- 1Department of Strength and Conditioning Assessment and Monitoring, Beijing Sport University, Beijing, China
- 2School of Physical Education, Xihua University, Chengdu, China
- 3School of Sport Sciences, Beijing Sport University, Beijing, China
- 4China Institute of Sport and Health Science, Beijing Sport University, Beijing, China
Introduction: An increasing number of studies have investigated the effect of exercise on flow-mediated dilation (FMD) in people with type 2 diabetes mellitus (T2DM), while the findings were controversial. The primary aim of this systematic review and meta-analysis was to investigate the effect of exercise on FMD in T2DM patients, and the secondary aim was to investigate the optimal type, frequency, session duration, and weekly time of exercise for T2DM patients.
Methods: Searches were conducted in PubMed, Cochrane Library, Scopus, Web of Science, Embase and EBSCO databases. The Cochrane risk of bias tool (RoB2) in randomized trial and Physiotherapy Evidence Database (PEDro) scale were used to assess the methodological quality of the included studies.
Results: From the 3636 search records initially retrieved, 13 studies met the inclusion criteria. Our meta-analysis revealed that exercise had a significant effect on improving FMD in T2DM patients [WMD, 2.18 (95% CI, 1.78-2.58), p < 0.00001, I2 = 38%], with high-intensity interval training (HIIT) being the most effective intervention type [HIIT, 2.62 (1.42-3.82); p < 0.0001; aerobic exercise, 2.20 (1.29-3.11), p < 0.00001; resistance exercise, 1.91 (0.01-3.82), p = 0.05; multicomponent training, 1.49 (0.15-2.83), p = 0.03]. In addition, a higher frequency [> 3 times, 3.06 (1.94-4.19), p < 0.00001; ≤ 3 times, 2.02 (1.59-2.45), p < 0.00001], a shorter session duration [< 60 min, 3.39 (2.07-4.71), p < 0.00001; ≥ 60 min, 1.86 (1.32-2.40), p < 0.00001], and a shorter weekly time [≤ 180 min, 2.40 (1.63-3.17), p < 0.00001; > 180 min, 2.11 (0.82-3.40), p = 0.001] were associated with larger improvements in FMD.
Conclusion: This meta-analysis provides clinicians with evidence to recommended that T2DM patients participate in exercise, especially HIIT, more than 3 times per week for less than 60 min, with a target of 180 min per week being reached by increasing the frequency of exercise.
Systematic review registration: https://www.crd.york.ac.uk/prospero, identifier CRD42023466575.
1 Introduction
Type 2 diabetes mellitus (T2DM) is a prevalent chronic metabolic disease usually due to defective insulin secretion from pancreatic β-cells and a blunted insulin response in insulin-sensitive tissues (1). Patients with T2DM exhibit hyperglycemia, excessive release of free fatty acids (FFAs), insulin resistance, and hyperinsulinemia (2). Endothelial dysfunction, one of the pathological features of T2DM (3), is usually defined as decreased nitric oxide (NO) bioavailability (4), which may be triggered by elevated oxidative stress, leading to increased reactive oxidative substances (ROS), thereby impairing vascular endothelial function (4, 5). In addition, endothelial dysfunction and atherosclerosis are important factors affecting vascular complications in T2DM patients (6).
Endothelial dysfunction leads to a significantly increased risk of chronic diseases such as cardiovascular diseases (CVDs) and its associated complications (7, 8), which is a major cause of morbidity and mortality in T2DM patients (9). A previous study showed that T2DM patients had a doubled risk of developing CVDs (10). Flow-mediated dilation (FMD) is the non-invasive gold standard method for assessing arterial endothelial function (11, 12). Several studies have demonstrated that brachial artery FMD serves as an independent predictor of cardiovascular events (13–15). Therefore, it is important to develop programs to improve endothelial function for the prevention and treatment of T2DM and its associated chronic diseases (16).
Exercise, diet, and medication are important tools in the treatment of T2DM (17). However, exercise interventions are more cost-effective and convenient than other interventions. Studies have shown that exercise can improve the health of T2DM patients, including cardiovascular function (18–20), inflammation (21), cognitive function, and metabolic health (22). A meta-analysis showed that exercise had a significant effect on FMD in different populations (23). With the consensus on exercise as a treatment for T2DM (17), the potential benefits of exercise on FMD in T2DM patients have attracted considerable attention (24–36). However, the type of intervention can have a different impact on FMD in T2DM patients. Of these, aerobic exercise is the most studied type of exercise for T2DM and usually involves exercises that mobilize whole-body muscle groups, such as running, swimming, and brisk walking (37). In addition, traditional aerobic exercise tends to use lower intensity exercise, which means that a longer duration may be required to achieve the corresponding exercise effect. Unfortunately, obesity is a common complication of T2DM, with 80% of T2DM patients having obesity (38). Due to limited mobility and peripheral neuropathy (39), it may be difficult for these patients to ensure good compliance when performing prolonged whole-body exercise (40). In such cases, using resistance exercise that stimulates localized muscle groups or using shorter high-intensity interval training (HIIT) sessions may be a better option (24, 40). However, the effect of exercise and other modalities on the efficacy of FMD in T2DM patients remains unclear.
Therefore, the primary aim of this systematic review and meta-analysis was to investigate the effect of exercise on FMD in T2DM patients, and the secondary aim was to investigate the optimal type, frequency, session duration, and weekly time of exercise for T2DM patients. We hypothesized that exercise would significantly improve FMD in T2DM patients, with HIIT being the most effective type of intervention, and that the frequency and session duration would influence the efficacy of the exercise intervention, with the optimal combination being a higher frequency (more than 3 times per week) and a shorter session duration (less than 60 min).
2 Methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA, 2020) (41) and was registered on PROSPERO (CRD42023466575).
2.1 Search strategy
We searched the PubMed, Web of Science, Embase, EBSCO, Scopus, and Cochrane library for randomized controlled trials (RCTs) relating to the effect of exercise on endothelial function in T2DM patients from the inception dates to 20 February, 2024 (Supplementary Table 1). Reference lists of relevant studies, including reviews and meta-analyses, were manually searched to identify additional relevant studies. The procedure was performed independently by two authors (BQ and YZ), and disagreement were resolved through discussion with the third author (LY).
2.2 Eligibility criteria
Inclusion criteria were: (1) RCTs; (2) using T2DM patients as subjects; (3) including an intervention and control groups; (4) using FMD as the outcome measure and the data were present as percentage.
Exclusion criteria were: (1) non-English articles; (2) conference abstracts; (3) animal studies; (4) reviews.
2.3 Data extraction
The data extraction was conducted by two authors (BQ and YZ), including: (1) surname of the first author, publication year, and sample size; (2) categorized variable: intervention type [aerobic exercise, HIIT, resistance exercise, and multicomponent training (a training modality that involves different physical capacities in the same exercise session) (42)] and continuous variables: duration, session duration, frequency, and weekly time; (3) participants’ age and disease duration; and (4) mean and standard deviation (SD) values reflecting changes in FMD, as described previously (43).
2.4 Methodological quality assessment
The version 2 of the Cochrane risk of bias tool (RoB2) in randomized trial and Physiotherapy Evidence Database (PEDro) scale were used to assess the methodological quality of included studies (44, 45). RoB2 was assessed mainly from 7 items: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases. For PEDro scale, 11 items were evaluated, where studies scoring < 4 points, 4-5 points, 6-8 points, and > 9 points are considered poor, average, good, and excellent quality, respectively (46).
2.5 Statistical analysis
Weighted mean differences (WMDs) and 95% confidence intervals (CIs) were used to estimate the effects of exercise on FMD in T2DM patients. For included studies reporting standard error (SE) or 95% CI, SD was calculated using the previously described formula (47). Heterogeneity was assessed using the I2 static, where I2 < 25% indicate no significant heterogeneity, 25% < I2 < 50% indicate low heterogeneity, 50% < I2 < 75% indicate moderate heterogeneity, and I2 > 75% indicate high heterogeneity (48). Data were pooled using fixed effects models or random effects models when I2 < 50% or I2 ≥ 50%, respectively (49). Subgroup analysis, meta-regression analysis, and sensitivity analysis were used to interpret the results if there was a high heterogeneity (I2 > 60%) (43).
For subgroup analyses, we examined the effect of intervention type (aerobic exercise, HIIT, resistance exercise, and multicomponent exercise), frequency (≤ 3 times and > 3 times), session duration (< 60 min and ≥ 60 min), and weekly time (≤ 180 min and > 180 min) on FMD in T2DM patients. Meta-regressions were conducted based on the participants’ age, disease duration, frequency, session duration, and weekly time. The forest plots were generated using Review manager software (Version 5.4; Cochrane Collaboration), and sensitivity analysis, meta-regressions, and funnel plot were performed using Stata software (Version 15.0, Stata Corp, College Station, Texas). Statistical significance was considered for outcomes with a p < 0.05.
