of tumor destruction (31). In previous studies, it was well accepted that the ΔTgon% thresholds of 20.0% and 25.0% were adopted for differentiating effective RT from non-effective RT (4, 32). To utmostly avoid the invalid RT, the threshold ΔTgon% of 25.0% was adopted in our study to screen clinicopathological features associated with biochemical remission. Moreover, it is noteworthy that, along with biochemical progression, we categorized biochemical stabilization into non-effective RT, owing to that DTC is an indolent tumor with a long duration of stable Tg level under TSH suppression. Thus, prospective randomized controlled studies are required to elucidate whether stable BR truly represents a benefit of RT.
Some limitations exist in our study. Firstly, some clinicopathological features potentially associated with BR, such as diagnostic 131I scan outcomes, 18F-FDG PET findings, and mutational statuses, were not included in the initial study design. Secondly, all patients received a dose of 7.4 GBq in each administration with an interval of nearly 6 months between neighboring courses regarding professional guidelines, and presumed impact of a former RT on the biochemical effect of the latter course could not be clearly determined and completely excluded (33, 34). Finally, T/Bmax may vary with the time interval between the administration of 131I and post therapeutic scans, as well as the activity and status of administrated 131I. Similarly, Tg levels may fluctuate with time interval after the administration of 131I, as well as be interfered by the presence of TgAb and/or TSH levels. We, for the sake of extensibility of this study, encourage institutions to establish their own cut-off values of T/Bmax and ΔTgon% derived from the latest RT to predict BR to the possible next RT.
Conclusion
Our prospective study demonstrated that T/Bmax combined with ΔTgon% derived from the latest course of RT may efficiently differentiate patients with 131I-avid DM-DTC who would biochemically benefit from next RT from those who would not, warranting management optimization of this entity.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
RS and LibC designed the study. RS, LinC, and YJ participated in patient data acquisition. RS, LinC, HF, and YS analyzed the data. RS and LibC wrote and revised the draft. All authors contributed to the article and approved the submitted version.
Funding
This study was sponsored by the National Natural Science Foundation of China (Grant No. 81671711) and Shanghai key discipline of medical imaging (Grant No. 2017ZZ02005).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Furuya-Kanamori L, Sedrakyan A, Onitilo AA, Bagheri N, Glasziou P, Doi SAR. Differentiated thyroid cancer: millions spent with no tangible gain? Endocr Relat Cancer (2018) (1):51–7. doi: 10.1530/ERC-17-0397
2. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. Jama (2017) (13):1338–48. doi: 10.1001/jama.2017.2719
3. Nixon IJ, Whitcher MM, Palmer FL, Tuttle RM, Shaha AR, Shah JP, et al. The impact of distant metastases at presentation on prognosis in patients with differentiated carcinoma of the thyroid gland. Thyroid (2012) (9):884–9. doi: 10.1089/thy.2011.0535
4. Zhang XY, Sun JW, Qiu ZL, Wang Y, Chen XY, Zhao JH, et al. Clinical outcomes and prognostic factors in patients with no less than three distant organ system metastases from differentiated thyroid carcinoma. Endocrine (2019 (2):254–65. doi: 10.1007/s12020-019-01999-6
5. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (2016) (1):1–133. doi: 10.1089/thy.2015.0020
6. Pace L, Klain M, Albanese C, Salvatore B, Storto G, Soricelli A, et al. Short-term outcome of differentiated thyroid cancer patients receiving a second iodine-131 therapy on the basis of a detectable serum thyroglobulin level after initial treatment. Eur J Nucl Med Mol Imaging (2006) (2):179–83. doi: 10.1007/s00259-005-1929-2
7. Jin Y, Van Nostrand D, Cheng L, Liu M, Chen L. Radioiodine refractory differentiated thyroid cancer. Crit Rev Oncol Hematol (2018) 125:111–20. doi: 10.1016/j.critrevonc.2018.03.012
