- 1Department of Pediatrics and Pediatric Endocrinology, Faculty of Medical Sciences in Katowice, Medical University of Silesia, Katowice, Poland
- 2Student Scientific Society, Department of Biophysics, Jagiellonian University Medical College, Kraków, Poland
- 3Department of Biochemistry, Faculty of Medical Sciences in Katowice, Medical University of Silesia, Katowice, Poland
Background: Turner syndrome (TS) presents a high risk of congenital heart defects and may predispose to both obesity and related metabolic complications. Hence the search for new markers as potential early predictors of the metabolic syndrome (MetS) and cardiovascular diseases appears warranted.
Objective: To assess MMP-1 (matrix metalloproteinase-1), MMP-2 (matrix metalloproteinase-2), MMP-9 (matrix metallopeptidase-9), BDNF (brain-derived neurotrophic factor), GDNF (glial cell line-derived neurotrophic factor), and VEGF (vascular endothelial growth factor) in non-MetS TS girls not treated with growth hormone (GH) vs. healthy short stature girls, and to assess the connection with basic metabolic parameters.
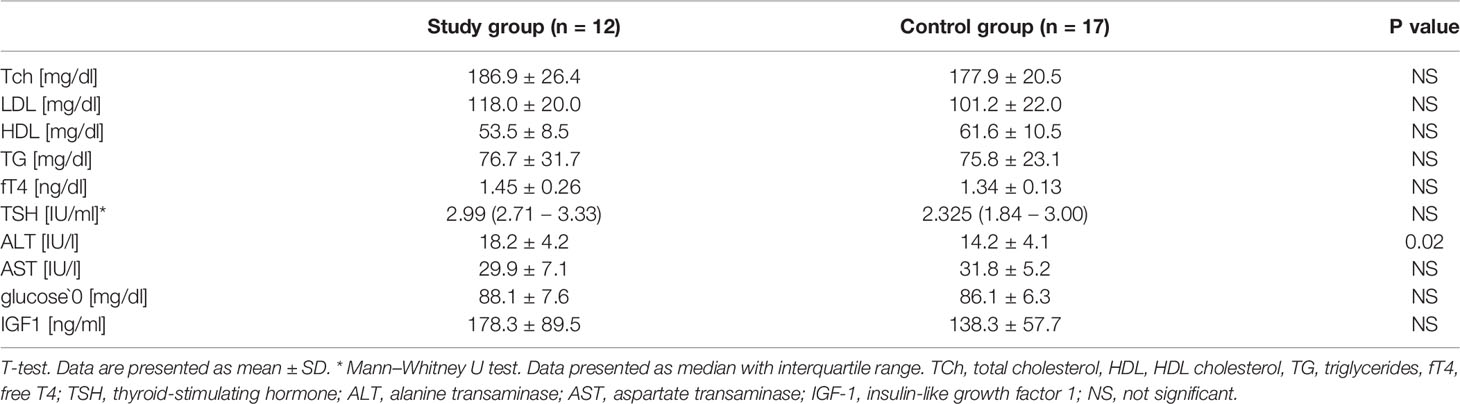
Method: The concentrations of circulating MMP-1, MMP-2, MMP-9, BDNF, GDNF and VEGF were measured in 12 patients with TS not treated with growth hormone. The control group was composed of 17 girls with non-pathologic short stature. The patients’ clinical and biochemical phenotypes were determined by weight, height, total cholesterol, HDL cholesterol, triglycerides, glucose, aminotransferases, IGF1, TSH and fT4.
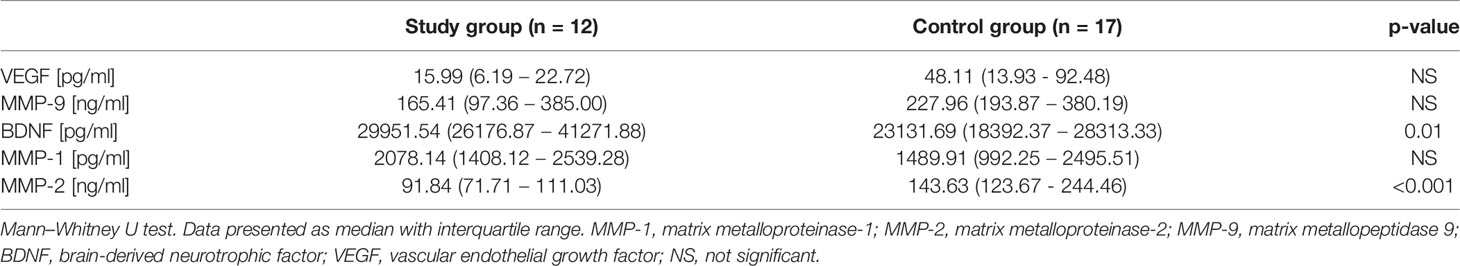
Results: There were no differences in mean age, weight, BMI Z-Score, or hSDS between the studied group and the controls; however, they differed in baseline values of ALT (18.2 ± 4.2 vs. 14.2 ± 4.1, p= 0.02), BDNF [29951.5 (26176.9 – 41271.9) vs. 23131.7 (18392.4 – 28313.3), p=0.01] and MMP-2 [91.8 (71.7 – 111.0) vs. 143.6 (123.7 - 244.5), p< 0.001]. BDNF correlated with ALT activity (r = 0.56 p = 0.002) and BMI Z-score (r = 0.38 p = 0.042), while MMP-2 correlated with HDL concentration (r = 0.48 p = 0.029) in all the patients. The analysis of the study group alone revealed significant positive correlations between MMP-9 and TSH (r = 0.74 p = 0.036), BDNF and both ALT (r = 0.73 p = 0.038) and TSH (r = 0.85 p = 0.008), and a negative correlation between MMP-1 and fT4 (r = -0.75 p = 0.032). The control group did not present any significant correlations.
