- 1Department of Community Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences (TUMS), Tehran, Iran
- 2Network of Interdisciplinarity in Neonates and Infants (NINI), Universal Scientific Education and Research Network (USERN), Tehran, Iran
- 3Student Research Committee, Alborz University of Medical Sciences, Karaj, Iran
- 4Endocrinology and Metabolism Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran
- 5Department of Clinical Biochemistry, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
- 6Non-Communicable Diseases Research Center, Endocrinology and Metabolism Population Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran
- 7Toxicology and Diseases Group (TDG), Pharmaceutical Sciences Research Center (PSRC), The Institute of Pharmaceutical Sciences (TIPS), Tehran University of Medical Sciences (TUMS), Tehran, Iran
- 8Metabolic Disorders Research Center, Endocrinology and Metabolism Molecular-Cellular Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran
Background: There is controversial data on the effects of prebiotic, probiotic, or synbiotic supplementations on overweight/obesity indicators. Thus, we aimed to clarify this role of biotics through an umbrella review of the trials’ meta-analyses.
Methods: All meta-analyses of the clinical trials conducted on the impact of biotics on overweight/obesity indicators in general populations, pregnant women, and infants published until June 2023 in PubMed, Web of Sciences, Scopus, Embase, and Cochrane Library web databases included. The meta-analysis of observational and systematic review studies without meta-analysis were excluded. We reported the results by implementing the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flowchart. The Assessment of Multiple Systematic Reviews-2 (AMSTAR2) and Grading of Recommendations Assessment, Development, and Evaluation (GRADE) systems were used to assess the methodological quality and quality of evidence.
Results: Overall, 97 meta-analysis studies were included. Most studies were conducted on the effect of probiotics in both genders. Consumption of prebiotic: 8-66 g/day, probiotic: 104 -1.35×1015 colony-forming unit (CFU)/day, and synbiotic: 106-1.5×1011 CFU/day and 0.5-300 g/day for 2 to 104 weeks showed a favorable effect on the overweight/obesity indicators. Moreover, an inverse association was observed between biotics consumption and overweight/obesity risk in adults in most of the studies. Biotics did not show any beneficial effect on weight and body mass index (BMI) in pregnant women by 6.6×105-1010 CFU/day of probiotics during 1-25 weeks and 1×109-112.5×109 CFU/capsule of synbiotics during 4-8 weeks. The effect of biotics on weight and BMI in infants is predominantly non-significant. Prebiotics and probiotics used in infancy were from 0.15 to 0.8 g/dL and 2×106-6×109 CFU/day for 2-24 weeks, respectively.
Conclusion: It seems biotics consumption can result in favorable impacts on some anthropometric indices of overweight/obesity (body weight, BMI, waist circumference) in the general population, without any significant effects on birth weight or weight gain during pregnancy and infancy. So, it is recommended to intake the biotics as complementary medications for reducing anthropometric indices of overweight/obese adults. However, more well-designed trials are needed to elucidate the anti-obesity effects of specific strains of probiotics.
1 Introduction
Over the last four decades, there has been a threefold acceleration in the global prevalence of obesity (1). In 2019, a systematic review and meta-analysis highlighted that 21.4% of elderly individuals in Iran were affected by obesity (2). As a global public health issue, it is linked to the prevalence of different chronic severe conditions, including diabetes, cardiometabolic diseases, hypertension, hyperlipidemia, and malignancy (3, 4). Although the pathogenesis of obesity and overweight is influenced by genetic and environmental factors, it is widely recognized that the primary cause of weight gain is a persistent imbalance between excessive energy intake and inadequate energy expenditure (5–7).
Despite various weight loss strategies being proposed, their long-term effectiveness has been limited. Consequently, there is an increasing demand for innovative methods to supplement existing strategy. The gut microbiota has recently emerged as a critical environmental factor in the development of obesity and its associated metabolic irregularities. Integrating an understanding of gut microbiota with traditional measures, such as a balanced diet and lifestyle modifications, is now recommended for effective weight management (8–12). The gut microbiome, a diverse microbial community in the human digestive system, plays a crucial role in shaping the host’s overall physiology by participating in various metabolic functions (13).
The International Scientific Association of Probiotics and Prebiotics (ISAPP) defines probiotics as live microorganisms offering health benefits upon ingesting in specific quantities. Similarly, prebiotics are characterized as substrates specifically utilized by microorganisms within the host, leading to health benefits. Additionally, synbiotics entail a combination of live microorganisms and substrates that are selectively utilized by microorganisms within the host, resulting in health benefits for the host (14–16). Probiotic and synbiotic supplementation have attracted attention for their potential in regulating gut microbiota and body weight. They can produce short-chain fatty acids that influence hormones responsible for appetite regulation and enhance the resting energy (17–19).
There is accumulating evidence that individuals who are overweight or obese exhibit a distinct profile of the gut microbiota, including reduced microbial gene richness and diversity (known as dysbiosis) compared with normal weight (10, 20, 21). These alterations have been linked to low-grade inflammation, impaired energy metabolism homeostasis, elevated body weight, and dysregulation of insulin signaling (22). Hence, targeting gut microbiota has recently been a promising strategy for treating obesity and related metabolic disorders. Controversy exists regarding the impact of prebiotic, probiotic, or synbiotic consumption on body weight change during gestational diabetes and pregnancy (23, 24), as well as infancy and toddler stages (25–27).
To this point, some studies indicated that supplementing infant formula with prebiotics for full-term infants increases weight gain. Additionally, toddlers consuming milk with synbiotics demonstrated improved growth and greater weight gain (25, 27). However, another systematic review reported that infant formula intake enriched with probiotics or synbiotics did not impact weight in infants and toddlers (26). A meta-analysis showed that probiotic and synbiotic supplements can improve newborn weight among gestational diabetes mellitus patients (23). However, another meta-analysis revealed no significant difference in mean weight at the end of the trial and in gestational weight gain between the intervention group and the control group (24).
Numerous systematic reviews with meta-analysis, though yielding conflicting results, have been conducted to assess the effects of biotics on anthropometric indices such as weight, body mass index (BMI), and waist circumference (WC) (9, 22, 25–29). Moreover, no review of the meta-analysis studies from these trials has comprehensively examined the effect of biotics on various obesity indices in both adults and infants. Some umbrella reviews were conducted in this regard (30–32). However, considering the divergent results in the existing literature, our study extensively searched all interventions to attain a more comprehensive understanding. This umbrella review of the trials’ meta-analysis studies aims to give a snapshot of the influence of prebiotic, probiotic, or synbiotic intake on body weight changes, irrespective of age and sex differences.
2 Methods and materials
This study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA), ensuring the reporting of preferred items for systematic reviews and meta-analyses (33).
2.1 Search strategy
A comprehensive search was conducted across multiple international web databases, including PubMed, Scopus, Web of Science, Embase, and Cochrane Library, to identify pertinent meta-analyses exploring the relationship between prebiotic, probiotic, or synbiotic supplementation and body weight. This search encompassed records up to June 2023. Furthermore, the bibliographies of research papers were examined to identify potential studies that may have been overlooked during the initial search. The PICO (Participant, Intervention, Comparison/Control, Outcome) search framework was employed to systematically explore articles on the effects of prebiotic, probiotic, or synbiotic supplementation on overweight/obesity indicators such as body weight gain, BMI, or WC. An English language criterion was applied for inclusion to ensure comprehensive coverage of relevant studies. To prevent the omission of pertinent research, a combination of MeSH terms and keywords was employed as the initial approach for data collection. Our exclusion criteria were meta-analyses of observational studies, in vivo/in vitro research, case reports, and systematic review studies without meta-analysis. Gray literature and conference abstracts were considered if they provided substantial data. Hand-searching of the reference list of the included studies was performed to find relevant studies. A comprehensive outline of the search strategy is available in Supplementary Table S1.
2.2 Study selection
Research studies were deemed eligible for inclusion if they met most of the following criteria: (1) Systematic review/meta-analysis of various types of clinical trials; (2) examined the sole or combination intake of prebiotic, probiotic, or synbiotic; (3) evaluated the impact of prebiotic, probiotic, or synbiotic supplementation on overweight/obesity indicators such as weight gain, BMI or WC; (4) compared the effects of the supplementation with either a placebo or a standard treatment as the control group; (5) encompassed participants of all age groups and genders; (6) included healthy individuals, or those with any medical condition.
2.3 Data extraction
FE and MRH independently assessed the validity of eligible studies by reviewing titles and abstracts, extracting outcomes, and evaluating the credibility of the included publications. Consensus was achieved through consultation with the corresponding authors (OTM and SE) in the discrepancies. Data points encompassed title, authors, publication year, geographic region of the study, population details (total number, age, gender, underlying condition), the number and design of included trials, intervention dose and duration, primary outcome, subgroup analyses, dose-response findings, and reported effective dosage. Three e-mails were frequently sent to the corresponding author to find the full text of inaccessible studies.
2.4 Quality assessment
FE and MRH independently assessed the quality of the studies, addressing discrepancies through consultation with OTM and SE. The evaluation employed the “A Measurement Tool to Assess Multiple Systematic Reviews–2” (AMSTAR2), a validated tool suitable for assessing the internal validity of intervention-focused systematic reviews (34). The evaluation results were tabulated in Supplementary Table S2. Moreover, The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) systems were used to assess the evidence quality (35), (Supplementary Table S3 and Table 1). Rating discrepancies were discussed and resolved, involving a third party if needed.
