- 1Department of Endocrinology and Metabolism, The People’s Hospital of Laibin, Guangxi, China
- 2Department of Nephrology, The People’s Hospital of Laibin, Guangxi, China
- 3Department of Endocrinology and Metabolism, Suqian First Hospital, Jiangsu, China
Objective: Diabetic peripheral neuropathy frequently occurs and presents severely in individuals suffering from type 2 diabetes mellitus, representing a significant complication. The objective of this research was to develop a risk nomogram for DPN, ensuring its internal validity and evaluating its capacity to predict the condition.
Methods: In this retrospective analysis, Suqian First Hospital’s cohort from January 2021 to June 2022 encompassed 397 individuals diagnosed with T2DM. A random number table method was utilized to allocate these patients into two groups for training and validation, following a 7:3 ratio. By applying univariate and multivariable logistic regression, predictive factors were refined to construct the nomogram. The model’s prediction accuracy was assessed through metrics like the ROC area, HL test, and an analysis of the calibration curve. DCA further appraised the clinical applicability of the model. Emphasis was also placed on internal validation to confirm the model’s dependability and consistency.
Results: Out of 36 evaluated clinicopathological characteristics, a set of four, duration, TBIL, TG, and DPVD, were identified as key variables for constructing the predictive nomogram. The model exhibited robust discriminatory power, evidenced by an AUC of 0.771 (95% CI: 0.714-0.828) in the training cohort and an AUC of 0.754 (95% CI: 0.663-0.845) in the validation group. The congruence of the model’s predictions with actual findings was corroborated by the calibration curve. Furthermore, DCA affirmed the clinical value of the model in predicting DPN.
Conclusion: This research introduces an innovative risk nomogram designed for the prediction of diabetic peripheral neuropathy in individuals suffering from type 2 diabetes mellitus. It offers a valuable resource for healthcare professionals to pinpoint those at elevated risk of developing this complication. As a functional instrument, it stands as a viable option for the prognostication of DPN in clinical settings.
Introduction
T2DM, a widespread chronic metabolic condition, is predominantly identified by elevated levels of glucose in the blood. This condition stems from a dual complication: the body’s resistance to insulin and a deficiency in insulin production (1). According to the International Diabetes Federation’s reports up to September 2021, the global incidence of diabetes in 2019 was estimated at 9.3% (involving 463 million people), with forecasts suggesting a rise to 10.2% (578 million people) by 2030 and further to 10.9% (encompassing 700 million people) by 2045. Notably, approximately 90% of these cases are identified as T2DM (2). Such staggering figures indicate a substantial impact on both healthcare infrastructures and the lives of those diagnosed with the disorder.
Patients with T2DM frequently experience DPN, a serious complication marked by a gradual reduction in nerve function from the extremities inward (3). This condition is prevalent in 30-50% of T2DM patients, leading to significant consequences like physical impairments and potentially intense neuropathic pain (3–5). Beyond negatively affecting life quality and heightening the likelihood of minor injuries, which could escalate to severe infections or even amputations (6), The presence of DPN is significantly linked to elevated mortality rates due to various causes, including cardiovascular issues, among individuals with diabetes (7). Despite this, awareness of DPN among diabetic individuals remains inadequate. There is a clear and urgent necessity for an inclusive, easily navigable tool that consolidates identified risk factors for DPN in T2DM, facilitating precise risk assessment for each patient.
Nomograms are increasingly recognized as effective tools in clinical risk assessment, given their proficiency in integrating various variables into a cohesive and visually comprehensible instrument. Our hypothesis is that a model based on nomograms, encompassing diverse clinical, demographic, and lab parameters, will develop into a comprehensive predictive model, effectively estimating DPN risk. The objective of this study is to bridge a significant research gap by creating and validating a nomogram for DPN prediction in T2DM patients, facilitating the early identification of patients at high risk and offering a dependable guide for initial clinical interventions.
Materials and methods
Study design and population
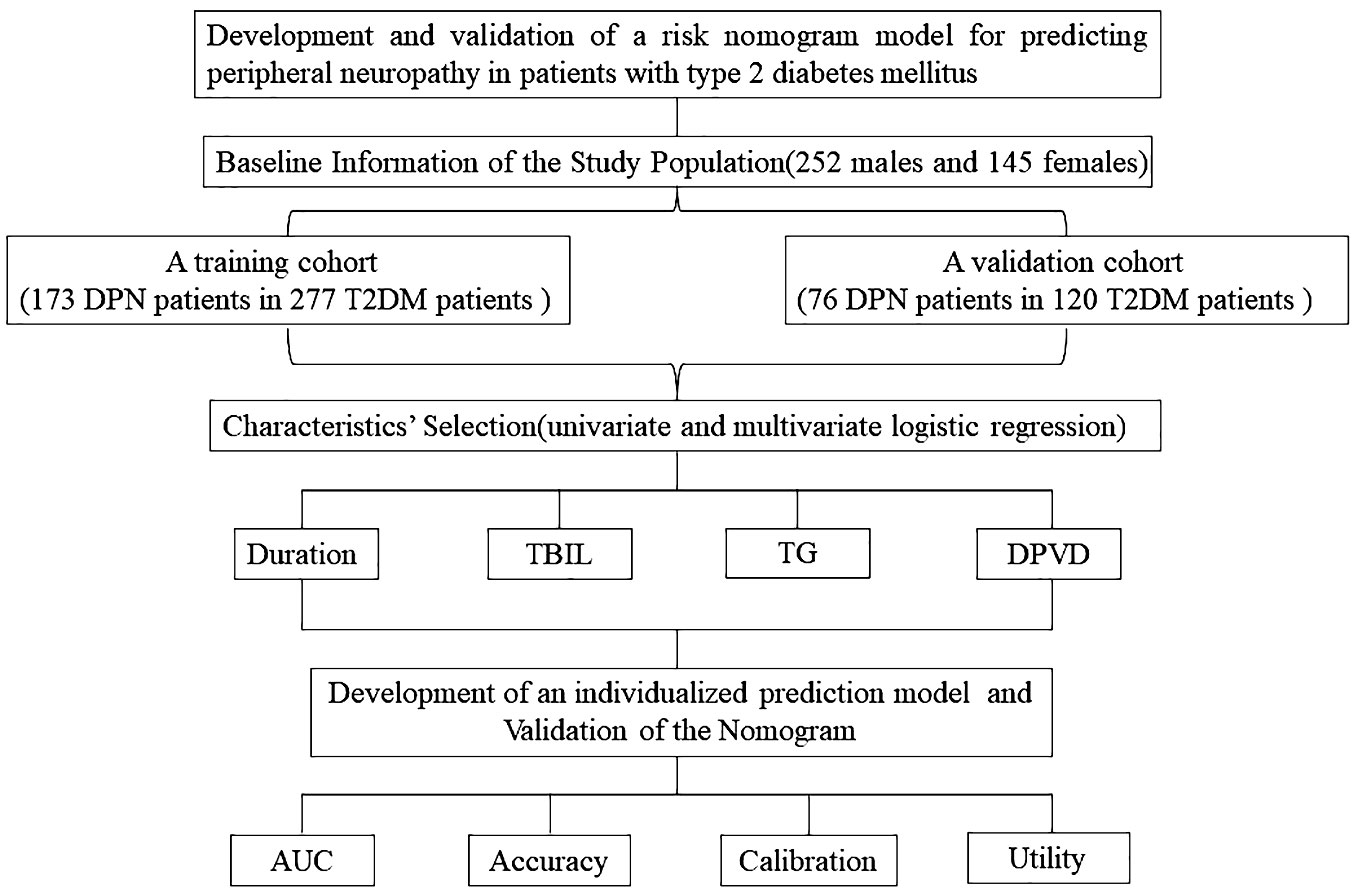
