- 1Reproductive Medicine Center, Henan Provincial People’s Hospital, People’s Hospital of Zhengzhou University, Zhengzhou, China
- 2Reproductive Medicine Center, Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology, Wuhan, China
- 3Reproductive Medicine Center, Jiangsu Provincial Hospital, Nanjing, China
- 4Reproductive Medicine Center, Northwest Women’s and Children’s Hospital, Xi’an, China
- 5Reproductive Medicine Center, Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
Objective: To investigate the effects of endometrial thickness (EMT) on pregnancy outcomes on hCG trigger day in fresh in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) cycles.
Methods: A total of 42,132 fresh cycles between 1 January 2013 and 31 December 2019 were included in this retrospective cohort study. Data were collected from five reproductive centers of large academic or university hospitals in China. All patients were divided into different groups according to their endometrial thickness on hCG trigger day. Multivariate regression analysis, curve fitting and threshold effect analysis were performed.
Results: After adjusting for age, body mass index, infertility type, number of embryos transferred, number of retrieved oocytes and COS (controlled ovarian stimulation) protocols, significant associations were found between endometrial thickness and clinical pregnancy rate (adjusted odds ratio [aOR]: 1.05; 95% confidence interval [CI]: 1.06–1.08, P < 0.0001), live birth rate (aOR: 1.04; 95% CI: 1.03–1.05, P < 0.0001) as well as miscarriage rate(aOR: 0.96; 95% CI: 0.94 – 0.98, P < 0.0001). When the endometrial thickness was less than 12mm, the clinical pregnancy rate and live birth rate were increased significantly by 10% and 9%(OR:1.10; 95%CI: 1.08-1.12, OR:1.09; 95%CI: 1.07-1.11), respectively, along with the increase of each millimeter increment of endometrial thickness. However, when the EMT ranged from 12-15 mm, were stable at the ideal level, that were not significantly associated with EMT growth. Additionally, clinical pregnancy rate and live birth rate were slightly reduced by 6% and 4% when EMT was ≥15mm. Meanwhile, the miscarriage rate was significantly declined by 8% (OR:0.92; 95%CI: 0.90-0.95)with each millimeter increment of EMT. And when EMT was thicker than 12mm, the miscarriage rate didn’t change any more significantly.
Conclusions: Endometrial thickness exhibits a curvilinear relationship with pregnancy outcomes in fresh embryo transfer cycles. Clinical pregnancy rate, live birth rate and miscarriage rate may achieve their optimal level when EMT ≥ 12 mm, but some adverse pregnancy outcomes would be observed when EMT ≥15 mm especially for clinical pregnancy.
Introduction
Steroid hormones secreted from the ovaries regulate cell division, differentiation and degeneration in human endometrium. A woman’s menstrual cycle is divided into menstrual phase, proliferation phase, and secretory phase (1). In the proliferation phases, the endometrium increases in preparation for embryo implantation (2). In the secretory phase, the functional layer of the endometrium thickens in preparation for embryo implantation (3).
Endometrial receptivity is widely accepted as one of the key factors affecting ART(assisted reproductive technology) outcomes. Generally, ultrasound examination is carried out as routine method of endometrium evaluation in IVF cycles in most reproductive medicine agencies due to its convenient and noninvasive property (4). Sonographic parameters, such as endometrial thickness (EMT), endometrial pattern, endometrial volume and endometrial or sub-endometrial blood flow are used to identify endometrial receptivity (5).
Previous studies have demonstrated that increased EMT on the day of embryo transfer (ET) resulted in better pregnancy outcomes after fresh cycles (6, 7). However, other studies showed that EMT on the day of ET is a poor predictor of IVF outcomes (8, 9). Although there have been numerous studies investigating the relationship between EMT and pregnancy outcomes, there is still no consensus. The inconsistency of the results may attribute to many potential confounders such as maternal age, stimulation protocol used, numbers of egg retrieved and embryos transferred.
Therefore, in order to investigate the relationship between EMT and pregnancy outcomes including clinical pregnancy rate, live birth rate, and miscarriage rate, we designed this study which included 42,132 cycles collected from five IVF centers in China between 1 January 2013 and 31 December 2019.
Materials and Methods
Study Cohort
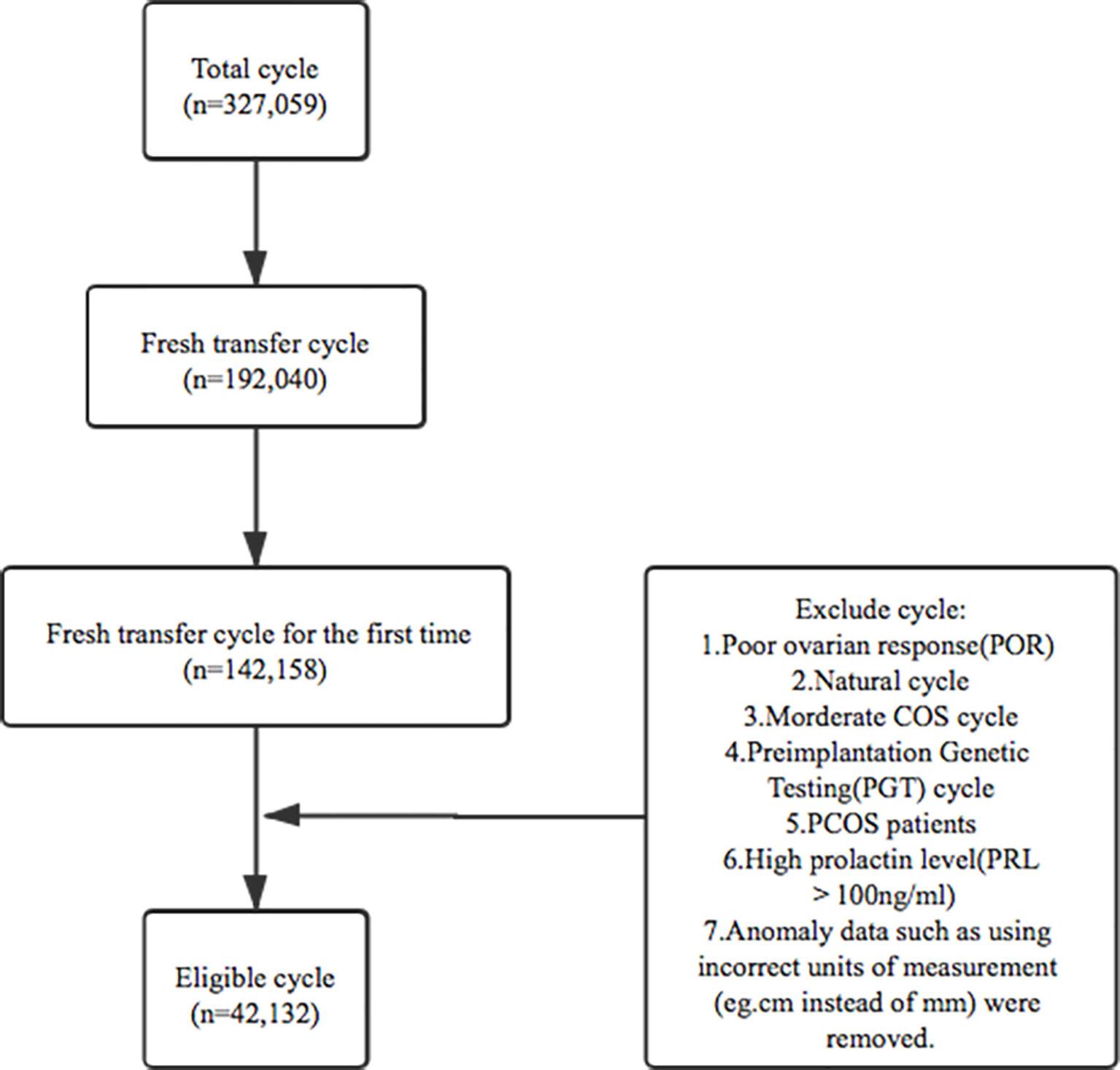
