- 1Department of Food Science and Nutrition, College of Life Sciences, Kuwait University, Kuwait City, Kuwait
- 2Biochemistry and Molecular Biology Department, Dasman Diabetes Institute, Kuwait City, Kuwait
- 3Department of Community Medicine and Behavioural Sciences, Faculty of Medicine, Kuwait University, Kuwait City, Kuwait
- 4Genetics and Bioinformatics Department, Dasman Diabetes Institute, Kuwait City, Kuwait
- 5Special Services Department, Dasman Diabetes Institute, Kuwait City, Kuwait
Insulin-like growth factor binding proteins (IGFBPs) are critical modulators of metabolism. In adults, IGFBPs are associated with obesity and insulin resistance. However, the association of IGFBPs with metabolic homeostasis in children and adolescents is not yet fully characterized. In this study we investigated the association of plasma IGFBPs (IGFBP-1, 3 and 7) with weight, central adiposity and cardiovascular disease markers Hs-CRP and Ox-LDL. A total of 420 adolescents (age 11-14 years) were recruited from public middle schools in Kuwait. IGFBPs were measured using bead-based multiplexing while Hs-CRP and Ox-LDL were measured using ELISA. Results showed that levels of IGFBP-1 were significantly lower in obese and overweight children when compared to normal weight children. Correlation analysis showed negative association between the level of IGFBP-1 and waist circumference to height (WC/Ht) ratio. IGFBP-1 level was also negatively associated with Hs-CRP. It was also observed that the levels of IGFBP-3 and IGFBP-7 were negatively correlated with Ox-LDL. Our data demonstrate a strong negative association of IGFBP-1 with overweight/obesity, and the inflammatory marker Hs-CRP. This was not seen with the levels of IGFBP-3 and 7. The association of IGFBP-1 with central adiposity (WC/Ht ratio) was stronger than its association with BMI-for-age z-score. Therefore we suggest that IGFBP-1 could potentially be used as a sensitive biomarker for obesity and its subsequent effects in screening and monitoring of obesity-related metabolic complications in adolescents.
Introduction
Childhood obesity is a public health concern. The Arabian Gulf region, including Kuwait, has one of the highest reported rates of childhood obesity (1). The prevalence of overweight and obesity among school children in Kuwait has been reported to be 45% (2, 3). Obesity has a complex etiology. The major contributing factors include both genetics and lifestyle behaviors such as poor dietary habits and lack of physical activity (4). Several reports show that childhood and adolescence obesity most likely persists into adulthood (5). Obesity is associated with many complications including dyslipidemia, hypertension, heart failure, and atherosclerotic cardiovascular diseases (6–8). Because of the increased health care cost to deal with these chronic diseases and the poor quality of life for patients, tackling obesity in early life should be a public health priority.
The need for a shift from reactive to predictive, preventive and personalized medicine (PPPM) is eminent (9, 10). While precision/personalized medicine has limited applications due to several challenges, tremendous effort is directed towards the advancement and use of this approach in the general clinical practice. One of the ways through which targeted therapy may be utilized is by screening for novel and easy-to-measure molecular biomarkers. The metabolic syndrome and its related pathologies, especially obesity, are in heightened need for such therapy options since they cause tremendous economic burdens on the healthcare systems (11). Identifying novel biomarkers, which could be used in detecting metabolic complications associated with obesity, is important for risk stratification and for monitoring and evaluating intervention programs. Personal variability related to genes, environment and lifestyle are taken into account when precision medicine approach is considered to be used (9).
Like insulin, insulin-like growth factors (IGFs) regulate diverse physiological functions related to growth, development and glucose homeostasis, which occur through common signaling pathways (12). When IGF-I and IGF-II were first described in the early fifties, they were presented as skeletal growth factors responsive to pituitary growth hormones (GH) and involved in the regulation of whole-body growth. Later investigations revealed that these molecules display homology to proinsulin and that they are regulated by multiple factors other than GH. The transport, metabolism and signaling by the IGFs are modulated by a family of binding proteins, which is comprised of six IGF binding proteins (IGFBP-1 – 6) as well as the IGFBP-related proteins (IGFBP-rP1, designated as IGFBP-7) (12–14). The different IGFBPs poses distinct features that allow them to specifically bind to certain receptors or translocate to various cellular compartments to mediate IGF-independent actions (14). Furthermore, different tissues produce different IGFBPs and their functions vary according to the metabolic conditions surrounding them (15). IGFBPs have been implicated in the development and pathogenesis of obesity and its related comorbidities like diabetes, metabolic syndrome and cardiovascular diseases (CVDs) through IGF-dependent as well as IGF-independent roles (12, 13).
Despite their significant sequence homology and their common ability to bind to IGFs with similar affinity, IGFBPs have unique structural features and distinct functions. This is mainly due to the different functional motifs of the family members. For examples, IGFBP-1 has an integrin-binding RGD (Arginine, Glycine, and Aspartate) motif and therefore can mediate cell migration. IGFBP-3 on the other hand does not have this RGD motif, but has many others including a nuclear localization sequence and a heparin binding domain (14).
The association of IGFBPs with obesity, metabolic syndrome and diabetes has been the subject of many investigations. Of these, IGFBP-1 has been consistently shown to be inversely associated with overweight and obesity (16–18), plasma insulin and glucose levels (19, 20), as well as fasting plasma leptin levels (21). Low serum IGFBP-1 levels have been reported to be predictive of the development of diabetes (22–24). The association of IGFBP-1 with metabolic homeostasis has been consistent across gender, different age groups and across various ethnicities (21, 25).
IGFBP-3 is the most abundant protein among all the IGFBPs and it transports more than 90% of IGF-I and IGF-II in circulation as a ternary complex with IGFs (12). However, its association with weight, metabolic syndrome and glucose homeostasis has not been consistent across studies. Some studies reported increased level of IGFBP-3 in overweight and obese subjects (16), while others reported no association with weight (26). In a prospective case-control study conducted on female nurses, a positive correlation of IGFBP-3 with the development of diabetes, BMI and waist circumference was reported (27).
IGFBP-7 is the most recent addition to the IGFBPs family. It has a similar amino acid sequence and structure to other human IGFBPs and can specifically bind to IGF-I and IGF-II. It is the least studied member of the IGFBP family in the context of its association with metabolic homeostasis. The available literature suggests that its serum levels are positively associated with BMI (28) and type 2 diabetes (29), fasting glucose levels (30), insulin resistance and metabolic syndrome (28, 31). A recent study reported higher levels of IGFBP-7 in coronary artery disease (CAD) patients compared to healthy subjects (32). In addition, IGFBP-3 was reported to be negatively associated with the levels of some inflammatory markers including C-reactive protein and interleukin-6 (33), whereas high levels of serum IGFBP-7 were associated with increased CRP levels (28).
The aim of this study was thus to investigate the association of IGFBP-1, 3 and 7 with weight status, waist circumference and well-established CVD risk factors, specifically Hs-CRP and Oxidized Low-Density Lipoprotein (Ox-LDL) in a group of healthy adolescents from Kuwait.
Materials and Methods
Study Participants
This is a cross-sectional study that was conducted in selected public middle schools from the State of Kuwait as previously described (34–36). Study participants were adolescents in the age range of 11–14 years. Data on socioeconomic status and other covariates were collected from the parents through a self-administered questionnaire, and from the adolescents using face-to-face interview.