This study aimed to develop and corroborate a nomogram for evaluating DPN risk in T2DM patients. Conducted as a cross-sectional analysis, it encompassed 397 Chinese individuals diagnosed with T2DM, who were enrolled at Suqian First Hospital from January 2021 through June 2022 (Figure 1). Inclusion criteria stipulated that participants must be a minimum of 18 years of age and diagnosed with T2DM, adhering to the criteria set forth by the American Diabetes Association in 2021 (8), and capable of independent communication. Exclusion criteria encompassed patients with incomplete clinical records or other neuropathic conditions not related to diabetes, such as neuropathies due to systemic toxicity (alcohol abuse), neurotoxic medications (chemotherapy), vitamin B12 deficiency, hypothyroidism, renal diseases, specific cancers (multiple myeloma, bronchogenic carcinoma), infections like HIV, chronic inflammatory demyelinating neuropathy, genetic neuropathies, and vasculitis-related neuropathies (9).
Neuropathy assessment
Diagnosis criteria included (1): confirmed diabetes history or signs of abnormal glucose metabolism; (2) onset of neuropathy concurrent with or following diabetes diagnosis; (3) definitive clinical or electrophysiological evidence of diabetic-related peripheral nerve damage; (4) exclusion of other peripheral neuropathy causes. Comprehensive neurological examinations were performed on all T2DM patients by expert neurologists for detailed evaluation. Experienced technicians conducted electrophysiological tests using electromyography equipment. Indicators of DPN included abnormal nerve conduction velocity or scores exceeding 7 on the MNSIQ (10). Assessment included recording the amplitude, distal latency, and velocity of nerve conduction for the compound muscle action potentials in the ulnar, median, tibial, and common peroneal nerves. Moreover, evaluations of the amplitude and nerve conduction velocity were conducted for the sensory nerve action potentials in the ulnar, median, and superficial peroneal nerves. Reference values were based on data from the healthy Chinese population. Doctors determined abnormal nerve conduction by identifying irregularities in one or more characteristics across at least two nerves.
Diabetic peripheral vascular disease
Diagnosis can be made if one or more of the following clinical symptoms occur and at least two of the three tests are positive based on comprehensive indicators:
1) Symptoms: (1) Intermittent claudication; (2) Resting pain; (3) Limb pain (with or without sensory abnormalities, paralysis, no pulse, pale skin); (4) Skin ulcer.
2) Inspection: (1) Percutaneous oxygen partial pressure measurement<40mmHg; (2) In ankle brachial index measurement, ABI<0.9 or ABI>1.3; (3) Ultrasound scan indicates vascular lesions.
Data collection
The study encompassed a comprehensive collection of initial clinical features, including BMI, WBC, MONO, LYM, NEUT, RBC, Hb, PLT, FT3, FT4, TSH, PT, APTT, PTINR, HbA1c, TC, TBIL, DBIL, AST, ALT, Scr, BUN, ALB, FPG, ALP, HDL-C, LDL-C, CHE, UA, TG, along with conditions like DPVD, DKD, and DPN.
Training and validation of the nomogram
In this study, 397 individuals diagnosed with T2DM were randomly assigned into two groups. The first group, comprising 277 participants, served as the training set, while the second group, consisting of 120 individuals, functioned as the validation set. This distribution followed a 7:3 ratio (Figure 1). The study employed both univariate and multivariate logistic regression methods to pinpoint key variables. The performance of these methods was appraised through the analysis of ROC curves. The predictive precision of the models for DPN was determined using the AUC. Calibration plots and the Hosmer-Lemeshow test were employed to confirm the nomogram’s accuracy. Additionally, we employed R software (version 4.1.3) for DCA to assess the nomogram models’ net benefit at varying probability thresholds in the datasets.
Statistical methods
The statistical analysis in this research utilized R software (version 4.2.1) alongside RStudio (version 2021.09.02). For analyzing continuous variables, their mean and standard deviation were calculated and represented as mean ± SD. In contrast, categorical variables were depicted using their frequency and percentage, denoted as n (%). Comparison between groups was performed using the Student’s t-test for continuous data and the χ² test for categorical data. When variables showed non-normal distribution, median and interquartile ranges were applied, with the Wilcoxon rank-sum test for comparing groups. We conducted both univariate and multivariate logistic regression analyses. Significant risk factors (P ≤ 0.05) were identified using forward-backward stepwise regression for developing a nomogram model. The ‘rms’ package facilitated the construction of the nomogram and calibration curve. Evaluation of characteristics was done using OR with 95% CI. P-values were computed bidirectionally for statistical relevance. The predictive model’s precision was assessed by ROC curve analysis, calibration plot generation, and DCA using the training and validation cohort data.
