- 1Department of Radiology, Medical Imaging Center, University Medical Center Groningen, University of Groningen, Groningen, Netherlands
- 2Department of Surgical Oncology, University Medical Center Groningen, University of Groningen, Groningen, Netherlands
- 3Department of Nuclear Medicine and Molecular Imaging, Medical Imaging Center, University Medical Center Groningen, University of Groningen, Groningen, Netherlands
- 4Department of Pathology, University Medical Center Groningen, University of Groningen, Groningen, Netherlands
- 5Department of Internal Medicine, Division of Endocrinology, University Medical Center Groningen, University of Groningen, Groningen, Netherlands
Background: The rising demand for 18F-fluorodeoxyglucose positron emission tomography with computed tomography (18F-FDG PET/CT) has led to an increase of thyroid incidentalomas. Current guidelines are restricted in giving options to tailor diagnostics and to suit the individual patient.
Objectives: We aimed at exploring the extent of potential overdiagnostics by performing a systematic review and meta-analysis of the literature on the prevalence, the risk of malignancy (ROM) and the risk of inconclusive FNAC (ROIF) of focal thyroid incidentalomas (FTI) on 18F-FDG PET/CT.
Data Sources: A literature search in MEDLINE, Embase and Web of Science was performed to identify relevant studies.
Study Selection: Studies providing information on the prevalence and/or ROM of FTI on 18F-FDG PET/CT in patients with no prior history of thyroid disease were selected by two authors independently. Sixty-one studies met the inclusion criteria.
Data Analysis: A random effects meta-analysis on prevalence, ROM and ROIF with 95% confidence intervals (CIs) was performed. Heterogeneity and publication bias were tested. Risk of bias was assessed using the quality assessment of diagnostic accuracy studies (QUADAS-2) tool.
Data Synthesis: Fifty studies were suitable for prevalence analysis. In total, 12,943 FTI were identified in 640,616 patients. The pooled prevalence was 2.22% (95% CI = 1.90% - 2.54%, I2 = 99%). 5151 FTI had cyto- or histopathology results available. The pooled ROM was 30.8% (95% CI = 28.1% - 33.4%, I2 = 57%). 1308 (83%) of malignant nodules were papillary thyroid carcinoma (PTC). The pooled ROIF was 20.8% (95% CI = 13.7% - 27.9%, I2 = 92%).
Limitations: The main limitations were the low to moderate methodological quality of the studies and the moderate to high heterogeneity of the results.
Conclusion: FTI are a common finding on 18F-FDG PET/CTs. Nodules are malignant in approximately one third of the cases, with the majority being PTC. Cytology results are non-diagnostic or indeterminate in one fifth of FNACs. These findings reveal the potential risk of overdiagnostics of FTI and emphasize that the workup of FTI should be performed within the context of the patient’s disease and that guidelines should adopt this patient tailored approach.
Introduction
18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) with computed tomography (CT) has become an important diagnostic tool in the assessment of malignancies and inflammatory diseases (1, 2). It is estimated that 2.2 million PET/CT scans were performed in the USA in 2019, with an estimated growth of 6% per year since 2013 (3). Due to this rise in imaging demand, incidentalomas are being discovered more often. Incidentalomas are incidentally found lesions unrelated to the clinical indication for 18F-FDG PET/CT (4). The incidence of 18F-FDG incidentalomas increases with age, which makes a further increase in incidence and financial impact likely due to population demographics change (5).
18F-FDG is a glucose analog that accumulates in metabolically active tissue like malignant tumors (6). Therefore, incidentalomas discovered on 18F-FDG PET/CT have a relatively high risk of malignancy (ROM) compared to incidentalomas detected by other imaging modalities (e.g. ultrasound). The overall prevalence of incidentalomas on whole body 18F-FDG PET/CT is 2.5% in patients with or without known or suspected cancer (4). Malignant lesions are most commonly found in the gastrointestinal tract, thyroid and lung (6).
Thyroid incidentalomas can be classified as either focal or diffuse. Diffuse 18F-FDG uptake in the thyroid is often caused by inflammatory disease, like (autoimmune) thyroiditis or Graves’ disease (7, 8). In contrast, focal 18F-FDG uptake is more likely caused by benign thyroid disease or malignancy, i.e. adenoma, thyroid carcinoma, metastasis of another origin or lymphoma. The most recent meta-analyses till 2014 showed focal thyroid incidentaloma (FTI) malignancy risks ranging from 34.6 to 37 percent (8–11).
Guidelines of the American Thyroid Association (ATA), American College of Radiology (ACR), European Thyroid Association (ETA) and British Thyroid Association (BTA) recommend ultrasound (US) guided fine needle aspiration cytology (FNAC) for patients with focal increased uptake in the thyroid gland as detected by 18F-FDG PET/CT (12–14). The guidelines are well-delineated and easy to adhere to, but seem to provoke a reflexive or habitual process that propel patients from incidental discovery of a thyroid nodule to FNAC and even surgery (15). Ultimately, this approach might contribute to a cascade effect of overdiagnostics and overtreatment, affecting the quality of life of these patients. Because the recommendations are strongly based on non-randomized retrospective studies, they are restricted in giving options and modifications to tailor diagnostics and to suit the individual patient with his or her specific characteristics and concerns.
Non-diagnostic or indeterminate results on cytopathology are assessed as undesirable yields of the diagnostic chain, resulting in repeat examinations and anxiety and uncertainty in patients. At the same time, doctors and patients seem to be indifferent or unaware of the impact of this potential hazard. Therefore, different from previous systematic reviews and meta-analyses, we looked beyond the prevalence and the ROM of FTI and also analyzed the risk of inconclusive FNAC (ROIF).
We aimed at exploring the extent of potential overdiagnostics by performing a systematic review and meta-analysis of the literature on the prevalence, ROM and ROIF of focal thyroid incidentalomas (FTI) on 18F-FDG PET/CT, thereby revealing opportunities to improve FTI management.
Methods
Literature Search
A systematic literature search was conducted using MEDLINE, Embase and Web of Science to identify relevant articles. Database keywords and text words were searched using thyroid neoplasms, PET and incidental findings including the subcategories and variants of these words as search terms. Similar terms were used for Embase and Web of Science (Supplemental Table 1). The search was restricted to articles published between January 2010 and June 2020, to provide an update to existing meta-analyses. Articles without an English abstract and conference abstracts were excluded. If insufficient data were reported, the authors were contacted to provide additional information. To expand our search, references of retrieved systematic reviews and meta-analyses were screened for additional studies.
The complete search yielded 1156 articles and is displayed in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) in Figure 1 (16).
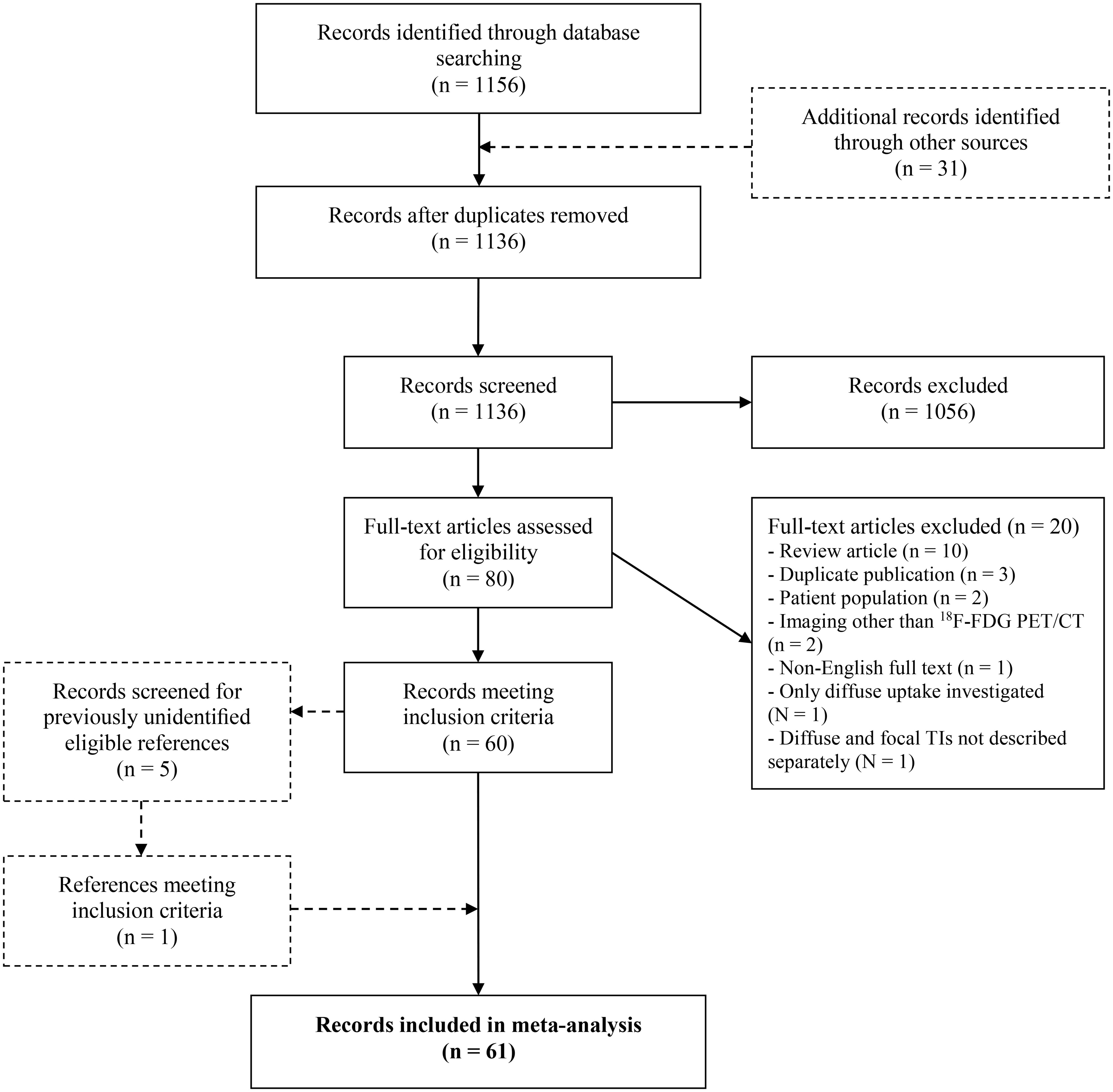
Figure 1 The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart. 18F-FDG PET/CT, 18F-Fluorodeoxyglucose Positron Emission Tomography with Computed Tomography; FTI, Focal Thyroid Incidentaloma.