Ethics Statement
The study was approved by The Ethics Committee at Ministry of Health, Kuwait (No: 2015/248), the Ethics Committee of the Health Sciences Centre, Kuwait University (No: DR/EC/2338) and the Ethical Review Committee at Dasman Diabetes Institute (RA2017-026). Written informed consent was obtained from the parents and verbal assent was obtained from all the study subjects. We certify that the work conducted in this research complies with the ethical standards recommended by the Helsinki Declaration.
Blood Collection and Biochemical Analyses
A sample of venous blood (5 mL) was collected from each child in EDTA-containing tubes. Plasma was separated and stored at −80 °C till analysis. IGFBPs levels were assessed using multiplexing immunobead array platform according to the manufacturer’s instructions (R & D Systems). Median fluorescence intensities were collected on a Bioplex-200 system and data were processed using the Bio-Plex Manager Software version 6 (Bio-Rad), with five-parametric curve fitting. Hs-CRP concentrations were determined using ELISA (Hycult Biotech, Cat. # HK369) following manufacturer’s instructions. Optimal dilution was found to be 1:1000. Ox-LDL concentrations were determined using ELISA (Immundiagnostik AG, Germany, Cat. # K 7810) following manufacturer’s instructions. Optimal dilution was found to be 1:10.
Anthropometric Measurements and Other Covariates
Standing height and bodyweight of the study participants were measured in a standardized manner, using digital weight and height scale (Detecto, Webb City, MO, USA) with the participants standing erect without shoes and wearing light clothes. BMI-for-age z-scores were calculated using WHO growth charts. Obesity was defined as BMI-for-age ≥ +3 Standard Deviation (SD), while overweight was defined as BMI-for-age > +2 SD and < +3 SD. Waist circumference (WC) was measured in the horizontal plane at the superior border of the right iliac crest to the nearest 0.1 cm with a non-stretchable tape by a trained data collector. Measurements were taken at the end of normal expiration; three readings were taken, and the average of the three was recorded. Care was taken to ensure that the tape was horizontal to the floor and touched the skin without compressing it. The ratio of waist circumference (cm) to height in centimeters (WC/Ht ratio) was calculated and the obesogenic waist was defined as a WC/Ht ratio of > 0.5 (37).
Statistical Analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) for Windows version 26 (IBM Corp., Armonk, N.Y., USA). Data were log-transformed and checked for normality using SPSS. Data for the IGFBP in different weight status groups and in male and female were presented as bar graphs showing mean with standard deviation (SD). Association between IGFBPs and weight status categories was assessed by both univariable and multivariable linear regression adjusting for age and sex. The association of IGFBPs with Hs-CRP and Ox-LDL (both log-transformed) was also assessed by linear regression analysis. Levels of IGFBPs were categorized into tertiles and the odds of overweight/obesity (combined) were determined in various tertiles of IGFBPs using binary logistic regression without and with adjusting for age and sex. Mean differences in the IGFBPs across weight status groups, age groups and gender were assessed by one-way ANOVA and t-test for independent samples. A p-value of < 0.05 was considered as statistically significant.
Results
Study Population Characteristics
Table 1 summarizes the characteristics of the participants that were involved in the study. Data were analyzed for 420 participants of whom 192 (45.7%) were male. Mean (SD) age was 12.4 (1.5) years. Of the total samples, 47% adolescents were normal weight, 21% were overweight and 32% were obese. Median (IQR) for IGFBP-1 was 5.7 (3.5, 10.0) ng/mL, while median (IQR) for IGFBP-3 and IGFBP-7 were 835.0 (688.5, 1007.8) and 17.7 (15.2, 20.0) ng/mL, respectively. Female adolescents had significantly lower level of IGFBP-1 compared to males (p<0.001) (Figure 1A), whereas the differences in plasma levels of IGFBP-3 and IGFBP-7 between male and female subjects were non-significant (data not shown). IGFBP-1 level was significantly higher in the age group 10-<12 year old when compared to 12-<13 years (p<0.01) and 13+ years group (p<0.001) (Figure 1B). On the other hand, no significant difference was observed among different age groups in IGFBP-3 and IGFBP-7 levels (data not shown).
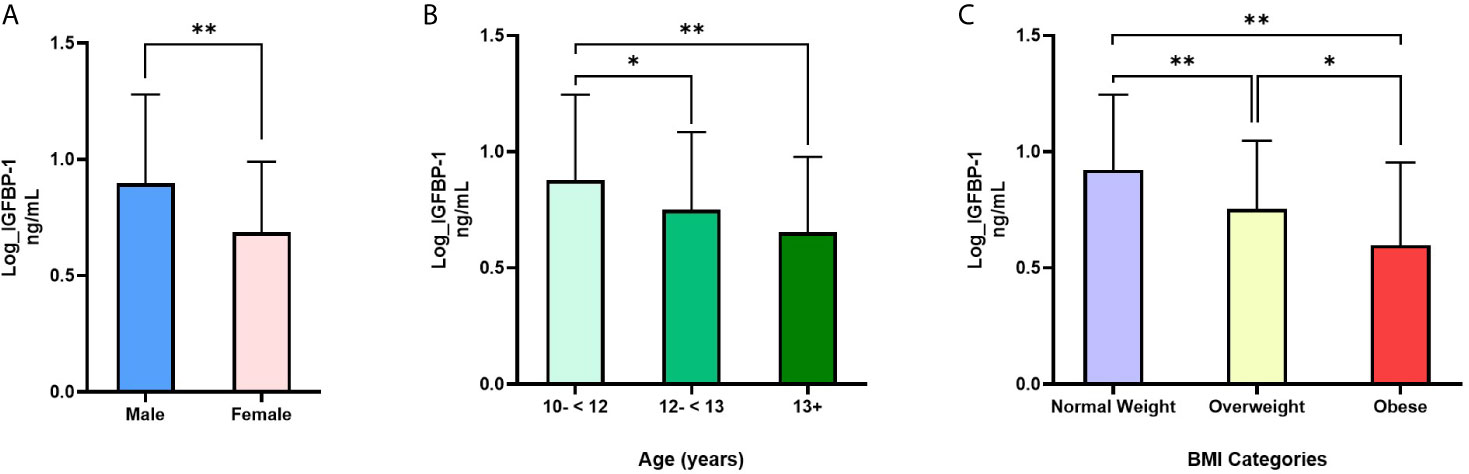
Figure 1 Differences in IGFBP-1 levels based on (A) sex, (B) age and (C) weight status: (A) IGFBP-1 are significantly lower in females compared to males; N=191 males, 228 females; t=6.18, p<0.001; (B) IGFBP-1 levels are significantly reduced with age; N=178 (10-<12), 143 (12-<13) and 98 (13+); (C) IGFBP-1 is reduced in overweight and obesity compared to normal weight; N= 198 normal weight, 88 overweight, 135 obese. Data is presented as means ± SD. Males and females were compared by t-test for independent samples, whereas, age groups and weight status groups were compared with one-way ANOVA. *p < 0.01; **p < 0.001.