Results
Baseline information of the study population
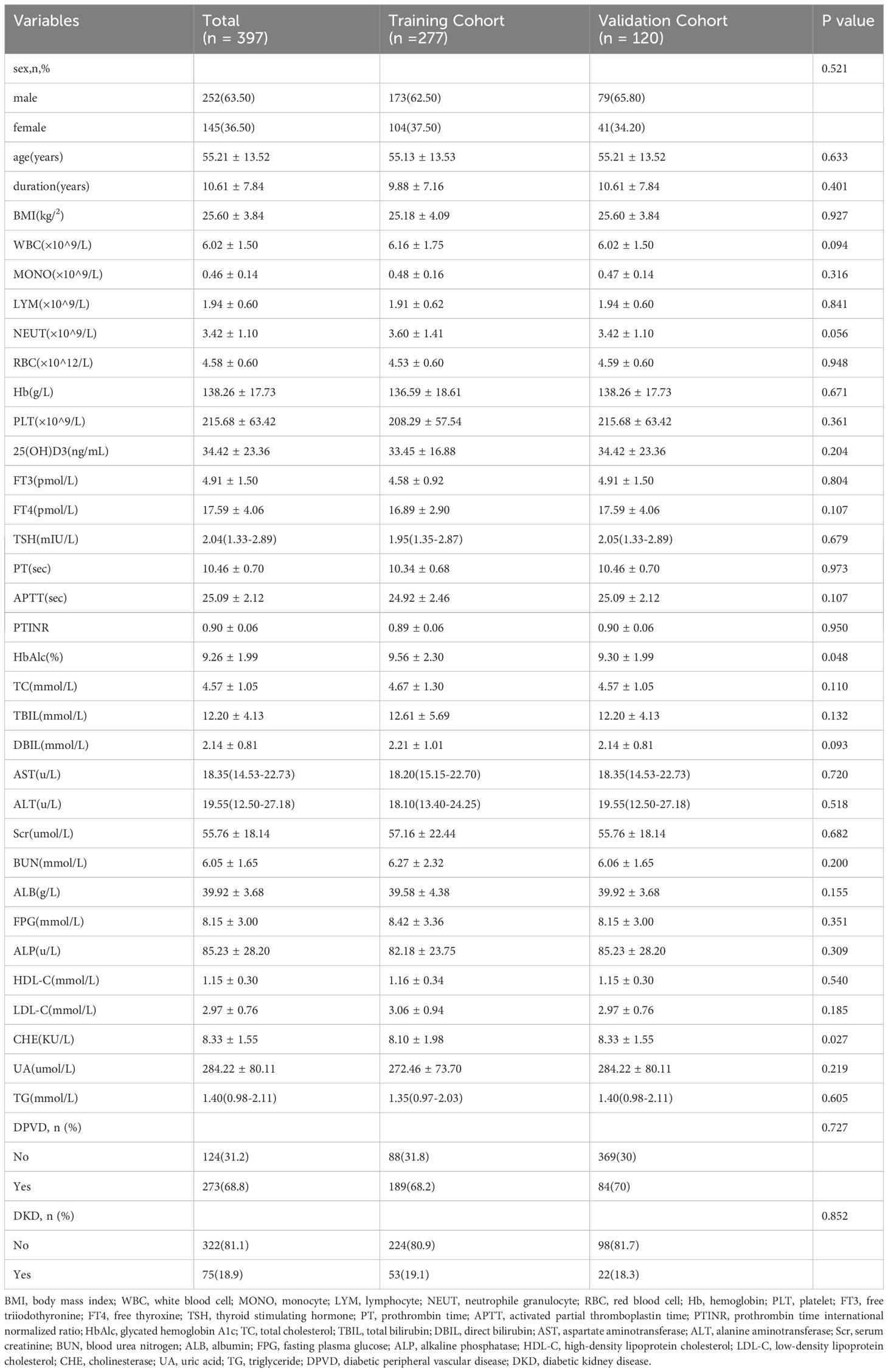
This research included 397 subjects, comprising 252 males and 145 females. These participants were segregated into two cohorts: a training cohort consisting of 277 T2DM patients, of which 173 also had DPN, and a validation cohort comprising 120 individuals with T2DM, 76 of whom also presented with DPN. Proportionally, 62.45% of the training cohort and 63.33% of the validation cohort were diagnosed with both T2DM and DPN. Notably, the training and validation groups showed similar demographic and clinical profiles, with the exception of HbA1c levels, as detailed in Table 1.
Characteristics’ selection
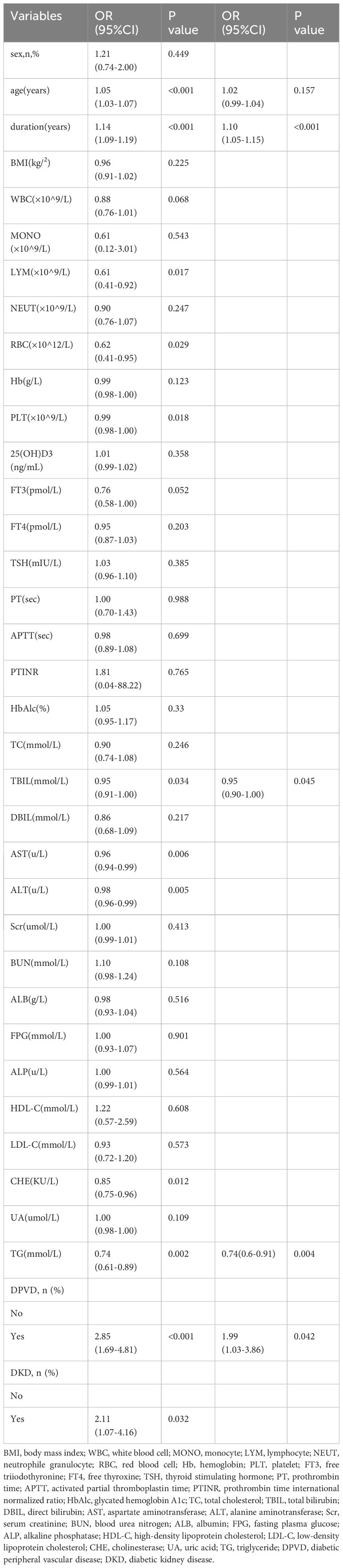
In this research, both univariate and multivariate logistic regression analyses were applied to assess the clinical variables in patients having T2DM and DPN, as detailed in Table 2. The forward-backward method of logistic regression analysis indicated that factors such as duration of disease, levels of TBIL, TG, and the presence of DPVD independently influenced the occurrence of DPN in individuals with T2DM, demonstrating significant associations (P < 0.05).
