- Center for Kidney Disease, The Second Affiliated Hospital, Nanjing Medical University, Nanjing, Jiangsu, China
Background: Patients on hemodialysis have a higher burden of cognitive impairment than individuals of the same age in the general population. Studies have found a link between cognition and skeletal muscle function. However, few studies have investigated these associations and the underlying mechanisms in patients on hemodialysis.
Methods: A total of 166 patients on hemodialysis were enrolled in this longitudinal study. Cognitive function was assessed by Montreal Cognitive Assessment (MoCA) scores. Skeletal muscle indicators were evaluated using Inbody S10. Plasma brain-derived neurotrophic factor (BDNF) concentrations were measured by enzyme-linked immunosorbent assay. The primary outcome was a change in the MoCA scores. A mediation analysis was performed to examine the indirect effect of skeletal muscle on cognitive decline through BDNF.
Results: Among the 166 patients, the average age was 49.9 ± 11.2 years. Of these patients with a median follow-up of 1,136 days, 133 participated in the study. We defined MoCA scores decreased by ≥2 points at 3 years from the baseline measurement as cognitive decline (CD). Compared to the cognitively unchanged group, patients with CD had significantly lower fat-free mass, soft lean mass, skeletal muscle mass, and skeletal muscle index (all P<0.05). After adjusting for potential confounders, skeletal muscle indicators were protective predictors of CD. A significant increase in plasma BDNF levels was observed in the CD group. Mediation analysis suggested that BDNF played a mediating role of 20-35% between cognitive impairment and skeletal muscle.
Conclusion: Skeletal muscle is a protective predictor of CD in patients undergoing dialysis. BDNF mediates the relationship between cognitive impairment and skeletal muscle function.
Introduction
Patients with chronic kidney disease (CKD) have a significantly higher risk for cognitive impairment than the general population. Renal failure is an independent risk factor for developing cognitive impairment and dementia. The prevalence of cognitive impairment ranges from approximately 10% to 40% in patients with different stages of CKD and is as high as 80% in patients undergoing hemodialysis (1, 2). Karakizlis et al. assessed the cognitive function of 408 patients who underwent regular hemodialysis and found that only 25% had normal cognitive function, whereas 50.5% had mild to moderate cognitive impairment and 24.5% had severe cognitive impairment (3).
The pathophysiological mechanisms by which cognitive impairment occurs in patients undergoing hemodialysis is complex and includes various factors related to vascular diseases, conventional cardiovascular factors, and dialysis (4). Previous studies have confirmed that crosstalk occurs between the skeletal muscles and brain (5). A study involving 281 elderly male patients confirmed a significant correlation between the radial and tibial muscle densities and cognitive function (6). The mechanisms underlying this correlation are important to elucidate.
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family that promotes neuronal development, cell survival, neurotransmission, and the maintenance of energy homeostasis. BDNF is expressed primarily in the hippocampus and cortex of the brain as well as in skeletal muscles (7). BDNF levels in the brain can enter the peripheral blood through the blood-brain barrier, and these levels are positively correlated with changes in serum BDNF levels. Therefore, peripheral BDNF levels may reflect changes in BDNF levels in the brain (8). Altered BDNF levels are associated with increased appetite, obesity, type 2 diabetes, schizophrenia, depression, and neurodegenerative diseases, including Alzheimer’s disease (9). Both BDNF-mediated synaptogenesis and neurogenesis contribute to enhanced cognition (10). Running and other types of aerobic exercises increase serum BDNF concentrations and enhance cognitive performance in human subjects (11).
This study aimed to analyze the correlation between changes in cognitive level and skeletal muscle mass in patients undergoing hemodialysis and explore the possible mechanism by which BDNF mediates skeletal muscle mass and cognitive disorders through a mediation analysis.
Methods
Study population
We conducted a prospective cohort study of patients received maintenance hemodialysis (HD) in the Second Affiliated Hospital of Nanjing Medical University. A total of 166 patients were enrolled on the non-dialysis day following the second dialysis treatment in a week. Inclusion criteria included participants who age ≥ 18 years and all participants had been on HD three times per week for 4h over 3 months. Exclusion criteria included: (1) Previous history of neurologic diseases (self-reported or medical records); (2) plans to transfer to other dialysis centers or receive kidney transplant within 1 year; (3) acute infection or any other condition that precludes the patients from immediate participation; (4) illiteracy, visual impairment or any other cause that leads to the inability to complete cognitive tests; (5) contraindications to magnetic resonance imaging; (6) malignancy with life expectancy <1 year. The study was reviewed and approved by the institutional research ethics committee of The Second Affiliated Hospital of Nanjing Medical University, and all participants provided written informed consent.
Data and sample acquisition
Interview questionnaire was administered by trained research staff on demographic and medical information. Routine clinical laboratory tests were performed on the morning of the day of enrollment.
Body composition assessment
A multi-frequency bioelectrical impedance analyzer, InBody S10 (Biospace, Seoul, Korea) was used to evaluated skeletal muscle mass including fat-free mass (FFM), soft lean mass (SLM), skeletal muscle mass (SMM), and skeletal muscle index (SMI).
Cognition test
Cognition test was operated by a trained research staff member in a quiet test room. Global cognition was assessed using the Chinese (Mandarin) translation of the MoCA (Version 7) (from http://www.mocatest.org). The total score of the MoCA is 30 points and it covers seven components including visuospatial/executive functions (trail-making test: 1 point, copy tube: 1 point and clock drawing task:3 points), naming (3 points), attention (forward digit span: 1 point, backward digit span: 1 point, vigilance: 1point and serial 7 subtraction: 3 points), language (sentence repetition: 2 points, verbal fluency: 1 point), abstraction (2 points), delayed recall (5 points) and orientation (6 points). Higher scores in MoCA mean better cognitive performances.