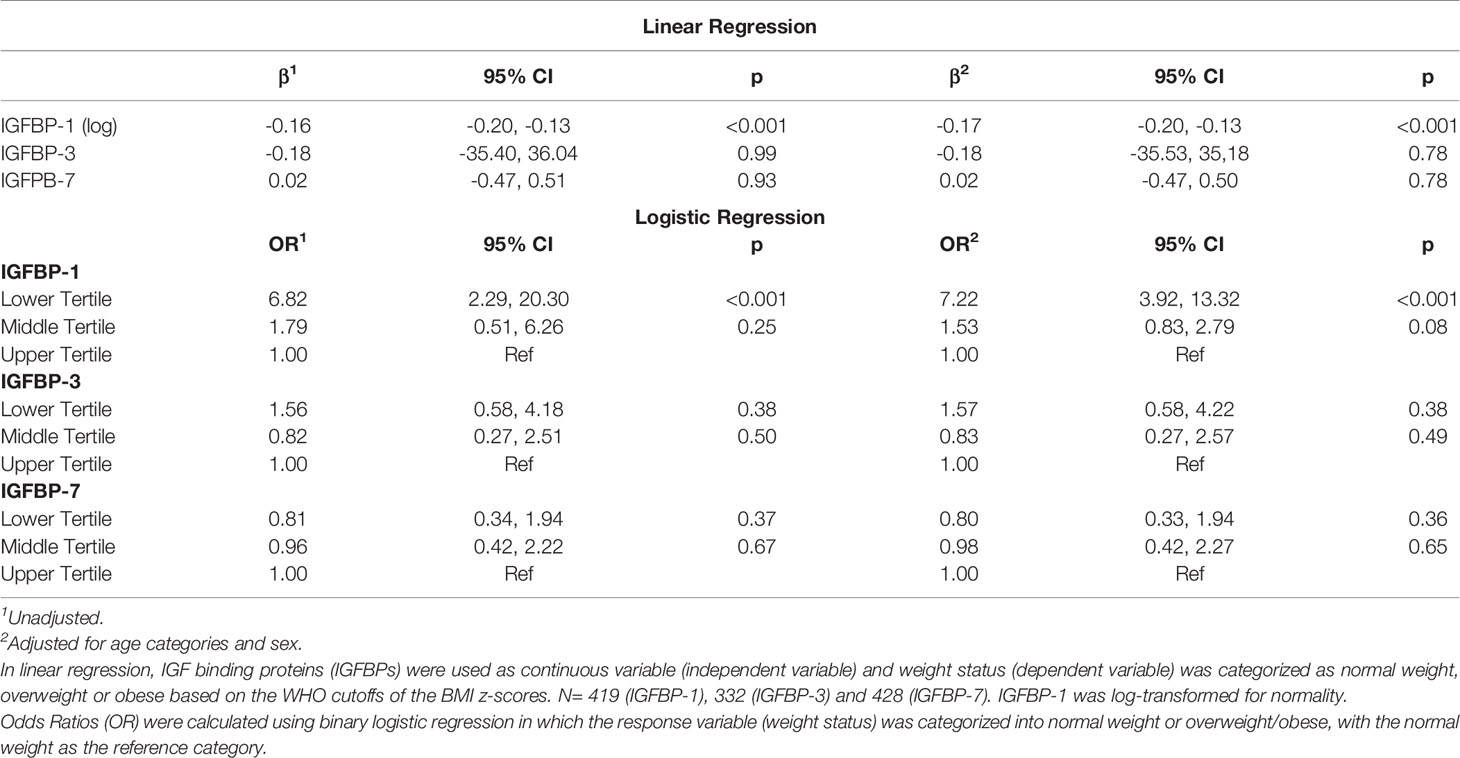
Association of IGFBPs and Weight Status
IGFBP-1 level was significantly lower in participants classified in the obese and overweight groups compared to normal weight children (p<0.01) (Figure 1C). No significant difference was observed in the levels of IGFBP-3 and IGFBP-7 when comparing different BMI categories (data not shown). Parallel to these results, the plasma level of IGFBP-1 was negatively associated with weight status (BMI categories) in univariable regression analysis [β (95% CI) = -0.16 (-0.20, -0.13)]. This association remained significant in the multivariable regression when adjusted for age and sex [β (95% CI) = -0.17 (-0.20, -0.13)]. The association of weight status with either IGFBP-3 or IGFBP-7 plasma levels was not significant either in multivariable regression, or in the adjusted multivariable regression (Table 2). In binary logistic regression, the odds of being overweight/obese (combined category) were significantly higher in the lower tertile of IGFBP-1 level when compared to the upper tertile (reference) [(OR (95% CI) = 6.82 (2.29, 20.30)] in the unadjusted model and this association sustained in the model adjusted for age and sex [(OR (95% CI) = 7.22 (3.92, 13.32)] (Table 2). IGFBP-3 and IGFBP-7 tertiles did not show significant association with overweight/obesity in binary logistic regression models, either unadjusted or adjusted.
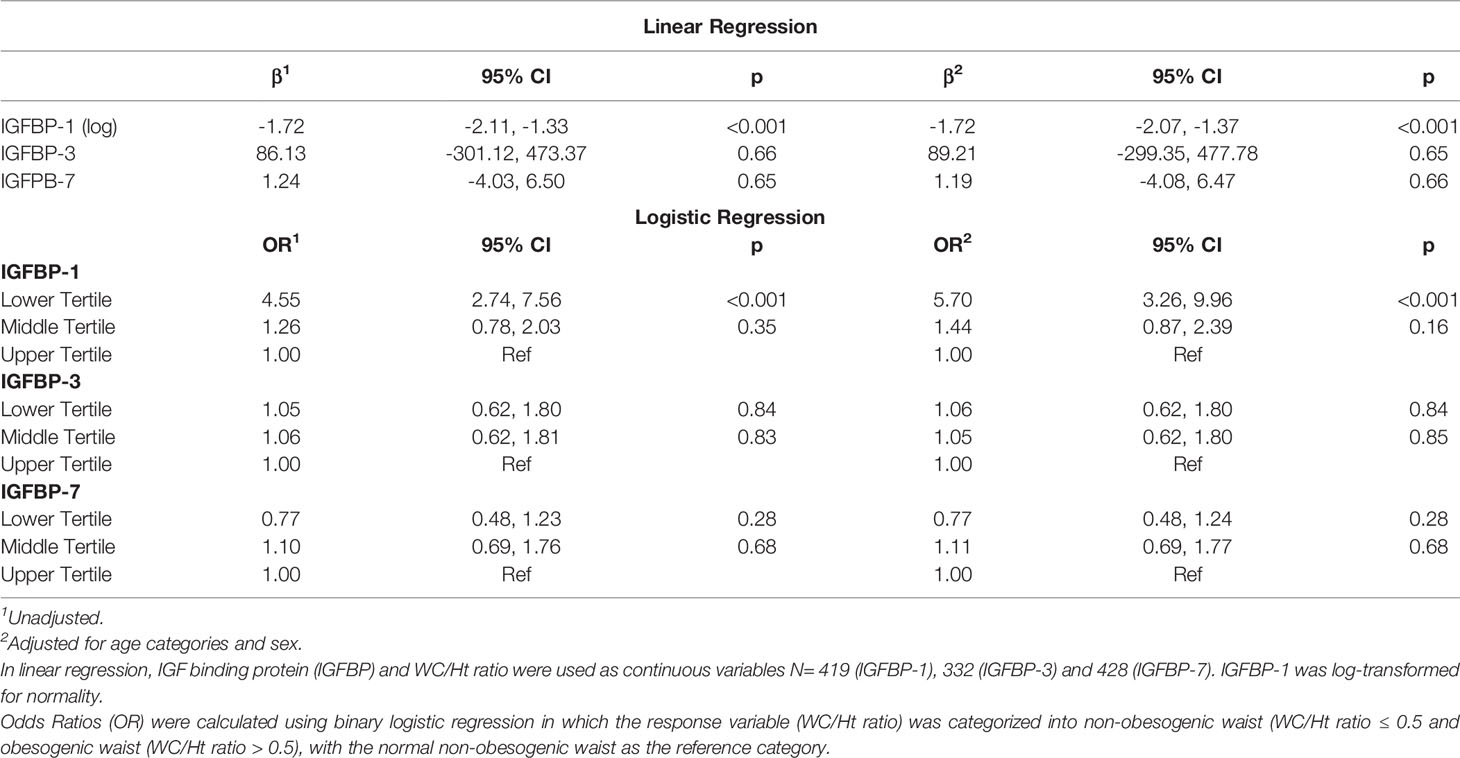
Association of IGFBPs With Waist Circumference
In the univariable regression analysis, the level of IGFBP-1 was negatively associated with WC/Ht ratio [β (95% CI) = -1.72 (-2.11, -1.33); p<0.001], and this association remained significant after adjusting for age and sex [β (95% CI) = -1.72 (-2.07, -1.37), p<0.001]. IGFBP-3 and IGFBP-7 levels did not show significant association with WC/Ht ratio either in the univariable or multivariable regression (Table 3). In the binary logistic regression, the odds of obesogenic waist, defined as a WC/Ht ratio of > 0.5, was significantly higher in the lower tertile of IGFBP-1 level when compared to the higher tertile [OR (95% CI) = 4.55 (2.74, 7.56)] in the unadjusted model. This remained significant in the model adjusted for age and sex [OR (95% CI) = 5.70 (3.26, 9.96)]. The tertiles of IGFBP-3 and IGFBP-7 levels did not show significant association with obesogenic waist in binary logistic regression models, whether they were adjusted or unadjusted (Table 3). When adjusted for age and sex, the negative association of IGFBP-1 level (log-transformed) with WC/Ht ratio [β (95% CI) = -1.73 (-2.08, -1.38)] was stronger than its association with the BMI-for-age z-scores [β (95% CI) = -0.11 (-0.13, -0.09)].