8. Klein Hesselink EN, Brouwers AH, de Jong JR, van der Horst-Schrivers AN, Coppes RP, Lefrandt JD, et al. Effects of Radioiodine Treatment on Salivary Gland Function in Patients with Differentiated Thyroid Carcinoma: A Prospective Study. J Nucl Med (2016) (11):1685–91. doi: 10.2967/jnumed.115.169888
9. Chen L, Shen Y, Luo Q, Yu Y, Lu H, Zhu R. Pulmonary fibrosis following radioiodine therapy of pulmonary metastases from differentiated thyroid carcinoma. Thyroid (2010) (3):337–40. doi: 10.1089/thy.2009.0266
10. Bikas A, Van Nostrand D, Jensen K, Desale S, Mete M, Patel A, et al. Metformin Attenuates 131I-Induced Decrease in Peripheral Blood Cells in Patients with Differentiated Thyroid Cancer. Thyroid (2016) (2):280–6. doi: 10.1089/thy.2015.0413
11. Sawka AM, Thabane L, Parlea L, Ibrahim-Zada I, Tsang RW, Brierley JD, et al. Second primary malignancy risk after radioactive iodine treatment for thyroid cancer: a systematic review and meta-analysis. Thyroid (2009) (5):451–7. doi: 10.1089/thy.2008.0392
12. Klain M, Pace L, Zampella E, Mannarino T, Limone S, Mazziotti E, et al. Outcome of Patients With Differentiated Thyroid Cancer Treated With 131-Iodine on the Basis of a Detectable Serum Thyroglobulin Level After Initial Treatment. Front Endocrinol (Lausanne) (2019) 10:146. doi: 10.3389/fendo.2019.00146
13. Hirsch D, Gorshtein A, Robenshtok E, Masri-Iraqi H, Akirov A, Duskin Bitan H, et al. Second Radioiodine Treatment: Limited Benefit for Differentiated Thyroid Cancer With Locoregional Persistent Disease. J Clin Endocrinol Metab (2018) (2):469–76. doi: 10.1210/jc.2017-01790
14. Califano I, Deutsch S, Lowenstein A, Cabezon C, Pitoia F. Outcomes of patients with bone metastases from differentiated thyroid cancer. Arch Endocrinol Metab (2018) (1):14–20. doi: 10.20945/2359-3997000000004
15. de la Fouchardiere C, Decaussin-Petrucci M, Berthiller J, Descotes F, Lopez J, Lifante JC, et al. Predictive factors of outcome in poorly differentiated thyroid carcinomas. Eur J Cancer (2018) 92:40–7. doi: 10.1016/j.ejca.2017.12.027
16. Wang R, Zhang Y, Tan J, Zhang G, Zhang R, Zheng W, et al. Analysis of radioiodine therapy and prognostic factors of differentiated thyroid cancer patients with pulmonary metastasis: An 8-year retrospective study. Med (Baltimore) (2017) (19):e6809. doi: 10.1097/MD.0000000000006809
17. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid (2009) (11):1167–214. doi: 10.1089/thy.2009.0110
18. Chen L, Luo Q, Shen Y, Yu Y, Yuan Z, Lu H, et al. Incremental value of 131I SPECT/CT in the management of patients with differentiated thyroid carcinoma. J Nucl Med (2008) (12):1952–7. doi: 10.2967/jnumed.108.052399
19. Yang X, Li J, Li X, Liang Z, Gao W, Liang J, et al. TERT Promoter Mutation Predicts Radioiodine-Refractory Character in Distant Metastatic Differentiated Thyroid Cancer. J Nucl Med (2017) (2):258–65. doi: 10.2967/jnumed.116.180240
20. Wu S, Wang H. Efficacy analysis of (131)I therapy and predictive value of preablation stimulated thyroglobulin for lung metastases from differentiated thyroid cancer. Ann Endocrinol (Paris) (2013) (1):40–4. doi: 10.1016/j.ando.2012.11.007
21. Cheng L, Liu M, Ruan M, Chen L. Challenges and strategies on radioiodine treatment for differentiated thyroid carcinoma. Hell J Nucl Med (2016) (1):23–32. doi: 10.1016/j.ando.2012.11.007
22. Gonzalez Carvalho JM, Gorlich D, Schober O, Wenning C, Riemann B, Verburg FA, et al. Evaluation of (131)I scintigraphy and stimulated thyroglobulin levels in the follow up of patients with DTC: a retrospective analysis of 1420 patients. Eur J Nucl Med Mol Imaging (2017) (5):744–56. doi: 10.1007/s00259-016-3581-4
23. Van Nostrand D, Aiken M, Atkins F, Moreau S, Garcia C, Acio E, et al. The utility of radioiodine scans prior to iodine 131 ablation in patients with well-differentiated thyroid cancer. Thyroid (2009) (8):849–55. doi: 10.1089/thy.2008.0419