This study was a retrospective cohort analysis of five reproductive centers in university-affiliated hospitals or large academic hospitals in China including the Sixth Affiliated Hospital of Sun Yat-sen University, Henan Provincial People’s Hospital, Jiangsu Provincial People’s Hospital, Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology and Northwest Women’s and Children’s Hospital. The inclusion criteria were: 1. patients undertaken in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) between 1 January 2013 and 31 December 2019; 2. controlled ovarian stimulation (COS) cycles; 3. fresh transfer cycle for the first time; 4. endometrium was thicker than 5mm on hCG trigger day. Patients were excluded if they met any of these following criteria:1.poor ovarian response(POR) cycle; 2.natural cycle; 3.moderate COS cycle; 4. preimplantation genetic testing(PGT) cycle; 5. PCOS patients; 6.high prolactin level(PRL>100ng/ml); 7. some clinics reported that their input data using incorrect units of measurement (cm instead of mm), since it was not clear how many cycles had been affected, data from these clinics were removed. After the corresponding standard screening (listed in Figure 1), a number of 42,132 eligible fresh cycles between 1 January 2013 and 31 December 2019 were included.
The study was reviewed and approved separately by the ethical committees in each hospital, including Reproductive Medicine Ethics Committee of Henan Provincial People’s Hospital(SYSZ-LL-2019110401), Medical Ethics Committee of Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology(TJ-IRB20210320), Medicine Ethics Committee of Jiangsu Provincial People’s Hospital (2020-SR-046), Medical Ethics Committee of the Sixth Affiliated hospital of Sun Yat-sen University (2020ZSLYEC-295), and Medical Ethics Committee of Northwest Women’s and Children’s Hospital (2019013), and the Ethics Committee of People’s Hospital of Zhengzhou University.
Patient Data Collection
For fresh IVF/ICSI embryo transfer cycles, the EMT on the day of hCG trigger was recorded. Analysis was restricted to fresh embryo transfer cycles after ovarian stimulation in accordance with clinical practice rules at the five centers.
All patients were grouped according to the EMT on hCG trigger day. There were nine groups for EMT between 5mm to 15mm with a 1 mm interval between each group, while all the cycles with EMT≥15mm were grouped together. In total, this study held eleven different patient groups with varying levels of pregnancy outcomes.
There were three main outcomes of interest, clinical pregnancy rate, live birth rate and miscarriage rate. Clinical pregnancy was defined as the presence of a gestational sac on first trimester ultrasound. Live birth was defined as the delivery of a live baby after 24 weeks of gestation. Miscarriage was defined as clinical intrauterine pregnancy loss before 12 weeks of gestation.
Prior to embryo transfer, each embryo was graded based on developmental speed, degree of fragmentation and the evenness of cleavage sphere. The embryos with 7-9 blastomere, uniform cytoplasm, regular morphology, fragmentation < 10% were considered to be high-quality embryos. Blastocyst scoring was performed following the Gardner scoring system [8], and embryos graded 3BB or higher were defined as good quality embryos.
Outcome and Statistical Analysis
Clinical pregnancy rate, live birth rate and miscarriage rate were calculated per fresh ET cycle. The calculation formula used were: clinical pregnancy rate = number of cycles with clinical pregnancy/number of fresh ET cycles* 100%; live birth rate = number of cycles with live births/number of fresh ET cycles*100%; miscarriage rate = number of cycles with miscarriage/number of cycles with clinical pregnancy* 100%.
Statistical analysis was performed using the Empower Stats software base on R language. Continuous variables were presented as mean ± SD or median (Q1-Q3), and categorical variables were presented as N (%). Comparisons of these variables between groups were performed using the one-way ANOVA and χ2 tests for categorical variables. Multivariate regression analysis, curve fitting and threshold effect analysis were performed on all cycles. Confounders were selected on the basis of their association with the outcomes of interest or a change in effect estimate of 10%. Smooth curve fitting was performed to assess if there was any non-linear relationship between EMT and pregnancy outcomes. A piece wise linear regression method was used to analyze the threshold effect between EMT and pregnancy outcomes. The statistical significance was defined as a P-value less than 0.05 in the results.