Development of an individualized prediction model
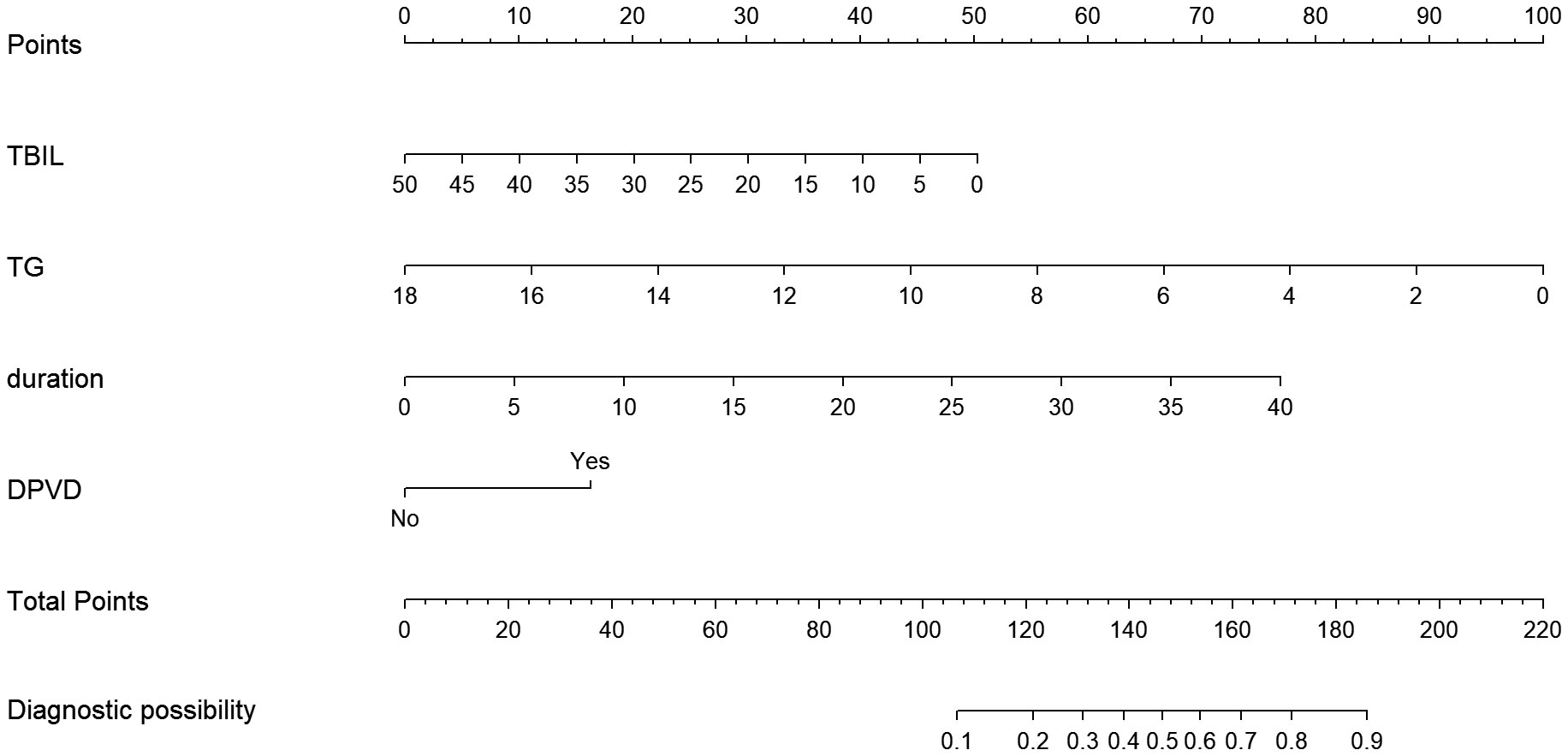
The study utilized univariate and multivariate logistic regression to pinpoint four key independent predictors. Among these, TBIL and TG acted as protective elements against DPN, whereas the remaining two were identified as risk contributors (refer to Table 2). This research led to the creation of a model encompassing these predictors, depicted in the form of a nomogram (refer to Figure 2). Each predictor’s score was assigned according to a specific scale within the nomogram, linked to its respective risk factor. Summing these scores yielded an overall score, which was then used to estimate the likelihood of DPN occurrence. This overall score ranged from 0 to 220, with the associated risk level varying between 0.1 and 0.9. Essentially, an elevated total score indicated an increased risk of DPN among T2DM patients.