Study Selection
Retrospective and prospective cohort studies providing information on the prevalence of FTI on 18F-FDG PET/CT and/or ROM of 18F-FDG-avid FTI in patients with no prior history of thyroid disease were considered for inclusion. After duplicates were eliminated, studies were screened for eligibility based on title, abstract, and subsequently on full text by two authors (J.F.d.L., H.E.W.) independently. Disagreements on article inclusion were resolved by consensus reading by the same reviewers. Studies were excluded if: (a) only thyroid incidentalomas with diffuse uptake patterns were investigated or the results of focal and diffuse uptake patterns were not described separately. If both focal and diffuse thyroid incidentalomas were included in a study and described separately, the FTI were considered for further analysis. (b) they concerned a retrospective analysis of surgically treated FTI. (c) they concerned duplicate publications. If so, the study with the largest patient population was included. (d) the full article was written in a non-English language. Finally, 61 studies were included for analysis.
Data Extraction
Data were extracted by three authors (J.F.d.L., M.J.H.M., H.E.W.) using a data extraction table. All full articles were analyzed by J.F.d.L. Duplicate data extraction was performed by either M.J.H.M. or H.E.W. Any discrepancies were resolved with consensus reading by a third reviewer (M.J.H.M. or H.E.W). The following data were collected for meta-analysis: the total number of 18F-FDG PET/CTs, the total number of FTI, the number of malignant and benign FTI and the number of FTI with a non-diagnostic or indeterminate cytopathology result after initial FNAC. Specific information regarding the pathological classification or description (based on either cytopathology and/or histopathology) of malignant FTI was collected as well. Hürthle cell and follicular carcinomas were considered as one group. Some studies had patients with multiple FTI. These FTI were considered as separate cases.
For analysis of ROM, FTI were classified according to cytopathology and histopathology. When both results were available, the histopathology result was used. The definition of a malignant cytopathology result was a description of “malignant” or “suspicious for malignancy” or, according to the Bethesda (B) classification, BV and BVI (17). Some studies used the British THY system as FNAC classification system. THY4 and BV were considered equally as well as THY5 and BVI, as described by the ETA (18). FTI with non-diagnostic/unsatisfactory (BI/THY1), atypia/follicular lesion of undetermined significance (AUS/FLUS) (BIII/THY3a) or (suspicious for) follicular neoplasm (BIV/THY3b) cytopathology were not defined as malignant or benign, unless histopathology or repeat cytopathology was done and decided otherwise (i.e. BII/BV/BVI and THY2/THY4/THY5). FTI were considered benign when they had benign cytopathology (BII/THY2) or histopathology. FTI classified according to ultrasound, scintigraphy or clinical follow-up were not considered for further analysis.
For analysis of ROIF, the number of FTI with initial BIII/indeterminate and BI/non-diagnostic cytopathology results were registered separately, independent from repeat FNAC or histopathology results used for ROM analysis.
Quality Assessment
The quality of included studies was assessed using the quality assessment of diagnostic accuracy studies, QUADAS-2 (19). All studies were independently assessed by two reviewers (J.F.d.L., H.E.W.) and disagreements were resolved with consensus reading. QUADAS-2 divides the risk of bias of study methodology into four domains: patient selection, index test, reference test and flow and timing. Studies were considered to have a high risk of bias in the patient selection domain when a non-consecutive or non-random sample was used or inappropriate exclusion criteria were used like “patients with inconclusive cytology”. Studies were considered to have a high risk of bias in the index test domain when the 18F-FDG PET/CTs were interpreted or adjusted with knowledge of the FNAC results. Studies were only classified as high risk in the reference test domain when studies did not use the Bethesda classification to report FNAC results. The patient flow domain was classified as high risk when less than 50% of included patients received FNAC and/or surgery or FNAC was only performed after suspicious US. Domains were considered to be of unclear risk when insufficient information was given to assess methodological quality properly.
QUADAS-2 was used to assess applicability as well. In all studies the patient selection, index test and reference standard met the inclusion criteria and the question of the review.
Statistical Analysis
Prevalence, ROM and ROIF of FTI were calculated using the data extracted from included studies. Regarding ROM, a proportion was calculated using the number of FTI investigated with FNAC and/or surgery as denominator and the number of FTI with malignant cytopathology or histopathology as nominator. ROIF was calculated using the number of initial FNAC as denominator and the number of non-diagnostic and indeterminate FNAC as nominator.
Data were pooled using a random effects model generated by the Cochrane Collaboration software, Review Manager (RevMan) 5.4 software. Heterogeneity was tested using the Chi-square test (p < 0.01) and Higgins and Thompson test to calculate the I2 statistic (20). As this demonstrated a heterogeneous study set, a random effects model was utilized to calculate pooled estimates. Publication bias was assessed using a funnel plot and weighed Egger’s regression test (21).
A forest plot was generated displaying the individual study prevalence, malignancy risk and percentage of indeterminate and non-diagnostic FNAC results with 95% Confidence Intervals (CIs) and the pooled estimates using the forestplot package in the R environment.
Subgroup analyses were done to identify sources of bias and heterogeneity in the data. Methodological quality and study characteristics (age, indication for PET, geography) were used to divide studies into subgroups. With regard to the latter, some parts of the world, i.e. South Korea, have a higher and faster increasing incidence of thyroid cancer, than other parts. Although this increase has mostly been attributed to overscreening and higher rates of diagnosis, a ‘true’ difference in incidence due to geographic variation in individual factors like obesity or genotype and environmental factors like iodine supplementation or radiation exposure also plays a role (22).
Results
Study Characteristics
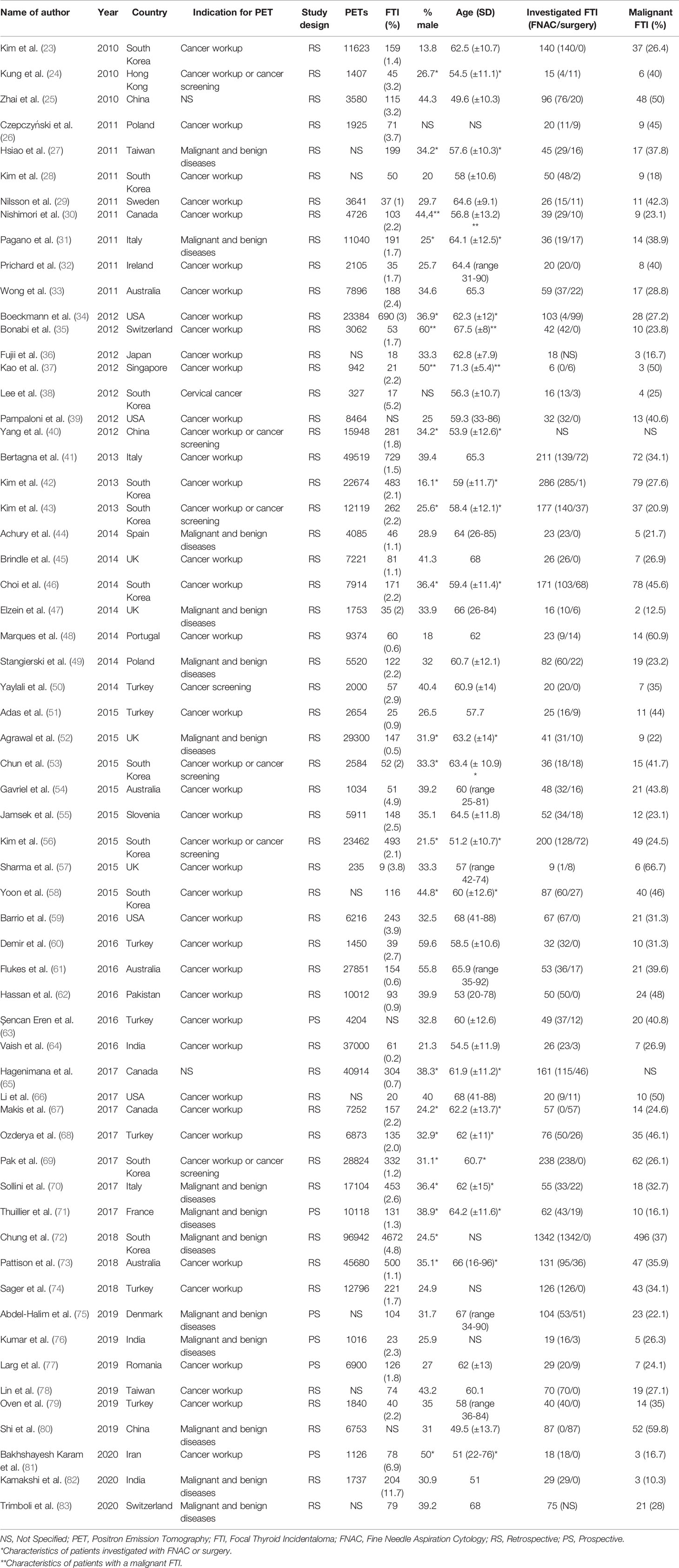
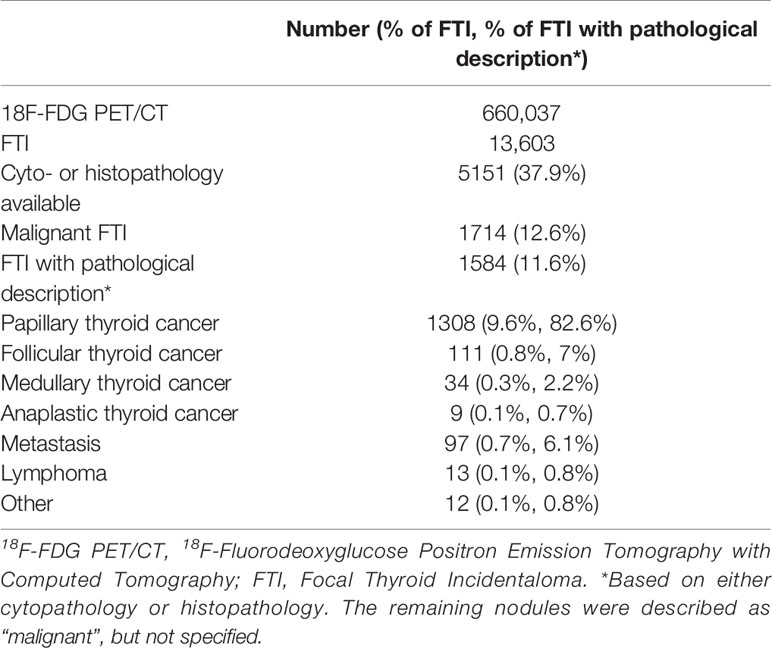
Sixty-one studies were included in final analysis. The study characteristics are shown in Table 1. Most studies had a retrospective (n=55) study design. Thirty-eight of the studies included only patients who underwent 18F-FDG PET/CT for non-thyroidal oncological indications. The other studies also used 18F-FDG PET/CTs conducted for benign diseases and cancer screening in healthy subjects or did not specify the nature of the indication of the requested 18F-FDG PET/CT scans that were included. In total, 660,037 18F-FDG PET/CTs were carried out and 13,603 FTI were identified. A brief overview of our data is shown in Table 2. Not all studies were suitable for both prevalence and malignancy risk analysis. Therefore, the data presented in the meta-analyses do not match the total number of 18F-FDG PET/CTs or the total number FTI.