3 Results
3.1 Study selection
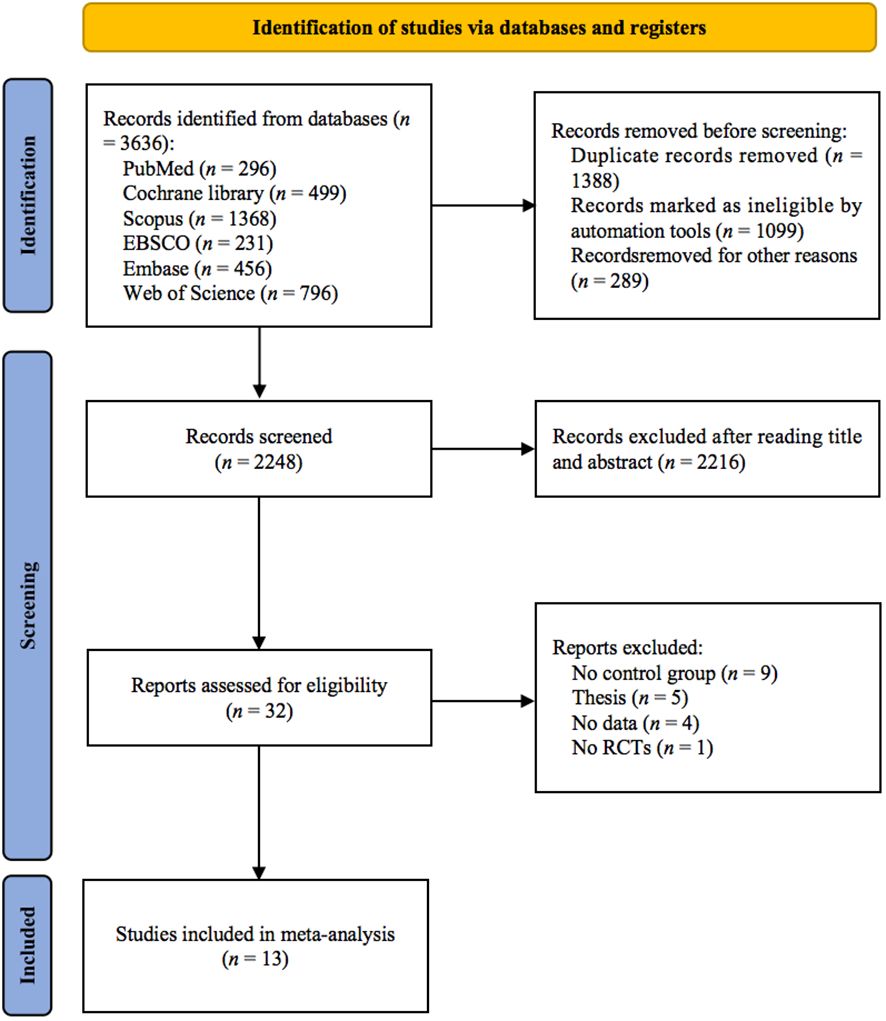
As shown in Figure 1, 3636 studies were identified from 6 databases. After excluding duplicates, 2248 studies remained, and after screening titles and abstracts, 32 studies remained. Nineteen studies were excluded for the following reasons: (1) no control group (n = 9); (2) thesis (n = 5); (3) no data (n = 4); and (4) no RCTs (n = 1). Finally, 13 studies (24–36) met the inclusion criteria.
3.2 Study characteristics
As shown in Supplementary Table 2, among the included studies, there were 290 T2DM patients in the 18 intervention groups and 233 T2DM patients in the 13 control groups. Among the included studies, 3 studies (24, 30, 34) involved only women, 1 study (29) involved only men, and 9 studies (25–28, 31–35) involved both men and women. The mean age of the participants ranged from 15.3 to 70.5 years. The mean age of participants in 2 studies (24, 32) was < 45 years, and 11 studies (25–31, 33–36) involved participants with mean age ≥ 45 years. The mean time from T2DM to intervention of participants ranged from 1.43 to 21.1 years. Most interventions specified aerobic exercise (n = 7) (26, 28–31, 34, 35), high-intensity interval training (HIIT, n = 5) (24, 26–28, 31), resistance exercise (n = 3) (27, 30, 36), and multicomponent training (n = 3) (25, 32, 33). For aerobic exercise, the total duration of intervention ranged from 8 to 12 weeks, with an average of 11.3 weeks, the frequency of intervention per week was 3 times, and minutes of intervention per session ranged from 30 to 62 minutes, with an average of 54 minutes. For HIIT, the total duration of intervention was 12 weeks, the frequency of intervention per week was 3 times, the number of intervals ranged from 3 to 11 times, with an average of 7 times, the interval time ranged from 1 to 3 minutes, with an average of 2 minutes, and the number of repetitions per session ranged from 4 to 12 times, with an average of 8 times. For resistance exercise, the total duration of intervention was 12 weeks and the frequency of intervention per week was 3 times. For multicomponent training, the total duration of intervention ranged from 12 to 24 weeks, with an average of 16 weeks, the frequency of intervention per week ranged from 3 to 5 times, and minutes of intervention per session ranged from 60 to 75 minutes, with an average of 67.5 minutes. The session duration ranged from 19 to 75 min, while 1 study (36) did not provide information on session duration. The frequency ranged from 3 to 5 times per week, and we calculated the weekly time based on frequency and session duration (42), which ranged from 90 to 300 min. The results of FMD in all included studies were presented as percentages.
3.3 Risk of bias
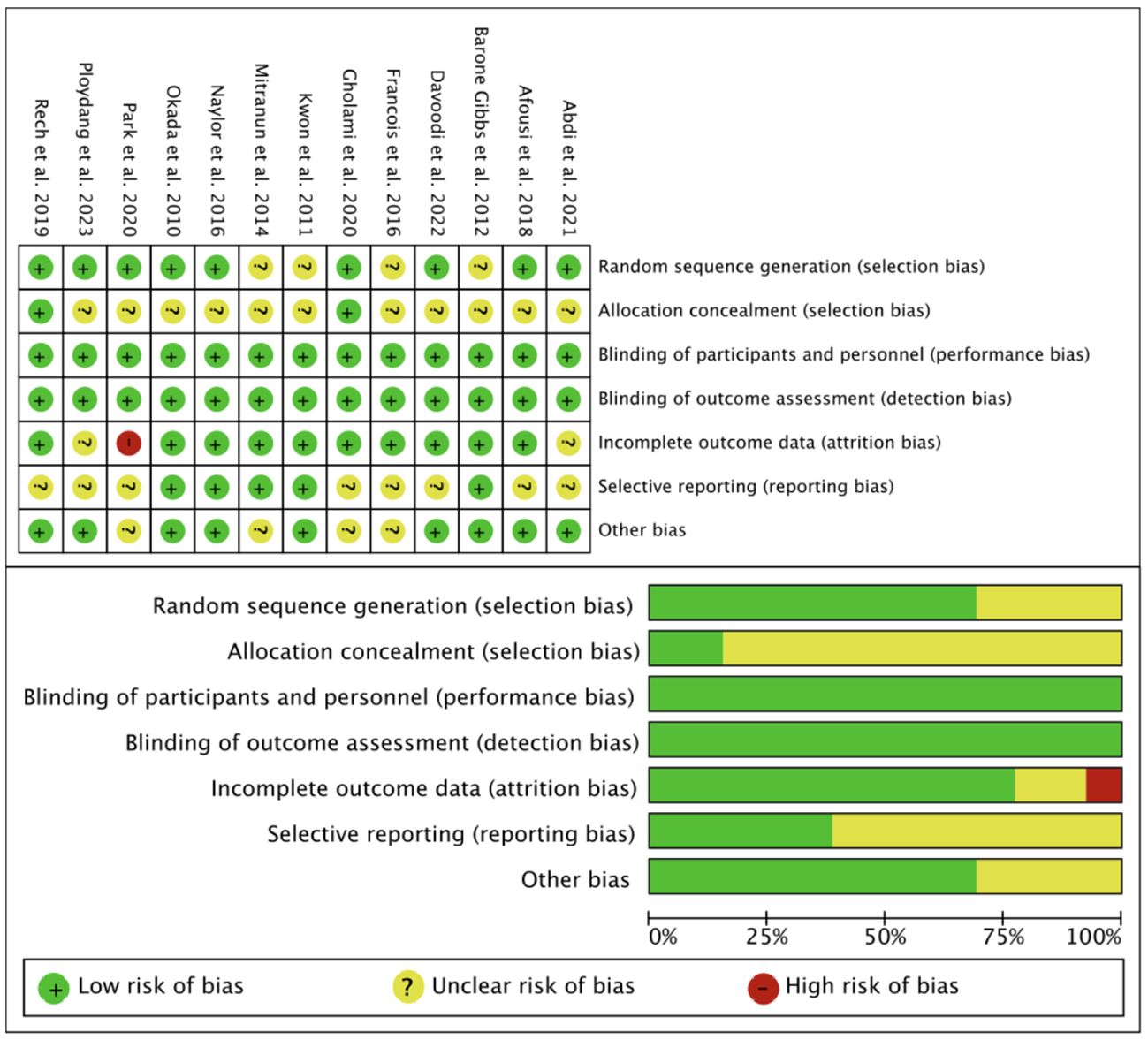
The RoB2 was used to assess the quality of the included studies in terms of selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias (Figure 2). The PEDro scale showed that of the 13 included studies, 1 were of excellent quality and 12 was of good quality (Supplementary Table 3).