Plasma BDNF measurement
Human plasma BDNF concentrations were determined with an enzymelinked immunosorbent assay kit (Elabscience, E-EL-H0010) according to the manufacturer’s recommended instructions. The detection range was 31.25-2000pg/ml and the detection sensitivity of BDNF was 18.75 pg/ml. All plasma samples were measured at a dilution of 1:5.
Plasma SIRT1 measurement
We detected plasma SIRT-1 using an enzyme-linked immunosorbent assay (ELISA) kit (Elabscience, E-EL-H1546). The detection range was 0.31–20 ng/ml and the sensitivity was 0.19 ng/ml. The intra and inter detection variability ranges are ≤ 8% and ≤ 12%, respectively. All plasma samples were measured at a dilution of 1:2.
Follow-up and outcome
With a median follow-up of 1,136 days, participants were administered cognitive test. The primary outcome was cognitive decline using the change of MoCA. The cognitive decline defined as the difference of MoCA score at the 3 year and baseline over -2.
Statistical analysis
Our data was analyzed using SPSS 25.0. Categorical variables were presented as frequencies and continuous variables are presented as mean ± standard deviation or median (interquartile range). Student t-test or Chi-squared test was used for the mean comparison between two independent groups. Univariate and multivariate Cox regressions were used to assess the relationship between cognition and muscle mass, with age, sex, body mass index, education year, diabetes, previous history of cardiovascular disease and stain included as covariates. Mediation analysis, which is a regression-based path analysis technique, was performed to examine the indirect effect of skeletal muscle on cognitive decline through BDNF. The statistical significance level was taken as 0.05.
Results
Baseline characteristics of the study population
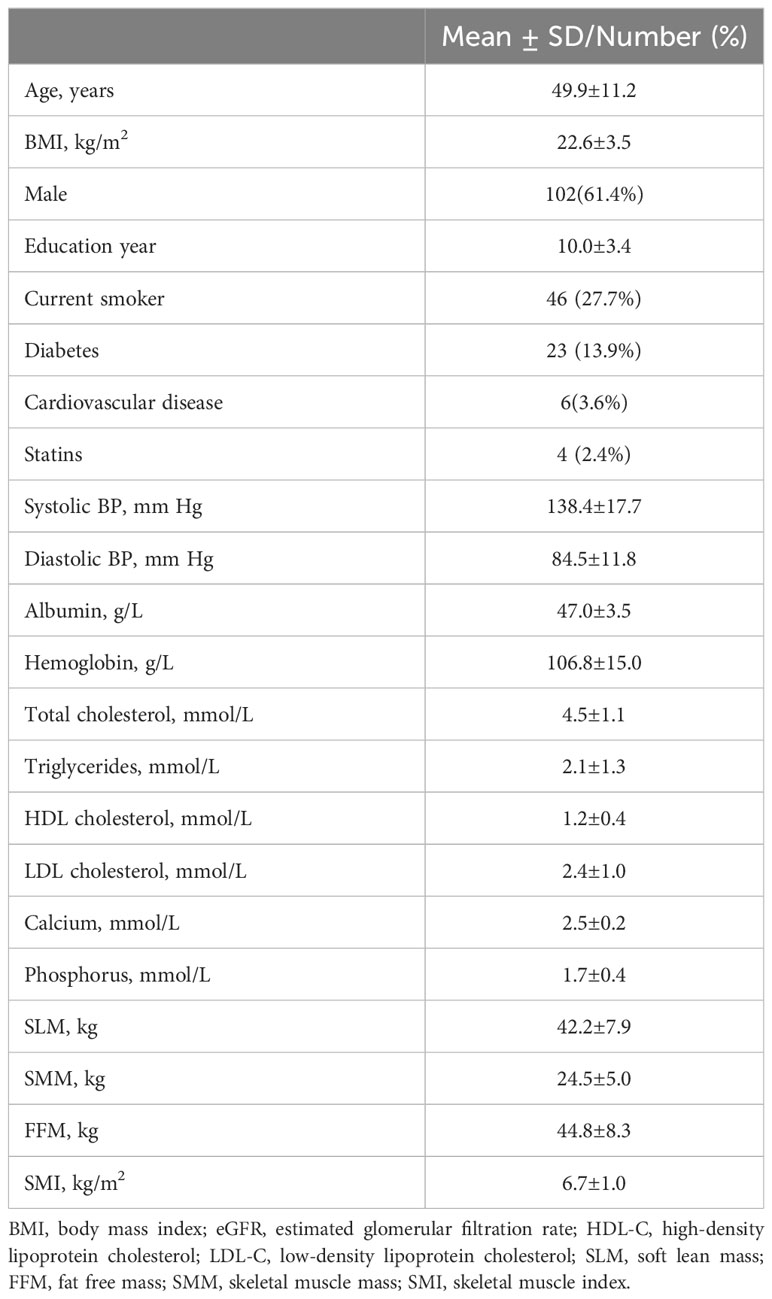
A total of 166 maintenance hemodialysis patients enrolled in the study between July 2017 and January 2018 were assessed at 3-year follow-up. The baseline characteristics of the patients are given in Table 1. The mean age was 49.9 ± 11.2 years, and there were 102 (61.4%) male participants. The average FFM was 44.8 ± 8.3 kg, SLM was 42.2 ± 7.9kg, SMM was 24.5 ± 5.0kg, and SMI was 6.7 ± 1.0kg/m2.