Association of IGFBPs With Markers of Oxidative Stress
In linear regression analysis, plasma level of Ox-LDL was not associated with IGFBP-1 level (β=0.06; p=0.40). However, the level of Ox-LDL was negatively associated with the levels of IGFBP-3 (β=-0.18; p=0.001) and IGFBP-7 (β=-0.02; p<0.001) (Figures 2A–C). On the other hand, plasma level of Hs-CRP was negatively associated with the level of IGFBP-1 (β=-0.30; p=0.001). No significant association was found between the level of Hs-CRP with either the level of IGFBP-3 or the level of IGFBP-7 in plasma (Figures 2D–F). We further analyzed mean differences of Ox-LDL and Hs-CRP levels across different tertiles of the levels of IGFBPs. The results are shown in Figure 3. The level of Ox-LDL was significantly higher in the lower tertiles of IGFBP-3 and IGFBP-7 levels compared to the middle and higher tertiles, whereas no differences were observed between Ox-LDL level in the middle and upper tertiles (Figures 3B, C). The level of Ox-LDL across the three tertiles of the level of IGFBP-1 was not significantly different (Figure 3A). On the other hand, the level of Hs-CRP across the three tertiles of IGFBP-3 and IGFBP-7 levels was not significantly different (Figures 3E, F). However, the level of Hs-CRP was significantly higher in the lower tertile of IGFBP-1 level when compared to the middle and upper tertiles. Furthermore, the level of Hs-CRP was not significantly different between the middle and upper tertiles of IGFBP-1 level (Figure 3D).
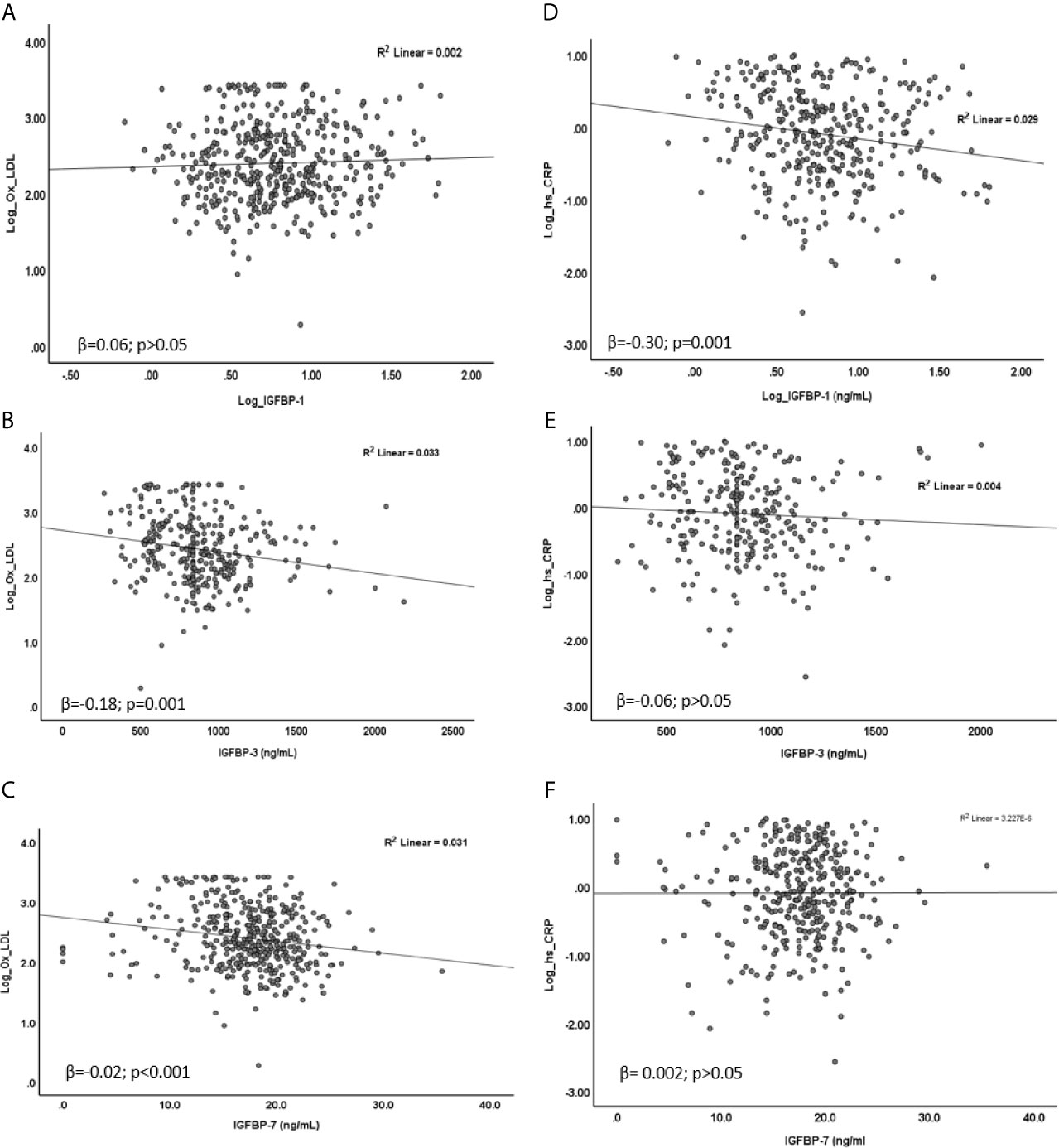
Figure 2 Association of Ox-LDL (A–C) and Hs-CRP (D–F) with IGFBPs. (A) Ox-LDL was not associated with IGFBP-1 (β = 0.06; p = 0.40), (B, C) Ox-LDL was negatively associated with IGFBP-3 (β = -0.18; p = 0.001) and IGFBP-7 (β = -0.02; p < 0.001). (D) Hs-CRP was negatively associated with IGFBP-1 (β = -0.30; p = 0.001) (E, F) No significant association of Hs-CRP was found with either IGFBP-3 (β = -0.06; p > 0.05) or IGFBP-7 (β = 0.002; p > 0.05). Data were analyzed by linear regression.