3.4 Meta-analysis results
3.4.1 Effects of exercise on FMD in T2DM patients
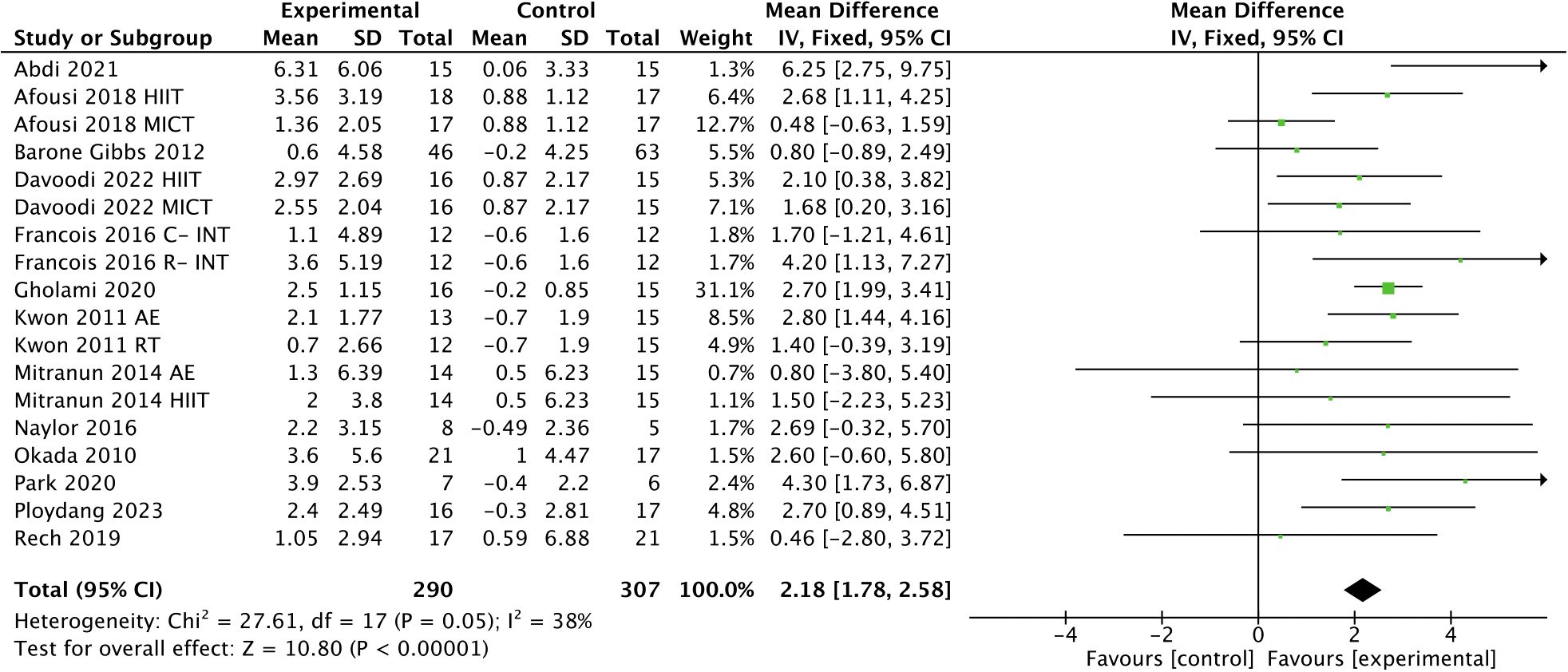
Exercise had a significant effect on improving FMD in T2DM patients [WMD, 2.18 (95% CI, 1.78-2.58); p < 0.00001; I2 = 38%; Figure 3].
3.4.2 Subgroup analysis
As shown in Figure 4, subgroup analysis showed that HIIT [WMD, 2.62 (95% CI, 1.42-3.82; p < 0.0001; I2 = 23%], aerobic exercise [WMD, 2.20 (95% CI, 1.29-3.11); p < 0.00001; I2 = 61%], resistance exercise [WMD, 1.91 (95% CI, 0.01-3.82); p = 0.05; I2 = 37%], and multicomponent training [WMD, 1.49 (95% CI, 0.15-2.83); p = 0.03; I2 = 0%] were effective in improving FMD in T2DM patients, with HIIT being the most effective intervention type.
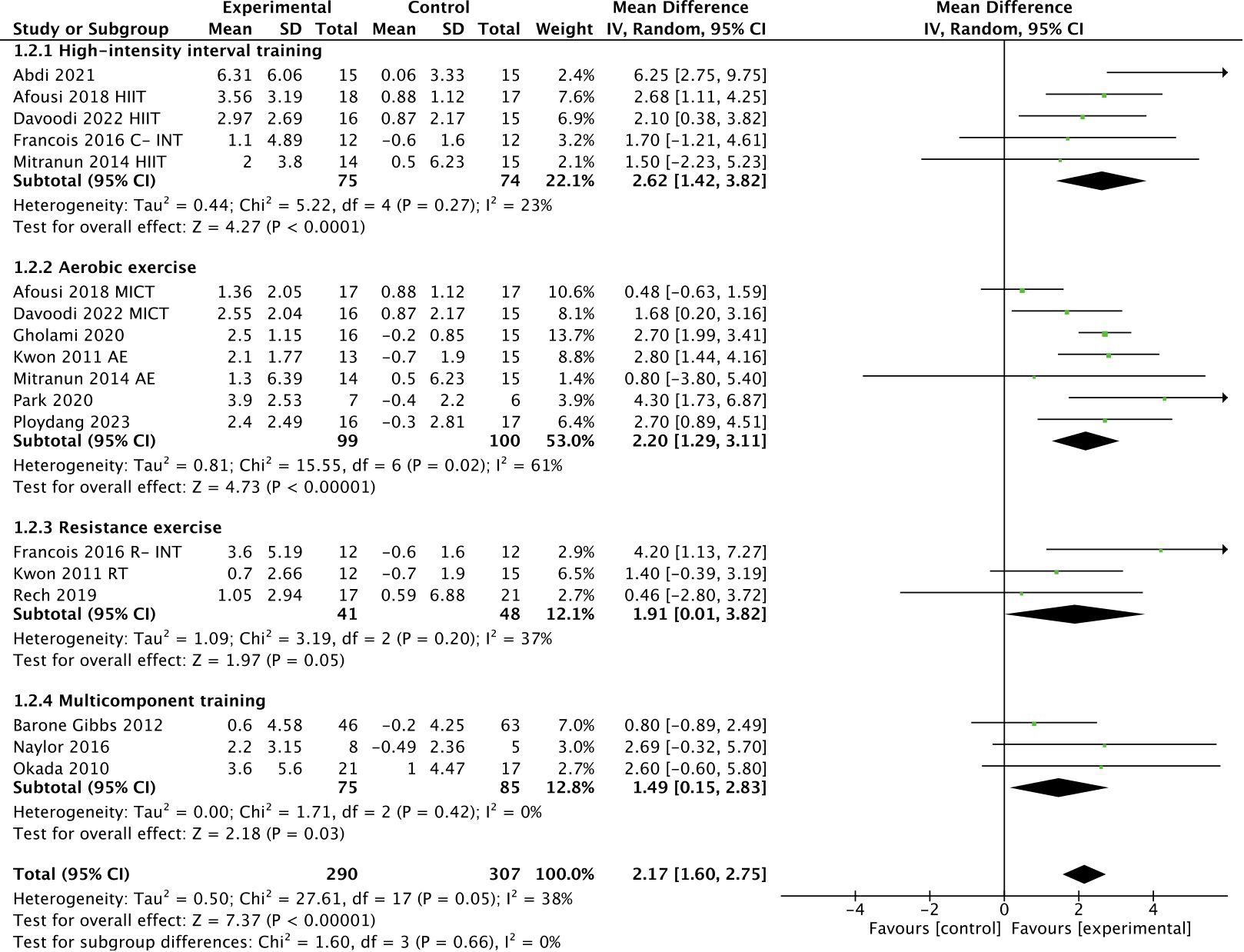
Figure 4 Meta-analysis results of the effect of different types of intervention on FMD in T2DM patients.
In addition, subgroup analyses indicated that a higher frequency [> 3 times, WMD, 3.06 (95% CI, 1.94-4.19); p < 0.00001; I2 = 0%; ≤ 3 times, WMD, 2.02 (95% CI, 1.59-2.45); p < 0.00001; I2 = 45%; Figure 5], a shorter session duration [< 60 min, WMD, 3.39 (95% CI, 2.07-4.71); p < 0.00001; I2 = 27%; ≥ 60 min, WMD, 1.86 (95% CI, 1.32-2.40); p < 0.00001; I2 = 24%; Figure 6], and a shorter weekly time (≤ 180 min, WMD, 2.40 [95% CI, 1.63-3.17); p < 0.00001; I2 = 0%; > 180 min, WMD, 2.11 (95% CI, 0.82-3.40); p = 0.001; I2 = 65%; Figure 7] were associated with larger improvements in FMD.