Conclusion: The higher concentrations of BDNF and lower of MMP-2 found in girls with TS without MetS compared to healthy girls with short stature, could have a major impact on the future “natural” development of the metabolic status. Our findings need further studies.
Introduction
Obesity and other components of the metabolic syndrome (MetS), such as hypertension, dyslipidemia and type 2 diabetes that occur in childhood, have been identified as significant determinants of cardiovascular diseases in adulthood (CVD) (1). Many studies indicate that metabolic disorders occur more frequently in the Turner Syndrome (TS) population and are noticeable as early as in childhood (2, 3). Additionally, heart defects, mainly aortic coarctation and bicuspid aortic valve, are more common in Turner syndrome (2). All these factors cause shorter life expectancy in the group of TS patients (4). It remains unclear whether cardiometabolic risks in TS are a consequence of intrinsic factors or the result of modifiable metabolic risk factors. Thus, the search for new markers as potential early predictors of the natural development of metabolic disorders/complications seems to be justified. Our previous pilot study regarded selected metabolic markers in TS girls undergoing growth hormone (GH) therapy (5). In this study, we re-examine brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factors (GDNF), vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) in a newly assembled group of patients, this time before the onset of GH-therapy (to exclude its potential influence) with a view to investigate their possible connection with basic metabolic parameters.
BDNF is a member of the nerve growth factor family, which plays an important role in brain metabolic and feeding regulation (6). BDNF affects glucose, lipid and energy metabolism and is considered an anorexigenic factor (7). Its concentration correlates negatively with body weight (8) and age (9) and is believed to be a factor for cardioprotection, neuroprotection and aging. Furthermore, patients diagnosed with MetS or acute coronary syndrome (10) have significantly lower plasma BDNF levels. These reports support the metabotropic deficit hypothesis (11), according to which, the absence of neurotrophins can predispose to the development of metabolic diseases. There is paucity of research on the role and plasma concentration of BDNF in patients with TS. However, in one study, BDNF plasma concentrations were proven to be significantly higher in the group of adult TS patients compared to the control group (12). Our pilot study also confirmed higher BDNF levels in girls with TS (5).
GDNF plays a neuroprotective role for neurons that participate in the regulation of energy intake and expenditure (13). The study on transgenic mice with overexpression of GDNF in glial cells revealed that they are protected from obesity, glucose intolerance, and resistance to insulin caused by high-fat meals (14). Like BDNF, GDNF seems to have a role in the suggested metabotropic hypothesis of metabolic disorders.
VEGF appears to have a metabolic effect, too. In obese people, the development of blood vessels is insufficient and lower concentrations of VEGF are observed, which results in hypoxia of adipocytes and local inflammation (15). Hypoxia is known to cause glucose intolerance (16), whilst ongoing low-grade inflammation leads to insulin insensitivity (17). However, the published data regarding the relationship between VEGF and metabolic processes are inconclusive. One study demonstrated a positive correlation between the concentrations of circulating VEGF levels and BMI in healthy male subjects (18), whilst another proved increased levels of VEGF in overweight and obese individuals, with higher levels in females (19). To date, no large studies on VEGF in TS in the context of obesity and carbohydrate disorders have been published. Our previous pilot work showed no differences in VEGF concentrations between TS girls treated with GH and short stature girls (5).
Matrix metalloproteinases (MMPs) are a group of enzymes that degrade extracellular matrix components with expression regulated by growth factors or ongoing inflammation (20). MMP-1 seems to be involved in the development of adipose tissue (21), while plasma levels of MMP-2 and MMP-9 are elevated in patients with the metabolic syndrome (22). In our previous study, MMP-1 was significantly higher in the study group, with a significant positive correlation with Z-score BMI; we found no differences in the levels of other MMPs (5).
Subjects and Methods
Twenty-nine patients were enrolled in this prospective study. The study group included 12 patients with Turner syndrome (TS), confirmed by karyotyping with routine G-banding according to the recommendations of the American College of Medical Genetics. The control group consisted of 17 girls with nonpathological/non-syndromic short stature (healthy, short stature girls, with normal karyotype, normal birth weight [non-SGA] and excluded endocrine causes of short stature). We have extended the control group compared to the pilot study (5).
Clinical Phenotype of Study Participants
The detailed anthropometrical analysis was based on weight and height measurements, along with body mass index (BMI) calculation, using the standard formula of weight (kg) divided by height (m) squared. Weight was measured with a Seca scale with a precision of 100 g, and height with a Harpenden stadiometer with a graduation of 0.1 cm. A BMI above the 97th percentile was classified as obesity, while a BMI between the 90th and 97th percentile as overweight based on the BMI chart for healthy girls (23). Height was expressed as standardized values (height standard deviation score (hSDS) based on Turner Syndrome Growth Chart by Ranke (24) and on the Polish growth chart for healthy girls (23). hSDS was calculated using the following formula: hSDS = child’s height − height for 50 pc/0.5 ∗ (height 50 pc − height 3 pc). Based on the age, sex, BMI, and appropriate reference standard, the BMI Z-score was calculated using the international (International Obesity Task Force; IOTF) body mass index (BMI) cut-offs (25). The patients’ bone age was determined based on the X-ray of the non-dominant hand using the Greulich- Pyle Atlas (26). The Tanner scale was used for puberty assessment (27). TS girls underwent cardiological evaluation to assess heart defects and detect hypertension before the onset of GH-therapy.
