- 1Department of Respiratory and Critical Care Medicine, Dianjiang People’s Hospital of Chongqing, Chongqing, China
- 2Department of Pharmacy, Dianjiang People’s Hospital of Chongqing, Chongqing, China
Background
Due to the cardiovascular and renal benefits of sodium-glucose cotransporter-2 inhibitors (SGLT2is) in patients with type 2 diabetes (T2D), those with heart failure (HF), and those with chronic kidney disease (CKD), SGLT2is have been recommended in these populations to prevent cardiorenal events (1–3). Two recent reviews (4, 5) mainly summarizing the results of mechanistic studies have reported that, apart from exerting the glucose-lowering and cardiorenal protection effects, SGLT2is can also decrease oxidative stress and endoplasmic reticulum stress, diminish proinflammatory and profibrotic pathways, stimulate mitochondrial biogenesis, restore mitochondrial health, and regulate the mTOR pathway. The aforementioned biological activities of SGLT2is seem to suggest the possibility that this drug class could be used for the prevention/treatment of other diseases except T2D, HF, and CKD. A comprehensive meta-analysis conducted by Qiu et al. (6), including a total of nine large-scale randomized controlled trials (RCTs) of SGLT2is, has evaluated 1,009 safety outcomes from 24 different body systems and, accordingly, has revealed some novel potentials of SGLT2is, such as the possible benefits of SGLT2is against some respiratory diseases including chronic obstructive pulmonary disease (COPD), asthma, and pneumonia. Nowadays, a great number of novel large-scale RCTs of SGLT2is are available, such as NCT03594110 [EMPA-KIDNEY] (7), NCT03619213 [DELIVER] (8), NCT03057951 [EMPEROR-Preserved] (9), NCT03521934 [SOLOIST-WHF] (10), and NCT03315143 [SCORED] (11). Therefore, we have carried out an updated meta-analysis to confirm the findings of Qiu et al. and examine the possibility of the novel applications of SGLT2is.
Methods
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (12). PubMed and ClinicalTrials.gov were searched to identify relevant papers published before September 8, 2023. We searched PubMed using the following search strategy: (Sodium-Glucose Transporter 2 Inhibitors[Mesh] OR “Sodium glucose transporter 2 inhibitor*”[TIAB] OR “Sodium glucose cotransporter 2 inhibitor*”[TIAB] OR “Sodium glucose co-transporter 2 inhibitor*”[TIAB] OR SGLT2*[TIAB] OR Gliflozin*[tiab] OR “Empagliflozin”[tiab] OR “Empagliflozin”[Supplementary Concept] OR “Dapagliflozin”[tiab] OR “Dapagliflozin”[Supplementary Concept] OR “Canagliflozin”[Mesh] OR “Canagliflozin”[tiab] OR “ertugliflozin”[tiab] OR “ertugliflozin”[Supplementary Concept] OR “sotagliflozin”[tiab] OR “LX4211”[tiab]) AND ((randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR randomly [tiab] OR drug therapy [sh] OR placebo [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh])).
In this meta-analysis, we included those RCTs that enrolled >500 subjects, compared SGLT2is with placebo or a non-SGLT2is drug, and reported various serious adverse events (SAEs) regarding various body systems. The outcomes of interest were the various SAEs which included trials reported at the website of ClinicalTrials.gov as long as they were reported by at least four of the included trials. We collected all of the outcome data from ClinicalTrials.gov. We conducted meta-analyses on eligible outcomes using a random-effects model (when I2 ≥50%) or a fixed-effects model (when I2 <50%). Risk ratio (RR) with its 95% confidence interval (CI) was regarded as effect size. P <0.05 was considered as statistically significant. All statistical analyses were done using the Stata/MP 16.0 software.