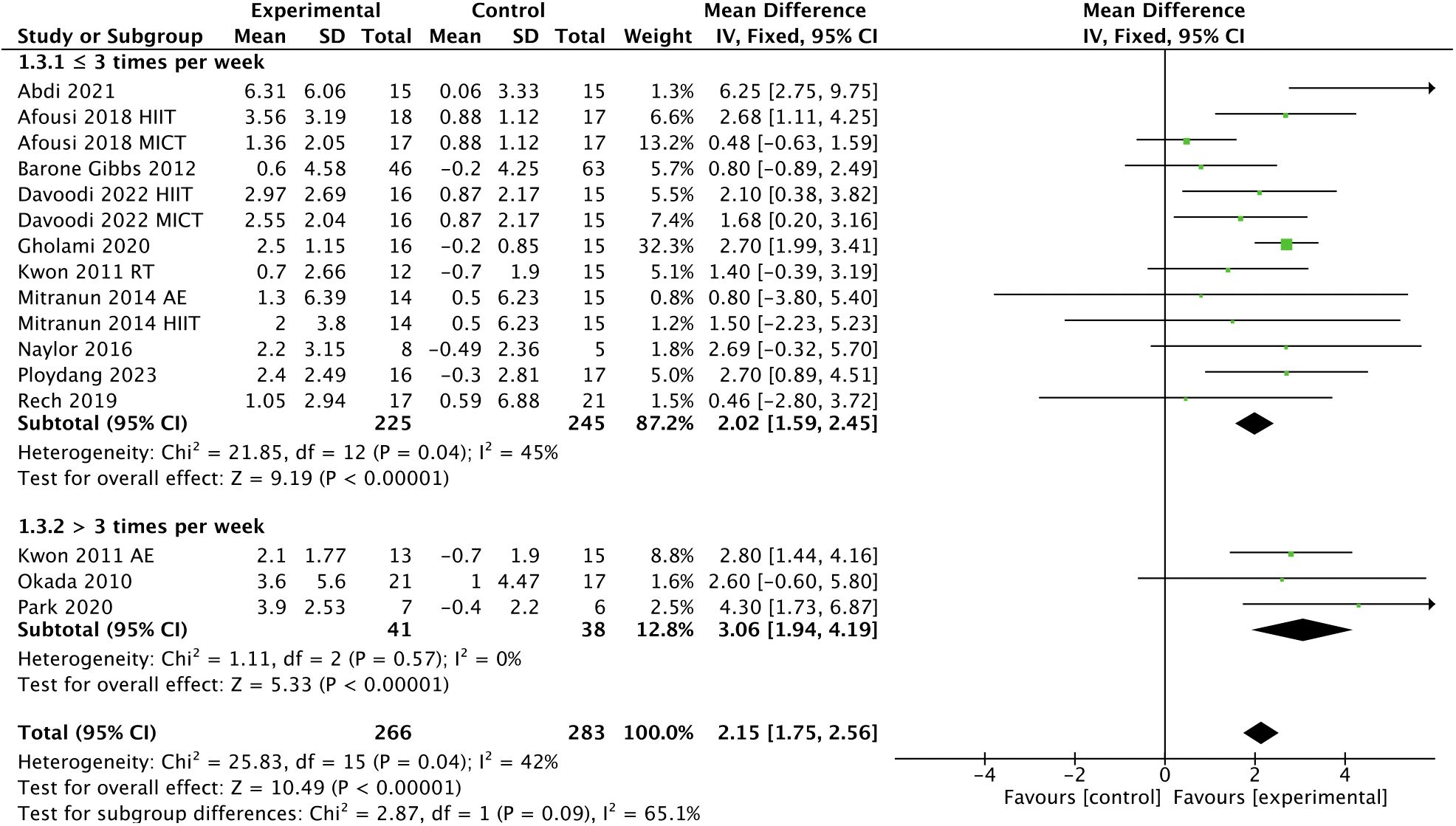
Figure 5 Meta-analysis results of the effect of the frequency of intervention on FMD in T2DM patients.
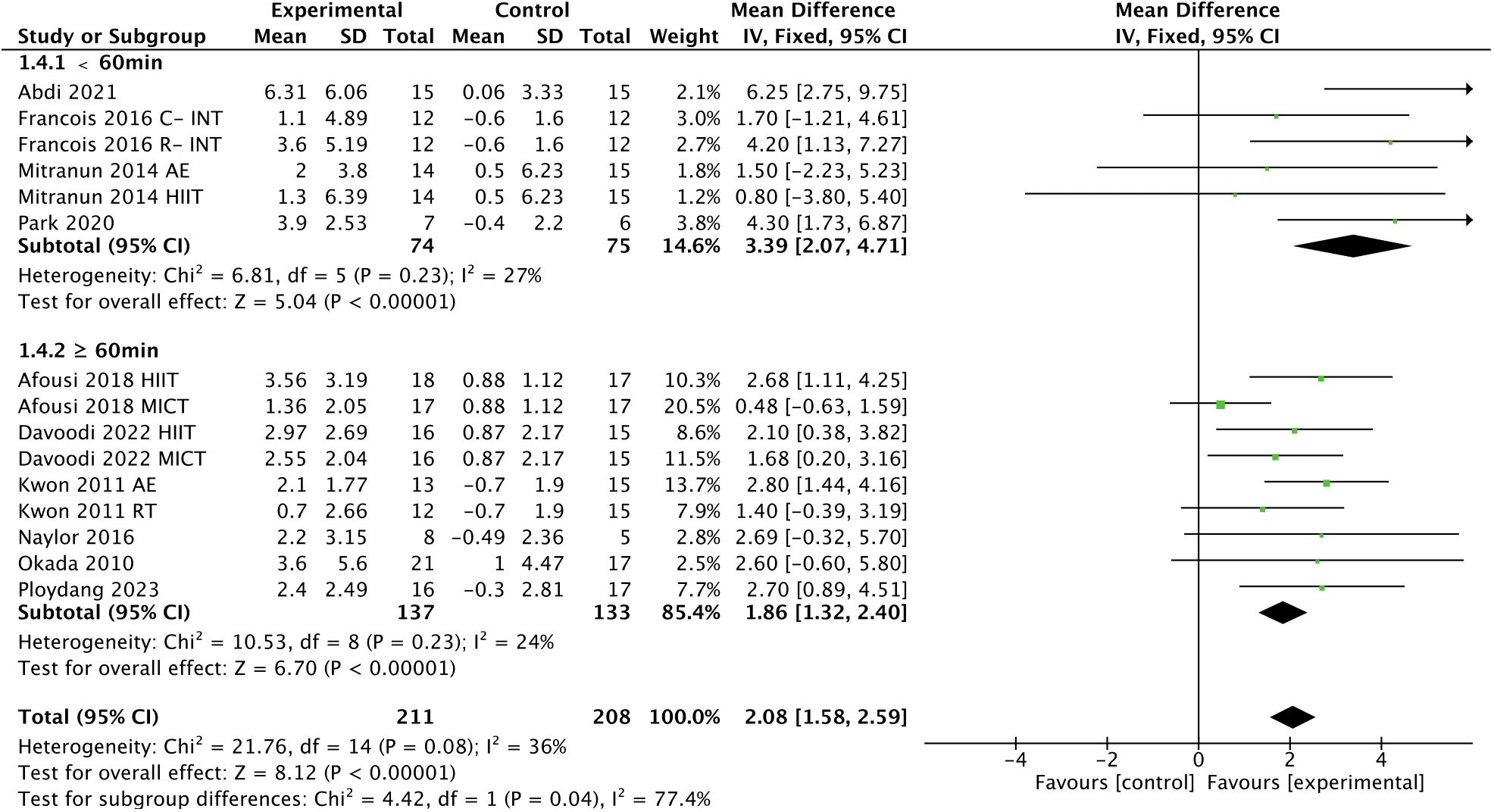
Figure 6 Meta-analysis results of the effect of the duration of intervention per session on FMD in T2DM patients.