3 Results
3.1 Studies characteristics
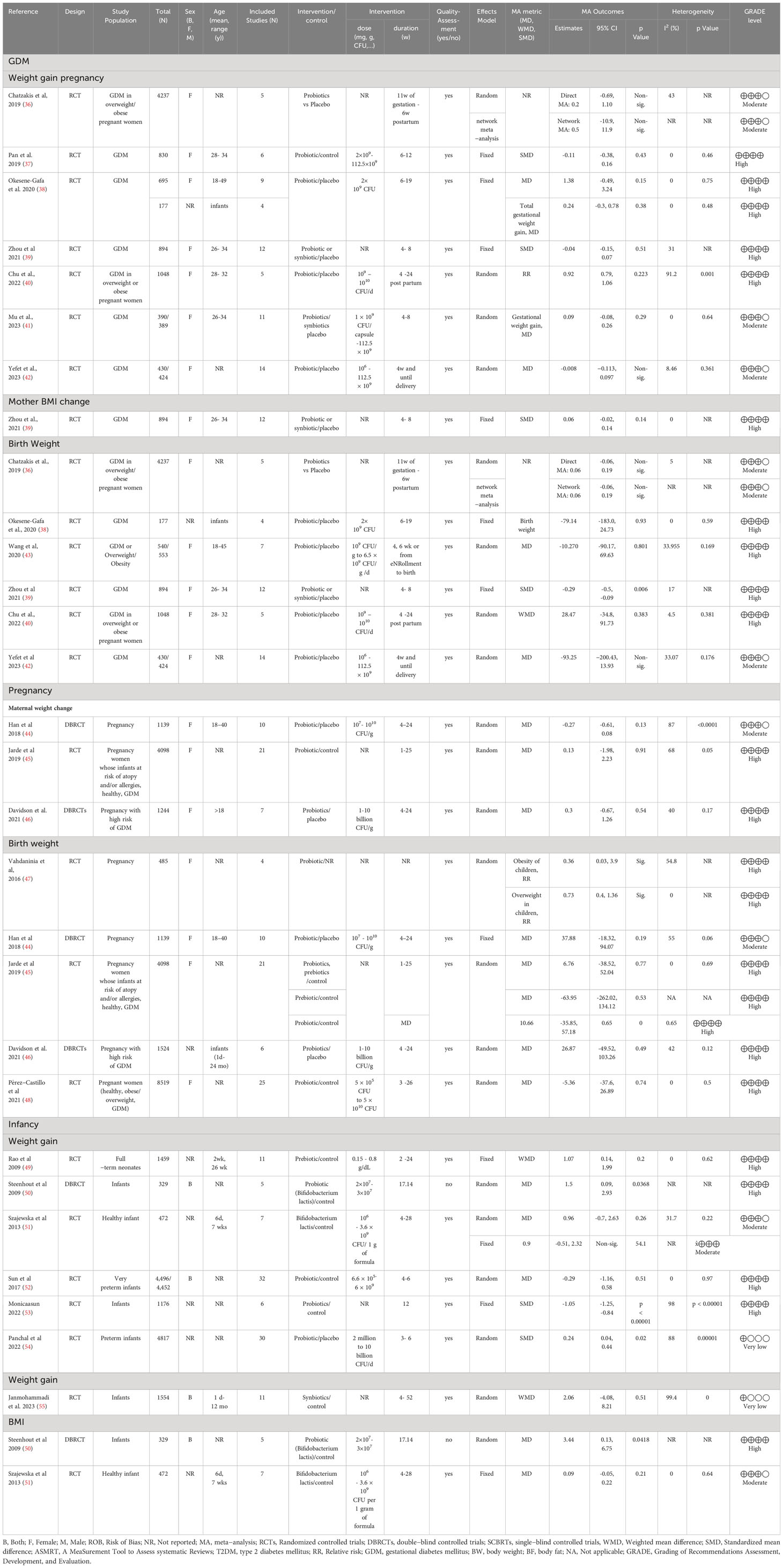
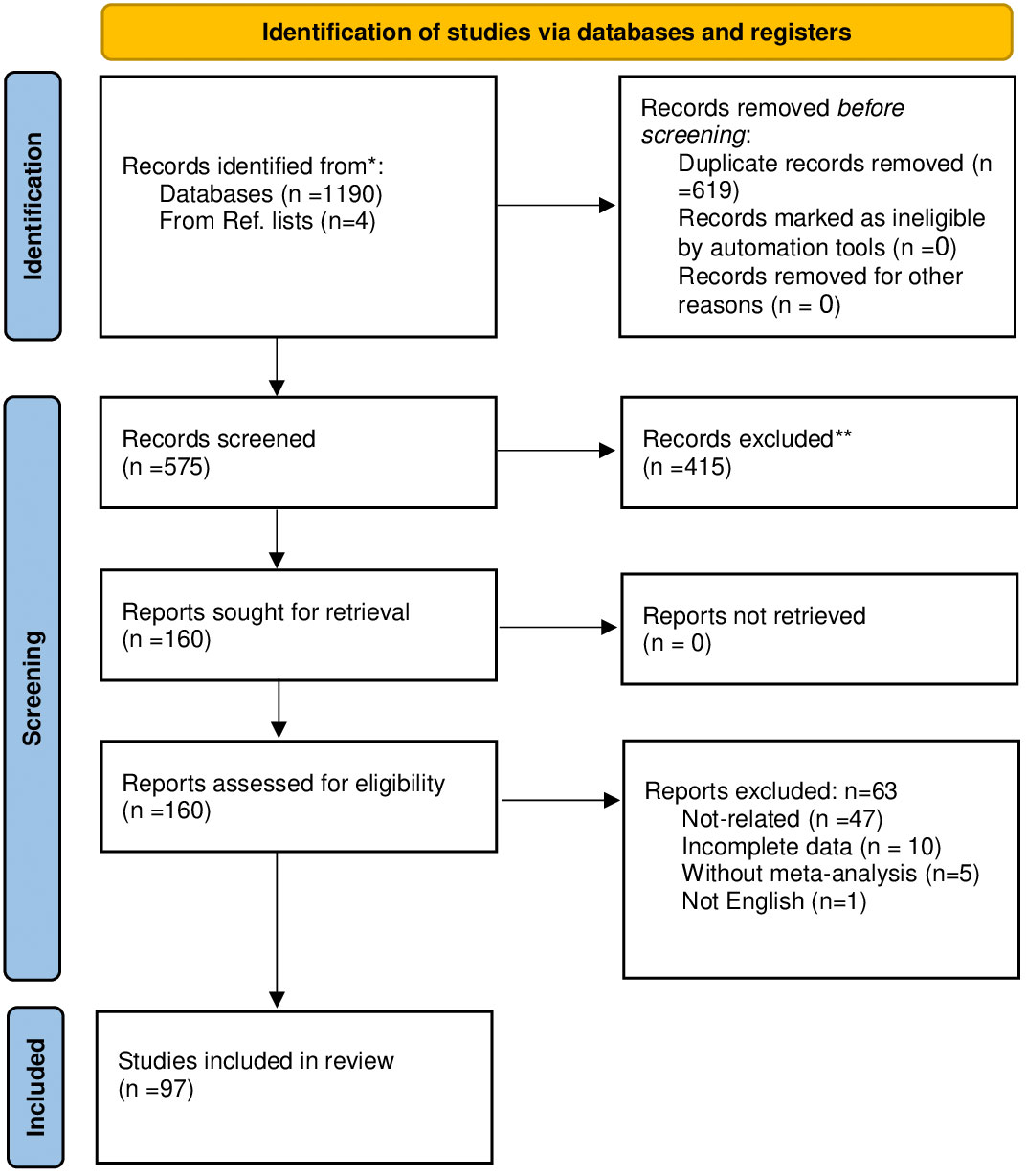
A total of 97 papers satisfied the inclusion criteria and were incorporated into the study. The search process for these studies under the PRISMA flowchart is outlined in Figure 1. Subsequently, based on the population studied, they were classified into three distinct groups: (1) general population, (2) pregnant, and (3) infants. Comprehensive details of the selected studies can be found in Supplementary Table S3 and Table 1.
Most included studies were meta-analyses of clinical trials centered on prebiotic, probiotic, or synbiotic intervention. However, one meta-analysis study focused on clinical trials besides observational studies exploring probiotic supplementation. These studies encompassed both genders and examined the effects of the biotics versus placebo.
The study populations ranged from 134 to 1,324,640 participants, spanning ages from 1 day to 87 years. Participants comprised healthy individuals as well as patients with various background conditions, including metabolic syndrome (MetS), type 2 diabetes mellitus (T2DM), dyslipidemia, obesity, and hypertension (HTN). Almost all of the included meta-analyses underwent quality assessment. Further details of the included studies are provided as follows.
3.2 Effects of prebiotic, probiotic, or synbiotic supplementation on overweight/obesity measurements in the general population
Overall, sixteen papers assessed the prebiotic effect on overweight/obesity variables. Among these trials, the most frequently studied conditions were non-alcoholic fatty liver disease (NAFLD) or obesity. The primary outcome measures in these studies were focused on body weight (56–63), BMI (56, 57, 59, 61–66), WC (62, 64, 67, 68), or body fat mass (BFM) (56, 57, 62). Prebiotic supplementation exhibited a significant reducing effect on body weight and BMI. Nonetheless, the impact of prebiotic consumption on body weight and BMI among patients with NAFLD and DM displayed inconsistency (64). Regarding body weight, 3 out of 5 studies demonstrated a weight-reducing effect (56, 58, 67). In the case of BMI, a reducing effect was observed in 2 out of 5 studies (65, 67). Similarly, investigations into the effects of prebiotic supplementation on BFM (56, 57) and WC (65, 67) showed a neutral effect on these measurements. The participants’ ages spanned from 1 day to 77 years, and both genders were represented. The administered doses and duration of the studies ranged from 0.007 × 109-150 ×109 colony-forming unit (CFU)/day to 0.88-66000 mg/d for 1-104 weeks. Notably, significant body weight and BMI reductions were observed with prebiotic treatment lasting more than 7 and 15 days, utilizing 8- 66 gr/day doses for both parameters.