Biochemical Phenotype of Study Participants
Morning fasting venous blood samples were collected to measure MMP-1 (matrix metalloproteinase-1), MMP-2 (matrix metalloproteinase-2), MMP-9 (matrix metallopeptidase-9), BDNF (brain-derived neurotrophic factor), GDNF (glial cell line derived neurotrophic factor), and VEGF (vascular endothelial growth factor). The concentrations of these markers were determined with sandwich ELISA, using kits distributed by the R&D systems.
Concentrations of total cholesterol (TCh), HDL cholesterol (HDL-chol) and triglycerides (TG) were analyzed enzymatically (Beckman Coulter, Brea, CA). An enzymatic test (hexokinase method) was used for the quantitative determination of glucose (Beckman Coulter). In order to assess the concentrations of TG, HDL-chol and fasting glucose in children aged 3 to 11 years old, the results were categorized into 3 groups according to the IDEFICS study (28): 0 - good result; 1 - observation necessary; 2 - intervention required. For older children, IDF percentile charts were used - values were classified as 0 (good result) or 1 (incorrect result) (29).
The concentrations of fT4 (free thyroxine), TSH (thyroid-stimulating hormone), ALT (alanine transaminase), AST (aspartate transaminase), and IGF-1 (insulin-like growth factor 1) were also determined. Serum concentrations of fT4 and TSH were measured with a chemiluminescent immunometric assay (IMMULITE 2000 Free T4 and IMMULITE 2000 Third Generation TSH, respectively; Siemens). Alanine and aspartate aminotransferase activity in the serum was assessed according to the International Federation in Clinical Chemistry (Beckman Coulter).
Statistical Analysis
Data processing and statistical analyses were performed using Statistica 13.3 PL software. p value < 0.05 was considered significant. Variables with a normal distribution were analyzed using T-tests and reported as mean ± standard deviation (SD), those without normal distribution were analyzed using the Mann–Whitney U test and reported as median with interquartile range.
The study was conducted according to the Declaration of Helsinki and approved by the Ethics Committee of the Medical University of Silesia [resolution number KNW/0022/KB1/162/15/16)]. Informed consent was obtained from each participant aged over 16, a parent, or a legal guardian.
Results