Follow-up and endpoints
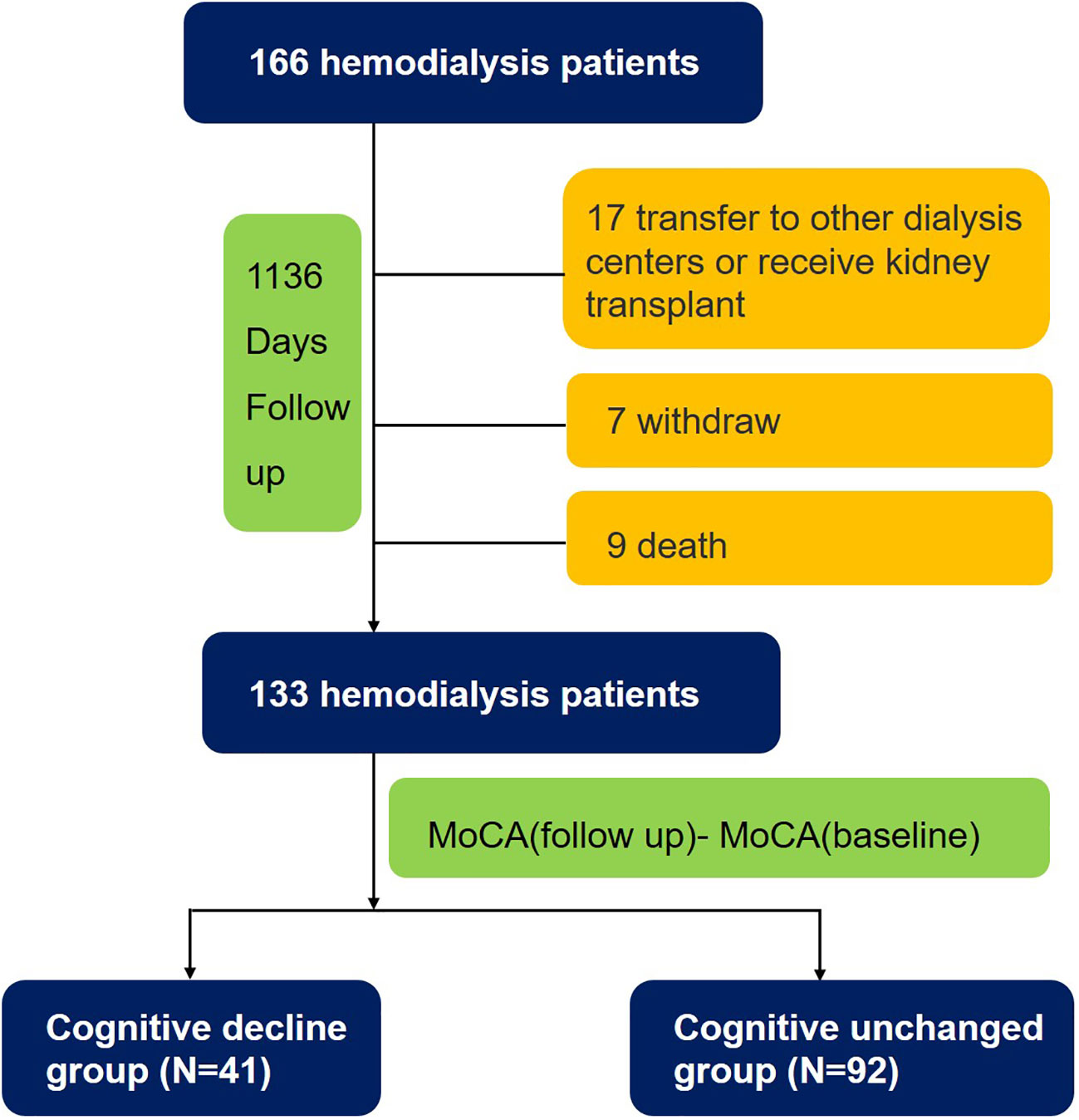
During a median follow-up period of 1,136 days, 9 subjects died, 24 subjects dropped out, and 133 (80.1%) subjects completed all tests. The patients were divided into two groups, the cognitively unchanged and cognitive decline groups, based on the difference in MoCA scores between the follow-up and baseline. Overall, 41 (31%) patients undergoing HD experienced cognitive decline (Figure 1). The mean MoCA scores of the patients at baseline and follow-up were 21.6 ± 3.8 and 21.6 ± 4.1 (Figure 2), respectively.

Figure 2 Total goal of MoCA at baseline and 3-year follow up. The mean MoCA score of patients in baseline and follow-up time was 21.6 ± 3.8 and 21.6 ± 4.3.
