- 1Department of Endocrinology and Metabolism, The National Health Committee (NHC) Key Laboratory of Diagnosis and Treatment of Thyroid Diseases, Institute of Endocrinology, The First Hospital of China Medical University, Shenyang, China
- 2Fuxin Central Hospital, Fuxin, China
Objective: This study was a prospective assessment of the epidemiological characteristics of metabolic syndrome (MetS) in cities in Northeast China. We explored the factors that affect the occurrence and outcome of MetS according to sex.
Design and Methods: This was a longitudinal survey assessing MetS status among 750 urban community residents in China. At baseline, the intra-abdominal fat area was measured by MRI, simple anthropometric parameters (body mass index (BMI), waist circumference (WC), etc.) were used to evaluate fat distribution; blood pressure and blood lipid profile were measured; an oral glucose tolerance test (OGTT) was used to detect blood glucose; questionnaires were used to investigate lifestyles. Follow-up was conducted after 1.5 years (follow-up rate was 66.93%) to analyze the incidence of MetS and the influencing factors of MetS outcomes according to sex.
Results: The 1.5-year cumulative incidence of MetS in the survey area was 25.40%. Men with visceral obesity were more likely to develop MetS than those with subcutaneous obesity (OR=9.778, p<0.05). Increased BMI (OR=1.379) and blood uric acid (BUA)>416 mmol/L (OR=2.318) were associated with the occurrence of MetS in men (all p<0.05). At the initial visit, BUA>356.9 mmol/L (OR=3.538), increased BMI (OR=1.212), and increased HbA1c (OR=2.577) were associated with the occurrence of MetS in women (all p<0.05). After 1.5 years, 25.37% of MetS patients no longer had MetS. Elevated diastolic blood pressure (DBP) (OR=1.097) and increased visceral fat (OR=1.023) at the initial visit made men with MetS less likely to recover from MetS (all p<0.05). Higher High-density lipoprotein cholesterol (HDL-C) at the initial visit made women with MetS more likely to recover from MetS (β: -3.509, OR=0.003, p<0.05).
Conclusion: There are different risk factors for MetS in different genders. Hyperuricemia is a risk factor for the onset of MetS in both men and women.
Introduction
Metabolic syndrome (MetS) is a group of metabolic disorders including obesity, elevated blood glucose, dyslipidemia and hypertension (1). Globally, approximately 25% of adults suffer from MetS, and this proportion is expected to continue to increase in the coming decades (2). MetS is a risk factor for cardiovascular and cerebrovascular diseases and diabetes and a substantial challenge to public health (3, 4).
There are many risk factors for the development of MetS (5). Among them, obesity, particularly central obesity, is a key risk factor and has become one of the core diagnostic indicators for MetS. However, waist circumference (WC) alone cannot adequately identify central obesity caused by increased subcutaneous fat and increased visceral fat. Studies have indicated that visceral fat is more likely to lead to insulin resistance than subcutaneous fat in the abdomen (6). Computed tomography (CT) and magnetic resonance imaging (MRI) are gold standards for differentiation. The roles of visceral and subcutaneous fat in the development and outcome of MetS still need to be further explored.
Both hyperuricemia and MetS are common chronic diseases, and studies have indicated that hyperuricemia and MetS are closely related (7–9). A cross-sectional study indicated that hyperuricemia was an important factor in MetS, but it was not discussed individually in men and women (8). A cross-sectional study from southwestern China demonstrated significant risk factors for MetS with hyperuricemia in people over 80 years of age (7). Nonetheless, cross-sectional studies cannot analyze cause and effect very well. A prospective study with up to 4.5 years of follow-up explored the relationship between new-onset MetS and hyperuricemia and suggested a bidirectional effect (9). However, the role of hyperuricemia in the recovery of MetS is unclear.
Increasing age is also a risk factor for MetS. Studies in the United States have shown that the prevalence of MetS increases significantly after 30 years of age (10). Sex also plays a key role in the development and outcome of MetS due to the presence of sex and sex-related determinants of each single component of MetS. A large Korean cross-sectional study showed that male MetS patients had significantly higher rates of MetS components such as hypertriglyceridemia (+5%), hypertension (+6%), and hyperglycemia (+11%) than female patients, whereas the incidence of central obesity (+12%) and low-density lipoproteinemia (+47%) was significantly higher in female MetS patients than in males (11). In addition, the role of sex in MetS is also age-related. In a cross-sectional study of 4,289 subjects, the overall prevalence of MetS was higher in men than in women (22.3% in men and 15.8% in women), but this association reversed after age 60 years (30.4% in men and 40.3% in women) (12). Evidence suggests that changes in women’s hormonal status during and after menopause affect the development of MetS (13). In addition, many studies have shown that sex may influence cardiovascular risk associated with MetS and response to therapeutic interventions and therefore requires our attention (14).
Studies have shown that MetS can be improved or remission. For example, lifestyle intervention and exercise can have a positive effect on the outcome of MetS and reduce the severity of its components (15, 16). In addition to dietary control and regular exercise, current treatment includes the treatment of antihypertensive, hypoglycemic, regulating dyslipidemia. Exploring which initial conditions are associated with better or worse MetS outcomes will help guide us to choose individualized treatment strategies based on patient conditions.
Because MetS is associated with many diseases, many prospective studies have used MetS as a risk factor to explore the occurrence, development and outcome of related diseases, but few studies have conducted prospective investigations on the development and outcome of MetS. This prospective longitudinal study investigated the prevalence of MetS in Chinese individuals and the risk factors for MetS according to sex and conducted a 1.5-year follow-up to explore the outcomes of patients with MetS to provide theoretical support for clinical practice.