A total of sixty-two papers were incorporated in assessing probiotics’ effect on obesity variables. All of them were clinical trial meta-analyses, and an additional one focused on clinical trials and observational studies. The research primarily concentrated on obese individuals with NAFLD aged one day to 85 years old. The main measured indicators were body weight (23, 28, 56, 59, 69–85), BMI (23, 28, 56, 59, 60, 65, 69–82, 84–105), WC (28, 68, 70, 72, 75, 79–81, 85, 87, 90, 93, 94, 99, 101), hip circumference (HC) (90), waist to hip ratio (WHR) (78, 80, 90, 94), body fat percent (BF%) (71, 75, 80, 85, 90, 94), and BFM (56, 61, 71, 72, 75, 80, 81, 90). Probiotic supplementation significantly reduced weight, BMI, WC, BF%, and BFM across most studies. However, investigations into the impact of probiotic intake on variables such as HC (90) and percentage of excess weight loss (%EWL) (76, 105) revealed neutral effects on these measurements. In summary, the dosages of probiotic supplements varied widely among the included studies in 1.0 × 104 to 1.35 × 1015 CFU/day, with administration durations ranging from 2 to 104 weeks. Notably, body weight and BMI showed significant reductions with probiotic treatment for over 15 days at doses ranging from 4.97 × 106 to 7 × 1011 CFU/day.
Also, twenty-five meta-analyses of clinical trials were included in the current study to examine the impact of synbiotics on overweight/obesity indices. Their main focus of the outcome measurements was on body weight (24, 28, 56, 106), BMI (28, 56, 91, 106, 107), WC (28, 68, 106, 107), and BFM (56). Synbiotic supplementation exhibited notable decreasing effects on WC in 4 out of 6 studies (68, 106–108), as well as on BFM in 1 out of 2 studies (109). However, the impact of synbiotic consumption on weight and BMI showed inconsistency. Among the included studies, 3 out of 7 reported a reduction in body weight (63, 106, 107), and 2 out of 6 studies observed a decrease in BMI (63, 91) following synbiotic supplementation. The study participants, spanning both genders and aged from 1 day to 85 years old, received varied treatment doses of synbiotics: 0.5-300 g/day and 106-1.5×1011 CFU/day over durations ranging from 2 to 104 weeks. Synbiotic treatment for more than 15 days and at doses ranging from 3.7 × 106 to 1 × 1011 CFU/day and 9-150 g/d led to a reduction in body weight. In addition, in some studies, the effect of consuming prebiotics, probiotics, and synbiotics was not reported separately, and their collective impact was stated in comparison with the control group. BMI (56, 110) and weight reduction (56, 111) were observed in some studies, but a neutral effect on BMI (112) and EWL% (113) was found in others.
3.3 Effects of prebiotic, probiotic, or synbiotic supplementation on overweight/obesity measurements in pregnancy population
The prebiotic, probiotic, or synbiotic did not have any beneficial effects during pregnancy on weight and BMI with treatment doses 6.6 × 105-1010 CFU/day of probiotics during 1-25 weeks and 1 × 109-112.5 × 109 CFU/capsule of synbiotic during 4-8 weeks. The effect of prebiotics on these anthropometric indices during pregnancy was assessed in only one study (114) in which the administered doses were not reported. The duration of prebiotic usage during pregnancy varied, ranging from 1 to 25 weeks, and was administered in differing doses. Vahdaninia et al. (115) showed a significant increase in overweight/obesity in children with probiotic supplementation in pregnant women (116). In addition, probiotic or synbiotic supplementation compared to placebo consumption (23) showed a negative significant effect on newborn weight in GDM with a neutral impact on gestational weight and BMI change, as well as mother weight and BMI at the end of the trial. In the rest of the included studies, there was no prebiotic, probiotic, or synbiotic supplementation effect on birth weight, gestational weight gain, and weight change for mothers during pregnancy.
3.4 Effects of prebiotic, probiotic, or synbiotic supplementation on overweight/obesity measurements in infants
The dosage administered for prebiotics and probiotics in infancy were 0.15 to 0.8 g/dL (2-24 weeks) and 2×106 -6 × 109 CFU/day (2-24 weeks), respectively. Synbiotics were used in infancy for ages 4 to 52 weeks, with doses varying across the studies. Overall, one study assessed the effects of prebiotic supplementation on weight gain (117), which showed a non-significant increase. Also, the consumption of probiotics observed a non-significant impact on weight gain, except in two studies (118, 119), which reported a reduction in weight gain, and another study (119), showed an increase in weight gain for a short term. Moreover, only one study (120), investigated the effect of synbiotics on weight gain, and the results were neutral.
3.5 Quality of methodology and evidence
The AMSTAR2 assessment revealed that 23 studies were categorized as having critically low quality, while 24 were rated as low quality. The primary limitations in these assessments were associated with item two, which concerns the registration protocol before conducting the review; item seven, which involves providing a list of excluded studies and justifying their exclusions; and item 10, which pertains to the reporting of funding sources for individual studies (refer to Supplementary Table S2 for details).
Among the six outcomes investigated in the general population, a significant proportion of studies that examined the impact on BMI (54.76%) or weight (53.06%) found evidence of moderate or high quality, with estimated significant positive effects on reducing BMI or weight. Studies focusing on weight predominantly exhibited moderate to high-quality evidence (53.06%). However, the quality of evidence for the three studies concerning the percentage of excess weight loss was notably low, and they did not show a significant reduction in excess weight among obese and morbidly obese participants.
For studies related to WC, the majority (70%) demonstrated high or moderate-quality evidence, indicating significant beneficial effects. In contrast, studies investigating HC and WHR were characterized by low-quality evidence in 83.3% of cases and did not show significant reductions in HC or WHR. Moreover, most studies examining the impact on BFM (62.5%) were backed by either high or moderate-quality evidence, and these studies revealed significant enhancements, as outlined in Supplementary Table S3.
In the context of GDM, pregnancy, and infancy populations, when considering the seven outcomes, 66.67% of the studies exhibited high-quality evidence, 27.78% were associated with moderate-quality evidence, and a mere 5.56% had very low-quality evidence, as indicated in Table 1. However, when assessing the impact of probiotics, prebiotics, or synbiotics compared to control or placebo on maternal or infant weight gain and BMI, the overall findings were non-significant for patients with GDM, pregnant women, and infants.
4 Discussion
Our study revealed that the prebiotics showed favorable impacts on body weight and BMI reduction across diverse populations beyond 15 days and by doses of 0.88-66 g/day, with any significant reduction of WC and BFM in adults. However, the probiotics at doses ranging from 104 to 1.35×1015 CFU/day for durations exceeding 7 and 15 days, respectively, decreased body weight and BMI. Moreover, the synbiotic had a favorable impact on weight, BMI, WC, and BFM at doses of 106-1.5×1011 CFU/day and 0.5-300 g/day for over 15 days. The prebiotic and probiotic had a neutral effect on weight gain and weight change in pregnant women by doses 6.6 × 105-1010 CFU/day of probiotics during 1-25 weeks’ treatment. The effect of biotics on weight and BMI in infants was mostly non-significant, except in two studies in which probiotics (2×106 -6 × 109 CFU/day for 2-24 weeks had significant but opposite effects on weight gain. Despite negatively affecting newborn weight, synbiotic supplementation in individuals with gestational diabetes mellitus (GDM) did not significantly influence weight gain, weight change, BMI change, or maternal weight and BMI at the end of the trial.
Prebiotics, probiotics, and synbiotics have shown potential anti-obesity effects through various mechanisms. Prebiotics are non-digestible fibers that serve as food for beneficial gut bacteria such as Bifidobacteria and Lactobacilli. By selectively promoting the growth of these bacteria, prebiotics contribute to a healthier gut microbiota composition. Dewulf et al. found that prebiotics prompt an increase in the proportions of Bifidobacterium and Faecalibacterium in the gut microbiota while reducing Body Fat Mass (BFM) in obese women (121). These beneficial bacteria produce short-chain fatty acids (SCFAs) during the fermentation of prebiotics. SCFAs have been shown to have positive effects on metabolism and inflammation. They can regulate appetite and promote the utilization of energy from food, potentially helping to prevent excess calorie storage. SCFAs interact with receptors on epithelial cells within the gut lining, elevating levels of glucagon-like peptide 1 (GLP-1) and peptide YY (PYY), thereby enhancing satiety (122, 123). A study using 21 g/d doses of inulin revealed increased PYY, GLP-1, leptin, satiety, reduced ghrelin, energy intake, body weight, and BFM (124, 125). Furthermore, prebiotic treatment upregulates peroxisome proliferator-activated receptor (PPAR)γ and PPARα expression (125) while concurrently downregulating sterol regulatory element-binding protein-1c (SREBP-1c) and fatty acid synthase expression, thus diminishing fatty acid production. This suggests that prebiotics may positively impact lipid metabolism by influencing gene expression (126). Additionally, prebiotics, which are viscous plant-derived oligosaccharides, have been found to delay gastric emptying and enhance feelings of satiety. This effect is attributed to their high soluble fiber content, which slows down the movement of food through the digestive tract. They can also interfere with dietary cholesterol uptake and bile acid reabsorption, leading to beneficial effects on lipid metabolism (127, 128).