Results
Baseline and Patient’s Characteristics
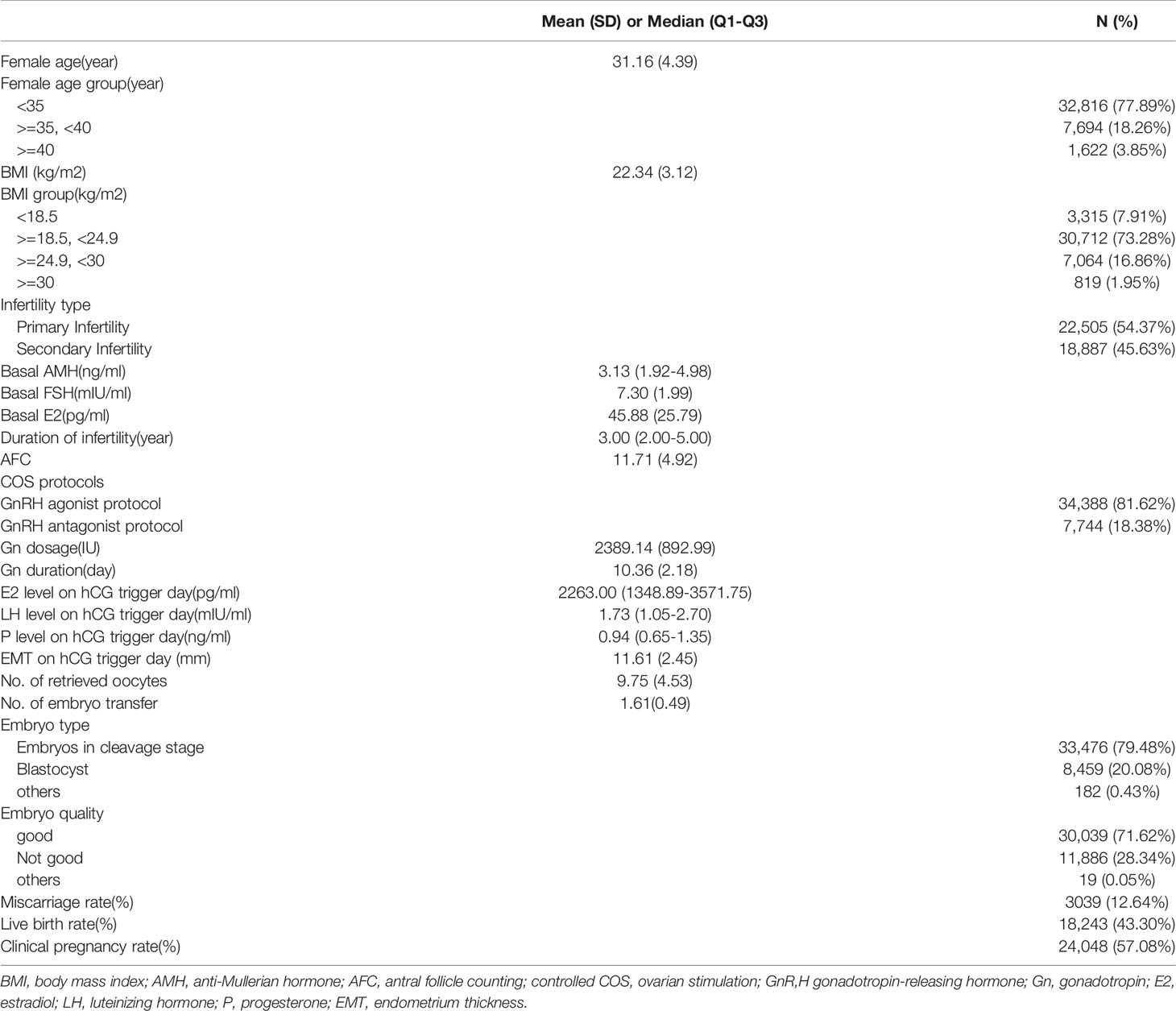
A total of 42,132 IVF/ICSI fresh embryo transfer cycles were included in our study. The baseline characteristics included female age, BMI, infertility type, basal AMH, basal FSH, basal E2, duration of infertility, AFC, COS protocols, Gn dosage, Gn duration, E2 level, LH level and P level on hCG trigger day, EMT, No. of oocytes retrieved, No. of embryos transferred, embryo type and embryo quality. The overall clinical pregnancy rate per embryo transfer cycle was 57.08% (24,048/42,132), the overall live birth rate per embryo transfer cycle was 43.30%(18,243/42,132), and the spontaneous abortion rate per pregnancy was 12.64% (3,039/24,048). Patient demographics and characteristics are shown in Table 1.
Comparison of Clinical Outcomes Between Different Groups
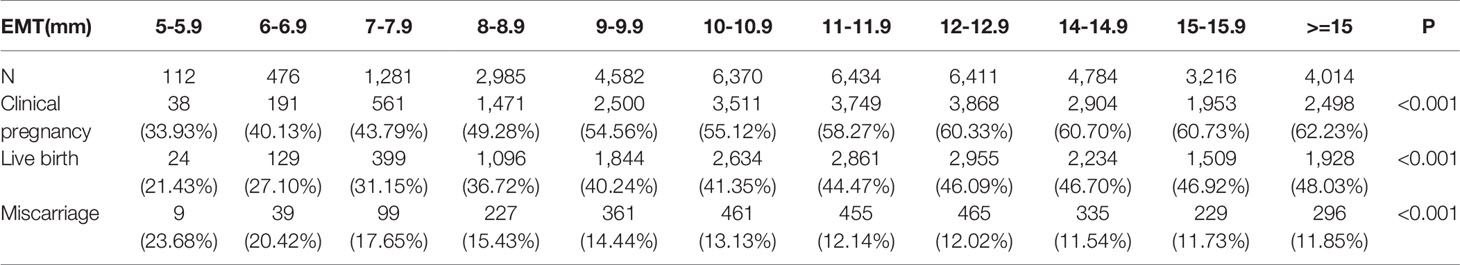
Patients were divided into eleven different groups according to their EMT on hCG trigger day. The clinical pregnancy rate and live birth rate were significantly different among groups with different EMT (P < 0.001). It was noteworthy that with the increased EMT, the percentage of clinical pregnancy and live birth also increased. Whereas, there was no significant difference in the miscarriage rate among groups (P > 0.05). The information was detailed in Table 2.