Risk factor of cognitive decline
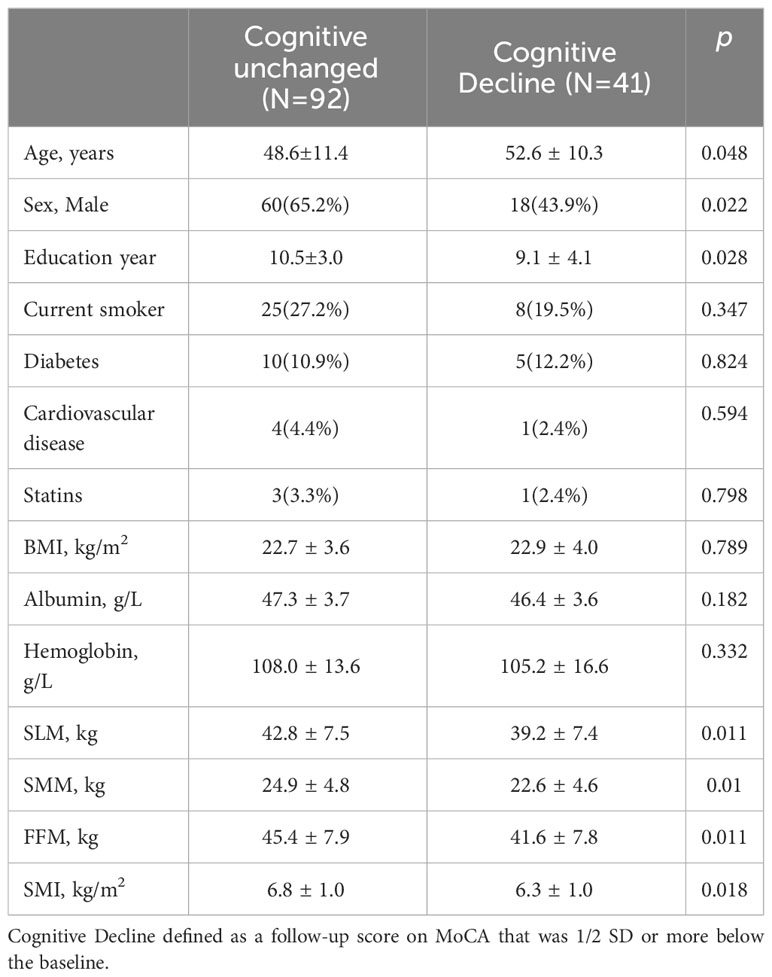
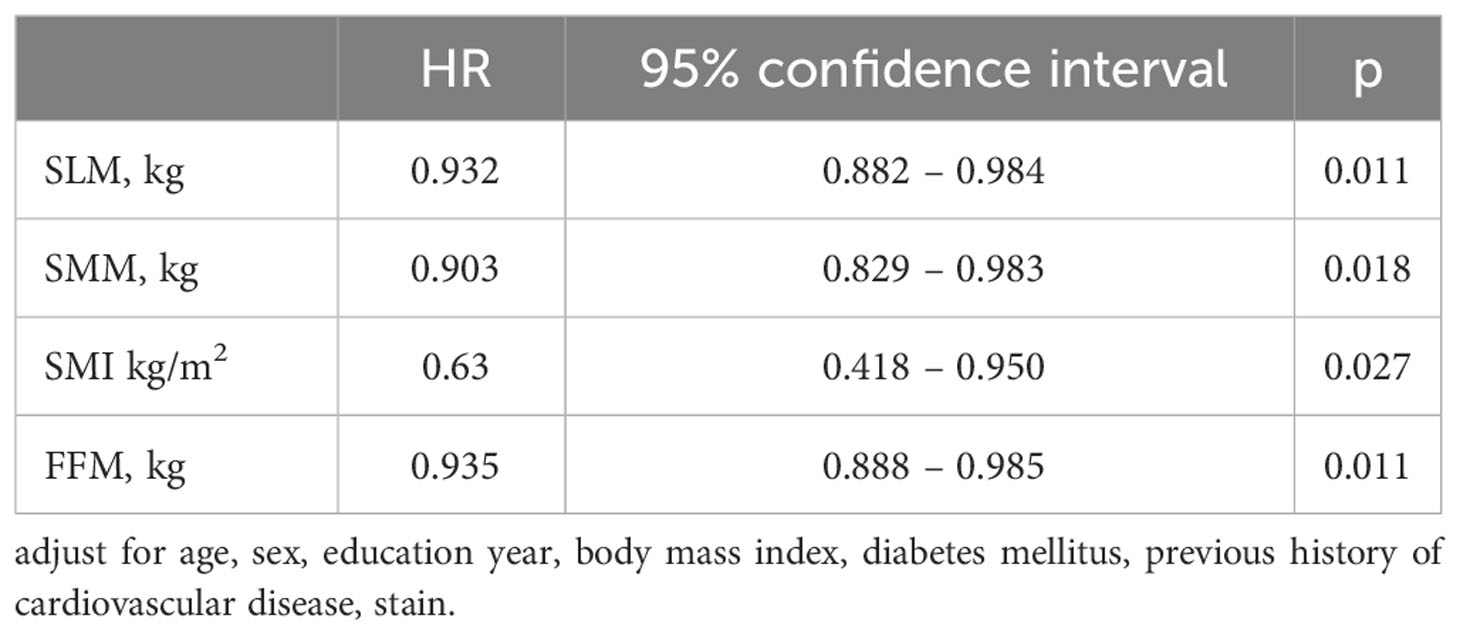
As shown in Table 2, compared to the cognitively unchanged group, the cognitive decline group were older (52.6 ± 10.3 vs 48.6 ± 11.4, P = 0.048) and had fewer years of education (9.1 ± 4.1 vs 10.5 ± 3.0, P=0.028). No significant differences were found in smoking, diabetes, BMI, albumin, or hemoglobin levels between the two groups. Patients in the cognitive decline group had lower SLM, SMM, FFM, and SMI (all P < 0.05) than those in the cognitively unchanged group. The adjusted HRs from the multivariate Cox models are presented in Table 3. SLM, SMM, SMI, and FFM were predictors of cognitive decline. Independent confounding variables included age, sex, years of education, body mass index, diabetes mellitus, previous history of cardiovascular disease, and stain (SLM, HR 0.932, P = 0.011; SMM, HR 0.903, P = 0.018; SMI, HR 0.63, P = 0.027; FFM, HR 0.935, P = 0.011).
Mediating role of BDNF between skeletal muscle mass and cognitive impairment
To explore the association between muscle function and cognition, we measured plasma levels of BDNF. The mean BDNF level of the cognitive decline group was 2313.46 (1039.42-3592.56) pg/mL, while the level in the cognitively unchanged group was 3182.87 (2343.05-4459.37) pg/ml (P = 0.001) (Figure 3).
