- 1College of Animal Science, Inner Mongolia Agricultural University, Hohhot, China
- 2Northern Agriculture and Livestock Husbandry Technical Innovation Center, Chinese Academy of Agricultural Sciences, Hohhot, China
- 3Institute of Animal Husbandry, Heilongjiang Academy of Agricultural Sciences, Harbin, China
Introduction: Melatonin can treat androgenetic alopecia in males. Goats can be used as animal models to study melatonin treatment for human alopecia. In this study, a meta-analysis of melatonin’s effects on goat hair follicles was pursued.
Methods: Literature from the last 20 years was searched in Scopus, Science Direct, Web of Science and PubMed. Melatonin’s effect on goat hair follicles and litter size were performed through a traditional meta-analysis and trial sequential analysis. A network meta-analysis used data from oocyte development to blastocyst. The hair follicle genes regulated by melatonin performed KEGG and PPI. We hypothesized that there are differences in melatonin receptors between different goats, and therefore completed melatonin receptor 1A homology modelling and molecular docking.
Results: The results showed that melatonin did not affect goat primary follicle or litter size. However, there was a positive correlation with secondary follicle growth. The goat melatonin receptor 1A SNPs influence melatonin’s functioning. The wild type gene defect MR1 is a very valuable animal model.
Discussion: Future studies should focus on the relationship between goat SNPs and the effect of embedded melatonin. This study will provide theoretical guidance for the cashmere industry and will be informative for human alopecia research.
Highlights
● Melatonin did not affect goat primary follicle and litter size. However, there was a positive correlation with secondary follicle growth.
● Goat melatonin receptor 1A SNPs affects melatonin to exert its function.
● Wild-type gene defect of MR1 is a very valuable animal model.
Introduction
Alopecia (hair loss) due to endocrine disorders has always plagued humans (1, 2). Animal models for studying hair follicles in humans are usually rats (3), mice (4), guinea pigs (5), rabbits (6), and dogs (7). In addition, the goat PLP2 (proteolipid protein 2) gene expressed only in the inner root sheath, suggesting that it may be associated with alopecia (8). Thus, goat hair follicle research can be applicable for human alopecia (9). It can be used as an animal model for human alopecia research (10).
Melatonin regulates physiological activity in the whole body. Melatonin-associated pathways possibly alleviate radiotherapy-induced alopecia (11). A recent meta-analysis showed evidence to support the use of melatonin to promote scalp hair growth, with melatonin being more effective in men with androgenetic alopecia (12). Moreover, melatonin regulates gene expression in the hair follicles of goats and affects hair follicle growth (13).
Melatonin affects oocyte development in humans (14); there has been a study on goat oocyte development (15). In addition, studies on melatonin, luzindole, and cysteamine mixed effects have also been reported (16, 17), and such research data are suitable for network meta-analysis.
Embedding melatonin under the skin of goats can increase cashmere production (18, 19). Melatonin can increase goat secondary follicle density and does not affect the growth of primary follicle density (18, 20). On the contrary, melatonin significantly reduces primary follicle density in goats (21, 22). It is necessary to analyze the effect of melatonin on goat hair follicles. This study provides theoretical guidance for the cashmere industry.
Methods
Database search strategy and study inclusion
Three scholars searched for related studies in Scopus, Science, Web of Science, and PubMed published between 01/01/2003 and 01/09/2023. Two searches were performed. In the first, we searched for effects of melatonin on goat hair follicle growth. The search terms were melatonin AND (hair follicle) AND (goat OR ram OR ewe OR ovine). This search strategy includes hair follicle data as well as data on regulatory genes. In the second search, we looked for effects of melatonin on goat oocyte development. The search keywords were melatonin AND (ovine OR goat OR ewe OR ram) AND (oocyte OR implant). Each of the three scholars worked independently and negotiated any disagreements. Literature mentioned random trials or what the author believes are individuals randomly selected from a population. Literature was included according to the following points: (1) The studied species included goats but were not restricted to goats. (2) The writing was in English. (3) Hair follicle growth or oocyte development was studied in the paper. The process of study inclusion is shown in Figure 1A.
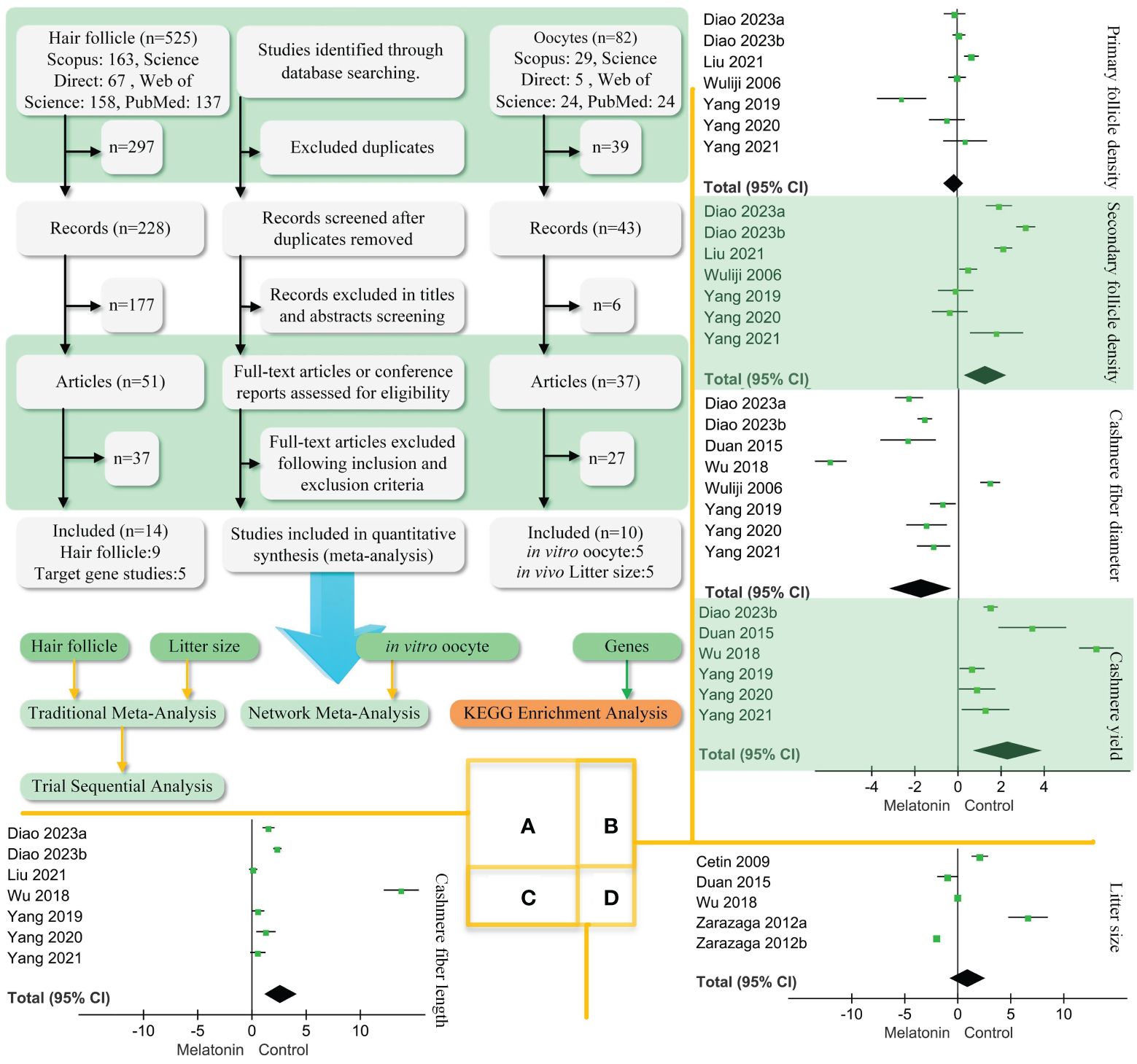
Figure 1 PRISMA diagram of the process of study selection and traditional meta-analysis. (A). We followed the steps to screen the retrieved literature and ended up with 22 articles. Two articles had both hair follicle findings and litter size. Hair follicle, litter size, in vitro oocyte, and gene data performance traditional and network meta-analysis. (B). Traditional meta-analysis of follicle density, cashmere fibre diameter, and yield. (C). Traditional meta-analysis of melatonin’s effect on cashmere fibre length. (D). Traditional meta-analysis of melatonin’s effect on goat litter size. Black diamond block represents 95% CI.
