- 1College of Basic Medicine, Naval Medical University, Shanghai, China
- 2Department of Traditional Chinese Medicine, Naval Medical University, Shanghai, China
Chronic fatigue syndrome (CFS) causes great harm to individuals and society. Elucidating the pathogenesis of CFS and developing safe and effective treatments are urgently needed. This paper reviews the functional changes in the hypothalamus-pituitary-adrenal (HPA) axis in patients with CFS and the associated neuroendocrine mechanisms. Despite some controversy, the current mainstream research evidence indicates that CFS patients have mild hypocortisolism, weakened daily variation in cortisol, a weakened response to the HPA axis, and an increase in negative feedback of the HPA axis. The relationship between dysfunction of the HPA axis and the typical symptoms of CFS are discussed, and the current treatment methods are reviewed.
Introduction
The term chronic fatigue syndrome (CFS) was first proposed by the U.S. Centers for Disease Control and Prevention in 1988 (1). To improve the definition and diagnostic criteria, the International CFS Study Group released two revisions in 1994 and 2003 (2, 3) and gradually established the widely used “gold standard”; that is, CFS is a syndrome characterized by chronic fatigue that is clinically assessed, unexplained, persistent or recurrent, new or with a definite onset, non-congenital, not due to ongoing labor, and not relieved. Additionally, the occupational ability, educational ability, social ability and personal life of affected individuals are substantially worse than those before the illness. For the diagnosis of CFS, four or more of the following symptoms persist or recur for at least 6 months, the appearance of which does not occur prior to fatigue symptoms: ① severe impairment of short-term memory and concentration, causing a substantial decrease in occupational ability, educational ability, social ability and personal life compared with those before disease onset; ② sore throat and tenderness of the neck or axillary lymph nodes; ③ muscle pain and multiple joint pain not accompanied by swelling; ④ type, manner, and severity of headache attacks different from those before; ⑤ inability to recuperate after sleep; ⑥ and persistent fatigue for more than 24 hours after activity. It is estimated that 836,000 to 2.5 million people are affected with CFS in the U.S., with as many as a quarter being homebound or bedridden (4). However, considering the number of people affected and the harm caused, CFS has not received due research attention (5). Notably, in 2018, an article in “Nature” called for the reinitiation of CFS studies (6).
Due to the enormous complexity of CFS, its pathogenesis is still unclear. Some of the researchers suggest immunological, neuroendocrine and metabolic pathways that causes CFS, which indicates significant immune dysregulation (7, 8). Neuroendocrine mechanisms have been the focus of some research. In 1991, several researchers focused on the hypothalamus-pituitary-adrenal (HPA) axis (9), and since then, the HPA axis has been a research hotspot in the field of neuroendocrine mechanisms. The HPA axis has three levels: the hypothalamus is the first level, and corticotropin releasing hormone (CRH) secreted by the hypothalamus activates the HPA axis; the pituitary gland is the second level, and CRH can promote the secretion of adrenocorticotropic hormone (ACTH) from the pituitary gland; and the adrenal gland acts as the third level and is regulated by ACTH, cortisol (CORT) secreted by the adrenal gland is the main functional product of the HPA axis. Additionally, CORT acts on the hypothalamus and the pituitary gland through a negative feedback mechanism, which inhibits the secretion of CRH and ACTH to stabilize CORT levels (10). The aim of this paper was to investigate the functional changes and impact of the HPA axis in patients with CFS and to review the current treatment methods.
HPA axis dysfunction in CFS patients
The plasma CORT levels in CFS patients were first reported by a researcher in 1981 (11). Since then, clinical factors have been gradually expanded to include urine CORT (12) and saliva CORT (13); additionally, because CORT secretion has diurnal variation and is pulsatile, observation times have also gradually changed from a single time point (14) to overall circadian rhythm (15).
Current mainstream research evidence supports that CFS patients have mild hypocortisolism, weakened daily variation in CORT, unresponsiveness of the HPA axis, and enhanced negative feedback from the HPA axis (16). Several studies have shown that, compared with those in healthy controls, the plasma (14, 17, 18), urine (12), and saliva (19) concentrations of CORT in CFS patients are significantly lower. In addition to observations at a single time point, changes in the circadian rhythm of the HPA axis have also been analyzed. Through the collection and comparison of saliva CORT concentrations at multiple time points in 24 hours, it was found that the CORT levels in CFS patients were lower in the morning and higher in the evening; that is, daily CORT variability was attenuated compared with that in normal controls (20). More intensive plasma collection revealed that the secretion rhythm of ACTH in CFS patients was significantly different from that in normal controls, with release decreasing during the physiological morning peak (21). These findings demonstrated an alteration in the secretion rhythm of the HPA axis. Using the Trier Social Stress Test (TSST), researchers found that, compared with healthy controls, CFS patients had a significantly reduced area under the ACTH response curve, with no significant difference in the area under the CORT response curve (22). Cortisol awakening response (CAR) was tested, and it was found that, compared with healthy controls, CFS patients with childhood trauma experienced a significantly reduced area under the CORT response curve and that their CORT response curves were flatter (23). In addition, women with CFS had significantly lower CAR levels than healthy women did, and the increase in the area under the response curve was also lower (24). An insulin tolerance test (ITT) showed that, in CFS patients, the area under the ACTH response curve was significantly reduced, while that of CORT was not significantly different from that for controls (25, 26). This finding confirmed that there was a blunted response of the HPA axis. The enhancement of negative HPA axis feedback has been tested using the dexamethasone suppression test (DST). After the administration of 0.5 mg dexamethasone, the saliva CORT output was significantly lower in CFS patients than in healthy controls (27),the saliva CORT response significantly decreased (28). After the administration of 5 mg of prednisolone, the saliva CORT levels and urine CORT metabolite levels were significantly lower in CFS patients than in individuals in the control group, and the percentage of inhibition was significantly higher in CFS patients than in individuals in the control group (29), demonstrating the enhancement of negative feedback on the HPA axis (Figure 1).