The clinical characteristics of all participants are presented in Table 1. The groups did not differ in chronological or bone age. There were no differences in mean weight, BMI Z-Score, or hSDS. Obesity was diagnosed in one patient in the study group, and overweight in two. No excess body weight was observed in the control group patients. A statistically significant difference in birth weight was found between the control group and the study group. According to the Tanner scale, 5 controls and 2 study patients started spontaneous puberty (no higher than Tanner stage B2). Heart defects were observed in 3 patients in the study group: coarctation of the aorta (1 patient) and bicuspid aortic valve (2 patients), whilst 3 patients in this group had confirmed hypertension.
The biochemical characteristics of both groups are presented in Table 2. Two patients in the study group had impaired fasting glucose, one hypertriglyceridemia and three hypercholesterolemia. In the control group, two girls had hypertriglyceridemia and one hypercholesterolemia. None of the patients had lower HDL concentrations. The analyzed groups differed in mean baseline values of ALT, which, although within normal ranges, were significantly higher in the study group.
The concentrations of the proposed markers in the two groups are shown in Table 3. No differences were found in VEGF, MMP-9, and MMP-1 concentrations, while BDNF and MMP-2 were significantly different in the two groups. GDNF concentrations were below the limit of detection.
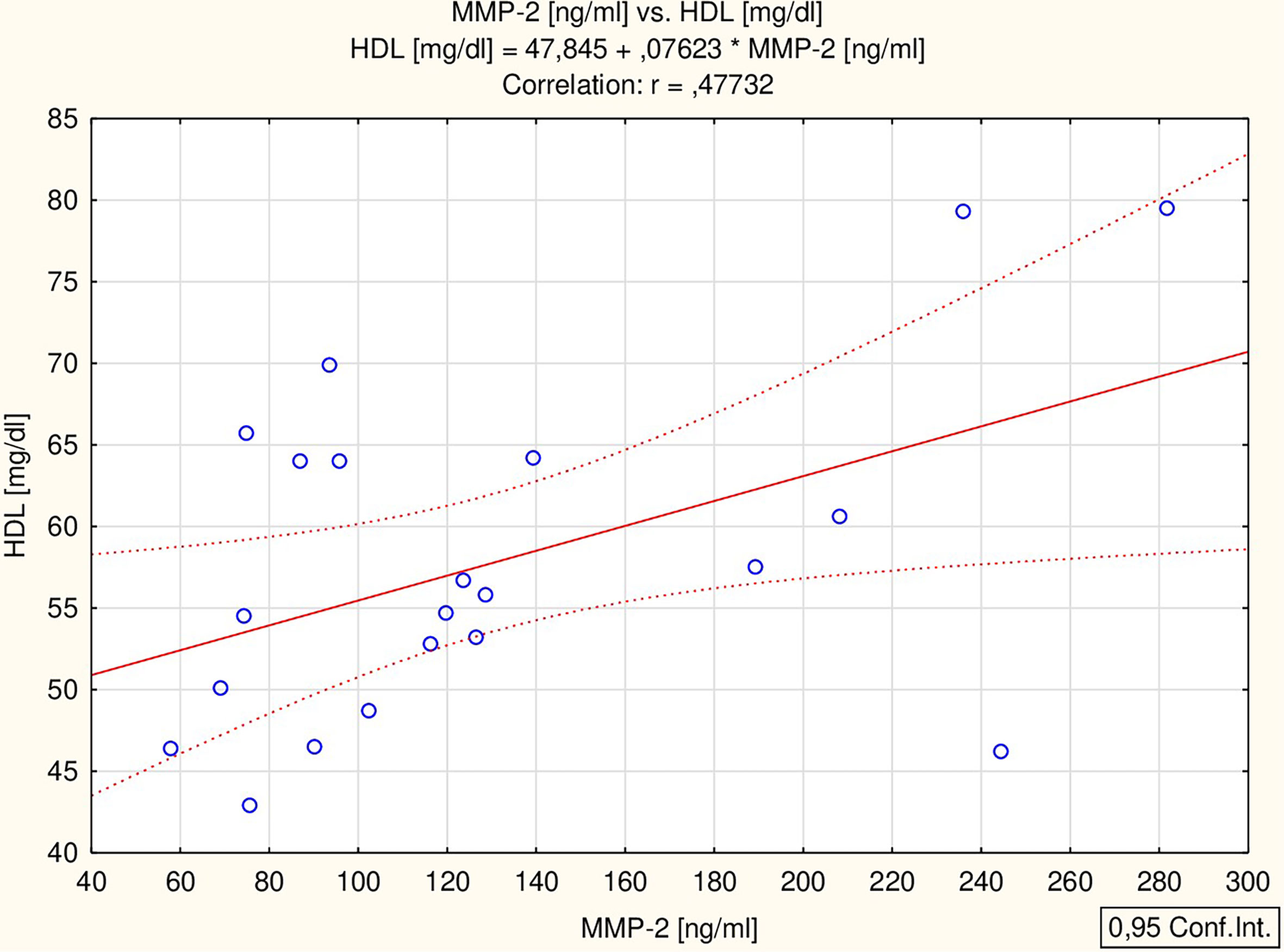
All the proposed markers were correlated to glucose, total cholesterol, HDL-chol, TG, ALT, IGF-1, TSH and ft4 concentrations, as well as BMI Z-score. BDNF correlated with ALT activity (r = 0.56 p = 0.002) and BMI Z-score (r = 0.38 p = 0.042) (Figure 1), while MMP-2 correlated with HDL concentration (r = 0.48 p = 0.029) (Figure 2) in all the patients. The analysis of the study group alone revealed significant positive correlations between MMP-9 and TSH (r = 0.74 p = 0.036), BDNF and both ALT (r = 0.73 p = 0.038) and TSH (r = 0.85 p = 0.008), and a negative correlation between MMP-1 and fT4 (r = -0.75 p = 0.032). The control group did not present any significant correlations.
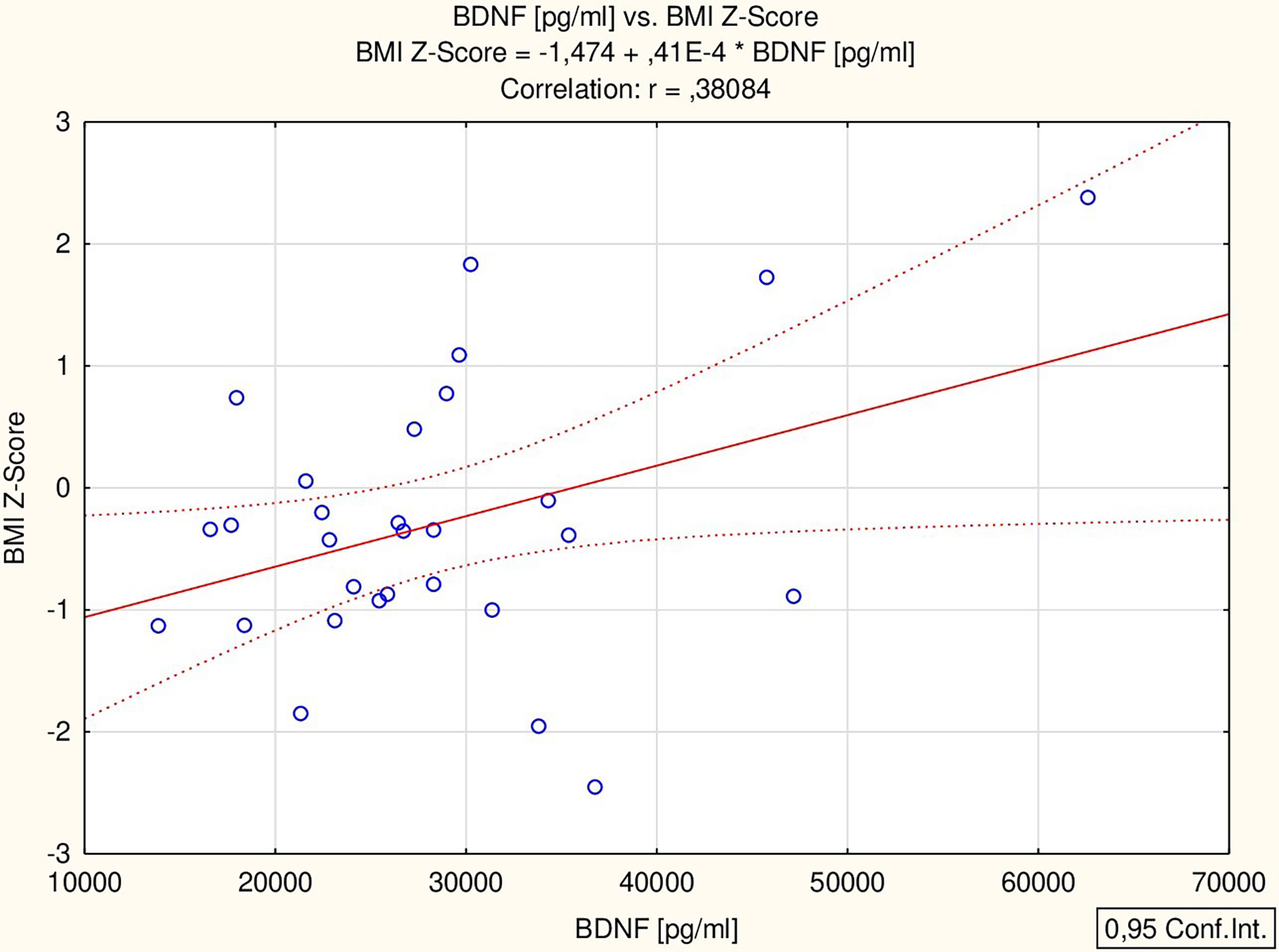
Figure 1 Correlation of BDNF with BMI Z-score; body mass index Z-score; BDNF, brain-derived neurotrophic factor.