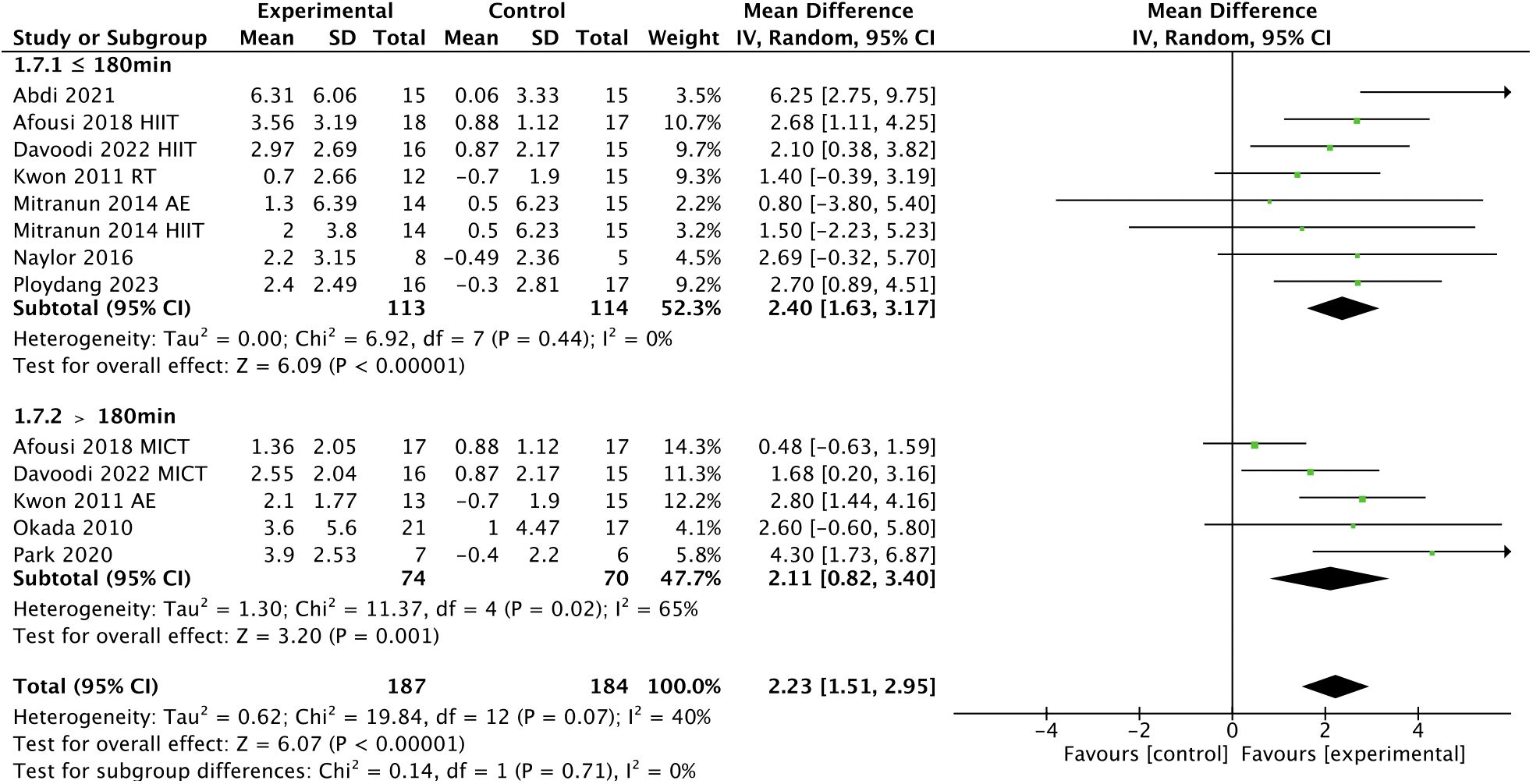
Figure 7 Meta-analysis results of the effect of the duration of intervention per week on FMD in T2DM patients.
3.5 Meta regression
Meta-regression analyses were performed on intervention duration, session duration, frequency, weekly time, participants’ age, and disease duration. No significant associations were observed between intervention duration (p = 0.128, Supplementary Figure 1), frequency (p = 0.144, Supplementary Figure 2), weekly time (p = 0.636, Supplementary Figure 3), session duration (p = 0.297, Supplementary Figure 4), age (p = 0.213, Supplementary Figure 5), or disease duration (p = 0.569, Supplementary Figure 6) and FMD.
3.6 Publication bias
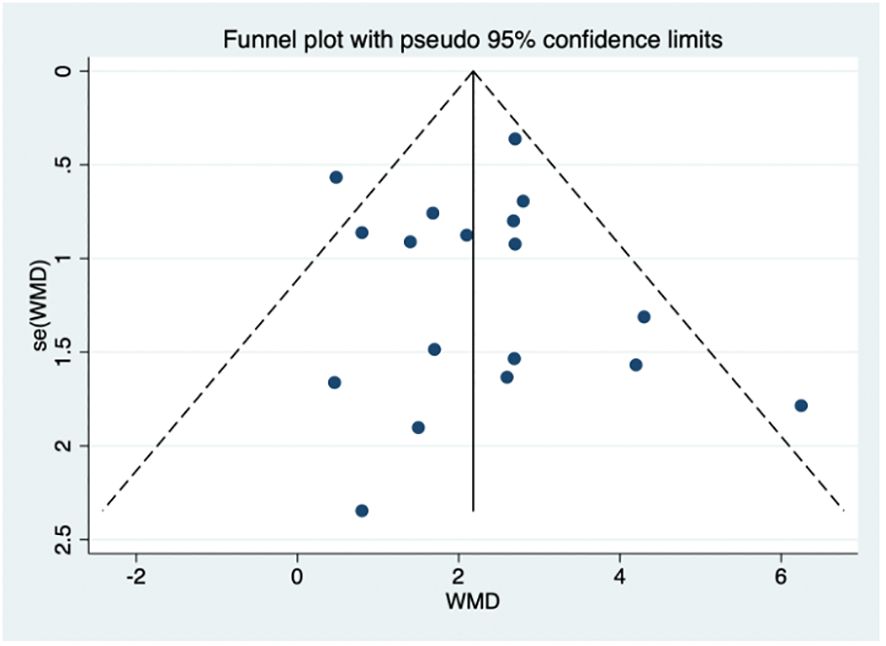
Possible publication bias was evaluated by the funnel plot (Figure 8). Visual inspection of the funnel plot suggested the absence of funnel plot asymmetry. Based on the results of egger’s test, small sample size studies were insufficient to affect the final results (p = 0.775, Supplementary Table 4).
3.7 Sensitivity analysis
Sensitivity analysis showed that there is no change in the direction or level of compatibility of the overall effect of exercise on FMD in T2DM patients when any of the included studies are omitted (Supplementary Figure 7).
4 Discussion
4.1 Effects of exercise on FMD in T2DM patients
In this study, we aimed to investigate the effect of exercise on FMD in T2DM patients and the optimal type, frequency, session duration, and weekly time of exercise for T2DM patients, and a total of 13 studies containing data from 523 patients were included. Our results showed that exercise significantly improved FMD in T2DM patients, which was consistent with the results of previous studies (50–52). In terms of WMD, exercise improved FMD by 2.18% in T2DM patients, which has significant clinical implications for individuals with T2DM. A previous meta-analysis showed that for every 1% increase in FMD, the risk of cardiovascular events was expected to decreased by 13% (53). Meanwhile, we noted the inclusion of studies combining exercise and dietary interventions in previous meta-analyses (50, 51), which may be due to the limited number of studies on the effect of exercise interventions alone on endothelial function in T2DM patients. Notably, our meta-analysis avoided this limitation by excluding studies combining exercise and dietary interventions (54–56), and none of the 13 included studies involved dietary interventions. This is because dietary interventions may have a confounding effect with exercise, thus masking the true efficacy of exercise in T2DM patients (57, 58).
Although the exact mechanisms remain incompletely elucidated, it can be hypothesized that the benefits of exercise on endothelial function can be amplified through the following mechanisms. First of all, shear stress plays a central role in regulating the inflammatory response of the vascular endothelium and the pathogenesis of atherosclerosis (59). Several studies have shown that exercise leads to an increase in blood flow, which in turn increases the shear stress of blood flow (60, 61), suggesting that the vascular endothelium is induced to synthesize more NO synthesis and increase the bioavailability of NO (62, 63). Secondly, both oxidative stress and inflammation are among the risk factors for vascular endothelial diseases (64, 65), and both are initiating factors for endothelial dysfunction. However, exercise has been shown to have anti-inflammatory properties and to reduce oxidative stress as a non-pharmacological intervention (66, 67). On the one hand, exercise reduces low-grade inflammation biomarkers and endothelial dysfunction biomarkers in plasma (68). In addition, exercise also affects oxidative stress by increasing the availability of antioxidant enzymes, thus improving endothelial function (62, 69). Furthermore, endothelial progenitor cells (EPCs) may also server as biomarkers of cardiovascular function (70), and Ribeiro et al. (71) showed that exercise increased the number and differentiation capacity of EPCs, which may contribute to vascular regeneration and angiogenesis. Thus, an increased in the number of EPCs may positively affect endothelial function (72, 73).
4.2 Subgroup analysis
Subgroup analysis of different types of intervention showed that HIIT, aerobic exercise, resistance exercise, and multi-component training were all effective in improving FMD in T2DM patients, with HIIT being the most effective intervention type, although aerobic exercise is widely used to improve chronic disease. This may be due to the fact that intensity is an important factor in FMD (28), and HIIT tends to be higher in intensity compared to aerobic exercise or other interventions. Thijssen et al. (74) showed that vascular blood flow and shear stress improved with increasing exercise intensity. Elevated vascular shear stress due to HIIT would lead to potassium channel activation and increased Ca2+ entering the vascular endothelium. Elevated intracellular Ca2+ concentration triggers activation of endothelial nitric oxide synthase (eNOS) (75, 76). In addition, HIIT may also lead to decreased catecholamine levels and α-adrenoceptor density (77, 78). Furthermore, adropin, as a regulator of eNOS synthase and NO release, has been implicated as a potential factor affecting endothelial function. A previous study showed that elevated adropin levels increased eNOS mRNA expression (79), indicating that elevated adropin may contribute to the reduction of exercise-induced atherosclerosis (80). Thus, elevated adropin may be considered a marker of improved endothelial function (24). Although the mechanism by which exercise leads to elevated adropin levels is unknown, it was observed in one study that a 12-week HIIT intervention significantly increased adropin levels in T2DM patients (24). Meanwhile, in another clinical trial using HIIT and MICT as interventions, a greater increase in adropin was observed in the HIIT group than in the MICT group (26). Moreover, a recent study (73) has shown that HIIT is superior to MICT in mobilizing circulating EPCs. All of these mechanisms appear to lead to greater NO production and increased NO bioavailability, thus well explaining the further improvement in FMD. Moreover, our result was consistent with a meta-analysis conducted by Ramos et al. (81), showing that HIIT was more effective in improving FMD compared to MICT.