Materials and Methods
Study Population
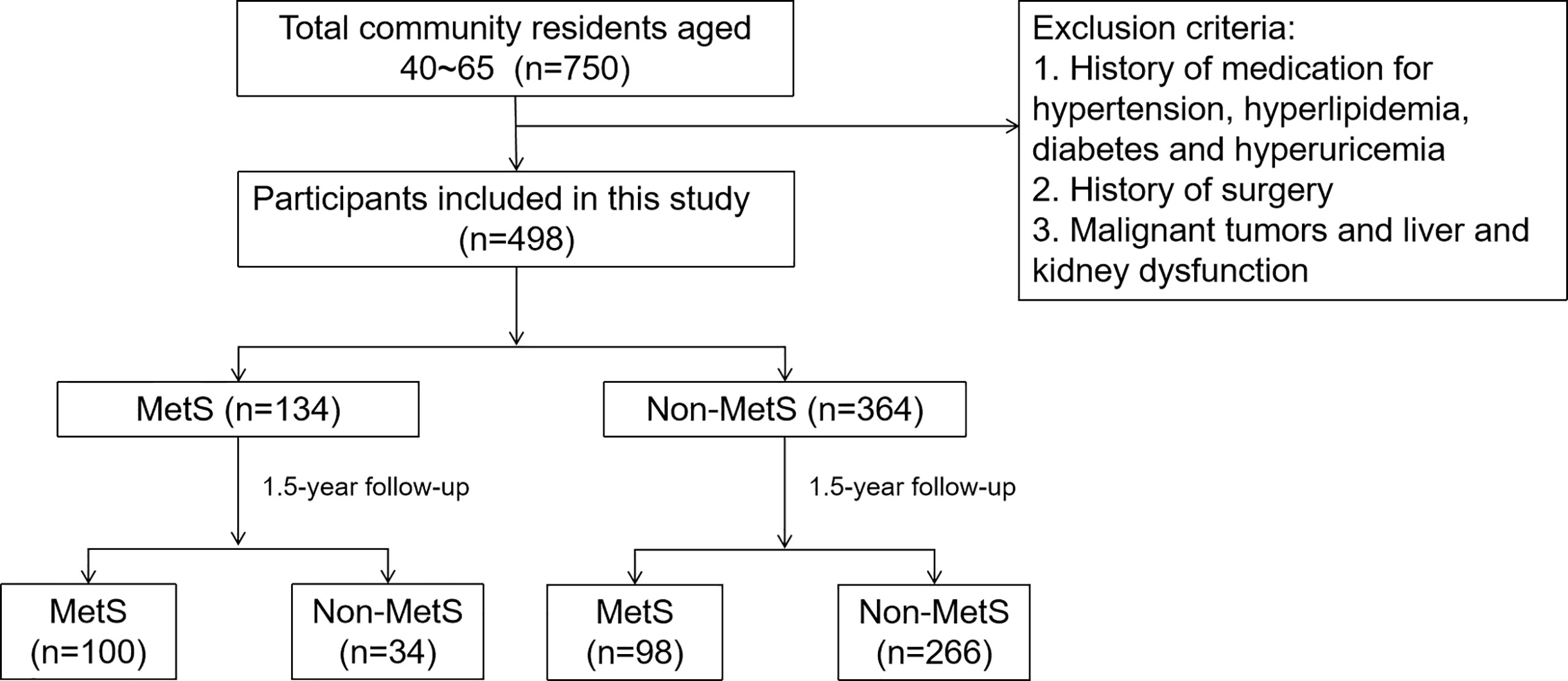
To assess the prevalence and outcome of MetS in the Chinese population, we conducted a community-based prospective study in Shenyang, Liaoning Province, China, in 2011. The study recruited 40- to 65-year-old residents of the area. A total of 750 subjects were included in this epidemiological survey and were followed up for 1.5 years. The exclusion criteria were (1) subjects with a history of medication for hypertension, hyperlipidemia, diabetes or hyperuricemia, (2) subjects with a history of surgery, and (3) subjects with malignant tumors or liver or kidney dysfunction during the baseline period. Ultimately, 498 participants were included. Among them, there were 134 patients with MetS and 364 patients with non-MetS (Figure 1).
Data Collection
A unified questionnaire was used to conduct health surveys for all study subjects at both baseline and the end of follow-up. The main contents of the questionnaire included general information (name, race, occupation, marital status, birth weight, etc.), personal behavior and lifestyle (alcohol consumption, physical activity, eating habits, etc.), family history of diseases (hypertension, diabetes, blood lipids, abnormality, stroke, coronary heart disease, obesity, etc.); and history of illness (diabetes, hypertension, dyslipidemia, etc.). A physical examination was performed to measure height, weight, waist circumference, hip circumference, blood pressure, and heart rate. The percentage of fat content was determined with a body fat meter. All subjects were in the prone position when they underwent an MRI scan of the abdomen between the 4th and 5th lumbar vertebrae at baseline and at the endpoint (field angle (FOV) 42 cm×42 cm, thickness 1 cm, six layers, GE, USA). Two experienced researchers used SLICE-O-MATIC version 4.2 software (Tomovision) to calculate the subcutaneous fat and visceral fat contents. Participants underwent a standard 75 g oral glucose tolerance test (OGTT). Venous blood samples were drawn, and fasting plasma glucose (FPG), 0.5-hour plasma glucose (0.5-h PG) and 2-hour plasma glucose (2-h PG) were measured using the hexokinase method. Fasting nonanticoagulated venous blood samples were taken to determine HbA1c, total cholesterol (TC), triglycerides (TGs), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) and blood uric acid (BUA) by enzymatic colorimetry.
Diagnostic Criteria
The 2005 IDF diagnostic criteria are used for the diagnosis of MetS in our study (17): prerequisites: (1) Central obesity: WC ≥90 cm in men; ≥80 cm in women; at least two of the following conditions: (2) SBP ≥130 mmHg, DBP ≥85 mmHg or antihypertensive treatment; (3) TGs ≥ 1.7 mmol/L and/or corresponding lipid-lowering treatment; (4) HDL-C < 1.03 mmol/L in men or <1.29 mmol/L in women and/or corresponding lipid-lowering therapy; and (5) FPG ≥ 5.6 mmol/L or diagnosis and treatment of diabetes. Patients treated with antihypertensive drugs, lipid-lowering drugs and hypoglycemic drugs were not included in this study.
Diagnosis of obesity type: Necessary conditions (18): Body mass index (BMI) ≥ 25.0 kg/m2 and waist circumference (WC) ≥ 85 cm for men or ≥ 80 cm for women. Visceral obesity: (1) Male: visceral fat area (VFA) ≥80 cm2; subcutaneous obesity: VFA <80 cm2; (2) Female: age ≥50 years, VFA ≥90 cm2; subcutaneous obesity: VFA <90 cm2; age <50 years old, VFA ≥70 cm2; subcutaneous obesity: VFA <70 cm2.
Statistical Analyses
All data were analyzed by SPSS 23.0 software. We used the Kolmogorov–Smirnov test for normality testing. Data are expressed as the mean ± standard deviation (SD), median (IQR) and count (percentage). The t-test was used for the mean comparison of variables that conformed to a normal distribution, and the rank-sum test was used for the variables that conformed to a skewed distribution. The chi-square test was used to compare the incidence and other rates of MetS. Univariate logistic analysis (enter) was used for selecting independent variables. Multivariate logistic regression (enter) was used for influencing factor analysis. P<0.05 indicated that a difference was statistically significant.