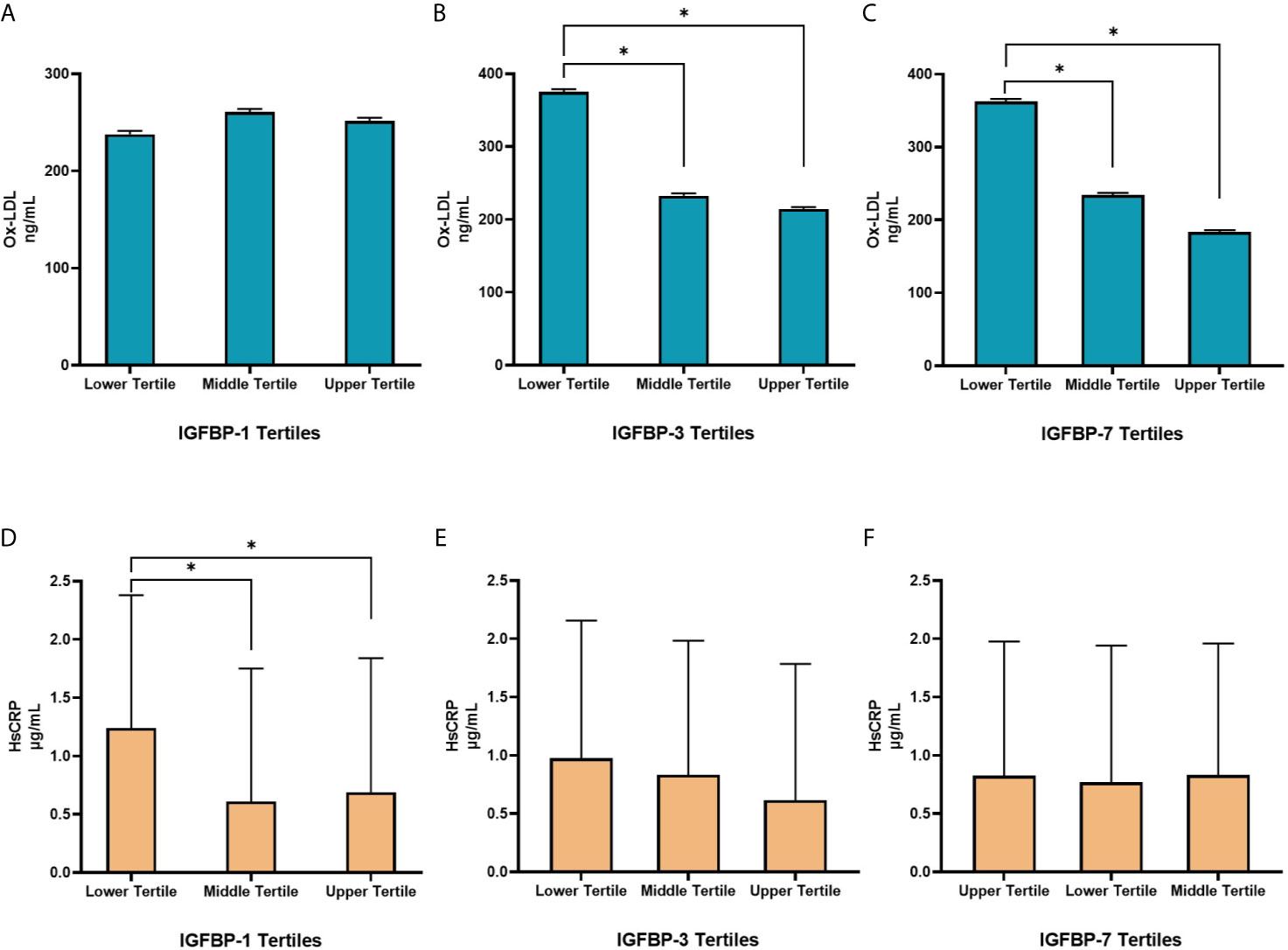
Figure 3 Distribution of Ox-LDL (A–C) and Hs-CRP (D–F) in different tertiles of IGFBPs. (A) Levels of Ox-LDL across the three tertiles of IGFBP-1 were not significantly different. (B, C) The levels of Ox-LDL were significantly higher in the lower tertiles of IGFBP-3 and IGFBP-7 compared to the middle and higher tertiles, no differences were observed between Ox-LDL levels in the middle and upper tertiles. (D) Levels of Hs-CRP were significantly higher in the lower tertile of IGFBP-1 compared to the middle and upper tertiles but were not different between the middle and upper tertiles. (E, F) Levels of Hs-CRP across the three tertiles of IGFBP-3 and IGFBP-7 were not significantly different. N=144 in each group. Data is presented as geometric means ± SD. *p < 0.01 by one way ANOVA with Bonferroni post-hoc comparison.
Discussion
In this study we investigated the association between obesity and the levels of various IGFBPs (particularly, IGFBP-1, 3 and 7) in a group of Kuwaiti adolescents. Our findings show that the plasma level of IGFBP-1 was decreased in children who were classified in the overweight or obese group. On the other hand, plasma levels of IGFBP-3 and IGFBP-7 were not affected. Furthermore, the level of IGFBP-1 was negatively associated with WC/Ht ratio. This association was stronger than the association of IGFBP-1 level with BMI-for-age z-scores. Interestingly, a strong negative association between the level of IGFBP-1 and Hs-CRP was also observed.
Childhood obesity is on the rising trajectory, especially in the Gulf region. Early predictors of obesity complications can contribute to the reduction in obesity-related metabolic consequences. Upper body obesity (truncal obesity) has been consistently shown to be more strongly associated with obesity-related comorbidities like diabetes and CVD. Thus, we investigated the association of truncal obesity, measured by WC/Ht ratio, with IGFBPs. Only IGFBP-1 was lower in the children with obesogenic WC/Ht ratio and the other two IGFBPs were not different in the two categories of WC/Ht ratio. To our knowledge, this is the first report to specifically correlate central obesity with IGFBP-1. The overall pattern of association was similar whether we categorized the study group based on weight using BMI or based on WC/Ht ratio. However, this correlation is stronger with WC/Ht ratio when compared to the BMI-for-age z-scores, which suggests that central body adiposity has a stronger influence on the level of IGFBP-1 than the overall body adiposity. Several other studies has supported this notion that central adiposity is more strongly associated with the adverse health consequences of obesity (38–41) and that WC/Ht ratio is a better indicator of adiposity than BMI (42–44).