Biotic supplementation may also influence the gut-brain axis, a bidirectional communication system between the gut and the brain. Some studies suggest they can modulate hormones such as ghrelin and peptide YY, which control hunger and satiety. Improved gut barrier function is another mechanism for the beneficial effects of biotics. Dysbiosis can weaken the gut barrier and contribute to metabolic disorders. Prebiotics support the growth of beneficial bacteria, which can enhance the integrity of the gut barrier. This reduces the absorption of endotoxins and other potentially harmful molecules, which could play a role in reducing inflammation and obesity-related complications (129). Obesity is linked to chronic low-grade inflammation, which contributes to metabolic disturbances. Probiotic supplementation can interact with the immune system, influencing the production of inflammatory cytokines. By fostering an anti-inflammatory environment both in the gut and systemically, specific probiotics may assist in reducing inflammation and enhancing insulin sensitivity (130).
One of the main challenges in determining the effectiveness of currently available probiotic preparations for weight control is the presence of different confounding factors (131). Some studies may have implemented relatively short durations, potentially insufficient for significant changes in anthropometric measurements to manifest. Studies conducted over longer terms might produce different outcomes.
Assessing the effectiveness of currently available probiotic preparations for weight control presents a significant challenge, primarily due to various confounding factors. These factors can include differences in the strains and formulations of probiotics used, variations in individual responses to probiotics, dietary habits, genetic predispositions, lifestyle factors, and personal gut microbiota composition. Additionally, the duration of probiotic supplementation, the specific target population, and the quality of study designs all contribute to the complexity of evaluating their impact on weight management. To draw meaningful conclusions about the effectiveness of probiotics in weight control, it is essential to account for and mitigate these confounding factors in research and analysis.
Here, we can discuss the abovementioned factors based on the available literature. Probiotics from the Bifidobacterium and Lactobacillus genera have notable effects on weight management. These genera are among the most widely studied for their anti-obesity, anti-inflammatory, and immunomodulatory properties (128, 132). However, changes in BMI remained negligible following intervention with the Probiotic Lactobacillus rhamnosus GG, indicating that the probiotic treatment didn’t significantly influence body weight in obese children with hepatic issues (133). Conversely, Lactobacillus gasseri BNR17 supplementation reduces visceral fat accumulation and WC in obese adults (134).
Regarding the duration of biotic supplementation, a meta-analysis found that administering synbiotics to infants for 3-52 weeks had no significant impact on weight. In children, supplementation for 8-104 weeks resulted in weight gain, whereas in adults, supplementation over 2-26 weeks led to weight loss (111). These findings imply that the impact of synbiotics on body weight could depend on the type of additive, the duration of the administration, and the host. The effectiveness and safety might differ based on microorganism strains and doses. Another meta-analysis, examining 3-24 weeks of synbiotic intake at doses of 4.97×106-1.5×1011, observed reduced waist circumference (WC) without significant weight or BMI changes. This hints at synbiotics’ potential to target abdominal adiposity (28). Supplementation with Lactobacillus rhamnosus GG before delivery and for six months postpartum reduced weight gain in 1-4-year-olds, but this effect didn’t persist after a decade (135). While the short-term of probiotics on weight management in children is promising, the long-term intervention is still unclear. Also, according to this umbrella review, probiotic supplementation did not affect gestational weight gain.
Studies investigating the effect of prebiotic, probiotic, or synbiotic consumption on weight changes during various life stages, including pregnancy (23, 24), infancy, and early childhood (25–27), have yielded inconsistent results. Some research indicated increased weight gain in full-term infants fed prebiotic-enriched formula, while others noted improved growth and weight gain in toddlers consuming synbiotic-fortified milk (25, 27). Conversely, supplementing infant formula with probiotics or synbiotics showed no significant weight effect in another systematic review (26). Additionally, a meta-analysis found no noteworthy differences in mean end-of-trial weight or gestational weight gain between intervention and control groups (24). Although, to the best of the author’s knowledge, this umbrella review represents the initial endeavor to comprehensively explore existing meta-analyses in the studied topic, irrespective of age and gender, it should be noted that our findings may not be generalizable to all populations and health conditions. The heterogeneity in the effects of prebiotic, probiotic, and synbiotic supplementation on overweight/obesity indicators arises from intricate interactions among several factors. Firstly, studies examining the effects of the abovementioned intervention on these indices often vary in terms of participant characteristics, intervention protocols, duration of supplementation, and outcome measures.
Diversity can lead to differing results, as the impact of the intervention may depend on the specific context and conditions of each study. Secondly, the composition of gut microbiota and metabolic responses can significantly differ among individuals. Genetic factors, dietary patterns, lifestyle, and pre-existing health conditions all play a role in this variation (135). Consequently, individuals’ responses to each supplementation may differ, leading to inconsistent outcomes across participants. Underlying health conditions like MetS, obesity, or diabetes can influence how the body reacts to supplementation by impacting metabolic pathways and gut microbiota composition, altering expected outcomes (136). Thirdly, variability in the study population, including age, gender, ethnicity, and baseline health status, can contribute to heterogeneous responses to biotic supplementation (136, 137). What works effectively in one population might produce different effects in another. More importantly, the specific strains of probiotics and types of prebiotics used in synbiotic formulations can influence the outcomes. Different strains and types of these components interact distinctly with the gut microbiota and host metabolism, resulting in a range of genus-specific effects on anthropometric measurements. Ensuring standardized viable bacterial cells in commercial probiotics is crucial for research and clinical studies. Accurate dosing allows scientists and healthcare professionals to replicate results and make meaningful comparisons between studies. It also enables more precise insights into the relationship between probiotics and health outcomes.
To accurately evaluate probiotic efficacy across health conditions, study design and analysis must account for these confounding factors. Finally, we should pay attention to the role of epigenetic factors when discussing the inconsistent effects of prebiotics and probiotics on birth weight and childhood weight gain. Epigenetics refers to changes in gene expression that are influenced by factors such as environment, lifestyle, and diet. Epigenetic mechanisms can be crucial in shaping how genes are activated or silenced, impacting various developmental outcomes. The effects of prebiotics and probiotics on birth weight and weight gain might be mediated through epigenetic modifications (138). However, these modifications can be intricate and multifaceted, influenced by genetic and environmental factors. Understanding the complex interplay between prebiotics, probiotics, epigenetics, and growth outcomes requires comprehensive research to decipher the specific epigenetic pathways influenced by these interventions and how they contribute to the observed effects on birth weight and weight gain.
Our study has particular strengths and limitations. The study’s main strength is the overview of the meta-analyses’ trials. As it is known, the meta-analysis of clinical trials is at the highest level of evidence-based medicine (139). The second strength is using standard tools to assess the quality of the methods in the included studies (AMSTAR2) and the strength of the evidence (GRADE). The study’s main limitation is disagreement among various meta-analyses because of variations in multiple factors. These include differences in sample sizes, participants’ health conditions, the quality ratings of studies, the dose of supplements provided, the techniques employed to measure anthropometric indices, the absence of evaluation of confounding variables, and failure to conduct subgroup analysis. These discrepancies contribute to inconsistencies among the meta-analyses.
5 Conclusion
In conclusion, this umbrella review highlights the potential role of probiotics as supplementary treatments in managing the anthropometric indices associated with overweight and obesity in adults. Although current findings in the literature are encouraging, they also reveal the complexity inherent in the interactions between the gut microbiota and the host. To fully realize the therapeutic potential of probiotics in this area, upcoming research should focus on enhancing the precision and consistency of study designs, standardizing intervention protocols for better comparison, and identifying the specific probiotic strains with the most effective anti-obesity properties. Furthermore, a deeper investigation into the mechanisms underlying these effects is essential. Advancements in these areas will provide more consistent, reliable, and detailed insights, thereby facilitating the development of more effective and tailored treatment strategies to combat obesity.