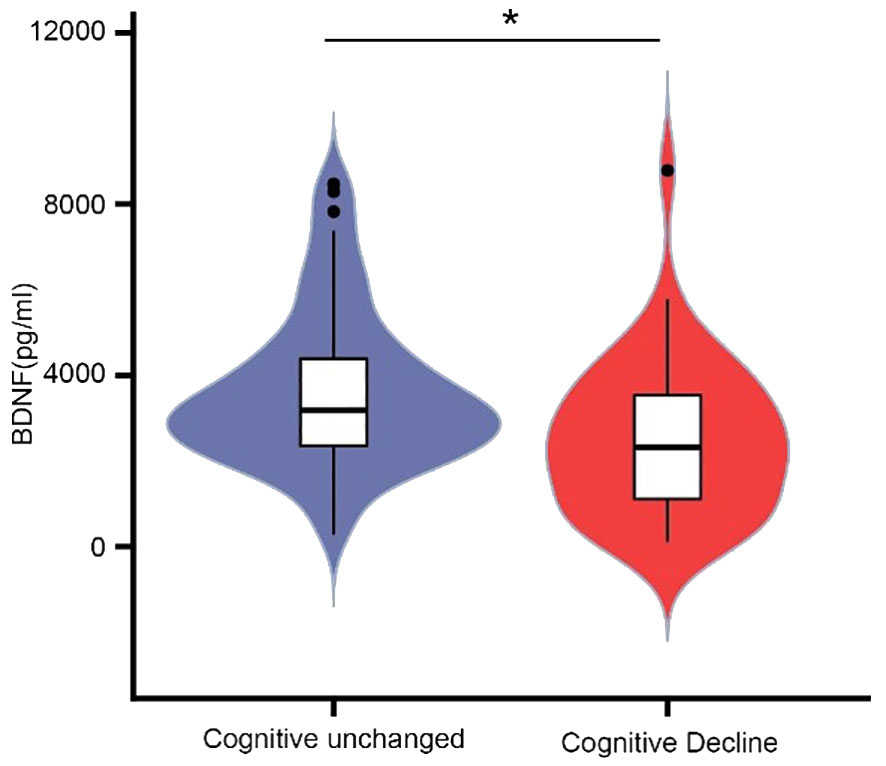
Figure 3 Comparison of plasma BDNF levels between cognitive unchanged and decline group. The mean BDNF level of the cognitive decline group was 2313.46(1039.42-3592.56) pg/mL, and 3182.87(2343.05-4459.37) for the unchanged group. * P<0.05.
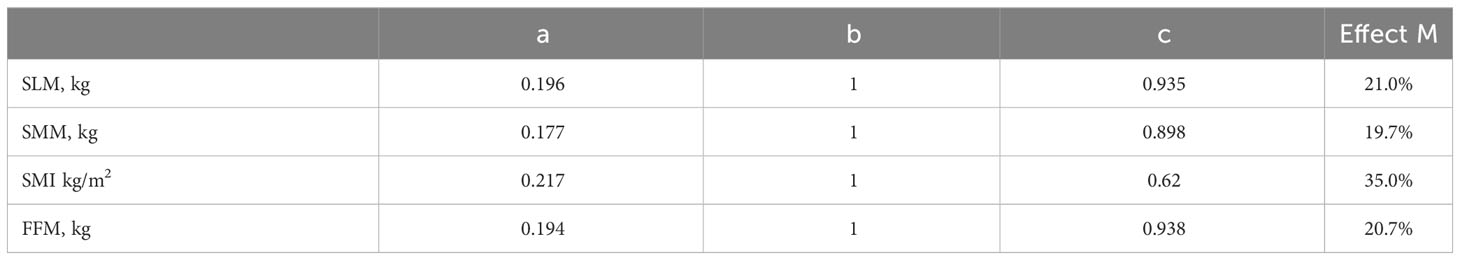
We hypothesized that BDNF levels may mediate the underlying mechanism of cognitive impairment in lower skeletal muscle mass. As shown in Figure 4, while the effect of skeletal muscle on BDNF was evaluated for the indirect or direct effect of skeletal muscle on cognitive decline was evaluated for c’ and c (all P < 0.05). The standardized regression coefficient (β) for the association between BDNF and cognitive decline (b) was 1 (P < 0.05). Therefore, BDNF had a partial direct effect on cognitive decline, and the contribution rates of the intermediary effect to the total effect were 21%, 19.7%, 35%, and 20.7% for SLM, SMM, SMI, and FFM, respectively (Table 4).
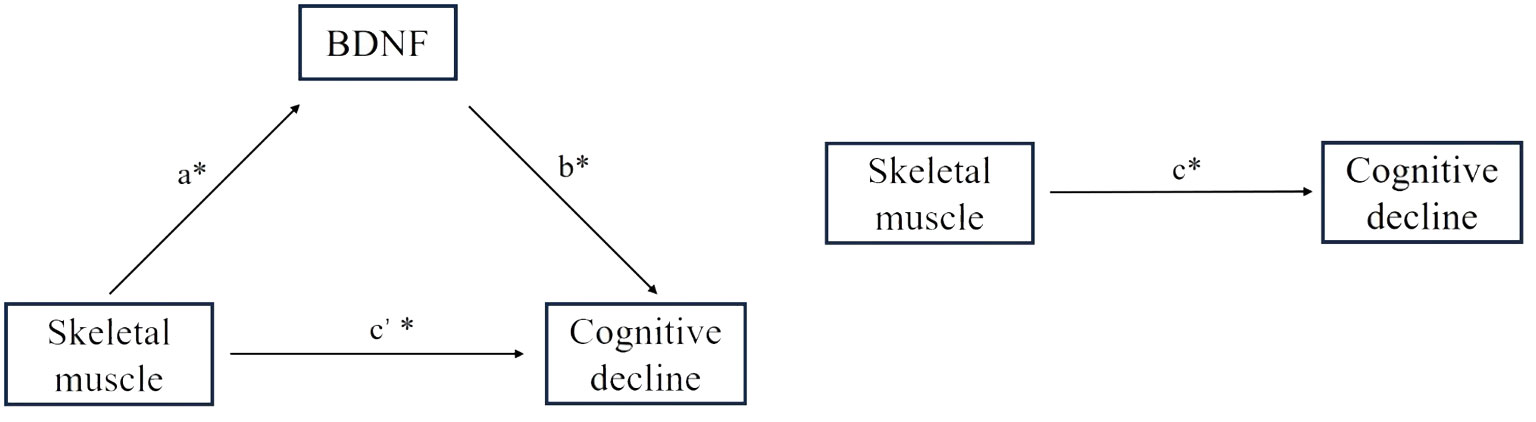
Figure 4 Effect of skeletal muscle on global cognitive decline through BDNF. a= Effect of skeletal muscle on BDNF; c’= Effect of indirect skeletal muscle on cognitive decline; c= Effect of direct skeletal muscle on cognitive decline; b= Effect of BDNF on cognitive decline. * P<0.05.