Results
As shown in Supplementary Figure S1 (flow diagram), we included a total of 26 papers reporting 27 large RCTs in total. These included trials that had their only ClinicalTrials.gov Identifiers: NCT03594110 [EMPA-KIDNEY] (7), NCT03619213 [DELIVER] (8), NCT03057951 [EMPEROR-Preserved] (9), NCT03521934 [SOLOIST-WHF] (10), NCT03315143 [SCORED] (11), NCT03242252 (13), NCT02033889 (14), NCT04157751 (15), NCT02384941 (16), NCT01042977 (17), NCT01031680 (18), NCT01164501 (19), NCT01106677 (20), NCT01177813 (21), NCT01106651 (22), NCT00528879 (23), NCT04350593 (24), NCT00673231 (25), NCT02065791 (26), NCT01032629 [CANVAS] (27), NCT01989754 [CANVAS-R] (27), NCT03057977 (28), NCT03036150 (29), NCT01986881 (30), NCT01131676 (31), NCT03036124 (32), and NCT01730534 (33). The detailed characteristics of the included 27 trials are provided in Supplementary Table S1, and these trials involved 100,995 subjects in total. We performed meta-analyses on a total of 1,080 kinds of diseases deriving from 25 kinds of body systems (Supplementary Table S2): blood and lymphatic system disorders (involving 17 diseases), cardiac disorders (involving 79 diseases), congenital, familial, and genetic disorders (involving two diseases), ear and labyrinth disorders (involving nine diseases), endocrine disorders (involving six diseases), eye disorders (involving 20 diseases), gastrointestinal disorders (involving 104 diseases), general disorders (involving 31 diseases), hepatobiliary disorders (involving 27 diseases), immune system disorders (involving four diseases), infections and infestations (involving 158 diseases), injury, poisoning, and procedural complications (involving 102 diseases), investigations (involving 16 diseases), metabolism and nutrition disorders (involving 32 diseases), musculoskeletal and connective tissue disorders (involving 51 diseases), benign, malignant, and unspecified neoplasms (involving 131 diseases), nervous system