Results
Baseline Characteristics of the Participants
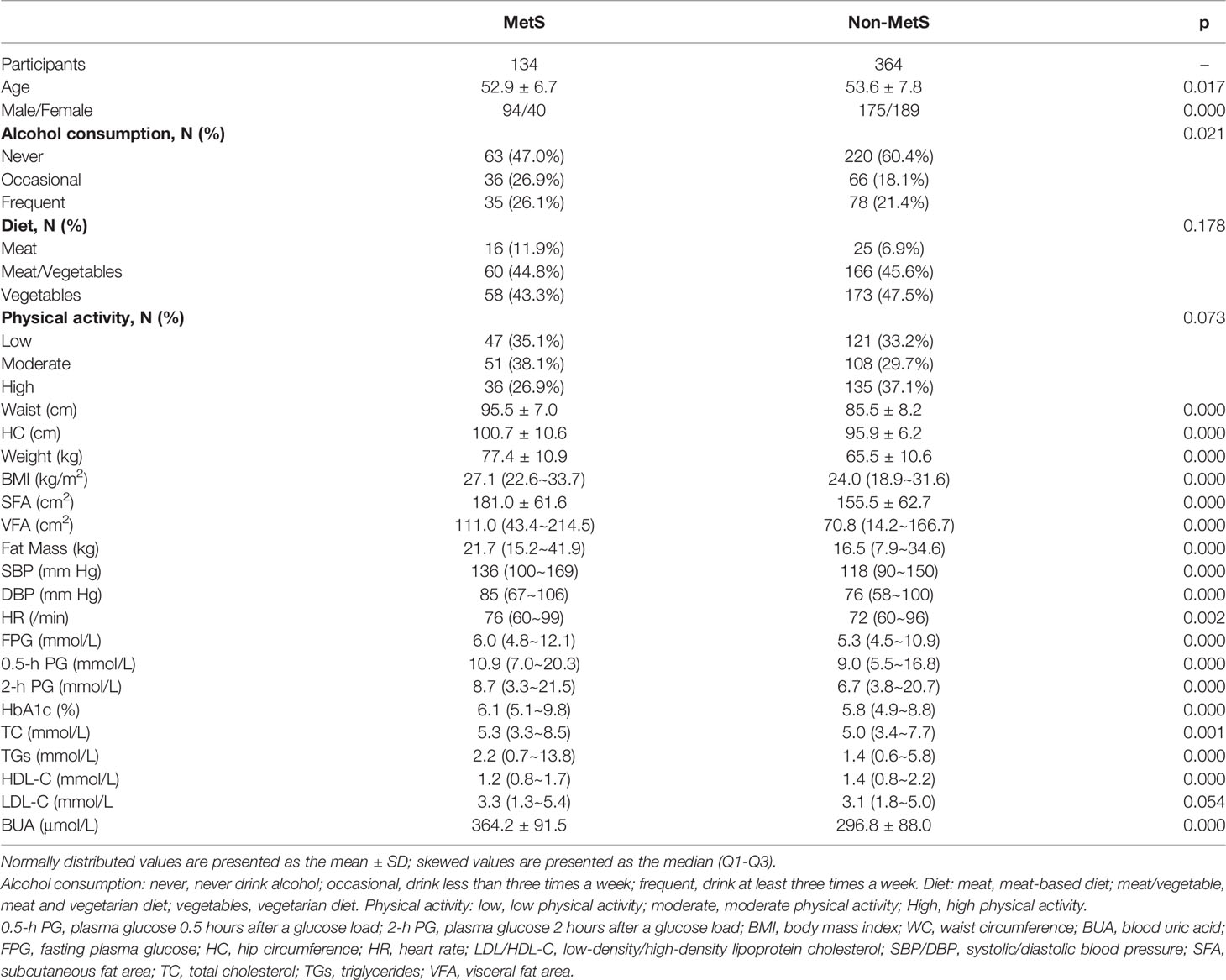
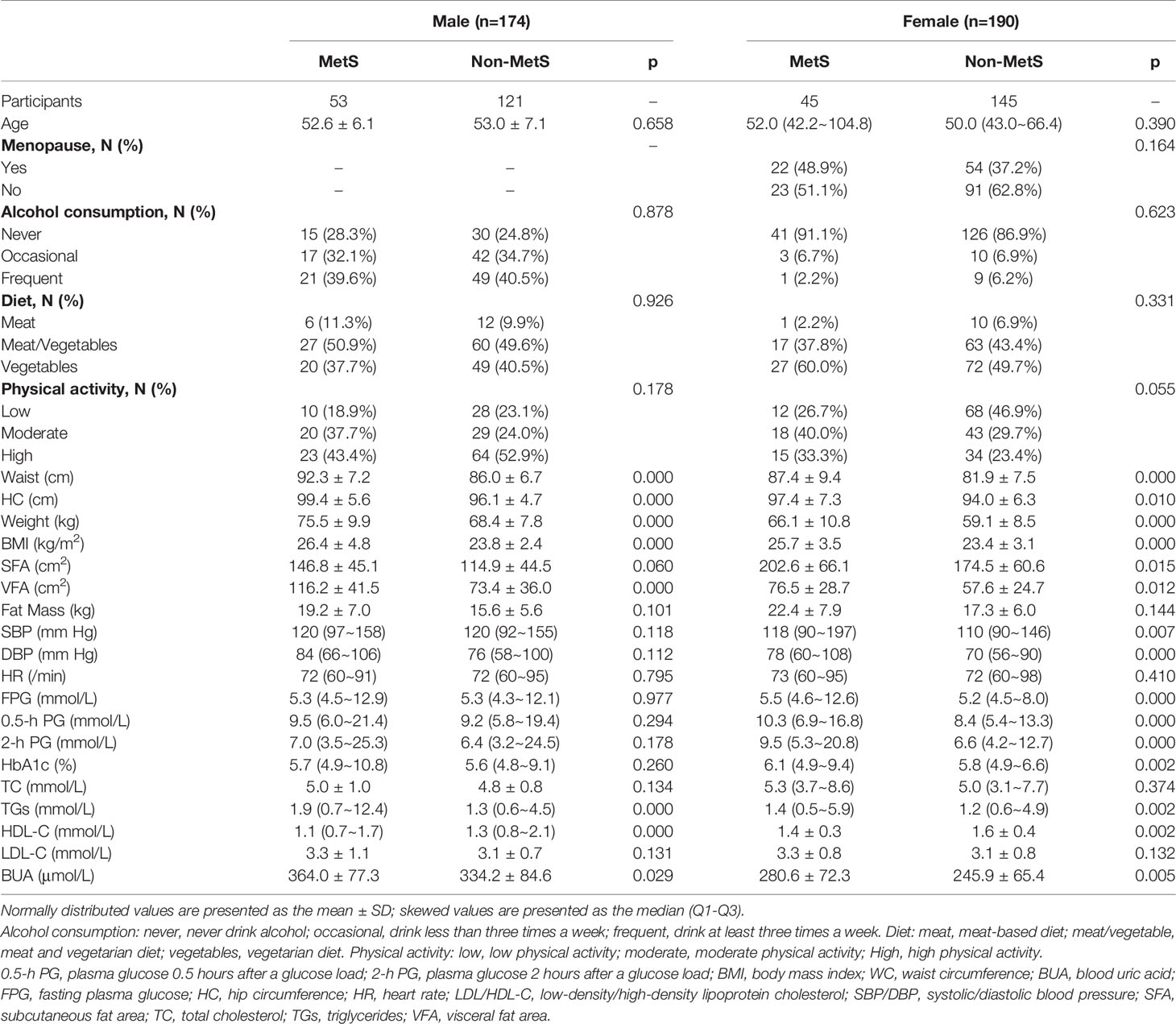
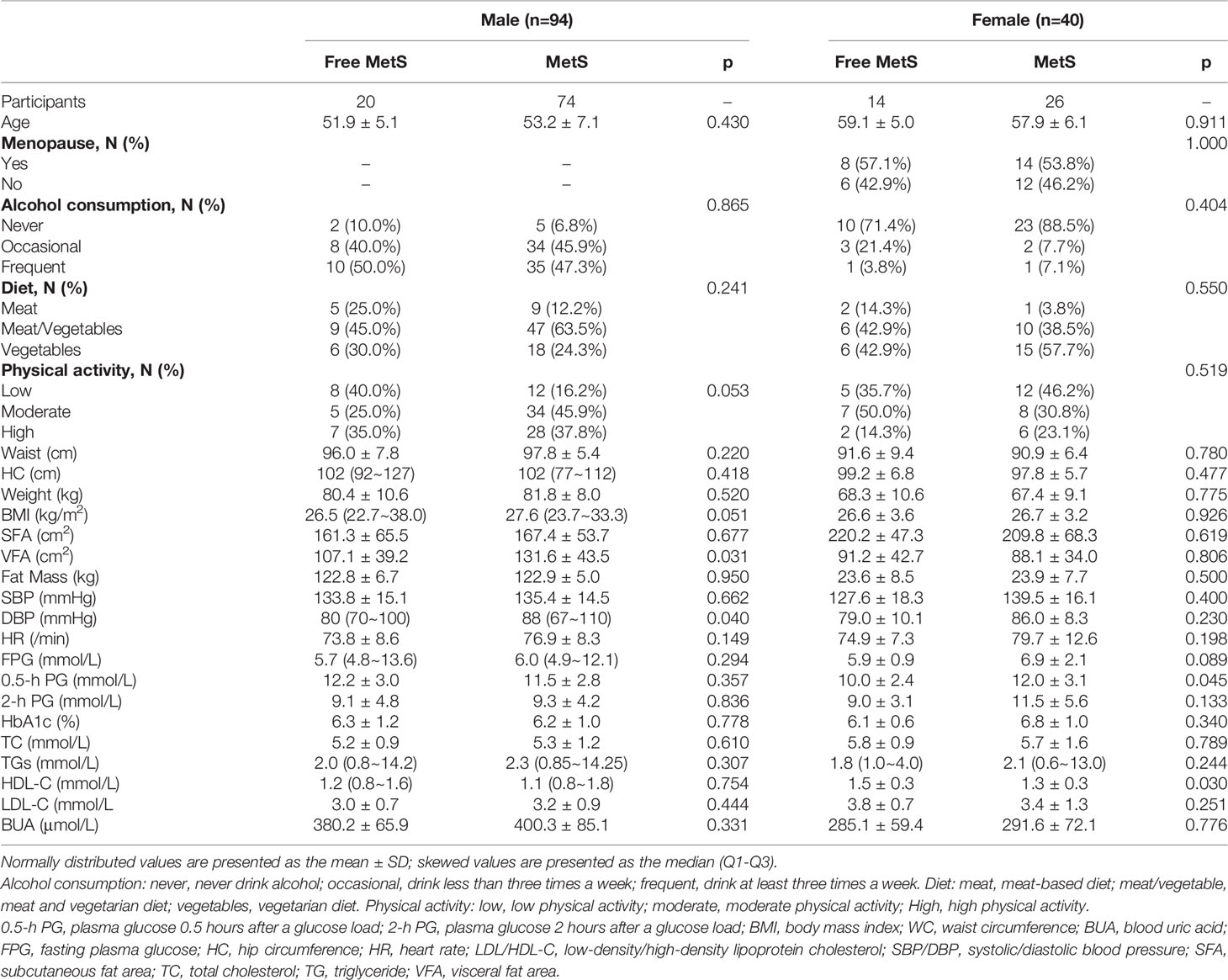
A total of 750 people were included in this study in 2011, and a total of 502 people were followed up at 1.5 years, with a follow-up rate of 66.93%. There was no significant difference in the follow-up rate according to sex (p>0.05). A total of 498 people (269 men and 229 women) were included in this study. Among them, there were 134 patients with MetS and 364 without MetS. At baseline, 94 men (18.87%) and 40 women (8.03%) were diagnosed with MetS (Table 1).
Influencing Factors of MetS
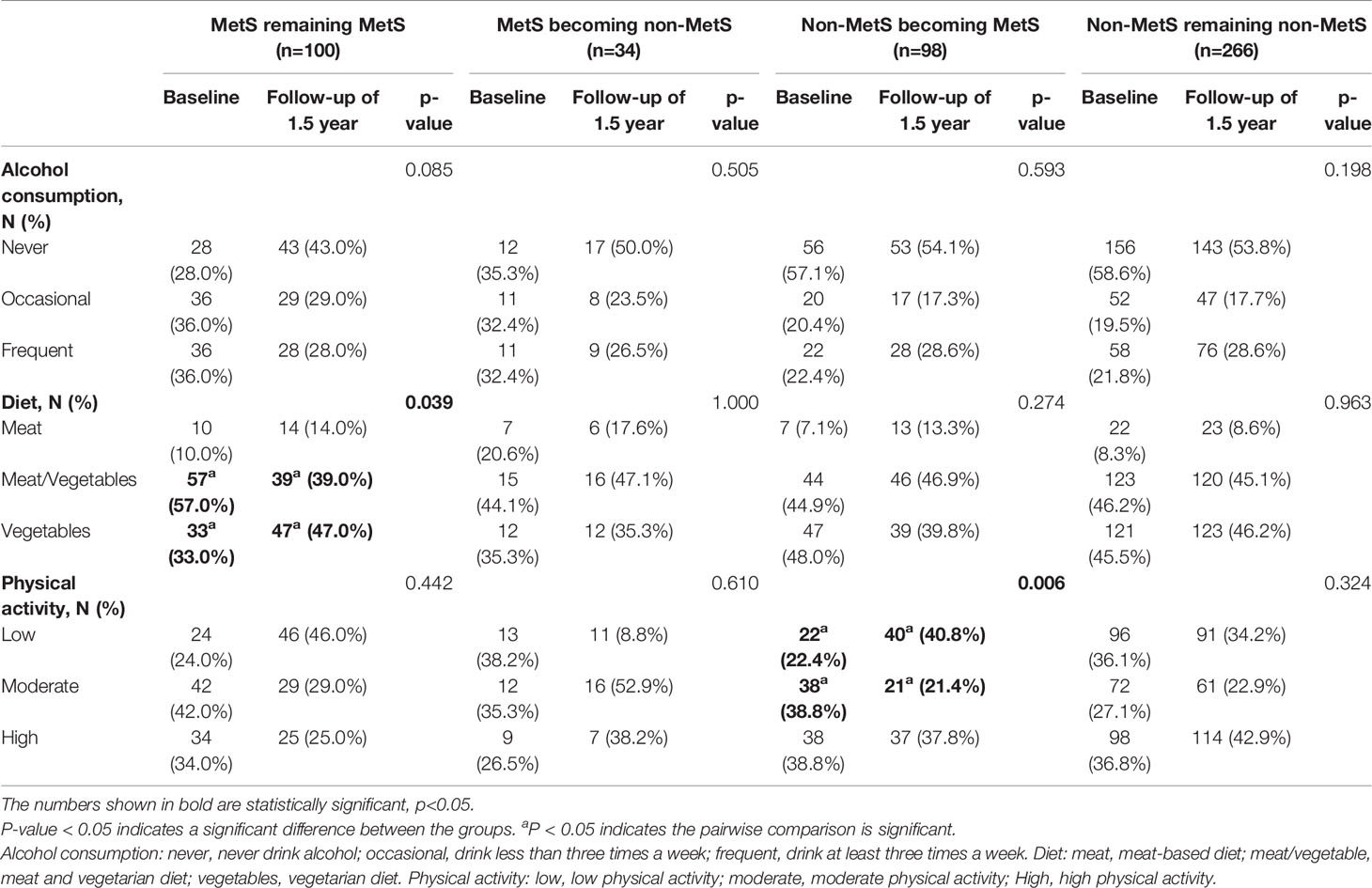