Univariate Analysis of Factors Associated With Clinical Outcomes
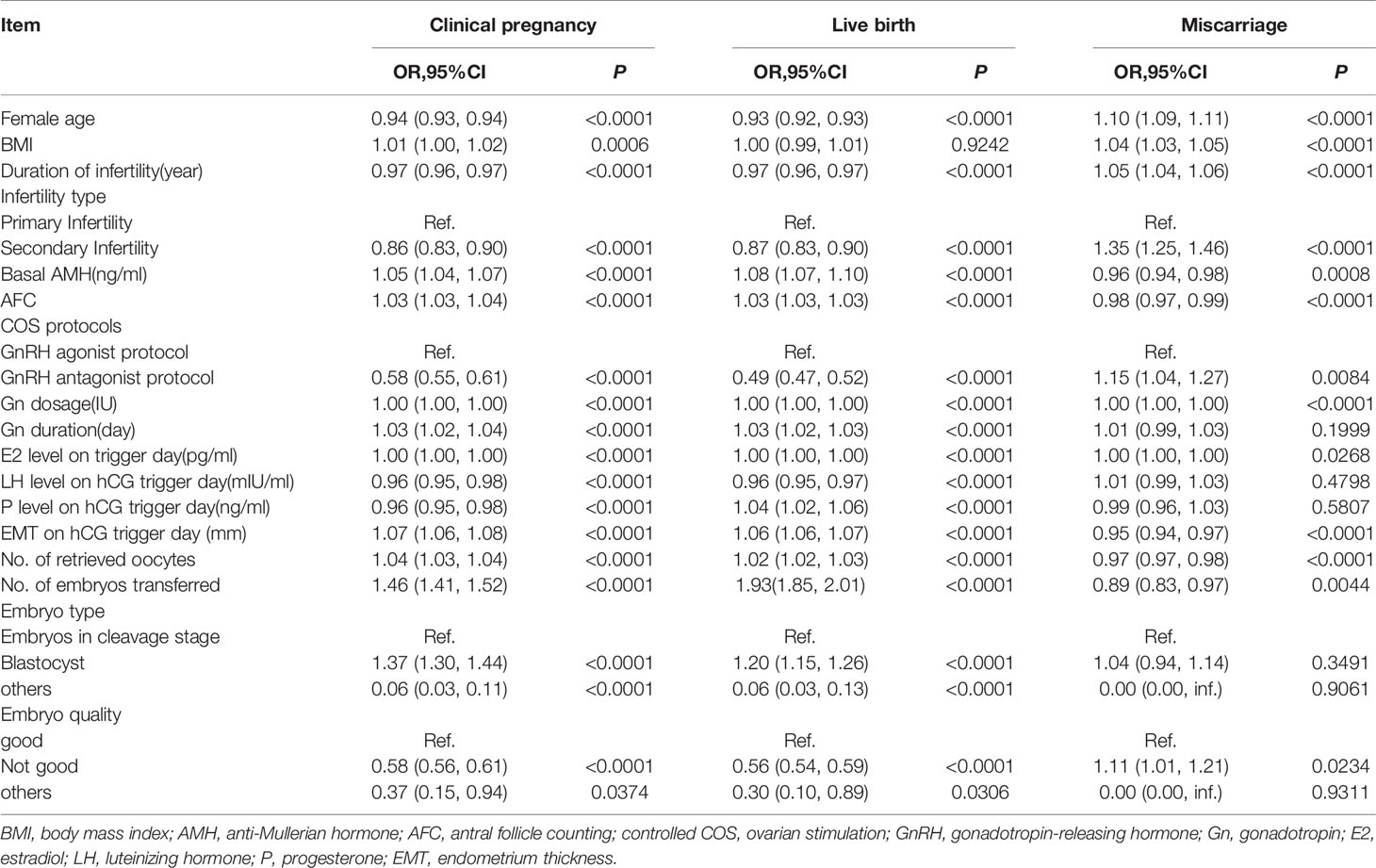
Univariate analysis showed that EMT was associated with an increased clinical pregnancy rate (OR=1.07, 95% CI: 1.06-1.08, P<0.0001) and increased live birth rate (OR=1.06, 95% CI: 1.06-1.07,P<0.0001). While the miscarriage rate was not significantly associated with EMT (OR=0.99, 95% CI: 0.97-1.00, P=0.0937). Female age, duration of infertility, LH levels, and P levels showed decreased effect on clinical pregnancy, live birth. However, basal AMH, AFC, Gn duration, No. of retrieved oocytes and No. of embryos transferred could increase the rate of pregnancy and live birth. The analysis data was showed in Table 3.
Associations Between EMT and Clinical Outcomes of Fresh Cycles Using Multivariable Logistic Regression Analysis
After adjusting for confounding variables such as age, BMI, infertility type, No. of embryos transferred, No. of retrieved oocytes and COS protocols. we performed a multivariable logistic regression analysis to evaluate the association between EMT and pregnancy outcomes. Significant associations were found between EMT and clinical pregnancy rate (adjusted odds ratio [aOR]: 1.05; 95% confidence interval [CI]: 1.06–1.08, P< 0.0001), live birth rate (aOR: 1.04; 95% CI: 1.03–1.05, P < 0.0001), and miscarriage rate (aOR: 0.96; 95% CI: 0.94 – 0.98, P < 0.0001).The result was exhibited in Table 4.
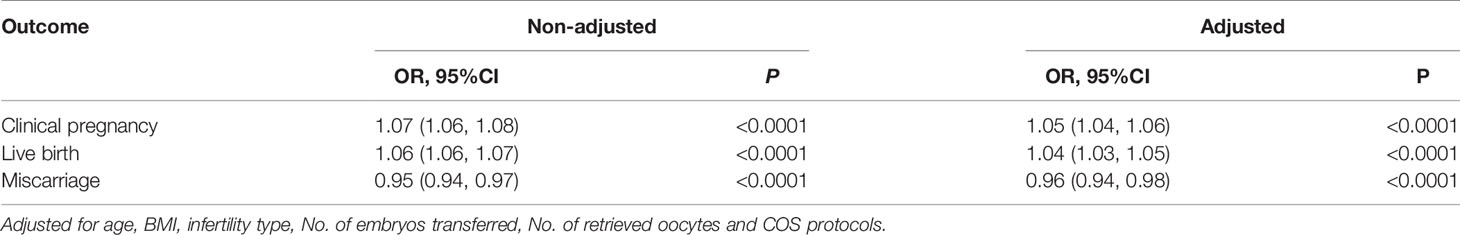
Table 4 Associations between EMT and pregnancy outcomes of fresh cycles using multivariable logistic regression analysis.
Curve Fitting Between EMT and Pregnancy Outcomes
Fitted curves with covariate adjustment, showing the relationship between EMT and clinical pregnancy rate, live birth rate and miscarriage rate, were presented in Figures 2, 3. As EMT increased, clinical pregnancy rate and live birth rate initially increased before declining at high levels (Figures 2, 3), shown like an inverted “U” shape. Meanwhile, the miscarriage rate first declined and then leveled off with EMT increasing (Figure 4).