Quality Assessment
The methodological quality of the included studies is summarized in Supplemental Table 2.
In the patient selection domain, 17 studies were considered as high risk due to inappropriate exclusion criteria (27, 39, 42, 46, 54, 56, 58, 59, 62, 63, 67, 69, 70, 72, 76, 80, 83). We considered another 6 studies to be of high risk because a non-consecutive sample of patients was enrolled (24, 28, 29, 31, 66, 79). One study included only patients with a history of cervical cancer and was therefore classified as high risk (38).
In the index test domain, 16 studies were classified as being of unclear risk of bias as it was not described how 18F-FDG PET/CTs were assessed (24, 30, 32, 35, 39, 45, 47, 51, 52, 61, 65, 66, 73–75, 79).
Thirty-six studies did not use the Bethesda classification to report FNAC results and were therefore classified as high risk in the reference test domain (23–29, 31–37, 39–41, 44, 45, 47, 48, 50–54, 57, 59, 60, 67, 70, 74, 79–81, 83).
In the patient flow domain, 27 studies were classified as high risk because not all included patients received FNAC and/or surgery (24, 26, 27, 30, 31, 33, 34, 37, 39, 41, 45, 47, 48, 50, 52, 55, 56, 59, 61, 63, 64, 67, 70, 71, 73, 81, 82). Another 8 studies were considered to have a high risk of bias because FNACs were performed only after suspicious US (25, 40, 44, 51, 62, 66, 72, 77).
Publication Bias
Publication bias was assessed using the malignancy risks reported in the included studies (Figure 2). An Egger’s regression showed no significant (t = 0.65, p = 0.52) funnel plot asymmetry.
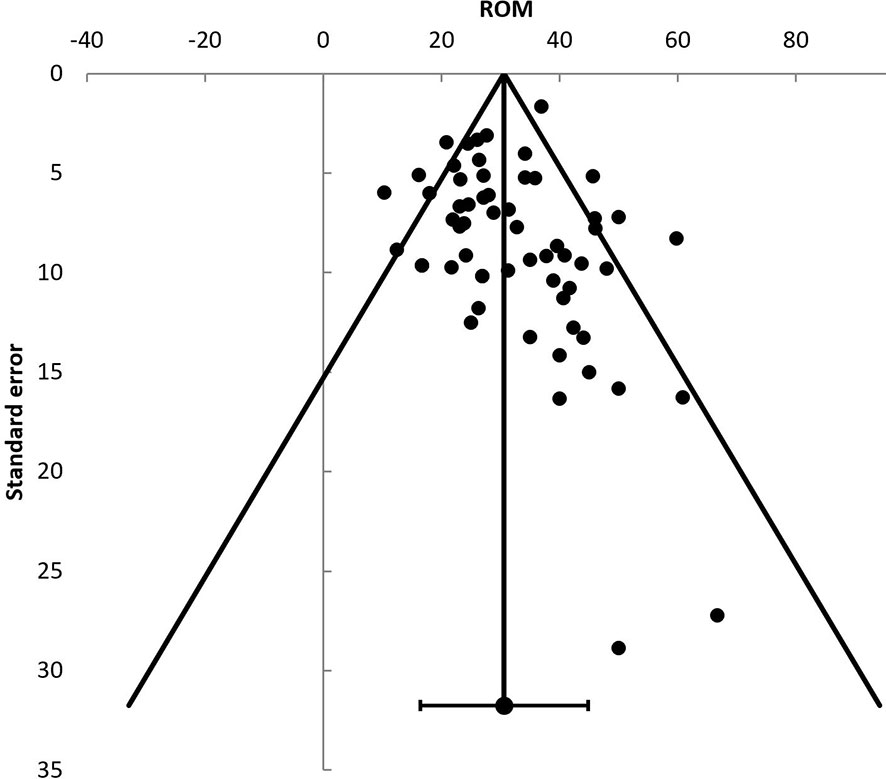
Figure 2 Funnel plot displaying individual studies risk of malignancy (ROM). ROM, risk of malignancy.
Prevalence
Fifty studies provided information regarding the prevalence of 18F-FDG-avid FTI. Of the 11 excluded studies, in 3 the prevalence data were not provided separately for focal and diffuse thyroid incidentalomas (39, 63, 80) and 8 did not report on the total number of 18F-FDG PET/CTs (27, 28, 36, 58, 66, 75, 78, 83).
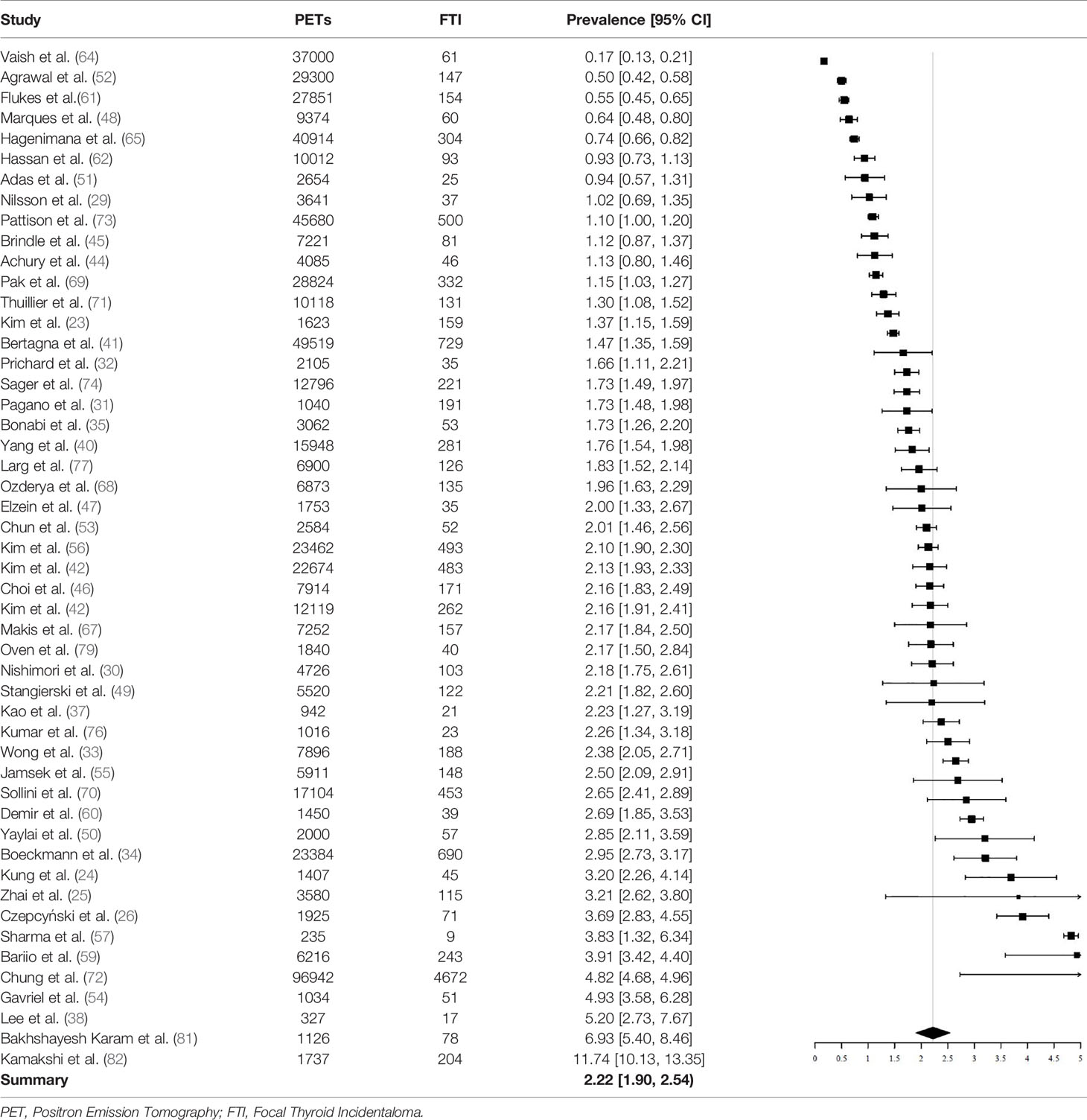
A total of 640,616 patients undergoing 18F-FDG PET/CT were described. In this population, 12,943 FTI were identified. FTI prevalence ranged between studies, from 0.16% to 11.74%. The pooled prevalence of 18F-FDG-avid FTI was 2.22% (95% CI = 1.90% - 2.54%, I2 = 99%) (Table 3).
Subgroup analysis on age showed that prevalence of FTI was significantly lower in studies with a mean age > 60 (N = 27) than in studies with a mean age < 60 (N = 16). The pooled proportion in the > 60 subgroup was 1.76% (95% CI = 1.50% - 2.01%, I2 = 98%) and in the < 60 subgroup 2.91% (95% CI = 2.24% - 3.58%, I2 = 98%).
Subgroup analysis based on the 18F-FDG PET/CT indication showed no difference between studies including exclusively patients undergoing 18F-FDG PET/CT for non-thyroidal oncological indications (N = 31, prevalence 2.07%, 95% CI = 1.73% - 2.40%), compared to studies also including 18F-FDG PET/CTs conducted for benign diseases and cancer screening (N = 19, prevalence, 2.44%, 95% CI = 1.80% - 3.07).
Subgroup analysis based on geographic location showed that studies carried out in South Korea (N = 9, prevalence 2.45%, 95% CI = 1.10% - 3.79%) did not have a significantly different pooled prevalence than studies carried out in other countries (N = 41, prevalence 2.09%, 95% CI = 1.83% - 2.35%).