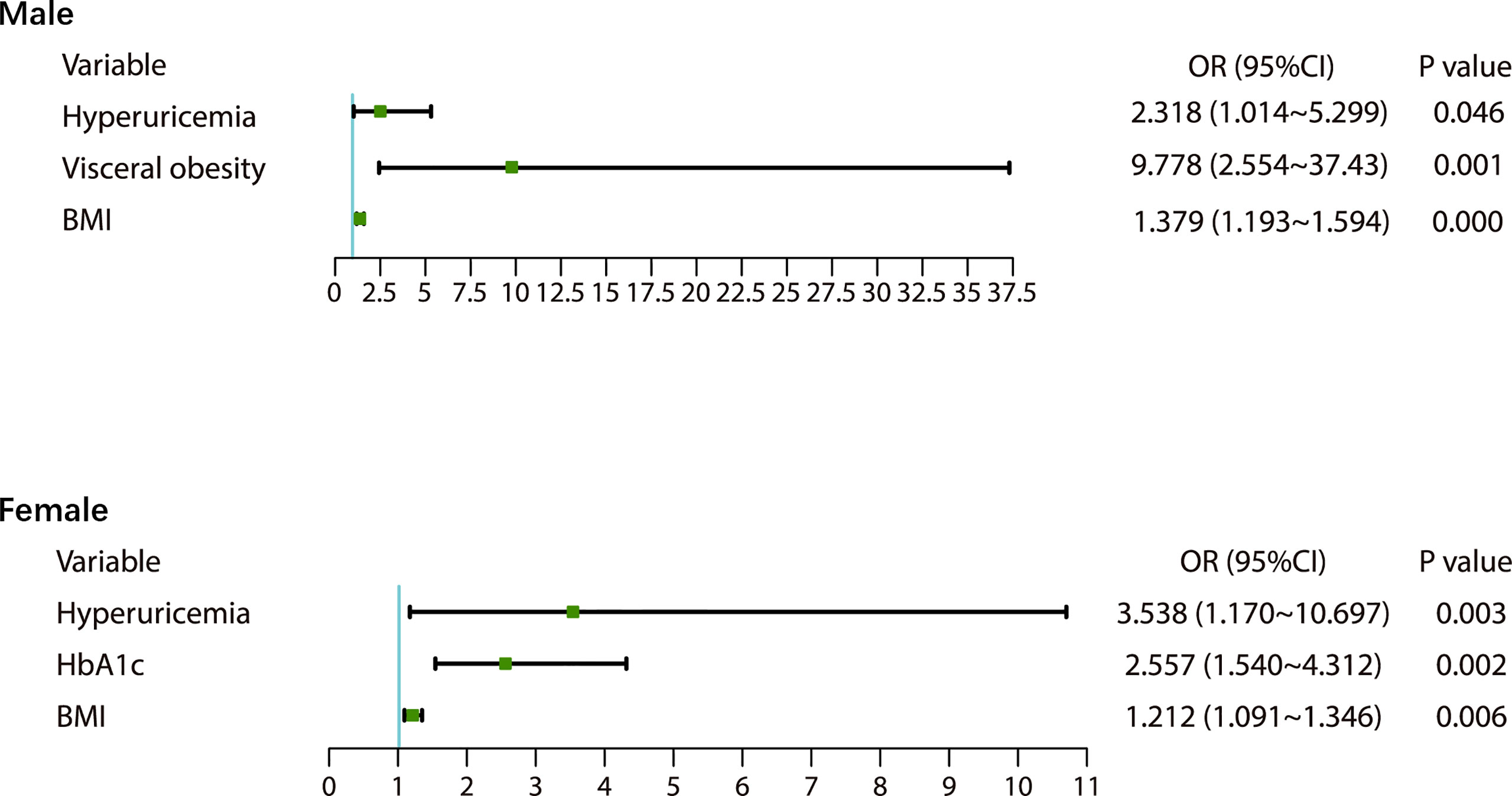
There were 98 new cases of MetS during the follow-up period. The cumulative incidence rate of MetS in the survey population was 25.40%; the incidence rate in males was 30.46% and the incidence in females was 23.68%. There was no significant difference in the incidence between men and women (p>0.05). The initial visit characteristics of the population separately are shown in Table 2. Alcohol consumption, diet and physical activity before and after follow-up are shown in Table 3. We found a significant increase in low-level physical activity and a significant decrease in moderate-level physical activity in patients with newly MetS (p< 0.05) (Table 3). Next, we analyzed risk factors that may have influenced the occurrence of MetS within 1.5 years in men and women separately (Figure 2). Through univariate logistic analysis, the independent variables affecting the incidence of MetS in men at the initial visit were identified. Multivariate logistic regression was performed with BUA, BMI and visceral obesity as independent variable. We found that BUA was >416 mmol/L (OR=2.318, 95% CI: 1.014-5.299, p<0.05) and high BMI (OR=1.379, 95% CI: 1.193-1.593, p<0.05) among men at the initial visit were associated with the occurrence of MetS (Figure 2). Men with visceral obesity were more likely to develop MetS than those with subcutaneous obesity (OR=9.778, 95% CI: 2.554-37.43, p<0.05) (Figure 2).