Data extraction
Study data were extracted as continue-type data that included the number of study individuals, observations, and SD (standard deviation) or SE (standard error). SE was converted to SD for extraction. If the data were in figures, the GetData Graph Digitizer (version 2.26) was used to obtain the data (23). When extracting data of melatonin’s effect on gene expression, only the genes mentioned in the study were obtained and the data uploaded to the database were not used.
Traditional and network meta-analysis
A traditional meta-analysis was used to analyze hair follicle and litter size data. Specifically, primary and secondary hair follicle densities, cashmere yield, cashmere fiber length, and litter size were treated as continuous data. Using Review Manager (version 5.4), the meta-analysis was performed according to the random model. Java (version 1.8.0) was used to perform the trial sequential analysis (TSA) on the above data.
Network meta-analysis data from the development of oocytes to blastocysts were used. R software (version 4.1.2) and the “coda,” “rjag,” and “gemtc” packages in JAGS (Just Another Gibbs Sampler, version 4.3.0) were used for the analysis. The results of the network meta-analysis were landscaped and processed using 3DMax software (version, 2023).
KEGG enrichment analysis and PPI network analysis
For the genes regulated by melatonin, DAVID (https://david.ncifcrf.gov) was used to procedure the KEGG enrichment analyses. The reference species was chosen as the goat Capra hircus. GraphPad Prism (version 9.0.2) was used to visualize the obtained data.
For the same genes regulated by melatonin procedure PPI (protein–protein interaction networks) construction analysis, STRING (https://cn.string-db.org/) was used to establish an original PPI network data. The data were visualized using Cytoscape (version 3.7.2).
Homology modelling of melatonin receptor 1A 3D structures and molecular docking
To elucidate the reasons for the discrepancies in the data reported in the various studies, we hypothesized that there are differences in melatonin receptors in different goats. SNPs (single-nucleotide polymorphisms) were previously reported to affect cashmere production in goats (24) through melatonin receptor 1A (25). Five SNPs (190, 424, 577, 589 C>T, and 421 T>C) NCBI data (gene number, AF419334) were downloaded. Each mutation was translated into an amino acid sequence, and a homology model was created based on the Swiss model (https://swissmodel.expasy.org/).
The ligand structure of melatonin was downloaded from PubChem. AutoDock (version 4.2.6) conducts protein and ligand docking and estimates the affinity. The docking results were analyzed using PyMOL (version 2. 6. 0).
Results
Study selection and characteristics
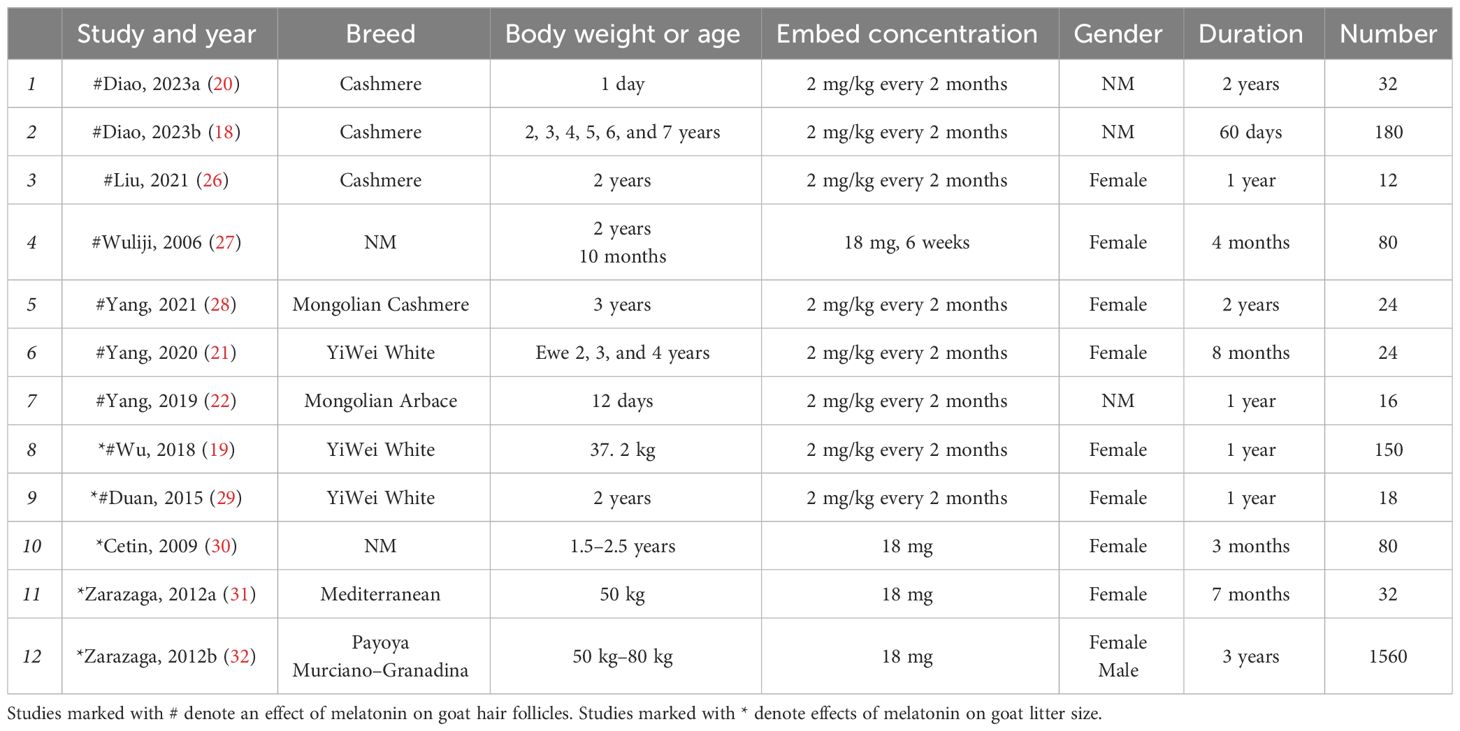
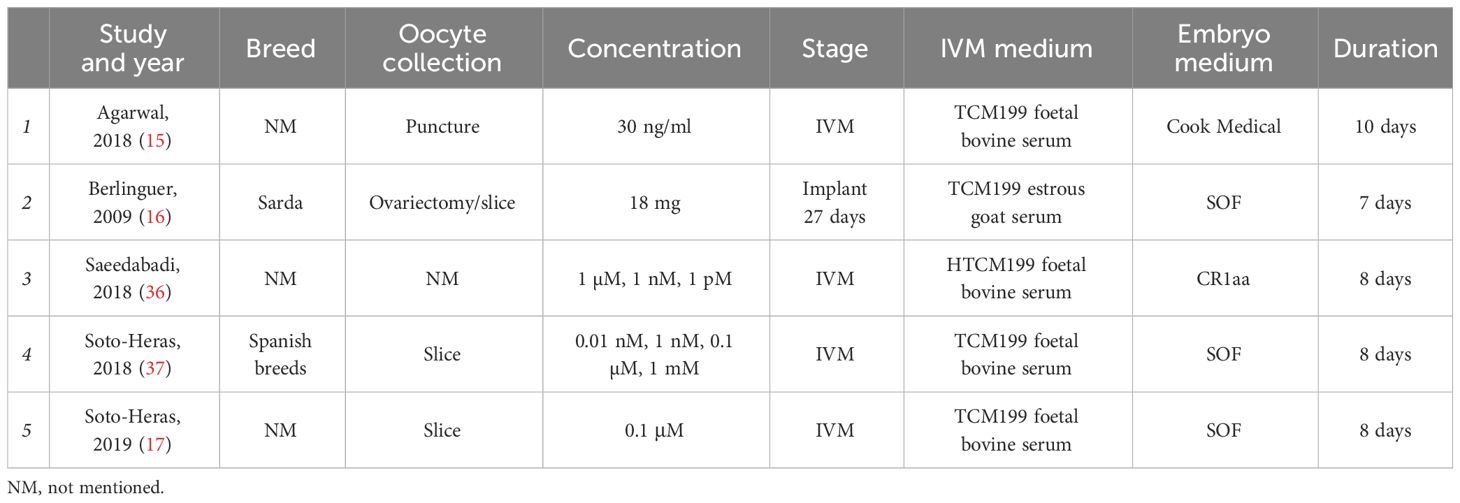
A total of 607 studies were retrieved, including 525 studies involving hair follicles and 82 studies on oocytes. Figure 1A shows that 22 papers were eventually obtained for this study (Table 1). The results of effects on goat hair follicle and litter size are listed in two papers that mentioned both hair follicles and litter size (19, 29). Melatonin regulator goat secondary hair follicle genes are listed in Table 2. Table 3 shows melatonin’s effect on goat oocyte development in vitro.