disorders (involving 100 diseases), product issues (involving five diseases), psychiatric disorders (involving 12 diseases), renal and urinary disorders (involving 39 diseases), reproductive system and breast disorders (involving 15 diseases), respiratory, thoracic, and mediastinal disorders (involving 47 diseases), skin and subcutaneous tissue disorders (involving 15 diseases), surgical and medical procedures (involving four diseases), and vascular disorders (involving 54 diseases).
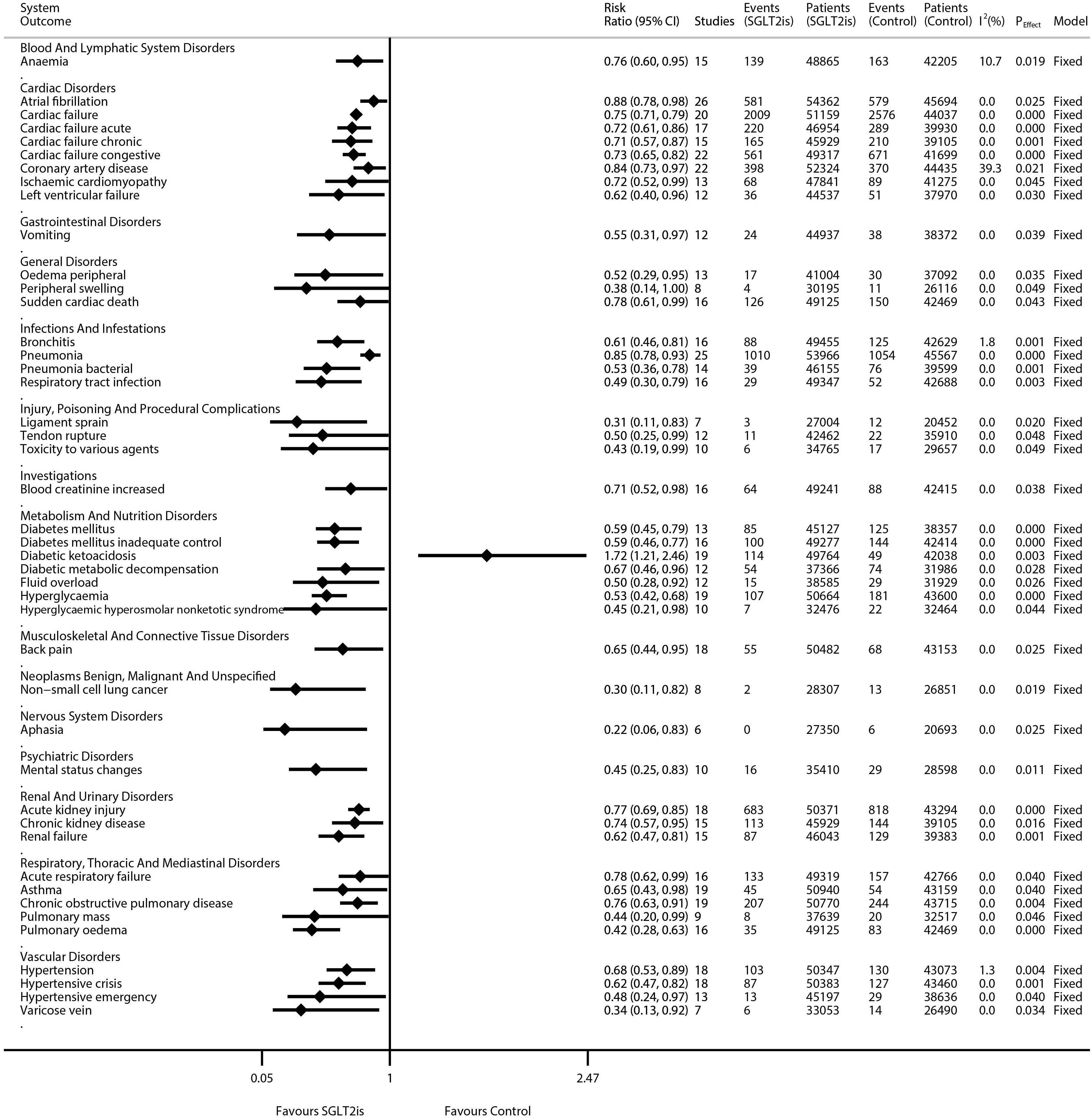
The results of the meta-analyses on 1,080 kinds of diseases are detailed in Supplementary Figure S2 (forest plots) and are summarized in Supplementary Table S2. Moreover, the statistically significant results were extracted and collated into Figure 1. SGLT2is were significantly associated with the decreased risks of eight kinds of cardiac disorders [e.g., atrial fibrillation (RR 0.88, 95% CI 0.78–0.98; P = 0.025; N of studies = 26; I2 = 0.0%), cardiac failure (RR 0.75, 95% CI 0.71–0.79; P = 0.000; N of studies = 20; I2 = 0.0%), and coronary artery disease (RR 0.84, 95% CI 0.73–0.97; P = 0.021; N of studies = 22; I2 = 39.3%)], four kinds of vascular disorders [e.g., hypertension (RR 0.68, 95% CI 0.53–0.89; P = 0.004; N of studies = 18; I2 = 1.3%), hypertensive crisis (RR 0.62, 95% CI 0.47–0.82; P = 0.001; N of studies = 18; I2 = 0.0%), and hypertensive emergency (RR 0.48, 95% CI 0.24–0.97; P = 0.040; N of studies = 13; I2 = 0.0%)], three kinds of renal disorders [e.g., acute kidney injury (RR 0.77, 95% CI 0.69–0.85; P = 0.000; N of studies = 18; I2 = 0.0%) and CKD (RR 0.74, 95% CI 0.57–0.95; P = 0.016; N of studies = 15; I2 = 0.0%)], and six kinds of metabolism disorders [e.g., diabetes mellitus (RR 0.59, 95% CI 0.45–0.79; P = 0.000; N of studies = 13; I2 = 0.0%), hyperglycemia (RR 0.53, 95% CI 0.42–0.68; P = 0.000; N of studies = 19; I2 = 0.0%), and hyperglycemic hyperosmolar nonketotic syndrome (RR 