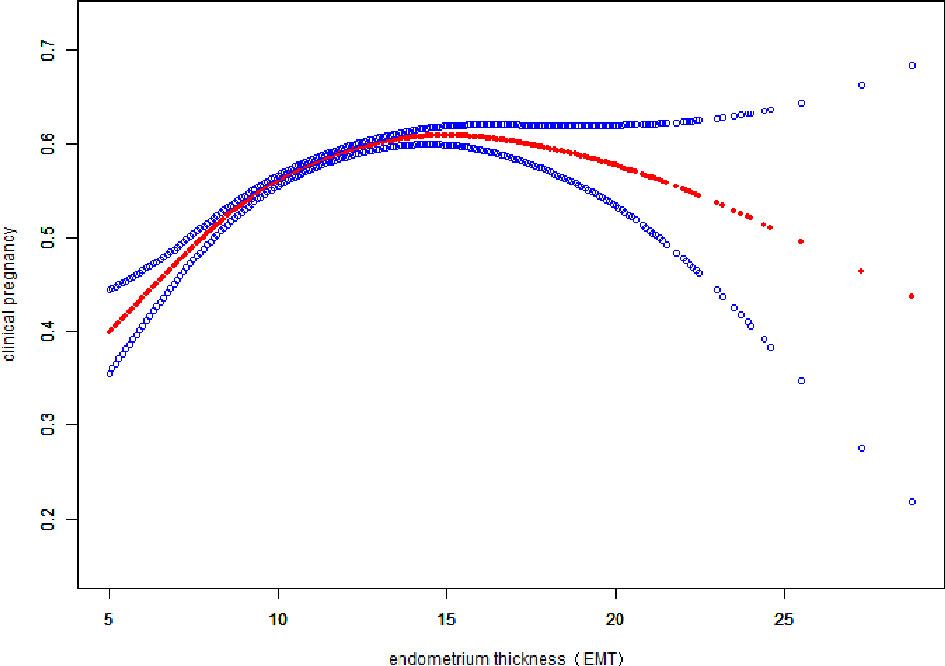
Figure 2 Association between EMT and clinical pregnancy rate. A threshold, nonlinear association between EMT and clinical pregnancy rate was found (P<0.001) in a generalized additive model (GAM). The solid red line represents the smooth curve fit between variables. Blue bands represent the 95% of confidence interval from the fit. The fitted line adjusted for age, BMI, infertility type, and the number type of embryos transferred, number. of retrieved oocytes, and COS protocols.
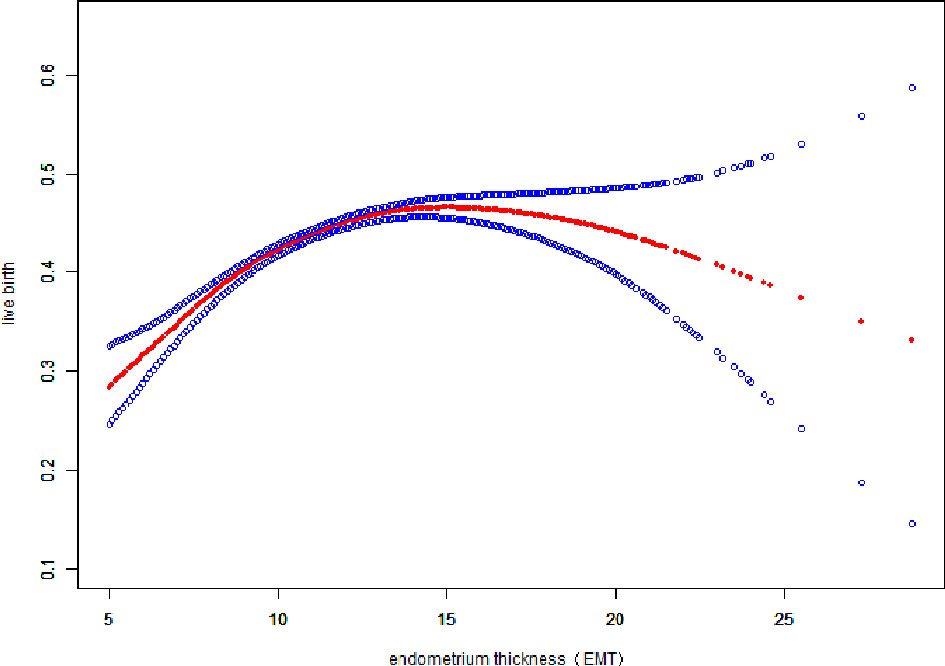
Figure 3 Association between EMT and live birth rate. A threshold, nonlinear association between EMT and live birth rate was found (P<0.001) in a generalized additive model (GAM). Solid red line represents the smooth curve fit between variables. Blue bands represent the 95% of confidence interval from the fit. The fitted line adjusts for age, BMI, infertility type, and the number type of embryos transferred, numbers of retrieved oocytes, and COS protocols.
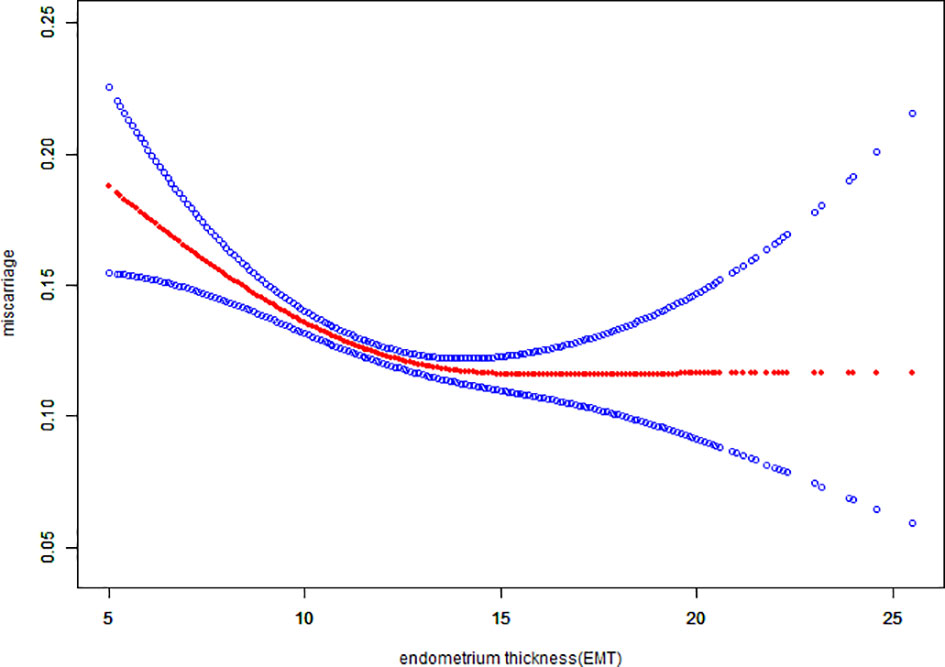
Figure 4 Association between EMT and miscarriage rate. A threshold, nonlinear association between EMT and miscarriage rate was found (P<0.001) in a generalized additive model (GAM). The solid red line represents the smooth curve fit between variables. Blue bands represent the 95% of confidence interval from the fit. The fitted line adjusted for age, BMI, infertility type, and the number type of embryos transferred, number. of retrieved oocytes, and COS protocols.