Finally, subgroup analysis using studies that were classified as “low risk” in the patient selection domain (studies with a consecutive design and appropriate exclusion criteria) (N = 34) versus studies that were classified as “high risk” (N = 16) did not result in significantly different pooled prevalences. The prevalence in the “low risk” subgroup was 1.98% (1.70% - 2.25%) and the prevalence was 2.57% (1.80% - 3.33%) in the “high risk” subgroup.
Malignancy Risk
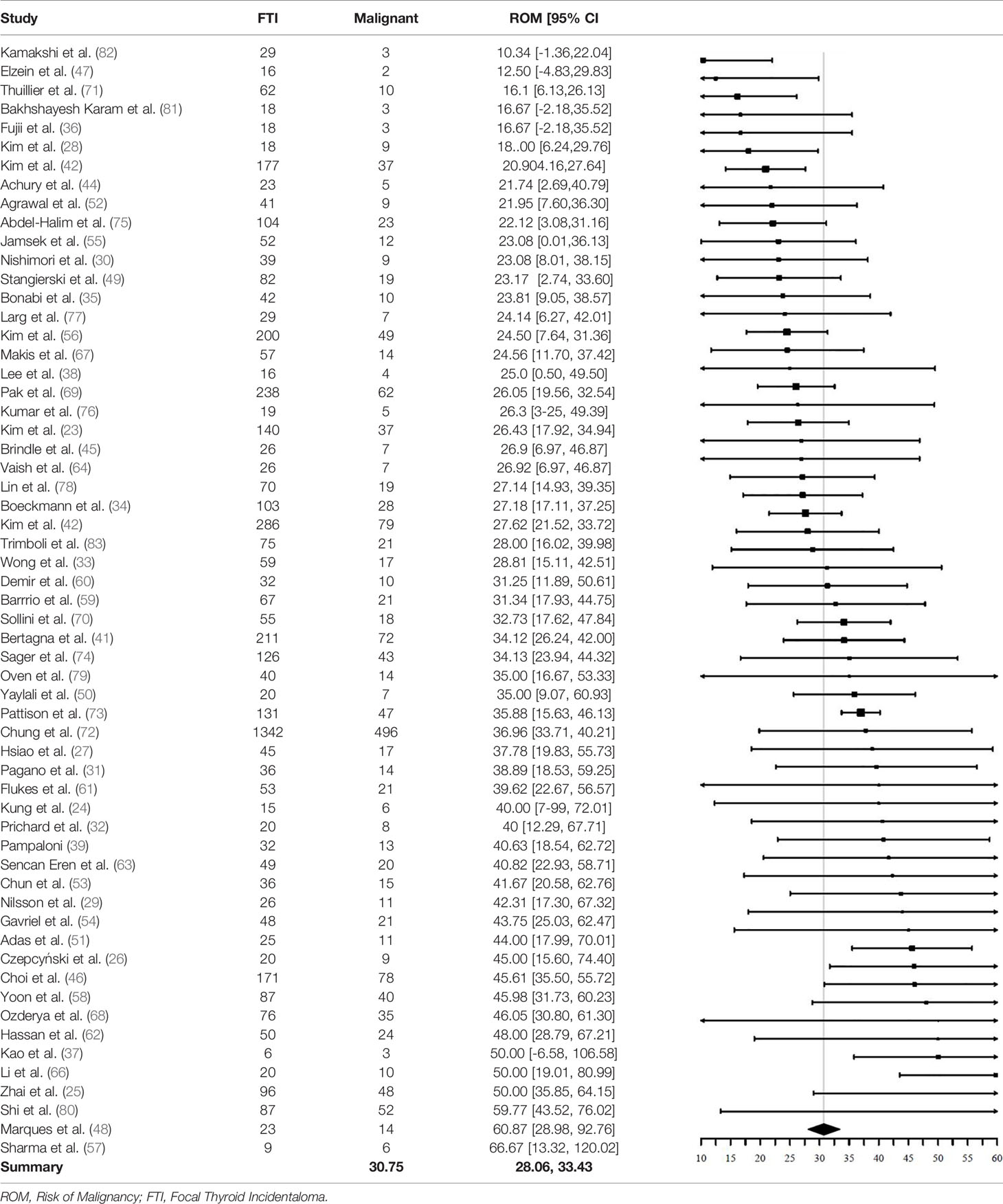
A total of 5151 FTI in 59 studies had cyto- or histopathology results available. One of two excluded studies did not provide sufficient information to calculate the ROM (65), the other did not adequately specify their eligibility criteria for further characterization with FNAC and only performed FNAC in a small part of their included patiënts (40).
Of the 5151 included FTI, 1714 FTI were malignant. The pooled ROM was 30.8% (95% CI = 28.1% - 33.4%, I2 = 57%) (Table 4). Of the 1714 malignant nodules, 1584 had a final pathological description available (based on either cytopathology or histopathology). The remaining 130 nodules were described as “malignant”, but not specified. Of these 1584 FTI with a pathological description available, 1462 (92%) were of thyroidal origin and 1308 (83%) were papillary thyroid cancer (PTC).
A significant difference in pooled ROM between age subgroups was not found. Studies with a mean age > 60 years (N = 33) showed a ROM of 30.5% (95% CI = 27.6% - 33.4%), similar to the ROM of 31.8% (95% CI = 25.8% - 37.7%) in studies with a mean age < 60 years (N = 19). The ROM was not significantly different between studies including only patients undergoing 18F-FDG PET/CT for non-thyroidal oncological indications (N = 38) and studies including also 18F-FDG PET/CTs with non-oncological indications and for cancer screening (N = 21). The ROM was 32.2% (95% CI = 29.2% - 35.1%, I2 = 31%) in the oncology subgroup and 28.5% (95% CI = 23.6% - 33.4%, I2 = 75%) in the subgroup with other indications.
Finally, a subgroup analysis based on QUADAS-2 was performed. Patient selection, reference test and flow and timing were tested independently with “low risk” and “high risk” as subgroups. No significant difference in pooled ROM could be demonstrated.
Inconclusive Cytopathology
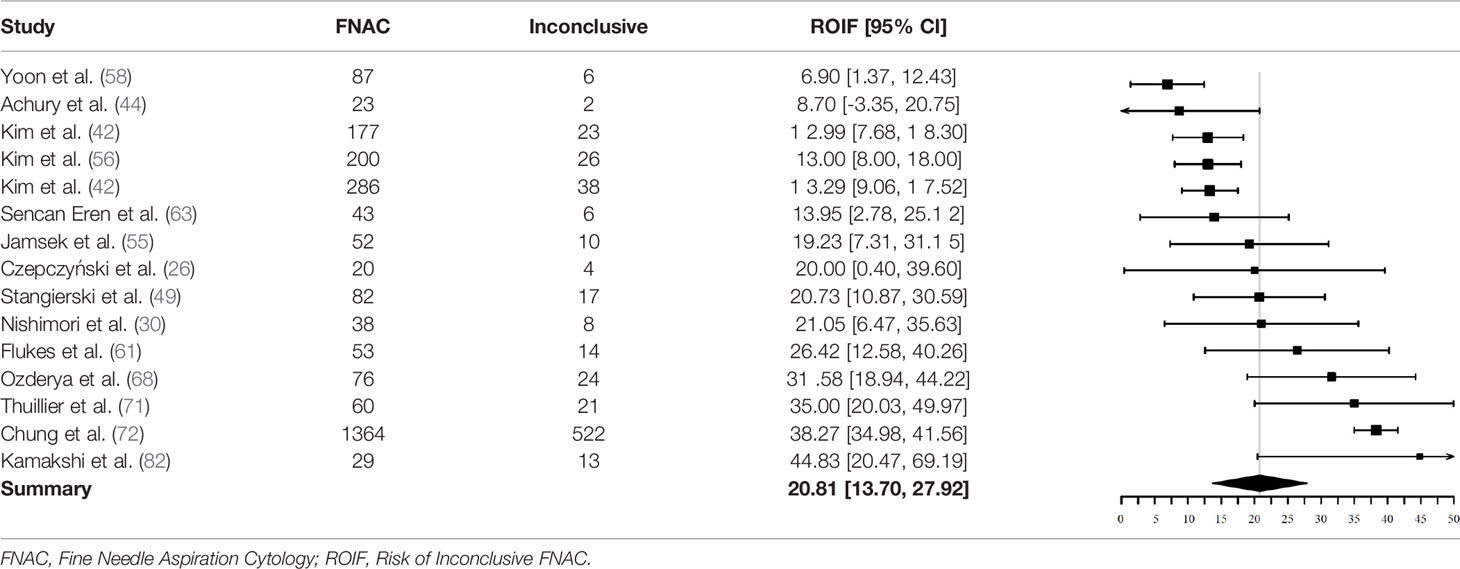
Fifteen studies could be used to investigate the pooled ROIF after initial FNAC. Reasons for exclusion were: (1) missing data of non-diagnostic and indeterminate FNAC results (N = 28) (23–25, 27–29, 32, 33, 35–37, 39, 40, 51, 53, 54, 59, 60, 62, 64, 65, 67, 69, 74, 77, 80, 81, 83). (2) inconsequent description of data of non-diagnostic or indeterminate FNAC results (N = 5) (41, 45, 52, 66, 73). (3) missing separate data of BIII and BIV or THY3a and THY3b categories (N = 6) (31, 47, 57, 70, 75, 76). (4) description of either non-diagnostic or indeterminate FNAC results and not both subgroups (N = 7) (34, 38, 46, 48, 50, 78, 79).
Two of the 15 included studies did not use the Bethesda classification to report FNAC results (26, 44). They reported inconclusive results as either “non-diagnostic” or “indeterminate”.
In total, 2590 nodules were included for analysis of ROIF. Of these, 734 had a non-diagnostic or indeterminate FNAC result (219 non-diagnostic, 515 indeterminate). The pooled proportion of inconclusive FNAC results was 20.8% (95% CI = 13.7% - 27.9%, I2 = 92%) (Table 5).
Discussion
The present systematic review and meta-analysis shows a pooled prevalence of 18F-FDG-avid focal thyroid incidentalomas (FTI) of 2.2%. Malignancy is found in about one third of the FTI, the vast majority being papillary thyroid cancer (PTC). Non-diagnostic or indeterminate FNAC results are seen in approximately 21% of FNACs, meaning diagnostic uncertainty and new decision making.