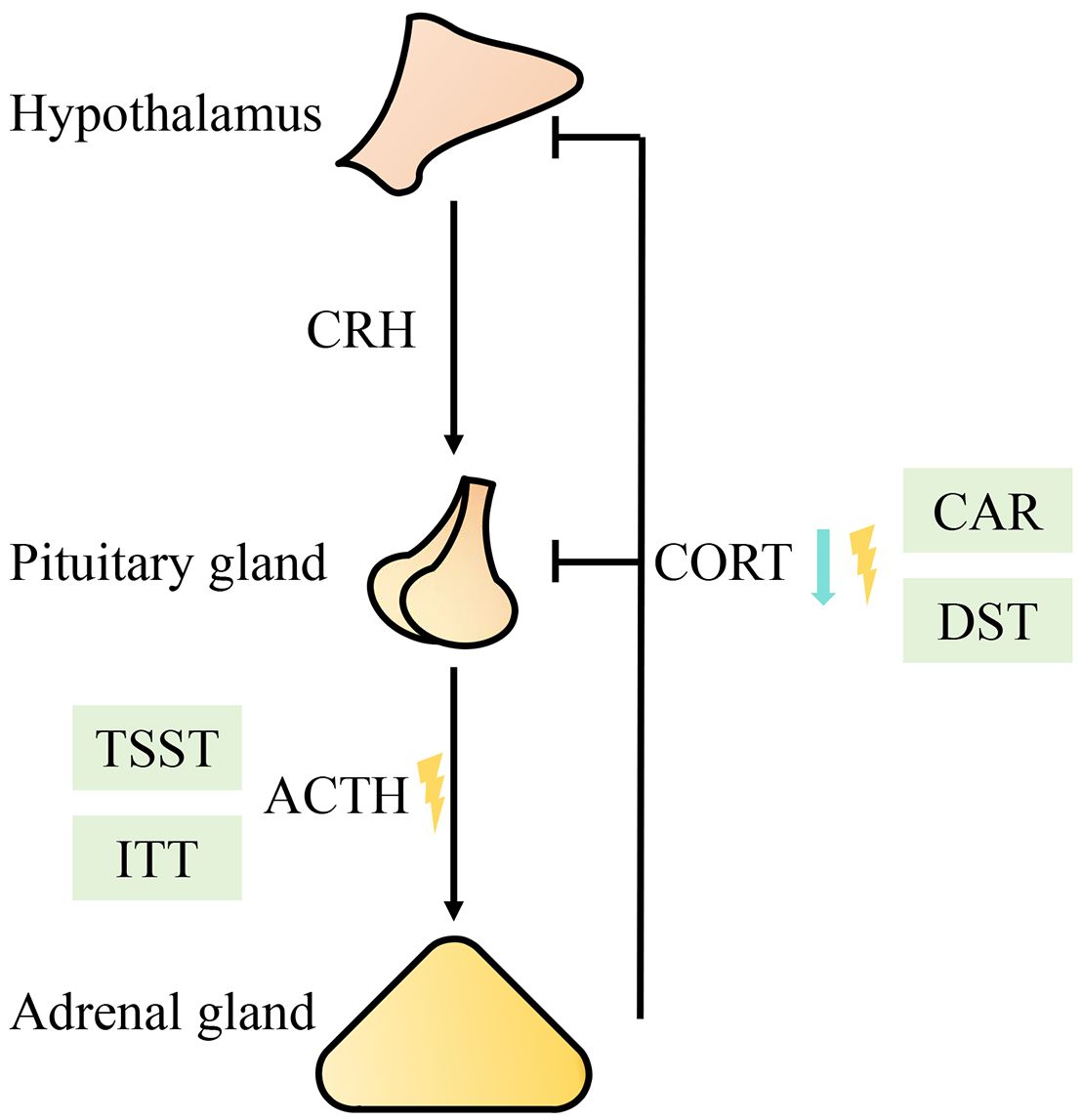
Figure 1 Diagram of functional changes in the HPA axis. The blue arrow represents a decreased level; the yellow lightning bolt represents a rhythm disorder. Abbreviations: CRH (corticotropin releasing hormone), ACTH (adrenocorticotropic hormone), CORT (cortisol), TSST (Trier Social Stress Test), CAR (cortisol awakening response), ITT (insulin tolerance test), DST (dexamethasone suppression test).