0.45, 95% CI 0.21–0.98; P = 0.044; N of studies = 10; I2 = 0.0%)]. SGLT2is were significantly associated with the decreased risks of five kinds of respiratory disorders [e.g., acute respiratory failure (RR 0.78, 95% CI 0.62–0.99; P = 0.040; N of studies = 16; I2 = 0.0%), asthma (RR 0.65, 95% CI 0.43–0.98; P = 0.040; N of studies = 19; I2 = 0.0%), and COPD (RR 0.76, 95% CI 0.63–0.91; P = 0.004; N of studies = 19; I2 = 0.0%)], and four kinds of infectious disorders [e.g., bronchitis (RR 0.61, 95% CI 0.46–0.81; P = 0.001; N of studies = 16; I2 = 1.8%) and pneumonia (RR 0.85, 95% CI 0.78–0.93; P = 0.000; N of studies = 25; I2 = 0.0%)]. SGLT2is were significantly associated with decreased risks of non-small cell lung cancer (NSCLC) (RR 0.30, 95% CI 0.11–0.82; P = 0.019; N of studies = 8; I2 = 0.0%), aphasia (RR 0.22, 95% CI 0.06–0.83; P = 0.025; N of studies = 6; I2 = 0.0%), mental status changes (RR 0.45, 95% CI 0.25–0.83; P = 0.011; N of studies = 10; I2 = 0.0%), anemia (RR 0.76, 95% CI 0.60–0.95; P = 0.019; N of studies = 15; I2 = 10.7%), and back pain (RR 0.65, 95% CI 0.44–0.95; P = 0.025; N of studies = 18; I2 = 0.0%). SGLT2is were significantly associated with an increased risk of diabetic ketoacidosis (RR 1.72, 95% CI 1.21–2.46; P = 0.003; N of studies = 19; I2 = 0.0%). Moreover, SGLT2is were not significantly associated with the incidences of 1,036 kinds of diseases, e.g., intestinal obstruction (RR 0.91, 95% CI 0.53–1.54; P = 0.716; N of studies = 17; I2 = 0.0%), acute pancreatitis (RR 0.92, 95% CI 0.63–1.34; P = 0.676; N of studies = 17; I2 = 0.0%), gastrointestinal hemorrhage (RR 0.88, 95% CI 0.67–1.16; P = 0.376; N of studies = 16; I2 = 0.0%), upper gastrointestinal hemorrhage (RR 0.75, 95% CI 0.50–1.12; P = 0.161; N of studies = 14; I2 = 0.5%), ischemic colitis (RR 1.11, 95% CI 0.53–2.36; P = 0.777; N of studies = 13; I2 = 0.0%), and gastric ulcer (RR 0.95, 95% CI 0.57–1.61; P = 0.859; N of studies = 13; I2 = 0%).