Out of the three patients with excessive BMI in the study group, two (one obese and one overweight) had hypercholesterolemia. No other metabolic disturbances were found.
Discussion
This cross-sectional study confirmed higher concentrations of BDNF and lower of MMP-2 in non-MetS girls with TS compared to healthy girls with short stature. These findings present a protective trend and may prove important in the context of the “natural” development of the metabolic status in Turner syndrome patients.
Similar to our previous study, we found differences in BDNF concentration between the analyzed groups. In the previous analysis, we also found a higher concentration of BDNF in girls with TS treated with GH compared to healthy short-stature girls (5). In both studies, the absence of correlation between BDNF and IGF-1 was confirmed. It should be noted that in our study, the groups were comparable in chronological and bone age as well as in weight, BMI Z-Score and hsds. The only clinical difference we found between the groups was birth weight. (Previously, we had observed differences in BMI Z-score between groups, which could account for the observed difference; however, in this study, the groups were comparable in terms of BMI Z-score). In both studies we showed a positive correlation between BDNF and BMI Z-score. Of the three highest BDNF values obtained, two were found in an obese and in an overweight patient.
According to the literature, difference in BDNF concentrations between the groups could also relate to estrogen-androgen imbalance in TS. This would be in line with the results of the study performed by Czyzyk et al., who described a two-fold increase in the concentration of BDNF in a group of adult TS patients compared to the controls. In their study, BDNF correlated positively with testosterone levels in TS women, and the authors hypothesized that androgens can be one of the main regulators of plasma BDNF levels (12). Additionally Apter et al. showed that differences in androgen levels are noticeable in TS as early as at the age of 13 (30). However, most of the patients participating in our study were in the pre-pubertal period and we have not conducted relevant studies to support this hypothesis.
Our analysis showed no significant differences between the study group and the control group in the concentration of MMP-1 and MMP-9; however, a difference was observed in the level of MMP-2. Additionally, MMP-2 significantly correlated with HDL concentration: patients with the highest concentration of MMP-2 had a high level of HDL.
Studies in humans have shown that the plasma levels of MMP-2 and MMP-9 are elevated in patients with the metabolic syndrome (22). Our population included only one obese patient in the study group. What is more, short stature girls and patients with TS did not differ in mean baseline concentration of glucose, HDL, and TG levels. We found that the HDL concentration was within the normal range in all the examined girls; fasting glucose concentration was impaired in two girls in the study group; hyper TG was observed in one girl in the study group and in two controls. This might explain the absence of differences in MMP-9 between the analyzed groups. Study group patients with and without impaired carbohydrate-lipid metabolism haven’t differed in MMP-9 concentrations.)
By contrast, no explanation was found for the differences in MMP-2 levels between the analyzed groups. In our study we haven’t noticed a difference in MMP-2 concentrations between girls with and without impaired carbohydrate-lipid parameters. The correlation between HDL and MMP-2 concentrations is difficult to explain. What is more, some results indicate the inhibition of MMP-2 activation as a mechanism for HDL-mediated cardioprotection (31) and suggest negative correlation between HDL and MMP-2 activity (32). However, it is known that the synthesis of MMP-2 is influenced by many factors, which makes the explanation of individual observations a difficult task. The concentration of MMP-2 is also known to correlate with parameters, such as the thickness of the intima-media complex (33) or blood pressure values (34), not analyzed in this study. In order to clarify this difference, a study on a larger group of TS patients, with analysis of additional factors, is needed.
MMP-1 is involved in adipose tissue remodeling in obesity (21). In our previous study we found higher concentrations of this metalloproteinase in the study group, but we also found higher Z-score BMI in the study group and a positive correlation between MMP-1 and Z-score BMI, possibly confirming a link between MMP-1 and body weight (5). However, a larger study performed by Papazoglou et al. in a group of more than 100 obese patients revealed no significant differences in MMP-1 concentrations between obese and lean participants (35). Further research is needed.