SIRT1 has been reported to regulate BDNF expression and we further examined plasma SIRT1 levels (12). Mean plasma SIRT1 levels of the cognitive decline group were 1.85 (1.01-2.96) ng/mL, compared with 2.01 (1.38-3.47) ng/mL for the cognitively unchanged group, which was close to statistical significance (P = 0.059). SIRT1 levels were positively associated with BDNF levels (r = 0.273, P < 0.001).
Discussion
This single-center prospective cohort study illustrated a correlation between decreased skeletal muscle mass and cognitive decline in patients undergoing hemodialysis. The results showed that 31% of the patients undergoing hemodialysis experienced cognitive decline during the 3-year follow-up period. Decreased muscle mass was found to be an independent risk factor of cognitive decline. In addition, patients with cognitive decline have significantly lower plasma BDNF concentrations than cognitively unchanged patients. Plasma BDNF levels account for 20%-35% of the association between skeletal muscle mass and cognitive function.
The kidneys and nervous system are interconnected through several pathways, and decreased renal function can lead to various pathological changes in the nervous system, including cognitive impairment (13). Cognitive impairment can occur in all stages of CKD. However, cognitive impairment mostly occurs in patients undergoing hemodialysis, affecting 70−90% of patients (1). The fluctuation in the prevalence of cognitive impairment is mainly due to the use of different cognitive assessment tools and criteria. Our study found that 31% of patients undergoing hemodialysis experienced a decline in total MoCA score of ≥2 points after the 3-year follow-up period compared with that at baseline, indicating cognitive decline. The criterion used in our study is inconsistent with other studies in that most used a cross-sectional MoCA score of < 26 as the criterion for cognitive impairment (14, 15). In contrast, our results were based on the patients’ baseline scores, and the change in individual scores at follow-up was used as the criterion to determine whether cognitive decline had occurred.
At present, the pathophysiological mechanisms underlying dementia and mild cognitive impairment (MCI) in patients with CKD are not fully understood; however, causes of cognitive impairment in CKD are certain to be multifaceted. The risk factors for dementia and MCI in the general population are common risk factors for cognitive impairment in people with CKD and include aging, low educational level, ethnicity, diabetes, hypertension, hyperlipidemia, anemia, obesity, chronic inflammation, and a number of genetic markers associated with cognitive impairment (16, 17). Our results confirmed that patients in the cognitive decline group were older, had a lower educational level, and consisted of a greater proportion of females than those in the group with normal cognitive function. However, no significant difference was observed for the proportion of patients with diabetes, possibly because both groups included only a small proportion of patients with diabetes.
Surprisingly, our results found a difference in skeletal muscle mass between the two groups and found that loss of muscle mass was an independent risk factor for the development of cognitive decline. In a cross-sectional study, Nourbashemi et al. examined the correlation between lean body mass and cognitive function in 7,105 women. The results showed that women in the lowest quartile of lean body mass had 1.43 times the risk of cognitive impairment than women in the highest quartile (18). Additionally, Kwak et al. demonstrated that older women with sarcopenia have extensive cranial nerve degeneration and a significantly higher risk of cognitive impairment than those in the control group (19, 20). Increasing skeletal muscle mass through exercise can significantly improve memory, processing speed, and executive function while decreasing the rate of cognitive decline in elderly people and children (21). However, Ishii et al. conducted a study involving an elderly population from a Japanese community, which yielded the opposite result, suggesting that lower extremity function, rather than skeletal muscle mass, was an independent risk factor for cognitive function (22). These conflicting results may be associated with the use of the Mini-Mental State Examination, which is less sensitive than the MoCA for assessing cognitive function. In addition, appendicular skeletal muscle mass was assessed by Ishii et al., whereas we evaluated the skeletal muscle of the whole body.
BDNF, a secreted growth factor belonging to the neurotrophin family, is widely involved in neurophysiological processes, including neurodevelopment, regulation of cytogenesis and synaptogenesis, and neuroprotection. It also influences memory and cognition. The human BDNF gene consists of 11 exons, and differential splicing allows the formation of different transcripts that are specific to different tissues and respond to different stimuli (23). Exercise can increase BDNF levels in the hippocampus of mice, thereby improving their performance in the Morris water maze test and learning-related behavioral tasks (24). Numerous clinical trials have also found that low plasma BDNF concentrations are associated with poor cognitive function in elderly women living in the community (25). Our findings are consistent with the aforementioned results, where we found that plasma BDNF concentrations were significantly lower in patients with cognitive decline than in those with unchanged cognitive function, suggesting that plasma BDNF levels are significantly associated with cognitive function in patients undergoing hemodialysis.
Muscle BDNF is primarily involved in autocrine and paracrine signal transduction, which promotes fat oxidation in muscle fibers and potential muscle development (26). Myokines secreted by muscle fibers not only act locally but also affect other organs and tissues, regulating peripheral metabolic and physiological functions through hormone-like signal transduction (27). Currently, multiple candidate molecules have been proposed to mediate brain-muscle communication. Skeletal muscles produce irisin, a myokine, by cleaving the fibronectin type III domain-containing protein 5 (FNDC5). Irisin binds to neuronal receptors and upregulates BDNF expression, thereby affecting neurogenesis and enhancing cognition (28). In contrast, the brain controls the relationship between skeletal muscle function and peripheral metabolism via direct innervation of target tissues and modulation of sympathetic tone through cortisol produced by the hypothalamic-pituitary-adrenal axis (29). The results of the mediation analysis further suggested that plasma BDNF accounts for 20–35% of the association between skeletal muscle mass and cognitive function. However, the signaling pathways through which BDNF mediates skeletal muscle and cognitive functions require further investigation using animal models.