Discussion
Up to now, this is the most comprehensive meta-analysis that assessed the safety outcomes of SGLT2is, given assessing 1,080 kinds of SAEs deriving from 25 kinds of body systems in total. Accordingly, we produced several key findings as follows: First, SGLT2is were significantly associated with the decreased risks of several kinds of cardiovascular disorders (e.g., cardiac failure, atrial fibrillation, and hypertensive crisis), renal disorders (e.g., acute kidney injury, and CKD), and metabolism disorders (e.g., diabetes mellitus, and hyperglycemia), but with an increased risk of diabetic ketoacidosis. These findings again confirm the known characteristics of SGLT2is in such a way that SGLT2is can exert the protective effects against relevant cardiorenal diseases and T2D but lead to common adverse effects, i.e., diabetic ketoacidosis. Second, SGLT2is were significantly associated with the decreased risks of several kinds of respiratory disorders (e.g., acute respiratory failure, asthma, and COPD), and infectious disorders (e.g., bronchitis and pneumonia). Similarly, a cohort study (34) showed that SGLT2is had a significant association with the lower risks of adverse respiratory events including respiratory failure, pulmonary edema, and pneumonia. These findings may suggest that SGLT2is could be used for the prevention of relevant respiratory and infectious diseases. Third, SGLT2is were significantly associated with a lower incidence of NSCLC. Similarly, two recent studies (35, 36) showed the benefits of SGLT2is against NSCLC. These may suggest a new application area of SGLT2is: as an adjuvant drug for the treatment of NSCLC. Fourth, our meta-analysis identified that SGLT2is were associated with lower incidences of aphasia and mental status changes. Meanwhile, Liebers et al. proposed the hypothesis that ketogenesis induced by SGLT2is could be an effective treatment for depressive disorders (37). Fifth, our meta-analysis identified that SGLT2is were associated with a lower incidence of anemia. Meanwhile, a newly published article (38) illustrated the possible mechanisms for the alleviation of anemia by SGLT2is. Sixth, our meta-analysis identified that SGLT2is were associated with a lower incidence of back pain, which may be due to the anti-inflammation and anti-oxidative stress activities of SGLT2is. Lastly, in this meta-analysis SGLT2is were not observed to have an association with the incidences of 1,036 kinds of diseases including various gastrointestinal diseases (e.g., intestinal obstruction, pancreatitis acute, and upper gastrointestinal hemorrhage). These findings suggest that SGLT2is are generally safe for various body systems, including the gastrointestinal system.
Compared with the meta-analysis of Qiu et al. (6), including nine trials involving a total of 33,124 subjects, our meta-analysis included more trials (27 trials) and involved more subjects (100,995 subjects). This suggested that our meta-analysis had a more sufficient statistical power to produce more credible findings. Moreover, Qiu et al. performed analyses only on the outcomes that were reported by at least three of the included trials, whereas we performed analyses only on the outcomes that were reported by at least four of the included trials. This suggested that we further reduced the possibility of the bias of meta-analysis results deriving from the limited number of included studies. On the contrary, this meta-analysis has two main weaknesses. First, just like the meta-analysis of Qiu et al. (6), our meta-analysis also evaluated a great number of outcomes, and therefore it was likely to lead to false positive findings. Second, the outcomes we performed analyses on in this study were the various SAEs reported by the included trials, not the primary outcomes of the included trials. Therefore, our findings in this study should be considered as exploratory but not confirmatory, and further verification is needed.
In conclusion, our meta-analysis confirms the benefits of SGLT2is against relevant cardiorenal diseases and T2D again, reveals the general safety of SGLT2is for various body systems including the gastrointestinal system, and, more importantly, suggests some novel potentials of SGLT2is: for the prevention/treatment of relevant respiratory and infectious diseases, NSCLC, depressive disorders, anemia, and back pain.
Author contributions
YC: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. HZ: Formal analysis, Writing – original draft, Writing – review & editing. RH: Formal analysis, Writing – original draft, Writing – review & editing. HC: Data curation, Writing – original draft, Writing – review & editing. GY: Data curation, Writing – original draft, Writing – review & editing. LG: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1376446/full#supplementary-material
Supplementary Table 2 | Meta-analyses of SGLT2is and 1,080 kinds of diseases.
Abbreviations
SGLT2is, sodium-glucose cotransporter 2 inhibitors; T2D, type 2 diabetes; HF, heart failure; CKD, chronic kidney disease; RCTs, randomized controlled trials; COPD, chronic obstructive pulmonary disease; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses; SAEs, serious adverse events; RR, risk ratio; CI, confidence interval; NSCLC, non-small cell lung cancer.