Hs-CRP and Ox-LDL are well established cardiometabolic risk factors. In this study we found a differential association pattern between the level of the various IGFBPs under study with markers of oxidative stress (Ox-LDL) and cardiovascular diseases (Hs-CRP). For instance, the level of IGFBP-1 showed a negative association with Hs-CRP but not Ox-LDL. On the other hand, the levels of both IGFBP-3 and 7 were significantly associated with Ox-LDL but not Hs-CRP. Obesity is a state of low-level chronic inflammation. Thus, high Hs-CRP level in individuals living with overweight or obesity is consistent with this notion. The functional as well as the causal relationship between IGFBP-1 and Hs-CRP could not be deduced from this study. Therefore, a prospective study will possibly help delineate if the increased level of Hs-CRP are the cause or the consequence of lower level of IGFBP-1 in children with obesity.
A study conducted in Kuwait reported significantly lower levels of IGF-1 and IGFBP-3 in patients with coronary heart disease (CHD) and significant correlation between the level of IGFBP-3 and some metabolic markers including cholesterol, triglyceride (TG) and high-density lipoprotein (HDL) (45). Recently, a cross-sectional observational study conducted on 84 children under 10 years of age from two schools in Colombia demonstrated an inverse correlation of both IGFBP-1 and IGFBP-2 levels with the level of TG, as well as a direct correlation with HDL level (46). The study also reported lower level of IGFBP-1 with obesity. However, to the best of our knowledge, this is the first study to report an association of IGFBPs with Hs-CRP and Ox-LDL in adolescents. This is an important finding, since both markers are essential for detecting low inflammatory processes. It was interesting to observe that while the levels of both IGFBP-3 and -7 were not affected by obesity, they correlated negatively with Ox-LDL level. This suggests that the role of some IGFBPs in mediating inflammation and CVD can be independent of weight status and body fat composition. Further investigations are required to understand the role that both IGFBP-3 and -7 play in the development of CVDs. Our data further confirm that the different IGFBPs have distinct functional significance.
In this study the level of IGFBP-1 was negatively associated with age, which was not observed with the levels of both IGFBP-3 and 7. Although the age range of the study participants was very narrow (11-14 years), the difference in the level of IGFBP-1 was significant among various age groups. This suggests that IGFBP-1 is a sensitive biomarker when compared to the other IGFBPs. The age-dependent changes in IGFBP-1 level could be explained by changes in either body fat composition or in the level of sex hormones. The level of sex hormones is also affected by body fat composition. Therefore, body fat composition appears to be the major common determinant of IGFBP-1 level. The lower level of IGFBP-1 in girls compared to boys can also be explained by body fat composition, as girls generally have higher body fat percentage compared to boys at this age. However, the effect of changes in hormones should not be ruled out. Contrary to our results presented in this study, a Turkish group reported that serum level of IGFBP-3 are steadily increased in prepubertal children with age (47). However, due to lack of information regarding the pubertal stage of the subjects enrolled in our study, a direct comparison cannot be made. Nonetheless, such discrepancies emphasize the importance of personalized medicine and group-specific interventions. Since therapies that could work with children from Turkish descendent might not necessarily work on children from Kuwaiti (Arab) descendent. Therefore, further studies that cover a larger population with a wider age range are necessary to determine the exact interplay between growth, the level of sex hormones, body fat composition and the levels of various IGFBPs.
To our knowledge, this is the first study that investigated the association of IGFBPs with markers of inflammation and oxidative stress (Hs-CRP and Ox-LDL). Also, for the first time, we investigated the association of IGFBPs with a measure of central body adiposity. Most studies in this field use BMI as a measure of adiposity. Although BMI is generally a good indicator of overall body fat composition, it does not discriminate between upper and lower body fat composition. It is well documented that upper body fat composition (central adiposity) is more closely associated with obesity-related comorbidities. Therefore, the fact that IGFBP-1 was more strongly associated with WC/Ht ratio than the BMI z-scores makes IGFBP-1 a sensitive biomarker for obesity-related metabolic abnormalities. Other strengths of this study include a large sample size, which was representative of the adolescent population in Kuwait, using several statistical approaches to minimize the bias in the association, and the narrow age range of the study subjects. The latter is particularly important, as it will minimize the age-dependent influence on the association between obesity and IGFBPs. Furthermore, this study is based on healthy subjects without any overt obesity-related complications. Thus it highlights the possible use of IGFBP-1 as a screening marker for metabolic disorders. However, the limitations of this study include, the cross-sectional design, which does not allow us to establish causality, and the narrow age range of the participants, which could be considered as both a strength and a limitation. The limitation could result from the inability to project a similar association between IGFBP-1 and obesity into other age groups. Finally, due to logistic restrictions, a fasting blood sample could not be obtained, and thus data on parameters of glucose homeostasis and lipid profile are lacking.
In conclusion, the study results presented demonstrate the importance of IGFBPs in childhood obesity and highlight the distinct functions of the different members of the IGFBPs family. Since, a strong negative association was detected between the level of IGFBP-1 with overweight and obesity in adolescents. This negative association was shown to be more pronounced with central adiposity when compared to overall increased body weight. Furthermore, IGFBP-1 was negatively associated with Hs-CRP, which is a marker of inflammation. On the other hand, the levels of both IGFBP-3 and 7 were not associated with body weight. Nevertheless, the levels of these proteins showed a significantly negative association with the level of Ox-LDL, which is a marker of oxidative stress. Together these data further illustrate the possibility of using IGFBP-1 as a sensitive marker for obesity-related inflammation and its related comorbidities. The identification of such markers, especially in younger subjects, is a step towards the advancement in the field of Predictive, Preventive and Personalized Medicine.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by The Ethics Committee at Ministry of Health, Kuwait (No: 2015/248), The Ethics Committee of the Health Sciences Centre, Kuwait University (No: DR/EC/2338), The Ethical Review Committee at Dasman Diabetes Institute (RA2017-026). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
Conceptualization, AR, MA-F, and JA. Methodology, MH, IA, and PC. Software, AR. Validation, MH, IA, and PC. Formal analysis, AR. Investigation, AR, MA-F, and JA. Resources, AR, MA-F, and JA. Data curation, AR, MH, IA, and PC. Writing—original draft preparation, MH and AR. Writing—review and editing, AR, MH, RA-S., FA-M, MA-F, and JA. Supervision, MA-F and JA. Project administration, AR, MA-F, and JA. Funding acquisition, AR, MA-F, and JA. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Dasman Diabetes Institute project No. RA 2017-026 and Kuwait University Project No. WF02/13.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Al Hammadi H, Reilly J. Prevalence of Obesity Among School-Age Children and Adolescents in the Gulf Cooperation Council (GCC) States: A Systematic Review. BMC Obes (2019) 6:3. doi: 10.1186/s40608-018-0221-5
2. Weiderpass E, Botteri E, Longenecker JC, Alkandari A, Al-Wotayan R, Al Duwairi Q, et al. The Prevalence of Overweight and Obesity in an Adult Kuwaiti Population in 2014. Front Endocrinol (Lausanne) (2019) 10:449. doi: 10.3389/fendo.2019.00449
3. Ministry of Health K. Kuwait_Nutrition_Surveillance_System: 2017 Annual Report. Kuwait, Ministry of Health, 2017 (2017) (Accessed 2 Jul 2019).