Sirtuin-1 (SIRT1) is a histone deacetylase expressed in the brain, liver, skeletal muscle, and adipose tissue. It is involved in learning, memory, and emotional regulation by activating BDNF transcription and regulating BDNF expression (30). A previous study reported that the plasma concentration of SIRT1 was positively correlated with BDNF levels in patients with depression (31). Liu et al. found that SIRT1 mediates the BDNF–TrkB signaling pathway and participates in the mechanism of rat brain damage caused by fluorosis (32). We also observed that SIRT1 levels were positively correlated with BDNF levels. However, the effect of SIRT1 on BDNF requires further investigation.
This study has some limitations. First, we examined plasma BDNF concentrations at baseline but did not examine BDNF concentrations during the 3-year follow-up period. Second, we included study participants with a mean age of 49.9 years, who were younger than the general populations of many developed countries, and thus the results cannot necessarily be extrapolated to other hemodialysis centers. Finally, our sample size was relatively small. Hence, further multicenter cohort studies with larger sample sizes are required.
In conclusion, our findings demonstrate that plasma BDNF concentrations mediate the effect of skeletal muscle mass on cognitive function in patients undergoing hemodialysis. This study provides a partial understanding of the complex mechanisms driving muscle-brain communication and offers a potential preventive and therapeutic option for reducing cognitive decline in patients undergoing hemodialysis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by The Second Affiliated Hospital, Nanjing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LW: Formal analysis, Writing – original draft, Software. XB: Data curation, Writing – review & editing. LL: Data curation, Writing – review & editing. QH: Methodology, Investigation. JX: Validation, Writing – review & editing. XC: Validation, Writing – review & editing. HY: Project administration, Writing – review & editing. JY: Funding acquisition, Writing – review & editing. LJ: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Natural Science Foundation of China Grants 81873618 to Junwei Yang; National Natural Science Foundation of China Grants 81870502 to Lei Jiang.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1324867/full#supplementary-material
Supplementary Figure 1 | Correlation between SIRT1 and BDNF. (A) The mean SIRT1 level of the cognitive decline group was 1.85(1.01-2.96) ng/mL, and 2.01 (1.38-3.47) for the unchanged group. P=0.059. (B) Pearson correlation between plasma SIRT1 level and BDNF. The SIRT1 level was logarithm transformed in Pearson’s correlation analysis due to skewed distribution.
References
1. Drew DA, Weiner DE, Sarnak MJ. Cognitive impairment in CKD: pathophysiology, management, and prevention. Am J Kidney Dis. (2019) 74:782–90. doi: 10.1053/j.ajkd.2019.05.017
2. van Zwieten A, Wong G, Ruospo M, Palmer SC, Barulli MR, Iurillo A, et al. Prevalence and patterns of cognitive impairment in adult hemodialysis patients: the COGNITIVE-HD study. Nephrol Dial Transplant. (2018) 33:1197–206. doi: 10.1093/ndt/gfx314
3. Karakizlis H, Bohl K, Ziemek J, Dodel R, Hoyer J. Assessment of cognitive impairment and related risk factors in hemodialysis patients. J Nephrol. (2022) 35:931–42. doi: 10.1007/s40620-021-01170-3
4. Pepin M, Ferreira AC, Arici M, Bachman M, Barbieri M, Bumblyte IA, et al. Cognitive disorders in patients with chronic kidney disease: specificities of clinical assessment. Nephrol Dial Transplant. (2021) 37:ii23–32. doi: 10.1093/ndt/gfab262
5. Pedersen BK. Physical activity and muscle-brain crosstalk. Nat Rev Endocrinol. (2019) 15:383–92. doi: 10.1038/s41574-019-0174-x
6. Sui SX, Williams LJ, Holloway-Kew KL, Hyde NK, Anderson KB, Tembo MC, et al. Skeletal muscle density and cognitive function: A cross-sectional study in men. Calcif Tissue Int. (2021) 108:165–75. doi: 10.1007/s00223-020-00759-3
7. Ahuja P, Ng CF, Pang BPS, Chan WS, Tse MCL, Bi X, et al. Muscle-generated BDNF (brain derived neurotrophic factor) maintains mitochondrial quality control in female mice. Autophagy. (2022) 18:1367–84. doi: 10.1080/15548627.2021.1985257
8. Amidfar M, de Oliveira J, Kucharska E, Budni J, Kim YK. The role of CREB and BDNF in neurobiology and treatment of Alzheimer’s disease. Life Sci. (2020) 257:118020. doi: 10.1016/j.lfs.2020.118020
9. Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab. (2014) 25:89–98. doi: 10.1016/j.tem.2013.10.006
10. Benarroch E. What muscle signals mediate the beneficial effects of exercise on cognition? Neurology. (2022) 99:298–304. doi: 10.1212/WNL.0000000000201049
11. Griffin EW, Mullally S, Foley C, Warmington SA, O’Mara SM, Kelly AM. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav. (2011) 104:934–41. doi: 10.1016/j.physbeh.2011.06.005