References
1. Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the american diabetes association (ada) and the european association for the study of diabetes (easd). DIABETOLOGIA. (2022) 65:1925–66. doi: 10.1007/s00125-022-05787-2
2. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 aha/acc/hfsa guideline for the management of heart failure: a report of the american college of cardiology/american heart association joint committee on clinical practice guidelines. CIRCULATION. (2022) 145:e895–e1032. doi: 10.1161/CIR.0000000000001063
3. Navaneethan SD, Zoungas S, Caramori ML, Chan J, Heerspink H, Hurst C, et al. Diabetes management in chronic kidney disease: synopsis of the kdigo 2022 clinical practice guideline update. Ann Intern Med. (2023) 176:381–7. doi: 10.7326/M22-2904
4. O'Keefe JH, Weidling R, O'Keefe EL, Franco WG. Sglt inhibitors for improving healthspan and lifespan. Prog Cardiovasc Dis. 81:2–9. doi: 10.1016/j.pcad.2023.10.003
5. Youssef ME, Yahya G, Popoviciu MS, Cavalu S, Abd-Eldayem MA, Saber S. Unlocking the full potential of sglt2 inhibitors: expanding applications beyond glycemic control. Int J Mol Sci. (2023) 24:6039. doi: 10.3390/ijms24076039
6. Qiu M, Zhao LM, Zhan ZL. Comprehensive analysis of adverse events associated with sglt2is: a meta-analysis involving nine large randomized trials. Front Endocrinol. (2021) 12:743807. doi: 10.3389/fendo.2021.743807
7. Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, Emberson JR, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. (2023) 388:117–27. doi: 10.1056/NEJMoa2204233
8. Solomon SD, McMurray J, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. (2022) 387:1089–98. doi: 10.1056/NEJMoa2206286
9. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. (2021) 385:1451–61. doi: 10.1056/NEJMoa2107038
10. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. (2021) 384:117–28. doi: 10.1056/NEJMoa2030183
11. Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. (2021) 384:129–39. doi: 10.1056/NEJMoa2030186
12. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
13. Cherney D, Ferrannini E, Umpierrez GE, Peters AL, Rosenstock J, Powell DR, et al. Efficacy and safety of sotagliflozin in patients with type 2 diabetes and stage 3 chronic kidney disease. Diabetes Obes Metab. (2023) 25:1646–57. doi: 10.1111/dom.15019
14. Gallo S, Charbonnel B, Goldman A, Shi H, Huyck S, Darekar A, et al. Long-term efficacy and safety of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy: 104-week vertis met trial. Diabetes Obes Metab. (2019) 21:1027–36. doi: 10.1111/dom.13631
15. Voors AA, Angermann CE, Teerlink JR, Collins SP, Kosiborod M, Biegus J, et al. The sglt2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. (2022) 28:568–74. doi: 10.1038/s41591-021-01659-1
16. Buse JB, Garg SK, Rosenstock J, Bailey TS, Banks P, Bode BW, et al. Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: the north american intandem1 study. Diabetes Care. (2018) 41:1970–80. doi: 10.2337/dc18-0343
17. Leiter LA, Cefalu WT, de Bruin TW, Xu J, Parikh S, Johnsson E, et al. Long-term maintenance of efficacy of dapagliflozin in patients with type 2 diabetes mellitus and cardiovascular disease. Diabetes Obes Metab. (2016) 18:766–74. doi: 10.1111/dom.12666
18. Cefalu WT, Leiter LA, de Bruin TW, Gause-Nilsson I, Sugg J, Parikh SJ. Dapagliflozin's effects on glycemia and cardiovascular risk factors in high-risk patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. Diabetes Care. (2015) 38:1218–27. doi: 10.2337/dc14-0315
19. Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes ENDO. (2014) 2:369–84. doi: 10.1016/S2213-8587(13)70208-0
20. Lavalle-González FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. DIABETOLOGIA. (2013) 56:2582–92. doi: 10.1007/s00125-013-3039-1