Validation of the nomogram
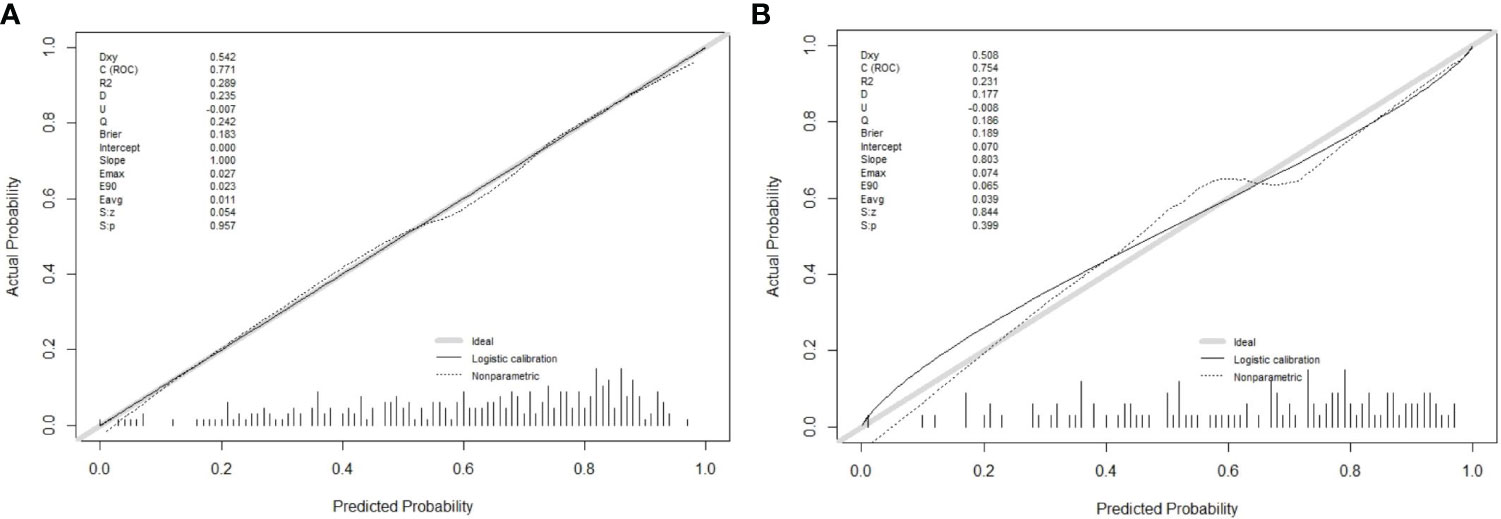
In this research, the nomogram exhibited significant predictive accuracy, with AUC values of 0.771 (95% CI: 0.714-0.828) for the training group (as shown in Figure 3A) and 0.754 (95% CI: 0.663-0.845) for the validation group (as indicated in Figure 3B). The model’s accuracy is visually represented in the calibration plots by a black line, where its closeness to the diagonal gray line reflects higher predictive precision. Both the training (illustrated in Figure 4A) and validation (shown in Figure 4B) cohort calibration plots demonstrated a strong correlation between the model’s estimated probabilities and the actual data. The calibration analysis, assessed via HL tests, indicated Chi-square values of 7.689 for the training cohort and 6.612 for the validation cohort, with P-values of 0.609 and 0.565, respectively, suggesting a reliable fit of the model. Additionally, DCA emphasized the nomogram’s clinical utility in estimating DPN risk across various probability thresholds, as evidenced in the training (Figure 5A) and validation (Figure 5B) cohorts.
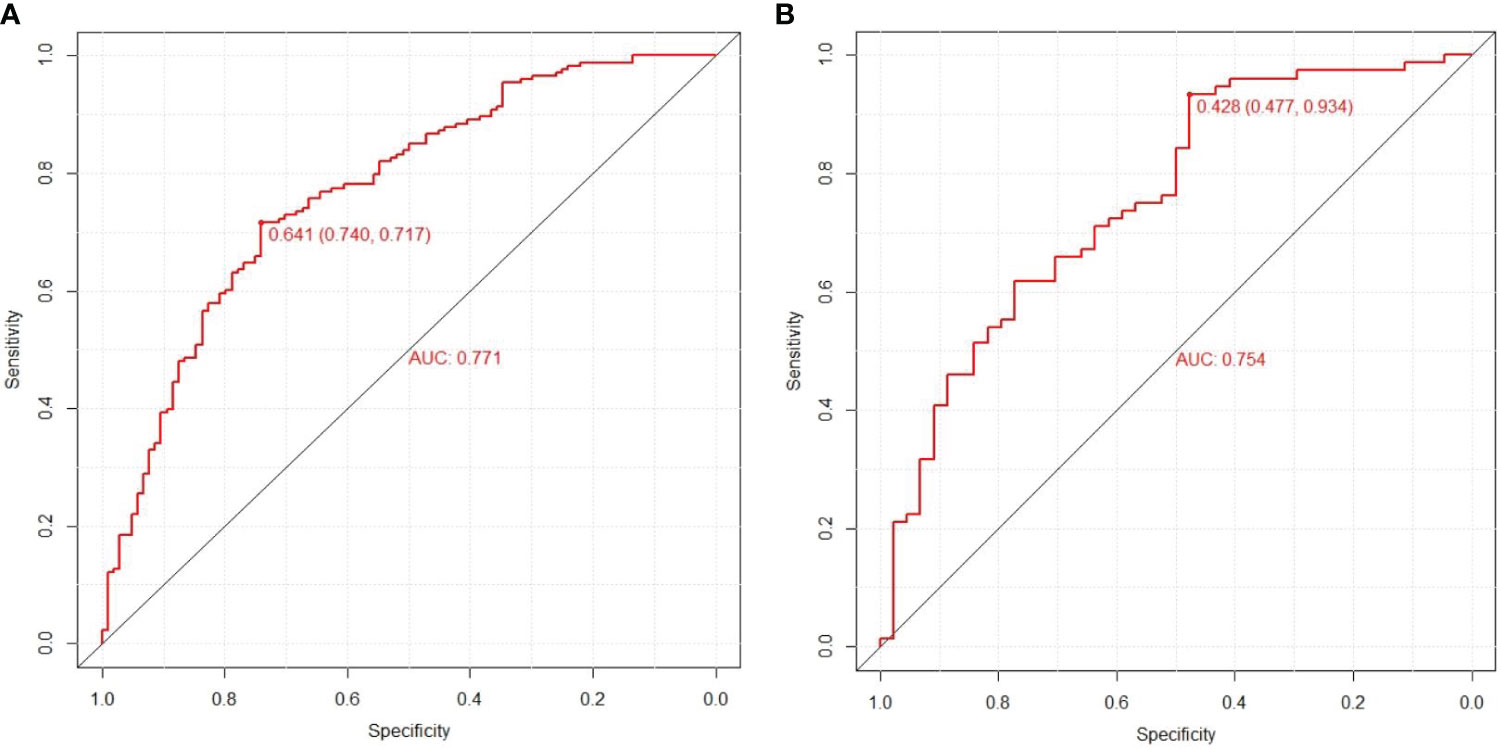
Figure 3 Prediction performance of the model. Receiver operating characteristic (ROC) curve plot in the training cohort (A); ROC curve plot in the validation cohort (B); AUC, the area under the ROC.