However, not all the study results are consistent with the mainstream conclusions. Several studies have shown that there is no significant difference between the CORT levels in CFS patients and those in healthy controls (30). Using the TSST, researchers found that childhood traumatic experience was not significantly associated with CORT output (31). There were no significant differences between a CFS group and a healthy control group in terms of CAR, daily CORT secretion curve, or CAR after DST (32). The differences between these studies may be explained by the study design and insufficient sample size. However, the mainstream conclusions still need verification through comprehensive studies with larger sample sizes (33).
The effects of HPA axis dysfunction in CFS patients
Because most studies tend to analyze patients who have been ill for many years, it is not clear whether HPA axis dysfunction is the cause or result of CFS (16). Therefore, some researchers have proposed that the changes in the HPA axis in CFS patients may be an incidental phenomenon that occurs later in the disease course rather than having special etiological significance (34). However, considering that HPA axis dysfunction may play an etiological and pathological role in CFS, it is still necessary to investigate the association of the HPA axis with typical symptoms of CFS (35).
Dysfunction of the HPA axis, especially hypocortisolemia, is often closely related to fatigue, pain, and increased pressure sensitivity. Various mechanism can explain such dysfunction. The release of different hormones may be reduced, thereby reducing the stimulation of receptors for each hormone. This may also be the result of the excessive secretion of a certain hormone, followed by the downregulated expression of the corresponding target receptor, increased sensitivity to the negative feedback of glucocorticoids, or increased relative resistance to CORT (36). Dysfunction of the HPA axis may also be mediated through immune mechanisms, especially HPA axis dysfunction characterized by hypocortisolism, which can weaken the ability of HPA axis hormones to suppress the immune system; therefore, relatively few physiological or psychological stress signals can be transmitted into inflammatory response through activating inflammasomes and the subsequent proinflammatory cytokines (37, 38). Cytokine-mediated inflammation may also explain the widespread pain and pain hypersensitivity in CFS patients (39). In addition, the circadian rhythm of melatonin (MT) secretion, the most important sleep regulation factor, is opposite to that of the HPA axis, and there is an interaction between the two (40). In CFS patients, changes in the circadian rhythm of the HPA axis have been observed; the amplitude of the circadian secretion of CORT is reduced, the peak phase of CORT secretion is advanced, and the peak phase of MT secretion is delayed. Abnormalities in the peak phases of the two disorders can lead to sleep disorders, providing a neuroendocrine explanation for CFS patients’ difficulty falling asleep at night and easy awakening early in the morning (41).
Treatment of CFS patients with modern medicine
The 2023 updated guidelines of the CDC noted that there is currently no cure or approved treatment for CFS, with the management of symptoms being the only treatment approach. The guidelines recommend treatment for post-exertional malaise, sleep, pain, anxiety, stress, depression, orthostatic intolerance, and memory and attention problems, with an emphasis on activity management (42). Activity management involves patients finding their mental and physical activity limits and then planning their activities and rest to remain within these limits to avoid post-exertional malaise. Limits vary among individuals; therefore, recording activities and symptoms can help patients discover their personal limits, especially in the early stage of the disease. In addition, patients should be reminded not to try to increase activities beyond their limit even though the activity management plan is working well because post-exertional malaise can recur (43).
The largest study on this topic provided favorable evidence for the efficacy and safety of cognitive behavioral therapy and graded exercise therapy (44, 45). However, when the data were re-analyzed, the results changed significantly (46). Subsequent studies have questioned their efficacy and safety (47). After comprehensive consideration, the CDC no longer recommends both for the treatment of CFS. Recently, new randomized controlled trials (RCTs) have been conducted to find more evidence, thus indicating that modern medicine is still making efforts to find a better treatment plan for CFS (48, 49).
Regarding HPA axis dysfunction, randomized controlled trials have shown beneficial effects of hydrocortisone for some patients, but the overall evidence of its efficacy is insufficient (50–54). Long-term treatment is associated with adverse effects, including Cushing’s syndrome, osteoporosis, extreme mood changes and seizures (55); therefore, hydrocortisone was not included in the CDC guidelines.
Treatment of CFS patients with traditional Chinese medicine
Due to limited treatment options, many CFS patients receive traditional medicine and alternative therapies, including traditional Chinese medicine (TCM) (56). The modern medical term “chronic fatigue syndrome” does not accurately correspond to the TCM literature. According to its clinical symptoms, “CFS” is often categorized as “asthenia” (57). The main characteristics of “fatigue” were first described during the Han Dynasty in one of the most important works of TCM: “Synopsis of the Golden Chamber” by Zhongjing Zhang (58). In summary, the etiology includes the following four aspects: ① damage to five internal organs (including qi, blood, yin, and yang) caused by exogenous invasion; ② fatigue (including physical, mental, and sexual fatigue); ③ emotional imbalance (happiness, anger, worry, obsessive thoughts, sadness, fear, and shock); ④ and improper diet (59). There are many methods used by TCM to treat CFS, including acupuncture, massage, cupping, and Chinese medicine, which can relieve pain and improve quality of life (58).