Regarding intervention frequency, our subgroup analysis showed that interventions performed more than 3 times per week had a greater improvement in FMD compared to interventions performed up to 3 times per week, which was in agreement with a previous study (30), showing that high-frequency interventions are more beneficial than low-frequency interventions for endothelial function in T2DM patients. This hypothesis is also supported by a meta-analysis conducted by Fuertes-Kenneally et al. (82), showing that a higher frequency of intervention per week was associated with a better effect on endothelial function improvement. However, we believe that the frequency of intervention may be influenced by other factors, such as session duration and weekly time.
It is reported that the effects of exercise on health have a dose-response relationship, and that it is not more exercise that is beneficial, but rather the appropriate load that determines the health benefits of exercise (83). Several studies have found that engaging in extraordinarily prolonged exercise does not seem to provide corresponding benefits to the body and can even trigger negative effects on cardiac function (84–86). The benefits of exercise on endothelial function seem to apply here as well. Our subgroup analysis showed greater improvements in FMD in T2DM patients with exercise conducted less than 60 min compared to 60 min or more per session. It has been shown that T2DM patients typically have lower exercise tolerance (39), which may make it difficult for them to perform prolonged exercise during each session. Therefore, it can be concluded that a longer exercise duration does not contribute to more improvement in T2DM patients, and that a single session of less than 60 min may be more favorable for adherence to exercise and associated health benefits in T2DM patients.
However, our previous study found that the use of frequency and session duration alone did not exclude the influence of other variables (49). Therefore, we considered introducing weekly time to provide new ideas for exercise prescription. The weekly time was calculated based on the frequency and session duration. The World Health Organization (WHO) recommends that people perform 150-300 min of moderate-intensity aerobic exercise, 75-150 minutes of vigorous-intensity aerobic exercise, or an equal combination of moderate- and vigorous-intensity each week (87). Our subgroup analysis showed that a shorter weekly time (≤ 180 min vs. > 180 min) were associated with a larger improvement in FMD, which may also be related to the exercise tolerance of T2DM patients, for which more than 180 min per week does not seem to provide additional physical benefits.
4.3 Strengths and limitations of this systematic review
In this systematic review and meta-analysis, we included studies on the effect of exercise interventions alone on FMD in T2DM patients, and excluded studies where exercise was combined with dietary interventions, which can better reflect the effect of exercise interventions. Our findings provide an optimal combination of exercise modalities for T2DM patients. Clinically, T2DM patients can improve endothelial function by engaging in exercise 3 times per week for less than 60 min each time, especially HIIT, to achieve the goal of 180 min of exercise per week.
However, the present study has some limitations that should be noted. Although previous studies have found that improvement in FMD in T2DM patients decreases as the duration of intervention increases, it was not possible to investigate the effect of duration on the degree of improvement in FMD because the duration of the interventions in the included studies was generally focused on 12 weeks. In addition, although our study found that HIIT is the most effective intervention type for improving FMD in T2DM patients. However, due to the limited number of studies using HIIT in T2DM patients, we were unable to examine the optimal design of HIIT interventions. Finally, the studies we included contained aerobic exercise, HIIT, resistance exercise, and multicomponent exercise, and we were unable to standardize the intensity of exercise, so we were unable to explore the effect of intensity on FMD in T2DM patients.
5 Conclusion
In this meta-analysis, exercise had beneficial effect in improving FMD in T2DM patients, with HIIT being the most effective intervention type. To improve endothelial function, this meta-analysis provides clinicians with evidence to recommended that T2DM patients participate in exercise, especially HIIT, more than 3 times per week for less than 60 min, with a target of 180 min per week being reached by increasing the frequency of exercise.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
BQ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. YZ: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft. XT: Data curation, Formal analysis, Writing – review & editing. XH: Data curation, Formal analysis, Writing – review & editing. LD: Data curation, Formal analysis, Writing – review & editing. YL: Data curation, Formal analysis, Writing – review & editing. LY: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Key R&D Program of China (2022YFC3600201) and the Chinese Universities Scientific Fund (2021QN001, 2022QN015).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1347399/full#supplementary-material
References
1. Roden M, Shulman GI. The integrative biology of type 2 diabetes. Nature. (2019) 576:51–60. doi: 10.1038/s41586-019-1797-8
2. Zhang H, Dellsperger KC, Zhang C. The link between metabolic abnormalities and endothelial dysfunction in type 2 diabetes: an update. Basic Res Cardiol. (2012) 107:237. doi: 10.1007/s00395-011-0237-1
3. Avogaro A, Albiero M, Menegazzo L, de Kreutzenberg S, Fadini GP. Endothelial dysfunction in diabetes: the role of reparatory mechanisms. Diabetes Care. (2011) 34 Suppl 2:S285–90. doi: 10.2337/dc11-s239
4. Jansson PA. Endothelial dysfunction in insulin resistance and type 2 diabetes. J Intern Med. (2007) 262:173–83. doi: 10.1111/j.1365-2796.2007.01830.x
5. Xu S, Ilyas I, Little PJ, Li H, Kamato D, Zheng X, et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol Rev. (2021) 73:924–67. doi: 10.1124/pharmrev.120.000096
6. Woodman RJ, Chew GT, Watts GF. Mechanisms, significance and treatment of vascular dysfunction in type 2 diabetes mellitus: focus on lipid-regulating therapy. Drugs. (2005) 65:31–74. doi: 10.2165/00003495-200565010-00003
7. De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. (2000) 130:963–74. doi: 10.1038/sj.bjp.0703393
8. Nakagawa T, Tanabe K, Croker BP, Johnson RJ, Grant MB, Kosugi T, et al. Endothelial dysfunction as a potential contributor in diabetic nephropathy. Nat Rev Nephrol. (2011) 7:36–44. doi: 10.1038/nrneph.2010.152
9. Robson R, Kundur AR, Singh I. Oxidative stress biomarkers in type 2 diabetes mellitus for assessment of cardiovascular disease risk. Diabetes Metab Syndr. (2018) 12:455–62. doi: 10.1016/j.dsx.2017.12.029
10. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet. (2010) 375:2215–22. doi: 10.1016/s0140-6736(10)60484-9
11. Anderson EA, Mark AL. Flow-mediated and reflex changes in large peripheral artery tone in humans. Circulation. (1989) 79:93–100. doi: 10.1161/01.cir.79.1.93
12. Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. (2007) 115:1285–95. doi: 10.1161/circulationaha.106.652859
13. Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. (2009) 120:502–9. doi: 10.1161/circulationaha.109.864801
14. Thijssen DHJ, Bruno RM, van Mil A, Holder SM, Faita F, Greyling A, et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J. (2019) 40:2534–47. doi: 10.1093/eurheartj/ehz350
15. Shechter M, Issachar A, Marai I, Koren-Morag N, Freinark D, Shahar Y, et al. Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. Int J Cardiol. (2009) 134:52–8. doi: 10.1016/j.ijcard.2008.01.021
16. Avogaro A, de Kreutzenberg SV, Fadini G. Endothelial dysfunction: causes and consequences in patients with diabetes mellitus. Diabetes Res Clin Pract. (2008) 82:S94–s101. doi: 10.1016/j.diabres.2008.09.021
17. Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: A consensus statement from the American diabetes association. Diabetes Care. (2006) 29:1433–8. doi: 10.2337/dc06-9910
18. Qiu Y, Fernández-García B, Lehmann HI, Li G, Kroemer G, López-Otín C, et al. Exercise sustains the hallmarks of health. J Sport Health Sci. (2023) 12:8–35. doi: 10.1016/j.jshs.2022.10.003