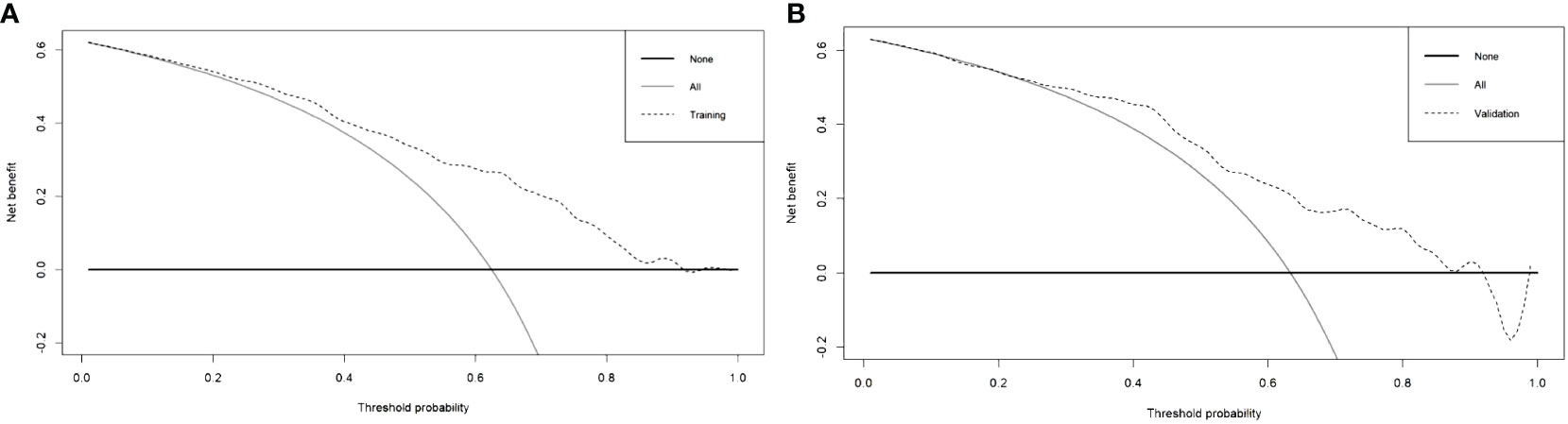
Figure 5 Decision curve analysis of training cohort (A) and validation cohort (B) for the risk of DPN in patients with T2DM.
Discussion
Considering the detrimental impact of DPN, it is crucial for healthcare professionals to focus on its management, aiming to reduce its prevalence among T2DM patients. Recent studies have indicated that nomograms are effective in intuitively predicting the risk of diseases. This research was centered on creating and validating a new nomogram, tailored for estimating the likelihood of DPN in individuals with T2DM. This nomogram is a significant asset in predicting and identifying individual risk factors related to DPN. In this study, logistic regression and forward-backward stepwise regression methods were utilized to examine the clinical data of T2DM patients with concurrent DPN. This analysis highlighted TBIL, TG, diabetes duration, and DPVD as crucial independent predictors for DPN. This study’s nomogram model included these four critical predictors. The model’s effectiveness was evidenced by generating ROC curves, with the training and validation cohorts showing AUCs of 0.771 (95% CI, 0.714, 0.828) and 0.754 (95% CI, 0.663, 0.845), respectively, indicating the model’s robust capacity for discrimination. The calibration curve further confirmed the model’s precision in estimating the risk of DPN. At the sama time, the area under the curve (AUC) of the column chart model designed by Wanli Zhang et al. was 0.763 in the training queue and 0.755 in the validation queue (11). Jing Yang et al. found serum creatinine (Scr), hypertension, glycosylated hemoglobin A1c (HbA1c), blood urea nitrogen (BUN), body mass index (BMI), triglycerides (TG), and diabetic peripheral neuropathy (DPN) Can serve as a key factor for this prediction model. The Harrell’s C-indexes were 0.773 (95% CI: 0.726-0.821) and 0.758 (95% CI: 0.679-0.837) in the training and validation sets, respectively (12). The accuracy of the prediction model in this study is almost comparable to that of existing prediction models, but the key factors of the prediction model in this study are only 4, which is less than the key factors of existing models. Therefore, overall, the predictive model of this study is superior to existing predictive models. Additionally, the DCA decision curves supported the model’s efficacy in prediction, underscoring its reliability as a tool for assessing DPN risk.
Numerous studies have highlighted that the length of time a patient has had T2DM, combined with DPN (13–16), is a crucial determinant. As the duration of diabetes increases, the likelihood of developing DPN also rises. Notably, individuals who have had T2DM for five years or more tend to experience gradual declines in their vibration perception threshold, reaction time, and balance (13). A particular cross-sectional study underscored a notable positive correlation between the length of diabetes and the prevalence of DPN, suggesting that DPN progression is closely tied to how long one has had diabetes, typically spanning 8 to 16 years (14). Amelia R et al. also observed that individuals diagnosed with DM for more than five years have a higher propensity for DPN (15). The prolonged suffering from DM escalates the risk and exacerbates nerve cell damage over time (15, 16). This finding helps clarify why the duration of diabetes was selected as a key variable in our study’s final model.