Although it is still not clear whether HPA axis dysfunction is the cause or an incidental phenomenon of CFS, the HPA axis is clearly a potentially important target for studying treatment strategies (35). Table 1 lists the 7 RCTs of TCM treatment methods that reported changes in HPA axis indicators (60–66). Six studies reported changes in CORT levels. The treatments included pestle needle therapy combined with electric acupuncture, Fuyang cupping, five-element gongdiao (positive mode) music combined with Lixujieyu decoction, acupoint sticking, Lixujieyu decoction, and electric acupuncture (60–62, 64–66). In a study of CFS patients treated with pestle needle therapy combined with electric acupuncture, the CORT level in patients was lower after treatment than before treatment (60). The remaining 5 studies reported that treatment effectively increased CORT levels (61, 62, 64–66) and increased the circadian secretion amplitude of saliva CORT and restored CORT peak phase that had tended to shift forward (66). Three studies reported changes in ACTH levels. The treatments included pestle needle therapy combined with electric acupuncture, scalp acupuncture, and acupoint sticking; all the reported treatment methods effectively increased ACTH levels (60, 63, 64). One study reported changes in MT, and treatment with electric acupuncture increased salivary MT levels and increased the variation in diurnal salivary MT secretion, restoring MT peak phases that had tended to shift backward (66). The occurrence of a CORT regulation exception in these clinical studies has sparked our thinking (60). A number of animal experiments have shown that CFS model rats exhibit hyperfunction of the HPA axis and that after TCM treatment, CORT levels significantly decrease (67–73). A study showed that when the body is subjected to a variety of noxious stimuli, HPA axis function increases, and the release of ACTH and CORT increases, thereby improving tolerance to stimulation and survival. When the regulatory ability is disrupted, HPA axis function is inhibited, adrenal function decreases, and the CORT concentration decreases (74, 75). Therefore, we speculate that the HPA axis has two disordered states, i.e., a hyperactive state and an inhibitory state, and that these states are related to the duration of stress. In most established animal models, the animals are in a state of HPA axis hyperactivity due to short modeling time. The patients in clinical studies are mostly chronically ill and more likely to be in an inhibitory state. In the absence of HPA axis inhibition, TCM treatment methods may not be limited to simply increasing or decreasing the level of a certain hormone but can resolve HPA axis dysfunction. That is, TCM therapeutic approaches have a bi-directional regulatory effect on HPA axis dysfunction in CFS. Although TCM treatment methods provide new possibilities for the treatment of CFS, as suggested by evidence-based research, the relevant RCT studies have deficiencies in terms of experimental design, random allocation concealment, blinding, and safety reporting, thus limiting the interpretation of evidence. Considering these limitations, it is necessary to conduct RCT studies with larger samples and more standardized treatments to provide evidence for the efficacy and safety of TCM treatment methods (76–78).
Summary and outlook
Given the negative impacts of CFS on individuals and society, there is an urgent need to elucidate the pathogenesis of CFS and develop safe and effective treatments. In this paper, we reviewed the changes in HPA axis function in CFS patients. Although controversial, the current mainstream research evidence still indicates that CFS patients have mild hypocortisolism, weakened diurnal variations in CORT, unresponsiveness of the HPA axis, and enhanced negative feedback from the HPA axis. We also discussed the association between HPA axis dysfunction and typical symptoms of CFS. Finally, the current treatment methods were reviewed. Due to the lack of modern medical treatments approved for CFS at this time, TCM treatment methods have the potential to become a new treatment strategy; in particular, TCM may have a two-way regulatory effect and resolve HPA axis dysfunction. However, due to limited evidence, it is necessary to conduct RCTs with larger sample sizes and more standardized protocols to provide additional data.
Author contributions