Variables affecting the incidence of MetS in women at the initial visit were identified through univariate logistic analysis. Multivariate logistic regression was performed with BUA, BMI and HbA1c as independent variable (Figure 2). We found that BUA>356.9 mmol/L (OR=3.538, 95% CI: 1.170-10.697, p<0.05), increased BMI (OR=1.212, 95% CI: 1.091-1.346, p<0.05) and increased HbA1c (OR=2.577, 95% CI: 1.540-4.312, p<0.05) were associated with the occurrence of MetS in women (Figure 2).
Factors Influencing MetS Recovery
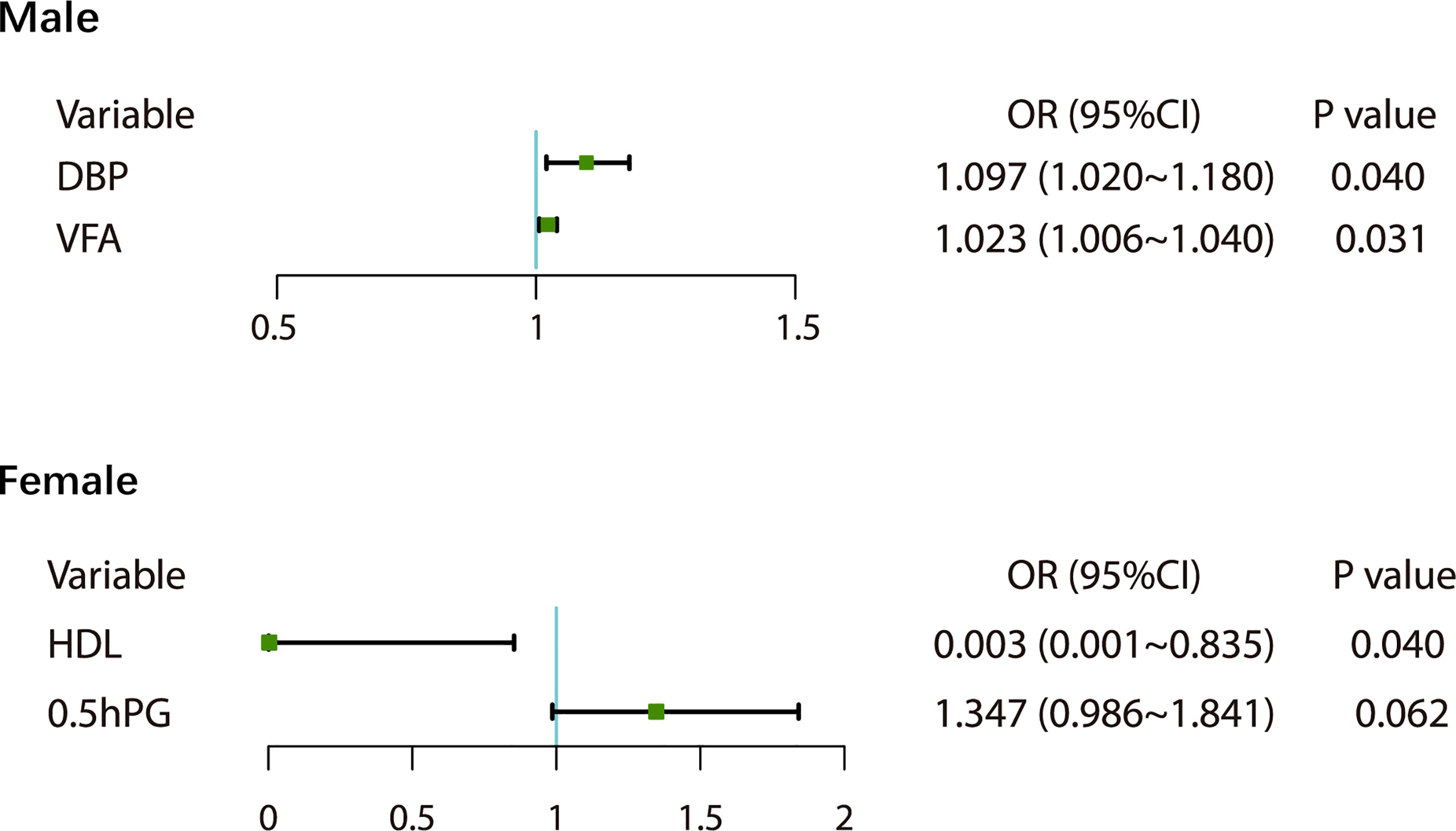
At baseline, 204 people with MetS were screened, of whom 134 were followed up, with a follow-up rate of 65.69%. After 1.5 years of follow-up and health guidance and lifestyle intervention, 25.37% of the 134 patients with MetS were classified as non-MetS, including 20 males (21.28%) and 14 females (35.00%). The initial visit characteristics of the population separately are shown in Table 4. We found that patients still with MetS after 1.5 years have higher vegetarian habits (p<0.05) (Table 3). Through univariate logistic analysis, it was found that the independent variables affecting the outcome of MetS among males were VFA and diastolic blood pressure (DBP), and Multivariate logistic regression was performed with “MetS to non-MetS” as the dependent variable. The results showed that men patients with increased DBP (OR=1.097, 95% CI: 1.020-1.180, p<0.05) and increased VFA (OR=1.023, 95% CI: 1.006-1.040, p<0.05) at the initial visit were unlikely to recover from MetS (Figure 3). Through univariate analysis, it was found that the independent variables affecting the outcome of MetS were high-density lipoprotein cholesterol (HDL-C) and OGTT0.5hPG. Multivariate logistic in the female group was performed with “MetS to non-MetS” as the dependent variable. Patients with higher HDL-C at the initial visit were significantly more likely to recover from MetS (β: -3.509, OR=0.003, 95% CI: 0.001-0.835, p<0.05), but elevated OGTT0.5hPG was not associated with the outcome of MetS in women (OR=1.347, 95% CI: 0.986~1.841, p>0.05) (Figure 3).
Discussion
In this prospective cohort study of the Chinese population, we found that within 1.5 years of follow-up, the cumulative incidence of MetS was 30.46% in men and 23.68% in women. Hyperuricemia is a risk factor for MetS in men, and men with visceral obesity are more likely to develop MetS than men with subcutaneous obesity. Women with elevated BMI, hyperuricemia and HbA1c are more likely to develop MetS. Elevated DBP and VFA make it difficult for men with MetS to convert to non-MetS. Elevated HDL-C makes women more likely to recover from MetS.
The association between hyperuricemia and MetS has been explored in many cross-sectional studies, but prospective studies are rare (7–9, 19, 20). Some studies suggest that MetS can increase the incidence of hyperuricemia (19–21); however, others suggest that hyperuricemia should be included as a component of MetS (7). The latest study that reviewed the China Health and Retirement Longitudinal Study (CHARLS) data from 2011 to 2015 concluded that there is a bidirectional relationship between MetS and hyperuricemia, which is in agreement with our research (9). In our study, the OR of female hyperuricemia patients with MetS was greater than that of males, which is in line with the study of American scholar Sui and Turkish scholar Onat et al. that pointed out that the relationship between hyperuricemia and MetS is stronger in females than in males (22). There is currently no direct evidence that lowering BUA levels improves MetS; therefore, larger randomized controlled trials are needed to identify uric acid as a target for MetS prediction and novel therapeutic interventions.