20. Chen H, Chen C, Spanos M, Li G, Lu R, Bei Y, et al. Exercise training maintains cardiovascular health: signaling pathways involved and potential therapeutics. Signal Transduct Target Ther. (2022) 7:306. doi: 10.1038/s41392-022-01153-1
21. Magalhães JP, Santos DA, Correia IR, Hetherington-Rauth M, Ribeiro R, Raposo JF, et al. Impact of combined training with different exercise intensities on inflammatory and lipid markers in type 2 diabetes: A secondary analysis from a 1-year randomized controlled trial. Cardiovasc Diabetol. (2020) 19:169. doi: 10.1186/s12933-020-01136-y
22. Zhang J, Tam WWS, Kanokwan H, Kusuyama J, Wu VX. Effectiveness of combined aerobic and resistance exercise on cognition, metabolic health, physical function, and health-related quality of life in middle-aged and older adults with type 2 diabetes mellitus: A systematic review and meta-analysis. Arch Phys Med Rehabil. (2023) 000:1–15. doi: 10.1016/j.apmr.2023.10.005
23. Ashor AW, Lara J, Siervo M, Celis-Morales C, Oggioni C, Jakovljevic DG, et al. Exercise modalities and endothelial function: A systematic review and dose-response meta-analysis of randomized controlled trials. Sports Med. (2015) 45:279–96. doi: 10.1007/s40279-014-0272-9
24. Abdi S, Tadibi V, Sheikholeslami-Vatani D. Effect of high-intensity interval training on endothelial function in type 2 diabetic females. Asian J Sports Med. (2021) 12:e113566. doi: 10.5812/asjsm.113566
25. Barone Gibbs B, Dobrosielski DA, Bonekamp S, Stewart KJ, Clark JM. A randomized trial of exercise for blood pressure reduction in type 2 diabetes: effect on flow-mediated dilation and circulating biomarkers of endothelial function. Atherosclerosis. (2012) 224:446–53. doi: 10.1016/j.atherosclerosis.2012.07.035
26. Davoodi M, Hesamabadi BK, Ariabood E, Izadi MR, Ghardashi-Afousi A, Bigi MAB, et al. Improved blood pressure and flow-mediated dilatation via increased plasma adropin and nitrate/nitrite induced by high-intensity interval training in patients with type 2 diabetes. Exp Physiol. (2022) 107:813–24. doi: 10.1113/ep089371
27. Francois ME, Durrer C, Pistawka KJ, Halperin FA, Little JP. Resistance-based interval exercise acutely improves endothelial function in type 2 diabetes. Am J Physiol Heart Circ Physiol. (2016) 311:H1258–h67. doi: 10.1152/ajpheart.00398.2016
28. Ghardashi Afousi A, Izadi MR, Rakhshan K, Mafi F, Biglari S, Gandomkar Bagheri H. Improved brachial artery shear patterns and increased flow-mediated dilatation after low-volume high-intensity interval training in type 2 diabetes. Exp Physiol. (2018) 103:1264–76. doi: 10.1113/ep087005
29. Gholami F, Nazari H, Alimi M. Cycle training improves vascular function and neuropathic symptoms in patients with type 2 diabetes and peripheral neuropathy: A randomized controlled trial. Exp Gerontol. (2020) 131:110799. doi: 10.1016/j.exger.2019.110799
30. Kwon HR, Min KW, Ahn HJ, Seok HG, Lee JH, Park GS, et al. Effects of aerobic exercise vs. Resistance training on endothelial function in women with type 2 diabetes mellitus. Diabetes Metab J. (2011) 35:364–73. doi: 10.4093/dmj.2011.35.4.364
31. Mitranun W, Deerochanawong C, Tanaka H, Suksom D. Continuous vs interval training on glycemic control and macro- and microvascular reactivity in type 2 diabetic patients. Scand J Med Sci Sports. (2014) 24:e69–76. doi: 10.1111/sms.12112
32. Naylor LH, Davis EA, Kalic RJ, Paramalingam N, Abraham MB, Jones TW, et al. Exercise training improves vascular function in adolescents with type 2 diabetes. Physiol Rep. (2016) 4:e12713. doi: 10.14814/phy2.12713
33. Okada S, Hiuge A, Makino H, Nagumo A, Takaki H, Konishi H, et al. Effect of exercise intervention on endothelial function and incidence of cardiovascular disease in patients with type 2 diabetes. J Atheroscler Thromb. (2010) 17:828–33. doi: 10.5551/jat.3798
34. Park LK, Parks EJ, Pettit-Mee RJ, Woodford ML, Ghiarone T, Smith JA, et al. Skeletal muscle microvascular insulin resistance in type 2 diabetes is not improved by eight weeks of regular walking. J Appl Physiol (1985). (2020) 129:283–96. doi: 10.1152/japplphysiol.00174.2020
35. Ploydang T, Khovidhunkit W, Tanaka H, Suksom D. Nordic walking in water on cerebrovascular reactivity and cognitive function in elderly patients with type 2 diabetes. Med Sci Sports Exerc. (2023) 55:1803–11. doi: 10.1249/mss.0000000000003216
36. Rech A, Botton CE, Lopez P, Quincozes-Santos A, Umpierre D, Pinto RS. Effects of short-term resistance training on endothelial function and inflammation markers in elderly patients with type 2 diabetes: A randomized controlled trial. Exp Gerontol. (2019) 118:19–25. doi: 10.1016/j.exger.2019.01.003
37. Boulé NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: A meta-analysis of controlled clinical trials. Jama. (2001) 286:1218–27. doi: 10.1001/jama.286.10.1218
38. Bloomgarden ZT. American diabetes association annual meeting, 1999: diabetes and obesity. Diabetes Care. (2000) 23:118–24. doi: 10.2337/diacare.23.1.118
39. Yang Z, Scott CA, Mao C, Tang J, Farmer AJ. Resistance exercise versus aerobic exercise for type 2 diabetes: A systematic review and meta-analysis. Sports Med. (2014) 44:487–99. doi: 10.1007/s40279-013-0128-8
40. Praet SF, van Loon LJ. Optimizing the therapeutic benefits of exercise in type 2 diabetes. J Appl Physiol (1985). (2007) 103:1113–20. doi: 10.1152/japplphysiol.00566.2007
41. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. Bmj. (2021) 372:n71. doi: 10.1136/bmj.n71
42. Li G, You Q, Hou X, Zhang S, Du L, Lv Y, et al. The effect of exercise on cognitive function in people with multiple sclerosis: A systematic review and meta-analysis of randomized controlled trials. J Neurol. (2023) 270:2908–23. doi: 10.1007/s00415-023-11649-7
43. Tao X, Chen Y, Zhen K, Ren S, Lv Y, Yu L. Effect of continuous aerobic exercise on endothelial function: A systematic review and meta-analysis of randomized controlled trials. Front Physiol. (2023) 14:1043108. doi: 10.3389/fphys.2023.1043108
44. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. Bmj. (2011) 343:d5928. doi: 10.1136/bmj.d5928
45. Zhen K, Zhang S, Tao X, Li G, Lv Y, Yu L. A systematic review and meta-analysis on effects of aerobic exercise in people with Parkinson's disease. NPJ Parkinsons Dis. (2022) 8:146. doi: 10.1038/s41531-022-00418-4
46. Zhang S, Zhen K, Su Q, Chen Y, Lv Y, Yu L. The effect of aerobic exercise on cognitive function in people with Alzheimer's disease: A systematic review and meta-analysis of randomized controlled trials. Int J Environ Res Public Health. (2022) 19:15700. doi: 10.3390/ijerph192315700
47. You Q, Yu L, Li G, He H, Lv Y. Effects of different intensities and durations of aerobic exercise on vascular endothelial function in middle-aged and elderly people: A meta-analysis. Front Physiol. (2021) 12:803102. doi: 10.3389/fphys.2021.803102
48. Wang Z, Qiu B, Gao J, Del Coso J. Effects of caffeine intake on endurance running performance and time to exhaustion: A systematic review and meta-analysis. Nutrients. (2022) 15:148. doi: 10.3390/nu15010148
49. Li G, Lv Y, Su Q, You Q, Yu L. The effect of aerobic exercise on pulse wave velocity in middle-aged and elderly people: A systematic review and meta-analysis of randomized controlled trials. Front Cardiovasc Med. (2022) 9:960096. doi: 10.3389/fcvm.2022.960096
50. Montero D, Walther G, Benamo E, Perez-Martin A, Vinet A. Effects of exercise training on arterial function in type 2 diabetes mellitus: A systematic review and meta-analysis. Sports Med. (2013) 43:1191–9. doi: 10.1007/s40279-013-0085-2
51. Qiu S, Cai X, Yin H, Sun Z, Zügel M, Steinacker JM, et al. Exercise training and endothelial function in patients with type 2 diabetes: A meta-analysis. Cardiovasc Diabetol. (2018) 17:64. doi: 10.1186/s12933-018-0711-2
52. Lee J-H, Lee R, Hwang M-H, Hamilton MT, Park Y. The effects of exercise on vascular endothelial function in type 2 diabetes: A systematic review and meta-analysis. Diabetol Metab Syndrome. (2018) 10:15. doi: 10.1186/s13098-018-0316-7
53. Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: A meta-analysis. Int J Cardiovasc Imaging. (2010) 26:631–40. doi: 10.1007/s10554-010-9616-1