This study can be considered as an update with inclusion of studies published in the last 10 years, using newer generations of PET/CT scanners. A major distinction from previous reviews is that we analyzed the risk of inconclusive FNAC (ROIF) with the purpose of estimating the encountered difficulties of the diagnostic chain. Both the risk of malignancy (ROM) and ROIF are key findings of our analyses and illustrate the necessity of tailoring the diagnostics of FTI to suit the preferences and context of the individual patient. The found ROIF (21%) is comparable to the ROIF found in a general population with thyroid nodules (23%) (84). Our findings concerning prevalence and ROM of FTI on 18F-FDG-PET/CT are similar to those in previous meta-analyses, which found FTI prevalences varying between 1.6% and 2.5% and ROM of 35-37% (8–11).
The substantial ROM along with the common finding and still rising number of FTI on 18F-FDG PET/CT seem to justify further diagnostics. However, the general approach to continue to ultrasound guided FNAC might contribute to overdiagnostics and overtreatment of benign nodules and (small) PTC. Similarly, the accompanying undiagnostic or indeterminate findings on cytopathology might require repeat FNACs or surgeries in one-fifth of patients. Additional undesirable consequences of this straightforward approach might be anxiety and interferences with definitive treatment planning, in particular in patients with other malignancies making up the main indication for 18F-FDG-PET/CT. Moreover, the impact of diagnosing a thyroid malignancy on overall survival in patients with other malignancies is questionable, not to mention the significant health care costs of incidentally detected findings (5, 73, 85, 86). Finally, the general recommendation ignores the importance of engaging patients in making decisions.
Given these points, the options of “inaction” or alternative action and active investigation according to current guidelines need to be explored evenly and the preferred option should be consistent with the patients’ wishes and preferences. The clinical context needs to be weighed carefully on the possible scenarios after FNAC and the clinical impact of an incidental thyroid cancer or metastasis with regard to treatment options, risk of complications and adverse effects and prognosis. Patients who are more engaged in their health care decision making are more likely to experience confidence in treatment decisions, satisfaction with treatment, and trust in their providers (87). Our study showed a strong preselection of patients eligible for FNAC and surgery, indicating that further investigations were performed only if the results had impact on treatment algorithms. Similarly, two other studies demonstrated that 18F-FDG PET/CT incidental findings could be managed appropriately in the clinical context and based on physician and patient decisions (88, 89).
When aiming at allocating FTI for FNAC, ultrasound classification systems might be valuable. They have been developed in order to improve the uniformity of the interpretation of the sonographic patterns and the stratification of thyroid nodules for FNAC. These ultrasound-based tools have been validated in the general population of patients with nodular goiter and an estimated cancer prevalence of 2-3% (90). As shown in the present study, thyroid cancer prevalence among patients with FTI at 18F-FDG PET/CT is significantly higher. Since the pretest risk of malignancy is hence higher for the latter the aim of the classification system will change likewise from saving unnecessary FNAC to detecting malignancy accurately. Four included studies aimed to assess the reliability of ultrasound classification systems in indicating FNAC and predicting malignancy in FTI on 18F-FDG PET/CT (58, 71, 72, 83). Three of them demonstrated, that the malignancy risk of FTI detected on 18F-FDG PET/CT in the low suspicion categories did not show an increase in malignancy when compared with the estimated malignancy risks of these categories suggested by the guidelines (58, 71, 72). The FTI belonging to these categories accounted for 30-37% of the total. Conversely, in two of the studies FTI detected on 18F-FDG PET/CT with intermediate to high suspicion showed an increase in malignancy in comparison with the estimated malignancy risks suggested by the guidelines (58, 72). Furthermore, Trimboli et al. compared three ultrasound classifications in indicating FNAC in FTI and showed that all had a good performance, possibly reducing unnecessary FNACs in 25-53% of the total (83). Though subject to limitations with regard to study design these preliminary results show that the implementation of ultrasound classification systems might contribute to less unnecessary FNACs in the low suspicious nodules, whereas the indications for FNACs of the intermediate or high suspicious nodules might be more evidenced. Guidelines are concordant in recommending against routine FNAC of nodules smaller than 1 cm, even if they are highly suspicious on ultrasound (12–14, 91).
Regarding the ROIF ultrasound classification systems might stratify thyroid nodules with BI, BIII and BIV. Guidelines recommend a repeat FNAC after a non-diagnostic initial FNAC. However, repeat US might be considered as well when initial European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults (EU-TIRADS) is 2 or 3 (92). In case of BIII and BIV clinical management is not that straightforward. Several studies evaluated the usefulness of the ultrasound classification systems in predicting malignancy of thyroid nodules with indeterminate cytology according to the Bethesda classification (93–98). The varying results between the studies are affected by differences in sonographic patterns, cytologic diagnose and ROM. Even so, the US classifications confirm more or less a gradation in the pretest risk of malignancy. Therefore, it might be possible to guide management after an indeterminate cytological diagnosis based on US patterns. In other words, an intermediate or high suspicious ultrasound in a nodule with indeterminate cytology should trigger repeat FNAC or surgery, whereas a nodule with benign appearance may need clinical follow-up. Guidelines have not recommended this sonographic pattern stratification of nodules with indeterminate cytology and decision making should be made from a multidisciplinary perspective (14).
A new technique to manage indeterminate nodules could be the use of molecular markers. For example, BRAF mutation analysis could guide towards accurate surgical therapy. These molecular tests require standardization of performance characteristics and appropriate calibration as well as analytic validation before clinical interpretation (18, 99). Therefore, the routine BRAF testing does not (yet) have a place in the clinical routine and is therefore not recommended (100).
Some considerations in the interpretation of the results of the present systematic review and meta-analysis should be mentioned. First, a threat to the validity of any meta-analysis is publication bias. Our analyses were not suggestive of publication bias.
Second, the prevalence of the included studies showed substantial heterogeneity. Only age was a significant discriminator with studies with a mean age younger than 60 years having a higher prevalence of FTI. This finding might seem surprising given the fact that the prevalence of thyroid nodules increases with age (101). However, at the same time the prevalence of malignant, and therefore FDG-avid, thyroid nodules decreases with age (102). The subgroups were not controlled for contributing factors, such as sex distribution, histopathology or cytopathology findings, clinical signs of thyroid malignancy or risk factors for developing thyroid cancer, hampering straightforward conclusions (103). Another contributing factor might have been the applied definition of focal increased uptake in the thyroid gland on 18F-FDG PET/CT. Most studies used visual and semiquantitative assessments, which might be prone to non-replicability and variability of results. Patient selection, 18F-FDG PET/CT indication and geographic influences were of minor significance at subgroup analysis.
Third, a major limitation in calculating the ROM was the high degree of preselection of FTI for cyto- or histopathology and the different reference standards for defining malignancy. Although FNAC is valuable by facilitating the diagnostic correlation with histopathology, cytopathology is not considered the gold standard (104–107). Nevertheless, in the present meta-analysis both cyto- and histopathology results were used equally for estimating the ROM. ROM was not calculated using only histopathology results, because most patients undergoing diagnostic surgery were preselected by FNAC. Follicular carcinomas, which are per definition not higher than Bethesda IV, were still included in analysis as Bethesda IV often led to diagnostic surgery.
Fourth, the ROM of the selected studies showed moderate heterogeneity. This might be caused by the retrospective design of most studies with higher risks of bias and non-replicability of methods and results. The visual assessment method for defining FTI on 18F-FDG PET/CT might also have contributed as the degree of focal uptake of FTI might be of predictive though not of conclusive value for malignancy (10, 34, 37, 41, 45, 49).
Finally, only one fourth of included studies were suitable for analysis of the ROIF. Pooling of data resulted in substantial heterogeneity. No sources of heterogeneity could be shown at subgroup analysis. Variability in ROIF might be accounted to the hospital setting (i.e. settings of local multidisciplinary guidelines and consultations and organization of patient flow pathways), the degree of experience of the radiologist performing the FNAC, the availability of a cytopathology technician for on-site assessment of the specimen adequacy and a pathologist for consulting a second-reading of the FNAC. The latter might be of decisive importance as intra- and interobserver variation exists in the distinction between BIII and BIV (108).
The present systematic review and meta-analysis shows that FTI are a common finding on 18F-FDG PET/CT. Nodules are malignant in approximately one third of cases with the majority being PTC. At the same time, cytology results are non-diagnostic or indeterminate in one fifth of FNACs. Before proceeding to active examination of the FTI, the clinical context and the preferences of the patient should be reviewed and balanced with the possible scenarios after FNAC and the clinical impact of diagnosing PTC.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
Conception or design of the work, JL and HW. Data collection, JL, MM, and HW. Data analysis and interpretation, JL, MM, AH, AB, SK, BH, TL, and HW. Drafting the article, JL, MM, and HW. Critical revision of the article, JL, MM, AH, AB, SK, TL, and HW. Final approval of the version to be published, JL, MM, AH, AB, SK, BH, TL, and HW. All authors contributed to the article and approved the submitted version.
Funding
The open access publication fee is funded by the Medical Imaging Center (MIC), which is a UMCG research facility. No other sources of funding were used.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.723394/full#supplementary-material
References
1. Farwell MD, Pryma DA, Mankoff DA. PET/CT Imaging in Cancer: Current Applications and Future Directions. Cancer (2014) 120(22):3433–45. doi: 10.1002/cncr.28860
2. Zhuang H, Codreanu I. Growing Applications of FDG PET-CT Imaging in Non-Oncologic Conditions. J BioMed Res (2015) 29(3):189–202. doi: 10.7555/JBR.29.20140081
3. IMV Medical Information Division. 2020 Pet Imaging Market Summary Report. Des Plaines, IL: IMV Medical Information Division (2020).