12. El Hayek L, Khalifeh M, Zibara V, Abi Assaad R, Emmanuel N, Karnib N, et al. Sleiman, lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J Neurosci (2019) 39:2369–82. doi: 10.1523/JNEUROSCI.1661-18.2019
13. Chillon JM, Massy ZA, Stengel B. Neurological complications in chronic kidney disease patients. Nephrol Dial Transplant. (2016) 31:1606–14. doi: 10.1093/ndt/gfv315
14. Davis DH, Creavin ST, Yip JL, Noel-Storr AH, Brayne C, Cullum S. Montreal Cognitive Assessment for the detection of dementia. Cochrane Database Syst Rev 7. (2021) 7:CD010775. doi: 10.1002/14651858.CD010775.pub3
15. Lu R, Xu C, Li Y, Yu L, Shao X, Xie K, et al. The incidence prognosis and risk factors of cognitive impairment in maintenance haemodialysis patients. Blood Purif. (2019) 47:101–8. doi: 10.1159/000493524
16. Viggiano D, Wagner CA, Martino G, Nedergaard M, Zoccali C, Unwin R, et al. Mechanisms of cognitive dysfunction in CKD. Nat Rev Nephrol. (2020) 16:452–69. doi: 10.1038/s41581-020-0266-9
17. Guerville F, De Souto Barreto P, Coley N, Andrieu S, Mangin JF, Chupin M, et al. Kidney function and cognitive decline in older adults: examining the role of neurodegeneration. J Am Geriatr Soc. (2021) 69:651–9. doi: 10.1111/jgs.16954
18. Nourhashemi F, Andrieu S, Gillette-Guyonnet S, Reynish E, Albarede JL, Grandjean H, et al. Is there a relationship between fat-free soft tissue mass and low cognitive function? Results from a study of 7,105 women. J Am Geriatr Soc. (2002) 50:1796–801. doi: 10.1046/j.1532-5415.2002.50507.x
19. Kwak SY, Kwak SG, Yoon TS, Kong EJ, Chang MC. Deterioration of brain neural tracts in elderly women with sarcopenia. Am J Geriatr Psychiatry. (2019) 27:774–82. doi: 10.1016/j.jagp.2019.02.018
20. Peng TC, Chen WL, Wu LW, Chang YW, Kao TW. Sarcopenia and cognitive impairment: A systematic review and meta-analysis. Clin Nutr. (2020) 39:2695–701. doi: 10.1016/j.clnu.2019.12.014
21. Isaac AR, Lima-Filho RAS, Lourenco MV. How does the skeletal muscle communicate with the brain in health and disease? Neuropharmacology. (2021) 197:108744. doi: 10.1016/j.neuropharm.2021.108744
22. Ishii H, Makizako H, Doi T, Tsutsumimoto K, Shimada H. Associations of skeletal muscle mass, lower-extremity functioning, and cognitive impairment in community-dwelling older people in Japan. J Nutr Health Aging. (2019) 23:35–41. doi: 10.1007/s12603-018-1110-9
23. Kowianski P, Lietzau G, Czuba E, Waskow M, Steliga A, Morys J. BDNF: A key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol Neurobiol. (2018) 38:579–93. doi: 10.1007/s10571-017-0510-4
24. Sasi M, Vignoli B, Canossa M, Blum R. Neurobiology of local and intercellular BDNF signaling. Pflugers Arch. (2017) 469:593–610. doi: 10.1007/s00424-017-1964-4
25. Krabbe KS, Mortensen EL, Avlund K, Pedersen AN, Pedersen BK, Jorgensen T, et al. Brain-derived neurotrophic factor predicts mortality risk in older women. J Am Geriatr Soc. (2009) 57:1447–52. doi: 10.1111/j.1532-5415.2009.02345.x
26. Delezie J, Handschin C. Endocrine crosstalk between skeletal muscle and the brain. Front Neurol. (2018) 9:698. doi: 10.3389/fneur.2018.00698
27. Sui SX, Williams LJ, Holloway-Kew KL, Hyde NK, Pasco JA. Skeletal muscle health and cognitive function: A narrative review. Int J Mol Sci 22. (2020) 22(1):255. doi: 10.3390/ijms22010255
28. Oudbier SJ, Goh J, Looijaard S, Reijnierse EM, Meskers CGM, Maier AB. Pathophysiological mechanisms explaining the association between low skeletal muscle mass and cognitive function. J Gerontol A Biol Sci Med Sci. (2022) 77:1959–68. doi: 10.1093/gerona/glac121
29. Severinsen MCK, Pedersen BK. Muscle-organ crosstalk: the emerging roles of myokines. Endocr Rev. (2020) 41:594–609. doi: 10.1210/endrev/bnaa016
30. Fagerli E, Escobar I, Ferrier FJ, Jackson CW, Perez-Lao EJ, Perez-Pinzon MA. Sirtuins and cognition: implications for learning and memory in neurological disorders. Front Physiol. (2022) 13:908689. doi: 10.3389/fphys.2022.908689
31. Fang X, Chen Y, Wang Y, Ren J, Zhang C. Depressive symptoms in schizophrenia patients: A possible relationship between SIRT1 and BDNF. Prog Neuropsychopharmacol Biol Psychiatry. (2019) 95:109673. doi: 10.1016/j.pnpbp.2019.109673
Keywords: cognitive function, skeletal muscle, BDNF, hemodialysis, MoCA
Citation: Wang L, Bian X, Liu L, He Q, Xu J, Chen X, Ye H, Yang J and Jiang L (2024) Association between cognitive function and skeletal muscle in patients undergoing maintenance hemodialysis. Front. Endocrinol. 15:1324867. doi: 10.3389/fendo.2024.1324867
Received: 20 October 2023; Accepted: 28 February 2024;
Published: 15 March 2024.
Edited by:
Jianmin Yang, Memorial Sloan Kettering Cancer Center, United StatesReviewed by:
Renqing Zhao, Yangzhou University, ChinaIan James Martins, University of Western Australia, Australia
Copyright © 2024 Wang, Bian, Liu, He, Xu, Chen, Ye, Yang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Ye, yehong@njmu.edu.cn; Junwei Yang, jwyang@njmu.edu.cn; Lei Jiang, jianglei@njmu.edu.cn
†These authors have contributed equally to this work
 Lulu Wang
Lulu Wang Xueqin Bian†
Xueqin Bian† Junwei Yang
Junwei Yang