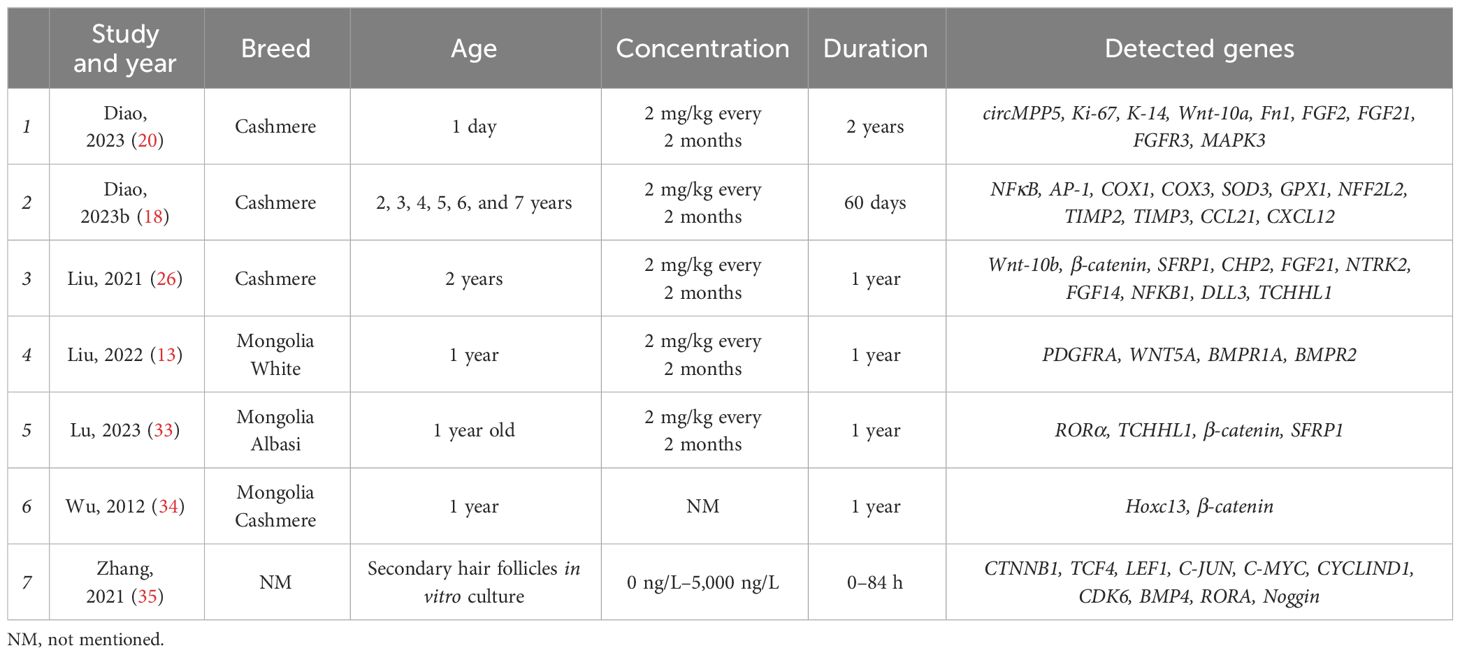
Table 2 Characteristics of selected studies’ melatonin regulator goat secondary hair follicle genes.
Traditional meta-analysis
Figures 1B, D show that melatonin did not correlate with primary follicle density and litter size in goats. Embedding melatonin under the skin of goats was positively correlated with secondary follicle density (SMD = 1.27, 95% CI = 0.31–2.22; p < 0. 001), cashmere yield (SMD = 2.35, 95% CI = 0.74–3.95; p < 0. 001), and fiber length (Figure 1C, SMD = 2.7, 95% CI = 1.14–4.25; p < 0. 001). However, there was a negative correlation to cashmere fiber diameter (SMD = −1.70, 95% CI = −3.1–(−0.31); p < 0. 001).
Network meta-analysis and TSA
The results of this network meta-analysis are shown in Figures 2A, B. This includes three two-arm and two three-arm studies. Adding melatonin to the in vitro culture medium was positively correlated with the development of goat oocytes to blastocysts. Neither melatonin plus luzindole nor cysteamine showed correlations in this process.
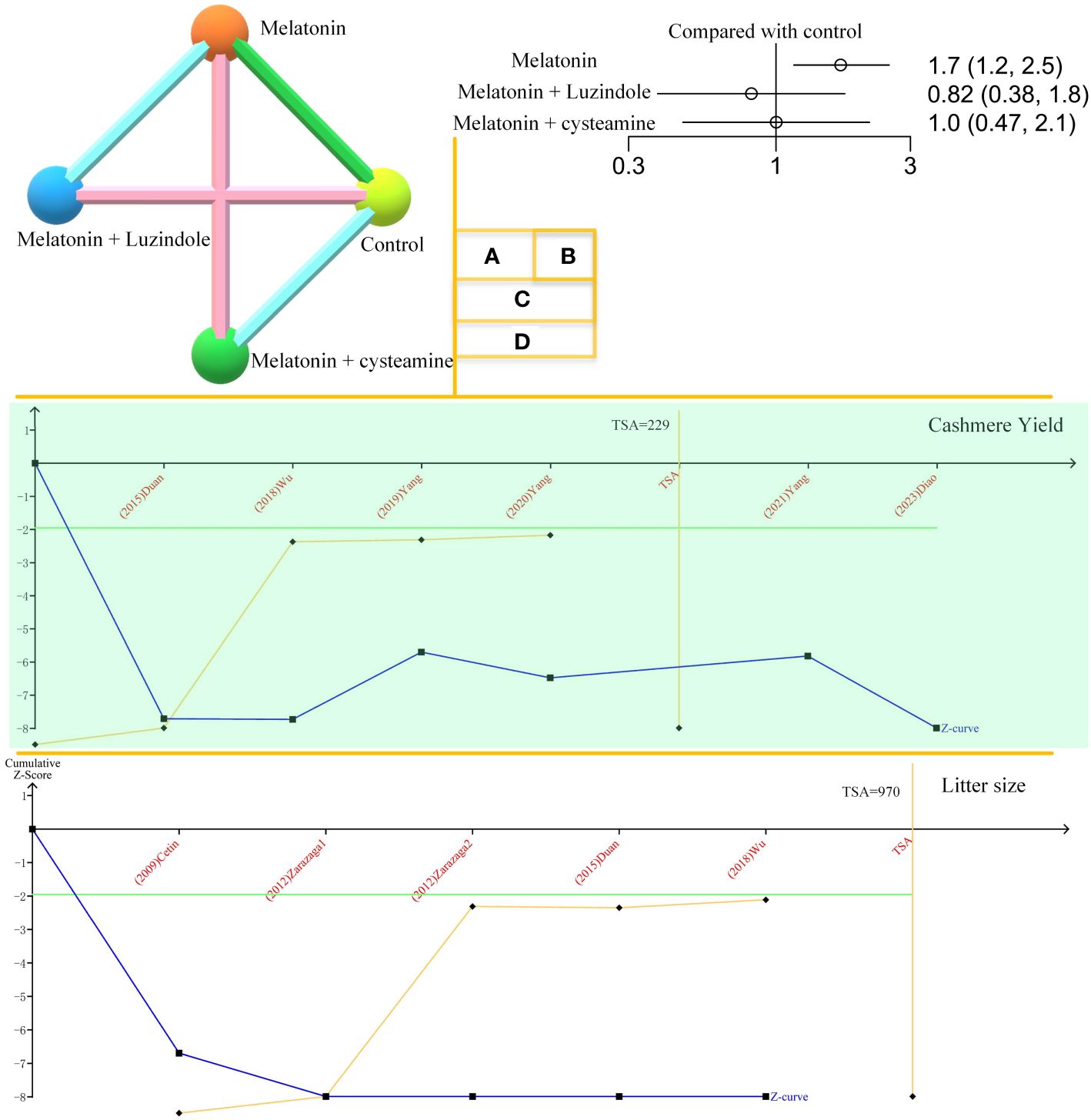
Figure 2 Network meta-analysis and TSA. (A). Network plot of melatonin’s effects on goat oocyte development in vitro. (B). Forest plot of melatonin’s effects on goat oocyte development in vitro. (C). Trial sequential analysis (TSA) of cashmere yield. The orange line represents the horizontal line in the traditional sense. The TSA mathematical expected value is 229. (D). TSA of melatonin’s effect on goat litter size.