4. O’sullivan JW, Muntinga T, Grigg S, Ioannidis JPA. Prevalence and Outcomes of Incidental Imaging Findings: Umbrella Review. BMJ (2018) 361:k2387. doi: 10.1136/bmj.k2387
5. Adams SJ, Rakheja R, Bryce R, Babyn PS. Incidence and Economic Impact of Incidental Findings on 18F-FDG PET/CT Imaging. Can Assoc Radiologists J (2018) 69(1):63–70. doi: 10.1016/j.carj.2017.08.001
6. Liu Y, Ghesani NV, Zuckier LS. Physiology and Pathophysiology of Incidental Findings Detected on FDG-PET Scintigraphy. Semin Nucl Med (2010) 40(4):294–315. doi: 10.1053/j.semnuclmed.2010.02.002
7. Lee JY, Choi JY, Choi Y, Hyun SH, Moon SH, Jang SJ, et al. Diffuse Thyroid Uptake Incidentally Found on 18F-Fluorodeoxyglucose Positron Emission Tomography in Subjects Without Cancer History. Korean J Radiol (2013) 14(3):501–9. doi: 10.3348/kjr.2013.14.3.501
8. Soelberg KK, Bonnema SJ, Brix TH, Hegedüs L. Risk of Malignancy in Thyroid Incidentalomas Detected by 18F-Fluorodeoxyglucose Positron Emission Tomography: A Systematic Review. Thyroid (New York N.Y.) (2012) 22(9):918–25. doi: 10.1089/thy.2012.0005
9. Nayan S, Ramakrishna J, Gupta MK. The Proportion of Malignancy in Incidental Thyroid Lesions on 18-FDG PET Study. Otolaryngology–Head Neck Surg (2014) 151(2):190–200. doi: 10.1177/0194599814530861
10. Bertagna F, Treglia G, Piccardo A, Giubbini R. Diagnostic and Clinical Significance of F-18-FDG-PET/CT Thyroid Incidentalomas. J Clin Endocrinol Metab (2012) 97(11):3866–75. doi: 10.1210/jc.2012-2390
11. Treglia G, Bertagna F, Sadeghi R, Verburg FA, Ceriani L, Giovanella L. Focal Thyroid Incidental Uptake Detected by ¹⁸F-Fluorodeoxyglucose Positron Emission Tomography. Meta-Analysis on Prevalence and Malignancy Risk. Nuklearmedizin (2013) 52(4):130–6. doi: 10.3413/Nukmed-0568-13-03
12. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (New York N.Y.) (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
13. Pencharz D, Nathan M, Wagner TL. Evidence-Based Management of Incidental Focal Uptake of Fluorodeoxyglucose on PET-Ct. Br J Radiol (2018) 91(1084):20170774. doi: 10.1259/bjr.20170774
14. Hoang JK, Langer JE MD, Middleton WD MD, Wu CC MD, Hammers LW DO, Cronan JJ MD, et al. Managing Incidental Thyroid Nodules Detected on Imaging: White Paper of the ACR Incidental Thyroid Findings Committee. J Am Coll Radiol (2015) 12(2):143–50. doi: 10.1016/j.jacr.2014.09.038
15. Jensen CB, Saucke MC, Francis DO, Voils CI, Pitt SC. From Overdiagnosis to Overtreatment of Low-Risk Thyroid Cancer: A Thematic Analysis of Attitudes and Beliefs of Endocrinologists, Surgeons, and Patients. Thyroid (2020) 30(5):696–703. doi: 10.1089/thy.2019.0587
16. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PloS Med (2009) 6(7):e1000100. doi: 10.1371/journal.pmed.1000100
17. Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid (2017) 27(11):1341–6. doi: 10.1089/thy.2017.0500
18. Paschke R, Cantara S, Crescenzi A, Jarzab B, Musholt TJ, Sobrinho Simoes M. European Thyroid Association Guidelines Regarding Thyroid Nodule Molecular Fine-Needle Aspiration Cytology Diagnostics. Eur Thyroid J (2017) 6(3):115–29. doi: 10.1159/000468519
19. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann Intern Med (2011) 155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
20. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring Inconsistency in Meta-Analyses. BMJ (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
21. Egger M, Davey Smith G, Schneider M, Minder C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
22. Kim J, Gosnell JE, Roman SA. Geographic Influences in the Global Rise of Thyroid Cancer. Nat Rev Endocrinol (2020) 16(1):17–29. doi: 10.1038/s41574-019-0263-x
23. Kim B, Na M, Kim I, Kim S, Kim Y. Risk Stratification and Prediction of Cancer of Focal Thyroid Fluorodeoxyglucose Uptake During Cancer Evaluation. Ann Nucl Med (2010) 24(10):721–8. doi: 10.1007/s12149-010-0414-6
24. Kung BT, Wong CP, Chu KS, AuYong TK, Tong CM. Cancer Risk of Focal Thyroid Incidentaloma in Patients Undergoing 18f-Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography Studies: Local Experience in a Single Centre. J Hong Kong Coll Radiol (2010) 13:120–4.
25. Zhai G, Zhang M, Xu H, Zhu C, Li B. The Role of 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Whole Body Imaging in the Evaluation of Focal Thyroid Incidentaloma. J Endocrinol Invest (2010) 33(3):151–5. doi: 10.1007/BF03346574
26. Czepczyński R, Stangierski A, Oleksa R, Janicka-Jedyńska M, Czarnywojtek A, Ruchała M, et al. Incidental 18f-FDG Uptake in the Thyroid in Patients Diagnosed With PET/CT for Other Malignancies. Nucl Med Rev (2011) 14(2):68–72. doi: 10.5603/NMR.2011.00018
27. Hsiao YC, Wu PS, Chiu NT, Yao WJ, Lee BF, Peng SL. The Use of Dual-Phase 18f-FDG PET in Characterizing Thyroid Incidentalomas. Clin Radiol (2011) 66(12):1197–202. doi: 10.1016/j.crad.2011.08.005
28. Kim S, Kim B, Jeon Y, Kim S, Kim I. Limited Diagnostic and Predictive Values of Dual-Time-Point 18f FDG PET/CT for Differentiation of Incidentally Detected Thyroid Nodules. Ann Nucl Med (2011) 25(5):347–53. doi: 10.1007/s12149-011-0468-0
29. Nilsson I, Arnberg F, Zedenius J, Sundin A. Thyroid Incidentaloma Detected by Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography: Practical Management Algorithm. World J Surg (2011) 35(12):2691–7. doi: 10.1007/s00268-011-1291-4
30. Nishimori H, Tabah R, Hickeson M, How J. Incidental Thyroid “Petomas”: Clinical Significance and Novel Description of the Self-Resolving Variant of Focal FDG-PET Thyroid Uptake. Can J Surg (2011) 54(2):83–8. doi: 10.1503/cjs.023209
31. Pagano L, Samà MT, Morani F, Prodam F, Rudoni M, Boldorini R, et al. Thyroid Incidentaloma Identified by 18F-Fluorodeoxyglucose Positron Emission Tomography With CT (FDG-PET/CT): Clinical and Pathological Relevance. Clin Endocrinol (2011) 75(4):528–34. doi: 10.1111/j.1365-2265.2011.04107.x
32. Prichard RS, Cotter M, Evoy D. Focal Thyroid Incidentalomas Identified With Whole-Body Fdg-Pet Warrant Further Investigation. (2011).
33. Wong C, Lin M, Chicco A, Benson R. The Clinical Significance and Management of Incidental Focal FDG Uptake in the Thyroid Gland on Positron Emission Tomography/Computed Tomography (PET/CT) in Patients With Non-Thyroidal Malignancy. Acta Radiologica (2011) 52(8):899–904. doi: 10.1258/ar.2011.110078
34. Boeckmann J, Bartel T, Siegel E, Bodenner D, Stack BC. Can the Pathology of a Thyroid Nodule be Determined by Positron Emission Tomography Uptake? Otolaryngology–Head Neck Surg (2012) 146(6):906–12. doi: 10.1177/0194599811435770
35. Bonabi S, Schmidt F, Broglie M, Haile S, Stoeckli S. Thyroid Incidentalomas in FDG-PET/CT: Prevalence and Clinical Impact. Eur Arch Otorhinolaryngol (2012) 269(12):2555–60. doi: 10.1007/s00405-012-1941-7
36. Fujii T, Yajima R, Yamaguchi S, Tsutsumi S, Asao T, Kuwano H. Is it Possible to Predict Malignancy in Cases With Focal Thyroid Incidentaloma Identified by 18F-Fluorodeoxyglucose Positron Emission Tomography? Am Surg (2012) 78(1):141–3. doi: 10.1177/000313481207800153
37. Kao YH, Lim SS, Ong SC, Padhy AK. Thyroid Incidentalomas on Fluorine-18-Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography: Incidence, Malignancy Risk, and Comparison of Standardized Uptake Values. Can Assoc Radiologists J (2012) 63(4):289–93. doi: 10.1016/j.carj.2011.04.003
38. Lee W, Kim B, Kim M, Choi S, Ryu S, Lim I, et al. Characteristics of Thyroid Incidentalomas Detected by Pre-Treatment [18f]FDG PET or PET/CT in Patients With Cervical Cancer. J gynecologic Oncol (2012) 23(1):43–7. doi: 10.3802/jgo.2012.23.1.43
39. Pampaloni MH, Win AZ. Prevalence and Characteristics of Incidentalomas Discovered by Whole Body FDG PETCT. Int J Mol Imaging (2012) 2012:476763. doi: 10.1155/2012/476763
40. Yang Z, Shi W, Zhu B, Hu S, Zhang Y, Wang M, et al. Prevalence and Risk of Cancer of Thyroid Incidentaloma Identified by Fluorine-18 Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography. J Otolaryngology-Head Neck Surg (2012) 41(5):327.
41. Bertagna F, Bertagna F, Treglia G, Treglia G, Piccardo A, Piccardo A, et al. F18-FDG-PET/CT Thyroid Incidentalomas: A Wide Retrospective Analysis in Three Italian Centres on the Significance of Focal Uptake and SUV Value. Endocrine (2013) 43(3):678–85. doi: 10.1007/s12020-012-9837-2
42. Kim H, Kim S, Kim I, Kim K. Thyroid Incidentalomas on FDG PET/CT in Patients With Non-Thyroid Cancer - A Large Retrospective Monocentric Study. Oncol Res Treat (2013) 36(5):260–4. doi: 10.1159/000350305
43. Kim BH, Kim SJ, Kim H, Jeon YK, Kim SS, Kim IJ, et al. Diagnostic Value of Metabolic Tumor Volume Assessed by 18F-FDG PET/CT Added to SUVmax for Characterization of Thyroid 18F-FDG Incidentaloma. Nucl Med Commun (2013) 34(9):868–76. doi: 10.1097/MNM.0b013e328362d2d7
44. Achury C, Estorch M, Domènech A, Camacho V, Flotats A, Jaller R, et al. Interpretation of Thyroid Incidentalomas in (18)F-FDG PET/CT Studies. Rev Esp Med Nucl Imagen Mol (2014) 33(4):205–9.
45. Brindle R, Mullan D, Yap BK, Gandhi A. Thyroid Incidentalomas Discovered on Positron Emission Tomography CT Scanning – Malignancy Rate and Significance of Standardised Uptake Values. Eur J Surg Oncol (2014) 40(11):1528–32. doi: 10.1016/j.ejso.2014.05.005
46. Choi JS, Choi Y-J, Kim EK, Yoon JH, Youk JH, Han KH, et al. A Risk-Adapted Approach Using US Features and FNA Results in the Management of Thyroid Incidentalomas Identified by 18F-FDG PET. Ultraschall der Med - Eur J Ultrasound (2014) 35(1):51–8. doi: 10.1055/s-0033-1335328
47. Elzein S, Ahmed A, Lorenz E, Balasubramanian SP. Thyroid Incidentalomas on PET Imaging – Evaluation of Management and Clinical Outcomes. Surgeon (2014) 13(2):116–20.