24. Webb RC, Howard RS, Stojadinovic A, Gaitonde DY, Wallace MK, Ahmed J, et al. The utility of serum thyroglobulin measurement at the time of remnant ablation for predicting disease-free status in patients with differentiated thyroid cancer: a meta-analysis involving 3947 patients. J Clin Endocrinol Metab (2012) (8):2754–63. doi: 10.1210/jc.2012-1533
25. Piccardo A, Arecco F, Puntoni M, Foppiani L, Cabria M, Corvisieri S, et al. Focus on high-risk DTC patients: high postoperative serum thyroglobulin level is a strong predictor of disease persistence and is associated to progression-free survival and overall survival. Clin Nucl Med (2013) (1):18–24. doi: 10.1097/RLU.0b013e318266d4d8
26. Robenshtok E, Grewal RK, Fish S, Sabra M, Tuttle RM. A low postoperative nonstimulated serum thyroglobulin level does not exclude the presence of radioactive iodine avid metastatic foci in intermediate-risk differentiated thyroid cancer patients. Thyroid (2013) (4):436–42. doi: 10.1089/thy.2012.0352
27. Kwon SY, Lee SW, Kong EJ, Kim K, Kim BI, Kim J, et al. Clinicopathologic risk factors of radioactive iodine therapy based on response assessment in patients with differentiated thyroid cancer: a multicenter retrospective cohort study. Eur J Nucl Med Mol Imaging (2019) (3):561–71. doi: 10.1007/s00259-019-04634-8
28. Suzuki C, Jacobsson H, Hatschek T, Torkzad MR, Boden K, Eriksson-Alm Y, et al. Radiologic measurements of tumor response to treatment: practical approaches and limitations. Radiographics (2008) (2):329–44. doi: 10.1148/rg.282075068
29. Qiu ZL, Song HJ, Xu YH, Luo QY. Efficacy and survival analysis of 131I therapy for bone metastases from differentiated thyroid cancer. J Clin Endocrinol Metab (2011) (10):3078–86. doi: 10.1210/jc.2011-0093
30. Liu L, Zhang X, Tian T, Huang R, Liu B. Prognostic value of pre-ablation stimulated thyroglobulin in children and adolescents with differentiated thyroid cancer. Thyroid (2020) (7):1017–24. doi: 10.1089/thy.2019.0585
31. Barres B, Kelly A, Kwiatkowski F, Batisse-Lignier M, Fouilhoux G, Aubert B, et al. Stimulated Thyroglobulin and Thyroglobulin Reduction Index Predict Excellent Response in Differentiated Thyroid Cancers. J Clin Endocrinol Metab (2019) (8):3462–72. doi: 10.1210/jc.2018-02680
32. Sabra MM, Grewal RK, Tala H, Larson SM, Tuttle RM. Clinical outcomes following empiric radioiodine therapy in patients with structurally identifiable metastatic follicular cell-derived thyroid carcinoma with negative diagnostic but positive post-therapy 131I whole-body scans. Thyroid (2012) (9):877–83. doi: 10.1089/thy.2011.0429
33. Padovani RP, Robenshtok E, Brokhin M, Tuttle RM. Even without additional therapy, serum thyroglobulin concentrations often decline for years after total thyroidectomy and radioactive remnant ablation in patients with differentiated thyroid cancer. Thyroid (2012) (8):778–83. doi: 10.1089/thy.2011.0522
34. Biko J, Reiners C, Kreissl MC, Verburg FA, Demidchik Y, Drozd V. Favourable course of disease after incomplete remission on (131)I therapy in children with pulmonary metastases of papillary thyroid carcinoma: 10 years follow-up. Eur J Nucl Med Mol Imaging (2011) (4):651–5. doi: 10.1007/s00259-010-1669-9
Keywords: thyroid cancer, radioiodine, target/background ratio, thyroglobulin, biochemical response
Citation: Sa R, Cheng L, Jin Y, Fu H, Shen Y and Chen L (2020) Distinguishing Patients With Distant Metastatic Differentiated Thyroid Cancer Who Biochemically Benefit From Next Radioiodine Treatment. Front. Endocrinol. 11:587315. doi: 10.3389/fendo.2020.587315
Received: 25 July 2020; Accepted: 21 October 2020;
Published: 16 November 2020.
Edited by:
Leonidas H. Duntas, National University of Athens, GreeceReviewed by:
Misa Imaizumi, Radiation Effects Research Foundation, JapanRafal Czepczynski, Poznan University of Medical Sciences, Poland
Copyright © 2020 Sa, Cheng, Jin, Fu, Shen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Shen, shenyan1050@126.com; Libo Chen, lbchen@sjtu.edu.cn
†These authors share first authorship
 Ri Sa
Ri Sa Lin Cheng
Lin Cheng Yuchen Jin
Yuchen Jin Hao Fu
Hao Fu Yan Shen
Yan Shen Libo Chen
Libo Chen