The TSA results for cashmere yield are shown in Figure 2C. The amount of information required for TSA is 229, and the Z-curve crosses the monitoring boundary. Melatonin’s effects on cashmere yield in goats are considered credible. Figure 2D shows the effect of melatonin on goat litter size. The curve crosses the monitoring boundary. However, the required information size was not reached. The results of the effect of melatonin on cashmere production in goats are considered credible. However, more research is needed to support this conclusion.
Gene enrichment analysis and PPI network analysis
The KEGG results of melatonin regulator genes are shown in Figure 3A. The 19 pathways with the highest p-values were selected, which included the MAPK signaling pathway, BMP (bone morphogenetic proteins) genes, and receptors, which are the pathways we have been focusing on (38). The results of the PPI network analysis are shown in Figure 3B. BMP proteins and receptor proteins are also included.
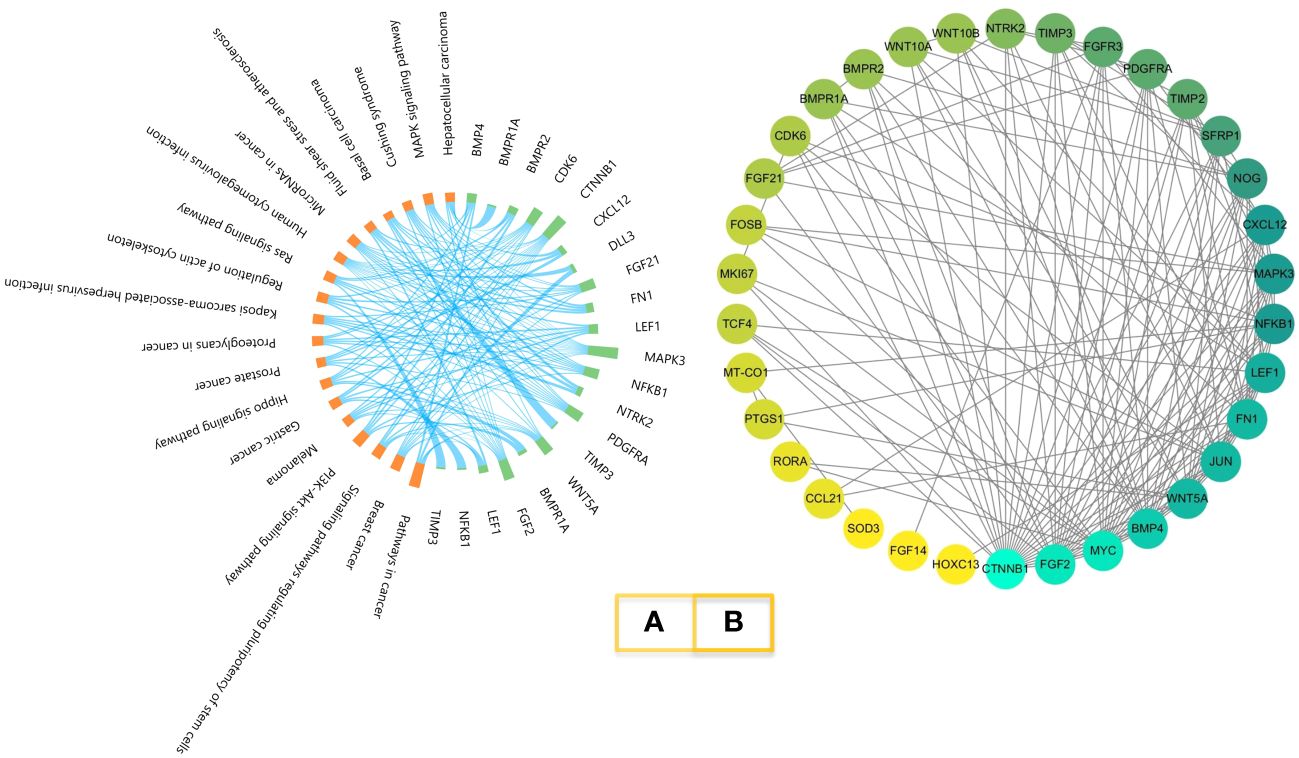
Figure 3 KEGG enrichment and PPI network. (A). Melatonin-regulated gene KEGG enrichment. Orange represents the pathway; green represents the genes. (B). PPI network of melatonin-regulated genes. Proteins are ordered by the numbers of interactions.
Homology modelling of melatonin receptor 1A 3D structures and molecular docking
In total, five SNPs (190, 424, 577, and 589 C>T and 421 T>C) were analyzed individually. The SNP 589 C>T has a terminator at amino acid 197 and is not able to translate a complete protein. SNPs 190, 424, 577 C>T, and 421 T>C do not affect the higher structure of melatonin receptor 1A. SNP 577 C>T is closer to the melatonin docking position, and thus we were simulating ligand docking. Figure 4 shows melatonin docking of 577 C (193Cys) and 577 T (193Arg), with no change in the docking pocket. A total of 195 Phe hydrogen bonds were 2.1 and 2.3 A. The binding energies were 6.53 and 6.35, respectively.