YZ: Writing – original draft, Writing – review & editing. LW: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Military TCM service capability enhancement project (2023ZY003).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Holmes GP, Kaplan JE, Gantz NM, Komaroff AL, Schonberger LB, Straus SE, et al. Chronic fatigue syndrome: a working case definition. Ann Internal Med. (1988) 108:387–9. doi: 10.7326/0003-4819-108-3-387
2. Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Internal Med. (1994) 121:953–9. doi: 10.7326/0003-4819-121-12-199412150-00009
3. Reeves WC, Lloyd A, Vernon SD, Klimas N, Jason LA, Bleijenberg G, et al. Identification of ambiguities in the 1994 chronic fatigue syndrome research case definition and recommendations for resolution. BMC Health Serv Res. (2003) 3:25. doi: 10.1186/1472-6963-3-25
4. Clayton EW. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: an IOM report on redefining an illness. Jama. (2015) 313:1101–2. doi: 10.1001/jama.2015.1346
5. Wadman M. For chronic fatigue syndrome, a ‘shifting tide’ at NIH. Sci (New York NY). (2016) 354:691–2. doi: 10.1126/science.354.6313.691
6. Maxmen A. A reboot for chronic fatigue syndrome research. Nature. (2018) 553:14–7. doi: 10.1038/d41586-017-08965-0
7. Gil A, Hoag GE, Salerno JP, Hornig M, Klimas N, Selin LK. Identification of CD8 T-cell dysfunction associated with symptoms in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and Long COVID and treatment with a nebulized antioxidant/anti-pathogen agent in a retrospective case series. Brain behavior Immun - Health. (2024) 36:100720. doi: 10.1016/j.bbih.2023.100720
8. Liu X, Liu S, Ren R, Wang X, Han C, Liu Z. A cross-sectional study exploring the relationship between symptoms of anxiety/depression and P50 sensory gating in adult patients diagnosed with chronic fatigue syndrome/myalgic encephalomyelitis. Front Neurosci. (2023) 17:1286340. doi: 10.3389/fnins.2023.1286340
9. Demitrack MA, Dale JK, Straus SE, Laue L, Listwak SJ, Kruesi MJ, et al. Evidence for impaired activation of the hypothalamic-pituitary-adrenal axis in patients with chronic fatigue syndrome. J Clin Endocrinol Metab. (1991) 73:1224–34. doi: 10.1210/jcem-73-6-1224
10. Holloway AL, Lerner TN. Hidden variables in stress neurobiology research. Trends Neurosci. (2024) 47:9–17. doi: 10.1016/j.tins.2023.10.006
11. Poteliakhoff A. Adrenocortical activity and some clinical findings in acute and chronic fatigue. J psychosomatic Res. (1981) 25:91–5. doi: 10.1016/0022-3999(81)90095-7
12. Scott LV, Dinan TG. Urinary free cortisol excretion in chronic fatigue syndrome, major depression and in healthy volunteers. J Affect Disord. (1998) 47:49–54. doi: 10.1016/s0165-0327(97)00101-8
13. Young AH, Sharpe M, Clements A, Dowling B, Hawton KE, Cowen PJ. Basal activity of the hypothalamic-pituitary-adrenal axis in patients with the chronic fatigue syndrome (neurasthenia). Biol Psychiatry. (1998) 43:236–7. doi: 10.1016/s0006-3223(97)00404-6
14. Cevik R, Gur A, Acar S, Nas K, Sarac AJ. Hypothalamic-pituitary-gonadal axis hormones and cortisol in both menstrual phases of women with chronic fatigue syndrome and effect of depressive mood on these hormones. BMC musculoskeletal Disord. (2004) 5:47. doi: 10.1186/1471-2474-5-47
15. MacHale SM, Cavanagh JT, Bennie J, Carroll S, Goodwin GM, Lawrie SM. Diurnal variation of adrenocortical activity in chronic fatigue syndrome. Neuropsychobiology. (1998) 38:213–7. doi: 10.1159/000026543
16. Papadopoulos AS, Cleare AJ. Hypothalamic-pituitary-adrenal axis dysfunction in chronic fatigue syndrome. Nat Rev Endocrinol. (2011) 8:22–32. doi: 10.1038/nrendo.2011.153
17. Turan T, Izgi HB, Ozsoy S, Tanrıverdi F, Basturk M, Asdemir A, et al. The effects of galantamine hydrobromide treatment on dehydroepiandrosterone sulfate and cortisol levels in patients with chronic fatigue syndrome. Psychiatry Invest. (2009) 6:204–10. doi: 10.4306/pi.2009.6.3.204
18. Gur A, Cevik R, Nas K, Colpan L, Sarac S. Cortisol and hypothalamic-pituitary-gonadal axis hormones in follicular-phase women with fibromyalgia and chronic fatigue syndrome and effect of depressive symptoms on these hormones. Arthritis Res Ther. (2004) 6:R232–8. doi: 10.1186/ar1163
19. Jerjes WK, Cleare AJ, Wessely S, Wood PJ, Taylor NF. Diurnal patterns of salivary cortisol and cortisone output in chronic fatigue syndrome. J Affect Disord. (2005) 87:299–304. doi: 10.1016/j.jad.2005.03.013
20. Nater UM, Youngblood LS, Jones JF, Unger ER, Miller AH, Reeves WC, et al. Alterations in diurnal salivary cortisol rhythm in a population-based sample of cases with chronic fatigue syndrome. Psychosomatic Med. (2008) 70:298–305. doi: 10.1097/PSY.0b013e3181651025
21. Di Giorgio A, Hudson M, Jerjes W, Cleare AJ. 24-hour pituitary and adrenal hormone profiles in chronic fatigue syndrome. Psychosomatic Med. (2005) 67:433–40. doi: 10.1097/01.psy.0000161206.55324.8a