In the past few years, there has been a growing focus on the role of TG levels in predicting DPN. Research increasingly shows that elevated triglycerides, or hypertriglyceridemia, heighten the risk of developing DPN by nearly four times, independently of blood glucose levels. This risk is often linked to the degeneration of small unmyelinated nerve fibers (17–20). Increasing levels of TG are considered a primary factor in the advancement of DPN, attributed to heightened levels of triglyceride metabolites (plasma free fatty acids). These acids instigate inflammatory reactions and oxidative stress within sensory neurons, potentially resulting in substantial cellular harm. Such damage encompasses endoplasmic reticulum stress, mitochondrial impairment, and permanent neuronal injury (19). Consequently, TG is a crucial factor in our predictive model. Additionally, this study has noted that increased TBIL levels might play a protective role in DPN’s development (21–24). The exact biological mechanisms connecting TBIL levels to DPN remain speculative. However, it is hypothesized that bilirubin’s strong antioxidant properties might offer direct nerve tissue protection by mitigating oxidative stress (21–24). Furthermore, bilirubin’s anti-inflammatory effects could contribute to reducing nerve damage (22, 23), and its neuroprotective impact might be linked to changes in enzyme activities within the bilirubin metabolism pathway (22, 23). Thus, incorporating TBIL levels into the model is essential for accurately predicting DPN.
Within our final model, three factors – duration, TG, and TBIL – are already established as being correlated with DPN based on prior research. Conversely, DPVD’s association with DPN is a novel discovery. This study is the inaugural one to identify DPVD as a predictive factor for DPN, possibly linked to several indirect influences, particularly oxidative stress and lipid metabolism (25, 26). Therefore, DPVD may be significantly associated with the risk of DPN in T2DM patients.
In conclusion, this research introduces a clear and concise risk assessment tool based on four clinical parameters, proving its effectiveness in forecasting the risk of DPN in T2DM patients. This model can be seamlessly integrated into clinical practice by healthcare professionals for assessing DPN risk in patients with T2DM. Furthermore, it offers a significant foundation for the creation of upcoming clinical trials that focus on thwarting the onset of DPN in individuals suffering from type 2 diabetes.
Nevertheless, there were certain constraints in this study. Primarily, it was a retrospective study based in a single center. For improved clinical relevance and to achieve broader external validation, it is advisable for subsequent research to adopt a prospective approach, incorporating data from various centers. Additionally, the sample size in this study was relatively small, and we relied solely on internal validation to assess the model’s accuracy and effectiveness. Future research should encompass larger sample sizes and incorporate a broader range of variables to corroborate our findings. Another limitation is that, for the sake of clinical practicality, the model only incorporated commonly used laboratory and clinical assessment indicators, excluding newer biomarkers such as serum periostin.
Conclusions
This research methodically developed and validated an innovative nomogram designed to predict DPN risk in T2DM patients. This easy-to-use scoring model equips medical practitioners with an efficient means to optimize the management of T2DM patients, providing a practical solution for quick and tailored clinical decision-making.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, frther inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the ethics committee of the Suqian First Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LL: Writing – original draft, Validation, Software, Methodology, Investigation. XL: Writing – original draft, Validation, Formal analysis. CC: Writing – original draft, Visualization, Data curation. QX: Writing – original draft, Methodology, Data curation. JL: Writing – review & editing, Writing – original draft, Visualization, Funding acquisition, Formal analysis, Data curation.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding for this research was supported by the Suqian Sci&Tech Program, under Grant Number KY202213.
Acknowledgments
We thank all patients who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BMI, Body Mass Index; WBC, White Blood Cell; MONO, Monocyte; LYM, Lymphocyte, NEUT, Neutrophile Granulocyte; RBC, Red Blood Cell; Hb, Hemoglobin; PLT, Platelet; FT3, Free Triiodothyronine; FT4, Free Thyroxine; TSH, Thyroid Stimulating Hormone; PT, Prothrombin Time; APTT, Activated Partial Thromboplastin Time; PTINR Prothrombin Time International Normalized Ratio; HbA1c, Glycated Hemoglobin A1c; TC, Total Cholesterol; TBIL, Total Bilirubin; DBIL, Direct Bilirubin; AST, Aspartate Aminotransferase; ALT, Alanine Aminotransferase; Scr, Serum Creatinine; BUN, Blood Urea Nitrogen; ALB, Albumin; FPG, Fasting Plasma Glucose; ALP, Alkaline Phosphatase; HDL-C, High-Density Lipoprotein Cholesterol; LDL-C, Low-Density Lipoprotein Cholesterol; CHE, Cholinesterase; UA, Uric Acid; TG, Triglyceride; DPVD, Diabetic Peripheral Vascular Disease; DKD, Diabetic Kidney Disease; DPN, Diabetic Peripheral Neuropathy; T2DM, Type 2 Diabetes Mellitus; ROC, Operating Characteristic Curve; AUC, Area Under the ROC Curve; HL, Hosmer-Lemeshow Test; DCA, Calibration Curve Analysis.