Author contributions
NR: Writing – original draft, Writing – review & editing. MH: Data curation, Methodology, Validation, Writing – review & editing. FE: Data curation, Methodology, Validation, Writing – review & editing. SK: Data curation, Methodology, Writing – review & editing. MB: Data curation, Methodology, Writing – review & editing. OT-M: Conceptualization, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing. SE: Investigation, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1277921/full#supplementary-material
References
1. Haththotuwa RN, Wijeyaratne CN, Senarath U. Obesity and Obstetrics (Second Edition). (2020), 3–8. doi: 10.1016/B978-0-12-817921-5.00001-1
2. Vaisi-Raygani A, Mohammadi M, Jalali R, Ghobadi A, Salari N. The prevalence of obesity in older adults in Iran: a systematic review and meta-analysis. BMC geriat. (2019) 19:1–9. doi: 10.1186/s12877-019-1396-4
3. Bray G, Kim K, Wilding J, Federation WO. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. (2017) 18:715–23. doi: 10.1111/obr.12551
4. Mohamadi A, Shiraseb F, Mirzababaei A, Hosseininasab D, Rasaei N, Clark CC, et al. Circulating inflammatory markers may mediate the relationship between healthy plant-based diet and metabolic phenotype obesity in women: A cross-sectional study. Int J Clin Pract. (2022) 2022:8099382. doi: 10.1155/2022/8099382
5. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. (2019) 15:288–98. doi: 10.1038/s41574-019-0176-8
6. Bazshahi E, Sheikhhossein F, Amini MR, Shab-Bidar S. The association of dietary energy density and the risk of obesity, type 2 diabetes and metabolic syndrome: A systematic review and meta-analysis of observational studies. Int J Clin Pract. (2021) 75:e14291. doi: 10.1111/ijcp.14291
7. Gholami F, Rasaei N, Samadi M, Yekaninejad MS, Keshavarz SA, Javdan G, et al. The relationship of genetic risk score with cardiometabolic risk factors: a cross-sectional study. BMC Cardiovasc Disord. (2022) 22:459. doi: 10.1186/s12872-022-02888-z
8. Parekh PJ, Arusi E, Vinik AI, Johnson DA. The role and influence of gut microbiota in pathogenesis and management of obesity and metabolic syndrome. Front endocrinol. (2014) 5:47. doi: 10.3389/fendo.2014.00047
9. Álvarez-Arraño V, Martín-Peláez S. Effects of probiotics and synbiotics on weight loss in subjects with overweight or obesity: A systematic review. Nutrients. (2021) 13:3627. doi: 10.3390/nu13103627
10. Muscogiuri G, Cantone E, Cassarano S, Tuccinardi D, Barrea L, Savastano S, et al. Gut microbiota: a new path to treat obesity. Int J Obes suppl. (2019) 9:10–9. doi: 10.1038/s41367-019-0011-7
11. Musso G, Gambino R, Cassader M. Obesity, diabetes, and gut microbiota: the hygiene hypothesis expanded? Diabetes Care. (2010) 33:2277–84. doi: 10.2337/dc10-0556
12. Sáez-Lara MJ, Robles-Sanchez C, Ruiz-Ojeda FJ, Plaza-Diaz J, Gil A. Effects of probiotics and synbiotics on obesity, insulin resistance syndrome, type 2 diabetes and non-alcoholic fatty liver disease: a review of human clinical trials. Int J Mol Sci. (2016) 17:928. doi: 10.3390/ijms17060928
13. Harmsen HJ, Wildeboer–Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, et al. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr. (2000) 30:61–7. doi: 10.1002/j.1536-4801.2000.tb02655.x
14. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. (2014) 11(8):506–14. doi: 10.1038/nrgastro.2014.66
15. Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol hepatol. (2017) 14:491–502. doi: 10.1038/nrgastro.2017.75
16. Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. (2020) 17:687–701. doi: 10.1038/s41575-020-0344-2
17. Yao H, Fan C, Fan X, Lu Y, Wang Y, Wang R, et al. Effects of gut microbiota on leptin expression and body weight are lessened by high-fat diet in mice. Br J Nutr. (2020) 124:396–406. doi: 10.1017/S0007114520001117
18. Byrne C, Chambers E, Morrison D, Frost G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int J Obes. (2015) 39:1331–8. doi: 10.1038/ijo.2015.84
19. Kimura I, Inoue D, Hirano K, Tsujimoto G. The SCFA receptor GPR43 and energy metabolism. Front endocrinol. (2014) 5:85. doi: 10.3389/fendo.2014.00085
20. Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. (2013) 500:541–6. doi: 10.1038/nature12506
21. Liu B-N, Liu X-T, Liang Z-H, Wang J-H. Gut microbiota in obesity. World J gastroenterol. (2021) 27:3837. doi: 10.3748/wjg.v27.i25.3837
22. Soltani S, Ashoori M, Dehghani F, Meshkini F, Clayton ZS, Abdollahi S. Effects of probiotic/synbiotic supplementation on body weight in patients with diabetes: a systematic review and meta-analyses of randomized-controlled trials. BMC endocr Disord. (2023) 23:1–13. doi: 10.1186/s12902-023-01338-x
23. Zhou L, Ding C, Wu J, Chen X, Ng DM, Wang H, et al. Probiotics and synbiotics show clinical efficacy in treating gestational diabetes mellitus: A meta-analysis. Prim Care Diab. (2021) 15:937–47. doi: 10.1016/j.pcd.2021.08.005
24. Mu J, Guo X, Zhou Y, Cao G. The effects of probiotics/synbiotics on glucose and lipid metabolism in women with gestational diabetes mellitus: A meta-analysis of randomized controlled trials. Nutrients. (2023) 15:1375. doi: 10.3390/nu15061375
25. Mugambi MN, Musekiwa A, Lombard M, Young T, Blaauw R. Synbiotics, probiotics or prebiotics in infant formula for full term infants: a systematic review. Nutr J. (2012) 11:1–32. doi: 10.1186/1475-2891-11-81
26. Indrio F, Gutierrez Castrellon P, Vandenplas Y, Cagri Dinleyici E, Francavilla R, Mantovani MP, et al. Health effects of infant formula supplemented with probiotics or synbiotics in infants and toddlers: systematic review with network meta-analysis. Nutrients. (2022) 14:5175. doi: 10.3390/nu14235175
27. Firmansyah A, Dwipoerwantoro PG, Kadim M, Alatas S, Conus N, Lestarina L, et al. Improved growth of toddlers fed a milk containing synbiotics. Nutr J. (2012) 11:81. doi: 10.1186/1475-2891-11-81
28. Suzumura EA, Bersch-Ferreira AC, Torreglosa CR, da Silva JT, Coqueiro AY, Kuntz MG, et al. Effects of oral supplementation with probiotics or synbiotics in overweight and obese adults: a systematic review and meta-analyses of randomized trials. Nutr Rev. (2019) 77:430–50. doi: 10.1093/nutrit/nuz001
29. Naseri K, Saadati S, Yari Z, Asbaghi O, Hezaveh ZS, Mafi D, et al. Beneficial effects of probiotic and synbiotic supplementation on some cardiovascular risk factors among individuals with prediabetes and type 2 diabetes mellitus: A grade-assessed systematic review, meta-analysis, and meta-regression of randomized clinical trials. Pharmacol Res. (2022) 182:106288. doi: 10.1016/j.phrs.2022.106288
30. Musazadeh V, Zarezadeh M, Ghalichi F, Ahrabi SS, Jamilian P, Jamilian P, et al. Anti-obesity properties of probiotics; a considerable medical nutrition intervention: Findings from an umbrella meta-analysis. Eur J Pharmacol. (2022) 928:175069. doi: 10.1016/j.ejphar.2022.175069
31. Musazadeh V, Mohammadi Anilou M, Vajdi M, Karimi A, Sedgh Ahrabi S, Dehghan P. Effects of synbiotics supplementation on anthropometric and lipid profile parameters: Finding from an umbrella meta-analysis. Front Nutr. (2023) 10:1121541. doi: 10.3389/fnut.2023.1121541
32. Shirvani-Rad S, Tabatabaei-Malazy O, Mohseni S, Hasani-Ranjbar S, Soroush AR, Hoseini-Tavassol Z, et al. Probiotics as a complementary therapy for management of obesity: A systematic review. Evid Based Complement Alternat Med. (2021) 2021:6688450. doi: 10.1155/2021/6688450
33. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J surge. (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
34. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:358. doi: 10.1136/bmj.j4008
35. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. (2011) 64:401–6. doi: 10.1016/j.jclinepi.2010.07.015
36. Chatzakis C, Goulis DG, Mareti E, Eleftheriades M, Zavlanos A, Dinas K, et al. Prevention of gestational diabetes mellitus in overweight or obese pregnant women: A network meta-analysis. Diabetes Res Clin Pract (2019) 158:107924. doi: 10.1016/j.diabres.2019.107924
37. Pan B, Liu X, Shi J, Chen Y, Xu Z, Shi D, et al. A meta-analysis of microbial therapy against metabolic syndrome: Evidence from randomized controlled trials. Front Nutr (2021) 8:775216. doi: 10.3389/fnut.2021.775216
38. Okesene-Gafa K, Moore A, Jordan V, McCowan L, Crowther C. Probiotics in women with gestational diabetes: Impact on pregnancy and metabolism. J Paediatrics Child Health (2019) 55:41. doi: 10.1111/jpc.14409_105
39. Zhou L, Ding C, Wu J, Chen X, Ng DM, Wang H, et al. Probiotics and synbiotics show clinical efficacy in treating gestational diabetes mellitus: A meta-analysis. Prim Care Diabetes. (2021) 15(6):937–47.
40. Chu X, Yan P, Zhang N, Feng L, Li X, Wang Y, et al. Probiotics for preventing gestational diabetes mellitus in overweight or obese pregnant women: A systematic review and meta-analysis. Clin Nutr ESPEN. (2022) 50:84–92.
41. Mu J, Guo X, Zhou Y, Cao G. The effects of Probiotics/Synbiotics on glucose and lipid metabolism in women with gestational diabetes mellitus: A meta-analysis of randomized controlled trials. Nutrients. (2023) 15(6):1375. doi: 10.3390/nu15061375
42. Yefet E, Bar L, Izhaki I, Iskander R, Massalha M, Younis JS, et al. Effects of probiotics on glycemic control and metabolic parameters in gestational diabetes mellitus: Systematic review and meta-analysis. Nutrients. (2023) 15(7):1633. doi: 10.3390/nu15071633
43. Wang CC, Tung YT, Chang HC, Lin CH, Chen YC. Effect of probiotic supplementation on newborn birth weight for mother with gestational diabetes mellitus or Overweight/Obesity: A systematic review and meta-analysis. Nutrients. (2020) 12(11):3477. doi: 10.3390/nu12113477
44. Han MM, Sun JF, Su XH, Peng YF, Goyal H, Wu CH, et al. Probiotics improve glucose and lipid metabolism in pregnant women: a meta-analysis. Ann Transl Med (2019) 7(5):99. doi: 10.21037/atm.2019.01.61
45. Jarde A, Lewis-Mikhael AM, Moayyedi P, Stearns JC, Collins SM, Beyene J, et al. Pregnancy outcomes in women taking probiotics or prebiotics: a systematic review and meta-analysis. BMC Pregnancy Childbirth. (2018) 18(1):14. doi: 10.1186/s12884-017-1629-5
46. Davidson SJ, Barrett HL, Price SA, Callaway LK, Dekker Nitert M. Probiotics for preventing gestational diabetes. Cochrane Database Syst Rev (2021) 4(4):CD009951. doi: 10.1002/14651858.CD009951.pub3
47. Vahdaninia M, Mackenzie H, Helps S, Dean T. The effectiveness of maternal dietary interventions during pregnancy on obesity in offspring: A systematic review and meta-analysis. Obes Rev (2016) 17:139–40.