22. Gaab J, Rohleder N, Heitz V, Engert V, SChad T, Schürmeyer TH, et al. Stress-induced changes in LPS-induced pro-inflammatory cytokine production in chronic fatigue syndrome. Psychoneuroendocrinology. (2005) 30:188–98. doi: 10.1016/j.psyneuen.2004.06.008
23. Heim C, Nater UM, Maloney E, Boneva R, Jones JF, Reeves WC. Childhood trauma and risk for chronic fatigue syndrome: association with neuroendocrine dysfunction. Arch Gen Psychiatry. (2009) 66:72–80. doi: 10.1001/archgenpsychiatry.2008.508
24. Nater UM, Maloney E, Boneva RS, Gurbaxani BM, Lin JM, Jones JF, et al. Attenuated morning salivary cortisol concentrations in a population-based study of persons with chronic fatigue syndrome and well controls. J Clin Endocrinol Metab. (2008) 93:703–9. doi: 10.1210/jc.2007-1747
25. Gaab J, Engert V, Heitz V, SChad T, Schürmeyer TH, Ehlert U. Associations between neuroendocrine responses to the Insulin Tolerance Test and patient characteristics in chronic fatigue syndrome. J psychosomatic Res. (2004) 56:419–24. doi: 10.1016/s0022-3999(03)00625-1
26. Gaab J, Hüster D, Peisen R, Engert V, Heitz V, SChad T, et al. Hypothalamic-pituitary-adrenal axis reactivity in chronic fatigue syndrome and health under psychological, physiological, and pharmacological stimulation. Psychosomatic Med. (2002) 64:951–62. doi: 10.1097/01.psy.0000038937.67401.61
27. Gaab J, Hüster D, Peisen R, Engert V, SChad T, Schürmeyer TH, et al. Low-dose dexamethasone suppression test in chronic fatigue syndrome and health. Psychosomatic Med. (2002) 64:311–8. doi: 10.1097/00006842-200203000-00015
28. Van Den Eede F, Moorkens G, Hulstijn W, Van Houdenhove B, Cosyns P, Sabbe BG, et al. Combined dexamethasone/corticotropin-releasing factor test in chronic fatigue syndrome. psychol Med. (2008) 38:963–73. doi: 10.1017/s0033291707001444
29. Jerjes WK, Taylor NF, Wood PJ, Cleare AJ. Enhanced feedback sensitivity to prednisolone in chronic fatigue syndrome. Psychoneuroendocrinology. (2007) 32:192–8. doi: 10.1016/j.psyneuen.2006.12.005
30. Inder WJ, Prickett TC, Mulder RT. Normal opioid tone and hypothalamic-pituitary-adrenal axis function in chronic fatigue syndrome despite marked functional impairment. Clin Endocrinol. (2005) 62:343–8. doi: 10.1111/j.1365-2265.2005.02220.x
31. Kempke S, Luyten P, De Coninck S, Van Houdenhove B, Mayes LC, Claes S. Effects of early childhood trauma on hypothalamic-pituitary-adrenal (HPA) axis function in patients with Chronic Fatigue Syndrome. Psychoneuroendocrinology. (2015) 52:14–21. doi: 10.1016/j.psyneuen.2014.10.027
32. Mommersteeg PM, Heijnen CJ, Verbraak MJ, van Doornen LJ. Clinical burnout is not reflected in the cortisol awakening response, the day-curve or the response to a low-dose dexamethasone suppression test. Psychoneuroendocrinology. (2006) 31:216–25. doi: 10.1016/j.psyneuen.2005.07.003
33. Van Den Eede F, Moorkens G, Van Houdenhove B, Cosyns P, Claes SJ. Hypothalamic-pituitary-adrenal axis function in chronic fatigue syndrome. Neuropsychobiology. (2007) 55:112–20. doi: 10.1159/000104468
34. Roberts AD, Papadopoulos AS, Wessely S, Chalder T, Cleare AJ. Salivary cortisol output before and after cognitive behavioural therapy for chronic fatigue syndrome. J Affect Disord. (2009) 115:280–6. doi: 10.1016/j.jad.2008.09.013
35. Tomas C, Newton J, Watson S. A review of hypothalamic-pituitary-adrenal axis function in chronic fatigue syndrome. ISRN Neurosci. (2013) 2013:784520. doi: 10.1155/2013/784520
36. Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. (2005) 30:1010–6. doi: 10.1016/j.psyneuen.2005.04.006
37. Raison CL, Miller AH. Malaise, melancholia and madness: the evolutionary legacy of an inflammatory bias. Brain behavior Immun. (2013) 31:1–8. doi: 10.1016/j.bbi.2013.04.009
38. Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. (2009) 27:229–65. doi: 10.1146/annurev.immunol.021908.132715
39. Van Houdenhove B, Luyten P. Customizing treatment of chronic fatigue syndrome and fibromyalgia: the role of perpetuating factors. Psychosomatics. (2008) 49:470–7. doi: 10.1176/appi.psy.49.6.470
40. Ling X, Zhimin L. Pineal melatonin and the hypothalamic-pituitary-adrenal axis. Shanghai Med J. (2009) 32:163–5.
41. Yihui Z, Fanrong L, Cisong C, Xi W. The study on pathological rhythm characteristics of chronic fatigue syndrome. Chin J Pract Internal Med. (2008) 28:444–6. doi: 10.3969/j.issn.1005-2194.2008.06.010
42. Treatment of ME/CFS. Centers for disease control and prevention(2021). Available online at: https://www.cdc.gov/me-cfs/treatment/.