Threshold Effect Analysis of EMT and Pregnancy Outcomes in Fresh Cycles Using Piece Wise Linear Regression Method
The threshold effect analysis of EMT and the pregnancy outcomes were presented in Table 5. The EMT was a non-linear significant predictor of pregnancy outcomes. When the EMT was less than 12mm, the clinical pregnancy rate and live birth rate were significantly increased by 10% and 9%(OR:1.10; 95%CI: 1.08-1.12, OR:1.09; 95%CI: 1.07-1.11), respectively, along with the increase of each millimeter increment of EMT. As the EMT ranged from 12 to 15 mm, the pregnancy outcomes came to the plateau phase at the ideal level. However, clinical pregnancy rate and live birth rate tended to decrease by 6% and 4% when EMT was ≥15mm. In addition, the miscarriage rate was significantly declined by 8% (OR:0.92; 95%CI: 0.90-0.95)with each millimeter increment of EMT. And when EMT was thicker than 12mm, the miscarriage rate didn’t change significantly any more.
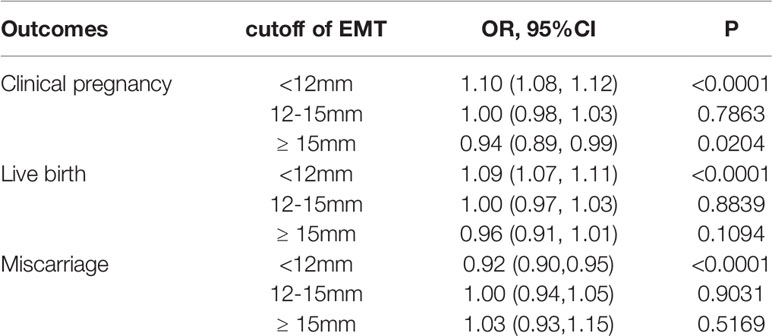
Table 5 Threshold effect analysis of EMT and clinical outcomes in fresh cycles using piece wise linear regression method.
Discussion
To the best of our knowledge, this is the largest multi-center study to demonstrate the relationship between EMT and pregnancy outcomes such as clinical pregnancy rate, live birth rate and miscarriage rate. We determined cut-off values for EMT in this large cohort including 42,132 IVF/ICSI fresh embryo transfer cycles. Our study found EMT to be a non-linear significant predictor of pregnancy outcomes, including clinical pregnancy, live birth rate and miscarriage rate. With the increase of EMT, clinical pregnancy rate and live birth rate initially increased before declining at high levels. Moreover, we also found that miscarriage rates could decreased with the growth of EMT in fresh cycles.
In most reproductive medicine centers, EMT and endometrial patterns are usually considered to be reliable and noninvasive measurements in endometrial receptivity evaluation. Endometrial receptivity is the key factor affecting the pregnancy outcomes of embryo transfer cycles (10). Since the EMT has been measured at different time points during previous studies, such as on the day of hCG administration, on the day of oocyte retrieval or on the day of embryo transfer (11–13), therefore there is no consensus on the relationship between EMT and pregnancy outcomes despite the relative abundance of studies.
As far as we know, most previous studies only focused on whether EMT affects pregnancy outcomes or not. Yuan et al. (6) showed EMT as one of the independent variables predictive of clinical pregnancy (OR:1.097; P<0.001), live birth (OR:1.078; P<0.001), spontaneous abortion (OR:0.948; P<0.001), and ectopic pregnancy (OR:0.851; P<0.001). Gallos et al. (14) considered EMT is strongly associated with pregnancy losses and live births in IVF. Ma et al. (15) also investigated that live birth rate and clinical pregnancy rate were positively correlated with increasing EMT. In addition, a meta-analysis (5) including 9 prospective and 21 retrospective investigations also indicated that compared with high EMT, low EMT was associated with decreased pregnancy rate (n = 30, OR: 0.61; 95%CI: 0.52 - 0.70; P < 0.001). However, there were still some mixed conclusions. In these studies of Craciunas et al. and Huang, J et al. (8, 9), biomarkers such as EMT had poor ability to predict clinical pregnancy. In order to obtain a more comprehensive relationship between EMT and pregnancy outcomes, we adjusted for confounding factors and conducted curve fitting and threshold effect analysis.
We performed multivariable logistic regression analysis to evaluate the association between EMT and pregnancy outcomes. Significant associations were found between EMT and clinical pregnancy rate (aOR: 1.05; 95% CI: 1.06–1.08, P<0.0001), live birth rate (aOR: 1.04; 95% CI: 1.03–1.05, P<0.0001) and miscarriage rate(aOR: 0.96; 95% CI: 0.94 – 0.98, P < 0.0001), similar to results of other studies (5, 16, 17). A curve fitting and threshold analysis further revealed a quantitative relationship between EMT and pregnancy outcomes. The correlation between EMT and pregnancy outcome tended to be an inverted “U” shape. Our data suggested that clinical pregnancy rate could reach 49.28% with EMT between 8-8.9mm, which was acceptable among most IVF centers. With every millimeter increase of endometrium up to 12 mm, the clinical pregnancy rate and live birth rate increased by 10% and 9%(OR:1.10; 95%CI: 1.08-1.12, OR:1.09; 95%CI: 1.07-1.11), respectively. When EMT attained to the range of 12-15 mm, clinical pregnancy rate and live birth rate were stable at the ideal level of 60.33% - 60.73% and 46.09% - 46.92%, which were not significant associated with EMT growth. As the EMT increased thicker than 15mm, clinical pregnancy rate and live birth rate tended to be slightly decreased by 6% and 4%. So we could speculate about the minimum threshold of EMT for optimal clinical pregnancy and live birth rate may at 12 mm.
In the study of K.E. Liu et al. (18), they reported that clinical pregnancy and live birth rates decreased (P < 0.0001) and pregnancy loss rates increased (P = 0.01) with each millimeter decline in endometrial thickness below 8 mm in 24,363 fresh cycles. Zhang T et al (4) performed binary logistic analysis and ROC curves in 1,512 IVF cycles and revealed the cut-off points of endometrial thickness on oocyte retrieval day was 8.75 mm (sensitivity of 84.6% and specificity of 22.8%) for live birth. Besides, Gallos et al. (14) considered that endometrial thickness is strongly associated with pregnancy losses and live births in IVF, and the optimal endometrial thickness threshold of 10 mm. Those cut-off values in previous studies were different to ours. What should be pointed out is that the present study used smooth curve fitting, segmented regression model, and then found the non-linear relationship and the threshold value of EMT. It’s a new statistical method applied to the field of EMT and this method should be used to find the exact association when there is a possible threshold effect.