48. Pérez-Castillo ÍM, Fernández-Castillo R, Lasserrot-Cuadrado A, Gallo-Vallejo JL, Rojas-Carvajal AM, Aguilar-Cordero MJ. Reporting of perinatal outcomes in probiotic randomized controlled trials. a systematic review and meta-analysis. Nutrients. (2021) 13(1):256. doi: 10.3390/nu13010256
49. Rao S, Srinivasjois R, Patole S. Prebiotic supplementation in full-term neonates: a systematic review of randomized controlled trials. Arch Pediatr Adolesc Med (2009) 163(8):755–64.
50. Steenhout PG, Rochat F, Hager C. The effect of bifidobacterium lactis on the growth of infants: a pooled analysis of randomized controlled studies. Ann Nutr Metab (2009) 55(4):334–40.
51. Szajewska H, Chmielewska A. Growth of infants fed formula supplemented with bifidobacterium lactis Bb12 or lactobacillus GG: a systematic review of randomized controlled trials. BMC Pediatr (2013) 13:185. doi: 10.1186/1471-2431-13-185
52. Sun J, Marwah G, Westgarth M, Buys N, Ellwood D, Gray PH. Effects of probiotics on necrotizing enterocolitis, sepsis, intraventricular hemorrhage, mortality, length of hospital stay, and weight gain in very preterm infants: A meta-analysis. Adv Nutr (2017) 8(5):749–63.
53. Monicaasun MR, Satya Lakshmi K, Sathyanath D, Muralidharan S. Efficacy of probiotics in the role of weight gain in infants. A systematic Rev meta analysis. NeuroQuantology. (2022) 20(8):5772–85.
54. Panchal H, Athalye-Jape G, Rao S, Patole S. Growth and neuro-developmental outcomes of probiotic supplemented preterm infants-a systematic review and meta-analysis. Eur J Clin Nutr (2023) 77(9):855–71.
55. Janmohammadi P, Nourmohammadi Z, Fazelian S, Mirzababaei A, Alizadeh S, Zarei M, et al. Does infant formula containing synbiotics support adequate growth in infants? a meta-analysis and systematic review of randomized controlled trials. Crit Rev Food Sci Nutr (2023) 63(6):707–18.
56. Kunnackal John G, Wang L, Nanavati J, Twose C, Singh R, Mullin G. Dietary alteration of the gut microbiome and its impact on weight and fat mass: a systematic review and meta-analysis. Genes. (2018) 9:167. doi: 10.3390/genes9030167
57. Qu H, Song L, Zhang Y, Gao Zy, Shi Dz. The effect of prebiotic products on decreasing adiposity parameters in overweight and obese individuals: a systematic review and meta-analysis. Curr Med Chem. (2021) 28:419–31. doi: 10.2174/0929867327666191230110128
58. Snelson M, Jong J, Manolas D, Kok S, Louise A, Stern R, et al. Metabolic effects of resistant starch type 2: A systematic literature review and meta-analysis of randomized controlled trials. Nutrients. (2019) 11:1833. doi: 10.3390/nu11081833
59. Jin H, Xu X, Pang B, Yang R, Sun H, Jiang C, et al. Probiotic and prebiotic interventions for non-alcoholic fatty liver disease: a systematic review and network meta-analysis. Benef Microbes. (2021) 12:517–29. doi: 10.3920/BM2020.0183
60. Li Z, Li Y, Pan B, Wang X, Wu Y, Guo K, et al. The effects of oral probiotic supplementation in postmenopausal women with overweight and obesity: a systematic review and meta-analysis of randomized controlled trials. Probiotics Antimicrob Proteins. (2022) 15(6):1–16. doi: 10.1007/s12602-022-10037-3
61. Thompson SV, Hannon BA, An R, Holscher HD. Effects of isolated soluble fiber supplementation on body weight, glycemia, and insulinemia in adults with overweight and obesity: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. (2017) 106:1514–28. doi: 10.3945/ajcn.117.163246
62. Mohammadi H, Ghavami A, Hadi A, Askari G, Symonds M, Miraghajani M. Effects of pro-/synbiotic supplementation on anthropometric and metabolic indices in overweight or obese children and adolescents: A systematic review and meta-analysis. Complement Ther Med. (2019) 44:269–76. doi: 10.1016/j.ctim.2019.05.008
63. Hadi A, Moradi S, Ghavami A, Khalesi S, Kafeshani M. Effect of probiotics and synbiotics on selected anthropometric and biochemical measures in women with polycystic ovary syndrome: a systematic review and meta-analysis. Eur J Clin Nutr. (2020) 74:543–7. doi: 10.1038/s41430-019-0434-9
64. Liu L, Li P, Liu Y, Zhang Y. Efficacy of probiotics and synbiotics in patients with nonalcoholic fatty liver disease: A meta-analysis. Dig Dis Sci. (2019) 64:3402–12. doi: 10.1007/s10620-019-05699-z
65. Stachowska E, Portincasa P, Jamioł-Milc D, Maciejewska-Markiewicz D, Skonieczna-Żydecka K. The relationship between prebiotic supplementation and anthropometric and biochemical parameters in patients with NAFLD—A systematic review and meta-analysis of randomized controlled trials. Nutrients. (2020) 12:3460. doi: 10.3390/nu12113460
66. Rao M, Gao C, Xu L, Jiang L, Zhu J, Chen G, et al. Effect of inulin-type carbohydrates on insulin resistance in patients with type 2 diabetes and obesity: A systematic review and meta-analysis. J Diabetes Res. (2019) 2019:5101423. doi: 10.1155/2019/5101423
67. Rahmani J, Miri A, Černevičiūtė R, Thompson J, de Souza NN, Sultana R, et al. Effects of cereal beta-glucan consumption on body weight, body mass index, waist circumference and total energy intake: A meta-analysis of randomized controlled trials. Complement Ther Med. (2019) 43:131–9. doi: 10.1016/j.ctim.2019.01.018
68. Vazquez-Marroquin G, Ochoa-Précoma R, Porchia LM, Pérez-Fuentes R, Nicolás-Toledo L, Rodríguez-Antolín J, et al. The effect of microbiome therapies on waist circumference, a measure of central obesity, in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. J Acad Nutr Dietet. (2023) 123(6):933–52.e1. doi: 10.1016/j.jand.2023.01.006
69. Park S, Bae J-H. Probiotics for weight loss: a systematic review and meta-analysis. Nutr Res. (2015) 35:566–75. doi: 10.1016/j.nutres.2015.05.008
70. Zhang Q, Wu Y, Fei X. Effect of probiotics on body weight and body-mass index: a systematic review and meta-analysis of randomized, controlled trials. Int J Food Sci Nutr. (2016) 67:571–80. doi: 10.1080/09637486.2016.1181156
71. Borgeraas H, Johnson L, Skattebu J, Hertel J, Hjelmesaeth J. Effects of probiotics on body weight, body mass index, fat mass and fat percentage in subjects with overweight or obesity: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. (2018) 19:219–32. doi: 10.1111/obr.12626
72. Koutnikova H, Genser B, Monteiro-Sepulveda M, Faurie J-M, Rizkalla S, Schrezenmeir J, et al. Impact of bacterial probiotics on obesity, diabetes and non-alcoholic fatty liver disease related variables: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. (2019) 9:e017995. doi: 10.1136/bmjopen-2017-017995
73. Tang Y, Huang J, Zhang WY, Qin S, Yang YX, Ren H, et al. Effects of probiotics on nonalcoholic fatty liver disease: a systematic review and meta-analysis. Ther Adv Gastroenterol. (2019) 12:1756284819878046. doi: 10.1177/1756284819878046
74. AbdelQadir YH, Hamdallah A, Sibaey EA, Hussein AS, Abdelaziz M, AbdelAzim A, et al. Efficacy of probiotic supplementation in patients with diabetic nephropathy: a systematic review and meta-analysis. Clin Nutr ESPEN. (2020) 40:57–67. doi: 10.1016/j.clnesp.2020.06.019
75. Companys J, Pla-Pagà L, Calderón-Pérez L, Llauradó E, Solà R, Pedret A, et al. Fermented dairy products, probiotic supplementation, and cardiometabolic diseases: a systematic review and meta-analysis. Adv Nutr. (2020) 11:834–63. doi: 10.1093/advances/nmaa030
76. Swierz MJ, Storman D, Staskiewicz W, Gorecka M, Jasinska KW, Swierz AM, et al. Efficacy of probiotics in patients with morbid obesity undergoing bariatric surgery: a systematic review and meta-analysis. Surg Obes Related Dis. (2020) 16:2105–16. doi: 10.1016/j.soard.2020.08.038