In this study, the incidence of MetS in men with visceral obesity was higher than that in men with subcutaneous obesity. Increased VFA also made men with MetS less likely to recover from MetS. Much evidence supports the hypothesis that visceral fat plays a role in the development of MetS (23–25). In addition, abdominal adipose tissue can secrete inflammatory factors, which are closely related to cardiovascular disease (26). Even the absence of clinical manifestations of obesity can lead to the occurrence of atherosclerosis (27, 28). WC cannot directly determine the content of visceral fat. CT and MRI have limited clinical applications. Therefore, it is necessary to actively use better indicators, such as the visceral adiposity index (VAI) or abdominal volume index (AVI), to improve the identification of visceral obesity (29). More attention should be given to predict the onset and outcome of MetS in men and guide the clinical adoption of more individualized diagnosis and treatment.
Due to the action of estrogen, women generally have higher levels of HDL-C than men (30), and fat is mainly distributed in the buttocks and legs, not the abdomen. During menopause, hormonal changes in women lead to increased visceral fat and elevated blood lipids, which are accompanied by insulin resistance, increased free fatty acid concentrations and increased hepatic lipase activity (31). Epidemiological studies have shown that the prevalence of MetS in older people has tripled in men and increased fivefold in women compared with middle-aged people (32). In a study of the components of MetS, female MetS patients were found to have higher body weight and WC and a higher prevalence of low-density lipoproteinemia (33). This finding is consistent with our results. This shows that the occurrence and prognosis of MetS in women may be driven by high-density lipoprotein. In addition, in this study, no significant differences in the menopause status of women with MetS and non-MetS groups were found, so this needs to be further discussed in future studies.
In this study, 750 people living in Northeast China were followed up for 1.5 years, with a follow-up rate of 66.93%. This prospective research survey with a large sample size can better explain the causal link between influencing factors and diseases. In addition, the age group included in this study was 40-65 years old, which is in the critical period of the body’s transition from middle age to old age. The incidence of MetS was highly variable, and it was more targeted and clinically valuable. In addition, this study is the first to explore the impact of different risk factors on disease outcomes, which is valuable for clinical guidance. This study also included a detailed questionnaire survey, which clearly asked the participants about the status of alcohol consumption, eating habits and physical activity, helping us to rule out confounding factors that might have contributed to the conclusions. In addition, this study surveyed chronic diseases and excluded the influence of medication for diabetes, hypertension hyperlipidemia and hyperuricemia on this study. This study also has certain limitations. The age and geographical limitations of the research subjects affect the generalizability of the research conclusions. The follow-up time of the study was 1.5 years, which could not fully reflect the whole process of the occurrence, development and evolution of MetS. Larger, longer-term prospective studies are needed to investigate the outcome of MetS in the future.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of China Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conception and design of study: ZS and YL; acquisition of data: YL and HW; analysis and/or interpretation of data: CZ, SF and YL; drafting the manuscript: CZ and SF; revising the manuscript critically for important intellectual content: YL and ZS. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Key Laboratory Project of Thyroid Diseases, National Health Commission (Grant No. 2019PT330001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, et al. The Metabolic Syndrome. Endocr Rev (2008) 29(7):777–822. doi: 10.1210/er.2008-0024
2. Tanner RM, Brown TM, Muntner P. Epidemiology of Obesity, the Metabolic Syndrome, and Chronic Kidney Disease. Curr Hypertens Rep (2012) 14(2):152–9. doi: 10.1007/s11906-012-0254-y
3. Grundy SM. Metabolic Syndrome: A Multiplex Cardiovascular Risk Factor. J Clin Endocrinol Metab (2007) 92(2):399–404. doi: 10.1210/jc.2006-0513
4. Gallagher EJ, Leroith D, Karnieli E. The Metabolic Syndrome–From Insulin Resistance to Obesity and Diabetes. Med Clin North Am (2011) 95(5):855–73. doi: 10.1016/j.mcna.2011.06.001
5. McCracken E, Monaghan M, Sreenivasan S. Pathophysiology of the Metabolic Syndrome. Clin Dermatol (2018) 36(1):14–20. doi: 10.1016/j.clindermatol.2017.09.004
6. Hayashi T, Boyko EJ, Leonetti DL, McNeely MJ, Newell-Morris L, Kahn SE, et al. Visceral Adiposity and the Prevalence of Hypertension in Japanese Americans. Circulation (2003) 108(14):1718–23. doi: 10.1161/01.Cir.0000087597.59169.8d
7. Huang G, Xu J, Zhang T, Cai L, Liu H, Yu X, et al. Hyperuricemia is Associated With Metabolic Syndrome in the Community Very Elderly in Chengdu. Sci Rep (2020) 10(1):8678. doi: 10.1038/s41598-020-65605-w
8. Wei CY, Sun CC, Wei JC, Tai HC, Sun CA, Chung CF, et al. Association Between Hyperuricemia and Metabolic Syndrome: An Epidemiological Study of a Labor Force Population in Taiwan. BioMed Res Int (2015) 2015:369179. doi: 10.1155/2015/369179
9. Chen WY, Fu YP, Zhou M. The Bidirectional Relationship Between Metabolic Syndrome and Hyperuricemia in China: A Longitudinal Study From CHARLS. Endocrine (2022) 76(1):62–9. doi: 10.1007/s12020-022-02979-z
10. Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The Metabolic Syndrome: Prevalence and Associated Risk Factor Findings in the US Population From the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med (2003) 163(4):427–36. doi: 10.1001/archinte.163.4.427