In addition, we noted that although clinical pregnancy and live birth rates were lower when the endometrial thickness was below 8mm. Clinical pregnancy and live birth rates were 33.93% to 43.79% and 21.43% to 31.15% with an endometrial thickness of 5 – 7.9mm in Table 2. Many studies had different definitions for thin endometrium and used different thresholds of 6-8 mm to define thin endometrium (6, 19–22).The reason for why a thin endometrium leads to implant failure or early pregnancy loss still remains unclear. For patients with a thin endometrium, endometrial receptivity may be compromised because of the alteration of implantation associated factors (23). A thinned or absent functional layer may subject the embryos to higher vascularity and oxygen concentrations from the basal endometrium (13, 23). As a result, the subsequent production of reactive oxygen species could generate a suboptimal uterine environment for implantation and placentation, which ultimately leads to impaired fetal growth (23, 24). However, a thickened endometrium (≥ 15mm, data displayed in Table 5) had an adverse effect on clinical pregnancy rate decreasing by 6% significantly. Thicken endometrium may associate with intrauterine pathologies such as polyps, fibroids or hyperplasia may have an adverse effect on the implantation and clinical pregnancy (25). Therefore, in women with an abnormally thickened endometrium, it is necessary to carry out further investigations, such as a hysteroscopy to make clear and treat any intra-uterine lesions (6).
The observations in our study were based on fresh embryo transfer in IVF stimulated cycles, and our conclusions might not apply to frozen embryo transfer in HRT cycles. BU.Z et al. (26) retrospectively analyzed data from 2997 patients undergoing their first FET cycles, after adjusting for age, body mass index (BMI), baseline follicle stimulating hormone (FSH), FET protocol and No. of embryos transferred, the associations between EMT and clinical pregnancy rate (aOR: 1.39; 95% CI: 1.10-1.77, p < 0.01) and live birth rate (aOR: 1.50; 95% CI: 1.16-1.95, p < 0.01) were significant with thickness of 9-13mm. In another retrospective study of 1,627 FET cycles on patients with different EMT, Zhang, Q. et al., (17). found a non-linear relationship between EMT and pregnancy outcomes and an EMT threshold of 10.9 mm. The effect size of the left and right sides of the threshold were 1.16 (95% CI 1.07-1.25) and 0.89 (95% CI 0.78-1.01), respectively. In our previous study (10), we found in FET cycles, live birth rate would be optimal when the EMT was within the range of 8.7-14.5 mm. We hypothesized that the different threshold value between fresh cycle and FET cycle might be caused by confounders such as high levels of estrogen, large amount of Gn dosage or pre-increasing LH surge that would affect endometrial receptivity to some degree. Thus in FET cycles, the endometrium is more receptive and EMT threshold value range is wider than those in fresh cycles.
However, there are some limitations in this study. Firstly, this study was retrospective and there might be some confounders that we did not control for. In addition, we prioritized a few select pregnancy outcomes and did not investigate neonatal outcomes such as gestational age and birth weight. Finally, the observations in our study were based on fresh cycles in IVF/ICSI treatment, the conclusions of which might not be able to apply to FET cycles. In the future, a well-designed and randomized clinical trial would be needed for further study.
In conclusion, EMT has a curvilinear relationship with pregnancy outcomes in fresh embryo transfer cycles. Clinical pregnancy rate and live birth rate may achieve the optimal level when EMT ≥ 12 mm, but some adverse pregnancy outcomes would be observed when EMT ≥15 mm especially for clinical pregnancy. These would be relevant evidence when considering the embryo transfer.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
CZ and XL supervised the entire study, including procedures, conception, design, and completion. SZ, LJ, YM, JS, and RH were responsible for collecting information. JX and SZ contributed to the analysis data and drafted the manuscript. XH participated in revising the article. JX and SZ contributed equally to this article. All authors contributed to the article and approved the submitted version.
Funding
The National Natural Science Foundation of China (U2004130); The Major Projects in Provincial and National Union Construction of Henan Medical Science Research Plan (SBGJ202001002); The Provincial Natural Science Foundation of Henan (202300410456).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the all the laboratory staff of the five reproductive centers for their contribution to this work. Besides, we acknowledge the patients who took part in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.788706/full#supplementary-material
References
1. Onogi S, Ezoe K, Nishihara S, Fukuda J, Kobayashi T, Kato K. Endometrial Thickness on the Day of the LH Surge: An Effective Predictor of Pregnancy Outcomes After Modified Natural Cycle-Frozen Blastocyst Transfer. Hum Reprod Open (2020) 2020(4):hoaa060. doi: 10.1093/hropen/hoaa060
2. Cha J, Sun X, Dey SK. Mechanisms of Implantation: Strategies for Successful Pregnancy. Nat Med (2012) 18(12):1754–67. doi: 10.1038/nm.3012
3. Marquardt RM, Kim TH, Shin JH, Jeong JW. Progesterone and Estrogen Signaling in the Endometrium: What Goes Wrong in Endometriosis? Int J Mol Sci (2019) 20(15):3822. doi: 10.3390/ijms20153822
4. Zhang T, Li Z, Ren X, Huang B, Zhu G, Yang W, et al. Endometrial Thickness as a Predictor of the Reproductive Outcomes in Fresh and Frozen Embryo Transfer Cycles: A Retrospective Cohort Study of 1512 IVF Cycles With Morphologically Good-Quality Blastocyst. Medicine (2018) 97(4):e9689. doi: 10.1097/md.0000000000009689
5. Gao G, Cui X, Li S, Ding P, Zhang S, Zhang Y. Endometrial Thickness and IVF Cycle Outcomes: A Meta-Analysis. Reprod Biomed Online (2020) 40(1):124–33. doi: 10.1016/j.rbmo.2019.09.005