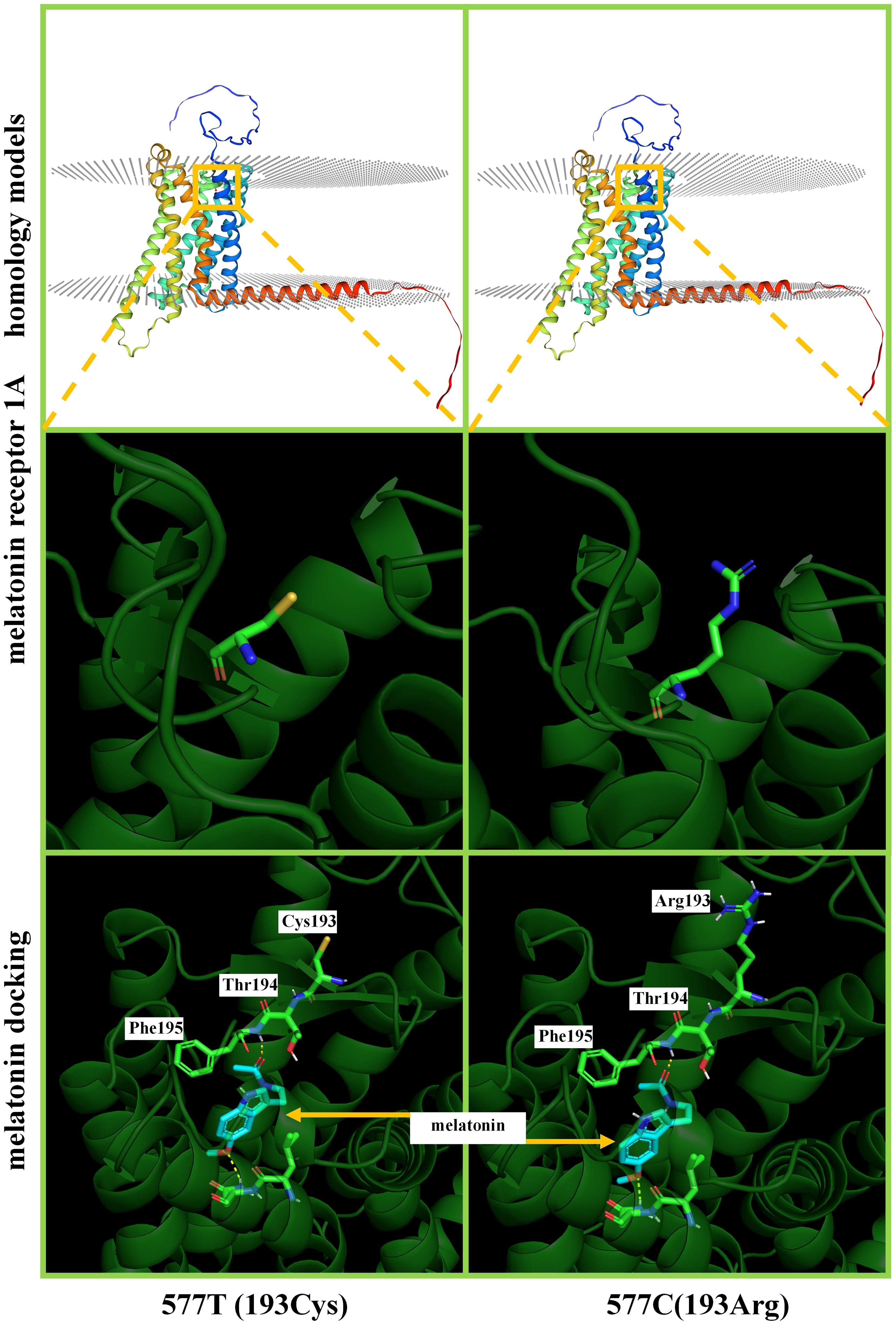
Figure 4 Homology modelling and ligand docking of melatonin receptor 1A structure. 577T and 577C represent the T>C mutation, which results in the amino acid chain 193Cys>Arg. The top and middle show that this mutation does not cause a change in the higher structure of the protein. Below is a magnification of melatonin receptor 1A structures and ligand docking binding pocket; the hydrogen bonding site is 195Phe, which does not involve the 193rd position on the amino acid chain.
Discussion
Androgenetic alopecia is a widespread problem, and hair follicle growth studies are key to treating alopecia (39, 40). Melatonin may be a potential treatment for androgenetic alopecia (12). Rats (3), mice (4), guinea pigs (5), rabbits (6), and dogs (7) are animal models for studying human hair follicles. Comparative studies on the impact of diacylglycerol O-acyltransferase 1 (DGAT1) on mouse and dog alopecia suggest that mice may be an especially sensitive species (41).
Melatonin affects hair regeneration
Melatonin promotes hair follicle growth in humans (12) and goats. Primary hair follicles are generally considered the hair that has already developed. The secondary follicles are the newly grown hairs. The results of the present study showed that there was no correlation between melatonin and goat primary follicle density. However, melatonin was positively correlated with goat secondary follicle density. This suggests that melatonin can promote hair regrowth.
Melatonin promotes the expression of MTNR1A (melatonin receptor 1A) in human and rex rabbit hair follicles (42, 43) and also enhances the expression of goat Wnt10b and beta-catenin (26). The signaling pathways involved in the regulation of hair follicle growth by melatonin are the PI3K/AKT signaling (43), Hippo, TGF-beta, MAPK signaling (13), and AKT/GSK3beta/beta-catenin signaling pathways (44). The results of the present study suggest that the MAPK signaling pathway is also at the forefront of KEGG enrichment. We have been focusing on the effects of the signaling pathways on reproduction (38). In addition, oral melatonin exerts a systemic effect on all cells, tissues, and organs, and it plays a key regulatory role in female reproduction (45). Therefore, the effect of embedded melatonin on goat litter size was considered in this study.
Melatonin and goat litter size
Adding melatonin in vitro can promote oocyte development in humans (14), mice (46), bovines (47), sheep (48), and swine (49). Our network meta-analysis results also showed that melatonin could promote goat oocyte development in vitro. However, melatonin did not affect goat litter size. There are three possible reasons for this. First, embedded melatonin enters the bloodstream then passes through the blood-follicle barrier (BFB), where the concentration changes dramatically. Second, melatonin affects sheep litter size, not through direct action, but by regulating hormonal changes in the whole body (48). Third, melatonin may play different roles at different stages of oocyte development. An example of a similarity is that follistatin inhibits oocyte maturation before meeting sperm. However, it promotes zygote development to blastocysts after fertilization (38).
We previously reported that melatonin can be positively correlated with sheep litter size (48). However, the results of our analysis did not correlate with goat litter size. We believe that this is because the purposes of the experiments were different and the treatments of embedded melatonin were different. Goats embedded with melatonin produced more cashmere if it was embedded for 6 months at 2 mg/kg every 2 months (18, 28). Sheep were generally embedded with melatonin 35 days to obtain more lambs (48).
Comparative analysis of melatonin on human and goat hair follicle growth promotion
Figure 5 compares the promotion of hair follicles by melatonin in humans and goats, which can provide more reference for goats as a model animal. Melatonin regulates both reproduction and alopecia in humans (12, 45). Promotion of secondary hair follicle growth has also been obtained in studies on goats (13). Melatonin plays a promotional role during in vitro maturation of goat and human oocytes (14, 17). Appropriate oral administration melatonin can enhance whole-body physiology. For example, it improves sleep and boosts immunity. However, excessive melatonin may lead to depression (50).