43. Bateman L, Bested AC, Bonilla HF, Chheda BV, Chu L, Curtin JM, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: essentials of diagnosis and management. Mayo Clinic Proc. (2021) 96:2861–78. doi: 10.1016/j.mayocp.2021.07.004
44. White PD, Goldsmith KA, Johnson AL, Potts L, Walwyn R, DeCesare JC, et al. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): a randomised trial. Lancet (London England). (2011) 377:823–36. doi: 10.1016/s0140-6736(11)60096-2
45. White PD, Goldsmith K, Johnson AL, Chalder T, Sharpe M. Recovery from chronic fatigue syndrome after treatments given in the PACE trial. psychol Med. (2013) 43:2227–35. doi: 10.1017/s0033291713000020
46. Wilshire CE, Kindlon T, Courtney R, Matthees A, Tuller D, Geraghty K, et al. Rethinking the treatment of chronic fatigue syndrome-a reanalysis and evaluation of findings from a recent major trial of graded exercise and CBT. BMC Psychol. (2018) 6:6. doi: 10.1186/s40359-018-0218-3
47. Geraghty K, Hann M, Kurtev S. Myalgic encephalomyelitis/chronic fatigue syndrome patients’ reports of symptom changes following cognitive behavioural therapy, graded exercise therapy and pacing treatments: Analysis of a primary survey compared with secondary surveys. J Health Psychol. (2019) 24:1318–33. doi: 10.1177/1359105317726152
48. Gaunt DM, Brigden A, Harris SRS, Hollingworth W, Jago R, Solomon-Moore E, et al. Graded exercise therapy compared to activity management for paediatric chronic fatigue syndrome/myalgic encephalomyelitis: pragmatic randomized controlled trial. Eur J Pediatr. (2024). doi: 10.1007/s00431-024-05458-x
49. van der Schaaf ME, Geerligs L, Toni I, Knoop H, Oosterman JM. Disentangling pain and fatigue in chronic fatigue syndrome: a resting state connectivity study before and after cognitive behavioral therapy. psychol Med. (2024), 1–14. doi: 10.1017/s0033291723003690
50. McKenzie R, O’Fallon A, Dale J, Demitrack M, Sharma G, Deloria M, et al. Low-dose hydrocortisone for treatment of chronic fatigue syndrome: a randomized controlled trial. Jama. (1998) 280:1061–6. doi: 10.1001/jama.280.12.1061
51. Cleare AJ, Heap E, Malhi GS, Wessely S, O’Keane V, Miell J. Low-dose hydrocortisone in chronic fatigue syndrome: a randomised crossover trial. Lancet (London England). (1999) 353:455–8. doi: 10.1016/s0140-6736(98)04074-4
52. Blockmans D, Persoons P, Van Houdenhove B, Lejeune M, Bobbaers H. Combination therapy with hydrocortisone and fludrocortisone does not improve symptoms in chronic fatigue syndrome: a randomized, placebo-controlled, double-blind, crossover study. Am J Med. (2003) 114:736–41. doi: 10.1016/s0002-9343(03)00182-7
53. Smith ME, Haney E, McDonagh M, Pappas M, Daeges M, Wasson N, et al. Treatment of myalgic encephalomyelitis/chronic fatigue syndrome: A systematic review for a national institutes of health pathways to prevention workshop. Ann Internal Med. (2015) 162:841–50. doi: 10.7326/m15-0114
54. Whiting P, Bagnall AM, Sowden AJ, Cornell JE, Mulrow CD, Ramírez G. Interventions for the treatment and management of chronic fatigue syndrome: a systematic review. Jama. (2001) 286:1360–8. doi: 10.1001/jama.286.11.1360
55. Buchman AL. Side effects of corticosteroid therapy. J Clin Gastroenterol. (2001) 33:289–94. doi: 10.1097/00004836-200110000-00006
56. Li Y, Yang J, Chau CI, Shi J, Chen X, Hu H, et al. Is there a role for traditional and complementary medicines in managing chronic fatigue? a systematic review of randomized controlled trials. Front Pharmacol. (2023) 14:1266803. doi: 10.3389/fphar.2023.1266803
57. Zhaohui Z, Lihua L. New progress of traditional Chinese medicine in the treatment of chronic fatigue syndrome. World Chin Med. (2021) 16:991–5. doi: 10.3969/j.issn.1673-7202.2021.06.026
58. Yan Y, Yidan W, Dongdong Z, Lin F. Research progress of chronic fatigue syndrome treated by Chinese and western medicine. Inf Traditional Chin Med. (2019) 36:122–5. doi: 10.19656/j.cnki.1002-2406.190128
59. Chen R, Moriya J, Yamakawa J, Takahashi T, Kanda T. Traditional chinese medicine for chronic fatigue syndrome. Evidence-Based complementary Altern medicine: eCAM. (2010) 7:3–10. doi: 10.1093/ecam/nen017
60. Luoji L, Quan L, Anmin H, Honglian P, Fengmei L. Discussion of the effect of Chuzhen combined with electroacupuncture on patients with chronic fatigue syndrome of Yang deficiency based on Fuyang theory. China’s Naturopathy. (2023) 31:35–9. doi: 10.19621/j.cnki.11-3555/r.2023.2111
61. Tian L, Xiaochen Z, Chenchen Z, Congcong W, Qing L, Tielang L. Effect of fuyang cupping on beishu functional belt on clinical efficacy and cortisol in patients with chronic fatigue syndrome. Guiding J Traditional Chin Med Pharmacol. (2023) 29:94–8. doi: 10.13862/j.cn43-1446/r.2023.05.020