54. Wycherley TP, Brinkworth GD, Noakes M, Buckley JD, Clifton PM. Effect of caloric restriction with and without exercise training on oxidative stress and endothelial function in obese subjects with type 2 diabetes. Diabetes Obes Metab. (2008) 10:1062–73. doi: 10.1111/j.1463-1326.2008.00863.x
55. Yezhova O, Melekhovets O, Olha S, Kovalenko Y, Ol’khovyk A, Melekhovets Y, et al. Impact of the multimodal physical program on the endothelium function in diabetic patients with obesity. Acta Balneologica. (2019) 61:11–6. doi: 10.36740/ABal201901102
56. Leehey DJ, Collins E, Kramer HJ, Cooper C, Butler J, McBurney C, et al. Structured exercise in obese diabetic patients with chronic kidney disease: A randomized controlled trial. Am J Nephrol. (2016) 44:54–62. doi: 10.1159/000447703
57. Hamdy O, Ledbury S, Mullooly C, Jarema C, Porter S, Ovalle K, et al. Lifestyle modification improves endothelial function in obese subjects with the insulin resistance syndrome. Diabetes Care. (2003) 26:2119–25. doi: 10.2337/diacare.26.7.2119
58. Schwingshackl L, Hoffmann G. Mediterranean dietary pattern, inflammation and endothelial function: A systematic review and meta-analysis of intervention trials. Nutr Metab Cardiovasc Dis. (2014) 24:929–39. doi: 10.1016/j.numecd.2014.03.003
59. Zakkar M, Angelini GD, Emanueli C. Regulation of vascular endothelium inflammatory signalling by shear stress. Curr Vasc Pharmacol. (2016) 14:181–6. doi: 10.2174/1570161114666151202205139
60. Goto C, Higashi Y, Kimura M, Noma K, Hara K, Nakagawa K, et al. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: role of endothelium-dependent nitric oxide and oxidative stress. Circulation. (2003) 108:530–5. doi: 10.1161/01.Cir.0000080893.55729.28
61. Niebauer J, Cooke JP. Cardiovascular effects of exercise: role of endothelial shear stress. J Am Coll Cardiol. (1996) 28:1652–60. doi: 10.1016/s0735-1097(96)00393-2
62. Di Francescomarino S, Sciartilli A, Di Valerio V, Di Baldassarre A, Gallina S. The effect of physical exercise on endothelial function. Sports Med. (2009) 39:797–812. doi: 10.2165/11317750-000000000-00000
63. Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, et al. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. (2000) 101:2896–901. doi: 10.1161/01.cir.101.25.2896
64. Steven S, Frenis K, Oelze M, Kalinovic S, Kuntic M, Bayo Jimenez MT, et al. Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxid Med Cell Longev. (2019) 2019:7092151. doi: 10.1155/2019/7092151
65. Higashi Y. Roles of oxidative stress and inflammation in vascular endothelial dysfunction-related disease. Antioxid (Basel). (2022) 11:20220930. doi: 10.3390/antiox11101958
66. Teixeira-Lemos E, Nunes S, Teixeira F, Reis F. Regular physical exercise training assists in preventing type 2 diabetes development: focus on its antioxidant and anti-inflammatory properties. Cardiovasc Diabetol. (2011) 10:12. doi: 10.1186/1475-2840-10-12
67. Hambrecht R, Adams V, Erbs S, Linke A, Kränkel N, Shu Y, et al. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. (2003) 107:3152–8. doi: 10.1161/01.Cir.0000074229.93804.5c
68. Vandercappellen EJ, Koster A, Savelberg H, Eussen S, Dagnelie PC, Schaper NC, et al. Sedentary behaviour and physical activity are associated with biomarkers of endothelial dysfunction and low-grade inflammation-relevance for (Pre)Diabetes: the maastricht study. Diabetologia. (2022) 65:777–89. doi: 10.1007/s00125-022-05651-3
69. El Assar M, Álvarez-Bustos A, Sosa P, Angulo J, Rodríguez-Mañas L. Effect of physical activity/exercise on oxidative stress and inflammation in muscle and vascular aging. Int J Mol Sci. (2022) 23:8713. doi: 10.3390/ijms23158713
70. Heinisch PP, Bello C, Emmert MY, Carrel T, Dreßen M, Hörer J, et al. Endothelial progenitor cells as biomarkers of cardiovascular pathologies: A narrative review. Cells. (2022) 11:1678. doi: 10.3390/cells11101678
71. Ribeiro F, Ribeiro IP, Alves AJ, do Céu Monteiro M, Oliveira NL, Oliveira J, et al. Effects of exercise training on endothelial progenitor cells in cardiovascular disease: A systematic review. Am J Phys Med Rehabil. (2013) 92:1020–30. doi: 10.1097/PHM.0b013e31829b4c4f
72. Koutroumpi M, Dimopoulos S, Psarra K, Kyprianou T, Nanas S. Circulating endothelial and progenitor cells: evidence from acute and long-term exercise effects. World J Cardiol. (2012) 4:312–26. doi: 10.4330/wjc.v4.i12.312
73. Ferentinos P, Tsakirides C, Swainson M, Davison A, Martyn-St James M, Ispoglou T. The impact of different forms of exercise on endothelial progenitor cells in healthy populations. Eur J Appl Physiol. (2022) 122:1589–625. doi: 10.1007/s00421-022-04921-7
74. Thijssen DH, Dawson EA, Black MA, Hopman MT, Cable NT, Green DJ. Brachial artery blood flow responses to different modalities of lower limb exercise. Med Sci Sports Exerc. (2009) 41:1072–9. doi: 10.1249/MSS.0b013e3181923957
75. Gerhold KA, Schwartz MA. Ion channels in endothelial responses to fluid shear stress. Physiol (Bethesda). (2016) 31:359–69. doi: 10.1152/physiol.00007.2016
76. Currie KD, McKelvie RS, Macdonald MJ. Flow-mediated dilation is acutely improved after high-intensity interval exercise. Med Sci Sports Exerc. (2012) 44:2057–64. doi: 10.1249/MSS.0b013e318260ff92
77. Casey DP, Schneider AC, Ueda K. Influence of chronic endurance exercise training on conduit artery retrograde and oscillatory shear in older adults. Eur J Appl Physiol. (2016) 116:1931–40. doi: 10.1007/s00421-016-3445-4
78. Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation. (2007) 115:3086–94. doi: 10.1161/circulationaha.106.675041
79. Lovren F, Pan Y, Quan A, Singh KK, Shukla PC, Gupta M, et al. Adropin is a novel regulator of endothelial function. Circulation. (2010) 122:S185–92. doi: 10.1161/circulationaha.109.931782
80. Fujie S, Hasegawa N, Sato K, Fujita S, Sanada K, Hamaoka T, et al. Aerobic exercise training-induced changes in serum adropin level are associated with reduced arterial stiffness in middle-aged and older adults. Am J Physiol Heart Circ Physiol. (2015) 309:H1642–7. doi: 10.1152/ajpheart.00338.2015
81. Ramos JS, Dalleck LC, Tjonna AE, Beetham KS, Coombes JS. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: A systematic review and meta-analysis. Sports Med. (2015) 45:679–92. doi: 10.1007/s40279-015-0321-z
82. Fuertes-Kenneally L, Manresa-Rocamora A, Blasco-Peris C, Ribeiro F, Sempere-Ruiz N, Sarabia JM, et al. Effects and optimal dose of exercise on endothelial function in patients with heart failure: A systematic review and meta-analysis. Sports Med Open. (2023) 9:8. doi: 10.1186/s40798-023-00553-z
83. Izquierdo M, Merchant RA, Morley JE, Anker SD, Aprahamian I, Arai H, et al. International exercise recommendations in older adults (Icfsr): expert consensus guidelines. J Nutr Health Aging. (2021) 25:824–53. doi: 10.1007/s12603-021-1665-8
84. Aengevaeren VL, VAN Kimmenade RRJ, Hopman MTE, VAN Royen N, Snider JV, Januzzi JL, et al. Exercise-induced changes in soluble st2 concentrations in marathon runners. Med Sci Sports Exerc. (2019) 51:405–10. doi: 10.1249/mss.0000000000001806
85. Aengevaeren VL, Hopman MTE, Thijssen DHJ, van Kimmenade RR, de Boer MJ, Eijsvogels TMH. Endurance exercise-induced changes in bnp concentrations in cardiovascular patients versus healthy controls. Int J Cardiol. (2017) 227:430–5. doi: 10.1016/j.ijcard.2016.11.016
86. Shave R, Baggish A, George K, Wood M, Scharhag J, Whyte G, et al. Exercise-induced cardiac troponin elevation: evidence, mechanisms, and implications. J Am Coll Cardiol. (2010) 56:169–76. doi: 10.1016/j.jacc.2010.03.037
Keywords: exercise, endothelial function, flow-mediated dilation, type 2 diabetes mellitus, systematic review, meta-analysis
Citation: Qiu B, Zhou Y, Tao X, Hou X, Du L, Lv Y and Yu L (2024) The effect of exercise on flow-mediated dilation in people with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Front. Endocrinol. 15:1347399. doi: 10.3389/fendo.2024.1347399
Received: 30 November 2023; Accepted: 11 March 2024;
Published: 26 March 2024.
Edited by:
Roberto Codella, University of Milan, ItalyReviewed by:
Giuseppe Battaglia, University of Palermo, ItalyEwan Thomas, University of Palermo, Italy
Copyright © 2024 Qiu, Zhou, Tao, Hou, Du, Lv and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laikang Yu, yulaikang@126.com
†These authors have contributed equally to this work and share first authorship
 Bopeng Qiu
Bopeng Qiu Yilun Zhou1†
Yilun Zhou1† Xifeng Tao
Xifeng Tao Xiao Hou
Xiao Hou Yuanyuan Lv
Yuanyuan Lv Laikang Yu
Laikang Yu