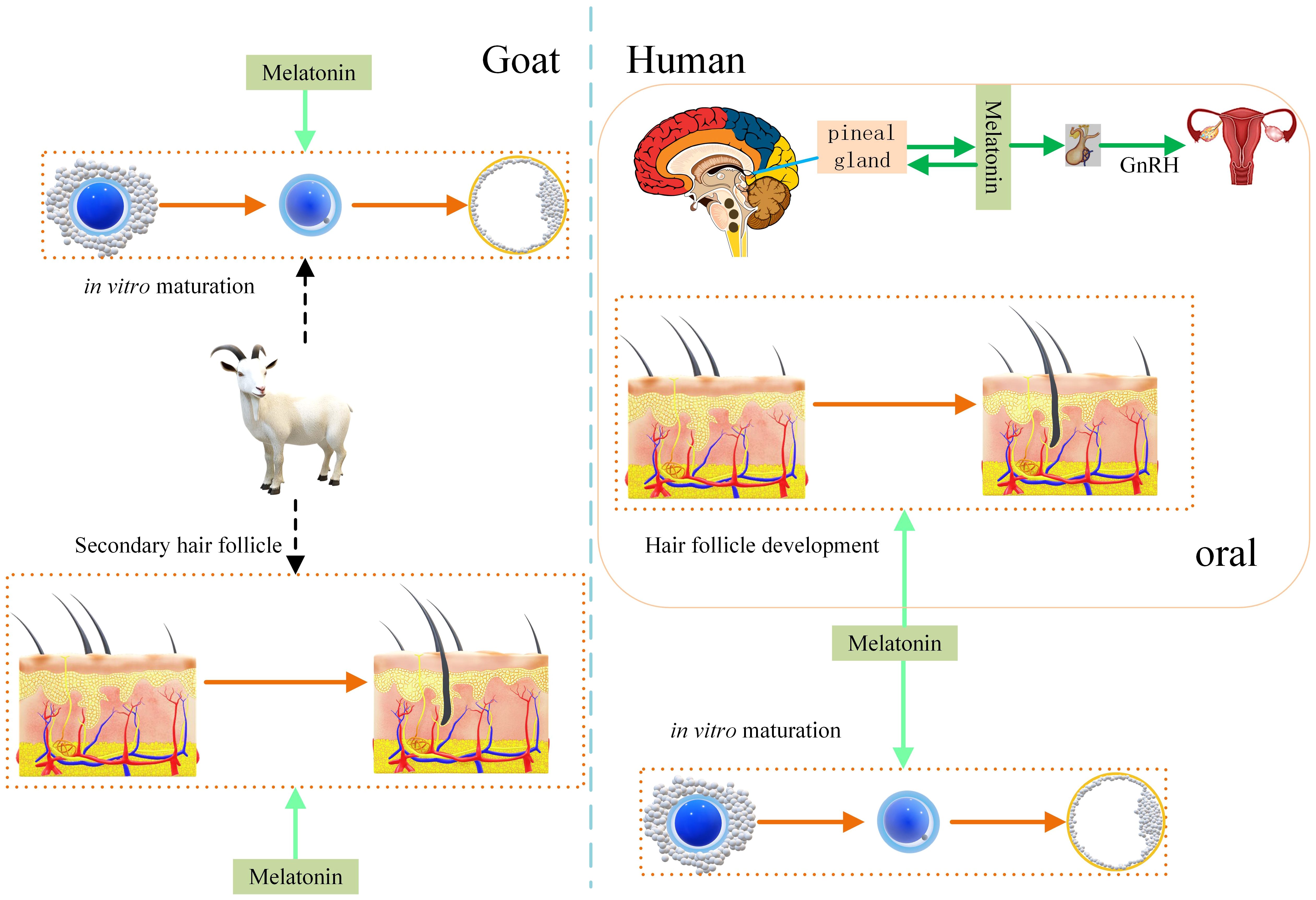
Figure 5 Effect of melatonin on hair follicle growth in humans and goats. Melatonin promotes hair follicle growth in humans and secondary hair follicle growth in goats. Melatonin promotes human and goat oocyte competence under in vitro culture conditions. That increase development rate of oocyte to embryo. Melatonin regulates the human reproductive system.
Effect of goat melatonin receptor 1A SNPs on experimental results
Melatonin regulates the body’s biological clock and sleep rhythms by binding to receptors. Melatonin receptors 1A and 1B (MT1 and MT2) are members of the family of G protein-coupled receptors (GPCRs)) (51). MT3 (quinone reductase 2) has low binding affinity with melatonin (52). Melatonin receptor 1A and 1B genes are differentially expressed at different locations in the brain and may perform different physiological functions (53). We investigated the SNPs and ligand docking of melatonin receptor 1A and found that 589 C>T has a terminator at the 197th amino acid. That leads to the loss of function of melatonin receptor 1A, which may lead to uncertain results if such goat individuals are mixed into experimental groups. This could also explain the inclusion of literature where opposite results were seen. Future studies should screen goat SNPs before embedding melatonin. More studies focus on SNPs of human melatonin 1A receptors (54). Goat wild-type genes defective for MR1 can be used as an animal model to study alopecia (24).
Limitations
Melatonin can interact with enzymes, molecular channels, transporters, and signaling molecules to perform physiological functions (45, 55). This study only considered melatonin receptor 1A, and this has some limitations. In addition, there is very limited research on human secondary follicles and melatonin. This will also be a limitation for goats as a research model for human alopecia.
Intestinal melatonin concentrations were 400 times higher than those of the pineal gland (56), with uncertainty in the results when the effect of intestinal flora was ignored. For our inclusion study, both experimental and control groups were under the same feeding management conditions, and we defaulted to a negligible effect of intestinal flora. For the same reason, whether oral administration of melatonin in humans and embedded melatonin behind the ears of goats would have the same effect also needs further investigation because oral magnesium sulphate administration (57) can achieve completely different pharmacological effects than injection (58).
Conclusion
Goats can be used as an animal model for human alopecia research. Melatonin does not affect goat primary follicle or litter size. However, there is a positive correlation with secondary follicle growth. Melatonin was positively correlated with the development competence of goat oocytes. Goat melatonin receptor 1A SNPs affect melatonin to exert its function. The wild-type gene defect of MR1 is a valuable animal model. Future studies should focus on the relationship between goat SNPs and the effect of embedded melatonin. This study can provide a reference for improving cashmere production and a suggestion for animal models of human alopecia.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding authors.
Author contributions
YR: Data curation, Writing – original draft. RM: Data curation, Writing – original draft. YZ: Funding acquisition, Writing – original draft. ZG: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by a project of the northern agriculture and livestock husbandry technical innovation center, Chinese academy of agricultural sciences (BFGJ2022002) and program for innovative research teams in universities of the Inner Mongolia Autonomous Region (NMGIRT2322). The funding agencies were not involved in the development of the study design or the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Popa A, Carsote M, Cretoiu D, Dumitrascu MC, Nistor CE, Sandru F. Study of the thyroid profile of patients with alopecia. J Clin Med. (2023) 12:1115. doi: 10.3390/jcm12031115
2. Akhbari M, Firooz A, Rahimi R, Shirzad M, Esmaealzadeh N, Shirbeigi L. The effect of an oral product containing Amla fruit (Phyllanthus emblica L.) on female androgenetic alopecia: A randomized controlled trial. J Ethnopharmacol. (2024) 318:116958. doi: 10.1016/j.jep.2023.116958
3. Elsebay SAG, Nada HF, Sultan NSS, El-Waseef D. Comparative histological and immunohistochemical study on the effect of platelet rich plasma/minoxidil, alone or in combination, on hair growth in a rat model of androgenic alopecia. Tissue Cell. (2022) 75:101726. doi: 10.1016/j.tice.2021.101726
4. Li Y, Kilani RT, Leung G, Ghahary A. Myeloid adherent cells are involved in hair loss in the alopecia areata mouse model. J Investig Dermatol Symp Proc. (2020) 20:S16–21. doi: 10.1016/j.jisp.2020.04.001
5. Kinoshita M, Fujimoto C, Iwasaki S, Kondo K, Yamasoba T. Oral administration of trkB agonist, 7, 8-dihydroxyflavone regenerates hair cells and restores function after gentamicin-induced vestibular injury in Guinea pig. Pharmaceutics. (2023) 15:493. doi: 10.3390/pharmaceutics15020493
6. Yao F, Zhao B, Hu S, Bai S, Jin R, Zhang C, et al. miR-129-5p participates in hair follicle growth by targeting HOXC13 in rabbit. Genes (Basel). (2022) 13:679. doi: 10.3390/genes13040679
7. Colodel MM, Pietroluongo B, Lucas R, Gomes KB, Grandi F. Follicular lipidosis in a dachshund dog. Vet Dermatol. (2017) 28:410–e99. doi: 10.1111/vde.12424
8. Jin M, Zhang Y, Piao J, Zhao F, Piao J. Expression of lipid-protein gene PLP2 in Liaoning cashmere goat. Anim Biotechnol. (2019) 30:279–86. doi: 10.1080/10495398.2018.1485682
9. Wang J, Sui J, Mao C, Li X, Chen X, Liang C, et al. Identification of key pathways and genes related to the development of hair follicle cycle in cashmere goats. Genes (Basel). (2021) 12:180. doi: 10.3390/genes12020180
10. Yang F, Li R, Zhao C, Che T, Guo J, Xie Y, et al. Single-cell sequencing reveals the new existence form of dermal papilla cells in the hair follicle regeneration of cashmere goats. Genomics. (2022) 114:110316. doi: 10.1016/j.ygeno.2022.110316
11. Lin SJ, Yue Z, Paus R. Clinical pathobiology of radiotherapy-induced alopecia: A guide toward more effective prevention and hair follicle repair. J Invest Dermatol. (2023) 143:1646–56. doi: 10.1016/j.jid.2023.02.041
12. Babadjouni A, Reddy M, Zhang R, Raffi J, Phong C, Mesinkovska N. Melatonin and the human hair follicle. J Drugs Dermatol. (2023) 22:260–4. doi: 10.36849/JDD
13. Liu Z, Liu Z, Mu Q, Zhao M, Cai T, Xie Y, et al. Identification of key pathways and genes that regulate cashmere development in cashmere goats mediated by exogenous melatonin. Front Vet Sci. (2022) 9:993773. doi: 10.3389/fvets.2022.993773