4. Alrashidi M, Shahwan-Akl L, James J, Jones L. Contributing Factors to Childhood Overweight and Obesity in Kuwait. American Research Institute for Policy DevelopmentInternational Journal of Health Sciences. (2015) 3:133–55. doi: 10.15640/ijhs.v3n1a8
5. Kansra AR, Lakkunarajah S, Jay MS. Childhood and Adolescent Obesity: A Review. Front Pediatr (2020) 8:581461. doi: 10.3389/fped.2020.581461
6. Carr MC, Brunzell JD. Abdominal Obesity and Dyslipidemia in the Metabolic Syndrome: Importance of Type 2 Diabetes and Familial Combined Hyperlipidemia in Coronary Artery Disease Risk. J Clin Endocrinol Metab (2004) 89(6):2601–7. doi: 10.1210/jc.2004-0432
7. Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and Cardiovascular Disease: Pathophysiology, Evaluation, and Effect of Weight Loss: An Update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease From the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation (2006) 113(6):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016
8. Weiss R, Kaufman FR. Metabolic Complications of Childhood Obesity: Identifying and Mitigating the Risk. Diabetes Care (2008) 31 Suppl 2:S310–6. doi: 10.2337/dc08-s273
9. Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, et al. Medicine in the Early Twenty-First Century: Paradigm and Anticipation - EPMA Position Paper 2016. EPMA J (2016) 7(1):23. doi: 10.1186/s13167-016-0072-4
10. Golubnitschaja O, Kinkorova JF, Costigliola V, Costigliola V. Predictive, Preventive and Personalised Medicine as the Hardcore of ‘Horizon 2020’: EPMA Position Paper. EPMA J (2014) 5(1):6. doi: 10.1186/1878-5085-5-6
11. Bubnov RA-O, Babenko L, Lazarenko L, Kryvtsova M, Shcherbakov O, Zholobak N, et al. Can Tailored Nanoceria Act as a Prebiotic? Report on Improved Lipid Profile and Gut Microbiota in Obese Mice. EPMA J (2019) 10(4):317–35. doi: 10.1007/s13167-019-00190-1
12. Haywood NJ, Slater TA, Matthews CJ, Wheatcroft SB. The Insulin Like Growth Factor and Binding Protein Family: Novel Therapeutic Targets in Obesity & Diabetes. Mol Metab (2019) 19:86–96. doi: 10.1016/j.molmet.2018.10.008
13. Firth SM, Baxter RC. Cellular Actions of the Insulin-Like Growth Factor Binding Proteins. Endocr Rev (2002) 23(6):824–54. doi: 10.1210/er.2001-0033
14. Allard JB, Duan C. IGF-Binding Proteins: Why Do They Exist and Why Are There So Many? Front Endocrinol (Lausanne) (2018) 9:117. doi: 10.3389/fendo.2018.00117
15. Holly J, Perks C. The Role of Insulin-Like Growth Factor Binding Proteins. Neuroendocrinology (2006) 83(3-4):154–60. doi: 10.1159/000095523
16. Frystyk J, Vestbo E, Skjaerbaek C, Skjaerbaek C, Mogensen CE, Mogensen Ce, et al. Free Insulin-Like Growth Factors in Human Obesity. Metabolism (1995) 44(10 Suppl4):37–44. doi: 10.1016/0026-0495(95)90219-8
17. Fontana L, Villareal DT, Das SK, Smith SR, Meydani SN, Pittas AG, et al. Effects of 2-Year Calorie Restriction on Circulating Levels of IGF-1, IGF-Binding Proteins and Cortisol in Nonobese Men and Women: A Randomized Clinical Trial. Aging Cell (2016) 15(1):22–7. doi: 10.1111/acel.12400
18. Song Z, Dai X, Yu H, Luo Q, Zhang H, Wu LA-O. Increased Serum IGFBP-1 and Reduced Insulin Resistance After Roux-En-Y Gastric Bypass in Chinese Patients With Type 2 Diabetes: A 6-Month Follow-Up. Obes Surg (2018) 28(10):3165–71. doi: 10.1007/s11695-018-3242-8
19. Heald AH, Cruickshank JK, Riste LK, Cade JE, Anderson S, Greenhalgh A, et al. Close Relation of Fasting Insulin-Like Growth Factor Binding Protein-1 (IGFBP-1) With Glucose Tolerance and Cardiovascular Risk in Two Populations. Diabetologia (2001) 44(3):333–9. doi: 10.1007/s001250051623
20. Gokulakrishnan K, Velmurugan K, Ganesan S, Mohan V. Circulating Levels of Insulin-Like Growth Factor Binding Protein-1 in Relation to Insulin Resistance, Type 2 Diabetes Mellitus, and Metabolic Syndrome (Chennai Urban Rural Epidemiology Study 118). Metabolism (2012) 61(1):43–6. doi: 10.1016/j.metabol.2011.05.014
21. Liew CF, Wise SD, Yeo KP, Lee KO. Insulin-Like Growth Factor Binding Protein-1 is Independently Affected by Ethnicity, Insulin Sensitivity, and Leptin in Healthy, Glucose-Tolerant Young Men. J Clin Endocrinol Metab (2005) 90(3):1483–8. doi: 10.1210/jc.2004-1501
22. Lewitt MS, Hilding A, Ostenson CG, Efendic S, Brismar K, Hall K. Insulin-Like Growth Factor-Binding Protein-1 in the Prediction and Development of Type 2 Diabetes in Middle-Aged Swedish Men. Diabetologia (2008) 51(7):1135–45. doi: 10.1007/s00125-008-1016-x
23. Petersson U, Ostgren CJ, Brudin L, Brismar K, Nilsson PM. Low Levels of Insulin-Like Growth-Factor-Binding Protein-1 (IGFBP-1) are Prospectively Associated With the Incidence of Type 2 Diabetes and Impaired Glucose Tolerance (IGT): The Soderakra Cardiovascular Risk Factor Study. Diabetes Metab (2009) 35(3):198–205. doi: 10.1016/j.diabet.2008.11.003
24. Lewitt MS, Hilding A, Brismar K, Efendic S, Ostenson CG, Hall K. IGF-Binding Protein 1 and Abdominal Obesity in the Development of Type 2 Diabetes in Women. Eur J Endocrinol (2010) 163(2):233–42. doi: 10.1530/EJE-10-0301
25. Rajpathak SN, McGinn AP, Strickler HD, Rohan TE, Pollak M, Cappola AR, et al. Insulin-Like Growth Factor-(IGF)-Axis, Inflammation, and Glucose Intolerance Among Older Adults. Growth Horm IGF Res (2008) 18(2):166–73. doi: 10.1016/j.ghir.2007.08.004
26. Frystyk J, Brick DJ, Gerweck AV, Utz AL, Miller KK. Bioactive Insulin-Like Growth Factor-I in Obesity. J Clin Endocrinol Metab (2009) 94(8):3093–7. doi: 10.1210/jc.2009-0614
27. Rajpathak SN, He M, Sun Q, Kaplan RC, Muzumdar R, Rohan TE, et al. Insulin-Like Growth Factor Axis and Risk of Type 2 Diabetes in Women. Diabetes (2012) 61(9):2248–54. doi: 10.2337/db11-1488
28. Lopez-Bermejo A, Khosravi J, Fernandez-Real JM, Hwa V, Pratt KL, Casamitjana R, et al. Insulin Resistance Is Associated With Increased Serum Concentration of IGF-Binding Protein-Related Protein 1 (IGFBP-Rp1/MAC25). Diabetes (2006) 55(8):2333–9. doi: 10.2337/db05-1627