GDNF concentration was below the limit of detection. In our previous study, the concentration of GDNF was low, but detectable in plasma. However, we found no differences between the analyzed groups at that time (5). According to some studies, plasma GDNF concentrations seem to poorly reflect its concentrations in tissues which are involved in metabolic regulation (14).
We observed no differences in VEGF concentrations between the analyzed groups. As mentioned above, the available studies on the association of VEGF and metabolic disorders have given contradictory results – some studies revealed higher levels of VEGF in overweight and obese patients (18, 19), whilst other studies proved the opposite (15, 36). In our previous study, we found no difference between the TS group and control group (5), which is further confirmed by this study. It is worth mentioning, however, that many factors affect the concentration of VEGF. This factor is involved in many physiological and pathological conditions such as lymhangiogenesis, embryogenesis, wound healing, inflammation, tumor metastasis, cardiovascular diseases, or rheumatoid arthritis (37). VEGF exerts an influence mainly locally, as in the case of wound healing or atherosclerotic process. Nevertheless, despite the local effect on tissues, its serum concentration is increased in several types of cancers (38) as well as there are significant familial correlations of plasma VEGF concentration between genetically related individuals (39). Given individual variability of VEGF’s circulating levels, more research is needed in this area to clearly establish the factors influencing its concentration in plasma and avoid incorrect associations.
We are aware of the limitations of our study: it was performed on a small number of young patients. This is due to the fact that TS is classified as a rare disease, and it takes time to collect a large number of GH-untreated patients. Future studies could include the correlation between the concentrations of metabolic markers with blood pressure values obtained from Holter RR recordings. It could also be useful to compare the levels of metabolic markers in TS girls and in normal weight, overweight and obese girls.
Despite of the above limitations, the study remains unique in the context of the analyzed group and the selected metabolic markers. Moreover, the presented markers also play roles other than those discussed here. The strength of this work lies in its well selected control group and novel take on biomarkers of metabolic risk. This study is a part of a series of markers determinations in patients with TS. The study performed before the onset of GH therapy allows to exclude its potential influence on carbohydrate and lipid metabolism that could be connected with shifts in markers concentration. It is known that in the TS group, the effect of GH therapy on carbohydrate-lipid parameters was found (40–42). Hence, this research seems to be valuable, because study group was composed of girls who had not been treated with GH so far. In our previous study, the influence of GH on the results could not be ruled out. In addition, we are currently conducting further research on marker stability and repeatability, the impact of rGH, the impact of BMI changes, and the appearance of MetS components.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the Medical University of Silesia (resolution number KNW/0022/KB1/162/15/16). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
EB and AG designed the study, prepared the database, and wrote the manuscript. JGi monitored the patients and collected samples for biochemical analysis. JGa analyzed the patient database and wrote the manuscript. TF collaborated in designing the work and performed laboratory analyses. MKF and GH performed laboratory analyses. All authors contributed to the article and approved the submitted version.
Funding
Financial resources were granted as part of financing tasks aimed at the development of doctoral studies and statutory work. The number of research funding contract is KNW-2-K27/D17/N and KNW-1-070/N/9/K (Medical University of Silesia).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors wish to thank all patients and their families for participating in this study. The authors also specially thank Sandra Lindon for the proofreading of this manuscript.
References
1. Morrison JA, Friedman LA, Gray-McGuire C. Metabolic Syndrome in Childhood Predicts Adult Cardiovascular Disease 25 Years Later: The Princeton Lipid Research Clinics Follow-Up Study. Pediatrics (2007) 120(2):340–5. doi: 10.1542/peds.2006-1699
2. Gravholt CH, Andersen NH, Conway GS, Dekkers OM, Geffner ME, Klein KO, et al. Clinical Practice Guidelines for the Care of Girls and Women With Turner Syndrome: Proceedings From the 2016 Cincinnati International Turner Syndrome Meeting. Eur J Endocrinol (2017) 177(3):G1–70. doi: 10.1530/EJE-17-0430
3. Caprio S, Boulware S, Diamond M, Rs S, To C, Rubin K, et al. Insulin Resistance: An Early Metabolic Defect of Turner’s Syndrome*. J Clin Endocrinol Metab (1991) 72(4):832–6. doi: 10.1210/jcem-72-4-832
4. Sybert VP, McCauley E. Turner’s Syndrome. N Engl J Med (2004) 351(12):1227–38. doi: 10.1056/NEJMra030360
5. Błaszczyk E, Lorek M, Francuz T, Gieburowska J, Gawlik A. Selected Metabolic Markers in Girls With Turner Syndrome: A Pilot Study. Int J Endocrinol (2018) 2018:1–6. doi: 10.1155/2018/9715790
6. Takei N, Furukawa K, Hanyu O, Sone H, Nawa H. A Possible Link Between BDNF and mTOR in Control of Food Intake. Front Psychol (2014) 5:1093(SEP). doi: 10.3389/fpsyg.2014.01093
7. Chaldakov GN, Tonchev AB, Aloe L. NGF and BDNF: From Nerves to Adipose Tissue, From Neurokines to Metabokines. Riv Psichiatr (2009) 44(2):79–87.
8. Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, et al. The Impact of Age, Weight and Gender on BDNF Levels in Human Platelets and Plasma. Neurobiol Aging (2005) 26(1):115–23. doi: 10.1016/j.neurobiolaging.2004.03.002
9. Ziegenhorn AA, Schulte-Herbrüggen O, Danker-Hopfe H, Malbranc M, Hartung H-D, Anders D, et al. Serum Neurotrophins–a Study on the Time Course and Influencing Factors in a Large Old Age Sample. Neurobiol Aging (2007) 28(9):1436–45. doi: 10.1016/j.neurobiolaging.2006.06.011
10. Manni L, Nikolova V, Vyagova D, Chaldakov GN, Aloe L, Aloe L, et al. Reduced Plasma Levels of NGF and BDNF in Patients With Acute Coronary Syndromes. Int J Cardiol (2005) 102(1):169–71. doi: 10.1016/j.ijcard.2004.10.041
11. Hristova M, Aloe L, Rosmond R, Björntorp P, Blalock E, Besedovsky H, et al. Metabolic Syndrome – Neurotrophic Hypothesis. Med Hypotheses (2006) 66(3):545–9. doi: 10.1016/j.mehy.2005.08.055
12. Czyzyk A, Casarosa E, Luisi M, Podfigurna-Stopa A, Meczekalski B, Genazzani AR. Brain-Derived Neurotrophic Factor Plasma Levels in Patients With Turner Syndrome. Gynecol Endocrinol (2014) 30(3):245–9. doi: 10.3109/09513590.2013.871513
13. Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gómez-Díaz R, López-Barneo J. Absolute Requirement of GDNF for Adult Catecholaminergic Neuron Survival. Nat Neurosci (2008) 11(7):755–61. doi: 10.1038/nn.2136
14. Mwangi SM, Nezami BG, Obukwelu B, Anitha M, Marri S, Fu P, et al. Glial Cell Line-Derived Neurotrophic Factor Protects Against High-Fat Diet-Induced Obesity. Am J Physiol Gastrointest Liver Physiol (2014) 306(6):G515–25. doi: 10.1152/ajpgi.00364.2013
15. Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, et al. Reduced Adipose Tissue Oxygenation in Human Obesity: Evidence for Rarefaction, Macrophage Chemotaxis, and Inflammation Without an Angiogenic Response. Diabetes (2009) 58(3):718–25. doi: 10.2337/db08-1098
16. Oltmanns KM, Gehring H, Rudolf S, Schultes B, Rook S, Schweiger U, et al. Hypoxia Causes Glucose Intolerance in Humans(2012). doi: 10.1164/rccm.200308-1200OC
17. Trayhurn P, Wood IS. Adipokines: Inflammation and the Pleiotropic Role of White Adipose Tissue. Br J Nutr (2004) 92(03):347. doi: 10.1079/BJN20041213
18. Loebig M, Klement J, Schmoller A, Betz S, Heuck N, Schweiger U, et al. Evidence for a Relationship Between VEGF and BMI Independent of Insulin Sensitivity by Glucose Clamp Procedure in a Homogenous Group Healthy Young Men. PloS One (2010) 5(9):e12610. doi: 10.1371/journal.pone.0012610
19. Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic Factors are Elevated in Overweight and Obese Individuals. Int J Obes (2005) 29(11):1308–14. doi: 10.1038/sj.ijo.0802987
20. Nagase H, Woessner JF. Matrix Metalloproteinases. J Biol Chem (1999) 274(31):21491–4. doi: 10.1074/jbc.274.31.21491
21. Samuvel DJ, Jin J, Sundararaj KP, Li Y, Zhang X, Lopes-Virella MF, et al. TLR4 Activation and IL-6-Mediated Cross Talk Between Adipocytes and Mononuclear Cells Synergistically Stimulate MMP-1 Expression. Endocrinology (2011) 152(12):4662–71. doi: 10.1210/en.2011-1026
22. Hopps E, Lo Presti R, Montana M, Noto D, Averna MR, Caimi G. Gelatinases and Their Tissue Inhibitors in a Group of Subjects With Metabolic Syndrome. J Investig Med (2013) 61(6):978–83. doi: 10.231/JIM.0b013e318294e9da
23. Palczewska INZ. Siatki Centylowe do Oceny Rozwoju Somatycznego Dzieci I Młodzieży. Warszawa: Polish Institute of Mother’s and Child’s Health (1999).
24. Ranke MB, Pfliiger H, Rosendahl W, Stubbe P, Enders H, Bierich JR, et al. Pediatrics Turner Syndrome: Spontaneous Growth in 150 Cases and Review of the Literature. Eur J Eur J Pediatr (1983) 141:81–88. doi: 10.1007/BF00496795
25. Cole TJ, Lobstein T. Extended International (IOTF) Body Mass Index Cut-Offs for Thinness, Overweight and Obesity. Pediatr Obes (2012) 7(4):284–94. doi: 10.1111/j.2047-6310.2012.00064.x
26. Greulich WW, Pyle SI. Radiographic Atlas Of Skeletal Development Of The Hand And Wrist. Am J Med Sci (1959) 238(3):393. doi: 10.1097/00000441-195909000-00030
27. Marshall WA, Tanner JM. Variations in Pattern of Pubertal Changes in Girls. Arch Dis Child (1969) 44(235):291–303. doi: 10.1136/adc.44.235.291
28. Ahrens W, Moreno L, Mårild S, Molnár D, Siani A, De Henauw S, et al. Metabolic Syndrome in Young Children: Definitions and Results of the IDEFICS Study. Int J Obes (2014) 38(2):S4–14. doi: 10.1038/ijo.2014.130
29. Zimmet P, Alberti G, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The Metabolic Syndrome in Children and Adolescents. Lancet Elsevier Limited (2007) 369:2059–61. doi: 10.1016/S0140-6736(07)60958-1
30. Apter D, Lenko H-L, Perheentupa J, Söderholm A, Vihko R. Subnormal Pubertal Increases of Serum Androgens in Turner’s Syndrome. Horm Res (1982) 16(3):164–73. doi: 10.1159/000179498
31. Bellosta S, Gomaraschi M, Canavesi M, Rossoni G, Monetti M, Franceschini G, et al. Inhibition of MMP-2 Activation and Release as a Novel Mechanism for HDL-Induced Cardioprotection. FEBS Lett (2006) 580(25):5974–8.