21. Roden M, Weng J, Eilbracht J, Delafont B, Kim G, Woerle HJ, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes ENDO. (2013) 1:208–19. doi: 10.1016/S2213-8587(13)70084-6
22. Januzzi JJ, Butler J, Jarolim P, Sattar N, Vijapurkar U, Desai M, et al. Effects of canagliflozin on cardiovascular biomarkers in older adults with type 2 diabetes. J Am Coll Cardiol. (2017) 70:704–12. doi: 10.1016/j.jacc.2017.06.016
23. Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. LANCET. (2010) 375:2223–33. doi: 10.1016/S0140-6736(10)60407-2
24. Kosiborod MN, Esterline R, Furtado R, Oscarsson J, Gasparyan SB, Koch GG, et al. Dapagliflozin in patients with cardiometabolic risk factors hospitalised with covid-19 (dare-19): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes ENDO. (2021) 9:586–94. doi: 10.1016/S2213-8587(21)00180-7
25. Wilding JP, Woo V, Soler NG, Pahor A, Sugg J, Rohwedder K, et al. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. (2012) 156:405–15. doi: 10.7326/0003-4819-156-6-201203200-00003
26. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink H, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. (2019) 380:2295–306. doi: 10.1056/NEJMoa1811744
27. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. (2017) 377:644–57. doi: 10.1056/NEJMoa1611925
28. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. (2020) 383:1413–24. doi: 10.1056/NEJMoa2022190
29. Heerspink H, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. (2020) 383:1436–46. doi: 10.1056/NEJMoa2024816
30. Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. New Engl J Med. (2020) 383:1425–35. doi: 10.1056/NEJMoa2004967
31. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. (2015) 373:2117–28. doi: 10.1056/NEJMoa1504720
32. McMurray J, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. (2019) 381:1995–2008. doi: 10.1056/NEJMoa1911303
33. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. (2019) 380:347–57. doi: 10.1056/NEJMoa1812389
34. Jeong HE, Park S, Noh Y, Bea S, Filion KB, Yu O, et al. Association of adverse respiratory events with sodium-glucose cotransporter 2 inhibitors versus dipeptidyl peptidase 4 inhibitors among patients with type 2 diabetes in South Korea: a nationwide cohort study. BMC Med. (2023) 21:47. doi: 10.1186/s12916-023-02765-2
35. Luo J, Hendryx M, Dong Y. Sodium-glucose cotransporter 2 (sglt2) inhibitors and non-small cell lung cancer survival. BRIT J CANCER. (2023) 128:1541–7. doi: 10.1038/s41416-023-02177-2
36. Biziotis OD, Tsakiridis EE, Ali A, Ahmadi E, Wu J, Wang S, et al. Canagliflozin mediates tumor suppression alone and in combination with radiotherapy in non-small cell lung cancer (nsclc) through inhibition of hif-1α. Mol Oncol. (2023) 17:2235–56. doi: 10.1002/1878-0261.13508
37. Liebers DT, Ebina W, Iosifescu DV. Sodium-glucose cotransporter-2 inhibitors in depression. HARVARD Rev PSYCHIAT. (2023) 31:214–21. doi: 10.1097/HRP.0000000000000374
Keywords: SGLT2is, respiratory diseases, NSCLC, anemia, back pain, gastrointestinal diseases
Citation: Cai Y, Zou H, Hu R, Chen H, Yang G and Gong L (2024) A comprehensive meta-analysis on safety outcomes reveals the novel potentials of SGLT2is, especially preventing respiratory diseases. Front. Endocrinol. 15:1376446. doi: 10.3389/fendo.2024.1376446
Received: 25 January 2024; Accepted: 15 April 2024;
Published: 29 April 2024.
Edited by:
Bert B. Little, University of Louisville, United StatesReviewed by:
Ichiro Horie, Nagasaki University Hospital, JapanCopyright © 2024 Cai, Zou, Hu, Chen, Yang and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: LiDan Gong, lidan20gong20@yeah.net
 YuJun Cai1
YuJun Cai1 HaiTao Zou
HaiTao Zou