6. Yuan X, Saravelos SH, Wang Q, Xu Y, Li TC, Zhou C. Endometrial Thickness as a Predictor of Pregnancy Outcomes in 10787 Fresh IVF-ICSI Cycles. Reprod biomedicine Online (2016) 33(2):197–205. doi: 10.1016/j.rbmo.2016.05.002
7. Kovacs P, Matyas S, Boda K, Kaali SG. The Effect of Endometrial Thickness on IVF/ICSI Outcome. Hum Reprod (Oxf Engl) (2003) 18(11):2337–41. doi: 10.1093/humrep/deg461
8. Huang J, Lin J, Gao H, Zhu J, Lu X, Song N, et al. Value of Endometrial Thickness Change After Human Chorionic Gonadotrophin Administration in Predicting Pregnancy Outcome Following Fresh Transfer In Vitro Fertilization Cycles. Arch Gynaecol Obstet (2021) 303(2):565–72. doi: 10.1007/s00404-020-05763-4
9. Craciunas L, Gallos I, Chu J, Bourne T, Quenby S, Brosens JJ, et al. Conventional and Modern Markers of Endometrial Receptivity: A Systematic Review and Meta-Analysis. Hum Reprod Update (2019) 25(2):202–23. doi: 10.1093/humupd/dmy044
10. Shaodi Z, Qiuyuan L, Yisha Y, Cuilian Z. The Effect of Endometrial Thickness on Pregnancy Outcomes of Frozen-Thawed Embryo Transfer Cycles Which Underwent Hormone Replacement Therapy. PloS One (2020) 15(9):e0239120. doi: 10.1371/journal.pone.0239120
11. Vaegter KK, Lakic TG, Olovsson M, Berglund L, Brodin T, Holte J. Which Factors are Most Predictive for Live Birth After In Vitro Fertilization and Intracytoplasmic Sperm Injection (IVF/ICSI) Treatments? Analysis of 100 Prospectively Recorded Variables in 8,400 IVF/ICSI Single-Embryo Transfers. Fertil Steril (2017) 107(3):641–8.e2. doi: 10.1016/j.fertnstert.2016.12.005
12. Gingold JA, Lee JA, Rodriguez-Purata J, Whitehouse MC, Sandler B, Grunfeld L, et al. Endometrial Pattern, But Not Endometrial Thickness, Affects Implantation Rates in Euploid Embryo Transfers. Fertil Steril (2015) 104(3):620–8.e5. doi: 10.1016/j.fertnstert.2015.05.036
13. Ribeiro VC, Santos-Ribeiro S, De Munck N, Drakopoulos P, Polyzos NP, Schutyser V, et al. Should We Continue to Measure Endometrial Thickness in Modern-Day Medicine? The Effect on Live Birth Rates and Birth Weight. Reprod Biomed Online (2018) 36(4):416–26. doi: 10.1016/j.rbmo.2017.12.016
14. Gallos ID, Khairy M, Chu J, Rajkhowa M, Tobias A, Campbell A, et al. Optimal Endometrial Thickness to Maximize Live Births and Minimize Pregnancy Losses: Analysis of 25,767 Fresh Embryo Transfers. Reprod Biomed Online (2018) 37(5):542–8. doi: 10.1016/j.rbmo.2018.08.025
15. Ma NZ, Chen L, Dai W, Bu ZQ, Hu LL, Sun YP. Influence of Endometrial Thickness on Treatment Outcomes Following In Vitro Fertilization/Intracytoplasmic Sperm Injection. Reprod Biol Endocrinol RB&E (2017) 15(1):5. doi: 10.1186/s12958-016-0222-5
16. Ye J, Zhang J, Gao H, Zhu Y, Wang Y, Cai R, et al. Effect of Endometrial Thickness Change in Response to Progesterone Administration on Pregnancy Outcomes in Frozen-Thawed Embryo Transfer: Analysis of 4465 Cycles. Front Endocrinol (2020) 11:546232. doi: 10.3389/fendo.2020.546232
17. Zhang Q, Li Z, Wang Y, Lu H, Yu Y. The Relationship and Optimal Threshold of Endometrial Thickness With Early Clinical Pregnancy in Frozen Embryo Transfer Cycles. Int J Gynaecol Obstet: Off Organ Int Fed Gynaecol Obstet (2021) 156(1):55–63. doi: 10.1002/ijgo.13606
18. Liu KE, Hartman M, Hartman A, Luo ZC, Mahutte N. The Impact of a Thin Endometrial Lining on Fresh and Frozen-Thaw IVF Outcomes: An Analysis of Over 40 000 Embryo Transfers. Hum Reprod (Oxford England) (2018) 33(10):1883–8. doi: 10.1093/humrep/dey281
19. Bozdag G, Esinler I, Yarali H. The Impact of Endometrial Thickness and Texture on Intracytoplasmic Sperm Injection Outcome. J Reprod Med (2009) 54(5):303–11.
20. Kumbak B, Erden HF, Tosun S, Akbas H, Ulug U, Bahçeci M. Outcome of Assisted Reproduction Treatment in Patients With Endometrial Thickness Less Than 7 Mm. Reprod Biomed Online (2009) 18(1):79–84. doi: 10.1016/s1472-6483(10)60428-2
21. Jimenez PT, Schon SB, Odem RR, Ratts VS, Jungheim ES. A Retrospective Cross-Sectional Study: Fresh Cycle Endometrial Thickness is a Sensitive Predictor of Inadequate Endometrial Thickness in Frozen Embryo Transfer Cycles. Reprod Biol Endocrinol RB&E (2013) 11:35. doi: 10.1186/1477-7827-11-35
22. Mahajan N, Sharma S. The Endometrium in Assisted Reproductive Technology: How Thin is Thin? J Hum Reprod Sci (2016) 9(1):3–8. doi: 10.4103/0974-1208.178632
23. Casper RF. It’s Time to Pay Attention to the Endometrium. Fertil Steril (2011) 96(3):519–21. doi: 10.1016/j.fertnstert.2011.07.1096
24. Zhang J, Liu H, Mao X, Chen Q, Si J, Fan Y, et al. Effect of Endometrial Thickness on Birthweight in Frozen Embryo Transfer Cycles: An Analysis Including 6181 Singleton Newborns. Hum Reprod (Oxf Engl) (2019) 34(9):1707–15. doi: 10.1093/humrep/dez103
25. Pritts EA, Parker WH, Olive DL. Fibroids and Infertility: An Updated Systematic Review of the Evidence. Fertil Steril (2009) 91(4):1215–23. doi: 10.1016/j.fertnstert.2008.01.051
Keywords: IVF/ICSI, clinical pregnancy rate, endometrial thickness, live birth rate (LBR), fresh embryo transfer (fresh ET)
Citation: Xu J, Zhang S, Jin L, Mao Y, Shi J, Huang R, Han X, Liang X and Zhang C (2022) The Effects of Endometrial Thickness on Pregnancy Outcomes of Fresh IVF/ICSI Embryo Transfer Cycles: An Analysis of Over 40,000 Cycles Among Five Reproductive Centers in China. Front. Endocrinol. 12:788706. doi: 10.3389/fendo.2021.788706
Received: 03 October 2021; Accepted: 10 December 2021;
Published: 24 January 2022.
Edited by:
Yang Xu, Peking University First Hospital, ChinaReviewed by:
Fahiel Casillas, Metropolitan Autonomous University, MexicoHilma Putri Lubis, University of North Sumatra, Indonesia
Copyright © 2022 Xu, Zhang, Jin, Mao, Shi, Huang, Han, Liang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyan Liang, liangxy2@mail.sysu.edu.cn; Cuilian Zhang, luckyzcl@qq.com
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
 Jianing Xu
Jianing Xu Shaodi Zhang
Shaodi Zhang Lei Jin
Lei Jin Yundong Mao
Yundong Mao Juanzi Shi
Juanzi Shi Rui Huang
Rui Huang Xiao Han
Xiao Han Xiaoyan Liang
Xiaoyan Liang Cuilian Zhang
Cuilian Zhang