77. Mirashrafi S, Hejazi TaGhanaki SZ, Sarlak F, Moravejolahkami AR, Hojjati Kermani MA, Haratian M. Effect of probiotics supplementation on disease progression, depression, general health, and anthropometric measurements in relapsing-remitting multiple sclerosis patients: a systematic review and meta-analysis of clinical trials. Int J Clin Pract. (2021) 75:e14724. doi: 10.1111/ijcp.14724
78. Moravejolahkami AR, Hojjati Kermani MA, Balouch Zehi Z, Mirenayat SMS, Mansourian M. The effect of probiotics on lipid profile & anthropometric indices in diabetic nephropathy; a systematic review and meta-analysis of clinical trials. J Diabetes Metab Disord. (2021) 20:893–904. doi: 10.1007/s40200-021-00765-8
79. Perna S, Ilyas Z, Giacosa A, Gasparri C, Peroni G, Faliva MA, et al. Is probiotic supplementation useful for the management of body weight and other anthropometric measures in adults affected by overweight and obesity with metabolic related diseases? A systematic review and meta-analysis. Nutrients. (2021) 13:666. doi: 10.3390/nu13020666
80. da Silva Pontes KS, Guedes MR, da Cunha MR, de Souza Mattos S, Silva MIB, Neves MF, et al. Effects of probiotics on body adiposity and cardiovascular risk markers in individuals with overweight and obesity: A systematic review and meta-analysis of randomized controlled trials. Clin Nutr. (2021) 40:4915–31. doi: 10.1016/j.clnu.2021.06.023
81. Daghmouri MA, Chaouch MA, Yang W, Akremi S, Jaoua H, Fadhel KB, et al. Probiotics in bariatric surgery ensure greater lipids and glycemic profile with no effect on anthropometric measurements and inflammatory markers: A systematic review and meta-analysis of RCT. Surg Open Digest Adv. (2022) 7:100061. doi: 10.1016/j.soda.2022.100061
82. Tabrizi R, Ostadmohammadi V, Akbari M, Lankarani KB, Vakili S, Peymani P, et al. The effects of probiotic supplementation on clinical symptom, weight loss, glycemic control, lipid and hormonal profiles, biomarkers of inflammation, and oxidative stress in women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Probiotics Antimicrob Proteins. (2019) 14(1):1–14. doi: 10.1007/s12602-019-09559-0
83. Li Y, Wu Y, Wu L, Qin L, Liu T. The effects of probiotic administration on patients with prediabetes: a meta-analysis and systematic review. J Trans Med. (2022) 20:1–13. doi: 10.1186/s12967-022-03695-y
84. Mayta-Tovalino F, Diaz-Arocutipa C, Piscoya A, Hernandez AV. Effects of probiotics on intermediate cardiovascular outcomes in patients with overweight or obesity: A systematic review and meta-analysis. J Clin Med. (2023) 12:2554. doi: 10.3390/jcm12072554
85. Thu MS, Ondee T, Nopsopon T, Farzana IA, Fothergill JL, Hirankarn N, et al. Effect of probiotics in breast cancer: a systematic review and meta-analysis. Biology. (2023) 12:280. doi: 10.3390/biology12020280
86. Ma Y-Y, Li L, Yu C-H, Shen Z, Chen L-H, Li Y-M. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J gastroenterol: WJG. (2013) 19:6911. doi: 10.3748/wjg.v19.i40.6911
87. Sun J, Buys N. Effects of probiotics consumption on lowering lipids and CVD risk factors: a systematic review and meta-analysis of randomized controlled trials. Ann Med. (2015) 47:430–40. doi: 10.3109/07853890.2015.1071872
88. Gao X, Zhu Y, Wen Y, Liu G, Wan C. Efficacy of probiotics in non-alcoholic fatty liver disease in adult and children: A meta-analysis of randomized controlled trials. Hepatol Res. (2016) 46:1226–33. doi: 10.1111/hepr.12671
89. Lavekar AS, Raje DV, Manohar T, Lavekar AA. Role of probiotics in the treatment of nonalcoholic fatty liver disease: a meta-analysis. Euroasian J hepato-gastroenterol. (2017) 7:130. doi: 10.5005/jp-journals-10018-1233
90. Dong Y, Xu M, Chen L, Bhochhibhoya A. Probiotic foods and supplements interventions for metabolic syndromes: a systematic review and meta-analysis of recent clinical trials. Ann Nutr Metab. (2019) 74:224–41. doi: 10.1159/000499028
91. Sharpton SR, Maraj B, Harding-Theobald E, Vittinghoff E, Terrault NA. Gut microbiome–targeted therapies in nonalcoholic fatty liver disease: A systematic review, meta-analysis, and meta-regression. Am J Clin Nutr. (2019) 110:139–49. doi: 10.1093/ajcn/nqz042
92. Xiao M-W, Lin S-X, Shen Z-H, Luo W-W, Wang X-Y. Systematic review with meta-analysis: the effects of probiotics in nonalcoholic fatty liver disease. Gastroenterol Res Pract. (2019) 2019:1484598. doi: 10.1155/2019/1484598
93. Wang Z-B, Xin S-S, Ding L-N, Ding W-Y, Hou Y-L, Liu C-Q, et al. The potential role of probiotics in controlling overweight/obesity and associated metabolic parameters in adults: a systematic review and meta-analysis. Evid Based Complement Alternat Med. (2019) 2019:3862971. doi: 10.1155/2019/3862971
94. Cao S, Ryan PM, Salehisahlabadi A, Abdulazeem HM, Karam G, Černevičiūtė R, et al. Effect of probiotic and synbiotic formulations on anthropometrics and adiponectin in overweight and obese participants: a systematic review and meta-analysis of randomized controlled trials. J King Saud University-Sci. (2020) 32:1738–48. doi: 10.1016/j.jksus.2020.01.011
95. Chi C, Li C, Wu D, Buys N, Wang W, Fan H, et al. Effects of probiotics on patients with hypertension: a systematic review and meta-analysis. Curr hypertens Rep. (2020) 22:1–8. doi: 10.1007/s11906-020-01041-5
96. Dixon A, Robertson K, Yung A, Que M, Randall H, Wellalagodage D, et al. Efficacy of probiotics in patients of cardiovascular disease risk: a systematic review and meta-analysis. Curr hypertens Rep. (2020) 22:1–27. doi: 10.1007/s11906-020-01080-y
97. Kocsis T, Molnár B, Németh D, Hegyi P, Szakács Z, Bálint A, et al. Probiotics have beneficial metabolic effects in patients with type 2 diabetes mellitus: a meta-analysis of randomized clinical trials. Sci Rep. (2020) 10:11787. doi: 10.1038/s41598-020-68440-1
98. López-Moreno A, Suárez A, Avanzi C, Monteoliva-Sánchez M, Aguilera M. Probiotic strains and intervention total doses for modulating obesity-related microbiota dysbiosis: a systematic review and meta-analysis. Nutrients. (2020) 12:1921. doi: 10.3390/nu12071921
99. Skonieczna-Żydecka K, Kaźmierczak-Siedlecka K, Kaczmarczyk M, Śliwa-Dominiak J, Maciejewska D, Janda K, et al. The effect of probiotics and synbiotics on risk factors associated with cardiometabolic diseases in healthy people—a systematic review and meta-analysis with meta-regression of randomized controlled trials. J Clin Med. (2020) 9:1788. doi: 10.3390/jcm9061788
100. Jafarabadi MA, Dehghani A, Khalili L, Barzegar A, Mesrizad M, Hassanalilou T. A meta-analysis of randomized controlled trials of the effect of probiotic food or supplement on glycemic response and body mass index in patients with type 2 diabetes, updating the evidence. Curr Diabetes Rev. (2021) 17:356–64. doi: 10.2174/1573399816666200812151029
101. Gkiourtzis N, Kalopitas G, Vadarlis A, Dionysopoulos G, Bakaloudi D, Tsekitsidi E, et al. The effect of probiotics on the treatment of non-alcoholic fatty liver disease in pediatric patients: a systematic review and meta-analysis. Clin Nutr ESPEN. (2021) 46:S653. doi: 10.1016/j.clnesp.2021.09.315
102. Huang Y, Wang X, Zhang L, Zheng K, Xiong J, Li J, et al. Effect of probiotics therapy on nonalcoholic fatty liver disease. Comput Math Methods Med. (2022) 2022:7888076. doi: 10.1155/2022/7888076
103. Wang Y, Wang Y, Sun J. The clinical effect of probiotics on patients with non-alcoholic fatty liver disease: a meta-analysis. Bioengineered. (2022) 13:14960–73. doi: 10.1080/21655979.2023.2185941
104. Saufani IA, Marliyati SA, Palupi E, Handharyani E. The effect of probiotic intake on metabolic syndrome: A meta-analysis. Malaysian J Med Health Sci. (2023) 19:11.