29. Gu HF, Gu T, Hilding A, Zhu Y, Karvestedt L, Ostenson CG, et al. Evaluation of IGFBP-7 DNA Methylation Changes and Serum Protein Variation in Swedish Subjects With and Without Type 2 Diabetes. Clin Epigenet (2013) 5(1):20. doi: 10.1186/1868-7083-5-20
30. Shao L, Huang Q, Luo M, Lai M. Detection of the Differentially Expressed Gene IGF-Binding Protein-Related Protein-1 and Analysis of Its Relationship to Fasting Glucose in Chinese Colorectal Cancer Patients. Endocr Relat Cancer (2004) 11(1):141–8. doi: 10.1677/erc.0.0110141
31. Liu Y, Wu M, Ling J, Cai L, Zhang D, Gu HF, et al. Serum IGFBP7 Levels Associate With Insulin Resistance and the Risk of Metabolic Syndrome in a Chinese Population. Sci Rep (2015) 5:10227. doi: 10.1038/srep10227
32. Lisowska A, Swiecki P, Knapp M, Gil M, Musial WJ, Kaminski K, et al. Insulin-Like Growth Factor-Binding Protein 7 (IGFBP 7) as a New Biomarker in Coronary Heart Disease. Adv Med Sci (2019) 64(1):195–201. doi: 10.1016/j.advms.2018.08.017
33. Mogul HR, Marshall M, Frey M, Burke HB, Wynn PS, Wilker S, et al. Insulin Like Growth Factor-Binding Protein-1 as a Marker for Hyperinsulinemia in Obese Menopausal Women. J Clin Endocrinol Metab (1996) 81(12):4492–5. doi: 10.1210/jcem.81.12.8954066
34. Al-Taiar A, Rahman A, Al-Sabah R, Shaban L, Al-Harbi A. Vitamin D Status Among Adolescents in Kuwait: A Cross-Sectional Study. BMJ Open (2018) 8(7):e021401. doi: 10.1136/bmjopen-2017-021401
35. Hammad MM, Abu-Farha M, Al-Taiar A, Alam-Eldin N, Al-Sabah R, Shaban L, et al. Correlation of Circulating ANGPTL5 Levels With Obesity, High Sensitivity C-Reactive Protein and Oxidized Low-Density Lipoprotein in Adolescents. Sci Rep (2020) 10(1):6330. doi: 10.1038/s41598-020-63076-7
36. Rahman A, Al-Taiar A, Shaban L, Al-Sabah R, Al-Harbi A, Mojiminiyi O. Plasma 25-Hydroxy Vitamin D Is Not Associated With Either Cognitive Function or Academic Performance in Adolescents. Nutrients (2018) 10(9):1997–210. doi: 10.3390/nu10091197
37. Gibson S, Ashwell M. A Simple Cut-Off for Waist-to-Height Ratio (0.5) can Act as an Indicator for Cardiometabolic Risk: Recent Data From Adults in the Health Survey for England. Br J Nutr (2020) 123(6):681–90. doi: 10.1017/S0007114519003301
38. Cox BD, Whichelow M. Ratio of Waist Circumference to Height Is Better Predictor of Death Than Body Mass Index. Bmj (1996) 313(7070):1487. doi: 10.1136/bmj.313.7070.1487
39. Thomas GN, McGhee SM, Schooling M, Ho SY, Lam KSL, Janus ED, et al. Impact of Sex-Specific Body Composition on Cardiovascular Risk Factors: The Hong Kong Cardiovascular Risk Factor Study. Metabolism (2006) 55(5):563–9. doi: 10.1016/j.metabol.2005.08.004
40. Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, et al. General and Abdominal Adiposity and Risk of Death in Europe. N Engl J Med (2008) 359(20):2105–20. doi: 10.1056/NEJMoa0801891
41. Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal Obesity and the Risk of All-Cause, Cardiovascular, and Cancer Mortality: Sixteen Years of Follow-Up in US Women. Circulation (2008) 117(13):1658–67. doi: 10.1161/CIRCULATIONAHA.107.739714
42. Schneider HJ, Friedrich N, Klotsche J, Pieper L, Nauck M, John U, et al. The Predictive Value of Different Measures of Obesity for Incident Cardiovascular Events and Mortality. J Clin Endocrinol Metab (2010) 95(4):1777–85. doi: 10.1210/jc.2009-1584
43. Ashwell M, Lejeune S, McPherson K. Ratio of Waist Circumference to Height May Be Better Indicator of Need for Weight Management. Bmj (1996) 312(7027):377. doi: 10.1136/bmj.312.7027.377
44. Hsieh SD, Yoshinaga H, Muto T. Waist-To-Height Ratio, a Simple and Practical Index for Assessing Central Fat Distribution and Metabolic Risk in Japanese Men and Women. Int J Obes Relat Metab Disord: J Int Assoc Study Obes (2003) 27(5):610–6. doi: 10.1038/sj.ijo.0802259
45. Akanji AO, Suresh CG, Al-Radwan R, Fatania HR. Insulin-Like Growth Factor (IGF)-I, IGF-II and IGF-Binding Protein (IGFBP)-3 Levels in Arab Subjects With Coronary Heart Disease. Scand J Clin Lab Invest (2007) 67(5):553–9. doi: 10.1080/00365510601173153
46. Vera S, Figueroa T, Aranzalez LH, Mockus I. Cardiovascular Disease Risk Markers in Children Under 10 Years of Age and Their Relationship With Serum Concentrations of IGF-1, IGFBP-1, IGFBP-2 and IGFBP-3. Rev la Facultad Med (2020) 68(1):51–8. doi: 10.15446/revfacmed.v68n1.69979
Keywords: adolescents, high sensitivity C-reactive protein, insulin-like growth factor binding proteins, obesity, oxidized low-density lipoprotein
Citation: Rahman A, Hammad MM, Al Khairi I, Cherian P, Al-Sabah R, Al-Mulla F, Abu-Farha M and Abubaker J (2021) Profiling of Insulin-Like Growth Factor Binding Proteins (IGFBPs) in Obesity and Their Association With Ox-LDL and Hs-CRP in Adolescents. Front. Endocrinol. 12:727004. doi: 10.3389/fendo.2021.727004
Received: 17 June 2021; Accepted: 15 July 2021;
Published: 28 July 2021.
Edited by:
Giulio Maltoni, Sant’Orsola-Malpighi Polyclinic, ItalyReviewed by:
Jelena Kotur-Stevuljević, University of Belgrade, SerbiaAdriana Franzese, University of Naples Federico II, Italy
Copyright © 2021 Rahman, Hammad, Al Khairi, Cherian, Al-Sabah, Al-Mulla, Abu-Farha and Abubaker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jehad Abubaker, jehad.abubakr@dasmaninstitute.org; Mohamed Abu-Farha, mohamed.abufarha@dasmaninstitute.org; mafarha@gmail.com
†These authors have contributed equally to this work and share first authorship
 Abdur Rahman
Abdur Rahman Maha M. Hammad
Maha M. Hammad Irina Al Khairi
Irina Al Khairi Preethi Cherian
Preethi Cherian Reem Al-Sabah
Reem Al-Sabah Fahd Al-Mulla
Fahd Al-Mulla Mohamed Abu-Farha
Mohamed Abu-Farha Jehad Abubaker
Jehad Abubaker