32. Miksztowicz V, Siseles N, Machulsky NF, Schreier L, Berg G. Increase in MMP-2 Activity in Overweight and Obese Women Is Associated With Menopausal Status. Climacteric (2012) 15(6):602–6. doi: 10.3109/13697137.2012.667174
33. Choudhary S, Higgins CL, Chen IY, Reardon M, Lawrie G, Vick GW, et al. Quantitation and Localization of Matrix Metalloproteinases and Their Inhibitors in Human Carotid Endarterectomy Tissues. Arterioscler Thromb Vasc Biol (2006) 26(10):2351–8. doi: 10.1161/01.ATV.0000239461.87113.0b
34. Belo VA, Luizon MR, Carneiro PC, Gomes VA, Lacchini R, Lanna CMM, et al. Effect of Metabolic Syndrome Risk Factors and MMP-2 Genetic Variations on Circulating MMP-2 Levels in Childhood Obesity. Mol Biol Rep (2013) 40(3):2697–704. doi: 10.1007/s11033-012-2356-7
35. Papazoglou D, Papatheodorou K, Papanas N, Papadopoulos T, Gioka T, Kabouromiti G, et al. Matrix Metalloproteinase-1 and Tissue Inhibitor of Metalloproteinases-1 Levels in Severely Obese Patients: What is the Effect of Weight Loss? Exp Clin Endocrinol Diabetes (2010) 118(10):730–4. doi: 10.1055/s-0030-1249671
36. Mirhafez SR, Pasdar A, Avan A, Esmaily H, Moezzi A, Mohebati M, et al. Cytokine and Growth Factor Profiling in Patients With the Metabolic Syndrome. Br J Nutr (2015) 113(12):1911–9. doi: 10.1017/S0007114515001038
37. Roy H, Bhardwaj S, Ylä-Herttuala S. Biology of Vascular Endothelial Growth factors. FEBS Lett (2006) 580(12):2879–87. doi: 10.1016/j.febslet.2006.03.087
38. Li L, Wang L, Zhang W, Tang B, Zhang J, Song H, et al. Correlation of Serum VEGF Levels With Clinical Stage, Therapy Efficacy, Tumor Metastasis and Patient Survival in Ovarian Cancer. Anticancer Res (2004) 24(3b):1973–9.
39. Berrahmoune H, Herbeth B, Lamont JV, Masson C, Fitzgerald PS, Visvikis-Siest S. Heritability for Plasma VEGF Concentration in the Stanislas Family Study. Ann Hum Genet (2007) 71(1):54–63. doi: 10.1111/j.1469-1809.2006.00298.x
40. Darendeliler F, Aycan Z, Cetinkaya E, Vidilisan S, Bas F, Bideci A, et al. Effects of Growth Hormone on Growth, Insulin Resistance and Related Hormones (Ghrelin, Leptin and Adiponectin) in Turner Syndrome. Horm Res Paediatr (2007) 68(1):1–7. doi: 10.1159/000098440
41. Radetti G, Pasquino B, Gottardi E, Contadin IB, Aimaretti G, Rigon F. Insulin Sensitivity in Turner’s Syndrome: Influence of GH Treatment. Eur J Endocrinol (2004) 151(3):351–4. doi: 10.1530/eje.0.1510351
Keywords: Turner syndrome, metabolic status, MMP-1/matrix metalloproteinase-1, MMP-2/matrix metalloproteinase-2, MMP-9/matrix metalloproteinase-9, BDNF/brain-derived neurotrophic factor, GDNF/glial cell line-derived neurotrophic factor, VEGF/vascular endothelial growth factor
Citation: Błaszczyk E, Gawlik J, Gieburowska J, Tokarska A, Kimsa-Furdzik M, Hibner G, Francuz T and Gawlik AM (2021) Brain-Derived Neurotropic Factor, Vascular Endothelial Growth Factor and Matrix Metalloproteinases as Markers of Metabolic Status in Non-Growth Hormone-Treated Girls With Turner Syndrome. Front. Endocrinol. 12:722199. doi: 10.3389/fendo.2021.722199
Received: 08 June 2021; Accepted: 09 August 2021;
Published: 27 August 2021.
Edited by:
Rodolfo A. Rey, Hospital de Niños Ricardo Gutiérrez (CONICET), ArgentinaReviewed by:
Gabriela Alicia Berg, University of Buenos Aires, ArgentinaNayely Garibay, General Hospital of Mexico, Mexico
Copyright © 2021 Błaszczyk, Gawlik, Gieburowska, Tokarska, Kimsa-Furdzik, Hibner, Francuz and Gawlik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aneta Monika Gawlik, agawlik@mp.pl
 Ewa Błaszczyk
Ewa Błaszczyk Jakub Gawlik
Jakub Gawlik Joanna Gieburowska
Joanna Gieburowska Agnieszka Tokarska1
Agnieszka Tokarska1 Grzegorz Hibner
Grzegorz Hibner Aneta Monika Gawlik
Aneta Monika Gawlik