14. Yang D, Mu Y, Wang J, Zou W, Zou H, Yang H, et al. Melatonin enhances the developmental potential of immature oocytes from older reproductive-aged women by improving mitochondrial function. Heliyon. (2023) 9:e19366. doi: 10.1016/j.heliyon.2023.e19366
15. Agarwal S, Kharche SD, Bhatiya AK. Melatonin improves in vitro maturation and subsequent embryo development of caprine oocytes*. Indian J Anim Sci. (2018) 88:801–4. doi: 10.56093/ijans.v88i7.81458
16. Berlinguer F, Leoni GG, Succu S, Spezzigu A, Madeddu M, Satta V, et al. Exogenous melatonin positively influences follicular dynamics, oocyte developmental competence and blastocyst output in a goat model. J Pineal Res. (2009) 46:383–91. doi: 10.1111/j.1600-079X.2009.00674.x
17. Soto-Heras S, Catala MG, Roura M, Menendez-Blanco I, Piras AR, Izquierdo D, et al. Effects of melatonin on oocyte developmental competence and the role of melatonin receptor 1 in juvenile goats. Reprod Domest Anim. (2019) 54:381–90. doi: 10.1111/rda.13378
18. Diao X, Duan C, Yao L, Qin J, He L, Zhang W. Melatonin promotes the development of secondary hair follicles in adult cashmere goats by activating the keap1-nrf2 signaling pathway and inhibiting the inflammatory transcription factors NFkappaB and AP-1. Int J Mol Sci. (2023) 24:3403. doi: 10.3390/ijms24043403
19. Wu Z, Duan C, Li Y, Duan T, Mo F, Zhang W. Short communication: Melatonin implantation during the non-growing period of cashmere increases the cashmere yield of female Inner Mongolian cashmere goats by increasing fiber length and density. Spanish J Agric Res. (2018) 16:e06SC01. doi: 10.5424/sjar/20181614-11053
20. Diao X, Yao L, Duan T, Qin J, He L, Zhang W. Melatonin promotes the development of the secondary hair follicles by regulating circMPP5. J Anim Sci Biotechnol. (2023) 14:51. doi: 10.1186/s40104-023-00849-w
21. Yang CH, Wu ZY, Li Y, Zhang W. Effect of melatonin administration to lactating cashmere goats on milk production of dams and on hair follicle development in their offspring. Animal. (2020) 14:1241–8. doi: 10.1017/S1751731119002726
22. Yang CH, Xu JH, Ren QC, Duan T, Mo F, Zhang W. Melatonin promotes secondary hair follicle development of early postnatal cashmere goat and improves cashmere quantity and quality by enhancing antioxidant capacity and suppressing apoptosis. J Pineal Res. (2019) 67:e12569. doi: 10.1111/jpi.12569
23. Zhou X, Cao Q, Orfila C, Zhao J, Zhang L. Systematic review and meta-analysis on the effects of astaxanthin on human skin ageing. Nutrients. (2021) 13:2917. doi: 10.3390/nu13092917
24. Gong H, Zhou H, Wang J, Li S, Luo Y, Hickford JGH. Characterisation of an ovine keratin associated protein (KAP) gene, which would produce a protein rich in glycine and tyrosine, but lacking in cysteine. Genes (Basel). (2019) 10:848. doi: 10.3390/genes10110848
25. Lai P, Wang PQ, Chu MX, Song WJ, Cai BJ. Polymorphism of the melatonin receptor genes and its relationship with seasonal reproduction in the Gulin Ma goat breed. Reprod Domest Anim. (2013) 48:732–7. doi: 10.1111/rda.12153
26. Liu J, Mu Q, Liu Z, Wang Y, Liu J, Wu Z, et al. Melatonin regulates the periodic growth of cashmere by upregulating the expression of wnt10b and beta-catenin in inner Mongolia cashmere goats. Front Genet. (2021) 12:665834. doi: 10.3389/fgene.2021.665834
27. Wuliji T, Litherland A, Goetsch AL, Sahlu T, Puchala R, Dawson LJ, et al. Evaluation of melatonin and bromocryptine administration in Spanish goats: III. Effects on hair follicle activity, density and relationships between follicle characteristics. Small Ruminant Res. (2006) 66:11–21. doi: 10.1016/j.smallrumres.2005.04.024
28. Yang CH, Duan CH, Wu ZY, Li Y, Luan YY, Fu XJ, et al. Effects of melatonin administration to cashmere goats on cashmere production and hair follicle characteristics in two consecutive cashmere growth cycles. Domest Anim Endocrinol. (2021) 74:106534. doi: 10.1016/j.domaniend.2020.106534
29. Duan C, Xu J, Sun C, Jia Z, Zhang W. Effects of melatonin implantation on cashmere yield, fibre characteristics, duration of cashmere growth as well as growth and reproductive performance of Inner Mongolian cashmere goats. J Anim Sci Biotechnol. (2015) 6:22. doi: 10.1186/s40104-015-0023-2
30. Cetin Y, Sagcan S, Gungor O, Ozyurtlu N, Uslu BA. Effects of CIDR-G and melatonin implants, and their combination on the efficacy of oestrus induction and fertility of Kilis goats. Reprod Domest Anim. (2009) 44:659–62. doi: 10.1111/j.1439-0531.2007.01043.x
31. Zarazaga LA, Celi I, Guzman JL, Malpaux B. Enhancement of the male effect on reproductive performance in female Mediterranean goats with long day and/or melatonin treatment. Vet J. (2012) 192:441–4. doi: 10.1016/j.tvjl.2011.09.012
32. Zarazaga LA, Gatica MC, Celi I, Guzman JL. Reproductive performance is improved during seasonal anoestrus when female and male Murciano-Granadina goats receive melatonin implants and in Payoya goats when females are thus treated. Reprod Domest Anim. (2012) 47:436–42. doi: 10.1111/j.1439-0531.2011.01899.x
33. Lu Z, Wu J, Wu J, Zhang T, Liu J, Mu Q, et al. Melatonin regulates the periodic growth of secondary hair follicles through the nuclear receptor RORalpha. Front Vet Sci. (2023) 10:1203302. doi: 10.3389/fvets.2023.1203302
34. Wu J-h, Zhang Y-j, Zhang J-x, Chang Z-l, Li J-q, Yan Z-w, et al. Hoxc13/β-catenin correlation with hair follicle activity in cashmere goat. J Integr Agric. (2012) 11:1159–66. doi: 10.1016/S2095-3119(12)60110-5
35. Zhang W, Wang N, Zhang T, Wang M, Ge W, Wang X. Roles of melatonin in goat hair follicle stem cell proliferation and pluripotency through regulating the wnt signaling pathway. Front Cell Dev Biol. (2021) 9:686805. doi: 10.3389/fcell.2021.686805
36. Saeedabadi S, Abazari-Kia AH, Rajabi H, Parivar K, Salehi M. Melatonin improves the developmental competence of goat oocytes. Int J Fertil Steril. (2018) 12:157–63. doi: 10.22074/ijfs.2018.5204
37. Soto-Heras S, Roura M, Catala MG, Menendez-Blanco I, Izquierdo D, Fouladi-Nashta AA, et al. Beneficial effects of melatonin on in vitro embryo production from juvenile goat oocytes. Reprod Fertil Dev. (2018) 30:253–61. doi: 10.1071/RD17170
38. Guo Z, Islam MS, Liu D, Liu G, Lv L, Yang Y, et al. Differential effects of follistatin on porcine oocyte competence and cumulus cell gene expression in vitro. Reprod Domest Anim. (2018) 53:3–10. doi: 10.1111/rda.13035
39. Ieremia E, Stefanato CM. The role of hair follicle counts and ratios in the histopathological assessment of androgenic alopecia, alopecia areata and telogen effluvium: does counting 'count'? Hum Pathol. (2023) 140:233–9. doi: 10.1016/j.humpath.2023.03.015
40. Gan Y, Du L, Wang H, Li K, Fan Z, Sun P, et al. A clinical trial of treating androgenic alopecia with mesenchymal stem cell suspension derived from autologous hair follicle. Plast Reconstr Surg. (2023). doi: 10.1097/PRS.0000000000010841 (this article has not yet been published in final form.)