48. Marques P, Ratão P, Salgado L, Bugalho MJ. Thyroid Carcinoma Detected by 18F-Fluorodeoxyglucose Positron Emission Tomography Among Individuals Without Prior Evidence of Thyroid Disease: Relevance and Clinicopathologic Features. Endocr Pract (2014) 20(11):1129–36. doi: 10.4158/EP14042.OR
49. Stangierski A, Woliński K, Czepczyński R, Czarnywojtek A, Lodyga M, Wyszomirska A, et al. The Usefulness of Standardized Uptake Value in Differentiation Between Benign and Malignant Thyroid Lesions Detected Incidentally in 18F-FDG PET/CT Examination. PloS One (2014) 9(10):e109612. doi: 10.1371/journal.pone.0109612
50. Yaylali O, Kirac FS, Yuksel D, Marangoz E. Evaluation of Focal Thyroid Lesions Incidentally Detected in Fluorine-18-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Images. Indian J Cancer (2014) 51(3):236–40. doi: 10.4103/0019-509X.146737
51. Adas M, Adas G, Koc B, Ozulker F. Incidental Thyroid Lesions on FDG-PET/CT: A Prevalence Study and Proposition of Management. Minerva endocrinologica (2015) 40(3):169.
52. Agrawal K, Weaver J, Ul-Hassan F, Jeannon J, Simo R, Carroll P, et al. Incidence and Significance of Incidental Focal Thyroid Uptake on 18F-FDG PET Study in a Large Patient Cohort: Retrospective Single-Centre Experience in the United Kingdom. Eur Thyroid J (2015) 4(2):115–22. doi: 10.1159/000431319
53. Chun AR, Jo HM, Lee SH, Chun HW, Park JM, Kim KJ, et al. Risk of Malignancy in Thyroid Incidentalomas Identified by Fluorodeoxyglucose-Positron Emission Tomography. Endocrinol Metab (Seoul) (2015) 30(1):71–7. doi: 10.3803/EnM.2015.30.1.71
54. Gavriel H, Gavriel H, Tang A, Tang A, Eviatar E, Eviatar E, et al. Unfolding the Role of PET FDG Scan in the Management of Thyroid Incidentaloma in Cancer Patients. Eur Arch Otorhinolaryngol (2015) 272(7):1763–8. doi: 10.1007/s00405-014-3120-5
55. Jamsek J, Zagar I, Gaberscek S, Grmek M. Thyroid Lesions Incidentally Detected by 18F-FDG PET-CT ― a Two Centre Retrospective Study. Radiol Oncol (2015) 49(2):121–7. doi: 10.2478/raon-2014-0039
56. Kim S, Chang S. Predictive Value of Intratumoral Heterogeneity of F-18 FDG Uptake for Characterization of Thyroid Nodules According to Bethesda Categories of Fine Needle Aspiration Biopsy Results. Endocrine (2015) 50(3):681–8. doi: 10.1007/s12020-015-0620-z
57. Sharma SD, Jacques T, Smith S, Watters G. Diagnosis of Incidental Thyroid Nodules on 18F-Fluorodeoxyglucose Positron Emission Tomography Imaging: Are These Significant? J laryngology otology (2015) 129(1):53–6. doi: 10.1017/S0022215114003107
58. Yoon JH, Cho A, Lee HS, Kim EK, Moon HJ, Kwak JY. Thyroid Incidentalomas Detected on 18F-Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography: Thyroid Imaging Reporting and Data System (TIRADS) in the Diagnosis and Management of Patients. Surgery (2015) 158(5):1314–22. doi: 10.1016/j.surg.2015.03.017
59. Barrio M, Czernin J, Yeh MW, Diaz MFP, Gupta P, Allen-Auerbach M, et al. The Incidence of Thyroid Cancer in Focal Hypermetabolic Thyroid Lesions: A 18fdg PET/CT Study in More Than 6,000 Patients. Nucl Med Commun (2016) 37(12):1290–6. doi: 10.1097/MNM.0000000000000592
60. Demir Ö, Köse N, Özkan E, Ünlütürk U, Aras G, Erdoğan MF. Clinical Significance of Thyroid Incidentalomas Identified by 18F-FDG PET/CT: Correlation of Ultrasonograpy Findings With Cytology Results. Nucl Med Commun (2016) 37(7):715–20. doi: 10.1097/MNM.0000000000000495
61. Flukes S, Lenzo N, Moschilla G, Sader C. Positron Emission Tomography-Positive Thyroid Nodules: Rate of Malignancy and Histological Features. ANZ J Surg (2016) 86(6):487–91. doi: 10.1111/ans.12834
62. Hassan A, Riaz S, Zafar W. Fluorine-18 Fluorodeoxyglucose Avid Thyroid Incidentalomas on PET/CT Scan in Cancer Patients: How Sinister are They? Nucl Med Commun (2016) 37(10):1069–73. doi: 10.1097/MNM.0000000000000557
63. Şencan Eren M, Özdoğan Ö, Gedik A, Ceylan M, Güray Durak M, Seçil M, et al. The Incidence of 18F-FDG PET/CT Thyroid Incidentalomas Andthe Prevalence of Malignancy: A Prospective Study. Turkish J Med Sci (2016) 46(3):840–7. doi: 10.3906/sag-1503-26
64. Vaish R, Venkatesh R, Chaukar DA, Deshmukh AD, Purandare NC, D’cruz AK. Positron Emission Tomography Thyroid Incidentaloma: Is it Different in Indian Subcontinent? Indian J Cancer (2016) 53(1):186–9. doi: 10.4103/0019-509X.180860
65. Hagenimana N, Dallaire J, Vallée É, Belzile M. Thyroid Incidentalomas on 18FDG-PET/CT: A Metabolico-Pathological Correlation. J Otolaryngol Head Neck Surg (2017) 46(1):22–8. doi: 10.1186/s40463-017-0200-8
66. Li Y, Cui M, Azar N, Nakamoto D, Michael CW. Cytological Evaluation by Fine Needle Aspiration Biopsy of Incidental Focal Increased Fluorodeoxyglucose Uptake in Thyroid on Positron Emission Tomography Scan. Diagn Cytopathol (2017) 45(6):501–6. doi: 10.1002/dc.23695
67. Makis W, Ciarallo A. Thyroid Incidentalomas on 18F-FDG PET/CT: Clinical Significance and Controversies. Mol Imaging Radionuclide Ther (2017) 26(3):93–100. doi: 10.4274/mirt.94695
68. Ozderya A, Ozderya A, Temizkan S, Temizkan S, Gul A, Gul A, et al. Correlation of BRAF Mutation and Suvmax Levels in Thyroid Cancer Patients Incidentally Detected in 18F-Fluorodeoxyglucose Positron Emission Tomography. Endocrine (2017) 55(1):215–22. doi: 10.1007/s12020-016-1128-x
69. Pak K, Shin S, Kim SJ, Kim K, Kim BS, Kim SJ, et al. Risk of Malignancy in Thyroid Incidentaloma is Not Increased in Overweight or Obese Patients, But in Young Patients. Nutr Cancer (2017) 69(4):580–4. doi: 10.1080/01635581.2017.1299877
70. Sollini M, Cozzi L, Pepe G, Antunovic L, Lania A, Di Tommaso L, et al. (18)F]FDG-PET/CT Texture Analysis in Thyroid Incidentalomas: Preliminary Results. Eur J Hybrid Imaging (2017) 1(1):3–8. doi: 10.1186/s41824-017-0009-8
71. Thuillier P, Roudaut N, Crouzeix G, Cavarec M, Robin P, Abgral R, et al. Malignancy Rate of Focal Thyroid Incidentaloma Detected by FDG PET-CT: Results of a Prospective Cohort Study. Endocrine Connections (2017) 6(6):413–21. doi: 10.1530/EC-17-0099
72. Chung SR, Choi YJ, Suh CH, Kim HJ, Lee JJ, Kim WG, et al. Thyroid Incidentalomas Detected on 18F-Fluorodeoxyglucose Positron Emission Tomography With Computed Tomography: Malignant Risk Stratification and Management Plan. Thyroid (New York N.Y.) (2018) 28(6):762–8. doi: 10.1089/thy.2017.0560
73. Pattison DA, Bozin M, Gorelik A, Hofman MS, Hicks RJ, Skandarajah A. (18)F-FDG-Avid Thyroid Incidentalomas: The Importance of Contextual Interpretation. J Nucl Med (2018) 59(5):749–55. doi: 10.2967/jnumed.117.198085
74. Sager S, Vatankulu B, Sahin O, Cınaral F, Uslu L, Baran A, et al. Clinical Significance of Standardized Uptake Values in Thyroid Incidentaloma Discovered by F-18 Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography. J Cancer Res Ther (2018) 14(5):989–93. doi: 10.4103/0973-1482.187247
75. Abdel-Halim CN, Rosenberg T, Bjørndal K, Madsen AR, Jakobsen J, Døssing H, et al. Risk of Malignancy in FDG-Avid Thyroid Incidentalomas on PET/CT: A Prospective Study. World J Surg (2019) 43(10):2454–8. doi: 10.1007/s00268-019-05043-6
76. Kumar A, Datta G, Singh H, Mukherjee P, Vangal S. Clinical Significance of Thyroid Incidentalomas Detected on Fluorodeoxyglucose Positron Emission Tomography Scan (Petomas): An Indian Experience. World J Nucl Med (2019) 18(3):273–82. doi: 10.4103/wjnm.WJNM_46_18
77. Larg M, Apostu D, Peștean C, Gabora K, Bădulescu IC, Olariu E, et al. Evaluation of Malignancy Risk in 18F-FDG PET/CT Thyroid Incidentalomas. Diagnostics (Basel) (2019) 9(3):92. doi: 10.3390/diagnostics9030092
78. Lin Y, Tsai Y, Lin KJ, Der Lin J, Wang C, Chen S. Computer-Aided Diagnostic Technique in 2-Deoxy-2-[18f]Fluoro-D-Glucose-Positive Thyroid Nodule: Clinical Experience of 74 Non-Thyroid Cancer Patients. Ultrasound Med Biol (2019) 45(1):108–21. doi: 10.1016/j.ultrasmedbio.2018.09.002
79. Oven B, Kilicoglu Z, Bilici A, Tatoglu M, Canberk S, Tilki M, et al. The Relationship Between Positron Emission Tomography-Computed Tomography Imaging and Histopathological Features of Thyroid Incidentalomas Detected During Follow-Up for Primary Malignancy. J Cancer Res Ther (2019) 15(3):589–95. doi: 10.4103/jcrt.JCRT_889_16
80. Shi H, Yuan Z, Yang C, Zhang J, Liu C, Sun J, et al. Role of Multi-Modality Functional Imaging in Differentiation Between Benign and Malignant Thyroid 18f-Fluorodeoxyglucose Incidentaloma. Clin Transl Oncol (2019) 21(11):1561–7. doi: 10.1007/s12094-019-02089-9
81. Bakhshayesh Karam M, Doroudinia A, Joukar F, Nadi K, Dorudinia A, Mehrian P, et al. Hypermetabolic Thyroid Incidentaloma on Positron Emission Tomography: Review of Laboratory, Radiologic, and Pathologic Characteristics. J Thyroid Res (2017) 2017:7176934. doi: 10.1155/2017/7176934
82. Kamakshi K, Krishnamurthy A, Karthik V, Vinodkumar P, Kumar R, Lakshmipathy K. Positron Emission Tomography-Computed Tomography-Associated Incidental Neoplasms of the Thyroid Gland. World J Nucl Med (2020) 19(1):36–40.