62. Zhixing C, Zhenxian Z, Lili W, Yuanshu Y. Clinical observation of five-element gongdiao (positive mode) music combined with Lixujieyu decoction in the treatment of chronic fatigue syndrome. Acta Universitatis Traditionis Medicalis Sinensis Pharmacologiaeque Shanghai. (2015) 29:18–22. doi: 10.16306/j.1008-861x.2015.03.006
63. Chunfeng G, Zhongren S. Clinical observation of scalp acupuncture in the treatment of chronic fatigue syndrome. Acta Chin Med Pharmacol. (2014) 42:65–6. doi: 10.19664/j.cnki.1002-2392.2014.02.023
64. Xinghua C, Haiqiong C, Limei Z, Yang W. Effect of acupoint sticking on chronic fatigue syndrome and endocrine function. Shanghai J Acupuncture Moxibustion. (2014) 33:618–20. doi: 10.13460/j.issn.1005-0957.2014.07.0618
65. Ye Z, Zhenxian Z, Min C, Lili W, Yang W. Effect of deficiency-regulating and depression-relieving formula on 5-HT and cortisol in chronic fatigue syndrome. Liaoning J Traditional Chin Med. (2012) 39:1295–6. doi: 10.13192/j.ljtcm.2012.07.102.zhangy.050
66. Yihui Z, Fangrong L, Xi W, Cisong C. Mechanism of electric acupuncture on saliva CS and MT circadian rhythm in chronic fatigue syndrome. Shandong Med J. (2007) 47:18–20. doi: 10.3969/j.issn.1002-266X.2007.28.007
67. Mingzhu P, Jian L, Bing R, Jun J, Huanan L. Effect of abdominal manipulation on remodeling of hippocampal neurons in chronic stress-induced chronic fatigue syndrome and its mechanism of negative feedback regulation of hippocampus-HPA axis. J Jilin Univ (Medicine Edition). (2021) 47:842–8. doi: 10.13481/j.1671-587X.20210404
68. Peng Z, Jianping Y, Xiaoqian J, Shiyun C, Jingjing L, Liansha H, et al. Effect of “Beishu points” Electroacupuncture stimulation on CRHmRNA, ACTH and CORT in rats with chronic fatigue sydome. Guiding J Traditional Chin Med Pharmacol. (2018) 24:32–5 + 41. doi: 10.13862/j.cnki.cn43-1446/r.2018.12.009
69. Xiaohong L, Bin Y, Xiaojun W, Mingyan X, Fanchao Z, Liuting W, et al. Effects of Xiaoyao powder on HPA axis, IL-13 and IL-17 in rats with chronic restraint stress of liver stagnation and spleen deficiency syndrome. Lishizhen Med Materia Med Res. (2017) 28:1815–6. doi: 10.3969/j.issn.1008-0805.2017.08.008
70. Yi T, Qi L, Li J, Le JJ, Shao L, Du X, et al. Moxibustion upregulates hippocampal progranulin expression. Neural regeneration Res. (2016) 11:610–6. doi: 10.4103/1673-5374.180746
71. Li Z, Ganghui J. Effects of moxibustion on behavior and hormone levels of hypothalamus-pituitary-adrenergic axis of the model rat with chronic fatigue syndrome. Shandong J Traditional Chin Med. (2014) 33:301–3. doi: 10.16295/j.cnki.0257-358x.2014.04.023
72. Hangzhou L, Mingzhuang L. The effect of acupoint catgut embedding on behavior and hormone levels of hypothalamic-pituitary-adrenocortical axis in rats with chronic fatigue. J Guiyang Coll Traditional Chin Med. (2009) 31:62–5. doi: 10.16588/j.cnki.issn1002-1108.2009.06.016
73. Yunfei C, Wenjia Y, Shengguang F, Xiaodan Z. Experimental study on hormones of HPA-axis with electro-acupuncture by CFS model rats. Chin Arch Traditional Chin Med. (2007) 25:1146–9. doi: 10.13193/j.archtcm.2007.06.59.chenyf.023
74. Xinghua C, Juan Y, Wei S, Chunzhi T. Effects of acupuncture on blood adrenocorticotrophic hormone and cortisol in chronic fatigue syndrome. Chin J Integr Med Cardio-/Cerebrovascuiar Dis. (2011) 9:1320–2. doi: 10.3969/j.issn.1672-1349.2011.11.024
75. Yanhui L, Qiaolin M, Bin H, Zhonglei W. Current state about researches on selection of experimental indexs mechanisms of acupuncture underlying improvement of chronic fatigue syndrome. Acupuncture Res. (2021) 46:980–4. doi: 10.13702/j.1000-0607.200998
76. Fang Y, Yue BW, Ma HB, Yuan YP. Acupuncture and moxibustion for chronic fatigue syndrome: A systematic review and network meta-analysis. Medicine. (2022) 101:e29310. doi: 10.1097/md.0000000000029310
77. Zhang Q, Gong J, Dong H, Xu S, Wang W, Huang G. Acupuncture for chronic fatigue syndrome: a systematic review and meta-analysis. Acupuncture medicine: J Br Med Acupuncture Soc. (2019) 37:211–22. doi: 10.1136/acupmed-2017-011582
Keywords: chronic fatigue syndrome, hypothalamus-pituitary-adrenal axis, dysfunction, modern medicine, traditional Chinese medicine
Citation: Zhang Y-D and Wang L-N (2024) Research progress in the treatment of chronic fatigue syndrome through interventions targeting the hypothalamus-pituitary-adrenal axis. Front. Endocrinol. 15:1373748. doi: 10.3389/fendo.2024.1373748
Received: 20 January 2024; Accepted: 28 March 2024;
Published: 10 April 2024.
Edited by:
Ben Nephew, Worcester Polytechnic Institute, United StatesReviewed by:
Pawel Zalewski, Nicolaus Copernicus University in Toruń, PolandCopyright © 2024 Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li-Na Wang, rena1022@163.com
 Yi-Dan Zhang
Yi-Dan Zhang Li-Na Wang2*
Li-Na Wang2*