41. Floettmann E, Lees D, Seeliger F, Jones HB. Pharmacological inhibition of DGAT1 induces sebaceous gland atrophy in mouse and dog skin while overt alopecia is restricted to the mouse. Toxicol Pathol. (2015) 43:376–83. doi: 10.1177/0192623314545657
42. Feng Y, Gun S. Melatonin supplement induced the hair follicle development in offspring rex rabbits. J Anim Physiol Anim Nutr (Berl). (2021) 105:167–74. doi: 10.1111/jpn.13417
43. Sevilla A, Cheret J, Slominski RM, Slominski AT, Paus R. Revisiting the role of melatonin in human melanocyte physiology: A skin context perspective. J Pineal Res. (2022) 72:e12790. doi: 10.1111/jpi.12790
44. Bae S, Yoon YG, Kim JY, Park IC, An S, Lee JH, et al. Melatonin increases growth properties in human dermal papilla spheroids by activating AKT/GSK3beta/beta-Catenin signaling pathway. PeerJ. (2022) 10:e13461. doi: 10.7717/peerj.13461
45. Olcese JM. Melatonin and female reproduction: An expanding universe. Front Endocrinol (Lausanne). (2020) 11:85. doi: 10.3389/fendo.2020.00085
46. Liu Y, Zhang Y, Wang Z, Teng Z, Zhu P, Xie M, et al. Melatonin improves the ability of spermatozoa to bind with oocytes in the mouse. Reprod Fertil Dev. (2023) 35:445–57. doi: 10.1071/RD23006
47. Silva BR, Barrozo LG, Nascimento DR, Costa FC, Azevedo VAN, Paulino L, et al. Effects of cyclic adenosine monophosphate modulating agents during oocyte pre-maturation and the role of melatonin on in vitro maturation of bovine cumulus-oocyte complexes. Anim Reprod Sci. (2023) 257:107327. doi: 10.1016/j.anireprosci.2023.107327
48. Chen Y, Shan X, Jiang H, Guo Z. Exogenous melatonin directly and indirectly influences sheep oocytes. Front Vet Sci. (2022) 9:903195. doi: 10.3389/fvets.2022.903195
49. Wang J, Wang XQ, Liu RP, Li YH, Yao XR, Kim NH, et al. Melatonin supplementation during in vitro maturation of porcine oocytes alleviates oxidative stress and endoplasmic reticulum stress induced by imidacloprid exposure. Anim (Basel). (2023) 13:2596. doi: 10.3390/ani13162596
50. Pereira JC Jr., Pradella Hallinan M, Alves RC. Secondary to excessive melatonin synthesis, the consumption of tryptophan from outside the blood-brain barrier and melatonin over-signaling in the pars tuberalis may be central to the pathophysiology of winter depression. Med Hypotheses. (2017) 98:69–75. doi: 10.1016/j.mehy.2016.11.020
51. Wu N, Carpino G, Ceci L, Baiocchi L, Francis H, Kennedy L, et al. Melatonin receptor 1A, but not 1B, knockout decreases biliary damage and liver fibrosis during cholestatic liver injury. Hepatology. (2022) 75:797–813. doi: 10.1002/hep.32233
52. Ng KY, Leong MK, Liang H, Paxinos G. Melatonin receptors: distribution in mammalian brain and their respective putative functions. Brain Struct Funct. (2017) 222:2921–39. doi: 10.1007/s00429-017-1439-6
53. Gobbi G, Comai S. Differential function of melatonin MT(1) and MT(2) receptors in REM and NREM sleep. Front Endocrinol (Lausanne). (2019) 10:87. doi: 10.3389/fendo.2019.00087
54. Cantarini M, Rusciano D, Amato R, Canovai A, Cammalleri M, Monte MD, et al. Structural Basis for Agonistic Activity and Selectivity toward Melatonin Receptors hMT1 and hMT2. Int J Mol Sci. (2023) 24:2836. doi: 10.3390/ijms24032863
55. Liu L, Labani N, Cecon E, Jockers R. Melatonin target proteins: Too many or not enough? Front Endocrinol (Lausanne). (2019) 10:791. doi: 10.3389/fendo.2019.00791
56. Esteban-Zubero E, Lopez-Pingarron L, Alatorre-Jimenez MA, Ochoa-Moneo P, Buisac-Ramon C, Rivas-Jimenez M, et al. Melatonin's role as a co-adjuvant treatment in colonic diseases: A review. Life Sci. (2017) 170:72–81. doi: 10.1016/j.lfs.2016.11.031
57. Barooti A, Kamran M, Kharazmi F, Eftakhar E, Malekzadeh K, Talebi A, et al. Effect of oral magnesium sulfate administration on blood glucose hemostasis via inhibition of gluconeogenesis and FOXO1 gene expression in liver and muscle in diabetic rats. BioMed Pharmacother. (2019) 109:1819–25. doi: 10.1016/j.biopha.2018.10.164
58. Fathy M, Abdel-Razik MA, Elshobaky A, Emile SH, El-Rahmawy G, Farid A, et al. Impact of pyloric injection of magnesium sulfate-lidocaine mixture on postoperative nausea and vomiting after laparoscopic sleeve gastrectomy: a randomized-controlled trial. Obes Surg. (2019) 29:1614–23. doi: 10.1007/s11695-019-03762-2
Keywords: alopecia, cashmere, molecular docking, network meta-analysis, single nucleotide polymorphism
Citation: Rong Y, Ma R, Zhang Y and Guo Z (2024) Melatonin’s effect on hair follicles in a goat (Capra hircus) animal model. Front. Endocrinol. 15:1361100. doi: 10.3389/fendo.2024.1361100
Received: 25 December 2023; Accepted: 18 March 2024;
Published: 02 April 2024.
Edited by:
Govindan Dayanithi, Centre National de la Recherche Scientifique (CNRS), FranceReviewed by:
Veronica Palmira Filippa, National University of San Luis, ArgentinaChunjin Li, Jilin University, China
Copyright © 2024 Rong, Ma, Zhang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanjun Zhang, imauzyj@163.com; Zhenhua Guo, gzhh00@163.com
†These authors have contributed equally to this work and share first authorship
 Youjun Rong1†
Youjun Rong1† Rong Ma
Rong Ma Zhenhua Guo
Zhenhua Guo