11. Lee S, Ko Y, Kwak C, Yim ES. Gender Differences in Metabolic Syndrome Components Among the Korean 66-Year-Old Population With Metabolic Syndrome. BMC Geriatr (2016) 16:27. doi: 10.1186/s12877-016-0202-9
12. Wang WS, Wahlqvist ML, Hsu CC, Chang HY, Chang WC, Chen CC. Age- and Gender-Specific Population Attributable Risks of Metabolic Disorders on All-Cause and Cardiovascular Mortality in Taiwan. BMC Public Health (2012) 12:111. doi: 10.1186/1471-2458-12-111
13. Krieger N. Genders, Sexes, and Health: What are the Connections–and Why Does it Matter? Int J Epidemiol (2003) 32(4):652–7. doi: 10.1093/ije/dyg156
14. Regitz-Zagrosek V, Oertelt-Prigione S, Prescott E, Franconi F, Gerdts E, Foryst-Ludwig A, et al. Gender in Cardiovascular Diseases: Impact on Clinical Manifestations, Management, and Outcomes. Eur Heart J (2016) 37(1):24–34. doi: 10.1093/eurheartj/ehv598
15. Yamaoka K, Tango T. Effects of Lifestyle Modification on Metabolic Syndrome: A Systematic Review and Meta-Analysis. BMC Med (2012) 10:138. doi: 10.1186/1741-7015-10-138
16. Ostman C, Smart NA, Morcos D, Duller A, Ridley W, Jewiss D. The Effect of Exercise Training on Clinical Outcomes in Patients With the Metabolic Syndrome: A Systematic Review and Meta-Analysis. Cardiovasc Diabetol (2017) 16(1):110. doi: 10.1186/s12933-017-0590-y
17. Ford ES. Prevalence of the Metabolic Syndrome Defined by the International Diabetes Federation Among Adults in the U.S. Diabetes Care (2005) 28(11):2745–9. doi: 10.2337/diacare.28.11.2745
18. Bao Y, Lu J, Wang C, Yang M, Li H, Zhang X, et al. Optimal Waist Circumference Cutoffs for Abdominal Obesity in Chinese. Atherosclerosis (2008) 201(2):378–84. doi: 10.1016/j.atherosclerosis.2008.03.001
19. Liu JH, Ma QH, Xu Y, Chen X, Pan CW. Metabolic Syndrome and 5-Year Incident Hyperuricemia Among Older Chinese Adults: A Community-Based Cohort Study. Diabetes Metab Syndr Obes (2020) 13:4191–200. doi: 10.2147/dmso.S278542
20. Kim IY, Han KD, Kim DH, Eun Y, Cha HS, Koh EM, et al. Women With Metabolic Syndrome and General Obesity Are at a Higher Risk for Significant Hyperuricemia Compared to Men. J Clin Med (2019) 8(6):837. doi: 10.3390/jcm8060837
21. Nagahama K, Inoue T, Kohagura K, Ishihara A, Kinjo K, Ohya Y. Hyperuricemia Predicts Future Metabolic Syndrome: A 4-Year Follow-Up Study of a Large Screened Cohort in Okinawa, Japan. Hypertens Res (2014) 37(3):232–8. doi: 10.1038/hr.2013.137
22. Onat A, Uyarel H, Hergenç G, Karabulut A, Albayrak S, Sari I, et al. Serum Uric Acid is a Determinant of Metabolic Syndrome in a Population-Based Study. Am J Hypertens (2006) 19(10):1055–62. doi: 10.1016/j.amjhyper.2006.02.014
23. Hwang YC, Hayashi T, Fujimoto WY, Kahn SE, Leonetti DL, McNeely MJ, et al. Visceral Abdominal Fat Accumulation Predicts the Conversion of Metabolically Healthy Obese Subjects to an Unhealthy Phenotype. Int J Obes (Lond) (2015) 39(9):1365–70. doi: 10.1038/ijo.2015.75
24. Lim KI, Yang SJ, Kim TN, Yoo HJ, Kang HJ, Song W, et al. The Association Between the Ratio of Visceral Fat to Thigh Muscle Area and Metabolic Syndrome: The Korean Sarcopenic Obesity Study (KSOS). Clin Endocrinol (Oxf) (2010) 73(5):588–94. doi: 10.1111/j.1365-2265.2010.03841.x
25. Rothney MP, Catapano AL, Xia J, Wacker WK, Tidone C, Grigore L, et al. Abdominal Visceral Fat Measurement Using Dual-Energy X-Ray: Association With Cardiometabolic Risk Factors. Obes (Silver Spring) (2013) 21(9):1798–802. doi: 10.1002/oby.20223
26. Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the Release of Adipokines by Adipose Tissue, Adipose Tissue Matrix, and Adipocytes From Visceral and Subcutaneous Abdominal Adipose Tissues of Obese Humans. Endocrinology (2004) 145(5):2273–82. doi: 10.1210/en.2003-1336
27. Ho JS, Cannaday JJ, Barlow CE, Willis B, Haskell WL, FitzGerald SJ. Comparative Relation of General, Central, and Visceral Adiposity Measures for Coronary Artery Calcium in Subjects Without Previous Coronary Events. Am J Cardiol (2009) 104(7):943–6. doi: 10.1016/j.amjcard.2009.05.047
28. Kim SK, Park SW, Hwang IJ, Lee YK, Cho YW. High Fat Stores in Ectopic Compartments in Men With Newly Diagnosed Type 2 Diabetes: An Anthropometric Determinant of Carotid Atherosclerosis and Insulin Resistance. Int J Obes (Lond) (2010) 34(1):105–10. doi: 10.1038/ijo.2009.210
29. Wang H, Liu A, Zhao T, Gong X, Pang T, Zhou Y, et al. Comparison of Anthropometric Indices for Predicting the Risk of Metabolic Syndrome and its Components in Chinese Adults: A Prospective, Longitudinal Study. BMJ Open (2017) 7(9):e016062. doi: 10.1136/bmjopen-2017-016062
30. Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and Pcos Society Disease State Clinical Review: Guide to the Best Practices in the Evaluation and Treatment of Polycystic Ovary Syndrome - Part 2. Endocr Pract (2015) 21(12):1415–26. doi: 10.4158/ep15748.Dscpt2
31. Santilli F, D'Ardes D, Guagnano MT, Davi G. Metabolic Syndrome: Sex-Related Cardiovascular Risk and Therapeutic Approach. Curr Med Chem (2017) 24(24):2602–27. doi: 10.2174/0929867324666170710121145
32. Vishram JK, Borglykke A, Andreasen AH, Jeppesen J, Ibsen H, Jørgensen T, et al. Impact of Age and Gender on the Prevalence and Prognostic Importance of the Metabolic Syndrome and its Components in Europeans. The MORGAM Prospective Cohort Project. PloS One (2014) 9(9):e107294. doi: 10.1371/journal.pone.0107294
Keywords: metabolic syndrome, hyperuricemia, visceral obesity, BMI, HDL-C (high density lipoprotein-cholesterol)
Citation: Zhang C, Fang S, Wang H, Shan Z and Lai Y (2022) Factors Related to Metabolic Syndrome Development and Recovery in Chinese Adults: A Prospective Longitudinal Study. Front. Endocrinol. 13:923650. doi: 10.3389/fendo.2022.923650
Received: 19 April 2022; Accepted: 12 May 2022;
Published: 13 June 2022.
Edited by:
Luca Busetto, Università degli Studi di Padova, ItalyReviewed by:
Małgorzata Wójcik, Jagiellonian University Medical College, PolandGianluca Gortan Cappellari, University of Trieste, Italy
Copyright © 2022 Zhang, Fang, Wang, Shan and Lai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongyan Shan, cmushanzhongyan@163.com; Yaxin Lai, laiyaxin811005@126.com
 Chenyu Zhang
Chenyu Zhang Sisi Fang2
Sisi Fang2 Yaxin Lai
Yaxin Lai