105. Zhang Y, Yan T, Xu C, Yang H, Zhang T, Liu Y. Probiotics can further reduce waist circumference in adults with morbid obesity after bariatric surgery: A systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. (2021) 2021:5542626. doi: 10.1155/2021/5542626
106. Arabi SM, Bahrami LS, Rahnama I, Sahebkar A. Impact of synbiotic supplementation on cardiometabolic and anthropometric indices in patients with metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. (2022) 176:106061. doi: 10.1016/j.phrs.2022.106061
107. Hadi A, Mohammadi H, Miraghajani M, Ghaedi E. Efficacy of synbiotic supplementation in patients with nonalcoholic fatty liver disease: A systematic review and meta-analysis of clinical trials: Synbiotic supplementation and NAFLD. Crit Rev Food Sci Nutr. (2019) 59:2494–505. doi: 10.1080/10408398.2018.1458021
108. Hadi A, Alizadeh K, Hajianfar H, Mohammadi H, Miraghajani M. Efficacy of synbiotic supplementation in obesity treatment: a systematic review and meta-analysis of clinical trials. Crit Rev Food Sci Nutr. (2020) 60:584–96. doi: 10.1080/10408398.2018.1545218
109. Peckmezian T, Garcia-Larsen V, Wilkins K, Mosli RH, BinDhim NF, John GK, et al. Microbiome-targeted therapies as an adjunct to traditional weight loss interventions: A systematic review and meta-analysis. Diabetes Metab Syndr Obes.. (2022) 15:3777–98. doi: 10.2147/DMSO.S378396
110. Loman BR, Hernández-Saavedra D, An R, Rector RS. Prebiotic and probiotic treatment of nonalcoholic fatty liver disease: a systematic review and meta-analysis. Nutr Rev. (2018) 76:822–39. doi: 10.1093/nutrit/nuy031
111. Dror T, Dickstein Y, Dubourg G, Paul M. Microbiota manipulation for weight change. Microb pathogen. (2017) 106:146–61. doi: 10.1016/j.micpath.2016.01.002
112. Bock PM, Telo GH, Ramalho R, Sbaraini M, Leivas G, Martins AF, et al. The effect of probiotics, prebiotics or synbiotics on metabolic outcomes in individuals with diabetes: a systematic review and meta-analysis. Diabetologia. (2021) 64:26–41. doi: 10.1007/s00125-020-05295-1
113. Zhu H, Ren Z, Zang Y, Hua H, Lu J, Xu Q, et al. Effects of microecological preparations on obese patients after bariatric surgery: A systematic review and meta-analysis. Evid Based Complement Alternat Med. (2020) 2020:8724546. doi: 10.1155/2020/8724546
114. Jarde A, Lewis-Mikhael AM, Moayyedi P, Stearns JC, Collins SM, Beyene J, et al. Pregnancy outcomes in women taking probiotics or prebiotics: a systematic review and meta-analysis. BMC Pregnancy Childbirth. (2018) 18:14. doi: 10.1186/s12884-017-1629-5
115. Vahdaninia M, Mackenzie H, Helps S, Dean T. The effectiveness of maternal dietary interventions during pregnancy on obesity in offspring: A systematic review and meta-analysis. Obes Rev (2016) 17:139–40.
116. Vahdaninia M, Mackenzie H, Dean T, Helps S. The effectiveness of ω-3 polyunsaturated fatty acid interventions during pregnancy on obesity measures in the offspring: An up-to-date systematic review and meta-analysis. Eur J Nutr. (2019) 58:2597–613. doi: 10.1007/s00394-018-1824-9
117. Rao S, Srinivasjois R, Patole S. Prebiotic supplementation in full-term neonates: a systematic review of randomized controlled trials. Arch Pediatr Adolesc Med. (2009) 163:755–64. doi: 10.1001/archpediatrics.2009.94
118. Monicaasun MR, Satya Lakshmi K, Sathyanath D, Muralidharan S. Efficacy of probiotics in the role of weight gain in infants. A systematic review and meta analysis. NeuroQuantology. (2022) 20(8):5772–85. doi: 10.14704/nq.2022.20.8.NQ44604
119. Panchal H, Athalye-Jape G, Rao S, Patole S. Growth and neuro-developmental outcomes of probiotic supplemented preterm infants—a systematic review and meta-analysis. Eur J Clin Nutr. (2023) 77(9):1–17. doi: 10.1038/s41430-023-01270-2
120. Janmohammadi P, Nourmohammadi Z, Fazelian S, Mirzababaei A, Alizadeh S, Zarei M, et al. Does infant formula containing synbiotics support adequate growth in infants? A meta-analysis and systematic review of randomized controlled trials. Crit Rev Food Sci Nutr. (2023) 63:707–18. doi: 10.1080/10408398.2021.1952548
121. Dewulf EM, Cani PD, Claus SP, Fuentes S, Puylaert PG, Neyrinck AM, et al. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut. (2013) 62:1112–21. doi: 10.1136/gutjnl-2012-303304
122. Posovszky C, Wabitsch M. Regulation of appetite, satiation, and body weight by enteroendocrine cells. Part 2: therapeutic potential of enteroendocrine cells in the treatment of obesity. Horm Res paedia. (2015) 83:11–8. doi: 10.1159/000369555
123. Delzenne NM, Cani PD, Daubioul C, Neyrinck AM. Impact of inulin and oligofructose on gastrointestinal peptides. Br J Nutr. (2005) 93:S157–S61. doi: 10.1079/BJN20041342
124. Parnell JA, Reimer RA. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am J Clin Nutr. (2009) 89:1751–9. doi: 10.3945/ajcn.2009.27465
125. Dehghan P, Gargari BP, Jafar-Abadi MA. Oligofructose-enriched inulin improves some inflammatory markers and metabolic endotoxemia in women with type 2 diabetes mellitus: a randomized controlled clinical trial. Nutrition. (2014) 30:418–23. doi: 10.1016/j.nut.2013.09.005
126. Bomhof MR, Saha DC, Reid DT, Paul HA, Reimer RA. Combined effects of oligofructose and Bifidobacterium animalis on gut microbiota and glycemia in obese rats. Obesity. (2014) 22:763–71. doi: 10.1002/oby.20632
127. Frost G, Brynes A, Dhillo W, Bloom S, McBurney M. The effects of fiber enrichment of pasta and fat content on gastric emptying, GLP-1, glucose, and insulin responses to a meal. Eur J Clin Nutr. (2003) 57:293–8. doi: 10.1038/sj.ejcn.1601520
128. Ferrarese R, Ceresola E, Preti A, Canducci F. Probiotics, prebiotics and synbiotics for weight loss and metabolic syndrome in the microbiome era. Eur Rev Med Pharmacol Sci. (2018) 22:7588–605. doi: 10.26355/eurrev_201811_16301
129. Neves AL, Coelho J, Couto L, Leite-Moreira A, Roncon-Albuquerque R Jr. Metabolic endotoxemia: a molecular link between obesity and cardiovascular risk. J Mol Endocrinol. (2013) 51:R51–64. doi: 10.1530/JME-13-0079
130. Cristofori F, Dargenio VN, Dargenio C, Miniello VL, Barone M, Francavilla R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: A door to the body. Front Immunol. (2021) 12:578386. doi: 10.3389/fimmu.2021.578386
131. Fåk F, Bäckhed F. Lactobacillus reuteri prevents diet-induced obesity, but not atherosclerosis, in a strain dependent fashion in Apoe–/– mice. PLoS One. (2012) 7(10):e46837. doi: 10.1371/journal.pone.0046837
132. Cerdó T, García-Santos JA G, Bermúdez M, Campoy C. The role of probiotics and prebiotics in the prevention and treatment of obesity. Nutrients. (2019) 11:635. doi: 10.3390/nu11030635
133. Vajro P, Mandato C, Licenziati MR, Franzese A, Vitale DF, Lenta S, et al. Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J Pediatr Gastroenterol Nutr. (2011) 52:740–3. doi: 10.1097/MPG.0b013e31821f9b85
134. Kim J, Yun JM, Kim MK, Kwon O, Cho B. Lactobacillus gasseri BNR17 supplementation reduces the visceral fat accumulation and waist circumference in obese adults: A randomized, double-blind, placebo-controlled trial. J Med Food. (2018) 21:454–61. doi: 10.1089/jmf.2017.3937
135. Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. (2014) 7:17–44. doi: 10.3390/nu7010017
136. Vallianou N, Stratigou T, Christodoulatos GS, Dalamaga M. Understanding the role of the gut microbiome and microbial metabolites in obesity and obesity-associated metabolic disorders: current evidence and perspectives. Curr Obes Rep. (2019) 8:317–32. doi: 10.1007/s13679-019-00352-2
137. Borrello K, Lim U, Park SY, Monroe KR, Maskarinec G, Boushey CJ, et al. Dietary intake mediates ethnic differences in gut microbial composition. Nutrients. (2022) 14:660. doi: 10.3390/nu14030660
138. Wankhade UD, Zhong Y, Kang P, Alfaro M, Chintapalli SV, Thakali KM, et al. Enhanced offspring predisposition to steatohepatitis with maternal high-fat diet is associated with epigenetic and microbiome alterations. PloS One. (2017) 12:e0175675. doi: 10.1371/journal.pone.0175675
139. Evidence-Based Medicine: Levels of Evidence (2023). Available online at: https://pct.libguides.com/ebm/levels-of-evidence.
Keywords: overweight, obesity, prebiotics, probiotics, synbiotics, meta-analysis
Citation: Rasaei N, Heidari M, Esmaeili F, Khosravi S, Baeeri M, Tabatabaei-Malazy O and Emamgholipour S (2024) The effects of prebiotic, probiotic or synbiotic supplementation on overweight/obesity indicators: an umbrella review of the trials’ meta-analyses. Front. Endocrinol. 15:1277921. doi: 10.3389/fendo.2024.1277921
Received: 15 August 2023; Accepted: 27 February 2024;
Published: 20 March 2024.
Edited by:
Shan Gao, Capital Medical University, ChinaReviewed by:
Pugazhendhi Srinivasan, University of Kansas Medical Center, United StatesAmir Saber, Kermanshah University of Medical Sciences, Iran
Copyright © 2024 Rasaei, Heidari, Esmaeili, Khosravi, Baeeri, Tabatabaei-Malazy and Emamgholipour. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ozra Tabatabaei-Malazy, tabatabaeiml@sina.tums.ac.ir; Solaleh Emamgholipour, semamgholipour@sina.tums.ac.ir
†These authors have contributed equally to this work and share first authorship
‡ORCID: Ozra Tabatabaei-Malazy, orcid.org/0000-0003-0188-9721
Solaleh Emamgholipour, orcid.org/0000-0002-9949-5773
 Niloufar Rasaei
Niloufar Rasaei Mohammadreza Heidari
Mohammadreza Heidari Fataneh Esmaeili
Fataneh Esmaeili Sepehr Khosravi
Sepehr Khosravi Maryam Baeeri
Maryam Baeeri Ozra Tabatabaei-Malazy
Ozra Tabatabaei-Malazy Solaleh Emamgholipour
Solaleh Emamgholipour