References
1. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care. (2023) 46:S19–40. doi: 10.2337/dc23-S002
2. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res And Clin Pract. (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843
3. Tentolouris A, Eleftheriadou I, Tentolouris N, Papanas N. Highlights from the 82nd ADA scientific sessions and the 58th EASD meeting on diabetic neuropathy. Int J Lower Extremity Wounds. (2023) 22:15347346231177569. doi: 10.1177/15347346231177569
4. Selvarajah D, Kar D, Khunti K, Davies MJ, Scott AR, Walker J, et al. Diabetic peripheral neuropathy: advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. (2019) 7:938–48. doi: 10.1016/S2213-8587(19)30081-6
5. Elafros MA, Andersen H, Bennett DL, Savelieff MG, Viswanathan V, Callaghan BC, et al. Towards prevention of diabetic peripheral neuropathy: clinical presentation, pathogenesis, and new treatments. Lancet Diabetes Endocrinol. (2022) 21:922–36. doi: 10.1016/S1474-4422(22)00188-0
6. Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, et al. Diabetic neuropathy. Nat Rev Dis Primers. (2019) 5:42. doi: 10.1038/s41572-019-0097-9
7. Hicks CW, Wang D, Matsushita K, Windham BG, Selvin E. Peripheral neuropathy and all-cause and cardiovascular mortality in U. S. Adults: A Prospective Cohort Study. Ann Internal Med. (2021) 174:167–74. doi: 10.7326/M20-1340
8. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. (2021) 44:S15–33. doi: 10.2337/dc21-S002
9. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 12. Retinopathy, neuropathy, and foot care: standards of care in diabetes-2023. Diabetes Care. (2023) 46:S203–15. doi: 10.2337/dc23-S012
10. Didangelos T, Karlafti E, Kotzakioulafi E, Kontoninas Z, Margaritidis C, Giannoulaki P, et al. Efficacy and safety of the combination of superoxide dismutase, alpha lipoic acid, vitamin B12, and carnitine for 12 months in patients with diabetic neuropathy. Nutrients. (2020) 12:3254. doi: 10.3390/nu12113254
11. Zhang WL, Chen LL. A nomogram for predicting the possibility of peripheral neuropathy in patients with type 2 diabetes mellitus. Brain Sci. (2022) 12:1328. doi: 10.3390/brainsci12101328
12. Yang J, Jiang S. Development and validation of a model that predicts the risk of diabetic nephropathy in type 2 diabetes mellitus patients: A cross-sectional study. Int J Gen Med. (2022) 15:5089–101. doi: 10.2147/IJGM.S363474
13. Khan N, Ahmad I, Noohu MM. Association of disease duration and sensorimotor function in type 2 diabetes mellitus: beyond diabetic peripheral neuropathy. Somatosensory motor Res. (2020) 37:326–33. doi: 10.1080/08990220.2020.1830757
14. Hindi E, Almusally BA, Bawareth R, Alhamadah W, Alfaraj R, Almwled A, et al. Diabetic polyneuropathy in type 1 and type 2 diabetes mellitus: A cross-sectional study. Cureus. (2022) 14:e30004. doi: 10.7759/cureus.30004
15. Amelia R, Wahyuni AS, Yunanda Y. Diabetic Neuropathy among Type 2 Diabetes Mellitus Patients at Amplas Primary Health Care in Medan City. Open Access Macedonian J Med Sci. (2019) 7:3400–3. doi: 10.3889/oamjms.2019.433
16. Hussain G, Rizvi SA, Singhal S, Zubair M, Ahmad J. Cross sectional study to evaluate the effect of duration of type 2 diabetes mellitus on the nerve conduction velocity in diabetic peripheral neuropathy. Diabetes Metab syndrome. (2014) 8:48–52. doi: 10.1016/j.dsx.2013.02.003
17. Rumora AE, Guo K, Alakwaa FM, Andersen ST, Reynolds EL, Jørgensen ME, et al. Plasma lipid metabolites associate with diabetic polyneuropathy in a cohort with type 2 diabetes. Ann Clin Trans Neurol. (2021) 8:1292–307. doi: 10.1002/acn3.51367
18. O’Brien PD, Guo K, Eid SA, Rumora AE, Hinder LM, Hayes JM, et al. Integrated lipidomic and transcriptomic analyses identify altered nerve triglycerides in mouse models of prediabetes and type 2 diabetes. Dis Models Mech. (2020) 13:dmm042101. doi: 10.1242/dmm.042101
19. Zhen Q, Yao N, Chen X, Zhang X, Wang Z, Ge Q. Total body adiposity, triglycerides, and leg fat are independent risk factors for diabetic peripheral neuropathy in chinese patients with type 2 diabetes mellitus. Endocrine Pract. (2019) 25:270–8. doi: 10.4158/EP-2018-0459
20. Smith AG, Singleton JR. Obesity and hyperlipidemia are risk factors for early diabetic neuropathy. J Diabetes its complications. (2013) 27:436–42. doi: 10.1016/j.jdiacomp.2013.04.003
21. Mao F, Zhu X, Lu B, Li Y. The association between serum bilirubin level and electrochemical skin conductance in chinese patients with type 2 diabetes. Int J Endocrinol. (2018) 2018:6253170. doi: 10.1155/2018/6253170
22. Kim ES, Lee SW, Mo EY, Moon SD, Han JH. Inverse association between serum total bilirubin levels and diabetic peripheral neuropathy in patients with type 2 diabetes. Endocrine. (2015) 50:405–12. doi: 10.1007/s12020-015-0583-0
23. Jin J, Wang W, Gu T, Chen C, Sun J, Chen W, et al. Low serum bilirubin levels contribute to the presence and progression of distal symmetrical polyneuropathy in Chinese patients with type 2 diabetes. Diabetes metabolis. (2019) 45:47–52. doi: 10.1016/j.diabet.2018.02.007
24. Yan P, Zhang Z, Miao Y, Xu Y, Zhu J, Wan Q. Physiological serum total bilirubin concentrations were inversely associated with diabetic peripheral neuropathy in Chinese patients with type 2 diabetes: a cross-sectional study. Diabetol Metab syndrome. (2019) 11:100. doi: 10.1186/s13098-019-0498-7
25. Viigimaa M, Sachinidis A, Toumpourleka M, Koutsampasopoulos K, Alliksoo S, Titma T. Macrovascular complications of type 2 diabetes mellitus. Curr Vasc Pharmacol. (2020) 18:110–6. doi: 10.2174/1570161117666190405165151
Keywords: diabetic peripheral neuropathy, risk factor, model, prediction, nomogram
Citation: Luo L, Long X, Cheng C, Xu Q and Li J (2024) Development and validation of a risk nomogram model for predicting peripheral neuropathy in patients with type 2 diabetes mellitus. Front. Endocrinol. 15:1338167. doi: 10.3389/fendo.2024.1338167
Received: 14 November 2023; Accepted: 16 April 2024;
Published: 29 April 2024.
Edited by:
Åke Sjöholm, Gävle Hospital, SwedenReviewed by:
Mengmeng Zhai, Alliance Pharma, United StatesMichael Edwin Edmonds, King’s College Hospital NHS Foundation Trust, United Kingdom
Copyright © 2024 Luo, Long, Cheng, Xu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Li, jsleejing@163.com
†These authors have contributed equally to this work and share first authorship
 Lingguang Luo1†
Lingguang Luo1† Jing Li
Jing Li