83. Trimboli P, Knappe L, Treglia G, Ruberto T, Piccardo A, Ceriani L, et al. FNA Indication According to ACR-TIRADS, EU-TIRADS and K-TIRADS in Thyroid Incidentalomas at 18F-FDG PET/CT. J Endocrinological Invest (2020). doi: 10.1007/s40618-020-01244-2
84. Bongiovanni M, Spitale A, Faquin WC, Mazzucchelli L, Baloch ZW. The Bethesda System for Reporting Thyroid Cytopathology: A Meta-Analysis. Acta Cytol (2012) 56(4):333–9. doi: 10.1159/000339959
85. Lubitz CC, Kong CY, McMahon PM, Daniels GH, Chen Y, Economopoulos KP, et al. Annual Financial Impact of Well-Differentiated Thyroid Cancer Care in the United States. Cancer (2014) 120(9):1345–52. doi: 10.1002/cncr.28562
86. Ding A, Eisenberg JD, Pandharipande PV. The Economic Burden of Incidentally Detected Findings. Radiol Clin North Am (2011) 49(2):257–65. doi: 10.1016/j.rcl.2010.11.004
87. Kane HL, Halpern MT, Squiers LB, Treiman KA, McCormack LA. Implementing and Evaluating Shared Decision Making in Oncology Practice. CA Cancer J Clin (2014) 64(6):377–88. doi: 10.3322/caac.21245
88. Beatty JS, Williams HT, Aldridge BA, Hughes MP, Vasudeva VS, Gucwa AL, et al. Incidental PET/CT Findings in the Cancer Patient: How Should They be Managed? Surgery (2009) 146(2):274–81. doi: 10.1016/j.surg.2009.04.024
89. Wang G, Lau EW, Shakher R, Rischin D, Ware RE, Hong E, et al. How do Oncologists Deal With Incidental Abnormalities on Whole-Body Fluorine-18 Fluorodeoxyglucose PET/Ct? Cancer (2007) 109(1):117–24. doi: 10.1002/cncr.22370
90. Castellana M, Grani G, Radzina M, Guerra V, Giovanella L, Deandrea M, et al. Performance of EU-TIRADS in Malignancy Risk Stratification of Thyroid Nodules: A Meta-Analysis. Eur J Endocrinol (2020) 183(3):255–64. doi: 10.1530/EJE-20-0204
91. Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J Am Coll Radiol (2017) 14(5):587–95. doi: 10.1016/j.jacr.2017.01.046
92. Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur Thyroid J (2017) 6(5):225–37. doi: 10.1159/000478927
93. Barbosa TLM, Junior COM, Graf H, Cavalvanti T, Trippia MA, da Silveira U, et al. ACR TI-RADS and ATA US Scores Are Helpful for the Management of Thyroid Nodules With Indeterminate Cytology. BMC endocrine Disord (2019) 19(1):112. doi: 10.1186/s12902-019-0429-5
94. Rocha TG, Rosario PW, Silva AL, Nunes MB, Silva TH, de Oliveira ,PHL, et al. Ultrasonography Classification of the American Thyroid Association for Predicting Malignancy in Thyroid Nodules >1cm With Indeterminate Cytology: A Prospective Study. Horm Metab Res (2018) 50(8):597–601. doi: 10.1055/a-0655-3016
95. Ahmadi S, Herbst R, Oyekunle T, Jiang X’, Strickland K, Roman S, et al. Using the Ata and Acr Ti-Rads Sonographic Classifications as Adjunctive Predictors of Malignancy for Indeterminate Thyroid Nodules. Endocr Pract (2019) 25(9):908–17. doi: 10.4158/EP-2018-0559
96. Chaigneau E, Russ G, Royer B, Bigorgne C, Bienvenu-Perrard M, Rouxel A, et al. TIRADS Score is of Limited Clinical Value for Risk Stratification of Indeterminate Cytological Results. Eur J Endocrinol (2018) 179(1):13–20. doi: 10.1530/EJE-18-0078
97. Wu H, Zhang B, Cai G, Li J, Gu X. American College of Radiology Thyroid Imaging Report and Data System Combined With K-RAS Mutation Improves the Management of Cytologically Indeterminate Thyroid Nodules. PloS One (2019) 14(7):e0219383. doi: 10.1371/journal.pone.0219383
98. Valderrabano P, McGettigan MJ, Lam CA, Khazai L, Thompson ZJ, Chung CH, et al. Thyroid Nodules With Indeterminate Cytology: Utility of the American Thyroid Association Sonographic Patterns for Cancer Risk Stratification. Thyroid (2018) 28(8):1004–12. doi: 10.1089/thy.2018.0085
99. Trimboli P, Scappaticcio L, Treglia G, Guidobaldi L, Bongiovanni M, Giovanella L. Testing for BRAF (V600E) Mutation in Thyroid Nodules With Fine-Needle Aspiration (FNA) Read as Suspicious for Malignancy (Bethesda V, Thy4, TIR4): A Systematic Review and Meta-Analysis. Endocr Pathol (2020) 31(1):57–66. doi: 10.1007/s12022-019-09596-z
100. Oczko-Wojciechowska M, Kotecka-Blicharz A, Krajewska J, Rusinek D, Barczyński M, Jarząb B, et al. European Perspective on the Use of Molecular Tests in the Diagnosis and Therapy of Thyroid Neoplasms. Gland Surg (2020) 9(Suppl 2):S69–76. doi: 10.21037/gs.2019.10.26
101. Dean DS, Gharib H. Epidemiology of Thyroid Nodules. Best Pract Res Clin Endocrinol Metab (2008) 22(6):901–11. doi: 10.1016/j.beem.2008.09.019
102. Kwong N, Medici M, Angell TE, Liu X, Marqusee E, Cibas ES, et al. The Influence of Patient Age on Thyroid Nodule Formation, Multinodularity, and Thyroid Cancer Risk. J Clin Endocrinol Metab (2015) 100(12):4434–40. doi: 10.1210/jc.2015-3100
103. Moon JH, Hyun MK, Lee JY, Shim JI, Kim TH, Choi HS, et al. Prevalence of Thyroid Nodules and Their Associated Clinical Parameters: A Large-Scale, Multicenter-Based Health Checkup Study. Korean J Intern Med (2018) 33(4):753–62. doi: 10.3904/kjim.2015.273
104. Yang J, Schnadig V, Logrono R, Wasserman PG. Fine-Needle Aspiration of Thyroid Nodules: A Study of 4703 Patients With Histologic and Clinical Correlations. Cancer (2007) 111(5):306–15. doi: 10.1002/cncr.22955
105. Wong LQ, Baloch ZW. Analysis of the Bethesda System for Reporting Thyroid Cytopathology and Similar Precursor Thyroid Cytopathology Reporting Schemes. Adv Anat Pathol (2012) 19(5):313–9. doi: 10.1097/PAP.0b013e3182666398
106. Melo-Uribe MA, Sanabria Á, Romero-Rojas A, Pérez G, Vargas EJ, Abaúnza MC, et al. The Bethesda System for Reporting Thyroid Cytopathology in Colombia: Correlation With Histopathological Diagnoses in Oncology and Non-Oncology Institutions. J Cytol (2015) 32(1):12–6. doi: 10.4103/0970-9371.155224
107. Yaprak Bayrak B, Eruyar AT. Malignancy Rates for Bethesda III and IV Thyroid Nodules: A Retrospective Study of the Correlation Between Fine-Needle Aspiration Cytology and Histopathology. BMC Endocr Disord (2020) 20(1):48–9. doi: 10.1186/s12902-020-0530-9
Keywords: thyroid, incidentaloma, FDG (18F-fluorodeoxyglucose)-PET/CT, thyroid nodule, thyroid cancer
Citation: de Leijer JF, Metman MJH, van der Hoorn A, Brouwers AH, Kruijff S, van Hemel BM, Links TP and Westerlaan HE (2021) Focal Thyroid Incidentalomas on 18F-FDG PET/CT: A Systematic Review and Meta-Analysis on Prevalence, Risk of Malignancy and Inconclusive Fine Needle Aspiration. Front. Endocrinol. 12:723394. doi: 10.3389/fendo.2021.723394
Received: 10 June 2021; Accepted: 20 September 2021;
Published: 20 October 2021.
Edited by:
Dario Giuffrida, Mediterranean Institute of Oncology (IOM), ItalyReviewed by:
Carlotta Giani, University of Pisa, ItalyCarl Christofer Juhlin, Karolinska Institutet (KI), Sweden
Lutske Lodewijk, University Medical Center Utrecht, Netherlands
Alessandro Prete, University of Pisa, Italy
Copyright © 2021 de Leijer, Metman, van der Hoorn, Brouwers, Kruijff, van Hemel, Links and Westerlaan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: H. E. Westerlaan, h.e.westerlaan@umcg.nl
 J. F. de Leijer
J. F. de Leijer M. J. H. Metman2
M. J. H. Metman2 A. van der Hoorn
A. van der Hoorn S. Kruijff
S. Kruijff T. P. Links
T. P. Links H. E. Westerlaan
H. E. Westerlaan